


Taxonomic implications of morphometric analysis of earless seal limb bones
MORGAN CHURCHILL and MARK D. UHEN
Churchill, M. and Uhen, M.D. 2019. Taxonomic implications of morphometric analysis of earless seal limb bones. Acta Palaeontologica Polonica 64 (2): 213–230.
Fossil Phocidae (earless seals) are mostly known from isolated postcranial material, forcing researchers to rely upon humeri and femora for the diagnosis of taxa and reconstruction of phylogeny. However, the utility of these elements has never been rigorously tested. Here, we provide the first quantitative analysis of morphometric data from the humerus and femur, incorporating measurement data from all extant genera as well as several fossil taxa. Principle components analysis (PCA) found that genera clustered together on PC1 and PC2, although there was poor segregation of taxa and extensive overlap with genera in adjacent regions of the morphospace. Discriminant function analysis (DFA) was able to sort fossil taxa into different subfamilies, but performed poorly at lower taxonomic levels. A preliminary review of phylogenetic characters found that while some characters performed well at distinguishing different subfamilies, many characters were poorly defined and not quantified, possessed greater individual variation than past studies suggested, or were more variable in fossil taxa. Our analyses suggest that the utility of isolated humeri and femora for diagnosis of new taxa has been greatly exaggerated, and that extreme caution should be applied to interpretations of taxonomy of fossil material based on isolated elements. Future research should instead focus on study of associated skeletons and cranial material. A thorough revision of fossil phocid taxonomy is needed, and many described taxa are likely to be nomina dubia and of limited use in phylogenetic analysis.
Key words: Mammalia, Phocidae, Monachinae, Pinnipedia, morphometrics, taxonomy, femur, humerus.
Morgan Churchill [churchim@uwosh.edu], Department of Biology, University of Wisconsin, 800 Algoma Blvd., Oshkosh WI 54901, USA.
Mark D. Uhen [muhen@gmu.edu], Department of Atmospheric, Oceanic, and Earth Sciences, George Mason University, 4400 University Dr., Fairfax, VA 22030, USA.
Received 12 February 2019, accepted 29 March 2019, available online 26 April 2019.
Copyright © 2019 M. Churchill and M.D. Uhen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Phocidae (earless seals) are represented today by 19 species which are found throughout the world’s oceans and in several freshwater lakes (Committee on Taxonomy 2018). They represent the most morphologically and ecologically diverse family of pinnipeds, a group which also includes sea lions, fur seals, walruses, and their fossil relatives. Two subfamilies of earless seal are strongly supported in recent phylogenetic analyses; the Monachinae (southern seals) with three tribes that include monk seals (Monachini), elephant seals (Miroungini), and Antarctic seals (Lobodontini); and the Phocinae (northern seals), which consist of three tribes, the monospecific bearded seal (Erignathini) and hooded seal (Cystophorini), and the more diverse Phocini, which include ribbon, harp, gray, harbor, and ringed seals (Berta and Wyss 1994; Davis et al. 2004; Higdon et al. 2007; Fulton and Strobeck 2010b; Dewaele et al. 2017a, 2018a). Molecular clock estimates place the divergence between these two subfamilies at ~22 Ma (Higdon et al. 2007; Fulton and Strobeck 2010a), which is also supported by the age of the oldest fossil occurrences of members of each clade (Koretsky and Domning 2014; Dewaele et al. 2017b). Generally, the North Atlantic region is considered the initial center of origin and diversification for both subfamilies (Koretsky and Barnes 2006; Fulton and Strobeck 2010a; Berta et al. 2018), with dispersal into the Southern Hemisphere during the middle Miocene (Muizon and Bond 1982).
There are currently 47 accepted species of fossil phocid seal in 36 genera (Paleobiology database, accessed January 2019, https://paleobiodb.org/). Of these described taxa, six species are known from South America, one is known from South Africa, ten are known from the Atlantic seaboard of North America, and 35 are known from Europe and North Africa. However, while fossil pinniped taxa in other clades have been described from material including partial and complete skulls and skeletons (Berta et al. 2018), the majority of fossil phocid taxa, especially those from the Northern Hemisphere, are known from isolated or fragmentary postcranial elements. Humeri and femora are particularly well represented in the fossil record for Phocidae, which has led to a heavy reliance on these skeletal elements for taxonomy (Koretsky 2001; Dewaele et al. 2018b). Currently, seventeen widely recognized fossil phocid taxa have holotypes consisting of partial or complete isolated humeri, while an additional five taxa have holotypes comprising partial and complete isolated femora. This represents ~46% of named extinct phocid taxa, and represents the 55% of the diversity known from the North Atlantic and Paratethys seaway. Because of the limited associated fossil material available for study, some researchers have employed an “ecomorphotype” hypothesis to link these isolated skeletal elements together (Van Beneden 1877; Koretsky 2001; Rahmat and Koretsky 2016), increasing the pool of morphological character information available for a given taxon. These “sets” of isolated skeletal elements are then used in phylogenetic analyses as well as to assess faunal change, biogeography, and ecology of fossil phocids (Koretsky 2001; Koretsky et al. 2012, 2015; Koretsky and Rahmat 2013).
Although researchers continue to rely upon humeri and femora, no one has rigorously quantified the morphological differences between these limb elements of different modern taxa, and their overall utility in diagnosing fossil taxa. Morphometric analysis has proven useful in assessing the taxonomy of fossil mammal taxa, and has been used with great success in groups as diverse as South African earless seals (Govender et al. 2012), Australasian fur seals and sea lions (Churchill and Boessenecker 2016), dogs (Drake et al. 2015), raccoons (Rodriguez et al. 2016), and rodents (Boroni et al. 2017). If the diagnostic differences between these clades are overstated, this may call into question how much we truly understand about the evolutionary history of the clade, and render many fossil taxa names as nomina dubia. The research carried out below is a preliminary assessment of morphometric variation in humerus and femur morphology, and the utility of these elements in differentiating fossil taxa.
Institutional abbreviations.—USNM, National Museum of Natural History, Washington D.C., USA.
Other abbreviations.—DFA, discriminant function analysis; LLF, length of the lateral side of the femur; MLF, length of the femur on the medial side; PCA, principal component analysis.
Material and methods
Sampling.—To assess the degree of morphological variation present within phocid limb bones, we collected measurements from 66 humeri and 78 femora. Our sampling included representatives of all modern genera and all extant species with the exception of Mirounga leonina, as recognized in the current taxonomic checklist of the Society for the Study of Marine Mammals (Committee on Taxonomy 2018). Most modern taxa are represented by no fewer than three individuals, although a few rarer species (e.g., Monachus monachus, Pusa caspica, and Pusa siberica) are represented by single individuals. Sampling was limited to adult individuals with fully fused epiphyseal plates. For many specimens examined, sex was unknown. Sexual dimorphism is significant for some phocid taxa (e.g., Mirounga), and when possible we tried to include both male and female individuals for these species. For modern taxa, we only examined specimens where both a humerus and femur was preserved for the same individual.
Table 1. Fossil taxa, with age and locality information, included in this study.
|
Taxon |
Subfamily |
Skeletal |
Locality |
Age |
References |
|
Piscophoca pacifica |
Monachinae |
humerus |
Sud-Sacaco West horizon, Pisco Formation, Peru |
Messinian–Zanclean (6.59–5.93 Ma) |
|
|
Piscophoca sp. |
Monachinae |
humerus |
Pisco Formation, Peru |
Messinian–Zanclean (11.6–3.6 Ma) |
|
|
Acrophoca longirostris |
Monachinae |
humerus |
Sud-Sacaco West horizon, Pisco Formation, Peru |
Messinian–Zanclean (6.59–5.93 Ma) |
|
|
Callophoca obscura |
