

A new kogiid sperm whale from northern Italy supports psychrospheric conditions in the early Pliocene Mediterranean Sea
ALBERTO COLLARETA, FRANCO CIGALA FULGOSI, and GIOVANNI BIANUCCI
Collareta, A., Cigala Fulgosi, F., and Bianucci, G. 2019. A new kogiid sperm whale from northern Italy supports psychrospheric conditions in the early Pliocene Mediterranean Sea. Acta Palaeontologica Polonica 64 (3): 609–626.
Among living cetaceans, dwarf and pygmy sperm whales (Kogia) are the only members of the family Kogiidae, regarded as diminutive and elusive relatives of the great sperm whale Physeter. Kogiids are known as fossils by several skulls, teeth, and ear bones from Neogene deposits of the Northern Hemisphere and Peru. We report on a fossil kogiid specimen collected at Sant’Andrea Bagni (northern Italy) from Zanclean marine mudstone; these deposits also yielded a rich deep-water elasmobranch assemblage depicting the presence of Atlantic-derived psychrospheric waters. The kogiid specimen, consisting of a partial cranium, one detached tooth, one vertebra, and one fragmentary rib, is here referred to Pliokogia apenninica gen. et sp. nov. Pliokogia is mostly characterised by a long and dorsally flattened rostrum and by the presence of two well-distinct fossae on the right side of the supracranial basin, including an elongated peripheral maxillary fossa on the posterior portion of the right maxilla. Our phylogenetic analysis recovers Pliokogia as a member of the subfamily Kogiinae, which includes Kogia, Koristocetus, Nanokogia, and Praekogia. A low temporal fossa and the absence of dental enamel suggest that, like extant Kogia, Pliokogia was a suction feeder. Since living kogiids do not inhabit the Mediterranean waters, and considering that they feed on deep-water prey in open-sea areas, the association of Pliokogia with a psychrospheric elasmobranch assemblage with Atlantic affinities is noteworthy. Indeed, in early Pliocene times, the Gibraltar connection was controlled by estuarine dynamics, thus allowing the entrance of deep-water organisms (including the putative prey of Pliokogia) in the Mediterranean Basin. The subsequent abandonment of the Mediterranean Sea by kogiids might therefore be related to the definitive establishment of the present-day antiestuarine circulation at Gibraltar, which likely led to a limited deep nutrient supply and resulted in the strong depletion of most Mediterranean deep-water ecosystems.
Key words: Mammalia, Cetacea, Odontoceti, Physeteroidea, Kogiinae, oceanisation, Pliocene, Northern Apennines.
Alberto Collareta [alberto.collareta@unipi.it] and Giovanni Bianucci [giovanni.bianucci@unipi.it], Dipartimento di Scienze della Terra, Università di Pisa, via Santa Maria 53, 56126 Pisa, Italy.
Franco Cigala Fulgosi [cigalaff@libero.it], Strada Martinella 292, 43124 Parma, Italy.
Received 2 December 2018, accepted 2 April 2019, available online 23 July 2019.
Copyright © 2019 A. Collareta et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Among modern toothed whales (Cetacea: Odontoceti), pygmy and dwarf sperm whales, Kogia breviceps (de Blainville, 1838) and Kogia sima (Owen, 1866), respectively, are the only living members of the physeteroid family Kogiidae, known as diminutive relatives of the great sperm whale (Physeteridae: Physeter macrocephalus Linnaeus, 1758), with whom they share the presence of a spermaceti organ in the supracranial region of the head (e.g., McAlpine 2002). These small-sized marine mammals are characterised by a square head, small lower jaws, and a short rostrum—the shortest snout among extant cetaceans (Werth 2006). Despite being globally distributed, kogiids are still among the less known families of marine mammals, and most of our understanding of their anatomy and life history comes from isolated and often serendipitous observations on beached individuals.
The fossil record of Kogiidae is currently represented by some skulls, teeth, and more abundant isolated ear bones from Neogene deposits of the Northern Hemisphere (e.g., Barnes 1973, 1998; Pilleri 1987; Whitmore 1994; Bianucci 1996, 1997; Cigala Fulgosi 1996; Luo and Marsh 1996; Bianucci et al. 1998, 2011; Bianucci and Landini 1999; Lambert 2008; Whitmore and Kaltenbach 2008; Vélez-Juarbe et al. 2015, 2016; Lambert et al. 2017b), with the remarkable exception of the late Miocene record from the Pisco Formation of southern Peru, which includes several skulls of Scaphokogia (a highly autapomorphic genus characterised by a pachyostotic, semicylindrical, downwards-deflected rostrum and a spoon-shaped dorsal surface of the neurocranium) besides a few specimens displaying less derived cranial morphologies (Muizon 1988; Bianucci et al. 2016; Collareta et al. 2017b; Di Celma et al. 2017). Although Kogia does not inhabit the present-day Mediterranean Sea (e.g., McAlpine 2017), kogiids are known from the Mediterranean region for a few late Neogene fossil specimens, namely: (i) an isolated left periotic from the Tortonian of Melleha (Malta), identified as belonging to Kogiidae indet. (Bianucci et al. 2011); (ii) some teeth, periotics, and tympanic bullae from the Pliocene of Orciano Pisano (Tuscany, central Italy) (Pilleri 1987; Bianucci 1996); and (iii) the holotype and only known specimen of Kogia pusilla (Pilleri 1987), consisting of an incomplete skull from the upper Pliocene of Monte Voltraio (Tuscany, central Italy) (Bianucci 1997; Bianucci et al. 1998; Bianucci and Landini 1999). Moreover, a partial kogiid skeleton was collected by one of us (FCF) from lower Pliocene (i.e., Zanclean) mudstone exposed at Sant’Andrea Bagni (Emilia-Romagna, Italy) and cursorily mentioned in a work on the remarkable deep-water elasmobranch assemblage from the same site (Cigala Fulgosi 1996). This cetacean fossil is here fully described, figured, and referred to a new genus and species of Kogiinae, the kogiid subfamily that includes the present-day Kogia. We then undertake a parsimony analysis of the phylogenetic relationships of this new taxon and briefly discuss its palaeobiological significance.
Institutional abbreviations.—IRSNB, Institut Royal des Sciences Naturelles de Belgique, Bruxelles, Belgium; MAS, Museo Arqueologico de Salango, Salango, Ecuador; MGPUF, Museo di Storia Naturale, Sezione di Geologia e Paleontologia, Università degli Studi di Firenze, Firenze, Italy; MNHN, Muséum National d’Histoire Naturelle, Paris, France; MSNC, Museo Civico di Storia Naturale di Comiso, Comiso, Italy; MSNUP, Museo di Storia Naturale dell’Università di Pisa, Calci, Italy; MUSM, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Lima, Peru; MUSNAF, Museo di Storia Naturale dell’Accademia dei Fisiocritici, Siena, Italy; USNM, National Museum of Natural History, Smithsonian Institution, Washington DC, USA.
Other abbreviations.—CNPL, Calcareous Nannofossil Plio-Pleistocene zonation of Backman et al. (2012); MNN, Mediterranean Neogene Nannoplankton zonation of Rio et al. (1990); MPL, Mediterranean Pliocene foraminiferal zonation of Cita (1973, 1975) and Sprovieri (1992).
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:D41EA0A5-E65D-48CB-AB8F-0EE1725247EC
Material and methods
Kogiid specimens analysed for comparison and anatomical terminology.—In addition to MSNUP I-17603, we have directly examined for comparison the following fossil and extant Kogiidae: Aprixokogia kelloggi Whitmore and Kaltenbach, 2008 (USNM 187015); Kogia breviceps (Blainville, 1838) (MAS 4000; MNHN 1976-37; USNM 283625); Kogia pusilla (Pilleri, 1987) (MGPUF 1540V); Kogia sima (Owen, 1866) (MSNC 3450; MUSNAF Mam410); Koristocetus pescei Collareta, Lambert, Muizon, Urbina, and Bianucci, 2017 (MUSM 888); Scaphokogia cochlearis Muizon, 1988 (MNHN PPI 229, MUSM 971, MUSM 1998); Scaphokogia sp. (MUSM 972); Scaphokogiinae sp. (MUSM 3291; MUSM 3405); Thalassocetus antwerpiensis Abel, 1905 (IRSNB M.525). The anatomical terminology for the skull follows Mead and Fordyce (2009), except when explicitly stated otherwise.
Phylogenetic analysis.—The phylogenetic analysis was undertaken with PAUP* (Swofford 2001) using a modified version of the character/taxon matrix of Collareta et al. (2017b) (for the list of characters and the character/taxon matrix see the SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app64-Collareta_etal_SOM.pdf). We used the tree-bisection–reconnection algorithm and the heuristic search option, considering all characters as unordered and unweighted. We used the definitions proposed by Bianucci and Landini (2006) and then reaffirmed by Lambert et al. (2017a) for Physeteroidea, Physeteridae, and Kogiidae. Following Collareta et al. (2017b), we define Kogiinae Gill, 1871 as the most inclusive clade including Kogia but not Scaphokogia, whereas we define Scaphokogiinae Muizon, 1988 as the most inclusive clade including Scaphokogia but not Kogia.
Geological and stratigraphic setting
The cetacean fossil described in the present work was collected from a small badland area at the margins of the village of Sant’Andrea Bagni (Parma Province, Northern Apennines, northern Italy), a site where lower Pliocene (i.e., Zanclean) marine mudstone (“Argille Azzurre” sensu lato) are exposed along a short section, about 25 m thick (Fig. 1A). The stratigraphy of this exposure, consisting of monotonous silty argillaceous pelites that exhibit indistinct bedding, has been described in detail by Cigala Fulgosi (1986, 1996) and Channel et al. (1994). The kogiid specimen here described was collected a few metres above the base of the section measured by Channel et al. (1994) and figured by Cigala Fulgosi (1996: fig. 2). These sediments have also yielded a rich deep-water elasmobranch assemblage (including teeth and scales attributed to rare squalean sharks such as Centrophorus squamosus [Bonnaterre, 1788], Chlamydoselachus sp., Deania sp., Pristiophorus sp., Scymnodalatias aff. garricki Kukuev and Konovalenko, 1988, Scymnodon ringens Barbosa du Bocage and Brito Capello, 1864, and Zameus squamulosus [Günther, 1877]) (Cigala Fulgosi 1986, 1996). On the whole, the chondrichthyan assemblage from Sant’Andrea Bagni depicts the presence of psychrospheric (i.e., belonging to colder, deeper part of the oceans, known as the psychrosphere; Bruun 1957) water masses of Atlantic origin in the Adriatic palaeo-area during an early Pliocene phase of remarkable “oceanisation” of the Mediterranean Basin (Cigala Fulgosi 1996), as already hypothesised on the basis of the fossil record of ostracod crustaceans (Benson 1972a, b, 1975) and scleractinian corals (Russo 1980). Teeth of bony fish, spines of cidaroid urchins, rare very small pyritised molluscs, and scattered remains of solitary corals were also collected at the study site (Cigala Fulgosi 1986, 1996); the latter include small-sized, cup-shaped, relatively flat corallites that might belong to the extant species Caryophyllia aradasiana Seguenza, 1864 (= Caryophyllia calveri Duncan, 1873; Vertino and Di Geronimo 2003).
Integration of foraminiferal biostratigraphy, calcareous nannoplankton biostratigraphy, and magnetostratigraphy allows a precise and robust bracketing of the time of deposition of the fossiliferous horizons cropping out at Sant’Andrea Bagni (Cigala Fulgosi 1986, 1996; Channel et al. 1994; Fig. 1B). In particular, the foraminiferal assemblage was attributed to the MPL 2 biozone, which is currently referred to the 5.08–4.52 Ma time span (Violanti 2012), whereas the calcareous nannoplankton assemblage was attributed to the MNN 12 biozone, corresponding to the more recently instituted CNPL 4 biozone, whose bounding bioevents have been calibrated at 5.53 Ma and 5.04 Ma, respectively (Backman et al. 2012). Confirming these biostratigraphical results, Channell et al. (1994) assigned the lower part of the section exposed at Sant’Andrea Bagni to the Thvera Subchron of the Gilbert Chron; the Thvera Subchron is currently referred to the 5.235–4.997 Ma time span (Ogg 2012). All together, these results indicate that the geological age of the cetacean specimen described herein and the co-occurring fish assemblage can be constrained between 5.08 and 5.04 Ma—a surprisingly precise age estimate.
