

Middle Triassic dipterid ferns from west-central Argentina and their relationship to palaeoclimatic changes
JOSEFINA BODNAR, JUAN MARTÍN DROVANDI, EDUARDO MANUEL MOREL, and DANIEL GUSTAVO GANUZA
Bodnar, J., Drovandi, J.M., Morel, E.M., and Ganuza, D.G. 2018. Middle Triassic dipterid ferns from west-central Argentina and their relationship to palaeoclimatic changes. Acta Palaeontologica Polonica 63 (2): 397–416.
Dipterid ferns possess robust fossil record from Mesozoic times on, and they are considered to be a reliable indicator of warm to subtropical humid or seasonal paleoclimatic conditions. In this contribution, we revised and described new samples of fossil Dipteridaceae, from the Barreal and Cortaderita formations (Sorocayense Group), Middle Triassic (Anisian–Ladinian), central-western Argentina. We found out that three species of Dictyophyllum, one of Hausmannia, and one of Thaumatopteris are present in the Sorocayense Group. We erected a new species Dictyophyllum menendezi sp. nov., which is characterized by petiolate fan-shaped fronds, rachis dividing into two arms, each one bearing 5 or 6 oblanceolate pinnae, basal lamina of adjacent pinnae fused forming a wide web, pinnae margin entire to undulate, primary veins catadromous to isodromous, secondary veins subopposite, tertiary veins subopposite to alternate, falcate or with a zig-zag pattern, dichotomizing four times to form a fine reticulate mesh of polygonal irregular areoles. Temporal changes in the diversity of Dipteridaceae were identified in Sorocayense Group. The first record and the maximum species richness occur at the top of the Barreal Formation (late Anisian) with three species. The lower member of the Cortaderita Formation (early Ladinian) presents two species. Dipteridaceae fossils are not registered in the upper member of the Cortaderita Formation (late Ladinian). An important diversification pulse of the family is registered during the late Norian–Rhaetian in other areas of Argentina (Paso Flores, Malargüe, and Atuel depocenters). The flourishing of Dipteridaceae during the late Anisian–early Ladinian and the late Norian–Rhaetian could be related to the increasing humidity episodes of the subtropical seasonal climate that prevailed during the Triassic. Furthermore, Dipteridaceae appear important in the establishment of plant communities, being part of early stages of floristic succession.
Key words: Monilophyta, Polypodiopsida, Gleicheniales, Thaumatopteris, Dictyophyllum, Hausmannia, Triassic, Sorocayense Group, Argentina.
Josefina Bodnar [jbodnar@fcnym.unlp.edu.ar], Eduardo Manuel Morel [emorel@fcnym.unlp.edu.ar], and Daniel Gustavo Ganuza [dganuza@fcnym.unlp.edu.ar], División Paleobotánica, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s/n, B1900FWA La Plata, Buenos Aires, Argentina.
Juan Martín Drovandi [drovandijuan@gmail.com], Instituto y Museo de Ciencias Naturales, Universidad Nacional de San Juan, Av. España 400 (norte), J5400DNQ San Juan, San Juan, Argentina.
Received 15 January 2018, accepted 25 April 2018, available online 4 June 2018.
Copyright © 2018 J. Bodnar et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The family Dipteridaceae (order Gleicheniales, class Polypodiopsida) comprises 11 living species distributed into 2 genera (Dipteris and Cheiropleuria), which nowadays range from India, southeast Asia, eastern and southern China, central and southern Japan, and Malesia, to Melanesia and western Polynesia (Samoa) (Kato et al. 2001; Pryer et al. 2004). They are characterized by long-creeping rhizomes; leaf blades cleft into two or often more subequal parts; veins highly reticulate, with included veinlets; sori exin-dusiate scattered over the surface; or leaves dimorphic, and the fertile ones covered with sporangia (Smith et al. 2006, 2008; Christenhusz et al. 2011). Dipteris includes one species, D. lobbiana, which grows on streambanks where the canopy is open, and other species, as D. conjugata, which are colonisers of disturbed sites and exposed ridges (Cantrill 1995).
In contrast to their restricted modern distribution, the Dipteridaceae family was well established during the Mesozoic, forming an important element of many Mesozoic floras in Gondwana, Eurasia, and North America, ranging from the Middle Triassic to the Cretaceous (Tidwell and Ash 1994; Cantrill 1995). About seven fossil genera have been recognized, including Hausmannia Dunker, 1846, Clathropteris Brongniart, 1828, Dictyophyllum Lindley and Hutton, 1834, Thaumatopteris Goeppert, 1841, Goeppertella Ôishi and Yamasita, 1936 emend. Arrondo and Petriella, 1982, Camptopteris Presl in Sternberg, 1838 emend. Nathorst, 1878, and Polyphacelus Yao, Taylor, and Taylor, 1991 emend. Bomfleur and Kerp, 2010 (Ôishi and Yamasita 1936; Bomfleur and Kerp 2010).
The Middle Triassic record of the family is scarce and is represented by Dictyophyllum davidi from Australia (Webb 1982), Thaumatopteris sp. from Italy (Kustatscher et al. 2014), and several species from Argentina (Stipanicic and Menéndez 1949), which are the object of the present revision.
We report here newly discovered dipterid fern fossils from the Middle Triassic of Sorocayense Group, Cuyo Basin, at western Precordillera, San Juan province, central-western Argentina. Additionally, we revise taxonomically the previously described fossils from the same lithostratigraphic unit. Finally, we analyse the changes in the diversity of Dipteridaceae during the Triassic of Argentina and examine their correlation with the palaeoclimatic conditions.
Institutional abbreviations.—BAPb, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (National Palaeobotanical Collection), Ciudad Autónoma de Buenos Aires, Argentina; LPPB, Museo de La Plata (Palaeobotanical Collection), La Plata, Argentina; PBSJ, Instituto y Museo de Ciencias Naturales de San Juan (Palaeobotanical Collection), San Juan, Argentina.
Other abbreviations.—EF, fossiliferous stratum.
Geological setting
The Cuyo or Cuyana basin (central-western Argentina), along with the Ischigualasto-Villa Unión and Marayes-El Carrizal basins, belongs to a series of continental extensional basins that developed during the early Mesozoic at the western edge of Pangea (e.g., Uliana and Biddle 1988; Ramos and Kay 1991; López Gamundí 1994).
The sediments from the northern part of the Cuyo Basin, in San Juan Province, are represented by the Sorocayense and Rincón Blanco groups. This area is known as Rincón Blanco half-graben (Barredo and Ramos 2010). According to these authors, the Sorocayense Group was accumulated along a passive margin of a half-graben. Conversely, the sediments from the Rincón Blanco Group were deposited in the active margin of the half-graben, which was inferred from the recognition of thicker basal successions and volcanic activity (López Gamundí 1994; Barredo and Ramos 2010; Barredo 2012).
The Sorocayense Group (Mésigos 1953) outcrops at the Barreal-Calingasta Depocenter (Stipanicic 1972; López Gamundí 1994), and, in the southern part, it comprises three formations: Barreal (Middle Triassic), Cortaderita (Middle Triassic), and Cepeda (Late Triassic) (Groeber and Stipanicic 1953). Except for the Cepeda Formation, the other units of the Sorocayense Group bear a continuous series of plant-bearing strata.
The Barreal Formation is characterized by deposits of siliciclastic, volcaniclastic, and pyroclastic nature. The lower part is composed of horizontal and cross-stratified polymictic conglomerate that is interlayered with minor lenticular bodies of sandstone and edafized fine-grained sandstone and siltstone. Towards the top, this unit comprises grey claystone, siltstone, and silty sandstone beds—with abundant plant impression-compressions and permineralized tree-trunks—interlayered with few lenticular and tabular bodies of massive sandstone and cross-stratified fine conglomerate. Throughout the formation, although more commonly present at the top, there are pink and greenish tuff and bentonite layers that bear abundant plant fossil material. The Barreal Formation was interpreted as deposits of high sinuosity gravel-sand meandering fluvial systems, with an increase in ash-fall deposits towards the top, which could have caused the damming of the depositional system (Bodnar et al. in press). With respect to the age, this formation was assigned to Anisian (early Middle Triassic) due to its fossiliferous content and the correlation with units from the Rincón Blanco Group (Morel et al. 2003; Bodnar et al. in press).
The Cortaderita Formation has served as a topic of interest for many researchers because of its accessibility, quality of the outcrops, and fossil richness (e.g., Du Toit 1927; Cuerda 1945; Stipanicic 1972; Groeber and Stipanicic 1953; Menéndez 1956; Bonetti 1963, 1968, 1972; Lutz and Herbst 1992; Spalletti 2001; Zamuner et al. 1999; Bodnar 2008; Bodnar et al. 2015). The Cortaderita Formation has been divided in two members due to lithological differences (Bodnar et al. in press). The lower member consists of yellowish cross-stratified conglomerate and sandstone in lenticular bodies, interlayered with greyish massive bentonitic and edafized siltstone, muddy sandstone, and greenish bentonite. These fine facies are highly bioturbated with root traces and host abundant plant fossil remains (Bodnar 2010; Bodnar et al. in press). It has been concluded that this section has been deposited in a mid-high sinuosity anastomosed fluvial system, with gravel and sandy amalgamated channels, with well-developed floodplains (Bodnar et al. in press). The upper member is composed of horizontal and cross-laminated and fine- to coarse-grained pink to purplish sandstone, bearing numerous permineralized tree-trunks, and grey siltstone and claystone with abundant tuffaceous clasts and numerous plant impressions. It was proposed that the upper member corresponds to high-energy sandy braided fluvial systems (Spalletti 2001; Bodnar et al. in press). On the basis of the stratigraphic correlation and the preserved floras, it was inferred that the lower member was deposited during the early Ladinian and the upper member during the late Ladinian (Bodnar et al. in press).
The Cepeda Formation is characterized by red conglomerate and cross-stratified reddish sandstone in its lower part, and tabular beds of massive reddish sandstone and massive yellowish and greenish tuffaceous siltstone towards the top (Spalletti 2001). The lower part of the formation was interpreted as distributary fluvial systems, and the part as an ephemeral fluvial system (Bodnar et al. in press). The Cepeda Formation was assigned to the Carnian with doubt (Bodnar et al. in press).
Material and methods
Plant fossils were collected from the Barreal and Cortaderita formations (Middle Triassic, Sorocayense Group) at Cortaderita and La Tinta creeks, 8 km east of Barreal city, situated in the western Precordillera, San Juan Province, central-western Argentina, between W 69°26’12,2” S 31°37’45,8” and W 69°23’52,1” S 31°39”00,1” (Fig. 1).
Twelve fossiliferous strata (EF) were identified in the Sorocayense Group, three in the Barreal Formation (EF1–3), five in the lower member of the Cortaderita Formation (EF4–8), and four in the upper member of the Cortaderita Formation (EF9–12). Although some fossil plants were found in the Cepeda Formation by Herbst (1995), we were not able to locate the precise stratigraphic position of these discoveries (Fig. 2). All the fossiliferous strata bear well preserved plant fossils, including permineralized trunks and impressions-compressions of leaves and reproductive structures (Bodnar et al. in press), but only three contain dipteridaceous remains (EF3, 4, 7; Fig. 2). The studied fossil remains consist of impressions-compressions of fern leaves and were preserved in tuffaceous and bentonitic grey claystone, siltstone and silty sandstone, and greenish tuff and bentonite layers (Fig. 2).
The leaf impressions-compressions were mechanically cleaned with the help of chisels, needles, and pneumatic pencil. Specimens were studied through the DM 2500 stereoscope microscope. Photographs of the specimens were taken with a Canon EOS Rebel T3i camera. Fine details of the impressions-compressions specimens were photographed with Leica DC 150 system and Canon Powershot S40 camera.
Descriptions were made according to the terminology of Ôishi and Yamasita (1936), Tryon (1960), Font Quer (1982), Webb (1982), and Herbst (1992a). The International Code of Nomenclature for algae, fungi, and plants (Melbourne Code; McNeill et al. 2012) was followed in the nomenclatural treatment of the fossils.
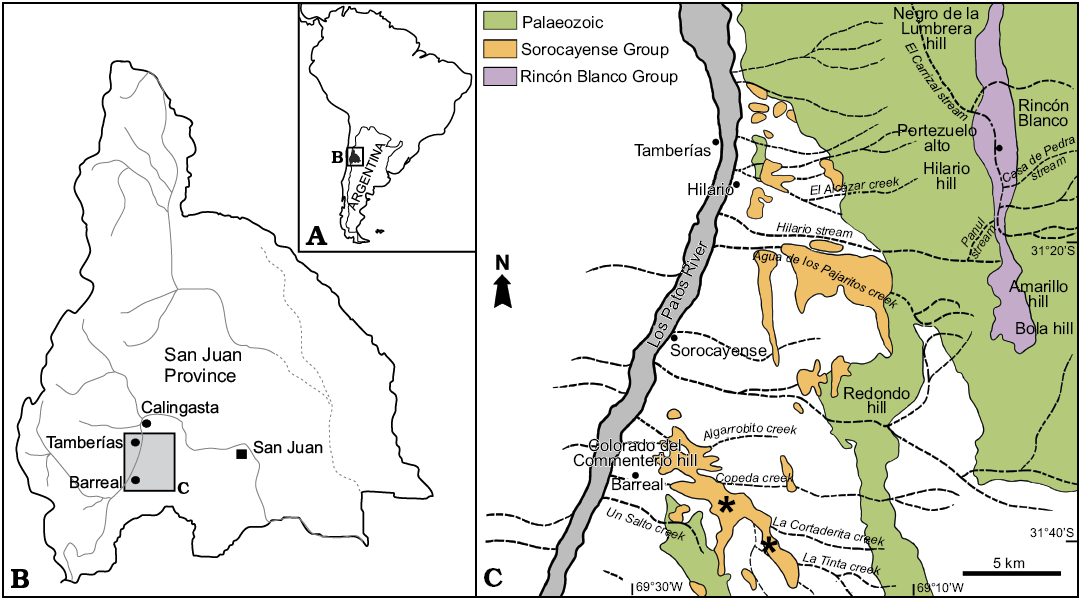
Fig. 1. Geographic location of study area in the San Juan Province, Argentina (A, B). Geologic map showing the sampled localities (asterisks) at Cortaderita and La Tinta creeks, near Barreal (C).

