


Chamber arrangement versus wall structure in the high-rank phylogenetic classification of Foraminifera
ZOFIA DUBICKA
Dubicka, Z. 2019. Chamber arrangement versus wall structure in the high-rank phylogenetic classification of Foraminifera. Acta Palaeontologica Polonica 64 (1): 1–18.
Foraminiferal wall micro/ultra-structures of Recent and well-preserved Jurassic (Bathonian) foraminifers of distinct foraminiferal high-rank taxonomic groups, Globothalamea (Rotaliida, Robertinida, and Textulariida), Miliolida, Spirillinata and Lagenata, are presented. Both calcite-cemented agglutinated and entirely calcareous foraminiferal walls have been investigated. Original test ultra-structures of Jurassic foraminifers are given for the first time. “Monocrystalline” wall-type which characterizes the class Spirillinata is documented in high resolution imaging. Globothalamea, Lagenata, porcelaneous representatives of Tubothalamea and Spirillinata display four different major types of wall-structure which may be related to distinct calcification processes. It confirms that these distinct molecular groups evolved separately, probably from single-chambered monothalamids, and independently developed unique wall types. Studied Jurassic simple bilocular taxa, characterized by undivided spiralling or irregular tubes, are composed of miliolid-type needle-shaped crystallites. In turn, spirillinid “monocrystalline” test structure has only been recorded within more complex, multilocular taxa possessing secondary subdivided chambers: Jurassic Paalzowella and Recent Patellina. More integrated molecular and structural studies are needed in order to better understand taxonomic position and phylogeny of tubular taxa. Unilocular and multichambered Lagenata (Lagenidae and Nodosariidae, respectively) show identical test micro and ultra-structure which suggests their close phylogenetic relationship and questions most recent theories of their separate evolutionary history and origins. A comparison of Recent, Cretaceous, and Jurassic foraminiferal test structure indicates that test characteristics at particular higher-rank taxonomic levels change very little over time and thus can serve as good proxies for the taxonomic designations of fossil taxa, when their state of preservation is appropriate for microstructural observations.
Key words: Foraminifera, biomineralisation, test structure, high-rank classification, Jurassic, Poland.
Zofia Dubicka [z.dubicka@uw.edu.pl], University of Warsaw, Faculty of Geology, al. Żwirki i Wigury 93, 02-089 Warszawa, Poland.
Received 2 November 2018, accepted 4 January 2019, available online 28 January 2019.
Copyright © 2019 Z. Dubicka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The traditional foraminiferal higher-level taxonomic system was based on the mineralogy and structure of the test, while lower-level ranks were classified mainly according to growth plan (Loeblich and Tappan 1964, 1984, 1987, 1992; Sen Gupta 2003). However, the commonly accepted traditional wall structure characteristics of specific foraminiferal groups were largely defined based on low-resolution microscopy and/or on recrystallized fossil specimens with obscured test composition, which precluded any record of the original biocrystals (see discussion in Mikhalevich 2009; Dubicka and Gorzelak 2017). Moreover, more recent molecular studies of Foraminifera (Darling et al. 1997, 2009; Pawlowski 2000; Habura et al. 2006; Schweizer et al. 2008; Ujiié et al. 2008; Pawlowski et al. 2013; Holzmann and Pawlowski 2017) have revealed that the classical test structure-based higher-level classification of Foraminifera is rather speculative and, in several points, invalid. In a seminal work on Foraminifera classification based on molecular data, Pawlowski et al. (2013) established a higher-level (supraordinary) taxonomic system in which multi-chambered foraminifers (polythalamous) were grouped into two classes, Tubothalamea and Globothalamea, and mentioned that Lagenida probably constitutes a distinct class as well. This classification is additionally supported by the differing morphology of these three clades. Globothalameans possess globular chambers and minimal distances between successive apertures; tubothalameans possess tubular chambers and maximal distances between apertures; lagenids, like Tubothalamea, are characterised by maximal distances between apertures (related with terminal chamber formation), but their chambers are rather globular. Accordingly, Pawlowski et al. (2013; see also Hohenegger 2018) postulated that the mode of coiling and foraminal distance corresponded more closely than wall composition to the branching pattern in the evolution of high-rank foraminiferal groups, supporting previous studies by Mikhalevich (1980, 1992, 2000, 2009, 2013). On the other hand, Rigaud and Martini (2016), based on a comparison of taxa with entirely porcelanous tests with those with agglutinated-porcelanous walls, postulated that general test morphology is taxonomically less important than key wall microstructural characteristics in tubular foraminifers. Moreover, most recent studies of the wall micro/ultra-structure (the term “wall ultra-structure” is used for the arrangement of and relations between the parts, elements and crystallites of foraminiferal test observed at very high magnification, at the nanoscale) of extant and fossil foraminiferal tests (Dubicka et al. 2018) shed some light on this debate. Namely, shells of Miliolida (Tubothalamea), Rotaliida (Globothalamea), and Lagenata, which, on the phylogenetic tree, represent separate multi-chambered clades (Lagenata also includes unilocular forms of the family Lagenidae) differ in being composed by morphologically distinct primary crystallites. Specifically, a miliolid test is mainly composed of needle-shaped primary crystallites up to 1 µm in length (see also Parker 2017; and literature cited there); a rotaliid test is composed of roughly nodular primary nanograins (up to 100 nm in diameter); and a lagenid test is composed of relatively large interlocked single-crystal fibres (up to 50 μm in length) with inner pores (ca. 150‒200 nm in diameter) extending along the entire length of each crystal. Dubicka et al. (2018) documented that calcite foraminiferal test composition is still consistent with the higher-level taxonomic system of Pawlowski et al. (2013), in which multilocular foraminifers are divided into three separate classes. Nevertheless, to determine whether test structures factually correspond to the molecular phylogenetic classification, solid documentation of additional taxonomic groups is needed. Therefore in this paper, I extend the studies of micro- and nanostructures in foraminifers published by Dubicka et al. (2018), and present the structures of various orders within the two distinctive foraminiferal multichambered classes Globothalamea and Tubothalamea, such as Rotaliida (both benthic and planktonic), Robertinida, Textulariida (in the sense of Pawlowski et al. 2013), Miliolida, and Spirillinida, as well as Lagenata (both unilocular Lagenidae and multilocular Nodosariidae) (Mikhalevich 2013; Rigaud et al. 2016). Moreover, I provide a comparison of entirely calcareous foraminiferal tests and calcareous cemented agglutinated tests in both the Tubothalamea and Globothalamea classes; agglutinated tests of foreign particles have never (or not convincingly) been documented in Lagenata. Both Recent and Bathonian (Middle Jurassic) specimens were investigated. Wall structure of Jurassic foraminifers have been published in a few papers only (Gronlung and Hansen 1976), however, no highly magnified ultra-structural observation has been presented previously.
Institutional abbreviations.—MWGUW, S.J. Thugutt Geological Museum, Faculty of Geology, University of Warsaw, Warsaw, Poland.
Material and methods
Selected extant and fossil (Bathonian, Middle Jurassic) representatives of Rotaliida, Robertinida, Textulariida, Spirillinida, Miliolida, and Lagenata were studied. Recent foraminiferal tests were collected from a beach at Ronsard Bay near Cervantes (Western Australia; see Haig 2002) except for Procerolagena gracilis (Williamson, 1848) which was selected from the Wojciech Majewski’s collection from Admiralty Bay, King George Island, West Antarctica (Majewski 2005). Fossil material was derived from Bathonian black clays of the Ore-Bearing Częstochowa Clay Formation, which is well-exposed in the Częstochowa Region, central Poland (Matyja and Wierzbowski 2006; Gedl and Kaim 2012). The material is characterised by excellent preservation of pristine test structure (i.e., preservation of characteristic test structures in different taxonomic groups, open pores, and lack of any evidence of dissolution or recrystallization)and mineralogy as exemplified by unequivocal evidence of original aragonite mineralogy of Epistomina (Robertinida) (see below). Selected taxa of the Middle Jurassic Lagenata (multichambered uniserial Nodosaria pulchra [Franke, 1936] and planispiral Lenticulina mamillaris [Terquem, 1886] and unilocular Lagena globosa [Montagu, 1803]), Rotaliida (planktonic Globuligerina bathoniana [Pazdrowa, 1969], and benthic Bulimina sp.), Robertinida (Epistomina nuda Terquem, 1883, and Epistomina regularis, Terquem, 1883), Miliolida (Ophthalmidium carinatum Pazdro, 1958; ?Cornuspira radiata [Terquem, 1886]; Planiinvoluta sp.) and Spirillinida (Paalzowella pazdroe Bielecka and Styk, 1969) species as well as Recent Rotaliida (benthic Elphidium crispum [Linnaeus, 1758] and planktonic Globigerinoides sp.), Miliolida (Quinqueloculina arenata Said, 1949), Spirillinida (Patellina sp.), Textulariida (Textularia sp., Gaudryina sp.) and Lagenata (Procerolagena gracilis Williamson, 1848) species (Fig. 1) were screened using a Zeiss Σigma VP field emission scanning electron microscope (FESEM) at the Faculty of Geology, University of Warsaw. The examined specimens were coated with 4–10 nm of gold.
