

Sieve-type normal pore canals in Jurassic ostracods: A review with description of a new genus
ALAN R. LORD, M. CRISTINA CABRAL, and DAN L. DANIELOPOL
Lord, A.R., Cabral, M.C., and Danielopol, D.L. 2020. Sieve-type normal pore canals in Jurassic ostracods: A review with description of a new genus. Acta Palaeontologica Polonica 65 (2): 313–349.
Sieve-type normal pore canals (StPC) occur commonly in living and fossil cytheroid ostracods but their biological function(s) and evolutionary history are poorly known. The new genus Minyocythere and its four species: Minyocythere macroporosa sp. nov., M. angulata sp. nov., M. maculosa, and M. tuberculata from the Middle Jurassic have StPC prominently developed, display a range of normal pore canals, and provide a context for review of the geological record and palaeobiological potential of these structures, and their application as a taxonomic tool compared with classical approaches. The related Cretaceous genus Dolocythere is reviewed and Dolocythere amphistiela sp. nov. described. The significance of StPC for comparative morphology, systematics, palaeobiology and environmental interpretation are discussed. The range of normal pore canals observed, including StPC, is greater than previously described and several types can occur on one animal implying different life functions. The potential of normal pore canals especially StPC for systematic use is established although good preservation is essential. The functional significance of normal pore canals and their setae must be verified with living material before their evolutionary history can be deduced and their application to palaeoenvironmental interpretation and modern environmental monitoring enhanced.
Key words: Ostracoda, Cytheroidea, normal pore canals, systematics, Jurassic, Cretaceous, Europe.
Alan R. Lord [alan.lord@senckenberg.de, ORCID ID: http://orcid.org/0000-0002-0008-7746], Senckenberg Forschungsinstitut Frankfurt, Senckenberganlage 25, D-60325 Frankfurt-am-Main, Germany.
M. Cristina Cabral [mccabral@fc.ul.pt, ORCID ID: http://orcid.org/0000-0001-8717-4043], Universidade de Lisboa, Faculdade de Ciências, Departamento de Geologia e Instituto Dom Luiz (IDL), Campo Grande, C6, 4º, 1749-016 Lisboa, Portugal.
Dan Luca Danielopol [dan.danielopol@uni-graz.at, ORCID ID: http://orcid.org/0000-0003-3968-5564], Karl-Franzens University, Institute of Earth Sciences, Department of Geology and Palaeontology, Heinrichstrasse 26, A-8010 Graz, Austria.
Received 4 May 2019, accepted 18 September 2019, available online 25 February 2020.
Copyright © 2020 A.R. Lord et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
In the course of work on the significance of normal pore canals in living and fossil ostracods one of us (DLD) noticed the striking images of sieve-type normal pore canals (StPC) in Middle Jurassic ostracods published by Triebel (1941: pl. 7: 71, 72a, b). Examination of the material in the Senckenberg Museum revealed the presence of a new taxon, recognized by Erich Triebel but unpublished, and led to a number of questions including when did StPC first evolve, what was their adaptive significance, why did not all ostracods develop this feature and, finally, what is their taxonomic importance? At present not all these questions can be adequately addressed because we lack key information for many basic aspects of the morphology and biology of the living animal. Therefore, we adopted a more modest agenda, with a general discussion on the variety of normal pore canals (NPC) in the Ostracoda, continuing with detailed descriptions of StPC in the Mesozoic material under study, and discussion of their significance for comparative morphology, systematics, palaeobiology and environmental interpretation.
During the early work MCC and ARL developed the comparative systematics described below using classical methods of ostracod taxonomy with fossil material, i.e., carapace characteristics of three dimensional shape, surface morphology and ornament, internal features of hingement, adductor muscle scars pattern and marginal features, and ontogenetic development, sexual dimorphism and dimensions. The systematic framework at generic and species levels was in place in draft while DLD was analysing the NPC especially the StPC. Thus, the two approaches over time became an experiment, a test: would the NPC analysis confirm or refute the recognition of new taxa defined by classical methodology, or have nothing useful to contribute to the debate? In this sense the present paper develops the philosophy behind the work described by Danielopol et al. (2018) on the Timiriaseviinae by updating the observational methodology, by testing taxonomic decisions using classical methods versus pore canal characteristics, and by emphasizing that understanding of normal pore canals and their evolutionary interpretation depends on understanding their function in the living animal.
A review of the full fossil record of NPC, particularly StPC, is beyond the scope of this work. We hope, however, to stimulate further research on NPC, especially StPC, namely on their biological function and role as adaptive mechanisms to specific environments.
Institutional abbreviations.—BGR, Bundesanstalt für Geowissenschaften und Rohstoffe, Hannover, Germany; BMNH, British Museum (Natural History), London, UK; SMF, Senckenberg Forschungsinstitut Frankfurt, Frankfurt-am-Main, Germany (in catalogue Xe).
Other abbreviations.—A-1–A-4, refers to estimated growth (moult) stages; AMS, adductor muscle scars; C, carapace; DI, Distance Index; H, height; L, length; LV, left valve; NPC, normal pore canals; RV, right valve; SeP-SI, Setal Pore Size Index (ratio of the SeP diameter to the StPC diameter); SI, Size Index (ratio of the pore diameter to the valve’s length); StPC, sieve-type normal pore canals; StPC-m (micro StPC), small sieve-type normal pore canals with a large subcentral setal pore and reduced sieve plate; StPC-M (macro StPC), large round or rarely elongate sieve-type normal pore canals with a small excentric setal pore; V, valve.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:500FB01D-AE30-4616-8666-818D2E67C720
Historical background
Ostracod valves are penetrated by pores through which setae (sensory bristles, also called sensilla, singular: sensillum) connect the animal’s body with the external milieu. Those canals running between the calcified outer and calcified inner lamellae close to the free margin, especially on the anterior and posterior sides of the valves, are referred to as marginal pore canals (cf. Yamada 2007), whereas those passing through the main shell or calcified outer lamella are called normal pore canals and are the subject of this paper. NPC can be described most basically as of simple or sieve type, with the former a simple tube in the calcified valve whereas the latter comprise a number of perforations as seen on the outer surface. Both marginal and normal pore canals and their setae have a sensory function, although this is not well understood (see below). Sieve NPC were first described by Müller (1894) from Recent material from the Bay of Naples and Triebel (1941) figured the first fossil example from the Jurassic of Germany. Subsequently Hartmann (1963, 1964) used sieve-type NPC as a diagnostic feature for certain subfamilies of the Cytheridae.
NPC were first described with the advantage of scanning electron microscopy (SEM) by Sandberg and Plusquellec (1969) although Sandberg had earlier (Sandberg 1964) established the value of NPC for taxonomic purposes in the genus Cyprideis Jones, 1857. Sandberg and Plusquellec (1969) recognized the complexity of NPC, that simple and sieve NPC can occur in the same animal, that associated setae may be fine and long or thick and short suggesting different functions, and that sieve plates with two different kinds of setae can occur in the same animal. Plusquellec and Sandberg (1969: pl. 8: 2, 3) used the size, number and distribution of NPC to distinguish genera in the Campylocytherinae and figured different types of StPC in one carapace.
Normal pore canals have been classified by Puri and Dickau (1969) and Puri (1974) as follows (Fig. 1A): (i) simple pore (hole) with a sensory seta—Type A’, (ii) simple pore with a lip and sensory seta—Type A”, (iii) sieve plate consisting of holes but without a defined central or subcentral pore with seta—Type B, (iv) sieve plate with central pore and seta (defined as “Type A superimposed on Type B”, Puri and Dickau 1969: 366)—Type C, (v) a sieve plate and a separate, single pore with a sensory hair (“combination of Type A and Type B”, Puri and Dickau 1969: 366)—Type D.
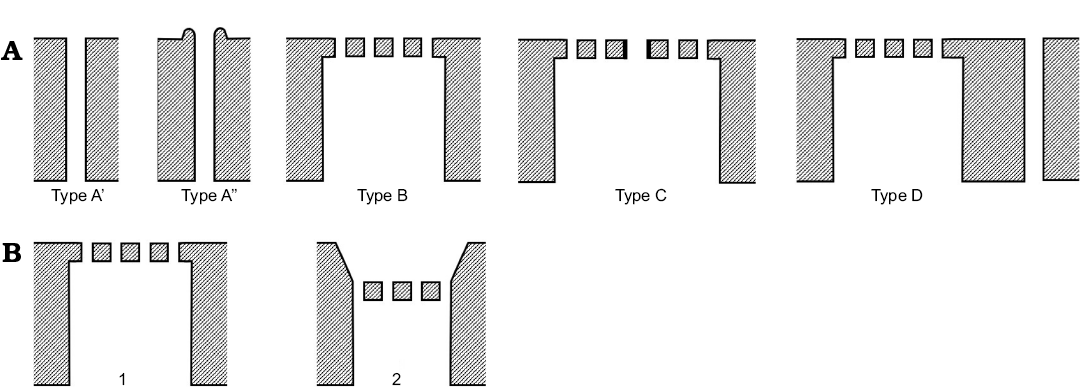
Fig. 1. Schematic cross-sections of ostracod valves, exterior to top, interior to bottom. A. NPC classification of Puri and Dickau (1969). B. StPC with sieve plate flush with external surface (1) and sieve plate in funnel-shaped depression (2).
