

A new record of a giant neoepiblemid rodent from Peruvian Amazonia and an overview of lower tooth dental homologies among chinchilloids
MYRIAM BOIVIN, PIERRE-OLIVIER ANTOINE, ALDO BENITES-PALOMINO, LAURENT MARIVAUX, and RODOLFO SALAS-GISMONDI
Boivin, M., Pierre-Olivier, A., Benites-Palomino, A., Marivaux, L., and Salas-Gismondi, R. 2019. A new record of a giant neoepiblemid rodent from Peruvian Amazonia and an overview of lower tooth dental homologies among chinchilloids. Acta Palaeontologica Polonica 64 (3): 627–642.
We report here a new record of the giant caviomorph Phoberomys corresponding to a fragmentary mandible from the Monte Salvado area, Peruvian Amazonia (Madre de Dios Department). We describe this specimen and compare it with the material previously attributed to Phoberomys. The mandibular fragment is referred to as Phoberomys sp. Found as float on a bank of the Río Las Piedras, it has been hypothetically assigned a late Miocene age, due to the local/regional stratigraphic and lithologic context. This specimen constitutes the second record of Phoberomys in Peru. For the first time, the pattern of p4s and lower molars in Phoberomys was analyzed and compared to a large taxonomic sample (including Paleogene–Recent chinchilloids and other caviomorphs) in order to progress the understanding of the homology of dental structures in this genus. For p4s and lower molars, the position of the protoconid in Phoberomys and other chinchilloids (Drytomomys sp., Potamarchus, Eumegamys, Gyriabrus, Isostylomys, and Tetrastylus) is ambiguous, and as a result we propose two alternative homology hypotheses for these taxa: protoconid within the first and second laminae or within the third lamina on juvenile specimens. The knowledge of a comprehensive ontogenetic sequence in extinct and extant chinchilloids, associated with more complete palaeontological records, would likely allow for a clarification of these homology ambiguities.
Key words: Mammalia, Rodentia, Phoberomys, mandible, Palaeogene, South America, Peru, Monte Salvado.
Myriam Boivin [myriam.boivin@univ-nantes.fr], Laboratoire de Planétologie et Géodynamique (LPG), UMR 6112, CNRS, Université de Nantes, Bât. 4, 2 Chemin de la Houssinière, F-44300 Nantes Cedex 3, France.
Pierre-Olivier Antoine [pierre-olivier.antoine@umontpellier.fr] and Laurent Marivaux [laurent.marivaux@umontpellier.fr], Laboratoire de Paléontologie, Institut des Sciences de l’Evolution de Montpellier (ISEM), UMR 5554 CNRS, IRD, EPHE, Université de Montpellier, Place Eugène Bataillon, F-34095 Montpellier Cedex 5, France.
Aldo Benites-Palomino [aldomar1955@gmail.com] and Rodolfo Salas-Gismondi [rsalasgismondi@gmail.com], Departamento de Paleontología de Vertebrados, Museo de Historia Natural, Universidad Nacional Mayor San Marcos (UNMSM, DPV-MUSM), Av. Arenales 1256, Lima 11, Peru.
Received 13 February 2019, accepted 29 March 2019, available online 21 May 2019.
Copyright © 2019 M. Boivin et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The caviomorph rodents (e.g., spiny rats, guinea pigs, chinchillas, and American porcupines) constitute one of the most successful groups of placental mammals from South America. Their Recent specific richness amounts to ca. 11% of the worldwide rodent diversity (ca. 250 species; e.g., Upham and Patterson 2015). They are characterized by a large array of ecomorphologic adaptations, especially of locomotion and diet, illustrating several lifestyles (terrestrial, fossorial, arboreal, and semi-aquatic; e.g., Patton et al. 2015). They present a huge morphological diversity depicting a wide disparity of body mass (ca. 50 g, plains viscacha rat; ca. 65 kg, capybara). During their evolutionary history, several groups from all four extant superfamilies (Hydrocheriinae Gray, 1825 among Cavioidea; Erethizontidae Bonaparte, 1845 among Erethizontoidea; Dinomyidae Peters, 1873 and Neoepiblemidae Kraglievich, 1926 among Chinchilloidea; “Heptaxodontinae” Anthony, 1917 among Octodontoidea and Chinchilloidea; see MacPhee 2011) showed trends toward the achievement of large to giant sizes (Biknevicius et al. 1993; Vucetich and Deschamps 2015; Vucetich et al. 2015). Within chinchilloids (i.e., chinchillas and their allies), the mean body mass of the dinomyid Josephoartigasia Mones, 2007 would have ranged 350–2584 kg (Rinderknecht and Blanco 2008; Millien 2008; Vucetich et al. 2015), and that of the neoepiblemid Phoberomys Kraglievich, 1926 would be 200–655 kg (Sánchez-Villagra et al. 2003; Hopkins 2008; Millien and Bovy 2010; Vucetich et al. 2015). This would make them the largest known rodents of all times.
The Neoepiblemidae are documented from the early Miocene up to the Pliocene, in Argentina (e.g., Ameghino 1886; Kramarz 2002; Rasia and Candela 2018), Brazil (e.g., Patterson 1942; Kerber et al. 2017), Chile (Flynn et al. 2002), Peru (Kretzoi and Vörös 1989; Tejada-Lara et al. 2015; Antoine et al. 2016), Colombia (Moreno-Bernal et al. 2012), and Venezuela (e.g., Mones 1980; Vucetich et al. 2010; Carrillo and Sánchez-Villagra 2015). In addition to Phoberomys, other fossil genera have often been related to Neoepiblema Ameghino, 1889 within the Neoepiblemidae: Eusigmomys (Ameghino, 1904) Ameghino, 1905 (e.g., Bondesio and Bocquentin Villanueva 1988; Negri and Ferigolo 1999; Sánchez-Villagra et al. 2003; but see Vucetich 1984); Perimys Ameghino, 1887 (e.g., McKenna and Bell 1997; Flynn et al. 2002; Kramarz 2002; Vucetich et al. 2010); Perumys Kretzoi and Vörös, 1989; and Doryperimys Kramarz et al. 2015. The genus Eusigmomys is monospecific. Its type species, E. oppositus (Ameghino, 1904), was described based on only one specimen, an upper molar from the Fénix River in Argentina (Río Frías Formation, middle Miocene; Rovereto 1914; Pascual and Díaz de Gamero 1969). As illustrated in Rovereto (1914: 35, fig. 11), this specimen displays a S-shaped like pattern on occlusal view, as noted by Pascual and Díaz de Gamero (1969). The specimen was considered as lost (Vucetich 1980, 1984), but Rasia and Candela (2018) recently analyzed an upper molar housed in the MACN (i.e., MACN-A 11189), which is very similar to the holotype of E. oppositus. This kind of S-shaped pattern does not match with neoepiblemid upper teeth, and is more characteristic to upper teeth of some dinomyids, such as Simplimus or Scleromys (Rasia and Candela 2018). Besides, the flexi are broader in neoepiblemids than those of MACN-A 11189 (Luciano Rasia, personal communication 2018). Perumys gyulavarii from the Upper Pisqui River in the Nuevo Edén area (Peruvian Amazonia; late? Pliocene) was described by Kretzoi and Vörös (1989) based on a single tooth (possibly a P4; Kerber et al. 2017). However, due to its large size, it would preferably be a representative of Phoberomys (Kerber et al. 2017; Table 1). This tooth would be the only record of Phoberomys in Peru, the other mentions of neoepiblemids being material assigned to Neoepiblema (Tejada-Lara et al. 2015; Antoine et al. 2016). Doryperimys appears morphologically close to Perimys (Kramarz et al. 2015), and two recently published cladistic analyses (Kerber et al. 2018; Rasia and Candela 2018) support close relationships of Perimys with Neoepiblema and Phoberomys. Therefore, after their revision of the Neoepiblemidae, Rasia and Candela (2018) recognized four undisputed genera in this family: Neoepiblema, Phoberomys, Perimys, and Doryperimys.
Table 1. Records of the extinct neoepiblemid rodent Phoberomys from the literature and history of their synonymies or suggested synonymies.