Fig. 2. Sedimentological section of the Sorocayense Group (Middle–Late Triassic) in the Barreal area (Cortaderita and La Tinta creeks), showing the stratigraphic distribution of the dipterid ferns and their correlation with the inferred climates.
Systematic palaeontology
Class Polypodiopsida Cronquist, Takhtajan, and Zimmerman, 1966
Order Gleicheniales Schimper, 1869
Family Dipteridaceae Seward and Dale, 1901
Type genus: Dipteris Reinwardt, 1828.
Remarks.—The leaves of fossil Dipteridaceae are characterized by the frond rachis that initially dichotomizes into two arms, each of which may then exhibit unequal dichotomies in an anadromic direction (i.e., toward the median plane of the frond; Cantrill 1995). The manner of division of the fronds, mode of disposition of pinnae, degree of dissection of laminae, and soral characters were utilized by Ôishi and Yamasita (1936) to recognize the subfamilies Dipteroideae, Goeppertelloideae, and Camptopteroideae. The Goeppertelloideae subfamily contains one fossil genus, Goeppertella, and is distinctive in being the only subfamily that has bipinnate pinnae and rachial pinnulae (Ôishi and Yamasita 1936; Arrondo and Petriella 1982). The Camptopteroideae, comprising the fossil genus Camptopteris, is similar to Dipteroideae, except the fact that the pinnae are spirally disposed on the rachial arms (Nathorst 1906). In the Dipteroideae, the frond lamina undergoes an initial dichotomy producing two equal halves, which can be entire or pinnate (Ôishi and Yamasita 1936; Cantrill 1995). Boundaries between fossil taxa of this subfamily have been subject to much discussion (Herbst 1992a, b; Cantrill 1995). Ôishi and Yamasita (1936) first included the genera Dipteris, Hausmannia (with two subgenera Hausmannia and Protorhipis), Clathropteris, Dictyophyllum, and Thaumatopteris in the Dipteroideae. Webb (1982) synonymized Thaumatopteris with Dictyophyllum, and afterwards Herbst (1992a, b) included the genera Clathropteris, Dictyophyllum, and Thaumatopteris as subgenera within Dictyophyllum. However, these proposals have found little acceptance, and the genera Thaumatopteris and Clathropteris are still widely used till date (e.g., Zhou 1995; Popa 1999; van Konijnenburg-van Cittert 2002; Wang 2002; Popa et al. 2003; Guignard et al. 2009; Schweitzer et al. 2009; Taylor et al. 2009; Bomfleur and Kerp 2010; Kustatscher et al. 2014; Choo et al. 2016).
Here, we consider Hausmannia, Clathropteris, Dictyophyllum, and Thaumatopteris as independent genera, without any subgeneric divisions. Hausmannia has a leaf lamina that dichotomizes once into two fan-shaped halves and is not pinnate, although the lamina is commonly greatly dissected (Cantrill 1995). Clathropteris is distinguished by the lamina that is divided for more than two-thirds of the total length and the conspicuous orthogonal venation pattern formed by secondary and tertiary veins (e.g., Ôishi and Yamasita 1936; Bomfleur and Kerp 2010; Choo et al. 2016). Dictyophyllum is characterized by a once-pinnate lamina and polygonal but not orthogonal areoles (e.g., Ôishi and Yamasita 1936). In Thaumatopteris, the pinnae are disposed in a funnel shape at the top of the petiole and are deeply dissected, often giving rise to pinnulae (e.g., Ôishi and Yamasita 1936).
Genus Dictyophyllum Lindley and Hutton, 1834
Type species: Dictyophyllum rugosum Lindley and Hutton, 1834; Jurassic of Great Britain.
Dictyophyllum castellanosii Stipanicic and Menéndez, 1949
Fig. 3.
1949 Dictyophyllum castellanosii sp. nov.; Stipanicic and Menéndez 1949: 9–11, pl. 3: 1–4, text-fig. 2.
1992 Dictyophyllum (Dictyophyllum) castellanosii Stipanicic and Menéndez, 1949; Herbst 1992a: 25–26.
Material.—BAPb 6227, part and BAPb 6228, counterpart; EF3, Barreal Formation, Sorocayense Group, Anisian (Middle Triassic), Cortaderita Creek, Barreal-Calingasta Depocenter, San Juan Province, Argentina.
Description.—The revised material corresponds to the type specimens of the species. They are part and counterpart of a fan-shaped once-pinnate frond of 7 cm in diameter. The rachis dichotomizes into two arms, each one bearing 4 or 5 linear-lanceolate pinnae. The basal web of lamina unites adjacent pinnae for a length of 1 cm. The free part of pinnae reaches 6 cm in length and 0.8 cm in width. The margin of the pinnae is lobate-serrate. The lobes are ovate-acuminate. The primary veins of the pinnae are slightly sinuous. Secondary veins are subopposite, departing at angles of 45–52° from the primary veins. Tertiary veins are subopposite, and depart from secondary veins at an acute angle. They dichotomize succesive times to form a reticulate mesh of polygonal areoles. First order areoles are elongated, and second order areoles are isodiametric. Sori and sporangia are not observed. See Stipanicic and Menéndez (1949) for full description.
Remarks.—Stipanicic and Menéndez (1949) erected this species on the basis of one specimen (BAPb 6227) and its counterpart (BAPb 6228). This taxon is similar to species of Dictyophyllum having a lobate, but not pinnatifid, pinna margin, i.e., D. exquisitum Sun, 1981, D. nathorstii Zeiller, 1903, and D. serratum (Kurr in Schimper, 1869) Frentzen, 1922 (from the Upper Triassic of Asia), and D. ellenbergii Fabre and Greber, 1960 (from the Upper Triassic of South Africa). However, D. castellanosii can be clearly distinguished from the Asiatic species (see Table 1) due to the number of pinnae per arm (up to 5 in the Argentinean taxon and more than 5 in the remaining ones) and the shape of the pinna lobes (ovate-acuminate in D. castellanosii; triangular or falcate, but not acuminate, in the other species). On the other hand, D. castellanosii is different from D. ellenbergii due to the venation areoles, which have an irregular shape in the last species. Unfortunately, we did not find more samples of the species in the fossiliferous strata of the Sorocayense Group. Until today, D. castellanosii has not been recorded in other localities of Argentina or elsewhere.
Stratigraphic and geographic range.—Anisian of Barreal Formation, Sorocayense Group, San Juan Province, Argentina.

Fig. 3. Dipterid fern Dictyophyllum castellanosi Stipanicic and Menéndez, 1949, from Barreal Formation (Anisian, Middle Triassic) of Barreal-Calingasta, San Juan Province, Argentina. BAPb 6227, part (A) and BAPb 6228 counterpart (B). Scale bars 20 mm.
Dictyophyllum menendezii sp. nov.
Figs. 4, 5.
Etymology: In honour to Carlos Alberto Menéndez (1921–1976), Argentinean palaeobotanist, for his important studies on Mesozoic floras of Argentina.
Type material: Holotype: PBSJ 832, half of a fan-shaped once-pinnate frond, with entire pinnae and clear venation. Paratypes: PBSJ 833, fragment of a fan-shaped once-pinnate frond, with the widest pinnae and clear venation; PBSJ 836, two pinnae, with undulate margin towards their apex; PBSJ 840, specimen with the maximum number of pinnae per rachial arm; PBSJ 843 sample with apical part of petiole preserved; all from type locality.
Type locality: Cortaderita Creek, Barreal-Calingasta Depocenter, San Juan Province, Argentina.
Type horizon: EF4, lower member of Cortaderita Formation, Sorocayense Group, early Ladinian (Middle Triassic).
Material.—Type material and additional seven specimens (PBSJ 834, 835, 837–839, 841, 842) from the type locality.
Diagnosis.—Petiolate fronds bilaterally symmetrical and fan-shaped. Rachis dividing into two opposite arms, each arm bearing 5 or 6 oblanceolate pinnae. The lowest part of the lamina of adjacent pinnae is fused, forming a wide web. Margin of the pinnae entire to undulate. Primary veins catadromous to isodromous, striate and straight. Secondary veins subopposite, smooth and falcate to sinuous. Tertiary veins subopposite to alternate, falcate or with a zig-zag pattern, dichotomizing four times to form a fine reticulate mesh of polygonal irregular areoles.
Description.—Leaf fragments of triangular or fan shape of 6 to 8 cm in length or diameter (Fig. 4A1, B). The fronds are once-pinnate, bilaterally symmetrical, and fan-shaped (Fig. 5A). The precise maximum size is unknown but is probably 8–10 cm in diameter. Only the most apical part of the petiole is preserved; its length and width cannot be determined (Fig. 4E).
The rachis dichotomizes into two opposite arms (Fig. 4C, E), each arm bearing 5 or 6 oblanceolate pinnae (Fig. 4B, C). A basal web of lamina unites adjacent pinnae for a length of up to 3 cm (Fig. 4A1, B). The free part of pinnae reaches 8 cm in length and 2 cm in width, in the larger specimens. The lamina of the pinnae is glabrous, and their margin is entire in most of its length, but it can be undulate near the apex (Fig. 4D).
Each rachial arm gives rise to a series of 5 or 6 catadromous to isodromous primary veins, each representing the main vein of the pinnae. Primary veins are longitudinally striated and straight (Fig. 4A2–A4), and they extend from the rachial arm to the pinna apex. Their width reaches up to 1 mm near the base and 0.5 mm near the apex. Secondary veins are subopposite, departing at angles of ~70° from the primary veins. They are smooth and falcate to sinuous (Fig. 4A3, A4). In the fused basal portion of the frond lamina, secondary veins acquire a zig-zag pattern and converge between them (Fig. 4A3). Their width varies 0.1–0.3 mm. Tertiary veins are subopposite to alternate, and they depart from secondary veins at 50–90°. They are falcate or have a zig-zag pattern, and dichotomize four times to form a fine reticulate mesh of polygonal irregular areoles (Figs. 4A2–A4, 5B). First order areoles are elongated (rectangular, pentagonal, or hexagonal), up to 1.5 mm wide and 3.2 mm long. Second order areoles are pentagonal to rhomboidal, up to 0.9 mm wide and 2.3 mm long. In many cases, second order areoles are not present or free veinlets occur inside the first order areoles (Fig. 5B). Sori and sporangia are not observed.
Remarks.—The studied material show the distinctive features of the genus Dictyophyllum, i.e., once-pinnate leaf, pinnae not disposed in a funnel-shape and not spirally arranged, polygonal but not orthogonal areoles (Ôishi and Yamasita 1936). In comparison with the known species of the genus from the Sorocayense Group, Dictyophyllum menendezi sp. nov. is similar in terms of the general size of the frond but differs in the length of basal lamina that unites adjacent pinna (which is greater in the new species), the entire to undulate pinna margin (lobate-serrate in D. castellanosii and dentate-lobate to pinnatifid in D. tenuifolium), and the irregular venation areoles (Table 1, Figs. 3, 4A2–A4, 5B, 6A, D–F). Conversely, Dictyophyllum menendezi sp. nov. shows similitude with D. ellenbergi in its basal web of the pinnae, which are concrescent for comparable lengths, in the number of pinnae per arm, and in the irregular shape of the areoles (Table 1). However, the South African species differs from the new taxon due to the maximum length and margin of the pinnae. The last character, which is entire to undulate in Dictyophyllum menendezi sp. nov., clearly contrasts with all the previously described species of Dictyophyllum, in which the pinna margin forms distinct lobes. In the few cases where the pinna lobes are not so evident (i.e., D. davidi Walkom, 1917 and D. fuenzalidai Herbst, 2000), the margin is dentate, being different from the rounded undulations present in the new species. From the exposed information, we propose a new specific taxon for the specimens from the Cortaderita Formation.
Stratigraphic and geographic range.—Type locality and horizon only.