This was pioneering work, especially as the authors tried to relate normal pore types to supra-specific classificatory levels and Puri (1974) made transmission electron microscope images of sections of valves with soft tissues. However, the classification is in some respects simplistic. For example, Type D with a separate sieve plate and single pore (Puri and Dickau 1969: pl. 3: 1b) could be interpreted as individual Type A” and Type C pores. Similarly, the classification does not recognize a difference between a central, sub-central or peripheral main pore in a Type C sieve plate although all are figured. There are clearly transitional types, for example, Puri (1974: pl. 8: 2b) figures a Type A” pore with a single row of surrounding pores as Type C, and a similar example (Puri 1974: pl. 9: 2) is described as “Type C primitive”. The present work found NPC not recognized by Puri and Dickau (1969) and a new overview of NPC is needed.
Hanai (1970), Puri (1974), Keyser (1980) followed by Tsukagoshi and Ikeya (1987), Ikeya and Tsukagoshi (1988) and Tsukagoshi (1990) documented the potential of NPC for the systematics of various groups of cytheroids. Hanai (1970) initiated this approach with the arrangement and the shape of the StPC of various schizocytherine ostracods. Tsukagoshi and Ikeya (1987) highlighted the value for Recent cytheroids of a classification of the normal pores based on the combination of the structure of the pores and the type of sensilla emerging from some of these pores. Five structural classes were proposed which incorporate two basic types of NPC, a simple tubular one without an external rim, and one with rim; this latter can be further differentiated following the degree of development of the rim. A StPC was described and this structural unit can be also subdivided by its shape into round and elongate. Their classificatory system differs from that of Puri and Dickau (1969) who recognize three basic pore-types (see above), although here a type without a sensillum (Type B of Puri and Dickau) and one with a sensillum within the sieve plate area (Type C of Puri and Dickau) are also found. Tsukagoshi and Ikeya (1987) studied species of Cythere Müller, 1785 and mapped the distribution of the StPC as a criterion for species differentiation. This approach was developed further by Tsukagoshi (1990) who followed the ontogenetic development of NPC which allowed him to reconstruct the phylogeny of eleven living and three extinct species of Cythere. The morphological data on StPC of Tsukagoshi and Ikeya (1987) and Tsukagoshi (1990) will be used below for comparative purposes with our own data as they were obtained from ostracods belonging to the family Cytheridae, as is our material.
Another way of looking at StPC was adopted by Rosenfeld and Vesper (1977) who analysed shape variability in relation to salinity in the euryhaline taxon Cyprideis torosa (Jones, 1850), recognizing three classes (round, oblong, irregular) the relative proportions of which show a logarithmic relationship to salinity in the oligohaline to mesohaline range. This approach has attracted much attention, not least for the difficulties of the subjective shape classification (see discussion in introduction of Frenzel et al. 2017).
Sylvester-Bradley and Benson (1971), in their seminal work on description of ostracod external carapace features with the SEM, cited Puri and Dickau (1969) and introduced some new terms: funnel-pore (Type A” of Puri and Dickau; Type A2 of Danielopol et al. 2018); celate pores (reflecting overgrowth of NPC by celation); flush sieve plates (exterior of sieve plate level with valve surface); intramural pore (NPC penetrating the mural wall of a fossa); perforate spines (with a NPC); rimmed pore-canals (apparently same as funnel-pore). However, Puri (1974) failed to cite Sylvester-Bradley and Benson (1971) and his paper is essentially an expansion of Puri and Dickau (1969). In parallel with the work of Puri and Dickau (1969) and Sandberg and Plusquellec (1969), the SEM was also used by Omatsola (1970) to figure NPC in Recent cytheroid ostracods.
An important development was the observation of NPC in the interior of the calcified outer lamella and in cross-section. Rome figured (1947: fig. 4) a diagrammatic section of a NPC with the soft tissue parts. In the 1980s Keyser (1980, 1981, 1983) and Okada (1982, 1983) used transmission and scanning electron microscopy and optical thin-sections to study the structure of sieve pores in the calcified outer lamella and the biological nature of the setae, and demonstrated that the StPC tubuli were built by an invagination of the epicuticle within the calcified outer lamella. In living specimens these tubes are closed to the interior, i.e., the tubuli do not traverse the entire calcified outer lamella, and terminate on a thin calcified lamella located within a larger proximal canal. During fossilisation the epicuticle is lost revealing the calcite sieve plate and tubuli (although some of our material appeared to have a calcite film which we interpret as secondary). An important conclusion is that while NPC have traditionally been overlooked by observers in fossil material it is necessary to study them both internally and externally, and where possible in section, with high-resolution electron microscopy.
Okada (1982, 1983) coined the term “sensillum pore” for NPC with seta containing two cilia which are distal extensions of nerve cells, indicating that the seta is a receptor of some kind(s). Sensillum pores are simple (Okada 1983: fig. 1: 5) or StPC (Okada 1983: fig. 1: 1–3). The invagination of the valve’s epicuticule in the minute canals surrounding a sensillum pore form, in the case of living cytheroids, a closed sieve plate where tubules have a digitiform shape. On valves of dead specimens where the epicuticule has decayed the sieve plates are clearly visible with minute tubuli which traverse the calcified outer lamella and open on the inner side of the valve in a bell shaped space. Okada (as cited above) introduced the term “exocrine pore” based on the species Bicornucythere bisanensis (Okubo, 1975) for pore structures lacking seta and with a substantial cavity below the calcified valve surface. Olempska (2008: 720, fig. 2: 4, 5) describes exocrine pores as “secretory” and illustrates pores from palaeocopid “ostracods” that closely resemble exocrine pores in morphology.
Kamiya and Hazel (1992) reported species-specific differences in number and occurrence of “smooth” bristles (seta), “twisted” bristles and “microhairs” in two species of Loxoconcha Sars, 1866. Kamiya (1989: 46) in a study of the same two Loxoconcha species comments “it is likely that ostracods have […] many mechano-receptors with bristles, whose number is fixed within species but flexible within genera, besides comparatively few (chemo-receptor?) pores (with/without bristles) whose number is definite within genera or families.”
Pore clusters are another type of normal pore differing from StPC (Maddocks and Steineck 1987), which were well described by Ishizaki and Gunther (1974) for the case of reticulate valves of Eucytherura Müller, 1894 species. Simple normal pores which cover the whole sollum of a reticulum of the valve represent the surface opening of canals which traverse the calcified outer lamella and open directly on the surface of the inner side of the valve. The canals of pore clusters have a diameter similar to those of simple pores dispersed on the muri of the valve. In this respect pore clusters differ from StPC where the canals (tubuli) generally have a diameter smaller than those of simple NPC. Pore clusters, we hypothesize, represent poorly developed StPC, possibly a primitive morphological state. Pore clusters were described for Microceratina Swanson, 1980 as well as Eucytherura. For the former Namiotko et al. (2004: 51, pl. 1) noted that in the NPC “three subcircular holes” resembling a poorly developed StPC exist. Moreover, Ishizaki (1973) reported the presence of pore clusters on the valves of Puncioidea ostracods, a group considered a phylogenetically primitive Podocopa group (Swanson 1989; Horne 2005).
StPC can be recognized as what Lewontin (2001) named quasi-independent structural units. This means that StPC display a stable structural configuration represented by the tubuli and well-defined shapes. The position and the spatial distribution of StPC are well integrated within the general morphology of the valve. We can characterize qualitatively and quantitatively the external shape and size of such structural units because they are visible on both sides of the valve. We assume that in principle the morphological diversity observed of StPC resulted from the combination of internal developmental processes and the dynamic reaction with the external environment within which the ostracods live. This intuitive perspective remains to be clarified in future work. In our opinion the diversity of the form and structure of StPC represent character states potentially useful as diagnostic traits for taxonomic studies, though with fossil material only partial evidence exists. In living ostracods setae can be long, short, fat, thin, tapering or paired, and the semiterrestrial Terrestricythere Schornikov, 1969 exhibits brush-like sensilla ventrally and some exceptionally long sensilla laterally that almost certainly reflect its specialised lifestyle (Horne et al. 2004); there are also sieve plates that lack setae. In other words when these features are combined with the morphological variations we observe in the calcified lamella then NPC reflect potentially highly complex biological systems of great evolutionary interest and importance for systematics.
Function of normal pore canals
Authors have hypothesised a variety of functions for NPC and their setae (when present), including StPC, however, we are unaware of any definitive study unambiguously establishing the function of normal (or marginal) pore canals in any living ostracods. From the literature:
Photoreceptors.—Müller (1894) when first describing StPC suggested that the structures might be light-sensitive. The idea is generally rejected as there are deep-sea and aquatic subterranean species with StPC living outside the photic zone, however, it is worth bearing this possibility in mind for shallow dwelling marine and non-marine species. Such a function would appear superfluous in taxa with evidence of antero-dorsal ocular structures.