|
Taxon |
Remains |
Locality and age |
Official or suggested |
Author(s) of synonymy |
Additional |
Noted here as |
|
Phoberomys burmeisteri (Ameghino, 1886) |
dental and cranial |
eastern margin of the Paraná River, Argentina, Ituzaingó Formation, “Mesopotamiense”, late Miocene |
Megamys
burmeisteri |
|
|
Phoberomys
|
|
“Euphilus burmeisteri” (Ameghino, 1886) |
||||||
|
Phoberomys burmeisteri (Ameghino, 1886) |
||||||
| |
|
|
||||
|
Dabbenea
lozanoi |
|
|
||||
|
Phoberomys
lozanoi |
|
|||||
|
Phoberomys burmeisteri (Ameghino, 1886) |
|
|||||
| |
|
|
||||
|
Dabbenea
insolita |
|
|
||||
|
Phoberomys
insolita |
|
|||||
|
Phoberomys burmeisteri (Ameghino, 1886) |
|
|||||
| |
|
|
||||
|
Dabbenea (Prodabbenea?) minima Kraglievich, 1940 |
|
|
||||
|
Phoberomys
minima |
|
|||||
|
Phoberomys burmeisteri (Ameghino, 1886) |
|
|||||
| |
|
|
||||
|
Phoberomys praecursor Kraglievich, 1932 |
|
|
||||
|
Phoberomys burmeisteri (Ameghino, 1886) |
|
|||||
| |
|
|
||||
|
Phoberomys sp. |
||||||
|
|
|
|
||||
|
Phoberomys |
||||||
|
Phoberomys pattersoni (Mones, 1980) |
dental, cranial and postcranial |
several localities in
Venezuela, Urumaco Formation, |
Dabbenea
pattersoni |
|
|
Phoberomys
|
|
Phoberomys
pattersoni |
||||||
|
Phoberomys cf. pattersoni |
upper teeth and cranial |
Tío Gregorio, Venezuela,
Urumaco Formation, |
|
|
Phoberomys cf. pattersoni |
|
|
Phoberomys sp. |
dental, cranial and postcranial |
El Mamón, Venezuela Urumaco Formation, late Miocene |
Phoberomys pattersoni (Mones, 1980) |
|
Phoberomys sp. 1 |
|
|
cf. Phoberomys |
|
|||||
|
Phoberomys sp. |
|
|||||
|
Phoberomys sp. |
dental |
near El Mamón, |
Phoberomys pattersoni (Mones, 1980) |
|
Phoberomys sp. 2 |
|
|
Phoberomys sp. |
|
|||||
|
Phoberomys sp. |
upper teeth and cranial |
Norte El Hatillo, Venezuela, Urumaco Formation, late Miocene |
|
|
Phoberomys sp. 3 |
|
|
Taxon |
Remains |
Locality and age |
Official or suggested |
Author(s) of synonymy |
Additional |
Noted here as |
|
Phoberomys sp. |
dental and postcranial |
Juruá River and Patos locality, Acre Region, Brazil, Solimões Formation, late Miocene |
Phoberomys burmeisteri (Ameghino, 1886) |
|
Phoberomys sp. 4 |
|
|
Phoberomys sp. |
|
|||||
| |
|
|
||||
|
Phoberomys minima (Kraglievich, 1940) |
|
|||||
|
Phoberomys sp. |
|
|||||
|
Phoberomys sp. A |
lower teeth and cranial |
included NW San Rafael locality, Venezuela, Urumaco Formation, late Miocene |
|
|
Phoberomys sp. A |
|
|
Phoberomys sp. B |
upper teeth and cranial |
NW San Rafael and El Picache localities, Venezuela, Urumaco Formation, late Miocene |
|
|
Phoberomys sp. B |
|
|
Phoberomys |
P4 |
Upper Pisqui River, Nuevo Edén area, Peru, late? Pliocene |
Perumys
gyulavarii |
|
Phoberomys |
|
|
cf. Phoberomys |
postcranial |
El Hatillo, Venezuela, Urumaco Formation, late Miocene |
|
|
cf. Phoberomys 1 |
|
|
cf. Phoberomys |
postcranial |
El Hatillo, Venezuela, Urumaco Formation, late Miocene |
|
|
cf. Phoberomys 2 |
|
|
Neoepiblema |
lower teeth |
upper Purus River, Acre Region, Brazil, late Miocene |
Phoberomys
bordasi |
Kerber et al. 2017, 2019; followed by Rasia and Candela 2018 and this work |
|
Neoepiblema |
At least two species of Phoberomys are currently recognized: P. burmeisteri (Ameghino, 1886) and P. pattersoni (Mones, 1980) (see Rasia and Candela 2018 and citations therein; Table 1). Phoberomys burmeisteri is from the Ituzaingó Formation (eastern margin of the Paraná River, Argentina; “Mesopotamiense”, late Miocene; Ameghino 1886; Kraglievich 1926; Rasia and Candela 2018). Several other species were previously described from this formation: P. praecursor (Kraglievich, 1932), P. insolita (Kraglievich, 1940), P. lozanoi (Kraglievich, 1940), and P. minima (Kraglievich, 1940). Nevertheless, Rasia and Candela (2018) concluded that they are all synonyms of P. burmeisteri. Phoberomys pattersoni is recorded in several late Miocene localities of the Urumaco Formation in Venezuela (Mones 1980; Bondesio and Bocquentin Villanueva 1988; Carrillo and Sánchez-Villagra 2015). It is documented by several tooth rows and postcranial remains (Mones 1980; Bondesio and Bocquentin Villanueva 1988; Sánchez-Villagra et al. 2003; Horovitz et al. 2006; Carrillo and Sánchez-Villagra 2015). Giant neoepiblemid specimens from Brazil (Solimões Formation, Acre Region) and Venezuela (Urumaco Formation) are assigned to Phoberomys, but remain in open nomenclature (Table 1): cf. Phoberomys (Horovitz et al. 2006), Phoberomys sp. (Horovitz et al. 2006; Carrillo and Sánchez-Villagra 2015; Kerber et al. 2017), Phoberomys sp. A (Carrillo and Sánchez-Villagra 2015), Phoberomys sp. B (Carrillo and Sánchez-Villagra 2015), and Phoberomys cf. pattersoni (Horovitz et al. 2006). Patterson (1942) described P. bordasi from the Acre Region (Brazil; late Miocene), but according to Kerber et al. (2017, 2019), later followed by Rasia and Candela (2018), it shows more affinities with Neoepiblema.
A new record of the giant rodent Phoberomys is reported here. It corresponds to a fragmentary mandible from the Monte Salvado area, Peruvian Amazonia (Madre de Dios Department; Fig. 1). In this paper, we provide a description of this specimen and we compare it with the material previously attributed to Phoberomys. We discuss the homologies of lower teeth in Phoberomys based on juvenile specimens and a comparison with a large taxonomic sample (including chinchilloids and other caviomorphs), and propose for the first time an associated nomenclature.
Institutional abbreviations.—FMNH, Field Museum of Natural History, Chicago, USA; IGM, Instituto Nacional de Investigaciones en Geociencias, Minería y Química, Museo Geológico, Bogotá, Colombia; LACM, Museum of Natural History Los Angeles County, Los Angeles, USA; MACN, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina; MHNC, Museo de Historia Natural de la Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru; MLP, Museo de Ciencias Naturales de La Plata, La Plata, Argentina; MNHN, Museo Nacional de Historia Natural, Montevideo, Uruguay; PU, Princeton University, Princeton, USA (specimens are today deposited at the Yale Museum of Natural History, New Haven, USA); UCMP, University of California Museum of Paleontology, Berkeley, USA; UFAC, Laboratório de Pesquisas Paleontológicas, Universidade Federal do Acre, Rio Branco-AC, Brazil; UFAC-CS, Laboratório de Pesquisas Paleontológicas, Universidade Federal do Acre, Campus Floresta, Cruzeiro do Sul, Brazil.
Other abbreviations.—HI, hypsodonty index of tooth. We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Geological setting
The concerned mandible (MHNC-MS-001) was found floating on a bank of the Río Las Piedras, in the vicinity of the Monte Salvado Native Community (Fig. 1). It is therefore difficult to ascertain the stratigraphic age of this specimen. Nevertheless, it may have originated from the upper Miocene–Pliocene Madre de Dios Formation, which crops out extensively around the river beds in that area (Romero Pittman et al. 1998; Campbell et al. 2001; Roddaz et al. 2010). This formation is considered as a lateral equivalent of the Solimões Formation in Acre, Western Brazil (e.g., Bissaro-Júnior et al. 2019). The matrix of MHNC-MS-001 was a beige marly limestone, compatible with a lacustrine floodplain depositional environment. That lithological facies matches perfectly the lowermost horizons of the Madre de Dios Formation (“Unit A”; light grey to beige shale and limestone lenses, with pedogenetic calcareous nodules; Romero Pittman et al. 1998: 54, fig. 4) overlying the so-called Acre Conglomerate (Campbell et al. 2001). This conglomerate, further known to yield vertebrate assemblages in a wide array of river banks all around (Romero Pittman et al. 1998: 54, fig. 4; Campbell et al. 2001; Negri et al. 2010; Ribeiro et al. 2013), unconformably overlies middle–upper Miocene levels assigned to the Ipururo Formation. The latter deposits are dominated by red siltstone, sandstone and conglomeratic channels (“Red Beds”; Campbell et al. 2001), which discards any assignment of MHNC-MS-001 to the underlying formation. As for other deposits assigned to the Madre de Dios Formation and attributed to the overlying Units B and C, they have a terrigenous origin and they lack carbonate components (Campbell et al. 2001); they typically consist of unconsolidated and oxidized sand and gravel or sandy clay (Romero Pittman et al. 1998), which is neither compatible with the specimen matrix.
A volcanic ash topping the Acre Conglomerate ca. 150 km to the NW of the Monte Salvado territory was dated at 9.01 ± 0.28 Ma by using 40Ar/39Ar radioisotopy on feldspar grains (“Cocama Ash”; Campbell et al. 2001). It was allocated to the Unit A of the Madre de Dios Formation, which may correspond to the level yielding MHNC-MS-001. Another ash was dated at 3.12 ± 0.02 Ma along the Río Las Piedras, 50 km SE to the Monte Salvado area (“Las Piedras Ash”, late Pliocene); it is situated well above in the regional section, in the Unit C of the Madre de Dios Formation (Campbell et al. 2001).
In other words, considering the local and regional stratigraphic context and even if a Pliocene age cannot be fully discarded, a late Miocene age, younger than 9 Ma, can be hypothesized for MHNC-MS-001. This age would be consistent with the biostratigraphic age of the Acre Local Fauna, which has repeatedly yielded Phoberomys remains in Brazil (Huayquerian South American Land Mammal Age; Negri et al. 2010; Ribeiro et al. 2013; Carrillo and Sánchez-Villagra 2015; Kerber et al. 2017). Interestingly, Bissaro-Júnior et al. (2019) have recently provided maximum ages, through U/Pb datings on detrital zircon grains, for two major Neoepiblemidae-yielding localities from Acre, namely Talismã (10.8 ± 0.5 Ma) and Niterói (8.1 ± 0.5 Ma), further supporting the late Miocene age of the Acre assemblages.
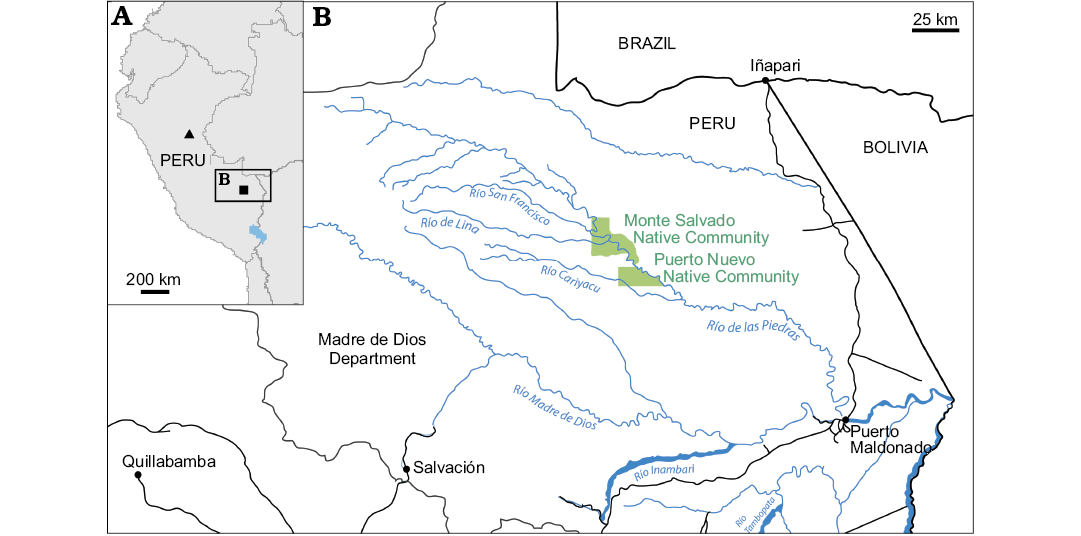
Fig. 1. A. General map of Peru showing geographic location of Phoberomys-yielding localities: Monte Salvado, Madre de Dios Department (square) and Pisqui River, Nuevo Edén area, Loreto Department (triangle). B. Location map of the Monte Salvado Native Community area in Peruvian Amazonia, where the fragmentary left mandible MHNC-MS-001 was found. Based on data from the Instituto Geográfico Nacional del Perú.
