


Sequence of post-moult exoskeleton hardening preserved in a trilobite mass moult assemblage from the Lower Ordovician Fezouata Konservat-Lagerstätte, Morocco
HARRIET B. DRAGE, THIJS R.A. VANDENBROUCKE, PETER VAN ROY, and ALLISON C. DALEY
Drage, H.B., Vandenbroucke, T.R.A., Van Roy, P., and Daley, A.C. 2019. Sequence of post-moult exoskeleton hardening preserved in a trilobite mass moult assemblage from the Lower Ordovician Fezouata Konservat-Lagerstätte, Morocco. Acta Palaeontologica Polonica 64 (2): 261–273.
Euarthropods have a tough exoskeleton that provides crucial protection from predation and parasitism. However, this is restrictive to growth and must be periodically moulted. The moulting sequence is well-known from extant arthropods, consisting of: (i) the long inter-moult stage, in which no changes occur to the hardened exoskeleton; (ii) the pre-moult stage where the old exoskeleton is detached and the new one secreted; (iii) exuviation, when the old exoskeleton is moulted; and (iv) the post-moult stage during which the new exoskeleton starts as soft, thin, and partially compressed and gradually hardens to the robust exoskeleton of the inter-moult stage. Trilobite fossils typically consist of inter-moult carcasses or moulted exuviae, but specimens preserving the post-moult stage are rare. Here we describe nine specimens assigned to Symphysurus ebbestadi representing the first group of contemporaneous fossils collected that preserve all key stages of the moulting process in one taxon, including the post-moult stage. They were collected from a single lens in the Tremadocian part of the Fezouata Shale Formation, Morocco. Based on cephalic displacement and comparison to other trilobite moults, one specimen appears to represent a moulted exoskeleton. Four specimens are typical inter-moult carcasses. Four others are wrinkled and flattened, with thin exoskeletons compared to inter-moult specimens, and are considered post-moult individuals. These S. ebbestadi specimens illuminate the preservation and morphology of the post-moulting stage, characterised by strong anterior-posterior exoskeleton wrinkling, as well as overall body flattening and reduced visibility of thoracic articulations. Being found in the same lens, these specimens likely represent the first preserved in-the-act mass moulting event. The displayed sequence of moulting suggests the moulting process in trilobites was comparable to modern arthropods, and conserved within euarthropod evolutionary history.
Key words: Trilobita, mass moult, soft-shelled, post-moult, moulting, exoskeleton, Ordovician, Morocco.
Harriet B. Drage [harriet.drage@zoo.ox.ac.uk], Department of Zoology, University of Oxford, South Parks Road, OX1 3PS, UK; and Oxford University Museum of Natural History, Parks Road, OX1 3PW, UK.
Thijs R.A. Vandenbroucke [thijs.vandenbroucke@ugent.be] and Peter Van Roy [Peter.VanRoy@ugent.be], Department of Geology, Ghent University, Campus Sterre, 9000 Ghent, Belgium.
Allison C. Daley [allison.daley@unil.ch], Institute of Earth Sciences, University of Lausanne, Géopolis, CH-1015 Lausanne, Switzerland.
Received 12 Decmeber 2018, accepted 18 March 2019, available online 23 May 2019.
Copyright © 2019 H.B. Drage et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Trilobites had strongly reinforced exoskeletons that protected them from predation but, like other euarthropods, needed to be moulted periodically to allow growth and development (see Daley and Drage 2016). These moults are readily preserved in the fossil record, and are particularly numerous for trilobites compared to other euarthropods because of their calcitic exoskeleton composition which, contrary to other biomineralising euarthropods, was not reabsorbed prior to moulting (Henningsmoen 1975; Daley and Drage 2016; Drage and Daley 2016; Drage et al. 2018a). The moulting sequence is inferred to be much the same as for other euarthropods (Henningsmoen 1975), including a pre-moult phase, followed by an exuviation event where the old exoskeleton is actually shed, then post-moulting expansion, biomineralisation, and hardening of the new exoskeleton (Ewer 2005). However, despite this assumption, little is known about the process of moulting in trilobites with the exception of the moment of exuviation and its associated behaviours, and the resultant moult configurations preserved in the fossil record (see Henningsmoen 1975; Whittington 1980, 1990; Daley and Drage 2016; Drage et al. 2018a, b). Only two euarthropods have been confidently considered individuals preserved during the actual process of exuviation (Garcia-Bellido and Collins 2004; Yang et al. 2019), neither representing trilobites. A specimen of the trilobite Trimerocephalus from the Upper Devonian of the Holy Cross Mountains was putatively preserved during the process of exuviation (Błażejowski et al. 2015). However, this was later shown to be two overlapping carcasses based on the morphology, configuration, and preservational context of these two individuals (Drage and Daley 2016), which are found at a locality where clusters and queues of trilobite carcasses are common (Radwański et al. 2009).
Aspects of the post-moult stage, also referred to as the soft-shell stage (Speyer and Brett 1985), have been described in respect to several trilobite species. Putative soft-shelled post-moult individuals have been described for Phacops rana milleri (Middle Devonian of Ohio, USA; Erdtmann 1978; Miller and Clarkson 1980), Phacops rana rana (Middle Devonian Hamilton Group, USA; Speyer 1983, 1987; Speyer and Brett 1985), and Greenops boothi (Hamilton Group; Speyer and Brett 1985). The P. rana subspecies were described as having a much thinner exoskeleton of a lighter colour than other trilobites with normally preserved exoskeletons found in the same bed, and their cuticles were often flattened and wrinkled. Importantly, these soft-shelled individuals were always larger in size than the clusters of other individuals found in the same beds, suggesting preservation of the post-moult stage. Miller and Clarkson (1980) cross-sectioned cuticles of suspected post-moult Phacops rana milleri individuals, which showed wrinkling and flattening, and also revealed thinner exoskeletons than their concurrent conspecific counterparts. They therefore determined that these individuals were preserved during the post-moult phase prior to hardening and thickening of the new exoskeleton, which caused wrinkling and allowed for a study of how microstructures in the cuticle and eyes changed as the exoskeleton hardened immediately after moulting. These post-moult stage specimens are extremely rare for trilobites, and have not been described for other extinct euarthropods, presumably because the individual is particularly at risk of predation owing to the softness of the new exoskeleton, and so there is evolutionary pressure to have the post-moult hardening phase as brief as possible (Henningsmoen 1975; Whittington et al. 1997). For example, lobsters occupy the post-moult stage for 5% or less of their total time because of this vulnerability (Phillips et al. 1980). In addition, post-moult individuals, displaying poor biomineralisation or sclerotisation, have a sharply reduced preservation potential, as they are essentially soft-bodied and hence require exceptional preservational circumstances to stand any chance of fossilisation. Publications on the structure of trilobite exoskeletons are limited but have important implications for understanding moulting processes. For example, trilobite taxa differ extensively in the structures and thicknesses of their exoskeletons (Teigler and Towe 1975; Fortey and Wilmot 1991), but it is unclear if this affected their moulting behaviour.
Nine specimens of Symphysurus ebbestadi (identified based on Gutiérrez-Marco et al. 2018), collected from a lens in the Lower Ordovician (upper Tremadocian) at the main level of exceptional preservation of the Fezouata Konservat-Lagerstätte, Tigzigzaouine area, Morocco (Van Roy 2006; Van Roy et al. 2010, 2015a, b; Martin et al. 2016a, b; Lefebvre et al. 2016, 2017), are described that display each of the moulting stages for trilobites (see Henningsmoen 1975). The morphology is described and compared for individuals representing a moulted exoskeleton, typical inter-moult specimens, and post-moult stage specimens. Thin sections demonstrate notable differences in exoskeleton thickness between specimens representing these stages. The material of S. ebbestadi represents a significant contribution to the trilobite fossil record of exoskeleton moulting, providing concrete indications of the preservation of post-moult stage arthropods.
Institutional abbreviations.—MGL, Museé Cantonal de Géologie Lausanne, Switzerland; YPMIZ, Yale Peabody Museum, New Haven, USA.
