
A new assessment of the Late Devonian antiarchan fish Bothriolepis leptocheira from South Timan (Russia) and the biotic crisis near the Frasnian–Famennian boundary
ERVĪNS LUKŠEVIČS, PAVEL BEZNOSOV, and VALDEMĀRS STŪRIS
Lukševičs, E., Beznosov, P., and Stūris, V. 2017. A new assessment of the Late Devonian antiarchan fish Bothriolepis leptocheira from South Timan (Russia) and the biotic crisis near the Frasnian–Famennian boundary. Acta Palaeontologica Polonica 62 (1): 97–119.
The species of the Late Devonian, earliest Famennian placoderm fish Bothriolepis jeremejevi has been downranked to subspecies Bothriolepis leptocheira jeremejevi comb. nov. in a result of detailed morphological studies of the type specimens and abundant new material from the type locality of the Sosnogorsk Formation, South Timan, Komi Republic, Russia. This taxon closely resembles Bothriolepis leptocheira leptocheira from Scotland, B. leptocheira curonica from Latvia, and B. leptocheira ssp. from Severnaya Zemlya differing only in small deviations of the size, proportions and shape of some plates of the armour. The composition of vertebrate assemblages from Scotland, Latvia, and Severnaya Zemlya containing B. leptocheira demonstrates a reduced diversity of antiarch placoderm, acanthodian, and sarcopterygian fishes; however, the Sosnogorsk assemblage differs in a larger diversity containing very primitive tetrapod and diversified sarcopterygian fishes including dipnoans and porolepiforms but lacking acanthodians. Wide distribution of a single species of Bothriolepis and usually diminished diversity of vertebrates suggest that the earliest Famennian vertebrate assemblages conform the survival faunas of the latest Frasnian–earliest Famennian biotic crisis.
Key words: Placodermi, diversity, variability, Devonian, Frasnian, Famennian, Russia, South Timan.
Ervīns Lukševičs [ervins.luksevics@lu.lv] and Valdemārs Stūris [valdemars.sturis@gmail.com], Department of Geology, Faculty of Geography and Earth Sciences, University of Latvia, Rainis Boulevard 19, Riga LV-1586, Latvia.
Pavel Beznosov [beznosov@geo.komisc.ru], Institute of Geology, Komi Science Centre, Ural Branch of the Russian Academy of Sciences, 54, Pervomayskaya St., 167982 Syktyvkar, Russia.
Received 9 July 2016, accepted 8 December 2016, available online 20 February 2017.
Copyright © 2017 E. Lukševičs et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The antiarch Bothriolepis has a widespread distribution in the territory of the East European platform and its surroundings, occurring at many localities in the Main Devonian Field (Lukševičs 2001), Central Devonian Field (Moloshnikov 2008), Poland (Szrek 2004), Severnaya Zemlya archipelago (Lukševičs 1999a, b), Timan (Ivanov and Lukševičs 1996), United Kingdom (Miles 1968), and Belgium (Olive 2015). First Bothriolepis specimens from South Timan were collected by the Timan expedition led by Theodosy Chernyshov in 1889–1890, and later studied in Saint-Petersburg by Rohon (1900), who described Bothriolepis jeremejevi, a new species among the material from the outcrop along the Izhma River. The description of two disarticulated and poorly preserved bones was associated with rather poor illustrations, therefore Gross (1932) designated this species as nomen dubium. However, Gross (1932) selected the specimen, determined and illustrated by Rohon (1900: fig. 18) as the anterior median dorsal plate, as the lectotype of B. jeremejevi. This specimen has not been found within the Theodosy Chernyshov’s collections, but the second specimen, namely fragmentary central ventral plate 1 (Rohon 1900: fig. 19) has been found by Alexander Ivanov in 1994 within the collection of Paleontological Institute of Russian Academy of Sciences, Moscow. Examination of this specimen and additional material, as well as comparison of bothriolepid material from other localities suggested that the material from the site at the Izhma River belongs to Bothriolepis leptocheira. This is the most widely distributed species of bothriolepid antiarchs from the lowermost Famennian, previously reported from Scotland (Miles 1968), Latvia (Lukševičs 2001), and Severnaya Zemlya (Lukševičs 1999a, b); B. cf. leptocheira has been reported from Central Russia (Moloshnikov 2008). However, the material of B. leptocheira from South Timan slightly differs from specimens from Scotland, Latvia, and Severnaya Zemlya, allowing the establishment of a separate subspecies Bothriolepis leptocheira jeremejevi comb. nov. Differences between several subspecies mainly regard small deviations of quantitative variables (indices), but no qualitative differences can be observed which could be used for distinguishing separate species.
Almost century long collecting in the Sosnogorsk locality has yielded an extensive collection of bothriolepid remains consisting of several hundred specimens. However, the largest part of the material kept within the collections of Institute of Geology of Komi Science Centre, Syktyvkar, has not been described yet; some specimens show very good preservation and the material contains several plates of the head armour previously unknown in the Scottish and Latvian material of Bothriolepis leptocheira. These new elements allow us to significantly improve the description of this species and specify previous reconstructions of the body armour, as well as briefly discuss variability of this taxon, traces of predation and parasite marks on the fish bones.
We provide here the description of Bothriolepis leptocheira jeremejevi (Rohon, 1900) from the outcrop along the Izhma River opposite the town of Sosnogorsk, South Timan, Komi Republic, Russia (Fig. 1). Judging from the composition and colour of the rock, colour and preservation of fossils, and large dimensions of the outcrop, this is surely the same locality from which the type specimens of B. jeremejevi Rohon, 1900 were gathered. The vertebrate assemblage comes from dolomitised limestone unit approximately 400–650 mm thick, which is widely known as “fish dolomite”, about 7 m above the probable Frasnian–Famennian boundary (John E.A. Marshall, personal communication 2009), and about 4 m below the contact with the base of the Izhma Formation (Fig. 2). The age of the fossil-bearing layer has been suggested to be the earliest Famennian (Obukhovskaya et al. 2000; Marshall et al. 2011).
The Sosnogorsk vertebrate assemblage also includes sarcopterygians and primitive tetrapod (Beznosov et al. 2011). The composition and proportions of faunal elements of the Sosnogorsk assemblage show remarkable similarities, but also differences from the lowermost Famennian assemblages of vertebrates from other localities of the Baltica province. Indications of reduced biological diversity around the Frasnian–Famennian boundary are briefly discussed here.
Institutional abbreviations.—CNIGR, the Central Geological Museum, Saint-Petersburg, Russia; CPC, the Commonwealth Palaeontological Collection, Bureau of Mineral Resources, Canberra, Australia; IG KSC, Institute of Geology, Komi Science Centre, Ural Branch of the Russian Academy of Sciences, Syktyvkar, Russia; LDM, the Natural History Museum of Latvia, Rīga, Latvia; PIN, the Paleontological Institute of Russian Academy of Sciences, Moscow, Russia; RSM, Royal Scottish Museum, Edinburgh, Scotland.
Other abbreviations.—ADL, anterior ventro-lateral plate; AMD, anterior median dorsal plate; AVL, anterior ventro-lateral plate; B, breadth; Ba, breadth of anterior part; Cd, dorsal central plate; cir, semicircular pit-line groove; cit1, crista transversalis interna anterior; cit2, transverse thickening on AVL; Cv, ventral central plate; FA, fluctuating asymmetry; L, length; La, lateral plate; Ml, lateral marginal plate; Mm, mesial marginal plate; MV, median ventral plate; MxL, mixilateral plate; Nu, nuchal plate; PMD, posterior median dorsal plate; Pmg, postmarginal plate; Pn, paranuchal plate; Pp, postpineal plate; Prm, premedian plate; PVL, posterior ventro-lateral plate; SO, suborbital plate.
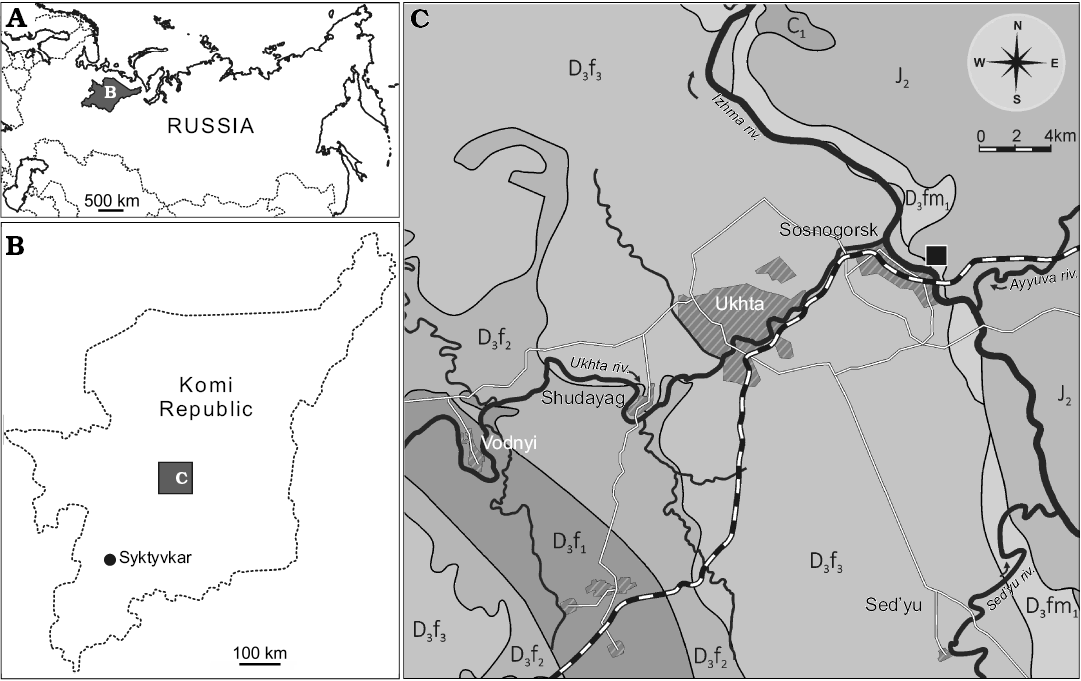
Fig. 1. Geographical and geological setting of the type locality of Bothriolepis leptocheira jeremejevi (Rohon, 1900): sketch-maps of Russia (A) and Komi Republic (B) and geological map of Ukhta-Sosnogorsk area, from Yukhtanov et al. (2008), amended (C). The Natural Geological Monument “Sosnovskiy” (Sosnogorsk fossil site) is marked by the black square. Abbreviations: D3f1, Upper Devonian, lower Frasnian; D3f2, Upper Devonian, middle Frasnian; D3f3, Upper Devonian, upper Frasnian; D3fm1, Upper Devonian, lower Famennian; C1, Carboniferous, Mississippian; J2, Middle Jurassic.
Geological setting
Fossil remains of Bothriolepis were collected from the type section of the Sosnogorsk Formation, the Natural Geological Monument “Sosnovskiy” (outcrop 20) on the right bank of the Izhma River opposite Sosnogorsk (63°35’56” N, 53°56’17” E), ENE from Ukhta, South Timan, Komi Republic, Russia by Natalya Belyaeva, Vitaliy Sorokin, Alexander Ivanov, Anatoliy Plyakin, Vjacheslav Kamashev, Pavel Beznosov, Per E. Ahlberg, Ervīns Lukševičs, Valdemārs Stūris, and others during expeditions in 1920, 1980, 1998, 2002, 2008–2012, as well as focused collecting during last decade. The upper part of the Sosnogorsk Formation (Fig. 2) consists of shallow-water carbonates most probably of lagoonal origin (Beznosov et al. 2011). Vertebrate fossils bearing strata, the bed No. 40 (sensu Beznosov 2009), are composed of a yellowish dolomitised limestone, widely known as the “fish-dolomite” (Kushnareva 1977). This limestone body, varying in thickness from about 400 to 650 mm, is varying in structure even in the 15–20 m2 small area in the type site. However, this limestone is rather pure without siliciclastic admixture. The bed No. 40 consists of several tempestites (the lowermost one is not continuous through the section), yielding numerous carbonate incrustations and fructifications of charophyte algae, including gyrogonites of Trochiliscus Pander, 1856 emended by Karpinsky, 1906 and utricles of Sycidium Sandberger, 1849 (Hecker 1983; determined by Artemiy Anfimiv, Institute of Geology and Geochemistry, Uralian Branch of Russian Academy of Sciences, Yekaterinburg, Russia), as well as abundant vertebrate fossils. Vertebrate fossils show uneven distribution, including vertical orientation in the middle part of the bed, moderately high fragmentation, reorientation, and sorting, as well as a small degree of corrosion and abrasion. Very rare articulated specimens of B. leptocheira jeremejevi and Holoptychius sp. occur only in the uppermost part and are exposed in life position (ventral side rest on the bottom). The limestone is bioturbated to various degrees, but locally shows fine lamination in the upper part. The bed No. 40 contains J- and Y-shaped burrows, horizontal networks and 3-D mazes, karst features, as well as possible rhizocretes in its middle and upper part. Trace fossils represented by the horizontal networks or three dimensional mazes of irregularly branching burrows, 3–4 mm in diameter, were provisionally referred to Thalassinoides isp., and vertical burrows to Balanoglossites isp. (Lukševičs et al. 2013). Besides these relatively large traces, tiny vermiculating horizontal objects resembling Pilichnus sp. were found in the middle part of this bed. Ventral walls of the trunk armour in IG KSC 155/10-3 are underlined by many traces of possible scavenging invertebrates. The uppermost part of the bed bears clear evidences of karst processes.
The earliest Famennian age was assigned to the underlying and overlying clayey deposits of the Sosnogorsk Formation by Obukhovskaya and Kuzmin (1993), Obukhovskaya et al. (2000), and Marshall et al. (2011), based on the presence of a miospore association characteristic of the Volgograd Regional Stage (RS) and corresponding to the Corbulispora viminea–Geminospora vasjamica (VV) Zone (Obukhovskaya et al. 2000). Fossil fauna of the “fish dolomite” consist of a variety of typical Late Devonian vertebrate representatives. Besides antiarchan placoderm, the remains of lobe-finned fishes, including porolepiforms Holoptychius sp. and Duffichthys sp., and dipnoans cf. Jarvikia sp. and Rhinodipteridae gen. indet., as well as remains of a new tetrapod taxon have been reported (Beznosov et al. 2012). However Bothriolepis remains form about 92% of total vertebrate macrofossils (Lukševičs et al. 2010).
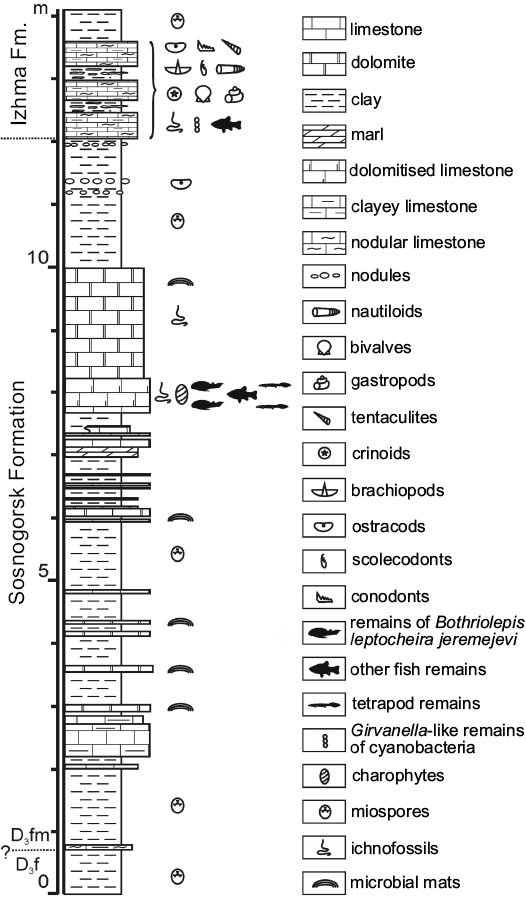
Fig. 2. Lithology and stratigraphy of the Sosnogorsk locality. “Fish-dolomite”, bed No. 40 is located about 8 m above the base of the section. Abbreviations: D3f, Upper Devonian, Frasnian; D3fm, Upper Devonian, Famennian.
Material and methods
The main part of Bothriolepis leptocheira jeremejevi material is stored in the collections of IG KSC, and some are stored at LDM, PIN, and CNIGR. In order to extract fish bones from limestone, 7–10% acetic acid dissolution was applied. Measurements were made with a Vernier calliper to the nearest 0.1 mm. Terminology of bones mainly follows that used by Miles (1968) and names proposed by Young (1984) for bothriolepid cheek and mouth plates are adopted here.