Material and methods
The mandible (MHNC-MS-001) is housed in the Museo de Historia Natural de la Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru (MHNC) since 2015. However, in August 2016, it was sent to the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM), through Marcelo Stucchi, for identification, and a cast was then prepared at this occasion (MUSM 3739). The fossil specimen has been returned to the MHNC.
For each tooth, measurements were taken with a digital caliper. We followed the protocol of Kerber et al. (2017) for the measurements of the maximum anteroposterior length (mesio-distal length sensu Kerber et al. 2017) and maximum linguolabial width (linguo-labial width sensu Kerber et al. 2017). For the lower molars, the anterior width, medium width, and posterior width correspond to the maximum width at the level of the first, second, and third laminae, respectively. For the p4, we divided the tooth into three equal parts along its length, and measured the maximum width of each part. All the dental measurements are given in Table 2.
Table 2. Dental measurements (in mm) of MHNC-MS-001. As the anterior part of p4 is greatly missing, the anterior width was not measured. Due to the state of preservation of the specimen, all measurements should be considered and used with caution.
|
Tooth |
Maximum
|
Maximum
|
Anterior
|
Medium
|
Posterior
|
|
p4 |
30.8 |
19.1 |
– |
15.7 |
18.9 |
|
m1 |
26.1 |
20.2 |
13.5 |
18.2 |
18.4 |
|
m2 |
27.5 |
20.5 |
17.5 |
20.5 |
19.6 |
|
m3 |
34.7 |
21.5 |
20.5 |
20.7 |
21.5 |
In caviomorphs, the presence of laminae or lobes, resulting from the fusion of dental structures and their enlargement, is found in high-crowned taxa (e.g., chinchilloids, cavioids). However, the crown height of teeth cannot be measured directly on the MHNC-MS-001 specimen, and thus the hypsodonty index of tooth (Janis 1986) could not be calculated. Moreover, without X-ray analyses, it was not possible to evaluate the presence or absence of tooth roots on the MHNC-MS-001 specimen. Therefore, we could not define if lower teeth are mesodont (HI = 1), protohypsodont (HI >1, with roots), or euhypsodont (HI >1, without root). So, in the following text (Systematic Palaeontology section), we decided to use the term “high-crowned” to define the crown height of the MHNC-MS-001 teeth. Given that neoepiblemids are characterized by euhypsodont teeth (Mones 1968, 1982; Koenigswald 2011), the lower teeth of the MHNC-MS-001 specimen could be expected to be euhypsodont.
The terminology used here for the rodent mandible follows the nomenclature proposed by Woods and Howland (1979) and Pérez (2010). The caviomorph taxa used for comparisons in the Systematic Palaeontology section are listed in Table 1. The material from the Acre Region assigned to P. bordasi by Patterson (1942) shows a smaller size with respect to ascertained Phoberomys remains, similar to that of Neoepiblema, and a p4 bearing three laminae. Based on these features, Kerber et al. (2017, 2019) suggested that this material was more closely related to Neoepiblema. Like Rasia and Candela (2018), we agree with such a generic assignment.
For the recognition
of dental homologies, we followed Boivin and Marivaux (2018)
using different criteria: topology/connectivity between structures (Rieppel 1988, 1994), the position of structures
relative to each other, and their orientation, shape, and size (in
surface and height). Several comparisons have allowed the proposition of
hypotheses regarding the lamina homologies of lower teeth in Phoberomys:
– an analysis of the pre-existing material of Phoberomys,
and notably the comparison between the juvenile specimen assigned to P. burmeisteri (MACN-Pv 2645, Rasia
and Candela 2018: 5, fig. 4F, G) and lower rows of adult Phoberomys;
– comparisons of lower teeth of Phoberomys
with those of other chinchilloids and caviomorphs. For these
comparisons, we used the same material as sampled by Boivin and Marivaux
(2018), to which specimens of other chinchilloids were
added (see SOM, Supplementary Online Material available at
http://app.pan.pl/SOM/app64-Boivin_etal_SOM.pdf). Some
chinchilloids characterized by lower teeth with a bilophodont pattern
(i.e., Pliolagostomus, Prolagostomus,
and Lagostomus) were not considered here
due to the difficulty in the recognition of the dental structures.
The nomenclature used to name dental structures is based on Boivin and Marivaux (2018). In the Systematic Palaeontology section below, we identified the laminar cristids of lower teeth in neoepiblemids by a number (e.g., first, second) with respect to their position on the tooth from mesial to distal.
Systematic palaeontology
Order Rodentia Bowdich, 1821
Infraorder Hystricognathi Tullberg, 1899
Parvorder Caviomorpha Wood and Patterson in Wood, 1955
Superfamily Chinchilloidea Bennett, 1833
Family Neoepiblemidae Kraglievich, 1926
Genus Phoberomys Kraglievich, 1926
1886 Megamys Laurillard in d’Orbigny, 1842; Ameghino 1886: 39. part.
1891 Euphilus Ameghino, 1889; Ameghino 1891: 246. part.
1926 Phoberomys Kraglievich, 1926; Kraglievich 1926: 127.
1988 Dabbenea Kraglievich, 1926; Bondesio and Bocquentin-Villanueva 1988: 33.
2017 Perumys Kretzoi and Vörös, 1989; Kerber et al. 2017: 7.
Type species: Megamys burmeisteri Ameghino, 1886; “Mesopotamiense”, late Miocene, eastern margin of the Paraná River, Ituzaingó Formation, Argentina.
Included species: Phoberomys burmeisteri and Phoberomys pattersoni.
Stratigraphic and geographic range.—Late Miocene–?Pliocene of Argentina, Brazil, Peru, and Venezuela.
Phoberomys sp.
Fig. 2, 3.
Material.—MHNC-MS-001, left mandibular fragment with incisor (portion) and p4 (portion)–m3, from Monte Salvado Native Community, Madre de Dios Department, Peru. Although found as float, based on its matrix, this specimen most likely originates from the lower unit of the Madre de Dios Formation, late Miocene in age (see Geological setting).
Measurements.—See Table 2.
Description.—Dentary: MHNC-MS-001 is a left mandibular fragment preserving m1–m3 and the distal portion of p4 (Figs. 2, 3). It is undistorted but fractured at several points. The body of the mandible is anteriorly broken at the level of the posterior part of the lower diastema. Posteriorly, the angular apophysis and most of the ascending ramus, including the mandibular condyle, are missing. The coronoid process is broken at its base posterodorsally.
The mandibular body is robust. The mandibular symphysis is stout, broken anteriorly, and ends at the level of m1. Labially, the notch for the insertion of the tendon of the zygomatico-mandibularis pars infraorbitalis is wide, below m1–m2, and ventrally situated on the labial edge of the mandible. The anterior tip of the masseteric crest and that of the lateral crest end below the m2, and they link the notch for the insertion of the tendon of the zygomatico-mandibularis pars infraorbitalis, at the level of its posteroventral and posterodorsal regions, respectively. The masseteric crest is posteriorly broken. It is posteroventrally directed and prominent in its anterior part. It is sub-horizontal and more reduced toward its posterior region. The lateral crest, posterodorsally directed, is markedly oblique. Its posterior part is not visible. The anterior part of the horizontal crest is absent. By contrast, its posterior part is conspicuous, although broken, and it delimits ventrally the fossa for the insertion of the zygomatico-mandibularis muscle. This fossa is moderately deep. The preserved part of the ascending ramus, which runs toward the coronoid process, begins below the m3. The retromolar fossa, posteriorly located with respect to the m3, is well developed. Lingually, the alveolar sheath of the lower incisor is partially broken, showing the lower incisor at two locations. The bottom of this alveolar sheath is situated at the level of the distal portion of the m3.
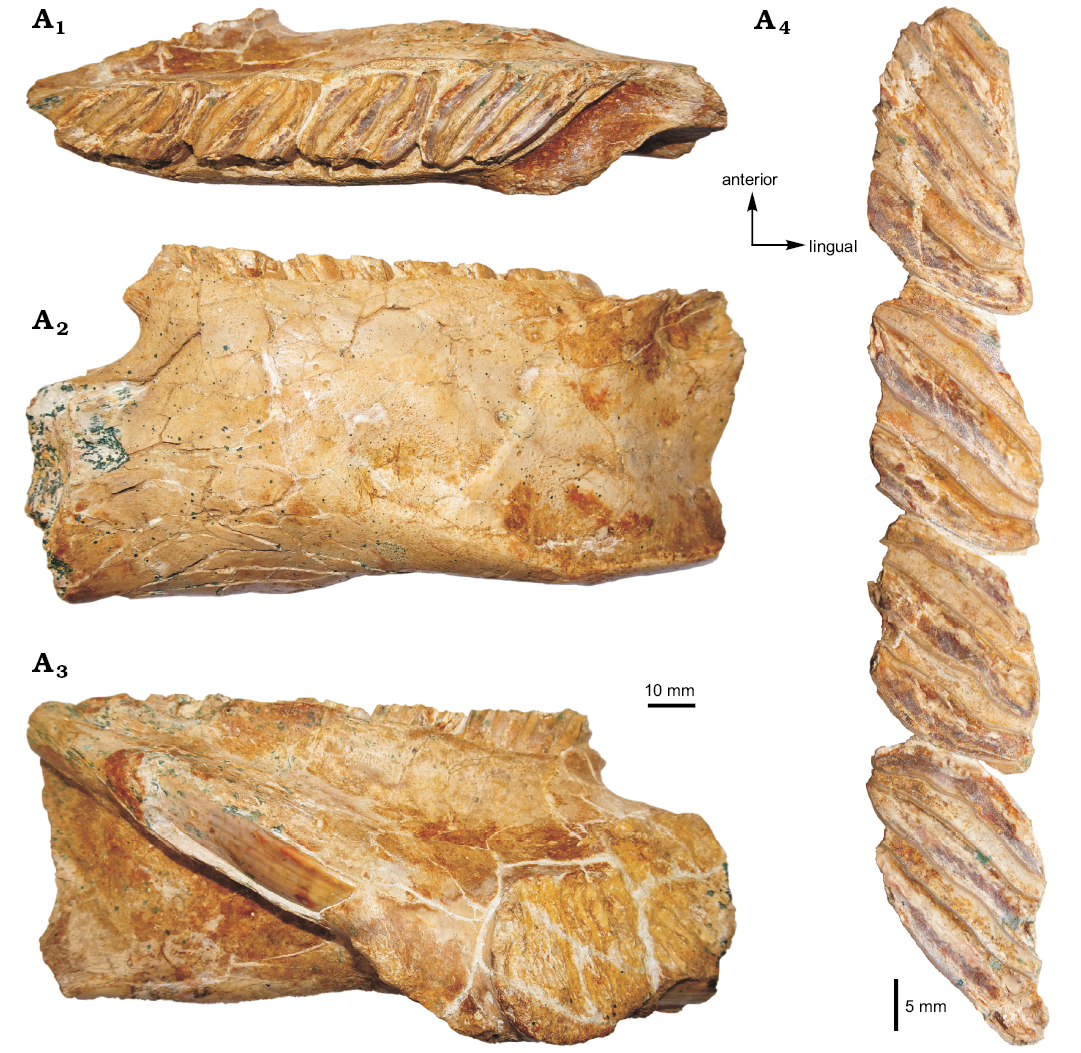
Fig. 2. Photograph of the MHNC-MS-001 attributed to caviomorph rodent Phoberomys sp., from Monte Salvado, Peruvian Amazonia, late Miocene or Pliocene; fragmentary left mandible in occlusal (A1), labial (A2), and lingual (A3) views, p4–m3 in occlusal view (A4).
