

Reconstruction of the cranial musculature of the paraceratheriid rhinocerotoid Pappaceras meiomenus and inferences of its feeding and chewing habits
HAI-BING WANG, BIN BAI, YAN-XIN GONG, JIN MENG, and YUAN-QING WANG
Wang, H.-B., Bai, B., Gong, Y.-X., Meng, J., and Wang, Y.-Q. 2017. Reconstruction of the cranial musculature of the paraceratheriid rhinocerotoid Pappaceras meiomenus and inferences of its feeding and chewing habits. Acta Palaeontologica Polonica 62 (2): 259–271.
The paraceratheriid Pappaceras is the earliest unequivocal rhinocerotoid genus to date, for which the osteological morphology is relatively unique compared to other perissodactyls. Due to the poor preservation condition, paleobiological aspects of Pappaceras (or forstercooperiines), such as chewing and feeding behavior, still remain unknown. Under the Extant Phylogenetic Bracket, the cranial musculature of the newly erected Pappaceras meiomenus has been reconstructed using two-dimensional illustrations, drawings and interpretations of the position and general morphology of cranial muscles for which origins and insertions on the skull are visible. In this study, eight muscles are reconstructed, described and compared to the corresponding muscles known or inferred in other perissodactyls, including the m. levator nasolabialis, the m. levator labii superior, the m. caninus, the m. zygomaticus, the m. masseter, the m. temporalis, the m. buccinator and the m. pterygoid. The reconstruction of the masticatory muscles suggests that Pappaceras meiomenus is strictly herbivorous, probably folivorous, with a primary component of vertical biting. The relatively well-developed m. pterygoid (particularly the m. pterygoideus medialis) indicates that Pappaceras meiomenus is similar to hyracodontids, having more advantages in rotary chewing than other non-hyracodontid rhinocerotoids. The configuration of basicranial features shows differentiation between non-hyracodontids and hyracodontids, demonstrating that the well-developed, specialized postglenoid process and the wide glenoid fossa, along with the postcotyloid process of the mandible, serve as a strong fulcrum during the power stroke in non-hyracodontids. Based on its rostral morphology, we suggest that Pappaceras meiomenus was a general browser. The morphology of its incisors and canines further indicate the ability to feed on hard plants, using the postulated puncture-crushing and grinding function.
Key words: Mammalia, Perissodactyla, Paraceratheriidae, Pappaceras meiomenus, chewing, Eocene, China.
Hai-Bing Wang [wanghaibing@ivpp.ac.cn], Bin Bai [baibin@ivpp.ac.cn], Yan-Xin Gong [gongyanxin@ivpp.ac.cn], Yuan-Qing Wang [wangyuanqing@ivpp.ac.cn] Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, 100044, China and College of Earth Sciences, University of Chinese Academy of Sciences, Beijing, 100049, China.
Jin Meng [jmeng@amnh.org], Division of Paleontology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, USA; Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, 100044, China.
Received 24 December 2016, accepted 8 March 2017, available online 13 April 2017.
Copyright © 2017 H.-B. Wang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The musculature is functionally fundamental for exploring biomechanics in extant animals. Unfortunately, soft tissues, like muscles, are rarely preserved in the fossilization process, so the exploration of biomechanical processes in extinct animals is challenging. Apart from the incompleteness of preserved materials, most muscles leave weak impressions on the cranium, which are commonly even less developed than those on the long bones. In addition, even when specimens are well preserved, myological reconstruction for fossil taxa is still highly speculative in nature, particularly tracing specific details of fiber orientation and relationships between adjacent structures. However, substantial clues about form, function, and behavior that are held in extant and extinct animals, lay the groundwork for inference of muscular arrangement and function as summarized by Lauder (1995), Witmer (1995), and Benton (2010). For instance, the rostral reconstruction of tapirs and horses that are specialized compared to primitive rhinocerotoids has been relatively well studied in extant and extinct taxa by several authors (e.g., Witmer et al. 1999; Bernardes et al. 2013). The musculature can be reconstructed using osteological markers, such as processes, ridges and rough areas that remain visible over the course of fossilization. Myological reconstructions have been widely conducted for crania of various fossil amphibians, dinosaurs and mammals (e.g., Bernardes et al. 2013; Lautenschlager 2013; Sanchez et al. 2013; Witzmann and Schoch 2013; Sharp 2014; Ercoli et al. 2016). Such studies have provided valuable insights (such as morphology and arrangement of adductor muscles) into paleobiological aspects of fossil taxa (such as bite force and feeding performance) (Lautenschlager 2013; Sharp 2014). The correlation between cranial musculature and feeding performance has been proposed for both extant and extinct animals (e.g., Qiu and Wang 2007; Cox and Baverstock 2016). These studies exemplify the paleobiological links between osteology and myology. Furthermore, reconstruction of cranial musculature, together with evidence from dentition and craniomandibular osteology, can be potentially helpful for inferring ecological aspects of extinct animals.
Previously, Pappaceras was considered a synonym of Forstercooperia (Wood 1938, 1963; Radinsky 1967; Lucas et al. 1981), but the latest study on forstercooperiines clarifies that Pappaceras is a valid genus and separated from Forstercooperia (Wang et al. 2016). To date, Pappaceras, recovered from late early Eocene sediments in Asia, represents the earliest unequivocal rhinocerotoid that is Arshantan in age (Wang et al. 2010, 2016). It is an early representative of paraceratheriid forstercooperiines, of which the origin and evolution are geographically restricted in Asia (Qiu and Wang 2007; Wang et al. 2016). Interestingly, the newly reported Pappaceras meiomenus H.B. Wang, Bai, Meng, and Y.Q. Wang, 2016 exhibits a number of cranial features primitively similar to basal tapiromorphs, but it also displays unique craniodental characteristics that resemble those of ancestral paraceratheriids (Wang et al. 2016), suggesting that this species possesses unique dietary habits.
There are few myological reconstructions of perissodactyls, but work has been done on extinct and extant horses (e.g., Sisson 1914; Gregory 1920; Bernardes et al. 2013), extant rhinoceroses (e.g., Beddard and Treves 1898; Groves 1972), and tapirs (e.g., Gregory 1920; Bressou 1961; Witmer et al. 1999). Only a few anatomical studies focusing on muscular reconstruction of fossil rhinocerotoids are present in the literature (Borsuk-Białynicka 1973; Qiu and Yan 1982; Qiu and Wang 2007). They serve as major resources for this study. Particularly, the myological reconstructions for paraceratheriines Juxia sharamurenensis and Paraceratherium lepidum by Qiu and Wang (2007) are comparable to the present work, as Pappaceras is phylogenetically and morphologically closely related to paraceratheriines (Lucas and Sobus 1989; Qiu and Wang 2007; Wang et al. 2016). There are also musculature reconstructions for Coelodonta antiquitatis by Borsuk-Białynicka (1973) and for Chilotherium cornutum by Qiu and Yan (1982). In the present study, the main cranial muscles that can be identified with confidence will be reconstructed, described and compared with those of other perissodactyls. Inferences on the feeding and chewing habits of Pappaceras meiomenus will be discussed on the basis of the combination of muscular, dental and osteological features.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York City, USA; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China.
Other abbreviations.—HSB, Hunter-Schreger Bands; m., muscle; MWC, width of the rostrum across the upper canines; PMW, maximum anterior width of the premaxilla.
