

New data on the anatomy of fuxianhuiid arthropod Guangweicaris spinatus from the lower Cambrian Guanshan Biota, Yunnan, China
HONG CHEN, DAVID A. LEGG, DA-YOU ZHAI, YU LIU, and XIAN-GUANG HOU
Chen, H., Legg, D.A., Zhai, D.-Y., Liu, Y., and Hou, X.-G. 2020. New data on the anatomy of fuxianhuiid arthropod Guangweicaris spinatus from the lower Cambrian Guanshan Biota, Yunnan, China. Acta Palaeontologica Polonica 65 (1): 139–148.
The fuxianhuiid arthropod Guangweicaris spinatus, from the lower Cambrian (Series 2, Stage 4), Guanshan Biota (Wulongqing Member, Canglangpu Formation), located in the vicinity of Kunming (Yunnan Province, southwest China), is redescribed based on new specimens and a re-examination of previously described material. A more complete overview of its morphology is given. Newly recognised features include: (i) a medial cephalic bulge; (ii) a tripartite hypostome; (iii) a pair of specialized post-antennal appendage (SPA); (iv) a putative telson; (v) two pairs of spines on the posteroventral margin of the terminal abdominal segment. This information is used to provide an emended diagnosis of the family Fuxianhuiidae, and the genus Guangweicaris.
Key words: Arthropoda, Euarthropoda, Fuxianhuiida, Fuxianhuiidae, Guanshan Biota, Cambrian, Asia.
Hong Chen [chenhong3397258@126.com], Yunnan Key Laboratory for Palaeobiology, Yunnan University, Kunming 650091, China; MEC International Laboratory for Palaeobiology and Palaeoenvironment, Yunnan University, Kunming 650091, China; School of Biological Sciences and Technology, Liupanshui Normal University, Liupanshui, Guizhou 553004, China.
David A. Legg [david.legg@manchester.ac.uk], School of Earth and Environmental Sciences, University of Manchester, Manchester M13 9PL, UK.
Da-You Zhai [dyzhai@ynu.edu.cn], Yu Liu [yu.liu@ynu.edu.cn], and Xian-Guang Hou [xghou@ynu.edu.cn], Yunnan Key Laboratory for Palaeobiology, Yunnan University, Kunming 650091, China; MEC International Laboratory for Palaeobiology and Palaeoenvironment, Yunnan University, Kunming 650091, China.
Received 26 May 2018, accepted 28 November 2019, available online 28 February 2020.
Copyright © 2020 H. Chen et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The fuxianhuiids occupy a crucial position in the upper stem-lineage of euarthropods (Legg et al. 2012, 2013; Legg 2013; Legg and Caron 2014; Ortega-Hernández 2016; Yang et al. 2018), and have gained considerable notoriety in recent years, due in large part to the discovery of exceptionally preserved specimens possessing putative nervous tissues (Ma et al. 2012, 2015; Yang et al. 2016), and vascular remains (Ma et al. 2014; but see also Liu et al. 2018, for a critique of this interpretation). Most members of this group have been described to possess a head with a wide carapace, covering a hypostome, a pair of antennae, and a pair of specialized post-antennal appendages (SPAs); a trunk differentiated into a thorax bearing multipodomerous appendages, and a limbless abdomen ending in a telson flanked by a pair of lateral processes. The thorax is divided into the prothorax consisting of a series of anteriorly reduced tergites and the wider opisthothorax bearing pleura (Chen et al. 2018). The prothorax is usually covered by the posterior margin of the carapace.
Although temporally and geographically restricted to the lower Cambrian (Series 2, Stage 3 to Stage 4), of Yunnan, southwest China, at least seven species of fuxianhuiid have been unequivocally recognised (Chen et al. 2018: table 1). The youngest of these is Guangweicaris spinatus Luo, Fu, and Hu in Luo et al., 2007, from the Cambrian (Series 2, Stage 4) Guanshan Biota. This species occurs within the Wulongqing Member of the Canglangpu Formation, which outcrops in the vicinity of Kunming. Although known from a large number of specimens (at least 150), this taxon is probably the poorest known of all the fuxianhuiids. To rectify this, a study of G. spinatus, including the type material, and a large collection of new specimens, was undertaken.
Institutional abbreviations.—ELI, Early Life Institute, Xi’an, China; NIGPAS, Nanjing Institute of Geological and Palaeontological Sciences, Nanjing, China; YIGS, Yunnan Institute of Geological Sciences, Kunming, China; YKLP, Yunnan Key Laboratory for Palaeobiology, Kunming, China.
Other abbreviations.—Kgs, locality Shitang hill, Gaoloufang, Kumming, China; SPA, specialized post-antennal appendage.
Material and methods
All currently known specimens of Guangweicaris spinatus, except for those deposited at the ELI (Liu et al. 2016; Wu and Liu 2019), were examined and photographed using a Canon EOS 5DSR with an MP-E 100 mm objective lens. These include a total of 51 new specimens, all deposited in the YKLP, five specimens described by Yang et al. (2008), also in the YKLP, and three specimens from the NIGPAS. Additionally, the type material of this species, deposited in the YIGS, was also examined (see SOM: fig. 1, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Chen_etal_SOM.pdf). Measurements of specimens were taken using callipers and refined using ImageJ (Schneider et al. 2012). Anatomical terminology follows that proposed in Chen et al. (2018).
All newly studied specimens were derived from the Gaoloufang section of the Wulongqing Member (Canglangpu Formation), situated near Guangwei village in the vicinity of Kunming, Yunnan, China. Associated trilobites, specifically Palaeolenus douvillei and P. mansuyi, indicate placement within the Palaeolenus Trilobite Zone, which is between 513 and 512 million years old (Hou et al. 2017). The latter occurs within the upper Canglangpuan regional stage, which correlated to lower Stage 4 of Cambrian Series 2.
Systematic palaeontology
Euarthropoda Lankester, 1904
Order Fuxianhuiida Bousfield, 1995
Family Fuxianhuiidae Hou and Bergström, 1997
Emended diagnosis.—Fuxianhuiid with a subtrapezoidal cephalic carapace at least 2–2.8 wider than long, covering a prothorax composed of three tergites. The remaining trunk is divided into an anterior, limb-bearing, opisthothorax with well developed, subtriangular pleural margins, and a caudal, limbless abdomen, the posteriormost segment of which is elongate and subtriangular. Endopods with a rounded termination (emended from Yang et al. 2018).
