

The gaudryceratid ammonoids from the Upper Cretaceous of the James Ross Basin, Antarctica
MARÍA E. RAFFI, EDUARDO B. OLIVERO, and FLORENCIA N. MILANESE
Raffi, M.E., Olivero, E.B., and Milanese, F.N. 2019. The gaudryceratid ammonoids from the Upper Cretaceous of the James Ross Basin, Antarctica. Acta Palaeontologica Polonica 64 (3): 523–542.
We describe new material of the subfamily Gaudryceratinae in Antarctica, including five new species: Gaudryceras submurdochi Raffi and Olivero sp. nov., Anagaudryceras calabozoi Raffi and Olivero sp. nov., Anagaudryceras subcompressum Raffi and Olivero sp. nov., Anagaudryceras sanctuarium Raffi and Olivero sp. nov., and Zelandites pujatoi Raffi and Olivero sp. nov., recorded in Santonian to Maastrichtian deposits of the James Ross Basin. The early to mid-Campanian A. calabozoi Raffi and Olivero sp. nov. exhibits a clear dimorphism, expressed by marked differences in the ornament of the adult body chamber. Contrary to the scarcity of representative members of the subfamily Gaudryceratinae in the Upper Cretaceous of other localities in the Southern Hemisphere, the Antarctic record reveals high abundance and diversity of 15 species and three genera in total. This highly diversified record of gaudryceratins is only comparable with the Santonian–Maastrichtian Gaudryceratinae of Hokkaido, Japan and Sakhalin, Russia, which yields a large number of species of Anagaudryceras, Gaudryceras, and Zelandites. The reasons for a similar, highly diversified record of the Gaudryceratinae in these distant and geographically nearly antipodal regions are not clear, but we argue that they probably reflect a similar paleoecological control.
Key words: Ammonoidea, Phylloceratida, Gaudryceratinae, Lytoceratoidea, Cretaceous, Antarctica.
María E. Raffi [eugeniaraffi@gmail.com] and Eduardo. B. Olivero [emolivero@gmail.com], Centro Austral de Investigaciones Científicas (CADIC), CONICET, Bernardo Houssay 200, CP9410, Ushuaia, Argentina and Instituto de Ciencias Polares, Ambiente y Recursos Naturales, Universidad Nacional de Tierra del Fuego, Ushuaia, Argentina.
Florencia N. Milanese [fnmilanese@gmail.com], Universidad de Buenos Aires, Facultad de Ciencias Exactas y Naturales, Departamento de Cs. Geológicas, Instituto de Geociencias Básicas, Aplicadas y Ambientales de Buenos Aires (IGEBA), CONICET, Buenos Aires, Argentina.
Received 20 October 2018, accepted 18 April 2019, available online 29 August 2019.
Copyright © 2019 M.E. Raffi et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Lytoceratid ammonites are generally not well represented in the Upper Cretaceous of the Southern Hemisphere (e.g., Wright and Kennedy 1984; Matsumoto 1995; Hoffmann 2010), and it is repetitively stated that the studied lytoceratid collection has no more than a few specimens (e.g., Howarth 1966; Kennedy and Klinger 1979). In contrast to that common belief, the specimens of lytoceratid genus Gaudryceras are extremely abundant and diversified in the Santonian–Campanian of the James Ross Basin (Raffi and Olivero 2016), where five species of Gaudryceras characterize four distinct stratigraphic horizons. Interestingly, while the Santonian–early Campanian species of Gaudryceras have a cosmopolitan or IndoPacific biogeographic distribution, the mid- to late Campanian ones are mostly restricted to the James Ross Basin. Contrary to the well-documented Maastrichtian distribution of species of Gaudryceras in the Northern Hemisphere (see Matsumoto 1995; Shigeta and Nishimura 2013 and references therein), Gaudryceras disappears in Antarctica during the late Campanian (Raffi and Olivero 2016). The biogeographic affinities and extinctions of the Antarctic species of Gaudryceras thus follow the same pattern already established for most of the Antarctic ammonite faunule, particularly for the families Nostoceratidae, Baculitidae, Scaphitidae, and Kossmaticeratidae (see Olivero and Medina 2000; Olivero 2012a, b; Raffi and Olivero 2016).
As for the other genera of Late Cretaceous Gaudryceratinae, only two species of Anagaudryceras and one species of Zelandites were reported for Antarctica so far: Anagaudryceras seymouriense Macellari, 1986 and Zelandites varuna (Forbes, 1846) from the late Maastrichtian of the López de Bertodano Formation, Seymour Island and Anagaudryceras politissimum (Kossmat, 1895) by Kilian and Reboul 1909 (= Anagaudryceras mikobokoense Collignon, 1956) from the Karlsen Cliffs Member, Snow Hill Island Formation.
In this work, we study an abundant new collection of more than 100 specimens of Gaudryceratinae from the James Ross Basin. The material was collected by the authors during several Antarctic field seasons from lower Campanian to upper Maastrichtian deposits of the Santa Marta, Rabot, Snow Hill Island, and López de Bertodano formations. Thus, the main aim of this work is to complete the systematic, biostratigraphic and paleobiogeographic study of the Santonian–Maastrichtian Gaudryceratinae from the James Ross Basin.
Institutional abbreviations.—CADIC PI, Invertebrate paleontology collection of the Centro Austral de Investigaciones Científicas, Ushuaia, Argentina; CALTECH, California Institute of Technology, Pasadena, California, USA; CITSE, Centro de Investigaciones y Transferencia de Santiago del Estero, Santiago del Estero, Argentina; CONICET, Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina; ENAP-ACM, Collection by Antonio Cañón Martínez for the Empresa Nacional de Petróleo Chilena, Punta Arenas, Chile; HMG, Hobetsu Museum, Mukawa, Hokkaido, Japan; NMNS, National Museum of Nature and Science, Tsukuba, Japan; OSU, Orton Geological Museum, Columbus, Ohio, USA; VC, Victoria University of Wellington (cephalopods collection), Wellington, New Zealand.
Other abbreviations.—D, diameter; U, umbilical diameter as % of D; Wb, whorl breadth at a given D; Wh, whorl height at a given D. N, Natalites Sequence; NG, Neograhamites and Gunnarites Sequence; MG, Maorites and Grossouvrites Sequence.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:AE289808-16F2-4F5D-B0D3- AD9376BC6BA0
Geological settings
The James Ross Basin is a back-arc basin located to the east of the Antarctic Peninsula with its greatest areal exposures in the James Ross, Snow Hill, Seymour, and Vega islands (Fig. 1). The c. 3 km-thick marine succession of the Santonian–Danian Marambio Group (Ineson 1989; Macellari 1988; Olivero and Medina 2000) represents prograding shelf settings punctuated by three major sedimentary cycles, which were correlated across the basin by 15 Santonian–Maastrichtian ammonite assemblages (Olivero and Medina 2000; Olivero et al. 2008; Olivero 2012a, b). The names of these sequences were derived from their most common kossmaticeratid ammonites: N for Natalites, NG for Neograhamites and Gunnarites, and MG for Maorites and Grossouvrites. The N Sequence comprises the Santonian–lower Campanian Santa Marta Formation (Olivero et al. 1986), restricted to northwest James Ross Island and exposed in Brandy Bay, Dreadnought Point and in the vicinity of the Dobson Dome (Fig. 1B). The Formation is subdivided into the Alpha and Beta members (Olivero et al. 1986 and Scasso et al. 1991), that are approximately equivalent to the Lachman Crags Member (Crame et al. 1991; Pirrie et al. 1997), and the mid- to upper Campanian Rabot Formation (Lirio et al. 1989) which is restricted to the southeast of the James Ross Island and exposed in Rabot Point, Redonda Point, and northwesten Cape Hamilton (Figs. 1, 2). The Rabot Formation is divided into three informal members well represented in its type locality at Rabot Point with an exposed thickness of 350 m (Lirio et al. 1989; Martinioni 1992).
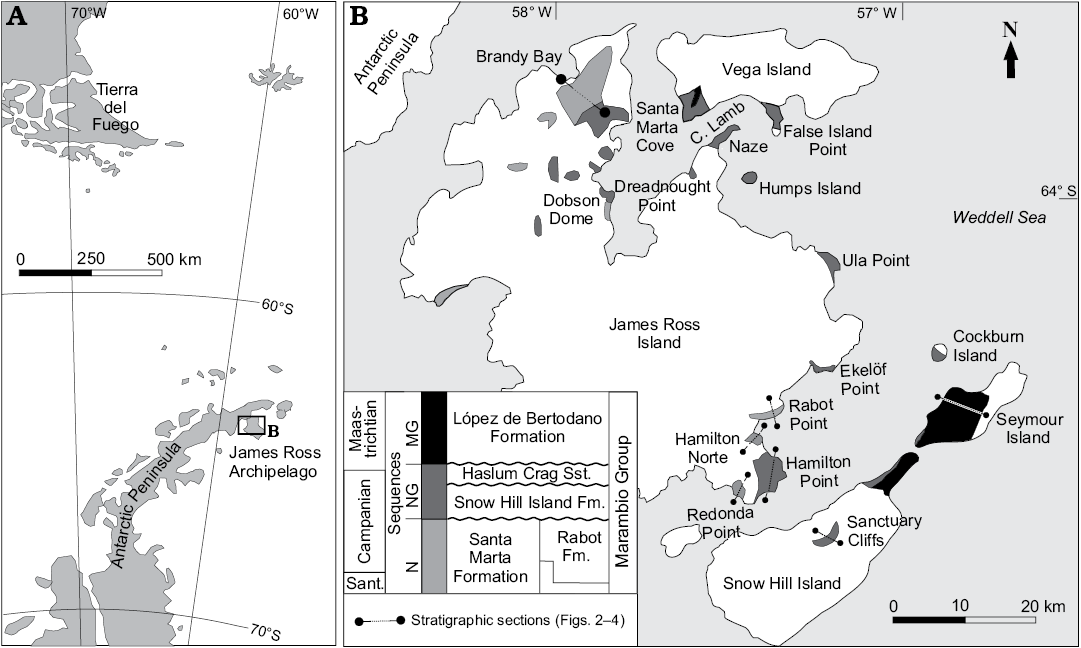
Fig. 1. Location map (A) and geological sketch (B) of the James Ross Basin, Antarctica. Abbreviations: C. Lamb, Cpe Lamb; Fm., Formation; MG, Maorites and Grossouvrites Sequence; N, Natalites Sequence; NG, Neograhamites and Gunnarites Sequence; Sant., Santonian; Sst., sandstone.
The widely exposed NG Sequence comprises the Hamilton Point, Sanctuary Cliffs, Karlsen Cliffs, Cape Lamb, and Gamma members of the Snow Hill Island Formation and the Haslum Crag Sandstone (Olivero 2012b and references therein). The Hamilton Point Member of the Snow Hill Island Formation (Pirrie et al. 1997) is about 500 m thick and it is exposed at Hamilton Point and Ekeloff Point (Fig. 1). The Hamilton Point Member is laterally equivalent to the Gamma Member of the Snow Hill Island Formation (Olivero 2012a, b), exposed in Santa Marta Cove and Dreadnought Point. The overlaying mud-dominated Sanctuary Cliffs Member, around 200 m thick, is exposed in the eponymous nunatak in southern Snow Hill Island (Figs. 1, 3A).
The MG Sequence is conformed by the López de Bertodano Formation (Rinaldi et al. 1978) cropping out mostly in Seymour and Snow Hill islands, with minor exposures at Cape Lamb, Vega Island (Figs. 1, 3B). The stratigraphic distribution of the ammonite species described in this paper is summarized in Figs. 2, 3.
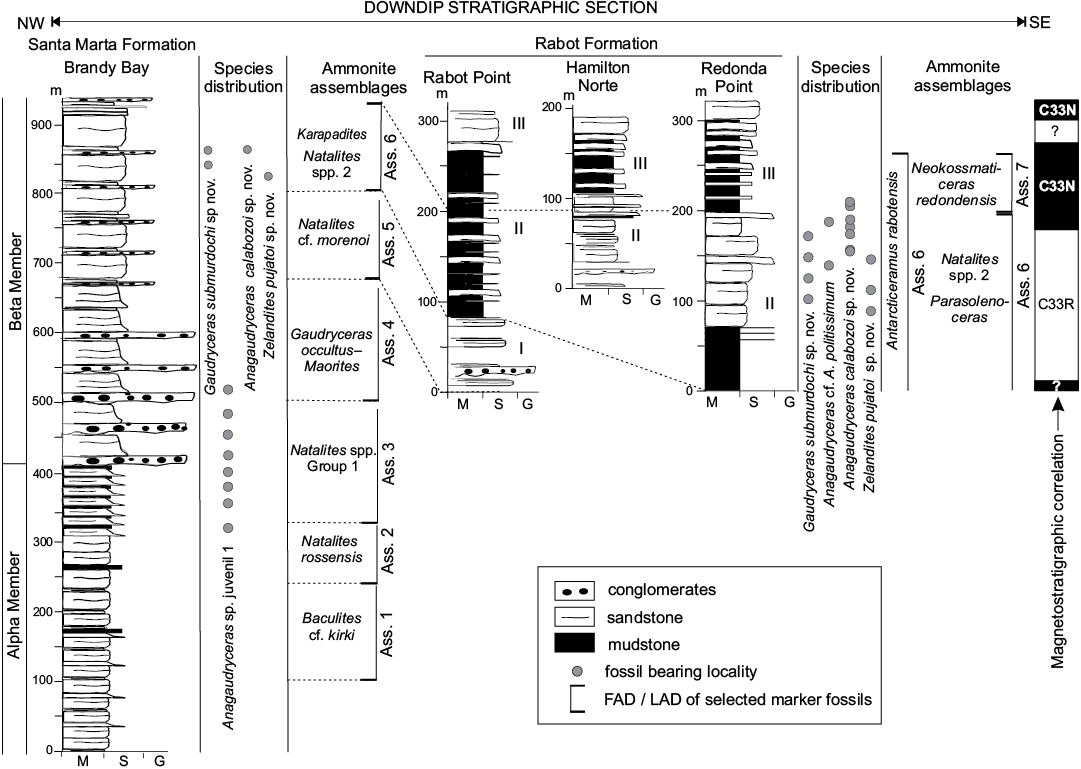
Fig. 2. Biostratigraphy of lytoceratid ammonites from the Santa Marta and Rabot formations, James Ross Island, early–mid-Campanian. Ammonite assemblages by Olivero (2012b), magnetostratigraphic correlation by Milanese et al. (2017a, b). Abbreviations: Ass., ammonite assemblage; G, gravel; I–III, informal names of the members of the Rabot Formation; M, mud; S, sand.