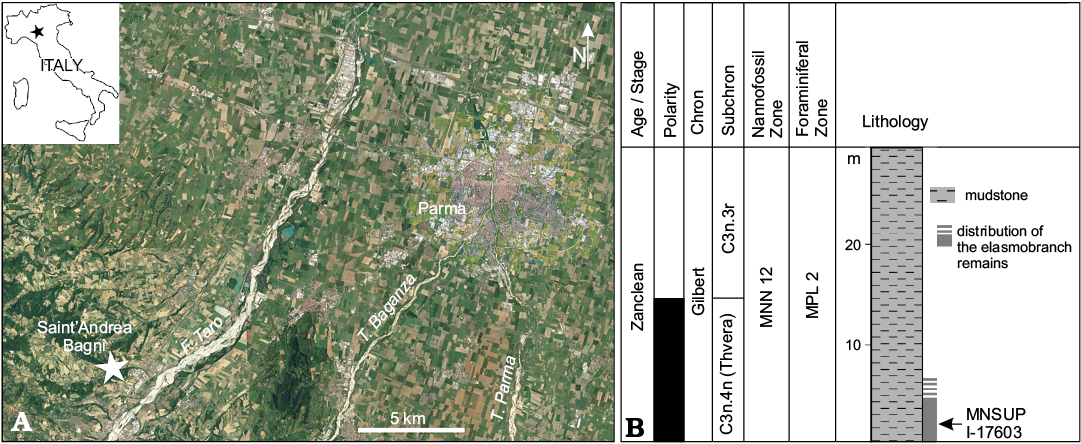
Fig. 1. A. Geographical position (star) of the lower Pliocene site of Sant’Andrea Bagni, Northern Apennines, northern Italy, where the holotype of Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603) was discovered (image from Google Earth). B. Schematic stratigraphic section of the Pliocene succession exposed at Sant’Andrea Bagni (redrawn and modified after Channel et al. 1994).
Systematic palaeontology
Cetacea Brisson, 1762
Pelagiceti Uhen, 2008
Neoceti Fordyce and Muizon, 2001
Odontoceti Flower, 1867
Physeteroidea Gray, 1821
Family Kogiidae Gill, 1871
Remarks.—Within the family Kogiidae, Muizon (1988) established the monotypic subfamily Scaphokogiinae for inclusion of the then new highly autoapomorphic genus Scaphokogia. In doing so, Muizon (1988) collected the genera Kogia and Praekogia under the subfamily name Kogiinae (established by Gill 1871 according to the “Principle of Coordination” of the International Code of Zoological Nomenclature; ICZN 1999: article 36.1). For purposes of completeness, the kogiid subfamily Kogiinae, to which the new taxon herein described belongs, is here provided with a diagnosis on the basis of the results of our cladistic analysis (see Phylogeny below).
Subfamily Kogiinae Gill, 1871
Diagnosis.—Kogiids in which the posterior end of the right antorbital notch opens onto the supracranial basin.
Genus Pliokogia nov.
ZooBank LSID: urn:lsid:zoobank.org:act:1DEA564E-280B-4E35-A 193-655DCE48CE61
Type and only known species: Pliokogia apenninica sp. nov.
Etymology: From the combination of Pliocene, in reference to the geological age of the holotype, and Kogia, generic name of the extant members of Kogiidae. Gender feminine.
Diagnosis.—Same as for the type species until other species are described.
Stratigraphic and geographic range.—Early Pliocene (5.08–5.04 Ma, early Zanclean; see above for more details) of northern Italy (palaeo-Adriatic area).
Pliokogia apenninica sp. nov.
Figs. 2–8.
1996 “a fragmented skull of kogiinae (Cetacea: Odontoceti)”; Cigala Fulgosi 1996: 303.
ZooBank LSID: urn:lsid:zoobank.org:act:27C92259-D30E-4F02-BB6F- CB13067302EA
Etymology: From Apennines, the mountain range that includes the type horizon.
Holotype: MSNUP I-17603, a partial cranium, one detached tooth, one vertebra, and one fragment of rib.
Type locality: Sant’Andrea Bagni, about 20 km WSW of the town of Parma, Parma Province, Italy. Indicative geographic coordinates: 44°43’ N, 10°05’ E. Precise locality data are available on request from the authors.
Type horizon: Zanclean marine mudstone (“Argille Azzurre” sensu lato): characterised by the presence of fossilised teeth and denticles of deep-water squalean sharks such as Centrophorus squamosus, Chlamydoselachus sp., Deania sp., Pristiophorus sp., Scymnodalatias aff. garricki, Scymnodon ringens, and Zameus squamulosus (Cigala Fulgosi 1986, 1996). The geological age of the type horizon has been constrained between 5.08 and 5.04 Ma by means of foraminiferal biostratigraphy, calcareous nannoplankton biostratigraphy, and magnetostratigraphy (Channel et al. 1994; Cigala Fulgosi 1996).
Diagnosis.—Pliokogia apenninica is a small-sized physeteroid, similar in skull length to Kogia breviceps. It is recognised as a member of Kogiidae based on the following features: estimated bizygomatic width much smaller than 400 mm, presence of a sagittal facial crest, and external nares greatly asymmetric (Lambert et al. 2017a). It is recognised as a member of Kogiinae by the right antorbital notch whose posterior end opens onto the supracranial basin (this work).
The cranium of Pliokogia apenninica differs from other kogiids by the following presumed autapomorphies: (i) presence of a proportionally long rostrum, accounting for more than three fifths of the reconstructed total length of the cranium, whose flat dorsal surface is not invaded by the supracranial basin; (ii) presence of two well-distinct fossae on the right side of the supracranial basin, including an elongated peripheral maxillary fossa on the caudal portion of the right maxilla, posterior and posteromedial to the corresponding antorbital notch; and (iii) presence of a sagittal vomerine sulcus, reflected in a V-shaped transverse section of the dorsal surface of the vomer along the mesorostral groove.
Pliokogia apenninica further differs from Aprixokogia, Koristocetus, Nanokogia, and Scaphokogia by the thicker and blunter lateral maxillary crest. It further differs from Aprixokogia and Koristocetus by the presence of a subdivided right posterior dorsal infraorbital foramen and by the lower temporal fossa. It further differs from Aprixokogia, Scaphokogia, and Thalassocetus by the right antorbital notch opening onto the supracranial basin. It further differs from Aprixokogia and Kogia by the smaller angle between the frontal–maxilla suture line and the coronal plane, along the supraorbital process, with skull in lateral view. It further differs from Aprixokogia and Thalassocetus by the slit-like geometry of the antorbital notches. It further differs from Aprixokogia by the smaller skull length. It further differs from Kogia, Nanokogia, and Praekogia by presenting no lateral expansion of the postnarial eminence of the right premaxilla. It further differs from Kogia by the proportionally smaller lacrimojugal complex. It further differs from Kogia breviceps, Kogia sima, and Koristocetus by the absence of any constriction of the right premaxilla at the level of the external bony nares. It further differs from Kogia breviceps and Kogia sima by the supracranial basin not being laterally expanded, by the thinner and not inflated lateral maxillary crest, and by the higher temporal fossa. It further differs from Kogia pusilla, Kogia sima, and Koristocetus by the greater skull length. It further differs from Kogia sima and Scaphokogia by the lack of a left premaxillary foramen. It further differs from Kogia sima by the presphenoid which does not extend anteriorly within the mesorostral groove. It further differs from Koristocetus and Nanokogia by the less obliquely oriented frontal groove. It further differs from Nanokogia and Scaphokogia by having a wider mesorostral groove in the posterior portion of the rostrum. It further differs from Praekogia by having the left premaxilla that does not reach the sagittal facial crest. It further differs from Scaphokogia by the rostrum having a dorsal surface that is not semicylindrical, the postnarial eminence that significantly contributes to the sagittal facial crest, the sagittal facial crest that is not significantly dislocated towards the left side of the skull, and the supracranial basin that is not spoon-shaped.
Description.—Cranium: The skull is incomplete, lacking the basicranium, the left part of the supracranial basin, the left lateral portion of the rostrum, all the ear bones, the mandibles, and all the teeth but one (Figs. 2–7). The dorsal aspect of the cranium is generally well preserved, although some surfaces are moderately abraded and the left part of the supracranial basin is lost (Fig. 2). In ventral and lateral views, the cranium is poorly preserved, which makes the relationships between the different bones locally unclear (Figs. 3, 4). Various fractures, sometimes filled by gypsum, are observed throughout the cranium.
The maximum preserved length of the cranium is 380 mm, which closely approximates the condylobasal length, here estimated at about 400 mm. Such a reconstructed skull length is similar to that of Kogia breviceps, smaller than that of Aprixokogia, and greater than those of Kogia pusilla, Kogia sima, Koristocetus, and possibly Nanokogia.
The neurocranium is dorsally concave, forming a wide supracranial basin (a key feature of Physeteroidea); it is also strongly asymmetric, exhibiting strongly uneven bony nares (i.e., the left naris is much wider than the right one) that are significantly displaced leftwards, as well as an obliquely directed sagittal facial crest (a diagnostic character of Kogiidae) (Fig. 2). With respect to extant Kogia, the skull of Pliokogia appears as strongly elongated anteroposteriorly (Figs. 2–5), due to the presence of a 250 mm long rostrum (measured between the level of the antorbital notches and the anteriormost tip of the skull). The ratio between the length of the splanchnocranium (i.e., the rostrum) and the condylobasal length is thus estimated at around 0.63 in Pliokogia, that is, indistinguishable from that estimated for K. pusilla (ca. 0.63, but the sole known skull is strongly distorted by diagenesis), vs. 0.51 in Nanokogia and about 0.45–0.50 in living kogiids and Koristocetus. As the rostrum of K. pusilla is markedly concave dorsally (Bianucci and Landini, 1999), the long and dorsally flat splanchnocranium of Pliokogia is unique among the living and extinct kogiids for which this feature is known. Sutures between adjacent cranial bones are fused in MSNUP I-17603, thus indicating that it belonged to a physically adult individual (e.g., Chen et al. 2011).
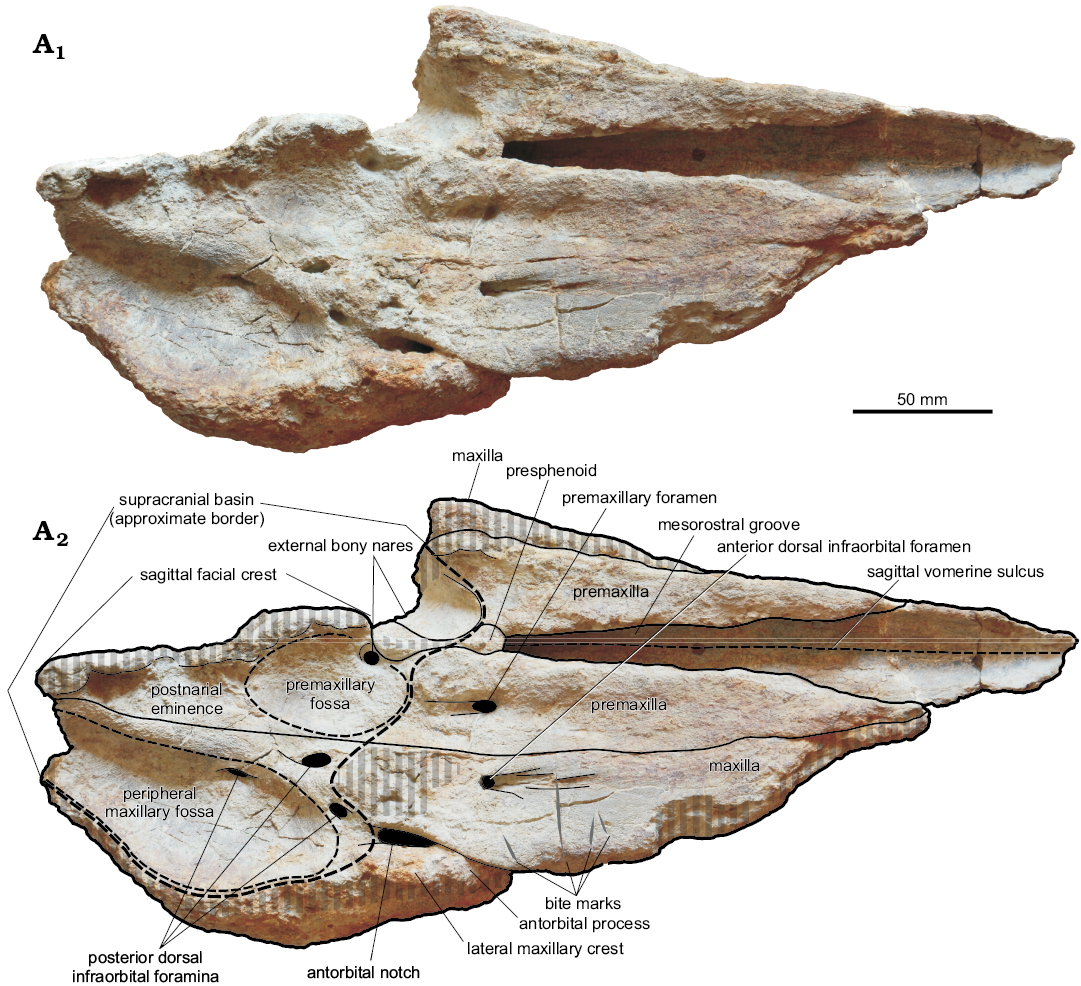
Fig. 2. Cranium, in dorsal view, of the kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy). Photograph (A1), explanatory drawing (A2). The black areas correspond to openings in the skull. The grey vertical stripes denote abraded or broken areas. Dashed lines are for the sagittal vomerine sulcus and the approximate border of the supracranial basin, premaxillary fossa, and peripheral maxillary fossa.
