

The Triassic eucynodont Candelariodon barberenai revisited and the early diversity of stem prozostrodontians
AGUSTÍN G. MARTINELLI, MARINA BENTO SOARES, TÉO VEIGA DE OLIVEIRA, PABLO G. RODRIGUES, and CESAR L. SCHULTZ
Martinelli, A.G., Soares, M.B., de Oliveira, T.V., Rodrigues, P.G., and Schultz, C.L. 2017. The Triassic eucynodont Candelariodon barberenai revisited and the early diversity of stem prozostrodontians. Acta Palaeontologica Polonica 62 (3): 527–542.
The dental anatomy of Candelariodon barberenai from the Dinodontosaurus Assemblage Zone (Pinheiros-Chiniquá Sequence, Santa Maria Supersequence, late Ladinian–early Carnian) of south Brazil, is redescribed. Candelariodon was originally classified as Eucynodontia incertae sedis and our analysis recovered this taxon deeply nested within Probainognathia, as the sister taxon of Potheriodon plus Prozostrodontia. The lower postcanine dentition of Candelariodon has several apomorphies shared with Prozostrodon, Santacruzgnathus, Brasilodon/Brasilitherium, and some basal mammaliaforms (Morganucodon, Megazostrodon), such as a lingual cingulum with discrete cusps e and g and two distinct morphologies in the tooth row. The reinterpretation of Candelariodon as a probainognatian cynodont more derived than Probainognathus and the rich Brazilian fossil record document an important adaptive radiation of non-mammaliaform prozostrodontians and closely related forms prior to the origin of the mammaliaform clade.
Key words: Cynodontia, Probainognathia, Prozostrodontia, Dinodontosaurus Assemblage Zone, South America, Brazil.
Agustín G. Martinelli [agustin_martinelli@yahoo.com.ar], Marina Bento Soares [marina.soares@ufrgs.br], Pablo G. Rodrigues [pablogr@bol.com.br], and Cesar L. Schultz [cesar.schultz@ufrgs.br], Laboratório de Paleontologia de Vertebrados, Departamento de Paleontologia e Estratigrafia, Instituto de Geociências, Universidade Federal do Rio Grande do Sul (UFRGS), Av. Bento Gonçalves 9500, Agronomia, 91540-000, Porto Alegre, RS, Brazil.
Téo Veiga de Oliveira [teovoli@yahoo.com.br], Divisão de Mamíferos do Museu de Zoologia da UEFS, Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana (UEFS), Av. Transnordestina s/n, Bairro Novo Horizonte, 44036-900, Feira de Santana, BA, Brazil.
Received 27 January 2017, accepted 19 April 2017, available online 14 August 2017.
Copyright © 2017 A.G. Martinelli et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Middle to Late Triassic continental tetrapod assemblages of southern Brazil and western Argentina have provided the most abundant and taxonomically diverse fossil record of non-mammaliaform probainognathian cynodonts worldwide (e.g., Abdala and Ribeiro 2010; Martinelli and Soares 2016). The plethora of forms described from these regions includes several species positioned near to the base of the mammaliaform clade (e.g., Santacruzgnathus abdalai, Therioherpeton cargnini, Prozostrodon brasiliensis, Brasilodon quadrangularis, Riograndia guaibensis, Irajatherium hernandezi, and Chaliminia musteloides) and illustrates the main evolutionary transformations in the skeleton towards the mammalian condition (e.g., Bonaparte and Barberena 2001; Bonaparte et al. 2005; Martinelli et al. 2005, 2016c; Martinelli and Rougier 2007; Ruta et al. 2013; Rodrigues et al. 2013, 2014; Ruf et al. 2014; Soares et al. 2014).
The Dinodontosaurus Assemblage Zone (AZ) of the Pinheiros-Chiniquá Sequence, Santa Maria Supersequence (Zerfass et al. 2003; Horn et al. 2014; Fig. 1), includes the oldest unambiguous cynodont records from the Triassic of Brazil (see Martinelli et al. 2016a for discussion on the taxonomy of cynodont remains from the Lower Triassic Sanga do Cabral Supersequence). The age of the Dinodontosaurus AZ is inferred on the basis of biostratigraphic correlations with the Chañares Formation of the Ischigualasto-Villa Unión Basin, western Argentina (Fiorelli et al. 2013; Marsicano et al. 2016) and radiometric dating of the overlying Santa Cruz Sequence (Philipp et al. 2013), both suggesting a late Ladinian–early Carnian age. Whereas the Chañares Formation yields two probainognathians, Chiniquodon theotonicus and Probainognathus jenseni (Abdala and Giannini 2002; Martinelli et al. 2016c), the Dinodontosaurus AZ includes five: Chiniquodon theotonicus (Abdala and Giannini 2002), Aleodon cromptoni (Martinelli et al. 2016b, 2017), Bonacynodon schultzi (Martinelli et al. 2016c), Protheriodon estudianti (Bonaparte et al. 2006; Martinelli et al. 2016c), and Candelariodon barberenai (Oliveira et al. 2011). The same disproportion is seen in traversodontid gomphodonts, being represented by one species in the Chañares Formation (Massetognathus pascuali, according to Abdala and Giannini 2000) and six in the Dinodontosaurus AZ (Luangwa sudamericana, Abdala and Sá-Teixeira 2004; a Scalenodon-like form, Melo et al. 2014; Massetognathus ochagaviae, M. pascuali, Barberena 1981b; Liu et al. 2008; Traversodon stahleckeri, Barberena 1981a; Liu and Abdala 2014; and Protuberum cabralense, Reichel et al. 2009).
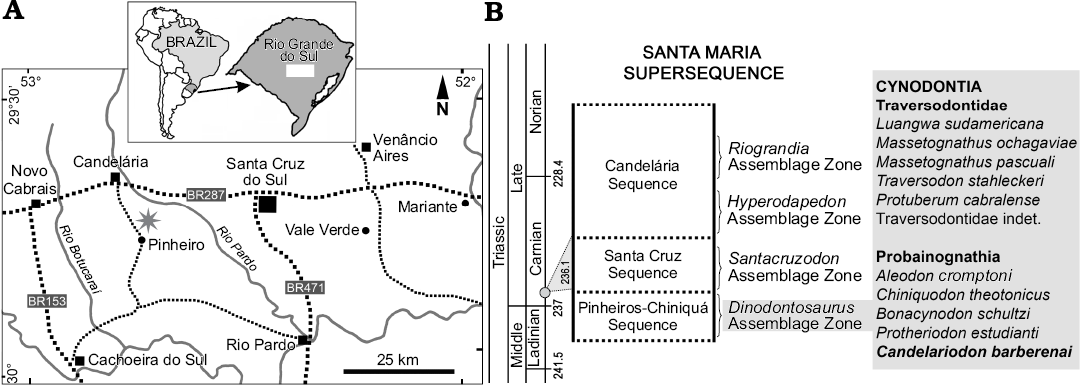
Fig. 1. Geographic (A, star indicates the type locality) and biostratigraphic (B) location of Candelariodon barberenai. The biostratigraphy follows Horn et al. (2014) and the dating (grey circle in B) corresponds to Philipp et al. (2013).
Of the above mentioned probainognathians from the Dinodontosaurus AZ, Protheriodon estudianti was included within brasilodontids (e.g., Bonaparte et al. 2006; Bonaparte 2013) or Prozostrodontia (Martinelli et al. 2016c), while the other species were classified as basal probainognathians (e.g., Hopson and Kitching 2001; Liu and Olsen 2010; Oliveira et al. 2010; Martinelli et al. 2016c). In particular, Candelariodon barberenai was classified as Eucynodontia incertae sedis (Oliveira et al. 2011). The authors also noted striking resemblances of one tooth with those of Aleodon brachyramphus, from the Manda Formation of Tanzania (Crompton 1955), and, to a lesser extent, with those seen in Cromptodon mamiferoides (Bonaparte 1972) from the Cerro de Las Cabras Formation of Argentina.
In this contribution we reinterpret the postcanine morphology of the holotype and only known specimen of Candelariodon barberenai and provide evidence that places this taxon phylogenetically close to the Prozostrodontia clade. Due to the age of the Dinodontosaurus AZ, the phylogenetic position of C. barberenai reinforces the hypothesis of a conspicuous diversification of non-mammaliaform prozostrodontians and closely related forms in older times, with at least two distinct morphotypes: one represented by Candelariodon and Prozostrodon and the other by tiny prozostrodontians such as Therioherpeton, Brasilodon, and possibly Protheriodon and dromatheriids, in addition to the disparate ictidosaur and tritylodontid (if this clade is considered within Probainognathia; see Luo 1994; Hopson and Kitching 2001; Abdala 2007; Liu and Olsen 2010) groups.
Institutional abbreviations.—MCT, Museu de Ciências da Terra, Rio de Janeiro, Brazil; MMACR-PV-T, Museu Municipal Aristides Carlos Rodrigues (Paleovertebrates-Triassic Collection), Candelária, Brazil; NHMUK, Natural History Museum (PV, Vertebrate Paleontology; R, Reptiles; M, Mammals), London, UK; PVL, Instituto Miguel Lillo (Vertebrate Paleontology Collection), Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina; UFRGS-PV-T, Universidade Federal Rio Grande do Sul (Vertebrate Paleontology, Triassic Collection), Porto Alegre, Brazil; UMZC, University Museum of Zoology, Cambridge, UK.
Other abbreviations.—AZ, Assemblage Zone; a–g, cusps of postcanine crowns (following Crompton 1974); c, lower canine; i, lower incisors; pc, lower postcanine teeth.
Material and methods
The holotype (MMACR PV-0001-T) of Candelariodon barberenai comes from the Dinodontosaurus AZ of the Pinheros-Chiniquá Sequence, Santa Maria Supersequence (Horn et al. 2014; Fig. 1). It corresponds to the lower portion of the traditional Santa Maria Formation (Gordon 1947) and the Santa Maria 1 Sequence of Zerfass et al. (2003). The outcrop that yielded MMACR PV-0001-T is located ~20 km south of Candelária city (state of Rio Grande do Sul, Brazil), in the Pinheiro (or Pinheiros) region (Fig. 1), an area in which several tetrapods characteristic of the Dinodontosaurus AZ have been discovered (e.g., Romer and Price 1944; Barberena 1977, 1981a, b; Barberena et al. 1985; Schultz et al. 2000; Bertoni-Machado et al. 2008; Martinelli et al. 2016b, c, 2017).
Candelariodon was compared to other probainognathians, based mainly on firsthand examination of specimens deposited in different institutions. Otherwise, bibliographic sources were used for comparisons. Brasilodon quadrangularis and Brasilitherium riograndensis were used along the text as two distinctive taxa, until the hypothesis of synonymy (Liu and Olsen 2010; Martinelli and Bonaparte 2011) is thoroughly tested (it is being done by the former author).
In order to test the phylogenetic affinities of MMACR PV-0001-T, this specimen was included in the data matrix of Liu and Olsen (2010), as modified by Martinelli et al. (2016c) with a few extra modifications on the scoring of Protheriodon, Prozostrodon, and Brasilitherium (Appendix 1). This modified data matrix was analyzed under equally-weighted parsimony using TNT 1.5 (Goloboff and Catalano 2016). A heuristic search of 100 replications of Wagner trees, followed by TBR branch-swapping algorithm (holding 10 trees per replication), was performed. All characters were treated as non-additive. Bremer support (Bremer 1994) and a bootstrap resampling analysis (Felsenstein 1985) were conducted. The modified data matrix is included in Appendix 2.
