

Origin attachments of the caudofemoralis longus muscle in the Jurassic dinosaur Allosaurus
ANDREA CAU and PAOLO SERVENTI
The caudofemoralis longus muscle (CFL) is the primary limb retractor among non-avian sauropsids, and underwent a dramatic reduction along the dinosaur lineage leading to birds. The osteological correlates of the CFL among fossil reptiles have been controversial, because, contrary to traditional interpretations, the extent of the muscle is not necessarily related to the distribution of the caudal ribs. In some Cretaceous dinosaurs, the extent of the CFL has been inferred based on the preserved bony septa between the CFL and other tail muscles. Here, we describe a series of tail vertebrae of the Jurassic dinosaur Allosaurus, each showing a previously-unreported feature: a sulcus, formed by a regular pattern of tightly packed horizontal slits, that runs vertically along the lateral surfaces of the centra and neural arches. These sulci are interpreted as the origin attachment sites of the CFL, allowing for direct determination of the muscle extent along the tail of this dinosaur. Anteriorly to the 18th caudal vertebra, the sulcus runs along most of the centrum and neural arch, then it progressively reduces its vertical extent, and disappears between caudals 24 and 32, a pattern consistent with previous CFL reconstructions in other theropods.
Introduction
The caudofemoralis longus (CFL) is a non-segmented muscle that originates from the tail vertebrae and inserts on the femur in the majority of sauropsid lineages (Gatesy 1990; Hutchinson 2001; Persons and Currie 2011b). The CFL is the most important hindlimb retractor in (non-avian) reptiles (Gatesy 1997), whereas in birds this muscle is extremely reduced or completely absent (Gatesy 1990). The progressive reduction of the osteological correlates of the CFL muscle have been documented along the avian stem, the grade including all fossil reptiles closer to extant birds than crocodiles (Hutchinson 2001). The reduction of the CFL represents one of the supports for the origin of birds from maniraptoran theropods (Gatesy 1990; Hutchinson 2001), and confirms a gradualistic scenario from the “reptile-like” locomotory module (mainly based on femoral retraction) of ancestral archosaurs to the “avian-like” module (mainly based on knee flexion; Gatesy 1990).
As pointed out by Persons and Currie (2011b), the inference on size and position of the CFL in fossil reptiles has been controversial, with different authors using alternative osteological correlates to infer the main features of this muscle. Persons and Currie (2011a) showed that, contrary to previous reconstructions (e.g., Romer 1923; Madsen 1976), the CFL does not attach on the lateral tips or onto the ventral surfaces of the caudal ribs (“transverse processes” of other authors: we follow the anatomical terminology of Persons and Currie 2011a, for the pleurapophyseal processes on the caudal vertebrae). Although the posterior termination of the ribs along the caudal series has been usually considered as the reference point for inferring the posterior extent of the CFL (e.g., Romer 1923), dissection of extant reptiles demonstrates that the two features do not necessarily co-vary along the tail of all taxa (Persons and Currie 2011b). In particular, given that the caudal ribs serve as lateral insertion points for the longissimus muscle (which is present along the full extent of the tail, contrary to the CFL), they may remain present well after the CFL muscle has terminated (Persons and Currie 2011b). These results have challenged the use of the caudal ribs as unambiguous osteological correlate for inferring the extent of the CFL.
Osteological correlates of the boundaries of the CFL have been reported among the tail vertebrae of some Cretaceous theropods. In ornithomimid and tyrannosaurid caudal series, sequential diagonal scarring on the lateral surfaces of the hemal spines has been interpreted as the tapering boundary between the insertions of the CFL and the ilioischiocaudalis muscle (Persons and Currie 2011b). In some abelisaurids, distinct ridges on the ventrolateral surfaces of the caudal ribs indicate that in a subclade of advanced ceratosaurians the CFL originated from a portion of the caudal ribs (Persons and Currie 2011a). In both studies mentioned (Persons and Currie 2011a, b), the extent of the CFL was inferred from osteological features (i.e., scars or septa) placed in the hemal spines or on the ribs, whereas no direct evidence of the CFL origin on the caudal centra has been reported. Here, we describe a series of caudal vertebrae of the Jurassic theropod Allosaurus Marsh, 1877 (see Madsen 1976) that show a type of osteological correlate of the CFL previously unreported among dinosaurs.