Bioerosional modifications can be locally observed on MSNUP I-17603. On the dorsal surface of the right maxilla, anterior to the antorbital notch, four prominent elongated incisions are present (Figs. 2, 6). These traces consist of substraight gouges with no serrations, disposed roughly orthogonal to the lateral margin of the bone, ranging in length between 11 mm and 32 mm. Their morphology cannot easily match any bioerosional feature originating from the action of marine invertebrates, being in turn perfectly consistent with the bone modifications due to biting by sharks provided with large, labiolingually flattened, smooth-edged tooth crowns (e.g., Bianucci et al. 2010, 2018; Govender 2015). In the light of the morphological-genetic classification scheme proposed by Cigala Fulgosi (1990) and then emended by Bianucci et al. (2010) and Collareta et al. (2017a), these traces can be interpreted as due to type I (i.e., production of a subrectilinear or weakly curved mark by impact of the tooth edge from above downward) or type II (i.e., production of a more or less elongated incision by dragging of the tooth edge in parallel with the dental axis) biting actions. In addition, an indeterminate circular bioerosion scar is present on the palatal surface of the left maxilla (Fig. 3).
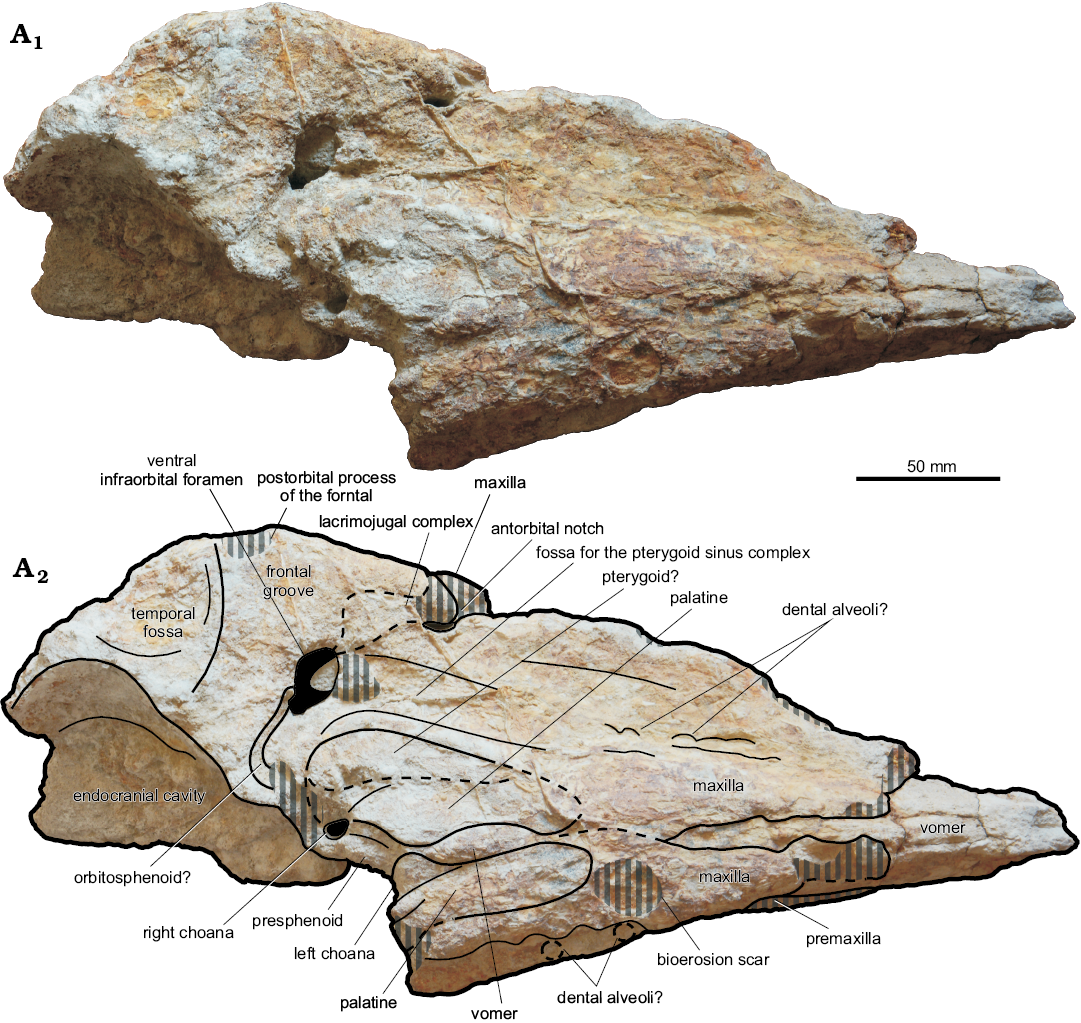
Fig. 3. Cranium, in ventral view, of the kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy). Photograph (A1), explanatory drawing (A2). The black-shaded areas correspond to openings in the skull. The grey vertical stripes denote abraded or broken areas. Dashed lines for approximating the position of several sutures and borders.
Premaxilla: Both premaxillae are lost in the anteriormost quarter of the rostrum; moreover, the left premaxilla is not preserved posterior to mid-length of the left bony naris. In dorsal view, the lateral margins of the premaxillae are substraight along the dorsal surface of the rostrum and weakly converge towards the anteriormost tip of the skull (Fig. 2). At the level of the bony nares, the premaxillae are subequal in transverse width; anterior to this level, the right premaxilla is slightly but distinctly wider than the left premaxilla throughout their preserved length along the rostrum. Medial to the premaxillae, and partially overhung on by them, the mesorostral groove opens approximately 40 mm anterior to the base of the rostrum. The mesorostral groove widens progressively towards the apex of the rostrum as the medial borders of the premaxillae weakly diverge forwards. The posterior half of the mesorostral groove is more widely open dorsally than in Nanokogia and Scaphokogia (a condition reminiscent of that observed in Aprixokogia, Kogia breviceps, and Koristocetus); however, moderate erosion of the medial margins of the premaxillae may have accentuated this character. In the posterior third of the rostrum, just anterior to the posterior end of the mesorostral groove, the dorsal surfaces of both premaxillae are slightly concave transversely (Fig. 5); they progressively flatten in the central third of the rostrum. At about two thirds of the length of the rostrum (measured from its base), the dorsal surface of the left premaxilla is gently convex, whereas the dorsal surface of the right premaxilla is flat; MSNUP I-17603 thus differs from Aprixokogia and Kogia spp., in which the dorsal surface of the rostrum is distinctly concave throughout. The lateral face of the left premaxilla (i.e., the premaxilla-maxilla suture) can be observed for most of the length of the rostrum thanks to the loss of the adjoining portion of the left maxilla; it is flat and subvertical throughout. As in Aprixokogia, Kogia breviceps, K. pusilla, Koristocetus, and Nanokogia, there are no foramina on the left premaxilla (Fig. 2). Conversely, a rather large (12 mm long and 8 mm wide) premaxillary foramen is present on the right premaxilla, at the level of the posterior end of the mesorostral groove (Figs. 2, 6); this foramen is followed posteriorly by a 15 mm long groove. Medial and posteromedial to the right premaxillary foramen, the dorsal surface of the right premaxilla is high and bulging. The external bony nares are situated at the level of the antorbital notches. As observed in all physeteroids, they are strongly asymmetrical and distinctly displaced towards the left side of the skull. Only the anterior and medial walls of the large (i.e., more than 20 mm wide) left naris are preserved; they suggest an oval-shaped opening, situated in an anteroposteriorly oriented, funnel-shaped depression (a feature reminiscent of Kogia spp. and Koristocetus). The right naris is significantly smaller than the left and roughly circular, having a diameter of 9 mm; its anterior, lateral, and posterior walls are formed by the right premaxilla. Differing from Kogia breviceps, K. sima, and Koristocetus, no constriction of the right premaxilla is observable at the level of the external bony nares. Posterior to this level, as observed in all Kogiidae described to date, the right premaxilla is expanded posteromedially as a strip of bone (i.e., the postnarial eminence sensu Whitmore and Kaltenbach 2008) that forms most of the right dorsolateral face of the sagittal facial crest (Figs. 2, 3, 5); in this respect, MSNUP I-17603 markedly differs from Scaphokogia, in which the right premaxilla constitutes only a small fraction of the short and strongly leftwards displaced sagittal facial crest. The lateral margin of the postnarial eminence is almost straight and posterodorsomedially oriented, thus differing from the condition observed in Aprixokogia, Kogia breviceps, K. sima, Nanokogia, and Praekogia. Differing from extant Kogia, the postnarial eminence of MSNUP I-17603 does not overhang laterally over the right side of the supracranial basin. The anterior portion of the postnarial eminence, lateral and posterolateral to the right naris, is shaped as a rather deep fossa (i.e., the premaxillary fossa sensu Barnes 1973), whose floor represents the lowest point of the exposure of the right premaxilla within the supracranial basin (see description of the maxilla below) and, more generally, on the whole dorsal surface of the skull. The position and extent of the premaxillary fossa recall a similar depression observed in Praekogia; in the latter taxon, however, this depression extends onto the adjoining lateral margin of the right maxilla, whereas in MSNUP I-17603 it is defined laterally by a steep wall just medial to the premaxilla-maxilla suture. The premaxillary fossa is oval-shaped, rather large (ca. 60 mm longitudinally long and 30 mm transversely wide), and parallels the lateral margin of the postnarial eminence. In our opinion, this depression is homologous to the fossa observed on the right dorsolateral face of the sagittal facial crest of Kogia spp., Koristocetus, and Thalassocetus (but see Barnes 1973 and Whitmore and Kaltenbach 2008 for different interpretations of the premaxillary fossa of Praekogia). If this homology is correct, the premaxillary fossa of MSNUP I-17603, being situated close to the sagittal plane of the skull, likely hosted the spermaceti chamber accommodating the fibrous case that surrounds the spermaceti organ of extant Kogiidae (Thornton et al. 2015). The sagittal facial crest is only partially preserved, lacking its dorsal (i.e., subhorizontal) edge; in turn, the lower portion of the anterior (i.e., subvertical) edge of the sagittal facial crest is preserved, being observed at the posterior termination of the dorsal exposure of the presphenoid, medial to the bony nares. The preserved portion of the sagittal facial crest, facing rightwards, is flat and slopes anterodorsally. The posterior termination of the sagittal facial crest reaches (or almost reaches) the nuchal crest, thus differing from the condition observed in Aprixokogia. The premaxillae are not exposed on the ventral surface of the cranium (Fig. 3); however, they are not preserved at (and close to) the anteriormost tip of the skull. The premaxilla is observed, in ventral view, on skulls of the extant kogiids Kogia breviceps and K. sima.