Systematic palaeontology
Therapsida Broom, 1905
Cynodontia Owen, 1861
Eucynodontia Kemp, 1982
Probainognathia Hopson, 1990
Genus Candelariodon Oliveira, Schultz, Soares, and Rodrigues, 2011
Type species: Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011; monotypic, see below.
Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011
Figs. 2, 3A, 4, 5A.
Holotype: MMACR PV-0001-T, almost complete left branch and anterior half of the right branch of the dentary, fused at the symphysis, bearing partial dentition, and an isolated posterior lower left postcanine tooth (Oliveira et al. 2011; Figs. 2, 3).
Type locality: ~20 km south of Candelária city, Pinheiro (or Pinheiros) region, Rio Grande do Sul, Brazil (Fig. 1).
Type horizon: Dinodontosaurus AZ of the Pinheros-Chiniquá Sequence, Santa Maria Supersequence, late Ladinian–early Carnian.
Emended diagnosis.—Probainognathian cynodont with the following combination of features (autapomorphies marked with an asterisk): tall horizontal ramus of the dentary and coronoid process; no angular process; fused mandibular symphysis; lower incisor alveolar level positioned above the level of the postcanine crowns; last lower incisor with strongly posteriorly curved crown; large lower canine without serrated edges; pc2–3 with large cusp a, tiny cusp b, small cusp c, accessory cusp g, faint lingual cingulum, and absence of cusp d*; pc2–4 transversely broader at distal half of the crown than mesially; posterior postcanines (pc5–8) mesiodistally longer than preceding ones; pc5 with continuous lingual cingulum, bearing accessory cusp g and at least cusps a, c, and d*; posterior postcanines with large, slightly posteriorly curved cusp a, small cusp b, cusp c larger than b, cusp d, accessory cusp e, and with non-continuous cingulum, absence of cusp g*.
Description.—Dentary: The dentary has a tall horizontal ramus and well-developed coronoid and articular processes (Fig. 2). The horizontal ramus has an almost straight ventral edge, parallel to the alveolar line. It is slightly convex dorsoventrally and about three times taller than the crown height of pc4 in lateral view. Hence, the horizontal ramus is relatively stout as usually found in probainognathians (e.g., Probainognathus jenseni, PVL 4445; Bonacynodon schultzi, MCT-1716-R; Prozostrodon brasiliensis, UFRGS-PV-248-T, Fig. 3B; Botucaraitherium belarminoi, MMACR-PV-003-T; Riograndia guaibensis, UFRGS-PV-0596-T). It differs from the low horizontal ramus of Protheriodon estudianti (UFRGS-PV-0962-T), Santacruzgnathus abdalai (UFRGS-PV-1121-T), and Brasilitherium riograndensis (UFRGS-PV-1043-T; Fig. 3C). A large mental foramen is observed at mid-height below pc2 and there are other small foramina nearby. The coronoid process is tall, with its dorsal edge broken off and the anterior margin wider at its base. It presents a large masseteric fossa on its lateral surface that extends anteriorly to about the level of the pc5 (Figs. 2A, 3A), as observed in most probainognathians (Hopson and Kitching 2001). The articular process of the dentary extends far posteriorly, but it is not completely preserved. Its ventral edge projects posterodorsally with the posteriormost tip positioned above the postcanine level, lacking an angular process, as in Prozostrodon, Brasilitherium, and some early mammaliaforms, such as Haramiyavia (Luo et al. 2015). Medially, the postdentary trough is reduced and at the inflection of the coronoid process there is no clear evidence of a facet or remnant of coronoid bone.
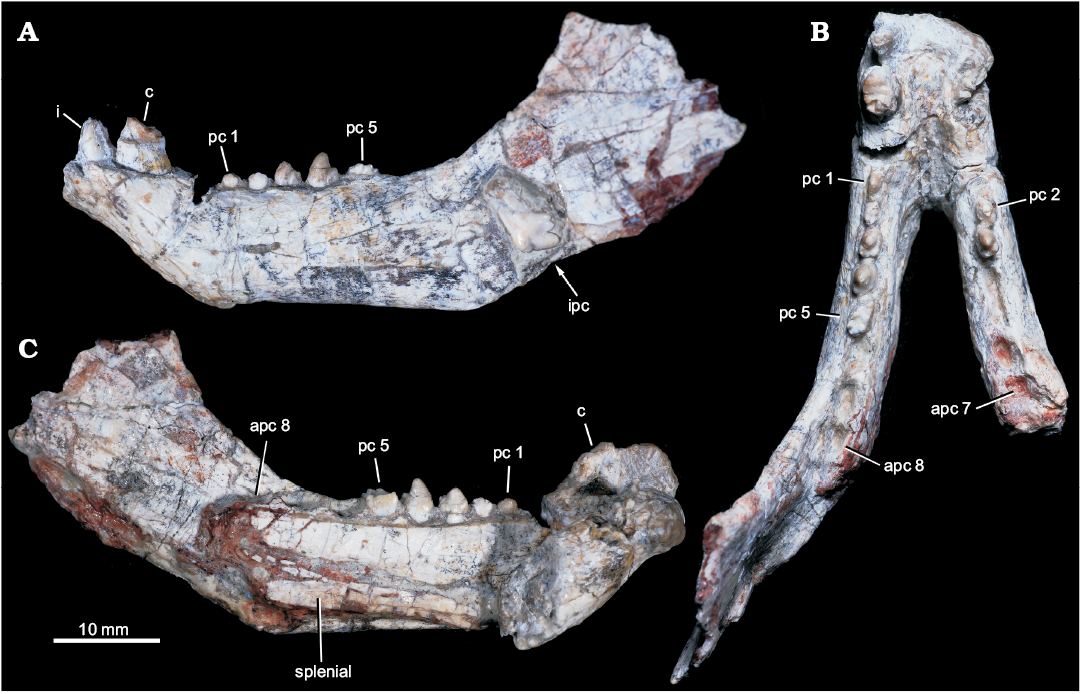
Fig. 2. Probainognathian eucynodont Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011 (holotype, MMACR PV-0001-T) from the Middle–Late Triassic of Rio Grande do Sul state, Brazil. Lower jaw with partial dentition in left lateral (A), occlusal (B), and right medial (with part of right dentary detached) (C) views. Abbreviations: apc, postcanine alveolus; c, lower canine; i, lower incisor; ipc, isolated postcanine; pc, lower postcanine.
The straight ventral border of the dentary bends abruptly anteriorly at the level of pc1, forming an angle of ~140° and delimiting the posteroventral edge of symphysis (Fig. 2A). It is anterodorsally to posteroventrally inclined, being about twice as long as wide. There is no evidence of suture at the symphysis, indicating that both dentaries are fused, which is the condition in most non-prozostrodontian cynodonts (Hopson and Kitching 2001; Abdala 2007). The anterodorsal development of the dentary places the alveolar edge of incisors and canine above the level of the tip of postcanine crowns. This condition is also seen in Prozostrodon (Fig. 3B), Brasilitherium (Fig. 3C), and, to a lesser degree, in Microconodon and Dromatherium (Simpson 1926; Sues 2001). In Botucaraitherium, this portion is partially broken but the canine seems to be positioned in a higher position than the postcanine line.
Splenial: The right and left splenials are preserved on each ramus. It is a laminar bone that covers the entire Meckelian groove. It extends parallel to the ventral edge of the dentary and reaches the mandibular symphysis (Fig. 2C), where it contacts its counterpart. The splenials are as developed as in Prozostrodon and other non-prozostrodontian probainognathians, being slightly dorsoventrally taller than in Brasilitherium and Riograndia.
Incisors: The lower incisor number is unknown in Candelariodon. The last left incisor is the only preserved (Fig. 2A). It is small in comparison to the canine and positioned next to it, without diastema. The crown is sub-conical, strongly curved posteriorly, with a thick layer of enamel. The distal edge seems to form a distal ridge of enamel. There is a small wear facet on the labial surface of the tip. The curved crown, with a strongly convex labial surface and ridged distal edge, is a morphology also seen in Prozostrodon.
Canine: Both canines are poorly preserved. The right canine only preserves the root and the left one less than half of the crown (Fig. 2A, B). They are the largest teeth, oval in cross section, being about two times longer than width. There is no evidence of serrated edges. The preserved portion of the left canine has a concave distal edge, indicating a posteriorly curved crown, as is seen in Prozostrodon (Fig. 3B). There is a diastema between canine and postcanines, being slightly longer on the right side as evidenced by the loss of the right pc1 (but not the left pc1; see below).
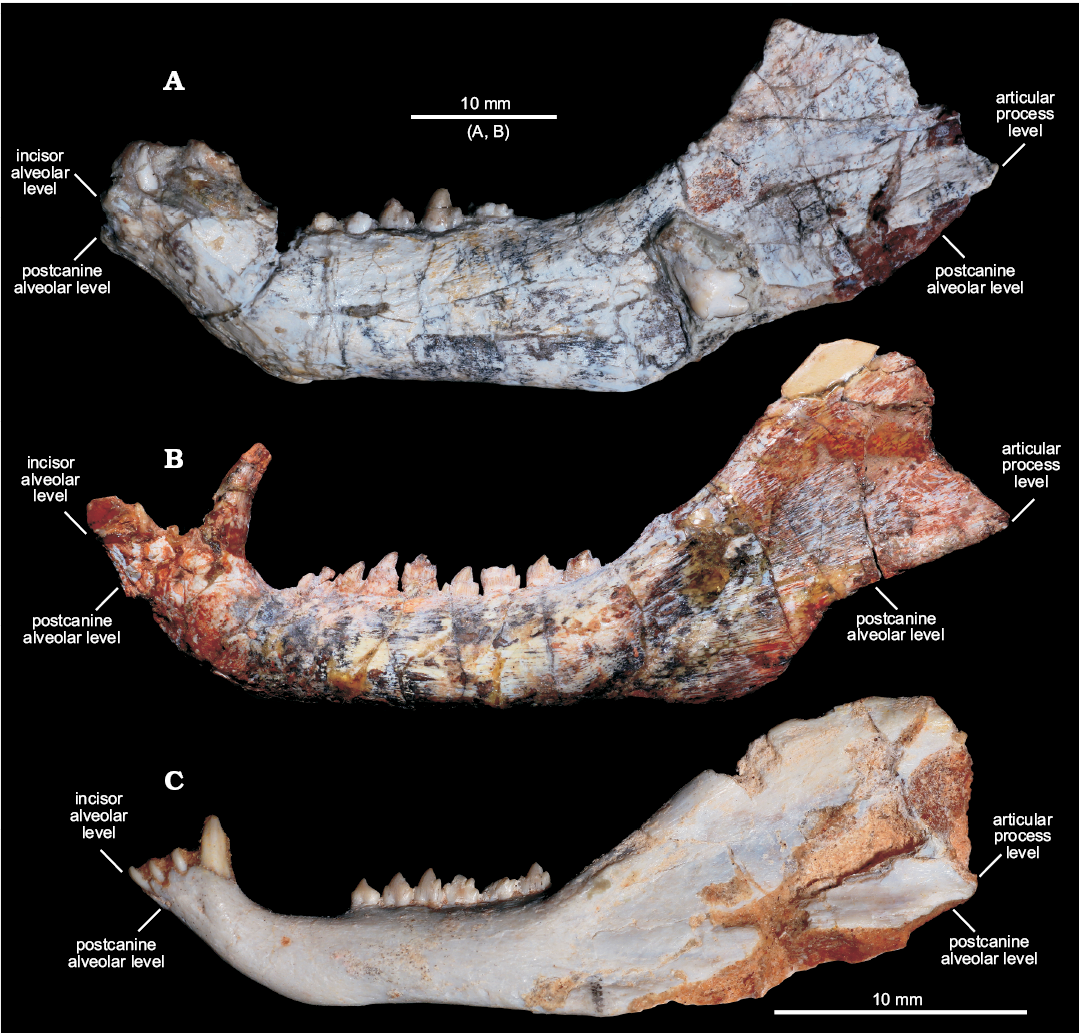
Fig. 3. Comparisons of dentaries of selected cynodonts in lateral view. A. Left dentary of Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011 from the Middle–Late Triassic of Rio Grande do Sul state, Brazil (holotype, MMACR PV-0001-T). B. Right dentary (inverted) of Prozostrodon brasiliensis (Barberena, Bonaparte, and Teixeira, 1987) from the Late Triassic of Rio Grande do Sul state, Brazil (holotype, UFRGS-PV-248-T). C. Left dentary of Brasilitherium riograndensis Bonaparte, Martinelli, Schultz, and Rubert, 2003 from the Late Triassic of Rio Grande do Sul state, Brazil (UFRGS-PV-1043-T). Abbreviations: aple, articular process level; ile, incisor alveolar level; pcle, postcanine alveolar level.