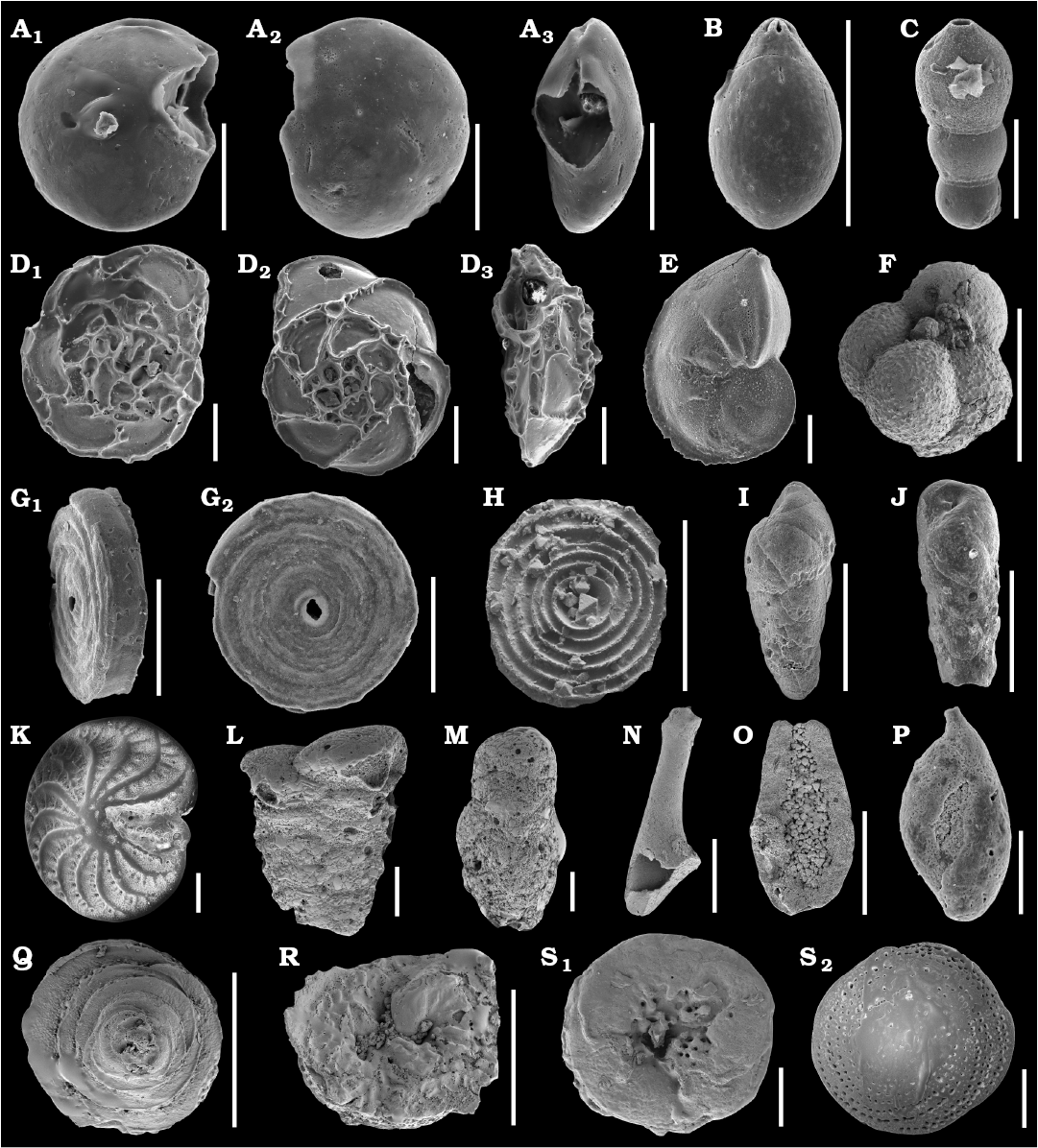
Fig. 1. SEM images of studied Jurassic foraminifers from the Bathonian clays of the Ore-Bearing Częstochowa Clay Formation, Poland (A–J, N–R) and Recent foraminifers from Ronsard Bay, Western Australia (K–M, Q–S). A. Epistomina nuda Terquem, 1883, MWGUW ZI/67/58/06, in ventral (A1), dorsal (A2), and lateral (A3) views. B. Lagena globosa (Montagu, 1803), MWGUW ZI/67/08/2.11, in lateral view. C. Nodosaria pulchra (Franke, 1936), MWGUW ZI/67/08/1.05, in lateral view. D. Epistomina regularis Terquem, 1883, MWGUW ZI/67/58/07, in ventral (D1), dorsal (D2), and lateral (D3) views. E. Lenticulina mamillaris (Terquem, 1886), MWGUW ZI/67/08/2.02, in lateral view. F. Globuligerina bathoniana (Pazdrowa, 1969), MWGUW ZI/67/61/12, in lateral view. G, H. Cornuspira radiata (Terquem, 1886). G. MWGUW ZI/67/08/2.09, in oblique (G1) and front (G2) views. H. MWGUW ZI/67/MG1/50, in spiral, inside of the test view. I, J. Bulimina sp. I. MWGUW ZI/67/57/16, in lateral view. J. MWGUW ZI/67/57/18, in lateral view. K. Elphidium crispum (Linnaeus, 1758), MWGUW ZI/67/55/26, in spiral view. L. Textularia sp., MWGUW ZI/67/55/24, in lateral view. M. Gaudryina sp., MWGUW ZI/67/61/16, in lateral view. N, O. ?Planiinvoluta sp. N. MWGUW ZI/67/61/24, in lateral view. O. MWGUW ZI/67/61/25, in inside of the test view. P. Ophthalmidium carinatum Pazdro, 1958, MWGUW ZI/67/08/5.03, in lateral view. Q, R. Paalzowella pazdroae Bielecka and Styk, 1969. Q. MWGUW ZI/67/61/05, in dorsal (spiral) view. R. MWGUW ZI/67/61/07, in ventral (umbilical) view. S. Patellina sp., MWGUW ZI/67/61/22, in ventral (umbilical) (S1) and dorsal (spiral) (S2) views. Scale bars 100 µm.
Mineralogy of Jurassic supposedly aragonitic (Epistomina nuda and Epistomina regularis [Robertinida]) and calcitic (Paalzowella pazdroe [Spirillinata] and Lenticulina mamillaris [Lagenata]) species has been determined using a Raman spectroscopy at the Faculty of Chemistry, University of Warsaw in order to assess their state of preservation. The analysis have been conducted with a dispersive Raman spectrometer (LabRam HR800 dual microscope, Horiba Jobin-Yvon) coupled with an Olympus BX61 confocal microscope, provided with a 50× objective, using a diode-pumped, frequency-doubled Nd:YAG laser (532 nm, Pmax = 100 mW on the head). The spectrometer was equipped with a Peltier-cooled CCD detector (1024 × 256 pixels). The calibration standard used was the well-known 520 cm−1 Raman band of a silicon chip. This method allowed identification of calcium carbonate polymorphs (calcite and aragonite) by comparing obtained peak assignments (Fig. 2) with literature references (e.g., Urmos et al. 1991; Taylor et al. 2008; Parker et al. 2010; Clode et al. 2011). The most convenient signals are grouped in the 100–300 cm−1 region.
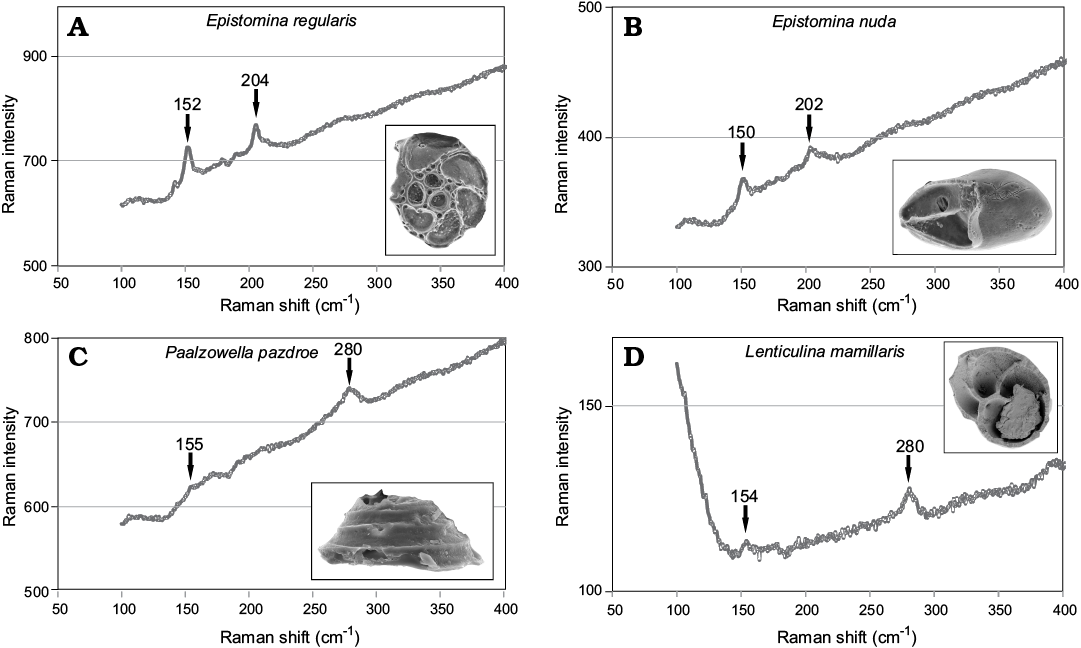
Fig. 2. A, B. Typical spectra of aragonite with well visible peaks at ca. 155 cm−1 and 204 cm−1 collected from Robertinida shells from Bathonian clays of Gnaszyn, Poland. A. Epistomina regularis Terquem, 1883, MWGUW ZI/67/63/18. B. Epistomina nuda Terquem, 1883, MWGUW ZI/67/63/08. C, D. Typical spectra of calcite with well visible peaks at ca. 155 cm−1 and 280 cm−1 obtained from tests from Bathonian clays of Gnaszyn, Poland. C. Spirillinata, Paalzowella pazdroe Bielecka and Styk, 1969, MWGUW ZI/67/63/07. D. Lagenata, Lenticulina mamillaris Terquem, 1883, MWGUW ZI/67/63/20.
Results
Recent benthic Elphidium crispum and planktonic Globigerinoides sp. as well as Jurassic benthic Bulimina sp. and planktonic Globuligerina bathoniana are composed entirely of nanograins with diameters up to 100 nm (Figs. 3, 4) grouped into higher-level aggregates. Nanograins can be additionally arranged to some extent in some even higher-order unites such as rows or columns as exemplified by Bulimina whose textural nanograins are assembled into elongated bundles.
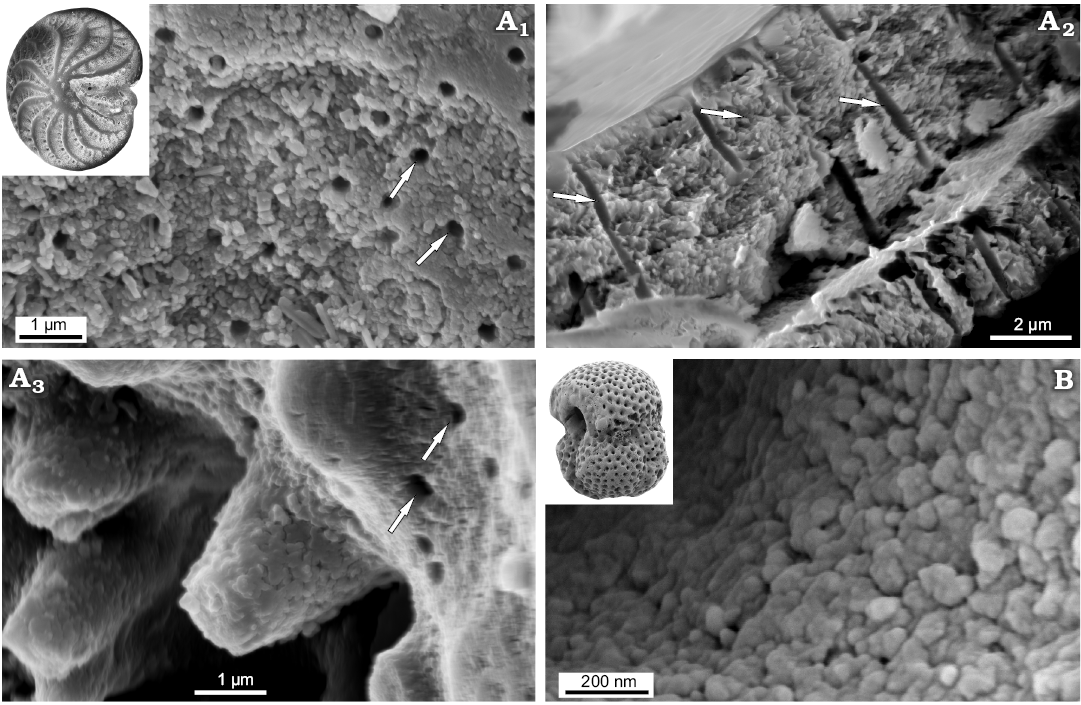
Fig. 3. FESEM images of the test structure in Recent rotaliid foraminifers from Ronsard Bay, Western Australia: benthic Elphidium crispum Linnaeus, 1758, MWGUW ZI/67/55/26 (A) and planktonic Globigerinoides sp., MWGUW ZI/67/61/15 (B). Front view of the test surface (A1, B); cross section of the wall (A2); front view of pustules, showing the nanogranular test structure (A3). Arrows indicate pores.