Geological setting
The Fezouata Shale Formation is an extensive, predominantly transgressive unit of fairly monotonous deposits of blue to green mudstone and siltstone with occasionally localised thin sandstone interbeds, ranging in age from the early Tremadocian to the late Floian. They were deposited at a high, southern sub-polar palaeolatitude (c. 65° S; Martin et al. 2016a). Historically, the Fezouata Shale was divided into the Lower (Tremadocian) and Upper (Floian) Fezouata formations, based on the occurrence of a glauconitic horizon separating the two (Destombes et al. 1985). This horizon, however, is not visible over a large part of the outcrop area, including the Ternata Plains near Zagora where the units reach their greatest thickness, and generally, they can only be distinguished based on fossil content. For this reason, Martin et al. (2016a) rejected the largely artificial division in Lower and Upper Fezouata formations, uniting both as the Fezouata Shale Formation.
The Fezouata Shale Formation provides a unique window on the Fezouata Biota, which represents the only diverse, open marine, exceptionally preserved fauna from the Ordovician (Van Roy 2006; Van Roy et al. 2010, 2015a, b). While exceptional fossil preservation was originally believed to occur throughout the Fezouata Shale Formation (Van Roy 2006; Van Roy et al. 2010), more recent work identified two specific intervals with exceptional preservation within the unit (Martin et al. 2016a). The lower interval, which is by far the most productive, is about 70 m thick and has a latest Tremadocian age (Araneograptus murrayi Biozone, c. 478–479 Ma, near the top of the Lower Fezouata Formation of Destombes et al. 1985; Gutiérrez-Marco and Martin 2016; Martin et al. 2016a). A second, c. 15 m thick interval with exceptional preservation occurs higher up, and has a mid Floian age (Gutiérrez-Marco and Martin 2016; Martin et al. 2016a). The occurrence of exceptional preservation within these intervals is not continuous, but spatially limited to lenticular bodies that may range in diameter from less than a metre to several tens of metres, and vary in thickness between less than 10 cm for the smallest lenses to over a meter for the largest bodies. They occur locally in high densities and often show marked differences in both diversity and faunal composition, even between adjacent collecting sites (Van Roy et al. 2015a). These lenses probably represent palaeo relief of the sea floor, being filled in by storm-generated sediments (Martin et al. 2016a; Vaucher et al. 2016). It was originally believed that the deposits preserving the Fezouata Biota formed in relatively deep water (Van Roy et al. 2010), but Martin et al. (2016a) and Vaucher et al. (2016) provided evidence for a relatively shallow open-marine setting around storm-wave base, representing possibly even less than 30 m of water depth (Bernard Pittet personal communication 2014). However, Gutiérrez-Marco et al. (2018) advocate a deeper water environment, based on the preserved trilobite and graptolite assemblages.
Non-biomineralised tissues in the Fezouata Biota are predominantly preserved within the mud- and siltstone as iron oxide pseudomorphs after pyrite, lending the fossils their vivid colours (Vinther et al. 2008). It seems, however, likely that organic carbon was also originally preserved, but was removed as the result of late deep weathering (Van Roy et al. 2015a). Although flattened, many specimens of non-biomineralised organisms preserved within the mudstone retain some three-dimensionality. Originally biomineralised parts have been replaced with clay minerals (Vinther et al. 2008; Van Roy et al. 2015a). In addition to the main mode of preservation in the shale, large organisms are also preserved within siliceous concretions, retaining their three-dimensionality (Van Roy and Briggs 2011; Gaines et al. 2012; Van Roy et al. 2015a, b).
Material and methods
The material for this paper (MGL 102127–102135) was collected in early 2004 from a single excavation in the Tigzigzaouine area, c. 20 km north of Zagora, Morocco (exact locality data curated with the specimens). This excavation falls within the lower c. 70 m thick level of exceptional preservation in the Fezouata Shale Formation (Araneograptus murrayi Biozone, 478–479 Ma; Martin et al. 2016a; Gutiérrez-Marco and Martin 2016). The material was briefly discussed and some of it figured by Van Roy (2006: 32–33), who considered the specimens to represent a moult sequence.
The specimens were photographed using a Leica M165C Z-Stack microscope controlled remotely by the Leica Application Suite program, and vertically stacked images were merged using Helicon Focus. Figures were produced using Adobe Illustrator and Photoshop CS6. Several specimens were photographed in parts, and these merged in Adobe Photoshop CS6 using the automated photomerge tool. Measurements of specimens were taken using digital callipers and are provided in SOM 1 (Supplementary Online Material available at http://app.pan.pl/SOM/app64-appetal_SOM.pdf). MGL 102127, 102130, 102133, and 102134 were sliced with a diamond saw through the cephalon, thorax, and pygidium. Slices were examined and photographed using an Olympus SZX10 microscope mounted with an Olympus SC50 digital camera. Exoskeletal material was distinguished from surrounding rock based on the colour of the material, and straight-line transverse measurements of exoskeletal thickness were obtained from digital photographs using ImageJ2 (Rueden et al. 2017), with measurements taken from the outer to inner layer of the exoskeleton in cross-section. For each specimen, multiple exoskeletal thickness measurements were made along the structure, producing a range of sizes.
Results
Description of specimens.—Nine complete or partial body fossils are here figured from the Fezouata Shale Formation. The specimens include five complete or mostly-complete trilobites (Fig. 1A–C, F, H), two with reasonably complete anterior halves (Fig. 1D, G), and two fragmentary specimens (Fig. 1E, I). All of the specimens share the same morphological characteristics. MGL 102128 and 102132–102134 (Fig. 1B, F–H, respectively) show incidences of wrinkling of the exoskeleton. The extent of wrinkling varies between the specimens, with MGL 102132 (Fig. 1F) being severely wrinkled, and MGL 102128 (Fig. 1B) less so. In particular, MGL 102132 has extremely prevalent wrinkling, unconfined to the lateral margins and found across the entire dorsal exoskeleton (Fig. 2A). MGL 102134 (Fig. 2B) is slightly more convex, with less obvious wrinkling in the axial portion of the pygidium, while MGL 102128 and 102133 (Fig. 2C, D, respectively) show lesser degrees of wrinkling, mostly confined to the pleural parts of the thorax, and have reasonably convex cephala. However, in all of these specimens the wrinkling is reasonably confined to the lateral (i.e., pleural) parts of the exoskeletons, usually on all tagma but strongly visible on the thoracic segments and pygidium. Wrinkles are elongated parallel to the anterior-posterior axis of the body on the thoracic segments, and curved around parallel to the posterior margin of the pygidium. MGL 102127 and 102129–102131 (Fig. 1A, C–E) show no wrinkling of the exoskeleton, and are generally very effaced with only terrace ridges. The four wrinkled specimens also show flattening of the exoskeleton in comparison to the non-wrinkled specimens. They generally have reasonably flat cephala and pygidia, as well as the axial portion of the thorax, which in the other specimens are notably convex and well-rounded (Fig. 1). This flattening also means some morphological aspects are less visible; the thoracic segments in particular are often indistinct, such as on MGL 102132 (Fig. 1F), where the articulations are less obvious than in the convex specimens.

Fig. 1. Trilobite referred to Symphysurus ebbestadi Gutiérrez-Marco, Rábano, and García-Bellido, 2018, from the early Ordovician of Morocco (Tigzigzaouine area), in dorsal views, under standard lighting. A. MGL 102127. B. MGL 102128. C. MGL 102129. D. MGL 102130; D2 close up of thorax axial rings in D1, showing the clear terrace ridges. E. MGL 102131. F. MGL 102132. G. MGL 102133. H. MGL 102134. I. MGL 102135. Scale bars 5 mm.

Fig. 2. Wrinkled specimens of trilobite Symphysurus ebbestadi Gutiérrez-Marco, Rábano, and García-Bellido 2018, from the early Ordovician of Tigzigzaouine area, Morocco, photographed under low-angle incident lighting, in order to emphasise the three-dimensional surface texture of their exoskeletons. Specimens are organised in relative order of exoskeleton hardening, from that with the most wrinkled and soft exoskeleton (A) to the least wrinkled (D) before being fully hardened. A. MGL 102132. B. MGL 102134. C. MGL 102128. D. MGL 102133. Scale bars 5 mm.
