

The Eocene South American metatherian Zeusdelphys complicatus is not a protodidelphid but a hatcheriform: Paleobiogeographic implications
LEONARDO M. CARNEIRO and ÉDISON VICENTE OLIVEIRA
Carneiro, L.M. and Oliveira, É.V. 2017. The Eocene South American metatherian Zeusdelphys complicatus is not a protodidelphid but a hatcheriform: Paleobiogeographic implications. Acta Palaeontologica Polonica 62 (3): 497–507.
Zeusdelphys complicatus is one of the most enigmatic metatherians from the Itaboraí Basin. The type and only known specimen was previously regarded as the upper dentition of Eobrasilia; an M4 of a new taxon; an M3 of a Kollpaniidae (now regarded as a group of “condylarths”); a probable M1 of an incertae sedis taxon; and as an M1 of a Protodidelphidae. Herein, we present a morphological review of the dental structures of Zeusdelphys complicatus, presenting new interpretations and comparing it with other North and South American taxa. We also perform a phylogenetic analysis in order to test the affinities of Zeusdelphys and the validity of most studied characters. The results recovered Zeusdelphys complicatus as more closely related to Hatcheritherium alpha than to any other metatherian. Glasbiidae were recovered as the sister lineage of Protodidelphidae within Didelphimorphia, as true marsupials. Ectocentrocristus was recovered as the sister taxon of Zeusdelphys + Hatcheritherium, as a Hatcheriformes. The analysis recovered this suborder as an independent lineage from Polydolopimorphia, being more closely related to “Alphadontidae”. The affinities with Protodidelphidae are a result of convergent evolution, as Zeusdelphys is more closely related to Hatcheritherium alpha from the Late Cretaceous of North America. The results support a North American origin for Hatcheriformes. The presence of strong sea-level lowstands and islands in the Caribbean Plate during the Late Cretaceous provide valid data to support a faunal interchange between Americas during the latest Late Cretaceous. Based on the results, Zeusdelphys represents a South American early Eocene surviving Hatcheriformes.
Key words: Mammalia, Metatheria, Hatcheriformes, Zeusdelphys, paleobiogeography, systematics, Eocene, Itaboraí Basin.
Leonardo M. Carneiro [leonardo.carneiro8@gmail.com], PALEOLAB (Laboratório de Paleontologia), Departamento de Geologia, Centro de Tecnologia e Geociências, Universidade Federal de Pernambuco, UFPE, Av. Acadêmico Hélio Ramos s/n, CEP 50740-530, Recife, PE, Brazil, and Laboratório de Paleontologia e Paleoecologia da Sociedade de História Natural, Travessa Florêncio Augusto Chagas nº 8B, 2560-230 Torres Vedras, Portugal.
Édison Vicente Oliveira [vicenteedi@gmail.com], PALEOLAB (Laboratório de Paleontologia), Departamento de Geologia, Centro de Tecnologia e Geociências, Universidade Federal de Pernambuco, UFPE, Av. Acadêmico Hélio Ramos s/n, CEP 50740-530, Recife, PE, Brazil.
Received 9 February 2017, accepted 1 May 2017, available online 6 July 2017.
Copyright © 2017 L.M. Carneiro and É.V. Oliveira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Zeusdelphys complicatus Marshall, 1987, currently considered the largest “opossum-like” species from Itaboraí, is recognized only by a single, isolated upper molar (M1). The tooth of this species was firstly considered to be the upper dentition of Eobrasilia coutoi Simpson, 1947, as it was compatible in size (Marshall 1984). Three years later, Marshall (1987) considered this tooth as an M4 of a new genus et species, Zeusdelphys complicatus, with the generic name identifying the largest and most important Itaboraí taxon due to its size, and “complicatus” in reference to the wrinkled enamel. Marshall et al. (1990) considered Zeusdelphys as a basal Paucituberculata, in the subfamily Kollpaniidae (currently, this family is considered as a subfamily of “condylarths”), reconsidering this tooth as an M3. Goin et al. (1998) identified this tooth as an M1; however, due to the autapomorphic state of this taxon, the authors considered it as an incertae sedis Didelphimorphian. Oliveira and Goin (2011) recovered Zeusdelphys as a Protodidelphidae, more closely related to Protodidelphis and Carolocoutoia than to Guggenheimia. The same conclusion was recovered in Oliveira et al. (2016). Zeusdelphys was grouped with Protodidelphidae based on the presence of wrinkled enamel, absence of StC, bunoid molars, large and inflated StB and StD, eccentric protocone, and absence of conules.
Oliveira and Goin (2011) superficially discussed the shared similarities between Zeusdelphys complicatus and Hatcheritherium alpha Clemens, 1966, from the Lancian NALMA (Late Cretaceous) of North America (Clemens 1966). Following the authors, both taxa share an open centrocrista, which connects to a centrally placed cuspule; but this taxon could not be assigned to Polydolopimorphia as it lacks a more developed protocone, a metaconule, and the transversally twinning of stylar cusp B (StB) and stylar cusp D (StD) with para- and metacone, respectively. However, Zeusdelphys and Hatcheritherium share all mentioned characters, including a labial compression of StB and StD, the presence of three developed accessory cusps on the postmetacrista, broad anterobasal cingulum, and the presence of lingual and labial accessory crests on the distal and mesial borders of StB and StD, respectively.
The affinities of Hatcheritherium and Glasbius as Hatcheriformes, and the inclusion of Ectocentrocristus as a basal Polydolopimorphia were not supported in recent phylogenies. The grouping of these taxa with these lineages was made a priori (i.e., without a phylogenetic analysis supporting it) by Case et al. (2005). Williamson et al. (2012, 2014) recovered Glasbius as an independent taxon from Hatcheritherium, which puts in doubt the affinities of both taxa as a monophyletic lineage; and Ectocentrocristus was recovered in both studies as a Herpetotheriidae. In addition, Zeusdelphys and Hatcheritherium have never been included together in a phylogenetic analysis.
Herein, we present a comparative study of dental characters of Zeusdelphys, Hatcheritherium, Ectocentrocristus, Glasbiidae, Protodidelphidae, “Alphadontidae”, and Polydolopimorphia in order to try to elucidate the affinities of Zeusdelphys.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York, USA; MCT, Museu de Ciências da Terra, Rio de Janeiro, Brazil; UM, University of Minnesota, Minneapolis, USA; UW, University of Wyoming, Laramie, USA; YPM, Yale University Peabody Museum, New Haven, USA.
Other abbreviations.—EECO, early Eocene Climatic Optimum; M, upper molars; NALMA, North American Land Mammal Age; PETM, Paleocene–Eocene Thermal Maximum; SALMA, South American Land Mammal Age; StB–D, stylar cusps B–D.
Material and methods
The new matrix was constructed based on characters that have never been proposed or studied in any phylogeny published so far (e.g., pyramidal cusps, vertical posterior edge of StD and bifurcated medium crest on the StD). The codification of the characters was based on the conclusions of previous studies (Fox 1987; Marshall et al. 1990; Johanson 1996; Cifelli and Muizon 1997; Oliveira and Goin 2011; Oliveira et al. 2016), though with changings in codification when necessary (e.g., mixing of characters in a single one). The matrix is mainly based on dental characters from upper and lower dentition of fossil and living Metatheria.