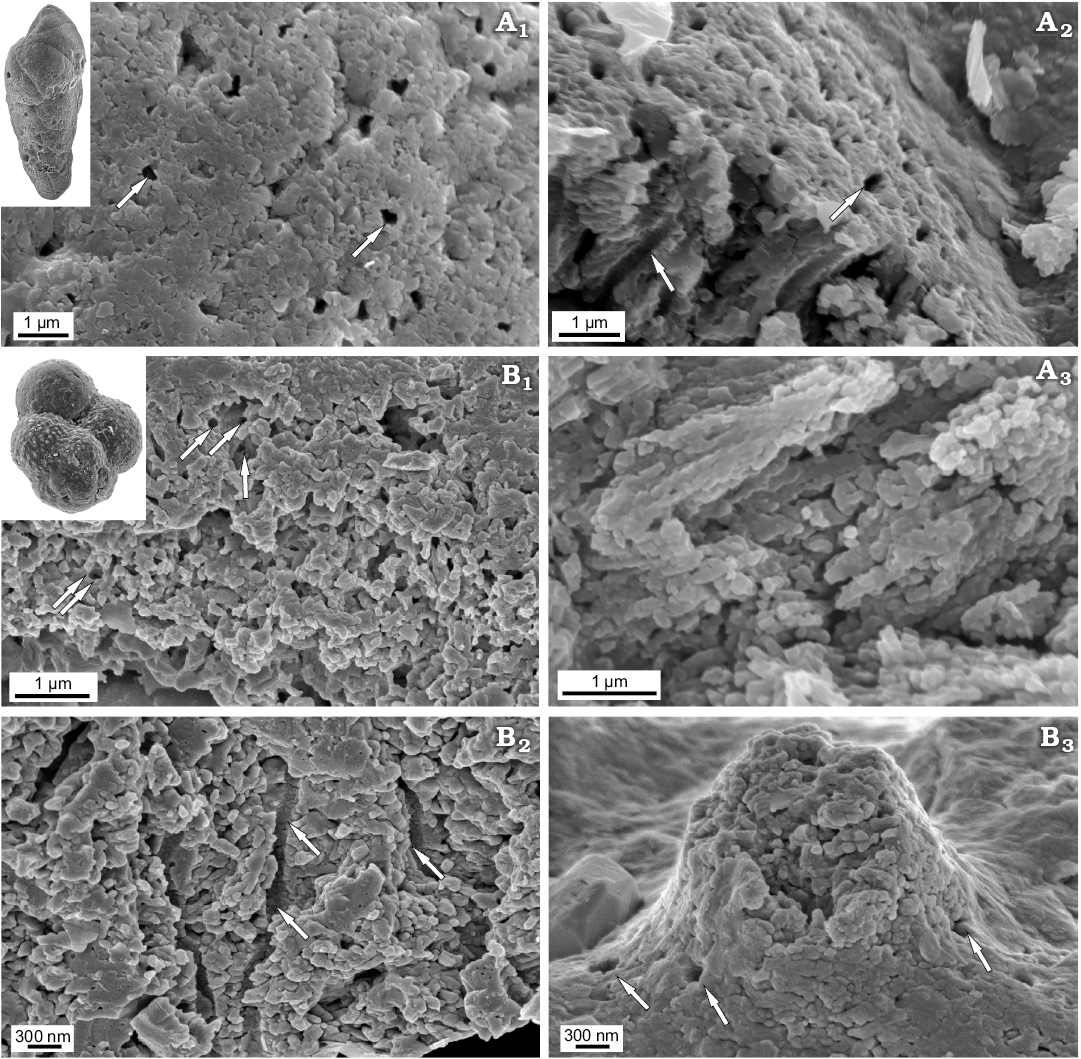
Fig. 4. FESEM images of the test structure in rotaliid foraminifers from the Jurassic of Gnaszyn, Poland: benthic Bulimina sp., MWGUW ZI/67/57/16 (A) and planktonic Globuligerina bathoniana Pazdrowa, 1969, MWGUW ZI/67/61/12 (B). Front view of the test surface (A1, B3); oblique cross-sectional view of the wall (A2, B1); cross section of the wall (A3, B2); front view of pustule, showing its entirely nanogranular test texture (B3). Arrows indicate pores.
Newly studied Jurassic (Bathonian) representatives of aragonitic Robertinida Epistomina nuda and E. regularis are characterised by lamellar tests composed of a few finely perforated internal layers ranging from 5‒30 μm in thickness and an inner lamina (~500 nm) occurring inside the test. Very thin outer lamina (~50 nm) which was noticed on the top of the test surface of Recent robertinid taxa Hoeglundina by Nakajima et al. (2017) is hardly visible in the studied fossil material. Both the interior test wall (internal layers) and inner test lamina of the studied Epistomina are made up of nodular nanograins (20‒100 nm in diameter) (Figs. 5, 6) identical to that of Rotaliida. In turn, the test structure when observed at a slightly lower magnification, differs significantly from representatives of Rotaliida. Namely, studied robertinids display test composition that is much better organised, especially clearly visible in the test cross section. Specifically, each internal layer is composed of pillar units (ca 1 μm in width) aligned in the same direction, perpendicular to the test surface, and separated from each other by ca. 500 nm (Fig. 6A5, A6). Inside each pillar unit, inner pores, ca. 100‒200 nm in diameter, extend along the entire unit, resulting in a fine test perforation. The smooth outer and inner layer surfaces, visible at low magnification, result from tightly-packed crystallite arrangements.
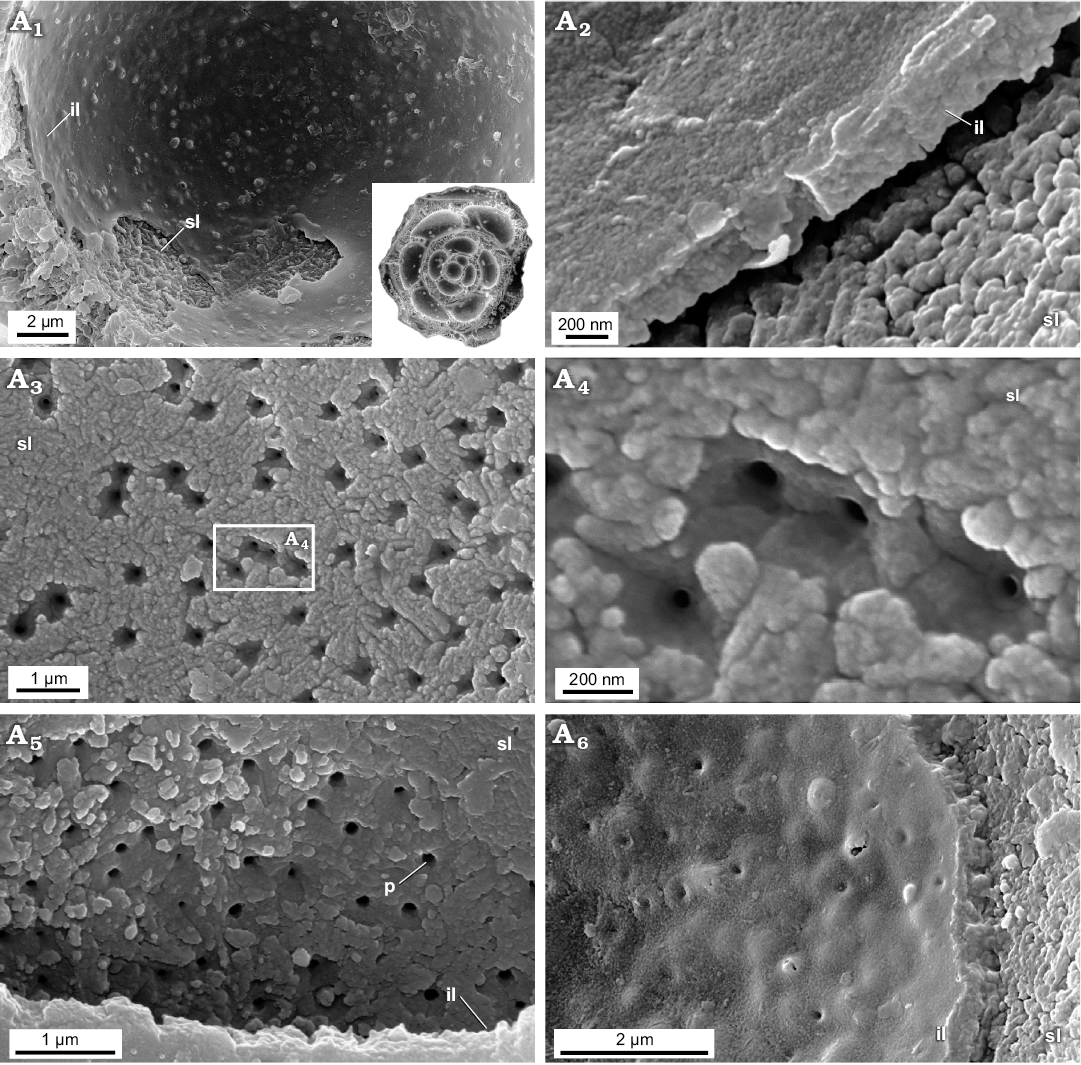
Fig. 5. FESEM images of the test structure in Epistomina nuda Terquem, 1883, MWGUW ZI/67/54/20 (Robertinida, Globothalamea) from the Jurassic of Gnaszyn, Poland; views from the chamber interior showing the finely porous and nanogranular texture of both the surface of the internal layer and the inner lamina. Front view of the chamber interior (A1); oblique cross-sectional view of inner lamina (A2); front view of the surface of internal layer (A3, A5); significantly magnified view of the internal layer surface (A4); oblique view of the inner lamina (A6). Abbreviations: il, inner lamina; p, pore; sl, surface of internal layer (columnar domain).
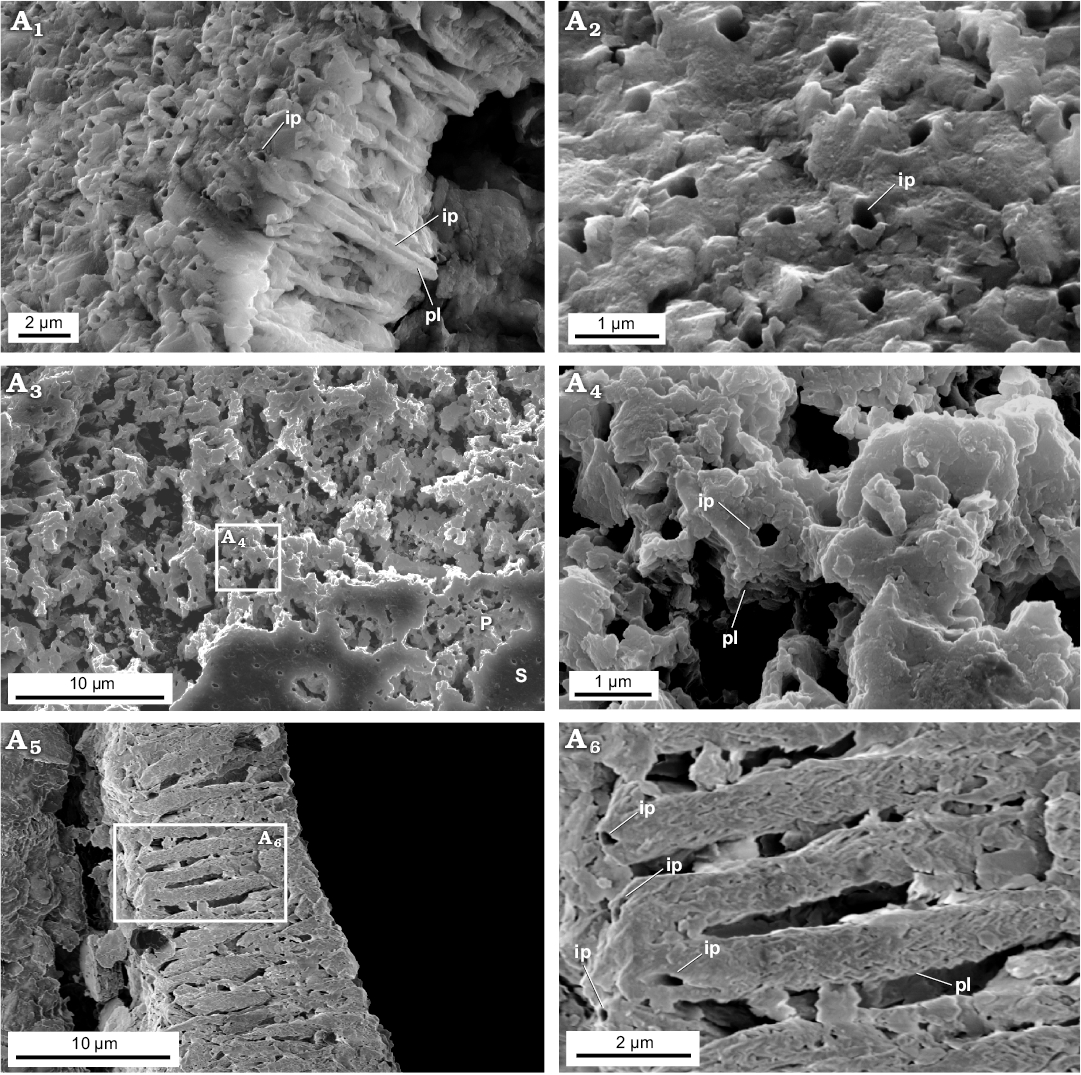
Fig. 6. FESEM images of the test structure in Epistomina nuda Terquem, 1883, MWGUW ZI/67/54/20 (Robertinida, Globothalamea) from the Jurassic of Gnaszyn, Poland. Side view of the test showing finely perforated test surface and cross section of columnar domain (A1); front view of the test surface (A2); front view of the outer test surface showing a well-preserved, smooth (S) and slightly abraded (P) outer test surface (A3), significantly magnified view of abraded test surface exposing columnar domain (A4); cross section of the test showing pillar unit composition (columnar domain) (A5); detail of the columnal domain (A6). Abbreviations: ip, inner pores, pl, pillar.