Fig. 3. Biostratigraphy of Anagaudryceras in the Sanctuary Cliffs Member, Snow Hill Island Formation, Antarctica, early Maastrichtian (A) and the López de Bertodano Formation, Seymour Island, late Maastrichtian (B). Ammonite assemblages by Olivero (2012b), magnetostratigraphic correlation for the Snow Hill Island Formation by Milanese et al. (2017a, b) and for the López de Bertodano Formation by Tobin et al. (2012). Abbreviations: A., Anagaudryceras; Ass., ammonite assemblage; Fm., Formation; G, gravel; M, mud; S, sand; Sst., sandstone.
Material and methods
Most of the described material was collected by EBO during several Antarctic field seasons since 1986, and by MER and EBO in the 2011–2015/2017 seasons from Brandy Bay, Redonda Point, Rabot Point, Hamilton Point, Sanctuary Cliffs, and Seymour Island localities. In the Santa Marta and Rabot formations, most of the specimens were found as deposit concentrations within or around pachydiscid body chambers (Olivero 2007; see also Maeda 1991) or large specimens of the inoceramid bivalve Antarcticeramus rabotensis Crame and Luther, 1997 (Olivero and Raffi 2018). In the Sanctuary Cliffs Member of the Snow Hill Island Formation, the specimens are preserved in concretionary accumulations dominated by suites of lytoceratids and gastropods.
For technical terms, dimensions and morphological descriptions we follow Matsumoto (1988, 1995) and Korn (2010), with slight changes in terminology (Table 1).
Table 1. Technical terms, dimensions, and morphological descriptions. Abbreviations: Wb, whorl breadth; Wh, whorl height.
|
Size of shell (diameter) |
Width of umbilicus |
Whorl compression |
|
very small < 25 mm 25 mm < small< 50 mm 50 mm < moderate < 100 mm 100 mm < large < 200 mm 200 < very large < 500 mm huge or gigantic > 500 mm |
very narrow = less than 8% narrow = 8% to 17% fairly narrow = from 17% to 30% moderate = from 30% to 40% fairly wide = from 40% to 50% wide = from 50% to 65% very wide = more than 65% |
Wb/Wh <0.6 = very compressed 0.6 < Wb/Wh <1 = fairly compressed Wb/Wh aprox. = 1 as high as broad 1 < Wb/Wh <1.5 = fairly depressed Wb/Wh > 1.5 = very depressed |
For systematic nomenclature and interpretation for high rank taxonomy we follow the proposal of Hoffmann (2010). Supplementary Online Material (available at http://app.pan.pl/SOM/app64-Raffi_etal_SOM.pdf) of Gaudryceras submurdochi Raffi and Olivero sp. nov. from Antarctica can be found in SOM: fig. 1 (Plot of Wb/Wh against D) and SOM: fig. 2 (Plot of U against D), of Anagaudryceras cf. A. politissimum can be found in SOM: fig. 3 (Plot of Wb/Wh against D). A picture of a huge specimen of Anagaudryceras seymouriense in SOM: fig. 4. Aditional material of the dimorphic pair of Anagaudryceras calabozoi Raffi and Olivero sp. nov. can be found in SOM: fig. 5 (Plot of Wb/Wh against D) and SOM: fig. 6 (Plot of D against U). Also a plot of Wb/Wh against D of Antarctic species of Anagaudryceras can be found in SOM: fig. 7.
Systematic palaeontology
(by M.E. Raffi and E.B. Olivero)
Order Phylloceratida Arkell, 1950
Superfamily Lytoceratoidea Neumayr, 1875
Family Tetragonitidae Hyatt, 1900
Subfamily Gaudryceratinae Spath, 1927
Genus Gaudryceras Grossouvre, 1894
Type species: Ammonites mitis Hauer, 1866, by the subsequent designation of Boule et al. (1906); Gosau Beds of Strobl, near Ischl, Austria, Coniacian.
Diagnosis.—See Kennedy and Klinger 1979: 128 emended by Hoffmann 2015: 16.
Remarks.—The genus has been discussed in our previous work (Raffi and Olivero 2016), here we add the early Campanian species Gaudryceras submurdochi Raffi and Olivero sp. nov. to the Antarctic conspicuous Gaudryceras fauna. Gaudryceras submurdochi Raffi and Olivero sp. nov. is included within the group of Gaudryceras tenuiliratum Yabe, 1903. We initially considered this species in the genus Vertebrites. However, in our new Antarctic Gaudryceras the Vertebrites-like ornamentation (sensu Matsumoto and Yoshida 1979) is accompanied by marked changes in whorl compression and evolution during ontogeny that clearly differs from the typically Vertebrites serpenticone whorl type.
According to Matsumoto and Yoshida (1979) and Hoffmann (2010), the Vertebrites-like ornamentation appears in many species of Gaudryceras. Besides, the extra lobe in the internal suture line of Vertebrites murdochi Marshall, 1926, reported for the first time by Marshall (1926), was misinterpreted and its suture correspond to that of Gaudryceras (see Hoffman 2010: 73 and the bibliography therein). In the last complete review of the family Tetragonitidae, Hoffmann (2010) mentioned Vertebrites murdochi as an endemic paedomorphic member of Gaudryceras rejecting a generic or subgeneric identity of Vertebrites. In this oportunity we desist to give opinion on the validity of Vertebrites as an independent genus, but we concur with Henderson and McNamara (1985) that larger specimens of Vertebrites murdochi are needed to asses its present generic status.
Thus, in addition to the five Antarctic species of Gaudryceras described in Raffi and Olivero (2016), the new species Gaudryceras submurdochi Raffi and Olivero sp. nov. is described for the early Campanian (possible up to the earliest mid-Campanian) of the Santa Marta and Rabot formations. The new material consists of relatively small-sized shells, and many specimens less than 30–40 mm in diameter seem to be juveniles with partly preserved body chambers. Nonetheless, there are three small shells preserving the phragmocone and part of the body chamber with diameters up to 50 mm, which we interpret as adult shells.
Stratigraphic and geographic range.—The genus ranges from the Upper Albian to the Maastrichtian. Its geographical distribution includes Antarctica, New Zealand, Madagascar, South Africa, Angola, north Africa, the Middle East, central and southern Europe, southern India, Japan, Sakhalin, Kamchatka, Alaska, British Columbia, California, Mexico, Chile, and southern Patagonia.
Gaudryceras submurdochi Raffi and Olivero sp. nov.
Fig. 4.
2018 Gaudryceras aff. murdochi (Marshall,1926); Olivero and Raffi 2018: 85.
ZooBank LSID: urn:lsid:zoobank.org:act:C48CBFAB-B76E-44F3-8FC9- 9B85C682BCC8
Etymology: From Latin sub, somewhat; meaning that the new species is somewhat similar to Vertebrites murdochi, the type species of the genus Vertebrites.
Holotype: CADIC PI 415, moderate shell (D 80 mm) with complete phragmocone and incomplete body chamber (Fig. 4A).
Type locality: Redonda Point locality, southeastern James Ross Island, Antarctica.
Type horizon: Informal Member II of the Rabot Formation, Ammonite Assemblage 6 Natalites spp. Group 2; Campanian (Cretaceous).
Material.—A total of 31 specimens that includes 28 small shells partly preserving the phragmocone and body chamber (CADIC PI 418–445) and 3 mostly complete large shells (CADIC PI 415–417). All from type locality and horizon except CADIC PI419, CADIC PI423, CADIC PI431, which are from Brandy Bay locality, Santa Marta Formation and CADIC PI 417 from Hamilton Norte locality, Rabot Formation.
Diagnosis.—Moderate shell, early whorls similar to “Vertebrites” murdochi Marshall, 1926 in ribbing style and shell shape but with less depressed whorl section and denser and more marked constrictions and collars. Body chamber with compressed whorl section ornamented with single, slightly sinuous flat ribs, bearing numerous constrictions preceded by strong collars.
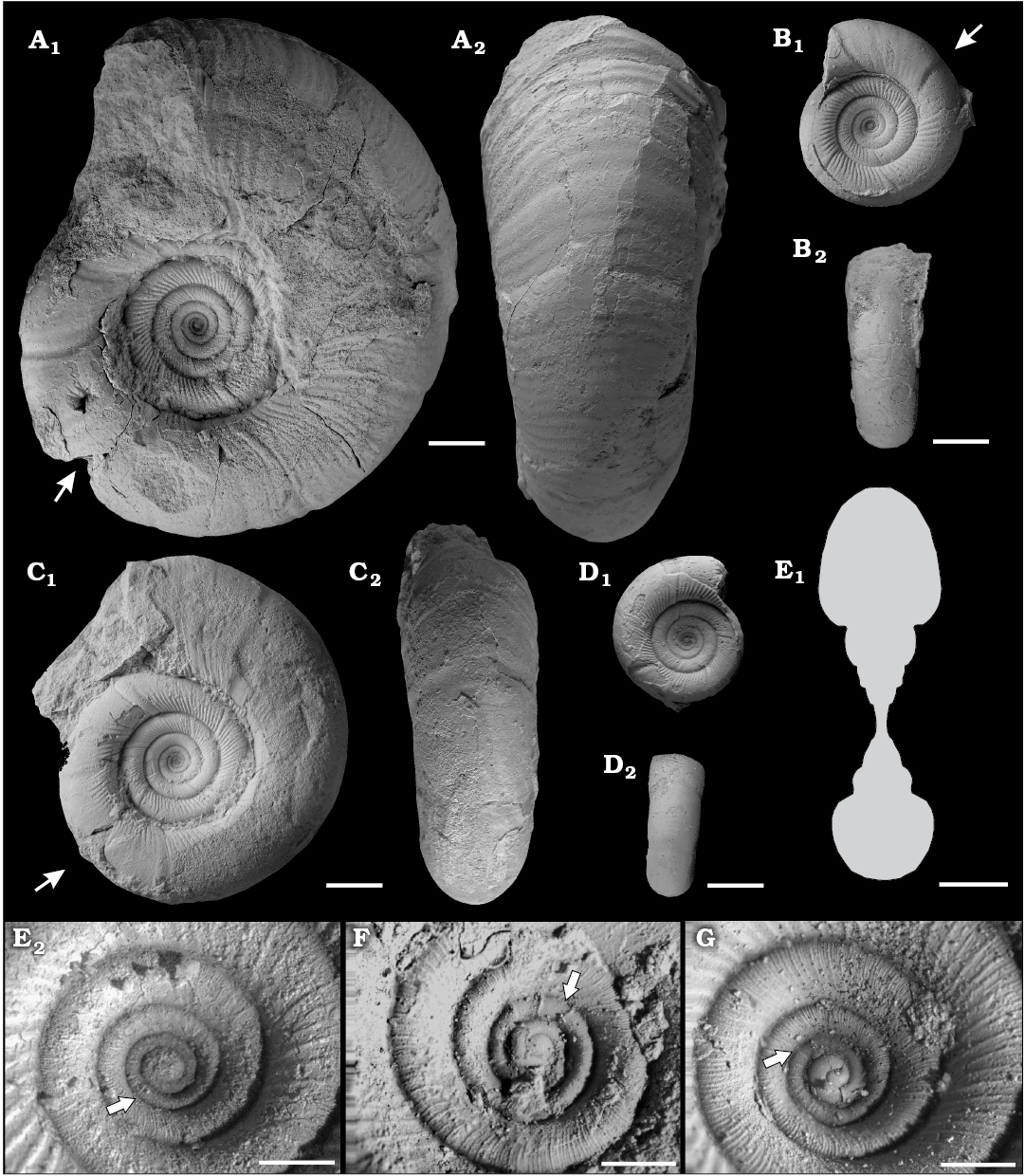
Fig. 4. The gaudryceratid ammonoid Gaudryceras submurdochi Raffi and Olivero sp. nov., early Campanian (Upper Cretaceous), Antarctica, from Rabot Formation, Redonda Point locality (A–D, F) and Hamilton Norte locality (E) and from Santa Marta Formation, Brandy Bay locality (G). A. CADIC PI 416, holotype, phragmocone and part of the body chamber in lateral (A1) and ventral (A2) views. B. CADIC PI 428, phragmocone and part of the body chamber in lateral (B1) and ventral (B2) views. C. CADIC PI 421, phragmocone and part of the body chamber in lateral (C1) and ventral (C2) views. D. CADIC PI 442, phragmocone in lateral (D1) and ventral (D2) views; the arrows mark the beginnig of the body chamber. E. CADIC PI 417, transversal section to a diameter of 57.5 mm (E1), neanoconch ornamentation (E2). F, G. Neanoconch ornamentation. F. CADIC PI 422. G. CADIC PI 431. The arrows point to major ribs in the neanoconch. Scale bars 1 mm, except E2, F, G 10 mm.
Description.—Early growth stages (D up to 40–45 mm): Neanoconch ornamented with more than 10 sharp, strong flares. The coiling is fairly evolute and serpenticone, the whorl section fairly depressed (Wb/Wh ~1.1–1.2; Table 2). The umbilicus is fairly wide to wide (U ~45–55%; Table 2), umbilical wall very short and convex, merging with a broadly rounded umbilical shoulder. Flank convex merging into a broadly rounded venter. Whorl section and coiling becoming progressively less depressed and more involute, respectively, through ontogeny. Ribs coarse and strongly prorsiradiate on main flank, just at or slightly below the whorl contact the ribs split into numerous fine lirae or subcostae, which are not preserved on the internal mold. In a half whorl, there are about 25–30 main ribs near the umbilical shoulder and more than 75–100 on the venter. Four to five deeply incised constrictions preceded by strong, very wide collars per whorl. The constrictions are best marked in the internal mold and the collars are only visible when the test is preserved. Suture line is typical for the genus.
Later growth stage (D up to 80 mm): The coiling is progressively more involute, with the umbilicus of the largest shell (D 80 mm) of moderate width (U 34%; Table 2). Whorl section nearly as high as broad to fairly compressed (Wb/Wh ~1.02–0.81; Table 2). The dense splitting of the ribs is progressively suppressed and only single ribs are preserved in the last part of the phragmocone and body chamber. Single ribs are first prorsiradiate and then change to moderately sinuous on flank and slightly projected on venter. Body chamber with 6–7 sinuous, well-marked constrictions and collars per half whorl.
