

Conellae, enigmatic structures on cephalopod shells—shapes, distribution, and formation
RENÉ HOFFMANN, ALEKSANDR MIRONENKO, and HELMUT KEUPP
Hoffmann, R., Mironenko, A., and Keupp, H. 2019. Conellae, enigmatic structures on cephalopod shells—shapes, distribution, and formation. Acta Palaeontologica Polonica 64 (4): 815–830.
Conellae, enigmatic cone-shaped structures which can be found on the surface of internal moulds of cephalopod shells (predominantly of ammonoids), are regarded herein as the product of remote (biologically induced) biomineralization formed in closed-off cavities during lifetime and might be primarily composed of vaterite, aragonite, or calcite. To date conellae have been interpreted in many different ways: (i) as organisms (gastropods, cirriped crustaceans, or disciniscid brachiopods), (ii) pre-diagenetic syn vivo features, i.e., biologically controlled or induced, the product of remote biomineralization, (iii) and diagenetic, i.e., abiogenic origin and post-mortem. The proposed processes of conellae formation seem insufficient to explain conellae related phenomena. Further, their assumed primary aragonitic or calcitic mineralogy are reviewed and based on new material critically assessed. The stratigraphic range of conellae extends from the Middle Ordovician and probably to modern Nautilus. Predominantly, conellae can be found on internal moulds along the keel, ribs or nodes, umbilical shoulder, at the transition between phragmocone and body chamber, and can be associated with repaired scars. However, conellae are also common on the smooth body chambers of large macroconchs of Jurassic ammonites. Conellae, which are located on ammonite body chambers, are filled with the same material found in the body chamber and can contain small burrows, sand grains, or coprolites. Some of these conellae are partially covered with nacreous shell material. Limonitic conellae were also found on the limonitic internal moulds of orthocone nautiloids. Moreover, disciniscid brachiopods found on inoceramid bivalves were re-identified herein as conellae. A short guide for conellae identification has been provided herein.
Key words: Cephalopoda, Ammonoidea, Nautilus, conellae, remote biomineralization, palaeopathology, Phanerozoic.
René Hoffmann [rene.hoffmann@rub.de], Institute of Geology, Mineralogy, and Geophysics, Ruhr-Universität Bochum, Universitätsstrasse 150, 44801, Bochum, Germany.
Aleksandr Mironenko [paleometro@gmail.com], Geological Institute of RAS, Pyzhevski Lane 7, Moscow 119017, Russia.
Helmut Keupp [keupp@zedat.fu-berlin.de], Institute of Geosciences, Freie Universität Berlin, Malteserstrasse 74-100, 12249, Berlin, Germany.
Received 20 may 2019, accepted 7 August 2019, available online 7 November 2019.
Copyright © 2019 R. Hoffmann et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Conellae, small cone-shaped structures often found on the surface of ammonite internal moulds, were first described by Raspail (1829: pl. 6: 36) and Quenstedt (1843: 178, 1851: 178, 1881–1884). Quenstedt (1881–1884) assigned these cone-shaped structures tentatively, but not beyond doubt, as patelloid gastropods; a view held also later by Busse (1976). Some workers confused conellae with barnacle-like epizoans providing generic names such as Probalanus or Pyramidobalanites, (e.g., Bogolubov 1926; Maubeuge 1949; Gerasimov 1955) or disciniscid brachiopods (Landman et al. 2016: table 1). Other authors regard conellae as purely inorganic calcite crystal growth comparable to cone-in-cone structures (Reis 1902; Schmidt 1933). Oppel (1853: 33) was the first to propose that conellae are a diagenetic product of the shell, which was also suggested by Denckmann (1887). Conellae have also been investigated in detail by Hölder and Mosebach (1950), Hölder (1952a, b, 1954a, b, 1956, 1970, 1973, 1980), Guex (1967), and Erben et al. (1969), Erben and Reid (1971), Erben (1972), and Birkelund (1981) who regarded them as a pre-diagenetic, i.e., syn vivo, feature. While the majority of conellae occur on the surface of internal moulds of Jurassic ammonites, a few had been reported for Cretaceous and Triassic ammonites: Denckmann (1887) and Hölder and Mosebach (1950) for Turrilites, Lehmann (1993) and Frerichs (2007) for Austiniceras, Busse (1976), Gensel (1990), Rein (1989, 1993, 2017), and Rein and Krause (1994) for Triassic Ceratites (see SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app64-Hoffmann_etal_SOM.pdf). Reports of conellae associated with the siphuncle and a hollow keel are of interest here because these conellae are outside the hollow keel (i.e., inside the chamber lumen as shown by Westermann 1971 for Sonninia). A list of ammonite taxa with conellae is provided by Hölder and Mosebach (1950), which comprises 25 genera. Predominantly, conellae can be found on internal moulds along the keel, ribs or nodes, umbilical shoulder, attachment area between suture line and the inner surface of the conch wall, and at the transition between phragmocone and body chamber (Hölder 1952b). The close association between shell injuries and the distribution of conellae was first mentioned by Guex (1967) (see also Keupp 1976). Conellae also occur in belemnites and internal moulds of nautilids (Hölder 1973; Seilacher and Gishlick 2015).
Most cone-shaped conellae described to date are composed of calcite, whereas some of them are filled with material similar to the host matrix (Gerasimov 1955). Rarely, rounded aragonitic conellae occur, while calcitic conellae have an angular outline. Roundish, aragonitic conellae represent spherulitic arrangements of assumed primary aragonite crystals. The surface of calcitic conellae form concentric rings displaying terrace-like structures, radial lines, or can be smooth. The concentric rings, related with organic layers, presumably represent imprints of shell layers. The radial lines are the result of cleavage planes of the rhomboedric calcite. They can occur as single elements, aggregates or as rug-like structures covering internal moulds. Isolated calcitic conellae can display 4 or 6 surfaces, and rarely 3 or 5 surfaces. Sometimes conellae are polygonal to roundish in cross section with a rounded tip. Based on thin sections of conellae found in the hollow keel of Harpoceras eseri Hölder and Mosebach (1950) described organic-rich layers, about 20 µm thick, that cut through all conellae. In addition, small (2 µm) opaque inclusions of organic matter occur. In rare cases, for example, during silicification conellae retain their original roundish morphology as reported for Stephanoceras by Hölder (1973). Both types of conellae (roundish or angular) are reported for the alveolar region of belemnite rostra.
Existing models to explain the formation of conellae seem to be insufficient. These difficulties motivated us to conduct an in depth study of the conellae-structure which should form the foundation for future research on the topic. Herein we provide (i) a revised model for conellae formation, (ii) evidence for their occurrence outside Cephalopoda, (iii) further occurrences of conellae on different cephalopod shell structures, and (iv) an extended stratigraphic range for the occurrence of conellae.
Proposed conellae formation processes.—Different processes have been proposed for the formation of conellae. (i) Hölder and Mosebach (1950), Hölder (1952b) suggested that conellae were primarily composed of aragonite crystals that formed charcoal-pile like spherulitic structures (= Meilerstellung of Hölder 1950, 1952b). During diagenetic transformation of aragonite to calcite, the former roundish conellae become angular displaying the typical cleavage faces of the calcite crystals. Moreover, they found remnants (honeycomb structures) of the primary aragonitic hexagonal crystals that formed a single conellae. Hölder and Mosebach (1950: 392), based on the organic-rich layers, argued for an organic process during the formation of conellae during a late stage of shell secretion. Specifically, conellae (e.g., in the position of the hollow keel) form during siphuncular sheet secretion and are related to the formation of the inner prismatic layer. This model explains the general lack of conellae on the body chamber the authors described, and where the secondary shell is missing (see discussion below). Hölder and Mosebach (1950) proposed that conellae are formed by the mantle epithelium secreting a secondary conellae-forming substance. However, later Hölder (1952a, b) regarded conellae as purely diagenetic features that form due to dissolution at rhombohedra cleavage surface of calcite crystals of the recrystallized, now calcitic shell wall (i.e., they have been regarded as post-diagenetic in origin). The observation of Hölder and Mosebach (1950) that oysters (Plicatula), serpulids, or bryozoans grow on such conellae excluded a late diagenetic origin for such cone-shaped structures. Hölder (1954a, b: 423) also assumed the presence of conellae-like structures in modern oysters and other bivalves (Cardium).
(ii) Erben et al. (1969) analysed the shell ultrastructure of modern Allonautilus scrobiculatus and found in juvenile shells primary aragonitic conellae (about 3 µm) within the inner prismatic layer. Erben et al. (1969) also documented conellae in the outer prismatic layer of Allonautilus. Furthermore, these structures are regarded as pre-diagenetic features which mean they form syn vivo supporting the original hypothesis of Hölder and Mosebach (1950). The specific spherulitic arrangement of aragonite crystals in conellae clearly deviate from the typical parallel arrangement of the aragonite crystals forming the prismatic shell layers (Erben et al. 1969; Hölder and Mosebach 1950; Hölder 1952b). Later diagenetic dissolution of the aragonitic shell brings the conellae to the surface (Erben 1972). Based on the calcium carbonate polymorph Erben and Reid (1971) observed distinction between aragonitic conellae, observed in modern Nautilus and the pristine shell of the Upper Jurassic ammonite Pavlovia, and the recrystallized, calcitic structures described by Hölder and Mosebach (1950). However, Erben and Reid (1971) noted on different orientations of aragonitic conellae. They proposed that, during the process of conellae growth, starting from single nucleation sites, neighbouring spherulites come into contact resulting in the formation of pyramid-like piles (see Shtukenberg et al. 2012 for spherulite formation). The space between these primary pyramids, with their tips pointing outwards, is filled with prismatic crystals automatically acquiring an inverted pyramid-shape (tips pointing inwards). Erben and Reid (1971) described the microstructure of the inner prismatic layer for such cases as composed of pyramidal and inverse-pyramidal piles of prismatic micro-crystals.
