

New Paleogene mantises from the Oise amber and their evolutionary importance
THOMAS SCHUBNEL and ANDRE NEL
Schubnel, T. and Nel, A. 2019. New Paleogene mantises from the Oise amber and their evolutionary importance. Acta Palaeontologica Polonica 64 (4): 779–786.
Mantodea are rather scarce in the fossil record, especially those belonging to the mantise crown group. Four fossil mantids are described from the lowermost Eocene amber of Oise (France), two Chaeteessidae considered as “genus and species incertae sedis”, and two Mantoididae, described as a new genus and species Pseudomantoida extendidera. We also describe a new specimen of Arvernineura insignis from the Paleocene of Menat (France), confirming the attribution of this taxon to the Chaeteessidae. These fossils are of great interest for future dating of the crown group Mantodea, being the oldest Chaeteessidae and Mantoididae. We propose a new genus name Louispitonia nom. nov. in replacement of Archaeophlebia Piton, 1940 preoccupied by Archaeophlebia Ris, 1909 (Odonata) with Archaeophlebia enigmatica as its type species.
Key words: Insecta, Mantodea, Chaeteessidae, Mantoididae, Paleocene, Eocene, France.
Thomas Schubnel [thomas.schubnel@wanadoo.fr] and Andre Nel [anel@mnhn.fr] (corresponding author), Institut Systématique Evolution Biodiversité (ISYEB), Muséum national d’Histoire naturelle, CNRS, Sorbonne Université, Université des Antilles, EPHE, 57 rue Cuvier, CP 50, 75005 Paris, France.
Received 26 April 2019, accepted 3 July 2019, available online 14 October 2019.
Copyright © 2019 T. Schubnel and A. Nel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Mantises (order Mantodea) are one of the smaller orders of insects with only 2500 described species (Patel and Singh 2016). Moreover, fossils of Mantodea are uncommon and the majority of them cannot be accurately associated with extant lineages of Mantodea (Cui et al. 2018). It is linked to two facts. First, the taxonomy of Mantodea is complicated, incongruent with phylogeny (Svenson and Whiting 2009, but see Schwarz and Roy 2019), characters are difficult to observe and often unpreserved in fossils (Ross 2019), while the general morphology of extant Mantodea is highly convergent in several lineages. Second, Mantodea are large insects, with body sizes ranging between 1 to 17 cm. Consequently, amber preserved mantises are almost exclusively nymphs, with some possible ootheca (Li and Huang 2019), while compressed fossils are isolated wings, where taxonomic characters are generally not present or unusable (Wieland 2013: figs. 414–417).
Therefore, even if there are more than ten currently known genera of Mesozoic fossil Mantodea (Delclòs et al. 2016), only one of them (Ambermantis Grimaldi, 2003) is currently associated with the crown group Mantodea but only in one of the many phylogenetic analyses (Grimaldi 2003). Furthermore Delclòs et al. (2016) considered Ambermantis as an incertae sedis. So it is probable that none of them could reliably been associated with the extant lineages of Mantodea (although undescribed material may help to resolve this problem, e.g., Ehrmann 1999; Xia et al. 2015). All ancient Cenozoic mantises from Palaeocene of Menat (France) are also considered by Cui et al. (2018) as doubtful. These authors reassigned Prochaeradodis enigmaticus Piton, 1940 to the Blattodea, while this taxon was previously considered as one of the oldest known species of crown Mantodea (see Legendre et al. 2015). Cui et al. (2018) also criticized the placement of Arvernineura insignis Piton, 1940 into the Chaeteessidae and concluded that: “there are no formally described and well-assigned fossil crown Mantodea suitable for date calibration” (Cui et al. 2018: 361).
Moreover, “stem sister group” of Mantodea (non-mantises Dictyoptera more related to Mantodea than Blattodea) are also not known. Several candidate taxa have been proposed. Most of them are controversial and/or unreliable: Palaeozoic Paoliida have been proposed as “ancestors” of mantises (Béthoux and Wieland 2009), but Prokop et al. (2014) refuted this hypothesis and considered the Paoliida as sister group of the Dictyoptera on the basis of their wing venation; several Mesozoic “roachoids” have been considered as sister group(s) of Mantodea, such as Raphidiomimidae and Manipulatoridae (Vršanský and Bechly 2015), or Raptoblatta (Dittmann et al. 2015), on the base of unreliable characters and without any phylogenetic analysis. As a conclusion, only Alienoptera can be considered as a sister group of Mantodea, but most of them date from Cretaceous (Vršanský et al. 2018), with only one potential species known from the middle Permian (Nel et al. 2014).
In this context, the recent description by Ross (2019) of a wing of Mantodea would represent one of the first fossil usable calibration data point, but is not sufficient to accurately calibrate entire order. Here, the description of a new species of Mantoididae, and the interpretation of a new specimen of Arvernineura insignis allow us to increase the number of crown mantodean fossils appropriate for data calibration.
Institutional abbreviations.—MNHN, Muséum national d’Histoire naturelle, Paris; MNT NEL, collection Nel André, Musée de la Paléontologie, Menat, France.