Table 2. Dimensions (in mm) of Gaudryceras submurdochi sp. nov. Abbreviations: D, diameter; U, umbilical diameter; %U, umbilical diameter as % of D; Wb, whorl breadth at a given D; Wh, whorl height at a given D.
| |
D |
Wh |
Wb |
Wb/Wh |
U |
%U |
|
CADIC PI 415 |
80.4 |
38.1 |
31.0 |
0.8 |
27.0 |
33.6 |
|
CADIC PI 416 |
60 |
22 |
21.8 |
1 |
27.0 |
45.0 |
|
CADIC PI 417 |
57.6 |
22.6 |
18.8 |
0.8 |
24.5 |
42.4 |
|
CADIC PI 418 |
49.7 |
18.9 |
18.2 |
1.0 |
21.2 |
42.7 |
|
CADIC PI 419 |
46.6 |
17.7 |
18.1 |
1.0 |
22.8 |
48.9 |
|
CADIC PI 420 |
44 |
17.1 |
15.5 |
0.9 |
19.6 |
44.5 |
|
CADIC PI 421 |
38.6 |
12.7 |
|
|
|
|
|
CADIC PI 422 |
38 |
12.5 |
14.3 |
1.1 |
19.7 |
51.8 |
|
CADIC PI 423 |
36.7 |
12.2 |
13.6 |
1.1 |
17 |
46.3 |
|
CADIC PI 424 |
36.5 |
12.1 |
13.1 |
1.1 |
18.2 |
49.9 |
|
CADIC PI 425 |
36.3 |
12.8 |
14.5 |
1.1 |
17.9 |
49.3 |
|
CADIC PI 426 |
34.5 |
10.0 |
12.5 |
1.2 |
17.7 |
51.3 |
|
CADIC PI 427 |
32.7 |
10.8 |
12 |
1.1 |
15.9 |
48.5 |
|
CADIC PI 428 |
30.5 |
9.0 |
11.4 |
1.3 |
15.7 |
51.3 |
|
CADIC PI 429 |
27.6 |
8.8 |
9.8 |
1.1 |
13.8 |
50 |
|
CADIC PI 430 |
25.8 |
8 |
9.6 |
|
12.7 |
|
|
CADIC PI 431 |
24.7 |
8.1 |
9.6 |
1.2 |
12.5 |
50.6 |
|
CADIC PI 432 |
24.2 |
7.5 |
8.4 |
1.1 |
13.2 |
54.5 |
|
CADIC PI 433 |
23.3 |
7.7 |
9 |
|
11.2 |
|
Remarks.—The ribbing style, coiling, and whorl sections of the early growth stages are very similar to that of Vertebrites murdochi from the Upper Cretaceous of New Zealand (Marshall 1926) and possibly New Caledonia (Henderson 1970), but Gaudryceras submurdochi Raffi and Olivero sp. nov. has less depressed whorl section at corresponding diameters (SOM: fig. 1), its adult body chamber is more involute (SOM: fig. 2) and is ornamented with single, slightly sinuous ribs, constrictions and collars. Vertebrites is doubtfully recognized as a separate gaudryceratid genus or subgenus (Henderson and McNamara 1985) and this is based mainly on the assumption that Vertebrites murdochi may represent an adult specimen, preserving in its last whorl the typical wire-like ornament, depressed whorl section, and serpenticone coiling characterizing its early whorls. We concur with Henderson and McNamara (1985) that larger specimens of Vertebrites murdochi are needed to confirm its present generic status. In addition, independently of the validity of the genus Vertebrites, the change during ontogeny from evolute coiling and depressed whorl section to more involute coiling and compressed whorl section in G. submurdochi sp. nov. clearly indicate that this species correspond to the genus Gaudryceras (see Kennedy and Klinger 1979; Hoffmann 2010).
The inner whorls of Gaudryceras hamanakense Matsumoto and Yoshida, 1979 from the Maastrichtian of Hokkaido, Japan and Sakhalin, Russia (Matsumoto and Yoshida 1979; Maeda et al. 2005) are similar to G. submurdochi sp. nov., but the former has finer ribs that continue into the body chamber. At corresponding diameters, G. hamanakense is also more depressed than G. submurdochi sp. nov.
Stratigraphic and geographic range.—Early Campanian of the Beta Member, Santa Marta Formation, and early Campanian (possible up to the earliest mid-Campanian) of the Rabot Formation, James Ross Island, Antarctica. Ammonite Assemblage 6 Natalites spp. Group 2.
Genus Anagaudryceras Shimizu, 1934
Type species: Ammonites sacya Forbes, 1846, by original designation of Shimizu (1934: 67), subjective synonym of Ammonites buddha Forbes (1846:112, pl. 14: 9), Albian of Southern India.
Remarks.—For synonymy and diagnosis see Hoffmann 2015: 17 and references therein.
The original type material, Ammonites sacya Forbes, 1846, designated by Shimizu (1934) is a poorly preserved juvenile specimen that put on display nomenclatural problems and the validity of Anagaudryceras as genus. Wright and Matsumoto (1954) conducted a comprehensive review of the genus, with subsequent interpretations by several authors (Matsumoto 1959; Wiedmann 1962; Howarth 1965; Haggart 1989; Kennedy and Klinger 1979; and Hoffman 2010). Stoliczka (1865) synonimized Ammonites buddha with Ammonites sacya. Afterward, Whiteaves (1884) and Kossmat (1895) treated Ammonites buddha as subjective junior synonym. Subsequent authors (eg., Matsumoto 1959, 1995; Henderson and McNamara 1985; Hoffman 2010) accepted the synonymy and treated Ammonites buddha as the adult stage of Ammonites sacya. However, we follow Matsumoto (1995) who according to the ICZN Article 24 (precedence of the names or acts is fixed by the First Reviser, in this case Stoliczka 1865) proposed to call this species A. sacya.
Arkell et al. (1957), Luppov and Drushchits (1958) and Wright et al. (1996) treated Anagaudryceras as an independent genus. Wiedmann (1962) placed the genus Anagaudryceras with Gaudryceras in synonymy. Although, Gaudryceras and Anagaudryceras present a similar type of suture (Schindewolf 1961), Anagaudryceras has finer and weaker ornamentation compared to Gaudryceras what is sufficient to a generic distinction (Howarth 1965). Kennedy and Klinger (1979) argued that if the synonymy between Ammonites sacya and Ammonites buddha is valid, Anagaudryceras is different enough from other gaudryceratids to be treated as separate genus. We concur with this statement and disagree with the taxonomic interpretation of Wiedmann (1962).
Kennedy and Klinger (1979) grouped the species of Anagaudryceras in two main groups: the group of Anagaudryceras buddha (= A. sacya) with strong ribs in the body chamber; and the group of Anagaudryceras involvulum (Stoliczka, 1865), with a weak ornamentation throughout ontogeny, weak constrictions and without ribs in the body chamber. However, Matsumoto (1995) does not accept this grouping, neglecting particularly the group of A. involvulum on the basis that many of the included species bear narrow and strong ribs separated by wide interspaces in the body chamber. He concluded that the succession of Albian–Turonian species with band-like ribs separated by narrow grooves (e.g., A. sacya) followed by Coniacian to Maastrichtian species with narrow and strong ribs separated by wide interspaces suggests an evolutionary change (Matsumoto 1995). However, the macroconch of Anagaudryceras calabozoi Raffi and Olivero sp. nov., from the mid-Campanian of the Rabot Formation, has band-like ribs at the adult body chamber gainsaying Matsumoto’s (1995) concept.
Stratigraphic and geographic range.—The genus is known from middle Albian to Maastrichtian. The geographic distribution includes Antarctica, New Zealand, Zululand, Madagascar, Angola, north Africa, France, Germany, Austria, Romania, southern India, Japan, Sakhalin, Kamchatka, Alaska, British Columbia, and California
Anagaudryceras sp. juvenile 1
Fig. 5C, D.
Material.—11 internal molds preserving patches of the shell and including the phragmocone and part of the body chamber (CADIC PI 533–542, 548). From early Campanian (Cretaceous), upper part of the Ammonite Assemblage 2 Natalites rossensis to lower part of Ammonites Assemblage 4 Grossouvrites occultus–Maorites, upper part of the Alpha Member and lower part of the Beta Member, Santa Marta Formation, James Ross Island, Antarctica.
Description.—Evolute coiling and fairly compressed (Wb/Wh ~0.82–0.94; Table 3) whorl section. Wide umbilicus (U ~35%; Table 3), slightly gradual umbilical wall, and rounded umbilical shoulder. The flanks converge to a well defined ventrolateral shoulder and rounded and slightly arched venter. The shell becoming less evolute as the diameter increases. Early-whorl ornamentation almost imperceptible to the naked eye, but consists of very fine and prorsiradiate lirae accompanied by slightly sinuous constrictions preceded by collars. At the body chamber the ornamentation becomes stronger with several collars and intercalate ribs.
Table 3. Dimensions (in mm) of Anagaudryceras sp. juvenile 1 and Anagaudryceras cf. politissimum. Abbreviations: D, diameter; U, umbilical diameter; %U, umbilical diameter as % of D; Wb, whorl breadth at a given D; Wh, whorl height at a given D.
| |
D |
Wb |
Wh |
Wb/Wh |
U |
%U |
|
Anagaudryceras sp. juvenile 1 |
||||||
|
CADIC PI 533 |
38 |
15 |
18.4 |
0.82 |
11 |
28.9 |
|
CADIC PI 534 |
31.8 |
12.6 |
14.2 |
0.89 |
11 |
34.6 |
|
CADIC PI 535 |
28.8 |
|
13 |
|
8.8 |
30.6 |
|
CADIC PI 536 |
26 |
9.8 |
10.6 |
0.92 |
8.4 |
32.3 |
|
CADIC PI 537 |
25.8 |
10.5 |
9.2 |
1.14 |
9.4 |
36.4 |
|
CADIC PI 538 |
25.6 |
10 |
10.6 |
0.94 |
9 |
35.2 |
|
CADIC PI 539 |
26.3 |
9.2 |
11.3 |
0.81 |
8.9 |
33.8 |
|
CADIC PI 540 |
18.6 |
8.5 |
8.3 |
1.02 |
6 |
32.3 |
|
CADIC PI 541 |
25.6 |
9.1 |
9.5 |
0.96 |
10 |
39.1 |
|
CADIC PI 542 |
16.5 |
7.3 |
5.5 |
1.33 |
7.1 |
43.0 |
|
CADIC PI 548 |
21 |
9 |
8.8 |
1.02 |
7.6 |
36.2 |
|
Anagaudryceras cf. A. politissimum |
||||||
|
CADIC PI 454 |
70 |
20.4 |
27.5 |
0.74 |
25.7 |
36.7 |
|
CADIC PI 455 |
68.8 |
19.8 |
27.9 |
0.71 |
24.4 |
35.5 |
|
CADIC PI 180 |
68.7 |
21.6 |
27 |
0.8 |
25 |
36.4 |
Remarks.—Even though our specimens are small juveniles, their style of ornamentation differ from the other species of Anagaudryceras described herein. Anagaudryceras subtilineatum (Kossmat, 1895) from the Campanian of India present fine lirae and infrequent weak constrictions preceded by collars but is fairly depressed (Wb/Wh ~1.4) at diameters of 15–45 mm (see Kossmat 1895: 123; Kennedy and Klinger 1979: 156, and Henderson and McNamara 1985: 45).
Anagaudryceras cf. A. politissimum (Kossmat, 1895)
Figs. 5A, B, 6C.
Material.—Three internal molds preserving patches of the shell and including the phragmocone and part of the body chamber (CADIC PI 180, 454, 455). From early Campanian of the Rabot Formation, Ammonite Assemblage 6 Karapadites–Natalites spp. Group 2, Redonda Point, Member II, James Ross Island, Antarctica.
Description.—The coiling is fairly evolute, the whorl section is fairly compressed (Wb/Wh ~0.71–0.74; Table 3). Wide umbilicus (U ~35%; Table 3) with a slightly gradual umbilical wall and rounded umbilical shoulder. The flanks converge to a well-defined ventrolateral shoulder and rounded and slightly arched venter. The ornamentation is almost imperceptible to the naked eye, but consists of fine lirae accompanied by sinuous constrictions preceded by flat collars.
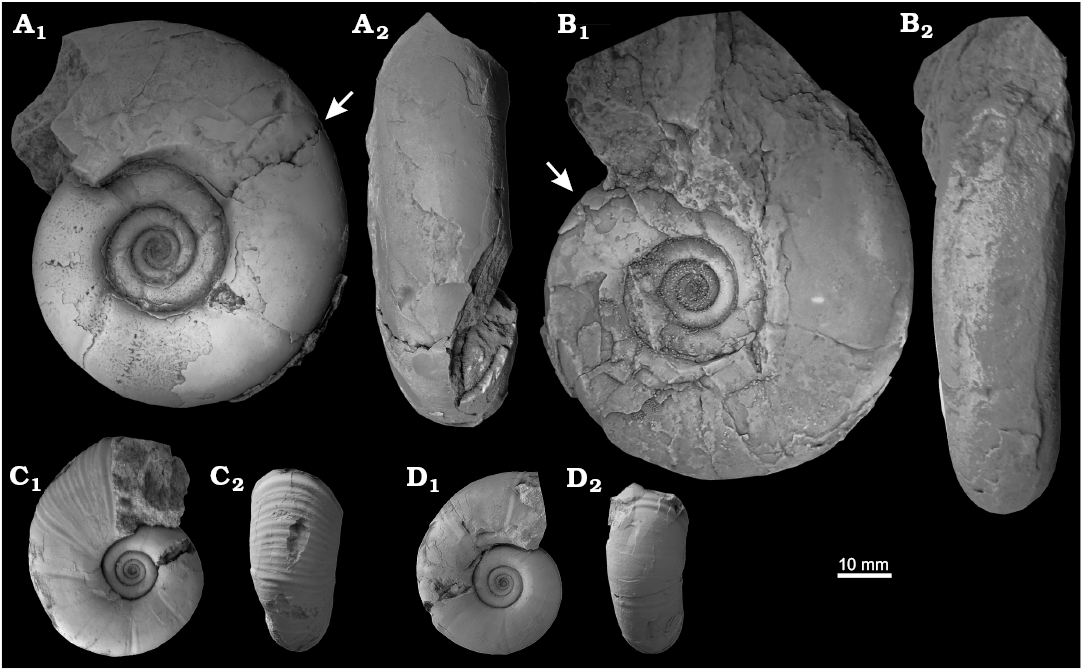
Fig. 5. The gaudryceratid ammonoid Anagaudryceras, early Campanian (Upper Cretaceous) of Antarctica, from Rabot Formation, Redonda Point locality (A, B) and from Santa Marta Formation, Brandy Bay locality (C, D). A, B. Anagaudryceras cf. A. politissimum (Kossmat, 1895). A. CADIC PI 455. B. CADIC PI 454. C, D. Anagaudryceras sp. juvenile 1. C. CADIC PI 533. D. CADIC PI 534. In lateral (A1–D1) and ventral (A2–D2) views. Arrows mark the beginning of the body chamber.