Material and methods
The holotype of Pappaceras meiomenus (IVPP V20254), a complete cranium housed in the collection of Institute of Vertebrate Paleontology and Paleoanthropology, was used as the cranial model for the myological reconstruction of this species. The detailed morphology of the holotype of Pappaceras meiomenus can be found in Wang et al. (2016). Given that several muscles have their origin and insertion on the cranium and mandible respectively, AMNH 26677 was used as the mandibular model for the present work. This specimen is assigned to Pappaceras meiomenus in the latest systematic revision of forstercooperiines (HBW, BB, JM, and YQW unpublished material). It is the most complete known mandibular material of Pappaceras meiomenus to date and has also been described in detail by Lucas et al. (1981). Specimens of Hyracodon nebraskensis (AMNH 12460, cranium and mandibles), Subhyracodon occidentalis (AMNH 534, cranium and mandibles) and Pappaceras confluens (AMNH 26660, mandible only) were used for comparison. Except for IVPP V20454, all specimens used in the present study are currently housed in the collection of the American Museum of Natural History. Only part of the traceable cranial muscles was chosen for description and comparison, and muscles that possess postcranial insertions were excluded in the present work because postcrania still remain unknown in Pappaceras meiomenus. The cranium (IVPP V20254) was imaged using micro-CT at the Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China. The scan was performed at 430 kV and 1.5 mA, and then imported as stacked images into Mimics v.15 to create and export a 3D surface model of the cranium. Given the few references on the myological reconstruction of rhinocerotoids and the considerable distinction between Pappaceras meiomenus and extant rhinoceroses, the Extant Phylogenetic Bracket was used as a general rule to provide a most-likely model of muscular arrangement for fossil taxa by examining their closest related living taxa (Witmer 1995). Under the frame of the Extant Phylogenetic Bracket, the myology of other perissodactyls, such as tapirs and rhinocerotids, was extensively taken into account for musculature reconstruction (Fig. 1, Table 1). However, the myology of horses is a valuable alternative to those of rhinocerotids, because of the general similarity of osteological features between Pappaceras meiomenus and horses (such as dolichocephalic skull and complete anterior dentition) and the relative lack of myological studies of rhinocerotids (Fig. 1, Table 1). Based on osteological markers from the skull, the extent of each muscle attachment was inferred and compared to those in other perissodactyls. Two-dimensional illustrations, drawings, and interpretation were provided to mark the positions and general forms of muscles. Illustrations of cranial muscles (Fig. 2–5) were created in Illustrator CS5 and the reconstruction of the skull (Fig. 6) was created in Photoshop CS5. Terminology follows Witmer et al. (1999).
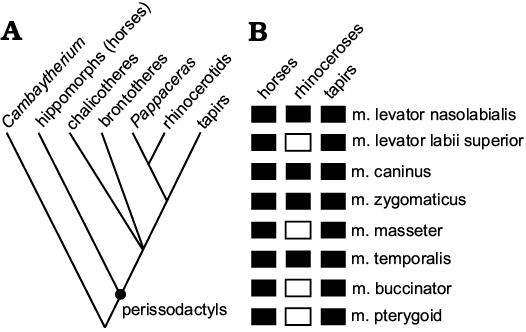
Fig. 1. Extant phylogenetic bracket (EPB) from which the cranial muscles in Pappaceras meiomenus are hypothesized. A. Simplified phylogeny of perissodactyls based on recent studies (Rose et al. 2014; Wang et al. 2016). B. Data for cranial muscles from extant perissodactyls, mainly taken from Beddard and Treves (1898), Boas and Paulli (1908), Sisson (1914), Gregory (1920), Bressou (1961), Witmer et al. (1999), Clifford (2003) and Bernardes et al. (2013). Solid rectangles denote the presence of references regarding certain muscle in extant perissodactyls, whereas open rectangles indicate a lack of references.
Table 1. Anatomical description of the cranial muscles present in extant horses, tapirs and rhinoceroses.
|
Muscles |
Tapirs (Tapirus) |
Horses (Equus) |
Rhinoceroses (Rhinoceros or Dicerorhinus) |
||||||
|
origin |
insertion |
|
origin |
insertion |
|
origin |
insertion |
|
|
|
m. levator nasolabialis |
lateral margin of the nasal |
lateral and ventral border of nostril |
well developed |
lateral margin of nasal and part of frontal |
superior angle of upper lip and lateral border of nostril |
weak |
anterior part of facial crest |
lateral margin of nostril |
moderately developed |
|
m. levator labii superior |
ridge of lacrimal, covered by m. levator nasolabialis |
upper lip |
well developed |
suture of lacrimal, zygomatic and maxilla |
upper lip |
moderately developed |
|
|
|
|
m. caninus |
ridge near the suture of maxilla and zygomatic |
lateral part of the nostril |
well developed |
border of rostral part of facial crest |
lateral part of nostril |
weak |
anterior part of the facial crest |
lateral part of nostril |
weak |
|
m. zygomaticus |
anterior part of zygomatic arch |
angle of mouth |
well developed |
beneath facial crest, under fascia covering m. masseter |
angle of mouth, uniting with m. buccinator |
weak |
anterior pat of zygomatic arch |
margin of lower lip |
well developed |
|
m. masseter superficialis |
facial crest and ventral part of zygomatic arch |
lateroventral surface of ascending ramus |
well developed |
facial crest and a tendon of zygomatic arch |
lateroventral surface of ascending ramus |
well developed |
|
|
|
|
m. masseter profundus |
posteroventral part of zygomatic arch |
lateral surface of ascending ramus |
weak |
facial crest and a tendon of zygomatic arch |
posteroventral border of mandible |
moderately developed |
|
|
|
|
m. temporalis |
sagittal crest |
coronoid process |
well developed |
sagittal crest |
coronoid process |
well developed |
sagittal crest |
coronoid process |
moderately developed |
|
m. buccinator |
buccinator fossa |
angle of mouth |
well developed |
maxillary part of buccinator fossa |
angle of mouth |
moderately developed |
|
|
|
|
m. pterygoideus lateralis |
lateral surface of alisphenoid |
anteromedial aspect of mandibular angle |
weak |
lateral surface of alisphenoid |
anteromedial aspect of mandibular angle |
weak |
|
|
|
|
m. pterygoideus medialis |
suture of alisphenoid and palatine |
medial surface of ascending ramus |
moderately developed |
suture of alisphenoid and palatine |
medial surface of ascending ramus |
moderately developed |
|
|
|
Results
In this section, eight muscles are described separately and compared to those of perissodactyls closely related to Pappaceras meiomenus. The origin and insertion of each muscle, as well as the inferred extent of each muscle, are given in Table 2.
Table 2. Cranial muscles of Pappaceras meiomenus reconstructed based on inferred correspondences.
|
Muscles |
Origin |
Insertion |
Inferred extent |
|
m. levator nasolabialis |
suture of nasal, frontal and lacrimal |
superior angle of upper lip |
moderately developed, as in Dicerorhinus |
|
m. levator labii superior |
suture of maxilla, zygomatic and lacrimal |
upper lip with a long tendon |
moderately developed, as in Equus |
|
m. caninus |
ventral part of orbit (flange and rough surface) |
lateral part of nostril |
weak in comparison to Tapirus |
|
m. zygomaticus |
lateroventral part of zygomatic arch (flange and rough surface) |
angle of mouth |
weak in comparison to Tapirus and Dicerorhinus |
|
m. masseter superficialis |
ventral part of zygomatic arch (flange and rough surface) |
lateroventral surface of ascending ramus |
well developed, as in Tapirus and Equus |
|
m. masseter profundus |
posteroventral part of zygomatic (flange and rough surface) |
masseteric fossa |
well developed in comparison to Tapirus and Equus |
|
m. temporalis |
sagittal crest (relatively well-developed) |
coronoid process of mandible (well-developed) |
well developed, as in Tapirus and Equus |
|
m. buccinator |
depression above canine-premolar diastema |
angle of mouth |
moderately developed, as in Equus |
|
m. pterygoideus lateralis |
lateral surface of alisphenoid (uneven surface) |
anteromedial part of the mandibular angle |
well developed in comparison to Tapirus and Equus |
|
m. pterygoideus medialis |
suture of the alisphenoid and palatine (a concave plate with rough ventral surface) |
medial surface of ascending ramus (concave medial surface of ascending ramus) |
well developed in comparison to Tapirus and Equus |
M. levator nasolabialis.—In extant tapirs, the m. levator nasolabialis is distinctively well developed due to the presence of a proboscis, arising from the lateral margin of the nasal, with its lateral outline being broad and sheet-like, and overlain anteroventrally by the levator anguli oculi medialis (Boas and Paulli 1908; Witmer et al. 1999). In the extant Dicerorhinus sumatrensis, the m. levator nasolabialis arises from the anterior part of the well-developed facial crest and inserts on the lateral margin of the nostril; this muscle is relatively massive (Beddard and Treves 1898; Qiu and Wang 2007). The m. levator nasolabialis is thin and directly lies under the skin and on the lateral surface of the nasal region in extant horses (Sisson 1914). The m. levator nasolabialis of Pappaceras meiomenus has been reconstructed as arising from the suture of the nasal, frontal and lacrimal, terminating around the superior angle of the upper lip. The shallow preorbital fossa may accommodate the m. levator nasolabialis. Given the anatomical correlation between the position of the muscle and the fossa, as previously proposed (Holbrook and Lucas 1997), the m. levator nasolabialis could be a relatively well-developed and thick muscle in Pappaceras meiomenus, despite a weak facial crest.