Chemoreceptors.—Rome (1947: 95, 139) discusses this potential function in Herpetocypris reptans (Baird, 1835). The most obvious application is in detecting the level of dissolved oxygen in the water but there could be more subtle signals to monitor such as water pH and especially alkalinity. The now discredited idea of a relationship between vestibule size in Krithe Brady, Crosskey, and Robertson, 1874 and level of bottom water oxygenation (Peypouquet 1979; Donze et al. 1982) does not preclude either marginal or normal pore canals and setae having a sensory function in relation to oxygen or for that matter pore canals, especially StPC, having a function for processing oxygen from the water. Hanai (1982: 12) cites chemoreceptor functions in Cythere omotenipponica (Hanai, 1959) and in Cypridina hilgendorfi Müller, 1890 but was not able to be precise as to the site(s) of the receptor functions. Kamiya (1989) in a study of Loxoconcha species notes “twisted” bristles (seta) as possible chemoreceptors and “smooth” bristles as possible mechano-receptors.
The much-studied relationship between StPC shape in Cyprideis torosa and salinity first reported by Rosenfeld and Vesper (1977) indicates a chemical relationship but the nature of this is not clear (see DeDeckker and Lord 2017).
Thermoreceptors.—Keyser (1981, 1983: 654) for Aurila convexa (Baird, 1850).
Mechanoreceptors.—Rome (1944, 1947). Keyser (1981, 1983: 654) for Aurila convexa. Müller (1894) and Sandberg and Plusquellec (1969) noted two kinds of setae, fine and long as opposed to large and short, and speculated that these represent delicate touch and coarse touch receptors respectively. Müller noted that the fine setae could be responsive to water movement or even sound.
Excretory.—Van Morkhoven (1962: 68) states “some pores may also serve as openings for glands inside the duplicatures”, which could refer to both normal and marginal pores, but without discussion as to whether such glands have an excretory or a receiving function, or both. Keyser (1981, 1983) postulated an excretory activity through the StPC tubuli during moulting which protects the sensory setae, and Keyser (1982) working on Hirschmannia viridis (Müller, 1785) identified a substance released during ecdysis that might protect the setae from corrosive ecdysal fluid or be part in formation of the cuticle of the setae.
Osmoregulation.—Ionic exchange between the outer aequeous environment and the animals inner osmotic medium (haemolymph) which, depending on the nature of the host water and the age of the animal, may be excretory or secretory (Aladin and Potts 1996).
Buoyancy function.—Athersuch (1976: 288) suggested a possible buoyancy function via water drawn into or expelled from the carapace, a point noted by Horne (1982) who observed sieve pores free of cement in ostracods within a Sabellaria reef.
Secretion organs.—According to Keyser (1983: 654) “the sieve pores seem to function the whole lifetime as secretion organs” in A. convexa (and all hemicytherids?).
Environmental function.—Hanai et al. (1985) suggested that StPC tubuli have a wider diameter in ostracods living in high energy environments as opposed to animals living in low energy muddy environments where small tubuli diameter might help “to keep off dirt”.
Control function.—In StPC with a large pore with a seta, might this control the function of associated tubuli?
The taxonomic significance of NPC, simple or StPC, is reviewed below (see Discussion) but the fact that they have been used as presence/absence features to discriminate taxa at various systematic levels (e.g., Hartmann 1963) shows that some workers have considered them of value.
The fossil record of StPC
StPC were reported by Schallreuter (1977) in the Lower Ordovician palaeocopid species Miehlkella cribroporata Schallreuter, 1977 and subsquently in Klimphores planus Schallreuter, 1966 (Schallreuter 1980) and Vaivanovia hiddenseensis Schallreuter, 1966 (Schallreuter 1983). The StPC in these taxa are relatively simple and consist either of a single circle of tubuli (Miehlkella type) or a group of more numerous tubuli (Klimphores type) (Schallreuter 1983: fig. 2). Olempska (2008) described and figured a variety of NPC in beyrichioidean palaeocopids but none are StPC. In any case the biological affinities of the Palaeocopida are at present unknown, it being a heterogeneous artificial unit, and they may not all be ostracods, in which case some could represent an important comparative clade. Thus, here we focus on podocopid ostracods and specifically on the Superfamily Cytheroidea. Gramm (1977) and Gramm and Egorov (1986) described StPC in the early Carboniferous genus Editia Brayer, 1952 which, given the presence of merodont hingement, a calcified inner lamella, an eye spot and adductor muscle scars comprising a vertical row of five scars, is a convincing early representative of the Cytheroidea; the StPC are described as Type C of Puri and Dickau (1969). Gramm (1977: 144) comments that StPC are restricted to the Cytheroidea. This is not confirmed by the data as far as we know, although the presence of a visible membrane around the sensillum in the case of the Type A” pores of Candona neglecta Sars, 1887 (Cypridoidea) figured by Meisch and Wouters (2004: fig. 2E–G) is worth noting.
The evolutionary traits and patterns of late Palaeozoic–early Mesozoic cytheroid ostracods are, however, poorly known and the Editidae clade may represent an early and unsuccessful experiment with StPC. The earliest StPC known to us personally are Jurassic in age: Phraterfabanella tridentinensis Whatley and Boomer in Boomer et al., 2001 (?Upper Triassic, Rhaetian–Lower Jurassic, Sinemurian; brackish to low-salinity), Camptocythere Triebel, 1950 (Toarcian–Aalenian; marine) and Aphelocythere Triebel and Klingler, 1959 (Upper Toarcian–Lower Aalenian; marine). StPC become common in the Middle Jurassic (e.g., Klieana levis Oertli, 1957 [Bate 1978: pl. 2: 2]; Progonocythere stilla Sylvester-Bradley, 1948 [Bate 1978: pl. 2: 15], Progonocythere polonica Błaszyk, 1959 [Bate 1978: pl. 3: 2], Lophocythere batei Malz, 1975 [Bate 1978: pl. 7: 1]) and much more common in younger Mesozoic and Cenozoic taxa.
Figure 2A, B provides images of topotypic material of Phraterfabanella tridentinensis from the Sinemurian Calcari Grigi of the Trento Platform, NE Italy with StPC, which supplements the published illustrations of Boomer et al. (2001: pl. 1: 11 and pl. 3). In this species, with the earliest StPC known to us, two types of StPC are present in one valve (Fig. 2A2–A5). The related Phraterfabanella boomeri Cabral and Colin in Cabral et al., 2015 (Lower–Upper Sinemurian, Portugal) (Fig. 2C herein) has large normal pore canals but StPC were not discussed in the original description. New illustrations of P. boomeri reveal the presence of StPC, poorly preserved but confirming the generic assignment to Phraterfabanella Whatley and Boomer in Boomer et al., 2001. In the light microscope both species show surface depressions where StPC are located, however, in the SEM the presence of sieve pores is not so clear. The critical role of preservation in the identification and categorization of these features is demonstrated by comparing images of Phraterfabanella tridentinensis in Fig. 2A3 and A4 and Phraterfabanella boomeri in Fig. 2C4 and C5.
We further document “early” StPC with images of Aphelocythere (Fig. 3A) and two species of Camptocythere (Fig. 3B–D). Of particular interest is the presence in Camptocythere of large StPC with both round and elongate shapes in a single valve (Fig. 3D2–D4), a phenomenon much studied by workers with Cyprideis torosa.