Remarks.—The ornamentation style, compressed whorl section and slightly arched venter of the three specimens discussed here closely resemble Anagaudryceras politissimum (Kossmat, 1895) from the Turonian–Santonian of India, Santonian of Zululand and Maastrichtian of Madagascar (Kossmat 1895; Kennedy and Klinger 1979). Killian and Reboul (1909: 14, pl. 1: 7) refered A. politissimum from Snow Hill Island (Karlsen Cliffs Member), however, this material is less compressed and has a narrower venter. Ifrim et al. (2004: 1590–1592; text-figs. 3I, J, 6D, E, I) referred juveniles specimens from northeastern Mexico to A. politissimum, but they are too small (D ~8.6–25 mm) for proper identification. Anagaudryceras cf. A. politissimum closely resambles A. politissimum from Central Chile (Salazar et al. 2010) in its style of ornamentation but is less compresed (SOM: fig. 3). Anagaudryceras yamashitai (Yabe, 1903), from the Coniacian–?early Campanian of Hokkaido, has similar whorl section and ornamentation style of thin and flexuous lirae to Anagaudryceras cf. A. politissimum. However, A. yamashitai presents neither collars nor constrictions, which are typical of A. politissimum (Kennedy and Klinger 1979). Thus, our specimens have consistent ornamentation similarities with A. politissimum, but they are more compressed than Kossmat’s holotype (SOM: fig. 3).
Anagaudryceras seymouriense Macellari, 1986
2015 Gaudryceras cf. seymouriense (Macellari, 1986); Shigeta et al. 2015: 115, fig. 8.
For a complete synonymy list see Klein et al. (2009).
Holotype: OSU 38333, complete phragmocone of 236 mm of D, preserving patches of the original shell, designated by Macellari (1986: figs. 9.1–9.2).
Type locality: Seymour Island, Antarctica.
Type horizon: Late Maastrichtian (cretaceous), Unit 8, López de Bertodano Formation.
Material.—One almost complete specimen with complete phragmocone and incomplete body chamber (CADIC PI 446), three phragmocones (CADIC PI 447–449) and three fragments of body chamber (CADIC PI 450–452). From the type locality and horizon.
Description.—Early growth stage (D up to 40 mm): The coiling is evolute with fairly depressed (Wb/Wh ~1.13–1.28) whorl section and greatest width occurring slightly below mid flank. The umbilicus is wide, with a slightly vertical wall and rounded umbilical shoulder. The flanks converge to a rounded ventrolateral shoulder and slightly rounded venter. The ornamentation consists of very fine flexous lirae that arise straight from the umbilical seam, bend forward to the umbilical shoulder and bends backward at the ventrolateral shoulder. In addition, there are intercalate lirae that reach to mid flank. Collars with superimposed lirae are occasionally observed. The neanoconch is smooth.
Mid growth stage (D 40–90 mm): As the diameter increases, the coiling becomes less evolute and the whorl section becomes compressed (Wb/Wh ~0.91). Subparallel flanks that converge to a slightly rounded ventrolateral shoulder and arched venter. In addition to the lirae are collars (constrictions of Macellari 1986).
Later growth stage (D more than 90 mm): As diameter increases the coiling becomes more involute with a narrower umbilicus (Table 4), and the ornamentation more delicate. The very fine lirae arise almost rectiradiate from the umbilical seam and prorsiradiate beyond the umbilical shoulder. Huge specimens, diameter larger than 600 mm, preserving the shell show wide flat-top ribs at the body chamber (see SOM: fig. 4).
Remarks.—Macellari (1986) mentioned that A. seymouriense has intermediate characters between Gaudryceras and Anagaudryceras, but places it within the genus Anagaudryceras by its weak ornamentation and change in whorl-shape (from a depressed evolute to a compressed and involute shell), although it presents prominent constrictions at huge diameters. Shigeta et al. (2015) placed A. seymouriense within the genus Gaudryceras because of its ornamentation style. However, the Macellari’s (1986) species perfectly match all the Anagaudryceras features: early rounded to depressed whorl section, which may become compressed in later growth stages; early and middle growth stages with very fine lirae and periodic collar ribs and frequent ornamentation changes on the body chamber (Kennedy and Klinger 1979) and thus, we kept it in Anagaudryceras. Furthermore, our material preserves the original aragonite shell, and the ornamentation in all growth stages does not resemble any known species of Gaudryceras.
Stratigraphic and geographic range.—Late Maastrichtian of the López de Bertodano Formation, Ammonite Assemblages 12 to 14, Seymour Island, Antarctica, Maastrichtian of Hobetsu area, Japan and Maastrichtian of Makarov area and Naiba area, Russia.
Anagaudryceras calabozoi Raffi and Olivero sp. nov. [M and m]
Figs. 6A, B, 7, 8, 9A.
1992 Anagaudryceras subsacya (Marshall, 1926); Marenssi et al. 1992: 92.
2018 Anagaudryceras sp.; Olivero and Raffi 2018: 86, fig. 7H.
ZooBank LSID: urn:lsid:zoobank.org:act:366EF942-7214-40C1-9FB0- 0E0E52085EC5
Etymology: In memory of Fernando Calabozo (1986–2016), an outstanding Argentinian geologist of Antarctica and friend, whose early death is much regretted.
Type material: Holotype (CADIC PI 411), large macroconch (D 102 mm) with complete phragmocone and incomplete body chamber (Fig. 7A). Paratype (CADIC PI 472), moderate-large microconch (D max 93.7 mm) with complete phragmocone and incomplete body chamber (Fig. 8A).
Type locality: Redonda Point locality, southeastern James Ross Island, Antarctica.
Type horizon: Mid-Campanian (Cretacaous), from the base of the informal Member III of the Rabot Formation, Ammonite Assemblage 7.
Material.—A total of 53 specimens that include 10 internal molds of macroconchs [M] with phragmocone and body chamber, preserving patches of the shell (CADIC PI 296, 411, 456–463) and 8 fragments of internal molds [M] (CADIC PI 464–471), 11 internal molds of microconchs [m] with phragmocone and body chamber preserving patches of the shell, (CADIC PI 472−481, 493), 12 fragments of body chamber (CADIC PI 494–505); 12 internal molds of juvenile specimens with phragmocone and part of the body chamber preserving patches of the shell (CADIC PI 302, 482−492). All from type locality and horizon. Fragment of body chamber collected 1956–1975 by Antonio Cañon Martinez in Magallanes Basin Chile (ACM-118).
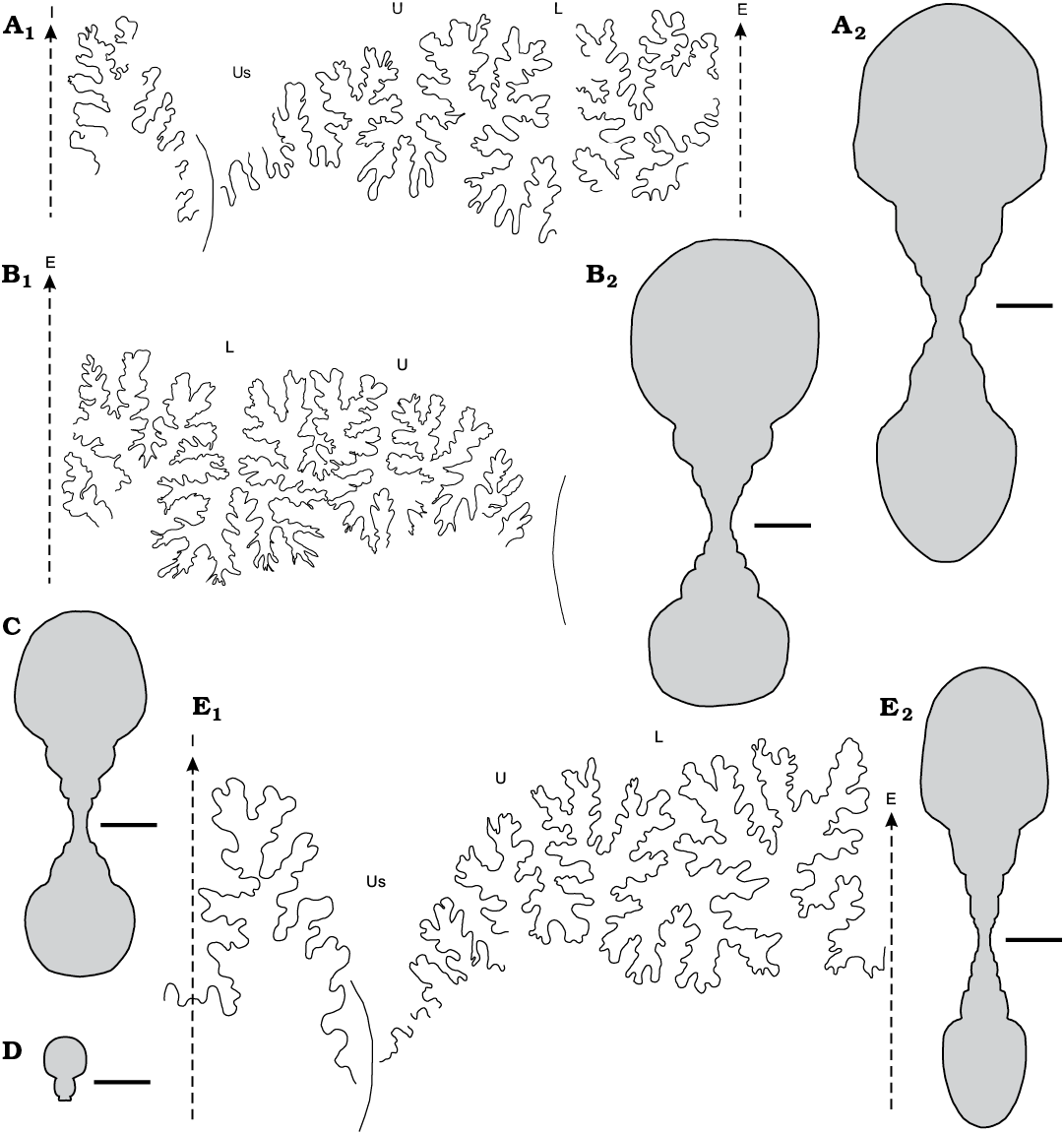
Fig. 6. The gaudryceratid ammonoid Anagaudryceras spp., from the Campanian (Cretaceous), Antarctica, Rabot Formation, Redonda Point locality (A–C), and from the late Campanian–early Maastrichtian (Cretaceous), Antarctica, Snow Hill Island Formation, Sanctuary Cliffs locality (D, E). A, B. Anagaudryceras calabozoi Raffi and Olivero sp. nov. A. CADIC PI 411, holotype, adult macroconch; suture line (A1), transversal section (A2). B. CADIC PI 472, adult microconch; suture line (B1), transversal section (B2). C. Anagaudryceras cf. A. politissimum (Kossmat, 1895), CADIC PI 455, transversal section. D. Anagaudryceras sanctuarium Raffi and Olivero sp. nov., CADIC PI 604, transversal section. E. Anagaudryceras subcompresum Raffi and Olivero sp. nov., CADIC PI 506, holotype, adult specimen; suture line (E1), transversal section (E2). Scale bars 10 mm. Abbreviations: E, external lobe; I, internal lobe; L, lateral lobe; U, umbilical lobe; Us, septal lobe.
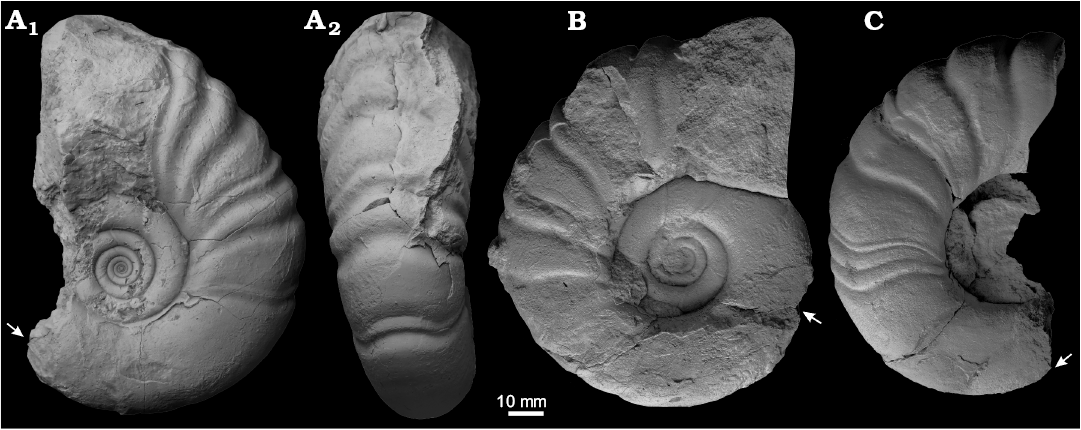
Fig. 7. Macroconchs of the gaudryceratid ammonoid Anagaudryceras calabozoi Raffi and Olivero sp. nov., from early Campanian (Cretaceous), Antarctica, Rabot Formation, Redonda Point locality (A) and Hamilton Norte locality (B, C). A. CADIC PI 411, holotype, phragmocone and part of the body chamber in lateral (A1) and ventral (A2) views. B. CADIC PI 456, phragmocone and part of the body chamber in lateral view. C. CADIC PI 464, phragmocone and part of the body chamber in lateral view. Arrows mark the beginning of the body chamber.
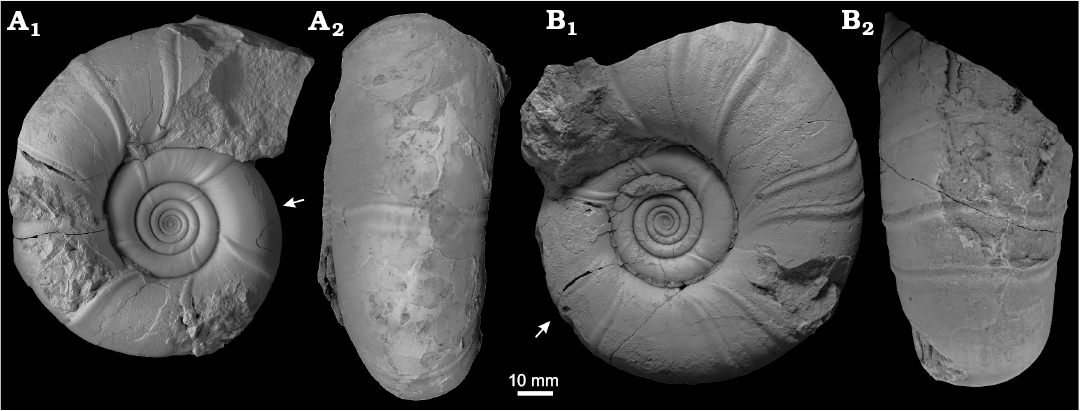
Fig. 8. Microconchs of the gaudryceratid ammonoid Anagaudryceras calabozoi Raffi and Olivero sp. nov. from early Campanian (Cretaceous), Antarctica, Rabot Formation, Redonda Point locality. A. CADIC PI 472, holotype, phragmocone and part of the body chamber in lateral (A1) and ventral (A2) views. B. CADIC PI 473, phragmocone and part of the body chamber in lateral (B1) and ventral (B2) views. Arrows mark the beginning of the body chamber.