Institutional abbreviation.—MUP, Museo Universitario Paleontologico di Modena, Modena, Italy.
Other abbreviations.—CFL, caudofemoralis longus muscle.
Systematic palaeontology
Dinosauria Owen, 1842
Allosauridae Marsh, 1878
Genus Allosaurus Marsh, 1877
Type species: Allosaurus fragilis Marsh, 1877; Fremon County, Colorado, USA; Morrison Formation, Kimmeridgian–Tithonian, Late Jurassic.
Allosaurus fragilis Marsh, 1877
Fig. 1.
Material.—MUP 40CA11, MUP 41CA12 2744, MUP 44CA15 3926, MUP 45CA16, MUP 46CA17, MUP 49CA20 PC69, MUP 52CA23, MUP 61CA32 1484, MUP 64CA35 2193, MUP 66CA37 407, MUP 64CA39 PC78, series of partially-preserved middle and posterior caudal vertebrae (Fig. 1, Table 1) from the Cleveland Lloyd Quarry of Utah (USA), Morrison Formation, Kimmeridgian–Tithonian (Madsen 1976; Gates 2005).
Table 1. Morphological features and positional identification criteria of the caudal vertebrae described in this study.
|
Specimen |
Lateral sulcus |
Morphological features for positional inference |
Inferred position along caudal series |
|
MUP
40CA11 |
Clearly visible on most of both sides of centrum and ventral to rib bases. Curved posterodorsally. |
Centrum 1.4 times anteroposteriorly longer than dorsoventrally tall. Rib base extended for half neurocentral suture |
10th –13th |
|
MUP 41CA12 2744 (Fig. 1B) |
Clearly visible on most of both sides of centrum. Oriented vertically. |
Centrum 1.2 times longer than tall. Rib base extended for more than half neurocentral suture |
9th–12th |
|
MUP 44CA15 3926 (Fig. 1C) |
More clearly visible on right side. Curved posterodorsally. |
Centrum 1.6 times longer than tall. Ribs long and posterolaterally oriented. Accessory neural spine present. |
14th–17th |
|
MUP 45CA16 |
More clearly visible on right side. Extended vertically along dorsal ¾ of centrum. |
Centrum 1.6 times longer than tall. Rib base extended for half neurocentral suture. Robust prezygapophyseal base. |
14th–18th |
|
MUP 46CA17 |
More clearly visible on left side. Curved posterodorsally from ventral third of centrum to postzygapophyseal pedicel. |
Centrum 1.8 times longer than tall. |
16th–20th |
|
MUP 49CA20 PC69 |
More clearly visible on left side. Curved posterodorsally from ventral third of centrum to postzygapophyseal pedicel. |
Centrum 1.8 times longer than tall. Neural spine and rib base restricted to posterior half of neural arch. Robust prezygapophyseal bases. |
18th–21th |
|
MUP 52CA23 |
Visible on left side. Inclined posterodorsally along anterior half of centrum. |
Centrum 1.9 times longer than tall. |
21th–24th |
Description.—The material described herein includes a series of partially-preserved middle and posterior caudal vertebrae. No chevrons are included in this material. In a subset of the caudal vertebrae (Fig. 1, Table 1), a shallow sulcus runs along both lateral surfaces of the centrum and, less frequently, it extends along the lateral surfaces of the neural arch. In the most anterior vertebrae where it is visible (placed at about the 9th–14th caudal position), the sulcus extends slightly posterodorsally from the ventral half of the lateral surface of the centrum, just anterior to level of the minimum width of the centrum, and reaches the level of the rib (Fig. 1A, B). In vertebrae placed more posteriorly (about at the 15th–20th caudal position), the sulcus describes a gentle curve that reaches the neurocentral suture (Fig. 1C, D) and extends to the posterior half of the lateral surface of the neural arch, between the rib and the postzygapophysis (Fig. 1E). In the most posterior vertebra where it is visible (placed about at the 21st–24th position), the sulcus is visible exclusively on one side of the vertebra (Fig. 1F). Furthermore, in that vertebra, the extent of the sulcus is limited compared to more anterior vertebrae: instead of reaching the ventral surface of centrum, its ventral end is placed about at one third of centrum height, and dorsally it barely reaches the neurocentral suture (Fig. 1F). The sulcus does not extend along the ventral surface of the caudal ribs in any vertebra. None of the posterior caudal vertebrae of this series (e.g., MUP 61CA32 1484, MUP 64CA35 2193, MUP 66CA37 407, MUP 64CA39 PC78, all placed posterior to the 25th position) show evidence of this sulcus. The sulcus is often barely visible under direct illumination, but is clearly visible under low-angle light. Closer examination of the sulci shows that each is formed of a series of horizontally-oriented slits (still filled by sediment) that are regularly spaced vertically (about three sulci every mm; Fig. 1A3). Each slit appears as a distinct incision of the otherwise smooth surface of the bone: no irregular margins nor redirected bone fibers (sensu Currie and Jacobsen 1995) are present along the sulcus. These slits are more marked on the centrum, and disappear toward the neural arch: when it extends along the neural arch, the sulcus is mainly developed as a shallow unornamented depression.