The characters of Zeusdelphys were recovered analysing the original material (type specimen), casts and the SEM picture present in Oliveira and Goin (2011). The North American taxa were studied based on their original descriptions and revisions, stereo and SEM pictures present in the literature (Clemens 1966; Case et al. 1990; Cifelli 1990; Johanson 1996; Davis 2007). It is important to comment that SEM pictures allow the identification of several structures that are not visible in stereo pictures or stereomicroscopes (e.g., three accessory cusps associated with the postmetacrista in several “Alphadontidae” and vertical edge of StD in several “Alphadontidae” and Ectocentrocristus foxi, described and commented below). The SEM pictures represent the most trustful way to study tiny metatherians teeth, as several structures and details of the crown can be securely identified with these pictures.
We conducted a traditional search using TNT 1.1 (Goloboff et al. 2008) with 1000 replications and 1000 random seeds, saving 10 trees for each replication. The morphological matrix is available at supplementary materials. For Bremer supports and tree scores we used TNT 1.1. The tree indexes were calculated using PAUP 4.0a152. The phylogeny presents 79 characters and 37 metatherian taxa, including 5 extant genera, from the Cretaceous and Cenozoic of North America and the Southern hemisphere (i.e., South America, Antarctica, and Australia).
The best way to test the validity of a character is testing it in a phylogenetic analysis (Simões et al. 2017). The idea that two or more different morphologies are not homologous can only be set after a phylogenetic analysis. The presence of highly homoplastic characters, if pointed like this by the analysis, will be “weighted” (not to be confused with “imply weighting” mechanism of phylogenies) with other characters in order to properly elucidate the evolutionary trends of a lineage. This study is based on the results of the phylogenetic analysis, as a priori considerations are not accepted in a systematic study.
Systematic palaeontology
Mammalia Linnaeus, 1758
Metatheria Huxley, 1880
“Alphadontia” sensu Archer (1984)
Hatcheriformes Case, Goin, and Woodburne, 2005
Family incertae sedis
Genus Zeusdelphys Marshall, 1987
Type species: Zeusdelphys complicatus Marshall, 1987; monotypic, see below.
Zeusdelphys complicatus Marshall, 1987
Fig. 1.
1987 Zeusdelphys complicatus sp. nov.; Marshall 1987: 124, fig. 45.
2011 Zeusdelphys complicatus Marshall, 1987; Oliveira and Goin 2011: 121, fig. 14.
Holotype: MCT 2830-M, M1 tooth.
Type locality: Municipality of São José de Itaboraí, Rio de Janeiro, Brazil (Bergqvist et al. 2009).
Type horizon: Fresh water travertine deposits of Itaboraí Formation, early Eocene, Itaboraian SALMA (ca. 53–50 Ma; Woodburne et al. 2014).
Material.—Holotype is the only known specimen.
Diagnosis.—Differs from other Metatheria in the following combination of characters: large, lingually shifted and centrally placed StC; pyramidal shape of StB and StD; presence of labial and lingual accessory crests of StB and StD; developed labial cingulum, oblique crest that separates the parastyle from the anterobasal cingulum; broad and well developed anterobasal cingulum; lingual border of the metacone markedly more lingual in position than the lingual edge of the paracone; presence of three large supernumerary cusps on the postmetacrista; reduced stylar shelf and postmetacrista; very compressed talon, and protocone not eccentric. Differs from Protodidelphidae and Glasbiidae in the absence of eccentric protocone, basal expansions of upper molars, the presence of pyramidal StB and StD, lingually shifted StC, and larger size of StC (Fig. 1).
Description.—The molar is 8.30 mm in length and 8.47 mm in width; it presents relatively low cusps, wrinkled enamel, pyramidal shape of StB and StD; concave mesial borders of StB and StD; StB and StD with labial and lingual accessory crests; bifurcated medium crest of StD; large and lingually shifted StC; developed StE; three well-developed accessory cusps on the postmetacrista; reduced postmetacrista; developed labial expansion; discontinuous centrocrista (premetacrista oblique oriented and postparacrista straight); pyramidal para- and metacone; paracone much smaller than the metacone; broad and mesially expanded anterobasal cingulum; compressed and relatively broad talon; reduced conules; not eccentric and mesiodistally expanded protocone.
Remarks.—When compared to most of Late Cretaceous and Paleogene metatherians, Zeusdelphys complicatus is more similar to Hatcheritherium alpha from the Late Cretaceous of USA than to any other metatherian, as both share the presence of three developed cusps on the postmetacrista, discontinuous centrocrista (i.e., the premetacrista is oblique oriented, while the postparacrista is straight), wide and mesially expanded anterobasal cingulum, compressed talon, large StC, and pyramidal shape of StB and StD.
Stratigraphic and geographic range.—Type locality and horizon only.
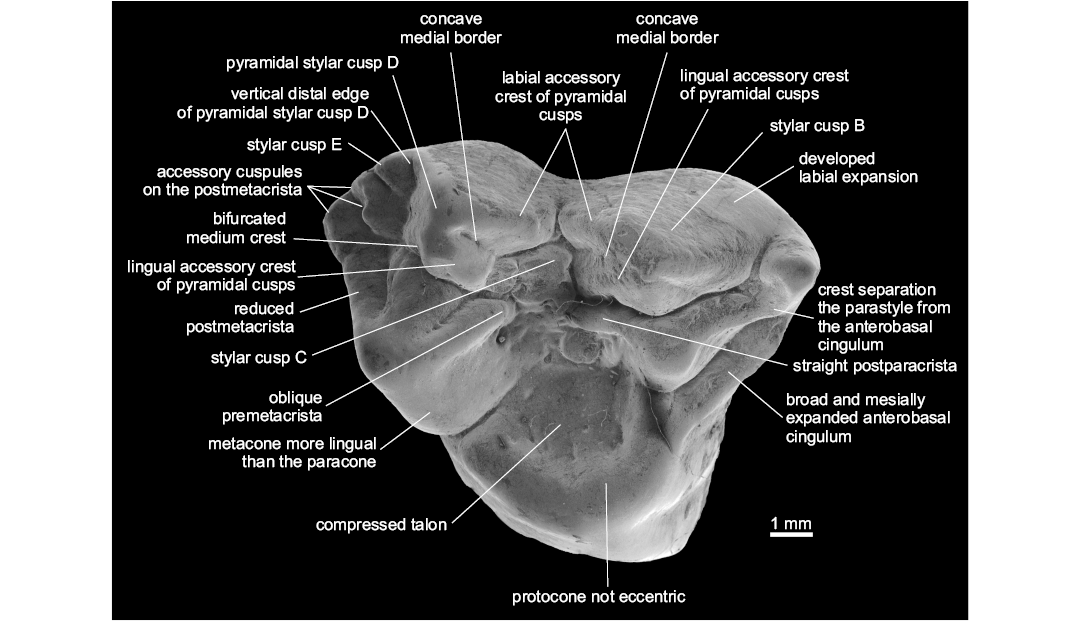
Fig. 1. Type specimen of hatcheriform Zeusdelphys complicatus Marshall, 1987 (MCT 2830-M) from Itaboraí Formation, Brasil; early Eocene (Woodburne et al. 2014); M1 in occlusal view showing the autapomorphies of this species and Hatcheriformes characters.
Phylogenetic analysis
The analysis found a single most parsimonious tree (tree score = 103; CI = 0.7573; HI = 0.2427; RI = 0.9004) (Fig. 2). Following the results, Zeusdelphys complicatus is recovered as the sister taxon of Hatcheritherium alpha. This result supports the previous hypothesis of Oliveira and Goin (2011), which mentioned similarities of both taxa. However, the results do not support the assignment of Zeusdelphys as a Protodidelphidae, as proposed by Oliveira and Goin (2011), and Oliveira et al. (2016).