Remarks.—A sister-taxon relationship between Guangweicaris and Fuxianhuia is well established (Yang et al. 2008, 2018; Legg et al. 2013), as is a sister-taxon relationship between Fuxianhuiidae and Chengjiangocarididae (Legg et al. 2013; Yang et al. 2018) and so does not need discussing further, however, the diagnostic features of the Fuxianhuiidae, as presented in Yang et al. (2018) are in need of emendation based on new observations made herein. The latter stated the length to width ratio of the head shield (carapace herein sensu Chen et al. 2018), to be 1 : 4. Whilst this is true of some specimens attributed to species of Fuxianhuia (DAL personal observation), this is not the case for Guangweicaris, which has a ratio closer to 1 : 2.5. This is still greater than that observed in chengjiangocaridids, however, and so is emended rather than deleted from the diagnosis of this group. Likewise, Yang et al. (2018) stated that the endopods of fuxianhuiids did not extend beyond the pleural margins of the parent tergite and/or carapace, however, this feature could not be actually and accurately determined for Guangweicaris as appendages are rare, and when present, appear to extend beyond the tergite lateral margins, although it is unclear if this is a genuine feature or due to post-mortem detachment.
Genus Guangweicaris Luo, Fu, and Hu in Luo et al., 2007
Type species: Guangweicaris spinatus Luo, Fu, and Hu in Luo et al., 2007; Gaoloufang section, China, Cambrian Series 2, Stage 4, by monotypy.
Emended diagnosis.—As for type species by monotypy, see below.
Remarks.—The diagnosis of Yang et al. (2008), although accurate, is lacking a number of features that have been discovered herein, in particular, the presence of a cephalic bulge, a pair of SPAs and a tripartite hypostome; subpentagonal spines on the opisthothoracic appendage podomeres, a set of posteroventral spines on the terminal abdominal segment, and a subtriangular telson.
Guangweicaris spinatus Luo,
Fu, and Hu
in Luo et al., 2007
Figs. 1–5.
1999 Habelia? sp.; Luo et al. 1999: 39, pl. 31: 10, 11.
2007 Guangweicaris spinatus sp. nov.; Luo et al. 2007: 6–7, pl. 1: 1–6, pl. 2: 1–6.
2008 Guangweicaris spinatus Luo, Fu and Hu, 2007; Luo et al. 2008: 84–85, pl. 24: 1–6.
2008 Guangweicaris spinatus Luo, Fu and Hu, 2007; Yang et al. 2008: 117–119, text-figs. 2–4, pl. I: A–F.
2010 Guangweicaris spinatus Luo, Fu and Hu, 2007; Hu et al. 2010: 1769, text-fig. 4a.
2013 Guangweicaris spinatus Luo, Fu and Hu, 2007; Hu et al. 2013: 106–109, text-figs. 133–138.
2013 Guangweicaris spinatus Luo, Fu and Hu, 2007; Edgecombe and Legg 2013: 396.
2016 Guangweicaris spinatus Luo, Fu and Hu, 2007; Liu et al. 2016: 1940, text-fig. 2e, f.
2018 Guangweicaris spinatus Luo, Fu and Hu, 2007; Chen et al. 2018: 553.
2019 Guangweicaris spinatus Luo, Fu and Hu, 2007; Wu and Liu 2019: 543–548, text-figs. 1–3.
Type material: Holotype: YIGS Kgs-1-26 (SOM: fig. 1A), two prothoracic segments, five opisthothoracic segments, and seven abdominal segments, without preserving the carapace and tail. Paratypes: YIGS Kgs-1-36, 37, 62. In addition to the type material, Luo et al. (2007) noted 101 specimens of this species, the majority of which are poorly preserved and incomplete, some of them are figured herein (Figs. 1B, C, 2A; SOM: fig. 1B–E).
Type locality: Specimens were collected from a short interval, roughly 5 m thick, of yellowish-brown mudstone, located in the vicinity of Guangwei village within the Gaoloufang section, Kunming, Yunnan Province, southwest China.
Type horizon: Palaeolenus Trilobite Zone of the Wulongqing Member (Canglangpu Formation), Cambrian Series 2, Stage 4, which is dated 513–512 million years old (Hou et al. 2017).