(iii) For the formation of cone-shaped conellae, Keupp (2000: 29–30; 2012: 115, fig. 122), following Bandel and Hemleben (1975), favours uncontrolled internal mineral growth. Specifically, injuries cause the formation of shell irregularities with interspaces filled with organic material that is easily decomposed and leaves pores or interspaces (Guex 1967, 1968). These interspaces favour the formation of uncontrolled mineral growth resulting in conellae. This observation explains the occurrence of conellae in taxa that otherwise do not develop these structures. Keupp (2012: 115) regarded conellae as primary calcitic structures following the first hypothesis put forward by Bandel and Hemleben (1975: 318) to explain the conellae-phenomenon. The second hypothesis put forward by Bandel and Hemleben (1975) refers to the differences in size and form of inorganically grown aragonite crystals. Inorganic crystals are larger compared to biominerals such as prisms or tablets. Those differences may play a role during diagenetic alteration processes. Potentially, larger crystals are less affected by dissolution processes or may be transferred more easily into calcite compared to the smaller, organic-rich biogenic aragonite crystals. This model significantly differs in the lack of biogenic control by the mantle tissue during their formation. Furthermore, all models require a closed-system during the dissolution and recrystallisation processes taking place (Bandel and Hemleben 1975).
(iv) For mineralization processes taking place without direct influence of living tissue Chinzei and Seilacher (1993) and Seilacher and Chinzei (1993) coined the term remote biomineralization providing main examples from modern oysters and cephalopods (Sepia, Spirula). Remote biomineralization is opposed to contact mineralization (i.e., biomineralization sensu stricto). Products of remote biomineralization, sometimes referred to as secondary or fill skeleton, are the result of self-organizing fabricational processes under weak functional control. Remote biominerals form in closed-off cavities, deviate considerably in their mineralization pattern from biomineralized hardparts and are variable in morphology, but show recurrent patterns in unrelated forms, and become easily recrystallized during early phases of diagenesis. In this context, Seilacher and Chinzei (1993: 364, fig. 1b, c) compared the morphology of the chalky layer of Spirula with conellae in ammonite shells. Additional examples of remote biomineralization were described by Kemperman and Gittenberger (1988) for the hollow ribs of clausiliid gastropods, and by Checa (2000) for the bivalves Strigilla and Solecurtus, which both share divaricate ribs. The deposition of the free growing aragonite prisms take place in closed spaces without contact with the mantle, although directly influenced by the fluids deriving from it. Crystal growth proceeds by epitaxy, on the substrate provided by fibres of composite prisms (Checa 2000). As it currently stands, processes summarized under the term remote biomineralization by Chinzei and Seilacher (1993) and Seilacher and Chinzei (1993), are described as biologically induced.
Analogue examples or phenomena tentatively excluded from true conellae.—Below we provide examples for analogue conellae-like structures or structures that were described as conellae but are tentatively excluded herein. Accordingly, these types are not further discussed herein. Conellae-like structures occur in belemnites where they are often found on the alveolar wall with the typical charcoal-pile like morphology, e.g., in the Upper Cretaceous genera Actinocamax, Gonioteuthis, and Belemnitella (see Christensen in Bergström et al. 1973, Hölder 1973). Moreover, Wanner (1907), Stolley (1929), and Hölder and Mosebach (1950) described the presence of large conellae at the alveolar wall of the Middle Jurassic Hibolithes ingens from Timor. Both, the roundish- and the angular-type occur in belemnites but it remains unclear whether those were primary aragonitic or calcitic. Seilacher and Gishlick (2015) described belemnite rostra as the product of self-organization. Because rostra grow from the inside out their conellae start to grow near at the apical line and fuse outwards into a solid layer. For Chitinobelus from the Toarcian Posidonia Shales (Southern Germany) they describe true conellae where they sit on the phragmocone (Seilacher and Gishlick 2015: 334–335, pl. 20.2). Engeser and Reitner (1983: 497) argue that the conellae formed in places where higher amounts of organic material prevented the aragonite from early dissolution.
Based on the reports of Barskov (1970) and Spaeth (1971) suggesting the presence of aragonite in belemnite rostra, Hölder (1973) argued that the belemnite rostrum is homologous to the inner prismatic layer of ammonoids. We herein reject the idea of Hölder (1973), and regard the belemnite phragmocone and the ammonoid phragmocone as homologous structures (Fuchs 2019). Bandel and Spaeth (1988) explained that aragonite in belemnites was erroneously reported by Spaeth (1971) and Spaeth et al. (1971). Recently, Ippolitov et al. (2018) provided convincing data for the presence of aragonite in megateuthid belemnite rostra. Nevertheless, it remains unclear whether that aragonite is a primary or secondary feature. Finally, the calcitic rostrum covers the aragonitic phragmocone (i.e., it would rather represent the outer prismatic layer).
Difficult to distinguish from true conellae are structures found on the shell surfaces of Devonian Platyclymenia and Sporadoceras described by Hölder (1973). These small, shallow angular conellae-like structures had smooth surfaces. Because ammonite shells were completely recrystallized we regard this conellae-like structure as a purely abiogenic post-diagenetic phenomenon with a different formation process, such as epitaxy.
Another phenomenon compared with conellae are pits on ammonoid internal moulds observed by House (1960). Because of their different orientation (tip of conellae points outwards while tip of pits points inwards), and significant differences in size House (1960) concluded that (i) the pits were formed syn vivo, and (ii) the conellae were too small and a result of diagenetic dissolution.
A third phenomenon was described from aragonitic bivalves by Seilacher (1990) and Seilacher and Gishlick (2015). Those bivalves form triangular structures of calcite resulting in divaricate patterns which can be used as windows in photosymbiotic species (Corculum). The triangles are, parallel to the shell surface and formed syn vivo, in which they differ from conellae.
Usdowski (1963) described single cones resembling conellae from the “Nagelkalk”. Similarly, Quenstedt (1881–1884) regarded the problematic gastropod Conella as an inorganic formation comparable to the structures found in the “Nagelkalk”. Additional abiogenic structures such as stylolithes and cone-in-cone structures (= Duten- or Tütenmergel in German) are compared with conellae by Hölder and Mosebach (1950). For different hypotheses to explain the formation of cone-in-cone structures the reader is referred to Brown (1954), Kimpe (1955), Lugli et al. (2005), and Kershaw and Guo (2016).
In recent years experimental studies were designed to understand the formation processes of complex biominerals. For example, Rudloff and Cölfen (2004) and Oaki et al. (2012) reported on the formation of cone-shaped macrocrystals of 5–50 µm height. These crystals, formed by vaterite, aragonite, or calcite, show the typical conellae morphology but are hollow, i.e., they rather represent a tepee instead of a massive pyramid. Furthermore, these crystals grow at the fluid-air interface pointing with their tips downwards. Oaki et al. (2012) found that the formation and shape of these hollow-cone carbonate crystals largely depends on molecular control.
Institutional abbreviations.—GIN MPC, Geological Institute of Russian Academy of Sciences, Moscow Paleontological Collection, Moscow, Russia; RUB-Pal, Ruhr-Universität Bochum Palaeontology, Bochum, Germany; SNSB-BSPG, Staatliche Naturwissenschaftliche Sammlungen Bayerns, Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany.
Material and methods
The material examined in this study ranges stratigraphically from Ordovician nautiloids to lowermost Cretaceous ammonites, and the modern Nautilus pompilius (Table 1). All conellae were found on cephalopod remains from Russia, Morocco, and Germany. Photographs were taken with (Nikon D5500 DSLR camera with Tamron 90 mm, f/2.8 Di VC USD Macro 1:1 F017 lens). SEM-images were taken with a Zeiss Gemini2 Merlin HR-FESEM in Germany and TESCAN VEGA-II XMU SEM in Paleontological institute of Russian Academy of Sciences in Moscow.
Table 1. Conellae bearing cephalopod remains used in this study in chronological order beginning with the latest records.
|
Stratigraphy |
Locality |
Taxon |
Position |
Morphotype |
Material |
|
Recent |
Phillipines |
Nautilus pompilius |
body chamber |
aggregates |
aragonite |
|
Late Volgian |
Kuntcevo, Moscow, Russia |
Craspedites sp. |
body chamber |
single |
sediment-filled (phosphorite) |
|
Middle Volgian |
Borsheva, Moscow region, Russia |
Virgatites virgatus |
internal mould of the phragmocone |
single, loose aggregates along ribs |
sediment-filled (phosphorite) |
|
Late Oxfordian |
Rybaki, Moscow region, Russia |
Amoeboceras serratum |
last septum, internal mould of the body chamber flanks and keel |
single, aggregates |
calcite and sediment-filled |
|
Late Callovian |
Peski, Moscow region, Russia |
Quenstedtoceras lamberti |
internal mould of the body chamber (annular elevation) |
groups |
calcite |
|
Middle Callovian (Catasigaloceras enodatum Zone) |
Nikitino, Ryazan region, Russia |
Rondiceras,
|
internal mould of the body chamber flanks |
single or in small groups |
sediment-filled |
|
Early Jurassic |
Buttenheim, |
Pleuroceras |
flank |
tightly packed |
calcite |
|
Late Devonian |
Morocco |
orthocone nautiloids |
septum-to-conch-attachment area |
single, loose aggregates |
limonite |
|
Middle Ordovician, |
East Baltic, Leningrad region, Russia |
Endoceras sp. (Endocerida, Nautiloidea) |
surface of endosiphuncle and spiculum internal mould |
single or tightly packed aggregates |
calcite |
Results
We found that Raspail (1829: pl. 6: 36) and Quenstedt (1843: 178) in the first issue of his “Flözgebirge” referred to pyramid-like structures that would later be named conellae.