Taxonomy.—All specimens are referred to the nileid asaphid species Symphysurus ebbestadi Gutiérrez-Marco, Rábano, and García-Bellido, 2018 following the full genus description in Goldfuss (1843) and Fortey (1986), further descriptions in (Ebbestad 1999), and species description in Gutiérrez-Marco et al. (2018). Symphysurus Goldfuss, 1843 has been previously described from complete or disarticulated fossils of the Lower Ordovician Moroccan Fezouata Shale Formation (Symphysurus sp., Destombes 1967; S. angustatus, Martin et al. 2016b), and similar material has been assigned to the new and distinct species S. ebbestadi by Gutiérrez-Marco et al. (2018). The material here matches this description, with specimens being convex, with a large forwardly-expanding glabella, semi-circular eye ridges, and a high level of effacement of all features. The occipital furrow and median tubercle, the latter often used to diagnose the relevant family Nileidae and considered to function as a light-sensitive organ (Fortey and Clarkson 1976; Fortey 1986), are not obvious. The pygidium is subisopygous and semicircular. The thorax consists of eight fulcrate segments. The entire exoskeleton is covered with particularly clear terrace ridges, which vary in length and direction (Fig. 1D2), in all but the least well-preserved specimens. These occur on all tagma (in contrast to the description of Fortey 1986), but are particularly prevalent running parallel to tergite margins and on axial rings. Librigenal morphology is difficult to interpret due to the preservation of the specimens. No ventral surfaces are exposed. The cephalic : thoracic : pygidial ratio is around 1 : 1.1 : 1.3. Additional Symphysurus material collected from the Fezouata Shale Formation was assigned to S. sicardi (Bergeron 1895), but this is characterised by the near absence of terrace ridges (Gutiérrez-Marco et al. 2018), which are clearly present on the material figured here (Fig. 1D2).
Interpretation of specimens.—Eight specimens (MGL 102128–102135) appear to be carcasses. MGL 102128 displays two presumably complete trilobite individuals overlapping each other, facing opposing directions (Fig. 1B). MGL 102127 (Fig. 1A) represents a putative moulted exoskeleton. This is based on comparison of the exoskeleton configuration to other trilobite moults generally (Drage et al. 2018a), but also close relatives. For example, the suggested moults of Paciphacops figured in Rustán et al. (2011: fig. 2) consistently show an outstretched thoracopygon with the cephalon rotated backwards, with some sediment between the cephalon and thorax and a displaced doublure/hypostome. These were interpreted as moults with an anterior exuvial gape that did not quite disarticulate the entire cephalon at the dorsal part of the cephalothoracic joint. Other specimens of similar forms were also found in the Natural History Museum, London (e.g., NHMUK 58934). MGL 102127 displays the same moult configuration, with the cephalon displaced dorsally. Four of the eight carcass specimens of S. ebbestadi show ordinary exoskeleton morphology for the species, but four carcasses show the wrinkling, flattening, and indistinctiveness of features that is atypical for trilobites (MGL 102128 and 102132–102134). These four wrinkled carcasses are interpreted as “soft-shelled” trilobites (Speyer and Brett 1985), preserved during the post-moult stage in which the new exoskeleton has not fully decompressed and hardened, and during which the thin and soft exoskeleton is vulnerable to deformation.
Cuticular structure.—When viewed in transverse section and measured from outer to inner layer, cuticle thickness and structure in the cephalon (Fig. 3A1–D1) and the thorax (Fig. 3A2–D2) show differences between the moulted exuvia (Fig. 3A), the wrinkled post-moult individuals (Fig. 3C, D), and the inter-moult specimen with a fully-hardened exoskeleton (Fig. 3B). Although most fossils from the Fezouata Shale Formation have undergone some recrystallistion, replacement by clay minerals, and pyritisation (Vinther et al. 2008; Botting 2016; Gutiérrez-Marco and Martin 2016; Ortega-Hernández et al. 2016; Kouraiss et al. 2018), there is no obvious evidence for varying modes of exoskeleton recrystallisation, with the thin sections showing comparable structures between all of the S. ebbestadi specimens. Further, the specimens, having been found in the same lens, were very likely subjected to the same preservation mechanisms. Thus, their exoskeleton thicknesses should be similarly affected by taphonomic aspects like recrystallisation, allowing for an exploration of their relative differences in exoskeleton thickness.
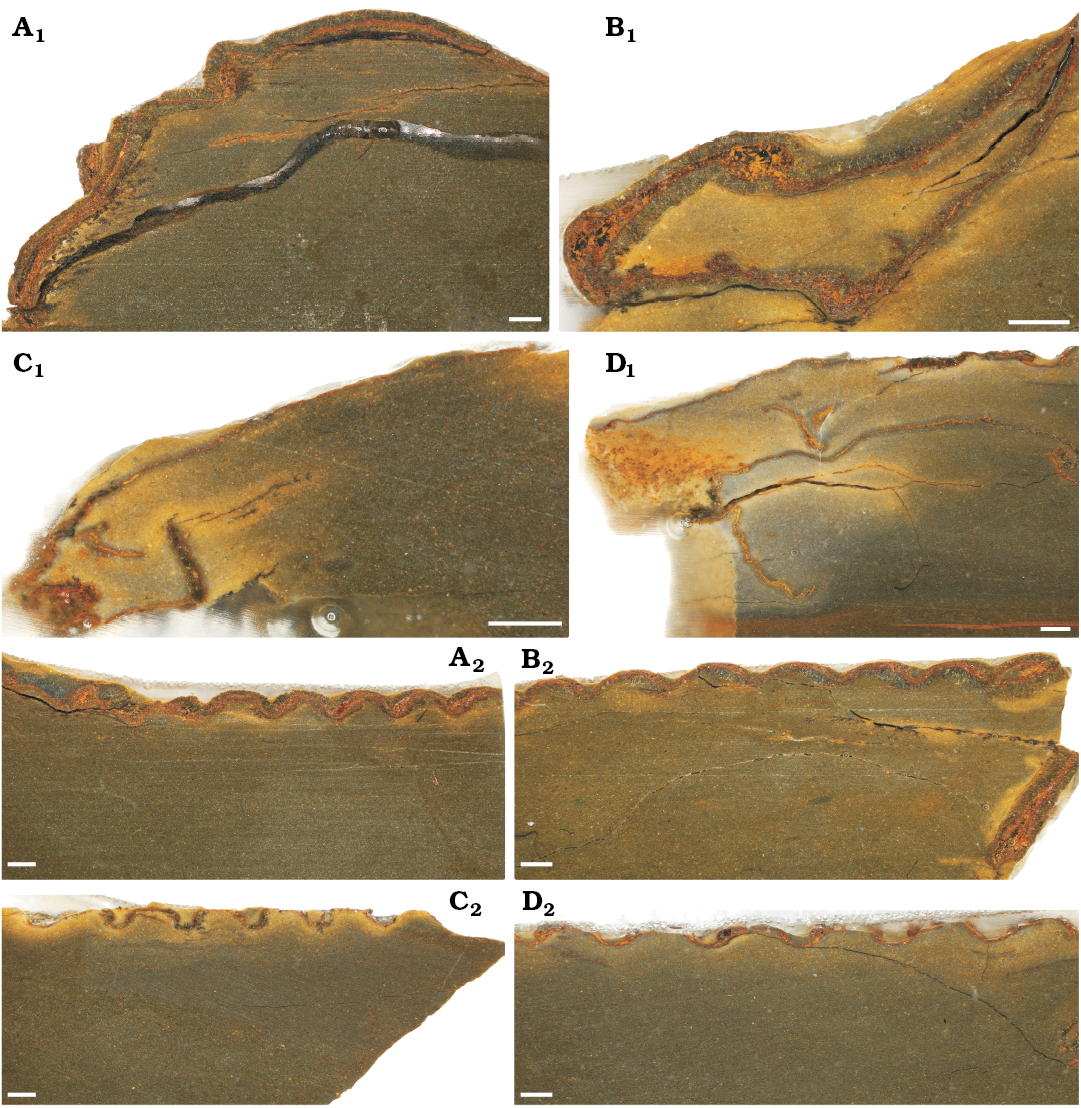
Fig. 3. Thin sections showing the cuticular structure of trilobites Symphysurus ebbestadi Gutiérrez-Marco, Rábano and García-Bellido 2018, from the early Ordovician of Tigzigzaouine area, Morocco. A. MGL 102127, the putative moult. B. MGL 102130, a fully-hardened individual. C. MGL 102133, individual with medium levels of wrinkling. D. MGL 102134, the most wrinkled individual. A1–D1, anterodorsal sections through the cephalon (except C1, transverse section); A2–D2, anterodorsal sections through the thorax. Scale bars 1 mm.