Maxilla: Both maxillae are incompletely preserved. The right maxilla is not preserved in the anteriormost fourth of the rostrum, and the ventral tip of the antorbital process is also lost (Figs. 3, 4). The left maxilla is mostly lost, except for part of its palatal surface. In dorsal view, the dorsal exposure of the maxilla tapers transversely towards the anterior termination of the skull (Fig. 2). Through the rostrum, the lateral margin of the right maxilla is anteromedially directed; it is not substraight as seen in Kogia breviceps and K. sima, but rather exhibits a marked constriction at about three fifths of the rostrum length, thus recalling the condition observed in Koristocetus and, especially, Nanokogia. The right maxilla is slightly but constantly wider than the adjoining premaxilla along the rostrum; however, the former bone is not preserved at (and close to) the anterior end of the rostrum, a region where the right maxilla is wider than the premaxilla in some extinct kogiids (e.g., Nanokogia). Medial and posteromedial to the right antorbital notch, the dorsal surface of the right maxilla is high, bulging, and rugose; then it flattens progressively forwards. The antorbital notch is deep, narrow, teardrop-shaped, and slit-like in its anterior portion, thus recalling Kogia spp. in this respect. The posterior end of the right antorbital notch opens onto the supracranial basin, a condition reminiscent of that observed in Kogia spp., Koristocetus, Nanokogia, and Praekogia. Lateral to the antorbital notch, the right lateral maxillary crest reaches its maximum height and width (Figs. 4, 5); it is high and transversely thick, not as inflated as in Kogia breviceps and K. sima, but in contrast to the thin lateral maxillary crest of Aprixokogia, Koristocetus, Nanokogia, and Scaphokogia. The right lateral maxillary crest does not overhang either the supracranial basin or the orbital region; it points towards the apex of the rostrum and forms part of the right lateral margin of the supracranial basin. Due to the loss of the left maxilla and premaxilla posterior to the left bony naris, only the right portion of the supracranial basin (i.e., right to the sagittal facial crest), is preserved in MSNUP I-17603. As in all Kogiidae except for Scaphokogia, the supracranial basin generally faces anterodorsally (Fig. 5). Similar to Koristocetus, Nanokogia, and Scaphokogia, the supracranial basin does not extend into the region above the rostrum (Fig. 2); conversely, in Aprixokogia and Kogia spp. the anterior part of the supracranial basin is open onto the dorsal surface of the rostrum. Similar to Nanokogia, the right posterolateral edge of the supracranial basin slightly overhangs the underlying temporal region (see description of the frontal bone below). The preserved lateral and posterolateral margins of the ascending process of the right maxilla suggest a roughly oval outline for the supracranial basin, thus recalling the condition observed in Nanokogia and Praekogia rather than the more circular and posterolaterally expanded supracranial basin of extant Kogia. Posterior and posteromedial to the right antorbital notch, and posterolateral to the premaxillary fossa, a major depression is observed on the facial surface of the right maxilla, in the right posterolateral region of the supracranial basin (Figs. 2, 5). This depression, for which the name “peripheral maxillary fossa” is here proposed, is limited anteriorly by the bulging region of the maxilla medial to the right antorbital notch, medially by a broad and substraight ridge running just lateral to the right border of the postnarial eminence, and laterally by the right lateral maxillary crest. The peripheral maxillary fossa is almost completely preserved, its posteromedial termination (sited where the sagittal facial crest and the nuchal crest likely joined to each other) being lost; it is lobe-shaped, larger than the premaxillary fossa (i.e., ca. 110 mm long and 55 mm wide), and deeply excavated. The floor of this depression, which displays a fibrous aspect, includes the lowest point of the exposure of the right maxilla within the supracranial basin. The peripheral maxillary fossa of MSNUP I-17603 is strongly reminiscent of a similar kidney-shaped depression located in the right posterolateral region of the supracranial basin of Aprixokogia. The latter was interpreted by Withmore and Kaltenbach (2008) as homologous to the larger right maxillary fossa of modern kogiids, which occupies most of the facial aspect of the right maxilla and expands onto the caudal termination of the right antorbital notch (thus differing from the condition observed in Pliokogia). In extant Kogia, this fossa hosts the vocal chamber where the phonic lips and vocal caps (two soft-tissue structures, part of the echolocating system of Kogia breviceps and K. sima) are situated (Thornton et al. 2015). If this homology and the above interpretation of the premaxillary fossa of MSNUP I-17603 are correct, then the topology of the echolocating system of this fossil kogiid would have been likely similar to that of the extant species K. breviceps and K. sima (see e.g., Thornton et al. 2015: figs. 2–5). Several dorsal infraorbital foramina are observed on the right maxilla (Figs. 2, 6). The right anterior dorsal infraorbital foramen is located 40 mm anterior to the posteriormost end of the right antorbital notch, near the maxilla-premaxilla suture; it is anteroposteriorly elongated, 5 mm wide, and followed anteriorly by a deep, 45 mm long groove extension. As observed in Kogia breviceps, K. sima, Nanokogia, Praekogia, Scaphokogia, and Thalassocetus, the right posterior dorsal infraorbital foramen is subdivided, and at least three large openings are observed on the supracranial basin posteromedial to the right antorbital notch. The anteriormost opening is located 10 mm posteromedial to the right antorbital notch; it is elliptical, anterolaterally oriented, 8 mm long and 4 mm wide, and bears no groove extension. Medial and slightly posterior to this foramen, another elliptical foramen is observed 5 mm lateral to the maxilla-premaxilla suture, close to the lateralmost margin of the premaxillary fossa. This foramen is anteroposteriorly elongated, 11 mm long and 7 mm wide, and is followed posteromedially by a short and wide groove; it is connected by a wide branch of the infraorbital canal with the underlying right ventral infraorbital foramen (see below). The posteriormost opening observed on the dorsal surface of the right maxilla is located 60 mm posterolateral to the right bony naris. It opens into the left portion of the peripheral maxillary fossa, 5 mm lateral to its medial border. This foramen is anteroposteriorly elongated, 15 mm long and 5 mm wide, and is not followed by a groove. Medial to the right antorbital notch, where the poorly preserved dorsal surface of the maxilla is rough and bulging, the strong abrasion of the cortical portion of the bone prevents the unambiguous detection of foramina, but three or four small (i.e., smaller than 5 mm in diameter) additional openings might be present in this area. In lateral view (Figs. 4, 5), the right lateral maxillary crest exhibits a high and subvertical face that comprises the supraorbital and antorbital processes of the maxilla (the anteroventral tip of the latter is missing). Medial to the anterior portion of the lateral maxillary crest, the lateral surface of the rostral portion of the right maxilla is high and adpressed to the right antorbital process. Anterior to this level, the lateral margin of the maxilla becomes slightly thinner dorsoventrally and regularly rounded. In ventral view (Fig. 3), the palatal surface of the maxilla is poorly preserved. Throughout the rostrum, the ventral surface of the maxilla is significantly convex transversely in its medial part, whereas it becomes flat to weakly concave close to the margins of the rostrum. A few badly defined circular depressions, having a mean diameter of ca. 8 mm, are locally observed on both maxillae, where the bone surface is better preserved. These depressions are reminiscent of shallow dental alveoli such as those observed on the bony palate of Koristocetus; in turn, deeper and large upper dental alveoli are present in Aprixokogia, whereas a relict alveolar groove is observed in Kogia spp., Nanokogia, and Scaphokogia. Along the right maxilla, these putative alveoli are situated roughly at mid distance between the lateral edge of the rostrum and the sagittal plane of the skull, where the flat and convex portions of the palatal surface of the maxillae meet each other. Among the individuated depressions, the posteriormost ones are observed on the left maxilla, in the posterior third of the rostrum. It is unclear, however, whether these putative alveoli were well distinct from each other all along the rostrum. Moreover, although the preservation state of the palatal surface of the rostrum might partially obliterate their original depth, it seems unlikely that, in the living animal, the maxillary dental alveoli would have been deep enough to bear functional teeth, especially when considering the slender and elongated morphology that characterises the sole preserved tooth of Pliokogia (see below). The right and left maxillae are separated medially by the ventral exposure of the vomer, except for the mid part of the palate, where the maxillae contact each other medially for about 50 mm, thus recalling the condition observed in Nanokogia. Due to the poor preservation state of the ventral surface of the skull, the presence of palatine foramina and sulci could not be ascertained. In ventral view, the right antorbital notch penetrates less posteriorly than observed in dorsal view, i.e., the notch itself is shorter posteriorly than it appears on the dorsal view. Posterior to the base of the rostrum, the maxilla projects lateral to the palatine and medial to the lacrimojugal complex and frontal to form the anterior and anteromedial walls of the ventral infraorbital foramen. The latter is elliptical, anterolaterally directed, and very large (i.e., 29 mm long and 15 mm wide), although its size could have been exaggerated by peripheral erosion of its walls. Anterior to the ventral infraorbital foramen and medial to the antorbital notch, a rather deep depression is observed on the posterior portion of the right maxilla. This fossa is anteroposteriorly stretched, ca. 55 mm long, and parallels the adjoining palatine, thus recalling the condition observed in Scaphokogia. Similar depressions, located anterior or anterolateral to the ventral infraorbital foramen, have also been observed in Kogia breviceps, K. sima, Koristocetus, and Nanokogia. Following the interpretation proposed by Vélez-Juarbe et al. (2015) and Collareta et al. (2017a), we regard this fossa as related to the anteriormost portion of the pterygoid sinus complex.
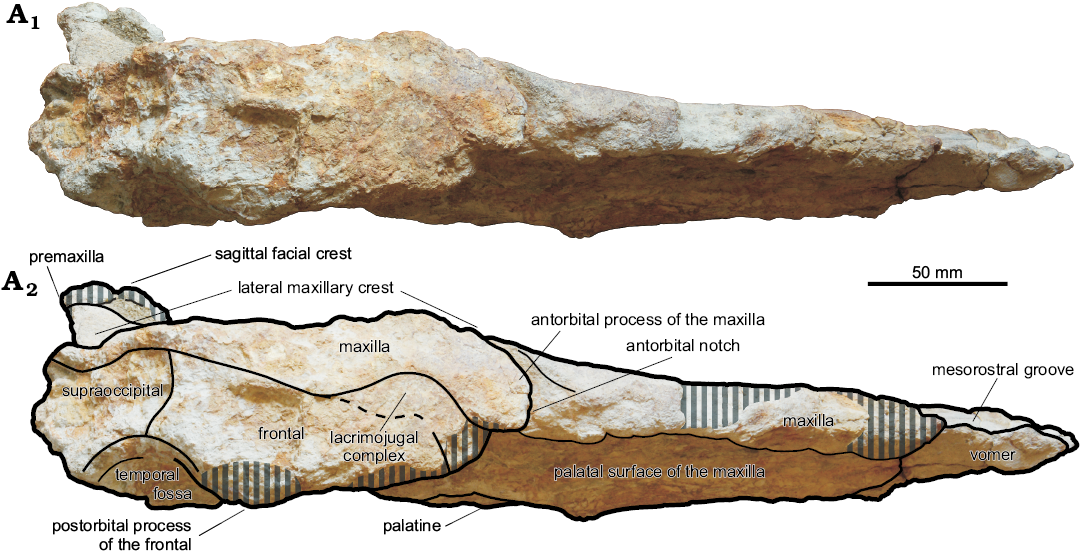
Fig. 4. Cranium, in lateral view, of the kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy). Photograph (A1), explanatory drawing (A2). The grey vertical stripes denote abraded or broken areas. Dashed line for approximating the position of the frontal-lacrimojugal complex suture.

Fig. 5. Cranium of the kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy); in anterodorsal (A1) and right anterodorsolateral (A2) views.
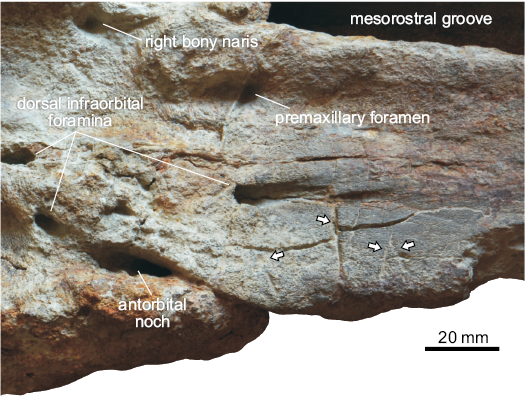
Fig. 6. Close-up of the dorsal surface of the cranium of the kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy), showing the shark bite marks that affect the right maxilla (arrows).
Nasal: The right nasal is absent. The same condition is observed in the vast majority of physeteroids, with the significant exception of the late Miocene Peruvian species Acrophyseter robustus (Lambert et al. 2017a). Due to the loss of the posterior wall of the left bony naris (Fig. 2), it is not possible to ascertain whether MSNUP I-17603 possessed the left nasal (absent in all kogiids known to date).