Monachinae |
humeri, femora |
Yorktown Formation, |
Zanclean (5–4.4 Ma) |
|
|
Auroraphoca atlantica |
Monachinae |
humerus |
Yorktown Formation, |
Zanclean (5–4.4 Ma) |
|
|
Pontophoca sarmatica |
Monachinae |
femur |
Chisinau, Moldova |
Serravalian (11.9–11.2 Ma) |
|
|
Leptophoca proxima |
Phocinae |
femur |
Calvert Formation, |
Burdigalian–Serravalian |
|
|
Leptophoca “amphiatlantica” |
Phocinae |
femur |
Calvert and St. Marys |
Langhian (16–13.07 Ma) |
|
|
Phocanella pumila |
Phocinae |
humeri, femora |
Yorktown Formation, |
Zanclean (5–4.4 Ma) |
|
|
Monachopsis pontica |
Phocinae |
femur |
Dobrogea, Romania |
Serravalian (13.7–11.6 Ma) |
|
|
Cryptophoca maeotica |
Phocinae |
femur |
Chisinau, Moldova |
Serravalian (11.9–11.2 Ma) |
|
|
Praepusa pannonica? |
Phocinae |
femur |
Chisinau, Moldova |
Serravalian (11.9–11.2 Ma) |
|
|
Praepusa vindobonensis |
Phocinae |
femur |
Dobrogea, Romania |
Serravalian (13.7–11.6 Ma) |
In addition to extant taxa, we also measured twelve humeri and ten femora from fossil phocids. None of the fossil specimens examined for this study included a associated set of humerus and femur; most specimens represent isolated limb bone elements. Specimens represented within the fossil sample set include a broad sample of known taxonomic diversity within the clade (Table 1). Monachinae taxa examined include Acrophoca longirostris, Piscophoca pacifica, and Piscophoca sp. from the Pisco Formation of Peru (Muizon 1981); Callophoca obscura (Koretsky and Ray 2008) and Auroraphoca atlantica (Dewaele et al. 2018b) from the Yorktown Formation of Virginia and North Carolina; and Pontophoca sarmatica from Chisinau, Moldova (Koretsky and Grigorescu 2002). Phocine taxa include Leptophoca proxima from the St. Marys Formation of Maryland (Dewaele et al. 2017b); Phocanella pumilla from the Yorktown Formation of Virginia and North Carolina (Koretsky and Ray 2008); Cryptophoca maeotica and Praepusa pannonica from Chisinau, Moldova (Koretsky 2001), and Monachopsis pontica and Praepusa vindobonensis from Dobrogea, Romania (Koretsky 2001). Of the above taxa examined, only casts were available for Piscophoca, Pontophoca, Monachopsis, Cryptophoca, and Praepusa. Sixteen measurements from both the humerus and femur were collected from each specimen (Fig. 1), using a set of digital calipers, with all values recorded to the nearest 0.01 mm. A list of all specimens can be found in SOM 1, while measurements for all specimens can be found in SOM 2 (Supplementary Online Material available at http://app.pan.pl/SOM/app64-Churchill_Uhen_SOM.pdf)
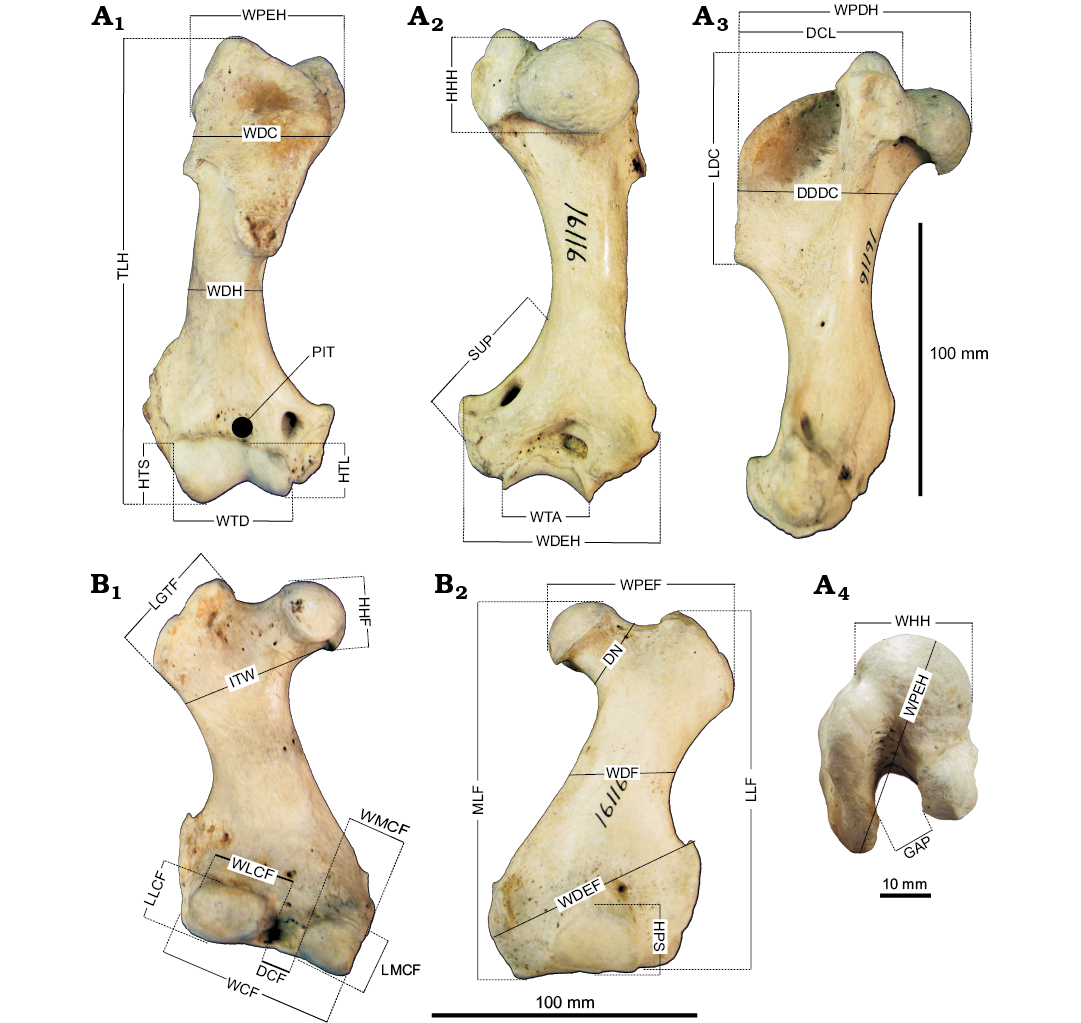
Fig. 1. Measurements of the earless seal humerus (A) and femur (B) used in morphometric analysis exemplified by Erignathus barbatus (USNM 16116). In anterior (A1, B2), posterior (A2, B1), medial (A3), and proximal (A4) views. Abbreviations: DCF, distance between condyles; DDDC, diameter of diaphysis at deltopectoral crest; DN, diameter of the neck of the femur; GAP, distance between head of humerus and deltopectoral crest; HHF, height of the head of the femur; HHH, height of head of humerus; HPS, height of the patellar surface; HTL, height of capitulum; HTS, height of trochlea; ITW, intertrochlear width of the femur; LDC, length of deltopectoral crest; LGTF, length of the greater trochanter; LLF, length of the lateral side of the femur; LLCF, length of the lateral condyle; LMCF, length of the medial condyle; MLF, length of the femur on the medial side; PIT, depth of coronoid fossa; SUP, length of supinator ridge; TLH, total length of humerus; WCF, width across condyles; WDC, width of deltopectoral crest; WDEF, maximum width of distal diaphysis; WDEH, maximum width of distal epiphysis; WDF, minimum width of diaphysis; WDH, minimum width of diaphysis; WHH, width of humeral head; WLCF, width of lateral condyle; WMCF, width of medial condyle; WPDH, width of proximal epiphysis, humeral head to lesser tubercle; WPEF, maximum width of proximal diaphysis; WTA, width of trochlea in anterior view; WTD, width of trochlea and capitulum in posterior view.
Morphometric analysis.—To quantify the morphological variation inherent in phocid humeri and femora, we performed a principal component analysis (PCA) using a covariance matrix produced from the limb measurement data. Three sets of analyses in total were performed. The first set of analyses incorporated data only from modern taxa and made use of both humeral and femoral measurements. We also performed separate analyses of just the humeral measurements, and just the femoral measurements. Each of these latter two analyses incorporated both extant and fossil taxa. We also performed discriminant function analysis (DFA) to determine how accurately our modern limb bone data set could assign fossil taxa to each of the two major subfamilies of earless seal, the Monachinae and the Phocinae, as well as the six tribes recognized for extant seals. All PCA and DFA analyses were implemented in R 2.12.1 (R Core Team 2013).
Results
Principal Components Analyses.—When measurement data from both femora and humeri of extant phocids are analyzed using PCA, 4 components are needed to explain ~93 of the variation observed (Fig. 2). The first component explains the majority of the variation (~81%), and represents variation in body size (Fig. 2A). Extremely large taxa such as male Mirounga have extremely positive scores, while small taxa such as Pusa have extremely negative scores. With the exception of taxa displaying sexual dimorphism (e.g., Mirounga), most species occupy a fairly narrow morphospace along this axis, although there is overlap between species of similar size.
The remaining components explains more trivial amounts of variation in limb bone morphology. The second component accounts for ~7% of the variation observed and represents size of the intercondyloid fossa of the femur and depth of the coronoid fossa of the humerus (Fig. 2A). Taxa with more positive scores, including Erignathus, Leptonychotes, and some Cystophora have a proportionally wide intercondyloid notch and shallow coronoid fossa, while taxa with more negative scores such as Mirounga have a narrow intercondyloid notch and deep coronoid fossa. Most taxa have values intermediate between these two extremes, leading to poor separation of taxa along this axis. Principal component three explains ~4% of the variation and represents changes in size of the patellar surface and length of the femur on the lateral side (Fig. 2B), with taxa with shorter femur lengths and proportionally smaller patellar surfaces having more negative scores. Within PC3, Erignathus is clearly segregated from all other phocids as having the most positive PC3 scores, while members of the clade Lobodontini have the most negative PC3 scores, and form a cluster with minimal overlap between other monachine seals and phocines. Principal component four explains only ~2 of the variation in the dataset, and poorly segregates taxa (Fig. 2B). This component seems to represent variation in size of the medial condyle of the femur and width of the diaphysis of the femur, with taxa with positive scores having a proportionally longer medial condyle and narrower diaphysis, and vice versa for taxa with negative scores.
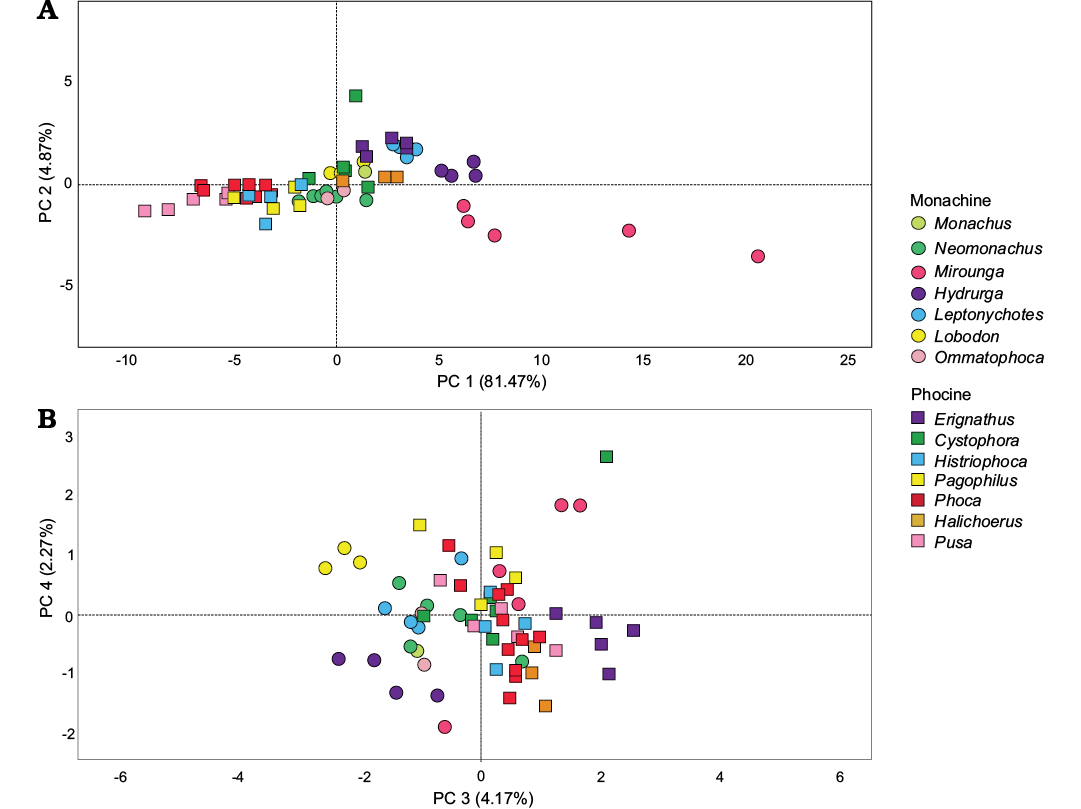
Fig. 2. Results of PCA for the combined dataset of both humeral and femoral measurement data, for extant earless seal taxa. A. PC1 vs. PC2. B. PC3 vs. PC4.
When only the humerus is examined, four components are needed to explain ~95% of the variation (Fig. 3). As with the combined limb dataset, the overwhelming majority of the variation (82%) in PC1 can be explained by variation in size of the humerus, with male Mirounga again having the most positive PC1 scores and Pusa has the most negative (Fig. 3A). Among fossil taxa, Callophoca specimens possess the most positive PC1 scores, with one individual (USNM 263656) overlapping in morphospace with Hydrurga and female Mirounga. Two other individuals (USNM 412266 and USNM 421543) are widely separated from this individual with more negative scores, and overlap in morphospace with Leptonychotes and Halichoerus. Auroraphoca, Acrophoca, and Piscophoca are close together and possess positive scores. These three taxa all overlap with a range of taxa including Halichoerus, Erignathus, Cystophora, Neomonachus, Monachus, and Leptonychotes. Phocanella specimens cluster relatively close together, overlapping in morphospace with Pagophilus, Histriophoca, and Phoca.
