

Filling the Corallian gap: New information on Late Jurassic marine reptile faunas from England
DAVIDE FOFFA, MARK T. YOUNG, and STEPHEN L. BRUSATTE
Foffa, D., Young, M.T., and Brusatte, S.L. 2018. Filling the Corallian gap: New information on Late Jurassic marine reptile faunas from England. Acta Palaeontologica Polonica 63 (2): 287–313.
Two of the best known Mesozoic marine reptile assemblages can be found in units deposited in the Jurassic Sub-Boreal Seaway of the UK: the late Middle Jurassic Oxford Clay Formation (OCF) and Late Jurassic Kimmeridge Clay Formation (KCF). They record two very differently structured faunas, but understanding the turnover between them is hampered by a gap in the fossil record that spans much of the Oxfordian, the so-called “Corallian gap”. We provide a comprehensive review of specimens from the Corallian Group (CG) of the UK, which includes the first descriptions of several fossils, particularly teeth. We demonstrate that there is a severe reduction in observed marine reptile diversity during the Oxfordian, with several Callovian taxa well known from the OCF not persisting into the Corallian strata, including small-to-mid-sized pliosaurids and longirostrine teleosaurids. We do, however, find evidence that at least one member of each key OCF lineage (plesiosauroids, pliosaurids, ichthyosaurs, and thalattosuchians) survived into the Corallian interval, and that one keystone KCF lineage (the Torvoneustes line of metriorhynchid thalattosuchians) was present during this time, indicating an earlier radiation of this group than previously thought. We suggest that faunal turnover between the OCF and KCF may have been driven by environmental perturbations during the Oxfordian, which selectively removed small bodied pliosaurids and longirostrine teleosaurids from the Jurassic Sub-Boreal Seaway, but less affected metriorhynchids, plesiosauroids, and ophthalmosaurid ichthyosaurs. The preferential removal of taxa from the sub-Boreal realm may have helped facilitate the radiation of lineages that became dominant during the Late Jurassic.
Key words: Plesiosauria, Teleosauridae, Ophthalmosauridae, “Pliosaurus” grossouvrei, Torvoneustes, faunal turnover, Corallian gap, Jurassic, UK.
Davide Foffa [davide.foffa@ed.ac.uk], Mark T. Young [mark.young@ed.ac.uk], and Stephen L. Brusatte [Stephen.Brusatte@ed.ac.uk], School of GeoSciences, University of Edinburgh, Grant Institute, James Hutton Road, Edinburgh, Scotland EH9 3FE, UK.
Received 5 January 2018, accepted 22 February 2018, available online 11 May 2018.
Copyright © 2018 D. Foffa et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Jurassic was a crucial time for marine reptile evolution (Pyenson et al. 2014; Kelley and Pyenson 2015). This period directly followed a severe extinction event at the Triassic–Jurassic (T–J) boundary that caused a major faunal turnover in marine ecosystems and the demise of several groups, including basal sauropterygians and non-parvipelvian ichthyosaurs (Fischer et al. 2014; Stubbs and Benton 2015; Kelley and Pyenson 2015). Just a few million years after the T–J event, surviving groups and new lineages (thalattosuchian crocodylomorphs, plesiosaurs, and parvipelvian ichthyosaurs) radiated to occupy some vacant and previously unexplored niches, while other taxa synchronously declined (Motani 2005; Benson et al. 2013; Benson and Druckenmiller 2014; Stubbs and Benton 2015). These events established the classic marine reptile fauna that characterized Europe, and much of the world, during roughly the first half of the Age of Dinosaurs.
The fossil record of Jurassic marine tetrapods is rich, but is often based on a relatively small number of (particularly European) Lagerstätten, meaning that our knowledge on the systematics and evolution of these animals may be temporally and spatially biased (Benson et al. 2010). In the UK these fossil-rich formations are common and include the Lower Jurassic deposits of the Blue Lias Formation (Weedon et al. 2017), the Middle–Late Jurassic OCF (Callovian–early Oxfordian, ~165–161 Ma) and the Late Jurassic KCF (Kimmeridgian–Tithonian, ~157–148 Ma) (Benson et al. 2010). Nevertheless, these Lagerstätten are discontinuously distributed through time, affecting our understanding of marine fauna evolution, especially at stage-level resolution. Thus, some critical intervals are less well-understood than others, and this is particularly frustrating when these “gaps” were times of major environmental perturbations, often connected to dramatic faunal turnover. An example of one of these poorly known, but important, stages is the Oxfordian (early Late Jurassic)—this stage essentially separates the well-known fossil records of the OCF and KCF Lagerstätten.
The Oxfordian witnessed a drastic decline of pliosaurid taxic and morphological diversity; a shift in thalattosuchian crocodylomorphs from a (numerically but not taxonomically) piscivorous-dominated metriorhynchine fauna to a hypercarnivorous-dominated geosaurine fauna; and the radiation of platypterygiine ichthyosaurs (Young et al. 2012b; Benson and Druckenmiller 2014; Young 2014; Fischer et al. 2015, 2016, 2017). This stage was also characterised by strong fluctuations in sea water depth and temperature, late Callovian–early Oxfordian cooling followed by middle–late Oxfordian warming, that drastically affected the distribution of facies and invertebrate fossils in Tethyan and peri-Tethyan areas (Dromart et al. 2003a, b; Cecca et al. 2005). In the sub-boreal area, such dramatic changes occurred across a ~8 million year interval deposited in between the strata of the OCF and KCF, concordant with radical changes in the taxonomy and diversity of every marine reptile lineage (Young et al. 2012a, b; Benson and Druckenmiller 2014; Young 2014; Fischer et al. 2015, 2016, 2017). Unfortunately, Oxfordian vertebrate fossils from the UK are rare and have received only scant attention, leaving numerous unanswered questions on the mode and timing of this marine reptile faunal turnover.
A better understanding of the rare Oxfordian fauna promises to shed light on the extinction, radiation, and turnover of marine reptile groups during this time. Thus, we here review what is currently known of the marine reptile fossil record of the Oxfordian beds of the Jurassic Sub-Boreal Seaway in the UK, based on a comprehensive survey of museum collections. Specifically, we focus on the least investigated part of the Oxfordian succession, called the “Corallian Group” (CG, late lower Oxfordian–upper Oxfordian, ~161.5–157.3 Ma). There has been very little study of the vertebrate fossils of these strata, despite the observation that dramatic changes in depositional settings occurred during this interval (Cecca et al. 2005). In doing so, we exclude the youngest part of the lower Oxfordian (Stewartby and Weymouth members of the OCF), which has been thoroughly investigated (e.g., Martill and Hudson 1991).
The bulk of our sample is hosted in several key collections: BRSMG, CAMSM, DORCM, MANCH, NHMUK, OUMNH, and YORYM. To date the most comprehensive effort to catalogue the rare fossils from these strata was made by Benton and Spencer (1995), who listed a number of fossil tetrapods found in the Corallian Group and upper OCF. However, the identification of several specimens in this work is clouded by outdated taxonomy and unresolved systematics, as the phylogenetics of many of the major marine reptile groups had yet to be studied in detail at the time. Here, we provide a fresh look at the Oxfordian marine reptiles of the UK, based on a re-examination of published specimens and study of new specimens, all of which are described in a phylogenetic and evolutionary context. This will help to better understand the changes in the Jurassic Sub-Boreal Seaway faunas during this interval of time.
Institutional abbreviations.—BRSMG, Bristol Museum and Art Gallery, Bristol, UK; CAMSM, Sedgwick Museum, Cambridge, UK; DORCM, Dorset County Museum, Dorchester, UK; GLAHM, Hunterian Museum, Glasgow, UK; MJML, Museum of Jurassic Marine Life—the Steve Etches Collection, Kimmeridge, UK; NHMUK, Natural History Museum, London, UK; OUMNH, Oxford University Museum of Natural History, Oxford, UK; SMNS, Staatliches Museum für Naturkunde Stuttgart, Germany; YORYM, Yorkshire Museum, York, UK.
Other abbreviations.—ABL, apicobasal length; ACF, Ampthill Clay Formation; AL, apicobasal length; CBL, crown base length; CBW, crown base width; CG, Corallian Group; FAD, first appearance date; KCF, Kimmeridge Clay Formation; LAD, last appearance date, OCF, Oxford Clay Formation.
Geological setting
Oxfordian sedimentary rocks in Britain crop out in patches along the continuous succession of the Jurassic System running from the South-West to the North-East of England, with isolated exposure in Northern Scotland and the Inner Hebrides (Figs. 1, 2). In the UK, the Oxfordian is represented by the upper OCF (Stewartby and Weymouth Members), the CG (a series of silico-clastic to carbonate rock units; Cope 2006), and the Ampthill Clay Formation (variably middle to upper Oxfordian) in Yorkshire, Oxfordshire, and Wiltshire. The CG represents a prominent part of the Oxfordian stage, and roughly spans from the Cordatum cordatum to the Ringsteadia pseudocordata evoluta ammonite subzones (although the OCF–CG boundary is marginally older in Yorkshire: Quenstedticeras mariae precordatum Ammonite Subzone, lower Oxfordian) (Coe 1995; Wright 2009). At least six major corrosive unconformities have been detected through the entire CG succession, the first (O1 in Yorkshire/O2 elsewhere) and the last of which (O6) represent the lithostratigraphic lower and upper boundaries of the CG with the OCF and the KCF, respectively (Coe 1992, 1995; Figs. 1, 2).
The CG succession is a complex succession of carbonate and silico-clastic units deposited in a shallow-marine environment during a prominent cycle of regression-transgression (Coe 1995; Figs. 1, 2). The long-term regression is represented by the transition from Callovian–early Oxfordian marine clay (OCF), to the mixed silico-clastics/carbonate deposits of the middle Oxfordian, and eventually carbonates. The long-term transgression is thought to have begun at the Perisphinctes parandieri Ammonite Subzone (middle Oxfordian), and later culminated in the deposition of dark mudstone in the deep basin of the KFC (Coe 1995). Short-term sea-level cycles are superimposed on this long-term transgression-regression cycle and are well represented in two main basins, the Wessex Basin (Dorset, Wiltshire, and Oxfordshire successions) and Cleveland Basin (Yorkshire succession) (Coe 1995). Although the facies in these localities formed synchronously, they vary laterally and vertically, most likely related to tectonic influence and proximity to sediment source (see Coe 1995 for more details). Fortunately, they are also stratigraphically well correlated and accurately represent the variety of depositional environments present in the Jurassic Sub-Boreal Seaway at the time (Figs. 1, 2).
Unlike in the overlying KCF and underlying OCF, vertebrate fossils are rare in the Corallian beds and mostly are isolated teeth, with exceptionally rare skeletal materials (see Benton and Spencer 1997; Young 2014; Foffa et al. 2015). Unfortunately, most of the fossils were collected before a precise stratigraphy was established. Several of the most prolific sites were quarries that are now abandoned or covered by buildings, so fine stratigraphic information is not retrievable. However, even if the available locality information for specimens is sometimes vague, it is often sufficient to be reconciled with the current stratigraphy, at least to formation/member level. Figures 1 and 2 summarise the principal units within the Corallian succession of the relevant stratigraphic units of the Oxfordian Wessex and Cleveland Basins. Further details on the lithostratigraphy of these basins are beyond the scope of this study, but can be found in the works of Cope (2006) and Wight (2009, 2014).
The rarity of fossils in the Oxfordian strata is well known and has been considered a genuine signal rather than a taphonomic/collection bias (Benton and Spencer 1995). Indeed, we agree with this assessment for a simple reason. Several of the Oxfordian fossil-bearing localities (in Yorkshire, Dorset, Wiltshire, Oxfordshire) are/were limestone and/or sandstone sites that have been historically exploited for building materials, aquifers, and oil exploration (Cox 2001). So, not unlike with the clay quarries of the OCF and KCF, there has been ample opportunity to sample vertebrate fossils in the Oxfordian strata, even when considering a lithological bias. Nevertheless, the OCF and KCF have yielded abundant specimens, whereas the Oxfordian strata have not.
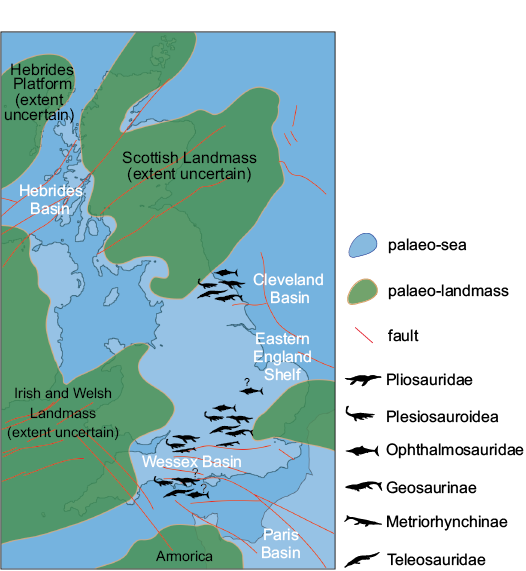
Fig. 1. Palaeogeographical map of Mid-Oxfordian in the UK (from Bradshaw et al. 1992 and Coe 1995).

Fig. 2. Stratigraphic columns showing the Oxfordian geological succession the main Corallian fossiliferous counties (from Cope 2000; Wright 2006, 2009; Coe 1995). Abbreviations: Fm., Formation; L. Calc., Lower Calcareous; Mb., Member; W.L.M, Wheatley Limestone Member; ? uncertain occurrence/lost specimen; * from Bedsfordshire. For explanations of animal silhouettes please see Fig. 1.
Material and methods
Most of the specimens described here are isolated teeth, which are challenging to classify for various reasons. Firstly, morphological convergence: different groups of marine reptiles exhibited extensive convergence in shape, size, and texture of their tooth crowns through their evolutionary history (Massare 1987). However, similarities are often limited to general features and do not affect the finest details, enabling higher-level taxonomic identifications even if they admittedly make species-level identifications more difficult. Most problematic, all marine reptile groups exhibit some degree of heterodonty, or at least anisodonty, meaning that the teeth often change drastically in shape and size across the tooth row of a single animal (Noè 2001; Andrade et al. 2010; Sassoon et al. 2015). Luckily, these changes are traceable in complete specimens, so it is often possible to provide detailed diagnoses by combining these features with the characters that stay unchanged along the tooth row. For these reasons, teeth are usually diagnostic for most marine reptile groups up to low taxonomic levels. When an assignment to a low taxonomic level was not possible, we subdivided our samples into morphotypes, and then discussed their likely phylogenetic affinities.
Specimen descriptions are based on standard characters that are commonly used in the literature, and can be found in the most recent phylogenetic datasets (Fischer et al. 2015, 2016, 2017; Young et al. 2016; Foffa et al. 2017). We particularly focused on shape (AL, CBL, CBW and ratios among them, ABL/CBL, curvature); ornamentation (enamel ridge morphology, coarseness, distribution and variation around and along the crown; texture of the dentine); wear and breakage (presence/absence/type of breakage and wear); carinae and denticles (presence/absence/type of serration morphology).
The phylogenetic tree structure and taxonomic and age (FAD and LAD) data for Fig. 14 come from pruned versions of Fischer et al. (2015, 2017) and Foffa et al. (2017). Given the local nature of the dataset, we did not consider ghost lineages. The figure was produced using the “strap” package for R (R Core Team 2013; Bell and Lloyd 2015).
Results
Plesiosauria
Plesiosaurian remains are fairly common amongst Corallian vertebrates (Figs. 3–5). The majority of specimens that can be confidently referred to the group are large pliosaurid teeth (Figs. 4, 5). These are most commonly found in the lower and middle part of the Oxfordian of Yorkshire (Coral Rag Member of the Coralline Oolite Formation and Lower Calcareous Grit Formation) and Oxfordshire (Stanford Formation, “Coral Rag”) (Figs. 1, 2). Large teeth have often been referred to the genera Pliosaurus, Liopleurodon, or Plesiosaurus in the past. Rare plesiosauroid teeth can also be found (Fig. 3A, B), as well as a handful of damaged and undiagnostic postcranial remains. Often the identifications of these specimens were made at the time of discovery and never revisited, and upon review we found no evidence supporting most of them. In other cases, when the specimens were referred to taxonomically problematic taxa (e.g., “Pliosaurus” grossouvrei), we revisited the validity of these taxa and discussed the affinities of the specimens, case-by-case.
Corallian plesiosauroid fossil are mostly isolated cryptoclidid teeth (Fig. 3A, B). They are found in both the Wessex and Cleveland Basins, with specimens primarily found in the Lower Calcareous Grit Formation (lower to middle Oxfordian) in Wiltshire and in the Coral Rag Member of the Coralline Oolite Formation in Yorkshire and contemporaneous “Coral Rag” (equivalent to the Wheatley Limestone Member–middle Oxfordian) of the Stanford Formation of Oxfordshire (Figs. 1, 2). Most findings have been referred to Plesiosaurus (a Lower Jurassic genus) or Muraenosaurus without further explanation (Benton and Spencer 1995). One of the youngest occurrences of plesiosauroids in the Oxfordian is a dorsal vertebra associated with two propodial fragments from Weymouth Dorset (DORCM G197) assigned to Muraenosaurus (Benton and Spencer 1995). There are, however, no known diagnostic characters in Muraenosaurus vertebrae, and the propodial fragments are too damaged to bear genus-level taxonomic utility, so the specimen should be referred to Plesiosauroidea indet. based on the proportions of the dorsal centrum.
Cf. Muraenosaurus: Two previously undescribed plesiosauroid teeth bear strong similarities with the Callovian cryptoclidid Muraenosaurus. BRSMG Cd5593 (Fig. 3A) from the Lower Calcareous Grit Formation (lower to middle Oxfordian, “lower Grit of the Coral Rag”) of Seend (Wiltshire), and OUMNH J.47559 (Fig. 3B) from the Wheatley Limestone Member (middle Oxfordian, “Coral Rag”) at Windmill quarry in Headington (Oxfordshire). Both teeth are still partially embedded in the matrix and their lingual side is inaccessible, making precise identification difficult. However, some accessible features on the exposed crown sides help to clarify their taxonomic affinities.
BRSMG Cd5593 is a large (AL ~33 mm) crown with a narrow base (CBR ~4.3). The single-cusped, lingually-curved crown is still attached to a conspicuous portion of the root. Although the tooth is still embedded in the rock (a yellow-to-grey calcareous bioclastic sandstone), the mesial and distal edges are visible, and bear enamel ornamentation that consists of a series of discontinuous ridges that are weakly divergent from one other (Fig. 3A2, A3).
OUMNH J.47559 is similar to BRSMG Cd5593. It is a single cusped ~34 mm long crown still attached to a substantial part of its root (Fig. 3B). The matrix surrounding the crown is an oolitic sandstone, yellow-to-grey in colour, typical of the Wheatley Limestone Member. The labial side is smooth and unornamented, whilst the other sides are unobservable.