In order to evaluate the intraspecific variability, the method first proposed by Cherepanov (1986) has been used. This method consists of the estimation of relative pair differences between all individual specimens using indices of measured plates as quantitative morphological features (Lukševičs 1995); it allows the comparison of various populations, subspecies or species. For the population/species relative differences calculated after one variable are:
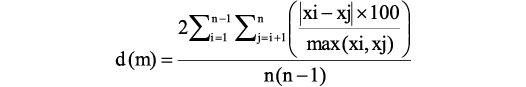
where: d (m), the mean difference between all individuals based on one variable m; xi and xj, value of variable m in individuals i and j; n, number of individuals.
The degree of mean differences between individuals from a set of variables d(k) is calculated as a sum of all d (m) divided by the number of variables k; e.g., dPMD is calculated as a sum of dB/L PMD, dL/Ba PMD and dB/Ba PMD divided by three. Plates of the head shield, trunk armour, and pectoral fin armour of seven bothriolepid species from the East European platform (Bothriolepis cellulosa [Pander in Keyserling, 1846], B. ciecere Lyarskaya, 1974, B. evaldi Lyarskaya, 1986, B. jani Lukševičs, 1986, B. maxima Gross, 1933, B. ornata Eichwald, 1840, and B. traudscholdi Jaekel, 1927), as well as plates of Bothriolepis leptocheira curonica and B. leptocheira jeremejevi were measured and 16 indices (d16) were calculated: seven indices for the head shield (two indices for premedian, four indices for nuchal and one index for lateral plates), eight indices for plates of the trunk armour (five indices for anterior median dorsal, and three indices for posterior median dorsal plates), and one index characterising the proportions of the ventral central plate 1 of the pectoral fin armour.
Systematic palaeontology
Euantiarcha Janvier and Pan, 1982
Bothriolepidoidei Miles, 1968
Bothriolepididae Cope, 1886
Genus Bothriolepis Eichwald, 1840
Type species: Bothriolepis ornata Eichwald, 1840; Priksha River, Novgorod region, Russia; the upper Famennian Lnyanka Formation.
Diagnosis.—Bothriolepididae in which the AMD plate is broadest across its lateral corners, and normally overlaps the ADL and is overlapped by the MxL plate. The MxL plate is broadest through its dorsal corner, with its lateral lamina of similar extent to the lateral lamina of the ADL plate, and not forming extensive contact with the AVL plate.
Bothriolepis leptocheira Traquair, 1893
1869 Pterichthys major; Geikie 1869 (after Miles 1968): 12–13.
1888 Bothriolepis major Agassiz; Traquair 1888 (after Miles 1968): 510.
1893 Bothriolepis leptocheirus sp. nov.; Traquair 1893: 285–286.
1900 Bothriolepis jeremejevi sp. nov.; Rohon 1900: 30, fig. 18, 19.
1917 Bothriolepis leptocheira Traquair, 1893; Evans 1917 (after Miles 1968): 112.
1932 Bothriolepis jeremejewi Rohon, 1900; Gross 1932: 34.
1942 Bothriolepis curonica sp. nov.; Gross 1942: 420, 421, abb. 10.
1948 Bothriolepis curonica Gross, 1942; Stensiö 1948: 615.
For a full list of synonyms before 1965 see Miles (1968).
Lectotype: RSM 1859.33.19A, AVL plate selected by Gross (1932: 26), illustrated by Traquair (1906: pl. 29: 3).
Type locality: Bracken Bay, Ayrshire (Heads of Ayr), Scotland;
Type horizon: Upper Old Red Sandstone, Upper Devonian.
Emended diagnosis.—Differs from all other Bothriolepis in possessing long supraoccipital groove extending to the Pn plates, sometimes fused with a long middle pit-line, and rather slender proximal segment of the pectoral appendage, which is more than 5 times as long as it is broad. Characterised by a rather large size with a median dorsal armour length of at least 240 mm. Preorbital recess of trifid type. The orbital fenestra is relatively small and narrow. Prm broad, orbital margin is much shorter than rostral margin. Nu with rather short orbital facets. AMD relatively narrow, B/L index about 0.8 (N = 22), with a relatively short anterior margin. PMD broad with narrow anterior margin. Median dorsal ridge poorly developed in posterior part of PMD. Dorso-lateral and ventro-lateral ridges are well-marked. Both AVL and PVL are elongated. Cv4 and Cd5 plates are present in the pectoral fin armour. Ornamentation is fine and basically of reticular type.
Stratigraphic and geographic range.—This species has also been reported from western Latvia, where the material has been collected in the right bank of the river Imula, near Bienes hamlet, and in the left bank of the river Amula, 1 km upstream from water-mill Kalnamuiža; Purviņi Member of the Eleja Formation, lowermost Famennian (Lukševičs 2001). A possible new subspecies of B. leptocheira occurs in the Malyutka Formation of Severnaya Zemlya, outcrops along the Matusevich River (Lukševičs 1999a, b). The material described in this paper comes from the geological monument “Sosnovskiy” (Sosnogorsk locality) at the right bank of the river Izhma opposite Sosnogorsk, Komi Republic, Russia; Sosnogorsk Formation, lowermost Famennian.
Bothriolepis leptocheira jeremejevi (Rohon, 1900) comb. nov.
Figs. 3–14.
1900 Bothriolepis jeremejevi sp. nov.; Rohon 1900: 30, fig. 18, 19.
1932 Bothriolepis jeremejewi Rohon, 1900; Gross 1932: 34.
Lectotype: PIN 1350/12 fragmentary Cv1, illustrated by Rohon (1900: fig. 19).
Type locality: Outcrop on the banks of the river Izhma, opposite Sosnogorsk, South Timan, Komi Republic, Russia.
Type horizon: Sosnogorsk Formation, Volgograd Regional Stage, lower Famennian, Upper Devonian.
Nomenclatorial remarks: Gross (1932: 26) selected the AMD plate 473 from the Cil’ma River (North Timan) as a of Bothriolepis jeremejewi Rohon, 1900 (erroneous spelling by Gross) illustrated by Rohon (1900: fig. 18). This specimen was most probably found in the lower Frasnian deposits and belongs to Asterolepis radiata (Rohon, 1900); it has never been found neither in CNIGR nor in PIN collections since the beginning of 1920-ies. However, the second specimen illustrated by Rohon (1900: fig. 19), 318b from the river Izhma (South Timan) has been identified by Alexander Ivanov in 1994 in the collection of PIN. Rohon (1900) erroneously identified it as the distal segment of the pectoral fin; in fact this is fragmentary Cv1 PIN 1350/12 from the outcrop on the banks of the river Izhma, judging from the original label. This specimen is about 40 mm long light brown plate embedded in the light yellow limestone; colour and preservation of the bone, as well as character of the rock are typical for the “fish dolomite” from the locality on the right bank of the river Izhma opposite Sosnogorsk, South Timan, Russia. Therefore the PIN 1350/12 illustrated by Rohon (1900: fig. 19) is designated here as the lectotype of Bothriolepis leptocheira jeremejevi (Rohon, 1900).
Material.—PIN 208, part of the head shield, two fragmentary Cv1, Nu, AMD, proximal segment of the pectoral appendage (on a single slab of limestone); IG KSC 155 (more than 160 disarticulated but mainly complete bones prepared out of the rock, some semiarticulated head shields, and three specimens demonstrating almost complete ventral wall of the trunk armour on several slabs), and IG KSC 71/И (many blocks with disarticulated fish remains, usually each block containing several specimens; only small part of all specimens is prepared); CNIGR 1226/1073, PVL; 1226/1074, PMD; 1226/1078, AMD (for complete list of specimens from IG KSC see the Appendix 1). All material from the type locality and horizon.
Description.—Bothriolepis leptocheira is well-represented in limestone from the Sosnogorsk locality by some articulated head shields and ventral walls of the trunk armour, some articulated proximal segments of the pectoral fin, as well as by numerous disarticulated plates of the head and trunk shields, and pectoral appendage. Most are from individuals of moderate size; however, there are remains from a very large, as well as from very small individuals. The plates are usually slightly flattened, sometimes deformed and bear cracks, most probably due to compaction of the fossil-bearing limestone.
The head shield (Fig. 3) is moderately broad, with an average B/L index 1.24 (n = 2), slightly narrower than the head shield of Bothriolepis leptocheira curonica from Latvia, but almost similar to that of B. ornata (Lukševičs 2001). The rostral margin is moderately convex and only slightly longer than the posterior margin. The head shield is weakly vaulted both rostrocaudally and transversely, as in Scottish material (Miles 1968), but the anterior part of the Prm and La plates is strongly curved. The antero-lateral corner, the prelateral notch (nprl; Figs. 4C, D2, 5) and the lateral process are usually weakly defined, as in B. leptocheira from Scotland and Latvia. The orbital fenestra is well seen in IG KSC 155/162 (Fig. 3C) and IG KSC 155/45 representing individual of moderate size; it is relatively small as in other subspecies of B. leptocheira, and with L/B index of 0.53. The preorbital recess (prh) is of trifid type, with the lateral horns less extended laterally than these in B. maxima or B. gigantea, but more broad at their base (Figs. 4, 5). The borders of the otico-occipital depression (ood; Figs. 6, 7) on the internal surface of the head shield are well defined by the paramarginal crista (cr.pm) on the paranuchal plates (Figs. 6, 7), which are rather low on the lateral plates (Figs. 4, 5). The antero-lateral corners of the otico-occipital depression (the imprints of the anterior postorbital processes of the endocranium, pr.po; Fig. 5F2) are moderately narrow at the base in comparison with these in B. ornata. In most cases the anterior postorbital processes do not extend in front of the anterior margin of the orbital fenestra, contrary to these in Scottish material (Miles 1968: 76).
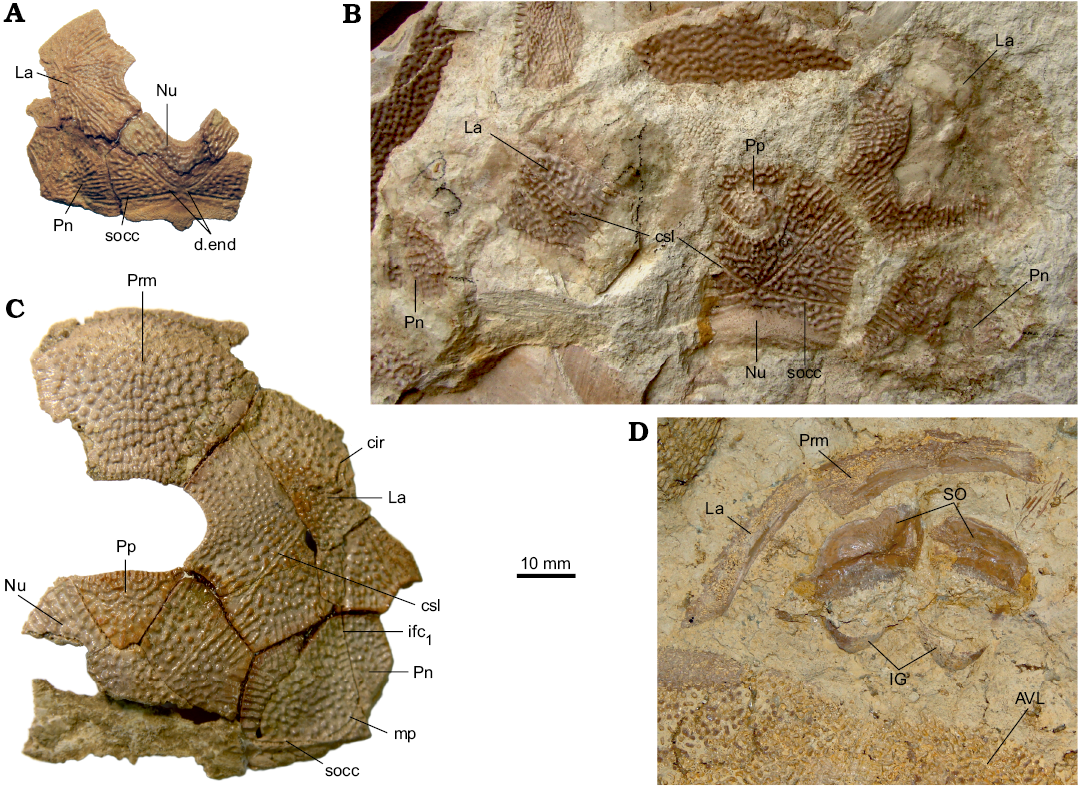
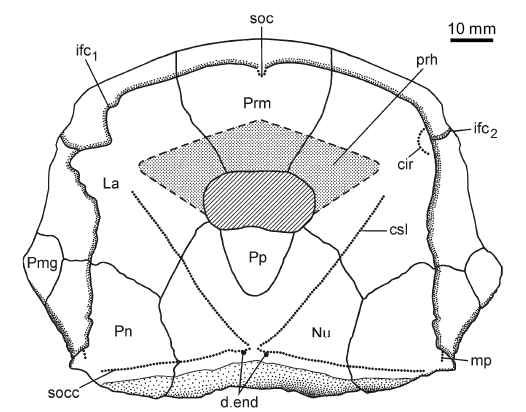
Fig. 3. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, separate plates and parts of articulated head shields. A. Articulated Nu, left Pn and left La plates IG KSC 155/90 in dorsal view. B. Disarticulated plates of the head shield IG KSC 155/150 in dorsal view. C. Part of articulated head shield IG KSC 155/162 in dorsal view. D. Imprint of the ventral wall of trunk armour and some skeletal elements of the head armour IG KSC 155/10-3 in ventral view showing suborbital and infragnathal bones. Abbreviations: AVL, anterior ventro-lateral plate; cir, semicircular pit-line groove; csl, central sensory line groove; d.end, opening of canal for endolymphatic duct; ifc1, principal section of infraorbital sensory line; IG, inferognathal; La, lateral plate; Nu, nuchal plate; mp, middle pit-line groove; Pn, paranuchal plate; Pp, postpineal plate; Prm, premedian plate; SO, suborbital plate; socc, supraoccipital cross-commissural pit-line groove.
The premedian plate (Prm; Figs. 3C, 4H, 5H) is slightly wider than long, with a B/L index of 1.07–1.37. It is slightly narrower than the Prm of B. leptocheira from Latvia (1.23–1.48; Lukševičs 2001), but approximately coincides with proportions of Prm in Scottish material of B. leptocheira (Miles [1968: 76] has mentioned B/L index of 1.14, 1.20, and 1.28). Prm is weakly arched in a lateral direction, but rather strongly vaulted in the parasagittal section with curvature in the anterior most part. The rostral margin is convex, it is 2.5–3.2 times longer than the slightly concave or straight orbital margin, similar to B. leptocheira from Latvia and Scotland (for comparison see Lukševičs 2001: figs. 32–34 and Miles 1968: pl. 20: 1, 2), and bears slightly defined median rostral projection (IG KSC 155/114; Fig. 4H2) seen only in anterior view. Also the position of the infraorbital sensory groove (ifc1) that crosses the plate close to its rostral margin and the well-defined anterior section of the supraorbital sensory line (soc) are similar characteristics in all three subspecies of B. leptocheira.
The lateral plate (La; Figs. 3, 4A–G, 5A–G, I) is short and broad with an overall shape and proportions resembling these of B. leptocheira curonica. The infraorbital sensory groove crosses the plate in its anterior part, not far from the anterior margin and along its lateral margins. The path of the sensory groove significantly varies in various specimens, e.g., on La plate IG KSC 155/15 (Figs. 4A, 5A) it displays a strong deviation from its usual path (Fig. 4B–G) because it partly follows the course of the semicircular pit-line groove, which is missing in this specimen (IG KSC 155/15). The plate is rather strongly vaulted in its anterior most part in large individuals (IG KSC 155/66; Fig. 4E2), but almost flat in small individuals (IG KSC 155/69; Fig. 4F). A moderately small, but deep pit (p) is positioned just posterior to the spiracular groove (spg; Fig. 5F2). The spiracular groove is moderately long and shallow. It is widest at the lateral margin of the head shield and narrows only slightly close to the pit. The anterior and posterior crests delimiting the spiracular groove are well developed along their entire extent. The anterior attachment for the submarginal plate (a1Sm; Fig. 5F2) is strongly developed transverse structure, which borders the anterior crest of the spiracular groove. This attachment is very close to the more anteriorly located triangular attachment area for the prelateral plate (a.PrL; Fig. 5F2). The posterior attachment for the submarginal plate (a2Sm) constitutes an elongate boss (Fig. 5F2), widening posteriorly. The attachment reaches anteriorly to the posterior crest delimiting the spiracular groove.