Principal component two explains ~7% of the variation observed in humerus anatomy (Fig. 3A). Taxa with positive scores have deeper coronoid fossae, as well as wide deltopectoral crests, which are narrowly separated from the lesser tubercle of the humerus. In contrast, taxa with negative scores have shallower coronoid fossae and narrower deltopectoral crests which are well separated from the head of the humerus. Taxa with more positive scores include Mirounga and most phocines. Taxa with negative scores include most of the remainder of the monachines, with Monachus, Lobodon, Leptonychotes, and Hydrurga having the most negative scores. Phocanella, Auroraphoca, and Callophoca (USNM 263656) possess positive PC2 scores, although they overlap with a wide variety of other phocids. Acrophoca, Piscophoca, and the remainder of Callophoca all possess negative scores, although not as negative as that observed for most lobodontines.
The remaining principal components explain increasingly trivial amounts of variation (Fig. 3B). Principal component three explains ~4% of the variation. Taxa with positive PC3 scores possess deeper coronoid fossae and narrower deltopectoral crests, while taxa with negative PC3 scores possess shallower coronoid fossae and wider deltopectoral crests. Principal component four explains ~2% of the variation and represents variation in the spacing between the tubercles of the humerus, with taxa with positive scores having greater spacing. Generally, for both PC3 and PC4, taxa are poorly segregated by either genus or higher level classification.
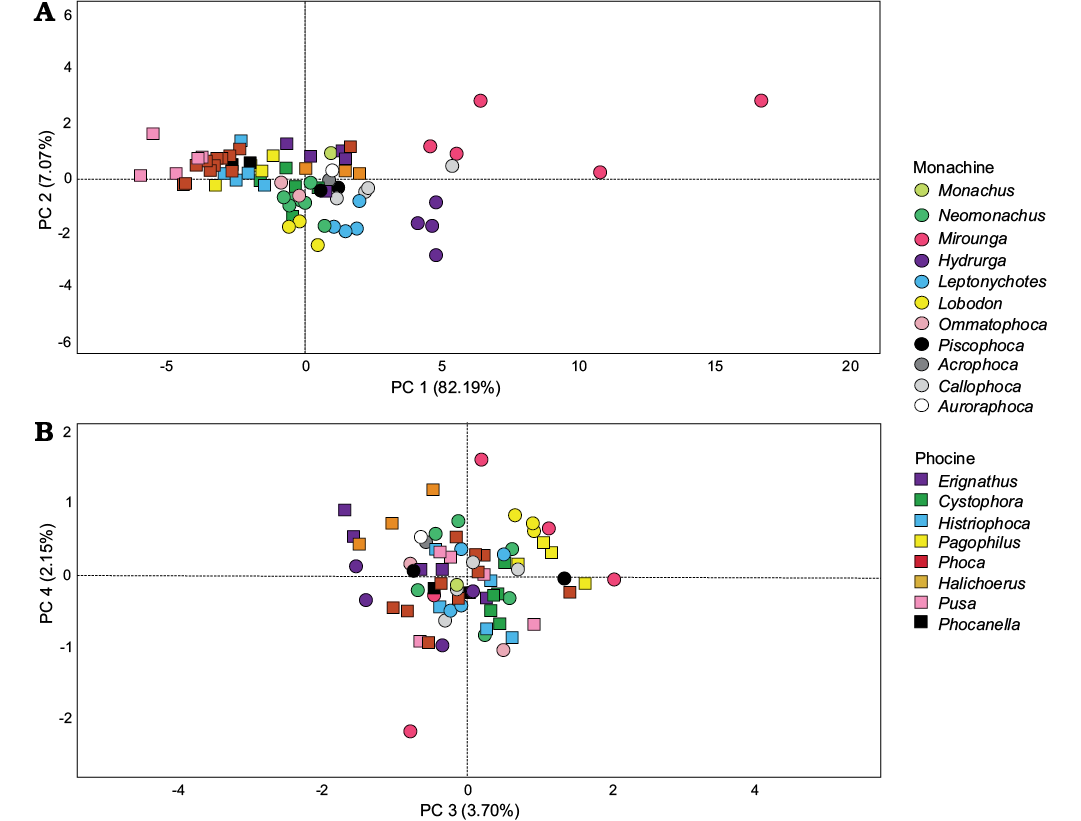
Fig. 3. Results of PCA for the humerus measurement data, including extant and fossil earless seal taxa. A. PC1 vs. PC2. B. PC3 vs. PC4.
When only the femur is examined, the first four components explain 95% of the variation observed (Fig. 4). As with the humerus, size explains ~84% of the variation, with the larger monachines having more positive scores than the smaller phocines (Fig. 4A). Among fossil taxa, Callophoca specimens have the most positive scores, and cluster together. Their PC1 scores are lower than monachines such as Leptonychotes, Hydrurga, and Mirounga, and they overlap with the larger phocine taxa such as Erignathus, Cystophora, and Halichoerus. Phocanella is tightly clustered towards the center of the spread of scores, with individuals with both slightly negative and slightly positive scores. Leptophoca is represented in the dataset by two putative species which are widely separated on the PC1 axis, with Leptophoca proxima having significantly more positive PC1 scores than L. amphiatlantica. The former clusters near Phocanella, while the latter occupies a position with the Phoca morphospace, and near the small fossil monachine Pontophoca. Monachopsis and Cryptophoca have similar PC1 scores and overlap with phocine taxa including Pagophilus, Histriophoca, and Phoca. Praepusa possesses the most negative PC1 score in this study, even more negative than the extant genus Pusa, which includes the smallest seals alive today.
Principal component two explains 6% of the variation and represents width of the intercondyloid notch (Fig. 4A), with a wide notch represented by more positive scores. Poor segregation of taxa occurs along this axis. This largely distinguishes one individual Cystophora (USNM 55041) from all other phocids in the analysis, although Mirounga is also somewhat separated from other taxa out on the basis of negative scores for this component, and Hydrurga has somewhat more positive scores than most phocids. Otherwise there is broad overlap in PC2 scores. Most fossil taxa included in the analysis occupy the middle region of the morphospace, although at least one Callophoca (USNM 437683) has a somewhat high PC2 score and overlaps with Hydrurga.
Principal component three explains ~3.5% of the variation and represents change in the size of the intercondyloid notch and length of the lateral side of the femur (Fig. 4B). Taxa with positive scores have wide intercondyloid notches, but a relatively short femur, while taxa with negative scores have a narrow intercondyloid notch and taller femur. This component seems to mostly distinguish monachine from phocine taxa, although with some overlap, with monachines having more positive scores. Taxa that appear to overlap both clades include Monachus, Neomonachus, Phoca, Pusa, and Cystophora. Among fossil taxa, Callophoca, Pontophoca, and Praepusa both have positive scores, while Phocanella, Monachopsis, Cryptophoca, and Leptophoca have negative scores.
Principal component four represents only 1.5% of the variation, and represents length of the medial condyle, size of the patellar surface, and width of the diaphysis (Fig. 4B). Taxa with positive scores possess a long medial condyle but short patellar surface and narrow diaphysis. Taxa with negative scores possess a short medial condyle but a tall patellar surface and wide diaphysis. Within the PCA plot, Hydrurga has the most positive scores and Mirounga and Cystophora the most negative. Other taxa occupy a middle ground between these extremes. Among the fossil taxa, Leptophoca proxima has the most positive PC4 score, and is in between Hydrurga and Phoca, although closer to the latter. Callophoca has the most negative scores, broadly overlapping with Histriophoca and Cystophora. The other taxa occupy the central portion of the total morphospace between these extremes.
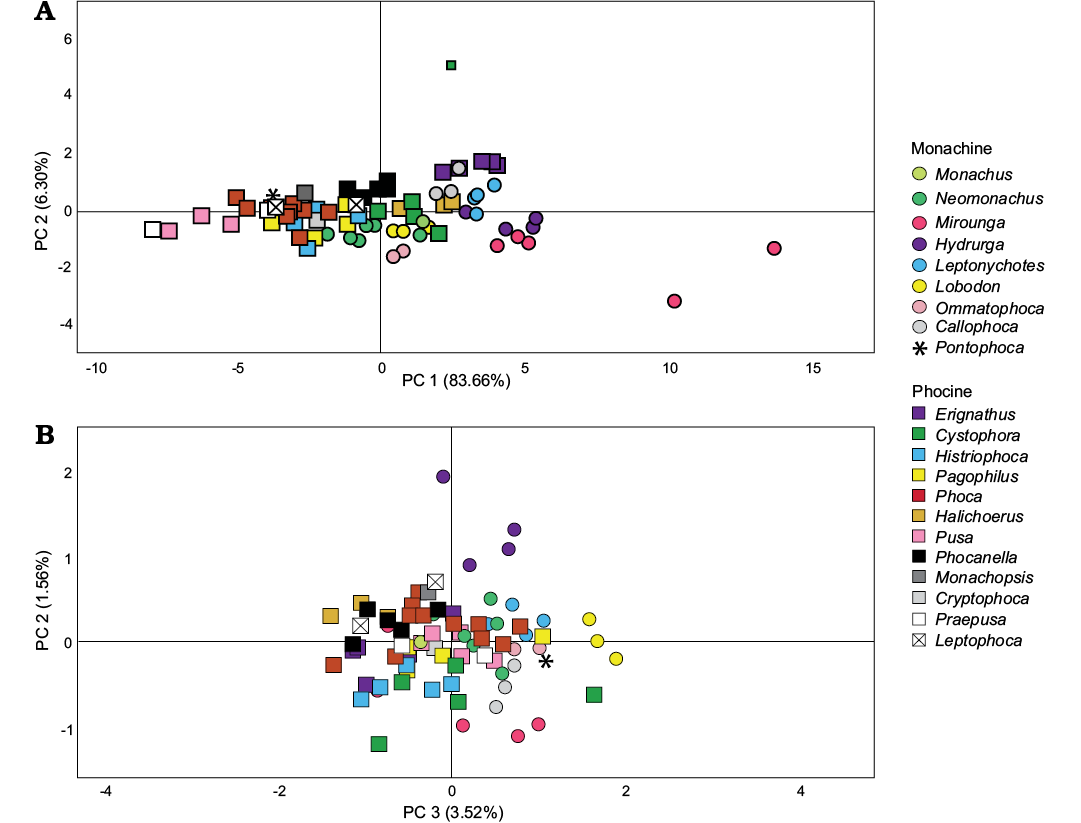
Fig. 4. Results of PCA for the femur measurement data, including extant and fossil earless seal taxa. A. PC1 vs. PC2. B. PC3 vs. PC4.
Discriminant Function Analysis.—Discriminant function analysis using data from the humerus performed well in classifying fossil taxa by subfamily (Table 2). Piscophoca, Acrophoca, Callophoca, and Auroraphoca were all classified as monachines, and Phocanella was classified as a phocine, all with posterior probabilities of 0.99 or greater. Posterior probabilities were also high for classification to tribe, although with greater discrepancies between specimens. Piscophoca, Acrophoca, Callophoca, and Auroraphoca were all classified as monk seals (Monachini), all with posterior probabilities greater than 0.98. The two specimens of Phocanella pumila, however, were classified as belonging to different tribes. One specimen, USNM 329059, was classified as belonging to Phocini with a posterior probability of 1.0. The other specimen, USNM 171151, was classified as a hooded seal (Cystophorini), but only with a posterior probability of 0.94.
