

The first identification of fossil Mesophyllum in accordance to the modern taxonomic concepts in coralline algae
JURAJ HRABOVSKÝ, DANIELA BASSO, and GIOVANNI COLETTI
Hrabovský, J., Basso, D., and Coletti, G. 2019. The first identification of fossil Mesophyllum in accordance to the modern taxonomic concepts in coralline algae. Acta Palaeontologica Polonica 64 (4): 897–909.
Despite their common occurrence, the potential of coralline algae is not yet fully exploited in paleoecological reconstructions. The reasons are mainly grounded in the taxonomic inconsistency caused by poor preservation or insufficient knowledge of the type material of many species, and confusion derived from the difficult recognition of the coralline three-phased life cycle in the fossil record. Specimens of fossil coralline algae from newly collected samples, and historical Schaleková’s collection of middle Miocene Paratethyan limestone were studied under optical and scanning electron microscopes, revealing the occurrence of the asexuate, male gametangial, and carposporangial conceptacles of Mesophyllum crassiusculum here documented for the first time. Based on the recent emendation of Mesophyllum and consequent circumscription of the genera Mesophyllum sensu stricto and Melyvonnea, this is the first and oldest finding of a fossil Mesophyllum sensu stricto. Moreover, we provide further evidence of the preservation potential of important diagnostic characters, such as the shape of epithallial and subepithallial cells, the shape of the conceptacle roofs, the number and shape of pore canals lining cells in the multiporate roof of the asexuate conceptacle chambers. The identification of M. crassiusculum in the middle Miocene of central Paratethys would deserve further biogeographic and paleoclimatic considerations that, however, are prevented by the incomplete exploration of the Paratethyan fossil record and the need of revision of other important type collections of closely related species.
Key words: Rhodophyta, Corallinophycidae, Mesophyllum, life cycle phases, taxonomy, Miocene, Slovakia.
Juraj Hrabovský [geoljuhr@savba.sk], Earth Science Institute of the Slovak Academy of Sciences, Dúbravská cesta 9, 840 05 Bratislava, Slovakia.
Daniela Basso [daniela.basso@unimib.it] and Giovanni Coletti [g.coletti@campus.unimib.it], Department of Earth and Environmental Sciences, University of Milano-Bicocca, P.za della Scienza 4, 20126 Milano, Italy.
Received 21 January 2019, accepted 10 April 2019, available online 31 July 2019.
Copyright © 2019 J. Hrabovský et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Coralline algae often dominate Miocene limestone beds from the central Paratethys. Corals, bryozoans, mollusks, polychaetes are less common but locally they can be the dominant components of the biofacies (Pisera 1985; Studencki 1988a; Riegl and Piller 2000; Doláková et al. 2008; Sremac et al. 2016). Skeletal assemblages and facies of these “Corallinacean Limestones” show similarities with both those of the modern Mediterranean and Red Sea (Seneš and Ondrejčíková 1991; Basso 1998; Riegl and Piller 2000; Basso et al. 2008) suggesting a complex depositional environment spanning potentially through different climatic zones (Seneš 1988, 1990; Seneš and Ondrejčíková 1991; Baráth 1992; Baráth et al. 1994; Pisera 1996; Riegl and Piller 2000). For the purpose of the paleoenvironmental analysis of these coralline-dominated limestone beds, the study of the algal assemblage (including its taxonomic composition at the lowest possible rank but also the distribution and the growth form of the different taxa) is fundamental (Basso 1998; Basso et al. 2008; Coletti et al. 2018).
Middle Miocene coralline algae from the central Paratethys were investigated mostly in the second half of the 20th century (Maslov 1956; Schaleková 1962, 1969, 1973, 1978; Schaleková and Čierna 1985; Golonka 1981; Pisera 1985; Studencki 1988b; Zdražílková 1988; Pisera and Studencki 1989). Since then, coralline taxonomy went through major changes and new diagnostic characters were introduced, affecting the taxonomy of some of the most common algae of these deposits with Mesophyllum as a good example (Studencki 1988a, 1999; Doláková et al. 2008; Sremac et al. 2016). Paleophycologists who first studied the corallines of the central Paratethys followed Lemoine’s (1928) diagnosis for Mesophyllum, based on the occurrence of multiporate sporangial conceptacles and coaxially arranged ventral core filaments (= hypothallus). Presently, coaxial hypothallus and multiporate asexuate conceptacles are known to occur also in Synarthrophyton patena (Hooker and Harvey, 1849) Townsend, 1979 (Townsend 1979; Woelkerling and Harvey 1992), and may also occur uncommonly in some areas of the thallus of some species of Lithothamnion Heydrich, 1897, Phymatolithon Foslie, 1898 and Clathromorphum Foslie, 1898 (Keats et al. 2000; Basso et al. 2004; Merwe and Maneveldt 2014). Therefore, the definition of Mesophyllum was emended, incorporating reproductive characteristics of the male conceptacles (= Mesophyllum sensu lato; Adey 1970), thus allowing for a clear separation from Synarthrophyton (Lebendik 1978; Woelkerling and Harvey 1992). More recently, two new genera, Capensia Athanasiadis, 2017 and Melyvonnea Athanasiadis and Ballantine, 2014 were established upon the study and critical revision of a large number of type collections of modern Mesophyllum sensu lato species (Athanasiadis and Ballantine 2014; Athanasiadis 2017). The latter revision resulted in a profound further update of Mesophyllum diagnosis (= Mesophyllum sensu stricto; Athanasiadis and Ballantine 2014) that presently includes the morphology of the asexuate conceptacle pore-canal lining cells (PCLC), the cystocarpic conceptacles morphology, dioecy, growth form and habitat (Athanasiadis 1999, 2001, 2007; Athanasiadis and Ballantine 2014; Athansiadis 2017). The morphology of PCLC provides an important element to discriminate Mesophyllum sensu stricto from Synarthrophyton and Melyvonnea (Athanasiadis 2001; Athanasiadis and Ballantine 2014). Capensia fucorum (Esper 1796) Athanasiadis 2017 is the only species of the genus and contrary to Mesophyllum, is a hemiparasitic species (Athanasiadis 2017).
There are almost no data on either, gametangial conceptacles and PCLC of fossil Mesophyllum, with the exception of Mastrorilli (1968) and Coletti et al. (2018) for gametangial conceptacles and Hrabovský et al. (2015) for carposporangial conceptacles and pore lining cells. Therefore, the fossil records of Mesophyllum needs revision, in order to verify possible misidentifications of genus attribution after the recent diagnostic update (Athanasiadis 2017).
Recent researches proved that carposporangial and gametangial conceptacles are more common than previously believed, and features like the PCLC can be preserved in the fossil record (Basso et al. 1996; Braga et al. 2015; Hrabovský et al. 2015; Chelaru and Bucur 2016; Coletti et al. 2016). In this paper, we use for the first time the modern diagnosis of Mesophyllum, based on the above mentioned characters, for the identification of its middle Miocene representatives from the central Paratethys (Slovakia).
Institutional abbreviations.—AHFH, University of Southern California, Los Angeles, USA; NHM, Natural History Museum, Bratislava, Slovakia; TRH, Norwegian University of Science and Technology, Trondheim, Norway.
Other abbreviations.—D, diameter; H, height; L, length; PCLC, pore canal lining cells; SD, standard deviation; TS, thin section(s).
Material and methods
The following features, listed in the modern definition of Mesophyllum (Athanasiadis and Ballantine 2014) and possessing some fossilization potential, have been investigated: (i) predominantly coaxially arranged hypothallus (= ventral core filaments); (ii) single rectangular, generally flattened or rounded, but not flared epithallial cell; (iii) subepithallial meristematic cells mostly as long as or longer than cells immediately subtending them; (iv) multiporate sporangial conceptacles; (v) filaments lining the pore canals generally composed of more than 5 cells, similar, slightly elongated or smaller in length than other roof cells; (vi) sporangial pore canal lining cells thinner/wider than other roof cells; (vii) dioecious gametophytes; (viii) type of male conceptacle development (sensu Johansen 1981); (ix) carposporangial conceptacles with central pedestal; (x) predominantly encrusting growth form with simple and unbranched protuberances (Lebednik 1978; Woelkerling and Harvey 1992, 1993; Athanasiadis et al. 2004; Athanasiadis 2007; Athanasiadis and Ballantine 2014). Growth form description follows Woelkerling et al. (1993).
All studied specimens are stored in Natural History Museum. Specimens from two different collections were described. The first includes newly collected samples from Modrý Majer (nine thin sections, two acetate peels, and three polished cuts for SEM study). Bi/tetrasporophyte NHM B1857/1a and male gametophyte NHM B1857/1b were identified in TS 918-1 and a carpogonial-carposporangial plant NHM B1858 was identified in TS 918-7. The counterpart cut of TS 918-1, including tetrasporophyte NHM B1857/2a and gametophyte NHM B1857/2b, was observed under SEM. The Schaleková Collection includes tens of thin sections, twenty-seven of them come from Modrý Majer, but they do not contain M. crassiusculum. We found the species in TS IIIb455 collected in Kosihovce under the name Mesophyllum galettoi Mastrorilli, 1968 (Schaleková 1978; Schaleková and Čierna 1985). Specimen from Schaleková collection (Schaleková and Čierna 1985) is labelled NHM B1859.