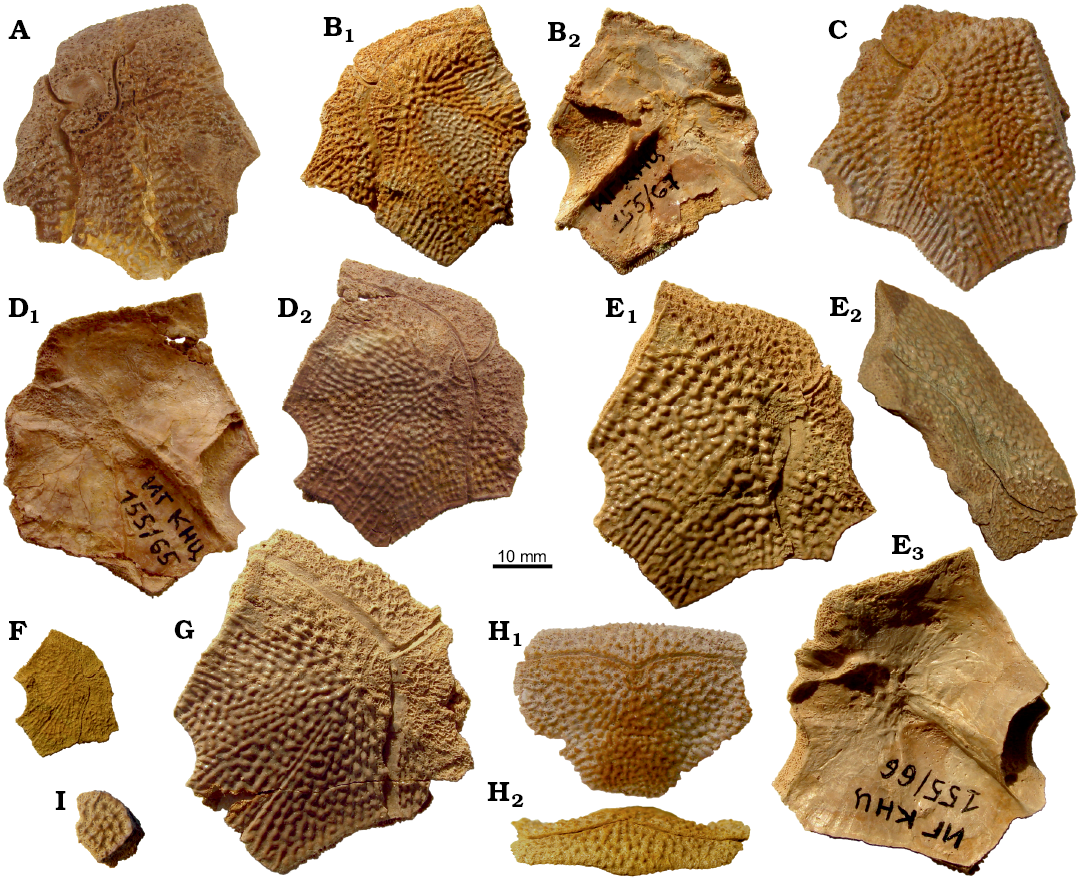
Fig. 4. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, lateral (A–G), premedian (H), and left postmarginal (I) plates of the head shield. A. IG KSC 155/15 in dorsal view. B. IG KSC 155/67 in dorsal (B1) and visceral (B2) views. C. IG KSC 155/129 in dorsal view. D. IG KSC 155/65 in visceral (D1) and dorsal (D2) views. E. IG KSC 155/66 in dorsal (E1), right lateral (E2), and visceral (E3) views. F. IG KSC 155/69 in dorsal view. G. IG KSC 155/64 in dorsal view. H. IG KSC 155/114 in dorsal (H1) and anterior (H2) views. I. Plate disconnected from the partial head shield IG KSC 155/161, in dorsal view.
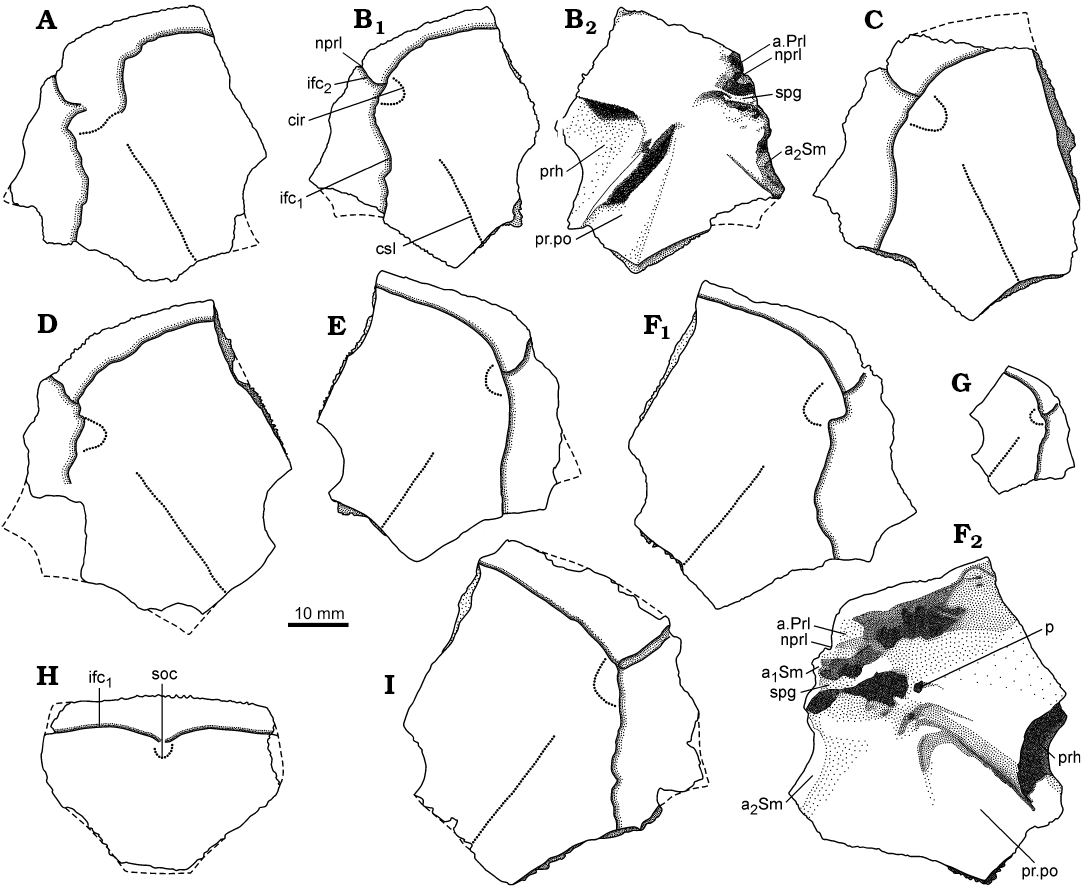
Fig. 5. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), lateral (A–G, I) and premedian (H) plates of the head shield. A. IG KSC 155/15 in dorsal view. B. IG KSC 155/67 in dorsal (B1) and visceral (B2) views. C. IG KSC 155/129 in dorsal view. D. IG KSC 155/74 in dorsal view. E. IG KSC 155/65 in dorsal view. F. IG KSC 155/66 in dorsal (F1) and visceral (F2) views. G. IG KSC 155/69 in dorsal view. H. IG KSC 155/114 in dorsal view. I. IG KSC 155/64 in dorsal view. Abbreviations: a1Sm and a2Sm, anterior and posterior attachment areas on lateral plate for submarginal plate; a.Prl, attachment area on lateral plate for prelateral plate; cir, semicircular pit-line groove; csl, central sensory line groove; ifc1, principal section of infraorbital sensory line; ifc2, branch of infraorbital sensory line diverging on La; nprl, prelateral notch of head shield; p, lateral pit of head shield; prh, preorbital recess of head shield; pr.po, antero-lateral corner of otico-occipital depression; soc, anterior section of the supraorbital sensory line; spg, spiracular groove of head shield.
The postpineal plate (Pp; Figs. 6–8) is, as usually observed in Bothriolepis, slightly wider than it is long and has an L/B index varying from 0.96 to 0.97, it is slightly less elongated than in the material of B. leptocheira from Scotland (Miles 1968: pl. 20: 4) and Latvia (Lukševičs 2001: fig. 34). The rounded anterior margin of Pp protrudes more into the orbital fenestra of small specimens, e.g., IG KSC 155/155 (Fig. 6C) than in larger specimens (Figs. 6A, B). IG KSC 155/146 is an asymmetrical plate with wider right part of the bone and pointed, rather than rounded posterior angle (Fig. 6B). Two large, rounded pits (g; Fig. 7B2) on the internal surface of the postpineal are separated by a low median ridge.
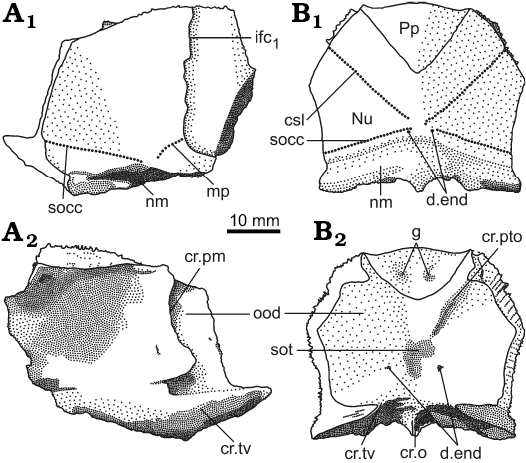
The nuchal plate (Nu; Figs. 3, 6–8) has an L/B index of about 0.83 (Miles 1968 mentioned an L/B index of 0.83 for Scottish material and Lukševičs 2001 mentioned an L/B index of 0.84 for material from Latvia). The plate is always broadest across the lateral corners. The anterior division of the lateral margin is usually convex and a little shorter than the concave posterior division (Figs. 6D–H, K, 7B). The orbital facets are very short, tapering to a point in IG KSC 155/53 and IG KSC 155/154 (Fig. 6F, G). The posterior margin is usually strongly concave; however, IG KSC 155/54-1 and IG KSC 155/56 demonstrate convex posterior margin (Fig. 6H, I). The median process is more pronounced in specimens with a concave posterior margin. The external openings for the endolymphatic ducts (d.end) are closely set to one another, in a distance of 2.9 mm in IG KSC 155/53. The supraoccipital cross-commissural pit-line groove (socc) is usually well defined, however, in IG KSC 155/53 (Fig. 6F) it is seen only as a line of pores along the posterior margin. Internally, the openings for the endolymphatic ducts are larger than their external openings, divided by the large supraotic thickening (sot; Fig. 7B2). The transverse nuchal crista (cr.tv) is high and well developed. Posteriorly, the median occipital crest of the head shield (cro; Figs. 6, 7B2) is usually well developed.
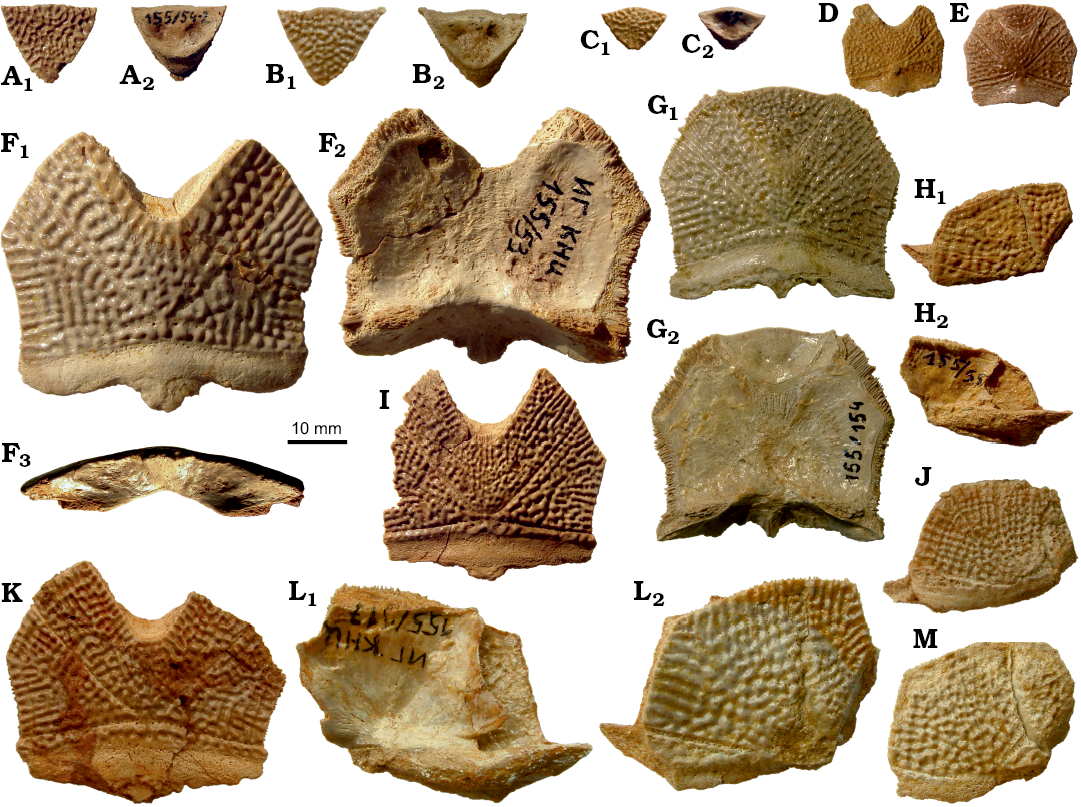
Fig. 6. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, postpineal (A–C, E, G), nuchal (D–G, I, K–M), and paranuchal (H, J, L, M) plates of the head shield. A. IG KSC 155/54-2, disconnected from the Nu IG KSC 155/54-1, in dorsal (A1) and visceral (A2) views. B. IG KSC 155/146 in dorsal (B1) and visceral (B2) views. C. IG KSC 155/155 in dorsal (C1) and visceral (C2) views. D. IG KSC 155/58 in dorsal view. E. Articulated plates IG KSC 155/118 in dorsal view. F. IG KSC 155/53 in dorsal (F1), visceral (F2), and posterior (F3) views. G. Articulated plates IG KSC 155/154 in dorsal (G1) and visceral (G2) views. H. IG KSC 155/55 in dorsal (H1) and visceral (H2) views. I. IG KSC 155/54-1 from the same individual as plate in A, in dorsal view. J. IG KSC 155/119 in dorsal view. K. IG KSC 155/56 in dorsal view. L. IG KSC 155/117 in visceral (L1) and dorsal (L2) views. M. IG KSC 155/125 in dorsal view.
The paranuchal plate (Pn; Figs. 3, 6, 7) is of moderate breadth, with an L/B index of about 0.92, whereas in B. leptocheira curonica it is broader, the index reaching 0.86 (Lukševičs 2001); Miles (1968) mentioned that Pn of B. l. leptocheira is poorly known and in the restoration (Miles 1968: text-fig. 36) it has an L/B index of about 0.80. The lateral division of Pn composes 39–44% (41.9 on the average) of the general breadth of the plate, thus being slightly broader than in B. leptocheira curonica with 38.7% on the average (Lukševičs 2001). A long supraoccipital cross-commissural pit-line groove (socc) is present in all examined specimens; it terminates approximately at the level of the middle of the plate posterior margin. B. hydrophila (Agassiz, 1844) from the Upper Old Red Sandstone of Dura Den, Scotland, also possess a long supraoccipital groove; however, it never extends to the Pn plate. The middle pit-line groove (mp) is always present, as it is in B. leptocheira leptocheira and B. leptocheira curonica (Lukševičs 2001); sometimes it is rather long, but it is not fused with the supraoccipital pit-line groove, contrary to B. leptocheira curonica where it is fused in some specimens (Lukševičs 2001: fig. 33A, B). Internally, the paramarginal crest (cr.pm) is very distinct; it is of semilunar shape in IG KSC 155/117 (Fig. 7A). The postero-lateral angle of the otico-occipital depression is complicated by two postero-lateral projections. Paranuchal trochlea does not reach the lateral margin of the plate.
 |
 |
|
Fig. 7. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), paranuchal (A) and postpineal and nuchal (B), plates of the head shield. A. IG KSC 155/117 in dorsal (A1) and visceral (A2) views. B. Articulated plates IG KSC 155/154 in dorsal (B1) and visceral (B2) views. Abbreviations: cr.o, median occipital crest of head shield; cr.pm, paramarginal crest of head shield; cr.pto, postorbital crest of head shield; cr.tv, transverse nuchal crest of head shield; csl, central sensory line groove; d.end, opening of canal for endolymphatic duct; g, paired pits on Pp; ifc1, principal section of infraorbital sensory line; mp, middle pit-line groove; nm, obtected nuchal area of head shield; Nu, nuchal plate; ood, otico-occipital depression of head shield; Pp, postpineal plate; socc, supraoccipital cross-commissural pit-line groove; sot, supraotic thickening of head shield. |
Fig. 8. Reconstruction of the head shield of antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), based on partial head shield (IG KSC 71/1-87), left (IG KSC 155/15), and right (IG KSC 155/65) lateral plates. Abbreviations: cir, semicircular pit-line groove; csl, central sensory line groove; d.end, opening of canal for endolymphatic duct; ifc1, principal section of infraorbital sensory line; ifc2, branch of infraorbital sensory line diverging on La; La, lateral plate; mp, middle pit-line groove; Nu, nuchal plate; Pmg, postmarginal plate; Pn, paranuchal plate; Pp, postpineal plate; Prm, premedian plate; prh, preorbital recess of head shield; soc, anterior section of the supraorbital sensory line; socc, supraoccipital cross-commissural pit-line groove. |
The postmarginal plate IG KSC 155/161 (Fig. 4J) is shorter and broader than it is in B. leptocheira curonica (Lukševičs 2001: figs. 32A, B, F, 33A, B, F), more closely resembling B. leptocheira leptocheira (Miles 1968: text-fig. 36) by its irregular quadrangular shape with an almost straight postero-mesial margin and strongly convex antero-mesial margin.