In contrast to the humerus, the femur did not perform as well in classifying specimens by subfamily or tribe. Callophoca was classified as a monachine, but posterior probabilities ranged from 0.63 to 0.99. Pontophoca was also classified as monachine, with a posterior probability of 1.0. Monachopsis, Cryptophoca, Praepusa, Leptophoca, and Phocanella were all classified as phocines with posterior probabilities of 1.0. At the tribal level, specimens of Callophoca were identified as Antarctic seals (lobodontini) and Pontophoca as a monk seal (monachini), all with posterior probabilities of 1.0. Monachopsis, Cryptophoca, Praepusa, Leptophoca, and Phocanella were all classified as belonging to the tribe Phocini, all with posterior probabilities of greater than 0.99, with Leptophoca ampiatlantica having a posterior probability of 0.99 and all other specimens having a posterior probability of 1.0.
Table 2. Discriminant function analysis classification of fossil seals by subfamily and tribe. Taxa with discordance in classification, due to differences in results from humeral and femoral data, indicated in bold. Percents represent probability of correct classification; na, not available.
|
Taxon |
Specimen |
Subfamily |
Tribe |
||
|
humerus |
femur |
humerus |
femur |
||
|
Piscophoca pacifica |
USNM 360406 (cast: MNHN SAS564) |
Monachinae (99.99%) |
na |
Monachini (98.6%) |
na |
|
Piscophoca sp. |
USNM 443879 |
Monachinae (99.99%) |
na |
Monachini (99.67%) |
na |
|
Acrophoca longirostris |
USNM 360407 (cast: MNHN SAS563) |
Monachinae (99.99%) |
na |
Monachini (98.6%) |
na |
|
Callophoca obscura |
USNM 263656 |
Monachinae (99.99%) |
na |
Monachini (99.99%) |
na |
|
USNM 412298 |
Monachinae (99.99%) |
na |
Monachini (99.99%) |
na |
|
|
USNM 412266 |
Monachinae (99.99%) |
na |
Monachini (99.99%) |
na |
|
|
USNM 421543 |
Monachinae (99.99%) |
na |
Monachini (99.32%) |
na |
|
|
USNM 437849 |
na |
Monachinae (99.13%) |
na |
Lobodontini (99.77%) |
|
|
USNM 244061 |
na |
Monachinae (62.82%) |
na |
Lobodontini (99.99%) |
|
|
USNM 437683 |
na |
Monachinae (78.85%) |
na |
Lobodontini (99.99%) |
|
|
Auroraphoca atlantica |
USNM 481812 |
Monachinae (99.99%) |
na |
Monachini (99.9%) |
na |
|
Pontophoca sarmatica |
USNM 214980 (cast: LPB259 III) |
na |
Monachinae (99.99%) |
na |
Monachini (99.99%) |
|
Leptophoca proxima |
USNM 559330 |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
Leptophoca amphiatlantica |
USNM 321926 |
na |
Phocinae (99.99%) |
na |
Phocini (98.83%) |
|
Phocanella pumila |
USNM 329059 |
Phocinae (99.94%) |
na |
Phocini (99.93%) |
na |
|
USNM 171151 |
Phocinae (99.98%) |
na |
Cystophorini (94.27%) |
na |
|
|
USNM 305283 |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
|
USNM 329060 |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
|
USNM 175217 |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
|
USNM 181649 |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
|
USNM 481569 |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
|
Monachopsis pontica |
USNM 214975 (cast: LPB 21) |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
Cryptophoca maeotica |
USNM 214979 (cast: LPB 259 II) |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
Praepusa pannonica? |
USNM 214978 (cast: LPB 5) |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
|
Praepusa vindobonensis |
USNM 214933 (cast: LPB 158) |
na |
Phocinae (99.99%) |
na |
Phocini (99.99%) |
Discussion
Assessment of past qualitative characters of the humerus and femur.—Within the Phocidae, the split between Monachinae and Phocinae is the most ancient (Higdon et al. 2007; Fulton and Strobeck 2010a), with nearly all known fossil forms being placed within one of these two clades, with only a few controversial taxa potentially placed outside the crown group (e.g., Devinophoca; Koretsky and Holec 2002). Given this ancient diversification, we might reasonably expect that divergence in limb bone morphology between these two clades may also be significant, and indeed several morphological characters have been used in prior studies that seem to reasonably refer isolated limb bones to one of these two major groups. Nine characters of the humerus and seven characters of the femur have been variously used in recent rigorous phylogenetic studies for this purpose (Bininda-Emonds and Russell 1996; Amson and Muizon 2013; Berta et al. 2015; Dewaele et al. 2017a, 2018a) and are reviewed below. A full appraisal of all phylogenetic characters related to the humerus and femur that may define smaller clades or distinguish individual taxa is beyond the scope of this study; we focus on these characters as we would expect differences at the subfamily level to be far more extreme than those that might separate more recently diverged subclades, such as Miroungini and Lobodontini for instance.
Entepicondylar foramen (King 1966; Berta and Wyss 1994; Bininda-Emonds and Russell 1996; Cozzuol 2001; Amson and Muizon 2013; Berta et al. 2015; Dewaele et al. 2018a).—The entepicondylar (or supracondylar; Berta et al. 2015) foramen is absent in all extant monachines, although some fossil monachine taxa such as Frisiphoca, Noriphoca, and Homiphoca possess this feature. While the feature is useful, it should be noted that variation in possession of the entepicondylar foramen has been observed in some extant phocines such as Halichoerus and Pagophilus (Bininda-Emonds and Russell 1996), with some individuals either completely lacking this foramen on both limbs or only on possessing an entepicondylar foramen on a single limb. Its widespread presence in different fossil monachines casts uncertainty on its utility in isolation to separate members of the two phocine subfamilies.
Supinator ridge (Berta and Wyss 1994; Bininda-Emonds and Russell 1996; Cozzuol 2001; Amson and Muizon 2013; Berta et al. 2015; Dewaele et al. 2017a, 2018a).—Another qualitative character is the development of the supinator ridge, also known as the epicondylar crest. In general, monachines lack a distinct supinator ridge, while it is weakly to strongly developed in phocines. Exceptions to the general trend within monachines include Neomonachus, which has a somewhat weakly developed supinator ridge, and Frisiphoca affine, which has a strongly developed ridge. Phocines have a well-developed supinator ridge for the most part, although this ridge is more weakly developed in Erignathus and Cystophora. Presence of a strongly developed ridge is thus likely a synapomorphy for Phocini, but taxa with a more weakly developed ridge may not be easily distinguished at the subfamily level.
Intertubercular groove (Berta et al. 2015; Dewaele et al. 2017a).—The intertubercular or bicipital groove is also a feature of interest on the humerus, although an anatomical landmark whose morphology has been confusingly described in phylogenetic studies. Berta et al. (2015) include a two state character representing the bicipital groove, with an absent or shallow groove considered the ancestral condition retained by Erignathus, Cystophora, Neomonachus tropicalis, Homiphoca, and Leptophoca. The derived character state, presence of a deep groove, characterizes Monachus, Neomonachus schauinslandi, Mirounga, lobodontine seals, and the fossil taxa Pliophoca, Callophoca, Acrophoca, and Piscophoca. However the coding of this character seems to be in error ({first name in full} Berta personal communication 2018), and taxa that were coded for a shallow or absent groove should instead be considered as having a deep groove, and vice versa. Dewaele et al. (2017a) also uses this character, although referring to it as an intertubercular groove. They define three states, with the ancestral condition being retention of a narrow and deep groove (state 0), an undefined intermediate state (state 1), and a broad and shallow groove (state 2). In the latter character matrix, Ommatophoca, Devinophoca, and most phocines possess a deep groove, while a broad and shallow groove characterizes Leptonychotes, Lobodon, Hydrurga, Monachus, and Mirounga. The intermediate condition is possessed by Piscophoca, Cystophora, Erignathus, Pagophilus, Praepusa vindobonensis, Praepusa boeska, and Leptophoca. Unfortunately, this feature exists on a continuum, and it’s unclear exactly how deep or shallow a feature must be to be “intermediate”. We had one measurement, the maximum distance between the greater and lesser tubercle, which appears to capture some of this variation relevant to this feature. Overall this measurement performed poorly separating taxa when only measurement ratios are examined. Some species have exceptionally broad separation between tubercles (Monachus, Leptonychotes, Lobodon), and other taxa have particularly narrow separation (Mirounga).However, most taxa (including the fossil specimens included in this study) showed extensive individual variation and a broad range of values between these extremes. Nevertheless, this feature was identified as significantly contributing to variation in PCs 2 and 4 of our PCA analysis of the humerus. While this character may have limited ability to differentiate phocid humeri by subfamily, it may still have some phylogenetic importance if quantified with a larger sample of phocid specimens.
Height of the lesser tubercle in relation to the humeral head (Berta and Wyss 1994; Koretsky 2001; Cozzuol 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Amson and Muizon 2013; Berta et al. 2015; Dewaele et al. 2017a, 2018a).—Another character used to describe the morphology of the tubercles is the height of the lesser tubercle in relation to the head. Dewaele et al. (2017a) defined two states for this character, the ancestral condition where the lesser tubercle extended to the same level or higher as the head of the humerus, and a derived condition where the lesser tubercle extended only to below the level of the head. The derived condition was found to only be present in all extant phocines as well as Lobodon, Hydrurga, and Ommatophoca. All fossil phocids retain the ancestral condition. While we did not quantify this character in our analysis, we largely agree with past character coding, although it is of probably limited use in separating monachine or phocine taxa only known from isolated elements.
Length of the deltopectoral crest (Wyss 1988; Bininda-Emonds and Russell 1996; Koretsky 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Amson and Muizon 2013; Berta et al. 2015; Dewaele et al. 2017a, 2018a).—Morphology of the deltopectoral crest is variable within phocid seals, with length of the deltopectoral crest as well as how the crest contacts the diaphysis used as phylogenetic characters in different matrices. Bininida-Emonds and Russell (1996), modifying the character of Wyss (1988), included this character in their analysis with three states, a short deltopectoral crest that is less than or equal to half the length of the humerus (state 0); a long deltopectoral crest that is greater than half the length of the humerus in length (state 1); and complete absence of the crest (state 2; this state is not found in pinnipeds). Later authors largely followed suite. Using these character states, extant monachines (with the exception of some individuals of Mirounga and Hydrurga) were then considered as having long deltopectoral crests, while phocine taxa (except for the fossil forms Leptophoca, Praepusa, and Kawas) were coded as possessing short deltopectoral crests.
Quantifying this character and comparing it to total humerus length, we found very different patterns. Most phocids have long deltopectoral crests that comprise over 50% of their total humerus length, with Monachus (72.7%), Neomonachus (66–72%), and Mirounga (60–70%) having the longest crests. Hydrurga (54–68%), Leptonychotes (61–66%), Lobodon (58–63%), Pagophilus (59–65%), and Halichoerus (60–68%) possess deltopectoral crests of intermediate length. All other taxa (Ommatophoca, Erignathus, Cystophora, Histriophoca, Phoca, and Pusa) have relatively short deltopectoral crests (61–47%). Among fossil taxa examined, Acrophoca (69.06%), Auroraphoca (74.62%), and Piscophoca (76.60–76.66%) had long crests. Both Callophoca (43.72–73.33%) and Phocanella (62.69–73.81%) were more variable, although they generally also had long deltopectoral crests. Discrepancies between our quantitative data and past studies may largely be a result of the difficulty in some taxa of determining distally where exactly the deltopectoral crest “ends”, given its gradual merging on the shaft. Length of the deltopectoral crest was not found to be a major source of variation in our PCA, however variation in this feature may be better captured using 3d morphometric methods.