Postcanine teeth: There are empty alveoli for the right pc1, pc4–7 and left pc6–8. Complete to fairly complete tooth crowns are represented in the right pc2–3 and left pc1–5, plus an isolated tooth laying on the lateral surface of the coronoid process of the dentary, here interpreted as a posterior left lower postcanine (see below) (Figs. 2A, B, 4, 5).
The postcanine tooth rows slightly diverge posteriorly and the last tooth is placed medial of the anterior base of the coronoid process (Fig. 2C). As indicated by alveolar dimensions, the postcanine teeth increase in size gradually to the rear. The right dentary is incomplete and only has seven tooth positions, with the position for pc8 only partially preserved. The right pc1 alveolus is small in comparison to the left one and is positioned very close to anterior wall of pc2 alveolus (Fig. 4). This condition together with the fact that the right canine-postcanine diastema is longer than the left one suggests that the anterior postcanines were lost during ontogeny, increasing the size of the diastema, as seen in some prozostrodontians (e.g., Prozostrodon, Brasilodon, Sinoconodon) and some gomphodonts (e.g., Diademodon, Exaeretodon) (Hopson 1971; Crompton and Luo 1993; Luo et al. 2004; Martinelli and Bonaparte 2011). Differences between the right and left postcanine tooth rows are common in eucynodonts. For example, in Prozostrodon (Fig. 6C) the left pc1 is absent and there is a substantial discrepancy in size between the large left and small right pc2.
The left pc1 is the smallest of the series. The crown is badly preserved, hampering the recognition of discrete cusps. However, it seems simpler and transversely narrower than pc2 (Fig. 4B–D).
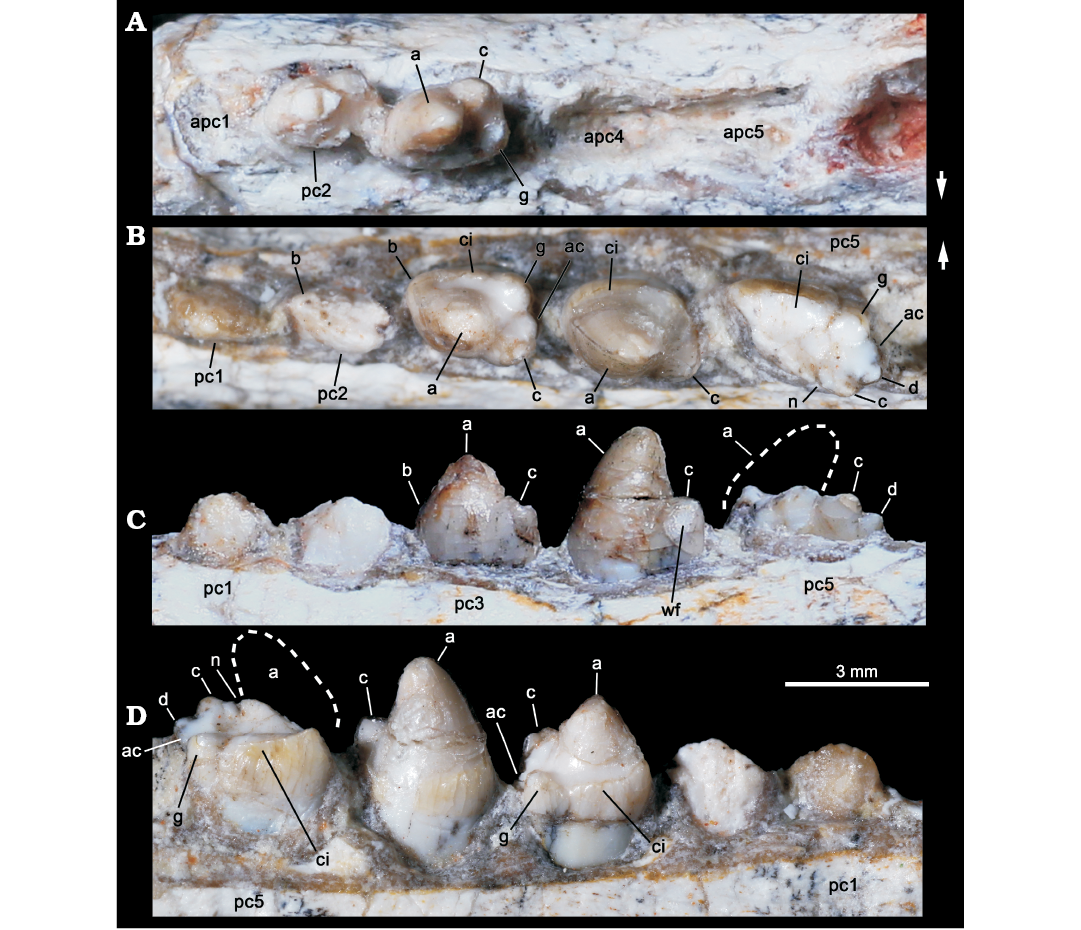
Fig. 4. Probainognathian eucynodont Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011 (holotype, MMACR PV-0001-T) from the Middle–Late Triassic of Rio Grande do Sul state, Brazil. Detail of the lower postcanine dentition. Right tooth row in occlusal view (A) and left tooth row in occlusal (B), labial (C), and lingual (D) views. The dashed line represents the shape of cusp a. Arrows indicate lingual side. Abbreviations: a–g, cusps of the crown; ac, accessory cusp; apc, postcanine alveolus; ci, cingulum; n, notch between a/c cusps; wf, wear facet.
The left pc2 crown is also broken but the crown shape can be discerned on the right one. The right pc2 has a prominent cusp a, with its tip broken off, followed by a reduce cusp c, which is slightly labially displaced (Fig. 4A). There is an evidence of a sharp mesial edge that seems to indicate the presence of a reduced cusp b. In addition, there is an evidence of a small distolingual cusp, which is interpreted as cusp g (following Crompton 1974). Due to the position of cusps c and g, the distal half of the crown is transversely wider than the mesial half. Such morphology is seen in middle postcanine teeth of sub-adult individuals of Brasilitherium (e.g., UFRGS-PV-603-T; Fig. 5C). In Prozostrodon there is also an accessory cusp on the distolingual corner of the crown of the anteriormost teeth, but due to the presence of other accessory cusps on the mesiolingual edge, the crown width is more homogeneous. Because the lingual cingular cusps are almost similar in size, the identification of a putative cusp g is not possible in Prozostrodon (Fig. 5B). Moreover, the anterior postcanine teeth of Prozostrodon have a cusp b, although very reduced, and the main cusp a is less bulbous and slightly posteriorly curved (Fig. 6C).
Both right and left pc3 of Candelariodon are well preserved. They have a pattern similar to pc2 but with accentuated features (Figs. 4, 5). The main cusp a is large, with a strongly convex mesial edge that descends to a very small cusp b, lingually located. The distal edge of cusp a is shorter than the mesial one and almost straight. It contacts the mesial edge of cusp c, without defining a conspicuous (“carnassial”) notch. The cusp c is considerably larger than cusp b and labially displaced. The distolingual accessory cusp g is slightly smaller than cusp c, but considerably larger than cusp b. In the left pc3 there is a distal accessory cusp (Fig. 4B) that is not seen in the right pc3. This distal accessory cusp is not interpreted as the cusp d based on the morphology seen in pc5 (see below). Between the distal accessory cusp and cusp g there is a “v” shaped notch, deeper than the one between accessory cusp and cusp c. One of the most conspicuous features of the pc3 is the presence of a faint, continuous cingulum connecting cusps b and g (Figs. 4B, 5A). There are two tiny notches at mid-length of the cingulum that do not define discrete crenulations or cusps. In occlusal view, pc3 has a width/length ratio of 0.7.
The pc4 has a large cusp a and small cusp c. The cusp b and accessory distolingual cusp g are not seen (Figs. 4B, 5A). However, this tooth has the same width/length ratio (= 0.69) as pc3. The lack of cusp g could be the result of breakage or perhaps due to wear (food processing and not tooth-to-tooth occlusion) on the crown. Based on the fact that the cusp g is smaller in the following teeth (i.e., pc5 and isolated posterior tooth, which have more sectorial shape), a less developed cusp g should be expected in pc4 than in pc3. A distal accessory cusp and cusp d is not discerned in pc4. In this postcanine, the lingual cingulum is better developed on the more mesial portion of the crown.
The pc5 is badly preserved with most of its mesiolabial portion broken off (Figs. 4B–D, 5A2, A3). Based on the preserved portion and the size of the alveolus, it is considered a mesiodistally larger and transversely narrower tooth than pc4–3. Oliveira et al. (2011) considered the crown of pc5 as having two mesiodistal rows of four cusps each. They considered the labial row to be made up of cusps b’, a’, c’, d’, and the lingual row of cusps b”, a”, c”, d”, separated by a mesiodistally oriented groove. That morphology was found fairly similar to the condition seen in the probainognathian Aleodon brachyramphus (Oliveira et al. 2011), which is characterized by having a row of sectorial labial cusps (homologous to the sectorial cusps of other probainognathians) and a broad lingual cingulum (i.e., cingular platform, sometimes with evident crenulations or tiny cusps), much more developed than in other known probainognathians (UMZC T906; Crompton 1955; Abdala and Giannini 2002). As shown in Figs. 4, 5, it is evident that most of the mesiolabial region of the crown is broken off. This is evident by the lack of the enamel layer that is continuous in the other parts of the crown, as also seen in the remaining postcanine teeth, and the eroded surface. Therefore, the bulk of cusp a and the entire cusp b are not preserved. Nonetheless, the notch between the distal ridge of cusp a and the mesial ridge of cusp c is still present (Figs. 4B, 5A3) and also adds supports to our interpretation. The cusp c is slightly labial, as in the remaining postcanine teeth, and smaller than the supposed size of cusp a. Distally, there is another cusp in line with cup c that is considered as cusp d. In addition, the pc5 has the accessory distolingual cusp g, which is present in previous teeth. Consequently, the morphology of pc5 is more complex than that of the preceding teeth, and it indicates that a discrete cusp d first appears in the pc5. Thus, the cusp interpreted as d in pc2–4 by Oliveira et al. (2011) is here considered as the accessory distolingual cusp g, which is kept all along the postcanine teeth (in pc1 and pc4 it is not observed due to preservation).
