
The dorsal shell wall structure of Mesozoic ammonoids
GREGOR RADTKE and HELMUT KEUPP
Radtke, G. and Keupp, H. 2017. The dorsal shell wall structure of Mesozoic ammonoids. Acta Palaeontologica Polonica 62 (1): 59–96.
The study of pristine preserved shells of Mesozoic Ammonoidea shows different types of construction and formation of the dorsal shell wall. We observe three major types: (i) The vast majority of Ammonoidea, usually planispirally coiled, has a prismatic reduced dorsal shell wall which consists of an outer organic component (e.g., wrinkle layer), which is the first layer to be formed, and the subsequently formed dorsal inner prismatic layer. The dorsal mantle tissue suppresses the formation of the outer prismatic layer and nacreous layer. With the exception of the outer organic component, secretion of a shell wall is omitted at the aperture. A prismatic reduced dorsal shell wall is always secreted immediately after the hatching during early teleoconch formation. Due to its broad distribution in (planispiral) Ammonoidea, the prismatic reduced dorsal shell wall is probably the general state. (ii) Some planispirally coiled Ammonoidea have a nacreous reduced dorsal shell wall which consists of three mineralized layers: two prismatic layers (primary and secondary dorsal inner prismatic layer) and an enclosed nacreous layer (secondary dorsal nacreous layer). The dorsal shell wall is omitted at the aperture and was secreted in the rear living chamber. Its layers are a continuation of an umbilical shell doubling (reinforcement by additional shell layers) that extends towards the ventral crest of the preceding whorl. The nacreous reduced dorsal shell wall is formed in the process of ontogeny following a prismatic reduced dorsal shell wall. (iii) Heteromorph and some planispirally coiled taxa secrete a complete dorsal shell wall which forms a continuation of the ventral and lateral shell layers. It is formed during ontogeny following a prismatic reduced dorsal shell wall or a priori. The construction is identical with the ventral and lateral shell wall, including a dorsal nacreous layer. The wide distribution of the ability to form dorsal nacre indicates that it is a plesiomorphic trait which either was passed on from gyrocone ammonoid ancestors or (re-)developed in post-Triassic ammonoids.
Key words: Ammonoidea, internal structure, dorsal shell wall, wrinkle layer, spiral ornament, Ritzstreifen, Mesozoic.
Gregor Radtke [gradtke@zedat.fu-berlin.de] and Helmut Keupp [keupp@zedat.fu-berlin.de], Department of Earth Sciences, Freie Universität Berlin, Malteserstraße 74-100, Building D, 12249 Berlin, Germany.
Received 11 March 2016, accepted 5 January 2017, available online 1 March 2017.
Copyright © 2017 G. Radtke and H. Keupp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Ammonoid conchs are built of a conservatively constructed aragonitic shell wall. In simplified terms their shell wall consists of four layers: an outer organic periostracum, an outer prismatic layer, a middle nacreous layer, and an inner prismatic layer (Fig. 1A; e.g., Birkelund 1967, 1980; Erben et al. 1968, 1969; Kulicki 1979, 1996; Keupp 2000; Doguzhaeva et al. 2010; Kulicki et al. 2016; Radtke and Keupp 2016; Radtke et al. 2016). In most cases, the periostracum is not preserved; it was probably shed during lifetime (Checa 1994; Keupp 2000). This general configuration can be modified, for example with omitted or additional shell layers (Howarth 1975; Birkelund 1980; Doguzhaeva and Mutvei 1989, 1991). Different portions of the shell-secreting mantle form the individual shell layers. It is assumed that the outer organic periostracum and the outer prismatic layer were secreted at the aperture by the oral edge of the mantle. Also the middle nacreous layer was formed near the aperture but more adapically. The adapical parts of the mantle secreted the inner prismatic layer in the rear of the living chamber, maybe in connection with the formation of the nacreous layer of the septa (e.g., Blind 1975; Howarth 1975).
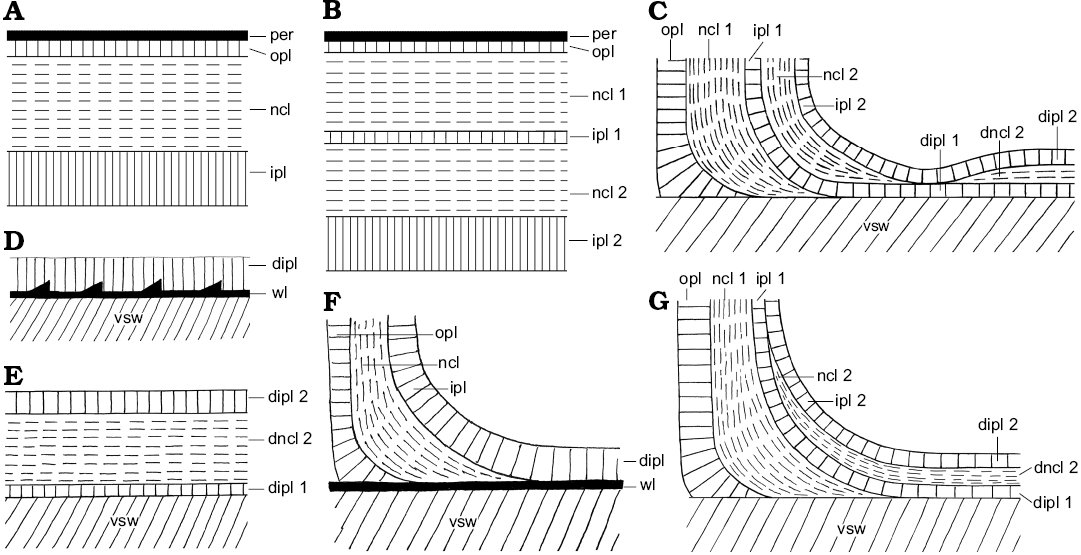
Fig. 1. Schematic construction of the ventral and dorsal shell wall (A, B, D, E, median section, growth direction right, centrifugal; C, F, G, transversal section, centrifugal). A. Simple ventral shell wall. B. Ventral shell wall with a doubling. C, E, G. Nacreous reduced dorsal shell wall. D, F. Prismatic reduced dorsal shell wall. Abbreviations: dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl, dorsal nacreous layer; dncl 1/2, primary/secondary dorsal nacreous layer; ipl, inner prismatic layer; ipl 1/2, primary/secondary inner prismatic layer; ncl, nacreous layer; ncl 1/2, primary/secondary nacreous layer; opl, outer prismatic layer; per, periostracum; vsw, ventral shell wall of the preceding whorl; wl, wrinkle layer.
Most ammonoids form a planispirally coiled conch by flanging the outer whorl on the preceding whorl. In general, during that process, ammonoids omit a dorsal shell wall in the contact area of the whorls (Fig. 2A) and the dorsal shell wall is reduced in this way. Mineralized shell material is formed only in the rear parts of the living chamber (Fig. 2A2) and consists often only of the inner prismatic layer (Fig. 1D). The outer prismatic and the nacreous shell layer wedge out at the umbilical contact (Fig. 1F; Palframan 1967; Birkelund and Hansen 1968, 1974, 1975; Erben et al. 1968, 1969; Drushits and Khiami 1970; Walliser 1970; Erben and Reid 1971; Bayer 1974; Howarth 1975; Lehmann 1976, 1990; Drushits et al. 1977; Kulicki 1979, 1996; Doguzhaeva 1980, 1981, 2002; Birkelund 1980; Zakharov and Graboskaya 1984; Doguzhaeva and Mutvei 1986, 1991, 1993a, b; Bucher et al. 1996; Zakharov 1996; Kulicki and Tanabe 1999; Keupp 2000; Kulicki et al. 1999, 2001, 2002, 2016; Doguzhaeva et al. 2010; Doguzhaeva 2012).
Several ammonoid taxa have a wrinkle layer as an additional element of the dorsal shell wall, namely an outer component (Fig. 1D, F; Kulicki 1979; Doguzhaeva 1980, 1981; Zakharov and Grabovskaya 1984; Zakharov 1996; Kulicki and Tanabe 1999; Kulicki et al. 2001) which is probably an equivalent formation to the black layer of Nautilus. Similar to the black layer this layer forms a highly variable, fingerprint-like relief of small ridges and knobs (wrinkles) at the surface of the preceding whorl (e.g., Walliser 1970; House 1971; Senior 1971; Tozer 1972; Hölder 1973; Korn 1985; Keupp 2000).
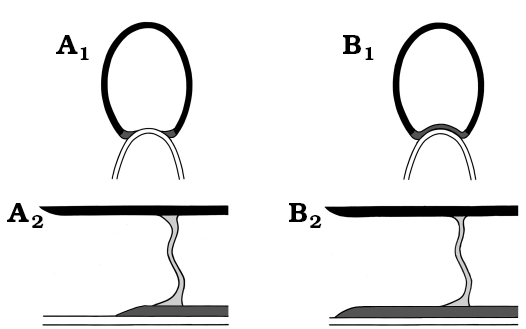
Given that the ancestors of planispirally coiled ammonoids had gyrocone conchs with whorls that are detached from each other and therefore do not support each other, it is likely that these ancestors formed a complete dorsal shell wall, which mean that the ventral, lateral, and dorsal shell wall form a continuum aperturally and adapically (Fig. 2B). Indeed, heteromorph ammonoids, taxa with a secondarily decoiled shell, reveal a uniformly three-layered shell tube ventrally, laterally and dorsally (Figs. 1A, 3A, B; Erben et al. 1969; Doguzhaeva and Mikhailova 1982; Landman 1987; Doguzhaeva and Mutvei 1989). A complete dorsal shell wall could be already formed right after the end of the embryonic ammonitella (first whorl and protoconch), as observed in the heteromorph Luppovia (Doguzhaeva and Mikhailova 1982) and Ptychoceras (Doguzhaeva and Mutvei 1989). Planispirally coiled taxa probably merely suppressed the secretion of the outer shell layers (i.e., dorsal outer prismatic layer and dorsal nacreous layer).
In this study, we aimed to determine whether the dorsal shell wall of ammonoids has any potential for phylogenetical and/or taxonomical purposes. Of particular interest is the question whether there are any systematically important similarities or differences in the internal structure, formation, or ontogeny (e.g., the morphological expression of the inner prismatic layer or the wrinkle layer). Primarily, this work is intended to clarify which ammonoid taxa form a complete dorsal shell wall. Do taxa, that form a reduced dorsal shell wall, have the general ability to form an optional complete dorsal shell wall during ontogeny or in reaction to some triggers (e.g., injuries, overgrowth of encrusters)? We also check whether the ability to form a complete dorsal shell wall is a requirement for the development of heteromorph taxa.
 |
 |
|
Fig. 2. Schematic drawing of general dorsal shell wall types (A1, B1, transversal section, centrifugal; A2, B2, median section, growth direction left, centrifugal). A. Reduced dorsal shell wall. The lateral shell wall wedges out at the contact with the preceding whorl. The dorsal wall is omitted at the aperture. B. Complete dorsal shell wall. The ventral, lateral, and dorsal shell walls form a continuum. The dorsal wall is present at the aperture. Colouring: black, ventral/lateral wall of the succeeding whorl; dark grey, dorsal wall of the succeeding whorl; light grey, septum of the succeeding whorl; white, ventral wall of the preceding whorl. |
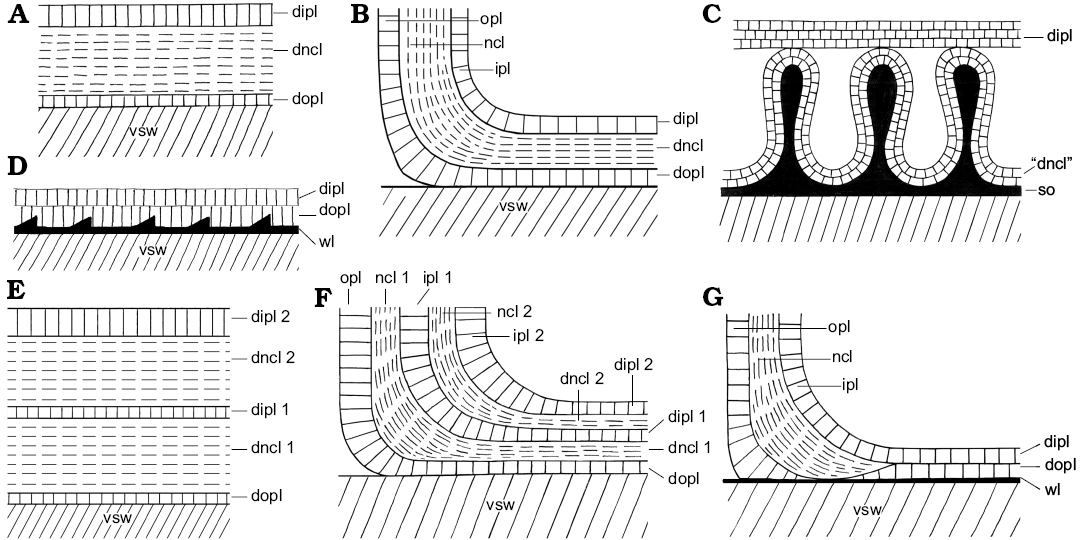
Fig. 3. Schematic construction of the dorsal shell wall (A, D, E, median section, growth direction right, centrifugal; B, C, F, G, transversal section, centrifugal). A, B. Complete dorsal shell wall. D, G. Seemingly complete dorsal shell wall. C. Complete dorsal shell wall of Amaltheidae. E, F. Reinforced complete dorsal shell wall. Abbreviations: dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl, dorsal nacreous layer; dncl 1/2, primary/secondary dorsal nacreous layer; dopl, dorsal outer prismatic layer; ipl, inner prismatic layer; ipl 1/2, primary/secondary inner prismatic layer; ncl, nacreous layer; ncl 1/2, primary/secondary nacreous layer; opl, outer prismatic layer; so, spiral ornament; vsw, ventral shell wall of the preceding whorl; wl, wrinkle layer.
|
Institutional abbreviations.—AMNH, American Museum of Natural History, New York City, USA; FU, Freie Universität, Berlin, Germany; BSPG, Bavarian State Collection for Palaeontology and Geology, Munich, Germany.
Other abbreviations.—cl, coating layer; D, diameter; dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl, dorsal nacreous layer; dncl 1/2, primary/secondary nacreous layer; dopl, dorsal outer prismatic layer; dspl, dorsal septal prismatic layer; dsw, dorsal shell wall; if, infilling; ipl, inner prismatic layer; ipl 1/2, primary/secondary inner prismatic layer; ncl, nacreous layer; ncl 1/2, primary/secondary nacreous layer; ol, organic layer; ooc, outer organic component; opl, outer prismatic layer; opl 1/2, primary/secondary outer prismatic layer; per, periostracum; PI, preservation index; s, septum; so, spiral ornament; sphpr, spherulitic-prismatic layer; spl, septal prismatic layer; vsw, ventral shell wall; wl, wrinkle layer.
Material and methods
This study is based on more than 290 well preserved shells of more than 200 different ammonoid taxa from different Triassic, Jurassic, and Cretaceous localities in England, France, Germany, Greenland, Japan, Madagascar, Russia, and the USA (see SOM: table A, Supplementary Online Material http://app.pan.pl/SOM/app62-Radtke_Keupp_SOM.pdf). The specimens are housed at BSPG (as part of H. Keupp’s collection) and AMNH. According to the SEM preservation index by Cochran et al. (2010), the examined shell material has a predominantly aragonitic preservation of a good (PI = 3) to poor (PI = 1) state. The shells of several taxa, mainly from the Triassic, were completely recrystallized without a preserved ultrastructure of the shell wall.
Freshly broken material and etched median and transversal sections were analyzed. Etched sections were polished with aluminum oxide and afterwards treated with 10% formic acid for 5–10 seconds. All samples were fixed on aluminum stubs with conductive carbon glue and sputtered with gold. Observations were made and pictures were taken with the scanning electron microscope Zeiss SUPRA 40VP at the palaeontological section of the FU.
Results and discussion
The ventral and lateral shell wall.—In all studied taxa with preserved shell, the ventral and lateral shell wall of the postembryonic conch consists of the typical three aragonitic shell layers: an outer prismatic layer, a middle nacreous layer and an inner prismatic layer (Figs. 1A, 4A1, B1, C, F1, 5A1, A2). An organic periostracum was only observed with certainty in some Phylloceratoidea (e.g., Phylloceras [Euphylloceras] cf. velledae) and Desmoceratoidea (e.g., Desmophyllites diphylloides) forming conspicuous extensions (see below). The outer and inner prismatic layers consist of parallel, elongated prisms that are perpendicular to the shell surface, i.e., regular simple prismatic microstructure. The median nacre layer is formed by stacks of polygonal aragonite plates, i.e., columnar nacre (Carter and Clark 1985; Carter et al. 1989).
The thickness of these mineralized layers can vary greatly within individual taxa. In particular, the members of the Perisphinctoidea seem to reduce the prismatic layers in late ontogenetic stages. Other taxa modify the shell wall by adding a secondary nacreous layer and secondary inner prismatic layer to the internal surface of the trilayered shell wall, e.g., Aconeceras sp. 1 (Haploceratoidea), Rondiceras sp. (Stephanoceratoidea), Speetoniceras sp., Aspidoceras sp. (both Perisphinctoidea), Beudanticeras sp. (Desmoceratoidea). These additional layers are called a shell doubling, i.e., the resulting shell wall has five mineralized layers (Fig. 1B; cf. Howarth 1975; Birkelund 1980; Doguzhaeva and Mutvei 1989, 1991).
The septa of all taxa attach to the inner surface of the inner prismatic layer. They are made of a layer of columnar nacre. Also prismatic structures can occur, particularly at the the contact of the septum with the shell wall, e.g., local thickenings of the inner prismatic layer or a proximal prismatic coating of the adoral septal surface (spl in Figs. 4C2, 5A3).

Fig. 4. Construction of the prismatic reduced dorsal shell wall (A–E, G, median section, growth direction to the left, centrifugal; F, transversal section, centrifugal). A. Phylloceras (Euphylloceras) sp., BSPG MAo-1769, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; A1, the dorsal shell wall consists of an outer wrinkle layer and a dorsal inner prismatic layer; A2, A3, organic wrinkles. B. Ptychophylloceras sp., BSPG MAn-4516, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; B1, the dorsal shell wall consists of an outer wrinkle layer and a dorsal inner prismatic layer; B2, organic wrinkle. C–E, G. Desmoceras (Desmoceras) latidorsatum (Michelin, 1838), early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar. C. BSPG MAo-1783; C1, the dorsal shell wall forms a wrinkle layer-complex; C2, the wrinkle layer is enriched with organic material. D. BSPG MAo-1839, organic wrinkle. E. BSPG MAo-1788, the relief of an injury of the preceding whorl (i.e., forma aegra substructa of Hölder (1973) is overgrown by the outer wrinkle layer and compensated by the dorsal inner prismatic layer. G. BSPG MAo-1782, the wrinkle layer of the dorsal shell wall becomes prismatic. F. Neosilesites ambatolafrensis Collignon, 1963, BSPG MAo-1780, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; F1, at the umbilical seam, the outer prismatic layer and the nacreous layer of the attaching whorl wedge out; only the inner prismatic layer continues towards the spiral plane; the wrinkle layer wedges out towards the umbilical seam; F2, organic wrinkle. Abbreviations: dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dspl, dorsal septal prismatic layer; ipl, inner prismatic layer; ipl 1/2, primary/secondary inner prismatic layer; ncl, nacreous layer; ncl 1/2, primary/secondary nacreous layer; opl, outer prismatic layer; s, septum; spl, septal prismatic layer; wl, wrinkle layer.

Fig. 5. Construction of the prismatic reduced dorsal shell wall (median section, growth direction to the left, centrifugal). A. Desmoceras (Desmoceras) latidorsatum (Michelin, 1838), BSPG MAo-1786, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; A1, the early dorsal shell wall consists of a smooth organic layer and (prismatic) septal mural parts; A2, later in ontogeny, the septal mural parts extend and seem to form the first dorsal inner prismatic layer; A3, the prismatic mural part of a nacreous septum can form an own layer which is separated from the ventral inner prismatic layer; the nacreous and prismatic materials of a septum merge. B. Neosilesites ambatolafiensis Collignon,1963, BSPG MAo-1779, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; the prismatic mural part of a nacreous septum can form an own layer which is separated from the dorsal inner prismatic layer; the nacreous and prismatic materials of a septum merge. C. Argonauticeras besairiei Collignon, 1949, BSPG MAo-1705, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; C1, the septal mural part seems to be the origin of the inner sub-layer of the dorsal inner prismatic layer; C2, close up of C1. Abbreviations: dipl, dorsal inner prismatic layer; dspl, dorsal septal prismatic layer; ipl, inner prismatic layer; ncl, nacreous layer; ol, organic layer; opl, outer prismatic layer; s, septum; spl, septal prismatic layer; wl, wrinkle layer.
The prismatic reduced dorsal shell wall.—The dorsal shell walls of this type consist basically of two components, an outer organic component, in most cases a wrinkle layer, which attaches to the previous whorl, and a dorsal inner prismatic layer which seals the inner surface of the outer organic component (Figs. 1D, 4A1, B1, C2, 6A, 7A1, 8A). With the exception of the outer component, dorsal shell material is omitted at the aperture, i.e., the inner prismatic layer wedges out towards the aperture in the rear living chamber (Figs. 2A2, 6).