For the sections through the cephala (Fig. 3A1–D1), parts of the doublure are visible for the inter-moult specimen and the post-moult individuals (MGL 102130, 102133, and 102134), but not for the moulted exuvia (MGL 102127). The doublure may have been displaced from the cephalon by specimen disturbance after exuviation occurred, or during exuviation as suggested for Paciphacops by Rustán et al. (2011). The exoskeleton is much thicker for the inter-moult (220–830 μm) and moulted (200–760 μm) specimens (MGL 102127 and 102130) than for the wrinkled post-moult individuals (MGL 102133 and 102134; 50–170 μm and 70–240 μm, respectively), when measured from the anterior to the posterior of the cephalon (only measured from the dorsal exoskeleton layer and not the doublure; Fig. 4A). The thoracic exoskeletons of the post-moult specimens (MGL 102133 and 102134; 69–140 μm and 44–100 μm, respectively) are also noticeably thinner than the moulted exuvia (MGL 102127; 210–340 μm) and the inter-moult individual (MGL 102130; 150–270 μm; Fig. 4B). The exoskeleton of the thorax generally seems to be thinner than that of the cephalon (Fig. 4).
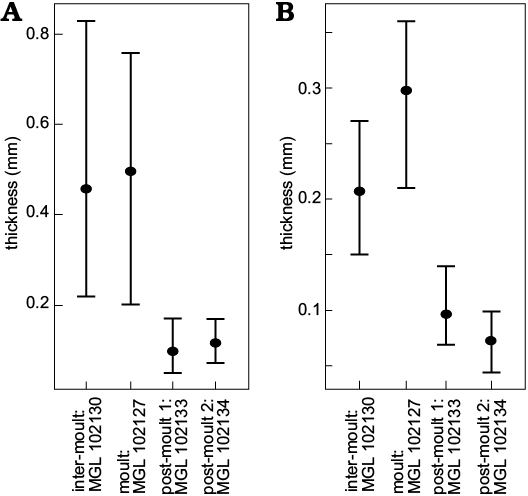
Fig. 4. Graphs showing means (points) and ranges of exoskeleton thickness for cephala (A) and thoraces (B) of the thin sectioned trilobites Symphysurus ebbestadi.
Composition of the exoskeleton also varies. All specimens had a reasonably clear orange-red layer, as well as at least one layer of grey crystalline material. The orange layer likely represents a layer of the exoskeleton that has been pyritised based on the colouration and the typical mode of preservation for Fezouata material (Van Roy et al. 2010, 2015; Gutiérrez-Marco and Martin 2016), and is apparently often seen in exoskeleton thin section (Wilmot 1990). This orange layer may represent the “prismatic” outer layer of Dalingwater et al. (1999; considered the exocuticle by Mutvei 1981), although this layer is not ubiquitous amongst trilobite taxa (Teigler and Towe 1975). The larger grey crystalline layer is likely the “principal layer” found in trilobite exoskeletons, which is always present, thickest, usually dark tan to almost black in colour (Wilmot 1990; considered the endocuticle by Mutvei 1981), and is the layer which tends to vary in thickness (Miller and Clarkson 1980). In the cephala of moulted exuvia and the inter-moult specimen (MGL 102127 and 102130), there was a grey crystalline layer either side of the orange layer (Fig. 3A1, B1), but the inside grey layer was usually lacking in the thinner exoskeletons of the post-moult specimens (MGL 102133 and 102134). The outer grey layer was also much thinner in the cephala of the post-moult specimens (MGL 102133 and 102134), although the orange layer is of reasonably comparable thickness to parts of the inter-moult individual and the moulted exuvia. It is primarily this outer grey layer that varies in thickness between the specimens. However, the level of preservation does not allow for much comparison of the crystalline structures of the two layers, but within the cephala the grey layer appears to consist of larger crystals orientated more dorso-ventrally than in the orange layer (Fig. 3A1, B1).
The thoracic exoskeleton has a similar structure to that of the cephalon, with a red-orange, presumably pyritised, layer and an outer grey layer (Fig. 3A2–D2). For the two specimens with thicker cuticles, particularly MGL 102130 (Fig. 3B2), the placement of the red layer seems to interchange with the grey layer, likely because the slice is taken at a slightly oblique angle to the thoracic segments. The specimens with thinner cuticles, particularly MGL 102133 (Fig. 3C2) seem to have a broader gap at the articulation point between the thoracic segments than the individuals with a thicker exoskeleton, which have segments with less of a gap (Fig. 3A2, B2). Slices through MGL 102133 and 102134 (Fig. 3C, D) all show flattening in cross-section of the morphological features, with both the cephala and the peaks of the thoracic segments appearing flatter than for the other specimens (Fig. 3A, B).
Discussion
Recognising trilobites at stages of the moulting process. —Comprehensive studies of trilobite moults have been provided by works such as Henningsmoen (1975), Whittington (1980), Daley and Drage (2016), and Drage et al. (2018a). Henningsmoen (1975) and Daley and Drage (2016) in particular outlined a number of criteria with which to identify trilobite moults and accurately distinguish them from carcasses. Carcasses represent individuals preserved during the long inter-moult stage. There have been very few trilobites described as having been preserved in the pre-moult or post-moult phases, with most fossils representing exuviae or inter-moult carcasses. However, in exceptional circumstances, figured trilobite specimens have been described as “soft-shelled” (Speyer and Brett 1985; or “paper-shelled”, Henningsmoen 1975), resulting from the individuals’ preservation during the short-lived post-moult phase. Derived from the few published descriptions of post-moult trilobites, several distinct criteria have been determined that identify soft-shelled trilobites.
(i) Flattening of the dorsal exoskeleton, so that it is less convex in lateral view than is usual for that taxon (Whittington 1975; Speyer and Brett 1985).
(ii) Obvious wrinkling of the cuticle (that is distinct from surface textures such as terrace ridges etc.). These are less visible in figures of the existing described “soft-shelled” trilobites (e.g., Speyer and Brett 1985: fig. 3D), but are nonetheless implicated as being a key characteristic. Exoskeletal wrinkling is clear in moulting and immediately post-moult extant euarthropod individuals, for example, the horseshoe crab in Fig. 5, which shows clear lateral wrinkling of the head shield (also see Fig. 2; Błażejowski et al. 2015: fig. 3).