Palatine: Because of the loss of most of the pterygoids, both palatines are largely exposed on the ventral surface of the skull, located posteromedially to the maxillae and anterolaterally to the choanae (Fig. 3). They are long, anterolaterally bent (i.e., reniform), gently convex transversely, and almost contact each other medially. Their medial margin is provided by the ventral exposure of the vomer. The left palatine contributes to the anterior border of the left choana. Otherwise, the palatines are not well defined.
Lacrimojugal complex: Only the right lacrimal is partly preserved (Figs. 3–5). In lateral view (Fig. 4), the lacrimal is large and roughly hook-shaped: indeed, it exhibits an elongate posterodorsal corner (posterodorsal process sensu Muizon 1988) that is wedged between the frontal and the maxilla; a similar feature has been observed, more or less pronounced, in all Kogiidae except for Aprixokogia (condition unknown in Thalassocetus). The posterodorsal process of the lacrimal is long and slender; it apparently extends more posteriorly than in Kogia, Koristocetus, and Nanokogia, thus recalling the condition observed in Praekogia. As observed in Kogia, Koristocetus, Nanokogia, and Scaphokogia, the lacrimal-maxilla suture is sigmoidal: it is distinctly convex dorsally in its anterior half and gently concave dorsally in its posterior half, thus roughly paralleling the dorsal profile of the right lateral maxillary crest. The anteroventral tip of the lacrimal bone is not preserved. In ventral view (Fig. 3), as observed in Nanokogia and Praekogia, the lacrimojugal complex extends posteromedially, towards the anterolateral termination of the ventral infraorbital foramen, but its boundaries are not well defined.
Frontal: Only the right frontal is partly preserved. This bone is mostly exposed in lateral and ventral views (Figs. 3, 4), its dorsal surface being indeed covered by the right maxilla. When viewed laterally (Fig. 4), the frontal-maxilla suture along the supraorbital process is inclined at about 20° with respect to the coronal plane, thus strongly differing from Aprixokogia and Kogia (in which much higher angles are observed) and resembling instead Thalassocetus in this respect. The preorbital and postorbital processes are not preserved, and only a central segment of the orbit roof is preserved. The frontal also comprises the upper portion of the roof of the temporal fossa. The latter is only incompletely preserved; its higher point is situated slightly higher than the highest point of the orbit but ca. 40 mm lower than the overlying margin of the supracranial basin, thus contrasting with both the high temporal fossa of Aprixokogia and Koristocetus and the low temporal fossa of extant Kogia, recalling instead the condition observed in Nanokogia. The outline of the preserved portion of the temporal crest could suggest that the temporal fossa did not strongly differ from that of Nanokogia. When viewed ventrally (Fig. 3), the supraorbital process displays a wide frontal groove (representing the distal extension of the optic canal) running from the unpreserved optic foramen towards the lateral termination of the supraorbital process of the frontal (i.e., the roof of the orbit). The frontal groove moderately widens towards its distal end; it is oriented anterolaterally at a smaller angle than observed in Koristocetus and Nanokogia.
Vomer: The vomer reaches the anterior end of the skull (Figs. 2–5). In dorsal view (Fig. 2), the vomer is widely exposed along the rostrum and forms the floor of the mesorostral groove. The dorsal surface of the vomer consists of two flat to slightly convex bony walls, facing dorsomedially, whose medial junction forms a straight, anteroposteriorly oriented sulcus, for which the name “sagittal vomerine sulcus” is here proposed; as such, the transverse section of the dorsal surface of the vomer along the mesorostral groove is V-shaped (Figs. 2, 5). The presence of such a sagittal vomerine sulcus appears to be unique of Pliokogia among Kogiidae. In ventral view (Fig. 3), the vomer is exposed as a narrow but distinctly carinated slit running between the maxillae for most of the rostrum length. At the base of the rostrum, the vomer is exposed between the palatines, where it displays a prominent, anteroposteriorly elongated ventral keel. As in most kogiids except Praekogia, the vomer does not cover the ventral surface of the presphenoid medial to the choanae.
Sphenoid: Following Ichishima (2016), we identify the bony septum that separates the external nares medially as belonging to the presphenoid. This septum (Fig. 2) is transversely thin but flares mediolaterally at the posterior termination of the mesorostral groove, where it is exposed for about 20 mm between the premaxillae. Differing from most physically mature specimens of Kogia sima and some specimens of K. breviceps, the presphenoid does not further extend anteriorly within the mesorostral groove. In ventral view (Fig. 3), the presphenoid is partially exposed between the choanae. A long and thin slice of bone, running parallel and contiguous to the anteromedial margin of the right frontal groove towards the posterior wall of the right ventral infraorbital foramen, is here regarded as possibly representing the orbitosphenoid, following the interpretation proposed by Collareta et al. (2017b) for a similar feature in Koristocetus.
Pterygoid: Only a small fraction of the pterygoids is tentatively identified on MSNUP I-17603. An anteroposteriorly elongated stripe of bone, taking place between the right palatine and the adjoining portion of the maxilla lateral and anterolateral to the right bony naris, is here interpreted as a portion of the right pterygoid (Fig. 3). This bone seems also to cover the lateral margin of the right palatine.
Occipital: A small portion of the supraoccipital is preserved, posterior to the apex of the temporal fossa and below the posterolateral margin of the supracranial basin (Fig. 4). It contacts the frontal anteriorly; the supraoccipital-frontal suture runs roughly subvertical.
Dentition: The only preserved tooth (Fig. 7) is slender, streamlined, delicate, and almost complete, lacking only the basalmost tip of the elongated root; it measures 35 mm in maximum preserved dorsoventral height and 5 mm in maximum transverse diameter. It displays a very weak curvature, weaker than generally observed in the teeth of extant Kogia and in more robust isolated fossil teeth, possibly belonging to Kogiidae, described and figured by Pilleri (1987: pl. 15) from the Italian Pliocene locality of Orciano Pisano. The uppermost 7 mm of the tooth are comprised of a dark-coloured cusp, having a roughly circular base (with a mean diameter of ca. 4 mm) and a conical outline. As in most extant adult individuals of Kogia breviceps and K. sima (Plön 2004), the sole preserved tooth of Pliokogia completely lacks enamel. The tooth apex exhibits a small, barely convex, slightly polished facet that is here interpreted as due to abrasive wear. This wearing facet is subvertically oriented, and as such, it provides the tooth apex with a spatula-like appearance. Similar abrasion surfaces, sometimes tilted medially or laterally, are often observed on the teeth of the great sperm whale Physeter macrocephalus, where they are explained by the repeated passage of water laden with prey items (Lambert et al. 2013); tooth wear modifications, mostly resulting in the loss of the neonatal enamel cap, have also been reported for extant kogiids, but their origin is still not clear (Plön 2004; Stephanie Plön, personal communication 2018).
Although the only preserved tooth of Pliokogia was found in close proximity of the tip of the rostrum of MSNUP I-17603 (personal observation by AC and FCF), the absence of deep alveoli on the ventral surface of the maxillae suggests its identification as a mandibular tooth; only mandibular teeth are observed in extant Kogiidae, but functional maxillary teeth were presumably present in Koristocetus (Collareta et al. 2017b). Pliokogia apenninica represents the first extinct kogiid species based on significant cranial material for which at least one tooth is known.

Fig. 7. Detached tooth of kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy), in four different views (A1–A4). The dashed line denotes the extent of the wear facet.
Ribs: A single fragmentary and slightly distorted anterior rib is preserved (Fig. 8A). The head, neck, tubercle, and distal termination of the rib are unfortunately lost. The shaft has a minimum preserved width of 30 mm and ranges from 7 mm to 12 mm in thickness. Based on its general shape and curvature at the angle, this bone is tentatively identified as the second right rib.
Vertebrae: Only one 21 mm long partial vertebra is preserved (Fig. 8B). This vertebra appears to have been deformed by diagenetic compression, and consequently its centrum is shaped as an oblique circular cylinder. The proximal portions of both pedicles are preserved: the left pedicle is relatively more complete than the right and points anterodorsolaterally. The position of the pedicles indicates that, originally, the neural arch was rather wide transversely. The general proportions and size of this vertebra, as well as its moderate anterolateral thickness and the presence of pedicles, suggest its identification as a thoracic. Both the anterior and the posterior vertebral epiphyses are fused with the centrum. Given that, in extant odontocetes, the progression of vertebral epiphyseal ankylosis seemingly terminates in the thoracic and lumbar regions (Galatius and Kinze 2003), this observation confirms that MSNUP I-17603 represents a full-grown cetacean individual.
Stratigraphic and geographic range.—Type locality and horizon only.

Fig. 8. Postcranial bones of the kogiid sperm whale Pliokogia apenninica gen. et sp. nov. (MSNUP I-17603, holotype), from the lower Pliocene of Sant’Andrea Bagni (Northern Apennines, northern Italy). A. Fragmentary right rib in dorsal (A1), posterior (A2), and ventral (A3) views. B. Fragmentary thoracic vertebra in anterior (B1), posterior (B2), and dorsal (B3) views.
Phylogeny
Our phylogenetic analysis resulted in thirty most parsimonious trees having tree length of 145, consistency index of 0.5379, and retention index of 0.7287. The strict consensus tree and the 50% majority-rule consensus tree are presented in Fig. 9. The former (Fig. 9A) displays basically the same relationships within physeteroids as the tree of Collareta et al. (2017a). In this tree, the base of Physeteroidea is marked by a polytomy involving: (i) Eudelphis from the Berchem Formation of Belgium; (ii) a clade including the four genera of macroraptorial stem physeteroids (namely, Acrophyseter, Brygmophyseter, Livyatan, and Zygophyseter); and (iii) a clade formed by “Aulophyseter” rionegrensis plus the crown-group physeteroids (i.e., Physeteridae + Kogiidae). The recovery of the macroraptorial sperm whales (sensu Lambert et al. 2017a) as a monophyletic group might suggest to erect a new family for them, pending further analyses. The strict consensus tree supports the referral of Pliokogia to the family Kogiidae. Also in agreement with the results of Collareta et al. (2017a), the strict consensus tree recognises three groups among kogiids: (i) a paraphyletic group including two early diverging forms from the North Atlantic realm, i.e., Aprixokogia and Thalassocetus; (ii) a clade coinciding with the extinct subfamily Scaphokogiinae, formed by Scaphokogia and two undescribed partial skulls from the Pisco Formation of southern Peru; (iii) a clade sister group to Scaphokogiinae and coinciding with the extant subfamily Kogiinae, which includes Koristocetus, Nanokogia, Praekogia, Kogia, and the newly described genus Pliokogia. The subfamily Kogiinae is thus comprised of all the members of Kogiidae in which the posterior end of the right antorbital notch opens onto the supracranial basin (character 10, state 1). Although Koristocetus is recovered as the earliest branching member of Kogiinae, the relationships among Kogia, Nanokogia, Praekogia, and Pliokogia are unresolved in the strict consensus tree, possibly reflecting the rather minor nature of the anatomical differences between these closely allied genera. The 50% majority-rule consensus tree (Fig. 9B) provides a more satisfactory result, being indeed able to fully resolve the relationships among the five genera of Kogiinae: Koristocetus, Nanokogia, Pliokogia, Praekogia, and Kogia are here recognised as subsequently branching genera. Therefore, the 50% majority-rule consensus tree recovers Pliokogia crownward of Koristocetus and Nanokogia, as sister group to Praekogia + Kogia, with which it forms a clade of Plio-Quaternary kogiids that includes the extant representatives of this family.

Fig. 9. Phylogenetic relationships of Pliokogia apenninica gen. et sp. nov. with other physeteroids. A. Strict consensus tree. B. 50% majority-rule consensus tree. Acrophyseter sp. refers to MUSM 2182, a partial skull from the site of Cerro Los Quesos (upper Miocene, Pisco Formation, Peru); Scaphokogiinae sp. refers to MUSM 3291 and MUSM 3405, two partial skulls from the sites of Cerro Los Quesos and Cerro Blanco, respectively (upper Miocene, Pisco Formation, Peru), representing a new form of Kogiidae sharing similarities with Scaphokogia cochlearis (see the SOM file for further details). Extinct genera and species are marked by a dagger (†).