A prismatic reduced dorsal shell wall is typical for the vast majority of planispirally coiled taxa and occurs throughout the whole Mesozoic in nearly all groups (Fig. 9; SOM: table A). Generally, even taxa which develop another dorsal shell wall type during ontogeny (see below) pass through an early ontogenetic stage of a prismatic reduced dorsal shell wall after hatching, e.g., heteromorphs like the Scaphitoidea.
 |
Fig. 6. Construction of the prismatic reduced dorsal shell wall (median section, growth direction to the left, centrifugal). Ptychophylloceras cf. dacquei Joly, 1976, BSPG MAn-4516, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar. The dorsal shell wall consists of an outer wrinkle layer and dorsal inner prismatic layer. The dorsal inner prismatic layer becomes thinner towards the aperture (A, B) and vanishes completely (C). Abbreviations: dipl, dorsal inner prismatic layer; ipl, inner prismatic layer; ncl, nacreous layer; opl, outer prismatic layer; s, septum; wl, wrinkle layer. |
The wrinkle layer.—The outer organic component is one of the integral parts of the prismatic reduced dorsal shell wall. The most common and distributed formation is the wrinkle layer (see SOM: table A). A genuine wrinkle layer can be observed in smooth or only weakly sculptured taxa of the Phylloceratoidea (e.g., Phylloceras [Euphylloceras] cf. velledae), Lytoceratoidea (e.g., Argonauticeras besairiei), Tetragonitoidea (e.g., Eogaudryceras [Eotetragonites] umbilicostriatus), Stephanoceratoidea (e.g., Quenstedtoceras lamberti) and Desmoceratoidea (e.g., Desmoceras [Desmoceras] latidorsatum), but occurs also in Eoderoceratoidea (e.g., Pleuroceras salebrosum), Hildoceratoidea (e.g., Leioceras opalinum), Haploceratoidea (e.g., Aconeceras sp. 1), Perisphinctoidea (e.g., Proplanulites sp., Divisosphinctes sp. 2), Hoplitoidea (e.g., Metaplacenticeras subtilistriatum), Douvilleiceratoidea (e.g., Douvilleiceras mammillatum), and Scaphitoidea (e.g., Scaphites whitfieldi).
The wrinkle layer has a fingerprint-like relief which is formed as a sequence of more or less regularly spaced faint ridges and/or knobs (Fig. 8B, C) which are usually triangular or trapezoidal in cross section (Figs. 4A2, A3, B2, D, F2, 7A3), i.e., the individual wrinkles. The ridges are restricted in radial length; up to 200 µm (Fig. 8B). The wrinkle layer attaches directly to the ventral shell wall of the preceding whorl, even covering its injuries (Figs. 4E, 7A5, 8A). The wrinkle layer extends throughout the whole living chamber (Fig. 6) but is restricted to the attachment area of the succeeding to the preceding whorl. The wrinkle layer either wedges out towards (Fig. 4F1), or often ends abruptly at (Fig. 10A), the umbilical seam. The wrinkle layer has no equivalent in the ventral/lateral (mineralized) shell layers (i.e., opl, ncl, and ipl) but these layers attach to the wrinkle layer at the umbilical seam (Figs. 1F, 4F1, 10A).
Well preserved wrinkles are “hollow” organic structures; they consist of a small organic core with a prismatic coating which is covered by an organic surrounding (Figs. 4A2, A3, D, F2, 7A3). Often the ultrastructure of the wrinkles is altered: the wrinkle layer or parts of it can be preserved as a granular layer (Fig. 8D), prismatic layer (Fig. 4G) or hollow space. Sometimes the wrinkle layer forms a thick homogeneous organic layer with an undulating relief (the wrinkles can be still differentiated; Fig. 4C2).
Our observations show that the wrinkle layer relief develops during ontogeny. The early appearance of the wrinkle layer is a relief-less, smooth organic layer that covers the previous whorl (Fig. 5A1). The first wrinkles occur suddenly between diameters of 2 to 10 mm (Fig. 5A2). These diameters probably correspond with the third to fourth whorl as derived from a few taxa with preserved ammonitella (first whorl and protoconch). On occasion, wrinkles cover the ammonitella, e.g., Eogaudryceras (Eotetragonites) umbilicostriatus (Tetragonitoidea). Ontogenetic young wrinkles in particular often seem to be entirely hollow (i.e., lacking prismatic portions) or consist only of organic material.

Fig. 7. Construction of the dorsal shell wall of Douvilleiceratoidea (A, median section, growth direction to the left, centrifugal; B, C, transversal section, centrifugal). Douvilleiceras mammillatum (Schlotheim, 1813), early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar. A. BSPG MAo-1808; A1, the juvenile dorsal shell wall consists of an outer wrinkle layer and a dorsal inner prismatic layer; A2, A3, organic wrinkles; A4, merged wrinkles; A5, the relief of an injury of the preceding whorl (forma aegra substructa of Hölder [1973]) is overgrown by the outer wrinkle layer and the dorsal inner prismatic layer; A6, dorsal shell wall close to the detachment (overgrowth of spines) from the ventral shell wall of the preceding whorl; the dorsal shell wall consists of an outer wrinkle layer, a primary dorsal inner prismatic layer, a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer; the primary dorsal nacreous layer is not formed yet but at complete detachment. B. BSPG MAo-1809, a reinforced complete dorsal shell is formed during detachment from the preceding whorl; the shell wall consists of a primary dorsal nacreous layer, a primary dorsal inner prismatic layer, a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer. C. BSPG MAo-1810; C1, the dorsal shell wall (dsw) detaches from the ventral shell wall (vsw) during overgrowth of the ventral relief; C2, Same as in B; C3, at the umbilical seam, the attaching shell wall vanishes towards the spiral plane; C4, close up of C3. Abbreviations: dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl 1/2, primary/secondary dorsal nacreous layer; dsw, dorsal shell wall; if, infilling; ipl, inner prismatic layer; ncl, nacreous layer; ncl 1, primary nacreous layer; opl, outer prismatic layer; opl 1/2, primary/secondary outer prismatic layer; vsw, ventral shell wall; wl, wrinkle layer; s, septum.
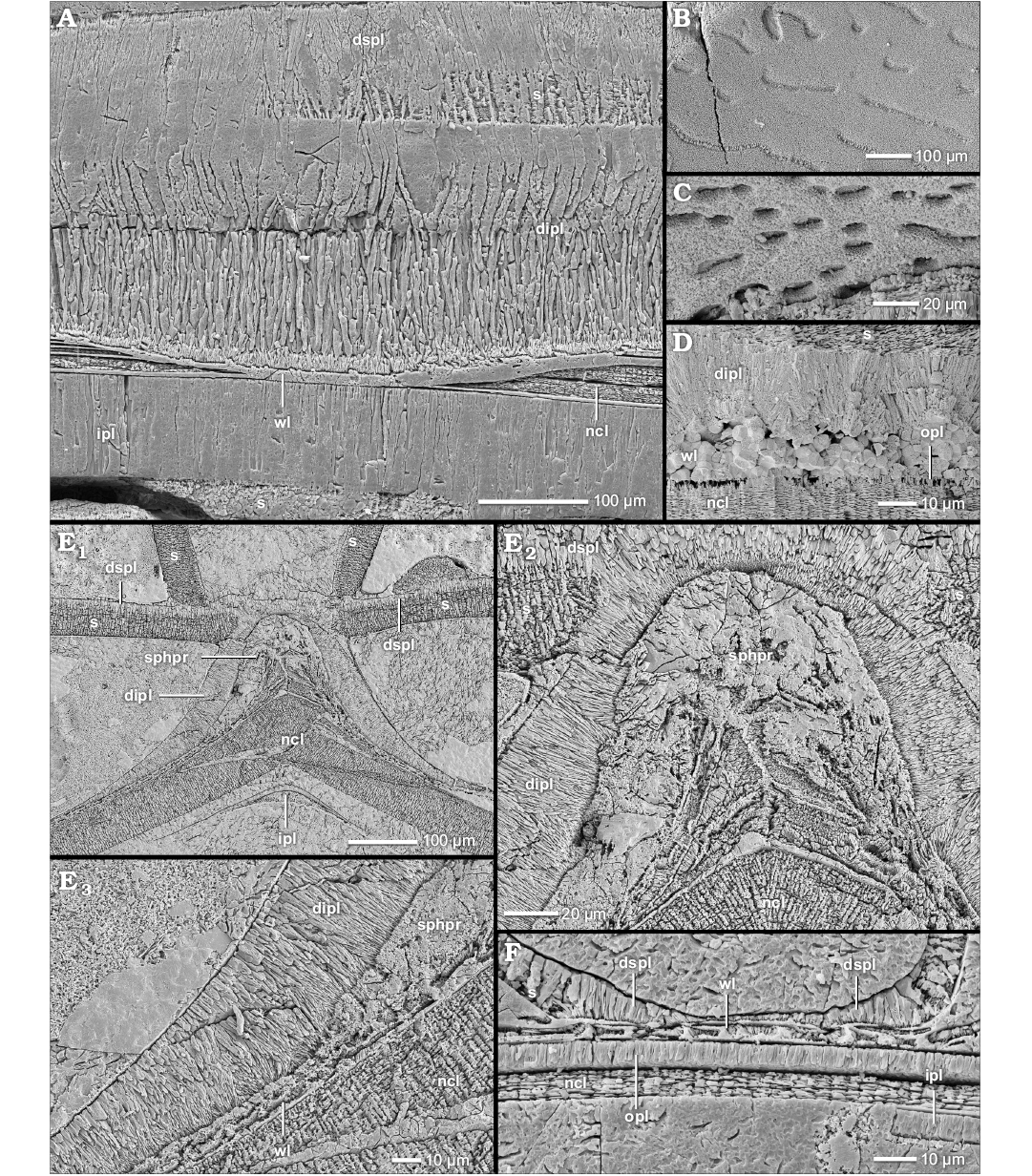
Fig. 8. Construction of the prismatic reduced dorsal shell wall (A–C, F, median, section, growth direction to the left, centrifugal; D, E, transversal section, centrifugal). A. Argonauticeras besairiei Collignon, 1949, BSPG MAo-1772, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; the dorsal shell wall consists of an outer wrinkle layer and a dorsal inner prismatic layer which has two sub-layers; the relief of an injury of the preceding whorl is overgrown by both layers. B. Calliphylloceras sp., BSPG MAn-4512, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; the wrinkle layer left imprints in the dorsal inner prismatic layer. C. Desmoceras (Desmoceras) latidorsatum (Michelin, 1838), BSPG MAo-1839, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; the same as in B. D. Cadoceras stupachenkoi Mitta, 1998, BSPG MAn-4790, early Callovian, Jurassic, Makaryev on Unzha River, Russia; the dorsal wrinkle layer is completely replaced by pyrite, i.e., diagenesis. E, F. Aconeceras sp. 1, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar. E. BSPG MAo-1851; E1, at the ventral crest of the preceding whorl, the wrinkle layer forms a thickening, i.e., spherulitic-prismatic layer; the dorsal inner prismatic layer is only present at the ventral crest of the preceding whorl and vanishes towards the flanks; E2, close-up of E1; the spherulitic-prismatic layer contains prismatic portions; E3, close-up of E1; the wrinkle layer transforms into the spherulitic-prismatic layer. F. BSPG MAo-1852; the juvenile dorsal shell wall consists of a wrinkle layer and septal mural parts. Abbreviations: dipl, dorsal inner prismatic layer; dspl, dorsal septal prismatic layer; ipl, inner prismatic layer; ncl, nacreous layer; opl, outer prismatic layer; s, septum; sphpr, spherulitic-prismatic layer; wl, wrinkle layer.
Derivates of the wrinkle layer.—During ontogeny, some taxa develop individual or special morphological expressions of the wrinkle layer or the outer organic component, respectively. For example, in the outer whorls of Douvilleiceras mammillatum (Douvilleiceratoidea) individual wrinkles (Fig. 7A2, A3) cannot be recognized any more and the prismatic portions of the wrinkles merge (Fig. 7A4). However, the organic cores of the wrinkles can be still distinguished. The results are elongated, large wrinkles or even a (discontinuous) prismatic-spherulitic layer.
In the Madagascan and Russian specimens of Aconeceras sp. 1 and 2 (Haploceratoidea), the wrinkle layer of the outer whorls forms a strong thickening at the ventral crest of the preceding whorl (sphpr in Fig. 8E1, E2). The overgrown flanks of the preceding whorl are covered by normal wrinkles (Fig. 8E3, F). At the ventral edges of the preceding whorl, both appearances merge (Fig. 8E3). The thickening is dominated by an organic composition but shows several disordered spherulitic-prismatic inclusions (Fig. 8E2); it is called the spherulitic-prismatic layer (cf. Doguzhaeva and Mutvei 1991, 1993b). Two other haploceratids, Taramelliceras externodosum and Sanmartinoceras sp., develop similar structures. However, thick prismatic portions dominate the layer in Sanmartinoceras sp. (Fig. 10B). Interestingly, Puzosia saintoursi (Desmoceratoidea) develops a similar organic-prismatic package of the wrinkle layer at local ridge-like thickenings (Fig. 11A1, A2).
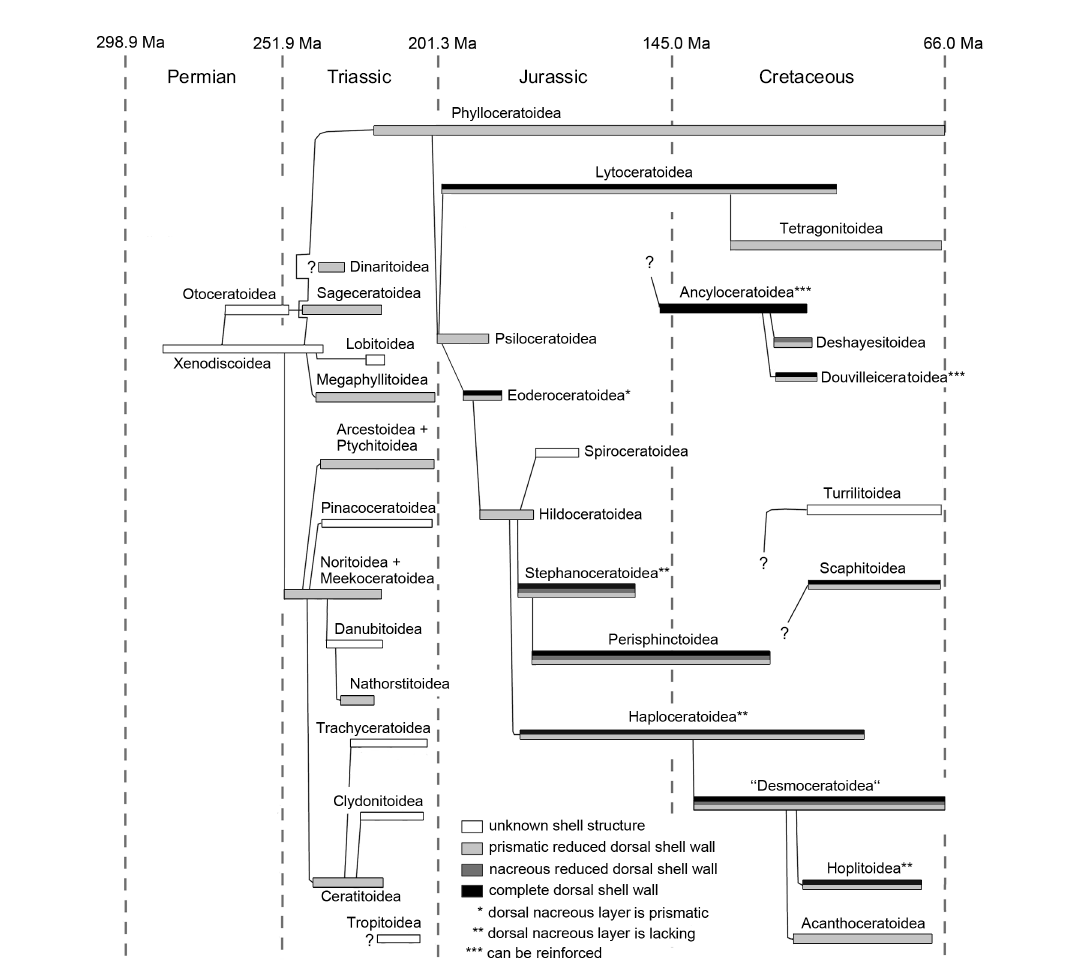 |
Fig. 9. Occurrences of dorsal shell wall types in Mesozoic ammonoid superfamilies (after Rouget et al. 2004; cf. Tables 1, 2, SOM: table A). The wide distribution of reduced dorsal shell walls in Mesocoic taxa suggests a plesiomorphy. In general, nacreous reduced dorsal shell walls or complete dorsal shell walls follow a stage of a prismatic reduced dorsal shell wall. The wide distribution of nacreous reduced dorsal shell walls and complete dorsal shell walls in Mesozoic ammonoid taxa suggests that the ability to form dorsal nacre is also a plesiomorph feature. Note: The dorsal nacreous layer of Eoderoceratoidea (Amaltheidae) has a prismatic appearance (*). The complete dorsal shell walls in Stephanocertatoidea, Haploceratoidea, and Hoplitoidea lack a dorsal nacreous layer, i.e., seemingly complete dorsal shell wall (**). The complete dorsal shell walls of Anclyceratoidea and Douvilleiceratoidea can be reinforced by additional pair of nacreous and prismatic layers, i.e., reinforced complete dorsal shell wall (***). |
Table 1. Ammonoid specimens which develop the nacreous reduced dorsal shell wall. <, > indicate that the proper onset of the nacreous reduced dorsal shell wall cannot be accurately determined due to hiatus in shell preservation or preparation (e.g., transversal section).
|
Taxon |
Collection |
Preservation index |
Age, period |
Location |
Diameter at which nacreous reduced dorsal shell wall begins to form (mm) |
|
Stephanoceratoidea |
|||||
|
Kepplerites
galilaeii |
BSPG MAn-4783 |
1–2 |
early Callovian, Jurassic |
Znamenka on Unzha River, Russia |
> 44 < 79 |
|
Kosmoceras (Kosmoceras) cf. duncani (Sowerby, 1816) |
BSPG MAn-4788 |
1–2 |
late Callovian, |
Dubki near Saratov, Russia |
< 90 (estimated) |
|
Perisphinctoidea |
|||||
|
Perisphinctes (Kranaosphinctes) mahabokensis (Collignon, 1959) |
BSPG MAn-4834 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
54 |
|
Perisphinctes (Kranaosphinctes) mahabokensis (Collignon, 1959) |
BSPG MAn-4835 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
< 210 |
|
Speetoniceras versicolor (Trautschold, 1865) |
BSPG MAo-1861 |
1–2 |
Aptian, Cretaceous |
Simbirsk, Uljanowsk, Volga Basin, Russia |
< 200 |
|
Aspidoceratidae: Aspidoceratinae |
|||||
|
Aspidoceras sp. |
BSPG MAn-4506 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
< 11 |
|
Aspidoceras sp. |
BSPG MAn-4507 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
8 |
|
Aspidoceras sp. |
BSPG MAn-4046b |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
7 |
|
Aspidoceras sp. |
BSPG MAn-3193 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
7 |
|
Epaspidoceras jeannetti (Collignon, 1959) |
BSPG MAn-4505 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
31 |
|
Euaspidoceras sp. 1 |
BSPG MAn-4750 |
2–3 |
late Callovian, |
Dubki near Saratov, Russia |
< 110 (estimated) |
|
Euaspidoceras sp. 2 |
BSPG MAn-4751 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
9 |
|
Mirosphinctes sp. 1 |
BSPG MAn-1769 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
7 |
|
Mirosphinctes sp. 2 |
BSPG MAn-4747 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
> 7 < 25 |
|
Pseudowaagenia sp. |
BSPG MAn-4502 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, SW Madagascar |
< 6 |
|
Desmoceratoidea |
|||||
|
Desmoceras (D.) latidorsatum (Michelin, 1838) |
BSPG MAo-1787 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
9 (secondary, local) |
|
Eupachydiscus sp. |
BSPG MAo-1831 |
2 |
Campanian, |
Teshio-Nakagawa area, Hokkaido, Japan |
54 |
|
Eupachydiscus sp. |
BSPG MAo-1832 |
2 |
Campanian, |
Teshio-Nakagawa area, Hokkaido, Japan |
< 430 (estimated) |
|
Eupachydiscus sp. |
BSPG MAo-1833 |
2 |
Campanian, |
Teshio-Nakagawa area, Hokkaido, Japan |
< 340 (estimated) |
|
Eupachydiscus sp. |
BSPG MAo-1834 |
2 |
Campanian, |
Teshio-Nakagawa area, Hokkaido, Japan |
< 470 (estimated) |
|
Puzosia
saintoursi |
BSPG MAo-1797 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
17–19 (secondary, local) 35–39 (secondary, local) |
|
Deshayesitoidea |
|||||
|
Colombiceras sp. |
BSPG MAo-1884 |
1–2 |
Aptian, |
Caucasus region, Russia |
< 25 |
Table 2. Ammonoid specimens which develop the complete dorsal shell wall. <, > indicate that the proper onset of the complete dorsal shell wall cannot be accurately determined due to hiatus in shell preservation or preparation (e.g., transversal section). *, the dorsal nacreous layer has a prismatic appearance; **, seemingly complete dorsal shell wall. (The complete dorsal shell wall lacks the dorsal nacreous layer). ***, reinforced complete dorsal shell wall. (The complete dorsal shell wall is reinforced by additional pair of nacreous and prismatic layers).
|
Taxon |
Collection |
Preservation index |
Age, period |
Location |
Diameter at which complete dorsal shell wall begins to form (mm) |
|
Lytoceratoidea |
|||||
|
Lobolytoceras
costellatum |
BSPG MAn-2061 |
2 |
late Oxfordian, |
Sakaraha, Morondava Basin, |
> 37 |
|
Lobolytoceras
costellatum |
BSPG MAn-3059 |
2 |
late Oxfordian, |
Sakaraha, Morondava Basin, |
< 620 |
|
Eoderoceratoidea |
|||||
|
Amaltheus
margaritatus |
BSPG MAn-4798 |
2 |
late Pliensbachian, Jurassic |
Buttenheim, Bavaria, |
< 72 |
|
Amaltheus
margaritatus |
BSPG MAn-4799 |
2 |
late Pliensbachian, Jurassic |
Buttenheim, Bavaria, |
< 140 |
|
Stephanoceratoidea |
|||||
|
Quenstedtoceras
henrici |
BSPG MAn-4768 |
1–2 |
early Callovian, Jurassic |
Dubki near Saratov, Russia |
< 15 |
|
Sigaloceras (Sigaloceras) calloviense (Sowerby, 1815)** |
BSPG MAn-4785 |
1–2 |
late Callovian, Jurassic |
Znamenka on Unzha River, Russia |
< 85 (estimated) |
|
Perisphinctoidea |
|||||
|
Choffatia (Grossouvria) sp. 2 |
BSPG MAn-4520 |
1–2 |
late Callovian, Jurassic |
Dubki near Saratov, Russia |
~ 22 |
|
Divisosphinctes
besairiei |
BSPG PA-10151 |
2–3 |
late Oxfordian, |
Sakaraha, Morondava Basin, |
39 (secondary formation) |
|
Haploceratoidea |
|||||
|
Hecticoceras (Sublunuloceras) sp. ** |
BSPG MAn-4739 |
1–2 |
late Callovian, Jurassic |
Dubki near Saratov, Russia |
< 47 (estimated) |
|
Desmoceratoidea |
|||||
|
Cleoniceras (Grycia) besairiei Collignon, 1949 |
BSPG PA-33582 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
65 (secondary formation) |
|
Hoplitoidea |
|||||
|
Metaplacenticeras subtilistriatum (Jimbo, 1894)** |
BSPG MAo-1824 |
2–3 |
Campanian, |
Teshio-Nakagawa area, |
> 10 |
|
Ancyloceratoidea |
|||||
|
Ancyloceratoidea indet.*** |
BSPG MAo-1813 |
2–3 |
Aptian, |
Shilovka near Volga River, Russia |
< 2nd shaft |
|
Douvilleiceratoidea |
|||||
|
Douvilleiceras mammilliatum (Schlotheim, 1813)*** |
BSPG MAo-1808 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
29 |
|
Douvilleiceras mammilliatum (Schlotheim, 1813)*** |
BSPG MAo-1809 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
> 16 |
|
Douvilleiceras mammilliatum (Schlotheim, 1813)*** |
BSPG MAo-1810 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
< 23 |
|
Douvilleiceras mammilliatum (Schlotheim, 1813)*** |
BSPG MAo-1811 |
2–3 |
early Albian, |
Ambatolafia, Mahajanga Basin, NW Madagascar |
24 |
|
Douvilleiceras sp.*** |
BSPG MAo-1812 |
2 |
Early Cretaceous |
Bally, Normandy, France |
< 23 |
|
Scaphitoidea |
|||||
|
Hoploscaphites
nicoletii |
AMNH-FI-99141 |
2 |
Maastrichtian, |
Fox Hills Formation (Loc. #3272), S Dakota, USA |
< 56 |
|
Hoploscaphites
nicoletii |
AMNH-FI-99143 |
2 |
Maastrichtian, |
Fox Hills Formation (Loc. #3272), S Dakota, USA |
< 16 |
|
Scaphites
whitfieldi |
AMNH-FI-99144 |
2 |
Touronian, |
Turner Sandy Member (Loc. #3190), Wyoming, USA |
< 22 |
The outer organic component of the dorsal shell wall of Scythian Hedenstroemia hedenstroemi (Sageceratoidea) thickens towards the ventral crest of the preceding whorl (ol in Fig. 10C). On top of the venter of the preceding whorl, it is up to twelve times thicker than at the shell flanks.