The pc5 also has a continuous cingulum, more transversely developed than that seen in pc3. It forms a shallow concavity between the main cusps a and c and its elevated lingual edge (Fig. 5A2, A3). Along the cingulum, at least two worn cusps are evidenced. This cingulum, however, is not comparable with the labial platform seen in the middle and posterior postcanine teeth of Aleodon, which form a large lingual platform (UMZC T906, NHMUK-PV-R- 9390; Crompton 1955). A continuous lingual cingulum is observed in Prozostrodon, Botucaraitherium, Brasilodon, and some tritheledontids (Pachygenelus, Diarthrognathus; Gow 1980). In Prozostrodon the lingual cingulum bears up to nine tiny, discrete cusps (Bonaparte and Barberena 2001) (Figs. 5, 6). That number is smaller in Botucaraitherium (Soares et al. 2014) and Brasilodon (Bonaparte et al. 2005).
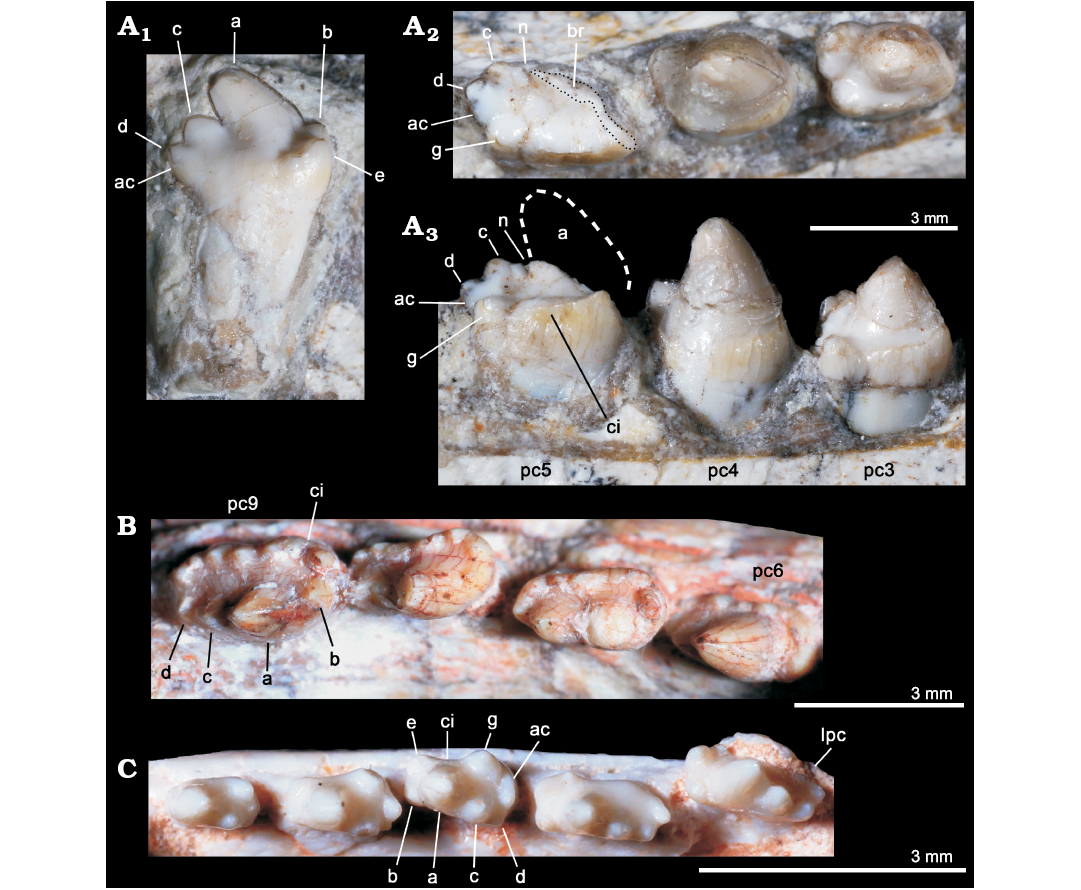
Fig. 5. Comparisons of postcanine teeth among selected eucynodonts. A. Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011 (holotype, MMACR PV-0001-T) from the Middle–Late Triassic of Rio Grande do Sul state, Brazil; detail of the lower postcanine dentition, including isolated tooth interpreted as a left posterior postcanine, in lingual view (A1) and left pc3–5 in occlusal (A2) and lingual (A3) views. B. Prozostrodon brasiliensis (Barberena, Bonaparte, and Teixeira, 1987) (holotype, UFRGS-PV-248-T) from from the Late Triassic of Rio Grande do Sul state, Brazil; last right lower postcanines in occlusal view. C. Brasilitherium riograndensis Bonaparte, Martinelli, Schultz, and Rubert, 2003 (UFRGS-PV-0603-T) from from the Late Triassic of Rio Grande do Sul state, Brazil; left lower postcanines in occlusal view. The dashed line represents the shape of cusp a. Abbreviations: ac, accessory cusp; a–g, cusps of the crown; br, broken surface; ci, cingulum; lpc, last postcanine tooth; n, notch between a/c cusps; pc, lower postcanine.
As consequence, the changes along the postcanine tooth row of Candelariodon are gradual, within a “triconodont-like” pattern, contrary to the original proposal of Oliveira et al. (2011) that recognized a drastic change of morphology only in pc5.
The isolated tooth was originally considered as an upper postcanine tooth (Oliveira et al. 2011), by comparing with gomphodonts (Diademodon and Andescynodon) and based on the original interpretation of pc5 that made difficult the allocation of a more sectorial tooth at the rear of the tooth series. We interpreted here this tooth as a posterior left postcanine that should have occupied one of the three last empty alveoli. This tooth is more sectorial (i.e., it is mesiodistally longer and transversely narrower) than the remaining ones (Fig. 5A1), as seen in some other prozostrodontians, such as Prozostrodon (Fig. 5B) and Brasilitherium (Fig. 5C). The crown of this isolated tooth of Candelariodon has a sectorial crest with main cusp a followed by cusps c and d, this latter being slightly lingually dislocated. Just lingual to the base of cusp d there is a small bulge that would be a remnant of the accessory distolingual cusp, present in more anterior teeth. The cusp a has a rounded tip and its main axis is posterodorsally inclined. The cusp b is small and low in position (Fig. 5A1). Lingually to it, there are two accessory cingular cusps, being the more mesial cusp e, as large as cusp b, and the other one relatively smaller. Differing from the pc5, the cingulum is not lingually complete, being restricted to the mesiolingual corner of the crown. This postcanine tooth has a single root, differing from the constricted root pattern seen in most, but not all (e.g., Pachygenelus; Gow 1980), prozostrodontians (Hopson and Kitching 2001; Liu and Olsen 2010).
There is no positive evidence to consider this isolated tooth as an upper postcanine. The changes in tooth crown morphology along the row are similar to that observed in Brasilitherium (e.g., UFRGS-PV-603-T; Bonaparte et al. 2003). Importantly, in Prozostrodon and Botucaraitherium the cuspidated cingulum is maintained in the last teeth, a condition not seen in Candelariodon.
The distribution of enamel on the postcanine teeth of Candelariodon is noteworthy. The external walls of the crown exhibit a thick layer of enamel with a yellowish coloration, but the inner walls of the cusps and cingulum have a whitish coloration, suggesting the lack of enamel or the presence of a very thin layer. This enamel pattern is clearly seen in both right and left pc3, left pc4–5, and the isolated tooth. Particularly the enamel, if present, is extremely thin in the lingual portion of the crown-root boundary of the isolated tooth, where the cingulum is absent.
Clear evidence of wear is seen in the left pc3, having apical wear on the main cusp a, and the left pc4, with an oval wear facet on the labial surface of cusp c (Figs. 4C, 5A2, A3).
Stratigraphic and geographic range.—Dinodontosaurus AZ of the Pinheros-Chiniquá Sequence, Santa Maria Supersequence, late Ladinian–early Carnian, Middle–Late Triassic. Pinheiro region, Rio Grande do Sul, Brazil.