Other abbreviations.—AA2, second anal vein; CuA, cubitus anterior vein; M, median vein; R, radial vein; RP, radius posterior vein; ScP, subcostal posterior vein.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:6D7139A4-DC49-457A-A295-16A285827681
Material and methods
A summary on the state of the art concerning the lowermost Eocene Oise amber can be found in Brasero et al. (2009). The middle Paleocene Menat fossil site (Menat Basin, Puy-de-Dôme, France) is a volcanic maar containing rather small palaeolake ca. 1 km in diameter, which contains sedimentary rocks (spongo-diatomite) with remains of diverse aquatic and terrestrial flora and fauna. The composition of faunal and floral remains suggests that this lake was surrounded by a forest and the palaeoenvironment was warm and humid (Wedmann et al. 2018). The age of the Menat fauna is currently estimated as 60–61 Ma (Wappler et al. 2009). Mantodea are infrequent at Menat, representing less than 1% of the entomofauna, dominated by Coleoptera (78%).
The fossils in amber are embedded in small clear pieces. They have been prepared using a diamond disk, examined using a Nikon binocular microscope SMZ 1500. Photographs have been taken with a Nikon D800 with Nikon SMZ25 or a AF-S Micro NIKKOR 60mm f/2.8G ED, and adjusted with Adobe Photoshop CS6. We follow the terminology of Brannoch et al. (2017) for the morphology of the Mantodea.
Systematic palaeontology
Class Insecta Linnaeus, 1758
Order Mantodea Burmeister, 1838
Familly Chaeteessidae Handlirsch, 1926
Included genera: Chaeteessa Burmeister, 1838, Louispitonia nom. nov. (new name for Archaeophlebia Piton, 1940), Arvernineura Piton, 1940, Lithophotina Cockerell, 190 and Megaphotina Gratshev and Zherikhin, 1993.
Genus Louispitonia nom. nov.
ZooBank LCID: urn:lsid:zoobank.org:act:134251D2-19FB-43FA-BA 4E-14F07DE56AD3
Etymology: Dedicated to the late Dr. Louis Piton (1909–1945), who made the first extensive study on the fossil flora and fauna of the Paleocene of Menat.
Type species: Archaeophlebia enigmatica Piton, 1940, original designation.
Included species: Louispitonia enigmatica (Piton, 1940) comb. nov.
Louispitonia enigmatica (Piton, 1940) comb. nov.
Holotype: MNHN-F-R06999, an incomplete hindwing (Piton 1940: fig. 12).
Type locality: South-east of the village of Menat, Menat Basin, Puy-de-Dôme, France.
Type horizon: Middle Paleocene.
Remarks.—Piton (1940) described an incomplete hindwing from the Paleocene of Menat that he named Archaeophlebia enigmatica, and attributed to the ephemeropteran family “Protoneuridae” Piton, 1940 (not the damselfly family Protoneuridae Tillyard, 1917). Nel and Roy (1996) revised it and attributed this fossil to the Chaeteessidae, but they were not aware that the genus name Archaeophlebia was preoccupied by the extant genus Archaeophlebia Ris, 1909 (Odonata: Libellulidae). Thus we propose a new genus name in replacement of Archaeophlebia Piton, 1940.
Stratigraphic and geographic range.—Only type locality.
Chaeteessidae indet.
Figs. 1, 2, 4A.
Material.—MNHN-F.A71139, PA 3309, head and fore legs; MNHN-F.A71142, PA 2420, fore legs. >From Le Quesnoy, Chevrière, region of Creil, Oise department, France; lowermost Eocene, Sparnacian, level MP7 of the mammal fauna of Dormaal.
Description.—MNHN-F.A71139: head 1.9 mm long, 2.3 mm wide, triangular, 1.2 times as wide as long; compound eyes globular; antennae filiform, long but incomplete; ocelli not visible; fore coxae without visible spines; fore femora 2.8 mm long, of semicircular section, without distinct carina and probably with six posteroventral spines; fore tibia 1.8 mm long, slightly curved toward posteroventral edge; 14 anteroventral spines, with eighth spine larger than ninth, apical spur not clearly define (Fig. 1). MNHN-F.A71142 is similar to MNHN-F.A71139 (Fig. 2).