Remarks.—The material described here was acquired from the University of Utah by the University of Modena-Reggio Emilia (Modena, Italy), and registered in 1967 to the collection of the Palaeontological Universitary Museum (MUP) in Modena (Alessandrini 2015). The complete material housed in MUP (including one left premaxilla, one left maxilla, one left postorbital, one right dentary, one surangular, several vertebrae, a scapulocoracoid, a humerus, additional forelimb elements, most of the pelvis, both femora and part of tibiae and pes) forms about 50% of a skeleton of Allosaurus fragilis. As outlined by Madsen (1976) and Gates (2005), most of the material from the Cleveland Llyod Quarry is disarticulated. Furthermore, no indication in the registered material indicates that the skeleton mounted in MUP is based on a single individual. Taphonomic and morphometric analyses on the cranial bones indicate that at least two individuals are included in the MUP material (Alessandrini 2015), supporting a composite status for the skeleton. Therefore, although each caudal vertebra is labeled according to its position along the vertebral series in the mounted skeleton, we refrain from considering all the material as belonging to the same individual. The position of each vertebra along the tail was inferred referring to the generalised Allosaurus skeleton illustrated in Madsen (1976: pls. 29–36), and also by comparison with well-preserved caudal series in other theropods (e.g., Brochu 2002). Here, we focus on those features of the lateral surface of the centra and neural arches of Allosaurus not described previously.
Stratigraphic and geographic range.—Brushy Basin Member, Morrison Formation, Cleveland-Lloyd Dinosaur Quarry, Utah.
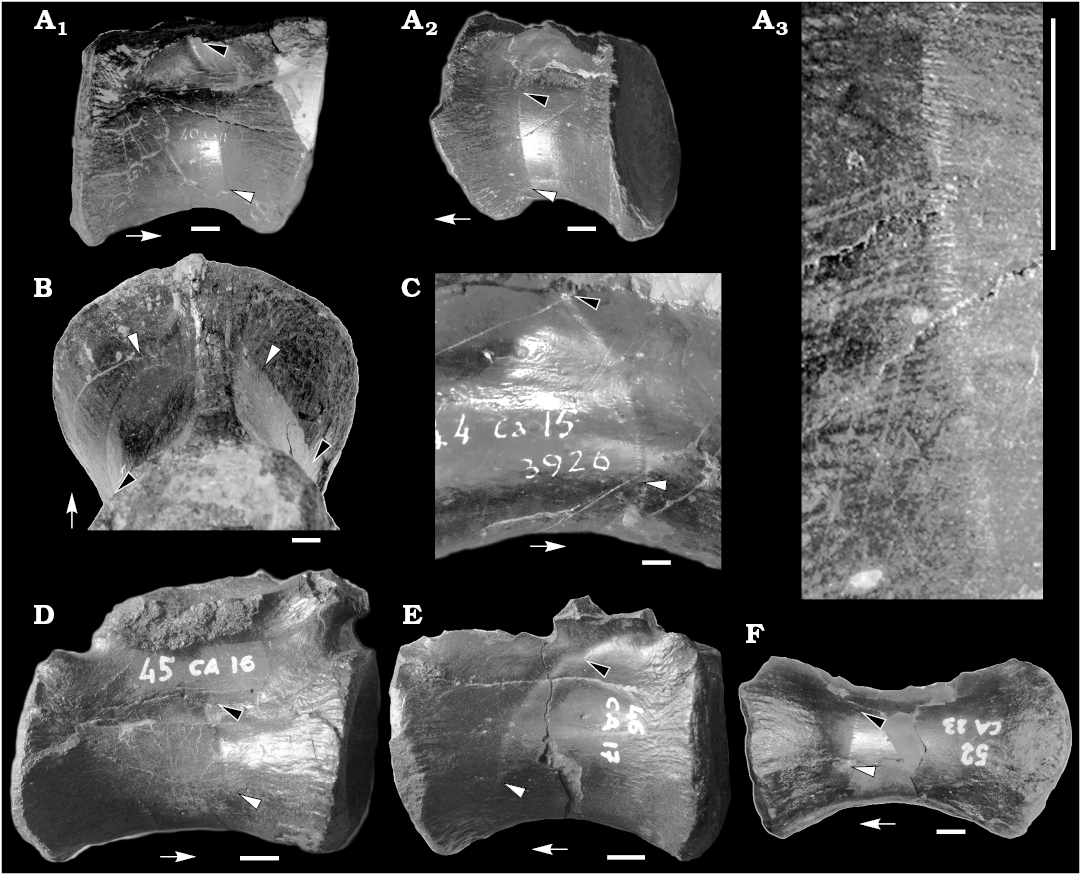