Beddard and Treves (1898) regarded the m. levator nasolabialis as the levator labii superioris alaeque nasi (Beddard and Treves 1898: 24, fig. 10). Later, the levator labii superioris alaeque nasi was hypothesized to consist of three groups by Gregory (1920: 272, fig. 8), despite the fact that the anterodorsal group in Gregory’s (1920) hypothesis was not well known. Qiu and Wang (2007) mentioned that the distinctively rough surface of the preorbital facial area indicates a well-developed m. levator nasolabialis in the Indian rhinoceros (Rhinoceros unicornis). Similar to the extant rhinoceroses, some fossil rhinocerotids likely maintained a well-developed m. levator nasolabialis based on diagnosed characters closely related to muscular attachment, for instance, the presence of a distinct crest above the dorsal border of the orbital in the woolly rhinocerotid Coelodonta antiquitatis (Borsuk-Białynicka 1973: 46, fig. 5A-b), and the distinct depression anterodorsal to the anterior border of the orbit in Chilotherium cornutum (Qiu and Yan 1982). For the giant rhino Paraceratherium lepidum, it is postulated that the origin of the m. levator nasolabialis is located at the anterodorsal part of the orbital and the concave surface superior to the orbital (Qiu and Wang 2007). In contrast, this muscle is considered thin in Juxia sharamurenensis, similar to those of living horses (Qiu and Wang 2007). Unlike the specialized condition of the cranium of Chilotherium cornutum, Paraceratherium lepidum, and extant tapirs, the anterodorsal portion of the preorbital region in Pappaceras meiomenus is similar to that of Juxia sharamurenensis, with a relatively smooth surface and without the trace of a distinct crest for muscular attachment. The m. levator nasolabialis in Pappaceras meiomenus is weaker than in Paraceratherium lepidum, Coelodonta antiquitatis, and Chilotherium cornutum. On the other hand, the m. levator nasolabialis in Pappaceras meiomenus may be much thicker than those of Juxia sharamurenensis and living horses (for which the muscular layer is relatively thin), in order to support the snout. The m. levator nasolabialis of Pappaceras meiomenus may be more anteriorly displaced than that of Juxia sharamurenensis, for which the deeper narial notch ends above the third upper premolar, based on the anatomical correlation analyses for rostral muscles of fossil and extant horses (Bernardes et al. 2013).
M. levator labii superior.—The extant tapirs possess a distinctive m. levator labii superior, which is attached to a well-developed ridge of the lacrimal, and the insertion of this muscle is fully covered by the m. levator nasolabialis (Boas and Paulli 1908; Bressou 1961; Witmer et al. 1999). According to Gregory’s illustration (Gregory 1920: 272, figs. 6, 7), this muscle was gradually thickened posteriorly in Tapirus terrestris. In extant horses, this muscle arises from the junction of the lacrimal, zygomatic and maxilla and inserts to the upper lip (Bernardes et al. 2013). However, it is still not clear in extant rhinoceroses despite the simplified illustrations (Beddard and Treves 1898; Gregory 1920: 272, fig. 8). Based on the relatively large facial exposure of the lacrimal and the rough surface around the suture of the maxilla, zygomatic, and lacrimal, the m. levator labii superior in Pappaceras meiomenus has been reconstructed as moderately well developed, originating from the suture of the maxilla, zygomatic and lacrimal and extending anteriorly to the upper lips with a long tendon.
In Coelodonta antiquitatis, this muscle was assumed to be distinctively large due to the large muscle attachment area present on the lateral surface of the visceral portion of the skull (Borsuk-Białynicka 1973: 46, fig. 5A-a). Qiu and Wang (2007) suggested that the m. levator labii superior in Juxia sharamurenensis should have been rather large and accommodated by a broad surface area. This muscle is considered to be rather massive in fossil hippidiforms, such as Onohippidium, which bears a distinct preorbital fossa immediately anterior to the orbital for muscular attachment. Similarly, extant tapirs possess a distinctive m. levator labii superior, which is attached to a well-developed ridge of the lacrimal, and the proximal insertion of this muscle is fully covered by the m. levator nasolabialis (Boas and Paulli 1908; Bressou 1961; Witmer et al. 1999).
It should be noted that although Pappaceras meiomenus has a shallow and relatively broad preorbital fossa on the facial area (mainly the maxilla), the location and form of the preorbital are considerably different from those of tapirs and some specialized horses, which display a narrow but relatively deep fossa adjacent to the anterior border of the orbital and the ridges of the lacrimal. The lack of a deep, narrow preorbital fossa indicates that the m. levator labii superior in Pappaceras meiomenus would be much less developed than that of Onohippidium and living tapirs that possess evidently prehensile upper lips. This muscle was considered well developed due to its broad proximal muscular attachment. It was proximally covered by the m. levator nasolabialis in Juxia sharamurenensis and Paraceratherium lepidum, despite the lack of evident trace of attachment from the cranial surface in Juxia sharamurenensis (Qiu and Wang 2007). If the reconstruction for Juxia sharamurenensis and Paraceratherium lepidum is correct, the m. levator labii superior in Pappaceras meiomenus would be similar to those of living horses, and slightly weaker than that of Juxia sharamurenensis and Paraceratherium lepidum. The proximal end of the m. levator labii superior in Pappaceras meiomenus is probably not fully covered by the m. levator nasolabialis (Fig. 1), as the origin of the m. levator nasolabialis is not particularly extensive, similar to the condition observed in living horses and unlike that of extant tapirs.
M. caninus.—In extant tapirs, the m. caninus originates as a tendinous band from a ridge on the maxilla near the suture of the maxilla and zygomatic and inserts to the lateral part of the nostril (Witmer et al. 1999). It is well developed and relatively extensive anteriorly, but separated from the m. levator labii superior by a connective tissue pad (Witmer et al. 1999). In living horses, the m. caninus is thin and triangular in lateral view and located on the lateral nasal region, arising from the border of the rostral portion of the well-developed facial crest and passing between the two branches of the levator nasolabialis (Boas and Paulli 1908; Sisson 1914; Bernardes et al. 2013). In living rhinoceroses, this muscle is illustrated as slender and weak, originating from the anterior part of the facial crest and extending anteriorly to the lateral part of the nostril, but lacks detailed description and comparison (Beddard and Treves 1898). Based on the distinct flange and rough surface on the ventral part of the orbit, and the lack of distinctive facial ridges or scars for muscular attachment around the infraorbital foramen, the m. caninus in Pappaceras meiomenus has been illustrated as a weak muscle, slender and thin, probably originating in the ventral part of the orbit, passing through the m. levator nasolabialis and anteriorly terminating at the m. orbicularis oris.
The m. caninus is a slender and weak muscle in extant Dicerorhinus sumatrensis, as illustrated by Beddard and Treves (1898). The m. caninus was reconstructed as a massive muscle attaching to a slightly concave and reniform muscle scar posterior to the infraorbital foramen in the extinct Coelodonta antiquitatis (Borsuk-Białynicka 1973: fig. 5A-c). On the basis of observation on the skull of Rhinoceros unicornis, Qiu and Wang (2007) proposed that the m. caninus would be relatively robust both in Coelodonta antiquitatis and Rhinoceros unicornis. However, this argument regarding the extent of muscular attachment requires further testing through detailed myology of extant rhinoceroses. In addition, they argued that the m. caninus might be attached to the ventral portion of the anterior zygomatic arch in Juxia sharamurenensis, and to the anterior portion of the orbit in Paraceratherium lepidum, considering the morphological differences on the facial area between the two taxa (Qiu and Wang 2007). The variation of muscular attachment site is highly possible across rhinocerotoid taxa, for which hypotheses made based on potential structures for muscular attachment are reasonable. We propose that Pappaceras meiomenus has the m. caninus attaching at the ventral portion of the orbital instead at the infraorbital foramen or the anterior portion of orbital like in Coelodonta antiquitatis, Rhinoceros unicornis, and extant horses.
M. zygomaticus.—In extant tapirs, the m. zygomaticus forms a distinct, powerful muscle, arising from the anterior part of the zygomatic arch, running forward in a downward convex arc and ending at the angle of the mouth (Boas and Paulli 1908: 67). In the Dicerorhinus sumatrensis, the m. zygomaticus was illustrated as a long and robust muscle with its origin at the lateroventral part of the zygomatic arch and the distal end at the angle of the mouth (Beddard and Treves 1898: 24, fig. 10). In living horses, the m. zygomaticus is thin, slender and completely separated from the platysma, and not directly attached to the surface of the cranium (Sisson 1914; Boas and Paulli 1908). It originates beneath the facial crest under the fascia covering the m. masseter and terminates at the angle of the mouth, uniting with m. buccinator (Bernardes et al. 2013). On the basis of the relatively slender zygomatic arch and the distinct rough ventral surface of the zygomatic, the m. zygomaticus in Pappaceras meiomenus has been reconstructed as arising from the anteroventral border of the zygomatic and terminating at the angle of the mouth, and this muscle would be moderately developed and less developed than those of extant tapirs and rhinoceroses.