The studied Recent representatives of the order Texturaliida Textularia sp. and Gaudryina sp. possess their tests agglutinated composed of relatively large (up to ca. 20 µm) foreign particles cemented with calcite. Calcareous cement of these taxa is porous, with pore diameters of ca. 500 nm‒5 µm, and is composed of roughly rounded nanograins less than 100 nm in diameter (Fig. 7A, B) similar to that of other representatives of Globothalamea.
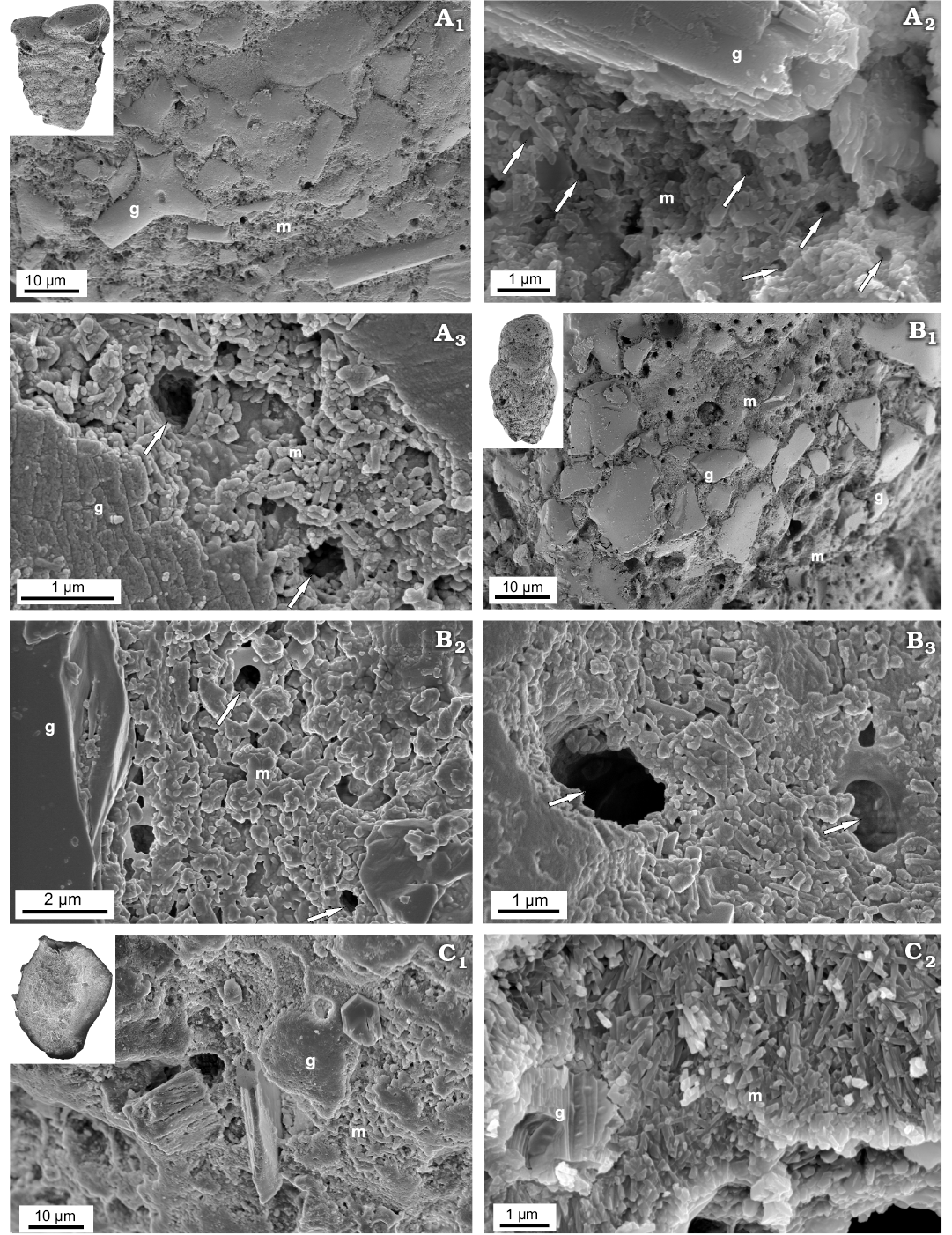
Fig. 7. FESEM images of the test structure in Recent calcareous cemented agglutinated textulariid (Globothalamea; A, B) and miliolid (Tubothalamea; C) foraminifers from Ronsard Bay, Western Australia. A. Textularia sp., MWGUW ZI/67/55/24, front view of the test, showing agglutinated grains and the calcareous nanogranular matrix (A1); details of test wall (A2, A3). B. Gaudryina sp., MWGUW ZI/67/61/16, front view of the test, showing agglutinated grains and the matrix (B1); details of nanogranular matrix (B2, B3). C. Quinqueloculina arenata Said, 1949, MWGUW ZI/67/57/02, front view of the test (C1); oblique cross-sectional view of test showing foreign particle partially embedded in the irregular meshwork of needle-shaped crystallites (C2). Abbreviations: g, foreign particle; m, calcareous matrix. Arrows indicate pores.
Jurassic Miliolid taxa Ophthalmidium which displays a miliolid type of coiling is mainly composed of needle-shaped crystallites (up to 1 µm in length and 200 nm in thickness) making up the wall inside, the so-called intrados. In turn, outer lamina (extrados) is made up of nanograins similar to that of the Recent representatives of the true miliolids, e.g., with miliolid chamber test arrangement. However, the extrados of the studied Ophthalmidium, which is composed of amalgamated irregular sheets (Fig. 8), slightly differs from Recent representatives in the shape and arrangement of polygons. The studied Bathonian morphologically simple taxa: bilocular planispirally coiled ?Cornuspira radiata and irregularly tubular Planiinvoluta sp. have their shells entirely composed of miliolid-type needle-shaped crystallites (up to 1 µm in length and 200 nm in thickness (Fig. 8B, C). Interestingly, the granular outer mineralised test surface (extrados) is not observed. The inner and outer test surfaces of ?C. radiata and Planiinvoluta sp. are composed of needle-shaped elements building up the wall inside (intrados).
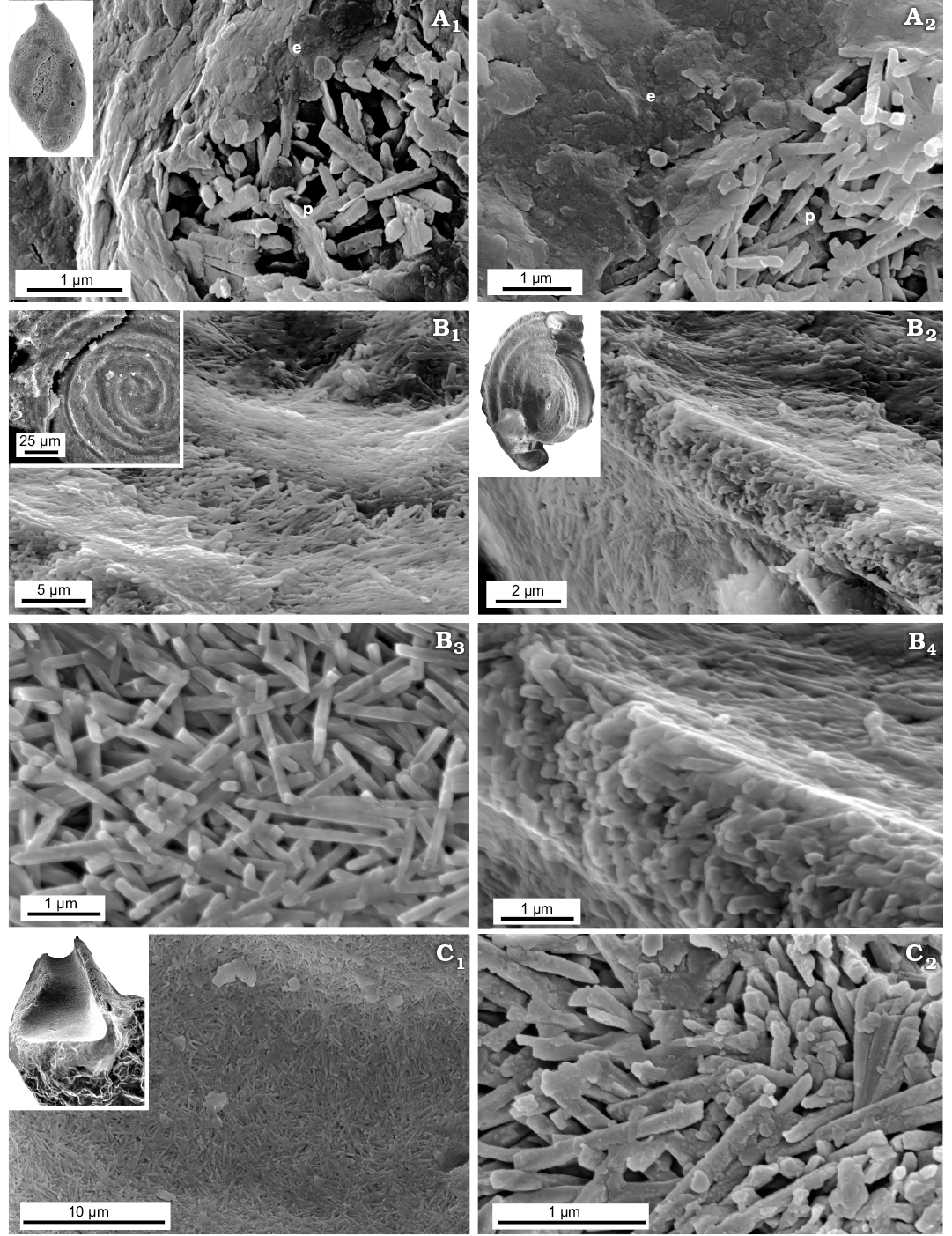
Fig. 8. FESEM images of the test structure in Tubothalamea from the Jurassic of Gnaszyn, Poland. A. Ophthalmidium carinatum Pazdro, 1958, MWGUW ZI/67/08/5.03; front views of the abraded test surface, showing the extrados and porcelain (A1, A2). B. ?Cornuspira radiata (Terquem, 1886), MWGUW ZI/67/55/11; front view of the test surface (B1, B3); oblique view of the test cross section, showing the test as being entirely composed of needle-shaped crystallites (B2, B4). C. Planiinvoluta sp., MWGUW ZI/67/57/13; view of the inner test surface (C1); side view of the test cross section, showing irregular meshwork of needle-shaped crystallites (C2). Abbreviations: e, extrados; p, porcelain.