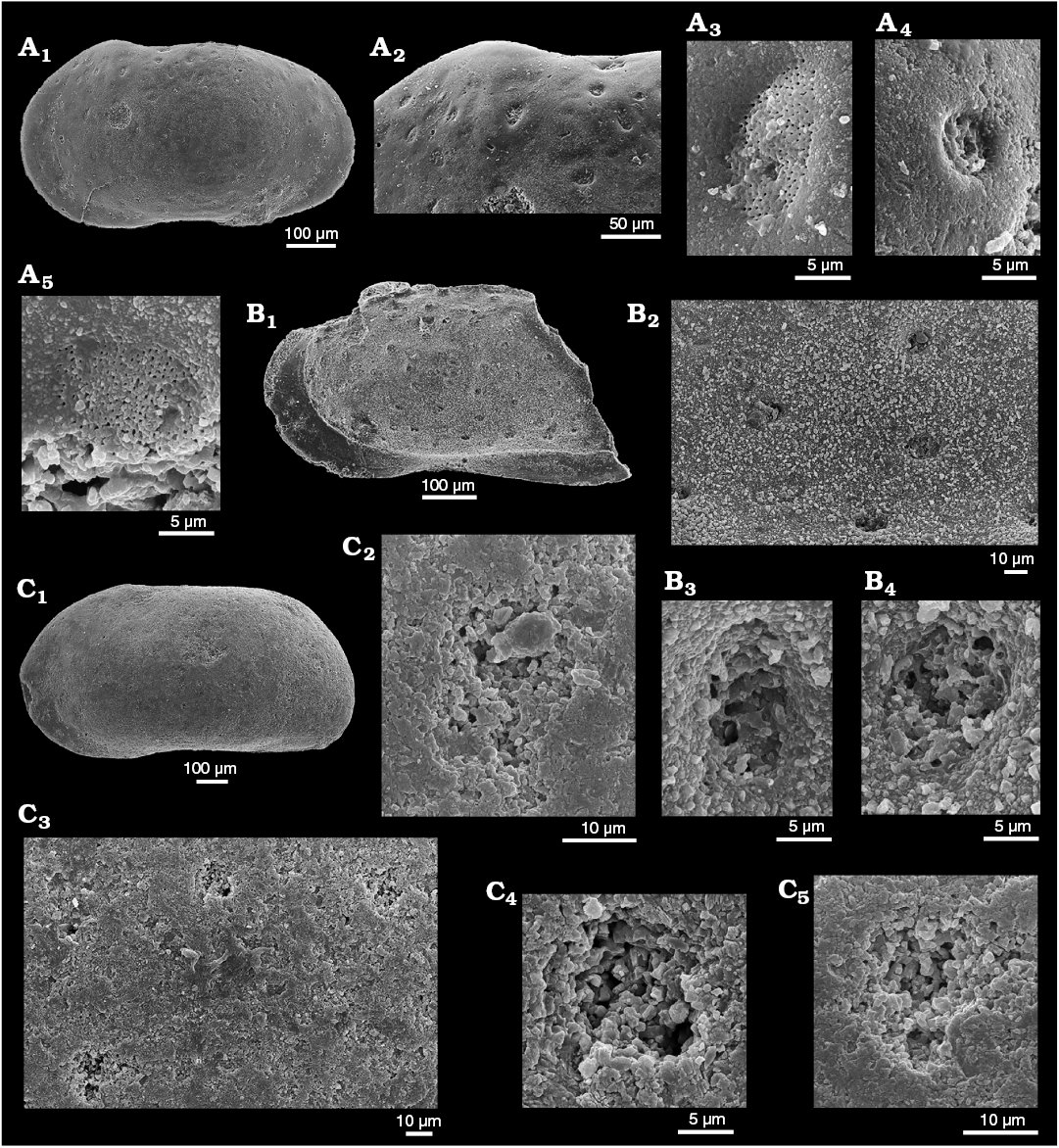
Fig. 2. Sieve-type normal pore canals (StPC) in Phraterfabanella spp. A, B. Phraterfabanella tridentinensis Whatley and Boomer in Boomer et al., 2001, from Rotzo Member, Calcari Grigi Formation, Trento Platform, Venetian Prealps, NE Italy, Upper Sinemurian, Lower Jurassic. A. SMF Xe 23715, female left valve, in external view (A1). Detail of antero-dorsal area (A2), macro StPC (StPC-M) (A3), micro StPC (StPC-m) (A4), and large StPC (A5). B. SMF Xe 23716, male? fragment of right valve, in internal view (B1), StPC-M in general view (B2) and details (B3, B4). C. Phraterfabanella boomeri Cabral and Colin, 2015, from Praia da Concha, Coimbra Formation, sample PCR-318, Portugal, Upper Sinemurian, Lower Jurassic. SMF Xe 23717, male carapace, in external left view (C1), StPC-M in general view (C3) and details (C2, C4, C5).
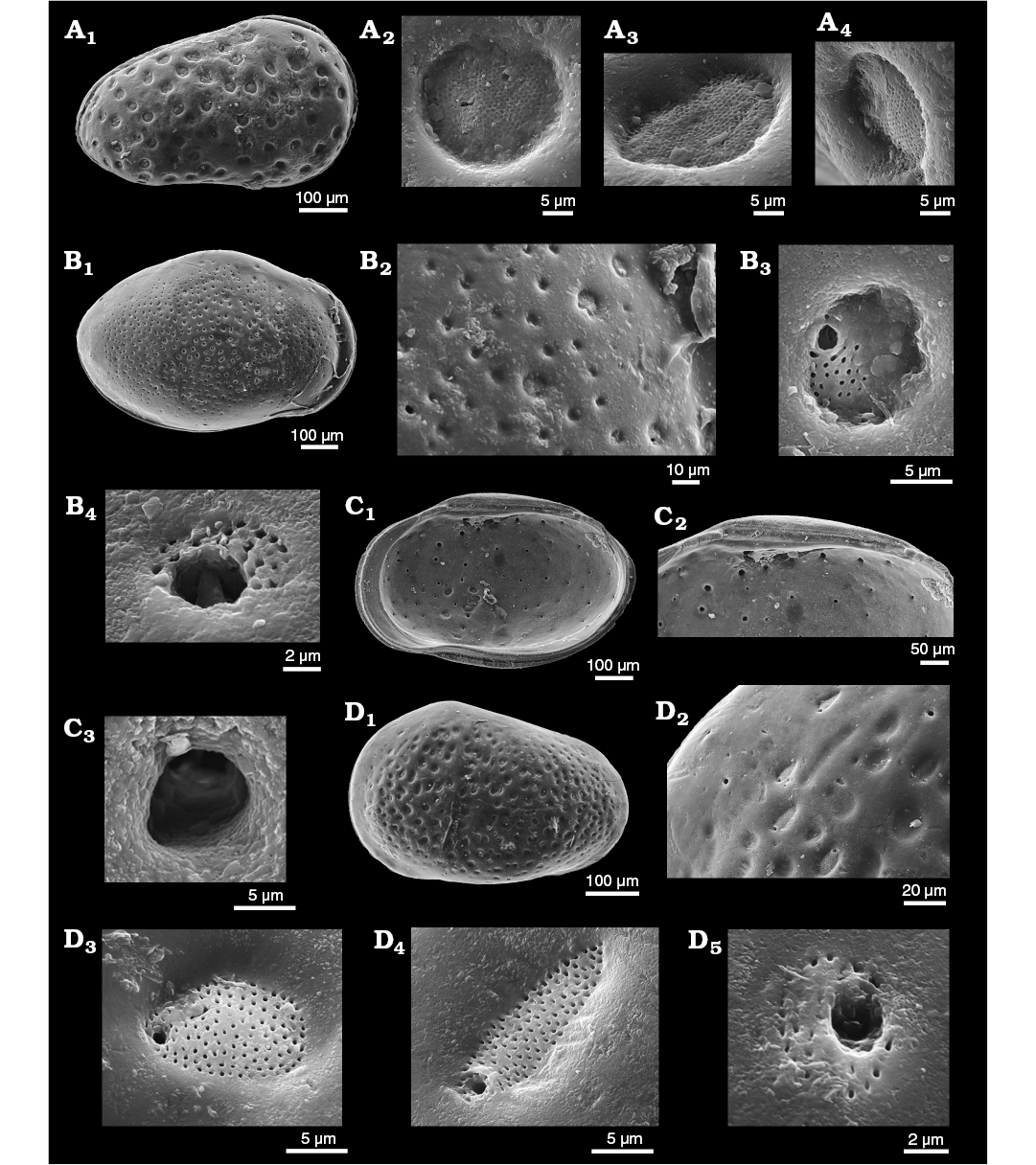
Fig. 3. Sieve-type normal pore canals (StPC) in Aphelocythere and Camptocythere spp. A. Aphelocythere perforata Plumhoff, 1963, from Borehole Wesendorf 51a, 1031–1035 m, NW Germany, Upper Aalenian. SMF Xe 5608 (Plumhoff Collection), carapace in external right view (A1), macro StPC (StPC-M) (A2–A4). B, C. Camptocythere praecox Triebel, 1950 from Borehole Hambühren WA 2, 341 m, NW Germany, Leioceras opalinum Zone, Lower Aalenian (Braun Jura α). B. Paratype, SMF Xe 1448, male right valve in external view (B1), mid-anterior area behind marginal rim, ornamentation and pores (large and small) (B2), StPC-M with a peripheral large pore (B3), micro StPC (StPC-m) (B4). C. Paratype, SMF Xe 1448, male right valve in internal view (C1), hinge (C2), StPC-m (C3). D. Camptocythere media Triebel, 1950 from Borehole Altencelle 1014, 212/16 m, NW Germany, Upper Aalenian (Braun Jura β). Paratype, SMF Xe 1513, female left valve in external view (D1), detail, antero-dorsal area, ornamentation and pores (large and small) (D2), round StPC-M (D3), elongate StPC-M (D4), StPC-m (D5).
Geological setting and material
North-west Germany.—Jurassic: Cored boreholes Hambühren WA2, Rodewald WA12, Fuhrberg 26, Lingen 330, Wesendorf 51a, Altencelle 1014; road cut Klein Schöppenstedt, near Braunschweig (Luppold 2012). Type material of Dolocythere tuberculata Luppold, 2012 in BGR collections. Cretaceous: cored boreholes Lingen 196, Rühme 53, Rodewald WA4, WA6, WA7, ZW Losser 1, Ziegelei Bekum. Type material of Dolocythere rara Mertens, 1956 in BGR collections. Mertens (1956: 193) records D. rara from the Cretaceous of several other boreholes not studied here.
Italy.—Jurassic: Topotypes of Phraterfabanella tridentinensis Whatley and Boomer in Boomer et al., 2001, from Rotzo Member, Calcari Grigi Formation, Trento Platform, Venetian Prealps.
Netherlands.—Jurassic: Borehole Oldenzaal 1, SMF archive material, clay facies.