Diagnosis.—Moderate to large shell, compressed whorl section with dense and prorsiradiate lirae, slightly bifurcated, accompanied by four or five collars per whorl. Neanoconch with five or less ribs accompanied by fine lirae. Macroconch body chamber with band-like ribs that become wider towards the venter, where they project aperturally. Microconch body chamber with two types of ornamentation, strong asymmetrical ribs, slightly flexuous, preceded by constrictions and almost imperceptible spiral ornamentation.
Description—Early and mid growth stage (D up to 65 mm): The coiling is evolute, with fairly depressed whorl section (Wb/Wh ~1.07–1.27; Table 4). The umbilicus is moderately wide (U ~45%; Table 4) and shallow, with rounded umbilical wall and umbilical shoulder. The flanks are subparallel and mildly rounded but slightly convergent to a moderate rounded venter. The ornamentation in the juvenile shell can be clearly differentiated in two stages. The neanoconch presents five or less ribs accompanied by fine lirae. Beyond the neanoconch, the ornamentation consists of fine, flexuous, prorsiradiate lirae with some bifurcation and intercalate lirae at the first third of the flank and into the ventrolateral shoulder. Accompanying the lirae and parallel to them, there are up to five collars per whorl. The ribs are markedly asymmetrical and projected slightly aperturally on the venter.
Later growth stage (D more than 65 mm): Macroconch [M], as the diameter increases, the whorl section becomes more compressed (Wb/Wh ~0.80; SOM: fig. 5; Table 4), with flanks that converge to a less rounded ventrolateral shoulder and slightly arched venter. The ornamentation in the body chamber consist of flexuous broad ribs (band-like ribs of Kennedy and Klinger 1979), that becomes wider towards the venter, where they project aperturally. Each rib is preceded by a marked constriction that runs parallel to the rib following its shape. As the shell diameter increases, the ribs become less distant. Microconch [m], the coiling becomes slightly more involute at 75–90 mm of diameter (U ~36%; Table 4; SOM: fig. 6), the whorl section is fairly depressed (SOM: fig. 5) or as high as broad (Wb/Wh ~1.18–1.03; Table 4), with umbilical and ventrolateral shoulders very rounded. At the body chamber there are strong asymmetrical ribs, slightly flexuous, preceded by constrictions and almost imperceptible longitudinal striation, consisting of an alternation of fine ridges and broad and shallow sinus.
Table 4. Dimensions (in mm) of Anagaudryceras seymouriense and Anagaudryceras calabozoi sp. nov. Type material is indicated in bold. Abbreviations: D, diameter; Dmax, maximum diameter; U, umbilical diameter; %U, umbilical diameter as % of D; Wb, whorl breadth at a given D; Wh, whorl height at a given D.
| |
Dmax |
D |
Wb |
Wh |
Wb/Wh |
U |
%U |
|
Anagaudryceras seymouriense |
|||||||
|
CADIC PI 446 |
350 |
250 |
116.5 |
123 |
0.94 |
52 |
20.8 |
|
Anagaudryceras calabozoi sp. nov. |
|||||||
|
CADIC PI 456 [M] |
105.7 |
105.7 |
35 |
43 |
0.81 |
|
0.0 |
|
CADIC PI 457 [M] |
105 |
105 |
|
|
|
|
0.0 |
|
CADIC PI 411 [M] |
102 |
102 |
35 |
40 |
0.88 |
34 |
33.3 |
|
CADIC PI 458 [M] |
99.4 |
99.4 |
33 |
41.2 |
0.80 |
33.1 |
33.3 |
|
CADIC PI 296 [M] |
93.7 |
93.7 |
30.8 |
38 |
0.81 |
31.4 |
33.5 |
|
CADIC PI 459 [M] |
93 |
93 |
27 |
37 |
0.73 |
31.8 |
34.2 |
|
CADIC PI 460 [M] |
91 |
91 |
28 |
35 |
0.80 |
32 |
35.2 |
|
CADIC PI 461 [M] |
81.8 |
81.8 |
27.8 |
31 |
0.90 |
28.8 |
35.2 |
|
CADIC PI 462 [M] |
|
|
28 |
34 |
0.82 |
|
|
|
CADIC PI 463 [M] |
|
|
31 |
39 |
0.79 |
|
|
|
CADIC PI 472 [m] |
93.7 |
80.2 |
33.4 |
28.3 |
1.18 |
32 |
39.9 |
|
CADIC PI 473 [m] |
91.6 |
91.6 |
39.4 |
37.2 |
1.06 |
33.3 |
36.4 |
|
CADIC PI 474 [m] |
85.3 |
95.3 |
34.2 |
33.3 |
1.03 |
30.7 |
32.2 |
|
CADIC PI 475 [m] |
92.9 |
92.9 |
35.3 |
37.4 |
0.94 |
30.5 |
32.8 |
|
CADIC PI 476 [m] |
96.5 |
93 |
33.7 |
38.8 |
0.87 |
33.4 |
35.9 |
|
CADIC PI 477 [m] |
83 |
71.4 |
28.3 |
26.1 |
1.08 |
29.9 |
41.9 |
|
CADIC PI 478 [m] |
74.8 |
74.8 |
31.4 |
27.1 |
1.16 |
30.9 |
41.3 |
|
CADIC PI 479 [m] |
84.8 |
82.7 |
32.7 |
30.9 |
1.06 |
31.7 |
38.3 |
|
CADIC PI 480 [m] |
76.3 |
76.3 |
26.7 |
28.8 |
0.93 |
27.7 |
36.3 |
|
CADIC PI 481 [m] |
|
65.3 |
28.8 |
27 |
1.07 |
28 |
42.9 |
|
CADIC PI 493 [m] |
69 |
69 |
|
|
|
27 |
39.1 |
|
CADIC PI 482 |
|
62.2 |
26.7 |
21.4 |
1.25 |
26.2 |
42.1 |
|
CADIC PI 302 |
61.6 |
57.9 |
20.4 |
21.4 |
0.95 |
25.8 |
44.6 |
|
CADIC PI 483 |
65.9 |
65.9 |
27.2 |
24 |
1.13 |
28 |
42.5 |
|
CADIC PI 384 |
63 |
61 |
25 |
21.8 |
1.15 |
26.8 |
43.9 |
|
CADIC PI 485 |
48.7 |
48.7 |
20.4 |
16.8 |
1.21 |
21.9 |
45.0 |
|
CADIC PI 486 |
50 |
50 |
22.9 |
19.5 |
1.17 |
21.7 |
43.4 |
|
CADIC PI 487 |
49.5 |
46 |
19 |
15.8 |
1.20 |
20.5 |
44.6 |
|
CADIC PI 488 |
46.5 |
46.5 |
17.2 |
15.8 |
1.09 |
23.1 |
49.7 |
|
CADIC PI 489 |
39.6 |
39.6 |
16 |
13 |
1.23 |
18.3 |
46.2 |
|
CADIC PI 490 |
35.8 |
35.8 |
14.5 |
11.4 |
1.27 |
16.9 |
47.2 |
|
CADIC PI 491 |
40.5 |
40.5 |
16.7 |
14.9 |
1.12 |
18.2 |
44.9 |
|
CADIC PI 492 |
40 |
40 |
16.2 |
13 |
1.25 |
19.2 |
48.0 |
Remarks.—The most notable aspect of our collection is that the two markedly different morphotypes of Anagaudryceras have the same stratigraphic range, and have more or less identical early developmental stage but differs in adult morphology. These facts, plus a similar ratio of specimens of each morphotype, are strong evidences of sexual dimorphism. The micro- and macroconchs of Anagaudryceras calabozoi Raffi and Olivero sp. nov. are morphologically almost identical and cannot be discriminated from each other at 60–65 mm shell diameter or smaller. Unfortunately, the preservation of the peristome in Antarctic lytoceratids is extremely rare, and consequently we do not have lappets that would confirm the microconch condition. Nonetheless our specimens present a remarkable differentiation in adult sizes.
Anagaudryceras calabozoi Raffi and Olivero sp. nov. has intermediate morphological characters between Anagaudryceras sacya and Anagaudryceras subsacya (Marshall, 1926). A. sacya has six ribs per whorl, covered by fine lirae in the inner whorls and, flexuous, flattened band-like ribs on the mature body chamber. This completely different style of ornamentation at the adult body chamber, concurs with macroconch ornamentation of A. calabozoi Raffi and Olivero sp. nov. but ribs are wider. A. subsacya, from the Santonian–Campanian of Natal and early to mid-Campanian of New Zealand, also presents two types of ornamentation. However, its internal whorls have five ribs that increase in number in the body chamber, with a slight aperturally projection on the venter. Anagaudryceras compressum Shigeta and Nishimura, 2014, from the lower Maastrichtian of Hokkaido, also has band-like ribs in the adult body chamber. However, they are narrower and less flexuous, and have a more compressed whorl section (Wb/Wh ~0.65). Similar flattened but wider ribs are observed on the last whorl of A. matsumotoi Morozumi, 1985, from the upper Maastrichtian (Maeda et al. 2005; Shigeta and Nishimura 2014). The Maastrichtian Anagaudryceras lueneburgense (Schlüter, 1872) has dense flexuous ribs accompanied by constriction at the adult body chamber, but these are less broad than in A. calabozoi Raffi and Olivero sp. nov. (see Birkelund 1993: pl. 1: 3–5; Kennedy and Summesberger 1986: pl. 3: 6, pl. 15: 4). Anagaudryceras subtililineatum differs from the juvenile stages of A. calabozoi Raffi and Olivero sp. nov. by the more depressed whorl section (Wb/Wh ~1.40) at same diameters. Also, A. calabozoi Raffi and Olivero sp. nov. presents bifurcate and intercalate lirae. Anagaudryceras mikobokense Collignon, 1956 from the Maastrichtian of Madagascar also resembles A. calabozoi Raffi and Olivero sp. nov. in present radial striae and flexuous ribs, but this are not asymmetrical and without associated constrictions. The same character is present in Anagaudryceras aurarium (Anderson, 1938) but the latter species is ornamented by distant constrictions separating very broad flattened ribs and it is more involute (U ~29%) and compressed (Wb/Wh ~0.75) than A. calabozoi Raffi and Olivero sp. nov. at same diameters.
Anagaudryceras pulchrum (Crick, 1907) closely resembles A. calabozoi Raffi and Olivero sp. nov. in its ornamentation style with a shell covered by thin sinuous lirae associated with 4 or 5 lirated and asymmetrical ribs followed by constrictions. Still, A. pulchrum is devoid of longitudinal striation. The topotype material mentioned by Marenssi et al. (1992) for the Rabot Formation as A. subsacya, probably corresponds to the microconch of A. calabozoi Raffi and Olivero sp. nov.
According to Matsumoto (1995), ancestral characters in ammonites apparently reappear at the last growth stage of descendants. In Antarctica, the record of the A. sacya (= A. buddha of Medina et al. 1982 and Ineson et al. 1986) from the deep-marine Albian Lower Kotick Point Formation, Gustav Group supports the evolutionary concept of Matsumoto (1995), reinforcing the idea that the band-like ornamentation is a basal feature, probably expressed in different species controlled by environmental factors. In this case environmental factors could be the similar habitat-depth for both the Albian A. sacya and the Campanian A. calabozoi Raffi and Olivero sp. nov (see Olivero and Raffi 2018). The presence of different states of this feature (band-like rib) may suggest a close phylogenetic relationship between these taxa (see also Shigeta and Nishimura 2014), and probably our new species is a descendant of A. sacya.
Stratigraphic and geographic range.—Late early to mid-Campanian, around the C33R/C33N magnetic chron boundary (Milanese et al. 2017a, b), Rabot and Santa Marta Formation, Ammonite Assemblage 6 Natalites spp. Group 2 and Ammonite Assemblage 7 Neokossmaticeras redondensis, James Ross Island, Antarctica. Campanian of the Cerro Toro Formation, Lake Pehoe locality, Magallanes Basin, Chile.
Anagaudryceras subcompressum Raffi and Olivero sp. nov.
Fig. 9B−F.
ZooBank LSID: urn:lsid:zoobank.org:act:81F09A97-D667-477F-A6EE-56FC0D1964CC
Etymology: From Latin sub, somewhat; meaning that the new species is somewhat similar to the type species of Anagaudryceras compressum.
Holotype: CADIC PI 506, moderate shell (D max 79 mm) with complete phragmocone and incomplete body chamber (Fig. 9C).
Type locality: Sanctuary Cliffs Nunatak, southern Snow Hill Island, Antarctica.
Type horizon: Maastrichtian (Cretaceous), the Sanctuary Cliffs Member, Snow Hill Island Formation, Ammonite Assemblage 8.2.
Material.—27 internal molds of phragmocone and body chamber, some of them preserve patches of the shell (CADIC PI 506–532). From the type locality and horizon.

Fig. 9. The gaudryceratid ammonoid Anagaudryceras, from early Campanian−Maastrichtian (Cretaceous), Antarctica, Rabot Formation, Punta Redonda (A, H–J), Snow Hill Island Formation, Sanctuary Cliffs (B–G), and López de Bertodano Formation, Seymour Island (K). A. Anagaudryceras calabozoi Raffi and Olivero sp. nov., CADIC PI 486, neanoconch, the arrow marks the last rib of the neanoconch. B–F. Anagaudryceras subcompressum Raffi and Olivero sp. nov. B. CADIC PI 508, phragmocone and part of the body chamber, in lateral (B1) and ventral (B2) views. C. CADIC PI 506, holotype, phragmocone and part of the body chamber, in lateral (C1) and ventral (C2) views. D. CADIC PI 511, phragmocone, in lateral (D1) and ventral (D2) views. E. CADIC PI 527, phragmocone and part of the body chamber, in lateral (E1) and ventral (E2) views. F. CADIC PI 509, phragmocone and part of the body chamber, in lateral view. G. Anagaudryceras sanctuarium Raffi and Olivero sp. nov., CADIC PI 604, phragmocone and part of the body chamber, in lateral view. H–J. Zelandites pujatoi Raffi and Olivero sp. nov. H. CADIC PI 543, holotype, phragmocone and part of the body chamber, in lateral view. I. CADIC PI 190, phragmocone, in lateral view. J. CADIC PI 545, phragmocone and part of the body chamber, in lateral view. K. Anagaudryceras sp. juvenile 2, CADIC PI 452, phragmocone, in lateral (K1) and ventral (K2) views. Arrows in B1, C1, F, G, H, and I mark the beginning of the body chamber.