Additional specimens showing the same morphology have been found. YORYM:2006.1038 in the Lower Calcareous Grit (contemporaneous with the upper OCF, lower Oxfordian) of Appleton (North Yorkshire) and YORYM: 2006.310, 2006.311 in the Coral Rag Member of the Coralline Oolite Formation (middle Oxfordian, “Coral Rag”) at North Grimston (Yorkshire).
The morphology of these specimens, particularly the presence of enlarged teeth and the absence of ornamentation on the lingual side of the crown, is typical of some Callovian cryptoclidid plesiosauroids such as Muraenosaurus leedsi, Tricleidus seeleyi (but not Cryptoclidus eurymerus), and isolated and still taxonomically indeterminate teeth found in the Kimmeridgian (CAMSM J.30069–J.30071) (Andrews 1910; Brown 1981) (Fig. 3C–G). The teeth have very high CBR (~5) values. Among Jurassic marine reptiles only plesiosaurians have such high CBR (> 4) ratios combined with such large AL and root lengths. The differences among Callovian and Kimmeridgian–Tithonian plesiosaur dentitions mostly concern their absolute size and the ornamentation pattern on the enamel. These are reported by Andrews (1910) and Brown (1981), who provided the most detailed dental description of the Middle and Late Jurassic British plesiosauroids with preserved teeth: Muraenosaurus leedsi, Cryptoclidus eurymerus, Tricleidus seeleyi, and Kimmerosaurus langhami (Fig. 3C–G).
Muraenosaurus and Tricleidus have enlarged anterior maxillary and dentary teeth relative to the rest of the tooth row (heterodonty), while Cryptoclidus dentition is considered more uniform, with variation concerning mostly the size rather than the shape of the teeth (anisodonty). Thus, the largest teeth of Cryptoclidus are rarely longer than 25 mm from base to tip of the crown, while in Muraenosaurus the largest fangs can easily reach and surpass 30 mm in AL. The distribution of enamel ridges around the crown is also different in each genus, ranging from only 5–7 well separated ridges on Cryptoclidus to the densely ornamented teeth of Tricleidus (Brown 1981). Muraenosaurus crowns are more similar to the latter in that ridges are present on all surfaces of the teeth (although they are less densely packed on the lingual side) (Brown 1981). In light of these descriptions, we note striking similarities especially between the CG specimens and Muraenosaurus teeth (see Brown 1981: fig. 19), particularly in the case of BRSMG Cd5593 (Fig. 3A, B, F). We can also confidently determine that none of the above described Corallian specimens belong to Cryptoclidus, whose teeth are too small and only bear a few, well-separated ridges. The CG specimens are also very different from the small, gracile, hooked, laterally compressed, and unornamented teeth of Kimmerosaurus langhami (Brown 1981; Brown et al. 1986; Fig. 3G).
Our identification of the CG teeth as belonging to a Mureanosaurus-like taxon shows that this classic group of Callovian cryptoclidids persisted into the Corallian gap. Furthermore, Brown (1981) reported two isolated teeth from after the Corallian gap (CAMSM J.30069–J.30071 from the KCF and CAMSM J.13270a from the Portland Stone Formation; Fig. 3E) that resemble Mureanosaurus based on the strong ornamentation on the lingual side and lack of ornamentation on the labial side. Thus, plesiosaurs with Mureanosaurus-type dentition survived in the Jurassic Sub-Boreal Seaway during the Oxfordian and younger time intervals.
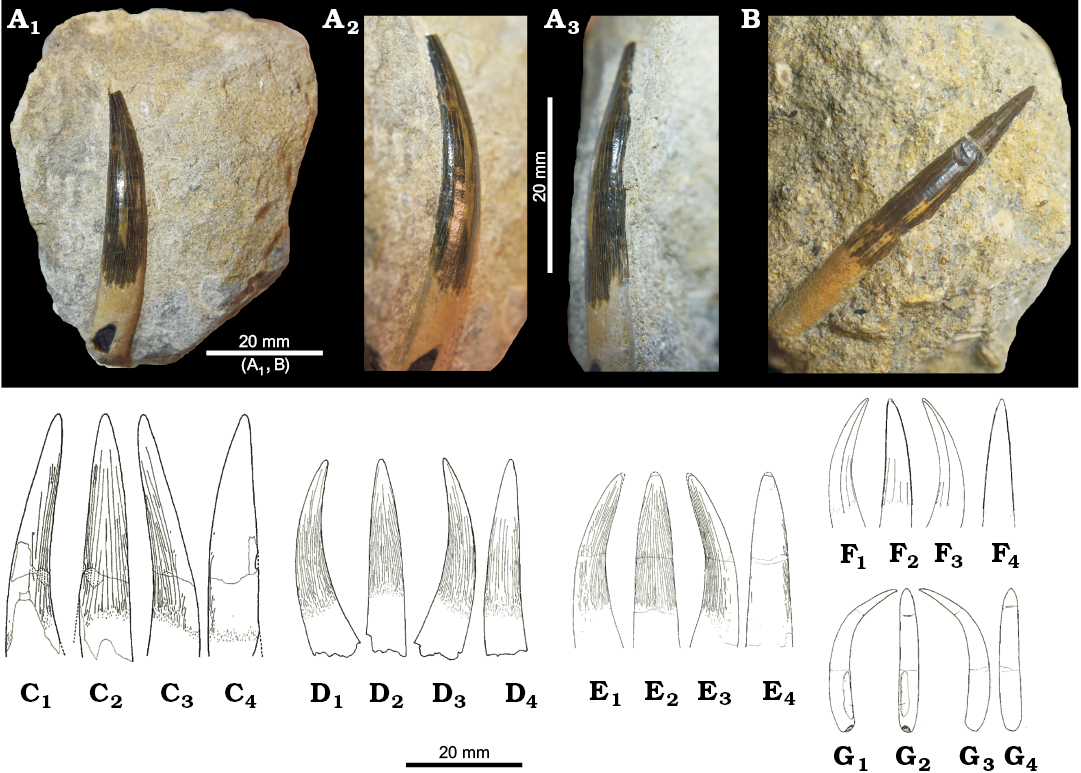
Fig. 3. Teeth of Plesiosauroidea. A. BRSMG Cd5593, cf. Muraenosaurus, from Seend (Whiltshire), lower to middle Oxfordian. B. OUMNH J.47559, Cryptroclididae indet., from Headington (Oxfordshire), middle Oxfordian. C. NHMUK PV R 2861, Muraenosaurus leedsi Seeley, 1874, from Peterborough (Cambridgeshire), Callovian. D. NHMUK PV R3539, Tricleidus seeleyi Andrews, 1909 from Peterborough (Cambridgeshire), Callovian. E. CAMSM J.30070, Cryptoclididae indet. from Ely (Cambridgeshire), Kimmeridgian. F. NHMUK PV R8621, Cryptoclidus eurymerus (Phillips, 1871) from Peterborough (Cambridgeshire), Callovian. G. NHMUK PV R8431, Kimmerosaurus langhami Brown, 1981 from Endcombe Bay (Dorset), Tithonian. A1, B, C4–G4, labial, A2, A3, C1–G1, C3–G3, axial, and C2–G2, lingual views. Note the similarities in the distribution and shape of ornamentation in A and E. C–G modified from Brown (1981).
Pliosauridae
We report a vast collection of large pliosaurid teeth from the Corallian deposits of Yorkshire, Oxfordshire, Wiltshire, and Dorset (Figs. 4, 5). Specimens are known from a variety of formations, namely the Lower Calcareous Grit Formation, Coral Rag Member of the Coralline Oolite Formation, and upper Corallian of Dorset, spanning from the lower to upper Oxfordian. Several specimens have been known since the 19th century. This material has received little attention and specimen identifications have lagged behind recent advancements in plesiosaur taxonomy and systematics (Noé 2001; Benson et al. 2010; Benson and Druckenmiller 2014; Fischer et al. 2015, 2017). The vast majority of known teeth have been identified as either Pliosaurus or “Pliosaurus” grossouvrei (Liopleurodon grossouvrei). However, in their recent reviews of Pliosaurus, Knutsen et al. (2012), and Benson et al. (2013) showed that a trihedral (or “sub-trihedral” in the case of Pliosaurus kevani) tooth cross-sections is diagnostic of the Late Jurassic genus Pliosaurus, meaning that none of the Corallian specimens we describe can be referred to this genus because they lack this tooth type. It is also worth noting, for completeness, that sub-trihedral teeth are present in Gallardosaurus iturraldei and Stenorhynchosaurus munozi, and that this is a plesiomorphic condition in Brachaucheninae (Gasparini 2009; Fischer et al. 2015; Páramo et al. 2016).
Type 1 “Pliosaurus” grossouvrei.—Teeth of this type are characterised by moderate to high CBR and strong ornamentation, which is almost exclusively present on the lingual side, and consists of high relief (“coarse ridges” in plesiosaurian literature) and continuous enamel ridges that are triangular in cross-section. Only a few ridges reach the apical half of the crown (with maximum 3–4 continuing until the apex), while most of the others terminate in the basal half of the crown (Figs. 4A, B, 5A, B). The ridges are less densely packed than in Liopleurodon ferox (Noè 2001; DF personal observations, e.g., NHMUK PV R2680, PV R3536; Fig. 4C), and the ornamentation on the labial side is absent in the largest crowns (BRSMG Cd5588), but may consist of a few very short ridges at the crown-root contact in mid-size crowns (e.g., YORYM:2007.2987.2). The large sample of teeth of this type offers a chance to discuss character variation along the tooth row, which we outline here.
BRSMG Cd5588 is a large caniniform crown (AL 63.2 mm, CBR ~2.54) still attached to an incomplete root and embedded in a bioclastic grey/yellow limestone (Fig. 4A). It was found at North Grimston, near Malton (Yorkshire), in the “Upper Oolite, Corallian” (likely corresponding to the Upper Corallian Oolite, middle Oxfordian). Unfortunately, only the labial side and the apex of the crown are exposed from the matrix. Fortunately, however, because of the distinguishability of pliosaurid teeth, it is still possible to confidently rule out some taxonomic relationships for this specimen. The size and shape of the crown and root are characteristic of an enlarged anterior tooth (from the maxilla or anterior dentary, or one of the middle premaxilla) of a large bodied pliosaurid. Both Oxfordian and Kimmeridgian pliosaur affinities can likely be excluded for this specimen because of its lack of the triangular/subtriangular cross section typical of Gallardosaurus iturraldei, Pliosaurus spp., and Stenorhynchosaurus munozi (Gasparini 2009; Benson et al. 2013; Pàramo et al. 2016). Liopleurodon ferox, Simolestes vorax, and “Pliosaurus” andrewsi are the only Callovian pliosaurids known for having teeth of comparable size, and therefore the CG specimens likely belong to one of these taxa. Amongst these, the CBR of “Pliosaurus” andrewsi is notably lower than in the other taxa: 1.67 (1.39–2.06) for “Pliosaurus” andrewsi; 2.56 (2.30–3.10) for Liopleurodon ferox; 2.67 (2.32–3.2) for Simolestes vorax larger teeth (Noè 2001). “Pliosaurus” andrewsi also differs from other Callovian taxa in having a unique type of tooth wear, in which abrasion on the crown is considerably more extensive than in any other plesiosaur (Tarlo 1960; Judyth Sassoon personal communication 2017). BRSMG Cd5588 has high relief ornamental ridges, four of which reach the sharp apex, which makes it more similar to Liopleurodon than to Simolestes vorax or “Pliosaurus” andrewsi.
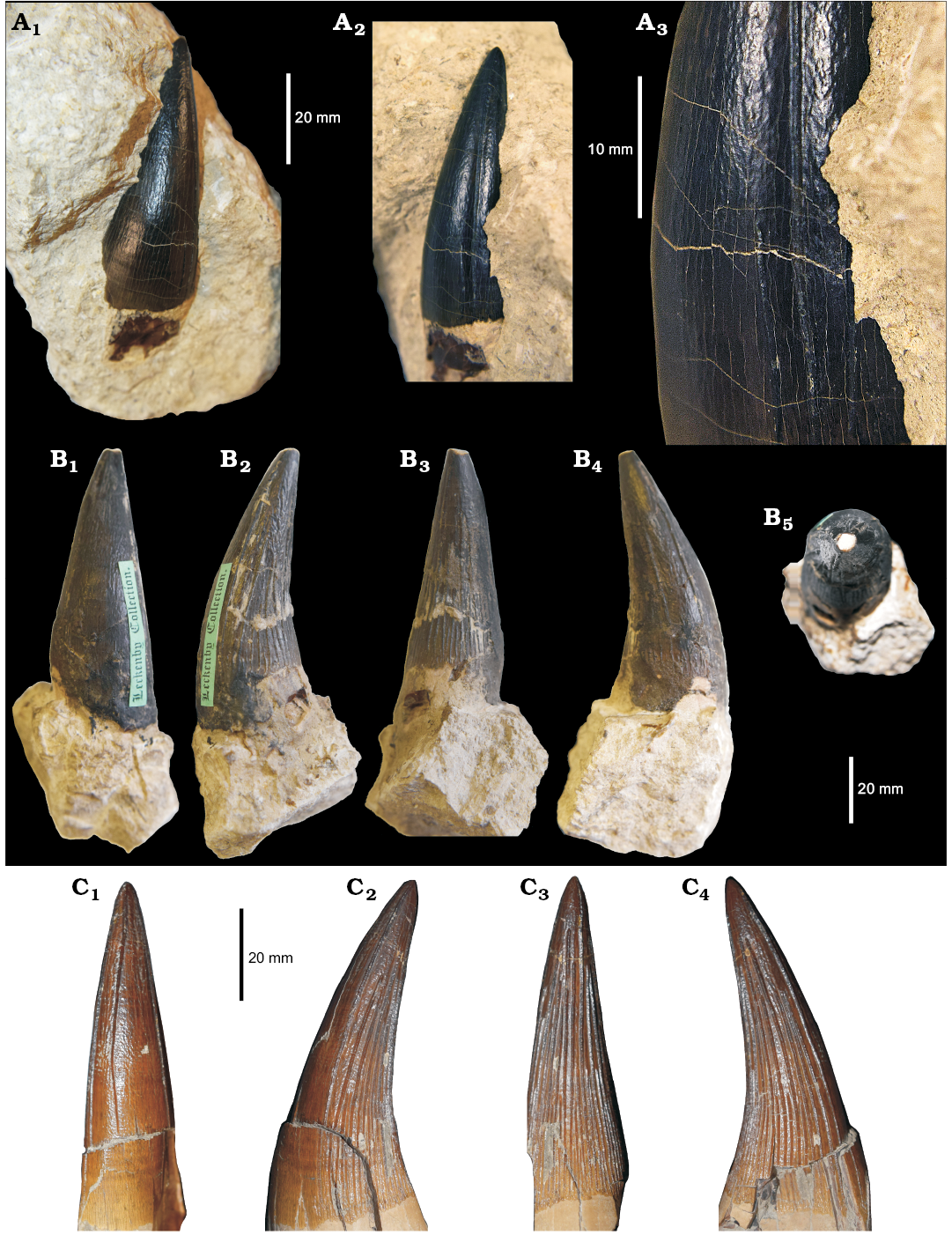
Fig. 4. Teeth of the pliosaurid reptile “Pliosaurus” grossouvrei Sauvage, 1873 (A, B) and Liopleurodon ferox Sauvage, 1873 (C). A. BRSMG Cd5588, from North Grimston (Yorkshire), middle Oxfordian, in labial (A1) and axial (A2) views, and close up of axial side (A3). B. CAMSM J.13303, from unknown “Corallian” locality, Oxfordian, in labial (B1), axial (B2, B4), lingual (B3), and apical (B5) views. C. NHMUK PV R2680, from Peterborough (Cambridgeshire), Callovian, in labial (C1), axial (C2, C4), and lingual (C3) views.
Several other teeth belong to this same morphotype, based on the shape, texture, and ornamentation of the crowns. These include: NHMUK PV OR 47044 (Fig. 4A), NHMUK PV OR 47971 (Fig. 5B), and a collection of 6 teeth under the accession number NHMUK PV OR 36335, from the Coral Rag of Malton (Yorkshire) (Lydekker 1889; Fox-Strangways 1892). Together, these specimens are important because they exemplify the variation in ornamentation and proportions along the tooth row (Fig. 5). NHMUK PV OR 47044 and PV OR 47971 are in fact slightly smaller and stouter than the largest teeth belonging to this morphotype, with CBR values (~2.16) closer to the “Pliosaurus” andrewsi average. NHMUK PV OR 47044 and PV OR 47971 were originally assigned by Lydekker (1889) to Liopleurodon grossouvrei (Sauvage 1873), which has since been incorporated into “Pliosaurus” andrewsi (Tarlo 1960), based on similarities with the holotype figured by Sauvage. We disagree with this assignment (see Discussion), and in our opinion, these teeth probably belong to a new taxon. Other specimens of this morphotype include: CAMSM J.13302, erroneously identified as a Pliosaurus sp. tooth from the Lower Calcareous Grit of Scarborough (Yorkshire); YORYM:2007.2987.1 and 2007.2987.2 from the Coral Rag of Yorkshire; YORYM:2016.304 from the Coralline Oolite of Slingsby (Yorkshire); OUMNH J.52357 from Bullington (Oxfordshire); CAMSM J.13297–J.13299 from the Coral Rag of North Grimston (Yorkshire) (Seeley 1869 referred these specimens to Pliosaurus Jb2 23, Jb2 21, Jb2 22 [sic!] in the old CAMSM catalogue; Drake and Sheppard 1909); and CAMSM J.13303 from an unknown “Corallian” locality. A considerable number of teeth from the Coralline Oolite of Slingsby (North Yorkshire) in YORYM also belong to this morphotype (e.g., YORYM:2007.2985.1, 2007.2985.2, 2016.298, 2016.299, and associated unnumbered teeth). This latter collection is particularly interesting as the teeth cover the whole spectrum of shape and sizes that are expected from pliosaurid jaws (see Taylor and Cruickshank 1993; Noè 2001; Sassoon et al. 2015).