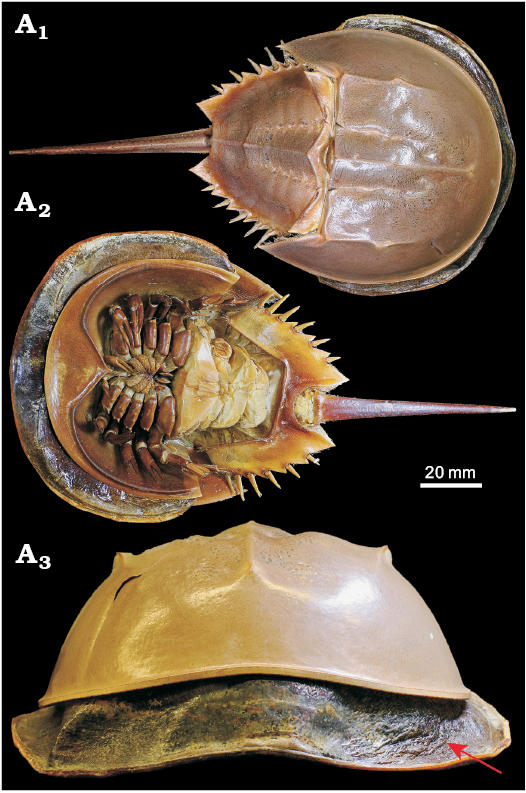
Fig. 5. Mid-moult specimen of Limulus polyphemus Linnaeus, 1758 (YPMIZ 55597), Recent, USA, in dorsal (A1), ventral (A2), and anterior (A3) views. The moult is fully hardened and shows a robust convex exoskeleton, whereas the carcass partially emerged but trapped within the old exoskeleton shows extensive lateral wrinkling of its new exoskeleton (arrowed). Photo Russell Bicknell.
(iii) Indistinct features of the dorsal exoskeleton. For example, structures such as the occipital ring, axial rings, or furrows of the rachis are less well defined than is usual for that species (e.g., Speyer and Brett 1985; Speyer 1987: fig. 7E).
(iv) Associated arthropod material within the same bed, ideally trilobites of the same species, which do not show criteria i–iii; i.e., putative soft-shelled trilobites are found in direct association with specimens that are normally convex, with no wrinkling, and distinct morphological features (Miller and Clarkson 1980). This rules out taphonomic causes of exoskeletal deformation.
(v) In addition, thin sectioning may show these putative soft-shelled trilobites have thinner exoskeletons than the “normal” trilobite carcasses found in association with them (Figs. 3, 4; Miller and Clarkson 1980; Mutvei 1981; Speyer and Brett 1985), possibly showing a reduced “principal layer”.
These features are interpreted as resulting from preservation prior to decompression and expansion of the new larger exoskeleton following moulting from the smaller restrictive one (producing wrinkling), and potentially deformation during burial prior to hardening (and biomineralisation) of the new soft exoskeleton (flattening and indistinct features). In comparison, the moulting horseshoe crab in Fig. 5 shows that the larger exoskeleton is clearly soft and flexible in order to emerge from the restrictive old exoskeleton, with the wrinkles disappearing when the new exoskeleton decompresses and hardens. Owing to the short-lived nature of moulting events, very few trilobite individuals have been described as preserved in the immediate post-moult phase. Previously described putative soft-shelled post-moult individuals (Erdtmann 1978; Miller and Clarkson 1980; Speyer 1983, 1987; Speyer and Brett 1985) also generally match the five criteria listed above, with wrinkling and flattening resulting from recent moulting. In all existing descriptions of thin-shelled, wrinkled, and flattened trilobites the individuals co-occur contemporaneously with other individuals of the same species that lack these features (Speyer 1987). It is this contextual information that allows for a supported interpretation of a freshly-moulted soft-shell trilobite. Further examples of contemporaneous undeformed arthropods (i.e., with no wrinkling or flattening) allow us to confidently exclude a non-biological cause for the occurrence of criteria i–iii. In addition to the biological causes discussed above, wrinkling of post-moult specimens may be further accentuated by burial and associated deformation of the particularly soft and thin new exoskeleton, and not from otherwise unrelated tectonic deformation (again, when also found in association with non-wrinkled contemporary specimens). Further, compaction deformation more broadly in trilobites tends to occur as brittle deformation rather than plastic (Webster and Hughes 1999), and thus a soft-shell exoskeleton would usually be necessary for wrinkling to be related to burial.
The exoskeletons of the two non-wrinkled sectioned specimens (the putative moult, MGL 102127, Figs. 1A, 3A; and one inter-moult specimen, MGL 102130, Figs. 1D, 3B) are comparatively thicker than in most studies of trilobite exoskeleton structure. For example, the exoskeleton was between 220–300 μm in Homalonotus rhinotropis (Dalingwater et al. 1999), and up to 500 μm in Phacops rana milleri (Miller and Clarkson 1980). Fortey and Wilmot (1991) found a huge spectrum in trilobite exoskeleton thicknesses, with a 40-fold difference depending on palaeoenvironment as nearshore taxa had notably thick exoskeletons and those offshore being much thinner. This correlation between environment and exoskeletal thickness is also paralleled in extant arthropods, with the desert scorpion Hadrurus arizonensis having a cuticle that is about twice as thick as that of Euscorpius italicus, which inhabits less arid regions (Pavan 1958; Filshie and Hadley 1979; Hadley and Filshie 1979; Hjelle 1990). The thinnest trilobite exoskeleton found by Fortey and Wilmot (1991) was of the cranidium of Svalbardites at 8 μm, although the thickest was 350 μm, with those <60 μm being considered “thin”, and those >130 μm “thick”. In contrast, the two thicker specimens studied here (Fig. 3A1, B1) gave measures upwards of 700–800 μm in the cephalon, therefore seeming comparably thicker. These are the putative moult and inter-moult specimens, which did not particularly differ in the ranges of their exoskeleton thicknesses (200–760 μm and 150–830 μm, respectively; Fig. 4). Mutvei (1981) also found that moults and carcasses did not differ and could not be distinguished at the exoskeletal level (see also Brandt 1993). This is supported by other studies finding no evidence for exoskeleton reabsorption pre-moulting, as is found in most crustaceans (Miller and Clarkson 1980; Feldmann and Tshudy 1987; Speyer 1987). However, the two sectioned wrinkled specimens described here (MGL 102133 and 102134) not only have much thinner exoskeletons than MGL 102127 and 102130, but are comparably thinner than described for other trilobites. These were between 50–170 μm (Figs. 1G, 3C, 4) and 44–240 μm (Figs. 1H, 3D, 4), which puts their minima (and particularly their thoracic exoskeletal widths) within the “thin” category of Fortey and Wilmot (1991).
Studies of the relative effects of diagenesis on thin section ultrastructure of trilobite cuticles are scarce (Whittington et al. 1997), and this hinders comparisons between the specimens of S. ebbestadi figured here with other trilobite species invariably preserved under different taphonomic regimes (e.g., Fortey and Wilmot 1991; Dalingwater et al. 1999). For example, the middle orange-coloured layer of the exoskeleton (likely to be pyritic as for other Fezouata biota; Gutiérrez-Marco and Martin 2016) and the grey layers (potentially formed by replacement of calcite with clay minerals, as for the calcitic plates of Plumulites bengtsoni; Vinther et al. 2008), may have undergone associated thickness changes during pyritisation and clay-replacement which, while occurring to all specimens figured here and so not affecting comparisons between them, may affect comparisons with the other trilobite species from different localities discussed above (that have not undergone these preservational modes). Similarly, the outer cuticle layer of the S. ebbestadi specimens may be thinner than expected owing to the ubiquitous weathering found in the Fezouata Shale Formation (Gutiérrez-Marco and Martin 2016). Further analysis of the effects of different preservational regimes on the thickness and ultrastructure of trilobite exoskeletons would be invaluable to future studies of exoskeleton moulting and taphonomy.
A complete trilobite moulting sequence from the Fezouata Shale Formation.—The nine specimens of S. ebbestadi represent the first full exoskeleton moulting sequence for a trilobite species. Arthropods spend most of their time in the inter-moult stage, between the relatively rapid moulting events, and thus individuals are preserved during this time in the vast majority of cases (with the exception of moulted exoskeletons, which are found in abundance for trilobites; Daley and Drage 2016). The S. ebbestadi specimens include one putative moult (Fig. 1A; similar to the configuration described by Rustán et al. 2011), four carcasses of standard inter-moult interval individuals ( (Figs. 1C–E, I; typical convex, effaced, and with no wrinkling), and four carcasses preserved during the post-moult soft-shell stage (Fig. 1B, F–H). The last of these are identifiable based on their previously described characteristics, in particular the strong lateral wrinkling of tagma (Fig. 2), and the notably thinner exoskeletons as measured in two of these specimens (Figs. 3, 4) compared to the putative moult and an inter-moult specimen. Thus, the nine contemporaneous specimens of S. ebbestadi collected from the same lens, contain all identifiable stages of the moulting process: a moulted exoskeleton, individuals in the long inter-moult stage, and individuals preserved immediately post-moulting. Pre-moult stage preserved carcasses will be almost impossible to distinguish, as this stage is also particularly short-lived, and characterised by the detachment of the old exoskeleton from the new cuticle, which would be extremely difficult to identify even in thin section of exceptionally preserved individuals.