Fig. 1. Caudal vertebrae of the theropod dinosaur Allosaurus fragilis Marsh, 1877 from Cleveland Lloyd Quarry of Utah (USA), Morrison Formation, Kimmeridgian–Tithonian. A. MUP 40CA11, ~10th–13th position, in right lateral (A1) and left lateral (A2) views. Detail showing the slits along the sulcus (A3). B. MUP 41CA12 2744, ~9th–12th position. C. MUP 44CA15 3926, ~14th–17th position. D. MUP 45CA16, ~14th–18th position. E. MUP 46CA17, ~16th–20th position. F. MUP 52CA23 (~21st–24th position). In lateral (A, C–F) and posteroventral (B) views. Black arrowheads indicate dorsalmost extent of CFL (caudofemoralis longus muscle) sucus, white arrowheads indicate ventralmost extent of CFL sulcus, arrows point toward anterior end of vertebra. Scale bars 10 mm.
Concluding remarks
These sulci are a biological feature of the vertebrae and are not due to scavenging or taphonomic factors, because: (i) they are consistently recovered as a single, continuous track placed in the same position along different vertebrae (i.e., they start from the anteroventral third of the centrum, extending dorsally or posterodorsally toward the neurocentral suture and the neural arch), and never occur in multiple groups or in other parts of the vertebra (in particular, in the posterior half of the centrum): this pattern supports serial homology among different sulci; (ii) in the best preserved vertebrae, the sulci are present symmetrically along both sides of the vertebra (Fig. 1A1, A2, B); (iii) the sulcus and the regular pattern of slits differ in shape and extent from feeding traces and denticle drag marks left on bones by scavengers (e.g., Fiorillo 1991; Currie and Jacobsen 1995; Forrest 2003; Rogers et al. 2003; Drumheller 2007; D’Amore et al. 2009; Fig. 1A3); (iv) we are not aware of any taphonomic process that produces a regular series of short longitudinal slits on bones.