Light Microscope AXIOZEISS scope A1 was used in analyses of TS. Figures were provided by AXIOCAM 105 Color. Morphometry was done in AxioVision Microscopy Software. Counterparts were studied with Scanning Electron Microscope JEOL JSM-6390LV. Preparation of the counterparts followed a method modified from Braga et al. (1993). Differently from Braga et al. (1993) the best results were obtained with 1% HCl and 40–60 seconds of etching. Addition of 2% HCl caused net dissolution of the surfaces.
Geological settings
Limestone samples were collected from a site known as Modrý Majer (Schaleková 1978), which is located in Belá Hills, in the vicinity of the Belá village, about 4 km NW from Štúrovo town, 6 km SE from the Gbelce town, and south from the spot height Veľký vrch (251 m a.s.l.) (Fig. 1). The site was previously studied by Seneš (1963) and Schaleková (1978) but it was already known from Matějka (1949). Belá Hills are located in the Southeastern Danubian Basin. The basin sedimentary record starts with the “Kiscellian” Formation deposited in the Želiezovce depression (Klučiar et al. 2016). The Bajtava, Špačince, and Pozba formations were deposited above the “Kiscellian” Formation during the Badenian (Klučiar et al. 2016). Upper Karpatian and lower Badenian terrestrial deposits locally lie directly on pre-Neogene basement (Kováč 2000). The hills are built by products of lower Badenian andesitic volcanoes above which algal reefs of the Špačince and Pozba formations developed (Schaleková 1978; Schaleková and Čierna 1985; Klučiar et al. 2016; Kováč et al. 2018). At the top there are clay, sand, conglomerate, organodetrital limestone, and tuffaceous clay beds of the Sarmatian Vráble Formation. Slopes and low-lands are filled by the Pliocene Volkov Formation and by Quaternary deposits.
Algal limestone developed during both lower and upper Badenian, thus, stratigraphically, they cover the whole Badenian including Bajtava, Špačince, and Pozba formations (Hók et al. 1999; Vass 2002; Klučiar et al. 2016; Kováč et al. 2018). A more accurate stratigraphical positioning of studied limestone beds should require a detailed micropaleontological research that is out of the scope of this study. However, some additional considerations are necessary. Klučiar et al. (2016) documented algal reefs in the lower Badenian Špačince Formation of the Danubian Basin, and formerly placed in the now abandoned “middle” Badenian or Wielichian substage. Vass (2002) proposed that coralline algal bioconstructions developed on the Levice Horst elevations during the upper Badenian and included them into the Pozba Formation, which was previously considered as “middle” to “upper Badenian” by Hók et al. (1999). Limestone is not marked on the geological map of the scale 1:50 000 although they were known since Matějka (1949) fieldwork. Their stratigraphic relationship with volcanic and volcanoclastic units gives further information. Coral and coralline algal assemblages occur above lower Badenian andesites and are covered by upper Badenian (12.56±0.1 Ma) volcanoclastics. Locally the limestone deposits are dated with nannoplankton to the upper Badenian. Therefore it seems to be highly probable that studied limestone beds belong to the upper Badenian Pozba Formation (personal communication Samuel Rybár 2018).
Outcropping limestone bed is about 1 meter thick and covers an area of about 50 square meters. The bed lacks a siliciclastic fraction and is dominated by corals and coralline algae, gastropods are less common (Schaleková 1978). Schaleková (1978) described also the presence of encrusting foraminifers, benthic foraminifers, serpulids, and ostracods. In the thin sections, echinoid spines and bryozoan colonies were observed as well. Coralline algae are represented by discoid-shaped rhodoliths with warty-protuberant to wavy-laminar growth morphology as well as encrusting growth-forms developing over the small patches of corals and over corals fragments.
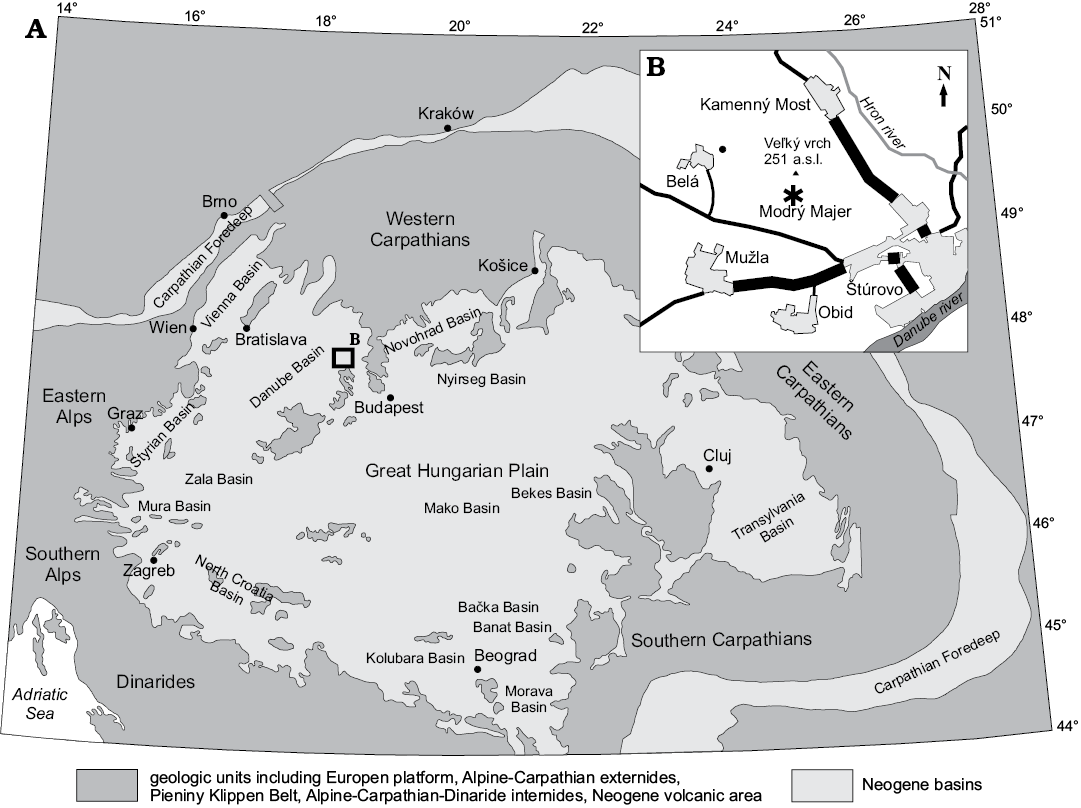
Fig. 1. The recent distribution of the central Paratethyan deposits with the location of the studied site. A. The central Paratethys basins (modified from Kováč 2000). B. Map of the study area, asterisk indicates sampling site Modrý Majer.
Systematic palaeontology
Phylum Rhodophyta Wettstein, 1901
Subphylum Eurhodophytina Saunders and Hommersand, 2004
Class Florideophyceae Cronquist, 1960
Subclass Corallinophycidae Le Gall and Saunders, 2007
Order Hapalidiales Nelson, Sutherland, Farr, and Yoon in Nelson et al., 2015
Family Mesophyllaceae Athanasiadis, 2016
Genus Mesophyllum Lemoine, 1928
Type species: Mesophyllum lichenoides (Ellis, 1768) Lemoine, 1928; Cornwall, England, Recent.
Mesophyllum crassiusculum (Foslie, 1902) Lebednik, 2004
Figs. 2–4.
1985 Mesophyllum galettoi Mastrorilli, 1968; Schaleková and Čierna 1985: 42.
Material.— Species was identified in the two thin sections NHM B1857/1a and NHM B1857/1b, and their counterparts studied on SEM NHM B1857/2a and NHM B1857/2b. One specimen was detcted in Schaleková collection (Schaleková and Čierna 1985)—thin section NHM B1858.
Description.—The species develops non-protuberant rhodoliths or encrusts other coralline algae or corals (Fig. 2A). The core of the rhodoliths usually consists of corals but carposporangial-carpogonial plants also encrust other fruticose protuberant coralline algae (e.g., Phymatolithon calcareum; Fig. 2B). Growth form is encrusting without prominent or branched protuberances. Applanate branches locally develop (Fig. 2C1). Two short warty protuberances 0.5×0.5 mm were also detected. Thickness of the superimposed thalli could reach few mm. Individual lamellae are 0.2–2 mm thick (Table 1).
