

Salvinialean megaspores in the Late Cretaceous of southern Patagonia, Argentina
PATRICIO E. SANTAMARINA, VIVIANA D. BARREDA, ARI IGLESIAS, and AUGUSTO N. VARELA
Santamarina, P.E., Barreda, V.D., Iglesias, A., and Varela, A.N. 2018. Salvinialean megaspores in the Late Cretaceous of southern Patagonia, Argentina. Acta Palaeontologica Polonica 63 (3): 607–616.
We report here two megaspores species related to the aquatic ferns of the order Salviniales from the Late Cretaceous Mata Amarilla Formation (Austral Basin), southern Santa Cruz Province, Argentina. We identified the species Arcellites disciformis and Balmeisporites cf. B. holodictyus. The presence of A. disciformis, in particular, is significant not only because it represents the first record for the Southern Hemisphere, indicating a bi-hemispheric distribution for the species, but also because it increases the diversity of this genus in Patagonia. The new findings of salvinialean megaspores highlight the importance of water ferns in the Late Cretaceous aquiferous enviroments of southern South America. The common occurrences of Arcellites and Balmeisporites, whether in shallow, fresh or brackish water facies, indicates aquatic paleoenvironment of the Mata Amarilla Formation, as was inferred also from the sedimentological evidence. Their presence also indicates that the lower and middle levels of the Mata Amarilla Formation can be attributed to the megaspore Zone M3 (Albian–Cenomanian) defined for the Cretaceous of Patagonia.
Key words: Salviniales, Hydropteridales, Arcellites, megaspores, Cenomanian, South America, Argentina.
Patricio E. Santamarina [santamarinape@gmail.com] and Viviana D. Barreda [vbarreda@macn.gov.ar], División Paleobotánica, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN-CONICET), Av. Angel Gallardo 470, Buenos Aires, C1405DJR, Argentina.
Ari Iglesias [ari_iglesias@yahoo.com.ar], Instituto de Investigaciones en Biodiversidad y Medioambiente (INIBIOMA, CONICET-UNCO), Quintral 1250, San Carlos de Bariloche, 8400, Argentina.
Augusto N. Varela [augustovarela@cig.museo.unlp.edu.ar], Centro de Investigaciones Geológicas (CIG, CONICET- UNLP), Diagonal 113 N°275, La Plata, 1900, Argentina.
Received 24 April 2018, accepted 20 June 2018, available online 26 July 2018.
Copyright © 2018 P.E. Santamarina et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Water ferns are a monophyletic clade of heterosporous ferns consisting of two extant families, Marsileaceae and Salviniaceae, placed in the order Salviniales (Smith et al. 2006) or Hydropteridales (Rothwell and Stockey 1994; Yamada and Kato 2002). They have a relatively simple vegetative structure coupled with a highly specialized reproductive arrangement, and live as rooted plants in moist or flooded places or as free-floating plants. Marsileaceae include three extant genera: Marsilea Linnaeus, 1753, Pilularia Linnaeus, 1753, and Regnellidium Lindman, 1904, and Salviniaceae comprise two extant genera: Azolla Lamarck, 1783, and Salvinia Séguier, 1754. These families are not easily related to the larger clade of leptosporangiate ferns, because the adaptation to the aquatic habitat has deeply influenced the morphology of the sporophyte and spores (Tryon and Tryon 1982; Tryon and Lugardon 1991). Megagametophytes are enclosed within the megaspores (generally dispersed in water), which have a complex spore wall stratification, including a specialized epispore (Tryon and Tryon 1982; Tryon and Lugardon 1991).
Salviniales have a widespread fossil record since the Late Jurassic, based both on macro and microfossils, including megaspores with their respective microspores in organic connection (e.g., Cookson and Dettmann 1958; Ellis and Tschudy 1964; Collinson 1980, 1991, 2001, 2002; Archangelsky et al. 1999; Lupia et al. 2000; Yamada and Kato 2002; Vajda and McLoughlin 2005; Villar de Seoane and Archangelsky 2008; Batten et al. 2011a, b; Cúneo et al. 2013).
In Patagonia, salvinialean megaspores have been widely recorded in Early Cretaceous units such as: the Springhill Formation (Berriasian–Barremian) (Baldoni and Taylor 1985; Baldoni and Batten 1997), the Baqueró Group (Aptian) (Gamerro 1975; Taylor and Taylor 1988; Villar de Seoane 1988; Archangelsky and Villar de Seoane 1989, 1990, 1991), the Kachaike Formation (Albian) (Baldoni 1987; Baldoni and Taylor 1987, 1988; Baldoni and Batten 1991; Villar de Seoane and Archangelsky 2008) and the Piedra Clavada Formation (Albian) (Villar de Seoane and Archangelsky 2008). Records from the Late Cretaceous are mainly restricted to the Campanian and Maastrichtian (Stough 1968; Archangelsky et al. 1999; Marenssi et al. 2004; Cúneo et al. 2013, 2014; Hermsen et al. 2014)
The Mata Amarilla Formation (Feruglio in Fossa Mancini et al. 1938; Leanza 1972) crops out around the town of Tres Lagos in southern Santa Cruz Province (Fig. 1), transitionally overlies the Piedra Clavada Formation (middle–late Albian; Riccardi et al. 1987; Archangelsky et al. 2008; Poiré et al. 2017) and is unconformably overlain by the La Anita Formation (Campanian; Varela et al. 2012a). It comprises three sections: lower, middle and upper (Varela et al. 2011, 2012a, b) that grade from fluvial to estuarine facies.
Here we report the presence of megaspores related to aquatic ferns from the lower and middle sections of the Mata Amarilla Formation at Cerro Waring (S49°31’16.8’’ W71°29’7.7’’) and Estancia Mata Amarilla (S49°37’5.9’’ W71°7’40.5’’) localities, southern Santa Cruz Province, Argentina (Figs. 1, 2). The paleobiogeographic and paleoenvironmental significance of these new fossil records is also discussed.