Here we report on the oldest record off conellae from the Middle Ordovician of the Leningrad region (Russia, Figs. 1, 2). The Ordovician conellae consist of calcite and are located on the pointed end of the endocerid siphuncle named spiculum, which is positioned inside the last endocone (see Cichowolski 2009: fig. 4D, E for details). Based on the location of the conellae, they formed inside these calcitic endocones, which are analogous to ammonoid conellae that appear within the aragonitic shell wall. The outer surface of these endocones is preserved in two specimens (Fig. 1). Most of the conellae in endocerids are grouped into dense aggregates with fused bases, but also isolated individual specimens occur. Endocerid conellae are rather high, with a rounded or oval base, their surface is covered with fine longitudinal (Fig. 1C1, D) and horizontal lines (Fig. 1A3, C2).
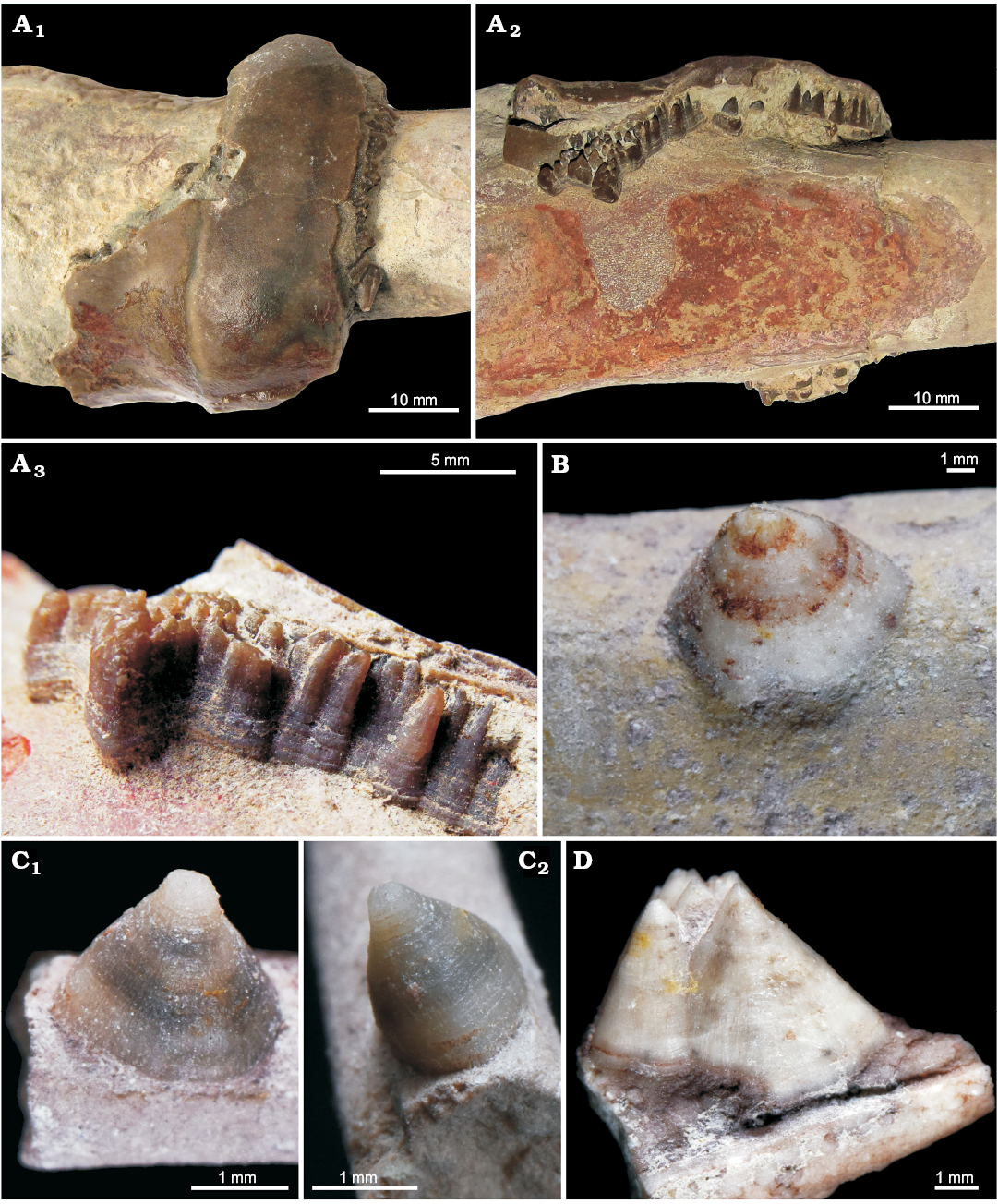
Fig. 1. Calcite conellae on internal moulds of siphuncle of Middle Ordovician endocerids from Leningrad region of Russia. A. GIN MPC 4/1, conellae in remnants of nautiloid siphuncle endocones; in top (A1) and lateral (A2) views, and close up, lateral view (A3). B. GIN MPC 4/2, single conella on the surface of pointed end of the endocerid siphuncle (spiculum). C. GIN MPC 4/3, single conella on the surface of spiculum, both with longitudinal and horizontal lines; in lateral (C1) and anterior (adapertural) (C2) views. D. GIN MPC 4/4, group of conellae on the spiculum internal mould.
Jurassic conellae from central Russia consist rarely of calcite (see Fig. 2 for Upper Oxfordian Amoeboceras serratum and Fig. 3 for the Upper Callovian Quenstedtoceras lamberti). The first example is of particular interest since for the first time conellae were found in central parts of ammonite septa. Specifically, the calcitic conellae are located within the last septum with its apex pointing towards the phragmocone, and in one case they are partially surrounded by preserved aragonitic nacre of the septum. All calcitic conellae on the ammonites studied herein are low, relatively wide and polygonal in cross section, sometimes with a stepped surface (Figs. 2, 3).

Fig. 2. Calcite conellae on Late Oxfordian Amoeboceras serratum (Sowerby, 1813) [M] internal moulds from Rybaki locality in the Moscow region, Russia. A. GIN MPC 4/5, pair of calcite conellae on the last septum with partially preserved aragonite; A2, detail. B. GIN MPC 4/6, single calcite conella on internal mould of the last septum; B2, detail. C. GIN MPC 4/7, calcite conellae in the rear part of phosphorite internal mould of the body chamber.

Fig. 3. Calcite conellae on the area of annular elevation of Late Callovian Quenstedtoceras lamberti (Sowerby, 1819) [M] (GIN MPC 4/8) from Peski locality in the Moscow region, Russia. Overview of the specimen (A1), note the transition from phragmocone to body chamber at about the middle of the preserved outer whorl. Close-up of tightly packed aggregates of conellae (A2, A3).
The majority of the conellae from Jurassic deposits of central Russia are found on the internal moulds of ammonite body chambers. Most of these conellae are filled with the same matrix of the ammonite internal mould (e.g., phosphorite, oolite marlstone, phosphatized sand; Figs. 4, 5). Most often they are found on the smooth body chambers of macroconchs, and, unlike most of the previously described conellae, they are rare on the ribbed ammonite phragmocone. Conellae are especially common on the smooth body chambers of large perisphinctids and on the body chambers of cardioceratid macroconchs, including: Cadoceras (see Gerasimov 1955: pl. 7: 19; Keupp 2012: fig. 227), Rondiceras, Quenstedtoceras, and Amoeboceras. Although in Amoeboceras conellae sometimes are localized in the area of the ventral keel in large quantities, most often they are found on the smooth walls of the body chambers of adult macroconchs.
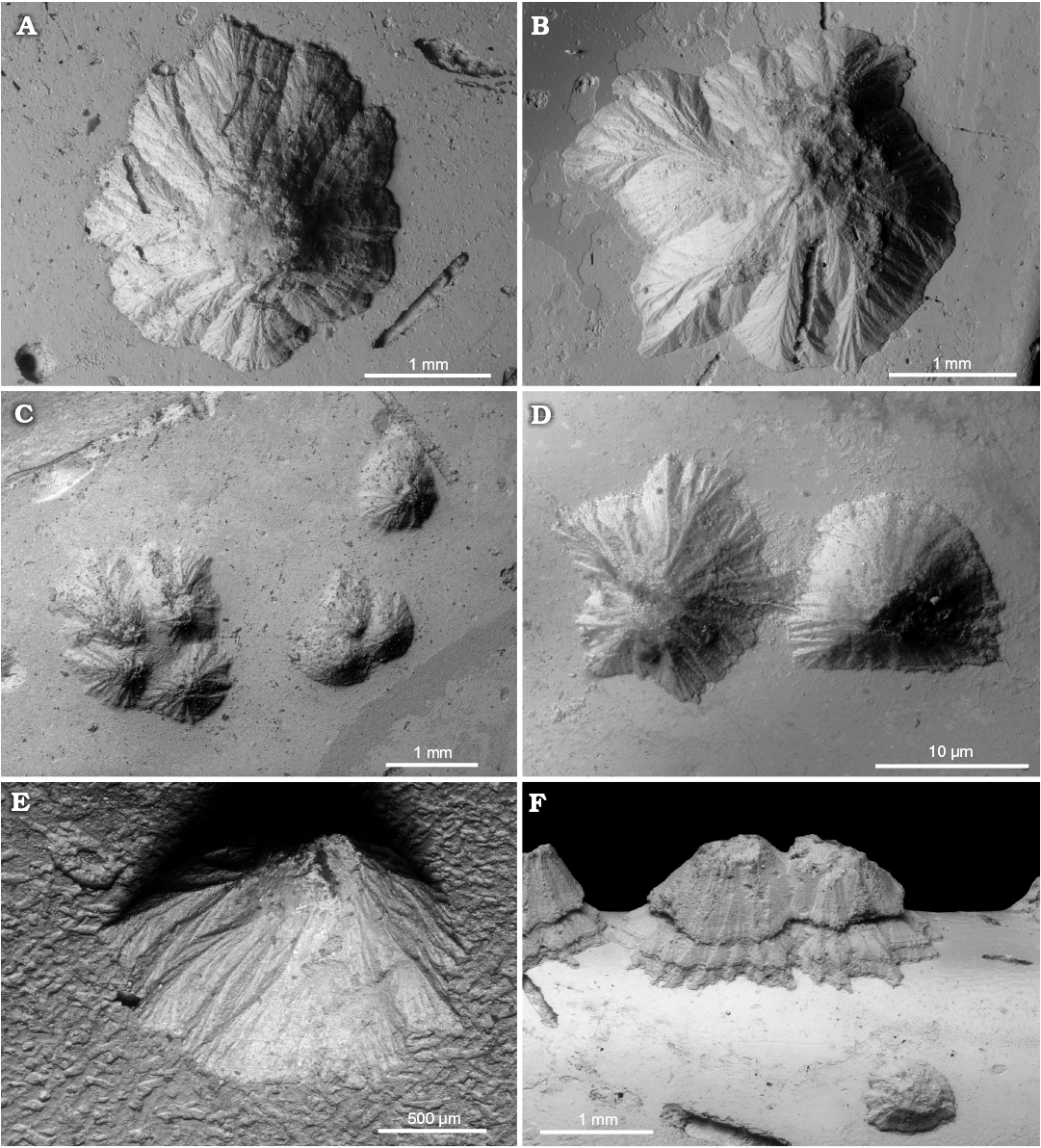
Fig. 4. Sediment-filled conellae on phosphorite internal moulds of Late Oxfordian Amoeboceras serratum (Sowerby, 1813) (body chambers) from Rybaki locality in the Moscow region, Russia. A. GIN MPC 4/9, typical conella of tepee-like shape. B. GIN MPC 4/10, conella of more complex shape, note irregular outline. C. GIN MPC 4/11, groups of sediment-filled conellae with different heights. D. GIN MPC 4/12, shape of the neighbouring conellae differ significantly. E. GIN MPC 4/13, lateral view of a single conella. F. GIN MPC 4/14, conellae on the ventral keel (body chamber), with seemingly two growth phases and a lower conella in front.