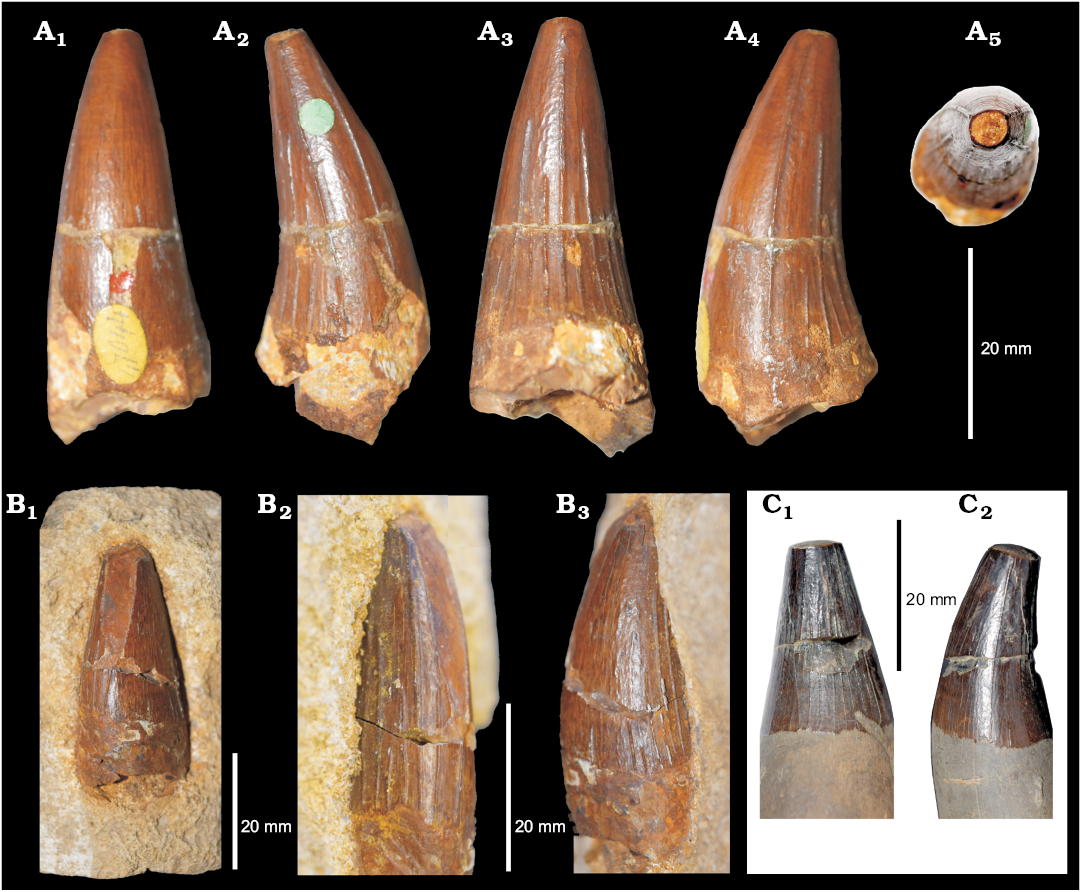
Fig. 5. Teeth of the pliosaurid reptile “Pliosaurus” grossouvrei Sauvage, 1873 (A, B) and “Pliosaurus” andrewsi Tarlo, 1960 (C). A. NHMUK PV OR 47044, from Malton (Yorkshire), middle Oxfordian, in labial (A1), axial (A2, A4), lingual (A3), and apical (A5) views. B. NHMUK PV OR 47971a, from Malton (Yorkshire), middle Oxfordian, in labial (B1), axial (B2), and oblique axial/labial (B3) views. C. NHMUK PV R3891, from Peterborough (Cambridgeshire), Callovian, in lingual (C1) and axial (C2) views.
Type 1 pliosaurid teeth differ from any Callovian taxon in CBR (intermediate between Liopleurodon ferox and “Pliosaurus” andrewsi) and in the ornamentation, which compared to all known Callovian pliosaurids: (i) is less dense; (ii) lacks continuous ridges crossing the labial side; and (iii) has ridges restricted to the basal half of the lingual side. Specifically, Type 1 pliosaurid teeth differ from “Pliosaurus” andrewsi in having a higher CBR and higher relief ornamentation, and additionally in lacking the characteristic, and extreme, type of abrasion, which is typical of the Callovian taxon (Tarlo 1960; Judyth Sassoon personal communication 2017)—although the latter feature cannot be used as a diagnostic feature. Type 1 crowns are demonstrably different from Peloneustes phylarchus and Marmornectes candrewi based on size, proportions, and ornamentation patterns (Andrews 1913; Ketchum and Benson 2011a, b). They are also distinct from Simolestes vorax (e.g., NHMUK PV R 3319, PV R 3170) based on a lower CBR ratio, and in having considerably fewer ornamental ridges that have a higher relief and are coarser compared to Simolestes (Andrews 1913; Noè 2001). The ornamentation of Type 1 pliosaurid teeth is less dense (fewer ridges) than in Liopleurodon ferox (e.g., NHMUK PV R 3546, PV R 2680), and the ornamental ridges rarely reach the apical half of the crown (Figs. 4, 5); furthermore, the CBR of the largest Corallian teeth is lower than in the largest Liopleurodon ferox fangs (Noè 2001). Finally, analogous arguments could be made for Pachycostasaurus dawni (PETMG R338), as this taxon has a similar ornamentation to Liopleurodon ferox with the difference that the former has considerably coarser ridges than the latter. However, in Pachycostasaurus the ornamental ridges do not reach the bottom of the enamel on the labial side, and are extended all around the crowns in some large crowns (Noè 2001). Unfortunately the only preserved complete tooth of Pachycostasaurus dawni comes from the middle portion of the jaw of a juvenile individual (Cruickshank et al. 1996; Noè 2001).
Despite the differences between Type 1 pliosaurids and all other known taxa, we refrain from naming a new taxon based on these isolated teeth because of the absence of more complete remains and the still unclear taxonomic content of “Pliosaurus” andrewsi (currently under revision: Judyth Sassoon personal communication 2017), and the dearth of more complete and diagnostic remains of “Pliosaurus” grossouvrei.
Indeterminate specimens.—Further non-diagnostic postcranial plesiosaurian specimens from Berkshire, Dorset, Oxfordshire, and Wiltshire are described and figured below (Fig. 6).
Dorset lost pliosaurid specimen: The only known Corallian pliosaurid specimen with cranial remains is unfortunately lost. The specimen is a partial lower jaw that was originally described and figured by Newton (1878) and later mentioned by Delair (1958: 60) who reported it as an undetermined teleosaur). It was found at Sandsfoot Castle (Weymouth, Dorset), in association with Goniomya literata and Pinna lanceolata (Strahan, 1898), “from the uppermost beds of the Coral Rag series West of Sandsfoot Castle, near Weymouth” (Delair 1958: 60). Some information is still obtainable from the description and illustration by Newton (1878). The specimen is ~300 mm long, but lacks large portions of the middle and posterior lower jaw. Crucially, the teeth are reported to have circular cross sections, and bearing ridges but with an unornamented labial side; five ridges are reported to reach the apex. These descriptions may indicate that the specimen is a pliosaurid with non-trihedral teeth, which would support the persistence of these species near the Oxfordian–Kimmeridgian boundary.
Plesiosauria indet.: A number of postcranial, axial, and appendicular remains can only be referred as to Plesiosauria indet. (Fig. 6). These include a juvenile propodial (OUMNH J.50338, Headington, Oxfordshire) (Fig. 6A); a fragment of a girdle element (OUMNH J.10349, perhaps pubis?, Headington, Oxfordshire) (Fig. 6B1, B2); and a cervical and a dorsal vertebra (OUMNH J.12340/1,2, Hatford Farington, Berkshire) (Fig. 6C1, C2).
CAMSM J.13308 is a posterior pliosaurid tooth from the Coral Rag of (?) Heddington, Wiltshire, which was originally and erroneously labelled as Plesiosaurus.
DORCM G.188 is reported in the specimen catalogue of the DORCM as “A dorsal centrum and oyster debris” from “garden 1/4 mile from Coombe Valley, Littlemoor Rd” Furzy Cliff (Dorset). It is assigned to Muraenosaurus sp. in the catalogue, but we find no diagnostic evidence for this and the specimen (which could be from the youngest Oxfordian beds of the OCF) and can only be referred to Plesiosauroidea indet. based on the proportions of the centrum (or perhaps Cryptoclididae indet., but only based on the stratigraphic range of cryptoclidids). Two additional vertebrae, OUMNH J.12340/1 and J.12340/2 from the Upper Jurassic “[Kimmeridge Clay or Corallian]” of Hatford, near Stanford-in-the-Vale (Oxfordshire), were labelled “Plesiosaurus sp. vertebra” and should also be referred as Plesiosauria indet.
Finally, two teeth, CAMSM J.13306 and J.13307, from the Coral Rag of Heddington (Oxfordshire) labelled “Plesiosaurus?” are too worn to be confidently assessed.
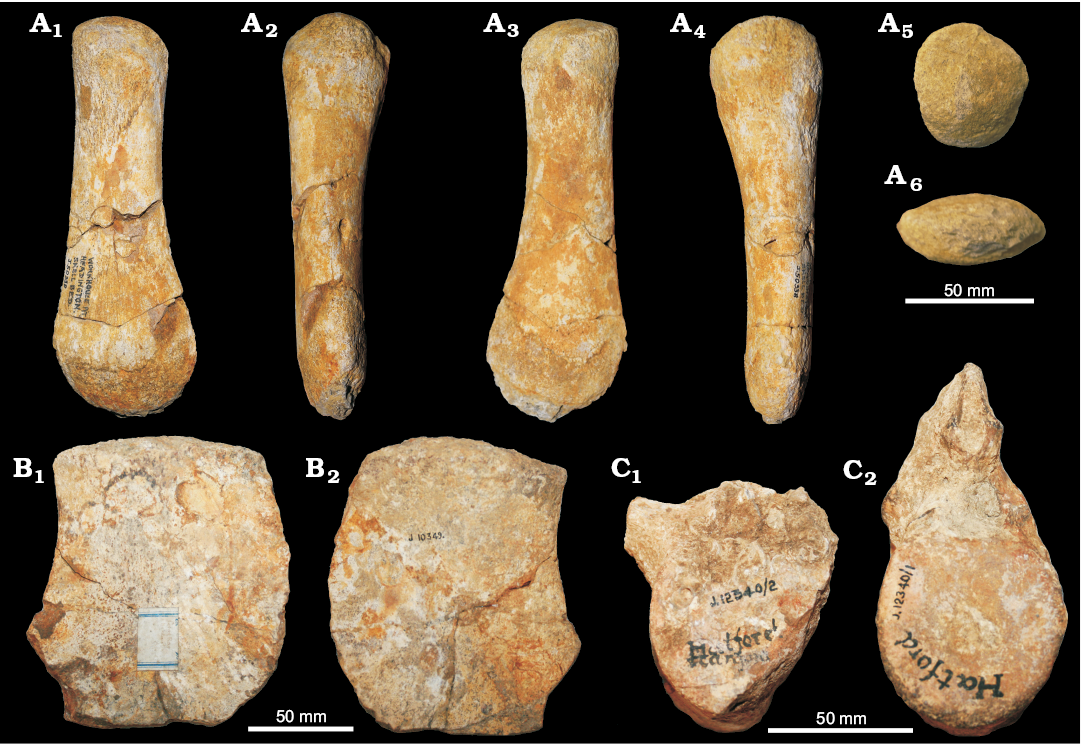
Fig. 6. Postcrania of Plesiosauria indet. A. OUMNH J.50338, ?femur of a juvenile plesiosaur, from Headington (Oxfordshire), ?middle Oxfordian, in lateral (A1), anterior (A2), medial (A3), posterior (A4), proximal (A5), and distal (A6) views. B. OUMNH J.10349, from Headington (Oxfordshire), ?middle Oxfordian, fragment of a girdle element in ventral (B1) and dorsal (B2) views. C. OUMNH J.12340/1,2, from Hatford Farington (Berkshire), pectoral (C1) and dorsal (C2) vertebrae.
Ichthyosauria
Ichthyosaurs are the rarest vertebrates in the Corallian beds. The taxonomy of Middle and Late Jurassic ichthyosaurs from the UK has only recently been rigorously revised, and only a few specimens (mostly isolated vertebrae, OUMNH J.52433–J.52435; CAMSM J.58841, J.10509, J.12051; and a coracoid, OUMNH J.50342) from the Corallian strata of Cambridgeshire, Bedfordshire, and Dorset) have been referred to ichthyosaurs in the latest comprehensive compendium (see Moon and Kirton 2016). We agree that none of these specimens is diagnostic at the generic level, and can only be used to attest to the presence of ichthyosaurs in the Corallian Group. As these specimens have recently been reviewed, we will not discuss them further.
In our survey, we found only one additional single tooth that can be tentatively assigned to Ophthalmosauridae. The specimen is listed together with four other reptilian teeth under the accession number NHMUK PV R 648, from the Coralline Oolite of Malton (Yorkshire) (Fig. 7A). All the teeth were labelled “Steneosaur?” or “tooth of crocodile”, a referral that we find impossible to verify because of the poor preservation of most of the specimens. One of those teeth belongs to an indeterminate ophthalmosaurid (likely cf. Ophthalmosaurus sp.) based on the shape and texture of the crown. There is no ornamentation, and what may be mistaken for ornamental ridges are instead broad, continuous, and smooth infoldings of the dentine (a typical feature in Neoichthyosauria; Schultz 1969, 1970; Kirton 1983; Moon and Kirton 2016). It is, however, worth noticing that this feature is phylogenetically homoplastic or may be present as similar, but potentially non-homologous conditions in other groups, as shown by Fischer et al. (2013, 2014), Moon and Kirton (2016), and Moon (2017). The crown is ~15 mm long and slightly curved, and the details of the roots, enamel-root contact, and crown tip are still hidden in the oolitic beige matrix.
We also report a single tooth, NHMUK PV R 6770, from the Corallian of Malton (Yorkshire), which was labelled as a tooth of “Ichthyosaurus sp.” (Fig. 7B). A careful examination revealed no diagnostic characters supporting the assignment of the specimen to any particular group, particularly Ichthyosaurus, which is an Early Jurassic taxon (Lomax and Massare 2017). Thus it can only be referred to Reptilia indet.
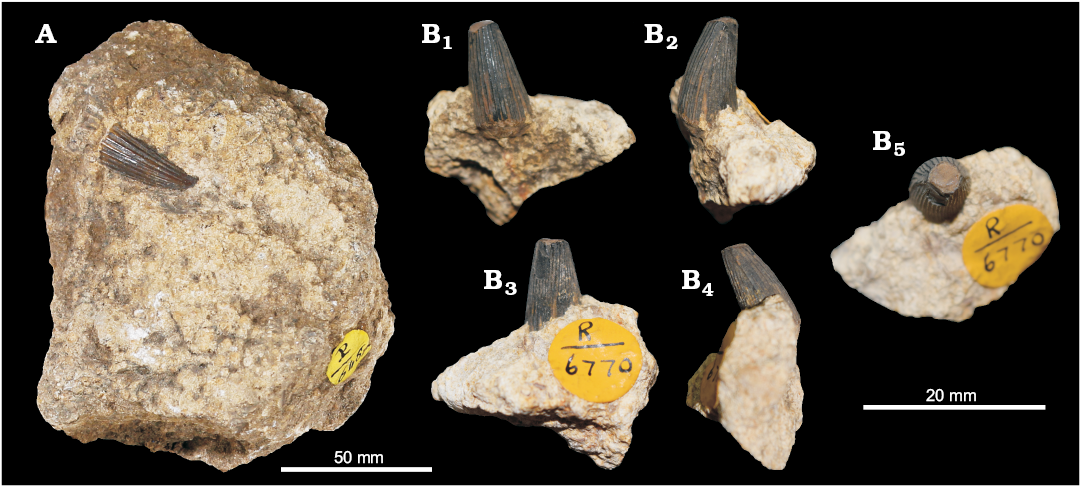
Fig. 7. Ichthyosaurid reptile cf. Ophthalmosaurus sp. (A) and Reptilia indet. (B) from Malton (Yorkshire), Oxfordian. A. NHMUK R 648, isolated crown, the exact view cannot be assessed. B. NHMUK PV R 6770, tooth in labial (B1), axial (B2, B4), lingual (B3), and apical (B5) views.
We report a new unambiguous Ophthalmosaurinae cranial remain: CAMSM J.29866, a small (~60 mm across) and well preserved basioccipital recovered from clay beds near Ampthill, Bedfordshire (Fig. 8). This specimen likely comes from the Ampthill Clay (middle–upper Oxfordian in Bedfordshire). The shape of the basioccipital closely matches that of the Ophthalmosaurus icenicus holotype and other ophthalmosaurines (see Moon and Kirton 2016); in particular, the size of the extracondylar area is more extensive than in Brachypterygius and other Late Jurassic ophthtalmosaurids (Moon and Kirton 2016), but slightly reduced in ophthalmosaurines compared to Lower Jurassic ichthyosaurs. Its smaller size compared to holotype of Ophthalmosaurus icenicus and other adult specimens (NHMUK PV R 2133, PV R 4522, GLAHM V1070) and slight shape differences (e.g., in the peg area), which are likely to be due to poor ossification (Benjamin C. Moon personal communication 2017), suggest that CAMSM J.29866 may belong to a juvenile specimen. Based on this we refer CAMSM J.29866 to Ophthalmosaurinae indet.
Additionally, Benton and Spencer (1995) reported two additional ichthyosaur specimens. The first consists of a series of 34 vertebrae that was found at Furzy Cliff (Weymouth, Dorset, perhaps in the Cardioceras cordatum Ammonite Zone), briefly mentioned by Cope (1974). It was later referred by Benton and Spencer (1995) to Ophthalmosaurus sp., but never formally described. Unfortunately, the whereabouts of this specimen is currently unknown, so this assignment is impossible to verify. Finally, DORCM G.173 from the “Corallian of Osmington” is a small dorsal vertebral centrum (~35 mm across) belonging to a small (perhaps juvenile) ophthalmosaurid. This specimen is labelled as “Ichthyosaurus sp.”; however vertebrae are poorly diagnostic in Neoichthyosauria, and the phylogenetic position of Malawania anachrous means that not all Middle–Late Jurassic ichthyosaurs can be assumed to belong to Ophthalmosauridae (Fischer et al. 2013). Thus, DORCM G.173 should instead be referred to Neoichthyosauria indet.