Distal merger of deltopectoral crest onto shaft of the humerus (Berta and Wyss 1994; Bininda-Emonds and Russell 1996; Dewaele et al. 2017a, 2018a).—The merging of the deltopectoral crest is generally treated as a separate two state character, with the crest either merging smoothly with the shaft (as in most Monachinae) or abruptly (as in Phocinae). This description is somewhat misleading; to some degree the deltopectoral crest merges “smoothly” in all taxa. What this character actually alludes to is the overall shape of the crest. In most monachine taxa the crest forms an arc, while the distal portion of the deltopectoral crest in phocine seals usually forms ~90° angle between the crest and shaft. Exceptions to these overall patterns in modern taxa include Leptonychotes, which possesses the phocine condition, and Monachus, which has a condition somewhat intermediate between both states, although it’s usually characterized as having a smooth merger of the crest to the shaft. Among fossil taxa, Acrophoca, Auroraphoca, Callophoca, and Piscophoca all had rounded crests, while Phocanella had a deltopectoral crest which distally forms an angle between crest and shaft. Overall, although there are exceptions, shape of the deltopectoral crest seems to perform well at distinguishing monachine and phocine seals.
Beyond length and shape, another aspect of the morphology of the deltopectoral crest, width of the deltopectoral crest (Fig. 5A), was found to be also useful. Overall, monachine taxa have narrow deltopectoral crests, with most taxa having crest ratios below 125% of the width of the diaphysis. Among phocines, only Cystophora (71–113%) and Pagophilus (86–129%) have deltopectoral crests of comparable width. Erignathus (100–183%), Phoca (88–203%), Halichoerus (156–162%), and Pusa (110–225%) possess particularly wide deltopectoral crests. Width of the deltopectoral crest as a character should be viewed cautiously however, as monachines also possess proportionally wider diaphyses than phocines (see below). Width of the deltopectoral crest was found to be a significant driver of variation for both PCs two and three of the humerus only analysis.
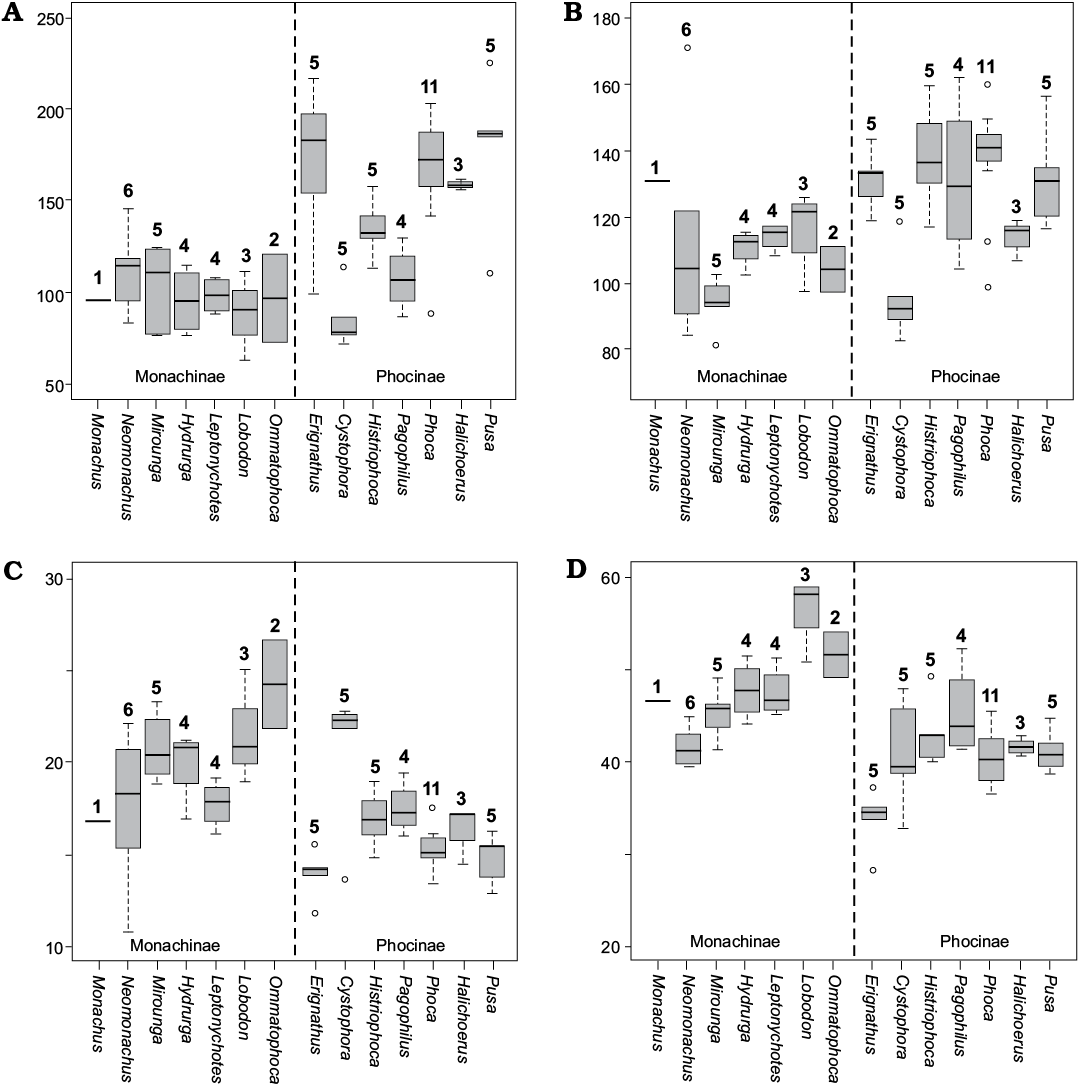
Fig. 5. Boxplots of humeral measurement ratios useful for distinguishing monachine from phocine seal taxa: width of deltoid crest (A), trochlea (B), proximal end of humerus (D) as % of diaphysis width, and width of diaphysis as % of humerus length (C). Subfamilies separated by dashed line. Measurements that fall outside the range of values represented by the upper and lower quartiles indicated by circles. Sample size for each genus listed above the boxplot.
Diameter of trochlea (Dewaele et al. 2017a).—Dewaele et al. (2017a) defined a two state character describing differences in the diameter or width of the trochlea. The ancestral condition of this character was defined as possession of a trochlea the same diameter as the “distal head”, with the derived state as possession of a trochlea with a diameter significantly greater than the “distal head”. It is not clear what is meant by distal head, but our study does indicate that when quantified major differences can be observed between monachine and phocine taxa for this character (Fig. 5C).
Monachinae in general all possess relatively narrow trochlea (>130%) in proportion to the width of the diaphysis (Fig. 5B). Taxa with the smallest trochlea include Neomonachus schauinslandi (85–94%) and Mirounga (82–103%). The phocine Cystophora also possesses an unusually small trochlea (83–119%). Taxa with somewhat larger trochlea include the remainder of Monachinae: Monachus (131%), Hydrurga (102–116%), Leptonychotes (108–117%), Lobodon (98–126%), and Ommatophoca (98–111%). Pagophilus also has a trochlea diameter within this range of values (105–162%). The remainder of phocines all are generally in possession of a fairly wide trochlea, with some outliers. These include Erignathus (119–143%), Histriophoca (117–160%), Phoca (99–159%), Halichoerus (107–119%), and Pusa (117–156%). Among the fossil taxa examined in this analysis, Acrophoca (84%), Auroraphoca (101–107%), Callophoca (85–105%), and Piscophoca (92–97%) all have a fairly narrow trochlea, while Leptophoca (160%) and Phocanella (112–144%) possess a wider trochlea.
Development of distal portion of shaft of humerus (Berta et al. 2015).—Berta et al. (2015) characterize variation in this portion of the humerus using a two state character. In the ancestral condition, the distal portion of the shaft is more developed transversely than the proximal portion of the shaft, while in the derived state, there is less development transversely. Comparing the overall morphology of the diaphysis, it is difficult to ascertain a consistent difference between the proximal and distal portions of the diaphysis between phocines and monachines, and the character itself if vaguely described.
However, when examining the diameter of the diaphysis as a ratio to the total humerus length, consistent differences can be seen in the robustness of the shaft within Phocidae (Fig. 5C). Neomonachus schauinslandi (20–23%), Mirounga (19–23%), Hydrurga (17–21%), Lobodon (19–25%), and Ommatophoca (22–27%) have a particular thick and robust diaphysis, with only Cystophora (14–23%) within the Phocinae having particularly thick diaphysis. The remainder of phocines (12–19%) all have a relatively thin diaphysis, along with Monachus (~17%), N. tropicalis (11–17%), and Leptonychotes (16–19%).
Collo-diaphyseal angle of femoral head (Amson and Muizon 2013; Dewaele et al. 2017a).—The relative angle of projection of the head of the femur varies within Phocidae. In the ancestral condition, the head is angled more medially, producing a high angle between the head and neck of the femur and the greater trochanter. In the derived condition, the head is angled more proximally than medially, resulting in a low angle between the head and neck of the femur and the greater trochanter. We had difficulty in assessing the character states of taxa for this character, given that differences in morphology of the neck, trochanter, and diaphysis can make it difficult to objectively classify taxa. Furthermore, this character is likely to be at least in part correlated with one of the following characters, elevation of greater trochanter relative to femoral head, given that a more proximally oriented femoral head will be more elevated relative to the trochanter than a more medial head. We encourage future quantification of this character through detailed angle measurements.
Trochanteric fossa (Berta and Wyss 1994; Bininda-Emonds and Russell 1996; Berta et al. 2015; Dewaele et al. 2018a).—Extant phocines possess a distinct trochanteric fossa, and the presence or absence of this fossa has been used in phylogenetic analyses of phocid relationships. Generally, this is treated as a two state character, with the presence of a trochanteric fossa the ancestral condition and its reduction the derived state, although Bininda-Emonds and Russell (1996) divided the derived state into shallow, medium, and deep, without quantification. There appears to be some degree of individual variation in development of this feature, which may explain discrepancies in character coding between Berta et al. (2015) and Dewaele et al. (2018a). Cursory examination of this feature in taxa included within this analysis found a well-developed, although sometimes variable, trochanteric fossa in nearly all extant phocines, as well as Monachus. Erignathus was found to be variable for this character, with some taxa having a weakly developed trochanteric fossa, while in other individuals it was absent. The remainder of extant monachines lack a trochanteric fossa. Among the fossil taxa included within this study, a well-developed trochanteric fossa is found in the fossil phocines Cryptophoca, Leptophoca, Monachopsis, Phocanella, and Praepusa. In addition to these phocine taxa, Pontophoca as well as two presumed specimens of Callophoca (USNM 437683 and USNM 437849) also possess a well-developed trochanteric fossa. One other specimen of Callophoca (USNM 244061) completely lacked a trochanteric fossa.