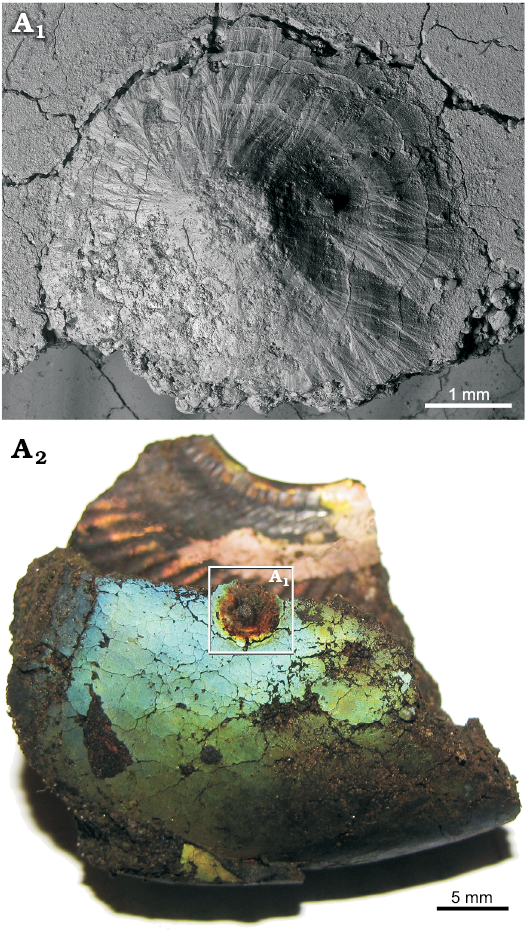
Fig. 5. A very large single sediment-filled conella on the phosphatized fragment of body chamber of Late Volgian macroconch of Craspedites sp. (GIN MPC 4/15) from Kuntsevo locality in the Moscow region, Russia. SEM image of conella showing the minute surface structure (A1), general view of the specimen (A2).
Often sediment-filled conellae contain various inclusions (e.g., thin burrows, grains of quartz sand, micro-coprolites [fecal pellets], and small fragments of unrecognizable phosphatized shells; Figs. 6, 7A2). The shape of conellae varies considerably from a simple cone with a rounded base to a very complex volcanic-like structure with very prominent and branched longitudinal ridges and furrows (Fig. 5). Some conellae are partially covered by remains of the aragonite shell (i.e., nacre tablets see Fig. 7A3, A5). The diameter of the conellae on Jurassic ammonites varies between 0.25 to 4.5 mm, but the majority of conellae are about 1 mm in diameter. Interestingly, no conellae were reported from calcitic aptychi found in the same deposits of conellae-bearing internal moulds (e.g., Mironenko 2018b).
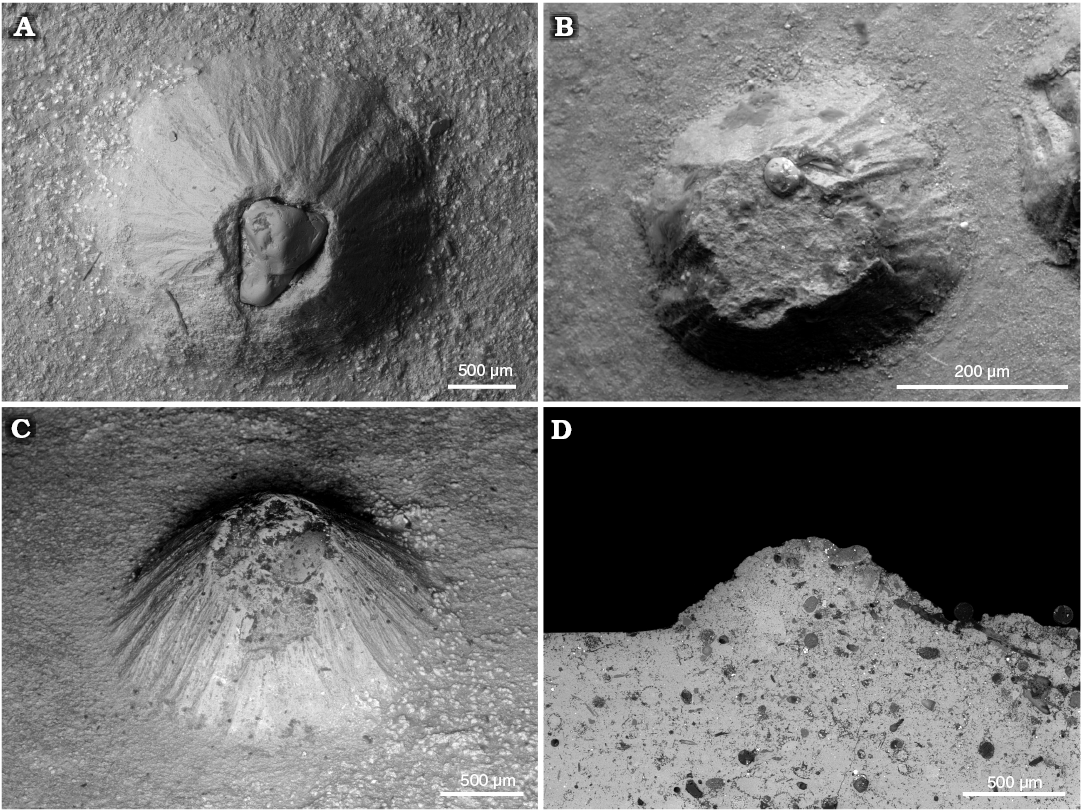
Fig. 6. Various inclusions in sediment-filled conellae. A, C. Sand grains in the conellae on the body chamber of Middle Callovian macroconch Rondiceras sp. from Nikitino locality, Ryazan region, Russia. A. GIN MPC 4/16, large sand grain on the top of conella. C. GIN MPC 4/17, small sand grain in the sediment which infilled the conella. B. Sand grain in the conella on phosphorite phragmocone of Late Volgian Virgatites virgatus (Buch, 1830) (GIN MPC 4/18) from Borsheva locality, Moscow region, Russia. D. Various algal microfossils in the internal mould of a body chamber and a conella, Amoeboceras serratum (Sowerby, 1813) [M] (GIN MPC 4/19) from Late Oxfordian, Rybaki locality in Moscow region, Russia.