Diagnosis.—Moderate shell with coiling markedly serpenticone and depressed whorl section. Inner whorls ornamented by prorsiradiate lirae and up to six prosiradiate constrictions per whorl. Adult body chamber with slightly flexuous constrictions associated with fine lirae almost imperceptible to the naked eye.
Description.—Early growth stage (D up to 40 mm): The coiling is evolute and strongly serpenticone, with small and vertical umbilical wall, and rounded umbilical shoulder. The whorl section is fairly depressed (Wb/Wh ~1.1–1.2; Table 5) up to 30 mm of diameter becoming compressed to larger diameters. The umbilicus represents more than the 50% of the shell (Table 5). The ornamentation consists of rectiradiate, fine and simple lirae that arise from the umbilical seam and become markedly prorsiradiate in the flank, with some intercalated lirae. Additional to the lirae are two or three constrictions per whorl and in the venter both lirae and constrictions project slightly adorally. The neanoconch is smooth. The internal mold is smooth with flat or even furrows representing the location of the constrictions in the shell. The suture is typical for the genus.
Mid growth stage (D up to 80 mm): The coiling becomes slightly less evolute and the umbilicus less wide (U ~35% of the shell at D ~80 mm; Table 5), with larger umbilical wall. The flanks converge to a gentle ventrolateral shoulder, mildly rounded and high venter. The whorl section becomes progressively more compressed (Wb/Wh ~0.92–0.72; Table 5). As the diameter increases the ornamentation becomes weaker. The body chamber, with more than 360°, is ornamentated by slightly flexuous and prorsiradiate constrictions. In addition to the constrictions there are fine flexuous lirae almost imperceptible to the naked eye.
Table 5. Dimensions (in mm) of Anagaudryceras subcompresum sp. nov. Abbreviations: D, diameter; Dmax, maximum diameter; U, umbilical diameter; %U, umbilical diameter as % of D; Wb, whorl breadth at a given D; Wh, whorl height at a given D.
| |
Dmax |
D |
Wb |
Wh |
Wb/Wh |
U |
%U |
|
CADIC PI 506 |
79 |
78.5 |
20.7 |
28.8 |
0.72 |
29.7 |
37.8 |
|
CADIC PI 507 |
|
50.2 |
14.2 |
17.7 |
0.80 |
21.5 |
42.8 |
|
CADIC PI 508 |
|
49.7 |
11.6 |
15 |
0.77 |
23.9 |
48.1 |
|
CADIC PI 509 |
49.2 |
46.3 |
12.4 |
14.4 |
0.86 |
|
|
|
CADIC PI 510 |
37.8 |
37.8 |
10.3 |
11 |
0.94 |
19.1 |
50.5 |
|
CADIC PI 511 |
37.7 |
37.7 |
11 |
11.5 |
0.96 |
19.3 |
51.2 |
|
CADIC PI 512 |
|
33.7 |
8.8 |
9.4 |
0.94 |
16.9 |
50.1 |
|
CADIC PI 513 |
38.6 |
38.6 |
11 |
11.6 |
0.95 |
19 |
49.2 |
|
CADIC PI 514 |
|
43 |
12.1 |
14 |
0.86 |
20.5 |
47.7 |
|
CADIC PI 515 |
31.1 |
31.1 |
8.6 |
8.2 |
1.05 |
16.9 |
54.3 |
|
CADIC PI 516 |
33 |
32.4 |
9.1 |
9.4 |
0.97 |
16.4 |
50.6 |
|
CADIC PI 517 |
35.3 |
30.8 |
9 |
8.5 |
1.06 |
12.2 |
39.6 |
|
CADIC PI 518 |
|
27.8 |
7.9 |
7.4 |
1.07 |
14.5 |
52.2 |
|
CADIC PI 519 |
28.2 |
28.2 |
8.2 |
7.7 |
1.06 |
15.4 |
54.6 |
|
CADIC PI 520 |
29.4 |
29.4 |
8.2 |
8.6 |
0.95 |
15 |
51.0 |
|
CADIC PI 521 |
32.2 |
32.2 |
8.3 |
8.8 |
0.94 |
18 |
55.9 |
|
CADIC PI 522 |
32.3 |
32.3 |
8 |
9.4 |
0.85 |
16.3 |
50.5 |
|
CADIC PI 523 |
29 |
29 |
7.6 |
7.8 |
0.97 |
15.3 |
52.8 |
|
CADIC PI 524 |
24.7 |
24.7 |
7 |
6.2 |
1.13 |
13.6 |
55.1 |
|
CADIC PI 525 |
32.5 |
32.5 |
|
7.8 |
0.00 |
|
0.0 |
|
CADIC PI 526 |
21.2 |
21.2 |
6.4 |
5 |
1.28 |
12 |
56.6 |
|
CADIC PI 527 |
24.4 |
24.4 |
7 |
6.3 |
1.11 |
13.7 |
56.1 |
|
CADIC PI 528 |
21.4 |
21.4 |
6.6 |
6 |
1.10 |
11.8 |
55.1 |
|
CADIC PI 529 |
18.4 |
18.4 |
5.8 |
4.3 |
1.35 |
10.9 |
59.2 |
|
CADIC PI 530 |
18.7 |
18.7 |
5.6 |
4.7 |
1.19 |
10.2 |
54.5 |
|
CADIC PI 531 |
19.3 |
19 |
5.9 |
4.5 |
1.31 |
10.6 |
55.8 |
|
CADIC PI 532 |
21.9 |
21.9 |
6.7 |
5.3 |
1.26 |
12.1 |
55.3 |
Remarks.—Anagaudryceras subcompressum Raffi and Olivero sp. nov. closely resembles A. compressum Shigeta and Nishimura, 2014 from the early Maastrichtian of Hokkaido, Japan. However, our new species differs in its less compressed whorl section (A. compressum has a Wb/ Wh ~0.65, D 73 mm), degree of involution and in the absence of band-like ribs in the adult stage. The whorl section and broad venter of the juvenile shells of Anagaudryceras tennenti Henderson, 1970, Haumurian age (the Haumurian is approximately equivalent to the Campanian–Maastrichtian; see Crampton et al. 2000) are very similar to those of A. subcompressum sp. nov., however, the Vertebrites-like ornament of the former is not present in A. subcompressum Raffi and Olivero sp. nov. In addition, A. tennenti has five collars per whorl in the adult stage and is more depressed (Wb/Wh ~1.10, D 48 mm).
Matsumoto in Matsumoto et al. (1985) described Anagaudryceras nanum from a juvenile specimen (D 24 mm) and Maeda et al. (2005) described new material from Hokkaido but did not give morphological measurements. However, these juveniles specimens with a very evolute shell present sinuous lirae and narrow constrictions followed by flares (or band-like ribs according to Maeda et al. 2005). Anagaudryceras mikobokense is ornamented by sinuous ribs without constrictions, also being more involute and less compressed at same diameters (Wb/Wh ~0.80−0.90, D 80 mm).
Besides, the whorl section and constrictions in the adult shell of Anagaudryceras yamashitai (Yabe, 1903), from the early Campanian of Hokkaido closely resemble A. subcompressum Raffi and Olivero sp. nov. However, the coiling is much more involute with an umbilicus of c. 25% of the total diameter of the shell. In addition, the suture line of Anagaudryceras subcompressum Raffi and Olivero sp. nov. has two bipartite saddles whereas Anagaudryceras yamashitai has three bipartite saddles at same diameters.
Stratigraphic and geographic range.—Early Maastrichtian of the Sanctuary Cliffs Member, Ammonite Assemblage 8.2, Snow Hill Island Formation, Antarctica. The first record of the species is just above the C32/C31 magnetic chron boundary (Milanese et al. 2017b).
Anagaudryceras sanctuarium Raffi and Olivero sp. nov.
Fig. 9G.
ZooBank LSID: urn:lsid:zoobank.org:act:DB4BE481-218C-4CFE-91E6-96514F6338CE
Etymology: Derived from the name of the outcrop area Sanctuary Cliffs Nunatak, where the species is well represented.
Holotype: CADIC PI 604, specimen (D 30 mm) with complete phragmocone and incomplete body chamber (Fig. 9G).
Type locality: Sanctuary Cliffs Nunatak, southern Snow Hill Island, Antarctica.
Type horizon: Campanian/Maastrichtian (Cretaceous), the lower part of the Sanctuary Cliffs Member, Snow Hill Island Formation, Ammonite Assemblage 8.2.
Material.—Three internal molds with phragmocone and part of the body chamber preserved (CADIC PI 604–606). From type locality and horizon.
Diagnosis.—Small Anagaudryceras of round whorl section with contrasting ornamentation during the ontogeny. Inner whorls with fine prorsiradiate lirae; adult body chamber strongly ornamented by dense collars with superimposed lirae.
Description.—Small and evolute shell, with round section, and wide umbilicus (Table 6) with round umbilical wall and umbilical shoulder. The flanks are subparallel and converge to a gentle ventrolateral shoulder and strongly rounded venter. The phragmocone is ornamented with fine prorsiradiate lirae that arise at the umbilical seam and cross straight on the venter. The body chamber ornament consists of dense lirae and wide and dense band-like collars with superimposed lirae. The neanoconch is smooth.
Table 6. Dimensions (in mm) of Anagaudryceras sanctuarium Raffi and Olivero sp. nov., Anagaudryceras sp. juvenile 2, and Zelandites pujatoi sp. nov. Abbreviations: D, diameter; U, umbilical diameter; %U, umbilical diameter as % of D; Wb, whorl breadth at a given D; Wh, whorl height at a given D.
| |
D |
Wb |
Wh |
Wb/Wh |
U |
%U |
|
Anagaudryceras sanctuarium |
||||||
|
CADIC PI 604 |
30 |
7.3 |
6.8 |
1.07 |
11.8 |
39.3 |
|
CADIC PI 605 |
25.5 |
|
|
|
12 |
47 |
|
CADIC PI 606 |
18.6 |
|
|
|
10 |
53 |
|
Anagaudryceras sp. juvenile 2 |
||||||
|
CADIC PI 453 |
36 |
12.1 |
12 |
1 |
15.9 |
44 |
|
Zelandites pujatoi sp. nov. |
||||||
|
CADIC PI 543 |
29 |
9.7 |
13.8 |
0.70 |
6.5 |
22.4 |
|
CADIC PI 544 |
27.2 |
9 |
13.3 |
0.68 |
6 |
22.1 |
|
CADIC PI 545 |
25.5 |
|
13 |
|
5.3 |
20.8 |
|
CADIC PI 190 |
24 |
11 |
9 |
0.8 |
7 |
29.1 |
Remarks.—Anagaudryceras sanctuarium Raffi and Olivero sp. nov. does not resemble any stage of the known species of Anagaudryceras. Anagaudryceras subcompressum Raffi and Olivero sp. nov. is much more compressed (SOM: fig. 7) and with strong constrictions in the first whorls. A. sanctuarium Raffi and Olivero sp. nov. could be confused with some small-sized species of the genus Parajaubertella Matsumoto, 1943. However, the typically globular round section of this genus does not match the specimens of A. sanctuarium Raffi and Olivero sp. nov. The small size of Anagaudryceras nanum Matsumoto in Matsumoto et al., 1985 from the early Campanian of Japan and Russia, is similar to that of our new species but its body chamber has very low, broad flexed band-like ribs separated by narrow constrictions.
Stratigraphic and geographic range.—Anagaudryceras sanctuarium Raffi and Olivero sp. nov. was recorded in a short stratigraphic interval just above the C32/C31 magnetic chron boundary, which is located approximately at the Campanian/Maastrichtian boundary (Milanese et al. 2017b). Ammonite Assemblage 8.2 Neograhamites cf. N. kiliani, Snow Hill Island, James Ross Archipelago, Antarctica.
Anagaudryceras sp. juvenile 2
Fig. 9K.
Material.—One internal mold of the phragmocone preserving patches of the shell (CADIC PI 453). From Maastrichtian (Cretaceous), López de Bertodano Formation, Ammonite Asemblage 11 Maorites tuberculatus, Seymour Island, Antarctica.
Description.—Small shell with evolute coiling , the whorl section is as high as broad (Wb/Wh ~1; Table 6). Wide umbilicus (U ~44 %; Table 6), with a gentle umbilical wall and rounded umbilical shoulder. The flanks converge to a rounded ventrolateral shoulder and rounded venter. The neanoconch is ornamented by four spaced ribs.The early-whorl ornamentation is almost imperceptible to the naked eye, but consists of strongly prorsiradiate hair-like striae. From the fourth whorl there are prorsiradiate constrictions and collars that are projected slightly aperturally on the venter.
Remarks.—Even though our specimen is a small juvenile, its style of ornamentation differs from the other species of Anagaudryceras described here. The inner whorls of A. seymouriense, from the upper part of the López de Bertonado Formation, differs in its smooth neanoconch and in the flexuous lirae with intercalate lirae that reach to mid flank. Otherwise, Anagaudryceras sp. juvenile 2 closely resambles A. subcompressum sp. nov. in the degree of involution but the ornamentation consists of prorsiradiate lirae with some constrictions and a more compressed whorl section.
Genus Zelandites Marshall, 1926
Type species: Zelandites kaiparaensis Marshall, 1926; Upper Cretaceous of New Zealand.
Remarks.—For synonymy and diagnosis see Hoffmann 2015: 18. The holotype of the type species Zelandites kaiparaensis Marshall, 1926 (Marshall 1926: pl. 31: 1) was lost, later Henderson (1970) designated from the original Marshall’s type series a poorly preserved specimen as a lectotype. Marshall (1926), and later Collignon (1956) and Matsumoto (1938), compared the neanic stage of Zelandites with Mesogaudryceras Spath, 1927 due to their similar morphological features. We do not concur with Hoffmann (2010) who interpreted Zelandites as the microconch of Anagaudryceras. Even though our specimens have a strongly ornamented neanoconch, a feature that clearly links Zelandites with Gaudryceras and Anagaudryceras, the ontogenetic development of Zelandites differs markedly from that of Anagaudryceras.
Zelandites is the lesser represented gaudryceratid genus in Antarctica, only three specimens of Z. varuna were described from the Maastrichtian of López de Bertodano Formation (Macellari, 1986) and now we add four specimens of Z. pujatoi Raffi and Olivero sp. nov. from the early Campanian of the Rabot and Santa Marta formations.
Zelandites pujatoi sp. nov.
Fig. 9H−J.
ZooBank LSID: urn:lsid:zoobank.org:act:11FB9232-8BEB-4252-8F0F-2D85943BBAF1
Etymology: In honor of General Hernán Pujato (1904–2003) founder of the Antarctic Institute.