The North American Ectocentrocristus is recovered as the sister lineage of Zeusdelphys + Hatcheritherium, within Hatcheriformes. This result does not support Polydolopiformes affinities, as proposed by Case et al. (2005). Williamson et al. (2012, 2014) recovered Ectocentrocristus as a Herpetotheriidae. The phylogenetic analysis recovers Zeusdelphys, Hatcheritherium, and Ectocentrocristus as Hatcheriformes. This suborder is recovered as the sister lineage of “Alphadontidae”, a paraphyletic family, as Turgidodon is apparently more closely related to Polydolopimorphia than to any “Alphadontidae”. For this review, “Alphadontidae” includes Alphadon, Albertatherium, and Nortedelphys.
Glasbiidae and Protodidelphidae are considered as sister lineages within Didelphimorphia. The phylogenetic relationship between Protodidelphidae and Polydolopimorphia was proposed by Marshall et al. (1990), but was refuted by Goin et al. (1998), and Goin and Candela (2010); this result is supported by our analysis. The exclusion of Glasbiidae from Hatcheriformes does not agree with Case et al. (2005), who considered this taxon along with Hatcheritherium as the members of Hatcheriformes.
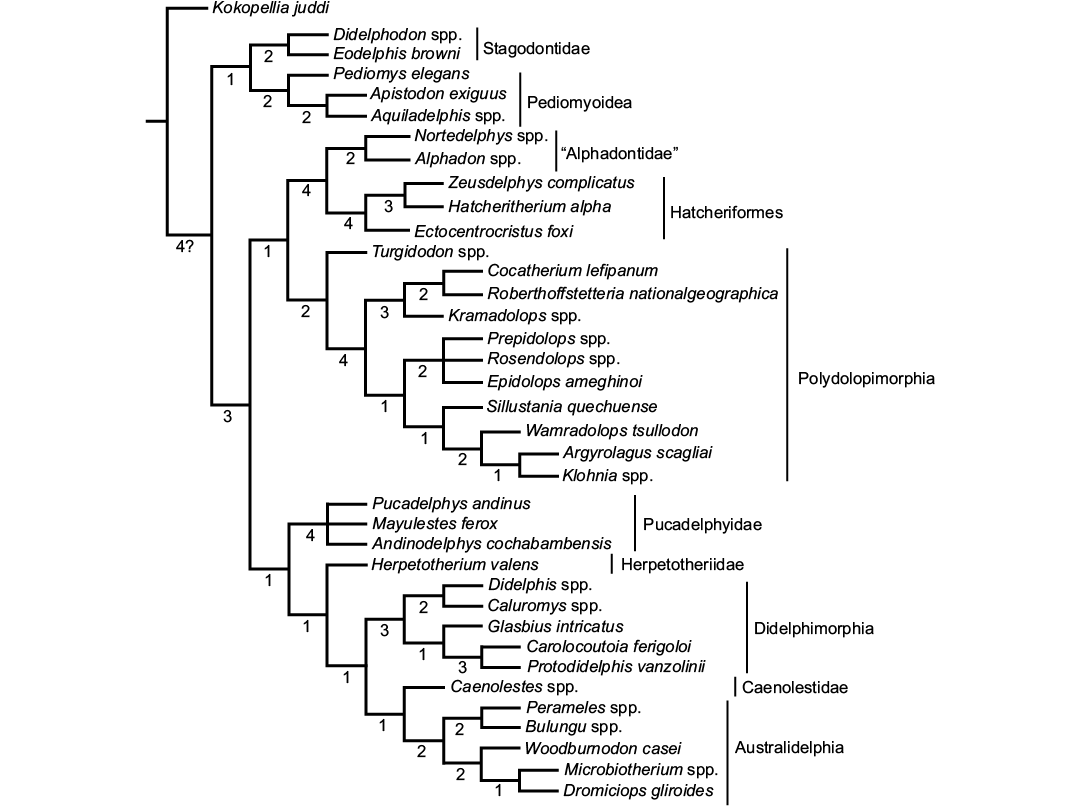
Fig. 2. Single most parsimonious tree found in the phylogenetic analysis. The metatherian lineages are identified by vertical bars. Numbers below the ramus indicate the Bremer Support.
Discussion and conclusions
Presence of three accessory cusps on the postmetacrista.— The presence of three accessory cusps associated with the postmetacrista is easily identifiable on the postmetacrista of Zeusdelphys and Hatcheritherium (Figs. 1, 3). The development of these structures is greater in Zeusdelphys complicatus than any other metatherian, which is the main autapomorphy of this species (character 23-1). In Hatcheritherium, these cusps are present in the labial third of the postmetacrista (Case et al. 2005), while these structures are appressed against each other in the labial edge in Zeusdelphys. The explanation for these two different morphologies is the strong reduction of the postmetacrista and the more labial positioning of the accessory cusps in the Brazilian taxon (Fig. 1). Hatcheritherium presents less developed cusps and a better developed postmetacrista, with less labially shifted cusps (Fig. 3).
Ectocentrocristus and Glasbiidae lack these accessory cusps (Figs. 3, 4). However, the phylogenetic analysis recovered Ectocentrocristus as a Hatcheriformes, while Glasbiidae was recovered as belonging to Didelphimorphia.
Interestingly, several “Alphadontidae like-taxa” also present similar structures (e.g., Nortedelphys, Alphadon, and Albertatherium), though with a variable degree of development (22-0+1) (Fig. 3). The tiny size of these structures in this family (with the exception of Nortedelphys minimus in which they are slightly better developed) associated with a worn postmetacrista on most preserved materials of “Alphadontidae” makes the identification of these cusps difficult, especially in stereomicroscopes or digital pictures. By contrast, SEM pictures provide a more reliable definition, allowing the identification of these cusps in much worn teeth.
In short, the presence of these structures is identifiable in Alphadon, Albertatherium, Nortedelphys, Hatcheritherium, and Zeusdelphys.