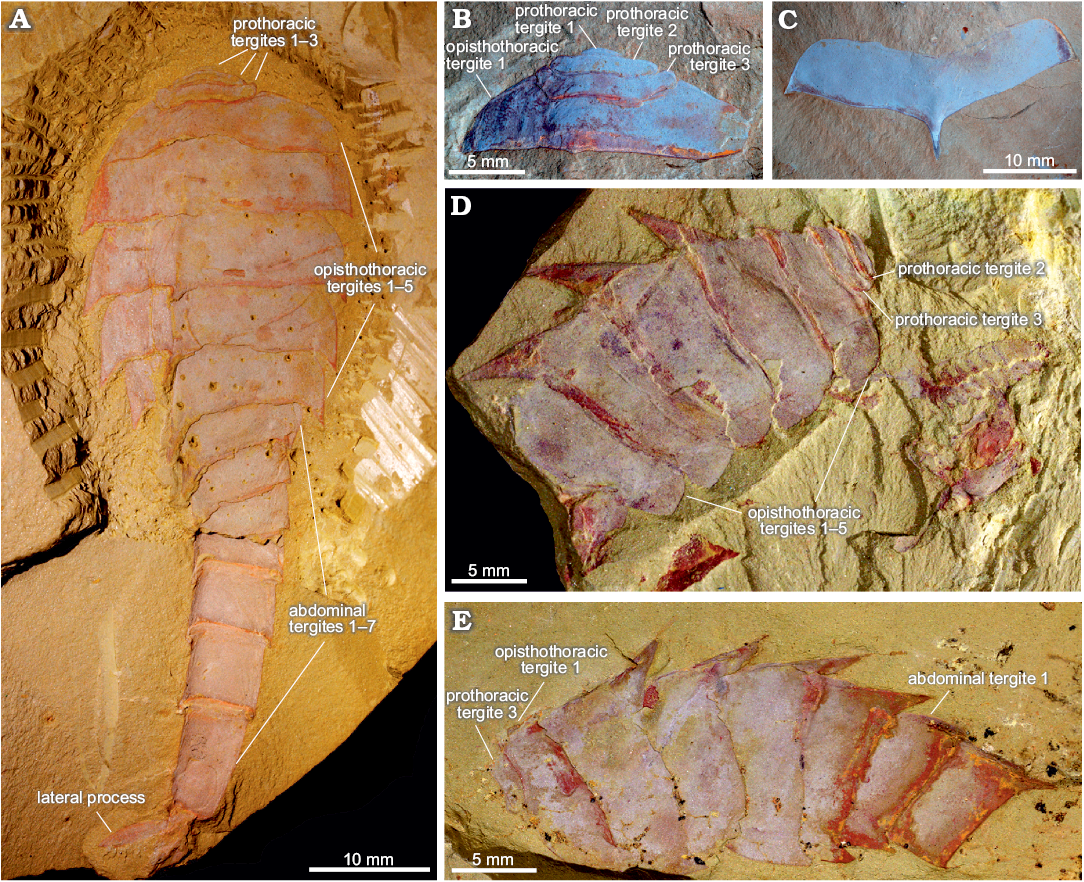
Fig. 1. Tergal morphology of fuxianhuiid arthropod Guangweicaris spinatus Luo, Fu, and Hu in Luo et al., 2007 from the lower Cambrian Guanshan Biota, China. A. YKLP 11564a, a nearly complete specimen in dorsal view, showing three prothoracic tergites, five opisthothoracic tergites and seven abdominal tergites. B. YIGS Kgs-1-36 (paratype), prothoracic tergites in dorsal view. C. YIGS Kgs-1-37 (paratype), isolated opisthothoracic tergite in dorsal view. D. YKLP 11140, last two prothoracic and five opisthothoracic tergites in lateral view, appendages are detached from the body. E. YKLP 11162b, last prothoracic, five opisthothoracic and first two abdominal tergites in lateral view.
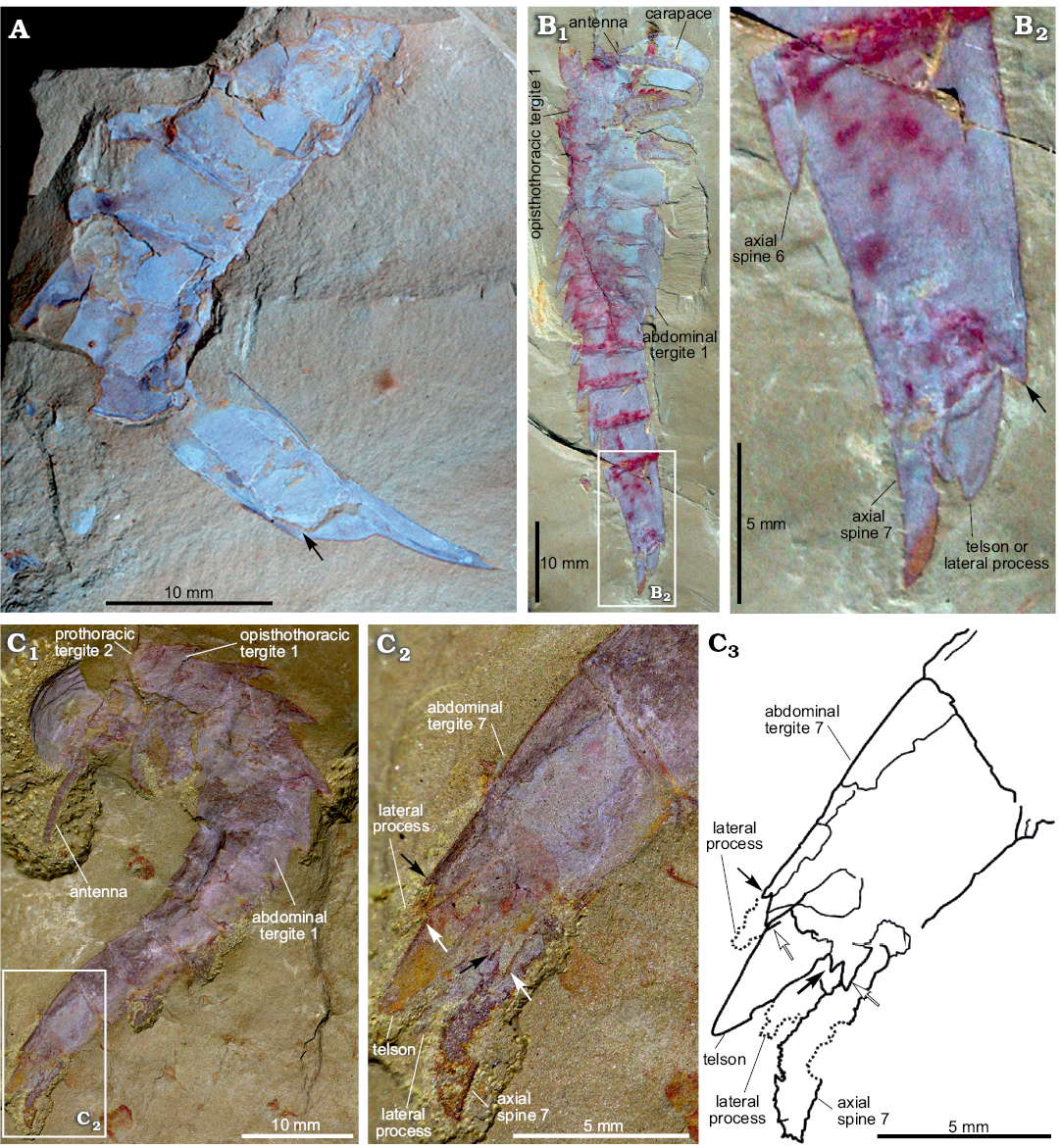
Fig. 2. Terminal abdominal tergite and associated structures of fuxianhuiid arthropod Guangweicaris spinatus Luo, Fu, and Hu, 2007 from the lower Cambrian Guanshan Biota, China. A. YIGS Kgs-1-62 (paratype), incomplete abdomen in lateral view. B. NIGPAS Kgs-1-137, nearly complete specimen in lateral view (B1), close-up (B2). Arrows indicate the position of the posteroventral spine. C. YKLP 11566, complete trunk and telson in lateral view (C1), close-up (C2), explanatory drawing (C3), white arrows, outer spines; black arrows, inner spines. Image B courtesy of Shixue Hu (Chengdu Centre of the Geological Survey of China, Chengdu, China).