Gaudryceras tenuiliratum (Tetragonitoidea) develops a thick, homogeneous organic layer (Fig. 10D) which is called the coating layer here (cf. Drushits et al. 1978; Birkelund 1980; Doguzhaeva and Mutvei 1993b; Kulicki 1996; Kulicki et al. 2001). It traces the relief of the preceding whorl. Furthermore, several taxa form only a smooth organic layer instead of a wrinkle layer, e.g., Rudolphtruempiceras planorbis (Dinaritoidea).

Fig. 10. Construction of the prismatic reduced dorsal shell wall (A–C, transversal section, centrifugal; D, median section, growth direction to the left, centrifugal). A. Umsinenoceras linguatuberculatum Kennedy, Wright, and Klinger, 1979, BSPG MAo-1844, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; A1, at the umbilical seam, the outer prismatic layer and the nacreous layer of the attaching whorl wedge out; the inner prismatic layer continues towards the ventral crest of the preceding whorl; all three layers attach to the wrinkle layer which ends abruptly at the umbilical seam; the resulting dorsal shell wall consists of an outer wrinkle layer and a dorsal inner prismatic layer; A2, A3, close up of A1. B. Sanmartinoceras sp., BSPG MAo-1854, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; the spherulitic-prismatic layer is dominated by prismatic portions. C. Hedenstroemia hedenstroemi Keyserling, 1845, BSPG MAm-1651, early Olenekian, Triassic, Buur River, Olenek River Basin, Siberia, Russia; C1, the dorsal shell wall cover of the shell flanks of the preceding whorl consists of a thin outer organic layer and several sub-layers of the dorsal inner prismatic layer; C2, at the ventral crest of the preceding whorl, the dorsal outer organic layer forms a thickening. D. Gaudryceras tenuiliratum Yabe, 1903, BSPG MAo-1875, Campanian, Cretaceous, Teshio-Nakagawa area, Hokkaido, Japan; the dorsal shell wall consists of an outer organic coating layer and a dorsal inner prismatic layer (D2, close-up of the coating layer in the same specimen). Abbreviations: cl, coating layer; dipl, dorsal inner prismatic layer; ipl, inner prismatic layer; ncl, nacreous layer; ol, organic layer; opl, outer prismatic layer; s, septum; sphpr, spherulitic-prismatic layer; wl, wrinkle layer.
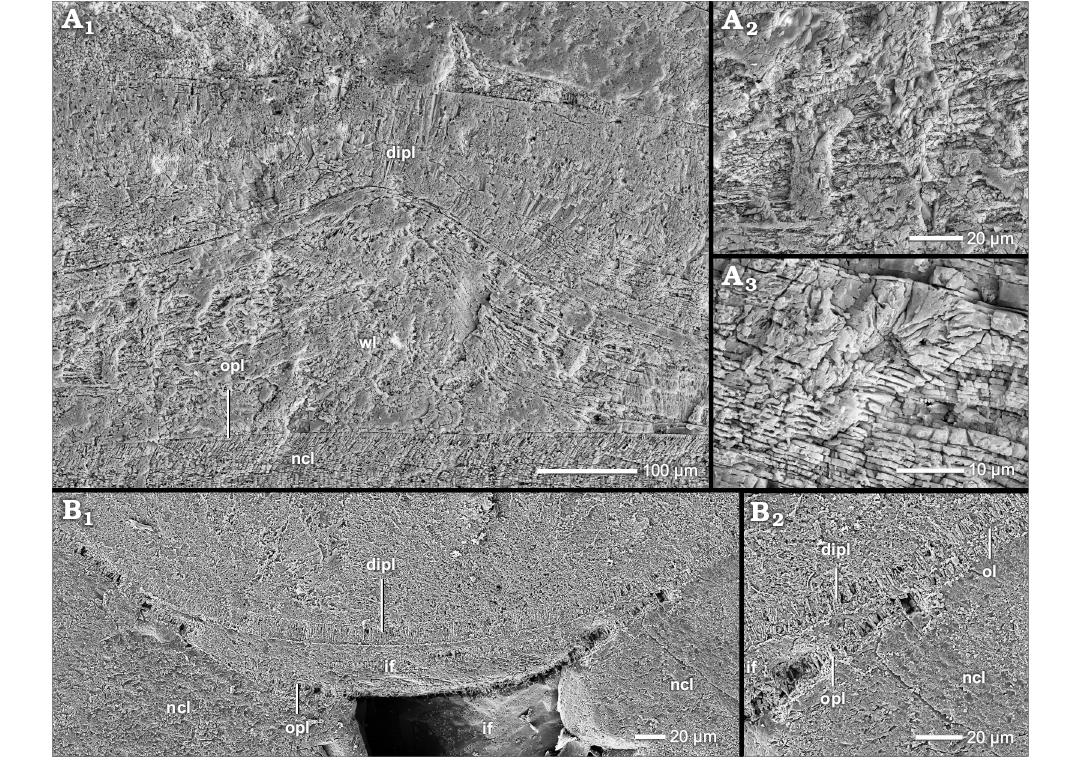
Fig. 11. Construction of the dorsal shell wall (median section, growth direction to the left, centrifugal). A. Puzosia saintoursi Collignon, 1963, BSPG MAo-1797, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; A1, the wrinkle layer forms an unusual cone-like thickening; the compensating thick dorsal inner prismatic layer forms nacreous inclusions; A2, close-up of A1; the organo-prismatic structure of the wrinkle layer thickening; A3, close-up of A1; the thickening of the dorsal inner prismatic layer shows nacreous inclusions. B. Perisphinctes (Kranaosphinctes) sp., BSPG MAn-4756, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; B1, the dorsal inner prismatic layer bridges the relief of two ribs forming a crescent hollow space; the ventral nacre layer of this shell portion is diagenetic altered; B2, close-up of B1. Abbreviations: dipl, dorsal inner prismatic layer; if, infilling; ncl, nacreous layer; opl, outer prismatic layer; wl, wrinkle layer.
The formation and function of the outer organic component.—The ammonoid preceding whorl was covered by an organic outer component of the ammonoid dorsal shell wall, e.g., a wrinkle layer, a spherulitic-prismatic layer, a thick coating layer or a smooth organic layer.
The wrinkle layer with its relief is the most peculiar and common character in our specimens. In contrast to some other opinions (e.g., Walliser 1970; House 1971; Senior 1971; Doguzhaeva 1980, 1981; Korn 1985) we conclude that the wrinkle layer is a distinct element of the dorsal shell wall (Tozer 1972; Kulicki et al. 2001; Klug et al. 2004; Keupp 2008; Mironenko 2015) which is not related to any other (mineralized) shell layer of the ventral/lateral shell wall (i.e., opl, ncl, and ipl).
We assign this layer to the dorsal shell wall due to the following characteristics: (i) The wrinkle layer occurs only dorsally covering the venter of the preceding whorl. Internal ventral and lateral wrinkle-like reliefs, e.g. “Ritzstreifen”, are formed by the inner prismatic layer (see below). (ii) It acts like a subsequent coating of the ventral shell wall (e.g., covering of injuries). (iii) The wrinkle layer has no connection to other shell layers (at least none was observed) and seems to be an individual element of the shell wall. However, we cannot exclude the possible assignment of the wrinkle layer to the dorsal periostracum.
Since the wrinkle layer attaches directly to the previous whorl and further layers attach to its internal surface, the wrinkle layer was the first of all dorsal shell layers to be secreted. Its wide extension within the living chamber, probably up to the aperture, indicates a formation at or at least near the aperture. Indeed, several authors have shown that the wrinkle layer extends beyond the aperture (e.g., Walliser 1970; House 1971; Tozer 1972; Korn 1985; Keupp 2000; Mironenko 2015). Current interpretations of the wrinkle layer assume a secretion of it in front of the aperture by a supracephalic mantle fold (e.g., Kulicki et al. 2001; Klug et al. 2004; Mironenko 2015). This explanation matches best with our observation and those of other authors, i.e., extension beyond the aperture.
The wrinkle layer or rather its relief is usually attributed to a roughness effect (e.g., Walliser 1970; Ristedt 1971; Doguzhaeva and Mutvei 1986; Lehman 1990; Keupp 2000) analogous to the black layer of Nautilus (Kulicki et al. 2001; Klug et al. 2004). The individual wrinkles had probably the function of a ratchet (e.g., asymmetric triangles in cross-section), so that the soft body had a better grip in the living chamber and beyond. This function is most probable because wrinkles are most prominent in weakly sculptured taxa. The delayed occurrence of the wrinkle layer relief indicates that it is not needed from the beginning of ontogeny. Its formation probably represents a more active life style that requires a movement of the soft body within the living chamber, i.e., the transition from planktonic to nectonic? In some ammonoids like Eogaudryceras (Eotetragonites) umbilicostriatus (Tetragonitoidea), this stage starts earlier than in other taxa, maybe corresponding to a temporal shift in ontogeny, i.e., heterochrony.
In particular, the wrinkle layer is liable to modification of its usual ridge- and knob-like appearance (cf. Korn et al. 2014; Mironenko 2015): The wrinkles of Douvilleiceras mammillatum merge in late ontogeny. The spherulitic-prismatic layer of several haploceratids (e.g., Aconeceras sp. 1 and 2, Taramelliceras externodosum, Sanmartinoceras sp.) replaces the normal wrinkle layer. It is equivalent to Doguzhaeva and Mutvei’s (1991, 1993b) spherulitic-prismatic layer of Aconeceras trautscholdi. They assigned this layer to the ventral shell wall secreted by an outer mantle epithelium, i.e., (semi-)internal shell. However, we show it is a derivate of the wrinkle layer, e.g., spatial (ventral vs. lateral cover) and ontogenetic transition of both morphological expressions. The thickening of the outer component at the venter of the preceding whorl in Hedenstroemia hedenstroemi could be an analogous formation as all these taxa are oxycones.
Gaudryceras tenuiliratum develops a prominent, thick, homogeneous layer. It probably represents the thick, smooth coating layer described by Drushits et al. (1978). However, a nacreous-like appearance, as observed by Drushits et al. (1978) and Birkelund (1980), is not preserved, nor are there indications of a lateral shell cover of the whole shell as typical for that layer. These are probably effects of diagenetic alterations in our specimens. Kulicki et al. (2001) identifies the coating layer as a late ontogenetic derivation of the wrinkle layer. Although we cannot observe wrinkles in G. tenuiliratum or in other members of the genus, a wrinkle layer can be at least identified in related genera, e.g., Eogaudryceras (Eotetragonites) umbilicostriatus or Tetragonites popetensis, proving a common feature in Tetragonitoidea which is therefore a likely precursory structure.
Several specimens lack a distinct wrinkle layer but have preserved a discrete, smooth organic layer instead. It is likely that it is an equivalent structure, or rather represents an initial state of the wrinkle layer since the early wrinkle layer is smooth as well. However, these layers also could be the ventral periostracum of the preceding whorl.
Since all of these structures are (more or less) derivates of the wrinkle layer, a similar formation can be assumed. Appearances of the wrinkle layer characterized by higher organic content, like the spherulitic-prismatic layer, the coating layer or simple thick “homogeneous” wrinkle layers (Fig. 4C2), may indicate a (temporary) higher production of organic compounds.
Phylogenetic and taxonomic implications of the outer organic component.—The wrinkle layer (and its derivates) is a frequent element of nearly all Jurassic and Cretaceous ammonoid superfamilies (SOM: table A). Its position in the shell wall and its extension as well as the ultrastructure of the wrinkles of the different taxa is more or less uniform. Therefore, we assume that it is a homologous shell feature and a plesiomorphy at least for Jurassic and Cretaceous taxa. The repeated development of similar structures is possible but since all these taxa are phylogenetically connected, it is most likely (by law of parsimony) that this feature appeared only once in the evolution of the Mesozoic ammonoids. Macroscopic and microscopic observations prove that wrinkle layers are common in Palaeozoic and Triassic taxa as well (e.g., Walliser 1970; House 1971; Tozer 1972; Doguzhaeva 1980, 1981; Korn 1985; Keupp 2000). These are probably homologous to the Jurassic and Cretaceous counterparts but their state must be evaluated in detail.
Our observations of the wrinkle ultrastructure match with the basal findings and descriptions of Doguzhaeva (1980: fig. 2A, B, 1981: fig. 2) and Kulicki et al. (2001) that include Carboniferous and Triassic taxa as well. However, these authors also report on prismatic and granular cores or completely prismatic wrinkles. Some wrinkles described show also a homogeneous (Doguzhaeva 1980, 1981: fig. 3B), lamellar (Zakharov and Grabovskaya 1984; Zakharov 1996), or predominantly prismatic (Doguzhaeva and Mutvei 1993b) ultrastructure. We suppose a secondary alteration of the wrinkle layer in all these cases of deviating appearance. Our findings suggest that the primary organic character of the wrinkle layer is prone to diagenetic alteration: e.g., hollow spaces (dissolution), granular layers (pyritization?), or prismatic layers (crystallization). In particular, the transition or parallel occurrence of pristine wrinkles and prismatic wrinkles is proved in some specimens (Fig. 4G).
The ultrastructure of wrinkles is unsuited for taxonomy. The various macroscopic patterns associated with the wrinkle layer are probably better suited for defining taxa (House 1971; Senior 1971; Tozer 1972). Korn (1985) assumed that differentiation of patterns is subjective too.
However, special deviations of the normal wrinkle layer (i.e., ridges and knobs) are unusual and are potentially characteristic for several taxa: e.g., (i) the merging wrinkles of Douvilleiceras mammillatum (Douvilleiceratoidea), (ii) the thick coating layer of Gaudryceras tenuiliratum (Tetragonitoidea), and (iii) the spherulitic prismatic layer of several genera of Haploceratoidea/Oppeliidea (e.g., Aconeceras sp. 1 and 2, Sanmartinoceras sp., and Taramelliceras externodosum).
All these structures are reoccurring features. Merging wrinkles are typical in all of our four specimens of D. mammillatum. The coating layer was identified several times (e.g., Drushits et al. 1978; Birkelund 1980; Doguzhaeva and Mutvei 1993b; Kulicki et al. 2001). The spherulitic prismatic layer was observed in several specimens by us and other authors (e.g., Doguzhaeva and Mutvei 1991, 1993b) but seems to be lacking in the related genus Hecticoceras. However, our specimens of this genus and those of other studies (e.g., Sprey 2002) are poorly preserved.
The dorsal inner prismatic layer.—The dorsal inner prismatic layer is the second and dominant component of the prismatic reduced dorsal shell wall (Figs. 1D, 4A1, B1, C2, 6A, 7A1). It attaches directly to the outer organic component (e.g., wrinkle layer). Nearly all observed ammonoid groups develop at least a short ontogenetic stage after the hatching that forms only this single aragonite layer dorsally (Fig. 9; SOM: table A).
In general, the innermost whorls lack a distinct dorsal inner prismatic layer. The early dorsal shell wall consists of (prismatic) septal mural parts and a thin, smooth outer organic component (dspl and ol in Fig. 5A1). Several of our specimens have not exceeded this ontogenetic stage due to their small diameter, e.g., Rudolphtruempiceras planorbis (Dinaritoidea), Pleuroceras solare (Eoderoceratoidea). In our specimens a continuous dorsal inner prismatic layer occurs between diameters of 2 to 8 mm. This is similar to the occurrence of the wrinkle layer (third to fourth whorl). However, its relief occurs mostly prior to the dorsal inner prismatic layer.
The dorsal inner prismatic layer is equivalent to its ventral/lateral counterpart, i.e., its continuation. The outer prismatic layer and middle nacreous layer of the ventral/lateral shell wall wedge out towards the spiral plane as they attach to the preceding whorl (Figs. 1F, 4F1, 10A). Only the inner prismatic layer coats the surface of the preceding whorl. Generally, the dorsal inner prismatic layer thickens towards the venter of the preceding whorl where it reaches maximal thickness: a circumstance best observed in phylloceratids and desmoceratids. In contrast to the wrinkle layer, the dorsal inner prismatic layer is restricted to the rear parts of the living chamber. It wedges out towards the aperture, leaving the wrinkle layer uncovered in the living chamber (Fig. 6). However, the ventral/lateral inner prismatic layer extends much further into the living chamber.
The dorsal inner prismatic layer can be thin, thick, single-layered or constructed of several sub-layers. Organic layers can separate the individual sub-layers. The morphological expression of the dorsal inner prismatic layer changes during ontogeny, e.g., changes in the thickness (normally an increase; Fig. 4A1, B1) or local appearance or disappearance of sub-layers (Fig. 4C). Sub-layering often occurs locally in several taxa but disappears afterwards, e.g., Phylloceras (Phylloceras) plicatum (Phylloceratoidea), Chamoussetia stuckenbergi (Stephanoceratoidea). With some exceptions, we cannot separate specific taxonomic groups by the internal structure or morphological expression of the dorsal inner prismatic layer (see below).
The dorsal inner prismatic layer acts as a sculptural compensator. It evens out the wrinkle layer relief (Figs. 1D, 4A1, B1, C2, 6A, B) and the sculpture of the preceding whorl (Fig. 10D1). In particular, the relief of rib concavities, constriction furrows or injuries (Figs. 4E, 8A) is compensated through local thickening. Often the dorsal inner prismatic layer becomes spherulitic or reveals a sub-layering. In the umbilical angle it usually forms a local thickening (Figs. 4F1, 10A1). Higher elevations of the sculpture can be bridged, leaving cavities (Fig. 11B). Sometimes the inner prismatic layer only adopts the relief of the preceding whorl.
The wrinkle layer complex.—A special kind of sub-layering of the dorsal inner prismatic layer can (sometimes) be observed during smoothing of the relief of the wrinkle layer (cf. Kulicki 1979; Zakharov 1996): e.g., in Phylloceratoidea, Amaltheidae, and Desmoceratoidea. A wrinkle layer complex consists of a sequence of four sub-layers: (i) the wrinkle layer, (ii) a primary dorsal inner prismatic sub-layer, (iii) an organic separation layer, and (iv) a secondary dorsal inner prismatic sub-layer (wl, dipl 1, ol, and dipl 2 in Fig. 4C1). The interspaces between individual wrinkles are filled by the first generation of the dorsal inner prismatic layer. This sub-layer and the tops of the wrinkles are coated with the thin organic layer on the inner surface. The secondary dorsal inner prismatic layer covers the arising undulating surface. Often, the organic separation layer is not present.
Unusual aspects of the dorsal inner prismatic layer.—Usually we cannot define specific taxonomic groups by the internal structure or morphological expressions of the dorsal inner prismatic layer. However, there are some exceptions: in Lobolytoceras costellatum, Protetragonites fraasi, and Argonauticeras besairiei (all Lytoceratoidea), the dorsal inner prismatic layer is usually subdivided into two very thick sub-layers (Fig. 8A). The thickness of the dorsal inner prismatic layer exceeds that of the entire ventral shell wall of the overgrown, preceding whorl by a factor of 1.5 to 2. A comparable thickness is not observed in any other of our taxa. Solely some Phylloceratoidea, e.g., Phylloceras (Euphylloceras) cf. velledae, Holcophylloceras polyolcum, Ptychophylloceras cf. dacquei, develop similar ratios (1:1) in outer whorls of large diameter (Fig. 4B1).