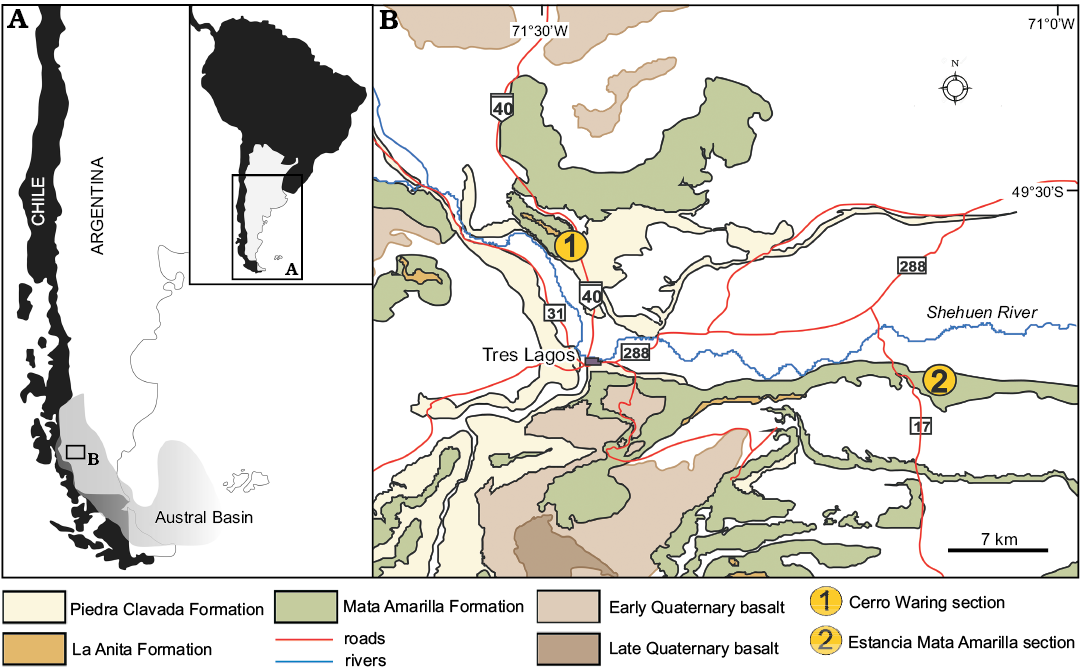
Fig. 1. Geographic (A) and geological (B) maps of the studied area (Tres Lagos Town, Santa Cruz Province, Argentina), showing the location of the studied sections (modified from Varela 2011).
Institutional abbreviations.—CIG, Centro de Investigaciones Geológicas, La Plata, Argentina; CONICET, Consejo Nacional de Investigaciones Científicas y Técnicas; INIBIOMA, Instituto de Investigaciones en Biodiversidad y Medioambiente, San Carlos de Bariloche, Argentina; MACN, Museo Argentino de Ciencias Naturales, Buenos Aires, Argentina; MPM-Pb, Museo Regional Provincial “Padre Jesús Molina” Paleobotanical collection, Rio Gallegos, Argentina; UNCO, Universidad Nacional del Comahue, San Carlos de Bariloche, Argentina; UNLP, Universidad Nacional de La Plata, La Plata, Argentina.
Geological setting
The Austral (or Magallanes) Basin, is located on the south-western end of the South American Plate (Fig. 1) and it is bordered to the south by the Scotia Plate covering an area of approximately 230.000 km2. In the studied area, the Austral Basin underwent three main tectonic stages (Varela 2014 and references therein): (i) a rift stage; (ii) a thermal subsidence stage; and (iii) a foreland stage. The rifting stage is related to the break-up of Gondwana, grabens and half-grabens were formed and filled with volcaniclastic and volcanic rocks intercalated with epiclastic sediments of the El Quemado and Tobífera formations. Subsequently, the thermal subsidence stage resulted in the deposition of the transgressive quartzose sandstone of the Springhill Formation, and the black mudstone and marl of the Río Mayer Formation. Towards the end of this stage, the Piedra Clavada Formation was deposited, representing a large passive-margin delta system. The foreland stage, in response to the regional change from extensive to compressive regime, resulted in the deposition of the continental Mata Amarilla Formation (Varela 2014). This unit is mainly composed of grey and blackish siltstone and claystone, alternating with whitish and yellowish-grey fine to medium grained sandstone (Varela et al. 2012b). Varela (2014) recognized three informal sections (lower, middle, and upper) on the bases of sedimentological and sequence stratigraphic analysis. The lower section consists of fine-grained intervals with paleosols interbedded with laminated shale and coquina, representing coastal plain and lagoon paleoenvironments. The middle section comprises sandstone and siltstone representing meandering fluvial channels and crevasse splay deposits (Varela 2011), intercalated with fine-grained floodplains and subordinate lacustrine deposits (Varela 2011). The upper section is dominated by fine-grained deposits, related to distal fluvial channels. U-Pb dating indicates a middle Cenomanian age (96.2 ± 0.7 Ma) for the middle section of the Mata Amarilla Formation (Varela et al. 2012a). Paleosol features and paleosol-derived climatic proxies suggest a subtropical temperate-warm (12 °C ± 2.1°C) and humid (1404 ± 108 mm/yr) climate with marked rainfall seasonality during the deposition of this unit (Varela et al. 2012b; 2018), in accordance with previous paleobotanical interpretations (Iglesias et al. 2007; Varela et al. 2016).
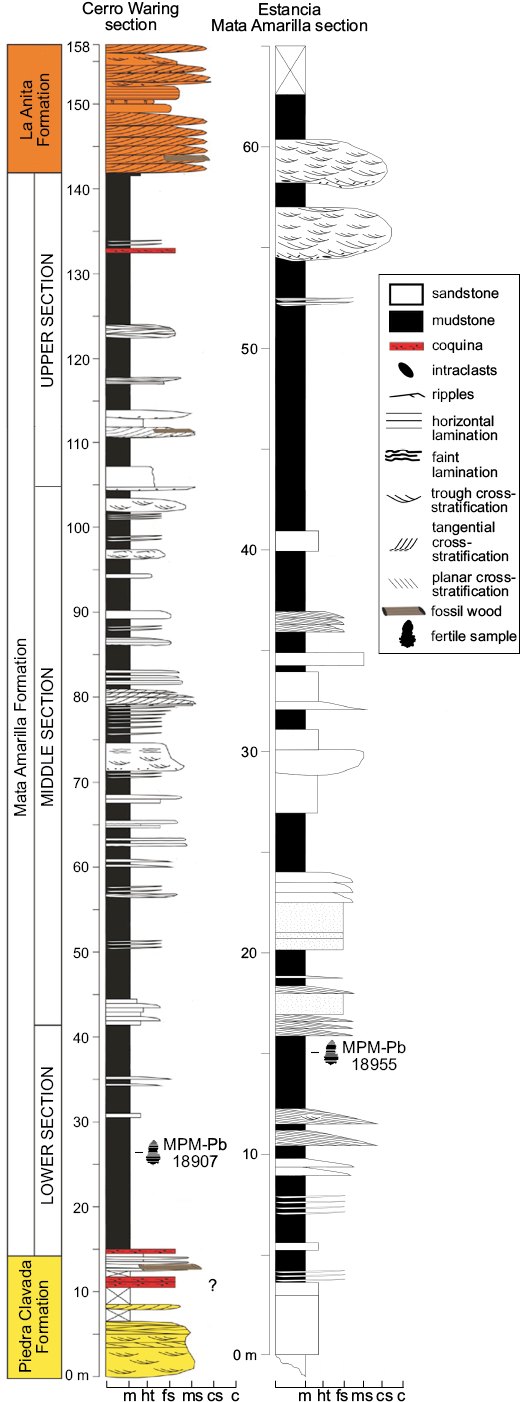
Fig. 2. Stratigraphic sections of the Mata Amarilla Formation at Cerro Waring and Estancia Mata Amarilla localities, showing samples location. Abbreviations: c, conglomerate; cs, coarse sandstone; fs, fine sandstone; ht, heterolitic; m, mudstone; ms, medium sandstone.