Palaeoecology
Extant kogiids inhabit tropical to temperate pelagic environments outside the Mediterranean Basin (e.g., McAlpine 2002). Dwarf sperm whales (Kogia sima) appear to be widely distributed in the offshore waters of tropical and warm-temperate regions, seemingly preferring low-latitude open-ocean areas (Caldwell and Caldwell 1989). Pygmy sperm whales (Kogia breviceps) are known from deep waters (outer continental shelf and beyond) in tropical to temperate zones of all oceans (McAlpine 2002); this species might prefer somewhat cooler waters than the dwarf sperm whale does. Dwarf and pygmy sperm whales are nevertheless sympatric species on large portions of their ranges; evidence for sympatric habits in extinct kogiids has also arisen from the fossil record of the Pliocene Yorktown Formation of North Carolina, USA (Vélez-Juarbe et al. 2015) and the upper Miocene strata of the Pisco Formation of Peru (Collareta et al. 2017b). Three modern records of K. sima exist for the Mediterranean region, all from the coasts of Italy (Baccetti et al. 1991; Bortolotto et al. 2003; Maio et al. 2017). All these records, consisting of strandings of isolated individuals, are interpreted as resulting from the entrance of Atlantic vagrants into the Mediterranean Basin; therefore, the latter is still considered outside the range of living kogiids (e.g., McAlpine 2017: fig. 2). By contrast, the Italian fossil record of Kogiidae (Pilleri 1987; Bianucci 1996, 1997; Cigala Fulgosi 1996; Bianucci et al. 1998; Bianucci and Landini 1999; this work) demonstrates that, during the Pliocene, kogiids were part of the Mediterranean cetacean fauna, both during the Zanclean and the Piacenzian. Interestingly, the fossil occurrence reported in the present paper comes from a palaeoenvironmental setting characterised by a high degree of “oceanisation”, that is, by the abundance of Atlantic-derived, strongly oceanic vertebrate taxa, which indicate strong and well-structured connections between the northern Atlantic ocean and the Adriatic palaeo-area (Cigala Fulgosi 1986, 1996). Therefore, even in the light of the habitat preferences of present-day Kogia, the recovery of a fossil kogiid from such a palaeoenviromental setting is not entirely surprising. Although floating cetacean carcasses can suffer substantial transport by marine or riverine currents before deposition on the seafloor (e.g., Schäfer 1972), it seems indeed reasonable to hypothesise that Pliokogia inhabited offshore, several hundred-metres-deep palaeonvironmental settings such as the Sant’Andrea Bagni area during deposition of the mudstone from which MSNUP I-17603 was collected.
As already observed, the dorsal surface of the rostrum of MSNUP I-17603 displays some unambiguous shark bite marks. Similar traces are known from several Pliocene cetacean specimens from the central Mediterranean region (e.g., Portis 1883; Cigala Fulgosi 1990; Bianucci et al. 2002, 2010; Freschi 2017). Due to their remarkable maximum length, unserrated nature, and substraight geometry, these bite marks are most likely referable to a large-sized lamniform such as Cosmopolitodus plicatilis (Agassiz, 1843), a possible synonym of Cosmopolitodus hastalis (Agassiz, 1838), which in turn is sometimes placed in the extant genus Carcharodon (e.g., Ehret et al. 2012; Kent 2018). Interestingly, teeth of Cosmopolitodus plicatilis are rather common in lower Pliocene deposits of Italy (see e.g., Marsili 2007a for a review), and evidence for Cosmopolitodus preying upon the extinct dolphin Astadelphis gastaldii (Brandt, 1874) from the Pliocene of Piedmont has been provided by Bianucci et al. (2010). As highlighted elsewhere (e.g., Cigala Fulgosi 1990; Ehret et al. 2009; Bianucci et al. 2010, 2018; Collareta et al. 2017a; Godfrey et al. 2018), it is virtually impossible to discriminate between active predation and scavenging when dealing with fragmentary fossil specimens such as the holotype of Pliokogia. Interestingly, the extant Carcharodon carcharias (Linnaeus, 1758) is known to include the pygmy sperm whale K. breviceps (which is similar to Pliokogia in body size) among its prey items (e.g., Long 1991). Moreover, Marsili (2007a) pointed out that Cosmopolitodus plicatilis thrived in the Mediterranean Sea at the Miocene–Pliocene transition, when marine mammals also boomed, and he proposed that the trophic spectrum of this extinct lamniform species was similar to that of the extant great white shark C. carcharias, that is, primarily focused on pinnipeds and small-sized cetaceans. In the light of these considerations, a predator-prey relationship between Cosmopolitodus and Pliokogia does not appear unlikely. At the same time, the placement of the bite marks affecting MSNUP I-17603 (i.e., on the cranium) does not compare favourably with the body regions of small-sized odontocetes primarily targeted by extant white sharks during their predatory attacks (e.g., the dorsum or the caudal peduncle); indeed, Carcharodon is believed to avoid detection by both the lateral visual field and the anteriorly directed biosonar of their odontocete prey (Long and Jones 1996).
Overall, extant Kogiidae appear to feed mostly on mid- and deep-water prey, thus recalling the diet habits of their much larger relative Physeter (McAlpine 2017). Studies of feeding habits, based on stomach contents of stranded animals, suggest that K. breviceps feeds in deep water, primarily on cephalopods and, less often, on deep-sea fish and shrimps (Dos Santos and Haimovici 2001; McAlpine et al. 1997). In Hawaiian waters, pygmy sperm whales appear to engage in foraging activity between 600 m and 1200 m, spanning both the mesopelagic and bathypelagic zones (West et al. 2009). Dwarf sperm whales may prefer to forage in slightly shallower waters of the continental shelf and slope (McAlpine 2017); for instance, stomach contents of K. sima from South African waters suggest that this species routinely dives to about 600 m, and possibly even deeper, to feed (Plön and Relton 2016, and references therein). Nevertheless, the feeding ecologies of the pygmy and dwarf sperm whales are similar, and both species occupy roughly equivalent trophic niches, at least off the US mid-Atlantic coast (Staudinger et al. 2014). In K. breviceps and K. sima, foraging relies on a strong suction feeding specialization based on a highly apomorphic craniomandibular architecture (featuring the proportionally shortest rostrum among living cetaceans) and hyoid apparatus (Caldwell and Caldwell 1989; McAlpine 2002; Bloodworth and Marshall 2005; Werth 2006; Bloodworth and Odell 2008; Hocking et al. 2017). With respect to extant Kogia, Pliokogia exhibits a distinctly longer rostrum, which can be interpreted in two ways: it could suggest that the foraging technique of this extinct form differed somewhat from that of the extremely short-snouted modern kogiids; alternatively, it might be related to a different extension of the melon (a lipid-rich structure located in the forehead of all odontocetes; Hooker 2018) with respect to K. breviceps and K. sima. Interestingly, the elongated rostrum of Pliokogia recalls that of Physeter, thus evoking the possibility that the feeding technique of the former genus integrated some elements of the highly derived suction feeding technique of the latter; however, the length of the rostrum might not be relevant to this feeding strategy, as Physeter sucks its prey directly into the oropharyngeal opening, i.e., at the posterior end of the oral cavity (Werth 2004, 2006). As a matter of fact, MSNUP I-17603 shares with the three living species of physeteroids a low temporal fossa, the lack of dental enamel, and the likely absence of functional maxillary teeth; coupled with the extremely slender and delicate aspect of the sole preserved tooth, these characters suggest that, similar to the dwarf and pygmy sperm whales, Pliokogia was a suction feeder rather than an active raptorial predator. Moreover, as already highlighted, the arrangement of the premaxillary fossa and peripheral maxillary fossa on the dorsal surface of the neurocranium of Pliokogia supports the hypothesis that the echolocating system of this extinct genus was well-developed and overall similar to that of extant kogiids, thus suggesting a similar specialization for deep diving and foraging at great depths. Given these observations, and considering also the palaeobathymetry estimates for the offshore mudstone exposed at Sant’Andrea Bagni (at least 400–900 m; Cigala Fulgosi 1996), we hypothesise that the diet of Pliokogia included deep-water prey, thus not substantially departing from that of Kogia and, possibly, Nanokogia (Vélez-Juarbe et al. 2015). Interestingly, the Sant’Andrea Bagni palaeoenvironment was characterised by oxygenated sea bottom conditions, as well as by an abundant and diverse benthic and benthomesopelagic fauna (Cigala Fulgosi 1986, 1996).
Since modern kogiids do not inhabit the Mediterranean Sea, and considering also that they are believed to forage on deep-water cephalopods and fish at or beyond the edge of the continental shelf, the association of Pliokogia with a psychrospheric elasmobranch assemblage with strong Atlantic affinities, such as that described by Cigala Fulgosi (1996) from Sant’Andrea Bagni, could stimulate some broader palaeoecological considerations. During the early Pliocene, the Gibraltar connection was likely deep and, at least at times, controlled by estuarine dynamics (e.g., Benson 1975; Van Harten 1984; Thunnel et al. 1987; McKenzie et al. 1990; Iaccarino et al. 1999). This situation likely allowed the passage of deep-water organisms (some of which constitute the core of the diet of extant Kogiidae, being possibly important also in the trophic spectrum of the Pliocene genus Pliokogia) from the Atlantic Ocean to the Mediterranean Sea and led to the establishment of rich deep-water ecosystems, supported by well-structured trophic systems, in the Mediterranean Basin (Cigala Fulgosi 1996, and references therein). Similar episodes of extensive “oceanisation” of the Mediterranean Basin, characterised by the presence of psychrospheric waters of Atlantic origin due to estuarine circulation in the Gibraltar area, apparently occurred again (if not continuously) during the late Pliocene and early Pleistocene, as indicated by a well-established fossil record of vertebrates and invertebrates (e.g., Di Geronimo and La Perna 1996, 1997a, b; Marsili 2007b; Cigala Fulgosi et al. 2009; Borghi et al. 2014; Sciuto 2014; Collareta et al. 2018). The eventual establishment of present-day antiestuarine circulation at Gibraltar, characterised by deep homothermy and a limited deep nutrient supply, likely led to the eradication of most Atlantic-derived deep-water ecosystems from the Mediterranean Sea (see also Cigala Fulgosi 1996, Marsili 2007b, and Borghi et al. 2014 at this regard). Although several modern Mediterranean toothed whales (e.g., Physeter macrocephalus and Ziphius cavirostris Cuvier, 1823) forage at mesopelagic and bathypelagic depths, the current diversity of Mediterranean deep-diving odontocetes might be smaller than in Pliocene times, and the absence of kogiids from the present-day Mediterranean Sea has been proposed to reflect a strong, basin-wide depletion of the teuthophagous trophic resource—an interpretation that highlights the prime importance of food availability in controlling the structure and composition of cetacean communities (Bianucci et al. 1998). In the light of these hypotheses, and if our reconstruction of the ecological preferences of Pliokogia is correct, the eventual disappearance of Kogiidae from the Mediterranean Basin could be related to the definitive establishment of threshold basin conditions at Gibraltar, resulting in a physical and biological barrier to the entrance of deep-water Atlantic species, including the putative prey of the Pliocene Mediterranean kogiids.
Conclusions
We describe MSNUP I-17603, a partial physeteroid skeleton from the early Pliocene (ca. 5 Ma) of northern Italy (Northern Apennines, Adriatic palaeo-area). This fossil specimen is designated as the holotype of Pliokogia apenninica, a new genus and species of Kogiidae. Pliokogia is mostly characterised by a long and dorsally flattened rostrum and by the presence of two well-distinct fossae on the right side of the supracranial basin, including an elongated peripheral maxillary fossa (here interpreted as accomodating the vocal chamber) on the posterior portion of the right maxilla. Our phylogenetic analysis recovers Pliokogia as a member of the subfamily Kogiinae, which also includes Kogia, Koristocetus, Nanokogia, and Praekogia. The palaeoecology of Pliokogia is then discussed with reference to its cranial features, the embedding sediments and associated faunal assemblage (which includes a number of deep-water elasmobranch taxa with strong Atlantic affinities), and relevant ecological literature on extant pygmy and dwarf sperm whales. We propose that the eventual disappearance of Kogiidae from the Mediterranean Basin might be related to the definitive establishment of threshold basin conditions at Gibraltar, which possibly resulted in the strong depletion of their putative prey (e.g., deep-sea squids and fish). In conclusion, while significantly expanding our knowledge on the early Pliocene Mediterranean biodiversity, the finding of Pliokogia suggests that our understanding of the past diversity, disparity, and distribution patterns of kogiid sperm whales is still far from being exhaustive.