In Aconeceras sp. 1 and 2, the dorsal inner prismatic layer only covers the venter of the preceding whorl, where it reaches an enormous thickness (Fig. 8E1). At the ventral edge of the preceding whorl, the layer wedges out. The lateral whorl cover is usually absent. In the related Sanmartinoceras sp. the dorsal inner prismatic layer is similarly developed but transforms into a thin layer that covers the flanks of the preceding whorl.
Hedenstroemia hedenstroemi forms a prominent sub-layered dorsal inner prismatic layer of up to three different sub-layers (Fig. 10C1).
The dorsal septal prismatic layer.—It appears that the septal mural parts affect the dorsal shell wall. The inner prismatic layer has a tendency to merge with the inserted septa on contact (spl and dspl in Figs. 4C2, 8E1, E2). Several times, the septa and the inner prismatic layer show gradual transitions between the nacre of the septal wall and prisms of the inner prismatic layer (cf. septal prismatic layer in Howarth 1975). These structural transitions affect only the adoral saddles of the septa. In cross-section, the inner prismatic layer forms wedges at the septal contact which vanish towards the mouth (spl and dspl in Figs. 4A1, C2, 5A3, B, C). Locally, this often increases the thickness of the (dorsal) inner prismatic layer. Furthermore, the proximal, adoral septal surface is often covered by a prismatic layer (spl and dspl in Figs. 4C2, 5A3, B, 8E1, E2).
It is likely that all these septal-prismatic formations are connected to the septal mural parts. In particular, in specimens of Phylloceratoidea, Tetragonitoidea, and Desmoceratoidea, several times a distinct prismatic layer can be distinguished that originates in the septa (Fig. 5A3, B). This septal prismatic layer coats the inner surface of the inner prismatic layer. It can be separated by a thin organic layer from the actual inner prismatic layer. In general, at the umbilical edge the separation is most obvious. However, the separation of the dorsal inner prismatic layer and the dorsal septal prismatic layer becomes vague and both layers merge towards the crest of the former whorl.
In Argonauticeras besairiei the inner sub-layer of the dorsal inner prismatic layer seems to originate in the septa and fuses with them (dspl in Fig. 5C; inner part of dipl in Fig. 8A). Sometimes it even seems that the inner sub-layer wedges out adorally like a septal mural part (Fig. 5C). Also Hedenstoemia hedenstroemi forms a septal prismatic layer; hence some of the prismatic sub-layers of the dorsal shell wall (Fig. 10C1) may represent septal mural parts. Since the entire unusually distributed dorsal inner prismatic layer of Aconeceras sp. 1 and 2 (and Sanmartinoceras sp.) is usually associated with the septal contact zone, and otherwise is absent, it is possible that their dorsal inner prismatic layer is only of septal origin (Fig. 8E1, E2).
In several phylloceratid, perisphinctid, and desmoceratid specimens (where the dorsal inner prismatic layer is still absent), the adoral septal mural parts of the juvenile whorls (dspl in Fig. 5A1) gradually elongate, bridge the distance between the single septa (dspl in Fig. 5A2) and ultimately fuse to the continuous dorsal inner prismatic layer in the course of shell growth. In one specimen of Eogaudryceras (Eotetragonites) umbilicostriatus (BSPG MAo-1775), the dorsal inner prismatic layer seems to originate at its 20th septum, i.e., its mural part.
The formation and function of the dorsal inner prismatic layer.—Apparently, the dorsal inner prismatic layer was the second layer to be formed in the prismatic reduced dorsal shell wall, i.e., it directly attaches to the outer organic component, e.g., wrinkle layer. Its restricted extension within the living chamber (it wedges out towards the aperture in the rear) indicates a secretion in the rear part of the living chamber. However, the ventral/lateral inner prismatic layer extends much further into the living chamber, implying a disparity between the ventral, lateral, and dorsal secretion zones for the inner prismatic layer. Reoccurring sub-layers (e.g., wrinkle layer complex) probably indicate an intermittent secretion process or at least (brief) interruption during mineralization.
The primary task of this layer seems to be to smooth out the internal relief of the living chamber. Apparently, smoothing of the shell interior was crucial for an ammonoid, probably to facilitate the attachment of the nacreous septa that are subsequently inserted adapically. The dorsal inner prismatic layer and the nacreous layer of the septa argue for two distinct secretion regimes of the adapical mantle epithelium, i.e., prismatic and nacreous.
However, at the septal contact, wedge-like thickenings of the dorsal inner prismatic layer or even a separate septal prismatic layer often merge with the nacre layer of the septa, therefore suggesting that adapical mantle portions are able to form both materials. These structures and transitions could represent diagenetic alteration (e.g., recrystallization, epitaxial crystal growth) but widespread and recurrent observations of these in different ammonoid taxa and fossil localities by us and in other studies (e.g., Hölder 1952; Birkelund and Hansen 1968, 1974; Blind 1975, 1976; Howarth 1975; Kulicki 1979, 1996; Birkelund 1980; Doguzhaeva and Mutvei 1986; Kulicki et al. 2016) argue for a distinct element of the shell wall, which Howarth (1975) first described as a discrete septal prismatic layer of the dorsal (and ventral/lateral) shell wall of Dactylioceratidae. We consider the septal prismatic layer as proven. The septal prismatic layer probably represents an additional secretion stage which begins with the formation of the septa, either as a discrete generation of the inner prismatic layer or as prismatic mural parts which can fuse with formerly secreted prismatic material.
In general, a strict chemical (i.e., specific matrix proteins) and spatial separated formation of prismatic and nacreous material by the molluscan mantle (i.e., distinct mantle portions with probably appropriate differentiated cells form distinct materials) is assumed and partly proven (e.g., in the pearl oyster Pinctada; Joubert et al. 2010; Marie et al. 2012; Funabara et al. 2014). Our observations of transition structures in ammonoid aragonitic shell wall indicate that at least the adapical mantle portions, that form the septa, were able to form both materials. A strict chemical and spatial separation is not necessarily given. This either means that two different cell types (i.e., prismatic vs. nacre secreting) existed simultaneously, that the concurrent cells were able to do multiple tasks (i.e., formation of different matrix proteins) or that both chemical secretion processes are closely linked (i.e., related or identical matrix proteins) in ammonoids. Blind (1975, 1976) already assumed that the mantle epithelium that secrets the septa could change its secretion mode depending on function. Cell secretion plasticity is at least known in Gastropoda (Fleury et al. 2008) and Bivalvia (Cuif et al. 2011). Carter and Clark (1985) even assume that the nacreous layer is derived from the aragonitic prismatic layer (cf. Bandel 1977; Fleury et al. 2008).
Phylogenetic and taxonomic implications of the prismatic reduced dorsal shell wall.—The vast majority of our Mesozoic ammonoids forms a prismatic reduced dorsal shell wall which is in accordance with former studies of the dorsal shell wall of planispirally coiled ammonoids (see Introduction, Fig. 9; SOM: table A). Because of the wide distribution of this dorsal shell wall type within the phylogenetic tree in combination with its usual early ontogenetic formation (subsequent, diverging ontogenetic changes are possible, see below), we assume that the prismatic reduced dorsal shell wall represents the primary state of dorsal shell wall construction at least in Mesozoic ammonoid taxa. Observations in members of the Goniatitina (Erben et al. 1968, 1969; Walliser 1970; Kulicki et al. 1999, 2001, 2002; Doguzhaeva 2002) imply similar conditions already in Devonian, Carboniferous, and Permian ammonoids (SOM: table A). Therefore, we presume that the prismatic reduced dorsal shell wall is a synapomorphic character of all coiled ammonoids. It is likely that in order to reduce weight, material consumption and formation effort, the dorsal shell was usually suppressed in planispiral ammonoids analogous to the planispiral Nautilidae.
However, variations in the outer organic component are probably taxon-specific (see above). In particular, the occurrence and morphological expression of the inner prismatic layer vary widely and may allow the distinction of individual taxa at the genus or species level or higher order taxa. Some phylloceratids have an extraordinarily thick dorsal inner prismatic layer, however, it cannot be observed in all of our specimens. With the exception of Argonauticeras besairiei, Lobolytoceras costellatum, and Protetragonites fraasi (all Lytoceratoidea), no other ammonoid in our study forms a comparably thick dorsal inner prismatic layer. The dorsal inner prismatic layer of the genus Aconeceras attracts attention through its unusual restriction to the ventral crest of the preceding whorl. Since our knowledge of the individual shell structures is still limited, these characteristics and findings have to be used with caution.
The nacreous reduced dorsal shell wall.—The dorsal shell wall of this type is three-layered consisting of two prismatic layers that enclose a nacreous layer (Figs. 1E, 12A–C, D1, D2, 13B, C1, 14A1). These layers do not form a continuum with the three layers of the ventral/lateral shell wall but rather correspond to an umbilical shell doubling that extends towards the ventral crest of the preceding whorl (Figs. 1G, 12D3). At the aperture, the dorsal shell wall is absent (Fig. 2A2). A nacreous reduced dorsal shell wall can be observed in some planispirally coiled genera of Stephanoceratoidea (e.g., Kepplerites galilaeii), Perisphinctoidea (e.g., Perisphinctes [Kranaosphinctes] mahabokensis), Desmoceratoidea (e.g., Eupachydiscus sp.) and Deshayesitoidea (e.g., Colombiceras sp.) (Fig. 9, Table 1; SOM: table A).

Fig. 12. Construction of the nacreous reduced dorsal shell wall (A, D3, transversal section, centrifugal, B, C, D1, D2, median section, growth direction to the left, centrifugal). A. Perisphinctes (Kranaosphinctes) mahabokensis (Collignon, 1959), BSPG MAn-4835, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; A1, the dorsal shell wall consists of a primary dorsal inner prismatic layer, a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer; A2, the secondary dorsal inner prismatic layer; A3, the primary dorsal inner prismatic layer. B. Kepplerites galilaeii (Oppel, 1862), BSPG MAn-4783, early Callovian, Jurassic, Znamenka on Unzha River, Russia; same as in A1. C. Mirosphinctes sp. 1, BSPG MAn-1769, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; the dorsal shell wall consists of a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer. D. Aspidoceras sp., BSPG MAn-4507, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; D1, D2, the same as in A1; D3, at the umbilical seam multiple new shell layers are formed; the inner layers of the (dorsal) nacreous layer (dncl 1–3) and of the (dorsal) inner prismatic layer (dipl 1–4) wedge out towards the spiral plane; the inner layers form the nacreous reduced dorsal shell wall. Abbreviations: dipl 1/2/3/4, primary/secondary/tertiary/quaternary dorsal inner prismatic layer; dncl 1/2/3/4, primary/secondary/tertiary/quaternary dorsal nacreous layer; dspl, dorsal septal prismatic layer; if, infilling; ipl, inner prismatic layer; ipl 1/2, primary/secondary inner prismatic layer; ncl, nacreous layer; ncl 1/2, primary/secondary nacreous layer; opl, outer prismatic layer; s, septum.
The morphological expression of the three layers of the nacreous reduced dorsal shell wall looks very similar to the proportions observed in the ventral/lateral shell wall simulating a connection (Figs. 12C, D1, 14A1), but the dorsal and ventral/lateral shell layers are not equivalent. The outer prismatic and nacreous layer of the ventral/lateral shell wall wedge out towards the spiral plane at the umbilical seam (Fig. 1G). The actual nacreous reduced dorsal shell wall begins as a (strong) umbilical shell doubling that reinforces the three-layered ventral/lateral shell wall with a secondary nacreous layer and secondary inner prismatic layer. The shell doubling continues as the dorsal shell wall towards the ventral crest of the preceding whorl (Fig. 1G). The three layers of the dorsal shell wall are equivalent to the (primary) inner prismatic layer, the secondary nacreous layer and the secondary inner prismatic layer of the ventral/lateral wall and the umbilical shell doubling, and therefore are called the primary dorsal inner prismatic layer, the secondary dorsal nacreous layer and the secondary dorsal inner prismatic layer of the dorsal shell wall. In the outer whorls of some taxa (e.g., Aspidoceras sp.), at the umbilical edge further additional inner prismatic and nacreous layers can develop but only the three innermost layers continue towards the ventral crest of the preceding whorl. The remaining outermost layers wedge out towards the spiral plane at the umbilical seam (dncl 1–3 and dipl 1–4 in Fig. 12D3).
Typically, the whole nacreous reduced dorsal shell wedges out towards the aperture in the rear living chamber adorally of the last septum (Figs. 2A2, 14A). The primary dorsal inner prismatic layer usually attaches directly to the preceding whorl. The nacreous reduced dorsal shell wall smooths the sculpture of the preceding whorl (Fig. 12C, D1). Often the whole package bridges the relief (Fig. 15A). In other cases, it thickens during compensation. In particular, the secondary dorsal nacreous layer thickens in the rib concavities, but thins at the rib crests (Figs. 12B, C, 15A).
The ontogenetic development shows that a nacreous reduced dorsal shell wall replaces a prismatic reduced dorsal shell wall (i.e., wl and ipl). With the exception of the Aspidoceratinae (see below), additional shell layers appear at diameters of 25 to 79 mm (Table 1). However, in part the values represent the first possible observations due to inadequate preservation of inner whorls, i.e., earlier occurrences are possible. Because of this preservation gap, data on the whorl number cannot be given.
The first nacreous structures are part of the outer portions of the (secondary) dorsal inner prismatic layer. The outer portion of the layer develops nacreous inclusions which originate in the prisms, i.e., “partitioning of the prisms” (Fig. 14B, C). With further growth a separate shell layer is clearly defined. The juvenile secondary dorsal nacreous layer is rather thin, consisting of few nacre lamellae, but thickens gradually during progressive growth and usually becomes the main component of the dorsal shell. Thus the dorsal shell wall of large Perisphinctes (Kranaosphinctes) mahabokensis (D = 210 mm, BSPG MAn-4835) is dominated by a very thick secondary dorsal nacreous layer (Fig. 12A1). The primary dorsal inner prismatic layer (Fig. 12A3) and secondary dorsal inner prismatic layer (Fig. 12A2) are subordinate components. Also in large specimens of Eupachydiscus sp. (D ≥ 300–450 mm, BSPG MAo-1832–1834) the secondary dorsal nacreous layer becomes an essential and dominant dorsal shell element (Fig. 13C1). However, the primary and secondary dorsal inner prismatic layers are of comparable thickness. In both taxa the primary dorsal inner prismatic layer develops two sub-layers and a more or less spherulitic-prismatic appearance (Figs. 12A3, 13B, C1). In Eupachydiscus, the inner sub-layer has a more palisade-like structure. The outer spherulitic sub-layer often appears granular. Also the secondary dorsal inner prismatic layer consists of two sub-layers in Eupachydiscus (Fig. 13C1).
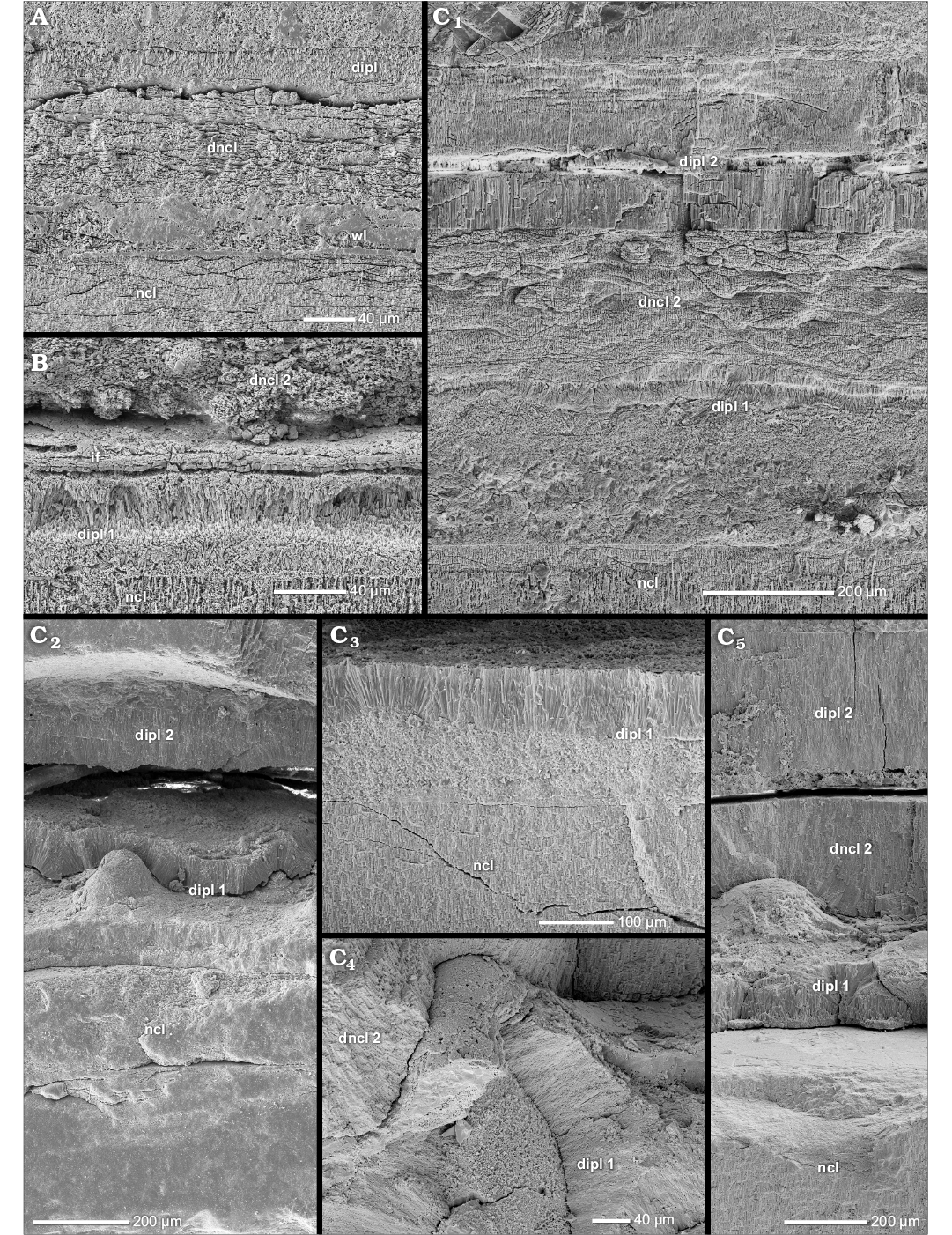
Fig. 13. Construction of a secondary complete dorsal shell wall and the nacreous reduced dorsal shell wall (A, median section, growth direction to the left, centrifugal; B, C, transversal section, centrifugal). A. Cleoniceras (Grycia) besairiei Collignon, 1949, BSPG PA-33582, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; in reaction to a forma aegra aptycha of Keupp (1977), the dorsal shell wall is secondarily complete; it consists of an outer wrinkle layer, a dorsal nacreous layer and a dorsal inner prismatic layer. B, C. Eupachydiscus sp., Campanian, Cretaceous, Teshio-Nakagawa area, Hokkaido, Japan. B. BSPG MAo-1832, the primary dorsal inner prismatic layer consists of two sub-layers. C. BSPG MAo-1834; C1, the dorsal shell wall consists of a primary dorsal inner prismatic layer, a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer; the primary and the secondary dorsal inner prismatic layer develop sub-layers; the primary dorsal inner prismatic layer shows a relief (i.e., “Ritzknoten”); C2, C3, umbilical-lateral, the primary inner prismatic layer forms cone-like elevations, i.e., “Ritzknoten”; C4, C5, the “Ritzknoten” reach up to the umbilical seam and the dorsum. Abbreviations: dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl, dorsal nacreous layer; dncl 2, secondary dorsal nacreous layer; if, infilling; ncl, nacreous layer; wl, wrinkle layer.
Interestingly in Eupachydiscus sp., the secondary dorsal nacreous layer wedges out near the umbilical seam (Fig. 1C). It reappears as a thin cover of the preceding whorl at its mid-flank. Towards the ventral crest of the preceding whorl the layer thickens. There, it is up to 10 times thicker than at the mid-flank.
A particularity of the outer whorls in Colombiceras sp. is that the outer prismatic layer of the ventral/lateral shell wall does not wedge out at the umbilical seam but extends up to the ventral edges of the preceding whorl. Therefore, the dorsal shell wall consists of four shell layers at the flanks of the preceding whorl (Fig. 14D) but three layers at its crest.

Fig. 14. Construction of the nacreous reduced dorsal shell wall (A–C, E, median section, growth direction to the right, centrifugal; D, transversal section, centrifugal). A. Aspidoceras sp., BSPG MAn-3193, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; the dorsal shell wall consists of a primary dorsal inner prismatic layer, a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer; the dorsal shell wall becomes thinner towards the aperture (A1–A3) and vanishes completely (A4). B. Eupachydiscus sp., BSPG MAo-1831, Campanian, Cretaceous, Teshio-Nakagawa area, Hokkaido, Japan; the early secondary nacreous layer is part of the secondary inner prismatic layer. C. Euaspidoceras sp. 2, BSPG MAn-4751, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; same as in B. D. Colombiceras sp., BSPG MAo-1884, Aptian, Cretaceous, Caucasus region, Russia; the dorsal shell wall cover of the flanks of the preceding whorl consists of a dorsal outer prismatic layer, a primary dorsal inner prismatic layer, a secondary dorsal nacreous layer (and a secondary dorsal inner prismatic layer). E. Desmoceras (Desmoceras) latidorsatum (Michelin, 1838), BSPG MAo-1787, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; the dorsal shell wall can develop nacreous material within the dorsal inner prismatic layer. Abbreviations: dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl 2, secondary dorsal nacreous layer; hbl, heringbone layer; if, infilling; ipl, inner prismatic layer; ncl, nacreous layer; opl, outer prismatic layer; wl, wrinkle layer.
The nacreous reduced dorsal shell wall of Aspidoceratinae.—Members of the Aspidoceratinae (Perisphinctoidea) form a nacreous reduced dorsal shell wall most frequently and significantly earlier in ontogeny than other taxa (Figs. 12C, D1, D2, 14A1, C, Table 1; SOM: table A). In most members of Aspidoceratinae (e.g., Aspidoceras sp., Euaspidoceras sp. 2, Mirosphinctes sp. 1 and 2, Pseudowaagenia sp.), the first nacreous structures occur at diameters between 6 and 9 mm. Nacre onset usually coincides with the beginning of the sculpture of the preceding whorl (Fig. 12C, D1), i.e., prismatic radial lirae (cf. Radtke and Keupp 2016). The nacreous reduced dorsal shell wall compensates the relief.