Table 1. Basic morpho-anatomical characteristics of fossil asexuate, male and carposporangial plants of Mesophyllum crassiusculum. Measurements are in μm when not indicated otherwise. Abbreviations: D, diameter; L, length; n, number of filaments; “+” presence and “–” absence character; “±” intermediate.
| |
Bi/tetrasporophyte |
Male gametophyte |
Carposporangial conceptacles |
|
Growth form |
encrusting |
encrusting |
encrusting |
|
± applanate branches |
± applanate branches |
± applanate branches |
|
|
short unbranched protuberances |
short unbranched protuberances |
short unbranched protuberances |
|
|
Thallus thickness |
0.2–1.4 mm |
0.4–1.5 mm |
0.3–2 mm |
|
Hypothallus |
|||
|
thickness, filaments (n) |
43–146, n = 8–22 |
45–185, n = 7–26 |
47–149, n = 6–27 |
|
arrangement of filaments |
coaxial in patches |
coaxial in patches |
coaxial in patches |
|
lateral fusion of cells |
+ |
+ |
+ |
|
hypothallial cells: L, D |
11–26, 5–11 |
10–29, 5–10 |
8–25, 4–8 |
|
hypothallial cells L/D ratio |
2–4 |
2–4 |
2–4 |
|
Perithallus |
|||
|
thickness |
121–401 |
237–485 |
110–870 |
|
pattern of zonation |
2–5 long vs. 1–2 short |
2–5 long vs. 1–2 short |
2–5 long vs. 1–2 short |
|
cell fusions |
+ |
+ |
+ |
|
perithallial cells: L, D |
4–16, 4–11 |
5–15, 4–10 |
6–17, 4–10 |
|
perithallial cells L/D ratio |
0.5–2 |
0.5–3 |
0.5–3 |
|
Meristematic cells |
|||
|
shape |
elongated |
elongated |
elongated |
|
meristematic cells: L, D |
8–12, 6–8 |
7–10, 6–7 |
8–10, 6 |
|
meristematic cells L/D ratio |
1–2 |
1–2 |
1–2 |
|
Epithallial cells |
|||
|
cells (n) |
1–2 |
1–2 |
1 |
|
epithallial cells: L, D |
3–5, 5–7 |
4–5, 5–7 |
3, 4–6 |
|
epithallial cells L/D ratio |
rounded, flattened |
rounded, flattened |
rounded |
|
trichocytes |
– |
– |
– |
Thallus is pseudoparenchymatous with dorsiventral organization, consisting of a single system of branching filaments (monomerous construction). Filaments of the hypothallus run in parallel with the substrate and can be arranged either coaxially or non-coaxially (Fig. 2C2). In the asexuate plants the hypothallus is 43–146 μm thick (Table 1). The hypothallial cells (n = 38) are 11–26 μm long (mean ± SD, 18±4 μm) and 5–11 μm in diameter (mean ± SD, 7±1.2 μm). Hypothallus filaments bend upward toward thallus surface and develop the peripheral portion of the thallus (perithallus). Cells (n = 180) are 4–16 μm long (mean ± SD, 9±2.3 μm) and 4–11 μm in diameter (mean ± SD, 7±1.5 μm) (Table 1). Cells within single filaments show cyclic variation in length. Sets of 2–5 long cells are alternated with 1–2 short cells. Such pattern results in irregular growth zones (Fig. 2C1). Cells are laterally joined by cell fusions (Fig. 2C3). Epithallial cells (n = 7) are rounded or flattened but not flared, measuring 3–4 μm in length (mean ± SD, 3±0.5 μm) and 5–7 μm in diameter (mean ± SD, 6±0.8 μm) (Fig. 2C4). One or two cells are present above meristematic cells. Meristematic cells (n = 12) are as long or longer than cells immediately subtending them and measure 8–12 μm in length (mean ± SD, 10±1.5 μm) and 6–8 μm in diameter (mean ± SD, 7±0.6 μm) (Fig. 2C4). The size of the vegetative characters observed in the sexuate plants are largely overlapping with those of the asexuate plants (Table 1).
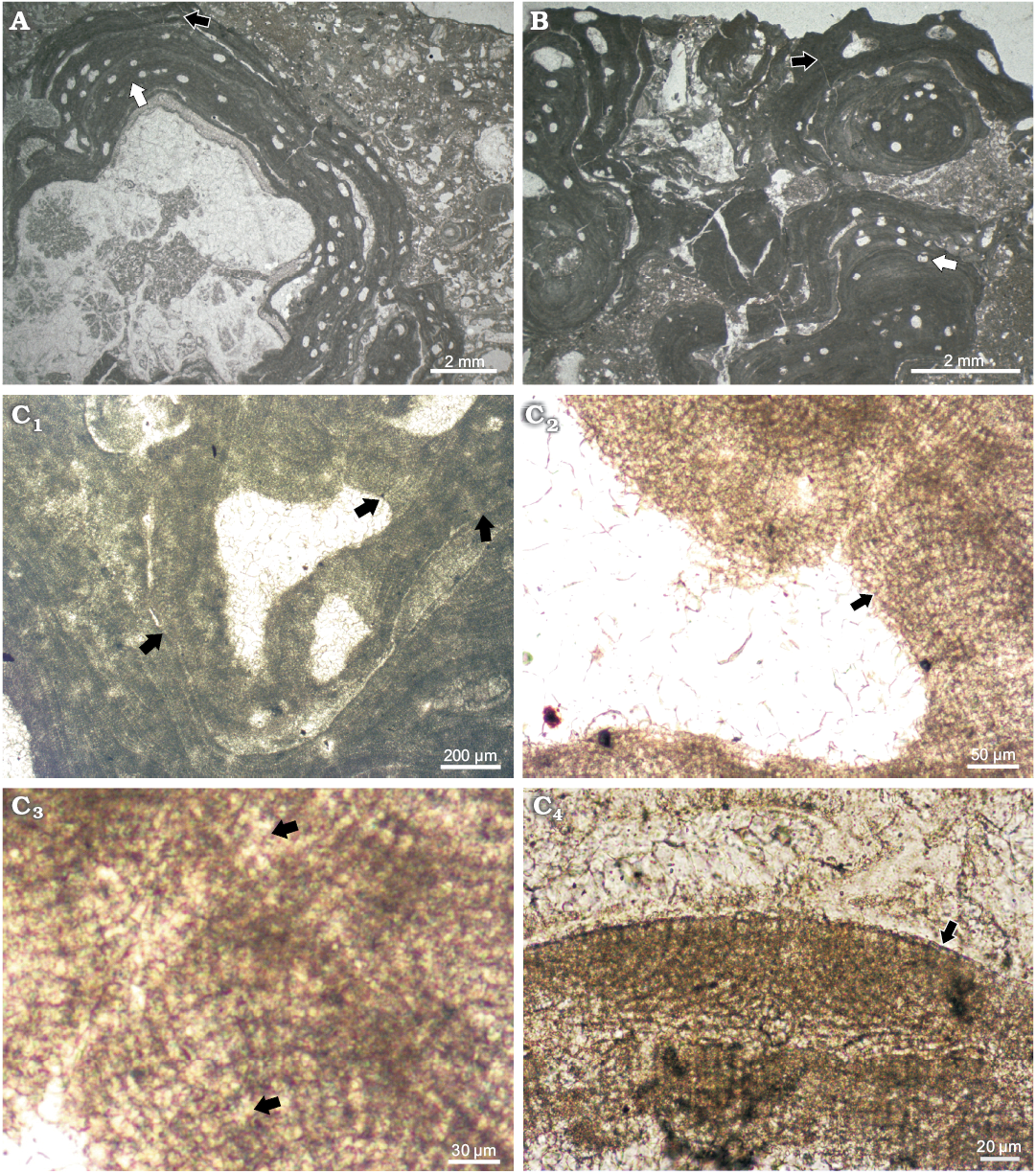
Fig. 2. Corallinae alga Mesophyllum crassiusculum (Foslie, 1902) Lebednik, 2004 from early Serravallian, Miocene, Modrý Majer, Slovakia. A. Bi/tetrasporophyte and male gametophyte, NHM B1857/1a and NHM B1857/1b (TS 918-1), respectively. An asexuate (tetra/bisporangial) plant (white arrow) overgrows a fragment of scleractinian coral colony. Growth form of coralline alga is encrusting. Male gametophyte overgrows tetrasporophyte (black arrow). B. Carposporophyte, NHM B1858 (TS 918-7). Carpogonial-carposporangial plant of M. crassiusculum (black arrow) overgrows a protuberant rhodolith of Phymatolithon calcareum (Pallas, 1766) (white arrow). Growth form of M. crassiusculum is encrusting and without protuberances. C. Bi/tetrasporophyte, NHM B1857/1a (TS 918-1). C1. Applanately branching thallus (arrows). C2. Pseudoparenchymatous thallus with coaxially to non-coaxially arranged hypothallus. Arrow points to the portion where coaxial hypothallus is best visible. C3. Magnified portion of the thallus from C2, arrows point to cell fusions in hypothallus and in the perithallus. C4. Flattened epithallial cells above meristematic cells located at the top of the embedded conceptacle.
Gametophyte encrusts tetrasporophyte. However, where margins of the two thalli meet, multiple overgrowths may occur. Presumed gametangial male conceptacle is of the type 1 (Johansen 1981) and the mode of projection complies with those of Mesophyllum and Lithothamnion (according to Lebednik 1978) (Fig. 3A). In fact, in Mesophyllum and Lithothamnion the initiation of the male conceptacle development takes place just below the meristematic cells in contrary to Phymatolithon in which initiation takes place deep in the vegetative filaments, thus male conceptacle is not prominent in the latter (Lebednik 1978: 393, 394, text-figs. 8–9, 11). Male conceptacles (n = 7) measure 185–310 μm in diameter (mean ± SD, 254±63.7 μm) and 42–60 μm high (mean ± SD, 51±9.1 μm). Pore canal is 77–108 μm long (mean ± SD, 89±16.4 μm) and up to 54 μm broad (Table 1). Some conceptacles are filled with dark material of uncertain origin (Fig. 3B).