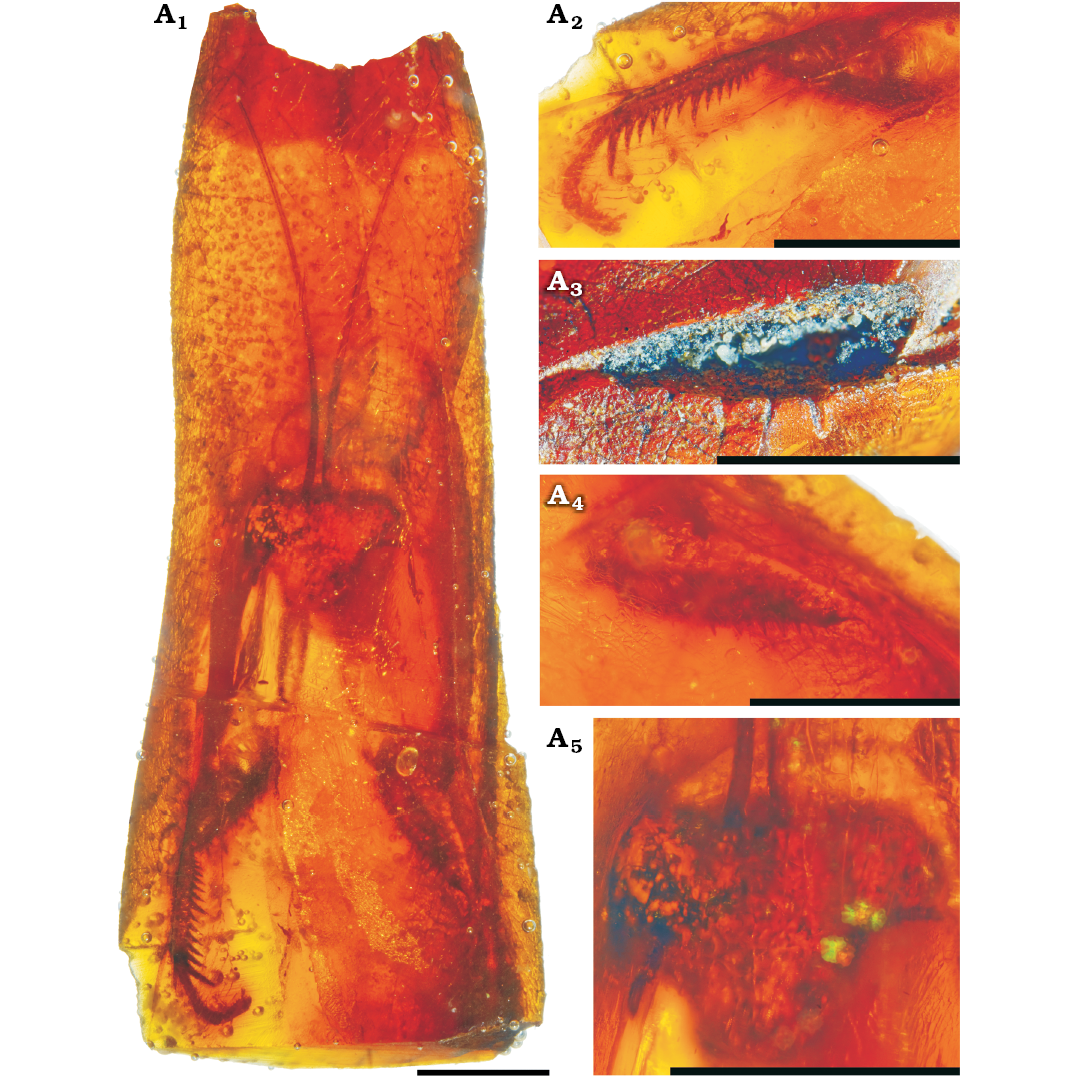
Fig. 1. Chaeteessidae gen. at sp. incertae sedis, MNHN-F.A71139, Le Quesnoy, France, Eocene. General habitus (A1), right fore tibia (A2), outer side of left fore femur (A3), inner side of left fore femur (A4), head (A5). Scale bars 2 mm.

Fig. 2. Fore legs of Chaeteessidae gen. at sp. incertae sedis, MNHN-F.A71142, Le Quesnoy, France, Eocene.
Genus Arvernineura Piton, 1940
Type species: Arvernineura insignis Piton, 1940; Menat, Menat Basin, Puyde-Dôme, France; Middle Paleocene.
Arvernineura insignis Piton, 1940
Figs. 3, 4C.
Material.—MNT NEL1656, a pair of forewings, nearly complete, and some foretibial and forefemoral spines. From South-east of the village of Menat, Menat Basin, Puy-de-Dôme, France; middle Paleocene.
Description.—Preserved length of wing 38.0 mm, width 6.0 mm; fore femoral spines hardly visible and difficult to count; the two fore femora superposed and some spines are detached from raw; six preserved spines on one fore tibia, and 10–11 preserved spines on second with seventh or eighth larger than others; bases of these spines disposed into a curved line, indicating a curvature of fore tibiae (Figs. 3, 4C).