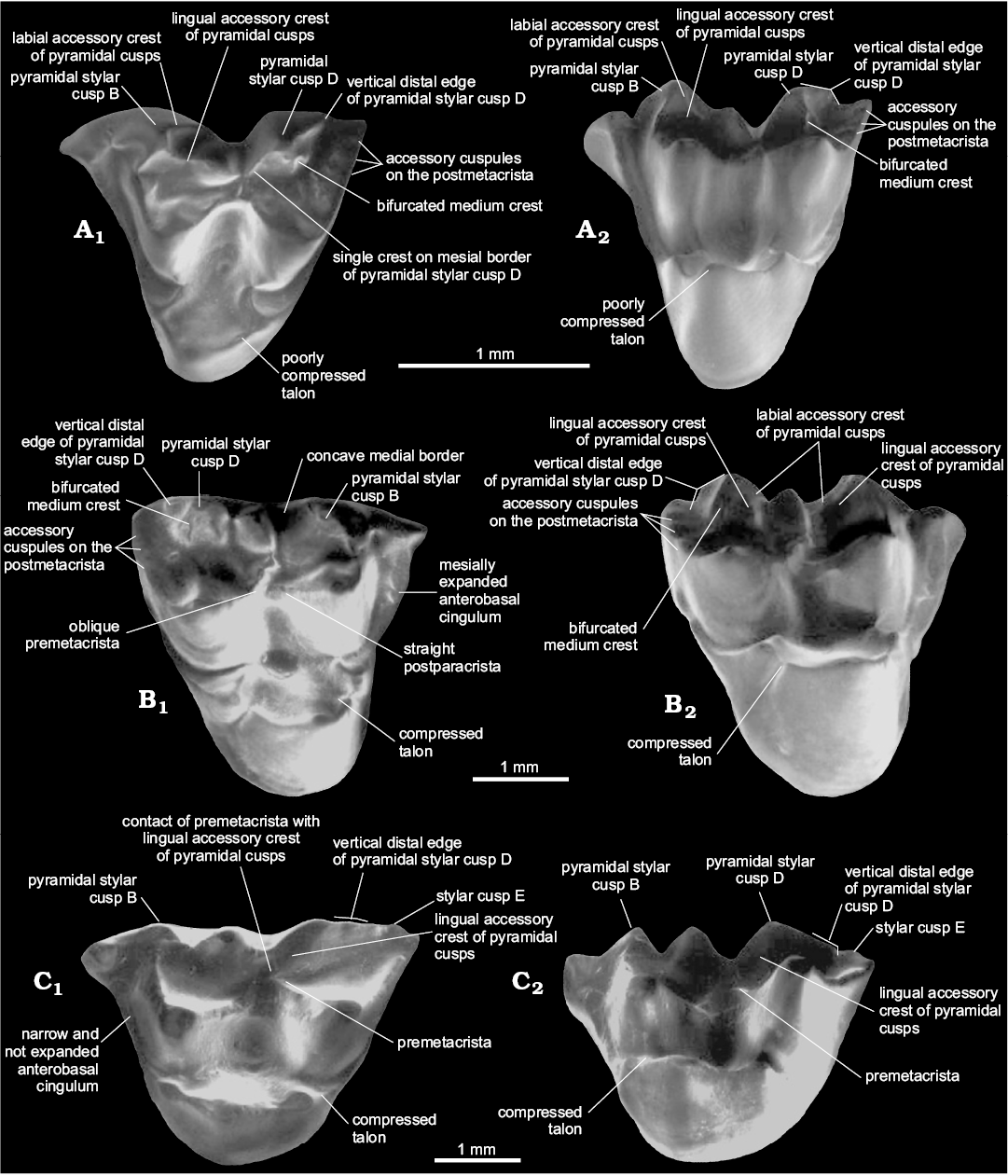
Fig. 3. Dental characters in selected species of Alphadontia. A. Nortedelphys minimus Case, Goin, and Woodburne, 2005 (UW 27031) from V-65127, Bug Creek Anthills, Hell Creek Formation, late Maastrichtian (Lancian NALMA), Montana, USA; LM1 in occlusal (A1) and lingual (A2) views. B. Hatcheritherium alpha Clemens, 1966 (YPM 14912) from Quarry 9, Lance Creek, Lance Formation, late Maastrichtian (Lancian NALMA), Wyoming, USA; RM1 in occlusal (B1) and lingual (B2) views. C. Ectocentrocristus foxi Rigby and Wolberg, 1987 (AMNH 77372) from the upper part of Judith River Formation, late Campanian (Judithian NALMA), Montana, USA; LM3 in occlusal (C1) and lingual (C2) views. Adapted from Case et al. (2005: figs. 4, 5, 9).
Discontinuous centrocrista.—The presence of an open centrocrista is identified in Polydolopimorphia, Hatcheriformes, and Zeusdelphys (Goin et al. 1998; Oliveira and Goin 2011). The condition present in Zeusdelphys is more similar to Hatcheritherium than to any other metatherian. Both taxa present a straight postparacrista and an oblique premetacrista, a unique pattern for Metatheria (Figs. 1, 3). Hatcheritherium presents the premetacrista merged with two small cuspules at the labiocentral portion of stylar shelf (Case et al. 2005), while Zeusdelphys presents the premetacrista merged with a large and lingually shifted StC, previously identified as a cusp non-homologous to the StC by Oliveira and Goin (2011).
The open centrocrista of Polydolopimorphia is different from the one of Hatcheritherium + Zeusdelphys: the former lineage presents a markedly opened centrocrista or an “arc-shaped” centrocrista, with invasive postparacrista and premetacrista on stylar shelf. For Hatcheritherium + Zeusdelphys, only the premetacrista is invasive on stylar shelf, as the postparacrista is straight (Figs. 1, 3). The postparacrista is short in Hatcheritherium and nearly vestigial in Zeusdelphys, which makes it difficult to identify this condition in the last taxon, but it is possible to identify a small and broad postparacrista with a straight trajectory in the type specimen (Fig. 1). We name the unique morphology of the centrocrista of Zeusdelphys + Hatcheritherium as discontinuous centrocrista.
The phylogenetic analysis does not recover the opened and “arc-shaped” centrocrista as homologous to the discontinuous centrocrista, which indicates that the condition of Hatcheritherium + Zeusdelphys is not plesiomorphic to the open state of Polydolopimorphia, representing then two independent evolutionary events (Fig. 2). The discontinuous centrocrista is identified in the phylogenetic analysis as a synapomorphy of Zeusdelphys + Hatcheritherium (32-2).
Ectocentrocristus foxi Rigby and Wolberg, 1987, shows an “arc-shaped” centrocrista, with the postparacrista and the premetacrista not in contact, creating a notch between both (Fig. 3). The condition is different from the one present in Polydolopimorphia, in which the centrocrista is markedly more open. Case et al. (2005) considered the centrocrista of Ectocentrocristus as representing a probable plesiomorphic state of Polydolopiformes, but they did not bring support to this hypothesis in a phylogenetic analysis.
In order to test the affinities of this taxon with Hatcheriformes and Polydolopimorphia, it was included in the phylogenetic analysis. The results recover its centrocrista state as an acquisition independent to the one present in Polydolopiformes, as both taxa were not recovered as a monophyletic lineage (Fig. 2).
The centrocrista of Glasbiidae and Protodidelphidae is more U-shaped than the one present in Polydolopimorphia and Hatcheriformes (Fig. 4). The phylogenetic analysis does not recover this morphology as an evidence for the grouping of these two taxa with Polydolopimorphia or Hatcheriformes; instead, it groups both families as a monophyletic lineage within Didelphimorphia. This morphology is identified in the analysis as an apomorphic V-shape centrocrista (30-1).
Based on the analysis, the morphology of the centrocrista excludes the Glasbiidae from Hatcheriformes, but groups it with Didelphimorphia (Fig. 2).
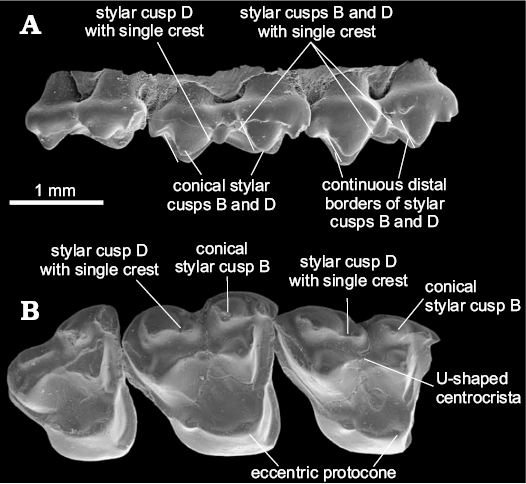
Fig. 4. Upper molars of Glasbius intricatus Clemens, 1966 (UM VP1593) from UCMP V5711, Lance Formation, late Maastrichtian (Lancian NALMA), Wyoming, USA; RP3–M3 in labial (A) and occlusal (B) views showing this taxon characters. Adapted from Davis (2007: fig. 23).
Pyramidal StB and StD.—In this review, we identify an important character regarding the upper dental elements of Nortedelphys, Alphadon, Albertatherium, Zeusdelphys, Hatcheritherium, and Ectocentrocristus: the presence of StB and StD stylar cusps with concave medial borders and accessory crests (Figs. 1, 3). In addition, these two cusps show a pyramidal shape in lingual view, a morphology identified as “pyramidal StB and StD”. The pyramidal shape of these structures can be easily identified in occlusal and lingual view. The pyramidal cusps could be slightly or strongly labiolingually compressed, as it is the case of Ectocentrocristus.