Material and methods
Two levels of the Mata Amarilla Formation contain well preserved water fern megaspores: level CW1-003 at the Cerro Warring section (sample MPM-Pb-18907), and level MAT3-MAL’A’ at the Estancia Mata Amarilla section (sample MPM-Pb-18955). Rock samples were treated with traditional palynological techniques and the residues were sieved through 200 µm and 25 µm meshes. For light microscopy observations, residues were dehydrated with alcohol, and mounted in UV-curable acrylate (Noetinger et al. 2017). Slides were observed under a Leica DM500 microscope and photographed with a Leica ICC50 HD camera. Specimen locations are referred to by using England Finder coordinates between brackets. For scanning electronic microscopy (SEM) and transmission electronic microscopy (TEM) observations, individual megaspores were picked from the 200 µm residue, under a light microscope at 10× magnification. For SEM, specimens were mounted on a cover glass and coated with gold-palladium; observations were made under a Philips XL30 TMP microscope at the Electronic Microscopy Service of the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN). Ultrathin sections were made for TEM, and observed under a Jeol 1200 EX II from the Central Service of Electronic Microscope of the Faculty of Veterinary Science, National University of La Plata. The specimens are stored at the Museo Regional Provincial “Padre Jesús Molina”, Rio Gallegos, Santa Cruz Province (MPM-Pb). Terminology used for describing fossil megaspores and spore wall structure follows Batten et al. (2011b). In particular, for Arcellites we use the term acrolamella in the sense of Batten et al. (2011b), who restricted that word to the aggregation of leaf-like, commonly twisted segments that enclose the triradiate suture of the megaspores.
Systematic palaeontology
Division Monilophyta Pryer, Schuettpelz, Wolf, Schneider, Smith, and Cranfill, 2004
Class Polypodiopsida Cronquist, Takhtajan, and Zimmerman, 1966
Order Salviniales Bartling in von Martius, 1835
Genus Arcellites (Miner, 1935) Ellis and Tschudy, 1964
Type species: Arcellites disciformis (Miner, 1935) Ellis and Tschudy, 1964; Cenomanian of the east coast of Disko Island, Greenland.
Arcellites disciformis (Miner, 1935) Ellis and Tschudy, 1964
Fig. 3.
1935 Arcellites disciformis sp. nov.; Miner 1935: 600, pl. 20: 61, 64–66.
1964 Arcellites disciformis (Miner, 1935); Ellis and Tschudy 1964: 75, pl. 1: 1–12, text-fig. 1.
Material.—16 specimens measured. Sample MPM-Pb-18907a (N40/1); MPM-Pb-18907b (R48/2); MPM-Pb-18907 SEM stub 1 (4 specimens), MPM-Pb-18907 SEM stub 2 (2 specimens), MPM-Pb-18907 SEM stub 3 (4 specimens), and MPM-Pb-18907 TEM (4 specimens). Cenomanian of Patagonia, Argentina. Cerro Waring locality, Mata Amarilla Formation.
Description.—Trilete megaspore with spherical body and long acrolamella at proximal face, covering the trilete mark (Fig. 3A1, B1). Megaspore body with 25 to 40 short appendages regularly distributed, with reticulated ends (Fig. 3C2). Body sculpture foveolate (Fig. 3A2, B2). Fovea perpendicular to surface (Fig. 3B3), rounded between appendages, and ovate to slender at their bases (Fig. 3A2). Acrolamella composed of leaf-like appendages twisted along their length, with fimbriate margins and smooth surface (Fig. 3C1). In SEM and TEM, the megaspore wall shows a tripartite structure composed of an outer exoexine, an inner exoexine and an intexine (Fig. 3B3, D). In TEM, the outer exoexine presents a coarsely granular aspect (Fig. 3D1), with granules that range in diameter from 0.3–0.4 µm. Towards the surface, outer exoexine becomes massive, and numerous pits penetrate it perpendicularly giving a palisade-like appearance. The inner exoexine is loosely and finely granulated (granules <0.2 µm in diameter). The intexine presents the most solid aspect of the three wall layers, and at high magnifications ultra-thin and irregular channels (<0.1 µm in diameter) are observed (Fig. 3D2).
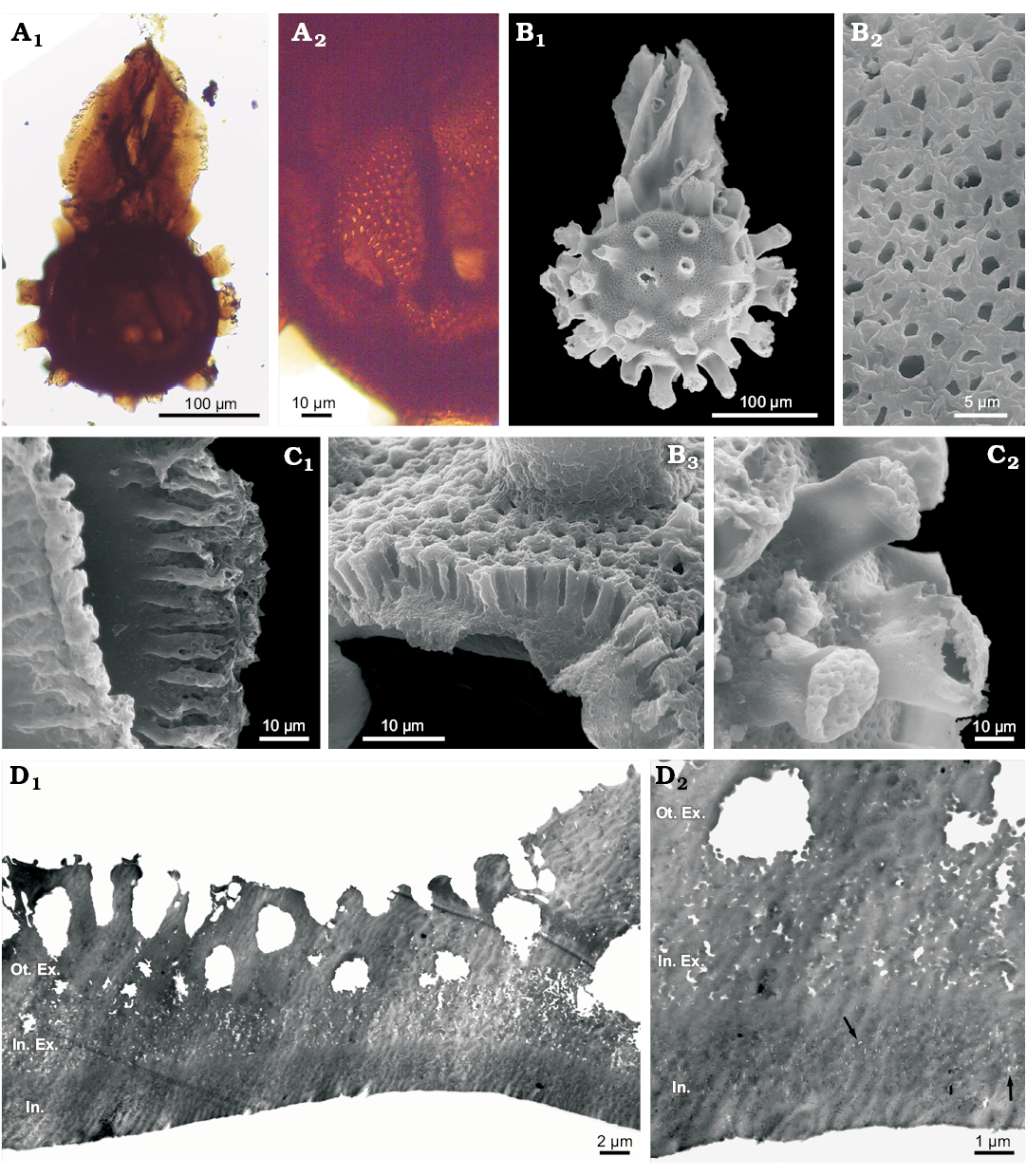
