


A new mammal from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania
PATRICK M. O’CONNOR, DAVID W. KRAUSE, NANCY J. STEVENS, JOSEPH R. GROENKE, ROSS D.E. MACPHEE, DANIELA C. KALTHOFF, and ERIC M. ROBERTS
O’Connor, P.M., Krause, D.W., Stevens, N.J., Groenke, J.R., MacPhee, R.D.E., Kalthoff D.C., and Roberts, E.M. 2019. A new mammal from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania. Acta Palaeontologica Polonica 64 (1): 65–84.
We here establish a new mammaliaform genus and species, Galulatherium jenkinsi (Mammalia), from the Upper Cretaceous Galula Formation in the Rukwa Rift Basin of southwestern Tanzania. This represents the first named taxon of a mammaliaform from the entire Late Cretaceous of continental Afro-Arabia, an interval of 34 million years. Preliminary study of the holotypic and only known specimen (a partial dentary) resulted in tentative assignation to the Gondwanatheria, a poorly known, enigmatic clade of Late Cretaceous–Paleogene Gondwanan mammals (Krause et al. 2003). The application of advanced imaging (µCT) and visualization techniques permits a more detailed understanding of key anatomical features of the new taxon. It reveals that the lower dentition consisted of a large, procumbent lower incisor and four cheek teeth, all of which were evergrowing (hypselodont). Importantly, all of the teeth appear devoid of enamel. Comparisons conducted with a range of Mesozoic and selected Cenozoic mammaliaform groups document a number of features (e.g., columnar, enamel-less and evergrowing teeth, with relatively simple occlusal morphology) expressed in Galulatherium that are reminiscent of several distantly related groups, making taxonomic assignment difficult at this time. Herein we retain the provisional referral of Galulatherium (RRBP 02067) to Gondwanatheria; it is most similar to sudamericids such as Lavanify and Bharratherium from the Late Cretaceous of Madagascar and India, respectively, in exhibiting relatively simple, high-crowned, columnar cheek teeth. Other features (e.g., enamel-less dentition) are shared with disparate forms such as the Late Jurassic Fruitafossor and toothed xenarthrans (e.g., sloths), here attributed to convergence. Revised analyses of the depositional context for the holotype place it as having lived sometime between the late Turonian and latest Campanian (roughly 91–72 million years ago). This enhanced geochronological context helps to refine the palaeobiogeographical significance of Galulatherium among Cretaceous mammals in general and those from Gondwanan landmasses specifically.
Key words: Mammaliaformes, Mammalia, Gondwanatheria, Galulatherium, Late Cretaceous, Tanzania.
Patrick M. O’Connor [oconnorp@ohio.edu, https://orcid.org/0000-0002-6762-3806], Ohio Center for Ecology and Evolutionary Studies, 228 Irvine Hall, Athens, Ohio 45701 USA; Department of Biomedical Sciences, Ohio University Heritage College of Osteopathic Medicine, 228 Irvine Hall, Athens, Ohio 45701, USA.
David W. Krause [David.Krause@dmns.org], Department of Earth Sciences, Denver Museum of Nature and Science, Denver, CO, 80205, USA; Department of Anatomical Sciences, Stony Brook University, Stony Brook, NY 11794-8081, USA.
Nancy J. Stevens [stevensn@ohio.edu], Ohio Center for Ecology and Evolutionary Studies, 228 Irvine Hall, Athens, Ohio 45701 USA; Department of Biomedical Sciences, Ohio University Heritage College of Osteopathic Medicine, 228 Irvine Hall, Athens, Ohio 45701, USA.
Joseph R. Groenke [groenke@ohio.edu], Department of Biomedical Sciences, Ohio University Heritage College of Osteopathic Medicine, 228 Irvine Hall, Athens, Ohio 45701, USA.
Ross D. E. MacPhee [macphee@amnh.org] Department of Mammalogy, American Museum of Natural History, New York, NY, 20212, USA.
Daniela C. Kalthoff [daniela.kalthoff@nrm.se], Department of Zoology, Swedish Museum of Natural History, Box 50007, 10405 Stockholm, Sweden.
Eric M. Roberts [eric.roberts@jcu.edu], Department of Geosciences, College of Science and Engineering, James Cook University, Townsville, QLD 4811, Australia.
Received 12 November 2018, accepted 08 January 2019, available online 13 March 2019.
Copyright © 2019 P.M. O’Connor et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The paucity of Cretaceous-age mammaliaforms from Africa, the Arabian Peninsula, and nearby islands such as Madagascar has long been a topic for discussion among paleobiologists. This gap, particularly obvious relative to the extensive record from Laurasia, has hampered large-scale biogeographic analyses and limited our understanding of the origin of major mammaliaform clades (e.g., gondwanatherians, haramiyidans). An intriguing mammalian dentary (see Krause et al. 2003) from the United Republic of Tanzania provides a novel glimpse into mammaliaform evolutionary history in Afro-Arabia during the close of the Mesozoic Era.
Collected during the inaugural field season of the Rukwa Rift Basin Project (RRBP) in 2002, the specimen (RRBP 02067) highlights the paleontological significance of the Red Sandstone Group (now formally referred to as the Galula Formation; Roberts et al. 2010) in the western arm of the East African Rift System. As noted in the original description (Krause et al. 2003), the specimen consists of a partial dentary and teeth and represents the most complete mammaliaform from the Cretaceous of continental Africa. This remains true nearly two decades later, with other Cretaceous mammaliaform records from continental Afro-Arabia consisting of: (i) isolated teeth of more than a dozen named monotypic genera of eutriconodonts, haramiyidans (originally thought to be multituberculates, but see Butler and Hooker 2005 and Huttenlocker et al. 2018), and cladotherians from the ?Berriasian Ksar Metlili fauna of the Anoual Syncline, eastern Morocco (see summaries in Sigogneau-Russell et al. 1998; Kielan-Jaworowska et al. 2004; and Haddoumi et al. 2016: table 6); (ii) isolated teeth and an edentulous jaw belonging to at least three taxa, only one of which has been named (Abelodon abeli, a peramurid “eupantotherian”), from the Barremian–Aptian of the Koum Basin, Cameroon (Brunet et al. 1988, 1990; Jacobs et al. 1988); and (iii) a mammaliaform caudal vertebra in the Draa Ubari fauna from an unnamed sandstone in western Libya (Nessov et al. 1998) originally thought to be of Santonian–Campanian age but now regarded as Cenomanian (Rage and Cappetta 2002). In addition, ichnofossils attributed to mammaliaform track-makers have been found in the Cenomanian of Tunisia (Contessi 2013), the Aptian of Angola (Jacobs et al. 2016; Mateus et al. 2017) and the Barremian–?Aptian of Morocco (Klein et al. 2018).
RRBP 02067 consists of a left dentary preserving a nearly complete dentition (Fig. 1). Krause et al. (2003) did not name the taxon represented by RRBP 02067 and conservatively referred the specimen to ?Gondwanatheria in the hope of recovery of more complete (and more diagnostic) materials. Although field-collecting efforts have yet to reveal additional specimens of the Rukwa mammaliaform, advances in high-resolution, high-energy µCT imaging and visualization permit more detailed anatomical study of the dentary of RRBP 02067 and more refined assessments of features such as cheek tooth count. Furthermore, the discovery of a number of new gondwanatherian taxa since 2003 (Bharattherium, Prasad et al., 2007; also see Wilson et al. 2007; Trapalcotherium Rougier et al. 2009; Greniodon Goin et al. 2012; Vintana Krause et al. 2014) provides a broader comparative context in which to evaluate gondwanatherian morphology. Finally, detailed geological and geochronological studies of the Galula Formation (Roberts et al. 2010; Widlansky et al. 2018) provide much improved age constraint for the fossil-bearing locality. Together, these advances reveal important anatomical detail and relevant comparative and temporal context for the specimen, prompting us to recognize and describe it as a new genus and species.
Institutional abbreviations.—MPEFCH, Museo Paleontológico “Egidio Feruglio”, Trelew, Chubut Province, Argentina; RRBP, Rukwa Rift Basin Project, Tanzania Antiquities Unit, Dar es Salaam, Tanzania.
Other abbreviations.—ch, lower cheek teeth; d, distal edge of incisor; ics, incisor cross-section; inc, lower incisor; ln, lingual edge of incisor. Anatomical orientation terms follow those defined in Krause (2014).
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:1D6D0537-594B-413B-98C1-5E AFC70212E8. Additional images and 3D data associated with this work can be found at Morphobank ID: 3259 (http://morphobank.org/permalink/?3259).
Methods
Computed tomography.—The dataset used for digital preparation and study was created in January of 2012 with an Xradia scanner at the High-Resolution X-ray Computed Tomography Facility at the University of Texas High-Resolution X-ray CT Facility. Data acquired from the scans (kV = 70; µA = 52.6; CaF2 filter) were used to create 8-bit jpg and 16-bit tiff slices using Xradia Reconstructor. The resultant reconstructed volume (401×801×1265 voxels with each voxel = 0.01585×0.01585×0.01585 mm) was segmented at Ohio University using Avizo 7.1 (VSG) and 9.2 (FEI). Resultant polygon (.surf) files generated from segmented voxel data were visualized with shaded, opaque, vertex normal, non-specular attributes for figure images. Images were captured in orthographic view and with default headlight, using the snapshot function, and 5×5 tiles were exported as tiff files.
In addition to the anatomical segmentation of voxel data, sub-segmentations of specimen fragments were also created. The polygon outputs of these fragment sub-segmentations were manipulated using the Transform Editor in Avizo for the purposes of a digital reconstruction approximating in-life morphology. These coordinate changes for position transformations were logged to ensure a repeatable result, and appear in SOM 1–4 (Supplementary Online Material available at http://app.pan.pl/SOM/app64-OConnor _etal_SOM.pdf).
Dentine microstructure and occlusal surface wear.—The posterior part of the tip of the second cheek tooth (ch2) was partially separated from the rest of the tooth along a previous fracture plane (Fig. 2A1, A4). This fragment was used to investigate dental tissue microstructure and for dental microwear analysis. The fracture plane on the mesial side of the small fragment exposes dental tissue in longitudinal aspect; this area was etched for two seconds with 2N HCl in an attempt to reveal microstructure features. The fragment was investigated with a Hitachi S-4300 SEM, located at the Swedish Museum of Natural History in Stockholm, at acceleration voltages of 5 kV and magnifications of 200–4500×. For dental microwear analysis, the occlusal surface of the fragment was cleaned with water and acetone to expose dental tissue and examined using a Zeiss Discovery V12 stereomicroscope at ×100 magnification.
Systematic palaeontology
Mammaliaformes Rowe, 1988
Mammalia Linnaeus, 1758
?Gondwanatheria Mones, 1987
?Sudamericidae Scillato-Yané and Pascual, 1984
Genus Galulatherium nov.
Etymology: From Galula, in reference to the named geological formation and local village near the stratotype section, and Neo-Latin therium, beast.
Type species: Galulatherium jenkinsi sp. nov., by monotypy, see below.
Diagnosis.—Same as for type species.
Galulatherium jenkinsi sp. nov.
Figs. 1–5.
ZooBank LCID: urn:lsid:zoobank.org:pub:1D6D0537-594B-413B-98C1-5EAFC70212E8
Etymology: In honour of the late Farish A. Jenkins, Jr. (1940–2012), pioneering researcher on synapsid evolution.
Holotype: RRBP 02067, partial left dentary with single incisor and four cheek teeth (only known specimen, Figs. 1–3). Formerly referred to as NMT 02067 in Krause et al. 2003, permanently deposited at the National Museum of Tanzania (Dar es Salaam) under agreement with the Tanzania Antiquities Unit.
Type locality: Locality RRBP TZ-07, approximately 35 km south of Lake Rukwa in the Songwe sub-basin, Rukwa Rift Basin, southwestern Tanzania. Approximate locality coordinates 8º56’ S, 33º12’ E, with additional details on file at Ohio University and the Tanzania Antiquities Unit.
Type horizon: Namba Member of the Galula Formation of the Red Sandstone Group. Independent lines of geological (Roberts et al. 2010) and biostratigraphic (O’Connor et al. 2006) data previously suggested a middle Cretaceous (Aptian–Cenomanian) age for the Galula Formation. Recent paleomagnetic reversal stratigraphy (Widlansky et al. 2018) further refines the age of the upper portion (i.e., Namba Member) of the formation, resolving the RRBP TZ-07 fossil-bearing locality as being of Turonian–Campanian age based on the presence of distinct reversals identified near the top of the section.
Diagnosis.—Mesozoic mammaliaform with the following characteristics: single, procumbent, evergrowing, enamel-less, laterally compressed lower incisor; incisor root extends only as far posteriorly as mesial edge of root of ch2, positioned ventromedial (or ventrolingual) to root of ch1; four, evergrowing, enamel-less, cylindrical cheek teeth; two posterior-most cheek teeth located medial to anterior edge of coronoid process; co-planarity of occlusal surfaces of three distal-most cheek teeth, providing a single occlusal working surface that faces dorsolingually; two diastemata, one separating incisor from ch1, another separating ch1 and ch2.
Differs from previously described gondwanatherians by complete absence of enamel on all teeth (not only individual sides of teeth as in Bharattherium and Lavanify) and presence of hypselodont (evergrowing) teeth. Shares with the gondwanatherian Sudamerica and an unnamed new gondwanatherian from Madagascar presence of four lower cheek teeth. Also similar to Sudamerica in presence of posteriorly canted cheek tooth crowns. Differs from Sudamerica and new gondwanatherian from Madagascar in having two diastemata (between incisor and ch1 and between ch1 and ch2). Further differs from Sudamerica in possessing concave ventral border of horizontal ramus of dentary, in lacking pronounced difference in height between diastema and gnathic portion of dentary, and in having mental foramen placed near mid-height of dentary, rather than in relatively dorsal position.
Description.—Lower jaw: The holotype of Galulatherium jenkinsi (RRBP 02067) consists of a partial left dentary preserving the open root of a single enlarged, procumbent incisor and the open roots and partial-to-complete crowns of four cheek teeth (Fig. 1). The tooth-bearing portion of the dentary is intact, reflecting a short, wide, and deep (i.e., very robust) element (Table 1). The ascending ramus is incomplete posteriorly and the cortical bone throughout has poor surface preservation. The incisor and mesial-most cheek tooth (ch1) are incomplete apically, broken off near their alveolar margins. In contrast, the three distal-most cheek teeth are essentially complete despite small fractures and minimal displacement of fragments near their apices; the apical fragments were repaired either during preparation (ch3, ch4) or have been digitally repositioned (ch2) (e.g., Fig. 5A2). The dentary is incomplete on the lateral surface, generally following the contour of ch1 (Fig. 1A1). Fortunately, both the ventral and medial margins of the specimen are intact, permitting a reliable reconstruction of the proportions of the element. Given the incomplete nature of the specimen, it remains somewhat unclear whether separate postdentary bones may have articulated with the dentary but the parts that are preserved provide no suggestion that they were present. Similarly, there is no trace of a Meckelian sulcus on the medial aspect of the dentary.