The StB and StD of Zeusdelphys and Hatcheritherium show some degree of labial flattening, though not reaching the greater compression of Ectocentrocristus, especially in the StD (Figs. 1, 3). The StB of “Alphadontidae” and Hatcheriformes present both labial and lingual accessory crests. This morphology is recovered in the phylogenetic analysis as a synapomorphy of Alphadontia (4-1). The “Alphadontidae” differs from Hatcheriformes in the stronger development of the labial accessory crest, which is recovered as a synapomorphy for this lineage (13-1) (Fig. 3).
The pyramidal condition of Ectocentrocristus can be attested by the presence of a vertical posterior edge on StD, and the merging of the lingual accessory crest with the premetacrista (Fig. 3). The contact of these two crests was previously discussed in Case et al. (2005: 469): “the premetacrista terminates at the base of the crista connecting the C and D stylar cusps…” Interestingly, the crest that contacts with the premetacrista is the lingual accessory crest of StD and not the labial accessory crest of StD, which contacts StC.
Zeusdelphys, Hatcheritherium, Ectocentrocristus, and Nortedelphys present a vertical distal edge on StD, which differs from remaining metatherians that present a more convex and continuous edge (Figs. 1, 3). Glasbius shows a continuous distal edge on StD (Fig. 4). The vertical edge of “Alphadontidae” is more evidently developed on M1–2, but it is less developed on M3; this is evident in Albertatherium (Johanson 1995), Nortedelphys, and Alphadon species. More bunoid species of “Alphadontidae”, such as Nortedelphys jasoni and Nortedelphys magnus, show less developed vertical edges on all upper molars, though a vestigial one can be identified in the M2 of the type specimen of N. magnus (i.e., UA 2846).
Hatcheritherium shows a pattern similar to most “Alphadontidae”, with M1 presenting a vertical edge markedly more developed than the one on M3. The easy identification of the vertical edge of StD on the M1 of Zeusdelphys and the lesser degree of development of the same structure on the M3 of Ectocentrocristus could indicate that the same pattern is also true for Hatcheriformes. This observation supports the correct identification of the locus of these teeth in previous studies (Clemens 1966; Case et al. 2005; Oliveira and Goin 2011). The presence of this vertical edge is identified in the phylogenetic analysis as a synapomorphy of “Alphadontidae” + Hatcheriformes (18-1).
The pyramidal StD of Zeusdelphys, Hatcheritherium, and Ectocentrocristus shows two medial accessory crests, one labial and another lingual; these two structures result in a “concavity” on mesial border of this cusp. These structures are observable in occlusal and lingual views (Figs. 1, 3). The “Alphadontidae” differs from Hatcheriformes in the absence of two accessory crests on the mesial border of StD in all upper molars, while the opposite trend is observed in Hatcheriformes. The “Alphadontidae”, such as Albertatherium, Alphadon, and Nortedelphys, show only a single accessory crest of pyramidal StD (Fig. 3).
Zeusdelphys, Hatcheritherium, and Nortedelphys minimus share the presence of a bifurcated medium accessory crest on StD. This morphology is observable in the M1 of Nortedelphys minimus. Interestingly, Nortedelphys minimus shows a single crest on mesial border of pyramidal StD, while Hatcheritherium and Zeusdelphys show the labial and the lingual accessory crests (Figs. 1, 3). The presence of two accessory crests on the mesial border of pyramidal StD is identified as a synapomorphy of Hatcheriformes (17-1), which excludes Nortedelphys from this lineage, and recovers this morphology as a homoplasy between these two lineages.
Nortedelphys intermedius (e.g., UCMP 53097) presents a small cusp tightly appressed against the posterior wall of pyramidal StD, but it is still possible to identify the vertical edge. Interestingly, the presence of a similar cusp is also identified in Ectocentrocristus foxi (e.g., AMNH 77372-type), Hatcheritherium alpha (e.g., YPM 14912-type), Nortedelphys minimus (e.g., UW 27031), Nortedelphys magnus (e.g., UALVP 2758 and UALVP 2452), Alphadon wilsoni (e.g., UALVP 2738), and Nortedelphys jasoni (e.g., UALVP 22678 and UALVP 2316). In some taxa, such as Zeusdelphys, Hatcheritherium, and Nortedelphys minimus, for example, this cusp is appressed against the posterior wall of pyramidal StD, as observed in Nortedelphys intermedius; while other taxa, such as Ectocentrocristus show this cusp closely spaced, but not appressed to StD. The same condition is also present in Zeusdelphys complicatus, reason why this taxon presents four and not three cusps on the labial edge of the metastylar shelf (Fig. 1).
The Glasbiidae and Protodidelphidae do not present pyramidal StB and StD, the distal vertical edge of StD, medium accessory crest of StD, and labial and lingual accessory crests of StB and StD (Fig. 4). The absence of these structures, considered as synapomorphies of Hatcheriformes in the phylogenetic analysis, excludes Glasbiidae and Protodidelphidae from Polydolopimorphia and Hatcheriformes. The posterior edge of StD does not form a vertical edge in Protodidelphidae and Glasbiidae.
The Glasbiidae presents a reduced disto-labial crest of StD in M3 (i.e., the one that contacts StE or the postmetacrista), which gives the impression that StD ends abruptly, resembling the pyramidal shape of Hatcheriformes. However, this morphology cannot be considered as homologous to the pyramidal StD of Hatcheriformes, as Glasbiidae lacks the accessory structures on mesial border. The absence of diagnostic features of Hatcheriformes and the explanation for the apparently pyramidal shape of StD in occlusal view (Fig. 4) exclude Glasbiidae from Hatcheriformes (Fig. 2).
Broad anterobasal cingulum and development of the labial cingulum.—The anterobasal cingulum of Zeusdelphys and Hatcheritherium is more anteriorly expanded than in any other metatherian lineage, which is recovered as a synapomorphy in the phylogenetic analysis (46-1) (Figs. 1, 3). The presence of a developed labial cingulum is conspicuous in Zeusdelphys (Fig. 1), several Glasbiidae (e.g., Glasbius; Fig. 4) and Protodidelphidae (e.g., Carolocoutoia and Protodidelphis), which can be considered as an evidence for their close relationship. Interestingly, the phylogenetic analysis considers the greater development of the labial cingulum as an independent evolutionary event between Zeusdelphys and Glasbiidae + Protodidelphidae (24-1). The extant Didelphis also presents a similar degree of development of labial cingulum.
Hatcheritherium alpha shows a poorly developed labial cingulum (Case et al. 2005), Guggenheimia presents a moderately developed labial cingulum (Paula Couto 1952, 1962, 1970); Alphadon (Johanson 1996), Nortedelphys (Case et al. 2005), Sillustania (Chornogubsky and Goin 2015), Ectocentrocristus (Sahni 1972; Rigby and Wolberg 1987; Case et al. 2005), and Roberthoffstetteria (Marshall et al. 1983; Muizon et al. 1984; Muizon 1992; Goin et al. 2003) do not develop a labial cingulum. The greater degree of development of this cingulum appears to be an adaptation to more frugivorous diet, a conclusion that agrees well with that proposed for Zeusdelphys and Protodidelphis (Zimicz 2012). Unfortunately, without a more focused study, this idea can only be treated as a plausible hypothesis.
The idea that this feature could indicate a close relationship between Zeusdelphys and Protodidelphidae is not supported in the phylogenetic analysis, but the same analysis recovers this morphology as an evidence for the sister-group relationship between Glasbiidae and Protodidelphidae (Fig. 2).