Fig. 3. Megaspores of the water fern Arcellites disciformis (Miner, 1935) Ellis and Tschudy, 1964; Cenomanian, town of Tres Lagos, Argentina. A. MPM-Pb-18907a (N40/1), general view under light microscope (A1), a detail of the pitted pattern (A2). B. MPM-Pb-18907 SEM stub 1, general view under SEM (B1), a detail of pitted surface under SEM (B2), a detail of wall in cross-section under SEM, showing the palisade structure of outer exoexine (B3). C. MPM-Pb-18907 SEM stub 3, detail of acrolamella under SEM, showing leaves with well-developed fimbriate margins (C1), detail of the reticulated tips of appendages (C2). D. MPM-Pb-18907 TEM, body of the spore showing wall-layers under TEM, with coarsely granulated outer exoexine, loosely and finely granulated inner exoexine and massive intexine (D1); detail showing ultra-thin channels (arrows) of intexine (D2). Abbreviations: Ot. Ex., outer exoexine; In. Ex., inner exoexine; Int., intexine.
Dimensions.—Total length (body and acrolamella) 367–378 µm, body diameter 255–278 µm (with appendages), length of appendages 21–51 µm, width of appendages 21.2–36.3 µm, fovea 0.9–2.5 × 0.9–2.2 µm (1.5 × 2.7 µm at base of appendages), acrolamella length 165.7–196.6 µm, acrolamella width 111–155 µm, exine thickness 10.7–18.3 µm, outer exoexine thickness 6–8 µm, inner exoexine thickness 2.3–5.7 µm, intexine thickness 2.4–4.6 µm.
Remarks.—Arcellites disciformis (Miner, 1935) Ellis and Tschudy, 1964 and Arcellites hexapartitus (Dijkstra, 1951) Potter, 1963 share a similar general morphology; Batten et al. (1996) compared and contrasted these species and listed a series of characters useful to separate them. Arcellites disciformis is characterized by the presence of: (i) leaf-like segments of the neck tightly twisted against each other, (ii) leaves of acrolamella with well-developed fimbriate margins, (iii) megaspore wall surface profusely pitted, (iv) appendages with reticulate tips and absence of surface swellings of the exoexine. The Patagonian specimens conform to the diagnosis of Arcellites disciformis and present all the morphological features that characterized the species (Hueber 1982; Batten et al. 1996).
The Argentinean specimens have similar dimensions to those reported for the Barremian–Aptian of Virginia, USA (Hueber 1982) and for the Albian–Cenomanian of Maryland, USA (Lupia 2015). However, they are smaller than those described for the Albian–Cenomanian of the Denver Basin, central USA (Ellis and Tschudy 1964) and for the Cenomanian of Alberta, Canada (Singh 1983). Differences in size may be related to dehydration during processing as previously noted by Hueber (1982).
Mays (2011) reported the presence of A. disciformis for the Cenomanian of Chatham Islands, New Zealand, but the illustrated specimens do not show the main morphological features that characterized the species (Batten et al. 1996) and might be related to A. hexapartitus.
Stratigraphic and geographic range.—Barremian–Cenomanian of USA (Schemel 1950; Hall 1963; Potter 1963; Ellis and Tschudy 1964; Hall and Peake 1968; Hueber 1982; Kovach and Dilcher 1988; Lupia 2015), Barremian–Cenomanian of Canada (Singh 1964, 1971, 1983; Hopkins and Sweet 1976; Sweet 1979), Albian–Cenomanian of Greenland (Miner 1935; Koppelhus and Pedersen 1993; Batten et al. 1996), Aptian–Cenomanian of Sudan (Kaska 1989), Turonian of France (Colin 1975), Aptian of Germany (Schultz and Noll 1987), Cenomanian of Patagonia (this work).
Genus Balmeisporites (Cookson and Dettmann, 1958) Dettmann, 1995
Type species: Balmeisporites holodictyus Cookson and Dettmann, 1958; Albian of Robe Bore, South Australia.
Balmeisporites cf. B. holodictyus Cookson and Dettmann, 1958
Fig. 4.
1958 Balmeisporites holodictyus sp. nov.; Cookson and Dettmann 1958: 42, pl. 2: 1–6, text-fig. 3.
Material.—32 specimens measured. MPM-Pb-18907a (J53/3, H32/4, K49/1, Q49/1, F39, D28/2, C33/3, C30/2, B40/2, H32/4, R33); MPM-Pb-18907b (Z33/4, W39/4, U49/2, J37/1, C38/2, E36/2); MPM-Pb-18907 SEM stub 1 (5 specimens); MPM-Pb-18955a (M35/3, W43, Y44/2, Y34/3); MPM-Pb-18955b (V44/3, J52/1, N42/3); MPM-Pb-18955c (G25/1); MPM-Pb-18955d (Z45, H25/1). Cenomanian of Patagonia, Argentina. Cerro Waring and Estancia Mata Amarilla localities, Mata Amarilla Formation.
Description.—Trilete megaspore with round to elliptical body. Laesurae with membranous, highly elevated lips (Fig. 4A, B). Lips finely granulated (Fig. 4C). Megaspore surface irregularly reticulate. Lumina polygonal to irregular in shape, larger near the equator (Fig. 4A, B), sculpture inside lumina scabrated. Muri of the reticulum narrow and low (Fig. 4B).