Holotype: CADIC PI 543, specimen (D 29 mm) with complete phragmocone and incomplete body chamber (Fig. 9H)
Type locality Redonda Point, southeast of the James Ross Island, Antarctica.
Type horizon: Early Campanian (Cretaceous), Member II, Rabot Formation, Ammonite Assemblage 6 Natalites spp. Group 2.
Material.—Six internal molds, preserving patches of the shell and including the phragmocone and part of the body chamber (CADIC PI 190, 543–547). From type locality and horizon.
Diagnosis.—Small shell, strongly involute (U ~22 %) and fairly compressed whorl section. Neanoconch with up to seven ribs, young stages with weak prorsiradiate lirae accompanied by at least 8 slightly flexuous constrictions that project aperturally on the venter.
Description.—Involute shell with fairly compressed section (Wb/Wh ~0.74; Table 6). The umbilicus is small with a short umbilical wall and a slightly rounded umbilical shoulder. Subparallel flanks with gentle ventrolateral shoulder and slightly sharp venter. Neanoconch with up to seven strong ribs. As the diameter increases the ornamentation becomes much finer, almost imperceptible to the naked eye and in addition to the lirae, there are more than 8(?) well-defined slightly flexuous constrictions that in the venter form a slight aperturall projection.
Remarks.—Zelandites pujatoi Raffi and Olivero sp. nov. is similar to Zelandites kaiparaensis Marshall, 1926 and Zelandites inflatus Matsumoto, 1959 in its style of ornamentation. Z. kaiparaensis has fine lirae and more than 8 constrictions per whorl, but the constrictions are rectiradiate across the venter. Z. inflatus also has slightly flexuous constrictions but are more spaciate than those in Z. pujatoi. In addition, our new species differ from both in the strong ornamented neanoconch and in the greater degree of involution (~22% of the shell diameter).
Z. varuna, recorded in Antarctica (Macellari 1986), has two or three constrictions per whorl and these are strongly incised in the flanks but disappearing toward the venter.
Stratigraphic and geographic range.—Early Campanian of the Rabot and Santa Marta Formations, Ammonites Assemblage 6 Natalites spp. Group 2, James Ross Island, Antarctica.
Concluding remarks
Contrary to the scarcity of the Gaudryceratinae in other Upper Cretaceous localities of the Southern Hemisphere, the Antarctic record reveals that the subfamily is very well represented by numerous specimens of the genera Gaudryceras, Anagaudryceras, and Zelandites. In a previous study we have recorded the Santonian–early Campanian Gaudryceras cf. G. strictum Kennedy and Bengtson in Kennedy et al., 2007 and G. santamartense Raffi and Olivero, 2016, the early to mid-Campanian G. brandyense Raffi and Olivero, 2016, G. rabotense Raffi and Olivero, 2016 and G. cf. G. mite (Hauer, 1866) (Raffi and Olivero 2016). Here we add the early Campanian Anagaudryceras sp. juvenile 1, the early to mid-Campanian Gaudryceras submurdochi Raffi and Olivero sp. nov., Anagaudryceras cf. politissum (Kossmat, 1895), A. calabozoi Raffi and Olivero sp. nov., and Zelandites pujatoi Raffi and Olivero sp. nov., and the early Maastrichtian A. subcompressum Raffi and Olivero sp. nov., A. sanctuarium Raffi and Olivero sp. nov., and Anagaudryceras sp. juvenile 2. In addition to the previous record of the late Maastrichtian A. seymouriense Macellari, 1986 and Z. varuna (Forbes, 1846) (Macellari 1986), the Late Cretaceous Antarctic Gaudryceratinae encompass 15 species. This is the richest and most diversified record at the specific level of the subfamily in the Santonian–Maastrichtian of the whole Southern Hemisphere. In the rest of the Austral regions, only South Africa records a Santonian–Campanian Gaudryceratinae diversity comparable to that of Antarctica, reaching 10 species belonging to the genera Gaudryceras, Anagaudryceras, Vertebrites, and Zelandites. However, the described gaudryceratid collection consists only of a few specimens (Kennedy and Klinger 1979). Interestingly, dominance of Gaudryceratinae is not extended over the total Santonian–Maastrichtian ammonite record in the James Ross Basin, where seven out of the 15 Santonian–Maastrichtian species of Gaudryceratinae are restricted to a relatively short stratigraphic range encompassing the late early Campanian–early to mid-Campanian interval, around the C33R–C33N paleomagnetic chron boundary. The stratigraphic record of the other Gaudryceratinae species is dispersed, discontinuous, and widely distributed in separarated stratigraphic horizons spanning the Santonian–early Campanian and the Maastrichtian. Consequently, with the exception of the Rabot Formation, generally only one or two species of Gaudryceratinae are present in the same stratigraphic interval (Figs. 2, 3). The Gaudryceratinae are extremely abundant and diversified only in the relatively short stratigratigraphic interval covering the Ammonite Assemblages 6 and 7, late early Campanian–basal mid-Campanian in the Rabot Formation (Olivero and Raffi 2018). In this stratigraphic interval centered around the C33R–C33N paleomagnetic chron boundary, the Gaudryceratinae includes seven species of Gaudryceras, Anagaudryceras and Zelandites (Raffi and Olivero 2016; this study).
This outstanding, highly diversified record is only rivaled by an even larger number of species of the genera Anagaudryceras, Gaudryceras, and Zelandites in the Santonian–Maastrichtian of Japan (cf. Matsumoto, 1995). The reasons for a similar, highly diversified record of the Gaudryceratinae in these distant, almost antipodal regions are not clear, but we argue that they probably reflect a similar paleoecological control.
In the Upper Cretaceous Yezo Group, Japan gaudryceratid ammonites are abundant and diversified around the oceanic regions; conversely, they are scarce in shallow epicontinental seas. It is thought that this contrasting abundance can be explained by the preferred original habitats of gaudryceratids, which are interpreted as oceanic areas located near the outer shelf (Matsumoto 1995; Westermann 1996).
Apparently, the new gaudryceratids described here have a geographical restricted occurrence and follow the same trend previously detected in the rest of the Antarctica fauna. The paleobiogeography of the Santonian–Maastrichtian Antarctic ammonite fauna was interpreted as being characterized by two main elements: (i) a cosmopolitan or Indo-Pacific Santonian–early Campanian fauna (Olivero 2012b; Olivero and Medina 2000; Raffi and Olivero 2016); and (ii) a strongly endemic mid-Campanian–Maastrichtian fauna, the appearance of which is associated with the earlier Antarctic extinction of several mollusk taxa that range into the Maastrichtian elsewhere in the world (see Macellari 1987; Olivero 2012b; Olivero and Medina 2000). This paleobiogeographical faunal turnover is concomitant with an austral decline of the seawater and terrestrial areas (Barreda et al. 2019). The effects of this lowering temperature are far more pronounced in the Antarctic kossmaticeratid ammonoids, reflecting the interpretation that they were stenothermal ammonites (Olivero 2012b). However, for the gaudryceratids it can not be ruled out that the generalized somerization of the basin during the Campanian−early Maastrichtian could have add an additional effect conditioning their geographical distribution.
In the James Ross Basin gaudryceratids are extremely abundant and diversified in the Rabot Formation, whereas ornate kossmaticeratids are dominant in the age-equivalent deposits of the Beta Member of the Santa Marta Formation. Ornate kossmaticeratids are dominant in shallow, inner shelf deposits located to the northwest of the basin (Brandy Bay section, Fig. 2), whereas gaudryceratids are dominant in relatively deeper mid to outer shelf deposits located to the southeast of the basin (Rabot, Hamilton, and Redonda points sections, Fig. 2); reflecting different depositional settings and ammonite habitats (Olivero and Raffi 2018). Consequently, they conclude that Gaudryceratinae dominate in offshore oceanic-influenced settings, suggesting that they have a mesopelagic, planktic mode of life. On this basis, we argue that the outstanding abundant and highly diversified Gaudryceratinae record in the Upper Cretaceous of Japan and Antarctica probably reflects a similar paleoecological control, dominated by oceanic-influenced settings, which apparently characterized the preferred habitat of the Gaudryceratinae.
Ontogenetic differences in morphology and size between the inmature and mature shells of the same species is not common in gaudryceratids. To the authors’ knowledge it was only described for the dimorphic pair Gaudryceras denseplicatum (Jimbo, 1894)–G. intermedium Yabe, 1903, which was interpreted by Hirano (1978) as sexual antidimorphs (see also Matsumoto 1995). Another, albeit doubtfull, case of sexual dimorphism in gaudryceratids was proposed by Hoffmann (2010), who interpreted Zelandites as the microconchs of Anagaudryceras. However, the ontogeny of the immature shell of Zelandites, characterized at some stages at least by a relatively compressed and involute shell, has no parallel with the relatively depressed and more evolute immature shells of Anagaudryceras, confirming that they are different genera (cf. Kennedy and Klinger 1979; Matsumoto 1995).
In the studied Antarctic collection, a large number of specimens referred to Anagaudryceras calabozoi Raffi and Olivero sp. nov. are characterized by similar inmature shells but differ in the ornamentation of the body chamber of the adult shell. We interpreted these specimens as sexual antidimorphs, and such an interpretation is supported by the similar ratio of micro- and macroconchs, which are recorded in the same stratigraphic levels.
Acknowledgements
We thank the Instituto Antártico Argentino and the logistic facilities of the Fuerza Aérea Argentina during Antarctic field seasons. Alvar Sobral (CITSE, CONICET), Matías Vaca (Universidad Nacional de Córdoba, Spain), Marcos Rodriguez (Universidad Nacional de Tierra del Fuego, Antártida e Islas del Atlántico Sur, Ushuaia, Argentina), Erika Bedoya (CADIC, CONICET), Steven Skinner and Ross Mitchell (both CALTECH), Tomás Luppo (Instituto de Geociencias Básicas, Aplicadas y Ambientales de Buenos Aires, Argentina, CONICET) helped in the fieldwork. We wish to acknowledge thorough but constructive reviews by René Hoffmann (Institute of Geology, Mineralogy and Geophysics, Ruhr-Universität, Bochum, Germany) and Haruyoshi Maeda (The Kyushu University Museum, Fukuoka, Japan). This study was supported by previous grants by ANPCyT-DNA and CONICET (Argentina). The National University of Tierra del Fuego (PIDUNTdF-A) and CONICET (PUE-CADIC 2016) partially financed the study.
References
Anderson, F.M. 1938. Lower Cretaceous deposits in California and Oregon. Geological Society of America Special Paper 16: 1–429. Crossref
Arkell, W.J. 1950. A classification of the Jurassic ammonites. Journal of Paleontology 24: 354−364.
Arkell, W.J., Kummel, B., and Wright, C.W. 1957. Mesozoic Ammonoidea. In: R.C. Moore (ed.), Treatise on Invertebrate Palaeontology, Part L, Mollusca 4. Cephalopoda, Ammonoidea, 80−465. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Barreda, V.D., Palazzesi, L., and Olivero, E.B. 2019. When flowering plants ruled Antarctica: evidence from Cretaceous pollen grains. New Phytologist 223: 1023–1030. Crossref
Birkelund, T. 1993. Ammonites from the Maastrichtian White Chalk of Denmark. Bulletin of the Geological Society of Denmark 40: 33−81.
Boulé, M., Lemoine, P., and Thevenin, A. 1906. Paléontologie de Madagascar 3. Céphalopodes crétacés des environs de Diego-Suarez. Annales de Paléontologie 4: 173−192.
Collignon, M. 1956. Ammonites néocrétacées du Menabe (Madagascar). 4 Les Phylloceratidae; 5 Les Gaudryceratidae; 6 Les Tetragonitidae. Annales Géologiques du Service des Mines, Madagascar 23: 1−106.
Crame, J.A. and Luther, A. 1997. The last inoceramid bivalves in Antarctica. Cretaceous Research 18: 179−195. Crossref
Crame, J.A., Pirrie, D., Riding, J.B., and Thomson, M.R.A. 1991. Campanian–Maastrichtian (Cretaceous) stratigraphy of the James Ross Island area, Antarctica. Journal of the Geological Society 148: 1125–1140. Crossref
Crampton, J., Mumme, T., Raine, I., Roncaglia, L., Schioler, P., Strong, P., Turner, G., and Wilson, G. 2000. Revision of the Piripauan and Haumurian local stages and correlation of the Santonian–Maastrichtian (Late Cretaceous) in New Zealand. New Zealand Journal of Geology and Geophysics 43: 309−333 Crossref
Crick, G.C. 1907. Cretaceous fossils of Natal collected by Mr. William Anderson, Government Geologist. Part 3: 1. The Cephalopoda from the deposit at the north end of false bay, Zululand. 2. The Cephalopoda from the Tributaries of the Manuan Creek, Zululand. 3. Note on a Cretaceous ammonite from the Mouth of the Umpenyati River, Natal. Report of the Geological Survey of Natal and Zululand 3: 163−250.
Forbes, E. 1846. Report on the Cretaceous fossil invertebrates from Southern India, collected by Mr. Kaye and Mr. Cunliffe. Transactions of the Geological Society of London (2) 7: 97−174. Crossref
Grossouvre, A. de. 1894. Recherches sur la Craie supérieure. Deuxième partie: Paléontologie, Les ammonites de la Craie supérieure. Mémoires du Service de la Carte Geologique Detaillée de la France, Imprimerie Nationale 1893: 1−264. Crossref
Haggart, J.W. 1989. New and revised ammonites from the Upper Cretaceous Nanaimo Group of British Columbia and Washington State. Geological Survey of Canada 396: 181−221 Crossref
Hauer, F.R. von 1866. Neue Cephalopoden aus den Gosaugebilden der Alpen. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften 53: 300−308.
Henderson, R.A. 1970. Ammonoidea from the Mata Series (Santonian−Maastrichtian) of New Zealand. Special Papers in Palaeontology 6: 1−82.
Henderson, R.A. and McNamara, K.J. 1985. Maastrichtian non-heteromorph ammonites from the Miria Formation, Western Australia. Palaeontology 28: 35−88.
Hirano, H. 1978. Phenotypic substitution of Gaudryceras (a Cretaceous ammonite). Transactions and Proceedings of the Palaeontological Society of Japan, New Series 190: 235−258
Hoffmann, R. 2010. New insights on the phylogeny of the Lytoceratoidea (Ammonitina) from the septal lobe and its functional interpretation. Revue de Paléobiologie 29: 1−156.
Hoffmann, R. 2015. Lytoceratoidea. In: P. A. Selden (ed.), Treatise Online Part L, Revised, Volume 3B, 1−34. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Howarth, M.K. 1965. Cretaceous ammonoids and nautiloids from Angola. Bulletin British Museum Natural History 10: 337−412.