Both Recent Patellina sp. and Bathonian Paalzowella pazdroe, which are traditionally placed within the order Spirillinida despite that they are characterized by more complex test (conical, multichambered test composed of elongate tubular chambers of about one whorl in the youngest part of the test and then with two chambers per whorl with characteristic septal pattern) display another wall-type. Namely, their tests are built of a few layers (Fig. 9A6, A7) which appear to be made of a single crystal, with well visible cleavage surfaces (Fig. 9A2, B1, B2). Significantly magnified images of the test cross-section reveals spherical grains, a few nm in size, which look like the smallest building units observable at the nanoscale. The very small pore-like features (around 10 nm) illustrated in Fig. 9A2 possibly would be structural deficiencies.
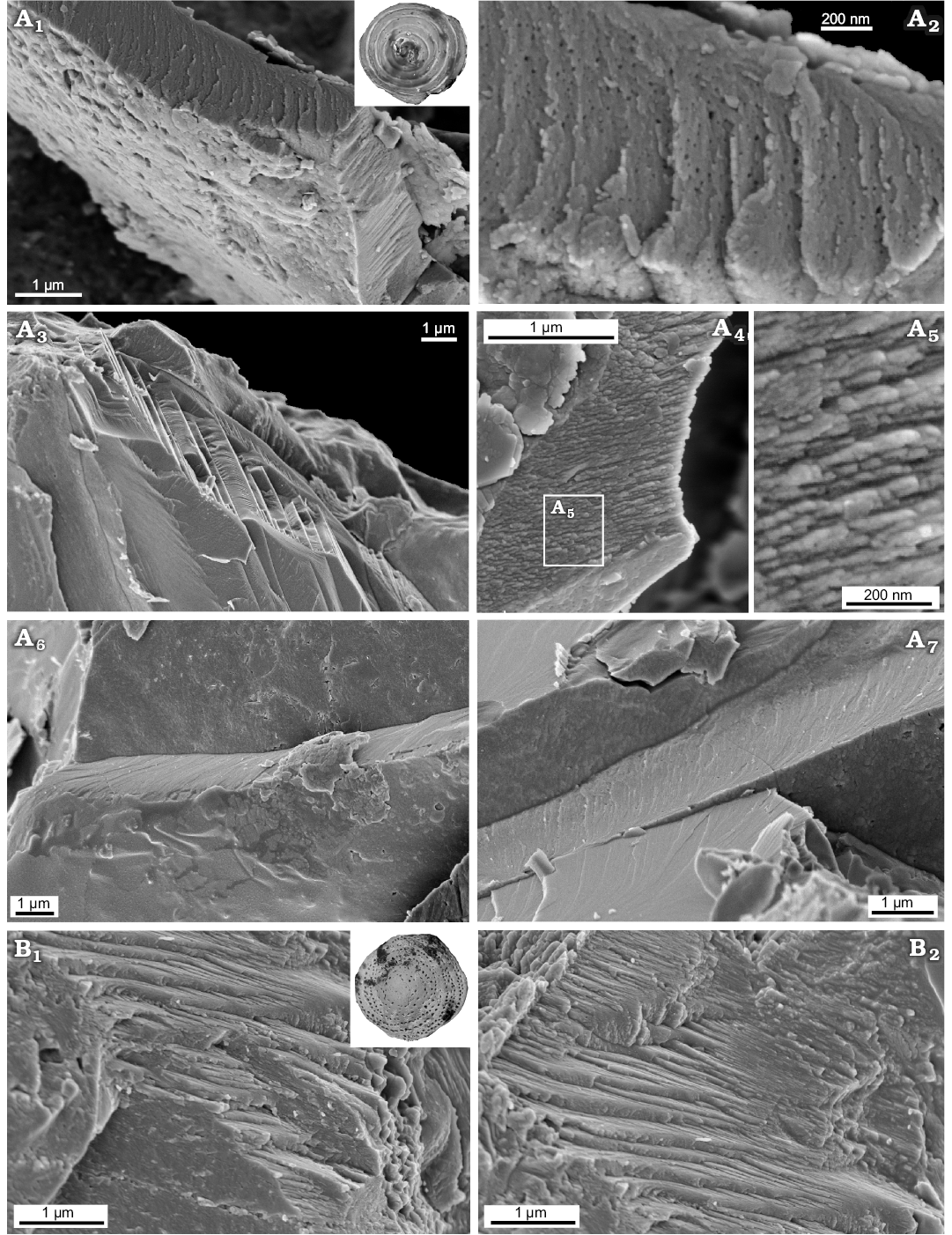
Fig. 9. FESEM images of “monocrystalline” test structure in Spirillinata foraminifers from the Jurassic of Gnaszyn, Poland (A) and Recent from Ronsard Bay, Western Australia (B). A. Paalzowella pazdroe Bielecka and Styk, 1969, MWGUW ZI/67/61/27; view of the test cross-section (A1); significantly magnified view of the test cross-section (A2, A4, A5); oblique cross-sectional view of the test showing “monocrystalline” test structure (A3); oblique cross sections of the test showing test composed of a few layers (A6, A7). B. Patellina sp., MWGUW ZI/67/61/22; oblique cross sections of the test showing prominent calcite cleavage (B1, B2).
Recent unilocular Lagenidae Procerolagena gracilis and Jurassic unilocular Lagena globosa and plurilocular Nodosariidae planispiral Lenticulina mamillaris and uniserial Nodosaria pulchra, show that both single- and multichambered representatives of Lagenata display an identical wall type. All these taxa display fibrous texture (Fig. 10; see also Dubicka et al. 2018) composed of relatively large interlocked single-crystal fibres oriented perpendicular to test surfaces with an inner pore (ca. 200 nm in diameter) extending along each unit.
The Raman spectra obtained from the studied foraminiferal tests show clear differences. The distinct diagnostic bands for aragonite at ca. 204 cm−1 and 155 cm−1 have been collected from Epistomina regularis and Epistomina nuda (Robertinida). In turn, the collected spectra of the test samples of Lenticulina mamillaris (Lagenata) and Paalzowella pazdroe (Spirillinata) display clear diagnostic peaks for calcite at ca. 155 cm−1 and 280 cm−1 (Fig. 2).
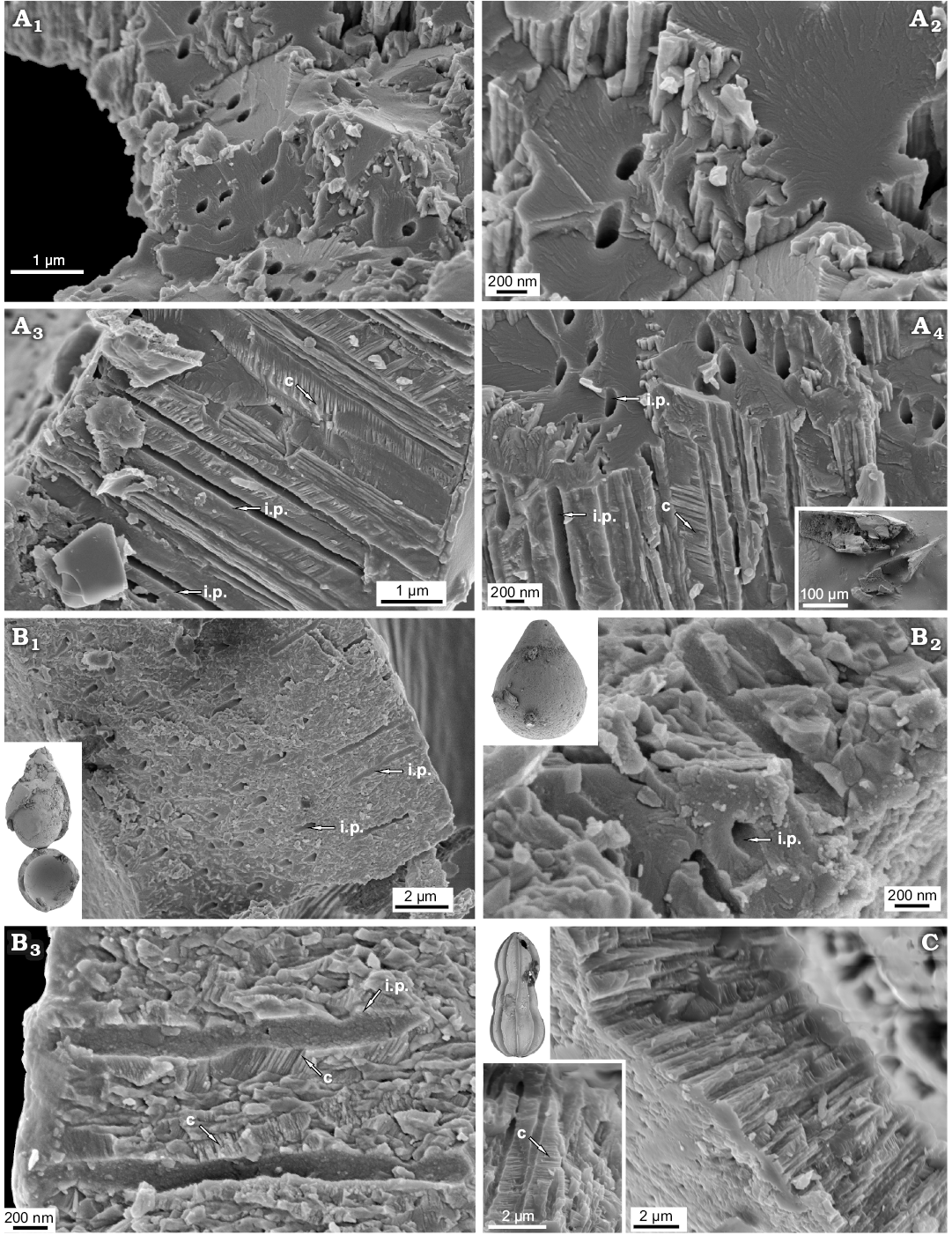
Fig. 10. FESEM images of the test structure in lagenid foraminifers from Recent, Admiralty Bay, King George Island, West Antarctica (A) and from the Jurassic of Gnaszyn, Poland (B, C). A. Unilocular Procerolagena gracilis Williamson, 1848, MWGUW ZI/67/44/02. B. Unilocular Lagena globosa Montagu, 1803, MWGUW ZI/67/61/09. C. Uniserial Nodosaria pulchra Franke, 1936, MWGUW ZI/67/61/26. Oblique cross-sectional views (A1, A2, A4, B1, B2, C); transverse cross-sectional views, showing single-crystal interlocked bundle structures, inner pores which extend along the entire length of the bundles as well as prominent calcite cleavage (A3, B3). Abbreviations: c, prominent calcite cleavage; ip, inner pore.
Discussion
The class Globothalamea of Pawlowski et al. (2013, 2014) comprises multichambered calcareous or agglutinated foraminifers with globular or crescent-shaped chambers (at least in the early stage of ontogeny) and minimal distances between successive apertures. Globothalamea encompasses the following orders: calcitic Rotaliida, aragonitic Robertinida, agglutinated Textulariida, and calcitic Carterinida. The tests of Rotaliida (including the previously distinguished orders Buliminida, Globigerinida, and Rotaliida in the sense of Loeblich and Tappan 1987) of Recent (Fig. 3) and Cretaceous taxa (Dubicka et al. 2018; see also Wood 1949; Krasheninnikov 1956; Towe and Cifelli 1967; Debenay et al. 1996, 2000), as well as of newly studied Jurassic planktic (Globuligerina bathoniana) and benthic (Bulimina sp.) species are composed entirely of granular nanograins with diameters up to 100 nm (Figs. 3, 4) which are grouped into higher-level aggregates. These aggregates can be arranged to some extent in some rows, needles, columns, or tablets (Debenay 2000) and that results in translucent to light rotaliid tests (hyaline walls; e.g., Hohenegger 2009). It should be noted that the earliest planktonic foraminifers are believed to have possessed aragonitic walls (Werneli 1988; Hillebrandt 2012). Aragonitic test composition imposes an interpretation that aragonitic Jurassic planktonic foraminifers separates from younger calcitic rotaliid planktonic species and are placed within Robertinida (e.g., Rigaud et al. 2015). The other authors postulated that traditional suborder Globigerinina includes both calcitic and aragonitic taxa and would be rather monophyletic group (Simmons et al. 1997; Görög and Werneli 2003; Sen Gupta 2003). Nevertheless, further studies, especially at a higher level in skeletal hierarchy, are necessary in order to understand the relationship between text structure and particular taxonomic groups of such diversified rotaliids.