Table 1. Comparative table of the most representative species of the genus Dictyophyllum. *Some samples of Dictyophyllum atuelense were transferred to Goeppertella by Rees and Cleal (2004). Abbreviations: L, length; Lbl, length of basal lamina uniting adjacent pinnae; N, number on each arm.
|
Species |
Location and age |
Lbl |
Pinnae |
Pinna |
Angle of |
Shape |
Shapeof |
Shape of 2° order areoles |
Shape |
References |
||
|
L |
N |
Shape |
||||||||||
|
Dictyophyllum atuelense* |
Argentina and Antarctica,
|
unknown |
10 |
unknown |
unknown |
pinnatipartite |
75–90° |
straight to falcate |
pentagonal to heptagonal elongated |
pentagonal to heptagonal isodiametric |
unknown |
|
|
Dictyophyllum bremerense |
Australia and South Africa, Upper Triassic |
up to 3.5 |
>14 |
7 |
unknown |
pinnatifid |
55–70° |
triangular |
rectangular to polygonal elongated |
rectangular to polygonal |
rounded or elongated |
|
|
Dictyophyllum castellanosii |
Argentina, |
1 |
4.5–6 |
4–5 |
linear-lanceolate |
lobate-serrate |
45–52° |
ovate-acuminate |
polygonal elongated |
polygonal isodiametric |
unknown |
|
|
Dictyophyllum davidi |
Australia, |
1–3 |
6–20 |
5–8 |
lanceolate |
dentate to pinnatifid |
45–65° |
triangular |
square, rhomboidal to heptagonal, elongated to isodiametric |
indistinct |
rounded or elongated |
|
|
Dictyophyllum ellenbergii |
South Africa, Upper Triassic |
up to 3.5 |
up to 20 |
5 |
unknown |
lobate |
65° |
triangular to ovate |
polygonal irregular |
polygonal irregular |
circular or elongated |
|
|
Dictyophyllum exile |
Laurasia, Upper Triassic
to |
0.2–1 |
>17.5 |
>17 |
linear to linear-oblanceolate |
lobate to pinnatifid |
60–90° |
triangular |
polygonal |
polygonal |
unknown |
|
|
Dictyophyllum exquisitum |
China, Upper Triassic |
0.5–1 |
5.5–7 |
6–7 |
linear- lanceolate |
lobate |
50° |
triangular to falcate, apiculate |
polygonal |
polygonal |
unknown |
|
|
Dictyophyllum fuenzalidai |
Chile, Upper Triassic |
unknown |
35–40 |
unknown |
unknown |
dentate |
80–90° |
triangular-falcate |
pentagonal- heptagonal isodiametric |
polygonal |
unknown |
|
|
Dictyophyllum
menendezii |
Argentina, |
up to 3 |
up to |
5–6 |
oblanceolate |
entire to undulate |
70–75° |
– |
rectangular, pentagonal, hexagonal, irregular, elongated |
pentagonal, rhomboidal, irregular |
unknown |
this work |
|
Dictyophyllum nathorstii |
Asia, Upper Triassic |
4–8 |
30–40 |
20–25 |
linear to lanceolate |
lobate |
60° |
triangular to falcate, apiculate or obtuse |
polygonal |
polygonal |
circular or variable |
|
|
Dictyophyllum nilssonii |
Greenland, Alaska, Sweden, Great Britain, China, Upper Triassic–Lower Jurassic |
2–5 |
>13 |
6–9 |
linear |
lobate to pinnatipartite |
60–90° |
falcate |
polygonal irregular |
polygonal irregular |
unknown |
|
|
Dictyophyllum rugosum |
Great Britain, Middle Jurassic |
1–2 |
>50 |
4–5 |
lanceolate |
lobate to pinnatipartite or pinnatifid |
80° |
triangular to linear-falcate |
polygonal irregular |
polygonal irregular |
rounded |
|
|
Dictyophyllum serratum |
China, Upper Triassic |
1–2 |
12–15 |
5–13 |
linear to lanceolate |
lobate |
60–70° |
falcate |
polygonal |
polygonal |
unknown |
|
|
Dictyophyllum tenuifolium |
Argentina, Chile, Antarctica, Middle to Late Triassic, and Lower Jurassic |
1.5 |
6–15 |
5–7 |
oblanceolate |
dentate-lobate to pinnatifid |
60–90° |
triangular |
pentagonal to heptagonal elongated |
rhomboidal and pentagonal isodiametric |
circular |
Stipanicic and Menéndez 1949; Bonetti and Herbst 1964; Morel et al. 1994; Herbst 2000; Herbst and Troncoso 2012; this work |
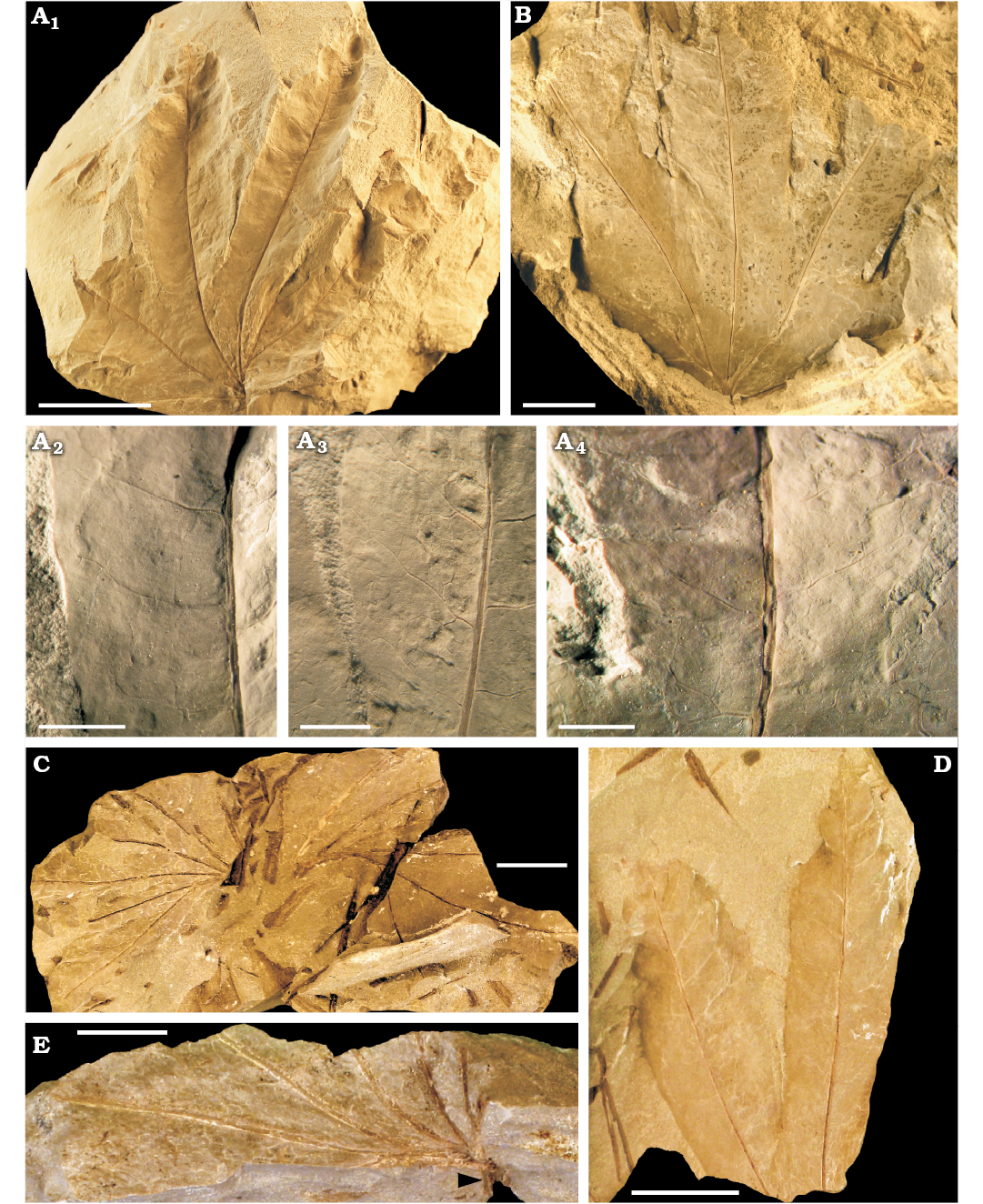
Fig. 4. Dipterid fern Dictyophyllum menendezi sp. nov. from Cortaderita Formation (Ladinian, Middle Triassic) of Barreal-Calingasta, San Juan Province, Argentina. A. PBSJ 832, general view of the frond (A1), details of secondary and tertiary veins, and areoles (A2, A3, A4). B. PBSJ 833. C. PBSJ 840. D. PBSJ 836. E. PBSJ 843, sample with apical part of petiole preserved (arrow). Scale bars: A1, D, 20 mm; B, C, E, 10 mm; A2, A3, A4, 5 mm.

Fig. 5. Dipterid fern Dictyophyllum menendezi sp. nov. from Cortaderita Formation (Ladinian, Middle Triassic) of Barreal-Calingasta, San Juan Province, Argentina. A. Diagramatic reconstruction of the leaf. B. Sketch drawing of pinnae venation.
Dictyophyllum tenuifolium Stipanicic and Menéndez, 1949 emend. Bonetti and Herbst, 1964
Fig. 6A, D–F.
1949 Dictyophyllum tenuifolium sp. nov.; Stipanicic and Menéndez 1949: 11–12, pl. 4: 1–3, pl. 5: 1–2, pl. 6: 1, 2, 4.
1964 Dictyophyllum tenuifolium Stipanicic and Menéndez, 1949 emend.; Bonetti and Herbst 1964: 275, pl. 1: 1–5, text-figs. a, b.
1992 Dictyophyllum (Dictyophyllum) tenuifolium (Stipanicic and Menéndez, 1949) emend. Bonetti and Herbst, 1964; Herbst 1992a: 27–28.
Neotype: BAPb 8174, here designated, fragments of four pinnae, with the lamina margin preserved and distinct venation.
Type locality: Paso Flores Depocenter, Neuquén Province, Argentina.
Type horizon: Paso Flores Formation, late Norian–Rhaetian (Upper Triassic).
Material.—PBSJ 412a, b, c, EF4, lower member of Cortaderita Formation, early Ladinian (Middle Triassic), Cortaderita Creek, Barreal-Calingasta Depocenter, San Juan Province, Argentina, and PBSJ 1051, EF7, lower member of Cortaderita Formation, early Ladinian (Middle Triassic), La Tinta Creek, Barreal-Calingasta Depocenter, San Juan Province, Argentina.
Description.—Fragments of up to 6.5 cm long and 3 cm wide of once-pinnate, bilaterally symmetrical, and fan-shaped fronds. The precise maximum size is unknown, but is probably 8 cm in diameter. Petiole is not preserved. The lamina is divided, forming at least 8 linear-lanceolate pinnae. A basal web of lamina unites adjacent pinnae for a length of up to 1.5 cm (Fig. 6A). The free part of pinnae reaches 5 cm in length and 1.5 cm in width, in the most complete specimens. The lamina of the pinnae is glabrous and their margin is lobate to serrate with deep irregular lobes, of triangular shape and acute apex.
The rachis gives rise to a series catadromous to isodromous primary veins; each one represents the main vein of the pinnae. Primary veins are longitudinally striate and straight, 0.3–0.5 mm wide. Secondary veins are smooth and alternate to sub-alternate, departing from the primary veins at an angle between 45° and 60° and extending up to the lobule apex. In the basal part of the pinna, secondary veins are sinuous and weakly marked; while they are straight to falcate and conspicuous in the free part of the pinna. Tertiary veins are alternate to sub-alternate and depart from secondary veins at 45–60°. They are straight near the primary vein and sinuous near the lobule apex. They dichotomize three times to form a fine reticulate mesh of polygonal areoles. First order areoles are elongated, 0.8–2 mm wide and 0.9–4.1 mm long, decreasing their size towards the pinna margin. Second order areoles are isodiametric, 0.6–0.9 mm of diameter. Sori and sporangia are not observed.
Remarks.—Dictyophyllum tenuifolium has been erected by Stipanicic and Menéndez (1949) based on fossils coming from the Barreal Formation, but they did no designate a holotype, and, unfortunately, the original specimens on which the diagnosis was established are lost. According to a recent revision of the fossil levels and localities studied by Stipanicic and Menéndez (1949), the type horizon is actually located within the Cortaderita Formation and not the Barreal Formation (Bodnar 2010; Bodnar et al. in press). Bonetti and Herbst (1964) emended the specific diagnosis after studying new fossils from the Paso Flores Formation (also Triassic of Argentina), but they have not designated a neotype. From the material published by Bonetti and Herbst (1964), we only found three specimens in the National Palaeobotanical Collection of the Museo Argentino de Ciencias Naturales (BAPb 8169, BAPb 8175, BAPb 8174). Among them, we selected the specimen BAPb 8174 as the neotype of the species, since it is the most complete one and presents all the characters included in the emended diagnosis of Bonetti and Herbst (1964). D. tenuifolium is characterized by once pinnate lamina, with 5–7 oblanceolate pinnae per rachial arm, pinna margin dentate to pinnatifid, polygonal areoles and circular sori. Even though the samples studied here do not show reproductive characters, the remaining traits coincide with the characterization of the species. Stipanicic and Menéndez (1949) and Bonetti (1963) mentioned the presence of this species in the Upper Triassic of Sweden, based on the transfer of the samples determined as Dictyophyllum sp. cf. D. exile by Johansson (1922). However, these specimens have been recently assigned to D. exile by Pott and McLoughlin (2011). Thus, D. tenuifolium has, until now, been only recorded in the Middle and Upper Triassic of Argentina.
Stratigraphic and geographic range.—Early Ladinian of the the lower member of Cortaderita Formation, Sorocayense Group, San Juan Province, Argentina; and late Norian–Rhaetian of the Paso Flores Formation, Neuquén Province, Argentina.

Fig. 6. Dipterid ferns from Barreal-Calingasta, San Juan Province, Argentina. A, D–F. Dictyophyllum tenuifolium Stipanicic and Menéndez, 1949 emend. Bonetti and Herbst, 1964; Cortaderita Formation (Ladinian, Middle Triassic). A. PBSJ 412a. D. PBSJ 1051. E. PBSJ 412b. F. PBSJ 412c. B, C. Hausmannia faltisiana Stipanicic and Menéndez, 1949 emend. herein; Barreal Formation (Anisian, Middle Triassic). B. BAPb 6248. C. BAPb 6254. Scale bars: A, C, D, 10 mm; B, 20 mm; E, F, 5 mm.
Genus Hausmannia Dunker, 1846
Type species: Hausmannia dichotoma Dunker, 1846; Lower Cretaceous, Germany.
Hausmannia faltisiana Stipanicic and Menéndez, 1949 emend. herein
Fig. 6B, C.
1949 Hausmannia (Protorhipis) faltisiana sp. nov.; Stipanicic and Menéndez 1949: 8–9, pl. 1: 4, pl. 2: 1–5; text-fig. 1.
1949 Hausmannia (Protorhipis) dentata Ôishi 1932; Stipanicic and Menéndez 1949: 6–7, pl. 1: 1–3.
1992 Hausmannia faltisiana Stipanicic and Menéndez, 1949; Herbst 1992a: 47–48.
Type material: Lectotype: BAPb 6249, part and BAPb 6254, counterpart (designated by Herbst 1992a). Paralectotypes: BAPb 6248, part and BAPb 6256, counterpart (designated by Herbst 1992a); all from type locality.
Type locality: Cortaderita Creek, Barreal-Calingasta Depocenter, San Juan Province, Argentina.
Type horizon: EF3, Barreal Formation, Sorocayense Group, Anisian (Middle Triassic).
Material.—Type material only.