The suborbital plates (SO) have not been previously found in this species. The right and left SO are preserved only from one individual: in IG KSC 155/10-3 these are seen in visceral (internal) view (Fig. 3D), and the IG KSC 155/10-2 (Fig. 11A) shows their cast. The shape of the plate is rhombic with a slightly convex anterior and an almost straight posterior margin. The lateral margin is slightly concave, forming a prominent postero-lateral corner. The mesial margin is oblique therefore the split between pair of suborbital plates widens posteriorly. The ventral margin seems to bear rather small tubercles showing weakly defined denticulation. The visceral surface is dominated by a pronounced transverse ridge, which begins near the dorso-mesial corner, and extends obliquely, towards the middle of the lateral edge of the plate. It forms a posteriorly oriented convex curve. The height of this ridge varies: large close to the mesial margin and becoming lower laterally. The morphology of the visceral surface of SO is almost similar to those described for other species of Bothriolepis: B. canadensis (Stensiö 1948), Bothriolepis sp. from Gogo (Young 1984), and B. yeungae Johanson (1998).
The S-shaped infragnathals of B. leptocheira are also described here for the first time; they are preserved in the IG KSC 155/10-2 and IG KSC 155/10-3 (Figs. 3D, 11A, 14). The lateral projection of the right infragnathal lies very close to the tip of posterolateral corner of SO (Fig. 3D). Infragnathal is strongly curved in the mesial part and tapers mesially.
The trunk armour is narrow, relatively low, with a rather flattened dorsal wall, and the lateral wall more than three times as long as it is high. The length of the dorsal wall, estimated from the largest specimens of AMD and PMD plates, probably, slightly exceeds 200 mm. Thus it is larger than in B. leptocheira from Scotland (Miles [1968] estimated the dorsal wall reached a length of some 140 mm), but smaller than in B. leptocheira curonica from Latvia estimated by Lukševičs (2001) as reaching 240 mm. The median dorsal ridge is weakly defined only in the posterior part of the PMD. The dorso-lateral and ventro-lateral ridges are well marked. The ventral wall of the trunk armour is not completely flat as it was demonstrated in the 3D model of B. canadensis (Béchard et al. 2014), but it is slightly convex both in transverse and longitudinal directions. The ventral wall is moderately wide with an estimated B/L index of 0.6 in IG KSC 155/10-3 (Figs. 3D, 14), exceeding the estimated B/L index of about 0.53 in Latvian material of B. leptocheira (Lukševičs 2001). However, this difference could be explained by the use of separate AVL and PVL plates of moderate size to estimate the B/L index of the ventral wall of B. leptocheira curonica. The ventral wall is slightly narrower than that restored for B. prima and B. obrutschewi (Karatajūte-Talimaa 1966: figs. 3, 10), but almost perfectly similar in the proportions to B. canadensis (Béchard et al. 2014: fig. 3).
The anterior median dorsal plate (AMD; Fig. 9) is weakly arched, relatively narrow, with a B/L index of about 0.78 in the Sosnogorsk material. The index is 0.83 in Latvian material (Lukševičs 2001: 549), and about 0.77 in Scottish material (Miles 1968: 78). The anterior margin is weakly convex, sometimes slightly concave in its middle (IG KSC 155/44; Fig. 9G), relatively narrow, and about 1.3 times longer than the posterior margin. The antero-lateral and lateral corners, and the postlevator processes (pr.pl) are well defined, especially in larger specimens. The posterior division of the lateral margin is 1.4–1.6 times shorter than the anterior division. There is no median dorsal ridge. Overlap areas for ADL and MxL are sometimes normally developed as typical for Bothriolepis (IG KSC 155/44; Fig. 9G), but usually demonstrate the so called Remigolepis-type overlapping: IG KSC 155/140 and IG KSC 155/42 (Fig. 9E, F) show the anterior ¼–⅓ of the posterior lateral margin overlapping MxL (cf.MxL) and remaining ¾ or ⅔ of that margin are overlapped by MxL. The same feature is also seen in IG KSC 155/108, where the AMD overlaps MxL to 1/6 of the common suture (Fig. 9B), and IG KSC 155/5, where the cf.MxL is even smaller (Fig. 9A). Unusual are sutural connections of AMD and ADL in IG KSC 155/44, where the posterior ⅓ of the anterior lateral margin of AMD is overlapped by ADL (oa.ADL; Fig. 9G). Internally, the levator fossa (f.retr) is longer than it is wide; it is delineated by the low and narrow postlevator thickenings (alr). The low and short medial crest (mc; Fig. 9A, B) is more than twice shorter than the levator fossa. The supranuchal area (sna) is well defined and slightly broader medially from the antero-lateral corner. The centre of the anterior ventral pit (pt1; Fig. 9A, B, D) is located slightly anteriorly to the tergal angle (dma). The median ventral ridge (mvr) is relatively low; in IG KSC 155/113 (Fig. 9D) it is extremely short and divides into two crests which continue as a moderately deep median ventral groove (grm).
The posterior median dorsal plate (PMD; Fig. 9H–N) is broad in comparison with the AMD. Its B/L index varies from 0.84 to 1.00, 0.92 on the average in the Sosnogorsk material, whereas it reaches about 0.95 in the material from Scotland (Miles 1968) and varies from 0.88 to 1.01, 0.95 on the average, in the material from Latvia (Lukševičs 2001). The anterior margin is rather narrow, strongly convex and pointed in small individuals, and convex but rounded in large individuals. The posterior margin is well convex, 1.7–2.7 times longer than the anterior margin in the material from Sosnogorsk, but only 2–2.5 in the Scottish and Latvian material. The posterior corner of the PMD is not pronounced as in the Scottish (Miles 1968: text-fig. 38) and Latvian material (Lukševičs 2001: fig. 35C). The median dorsal ridge (dmr) is present only in the posterior third of the plate in medium-sized individuals, and only in the posterior quarter in the largest PMD IG KSC 155/142, or even absent in some large PMD; however, in the smallest individual IG KSC 155/148 reaching only 19 mm in length, it is seen along the whole plate. IG KSC 155/7 differs from all other specimens in possessing two distinct abnormalities which are most probably related to one another: (i) the posterior margin bears 5–6 mm wide unornamented area (pua) that superficially resembles the obtected nuchal area of the head shield; (ii) a branch of the sensory line canal (dlg2) is clearly seen along the same posterior margin of the left side of the plate (Fig. 9H) in the position where this canal has been reported in B. canadensis (Graham-Smith 1978: fig. 2e). The canal crosses the lateral margin of the PMD close to its posterior margin, runs parallel to the posterior margin and a few milimeters from the dorsal median ridge turns posteriorly and becomes deeper than in the most part of its course, “diving” into the bone. The 5–6 mm narrow belt between the canal and pua is ornamented by separate round and high tubercles, in some cases two or three of them fused into very short ridges, while the rest of the surface bears mainly vermiculating ridges and only several separate tubercles close to the canal. The ventral surface of the PMD shows a relatively low posterior transverse thoracic crest (cr.tp), in some specimens (e.g., IG KSC 155/1; Fig. 9J) forming a distinct shelf on the short area posterior to the crest. There is a wide median ventral groove (grm) and a peculiar posterior ventral pit (pt2; Fig. 9J, N), which is funnel-shaped, quite elongated and oblique in sagittal view so that the anterior wall is very low and the posterior wall is rather high.
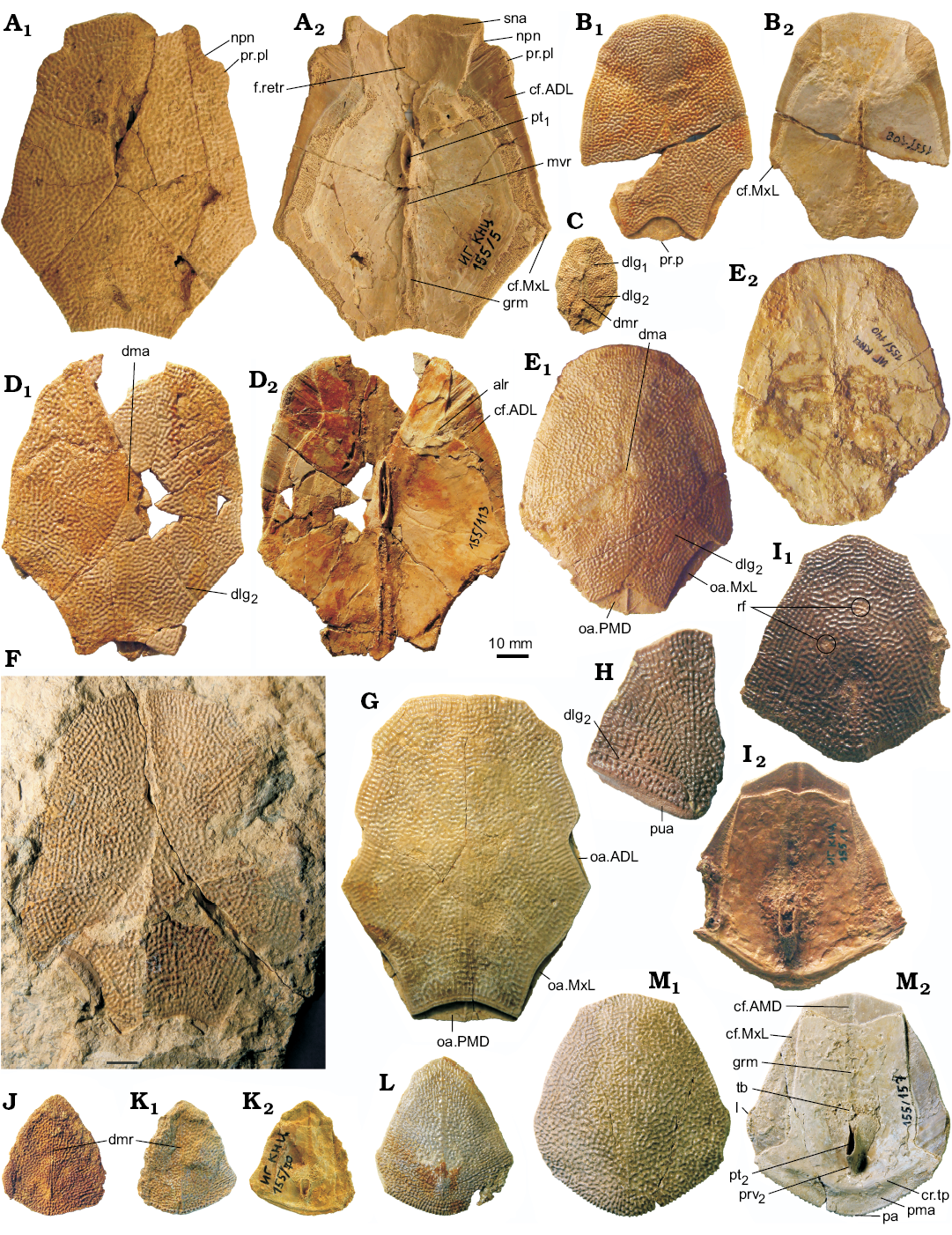
Fig. 9. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, anterior median dorsal (A–G) and posterior median dorsal (H–M) plates of the trunk armour. A. IG KSC 155/5 in dorsal (A1) and visceral (A2) views. B. IG KSC 155/108 in dorsal (B1) and visceral (B2) views. C. IG KSC 155/97 in dorsal view. D. IG KSC 155/113 in dorsal (D1) and visceral (D2) views. E. IG KSC 155/140 in dorsal (E1) and visceral (E2) views. F. Impression of the dorsal surface of IG KSC 155/42. G. IG KSC 155/44 in dorsal view. H. Fragment of IG KSC 155/7 in dorsal view. I. IG KSC 155/1 in dorsal (I1) and visceral (I2) views. J. IG KSC 155/71 in dorsal view. K. Slightly deformed IG KSC 155/70 in dorsal (K1) and visceral (K2) views. L. IG KSC 155/158 in dorsal view. M. IG KSC 155/157 in dorsal (M1) and visceral (M2) views. Abbreviations: ADL, anterior dorso-lateral plate; alr, postlevator thickening; AMD, anterior median dorsal plate; cf.ADL, cf.AMD, and cf.MxL, area overlapping ADL, AMD or MxL respectively; cr.tp, posterior transversal internal crest; dlg1 and dlg2, anterior and posterior oblique dorsal sensory line groove; dma, tergal angle; dmr, dorsal median ridge; f.retr, levator fossa; grm, ventral median groove; l, lateral corner; mvr, median ventral ridge; MxL, mixilateral plate; npn, postnuchal notch; oa.ADL, oa.MxL and oa.PMD, area overlapped by ADL, MxL or PMD respectively; pa, posterior corner; pma, posterior marginal area; PMD, posterior median dorsal plate; pr.p, posterior process of AMD; pr.pl, external postlevator process; prv2, posterior ventral process of dorsal wall of trunk armour; pt1 and pt2, anterior and posterior ventral pit; pua, posterior unornamented area of PMD; rf, “round fossula”; sna, supranuchal area; tb, ventral tuberosity.
The dorsal lamina of ADL is of moderate breadth, about 2.6–3.3 times (2.9 on the average) as long as it is broad (Fig. 10A–G), thus the proportions of the ADL of B. leptocheira jeremejevi are more similar to those of B. leptocheira curonica (e.g., LDM 98/58; Lukševičs 2001: figs. 36B, 37A) and ADL is shorter than it is 3.5 times longer and broader in B. leptocheira leptocheira RSM 1859.33.632D (Miles 1968: pl. 20: 3). The postnuchal ornamented corner (pnoa) is prominent in all studied specimens. The width of the dorsal lamina is usually slightly larger than the height of the lateral lamina. The dorso-lateral ridge (dlr) is well pronounced. Usually, the AMD overlaps the mesial margin of the ADL, but in IG KSC 155/116 (Fig. 10A2) the ADL overlaps the AMD (cf.AMD) along the posterior third part of the common suture. The outline of the mesial margin of this specimen greatly complements the lateral margin of AMD plate IG KSC 155/44 (Fig. 9G). Thus, this feature is not remarkably rare in B. leptocheira jeremejevi. The lateral lamina of ADL is 4.7 times as long as it is high in IG KSC IG 155/112 (Fig. 10G) and more than 6 times as long as it is high in IG KSC IG 71/i-68; the lateral lamina of ADL is slightly higher in the material from Latvia (Lukševičs 2001: fig. 37B), and Scottish material does not show the lateral lamina well (Miles 1968: 78). The processus obstans (pro) is rather deep, but the course of the main lateral line groove (lcg) conforms the usual pattern.
The dorsal lamina of MxL is slightly less than twice as long as it is broad (Fig. 10H–J). The dorsal corner is clearly seen. The lateral lamina is moderately low, about 2–2.5 times as long as it is high, but relatively higher than in material from Latvia where it is about three times as long as it is high (Lukševičs 2001: fig. 37E). The posterior oblique sensory line groove (dlg2) usually terminates in some distance from the lateral margin (e.g., IG KSC 155/82); as in B. leptocheira curonica LDM 98/53 (Lukševičs 2001: fig. 36C), IG KSC 155/115 shows a dlg2 crossing the dorso-lateral ridge (Fig. 10I). The separate dorso-ventral pit-line groove (dxp) is present in a medium sized IG KSC 155/144 (Fig. 10H), similarly to B. prima Gross, 1942 (Karatajūte-Talimaa 1966: fig. 3), B. obrutschewi Gross, 1942 (Karatajūte-Talimaa 1966: fig. 10), and B. evaldi Lyarskaya, 1986 (Lyarskaya 1986). The dorso-lateral ridge (dlr) is well defined both in the ADL and MxL plates of small and medium sized specimens, as it is also seen in material from Latvia; Miles (1968: 78) claimed that “there is no clear development of the dlr on either the MxL or ADL”; in fact dlr is not clearly seen, particularly in MxL, because of preservation of the Scottish material as internal impressions.