Portugal.—Jurassic: Topotype of Phraterfabanella boomeri Cabral and Colin, 2015, from Coimbra Formation, Praia da Concha, marl facies.
United Kingdom.—Jurassic: Topotypic material of Dolocythere maculosa Bate, 1963, from South Cave, North Humberside.
Methods
The procedures for studying StPC we use here were mainly described in Danielopol et al. (2018), but with modifications reflecting specific aspects of the present material. High-magnification, high-resolution scanning electron microscopy is essential as simple NPC can be very small and even StPC can be below 5μm diameter with the pores of a sieve-plate (tubuli) about 0.3–0.6 μm diameter (this work). There are also observational problems in relation to preservation (diagenetic dissolution, overgrowth and recrystallisation) and sediment on the valve surface. Whenever possible we have imaged with the SEM both exterior and interior of each valve. SEM StPC images were examined with Adobe Photoshop CS3 Extended, Version 10.0 at high-magnification (600–1000 dpi). We also include a number of light micrographs of marginal zones made in the 1940s and 1950s by Erich Triebel that are of a quality unrivalled almost to the present day (Fig. 9).
Considering the size of the StPC we recognized two types: StPC-m, small sieve-type pore canals with a large subcentral setal pore and reduced sieve plate; StPC-M, large round or rarely elongate pore canals with a small excentric setal pore. Our intuition is that StPC-m and StPC-M represent two different morphologic and functional entities. We can recognize several traits in the StPC: (i) the sieve plate traversed by tubuli; (ii) the setal pore (SeP) belonging to the original sensillium; (iii) the tubuli, minute pores traversing the calcitic wall of the valve; (iv) the position of the setal pore within the sieve-plate.
The sieve-plate of a StPC is positioned either directly on the surface of the exterior of the valve or depressed in a funnel-like structure (Fig. 1B). In the latter case we considered it necessary to delineate the periphery of the sieve plate. Therefore the values of the diameter of the sieve plate given here refer to the plate sensu stricto measured as precisely as possible and not to the upper aperture of the funnel.
Representation of the sieve-plate is characterized by its shape and its size. For the former we recognize two shapes: round (R) and oblong (O). For round sieve plates we used the linear dimension of their diameter and in some cases also their area. Oblong sieve plates were defined using the rule-of-thumb of Rosenfeld and Vesper (1977), namely the calculation of the ratio height/width with its critical value of 1.5, with oblong shape defined by a value above 1.5 (see also Danielopol et al. 2018: 21).
The setal pore and the pores of the tubuli belonging to a StPC are generally round in shape and their size is expressed by the linear dimension of the diameter. The position of the setal pore within the sieve plate was noted as Peripheral, when its location approaches the margin of the StPC or subcentral, when the pore is located close to the centre of the sieve plate.
The size of the sieve-plate is expressed in absolute dimensions and also by its relative size. The morphological trait of StPC relative size is the ratio between the diameter of the plate and the length of the valve supporting the StPC. Danielopol et al. (2018) named this dimension the Size Index (SI) of the StPC. The advantage of using dimensions expressed as a ratio scale is that it allows comparison of these traits from valves with different lengths. The ratio scale, as compared to the real size scale on which we measure the absolute value of the pore, has a natural zero point (Schneider 1994) and this avoids the problem of dependence of the pore size related to the different valve dimensions. This procedure also better reflects pore size as part of the biological development of the valve from initiation to end point.
The size of the setal pore is also expressed in both absolute and relative terms. For the latter we used the ratio of the pore diameter to the diameter of the supporting StPC. We name this new descriptor, Setal Pore Size Index (SeP-SI). The SI and the SeP-SI descriptors are useful for the comparative characterization of the two types of sieve plates, the StPC-M and StPC-m. Additional characterization of the StPC is related to the number and position of tubuli within one sieve plate, i.e., randomly placed on the whole surface or in a few approximately concentric rows. Considering the number of tubuli in a sieve plate, Danielopol et al. (2018) showed in the Limnocytheridae that we can distinguish two classes, a “low” one when the number of tubuli is less than about 60 and a “large” one exceeding this approximate number.
The size of the minute pores (tubuli) of the sieve plate is expressed as their diameter, and measured either on the external or the internal side of the valve, depending on their visibility. Tubuli analysis requires very well preserved material, high-resolution electron microscopy, and patience.
If we consider that the structure of the StPC-module plays an active role in the adaptive interaction between the animal and the surrounding environment, then beside the size of the sieve plate and of the setal pore it is important to approximate the density and the dispersion of the StPC on the lateral side of the valve. In our study of the StPC of Timiriseviinae we quantified the density of these pore structures by a cartographic method developed by MCC and Telmo Nunes (Danielopol et al. 2018). In the present work we express the density of the visible StPC as a “low” class with about 70–75 entities, a “large” class with about 100 pores per valve and a “very large” class where the number of StPC significantly exceeded the latter class.
We used the Distance Index (DI) described in Danielopol et al. (2018) for the spatial dispersion of the StPC on the valves. The data are extracted from the length between pores placed in a triangular shape. A mean value between the three linear distances is calculated and the value expressed under two distance categories, a “wide” one (larger than 10 µm) and a “narrow” one (less than 10 µm).
The counts of the traits described above form sample statistics for which we computed the median accompanied by the min-max values.
All dimensions are in mm unless otherwise stated.
Systematic palaeontology
Class Ostracoda Latreille, 1806
Order Podocopida Sars, 1866
Suborder Cytherocopina Gründel, 1967
Superfamily Cytheroidea Baird, 1850
Family Cytheridae Baird, 1850
Remarks.—The Middle Jurassic species Dolocythere maculosa Bate, 1963, recognized here as a species of Minyocythere gen. nov., was originally placed by that author in the mid-Cretaceous genus Dolocythere Mertens, 1956 and it is therefore necessary to discuss the suitability of that genus for the new Middle Jurassic species we describe below which are clearly congeners with D. maculosa.
Dolocythere rara Mertens, 1956 is the type species of Dolocythere. We figure paratypes of D. rara (Fig. 18A, B) together with Senckenberg material that is conspecific in our opinion (Fig. 18C–F). There are clear differences between Albian Dolocythere rara and our Middle Jurassic Minyocythere gen. nov. in, for example, StPC, in muscle scar pattern and in the shape of the mandibular depression (median depression = fulcral point of Van Morkhoven 1962) (Figs. 4, 5). Mertens (1956) cites dimorphism (see Appendix 1 for generic diagnosis) but does not mention this for D. rara in text or figures. We have inspected numerous adults and juveniles of D. rara of Aptian–Albian age from NW Germany (Erich Triebel collection in SMF) and find that putative males are difficult to recognize and rather rare. It is clear that revision of the genus is necessary and more than one species is involved, thus we describe Dolocythere amphistiela sp. nov. as distinct from D. rara.
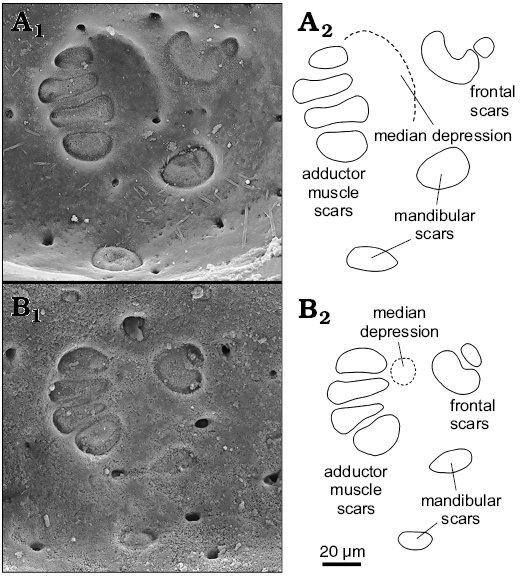
Fig. 4. Comparison of muscle scars of Dolocythere Mertens, 1956 and Minyocythere gen. nov. A. Dolocythere rara Mertens, 1956 from Borehole Lingen 196, 697–704 m, NW Germany, Lower Albian. Paratype, BGR T.-K. 1300 (Mertens Collection, Hannover), female left valve in internal view. B. Minyocythere angulata sp. nov. from Borehole Hambühren WA2, 203 m, NW Germany, Upper Aalenian (Braun Jura β). Paratype, SMF Xe 23738, female left valve in internal view. A1, B1, photographs; A2, B2, explanatory drawings.