The reconstruction of the m. zygomaticus in Juxia sharamurenensis was illustrated as a well-developed muscle based on the rough ventral surface of its zygomatic arch (Qiu and Wang 2007: 191, fig. 33). Compared to Juxia sharamurenensis, Pappaceras meiomenus also possesses a rough ventral surface on the zygomatic, but the zygomatic arch of the latter is much more slender than the former, suggesting a weaker condition of the m. zygomaticus in Pappaceras meiomenus.
M. masseter.—In extant horses and tapirs, the m. masseter is divided into two layers: deep and superficial (Beddard and Treves 1898; Sisson 1914). The superficial layer of the m. masseter is commonly extensive in horses, tapirs, and rhinoceroses, arising from the facial crest and the ventral portion of the zygomatic arch and inserting on the lateroventral surface of the ascending branch of the mandible. The deep layer of the m. masseter is extensive but thin in living horses, arising from the zygomatic arch and facial ridge, and passing straight to the posteroventral border of the mandible (Sisson 1914), while it is small and weakened in tapirs, arising from the posteroventral portion of the zygomatic and terminating at the lateral surface of the ascending branch of the mandible (Qiu and Wang 2007). These differences are consistent with the mandibular morphology, in which the lateral surface of the ascending branch, particularly near the mandibular angle, is rough with various ridges for extant horses and rhinoceroses, but smooth for tapirs. The literature lacks a detailed description of the m. masseter of extant rhinoceroses. The m. masseter in Pappaceras meiomenus has been reconstructed as possessing deep and superficial layers, analogous to those in extant tapirs and horses. Given that the masseteric fossa is relatively deep and narrow in Pappaceras meiomenus, it is possible that this fossa potentially accommodates a thick, deep layer of the m. masseter. The deep layer of the m. masseter in Pappaceras meiomenus is probably thick but not extensive, arising from the ventral part of the zygomatic arch and inserting to the masseteric fossa in the lateral surface of the mandible. The superficial layer of the m. masseter in Pappaceras meiomenus originates at the ventral part of the zygomatic arch and inserts to the lateral surface of the ascending ramus. It is probably relatively well developed based on the distinct mandibular angle, with the posterior border of the mandible anteriorly inclined, and the rough lateral surface of the ascending ramus, which are closely associated with the attachment of the superficial layer of the m. masseter.
Despite the lack of detailed description of the m. masseter in extant rhinoceroses, there are a few studies regarding the inferred reconstruction of the m. masseter for fossil rhinocerotoids, such as Coelodonta antiquitatis, Juxia sharamurenensis and Paraceratherium lepidum, proposing that the superficial layer of the m. masseter attaches to the slightly concave surface below the masseteric fossa is extensive and thin, as in extant tapirs (Borsuk-Białynicka 1973; Qiu and Wang 2007). Furthermore, Qiu and Wang (2007) also proposed that the superficial layer of the m. masseter is much more developed in extant rhinoceroses than in living horses and tapirs on the basis of the robust zygomatic arch and distinctive ridges around the mandibular angle. The deep layer of the m. masseter, which attaches to the masseteric fossa in extant rhinoceroses, is small and thick in cross section (Beddard and Treves 1898; Borsuk-Białynicka 1973; Qiu and Wang 2007). Unlike that of rhinoceroses, the zygomatic arch in Pappaceras meiomenus is much more slender than in Juxia sharamurenensis and Paraceratherium lepidum, with a flange on the rough ventral surface and weak facial ridges (rudimentary to the facial crest of derived rhinocerotoids and living horses) (Figs. 1, 2). This suggests that the superficial layer of the m. masseter in Pappaceras meiomenus is less developed than in the aforementioned rhinocerotoids. The masseteric fossa provides muscular attachment for the deep layer of the m. masseter (Borsuk-Białynicka 1973; Qiu and Wang 2007). The deep layer of the m. masseter in the extant rhinoceroses is weakened and coupled with the strengthened superficial layer (Qiu and Wang 2007). Our observation shows that the masseteric fossa posterior to the anterior border of the ascending ramus is consistently present in Pappaceras meiomenus, Juxia sharamurenensis, Paraceratherium lepidum, and Coelodonta antiquitatis, while it is absent in living horses and rhinoceroses. If the correlation between the masseteric fossa and the deep layer of the m. masseter is correct, the deep layer of the m. masseter in Pappaceras meiomenus is not extensive, but thicker compared to extant rhinoceroses.
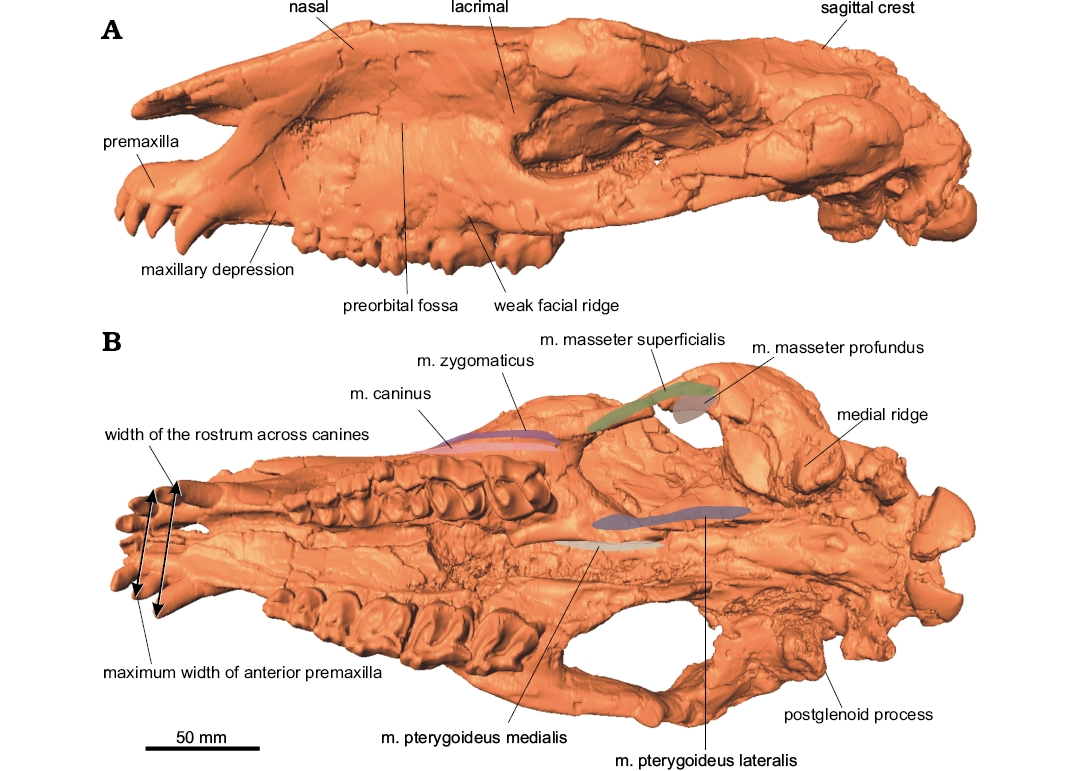
Fig. 2. Surface model of the cranium of the paraceratheriid rhinocerotoid Pappaceras meiomenus H.B. Wang, Bai, Meng, and Y.Q. Wang, 2016 (IVPP V20254) from the late Early Eocene Arshanto Formation, Erlian Basin, Nei Mongol, China, in lateral (A) and ventral (B) views.
M. temporalis.—The m. temporalis conforms to the temporal fossa, arising from the sagittal crest and terminating lingually and labially around the coronoid process of the mandible in extant ungulates. Attachment of the m. temporalis is massive in Pappaceras meiomenus, judging from the well-developed sagittal crest on the surface of the temporal fossa. This is similar to that of Juxia sharamurenensis despite a smaller attachment area, and different from those of Paraceratherium lepidum and extant rhinoceroses, which lack a well-developed sagittal crest or a pair of high frontoparietal ridges. By comparison, the muscular attachment of the m. temporalis in Paraceratherium lepidum would be less extensive than in Juxia sharamurenensis because of the absence of a well-developed sagittal crest (Qiu and Wang 2007). The coronoid process in Pappaceras meiomenus is robust, similar to that in Juxia sharamurenensis. The m. temporalis is well developed in Pappaceras meiomenus, at least comparatively stronger than many perissodactyls that lack sagittal crests.