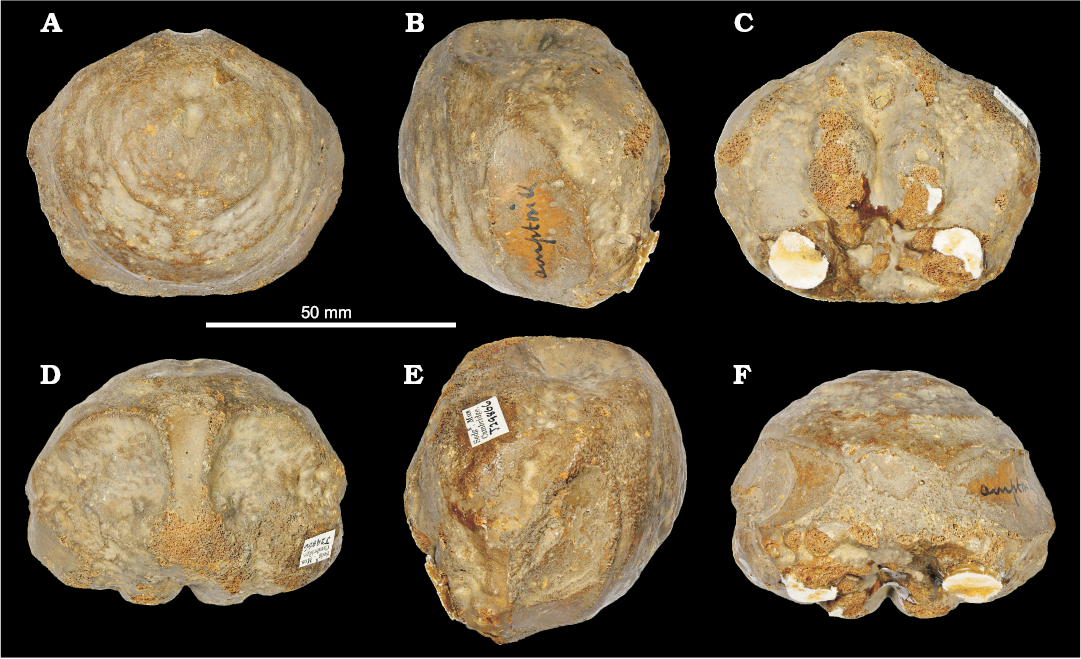
Fig. 8. Ichthyosaurid reptile Ophthalmosaurinae indet. (CAMSM J.29866), basioccipital from Ampthill (Bedfordshire), Oxfordian, in posterior (A), lateral (B, E), anterior (C), dorsal (D), and ventral (F) views.
Thalattosuchia
Thalattosuchia has also long suffered from numerous systematic and taxonomic issues, until recent comprehensive phylogenetic analyses (Young et al. 2012b, 2016). Excluding a few recent discoveries (Young 2014; Foffa et al. 2015), the majority of the known Corallian specimens consist of isolated, and often worn, teeth, which have generally been assigned to historically problematic taxa (e.g., “Steneosaurus”, “Teleosaurus”, and “Metriorhynchus”) (Benton and Spencer 1995). We here review a collection of historic and previously undescribed specimens, and report some newly located cranial and postcranial elements. This demonstrates that the misidentification of specimens had led to a severe underestimate of thalattosuchian diversity in the CG.
Teleosaurids.—The group of long-snouted, nearshore marine and lagoonal thalattosuchians were considerably diverse in the Callovian of the Jurassic Sub-Boreal Seaway of the UK. After the Callovian, a single lineage of teleosaurids was dominant during the Oxfordian, Machimosaurini (Young et al. 2014b; Foffa et al. 2015). All Oxfordian findings, in fact, belong to this group (Lemmysuchus obtusidens + Machimosaurus), which can be easily recognised by their unique tooth morphology (Young et al. 2014a, b; Foffa et al. 2015; Fanti et al. 2016; Jouve et al. 2016; Johnson et al. 2017). Compared to longirostrine teleosaurids, Machimosaurini teeth have a low CBR ratio and blunt apices; are extensively and uniformly ornamented around the crown, with an anastomosed ornamentation pattern near the apex; and feature either true, or sometimes false, serrations (Young et al. 2014a, b; Foffa et al. 2015; Fanti et al. 2016; Johnson et al. 2017).
The most complete teleosaurid from the Corallian strata of the UK is DORCM G.03939, which was referred to “Steneosaurus” cf. obtusidens (now Lemmysuchus cf. obtusidens; see Foffa et al. 2015 and Johnson et al. 2017), from the Lower Calcareous Grit in Nothe (Weymouth, Dorset). This specimen and its implications have been recently described by Foffa et al. (2015), so here we will instead focus on additional specimens from the Corallian gap, all of which are isolated teeth belonging to taxa closely related to Machimosaurus (following the systematics and taxonomy of Young et al. 2014a).
The teeth of Lemmysuchus obtusidens and the species of Machimosaurus have been described in detail (Young et al. 2014a, b; Foffa et al. 2015; Johnson et al. 2017), which enables careful comparison with isolated dental specimens. OUMNH J.40669 (Fig. 9A), labelled as “Teleosaurus sp.” from the “Corallian beds” of an unknown locality and OUMNH J.50169 (Fig. 9B) from Coral Rag at Headington (Oxfordshire), identified as “crocodile? tooth” (sic!) in the museum catalogue—are the best examples of machimosaurin teeth of this morphotype from the Corallian gap. The crowns of this type are robust and conical, with a round cross section. The apex of the crown is rounded (apomorphy of Machimosaurini, but also characteristic of the metriorhynchid Torvoneustes) (Wilkinson et al. 2008; Young et al. 2013a, 2014a, b, 2015; Johnson et al. 2017). Furthermore, general teleosaurid affinities of these specimens are clearly demonstrated by the regular conical shape of the crown, the ornamentation (dense and uniformly distributed around the crown), the presence of true and “false” serration morphologies (not present in Torvoneustes), and a distinct machimosaurini-like carinae morphology visible on the apical third, on only one side of the crowns (carinae are visible all along the mesial-distal margins in Metriorhynchidae, with the carinae of Torvoneustes being particularly well-developed).
The same features are also visible on NHMUK PV OR 47971(III) from the “Coral Rag” of Wheatley, Oxfordshire; YORYM:2016.297 and 2016.300 from the Coral Rag of Yorkshire. Finally, it is possible that NHMUK PV R 6770 from the “Corallian” of Malton, Yorkshire may also belong to this morphotype based on the ornamentation and “laterally undulating” texture of the enamel ridges on the crown.
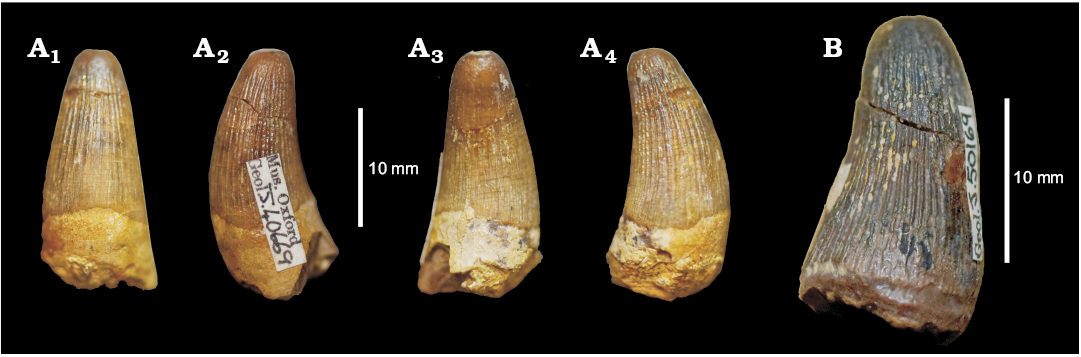
Fig. 9. Teeth of Machimosaurini indet. from Corallian. A. OUMNH J.40669, unkown locality, “Corallian beds”, Oxfordian, in labial (A1), axial (A2, A4), and lingual (A3) views. B. OUMNH J.50169, from Headington (Oxfordshire), middle Oxfordian, in lingual/axial oblique view.
Metriorhynchidae.—Metriorhynchid teeth are the most commonly found marine vertebrates in the Corallian Group. To date, the most complete and best studied specimen is an incomplete snout including nasals and parts of the maxillae (MANCH L6459) (Young 2014). This specimen was collected in the middle Oxfordian strata at Headington, Quarry Fields (Oxfordshire), originally described by Watson (1911), and mentioned by Benton and Spencer (1995). It was recently re-examined by Young (2014) and identified as cf. Torvoneustes, an assessment which we agree with. As this specimen has been described recently, we will not comment on it further.
The clearest evidence of metriorhynchids inhabiting the Jurassic Sub-Boreal Seaway during the Corallian are teeth that have been recovered across England since the 19th century. After careful examination, we group them into three different morphotypes.
Torvoneustes sp. Type 1: Several isolated teeth belonging to this type have been found in the Coral Rag of Yorkshire (e.g., Malton, North Grimston), and also in the synchronous strata of Headington (Oxfordshire) (Figs. 1, 2, 10A, B). Despite their abundance in the north, we found no Corallian evidence of this morphology in the Southern deposits of Dorset or Wiltshire. A large collection of this morphotype is hosted in the Yorkshire Museum. Among all of the specimens in that we examined, not only are the YORYM specimens the best preserved of this morphotype, but they also show some of the variability along the tooth row. For these reasons we will describe them first and use them as reference for comparisons with other specimens.
YORYM:2016.309 (Fig. 10A) is the largest (AL ~26.5 mm) and best-preserved tooth of the Type 1 morphotype. The CBR is ~1.9–2.2, lower than in most geosaurins (Young et al. 2012a, 2016). The crowns of Type 1 are all weakly labially curved, and poorly laterally compressed (if at all). Two well-developed carinae divide a strongly convex lingual side from a less convex labial surface. On the mesial and distal sides, the carinae are well preserved and become more prominent along the apical third of the crown. No carinal flange can be seen. The carinae have denticles that are particularly clear along the apical half of the crown; these appear to be poorly formed, irregularly spaced, and can only be seen using visual aids (e.g. magnification hand-lens or macrophotographs). The labial side of the crown is normally less densely ornamented than the lingual side, and the ornamentation consists of medium relief apicobasal ridges, which are more continuous near the base but become short segments arranged in an anastomosed pattern in the apical half of the crown (Fig. 10A4, A5). In proximity to the apex, the enamel ornamentation of the lingual side contacts the carinae, forming “false” serrations similar to those of Torvoneustes carpenteri and T. mexicanus (Wilkinson et al. 2008; Young et al. 2013b; Barrientos-Lara et al. 2016; Fig. 10C–E). Type 1 teeth have a rounded apex and are poorly compressed, similar to Torvoneustes teeth (Young et al. 2013b, 2014b), and the tip is sometimes worn below the enamel level. Type 1 crowns are weakly distally curved, a feature that is common in Metriorhynchidae but not as pronounced as in the large-bodied metriorhynchid Plesiosuchus manselii (Young et al. 2012b).
Crowns belonging to Type 1 have been found across the Corallian strata of the Northern Wessex Basin, and more commonly in the Cleveland Basin. Many additional teeth can be assigned to Type 1, based on weak lateral compression, pattern of ornamentation, and the details of the carinae/denticles. These specimens come from the Coral Rag of the Corallian Oolite of Malton (Yorkshire): BRSMG Cd5591, Cd5592; CAMSM J.13309, J.13310 (Fig. 10B), YORYM:2016.306–2016.309; from the Coral Rag of Wheatley Formation of Headington (Oxfordshire): OUMNH J.52428, J.47587a, J.47560; and an unknown locality: CAMSM J.13305. Some of these crowns slightly differ from the previously described teeth, with the main differences concerning the density of ornamentation on the lingual side. However, such variation is within the spectrum observed along the tooth row of the holotype Torvoneustes carpenteri (BRSMG Ce17365; DF personal observations) and other member of the same clade (e.g., “Mr. Passmore’s crocodile”, OUMNH J.1583), or can be explained as the result of different development stages (e.g., young replacement teeth are less well ornamented, and more laterally compressed, than mature ones; DF and MTY personal observations).
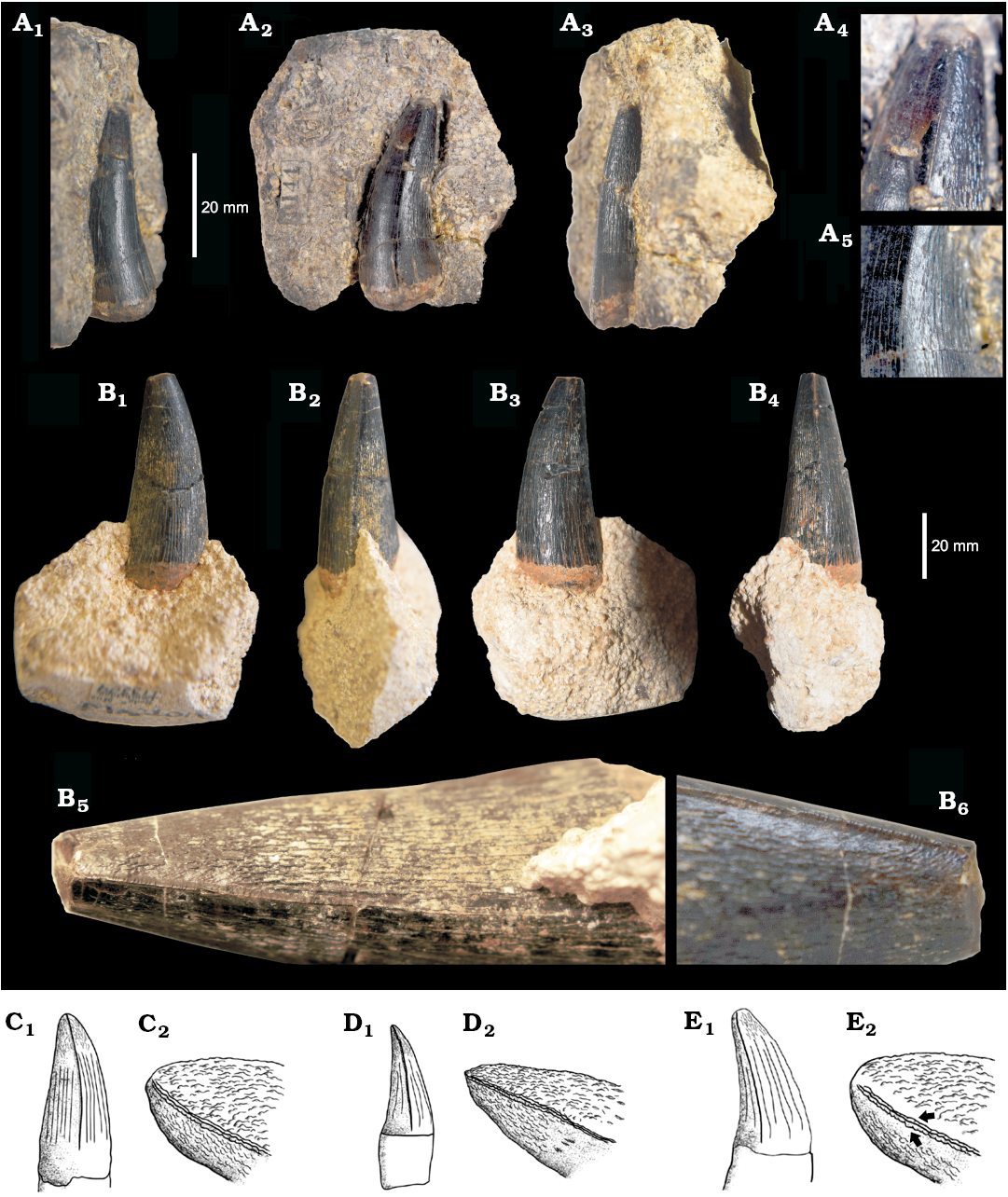
Fig. 10. Teeth of the metriorhynchid reptile Torvoneustes sp. A. YORYM:2016.309, from Malton (Yorkshire), middle Oxfordian, in labial (A1), axial (A2), and lingual (A3) views, and close ups of the carina and ornamentation (A4, A5, not to scale). B. CAMSM J.13309, from Malton (Yorkshire), middle Oxfordian, in labial (B1), axial (B2, B4), lingual (B3) views, and close ups of the carinae (B5, B6, not to scale). C–E. Comparative line drawings (C, Torvoneustes carpenteri; D, T. mexicanus; E, T. coryphaeus) teeth (C1–E1, not to scale) and details of ornamentation (C2–E2, not to scale), the arrows in E2 show that the ornamentation does not interact with the carinae (modified from Barrientos-Lara et al. 2016).
Interestingly, some of the tips of these teeth (e.g., CAMSM J.13305, OUMNH J.47587a) are broken or polished and worn to an unusual level for Metriorhynchidae, reminiscent of certain teleosaurid teeth (e.g., DORCM G.03939, for comparisons see figures in Foffa et al. 2015). This perhaps suggests a durophagous diet, as hypothesised for Torvoneustes among metriorhynchids (Young et al. 2014b).
Several of these specimens, such as YORYM:2016.306, 2016.309; CAMSM J.13309, J.13310, were all originally labelled as Dakosaurus. CAMSM J.13309 and J.13310 were both mentioned by Seeley (1864), with old catalogue numbering “J.b.2.24”. We remark that Type I teeth lack several features present in Dakosaurus teeth: largely smooth unornamented enamel, and prominent carinae with well developed and contiguous macro-denticles (macroziphodonty sensu Young and Andrade 2009), which is an autapomorphy of the Dakosaurus (Young et al. 2012a, b, 2014b). Conversely, we observe that Type 1 teeth are similar to an isolated crown found at Ecqueville near Octeville-sur-Mer (Seine-Maritime), France in the Aulacostephanus eudoxus Ammonite Zone (upper Kimmeridgian) (Lepage et al. 2008). This tooth was also assigned to Dakosaurus sp., but for the reasons mentioned above it also most likely belongs to a geosaurin taxon of the clade comprising (Torvoneustes + “M.” hastifer + “Mr. Passmore’s crocodile”).
cf. Tyrannoneustes Type 2: We identify a single tooth from YORYM:2016.308 (Fig. 11A) as being very similar to adult crowns in Tyrannoneustes lythrodectikos (NHMUK PV R 3939, PETMG R176; Foffa and Young 2014; Fig. 11B). The tooth is strongly curved posteriorly and only weakly in the lingual direction. As in Tyrannoneustes, the crown bears carinae with no clearly discernible denticles. The enamel is smooth and mostly unornamented, except for the apex, where the enamel is curiously roughened in a very low relief “pebbled” texture. Not only is the one tooth of YORYM:2016.308 probably the first occurrence of Tyrannoneustes in the Oxfordian of the UK (Young et al. 2013a reported isolated teeth from the Callovian–Oxfordian of Poland and France), but also the youngest global occurrence of the genus.