Table 1. Measurements (in mm) of the lower jaw
and dentition of the Galulatherium jenkinsi
holotype (RRBP 02067).
* incomplete measurement; M-L, mediolateral; M-D, mesiodistal; na,
unable to measure.
|
Dentary |
|
Individual tooth |
Incisor |
ch1 |
ch2 |
ch3 |
ch4 |
||||||
|
Max length (apicobasal) |
9.9 |
5.39 |
8.37 |
6.85 |
4.94 |
||||||||
|
Max length |
19.54* |
|
M-L |
M-D |
M-L |
M-D |
M-L |
M-D |
M-L |
M-D |
M-L |
M-D |
|
|
Height at symphysis |
7.13 |
Apex of crown |
na |
na |
na |
na |
1.47 |
2.07 |
na |
1.15 |
na |
0.93 |
|
|
Width at symphysis |
2.29* |
Alveolar rim |
1.86 |
2.66 |
1.07 |
1.38 |
1.72 |
2.12 |
1.62 |
1.18 |
1.39 |
0.91 |
|
|
Height at anterior coronoid |
9.53 |
1/2 root depth |
1.67 |
2.7 |
1.08 |
1.37 |
1.78 |
2.16 |
1.66 |
1.22 |
1.57 |
1.04 |
|
|
Width at anterior coronoid |
3.96 |
Root terminus |
1.88 |
2.69 |
1.00 |
1.30 |
1.71 |
2.20 |
1.71 |
1.17 |
1.56 |
1.07 |
|
The ventral border of the dentary is largely intact. By contrast, the dorsal border of the horizontal ramus is in poor condition except where the alveolar margin is preserved along the buccal edges of ch3 and ch4 (Fig. 1A1, A5). The coronoid process of the dentary is incomplete. However, the dorsal margin of its anterior portion is intact and sweeps posterodorsally, forming an angle of ~140° with the dorsal alveolar margin of the horizontal ramus beginning at the position of ch2. This is notable in that ch3 and ch4 are located along the medial surface of the emergent coronoid process. There is no sign (bone or scar) of a distinct coronoid articulation on the medial aspect of the anterior base of the coronoid process.
The anterior end of the dentary is relatively complete, partially preserving the large symphyseal region (Fig. 1A2). The posterior end of the dentary is incomplete, with the broken margin passing vertically through the mid-region of the prominent masseteric fossa laterally and just posterior to the mandibular foramen medially (Fig. 1). The fossa extends anteriorly on the horizontal ramus to a position ventral to ch3, and its ventral border is bounded by a low and broad crest. The medial surface of the dentary hosting the mandibular foramen is largely preserved, revealing a short anteroposterior sulcus leading to the ventral margin of the foramen (Fig. 1A2, A4). A distinctive, low, broad crest is also present along the coronoid process margin of the fossa, although it is not laterally flaring or hypertrophied as in some other Mesozoic mammaliaforms. There are multiple (~5) small foramina opening in the anterior region of the masseteric fossa, the largest positioned anteroventrally, with smaller foramina situated dorsal to it. This configuration resembles that seen in Gobiconodon sp. indet., as illustrated in Rougier et al. (2001: fig. 4). Based on µCT imaging, the largest canal in Galulatherium passes through the cortical surface and connects with the mandibular canal, suggesting homology with the masseteric foramen as described in a variety of Mesozoic and Recent mammals (see review in Davis 2012). The other foramina are smaller, do not appear to connect directly with the mandibular canal, and may represent nutrient foramina. Breakage on the ascending ramus does not permit determination of the extent or depth of the attachment point for the medial pterygoid m.; the only trace of it is a shallow depression that originates at roughly mid-height of the ascending ramus well posterior to the cheek-tooth row (Fig. 1A2).
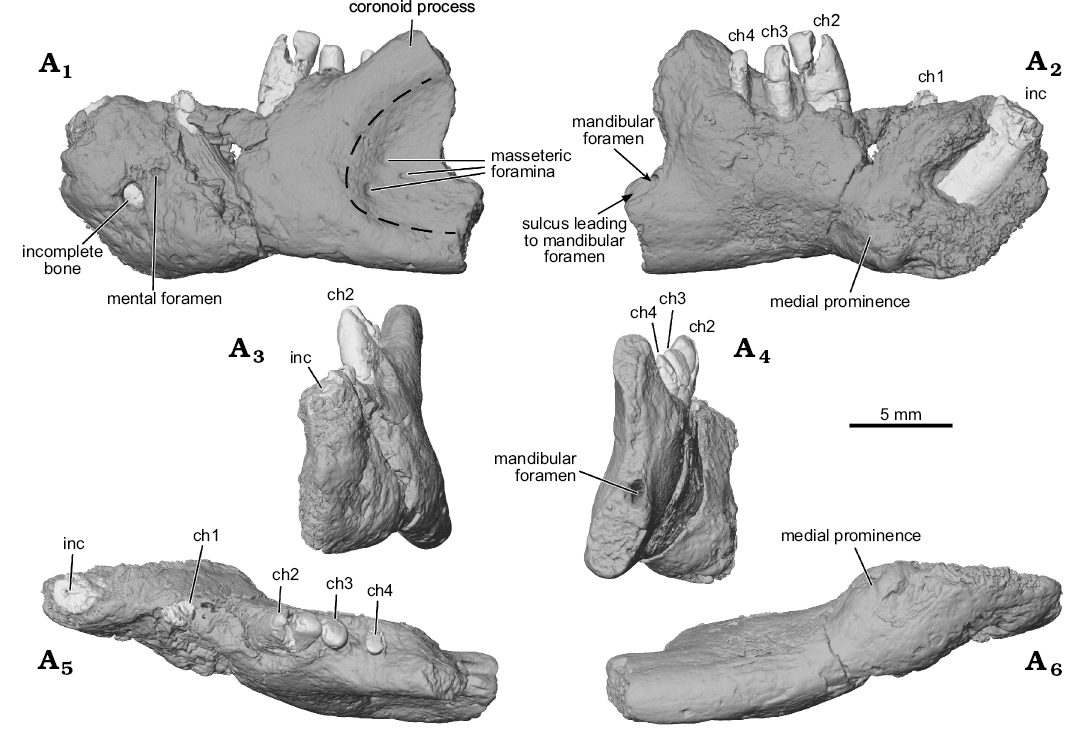
Fig. 1. Digital surface reconstructions from µCT scans of the ?gondwanatherian mammal Galulatherium jenkinsi sp. nov. (holotype, RRBP 02067) from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania; left dentary in lateral (A1), medial (A2), anterior (A3), posterior (A4), dorsal (A5), and ventral (A6) views. Dashed line estimates the anterior margin of the masseteric fossa. Abbreviations: ch, lower cheek teeth; inc, lower incisor.
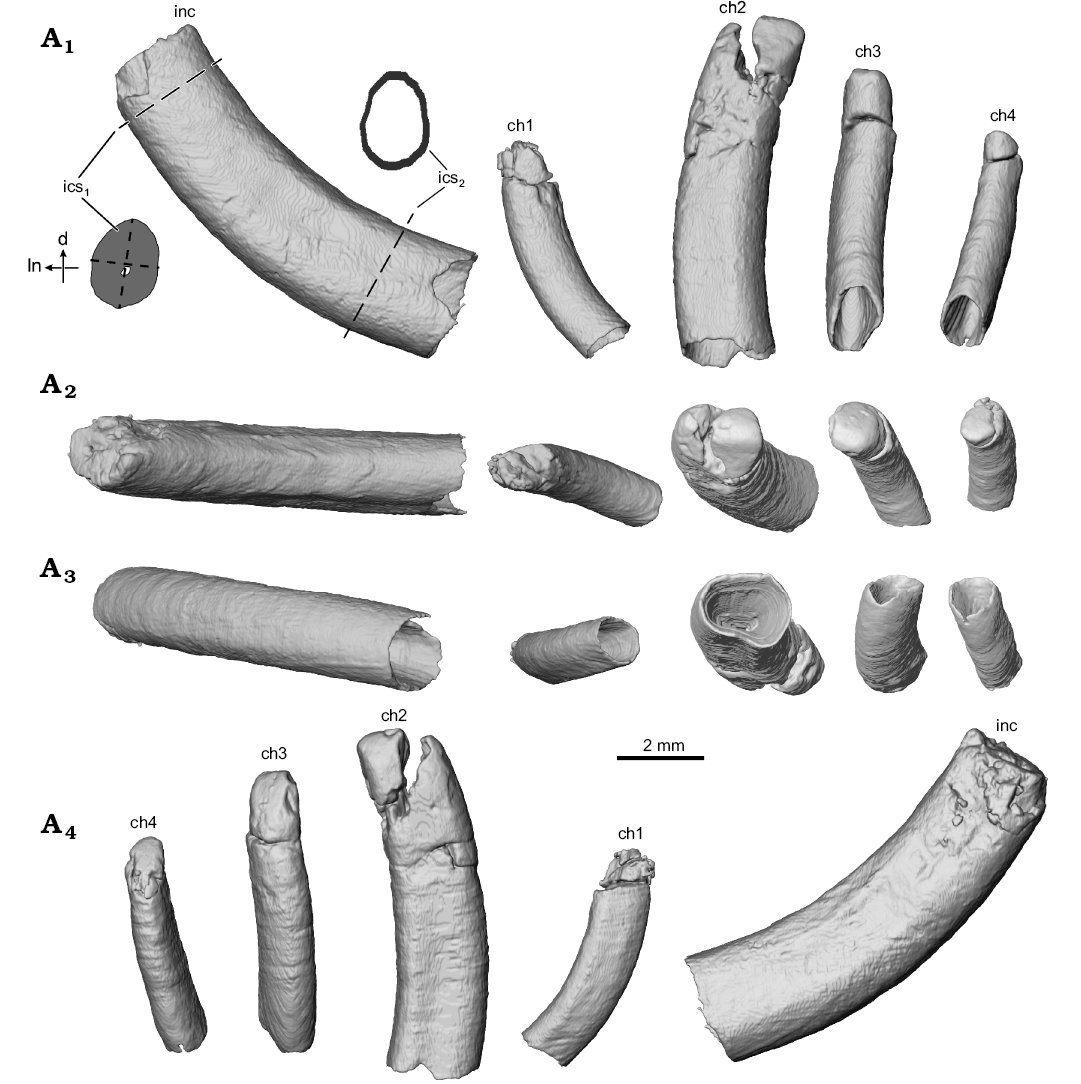
Fig. 2. Digital surface reconstructions from µCT scans of the ?gondwanatherian mammal Galulatherium jenkinsi sp. nov. (holotype, RRBP 02067) from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania; lower left dental series in buccal (A1), apical/occlusal (A2), abapical/ventral (A3), and lingual (A4) views. Abbreviations: ch, lower cheek teeth; d, distal edge of incisor; inc, lower incisor; ics1, incisor cross-section near alveolar margin; ics2, incisor cross-section near root tip; ln, lingual edge of incisor; dashed lines indicate the approximate locations from which incisor metrics were collected.
A mental foramen is present, positioned nearly midway on the lateral surface, interposed between the long axes of the incisor and ch1. The small, round opening identified as the mental foramen by Krause et al. (2003) represents an artefact of preservation where the surface of the dentary is broken immediately lateral to the incisor. The re-identified mental foramen, although somewhat indistinct at the bone surface, is revealed by µCT data that document the entire path of the mandibular canal from its opening anterolaterally at the mental foramen through to the mandibular foramen posteriorly. Relative to the dentition, the mandibular canal is located ventral to the open roots of all four cheek teeth (Figs. 3A2, 4A3–A5). Immediately anterior to ch2, the canal sweeps anterodorsally and generally follows the same curvature of the incisor to its termination at the mental foramen. At its posterior-most extent, the mandibular canal ends at a posteriorly/posteromedially facing mandibular foramen near the preserved posterior edge of the dentary. The mandibular foramen is positioned below the estimated mid-height of the dentary (estimated, as the dorsal margin of the coronoid process is incomplete). Given this configuration, it is also clear that the mandibular foramen is located at a position ventral to the alveolar plane. The remainder of the medial surface of the preserved dentary is relatively nondescript, with the exception of an exaggerated medial prominence that represents the posteroventral extent of the incisor root inside the dentary.
Gross dental morphology: As noted in Krause et al. (2003), teeth preserved in the type specimen of Galulatherium are columnar and singled rooted. Due to limited preparation of the fossil at the time of the initial description, it was not clear whether the taxon actually lacked tooth enamel, or if its absence reflected taphonomic processes. Constrained by the state of preservation, occlusal morphology was not characterized, other than to note the absence of synclines or furrows in the cheek teeth that are typical of some clades (e.g., gondwanatherians). Additional mechanical preparation, combined with high-resolution µCT and SEM of RRBP 02067 provides an opportunity for a more detailed assessment of dental morphology in Galulatherium.
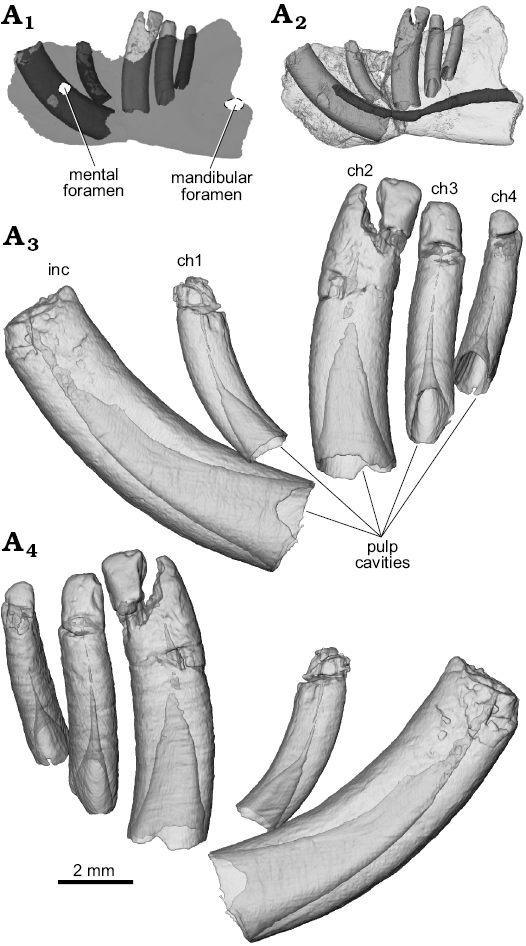
Fig. 3. Digital semi-transparent reconstructions from µCT scans of the ?gondwanatherian mammal Galulatherium jenkinsi sp. nov. (holotype, RRBP 02067) from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania; lower left dental series in buccal (A3) and lingual (A4) views to illustrate the extent of the pulp cavity within individual teeth. Ghosted left dentary (medium gray) with teeth in-situ (light gray crowns, dark gray roots) to highlight hypselodonty and relative positions of teeth within the dentary (A1). Digital semi-transparent reconstruction of left dentary with teeth in-situ to highlight path of mandibular canal (dark gray) (A2). Abbreviations: ch, lower cheek teeth; inc, lower incisor.