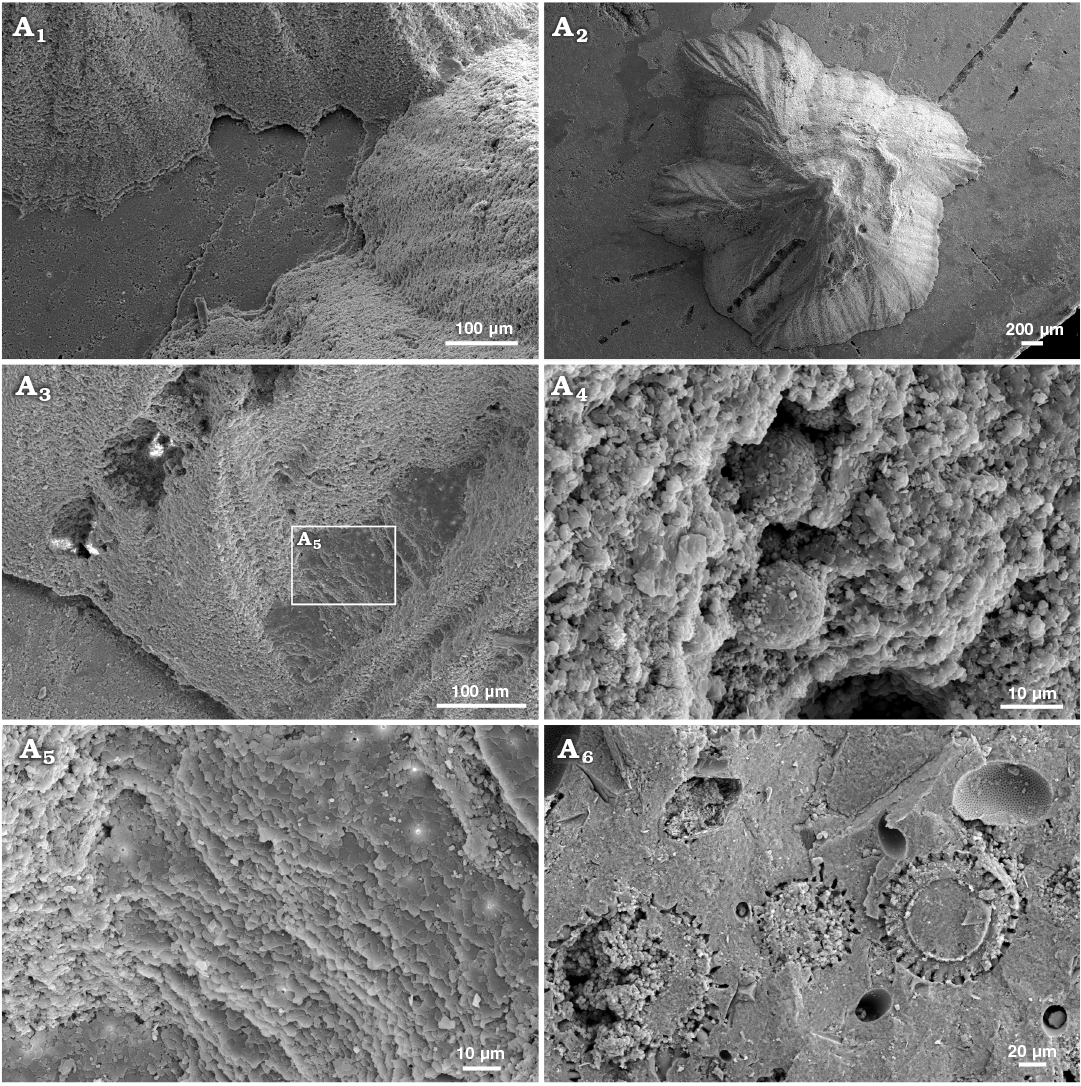
Fig. 7. Sediment-filled conellae on phosphorite internal moulds of Late Oxfordian Amoeboceras serratum (Sowerby, 1813) (RUB-Pal 7-11293, body chambers) from Rybaki locality in Moscow region, Russia. Group of conellae, note the small gap between conellae and internal mould (A1), conella with irregular outline showing a microboring, conella partially covered by nacre tablets suggesting that the conellae formed within the shell, was temporarily hollow and subsequently filled with sediment (A3, A5), framboid pyrite within the sediment fill of conellae (A4), matrix rich in radiolaria (so far unidentified) (A6).
We also found for the first time conellae on the internal mould of an orthoconic nautiloid phragmocone from the Devonian of Morocco. This specimen consists of limonite and originally likely was pyritic. Conellae on this specimen are partially single or arranged in rows, relatively wide and low, and with round bases (Fig. 8).
Interestingly, we found for the first time mm-sized conellae on the body chamber of modern Nautilus relatively close to the aperture (Fig. 9A). The conellae are white or brownish and occur in an injured area and display aggregates of different sizes. They are roundish in cross section, show horizontal layers and are composed of aragonite. The conellae are comparable in size with fossil examples. Note that ultrathin organic membranes are preserved suggesting a growth of the conellae from the shell surface towards the center of the fluid filled blister. For comparison we show a similarly injured ammonite (Cardioceras) with spherulitic conellae-like structures in the healed area (Fig. 9B).
Finally, we found in the report on Maastrichtian scaphitid ammonites infested by disciniscid brachiopods by Landman et al. (2016: fig. 4) the first evidence of conellae grown in inoceramid shells.
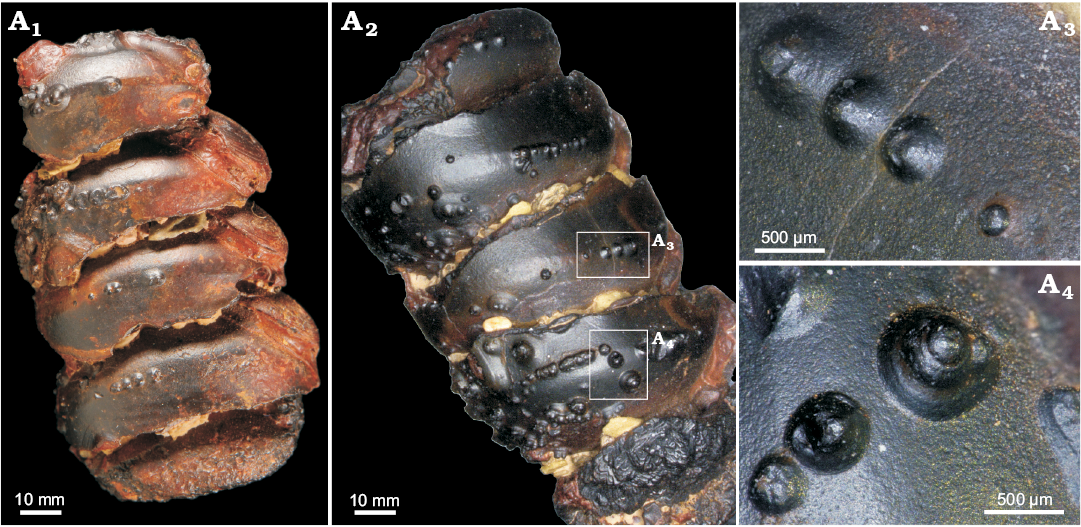
Fig. 8. Limonite conellae on the internal mould of Late Devonian orthoconic nautiloid (SNSB-BSPG 2014 XXI 81216) from Morocco. Overview on the preserved two specimens composed of five preserved chambers each (A1, A2), close up of limonitic conellae of different heights (A3, A4).
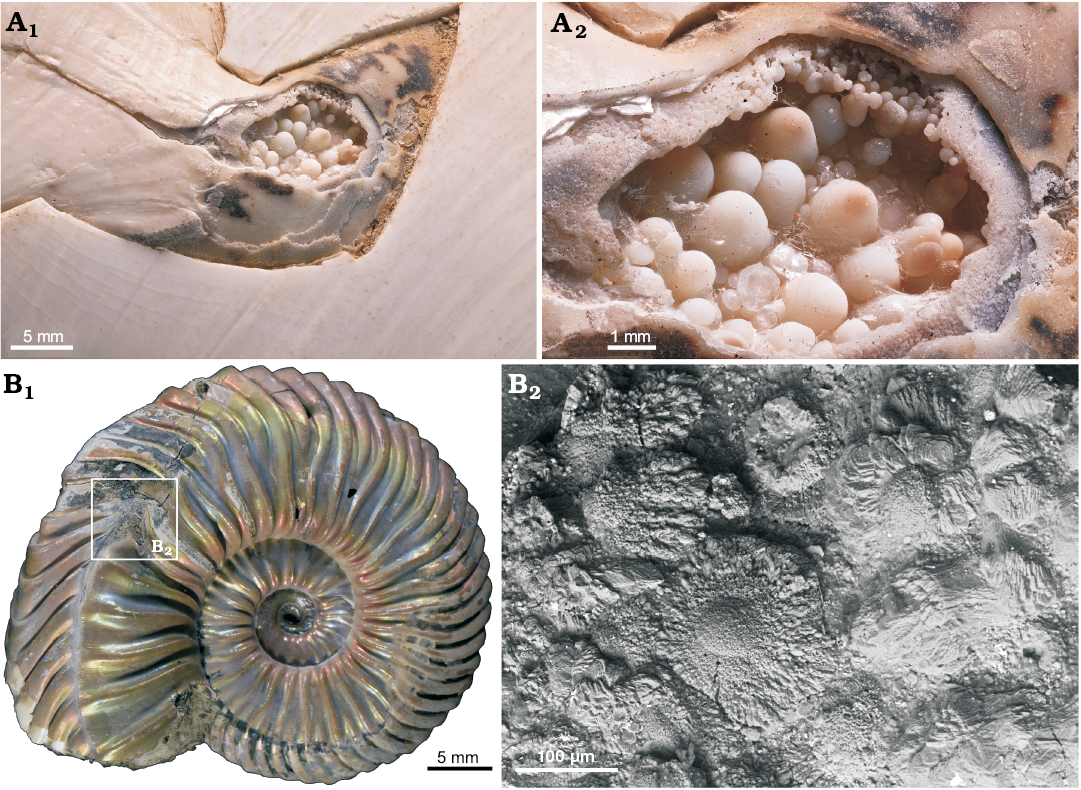
Fig. 9. A. Aragonitic conellae in an injured area close to the aperture of Recent Nautilus pompilius Linnaeus, 1758 (RUB-Pal 11292) from Philippines; the conellae are comparable in size with fossil examples (A1, A2). Note ultrathin organic membrane is preserved and a growth from the shell surface towards the center of the fluid filled blister is proposed. B. Aragonitic and calcitic conellae-like structures in an injured area (pathological band-slit: forma aegra verticata) of the Late Callovian ammonite Quenstedtoceras sp. (GIN MPC 4/20) from Dubki, Saratov region, Russia; overview (B1), SEM image showing the shell microstructure (B2).
Discussion
Based on our findings of conellae on endocerid spiculum and on ammonite septa without a prismatic shell layer, we reject a strict dependence of conellae formation on the presence of prismatic shell layers (e.g., Hölder and Mosebach 1950; Erben and Reid 1971). We also suggest that µm-sized conellae-like structures described for modern Nautilus by Erben et al. (1969), Erben and Reid (1971), and Erben (1972) have nothing to do with the mm-sized conellae described from Jurassic ammonites, because (i) conellae are often larger than the thickness of the inner prismatic layer, (ii) inverse-pyramids of the inner prismatic layer have never been observed, (iii) conellae from the outer prismatic layer have never been observed as well. Moreover, µm-sized cone-shaped tubercles are a constant element of the surface of ammonoid protoconch since the Triassic (e.g., Landman et al. 1996: fig. 12A, B; De Baets et al. 2015b) that do not lead to conellae formation. Based on their size and the presence of horizontal terrace-like structures we regard the cone-shaped structures observed on the injured shell area of modern Nautilus pompilius as true conellae sensu Quenstedt (1843). These document that conellae can form syn vivo in cephalopods.