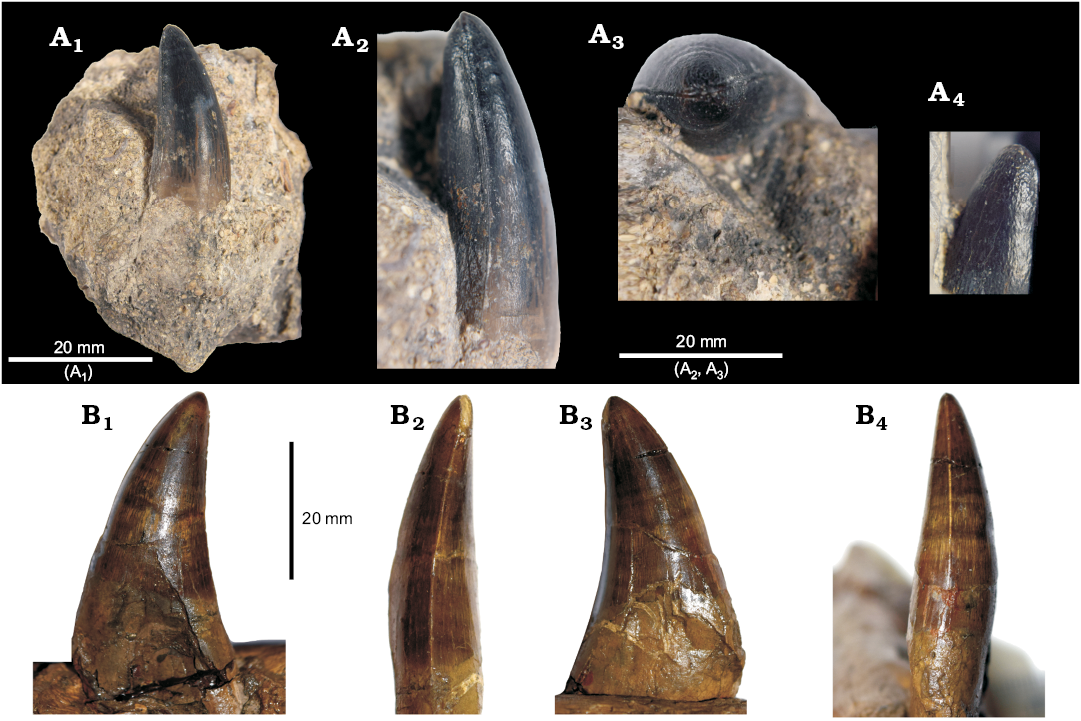
Fig. 11. Teeth of the metriorhynchid reptile Tyrannoneustes. A. YORYM:2016.308, cf. Tyrannoneustes, from Malton (Yorkshire), middle Oxfordian, in labial (A1), axial (A2), and apical (A3) views, detail of the apex of the crown (A4 , not to scale). B. NHMUK PV R3939, Tyrannoneustes lythrodectikos Young, Andrade, Brusatte, Sakamoto, and Liston, 2013a, from Peterborough (Cambridgeshire), Callovian, dentary tooth crushed at its base resulting more laterally compressed than in life, in labial (B1), axial (B2, B4), and lingual (B3) views.
cf. Metriorhynchus superciliosus Type 3: Teeth of this third type are very similar to the crowns of the Callovian metriorhynchine Metriorhynchus superciliosus (Fig. 12). Teeth belonging to this morphotype come from Oxfordshire, Wiltshire, and Yorkshire (Figs. 1, 2). They differ from the previous types in dimension, ornamentation (pattern, density, and relief), and carinae morphology. Most salient, Type 3 teeth have carinae that are smooth, linear, and lack denticles. The ornamentation is denser on the lingual side and consists of continuous apicobasal parallel ridges, which are low relief and, although they become shorter towards the apex of the crowns, do not create any “pebbled” or anastomosed pattern. The dentine in between the ridges is completely smooth.
Examples of this morphotype include: NHMUK PV OR 47044, smaller of the two specimens under the same accession number (Fig. 12), from the Calcareous Grit of Heddington (Wiltshire); OUMNH J.52429 from Headington, Oxfordshire; OUMNH J.40668 from the Coralline Oolite at Badton fields. Finally, CAMSM J.13300, found in North Grimston, near Malton, Yorkshire and mentioned several times in older literature (Fox-Strangways 1829; Seeley 1869 as J.b.2.24; Drake and Sheppard 1909), was assigned to Dakosaurus (Seeley 1869) but lacks all the diagnostic features of the genus (Young et al. 2012b) and shares the above described features of Type 3 specimens, so it most likely belongs to this morphotype.
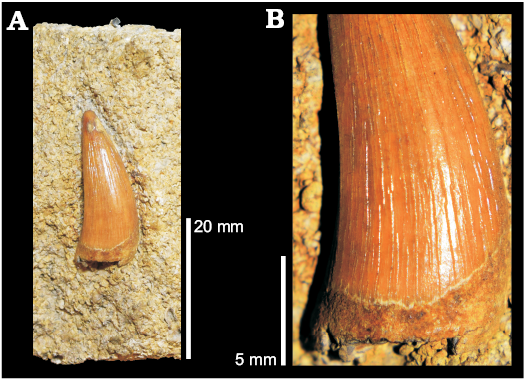
Fig. 12. Tooth of the metriorhynchid reptile Metriorhynchus superciliosus de Blainville, 1853 (NHMUK PV OR 47044) from Heddington (Wiltshire), Corallian, in labial view (A), close up of labial ornamentation (B).
Indeterminate specimens.—Further undescribed cranial and postcranial material of indeterminate teleosaurids and metriorhynchids is described and figured below (Fig. 13). Although, not identifiable to genus level, these specimens provide additional evidence of thalattosuchian diversity through time.
Geosaurinae indet.: CAMSM X.50300 (Fig. 13A) is one of the rare skull remains recovered from the Corallian Group. It was found at Garsington (Oxfordshire) from unrecorded strata, likely the Lower Calcareous Grit Formation (Horton et al. 1995). The specimen is part of the Strickland Collection at the Sedgwick Museum, erroneously labelled as “Plesiosaurus” in the catalogue. It was mentioned in the literature for the first time by Benton and Spencer (1995). Here we show that CAMSM X.50300 has as series of characters that unambiguously identify it as a large Geosaurini crocodylomorph.
CAMSM X.50300 is the posterior half of a right mandibular ramus that includes the surangular, angular, articular, prearticular, and potentially the posterior fragments of the dentary, splenial, and coronoid. The absence of alveoli suggests that the break occurred posterior to the end of the tooth row. A conspicuous amount of mustard-yellow sandy matrix still encrusts the anterior part of the lateral side, the glenoid fossa, and much of the medial side of the fossil. On the medial side there is a well-developed triangular pre-articular and a flat surface on the medial-ventral margin of the angular that we identify as the articular facet for the splenial on angular. This reaches posteriorly as far as ~100 mm anterior to the level of the coronoid process.
The lateral side of the specimen is better preserved. Here there are at least three diagnostic features that allow identification of CAMSM X.50300 as a geosaurin metriorhynchid: (i) the posterior part of the jaw is strongly curved upwards, which raises the articular and the glenoid fossa level to the coronoid process; consequently (ii) the posterior part of the mandibular ramus is very deep (~80–90 mm at the coronoid process); (iii) there is a strongly developed sulcus, corresponding to the “surangular-dentary groove” that runs on the surface of the surangular-dentary anterior to the coronoid process: this is deeply excavated in Geosaurini and shallower in Metriorhynchinae. All these features are apomorphies of Geosaurini within Metriorhynchidae (Young et al. 2012b, 2013a).
The posterior curvature of the post-symphyseal mandibular ramus varies in metriorhynchids. Different clades of geosaurines achieved a raised posterior articular surface (adaptation for increased gape and more advantageous lever mechanisms linked to macrophagy) in different ways (MTY personal observation). Plesiosuchina (Plesiosuchus+Suchodus subclade) is characterised by a distinct “kink” posterior to the mandibular symphysis and the ventral margin of the angular is poorly convex, appearing almost straight at the inflection point. In contrast, in Tyrannoneustes lythrodectikos (GLAHM V972, NHMUK PV R 3939) and “Mr. Passmore’s crocodile” (OUMNH J.1583) the curvature is very large and strongly convex. Finally, in Torvoneustes coryphaeus (MJML K1863), Dakosaurus maximus (SMNS 82043), and Geosaurus giganteus (NHMUK PV OR 30720) the angular curvature is weak. The ventral margin of CAMSM X.50300 angular is strongly curved as it is in Tyrannoneustes lythrodectikos (GLAHM V972, NHMUK PV R 3939).
Finally, CAMSM X.50300 is poorly ornamented on the lateral surface of the angular and surangular, a feature that convergently evolved several times in metriorhynchids by the Late Jurassic (at least 3 times in Geosaurini: Torvoneustes coryphaeus, Dakosaurus and Plesiosuchus, and Geosaurina; and in Metriorhynchinae: Gracilineustes and in the subclade Rhacheosaurini) (Young et al. 2012b, 2013a; Parilla-Bel et al. 2013; Foffa et al. 2017). Thus this feature cannot be considered unique to Geosaurinae. Nevertheless, it is possible to exclude CAMSM X.50300 from any Callovian geosaurines because they all (except Ieldraan melkshamensis) have conspicuously ornamented surangulars and angulars (Andrews 1913; Young et al. 2012b, 2013b; Foffa and Young 2014; Foffa et al. 2017). Additionally, we can rule out affinities between CAMSM X.50300 and a number of Late Jurassic geosaurin lineages. For example, in Geosaurus giganteus the surangular is poorly developed (Young and Andrade 2009), while it is large in CAMSM X.50300. We predict that further preparation of the specimen, especially in the articular area, would potentially help further classify the specimen (Plesiosuchus and Suchodus, for example, have a contiguous and deep glenoid condylar fossa, i.e., not two shallow fossae) (Young et al. 2012b).
Unfortunately, at this stage, the absence of diagnostic features does not allow us to identify the specimen to a lower taxonomic level than Geosaurini indet. (with remarkable similarities with Tyrannoneustes). Considering the presence of a Torvoneustes-like snout (MANCH L6459; see Young 2014) and teeth in the Corallian strata, CAMSM X.50300 may be a second Corallian geosaurin taxon of the same clade. Specifically, it is possible that CAMSM X.50300, although it lacks strong ornamentation, may be referred to cf. Tyrannoneustes based on the strongly upward-curved posterior half of the angular.
Metriorhynchidae indet.: Two specimens, OUMNH J.01430 and J.50341, can be referred to Metriorhynchidae indet. (Fig. 13B, C).
OUMNH J.01430 is a left articular found at Headington, Oxfordshire (Fig. 13B). The length, orientation, and shape of the retro-articular process can be readily distinguished from the long, well developed retroarticular processes of teleosaurids. Although plesiosaur retroarticular processes are also often poorly developed, they are rectangular in lateral and dorsal views (triangular in Metriorhynchidae). Furthermore, plesiosaur glenoid fossae are substantially different from those in thalattosuchians in being deeply excavated and bean-shaped in dorsal view. None of these features is present in OUMNH J.01430. The suggested identification of the fossil as Geosaurus sp. in the museum catalogue is also problematic, because no known Geosaurus (or closely related) species has a completely accessible articular/retroarticular process to compare with. Thus neither the genus Geosaurus nor any species within it can be diagnosed by specific features of this area (Young and Andrade 2009; Foffa et al. 2017). It is then likely that this specimen was originally assigned to this genus while its content was still confusingly inclusive (see Young and Andrade 2009). In absence of more diagnostic features, we refer this specimen to Metriorhynchidae indet.
OUMNH J.50341 is a vertebra found in the “Corallian Oolite (Coral Rag)” at Headington, Oxfordshire, and was wrongly labelled as “Teleosaurus?” and “crocodile dorsal vertebra” on its identification labels (Fig. 13C). This specimen is also referable to Metriorhynchidae. The vertebra is partially embedded into a yellow-grey sandy matrix that hides the posterior and the lateral sides of the centrum and neural spine. Consequently, a large part of the centrum, the prezygapophyses, and the right surface of the neural spine are visible, while the postzygapophyses are obscured. The transverse processes are both missing. One, on the left side, is truncated at its base in line with the neural arch, while an in-print in the matrix is all that is left of the right process. The centrum of OUMNH J.50341 bears no trace of the parapophyses or diapophyses, so we agree with its identification as a dorsal vertebra based on the well-known morphology of vertebrae in Crocodylomorpha. Distinguishing teleosaurids from metriorhynchids using dorsal vertebrae is challenging, as dorsals do not bear significant diagnostic characters. However, in the mid thoracic vertebrae of metriorhynchoids and Pelagosaurus the lateral margin of the prezygapophysis is fused with the transverse processes. In teleosaurids, in contrast, it appears as if the pre-zygapophyses are “laying on” the transverse processes (MTY personal observation). Considering this distinction, we can refer OUMNH J.50341 as to Metriorhynchidae indet.
A series of additional teeth (OUMNH J.47572, NHMUK PV OR 36336–39, PV OR 47497iv) will not be discussed because they are too poorly preserved or damaged to be assigned to anything less inclusive than Metriorhynchidae indet. These include specimens from Oxfordshire, Yorkshire, and Hampshire.
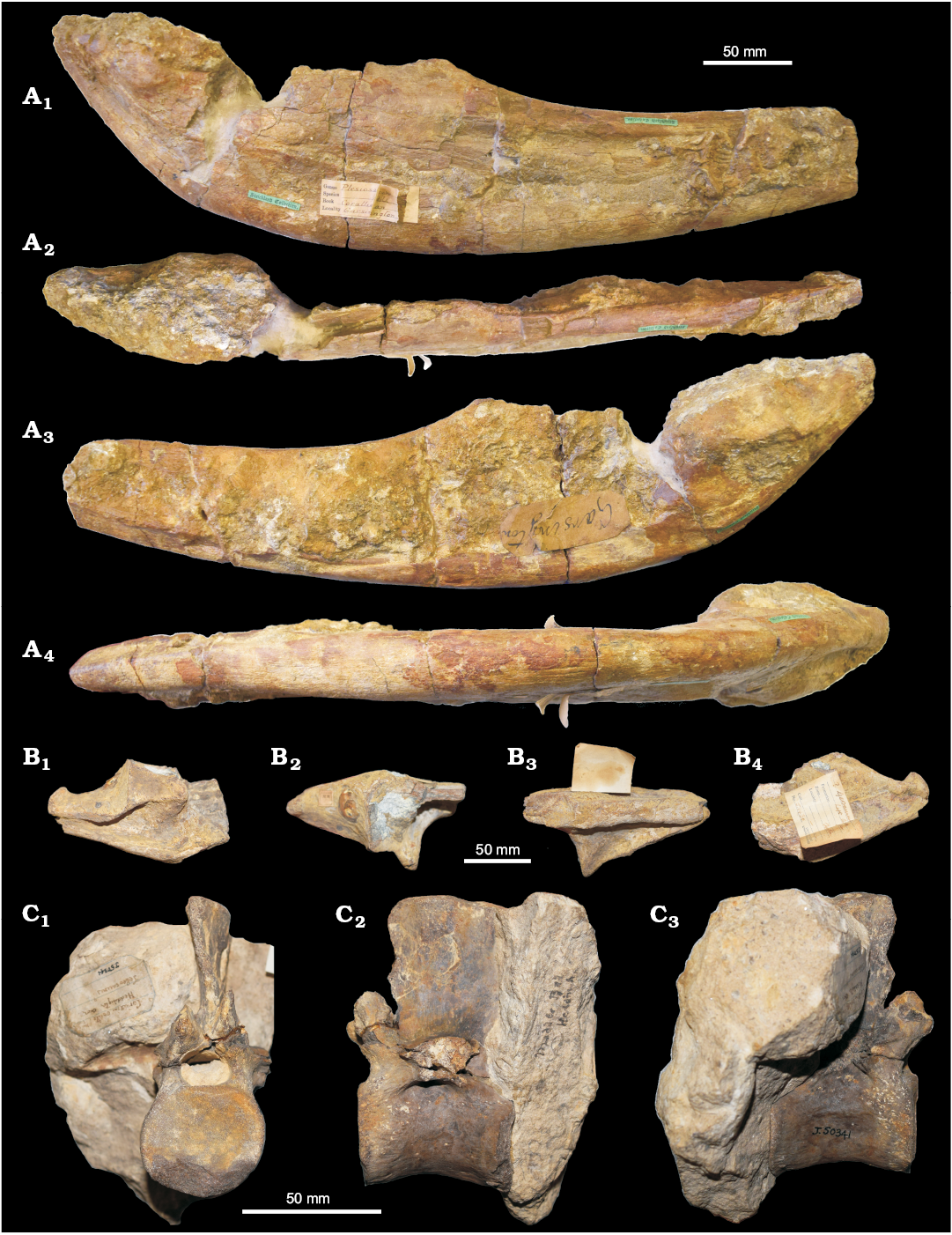
Fig. 13. Cranial and axial elements of Metriorhynchidae. A. CAMSM X.50300, Geosaurini indet. from Garsington (Oxfordshire), lower to middle Oxfordian, posterior fragment of lower jaw in lateral (A1), dorsal (A2), medial (A3), and ventral (A4) views. B. OUMNH J.1430, Metriorhynchidae indet. from Headington (Oxfordshire), ?middle Oxfordian, fragment of articular area and retroarticular process in lateral (B1), dorsal (B2), medial (B3), and ventral (B4) views. C. OUMNH J.50341, Metriorhynchidae indet. from Headington (Oxfordshire), ?middle Oxfordian, dorsal vertebra in posterior (C1), right lateral (C2), and left lateral (C3) views.
Discussion
Is “Pliosaurus” grossouvrei a valid species of Oxfordian pliosaurid?
The phylogenetic affinities of Callovian pliosaurid specimens are central to interpreting their relevance for understanding faunal evolution through the Oxfordian. Phylogenetic analyses suggest that at least one ghost lineage must have survived globally during the Oxfordian (Fischer et al. 2015, 2017). Unfortunately, attesting to the rarity of Oxfordian pliosaurids, only two species from this time interval have been described. These are Gallardosaurus iturraldei from the Caribbean (Gasparini 2009), and Anguanax zignoi from Northern Italy (Cau and Fanti 2014, 2015; Fischer et al. 2017). None of the specimens described here can be referred to either of these taxa, as they clearly belong to some large bodied, strongly heterodont pliosaurid, which Gallardosaurus and Anguanax are not. The only other taxon that has been identified from Oxfordian strata is “Pliosaurus” grossouvrei (Sauvage 1873; Tarlo 1960). The bibliographic record of this taxon is complicated, and is briefly reviewed here.
Liopleurodon grossouvrei was first erected by Sauvage (1873) to distinguish one tooth found at Charly (and curated at the École des Mines, Paris, France) from the Liopleurodon ferox holotype. The distinction was based on the absence of ridges on the labial side and the smooth enamel texture of the tooth. Only 16 years later, Lydekker (1889: 130–131) assigned teeth “in which the smooth surface is convex from side to side, and the carinae are not very prominent” to Pliosaurus grossouvrei. Several of the Coral Rag teeth from Yorkshire, Wiltshire, and Oxfordshire (e.g., NHMUK PV OR 36335-a–e, PV OR 47971-a, and PV OR 47044) were also assigned to this taxon, with little explanation other than general similarities with the Pliosaurus grossouvrei holotype specimen, and supposedly because they were found in the same stratigraphic level (Lydekker 1889; Fox-Strangways 1892). Almost a century later, in 1960, Tarlo revised the taxonomy of pliosaurids from Late Jurassic British formations, synonymising “Pliosaurus” grossouvrei with “Pliosaurus” andrewsi (holotype NHMUK PV R 3891, a controversial Callovian pliosaurid based on a skull and associated skeleton from the Peterborough Member of the OCF). The teeth of “Pliosaurus” andrewsi, however, are reported to be circular in cross-section, and thus they differ from the characteristic teeth of Pliosaurus, which are sub-triangular/triangular in cross section—see Pliosaurus diagnosis in Knutsen (2012), and Benson et al. (2013). Moreover, the teeth of the “Pliosaurus” andrewsi holotype have smooth enamel, and crown ornamentation consisting of loosely packed low-relief continuous ridges, with ornamentation on the lingual side scarce or absent (Fig. 5C). The latter feature is unreliable because it is known to vary along the tooth row in pliosaurids. Additionally, in “Pliosaurus” andrewsi the teeth were reported to “wear to a greater extent than in any other plesiosaurian” (Tarlo 1960: 164).