Emended diagnosis.—Petiolate fronds, orbicular, with a strongly lobate margin. Large regular lobes with triangular shape, rounded apex and entire to irregularly and slightly undulate margins. Number of lobes ranging 14–22. Base of the frond lamina composed of two equal, opposite rachial arms. Each arm gives rise to 7 to 11 catadromous to isodromous primary veins. Primary veins striate and straight to slightly sinuous. Secondary veins subopposite, departing at angles of 45–80° from primary veins. Secondary veins smooth and sinuous, dichotomizing three times to form a fine reticulate mesh of polygonal areoles. First order areoles regular and polygonal elongated, second order areoles slightly irregular polygonal.
Description.—The studied material are the original specimens described by Stipanicic and Menéndez (1949). They are two specimens with their counterparts, and correspond to orbicular petiolate fronds, of 4–6 cm in diameter. Only the apical part of the petiole is preserved. The frond lamina has a strongly lobate margin, forming 14–22 regular lobes. These lobes have a triangular shape, rounded apex and entire to irregularly and slightly undulate margin. They reach 1.7 cm of heigth and 0.8 cm of width. The base of the lamina is composed of two equal, opposite rachial arms; each one gives rise to 7 to 11 catadromous to isodromous primary veins. The primary veins are straight to slightly sinuous. 0.5 cm wide. The secondary veins are subopposite, and depart at angles of 45-80° from primary veins. They are smooth and sinuous, dichotomizing three times to form a fine reticulate mesh of polygonal areoles. First order areoles regular and polygonal elongated up to 4×7 mm in size, second order areoles slightly irregular polygonal, up to 1 mm in size. Sori and sporangia are not observed. See Stipanicic and Menéndez (1949) for full description.
Remarks.—Stipanicic and Menéndez (1949) described the fronds of two species of Hausmannia: H. (Protorhipis) dentata and the new species H. (Protorhipis) faltissiana. As noted afterwards by Herbst (1992a), the materials assigned to both taxa are very similar and belong to the same species: Hausmannia faltisiana, considering the fact that they share the characters defined by Stipanicic and Menendez (1949) (i.e., lamina orbicular, of 4–5.5 cm in diameter, strongly lobate margin, high and regular lobes with triangular shape, rounded apex and entire margin, primary veins straight, secondary veins departing at angles of 45–80°, first order areoles polygonal regular and elongated 2×4 mm in size, second order areoles polygonal less regular, 0.6×0.8 mm in size). Hausmannia faltisiana differs from H. dentata Ôishi, 1932 (described for the Upper Triassic of Japan; see Table 2) in shape and size of the lamina (semi-orbicular to reniform and with a maximum diameter of 12 cm in the second one), the margin of the lobes (undulate in H. dentata), the course of the primary veins (slightly sinuous in the last one), and the second order areolas (which are inconspicuous in H. dentata). Even though Stipanicic and Menéndez (1949) mentioned most of the characters of H. dentata in some specimens from Barreal Formation (BAPb 6248, 6256), when we revised the materials, they show more similarities with H. faltisiana (e.g., the diameter of the lamina between 5–7 cm and conspicuous second order areoles). The shape of the lamina (defined as semi-orbicular by Stipanicic and Menéndez 1949) is actually unknown due to fact that the samples are fragmentary and could possibly be orbicular as in H. faltisiana. Finally, although the margin of the lobes seems to be undulate as in H. dentata, the undulations are much scarcer, more irregular and shallow that in the Japanese species. In line with the proposal of Herbst (1992a), we transferred the samples determined as H. dentata to H. faltisiana, and emended the specific diagnosis of the last one in order to include the morphological variability in the margin of the lamina lobes.
Stratigraphic and geographic range.—Type locality and horizon only.
Table 2. Comparative table of the most representative species of the genus Hausmannia.
|
Species |
Location and age |
Lamina division |
Leaf margin |
Shape of 1° order areoles |
Shape of 2° order areoles |
Shape of sori |
References |
|
Hausmannia buchii |
Europe, India, |
entire |
dentate |
quadrangular to polygonal |
quadrangular to polygonal (?) |
unknown |
|
|
Hausmannia crenata |
China, Lower Jurassic |
dichotomously dissected into two halves |
shallowly crenulated to crenate |
polygonal or rhomboidal |
quadrangular |
unknown |
|
|
Hausmannia |
India, Upper Jurassic |
entire |
entire to undulate |
quadrangular or polygonal |
indistinct |
unknown |
|
|
Hausmannia deferraresi |
Argentina, Middle Triassic |
entire |
crenate |
polygonal irregular |
rectangular |
unknown |
|
|
Hausmannia dentata |
Japan, Upper Triassic |
entire |
sinuate to dentate, large and undulate teeth |
polygonal |
indistinct |
circular |
|
|
Hausmannia dichotoma |
Great Britain, Germany, France, India, Upper Jurassic–Lower Cretaceous |
dichotomously and deeply dissected |
dichotomously lobate |
subquadragular |
indistinct |
unknown |
|
|
Hausmannia emeiensis |
China, Upper Triassic |
entire |
crenate |
polygonal |
polygonal |
unknown |
|
|
Hausmannia indica |
India, Upper Jurassic |
entire (?) |
entire to undulate |
rectangular |
polygonal |
unknown |
|
|
Hausmannia faltisiana |
Middle Triassic, Argentina |
entire |
lobate,with large lobes |
polygonal elongated |
polygonal irregular |
unknown |
Stipanicic and Menéndez 1949; this work |
|
Hausmannia leeiana |
China, Jurassic |
entire |
entire or slightly undulate |
rectangular |
rectangular |
unknown |
|
|
Hausmannia morinii |
Canada, Lower |
dichotomous |
crenate |
square to rectangular to polygonal |
square to polygonal |
indistinct |
|
|
Hausmannia nariwaensis |
Japan, Upper Triassic |
entire |
entire or undulate |
square to polygonal |
square to polygonal |
circular |
|
|
Hausmannia papilio |
Argentina, Antarctica, Lower Jurassic, Lower Cretaceous |
dichotomous, with two halves |
entire to crenulate-undulate |
rectangular |
subquadrangular |
subcircular-circular |
Feruglio 1937; Herbst 1992a; Morel et al. 1994; Cantrill 1995 |
|
Hausmannia patagonica |
Argentina, Lower Cretaceous |
highly dissected |
palmatipartite |
rectangular |
rectangular |
unknown |
|
|
Hausmannia shebudaiensis |
China, Middle Jurassic |
dichotomous, with two halves |
undulate |
polygonal |
polygonal |
unknown |
|
|
Hausmannia sinensis |
China, Middle Jurassic |
dichotomous, with two halves |
entire or slightly undulate |
square to rectangular or polygonal |
square to rectangular or polygonal |
circular |
|
|
Hausmannia ussuriensis |
China, Russia, Late Triassic–Jurassic |
dichotomous, with two halves |
undulate |
polygonal |
polygonal |
unknown |
|
|
Hausmannia wanlongensis |
China, Early |
dichotomous, with two halves |
undulate |
polygonal |
polygonal |
circular |
Genus Thaumatopteris Goeppert, 1841
Type species: Thaumatopteris muensteri Goeppert, 1841; Upper Triassic, Germany.
Thaumatopteris barrealensis Stipanicic and Menéndez, 1949
Fig. 7.
1949 Thaumatopteris barrealensis sp. nov.; Stipanicic and Menéndez 1949: 16–17, pl. 8: 2, pl. 9: 1–4, text-fig. 4.
1949 Thaumatopteris pusilla (Nathorst) Ôishi and Yamasita, 1936; Stipanicic and Menéndez 1949: 12–14, pl. 7: 2; pl. 7: 1, 3, text-fig. 3.
1949 Thaumatopteris cf. pusilla (Nathorst) Ôishi and Yamasita, 1936; Stipanicic and Menéndez 1949: 14–15, pl. 8: 4.
1992 Dictyophyllum (Thaumatopteris) barrealensis (Stipanicic and Menéndez, 1949); Herbst 1992a: 31–32.
Material.—BAPb 6263, PBSJ 844–850, PBSJ 1043–1050; EF3, Barreal Formation, Anisian (Middle Triassic), Cortaderita Creek, Barreal-Calingasta Depocenter, San Juan Province, Argentina.
Description.—Fragments of up to 3.5 cm long and up to 3 cm wide of once pinnate, bilaterally symmetrical, and fan-shaped fronds. In the base of the frond where the petiole is inserted, the lamina acquires a funnel shape. The precise maximum size is unknown, but it probably reaches up to 5 cm in diameter. The lamina is divided, forming at least 8–10 lanceolate pinnae, 0.5–0.9 cm wide and 1.3–3 cm long. The lamina of the pinnae is glabrous, and their margin is pinnatisect, forming linear lanceolate to falcate pinnulae. Pinnulae are alternate to sub-opposite, and slightly decurrent, generating a winged rachis. They have entire margin and rounded to acute apex. Pinnulae measure 0.22–0.68 cm in length, 0.11–0.19 cm in width, being longer in the middle part of the pinnae than in the base and the apex. The apical pinnula is unique and deltoid to ovate.
The rachis divides into two equal opposite arms. Each arm gives rise to a series 4–5 catadromous to isodromous primary veins. They are longitudinally striated and straight to sinuous, with the width decreasing near the apex and the base, 0.2–0.3 mm wide. Secondary veins represent the midrib of the pinnulae and depart from primary veins at an angle between 40° and 50°, extending up to the pinnulae apex. They are smooth and straight to sinuous. Tertiary veins are sinuous, and alternate to sub-alternate, departing from the secondary veins at 45–50°. They dichotomize three times to form a fine reticulate mesh of polygonal (hexagonal?) areoles that are hardly discernible in most of the samples. Sori and sporangia are not observed.
Remarks.—Stipanicic and Menéndez (1949) described fronds of three species of the genus Thaumatopteris in the Barreal strata: T. pusilla (Nathorst, 1878) Ôishi and Yamasita, 1936, T. dunkeri (Nathorst, 1878) Ôishi and Yamasita, 1936, and the new species T. barrealensis; and other specimens assigned with doubt to T. pusilla. The species T. barrealensis, as defined by Stipanicic and Menéndez (1949), is characterized by petiolate fronds, with a lamina divided into at least 5 lanceolate pinnae, 6–8 cm long and 1.9–2 cm wide, primary veins striate, pinnae dissected in alternate to subalternate pinnulae inserted to the rachis at an angle of 50°, pinnulae linear-lanceolate with rounded apex and coalescent base, 1.1×3 cm in size, pinnula midrib from base to top, polygonal (hexagonal) elongated areoles, 0.7 mm in size, second order areoles pentagonal or rhomboidal. The new samples described in this work coincide with this diagnosis. The materials referred to T. pusilla and T. sp. cf. T. pusilla are very similar to T. barrealensis and only display minor differences in size. On the other hand, they differ from T. pusilla (Table 3) from the Upper Triassic of Sweden and Japan, as the Laurasian species has pinnulae that are more spaced on the rachis, their angle of insertion is wider than 70°, and their apex is obtusely rounded apex. For all this, we transfer the materials originally determined as T. pusilla and T. sp. cf. T. pusilla to T. barrealensis. We agree with Herbst (1992a) that the specimen determined as T. dunkeri is very fragmentary, and that preserved characters are insufficient to make a specific determination. However, the original specimen is lost, and we cannot revise it in more conclusive way.
Stratigraphic and geographic range.—Anisian of the Barreal Formation, Sorocayense Group, San Juan Province, Argentina.
Table 3. Comparative table of the most representative species of the genus Thaumatopteris.