Elevation of greater trochanter relative to femoral head (King 1966; Muizon 1982; Berta and Wyss 1994; Bininda-Emonds and Russell 1996; Koretsky 2001; Cozzuol 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Berta et al. 2015).—This character has variously been used to characterize phocines and monachines, with phocines generally having a greater trochanter that extends proximally past the level of the head, while monachines have a greater trochanter that extends to a level proximally below head. Bininda-Emonds and Russell (1996) discussed this character and added an additional intermediate state, where the greater trochanter reaches the same level as the head, and considered the monachine condition the ancestral character state. Extant taxa with a comparatively low greater trochanter include Mirounga, Hydrurga, Leptonychotes, Lobodon, and Ommatophoca. Taxa where the greater trochanter extends to the same level as the head include Monachus, Neomonachus, Erignathus, Pagophilus, and Histriophoca. Taxa where the greater trochanter extends proximally past the head include Cystophora, Phoca, Halichoerus, and Pusa. Note there is likely some individual variation in this character, with Bininda-Emonds and Russell (1996) noting the intermediate condition for Pusa siberica and Phoca largha, and in some taxa we observed (Hydrurga), the greater trochanter only barely extends past the level of the head. In examining the fossil taxa with femora included in this study, Callophoca either has a greater trochanter lower or at least at the same level as the femoral head, while the other monachine Pontophoca has a greater trochanter which extends past the level of the femoral head. All fossil phocines (Cryptophoca, Leptophoca, Monachopsis, Phocanella, and Praepusa) have greater greater trochanters that extend past the level of the femoral head, although the degree of this extension does vary between taxa, with Praepusa having minimal extension and Phocanella having a much higher degree of extension.
Measurements examined in our study which relate to this and the prior character are measurements of the femur on the lateral and medial sides. These measurements capture variation in both development of the greater trochanter as well as orientation of the femoral head. When these measurements are expressed as a ratio (LLF/MLF; Fig. 6A), phocines generally have greater values (92–102%) than monachines (82–93%), although there is overlap with Monachus (~94%) and Ommatophoca (92–96%). The monachine Callophoca generally shows low ratio values (88–90%), in common with extant monachines, although the presumptive fossil monachine Pontophoca has an unusually large value (100%). All of the fossil phocine taxa examined in this study, including Cryptophoca (~98%), Leptophoca (100–101%), Monachopsis (~100), Phocanella (99–106%), and Praepusa (99–102%), have low ratio values. Variation in the length of the femur related to this feature is a major driver of variation for PC 3 of the combined limb bone analysis and PC 2 of the femur analysis.

Fig. 6. Boxplots of femoral measurement ratios useful for distinguishing monachine from phocine seal taxa: length of femur medially (A), length of medial condyle (B), diameter of femur neck (C), and width of femur head (D) as % of femur length laterally. Subfamilies separated by dashed line. Measurements that fall outside the range of values represented by the upper and lower quartiles indicated by circles. Sample size for each genus listed above the boxplot.
Width of the distal end of the femur (Bininda-Emonds and Russell 1996; Koretsky 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Berta et al. 2015; Dewaele et al. 2017a, 2018a).—Dewaele et al. (2018a) identify a three state character relating to the comparative width of the distal and proximal epiphyses of the femur. In the ancestral condition, the distal epiphysis is wider than proximal, while the derived states include both epiphyses being of the same width (state 1), or the distal epiphysis narrower than the proximal epiphysis (state 2). Dewaele et al. (2018a) coded Monachinae and most fossil Phocinae as possessing the ancestral condition. Cystophora, Erignathus, Histriophoca, Pagophilus, Phoca, and the fossil taxon Praepusa vindobonenesis were coded as having state 1, while Acrophoca and Devinophoca were coded as possessing state 2.
We were able to quantify this character, but failed to find consistent differences in width of distal epiphyses compared to the proximal epiphysis between the subfamilies of Phocidae. Neomonachus (89–102%) and Erignathus (103–107%) have particularly wide distal epiphyses, while Monachus (~84%) and Lobodon (82–88%) have particularly narrow distal epiphyses. Most other taxa have development of distal epiphyses intermediate between these extremes. This suggests that overall this character is likely to be prone to extensive individual variation and to be of limited use in phylogenetic analyses.
Width of the diaphysis of femur (Koretsky 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Dewaele et al. 2018a).—Dewaele et al. (2018a) found that the thickness of the diaphysis of the femur varies between Monachinae and Phocinae, with monachines having the ancestral condition (state 0), a narrow diaphysis that is less than or equal to two-thirds of the width of the proximal epiphysis. Phocines, with the exception of Leptophoca and Nanophoca, have a femur diaphysis that is wide and more than two-thirds of the width of the proximal epiphysis.
In our analysis, we found that this measurement was useful in distinguishing taxa, but not at the subfamilial level, nor did our quantified results match up with the patterns described by Dewaele et al. (2018a). Mirounga (61–75%) and Ommatophoca (63–73%) were found to have the widest femoral diaphyses. Most of the other phocids had more intermediate values (46–64%), however Erignathus (44–48%) and Phoca (38–51%) have particularly narrow diaphyses. Among fossil taxa, Callophoca (52–65%) and Pontophoca (~64%) had intermediately sized diaphyses, thicker than the narrow diaphyses of fossil phocines, which ranged from 43–56% of the proximal epiphysis width. Thus this character appears to perform well at separating fossil members of these subfamilies, but less well with modern taxa. Variation in this feature is reflected in PC 4 of both the combined limb bone PCA and the Femur PCA
Size of femoral condyles (Koretsky 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Berta et al. 2015; Dewaele et al. 2018a).—This character is usually considered a two state character. Koretsky (2001) originally defined this character, with the medial and lateral condyles being of unequal size the ancestral condition (state 0), and in the derived condition the condyles were approximately equal in size (state 1). Later studies (Berta et al. 2015; Dewaele et al. 2018a) reversed the polarity of this character, treating the possession of femoral condyles of unequal size as the derived condition. No matter which is the ancestral condition, possession of condyles which are unequal in size characterizes all members of Phocinae (Koretsky 2001), but is also widely found in many monachinae taxa, including Monachus, Pliophoca, Mirounga, and Acrophoca (Berta et al. 2015; Dewaele et al. 2018a), making this character less useful in distinguishing the two subfamilies.
In our study, this character may be related to the length of the medial condyle of the femur, which was found, when compared to total femoral length directly (Fig. 6B), and inferred by the PCA analysis, as reliably separating monachine and phocine taxa. Monachines generally have long medial condyles (22–32% LLF), while phocines have shorter medial condyles (13–29% LLF). Within Monachinae, Monachus has the shortest medial condyle (~22% LLF), while Lobodon has the longest (31–31% LLF). Within Phocinae, Erignathus has the shortest medial condyle (13–20% LLF), while Pagophilus has the longest (21–29%). In contrast to extant taxa however, fossil monachines have comparatively short medial condyles (Callophoca 19–21% LLF; Pontophoca ~20% LLF). Fossil phocine taxa have fairly short medial condyles, although with some variation. Leptophoca (18–21% LLF), Monachopsis (~17% LLF), Phocanella (17–19% LLF), and Praepusa pannonica (~18% LLF) have especially short medial condyles, with Cryptophoca (~22% LLF) and Praepusa vindobonensis (~21% LLF) having somewhat longer medial condyles. This character was also found to be a significant source of variation for PC 4 of the combined limb bone as well as femur PCA analysis.
New potential characters of the humerus and femur.—In examining the range of measurement values for the humerus and femur, we identified within the measurement data three novel characters previously undiscussed in prior phylogenetic studies which may be useful in future studies. When compared to the total humeral length, the proximal end of the humerus, specifically the width across the humerus from the lesser tubercle to the head of the humerus, is expanded in most monachine taxa (Fig. 5A). Taxa with the greatest expansion of the proximal end of the humerus include Lobodon (51–60%) and Ommatophoca (49–54%). Less developed but still wide are Monachus (~47%), Mirounga (41–49%), Hydrurga (44–52%), and Leptonychotes (45–51%). Taxa with narrower proximal ends of the humerus include Neomonachus (40–45%) and all phocines (28–52%), although there is some overlap with the prior taxa for both Cystophora (33–48%) and Pagophilus (41–52%). Erignathus has the narrowest proximal end of the humerus (28–37%). When the fossil taxa examined in this analysis are compared to modern taxa, Acrophoca (~46%), Auroraphoca (40–46%), Callophoca (40–45%), and Piscophoca (47–49%) all have expanded proximal ends of the humerus, while the phocine Phocanella displays minimal expansion (46–49%).
For the femur, two measurements were found to be particularly useful in separating out monachine and phocine taxa. The diameter of the neck compared to length of the femur was found to vary within taxa, with almost all monachines having particularly thick necks (20–33% LLF) compared to the slender necks of phocine seals (16–24% LLF; Fig. 6C). There is some overlap in values however, with Monachus (~20% LLF), Neomonachus (21–26% LLF), and Leptonychotes (22–28% LLF) having thinner necks which overlap the range of values seen in phocine seals. Among fossil taxa, Callophoca (24–29% LLF) has a particularly thick femoral neck, and to a lesser degree so does Pontophoca (~22% LLF). Within presumed fossil phocines, a narrow neck is seen in Cryptophoca (~19% LLF), Leptophoca (15–18% LLF), Monachopsis (~15% LLF), Phocanella (13–19% LLF), and Praepusa (15–18% LLF). Thickness of the femoral neck has been used as a character in phylogenetic analyses in the past (Koretsky 2001; Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013; Berta et al. 2015; Dewaele et al. 2018a), however was never quantified, and often included in analyses with very sparse taxon sampling (Koretsky and Grigorescu 2002; Dewaele et al. 2018a), potentially obscuring its usefulness as a phylogenetically informative character for subfamilial separation.
Another informative character of the femur that may allow separation of phocine from monachine seals is width of the head of the femur (Fig. 6D). Extant monachine all have fairly wide femoral heads (25–35% LLF), with phocines possessing narrower heads (16–27% LLF), with only minor overlap between Pagophilus (19–26% LLF) and monachine taxa. This feature may be less useful among fossil phocids however. Among fossil monachines, Callophoca has a femoral head intermediate in size between both clades (23–28% LLF), while Pontophoca has a relatively narrow femoral head (~23% LLF). Presumed fossil phocines generally show narrow femoral heads, such as Cryptophoca (~19% LLF), Leptophoca (17–19% LLF), Monachopsis (~19% LLF), Phocanella (18–21% LLF), and Praepusa (18–22% LLF). While size of the femoral head does not seem to have been used as character in previous phylogenetic analyses, the overall morphology of the head has been used by Koretsky and colleagues (Koretsky and Grigorescu 2002; Koretsky and Rahmat 2013); while not directly linked, overall shape of the head is likely to influence the size of this feature. In these studies, the shape of the head is characterized as round (state 0), flattened in a proximo-distal direction (1), and compressed in a medial lateral direction (state 2). Shape of the femoral head, however, did not separate the seals of different subfamilies.