Dimensions.—Total length (body and lips) 125–197 µm, body diameter 90–142.9 µm, lumina diameter 7–20 µm, reticulum wall thickness 1–3.5 µm, length of lips 36–45 µm, width of lips 80–136 µm.
Remarks.—The Patagonian specimens broadly fit with the general diagnosis of B. holodictyus (Cookson and Dettmann 1958; Dettmann 1995) but differ in lacking the three wing-like outgrowths in each radial equatorial region. Some specimens show variations in the development of the reticulum (Fig. 4D, E), a feature also reported for specimens from the Aptian–Albian of Australia (Tosolini et al. 2002). Bearing in mind that we only recovered few poorly preserved specimens, we temporarily retain these megaspores within B. cf. B. holodictyus (Fig. 4D, E), although we cannot rule out that they belong to a different species. This species has been widely reported from the Barremian to Danian around the globe (Dettmann 1995).

Fig. 4. Megaspores of the water fern Balmeisporites cf. B. holodictyus Cookson and Dettmann, 1958; Cenomanian, town of Tres Lagos, Argentina. A. MPM-Pb-18907a (H32/4), general view under light microscope. B. MPM-Pb-18907 SEM stub 1, general view under SEM, showing the larger reticulum in the equatorial region. C. MPM-Pb-18907a (Q49/1), detail of finely granulated lips. D. MPM-Pb-18907a (F39), general view of one individual with variations in reticulum development. E. MPM-Pb-18907a (J53/3), detail of the variations in reticulum development.
Discussion
The record of Arcellites and Balmeisporites in the Cenomanian of southern Patagonia enlarges the distribution of salvinialean megaspores in the area. In particular, the presence of A. disciformis is significant because it represents the first Southern Hemisphere record; all previous reports being restricted to the Northern Hemisphere (Fig. 5). This new record indicates a bi-hemispheric distribution for the species. The genus Arcellites was previously represented in Patagonia by four species: A. santacrucensis Baldoni, 1987; A. humilis Villar de Seoane and Archangelsky, 2008; A. pentagonalis Villar de Seoane and Archangelsky, 2008; and Arcellites sp. A in Villar de Seoane and Archangelsky, 2008, ranging from Albian to Cenomanian in age (Baldoni 1987; Baldoni and Taylor 1988; Villar de Seoane and Archangelsky 2008). The new species record increases the diversity of the genus for the Cretaceous of Patagonia.
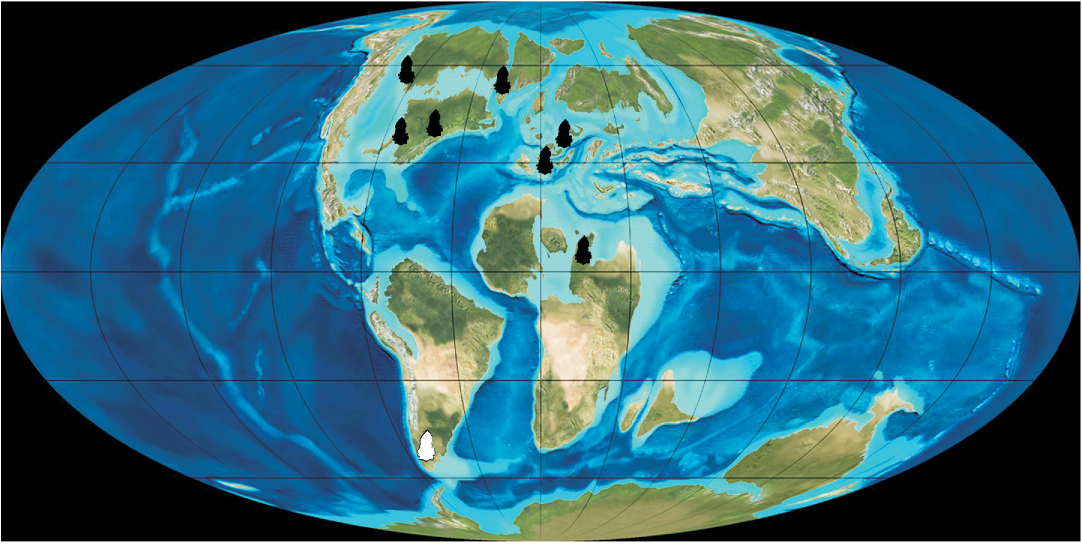
Fig. 5. Paleogeographic map of the Late Cretaceous (modified from Blakey 2010) showing the known distribution of Arcellites disciformis (black symbols), and the new Patagonian record (white symbol).
Arcellites has been related to Salviniales on the bases of both general morphology and association with microspores of Crybelosporites Dettmann, 1963. The acrolamella of Arcellites with six twisted segments is broadly comparable to that of recorded in megaspores of some extant members of Marsileaceae (Regnellidium and Pillularia) and the closely related extinct genus Molaspora Schemel, 1950 (Lupia et al. 2000; Cúneo et al. 2013; Friss et al. 2014). The wall ultrastructure of Arcellites, however, differs from that present in any extant species of Marsileaceae and, according to Collinson (1991), it may be related to an extinct family within Salviniales. Dispersed microspores of Crybelosporites have been related to aquatic ferns of Salvineales (Dettmann 1963), so that, the presence of microspores of Crybelosporites in the folds of the acrolamella of Arcellites species also suggests its relationship with the order (Cookson and Dettmann 1958; Hall 1963; Ellis and Tshudy 1964; Hall and Peake 1968; Hueber 1982; Li and Batten 1986; Tosolini et al. 2002; Lupia 2004, 2015; Friis et al. 2014). Crybelosporites is also associated with fossil megaspores of the genus Molaspora. Both taxa were found in situ in sporocarps related to Regnellidium in the Santonian of Georgia, USA (Lupia et al. 2000). The diversity and abundance of these megaspores in the Cretaceous of southern Patagonia is remarkable. The fact that Crybelosporites is related to both Molaspora and Arcellites supports evolutionary links between them, as well as with their parent plants (Zavialova and Batten 2018).
The presence of acrolamella and appendages resembling bladder-like swellings in Arcellites were interpreted as adaptations to floating and fertilization on the water surface (Ellis and Tschudy 1964). This was also supported by the common occurrences of Arcellites either in shallow fresh or brackish water facies (Cookson and Dettman 1958; Ellis and Tschudy 1964; Tosolini et al. 2002).
Balmeisporites is a widespread Cretaceous genus with a continuous history ranging from the Barremian to the Danian, and with a cosmopolitan distribution during the Albian–Cenomanian (Dettmann 1995). This genus was referred to a new fossil order of heterosporous plants following the finding of a fertile plant in the Cenomanian of western Siberia (Krasilov and Golovneva 2000), but this affinity needs to be revised. At present, most researchers have little doubt that Balmeisporites was derived from a water fern within the Salviniales, mainly on the bases of general morphology and spore wall characters (Hall 1974; Baldoni and Batten 1991; Dettmann 1995; Villar de Seoane and Archangelsky 2008; Lupia 2011).