Systematic review.—The results of this study indicate that the current definition of Hatcheriformes is paraphyletic, with Glasbiidae representing a Didelphimorphian lineage, being more closely related to Protodidelphidae than to any other metatherian lineage. Several studies did not support the assignment of Glasbiidae within Hatcheriformes or Polydolopimorphia (Forasiepi et al. 2014; Williamson et al. 2012; 2014), while others support the assignment of Glasbius as the sister lineage of Polydolopimorphia (Chornogubsky and Goin 2015). However, the studies that considered Glasbiidae as a basal lineage of Polydolopimorphia did not include any Protodidelphidae or Didelphidae in their phylogenetic analyses.
Interestingly, except for Williamson et al. (2012, 2014), no other study previously included Hatcheritherium and Glasbiidae in a phylogenetic analysis. This statement casts doubt on the conclusions of Case et al. (2005), who in fact did not present a phylogeny supporting this hypothesis. Our phylogeny supports Williamson et al. (2012, 2014) demonstrating that Glasbiidae and Hatcheritherium do not constitute a monophyletic lineage.
Ectocentrocristus should be included among Hatcheriformes, as it presents all main synapomorphies of this lineage: pyramidal shape of StB and StD; vertical posterior border of StD; presence of labial and lingual accessory crests on pyramidal StB and StD, which forms concave medial borders on these cusps; open centrocrista, with postparacrista and premetacrista not contacting at the center of the tooth; and absence of an eccentric protocone. The stylar cusps of Ectocentrocristus are more labiolingually compressed than in other Hatcheriformes, which is an autapomorphy of this genus.
The current status of Protodidelphidae is paraphyletic, as Zeusdelphys complicatus represents a Hatcheriformes. Following the analysis, Zeusdelphys is the sister taxon of Hatcheritherium alpha. Both, along with Ectocentrocristus, represents the Hatcheriformes, the sister lineage of “Alphadontidae” and not Polydolopimorphia. These results demonstrate that Polydolopimorphia represents an independent lineage from Hatcheriformes, excluding the suborder Hatcheriformes from Polydolopimorphia. The systematic state of Polydolopimorphia appears to be polyphyletic, as Hatcheriformes belongs to “Alphadontia”, along with “Alphadontidae”; Glasbiidae represents a family of Didelphimorphia; and Ectocentrocristus is a Hatcheriformes. In order to recover the monophyletic state of Polydolopimorphia, Ectocentrocristus, Hatcheriformes and Glasbiidae must be excluded from this order.
Zeusdelphys complicatus and Hatcheritherium alpha share the presence of a discontinuous centrocrista, pyramidal StB and StD with a strong labial compression, bifurcated medium accessory crest of StD, and broad and mesially expanded anterobasal cingulum. The phylogenetic analysis recovers as synapomorphies of Zeusdelphys + Hatcheritherium the following characters of the morphological matrix: bifurcated medium accessory crest (16-1), postmetacrista with three accessory cusps (22-1), and broad and mesially expanded anterobasal cingulum (46-1), which justifies the strong Bremer support of this lineage (Fig. 2). The analysis recovers the character presence of protoconal cingula (38-1) as an autapomorphy of Hatcheritherium; and the characters StC lingual to StB and StD (9-1), well-developed postmetacrista cusps (23-1), wrinkled enamel (53-1) and bunoid molars (54-1) as autapomorphies of Zeusdelphys.
These two taxa also share with Ectocentrocristus the presence of the pyramidal StB and StD, compressed talon, notched centrocrista, labial and lingual accessory crests, the vertical distal border of StD, and greater development of the medium accessory crest of StD. These characters are the main synapomorphies of the Hatcheriformes lineage. The phylogenetic analysis recovers as synapomorphies of Hatcheriformes the following characters of the morphological matrix: pyramidal StD (13-1), StD with lingual and labial accessory crests (17-1), compressed labial borders of para- and metacone (27-1) and open centrocrista (30-2). The great number of synapomorphies justifies the strong Bremer support (Fig. 2).
Alphadon and Nortedelphys share with Hatcheriformes the presence of a well-developed StC, three accessory cusps on the postmetacrista, pyramidal StB, and lingually oriented crest connecting the apex of StC with the centrocrista. “Alphadontidae” + Hatcheriformes represent the sister lineage of Polydolopimorphia + Turgidodon; with both sharing the presence of a frequently present distolabial cusp to StB, a synapomorphy for them. The phylogenetic analysis recovers as synapomorphies of “Alphadontia” the following characters of the morphological matrix: pyramidal StB (4-1), StB with accessory labial and lingual crests (5-1), medium accessory crest of StD (15-1) and StD with vertical posterior edge (18-1). Similar to Zeusdelphys + Hatcheritherium and Hatcheriformes lineages, the great number of synapomorphies recovered in the analysis explains the strong Bremer support of “Alphadontia” (Fig. 2). The “Alphadontidae” is supported by two synapomorphies: broad and well-developed parastyle (1-1) and StB with broad and well-developed labial accessory crest (6-1).
The inclusion of Zeusdelphys within Protodidelphidae probably was a result of a convergent evolution related to a more frugivorous diet. Oliveira and Goin (2011) considered Zeusdelphys as lacking a StC, but this taxon presents a well-developed cusp lingually shifted on stylar shelf. The presence of developed labial cingulum, wrinkled enamel, bunoid molars, relatively large size, developed protocone, and reduction of the conules are adaptations for a frugivorous diet (Zimicz 2012). Despite the presence of these shared adaptations between Protodidelphidae and Zeusdelphys, the phylogenetic analysis recovers these characters as independent adaptations, probably to increase the contribution of fruits to their diet.
The hypothesis that considers Zeusdelphys as a Protodidelphidae that converged to acquire all main synapomorphies of Hatcheritherium and Ectocentrocristus is not supported by the phylogenetic analysis, as it tested all main characters shared between Hatcheriformes, Polydolopimorphia, Glasbiidae, and Protodidelphidae. It is important to comment that phylogenetic analyses are currently considered as the most reliable way to test the affinities between different taxa, as the number of variables is too high to be considered without a statistic analysis (Simões et al. 2017). Based on this, the arguments that defend convergent evolution between Zeusdelphys and Hatcheritherium or the idea that taxa from two different continents could not represent a single lineage is not supported by the analysis.
The idea that Zeusdelphys should not be included in a phylogenetic analysis due to a possible character limitation, as it is restricted to a single specimen (i.e., the type), is not supported as well, as the present characters were strongly supported by the analysis. Besides, other studies also included Zeusdelphys in their phylogenetic analysis without methodological problems (Marshall 1987; Oliveira and Goin 2011; Oliveira et al. 2016). The greatest “problem” of these studies was the absence of Hatcheriformes in the analysis, which restricted the possibilities for the grouping of Zeusdelphys. The presence in our analysis of several metatherians lineages, including Glasbiidae, Hatcheriformes, Protodidelphidae, and Polydolopimorphia supported the grouping of Zeusdelphys within Hatcheriformes and not Protodidelphidae.
Paleobiogeography.—The phylogenetic analysis indicates a North American origin for “Alphadontia”, as “Alphadontidae” and Ectocentrocristus were recovered as the most basal lineages of this order, and both taxa are endemic from North America. The results also indicate that Zeusdelphys represents an invasive lineage from North to South America, as Hatcheritherium, a North American endemic taxon, is recovered as its sister lineage (Fig. 5). Following the phylogenetic results, the lineage of Zeusdelphys survived for at least 14 million years after the extinction of Hatcheritherium (Fig. 5).