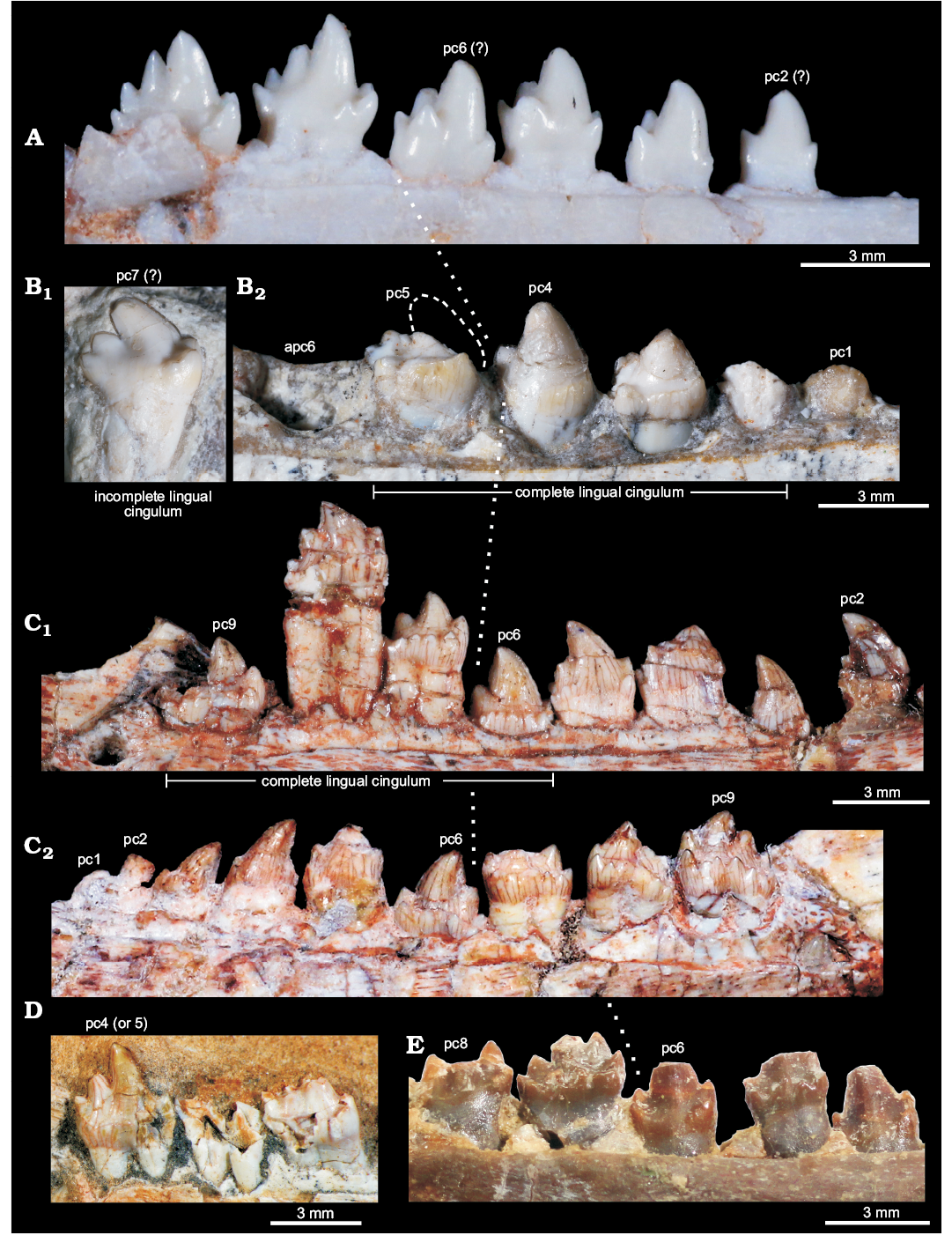
Fig. 6. Comparisons of postcanine teeth among selected eucynodonts. A. Brasilitherium riograndensis Bonaparte, Martinelli, Schultz, and Rubert, 2003 (UFRGS-PV-603-T) from the Late Triassic of Rio Grande do Sul, Brazil; left postcanines. B. Candelariodon barberenai Oliveira, Schultz, Soares, and Rodrigues, 2011 (holotype, MMACR PV-0001-T) from the Middle–Late Triassic of Rio Grande do Sul, Brazil; isolated left posterior postcanine (B1) and left pc1–5. C. Prozostrodon brasiliensis (Barberena, Bonaparte, and Teixeira, 1987) (holotype, UFRGS-PV-248-T) from the Late Triassic of Rio Grande do Sul, Brazil; left (C1) and right (C2) postcanine rows. D. Botucaraitherium belarminoi Soares, Martinelli, and Oliveira, 2014 (holotype, MMACR-PV-003-T) from the Late Triassic of Rio Grande do Sul, Brazil; last left postcanines. E. Thrinaxodon liorhinus Seeley, 1894 (NHMUK-PV-R3731) from the Early Triassic of South Africa; left postcanine row. All teeth are in lingual view. The dashed line in pc5 represents the shape of cusp a. The dotted line indicates the point where postcanine teeth change their morphology radically. Abbreviations: apc, alveolus of postcanine tooth; pc, postcanine tooth.
Discussion
Phylogenetic position of Candelariodon barberenai.—The present phylogenetic analysis resulted in four most parsimonious trees (tree length = 443 steps; consistency index = 0.47; retention index = 0.78), and the consensus tree is presented in Fig. 7. The resolution of the monophyletic groups is complete in Probainognathia (Fig. 7), recovering Candelariodon as the sister taxon of Protheriodon plus Prozostrodontia. Candelariodon plus the less inclusive clades is supported by the presence of a mediolaterally thick anterior margin of the coronoid process (character 86[1], unambiguous) and presence of lingual cingulum (character 115[0], ambiguous). This latter feature is unknown in Protheriodon and is also present in the basal cynodont Thrinaxodon (Crompton 1963; Abdala et al. 2013). Although the phylogenetic resolution of taxa crownward Probainognathus is still conflictive due to incompleteness of several taxa (e.g., Protheriodon, Prozostrodon, Therioherpeton), Candelariodon is deeply nested within Probainognathia, closely related to Protheriodon and prozostrodontians (Fig. 7). The inclusion of several putative dental features (relationships of main cusps, morphologic changes along postcanine tooth row, features on the cingulum) will be necessary to elucidate the inter-relationships of prozostrodontians and closely related forms. Up to now most analyses (including the one presented here) deal with a broad spectrum of disparate cynodonts (e.g., Hopson and Kitching 2001; Abdala 2007; Oliveira et al. 2010; Ruta et al. 2013; Martinelli et al. 2016c) and are focused on major relationships among main clades.
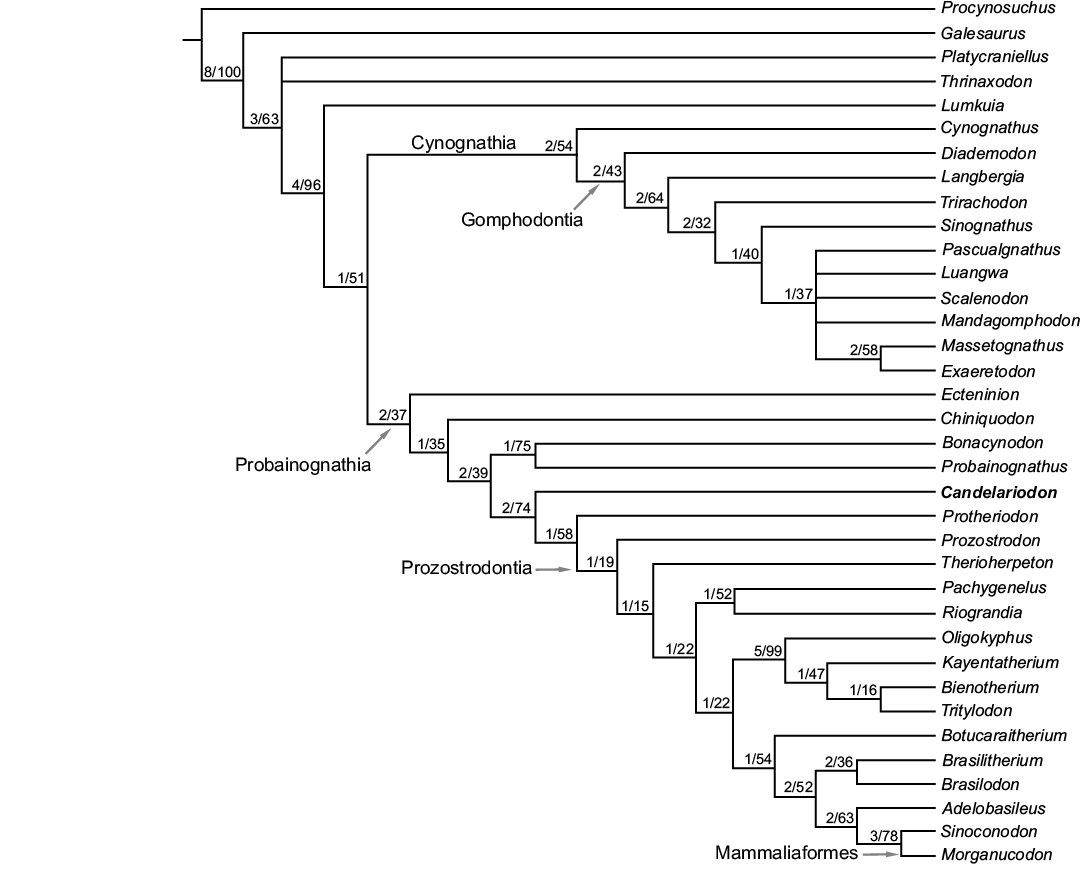
Fig. 7. Strict consensus tree of the four most parsimonious trees depicting the phylogenetic position of Candelariodon barberenai. The numbers at nodes indicate Bremer support and bootstrap values, respectively.
Dental and lower jaw features.—The complexity seen in the postcanine tooth row of Candelariodon, from the late Ladinian–early Carnian Dinodontosaurus AZ, is noteworthy when compared with coeval probainognathians, such as Chiniquodon, Bonacynodon, and Probainognathus (Fig. 8). With the exception of the chiniquodontid Aleodon, which has postcanine teeth with a well-developed lingual platform (Crompton 1955; Abdala and Giannini 2002), other basal probainognathians were more notably adapted to carnivory (e.g., Chiniquodon, Trucidocynodon, Ecteninion; Abdala and Giannini 2002; Martínez et al. 1996; Oliveira et al. 2010) or developed simple “triconodont-like” postcanines, without conspicuous lingual cingulum and constricted roots (e.g., Probainognathus, Bonacynodon; Romer 1970; Martinelli et al. 2016c) (Fig. 8). Nonetheless, the dentition of Candelariodon can be easily divided in two morphotypic patterns: (i) anterior teeth with a sub-square occlusal shape dominated by large cusp a and cusp c, plus an accessory well developed lingual cusp g, and a continuous, faint lingual cingulum; (ii) posterior teeth with an elongated crown, with a sectorial margin with cusps a to d, accessory lingual cusps (e and g), and continuous or truncated lingual cingulum.
In Candelariodon, the lingual cingulum in lower postcanines, including putative accessory cusps, is considered a derived feature among probainognathians, being present in Prozostrodon, Santacruzgnathus, Botucaraitherium, Brasilodon, Brasilitherium, Pachygenelus, Diarthrognathus (e.g., Gow 1980; Bonaparte and Barberena 2001; Bonaparte et al. 2003, 2005, 2012; Soares et al. 2014; Martinelli et al. 2016c), and early mammaliaforms (e.g., Megazostrodon, Morganucodon; Crompton 1974; Mills 1971; Parrington 1973; Gow 1986) (Figs. 5, 6, 8).
In the Early Triassic basal cynodont Thrinaxodon, posterior postcanine teeth of young individuals also develop a lingual cingulum similar to that present in probainognathians (Crompton and Jenkins 1968). The occurrence of such structures in Thrinaxodon and more derived forms highlights the plasticity of some dental features in cynodont evolution and the diversity of processes in tooth replacement mechanisms producing different kind of morphologies along ontogeny. However, the complexity along the tooth row of Candelariodon is not seen in Thrinaxodon, which in addition has less developed articular process of the dentary, reduced coronoid process, less developed masseteric fossa on the dentary, and more active tooth replacement, among several other plesiomorphies in the skull (e.g., Fourie 1974; Abdala et al. 2013; Jasinoski et al. 2015).
Candelariodon has well developed dentary as in other eucynodonts, with a tall coronoid process, large masseteric fossa, elongated articular process, thin and laminar splenial, and reduced postdentary trough (Figs. 2, 3). Its horizontal ramus is relatively tall, as commonly occurs in most eucynodonts. Among prozostrodontians, a relatively tall dentary is seen in ictidosaurs, tritylodontids, Prozostrodon (Sues 1986; Bonaparte and Barberena 2001; Martinelli et al. 2005; Martinelli and Rougier 2007; Soares et al. 2011), and, to a lesser extent, in Botucaraitherium (Soares et al. 2014), Sinoconodon (Crompton and Luo 1993; Luo 1994), and a few some early mammaliaforms (e.g., Haramiyavia; Luo et al. 2015). In contrast, Protheriodon, Santacruzgnathus, dromatheriids, Brasilitherium (Fig. 3), and some early mammaliaforms (Simpson 1926; Luo et al. 2001; Sues 2001; Bonaparte et al. 2003, 2005; Gill et al. 2014; Martinelli et al. 2016c) have slender and dorsoventrally low dentaries, with a very discrete Meckelian groove. The dentaries are fused in Candelariodon, as in most non-prozostrodontian eucynodonts (Hopson and Kitching 2001). In contrast, unfused mandibular symphysis is considered a synapomorphy of prozostrodontians (Liu and Olsen 2010). This condition is also reported in basal cynodonts, such as Procynosuchus, Thrinaxodon, and Galesaurus.