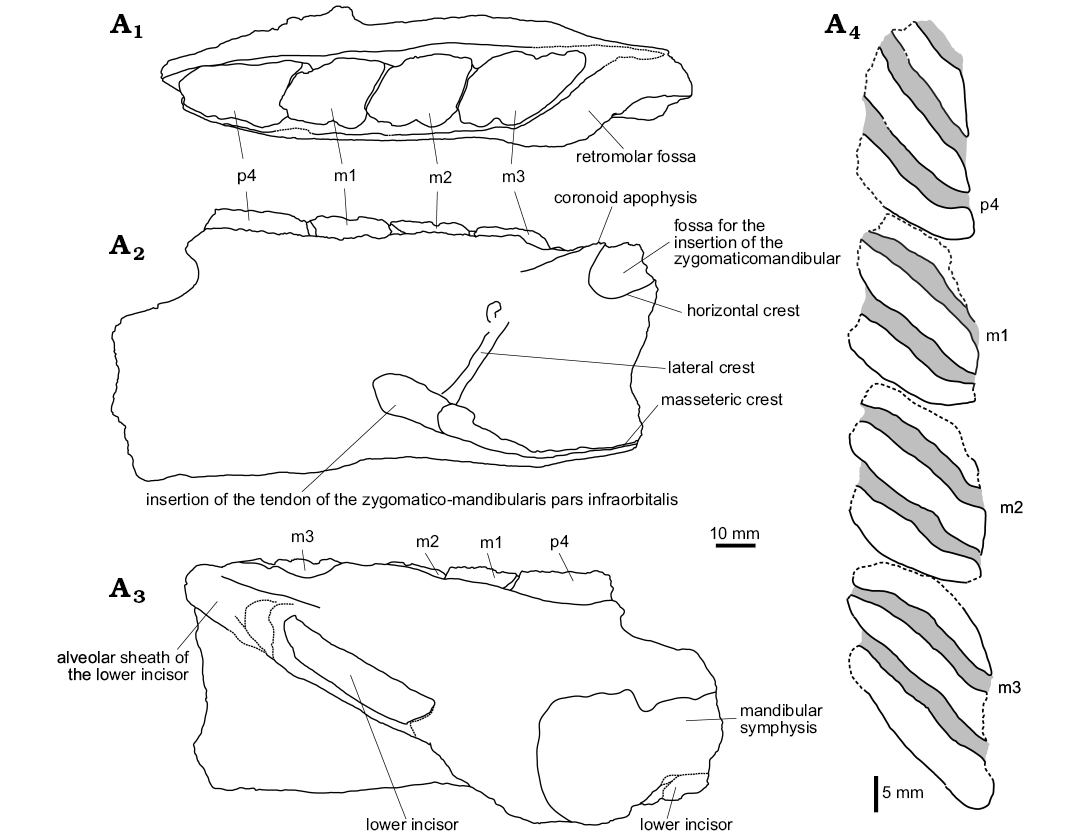
Fig. 3. Explanatory drawings of the MHNC-MS-001 attributed to caviomorph rodent Phoberomys sp., from Monte Salvado, Peruvian Amazonia, late Miocene or Pliocene; fragmentary left mandible in occlusal (A1), labial (A2), and lingual (A3) views, p4–m3 in occlusal view (A4). The dotted lines indicate incomplete parts.
Lower tooth row: The p4–m3 of MHNC-MS-001 are damaged mesially and distally. The m3 is also slightly broken on its lingual edge. The four teeth are high-crowned and taeniodont (i.e., absence of anterior arm of the hypoconid). The cuspids/stylids are not visible because they are subsumed within enlarged lophids, thereby forming laminar cristids (i.e., laminae). The latter are mesiolabially directed (slightly oblique forward with respect to the long axis of the tooth row). Compared with the size of teeth, each cristid displays a continuous and relatively thin enamel layer (without noticeable heterogeneous thickness on the leading and trailing edges), coating a thick dentine layer. The inter-cristid regions (i.e., flexids) are laminar and filled by cement.
On p4, despite the damage, three cristids and three inter-cristid cement layers are distinct on this tooth, suggesting that it likely displayed four cristids when it was complete (i.e., the first cristid is missing). Due to the fragmentary state of the p4, the presence or absence of labial connections between cristids cannot be determined. The lower molars display three cristids and two inter-cristid cement layers. Although the cristids have their lingual and labial tips mostly fractured, they seem to be connected neither lingually nor labially with each other. The m2 is slightly longer than m1, and these two teeth have a similar width. The m3 is much longer and slightly wider than m1 and m2.
Remarks.—Taeniodont and high-crowned lower teeth with laminar, oblique, and thick cristids suggest chinchilloid affinities for MHNC-MS-001. The lower teeth have a typical neoepiblemid occlusal pattern characterized by the presence of laminar and thick inter-cristid cement, as well as a continuous enamel layer (i.e., without heterogeneous thickness between leading and trailing edges). The huge size (Table 2) and the presence of a tetralophodont p4 suggest a generic assignment of the MHNC-MS-001 specimen to Phoberomys. The two included species, P. burmeisteri and P. pattersoni, have a similar dental size (Carrillo and Sánchez-Villagra 2015; Rasia and Candela 2018). They are differentiated by characters on M3 and p4 (see Mones 1980; Carrillo and Sánchez-Villagra 2015; Rasia and Candela 2018). The M3s of P. pattersoni have straighter laminae than those of P. burmeisteri. There are mesial indentations on the sixth or seventh laminae of M3s in P. burmeisteri, whereas the edges of the distal laminae are straight in P. pattersoni. In P. pattersoni, the two mesial laminae are labially united on p4, and the distal ones are free. Representatives of Phoberomys burmeisteri show this connection but they can also have a labial connection between the second and third laminae. Due to the poor preservation of MHNC-MS-001, the latter feature cannot be assessed. Besides, the specimen does not exhibit the morphological characters that would clearly differentiate it from P. burmeisteri, P. pattersoni, Phoberomys sp. 1, Phoberomys sp. 2, and Phoberomys sp. A from the Urumaco Formation (Venezuela; Bondesio and Bocquentin Villanueva 1988; Horovitz et al. 2006; Carrillo and Sánchez-Villagra 2015), and the UFAC 1817 m1 or m2 assigned to Phoberomys sp. 4 from the Solimões Formation (Brazil; Kerber et al. 2017). Teeth of Phoberomys sp. A (and Phoberomys sp. B) from the Urumaco Formation are smaller in size than those of MHNC-MS-001. In caviomorphs, some groups with hypsodont teeth show a wide range of dental size during ontogeny (i.e., teeth grow in length and width in addition to crown height), often associated with morphological variations (e.g., Kraglievich and Parodi 1940; Vucetich et al. 2005; Fields 1957; Nasif and Abdala 2015). Therefore, based on only one specimen, the size criterion is somewhat useless for differentiating MHNC-MS-001 from other species of Phoberomys (Carrillo and Sánchez-Villagra 2015; Rasia and Candela 2018). Lastly, MHNC-MS-001 being a fragmentary mandible, comparison with taxa only known by upper teeth or postcranial remains is de facto limited: the neoepiblemid from the Upper Pisqui River, Peru (originally described as Perumys gyulavarii Kretzoi and Vörös, 1989; see Kerber et al. 2017), and cf. Phoberomys sp. 1, cf. Phoberomys sp. 2, Phoberomys sp. 3, and Phoberomys sp. B from the Urumaco Formation (Horovitz et al. 2006; Carrillo and Sánchez-Villagra 2015). In light of these various points, MHNC-MS-001 is provisionally identified here as Phoberomys sp.
Homologies of lower teeth in Phoberomys and other chinchilloids
Lower
molars.—As in Neoepiblema, the lower molars of Phoberomys
have three laminae in adult specimens. The study of the material of Phoberomys from the Ituzaingó Formation led by
Rasia and Candela (2018) allowed for the recognition
of a juvenile specimen, the MACN-Pv 2645 m1 or m2. Based on this tooth,
Rasia and Candela (2018) highlighted two early
ontogenetic stages of Phoberomys burmeisteri.
The first was reconstructed
from the occlusal surface of the tooth and characterized by a
pattern with five laminae (Fig. 4A). The second was rebuilt from the
outline of the dental base, and it is characterized by four laminae
(Fig. 4B). During ontogeny, lower molars of P.
burmeisteri show transformations from a pentalophodont pattern
(Fig. 4A) to a tetralophodont pattern (Fig. 4B), and then to a
trilophodont pattern (Fig. 4C; Rasia and Candela 2018).