Additional material.—Since the initial description of this species, twelve additional specimens have been described and figured including YKLP 11564–11568 (Yang et al. 2008), NIGPAS Kgs-1-137, prior to preparation (Luo et al. 2008), and NIGPAS Kgs-6-108, NIGPAS Kgs-1-137, and an unnumbered NIGPAS specimen, after preparation (Hu et al. 2013), and ELI-LBSG-0006B, 0046A, 0047, 0022B (Wu and Liu 2019). To this we add 51 specimens: YKLP 11140–11188, 11201, 11202. All from Gaoloufang section, China, Cambrian Series 2, Stage 4. Another specimen assigned to Guangweicaris spinatus by Hu et al. (2013: 108, fig. 135) does not actually belong to this taxon, and may instead belong to the “trilobitomorph” Longquania bispinosa Luo and Hu in Luo et al., 2008 (see Remarks).
Diagnosis.—Fuxianhuiid possessing a wide carapace with a medial cephalic bulge; a pair of specialized post-antennal appendage and a tripartite hypostome; an opisthothorax composed of five segments, the posterior four of which possess an extensive posteromedial axial spine and posterolateral spinose extensions of the pleurae; opisthothoracic appendages composed of, at least, 11 podomeres, each bearing a subpentagonal spine; an abdomen consisting of seven tergites all bearing a posteromedial axial spine and a terminal abdominal segment bearing a subtriangular telson, two pairs of posteroventral spines, and a pair of lateral, phylliform processes (emended from Yang et al. 2008).
Description.—Carapace: The carapace (Figs. 3A1, 4A1, B, D1) is subreniform in outline with a broad anterior margin, and widely curved lateral margins, which abruptly change angle at their postero-lateral edge, resulting in an acute margin that then curves gently towards the posteromedial axis. The carapace is between 2.0 and 2.8 times wider than long (SOM: table 1), with most variation in aspect ratio caused by differences in burial orientation, which also causes the anterior margin to appear more strongly curved in some specimens (cf. Figs. 3A1 and 4B). Lateral wrinkling (see for examples Figs. 3A1 and 4D1), is no doubt the result of compaction, indicating the anterior-medial area of the carapace was somewhat bulbous. A medial hinge or suture is lacking. It is unclear, due to a lack of ventrally preserved specimens, if a marginal doublure is present. The posterior margin of the carapace entirely covers the prothoracic segments and overlaps the anterior margin of the first opisthothoracic tergite (Fig. 3A1).
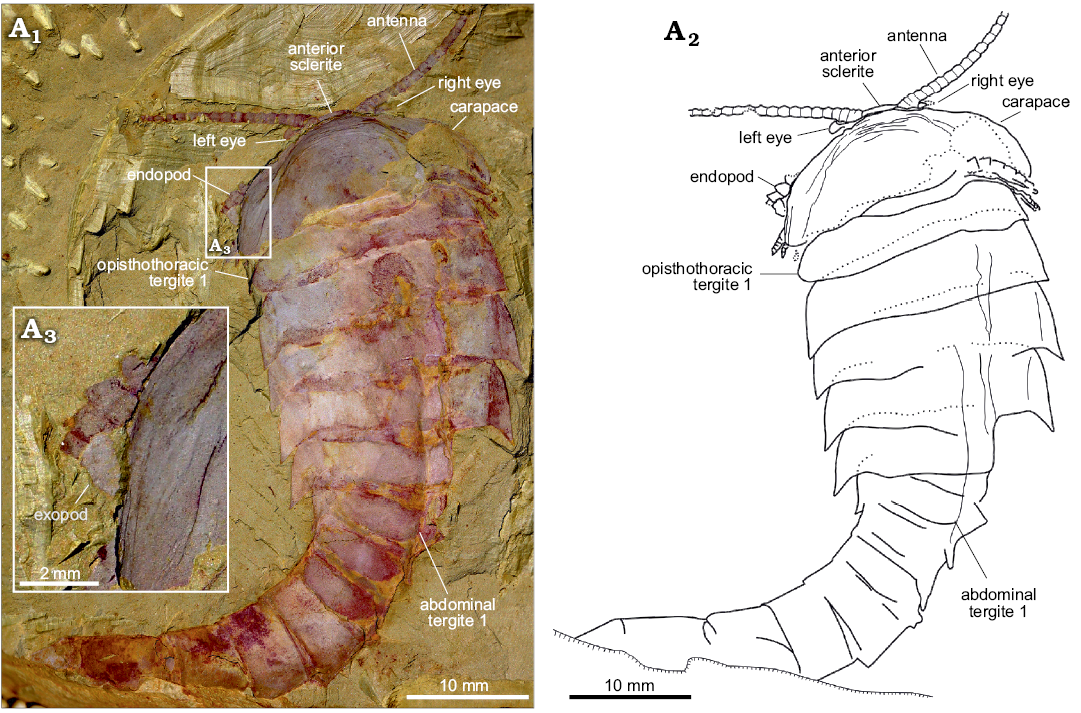
Fig. 3. An almost complete and exquisite specimen of fuxianhuiid arthropod Guangweicaris spinatus Luo, Fu, and Hu, 2007 (YKLP 11141) from the lower Cambrian Guanshan Biota, China. Specimen in dorsal view showing the antennae, eyes, carapace and trunk (A1), explanatory drawing (A2), close-up of the left margin of the carapace, showing the flap of an exopod (A3).