Based on recent studies, several South American metatherians represent Paleogene surviving taxa of Late Cretaceous North American lineages (Woodburne and Case 1996; Case et al. 2005; Oliveira and Goin 2012; Goin et al. 2016). These studies defend the Caribbean Plate as the main pathway for the arrival of North American lineages in South America, through the “Aves Ridge” (Pindell 1994). The late Maastrichtian (Lancian NALMA) is considered the most probable time span for the arrival of metatherians in South America (see FABI in Goin et al. 2016).
Interestingly, strong sea-level regressions are registered for the latest Late Cretaceous, including one around 67–66 Ma (Haq 2014). This sea-level regression was probably strong enough to create land connections between the Caribbean islands and South America, allowing the dispersal of North American lineages to South America. The idea of a faunal interchange between Americas during the latest Late Cretaceous is also known for other groups, such as dinosaurs (Bonaparte 1984; Pascual 2006; Pascual and Ortiz-Jaureguizar 2007) and “ungulates” (Muizon and Cifelli 2001).
The results of the phylogenetic analysis support a North American origin for Hatcheriformes, with Zeusdelphys representing one of the last members of this lineage. The ancestors of Zeusdelphys probably reached South America during the Late Cretaceous and survived until the early Eocene (Itaboraian SALMA) in Itaboraí (Fig. 5). Recent studies proposed that Itaboraí represents a faunal assemblage during the early Eocene Climatic Optimum (EECO) (Woodburne et al. 2014; Goin et al. 2016), after the Paleocene–Eocene Thermal Maximum (PETM), around 55.2 Ma (Bowen et al. 2014). This climatic event increased the presence of tropical forests related to warm temperatures and is considered the main event for the evolution of Metatheria during the Paleogene (Woodburne et al. 2013; Goin et al. 2016). The large size and the bunoid adaptations of Zeusdelphys identify this taxon as a specialized metatherian, probably with a strict frugivorous diet (Zimicz 2012). The reduction in global temperatures during the middle and late Eocene can be considered as the main responsible for the extinction of Zeusdelphys and many other frugivorous metatherian lineages (Goin et al. 2016).
In short, Zeusdelphys complicatus represents an early Eocene South American surviving lineage of Hatcheriformes, which indicates that a land connection between North and South American existed during the Late Cretaceous.
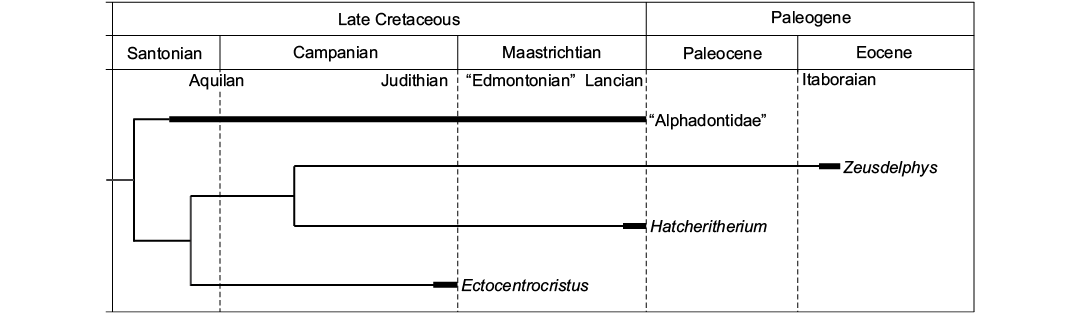
Fig. 5. Temporal and geographical distribution of Hatcheriformes, based on the phylogeny presented in Fig. 2. The wide bars indicate the recorded temporal range of Hatcheriformes taxa. NALMAs: Aquilan, Judithian, “Edmontonian”, and Lancian; SALMA: Itaboraian.
Acknowledgements
We would like to thank Rodrigo Machado (MCT), who gave the permission for the study of the material; the Centro de Microscopia Eletrônica de Varredura do Departamento de invertebrados do Museu Nacional for allowing us to use of the MEV and Camila S.M.A. Messias (Museu Nacional – Universidade Federal do Rio de Janeiro, Brazil) for photographing the material; Lílian P. Bergqvistae (Universidade Federal do Rio de Janeiro, Brazil), for her support with Itaboraí material; and the Willi Henning Society for sponsoring the construction and allowing the free use of TNT. We thank the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for supporting the project. We thank the Universidade Federal de Pernambuco for providing the equipment and laboratories that allowed this study. We also thank the referees for their contribution to this study.
References
Archer, M. 1984. Origins and early radiations of marsupials. In: M. Archer, and G. Clayton (eds.), Vertebrate Zoogeography and Evolution in Australasia, 586–629. Hesperian Press, Carlisle.
Bergqvist, L.P, Mansur, K., Rodrigues, M.A., Rodrigues-Francisco, B.H., Perez, R., and Beltrão, M.C. 2009. Bacia São José de Itaboraí, RJ. Berço dos mamíferos no Brasil. In: M. Winge, C. Schobbenhaus, C.R.G. Souza, A.C.S. Fernandes, M. Berbert-Born, and E.T. Queiroz (eds.), Sítios Geológicos e Paleontológicos do Brasil, 1–15. CPRM, Brasilia.
Bonaparte, J.F. 1984. El intercambio faunístico de vertebrados continentales entre América del Sur y del Norte a fines del Cretácico. Memória do III Congreso Latinoamericano de Paleontologia: 438–450.
Bowen, G.J., Maibauer, B.J., Kraus, M.J., Röhl, U., Westerhold, T., Steimke, A., Gingerich, P.D., Wing, S.L., and Clyde, W.C. 2014. Two massive, rapid releases of carbon during the onset of the Palaeocene–Eocene thermal maximum. Nature Geosciences 8: 44–47. Crossref
Case, J.A., Goin, F.J., and Woodburne, M.O. 2005. “South American” marsupials from the Late Cretaceous of North America and the origin of marsupial cohorts. Journal of Mammalian Evolution 12: 461–494. Crossref
Chornogubsky, L. and Goin, F.J. 2015. A review of the molar morphology and phylogenetic affinities of Sillustania quechuense (Metatheria, Polydolopimorphia, Sillustaniidae), from the early Paleogene of Laguna Umayo, southeastern Peru. Journal of Vertebrate Paleontology 35: e983238. Crossref
Cifelli, R.L. 1990. Cretaceous mammals of southern Utah. I. Marsupials from the Kaiparowits Formation (Judithian). Journal of Vertebrate Paleontology 10: 295–319. Crossref
Cifelli, R.L. and Muizon, C. 1997. Dentition and jaw of Kokopellia juddi, a primitive marsupial or near-marsupial from the medial Cretaceous of Utah. Journal of Mammalian Evolution 4: 241–258. Crossref
Clemens, W.A. 1966. Fossil mammals of the type Lance Formation, Wyoming. Part II. Marsupialia. Publications in Geological Sciences 62: 1–122.
Davis, B.M. 2007. A revision of “pediomyid” marsupials from the Late Cretaceous of North America. Acta Palaeontologica Polonica 52: 217–256.
Forasiepi, A.M., Goin, F.J., Abello, M.A., and Cerdeño, E. 2014. A unique, Late Oligocene shrew-like marsupial from western Argentina and the evolution of dental morphology marsupial. Journal of Systematic Palaeontology 12: 549–564. Crossref
Fox, R.C. 1987. Palaeontology and the early evolution of marsupials. In: M. Archer (ed.), Possums and Opossums: Studies in Evolution, 161–169. Surrey Beatty & Sons Pty Ltd and the Royal Zoological Society of New South Wales, Sydney.