Acknowledgements
We are grateful to Chiara Sorbini (MSNUP), Rodolfo Salas-Gismondi and Rafael Varas-Malca (both MUSM), Olivier Lambert (IRSNB), Christian de Muizon, Daniel Robineau, and Christine Lefèvre (all MNHN), Elena Cioppi and Stefano Dominici (both MGPUF), Gianni Insacco (MSNC), James G. Mead, Nicholas D. Pyenson, and Charles W. Potter (all USNM), Giuseppe Manganelli (Dipartimento di Scienze Fisiche, della Terra e dell’Ambiente, Università di Siena, Italy), and Fabrizio Cancelli (MUSNAF) for providing access to specimens under their care. Special thanks are due to Olivier Lambert, Christian de Muizon, Aldo Marcelo Benites Palomino (MUSM), Felix Georg Marx (Université de Liège, Belgium), Stephanie Plön (Nelson Mandela University, Port Elizabeth, South Africa), Walter Landini (Dipartimento di Scienze della Terra, Università di Pisa, Italy), and Simone Casati (Museo Geopaleontologico G.A.M.P.S., Scandicci, Italy) for fruitful discussions about the palaeobiology of Kogiidae, the trophic ecology of marine mammals, and the palaeoenvironmental scenarios of the Mediterranean Sea during the Pliocene. This paper greatly benefited from constructive comments and thoughtful suggestions by Yoshihiro Tanaka (Osaka Museum of Natural History, Japan), Travis Park (Natural History Museum, London, UK), Jorge Vélez-Juarbe (Natural History Museum of Los Angeles County, Los Angeles, USA), and Olivier Lambert: thank you very much! AC received support from the SYNTHESYS Project http://www.synthesys.info/ (Project Number FR-TAF-6129), which is financed by European Community Research Infrastructure Action under the FP7 “Capacities” Program.
References
Baccetti, N., Cancelli, F., and Renieri, T. 1991. First record of Kogia simus (Cetacea, Physeteridae) from the Mediterranean Sea. Mammalia 55: 152–154.
Backman, J., Raffi, I., Rio, D., Fornaciari, E., and Pälike, H. 2012. Biozonation and biochronology of Miocene through Pleistocene calcareous nannofossils from low and middle latitudes. Newsletters on Stratigraphy 45: 221–244. Crossref
Barnes, L.G. 1973. Praekogia cedrosensis, a new genus and species of fossil pygmy sperm whale from Isla Cedros, Baja California, Mexico. Contributions in Science of the Natural History Museum of Los Angeles County 247: 1–20.
Barnes, L.G. 1998. The sequence of fossil marine mammal assemblages in Mexico. Avances en Investigación, Paleontología de Vertebrados, Publicación Especial 1: 26–79.
Benson, R.H. 1972a. Ostracodes as indicators of threshold depth in the Mediterranean during the Pliocene. In: D.J. Stanley (ed.), The Mediterranean Sea, 63–72. Dowden, Hutchinson and Ross, Stroudsbury.
Benson, R.H. 1972b. Psychrospheric and continental Ostracoda from ancient sediments in the floor of the Mediterranean. Initial Reports of the Deep Sea Drilling Project 13: 1002–1008. Crossref
Benson, R.H. 1975. Ostracodes and Neogene history. In: T. Saito and L.H. Burckle (eds.), Late Neogene Epoch Boundaries, 41–48. American Museum of Natural History, New York.
Bianucci, G. 1996. I cetacei fossili del Museo di Storia Naturale dell’Universita di Pisa. Atti della Societa Toscana di Scienze Naturali, Memorie, Serie A 103: 63–68.
Bianucci, G. 1997. The Odontoceti (Mammalia, Cetacea) from Italian Pliocene. The Ziphiidae. Palaeontographia Italica 84: 163–192.
Bianucci, G. and Landini, W. 1999. Kogia pusilla from the Middle Pliocene of Tuscany (Italy) and a phylogenetic analysis of the family Kogiidae (Odontoceti, Cetacea). Rivista Italiana di Paleontologia e Stratigrafia 105: 445–453.
Bianucci, G. and Landini, W. 2006. Killer sperm whale: a new basal physeteroid (Mammalia, Cetacea) from the Late Miocene of Italy. Zoological Jounal of the Linnean Society 148: 103–131. Crossref
Bianucci, G., Bisconti, M., Landini, W., Storai, T., Zuffa, M., Giuliani, S., and Mojetta, A. 2002. Trophic interactions between white sharks (Carcharodon carcharias) and cetaceans: a comparison between Pliocene and recent data. In: M. Vacchi, G. La Mesa, F. Serena, and B. Sèret (eds.), Proceedings 4ht Meeting of the European Elasmobranch Association, 33–48. Imprimerie F. Paillart, Abbeville.
Bianucci, G., Collareta, A., Bosio, G., Landini, W., Gariboldi, K., Gioncada, A., Lambert, O., Malinverno, E., Muizon, C. de, Varas-Malca, R., Villa, I.M, Coletti, G., and Urbina, M. 2018. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, southern Peru). Palaeogeography, Palaeoclimatology, Palaeoecology 511: 256–279. Crossref
Bianucci, G., Di Celma, C., Collareta, A., Landini, W., Post, K., Tinelli, C., Muizon, C. de, Bosio, G., Gariboldi, K., Gioncada, A., Malinverno, E., Cantalamessa, G., Altamirano-Sierra, A., Salas-Gismondi, R., Urbina, M., and Lambert, O. 2016. Fossil marine vertebrates of Cerro Los Quesos: Distribution of cetaceans, seals, crocodiles, seabirds, sharks, and bony fish in a late Miocene locality of the Pisco Basin, Peru. Journal of Maps 12: 1037–1046. Crossref
Bianucci, G., Gatt, M., Catanzariti, R., Sorbi, S., Bonavia, C.G., Curmi, R., and Varola, A. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios 44: 549–585. Crossref
Bianucci, G., Sarti, G., Catanzariti, R., and Santini, U.1998. Middle Pliocene cetaceans from Monte Voltraio (Tuscany, Italy). Biostratigraphical, paleoecological and paleoclimatic observations, Rivista Italiana di Paleontologia e Stratigrafia 104: 123–130.
Bianucci, G., Sorce, B., Storai, T., and Landini, W. 2010. Killing in the Pliocene: shark attack on a dolphin from Italy. Palaeontology 53: 457–470. Crossref
Bloodworth, B.E. and Marshall, C.D. 2005. Feeding kinematics of Kogia and Tursiops (Odontoceti: Cetacea): characterization of suction and ram feeding. Journal of Experimental Biology 208: 3721–3730. Crossref
Bloodworth, B.E. and Odell, D.K. 2008. Kogia breviceps (Cetacea: Kogiidae). Mammalian Species 812: 1–12. Crossref
Borghi, E., Garilli, V., and Bonomo, S. 2014. Plio-Pleistocene Mediterranean bathyal echinoids: evidence of adaptation to psychrospheric conditions and affinities with Atlantic assemblages. Palaeontologia Electronica 17 (3): 1–26. Crossref
Bortolotto, A., Papini, L., Insacco, G., Gili, C., Tumino, G., Mazzariol, S., Pavan, G., and Cozzi, B. 2003. First record of a dwarf sperm whale, Kogia sima (Owen, 1866) stranded alive along the coasts of Italy. In: 31st Symposium of the European Association for Aquatic Mammals. European Association for Aquatic Mammals, Tenerife.
Bruun, A.F. 1957. Deep sea and abyssal depths. Geological Society of America Memoirs 67 (1): 641–673. Crossref
Caldwell, D.K. and Caldwell, M.C. 1989. Pygmy sperm whale—Kogia breviceps (de Blainville, 1838); dwarf sperm whale—Kogia simus Owen, 1866. In: S.H. Ridgway and R.J. Harrison (eds.), Handbook of Marine Mammals. Volume 4, River Dolphins and the Larger Toothed Whales, 235–260. Academic Press, London.
Channel, J., Poli, M., Rio, D. Sprovieri R., and Villa, G. 1994. Magnetic stratigraphy and biostratigraphy of Pliocene “argille azzurre” (Northern Apennines, Italy). Palaeogeography, Palaeoclimatology, Palaeoecology 110: 83–102. Crossref
Chen, I., Chou, L.-S., Chen, Y.-J., and Watson, A. 2011. The maturation of skulls in postnatal Risso’s dolphins (Grampus griseus) from Taiwanese waters. Taiwania 56: 177–185.
Cigala Fulgosi, F. 1986. A deep water elasmobranch fauna from a lower Pliocene outcropping (Northern Italy). In: T. Uyeno, R. Arai, T. Taniuchi, and K. Matsuura (eds.), Indo-Pacific Fish Biology. Proceedings of the Second International Conference on Indo-Pacific Fishes, 133–139. Ichthyological Society of Japan, Tokyo.
Cigala Fulgosi, F. 1990. Predation (or possible scavenging) by a great white shark on an extinct species of bottlenosed dolphin in the Italian Pliocene. Tertiary Research 12: 17–36.
Cigala Fulgosi, F. 1996. Rare oceanic deep water squaloid sharks from the lower Pliocene of the Northern Apennines (Parma Province, Italy). Bollettino della Società Paleontologica Italiana 34: 301–322.
Cigala Fulgosi, F., Casati, S., Orlandini, A., and Persico, D. 2009. A small fossil fish fauna, rich in Chlamydoselachus teeth, from the Late Pliocene of Tuscany (Siena, central Italy). Cainozoic Research 6: 3–23.
Cita M.B. 1973. Pliocene biostratigraphy and chronostratigraphy. Initial Reports of the Deep Sea Drilling Project 13: 1343–1379. Crossref
Cita, M.B. 1975. Studi sul Pliocene e sugli strati di passaggio dal Miocene al Pliocene. VIII. Planktonic foraminiferal biozonation of the Mediterranean Pliocene deep sea record. A revision. Rivista Italiana di Paleontologia e Stratigrafia 81: 527–544.
Collareta, A., Casati, S., and Di Cencio, A. 2018. The porbeagle shark, Lamna nasus (Elasmobranchii: Lamniformes), from the late Pliocene of the central Mediterranean Basin. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 287: 307-316. Crossref
Collareta, A., Lambert, O., Landini, W., Di Celma, C., Malinverno, E., Varas-Malca, R., Urbina, M., and Bianucci, G. 2017a. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeography, Palaeoclimatology, Palaeoecology 469: 84–91. Crossref
Collareta, A., Lambert, O., Muizon, C. de, Urbina, M., and Bianucci, G. 2017b. Koristocetus pescei gen. et sp. nov., a diminutive sperm whale (Cetacea: Odontoceti: Kogiidae) from the late Miocene of Peru. Fossil Record 20: 259–278. Crossref
Di Celma, C., Malinverno, E., Bosio, G., Collareta, A., Gariboldi, K., Gioncada, A., Molli, G., Basso, D., Varas-Malca, R., Pierantoni, P. P., Villa, I. M., Lambert, O., Landini, W., Sarti, G., Cantalamessa, G., Urbina, M., and Bianucci G. 2017. Sequence stratigraphy and paleontology of the upper Miocene Pisco Formation along the western side of the lower Ica Valley (Ica Desert, Peru). Rivista Italiana di Paleontologia e Stratigrafia 123: 255–273.
Di Geronimo, I. and La Perna, R. 1996. Bathyspinula excisa (Philippi, 1844) (Bivalvia, Protobranchia): a witness of the Plio-Quaternary history of the deep Mediterranean benthos. Rivista Italiana di Paleontologia e Stratigrafia 102: 105–118.
Di Geronimo, I. and La Perna, R. 1997a. Homalopoma emulum (Seguenza) a bathyal cold stenothermic gastropod in the Mediterranean Pleistocene. Geobios 30: 215–223.
Di Geronimo, I. and La Perna, R. 1997b. Pleistocene bathyal molluscan assemblages from Southern Italy. Rivista Italiana di Paleontologia e Stratigrafia 103: 389–426. Crossref
Dos Santos, R.A. and Haimovici, M. 2001. Cephalopods in the diet of marine mammals stranded or incidentally caught along southeastern and southern Brazil (21–34° S). Fisheries Research 52: 99–112. Crossref
Ehret, D.J., MacFadden, B.J., Jones, D., DeVries, T.J., and Salas-Gismondi, R. 2009. Caught in the act: trophic interactions between a 4-million-year-old white shark (Carcharodon) and mysticete whale from Peru. Palaios 24: 329–333. Crossref
Ehret, D.J., MacFadden, B.J., Jones, D., DeVries, T.J., Foster, D.A., and Salas-Gismondi, R. 2012. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the upper Neogene Pisco Formation of Peru. Palaeontology 55: 1139–1153. Crossref
Freschi, A. 2017. New Carcharodon scavenging evidence on Pliocene whale bones remains from Northern Appennines. Quaderni del Museo Civico di Storia Naturale di Ferrara 5: 33–36.