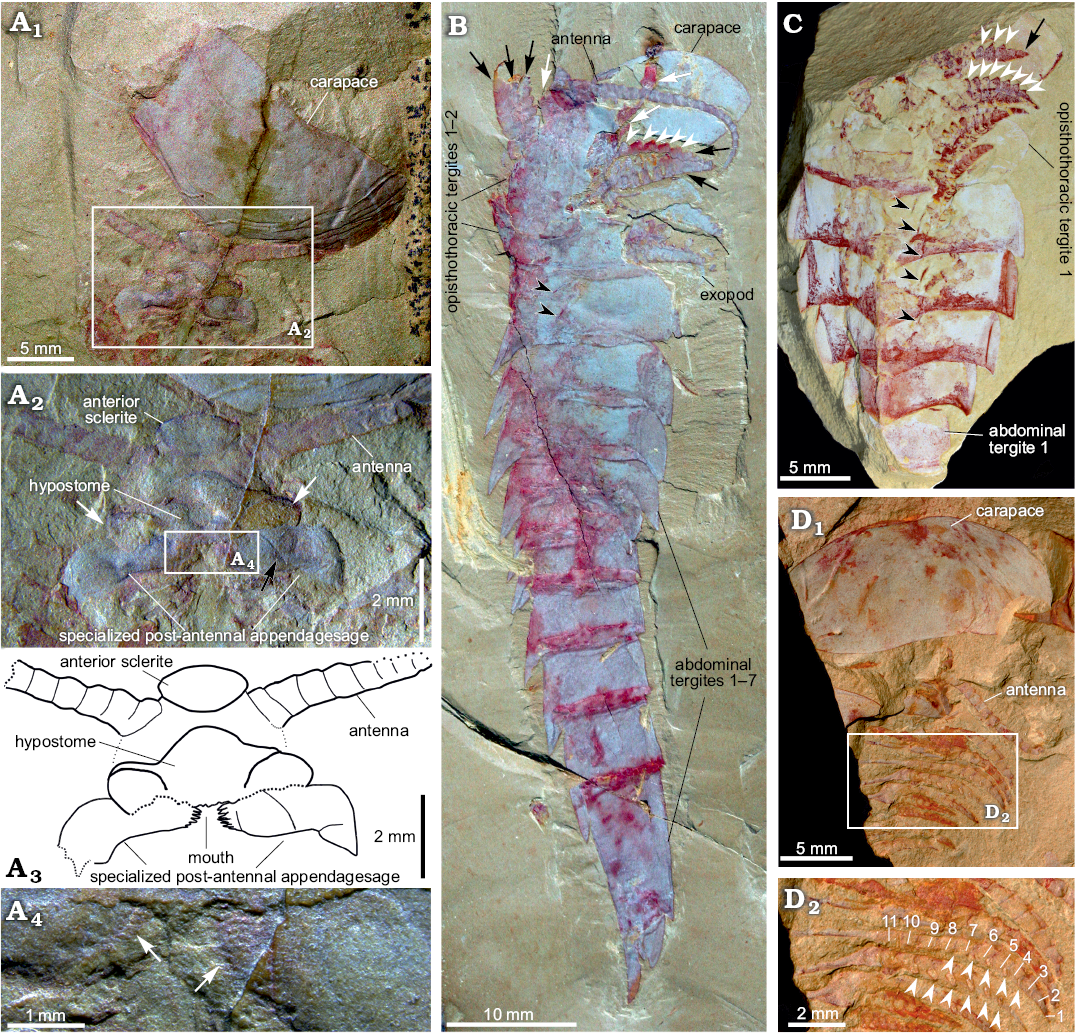
Fig. 4. Carapace and appendages of fuxianhuiid arthropod Guangweicaris spinatus Luo, Fu and Hu, 2007 from the lower Cambrian Guanshan Biota, China. A. YKLP 11201, detached carapace in ventral view and the organization of head appendages (A1); close-up of the head appendages (A2, photograph; A3, explanatory drawing); white arrows, the lateral lobes of the hypostome; black arrow, the possible section in SPA; close-up of the spinose medial margin of the SPA (A4), white arrows indicate the spinose medial margin. B. NIGPAS Kgs-1-137, nearly complete body with well-preserved appendages in lateral view; white arrows, the anterior appendages; black arrows, the appendages belonging to the first and second opisthothoracic segments; white arrowheads, lateral subpentagonal spines of an endopod; black arrowheads, the attachments of the proximal podomeres of appendages. C. YKLP 11202, opisthothoracic appendages in dorsal view; black arrow, the terminal subtriangular podomere; white arrowheads, lateral subpentagonal spines of an endopod; black arrowheads, the attachments of the proximal podomeres of appendages. D. NIGPAS Kgs-6-108 the carapace, antennae and trunk appendages in ventral view (D1), close-up of trunk appendages showing 11 podomeres in the endopod of one biramous appendage (D2), arrowheads point to hollow nodes that indicate the insertions of the spines along the inner margin of the endopod. Images B, C courtesy of Shixue Hu (Chengdu Centre of the Geological Survey of China, Chengdu, China).
Trunk: The trunk is divided into three distinct sections, or pseudotagmata: a prothorax composed of three segments, an opisthothorax bearing five segments, and finally an abdomen of seven segments (Fig. 1A). The anterior-most prothoracic tergite is the least commonly preserved; it is very small, measuring about half the length and a third the width of the second tergite (SOM: table 2), and almost semi-circular in outline (Fig. 1A, B). The second tergite is subtrapezoidal with a slightly bowed anterior, and expansive and rounded lateral margins (Fig. 1A, B, D). The third and final prothoracic tergite curves around the second tergite resulting in an almost trapezoidal outline with anteriorly deflected posterolateral margins (Fig. 1A, B, D, E). The second tergite is roughly two thirds the length, and three quarters the width of the third prothoracic tergite (SOM: table 2). The third prothoracic tergite is nestled within the anteriomedial margin of the first opisthothoracic tergite, and is the widest prothothoracic tergite (Fig. 1A, B, D, E).