Three chronostratigraphic zones were defined for the Berriasian–Cenomanian of southern Patagonia characterized by its megaspore content (Villar de Seoane and Archangelsky 2008). These are: M1 (Berriasian–Barremian), M2 (Aptian), and M3 (Albian–?Early Cenomanian). Based on the presence of these species, the lower and middle levels of the Mata Amarilla Formation may be referred to the Zone M3, which is characterized by the presence of B. holodictyus, several species of Arcellites, along with lycopsid megaspores. Records of A. disciformis are mostly restricted to the Albian–Cenomanian in the Northern Hemisphere (except for the Barremian–Aptian of Virginia, USA), suggesting that it may have potential as biostratigraphic marker for the Cenomanian in Argentina. The presence and abundance of these water ferns in the lower and middle sections of the Mata Amarilla Formation supports a local aquatic paleoenvironment as was also suggested by sedimentological evidence (Varela 2011), with fresh or brackish water bodies under warm and humid conditions.
Conclusions
The new record of Arcellites disciformis and Balmeisporites cf. B. holodictyus in the Mata Amarilla Formation extends the distribution of water ferns in the Cenomanian of southern Patagonia. In particular, the finding of A. disciformis is important since it represents the first record of the species for the Southern Hemisphere; all previous reports came from the Northern Hemisphere. This new finding supports a bi-hemispheric distribution for the species and also increases the diversity of the genus Arcellites in the Cretaceous of Patagonia.
These new findings allow correlating the lower and middle sections of the Mata Amarilla Formation with the megaspore Zone M3 (Albian–Cenomanian) defined for southern Patagonia. They also support an aquatic environment for this unit with fresh or brackish water bodies developed under warm and humid conditions.
Overall, the present findings enlarge the importance of salvinealean megaspores in Late Cretaceous ecosystems of southernmost South America. Further investigation in yet unexplored areas would help to better understand the evolutionary history of these water ferns.
Acknowledgements
The authors want to thank Sebastián Mirabelli, Fabián Tricárico, and Orlando Cárdenas (all MACN), Susana Jurado and Fernanda Faisal (UNLP), and Isabel Farias (all Buenos Aires, Argentina) for their technical assistance; the Secretaría de Cultura de la Provincia de Santa Cruz, Waring, Piccinini and Nacer families for their field work permission and priceless help at the field. We also thank David Batten (University of Manchester, UK) and Anne-Marie Tosolini (University of Melbourne, Australia) for the helpful comments that improve this paper. Support was provided through the Consejo Nacional de Investigaciones Científicas y Técnicas, and grants PIP-2014-0259 and PIP-2013-0388.
References
Archangelsky, A., Archangelsky, S., Poiré, D., and Canessa, N. 2008. Registros palinológicos en la Formación Piedra Clavada (Albiano) en su área tipo, provincia de Santa Cruz, Argentina. Revista del Museo Argentino de Ciencias Naturales, nueva serie 10: 185–198.
Archangelsky, A., Phipps, C.J., Taylor T.N., and Taylor, E.L. 1999. Paleoazolla, a new heterosporous fern from the Upper Cretaceous of Argentina. American Journal of Botany 86: 1200–1206. Crossref
Archangelsky, S. and Villar de Seoane, L.M. 1989. Ultraestructura de dos nuevas Megasporas Cretácicas de Santa Cruz, Argentina. Boletín de la Asociación Latinoamericana de Paleobotánica y Palinología 12: 13–25.
Archangelsky, S. and Villar de Seoane, L.M. 1990. Morfología y estructura de megasporas cretácicas de Patagonia. República Argentina. Revista Española de Micropaleontología 22: 419–450.
Archangelsky, S. and Villar de Seoane, L.M. 1991. Notas sobre la flora fósil de la Zona de Ticó, provincia de Santa Cruz. XI. Morfología y estructura de tres megasporas. Ameghiniana 28: 353–364.
Baldoni, A.M. 1987. Dos nuevas especies de megasporas de la Formación Kachaike, Cretácico Inferior de Santa Cruz, Argentina. Anais do X Congreso Brasileiro de Paleontología, Rio de Janeiro, 669–689.
Baldoni, A.M. and Batten, D.J. 1991. Megaspores from the Lower Cretaceous Kachaike Formation, Santa Cruz Province, Argentina. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 182: 377–393. Crossref
Baldoni, A.M. and Batten, D.J. 1997. Cretaceous megaspores from two boreholes in the Austral Basin, Santa Cruz Province, Argentina, and their stratigraphic and palaeoenvironmental significance. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 205: 97–110.
Baldoni, A.M. and Taylor, T.N. 1985. Megasporas cretácicas de la Formación Springhill en el subsuelo de Argentina y Chile Austral. Ameghiniana 21: 151–167.
Baldoni, A.M. and Taylor, T.N. 1987. Ultraestructura de la pared de Paxillitriletes kachaikense una nueva especie en el Cretácico Inferior de la Provincia de Santa Cruz, Argentina. Actas del VII Simposio Argentino de Paleobotánica y Palinología, Buenos Aires, 89–91.
Baldoni, A.M. and Taylor, T.N. 1988. Ultraestructura de una nueva especie de Arcellites en el Cretácico Inferior de la Provincia de Santa Cruz, Argentina y sus vinculaciones con la Familia Marsileaceae. IV Congreso Argentino de Paleontología y Bioestratigrafía, Mendoza, Actas 3: 15–22.
Batten, D.J., Dutta, R.J., and Knobloch, E. 1996. Differentiation, affinities and palaeoenvironmental significance of the megaspores Arcellites and Bohemisporites in Wealden and other Cretaceous successions. Cretaceous Research 17: 39–65. Crossref
Batten, D.J., Collinson, M.E., and Brain, A.P. 2011a. Megaspores and microspores of the extant and Paleogene marsileaceous fern Regnellidium and Cretaceous Molaspora: evolutionary and phytogeographic implications. International Journal of Plant Sciences 172: 1087–1100. Crossref
Batten, D.J., Zavattieri, A.M., and Collinson, M.E. 2011b. Megaspores from the upper Maastrichtian of the eastern Spanish Pyrenees and their biostratigraphic, palaeogeographic and palaeoenvironmental significance. Review of Palaeobotany and Palynology 167: 156–172. Crossref
Blakey, R. 2010. Global Late Cretaceous (90 Ma) Paleogeographic Map. Colorado Plateau Geosystems, http://deeptimemaps.com/global-map-series/
Colin, J.P. 1975. Quelques megaspores de Cenomanien et du Turonien supérieur du Sarladais (Dordogne, S.O. France). Revista Española de Micropaleontologia 7: 15–23.
Collinson, M.E. 1980. A new multiple floated Azolla from the British Eocene with a brief review of the genus. Palaeontology 23: 213–299.