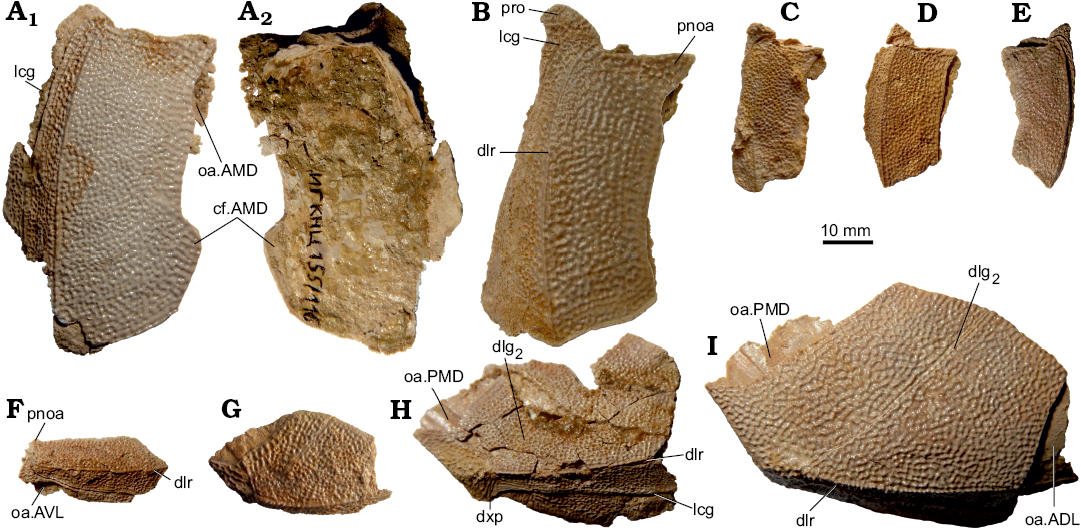
Fig. 10. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, anterior dorso-lateral (A–D, F, left; E, right) and left mixilateral (G–I, right) plates of the trunk armour. A. IG KSC 155/116 in dorsal (A1) and visceral (A2) views. B. IG KSC 155/111. C. IG KSC 155/61. D. IG KSC 155/60. E. IG KSC 155/136. F. IG KSC 155/112. G. IG KSC 155/110. H. Incomplete IG KSC 155/144. I. IG KSC 155/115. B–E, G–I, in dorsal views; F, in lateral view. Abbreviations: ADL, anterior ventro-lateral plate; AMD, anterior median dorsal plate; AVL, anterior ventro-lateral plate; cf.AMD, area overlapping AMD; dlg2, posterior oblique dorsal sensory line groove; dlr, dorso-lateral ridge of trunk armour; dxp, dorso-ventral pit-line groove; lcg, main lateral line groove; oa.ADL, oa.AMD, oa.AVL, oa.PMD, area overlapped by ADL, AMD, AVL or PMD respectively; PMD, posterior median dorsal plate; pnoa, postnuchal ornamented corner of ADL; pro, processus obstans of trunk armour.
The semilunar plate (SM) is not known, but from the configuration of the anterior margin of the AVL plates, the SM is of average proportions and normal shape (Fig. 14).
The ventral lamina of the AVL is 1.6–2 times as long as it is broad (Fig. 11). The anterior margin of the ventral lamina is rounded without clearly defined corners, similar to B. leptocheira leptocheira (Miles 1968: 79, text-fig. 39) and B. leptocheira curonica (Lukševičs 2001: figs. 37F, G, 38A). The subcephalic division comprises 21–30% (24% on average) of the total length of the ventral lamina (about 20% in the Scottish material [Miles 1968]; about 21–25% with 22.5% on average in the Latvian material [Lukševičs 2001: 551]). The lateral lamina is not preserved within the material from Scotland. The lateral lamina is low, and the ventral lamina is 3.8 times as broad as the lateral lamina is high in B. leptocheira curonica LDM 98/30. The lateral lamina is relatively higher in the Sosnogorsk material, e.g., IG KSC 155/122 (Fig. 11D) shows the ventral lamina is 2.9 times as broad as the lateral lamina is high. The right AVL overlaps the left AVL by a very narrow overlap area. The pectoral fin attachment area is usually not well preserved in the studied Sosnogorsk material, except on AVL IG KSC 155/122 (Fig. 11D), which shows the typical morphology of Bothriolepis. Internally, the crista transversalis interna anterior (cit1; Fig. 11A) is developed as a high, thin ridge running at an oblique angle across half the width of the anterior portion of the plate. The transverse thickening (cit2) is more prominent in comparison with cit1 as it is seen in the IG KSC 155/10-2 (Fig. 11A).
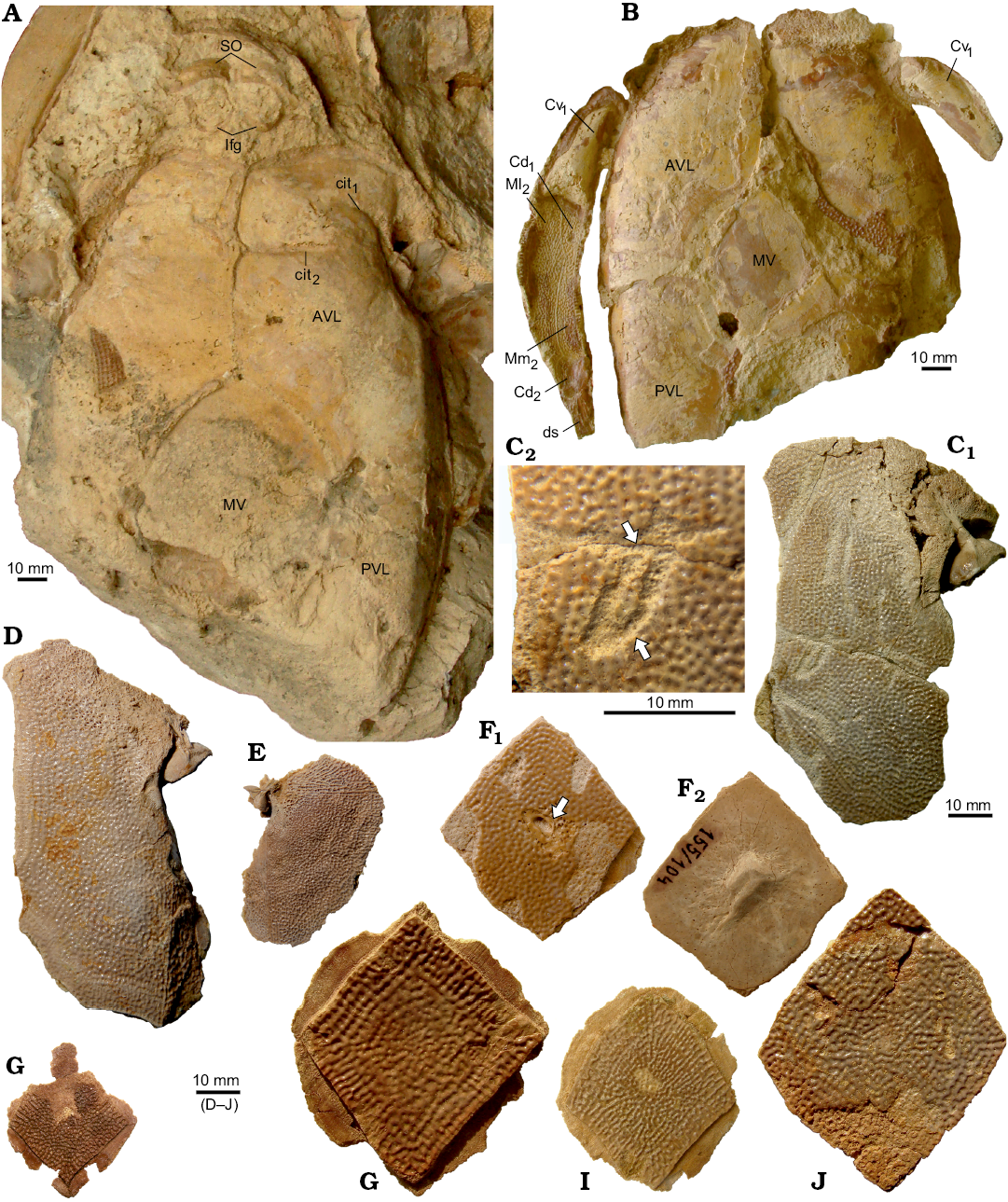
Fig. 11. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, partial ventral wall (A, B), anterior ventro-lateral (AVL) (C, D, left; E, right), and median ventral (F–J) plates of the trunk armour, and elements of pectoral fin. A. Impression of partial ventral wall of the trunk armour, inferognathals, and suborbitals IG KSC 155/10-2 in ventral view. B. Partial ventral wall of the trunk armour with articulated pectoral fin armour IG KSC 155/10-1 in ventral view. C. IG KSC 155/151 (C1) and enlargened part of the ventral lamina with pathologies (C2, arrows). D. IG KSC 155/122 in ventral view. E. IG KSC 155/123 in ventral view. F. IG KSC 155/104 with the bite mark (arrow) in ventral (F1) and visceral (F2) views. G. IG KSC 155/76 in ventral view. H. IG KSC 155/77 in ventral view. I. IG KSC 155/133 in ventral view. J. IG KSC 155/106 in ventral view. Abbreviations: AVL, anterior ventro-lateral plate; Cd1–2, dorsal central plates 1 and 2; Cv1, ventral central plate 1; cit1, crista transversalis interna anterior; cit2, transverse thickening on the visceral surface of AVL; ds, distal segment of the pectoral fin armour; Ifg, inferognathal; Ml2, lateral marginal plate 2; Mm2, mesial marginal plate 2; MV, median ventral plate; PVL, posterior ventro-lateral plate; SO, suborbital plate.
The posterior ventro-lateral plate (PVL) has variable proportions (Fig. 12). It is relatively narrow with a ventral lamina that is 2.1–2.7 times as longer than it is broad. The subanal division is rather narrow and relatively longer than in the Latvian material where it occupies about one third (28–38%) of the total PVL plate length. This is comparable with the 33% in B. leptocheira leptocheira (Miles 1968: 80), but slightly different from B. leptocheira curonica (Luksevics 2001: figs. 38B, C, 39C) where it varies between 22% and 32%. The lateral lamina is moderately high in IG KSC 155/4 (Fig. 12A). The ventro-lateral ridge (vlr) is clearly defined both in the AVL and PVL. The left PVL overlaps the right PVL plate.
The median ventral plate (MV; Fig. 11F–K) is not known in the material from Scotland (Miles 1968: 80) and Latvia (Lukševičs 2001: 552), whereas it is well represented in the Sosnogorsk material by several disarticulated specimens and also found in partially articulated ventral walls of the trunk armour. This plate is slightly elongated with an L/W index of about 1.2 on average. The shape of AVL and PVL plates in B. leptocheira curonica (Lukševičs 2001: 552) suggests a moderately small size of the MV; whereas in B. leptocheira jeremejevi, it is rather large (Fig. 11A, F–K). Almost all MV plates are from relatively large individuals, the plate reaching 30 to 61 mm in length. Some plates are asymmetrical in shape, e.g., IG KSC 155/77 (Fig. 11H) and 155/106 (Fig. 11K). Usually all margins of the plate are slightly convex or almost straight, but in IG KSC 155/133 both posterior lateral margins are concave (Fig. 11J). Overlap areas usually are rather narrow (Fig. 11H, J).
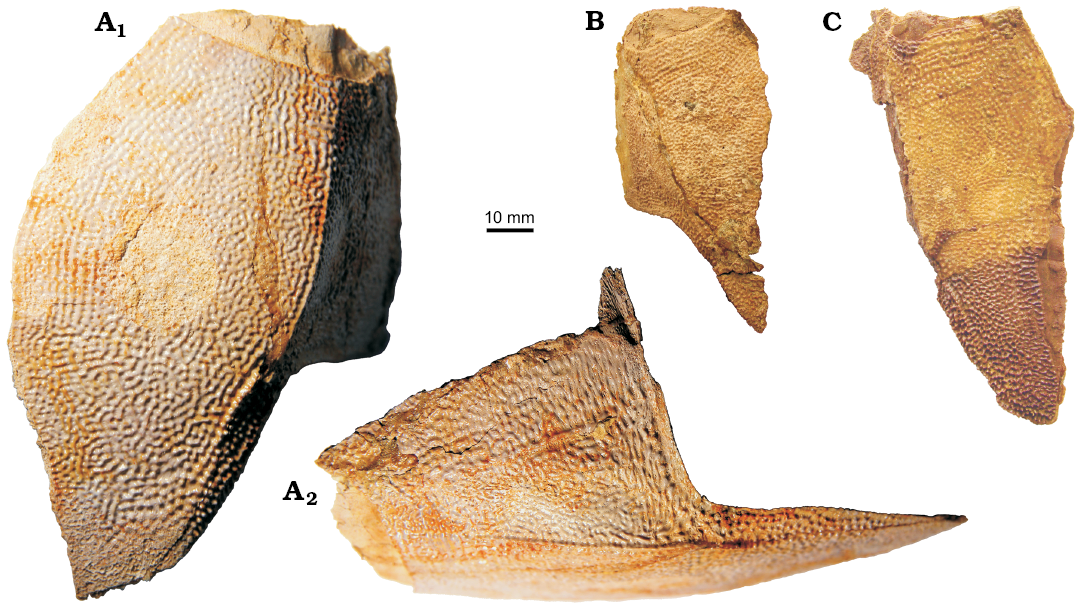
Fig. 12. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, posterior ventro-lateral plates, left (A) and right (B, C) of the trunk armour. A. IG KSC 155/4 in ventral (A1) and lateral (A2) views. B. IG KSC 155/16 in ventral view. C. IG KSC 155/78 in ventral view.
The pectoral fin is represented in the Sosnogorsk material by several disarticulated bones, at least three specimens showing articulated plates of the proximal segment associated with the AVL (e.g., Fig. 11B), and one articulated distal segment (Fig. 13E). The proximal segment bears small rarely set lateral and mesial spines. The lateral spines are larger than the mesial ones (which are even not seen in figured specimens) as in B. leptocheira curonica (Lukševičs 2001: figs. 38A, 39B); Miles (1968) suggested the mesial spines were perhaps absent in B. leptocheira leptocheira. The proximal segment is very long and narrow, it is 4.6 times longer than it is broad in IG KSC 155/10. It is slightly less elongated than in the Scottish material (5.5 to 6 times as long as it is broad; Miles 1968: 80) and in the Latvian material (4.8–6 [5.5 on the average] times as long as it is broad; Lukševičs 2001: 553). The Cd1 has an L/B index varying from 2.6 to 2.9 (2.7–3.7, 3.3 on average in the Latvian material; Lukševičs 2001: 553); measurements were made during the field work in 2009. The Cv1 is gently longer than the Cd1 and slightly more elongated with an L/B index of 3.5–4.1 (3.9 on the average) (Fig. 13A, B). The Cd2 is slightly, about 1.2 times, longer than it is broad in IG KSC 155/10. Similarly to that of B. leptocheira curonica, the distal segment (Fig. 13E) is adorned with small and rounded marginal spines; the lateral spines are large, sharp and proximally directed. The distal segment is very long and narrow, with an L/B index of 5.4 in both IG KSC 155/21 and IG KSC 155/22 (L/B index of 5.7 in LDM 89/4). The Cd5 plate is present in the material from all three countries.
The ornamentation is fine and basically of the reticular type (Miles 1968: 80) in small individuals, changing into vermiculating type in the medium-sized fish, and even in the tubercular type on the largest specimens (e.g., Prm; IG 155/114), instead of becoming smooth in quite large individuals from Latvia. The network of anastomosing ridges is usually broken into radially arranged shorter ridges on the head shield plates. On the posterior margin of the PMD, on the subcephalic and subanal divisions of the AVL and PVL, the anastomoses between the ridges reduce, and nodose short ridges are present. The ornamentation on MV depends on the size of the plate: it is of reticular type in the smallest plates and becomes of the vermiculating type, i.e., short radiating nodose ridges, along the margins of the largest plates, whereas it remains of the reticular type in the central part. The ornamentation of the pectoral appendage is reticulate in general. On the anterior part of the Cd1 and Cv1 the ornament is radially arranged, whereas on the distal part of the proximal segment the ornamentation becomes smooth. The longitudinal striation in the ornament of the dorsal side of the distal segment is well shown in IG KSC 155/21 (Fig. 13E1).