|
Species |
Location and age |
Pinnae |
Pinna margin |
Angle of lobes/ pinnulae to rachis |
Lobe/pinnula shape |
Lobe/ |
Shape of 1° order areoles |
Shape of 2° order areoles |
Shape of sori |
References |
|
|
number on each arm |
shape |
||||||||||
|
Thaumatopteris apertum |
Argentina, |
unknown |
unknown |
pinnatipartite |
80–90° |
triangular elongated |
slightly crenulated |
polygonal |
– |
unknown |
|
|
Thaumatopteris barrealensis |
Argentina, |
4–5 |
linear |
pinnatisect |
50° |
linear-lanceolate, rounded apex |
entire |
hexagonal |
pentagonal or rhomboidal |
unknown |
Stipanicic
and Menéndez 1949; |
|
Thaumatopteris brauniana |
Laurasia, Late Triassic–Lower Jurassic |
unknown |
lanceolate |
pinnatisect |
70–90° |
sub-triangular to linear |
entire or undulate |
hexagonal elongated |
– |
rounded |
|
|
Thaumatopteris contracta |
China, Late Triassic |
unknown |
unknown |
pinnatisect |
80–90° |
linear |
entire or slightly undulate |
pentagonal |
– |
non-soral |
|
|
Thaumatopteris chihuiensis |
Argentina, Late Triassic |
unknown |
linear lanceolate |
pinnatipartite to pinnatisect |
55–75° |
linear to falcate |
entire, crenate to pinnatifid |
polygonal elongated |
polygonal |
unknown |
|
|
Thaumatopteris dunkeri |
Sweden, Poland, China, Late Triassic–Lower Jurassic |
unknown |
linear |
pinnatipartite to pinnatisect |
65–80° |
linear-lanceolate |
entire |
pentagonal |
indistinct |
unknown |
|
|
Thaumatopteris eximia |
Argentina, |
unknown |
unknown |
pinnatipartite |
50–60° |
linear to lanceolate |
entire to lobate |
polygonal irregular |
polygonal irregular |
unknown |
|
|
Thaumatopteris fuchsi |
China, Upper Triassic |
unknown |
unknown |
pinnatisect |
60° |
linear, acuminate |
entire |
pentagonal |
– |
unknown |
|
|
Thaumatopteris nodosa |
China, Late Triassic |
unknown |
unknown |
pinnatipartite |
80–90° |
elongated triangular |
entire |
rhomboidal to pentagonal |
– |
unknown |
|
|
Thaumatopteris pusilla |
Sweden, Japan, Late Triassic |
5 |
linear-lanceolate |
lobate to pinnatipartite |
50–70° |
linear with obtusely rounded apex |
entire |
polygonal |
– |
unknown |
|
|
Thaumatopteris remauryi |
China, Upper Triassic |
unknown |
unknown |
pinnatipartite |
70–90° |
elongated triangular or linear |
entire or undulate |
pentagonal |
– |
non-soral (?) |
|
|
Thaumatopteris rocablanquensis |
Argentina, |
unknown |
unknown |
pinnatipartite to pinnatisect |
75–90° |
linear to falcate |
pinnatifid |
pentagonal to hexagonal |
quadrangular to polygonal |
rounded |
|
|
Thaumatopteris rothi |
Argentina, Chile, Upper Triassic– Lower Jurassic |
unknown |
unknown |
pinnatisect |
60–85° |
linear-lanceolate |
undulate |
polygonal |
– |
unknown |
|
|
Thaumatopteris shirleyi |
Australia and South Africa, Late Triassic |
unknown |
unknown |
pinnatipartite |
60° |
lanceolate |
crenate to serrate |
polygonal |
polygonal |
unknown |
|
|
Thaumatopteris tenuiserrata |
Argentina, Late Triassic |
unknown |
unknown |
pinnatisect |
60° |
linear |
serrate |
polygonal isodiametric to elongated |
polygonal isodiametric to elongated |
rounded |
|
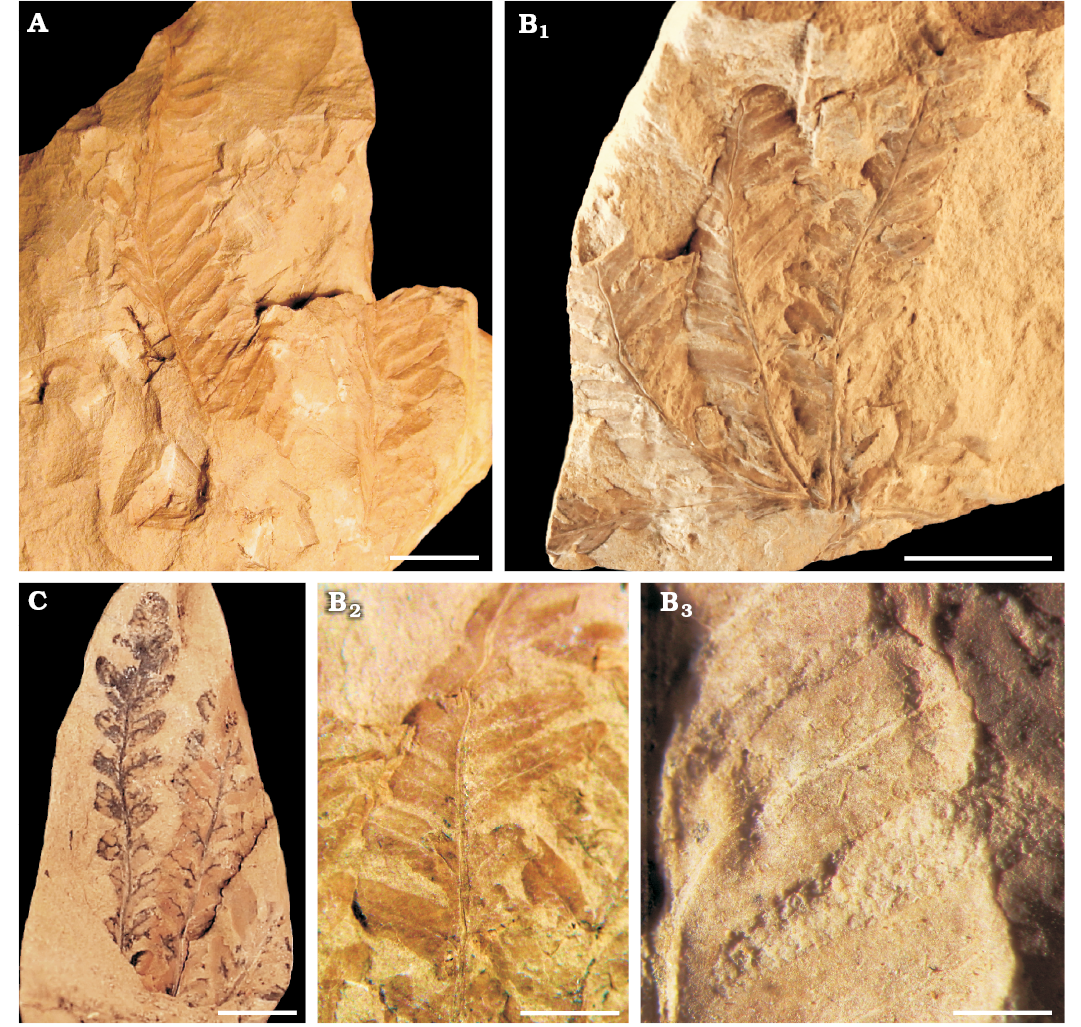
Fig. 7. Dipterid fern Thaumatopteris barrealensis Stipanicic and Menéndez, 1949 from Barreal Formation (Anisian, Middle Triassic) of Barreal-Calingasta, San Juan Province, Argentina. A. BAPb 6263. B. PBSJ 844; general view of the leaf (B1), detail of the pinnulae (B2), detail of the pinnula venation (B3). C. PBSJ 849. Scale bars: A, B1, 10 mm; C, 5 mm; B2, 3 mm; B3, 1 mm.
Spatio-temporal distribution and diversity of the Dipteridaceae in Argentina
In Argentina, during the Triassic, the Dipteridaceae had a restricted distribution, appearing only in some areas of the central-western region and northern Patagonia, i.e., the Barreal-Calingasta Depocenter in the San Juan Province, Malargüe Depocenter in Mendoza Province, and Paso Flores Depocenter in Neuquén and Río Negro provinces.
In the Sorocayense Group, at the Barreal-Calingasta Depocenter, twelve fossiliferous strata (EF1–12) were identified. We evaluated the diversity of the fern family along the Sorocayense Group by analysing the number of individuals (abundance) and species (species richness) present in each stratum (Fig. 2; Table 4). The EF3 of the Barreal Formation shows the maximum diversity of Dipteridaceae, with the highest number of species and individuals (Table 4). We inferred the following changes in the Dipteridaceae diversity along the Sorocayense Group: (i) In the lower part of the Barreal Formation (EF1 and EF2, early Anisian), dipteridaceous ferns are not present; (ii) at the top of the Barreal Formation (EF3, late Anisian), the first record of the group in the depocenter and the maximum species richness occur with three species (Dictyophyllum castellanosi, Haumannia faltisiana, and Thaumatopteris barrealensis); (iii) the base of the lower member of the Cortaderita Formation contains two species (EF4, early Ladinian), Dictyophyllum menendezi sp. nov. and D. tenuifolium, being the first one significantly more abundant; (iv) in the upper part of the lower member of the Cortaderita Formation (EF7, early Ladinian), only one species occurs (D. tenuifolium) with low abundance (one individual); (v) towards the top of the lower member of the Cortaderita Formation (from EF8, early Ladinian), Dipteridaceae fossils disappear from the depocenter. EF8 (lower member) and EF9–12 (upper member, late Ladinian) are characterized by corystosperms, peltasperms, cycadales, ginkgoales, and gnetales. However, dipteridaceous fossils were not recorded in any of them (Bodnar et al. in press); (vi) in the uppermost unit of the Sorocayense Group (Cepeda Formation) only an osmundaceous fern and an isolated trunk of corystosperm were recorded, from a fossiliferous stratum which could not be located.
Table 4. Number of specimens of each dipterid species recorded in the fossiliferous strata of Sorocayense Group; EF, fossiliferous stratum. EF3 is located in Barreal Formation, EF4 and EF7 are placed in Cortaderita Formation.
|
Taxa |
EF3 |
EF4 |
EF7 |
|
Dictyophyllum castellanosii |
1 |
0 |
0 |
|
Dictyophyllum menendezi sp. nov. |
0 |
10 |
0 |
|
Dictyophyllum tenuifolium |
0 |
1 |
1 |
|
Hausmania faltisiana |
2 |
0 |
0 |
|
Thaumatopteris barrealensis |
12 |
0 |
0 |
|
Total number of species |
3 |
2 |
1 |
|
Total number of specimens |
15 |
11 |
1 |
It should be noted that the fossils from the Barreal Formation constitute the earliest occurrence of the Dipteridaceae from Argentina and South America.
We analysed the records of the family from Barreal-Calingasta alongside those from other areas of Argentina, i.e., the Llantenes and Chihuido formations at the Malargüe Depocenter (Menéndez 1951; Herbst 1993), the Paso Flores Formation at the homonymous depocenter (Bonetti and Herbst 1964; Morel et al. 1992; Herbst 1993), and the Arroyo Malo Formation at the Atuel Depocenter (Riccardi et al. 1997), with the objective to establish temporal changes in their diversity during the Triassic. Llantenes, Chihuido, and Paso Flores formations were inferred to be Late Triassic (late Norian–Rhaetian) (Morel et al. 2003), whereas the Arroyo Malo Formation was interpreted as latest Triassic (Rhaetian) (Riccardi et al. 1997). Although the early Late Triassic (Carnian–early Norian) floras of Argentina are well known, dipterid ferns are not registered in any formation of this age (e.g., Potrerillos, Cacheuta, Carrizal, and Ischigualasto formations; see Zamuner et al. 2001; Morel et al. 2010, 2011, 2015). After analysing the species richness of this fern family in accordance with the lithostratigraphic unit age (Table 5, Fig. 8), we can establish two peaks of Dipteridaceae diversity during the Triassic in Argentina: one in the Middle Triassic (late Anisian–early Ladinian) and the other in the Late Triassic (late Norian–Rhaetian), with three species of Thaumatopteris, one of Dictyophyllum, one of Clathropteris and one species of the genus Goeppertella.
There are some differences between the Middle Triassic and the Late Triassic dipteridaceous assemblages. In general, the Middle Triassic dipterid leaves are smaller (4–10 cm in diameter) than those from the Late Triassic (9–50 cm in diameter). Only one species from the Middle Triassic (D. tenuifolium) persists during the Late Triassic. The genus Hausmannia is not present in the Late Triassic floras of Argentina, while Clathropteris and Goeppertella are not present in the Middle Triassic.
Table 5. Chronological distribution of Triassic species of Dipteridaceae from Argentina. The number of recorded specimens of each species is indicated in rows. Abbreviations: ET, Early Triassic; A, Anisian; L, Ladinian; C, Carnian; LN–R, Late Norian–Rhaetian.
|
Taxa |
ET |
A |
L |
C |
LN–R |
|
Clathropteris sp. |
0 |
0 |
0 |
0 |
1 |
|
Dictyophyllum castellanosii |
0 |
2 |
0 |
0 |
0 |
|
Dictyophyllum menendezi sp nov. |
0 |
0 |
10 |
0 |
0 |
|
Dictyophyllum tenuifolium |
0 |
0 |
3 |
0 |
12 |
|
Goeppertella stipanicicii |
0 |
0 |
0 |
0 |
3 |
|
Hausmania faltisiana |
0 |
2 |
0 |
0 |
0 |
|
Thaumatopteris barrealensis |
0 |
12 |
0 |
0 |
0 |
|
Thaumatopteris chihuiensis |
0 |
0 |
0 |
0 |
10 |
|
Thaumatopteris rothi |
0 |
0 |
0 |
0 |
3 |
|
Thaumatopteris tenuiserratum |
0 |
0 |
0 |
0 |
11 |
|
Total number of species |
0 |
3 |
2 |
0 |
6 |
|
Total number of specimens |
0 |
16 |
13 |
0 |
40 |
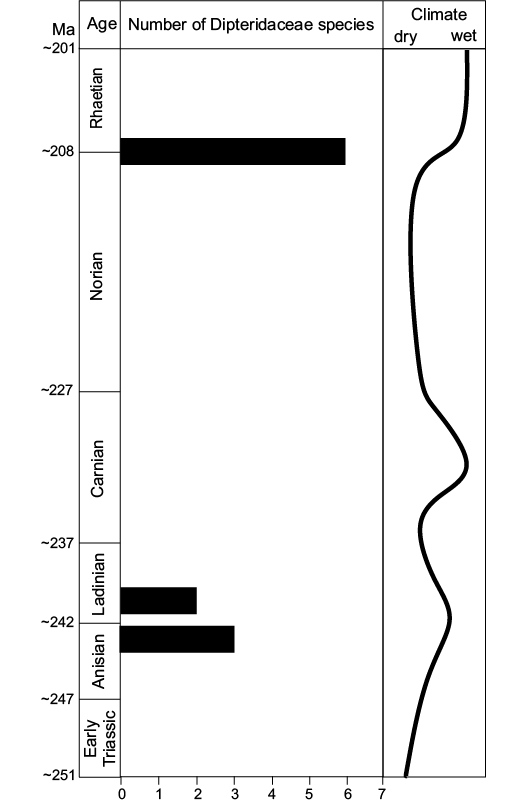
Fig. 8. Distribution of the dipterid ferns along the Triassic of Argentina, and their correlation with the inferred climates.
Palaeoclimatic and palaecological implications
During the Mesozoic, the Dipteridaceae mainly occupied humid localities in the temperate warm and subtropical zones (van Konijnenburg-van Cittert 2002). Moreover, as noted by Cantrill (1995), the distribution of fossil Dipteridaceae is broadly congruent with the inferred patterns of high storminess and large seasonal rainfall (monsoonal climates); both are characteristic conditions in which modern Dipteris occur today. Otherwise, fossil representatives of the Dipteridaceae are generally interpreted as opportunistic plants that colonize disturbed habitats, such as stream sides, riverbanks, and exposed ridges and clearings, by analogy with the many extant Dipteris species (Cantrill 1995; van Konijnenburg-van Cittert 2002; Stockey et al. 2006; Bomfleur and Kerp 2010).
During the Triassic, in Argentina, the Dipteridaceae occurs in basins of central-western and northern Patagonia (i.e., Barreal-Calingasta, Paso Flores, Malargue, and Atuel depocenters), which were located at palaeolatitudes between 40–55°S (Spalletti et al. 1999; Artabe et al. 2003). Conversely, they are not recorded in basins situated at palaeolatitudes beyond 60°S (i.e., El Tranquilo Basin), which led Artabe et al. (2003) to interpret that the seasonal climates were developed at a region between 30–60°S palaeolatitudes. In those basins, the Dipteridaceae grew in herb-shrub and tree communities developed in point and mid-channels bars, and floodplains of braided and meandering fluvial systems (Artabe et al. 2001).