The current analysis confirms that all of the teeth in Galulatherium are indeed columnar and were evergrowing, as all exhibit open roots (Figs. 2–4; Table 1). Each open root cavity extends as an apically tapering cone, such that a small circular opening is preserved at the broken apex of the incisor and ch1 (Fig. 4). As expected, the tapering cone does not reach the occlusal surface in those teeth (ch2–ch4) with complete crowns, but the small canal extending apically does. All teeth are inferred to lack an outer enamel coating because the radio-opaque layer covering the tooth surface indicative of enamel is not apparent, thus confirming the initial observation by Krause et al. (2003). However, alternating banding of density within the dentine is present on all teeth. Such banding, as revealed through µCT, appears as distinct circumferential laminations when examined via cross-sectional views of teeth (Fig. 4). This banding appears to represent serial deposition of dentine during tooth development. Such banding minimally represents differential density of the dentine, yet whether this reflects different degrees of mineralization or some other factor (e.g., differential signal attenuation based on dentine organization) remains unclear. Similar dentine banding is apparent in at least some multituberculates (e.g., Lambdopsalis; Mao et al. 2015: fig. 16), but whether this bears any phylogenetic or functional signal awaits a broader comparative survey. Our interpretation is that the teeth are exclusively composed of orthodentine, with the differential density banding likely representing either some non-daily cyclicity in deposition or some previously unknown diagenetic process.
Whereas the apices of the incisor and ch1 crowns are not complete, additional preparation revealed intact crowns on ch2–ch4, as discussed further below. The crowns of the three distal-most cheek teeth are posteriorly canted relative to the main axis of the horizontal ramus (Fig. 1). Also, the tightness of fit between individual teeth and their enclosing alveoli varies along the tooth row. There is a relatively tight fit only between the incisor and its alveolus; in the others, there is a distinct gap between the tooth and the external margin of the alveolus. This is particularly notable along the lingual margins of ch2–ch4 (Fig. 4). This enlarged space was evidently filled diagenetically with a crystalline matrix of unknown origin or composition. Digital reconstruction of the infilled material in the gap between the alveolus and tooth reveals that the exterior surface of the tooth is relatively smooth, but that the peripheral surface of the alveolar bone is coarse and almost corrugated (Fig. 1A6; note material reconstructed around periphery of ch1 and visible through the broken cortical surface on the lateral margin). The infilling occupies the space that would have been occupied by periodontium (i.e., cementum, periodontal ligament, associated neurovasculature; Nanci and Somerman 2003), with its volume notably increasing abapically, as in some other mammals (e.g., geomyid rodents; SOM 1–3).
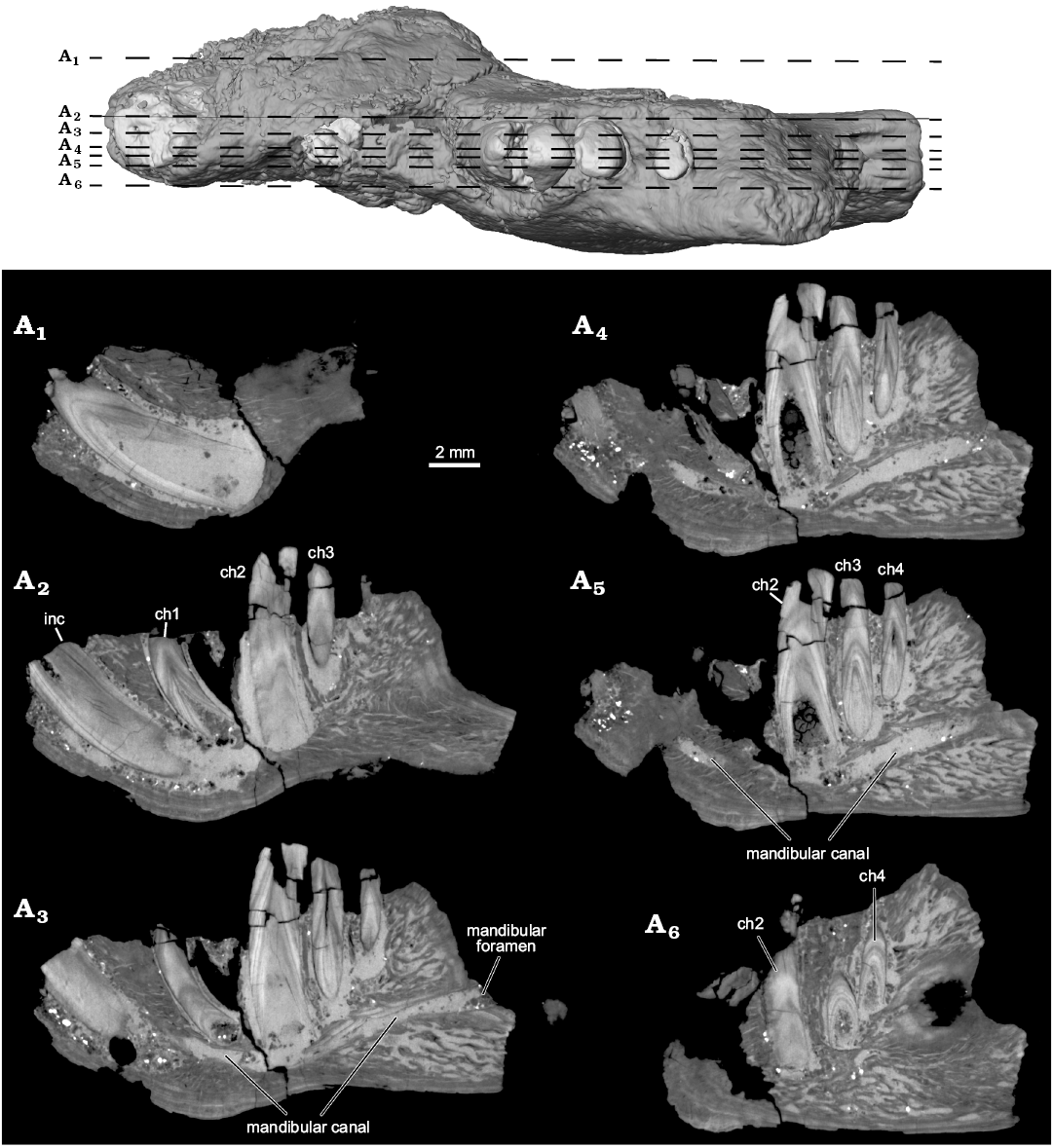
Fig. 4. Selected µCT slice images highlighting internal anatomy of the left dentary of the ?gondwanatherian mammal Galulatherium jenkinsi sp. nov. (holotype, RRBP 02067) from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania. Sagittal slices with top image corresponding to locations of slices through dataset. YZ150 (A1), YZ200 (A2), YZ225 (A3), YZ245 (A4), YZ253 (A5), YZ285 (A6). Abbreviations: ch, lower cheek teeth; inc, lower incisor.
One of the most significant observations in the present study relates to the total number of teeth in RRBP 02067. Krause et al. (2003) identified an incisor, a cheek tooth immediately posterior to the incisor, and three additional cheek teeth arranged along the posterior portion of the horizontal ramus. Based on both a radiograph of the specimen and visual observation of the broken alveolar margin, they also identified a partial root of what they considered another cheek tooth (Krause et al. 2003: figs. 3, 4). This was interpreted as the second of five cheek teeth, giving Galulatherium a total tooth count of six teeth (one incisor, five cheek teeth). Based on high-resolution µCT scans, we observe no evidence of a partial/broken root in this position (i.e., purported ch2 of Krause et al. 2003). Indeed, there is a conical radiopacity at this position in the dentary with its approximately circular base near the alveolar margin and the apex directed ventrally. However, this material is not consistent with that of any other teeth in the jaw based on structure, density, or morphology. Specifically, the radio-opaque material comprising the cone (i) lacks dentine laminations characteristic of all other teeth; (ii) lacks an open root characteristic of all other teeth; and (iii) lacks a smooth contour at both the alveolar margin and along the depth of the cone, the former of which is characteristic of all other teeth. Our current interpretation is that it represents a displaced fragment of cortical bone lodged in this position, or perhaps even a remnant of the dorsal alveolar margin. As such, we conclude that Galulatherium possessed one incisor and four (not five) cheek teeth, as described in the next section.
Incisor: A large, procumbent, laterally compressed incisor is present in Galulatherium. Although the extra-alveolar portion of the tooth is not complete, we estimate that the emergent crown would have intersected at an angle of ~130° with the long axis of the horizontal ramus (Fig. 1A2). The tooth is gently curved, with convex anteroventral and concave posterodorsal margins of which the latter is slightly less broad (buccolingually) than the former (Fig. 2). Both lingual and buccal surfaces of the tooth are nearly flat, forming a slightly curved sulcus that follows the apical-abapical contour of the tooth (Fig. 1). The root cavity is completely open, showing no indication of narrowing abapically. In fact, the posterior-most circumferential edges of the root narrow to less than one millimeter in thickness (Fig. 4). The internal contour of the pulp cavity is conical and extends apically to a narrow canal that is intersected by the broken apex of the tooth (Fig. 3). Narrowing of the pulp cavity begins gradually from the terminus of the root, ultimately being reduced to the canal as it approaches level of the alveolar margin.
As noted above, there is no evidence for enamel on any external surface of the incisor using either light microscopy or µCT (based on differential density). The alveolus for the incisor tightly conforms (circumferentially) to the tooth at the alveolar rim, but gradually increases its circumference relative to the tooth toward the abapical end. This space is occupied by what we interpret as diagenetically-infilled crystalline matrix noted above, appearing denser than the tooth or even the cortical bone.
Cheek teeth: The first cheek tooth (ch1) is generally similar in overall morphology to the incisor in being columnar, open-rooted, and laterally compressed. It is significantly smaller (Table 1), less laterally compressed, and less procumbent (~113° relative to the main axis of the horizontal ramus) than the incisor, separated from the latter by a diastema of ~2.6 mm at the alveolar margin. As in the case of the incisor, the crown of ch1 is broken near the alveolar margin, with only minimal fragments preserved. Unfortunately, these fragments are insufficiently preserved to confidently characterize the shape of the crown, hence we refer to it as ch1 rather than assigning it to a more precise tooth position. Notably, µCT imaging reveals a different sub-alveolar and likely emergent trajectory for ch1, relative to the incisor or the more posteriorly positioned cheek teeth (Fig. 3), suggesting a degree of morphological differentiation along the tooth row in Galulatherium. Other characteristics (e.g., open root, dentine lamination, absence of enamel) described for the incisor apply also to ch1. As for the incisor, the ch1 alveolar margin tightly conforms to the tooth margin, whereas the alveolus itself increases in diameter toward the terminus of the root and is filled with matrix. This expansion is more notable on the distal side than on the mesial, buccal, or lingual sides of the tooth, also similar to that observed for the incisor (Fig. 4A2). The mesial margin of ch1 is gently convex whereas the distal margin of the tooth is gently concave. The buccal surface of the tooth exhibits a relatively smooth contour. In contrast, the lingual surface of the tooth exhibits a shallow sulcus along the length of the tooth, similar to that of the incisor.
The second cheek tooth (ch2) is distinctly larger than ch1 and separated from it by a slightly longer diastema (2.7 mm) than the one separating the incisor from ch1. In overall dimensions, ch2 is the largest of the cheek teeth and the first in the series of three distal teeth (ch2–ch4) that together appear to have congruent and co-planar occlusal morphology. Similar to the more mesial teeth in the dentary, ch2 is gently curved (convex mesially, concave distally), columnar, somewhat laterally compressed, and hypsodont with an open root (Figs. 2, 3, 4A2–A5). As noted for the incisor, the lingual and buccal surfaces of ch2 are nearly flat with a slight longitudinal sulcus running the length of the tooth. The longitudinal sulcus is slightly more noticeable along the lingual surface than the buccal surface (Fig. 2A4). Horizontal cross-sectional shape shifts slightly from dorsal to ventral through the tooth, with the crown being more-or-less elliptical and the root becoming more sub-quadrangular. The crown has undergone additional preparation since the Krause et al. (2003) study, revealing additional detail of the occlusal surface. The dorsodistal half of ch2 is broken and slightly displaced, but remains in contact along the distal edge of the tooth. Fortunately, the displaced tip can be digitally repositioned based on congruity of the broken edges of the fragment and the remainder of the tooth (Fig. 5A2). The tip of the crown is relatively simple, represented by a mesiodistally oriented crest and sloping planar surfaces facing both dorsolingually and dorsobuccally, with the former being relatively larger in surface area than the latter (Figs. 1, 2). The ch2 is tightly approximated to its alveolus along the buccal and distal sides, with minimal presence of the intervening crystalline infill. By contrast, the mesial and lingual surfaces of the tooth exhibit a relatively thick (~1 mm) layer of this material surrounding the tooth. Similar to more mesial teeth in the series, the volume of the infill increases ventrally along the tooth near the termination of the root (Fig. 4A2).