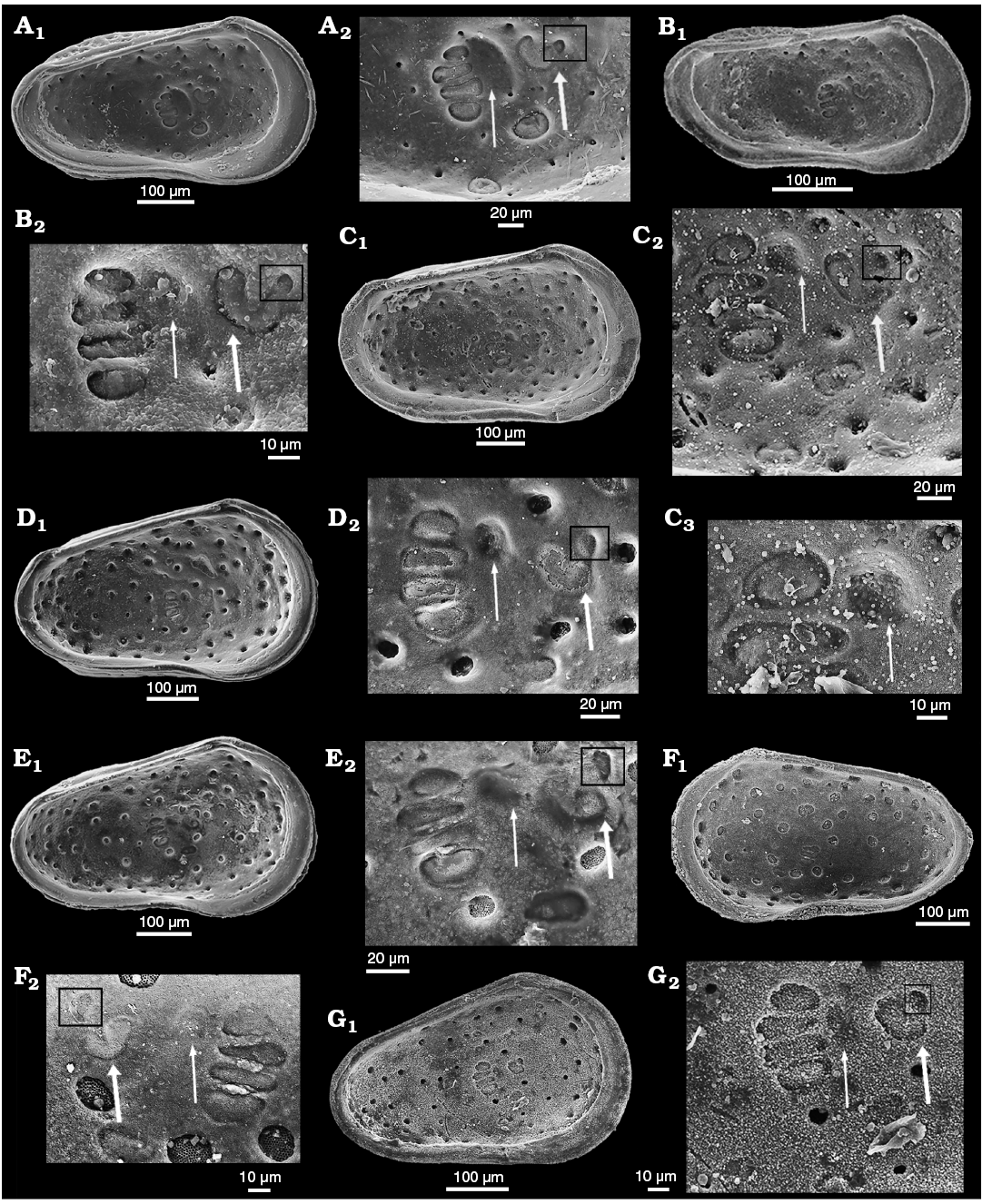
Fig. 5. Comparison of valve morphology and muscle scars in cytherid ostracods Dolocythere (A, B) and Minyocythere (C–G). A. Dolocythere rara Mertens, 1956 from Borehole Lingen 196, 697–704 m, NW Germany, Lower Albian. Paratype, BGR T.-K. 1300 (Mertens Collection, Hannover), female left valve in internal view (A1), detail (A2). B. Dolocythere amphistiela sp. nov. from Borehole Rodewald WA4, 187 m, NW Germany, lower Middle Albian. Paratype, SMF Xe 23765, male left valve in internal view (B1), detail (B2). C, D. Minyocythere maculosa (Bate, 1963). C. Topotype, SMF Xe 23743 from Bajocian, Basement Beds; Everthorpe Quarry, South Cave, UK, female left valve in internal view (C1), detail (C2), median depression (C3). D. SMF Xe 23746 from Borehole Hambühren WA2, 171–174 m, NW Germany, Witchellia laeviuscula Zone (Braun Jura γ), Lower Bajocian, female left valve in internal view (D1), detail (D2). E. Minyocythere macroporosa gen. et sp. nov. from Borehole Hambühren WA2, 166 m, NW Germany, Witchellia laeviuscula Zone (Braun Jura γ), Lower Bajocian. Holotype, SMF Xe 23721, male left valve in internal view (E1), detail (E2). F. Minyocythere sp. cf. M. macroporosa gen. et sp. nov. from Borehole Rodewald WA12, 386 m, NW Germany, Upper Aalenian (Braun Jura β upper). SMF Xe 23724, male right valve in internal view (F1), detail (F2). G. Minyocythere angulata gen. et sp. nov. from Borehole Hambühren WA2, 192–198 m, NW Germany, Upper Aalenian (Braun Jura β). Paratype, SMF Xe 23741, male left valve in internal view (G1), detail (G2). Thin white arrows point to median depression; thick white arrows point to frontal scar; black square indicates detail of frontal scar.
Minyocythere shows differently developed muscle scars and especially the mandibular fulcral depression, both genera have lophodont hingement but less strongly developed in Minyocythere than in Dolocythere and with an accommodation groove on LV. Both genera have StPC but Minyocythere has large and easily recognizable StPC (StPC-M herein; “caps” of Luppold 2012) whereas Dolocythere does not. Minyocythere has two of these StPC-M in a stable antero-dorsal position (Fig. 6) which we consider probably a diagnostic character for Minyocythere as it is common to the four (five?) known species of the genus and it is not present in Dolocythere. Minyocythere macroporosa sp. nov. and M. angulata sp. nov. show pre-adult sexual dimorphism (= “precocious sexual dimorphism”), a feature that is well-known in ostracods (living examples: Heterocythereis albomaculata (Baird, 1838) and Loxoconcha elliptica Brady, 1868 [Athersuch et al. 1989: 23–24], Keijcyoidea infralittoralis Tsukagoshi, Okada, and Horne, 2006 [Okada et al. 2008]; fossil examples: four species of Glyptocythere Brand and Malz, 1962, Glabellacythere Wienholz, 1967, Lophocythere Sylvester-Bradley, 1948 [Whatley and Stephens 1977], Praeschuleridea decorata Bate, 1968 [Bate 1968]). The important aspects for fossil material are: (i) to check internal features carefully to assess full maturity (see Athersuch et al. 1989: fig. 16 with A-1 dimorphs in L. elliptica demonstrating simple calcified inner lamella features of the penultimate moult) and (ii) the problem of time-averaging in micropalaeontological samples which may conceal ecological fluctuations reflected in size variation in mature animals over time. In this context larger adults may appear as post-maturation moults although this seems rare, for example, Whittaker in his study of The Fleet, Dorset, UK (personal communication to ARL; 1968, 1975) cites Paradoxostoma pulchellum Sars, 1866 with 1700 live adults collected over three years but only one was 15–20% larger than other adults, and Hirschmannia viridis (Müller, 1785) with 1500 live and dead adults over three years with only one 15–28% larger.
Our conclusion is that the suite of Middle Jurassic species: Minyocythere macroporosa sp. nov., M. angulata sp. nov., M. maculosa (Bate, 1963) and M. tuberculata (Luppold, 2012) represent a new genus that we erect as Minyocythere gen. nov. and which differs from Dolocythere in development of hingement, in details of the muscle scar pattern, in the nature of the mandibular depression and in the morphological diversity of the NPC.
Genus Minyocythere nov.
ZooBank LCID: urn:lsid:zoobank.org:act:00137DD9-13E3-4ABB-8367-8F1865C9D53D
Type species: Minyocythere macroporosa sp. nov., Borehole Hambühren WA2, NW Germany; Early Bajocian.
Species included: Minyocythere angulata sp. nov., M. maculosa (Bate, 1963), M. tuberculata (Luppold, 2012).
Etymology: From Greek, μινυο (minyo), of small dimension and κυθερα, κυθερης (Cythera, Cytherias [Kuthêra, Kuthêrias]), different forms of a surname of Aphrodite, derived from the town of Cythera in Crete, or from the island of Cythera, where the goddess was said to have first emerged, and where she had a celebrated temple.
Diagnosis.—A genus of the Family Cytheridae characterized by subtriangular to subrectangular shape, modified lophodont hingement with accommodation groove on LV and up to three types of normal pore canal especially large round or rarely elongate sieve-type pore canals.
Remarks.—Phraterfabanella Whatley and Boomer in Boomer et al., 2001 (Sinemurian), Camptocythere Triebel, 1950 and Aphelocythere Triebel and Klingler, 1959 (both Toarcian–Aalenian) (diagnoses in Appendix 1) are the earliest examples known to us of StPC in cytheroid ostracods (see “The fossil record of StPC” above). Some species of Aphelocythere, e.g., A. perforata Plumhoff, 1963 (Fig. 3A1) closely resemble the younger Minyocythere gen. nov. externally but they are easily differentiated by hingement which is merodont in the former and lophodont in the latter. Differences between Minyocythere and Dolocythere are discussed above.