Remarks.—These mantises in amber have a character on the fore tibiae typical of the extant Chaeteessa: a large median spine, larger and thicker than those more basal and distal of antero-ventral raw (Fig. 4). It seems to be absent in all other extant and fossils mantises with known fore legs (TS personal observation; for figures of fossil specimens see Grimaldi 2003: figs. 3, 7–15). This is a putative synapomorphy of the Chaeteessidae. Therefore, it refutes Cui et al. (2018) who doubted about the placement of Arvenineura into the Chaeteessidae. Also Cui et al. (2018) stated that the shape of the “stigma” is the same as in Arvenineura insignis and in Cretophotina tristriata Gratshev and Zherikhin, 1993. However, if we follow the wing nomenclature of Béthoux and Wieland (2009) (also used in Brannoch et al. 2017), we notice that the “stigma” of A. insignis covers a part of the RP+M and of the CuA areas, as in extant Chaeteessa (Nel and Roy 1996), while that of Cretophotina tristriata covers only the CuA area (Gratshev and Zherikhin 1993; Grimaldi 2003). This character does not allow to separate A. insignis from the extant Chaeteessa. Also the term “stigma” is not appropriate to designate this structure. This structure, a highly sclerotized vein, was referred by Grimaldi (2003) as the “pseudovein”, while in mantises, the stigma generally refers to another sclerotized area of fore wing, often white, smaller and present more basally than the “pseudovein”. Furthermore, some mantises have these two structures well separated on their fore wings. The general shape of the wing of A. insignis is also closer to that of Chaeteessa rather than of Cretophotina tristriata (Nel and Roy 1996; Grimaldi 2003). Lastly, A. insignis has a relatively broad area between the fore wing veins ScP and R, as in Chaeteessa, while all other extant and most fossil mantises (including Cretophotina tristriata) have a narrower area between these veins. Based on all these characters of wing and fore leg, we assume the placement of A. insignis into the Chaeteessidae, and its suitability as a crown Mantodea for date calibration.
Although the two specimens of Chaeteessidae of Oise amber could be associated without doubt to this family, we are not able to determinate if they are of the same species. Also, we are not able to assign these specimens to a precise genus of Chaeteessidae due to the lack of characters. Arvenineura is closer to these fossils spatially and temporally than Chaeteessa, but this does not represent an adequate evidence to associate them to this genus. Consequently, more material is needed to formally describe this (or these) species of Chaeteessidae.
Stratigraphic and geographic range.—Only type locality.

Fig. 3. Habitus of chaeteessid mantodean Arvernineura insignis Piton, 1940, MNT NEL1656, Menat, France, Paleocene.
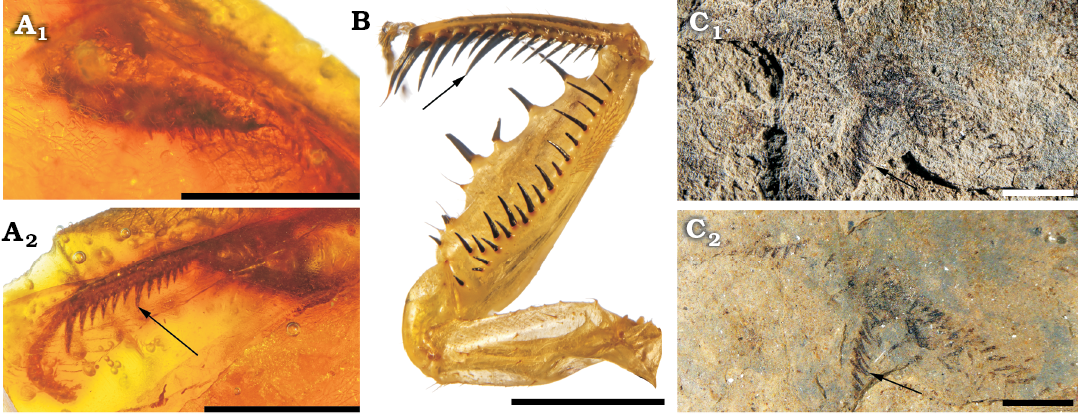
Fig. 4. Fore legs of chaeteessid mantodeans. A. Chaeteessidae gen. at sp. incertae sedis, MNHN-F.A71139, Le Quesnoy, France, Eocene; inner (A1) and outer (A2) side of left fore femur. B. Fore legs of Chaeteessa valida (Perty, 1833), Recent, MNHN collection. C. Habitus of Arvernineura insignis Piton, 1940, MNT NEL1656, Menat, France, Paleocene; photograph with low-angle light (C1), in alcohol (C2). Arrows correspond to tibial major median spines. Scale bars 2 mm.
Familly Mantoididae Giglio-Tos, 1927
Included genera: Mantoida Newman, 1838, Paramantoida Agudelo, 2014, Pseudomantoida gen. nov.
Genus Pseudomantoida nov.
ZooBank LSID: urn:lsid:zoobank.org:act:F11EE9A4-6E7B-4970-A9C1-4A25CA4105DE
Etymology: Named after its resemblance to Mantoida.
Type species: Pseudomantoida extendidera sp. nov., by monotypy, see below.
Diagnosis.—The same as for the monotypic type species.
Pseudomantoida extendidera sp. nov.
Fig. 5.
ZooBank LSID: urn:lsid:zoobank.org:act:365BADE6-6E90-4154-9A7F-C3B3D6396747
Etymology: From Latin extend, extend and Greek δέρη, neck; in reference to the extension of the pronotum above the forewings.