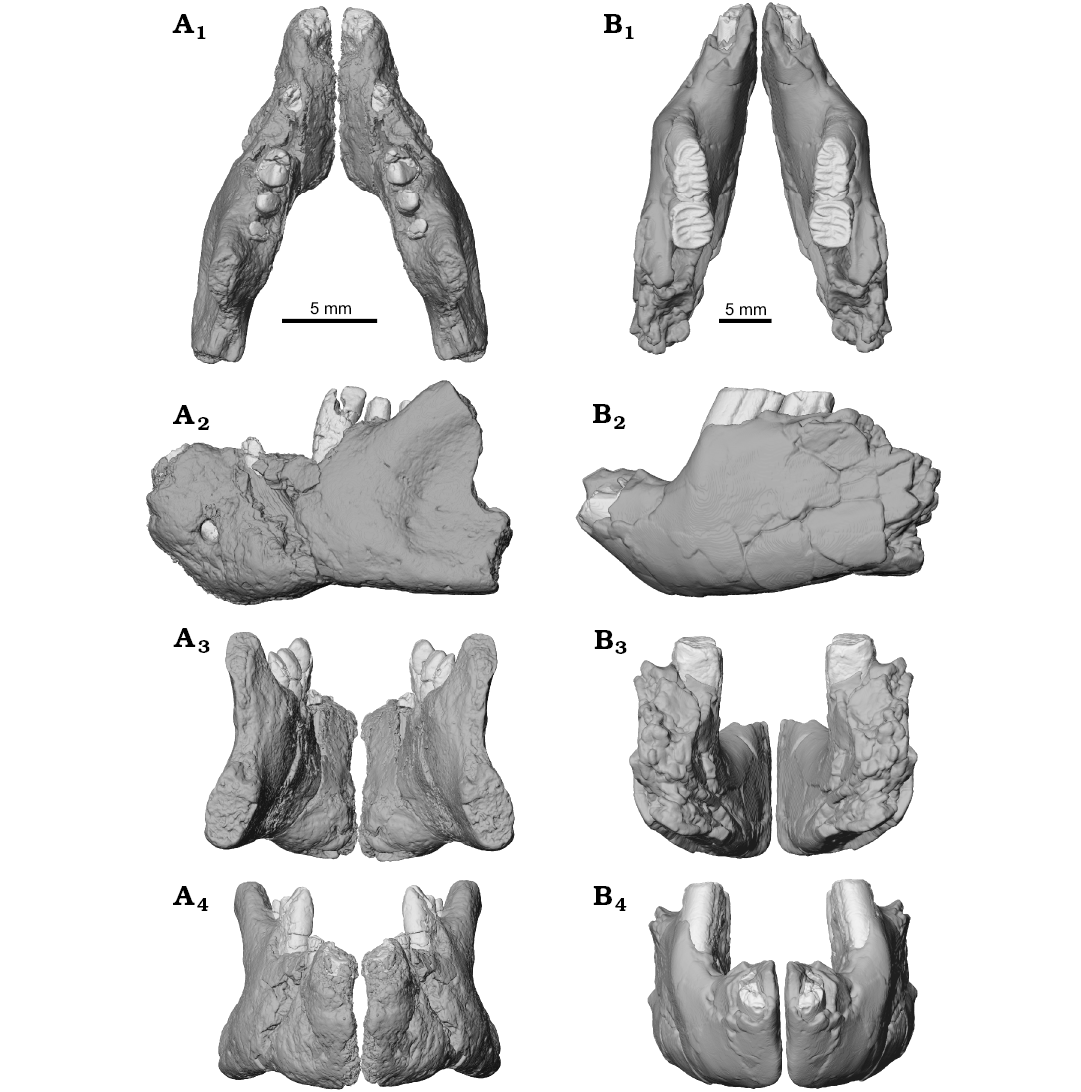
Fig. 5. Lower jaw reconstruction of the ?gondwanatherian mammal Galulatherium jenkinsi sp. nov. (holotype, RRBP 02067, A) from the Turonian–Campanian (Upper Cretaceous) Galula Formation, southwestern Tanzania and gondwanatherian mammal Sudamerica ameghinoi Scillato-Yané and Pascual, 1984 (holotype, MPEFCH 534, B) from the Paleocene Salamanca Formation, Punta Peligro, Chubut Province, Argentina; in dorsal (A1, B1), left lateral (A2, B2), posterior (A3, B3), and anterior (A4, B4) views. The preserved left dentaries of both Galulatherium and Sudamerica have been digitally mirrored to approximate the conformation of the anatomy from the contralateral side. The displaced apical ends of ch2–ch4 in Galulatherium have been digitally repositioned (see SOM 4 for details regarding repositioning).
Cheek teeth three (ch3) and four (ch4) are generally similar to ch2 in morphology (e.g., columnar, hypsodont, open-rooted), but with the cheek teeth decreasing in diameter and height distally along the tooth row (Table 1). Whereas the incisor and ch1 show pronounced lateral compression, both ch3 and ch4 are rotated in the dentary such that they are slightly more compressed along their mesiodistal axes (Fig. 4, Table 1), with occlusal surfaces facing dorsolingually (similar to the dominant surface of ch2). The openings of the root tips of both ch3 and ch4 face ventrobuccally. The cone-shaped pulp cavity extends apically to a position near the alveolar margin. As elsewhere in the series, a small canal extends farther through the dentine, from the conical pulp cavity to the apical surface in each tooth crown. Similar to all other teeth in the dentary, ch3 and ch4 are curved throughout their length such that one surface of the tooth is gently convex and the other gently concave. In contrast to more mesial teeth, however, both ch3 and ch4 are rotated within the dentary such that the convex surface faces lingually while the concave surface faces buccally.
Whereas the occlusal surface in ch2 appears somewhat chisel shaped, with both dorsolingually- and dorsobuccally-facing oblique surfaces (Fig. 2A2), the canted occlusal surfaces in both ch3 and ch4 face dorsolingually and, collectively, appear to form a functional occlusal surface that is co-planar with the dorsolingual facet of ch2 (Fig. 1A4). Alveolar morphology of ch3 and ch4 is difficult to characterize due to preservation but, similar to other teeth, it clasps the teeth more tightly near their alveolar margins than abapically. Moreover, the distobuccal edge of ch3 and mesiobuccal edge of ch4 nearly contact one another at their ventral ends, suggesting the possibility that the alveoli of these two teeth were confluent inside the dentary below the alveolar level. Another notable feature is that the ventral-most portion (= terminus) of ch4 contacts the inner aspect of cortical bone of the dentary.
Dental tissue histology and occlusal wear: As noted previously, we find no evidence (optical or density-based) for the presence of enamel on any of the teeth preserved in Galulatherium. The displaced section of the crown of ch2 was removed to examine both dentine microstructure and conduct occlusal surface wear analysis. Unfortunately, we could not detect neither microstructural information from the etched surface of the dentine, nor scars on the occlusal surface representing genuine microwear features. We presume that microstructure was not preserved or obscured by unknown process(es).
Remarks.—The holotypic and only known specimen (RRBP 02067) of Galulatherium jenkinsi presents a mosaic of primitive and derived morphological features not previously recorded in any known mammaliaform, extinct or extant. It was tentatively referred to Gondwanatheria by Krause et al. (2003) primarily because it preserved distinctively hypsodont cheek teeth. Here we test that hypothesis using additional anatomical information revealed by µCT examination of Galulatherium, together with a broader comparative sample of gondwanatherians described since 2003. As current working hypotheses suggest that gondwanatherians are members of, or closely related to, Allotheria (Gurovich and Beck 2009; Krause et al. 2014; but see Han et al. 2017; Huttenlocker et al. 2018), we compare Galulatherium to other allotherian or purported allotherian taxa, including multituberculates and haramiyidans. This study further reveals dental features (primarily, lack of enamel and columnar teeth with open root ends) that invite comparisons with toothed xenarthrans (e.g., armadillos and tree sloths), tubulidentates (aardvarks), palaeanodonts (thought to belong to either Xenarthra or Pholidota; see, e.g., Rose et al. 2005; Gunnell and Rose 2008; Gaudin and Croft 2015; and references therein), and the enigmatic Late Jurassic mammal Fruitafossor (Luo and Wible 2005).
Comparisons with gondwanatherians: Our comparisons of the mandibular morphology of Galulatherium to that of gondwanatherians are limited because there is only a single published specimen of a gondwanatherian dentary that reveals any significant anatomical information, a horizontal ramus from the Paleocene of Argentina (MPEFCH 534) allocated to Sudamerica ameghinoi (Fig. 5; Pascual et al. 1999). Taxonomic allocations of other dentary specimens referred to various gondwanatherian taxa (e.g., Bonaparte 1990; Kielan-Jaworowska and Bonaparte 1996; Goin et al. 2006) are controversial, thwarting conclusive comparisons (e.g., see review in Krause 2014). In any case, these specimens are too fragmentary to reveal significant morphology not already represented in MPEFCH 534.
The dentary of Sudamerica ameghinoi (MPEFCH 534) has not preserved any part of the ascending ramus but preserves the horizontal ramus containing a single, fragmentary (root only), enlarged incisor, two complete mesial cheek teeth, and alveoli for two distal cheek teeth (Fig. 5B). The dentaries of Sudamerica and Galulatherium are therefore similar to the degree that they exhibit the same lower dental formula, i.e., one incisor and four cheek teeth. The dentary of Galulatherium is likewise short and deep, with an unfused mandibular symphysis, a sizeable diastema between the incisor and ch1, a single mental foramen placed far anteriorly (below the diastema), a robust coronoid process with its base situated lateral to the distal-most cheek teeth, and an incisor root that lies medial and oblique to the cheek tooth row. One additional point of similarity between the two specimens relates to the posterodorsally canted cheek teeth. By contrast, the only notable differences between the two dentaries concern: (i) the presence of a second diastema (between ch1 and ch2) in Galulatherium, absent in Sudamerica; (ii) the ventral border, which is concave in Galulatherium and flat or slightly convex in Sudamerica; (iii) the shape of the anterior portion, which is not stepped in lateral view between the incisor and ch1 in Galulatherium but markedly so in Sudamerica; and (iv) the location of the mental foramen, which in Galulatherium it is only slightly above mid-height dorsoventrally whereas in Sudamerica it lies in a more dorsal position, well above mid-height.
The single lower incisors in the dentary fragments of both Galulatherium and Sudamerica are much enlarged, laterally compressed, open-rooted, procumbent, and emplaced such that the crown exits the alveolus at an angle of approximately 130–135° relative to the long axis of the horizontal ramus of the dentary. The lower incisor root of Galulatherium extends posteriorly in the jaw only to the level of ch2 whereas in Sudamerica the root is much longer and passes posteriorly beneath all four cheek teeth (Pascual et al. 1999: fig. 1B, C).
In addition to the incisor preserved in the dentary (MPEFCH 534) of S. ameghinoi, isolated lower incisors have been assigned to a number of gondwanatherian taxa: Ferugliotherium windhauseni (see Krause et al. 1992: figs. 1A, 1B; Krause and Bonaparte 1993: fig. 2; Gurovich 2006: appendix B-46), Gondwanatherium patagonicum (an incisor originally described as belonging to F. windhauseni by Bonaparte 1990, was later, on the basis of size, assigned to G. patagonicum by Krause et al. 1992: fig. 5; see also Krause and Bonaparte 1993: fig. 2 and Gurovich 2006: appendix B-32), an indeterminate sudamericid cf. Sudamerica ameghinoi from the Antarctic Peninsula (see Goin et al. 2006: fig. 2), Bharattherium bonapartei (see Wilson et al. 2007: fig. 4D, E) from India, an indeterminate gondwanatherian from India (Wilson et al. 2007: fig. 5D, E), and an indeterminate sudamericid from Madagascar (Krause 2013: fig. 7). Close examination reveals another important difference between Galulatherium and specimens referred to Gondwanatheria: the incisor in Galulatherium appears to be completely devoid of enamel. In contrast, all lower incisors confidently assigned to Gondwanatheria exhibit a ventrally restricted band of enamel that extends more dorsally on the lateral than on the medial side (a condition that has evolved independently in a broad range of mammals, Koenigswald 1988).
Finally, lower incisors allocated to Gondwanatheria appear to exhibit a generally higher degree of lateral compression than is observed in Galulatherium, although this feature varies across the sample. In Galulatherium the incisor measures 2.66 mm in long-axis diameter (i.e., height in Krause et al. 2003) and 1.86 mm in short-axis diameter (i.e., width in Krause et al. 2003) just ventral to the alveolar margin (i.e., the most superficial point that an intact external tooth surface is present), yielding a cross-sectional aspect ratio of 1.43 (Table 1; see dashed lines on inset in Fig. 2 for an approximation of where the measurements were taken). Additional preparation and measurements derived from high-resolution µCT allows a more accurate estimation of the tooth metrics in RRBP 02067. In Sudamerica the cross-sectional aspect ratio is 2.5, revealing a much more laterally compressed tooth. Cross-sectional aspect ratios for isolated specimens are as follows: cf. Sudamerica ameghinoi, 2.39; Gondwanatherium patagonicum, 2.03; Ferugliotherium windhauseni, 1.85; indeterminate sudamericid from Madagascar, 1.62 (Krause 2013).
Gondwanatherians, at least as presently constituted, exhibit a broad range of morphological variability in the cheek tooth series. Molariform teeth of the ferugliotheriids Ferugliotherium (Krause et al. 1992) and Trapalcotherium (Rougier et al. 2009) are brachyodont and have longitudinal rows of multiple cusps connected by transverse lophs, differing considerably from the teeth of Galulatherium, but also from the teeth of sudamericids, most obviously in their low-crowned condition. Sudamericids include Sudamerica, Gondwanatherium, Bharattherium, Lavanify, and Vintana (e.g., Pascual et al. 1999; Prasad et al. 2007; Gurovich 2008; Krause 2013, 2014; Krause et al. 2014). Although quite varied in their morphology, with the exception of the heavily worn teeth of Greniodon, they are united in the possession of tall, hypsodont, cheek teeth with vertical furrows, infundibula, and cementum-filled enamel islets. The cheek teeth of Greniodon, an enigmatic gondwanatherian originally not assigned by Goin et al. (2012) to either the Ferugliotheriidae or Sudamericidae but later found to nest within Sudamericidae by Krause et al. (2014), are heavily worn; they were described by Goin et al. (2012) as having complex occlusal morphology and protohypsodont teeth. Perhaps the strongest difference in cheek tooth morphology between Galulatherium and sudamericids is the fact that the former is completely enamel-less.
Although there are marked differences between the cheek teeth of Galulatherium and gondwanatherians, there are interesting similarities as well. Two of the sudamericid genera, Lavanify and Bharattherium, exhibit resemblances to the cheek teeth of Galulatherium in three important ways: (i) Bharattherium and Lavanify both exhibit cheek teeth that are enamel-less on one side of at least one locus (Krause et al. 1997; Prasad et al. 2007), minimally demonstrating that a partial enamel-less condition occurred among gondwanatherians; (ii) unlike the rectangular teeth of other gondwanatherians, the length and width of the cheek teeth of Lavanify and Bharattherium are more nearly equal, thus more closely resembling the peg-like condition of the cheek teeth of Galulatherium; and (iii) relative to their occlusal area, cheek teeth of Bharattherium and Lavanify are exceptionally high-crowned and curved along their length, again approaching the tall, curved, peg-like condition of the cheek teeth of Galulatherium.