We consider the sulci and associated slits as the impressions of the CFL origins on the caudal vertebrae. This interpretation is consistent with the extent and development of the CFL among other tetanuran theropods reconstructed by Persons and Currie (2011b): (i) this sulcus is absent in the cranial and appendicular bones and in the pre-caudal vertebrae of the MUP Allosaurus skeleton, suggesting that it is the osteological correlate of a tail muscle; (ii) contrary to other tail muscles (Persons and Currie 2011b), both sulcus and CFL are more developed toward the anterior end of the tail and are absent in posterior caudals; (iii) both sulcus and CFL originate from the lateral surface of the centra and eventually extend to the neural arches, but marginally (if not at all) along the proximoventral surface of the ribs (Persons ad Currie 2011a, b). Following this interpretation, the slits along the sulci may represent the origin points of the single CFL muscle fibers on the vertebrae: it is noteworthy that the horizontal orientation of the slits recalls that of the muscle fibers in extant reptiles, and is confirmed among fossils in the exceptionally-preserved CFL of Scipionyx Dal Sasso and Signore, 1998 (see Dal Sasso and Maganuco 2011).
Based on the extent and distribution of the sulci along the vertebrae described here, we propose a reconstruction of the CFL muscle in Allosaurus (Fig. 2). The posteriormost extent of the muscle is placed between the 23th and 32th caudal vertebra (absence of vertebrae from this part of the tail in the MUP material prevents us from a more accurate placement of CFL end). In the posteriormost vertebrae where the muscle originates, it exclusively contacts the centrum. Moving toward the anterior end of the tail, the muscle progressively expands its dorsoventral contact with the vertebrae. Approximately at the level of the 17th caudal vertebra, the CFL originates on both neural arch (ventral to the ribs) and centrum. The reconstructed extent of the CFL in Allosaurus is comparable to other large-bodied tetanurans (Persons and Currie 2011b).
The sulcus described here is relatively poorly visible under direct light, and may be unnoticed even in well-preserved specimens. This may explain why this feature is currently unreported in the palaeontological literature. We predict that re-examination of previously described caudal series may reveal the presence of CFL origin attachment sulci in other fossil reptiles. The analysis of the extent, development and distribution of this feature in the extinct sauropsids may provide additional information on the evolution of the CFL among Reptilia.
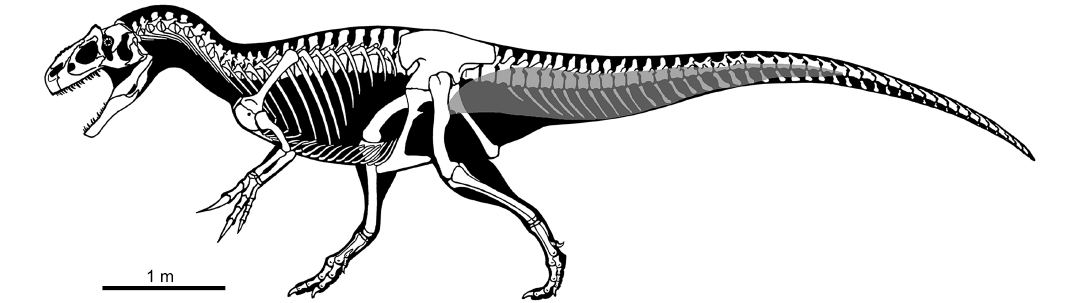
Fig. 2. Skeletal reconstruction of Allosaurus in lateral view, with reconstruction of caudofemoralis longus muscle (grey). Modified from drawing by Scott Hartman.
Acknowledgements.—We thank John Hutchinson (The Royal Veterinary College, London, UK) for useful suggestions on the interpretation of the sulcus, and Scott Hartman (University of Wisconsin-Madison, USA) for kindly providing the skeletal drawing of Allosaurus used in Figure 2. We thank Editor Stephen Brusatte (University of Edinburgh, UK), Susannah Maidment (University of Brighton, UK) and an anonymous reviewer for providing useful advice to this manuscript.
References
Alessandrini, G. 2015. Allosaurus fragilis nelle collezioni del Museo di Paleontologia dell’Università di Modena e Reggio-Emilia: analisi e descrizione. 35 pp. Unpublished Thesis, University of Bologna, Bologna.