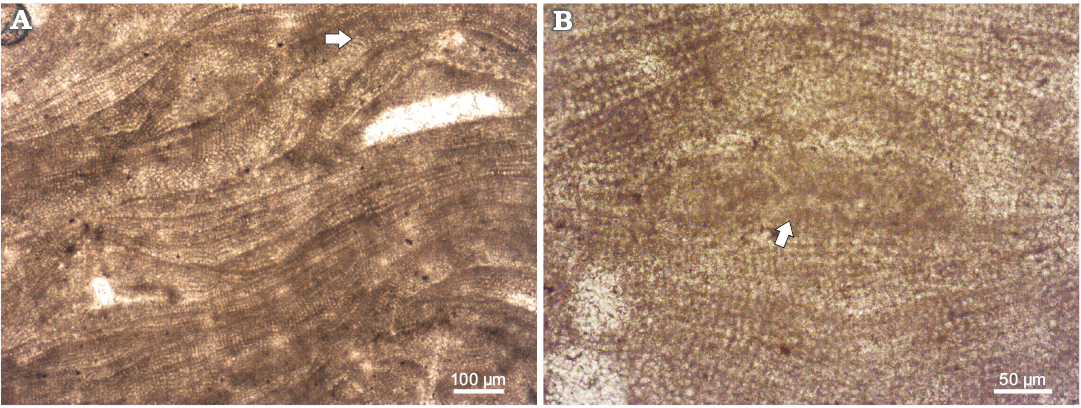
Fig. 3. Coralline alga Mesophyllum crassiusculum (Foslie, 1902) Lebednik, 2004 from early Serravallian, Miocene, Modrý Majer, Slovakia, male gametophyte, NHM B1857/1b (TS 918-1). A. Conceptacles of the type 1 (Johansen, 1981) were protruding above thallus surface during their maturity. Note the coaxial arrangment of the hypothallus; arrow points to the coaxial hypothallus. B. Conceptacle filled with material of unknown origin (arrow).
Carposporangial conceptacles are large and occur on other plant than male conceptacles (Fig. 4A). The presence of male and carposporangial conceptacles on distinct plants suggests that gametophytes are most likely dioecious. Chambers (n = 4) are dumbbell shaped, 519–689 μm in diameter (mean ± SD, 604±119.9 μm) and 155–229 μm in height (mean ± SD, 192±52.3 μm) measured aside of a well-developed central pedestal (H2 according Basso et al. 1996) (Fig. 3A, Table 1). Pore length is 108–178 μm (mean ± SD, 143±49.6 μm) and its width is 112–143 μm (mean ± SD, 127±22.6 μm).
Multiporate sporangial conceptacles are rounded to oval (Fig. 4B1). Conceptacles protrude above the thallus surface and are overgrown by thallus growth from the sides of the conceptacle. Chambers (n = 7) are 179–418 μm in diameter (mean ± SD, 326±59.2 μm) and 90–199 μm high (mean ± SD, 171±16.3 μm). D/H ratio is 1.6–2.4. The roofs are 42–69 μm thick and are formed by 6–9 cells that tend to be shorter towards the apex (Fig. 4B2, C1; Table 1). Pore canals are mostly lined by cells similar in size and shape to the other roof cells (Fig. 4B2, C2). Locally specialized thinner cells were observed (Fig. 4B2, C2). At least 6 cells lining each pore canal were observed under SEM (Fig. 4B2). Pore canal cells thinner than those of the roof were observed also in the thin section (Fig. 4C2). At the top of the pore canals epithallial cells and PCLC appear at the same level, indicating that rosette cells around the pore canal are not sunken (Fig. 4B1). Conceptacles are embedded and locally filled with adventitious cells (Fig. 4C3). Remains of these cells are present in some chambers (Fig. 4C1). The roof is either flat or convex, with no peripheral rim and sunken pore plates. Old conceptacles become buried when covered by successive thallus growth (Fig. 4C3).
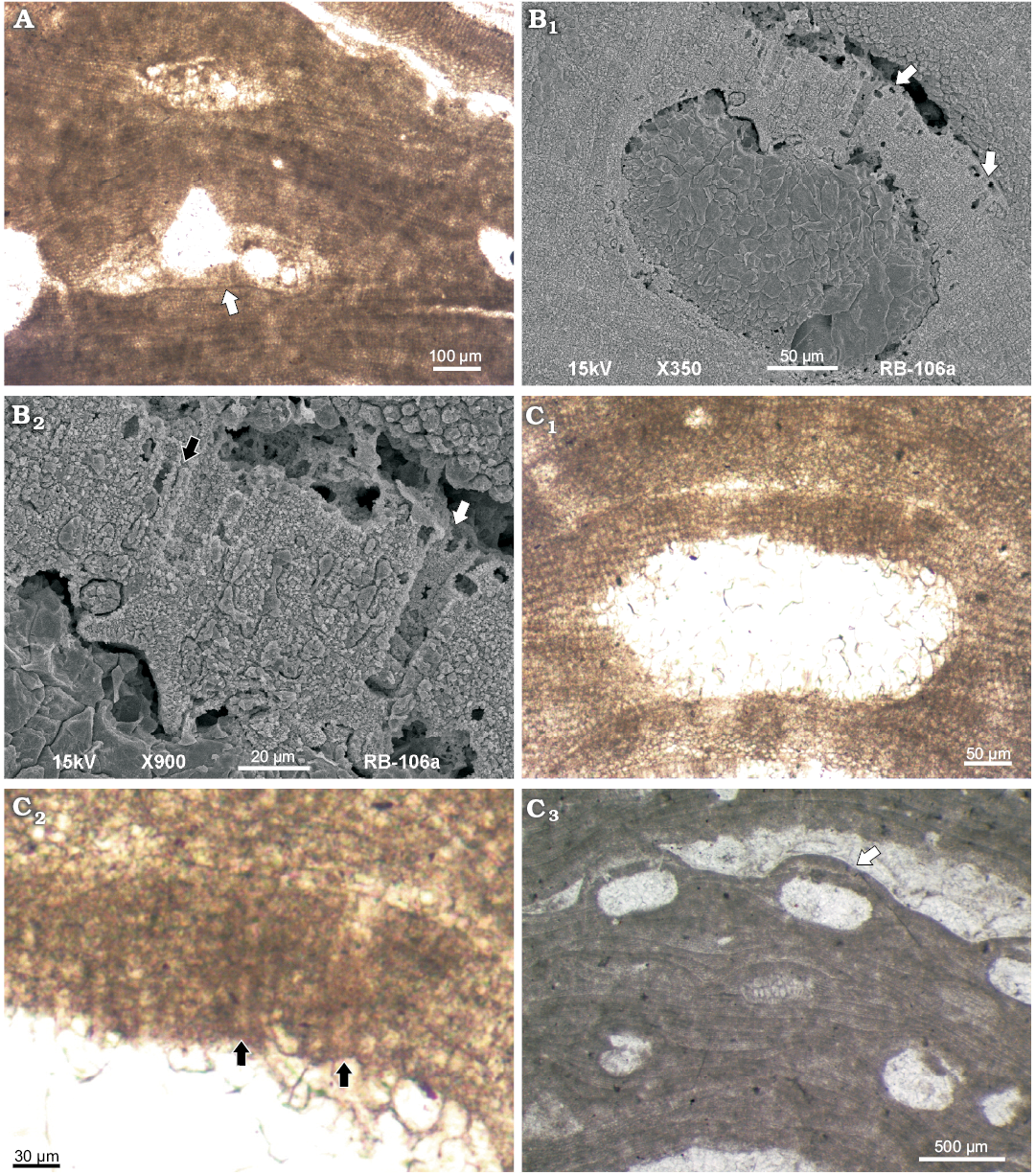
Fig. 4. Coralline alga Mesophyllum crassiusculum (Foslie, 1902) Lebednik, 2004 from early Serravallian, Miocene, Modrý Majer, Slovakia. A. Carposporophyte, NHM B1858 (TS 918-7), carposporangial conceptacle with central pedestal (arrow). B. Bi/tetrasporophyte, NHM B1857/2a (counterpart of the TS 918-1). B1. Multiporate sporangial conceptacle, arrows point to the rounded epithallial cells located at the top of conceptacle roof. Rounded cell at the margin of the pore canal marks the surface of the roof. Adjacent pore canal cells are therefore considered as rosette cells. Note that rosette cells are not sunken. B2. Asexuate conceptacle with roof filaments and pore canal filaments consisting of at least 6 cells, arrows point to thinner (black arrow) and wider (white arrow) pore canal cells. C. Bi/tetrasporophyte, NHM B1857/1a (TS 918-1). C1. Asexuate multiporate conceptacle with roof filaments consisting of up to 7 cells. C2. Detail of C1 with pore lining cells (arrows), the cells are same or wider (top of the pore canal) than adjacent roof cells. C3. Embedded asexuate conceptacles (arrow). Note chambers filed with adventitious cells. The roofs are convex to flat, lacking peripheral rim.
Remarks.—We have identified M. crassiusculum from the newly collected middle Miocene material. The asexuate (bi/tetrasporangial) specimens fully correspond with the type (Athanasiadis et al. 2004; Athanasiadis 2007). During the revision of the historical collection of Anna Schaleková we have identified one specimen of M. crassiusculum formerly described as M. galettoi (Schaleková and Čierna 1985). This specimen matches with both, Modrý Majer specimen and M. crassiusculum type (Fig. 5, Table 2).
Stratigraphic and geographic range.—Early Langhian of the central Paratethys (this work) to Recent of the Pacific Ocean (Athanasiadis et al. 2004; Athanasiadis and Ballantine 2014).