Comparisons with multituberculates: In comparing the known morphology of Galulatherium, Krause et al. (2003: 325) stated that, “Among Mesozoic mammals … only gondwanatherians, and taeniolabidoid and djadochtatheroidean multituberculates, possess a large, procumbent, laterally compressed lower central incisor and dentaries with the following suite of features, also exhibited by [RRBP] 02067: body short and deep, unfused mandibular symphysis, distinct diastema, and coronoid process originating far anteriorly (see Pascual et al. 1999).” These observations still hold but additional comparisons can now be made, in part because of the anatomy of Galulatherium revealed by the approaches (µCT and SEM) employed in this paper and in part because of added morphological character development in recent analyses of early mammals (e.g., Luo et al. 2011, 2015, 2017; Krause et al. 2014; Han et al. 2017; Huttenlocker et al. 2018). The dentary of Galulatherium resembles that of multituberculates in possessing a single mental foramen on the horizontal ramus below the diastema, the incisor lies oblique to the cheek tooth row, and the base of the robust coronoid process originates lateral to the distal-most cheek teeth. Galulatherium and multituberculates are similar in lacking a groove for the replacement dental lamina (a plesiomorphic feature of basal mammaliaforms), and both are different from extant xenarthrans in lacking the rostral mandibular spout (a derived xenarthran feature).
The lower incisor of Galulatherium differs from those of taeniolabidoid and djadochtatheroidean multituberculates in the same ways that it differs from those of gondwanatherians: it lacks enamel and is less laterally compressed (height [i.e., mesiodistal length]:width ratio = 1.43). Lateral compression in taeniolabidoid and djadochtatheroidean multituberculates is not frequently quantified but reveals height:width (i.e., cross-sectional aspect) ratios ranging at least between 1.67–2.72: Microcosmodon rosei, 2.00–2.33, Krause (1980); ?Djadochtatherium matthewi (deciduous), 1.67, Kielan-Jaworowska and Hurum (1997); and Neoliotomus ultimus (average), 2.72, Krause (1982).
Lower cheek teeth of multituberculates look nothing like those of Galulatherium. Rather than being single-rooted, peg-like, enamel-less, hypsodont, and evergrowing, they are generally two-rooted (with tapering ends indicating determinate growth) and have low, complex, enameled crowns. Furthermore, the lower cheek tooth series is clearly divided into laterally compressed, peg-like and/or blade-like premolars and molars with two longitudinal rows of cusps, again in contrast with the generally homodont condition in Galulatherium. In addition, although one or more of the mesial lower premolars of multituberculates can be single-rooted and peg-like, in all cases they are enamel-covered. Finally, Arginbaatar, from the Early Cretaceous of Mongolia, is a unique taxon exhibiting a small ventrodistal area on both buccal and lingual surfaces of p4 that is enamel-less, but this appears to be related to a highly unusual form of eruption as the tooth rotates into place (Kielan-Jaworowska et al. 1987).
Krause et al. (2003: 325) noted that: “Taeniolabidoid and djadochtatherioidean multituberculates are restricted to the Late Cretaceous and Paleogene of Laurasia (Kielan-Jaworowska and Hurum 2001), whereas gondwanatherians are roughly contemporaneous but known only from a few sites in Gondwana.” More recently, a number of multituberculates have been reported from Gondwanan landmasses although some of these occurrences have been disputed (see review in Krause et al. 2017). Records of multituberculates from the Mesozoic of the African mainland remain unresolved, as taxa from the Early Cretaceous of Morocco previously referred to the Multituberculata (Hahnodontidae, including Hahnodon and Denisodon) have more recently been considered by Butler and Hooker (2005) to be haramiyidans (also see Huttenlocker et al. 2018).
Comparisons with haramiyidans: Haramiyidans are an enigmatic and controversial clade of Mesozoic mammaliaforms that some workers (e.g., Rowe 1988; Jenkins et al. 1997; Zhou et al. 2013; Luo et al. 2015, 2017; Huttenlocker et al. 2018) regard as stem mammaliaforms, falling outside of Mammalia, although others regard them as allotherian mammals (e.g., Simpson 1928; Hahn 1973; Hahn et al. 1989; Sigogneau-Russell 1989; Miao 1993; Butler and MacIntyre 1994; Kermack et al. 1998; Luo et al. 2002, 2007a, b, 2011; Luo and Wible 2005; Hahn and Hahn 2006; Rowe et al. 2008; Ji et al. 2009; Meng et al. 2011; Zheng et al. 2013; Bi et al. 2014; Krause et al. 2014a; Han et al. 2017). The earliest haramiyidan, Haramiyavia, from the Late Triassic of Greenland, has a dentary that bears little resemblance to that of Galulatherium (Jenkins et al. 1997; Luo et al. 2015), being far more primitive in possessing a long, slender, and shallow dentary morphology while retaining a Meckelian sulcus and coronoid bone, and in lacking a sizeable diastema between the incisor and cheek teeth, a ventral border of the masseteric fossa, and an anteroventral extension of the masseteric fossa onto the horizontal ramus. The holotypic specimen of Haramiyavia has two mental foramina on the right side (with one positioned ventral to the canine and one more anteriorly) and one on the left (ventral to the canine) (Jenkins et al. 1997: fig. 1c; Luo et al. 2015: fig. 1A); by contrast, the mental foramen in Galulatherium lies ventral to the diastema. Finally, Luo et al. (2015) score the anterior base of the coronoid process in Haramiyavia as “partially medial” to the last cheek tooth but this overlap is insignificant relative to that seen in Galulatherium.
In strong contrast, recently described dentaries belonging to a suite of genera from the Middle–Late Jurassic of China (Arboroharamiya, Zheng et al. 2013; Meng et al. 2014; Han et al. 2017; Shenshou, Bi et al. 2014; Xianshou, Bi et al. 2014; Vilevolodon, Luo et al. 2017), and known collectively as euharamiyidans (or eleutherodontids), are highly derived. Their dentaries, like that of Galulatherium, are short and deep and possess a reduced or absent Meckelian sulcus (scored as reduced by Luo et al. 2017 but absent by Han et al. 2017 in Arboroharamiya, Shenshou, and Xianshou), an unfused mandibular symphysis, and a sizeable diastema between the distal incisor and cheek teeth. They are also similar to Galulatherium in lacking a replacement dental lamina. The coronoid processes in Galulatherium and euharamiyidans exhibit robust bases that begin anteriorly lateral to the distal-most cheek teeth and that are reclined at approximately 135–145°. Also, as in Galulatherium, the mandibular foramen in euharamiyidans is located in the medial pterygoid fossa, below the alveolar plane, and there are low anterodorsal and anteroventral crests demarcating the masseteric fossa. Incidentally, there is a discrepancy regarding the degree of development of the anteroventral crest between Luo et al. (2015), who described it as low and broad, and Luo et al. (2017), who characterized it as well-defined and thin). Han et al. (2017) regard the crest as low and broad, extending onto the horizontal ramus, although the euharamiyidan condition (with the possible exception of Vilevolodon; Luo et al. 2017: fig. 1b) is much more anterior than in the Tanzanian mammal. The mental foramen in Galulatherium is positioned ventral to the diastema on the lateral surface of the dentary. The position of the foramen appears to vary among euharamiyidans. In Vilevolodon, a mental foramen is present in the diastema region (Luo et al. 2017: extended data fig. 2) but in Arboroharamiya allinhopsoni (Han et al. 2017: fig. 2a) and Shenshou lui (Han et al. 2017: supplementary information, p. 37, lefthand figure) the foramen is illustrated as lying farther posteriorly, below p4.
Luo et al. (2015) list a lower dental formula of 3.1.4.3 for Haramiyavia, which thus differs substantially from the condition in Galulatherium, which has only a single incisor, no canine, and four cheek teeth. The lower incisors of Haramiyavia decrease in size from mesial to distal but are small, semi-procumbent, and with closed root tips, also contrasting strongly with the massive, procumbent, evergrowing incisor in Galulatherium. The lower incisors of euharamiyidans are reduced to a single, much enlarged, procumbent tooth that is covered with enamel (i.e., not restricted as in gondwanatherians and in taeniolabidoid and djadochtatheroidean multituberculates, or absent as in Galulatherium). Moreover, incisor roots are closed in those euharamiyidans in which this feature could be examined (Zheng et al. 2013; Bi et al. 2014; Han et al. 2017; Luo et al. 2017), again in contrast to the situation in Galulatherium. Furthermore, the shape of the lower incisor crowns of euharamiyidans appears to differ from that of Galulatherium in being very elongate and, in side view, strongly tapering to a pointed apex. Unfortunately, explicitly comparable height and width measurements of euharamiyidan lower incisors (taken at the alveolus) are not available, thus precluding an evaluation of degree of lateral compression. However, available illustrations suggest at least some degree of compression.
Haramiyidan cheek teeth are as different from those of Galulatherium as are those of multituberculates. Although the premolars of Haramiyavia are quite simple teeth, they increase in coronal complexity from mesial to distal, with p2–p4 each having two roots. The three lower molars have complex, low crowns, each with multiple cusps aligned in longitudinal rows. Euharamiyidans have fewer cheek teeth than Haramiyavia but they are clearly heterodont, divided into premolars and molars, and also have complex crowns completely unlike those of Galulatherium, the molars bearing longitudinal rows of multiple cusps (as in multituberculates).
Comparisons with xenarthrans: Although architecturally simple, almost all xenarthran teeth lack enamel and are composed of structurally different kinds of dentines (Kalthoff 2011). Extant tree sloth teeth are usually roundish whereas those of fossil ground sloths are kidney- or 8-shaped; occlusal surfaces are either more or less flat (most mylodontids) or markedly lophodont (megatheriids). Instead of having greatly enlarged lower incisors as in Galulatherium, sloths lack incisors entirely. Cheek teeth (5/4, 4/4) are typically evergrowing and homodont, although in some groups (e.g., megalonychids, extant Choloepus) the mesial-most tooth may be trenchant and projecting (hence “caniniform”). Mandibles, especially in Neogene taxa, may be elongated into an edentulous “spout” of uncertain function (McDonald and De Juliis 2008). However, there are many variations on this theme. For example, the highly specialized Pleistocene/Holocene Antillean sloth, Megalocnus rodens, exhibits an enlarged scalpriform tooth emerging from the front of the mandible that occludes with its maxillary antagonist. However, this tooth is presumably the homolog of the caniniform of other folivoran taxa and is in any case morphologically unlike that of Galulatherium. The Megalocnus dentary is quite short, and in that regard vaguely like that of the Tanzanian mammal, but proportionately much more robust (Paula Couto 1967). The well-circumscribed masseteric fossa interpreted as is another convergence that likely says more about shared functionality than shared phylogeny.
Comparisons with tubulidentates: Although molecular evidence places Tubulidentata within Afrotheria (Seiffert 2007), the only fossils confidently attributed to the order are no older than early Miocene and, in any case, represent only slightly more primitive versions of the living aardvark Orycteropus (cf. Lehmann 2009). Aardvark dentaries are long and shallow, probably adaptations to a myrmecophagous diet, although, in contrast to other committed myrmecophages, with a tall ascending ramus (Patterson 1978). All tubulidentates have a strongly reduced number of teeth and lack teeth at the rostral end of the dentary. Teeth are peg-like (premolars) or 8-shaped (molars), enamel-less, and evergrowing, and they cannot be homologized with individual loci in less specialized dentitions. Structurally, tubulidentate teeth are unique within Mammalia (Shoshani et al. 1988; Lehmann 2009): peg-like, homodont, enamel-less, and evergrowing. They are composed of up to 1500 hexagonal dentine columns (“tubulodentine”) encased in cementum sleeves. Each tubule is a tiny elongate polygon made up of a tiny pulp cavity surrounded by a bundle of radiating tubules (Cuvier 1823; Ungar 2010). In these features, aardvark dentine differs organizationally from dentine in all other mammals including epoicotheriids, folivoran xenarthrans, and Galulatherium. As there are no morphological intermediates or identifiable precursors to the teeth of orycteropodids, the origin of tubulidentate dental microstructure remains obscure. However, there is no reason to believe that, were the evolutionary sequence actually known, it would converge on anything resembling the extremely simplified cheek tooth pattern of RRBP 02067.
Comparisons with palaeanodonts: Paleogene palaeanodonts, whether considered as xenarthrans, pholidotans, or of uncertain phylogenetic affinities, offer another point of comparison for Galulatherium. Palaeanodonts are generally divided into the metacheiromyids and epoicotheriids, both of which include members that variably exhibit dental characteristics at least superficially similar to those observed in the Galulatherium type specimen. These comparisons concern tooth spacing, reductions in total number of cheek teeth, and decreased complexity of individual cheek teeth (e.g., single roots, simple occlusal morphology, enamel reduced or absent).
The early-branching late Wasatchian Tubulodon taylori has a long and slender, shallow dentary with double-rooted, brachyodont cheek teeth (Jepsen 1932). The tooth row terminates well anterior to the ascending ramus; the mental foramen is large and situated slightly beneath the mesial root of the p4 in the lower (ventral) third of the jaw. Cheek teeth of T. taylori exhibit crowns entirely covered by prismatic enamel (Kalthoff et al. 2011).
In contrast, the middle Wasatchian metacheiromyid Palaeanodon ignavus lacks enamel on its peg-like teeth, a situation that appears true for the entire family. Metacheiromys exhibits a long, shallow dentary, a mental foramen beneath the second cheek tooth, and at least one small, semi-procumbent incisor followed by a massive canine (Simpson 1931). The two cheek teeth are interpreted as having been cylindrical and upright, based on the alveolar morphology that would have housed them. The canines exhibit enamel only on some surfaces (e.g., the “outer faces” of the upper canine; Simpson 1931: 321), other surfaces presumably being enamel-less. The two lower cheek tooth alveoli are superficially similar to the cheek tooth alveoli in RRBP 02067, although tooth composition (e.g., enamel-covered vs. enamel-less) and occlusal morphology remain unclear. Mylanodon, another metacheiromyid, also exhibits a long, shallow dentary, with a reduced number of cheek teeth that exhibit relatively simple crowns, and at least some of which (p2, p3, and m2) that are single rooted (Secord et al. 2002). Perhaps the most notable similarity to Galulatherium is that all post-canine teeth are devoid of enamel (Secord et al. 2002). However, the long, shallow dentary that hosts at least some double-rooted cheek teeth differs considerably from the condition in Galulatherium.
Generally similar to metacheiromyids, representative epoicotheriids (e.g., Amelotabes) also preserve long and shallow dentaries (even though the dentary in Amelotabes is considered slightly more robust than in other palaeanodonts; Rose 1978). Cheek teeth in Amelotabes are characterized by relatively thin enamel, but with tapering roots that are closed. The presence of enameled cheek teeth with closed roots in a long, shallow dentary contrasts sharply with the enamel-less, evergrowing cheek teeth and short, moderately high dentary observed in Galulatherium. As another example within Epoicotheriidae, Alocodontulum also exhibits a shallow dentary that preserves one small incisor, a large canine, three premolars, and three molars (Rose et al. 1992). The number of roots varies through the post-canine series, with both p2 and p3 being single rooted (with the single root of p3 mesiodistally elongated); p4 through m2 are double-rooted, with m3 again exhibiting a single root. The crown morphology in cheek teeth, when preserved, is relatively simple and bears two distinct working surfaces that are separated by a transverse ridge (Rose et al. 1992: fig. 2), unlike the mesiodistally-oriented ridge as noted in ch2 of Galulatherium (Fig. 3A4). Taken together, the distinct canine and the multiple, double-rooted cheek teeth with characteristic occlusal morphology clearly set this form apart from Galulatherium despite some intriguing similarities (e.g., some single-rooted, relatively simple cheek teeth) that document the kinds of morphological adaptations that characterized these extinct radiations of early mammals and their kin.