From this study, we can conclude that the distribution of Dipteridaceae in Argentina was limited not only geographically but also temporally. Dipterid fossils were not found in the Early Triassic floras, although it should be noted that the taphofloras of that age are still controversial (e.g., Quebrada de los Fósiles Formation, Coturel et al. 2016; and Vera Formation, Artabe 1985a, b; Luppo et al. 2018). This family was well represented in the Middle Triassic, declining towards the end of that epoch and the beginning of the Late Triassic, to being inconspicuous during the Carnian age. The Dipteridaceae ferns reach their maximum diversity in the late Norian–Rhaetian interval. Since they are considered reliable palaeoclimatic indicators, it would be expected that their temporal distribution pattern was related to climate changes during the Triassic.
After the end-Permian mass extinction, most of the communities failed to radiate during the Early Triassic suggesting that the harsh conditions that caused the extinction, and particularly the intense hot-house climate, probably persisted at this time (Hallam 1991; Dickins 1993). According to Spalletti et al. (2003: 127, fig. 8), southwestern Gondwana was characterized by a “strongly arid phase” before the establishment of subtropical seasonal regime in the beginning of Middle Triassic epoch.
The climate of the Middle Triassic has been relatively little studied (Preto et al. 2010). Although an arid equatorial climatic belt is indicated by macrofloras and palynomorphs (Visscher and van der Zwan 1981; Ziegler et al. 1993), local humid episodes occur in different areas (Preto et al. 2010) as western Antarctic (Parrish et al. 1982) and Europe (e.g., Kustatscher et al. 2010; Kustatscher and van Konijnenburg-van Cittert 2005). In southwestern extratropical Gondwana, the appearance of the Dicrioidium flora took place during the Anisian (Morel et al. 2003). In central-western Argentina, a dry subtropical strongly seasonal climate was inferred for the Middle Triassic (Spalletti et al. 2003). Particularly, in the Barreal-Calingasta Depocenter, the development of vertisols in the Sorocayense Group indicates rainfall seasonality (Bodnar et al. in press). The presence of xeromorphic features in the dominant gymnosperms (i.e., corystosperms, peltasperms, cycads) suggests dry conditions, but with humid episodes, as indicated by the occurrence of lycophytes in the upper part of Barreal Formation, and mosses and hornworts in the lower member of the Cortaderita Formation (Bodnar 2010; Bodnar et al. in press). The dipterid ferns found in the Barreal and Cortaderita formations have relatively small leaves, which may suggest that the humidity was not optimal for the development of the typical large leaves of the extant Dipteris. The presence of Hausmannia in the Barreal Formation, a taxon considered to have adapted to more stress-related environments and more arid circumstances (van Konijnenburg-van Cittert 2002; Stockey et al. 2006), supports the conclusion of sub-humid palaeoclimates. In the upper member of the Cortaderita Formation, the occurrence of aridisols and a declining diversity of the palaeofloras suggest an intensification of dry conditions, such as the Dipteridaceae (and other ferns) could not succeed overcome (Bodnar 2010; Bodnar et al. in press).
The Late Triassic should be, according to numerous authors (e.g., Robinson 1973; Wang 2009), marked by a maximum expression of monsoonal climate. In southwestern Gondwana, the maximum diversification of Dicroidium flora took place in the late Ladinian–Carnian interval (Morel et al. 2003; Spalletti et al. 2003). Sedimentological and floristic evidences from Argentina indicate the development of semi-arid climates during this lapse of time (Spalletti et al. 2003; Colombi and Parrish 2008). In the Barreal-Calingasta Depocenter, the palaeoenvironments of the lower part of Cepeda Formation (Carnian?), would have evolved under dry palaeoclimates (Bodnar et al. in press). It is noteworthy that the Dipteridaceae were not present in the Argentinean Carnian megafloras (i.e., Paramillo, Potrerillos, Cacheuta, Ischigualasto, and Carrizal formations), while other fern families (e.g., Osmundaceae) were widespread and abundant (Zamuner et al. 2001; Morel et al. 2010, 2011, 2015). These ferns inhabited environments very similar to those where the Dipteridaceae developed, i.e., bars and flood-plains of meandering and braided rivers (Artabe et al. 2001).
However, the Late Triassic is interrupted by a significant episode of more humid conditions at the boundary between the early and late Carnian. This event, called the “Carnian Pluvial Event” (Visscher et al. 1994) or the “Carnian Humid Episode” (Ruffel et al. 2016), may be the peak of the Pangaean mega-monsoon (Preto et al. 2010). The extensive occurrence of humid climate indicators in Carnian sediments may represent this peak (Parrish 1993; Colombi and Parrish 2008). The beginning and cessation of this episode seemed to be broadly synchronous with significant biotic changes, including both extinctions and diversifications (Simms and Ruffell 1989, 1990). Although the general climate in Argentina would have been semi-arid, the Carnian humid event is documented as a short-duration episode in the Ischigualato Formation, Ischigualasto-Villa Unión Basin (Colombi and Parrish 2008; Césari and Colombi 2016). The palynofloras exhibit an increase of hygrophytic plants in that formation (Césari and Colombi 2016). In another depocenter of Argentina (upper section of the Potrerillos Formation, Cacheuta Depocenter, Cuyo Basin), the presence of mosses and abundant ferns with large fronds, including Marattiales and Osmundales, is indicative of more humid conditions (Morel 1994; Artabe et al. 2001). Nevertheless, as mentioned above, megafossil remains of Dipteridaceae were not registered in both depocenters. After analyzing the palynofloras of Ischigualasto and Potrerillos formations, it appears that spores definitely related to dipteridaceous ferns do not occur (Rojo and Zavattieri 2005; Césari and Colombi 2016). In other areas of southwestern Gondwana, this family is recorded in Carnian deposits from South Africa (Fabre and Greber 1960; Anderson and Anderson 2008).
The available evidence indicates a return or intensification of dry conditions at the Carnian–Norian boundary (Simms and Ruffell 1989, 1990). This climatic change appears to be significant for the Ischigualasto-Villa Unión and Cuyo basins (Spalletti et al. 2003; Colombi and Parrish 2008). Then again, Dipteridaceae fossil remains were not found in early Norian strata of central-western Argentina (e.g., the lower section of Rincón Blanco Formation, Cacheuta Depocenter, Cuyo Basin; Spalletti et al. 1995). During the late Norian–Rhaetian interval, the Gondwanan floras show a significant turnover: the Dicroidium flora decline (Morel et al. 2003), and the evergreen subtropical forests dominated by corystosperms are replaced by the deciduous subtropical forests dominated by conifers and ginkgoleans (Spalletti et al. 2003).
Hallam (1985) and Ahlberg et al. (2002) suggested a shift to more humid conditions at the base of the Rhaetian. In southwestern Gonwana, the subtropical seasonal regimen persists, but in some localities with marine influence, a subtropical humid climate has developed (Spalletti et al. 2003). In Argentina, the Dipteridaceae family increases its importance in the interval late Norian–Rhaetian, with the development of individuals with large leaves, which could indicate that the humidity regimen was more benign than that in the Middle Triassic.
In summary, the climate in Argentina was subtropical seasonal during the Triassic, with changes in humidity, varying from arid, semi-arid, sub-humid to humid. Dry conditions were developed: in the Early Triassic, between the late Ladinian and the early Carnian, and from the Carnian–Norian boundary to middle Norian (Fig. 8). We can define three intervals that presented an increase in the humidity: late Anisian–early Ladinian, middle Carnian, and late Norian–Rhaetian. The Dipteridaceae flourished in the Argentinan palaeocommunities during two of these intervals, but not in middle Carnian (Fig. 8). This suggests that the climatic conditions were not the only factor which determined the spreading of Dipteridacaeae in Argentina.
As mentioned above, some representatives of the family are opportunistic plants colonizing disturbed habitats. Considering the evolution of Gondwanan floras, it appears that the increase of the diversity of this fern family occurred when the plant communities were recovering or going through an important change. The first case is represented by the late Anisian–early Ladinian, when the subtropical seasonal forests of corystosperms were being stabilized after the strong arid phase of the Early Triassic–early Anisian. The second situation took place in the late Norian–Rhaetian when the evergreen corystosperm forests were replaced by deciduous conifer and ginkgoalean forests. The Dipteridaceae family declined during periods of community stability, represented in the Triassic of Argentina by the peak of the Dicroidium flora.
Conclusions
The Dipteridaceae from Sorocayense Group are one of the earliest occurrences of the family. In particular, Dictyophyllum castellanosii, Hausmannia faltisiana, and Thaumatopteris barrealensis from the Barreal Formation are, together with D. davidi from Australia, the oldest representatives of the Dipteridaceae, since they date from the Anisian (D. davidi from the Anisian–Ladinian).
When Stipanicic and Menéndez (1949) first described this fossil assemblage, they cited seven species in the Barreal Formation and none in the Cortaderita Formation. After the present taxonomic revision and description of new samples, it is concluded that five species of Dipteridaceae are present in the Sorocayense Group (three in the Barreal Formation and two in the Cortaderita Formation), including the new species Dictyophyllum menendezi sp. nov. In comparison with other Middle Triassic occurrences in which only one species was found in each taphoflora, the dipteridaceous fern assemblages from the Sorocayense Group are the most diverse.
In Argentina, the Dipteridaceae have two intervals of higher diversity: late Anisian–early Ladinian and late Norian–Rhaetian. Their diversification seems related to increasing humidity episodes and the establishment of plant communities after ecological disturbance.
Acknowledgements
We thank Georgina del Fueyo and Luis Lezama (Buenos Aires, Argentina) for access to National Palaeobotanical Collection under their care. This paper improved significantly due to the remarks and suggestions made by the reviewers Evelyn Kustatscher (Naturmuseum Südtirol, Bolzano, Italy) and Maria Barbacka (W. Szafer Institute of Botany, Kraków, Poland). This study was supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2011-2450; PICT 2014-2751) and Universidad Nacional de La Plata (Projects N686, N807).
References
Ahlberg, A., Arndorff, L., and Guy-Ohlson, D. 2002. Onshore climate change during the Late Triassic marine inundation on the Central European Basin. Terra Nova 14: 241–248. Crossref
Anderson, H.M. and Anderson, J.M. 2008. Molteno ferns: Late Triassic biodiversity in southern Africa. Strelitzia 21: 1–258.
Andrae, K.T. 1855. Die fossile Flora Siebenbürgens und des Banats. Abhandlungen der Kaiserlich-königlichen Geologischen Reichanstalt 2: 1–48.
Arrondo, O.G. and Petriella, B. 1982. Revisión del género Goeppertella Ôishi y Yamasita emend (Goeppertelloideae–Dipteridaceae). Ameghiniana 19: 67–78.
Artabe, A.E. 1985a. Estudio sistemático de la tafoflora triásica de Los Menucos, provincia de Río Negro, Argentina. Cycadophyta, Ginkgophyta y Coniferophyta. Ameghiniana 22: 159–180.
Artabe, A.E. 1985b. Estudio sistemático de la tafoflora triásica de Los Menucos, provincia de Río Negro, Argentina. Sphenophyta, Filicophyta, Pteridospemophyta. Ameghiniana 22: 3–22.
Artabe, A.E., Morel, E.M., and Spalletti, L.A. 2001. Paleoecología de las floras triásicas argentinas. In: A.E. Artabe, E.M. Morel, and A.B. Zamuner (eds.), El Sistema Triásico de Argentina, 199–225. Fundación Museo de La Plata “Francisco Pascasio Moreno”, La Plata.
Artabe, A.E., Morel, E.M., and Spalletti, L.A. 2003. Caracterización de las provincias fitogeográficas triásicas del Gondwana Extratropical. Ameghiniana 40: 387–405.
Barredo, S.P. 2012. Geodynamic and tectonostratigrafic study of a continental rift: The Triassic Guyana Basin, Argentina. In: E. Sharkov (ed.), Tectonics—Recent Advances, 99–130. IntechOpen, London.
Barredo, S.P. and Ramos, V.A. 2010. Características tectónicas y tectosedimentarias del hemigraben Rincón Blanco, Cuenca Cuyana: una síntesis. Revista de la Asociación Geológica Argentina 66: 133–145.
Bodnar, J. 2008. Rhexoxylon cortaderitaense (Menéndez) comb. nov., a species of permineralized stems newly assigned to the Corystospermaceae, from the Triassic of Argentina. Alcheringa 32: 171–190. Crossref
Bodnar, J. 2010. La paleoflora triásica de la Formación Cortaderita en la quebrada homónima, cuenca de Barreal-Calingasta, provincia de San Juan, Argentina. 283 pp. Unpublished Ph.D. Thesis, University Nacional de La Plata, La Plata.
Bodnar, J., Iglesias, A., Colombi, C.E., and Drovandi, J.M. (in press). Stratigraphical, sedimentological and paleofloristic characterization of Sorocayense Group (Triassic) in Barreal Area, San Juan Province, Argentina. Andean Geology.
Bodnar, J., Ruiz, D., Artabe, A.E., Morel, E.M., and Ganuza, D.G. 2015. Voltziales y Pinales (= Coniferales) de la Formación Cortaderita (Triásico Medio), Argentina, y su implicancia en la reconstrucción de las coníferas triásicas. Revista Brasileira de Paleontologia 18: 141–160. Crossref
Bomfleur, B. and Kerp, H. 2010. The first record of the dipterid fern leaf Clathropteris Brongniart from Antarctica and its relation to Polyphacelus stormensis Yao, Taylor & Taylor nov. emend. Review of Palaeobotany and Palynology 160: 143–153.
Bonetti, M.I.R. 1963. Contribución al conocimiento de la flora fósil de Barreal, departamento de Calingasta (provincia de San Juan). 260 pp. Unpublished Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires.