Howarth, M.K. 1966. Ammonites from the Upper Cretaceous of the James Ross Island Group. British Antarctic Survey Bulletin 10: 55−69.
Hyatt, A. 1900. Cephalopoda. In: K.A. von Zittel (translation C.R. Eastman,), Textbook of Paleontology, 502−604 pp. Macmillan, London.
Ifrim, C., Stinnesbeck, W., and López Oliva, J.G. 2004. Maastrichtian cephalopods from cerralvo, north-eastern Mexico. Palaeontology 47: 1575–1627. Crossref
Ineson, J.R. 1989. Coarse-grained submarine fan and slope apron deposits in a Cretaceous back-arc basin, Antarctica. Sedimentology 36: 739−819. Crossref
Ineson, J.R., Crame A., and Thomson, M.R.A. 1986. Lithostratigraphy of the Cretaceous strata of West of James Ross Island, Antarctica. Cretaceous Research 7: 141−159. Crossref
Jimbo, K. 1894. Beiträge zur Kenntnis der Fauna der Kreideformation von Hokkaido. Palaeontologische Abhandlungen 2: 147−194.
Kennedy, W.J. and Cobban, W.A. 1976, Aspects of ammonoid biology, biogeography and biostratigraphy. Special Papers in Palaeontology 17: 1–94.
Kennedy, W.J., and Summesberger, H. 1986. Lower Maastrichtian ammonites from Neuberg, Steiermark, Austria. Beiträge zur Paläontologie Österreich 12: 181−242.
Kennedy, W.J. and Klinger, H.C. 1979. Cretaceous faunas from Zululand and Natal, South Africa. The ammonite family Gaudryceratidae. Bulletin of the British Museum (Natural History) Geology 31: 121−174.
Kennedy, W.J., Crame, J.A., Bengtson, P., and Thomson, M.R.A. 2007. Conician ammonites from James Ross Island, Antarctica. Cretaceous Research 28: 509−531. Crossref
Kilian, W. and Reboul, P. 1909. Les Cèphalopodes Neocrètaceès des Iles Seymour et Snow Hill. In: F. Stephani (ed.), Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901−1903, 1–75. Lithographisches Institute des Generalstabs, Stockholm.
Klein, J., Hoffmann, R., Joly, B., Shigeta, Y., and Vasicek, Z. 2009. Lower Cretaceous Ammonites IV: Boreophylloceratoidea, Phylloceratoidea, Lytoceratoidea, Tetragonitoidea, Haploceratoidea including the Upper Cretaceous representatives. In: W. Riegraf (ed.), Fossilium Catalogus I: Animalia Vol 146. 416 pp. Backhuys Publishers, Leiden.
Korn, D. 2010. A key for the description of Palaeozoic ammonoids. Fossil Record 13: 5–12. Crossref
Kossmat, F. 1895. Untersuchungen über die Südische Kreideformation. Beitraege zur Paläeontologie und Geologie 9: 97−203.
Lirio, J.M., Marenssi, S.A., Santillana, S.N., Marshall, P.A., and Rinaldi, C.A. 1989. Marambio Group at the South Eastern Part of James Ross Island, Antarctica. Instituto Antártico Argentino 371: 1−46.
Luppov, N. and Drushchits, V.V. 1958. Mollusca. Cephalopoda 2. Ammonoidea (Ceratites and Ammonites) and Endocochlia. In: Y. Orlov (ed.), Fundamentals of Paleontology. 360 pp. Academy of Sciences USSR, Moscow.
Macellari, C.E. 1986. Late Campanian–Maastrichtian ammonite fauna from Seymour Island (Antarctic Peninsula). Memoirs of the Paleontological Society 18: 1−55. Crossref
Macellari, C.E. 1987. Progressive endemism in the Late Cretaceous ammonite family Kossmaticeratidae and the breakup of Gondwanaland. In: G.D. McKenzie (ed.), Gondwana Six: Stratigraphy, Sedimentology, and Paleontology. Geophysical Monograph 41: 85−92. Crossref
Macellari, C.E. 1988. Stratigraphy, sedimentology, and paleoecology of Upper Cretaceous/Paleocene Shelf-deltaic sediments of Seymour Island. Geological Society of America 169: 25−53 Crossref
Maeda, H., 1991. Sheltered preservation: a peculiar mode of ammonite occurrence in the Cretaceous Yezo Group, Hokkaido, north Japan. Lethaia 24: 69−82 Crossref
Maeda H., Shigeta, Y., Fernando, A.G.S., and Okada, H. 2005. Stratigraphy and Fossil Assemblages of the Upper Cretaceous System in the Makarov Area, Southern Sakhalin, Russian Far East. In: Y. Shigeta and H. Maeda (eds.), The Cretaceous System in the Makarov Area, Southern Sakhalin, Russian Far East. National Science Museum Monographs 31: 25–120
Marenssi, S.A., Lirio, J.M., Santillana, S.N., Martinioni, D.R., and Palamarczuc, S. 1992. El Cretácico Superior del sudeste de la isla James Ross, Antártida. In: C.A. Rinaldi (ed.), Geología de la isla James Ross, 77−88. Instituto Antártico Argentino, Buenos Aires.
Marshall, P. 1926. The Upper Cretaceous ammonites of New Zealand. Transactions of the New Zealand Institute 56: 129−210.
Martinioni, D.R 1992. La Formación Rabot (Cretácico Superior, isla James Ross, Antártida): Un ciclo transgresivo-regresivo de plataforma con dominio de procesos de tormenta. In: C.A. Rinaldi (ed.), Geología de la isla James Ross, 101−123. Instituto Artartico Argentino, Buenos Aires.
Matsumoto, T. 1938. A biostratigraphic study on the Cretaceous deposits of the Naibutu Valley, South Karahuto. Proceedings of the Imperial Academy of Japan 14: 190−194. Crossref
Matsumoto, T. 1943. A note on the Japanese ammonites belonging to the Gaudryceratidae. Proceedings of the Imperial Academy 18: 666−670. Crossref
Matsumoto, T. 1959. Upper Cretaceous ammonites of California. Part 2. Memoirs of the Faculty of Science, Kyushu University, Series D, Geology, Special Volume 1: 1−172.
Matsumoto, T. 1988. Notes on some Cretaceous ammonites from South Sakhalin held at Tohoku University, Sendai. Science Report of the Tohoku University, 2nd series (Geology) 59: 177–190.
Matsumoto, T. 1995. Notes on gaudryceratid ammonites from Hokkaido and Sakhalin. Palaeontological Society of Japan Special Papers 35: 1–152.
Matsumoto, T. and Yoshida, S. 1979. A new gaudryceratid ammonite from eastern Hokkaido. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 114: 65−76.
Matsumoto, T., Miyauchi, T., and Kanie, Y. 1985. Some gaudryceratid ammonites from the Campanian and Maastrichtian of Hokkaido Part 2. Science Reports of the Yokosuka City Museum 33: 19−36.
Medina, F.A., Rinaldi, C.A., Del Valle, R.A., and Baldoni, A.M. 1982. Edad de la Formación Lower Kotick Point en la isla James Ross, Antartida. Ameghiniana 19: 263−272.
Milanese, F.N., Olivero, E.B., Kirschvink, J.L. and Rapalini, A.E. 2017a. Magnetostratigraphy of the Rabot Formation, Upper Cretaceous, James Ross Basin, Antarctic Peninsula. Cretaceous Research 72:172−187. Crossref
Milanese, F.N., Olivero, E.B., Rapalini, A.E., Kirschvink, J.L., Raffi, M.E., Franceschinis, P.R., and Gallo, LC. 2017b. Marco cronológico absoluto del Cretácico Superior de la cuenca James Ross. In: XX Congreso Geológico Argentino, 7−11 August, Tucumán, Argentina. Acta de la Sesión Técnica 1: Estratigrafía, 83−86. Asociación Geológica Argentina, Buenos Aires.
Morozumi, Y. 1985. Late Cretaceous (Campanian and Maastrichtian) ammonites from Awaji Island, southwest Japan. Bulletin of the Osaka Museum of Natural History 39: 1–58.
Neumayr, M. 1875. Die Ammoniten der Kreide und die Systematik der Ammonitiden. Zeitschrift der Deutschen Geologischen Gesellschaft 27: 854−892.
Olivero, E.B. 2007. Taphonomy of ammonites from the Santonian lower Campanian Santa Marta Formation, Antarctica: sedimentological controls on vertically embedded ammonites. Palaios 22: 586−597. Crossref
Olivero, E.B. 2012a. New Campanian kossmaticeratid ammonites from the James Ross Basin, Antarctica, and their possible relationships with Jimboiceras? antarcticum Riccardi. Revue de Paléobiologie 11: 133−149.
Olivero, E.B. 2012b. Sedimentary cycles, ammonite diversity and palaeoenvironmental changes in the Upper Cretaceous Marambio Group, Antarctica. Cretaceous Research 34: 348−366. Crossref
Olivero, E. B., and Medina, F. 2000. Patterns of Late Cretaceous ammonite biogeography in southern high latitudes: the family Kossmaticeratidae in Antarctica. Cretaceous Research 21: 269–279. Crossref
Olivero, E.B. and Raffi, M.E. 2018. Onshore-offshore trends in Campanian ammonite facies from the Marambio Group, Antarctica: Implications for ammonite habitats. Cretaceous Research 78: 79−89 Crossref
Olivero, E.B., Ponce, P.P., and Martinioni, D.R. 2008. Sedimentology and architecture of sharp-based tidal sandstones in the upper Marambio Group, Maastrichtian of Antarctica. Sedimentary Geology 210: 11–26. Crossref
Olivero, E.B., Scasso, R.A., and Rinaldi, C.A. 1986. Revision of the Marambio Group, James Ross Island, Antarctica. Instituto Aantártico Argentino 331: 1−29.
Pirrie, D., Crame, J.A., Lomas, S.A., and Riding, J.B. 1997. Late Cretaceous stratigraphy of the Admiralty Sound region, Jamess Ross Basin, Antarctica. Cretaceous Research 18: 109−137. Crossref
Raffi, M.E., and Olivero, E.B. 2016. The Ammonite genus Gaudryceras from the Santonian–Campanian of Antarctica: Systematics and Biostratigraphy. Ameghiniana 53: 375–396. Crossref
Rinaldi, C.A., Massabie, A., Morelli, J., Rosenman, H.L., and del Valle, R.A. 1978. Geología de la isla Vicecomodoro Marambio. Instituto Antártico Argentino 217: 1–44.
Salazar, C., Stinnesbeck, W., and Quinzio- Sinn, L. A. 2010. Ammonites from the Maastrichtian (Upper Cretaceous) Quiriquina Formation in central Chile. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 257 (2): 181–236. Crossref
Scasso, R.A., Olivero, E.B., and Buatois, L.A. 1991. Lithofacies, biofacies and ichnoassemblage evolution of a shallow submarine volcaniclastic fan-shelf depositional system (Upper Cretaceous, James Ross Island, Antarctica). Journal of South American Earth Sciences 4: 239−260. Crossref
Schlüter, C.1872. Cephalopoden der oberen deutschen Kreide. Palaeontographica 22: 25−120.
Schindewolf, O.H. 1961. Studien zur Stammesgeschichte der Ammoniten. Lieferung 1. Abhandlungen der Akademie der Wissenschaften und der Literatur in Mainz, Mathematisch-Naturwissenschaftliche Klasse 1960: 639−743.
Shimizu, S. 1934. Amonites [in Japanese]. In: S. Shimizu and T. Obata (eds.), Cephalopoda. 137 pp. Iwanami’s Lecture Series of Geology and Paleontology, Japan.
Shigeta, Y. and Nishimura, T. 2013. A new species of Gaudryceras (Ammonoidea, Gaudryceratidae) from the lowest Maastrichtian of Hokkaido, Japan and its biostratigraphic implications. Paleontological Research 17: 47−57. Crossref
Shigeta, Y. and Nishimura, T. 2014. A new species of Anagaudryceras (Ammonoidea, gaudryceratidae) from the lowest Maastrichtian of Hokkaido, Japan. Paleontological Research 18: 176−185 Crossref
Shigeta, Y., Nishimura, T., and Nifuku, K. 2015. Middle and late Maastrichtian (latest Cretaceous) ammonoids from the Akkeshi Bay area, eastern Hokkaido, northern Japan and their biostratigraphic implications. Paleontological Research 19: 107–127. Crossref
Spath, L. 1927. Revision of the Jurassic cephalopod fauna of Kachh (Cutch). Part 1. Memoirs of the Geological Survey of India 9: 1−71.
Stoliczka, F. 1865. Ammonitidae with revision of the Nautilidae. In: H. Blanford (ed.), The Fossil Cephalopoda of the Cretaceous Rocks of Southern India (1863−1866). Memoirs of the Geological Survey of India 3: 107−154.
Tobin T.S., Ward, P.D., Steig, E.J., Olivero, E.B., Hilburn, I.A., Mitchel, R.N., Diamond, M.R., Raub, T.D., and Kirschvink, J.L. 2012. Extinction patterns, δ18O trends, and magnetostratigraphy from a southern high-latitude Cretaceous–Paleogene section: Links with Deccan volcanism. Palaeogeography, Palaeoclimatology, Palaeoecology 350–352: 180–188. Crossref
Westermann, G.E.G. 1996. Ammonoid life and habit. In: N.H. Landman, K. Tanabe, and R.A. Davies (eds.), Ammonoid Paleobiology, 607−707. Plenum Press, New York. Crossref
Whiteaves, J.F. 1884. On the fossils of the coal-bearing deposits of the Queen Charlotte Island collected by Dr. G.M. Dawson in 1878. Mesozoic Fossils, Geological Survey of Canada 1: 191−262. Crossref
Wiedmann, J. 1962. Ammoniten aus der vascogotischen Kreide (Nordspanien) 1. Phylloceratina, Lytoceratina. Palaeontographica Abteilung A 118: 119−237.
Wright, C.W. and Kennedy, W.J. 1984. The Ammonoidea of the Lower Chalk. Monograph. Palaeontographica Society 1: 1−126.
Wright, C.W. and Matsumoto, T. 1954. Some doubtful Cretaceous ammonite genera from Japan and Saghalien. Memoirs of the Faculty of Science, Kyushu University, Series D, Geology 4: 107−134.
Wright, C.W., Callomon, J.H., and Howarth, M.K. 1996. Cretaceous Ammonoidea. In: R.C. Moore (ed.), Treatise on Invertebrate Palaeontology, Part L Mollusca Revised. 362 pp. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Yabe, H. 1903. Cretaceous Cephalopoda from the Hokkaido (Part 1). The Journal of the College of Science, Imperial University of Tokyo 18: 1−55.
Acta Palaeontol. Pol. 64 (3): 523–542, 2019
https://doi.org/10.4202/app.00560.2018