In contrast to calcitic Rotaliida, Robertinida have shells made of aragonite (Loeblich and Tappan 1987; Nakajima et al. 2017). The test of Robertinida has been descried by several authors (Rigaud et al. 2015; and literature cited there) as made of radially arranged aragonite needles observed by light microscopy. Unfortunately, most of these aragonitic fossil foraminifers were recrystallized into sparitic calcite. SEM studies of Recent robertinids (Hoeglundina elegans) of Nakajima et al. (2017) and this study of pristine preserved Jurassic (Bathonian) Epistomina (E. nuda and E. regularis) indicate that, similarly to rotaliids (see Dubicka et al. 2018), aragonitic robertinids are characterised by lamellar tests entirely composed of nanograins comparable to Rotaliida nanoparticles. Nakajima et al. (2017) additionally suggested that these nanograins consist of 5-nm spherical subunits. The nanogranular composition of Robertinida supports its affinity with the class Globothalamea. On the other hand Robertinids differ from Rotaliida in possessing well-organised columnar domains (visible when observed at a slightly lower magnification) forming a few finely perforated internal lamellas (see also Reiss and Schneidermann 1969). This variation in the composition of the higher-order textural properties supports the molecular phylogenetic tree (Bowser et al. 2006; Pawlowski et al. 2013) in which Robertinida branches off from all Rotaliida. Furthermore, the distinctive test structure of the two orders, which existed as early as in the Middle Jurassic, does not support the classical opinion that all calcitic Rotaliida, including buliminids and planktonic foraminifers, originated directly from aragonitic robertinids (e.g., Fuchs 1973, 1975; Tappan and Loeblich 1988; Simmons et al. 1997). This theory has also been challenged by molecular studies (e.g., Darling et al. 1997, 2009; De Vargas et al. 1997; Bowser et al. 2006; Schweizer et al. 2008; Ujiié et al. 2008; Pawlowski et al. 2013) and early morphology-based evolution reconstructions of Robertinids (Hillebrandt 2010, 2012; Rigaud et al. 2015; Rigaud and Blau 2016), which indicated a polyphyletic Mesozoic origin for calcitic and aragonitic Globothalamea. Nevertheless, the origin of the early rotaliids, both benthic and planktonic, is still unclear (Hart et al. 2003; Rigaud and Blau 2016) and demands further study.
“Textulariida”, in the sense of Pawlowski et al. (2013), encompasses multichambered agglutinated foraminifers with tests composed of foreign particles attached to an organic lining or cemented by low magnesium calcite; term “Textulariida” is used by Pawlowski et al. (2013: 8) “only partially in the sense of the definition given by Loeblich and Tappan (1987), and includes the most of the Lituolida, the Loftusiida, and the Textularida (sensu stricto), of Kaminski (2004)”. On the molecular phylogenetic tree, this group, despite being agglutinated, forms a sister group of Rotaliida; both branches are numbered among globothalameans (Pawlowski et al. 2013). Interestingly, some rotaliids such as cibicidids are also capable of agglutinating particles (Alexander and DeLaca 1987). Besides Roberts and Murray (1995) documented aragonite as biomineralized cement in the agglutinated foraminiferan Textularia crenara. It is noteworthy that, according to laboratory culture experiments, the calcareous cement of textulariids (Clavulina, Textularia, and Valvulina) is biomineralised by the foraminifer itself (Bender and Hemleben 1988). Additionally, my study of the test wall of Recent Textularia sp. and Gaudryina sp. reveals that the calcareous cement of these taxa is porous, and is composed of roughly rounded nanograins (Fig. 7A, B; see also Bender and Hemleben 1988: pl. 2: 7, 8) similar to that of other representatives of the class Globothalamea. The nanogranular structure of textulariid cements agrees with the classification of Textulariida as a part of Globothalamea.
To sum up, the test structure of the studied representatives of the three orders of the class Globothalamea, namely, calcitic Rotaliida, aragonitic Robertinida, and calcite-agglutinated Textulariida, differs at the higher level of the skeletal hierarchy; however, all secrete calcite/aragonite in the form of comparable rounded nanoparticles (Table 1).
Table 1. Foraminiferal classification based on ultra-structural characteristic of the test.
|
Higher-level |
Primary crystallites |
Type of coiling |
Lower-level taxonomic groups (orders) |
Comments |
|
Tubothalamea |
needle-shaped, up to 1 µm in length |
tubular chambers (at least in early stage of ontogeny) and maximal distance between apertures |
Miliolida |
miliolid-like coil (smaller
foraminifera); well-developed nanogranular extrados; |
|
“cornuspirids” |
straight, irregularly coiled or
spiralling tube; |
|||
|
Globothalamea |
roughly globular nanograins |
globular or crescent-shaped chambers (at least in early stage of ontogeny) and minimal distance between successive apertures |
Rotaliida |
test entirely composed of globular nanograins arranged to some extent in some rows, needles or tablets |
|
Textulariida |
foreign particles cemented by calcareous matrix composed of globular nanograins |
|||
|
Robertinida |
wall composed of perforated pillar units |
|||
|
Lagenata |
relatively large single-crystal fibres oriented perpendicularly to test surfaces, with inner pores extending along the entire length of the bundle |
globular or crescent-shaped chambers and terminal chamber formation |
|
both single- and multichambered forms |
|
Spirillinata |
monocrystalline wall-type |
cone-shaped, multichambered test with elongate spiral chamber followed by semicircular chambers with pseudosepta |
|
|
In contrast, the class Tubothalamea of Pawlowski et al. (2013) is composed of species belonging to two distinctive orders, Miliolida and Spirillinida, and, generally speaking, encompasses foraminifers whose chambers are primarily (at least in early representatives of the groups) tubular, as opposed to globular or crescent-shaped globothalamean chambers. Furthermore, miliolid wall (the so called porcelaneous wall) texture is made up of primary crystallites different from those of all studied globothalameans and lagenids (see Dubicka et al. 2018). Specifically, miliolid crystallites are needle-shaped elements up to 1 µm long and 200 nm wide (Parker 2017; Dubicka et al. 2018 and literature cited therein). This difference is consistent with the distinctive mode of biomineralisation of porcelain- and hyaline-type foraminifers (e.g., Debenay et al. 2000; Erez 2003; De Nooijer et al. 2009, 2014). At the higher level of textural hierarchy, miliolid tests display (Towe and Cifelli 1967; Debenay et al. 2000; Parker 2017; Dubicka et al. 2018) three distinctive constituents, including (i) the porcelain, which constitutes the main body of the test, composed of randomly oriented needle-shaped crystallites; (ii) the intrados, which constitutes the inner test surface, composed of tightly-spaced needle-shaped crystallites identical to those of the porcelain; and (iii) the extrados, which constitutes the outer test surface, composed of aligned or non-aligned polygons composed entirely of round nanograins. This may indicate that whereas needle-shaped crystallites are formed inside cytoplasmic vesicles and then transported outside the test (see Angell 1980; Hemleben et al. 1986; Debenay et al. 2000; Toyofuku et al. 2000; Erez 2003), rounded nanograins may be initially produced at the organic membrane, forming a new chamber, similarly to the hyaline type of biomineralisation (for hyaline biomineralisation mechanism see e.g., Ter Kuile et al. 1989; Debenay et al. 2000; De Nooijer et al. 2009, 2014). The shape of calcite polygons composing the extrados and the degree of polygon alignment vary significantly; thus these features represent the main differences between the wall textures of distinctive miliolid taxa. Six principal types of extrados have been distinguished within Recent Miliolida by Parker (2017). The Bathonian miliolid taxa Ophthalmidium studied herein are consistently composed of needle-shape primary crystallites (Fig. 8A1, A2) and display a tripartite test structure similar to that of modern miliolids. Moreover, the extrados is composed of round nanograins, as in the case of Recent Miliolida (Dubicka et al. 2018). However, the structure of the extrados of the studied Ophthalmidium, composed of amalgamated irregular sheets, differs from all the extrados types described by Parker (2017). Microstructural observations of other species, especially from different geological periods, may reveal a greater variation in extrados types than currenty recognized. Interestingly, the calcareous cement of agglutinated miliolid coiling foraminifers (e.g., Agglutinella soriformis, Quinqueloculina cf. Q. agglutinas, Rudoloculina hooperi) already published (Mikhalevich et al. 1986; Hottinger et al. 1993; Guilbault and Patterson 1998; Rigaud and Martini 2016; Parker 2017; Dubicka et al. 2018), as well as that studied in this paper (Quinqueloculina arenata), has turned out to be composed of the same type of needle-shaped crystallites (Fig. 7C1, C2) as those found in entirely calcareous foraminiferal porcelain. Data from this test structure are consistent with the molecular studies of Fahrni et al. (1997) and Habura et al. (2006), which clearly show that agglutinated-porcelanous miliolid coiling taxa such as Miliammina, and possibly other members of Rzehakinidae, belong to the order Miliolida, as opposed to their former assignment in the order Lituolida (Habura et al. 2006; for Lituolida definition see e.g., Loeblich and Tappan 1987; Sen Gupta 2003).
The second order Spirillinida of the class Tubothalamea forms a sister clade to Miliolida according to molecular studies (Pawlowski et al. 2013). Conventionally Spirillinida is defined (Loeblich and Tappan 1987; Sen Gupta 2003; Pawlowski et al. 2013) as a group of foraminifers having a proloculus followed by a tubular undivided second chamber or with a few chambers, terminal aperture and a wall of low magnesium calcite, optically composed of a single crystal or few to a mosaic of crystals, the so-called monocrystalline texture, in Spirillinidae and Patellinidae (wall agglutinated in Ammodiscidae; Pawlowski et al. 2013). However, what “monocrystalline” test texture depicts is still far from being understood. Towe and Cifelli (1967: 744) indicated that the single crystal concept, applied to spirillinid test wall, can be interpreted in different ways depending on the different optical instruments used for study and “little or no consideration has been given to the possible ways that such crystals may form and grow in the calcified walls”. The spirillinid wall has been observed mostly using light microscopy. No studies showing significantly magnified view of monocrystalline test composition have been published. Regrettably, scanning electron microscopy (SEM) studies of Patellina corrugata (Berthold 1976) are rather of low magnification (maximum magnification of around 5000×) precluding record of wall ultrastructure. Furthermore, with regard to the monocrystalline texture concept, Towe and Cifelli (1967: 744) pointed out that: “what appears to be uniform crystals in the polarizing microscope are often seen to be mosaics of much smaller units in the electron microscope. It is not normally possible to predict from what is seen in the polarizing microscope what the nature of these aggregates will be at higher resolution.” In general, calcareous Mesozoic–Recent foraminifers of simple bilocular coiled test morphology can either belong to Miliolida (specifically to Cornuspirinae), Spirillinida or Involutinida (see Loeblich and Tappan 1987) and have either porcelaneous, hyaline calcitic or hyaline aragonitic tests, respectively (Hohenegger 2018). As an example, the test structure of the studied Bathonian bilocular planispirally coiled foraminifers, which were commonly described as “Spirillina radiata” (see e.g., Bielecka and Styk 1981; Bielecka et al. 1988; Smoleń 2012), has a shell consisting of miliolid-type needle-shaped crystallites (Fig. 8B). Therefore, this form should be rather assigned to Miliolida or some distinct group within Tubothalamea, possibly the porcelaneous genus Cornuspira. Interestingly, while the wall interior of ?C. radiata is very similar to that of unquestionable miliolids, the extrados (the outer mineralised surface, see Parker 2017) typical of Miliolida is not observed. The inner and outer test surfaces of ?C. radiata appear to be similar, being composed of needle-shaped elements building up the wall inside. Therefore, apparently there is a lack of biomineralisation stage involving formation of calcium carbonate outside the cell. The miliolid test crystallites are also exhibited by studied primitive tubular Bathonian encrusting foraminifers (Fig. 8C) tentatively assigned to the genus Planiinvoluta.