Conclusions
Overall, our study has produced mixed results on the utility of the humerus and femur in distinguishing phocid taxa and higher level clades. When considering subfamily divisions, DFA generally placed fossil taxa within subfamilies as suggested by prior studies (Muizon 1982; Koretsky 2001; Koretsky and Grigorescu 2002; Koretsky and Ray 2008; Amson and Muizon 2013; Berta et al. 2015; Dewaele et al. 2018a), although overall the humerus performed slightly better than the femur. A review of phylogenetic characters used in past studies, while revealing many characters to be poorly quantified or prone to variation, did identify approximately seven characters of the humerus and four characters of the femur that performed fairly well at distinguishing members of these two subclades. In contrast to these findings, the PCA generally did a much poorer job of sorting taxa, with extensive overlap and body size differences explaining the majority of the variation. This suggests a very strong relationship between allometry and morphology of the forelimb bones. Generally speaking, extant Monachinae are much larger than extant Phocinae, and at least some of the morphological variation observed may be a consequences of this difference in body size. Allometry is known to have a strong effect on morphology (Gould 1966), and as body size changes the size and development of features of the skeleton, also changes in response to differences in strain that may be placed on skeletal elements from increases or decreases in body mass. For instance, a larger animal may be expected to have a more robust diaphysis, which in turn is likely to affect the ratios examined in our survey of individual limb bone characters and may drive differences in the overall morphology of individual limb bones.
Furthermore, we observed that many important characters, while useful in separating modern taxa, are often more variable within fossil taxa, which often possess a mosaic of traits possessed today by taxa of completely different clades or may have intermediate conditions not found in modern taxa. This increases the likelihood, especially for taxa only known by isolated limb bone materials, to be incorrectly assigned to the appropriate subfamily, and with more referred fossil material we may eventually discover that many presumed monachine and phocine taxa may actually represent stem taxa outside the crown group.
While referral of isolated humeri and femora to extant subfamilies appears to be possible even considering the above caveats, identification to more specific taxonomic groups is much more challenging. The DFA often gave conflicting referrals based on the skeletal elements included, with Callophoca identified as belonging to the Monachini tribe when referred humeri were used, while referred femora were placed within the Lobodontini; previous studies have either suggested this taxon is a member of the Miroungini (Amson and Muizon 2013) or a stem monachine seal (Berta et al. 2015; Boessenecker and Churchill 2016). Referral of these specimens to different taxonomic groups also seriously undermines the utility of Koretsky’s ecomorphotype hypothesis (Koretsky 2001), which uses sets of traits to link unassociated skeletal elements from different regions of the skeleton together. A similar issue with tribal identification was found with Phocanella, with otherwise similar humeri referred to this taxon being placed in different tribes. Many taxa were also placed within tribes with high confidence by the DFA that seem contrary to prior studies, including placement of Leptophoca within Phocini and Pontophoca within Monachini; both taxa are generally considered to be stem representatives of Phocinae (Dewaele et al. 2017a, b) and Monachinae (Koretsky and Grigorescu 2002), respectively. While the DFA only had the option of placing these taxa into existing tribal bins, the high support values given for these identifications raise concerns on how morphologically distinct the limb elements of these tribes are. This lack of morphological distinction is also supported by the patterns observed in the PCA. For the most part, within PCA plots of either the femur or humerus, phocid tribes did not form discrete and distinct clusters separate from other tribes, although some tribes such as Erignathini and Miroungini can be somewhat distinguished. Overall, the measurement data gathered here was of little use in differentiating tribes of phocid seal.
Several factors may explain this. One important factor is sexual dimorphism. Since body size differences explain the overwhelming majority of the variation in the measurement dataset, sex-based differences in body size, as seen in extant taxa such as Mirounga and Halichoerus, will have a significant impact on identification. Indeed, Callophoca has been argued to display sexual dimorphism (Koretsky and Ray 2008), to the extent that this taxon has often considered a close relative to Mirounga (Muizon 1982; Koretsky and Ray 2008; Amson and Muizon 2013), the extant genus with the most extreme expression of this trait.
Another important consideration is the utility of tribal delimitations within Phocidae at all. Phocid tribes are based on extant taxa and for the most part (with the exception of Phocini and Lobodontini) only include one or two modern genera. These taxonomic units are thus of limited use in DFA, versus larger more inclusive clades with broader morphological variation. Secondly, as they represent narrowly defined clades, it’s likely that fossil taxa exhibit morphology which falls outside of the range of variation present in modern members of these clades, and thus accurate identification to this level of taxonomy is not possible. There do appear to be several fossil taxa, known from more complete material, which can be confidently referred to modern tribes. These include placement of Pliophoca within Monachini (Berta et al. 2015; Dewaele et al. 2018a), and potentially Acrophoca, Piscophoca, Hadrokirus, and Homiphoca within or at least closely related to Lobodontini (Amson and Muizon 2013; Dewaele et al. 2017a). Taxonomic referrals of taxa represented only by isolated limb bones or fragmentary material to specific tribes are likely to be much more dubious. These include Callophoca to Miroungini (Muizon 1982; Amson and Muizon 2013; Boessenecker and Churchill 2016) and Platyphoca to Cystophorini (Koretsky and Rahmat 2013). Based on our findings, we strongly advise against any referral of isolated humeri and femora from phocids to specific tribes.
Perhaps the most important question however is how diagnostic isolated femora and humeri are for specific genera. We found that within the PCA, modern genera form tight clusters. However many of these clusters overlapped with clusters of multiple other genera, leading to poor sorting of taxa. For instance, if we look at the genus Phoca in the plot of PC1 vs PC2 of the combined limb dataset, one of the taxa with the best sample size in our analyses, we find overlap of morphospace with Histriophoca, Pagophilus, and Pusa. Similar patterns are evident in separate analyses of the humerus and femur, although overall the humerus appeared to show a greater degree of segregation and may be more diagnostic than the femur. Separation of taxa is even worse with other PC components. This is not always the case when looking at morphometric data from pinnipeds; for instance, genera were easily distinguished and placed into separate morphospaces in a morphometric study of cranial variation in Australasian fur seals and sea lions, which used similar methods (Churchill and Boessenecker 2016)
Assessment of morphospace for fossil taxa is much more difficult to perform in this dataset. Unfortunately many fossil taxa included in this study are represented by single specimens. Genera represented by multiple specimens for at least one or both limb bones include Piscophoca (humerus), Callophoca (both), Phocanella (both), Praepusa (femur), and Leptophoca (femur). Of these taxa, only Piscophoca and Phocanella form particularly tight clusters, with Piscophoca forming a tight cluster with Acrophoca. One specimen of Callophoca within the humerus dataset, USNM 263656, has a far more positive score on PC 1 than other specimens of Callophoca in the analysis, reflecting a major difference in body from the other three specimens, which otherwise form a tight cluster. This may be a result of the reported extreme sexual dimorphism suggested for this taxa, with this specimen representing a male and the other three specimens female. However, while sexual dimorphism has been used to explain this difference, a thorough quantification of sexual dimorphism in limb bone morphology is still lacking for fossil phocids, although some work has been spent in examining sexual dimorphism in fossil pinniped crania (Cullen et al. 2014), suggesting avenues for future research. Potentially, this difference in size may reflect also difference in taxonomy, rather than sex, suggesting the material examined may in fact belong to different species. Callophoca femora included in this study, in contrast to the humeri, form a relatively compact cluster, suggesting that they represent a single sex or species. Within the femur dataset, Praepusa is represented by two separate species, P. vindobonensis and P. pannonica. These taxa are widely separated on the basis of size in PC1, although are relatively close together on the PC2 axis. Leptophoca is represented in this study by both L. proxima and L. amphiatlantica. The latter taxa was recently synonymized with L. proxima on the basis of extremely similar femoral morphology (Dewaele et al. 2017b). Thus, it’s a bit surprising that they do not form a tighter cluster. Whether this suggests sexual dimorphism in Leptophoca or multiple species is impossible to say at this point. The range of variation in PC1 scores for both genera are however comparable to the variation observed in modern genera such as Pusa and Phoca.
The overall results of the morphometric analysis, as well as problems encountered while reviewing prior characters used in phylogenetic analyses of phocid seals, raises troubling concerns over the usage of humeri and femora as holotypes. New taxa have been consistently named using these elements, as they have generally been considered the most reliable postcranial elements for identification (Ray 1976; Koretsky 2001; Dewaele et al. 2018a). Despite their usage in taxonomy, no study has rigorously assessed variation in these skeletal elements or how diagnostic this variation is for the purposes of identifying different species. Dewaele et al. (2018a) commented on this regarding the humeri, but used comparisons of just six monachine humeri of two extant species (Hydrurga and Leptonychotes) as the basis of arguing they were valid skeletal elements for the purposes of naming new taxa. However the sample size he examined was too small and limited for any meaningful conclusions to be made, nor was there a rigorous attempt to quantify the variation observed. In our own analysis, these taxa are easily distinguished in a PCA from one another in the PCA, however, such clear differences are not seen between Leptonychotes and other taxa, nor in overall segregation of phocid taxa.
Taxa based on isolated limb bones also have been incredibly difficult to use in phylogenetic reconstructions, with their inclusion often leading to poor resolution and low support values (Dewaele et al. 2018a; MC personal observation), making it difficult to ascertain the role they played in seal evolution and biogeography. Attempts to work around this problem, such as the “ecomorphotype” method employed by Koretsky (2001), have often worsened the situation, resulting in referrals of different isolated elements to the same taxa, often using subjective and qualitative comparisons (Dewaele et al. 2017b, 2018a), and only making phocid taxonomy more confusing. The findings of this study cast further doubt on the usage of this method.
Our study calls into question how distinctive the morphology of these limb elements is, which in turns completely calls into question the validity of many fossil phocid taxa, and whether these named species, would be better treated as nomina dubia. Of course, the findings of this study are based entirely on analysis of linear measurements, and may not completely capture more qualitative aspects of morphology. More complex 3D morphometric methods may perform better at sorting phocid humeri and femora into different taxa. And between the different limb bones, humeri appear to better differentiate taxa than femora. Although beyond the scope of this study, we can infer that complete limb bones are likely to be more diagnostic than partial humeri or femora.
However, pending further evaluation of the utility of isolated humeri and femora in taxonomy, perhaps it would be best to treat these morphologically distinct limb bone elements as “morphotypes” (e.g., Morphotype A, Morphotype B, etc.) outside of zoological nomenclature. Alternatively, workers who wish to continue using taxa known only from isolated limb elements to stay consistent with past literature should consider applying shutter quotes around taxon names (e.g., “Phocanella pumila” and “Auroraphoca atlantica”). Such treatment could still provide important information on overall diversity and faunal change through time, while lacking the baggage associated with a scientific name, such as how many species or genera are present, whether the differences observed reflect sexual dimorphism, and if those taxa are the same as species found during other time periods and from other regions. There would also be less of an emphasis on reconstructing where these morphotypes fall within phocid phylogeny, which may cause further confusion in understanding broader patterns of trait evolution and biogeography within the group. Phocid taxonomic research should instead focus on taxa represented by cranial material and associated skeletal remains, with morphotypes assigned to these taxa when overlapping skeletal elements are discovered. Only then will we have a much better understanding of the evolution of earless seals.