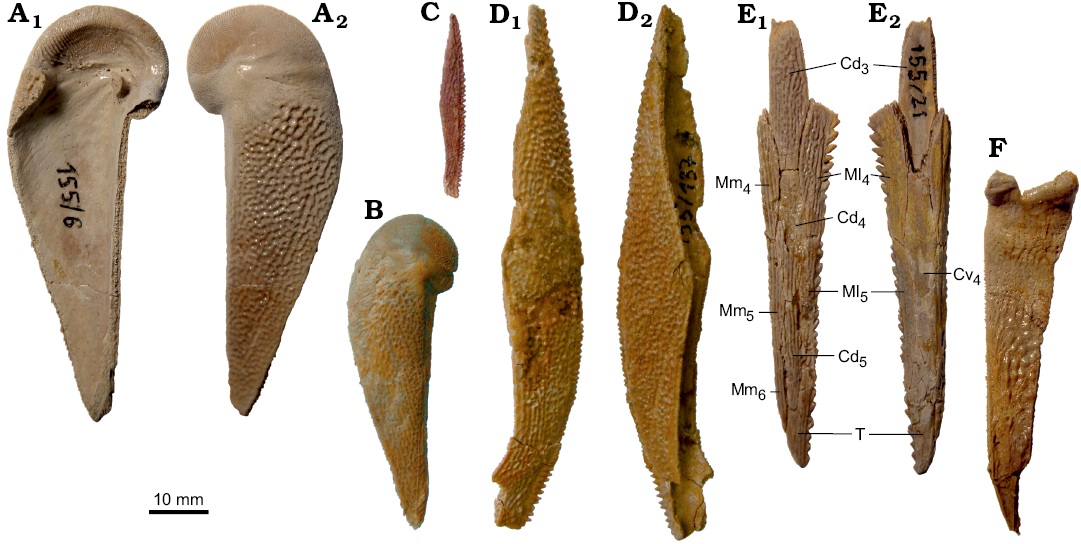
Fig. 13. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), Sosnogorsk locality, Sosnogorsk Formation, lowermost Famennian, plates of the pectoral fin armour. A. Left Cv1 plate IG 155/6 in visceral (A1) and ventral (A2) views. B. Right Cv1 plate IG KSC 155/134 in ventral view. C. Left Ml2 plate IG KSC 155/149 in ventral view. D. Right Ml2 plate IG 155/137 in dorsal (D1) and ventral (D2) views. E. Right distal segment of the pectoral fin armour IG KSC 155/21 in dorsal (E1) and ventral (E2) views. F. Mm1 plate IG KSC 155/85 in mesial view. Abbreviations: Cd3–5, dorsal central plates 3 to 5; Cv4, ventral central plate 4; Ml4–5, lateral marginal plates 4 and 5; Mm4–6, mesial marginal plates 4 to 6; T, terminal plate.
Remarks.—This species was established by Traquair (1893) based on the material from Scotland and well-described by Stensiö (1948) and Miles (1968). Gross (1942) erected Bothriolepis curonica as a separate species from the lowermost Famennian of Latvia based on a small set of bones collected in 1934 in the Bienes locality. This description was repeated by Stensiö (1948) in Addenda to his monograph without further comments. Since then, the collection of the remains of this fish was significantly added by Vitaliy Sorokin and Lyubov Lyarskaya in 1975–1982 and by EL in 2013, who collected specimens from the Kalnamuiža locality in western Latvia. Specimens described by Gross (1942) and material collected from the Kalnamuiža site in 1970–80ies were briefly described and figured (Lukševičs 1987), accenting the close morphological resemblance of B. curonica with B. leptocheira. A direct comparison of the specimens belonging to these species has indicated that they are conspecific (Lukševičs 2001). Nevertheless, several features supported designation of the Latvian material to the subspecies Bothriolepis leptocheira curonica Gross, 1942, which is morphologically very close to Bothriolepis leptocheira leptocheira from Scotland (Miles 1968).
B. leptocheira jeremejevi (Rohon, 1900) differs from the nominal subspecies B. leptocheira leptocheira from Scotland in its: (i) larger size; (ii) slightly narrower Pn plate; (iii) more acute posterior angle of PMD plate; (iv) slightly shorter ADL. It differs from B. l. curonica in its: (i) slightly narrower Prm and Pn plates, shorter and broader Pmg plate; (ii) slightly narrower AMD plate; (iii) relatively higher lateral wall of MxL and AVL plates; (iv) relatively shorter pectoral fin armour and separate plates of the fin; (v) coarser ornamentation. Overall proportions of the plates of the trunk armour and of the bones of the pectoral fin, distribution and path of sensory line canals as well as ornamentation of B. leptocheira jeremejevi are rather similar to those of B. leptocheira from Scotland and from the earliest Famennian of Latvia. The dissimilarities between the three subspecies mainly concern ornamentation, slightly differing proportions of some plates, relatively shorter but higher trunk armour, and shorter pectoral fins of B. leptocheira jeremejevi in comparison with B. leptocheira curonica.
Stratigraphic and geographic range.—Known only from the type locality and horizon.
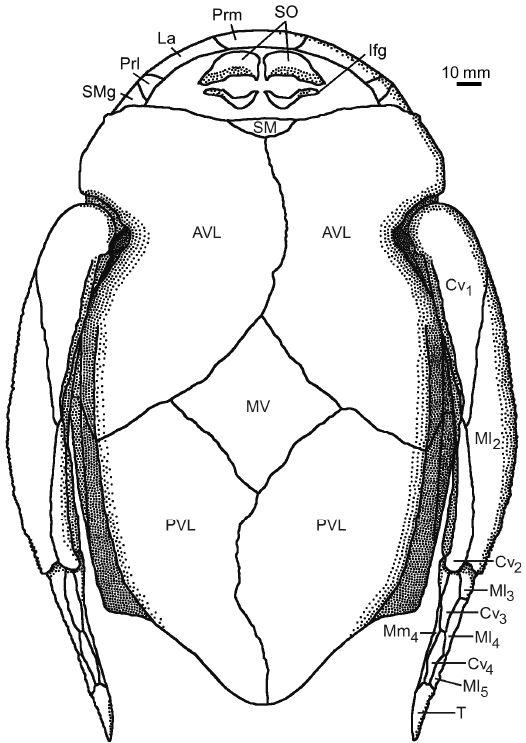 |
Fig. 14. Antiarchan fish Bothriolepis leptocheira jeremejevi (Rohon, 1900), reconstruction of the head, the trunk armour and the pectoral fins in ventral view, based on IG KSC 155/10-1, IG KSC 155/10-2, and IG KSC 155/10-3. Abbreviations: AVL, anterior ventro-lateral plate; Cv1–4, ventral central plate 1 to 4; Ifg, inferognathal plate; La, lateral plate; Ml2–5, lateral marginal plates 2 to 5; Mm4, mesial marginal plate 4; MV, median ventral plate; Prl, prelateral plate; Prm, premedian plate; PVL, posterior ventro-lateral plate; SM, semilunar plate; SMg, submarginal plate; T, terminal plate. |
Discussion
Systematics.—Within Bothriolepis species from Scotland, Baltic, and Russia, Bothriolepis leptocheira is particularly well diagnosed by a very short orbital margin of Prm, long and narrow AMD, and slender pectoral appendage (Miles 1968; Lukševičs 2001). Among the other species of Bothriolepis, B. leptocheira is morphologically closest to B. sosnensis Moloshnikov, 2003 from the Zadonsk Formation of Central Russia (Moloshnikov 2003, 2008), and B. jarviki Stensiö, 1948 from Greenland (Stensiö 1948). B. sosnensis rather closely resembles B. leptocheira by (i) shape and proportions of Nu plate, with very short anterior margins contacting the La plate; (ii) presence of supraoccipital cross-commissural pit-line groove (socc) on the Nu plate; (iii) shape and proportions of AMD and PMD plates; (iv) presence of a distinct dorso-lateral ridge on the ADL and MxL plates; (v) the so-called Remigolepis-type of overlapping of the AMD and MxL; (vi) basically reticular type of ornamentation. B. sosnensis differs from B. leptocheira by (i) significantly smaller size; (ii) the position of the infraorbital sensory groove (ifc1) rather far from the lateral and anterior margin of the La plate; (iii) presence of two superficial fossae (sop) in the centre of the supraotic thickening on the visceral surface of Nu plate; (iv) shape of the anterior margin of the dorsal lamina of ADL plate. Most probably, the PMD plate of B. sosnensis is shorter than that restored by Moloshnikov (2008: fig. 30g). Differences between B. leptocheira and B. sosnensis are insignificant, resembling differences between B. leptocheira leptocheira, B. leptocheira curonica, and B. leptocheira jeremejevi, thus most probably correspond to the subspecies level. Unfortunately, B. sosnensis is represented by a limited number of specimens, mostly fragmented and with heavily rounded edges, therefore, despite that it is impossible to provide specific diagnosis of B. sosnensis differing from the diagnosis of B. leptocheira, it is also difficult to make more detailed comparison and decide on the conspecific status of these two taxa.
B. jarviki Stensiö, 1948 from Greenland (Stensiö 1948) also closely resembles B. leptocheira. Both species are similar in (i) their size; (ii) shape and proportions of La and Nu plates of the head shield; (iii) the shape and proportions of the AMD; (iv) slender pectoral fin. However, B. jarviki differs from B. leptocheira in the shape and proportions of Prm, Pp, Nu, PMD, and the ornamentation.
Moloshnikov (2008) described the Prm and PMD plates of B. cf. leptocheira from the same locality in Central Russia where B. sosnensis comes from. B. cf. leptocheira from Central Russia differs from all subspecies of B. leptocheira by the proportions of the Prm, e.g., longer posterior margin and differing shape of lateral margins, the position of the infraorbital sensory groove (ifc1) in a larger distance from the anterior margin of the Prm plate, extremely elongated and narrow PMD plate with a rather pronounced posterior angle and tuberculate-ridged ornamentation (Moloshnikov 2008: text-fig. 33, pl. 3: 4, 5). Most probably, specimens figured by Moloshnikov (2008) do not belong to B. leptocheira.
Variability.—The extensive collection of Bothriolepis leptocheira jeremejevi provides possibilities to analyse the age and other variations of skeletal elements. It is impossible to measure the exact body size of individual fish, although it is possible to evaluate the approximate length of fish using the measurements of individual bones and knowing their proportional length in the complete body (e.g., known in Bothriolepis canadensis; Béchard et al. 2014). Judging from the size of individual skeletal elements, the smallest fish in the collection is represented by PMD IG KSC 155/148, which reaches only 19 mm; using the proportions of PMD and full body in B. canadensis (Béchard et al. 2014: fig. 1) for the evaluation of the body size of this small fish one might evaluate its length between 15 and 170 mm. The largest PMD IG KSC 155/142 is 86.4 mm long; thus, 4.5 times bigger than the smallest one; hence this presumably adult fish could reach 700–800 mm in length. Concerning the other skeletal elements, the difference between the largest and smallest specimens is even lesser (Table 1). Juvenile specimens described for Bothriolepis askini Young, 1988 demonstrate a much greater difference between the smallest and largest specimens, e.g., AMD CPC 25997 (Young 1988: fig. 10B) is about 59 mm long, whereas AMD CPC 26181 is under 5 mm in length (Young 1988: 43), thus, the largest AMD (presumably from an adult individual) exceeds the smallest (from a juvenile individual) almost 12 times. Besides, the morphology of juvenile Bothriolepis differs in many respects from adults (Stensiö 1948; Werdelin and Long 1986; Downs et al. 2011). Taking into consideration that the differences between the smallest and largest specimens of B. leptocheira jeremejevi do not reach differences characteristic for comparison of juvenile and well-grown specimens in other species, the anterior oblique sensory line groove (dlg1) which is typical for juvenile individuals is not represented on the AMD, the median dorsal ridge (dmr) is weakly defined, and also based on the three-layered histological structure of bones well seen along the broken edges, the conclusion may be drawn that all studied specimens of B. leptocheira jeremejevi belong to subadult or adult fish individuals. As it was demonstrated for Bothriolepis sp. from the Catskill Formation, Pennsylvania (Downs et al. 2011) and Asterolepis ornata from the Lode Formation, Lode clay pit, Latvia (Kuršs et al. 1999), these antiarchan fishes most probably utilized common nursing ground. It is possible, that juvenile specimens are absent in the material of B. leptocheira jeremejevi due to the poor preservation potential of tiny bones or due to dissolution of fine ossification during the acid preparation, but the most probable hypothesis is that the nursing place of these antiarchan fishes was located somewhere else. However, several features differ well in the smallest and largest specimens, e.g. the anterior margin of the PMD is rather pointed in the smallest specimens (IG KSC 155/70, IG KSC 155/71), and less convex in larger individuals, proportions of Pp and Pn also change: Pp becomes narrower and Pn becomes wider with age. Variation of the shape and proportions of the trunk armour plates, e.g., of the AMD (Fig. 9) also could partially be explained by the changes with age: external postlevator process and postnuchal notch are more pronounced in larger specimens (Fig. 9A, G) than in smaller ones (Fig. 9B, E). The shape of the AMD plate depends also on the type of sutural connections with the ADL and MxL plates. Unusual sutural connections of AMD with ADL and MxL plates are demonstrated as a rather typical for the large specimens of various Bothriolepis species, e.g., B. canadensis (Stensiö 1948), B. ornata and B. ciecere (Lukševičs 2001); most probably, unusual shape and proportions of the AMD IG KSC 155/44 (Fig. 9G) can be explained mainly by a very atypical sutural connections with the ADL.
Several specimens of B. leptocheira jeremejevi such as Pp IG KSC 155/146 (Fig. 6B), AMD IG KSC 155/140 (Fig. 9E), and PMD IG KSC 155/73 clearly show subtle asymmetry. Other kind of variations concerns the path of the sensory line canals: the dlg2 on PMD IG KSC 155/7 (Fig. 9H); the dlg2 on MxL IG KSC 155/127 is slightly displaced and situated more caudally than usually; La plate IG KSC 155/15 (Figs. 4A, 5A) shows an abnormal curvature of ifc1 and cir. Sum of small, random deviations from symmetry of bilaterally symmetrical pairs of attributes within the populations usually have been characterised as fluctuating asymmetry (Palmer 1994). It was postulated that very distinctive fluctuating asymmetry (FA) of various morphological traits appears both in the conditions of very close relative breeding, or more harsh ecological conditions due to environmental stress (e.g., Palmer and Strobeck 2003). FA as a measure of developmental stability of populations/species is widely used in the studies of modern animals and plants (see e.g., Palmer and Strobeck 2003 for an overview; the number of publications exploded during the last decade). Evidences of both morphological and behavioural asymmetries are well represented in the fossil record starting even from the Archaean (Babcock 2005). There are not many works concerning asymmetry in fossil fishes (Cloutier 2010); one of the rare examples of quantification of FA in fossil fishes is the Devonian dipnoan Scaumenacia curta from Miguasha (Cloutier 1997). Subtle asymmetries have also been reported for some Devonian placoderm fishes: arthrodires (Trinajstic and Dennis-Bryan 2009) and antiarchs (Graham-Smith 1978; Lukševičs 2001), but FA was not quantified in these papers. The high proportion of asymmetrical skull of fishes belonging to the placoderm families Incisoscutidae and Camuropiscidae from the Gogo Formation in Western Australia had been interpreted as a response to stressful environmental factors (anoxia) during the development of these species (Trinajstic and Dennis-Bryan 2009). However, since a “normal” level of asymmetry in placoderms is unknown, it is almost impossible to correctly interpret the relationships between the developmental stability, degree of asymmetry and environmental stress (see also Cloutier 2010).
Estimation of the variability of several morphological features in eight species of Bothriolepis from the East European platform, represented by collections large enough for statistical analysis was attempted using Cherepanov’s method (Cherepanov 1986; see Lukševičs 1995 for methodology applied for the fossil fishes). The statistical analysis revealed that the variability of Bothriolepis leptocheira jeremejevi only slightly exceeds the variability of B. leptocheira curonica and the mean variability of all analysed species (d16 for all species reaches 8.61; for variability of separate taxa see Table 2). The analysis shows that four species demonstrate a larger variability than B. leptocheira jeremejevi, Frasnian species B. cellulosa and B. evaldi, and Famennian B. ornata and B. jani. Therefore the observations of subtle asymmetries and estimation of variability do not contradict each other but there are not enough data to make any conclusions on the impact of environmental conditions on the morphological stability of B. leptocheira jeremejevi. Further studies of fluctuating asymmetry, as well as of the variability of various bothriolepid fishes, are needed.