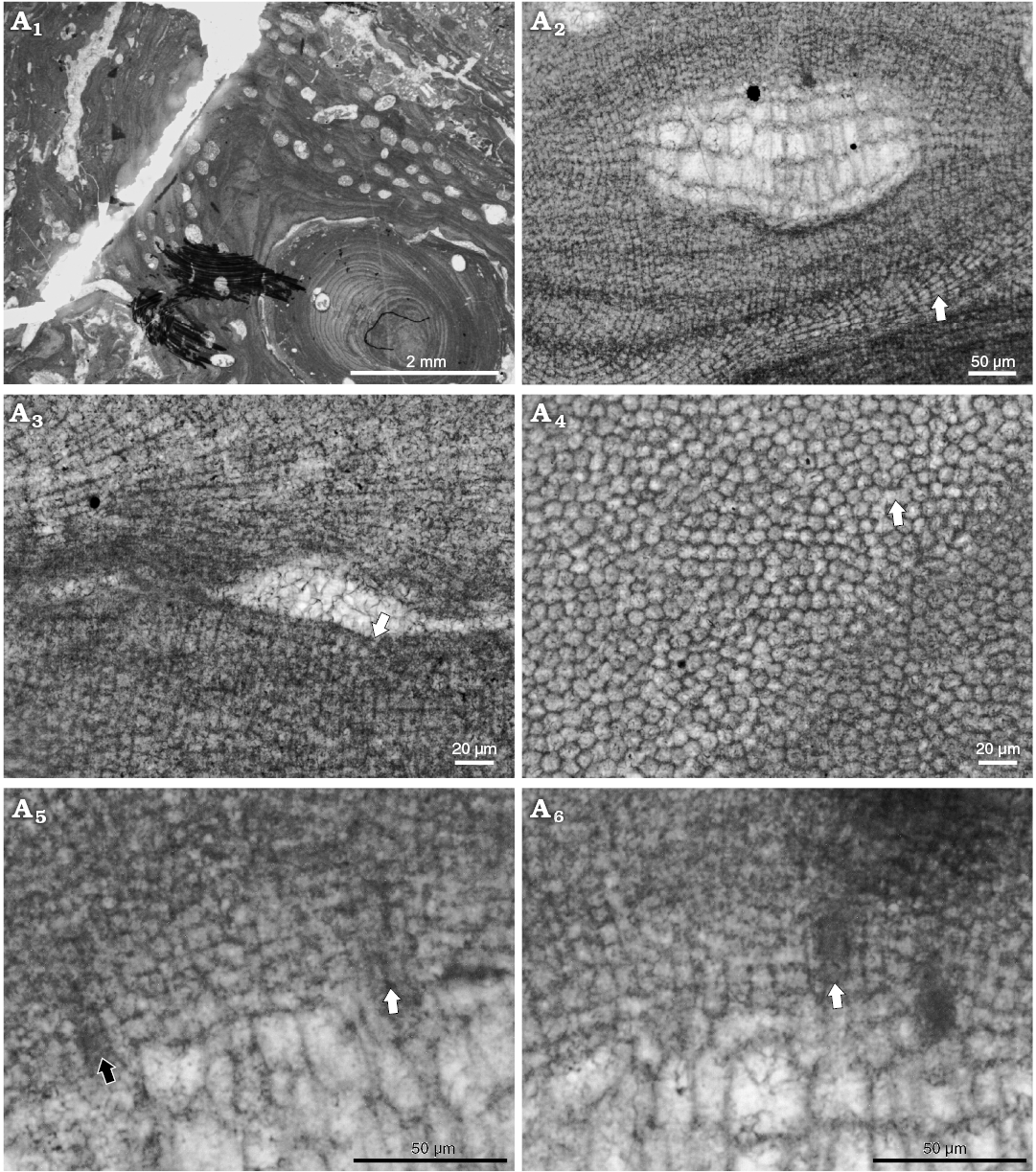
Fig. 5. Coralline alga Mesophyllum crassiusculum (Foslie, 1902) Lebednik, 2004 bi/tetrasporophyte, early Langhian, Miocene, Kosihovce, Slovakia. Schaleková’s collection, NHM B1859 (TS IIIb455). A1. Thallus morphology, growth form is encrusting with weak protuberances. A2. Coaxial to non-coaxial hypothallus (arrow). A3. Epithallial cells rounded or flattened (arrow). A4. Lateral cell fusions of the cells in adjacent filaments (arrow). A5. Pore canal anatomy, lining cells are same as adjacent roof cells (black arrow) or thinner near the base (white arrow). A6. Pore canal anatomy, lining cells are thinner than adjacent roof cells in some portions of the pore canal filaments. Arrows point to the center of the pore canal.
Discussion
The studied material corresponds to Mesophyllum crassiusculum as described by Athanasiadis et al. (2004) and Athanasiadis (2007) (Figs. 2, 3; Table 2). The pore canal cells shape, their number and the morphology of the roof of multiporate sporangial conceptacles fit within the description of the living plants of M. crassiusculum, although a complete comparison is prevented by the absence of data on gametophyte and carposporangial conceptacles in the Recent material (Athanasiadis et al. 2004; Athanasiadis 2007).
Table 2. Mesophyllum crassiusculum (Foslie, 1902) Lebednik, 2004 comparison from the Recent and middle Miocene. Measures are in μm when not indicated otherwise. Abbreviations: CP, central Paratethys; D, diameter; H, height; L, length; ND, no data; “+” presence, “–” absence, and “±” intermediate character.
|
Reference |
this work |
this work |
|||
|
Specimen |
TRH #205 |
AHFH #70353 |
NHM B1857, B1858 |
NHM B1859 |
|
|
Distribution |
California, USA |
California, USA |
CP (Modrý Majer, Slovakia) |
CP (Kosihovce, |
|
|
Habitat |
littoral |
littoral–sublittoral (49 m) |
littoral |
littoral |
|
|
Substratum |
epilithic, rhodolith |
epilithic |
epilithic, rhodolith |
epilithic, rhodolith |
|
|
Thallus growth form |
encrusting |
encrusting |
encrusting |
encrusting |
|
|
Thallus diameter |
up to 7 cm |
ND |
ND |
ND |
|
|
Ventral outgrowths |
– |
ND |
ND |
ND |
|
|
Peripheral protuberances |
± |
± |
± |
± |
|
|
Superimposing growth |
+ |
+ |
+ |
+ |
|
|
Lamella thickness |
150–2500 |
ND |
200–1400 |
150–2000 |
|
|
Hypothallus thickness |
52–300 |
ND |
43–150 |
56–144 |
|
|
Perithallus thickness |
50–80 |
ND |
121–401 |
up to 400 |
|
|
Predominantly coaxial hypothallus |
+ |
+ |
± |
± |
|
|
Stratified perithallus |
± |
ND |
±/– |
±/– |
|
|
Elongated meristematic cells |
+ |
ND |
+ |
+ |
|
|
Terminal trichocytes |
– |
ND |
– |
– |
|
|
Hypothallus cells: L, D |
10–31, 5–13 |
15–30, 8–12 |
11–26, 5–11 |
12–20, 4–8 |
|
|
Perithallus cells: L, D |
4–23, 4–9 |
ND |
4–16, 4–11 |
7–13, 5–11 |
|
|
Epithallial cells |
number of cells |
1–3 |
ND |
1–2 |
1 |
|
L, D |
2–5, 3–9 |
ND |
3–5, 5–7 |
2–4, 3–5 |
|
|
shape |
flattened |
flattened |
rounded, flattened |
rounded, square |
|
|
Asexuate (multiporate) conceptacles |
external diameter |
300–636 |
450–750 |
350–630 |
333–498 |
|
external height |
up to 160 |
120–250 |
75–120 |
75–100 |
|
|
chamber D, H |
250–410, 130–190 |
300–450, 180–240 |
275–418, 148–199 |
227–405, 135–202 |
|
|
convex roof |
+ |
+ |
+ |
+ |
|
|
roof/pore plate thickness |
40–70 |
42–60 |
42–69 |
44–70 |
|
|
roof/pore plate cells |
6–10 |
8–10 |
6–9 |
6–9 |
|
|
pore plate diameter |
170–300 |
260–470 |
ND |
ND |
|
|
number of pores |
36–50 |
ND |
ND |
ND |
|
|
conical canal |
– |
– |
– |
– |
|
|
pore canal diameter |
10–14 |
c.10 |
10–13 |
10–16 |
|
|
embedded conceptacles |
+ |
ND |
+ |
+ |
|
|
conceptacles degenerate |
– |
ND |
ND |
ND |
|
|
peripheral rim |
– |
– |
– |
– |
|
|
Pore canal |
rosette cells |
6–10 |
6–8 |
ND |
ND |
|
rosette cells sunken |
– |
– |
– |
– |
|
|
cells per filament |
6–8 |
8–10 |
6–9 |
6–9 |
|
|
similar to contiguous |
+ |
– |
+ |
+ |
|
|
smaller than contiguous |
– |
– |
– |
– |
|
|
thinner and wider |
– |
+ |
+ |
+ |
|
|
Bi/tetrasporangia: L, D |
110–200, 25–70 |
120–175, 60–100 |
ND |
ND |
|
|
Male conceptacles |
external diameter |
ND |
ND |
324–429 |
ND |
|
external height |
ND |
ND |
87–115 |
ND |
|
|
chamber D, H |
ND |
ND |
162–372, 42–90 |
ND |
|
|
roof thickness |
ND |
ND |
27–45 |
ND |
|
|
pore canal diameter |
ND |
ND |
57–69 |
ND |
|
|
Carposporangial conceptacles |
external diameter |
ND |
ND |
ND |
ND |
|
external height |
ND |
ND |
ND |
ND |
|
|
chamber D, H |
ND |
ND |
470–689, 127–256 |
ND |
|
|
chamber shape |
ND |
ND |
dumbbell |
ND |
|
|
roof thickness |
ND |
ND |
133–176 |
ND |
|
|
pore canal diameter |
ND |
ND |
129–132 |
ND |
|
The specimen from the Schaleková’s Collection published in Schaleková and Čierna (1985) was firstly identified as Mesophyllum galettoi Mastrorilli, 1968. However, our investigation revealed morpho-anatomical characteristics similar to the Recent M. crassiusculum.