Epaspidoceras jeanetti seems to be an exception to this common observation. Three (of four) specimens maintain the state of a prismatic reduced dorsal shell wall, even up to a diameter of 72 mm (BSPG MAn-4503). Nevertheless, this species was probably also able to form a nacreous reduced dorsal shell during ontogeny. At least one specimen (BSPG MAn-4505) develops this dorsal shell type at a diameter of 31 mm. Conch fragments of a large Euaspidoceras sp. 1 (BSPG MAn-4750) seem to prove at least an ontogenetic late existence at a diameter of 110 mm.
In contrast to other taxa, where the nacreous reduced dorsal shell wall corresponds only to an umbilical shell doubling, the shell doubling can coat the whole interior of the conch in Aspidoceratinae (Fig. 12D1).
The formation and function of the nacreous reduced dorsal shell wall.—The nacreous reduced dorsal shell has to be secreted in the rear living chamber since it does not extend up to the aperture, i.e., it wedges out. Ventral and lateral shell doublings were probably similarly formed but could adorally extend much further into the living chamber as associated muscle scars imply (Doguzhaeva and Mutvei 1991).
The adapical mantle portions, that formed this dorsal shell wall type, had the ability to form prismatic and nacreous material. Our observations imply that the secondary dorsal nacreous layer appears to be derived from the (secondary) dorsal inner prismatic layer (i.e., partitioning) of a prismatic reduced dorsal shell wall. We assume that this early stage shows a rearrangement of the adapical mantle. Its cells seem to develop new secretion abilities. The clear separation of all three layers in later ontogeny implies the emergence of distinct apical mantle sections for each layer.
The nacreous reduced dorsal shell wall is a product of ongoing ontogeny replacing a prismatic reduced dorsal shell wall. Apparently, the taxa have to reach a certain size (D = 6–79 mm) or age. However, size alone seems not to be the determining trigger; there is no general ontogenetic pattern of occurrence in size. Even large specimens of e.g., Argonauticeras besairiei (BSPG MAo-1802, D = 102 mm), Phylloceras (Euphylloceras) cf. velledae (BSPG MAo-1880, D = 106 mm), or Divisosphinctes sp. 1 (BSPG MAn-4499, D = 74 mm) can lack a nacreous reduced dorsal shell wall.
An important trigger seems to be the relief of the preceding whorl. The nacreous reduced dorsal shell wall of aspidoceratids commences with the formation of prismatic radial lirae (cf. Radtke and Keupp 2016). It also smooths out the sculpture of Kepplerites galilaeii, Perisphinctes (Kranaosphinctes) mahabokensis, and Colombiceras sp. which develop prominent ribs. It can be assumed that relief smoothing facilitates the attachment of the septa. However, the prismatic reduced dorsal shell wall adopts the same function but the nacreous reduced dorsal shell wall was probably much more robust due to its nacreous character. Nacre exhibits an extremely high resistance to fracture (Jackson et al. 1988).
Likely occurrences of the nacreous reduced dorsal shell wall.—Some rather inconspicuous formations of dorsal nacre are probably expressions of the nacreous reduced dorsal shell wall. All these formations are associated with, or are rather a part of, the dorsal inner prismatic layer. Often both materials merge with each other. An assignment to the septa can be excluded. At least we assume a similar place of secretion as the nacreous reduced dorsal shell wall.
At two points, at a distance of 360°, the dorsal inner prismatic layer of one specimen of Puzosia saintoursi (Desmoceratoidea, BSPG MAo-1797) develops nacre-like structures (Fig. 11A1, A3) which in the following shell portions repeatedly disappear and reappear. Similar to Eupachydiscus sp., P. saintoursi can form an umbilical shell doubling which probably is responsible for the formation of dorsal nacre. Also one specimen of Desmoceras (Desmoceras) latidorsatum (Desmoceratoidea, BSPG MAo-1787) develops similar nacreous portions (Fig. 14E).
One large specimen of Kosmoceras cf. duncani (Stephanoceratoidea, BSPG MAn-4788, D = 90 mm) forms inclusions of nacreous material within thickenings of the dorsal inner prismatic layer (Fig. 15B) which weaken the relief of ventro-lateral spines of the preceding whorls. Fragments of the dorsal shell wall of a large Speetoniceras versicolor (Perisphinctoidea, BSPG MAo-1861, D = 200 mm) show prismatic material which is interbedded with patches of nacre (Fig. 15C).
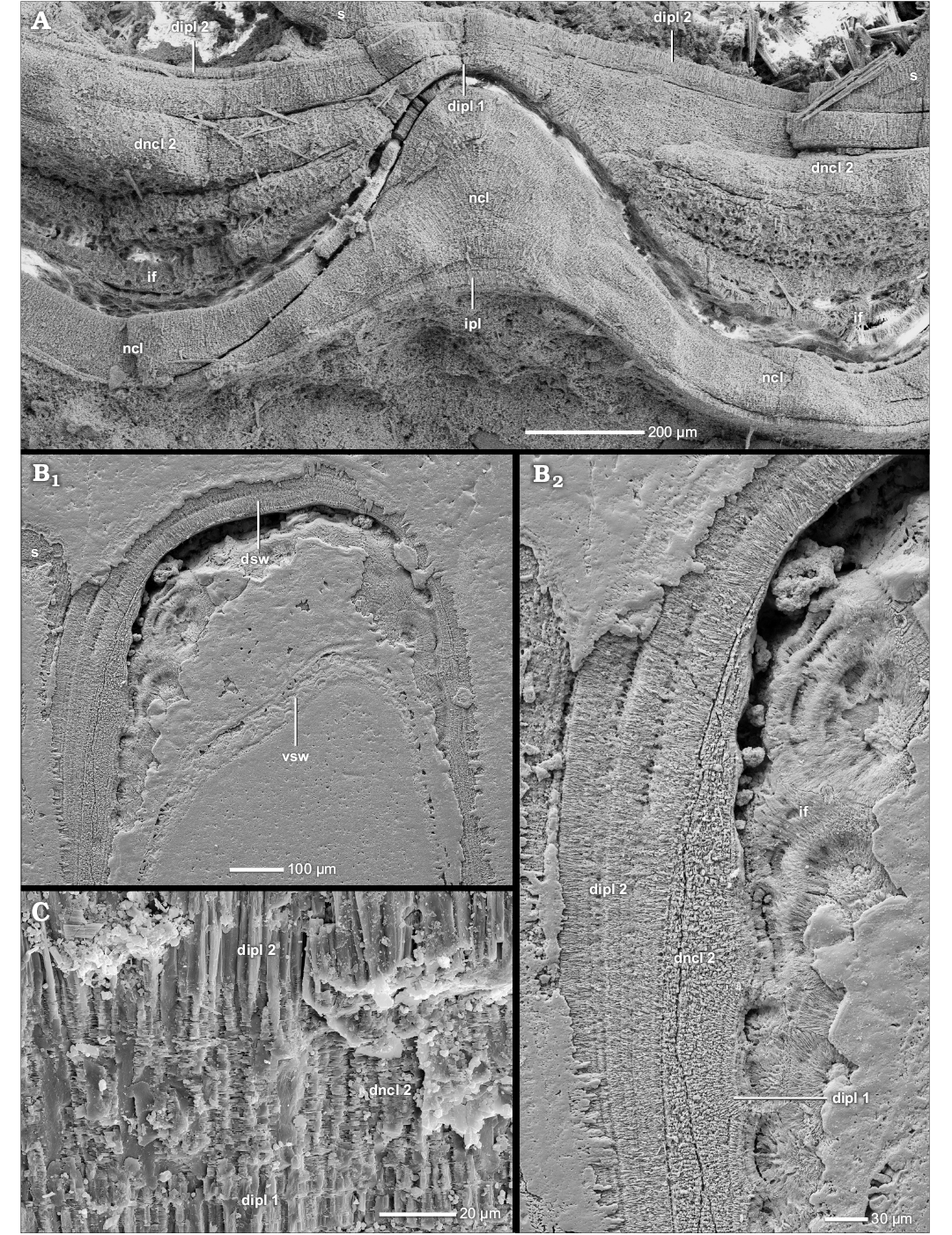
Fig. 15. Construction of the nacreous reduced dorsal shell wall (A, median section, growth direction to the left, centrifugal; B, C, transversal section, centrifugal). A. Kepplerites galilaeii (Oppel, 1862), BSPG MAn-4783, early Callovian, Jurassic, Znamenka on Unzha River, Russia; a thickening of the secondary dorsal nacreous layer compensates the rib relief; the layer thickens in the rib concavitie, but thins at the rib crest (compare Fig. 12B). B. Kosmoceras (Kosmoceras) cf. duncani (Sowerby, 1816), BSPG MAn-4788, late Callovian, Jurassic, Dubki near Saratov, Russia; B1, the spines (vsw) are overgrown by a thick dorsal shell wall (dsw) which forms nacreous portions; B2, close-up of B1; at the left flank of the spine a nacreous portion occurs in the dorsal shell wall. C. Speetoniceras versicolor (Trautschold, 1865), BSPG MAo-1861, early Aptian, Cretaceous, Simbirsk, Ulyanovsk, Volga Basin region, Russia; the dorsal inner prismatic layer develops inclusions of nacre. Abbreviations: dipl 1/2, primary/ secondary dorsal inner prismatic layer; dncl 2, secondary dorsal nacreous layer; dsw, dorsal shell wall; if, infilling; s, septum; vsw, ventral shell wall.
The complete dorsal shell wall.—Several heteromorph and planispirally coiled taxa develop a complete dorsal shell wall (Fig. 9, Table 2; SOM: table A) that corresponds to its ventral and lateral equivalents (Figs. 2B, 3A, B). A complete dorsal shell wall can be observed in heteromorph taxa of Scaphitoidea (e.g., Hoploscaphites nicoletti) and Ancyloceratoidea (e.g., Ancyloceratoidea indet.) as well as in some planispirally coiled taxa of Lytoceratoidea (e.g., Lobolytoceras costellatum), Eoderoceratoidea (e.g., Amaltheus margaritatus), Stephanoceratoidea (e.g., Quenstedtoceras henrici), Perisphinctoidea (e.g., Coffatia [Grossouvria] sp. 2), Haploceratoidea (e.g., Hecticoceras (Sublunuloceras) sp.), Desmoceratoidea (e.g., Cleoniceras (Grycia) besairiei), Hoplitoidea (e.g., Metaplacenticeras subtilistriatum), and Douvilleiceratoidea (e.g., Douvilleiceras mammilliatum).
In this dorsal shell wall type, the three shell layers of the ventral, lateral, and dorsal shell wall form a more or less continuous conch tube around the aperture (Figs. 2B, 3A, B). The wall consists of the same outer prismatic layer, median nacre layer, and inner prismatic layer all around (Figs. 16, 17A, 18). Our heteromorphs show that at least the dorsal nacreous layer extends up to the aperture (Fig. 18D). There, it ends simultaneously with its ventral and lateral counterparts. The dorsal inner prismatic layer is only formed in the rear parts of the living chamber. However, several taxa developed structural variations differing from the common case (see below).
 |
Fig. 16. Construction of the complete dorsal shell wall (transversal section, centrifugal). Lobolytoceras costellatum (Pavia, 2002), BSPG MAn-3059, late Oxfordian, Sakaraha, Morondava Basin, SW Madagascar. A. The dorsal shell wall consists of a dorsal outer prismatic layer, a dorsal nacreous layer and a dorsal inner prismatic layer. B. Close-up of A; the thick dorsal inner prismatic layer consists of two sub-layers. C. Close-up of A; the dorsal outer prismatic layer. Abbreviations: dipl, dorsal inner prismatic layer; dncl, dorsal nacreous layer; dopl, dorsal outer prismatic layer. |
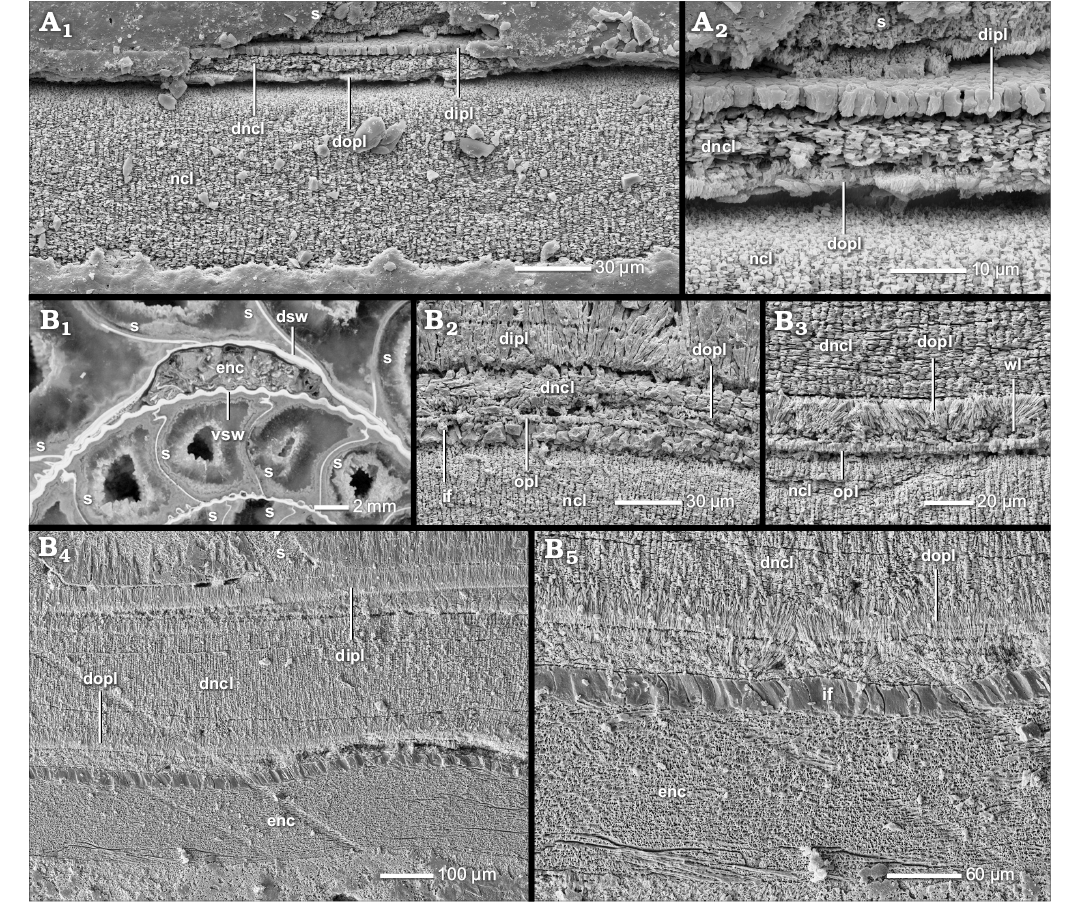 |
Fig. 17. Construction of the complete dorsal shell wall (median section, growth direction to the left, centrifugal). A. Choffatia (Grossouvria) sp. 1, BSPG MAn-4520, late Callovian, Jurassic, Dubki near Saratov, Russia; A1, the dorsal shell wall consists of a dorsal outer prismatic layer, a dorsal nacreous layer and a dorsal inner prismatic layer; A2, close-up of A1. B. Divisosphinctes besairiei Collignon, 1960, BSPG PA-10151b, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; B1, the ventral shell wall (vsw) is overgrown by an encruster (enc) which is in turn overgrown by the succeeding whorl (dsw); B2, the thin early complete dorsal shell is formed in contact with the ventral shell wall; it consists of (a wrinkle layer), a dorsal outer prismatic layer, a dorsal nacreous layer and a dorsal inner prismatic layer; B3, the dorsal outer prismatic layer; B4, the older, thick, detached dorsal shell wall consists of the same three layers as in B2; B5, close-up of B4. Abbreviations: dipl, dorsal inner prismatic layer; dncl, dorsal nacreous layer; dopl, dorsal outer prismatic layer; dsw, dorsal shell wall; enc, encruster; if, infilling; ncl, nacreous layer; opl, outer prismatic layer; s, septum; vsw, ventral shell wall; wl, wrinkle layer.
|
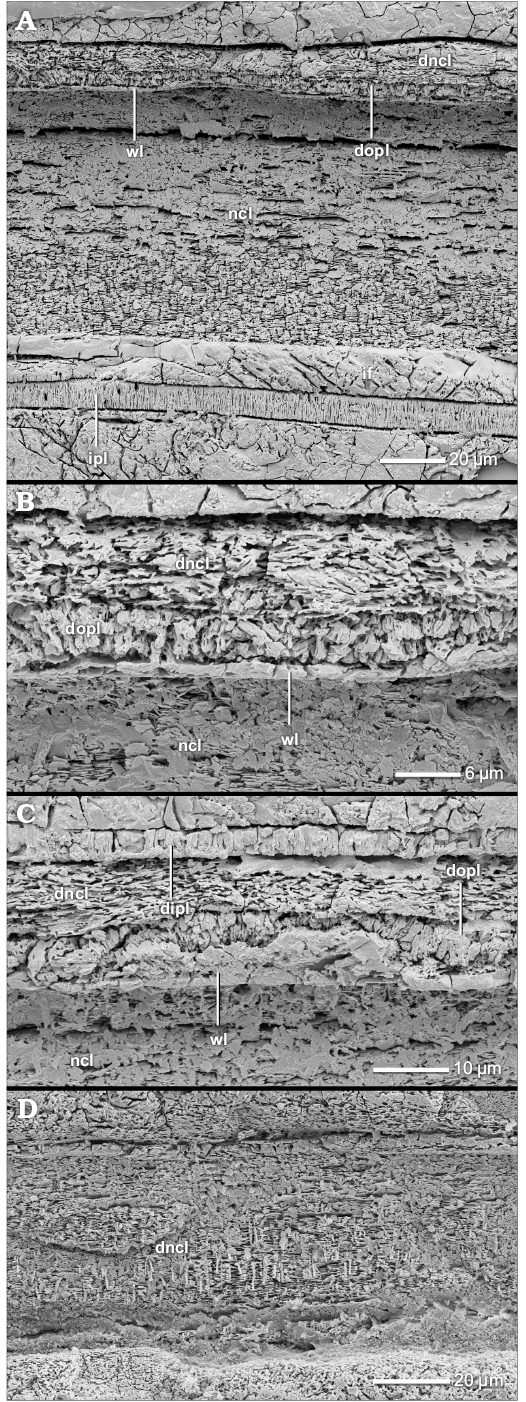 |
Fig. 18. Construction of the complete dorsal shell wall (transversal section, centrifugal). Hoploscaphites nicoletii (Morton, 1842), AMNH-FI-99143, Maastrichtian, Cretaceous, Fox Hills Formation (Loc. 3272), S Dakota, USA. A–C. Prior to the detachment of the living chamber, the dorsal shell wall is already complete and consists of a wrinkle layer, a dorsal outer prismatic layer, a dorsal nacreous layer and a dorsal inner prismatic layer. B. Close-up of A. D. In the detached living chamber, at least a dorsal nacreous layer is formed. The dorsal outer prismatic layer and the dorsal inner prismatic layer are not preserved. Abbreviations: dipl, dorsal inner prismatic layer; dncl, dorsal nacreous layer; dopl, dorsal outer prismatic layer; if, infilling; ipl, inner prismatic layer; ncl, nacreous layer; wl, wrinkle layer. |
The complete dorsal shell wall of Scaphitoidea.—Complete dorsal shell walls are favoured in heteromorph conchs like those of Scaphitpidea (Fig. 18). Our observations show that the dorsal shell wall of scaphitoids undergo an ontogenetic development. The planispiral, juvenile conch (phragomocone) is characterized by a prismatic reduced dorsal shell wall and can even form a wrinkle layer (Fig. 18B, C). A complete dorsal shell wall is accompanied by the detachment of their hook-like living chamber. Usually, already prior to the detachment, a first very thin dorsal nacreous layer and sometimes a first thin dorsal outer prismatic layer appear attaching to the preceding whorl, and internally sealed by the dorsal inner prismatic layer (Fig. 18A–C). We find diameter values of ca. 16 to 56 mm (Table 2). Observations in Hoploscaphites nicolletti show that the primary formation of the dorsal nacreous layer is not coeval in the whole conch tube. The dorsal nacre layer thus becomes thinner from the ventral crest of the preceding whorl towards its ventral edge and completely disappears at its flanks. The dorsal shell wall that covers the lateral shell portions consists of a dorsal inner prismatic layer only.
However, there are individual differences occuring in the ontogeny of particular taxa. For example, in Scaphites whitfieldi the first thin nacreous portions occur in the “phragmocone” (D = 22 mm). Then, ¼ whorl later, shortly before the detachment of the hooked living chamber, the dorsal nacreous layer thickens episodically (D = 27 mm): A new nacre package is attached repeatedly from within, i.e., imbrications (dncl A–C in Fig. 19A). However, the older nacre packages persist and the overall shell thickness increases. The thickening starts at a distance of ca. 4 septal spaces (adult septa compression) in front of the living chamber and ends with the last septum (or rather the development of the adult aperture).
In H. nicoletti, the dorsal nacreous layer begins in the adult living chamber. The diameter of the initiation of the dorsal nacreous layer changes depending on the beginning of whorl detachment. It varies between 16 and 56 mm (sexual dimorphism). In the rear living chamber, as well as in the phragmocone, only a prismatic reduced dorsal shell wall is formed. A short distance before the detachment of the hook (distance equivalent of 1–3 septal spaces) the first nacreous lamellae appear (Fig. 18A–C). The layer becomes successively thicker towards the aperture (Fig. 18D).