The presence of sediment-filled casts of conellae on ammonite body chambers (e.g., Keupp 2012: fig. 122 for Pleuroceras), suggests that the conellae were hollow prior to sediment infilling. That observation is particularly interesting, because the original shell aragonite is partially preserved around the conellae, and suggests that conellae were originally filled with material that was dissolved before the shell wall material or that conellae were originally hollow. In turn, we assume that these conellae were primarily composed of aragonite or vaterite or were originally hollow structures. The recent experiments of Rudloff and Cölfen (2004) and Oaki et al. (2012) show the possibility of existence of hollow-cone conellae-like carbonate crystals. Although these results were obtained in vitro, the occurrence of such structures in nature cannot be excluded. Therefore, it is theoretically possible that conellae were originally hollow aragonite or vaterite structures.
Conellae, which formed inside the endocerid endocones, however, could have a primary calcitic composition based on the absence of sediment in them. Regarding the Devonian conellae, which consist of limonite (originally of pyrite), it is suggested that these were dissolved contemporaneously with the shell during pyrite formation. Accordingly, they could have been composed of either aragonite or calcite.
Conellae growth direction needs consideration too. Erben and Reid (1971) and Erben (1972) argued that the apex of the conellae represents the starting point of its formation and the broader base due to spherulitic growth of the crystals represents its end. If this is correct, we tentatively suggest that conellae growth starts with the formation of the outer prismatic layer. That would explain larger conellae and the presence of nacre tablets on their surface. However, conellae, which are located close to each other on the same specimen often have different heights (Figs. 4C, F, 8A3, A4; Hölder 1973: fig. 7b). This fact indicates, in case they started to grow from the apex, that the starting points of the formation of conellae were located at various heights within the shell, and not always in its outer prismatic layer. Conellae that grow within the hollow keel when sealed off on the other hand may start to grow with a broader base and the apex might represent the end of conellae growth within the remaining body fluid. The latter mode of formation is suggested for the conellae observed in modern Nautilus.
As an example for remote biomineralization, it seems likely that conellae, depending on the constituting fluid, may had have different primary mineralogy (vaterite, aragonite, and calcite). Specifically, primary vaterite and aragonite conellae could be easily transformed into other material, such as calcite or silica (Hölder and Mosebach 1950; Hölder 1973; and herein) or dissolved during diagenesis (Fig. 10). Whether replacement or dissolution of conellae material takes place probably depends on composition and pH of the diagenetic fluid, and on the position of the conellae. The position of conellae on the ammonite shell may influence whether these become filled with sediment (on the body chamber) or replaced by calcite (on the phragmocone).
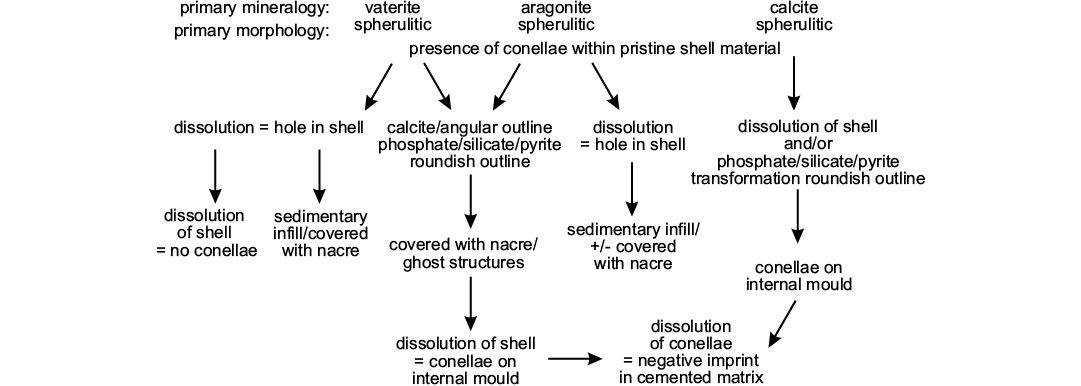
Fig. 10. Scheme illustrating potential diagenetic pathways for conellae formation and dissolution.
Bayer (1970: 32–33) observed dark bands for muscle attachments on pyrite internal moulds situated at the umbilical shoulder (= Nabelbandstrukturen of Jordan 1968). A different pyrite internal mould shows, in addition to these muscle attachment bands, similar darker areas where the shell was injured. Accordingly, Bayer (1970) argued that in both cases a similar material was used by the animal to ensure muscle attachment and to repair the injured shell. Along similar lines of evidence, Hölder (1952a, b, 1954a, b, 1956) argued that conellae found at the flanks of Arietites, perisphinctids, and oppeliids represent remains of the muscle attachment material. Further support for this, comes from conellae located at the muscle attachment bands on pyrite internal moulds (Oechsle 1958; Hölder 1952a, b, 1954a, b, 1956). It is possible that conellae are associated with areas of muscles attachment due to the thickening of the shell in these areas, such as in keels and ribs. Our observation of the numerous conellae on annular elevation of Quenstedtoceras confirms this hypothesis, since this feature is an area of soft tissue attachment (e.g., Mironenko 2015). The report of Riegraf et al. (1984) on conellae found associated with the crop content of Lower Jurassic ammonites from the Posidonia Shale supports the notion these features are associated with higher amounts of organic matter typical for injured area, muscle attachment sites and presumed soft tissue preservation (see Schweigert and Dietl 1999 for an Upper Jurassic Physodoceras). However, in most specimens examined for this study, the location of conellae are not related to muscle scars.
Bayer (1970) speculated that group specific distribution of conellae mainly depended on slight variation of the chemical composition of the shell material. We regard this case as an example of structures remotely mineralizing from a body fluid. The objection that the chemical composition of the shell material can explain the distribution of conellae among ammonite taxa is rejected. However, the notion that organic-rich material along muscle attachment bands as well as at injured area might have played a role during conellae formation is accepted (see Keupp 1976, 2012). In contrast to the favoured interpretation of conellae as the product of remote biomineralization is the fact that none of the remotely mineralized structures formed from aragonite or calcite crystals is morphologically similar to them (Bandel and Hemleben 1975; Seilacher and Chinzei 1993; Chinzei and Seilacher 1993; Kemperman and Gittenberger 1988; Checa 2000). The single exception is the shell of the modern Nautilus that forms conellae in an injured area.
Instead of diagenetic features of the shell or products of remote biomineralization some authors described conellae as remains of organisms, e.g., cirripeds, gastropods, or brachiopods. The actual disciniscid brachiopods attached to a Hoploscaphites have parts of their calcitic shell preserved, a rounded, subcentral apex, an elongated shell, growth on the aragonitic ammonite shell, and have minute concentric growth lines. In contrast, the structures regarded as disciniscid brachiopods described from inoceramid shells are partially intergrown (Landman et al. 2016: fig. 6.2b, c), have no shell material preserved, a central apex, growth within the shell and on the surface of the internal mould, and have a combination of concentric and radial lines (Landman et al. 2016: fig. 7.1d) covering the surface. Accordingly, we regard these structures not as disciniscids but as the first evidence of true conellae found on the internal mould of inoceramid bivalves. Cirriped shells can be distinguished from conellae because cirripeds have a central angular opening and form a net-like structure at their base. Another possible interpretation, regards sediment-filled conellae as boreholes of parasites or commensals, which lived inside these conellae. These parasites used the ammonite shell as shelter. According to this hypothesis, parasites exposed their gills through the open apex of the conellae for filter feeding and respiration. Also the broader bases of the conellae were originally open allowing the parasite direct access to the ammonite soft body. That interpretation is rejected here for the following reasons: conellae are arranged chaotically, without any relation to ammonite anatomy or swimming behaviour, and have different heights. Not all conellae exceed the shell thickness, i.e., did not reached the shell surface. Moreover, a reaction of the ammonite host to the presence of conellae has never been observed, whereas both ammonoids and nautiloids had various reactions to parasite infestations (De Baets et al. 2011, 2013, 2015a; Turek and Manda 2016; Mironenko 2018b).
Given the difficulties to identify true conellae, we provide a short identification guide: (i) never occur on shell surfaces, but on internal moulds (septal-attachment area, ribs, body chamber, last septum, siphuncle); (ii) preferably occur on moulds of ammonites with strong ribs, hollow keel, or nodes/tubercles or on injured areas or muscle attachment sites, but are also very common on smooth surface of macroconch body chambers; (iii) occur exclusively on mollusc internal moulds, predominantly on cephalopod remains (so far); (iv) mostly 1 mm in size (diameter and height); (v) their unknown primary composition can be modified to secondary aragonite, calcite, silica or infilled with pyrite, phosphorite, marlstone, etc.; (vi) occur as single-, loose- or dense aggregates, or as rug-like structures; (vii) base roundish or angular often with 4–6 edges (or more); (viii) with horizontal terrace-like or radial lines; (ix) apex of single conella is centrally located; (x) largely (but not exclusively) restricted to the Mesozoic; (xi) independent of facies (bituminous calcareous deposits, marly carbonates, clay, oolitic, white carbonates) but their preservation probably depends on facies and position on the shell; (xii) show distinct, internal-parallel layers enriched in organic matter that correspond with the horizontal layers visible on the surfaces.
Conclusions
Given the variable morphology and occurrence on cephalopod internal moulds as products of remote biomineralization (biologically induced), we assume no particular function for conellae. Conellae reported for belemnites or from shell surfaces are tentatively excluded. Information provided herein should form the foundation of detailed petrographic work, geochemical analysis applying state-of-the-art methods to disentangle the conellae-phenomenon.