Most of the morphoanatomical characteristics of the studied sporophyte and male gametophyte perfectly correspond to those of the specimens described by Coletti et al. (2018: supplementary material) as Mesophyllum roveretoi Conti, 1943, in the lower Miocene of the Sommières Basin (Southern France). Coletti et al. (2018) also describe a female gametophyte but in this case the comparison is more difficult because their specimen mainly reveals unfertilized conceptacles (Coletti et al. 2018: supplementary material). Because they do not present data on the epithallial cells or on the cells around the pore canal, a proper comparison is not possible. The similarity between M. roveretoi, M. galettoi, and M. crassiusculum suggests that a revisions of the original material of Conti (1943) and Mastrorilli (1968) is necessary.
Regarding the ecology of this species, according to Athanasiadis and Ballantine (2014) the genus Mesophyllum generally inhabits Recent temperate seas, and its occurrence in the tropics, as is the case of M. crassiusculum reaching the coast of California south to the Pacific Mexico, is presently accompanied by the absence of all reproductive phases excluding the sporophyte. This in turn would suggest temperate climate during the late Badenian in the study area, a hypothesis supported by Studencki (1988a, 1999) after his studies of the coralline algae from Poland. To the contrary, tropical climate during the Badenian was commonly proved by the presence of colonial corals (e.g., Baráth 1992; Riegl and Piller 2000; Saint Martin et al. 2007; this paper), whose patches or small frameworks also presently co-occur with the modern M. crassiusculum (Glynn et al. 2017). Sub-tropical climate is documented in central Paratethys in the upper Badenian and even in the Sarmatian (Kováč et al. 2005, 2007; Harzhauser and Piller 2007). However, a broader discussion of paleoclimate based solely upon Mesophyllum occurrences would require the full revision of the fossil records and it is not yet possible because of insufficient data.
Identification of Mesophyllum sensu lato gametophytes and carposporophytes is problematic when dealing with the fossil record (Hrabovský et al. 2015). Other genera, such as Neogoniolithon Setchell and Mason, 1943, Spongites Kützing, 1841, Pneophyllum Kützing, 1843, and Chamberlainium Caragnano, Foetisch, Maneveldt, and Payri, 2018 possess both a coaxial hypothallus and uniporate spermatangial conceptacle chambers that could be confused with Mesophyllum carposporangial conceptacle chambers (Johansen 1981; Braga et al. 1993; Caragnano et al. 2018). However, the occurrence of a central pedestal associated to a coaxial hypothallus is a unique combination in Mesophyllum sensu stricto. This structure develops from the decalcification and dissolution of floor cells around the carpogonial branches (which make room for the carposporangia; Athanasiadis et al. 2004). This results in a distinct morphological elevation in the central part of the carposporangial chamber of Mesophyllum (Athanasiadis et al. 2004; Athanasiadis and Ballantine 2014). Neogoniolithon sporophytes from Atlantic coast of Mexico predominantly possess flat floors whereas the others have just an ill-defined inflexion of the chamber floor, differently from the distinctive “central pedestal” in Mesophyllum carposporangial conceptacles as well as from our samples (Athanasiadis et al. 2004; Quaranta et al. 2007; Mateo-Cid et al. 2014).
The absence of trichocytes in the studied material is another important evidence that supports their placement in Mesophyllum rather than Neogoniolithon. Recent molecular taxonomy suggests trichocytes importance in coralline algal systematic (Basso et al. 2014; Rösler et al. 2016; Caragnano et al. 2018). Trichocytes are commonly reported in modern and fossil Neogoniolithon species from temperate and tropical seas (Basso and Rodondi 2006; Kato et al. 2011; Mateo-Cid et al. 2014; Rösler et al. 2015, 2016; Villas-Boas et al. 2015; Caragnano et al. 2018) contrarily to Mesophyllum s.s. species that do not possess trichocytes (Athanasiadis and Ballantine 2014).
There are no morphological characters allowing the distinction of fossil male plants of Neogoniolithon and Mesophyllum. However, the following observations suggest that the specimens analyzed in this study are indeed Mesophyllum gametophyte rather than Neogoniolithon plants: (i) overlapping vegetative morphoanatomical features with those of the sporophyte, (ii) co-occurrence of sporophyte and gametophyte of Mesophyllum in the same thin section, (iii) absence of trichocytes, (iv) absence of sporangial plants of Neogoniolithon in the same thin sections.
Actually, the observed carpogonial-carposporangial, spermatangial and asexuate plants present the same: (i) growth form, (ii) thickness of the lamellate branches, (iii) perithallus zonation, (iv) ventral core thickness, (v) cells dimensions, (vi) lateral cells connections, (vii) meristematic cells elongation, (viii) epithallial cells shape, and (ix) absence of trichocytes (Table 1).
For these reasons, we tentatively describe the observed specimens as different life cycle phases of Mesophyllum crassiusculum. The discovery of M. crassiusculum gametophytes in modern plants will hopefully provide further evidence supporting our conclusions, but for the moment there is no other reports of specimens corresponding with our gametophyte from the Slovakian part of the central Paratethys (Schaleková 1962, 1969, 1973, and 1978; Schaleková and Čierna 1985; Hrabovský 2013).
This is not the first example of description of fossil gametangial plant and other examples are known from the literature e.g., in Lithophyllum racemus (Lamarck, 1816) Foslie, 1901 (Basso et al. 1996), Phymatolithon calcareum (Pallas, 1766) Adey and McKibbin, 1970 (Basso et al. 1997), Mesophyllum roveretoi Conti, 1943 (Mastrorilli 1968), Mesophyllum cf. printzianum (Hrabovský et al. 2015), Lithothamnion ponzonense Conti, 1943 (Conti 1943) or Sporolithon preerythraeum (Airoldi, 1932) Vannucci, Piazza, Fravega, and Basso, 2000 (Vannucci et al. 2000; Chelaru and Bucur 2016). Both, spermatangial and carpogonial plants were described in Chelaru and Bucur (2016) for Spongites fruticulosus and Sporolithon sp. 1. Chelaru and Bucur (2016) provided examples of gametophyte for two Lithothamnion species but did not specify whether male or female. Uncertain Lithophyllum sp. 2 gametophyte was documented in Hrabovský et al. (2015). Almost complete life cycle with tetrasporophyte, male gametophyte, and carposporangial conceptacles were described for Sporolithon lvovicum (Maslov, 1956) Bassi, Braga, Zakrevskaya, and Petrovna Radionova, 2007 (Hrabovský et al. 2015) and Hydrolithon braganum Woelkerling, Bassi, and Iryu, 2012 (Woelkerling et al. 2012). Neogoniolithon contii (Mastrorilli, 1967) Quaranta, Vannucci, and Basso, 2007 has complete life cycle documented (Quaranta et al. 2007). However, further research in this direction is still necessary in order to fill one of the main gaps of knowledge in fossil coralline algae.
Conclusions
The gametophytic, carposporophytic, and asexuate phases of Mesophyllum crassiusculum are documented from middle Miocene material collected in the central Paratethys. This is the first report of this species in the fossil record, based on the complete matching with the morphology and anatomy of the modern asexuate counterpart. For the first time, the identification was based on the emended Mesophyllum diagnosis (Athanasiadis and Ballantine 2014) that includes sporophyte conceptacles pore canal anatomy, carposporangial conceptacles floor morphology and dioecy. The number and shape of the pore canals lining cells in the multiporate conceptacle roof are shown to be a character with fossilization potential, very useful when preserved, for fossil coralline systematics. The carposporangial Mesophyllum sensu stricto can be identified in the fossil on the basis of the unique combination of cell fusions, coaxial hypothallus, non-flared epithallial cells, and “central pedestal” at the center of the conceptacle floor. These results enrich the list of the fossil corallines for which the gametophyte/carposporophyte have been described. Last but not least, our results highlight once more the necessity of further revisions of historical collections to improve the knowledge about the distribution, paleoecology and paleobiogeography of fossil coralline algae.
Acknowledgements
We are thankful to Samuel Rybár (Comenius University, Bratislava, Slovakia) for his comments on Modrý Majer limestone stratigraphy and to Natália Hudáčková (Comenius University, Bratislava, Slovakia) for providing historical collection of Anna Schaleková. We thank reviewers Ioan Bucur (The Babeş-Bolyai University, Cluj-Napoca, Romania) and Bruno Granier (The University of Kansas, Lawrence, USA) for the review of our manuscript. Research was supported with VEGA project 2/0122/18 of the Grant Agency for MŠVVaŠ and SAV. This is a scientific contribution of Project MIUR – Dipartimenti di Eccellenza 2018–2022.
References
Adey, W.H. 1970. A revision of the Foslie crustose coralline herbarium. Det Kongelige Norske Videnskabers Selskabs Skrifter 1: 1–46.