General differences between Galulatherium and the assemblage of palaeanodonts reviewed above include the following: (i) short, high dentary of Galulatherium contrasts sharply with the generalized, elongate, shallow dentaries of palaeanodonts (including the relatively robust dentary of Amelotabes; Rose 1978); (ii) presence of an enlarged, procumbent lower incisor in Galulatherium, as compared to the generally small/reduced incisor of palaeanodonts; (iii) absence of an enlarged, or even regionally differentiated, lower canine in Galulatherium, contra virtually all palaeanodont specimens for which the lower dentition is well known; (iv) reduced number of cheek teeth (four in total) in Galulatherium that are single-rooted and lack enamel, with the variety of palaeanodonts exhibiting variable numbers of cheek teeth (but with all taxa having more than four), at least some of which are double-rooted.
An examination of earlier-branching members (e.g., Arcticanodon, Escavadodon) of this group reveal that certain features (e.g., long, shallow dentary; distinct, enlarged canine) likely represent mammalian plesiomorphies that are expressed throughout the group (Rose et al. 2004). Perhaps most revealing in this regard is the presence of a relatively complex, tribosphenic cheek tooth preserved in Escavadodon, representing one of the earliest palaeanodonts from the early Paleocene (Rose and Lucas 2000). Importantly, any trends in mandibular/dental morphology (e.g., reduction in cheek tooth number, variable expression of single-rooted cheek teeth, decreasing crown complexity, loss of enamel) that are superficially similar to morphology preserved in Galulatherium represent apomorphies of selected palaeanodont subclades, and thus, are clearly convergent features between Galulatherium and the group. Interestingly, a number of the features (both craniodental and postcranial) expressed in palaeanodonts have been invoked in support of dietary interpretations related to insectivory in general, and myrmecophagy more specifically (Rose et al. 2004).
Comparisons with Fruitafossor: Galulatherium and Fruitafossor are unique among Mesozoic mammaliaforms in possessing tubular, enamel-less cheek tooth crowns, each supported by a single, open-ended root. Yet the dentaries of Galulatherium and Fruitafossor exhibit many more differences than similarities. Although both taxa lack a postdentary trough, a groove for the replacement dental lamina, a fused mandibular symphysis, and a rostral mandibular spout (all of which can be considered to be primitive characteristics), the dentary of Galulatherium differs from that of Fruitafossor in exhibiting the following features: horizontal ramus relatively short and deep; mandibular symphysis relatively robust, presence of sizeable diastemata (between the incisor and ch1 and between ch1 and ch2), absence of Meckelian sulcus (and, thereby, having a complete functional disconnection between the middle ear and lower jaw), mandibular foramen located in pterygoid fossa (rather than within Meckelian sulcus, well anterior to pterygoid fossa), a masseteric (= labial mandibular) foramen, absence of coronoid bone, coronoid process much more robust and with anterior base medial to distal-most cheek teeth, masseteric fossa extending anteriorly onto the horizontal ramus, only a single (rather than three or four) mental foramina placed far anteriorly on horizontal ramus, and a more reclined anterior border of the coronoid process (~140° as opposed to ~120° in Fruitafossor; measured in Luo and Wible 2005: fig. 1).
Galulatherium and Fruitafossor are superficially similar in tooth morphology, with both exhibiting single-rooted, hypsodont, and enamel-less cheek teeth. However, Galulatherium differs fundamentally from Fruitafossor in exhibiting a much enlarged, procumbent incisor (as opposed to three small, peg-like incisors), in lacking a canine, and in having fewer cheek teeth (four cheek teeth vs. six, the latter of which were identified as three premolars and three molars by Luo and Wible 2005). Moreover, the distal three cheek teeth in Galulatherium decrease in size, as opposed to being subequal in size as in Fruitafossor (but note that Luo and Wible 2005 score the ultimate molar as being smaller than the penultimate one). Galulatherium also exhibits obliquely-canted occlusal surfaces on its cheek tooth crowns (as opposed to flat, apical wear), with the anterior cheek teeth being laterally compressed (as opposed to mesiodistally compressed). Luo and Wible (2005) concluded that the dental similarities between Fruitafossor and xenarthrans or any other placental group (e.g., tubulidentates) were the result of evolutionary convergence. We likewise conclude that the resemblances between Fruitafossor and Galulatherium are best regarded as the result of convergence.
Summary of comparisons: The comparisons above indicate that the structure of the dentary and teeth of Galulatherium are highly derived and convergent upon morphologies seen in a number of mammalian higher taxa, both extinct and extant. The overall structure of the dentary of Galulatherium is generally consistent with that seen in gondwanatherians and multituberculates in particular, but also with euharamiyidans. The relative size and positioning and, to some extent, the structure of the lower incisor is also not unlike that of gondwanatherians and at least some multituberculates. However, xenarthrans and tubulidentates lack teeth that can be positionally homologized with incisors, and those of haramiyidans differ in being elongate, strongly tapered, and not evergrowing. When preserved in palaeanodonts, incisors tend to be small and procumbent, rather than the enlarged procumbent incisor exhibited by Galulatherium.
Yet the overall morphology of the cheek teeth in Galulatherium is much simpler than that observed in multituberculates, haramiyidans, palaeanodonts and at least most gondwanatherians (excepting perhaps Lavanify and Bharattherium), instead sharing features with taxa likewise exhibiting highly reduced dentitions, particularly xenarthrans and tubulidentates. Those taxa currently assigned to Gondwanatheria evince a broad range of dental morphologies. Thus, if Galulatherium retains its gondwanatherian status, it expands the groups’ morphological range considerably in that its cheek teeth are enamel-less. In short, Galulatherium is a little like a lot of different mammaliaform taxa, but not very much like any one of them. However, Bharattherium and Lavanify exhibit hypsodont, columnar cheek teeth that are enamel-less on at least one side (Krause et al. 1997; Prasad et al. 2007), perhaps offering the single best comparison at this point in time.
Stratigraphic and geographic range.—Type locality and horizon only.
Concluding remarks
The discovery of RRBP 02067 in 2002 reigns as a hard-won victory in the context of Gondwanan Mesozoic vertebrate paleontology, as it still represents the most complete mammaliaform from the entire Cretaceous of continental Africa. Established here as the new genus and species Galulatherium jenkinsi, it also gains distinction as the first named mammaliaform from the Late Cretaceous of continental Africa. We therefore summarize below our new insights about Galulatherium gained since the original description of RRBP 02067.
Morphological considerations of the lower jaw and teeth.—There are several aspects of the lower jaw and dentition of Galulatherium that are noteworthy, particularly those related to the short, deep dentary and enamel-less, hypselodont teeth. These are even more interesting when considering that Galulatherium lived in the Late Cretaceous. When originally described (Krause et al. 2003), RRBP 02067 was noted as having a relatively robust dentary, a single, large, procumbent incisor, and five hypsodont cheek teeth. Our reanalysis of the specimen, primarily resulting from new observations and perspectives derived from µCT imaging, allow us to identify additional features of the dentary and cause us to reconsider and refine aspects of dental morphology related to cheek tooth count, dental tissues making up the teeth, and the specific nature of the high-crowned cheek teeth.
First, high-resolution µCT analysis reveals detailed information regarding the presence of masseteric foramina (not originally noted) and position and size of both the mental (incorrectly identified in original description) and mandibular (not originally noted) foramina, not to mention the size and course of the entire mandibular canal. The mandibular canal of Galulatherium appears to be quite large, similar to that of extant and fossil monotremes (e.g., Rowe et al. 2008; Rich et al. 2016), but a full assessment is not possible because quantitative comparative data are not available for this feature.
Second, we can also identify the presence of only four, not five, cheek teeth in Galulatherium, similar to the condition in a number of Mesozoic mammals including gondwanatherians for which the lower dentition is known (e.g., Sudamerica; a new, yet unnamed taxon from the Late Cretaceous of Madagascar; Pascual et al. 1999; Krause et al. 2018). We also confirm just a single lower incisor in the dentary.
Third is the fact that all teeth in the dentary of Galulatherium appear to have been hypselodont (i.e., evergrowing). Hypsodonty (protohypsodonty of Mones 1987) appears to have been ubiquitous among sudamericid gondwanatherians (as opposed to the brachiodonty of the ferugliotheriids Ferugliotherium and Trapalcotherium) but it is not known if any sudamericids had hypselodont (euhypsodont of Mones 1987) teeth at all positions. Sudamerica and Vintana, and perhaps Gondwanatherium, for instance, appear to have had hypselodont incisors but not cheek teeth (Krause et al. 1992; Koenigswald et al. 1999; Krause 2014). By contrast, the lower incisor and all four cheek teeth of Galulatherium were fully hypselodont. Hypselodonty among crown mammals has arisen independently in multiple lineages, including selected Marsupialia (e.g., all tooth positions in Vombatus among diprotodontian marsupials) and with variable expression in Artiodactyla, Notoungulata, Carnivora, Cetacea, Glires, and Afrotheria among Placentalia (Koenigswald 2011; Renvoisé and Michon 2014). Interestingly, it is unclear/unknown how common this condition may be at non-incisor loci among non-mammalian mammaliaforms, but Galulatherium represents the first clear and perhaps the earliest-occurring example of jaw-wide hypselodonty among mammaliaforms (the condition is unknown for the incisors of the Late Jurassic Fruitafossor, Luo and Wible 2005); jaw-wide hypselodonty is rare among Mammalia (e.g., Vombatus among diprotodontian marsupials, some rodents, lagomorphs; Koenigswald 2011; Renvoisé and Michon 2014). However, given the increasingly widespread use of high-resolution µCT in studies of extinct mammaliaforms, this condition may be more common than previously considered.
Fourth, as noted by Krause et al. (2003), it was unclear whether RRBP 02067 was truly enamel-less, or whether the enamel was so thin as to not be preserved or if it had somehow been lost during preservation. Based on work conducted to date, there is no evidence based on either novel µCT data (e.g., density differential among the dental tissues; Fig. 4) or visual inspection that RRBP 02067 ever had an enamel layer on its dentition. Moreover, it is unclear in what specific way (e.g., thickness, spatial configuration, etc.) cementum would have been associated with the teeth of Galulatherium. Similar to other taxa with hypselodont teeth, we would expect at least some amount of cementum near where a tooth was in tightest apposition to the alveolus to participate as part of the periodontium (e.g., Tummers and Thesleff 2008). Also, we cannot rule out that some taphonomic process (e.g., etching/dissolution by traveling through a digestive system; e.g., Fisher 1981) may have selectively eroded non-dentine tissues in Galulatherium. However, given the relatively tight conformation of the teeth with the alveolar margin of the dentary, not to mention the relatively uniform cross-sectional size dimensions along the entire height of the cheek teeth (i.e., no “waisting”; Table 1), we consider any non-dentine dental tissues to be relatively minor components of the total tooth volume.
Dentine-cementum dominant dentitions among extant mammals are represented in select clades of eutherian mammals (e.g., folivorans [sloths] among xenarthrans; physeteroids [sperm whales] among cetaceans; Meredith et al. 2009). A number of mammaliaforms variably express an enamel-less condition along the tooth row (e.g., Fruitafossor, palaeanodonts, Elephantidae) or even within a single locus (e.g., palaeanodonts, rodent incisors). Even rarer is the combination of a dentine/cementum-only dentition combined with hypselodonty, being restricted to elephant incisors, narwhal and walrus canines, and cheek teeth of selected other groups (e.g., sloths, aarvarks, some whales) among extant taxa (Renvoisé and Michon 2014). Thus, the presence of an enamel-less, hypselodont dentition in the Late Cretaceous Galulatherium represents the earliest occurrence of this combination of derived dental features among mammaliaforms (pending additional information on Fruitafossor). It also appears that Galulatherium is unique among mammaliaforms in exhibiting enamel-less, hypselodont teeth at every locus in the lower jaw, a trait shared, likely convergently, with toothed xenarthrans and aardvarks. Whether a transitory enamel cap would have been present at an earlier ontogenetic stage in Galulatherium, as reported in select extant mammals (e.g., sperm whales, walrus tusks; Koenigswald et al. 1999; Meredith et al. 2009), is unknown at this time. A consideration of the evolution of enamel loss in various mammalian (and other tetrapod) lineages has received recent attention, both with larger-scale pattern analyses (e.g., Davit-Béal et al. 2009; Koenigswald 2011; Renvoisé and Michon 2014) as well as at developmental-genetic scales (e.g., Tummers and Thesleff 2008, 2009; Meredith et al. 2009; Hautier et al. 2016).
Functional modeling of the jaw and teeth.—The co-planar occlusal surfaces modeled for the last three cheek teeth of RRBP 02067 (i.e., ch2–ch4, each with a dorsolingually facing wear surface; Fig. 1) suggest three possible primary power stroke modalities, none of which is mutually exclusive. A relatively simple orthal (i.e., dorsoventral) power stroke is consistent with the occlusal morphology of the cheek teeth. However, both a proal and palinal power stroke would also be possible with this configuration assuming that the upper cheek teeth have direct occlusal complementarity (i.e., with a single, coplanar, ventrobuccally facing surface) with the lower cheek teeth. It is also not possible to rule out a transverse component to the power stroke. However, the presence of a mesiodistally-oriented primary ridge along ch2–ch4 (Fig. 1) would likely limit significant transverse excursion. Of course the simplest way to directly distinguish among these possibilities would be via an analysis of occlusal surface microwear. As noted above, however, SEM imaging of the occlusal surface on a fragment of ch2 (i.e., the largest tooth with the single largest occlusal surface) did not reveal any clear and genuine dietary microwear features. Moreover, given the incomplete nature of the posterior region of the dentary and the absence of a cranium, important functional components of the masticatory apparatus (e.g., condylar position/size, position of the coronoid process, muscle vectors) are not accessible.