Conellae, form in closed-off cavities during lifetime, and clearly deviate in their mineralization pattern from biomineralized hardparts, are variable in morphology, but show recurrent patterns in unrelated forms, and become easily recrystallized during early phases of diagenesis. Their primarily mineralogy comprises vaterite, aragonite, and calcite. Conellae formation is not limited to prismatic shell layers. Described micrometer-sized conellae from modern Nautilus are excluded from true conellae. At least sediment-filled conellae were hollow during body chamber infill, whereas shell layers around them were preserved at that time. Different growth directions are recognized: conellae formed within the shell grow from their tip and broaden towards the base, conellae that grow in closed-off fluid filled spaces start grow at their base and conellae grow ends with the apex. Occurrence of conellae largely depends on the presence of higher amounts of organic matter such as in injured areas, muscle attachment sites, and stomach area. Disciniscid brachiopods described previously in the literature are reinterpreted and now regarded here as first evidence of conellae found on inoceramid bivalve shells, i.e., not cephalopod shells (see Landman et al. 2016). The stratigraphic distribution of conellae ranges from Recent back to the Middle Ordovician.
Acknowledgements
The authors are very grateful to amateur paleontologists Nikolay Semyonov (Saint-Petersburg, Russia) and Sergey Medov (Moscow, Russia) for donation of the specimens with the conellae. The authors are also thankful to Yuri Yashunsky (Geological Institute of RAS, Moscow, Russia) for help in making sections of specimens and Roman Rakitov (Paleontological Institute of RAS, Moscow, Russia) for assistance in working with SEM. The research was supported for AAM by the program of the Presidium of Russian Academy of Sciences No. 16 (project 0135-2018-0050). The constructional reviews of Kenneth de Baets (GeoZentrum Nordbayern, Erlangen, Germany) and Joshua Slattery (University of South Florida, Tampa, USA) improved significantly a former version of the manuscript.
References
Bandel, K. and Hemleben, C. 1975. Anorganisches Kristallwachstum bei lebenden Mollusken. Paläontologische Zeitschrift 49: 298–320. Crossref
Bandel, K. and Spaeth, C. 1988. Structural differences in the ontogeny of some belemnite rostra. In: J. Wiedmann and J. Kullmann (eds.), 2nd International Cephalopod Symposium—Cephalopods Present and Past, 247–271. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Barskov, I.S. 1970. Structure of the belemnitid rostrum [in Russian]. Paleontologičeskij žurnal 1970 (4): 557–559.
Bayer, U. 1970. Anomalien bei Ammoniten des Aaleniums und Bajociums und ihre Beziehung zur Lebensweise. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 135: 19–41.
Bergström, J., Christensen, W.K., Johansson, C., and Norling, E. 1973. An extension of Upper Cretaceous rocks to the Swedish west coast at Särdal. Bulletin of Geological Society of Denmark 22: 83–154.
Birkelund, T. 1981. Ammonoid shell structure. In: M.R. House and J.R. Senior (eds.), The Ammonoidea, 177–214. Academic Press, London.
Bogolubov, N.N. [Bogolûbov N.N.] 1926. Traces of Balanidae in the Jurassic of Moscow [in Russian] . Ežegodnik Russkogo Paleontologičeskogo Obšestva 4: 145–150.
Brown, R.W. 1954. How does cone-in-cone material become emplaced? American Journal of Science 252: 372–376. Crossref
Busse, E. 1976. Eine Napfschnecke (Gastropoda, Cyclobranchia, Patellaceae) im Oberen Muschelkalk (Mittlere Ceratitenschichten/Ladin). Niederhessens Geologisches Jahrbuch Hessen 104: 5–7.
Checa, A.G. 2000. Remote biomineralization in divaricate ribs of Strigilla and Solecurtus (Tellinoidea: Bivalvia). Journal of Molluscan Studies 66: 457–466. Crossref
Chinzei, K. and Seilacher, A. 1993. Remote Biomineralization I: Fill skeletons in vesicular oyster shells. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 190: 349–361.
Cichowolski, M. 2009. A review of the endocerid cephalopod Protocyptendoceras from the Floian (Lower Ordovician) of the Eastern Cordillera, Argentina. Acta Palaeontologica Polonica 54: 99–109. Crossref
De Baets, K., Keupp, H., and Klug, C. 2015a. Parasites of ammonoids. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology. Topics in Geobiology 43, 837–875. Dordrecht, Springer. Crossref
De Baets K., Klug, C., and Korn, D. 2011. Devonian pearls and ammonoid-endoparasite co-evolution. Acta Palaeontologica Polonica 56: 159–180. Crossref
De Baets, K., Klug, C., Korn, D., Bartels, C., and Poschmann, M. 2013. Emsian Ammonoidea and the age of the Hunsrück Slate (Rhenish Mountains, Western Germany): Palaeontographica A 299: 1–113. Crossref
De Baets, K., Landman, N.H., and Tanabe, K. 2015b. Ammonoid embryonic development. In: C. Klug, D. Korn, K. De Baets, I. Kruta, and R.H. Mapes (eds.), Ammonoid Paleobiology: From Anatomy to Ecology. Topics in Geobiology 43, 113–205. Dordrecht, Springer. Crossref
Denckmann, A. 1887. Über die geognostischen Verhältnisse der Umgebung von Dörnten nördlich Goslar mit besonderer Berücksichtigung der Fauna des oberen Lias (Atlas). Abhandlungen zur geologischen Specialkarte von Preussen und den Thüringischen Staaten 8: 1–108.
Engeser, T. and Reitner, J. 1983. Chitinobelus acifer Fischer 1981, ein Belemnoteuthide (Coleoidea) mit Epirostrum. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 165: 496–501.
Erben, H.K. 1972. Die Mikro- und Ultrastruktur abgedeckter Hohlelemente und die Conellen des Ammoniten-Gehäuses. Paläontologische Zeitschrift 46: 6–19. Crossref
Erben, H.K. and Reid, R.E.H. 1971. Ultrastructure of shell, origin of conellae and siphuncular membranes in an ammonite. Biomineralisation 3: 22–31.
Erben, H.K., Flajs, G., and Siehl, A. 1969. Die frühontogenetische Entwicklung der Schalenstruktur ectocochleater Cephalopoden. Palaeontographica Abteilung A 132: 1–54.
Frerichs, U. 2007. Ammonit aus Vöhrum: Doch keine Ammoniten-Bisse, sondern Conellen? Arbeitskreis Paläontologie Hannover 35: 23–26.
Fuchs, D. 2019. Homology problems in cephalopod morphology: deceptive (dis)similarities between different types of “caecum”. Swiss Journal of Palaeontology [published online]. Crossref
Gensel, P. 1990. Conellen an Ceratiten des Hauptmuschelkalks (Trias) von Weimar. Veröffentlichungen des Naturhistorischen Museums Schleusingen 5: 26–30.
Gerasimov, P.A. 1955. Rukovodâŝie iskopaemye mezozoâ centralʹnyh oblastej evropejskoj časti SSSR. Čast II: Iglokožie, rakoobraznye, červi, mšanki i korally ûrskih otloženij. 92 pp. Gosgeoltehizdat, Moskva.
Guex, J. 1967. Contribution à l’étude des blessures chez les ammonites. Bulletin des Laboratoires de Géologie, Minéralogie, Géophysique et du Musée Géologique de l’Université de Lausanne 165: 1–16.
Guex, J. 1968. Sur deux conséquences particulières des traumatismes du manteau des ammonites. Bulletin des Laboratoires de Géologie, Minéralogie, Géophysique et du Musée Géologique de l’Université de Lausanne 175: 1–6.
Hoffmann, R., Lemanis, R.E., Falkenberg, J., Schneider, S., Wesendonk, H., and Zachow, S. 2018. Integrating 2D and 3D shell morphology to disentangle the palaeobiology of ammonoids: a virtual approach. Palaeontology 61: 89–104. Crossref
Hölder, H. 1950. Die Conellen auf Ammonitensteinkernen als Schalenrelikte fossiler Cephalopoden. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 92: 367–414.
Hölder, H. 1952a. Der Hohlkiel der Ammoniten und seine Entdeckung durch F.A. Quenstedt. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg 108: 37–50.
Hölder, H. 1952b. Über Gehäusebau, insbesondere Hohlkiel jurassischer Ammoniten. Palaeontographica A 102: 18–48.
Hölder, H. 1954a. Paläontologische Nachlese zur Conellen Frage. Neues Jahrbuch für Geologie und Paläontologie Monatshefte 1954 (9): 418–426.
Hölder, H. 1954b. Über die Sipho-Anheftung bei Ammoniten. Neues Jahrbuch für Geologie und Paläontologie Monatshefte 1954 (8): 373–379.
Hölder, H. 1956. Über Anomalien bei jurassischen Ammoniten. Paläontologische Zeitschrift 30: 95–107. Crossref
Hölder, H. 1970. Anomalien an Molluskenschalen, insbesondere Ammoniten, und deren Ursachen. Paläontologische Zeitschrift 44: 182–195. Crossref
Hölder, H. 1973. Miscellanea cephalopodica. Münstersche Forschungen zur Geologie und Palaeontologie 29: 39–76.
Hölder, H. 1980. Conellen als Relikte von Cephalopoden-Schalen-Objekte einer naheliegenden Verwechslung. Geologisches Jahrbuch Hessen 108: 5–9.
Hölder, H. and Mosebach, R. 1950. Die Conellen auf Ammonitensteinkernen als Schalenrelikte fossiler Cephalopoden. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 92: 367–414.
House, M.R. 1960. Abnormal growths in some Devonian goniatites. Palaeontology 3: 129–136.