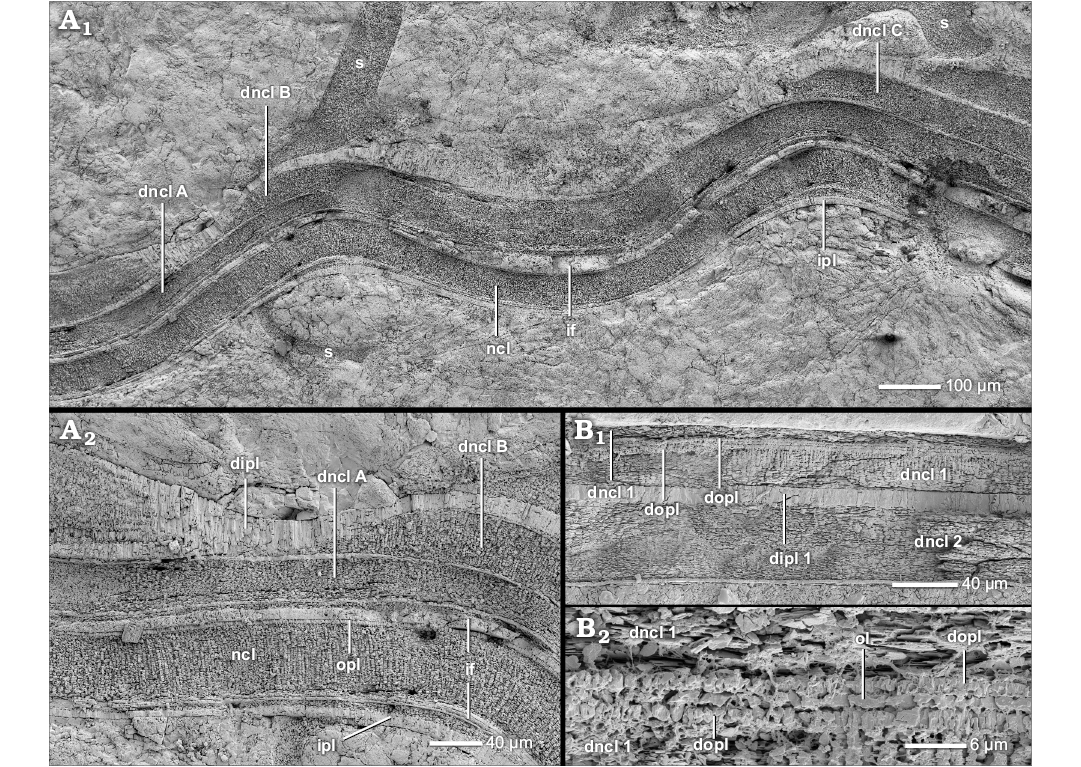
Fig. 19. Construction of the complete dorsal shell wall (A, median section, growth direction to the right, centrifugal; B, transversal section, centrifugal). A. Scaphites whitfieldi Cobban, 1951, AMNH-FI-99144, Touronian, Cretaceous, Turner Sandy Member (Loc. 3190), Wyoming, USA; A1, shortly before complete detachment of the living chamber, the dorsal nacreous layer is repeatedly reinforced by new generations of the dorsal nacreous layer (dncl A–C); A2, close-up of A1. B. Ancyloceratoidea indet., BSPG MAo-1813, Aptian, Cretaceous, Shilovka near Volga River, Russia; B1, contact of both dorsal shell portion of two succeeding shafts; the dorsal shell wall consists of a dorsal outer prismatic layer, a primary dorsal nacreous layer, a primary dorsal inner prismatic layer and a secondary dorsal nacreous layer; the secondary dorsal inner prismatic layer is not preserved; B2, close-up of B1. Abbreviations: dipl, dorsal inner prismatic layer; dipl 1/2, primary/secondary dorsal inner prismatic layer; dncl, dorsal nacreous layer; dncl 1/2, primary/secondary dorsal nacreous layer; dncl A–C, generations of the dorsal nacreous layer; if, infilling; ipl, inner prismatic layer; ncl, nacreous layer; ol, organic layer; s, septum.
The complete dorsal shell wall of planispirally coiled ammonoids.—A complete dorsal shell wall can also be observed in planispirally coiled ammonoid taxa (Fig. 9, Table 2; SOM: table A) like Lobolytoceras costellatum (Lytoceratoidea; Fig. 16) and Choffatia (Grossouvria) sp. 2 (Perisphinctoidea; Fig. 17A). In both taxa we observe a continuum of the ventral, lateral, and dorsal shell layers (Fig. 3B). At least the nacreous layer and the inner prismatic layer extend up to the ventral crest of the preceding whorl. On occasion, a dorsal outer prismatic layer can be observed (Figs. 16C, 17A2).
Similar to the scaphitids, a complete dorsal shell wall develops after a prismatic reduced dorsal shell wall. The first thin nacreous structures occur as an outer cover of the dorsal inner prismatic layer around a diameter of 66 mm in L. costellatum and 22 mm in Choffatia (Grossouvria) sp. 2. Unfortunately, the inner whorls of these specimens are lacking, therefore a connection with whorl number cannot be determined. In this early stage, the dorsal nacreous layer is thin but becomes thicker with progressing ontogeny. Thus in large specimens of L. costellatum (BSPG MAn-3059, D = 620 mm), the dorsal nacreous layer is of similar dimensions to the thick dorsal inner prismatic layer (Fig. 16A, B).
The specimen of Choffatia (Grossouvria) sp. 2 is probably an isolated case. At least two larger specimens of Choffatia (Grossouvria) sp. 1 maintain a prismatic reduced dorsal shell wall up to a diameter of 60 mm.
Secondarily formed complete dorsal shell walls.—On two occasions we observed the formation of a (secondary) complete dorsal shell wall as a result of an external trigger (e.g., encrusting, injury) in planispirally coiled taxa (Table 2). It is preceded by a prismatic reduced dorsal shell wall.
One specimen of Divisosphinctes besairiei (Persphinctoidea, BSPG PA-10151b) develops a complete dorsal shell wall during overgrowth of a ventrally attached oyster (Fig. 17B1; cf. Keupp 2012: fig. 238 left). The dorsal shell wall detaches from the preceding whorl to overgrow the oyster, forming cavities adorally and abapically. The dorsal shell wall forms a continuum with the ventral/lateral shell portions and their internal structures are identical (Fig. 17B2, B4). The complete dorsal wall begins adapically to the oyster while the dorsal shell wall still attaches to the preceding whorl. At a length of approximately 9 septal spaces adapically to, or rather before, the encruster (D = 39 mm), the first nacreous lamellae (1–3) occur. The early dorsal outer prismatic layer is absent or later not well defined (Fig. 17B2) but becomes rather clearer with progressing growth (Fig. 17B3–B5). The complete dorsal shell can be observed for a length of at least 9 septal spaces behind (or adorally of) the oyster (as far as the preserved end of the specimens’ shell). However, the dorsal nacre of this late stage is only formed in rib interspaces of the preceding whorl. There the dorsal shell wall thickens to smooth the relief of the ribs.
In one specimen of Cleoniceras (Grycia) besairiei (Desmoceratoidea, BSPG PA-33582) a secondarily formed dorsal nacreous layer, set between the wrinkle layer and the dorsal inner prismatic layer, reinforces a prismatic reduced dorsal shell wall (Fig. 13A). The dorsal nacreous layer is formed simultaneously, or rather in reaction, to a forma aegra aptycha (Keupp 1977, 2012; Drobniewski 2014), an internal shell lamella which underpins a broken shell portion and is formed after a withdrawal of the mantle edge (Drobniewski 2014; personal observations GR and HK). We assume the contemporaneous formation of both features.
The formation and function of the complete dorsal shell wall.—We assume that the above mentioned complete dorsal shell walls were formed in a similar way. Our observations imply that the ventral, lateral, and dorsal shell walls were secreted more or less contemporaneously by the same mantle portions: All shell layers, at least the nacreous layer and inner prismatic layer, were observed to form a continuum ventrally, laterally and dorsally. At least the dorsal, lateral and ventral nacreous layers form the aperture in heteromorphs, indicating a secretion near the mantle edge. It results from the secretion sequence (opl → ncl → ipl) that the dorsal outer prismatic layer has to be formed at the aperture as well. However, the dorsal inner prismatic layer was secreted by adapical mantle portions as inferred from evidence in the rear living chamber only. For planispirally ammonoids the same processes are likely.
Complete dorsal shell walls of heteromorph and planispiral taxa follow a prismatic reduced dorsal shell wall in ontogeny. Actually, size or age seems to be a determining factor. However, there is no general ontogenetic pattern of occurrence. Even closely related taxa of similar or larger size do not have a complete dorsal shell wall: e.g., Argonautitceras besairiei (Lytoceratoidea, BSPG MAn-1802, D = 102 mm) or Divisosphinctes sp. 1 (Perisphinctoidea, BSPG MAn-4499, D = 74 mm). It is likely that beside the age and size another factor has to be considered.
In heteromorph Scaphitoidea, the detachment of the dorsal shell wall from the preceding whorl induces the formation of a complete dorsal shell. Only apertural secreted portions of the dorsal shell wall can ensure integrity of the whole shell tube during detachment because the preceding whorl does not support the succeeding one. This is a general observation in heteromorphs (Erben et al. 1969; Doguzhaeva and Mikhailova 1982; Landman 1987; Doguzhaeva and Mutvei 1989). In our specimens of Hoploscaphites nicolletti and Scaphites whitfieldi the formation already begins prior to detachment. Considering the length of the living chamber, it is likely that these initial, thin stages of the dorsal nacre layer were formed in aboral portions while at the aperture the detachment of the whorl had already begun. This presumes large secretion zones at least in some taxa, i.e., Scaphites whitfieldi. Similar to heteromorphs, the secondary complete dorsal shell wall can be formed during detachment from the preceding whorl during overgrowth of encrusters in Divisosphinctes. Probably early occurences of a thin complete dorsal shell wall (ca. 9 septal chambers prior to the detachment) reflect a broad extension of the secretion zone of the mantle as well. Whorl detachment probably also accounts for the complete dorsal shell wall of Lobolytoceras costellatum. The whorls of its serpenticone, planispirally shell have only a small contact area. However, Protetragonites fraasi does not form a complete dorsal shell wall although its contact area is smaller.
The reinforced complete dorsal shell wall of Ancyloceratoidea and Douvilleiceratoidea.—Several members of the heteromorph Ancyloceratoidea (e.g., Ancyloceratoidea indet.) and planispiral Douvilleiceratoidea (e.g., Douvilleiceras mammilliatum) combine the characteristics of the complete dorsal shell wall and a nacreous reduced dorsal shell wall (Table 2; SOM: table A). The dorsal shell wall of studied adult specimens consists of up to five shell layers: a dorsal outer prismatic layer, a primary dorsal nacreous layer and a primary dorsal inner prismatic layer, i.e., the complete dorsal shell wall; which are reinforced by a second generation of the dorsal nacreous and the dorsal inner prismatic layer, i.e., the nacreous reduced dorsal shell wall (Figs. 3E, F, 7C2, 19B). In other words the complete dorsal shell wall displays shell doubling.
In our specimen of Ancyloceratoidea indet., the reinforced complete dorsal shell wall is present in both preserved, juvenile shafts (Fig. 19B). However, in specimens of D. mammilliatum the transition of a prismatic reduced dorsal shell wall (i.e., wl and dipl) (Fig. 7A1, A4) into a reinforced complete dorsal shell wall can be observed (Fig. 7C2). A thin primary dorsal nacreous layer is established at diameters of 21 to 29 mm (Table 2). With further growth the primary dorsal nacreous layer becomes thicker, the primary dorsal inner prismatic layer becomes thinner and the both inner layers (i.e., dncl 2 and dipl 2) are added.
In Ancyloceratoidea indet., all shell layers form a continuum encircling the whole conch tube dorsally, laterally and ventrally (Fig. 3E, F). However, the dorsal shell wall of the genus Douvilleiceras (e.g., D. mammilliatum, Douvilleiceras sp.) shows some special characteristics. For example, the dorsal outer prismatic layer is absent. A further peculiarity, in Douvilleiceras, a direct connection of the ventral/lateral and the dorsal shell portions cannot be observed; instead the lateral shell wall wedges out at the umbilical seam (Fig. 7C3, C4). With the exception of the wrinkle layer, the dorsal shell wall does not cover the whole overgrown portions of the preceding whorl but only the ventral and adjacent portions.
The internal structure can change from the ventral crest of the preceding whorl towards the umbilicus: The dorsal shell wall of Douvilleiceras overgrows a row of ventral spines (a row of ventro-lateral spines is uncovered). Usually the dorsal shell wall detaches from the preceding whorl during overgrowth whereby cavities are formed (Fig. 7C1). Only at these detached portions does the dorsal shell form the described complete dorsal shell wall with shell doubling (Fig. 7B, C2). When the dorsal shell wall attaches to the preceding whorl, the two outer layers (i.e., dncl 1 and dipl 1) wedge out towards the umbilicus and the two inner layers (i.e., dncl 2 and dipl 2) continue and cover the ventro-lateral flanks (Fig. 7A6, B). Occasionally, the dorsal shell wall can appear as trilayered through the local disappearance of only the primary dorsal nacreous layer. The primary dorsal inner prismatic layer forms a “dorsal outer prismatic layer” in these cases (Fig. 7A6).
The formation and function of the reinforced complete dorsal shell wall.—The reinforced complete dorsal shell walls combine characteristics of a normal complete dorsal shell wall (i.e., dopl and dncl 1) and a nacreous reduced dorsal shell wall (i.e., dipl 1, dncl 2, and dipl 2). Accordingly, we assume that during their formation adoral and adapical mantle portions were involved. The outer layers of the dorsal shell wall (i.e., dopl and dncl 1) were formed at the aperture. The inner layers (i.e., dipl 1, dncl 2, and dipl 2) were secreted in the rear living chamber, i.e., equivalent to the formation of a nacreous reduced dorsal shell wall. Douvilleiceras suppress the formation of an outer prismatic layer dorsally.
As in a normal complete dorsal shell wall, detachment from the preceding whorl/shell section seems to induce the formation of a reinforced complete dorsal shell wall. This is more or less obvious in our shafted specimen of Ancyloceratoidea indet. Also other shafted ancyloceratids like Ptychoceras (Doguzhaeva and Mutvei 1991, 1993a, b) have a similar construction of the dorsal and ventral shell wall. It is possible that the strong sculpture of Douvilleiceras, e.g., spines, and the accompanying detachments of the whorl cause the formation of the reinforced complete dorsal shell wall. It smooths out the relief, probably to facilitate the attachment of the septa. Furthermore, the spines may injure the animal’s soft body during overgrowth. The formation of a dorsal shell wall at the aperture could prevent it.
The complete dorsal shell wall of Amaltheidae.—The juvenile whorls of the Amaltheidae (Eoderoceratoidea) form a prismatic reduced dorsal shell wall (i.e., wl and dipl) including a prominent wrinkle layer. In the outer whorls of large Amaltheus margaritatus (BSPG MAn-4798, 4799, D > 72 mm), the dorsal shell wall consists of an outer spiral ornament (Fig. 20A; cf. Hölder 1973; Walliser 1970; Birkelund 1980), replacing the wrinkle layer, and an inner bunch of prismatic sub-layers corresponding to the dorsal nacreous layer and dorsal inner prismatic layer (Figs. 3C, 20B1, B2). The whole dorsal shell wall is almost 2.5 times thicker than the overgrown shell wall of the preceding whorl.
The spiral ornament is a conspicuous coating layer of the preceding whorl which forms raised spiral lines (Fig. 20A). It can be only observed in the overgrown area of the preceding whorl. Each raised line appears as a long pin-like structure in cross section (Figs. 3C, 20B1, B2). A pin has a broad base, forms a thinner shaft and ends in a pinhead. Nearly identical pin-like structures can be observed in the outer whorls (D > 46 mm) of a Pleuroceras salebrosum specimen (BSPG MAn-4804) too: In its living chamber we observe isolated structures at the venter of the preceding whorl and near the umbilical edge (Fig. 20C).
In Amaltheus margaritatus the pins (or spiral lines) of the spiral ornament are enclosed by multiple prismatic to spherulitic sub-layers which bend around the pins (dncl in Figs. 3C, 20B2). A further inner bunch of prismatic sub-layers, each a few micrometer thick, bridges the relief of the pins leaving cavities between the pins (dipl in Figs. 3C, 20B1, B2). These outer and inner sub-layers are the dorsal continuation of the ventral/lateral nacreous layer and inner prismatic layer, respectively. Only the inner sub-layers, which bridge the spiral ornament (pins), are the continuation of the inner prismatic layer of the ventral/lateral shell wall (dipl in Fig. 20B1, B2). The outer prismatic bands, which encrust the pins, originate in the nacreous layer of the ventral/lateral shell wall (dncl in Fig. 20B2, B3). The ventral/lateral nacreous layer thins at the whorl contact and extends towards the spiral plane along the surface of the preceding whorl up to its mid-flank, where the layer gradually transforms into several prismatic sub-layers (Fig. 20B2, B3) which continue towards the ventral crest of the preceding whorl, i.e., dorsal nacreous layer.

Fig. 20. Construction of the complete dorsal shell wall of Amaltheidae (A, lateral view, growth direction to the bottom; B, C, transversal section, centrifugal). A. Amaltheus cf. margaritatus de Monfort, 1808, BSPG MAn-100, late Pliensbachian, Jurassic, Eype Mouth, Dorset, England; a spiral ornament covers the overlap area of two whorls; the succeeding whorl was removed. The coated venter of the preceding whorl shows the typical pattern of several spiral lines. B. Amaltheus margaritatus de Monfort, 1808, BSPG MAn-4798, late Pliensbachian, Jurassic, Buttenheim, Bavaria, SE Germany; B1, the dorsal shell wall consists of an outer spiral ornament and inner bunches of prismatic sub-layers that correspond to the dorsal nacreous layer and the dorsal inner prismatic layer; B2, the dorsal nacreous layer transforms into prismatic layers; B3, close-up of B2. C. Pleuroceras salebrosum Hyatt, 1867, BSPG MAn-4804, late Pliensbachian, Jurassic, Buttenheim, Bavaria, SE Germany; the dorsal shell wall forms a spiral ornament. Abbreviations: dipl, dorsal inner prismatic layer; dncl, dorsal nacreous layer; if, infilling; ipl, inner prismatic layer; ncl, nacreous layer; s, septum; so, spiral ornament.
The formation and function of the complete dorsal shell wall of Amaltheidae.—Extension and position of the dorsal spiral ornament are similar to those of the wrinkle layer. Furthermore, the amaltheid juvenile wrinkle layer and adult spiral ornament seem to replace each other (e.g., Pleuroceras salebrosum) and both structures are not simultaneously present at the same whorl section (e.g., Amaltheus margaritatus), i.e., spatial and ontogenetic separation. Therefore, it is likely that the spiral ornament represents an ontogenetic derivation of the wrinkle layer (e.g., Walliser 1970; Hölder 1973). According to Birkelund (1980), a wrinkle layer can cover the spiral ornament, an observation we can reject (see immediately above). Additionally, Birkelund (1980) mentions an Amaltheus whose living chamber is covered by the spiral ornament, and assigns it therefore to the ventral/lateral shell wall. We doubt this assertion, since to our knowledge and according to our observations the spiral ornament is only preserved in formerly overgrown portions. Birkelund (1980) probably misinterpreted the often observed prominent spiral sculpture (an undulation of the shell wall) and/or spiral colour pattern of the ventral/lateral shell wall as equivalent expressions of the spiral ornament. Hölder (1973) assumed an internal folding of the spiral ornament layer (= “Leistenschicht”), indicated by undulating shell lamellae. He probably observed the undulated prismatic sub-layers of the dorsal nacreous layer (and inner prismatic layer).
We assume that the spiral ornament, like the wrinkle layer, was secreted by a supracephalic mantle fold at or beyond the aperture. It probably had a similar function as a common wrinkle layer. The spiral lines (and corresponding furrows) may serve in the attachment of the dorsal mantle. The dorsal nacreous and inner prismatic layers weaken the relief of the spiral ornament, probably to facilitate the attachment of the septa.
Apparently, the ventral, lateral, and dorsal nacreous layer and the ventral, lateral, and dorsal inner prismatic layer form a continuum. A secretion similar to a normal complete dorsal shell wall can be assumed: the dorsal inner prismatic layer in the rear living chamber and the dorsal nacreous layer near the aperture. The dorsal shell wall is probably not completely omitted at the aperture. The multi-layering of the dorsal inner prismatic layers is already known from the prismatic reduced dorsal shell wall and probably indicates an intermittent secretion process or at least (brief) interruption during mineralization. However, the dorsal nacreous layer shows a structural alteration from the umbilicus (nacre) towards the crest of the preceding whorl (prismatic). We exclude diagenetic alteration because two specimens develop these structures, each at a different whorl size, and their nacre is usually preserved. The prismatic multi-layering probably reflects the original nacreous lamellae. We assume that the mantle portion that forms the nacre ventrally, laterally and dorsally has changed its secretion ability from nacreous to prismatic dorsally. Apparently, the mantle tissue was able to locally change its secretion ability, i.e., cell secretion plasticity (Fleury et al. 2008; Cuif et al. 2011).
The seemingly complete dorsal shell wall.—Some specimens of Stephanoceratoidea (e.g., Quenstedtoceras henrici, Sigaloceras (Sigaloceras) calloviense), Haploceratoidea (e.g., Hecticoceras [Sublunuloceras] sp.), and Hoplitoidea (e.g., Metaplacenticeras subtilistriatum) form a dorsal outer prismatic layer as a further dorsal shell wall component that basically displays all characteristics of a prismatic reduced dorsal shell wall (i.e., wl and dipl) (Fig. 9, Table 2; SOM: table A). The dorsal shell wall is therefore three-layered (i.e., wl, dopl, and dipl).
The dorsal outer prismatic layer is set between the wrinkle layer and the dorsal inner prismatic layer (Figs. 3D, G, 21A–C). It evens out the wrinkle layer’s relief (Fig. 21D). The dorsal outer prismatic layer only extends from the left to the right umbilical margin. It neither represents a direct continuation of the ventral/lateral outer prismatic layer nor is it a sub-layer of the dorsal inner prismatic layer: After the ventral/lateral outer prismatic layer wedges out at the umbilical seam (Figs. 3G, 21A), the dorsal outer prismatic layer immediately appears as a new thin lamella, which thickens towards the spiral plane (Figs. 3G, 21A). Both the ventral/lateral nacreous layer and inner prismatic layer attach to the dorsal outer prismatic layer at the umbilical seam (Figs. 3G, 21A, B). However, the nacreous layer vanishes completely and only the inner prismatic layer continues as an inner cover of the dorsal outer prismatic layer towards the ventral crest.