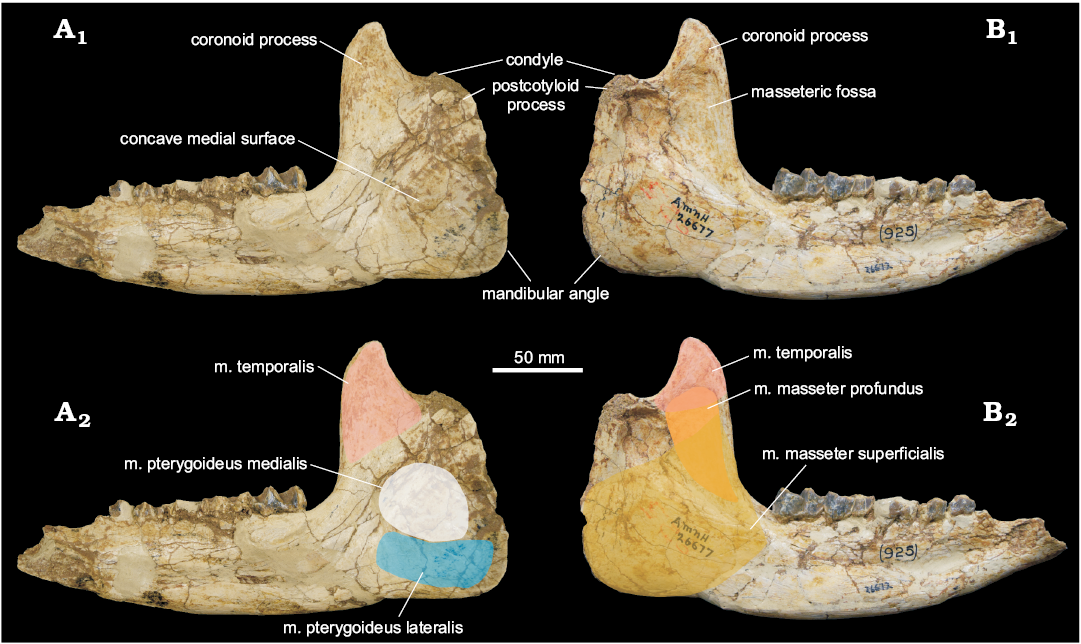
Fig. 3. Mandible of the paraceratheriid rhinocerotoid Pappaceras meiomenus H.B. Wang, Bai, Meng, and Y.Q. Wang, 2016 (AMNH 26677) from the late Early Eocene Arshanto Formation, Erlian Basin, Nei Mongol, China, in medial (A) and lateral (B) views. A1, B1, photographs, A2, B2, interpretations of muscular attachment.
M. buccinator.—In extant tapirs, the m. buccinator originates at the buccinator fossa, which is usually enlarged in proboscis-bearing mammals for controlling movements of the nasal and oral vestibules, and it inserts to the angle of the mouth (Clifford 2003; Bernardes et al. 2013). In living horses, this muscle lies in the lateral wall of the mouth, arising from the maxillary portion of the buccinator fossa and terminating at the angle of the mouth (Sisson 1914; Bernardes et al. 2013). The m. buccinator of living rhinoceroses has not been described. Despite the lack of a distinct buccinator fossa, a distinctive depression above the maxillary canine-premolar diastema is present in Pappaceras meiomenus (Fig. 2). This condition is similar to the maxillary portion of the buccinator fossa in horses but much shallower than those of proboscis-bearing mammals. The m. buccinator of Pappaceras meiomenus originates at the maxillary depression and inserts at the angle of the mouth.
The m. buccinator of Pappaceras meiomenus could be much larger than those of most rhinocerotoids (e.g., Juxia sharamurenensis and Coelodonta antiquitatis), because of the lack of a maxillary depression, except for amynodontids and Uintaceras, which possess distinctive maxillary depressions. It is also smaller than those of fossil hippidiforms, in which the buccinator fossa is considerably more concave and extensive (Bernardes et al. 2013). The m. buccinator in Pappaceras meiomenus would provide for moderate mobility of the muzzle along with the m. levator labii superior.
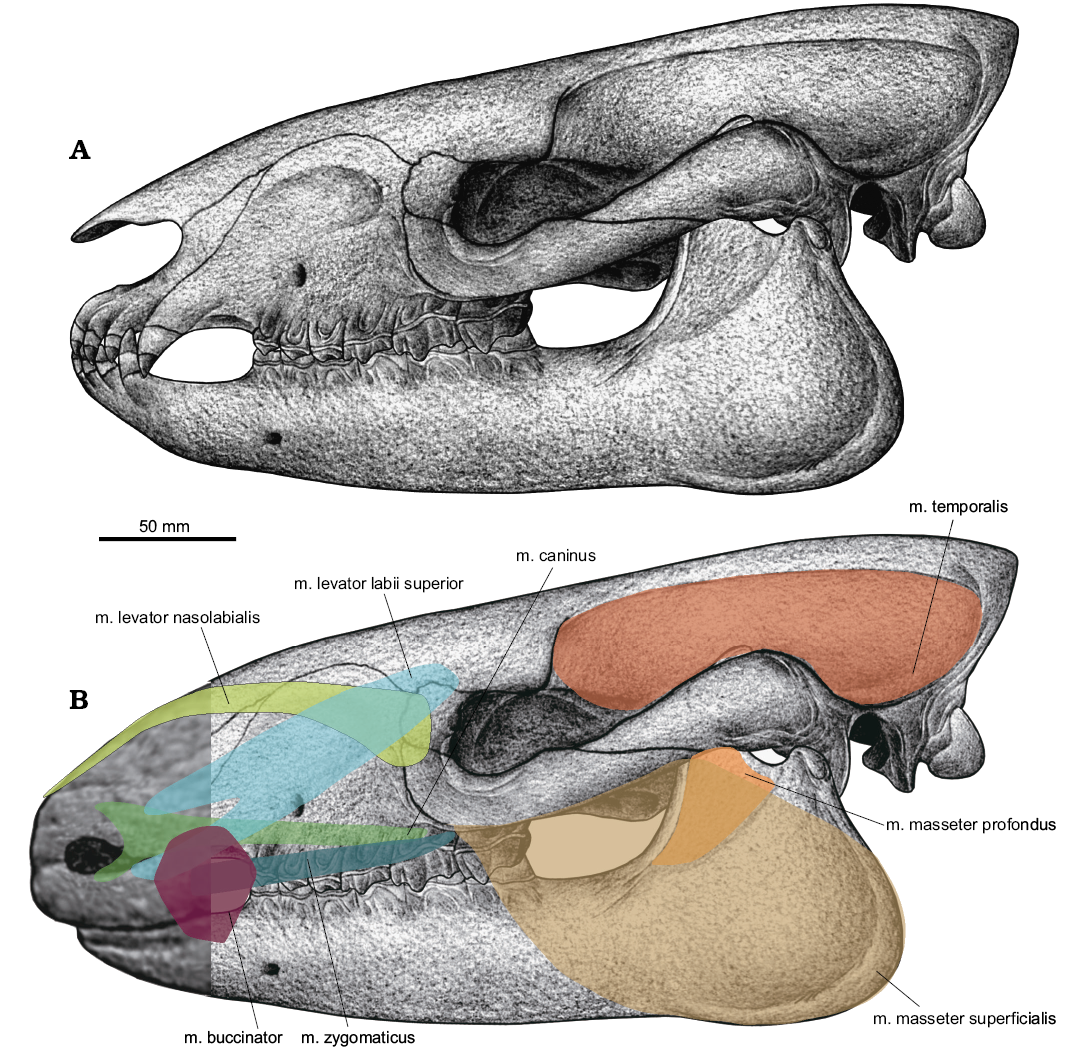
Fig. 4. Illustration of the cranial muscles in the paraceratheriid rhinocerotoid Pappaceras meiomenus in lateral view. A. Illustration of the cranium base on Wang et al. (2016). B. Interpretation of the reconstructed musculature; shaded area shows position of several facial muscles relative to the nose and lips.