Of the putative post-moult specimens, the degree of lateral wrinkling and flattening varies. MGL 102132 has extremely prevalent wrinkling (Fig. 2A), MGL 102134 (Fig. 2B) is slightly more convex with less obvious wrinkling, and MGL 102128 and 102133 (Fig. 2C, D, respectively) show lesser degrees of wrinkling still. Further, the thoracic cuticle thickness of MGL 102134 (Fig. 2B) is slightly thinner than that of the less wrinkled MGL 102133 (Fig. 2D), with their ranges being 44–100 μm for MGL 102134 (Figs. 2B, 3D) and 69–140 μm for MGL 102133 (Figs. 2D, 3C). Their cephalic thicknesses, however, were similar (50–170 μm for MGL 102133 and 70–240 μm for MGL 102134), but both were notably thinner than for the non-post-moult individuals (Figs. 3, 4). The differences in the degree of the post-moult criteria outlined above suggest that these specimens varied in how susceptible their exoskeletons were to compression during preservation, and how much they had undergone post-moult inflation of the new exoskeleton. This therefore suggests that the four post-moult specimens were preserved at different points of exoskeleton hardening after different amounts of time had passed following exuviation (also considered in the case of specimens of Phacops rana milleri described by Miller and Clarkson 1980).
The collection of individuals from all stages of the moulting process from the same lens of the Fezouata Shale Formation suggests a mass moulting event that may have been preserved during-the-act. Specifically, the presence of variously hardened post-moult trilobites together suggests that the mass moulting event was ongoing. Mass moulting is a behaviour commonly seen in modern arthropods (e.g., Tarling and Cuzin-Roudy 2003; Haug et al. 2013), with its origins seemingly very early in the fossil record of Arthropoda (Daley and Drage 2016) and clear mass moult assemblages having been described for various trilobite groups (Speyer and Brett 1985; Karim and Westrop 2002; Paterson et al. 2007; Ebbestad et al. 2013). This appears to be a protective behaviour, and has been suggested to accompany mating events in some arthropods (Speyer and Brett 1985; Braddy 2001; Vrazo and Braddy 2011, although see Tetlie et al. 2008). However, all so-far described trilobite mass moults have been preserved following cessation of the event. This means that typically only exuviae are found, as the recently-moulted individuals have moved away from the preserved accumulation. An exception is the carcass congregations of Phacops rana rana described by Speyer and Brett (1985), which are interpreted as having been preserved after a mass-moulting event, except in this case the freshly moulted individuals are suggested to have remained in place following moulting for reproduction. The Fezouata S. ebbestadi specimens, in contrast, originated from the same lens, with post-moult and pre-moult individuals co-occurring with at least one moulted exoskeleton. This represents the first occurrence of a probable trilobite mass moulting event preserved in-the-act, rather than post-event when soft-shelled individuals would not be present. Understanding the process and occurrence of mass moulting events in the fossil record is key to our knowledge of euarthropod behaviour. Mass moulting has distinct advantages in modern groups, and may therefore indicate similar ecological causes in the fossil record, for example an increased prevalence of predation within the locality.
Moulting sequence in extinct euarthropods is analogous to extant groups.—The description of the S. ebbestadi specimens as representing a post-moult sequence of hardening preserved during a mass moulting event adds to a growing body of evidence that the process of exoskeleton moulting has been strongly conserved throughout euarthropod evolutionary history, not just associated behaviours like mass moulting (e.g., Haug et al. 2013; Daley and Drage 2016). Although thin section work on trilobite exoskeletons has suggested a number of differences in structure to that of modern arthropods (which also vary themselves; see Krishnan 1953; Dalingwater 1973; Dalingwater et al. 1999), the results presented here support consistency in the biochemical and mechanical processes of moulting throughout euarthropod evolution. Modern arthropods follow a well-known sequence of pre-moult preparation, ecdysis, and post-moult inflation and hardening before returning to the long inter-moult stage (Skinner 1962; Ewer 2005). The specimens of S. ebbestadi found contemporaneously represent each of these stages (although the pre-moult stage may be impossible to identify due to preservation). This strongly suggests, more than previous studies in which only singular post-moult trilobites were figured (Erdtmann 1978; Miller and Clarkson 1980; Mutvei 1981; Speyer and Brett 1985), that trilobites followed the same sequence of moulting described in modern arthropods, as hypothesised by earlier workers (Henningsmoen 1975). The existence of a comparable moulting sequence between Early Ordovician trilobites and modern arthropods argues that the process of moulting is strongly conserved within euarthropods and has changed little over more than 400 million years of evolution. This is reasonable given the unparalleled importance, and riskiness, of exoskeleton moulting in the life histories of all euarthropods (Dalingwater et al. 1999; Daley and Drage 2016). Identification of specimens displaying the full moulting sequence in stem euarthropods would allow for further validation of the moulting process being conserved throughout Euarthropoda. The results do, however, support earlier evidence that unlike modern crustaceans, which also have thick calcitic exoskeletons, reabsorption of the exoskeletal material had not yet evolved. To date, no evidence has supported reabsorption prior to moulting in trilobites, with trilobite moults and inter-moult carcasses having comparable exoskeleton thicknesses (Miller and Clarkson 1980; Mutvei 1981; Feldmann and Tshudy 1987; Speyer 1987; Brandt 1993), while this is not the case in extant arthropods that show exoskeleton reabsorption (Ewer 2005; Nijhout 2013). The reasons for this are unclear, as the recurrent production of calcitic exoskeletons would have been extremely energetically expensive, and thus reabsorption is critically important in modern crustaceans (Skinner 1962; Luquet and Marin 2004). However, evolutionary uncertainties such as this highlight the need for further analysis of both primary and diagenetic cuticular ultrastructures in thin sections of early euarthropod exoskeletons (Whittington et al. 1997).
Conclusions
Nine figured trilobite specimens collected from a lens of late Tremadocian age in the Fezouata Shale Formation, Morocco, are assigned to Symphysurus ebbestadi based on a recent description by Gutiérrez-Marco et al. (2018). These are considered to reflect all described stages of the exoskeleton moulting process. One specimen is a putative moulted exoskeleton with the cephalon displaced dorsally and no visible doublure. Several others show morphology typical of the species, with a notably convex and effaced dorsal exoskeleton, and are therefore considered to represent the inter-moult stage, as for most trilobite carcasses (Henningsmoen 1975; Daley and Drage 2016). Four specimens represent the short-lived post-moult stage, indicated by the presence of characteristics associated in the literature with deformation following burial and compression of the new soft exoskeletons (wrinkling, flattening, less distinct features). Thin sections of two post-moult specimens, the putative moult, and an inter-moult specimen show notable differences in exoskeleton thickness, with the former two being much thinner in both the cephalon and thorax than the latter two. The discovery of these specimens within the same lens suggests that they likely represent preservation of an in-progress mass moulting event. The specimens of S. ebbestadi also provide more detail on the morphological characteristics of post-moult trilobites, giving an important example for the future identification of post-moult extinct arthropods and the study of the fossil record of moulting, and suggest the sequence of moulting has been conserved in the evolutionary history of euarthropods.