Athanasiadis, A. 1999. Mesophyllum macedonis, nov. sp. (Rhodophyta, Corallinales), a putative Tethyan relic in the North Aegean Sea. European Journal of Phycology 34: 239–252. Crossref
Athanasiadis, A. 2001. Lectotypification of Leptophytum arcticum (Corallinales, Rhodophyta) and a study of its relationships within the Melobesioideae. Nordic Journal of Botany 21: 93–112. Crossref
Athanasiadis, A. 2007. Revision of Dawson’s collections referred to Lithothamnion lamellatum (Melobesioideae, Corallinales, Rhodophyta). Nova Hedwiga 85: 195–242. Crossref
Athanasiadis, A. 2017. Capensia fucorum (Esper) gen. et. comb. nov. (Mesophyllaceae, Corallinales, Rhodophyta), a hemiparasite on Gelidium from South Africa. Botanica Marina 60: 555–565. Crossref
Athanasiadis, A. and Ballantine, D.L. 2014. The genera Melyvonnea gen. nov. and Mesophyllum s.s. (Melobesioideae, Corallinales, Rhodophyta) particularly from the central Atlantic Ocean. Nordic Journal of Botany 32: 385–436. Crossref
Athanasiadis, A., Lebednik, P.A., and Adey, W.H. 2004. The genus Mesophyllum (Melobesioideae, Corallinales, Rhodophyta) on the northern Pacific coast of North America. Phycologia 43: 126–165. Crossref
Baráth, I. 1992. Upper Badenian reef complex on the eastern margin of the Vienna Basin. Knihovníčka ZPN 15: 177–197.
Baráth, I., Nagy, A., and Kováč, M. 1994. Sandberské vrstvy—vrchnobádenské marginálne sedimenty východného okraja Viedenskej panvy. Geologické práce, Správy 99: 59–66.
Basso, D. 1998. Deep rhodolith distribution in the Pontian Islands, Italy: a model for the paleoecology of a temperate sea. Palaeogeography, Palaeoclimatology, Palaeoecology 137: 173–187. Crossref
Basso, D. and Rodondi, G. 2006. A Mediterranean population of Spongites fruticulosus (Rhodophyta, Corallinales), the type species of Spongites, and the taxonomic status of S. stalactitica and S. racemosa. Phycologia 45: 403–16. Crossref
Basso, D., Caragnano, A., and Rodondi, G. 2014. Trichocytes in Lithophyllum kotchyanum and Lithophyllum spp. (Corallinales, Rhodophyta) from the Indian Ocean. Journal of Phycology 50: 711–717. Crossref
Basso, D., Fravega, P., and Vannucci, G. 1996. Fossil and living Corallinaceans related to the Mediterranean endemic species Lithophyllum racemus (Lamarck) Foslie. Facies 35: 275–292. Crossref
Basso, D., Fravega, P., and Vannucci, G. 1997. The Taxonomy of Lithothamnium ramosissimum (Gümbel non Reuss) Conti and Lithothamnium operculatum (Conti) Conti (Rhodophyta, Corallinaceae). Facies 37: 167–182. Crossref
Basso, D., Rodondi G., and Mari, M. 2004. A comparative study between Lithothamnion minervae and the type material of Millepora fasciculata (Corallinales, Rhodophyta). Phycologia 43: 215–223. Crossref
Basso, D., Vrsaljko, D., and Grgasović, T. 2008. The coralline flora of a Miocene maërl: the Croatian „Litavac“. Geologica Croatica 61: 333–340.
Braga J.C., Bassi D., Aguirre J., Zakrevskaya E., and Radionova, E.P. 2015. Re-assessment of the type collections of Maslov’s species of Hapalidiales (Rhodophyta). Species originally attributed to Lithothamnium, Mesophyllum and Palaeothamnium. Spanish Journal of Palaeontology 30: 189–208.
Braga, J.C., Bosence, D.W.J., and Steneck, R.S. 1993. New anatomical characters in fossil coralline algae and their taxonomic implications. Palaeontology 36: 535–547.
Caragnano, A., Foetisch, A., Maneveldt, G.W., Millet, L., Liu, L.-C., Lin, S.-M., Rodondi, G., and Payri, C.E. 2018. Revision of the Corallinaceae (Corallinales, Rhodophyta): Recognizing Dawsoniolithon gen. nov., Parvicellularium gen. nov. and Chamberlainoideae subfam. nov. containing Chamberlainium gen. nov. and Pneophyllum. Journal of Phycology 54: 391–409. Crossref
Chelaru, R. and Bucur, I. 2016. The taxonomy of middle Miocene red algae from the Gârbova de Sus Formation (Transylvania Basin, Romania). Carnets de Geologie 16 (11): 307–336. Crossref
Coletti, G., Basso, D., and Corselli, C. 2018. Coralline algae as depth indicators in the Sommières Basin (early Miocene, Southern France). Geobios 51: 15–30. Crossref
Coletti, G., Hrabovský, J., and Basso, D. 2016. Lithothamnion crispatum: long-lasting species of non-geniculate coralline algae (Rhodophyta, Hapalidiales). Carnets de Geologie 16 (3): 27–41. Crossref
Conti, S. 1943. Contributo allo studio delle Corallinaceae de terziario italiano. II: Le Corallinaceae del Miocene del Bacino Ligure-Piemontse. Palaeontographia Italica 41: 37–61.
Doláková, N., Brzobohatý, R., Hladilová, Š., and Nehyba, S. 2008. The red-algal facies of the Lower Badenian limestones of the Carpathian Foredeep in Moravia (Czech Republic). Geologica Carpathica 59: 133–146.
Glynn, P.W., Alvarado, J.J., Banks, S., Cortés, J., Feingold, J.S., Jiménez, C., Maragos, J.E., Martínez, P., Maté, J.L., Moanga, D.A., Navarrete, S., Reyes-Bonilla, H., Riegl, B., Rivera, F., Vargas-Ángel, B., Wieters, E.A., and Zapata, F.A. 2017. Eastern Pacific coral reef provinces, coral community structure and composition: an overview. In: P.W Glynn, D. Manzello, and I.C. Enochs (eds.), Coral Reefs of the Eastern Tropical Pacific, Vol. 8, 107–176. Springer, Dordrecht. Crossref
Golonka, J. 1981. Glony i biosedymentacja wapieni mioceńskich okolic Rzeszowa. Biuletyn Instytutu Geologicznego 332: 5–46.
Harzhauser, M. and Piller, W.E. 2007. Benchmark data of a changing sea—palaeogeography, palaeobiology and events in the Central Paratethys during the Miocene. Palaeogeography, Palaeoclimatology, Palaeoecology 253: 8–31. Crossref
Hók, J., Kováč, M., Nagy, P., and Šujan, M. 1999. Geology and tectonics NW part of the Komjatice depression. Slovakian Geological Magazine 5: 187–199.
Hrabovský, J. 2013. Non-geniculate coralline algae (Corallinales, Sporolithales, Rhodophyta) from lithothamnion limestones of the locality Vrchná Hora at the town of Stupava (Vienna Basin, Slovakia). Mineralia Slovaca 45: 23–34.
Hrabovský, J., Basso, D., and Doláková, N. 2015. Diagnostic characters in fossil coralline algae (Corallinophycidae: Rhodophyta) from the Miocene of southern Moravia (Carpathian Foredeep, Czech Republic). Journal of Systematic Palaeontology 14: 499–525. Crossref
Johansen, H.W. 1981. Coralline algae, A first synthesis. 239 pp. CRC press, Boca Raton.
Kato, A., Baba, M., and Suda, S. 2011. Revision of the Mastophoroideae (Corallinales, Rhodophyta) and polyphyly in nongeniculate species widely distributed on Pacific coral reefs. Journal of Phycology 47: 663–672. Crossref
Keats, D.W., Maneveldt, G., and Chamberlain, Y.M. 2000. Lithothamnion superpositum Foslie: a common crustose red alga (Corallinaceae) in South Africa. Cryptogamie, Algologie 21: 381–400. Crossref
Kováč, M. 2000. Geodynamic, Paleogeographic and Structural Evolution of the Carpathian-Pannonian Region in the Miocene: New View on the Neogene Basins of Slovakia. 202 pp. Veda, Bratislava.
Kováč, M., Andreyeva-Grigorovich, A., Bajraktarević, Z., Brzobohatý, R., Filipescu, S., Fodor, L., Harzhauser, M., Nagymarosy, A., Oszczypko, N., Pavelić, D., Rögl, F., Saftić, B., Sliva, Ľ., and Studencka, B. 2007. Badenian evolution of the Central Paratethys Sea: paleogeography, climate and eustatic sea-level changes. Geologica Carpathica 58: 579–606.
Kováč, M., Fordinál, K., Grigorovich, A.S., Halásová, E., Hudáčková, N., Joniak, P., Pipík, R., Sabol, M., Kováčová, M., and Sliva, Ľ. 2005. Western Carpathians fossil ecosystems and their relationship to paleoenvironment in the context of the Neogene development of the Eurasian continent. Geologické práce, Správy 111: 61–121.
Kováč, M., Rybár, S., Halásová, N., Hudáčková, N., Šarinová, K., Šujan, M., Baranyi, V., Kováčová, M., Ruman, A., Klučiar, T., and Zlinská, A. 2018. Changes in Cenozoic depositional environment and sediment provenance in the Danube Basin. Basin Research 30: 97–131. Crossref
Klučiar, T., Kováč, M., Vojtko, R., Rybár, S., Šujan, M., and Králiková, S. 2016. The Hurbanovo-Diösjenő Fault: A crustal-scale weakness zone at the boundary between the Central Western Carpathians and Northern Pannonian Domain. Acta Geologica Slovaca 8: 59–70.