Based on the texture of the enamel and the thickness of the ornamental ridges we find it unlikely that the Corallian pliosaurid we assigned to Type 1 belongs to “Pliosaurus” andrewsi, although we notice some similarities in the stoutness of the crowns and distribution of the ornamentation between these taxa. Thus, all the Type 1 Corallian pliosaurid teeth we describe cannot be assigned to any named taxon, except for possibly “Pliosaurus” grossouvrei. In this context, we suggest that based on unique dental features (stout dentitions, “vermiculated” dentine texture, high-relief/coarse ornamental ridges) “Pliosaurus” grossouvrei is probably a valid taxon, distinct from “Pliosaurus” andrewsi. However, considering the unclear taxonomy of “Pliosaurus” andrewsi we refrain for the moment from erecting a new taxon that would uniquely be based on sporadic dental material. Furthermore, comprehensive re-description of “Pliosaurus” andrewsi (Judyth Sassoon, personal communications 2017) and Late Jurassic materials are in preparation and would help clarify this matter.
Late Jurassic local decline of Pliosauridae and Teleosauridae, and the diversification of Geosaurini and Ophthalmosauridae
New information provided by our redescription of the Corallian gap specimens helps to clarify the timing of key faunal turnover episodes, particularly the demise and radiation of major lineages.
Plesiosauroidea.—It is unclear how strongly plesiosauroids were affected by faunal turnover during the Oxfordian. Many excellent cryptoclidid plesiosauroid skeletons are found prior to the Corallian gap, in the Callovian strata of the Peterborough Member of the OCF. Unfortunately, Kimmeridgian–Tithonian plesiosauroids from after the Corallian gap are known only from very fragmentary material, with the most likely preserved bones (vertebrae and limb bones) being poorly diagnostic, and several contemporaneous taxa are known from non-overlapping elements (for further explanations see Benson and Bowder 2014; Roberts et al. 2017). Here, we have been able to identify one major morphotype of cryptoclidid teeth (similar to Muraenosaurus) from the Corallian gap, which suggests that cryptoclidids were continuously present in the Jurassic Sub-Boreal Seaway through the Middle–Late Jurassic transition. Although much remains unknown about plesiosauroid evolution during the Oxfordian, it is clear that overall diversity remains essentially unchanged when comparing the Callovian to the Kimmeridgian–Tithonian (4–5 taxa in the OCF and 4–5 taxa in the KCF). It might be that faunal turnover during the Oxfordian most severely affected the apex of the food web, with plesiosauroids less susceptible to changes. Or, it could be that Oxfordian plesiosauroids were affected in other ways that currently remain obscured by the poor Corallian gap record.
Pliosauridae.—Pliosaurids are perhaps the group that underwent the most drastic turnover from the Callovian to the Kimmeridgian (Figs. 4, 5, 11A1). Pliosaurids were diverse and abundant in the Callovian, with 5–7 known genera (including undescribed probable new taxa), which differ in body size and inferred ecology (Andrews 1909; Ketchum and Benson 2011a, b; Benson and Druckenmiller 2014; Foffa et al. 2014; Fischer et al. 2017). Conversely, in the Kimmeridgian, Pliosaurus (~6 species) is globally the only described pliosaurid genus, before the first occurrence of Brachaucheninae in the Early Cretaceous (Benson and Druckenmiller 2014; Benson et al. 2013; Fischer et al. 2017). However, the yet unreported presence of conical teeth in Kimmeridgian and Tithonian collections of the UK suggests that Late Jurassic pliosaurid diversity has hitherto been underestimated (DF personal observation), an issue that will be the subject of future work. Regardless, there is a clear difference between Callovian and Kimmeridgian pliosaurid faunas, but the nature of this turnover has been obscured by the Corallian gap in between. Our survey of Corallian fossils suggests that all pliosaurid teeth in the Corallian strata belong to a single large-bodied taxon (Figs. 14A, 15). This indicates that most, or all, small-to-middle-sized pliosaurids disappeared from the Jurassic Sub-Boreal Seaway by the Oxfordian. We suggest that this is a genuine biological pattern rather than a sampling bias, for two main reasons. First, our sample of pliosaurid teeth includes all portions of a tooth row, from large anterior fangs to posterior hooked teeth through intermediate morphologies, all indicative of large-bodied pliosaurids. Secondly, smaller and more fragile plesiosaur and thalattosuchian teeth can be found in the same Corallian strata from which pliosaurid fangs are reported; this is sufficient to indicate that the supposed absence of teeth of small-to-middle-sized pliosaurids, is a genuine signal of their rarity, rather than a taphonomic artefact.
It has been shown that the Corallian pliosaurid teeth belonged to a large bodied pliosaurid (possibly a new taxon with similarities with Liopleurodon and “Pliosaurus” andrewsi). This, combined with the unlikeliness of preservation biases towards large crowns, suggests that the drastic decline in pliosaurid ecomorphological diversity of the KCF compared to OCF had already started by the Oxfordian (Figs. 14A, 15). Callovian pliosaurids are found in the Peterborough Member of the OCF, and include taxa of disparate size, shape, and supposed ecology. Such diversity collapses across the Oxfordian, with the disappearance of small-to-middle-sized, longirostrine, and brevirostrine taxa. Our study is further evidence that the transition from a piscivorous small-to-middle-sized pliosaurid-dominated Callovian fauna to a generalist hypercarnivore giant Pliosaurus-dominated fauna was sudden, and that the transition involved the abrupt removal of medium-small bodied specialist taxa.
Ichthyosauria.—Amongst the principal marine reptile lineages, ichthyosaurs are the least taxonomically diverse and numerically abundant in the Corallian. In a recent revision of Middle and Late Jurassic ichthyosaurs from the UK, Moon and Kirton (2016) established that Ophthalmosaurus icenicus is the only valid ichthyosaur genus in the OCF, prior to the Corallian gap. Until now, only one Oxfordian specimen of ichthyosaur (CAMSM J.68689, from the Quenstedtoceras mariae Ammonite Zone, in the upper OCF of Warboys, Cambridgeshire) could be referred to Ophthalmosaurus icenicus. Ophthalmosaurus is a globally widespread taxon and has an unusually long stratigraphic record, with diagnostic material known from the Callovian–Tithonian of England (in OCF, KCF, and now CG), France, and Mexico. Globally, ophthalmosaurid diversity is known to increase during the Kimmeridgian–Tithonian with the radiation of Platypterygiinae (Fischer et al. 2016). The same trend is detected in the British fossil record, with ophthalmosaurid diversity higher in the Late Jurassic, after the Corallian gap, than in the Middle Jurassic (Fig. 11A2). Ophthalmosaurus icenicus, Nannopterygius enthekiodon, a taxon not often included in phylogenetic analyses but very likely belonging to Ophthalmosauridae (Moon 2017), and Brachypterygius mordax (McGowan 1976) can all be found in the KCF strata. In addition to these taxa, a few other yet undescribed KCF specimens likely represent at least a new taxon (MJML K1009, K1747) along with the third known Nannopterygius specimen (MJML K1885), respectively (DF personal observation). Thus, the Late Jurassic increased diversity in the KCF strata could be a genuine local representation of the global diversification of Platypterygiinae.
A possible explanation for the rarity of ichthyosaurs compared to other marine reptile lineages in the Corallian is the hypothesis that ophthalmosaurids preferred deeper basin habitats, as testified by several characteristics of their body plan and physiology (e.g., large eyeballs, cancellous bone) (Motani et al. 1999; Talevi and Fernández 2012). Indeed, abundant ichthyosaur specimens can be found in both the OCF and KCF, and in the Oxfordian beds that most likely were formed in deeper environments (Ampthill Clay) (Danise et al. 2014). In contrast, ichthyosaur specimens are extremely rare in the typical near-shore and shallow water deposits that make up the majority of the Corallian succession of the UK.
Teleosauridae.—At least four different teleosaurid taxa are found in the Peterborough Member (Callovian) of the OCF, and these are known from several specimens (Andrews 1910; Johnson et al. 2015, 2017; Figs. 14A, 15). Such taxic diversity and specimen abundance appears to be lost by the early Oxfordian, as shown by the limited and low diversity assemblage of specimens described from the Corallian gap above, and never recovered subsequently in the Jurassic Sub-Boreal Seaway, as teleosaurids are incredibly rare and exhibit low diversity in the overlying KCF, and in other UK Kimmeridgian–Tithonian units. In fact, excluding NHMUK PV OR 43086 and DORCM G. 5067i–v, the Kimmeridgian “Teleosaurus” megarhinus (Hulke, 1871), an enigmatic taxon which is currently being re-described, and a yet undescribed teleosaurid skull fragment (MJML K263), the only teleosaurids found in the Late Jurassic of the UK belong to the macrophagous/durophagous Machimosaurini subclade. This lineage can also be found in the underlying Corallian beds, as demonstrated by the lower jaw DORCM G.03939 (Foffa et al. 2015) and several teeth described here.
Not only are teleosaurids rare in Late Jurassic strata in the UK, but the only known KCF specimens, most of which are from the Kimmeridgian or Tithonian beds of Dorset, are extremely fragmentary. These fossils are almost exclusively teeth (e.g., NHMUK PV R 1774, Young and Steel 2014) and osteoderms (e.g., MJML K2158 and BRSMG Ce9826, Kimmeridgian and Tithonian of Dorset; CAMSM J.29481, Cambridgeshire; OUMNH J.77970 and J.77971, Oxfordshire), along with some scarce cranial material (NHMUK PV OR 43086, DORCM G. 5067i–v, MJML K263, Kimmeridgian of Dorset) (Seeley 1869; Young and Steel 2014). This is in contrast with continental Europe, where Kimmeridgian teleosaurid material is taxonomically more diverse and numerically more abundant (Vignaud et al. 1993; Young et al. 2014a, b; Foffa et al. 2015).
The stratigraphic and geographic distribution of the specimens that we describe demonstrate that machimosaurin teleosaurids were continuously present through the Oxfordian in the UK, but predominantly in near shore, shallow, warm, and potentially high-energy waters. This hints that taxa belonging to this group had a wider environmental/energy tolerance than other teleosaurids (Hua 1999; Young et al. 2014a, b). As a corollary, it is possible that the absence of other teleosaurids in the Corallian and Kimmeridgian strata of the UK may reflect ecological constraints for these taxa. Perhaps the rarity of longirostrine teleosaurids in coastal-marine Late Jurassic units of the UK may be due to unfavourable near shore conditions compared to the rest of Europe. In this scenario, only the machimosaurin teleosaurids with higher environmental tolerance would have survived undisturbed through the Oxfordian in the Jurassic Sub-Boreal Seaway. Possible support for this hypothesis comes from the wide palaeoenviornmental distribution of machimosaurins (see Young and Steel 2014: table 1; Foffa et al. 2015), and the contemporaneous occurrence of longirostrine teleosaurids with machimosaurins in near-shore deposits of France and elsewhere in Europe. The only exception to this observation is “Teleosaurus” megarhinus (a Kimmeridgian taxon from deep water units of the UK and near shore deposits of southern France; Vignaud et al. 1993). It is thus possible that the spread of adverse environmental conditions may have contributed to the selective decline of longirostrine teleosaurids through the Callovian–Oxfordian in the UK. Regardless of the explanation, however, the pattern is clear: machimosaurins were the only teleosaurid linage that was continuously present in the Jurassic Sub-Boreal Seaway from the Callovian to the Kimmeridgian.
Metriorhynchidae.—The metriorhynchid fauna also dramatically changed during the Oxfordian, transitioning from the metriorhynchine-dominated Callovian assemblage of the OCF to the geosaurine-dominated Kimmeridgian and Tithonian fauna of the KCF (Young 2014). Unlike with teleosaurids, our survey finds a relatively diverse metriorhynchid fauna in the Jurassic Sub-Boreal Seaway during the Corallian (Figs. 14A, 15). Uniquely among marine tetrapod groups of this interval, CG metriorhynchids are a mixture of taxa with Callovian affinities (e.g., cf. Tyrannoneustes sp. Type 2 and cf. Metriorhynchus superciliosus Type 3) and others with Kimmeridgian affinities (e.g., Torvoneustes sp. Type 1). The presence of the typical Late Jurassic taxon Torvoneustes in the Corallian beds, combined with the rarity of metriorhynchines, may indicate that metriorhynchid faunal turnover happened earlier and more gradually than previously thought, perhaps concordant with environmental perturbations during the Oxfordian. This is in accord with the recent realization that the classical Late Jurassic Geosaurini began their diversification in the Middle Jurassic, before reaching high diversity and numerical abundance in the Kimmeridgian and Tithonian of Europe (Young et al. 2012a; 2014; Foffa et al. 2017).
Within the metriorhynchid subclade Geosaurini, the Torvoneustes lineage seems to have been the first to become truly common in the early Late Jurassic, and was then later followed in the Kimmeridgian–Tithonian by the lineages of Geosaurus, Plesiosuchus, and Dakosaurus. Interestingly, this may indicate an environmental preference (or higher tolerance) of the genus Torvoneustes to shallower waters (e.g., Torvoneustes coryphaeus, the Oxfordian cf. Torvoneustes sp., and all the new specimens here reported were found from shallow sub-tidal deposits).
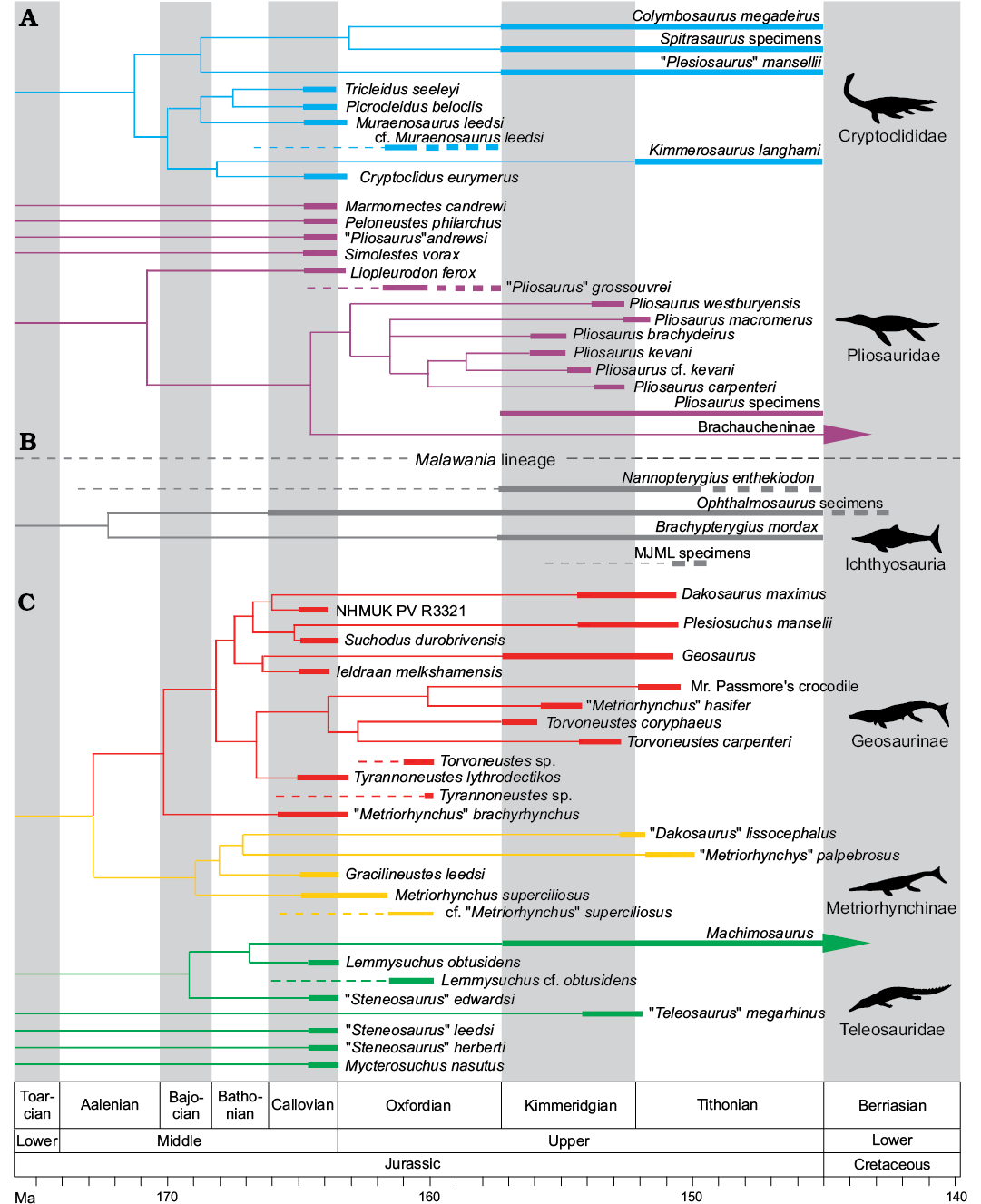
Fig. 14. Time calibrated phylogenetic tree of the main Jurassic Sub-Boreal Seaway marine reptile lineages stratigraphic ranges. A. Plesiosauria (modified from Fischer et al. 2015, 2017). B. Ichthyosauria (modified from Fischer et al. 2014). C. Thalattosuchia (Young et al. 2016; Foffa et al. 2017). MJML, Museum of Jurassic Marine Life—the Steve Etches Collection, Kimmeridge, UK.
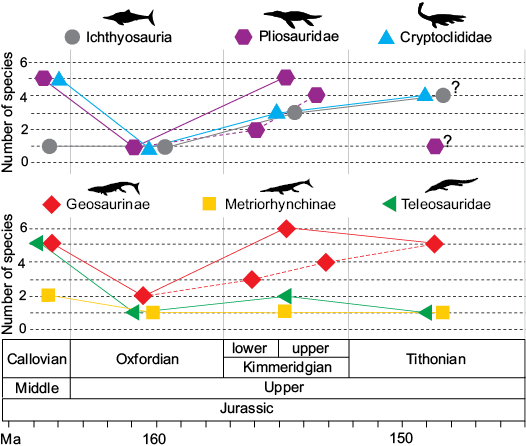
Fig. 15. Jurassic Sub-Boreal Seaway marine reptile species count.
Did environmental change induce a faunal turnover?
It is possible that environmental perturbations at or near the Callovian–Oxfordian boundary caused the observed faunal shift from the OCF and KCF. If this is the case, the intervening Oxfordian marine reptile fauna from the UK would be a primary source of information on the tempo and mode of these changes, and could be used to test specific hypotheses.