These transformations would be explained by fusions between mesial
laminae (Rasia and Candela 2018). In this species, the
cuspids/stylids are subsumed within lophids, and as such they form
laminae even in early ontogenetic stages (Fig. 4A, B). Hence,
cuspids/stylids and lophids cannot be directly recognized, but the
comparison with other chinchilloids and other caviomorphs allows to
propose hypotheses regarding an approximate position of these structures
on the tooth with pentalophodont pattern (Fig. 4A). We could
successively recognize (Fig. 4A2)
that:
– the first lamina would include the metaconid + the
metalophulid I + a part of the protoconid;
– the composition of the second lamina is ambiguous and would
depend on its morphology in earlier ontogenetic stages. If the first and
second laminae would be also lingually linked in the earlier ontogenetic
stages, then the metaconid would be common to both laminae. In this
case, the second laminae would correspond to a part of the metaconid +
the metaconid cristid. However, if this lamina is lingually separated
from the first, it would be a neolophid, developed from a neoconid;
– the third lamina would include the second transverse cristid
+ a part of the protoconid. The second transverse cristid could
correspond either to a posterior arm of the protoconid, or to a
neomesolophid, or to a posterior arm of the protoconid + a neomesolophid
(Boivin and Marivaux 2018). In the last two cases, a
mesostylid would be present, associated to the neomesolophid;
– the fourth lamina would include the entoconid + the
hypolophid + the ectolophid;
– the fifth lamina would include the posterolophid + the
hypoconid + the anterior outgrowth of the hypoconid.

Fig. 4. Explanatory drawings of occlusal morphologies of lower molars at three ontogenetic stages in Phoberomys. A. Pentalophodont pattern in a juvenile specimen: MACN-Pv 2645, right m1 or 2 (occlusal surface) of Phoberomys burmeisteri; the laminae (A1), homology hypothesis 1 (A2), and homology hypothesis 2 (A3). B.Tetralophodont pattern in a juvenile specimen: MACN-Pv 2645, right m1 or 2 (outline pattern of the dental base) of P. burmeisteri. C. Trilophodont pattern in an adult specimen: MACN-Pv 3475, right m1 or 2 of P. burmeisteri. The direction of the arrow indicates the direction of development associated with an increase of the ontogenetic growth and dental wear. Note that the position of the fused structures is speculative. Based on Rasia and Candela 2018: fig. 4.
The inclusion of the
ectolophid in the fourth lamina is based on the comparison with
Palaeogene and Miocene chinchilloids such as Eoincamys
(Fig. 5A), Incamys (Fig. 5B), Eoviscaccia (Fig. 5C), Scleromys
(Fig. 5D), Drytomomys aequatorialis (Fig.
5E), and Microscleromys (Fig. 5F; see Boivin
2017 and Boivin et al. 2019). These taxa are
characterized by tetralophodont/trilophodont lower molars, with oblique
loph(-id)s. It is worth noting that on their lower molars, the
ectolophid is often aligned with the hypolophid (i.e.,
mesiolabially-distolingually oriented). The mesiolabial-distolingual
alignment of these structures forms a central and oblique (diagonal)
cristid connecting the entoconid to the protoconid. The second cristid
is limited to a neomesolophid stemming from the mesostylid and often
mesiolabially linked to the metalophulid I. On some pristine specimens
of Eoviscaccia (MACN CH 1879; Fig. 5C), Scleromys (UCMP 40550; Fig. 5D), Drytomomys
(UCMP 41636; Fig. 5E), and Microscleromys
(IGM 250303; Fig. 5F), the metalophulid I is disconnected to the
protoconid. A connection between these two structures is then generated
with wear. The possibility exists that the protoconid could be
exclusively connected to the entoconid via the ectolophid and hypolophid
in Miocene and Pliocene chinchilloids displaying tetralophodont or
pentalophodont lower molars (at least at early ontogenetic stages) like
in Phoberomys (Fig. 4A1),
Drytomomys sp. (described by Kerber
et al. 2017; Fig. 5G), Potamarchus (Fig. 5H),
Eumegamys (Fig. 5I),
Gyriabrus (Fig. 5J), Isostylomys
(Fig. 5K) or Tetrastylus (Fig. 5L).
Therefore, according to this second hypothesis (Figs. 4A3,
5G3, H3–L3):
– the first lamina would include the metaconid + the
metalophulid I;
– as for the first hypothesis, the composition of the second
lamina is ambiguous: it would include either a part of the metaconid +
the metaconid cristid, or a neolophid + a neoconid;
– the third lamina would include a mesostylid + the
neomesolophid. The neomesolophid would be connected at the labial
extremity of the metalophulid I;
– the fourth lamina would include the entoconid + the
hypolophid + the ectolophid + the protoconid;
– as for the first hypothesis, the fifth lamina would include
the posterolophid + the hypoconid + the anterior outgrowth of the
hypoconid.
However, this
second hypothesis appears unlikely because:
– a substantial distal displacement of the protoconid should
be expected in these taxa. This hypothesis would be less parsimonious
than the first one;
– in the two earliest ontogenetic stages of Dinomys
figured by Nasif and Abdala (2015: 11, 13,
fig. 9 [FMNH 147996], fig. 11a [MACN 12962]), the protoconid is either
slightly or strongly linked to the second transverse cristid, and
clearly separated from the hypolophid + the ectolophid (Fig. 5M). In the
following ontogenetic stages, the protoconid is strongly connected to
the second transverse cristid and to the metalophulid I (Fig. 5N; Nasif
and Abdala 2015: 13, fig. 11c [MACN 12961]).

Fig. 5. Photographs (A1, G1–L1, O1, P1), explanatory drawings (B1–F1, M1, N1), and interpretative schematic drawings (A2–P2, G3–L3) of occlusal morphologies of lower molars in some chinchilloids; homology hypothesis 1 (A2–P2); homology hypothesis 2 (G3, H3–L3). A. Eoincamys pascuali, LACM 143299 (based on Frailey and Campbell 2004: 112, appendix 2). B. Incamys bolivianus, PU 21726 (based on Patterson and Wood 1982: 423, fig. 19b). C. Eoviscaccia australis, MACN CH 1862 (based on Kramarz 2001: 238, fig. 1A). D. “Scleromys” colombianus, UCMP 40550 (based on Fields 1957: 318, fig. 14b). E. Drytomomys aequatorialis, UCMP 41636 (based on Fields 1957: 328, fig. 16a). F. Microscleromys cribriphilus, IGM 250303 (based on Walton 1997: 393, fig. 24.2L). G. Drytomomys sp., UFAC 2742 (Kerber et al. 2017: 59, fig. 1h). H. Potamarchus murinus, UFAC 1820 (Kerber et al. 2016: 196, fig. 4B2). I. Eumegamys paranensis, MLP 15-245 (Nasif et al. 2013: 152, fig. 2.15). J. Gyriabrus holmbergi, MLP 15-252 (Nasif et al. 2013: 152, fig. 2.9). K. Isostylomys laurillardi, MNHN 2687 (Rinderknecht et al. 2018: 252, fig. 5). L. Tetrastylus laevigatus, MLP 52-X-1-59 (Nasif et al. 2013: 152, fig. 2.12). M. Dinomys branickii, MACN 12962 (Nasif and Abdala 2015: 13, fig. 11a). N. Dinomys branickii, MACN 12961 (Nasif and Abdala 2015: 13, fig. 11c). O. Chinchilla lanigera, MLP 11.VILL.99.41. P. Lagidium sp., MLP 22-IV-47-2. Note that the position of the fused structures is interpreted. Not to scale.
In the absence of early ontogenetic stages with visible cuspids in Phoberomys, Drytomomys sp. (Kerber et al. 2017), Potamarchus, Eumegamys, Gyriabrus, Isostylomys, and Tetrastylus, the second hypothesis cannot be entirely ruled out. These two homology hypotheses, which differ by the protoconid position, may occur in different chinchilloid taxa.
On the occlusal surface of MACN-Pv 2645 (Fig. 4A1), the first and second laminae are partially fused and they are only separated by two small fossettids. Hence, the transformation from a pentalophodont pattern to a tetralophodont pattern would be explained by a complete fusion of the first and second laminae. From the outline of the dental base of MACN-Pv 2645 (Fig. 4B), a partial fusion between the mesialmost lamina (1st + 2nd laminae) and the third one is observed: both laminae are strongly connected labially and linked lingually. The transformation from a tetralophodont pattern to a trilophodont pattern would be explained by a complete fusion of the 1st/2nd laminae with the third one (Fig. 4C).
p4s.—The analysis of dental homologies on p4 in chinchilloids reveals the same ambiguities regarding the position of the protoconid in some chinchilloids with tetralophodont or pentalophodont p4s (at least at early ontogenetic stages), such as Phoberomys (Fig. 6P), Potamarchus (Fig. 6K), Eumegamys (Fig. 6L), Gyriabrus (Fig. 6M), Isostylomys (Fig. 6N), and Tetrastylus (Fig. 6O). In these taxa, the protoconid is either disconnected (homology hypothesis 1; Fig. 6K2–P2) or connected to the ectolophid-hypolophid (homology hypothesis 2; Fig. 6K3–P3). In the former case, the second lamina on tetralophodont p4s (Fig. 6O2–P2) and the third lamina on pentalophodont p4s (Fig. 6K2–N2) would correspond to the mesostylid + the second transverse cristid + the protoconid. In the second case, the second lamina on tetralophodont p4s (Fig. 6O3–P3) and the third lamina on pentalophodont p4s (Fig. 6K3–N3) would correspond to the mesostylid + the neomesolophid.
Due to (i) the inclusion of several structures (i.e., cuspids/stylids and lophids) in each lamina, (ii) the ambiguity in the identification of some structures (i.e., position of the protoconid), and (iii) the fact that precise limits between these structures are undiscernible, it is preferable to describe teeth of Phoberomys in terms of laminae than of cuspids/stylids and lophids. The knowledge of a comprehensive ontogenetic sequence in extinct and extant chinchilloids, associated with more complete palaeontological records would certainly allow to clarify the ambiguities regarding these dental homologies.