Lebednik, P.A. 1978. Development of male conceptacles in Mesophyllum Lemoine and other genera of the Corallinaceae (Rhodophyta). Phycologia 17: 388–395. Crossref
Lemoine, M. 1928. Un nouveau genre de Mélobésiées: Mesophyllum. Bulletin de la Société Botanique de France 75: 251–254. Crossref
Maslov, V.P. 1956. Fossil calcareous algae of USSR [in Russian]. Trudy Instituta geologičeskih nauk Akademii Nauk 160: 1–301.
Mastrorilli, V.I. 1967. Lithophyllum contii: nuova specie di Corallinacea diffusa nella formazione oligocenica di Bric Mazzapiede, presso Prasco (Acqui). Atti dell’Instituto di geologia della Università di Genova 4: 475–488.
Mastrorilli, V.I. 1968. Nuovo contributo allo studio delle corallinacee dell’Oligocene Ligure-Piemontese: I repertir della Tavoletta Ponzone. Atti dell’Instituto di geologia della Università di Genova 5: 154–406.
Matějka, A. 1949. Overiew of the Geology of the Low Danube Lowland (Explanation to the geological map 1:200 000). Geologický ústav Dionýza Štúra, Bratislava.
Mateo-Cid, L.E., Mendoza-González, A.C., and Gabrielson, P.W. 2014. Neogoniolithon (Corallinales, Rhodophyta) on the Atlantic coast of Mexico, including N. siankanensis sp. nov. Phytotaxa 190: 64–93. Crossref
Merwe, E. van der and Maneveldt, G.W. 2014. The genus Phymatolithon (Hapalidiaceae, Corallinales, Rhodophyta) in South Africa, including species previously ascribed to Leptophytum. South African Journal of Botany 90: 170–192. Crossref
Pisera, A. 1985. Paleoecology and lithogenesis of the Middle Miocene (Badenian) algal-vermetid reefs from the Roztocze Hills, south-eastern Poland. Acta Geologica Polonica 35: 89–155.
Pisera, A. 1996. Miocene reefs of the Paratethys: a review. In: E.K. Franseen, M. Esteban, W.C. Ward, and J. Rouchy (eds.), Models for Carbonate Stratigraphy from Miocene Reef Complexes of Mediterranean Region. Concepts in Sedimentology and Paleontology, vol. 5, 97–104. SEPM Society for Sedimentary Geology, Tulsa. Crossref
Pisera, A. and Studencki, W. 1989. Middle Miocene rhodoliths from the Korytnica Basin (Southern Poland): environmental significance and paleontology. Acta Palaeontologica Polonica 34: 179–209.
Quaranta, F., Vannucci, G., and Basso, D. 2007. Neogoniolithon contii comb. nov. based on the taxonomic re-assessment of Mastrorilli’s original collections from the Oligocene of NW Italy (Tertiary Piedmont Basin). Rivista Italiana di Paleontologia e Stratigrafia 113: 43–55.
Riegl, B. and Piller, W.E. 2000. Biostromal coral facies—A Miocene example from the Leitha Limestone (Austria) and its actualistic interpretation. Palaios 15: 399–413. Crossref
Rösler, A., Perfectti, F., Peña, V., and Braga, J.C. 2016. Phylogenetic relationships of the Corallinalceae (Corallinales, Rhodophyta): Taxonomic implications for reef-building corallines. Journal of Phycology 52: 412–431. Crossref
Rösler, A., Pretković, V., Novak, V., Renema, W., and Braga, J.C. 2015. Coralline algae from the Miocene Mahakam delta (East Kalimantan, Southeast Asia). Palaios 30: 83–93. Crossref
Saint Martin, J.P., Merle, D., Cornee, J.-J., Filipescu, S., Saint Martin, S., and Bucur, I. 2007. The Badenian (Middle Miocene) coral build-ups of the western border of the Transylvanian Basin (Romania). Comptes Rendus Palevol 6: 37–46. Crossref
Schaleková, A. 1962. Fytogénne vápence mezozoika a terciéru Slovenska. 196 pp. Ph.D. Thesis, Registratúrne Stredisko PRIF UK (Registry), Comenius University, Bratislava.
Schaleková, A. 1969. Contribution to the knowledge of red algae in the Leitha Limestone at the locality Sandberg near Devínska Nová Ves (southwestern Slovakia). Acta Geologica et Geographica Universitatis Comenianae 18: 93–102.
Schaleková, A. 1973. Oberbadenische corallinaceen aus dem Steinbruch Rohožník-Vajar an dem Westhang der Kleinen Karpaten. Acta Geologica et Geographica Universitatis Comenianae 26: 211–227.
Schaleková, A. 1978. Riasové (Lithothmniové) vápence v bádene Viedenskej, Dunajskej a Juhoslovenskej panve Západných Karpát. 150 pp. Candidate Thesis. Registratúrne Stredisko PRIF UK (Registry), Comenius University, Bratislava.
Schaleková, A., and Čierna, E. 1985. Algenflora des unteren Badeniens (Corallinaceae, Bacillariophyceae) aus der Umgebung von Kosihovce (Südslowakisches Becken, Westkarpaten). Acta Geologica et Geographica Universitatis Comenianae Geologica 39: 35–49.
Seneš, J. 1963. Miocene of the eastern Danube Basin margin. Geologické práce, Správy 27: 75–87.
Seneš, J. 1988. Principles of study of Adriatic shelf ecosystems from the viewpoint of applications in geology. Geologický Zborník, Geologica Carpathica 39: 285–300.
Seneš, J. 1990. Some infra- and circalittoral ecosystems of the eastern part of the south Adriatic shelf. Geologický Zborník, Geologica Carpathica 41: 199–228.
Seneš, J., and Ondrejčíková, A. 1991. Proposal for the terminology of fossil marine benthic shelf ecosystems. Geologica Carpathica 42: 231–240.
Sremac, J., Bošnjak Makovec, M., Vrsaljko, D., Karaica, B., Tripalo, K., Fio Firi, K., Majstorović Bušić, A., and Marjanac, T. 2016. Reefs and bioaccumulations in the Miocene deposits of the North Croatian Basin—Amazing diversity yet to be described. Rudarsko-Geološko-Naftni Zbornik 31: 19–29. Crossref
Studencki, W. 1988a. Facies and sedimentary environment of the Pinczow Limestones (Middle Miocene; Holy Cross Mountains, Central Poland). Facies 18: 1–26. Crossref
Studencki, W. 1988b. Red algae from the Pińczów limestones (Middle Miocene, Świętokrzyskie Mountains, Poland). Acta Palaeontologica Polonica 33: 3–57.
Studencki, W. 1999. Red-algal limestones in the Middle Miocene of the Carpathian Foredeep in Poland: facies variability and palaeoclimatic implications. Geological Quarterly 43: 395–404.
Townsend, R.A. 1979. Synarthrophyton, a new genus of Corallinaceae (Craptonemiales, Rhodophyta) from the southern hemisphere. Journal of Phycology 15: 251–259. Crossref
Vannucci, G., Piazza, M., Fravega, P., and Basso, D. 2000. Revision and re-documentation of M. Airoldi’s species of Archaeolithothamnium from the Tertiary Piedmont Basin (NW Italy). Rivista Italiana di Paleontologia e Stratigrafia 106: 191–202.
Vass, D. 2002. Lithostratigraphy of the Western Carpathians: Neogene and Budín Paleogene. 202 pp. Vydavateľstvo ŠGÚDŠ, Bratislava.
Villas-Bôas, A.B., Riosmena-Rodriguez, R., Tâmega, F.T.S., Amado-Filho, G.M., Maneveldt, G.W., and Figueiredo, M.A.O. 2015. Rhodolith-forming species of the subfamilies Neogoniolithoideae and Hydrolithoideae (Rhodophyta, Corallinales) from Espírito Santo State, Brazil. Phytotaxa 222: 169–184. Crossref
Wettstein, R. von 1901. Handbuch der systematischen Botanik. Vol. 1. 201 pp. Franz Deuticke, Leipzig.
Woelkerling, W.J., Bassi, D., and Iryu, Y. 2012. Hydrolithon braganum sp. nov. (Corallinaceae, Rhodophyta), the first known exclusively fossil semi-endophytic coralline red algae. Phycologia 51: 604–611. Crossref
Woelkerling, W.J., Campbell, S.J., and Harvey, A.S. 1993. Growth-forms in non-geniculate coralline red algae (Corallinales, Rhodophyta). Australian Systematic Botany 6: 277–293. Crossref
Woelkerling, W.J. and Harvey, A. 1992. Mesophyllum incisum (Corallinaceae, Rhodophyta) in Southern Australia: Implications for generic and specific delimitation in the melobesioidese. European Journal of Phycology 27: 381–399. Crossref
Woelkerling, W.J. and Harvey, A. 1993. An account of Southern Australian species of Mesophyllum (Corallinalceae, Rhodophyta). Australian Systematic Botany 6: 571–637. Crossref
Zdražílková, N. 1988. Corallinaceae of the Lower Badenian from the Carpathian Foredeep. Časopis pro mineralogii a geologii 33: 187–198.
Acta Palaeontol. Pol. 64 (4): 897–909, 2019
https://doi.org/10.4202/app.00591.2019