The Callovian–Oxfordian environmental perturbations are summarised by a model based on geochemical, geological, and invertebrate fossil data such as distribution of reef facies, and statistical occurrences of Boreal (cardioceratid and kosmoceratid) and Tethyan (oppeliid and peltoceratoid) ammonite assemblages, the latter used as palaeo-temperature markers (Anderson et al. 1995; Podlaha et al. 1998; Riboulleau et al. 1998; Abbink et al. 2001; Wierzbowski 2004; Malchus and Steuber 2002; Lécuyer et al. 2003; Cecca et al. 2005). A principal merit of this model is that it provides a synthetic explanation for the geological and biotic shifts during the Oxfordian.
The first phase, early Oxfordian, was characterised by the continuation of cool temperatures from the late Callovian. The cooling occurred after sea temperature and levels reached optima in the middle Callovian. A substantial, ~1 My cooling event (Dromart et al. 2003a, b) is consistent with a crisis of carbonate production (absence of coral reefs) and a drop in sea-levels in the early–late Callovian, predating extensive capture of organic matter in marine sediments (e.g., Peterborough Member of OCF, an inverse “greenhouse” effect) (Dromart et al. 2003a, b). This was followed by the beginning of a sea-level rise at the Callovian–Oxfordian boundary (= Middle–Late Jurassic boundary). Boreal ammonites were common at low latitudes (southern France) since the late Callovian, suggesting that cooler conditions in Europe extended southwards up to the northern boundary of the Tethys (Cecca et al. 2005).
The second phase took place in the middle Oxfordian: a possible thermal maximum and “greenhouse” period, with relatively arid conditions at mid latitudes (25–40°N) that favoured coral reefs. These climatic conditions would have caused poor nutrient supply in the Jurassic Sub-Boreal Seaway and could explain the restoration of oligotrophic reef formations at mid latitudes. However, humid conditions are also necessary to explain the blooming of radiolarians in the deep basins of Southern Europe. This, and geochemical evidence, points to the installation of strong climatic belts across Tethyan and Boreal Europe (Cecca et al. 2005). These environmental settings must have been favourable to a Tethyan ammonite fauna that by the early part of middle Oxfordian extended as far north as Scotland, indicating the eurythermal character of these taxa. However, at the same time Boreal ammonites had already almost disappeared at the low latitudes of the Paris Basin, indicating a northward shift of their tolerance zone (Cecca et al. 2005).
Long term warming plateaued during the third phase, in the late Oxfordian and early Kimmeridgian, when the latitudinal stratification of climatic belts across Europe persisted. More humid conditions developed, and higher nutrient water supply is hypothesised for the latest part of the Oxfordian. These climates would have supported expansion of mesotrophic and eutrophic reef facies across northern and northern-continental Europe. The success of reefs in this phase contrasts with the shrinking distributions of regional ammonite faunas. In particular, the decline of Boreal ammonites continued at lower latitudes, while they became dominant in the Jurassic Sub-Boreal Seaway (particularly as shown by diverse and abundant fossils in Yorkshire), probably because of the southward shift of the Tethyan ammonites’ palaeogeographical limit. The Kimmeridgian would have been a time of high atmospheric carbon dioxide, warm and wet climate, high global sea-levels and moderate-to-high primary productivity in the KCF basins.
In summary, geochemical, geological, and fossil data show that the Oxfordian was indeed a time of shifting, and expanding and contracting, latitudinal climatic belts across Europe. The influences of these oscillating climates on the marine biota and the lithologies of their entombing sediments are evident in the variable distribution of coral reefs and marine invertebrates. Thus, it is possible that these climatic changes could have also affected the marine reptile assemblage and may have driven the turnover between the OCF and KCF that has long been noted. Overwhelming evidence from vertebrate fossils is elusive because of the incompleteness of the fossil record, most frustratingly the Corallian gap. However, we note that some, still isolated, anachronous marine reptile specimen occurrences hint that there may have been environmental control over marine reptile distribution during the Middle–Late Jurassic in the Jurassic Sub-Boreal Seaway.
The very diverse OCF marine reptile fauna lived in a temperate, shallow, nutrient rich sea during the late Callovian. By contrast, the extensive building of oligotrophic reefs at mid latitudes indicates that the Jurassic Sub-Boreal Seaway waters became nutrient-depleted in the middle Oxfordian (Cecca et al. 2005). If indeed the Oxfordian rarity of marine reptiles is a genuine biological signal, it is possible that the collapse in diversity and abundance is linked with the environmental perturbations at the Callovian–Oxfordian. Specifically, small-to-middle-sized pliosaurids and several lineages of thalattosuchians, perhaps with more restricted diet, may have been more susceptible to habitat shifts caused by changes in sea-level, temperatures, and primary productivity.
It has been argued that late Callovian-type conditions persisted at low–mid-latitudes (Paris Basin) until the early Oxfordian (Lécuyer et al. 2003). It is possible, then, that some marine reptile lineages were pushed away from sub-boreal latitudes by these environmental changes, and migrated southwards. Specifically, the warming-induced decrease in productivity may have contributed in making near shore environments unsuitable to sustain complex ecosystems like those of the late Callovian. Considering the observed delay of these changes in the rest of Europe (see above, Lécuyer et al. 2003), the occurrence of Callovian-like taxa (e.g., Liopleurodon ferox and Peloneustes-like taxa; Bigot 1939; Beckary 1992; Bardet 1993; Bardet et al. 1993; Noè 2001; Ketchum and Benson 2011a) would not be surprising in the Paris Basin during the very early part of the Oxfordian (compared with the southward occurrences of Boreal ammonites). Similarly, the occurrence of typical Callovian taxa at lower European latitudes (e.g., Anguanax zignoi, a Tethyan taxon related to Marmornectes and Thalassophonea; Cau and Fanti 2015; Fischer et al. 2015, 2017), but outside the reef-building range, may be expected in the middle–late Oxfordian, because of the persistence of humid climate and relatively high productivity in southern seawaters. At the same time sub-boreal latitudes were suffering arid and nutrient-depleted conditions that could perhaps only sustain the low diversity Corallian marine reptile fauna (see also contemporaneous unchanged southern limits of the boreal ammonite distribution and southward shrinking of the Tethyan ammonite belt).
Interestingly, non-machimosaurin teleosaurids also apparently disappeared from the Jurassic Sub-Boreal Seaway at the Callovian–Oxfordian boundary. After their disappearance from mid latitudes during the Oxfordian, longirostrine teleosaurids can be found again in near shore Kimmeridgian deposits of France (e.g. “Steneosaurus” bouchardi, “Steneosaurus” deslongchampsianus, and “Steneosaurus” priscus). It is worth noting that crocodylomorphs had a lower temperature and latitudinal gradient tolerance and/or had lower metabolism than other marine reptiles (Bernard et al. 2010; Mannion et al. 2015, Tennant et al. 2016: supplementary material fig. 5). This could also explain the absence of thalattosuchians in the Late Jurassic Boreal Lagerstätten of Svalbard, while numerous metriorhynchid and teleosaurid taxa were still thriving in the more temperate waters of sub-boreal and continental Europe (Maxwell 2010; Roberts et al. 2014; Delsett et al. 2017). It is then possible that, differently from other marine reptile groups, these taxa were more susceptible to coastal environmental changes because of their amphibious life-style. In other words, semi-aquatic teleosaurids would have been more severely affected by the development of arid climates at Sub-Boreal latitudes at the beginning of the Oxfordian. The southern expansion of the arid belt would have pushed further southwards the teleosaurid habitable zone, essentially relegating these taxa to the Tethys. Once more humid conditions had been restored across Europe, longirostrine teleosaurids would have been able to re-colonise continental Europe at the beginning of the Kimmeridgian. However, by this time the Jurassic Sub-Boreal Seaway would have been environmentally unsuitable (too deep a basin) for near-shore taxa.
Admittedly, the European Oxfordian marine reptile fossil record is still too patchy to rigorously test the hypothesized scenario outlined above. Thus, future discoveries, especially from the Oxfordian Tethyan area, could be used to support or falsify a climatic control over marine reptile distribution. In general, we hypothesize that Middle–Late Jurassic perturbations would have likely affected different marine reptile lineages in different ways, based on their environmental preferences and physiology. A thorough investigation of these factors, combining appropriate proxies for sea-level, temperature, productivity, and palaeoclimate and updated marine reptile diversity and occurrences in the Jurassic Sub-Boreal Seaway and neighbouring areas, is in progress, and is needed to clarify how environmental changes influenced the marine biota, and possibly drove turnover, during the Middle–Late Jurassic.
Conclusions
We suggest that the fluctuation of climatic belts during the Oxfordian may have strongly influenced marine reptile faunas across Europe. The complex, highly diverse, stratified ecosystem of the Callovian may have been disrupted by the development of an arid climatic belt across the Jurassic Sub-Boreal Seaway. The remains of this fauna may have pushed southwards in the early Oxfordian, as supported by the occurrence of Callovian-type taxa in the early Oxfordian of northern France and later in the Tethyan area. It is not clear what may have favoured the re-installation of high-diversity ecosystems in the Kimmeridgian–Tithonian. However, the organic-rich mudrocks of the KCF were deposited in a moderate-to-rich nutrient environment, during a period of warm and wet climate, and high global sea-level (Cox 2001), in striking contrast with the arid and nutrient-depleted conditions that characterised most of the Oxfordian.
Acknowledgements
DF is grateful to Michael J. Benton (University of Bristol, UK) for providing information and notes on Oxfordian specimens; to Aubrey J. Roberts (University of Bath, UK) for help, discussion, and photos of comparative material. DF thanks Deborah Hutchinson and Isla Gladstone (both BRSMG), Matthew Riley (CAMSM), Paul Tomlinson (DORCM), Neil Clark (GLAHM), Steve Etches (MJML), Lorna Steel (NHMUK), Eliza Howlett and Hilary Ketchum (both OUMNH) and Emma Jarvis, Sarah C. King, and Stuart Ogilvy (all YORYM) for access and guidance during DF’s visit to museum collections. We thank the editor and reviewers Benjamin C. Moon (University of Bristol, UK) and Valentin Fischer (University of Liège, Belgium) for the constructive comments that greately improved the quality of the manuscript. DF designed and developed the study, observed all specimens, and wrote the manuscript; MTY helped design the study and commented on the manuscript; SLB oversaw the study and commented and edited the manuscript. DF’s museum visits were funded by the Small Grant Scheme “2015 Wood Award” (PASW201402), the Systematics Research Fund, the Richard Owen Research Fund by the Palaeontographical Society. SLB is supported by a Marie Curie Career Integration Grant (630652). SLB and MTY are supported by a Leverhulme Trust Research Project Grant (RPG-2017-167).
References
Abbink, O., Targarona, J., Brinkhuis, H., and Visscher, H. 2001. Late Jurassic to earliest Cretaceous palaeoclimatic evolution of the southern North Sea. Global and Planetary Change 30: 231–256. Crossref
Anderson, T.F., Popp, B.N., Williams, A.C., Ho, L.Z., and Hudson, J.D. 1995. The stable isotope records of fossils from the Peterborough Member, Oxford Clay Formation (Jurassic), UK: Palaeoenvironmental implications. Journal of the Geological Society 151: 125–138. Crossref
Andrade, M.B., Young, M.T., Desojo, J.B., and Brusatte, S.L. 2010. The evolution of extreme hypercarnivory in Metriorhynchidae (Mesoeucrocodylia: Thalattosuchia): evidence from microscopic denticle morphology and a new tri-faceted Kimmeridgian tooth from Germany. Journal of Vertebrate Paleontology 30: 1451–1465. Crossref
Andrews, C.W. 1910. A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay. Part One. xxiii + 205 pp. British Museum (Natural History), London.
Andrews, C.W. 1913. A Descriptive Catalogue of the Marine Reptiles of the Oxford Clay. Part Two. xxiv + 206 pp. British Museum (Natural History), London.
Bardet, N. 1993. Pliosaurs and plesiosaurs from the Middle Jurassic (Callovian) of Normandy. Revue de Paléobiologie 7: 1–17.
Bardet, N., Pennetier, G., Pennetier, E., and Queromain, J. 1993. Presence du Pliosaure Liopleurodon ferox Sauvage dans le Jurassique moyen (Callovien) de Villers-sur-Mer, Normandie. Bulletin trimestriel de la Societé géologique de Normandie et des Amis du Museum du Havre 80: 11–14.
Barrientos-Lara, J.I., Herrera, Y., Fernández, M.S., and Alvarado-Ortega, J. 2016. Occurrence of Torvoneustes (Crocodylomorpha, Metriorhynchidae) in marine Jurassic deposits of Oaxaca, Mexico. Revista Brasileira de Paleontologia 19: 415–424. Crossref
Beckary, S.1992. Le pliosaure de Marquise. Annales de la Société géologique du Nord 1: 153–155.
Bell, M.A. and Lloyd, G.T. 2015. Strap: an R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58: 379–389. Crossref
Benson, R.B.J. and Bowdler, T. 2014. Anatomy of Colymbosaurus megadeirus (Reptilia, Plesiosauria) from the Kimmeridge Clay Formation of the U.K., and high diversity among Late Jurassic plesiosauroids. Journal of Vertebrate Paleontology 34: 1053–1071. Crossref
Benson, R.B.J. and Druckenmiller, P.S. 2014. Faunal turnover of marine tetrapods during the Jurassic–Cretaceous transition. Biological Reviews 89: 1–23. Crossref
Benson, R.B.J., Butler, R.J., Lindgren, J., and Smith, A.S. 2010. Mesozoic marine tetrapod diversity: mass extinctions and temporal heterogeneity in geological megabiases affecting vertebrates. Proceedings Royal Society B 277: 829–834. Crossref
Benson, R.B.J., Evans, M., Smith, A.S., Sassoon, J., Moore-Faye, S., Ketchum, H.F., and Forrest, R. 2013. A giant pliosaurid skull from the Late Jurassic of England. PLoS ONE 8: e65989. Crossref
Benton, M.J. and Spencer, P.S. 1995. Fossil Reptiles of Great Britain. 386 pp. Chapman and Hall, London. Crossref
Bernard, A., Lecuyer, C., Vincent, P., Amiot, R., Bardet, N., Buffetaut, E., Cuny, G., Fourel, F., Martineau, F., Mazin, J.-M., and Prieur, A. 2010. Regulation of body temperature by some Mesozoic marine reptiles. Science 328: 1379–1382. Crossref
Bigot, A. 1939. Sauropterygians du Jurassique de Calvados. Bulletin de la Société géologique de France 5: 631–636.
Bradshaw, M.J., Cope, J.C.W., Cripps, D.W., Donovan, D.T., Howarth, M.K., Rawson, P.F., West, I.M., and Wimbledon, W.A. 1992. Jurassic. Geological Society, London, Memoirs 13, 107–129.
Brown, D.S. 1981. The English Upper Jurassic Plesiosauroidea (Reptilia) and a review of the phylogeny and classification of the Plesiosauria. Bulletin of the British Museum (Natural History), Geology 35: 253–347.
Brown, D.S., Milner, A.C., and Taylor, M.A. 1986. New material of the plesiosaur Kimmerosaurus langhami Brown from the Kimmeridge Clay of Dorset. Bulletin of the British Museum (Natural History), Geology 40: 225–234.
Cau, A. and Fanti, F. 2014. A pliosaurid plesiosaurian from the Rosso Ammonitico Veronese Formation of Italy. Acta Palaeontologica Polonica 59: 643–650.
Cau, A. and Fanti, F. 2015. High evolutionary rates and the origin of the Rosso Ammonitico Veronese Formation (Middle–Upper Jurassic of Italy) reptiles. Historical Biology 7: 952–962.
Cecca, F., Garin, B.M., Marchand, D., Lathuiliere, B., and Bartolini, A. 2005. Paleoclimatic control of biogeographic and sedimentary events in Tethyan and peri-Tethyan areas during the Oxfordian (Late Jurassic). Palaeogeography, Palaeoclimatology, Palaeoecology 222: 10–32. Crossref
Coe, A.L. 1992. The Oxfordian and Portlandian. In: A.L. Coe, S.P. Hesselbo, D.N. Parkinsson, and H.C. Jenkyins (eds.), Sequence Stratigraphy of the Dorset Jurassic, a Field Guide. Sequence Stratigraphy of European Basins, 37–81. CNRS-IFP, Dijon.
Coe, A.L. 1995. A comparison of the Oxfordian successions of Dorset, Oxfordshire and Yorkshire. In: P.D. Taylor (ed.), Field Geology of the British Jurassic, 151–172. Geological Society, London.
Cope, J.C.W. 1974. Upper Kimmeridgian ammonite faunas of the Wash area and a subzonal scheme for the lower part of the Upper Kimmeridgian. Bulletin of the Geological Survey of Great Britain 47: 29–37.
Cope, J.C.W. 2006. Jurassic: the returning seas. In: P.J. Brenchley, and P.F. Rawson (eds.), The Geology of England and Wales. 2nd Edition, 325–363. Geological Society, London. Crossref
Cox, B.M. 2001. General introduction to Oxfordian and Kimmeridgian stratigraphy. In: J.K. Wright and B.M. Cox (eds.), British Upper Jurassic Stratigraphy. Geological Conservation Review Series 21, 3–10. Joint Nature Conservation Committee, Peterborough.
Cruickshank, A.R.I., Martill, D.M., and Noè, L.F. 1996. A pliosaur (Reptilia, Sauropterygia) exhibiting pachyostosis from the Middle Jurassic of England. Journal of the Geological Society of London 153: 873–879. Crossref
Danise, S., Twitchett, R.J., and Matts, K. 2014. Ecological succession of a Jurassic shallow-water ichthyosaur fall. Nature Communications 5: 4789. Crossref
Delair, J.B. 1958. The mesozoic reptiles of Dorset. Proceedings of the Dorset Natural History and Archaeological Society 79: 47–72.