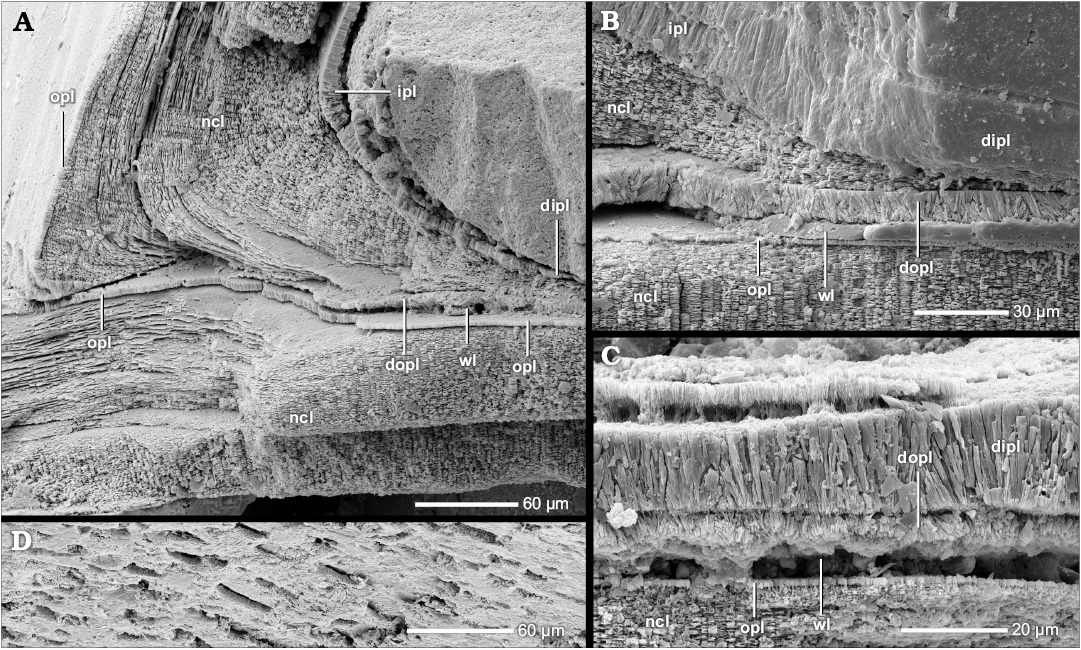
Fig. 21. Construction of the seemingly complete dorsal shell wall (transversal section, centrifugal). Quenstedtoceras henrici Douville, 1912, BSPG MAn-4768, early Callovian, Jurassic, Dubki near Saratov, Russia. A. At the contact of two whorls the outer prismatic layer and the nacreous layer of the attaching whorl wedge out but a “new” dorsal outer prismatic layer is formed towards the spiral plane which covers the wrinkle layer; the inner prismatic layer is continuous. B. The (dorsal) nacreous layer and the (dorsal) inner prismatic layer attach to the “new” dorsal outer prismatic layer which compensates the relief of the wrinkle layer. C. The dorsal shell wall consists of a wrinkle layer, a dorsal outer prismatic layer and a dorsal inner prismatic layer. D. The wrinkle layer left imprints in the dorsal outer prismatic layer. Abbreviations: dipl, dorsal inner prismatic layer; dopl, dorsal outer prismatic layer; ipl, inner prismatic layer, ncl, nacreous layer, opl, outer prismatic layer; wl, wrinkle layer.
The formation and function of the seemingly complete dorsal shell wall.—The wrinkle layer and the dorsal inner prismatic layer of this dorsal shell type were probably formed as described in the sections above. The function of the dorsal outer prismatic layer is similar to that of the dorsal inner prismatic layer, i.e., evening out the internal relief and sealing the soft tissue from possible encrusters. Kulicki (1979) highlights a similar layer in one specimen of Quenstedtoceras sp., but he interprets it as an alternative appearance of the wrinkle layer. We think this is a misinterpretation; both the dorsal outer prismatic and the wrinkle layer can be clearly separated in our specimens. This layer has to be secreted after the wrinkle layer but before the nacreous layer and inner prismatic layer. The latter two can be excluded as equivalents as well, i.e., no connection but attachment to the dorsal outer prismatic layer. There are no signs of a shell doubling at the umbilicus. An assignment to a primary (dorsal) inner prismatic layer can be therefore excluded as well. It is most likely that the dorsal outer prismatic layer of these specimens is equivalent to the actual (ventral/lateral) outer prismatic layer. Therefore, the layer was probably secreted at the aperture and the dorsal shell should be regarded as some kind of complete dorsal shell wall in which, however the dorsal nacreous layer was not formed. It is nevertheless unclear why the dorsal outer prismatic layer is interrupted at the umbilical seam.
Phylogenetic and taxonomic implications of nacreous reduced and complete dorsal shell walls.—The patchy occurrence of nacreous reduced and complete dorsal shell walls within the ammonoid phylogenetic tree (Fig. 9; SOM: table A) makes a clear assignment to a proper high-order group of Ammonoidea difficult. The prismatic reduced dorsal shell wall is still the most often and dominant type of dorsal shell. The other dorsal shell wall types are indeed a typical characteristic of individual genera or species but a generalization of a diagnostic feature for an entire high-order group (e.g., superfamily) is not advisable as long as only sporadic evidence exists. It is not certain if these examples are outliers or are representative of an entire group of ammonoids.
In spite of this, the scattered occurrence of nacreous reduced and complete dorsal shell walls gives rise to several possible interpretations:
(i) Since all these observations were made in individual genera or species, all of them could represent independent apomorphies of these low-order taxa. The entire subfamily of Aspidoceratinae (e.g., Aspidoceras sp. and Mirosphinctes sp. 1) seems to be unusual in this respect as all its members seem to have the ability to form a nacreous reduced dorsal shell wall.
However, this interpretation ignores secondary formations of nacreous reduced (e.g., Puzosia saintoursi, Desmoceras [Desmoceras] latidorsatum) as well as of complete dorsal shell walls (e.g., Divisosphinctes besairiei, Cleoniceras [Grycia] besairiei). We assume that the secondary formation ability is a general ammonoid feature and that primary nacreous reduced and complete dorsal shell walls (i.e., formed during ontogeny) are an expression of that feature. The ammonoid dorsal mantle tissue does not lose the ability to form additional shell layers, especially nacre, but suppresses it in general. The dorsal mantle had the same secretion abilities as the ventral and lateral portions. Coinciding occurrences of both dorsal shell wall types in Perisphinctoidea and Desmoceratoidea may even indicate that both types are connected. Since ventral and lateral shell doublings are commonly observed in ammonoids, e.g., Eoderoceratoidea, Haploceratoidea, Stephanoceratoidea, Perisphinctoidea, Desmoceratoidea (cf. Howarth 1975; Birkelund 1980; Doguzaheva and Mutvei 1989, 1991; this study), the nacreous reduced dorsal shell wall probably constitutes its homologous expression. Ventral, lateral as well as dorsal reinforcement of the shell wall (i.e., shell doubling) may represent a general ability. The reinforced complete dorsal shell wall of Ancyloceratoidea and Douvilleiceratoidea may reflect the ground plan of the entire ventral, lateral, and dorsal shell wall. Therefore, we assume that both dorsal shell wall expressions can be regarded as one feature representing the original dorsal shell wall.
The broad distribution of complete and nacreous reduced dorsal shell walls in the phylogenetic tree (Fig. 9; SOM: table A) indicates a plesiomorph ability to form these dorsal shell walls types, or rather dorsal nacre.
(ii) Based on our data collection (Fig. 9; SOM: table A), this ability seems to have evolved only in post-Triassic ammonoids after the separation from the Phylloceratoidea. As all occurrences are observed in taxa that are descendants of Psiloceratoidea, an origin within this group is likely although not proved yet. In Lytoceratoidea and Eoderoceratoidea (as well as in their descendants) it seems to be a symplesiomorphy. Due to its patchy occurrence we suppose a facultative feature that can be activated when needed.
(iii) Jurassic and Cretaceous samples are over-represented in our collection. Furthermore, the observed occurrences of dorsal nacre are less frequent compared to those of a prismatic reduced dorsal shell wall. There are probably pre-Jurassic and further Jurassic and Cretaceous samples not observed yet. We assume that the ability to form dorsal nacre is a relic feature of the gyrocone ancestors and therefore a plesiomorphy. A facultative feature is likely too.
In both variants, the plesimorphy of dorsal nacre complicates the usage for phylogeny and taxonomy at lower levels, e.g., superfamilies or families. We even anticipate that an ammonoid could generally pass through a stage of complete or nacreous reduced dorsal shell wall in a theoretical ontogenetic late stage when it reached a certain size or age (although not observed yet). Our observations of these dorsal shell wall types probably represent heterochrony. Nonetheless, we suggest using these features only as an additional taxonomic trait for genera and species (facultative feature) at the current state of knowledge.
Some groups seem to be exceptions and always form at least one expression:
(i) A complete dorsal shell wall is characteristic for the probably polyphyletic Jurassic and Cretaceous heteromorphs (Engeser and Keupp 2002), e.g., Ancyloceratoidea, Scaphitoidea. However, this is a secondary effect of whorl decoiling/detachment, i.e., lacking whorl support, and cannot be used as diagnostic feature.
Considering the dorsal wall structure only, the origin of heteromorphs could be in every ammonoid superfamily, i.e., facultative, plesiomorphic complete dorsal shell wall. However, proven occurrences of a complete dorsal shell wall and time-overlap in the geological record are only given in Lytoceratoidea (e.g., Lobolytoceras costellatum), Perisphinctoidea (e.g., Choffatia [Grossouvria] sp. 2, Divisosphinctes besairiei) and Desmoceratoidea (e.g., Cleoniceras [Grycia] besairiei). Despite the assumed general ability to form a complete dorsal shell wall among all ammonoids, these three superfamilies seem to be the most likely candidates as ancestors at the current state of knowledge (Fig. 9).
(ii) In Aspidoceratinae, a nacreous reduced dorsal shell wall is repeatedly formed and usually occurs very early in ontogeny (under 10 mm in diameter). We assume that the ontogenetically early occurrence indicates a characteristic heterochrony of this group.
(iii) The heteromorph superfamily Ancyloceratoidea and their planispiral descendants, Douvilleiceratoidea and Deshayesitoidea (Fig. 9; e.g., House 1993; Rouget et al. 2004; Yacobucci 2016), seemingly share the same characteristics of their dorsal shell wall, i.e., reinforced complete dorsal shell wall. This dorsal shell wall type may underline their close relation. The dorsal shell walls of Douvilleiceras (Douvileiceratoidea) and Colombiceras (Deshayesitoidea) can be interpreted as derived from Ancyloceratoidea. The dorsal shell wall of Douvilleiceras and Colombiceras are nearly identical to those of our Ancyloceratoidea indet. and Ptychoceras (Doguzhaeva and Mutvei 1991, 1993a, b) with the exception of a missing dorsal outer prismatic layer in Douvilleiceras and a missing primary dorsal nacreous layer in Colombiceras. Also Ptychoceras omits the outer prismatic layer in older shafts. However, the shell doubling does not encircle the whole shell tube in both derived taxa. Further, the dorsal outer prismatic layer of Colombiceras does not cover the ventral crest, i.e., nacreous reduced dorsal shell wall. Other taxa of Deshayesitoidea (e.g., Deshayesites sp.) develop only a prismatic reduced dorsal shell wall (SOM: table A). Indeed, in Luppovia (Ancyloceratoidea), a complete dorsal shell wall is described but it is not reinforced (Doguzhaeva and Mikhailova 1982). On the other hand, Ptychoceras as well as Douvilleiceras form primarily a similar, simple complete dorsal shell wall.
(iv) Nacreous reduced dorsal shell walls occur often in taxa that derived from Hildoceratoidea (Rouget et al. 2004) or Stephanoceratoidea (Yacobucci 2016), respectively. This could mean that the ability originates in these taxa (instead of Psiloceratoidea).
Replacement structures of the dorsal wrinkle layer.—Several elements of the ammonoid shell wall can develop structures that are similar in appearance to the wrinkle layer or rather have analogous functions. There are extensions of the periostracum and modifications of the (dorsal) inner prismatic layer. These shell elements replace the wrinkle layer during ontogeny, and the formation of the latter stops.
Periostracal extensions of the ventral shell wall.—The periostracum forms the outermost layer of the ventral and lateral shell wall. The layer usually has an organic composition, i.e., homogeneous appearance, and is followed by the outer prismatic layer at its internal surface. With some exceptions, the periostracum is not preserved. However, in several phylloceratids and desmoceratids, the periostracum forms conspicuous extensions that stick out from the ventral/lateral shell wall (Fig. 22). These extensions occur during late ontogeny and are not present in the innermost whorls. The wrinkle layer is contemporaneously abandoned and disappears. Both structures never appear simultaneously in the same shell portion. In Phylloceras (Phylloceras) plicatum, Phylloceras (Euphylloceras) cf. velledae, and Phylloceras (Euphylloceras) sp., the periostracum forms conspicuous projecting, scythe-like extensions in cross-section (Fig. 22A, B) causing the formation of organic radial lirae (Radtke and Keupp 2016). The newly formed lirae relief is much more pronounced than that of the dorsal wrinkles. The relief is smoothed out by the dorsal inner prismatic layer during overgrowth. It bridges the lira-concavity forming characteristic oval cavities (Fig. 22B). The lirae occur at diameters of 5 to 10 mm in those taxa. The dorsal wrinkle layer disappears accordingly at diameters of 12 to 24 mm of the succeeding whorl. Similar lirae, albeit made from (segments of) the outer prismatic layer, i.e., prismatic radial lirae (Radtke and Keupp 2016), are formed in several Aspidoceratinae, e.g., Aspidoceras sp., Pseudowaagenia sp. (Fig. 12D1).
Phyllopachyceras sp. (Phylloceratoidea) and Desmophyllites diphylloides (Desmoceratoidea) form thin, convex periostracal extensions in cross-section (Fig. 22C, D) instead of a wrinkle layer of the succeeding whorl in late ontogeny. They are present at diameters of at least 20 and 5 mm, respectively. Periostracal extensions that appear similar are formed by some Aspidoceratinae (Perisphinctoidea), i.e., a herringbone layer (Fig. 14A1, A4; Radtke and Keupp 2016).
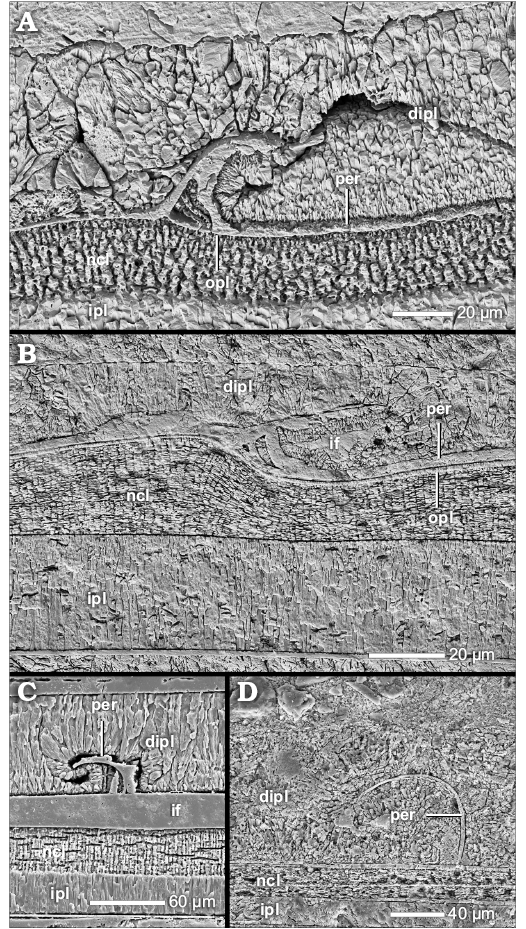 |
Fig. 22. Periostracal extension of the ventral shell wall (median section, growth direction to the right, centrifugal). A. Phylloceras (Euphylloceras) cf. velledae (Michelin, 1934), BSPG MAo-1770, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; a scythe-like extension of the ventral organic periostracum forms an organic radial lirae (Radtke and Keupp 2016); the dorsal inner prismatic layer of the succeeding whorl smoothes out the periostracum relief. B. Phylloceras (Euphylloceras) sp., BSPG MAo-1769, early Albian, Cretaceous, Ambatolafia, Mahajanga Basin, NW Madagascar; the same as in A. C. Phylloceras (Phylloceras) plicatum Neumayr, 1871, BSPG MAn-4509, late Oxfordian, Jurassic, Sakaraha, Morondava Basin, SW Madagascar; the ventral organic periostracum forms convex periostracal extensions; the dorsal inner prismatic layer of the succeeding whorl smoothes out the periostracum relief. D. Desmophyllites diphylloides (Forbes, 1846), BSPG MAo-1838, Campanian, Cretaceous, Teshio-Nakagawa area, Hokkaido, Japan; the same as in C. Abbreviations: dipl, dorsal inner prismatic layer; if, infilling; ipl, inner prismatic layer, ncl, nacreous layer, opl, outer prismatic layer, per, periostracum. |
Formation and function of periostracal extensions.—Periostracal extensions as mentioned above are identical with older findings in other phylloceratids (cf. Birkelund and Hansen 1974, 1975; Kulicki et al. 2001; Bucher et al. 2003; Radtke and Keupp 2016), e.g., Hypophylloceras and Calliphylloceras with organic radial lirae and Phyllopachyceras with convex arcs.
The assignment of these shell elements to the ventral shell wall is based on their internal structure. The scythe-appendage of the organic radial lirae implies a moderate withdrawal of the ventral/lateral mantle edge accompanied by an interruption in growth (Radtke and Keupp 2016). The scythe-appendage represents a first shell generation and the new periostracum generation is formed from within at the lira-crest. The formation of the scythe-appendage by the dorsal mantle seems to be rather difficult. For the convex arcs we assume a similar episodic growth. However, we cannot entirely exclude an assignment to the dorsal shell wall for this type.
The wrinkle layer is associated with a roughness effect (see above). In the special case of the periostracal extensions of the ventral shell wall, these form a new and usually much more pronounced relief than that of the wrinkles. With the formation of periostracal extensions, the relief of the wrinkle layer becomes obsolete and was probably therefore reduced.
Phylogenetic and taxonomic implications of periostracal extensions.—Our observations prove that ammonoids had the ability to form a periostracum but it is seldom preserved. Since the periostracum is a compartment of all molluscan shells, i.e., its template, it is likely that all ammonoids formed one to build their shells but usually shed it later on.
Due to the rarity of periostracal preservation and the conspicuous appearance, the periostracal extensions of Phylloceratoidea (e.g., scythe extensions, convex arcs) are likely taxon-specific but the record is incomplete. Radial lirae are a widely distributed shell element in Ammonoidea, at least in Phylloceratoidea, however. A uniform or at least similar formation can be generally assumed for radial lirae (cf. Bucher et al. 2003) but has to be proved yet. The relationship of radial lirae within Ammonoidea is likely but needs additional evidence, e.g., structural conformity. Radtke and Keupp (2016) assume at last a relation of organic radial lirae of Phylloceratoidea to prismatic radial lirae of Aspidoceratinae due to similarities in construction, e.g., episodic growth interruption, scythe appendages.
For the time being, the convex arc-like extensions are unique observations for the genera Phyllopachyceras and Desmophyllites, and they could be a result of convergent development. On the other hand, several authors assume a polyphyly of desmoceratids. Several families are believed to be direct descendants of Phylloceratoidea (Engeser and Keupp 2002). The structural consistence may indicate the relationship of at least Desmophyllites with Phylloceratoidea. So far, the herringbone layer of Aspidoceratinae is restricted to that group but although similar in appearance probably an analogous formation to the periostracal extensions of Phyllopachyceras and Desmophyllites (Radtke and Keupp 2016).
The “Ritzknoten”.—One large specimen of Eupachydiscus sp. (Desmoceratoidea, BSPG MAo-1834) does not form the dorsal wrinkle layer but instead secretes structures we named here “Ritzknoten” (German: scratch knobes). These are small, cone-like internal elevations (Fig. 13C2–C5) affecting at least the dorsal and umbilical-lateral portions of the ammonoid shell wall. Ventrally they seem not to be formed. The “Ritzknoten” originate in the dorsal and umbilical inner prismatic layer, respectively, i.e., the layers form a continuum.
Eupachydiscus sp. develops a nacreous reduced dorsal shell wall that originates in an umbilical shell doubling. The “Ritzknoten” are formed by the primary (dorsal) inner prismatic layer, in an umbilical-lateral as well as dorsal position (Fig. 13C2–C5). The actual relief is formed by a thickening of an outer sub-layer while an inner sub-layer adopts it (Fig. 13C3, C4). Its internal relief is evened out by the secondary (dorsal) inner prismatic layer (Fig. 13C2) or the secondary (dorsal) nacreous layer (Fig. 13C4, C5), respectively.
The formation and function of “Ritzknoten”.—The “Ritzknoten” are relief formations of the inner prismatic layer and therefore were probably formed in the rear living chamber. A direct relation to the wrinkle layer can be ruled out. It is likely that the “Ritzknoten” relief had a similar function (roughness effect) and the wrinkle layer therefore became obsolete and was reduced. However, the wrinkle layer extends up to the aperture but the “Ritzknoten” do not.
Keupp (2008) described another internal relief structure formed by the inner prismatic layer: “Ritzstreifen” (German: scratch lines, stripes), known also as “ventral wrinkle layer”. “Ritzstreifen” are common features in Palaeozoic ammonoids and usually form a ventral and lateral pattern of striae and/or pits at internal moulds which is probably caused by an internal relief (e.g., fine ridges and knobs) of the conch wall (e.g., Walliser 1970; House 1971; Senior 1971; Doguzhaeva 1980, 1981; Korn 1985). According to Keupp (2008), “Ritzstreifen” of Cretaceous Desmoceras (Pseudouhligella) intrapunctatum represent indeed ridges and knobs of the initial, still incomplete mineralized inner prismatic layer, i.e., whiskers, that are integrated into the complete layer afterwards.