The first opisthothoracic segment is over twice as wide as the preceding tergite, and between two and three times as long (SOM: table 2). It is almost semi-circular in outline (Fig. 1A, B). Its rounded lateral margins slope away from anterior tergal boundary at roughly 50°, before terminating at an acute posterolateral edge (Fig. 1A, B). Although the posterior margin of the first opisthothoracic tergite is often poorly preserved (Fig. 1D, E), presumable occurring during excavation and preparation, well preserved specimens indicate that it was relatively straight and featureless (e.g., Fig. 1B). The lateral margins of the second opisthothoracic tergite are contiguous with those of the preceding segment (Fig. 1A).
Isolated opisthothoracic tergites demonstrate that the anterior margins were relatively straight (Fig. 1C), with an observed medial deflection attributable to deformation caused by post-burial compression. With only a few exceptions, which can be attributed to taphonomic variance, the second opisthothoracic tergite is always the widest (SOM: table 3). The second opisthothoracic tergite is only a fraction larger than the adjacent tergites, with a more pronounced decrease in width occurring in tergites 4 and 5. The latter measures just two-thirds the width of the second tergite (SOM: table 3). Like the first opisthothoracic tergite, the lateral margins of the remaining opisthothoracic tergite are rounded, however, unlike the former their posterolateral margins are extended into a small subtriangular spine (Fig. 1A, C–E). The second opisthothoracic tergite, and all more posterior tergites, possess an extensive medial axial spine (Fig. 1A, C–E). These spines are often broken in dorsally preserved specimens, and are best observed in laterally preserved specimens, e.g., YKLP 11140 (Fig. 1D), and YKLP 11162 (Fig. 1E). These specimens reveal that the longest axial spines typically belong to the second opisthothoracic tergite, with successive tergal spines progressively decreasing in length. The fifth axial tergal spine measures roughly 60% the length of the first (SOM: table 4). Both the observed length of the axial spines and their angle of deflection, measured along the posterior spine margin, are controlled by their angle of burial, appearing shorter and more posteriorly arched in obliquely preserved specimens (cf. Fig. 1A, D). The angle of posterior deflection typically measuring between 130–150° (compared to the dorsum of parent tergite), with the more posterior spines showing a greater degree of displacement (SOM: table 4).
The abdomen is defined by an abrupt change in width compared to the preceding tergite, typically measuring 60–80% of the fifth opisthothoracic tergite (SOM: table 3). As with all post-prothoracic tergites, the first abdominal tergite possesses a posteromedial axial spine (Fig. 1A, E). The posterior margin of this tergite, and the following abdominal tergites, curve in a posteromedial orientation towards this spine (Fig. 1A). This spine is typically longer than that of the preceding tergite and with an increased posterior deflection (SOM: table 4). The terminal abdominal spine measured nearly three times the length of the first abdominal spine (SOM: table 4). Posterior spines also show a greater degree of posterior deflection, ranging 120–140° from the anterior tergites to the posterior (SOM: table 4). This is taken to the extreme in the terminal abdominal tergite, in which the main body of the tergite is more than twice as long as the preceding tergite (SOM: tables 3, 4), and the accompanying spine is orientated parallel to the dorsum of its parent tergite (Fig. 2). The terminal abdominal segment preserved in lateral aspect and demonstrates that at least one spinose process (Fig. 2A, B, arrows) occurs on the posteroventral margin. Such processes also appear to be present in YKLP 11566 (Fig. 2C), where two pairs of triangular spines consisting of an enlarged outer spine (Fig. 2C2, C3, white arrows), and a subordinate inner spine (Fig. 2C2, C3, black arrows), are present on the posteroventral margin.
Telson and associated structures: YKLP 11566 (Fig. 2C) is also significant in possessing a ventral medial, subtriangular extension, presumably a telson under the posterodorsal spine. This structure, and the associated spine, were previously identified as the furcae of a “tail” (lateral processes sensu Chen et al. 2018), by Yang et al. (2008), however, the medial extension is in fact flanked by two, albeit poorly preserved, phylliform projections, herein interpreted as lateral processes, comparable to those in other specimens (Fig. 2C). These processes are each associated with the aforementioned pair of triangular spines.
Eyes: The eyes, and associated structures, are preserved in a single specimen, YKLP 11141 (Fig. 3A1, A2). The eyes are small and ovoid, measuring just 1.25 mm, along their widest axis, compared to the carapace width, which is 21 mm. The left eye is the better preserved, although it is doubtful if an associated structure represents the right eye that appears to be attached to a small, subrectangular eyestalk (Fig. 3A2), although the base of this structure is poorly preserved. Anterior sclerite (Figs. 3A1, 4A1, A2) could be recognised and partially covered by a carapace. Unlike, other fuxianhuiids, it appears that the eyes did not project far beyond the anterior of the carapace.
Head appendages: Like other fuxianhuiids, G. spinatus possessed two pairs of post-ocular head appendages. Antennae are present in four specimens (Fig. 3A1, 4A1, B, D1), and it consists of at least 21 podomeres (Fig. 4B). The proximal podomeres are wider than long, with successive podomeres becoming smaller and gradually more elongate, resulting in a more rectangular shape in the distal segments, accompanied by a reversal in the length to width ratio when compared to the proximal elements (Figs. 3A1, 4B). Accessory antennal spines, or setae are not present in the described material. A tripartite hypostome and a pair of specialized post-antennal appendages forming the mouth part are well preserved in only one specimen (Fig. 4A), where the carapace is completely separated from the head appendages. Posterior to the anterior sclerite is a large boomerang-shaped region. The middle area of the hypostome is subrhombic, and the lobes on both sides (Fig. 4A2, white arrows) are suboval. The mouth opening is expected to situate at the posterior margin of the hypostome, where indications of tiny teeth can be observed (Fig. 4A2–A4). A pair of robust appendages, the so-called SPAs (Fig. 4A2, A3), are partly covered the hypostome. The medial margin of the basal podomere appears spinose (Fig. 4A2–A4). Possible section is in SPA (Fig. 4A2, black arrow). The proximal and middle part is transverse, and the distal part is curved backwards.