Collinson, M.E. 1991. Diversification of modern heterosporous pteridophytes. In: S. Blackmore and S.H. Barnes (eds.), Pollen and Spores. Patterns of Diversification. Systematics Association, Special Volume 44: 119–150.
Collinson, M.E. 2001. Cainozoic ferns and their distribution. Brittonia 53: 173–235. Crossref
Collinson, M.E. 2002. The ecology of Cainozoic ferns. Review of Palaeobotany Palynology 119: 51–68. Crossref
Cookson, I.C. and Dettmann, M.E. 1958. Cretaceous ‘‘megaspores’’ and a closely associated microspore from the Australian region. Micropaleontology 4: 39–49. Crossref
Cúneo, N.R., Gandolfo, M.A., Zamaloa, M.C., and Hermsen E. 2014. Late Cretaceous Aquatic Plant World in Patagonia, Argentina. PLoS ONE 9(8): e104749. Crossref
Cúneo, N.R., Hermsen, E., and Gandolfo, M. 2013. Regnellidium (Salviniales, Marsileaceae) macrofossils and associated spores from the Late Cretaceous of South America. International Journal of Plant Sciences 174: 340–349. Crossref
Dettmann, M.E. 1963. Upper Mesozoic microfloras from southeastern Australia. Proceedings of the Royal Society of Victoria 77: 1–148.
Dettman, M.E. 1995. Ultrastructure and biogeography of Balmeisporites Cookson and Dettmann, 1958. Review of Paleobotany and Palynology 89: 287–296. Crossref
Ellis, C.H. and Tschudy, R.H. 1964. The Cretaceous megaspore genus Arcellites Miner. Micropaleontology 10: 73–79. Crossref
Fossa Mancini, E., Feruglio, E., and Yussen de Campana, J.C. 1938. Una Reunión de geólogos de Y.P.F. y el problema de la Terminología Estratigráfica. Boletín de Informaciones Petroleras 171: 31–95.
Friis, E.M., Pedersen, K.R., and Marone, F. 2014. Arcellites punctatus sp. nov.: A new megaspore from the Early Cretaceous of Portugal studied using high resolution synchrotron radiation X-ray tomographic microscopy (SRXTM). Grana 53: 91–102. Crossref
Gamerro, J.C. 1975. Megasporas del Cretácico de Patagonia I. Ultraarquitectura de la pared megasporal en Hughesisporites patagonicus Archang. y Horstisporites feruglioi Archang. Ameghiniana 12: 97–108.
Hall, J.W. 1963. Megaspores and other fossils in the Dakota Formation (Cenomanian) of Iowa, (U.S.A.). Pollen et Spores 5: 425–443.
Hall, J.W. 1974. Cretaceous Salviniaceae. Annals of the Missouri Botanical Garden 61: 354–367. Crossref
Hall, J.W. and Peake, N.M. 1968. Megaspore assemblages in the Cretaceous of Minnesota. Micropaleontology 14: 456–464. Crossref
Hermsen, E.J., Gandolfo, M.A., and Cúneo, N.R. 2014. New marsileaceous fossils from the Late Cretaceous of South America and a reevaluation of Marsileaceaephyllum. Plant Systematics and Evolution 300: 369–386. Crossref
Hopkins, W.S. and Sweet, A.R. 1976. Miospores and megaspores from the Lower Cretaceous Muttagami Formation of Ontario. Geological Survey of Canada, Bulletin 256: 55–71. Crossref
Hueber, F.M. 1982. Megaspores and a palynomorph from the Lower Potomac Group in Virginia. Smithsonian Contributions to Paleobiology 49: 1–69. Crossref
Iglesias, A., Zamuner, A.B., Poiré, D.G., and Larriestra, F. 2007. Diversity, taphonomy, and palaeoecology of an angiosperm flora from the Cretaceous (Cenomanian–Coniacian) in Southern Patagonia, Argentina. Palaeontology 50: 445–466. Crossref
Kaska, H.V. 1989. A spore and pollen zonation of Early Cretaceous to Tertiary nonmarine sediments of central Sudan. Palynology 13: 79–90. Crossref
Kasks, E.B. and Pedersen, G.K. 1993. A palynological and sedimentological study of Cretaceous floodplain deposits of the Atane Formation at Skansen and Igdlunguaq, Disko, West Greenland. Cretaceous Research 14: 707–734.
Koppelhus, E.B. and Pedersen, G.K. 1993. A palynological and sedimentological study of Cretaceous floodplain deposits of the Atane Formation at Skansen and Igdlunguaq, Disko, West Greenland. Cretaceous Research 14: 707–734.
Kovach, W.L. and Dilcher, D.L. 1988. Megaspores and other dispersed plant remains from the Dakota Formation (Cenomanian) of Kansas, USA. Palynology 12: 89–119. Crossref
Krasilov, V.A. and Golovneva, L.B. 2000. A new order of heterosporous plants from the Late Cretaceous of the Kem’River, Western Siberia. Paleontological Journal 34: 84–92.
Leanza, A.F. 1972. Andes Patagónicos Australes. In: A.F. Leanza (ed.), Geología Regional Argentina, 689–706. Academia Nacional de Ciencia de Córdoba, Córdoba.
Li, W. and Batten, D.J. 1986. The Early Cretaceous megaspore Arcellites and closely associated Crybelosporites microspores from northeast Inner Mongolia, P.R. China. Review of Palaeobotany and Palynology 46: 189 –208. Crossref
Lupia, R. 2004. Megaspores and palynomorphs from the Lower Potomac Group of Maryland, U.S.A. International Journal of Plant Sciences 165: 651–670. Crossref
Lupia, R. 2011. Late Santonian megaspore floras from the Gulf coastal plain (Georgia, USA). Journal of Paleontology 85: 1–21. Crossref
Lupia, R. 2015. Mid-Cretaceous megaspore floras from Maryland, USA. Journal of Paleontology 89: 494–521. Crossref
Lupia, R., Schneider, H., Moeser, G.M., Pryer, K.M., and Crane, P.R. 2000. Marsileaceae sporocarps and spores from the Late Cretaceous of Georgia, U.S.A. International Journal of Plant Sciences 161: 975–988. Crossref
Marenssi, S., Guler, M.V., Casadío, S., Guerstein, G.R., and Papú, O. 2004. Sedimentology and bioestratigraphy of Maastrichtian deposits from Austral Basin, Argentina. Cretaceous Research 25: 907–918. Crossref
Mays, C. 2011. Mid-Cretaceous Greenhouse Environments and Floral Ecosystems of the South Polar Region (75–80° S): the Tupuangi Formation, Chatham Islands, Zealandia. 402 pp. Ph.D. Thesis, Monash University, Faculty of Science, School of Geosciences, Melbourne.