M. pterygoid.—In extant horses and tapirs, the m. pterygoid comprises the m. pterygoideus medialis and the m. pterygoideus lateralis. Generally, the medial pterygoid is a single muscle in living horses and tapirs (Sisson 1914; Bressou 1961), rather than being divided into a deep layer and a superficial layer in other mammals that have a distinct pterygoid fossa (a concave pocket lateral to the alisphenoid that is the origin of the superficial layer) (Sharp 2014). The medial pterygoid in living horses and tapirs originates from the area around the suture of the alisphenoid and palatine, and inserts to the medial surface of the ascending ramus, whereas the m. pterygoideus lateralis arises from the lateral surface of the alisphenoid and inserts to the anteromedial aspect of the mandibular angle (Bressou 1961; Qiu and Wang 2007; Sharp 2014). In Pappaceras meiomenus, the medial surface of the ascending ramus is large and considerably concave for a strong insertion of the medial pterygoid. Likewise, the origin site of the m. pterygoideus medialis in Pappaceras is distinctive and extensive, represented by a concave plate with a rough ventral surface around the suture of the alisphenoid and palatine (Figs. 2, 5B). The alisphenoid, despite being slightly damaged in this specimen, is uneven on the lateral surface with several ridges, probably for the attachment (origin) of the m. pterygoideus lateralis. However, the origin attachment area is smaller than that of the m. pterygoideus medialis. The m. pterygoid of Pappaceras meiomenus is composed of the well-developed m. pterygoideus medialis and the moderately developed m. pterygoideus lateralis. The m. pterygoideus medialis in Pappaceras meiomenus could be more extensive than in Juxia sharamurenensis and other primitive rhinocerotoids.
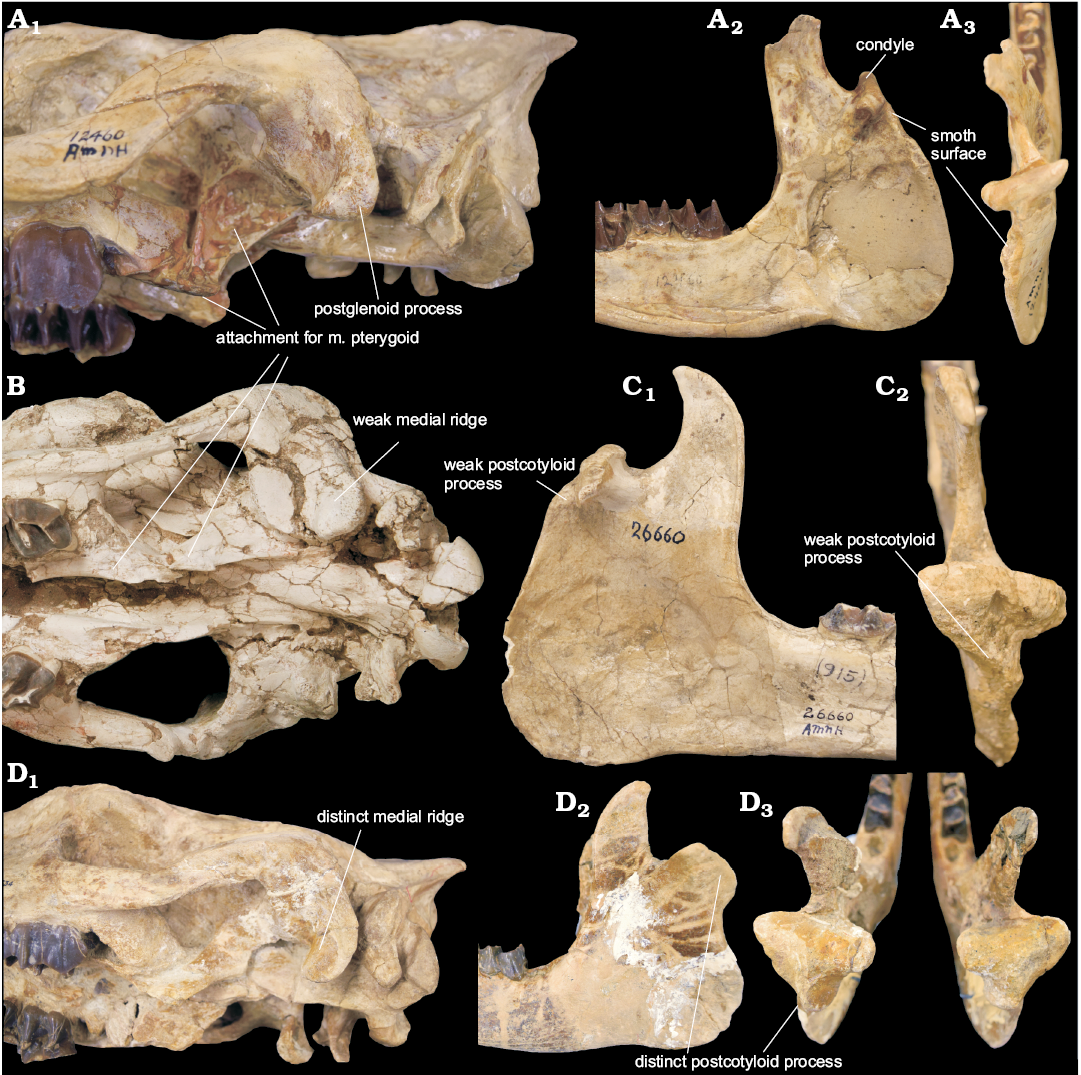
Fig. 5. Morphology of craniomandibular articulation in hyracodontids, Pappaceras, and rhinocerotids. A. Hyracodontid Hyracodon nebraskensis Leidy, 1850 (AMNH 12460) from Oligocene of Nebraska, USA. Cranium in lateroventral view (A1); right mandible in medial (A2) and dorsoposterior (A3) views. B. Paraceratheriid Pappaceras meiomenus H.B. Wang, Bai, Meng, and Y.Q. Wang, 2016 (IVPP V20254) from the late Early Eocene Arshanto Formation, Erlian Basin, Nei Mongol, China; cranium in ventral view. C. Paraceratheriid Pappaceras confluens Wood, 1963 (AMNH 26660) from the late Early Eocene Arshanto Formation, Erlian Basin, Nei Mongol, China; left mandible in medial (C1) and dorsoposterior (C2) views. D. Rhinocerotid Subhyracodon occidentalis Leidy, 1850 (AMNH 534) from Oligocene of South Dakota, USA; cranium in lateroventral view (D1), mandibles in lateral (D2) and dorsoposterior (D3) views.
Discussion
The function of the masticatory system is closely related to the morphology of the masseter, temporal, and pterygoid muscle groups. Many distinctions are apparent between the masticatory apparatuses of carnivorous and herbivorous mammals, considering their difference in food items and feeding behavior (Smith and Savage 1959; Fortelius 1985). Qiu and Wang (2007) discussed the feeding behavior of the herbivorous paraceratheriid Juxia sharamurenensis based on a reconstruction of the masticatory muscles. In Pappaceras meiomenus, the masseter muscle group, which has an essential role for processing vegetation, shows similarities and differences with the masseter of Juxia sharamurenensis. The features shared by Pappaceras meiomenus and Juxia sharamurenensis, such as the low ascending ramus of the mandible and well-developed masseteric fossa, are indicative of a well-developed m. masseter. However, Pappaceras meiomenus has a slender zygomatic arch, indicating a weak superficial layer of the m. masseter compared to Juxia sharamurenensis, which may result in less control during biting. The main action of the mandible in Juxia sharamurenensis is vertical biting without circular movement of the lower teeth against the upper ones as in derived grinders (Qiu and Wang, 2007). Similarly, it is unlikely that the rotary motion would be a major component of the power stroke in Pappaceras meiomenus, despite the distinctive muscular attachment of the well-developed m. pterygoid (especially the m. pterygoideus medialis). The evidence supporting this hypothesis is also based on the cranium and dentition. The presence of a flattened palate (the palatine process of maxilla and palatine) in Pappaceras meiomenus makes the circular movement difficult (Fig. 2), whereas in other cases a concave plate can provide an ellipsoid space between the roof of the mouth and the tongue during occlusion (Bernardes et al. 2013). Meanwhile, the cheek teeth of Pappaceras meiomenus, similar to those of Juxia sharamurenensis and other primitive perissodactyls, are brachydont and simply structured without distinct crochets and thickened enamel bands. Therefore, Pappaceras meiomenus is strictly herbivorous, primarily folivorous, eating leaves and stems rather than hard plants as suggested by Qiu and Wang (2007). In this aspect, Pappaceras meiomenus, similar to Juxia sharamurenensis, primarily possesses vertical biting to cut and grind food, although probably less efficiently than derived rhinocerotoids.