In turn, more complex representatives of Spirillinida, both Recent Patellina sp. and Bathonian Paalzowella pazdroe, which are characterized by cone-shaped, multichambered test rather than having undivided spiralling tube show another type of test texture which is apparently the real “monocrystalline” wall-type (Fig. 9) characterizing the true order Spirillinida. Textural studies of spirillinids additionally confirm a statement that the foraminiferal test structure is very stable taxonomic feature that can be used for high-rank taxonomic separation. Regrettably, however, molecular studies of Pawlowski et al. (2013) of the order Spirillinida were performed with a single calcareous species only, Spirillina sp., without test structure verification. Accordingly true spirillinids characterised by monocrystalline wall structure can represent distinct taxonomic group, possibly an independent class (Spirillinata), separate from Tubothalamea especially if Berthold (1976) pointed out that “the morphogenetic processes and test morphology of Patellina differs significantly from the general test morphology of Spirillina and that of other foraminifers”. Therefore it seems that more integrated molecular and textural studies on foraminifers having spiralling tube (undivided or secondary subdivided) are necessary in order to understand their phylogeny.
The order Lagenida is traditionally defined as a group of exclusively calcitic (low magnesium calcite) foraminifers which encompasses unilocular to plurilocular species with uniserial, biserial, or planispiral coiling tests and with terminal chamber formation (Loeblich and Tappan 1987, 1992; Pawlowski et al. 2013). Molecular data of Pawlowski et al. (2013), combined with a very distinctive lagenid test texture (see Dubicka et al. 2018) indicate that lagenids possibly represent an independent class Lagenata. In the alternatively-proposed classification by Mikhalevich (2012, 2013) most of the multichambered foraminifers, which traditionally would be assigned to Lagenida, have been classified into the new class Nodosariata. Most recently Rigaud et al. (2016), based on the study of test walls under polarized light, strongly postulated that Nodosariata is a monophyletic group which does not include the unilocular lagenids (Lagenidae). However, test structure studies of the plurilocular Dentalina communis by Dubicka et al. (2018) as well as this study of Recent unilocular Procerolagena gracilis and Jurassic unilocular Lagena globosa and plurilocular Lenticulina mamillaris and Nodosaria pulchra, show that both single- and multichambered lagenid tests display an identical, very peculiar, fibrous texture (Fig. 10; see also Dubicka et al. 2018) composed of interlocked single-crystal fibres oriented perpendicular to test surfaces with an inner pore (ca. 200 nm in diameter) extending along each unit. Therefore unilocular and more complex multichambered representatives of Lagenata, both uniserial and planispiral, are rather closely related and most likely have a common ancestor. Their taxonomic position traditionally placed within one higher-rank taxonomic unit seems to be correct.
Conclusions
This paper shows the test structure of Recent and Jurassic representatives of different foraminiferal high-rank taxonomic groups: Rotaliida, Robertinida, Textulariida, Miliolida, Spirillinata, and Lagenata. Both entirely calcareous and calcareous-agglutinated taxa of the class Globothalamea and Tubothalamea were studied.
Representatives of Rotaliida (both benthic and planktonic), Robertinida, and Textulariida, three different orders of the class Globothalamea, secret calcite as roughly rounded nanograins which may be arranged to a greater or lesser extent. Tests of the studied aragonite Robertinida differ from those of calcite Rotaliida in their possession of well-organised columnar domains made up of perforated pillar units and an inner lamina. This distinctive test texture of Robertinida contradicts the theory concerning the direct origination of Rotaliida from aragonitic robertinids.
The test structure of the studied Jurassic entirely calcareous true Miliolida (possessing complex miliolid chamber coiling tests) is composed of needle-shaped primary crystallites up to 1 µm long (the inside of the wall) as well as roughly globular nanograins forming the outer layer (extrados) similarly to their Recent counterparts. The calcareous cement of agglutinated, miliolid foraminifers are composed of miliolid-type needle-shaped primary crystallites what supports that these taxa belong to the order Miliolida, as opposed to their former assignment in the order Lituolida. Jurassic simple bilocular taxa characterized by undivided spiraling or irregular tubes that have been investigated in this study, are entirely composed of miliolid-type needle-shaped crystallites. There is a lack of nanogranular outer layer and thus apparently lack of biomineralisation stage involving formation of calcium carbonate outside the cell. These taxa possibly represent distinct taxonomic group of the class Tubothalamea, separate from Miliolida.
In turn, “monocrystalline” wall-type, which characterizes the order Spirillinida (possibly an independent class), has been recorded within more complex forms (possibly an independent class Spirillinata), both Recent and Jurassic, possessing secondary subdivided chambers. Accordingly, it seems that morphology-based classification of Pawlowski et al. 2013 does not satisfactorily work for tubular foraminifers as already mentioned in Rigaud and Martini (2016) and integrated molecular and textural studies are needed in order to better understand their taxonomic position and phylogeny.
Test walls of both single- and multichambered Lagenata (Lagenidae and Nodosariata respectively; for proposed taxonomic classification see Rigaud et al. 2016) display identical peculiar fibrous texture which indicate their close phylogenetic relationship and contradict the theory on their distinctive origin.
A comparison of Recent, Cretaceous and Jurassic foraminiferal test structure suggests that test characteristics at higher-rank taxonomic levels are very consistent over time and thus can serve as good proxies for the taxonomic designation of fossil forams (taxa), when adequately preserved.
This study indicates that not only chamber arrangement, but also test ultrastructure characteristics fit the molecular-based high-rank classification of Foraminifera and are more natural in true phylogenetic reconstruction as it is strictly related with genetically predetermined biomineralisation process. Test ultrastructure appears to be even more practical in taxonomic assignments when both larger and smaller foraminifers are considered.
Acknowledgments
I am grateful to journal reviewers Sylvain Rigaud (Nanyang Technological University, Singapore) and Roberto Rettori (Università degli Studi di Perugia, Italy) for their comments and corrections, which have markedly improved the final version of this paper. I thank Wojciech Majewski (Institute of Paleobiology PAS, Warsaw, Poland) for providing the specimens of extant Lagenidae. This work was funded by grant 2017/27/B/ST10/00687 from the National Science Centre, Poland.
References
Alexander, S.P. and DeLaca, T.E. 1987. Feeding adaptations of the foraminiferan Cibicides refulgens living epizoically and parasitically on the antarctic scallop Adamussium colbecki. Biological Bulletin 173: 136–159. Crossref
Angell, R.W. 1980. Test morphogenesis (chamber formation) in the foraminifer Spiroloculina hyaline Schultze. Journal of Foraminiferal Research 10: 89–101. Crossref
Bender, H. and Hemleben, C. 1988. Constructional aspects in test formation of some agglutinated foraminifera. Abhandlungen der Geologischen Bundesanstalt 41: 13–21.
Berthold, W.-U. 1976. Test morphology and morphogenesis in Patellina corrugata Williamson, Foraminiferida. Journal of Foraminiferal Research 6: 167–185. Crossref
Bielecka, W. and Styk, O. 1981. Biostratygrafia batonu i keloweju północno-zachodniej Polski na podstawie otwornic i małżoraczków. Prace Instytutu Geologicznego 100: 5–56.
Bielecka, W., Styk, O., Pazdro, O., and Kopik, J. 1988. Order Foraminiferida Eichwald 1830. In: L. Malinowska (ed.), Geology of Poland. Vol. III. Atlas of Guide and Characteristic Fossils. Part 2b. Mesozoic, Jurassic, 89–111. Wydawnictwo Geologiczne, Warszawa.
Bowser, S.S., Habura, A., and Pawlowski, J. 2006. Molecular evolution of Foraminifera. In: L. Katz and D. Bhattacharya (eds.), Genomics and Evolution of Microbial Eukaryotes, 78–93. University Press, Oxford.
Clode, P., Lema, K., Saunders, M., and Weiner, S. 2011. Skeletal mineralogy of newly settling Acropora millepora (Scleractinia) coral recruits. Coral Reefs 30: 1–8. Crossref
Darling, K.F., Thomas, E., Kasemann, S.A., Seears, H.A., Smart, C.W. and Wade, C.M. 2009. Surviving mass extinction by bridging the benthic/planktic divide. Proceedings of the National Academy of Sciences of USA 106: 12629–12633. Crossref
Darling, K.F., Wade, C.M., Kroon, D., and Leigh, A.J. 1997. Planktic foraminiferal molecular evolution and their polyphyletic origins from benthic taxa. Marine Micropaleontology 30: 251–266. Crossref
Debenay, J.P., Guillou, J.J., and Lesourd, M. 1996. Colloidal calcitein foraminiferal tests: Crystallization and texture of the test. Journal of Foraminiferal Research 26: 277–288. Crossref
Debenay, J.P., Guillou, J.J., Geslin, E., and Lesourd, M. 2000. Crystallization of calcite in foraminiferal tests. Micropaleontology 46 (Supplement 1): 87–94. Crossref
De Nooijer, L.J., Spero, H.J., Erez, J., Bijma, J. and Reichart, G.J. 2014. Biomineralization in perforate foraminifera. Earth-Sciences Reviews 135: 48–58. Crossref
De Nooijer, L.J., Toyofuku, T., and Kitazato, H. 2009. Foraminifera promote calcification by elevating their intracellular pH. Proceedings of the National Academy of Sciences of USA 106: 15374–15378. Crossref
De Vargas, C., Zaninetti, L., Hilbrecht, H. and Pawlowski, J. 1997. Phylogeny and rates of molecular evolution of planktonic foraminifera: SSU rDNA sequences compared to the fossil record. Journal of Molecular Evolution 45: 285–294. Crossref
Dubicka, Z. and Gorzelak, P. 2017. Unlocking the biomineralization style and affinity of Paleozoic fusulinid foraminifera. Scientific Reports 7: 15218. Crossref
Dubicka, Z., Owocki, K., and Gloc, M. 2018. Micro- and nanostructures of calcareous foraminiferal tests: Insight from representatives of Miliolida, Rotaliida and Lagenida. Journal of Foraminiferal Research 48: 142–155.
Erez, J. 2003. The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies. In: P.M. Dove, J.J.D. Yoreo, and S. Weiner (eds.), Reviews in Mineralogy and Geochemistry 54: 115–149. Crossref
Fahrni, J., Pawlowski, J., Richardson, S., Debenay, J.-P., and Zaninetti, L. 1997. Actin suggests Miliammina fusca (Brady) is related to porcellaneous rather than to agglutinated foraminifera. Micropaleontology 43: 211–214. Crossref
Fuchs, W. 1973. Ein Beitrag zur kenntnis der Jura-”Globigerinen” und verwandter Formen an Hand polnischen Materials des Callovien und Oxfordien. Verhandlungen der Geologischen Bundesanstalt 3: 445–489.
Fuchs, W. 1975. Zur Stammesgeschichte der Planktonforaminiferen und verwandter Formen im Mesozoikum. Jahrbuch der Geologischen Bundesanstalt, Wien 118: 193–246.
Gedl, P. and Kaim, A. 2012. An introduction to the palaeoenvironmental reconstruction of the Bathonian (Middle Jurassic) ore-bearing clays at Gnaszyn, Kraków-Silesia Homocline, Poland. Acta Geologica Polonica 62: 267–280. Crossref
Görög, Á. and Werneli, R. 2003. Palaeobiogeography of the Middle Jurassic protoglobigerinids (Foraminifera). Eclogae Geologicae Helvetiae 96: 237–248.