Holotype: MNHN-F.A71141 (PA 2378 2/2, the number in the working collection), an adult male with preserved head, prothorax, and fore legs in a hyaline piece of amber.
Type locality: Le Quesnoy, Chevrière, region of Creil, Oise department, France.
Type horizon: Lowermost Eocene, Sparnacian, level MP7 of the mammal fauna of Dormaal.
Material.—We tentatively attribute MNHN-F.A71140 to this taxon, but with doubt (Fig. 6).
Diagnosis.—Small mantises with short and bulky pronotum and fore legs, typical of Mantoididae; fore femorae with three posteroventral spines (0–2 or 4 in other Mantoididae, see Agudelo 2014). Lateral margin of pronotum rounded with an extension above bases of forewings.
Description.—Head 3.3 mm long, 3.8 mm wide, approximately as long as wide, triangular with vertex rounded; eyes globular protruding; three large ocelli (probably a male); clypeus without a ridge; antennae long and filiform, third antennomere 2.5 times as long as fourth.
Pronotum: 2.8 mm long, short, as long as wide; saddle-shaped, i.e., with lateral parts bent ventrad; metazona (1.8 mm) two times as long as prozona, but with a long extension above wings, measuring half of its length; outer margin of pronotum raised, forming a lateral carina without spines; lateral margin forming a soft angle in prozona and slightly S-shaped in metazona; central carina on all the length; ventral cervix not visible; scutellum not visible.
Legs: Fore coxae without spines; fore femora 3.8 mm long, thick and short, with a big carina on all length of its dorsal margin and a smaller carina close to the posteroventral raw of spines; three posteroventral spines, at least five same sized anteroventral spines and three discoidal spines; fore tibia with one posteroventral spine in apical position, at least seven anteroventral spines and one apical spur (Fig. 5).
Remarks.—The head shape of Pseudomantoida gen. nov. is quite similar to that of Mantoida, more globular than in other “more derived” Mantodea (Wieland 2013: fig. 70). The clypeus has no ridge, as in Chaeteessa, Metallyticus, and Mantoida (Wieland 2013: 44, 52). Also the very long antennae, probably longer than the body, is a character of Mantoida and Chaeteessa. The short prothorax of Pseudomantoida is shared by “Mantoida, Chaeteessa, Metallyticus, Amorphoscelinae, and Perlamantinae, in which the pronotum is almost square”, suggesting that this last character is homoplastic (Wieland 2013: 50). All these characters are currently considered as symplesiomorphies of the mantodean crown-group.
The pronotum of Pseudomantoida is saddle-shaped, as in Mantoida and Paramantoida, even its lateral parts are more bent ventrad than in these extant taxa (Fig. 5; Agudelo 2014). The fore tibia has only one posteroventral spine, which is a character only present in the Mantoididae, Amorphocelidae, and few other taxa. Generally the mantises have such spines more numerous. These characters could be putative synapomorphies of the Mantoididae. This fossil has also an apical claw on fore tibiae, and prominent ocelli, characters shared by the Mantoididae but not the Chaeteessidae (Agudelo 2014). Lastly, Pseudomantoida differs from Ambermantis Grimaldi, 2003 (type genus of the Cretaceous family Ambermantidae Grimaldi, 2003, a taxon sister group of the “Eumantodea” sensu Grimaldi [2003], crown-group of the Mantodea) in the shorter legs, the S-shaped posterior margin metazona, and different number of spines on fore femora. This last genus shares with Pseudomantoida a metazona nearly two times as long as prozona, with a long extension above wings, and outer margin of pronotum forming a lateral carina without spines. Notice that the forewing venation of Ambermantis is very similar to those of the extant Mantoididae, especially the anterior branch of the vein AA2 posteriorly pectinate with many branches (see Agudelo 2014). This character could represent a potential synapomorphy of the Ambermantidae with the Mantoididae.
Pseudomantoida extendidera appears to be very similar in size to the extant Paramantoida amazonica Agudelo, 2014 (head width 3.8–3.9 mm, prozona length 0.8–1.0 mm) (Agudelo 2014). Therefore, the total length of Pseudomantoida extendidera was probably also similar, i.e., 18–20 mm long.
Mantoida matthiasglinki Zompro, 2005 is currently considered to belong to the genus Mantoida and therefore to the Mantoididae. However, it has many characters strongly different from those of other Mantoididae (extant Mantoida and Paramantoida, and Pseudomantoida): cursorials legs are longer than those of Mantoididae (e.g., hind femorae as long as abdomen while they are clearly shorter in Mantoida and Paramantoida); fore legs also longer than those of Mantoididae, and with large spines on femoral and tibial posterioventral spines (small or reduced in Mantoididae). These characters would justify the attribution of this species to another family. The long legs and spines, associated with the very short pronotum, are also known in the Cretaceous Ambermantidae (Ambermantis) (Grimaldi 2003), which are very similar to M. matthiasglinki in their general habitus. However, the anal veins seem to be different; M. matthiasglinki has discoidal spines while these seem to be absent in Ambermantis. The long legs and spines present in both taxa can result from an adaptation to a particular life style, viz. fast-running predation on small preys. Notwithstanding, M. matthiasglinki needs a revision. Currently it cannot be accurately assigned to a precise family of mantises. Therefore, Pseudomantoida extendidera is the only one known fossil Mantoididae, thus of great interest for date calibration.