Phylogenetic considerations.—Galulatherium jenkinsi, at the time referred to as the “unnamed Tanzanian taxon”, was included in the phylogenetic analysis of Krause et al. (2014). Variations in scoring related to different hypotheses of cheek tooth homologies (whether two molars, following the arguments of Gurovich and Beck 2009, or four, the more traditional view, for Sudamerica, Vintana, and Galulatherium, the only gondwanatherian taxa for which the number of lower cheek teeth can be confidently determined or logically inferred) were included. Regardless, Galulatherium was consistently resolved as a sudamericid gondwanatherian along with Bharattherium, Gondwanatherium, Greniodon, Lavanify, Sudamerica, and Vintana, and more derived than the ferugliotheriids Ferugliotherium and Trapalcotherium. In some runs, sudamericids were recovered in a polytomy, whereas in others there was some resolution (e.g., Vintana as the sister taxon of Bharattherium + Lavanify) but, of most relevance for our purposes here, no matter what the variation in scoring, Galulatherium was determined to be a sudamericid. That said, the support values for Sudamericidae are low and, largely for that reason, we prefer to conservatively refer to Galulatherium as ?Gondwanatheria due to the fact that gondwanatherians as a group remain poorly known, represented primarily by isolated teeth and lower jaw fragments except for the relatively well preserved cranium of Vintana (Krause et al. 2014).
Age constraint for Galulatherium.—Increased resolution of age constraint and depositional setting of the Namba Member of the Galula Formation (Roberts et al. 2010; Widlansky et al. 2018) helps to better contextualize Galulatherium among Cretaceous mammals (regardless of the clade to which it ultimately belongs). Paleomagnetic reversal stratigraphy conducted through the Galula Formation reveals that at least the upper Namba Member (i.e., the sub-unit from which RRBP 02067 was recovered) can be confidently placed in the Upper Cretaceous based on the occurrence of alternating normal-reversed polarity events at locality RRBP TZ-07, the very locality from which Galulatherium is known. Although the exact position within the Upper Cretaceous can only be restricted to a span between the Turonian–Campanian, this significantly refines age estimates of just Cretaceous (e.g., Krause et al. 2003) or “middle Cretaceous” (i.e., Aptian–Cenomanian) for this unit that were previously based on coarse biostratigraphy using titanosaurian sauropods (Gorscak et al. 2017), notosuchian crocodyliforms (O’Connor et al. 2010), and long-distance correlative stratigraphy (O’Connor et al. 2006; Roberts et al. 2010). If Galulatherium is older than Campanian and if it is confirmed to be a gondwanatherian through subsequent discoveries and analyses, it will represent the oldest known member of the Gondwanatheria, pushing the origin of the clade back perhaps by as much as 10 million years.
Galulatherium jenkinsi remains as the most complete Late Cretaceous mammaliaform known from much of the former southern supercontinent. Given the relative paucity of Gondwanan mammals known from Africa and elsewhere in Gondwana, it is perhaps not surprising that this animal expresses a mixture of anatomical characteristics that preclude a simple placement among Mammaliaformes as we know them, thereby underscoring the need for additional sampling efforts in unexplored and underexplored rock units the world around.
Acknowledgements
We thank Donatius Kamamba, Elawisa Maro, Anthony Tibaijuka, Jesuit Temba, and Joseph Temu (all Tanzania Antiquities Unit, Dar es Salaam, Tanzania); Evelyne Mbede, Elisu Mshui, and Cassy Mtelela (all University of Dar es Salaam, Tanzania); and the Tanzania Commission for Science and Technology for administrative support. We are also grateful to Virginia Heisey (Stony Brook University, USA), Sebastian Egberts and Eric Lund (both Ohio University, Athens, USA) for mechanical preparation, and to Waymon Holloway (Ohio University) for preliminary digital preparation of RRBP 02067. For assistance in the field, we thank the Rukwa Rift Basin Project field team members since 2002. Thanks to Diego Pol (MPEFCH) for access to MPEFCH 534, and to Zhe-Xi Luo (University of Chicago, USA), Meng Jin (American Museum of Natural History, New York, USA), Louis Jacobs (Southern Methodist University, Dallas, USA), Lionel Hautier (Université Montpellier, France), Mariano Bond (Universidad de la Plata, La Plata, Argentina), Susan Williams (Ohio University), and Robert Asher (University of Cambridge, UK) for discussions and access to casts, photographs, and/or uCT data. We are also grateful to Zhe-Xi Luo (University of Chicago, USA) and Meng Jin (American Museum of Natural History) for providing thoughtful reviews of this manuscript. This research was supported by the National Science Foundation (EAR-0617561, EAR-1349825 to PMO and NJS, EAR-1525915 to PMO, and EAR-1664432 to DWK), the National Geographic Society (CRE), and Ohio University Heritage College of Osteopathic Medicine and Office of Research and Sponsored Programs.
References
Bi, S., Wang, Y., Guan, J., Sheng, X., and Meng, J. 2014. Three new Jurassic euharamiyidan species reinforce early divergence of mammals. Nature 514: 579–584.
Bonaparte, J.F. 1990. New Late Cretaceous mammals from the Los Alamitos Formation, northern Patagonia. National Geographic Research 6: 63–93.
Brunet, M., Coppens, Y., Dejax, J., Flynn, L.J., Heintz, E., Hell, J.V., Jacobs, L.J., Jehenne, Y., Mouchelin, G., Pilbeam, D., and Sudre, J. 1990. Nouveaux mammifères du Crétacé inférieur du Cameroun. Comptes Rendus de l’Académie des Sciences Série II 310: 1139–1146.
Brunet, M., Jacobs, L., Congleton, J., Coppens, Y., Dejax, J., Flynn, L., Hell, J., Jehenne, Y., Mouchelin, G., and Pilbeam, D. 1988. Première d.couverte d’un fragment de mandibule de Mammifère dans le Crétacé d’Afrique (Cameroun, Bassin de Koum). Comptes Rendus de l’Académie des Sciences Série 307: 1675–1680.
Butler, P.M. and Hooker J.J. 2005. New teeth of allotherian mammals from the English Bathonian, including the earliest multituberculates. Acta Palaeontologica Polonica 50: 185–207.
Butler, P.M. and MacIntyre, G.T. 1994. Review of the British Haramiyidae (?Mammalia,Allotheria), their molar occulsion and relationships. Philosophical Transactions of the Royal Society of London B 345: 433–458.
Contessi, M. 2013. First report of mammal-like tracks from the Cretaceous of North Africa (Tunisia). Cretaceous Research 42: 48–54.
Cuvier, G. 1823. Recherches sur les Ossements Fossiles. Vol. V, Part 1, 134–135. Chez G. Dufour et E. d’Ocagne, Paris.
Davis, BM. 2012. Micro-computed tomography reveals a diversity of peramuran mammals from the Purbeck Group (Berriasian) of England. Palaeontology 55: 789–817.
Davit-Béal, T., Tucker, A.S., and Sire, J.-Y. 2009. Loss of teeth and enamel in tetrapods: fossil record, genetic data and morphological adaptations. Journal of Anatomy 214: 477–501.
Fisher, D.C. 1981. Crocodilian scatology, microvertebrate concentrations, and enamel-less teeth. Paleobiology 7: 262–275.
Gaudin, T.J. and Croft, D.A. 2015. Paleogene Xenarthra and the evolution of South American mammals. Journal of Mammalogy 96: 622–634.
Goin, F.J., Reguero, M.A., Pascual, R., Koenigswald, W. von, Woodburne, M.O., Case, J.A., Vieytes, C., Marenssi, S.A., and Vizcaíno, S.F. 2006. First gondwanatherian mammal from Antarctica. In: J.E. Francis, D. Pirrie, and J.A. Crame (eds.), Cretaceous–Tertiary High-Latitude Palaeoenvironments, James Ross Basin, Antarctica G. Geological Society of London, Special Publications 258: 135–144. Geological Society of London, London.
Goin, F.J., Tejedor, M.F., Chornogubsky, L., López, G.M., Gelfo, J.N., Bond, M., Woodburne, M.O., Gurovich, Y., and Reguero, M. 2012. Persistence of a Mesozoic, non-therian mammalian lineage (Gondwanatheria) in the mid-Paleogene of Patagonia. Naturwissenschaften 99: 449–463.
Gorscak, E., O’Connor, P.M., Roberts, E.M., and Stevens, N.J. 2017. The second titanosaurian (Dinosauria: Sauropoda) from the middle Cretaceous Galula Formation, southwestern Tanzania with remarks on African titanosaurian diversity. Journal of Vertebrate Paleontology 37. [published online] Crossref
Gunnell, G.F. and Rose, K.D. 2008. “Edentata” summary. In: C.M. Janis, G.F. Gunnell, and M.D. Uhen (eds.), Evolution of Tertiary Mammals of North America: Volume 2: Small Mammals, Xenarthrans, and Marine Mammals, 127–134. Cambridge University Press, Cambridge.
Gurovich, Y. 2006. Bio-evolutionary Aspects of Mesozoic Mammals: Description, Phylogenetic Relationships and Evolution of the Gondwanatheria (Late Cretaceous and Paleocene of Gondwana). 621 pp. Ph.D. Dissertation, Universidad Nacional de Buenos Aires, Buenos Aires.
Gurovich, Y. 2008. Additional specimens of Sudamericid (Gondwanatheria) mammals from the early Paleocene of Argentina. Palaeontology 51: 1069–1089.
Gurovich, Y. and Beck, R. 2009. The phylogenetic affinities of the enigmatic mammalian clade Gondwanatheria. Journal of Mammalian Evolution 16: 25–49.
Haddoumi, H., Allain, R., Meslouh, S., Metais, G., Monbaron, M., Pons, D., Rage, J.-C., Vullo, R., Zouhri, S., and Gheerbrant, E. 2016. Guelb el Ahmar (Bathonian, Anoual Syncline, eastern Morocco): first continental flora and fauna including mammals from the Middle Jurassic of Africa. Gondwana Research 29: 290–319.
Hahn, G. 1973. Neue Zähne von Haramiyiden aus der Deutschen Ober-Trias und ihre Beziehungen zu den Multituberculaten. Palaeontographica, Abteilung A 142: 1–15.
Hahn, G. and Hahn, R. 2006. Evolutionary tendencies and systematic arrangement in the Haramiyida (Mammalia). Geologica et Palaeontologica 40: 173–193.
Hahn, G., Sigogneau-Russell, D., and Wouters, G. 1989. New data on Theroteinidae: their relations with Paulchoffatiidae and Haramiyidae. Geologica et Palaeontologica 23: 205–215.
Han, G., Mao, F., Bi, S., Wang, Y., and Meng, J. 2017. A Jurassic gliding euharamiyidan mammal with an ear of five auditory bones. Nature 551: 451–457.
Hautier, L., Gomes Rodrigues, H., Billet, G., and Asher, R.J. 2016. The hidden teeth of sloths: evolutionary vestiges and the development of a simplified dentition. Scientific Reports 6: 27763.
Huttenlocker, A.K., Grossnickle, D.M., Kirkland, J.I., Schultz, J.A., and Luo, Z.X. 2018. Late-surviving stem mammal links the lowermost Cretaceous of North America and Gondwana. Nature 558: 108–112.
Jacobs, L.L., Congleton, J.D., Brunet, M., Dejax, J., Flynn, L.J., Hell, J.V., and Mouchelin, G. 1988. Mammal teeth from the Cretaceous of Africa. Nature 336: 158–160.
Jacobs, L.L., Polcyn, M.J., Mateus, O., Schulp, A.S., Gonçalves, A.O., and Morais, M.L. 2016. Post-Gondwana Africa and the vertebrate history of the Atlantic Angolan coast. Memoirs of Museum Victoria 74: 343–362.
Jenkins, F.A., Gatesy, S.M. Shubin, N.H, and Amaral, W.W. 1997. Haramiyids and Triassic mammalian evolution. Nature 385: 715–718.
Jepsen, G.L. 1932. Tubulodon taylori, a Wind River Eocene tubulidentate from Wyoming. Proceedings of the American Philosophical Society 71: 255–274.
Ji, Q., Luo, Z.-X., Zhang, X., Yuan, C.-X., and Xu, L. 2009. Evolutionary development of the middle ear in Mesozoic therian mammals. Science 326: 278–281.
Kalthoff, D.C. 2011. Microstructure of dental hard tissues in fossil and recent xenarthrans (Mammalia: Folivora and Cingulata). Journal of Morphology 272: 641–661.
Kalthoff, D.C., Rose, K.D., and Koenigswald, W. von 2011. Dental microstructure in Palaeanodon and Tubulodon (Palaeanodonta) and bioerosional tunneling as a widespread phenomenon in fossil mammal teeth. Journal of Vertebrate Paleontology 31: 1303–1313.
Kermack, K.A., Kermack, D.M., Lees, P.M., and Mills, J.R.E. 1998. New multituberculate-like teeth from the Middle Jurassic of England. Acta Palaeontologica Polonica 43: 581–606.
Kielan-Jaworowska, Z. and Bonaparte, J.F. 1996. Partial dentary of a multituberculate mammal from the Late Cretaceous of Argentina and its taxonomic implications. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” e Instituto Nacional de Investigación de las Ciencias Naturales 145: 1–9.
Kielan-Jaworowska, Z. and Hurum, J.H. 1997. Djadochtatheria—a new suborder of multituberculate mammals. Acta Palaeontologica Polonica 42: 201–242.
Kielan-Jaworowska, Z. and Hurum, J.H. 2001. Phylogeny and systematics of multituberculate mammals. Palaeontology 44: 389–429.
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.-X. 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. 630 pp. Columbia University Press, New York.
Kielan-Jaworowska, Z., Dashzeveg, D., and Trofimov, B.A. 1987. Early Cretaceous multituberculates from Mongolia and a comparison with Late Jurassic forms. Acta Palaeontologica Polonica 38: 3–47.
Klein, H., Lagnaoui, A., Gierliński, G.D., Saber, H., Lallensack, J.N., Oukassou, M., and Charrière, A. 2018. Crocodylomorph, turtle and mammal tracks in dinosaur-dominated Middle–?Upper Jurassic and mid-Cretaceous ichnoassemblages of Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology 498: 39–52.
Koenigswald, W. von 1988. Enamel modification in enlarged front teeth among mammals and the various possible reinforcements of the enamel. In: D.E. Russell, J.-P. Santoro, and D. Sigogneau-Russell (eds.), Teeth Revisited: Proceedings of the VIIth International Symposium on Dental Morphology, Paris 1986. Mémoires du Muséum National d’Histoire Naturelle, Paris (série C) 53: 147–167.
Koenigswald, W. von 2011. Diversity of hypsodont teeth in mammalian dentitions—construction and classification. Palaeontographica Abteilung A 294: 63–94.
Koenigswald, W. von, Goin, F.J., and Pascual, R. 1999. Hypsodonty and enamel microstructure in the Paleocene gondwanatherian mammal Sudamerica ameghinoi. Acta Palaeontologica Polonica 44: 263–300.