Grønlund, H. and Hansen, H.J. 1976. Scanning electron microscopy of some recent and fossil nodosariid foraminifera. Bulletin of the Geological Society of Denmark 25: 121–134.
Guilbault, J.-P. and Patterson, R.T. 1998. Rudoloculina hooperi, a new miliolid with an agglutinated outer surface from the northeastern Pacific Ocean. Journal of Foraminiferal Research 28: 306–311. Crossref
Habura, A., Goldstein, S.T., Parfrey, L.W., and Bowser, S.S. 2006. Phylogeny and Ultrastructure of Miliammina fusca: Evidence for Secondary Loss of Calcification in a Miliolid Foraminifer. Journal of Eukaryotic Microbiology 53: 204–210. Crossref
Haig, D.W. 2002. Guidebook for Post-conference Excursion: Perth to Shark Bay 10–17 February 2002: Forams 2002, International Symposium on Foraminifera. 120 pp. The University of Western Australia, Perth.
Hart, M.B., Hylton, M.D., Oxford, M.J., Price, G.D., Hudson, W., and Smart, C.W. 2003. The search for the origin of the planktic Foraminifera. Journal of the Geological Society, London 160: 341–343. Crossref
Hemleben, C.H., Anderson, O.R., Berthold, W., and Spindler, M., 1986. Calcification and chamber formation in Foraminifera- a brief overview. In: B.S.C. Leadbeater and R. Riding (eds.), Biomineralization in Lower Plants and Animals. Systematics Association Special Volume 30: 237–249.
Hillebrandt, A. von 2010. Aragonitic Foraminifera (Robertinina) from the Triassic–Jurassic Boundary Interval of the Northern Calcareous Alps. Earth Sciences Frontiers, Special Issue 17: 70–72.
Hillebrandt, A. von 2012. Are the Late Triassic to Early Jurassic aragonitic Oberhauserellidae (Robertinina) the ancestors of planktonic Foraminifera? Neues Jahrbuch Geologie Paläontologie Abhandlungen 266: 199–215. Crossref
Hohenegger, J. 2009. Functional shell geometry of symbiont-bearing benthic Foraminifera. Journal of Coral Reef Studies 11: 81–89. Crossref
Hohenegger, J. 2018. Foraminiferal growth and test development. Earth-Science Reviews 185: 140–162. Crossref
Holzmann, M. and Pawlowski, J. 2017. An updated classification of rotaliid foraminifera based on ribosomal DNA phylogeny. Marine Micropaleontology 132: 18–34. Crossref
Hottinger, L., Halicz, E., and Reiss, Z. 1993. Recent Foraminiferida from the Gulf of Aqaba, Red Sea. Dela SAZU, Ljubljana 33: 1–179.
Kaminski, M.A. 2004. The year 2000 classification of the agglutinated Foraminifera. In: M. Bubík and M.A. Kaminski (eds.), Proceedings of the Sixth International Workshop on Agglutinated Foraminifera: Grzybowski Foundation Special Publication 8: 237–255.
Krasheninnikov, V.A. [Krašeninnikov, V.A.] 1956. Test microstructure of some Cenozoic foraminiers and methodology and observations in polarized light [in Russian]. Akademiâ Nauk SSSR, Voprosy Mikropaleontologii 1: 37–48.
Loeblich, A.R. Jr and Tappan, H. 1964. Treatise on Invertebrate Paleontology, Part C: Protista 2, Sarcodina, chiefly “Thecamoebians” and Foraminiferida. 900 pp. Geological Society of America, Boulder and University of Kanzas Press, Lawrence.
Loeblich, A.R. Jr and Tappan, H. 1984. Suprageneric classification of the Foraminiferida (Protozoa). Micropaleontology 30: 1–70. Crossref
Loeblich, A.R. Jr. and Tappan, H. 1987. Foraminiferal Genera and their Classification. 970 pp. Van Nostrand Reinhold, New York.
Loeblich, A.R. Jr and Tappan, H. 1992. Present status of foraminiferal classification. In: Y. Takayanagi and T. Saito (eds.), Studies in Benthic Foraminifera. Proceedings of the Fourth Symposium on Benthic Foraminifera, Sendai, 1990, 93–102. Tokai University Press, Tokyo.
Majewski, W. 2005. Benthic foraminiferal communities: distribution and ecology in Admiralty Bay, King George Island, West Antarctica. Polish Polar Research 6: 59–214.
Matyja, B.A. and Wierzbowski, A. 2006. Field Trip B1. Biostratigraphical framework from Bajocian to Oxfordian. Stop B1.7. Gnaszyn clay pit (Middle Bathonian–lowermost Upper Bathonian). In: A. Wierzbowski, R. Aubrecht, J. Golonka, J. Gutowski, M. Krobicki, B.A. Matyja, G. Pieńkowski, and A. Uchman (eds.), 7th International Congress on the Jurassic System, 6–18 September 2006, Kraków, Poland. Jurassic of Poland and adjacent Slovakian Carpathians. Field Trip Guidebook, 154–155. Polish Geological Institute, Warszawa.
Mikhalevich, V.I. 1980. Systematics and evolution of the Foraminifera in view of new data on their cytology and ultrastructure. Trudy Zoologičeskogo Instituta, AN SSSR 94: 42–61.
Mikhalevich, V.I. 1992. Makrosistema foraminifer. 43 pp. Ph.D. Thesis, Zoologičeskij institut Rossojskoj akademii nauk, St. Petersburg.
Mikhalevich, V.I. 2009. Taxonomic position of the superorder Fusulinoida Fursenko in the Foraminifera system. Paleontological Journal 43: 117–128. Crossref
Mikhalevich, V.I. 2000. The phylum Foraminifera d’Orbigny, 1826—foraminifers. In: A.F. Alimova, (ed.), Protista: Manual on Zoology, 533–623. Nauka, St. Petersburg.
Mikhalevich, V.I. 2013. New insight into the systematics and evolution of the foraminifera. Micropaleontology 59: 493–527.
Mikhalevich, V.I. [Mihalevič, V.I.], Rodionova, M.K., and Sinjakova, G.N. [Sinâkova, G.N.] 1986. The wall ultrastructure of the two agglutinated foraminiferal genera with the quinqueloculine type of the shell [in Russian]. Trudy Zoologičeskogo Instituta, AN USSR 144: 66–71.
Nakajima, K., Suzuki, M., Nagai, Y., Izumida, K., Oaki, Y., Toyofuku, T., Bijma,J., Nehrke,G., Raitzsch, M., Kenichiro Tani, K. and Imai, H. 2017. Hierarchical textures on aragonitic shells of the hyaline radial foraminifer Hoeglundina elegans. CrystEngComm 19: 7191–7196. Crossref
Parker, J.H. 2017. Ultrastructure of the test wall in modern porcelaneous foraminifera: Implications for the classification of the Miliolida. Journal of Foraminiferal Research 47: 136–174.
Parker, J.E., Thompson, S.P., Lennie, A.R., Potter, J., and Tang, C.C. 2010. A study of the aragonite-calcite transformation using Raman spectroscopy, synchrotron powder diffraction and scanning electron microscopy. CrystEngComm 12: 1590–1599. Crossref
Pawlowski, J. 2000. Introduction to the molecular systematics of foraminifera. Micropaleontology 46 (Supplement 1): 1–12.
Pawlowski, J., Holzmann, M., and Debeney, J.-P. 2014. Molecular phylogeny of Carterina spiculotesta and related species from New Caledonia. Journal of Foraminiferal Research 44: 434–439. Crossref
Pawlowski, J., Holzmann, M., and Tyszka, J. 2013. New supraordinal classification of Foraminifera: molecules meet morphology. Marine Micropaleontology 100: 1–10. Crossref
Reiss, Z. and Schneidermann, N. 1969. Ultramicrostructure of Hoeglundina. Micropaleontology 15: 135–144. Crossref
Rigaud, S. and Blau, J. 2016. New robertinid foraminifers from the Early Jurassic of Adnet, Austria and their evolutionary importance. Acta Palaeontologica Polonica 61: 721–734. Crossref
Rigaud, S. and Martini, R. 2016. Agglutinated or porcelaneous tests: where to draw the line? Journal of Foraminiferal Research 46: 333–344. Crossref
Rigaud, S., Martini, R., and Vachard, D. 2015. Early evolution and new classification of Robertinida (Foraminifera). Journal of Foraminiferal Research 45: 3–28. Crossref
Rigaud, S., Vachard, D., Schlagintweit, F., and Martini, R. 2016. New lineage of Triassic aragonitic Foraminifera and reassessment of the class Nodosariata. Journal of Systematic Palaeontology 14: 919–938. Crossref
Roberts, S. and Murray, J.W. 1995. Characterization of cement mineralogy in agglutinated foraminifera (Protista) by Raman spectroscopy. Journal of the Geological Society, London 152: 7–9. Crossref
Schweizer, M., Pawlowski, J., Kouwenhoven, T.J., Guiard, J., and Van der Zwaan, B. 2008. Molecular phylogeny of Rotaliida (Foraminifera) based on complete small subunit rDNA sequences. Marine Micropaleontology 66: 233–246. Crossref
Sen Gupta, B.K. 2003. Modern Foraminifera. 371 pp. Kluwer Academic Publishers, New York. Crossref
Simmons, M.D., Boudagher-Fadel, M.K., Banner, F.T., and Whittaker, J.E. 1997. The Jurassic Favusellacea, the earliest Globigerina. In: M.K. Boudagher-Fadel, F.T. Banner, and J.E. Whittaker (eds.), The Early Evolutionary History of Planktonic Foraminifera, 17–53. British Micropaleontological Society Publication Series, Chapman & Hall, London.
Smoleń, J. 2012. Faunal dynamics of foraminiferal assemblages in the Bathonian (Middle Jurassic) ore-bearing clays at Gnaszyn, Kraków-Silesia Homocline, Poland. Acta Geologica Polonica 62: 403–441. Crossref
Tappan, H. and Loeblich, A.R. Jr 1988. Foraminiferal evolution, diversification, and extinction. Journal of Paleontology 62: 695–714. Crossref
Taylor, P.D., Kudryavtsev, A.B., and Schopf, J.W. 2008. Calcite and aragonite distributions in the skeletons of bimineralic bryozoans as revealed by Raman spectroscopy. Invertebrate Biology 127: 87–97. Crossref
Ter Kuile, B., Erez, J., and Padan, E. 1989. Mechanisms for the uptake of inorganic carbon by two species of symbiont-bearing foraminifera. Marine Biology 103: 241–251. Crossref
Toyofuku, T., Kitazato, H., Kawahata, H., Tsuchiya, M., and Nohara, M. 2000. Evaluation of Mg/Ca thermometry in foraminifera: Comparison of experimental results and measurements in nature. Paleoceanography 15: 456–464. Crossref
Towe, K.M. and Cifelli, R. 1967. Wall ultrastructure in the calcareous foraminifera: crystallographic aspects and a model for calcification. Journal of Paleontology 41: 742–762.
Ujiié, Y., Kimoto, K., and Pawlowski, J. 2008. Molecular evidence for an independent origin of modern triserial planktonic foraminifera from benthic ancestor. Marine Micropaleontology 69: 334–340. Crossref
Urmos, J., Sharma, S.K., and Mackenzie, F.T. 1991. Characterization of some biogenic carbonates with Raman spectroscopy. American Mineralogist 76: 641–646.
Werneli, R. 1988. Les protoglobigérines (foraminifères) du Toarcien at de l’Aalénien du Domuz Dag (Taurus Occidental, Turquie). Eclogae Geologicae Helvetiae 81: 661–668.
Wood, A. 1949. The structure of the wall of the test in Foraminifera; its value in classification. Quarterly Journal of the Geological Society London 104: 229–255. Crossref
Acta Palaeontol. Pol. 64 (1): 1–18, 2019
https://doi.org/10.4202/app.00564.2018