Trunk appendages: Biramous trunk appendages are preserved in seven specimens, of which five are figured herein (Figs. 1D, 3A1, 4B–D). The endopod of every biramous trunk appendage is composed of 11 podomeres (Fig. 4D2). Most podomeres are subrhombic to subtrapezoidal, each possessing a medial, subpentagonal spine (Fig. 4B, C, white arrowheads). The distal podomere, by contrast, is in subtriangular shape and without a spine (Fig. 4C, black arrow). Due to different angles of compression, nodes instead of spines are observed in other specimens (Fig. 4D2, white arrowheads). The most proximal part of the appendage is only partly revealed, being hidden by the proceeding appendage. The anterior appendages (Fig. 4B, white arrows) are presumably derived from the prothoracic segments, with the lager, succeeding appendages (Fig. 4B, black arrows) presumably derived from the first and second opisthothoracic segments, akin to the arrangement seen in other fuxianhuiids (see for example Yang et al. 2013). More posterior appendages become shorter, progressively. The number of attachments at the proximal podomeres of appendages indicates that each opisthothoracic segment possesses two pairs of appendages (Fig. 4B, C, black arrowheads). Flaps indicating exopods can be observed in two specimens (Figs. 3A3, 4B). Apart from these, no more evidence for the exopod is observed in our material.
The new information presented herein was used to produce a new reconstruction of Guangweicaris spinatus (Fig. 5).

Fig. 5. Artistic reconstruction of Guangweicaris spinatus Luo, Fu, and Hu, 2007 from the lower Cambrian Guanshan Biota, China. Illustration by Xiaodong Wang (Yunnan Zhishui Corporation, Kunming, China).
Remarks.—A total of five specimens, which now form part of the type series of Guangweicaris spinatus, were originally attributed to Habelia sp. (Luo et al. 1999: pl. 31). Of these, three, Kg-f-1-55–57, were derived from the Gangtoucun section of the Wulongqing Member, whilst the others, Yl-f-1-12 and Yl-f-1-13, came from Lihuazhuang in Yiliang County. ELI-XLC-GS0042 figured by Liu et al. (2016: fig. 2e, f), and ELI-XLCG-0022B by Wu and Liu (2019: fig. 1B) was reported from the Xinglongcun section of the Wulongqing Formation. Whilst undertaking this study it became apparent that a specimen (Hu et al. 2013: 108, fig. 135) formerly attributed to G. spintus belongs to a different species. This specimen has several unique features that are not present in G. spintus: (i) a notch in the carapace; (ii) three slightly shortened segments exposed behind its carapace, with the third one bearing pleural processes; (iii) lack of narrower abdomen segments; (iv) long and thin pleurae; (v) lack of enlarged axial spines on the trunk tergites. These differences suggest an assignment of the specimen to Longquania bispinosa Luo and Hu in Luo et al., 2008, but further studies are required to confirm this suggestion.
Stratigraphic and geographic range.—Wulongqing Member, Canglangpu Formation, Cambrian Series 2, Stage 4, Yunnan province, China.
Discussion
The discovery and restudy of material attributed to Guangweicaris spinatus allows us to produce a more accurate depiction of its external morphology and provide a more detailed diagnosis of this taxon. Among our new findings, the tripartite hypostome and the SPAs in G. spinatus indicate that these structures may be present in all fuxianhuiids (even in Liangwangshania, HC personal observation), probably being a shared character of this group. Moreover, the SPAs can serve as a reliable diagnostic feature of fuxianhuiids. The basal podomeres of SPAs are covered by hypostomal flaps, and this would limit the movement of SPAs, thus reducing the possibility that they were used for active prey capture. The basal podomere of the SPAs carries gnathobasic edges with several spinose endites, are very like that in C. kunmingensis (Yang et al. 2018: fig. 2b, c), and this was very helpful for food processing. It has been reported that each prothorax segment of fuxianhuiids corresponds to one pair of appendages, such as F. protensa (Fu et al. 2018), C. kunmingensis (Yang et al. 2013) and Alacaris mirabilis (Yang et al. 2018). Due to the preservation of our specimens of G. spinatus, we are not able to clearly define the correspondence between the prothoracic segments and appendages. Nonetheless, according to the number of anterior appendages, we speculate that each prothoracic segment corresponds to one pair of appendages. Due to the burial direction, widths of the exposed appendage are different (see Fig. 4B and D2), which indicates that the appendages of G. spinatus are not cylindrical like those of F. protensa, but similar to the broad and thick oars, which are very suitable for swimming. The abdominal segments show a considerable degree of deflection, implying that the abdomen could have adjusted the direction of swimming. The dorsal axial spines on the opisthothoracic and abdominal segments, on the other hand, might have served as a defensive function.
Conclusions
The present study of G. spinatus based on both new and previously published specimens provides important new information in the head organizations, the body segmentation and trunk appendages of this species, and would improve our understanding of the autecology of G. spinatus and the phylogenetic relationships among fuxianhuiids. We suggest that further work can aim at synthesizing the morphological data of fuxianhuiids and generating more complete pictures of the phylogenetic connections among fuxianhuiids and other important evolutionary lineages of euarthropods, as well as the ecological positions of different fuxianhuiids in early Cambrian oceanic ecosystem, based on functional morphological and biostratigraphical analyses.
Acknowledgements
We thank referees Allison C. Daley (Institute of Earth Sciences, University of Lausanne, Switzerland) and Dongjing Fu (ELI) for their insightful and constructive comments. We also thank Shixue Hu (Chengdu Centre of the Geological Survey of China, China) for sharing the images of NIGPAS Kgs-1-137 and NIGPAS Kgs-6-108 and Guangxu Zhang (YKLP) for stimulating discussions. This study is supported by NSFC grants 41861134032 and 2015HC029 and Yunnan Provincial Research Grants 2018FA025 and 2018IA073. DAL is funded by a Dame Kathleen Ollerenshaw Research Fellowship from the University of Manchester. This work was funded in part by a Whittington Award PA-WA201701, awarded by the Palaeontological Association to DAL.
References
Bousfield, E.L. 1995. A contribution to the natural classification of Lower and Middle Cambrian arthropods: food-gathering and feeding mechanisms. Amphipacifica 2: 3–34.