Brochu, C.A. 2002. Osteology of Tyrannosaurus rex: insights from a nearly complete skeleton and high-resolution computer tomographic analysis of the skull. Society of Vertebrate Paleontology Memoir 7: 1–138. Crossref
Currie, P.J. and Jacobsen, A.R. 1995. An azhdarchid pterosaur eaten by a velociraptorine theropod. Canadian Journal of Earth Sciences 32: 922–925. Crossref
Dal Sasso, C. and Maganuco, S. 2011. Scipionyx samniticus (Theropoda: Compsognathidae) from the Lower Cretaceous of Italy (Osteology, ontogenetic assessment, phylogeny, soft tissue anatomy, taphonomy and palaeobiology). Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 37: 1–281.
Dal Sasso, C. and Signore, M. 1998. Exceptional soft-tissue preservation in a theropod dinosaur from Italy. Nature 392: 383–387. Crossref
D’Amore, D.C. and Blumenschine, R.J. 2009. Komodo monitor (Varanus komodoensis) feeding behavior and dental function reflected through tooth marks on bone surfaces, and the application to ziphodont paleobiology. Paleobiology 35: 525–552. Crossref
Drumheller, S.K. 2007. Experimental taphonomy and microanalysis of crocodylian feeding traces. Microscopy and Microanalysis 13 (Supplement 2): 510.
Fiorillo, A.R. 1991. Prey bone utilization by predatory dinosaurs. Palaeogeography Palaeoclimatology, Palaeoecology 88: 157–166. Crossref
Forrest, R. 2003. Evidence for scavenging by the marine crocodile Metriorhynchus on the carcass of a plesiosaur. Proceedings of the Geological Association 144: 363–366. Crossref
Gates, T.A. 2005. The Late Jurassic Cleveland-Lloyd Dinosaur Quarry as a Drought-Induced Assemblage. Palaios 20: 363–375. Crossref
Gatesy, S.M. 1990. Caudofemoral musculature and the evolution of theropod locomotion. Paleobiology 16: 170–186. Crossref
Gatesy, S.M. 1997. An electromyographic analysis of hindlimb function in Alligator during terrestrial locomotion. Journal of Morphology 234: 197–212. Crossref
Hutchinson, J.R. 2001. The evolution of femoral osteology and soft tissues on the line to extant birds (Neornithes). Zoological Journal of the Linnean Society 131: 169–197. Crossref
Madsen, J.H., Jr. 1976. Allosaurus fragilis—a revised osteology. Utah Geologic Survey Bulletin 109: 1–163.
Marsh, O.C. 1877. Notice of new dinosaurian reptiles from the Jurassic formation. The American Journal of Science and Arts, Series 3 14: 514–516.
Marsh, O.C. 1878. Notice of new dinosaurian reptiles. The American Journal of Science and Arts, Series 3, 15: 241–244. Crossref
Owen, R. 1842. Report on British fossil reptiles, part II. Reports of the British Association for the Advancement of Science 11: 60–204.
Persons, W.S. IV and Currie, P.J. 2011a. Dinosaur Speed Demon: The Caudal Musculature of Carnotaurus sastrei and Implications for the Evolution of South American Abelisaurids. PLoS ONE 6 (10): e25763. Crossref
Persons, W.S. IV and Currie, P.J. 2011b. The tail of Tyrannosaurus: reassessing the size and locomotive importance of the M. caudofemoralis in non-avian theropods. The Anatomical Record 294: 119–131.
Rogers, R.R., Krause, D.V., and Curry-Rogers, C. 2003. Cannibalism in the Madagascan dinosaur Majungatholus atopus. Nature 422: 515–518. Crossref
Romer, A.S. 1923. The pelvic musculature of saurischian dinosaurs. Bulletin of the American Museum of Natural History 48: 605–617.
Andrea Cau [cauand@gmail.com], Earth, Life and Environmental Sciences Department and Museo Geologico e Paleontologico “Giovanni Capellini”, Alma Mater Studiorum, Università of Bologna, Via Zamboni, 63, 40126, Bologna, Italy.
Paolo Serventi [paolo.serventi@unimore.it], Department of Chemical and Geological Sciences, University of Modena and Reggio Emilia, Via Giuseppe Campi, 103, 41125, Modena, Italy.
Received 21 March 2017, accepted 20 April 2017, available online 24 May 2017.
Copyright © 2017 A. Cau and P. Serventi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 62 (2):
273–277, 2017
https://doi.org/10.4202/app.00362.2017