Acknowledgements
Claudia Baumgartner (University of Lausanne, Switzerland) provided assistance with preparing sections of the specimens. Lourdes Rojas (YPMIZ) provided assistance with YPMIZ specimens, and Russell Bicknell (University of New England, Armidale, Australia) photographed the figured horseshoe crab specimen from the YPMIZ. We would also like to gratefully thank two reviewers, Błażej Błażejowski (Institute of Paleobiology, PAS, Warsaw, Poland) and Oldřich Fatka (Charles University, Prague, Czech Republic), for their comments on revising this manuscript. HBD is supported by a scholarship from the NERC Doctoral Training Partnership (NE/L002612/1). ACD received financial support from the Oxford University Museum of Natural History.
References
Bergeron, J. 1895. Notes paleontologiques. Crustaces. Bulletin de la Societe Geologique de France 23: 465–481.
Błażejowski, B., Gieszcz, P., Brett, C.E., and Binkowski, M. 2015. A moment from before 365 Ma frozen in time and space. Scientific Reports 5: 1–5. Crossref
Botting, J.P. 2016. Diversity and ecology of sponges in the Early Ordovician Fezouata Biota, Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 75–86. Crossref
Braddy, S.J. 2001. Eurypterid palaeoecology: palaeobiological, ichnological and comparative evidence for a “mass-moult-mate” hypothesis. Palaeogeography, Palaeoclimatology, Palaeoecology 172: 115–132. Crossref
Brandt, D.S. 1993. Ecydsis in Flexicalymene meeki (Trilobita). Journal of Paleontology 67: 999–1005. Crossref
Daley, A.C. and Drage, H.B. 2016. The fossil record of ecdysis, and trends in the moulting behaviour of trilobites. Arthropod Structure and Development 45: 71–96. Crossref
Dalingwater, J.E. 1973. Trilobite cuticle microstructure and composition. Palaeontology 16: 827–839.
Dalingwater, J.E., Siveter, D.E., and Mutvei, H. 1999. Cuticular microstructure of some Silurian homalonotid trilobites from Sweden. Journal of Paleontology 73: 256–262. Crossref
Destombes, J. 1967. Distribution et affinités des genres de trilobites de l’Ordovicien de l’Anti-Atlas (Maroc). Compte rendu sommaire des séances de la Société géologique de France 4: 133–134.
Destombes, J., Hollard, H., and Willefert, S. 1985. Lower Paleozoic rocks of Morocco. In: C.H. Holland (ed.), Lower Paleozoic of North-Western and West-Central Africa, 91–336. Wiley, New York.
Drage, H.B. and Daley, A.C. 2016. Recognising moulting behaviour in trilobites by examining morphology, development and preservation: Comment on Błażejowski et al. 2015. BioEssays 38: 981–990. Crossref
Drage, H.B., Holmes, J.D., García-Bellido, D.C., and Daley, A.C. 2018a. An exceptional record of Cambrian trilobite moulting behaviour preserved in the Emu Bay Shale, South Australia. Lethaia 51: 473–492.
Drage, H.B., Laibl, L., and Budil, P. 2018b. Post-embryonic development of Dalmanitina, and the evolution of facial suture fusion in Phacopina. Paleobiology 44: 638–659.
Ebbestad, J.O.R. 1999. Trilobites of the Tremadoc Bjørkäsholmen Formation in the Oslo Region, Norway. Fossils and Strata 47: 1–118.
Ebbestad, J.O.R., Rushton, A.W.A., Stein, M., and Weidner, T. 2013. A paradoxidid moult ensemble from the Cambrian of Sweden. GFF 135: 18–29. Crossref
Erdtmann, B.-D. 1978. Microstructure of “paper shell” stage (post-ecdysial cuticle) of the Middle Devonian trilobite Phacops rana from Silica Shale of Ohio. Geological Society of America, Abstracts with Programs 10: 252.
Ewer, J. 2005. How the ecdysozoan shed its coat. PLoS Biology 3: 1696–1699. Crossref
Feldmann, R. and Tschudy, D. 1987. Ultrastructure in cuticle from Hoploparia stokesi (Decapoda: Nepropidae) from the Lopez de Bertodano Formation (Late Cretaceous–Paleocene) of Seymour Island, Antarctica. Journal of Paleontology 61: 1194–1203. Crossref
Filshie, B.K. and Hadley, N.F. 1979. Fine structure of the cuticle of the desert scorpion, Hadrurus arizonensis. Tissue and Cell 11: 249–262. Crossref
Fortey, R.A. 1986. The type species of the Ordovician trilobite Symphysurus: systematics, functional morphology and terrace ridges. Paläontologische Zeitschrift 60: 255–275. Crossref
Fortey, R.A. and Clarkson, E.N.K. 1976. The function of the glabellar “tubercle” in Nileus and other trilobites. Lethaia 9: 101–106. Crossref
Fortey, R.A. and Wilmot, N. 1991. Trilobite cuticle thicknesses in relation to palaeoenvironment. Paläontologische Zeitschrift 65: 141–151. Crossref
Gaines, R.R., Briggs, D.E.G., Orr, P.J., and Van Roy, P. 2012. Preservation of giant anomalocaridids in silica-chlorite concretions from the Early Ordovician of Morocco. Palaios 27: 317–325. Crossref
García-Bellido, D.C. and Collins, D.H. 2004. Moulting arthropod caught in the act. Nature 429: 40. Crossref
Goldfuss, A. 1843. Systematische Übersicht der Trilobiten und Beschreibung einiger neuer Arten derselben. Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 1843: 537–567.
Gutiérrez-Marco, J.C. and Martin, É.L.O. 2016. Biostratigraphy and palaeoecology of Lower Ordovician graptolites from the Fezouata Shale (Moroccan Anti-Atlas). Palaeogeography, Palaeoclimatology, Palaeoecology 460: 35–49. Crossref
Gutiérrez-Marco, J.C., Rábano, I., and García-Bellido, D.C. 2018. The nileid trilobite Symphysurus from upper Tremadocian strata of the Moroccan Anti-Atlas: taxonomic reappraisal and palaeoenvironmental implications. Lethaia [published online: https://doi.org/10.1111/let.12297]. Crossref
Hadley, N.F. and Filshie, B.K. 1979. Fine structure of the epicuticle of the desert scorpion, Hadrurus arizonensis, with reference to the location of lipids. Tissue and Cell 11: 263–275. Crossref
Haug, J.T., Caron, J.-B., and Haug, C. 2013. Demecology in the Cambrian: synchronized molting in arthropods from the Burgess Shale. BMC Biology 11: 1–10. Crossref
Henningsmoen, G. 1975. Moulting in trilobites. Fossils and Strata 4: 179–200.
Hjelle, J.T. 1990. Anatomy and morphology. In: G.A. Polis (ed.), The Biology of Scorpions, 9–63. Stanford University Press, Stanford.
Karim, T. and Westrop, S.R. 2002. Taphonomy and palaeoecology of Ordovician trilobite clusters, Bromide Formation, South-central Oklahoma. Palaios 17: 394–402. Crossref
Kouraiss, K., El Hariri, K., El Albani, A., Azizi, A., Mazurier, A., and Vannier, J. 2018. X-ray microtomography applied to fossils preserved in compression: Palaeoscolescid worms from the Lower Ordovician Fezouata Shale. Palaeogeography, Palaeoclimatology, Palaeoecology 508: 48–58. Crossref
Krishnan, G. 1953. On the cuticle of the scorpion Palamneus swammerdami. Quarterly Journal of Microscopical Science 94: 11–21.
Lefebvre, B., El Hariri, K., Lerosey-Aubril, R., Servais, T., and Van Roy, P. 2016. The Fezouata Shale (Lower Ordovician, Anti-Atlas, Morocco): a historical review. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 7–23.