Chen, A., Chen, H., Legg, D.A., Liu, Y., and Hou, X. 2018. A redescription of Liangwangshania biloba Chen, 2005, from the Chengjiang Biota (Cambrian, China), with a discussion of possible sexual dimorphism in fuxianhuiid arthropods. Arthropod Structure and Development 47: 552–561. Crossref
Edgecombe, G.D. and Legg, D.A. 2013. The arthropod fossil record. In A. Minelli, G. Boxshall, and G. Fusco (eds.), Arthropod Biology and Evolution. Molecules, Development, Morphology, 393–415. Springer-Verlag, Berlin. Crossref
Fu, D., Ortega-Hernández, J., Daley, A.C., Zhang, X., and Shu, D. 2018. Anamorphic development and extended parental care in a 520 million-year-old stem-group euarthropod from China. BMC Evolutionary Biology 18: 147. Crossref
Hou, J., Hughes, N.C., Yang, J., Lan, T., Zhang, X., and Dominguez, C. 2017. Ontogeny of the articulated yiliangellinine trilobite Zhangshania typica from the lower Cambrian (Series 2, Stage 3) of southern China. Journal of Paleontology 91: 86–99.
Hou, X. and Bergström, J. 1997. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils & Strata 45: 1–116. Crossref
Hu, S., Zhu, M., Luo, H., Steiner, M., Zhao, F., Li, G., Liu, Q., and Zhang, Z. 2013. The Guanshan Biota [in Chinese]. 204 pp. Yunnan Science and Technology Press, Kunming. Crossref
Hu, S., Zhu, M., Steiner, M., Luo, H., Zhao, F., and Liu, Q. 2010. Biodiversity and taphonomy of the Early Cambrian Guanshan biota, eastern Yunnan. Science China: Earth Sciences 53: 1765–1773. Crossref
Lankester, E.R. 1904. The structure and classification of Arthropoda. Quarterly Journal of Microscopical Sciences 47: 523–582.
Legg, D.A. 2013. Multi-segmented arthropods from the Middle Cambrian of British Columbia (Canada). Journal of Paleontology 87: 493–501. Crossref
Legg, D.A. and Caron, J.B. 2014. New Middle Cambrian bivalved arthropods from the Burgess Shale (British Columbia, Canada). Palaeontology 57: 691–711. Crossref
Legg, D.A., Sutton, M.D., and Edgecombe, G.D. 2013. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nature Communications 4: 2458. Crossref
Legg, D.A., Sutton, M.D., Edgecombe, G.D., and Caron, J.B. 2012. Cambrian bivalved arthropod reveals origin of arthrodization. Proceedings of the Royal Society of London B: Biological Sciences 279: 4699–4704. Crossref
Liu, J., Han, J., Li, J., Wu, Y., Peng, J., Qi, N., Yang, Y., and Li, J. 2016. New localities and palaeoscolecid worms from the Cambrian (Stage 4, Series 2) Guanshan Biota in Kunming, Yunnan, South China. Acta Geologica Sinica 90: 1939–1945. Crossref
Liu, J., Steiner, M., Dunlop, J.A., and Shu, D. 2018. Microbial decay analysis challenges interpretation of putative organ systems in Cambrian fuxianhuiids. Proceedings of the Royal Society of London 285: 20180051. Crossref
Luo, H., Fu, X., Hu, S., Li, Y., Hou, S., You, T., Pang, J., and Liu, Q. 2007. A new arthropod, Guangweicaris Luo, Fu et Hu gen. nov. from the Early Cambrian Guanshan Fauna, Kunming, China. Acta Geologica Sinica 81: 1–7.
Luo, H., Hu, S., Chen, L., Zhang, S., and Tao, Y. 1999. Early Cambrian Chengjiang Fauna from Kunming Region, China [in Chinese with English summary]. 129 pp. Yunnan Science and Technology Press, Kunming.
Luo, H, Li, Y., Hu, S., Fu, X., Hou, S., Liu, X., Chen, C., Li, F., Pang, J., and Liu, Q. 2008. Early Cambrian Malong Fauna and Guanshan Fauna from Eastern Yunnan, China [in Chinese with English summary]. 134 pp. Yunnan Science and Technology Press, Kunming.
Ma, X., Cong, P., Hou, X., Edgecombe, G.D., and Strausfeld, N.J. 2014. An exceptionally preserved arthropod cardiovascular system from the early Cambrian. Nature Communications 5: 3560. Crossref
Ma, X., Edgecombe, G.D., Hou, X., Goral, T., and Strausfeld, N.J. 2015. Preservational pathways of corresponding brains of a Cambrian euarthropod. Current Biology 25: 2969–2975. Crossref
Ma, X., Hou, X., Edgecombe, G.D., and Strausfeld, N.J. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490: 258–261. Crossref
Ortega-Hernández, J. 2016. Making sense of “lower” and “upper” stem-group Euarthropoda, with comments on the strict use of the name Arthropoda von Siebold, 1848. Biological Reviews 91: 255–273. Crossref
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. Crossref
Wu, Y. and Liu J. 2019. Anatomy and relationships of the fuxianhuiid euarthropod Guangweicaris from the early Cambrian Guanshan Biota in Kunming, Yunnan, Southwest China revisited. Acta Palaeontologica Polonica 64: 543–548. Crossref
Yang, J., Hou, X., and Dong, W. 2008. Restudy of Guangweicaris Luo, Fu et Hu, 2007 from the Lower Cambrian Canglangpu Formation in Kunming area [in Chinese with English summary]. Acta Palaeontologica Sinica 47: 115–122.
Yang, J., Ortega-Hernández, J., Butterfield, N.J., Boyan, G.S., Hou, J., Lan, T., and Zhang, X. 2016. Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda. Proceedings of the National Academy of Sciences of the United States of America 113: 2988–2993. Crossref
Yang, J., Ortega-Hernández, J., Legg, D.A., Lan, T., Hou, J., and Zhang, X. 2018. Early Cambrian fuxianhuiids from China reveal origin of the gnathobasic protopodite in euarthropods. Nature Communications 9: 470. Crossref
Acta Palaeontol. Pol. 65 (1): 139–148, 2020
https://doi.org/10.4202/app.00508.2018