Krause, D.W. 1980. Multituberculates from the Clarkforkian Land-Mammal Age, late Paleocene–early Eocene, of western North America. Journal of Paleontology 54: 1163–1183.
Krause, D.W. 1982. Multituberculates from the Wasatchian Land-Mammal Age, early Eocene of western North America. Journal of Paleontology 56: 271–294.
Krause, D.W. 2013. Gondwanatheria and ?Multituberculata (Mammalia) from the Late Cretaceous of Madagascar. Canadian Journal of Earth Sciences 50: 324–340.
Krause, D.W. 2014. Dental Morphology of Vintana sertichi (Mammalia, Gondwanatheria) from the Late Cretaceous of Madagascar. Journal of Vertebrate Paleontology 34 (Supplement 1): 137–165.
Krause, D.W. and Bonaparte, J.F. 1993. Superfamily Gondwanatherioidea: A previously unrecognized radiation of multituberculate mammals in South America. Proceedings of the National Academy of Sciences 90: 9379–9383.
Krause, D.W., Gottfried, M.D., O’Connor, P.M., and Roberts, E.M. 2003. A Cretaceous mammal from Tanzania. Acta Palaeontologica Polonica 48: 321–330.
Krause, D.W., Hoffmann, S., and Werning, S. 2017. First postcranial remains of Multituberculata (Allotheria, Mammalia) from Gondwana. Cretaceous Research 80: 91–100.
Krause, D.W., Hoffmann, S., Wible, J.R., Kirk, E.C., Schultz, J.A., Koenigswald, W. von, Groenke, J.R., Rossie, J.B., O’Connor, P.M., Seiffert, E.R., Dumont, E.R., Holloway, W.L., Rogers, R.R., Rahantarisoa, L.J., Kemp, A.D., and Andriamialison, H. 2014. First cranial remains of a gondwanatherian mammal reveal remarkable mosaicism. Nature 515: 512–517.
Krause, D.W., Hoffmann, S., Wible, J.R., Rougier, J., and Hu, Y. 2018. Lower jaw morphology of a new gondwanatherian mammal from the Late Cretaceous of Madagscar. In: A. Farke, A. MacKenzie, and J. Miller-Camp (eds.), 78th Annual Meeting Society of Vertebrate Paleontology, Program and Abstracts, 162. Society of Vertebrate Paleontology, Albuquerque. [published online, http://vertpaleo.org/Annual-Meeting/Annual-Meeting-Home/SVP-2018-program-book-V4-FINAL-with-covers-9-24-18.aspx]
Krause, D.W., Kielan-Jaworowska, Z., and Bonaparte, J.F. 1992. Ferugliotherium Bonaparte, the first known multituberculate from South America. Journal of Vertebrate Paleontology 12: 351–376.
Krause, D.W., Prasad, G.V.R., Koenigswald, W. von, Sahni, A., and Grine, F.E. 1997. Cosmopolitanism among Gondwanan Late Cretaceous mammals. Nature 390: 504–507.
Lehmann, T. 2009. Phylogeny and systematics of the Orycteropodidae (Mammalia, Tubulidentata). Zoological Journal of the Linnean Society 155: 649–702.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Salvius, Stockholm.
Luo, Z.-X. and Wible, J.R. 2005. A late Jurassic digging mammal and early mammalian diversification. Science 308: 103–107.
Luo, Z.-X., Kielan-Jaworowska, Z., and Cifelli, R.L. 2002. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontologica Polonica 47: 1–78.
Luo, Z.-X., Chen, P.-J., Li, G., and Chen, M. 2007a. A new eutriconodont mammal and evolutionary development of early mammals. Nature 446: 288–293.
Luo, Z.-X., Gatesy, S.M., Jenkins Jr., F.A., Amaral, W.W., and Shubin, N.H. 2015. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proceedings of the National Academy of Sciences of the United States of America 112: E7101–E7109.
Luo, Z.-X., Ji, Q., and Yuan, C.-X. 2007b. Convergent dental adaptations in pseudo-tribosphenic and tribosphenic mammals. Nature 450: 93–97.
Luo, Z.-X., Meng, Q.-J., Grossnickle, D.M., Liu, D., Neander, A.I., Zhang, Y.-G., and Ji, Q. 2017. New evidence for mammaliaform ear evolution and feeding adaptation in a Jurassic ecosystem. Nature 548: 326–329.
Luo, Z.-X., Yuan, C.X., Meng, Q.J., and Ji, Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476: 442–445.
Mao, F., Wang, Q., and Meng, J. 2015. A systematic study of tooth enamel microstructures of Lambdopsalis bulla (Multituberculate, Mammalia) —Implications for multituberculate biology and phylogeny. PLoS One 10 (5): e0128243.
Mateus, O., Marzola, M., Schulp, A. S., Jacobs, L.L., Polcyn, M.J., Pervov, V., Gonçalves, A.O., and Morais, M.L. 2017. Angolan ichnosite in a diamond mine shows the presence of a large terrestrial mammaliamorph, a crocodylomorph, and sauropod dinosaurs in the Early Cretaceous of Angola. Palaeogeography, Palaeoclimatology, Palaeoecology 471: 220–232.
McDonald, H.G. and De Iuliis, G. 2008. Fossil history of sloths. In: S.F. Vizcaíno and W.J. Loughry (eds.), The Biology of the Xenarthra, 39–55. University Press of Florida, Florida.
Meng, J., Bi, S., Wang, Y., Zheng, X., and Wang, X. 2014. Dental and mandibular morphologies of Arboroharamiya (Haramiyida, Mammalia): A comparison with other haramiyidans and Megaconus and implications for mammalian evolution. PLoS One 9 (12): e113847.
Meng, J., Wang, Y., and Li, C.-K. 2011. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature 472: 181–185.
Meredith, R.W., Gatesy, J., Murphy, W.J., Ryder, O.A., and Springer, M.S. 2009. Molecular decay of the tooth gene enamelin (ENAM) mirrors the loss of enamel in the fossil record of placental mammals. PLoS Genetics 5(9): e1000634.
Miao, D. 1993. Cranial morphology and multituberculate relationships. In: F.S. Szalay, M.J. Novacek, and M.C. McKenna (eds.), Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials, 63–74. Springer, New York.
Mones, A. 1987. Gondwanatheria, un nuevo orden de Mamíferos Sudamericanos (Mammalia: Edentata: ?Xenarthra). Comunicaciones Paleontológicas del Museo de Historia Natural de Montevideo 18: 237–240.
Nanci, A. and Somerman, M. 2003. The periodontium. In: A. Nancy (ed.), Ten Cate’s Oral Histology: Development, Structure, and Function, 245–251. Harcourt Health Sciences, St. Louis.
Nessov, L.A., Zhegallo, V.I., and Averianov, A.O. 1998. A new locality of Late Cretaceous snakes, mammals and other vertebrates in Africa (western Libya). Annales de Paléontologie 84: 265–274.
O’Connor, P.M., Gottfried, M.D., Stevens, N.J., Roberts, E.M., Ngasala, S., Kapilima, S., and Chami, R. 2006. A new vertebrate fauna from the Cretaceous Red Sandstone Group, Rukwa Rift Basin, Southwestern Tanzania. Journal of African Earth Science 44: 277–288.
O’Connor, P.M., Sertich, J.J., Stevens, N.J., Roberts, E.M., Gottfried, M.D., Hieronymus, T.L., Jinnah, Z.A., Ridgely, R., Ngasala, S.E., and Temba, J. 2010. The evolution of mammal-like crocodyliforms in the Cretaceous Period of Gondwana. Nature 466: 748–751.
Pascual, R., Goin, F.J., Krause, D.W., Ritz-Jaureguizar, E., and Carlini, A.A. 1999. The first gnathic remains of Sudamerica: implications for gondwanathere relationships. Journal of Vertebrate Paleontology 19: 373–382.
Patterson, B. 1978. Pholidota and Tubulidentata. In: V.J. Maglio and H.B.S. Cooke (eds.), Evolution of African Mammals, 268–278. Harvard University Press, Cambridge.
Paula Couto, C. de 1967. Pleistocene edentates of the West Indies. American Museum Novitates 2304: 1–55.
Prasad, G.V.R., Verma, O., Sahni, A., Krause, D.W., Khosla, A., and Parmar, V. 2007. A new Late Cretaceous gondwanatherian mammal from central India. Proceedings of the Indian National Science Academy 73: 17–24.
Rage, J.-C., and Cappetta, H. 2002. Vertebrates from the Cenomanian, and the geological age of the Draa Ubari fauna (Libya). Annales de Paléontologie 88: 79–84.
Rich, T.H., Hopson, J.A., Gill, P.G., Trusler, P., Rogers-Davidson, S., Morton, S., Cifelli, R.L., Pickering, D., Kool, L., Siu, K., Burgmann, F.A., Senden, T., Evans, A.R., Wagstaff, B.E., Seegets-Villiers, D., Corfe, I.J., Flannery, T.F., Walker, K., Musser, A.M., Archer, M., Pian, R., and Vickers-Rich, P. 2016. The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri. Alcheringa 40: 475–501.
Renvoisé, E. and Michon, F. 2014. An evo-devo perspective on ever-growing teeth in mammals and dental stem cell maintenance. Frontiers in Physiology 5: 1–12.
Roberts, E.M., O’Connor, P.M., Stevens, N.J., Gottfried, M.D., Jinnah, Z.A., Ngasala, S., Choh, A.M., and Armstrong, R.A. 2010. Sedimentology and depositional environments of the Red Sandstone Group, Rukwa Rift Basin, southwestern Tanzania: New insight into Cretaceous and Paleogene terrestrial ecosystems and tectonics in sub-equatorial Africa. Journal of African Earth Sciences 57: 179–212.
Rose, K.D. 1978. A new Paleocene epoicotheriid (Mammalia), with comments on the Palaeanodonta. Journal of Paleontology 52: 658–674.
Rose, K.D. and Lucas, S.G. 2000. An early Paleocene palaeanodont (Mammalia, ?Pholidota) from New Mexico, and the origin of Palaeanodonta. Journal of Vertebrate Paleontology 20: 139–156.
Rose, K.D., Eberle, J.J., and McKenna, M.C. 2004. Arcticanodon dawsonae, a primitive new palaeanodont from the lower Eocene of Ellesmere Island, Canadian High Arctic. Canadian Journal of Earth Sciences 41: 757–763.
Rose, K.D., Emry, R.J., and Gingerich, P.D. 1992. Skeleton of Alocodontulum atopum, an early Eocene epoicotheriid (Mammalia, Palaeanodonta) from the Bighorn Basin, Wyoming. Contributions from the Museum of Paleontology, University of Michigan 28: 221–245.
Rose, K.D., Emry, R.J., Gaudin, T.J., and Storch, G. 2005. Xenarthra and Pholidota. In: K.D. Rose and J.D. Archibald (eds.), The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades, 106–126. Johns Hopkins University Press, Baltimore.
Rougier, G.W., Chornogubsky, L., Casadio, S., Arango, N.P., and Giallombardo, A. 2009. Mammals from the Allen Formation, Late Cretaceous, Argentina. Cretaceous Research 30: 223–238.
Rougier, G.W., Novacek, M.J., McKenna, M.C., and Wible, J.R. 2001. Gobiconodonts from the Early Cretaceous of Oshih (Ashile), Mongolia. American Museum Novitates 3348: 1–30.
Rowe, T. 1988. Definition, diagnosis, and origin of Mammalia. Journal of Vertebrate Paleontology 8: 241–264.
Rowe, T., Rich, T.H., Vickers-Rich, P., Springer, M., and Woodburne, M.O. 2008. The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proceedings of the National Academy of Sciences 105: 1238–1242.
Scillato-Yané, G.J. and Pascual, R. 1984. Un peculiar Paratheria, Edentata (Mammalia) del Paleoceno de Patagonia. In: Primeras Jornadas Argentinas de Paleontología de Vertebrados Resúmenes, 15.
Secord, R., Ginerich, P.D., and Bloch, J.I. 2002. Mylanodon rosei, a new metacheiromyid (Mammalia: Palaeanodonta) from the late Tiffanian (late Paleocene) of northwestern Wyoming. Contributions from the Museum of Paleontology, The University of Michigan 30: 385–399.
Seiffert, E.R. 2007. A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence. BMC Evolutionary Biology 7: 224.
Shoshani, J., Goldman, C.A., and Thewissen, J.G.M. 1988. Orycteropus afer. Mammalian Species 300: 1–8.
Sigogneau-Russell, D. 1989. Haramiyidae (Mammalia, Allotheria) en provenance du Trias Supérieur de Lorraine (France). Palaeontographica, Abteilung A 206: 137–198.
Sigogneau-Russell, D., Evans, S.E., Levine, J.F., and Russell, D.A. 1998. The Early Cretaceous microvertebrate locality of Anoual, Morocco: a glimpse at the small vertebrate assemblages of Africa. In: S.G. Lucas, J.I. Kirkland, and J.W. Estep (eds.), Lower and Middle Cretaceous Terrestrial Ecosystems. New Mexico Museum of Natural History and Science Bulletin 14: 177–182.
Simpson, G.G. 1928. A Catalogue of the Mesozoic Mammalia in the Geological Department of the British Museum. 216 pp. British Museum (Natural History), London.
Simpson, G.G. 1931. Metacheiromys and the Edentata. Bulletin of the American Museum of Natural History 59: 295–381.
Tummers, M. and Thesleff, I. 2008. Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns. Evolution and Development 10: 187–195.
Tummers, M. and Thesleff, I. 2009. The importance of signal pathway modulation in all aspects of tooth development. Journal of Experimental Zoolology 312B: 187–195.
Ungar, P.S. 2010. Mammalian Teeth: Origin, Evolution, and Diversity. 304 pp. Johns Hopkins University Press, Baltimore.
Widlansky, S.J., Clyde, W.C., O’Connor, P.M., Roberts, E.M., and Stevens, N.J. 2018. Paleomagnetism of the Cretaceous Galula Formation and implications for vertebrate evolution. Journal of African Earth Sciences 139: 403–420.
Wilson, G.P., Sarma, D.C.D., and Anantharaman, S. 2007. Late Cretaceous sudamericid gondwanatherians from India with paleobiogeographic considerations of Gondwanan mammals. Journal of Vertebrate Paleontology 27: 521–531.
Zheng, X., Bi, S., Wang, X., and Meng, J. 2013. A new arboreal haramiyid shows the diversity of crown mammals in the Jurassic Period. Nature 500: 199–202.
Zhou, C.-F., Wu, S., Martin, T., and Luo, Z.-X. 2013. A Jurassic mammaliaform and the earliest mammalian evolutionary adaptations. Nature 500: 163–168.
Acta Palaeontol. Pol. 64 (1): 65–84, 2019
https://doi.org/10.4202/app.00568.2018