Bonetti, M.I.R. 1968. Las especies del género Pseudoctenis en la flora triásica de Barreal (San Juan). Ameghiniana 5: 433–446.
Bonetti, M.I.R. 1972. Las “Bennettitales” de la flora Triásica de Barreal (San Juan). Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” 1: 307–322.
Bonetti, M.I.R. and Herbst, R. 1964. Dos especies de Dictyophyllum del Triásico de Paso Flores. Provincia del Neuquén, Argentina. Ameghiniana 3: 273–279.
Brongniart, A. 1828. Prodrome d’une histoire des végétaux fossiles. viii + 223 pp. Levrault, Paris
Cantrill, D.J. 1995. The occurrence of the fern Hausmannia Dunker (Dipteridaceae) in the Cretaceous of Alexander Island, Antarctica. Alcheringa 19: 243–254. Crossref
Césari, S.N. and Colombi, C. 2016. Palynology of the Late Triassic Ischigualasto Formation, Argentina: Paleoecological and paleogeographic implications. Palaeogeography, Palaeoclimatology, Palaeoecology 449: 365–384. Crossref
Choo, T.Y.S., Escapa, I.H., and Bomfleur, B. 2016. Monotypic colonies of Clathropteris meniscioides (Dipteridaceae) from the Early Jurassic of central Patagonia, Argentina: implications for taxonomy and palaeoecology. Palaeontographica, Abteilung B: Palaeobotany-Palaeophytology 294: 8–109. Crossref
Christenhusz, M.J.M., Zhang, X.-Ch., and Schneider, H. 2011. A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa 19: 7–54. Crossref
Colombi, C.E. and Parrish, J.T. 2008. Late Triassic environmental evolution in Southwestern Pangea: plant taphonomy of the Ischigualasto Formation. Palaois 23: 778–795. Crossref
Coturel, E.P., Morel, E.M., and Ganuza, D.G. 2016. Lycopodiopsids and equisetopsids from the Triassic of Quebrada de los Fósiles Formation, San Rafael Basin, Argentina. Geobios 49: 16–176. Crossref
Cronquist, A., Takhtajan, A., and Zimmerman, W. 1966. On the Higher Taxa of Embryobionta. Taxon 15: 129–134. Crossref
Cuerda, A.J. 1945. Estratigrafía y tectónica al este de Barreal, provincia de San Juan. 44 pp. Unpublished Ph.D. Thesis, Universidad Nacional de La Plata, La Plata.
Dickins, J.M. 1993. Climate of the Late Devonian to Triassic. Palaeogeography, Palaeoclimatology, Palaeoecology 100: 89–94. Crossref
Du Toit, A.L. 1927. A geological comparison of South America with South Africa. Carnegie Institution, Publication 381: 1–150.
Dunker, W. 1846. Monographie der norddeutschen Wealdenbildung. Ein Beitrag zur Geognosie und Naturgeschichte der Vorwelt. 83 pp. Oehme and Müller, Braunschweig.
Fabre, J. and Greber, C. 1960. Presence d’un Dictyophyllum dans la flore Molteno du Basutoland (Afrique Australe). Bulletin de la Société Géologique de France S7-II: 178–182.
Feng, S.N., Chen, G.X., Xi, Y.H., and Zhang, C.F. 1977. Plants [in Chinese]. In: Hubei Institute of Geological Sciences (ed.), Fossil Atlas of Middle-South China II, 622–674. Geological Publishing House, Beijing.
Feruglio, E. 1937. Dos nuevas de especies de “Hausmannia” de la Patagonia. Notas del Museo de La Plata 2: 125–136.
Font Quer, P. 1982. Diccionario de Botánica. 8ª reimpresión. 1244 pp. Editorial Labor, Barcelona.
Frenguelli, J. 1941. Las Camptopterídeas del Lias de Piedra Pintada en el Neuquén (Patagonia). Revista del Museo de La Plata, nueva serie, Paleontología 27: 27–57.
Frentzen, K. 1922. Beitrage zur Kenntnis der fossilen Flora des siidwestlichen Deutschland, III. Lettenkohlen- und Schilfsandstein. Jahresbericht Mitteilungen Oberrheinische Geologische Vereinigung, Neue Folge 11: 1–14.
Goeppert, H.R. 1841–1846. Die Gattungen der fossilen Pflanzen verglichen mit denen der Jetztwelt und durch Abbildungen erläutert. 151 pp. Henry & Cohen, Bonn.
Groeber, P. and Stipanicic, P.N. 1953. Triásico. In: P.F. Groeber, P.N. Stipanicic, and A.R.G. Mingramn (eds.), Mesozoico, Geografía de la República Argentina, Tomo II, 13–141. Sociedad Argentina de Estudios Geográficos GAEA, Buenos Aires.
Guignard, G., Wang, Y.D., Ni, Q., Tian, N., and Jiang, Z.K. 2009. A dipteridaceous fern and its in situ spores from the Early Jurassic in Hubei, China. Review of Palaeobotany and Palynolology 156: 104–115. Crossref
Gupta, K.M. 1955. Hausmannia indica sp. nov. Gupta, a dipteridaceous leaf from the Jurassic of Rajmahal Hills, Bihar (India). Proceedings of the National Academy of Sciences, India B 21: 147–148.
Hallam, A. 1985. A review of Mesozoic climates. Journal of the Geological Society 142: 433–445. Crossref
Hallam, A. 1991. Why was there a delayed radiation after the end-Permian mass extinction? Historical Biology 5: 257–262. Crossref
Harris, T.M. 1931. The fossil flora of Scoresby Sound East Greenland—Part 1: Cryptogams. (exclusive of Lycopodiales). Meddelelser om Grønland 85: 1–104.
Harris, T.M. 1961. The Yorkshire Jurassic Flora. 1 Tallophyta-Pteridophyta. 212 pp. Trustees of the British Museum (Natural History), London.
Herbst, R. 1964. La flora liásica de la zona del río Atuel, Mendoza, Argentina. Revista de la Asociación Geológica Argentina 19: 108–131.
Herbst, R. 1965. La Flora Fósil de la Formación Roca Blanca, provincia de Santa Cruz, Patagonia. Con consideraciones. Geológicas y Estratigráficas. Opera Lilloana 12: 1–101.
Herbst, R. 1979. Review of the Australian Dipteridaceae. Proceedings of the Linnean Society of New South Wales 103: 7–21.
Herbst, R. 1992a. Propuesta de clasificación de las Dipteridaceae, con un Atlas de las especies argentinas. D’Orbignyana 6: 1–71.
Herbst, R., 1992b. Propuesta de clasificación de las Dipteridaceae. Publicación Especial de la Asociación Paleontológica Argentina 2: 69–72.
Herbst, R. 1993. Dipteridaceae (Filicales) del Triásico del Arroyo Llantenes (provincia de Mendoza) y de Paso Flores (provincia del Neuquén), Argentina. Ameghiniana 30: 155–162.
Herbst, R. 1995. Millerocaulis stipabonettii nov. sp. (Osmundaceae, Filices) from the Late Traissic Cepeda Formation of San Juan Province, Argentina. Mededelingen Rijks Geologische Dienst 53: 13–19.
Herbst, R. 2000. Dipteridaceae (Filicales) del Triásico Superior de Chile. Revista Geológica de Chile 27: 65–81. Crossref
Herbst, R. and Troncoso, A. 2012. La flora Triásica de la Quebrada Doña Inés Chica, Región de Atacama, Chile. Gaea 8: 55–66. Crossref
Holmes, W.B.K. 2001. The Middle Triassic megafossil flora of the Basin Creek Formation, Nymboidea Coal Measures, New South Wales, Australia. Part 2: Filicophyta. Proceedings Linnean Society of New South Wales 123: 39–87.
Hsü, J., Chu, C.N., Chen, Y.H., Tuan, S.Y., Hu, Y.F., and Chu, W.C. 1979. Late Triassic Baoding Flora, SW Sichuan, China [in Chinese]. 130 pp. Science Press, Beijing.
Johansson, N. 1922. Die rhätische Flora der Kohlengruben bei Stabbarp und Skromberga in Schonen. Kungliga Svenska Vetenskapsakademiens Handlingar 63: 1–78.
Kato, M., Yatabe, Y., Sahashi, N., and Murakami, N. 2001. Taxonomic studies of Cheiropleuria (Dipteridaceae). Blumea 46: 513–525.
Kimura, T. and Kim, B.K. 1988. New taxa in the Late Triassic Daedong flora, South Korea. Part 1. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 152: 603–624.
Knowlton, F.H. 1916. A Lower Jurassic flora from the upper Matanuska Valley, Alaska. Proceedings of the United States National Museum 51: 451–460. Crossref
Kryshtofovich, A. 1923. Pleuromeia and Hausmannia in Eastern Siberia, with a summary of recent contributions to the paleobotany of the region. American Journal of Science 27: 200–208.
Kustatscher, E. and van Konijnenburg-van Cittert, J.H.A. 2005. The Ladinian Flora (Middle Triassic) of the Dolomites: palaeoenvironmental reconstructions and palaeoclimatic considerations. Geo.Alp 2: 31–51.
Kustatscher, E., Dellantonio, E., and van Konijnenburg-van Cittert, J.H.A. 2014. The ferns of the late Ladinian, Middle Triassic flora from Monte Agnello, Dolomites, Italy. Acta Palaeontologica Polonica 59: 741–755.
Kustatscher, E., van Konijnenburg-van Cittert, J.H.A., and Roghi, G. 2010. Macrofloras and palynomorphs as possible proxies for palaeoclimatic and palaeoecological studies: A case study from the Pelsonian (Middle Triassic) of Kühwiesenkopf/Monte Pràdella Vacca (Olang Dolomites, N-Italy). Palaeogeography, Palaeoclimatology, Palaeoecology 290: 71–80. Crossref
Li, P.J., Cao, Z.Y., and Wu, S.Q. 1976. Mesozoic plants from Yunnan [in Chinese]. In: Nanjing Institute of Geology and Palaeontology, Academia Sinica (ed.), Mesozoic Plants from Yunnan, 87–150. Science Press, Beijing.
Lindley, J. and Hutton, W. 1834. The Fossil Flora of Great Britain, Vol. 2. 223 pp. Ridgeway, London.
López Gamundí, O. 1994. Facies distribution in an asymmetric half-graben: the northern Cuyo Basin (Triassic), western Argentina. In: XIV International Sedimentological Congress, Abstracts, 6–7. Petrobrás, Recife.
Luppo, T., Lopez de Luchi, M.G, Rapalini, A.E., Martinez Dopico, C.I., and Fanning, C.M. 2018. Geochronologic evidence of a large magmatic province in northern Patagonia encompassing the Permian–Triassic boundary. Journal of South American Earth Sciences 82: 346–355. Crossref
Lutz, A.I. and Herbst, R. 1992. Una nueva especie de Rhexoxylon del Triásico de Barreal, San Juan Argentina. Publicación Especial de la Asociación Paleontológica Argentina 2: 73–76.
McNeill, J., Barrie, F.R., Buck, W.R., Demoulin, V., Greuter, W., Hawksworth, D.L., Herendeen, P.S., Knapp, S., Marhold, K., Prado, J., Prud’homme van Reine, W.F., Smith, G.F., Wiersema, J.H., and Turland, N.J. 2012. International Code of Nomenclature for Algae, Fungi and Plants (Melbourne Code) Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. XXX+240 pp. Koeltz Scientific Books, Koenigstein.
Menéndez, C.A. 1951. La flora mesozoica de la Formación Llantenes (provincia de Mendoza). Revista del Instituto Nacional de Investigaciones en Ciencias Naturales (Botánica) 2: 147–261.
Menéndez, C.A. 1956. Protophyllocladoxylon cortaderitaensis sp. nov. Tronco fósil del Triásico de Barreal (provincia de San Juan). Revista de la Asociación Geológica Argentina 11: 273–280.
Mésigos, M.G. 1953. El Paleozoico Superior de Barreal y su continuación austral, Sierra de Barreal, Provincia de San Juan. Revista de la Asociación Geológica Argentina 8: 65–109.
Morel, E.M. 1994. El Triásico del Cerro Cacheuta, Mendoza (Argentina). Parte I. Geología, contenido paleoflorístico y cronoestratigrafía. Ameghiniana 31: 161–176.
Morel, E.M., Artabe, A.E., and Spalletti, L.A. 2003. The Triassic floras of Argentina: Biostratigraphy, Floristic events and comparison with other areas of Gondwana and Laurasia. Alcheringa 27: 231–243. Crossref
Morel, E.M., Artabe, A.E., Ganuza, D.G., and Brea, M. 1994. Las plantas fósiles de la Formación Monte Flora, en Bahía Botánica, Península Antártica, Argentina 1. Dipteridaceae. Ameghiniana 31: 23–31.
Morel, E.M., Artabe, A.E., Ganuza, D.G., and Zuñiga, A. 2010. La paleoflora triásica del cerro Cacheuta, provincia de Mendoza, Argentina. Bryopsida, Lycopsida, Sphenopsida, Filicopsida y Gimnospermopsida (Corystospermales y Peltaspermales). Ameghiniana 47: 3–23. Crossref
Morel, E.M, Artabe, A.E., Ganuza, D., and Zúñiga, A. 2011. La Paleoflora Triásica del Cerro Cacheuta, provincia de Mendoza. Petriellales, Cycadales, Ginkgoales, Voltziales, Coniferales, Gnetales y Gimnospermas incertae sedis. Ameghiniana 48: 520–540. Crossref
Morel, E.M, Artabe, A.E, Ganuza, D.G, Bodnar, J., Correa, G., and Spalletti, L.A. 2015. El Triásico de la Formación Carrizal en el depocentro de Marayes (San Juan, Argentina): paleobotánica, tafonomía y bioestratigrafía. Revista de la Asociación Geológica Argentina, 72: 456–469.
Morel, E.M., Spalletti, L.A., Arrondo, O.G., and Ganuza, D.G. 1992. Los estratos plantíferos de la Formación Paso Flores (Triásico Superior) de las Lomas y Cañadón de Ranquel Huao, provincia del Neuquén, Argentina. Revista del Museo de La Plata (Nueva Serie) 9 Paleontología 58: 199–221.