Lefebvre, B., Gutiérrez-Marco, J.C., Lehnert, O., Nowak, H., Akodad, M., El Hariri, K., and Servais, T. 2017. Age calibration of the Lower Ordovician Fezouata Lagerstätte (Morocco). Lethaia 51: 296–311. Crossref
Liaennus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Salvius, Stockholm. Crossref
Luquet, G. and Marin, F. 2004. Biomineralisations in crustaceans: storage strategies. Comptes Rendus Palevol 3: 515–534. Crossref
Martin, É.L.O., Pittet, B., Gutiérrez-Marco, J.C., El Hariri, K.H., Lerosey-Aubril, R., Masrour, M., Servais, T., Vandenbroucke, T.R.A., Vannier, J., Van Roy, P., Vaucher, R., and Lefebvre, B. 2016a. Age and environmental setting of the Lower Ordovician Fezouata Biota (Zagora, Morocco). Gondwana Research 34: 274–283. Crossref
Martin, É.L.O., Vidal, M., Vizcaïno, D., Vaucher, R., Sansjofre, P., Lefebvre, B., and Destombes, J. 2016b. Biostratigraphic and palaeonvironmental controls on the trilobite associations from the Lower Ordovician Fezouata Shale of the Central Anti-Atlas, Morocco. Palaeogeography, Palaeoclimatology, Palaeoecology 460: 142–154. Crossref
Miller, J. and Clarkson, E.N.K. 1980. The post-ecdysial development of the cuticle and the eye of the Devonian trilobite Phacops rana milleri Stewart 1927. Philosophical Transactions of the Royal Society of London B 288: 461–480. Crossref
Mutvei, H. 1981. Exoskeletal structure in the Ordovician trilobite Flexicalymene. Lethaia 14: 225–234. Crossref
Nijhout, H.F. 2013. Arthropod developmental endocrinology. In: A. Minelli, G. Boxshall, and G. Fusco (eds.), Arthropod Biology and Evolution: Molecules, Development, Morphology, 123–148. Springer-Verlag, Berlin. Crossref
Ortega-Hernández, J., Van Roy, P., and Lerosey-Aubril, R. 2016. A new aglaspidid euarthropod with a six-segmented trunk from the Lower Ordovician Fezouata Konservat-Lagerstätte, Morocco. Geological Magazine 153: 524–536. Crossref
Paterson, J.R., Jago, J.B., Brock, G.A., and Gehling, J.G. 2007. Taphonomy and palaeoecology of the emuellid trilobite Balcoracania dailyi (Early Cambrian, South Australia. Palaeogeography, Palaeoclimatology, Palaeoecology 249: 302–321. Crossref
Pavan, M. 1958. Studi sugli Scorpioni, IV, Sulla birifrangenza e sella fluorescenza dell’epicuticola. Bolletino della Società entomologica italiana 87: 23–26.
Phillips, B.F., Cobb, J.S., and George, R.W. 1980. General biology. In: J.S. Cobb and B.F. Phillips (eds.), The Biology and Management of Lobsters, Volume 1: Physiology and Behavior, 2–72. Academic Press, Inc., New York. Crossref
Radwański, A., Kin, A., and Radwańska, U. 2009. Queues of blind phacopid trilobites Trimerocephalus: a case of frozen behaviour of Early Famennian age from the Holy Cross Mountains, Central Poland. Acta Geologica Polonica 59: 459–81.
Rueden, C.T., Schindelin, J., Hiner, M.C., Dezonia, B.E., Walter, A.E., Arena, E.T., and Eliceiri, K.W. 2017. ImageJ2: ImageJ for the next generation of scientific image data BMC Bioinformatics 18: 529. Crossref
Rustán, J.J., Balseiro, D., Waisfeld, B., Foglia, R.D., and Vaccari, N.E. 2011. Infaunal molting in Trilobita and escalatory responses against predation. Geology 39: 495–498. Crossref
Skinner, D.M. 1962. The structure and metabolism of a crustacean integumentary tissue during a molt cycle. Biological Bulletin 123: 635–647. Crossref
Speyer, S.E. 1983. Trilobite Clustering in the Hamilton Group of New York State. 113 pp. M.Sc. Thesis, University of Rochester, Rochester.
Speyer, S.E. 1987. Comparative taphonomy and palaeoecology of trilobite Lagerstätten. Alcheringa 11: 205–232. Crossref
Speyer, S.E. and Brett, C.E. 1985. Clustered trilobite assemblages in the Middle Devonian Hamilton Group. Lethaia 18: 85–103. Crossref
Tarling, G.A. and Cuzin-Roudy, J. 2003. Synchronization in the molting and spawning activity of northern krill (Meganyctiphanes norvegica) and its effect on recruitment. Limnology and Oceanography 48: 2020–2033. Crossref
Teigler, D.J. and Towe, K.M. 1975. Microstructure and composition of the trilobite exoskeleton. Fossils and Strata 4: 137–149.
Tetlie, O.E., Brandt, D.S., and Briggs, D.E.G. 2008. Ecdysis in sea scorpions (Chelicerata: Eurypterida). Palaeogeography, Palaeoclimatology, Palaeoecology 265: 182–194. Crossref
Van Roy, P. 2006. Non-trilobite Arthropods from the Ordovician of Morocco. xxvii, 230 pp. Unpublished Ph.D. Dissertation, Ghent University, Ghent.
Van Roy, P. and Briggs, D.E.G. 2011. A giant Ordovician anomalocaridid. Nature 473: 510–513. Crossref
Van Roy, P., Briggs, D.E.G., and Gaines, R.R. 2015a. The Fezouata fossils of Morocco – an extraordinary record of marine life in the Ordovician. Journal of the Geological Society 172: 541–549. Crossref
Van Roy, P. Daley, A.C., and Briggs, D.E.G. 2015b. Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps. Nature 522: 77–80. Crossref
Van Roy, P., Orr, P.J., Botting, J.P., Muir, L.A., Vinther, J., Lefebvre, B., El Hariri, K.H., and Briggs, D.E.G. 2010. Ordovician faunas of Burgess Shale type. Nature 365: 215–218. Crossref
Vaucher, R., Martin, É.L.O, Hormière, H., and Pittet, B. 2016. A genetic link between Konzentrat- and Konservat-Lagerstätten in the Fezouata Shale (Lower Ordovician, Morocco). Palaeogeography, Palaeoclimatology, Palaeoecology 460: 24–34. Crossref
Vinther, J., Van Roy, P., and Briggs, D.E.G. 2008. Machaeridians are Palaeozoic armoured annelids. Nature 451: 185–188. Crossref
Vrazo, M.B. and Braddy, S.J. 2011. Testing the “mass-moult-mate” hypothesis of eurypterid palaeoecology. Palaeogeography, Palaeoclimatology, Palaeoecology 311: 63–73. Crossref
Webster, M. and Hughes, N.C. 1999. Compaction-related deformation in Cambrian olenelloid trilobites and its implications for fossil morphometry. Journal of Paleontology 73: 355–371. Crossref
Whittington, H.B. 1975. Trilobites with appendages from the Middle Cambrian, Burgess Shale, British Columbia. Fossils and Strata 4: 97–136.
Whittington, H.B. 1980. Exoskeleton, moult stage, appendage morphology, and habits of the middle Cambrian trilobite Olenoides serratus. Palaeontology 23: 171–204.
Whittington, H.B. 1990. Articulation and exuviation in Cambrian trilobites. Philosophical Transactions of the Royal Society of London B 329: 27–46. Crossref
Whittington, H.B., Chatterton, B.D.E., Speyer, S.E., Fortey, R.A., Owens, R.M., Chang, W.T., Dean, W.T., Jell, P.A., Laurie, J.R., Palmer, A.R., Repina, L.N., Rushton, A.W.A., Shergold, J.H., Clarkson, E.N.K., Wilmot, N.V., and Kelly, S.R.A. 1997. Treatise on Invertebrate Paleontology, Part O: Trilobita, revised. The Geological Society of America, Inc., Boulder & University of Kansas, Lawrence.
Wilmot, N.V. 1990. Primary and diagenetic microstructures in trilobite exoskeletons. Historical Biology 4: 51–65. Crossref
Yang, J., Ortega-Hernández, J., Drage, H.B., Du, K.-S., and Zhang, X.-G. 2019. Ecdysis in a stem-group euarthropod from the early Cambrian of China. Scientific Reports [published online: https://doi.org/10.1038/s41598-019-41911-w]. Crossref
Acta Palaeontol. Pol. 64 (2): 261–273, 2019
https://doi.org/10.4202/app.00582.2018