Ippolitov, A., Barskov, I.S., Kosorukov, V.L., and Desai, B.G. 2018. Aragonite in belemnite rostra: new observations from the Lower Bathonian megateuthidids of Central Russia. In: A. El Hassani, R.T. Becker, S. Hartenfels, and F. Lüddecke (eds.), 10th International Symposium Cephalopods. Present and Past. Münstersche Forschungen zur Geologie und Paläontologie 110: 55–56.
Jordan, R. 1968. Zur Anatomie mesozoischer Ammoniten nach den Strukturelementen der Gehäuse-Innenwand. Beihefte zum Geologischen Jahrbuch 77: 1–64.
Kemperman, T.C.M. and Gittenberger, E. 1988. On morphology, function and taxonomic importance of the shell ribs in Clausiliidae (Mollusca: Gastropoda: Pulmonata), with special reference to those in Albinaria. Basteria 52: 77–100.
Kershaw, S. and Guo, L. 2016. Beef and cone-in-cone calcite fibrous cements associated with the end-Permian and end-Triassic mass extinctions: Reassessment of processes of formation. Journal of Palaeogeography 5: 28–41. Crossref
Keupp, H. 1976. Neue Beispiele für den Regenerationsmechanismus bei verletzten und kranken Ammoniten. Paläontologische Zeitschrift 50: 70–77. Crossref
Keupp, H. 2000. Ammoniten – Paläobiologische Erfolgsspiralen. 165 pp. Thorbecke Verlag, Stuttgart.
Keupp, H. 2012. Atlas zur Paläopathologie der Cephalopoden. Berliner Paläobiologische Abhandlungen 12: 1–390.
Kimpe, W.F.M. 1955. Unusual specimens of vertical cone-in-cone in Dutch coal. Association pout l’etude de la paleontologie et de la stratigraphie houilléres 21: 93–97.
Klinger, H.C. and Kennedy, W.J. 1977. Upper Cretaceous ammonites from a borehole near Richards Bay, South Africa. Annals of the South African Museum 72: 69–107.
Landman, N.H., Tanabe, K., and Shigeta, Y. 1996. Ammonoid embryonic development. In: N.H. Landman, K. Tanabe, and R.A Davis. (eds.), Ammonoid Paleobiology. Topics in Geobiology 13, 342–406. Plenum Press, New York. Crossref
Landman, N.H., Slattery, J.S., and Harries, P.J. 2016. Encrustation of inarticulate brachiopods on scaphitid ammonites and inoceramid bivalves from the Upper Cretaceous U.S. Western Interior. Acta Geologica Polonica 66: 645–662. Crossref
Lehmann, J. 1993. Conellen. Fossilien 3/93: 132–133. Crossref
Lugli, S., Reimold, W.U., and Koeberl, C. 2005. Silicified cone-in-cone structures from Erfoud (Morocco): a comparison with impact generated shatter cones. In: C. Koeberl and H. Henkel (eds.), Impact Tectonics, Impact Studies 6: 81–110. Crossref
Maubeuge, P.L. 1949. Sur la nature des „conelles“ (Quenstedt). Bulletin de la Société des sciences de Nancy 1: 1–3.
Mironenko, A.A. 2015. Soft-tissue preservation in the Middle Jurassic ammonite Cadoceras from Central Russia. Swiss Journal of Palaeontology 134: 281–287. Crossref
Mironenko, A.A. 2018b. Microstructure of aptychi of Upper Jurassic (Upper Oxfordian) ammonites from Central Russia. Lethaia 51: 75–85. Crossref
Mironenko, A.A. 2018a. First possible evidence of parasite infestation in Upper Devonian Discosorida (Nautiloidea). Swiss Journal of Palaeontology 137: 77–82. Crossref
Oaki, Y., Adachi, R., and Imai, H. 2012. Self-organization of hollow-cone carbonate crystals through molecular contral with an acid organic polymer. Polymer Journal 44: 612–619. Crossref
Oechsel, E. 1958. Stratigraphie und Ammonitenfauna der Sonninienschichten des Filsgebietes unter besonderer Berücksichtigung der sowerbyi-Zone (Mittlerer Dogger, Württemberg). Palaeontographica A 111: 47–129.
Oppel, A. 1853. Der mittlere Lias Schwabens. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg 10: 1–92.
Quenstedt, F.A. 1843. Das Flözgebirge Würtembergs mit besonderer Rücksicht auf den Jura. 558 pp. Laupp’sche Buchhandlung, Tübingen. Crossref
Quenstedt, F.A. 1851. Flözgebirge Würtembergs. 2nd Auflage. 578 pp. Laupp’sche Buchhandlung, Tübingen.
Quenstedt, F.A. 1881–1884. Petrefaktenkunde Deutschlands 7. Gastropoden, Erste Abtheilung, B, 1. 867 pp. Band Fues’s, Leipzig.
Quenstedt, F.A. 1885. Die Ammoniten des schwäbischen Jura. I. Der schwarze Jura (Lias). 436 pp. Schweizerbart, Stuttgart.
Raspail, F.V. 1829. Histoire naturelle des Bélemnites, accompagnée de la déscription et de la classification des espèces que M. Éméric, de Castellane, a recueillies dans les Basses Alpes de Provence. Première Partie. Annales des sciences d’observation 1: 271–331.
Rein, S. 1989. Über das Regenerationsvermögen der germanischen Ceratiten (Ammonoidea) des Oberen Muschelkalks (Mitteltrias). Veröffentlichung des Naturhistorischen Museums Schleusingen 4: 47–54.
Rein, S. 1993. Conellenbildung auf Ceratitensteinkernen. Veröffentlichungen des Naturkundemuseums Erfurt 1993: 44–55.
Rein, S. 2017. Die etwas andere biologische Organisation und Lebensweise der fossilen ectocochleater Cephalopoden des Oberen Muschelkalks (Mittel-Trias). Semana 32: 43–60.
Rein, S. and Krause, T.K. 1994. Aufbau und Diagenese der Conellen der Muschelkalkceratiten. Veröffentlichungen des Naturkundemuseums Erfurt 1994: 79–90.
Reis, O.M. 1902. Über Stylolithen, Dutenmergel und Landschaftskalk (Anthrakolith zum Theil). Geognostische Jahreshefte 14: 157–279.
Riegraf, W., Werner, G., and Lörcher, F. 1984. Der Posidonienschiefer—Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums. 195 pp. Ferdinand Enke Verlag, Stuttgart.
Rösner, T. and Schneider, C. 2011. Conellen an einem Nostoceras (Bostrychoceras) polyplocum aus Misburg. Arbeitskreis Paläontologie Hannover 39: 32–37.
Rudloff, J. and Cölfen, H. 2004. Superstructures of temporarily stabilized nanocrystalline CaCO3 particles: Morphological control via water surface tension variation. Langmuir 20: 991–996. Crossref
Säbele, D. and Schneider, C. 2013. Ammoniten. In: C. Schneider (ed.), Fossilien aus dem Campan von Hannover. Arbeitskreis Paläontologie Hannover 2013: 104–115.
Schairer, G. 1974. Quantitative Untersuchungen an Perisphinctidae (Ammonoidea) des untersten Unterkimmeridgium der Fränkischen Alb (Bayern). Zitteliana 3: 37–124.
Schmidt, M. 1933. Blatt Mössingen (Nr. 109). Erläuterungen zur Geologischen Spezialkarte. Württemberg 109: 1–184.
Schweigert, G. and Dietl, G. 1999. Zur Erhaltung und Einbettung von Ammoniten im Nusplinger Plattenkalk (Oberjura, Südwestdeutschland). Stuttgarter Beiträge zur Naturkunde Serie B 272: 1–31.
Seilacher, A. 1972. Divaricate patterns in pelecypod shells. Lethaia 5: 325–343. Crossref
Seilacher, A. 1990. Aberrations in bivalve evolution related to photo- and chemosymbiosis. Historical Biology 3: 289–311. Crossref
Seilacher, A. and Chinzei, K. 1993. Remote Biomineralization II: Fill skeletons controlling buoyancy in shelled cephalopods. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 190: 349–361.
Seilacher, A. and Gishlick, A.D. 2015. Morphodynamics. 531 pp. CRC Press, Boca Raton, Florida. Crossref
Spaeth, C. 1971. Aragonitische and calcitische Primärstrukturen im Schalenbau eines Belemniten aus der englischen Unterkreide. Paläontologische Zeitschrift 45: 33–40. Crossref
Spaeth, C., Hoefs, J., and Vetter, U. 1971. Some aspects of isotopic composition of belemnites and related paleotemperatures. Geological Society of America Bulletin 82: 3139–3150. Crossref
Shtukenberg, A.G., Punin, Y.O., Gunn, E., and Kahr, B. 2012. Spherulites. Chemical Reviews 112: 1805–1838. Crossref
Stolley, E. 1929. Über ostindische Jura Belemniten. In: J. Wanner (ed.), Palaeontologie von Timor, 16, Lieferg 29, 91–213. E. Schweizerbart, Stuttgart.
Turek, V. and Manda, Š. 2016. Early ontogeny, anomalous growth, and healed injuries in the Silurian nautiloid Ophioceras Barrande-Implications for hatching and the autecology of the Tarphycerida. Bulletin of Geosciences 91: 331–366. Crossref
Usdowski, H.E. 1963. Die Genese der Tutenmergel oder Nagelkalke (cone-in-cone). Beiträge zur Mineralogie und Petrographie 9: 95–110. Crossref
Wanner, J. 1907. Triaspetrefakten der Molukken und des Timorarchipels. Neues Jahrbuch Mineralogie, Geologie, Paläontologie, Beilagen Band 24: 161–220.
Westermann, G.E.G. 1971. Form, structure and function of shell and siphuncle in coiled Mesozoic ammonoids. Life Sciences Contributions, Royal Ontario Museum 78: 1–39. Crossref
Acta Palaeontol. Pol. 64 (4): 815–830, 2019
https://doi.org/10.4202/app.00640.2019