Table 1. Length (in mm) of the smallest and largest specimens of several plates of Bothriolepis leptocheira jeremejevi.
|
Plate |
Length of the specimen |
Index of the length of the largest/smallest specimen |
|
|
largest |
smallest |
||
|
ADL |
82.4 |
27.0 |
3.1 |
|
AMD |
109.4 |
29.2 |
3.8 |
|
MxL |
108.7 |
25.0 |
4.3 |
|
PMD |
86.4 |
19.0 |
4.5 |
|
Cv1 |
80.4 |
50.1 |
1.6 |
|
Ml2 |
94.7 |
31.6 |
3.0 |
Palaeoecology.—Many specimens from Sosnogorsk bear marks most probably produced by parasitic organisms, and some demonstrate healed scars made by predators (AMD IG KSC 155/5; Fig. 9A and MV IG KSC 155/104; Fig. 11F). AMD IG KSC 155/5 shows two pits in the outer surface, one about 7.5 mm and the other 4.8 mm in diameter. The shape of both pits resembles the cross-section of porolepiform Holoptychius tooth with two cutting edges. A rather large blister, about 12 mm in diameter, on the visceral surface under the largest bite mark confirms this interpretation. The anterior ventral funnel pit of the AMD is asymmetrical, slightly deviated to the right, further from the scar. IG KSC 155/104 demonstrates a well expressed bite mark on the outer surface and a corresponding blister on the visceral surface of the MV plate (Fig. 11F). This plate lost its normal ornamentation in a 10.8 × 6.8 mm2 large, oval area of the outer surface, possibly due to wound inflammation; the pit in the middle of this unornamented area is 5.3 × 2.7 mm2 large and most probably corresponds to the scar made by a predator tooth. Judging from the inclination of the pit walls and the position of the 15 × 9 mm2 large blister on the visceral surface, the tooth penetrated the MV plate under 50–60° angle to the ventral wall. Still, this scar was successfully healed. At least three of the nine MV plates bear damages on the outer surface, most probably done by scavengers or, less probably, during subaerial exposition of the fossil bearing sediments.
Some specimens have also a rather badly damaged surface of the bone, most probably caused by some trace maker organisms like crayfish or other arthropods. Some specimens were rather densely populated by possible parasites, e.g., PMD, IG KSC 155/1 (Fig. 9I) shows at least three fixation places of parasitic animals, two of them in the typical shape and size of “round fossulae” (Lukševičs et al. 2009), and a slightly larger one. Other infested specimens are: AMD, IG KSC 155/44; AVL, IG KSC 155/143, IG KSC 155/151, IG KSC 155/165; PVL, IG KSC 155/2; MV, IG KSC 155/104, IG KSC 155/106, IG KSC 155/153. Most of them are ventral plates of the trunk armour of well-grown individuals and show another type of possible parasite traces. AVL IG KSC 155/151 bears several elongated vermiculating, in places discontinuous or crossed, ca. 1.5–2 mm wide tracks on the external surface (Fig. 11C). The damaged bone tissue looks regenerated bearing pits and ridges characteristic for the network-type ornamentation typical for Bothriolepis, but with much smaller size of pits, justifying the track is not a post-mortem lesion. It is difficult to interpret such type of damage; this type of paleopathology might be the result of the attack of hypodermal mobile parasite like modern flatworms, or most probably the result of fungal infections, which in modern fishes are considered secondary to some other pathogens such as trauma, bacterial infection or parasites (Yanong 2003).
Paleobiogeography, taphonomy, and sedimentary environment.—Miles (1968) reported only Bothriolepis leptocheira and Holoptychius sp. from Bracken Bay, Roxburghshire, South of Scotland. Stratigraphic position of Bracken Bay Beds is not well established.
Lukševičs and Stinkulis (2015) provided the most recent list of vertebrate remains from the Eleja Formation of Latvia containing B. leptocheira, Holoptychius sp., medium-sized undetermined dipnoan and two acanthodian taxa represented by spines and Acanthodes-type scales. Panderichthys sp. from the Eleja Formation mentioned by Esin et al. (2000) has most probably been reported by Lyarskaya (in Lyarskaya and Lukševičs 1992) erroneously. Miospore assemblages from the Frasnian–Famennian boundary beds of Latvia, and Lithuania, studied in detail by various researchers (Žeiba and Savvaitova 1981), demonstrate the position of this boundary close to the boundary between the Amula Formation below and Eleja Formation (Šiauliai Formation in Lithuania) above. Facies analysis supports this position of the boundary (Lukševičs and Stinkulis 2015).
B. leptocheira ssp. from the Severnaya Zemlya Archipelago is the only fish species reported from sandstone rocks of the Malyutka Formation (Lukševičs 1999a, b). The stratigraphic position of the Malyutka Formation within the lowermost Famennian is based only on the distribution of B. leptocheira.
The composition of vertebrate assemblages of Bracken Bay, Eleja and Malyutka formations is very restricted, consisting of a small number of geographically very widely distributed taxa, possibly mirroring the biotic crisis known as the Kellwasser event (Sandberg et al. 1988; Carmichael 2014) close to the Frasnian–Famennian boundary in Scotland, Latvia, and the Severnaya Zemlya Archipelago. However, the Sosnogorsk assemblage is more diverse, containing B. leptocheira (B. jeremejevi in Beznosov et al. 2011, 2012), porolepiforms Holoptychius sp. and Duffichthys sp., lungfishes cf. Jarvikia and Rhinodipteridae gen. et sp. indet. (= cf. Andreyevichthys in Beznosov et al. 2011), and one of the most primitive Devonian, not yet described, tetrapod. This assemblage is restricted to the upper part of the Sosnogorsk Formation corresponding to the lowermost Famennian (Beznosov et al. 2011).
The bed No. 40 yields abundant charophyte algae Trochiliscus and Sycidium. As it was demonstrated by various authors, the extinct Silurian, Devonian, and Carboniferous charophyte algae Trochiliscus and related forms were usually associated with brackish or fresh water basins (e.g., Hecker 1983); however, Karpinskya oscolensis (Trochiliscaceae) has been recorded by Racki and Racka (1981) from the open marine settings of the Holy Cross Mountains. Uneven distribution, moderately high fragmentation, reorientation, sorting, and a small degree of corrosion and abrasion of vertebrate fossils, as well as a very rare articulated specimens of B. leptocheira and Holoptychius sp., that are exposed in life position, indicate a relatively calm hydrodynamic regime in very shallow water; complete drying of the basin also can’t be excluded. Modern Thalassinoides-like burrows are made by a variety of marine organisms, most usually decapods crustaceans such as thalassinid shrimps in intertidal and shallow subtidal environments; similar traces are also rather common in the Palaeozoic deposits (Myrow 1995).
The facies analysis indicates intertidal and shallow subtidal environment, and a taphonomic study suggests the accumulation of vertebrate remains under conditions of moderate turbulence, rather high or periodically high rates of carbonate sedimentation, and dysoxic environment, most probably associated with the high amount of rotting charophyte algae. Vertebrate remains from tempestites were most likely rewashed in storm events. Taken together with the presence of a late Frasnian barrier reef to the east of this site, which dried out before the earliest Famennian due to a sea regression (House et al. 2000), and overall similarity with the coeval assemblages from very distant areas such as Scotland, Latvia, the Severnaya Zemlya Archipelago, and probably Central Russia, the upper part of the Sosnogorsk Formation most probably represents a wide, shallow lagoon with changeable salinity in a back-reef zone. The carbon and oxygen isotope composition of the deposits as well as the distribution of strontium in the section also indicate changeable environment of a brackish to hypersaline basin (Maydl and Beznosov 2011) for the whole Sosnogorsk Formation. However, the “fish-dolomite” bed corresponds to the time of strongest connections of the Sosnogorsk lagoon to the open sea. Most probably fishes of the Sosnogorsk assemblage entered this mostly restricted lagoon during short transgressions and/or storm events. The connection to the open sea was soon reduced and then interrupted; as a result karst features developed on the top of the bed No. 40. Later massive dolomite bed No. 41 developed possibly reflecting a short period of higher temperature and enhanced evaporation; the top of the dolomite bed bears traces of erosion. An abundance of charophyte algae, subtle asymmetry of bones, and moderately high variability of Bothriolepis leptocheira probably could indicate a rather high environmental stress in the Sosnogorsk lagoon. However, diminished biodiversity and signs of ecological stress are characteristic for many fossil localities close to the Frasnian–Famennian boundary (Lukševičs and Stinkulis 2015; Marshall et al. 2011), indicating mass extinction of various marine organisms (e.g., Copper 2002) widely known as the Kellwasser Event (House 1985). Nevertheless, a relatively short distance of the Sosnogorsk lagoon to the open deep sea (nearly 40 km, according to Kuzmin et al. 1998) provided more favourable conditions for the still restricted number of survivors after the Frasnian–Famennian extinction, in comparison with the environments characterised by strongly limited assemblages from other coeval localities, e.g., Kalnamuiža site in Latvia (Lukševičs and Stinkulis 2015). The very wide geographical distribution of Bothriolepis leptocheira, represented by several local subspecies slightly differing in morphology but showing wide individual variations, most probably reveals the survival of fauna after the Kellwasser extinction event.
Conclusions
As the result of the detailed morphological studies of the abundant fossil material from the type locality of the Sosnogorsk Formation situated on the right bank of the river Izhma, South Timan, Komi Republic, Russia, the species B. jeremejevi Rohon, 1900 from the earliest Famennian, Late Devonian has been downranked to subspecific lavel. This antiarchan fish closely resembles B. leptocheira from the earliest Famennian or latest Frasnian of Scotland, Latvia, and Severnaya Zemlya differing only slightly in proportions of some bones. It resembles Bothriolepis sosnensis Moloshnikov, 2003 from the Zadonsk Formation of Central Russia by many features, mostly differing due to a significantly greater size of B. leptocheira, the position of the infraorbital sensory groove rather far from the lateral and anterior margin of the La plate, the presence of two superficial fossae in the centre of the supraotic thickening on visceral surface of the Nu plate, and shape of the anterior portion of the ADL plate. Most probably, specimens described and figured by Moloshnikov (2008) as B. cf. leptocheira from the Zadonsk Formation of Central Russia do not belong to B. leptocheira. The composition of vertebrate assemblages from Latvia, Scotland, and especially Severnaya Zemlya, containing B. leptocheira demonstrates the reduced diversity with one antiarch placoderm species, one acanthodian fish (from Latvia) and one (Scotland) or two sarcopterygian fishes (Latvia); however, the Sosnogorsk assemblage differs in a larger diversity containing very primitive tetrapod and diversified sarcopterygian fishes including dipnoans and two taxa of porolepiforms. Morphological studies show that all examined specimens of Bothriolepis leptocheira jeremejevi belong to subadult or adult individuals. Fossil remains of B. leptocheira jeremejevi demonstrate distinct subtle asymmetry, increased variability in the proportions and shape of individual skeletal elements and variations in the course of sensory line canals; many specimens show marks most probably produced by parasitic organisms, some demonstrate healed scars made by predators; however, since a “normal” level of asymmetry or infestation with parasites in this placoderm fish is unknown, it is almost impossible to correctly interpret the relationships between the developmental stability, degree of asymmetry, activities of parasites and environmental stress. Despite the distinct subtle asymmetry and wide variations of sensory canals, B. leptocheira shows moderate variability of selected morphometric features in comparison with other seven bothriolepidid species from the East European platform, which are represented by abundant material allowing the usage of statistical methods. Judging from the facies analysis, distribution of fossil remains and other taphonomic features, trace fossils, isotopic geochemical analyses of carbon, oxygen, and strontium, the upper part of the Sosnogorsk Formation most probably represents deposits originated in a wide shallow lagoon with changeable salinity in a back-reef zone. Wide geographical distribution of a single species of Bothriolepis and usually diminished diversity of other vertebrates suggest that the earliest Famennian vertebrate assemblages conforms to the survival faunas of the end Frasnian–earliest Famennian biotic crisis after the Kellwasser extinction event.
Acknowledgements
We are grateful to anonymous reviewer and especially to Sébastien Olive (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) for many comments and advices which allowed significantly improve the text; to Aija Zāns (Bangor University, Bangor, UK) for linguistic support; Anatoliy Plyakin (Ukhta University, Ukhta, Russia) and Vyacheslav Kamashev (Sosnogorsk, Russia) who provided well preserved specimens; Radek Mikuláš (Institute of Geology, Academy of Sciences of the Czech Republic, Praha, Czech Republic), Artemiy Anfimiv (Institute of Geology and Geochemistry, Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia) helped in identification of the trace fossils and charophytes. The authors thank everyone who has provided field assistance on the Sosnogorsk expeditions: Per E. Ahlberg (Uppsala University, Uppsala, Sweden), Jānis Lukševičs (University of Latvia, Riga, Latvia), Yuriy Gatovsky, Denis and Roman Khipeli, Oleg Gamolyuk, Lilia Kamaletdinova, Tatiana Rastvorova (all Institute of Geology, Komi Science Centre, Ural Branch of the Russian Academy of Sciences, Syktyvkar, Russia), very friendly local people and more than a dozen of diggers, who provided a long-term excavation during 2009–2012. This research was supported by the Uppsala University (we are grateful to Per E. Ahlberg for suggesting this support), the Committee for Research and Exploration of the National Geographic Society Grant No. 9099-12 (PB and EL); UNDP/GEF Project No. 00059042 (PB), Presidium of Ural Branch of RAS Project No. 15-18-5-37 (PB); Russian Academy of Sciences (EL); Latvian Science Council Grant No. 09.1032 and University of Latvia Grant “Sustainable use of nature resources in the context of climate changes” No. AAP2016/B041 (EL).
References
Babcock, L.E. 2005. Asymmetry in the fossil record. European Review 13 (S2): 135–143. Crossref
Béchard, I., Arsenault, F., Cloutier, R., and Kerr, J. 2014. The Devonian placoderm fish Bothriolepis canadensis revisited with three-dimensional digital imagery. Palaeontologia Electronica 17 (1, 2A): 1–19.
Beznosov, P.A. 2009. Sosnogorsk Formation—a new local stratigraphic unit of the Upper Devonian from South Timan [in Russian]. In: Geologiâ i mineral’nye resursy evropejskogo severo-vostoka Rossii: Materialy XV Geologičeskogo s’jezda Respubliki Komi. Vol. 2, 9–12. Geoprint, Syktyvkar.
Beznosov, P., Lukševičs, E., and Ahlberg, P.E. 2011. A unique vertebrate community from the Sosnogorsk Formation (Lower Famennian, South Timan). In: O. Lebedev and A. Ivanov (eds.), II International Obruchev Symposium “Palaeozoic Early Vertebrates”. St. Petersburg–Luga, August 1–6, 2011. Abstracts, 27. St. Petersburg State University, St. Petersburg.
Beznosov, P.A., Lukševičs, E.V., and Ahlberg, P.E. 2012. Diversity, habitat conditions and taphonomy of the Famennian vertebrates of Timan) [in Russian]. In: Geologiâ i mineral’nye resursy evropejskogo severo-vostoka Rossii: Materialy XV Geologičeskogo s’jezda Respubliki Komi. Vol. 2, 146–149. Geoprint, Syktyvkar.
Carmichael, S.K., Waters, J.A., Suttner, T.J., Kido, E., and DeReuil, A.A. 2014. A new model for the Kellwasser Anoxia Events (Late Devonian): Shallow water anoxia in an open oceanic setting in the Central Asian Orogenic Belt. Palaeogeography, Palaeoclimatology, Palaeoecology 399: 394–403. Crossref
Cherepanov, V. [Čerepanov, V. ] 1986. Evolûcionnaâ izmenčivost’ vodnyh i nazemnyh životnyh. 239 pp. Nauka, Novosibirsk.
Cloutier, R. 1997. Morphologie et variations du toit crânien du dipneuste Scaumenacia curta (Whiteaves) (Sarcopterygii), du Dévonien supérieur du Québec. Geodiversitas 19: 59–105.
Cloutier, R. 2010. The fossil record of fish ontogenies: Insights into developmental patterns and processes. Seminars in Cell & Developmental Biology 21: 400–413. Crossref
Cope, E.D. 1886. An interesting connecting genus of Chordata. The American Naturalist 20: 1027–1031. Crossref
Copper, P. 2002. Reef development at the Frasnian/Famennian mass extinction boundary. Palaeogeography, Palaeoclimatology, Palaeoecology 181: 27–65. Crossref
Downs, J.P., Criswell, K.E., and Daeschler, E.B. 2011. Mass mortality of juvenile antiarchs (Bothriolepis sp.) from the Catskill Formation (Upper Devonian, Famennian Stage), Tioga County, Pennsylvania. Proceedings of the Academy of Natural Sciences of Philadelphia 161: 191–203. Crossref
Eichwald, E. 1840. Die Tier- und Pflanzenreste des alten roten Sandsteins und Bergkalks im Nowgorodschen Gouvernement. Bulletin de l’Académie impériale des sciences de St.-Pétersbourg 7 (N6, 7): 78–91.
Esin, D., Ginter M., Ivanov A., Lebedev O., Luksevics E., Avkhimovich V., Golubtsov V., and Petukhova L. 2000. Vertebrate correlation of the Upper Devonian and Lower Carboniferous on the East European Platform. Courier Forschungsinstitut Senckenberg 223: 341–359.
Evans, J.W. 1917. The British Isles. In: J.W. Evans (ed.), Handbuch der Regionalen Geologie. 356 pp. Carl Winter, Heidelberg.
Geikie, A. 1869. Explanation of sheet 14. Ayrshire: southern district. Memoires of the Geological Survey of the United Kingdom 1: 1–27.