Delsett, L.L., Roberts, A.J., Druckenmiller, P.S., and Hurum, J.H. 2017. A new ophthalmosaurid (Ichthyosauria) from Svalbard, Norway, and evolution of the ichthyopterygian pelvic girdle. PLoS ONE 12: e0169971. Crossref
Drake, H.C. and Sheppard, T. 1909. Classified list of organic remains from the rocks of the East Riding of Yorkshire. Proceedings of the Yorkshire Geological Society 17: 4–71. Crossref
Dromart, G., Garcia, J.P., Gaumet, F., Picard, S., Rousseau, M., Atrops, F., Lecuyer, C., and Sheppard, S.M.F. 2003a. Perturbation of the carbon cycle at the Middle–Late Jurassic transition: geological and geochemical evidence. American Journal of Science 303: 667–707. Crossref
Dromart, G., Garcia, J.P., Picard, S., Atrops, F., Lécuyer, C., and Sheppard, S.M.F. 2003b. Ice age at the Middle–Late Jurassic transition? Earth and Planetary Science Letters 213: 205–220. Crossref
Gasparini, Z. 2009. A new Oxfordian pliosaurid (Plesiosauria, Pliosauridae) in the Caribbean Seaway. Palaeontology 52: 661–669. Crossref
Fanti, F., Miyashita, T., Cantelli, L., Mnasri, F., Dridi, J., Contessi, M., and Cau, A. 2016. The largest thalattosuchian (Crocodylomorpha) supports teleosaurid survival across the Jurassic–Cretaceous boundary. Cretaceous Research 61: 263–274. Crossref
Fischer, V., Appleby, R.M., Naish, D., Liston, J., Riding, J.B., Brindley S., and Godefroit, P. 2013. A basal thunnosaurian from Iraq reveals disparate phylogenetic origins for Cretaceous ichthyosaurs. Biology Letters 9 (4): 20130021. Crossref
Fischer, V., Arkhangelsky, M.S., Stenshin, I.M., Uspensky, G.N., Zverkov, N.G., and Benson, R.B.J. 2015. Peculiar macrophagous adaptations in a new Cretaceous pliosaurid. Royal Society Open Science 2: 150552. Crossref
Fischer, V., Bardet, N., Benson, R.J.B., Arkhangelsky, M.S., and Friedman, M. 2016. Extinction of fish-shaped marine reptiles associated with reduced evolutionary rates and global environmental volatility. Nature Communications 7: 10825. Crossref
Fischer, V., Bardet, N., Guiomar, M., and Godefroit, P. 2014. High diversity in Cretaceous ichthyosaurs from Europe prior to their extinction. PLoS ONE 9 (1): e84709. Crossref
Fischer, V., Benson, R.B.J., Zverkov, N.G., Soul, L.C., Arkhangelsky, M.S., Stenshin, I.M., Lambert, O., Stenshin, I.M., Uspensky, G.N., and Druckenmiller, P.S. 2017. Plasticity and convergence in the evolution of short-necked plesiosaurs. Current Biology 27: 1667–1676. Crossref
Foffa, D. and Young, M.T. 2014. The cranial osteology of Tyrannoneustes lythrodectikos (Crocodylomorpha: Metriorhynchidae) from the Middle Jurassic of Europe. PeerJ 2: e608. Crossref
Foffa, D., Cuff, A.R., Sassoon, J., Rayfield, E.J., Mavrogordato, M.N., and Benton, M.J. 2014. Functional anatomy and feeding biomechanics of a giant Upper Jurassic pliosaur (Reptilia: Sauropterygia) from Weymouth Bay, Dorset, UK. Journal of Anatomy 225: 209–219. Crossref
Foffa, D., Young, M.T., and Brusatte, S.L. 2015. Evidence of macrophagous teleosaurid crocodylomorphs in the Corallian Group (Oxfordian, Late Jurassic) of the UK. PeerJ 3: e1497. Crossref
Foffa, D., Young, M.T., Brusatte, S.L., Graham, M.R., and Steel, L. 2017. A new metriorhynchid crocodylomorph from the Oxford Clay Formation (Middle Jurassic) of England, with implications for the origin and diversification of Geosaurini. Journal of Systematic Palaeontology [published online, doi: 10.1080/14772019.2017.1367730]. Crossref
Fox-Strangways, C. 1892. The Jurassic Rocks of Britain, Part I. Yorkshire. Memoir of the Geological Survey of the United Kingdom. ix + 551 pp. HMSO, London.
Horton, A., Sumbler, M.G., Cox, B.M., and Ambrose, K. 1995. England and Wales. Memoir of the British Geological Survey 237.
Hua, S. 1999. Le crocodilien Machimosaurus mosae (Thalattosuchia, Teleosauridae) du Kimmeridgien du Boulonnais (Pas de Calais, France). Palaeontographica Abtteilung A 252: 141–170.
Hulke, J.W. 1871. Note on a fragment of a Teleosaurian snout from Kimmeridige Bay, Dorset. Quarterly Journal of the Geological Society of London 17: 442–443. Crossref
Johnson, M.M., Young, M.T., Steel, L., and Lepage, Y. 2015. Steneosaurus edwardsi (Thalattosuchia, Teleosauridae), the largest known crocodylomorph of the Middle Jurassic. Biological Journal of the Linnean Society 115: 911–918. Crossref
Johnson, M.M., Young, M.T., Steel, L., Foffa, D., Smith, A.S., Hua, S., Havlik, P., Howlett, E.A., and Dyke, G. 2017. Re-description of “Steneosaurus” obtusidens Andrews, 1909, an unusual macrophagous teleosaurid crocodylomorph from the Middle Jurassic of England. Zoological Journal of the Linnean Society 182: 385–418. Crossref
Jouve, S., Mennecart, B., Douteau, J., and Jalil, N.-E. 2016. The oldest durophagous teleosauroid (Crocodylomorpha, Thalattosuchia) from the lower Bathonian of central High Atlas, Morocco. Palaeontology 59: 863–876. Crossref
Kelley, N.P. and Pyenson, N.D. 2015. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science 348: aaa3716. Crossref
Ketchum, H.F. and Benson, R.B.J. 2011a. A new pliosaurid (Sauropterygia, Plesiosauria) from the Oxford Clay Formation (Middle Jurassic, Callovian) of England: evidence for a gracile, longirostrine grade of Early–Middle Jurassic pliosaurids. Special Papers in Palaeontology 86: 109–129.
Ketchum, H.F. and Benson, R.B.J. 2011b. The cranial anatomy and taxonomy of Peloneustes philarchus (Sauropterygia, Pliosauridae) from the Peterborough member (Callovian, Middle Jurassic) of the United Kingdom. Palaeontology 54: 639–665. Crossref
Kirton, A.M. 1983. A Review of British Upper Jurassic Ichthyosaurs. 478 pp. Unpublished Ph.D. Thesis, University of Newcastle-upon-Tyne, Newcastle-upon-Tyne.
Knutsen, E.M. 2012. A taxonomic revision of the genus Pliosaurus (Owen, 1841a) Owen, 1841b. Norwegian Journal of Geology 92: 259–276.
Lécuyer, C., Picard, S., Garcia, J.-P., Sheppard, S.M.F., Grandjean, P., and Dromart, G. 2003. Thermal evolution of Tethyan surface waters during the Middle–Late Jurassic: Evidence from δ18O values of marine fish teeth. Palaeoceanography 18: 1076. Crossref
Lepage, Y., Buffetaut, E., Hua, S., Martin, J.E., and Tabouelle, J. 2008. Catalogue descriptif, anatomique, géologique et historique des fossiles présentés à l’exposition Les Crocodiliens fossiles de Normandie. Bulletin de la Société géologique de Normandie et des Amis du Muséum du Havre 95: 5–152.
Lomax, D.R. and Massare, J.A. 2017. Two new species of Ichthyosaurus from the lowermost Jurassic (Hettangian) of Somerset, England. Papers in Palaeontology 3: 1–20.
LydekkerR. 1889. On the remains and affinities of five genera of Mesozoic reptiles. Quarterly Journal of the Geological Society 45: 41–59.Crossref
Mannion, P.D., Benson, R.B.J., Carrano, M.T., Tennant, J.P., Judd, J., and Butler, R.J. 2015. Climate constrains the evolutionary history and biodiversity of crocodylians. Nature Communications 6: 8438.Crossref
Malchus, N. and Steuber, T. 2002. Stable isotope records (O, C) of Jurassic aragonitic shells from England and NW Poland: palaeoecologic and environmental implications. Geobios 35: 29–39. Crossref
Massare, J.A. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. Journal of Vertebrate Paleontology 7: 121–137. Crossref
Martill, D.M. and Hudson, J.D. 1991. Fossils of the Oxford Clay. Palaeontological Association Field Guide to Fossils 4: 1–286.
Maxwell, E.E. 2010. Generic reassignment of an ichthyosaur from the Queen Elizabeth Islands, Northwest Territories, Canada. Journal of Vertebrate Paleontology 30: 403–415. Crossref
McGowan C. 1976. The description and phenetic relationships of a new ichthyosaur genus from the Upper Jurassic of England. Canadian Journal of Earth Sciences 13: 668–683. Crossref
Moon, B.C. 2017. A new phylogeny of ichthyosaurs (Reptilia: Diapsida). Journal of Systematic Palaeontology [published oline]. Crossref
Moon, B.C. and Kirton, A.M. 2016. Ichthyosaurs of the British Middle and Upper Jurassic. Part 1. Ophthalmosaurus. Monograph of the Palaeontographical Society 170 (647): 1–84. Crossref
Motani, R. 2005. Evolution of fish-shaped reptiles (Reptilia: Ichthyopterygia) in their physical environments and constraints. Annual Review of Earth and Planetary Sciences 33 (1): 395–420. Crossref
Motani, R., Rothschild, B.M., and Whal, W. Jr. 1999. Large eyeballs in diving ichthyosaurs. Nature 402: 747. Crossref
Newton, E.T. 1878. Notes on a crocodilian jaw from the Corallian rocks of Weymouth. The Quarterly Journal of the Geological Society of London 34: 398–400. Crossref
Noè, L.F. 2001. A Taxonomic and Functional Study of the Callovian (Middle Jurassic) Pliosauroidea (Reptilia, Sauropterygia). Volume 1: xxxii + 347 pp; Volume 2: xxxii + 182 pp. Ph.D. Dissertation, University of Derby, Derby.
Páramo, M.E., Gómez-Pérez, M., Noé, L.F., and Etayo, F. 2016. Stenorhynchosaurus munozi, gen. et sp. nov. a new pliosaurid from the Upper Barremian (Lower Cretaceous) of Villa de Leiva, Colombia, South America. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 40 (154): 84–103. Crossref
Parrilla-Bel, J., Young, M.T., Moreno-Azanza, M., and Canudo, J.I. 2013. The first metriorhynchid crocodylomorph from the Middle Jurassic of Spain, with implications for evolution of the subclade Rhacheosaurini. PLoS ONE 8 (1): e54275. Crossref
Podlaha, O.G., Mutterlose, J., and Veizer, J. 1998. Preservation of δ18O and δ13C in belemnite rostra from the Jurassic/early Cretaceous successions. American Journal of Science 298: 324–347. Crossref
Pyenson, N.D., Kelley, N.P., and Parham, J.F. 2014. Marine tetrapod macroevolution: physical and biological drivers on 250 Ma of invasions and evolution in ocean ecosystems. Palaeogeography, Palaeoclimatology, Palaeoecology 400: 1–8. Crossref
R Core Team 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www. R-project.org
Riboulleau, A., Baudin, F., Daux, V., Hantzpergue, P., Renard, M., and Zakharov, V. 1998. Évolution de la paléotempérature des eaux de la plate-forme russe au cours du Jurassique supérieur. Comptes Rendus de l’Académie des sciences de la terre et des planètes 326: 239–246.
Roberts, A.J., Druckenmiller, P.S., Deslett, L.L., and Hurum, J.H. 2017. Osteology and relationships of Colymbosaurus Seeley, 1874, based on new material of C. svalbardensis from the Slottsmøya Member, Agardhfjellet Formation of central Spitsbergen. Journal of Vertebrate Paleontology 37: e1278381 .Crossref
Roberts, A.J., Druckenmiller, P.S., Sætre, G.-P., and Hurum, J.H. 2014. A new Upper Jurassic ophthalmosaurid ichthyosaur from the Slottsmøya Member, Agardhfjellet Formation or central Spitsbergen. PLoS ONE 9: e103152. Crossref
Sassoon, J., Foffa, D., and Marek, R. 2015. Dental ontogeny and replacement in Pliosauridae. Royal Society Open Science 2: 150384. Crossref
Sauvage, H.E. 1873. Notes sur les reptiles fossiles. 4. Du genre Liopleurodon Sauvage. Bulletin de la Société géologique de France, Series 3 1: 377–380.
Schultz, H.-P. 1969. Die Faltenzähne der rhipidistiiden Crossopterygier, der Tetrapoden und der Actinopterygier – Gattung Lepisosteus; nebst einer Beschreibung der Zahnstruktur von Onychodus (struniiformer Crossopterygier). Palaeontographica Italica 65: 63–137.
Schultz, H.-P. 1970. Folded teeth and the monophyletic origin of tetrapods. American Museum Novitates 2408: 1–10.
Seeley H.G. 1869. Index to the Fossil Remains of Aves, Ornithosauria, and Reptilia, from the Secondary System of Strata Arranged in the Woodwardian Museum of the University of Cambridge. xxiii +143 pp. Deighton, Bell and Co., Cambridge.
Stubbs, T.L. and Benton, M.J. 2016. Ecomorphological diversifcations of Mesozoic marine reptiles: The roles of ecological opportunity and extinction. Paleobiology 42: 547–573. Crossref
Talevi, M. and Fernández, M.S. 2012. Unexpected skeletal histology of an ichthyosaur from the Middle Jurassic of Patagonia: implications for evolution of bone microstructure among secondary aquatic tetrapods. Naturwissenschaften 99: 241–244. Crossref
Tarlo, L.B. 1960. A review of the Upper Jurassic pliosaurs. Bulletin of the British Museum (Natural History) 4: 145–189.
Taylor, M.A. and Cruickshank, A.R.I. 1993. Cranial anatomy and functional morphology of Pliosaurus brachyspondylus (Reptilia: Plesiosauria) from the Upper Jurassic of Westbury, Wiltshire. Philosophical Transactions of the Royal Society B: Biological Sciences 341: 399–418. Crossref
Tennant, J.P., Mannion, P.D., and Upchurch, P. 2016. Sea level regulated tetrapod diversity dynamics through the Jurassic/Cretaceous interval. Nature Communications 7: 12737. Crossref
Vignaud, P., Lange-Badre, B., Hantzpergue, P., Dutrieux, M., and Maury, G. 1993. Découverte d’un crane de Teleosauridae dans la Zone à Eudoxus du Kimméridgien supérieur quercynois (France). Comptes Rendus de l’Académie des sciences. Série 2, Mécanique, physique, chimie, sciences de l’univers, sciences de la terre 317: 1509–1514.
Watson, D.M.S. 1911. Notes on some British Mesozoic crocodiles. Memoirs and Proceedings of the Manchester Literary and Philosophical Society 55: 1–13.
Weedon, G., Jenkyins, H., and Page, K. 2017. Combined sea-level and climate controls on limestone formation, hiatuses and ammonite preservation in the Blue Lias Formation, South Britain (uppermost Triassic–Lower Jurassic). Geological Magazine [published online, doi: 10.1017/S001675681600128X]. Crossref
Wierzbowski, H. 2004. Carbon and oxygen isotope composition of Oxfordian–Early Kimmeridgian belemnite rostra: palaeoenvironmental implications for Late Jurassic seas. Palaeogeography, Palaeoclimatology, Palaeoecology 203: 153–168. Crossref
Wilkinson, L.E., Young, M.T., and Benton, M.J. 2008. A new metriorhynchid crocodile (Mesoeucrocodylia: Thalattosuchia) from the Kimmeridgian (Upper Jurassic) of Wiltshire, UK. Palaeontology 51: 1307–1333. Crossref
Wright, J.K. 2009. The geology of the Corallian ridge (Upper Jurassic) between Gilling East and North Grimston, Howardian Hills, North Yorkshire. Proceedings of the Yorkshire Geological Society 57: 193–216. Crossref
Wright, J.K. 2014. A new section through the Corallian Group (Oxfordian, Upper Jurassic) rocks of Calne, Wiltshire, southern England. Proceedings of the Geologists’ Association 125: 83–95. Crossref
Young, M.T. 2014. Filling the “Corallian Gap”: re-description of a metriorhynchid crocodylomorph from the Oxfordian (Late Jurassic) of Headington, England. Historical Biology: An International Journal of Paleobiology 26: 80–90. Crossref
Young, M.T. and Andrade, M.B. 2009. What is Geosaurus? Redescription of G. giganteus (Thalattosuchia, Metriorhynchidae) from the Upper Jurassic of Bayern, Germany. Zoological Journal of the Linnean Society 157: 551–585. Crossref
Young, M.T. and Steel, L. 2014. Evidence for the teleosaurid crocodylomorph genus Machimosaurus in the Kimmeridge Clay Formation (Late Jurassic) of England, Historical Biology: An International Journal of Paleobiology 26: 472–479. Crossref
Young, M.T., Andrade, M.B., Brusatte, S.L., Sakamoto, M., and Liston, J. 2013a. The oldest known metriorhynchid super-predator: a new genus and species from the Middle Jurassic of England, with implications for serration and mandibular evolution in predacious clades. Journal of Systematic Palaeontology 11: 475–513. Crossref
Young, M.T., Andrade, M.B., Etches, S., and Beatty, B.L. 2013b. A new metriorhynchid crocodylomorph from the Lower Kimmeridge Clay Formation (Late Jurassic) of England, with implications for the evolution of dermatocranium ornamentation in Geosaurini. Zoological Journal of the Linnean Society 169: 820–848. Crossref
Young, M.T., Brusatte, S.L., Andrade, M.B., Beatty, L., and Desojo, J.B. 2012a. Tooth-on-tooth interlocking occlusion suggests macrophagy in the Mesozoic marine crocodylomorph Dakosaurus. The Anatomical Record 295: 1147–1158. crossref
Young, M.T., Brusatte, S.L., Andrade, M.B., Desojo, J.B., Beatty, B.L., Steel, L., Fernández, M.S., Sakamoto, M., Ruiz-Omeñaca, J.I., and Schoch, R.R. 2012b. The cranial osteology and feeding ecology of the metriorhynchid crocodylomorph genera Dakosaurus and Plesiosuchus from the Late Jurassic of Europe. PLoS ONE 7: e44985. Crossref
Young, M.T., Hua, S., Steel, L., Foffa, D., Brusatte, S.L., Thüring, S., Mateus, O., Ruiz-Omeñaca, J.I., Havlik, P., Lepage, Y., and Andrade, M.B. 2014a. Revision of the Late Jurassic teleosaurid genus Machimosaurus (Crocodylomorpha, Thalattosuchia). Royal Society Open Science 1: 140222. Crossref
Young, M.T., Steel, L., Brusatte, S.L., Foffa, D., and Lepage, Y. 2014b. Tooth serration morphologies in the genus Machimosaurus (Crocodylomorpha, Thalattosuchia) from the Late Jurassic of Europe. Royal Society Open Science 1: 140269. Crossref
Young, M.T., Hastings, A.K., Allain, R., and Smith, T.J. 2016. Revision of the enigmatic crocodyliform Elosuchus felixi de Lapparent de Broin, 2002 from the Lower–Upper Cretaceous boundary of Niger: potential evidence for an early origin of the clade Dyrosauridae. Zoological Journal of the Linnean Society [published online, doi: 10.1111/zoj.12452]. Crossref
Acta Palaeontol. Pol. 63 (2): 287–313, 2018
https://doi.org/10.4202/app.00455.2018