Stratigraphic and geographic range.—Only type locality.
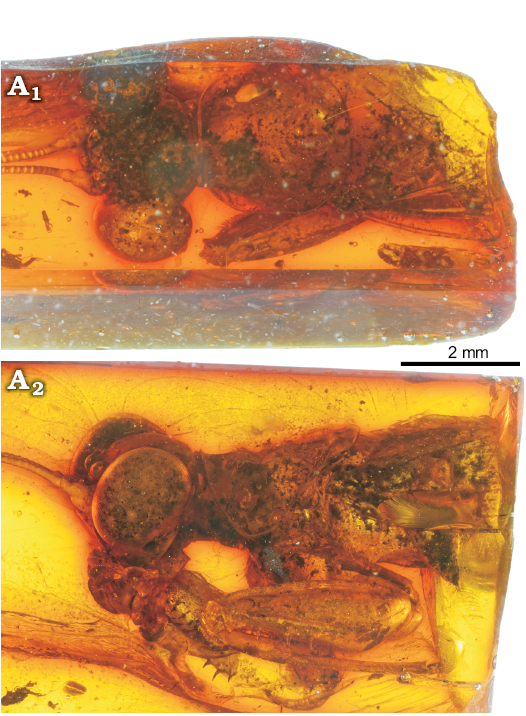
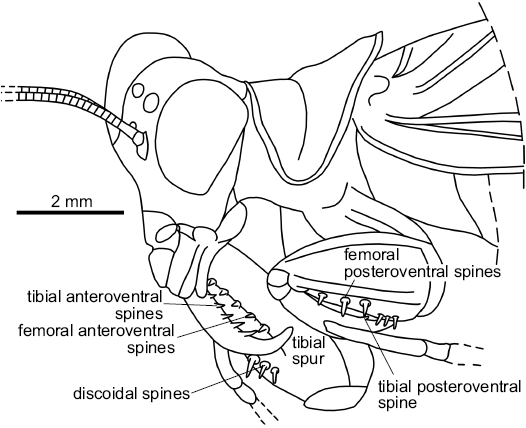
Fig. 5. Mantoidid mantis Pseudomantoida extendidera gen. et sp. nov., holotype MNHN-F.A71141, Le Quesnoy, France, Eocene. General view from above (A1) and left side (A2).

Fig. 6. Mantoidid mantis ?Pseudomantoida extendidera gen. et sp. nov., MNHN-F.A71140 Le Quesnoy, France, Eocene.
Concluding remarks
The new specimen of Arvernineura insignis confirms the placement of this species in the Chaeteessidae. Therefore, Arvernineura insignis is the oldest known reliable representative of this family. Pseudomantoida extendidera is the only reliable known fossil of the Mantoididae while Protohierodula crabbi is the oldest Artimantodea sensu Svenson and Whiting (2009). These three fossils are suitable for date calibration. Chaeteessidae are currently considered as sister group of all other crown Mantodea while Mantoididae are the sister group of the rest (Artimantodea) (Svenson and Whiting 2009). The three fossils allow dating three successive profound nodes in the phylogeny of Mantodea: with Arvernineura insignis (60 Myr) for the crown group of Mantodea, Pseudomantoida (53 Myr) for the crow group of (Mantodea minus Chaeteessidae), and Protohierodula (late Eocene) for (Artimantodea + Mantoididae). In our knowledge, these calibration points are the only accurate known points and are crucial to understand the evolution of the order.
The most diverse extant group among Mantodea is the Cernomantodea that have cyclopean ear detecting the ultra-sounds of bats (Yager and Svenson 2008). This group could have “replace” the Mantodea without earing system during the Paleogene. This hypothesis is congruent with the lack of Cernomantodea and the high diversity of Chaeteessidae in the Paleocene and Eocene. A similar phenomenon occurs with the Neuroptera: Chrysopidae, viz. the Chrysopinae have developed tympanic organ allowing them to detect the ultra-sounds of the bats. Their diversity greatly increased during the Neogene while the Nothochrysinae, without tympanic organ, were much more diverse during the Paleogene (Archibald et al. 2014). Insectivorous bats are recorded in the middle Eocene (those from of Messel having insects in their guts) (Habersetzer et al. 1994; Smith et al. 2012). It seems that the bats’ diversification had a significant impact on many insects that are relatively poor flyers. It also could indicate that Mantodea experienced a relatively recent diversification since the last 40 million years. This is congruent with the lack of morphological synapomorphies, typical of evolutionary radiation.