Consequently, the postcanine morphology of Candelariodon, together with other jaw features, supports the placement of this taxon close to the prozostrodontian clade (Fig. 7), and highlights the diversity of taxa crownward Probainognathus with conspicuous mammal-like features in the Middle–Late Triassic of Brazil (Fig. 8). Such unexpected diversity of disparate species (Candelariodon, Protheriodon, Prozostrodon, Therioherpeton, Riograndia, Irajatherium, Brasilitherium; Fig. 8) indicates for an adaptive radiation of a group of mammaliaform-like probainognathians, prior to the origin of the mammaliaform clade, that was only recently recognized as an important component of Late Triassic ecosystems.
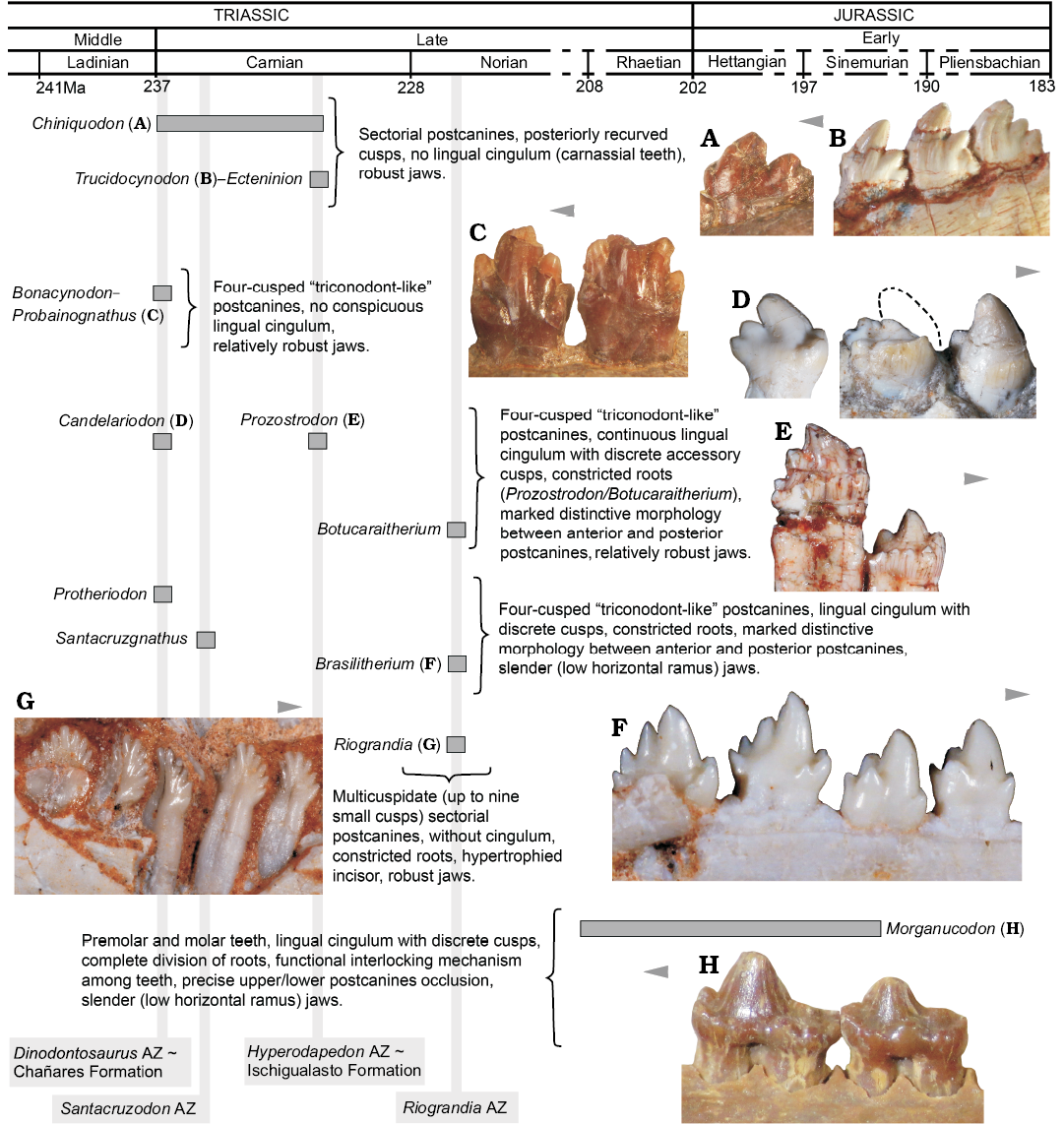
Fig. 8. Main dental features of selected non-mammaliaform probainognathians and one mammaliaform (Morganucodon) disposed in a time scale. A. Chiniquodon theotonicus, PVL 4444, left lower postcanine in labial view. B. Trucidocynodon riograndensis, UFRGS-PV-1051-T, left lower postcanines in labial view. C. Probainognathus jenseni, PVL 4445, right lower pc5–6 in lingual view. D. Candelariodon barberenai, MMACR PV-0001-T, left lower postcanines in lingual view. E. Prozostrodon brasiliensis, UFRGS-PV-0248-T, left lower postcanines in labial view. F. Brasilitherium quadrangularis, UFRGS-PV-0603-T, left lower postcanines in labial view. G. Riograndia guaibensis, UFRGS-PV-0833-T, left lower postcanines in lingual view. H. Morganucodon watsoni, NHMUK-PV-M-U273, right postcanines in lingual view. Arrows indicate mesial side of the dentition.
Conclusions
The holotype specimen of Candelariodon barberenai, a cynodont from the Middle–Late Triassic of south Brazil, was revisited and new conclusions about its dental anatomy and phylogeny were exposed. Its crown morphology has a suite of apormorphies, such as lingual cingulum with discrete cusps (e and g) and distinctive morphologies between anterior and posterior postcanine teeth, that are reminiscent of the pattern represented in non-mammaliaform prozostrodontians and basal mammaliaforms. This is also supported by a phylogenetic analysis that placed Candelariodon as the sister taxon of a clade formed by Protheriodon plus Prozostrodontia.
The radiation of probainognathians is clearly evident in the fossil record (e.g., Hopson and Kitching 2001; Bonaparte et al. 2005; Liu and Olsen 2010; Oliveira et al. 2010; Soares et al. 2011; Ruta et al. 2013; Martinelli and Soares 2016), with disparate morphotypes (e.g., ecteniniids, chiniquodontids, probainognathids, ictidosaurs, tritylodontids, dromatheriids, and “brasilodontids”) during the Middle–Late Triassic. For many years, tritheledontids and tritylodontids were the “most mammal-like” cynodont groups, diversified mostly during the Jurassic (see Luo 1994). Nonetheless, the new discoveries in Brazil and reinterpretations of already known fossils have demonstrated that non-mammaliaform prozostrodontians (e.g., Prozostrodon, Santacruzgnathus, Therioherpeton, Brasilodon) and very closely related forms (e.g., Candelariodon, Protheriodon) with triconodont-like dentition, and a morphological plan similar to some early mammaliaforms (e.g., Morganucodon, Megazostrodon) were extremely diverse during the Middle–Late Triassic. Consequently, the fossil record of non-mammaliaform probainognathians in the Triassic of Brazil is noteworthy and an unexpected amount of forms is being recovered showing a hidden and broad diversity by the late Middle and early Late Triassic.
Acknowledgements
We thank Carlos Nunes Rodrigues (MMACR) for loan of the Candelariodon holotype specimen. For access to collections we acknowledge Sandra Chapman, Pamela Gill, Pip Brewer (all NHMUK), Carlos Nunes Rodrigues, Belarmino Stefanello (all MMACR), Rodrigo Machado (MCT-DNPM), Jaime Powell and Rodrigo González (both PVL). Special thanks to Luiz Flavio Lopes (UFRGS) for skilfully taken photographs of Candelariodon, Prozostrodon, and Brasilitherium. The comments and suggestions made by the reviewers Christian Kammerer (Museum für Naturkunde, Berlin, Germany), Fernando Abdala (Instituto Miguel Lillo, San Miguel de Tucumán, Argentina), and the editor Mark D. Uhen (George Mason University, Fairfax, USA) have improved considerably the manuscript. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and the Paleontological Society International Research Program (PalSIRP)—Sepkoski Grant (2016) to AGM.
References
Abdala, F. 2007. Redescription of Platycraniellus elegans (Therapsida, Cynodontia) from the Lower Triassic of South Africa, and the cladistic relationships of eutheriodonts. Palaeontology 50: 591–618. Crossref
Abdala, F. and Giannini, N.P. 2000. Gomphodont cynodonts of the Chañares Formation: The analysis of an ontogenetic sequence. Journal of Vertebrate Paleontology 20: 501–506. Crossref
Abdala, F. and Giannini, N.P. 2002. Chiniquodontid cynodonts: systematic and morphometric considerations. Palaeontology 45: 1151–1170. Crossref
Abdala, F. and Ribeiro, A.M. 2010. Distribution and diversity patterns of Triassic cynodonts (Therapsida, Cynodontia) in Gondwana. Palaeogeography, Palaeoclimatology, Palaeoecology 286: 202–217.
Abdala, F. and Sá-Teixeira, A.M. 2004. A traversodontid cynodont of African affinity in the South American Triassic. Palaeontologia Africana 40: 11–22.
Abdala, F., Jasinoski, S.C., and Fernández, V. 2013. Ontogeny of the Early Triassic cynodont Thrinaxodon liorhinus (Therapsida): dental morphology and replacement. Journal of Vertebrate Paleontology 33: 1408–1431. Crossref
Barberena, M.C. 1977. Bioestratigrafia preliminar da Formação Santa Maria. Pesquisas 7: 111–129.
Barberena, M.C. 1981a. Novos materiais de Traversodon stahleckeri da Formação Santa Maria (Triássico do Rio Grande do Sul). Pesquisas 14: 149–162.
Barberena, M.C. 1981b. Uma nova espécie de Massetognathus (Massetognathus ochagaviae, sp. nov.) da Formação Santa Maria, Triássico do Rio Grande do Sul. Pesquisas 14: 181–195.
Barberena, M.C., Araújo, D.C., and Lavina, E.L. 1985. Late Permian and Triassic tetrapods of southern Brazil. National Geographic Research 1: 5–20.
Bertoni-Machado, C., Soares, M.B., Kislowski, F.F., and Dentzien-Dias, P.C. 2008. Uma peculiar tafocenose controlada por ação biogênica no Triássico Médio do Rio Grande do Sul, Brasil. Revista Pesquisas em Geociências 35: 57–69.
Bonaparte, J.F. 1972. Cromptodon mamiferoides, Galesauridae de la Formación Río Mendoza, Mendoza, Argentina (Therapsida–Cynodontia). Ameghiniana 9: 343–353.