Graham-Smith, W. 1978. On some variations in the latero-sensory lines of the placoderm fish Bothriolepis. Philosophical Transactions of Royal Society London B: Biological Science 282: 1–39. Crossref
Gross, W. 1932. Fossilium Catalogus. Pars 57: Antiarchi. 40 pp. W. Junk, Berlin.
Gross, W. 1942. Die Fischfaunen des baltischen Devons und ihre biostratigraphische Bedeutung. Korrespondenz-blatt des Naturforscher-Vereins zu Riga 64: 373–436.
Hecker, R. 1983. Tafonomičeskie i ekologičeskie osobennosti fauny i flory Glavnogo devonskogo polâ. 142 pp. Nauka, Moskva.
House, M.R. 1985. Correlation of mid-Palaeozoic ammonoid evolutionary events with global sedimentary perturbations. Nature 313: 17–22. Crossref
House, M.R., Menner, V.V., Becker, R.T., Klapper, G., Ovnatanova, N.S., and Kuz’min, V. 2000. Reef episodes, anoxia and sea-level changes in the Frasnian of the southern Timan (NE Russian Platform). Geological Society London Special Publications 178: 147–176. Crossref
Ivanov, A. and Lukševičs, E. 1996. Late Devonian vertebrates of the Timan. Daba un muzejs 6: 22–32.
Janvier, P. and Pan, J. 1982. Hyrcanaspis bliecki n. g. n. sp., a new primitive euantiarch (Antiarcha, Placodermi) from the Middle Devonian of north-eastern Iran, with a discussion on antiarch phylogeny. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 164: 364–392.
Johanson, Z. 1998. The Upper Devonian fish Bothriolepis (Placodermi: Antiarchi) from near Canowindra, New South Wales, Australia. Records of the Australian Museum 50 (3): 315–348. Crossref
Karatajūte-Talimaa, V.N. 1966. Bothriolepids of Šventoji Regional Stage of the Baltics [in Russian]. In: A. Grigelis (ed.), Paleontologiâ i stratigrafiâ Pribaltiki i Belorussji I (VI), 191–279. Mintis, Vilnius.
Karpinsky, A.P. [Karpinskij, A.P.] 1906. About Trochiliscus [in Russian]. Trudy Geologičeskogo Komiteta 27: 1–166.
Kuršs, V., Lukševičs, E., Upeniece, I., and Zupiņš, I. 1999. Upper Devonian clastics and associated fish remains in Lode clay quarry, Latvia (Part II) [in Latvian with English summary]. Latvijas ģeoloģijas vēstis 6: 10–17.
Kushnareva, T.I. [Kušnareva, T.I.] 1977. Famenskij ârus Timano-Pečorskoj provincji. 135 pp. Nedra, Moskva.
Kuzmin, A.V., Shuvalova, G.A. [Šuvalova, G.A.], Obukhovskaya T.G. [Obuhovskaâ T.G.], Avkhimovich, B.I. [Avhimovič, B.I.], Yudina, Yu.A. [Ûdina, Û.A.], and Moskalenko, M.N. 1998. Frasnian/Famennian boundary in the Izhma-Pechora Depression [in Russian]. Bulletin of Moscow Society of Naturalists, Geological Series 73 (4): 27–38.
Lukševičs, E. 1987. New information on Bothriolepis curonica Gross from the Upper Devonian of Latvia [in Russian with summaries in Latvian and English]. Priroda i muzej – muzeû prirody Latvijskoj SSR-140, 90–97. Zinātne, Riga.
Lukševičs, E. 1995. Variability in bothriolepid antiarchs (Placodermi) from the Main Devonian Field (East European Platform). In: H. Lelievre, S. Wenz, A. Blieck, and R. Cloutier (eds.), Premier vertébrés et vertébrés inférieurs. Geobios, Memoire Special 19: 117–120.
Lukševičs, E. 1999a. Placoderms (Antiarchi) [in Russian]. In: R.G. Matuhin and V.V. Menner (eds.), Stratigrafiâ silura i devona arhipelaga Severnaâ Zemlâ, 142–146. Siberian Institute of Geology, Geophysics and Mineral Resources, Novosibirsk.
Lukševičs, E. 1999b. Stratigraphic occurrence of vertebrate remains in the Upper Devonian of Severnaya Zemlya (Russia). Acta Geologica Polonica 49: 125–131.
Lukševičs, E. 2001. Bothriolepid antiarchs (Vertebrata, Placodermi) from the Devonian of the north-western part of the East European Platform. Geodiversitas 23: 489–609.
Lukševičs, E. and Stinkulis, Ģ. 2015. Signatures of biotic crisis in the Frasnian–Famennian boundary beds from Latvia. In: B. Mottequin, J. Denayer, P. Königshof, C. Prestianni, and S. Olive (eds.), IGCP596-SDS Symposium (Brussels, September 2015), Abstracts. STRATA, série 1, 16: 83–84.
Lukševičs, E., Beznosov, P.A., Majdl’, T.V., Ahlberg, P.E., and Stinkulis, G. 2010. Taphonomy of a Late Devonian vertebrate assemblage from Izhma River site, Sosnogorsk Formation, South Timan, Russia. In: 3rd International Palaeontological Congress, London, June 28–July 3 2010, Programme and Abstracts, 255.
Lukševičs, E., Beznosov, P., Mikuláš, R., and Mešķis, S. 2013. Late Devonian trace fossils from the Sosnogorsk Formation (South Timan, Komi Republic). Latvijas Universitātes 71. zinātniskā konference. Geogrāfija. Ģeoloģija. Vides zinātne. Abstracts, 332–334. University of Latvia, Riga.
Lukševičs, E., Lebedev, O., Mark-Kurik, E., and Karatajūtė-Talimaa, V. 2009. The earliest evidence of host-parasite interactions in vertebrates. Acta Zoologica 90 (Supplement 1): 335–343. Crossref
Lyarskaya, L. [Lârskaâ, L.] 1986. A new Bothriolepis (Antiarchi) from the Upper Devonian of Baltic states [in Russian]. In: A. Brangulis (ed.), Biofacii i fauna silurijskogo i devonskih bassejnov Pribaltiki, 123–130. Zinātne, Riga.
Lyarskaya, L. [Lârskaâ, L.] and Lukševičs, E. 1992. Composition and distribution of agnathan and vertebrate assemblages in the Silurian and Devonian deposits of Latvia [in Russian]. In: V.S. Sorokin (ed.), Paleontologiâ i stratigrafiâ fanerozoâ Latvii i Baltijskogo morâ, 46–62. Zinātne, Riga.
Marshall, J.E.A., Tel’nova, O.P., and Vetoshkina, O.S. 2011. Ecosystem crisis at the Frasnian–Famennian boundary (Southern Timan). Doklady Earth Sciences 440 (2): 1396–1398. Crossref
Maydl, T.V. and Beznosov, P.A. 2011. Carbon and oxygen isotope composition and the distribution of strontium in the Lower Famennian section of Izhma River (South Timan) [in Russian with English abstract]. Vestnik Instituta geologii Komi NTs UrO RAN 196 (4): 4–8.
Miles, R.S. 1968. The Old Red Sandstone antiarchs of Scotland: family Bothriolepididae. Palaeontographical Society Monograph 122: 1–130.
Moloshnikov, S.V. 2003. A new species of Bothriolepids Antiarch (Pisces, Placodermi) from the Zadonsk Formation (Upper Devonian) of the Central Devonian Field. Paleontological Journal 37: 184–187.
Moloshnikov, S.V. 2008. Devonian Antiarchs (Pisces, Antiarchi) from Central and Southern European Russia. Paleontological Journal 42: 691–773. Crossref
Myrow, P.M. 1995. Thalassinoides and the enigma of early Palaeozoic open-framework burrow systems. Palaios 10: 58–74. Crossref
Obukhovskaya, T.G. [Obuhovskaâ, T.G.] and Kuzmin, A.V. 1993. Spores and conodonts from the upper Frasnian–lower Famennian boundary beds in the Ukhta-Tebuk region [in Russian]. In: Paleontologičeskij metod v geologii, 35–51. Institute of Geology and Combustible Mineral Resources of Russian Academy of Sciences, Moskva.
Obukhovskaya, T.G., Avkhimovitch, V.I., Streel, M., and Loboziak, S. 2000. Miospores from the Frasnian–Famennian boundary deposits in Eastern Europe (the Pripyat Depression, Belarus and Timan-Pechora Province, Russia) and comparison with Western Europe (Northern France). Review of Paleobotany and Palynology 112: 229–246. Crossref
Olive, S. 2015. Devonian antiarch placoderms from Belgium revisited. Acta Palaeontologica Polonica 60: 711–731.
Palmer, A.R. 1994. Fluctuating asymmetry analyses: A primer. In: T.A. Markow (ed.), Developmental Instability: Its Origins and Evolutionary Implications, 335–364. Kluwer, Dordrecht. Crossref
Palmer, A.R. and Strobeck, C. 2003. Fluctuating asymmetry analyses revisited. In: M. Polak (ed.), Developmental Instability (DI): Causes and Consequences, 279–319. Oxford University Press, Oxford.
Pander, C. 1856. Monographie der fossilen Fische des silurischen Systems des russisch-baltischen Gouvernements. 91 pp. Buchdruckerei der Kaiserlichen Academie der Wissenschaften, St. Petersburg.
Racki, G. and Racka, M. 1981. Ecology of the Devonian charophyte algae from the Holy Cross Mts. Acta Geologica Polonica 31: 213–224.
Rohon, J.V. 1900. Die Devonischen Fische von Timan in Russland. Sitzungsberichte der Königliche Böhmischen Gesellschaft der Wissenschaften. Mathematisch-naturwissenschaltliche Classe (1899) 8: 3–77.
Sandberg, C.A., Ziegler, W., Dreesen, R., and Butler, J.L. 1988. Late Frasnian mass extinction: conodont event stratigraphy, global changes, and possible causes. Courier Forschungsinstitut Senckenberg 102: 263–307.
Stensiö, E.A. 1931. Upper Devonian vertebrates from East Greenland, collected by the Danish Greenland expedition in 1929 and 1930. Meddelelser øm Grønland 86: 3–213.
Stensiö, E.A. 1948. On the Placodermi of the Upper Devonian of East Greenland. II: Antiarchi: subfamily Bothriolepinae. Palaeozoologica Groenlandica 2: 1–622.
Szrek, P. 2004. The first articulated antiarch (Vertebrata, Placodermi) from the Upper Devonian of the Holy Cross Mountains (central Poland). Acta Geologica Polonica 54: 401–406.
Traquair, R.H. 1888. Notes on the nomenclature of the fishes of the Old Red Sandstone of Great Britain. Geological Magazine 5 (11): 507–517.
Traquair, R.H. 1893. Achanarras revisited. Proceedings of the Royal Phylosophical Society of Edinburgh 11: 283–286.
Traquair, R.H. 1906. The Fishes of the Old Red Sandstone of Britain. II: The Asterolepidae, 119–130. Palaeontographical Society, London.
Trinajstic, K. and Dennis-Bryan, K. 2009. Phenotypic plasticity, polymorphism and phylogeny within placoderms. Acta Zoologica 90: 83–102. Crossref
Werdelin, L. and Long, J.A. 1986. Allometry in the placoderm Bothriolepis canadensis and its significance to antiarch evolution. Lethaia 19: 161–169. Crossref
Yanong, R.P.E. 2003. Fungal diseases of fish. The Veterinary Clinics. Exotic Animal Practice 6: 377–400. Crossref
Young, G.C. 1984. Reconstruction of the jaws and braincase in the Devonian placoderm fish Bothriolepis. Palaeontology 27: 635–661.
Young, G.C. 1988. Antiarchs (placoderm fishes) from the Devonian Aztec siltstone, Southern Victoria Land, Antarctica. Palaeontographica A 202: 1–125.
Yukhtanov, P.P. [Ûktanov, P.P.], Antoshkina, A.I. [Antoškina, A.I.], Saldin, V.A., Tsyganko, V.S. [Cyganko, V.S.], Burtsev, I.N. [Burcev, I.N.], Mityusheva, T.P. [Mitûševa, T.P.], Beznosov, P.A., Ponomarev, D.V., Ryabinkina, N.N. [Râbinkina, N.N.], Sobolev, D.B., Andreicheva, L.N. [Andreičeva, L.N.], Sandula, A.N., Kalinin, E.P., Beznosova, T.M., Lukin, V.Yu. [Lukin, V.Û.], Gruzdev, D.A., Ploskova, S.I., Shadrin, A.N. [Šadrin, A.N.], and Maydl, T.V. [Majdl, T.V.] 2008. Geologičeskoe nasledie Respubliki Komi, Rossiâ. 360 pp. Institut geologii Komi, Syktyvkar.
Žeiba, S. and Savvaitova, L. 1981. Famennian [in Russian]. In: V. Sorokin (ed.), Devon i karbon Pribaltiki, 301–332. Zinātne, Riga.
Appendix 1
List of specimens of Bothriolepis leptocheira jeremejevi from IG KSC.
Partly articulated head and its imprint 155/45-1, 155/45-2; fragmentary head shield consisting of left La, Nu and Pp: 155/90; disarticulated plates of the head shield comprising two La, two Pn, Nu, and Pp: 155/150; fragmentary head shield consisting of the left La, Pmg, Pn, and Nu: 155/161; fragmentary head shield consisting of the right La and Pn, as well as Prm, Nu and Pp: 155/162; large block with the fragmentary head shield consisting of the right La, two Pn, Nu, and Pp, as well as Cv1, AVL and PVL: 71/И-87; left La: 155/15, 155/67, 155/68, 155/74, 155/126, 155/129; right La: 155/11, 155/64–155/66, 155/69, 155/96, 155/102, 155/138; Nu: 155/53, 155/56–155/58; slab with Nu, articulated Nu and Pp, as well as left ADL, 71/И-68; articulated Nu and Pp: 155/54-1 and 155/54-2, 155/118, 155/154; right Pn: 155/55, 155/117, 155/119, 155/125; Pp: 155/146, 155/155; Prm: 155/114; fragmentary Prm: 155/107; left ADL: 155/29, 155/59–155/63, 155/111, 155/112, 155/116, 155/131; right ADL: 155/18, 155/124, 155/128, 155/136; AMD: 155/5, 155/13, 155/19, 155/20, 155/23, 155/25, 155/26, 155/28, 155/32, 155/36, 155/42, 155/44, 155/81, 155/97, 155/108, 155/113, 155/121, 155/139, 155/140, 71/И-3; left AVL: 155/101, 155/109, 155/122, 155/143, 155/151; right AVL: 155/35, 155/87, 155/88, 155/93, 155/94, 155/100, 155/105, 155/123, 155/147, 155/164–155/166; fragmentary AVL wit articulated Cd1, Cv1 and Mm1: 155/103; fragmentary AVL or PVL: 155/89; MV: 155/76, 155/77, 155/104, 155/106, 155/133, 155/152, 155/153, 155/156; left MxL: 155/82, 155/120, 71/И-45; right MxL: 155/110, 155/115, 155/127, 155/132, 155/144, 155/163; fragmentary MxL: 155/3; PMD: 155/1, 155/17, 155/24, 155/27, 155/30, 155/31, 155/70–155/72, 155/95, 155/142, 155/148, 155/157–155/160, 71/И-7, 71/И-45 (on the same slab with MxL), 71/И-67; fragmentary PMD: 155/7, 155/73, 155/98; left PVL: 155/4, 155/75, 155/130, 155/141, 155/149, 71/И-13; right PVL: 155/16, 155/33, 155/78–155/80, 155/99; fragmentary PVL: 155/2, 155/91; right Cd1: 155/83; left Cv1: 155/6, 155/145; right Cv1: 155/14, 155/84, 155/134, 155/135; Cv2: 155/86; left Ml2: 155/92; right Ml2: 155/137; right Mm1: 155/85; distal segment of the pectoral fin: 155/21, 155/22; fragmentary bone of the pectoral fin: 155/12, 155/34; block with two ventral walls of the trunk armour, MV, AMD and fragments of bones: 155/9; block with a partially articulated ventral wall of the trunk armour, right pectoral appendage and left Cv1: 155/10-1; block with a partially articulated ventral wall of the trunk armour, left Cv1, inferognathals and fragments of the head shield bones: 155/10-2; block with three semiarticulated ventral walls of the trunk armour, imprint of 155/10-1 and 155/10-2: 155/10-3; block with AMD and Cv1: 155/37; block with separate AVL, Ml2 and MV: 155/38; block with separate AMD and PMD: 155/39; block with disarticulated bones of pectoral fin: 155/40; block with AMD and bones of pectoral fin: 155/41; block with separate AMD, MxL and distal segment of the pectoral fin: 155/43; block with several bones of the trunk and pectoral fin armour: 155/48; blocks with many disarticulated bones of the trunk armour: 155/49–155/51; block with the left ADL and distal segment of the pectoral fin: 155/52.