Miner, E.L. 1935. Paleobotanical examinations of Cretaceous and Tertiary Coals. American Midland Naturalist 16: 585–625. Crossref
Noetinger, S., Pujana, R.R., Burrieza, A., and Burrieza, H.P. 2017. Use of UV-curable acrylates gels as mounting media for palynological samples. Revista del Museo Argentino de Ciencias Naturales, nueva serie 19: 19–23.
Poiré, D.G., Iglesias, A., Varela, A.N., Richiano, S., Ibañez Mejías, M., and Strömberg, C.A.E. 2017. Edades U-Pb en zircones de tobas de la Fm. Piedra Clavada, Pcia. de Santa Cruz, Argentina: Un marcador Albiano tardío para la evolución tectónica y biológica de la Cuenca Austral. Actas del XX Congreso Geológico Argentino, San Miguel de Tucumán, 95–98.
Potter, D.R. 1963. An emendation of the sporomorph Arcellites Miner, 1935. Geology Notes 227: 227–230.
Riccardi, A.C., Aguirre Urreta, M.B., and Medina, F. 1987. Aconeceratidae (Ammonitina) from the Hauterivian–Albian of southern Patagonia. Palaeontographica 196: 105–185.
Rothwell, G.W. and Stockey, R.A. 1994. The role of Hydropteris pinnata gen. et sp. nov. in reconstructing the cladistics of heterosporous ferns. American Journal of Botany 81: 479–492. Crossref
Schemel, M.P. 1950. Cretaceous plant microfossils from Iowa. American Journal of Botany 37: 750–754. Crossref
Schultz, G. and Noll, H. 1987. Die Megasporen-Assoziation in den unterkretazischen Sedimenten einer Paläokartsthöhle bei Nehden im Sauerland (Rheinisches Schiefergebirge). Nachträge zu der bereits bekannten Vergesellschaftung. Palaeontographica Abteilung B 203: 83–107.
Singh, C. 1964. Microflora of the Lower Cretaceous Mannville Group, East-Central Alberta. Research Council of Alberta, Bulletin 15: 1–245.
Singh, C. 1971. Lower Cretaceous microfloras of the Peace River Area, Northwestern Alberta. Research Council of Alberta, Bulletin 28: 1–542.
Singh, C. 1983. Cenomanian microfloras from the Peace River area, Northwestern Alberta. Alberta Research Council, Bulletin 44: 1–330.
Smith, A.R., Pryer, K.M., Schuettpelz, E., Korall, P., Schneider, H., and Wolf, P.G. 2006. A classification for extant ferns. Taxon 55: 705–731. Crossref
Stough, J.B. 1968. Palynomorphs from South America. Part 1. New Late Cretaceous palynomorphs from southern South America. The University of Kansas Paleontological Contribution Papers 32: 1–8.
Sweet, A.R. 1979. Jurassic and Cretaceous megaspores. American Association of Stratigraphic Palynologists, Contribution Series 5: 1–30.
Taylor, W.A. and Taylor, T.N. 1988. Ultrastructural analysis of selected Cretaceous megaspores from Argentina. Journal of Micropalaeontology 7: 73–87. Crossref
Tosolini, A.M.P., McLoughlin, S., and Drinnan, A.N. 2002. Early Cretaceous megaspore assemblages from southeastern Australia. Cretaceous Research 23: 807–844. Crossref
Tryon, A.F. and Lugardon, B. 1991. Spores of the Pteridophyta: Surface, Wall Structure, and Diversity Based on Electron Microscope Studies. 648 pp. Springer-Verlag, New York. Crossref
Tryon, R.M. and Tryon, A.F. 1982. Ferns and Allied Plants. With Special Reference to Tropical America. 857 pp. Springer-Verlag, New York. Crossref
Vajda, V. and McLoughlin, S. 2005. A new Maastrichtian–Paleocene Azolla species from Bolivia, with a comparison of the global record of coeval Azolla microfossils. Alcheringa 29: 305–329. Crossref
Varela, A.N. 2011. Sedimentología y modelos deposicionales de la Formación Mata Amarilla, Cretácico de la Cuenca Austral, Argentina. 384 pp. Ph.D. Thesis, Universidad Nacional de La Plata, Facultad de Ciencias Naturales y Museo, La Plata.
Varela, A.N. 2014. Tectonic control of accommodation space and sediment supply within the Mata Amarilla Formation (lower Upper Cretaceous) Patagonia, Argentina. Sedimentology 62: 867–896. Crossref
Varela, A.N., Iglesias, A., Poiré, D.G., Zamuner, A.B., Richiano, S., and Brea, M. 2016. Petrified forests in the Austral Basin marks a Cenomanian forced regression heterogeneous surface. Geobiology 14: 293–313. Crossref
Varela, A.N., Poiré, D.G., Martin, T., Gerdes, A., Goin, F.J., Gelfo, J.N., and Hoffmann, S. 2012a. U-Pb zircon constraints on the age of the Cretaceous Mata Amarilla Formation, Southern Patagonia, Argentina: its relationship with the evolution of the Austral Basin. Andean Geology 39: 359–379. Crossref
Varela, A.N., Raigemborn, M.S., Richiano, S., White, T., Poiré, D.G., and Lizzoli, S. 2018. Late Cretaceous palaeosols as paleoclimate proxies of high-latitude Southern Hemisphere: Mata Amarilla Formation, Patagonia, Argentina. Sedimentary Geology 363: 83–85. Crossref
Varela, A.N., Richiano, S., and Poiré, D.G. 2011. Tsunami vs storm origin for shell bed deposits in a lagoon environment: an example from the Upper Cretaceous of Southern Patagonia, Argentina. Latin America Journal of Sedimentology and Basin Analysis 18: 63–85.
Varela, A.N., Veiga, G.D., and Poiré, D.G. 2012b. Sequence stratigraphic analysis of Cenomanian greenhouse palaeosols: A case study from southern Patagonia, Argentina. Sedimentary Geology 271: 67–82. Crossref
Villar de Seoane, L.M. 1988. Nota sobre una curiosa momificación del Cretácico Inferior de la Provincia de Santa Cruz. Boletín de la Asociación Latinoamericana de Paleobotánica y Palinología 11: 20–22.
Villar de Seoane, L.M. and Archangelsky, S. 2008. Taxonomy and biostratigraphy of Cretaceous megaspores from Patagonia, Argentina. Cretaceous Research 29: 354–372. Crossref
Yamada, T. and Kato, M. 2002. Regnellites nagashimae gen. et sp. nov., the oldest macrofossil of Marsileaceae, from the Upper Jurassic to Lower Cretaceous of western Japan. International Journal of Plant Sciences 163: 715–723. Crossref
Zavialova, N. and Batten, D.J. 2018. Species of the water-fern megaspore genus Molaspora from a Cenomanian deposit in western France: occurrence, sporoderm ultrastructure and evolutionary relationships. Grana 57 (5): 1–20. Crossref
Acta Palaeontol. Pol. 63 (3): 607–616, 2018
https://doi.org/10.4202/app.00491.2018