However, the chewing habit of Pappaceras meiomenus probably shows a higher level of rotary motion in comparison to most early rhinocerotoids, not unlike that of hyracodontids. This similarity lies in the relatively well-developed m. pterygoideus medialis, despite the m. pterygoideus medialis in Pappaceras meiomenus being weaker than in hyracodontids by the absence of a distinct pterygoid fossa (Fig. 5A1, B). In hyracodontids (e.g., Hyracodon), the pterygoid fossa is large and deep, located at the lateral surface of the alisphenoid (Fig. 5A1); it is likely that this fossa accommodates the superficial part of the m. pterygoideus medialis as in other mammals with pterygoid fossae (Sharp 2014), suggesting that the m. pterygoideus medialis in hyracodontids is well developed and comprises the deep and superficial parts. In rhinocerotids (e.g., Subhyracodon), no distinctive fossa or plate for the muscular attachment of the m. pterygoideus medialis is present around the junction of the alispenoid and pterygoid (Fig. 5D1). The m. pterygoideus medialis and the m. masseter superficialis are antagonistic; they control a combination of upward, forward and sideward movement, contributing to medial and lateral force, respectively (Fortelius 1985). This similarity of muscular attachment for the m. pterygoideus medialis indicates that Pappaceras meiomenus and hyracodontids have more potential in transverse (or medial) movement of the mandibles than other rhinocerotoids.
In contrast, the distinction of several basicranial features between Pappaceras meiomenus and hyracodontids suggests that the mechanisms of mandibular rotation across rhinocerotoids may be more complicated than previously thought, showing two kinds of configurations. One is represented by Pappaceras meiomenus and other non-hyracodontid rhinocerotoids, in which the glenoid fossa is wide open, and the postglenoid process is massive, bearing two articular facets usually divided by a medial ridge on the anterior surface (Fortelius 1985; Holbrook 2001). These features are closely associated with the presence of the postcotyloid process of the mandible. Pappaceras meiomenus possesses a broad glenoid fossa, a massive postglenoid process and a weak medial ridge (Figs. 2, 5B), along with a weak postcotyloid process of the mandible (Fig. 3) (also see Pappaceras confluens in Fig. 5C). It displays rudimentary features like most non-hyracodontids, especially rhinocerotids, in which the glenoid fossa is narrow but open, and the medial ridge is well developed, separating the anterior articulated surface into two facets, a lateral facet facing nearly laterally and a medial facet facing nearly anteriorly, as well as a well-developed postcotyloid process of the mandible, such as in Subhyracodon (Fig. 5D). The large postglenoid process and the broad glenoid fossa potentially provide a wide contact surface for the mandible and cranium in Pappaceras meiomenus, serving as a fulcrum for the power stroke (Fortelius 1985). Furthermore, the lateral facet of the postglenoid process, despite being confluent with the medial facet in Pappaceras meiomenus, probably functions more effectively as a fulcrum for mandibular rotation in amynodontids, rhinocerotids, and later paraceratheriids. The other configuration is represented in hyracodontids, in which the postglenoid process is relatively slender and extends anteroventrally, and the medial ridge and lateral facet of the postglenoid process are absent (Fig. 5D1). The postcotyloid process of the mandible is correspondingly absent (Fig. 5D2–D3). Furthermore, the glenoid fossa in hyracodontids is not as open as in other rhinocerotoids, forming a more hinge-like contact between the cranium and the mandibles (Fig. 5A). Given the presence of a distinctive pterygoid fossa, the m. pterygoideus medialis in hyracodontids (such as Hyracodon) is better developed than across rhinocerotoids, indicating a component of transverse movement of the mandibles. In that case, the weak postglenoid process as a fulcrum would benefit, to some extent, from the hingle-like articulation between the cranium and the mandibles. This configuration would be less efficient than the former for the power stroke of strictly herbivorous taxa.
Based on rostral morphology, Pappaceras meiomenus is a general browser compared to other herbivorous mammals. According to diet studies for herbivorous ruminants by Solounias et al. (1988) and Solounias and Moelleken (1993), and for horses by Bernardes et al. (2013), there is a strong correlation between shape of the premaxilla and feeding style. Browsers usually possess a pointed premaxilla, grazers have a wide premaxilla, and mixed feeders maintain an intermediate state. In that case, a simple approach has been proposed and applied in horses by Bernardes et al. (2013). He measured the shape of the premaxilla or the relative width of the premaxilla, assessing the ratio between the maximum anterior width of the premaxilla (PMW) and the width of the rostrum across the upper canines (MWC). Specifically, a ratio lower than 1.0 means that the PMW is narrower than the MWC, and reflects a transversely narrow and pointed anterior portion of the premaxilla. In Pappaceras meiomenus, the anterior portion of the premaxilla is slightly curved medially, and the upper canine is implanted close to the last upper incisor with a very short diastema (Fig. 2B). Therefore, the ratio in Pappaceras meiomenus is slightly lower than 1.0, suggesting that Pappaceras meiomenus is a general browser. With regard to the enamel structure and the configuration of the Hunter-Schreger Bands (HSB), Pappaceras meiomenus maintains transverse HSB on incisors and vertical HSB on cheek teeth (Wang et al. 2016), similar to the condition in most rhinocerotoids as summarized by Koenigswald et al. (2011).
The anterior dentition of Pappaceras meiomenus is unique among rhinocerotoids in terms of morphology and configuration. In Pappaceras meiomenus, the three upper incisors are sub-equal, conical, nearly vertically implanted and tightly arranged. The upper incisors narrowly interlock the lower incisors with two wear facets on each tooth. The mesial wear facet gradually decreases in size posteriorly from I1 to I3, whereas the distal facet becomes larger posteriorly. With regard to these features, Pappaceras meiomenus is different from extant rhinoceroses that lack the anterior dentition and instead use the upper lip to select food items (e.g., black rhinoceroses). It is also distinguishable from living horses that have relatively flat wear facets and tight, transversely positioned incisors. The incisors of Pappaceras meiomenus, nearly longitudinally oriented and relatively tightly arranged, indicate sideways feeding ability, roughly similar to Juxia sharamurenensis. Given the tiny diastemata between incisors in Pappaceras meiomenus, it can be distinguished from Juxia sharamurenensis, which has a distinctive combing action using sideways or backward movement of its head with loosely arranged incisors during feeding (Qiu and Wang 2007). It appears that the incisors of Pappaceras meiomenus are probably not capable of efficiently incising food (like living horses can), or assisting in grabbing food items using specialized first upper and lower incisors (as in derived paraceratheriids). Accordingly, Pappaceras meiomenus could be prone to using its conical incisors primarily as puncture-crushing organs, along with a grinding component, given the close interlocked condition and distinctive wear facets of the anterior dentition. In that case, the stubby, conical incisors of Pappaceras meiomenus, together with its large canines, would allow this animal to feed on hard plants, while the spatulate incisors of the contemporaneous Lophialetes and Hyrachyus would have helped with a puncture-crushing and grinding function.
 |
|
Conclusions
Based on osteological and myological correlation across perissodactyls, eight cranial muscles are reconstructed for Pappaceras meiomenus with a brief description and comparison with other perissodactyls. The reconstruction of masticatory muscles suggests Pappaceras meiomenus is probably folivorous, primarily possessing vertical biting. Given the relatively well-developed m. pterygoid (particularly the m. pterygoideus medialis), Pappaceras meiomenus is similar to hyracodontids to some extent. It also has more transverse (or medial) movement of the mandibles than other non-hyracodontid rhinocerotoids. The configuration of basicranial features, such as the large postglenoid process, the wide glenoid fossa and the weak postcotyloid process, indicates that Pappaceras meiomenus, as well as most rhinocerotoids except for hyracodontids, uses the well-developed, specialized postglenoid process along with the postcotyloid process of the mandible as a strong fulcrum during the power stroke. Alternatively, hyracodontids may have benefited from more hingle-like craniomandibular articulation, despite the lack of the specialization of postglenoid and postcotyloid processes. Morphology of the anterior dentition demonstrates that Pappaceras meiomenus is a general browser. Furthermore, its incisors and canines are probably great tools for feeding on hard plants by puncture-crushing and grinding.