Nathorst, A.G. 1878. Bidrag till Sveriges fossila flora. Floran vid Höganäs och Helsingborg. Kungliga Svenska Vetenskapsakademiens för Handlingar 16: 1–53.
Nathorst, A.G. 1906. Bemerkungen über Clathropteris meniscioides Brongniart und Rhizomopteris cruciata Nathorst. Kungliga Svenska Vetenskapsakademiens Handlingar 41: 1–14.
Ôishi, S. 1930. Notes on some fossil plants from the Upper Triassic Beds of Nariwa, Prov. Bitchu. Japanese Journal of Geology and Geography 7: 24–52.
Ôishi, S. 1932. The Rhaetic plants from the Nariwa District Prov. Bithcu (Okayama Prefecture), Japan. Journal of the Faculty of Science, Hokkaido Imperial University Series 4, Geology and Mineralogy 1: 257–379.
Ôishi, S. and Huzioka, K. 1938. Fossil Plants from Nariwa. A Supplement. Journal of the Faculty of Science, Hokkaido Imperial University, Series 4, Geology and Mineralogy 4: 69–101.
Ôishi, S. and Yamasita, K. 1936. On the fossil Dipteridaceae. Journal of the Faculty of Science, Hokkaido Imperial University Series 4, Geology and Mineralogy 3: 135–184.
Parrish, J.T. 1993. Climate of the Supercontinent Pangea. Journal of Geology 101: 215–233. Crossref
Parrish, J.T., Ziegler, A.M., and Scotese, C.R. 1982. Rainfall patterns and the distribution of coals and evaporites in the Mesozoic and Cenozoic. Palaeogeography, Palaeoclimatology, Palaeoecology 40: 67–101.
Popa, M.E. 1999. Aspects of Romanian Early Jurassic palaeobotany and palynology. Part III. Phytostratigraphy of the Getic Nappe. Acta Palaeontologica Romaniae 2: 377–386.
Popa, M.E., Barbu, O., and Codrea, V. 2003. Aspects of Romanian Early Jurassic palaeobotany and palynology. Part V. Thaumatopteris brauniana from Şuncuiuş. Acta Palaeontologica Romaniae 4: 361–367.
Pott, C. and McLoughlin, S. 2011. The Rhaetian flora of Rögla, northern Scania, Sweden. Palaeontology 54: 1025–1051. Crossref
Preto, N., Kustatscher, E., and Wignall, P.B. 2010. Triassic climates State of the art and perspectives. Palaeogeography, Palaeoclimatology, Palaeoecology 290: 1–10. Crossref
Pryer, K.M., Schuettpelz, E., Wolf, P.G., Schneider, H., Smith, A R., and Cranfill, R. 2004. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. American Journal of Botany 91: 1582–1598. Crossref
Ramos, V. and Kay, S. 1991. Triassic rifting and associated basalts in the Cuyo basin, central Argentina. In: R.S. Harmon and C.W. Rapela (eds.), Andean Magmatism and its Tectonic Setting. Geological Society of America, Special Paper 265: 79–91. Crossref
Rees, P.M. and Cleal, C.J. 2004. Lower Jurassic floras from Hope Bay and Botany Bay, Antarctica. Special Papers in Palaeontology 72: 1–90.
Reinwardt, C.G.C. 1828. Nova Plantarum indicarum genera. In: C.F. Hornschuch (ed.), Sylloge Plantarum Novarum itemque minus cognitarum, Tomus secundus, 3. Regensburgische Botanische Gesellschaft, Regensburg.
Riccardi, A., Damborenea, S.E., Manceñido, M.O, Scasso, S., Lanés, S., and Iglesia Llanos, M.P. 1997. Primer registro de Triásico marino fosilífero de la Argentina. Revista de la Asociación Geológica Argentina 52: 228–234.
Richter, P.B. 1906. Beiträge zur Flora der unteren Kreide Quedlinburgs. Pt 1. Die Gattung Hausmannia Dunker und einige seltenere Pflanzenreste. 22 pp. Engelmann, Leipzig.
Robinson, P.L. 1973. Palaeoclimatology and continental drift. In: D.H. Tarling and S.K. Runcorn (eds), Implications of Continental Drift to the Earth Sciences, 449–476. Academic Press, London.
Rojo, L.D. and Zavattieri, A.M. 2005. Estudio microflorístico de las formaciones Potrerillos y Cacheuta (Triásico) en el sur del cerro Cacheuta, Mendoza, Argentina. Parte 1. Ameghiniana 42: 3–20.
Ruffell, A., Simms, M., and Wignall, P. 2016. The Carnian Humid Episode of the late Triassic: a review. Geological Magazine 153: 271–284. Crossref
Schimper, W.P. 1869. Traité de Paléontologie Végétale 1. 738 pp. J.B. Baillière et fils, Paris.
Schweitzer, H.-J. 1978. Die räto-jurassischen Floren des Iran und Afganistans. 5. Todites princeps, Thaumatopteris brauniana und Phlebopteris polypodioides. Palaeontopraphica 168: 17–60.
Schweitzer, H.-J., Schweitzer, U., Kirchner, M., van Konijnenburg-van Cittert, J.H.A., van der Burgh, J., and Ashraf, R.A. 2009. The Rhaeto-Jurassic flora of Iran and Afghanistan. 14. Pteridophyta–Leptosporangiatae. Palaeontographica Abt. B 279: 1–108. Crossref
Seward, A.C. 1910. Fossil Plants: A Text-book for Students of Botany and Geology. Vol. 2. xxii + 624 pp. Cambridge University Press, Cambridge.
Seward, A.C. 1911. The Jurassic flora of Sutherland. Transactions of the Royal Society of Edinburgh 47: 643–709. Crossref
Seward, A.C. and Dale, E. 1901. On the structure and affinities of Dipteris, with notes on the geological history of the Dipteridinae. Philosophical Transactions of Royal Society of London 194: 1–187.
Shah, S.C. and Singh, G. 1964. Hausmannia crookshanki sp. nov. from Jabalpur Series of India. Current Science 33: 751–752.
Simms, M.J. and Ruffell, A.H. 1989. Synchroneity of climatic change in the late Triassic. Geology 17: 265–268. Crossref
Simms, M.J. and Ruffell, A.H. 1990. Climatic and biotic change in the Late Triassic. Journal of the Geological Society, London 147: 321–327. Crossref
Smith, A.R., Pryer, K.M., Schuettpelz, E., Korall, P., Schneider, H., and Wolf, P.G. 2006. A classification for extant ferns. Taxon 55: 705–731. Crossref
Smith, A.R., Pryer, K.M., Schuettpelz, E., Korall, P., Schneider, H., and Wolf, P.G. 2008. Fern classification. In: T.A. Ranker and C.H. Haufler (eds.), Biology and Evolution of Ferns and Lycophytes, 417–467. Cambridge University Press, Cambridge. Crossref
Spalletti, L.A. 2001. Modelo de sedimentación fluvial y lacustre en el margen pasivo de un hemigraben: el Triásico de la Precordillera occidental de San Juan, República Argentina. Revista de la Asociación Geológica Argentina 56: 189–210.
Spalletti, L.A., Artabe, A.E., and Morel, E.M. 2003. Geological factors and evolution of southwestern Gondwana Triassic plants. Gondwana Research 6: 119–134. Crossref
Spalletti, L., Artabe, A.E., Brea. M., and Ganuza, D. 1995. Ambientes de acumulación y paleoflora en capas rojas triásicas de la Cuenca Guyana. Mendoza. Revista de la Asociación Geológica Argentina 50: 175–188.
Spalletti, L.A., Artabe, A.E., Morel, E.M., and Brea, M. 1999. Biozonación paleoflorística y cronoestratigrafía del Triásico Argentino. Ameghiniana 36: 419–451.
Sternberg, K.M. 1838. Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt, Band II, Heft 7–8, 81–220. Gotlieb Hässe Söhne, Prague.
Stipanicic, P.N. 1972. Cuenca triásica de Barreal. In: A.F. Leanza (ed.), Geología Regional Argentina, 537–566. Academia Nacional de Ciencias, Córdoba.
Stipanicic, P.N. and Menéndez, C.A. 1949. Contribución al conocimiento de la flora fósil de Barreal (provincia de San Juan). I. Dipteridaceae. Boletín de Informaciones Petroleras, Buenos Aires 24: 44–73.
Stockey, R.A., Rothwell, G.W., and Little, S.A. 2006. Relationships among fossil and living dipteridaceae: anatomically preserved Hausmannia from the Lower Cretaceous of Vancouver Island. International Journal of Plant Sciences 167: 649–663. Crossref
Sun, G. 1981. Discovery of Dipteridaceae from the Upper Triassic of Eastern Jilin [in Chinese with English abstract]. Acta Palaeontologica Sinica 20: 459–467.
Surange, K.R. 1966. Indian Fossil Pteridophytes. Botanical Monograph 4. 209 pp. Council of Scientific & Industrial Research, New Delhi.
Sze, H.C., Li, X.X., Zhou, Z.Y., Ye, M.N., and Shen, G.L. 1963. Fossil Plants of China. Vol. 2. Mesozoic Plants of China [in Chinese]. 429 pp. Science Press, Beijing.
Taylor, E.L., Taylor, T.N., and Krings, M. 2009. Paleobotany. The Biology and Evolution of Fossil Plants. 1088 pp. Academic Press, Amsterdam.
Tidwell, W.D. and Ash, S.R. 1994. A review of selected Triassic to early Cretaceous ferns. Journal of Plant Research 107: 417–442. Crossref
Tryon, R. 1960. A glossary of some terms relating to the fern leaf. Taxon 9: 104–109. Crossref
Uliana, M.A. and Biddle, K.T. 1988. Mesozoic–Cenozoic paleogeographic and geodynamic evolution of Southern South America. Revista Brasileira de Geociencias 48: 172–190.
van Konijnenburg-van Cittert, J.H.A. 2002. Ecology of some Jurassic ferns in Eurasia. Review of Palaeobotany and Palynology 199: 113–124. Crossref
Visscher, H. and van der Zwan, C.J. 1981. Palynology of the circum-Mediterranean Triassic: phytogeographical and palaeoclimatological implications. Geologische Rundschau 70: 625–634. Crossref
Visscher, H., Van Houte, M., Brugman, W.A., and Poort, R.J. 1994. Rejection of a Carnian (Late Triassic) “pluvial event” in Europe. Review of Palaeobotany and Palynology 83: 217–226. Crossref
Walkom, A.B. 1917. Mesozoic floras of Queensland. Part 1 (continued). The flora of the Ipswich and Walloon Series, (c) Filicales etc. Publication of the Geological Survey of Queensland 257: 1–67.
Wang, P.X. 2009. Global monsoon in a geological perspective. Chinese Science Bulletin 54: 1113–1136. Crossref
Wang, Y. 2002. Fern ecological implications from the Lower Jurassic in Western Hubei, China. Review of Palaeobotany and Palynology 119: 125–141. Crossref
Wang, Y.D. and Zhang, H. 2010. Fertile organs and in situ spores of a new dipteridaceous fern (Hausmannia sinensis) from the Jurassic of northern China. Proceedings of the Royal Society B 277: 311–320. Crossref
Webb, J.A. 1982. Triassic species of Dictyophyllum from eastern Australia. Alcheringa 6: 79–91. Crossref
Wu, S.Q. 1999. Upper Triassic plants from Sichuan [in Chinese, with English abstract]. Bulletin of Nanjing Institute of Geology and Palaeontology. Academia Sinica 14: 1–69.
Yao, X., Taylor, T.N., and Taylor, E.L. 1991. Silicified dipterid ferns from the Jurassic of Antarctica. Review of Palaeobotany and Palynology 67: 353–362. Crossref
Ye, M.N., Liu, X.Y., Huang, G.Q., Chen, L.X., Peng, S.J., Xu, A.F., and Zhang, B.X. 1986. Late Triassic and Early–Middle Jurassic Fossil Plants from Northeastern Sichuan [in Chinese, with English abstract]. 141pp. Anhui Science and Technology Publishing House, Hefei.
Zamuner, A.B., Artabe, A.E., and Ganuza, D.G. 1999. A new Peltasperm (Gymnospermopsida) from the Middle Triassic of Argentina. Alcheringa 23: 185–191. Crossref
Zamuner, A.B., Zavattieri, A.M., Artabe, A.E., and Morel, E.M. 2001. Paleobotánica. In: A.E. Artabe, E.M. Morel, and A.B. Zamuner (eds.), El Sistema Triásico de Argentina, 143–184. Fundación Museo de La Plata “Francisco Pascasio Moreno”, La Plata.
Zeiller, R. 1903. Flore fossile des gîtes de charbon du Tonkin. 328 pp. Etudes desgîtes mineraux de la France, Paris.
Zheng, S.L. and Zhang, W. 1983. The Middle and Late Early Cretaceous floras in the Boli Basin of Heilongjiang Province, China [in Chinese, with English abstract]. Bulletin of the Shenyang Institute of Geology and Mineral Resources 7: 68–98.
Zhou, N., Wang, Y., Li, L., and Zhang, X. 2016. Diversity variation and tempo-spatial distributions of the Dipteridaceae ferns in the Mesozoic of China. Palaeoworld 25: 263–286. Crossref
Zhou, Z., 1995. Jurassic floras [in Chinese]. In: Li, X., Zhou, Z., Cai, C., Sun, G., Ouyang, S., and Deng, L. (eds.), Fossil Floras of China through the Geological Ages, 343–410. Guangdong Science and Technology Press, Guangzhou.
Ziegler, A.M., Parrish, J.M., Yao, J., Gyllenhaal, E.D., Rowley, D.B., Parrish, J.T., Nie, S., Bekker, A., and Hulver, M.L. 1993. Early Mesozoic phytogeography and climate. Philosophical Transactions of the Royal Society of London, Series B 341: 297–305. Crossref
Acta Palaeontol. Pol. 63 (2): 397–416, 2018
https://doi.org/10.4202/app.00459.2018