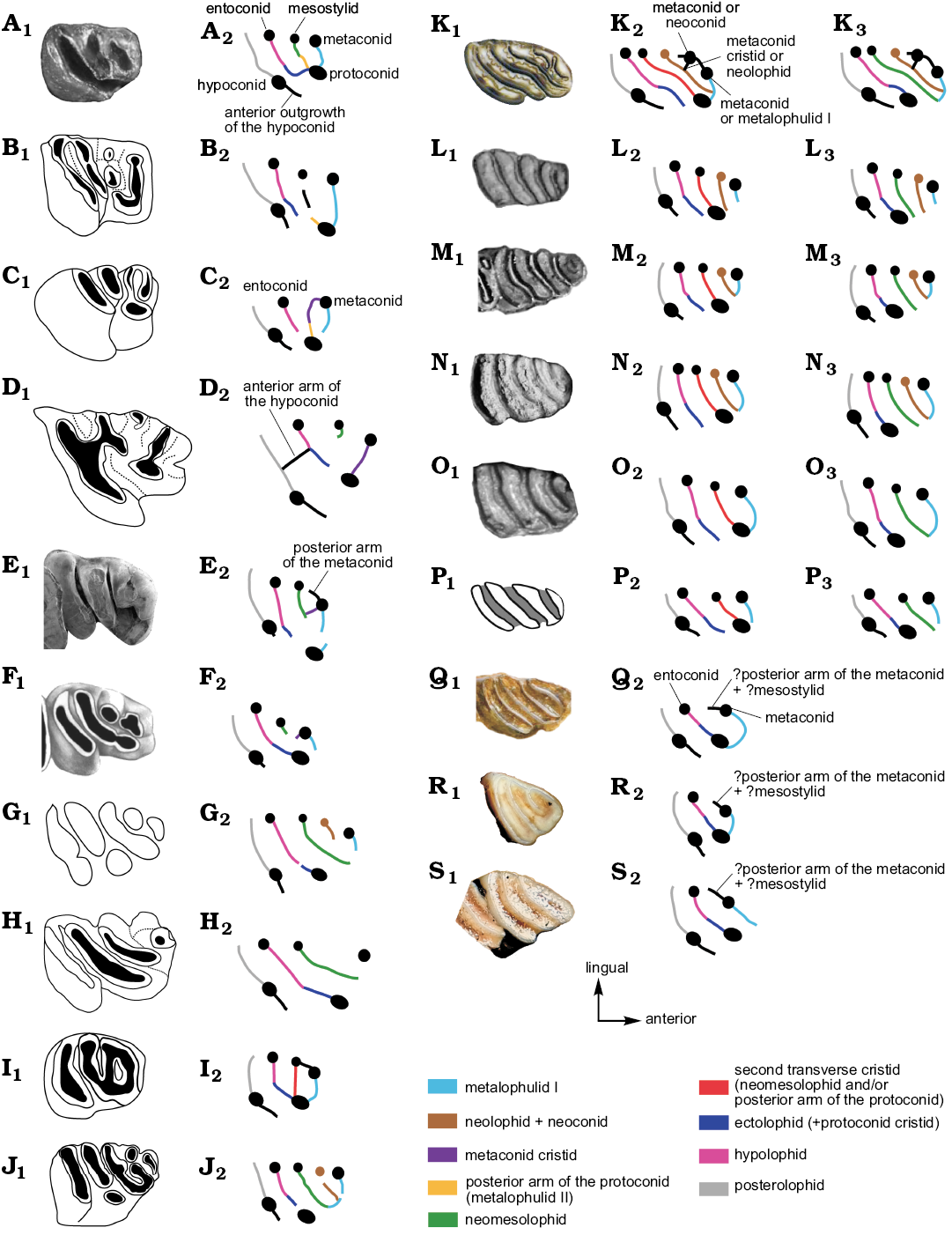
Fig. 6. Photographs (A1, E1, K1–O1, Q1–S1), explanatory drawings (B1–D1, F1–J1, P1), and interpretative schematic drawings (A2–S2, K3–P3) of occlusal morphologies on p4s in Phoberomys and other chinchilloids; homology hypothesis 1 (A2–S2); homology hypothesis 2 (K3–P3). A. Eoincamys pascuali, LACM 143301 (based on Frailey and Campbell 2004: 112, appendix 2). B. Incamys bolivianus, PU 20975 (based on Patterson and Wood 1982: 423, fig. 19b). C. Eoviscaccia australis, MACN CH 1875 (based on Kramarz 2001: 239, fig. 3A). D. Perimys intermedius, MACN Pv SC2037 (based on Kramarz 2002: 169, fig. 2C). E. Garridomys curnunuquem, MOZ-PV-938 (Kramarz et al. 2013: 253, fig. 4C). F. Scleromys quadrangulatus, MLP 82-VI-3-2 (Kramarz 2006: 18, fig. 3C). G. “Scleromys” colombianus, UCMP 39909 (based on Fields 1957: 318, fig. 14a). H. Drytomomys aequatorialis, UCMP 41636 (based on Fields 1957: 328, fig. 16a). I. Microscleromys paradoxalis, IGM 250308 (based on Walton 1997: 293, fig. 24.2H). J. “Simplimus indivisus”, MLP 15-244a (based on Vucetich 1984: 125, lamina Vj). K. Potamarchus cf. adaminae, UFAC-CS 057 (Kerber et al. 2017: 21, fig. S7B). L. Eumegamys paranensis, MLP 15-245 (Nasif et al. 2013: 152, fig. 2.15). M. Gyriabrus holmbergi, MLP 15-252 (Nasif et al. 2013: 152, fig. 2.9). N. Isostylomys laurillardi, MNHN 2187 (Rinderknecht et al. 2018: 252, fig. 5). O. Tetrastylus laevigatus, MLP 52-X-1-59 (Nasif et al. 2013: 152, fig. 2.12). P. Phoberomys burmeisteri, MACN-Pv 4729 (Rasia and Candela 2018: 5, fig. 4C). Q. Neoepiblema acreensis (=Neoepiblema ambrosettianus), UFAC 3525 (Kerber et al. 2017: 36, fig. S13E; Kerber et al. 2019). R. Chinchilla lanigera, MLP 11.VILL.99.41. S. Lagidium sp., MLP 22-IV-47-2. Note that the position of the fused structures is interpreted. Not to scale.
Conclusions
The neoepiblemid mandibular fragment from Monte Salvado, Madre de Dios, Peru is referred to as Phoberomys sp. Found as float on a bank of the Río Las Piedras, it has been hypothetically assigned a late Miocene age, due to the local/regional stratigraphic and lithologic context. This specimen constitutes the second record of Phoberomys in Peru, the first corresponding to one upper tooth from the Upper Pisqui River in the Nuevo Edén area (Kretzoi and Vörös 1989; Kerber et al. 2017). For the first time, the pattern of p4s and lower molars in Phoberomys was analyzed and compared to a large taxonomic sample (including Palaeogene–Recent chinchilloids and other caviomorphs) as a means of furthering the understanding of the homology of dental structures in this genus. For p4s and lower molars, the position of the protoconid in Phoberomys and other chinchilloids (Drytomomys sp., Kerber et al. 2017, Potamarchus, Eumegamys, Gyriabrus, Isostylomys, and Tetrastylus) is ambiguous, and we thus propose two alternative homology hypotheses for these taxa. The knowledge of a comprehensive ontogenetic sequence in extinct and extant chinchilloids, associated with more complete palaeontological records would likely allow for a clarification of these homology ambiguities. As the recognition of the dental homologies is a necessary prerequisite in any phylogenetic studies and in the understanding of dental character evolution, we encourage further discussions in that respect.
Acknowledgements
We are especially grateful to the Monte Salvado Native Community for having collected this specimen, Eddy Torres Rodríguez and Juvenal Silva Beltrán (both Sociedad Zoológica de Fránfort, Peru) for having provided it to the Museum in Cusco, José A. Ochoa Cámara (MHNC) and Marcelo Stucchi (Lima, Peru) for making possible its study. François Catzeflis, Suzanne Jiquel, and Bernard Marandat (all Institut des Sciences de l’Evolution de Montpellier, France), Christine Argot, Guillaule Billet, Violaine Nicolas, and Aurélie Verguin (all Muséum National d’Histoire Naturelle, Paris, France), César Chacaltana and Luz Marina Tejada-Medina (both Instituto Geológico Minero y Metalúrgico, Lima, Peru), Alejandro Kramarz (MACN), Marcelo Reguero and Itatí Olivares (both MLP), and Bernardino Mamani Quispe (Museo Nacional de Historia Natural, La Paz, Bolivia) kindly granted access to the osteological collections under their care. Many thanks to François Pujos (Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales, Mendoza, Argentina) for his help and longstanding investment in our collaboration with the Museo Nacional de Historia Natural, La Paz; Rubén Andrade Flores (Museo Nacional de Historia Natural, La Paz) and Céline Robinet (MLP) for their precious help during our journey and at the Museo Nacional de Historia Natural (La Paz, Bolivia); Lenna Defend (Institut des Sciences de l’Evolution de Montpellier, France) for the inventory and conditioning of the Université de Montpellier, France, collections of Salla rodents. We thank Lionel Hautier (Institut des Sciences de l’Evolution de Montpellier) for providing us the photographs of Salla collection specimens. We are grateful to Christine Bibal (Institut des Sciences de l’Evolution de Montpellier) for providing us essential bibliographic references. We are particularly indebted to Luciano Rasia (MLP) for fruitful discussions regarding diagnostic characters of neoepiblemids and the case of Eusigmomys, and for providing us a key Kraglievich’s work. Lastly, we also thank Leonardo Kerber (Centro de Apoio à Pesquisa Paleontológica da Quarta Colônia, Universidad Federal de Santa Maria, São João do Polêsine, Brazil), Luciano Rasia (MACN) and the Editor of Acta Palaeontologica Polonica Oliver Lambert (Institut Royal des Sciences Naturelles de Belgique, Brussels, Belgium), who provided formal reviews of this manuscript that enhanced the final version. This work was notably supported by the CoopIntEER CNRS/CONICET 252540 program, the ECOS-SUD/FONCyT program, the National Geographic Society (Grant #9679-15 to POA), and an “Investissements d’Avenir” grant managed by the French “Agence Nationale de la Recherche” (CEBA, ANR-10-LABX-0025-01).
References
Ameghino, F. 1886. Contribuciones al conocimiento de los mamíferos fósiles de los terrenos terciarios antiguos del Paraná. Boletín de la Academia Nacional de Ciencias en Córdoba 9: 5–228.
Ameghino, F. 1887. Enumeración sistemática de las especies de mamíferos fósiles coleccionados por Carlos Ameghino en los terrenos eocenos de Patagonia austral y depositados en el museo de La Plata. Boletín del Museo de La Plata 1: 1–26.
Ameghino, F. 1889. Contribución al conocimiento de los mamíferos fósiles de la República Argentina. Actas de la Academia Nacional de Ciencias de la República Argentina 6: 1–1027. Crossref
Ameghino, F. 1891. Mamíferos y aves fósiles argentinas. Especies nuevas, adiciones y correcciones. Revista Argentinian de Historia Natural 1: 240–259.
Ameghino, F. 1898. Sipnosis geológico-paleontológica. Segundo Censo de la República Argentina 1: 113–255.
Ameghino, F. 1904. Nuevas especies de mamíferos Cretáceos y Terciarios de la República Argentina. Anales de la Sociedad Científica Argentina 57: 327–341.