David Horne (personal communication 2019) commented on the similarity in valve shape between Minyocythere gen. nov. and Cytherissa Sars, 1925, the latter a living lacustrine taxon that displays a wide range of morphological variation (Danielopol et al. 1990). The hingement of both genera is similar, smooth and tripartite, and both have relatively narrow and simple marginal zones but differ in details of StPC and muscle scars in addition to the fact that Minyocythere is Jurassic, marine and extinct whereas Cytherissa is ?Paleogene, Pliocene to Recent (Colin and Carbonel 1990), living, and common in shallow and deep lake environments (Meisch 2000), and is a potential case of homeomorphy or convergent evolution. The question arises of family placement. On balance we believe Minyocythere to be closer to living Cythere and the Family Cytheridae than to Cytherissa and the Family Cytherideidae, and also close to Dolocythere which is considered a Cytheridae taxon, however, the definition of families of Jurassic ostracods is in need of revision.
Reference is made both above and below to Japanese work on NPC of the genus Cythere, especially by Tsukagoshi and Ikeya (1987), which provides an important comparative dataset. Cythere, a widespread living and fossil genus which gives its name to the family Cytheridae, has heavily calcified sub-quadrate valves ornamented with pits and/or ridges, with internally merodont hingement, broad calcified inner lamella without vestibules and fairly numerous marginal pore canals especially anteriorly, and is sexually dimorphic with females slightly higher than males. Although Cythere and Minyocythere fall in the same family they are not thought to be closely related.
An apparent progression in time of strength of ornament in Minyocythere species is evident: Minyocythere angulata (almost smooth), M. macroporosa (ranges from weak to strong depressions), M. maculosa (strong depressions), M. tuberculata (strong swollen curving ridges), which, perhaps surprisingly, matches the stratigraphic occurrence of these species in borehole Hambühren WA2 (although less well in borehole Rodewald WA 2 where occurrences are patchy) (discussed below).
Stratigraphic and geographic range.—Aalenian–Bajocian, Middle Jurassic; NW Europe.
Minyocythere macroporosa sp. nov.
Figs. 5E, 6A, 7, 8, 9C, D, 11A, 21.
1962 “Lophodentina”? sp. 99; Brand and Fahrion 1962: 136–137, pl. 20: fig. 6; table 9.
2012 Dolocythere tuberculata sp. nov.; Luppold 2012: pl. 4: 5–8.
non 2012 Dolocythere tuberculata sp. nov.; Luppold 2012: text-fig. 6a, ?b; pl. 4: 9–12, pl. 6: 15, 16.
ZooBank LCID: urn:lsid:zoobank.org:act:1D8AC905-2F98-418C-9467- E5CC136F984F
Etymology: In reference to the prominent StPC visible both externally and internally.
Type material: Holotype, SMF Xe 23721, LV male (Figs. 5E, 7D). Paratypes: SMF Xe 23718, C female (Fig. 7A); SMF Xe 23719, C male (Fig. 7B); SMF Xe 23720, C female (Fig. 7C); SMF Xe 23722, RV female (Fig. 7E); SMF Xe 23723, RV juvenile A-1? (Fig. 7F). All from the type locality, different depths.
Type locality: Borehole Hambühren WA2 (166 m depth), NW Germany.
Type horizon: Lower Bajocian, Witchellia laeviuscula Zone (Braun Jura γ).
Other material.—10 C, 97 V, 11 V juvenile, collective number SMF Xe 23767.
Diagnosis.—A species of Minyocythere characterized by its carapace tapering posteriorly with a postero-ventral swelling and surface of moderately developed foveolae and/or punctae within which NPC are sometimes located.
Dimensions (in mm).—Females: L = 0.550–0.624, H = 0.300–0.375 (SMF Xe 23718, L = 0.525, H = 0.324; SMF Xe 23720, L = 0.575, H = 0.350; SMF Xe 23722, L = 0.550, H = 0.350). Males: L = 0.525–0.588, H = 0.286–0.336 (SMF Xe 23721, L = 0.575, H = 0.350; SMF Xe 23719, L = 0.550, H = 0.324). Juvenile: SMF Xe 23723, L = 0.476, H = 0.300.
Description.—Exterior: Subtriangular to subrectangular in lateral view, greatest height at anterior cardinal angle, tapering posteriorly, greatest length at mid-height; anterior margin broadly rounded with marginal rims on both valves; dorsal margin medianly weakly concave; posterior low and symmetrically rounded in LV, ventrally inclined in RV; ventral margin weakly concave with slight postero-ventral swelling on both valves; LV and RV almost identical in shape and size, LV slightly larger than RV on all margins except dorsal. Sexual dimorphism present but relatively weakly expressed, with males appearing relatively elongate in lateral view than females, however, in dorsal view apparent males are slightly more inflated posteriorly whereas apparent females show greatest width at mid-length. Juveniles tend to be more triangular in lateral view. Dimorphism is recognisable in “pre-adult” moult stages (Fig. 8). Ocular structures not evident externally or internally. Surface rugose with weakly to moderately developed foveolae and punctae within which normal pore canals are located, the pores range from simple holes (Fig. 7D4) to simple sieve plates with 2–3 rows of tubuli (Fig. 7C5, D3, E5, F3) and to large sieve plates (with many tubuli, Fig. 7C3, D2, E4, F2) present in one animal; ornament tends to be more strongly developed near the anterior margin especially in males. Juveniles appear relatively smoother than adults, but also with StPC (Fig. 7F).
Simple NPC seldom observed at valve periphery (Fig. 6A1) and are of smaller diameter than StPC (Fig. 7D4; 4.5 μm diameter). StPC-M located in valve surface depressions. Mostly round (Figs. 6A, 7C3, D2, E4). Size varies, with those towards margins generally smaller than those located in centre of valve. Median diameter c. 11 μm and SI 0.02 (Table 1). Setal pore always round with diameter c. 1 μm (SeP-SI 0.09). StPC-M have a high number of tubuli, each with a diameter 0.3–0.4 μm, but difficult to count for preservational reasons, we estimate tubuli density in “large” class (Table 1). Maximum number of StPC-M counted is 53 in Fig. 6A1, “low” class. Pore dispersion on the valve is “wide” type (Figs. 6A1, 7B1, D1, E3). DI varies between 20–50 µm. On antero-dorsal area, the two diagnostic StPC-M round or elongate (Figs. 6A1, 7A, B1, B2, C1, D1, E3) seem to occupy a permanent position. StPC-m round with small diameter (c. 6 μm) and SI 0.01 (Table 1) (Figs. 6A, 7C5, D3, E5). Setal pore large with diameter c. 2 μm (SeP-SI c. 0.3). Number of tubuli visible about 20 in 2–3 rows. Number StPC-m counted 12, much fewer than StPC-M.