Bonaparte, J.F. 2013. Evolution of the Brasilodontidae (Cynodontia–Eucynodontia). Historical Biology 25: 643–653. Crossref
Bonaparte, J.F. and Barberena, M.C. 2001. On two advanced carnivorous cynodonts from the Late Triassic of Southern Brazil. Bulletin of the Museum of Comparative Zoology 156: 59–80.
Bonaparte, J.F., Martinelli, A.G., and Schultz, C.L. 2005. New information on Brasilodon and Brasilitherium (Cynodontia, Probainognathia) from the Late Triassic of southern Brazil. Revista Brasileira de Paleontologia 8: 25–46.
Bonaparte, J.F., Martinelli, A.G., Schultz, C.L., and Rubert, R. 2003. The sister group of mammals: small cynodonts from the Late Triassic of southern Brazil. Revista Brasileira de Paleontologia 5: 5–27. Crossref
Bonaparte, J.F., Soares, M.B. and Martinelli, A.G. 2012. Discoveries in the Late Triassic of Brazil improve knowledge on the origin of mammals. Historia Natural 2012 (2): 5–30.
Bonaparte, J.F., Soares, M.B., and Schultz, C.L. 2006. A new non-mammalian cynodont from the Middle Triassic of southern Brazil and its implications for the ancestry of mammals. Bulletin New Mexico Museum of Natural History & Science 37: 599–607.
Bremer, K. 1994. Branch support and tree stability. Cladistics 10: 295–304. Crossref
Broom, R. 1905. On the use of the term Anomodontia. Records of the Albany Museum 1: 266–269.
Crompton, A.W. 1955. On some Triassic cynodont from Tanganyika. Proceeding of the Zoological Society of London 125: 617–669.
Crompton, A.W. 1963. Tooth replacement in the cynodont Thrinaxodon liorhinus Seeley. Annals South African Museum 46: 479–521.
Crompton, A.W. 1974. The dentitions and relationships of the southern African Triassic mammals, Erythrotherium parringtoni and Megazostrodon rudnerae. Bulletin of the British Museum (Natural History), Geology 24: 397–437.
Crompton, A.W. and Jenkins, F.A. 1968. Molar occlusion in Late Triassic mammals. Biological Reviews 43: 427–458.
Crompton, A.W. and Luo, Z.-X. 1993. Relationships of the Liassic mammals Sinoconodon, Morganucodon oehleri, and Dinnetherium. In: F.S. Szalay, M.J. Novacek, and M.C. McKenna (eds.), Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials, 30–44. Springer-Verlang, New York.
Felsestein, J. 1985. Phylogenies and the comparative methods. The American Naturalist 125: 1–15. Crossref
Fiorelli, L.E., Ezcurra, M.D., Hechenleitner, E.M., Argañaraz, E., Taborda, J.R.A., Trotteyn, M.J., von Baczko, M.B., and Desojo, J.B. 2013. The oldest known communal latrines provide evidence of gregarism in Triassic megaherbivores. Scientific Reports 3: 1–7. Crossref
Fourie, S. 1974. The cranial morphology of Thrinaxodon liorhinus Seeley. Annals of the South African Museum 65: 337–400.
Gill, P.G., Purnell, M.A., Crumpton, N., Robson Brown, K., Gostling, N.J., Stampanoni, M., and Rayfield, E.J. 2014. Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature 512: 303–305. Crossref
Goloboff, P. and Catalano, S. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Gordon, M., Jr. 1947. Classificação das formações gondwânicas do Paraná, Santa Catarina e Rio Grande do Sul. Notas Preliminares e Estudos, DNPM/DGM 38: 1–20.
Gow, C.E. 1980. The dentitions of the Tritheledontidae (Therapsida: Cynodontia). Proceedings Royal Society of London B 208: 461–481. Crossref
Gow, C.E. 1986. A new skull of Megazostrodon (Mammalia, Triconodonta) from the Elliot Formation (Lower Jurassic) of Southern Africa. Palaeontologia Africana 26: 13–23.
Hopson, J.A. 1971. Postcanines replacement in the gomphodont cynodont Diademodon. Zoological Journal of Linnean Society 50 (Supplement 1): 1–21.
Hopson, J.A. 1990. Cladistic analysis of therapsid relationships. Journal of Vertebrate Paleontology 10 (Supplement 3): 28A.
Hopson, J.A. and Kitching, J.W. 2001. A probainognathian cynodont from South Africa and the phylogeny of nonmammalian cynodonts. Bulletin of the Museum of Comparative Zoology 156: 5–35.
Horn, B.L.D., Melo, T.M., Schutlz, C.L., Philipp, R.P., Kloss, H.P., and Goldberg, K. 2014. A new third-order sequence stratigraphic framework applied to the Triassic of the Paraná Basin, Rio Grande do Sul, Brazil, based on structural, stratigraphic and paleontological data. Journal of South American Earth Sciences 55: 123–132. Crossref
Jasinoski, S., Abdala F., and Fernandez, V. 2015. Ontogeny of the Early Triassic cynodont Thrinaxodon liorhinus (Therapsida): Cranial morphology. The Anatomical Record 298: 1440–1464. Crossref
Kemp, T.S. 1982. Mammal-like Reptiles and the Origin of Mammals. 363 pp. Academic Press, London.
Liu, J. and Abdala, F. 2014. Phylogeny and taxonomy of the Traversodontidae. In: C.F. Kammerer, K.D. Angielczyk, and J. Fröbisch (eds.), Early evolutionary history of the Synapsida, 255–279. Springer, London. Crossref
Liu, J. and Olsen, P.E. 2010. The phylogenetic relationships of Eucynodontia (Amniota, Synapsida). Journal of Mammalian Evolution 17: 151–176.
Liu, J., Soares, M.B., and Reichel, M. 2008. Massetognathus (Cynodontia, Traversodontidae) from the Santa Maria Formation of Brazil. Revista Brasileira de Paleontologia 11: 27–36. Crossref
Luo, Z.-X. 1994. Sister-group relationships of mammals and transformations of diagnostic mammalian characters. In: N.C. Frazer and H.D. Sues (eds.), In the Shadow of the Dinosaurs. Early Mesozoic Tetrapods, 98–128. Cambridge University Press, Cambridge.
Luo, Z.-X., Crompton, A.W., and Sun, A.-L. 2001. A new mammaliaform from the Early Jurassic and evolution of the mammalian characteristics. Science 292: 1535–1540. Crossref
Luo, Z.-X., Gatesy, S.M., Jenkins, F.A. Jr., Amaral, W.W., and Shubin, N.H. 2015. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. PNAS 112: E7101–E7109.
Luo, Z.-X., Kielan-Jaworowska, Z., and Cifelli, R.L. 2004. Evolution of dental replacement in mammals. Bulletin of the Carnegie Museum of Natural History. 36: 159–175. Crossref
Marsicano, C.A., Irmis, R.B., Mancuso, A.C., Mundile, R., and Chemale, F. 2016. The precise temporal calibration of dinosaur origins. PNAS 113: 509–513. Crossref
Martinelli, A.G. and Bonaparte, J.F. 2011. Postcanine replacement in Brasilodon and Brasilitherium (Cynodontia, Probainognathia) and its bearing in cynodont evolution. In: J. Calvo, J. Porfiri, B. González Riga, and D. Dos Santos (eds.), Dinosaurios y Paleontología desde América Latina, 179–186. Universidad Nacional de Cuyo Press, Mendoza.
Martinelli, A.G. and Rougier, G.W. 2007. On Chaliminia musteloides Bonaparte (Cynodontia, Tritheledontidae) and the phylogeny of the Ictidosauria. Journal of Vertebrate Paleontology 27: 442–460.
Martinelli, A.G. and Soares, M.B. 2016. Evolution of South American cynodonts. Contribuciones del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 6: 183–197.
Martinelli, A.G., Bonaparte, J.F., Schultz, C.L., and Rubert, R. 2005. A new tritheledontid (Therapsida, Eucynodontia) from the Late Triassic of Rio Grande do Sul (Brazil) and its phylogenetic relationships among carnivorous non-mammalian eucynodonts. Ameghiniana 42: 191–208.
Martinelli, A.G., Dias-Da-Silva, S., Pinheiro, F.L., Da-Rosa, Á.A.S., Schultz, C.L., Modesto, S.P., and Soares, M.B. 2016a. Cynodonts from the Sanga do Cabral Supersequence revisited: are they really present in the Early Triassic of Brazil? Reunião Anual da Sociedade Brasileira de Paleontologia, Paleo RS, Santa Maria. Caderno de Resumos: 68–69.
Martinelli, A.G., Kammerer, C.F., Melo, T.P., Paes Neto, V.D., Ribeiro, A.M., Da-Rosa, Á.A.S., Schultz, C.L., and Soares, M.B. 2016b. Rescued from the collections: the presence of the African cynodont Aleodon (Cynodontia, Probainognathia) in the Triassic of southern Brazil, hidden for over 30 years. In: Reunião Anual da Sociedade Brasileira de Paleontologia, Paleo RS, Santa Maria. Caderno de Resumos: 78–79.
Martinelli, A.G., Kammerer, C.F., Melo, T.P., Paes Neto, V.D., Ribeiro, A.M. Da-Rosa, Á.A.S., Schultz, C.L., and Soares, M.B. 2017. The African cynodont Aleodon (Cynodontia, Probainognathia) in the Triassic of southern Brazil and its biostratigraphic significance. PloS ONE 12 (6): e0177948. Crossref
Martinelli, A.G., Soares, M.B., and Schwanke, C. 2016c. Two new cynodonts (Therapsida) from the Middle–early Late Triassic of Brazil and comments on South American probainognathians. PloS ONE 11 (10): e0162945. Crossref
Martínez, R., May, C.L., and Forster, C.A. 1996. A new carnivorous cynodont from the Ischigualasto Formation (Late Triassic, Argentina), with comments on eucynodont phylogeny. Journal of Vertebrate Paleontology 16: 271–284. Crossref
Melo, T., Martinelli, A.G., and Soares, M.B. 2014. Novo cinodonte traversodontídeo da Zona-Associação de Dinodontosaurus (Sequência Santa Maria 1, Ladiniano) do Triássico do Rio Grande do Sul, Brasil. Paleontologia em Destaque, Edição Especial 2014: 82.
Mills, J.R.R. 1971. The dentition of Morganucodon. Zoological Journal of Linnean Society 50 (Supplement 1): 26–63.
Oliveira, T.V., Soares, M.B., and Schultz, C.L. 2010. Trucidocynodon riograndensis gen. nov. et sp. nov. (Eucynodontia), a new cynodont from the Brazilian Upper Triassic (Santa Maria Formation). Zootaxa 2382: 1–71.
Oliveira, T.V., Soares, M.B., Schultz, C.L., and Rogrigues, C.N. 2011. A new carnivorous cynodont (Synapsida, Therapsida) from the Brazilian Middle Triassic (Santa Maria Formation): Candelariodon barberenai gen. et sp. nov. Zootaxa 3027: 19–28.
Owen, R. 1861. Palaeontology, or a Systematic Summary of Extinct Animals and their Geological Relations. 463 pp. Adam and Black, Edinburgh. Crossref
Parrington, F.R. 1973. The dentition of earliest mammals. Zoological Journal of the Linnean Society 52: 85–95. Crossref
Philipp, R.P., Closs, H., Schultz, C.L., Basei, M., Horn, B.L.D., and Soares, M.B. 2013. Proveniência por U-Pb LA-ICP-MS em zircão detrítico e idade de deposição da Formação Santa Maria, Triássico da Bacia do Paraná, RS: evidências da estruturação do Arco do Rio Grande. Anais VIII Symposium International on Tectonics 2013: 154–157.