Since “Ritzknoten” form a relief of the fully mineralized inner prismatic layer and “Ritzstreifen” form it by an incomplete inner prismatic layer (i.e., whiskers), “Ritzknoten” and “Ritzstreifen” are apparently analogous formations. Following the formation model of “Ritzstreifen” of Keupp (2008), we propose the new term “Ritzknoten”.
The findings of “Ritzstreifen” and “Ritzknoten” are important to avoid misunderstandings as for the wrinkle layer. Several studies imply that the wrinkle layer covers the whole interior of the living chamber or conch wall, respectively (e.g., Walliser 1970; House 1971; Senior 1971; Doguzhaeva 1980, 1981; Korn 1985). This assumption is mainly based on taxa that form dorsal and ventral fingerprint pattern. Sometimes, even a dorsal and lateral transition of both reliefs can be observed at the internal mold (e.g., Walliser 1970; House 1971; Korn 1985). However, our observations of “Ritzknoten” and Keupp’s (2008) observations of “Ritzstreifen” prove that the previous interpretations of an enclosing wrinkle layer are better explainable by a ventral, lateral, and dorsal internal relief of the inner prismatic layer formed in the rear of the living chamber. The wrinkle layer, as we understand it, is formed at the aperture, coats only the preceding ventral whorl and does not form ventral and/or lateral internal reliefs (see above). We assume that ventral/lateral internal reliefs of the shell wall, like fine ridges and knobs, were formed by the inner prismatic layer in general and not by a wrinkle layer. Dorsally, one of the two layers (either wl or dipl) may cause an internal relief but they differ in their position of formation. In such cases where indeed ventral/lateral “Ritzstreifen”/“Ritzknoten” and dorsal wrinkle layers occur simultaneously, internal molds may display transitions between these structures even though they are not related.
Phylogenetic and taxonomic implications of “Ritzknoten” and “Ritzstreifen”.—Apparently, the inner prismatic layer can form internal reliefs in different ways that indicate a tendency for convergence of comparable structures. Palaeozoic taxa may form their “Ritzstreifen” similarly, but how they relate to the structures observed in their Mesozoic couterparts remains an open question.
Conclusions
The Ammonoidea had several modifications of ultrastructure of the shell wall what is reflected not only in the ventral and lateral shell portions but also in the dorsal shell wall. The dorsal shell wall can display great variability. The dorsal mantle tissue of Ammonoidea was able to secrete organic, prismatic, and nacreous material. The ability to form nacreous portions is a new observation in the dorsal shell wall (of planispirally coiled taxa). Adoral as well as adapical mantle tissues were involved. Furthermore, we assume that the mantle tissue maintains secretion plasticity, i.e., the mantle tissue was able to change its secretion abilities.
The majority of ammonoids, usually planispiral taxa, forms a prismatic reduced dorsal shell wall which is omitted at the aperture. It consists of an outer organic component, e.g., wrinkle layer, which is secreted by a supracephalic mantle fold in front of the aperture, and a dominant dorsal inner prismatic layer which is secreted in the rear living chamber. The wrinkle layer has no equivalent in the ventral/lateral wall but could be a derivate of the dorsal periostracum. The dorsal inner prismatic layer is a continuation of its ventral/lateral equivalent. Other layers of the ventral/lateral wall, i.e., outer prismatic layer and the nacreous layer, are suppressed. Usually all ammonoids go through such a stage, at least after hatching. However, some taxa form other dorsal shell wall types during ontogeny.
Several planispiral ammonoids form a nacreous reduced dorsal shell wall which is omitted at the aperture. It consists of a so-called primary dorsal inner prismatic layer, a secondary dorsal nacreous layer and a secondary dorsal inner prismatic layer. All three layers are continuations of an umbilical shell doubling (i.e., reinforcement of the shell wall) which is secreted in the rear living chamber where they wedge out towards the aperture. Prismatic and nacreous reduced dorsal shell walls are mainly used for smoothing the sculpture in planispirally coiled taxa, probably to facilitate the attachment of the septa.
Heteromorph as well as some planispirally coiled taxa form a complete dorsal shell wall at the aperture in continuation of the ventral and lateral shell wall’s outer prismatic layer, nacreous layer and inner prismatic layer. The (dorsal) outer prismatic layer and the (dorsal) nacreous layer are secreted at/near the aperture. The (dorsal) inner prismatic layer is restricted to the rear living chamber. Some taxa seem to form only the dorsal outer and inner prismatic layer but lack the dorsal nacreous layer, e.g., Quenstedtoceras henrici. Other taxa reinforced the trilayered shell wall by additional layers, i.e., a secondary nacreous and a secondary inner prismatic layer (shell doubling), e.g., Douvilleiceras mammilliatum.
The prismatic reduced dorsal shell wall probably represents the primary or plesiomorph state, respectively, in all Ammonoidea or at least in Mesozoic taxa. The ability to form dorsal nacre, hence to form nacreous reduced or complete dorsal shell walls, either represents a plesiomorphy retained from the gyrocone ammonoid ancestors, or (re-)developed in post-Triassic forms. Although a general ability to form complete dorsal shell walls is likely in all (Jurassic and Cretaceous) ammonoids, the Lytoceratoidea, Perisphinctoidea or Desmoceratoidea are probable ancestors for Jurassic and Cretaceous heteromorphs due to the proven occurrence of complete dorsal shell walls in combination with stratigraphic overlaps of these superfamilies with heteromorphs in earth’s history.
Acknowledgements
We would like to thank Yoshinori Hikida (Nakagawa Museum of Natural History, Hokkaido, Japan), Ulrich Kotthoff, Wolfgang Weitschat (both University of Hamburg, Germany), Neil H. Landman (American Museum of Natural History, New York, USA), Vasily V. Mitta (Russian Academy of Science, Moscow, Russia) and Günter Schweigert (State Museum of Natural History Stuttgart, Germany) for providing well preserved material for this study. Further, we would like to thank our reviewers, Antonio Checa (University of Granada, Spain), Kazuyoshi Moriya (Waseda University, Tokyo, Japan) and two anonymous referees for constructive comments that helped us to improve the manuscript. We thank Monika Bulang-Lörcher (Freie Universität, Berlin, Germany) for making the drawings, and Anja Kühnel and Giles Shephard (both Berlin, Germany) for proof-reading the manuscript. This work was funded by the Berlin Universities’ Elsa Neumann Stipend.
References
Bandel, K. 1977. Übergänge von der Perlmutter-Schicht zu prismatischen Schichttypen bei Mollusken. Biomineralization Research Reports 9: 28–47.
Bayer, U. 1974. Die Runzelschicht – ein Leichtbauelement der Ammonitenschale. Paläontologische Zeitschrift 48: 6–15. Crossref
Birkelund, T. 1967. Submicroscopic shell structures in early growth-stages of Maastrichtian ammonites (Saghalinites and Scaphites). Bulletin of the Geological Society of Denmark 17: 95–101.
Birkelund, T. 1980. Ammonoid shell structure. In: M.R. House and J.R. Senior (eds.), The Ammonoidea, 177–214. Academic Press, New York.
Birkelund, T. and Hansen, H.J. 1968. Early shell growth and structures of the septa and the siphuncular tube in some Maastrichtian ammonites. Bulletin of the Geological Society of Denmark 18: 95–101.
Birkelund, T. and Hansen, H.J. 1974. Shell ultrastructures of some Maastrichtian Ammonoidea and Coleoidea and their taxonomic implications. Kongelige Danske Videnskabernes Selskab Biologiske Skrifter 20: 1–34.
Birkelund, T. and Hansen, H.J. 1975. Further remarks on the post-embryonic Hypophylloceras shell. Bulletin of the Geological Society of Denmark 24: 87–92.
Blind, W. 1975. Über Entstehung und Funktion der Lobenlinie bei Ammonoideen. Paläontologische Zeitschrift 49: 254–267. Crossref
Blind, W. 1976. Die ontogenetische Entwicklung von Nautilus pompilius (Linné). Palaeontographica A 153: 117–160.
Bucher, H., Chirat, R., and Guex, J. 2003. Morphogenetic origin of radial lirae and mode of shell growth in Calliphylloceras (Jurassic Ammonoidea). Eclogae Geologicae Helvetiae 96: 495–502.
Bucher, H., Landman N.H., Klofak S.M., and Guex J. 1996. Mode and rate of growth in Ammonoids. In: N.H. Landman, K. Tanabe, and R.A. Davis (eds.), Ammonoid Paleobiology. Topics in Geobiology 13: 407–461. Plenum Press, New York. Crossref
Carter, J.G. and Clark, G.R. II 1985. Classification and phylogenetic significance of mollusk shell microstructures. In: T.W. Broadhead (ed.), Mollusk, Note for a Short Course. Studies in Geology 13, 50–71. University of Tennessee, Tennessee.
Carter, J.G., Bandel, K., de Buffrénil, V., Carlson, S.J., Castanet, J., Crenshaw, M.A., Dalingwater, J.E., Francillon-Vieillot, H., Géraudie, J., Meunier, F.J., Mutvei, H., de Ricqlès, A., Sire, J.Y., Smith, A.B., Wendt, J., Williams, A., and Zylberberg, L. 1989. Glossary of skeletal biomineralization. In: J.G. Carter (ed.), Skeletal Biomineralisation: Patterns, Processes and Evolutionary Trends, 609–671. Van Nostrand Reinhold, New York.
Checa, A. 1994. A model for the morphogenesis of ribs in ammonites inferred from associated microsculptures. Palaeontology 37: 863–888.
Cochran, J.K., Kallenberg, K., Landman, N.H., Harries, P.J., Weinreb, D. Turekian, K.K., Beck, A.J., and Cobban, W.A. 2010. Effect of diagenesis on the Sr, O, and C isotope composition of late Cretaceous molluscs from the Western Interior Seaway of North America. American Journal of Science 310: 69–88. Crossref
Cuif, J.-P., Dauphin, Y., Howard, L, Nouet, J., and Salome, M. 2011. Is the pearl layer a reverse shell? A re-examination of the theory of the pearl formation through physical characterizations of pearl and shell development stages in Pinctada margaritifera. Aquatic Living Resources 24: 411–424. Crossref
Doguzhaeva, L. 1980. New data on the shell wall structure in ammonoids. Doklady, Earth Science Sections 254: 238–241.
Doguzhaeva, L. 1981. The wrinkle-layer of the ammonoid shell. Paleontological Journal 15: 26–35.
Doguzhaeva, L. 2002. Adolescent bactritoid, orthoceroid, ammonoid and coleoid shells from the Upper Carboniferous and Lower Permian of the South Urals. Abhandlungen der Geologischen Bundesanstalt 57: 9–55.
Doguzhaeva, L. 2012. Functional significance of parabolae, interpreted on the basis of shell morphology, ultrastructure and chemical analyses of the Callovian ammonite Indosphinctes (Ammonoidea: Perisphinctidae), Central Russia. Revue de Paléobiologie, Genève 11: 89–101.
Doguzhaeva, L. and Mikhailova, J. 1982. The genus Luppovia and the phylogeny of Cretaceous heteromorphic ammonoids. Lethaia 15: 55–65. Crossref
Doguzhaeva, L. and Mutvei, H. 1986. Functional interpretation of inner shell layers in Triassic ceratid ammonids. Lethaia 19: 195–209. Crossref
Doguzhaeva, L. and Mutvei, H. 1989. Ptychoceras—a heteromorphic lytoceratid with truncated shell and modified ultrastructure (Mollusca: Ammonoidea). Paleontographica A 208: 91–121.
Doguzhaeva, L. and Mutvei, H. 1991. Organization of the soft body in Aconoeceras (Ammonitina), interpreted on the basis of shell morphology and muscle-scars. Paleontographica A 218: 17–38.
Doguzhaeva, L. and Mutvei, H. 1993a. Shell ultrastructure, muscle-scars, and buccal apparatus in ammonoids. Geobios Mémoire Special 15: 111–119. Crossref
Doguzhaeva, L. and Mutvei, H. 1993b. Structural features in Cretaceous ammonoids indicative of semi-internal or internal shells. In: M.R. House (ed.), The Ammonoidea: Environment, Ecology, and Evolutionary Change. Systematics Association Special Volume 47, 99–104. Clarendon Press, Oxford.
Doguzhaeva, L., Bengtson, S., and Mutvei, H. 2010. Structural and morphological indicators of mode of life in the Aptian lytoceratid ammonoid Eogaudryceras. In: K. Tanabe, Y. Shigeta, T. Sasaki, and H. Hirano (eds.), Cephalopods—Present and Past, 123–130. Tokai University Press, Tokyo.
Drobniewski, A. 2014. Vergleichende Untersuchungen zum paläopathologischen Phänomen der forma aegra aptycha bei Cleoniceras (Ammonoidea) aus der Unterkreide von Madagaskar. 185 pp. Unpublished M.Sc. Thesis, Freie Universität, Berlin.
Drushits, V.V . and Khiami, N. 1970. Structure of the septa, protoconch walls and initial whorls in early Cretaceous ammonites. Paleontological Journal 4: 26–38.
Drushits, V.V., Doguzhayeva, L.A., and Lominadze, T.A. 1977. Internal structural features of the shell of Middle Callovian ammonites. Paleontological Journal 11: 271–284.
Drushits, V.V., Doguzhayeva, L.A., and Mikhaylova, I.A. 1978. Unusual coating layers in ammonites. Paleontological Journal 12: 174–182.
Engeser, T. and Keupp, H. 2002. Phylogeny of the aptychi-possessing Neoammonoidea (Aptychophora nov., Cephalopoda). Lethaia 34: 79–96. Crossref
Erben, H.K. and Reid, R.E.H. 1971. Ultrastructure of shell, origin of conellae and siphuncular membranes in an ammonite. Biomineralisation 3: 22–31.
Erben, H.K., Flajs, G., and Siehl, A. 1968. Ammonoids: Early ontogeny of ultramicroscopial shell structure. Nature 219: 396–398. Crossref
Erben, H.K., Flajs, G., and Siehl, A. 1969. Die frühontogenetische Entwicklung der Schalenstruktur ectocochleater Cephalopoden. Palaeontographica A 132: 1–54.
Fleury, C., Marin, F., Marie, B., and Lebel, J.-M. 2008. Shell repair process in the green ormer Haliotis tuberculata: A histological and microstructural study. Tissue Cell 40: 207–218. Crossref
Funabara, D., Ohmori, F., Kinoshita, S., Koyama, H., Mizutani, S., Ota, A. Osakabe, Y., Nagai, K., Maeyama, K., Okamoto, K., Kanoh, S. Asakawa, S., and Watabe, S. 2014. Novel genes participating in the formation of prismatic and nacreous layers in the pearl oyster as revealed by their tissue distribution and RNA interference knockdown. PLoS ONE 9: e84706. Crossref
Hölder, H. 1952. Über Gehäusebau, insbesondere Hohlkiel Jurassicher Ammoniten. Palaeontographica A 102: 18–48.
Hölder, H. 1973. Miscelleana cephalopodica. Münsterländer Forschungshefte Geologie Paläontologie 29: 39–76.
House, M.R. 1971. The goniatite wrinkle-layer. Smithsonian Contributions to Paleobiology 3: 23–32.
House, M.R. 1993. Fluctuations in ammonoid evolution and possible environmental controls. In: M.R. House (ed.), The Ammonoidea: Environment, Ecology, and Evolutionary change. The Systematics Association Special Volume 47, 13–34. Clarendon Press, Oxford.
Howarth, M.K. 1975. The shell structure of the Liassic ammonite family Dactylioceratidae. Bulletin of the British Museum (Natural History) 26: 45–67.
Jackson, A.P., Vincent, J.F.V., and Turner, R.M. 1988. The mechanical design of nacre. Proceedings of the Royal Society B Biological Sciences 234: 415–440.
Joubert, C., Piquemal, D., Marie, B., Manchon, L., Pierrat, F., Zanella-Cléon, I., Cochennec-Laureau, N., Gueguen, Y., and Montagnani, C. 2010. Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: focus on biomineralization. BMC Genomics 11: 613. Crossref
Keupp, H. 1977. Paläopathologische Normen bei Amaltheiden (Ammonoidea) des Fränkischen Lias. Jahrbuch der Coburger Landesstiftung 1977: 263–280.
Keupp, H. 2000. Ammoniten – Paläbiologische Erfolgsspiralen. 165 pp. Thorbecke Verlag, Stuttgart.
Keupp, H. 2008. Desmoceras (Pseudouhligella) intrapunctatum n. sp. (Ammonoidea) aus dem unter-Albium von NW-Madagaskar mit Ritzstreifen. Paläontologische Zeitschrift 82: 437–447. Crossref
Keupp, H. 2012. Atlas zur Paläopathologie der Cephalopoden. Berliner paläobiologische Abhandlungen 12: 1–390.
Korn, D. 1985. Runzelschicht und Ritzstreifung bei Clymenien (Ammonoidea, Cephalopoda). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1985 (9): 533–541.
Korn, D., Klug, C., and Mapes, R. 2014. The coarse wrinkle layer of Palaeozoic ammonoids: New evidence from the Early Carboniferous of Morocco. Palaeontology 57: 771–781. Crossref
Klug, C., Korn, D., Richter, U., and Urlichs, M. 2004. The black layer in cephalopods from the German Muschelkalk (Triassic). Palaeontology 47: 1407–1425. Crossref
Kulicki, C. 1979. The ammonite shell: Its structure, development, and biological significance. Acta Palaeontologica Polonica 39: 97–142.
Kulicki, C. 1996. Ammonoid Shell Microstructure. In: N.H. Landman, K. Tanabe, and R.A. Davis (eds.), Ammonoid Paleobiology. Topics in Geobiology 13, 65–101. Plenum Press, New York. Crossref
Kulicki, C. and Tanabe, K. 1999. The ultrastructure of the dorsal shell wall of Mesozoic ammonoids. Berichte der Geologischen Bundesanstalt 46: 69.
Kulicki, C., Landman, N.H., Heany, M.J., Mapes, R.H., and Tanabe, K. 1999. Morphology of early whorls of goniatites from the Carboniferous Buckhorn Asphalt (Oklahoma) with aragonitic preservation. Berichte der Geologischen Bundesanstalt 46: 68.
Kulicki, C., Landman, N.H., Heany, M.J., Mapes, R.H., and Tanabe, K. 2002. Morphology of early whorls of goniatites from the Carboniferous Buckhorn Asphalt (Oklahoma) with aragonitic preservation. Abhandlungen der Geologischen Bundesanstalt Wien 57: 205–224.
Kulicki, C., Tanabe, K., Landman, N.H., and Kaim, A. 2016. Ammonoid shell microstructure. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology. Topics in Geobiology 43: 321–357. Springer, Dordrecht.
Kulicki, C., Tanabe, K., Landman, N.H., and Mapes R.H. 2001. Dorsal shell wall in ammonoids. Acta Palaeontologica Polonica 46: 23–42.
Landman, N.H. 1987. Ontogeny of Upper Cretaceous (Turonian–Santonian) scaphitid ammonites from the Western Interior of North America: systematics, developmental patterns, and life history. Bulletin of the American Museum of Natural History 185: 117–241.
Lehman, U. 1976. Ammoniten. Ihr Leben und ihre Umwelt. 171 pp. Ferdinand Enke Verlag, Stuttgart.
Lehman, U. 1990. Ammonoideen, Leben zwischen Skylla und Charybdis. In: H.K. Erben, G. Hillmer, and H. Ristedt (eds.), Haeckel-Bücherei 2, 1–258. Ferdinand Enke Verlag, Stuttgart.
Marie, B., Joubert, C., Tayalé, A., Zanella-Cléon, I., Belliard, C., Piquemal, D., Cochennec-Laureau, N., Marin, F., Gueguen, Y., and Montagnani, C. 2012. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proceedings of the National Academy of Sciences 109: 20986–20991. Crossref
Mironenko, A. 2015. Wrinkle layer and supracephalic attachment area: Implications for ammonoid paleobiology. Bulletin of Geosciences 90: 389–416. Crossref
Palframan, D.F.B. 1967. Mode of early shell growth in the ammonite Promicroceras marstonense SPATH. Nature 216: 1128–1130. Crossref
Radtke, G. and Keupp, H. 2016. Imbricational radial sculpture: A convergent feature within externally shelled cephalopods. Paleontology 59: 409–421. Crossref
Radtke, G., Hoffmann, R., and Keupp, H. 2016. Form and formation of flares and parabolae based on new observations of the internal shell structure in lytoceratid and perisphinctid ammonoids. Acta Palaeontologica Polonica 61: 503–517.
Ristedt, H. 1971. Zum Bau der orthoceriden Cephalopoden. Palaeontographica A 137: 155–195.
Rouget, I., Neige, P., and Dommergues, J.L. 2004. L’analyse phylogénétique chez les ammonites: état des lieux et perspectives. Bulletin de la Société Géologique de France 175: 507–512. Crossref
Senior, J.R. 1971. Wrinkle-layer structures in Jurassic ammonites. Palaeontology 14: 107–113.
Sprey, A.M. 2002. Early ontogeny of three Callovian ammonite genera (Binatisphinctes, Kosmoceras (Spinikosmoceras) and Hecticoceras) from Ryazan (Russia). Abhandlungen der Geologischen Bundesanstalt 57: 225–255.
Tozer, E.T. 1972. Observations on the shell structure of Triassic ammonoids. Palaeontology 15: 637–654.
Walliser, O.H. 1970. Über die Runzelschicht bei Ammonoidea. Göttinger Arbeiten für Geologie und Paläontologie 5: 115–126.
Yacobucci, M.M. 2016. Macroevolution and paleobiogeography of Jurassic–Cretaceous ammonoids. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology. Topics in Geobiology 44: 189–228. Springer, Dordrecht.
Zakharov, Y.D. 1996. Orthoceratid and ammonoid shell structure: Its bearing on cephalopod classification. Bulletin of the National Science Museum, Series C 1: 11–35.
Zakharov, Y.D. and Grabovskaya, V.S. 1984. The shell structure and stages in the development of the genus Zelandites (Lytoceratida). Paleontological Journal 18: 9–21.