The two extant families Chaeteeesidae and Mantoididae are strictly Neotropical; the presence of the former in Europe, North America, and Asia during the Paleocene and Eocene, and of the later in the Eocene of Europe could appear surprising, but several other similar cases of Paleogene faunal exchanges between the Western and Eastern Hemispheres and/or Paleogene widespread (even worldwide) distributions of taxa are known. For instance representatives of the extant African dragonfly subfamily Neophyinae Tillyard and Fraser, 1940 are recorded in the South American Paleogene but also in the latest Eocene of England (Nel and Fleck 2014). The extant Australian termite family Mastotermitidae has a Paleogene distribution in South and North America, Europe, Asia, Africa, and Australia. The Paleocene–Eocene was an exceptional period for Eurasiatic-North American interchanges (Brikiatis 2014). Nevertheless South America was well-separated from North America during the Paleocene–Eocene. It is possible that the Chaeteessidae and Mantoididae were present in North America and colonized the South America later. The other hypothesis is that both families were much older (Late Jurassic–Early Cretaceous) and colonized all the continents before the opening of the Northern part of the Atlantic Ocean. Future molecular dating and new discoveries of fossil Mantodea in North America and Africa could help to solve this question.
Acknowledgements
We thank Andrew Ross (Natural History Museum of Scotland, Edinburgh, UK) and an anonymous referee for their useful comments on the first version of this paper. We thank Gael De Ploëg and the Lafarge-Granulat company for help with the fossil sampling and the Langlois-Meurinne family for the authorization of working in their property. The authors had the pleasure to receive generous assistance from Bernard Duverger (President of the Communauté de Communes of Menat region), Clotilde Berger-Pompili and Mathilde Leygnac (directors of the EHPAD du pays de Menat), for their kind help and authorisations to collect fossil insects in a small but rich outcrop near the village of Menat (Puy-de-Dôme, France).
References
Agudelo A.A. 2014. A new genus and species of Mantoididae (Mantodea) from the Brazilian and Venezuelan Amazon, with remarks on Mantoida Newman, 1838. Zootaxa 3797: 194–206. Crossref
Archibald, S.B., Makarkin, V.N., Greenwood, D.R., and Gunnell, G.F. 2014. The Red Queen and court jester in green lacewing evolution: bat predation and global climate change. Palaios 29: 185–191. Crossref
Béthoux, O. and Wieland, F. 2009. Evidence for Carboniferous origin of the order Mantodea (Insecta: Dictyoptera) gained from forewing morphology. Zoological Journal of the Linnean Society 156: 79–113. Crossref
Brannoch, S.K., Wieland, F., Rivera, J., Klass, K.D., Béthoux, O., and Svenson, G.J. 2017. Manual of praying mantis morphology, nomenclature, and practices (Insecta, Mantodea). ZooKeys 696: 1–100. Crossref
Brasero, N., Nel, A., and Michez, D. 2009. Insects from the Early Eocene amber of Oise (France): diversity and palaeontological significance. Denisia 26: 41–52.
Brikiatis, L. 2014. The De Geer, Thulean and Beringia routes: key concepts for understanding early Cenozoic biogeography. Journal of Biogeography 41: 1036–1054. Crossref
Cui, Y., Evangelista, D.A., and Béthoux, O. 2018. Prayers for fossil mantis unfulfilled: Prochaeradodis enigmaticus Piton, 1940 is a cockroach (Blattodea). Geodiversitas 40: 355–362. Crossref
Dittmann, I.L., Hörnig, M.K., Haug, J.T., and Haug, C. 2015. Raptoblatta waddingtonae n. gen. et n. sp.—an Early Cretaceous roach-like insect with a mantodean-type raptorial foreleg. Palaeodiversity 8: 103–111.
Delclòs, X., Peñalver, E., Arillo, A., Engel, M.S., Nel, A., Azar, D., and Ross, A. 2016. New mantises (Insecta: Mantodea) in Cretaceous ambers from Lebanon, Spain, and Myanmar. Cretaceous Research 60: 91–108. Crossref
Ehrmann, R. 1999. Gottesanbeterinnen in Kopal und Bernstein (Insecta: Mantodea). Arthropoda 7: 2–10.
Gratshev, V.G. and Zherikhin, V.V. 1993. New fossil mantids (Insecta, Mantida [sic]). Paleontological Journal 27: 148–165.
Grimaldi, D.A. 2003. A revision of Cretaceous mantises and their relationships, including new taxa (Insecta, Dictyoptera, Mantodea). American Museum Novitates 3412: 1–47. Crossref
Habersetzer, J., Richter, G., and Storch, G. 1994. Paleoecology of early middle Eocene bats from Messel, FRG., aspects of flight, feeding and echolocation. Historical Biology 8: 1–4. Crossref
Legendre, F., Nel, A., Svenson, G.J., Robillard, T., Pellens, R., and Grandcolas, P. 2015. Phylogeny of Dictyoptera: dating the origin of cockroaches, praying mantises and termites with molecular data and controlled fossil evidence. Plos ONE 10: e0130127. Crossref
Li, X.-R. and Huang, D. 2019. A mantis-type ootheca from mid-Cretaceous Burmese amber (Insecta: Dictyoptera). Cretaceous Research 100: 134–137. Crossref
Nel, A. and Fleck, G. 2014. Dragonflies and damselflies (Insecta: Odonata) from the Late Eocene of the Isle of Wight. Earth and Environmental Science, Transactions of the Royal Society of Edinburgh 104: 283–306. Crossref
Nel, A. and Roy, R. 1996. Revision of the fossil “mantid” and “ephemerid” species described by Piton from the Palaeocene of Menat (France) (Mantodea: Chaeteessidae, Mantidae; Ensifera: Tettigonioidea). European Journal of Entomology 93: 223–234.