Reichel, M., Schultz, C.L., and Soares, M.B. 2009. A new traversodontid cynodont (Therapsida, Eucynodontia) from the Middle Triassic Santa Maria Formation of Rio Grande do Sul, Brazil. Palaeontology 52: 229–250. Crossref
Rodrigues, P.G., Ruf, I., and Schultz, C.L. 2013. Digital Reconstruction of the Otic Region and Inner Ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. Journal of Mammalian Evolution 20: 291–307. Crossref
Rodrigues, P.G., Ruf, I., and Schultz, C.L. 2014. Study of a digital cranial endocast of the non-mammaliaform cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian brain. Palaontologische Zeitschrift 88: 329–352. Crossref
Ruf, I., Maier, W., Rodrigues, P.G., and Schultz, C.L. 2014. Nasal anatomy of the non-mammaliaform cynodont Brasilitherium riograndensis (Eucynodontia, Therapsida) reveals new insight into mammalian evolution. The Anatomical Record 297: 2018–2030.
Romer, A.S. 1970. The Chañares (Argentina) Triassic reptile fauna. A chiniquodontid cynodont with incipient squamosal-dentary jaw articulation. Breviora 344: 1–18.
Romer, A.S. and Price, L.I. 1944. Stahleckeria lenzii, a giant Triassic Brazilian dicynodont. Bulletin of the Museum of Comparative Zoology 93: 463–491.
Ruta, M., Botha-Brink, J., Mitchell, S.A., and Benton, M.J. 2013. The radiation of cynodonts and the ground plan of mammalian morphological diversity. Proceedings of the Royal Society B, Biological Sciences 280: 20131865. Crossref
Schultz, C.L., Scherer, C.M.S., and Barberena, M.C. 2000. Biostratigraphy of southern Brazilian Middle–Upper Triassic. Revista Brasileira de Geociências 30: 491–494.
Simpson, G.G. 1926. Mesozoic Mammalia V. Dromatherium and Microconodon. American Journal of Science 12: 87–108. Crossref
Soares, M.B., Martinelli, A.G., and Oliveira, T.V. 2014. A new prozostrodontian cynodont (Therapsida) from the Late Triassic Riograndia Assemblage Zone (Santa Maria Supersequence) of southern Brazil. Anais da Academia Brasileira de Ciências 86: 1673–1691. Crossref
Soares, M.B., Schultz, C.L., and Horn, B.L.D. 2011. New information on Riograndia guaibensis Bonaparte, Ferigolo and Ribeiro, 2001 (Eucynodontia, Tritheledontidae) from the Late Triassic of southern Brazil: anatomical and biostratigraphic implications. Anais da Academia Brasileira de Ciências 83: 329–354. Crossref
Sues, H.-D. 1986. The skull and dentition of two tritylodontid synapsids from the Lower Jurassic of western North America. Bulletin of the Museum of Comparative Zoology 151: 217–268.
Sues H.-D. 2001. On Microconodon, a Late Triassic cynodont from the Newark Supergroup of Eastern North America. Bulletin of the Museum of Comparative Zoology 156: 37–48.
Zerfass, H., Lavina, E.L., Schultz, C.L., Garcia, A.J.V., Faccini, U.F., and Chemale F. Jr. 2003. Sequence stratigraphy of continental Triassic strata of Southernmost Brazil: a contribution to Southwestern Gondwana palaeogeography and palaeoclimate. Sedimentary Geology 161: 85–105. Crossref
Changes in character-states for Protheriodon estudianti:
Character 11. Changes from (?) to (1). The braincase has a moderate lateral expansion.
Character 22. Changes from (?) to (0). Low posteroventral process of the jugal, as seen in the right side of the skull.
Character 36. Changes from (0) to (1). Length of secondary palate represents more than 45% of skull length.
Character 107. Changes from (0) to (1). Constricted lower postcanine roots, as seen in posterior preserved teeth.
Change in character-states for Prozostrodon brasiliensis:
Character 17. Changes from (?) to (2). Low zygomatic arch based on the preserved anterior base of the arch.
Changes in character-states for Brasilitherium riograndensis:
Character 94. Changes from (0) to (0+1). The sample of specimens of this species shows changes in incisor number along ontogeny.
Character 95. Changes from (1) to (0+1). The sample of specimens of this species shows changes in incisor number along ontogeny.
Data matrix used in the phylogenetic analysis, based on Liu and Olsen (2010), plus the modifications of Martinelli et al. (2016c) and those from Appendix 1.
Procynosuchus delaharpeae 0000000100000000000000000000000000000000000000000000000000000001[01]00000000000000000000001000000000100010000000--00-0000000000000-00000000000000002
Galesaurus
planiceps
0100000000000000001000001000011000000000000000000000000000000000000000000000010000100 000000001100100000010000--00-1000000001100????00??0??0000000
Thrinaxodon
liorhinus
01[01]0000000000000000000000000001110000000000000000000000000000000000000001001010000 100000110001100000010000000--00-1000000001100-00000000000?0000?
Platycraniellus elegans
0110000000000000001100000000010110000000??00000000?0000000000000000000101??101000?101 0?0??0001?0?100000000000--0?-0?000??????????????????????????
Cynognathus
crateronotus
00000000000000001021002110000011100001011100010100?0000000000000100000111001011011201 011120001101000100010000--00-1001000000111000000110000000000
Diademodon
tetragonus
[01]0000000000000001022012111000111100000011100010100000000[01]000000000000000100101111 120101112113110100012001000100100?000100001111000000110000000000
Trirachodon
berryi
11100100[01]00000001121011111010011101002011100010100000002100000000000110111020110111 010111211311010001211100020110100101???01111000000????00000000
Sinognathus
gracilis
1020?100100000??11010011110?1?1?1010?0011000010100???????00000?000?01101110201101110101 1??113120000002?0?00?201100?0201??????????????????????????
Langbergia modisei
001000000000000001210111110100111010000110000101?0?000?21??0000000001?0?11????10111010 111211311010001200100020110100101?????1????????????????????
Pascualgnathus
polanskii
1020?100100000001122012111011?1110100001??000101?0???0?2?00000???0?0??????????[01]0?1201 011??113210000002[12][12]000-110100-12010???1101??0000??1000000000
Luangwa
drysdalli
??00?1000?0000010121001111????1110?00001???00101000000?2??00000???0?????? ?????1011201011 ??113110100012[12][12]000-202100-12010???110110000???1100000000
Massetognathus
pascuali
0111110010000000110101111101121110200001000001010000001211000000000011011102011011[12] 01011??11311021110211000-222100-12010000 0101101000??1100000000
Exaeretodon
argentinus
00111110100000111121012111011211101[01]00010?00010100010002110000000000?101????011011211011??113210010102[12][12 ]000-120100-122100000001101001111100000000
Scalenodon angustifrons
??10?1?0000000??1101012111?????1101?0??1??00010100?0000211000000?000??0?????0?[01]0?12??0 11??113110100012[12][12]000-2 02100-1201?????????? ????????????????
Mandagomphodon hirschoni
???0010?????001?11?????111011211101?0?0?0??00????????????1?000?0?????1??1?0?0?[01]??1???011 ??11 3220001002[12][12]000-222100-1201??????????????????????????
Lumkuia
fuzzi
??1000101000000?00000000010?1201101001010100010010?00000010000000000000010000110112010 00120001100000000010000--00-10000?????001????????????0?????
Chiniquodon theotonicus
11101010100000101011000001011[12]11112100011000010100000001000000000000?1?01???11101120 1011120001100000000010000--00-100000??000011010001110000?0000
Ectenion lunensis
001??0021000002000000000000?1[01]11100002011100010100?100010100000000001100110211101100 1011??00011000001[01]0010000--00-?00000???1??110??0?????0???????
Probainognathus
jenseni
011010021000001001011000010112111110000111000100000000110000 00000000110011021110210010 11120001100000000000000--00-10110000?000110???0??11000?0000
Bonacynodon schultzi
??1??0?21??000??01011000?10??211111000?????0?????0????????????0??000???????????0?0001?1112? 001?0000?100000000--00-?0110??????????????????????????
Therioherpeton
cargnini
?????0121?11122?2100??0????????1111???????????????????????????????????????????????????????000 ????????0?0?1100--00-?00000??1?00?????????1111110011
Riograndia guaibensis
20131012111112212100100000111201112120110001020000?0000102000010001121102?13???0003011 11??0012110011001001100--00-10100??????????????????????????
Pachygenelus monus
20131012111112212100100000011201112120110001020000100001020000101??121102213132020301 111120012210010001000010--00-002001???0001111101??1111111111
Prozostrodon brasiliensis
21?010?2????122121?????????112?1111?1????????????????????????? ???????????????????0301111??00 10000000111001100--00-001000???000?????0???1111110000
Botucaraitherium belarminoi
?????0??????????????????????????????????????????????????????????? ????????????????0???11????01? ?????001?001110--01-00??0??????????????????????????
Protheriodon estudianti
??0??0????1?????2000100???????011111???????????????????????????????????????????0?1?0??11????? 100001?00?000100--00-?010????????????? ??????????????
Brasilodon
quadrangularis
[01]000?0121121122120001000?00?1201111112110001021001?0?112220?00111011211022131320?030 1111??0011100001011001110--01-001[01][01]??????0111??11????????????
Brasilitherium
riograndensis
0000?0121121122120001000000?1201111102110001021011?01112220???11?0112110221313201030111 1??001[01][01]00001011001110--01-001[01]1????????????11????????1111
Tritylodon longaevus
102-1111110112211102001111011211102122110000110110110102 1211110110103110220312020031111 1??1132210-22-222-32-2-1100-03221??????210??????1?????1111
Oligokyphus
major
[12]??-1111?10112???102010110?1?2?1??21????????110?10110102?21111011000310022031202003111 11??1132110-22-222-32-2-1100-0322111100?2101111??1121111111
Bienotherium
yunnanense
102-11111101122111?201?111011211102122110000110110110?02?01111?110?031??22131?0200311111 ??1132110-22-222-32-2-1100-03221??????210??11??????111111
Kayentatherium
wellesi
102-11111?0112211102011111111201102122110000110110110102121111?110?03110221312020031111 1121132110-22-222-32-2-1100-0322?11100021011?1111121??1111
Adelobasileus
cromptoni
???????01121?2????????????????0??????2110001021011?21112210000101110??????????????????????? ?????????????????????????????????????????????????????
Sinoconodon rigneyi
0002?0101121122120001000?01?1211112102110011031011?21112221011101010????????0?302030111 2??2001000001001002210--00-10101???????????? ??????????????
Morganucodon spp.
0?02?0101121122120002?00001111111121021100110320111211122201121111112110221324302030111 2122221000001011002210--01-0010111111001111111??1121111111
Candelariodon
barberenai
?????????????????????????????????????????????????????????????????????????????????13??111??????? 00??00??00?0??--?0-00?????????????????????????????
Acta Palaeontol. Pol. 62 (3): 527–542, 2017
https://doi.org/10.4202/app.00344.2017