Galatius, A. and Kinze, C.C. 2003. Ankylosis patterns in the postcranial skeleton and hyoid bones of the harbour porpoise (Phocoena phocoena) in the Baltic and North Sea. Canadian Journal of Zoology 81: 1851–1861. Crossref
Gill, T. 1871. The sperm whales, giant and pygmy. The American Naturalist 4: 723–743. Crossref
Godfrey, S.J., Ellwood, M., Groff, S., and Verdin, M.S. 2018. Carcharocles-bitten odontocete caudal vertebrae from the Coastal Eastern United States. Acta Palaeontologica Polonica 63: 463–468. Crossref
Govender, R. 2015. Shark-cetacean trophic interaction, Duinefontein, Koeberg, (5 Ma), South Africa. South African Journal of Science 111 (11/12): 2014-0453. [published online, http://dx.doi.org/10.17159/sajs.2015/20140453] Crossref
Hocking D.P., Marx, F.G., Park, T., Fitzgerald, E.M., and Evans, A.R. 2017. A behavioural framework for the evolution of feeding in predatory aquatic mammals. Proceedings of the Royal Society of London Part B 284: 20162750. Crossref
Hooker, S.K. 2018. Toothed whales (Odontoceti). In: B. Würsig, J.G.M. Thewissen, and K. Kovacs (eds.), Encyclopedia of Marine Mammals, 3rd Edition, 1004–1010. Academic Press, San Diego. Crossref
Iaccarino, S., Castradori, D., Cita, M.B., Di Stefano, E., Gaboardi, S., McKenzie, J.A., Spezzaferri, S., and Sprovieri, R. 1999. The Miocene/Pliocene boundary and the significance of the earliest Pliocene flooding in the Mediterranean. Memorie della Società Geologica Italiana 54 (10): 109–131.
Ichishima, H. 2016. The ethmoid and presphenoid of cetaceans. Journal of Morphology 277: 1661–1674. Crossref
ICZN [International Commission on Zoological Nomenclature] 1999. International Code of Zoological Nomenclature, 4th Edition. International Trust for Zoological Nomenclature, London.
Kent, B.W. 2018. The Cartilaginous Fishes (Chimaeras, Sharks, and Rays) of Calvert Cliffs, Maryland, USA. In: S.J. Godfrey (ed.), The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland, USA. Smithsonian Contributions to Paleobiology 100: 233–227.
Lambert, O. 2008. Sperm whales from the Miocene of the North Sea: a re-appraisal. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre 78: 277–316. Crossref
Lambert, O., Bianucci, G., and Muizon, C. de 2017a. Macroraptorial sperm whales (Cetacea, Odontoceti, Physeteroidea) from the Miocene of Peru. Zoological Journal of the Linnean Society 179 (2): 404–474. Crossref
Lambert, O., Bianucci, G., and Muizon, C. de 2017b. Sperm and beaked whales, Evolution. In: B. Würsig, J.G.M. Thewissen, and K. Kovacs (eds.), Encyclopedia of Marine Mammals, 3rd Edition, 916–918. Academic Press, San Diego. Crossref
Lambert, O., Muizon, C. de, and Bianucci, G. 2013. The most basal beaked whale Ninoziphius platyrostris Muizon, 1983: clues on the evolutionary history of the family Ziphiidae (Cetacea: Odontoceti). Zoological Journal of the Linnean Society 167: 569–598.
Long, D.J. 1991. Apparent predation by a white shark Carcharodon carcharias on a pygmy sperm whale Kogia breviceps. Fishery Bulletin 89: 538–540.
Long, D.J. and Jones, R.E. 1996. White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean. In: A.P. Klimley and D.G. Ainley (eds.), Great White Sharks: the Biology of Carcharodon carcharias, 293–307. San Diego, Academic Press. Crossref
Luo, Z. and Marsh, K. 1996. Petrosal (periotic) and inner ear of a Pliocene kogiine whale (Kogiinae, Odontoceti): implications on relationships and hearing evolution of toothed whales. Journal of Vertebrate Paleontology 16: 328–348. Crossref
Maio, N., Pollaro, F., Gasparro, A., Petraccioli, A., Mezzasalma, M., Guariglia, M., Galiero, G., Di Nocera, F., Iaccarino, D., Santoro, M., Insacco, G., and Guarino, F.M. 2017. New record of dwarf sperm whale Kogia sima (Owen, 1866) from the Mediterranean Sea (Cetacea Kogiidae). Biodiversity Journal 8: 947–950.
Marsili, S. 2007a. Analisi sistematica, paleoecologica e paleobiogeografica della selaciofauna Plio-Pleistocenica del Mediterraneo. 246 pp. Unpublished Doctoral Dissertation, Università di Pisa, Pisa.
Marsili, S. 2007b. A new bathyal shark fauna from the Pleistocene sediments of Fiumefreddo (Sicily, Italy). Geodiversitas 29: 229–247.
McAlpine, D.F. 2002. Pygmy and dwarf sperm whales In: W.F. Perrin, B. Würsig, and J.G.M. Thewissen (eds.), Encyclopedia of Marine Mammals, 2nd Edition, 1007–1009. Academic Press, San Diego.
McAlpine, D.F. 2017. Pygmy and dwarf sperm whales In: B. Würsig, J.G.M. Thewissen, and K. Kovacs (eds.), Encyclopedia of Marine Mammals, 3rd Edition, 786–788. Academic Press, San Diego. Crossref
McAlpine, D.F., Murison, L.D., and Hoberg, E.P. 1997. New records for the pygmy sperm whale, Kogia breviceps (Physeteridae) from Atlantic Canada with notes on diet and parasites. Marine Mammal Science 13: 701–704. Crossref
McKenzie J.A., Sprovieri R., and Channel J.E.T. 1990. The terminal Messinian flood and earliest Pliocene paleoceanography in the Mediterranean: results from ODP Leg 127, Site 652, Tyrrhenian Sea. Memorie della Società Geologica Italiana 33: 1–53.
Mead, J.G. and Fordyce, R.E. 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology 627: 1–261. Crossref
Muizon, C. de 1988. Les Vertébrés de la Formation Pisco (Pérou). Troisième partie: Les Odontocètes (Cetacea, Mammalia) du Miocène. Travaux de l’Institut Français d’Études Andines 42: 1–244.
Ogg, J.G. 2012. Geomagnetic polarity time scale. In: F.M. Gradstein, J.G. Ogg, M.D. Schmitz, and G.M. Ogg (eds.), The Geologic Time Scale, 85–113. Elsevier, Amsterdam. Crossref
Pilleri, G. 1987. The Cetacea of the Italian Pliocene with a Descriptive Catalogue of the Species in the Florence Museum of Paleontology. 160 pp. Brain Anatomy Institute, Bern.
Plön, S. 2004. The Status and Natural History of Pygmy (Kogia breviceps) and Dwarf (K. sima) Sperm Whales off Southern Africa. 508 pp. Unpublished Doctoral Dissertation, Rhodes University, Grahamstown.
Plön, S. and Relton, C. 2016. A conservation assessment of Kogia spp. In: M.F. Child, L. Roxburgh, E. Do Linh San, D. Raimondo, and H.T. Davies-Mostert (eds.), The Red List of Mammals of South Africa, Swaziland and Lesotho, 1–6. South African National Biodiversity Institute and Endangered Wildlife Trust, Pretoria.
Portis, A. 1883. Nuovi studi sulle tracce attribuite all’uomo pliocenico. Memorie della Reale Accademia delle Scienze di Torino 35: 2–35.
Rio, D., Raffi, I., and Villa, G. 1990. Pliocene-Pleistocene calcareous nannofossil distribution patterns in the Western Mediterranean. Proceedings of the Ocean Drilling Program Scientific Results 107: 513–533. Crossref
Russo, A. 1980. The psychrospheric coral fauna from the Lower Pliocene of the Northern Italy. Acta Paleontologica Polonica 25: 613–617.
Schäfer, W. 1972. Ecology and Palaeoecology of Marine Environments. 538 pp. University of Chicago Press, Chicago.
Sciuto, F. 2014. First report on the palaeopsychrospheric ostracod genus Nemoceratina (Pariceratina) Gründel and Kozur, 1972 (Ostracoda, Bythocytheridae) from the Quaternary of the Mediterranean basin and description of a new species. Palaeontologia Electronica 17 (3): 1–10. Crossref
Sprovieri, R. 1992. Mediterranean Pliocene biochronology: an high resolution record based on quantitative planktonic foraminifera distribution. Rivista Italiana di Paleontologia e Stratigrafia 98: 61–100.
Staudinger, M.D., McAlarney, R.J., McLellan, W.A., and Ann Pabst, D. 2014. Foraging ecology and niche overlap in pygmy (Kogia breviceps) and dwarf (Kogia sima) sperm whales from waters of the US mid-Atlantic coast. Marine Mammal Science 30: 626–655. Crossref
Swofford, D.L. 2001. PAUP* 4.0: Phylogenetic Analysis Using Parsimony (* and other methods). Sinauer Associates, Sunderland.
Thornton, S.W., McLellan, W.A., Rommel, S.A., Dillaman, R.M., Nowacek, D.P., Koopman, H.N., and Ann Pabst, D. 2015. Morphology of the nasal apparatus in pygmy (Kogia breviceps) and dwarf (K. sima) sperm whales. The Anatomical Record 298: 1301–1326. Crossref
Thunell, R.C., Williams, D.F., and Howell, M. 1987. Atlantic-Mediterranean water exchange during the late Neogene. Paleoceanography 2: 661–678. Crossref
Van Harten, D. 1984. A model of estuarine circulation in the Pliocene Mediterranean based on new ostracod evidence. Nature 312: 359–361. Crossref
Vélez-Juarbe, J., Wood, A.R., and Pimiento, C. 2016. Pygmy sperm whales (Odontoceti, Kogiidae) from the Pliocene of Florida and North Carolina. Journal of Vertebrate Paleontology 36: e1135806. Crossref
Vélez-Juarbe, J., Wood, A.R., De Gracia, C., and Hendy, A.J. 2015. Evolutionary patterns among living and fossil kogiid sperm whales: evidence from the Neogene of Central America. PLOS ONE, 10: e0123909. Crossref
Vertino, A. and Di Geronimo, S. 2003. How many Caryophyllia species lived in the Plio-Pleistocene Mediterranean? Seguenza’s taxonomy revised. Berichte des Institutes für Geologie und Paläontologie der Karl-Franzens-Universität Graz 7: 113.
Violanti, D. 2012. Pliocene Mediterranean foraminiferal biostratigraphy: a synthesis and application to the paleoenvironmental evolution of Northwestern Italy. In: Ö Elitok (ed.), Stratigraphic Analysis of Layered Deposits, 123–160. InTech [available from: http://www.intechopen.com/books/stratigraphic-analysis-of-layered-deposits/pliocene-mediterranean-foraminiferal-biostratigraphy-a-synthesis-and-application-to-the-paleoenviro; accessed on November 19, 2018] Crossref
Werth, AJ. 2004. Functional morphology of the sperm whale tongue, with reference to suction feeding. Aquatic Mammals 30: 405–418. Crossref
Werth, A.J. 2006. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. Journal of Mammalogy 87: 579–588. Crossref
West, K.L., Walker, W.A., Baird, R.W., White, W., Levine, G., Brown, E., and Schofield, D. 2009. Diet of pygmy sperm whales (Kogia breviceps) in the Hawaiian Archipelago. Marine Mammal Science 25: 931–943. Crossref
Whitmore Jr., F.C. 1994. Neogene climatic change and emergence of the modern whale fauna of the North Atlantic Ocean. In: A. Berta and T.A. Deméré (eds.), Contributions in Marine Mammals Paleontology Honoring Frank C. Whitmore Jr. Proceedings of the San Diego Society of Natural History 29: 233–227.
Whitmore Jr., F.C. and Kaltenbach, J.A. 2008. Neogene Cetacea of the Lee Creek Phosphate Mine, North Carolina. Virginia Museum of Natural History Special Publication 14: 181–269.
Acta Palaeontol. Pol. 64 (3): 609–626, 2019
https://doi.org/10.4202/app.00578.2018