Ameghino, F. 1905. Reemplazamiento de un nombre genérico. Anales de la Sociedad Científica Argentina 59: 75.
Anthony, H.E. 1917. Two New Fossil Bats from Porto Rico. Bulletin of the American Museum of Natural History 37: 565–568.
Antoine, P.-O., Abello, M., Adnet, S., Altamirano Sierra, A.J., Baby, P., Billet, G., Boivin, M., Calderón, Y., Candela, A., Chabain, J., Corfu, F., Croft, D.A., Ganerod, M., Jaramillo, C., Klaus, S., Marivaux, L., Navarrete, R.E., Orliac, M.J., Parra, F., Pérez, M.E., Pujos, F., Rage, J.-C., Ravel, A., Robinet, C., Roddaz, M., Tejada-Lara, J.V., Vélez-Juarbe, J., Wesselingh, F.P., and Salas-Gismondi, R. 2016. A 60-million-year Cenozoic history of western Amazonian ecosystems in Contamana, eastern Peru. Gondwana Research 31: 30–59. Crossref
Bennett, E.T. 1833. On the Chinchillidae, a family of herbivorous Rodentia, and on a new genus referrible to it. Transactions of the Zoological Society of London 1: 35–64. Crossref
Biknevicius, A.R., McFarlane, D.A., and MacPhee, R.D. 1993. Body size in Amblyrhiza inundata (Rodentia, Caviomorpha), an extinct megafaunal rodent from the Anguilla bank, West Indies: estimates and implications. American Museum Novitates 3079: 1–25.
Bissaro-Júnior, M.C., Kerber, L., Crowley, J.L., Ribeiro, A.M., Ghilardi, R.P., Guilherme, E., Negri, F.R., Souza Filho, J.P., and Hsiou, A.S. 2019. Detrital zircon U-Pb geochronology constrains the age of Brazilian Neogene deposits from Western Amazonia. Palaeogeography, Palaeoclimatology, Palaeoecology 516: 64–70. Crossref
Boivin, M. 2017. Rongeurs paléogènes d’Amazonie péruvienne: anatomie, systématique, phylogénie et paléobiogéographie. 608 pp. Doctoral Dissertation, Université de Montpellier, Montpellier.
Boivin, M. and Marivaux, L. 2018. Dental homologies and evolutionary transformations in Caviomorpha (Hystricognathi, Rodentia): new data from the Paleogene of Peruvian Amazonia. Historical Biology [published online, https://doi.org/10.1080/08912963.2018.1506778] Crossref
Boivin, M., Marivaux, L., and Antoine, P.-O. 2019. L’apport du registre paléogène d’Amazonie sur la diversification initiale des Caviomorpha (Hystricognathi, Rodentia) : implications phylogénétiques, macroévolutives et paléobiogéographiques. Geodiversitas 41: 143–245. Crossref
Bonaparte, C.L.J.L. 1845. Catalogo methodico dei mammiferi Europei. 36 pp. Luigi di Giacomo Pirola, Milano. Crossref
Bondesio, P. and Bocquentin-Villanueva, J. 1988. Novedosos restos de Neoepiblemidae (Rodentia, Hystricognathi) del Mioceno tardío de Venezuela. Inferencias paleoambientales. Ameghiniana 25: 31–37.
Bowdich, T.E. 1821. An Analysis of the Natural Classifications of Mammalia: For the Use of Students and Travelers. 115 pp. J. Smith, Paris.
Campbell Jr, K.E., Heizler, M., Frailey, C.D., Romero-Pittman, L., and Prothero, D.R. 2001. Upper Cenozoic chronostratigraphy of the southwestern Amazon Basin. Geology 29: 595–598. Crossref
Candela, A.M. 2005. Los roedores del “Mesopotamiense” (Mioceno tardío, Formación Ituzaingó) de la provincia de Entre Ríos (Argentina). In: F.G. Aceñolaza (ed.), Temas de la Biodiversidad del Litoral Fluvial Argentino II, 37–48. Miscelánea, Tucumán.
Carrillo, J.D. and Sánchez-Villagra, M.R. 2015. Giant rodents from the Neotropics: diversity and dental variation of late Miocene neoepiblemid remains from Urumaco, Venezuela. Paläontologische Zeitschrift 89: 1057–1071. Crossref
Cione, A.L., Azpelicueta, M.M., Bond, M., Carlini, A.A., Casciotta, J., Cozzuol, M., de la Fuente, M., Gasparini, Z., Goin, F., Noriega, J., Scillato-Yané, G., Soibelzon, L., Tonni, E.P., Verzi, D., and Vucetich, M.G. 2000. Miocene Vertebrates from Entre Ríos, eastern Argentina. In: F. Azeñolaza and R. Herbst (eds.), El Neógeno de Argentina. INSUGEO, Serie de Correlación Geológica 14: 191–237.
Fields, R.W. 1957. Hystricomorph rodents from the late Miocene of Colombia, South America. University of California Publications in Geological Sciences 32: 273–404.
Flynn, J.J., Novacek, M.J., Dodson, H.E., Frassinetti, D., Mckenna, M.C., Norell, M.A., Sears, K.E., Swisher III, C.C., and Wyss, A.R. 2002. A new fossil mammal assemblage from the southern Chilean Andes: implications for geology, geochronology, and tectonics. Journal of South American Earth Sciences 15: 285–302. Crossref
Frailey, C.D. and Campbell, K.E. 2004. Paleogene rodents from Amazonian Peru: the Santa Rosa local fauna. In: K.E. Campbell (ed.), The Paleogene Mammalian Fauna of Santa Rosa, Amazonian Peru. Natural History Museum of Los Angeles County, Science Series 40: 71–130.
Gray, J.E. 1825. An outline of an attempt at the disposition of the Mammalia into tribes and families with a list of the genera apparently appertaining to each tribe. Annals of Philosophy, New Series 10: 337–344.
Hopkins, S.S.B. 2008. Reassessing the mass of exceptionally large rodents using tooth row length and area as proxies for body mass. Journal of Mammalogy 89: 232–243. Crossref
Horovitz, I., Sánchez-Villagra M., Martin T., and Aguilera, O. 2006. The fossil record of Phoberomys pattersoni Mones 1980 (Mammalia, Rodentia) from Urumaco (Late Miocene, Venezuela), with an analysis of its phylogenetic relationships. Journal of Systematic Palaeontology 4: 293–306. Crossref
Janis, C.M. 1986. An estimation of tooth volume and hypsodonty indices in ungulate mammals, and the correlation of these factors with dietary preference. In: D.E. Russell, J.P. Santoro, and D. Sigogneau-Russell (eds.), Teeth revisited. Proceedings of the 7th International Symposium on Dental Morphology. Mémoires du Muséum national d’Histoire naturelle, Série C 53: 367–387.
Kerber, L., Bissaro Júnior, M.C., Negri, F.R., De Souza-Filho, J.P., Guilherme, E., and Hsiou, A.S. 2018. A new rodent (Caviomorpha: Dinomyidae) from the upper Miocene of southwestern Brazilian Amazonia. Historical Biology 30: 985–993. Crossref
Kerber, L., Negri, F.R., Ribeiro, A.M., Nasif, N., Souza-Filho, J.P., and Ferigolo, J. 2017. Tropical fossil caviomorph rodents from the southwestern Brazilian Amazonia in the context of the South American faunas: systematics, biochronology, and paleobiogeography. Journal of Mammalian Evolution 24: 57–70. Crossref
Kerber, L., Negri, F.R., Ribeiro, A.M., Vucetich, M.G., and De Souza-Filho, J.P. 2016. Late Miocene potamarchine rodents from southwestern Amazonia, Brazil, with description of new taxa. Acta Palaeontologica Polonica 61: 191–203.
Kerber, L., Negri, F.R., and Sanfelice, D. 2019. Morphology of cheek teeth and dental replacement in the extinct rodent Neoepiblema Ameghino, 1889 (Caviomorpha, Chinchilloidea, Neoepiblemidae). Journal of Vertebrate Paleontology [published online https://doi.org/10.1080/02724634.2018.1549061] Crossref
Koenigswald, W.V. 2011. Diversity of hypsodont teeth in mammalian dentitions—construction and classification. Palaeontographica, Abteilung A 294: 63–94. Crossref
Kraglievich, L. 1926. Los grandes roedores terciarios de la Argentina y sus relaciones con ciertos géneros pleistocenos de las Antillas. Anales del Museo Nacional de Buenos Aires 34: 121–135.
Kraglievich, L. 1932. Diagnosis de nuevos géneros y especies de roedores cávidos y eumegámidos de la Argentina. Rectificación genérica de algunas especies conocidas y adiciones al conocimiento de otras. Anales de la Sociedad Científica de Argentina 64: 155–181, 211–237.
Kraglievich, L. 1940. Los roedores de la familia extinguida Neoepiblemidae. In: A.J. Torcelli and C.A. Marelli (eds.), Obras en Geología y Paleontología, Vol. 3, 741–764. Taller de Impresiones Oficiales, La Plata.
Kraglievich, L. and Parodi, L.J. 1940. Morfología normal y morfogénesis de los molares de los carpinchos. In: A.J. Torcelli and C.A. Marelli (eds.), Obras de Geología y Paleontología, Vol. 3, 437–484. Taller de Impresiones Oficiales, La Plata.
Kramarz, A.G. 2001. Registro de Eoviscaccia (Rodentia, Chinchillidae) en estratos colhuehuapenses de Patagonia, Argentina. Ameghiniana 38: 237–242.
Kramarz, A.G. 2002. Roedores chinchilloideos (Hystricognathi) de la Formación Pinturas, Mioceno temprano-medio de la provincia de Santa Cruz, Argentina. Revista del Museo Argentino de Ciencias Naturales, Nueva serie 4: 167–180. Crossref
Kramarz, A.G., Bond, M., and Arnal, M. 2015. Systematic description of three ne mammals (Notoungulata and Rodentia) from the early Miocene Cerro Bandera Formation, Northern Patagonia, Argentina. Ameghiniana 52: 585–597. Crossref
Kretzoi, M. and Vörös, I. 1989. On a new caviomorph rodent from Peru. Fragmenta Mineralogica et Palaeontologica 14: 111–116.
MacPhee, R.D.E. 2011. Basicranial morphology and relationships of Antillean Heptaxodontidae (Rodentia, Ctenohystrica, Caviomorpha). Bulletin of the American Museum of Natural History 363: 1–70. Crossref
McKenna, M.C. and Bell, S.K. 1997. Classification of Mammals: Above the Species Level, 631 pp. Columbia University Press, New York.