Goin, F.J. and Candela, A.M. 2010. A new early Eocene polydolopimorphian (Mammalia, Marsupialia) from Patagonia. Journal of Vertebrate Paleontology 16: 292–296. Crossref
Goin, F.J., Candela, A.M., and Muizon, C. 2003. The affinities of Roberthoffstetteria nationalgeographica (Marsupialia) and the origin of the polydolopine molar pattern. Journal of Vertebrate Paleontology 23: 869–876. Crossref
Goin, F.J., Oliveira, É.V., and Candela, A.M. 1998. Carolocoutoia ferigoloi nov. gen. and sp. (Protodidelphidae), a new Paleocene “opossum-like” marsupial from Brazil. Palaeovertebrata 27: 145–154.
Goin, F.J., Woodburne, M.O., Zimicz, A.N., Martin, G.M., and Chornogubsky, L. 2016. A brief history of South American Metatherians. In: P. Blondel, E. Guilyardi, J. Rabassa, and C. Horwood (eds.), Dispersal of Vertebrates from between the Americas, Antarctica, and Australia in the Late Cretaceous and Early Cenozoic, 77–124. Springer, New York. Crossref
Goloboff, P.A., Farris, J.S., and Nixon, K.C. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24: 774–786. Crossref
Haq, B.U. 2014. Cretaceous eustasy revisited. Global and Planetary Change 113: 44–58. Crossref
Johanson, Z. 1995. New information concerning the Late Cretaceous marsupial Albertatherium Fox, 1971. Journal of Vertebrete Paleontology 14: 595–602. Crossref
Johanson, Z. 1996. Revision of the Late Cretaceous North American marsupial genus Alphadon. Palaeontographica Abteilung A 242: 127–184.
Marshall, L.G. 1984. The lower jaw of Eobrasilia coutoi Simpson, 1947, a unique Didelphoid (not Borhyaenoid) marsupial from the Paleocene of Brazil. Journal of Paleontology 58: 173–177.
Marshall, L.G. 1987. Systematics of Itaboraian Age “opossum-like” Marsupials. In: M. Archer (ed.), Possums and Opossums: Studies in Evolution, 91–160. Surrey Beatty & Sons Pty Ltd. and the Royal Zoological Society of New South Wales, Sydney.
Marshall, L.G., Case, J., and Woodburne, M.O. 1990. Phylogenetic relationships of the families of marsupials. In: H.H. Genoways (ed.), Current Mammalogy, 433–505. Plenum Press, New York.
Marshall, L.G., Muizon, C., and Sigé, B. 1983. Late Cretaceous mammals (Marsupialia) from Bolivia. Geobios 16: 739–745. Crossref
Muizon, C. 1992. La fauna de Mamiferos de Tiupampa (Paleoceno Inferior, Formacion Santa Lucia), Bolivia. In: R. Suarez-Soruco (ed.), Fosiles y facies de Bolivia—I Vertebrados, 575–624. Revista Técnica de YPFB 12, Santa Cruz.
Muizon, C. and Cifelli, R.L. 2001. A new basal “Didelphoid” (Marsupialia, Mammalia) from the Early Paleocene of Tiupampa (Bolivia). Journal of Vertebrate Paleontology 21: 87–97. Crossref
Muizon, C., Marshall, L.G., and Sigé, B. 1984. The mammal fauna from the El Molino Formation (Late Cretaceous, Maastrichtian) at Tiupampa, southcentral Bolivia. Bulletin of the Muséum National d’Histoire Naturelle 6: 327–351.
Oliveira, É.V. and Goin, F.J. 2011. A reassessment of bunodont Metatherians from the Paleogene of Itaboraí (Brazil): systematics and age of the Itaboraian Salma. Revista Brasileira de Paleontologia 14: 105–136. Crossref
Oliveira, É.V. and Goin, F.J. 2012. Metatérios do início do Paleógeno no Brasil: diversidade e afinidades. In: N.C. Cáceres (ed.), Os Marsupiais do Brasil: Biologia, ecologia e conservação, 275–307. Universidade Federal de Mato Grosso do Sul, Campo Grande.
Oliveira, É.V., Zimicz, N., and Goin, F.J. 2016. Taxonomy, affinities, and paleobiology of the tiny metatherian mammal Minusculodelphis, from the early Eocene of South America. Science of Nature 103: 1–11. Crossref
Pascual, R. 2006. Evolution and geography: the biogeographic history of South American Land Mammals. Annals of the Missouri Botanical Garden 93: 209–230. Crossref
Pascual, R. and Ortiz-Jaureguizar, E. 2007. The Gondwanan and South American episodes: two major and unrelated moments in the history of the South American mammals. Journal of Mammalian Evolution 14: 75–137. Crossref
Paula Couto, C. 1952. Fossil Mammals from the beginning of the Cenozoic in Brazil Marsupialia: Didelphidae. American Museum Novitates 1567: 1–26.
Paula Couto, C. 1962. Didelfideos fosiles del Paleoceno de Brasil. Revista del Museo Argentino de Ciencias Naturales Ciencias Zoológicas 12: 135–166.
Paula Couto, C. 1970. News on the fossil marsupials from the Riochican of Brazil. Anais da Academia Brasileira de Ciências 42: 19–34.
Pindell, J.L. 1994. Evolution of the Gulf of Mexico and the Caribbean. In: S.K. Donovan and T.A. Jackson (eds.), Caribbean Geology: An Introduction, 13–39. The University of West Indies Publishers Association, Mona.
Rigby, J.K. and Wolberg, D.L. 1987. The therian mammalian fauna (Campanian) of Quarry 1, Fossil Forest study area, San Juan Basin, New Mexico. Geological Society of America Special Paper 209: 51–79. Crossref
Sahni, A. 1972. The vertebrate fauna of the Judith River Formation, Montana. Bulletin of the American Museum of Natural History 147: 321–412.
Simões, T.R., Caldwell, M.W., Palci, A., and Nydam, R.L. 2016. Giant taxon-character matrices: quality of character constructions remains critical regardless of size. Cladistics 33: 1–22.
Williamson, T.E., Brusatte, S.L., and Wilson, G.P. 2014. The origin and early evolution of metatherian mammals: the Cretaceous record. ZooKeys 465: 1–76. Crossref
Williamson, T.E., Brusatte, S.L., Carr, T.D., Weil, A., and Standhardt, B.R. 2012. The phylogeny and evolution of Cretaceous–Palaeogene metatherians: cladistic analysis and description of new early Palaeocene specimens from the Nacimiento Formation, New Mexico. Journal of Systematic Palaeontology 10: 625–651.
Woodburne, M.O. and Case, J.A. 1996. Dispersal, vicariance, and the Late Cretaceous to Early Tertiary land mammal biogeography from South America to Australia. Journal of Mammalian Evolution 3: 121–161. Crossref
Woodburne, M.O., Goin, F.J., Bond, M., Carlini, A.A., Gelfo, J.N., López, G.M., Iglesias, A., and Zimicz, A.N. 2013. Paleogene Land Mammal Faunas of South America; a response to global climatic changes and indigenous floral diversity. Journal of Mammalian Evolution 21: 1–73. Crossref
Woodburne, M.O., Goin, F.J., Raigemborn, M.S., Heizler, M., Gelfo, J.N., and Oliveira, É.V. 2014. Revised timing of the South American early Paleogene land mammal ages. Journal of South American Earth Sciences 54: 109–119. Crossref
Zimicz, A.N. 2012. Ecomorfología de los marsupiales paleógenos de América del Sur. 414 pp. Unpublished Ph.D. Thesis, Universidad nacional de La Plata, La Plata.
Acta Palaeontol. Pol. 62 (3): 497–507, 2017
https://doi.org/10.4202/app.00351.2017