Acknowledgements
For helpful comments on this manuscript, we would like to thank Olivier Lambert (Institut royal des Sciences naturelles de Belgique, Brussels, Belgium), Naoki Kohno (National Museum of Nature and Science, Tokyo, Japan), and Annalisa Berta (San Diego State University, San Diego, USA). For access to specimens, we would like to thank John Ososky, David Bohaska, Nicholas Pyenson, and Carlos Peredo (all USNM), as well as Eileen Westwig (American Museum of Natural History, New York, USA). For assistance with data collection, we would also like to thank Robert and Sarah Boessenecker (College of Charleston, Charleston, South Carolina, USA). Funding was provided by NSF-DEB 1349697.
References
Amson, E. and Muizon, C. 2013. A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seal phylogeny. Journal of Systematic Paleontology 12: 523–548. Crossref
Berta, A. and Wyss, A.R. 1994. Pinniped phylogeny. In: A. Berta and T.A. Deméré (eds.), Contributions in Marine Mammal Paleontology Honoring Frank C. Whitmore, Jr. Proceedings of the San Diego Society of Natural History 29: 33–56.
Berta, A., Churchill, M., and Boessenecker, R.W. 2018. The origin and evolutionary biology of pinnipeds: seals, sea lions, and walruses. Annual Review of Earth and Planetary Science 46: 203–228. Crossref
Berta, A., Kienle, S., Bianucci, G., and Sorbi, S. 2015. A re-evaluation of Pliophoca etrusca (Pinnipedia, Phocidae) from the Pliocene of Italy: phylogenetic and biogeographic implications. Journal of Vertebrate Paleontology 35: e889144. Crossref
Bininda-Emonds, O.R.P. and Russell, A.P. 1996. A morphological perspective on the phylogenetic relationships of the extant phocid seals (Mammalia: Carnivora: Phocidae). Bonner, Zoologische Monographien 41: 1–256.
Boessenecker, R.W. and Churchill, M. 2016. The origin of elephant seals: implications of a fragmentary late Pliocene seal (Phocidae: Miroungini) from New Zealand. New Zealand Journal of Geology and Geophysics 59: 544–550. Crossref
Boroni, N.L., Lobo, L.S., Romano, P.S.R., and Lessa, G. 2017. Taxonomic identification using geometric morphometric approach and limited data: and example using the upper molars of two sympatric species of Calomys (Cricetidae: Rodentia). Zoologia (Curitiba) 34: 1–11. Crossref
Churchill, M. and Boessenecker, R.W. 2016. Taxonomy and biogeography of the Pleistocene New Zealand sea lion Neophoca palatina (Carnivora: Otariidae). Journal of Paleontology 90: 375–388. Crossref
Committee on Taxonomy 2018. List of Marine Mammal Species and Subspecies. Society for Marine Mammalogy www.marinemammalscience.org (accessed 01.12.2018).
Cozzuol, M.A. 2001. A “northern” seal from the Miocene of Argentina: implications for phocid phylogeny and biogeography. Journal of Vertebrate Paleontology 21: 415–421. Crossref
Cullen, T.M., Fraser, D., Rybczynski, N., and Schröder-Adams, C. 2014. Early Evolution of sexual dimorphism and polygyny in Pinnipedia. Evolution 68: 1469–1484. Crossref
Davis, C.S., Delisle, I., Stirling, I., Siniff, D.B., and Strobeck, C. 2004. A phylogeny of extant Phocidae inferred from complete mitochondrial DNA coding regions. Molecular Phylogenetics and Evolution 33: 363–377. Crossref
Dewaele, L., Amson, E., Lambert, O., and Louwye, S. 2017a. Reappraisal of the extinct seal “Phoca” vitulinoides from the Neogene of the North Sea Basin, with bearing on its geological age, phylogenetic affinities, and locomotion. PeerJ 5: e3316. Crossref
Dewaele, L., Lambert, O., and Louwye, S. 2017b. On Prophoca and Leptophoca (Pinnipedia, Phocidae) from the Miocene of the North Atlantic realm: redescription, phylogenetic affinities, and paleobiogeographic implications. PeerJ 5: e3024. Crossref
Dewaele, L., Lambert, O., and Louwye, S. 2018a. A critical revision of the fossil record, stratigraphy, and diversity of the Neogene seal genus Monotherium (Carnivora, Phocidae). Royal Society Open Science 5: 171669. Crossref
Dewaele, L., Peredo, C.M., Meyvisch, P., and Louwye, S. 2018b. Diversity of late Neogene Monachinae (Carnivora, Phocidae) from the North Atlantic, with the description of two new species. Royal Society Open Science 5: 172437. Crossref
Drake, A.G., Coquerelle, M., and Colombeau, G. 2015. 3D morphometric analysis of fossil canid skulls contradict the suggested domestication of dogs during the late Paleolithic. Scientific Reports 5: 8299. Crossref
Ehret, D.J., MacFadden, B.J., Jones, D.S., DeVries, T.J., Foster, D.A., and Gismondi, R.S. 2012. Origin of the White Shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology 55: 1139–1153. Crossref
Fulton, T.L. and Strobeck, C. 2010a. Multiple fossil calibrations, nuclear loci and mitochondrial genomes provide new insight into biogeography and divergence timing for true seals (Phocidae, Pinnipedia). Journal of Biogeography 37: 814–829. Crossref
Fulton, T.L. and Strobeck, C. 2010b. Multiple markers and multiple individuals refine true seal phylogeny and bring molecules and morphology back in line. Proceedings of the Royal Society of London B 277: 1065–1070. Crossref
Gould, S.J. 1966. Allometry and size in ontogeny and phylogeny. Biological Review 41: 587–640. Crossref
Govender, R., Chinsamy, A., and Ackermann, R.R. 2012. Anatomical and landmark morphometric analysis of fossil phocid seal remains from Langebaanweg, West Coast of South Africa. Transactions of the Royal Society of South Africa 67: 135–149. Crossref
Higdon, J.W., Bininda-Emonds, O.R.P., Beck, R.M.D., and Ferguson, S.H. 2007. Phylogeny and divergence of the pinnipeds (Carnivora: Mammalia) assessed using a multigene dataset. BMC Evolutionary Biology 7: 216. Crossref
King, J.E. 1966. Relationships of the hooded and elephant seals (genera Cystophora and Mirounga). Journal of Zoology 148: 385–398. Crossref
Koretsky, I.A. 2001. Morphology and systematics of Miocene Phociane (Mammalia: Carnivora) from Paratethys and the North Atlantic region. Geologica hungarica 54: 1–109.
Koretsky, I.A. and Barnes, L.G. 2006. Pinniped evolutionary history and paleobiogeography. In: Z. Csiki (eds.), Mesozoic and Cenozoic Vertebrates and Paleoenvironments: Tributes to the Career of Prof. Dan Grigorescu, 143–153. Ars Docendi, Bucharest.
Koretsky, I.A. and Domning, D.P. 2014. One of the oldest seals (Carnivora: Phocidae) from the Old World. Journal of Vertebrate Paleontology 34: 224–229. Crossref
Koretsky, I.A. and Grigorescu, D. 2002. The fossil monk seal Pontophoca sarmatica (Alekseev) (Mammalia: Phocidae: Monachinae) from the Miocene of eastern Europe. Smithsonian Contributions to Paleobiology 93: 149–163.
Koretsky, I.A. and Holec, P. 2002. A primitive seal (Mammalia: Phocidae) from the early Middle Miocene of Central Paratethys. Smithsonian Contributions to Paleobiology 93: 163–178.
Koretsky, I.A. and Rahmat, S.J. 2013. First record of fossil Cystophorinae (Carnivora, Phocidae): Middle Miocene seals from the northern Paratethys. Rivista Italiana di Paleontologia e Stratigrafia 119: 325–350.
Koretsky, I.A. and Ray, C.E. 2008. Phocidae of the Pliocene of eastern USA. In: C.E. Ray, D.J. Bohaska, I.A. Koretsky, L.W. Ward, and L.G. Barnes (eds.), Geology and Paleontology of the Lee Creek Mine, North Carolina, IV. Virginia Museum of Natural History Publications 6: 81–140.
Koretsky, I.A., Peters, N., and Rahmat, S.J. 2015. New species of Praepusa (Carnivora, Phocidae, Phocinae) from the Netherlands supports east to west Neogene dispersal of true seals. Vestnik Zoologii 49: 57–66. Crossref
Koretsky, I.A., Ray, C.E., and Peters, N. 2012. A new species of Leptophoca (Carnivora, Phocidae, Phocinae) from both sides of the North Atlantic Ocean (Miocene seals of the Netherlands, part I). Deinsea 15: 1–12.
Muizon, C. 1981. Les vertébrés fossiles de la Formation Pisco (Pérou). Première parti: Deux nouveaux Monachina (Phocidae, Mammalia) du Pliocène de Sud-Sacaco. Recherche sur les grandes civilisations, Memoire 6: 1–150.
Muizon, C. 1982. Phocid phylogeny and dispersal. The Annals of the South African Museum 89: 175–213.
Muizon, C. and Bond, M. 1982. Le Phocidae (Mammalia) miocène de la formation Paraná (Entre Ríos, Argentine). Bulletin du Muséum National d’Histoire Naturelle Section C 4: 165–207.
R Core Team 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. http://www. R-project.org/.
Rahmat, S.J. and Koretsky, I.A. 2016. First record of postcranial bones in Devinophoca emryi (Carnivora, Phocidae, Devinophocinae). Vestnik Zoologii 50: 71–84. Crossref
Ray, C.E. 1976. Geography of phocid evolution. Systematic Zoology 25: 391–406. Crossref
Rodriguez, S.G., Morgan, C.C., Soibelzon, L.H., and Lynch, E. 2016. Intra- and interspecific variation in tooth morphology of Procyon cancrivorous and P. lotor (Carnivora, Procyonidae), and its bearing on the taxonomy of fossil South American procyonids. Hystrix, The Italian Journal of Mammalogy 27 (2) [published online: https://doi.org/10.4404/hystrix-27.2-11647].
Van Beneden, P.J. 1877. Description des ossements fossiles des environs d’Anvers. Première partie. Pinnipèdes ou amphithèriens. Annales du Musèe Royal d’Histoire Naturelle de Belgique 1: 1–88
Vangengeim, E.A., Lungu, A.N., and Tesakov, A.S. 2006. Age of the Vallesian lower boundary (continental Miocene of Europe). Stratigraphy and Geological Correlation 14: 655–667. Crossref
Vogt, P., Eshelman, R.E., and Godfrey, S.J. 2018. Calvert Cliffs: eroding mural escarpment, fossil dispensary, and paleoenvironmental archive in space and time. In: S.J. Godfrey (eds.), The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland USA, 3–44. Smithsonian Institution Scholarly Press, Washington D.C.
Wyss, A.R. 1988. On “retrogression” in the evolution of the Phocinae and phylogenetic affinities of the monk seals. American Museum Novitates 2924: 1–38.
Acta Palaeontol. Pol. 64 (2): 213–230, 2019
https://doi.org/10.4202/app.00607.2019