Millien,V. 2008. The largest among the smallest: the body mass of the giant rodent Josephoartigasia monesi. Proceedings of the Royal Society of London B 275: 1953–1955. Crossref
Millien, V. and Bovy, H. 2010. When teeth and bones disagree: body mass estimation of a giant extinct rodent. Journal of Mammalogy 91: 11–18. Crossref
Mones, A. 1968. Proposición de una nueva terminología relacionada con el crecimiento de los molares. Zoologia Platense 1: 13–14.
Mones, A. 1980. Un Neoepiblemidae del Plioceno Medio (Formación Urumaco) de Venezuela (Mammalia: Rodentia: Caviomorpha). Ameghiniana 16: 277–279.
Mones, A. 1982. An equivocal nomenclature: what means hypsodonty? Paläontologische Zeitschrift 56: 107–111. Crossref
Mones, A. 2007. Josephoartigasia, Nuevo nombre para Artigasia Francis & Mones, 1966 (Rodentia, Dinomyidae), non Artigasia Christie, 1934 (Nematoda, Thelastomatidae). Communicationes Paleontologicas, Museo Nacional de Historia Natural y Antropologia 36 (2): 213–214.
Moreno-Bernal, J., Federico, M., Carrillo, J., Vallejo-Pareja, M., and Jimenez-Campos, L. 2012. Neotropical Late Miocene-Early Pliocene vertebrates from the Castilletes Formation. Northern Colombia. In: Vertebrate Paleontology in the Northern Neotropics: Cradle and Museum of Evolution Across Geological Time, 72nd Annual Meeting of SVP (17 October 2012), Program and Abstracts. Journal of Vertebrate Paleontology (Supplement) 32: 145.
Nasif, N.L. and Abdala, F. 2015. Craniodental ontogeny of the pacarana Dinomys branickii Peters, 1873 (Rodentia, Hystricognathi, Caviomorpha, Dinomyidae). Journal of Mammalogy 96: 1224–1244. Crossref
Nasif, N.L., Candela, A.M., Rasia, L., Madozzo Jaén, M.C., and Bonini, R. 2013. Actualización del conocimiento de los roedores del Mioceno Tardío de la Mesopotamia argentina: aspectos sistemáticos, evolutivos y paleobiogeográficos. Publicación Electrónica de la Asociación Paleontológica Argentina 14: 147–163.
Negri, F.R. and Ferigolo, J. 1999. Anatomia craniana de Neoepiblema ambrosettianus (Ameghino, 1889) (Rodentia, Caviomorpha, Neoepiblemidae) do Mioceno superior-Plioceno, Estado do Acre, Brasil, e revisao das espécies do gênero. Boletim do Museu Paraense Emílio Goeldi, Ciências Terra 11: 3–81.
Negri, F.R., Bocquentin Villanueva, J., Ferigolo, J., and Antoine, P.-O. 2010. A review of Tertiary mammal faunas and birds from western Amazonia. In: C. Hoorn and F.P. Wesselingh (eds.), Amazonia, Landscape and Species Evolution: A Look into the Past, 245–258. Blackwell-Wiley, Hoboken. Crossref
d’Orbigny, A.D. 1842. Voyage dans l’Amérique Méridionale. 188 pp. Pitois-Levrault et C.e, Paris.
Pascual, R. and Díaz de Gamero, M.L. 1969. Sobre la presencia del género Eumegamys (Rodentia, Caviomorpha) en la Formación Urumaco del Estado Falcón (Venezuela). Su significación cronológica Asociación Venezolana de Geología, Minas y Petróleo, Boletín Informativo 12: 367–388.
Patterson, B. 1942. Two tertiary mammals from northern South America. American Museum Novitates 1173: 1–8.
Patton, J.L., Pardiñas, U.F., and d’Elía, G. (eds.) 2015. Mammals of South America. Vol. 2: Rodents. 384 pp. University of Chicago Press, Chicago. Crossref
Paula Couto, C. 1978. Fossil mammals from the Cenozoic of Acre, Brazil. 2. Rodentia Caviomorpha Dinomyidae. Iheringia, Série Geologia 5: 3–17.
Pérez, M.E. 2010. A new rodent (Cavioidea, Hystricognathi) from the middle Miocene of Patagonia, mandibular homologies, and the origin of the crown group Cavioidea sensu stricto. Journal of Vertebrate Paleontology 30: 1848–1859. Crossref
Peters, W. 1873. Über Dinomys eine merkwürdige neue Gattung der stachelschweinartigen Nagethiere aus den Hochgebirgen von Peru. Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin 1873: 551–552.
Rasia, L.L. and Candela, A.M. 2018. Reappraisal of the giant caviomorph rodent Phoberomys burmeisteri (Ameghino, 1886) from the late Miocene of northeastern Argentina, and the phylogeny and diversity of Neoepiblemidae. Historical Biology 30: 486–495. Crossref
Ribeiro, A.M., Madden, R.H., Negri, F.R., Kerber, L., Hsiou, A.S., and Rodrigues, K.A. 2013. Mamíferos fósiles y biocronología en el suroeste de la Amazonia, Brasil. In: D. Brandoni and J.I. Noriega (eds.), El Neógeno de la Mesopotamia argentina. Asociación Paleontológica Argentina, Publicación Especial 14: 207–221.
Rieppel, O. 1988. Fundamentals of Comparative Biology. 202 pp. Birkhäuser Verlag, Basel.
Rieppel, O. 1994. Homology, topology, and typology: the history of modern debates. In: B.K. Hall (ed.), Homology: the Hierarchical Basis of Comparative Biology, 63–100. Academic Press, New York. Crossref
Rinderknecht, A. and Blanco, R.E. 2008. The largest fossil rodent. Proceedings of the Royal Society of London B 275: 923–928. Crossref
Rinderknecht, A., Bostelmann, E., and Ubilla, M. 2018. Making a giant rodent: cranial anatomy and ontogenetic development in the genus Isostylomys (Mammalia, Hystricognathi, Dinomyidae). Journal of Systematic Palaeontology 16: 245–261. Crossref
Roddaz, M., Hermoza, W., Mora, A., Baby, P., Parra, M., Christophoul, F., Brusset, S., and Wesselingh, F.P. 2010. Cenozoic sedimentary evolution of the Amazonian foreland basin system. In: C. Hoorn and F.P. Wesselingh (eds.), Amazonia, Landscape and Species Evolution: A Look into the Past, 61–88. Blackwell-Wiley, Hoboken. Crossref
Romero Pittman, L., Morales Reyna, M.C., and Carpio, M. 1998. Geología de los Cuadrángulos de Río Acre 22-v, Iñapari 22-x, Qda. Mala 23-v, Iberia 23-x, San Lorenzo 23-y, Puerto Lidia 24-v, Río Manuripe 24-x, Mavila 24-y, Santa María 24-z, Valencia 25-z, Palma Real 26-z Y Río Heath 27-z. Boletín del INGEMMET A123: 1–199.
Rovereto, C. 1914. Los estratos araucanos y sus fósiles. Anales del Museo Nacional de Historia Natural de Buenos Aires 25: 1–340.
Sánchez-Villagra, M.R., Aguilera, O.A., and Horovitz, J. 2003. The anatomy of the world’s largest extinct rodent. Science 301: 1708–1710. Crossref
Sant’Anna-Filho, M.J. 1994. Roedores do Neógeno do alto Juruá, Estado do acre, Brasil. 167 pp. Master’s Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre.
Tejada-Lara, J., Salas-Gismondi, R., Pujos, F., Baby, P., Benammi, M., Brusset, S., De Franceschi, D., Espurt, N., Urbina, M., and Antoine, P.-O. 2015. Life in Protoamazonia: Middle Miocene mammals from the Fitzcarrald Arch (Peruvian Amazonia). Palaeontology 58: 341–378. Crossref
Tullberg, T. 1899. Uëber das System der Nagethiere, eine phylogenetische Studie. Nova Acta Regiae Societatis Scientarium Upsaliensis, Serie 3 18: 1–514. Crossref
Upham, N.S. and Patterson, B.D. 2015. Phylogeny and evolution of caviomorph rodents: a complete timetree for living genera. In: A.I. Vassallo and D. Antenucci (eds.), Biology of Caviomorph Rodents: Diversity and Evolution, 63–120. Sociedad Argentina para el Estudio de los Mamíferos (SAREM), Buenos Aires.
Vucetich, M.G. 1980. Los roedores Caviomorpha de la Edad Friasense (Colloncurense: Friasense). 170 pp. Doctoral Dissertation, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata.
Vucetich, M.G. 1984. Los roedores de la Edad Friasense (Mioceno Medio) de Patagonia. Revista del Museo de La Plata, Nueva Serie 8, Paleontología 50: 47–126.
Vucetich, M.G. and Deschamps, C.M. 2015. Roedores gigantes en el Museo de La Plata. Museo 27: 71–78.
Vucetich, M.G., Arnal, M., Deschamps, C.M., Pérez, M.E., and Vieytes, E.C. 2015. A brief history of caviomorph rodents as told by the fossil record. In: A.I. Vassallo and D. Antenucci (eds.), Biology of Caviomorph Rodents: Diversity and Evolution, 11–62. Sociedad Argentina para el Estudio de los Mamíferos (SAREM), Buenos Aires.
Vucetich, M.G., Carlini, A.A., Aguilera, O., and Sánchez-Villagra, M.R. 2010. The tropics as reservoir of otherwise extinct mammals: the case of rodents from a new Pliocene faunal assemblage from Northern Venezuela. Journal of Mammalian Evolution 17: 265–273. Crossref
Vucetich, M.G., Deschamps, C.M., Olivares, A.I., and Dozo, M.T. 2005. Capybaras, size, shape, and time: a model kit. Acta Palaeontologica Polonica 50: 259–272.
Walton, A.H. 1997. Rodents. In: R.F. Kay, R.H. Madden, R.L. Cifelli, and J.J. Flynn (eds.), Vertebrate Paleontology in the Neotropics. The Miocene Fauna of La Venta, Colombia, 392–409. Smithsonian Institution Press, Washington.
Wood, A.E. 1955. A revised classification of the rodents. Journal of Mammalogy 36: 165–187. Crossref
Woods, C.A. and Howland, E.B. 1979. Adaptive radiation of capromyid rodents: anatomy of the masticatory apparatus. Journal of Mammalogy 60: 95–116. Crossref
Acta Palaeontol. Pol. 64 (3): 627–642, 2019
https://doi.org/10.4202/app.00609.2019