Nel, A., Prokop, J., Grandcolas, P., Garrouste, R., Lapeyrie, J., Legendre, F., Anisyutkin, L.N., and Kirejtshuk, A.G. 2014. The beetle-like Palaeozoic and Mesozoic roachoids of the so-called “umenocoleoid” lineage (Dictyoptera: Ponopterixidae fam. nov.). Comptes Rendus Palevol 13: 545–554. Crossref
Patel, S. and Singh, R. 2016. Updated checklist and distribution of Mantidae (Mantodea: Insecta) of the World. International Journal of Research Studies in Zoology 2: 17–54. Crossref
Piton, L. 1940. Paléontologie du gisement éocène de Menat (Puy-de-Dôme), flore et faune. Mémoire de la Société d’Histoire Naturelle d’Auvergne 1: 1–303.
Prokop, J., Krzeminski, W., Krzeminska, E., Hörnschemeyer, T., Ilger, J.-M., Brauckmann, C., Grandcolas, P., and Nel, A. 2014. Late Palaeozoic Paoliida is the sister group of Dictyoptera (Insecta: Neoptera). Journal of Systematic Palaeontology 12: 601–622. Crossref
Ross, A.J. 2019. The Blattodea (cockroaches), Mantodea (praying mantises) and Dermaptera (earwigs) of the Insect Limestone (late Eocene), Isle of Wight, including the first record of Mantodea from the UK. Earth and Environmental Science. Transactions of the Royal Society of Edinburgh [published online] Crossref
Smith, T., Habersetzer, J., Simmons, N., and Gunnell, G. 2012. Systematics and paleobiogeography of early bats. In: G. Gunnell and N. Simmons (eds.), Evolutionary History of Bats: Fossils, Molecules and Morphology. Cambridge Studies in Morphology and Molecules: New Paradigms in Evolutionary Biology, 23–66. Cambridge University Press, Cambridge. Crossref
Schwarz, C.J. and Roy, R. 2019. The systematics of Mantodea revisited: an updated classification incorporating multiple data sources (Insecta: Dictyoptera). Annales de la Société entomologique de France 55: 101–196. Crossref
Svenson, G.J. and Whiting, M.F. 2009. Reconstructing the origins of praying mantises (Dictyoptera, Mantodea): the roles of Gondwanan vicariance and morphological convergence. Cladistics 25: 468–514. Crossref
Vršanský, P. and Bechly, G. 2015. New predatory cockroaches (Insecta: Blattaria: Manipulatoridae fam.n.) from the Upper Cretaceous Myanmar amber. Geologica Carpathica 66: 133–138. Crossref
Vršanský, P., Bechly, G., Zhang, Q., Jarzembowski, E.A., Mlynský, T., Šmídová, L., Barna, P., Kúdela, M., Aristov, D., Bigalk, S., Krogmann, L., Li, L., Zhang, Q. Zhang, H., Ellenberger, S., Müller, P., Gröhn, C., Xia, F., Ueda, K., Vďačný, P., Valaška, D., Vršanská, L., and Wang, B. 2018. Batesian insect-insect mimicry-related explosive radiation of ancient alienopterid cockroaches. Biologia 73: 987–1006. Crossref
Wappler, T., Currano, E.D., Wilf, P., Rust, J., and Labandeira, C.C. 2009. No post-Cretaceous ecosystem depression in European forests? Rich insect-feeding damage on diverse middle Palaeocene plants, Menat, France. Proceedings of the Royal Society, London B 276: 4271–4277. Crossref
Wedmann, S., Uhl, D., Lehman, T., Garrouste, R., Nel, A., Gomez, B., Smith, K., and Schaal, S.F.K. 2018. The Konservat-Lagerstätte Menat (Paleocene; France)—an overview and new insights. Geologica Acta 16: 1–31.
Wieland, F. 2013. The phylogenetic system of Mantodea (Insecta: Dictyoptera). Species, Phylogeny & Evolution 3: 3–222. Crossref
Xia, F., Yang, G., Zhang, Q., Shi, G., and Wang, B. 2015. Amber: Lives through Time and Space. 197 pp. Science Press, Beijing.
Yager, D.D. and Svenson, G.J. 2008. Patterns of praying mantis auditory system evolution based on morphological, molecular, neurophysiological, and behavioural data. Biological Journal of the Linnean Society 94: 541–568. Crossref
Acta Palaeontol. Pol. 64 (4): 779–786, 2019
https://doi.org/10.4202/app.00628.2019