Acknowledgements
We thank Allison Bronson, Camille Grohe (AMNH), Andrew Cuff (University of London College, UK), Jack Zhijie Tseng (University at Buffalo, USA) and Zhaoqun Zhang (IVPP) for constructive discussions; Xun Jin, Qian Li, Fangyuan Mao, Wei Zhou, Shijie Li, Qi Li, Yongxing Wang, and Yongfu Wang (all IVPP) for assistance in the field; Shijie Li (IVPP) for preparation of specimen; Yemao Hou (IVPP) for CT scanning; Yu Chen (IVPP) for drawing; Mai Reitmeyer and Tom Baione (AMNH) for the help with literature. The authors are grateful to Luke Holbrook (Rowan University, New Jersey, USA) and an anonymous reviewer for their constructive suggestions. The work was supported by National Basic Research Program of China (Grant No. 2012CB821904), the Geological Survey Projects of China Geological Survey Bureau (Grant No. 1212011120142), and the National Science Foundation of China (Grant No. 41002009). The fieldwork was also supported by the Special Fund for Fossil Excavation and Preparation from Chinese Academy of Sciences. HBW thanks the financial support of China Scholarship Council (CSC No. 201504910615).
References
Beddard, F.E. and Treves, F. 1898. On the anatomy of Rhinoceros sumatrensis. Proceedings of the Zoological Society of London 15: 7–25.
Benton, M.J. 2010. Studying function and behavior in the fossil record. PLoS Biology 8 (3): e1000321. Crossref
Bernardes, C., Sicuro, F.L., Avilla, L.S., and Pinheiro, A.E.P. 2013. Rostral reconstruction of South American hippidiforms (Mammalia, Perissodactyla, Equidae): New anatomical and ecomorphological inferences. Acta Palaeontologica Polonica 58: 669–678.
Boas, J.E.V. and Paulli, S. 1908. The Elephant’s Head: Studies in the Comparative Anatomy of the Organs of the Head of the Indian Elephant and Other Mammals. 127 pp. Gustav Fisher, Copenhagen.
Borsuk-Białynicka, M. 1973. Studies on the Pleistocene rhinoceros Coelodonta antiquitatis (Blumenbach). Palaeontologia Polonica 29: 5–94.
Bressou, C. 1961. La myologie du Tapir (Tapirus indicus L.). Mammalia 25: 358–400. Crossref
Clifford, A.B. 2003. Narial Novelty in Mammals: Case Studies and Rules of Construction. 128 pp. Ohio University, Ohio.
Cox, P.G. and H. Baverstock. 2016. Masticatory muscle anatomy and feeding efficiency of the American beaver, Castor canadensis (Rodentia, Castoridae). Journal of Mammalian Evolution 23: 191–200. Crossref
Ercoli, M.D., Álvarez, A., Busker, F., Morales, M.M., Julik, E., Smith, H.F., Adrian, B., Barton, M., Bhagavatula, K., Poole, M., Shahsavan, M., Wechsler, R., and Fisher, R.E. 2016. Myology of the head, neck, and thoracic region of the lesser grison (Galictis cuja) in comparison with the red panda (Ailurus fulgens) and other carnivorans: phylogenetic and functional implications. Journal of Mammalian Evolution 2016: 1–34 [published online].
Fortelius, M. 1985. Ungulate cheek teeth developmental, functional, and evolutionary interrelations. Acta Zoologica Fennica 180: 1–76.
Gregory, W.K. 1920. Studies in comparative myology and osteology. On the anatomy of the preorbital fossae of Equidae and other ungulates. Bulletin of American Museum of Natural History 42: 265–284.
Groves, C.P. 1972. Ceratotherium simum. Mammalian Species 8: 1–6. Crossref
Holbrook, L.T. 2001. Comparative osteology of early Tertiary tapiromorphs (Mammalia, Perissodactyla). Zoological Journal of the Linnean Society 132: 1–54. Crossref
Holbrook, L.T. and Lucas, S.G. 1997. A new genus of rhinocerotoid from the Eocene of Utah and the status of North American “Forstercooperia”. Journal of Vertebrate Paleontology 17: 384–396. Crossref
Koenigswald, W.V., Holbrook, L.T., and Rose, K.D. 2011. Diversity and evolution of Hunter-Schreger band configuration in tooth enamel of perissodactyl mammals. Acta Palaeontologica Polonica 56: 11–32. Crossref
Lauder, G.V. 1995. On the inference of function from structure. In: J.J. Thomason (ed.), Functional Morphology in Vertebrate Paleontology, 1–18. Cambridge University Press, Cambridge.
Lautenschlager, S. 2013. Cranial myology and bite force performance of Erlikosaurus andrewsi: a novel approach for digital muscle reconstructions. Journal of Anatomy 222: 260–272. Crossref
Lucas, S.G. and Sobus, J. 1989. The systematics of indricotheres. In: D.R. Prothero and R.M. Schoch (eds.), The Evolution of Perissodactyls, 358–378. Oxford University Press, New York.
Lucas, S.G., Schoch, R.M., and Manning, E. 1981. The systematics of Forstercooperia, a middle to late Eocene hyracodontid ( Perissodactyla: Rhinocerotoidea ) from Asia and western North America. Journal of Paleontology 55 (4): 826–841.
Qiu, Z.X. and Wang, B.Y. 2007. Paracerathere fossils of China. 387 pp. Science Press, Beijing.
Qiu, Z.X. and Yan, D. 1982. A horned Chilotherium skull from Yushe, Shansi. Vertebrata Palasiatica 20: 122–134.
Radinsky, L.B. 1967. A review of the rhinocerotoid family Hyracodontidae (Perissodactyla). Bulletin of American Museum of Natural History 136: 1–46.
Rose, K.D., Holbrook, L.T., Rana, R.S., Kumar, K., Jones, K.E., Ahrens, H.E., Missiaen, P., Sahni, A., and Smith, T. 2014. Early Eocene fossils suggest that the mammalian order Perissodactyla originated in India. Nature Communications 5: 5570. Crossref
Sanchez, S., Dupret, V., Tafforeau, P., Trinajstic, K.M., Ryll, B., Gouttenoire, P.-J., Wretman, L., Zylberberg, L., Peyrin, F., and Ahlberg, P.E. 2013. 3D microstructural architecture of muscle attachments in extant and fossil vertebrates revealed by synchrotron microtomography. Plos One 8 (2): e56992. Crossref
Sharp, A.C. 2014. Three dimensional digital reconstruction of the jaw adductor musculature of the extinct marsupial giant Diprotodon optatum. PeerJ 2: e514. Crossref
Sisson, S. 1914. The Anatomy of the Domestic Animals. 963 pp. W.B. Saunders Company, London.
Smith, J.M. and Savage, R.J.G. 1959. The mechanics of mammalian jaws. School Science Review 144: 289–301.
Solounias, N. and Moelleken, S.M. 1993. Dietary adaptation of some extinct ruminants determined by premaxillary shape. Journal of Mammalogy 74: 1059–1071. Crossref
Solounias, N., Teaford, M., and Walker, R. 1988. Interpreting the diet of extinct ruminants: the case of a non-browsing giraffid. Paleobiology 14: 287–300. Crossref
Wang, H.B., Bai, B., Meng, J., and Wang, Y.Q. 2016. Earliest known unequivocal rhinocerotoid sheds new light on the origin of Giant Rhinos and phylogeny of early rhinocerotoids. Scientific Reports 6: 39607. Crossref
Wang, Y.Q., Meng, J., Beard, C.K., Li, Q., Ni, X.J., Gebo, D.L., Bai, B., Jin, X., and Li, P. 2010. Early Paleogene stratigraphic sequences, mammalian evolution and its response to environmental changes in Erlian Basin, Inner Mongolia, China. Science China Earth Sciences 53: 1918–1926. Crossref
Witmer, L.M. 1995. The Extant Phylogenetic Bracket and the importance of reconstructing soft tissues in fossils. In: J.J. Thomason (ed.), Functional Morphology in Vertebrate Paleontology, 19–33. Cambridge University Press, Cambridge.
Witmer, L.M., Sampson, S.D., and Solounias, N. 1999. The proboscis of tapirs (Mammalia, Perissodactyla), a case study in novel narial anatomy. Journal of Zoology 249: 249–267. Crossref
Witzmann, F. and Schoch, R.R. 2013. Reconstruction of cranial and hyobranchial muscles in the Triassic temnospondyl Gerrothorax provides evidence for akinetic suction feeding. Journal of Morphology 274 (5): 525–542.
Wood, H.E. 1938. Cooperia totadentata, a remarkable rhinoceros from the Eocene of Mongolia. American Museum Novitates 1012: 1–20.
Wood, H.E. 1963. A primitive rhinoceros from the Late Eocene of Mongolia. American Museum Novitates 2146: 1–11.
Acta Palaeontol. Pol. 62 (2):
259–271, 2017
https://doi.org/10.4202/app.00336.2016