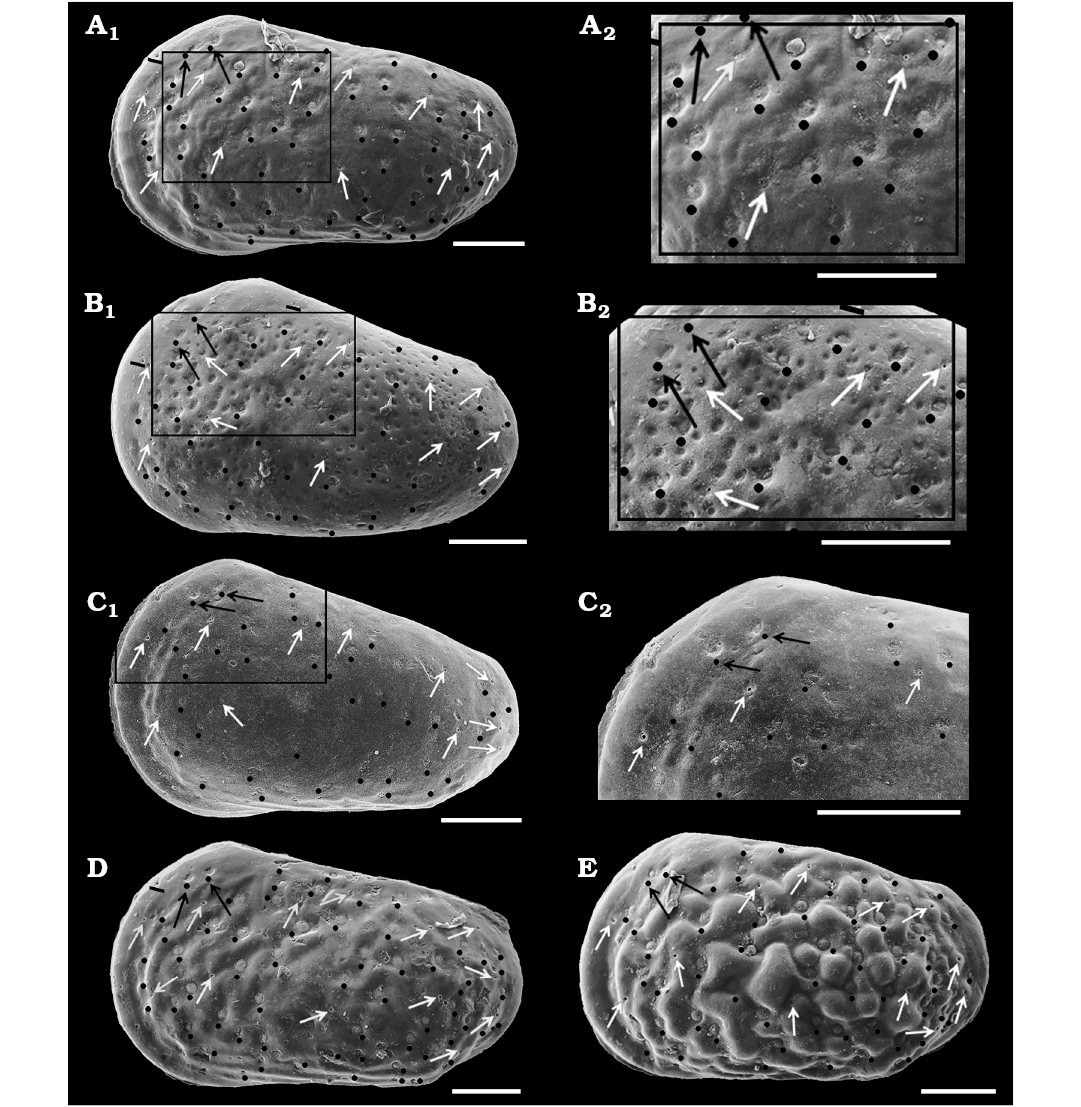
Fig. 6. Distribution of sieve-type normal pore canals (StPC) and simple normal pore canals (NPC) on the outer side of left valves of cytherid ostracod Minyocythere gen. et spp. nov. A. Minyocythere macroporosa sp. nov. from Borehole Hambühren WA2, 166 m, NW Germany, Witchellia laeviuscula Zone (Braun Jura γ), Lower Bajocian. Holotype, SMF Xe 23721, male left valve (A1), detail (A2), total area of A2, c. 45 µm2. B. Minyocythere angulata sp. nov. from Borehole Hambühren WA2, 203 m, NW Germany, Upper Aalenian (Braun Jura β). Holotype, SMF Xe 23737, male left valve (B1), detail (B2), total area of B2, c. 44 µm2. C. Minyocythere sp. cf. M. macroporosa sp. nov. from Borehole Rodewald WA12, 404.5 m, NW Germany, Witchellia laeviuscula Zone (Braun Jura γ upper), Lower Bajocian. SMF Xe 23725, male left valve (C1), detail (C2). D. Minyocythere maculosa (Bate, 1963) from Hambühren WA2, 171–174 m, NW Germany, Upper Aalenian (Braun Jura β). SMF Xe 23750, male left valve. E. Minyocythere tuberculata (Luppold, 2012) from Borehole Rodewald WA12, 404.5 m, NW Germany, Witchellia laeviuscula Zone (Braun Jura γ upper), Lower Bajocian. SMF Xe 23754, female left valve. Black dots refer to macro StPC (StPC-M); white arrows point to micro StPC (StPC-m); simple NPC underlined; black arrows indicate position of the two StPC-M characteristic of Minyocythere species. Scale bars 100 µm.
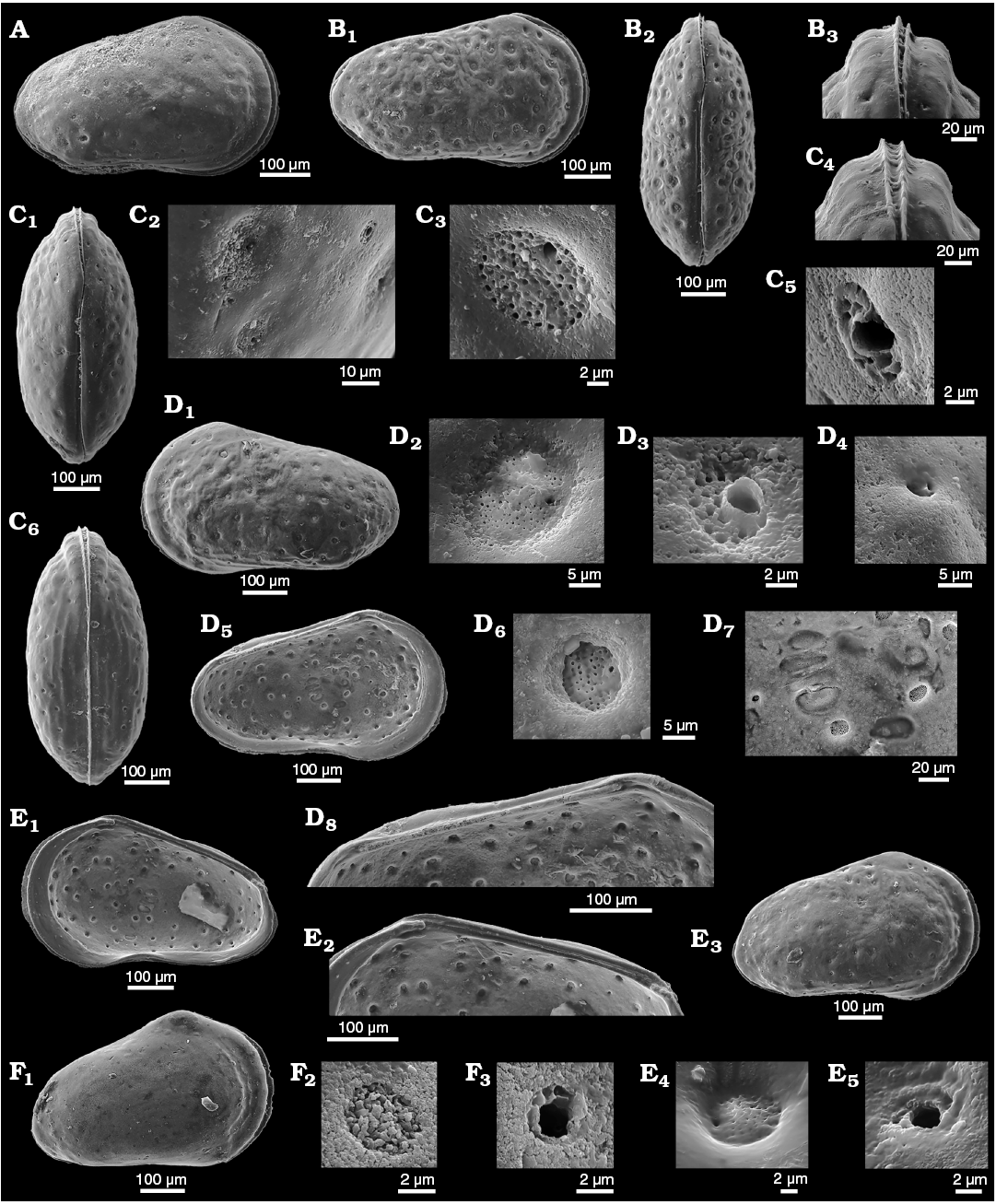
Fig. 7. Cytherid ostracod Minyocythere macroporosa sp. nov. from Borehole Hambühren WA2, 166–167 m (A–C), 166 m (D, E), 168–169 m (F), NW Germany, Witchellia laeviuscula Zone (Braun Jura γ), Lower Bajocian. A. Paratype, SMF Xe 23718, female carapace in right view. B. Paratype, SMF Xe 23719, male carapace in right (B1) and dorsal (B2) views, antero-dorsal margin (B3). C. Paratype, SMF Xe 23720, female carapace in dorsal (C1) and ventral (C6) views, pores (large and small) (C2), macro sieve-type normal pore canals (StPC-M) (C3), antero-dorsal margin (C4), and micro sieve-type normal pore canals (StPC-m) (C5). D. Holotype, SMF Xe 23721, male left valve in external (D1) and internal (D5) views, StPC-M (D2, D6), StPC-m (D3), simple pore (D4), muscle scars (D7), hinge (D8). E. Paratype, SMF Xe 23722, female right valve in internal (E1) and external (E3) views, hinge (E2), StPC-M (E4), StPC-m (E5). F. Paratype, SMF Xe 23723, A-1?, juvenile right valve in external view (F1), “large type” StPC (F2), “small type” StPC (F3).
Interior: Marginal zone well developed anteriorly, inner margin and line of concrescence coincide; marginal pore canals short, straight, widely spaced and arranged in a fan anteriorly, 9–10 anteriorly and c. 5 posteriorly (Fig. 9C, D). Muscle scars (Figs. 5E, 7D7) consist of a semi-vertical curved row of four adductor muscle scars (AMS), the dorsal and ventral ones rounded and the central ones more elongate, with two frontal scars one large and kidney-shaped and one small and round located above, with ventrally two oval mandibular scars; between the vertical row of scars and the frontal scars there is a round depression that represents the fulcral point (= median depression) of the mandible. Hinge tripartite, relatively weakly developed, modified lophodont: LV (Fig. 7D8) short terminal sockets and long smooth median bar, RV (Fig. 7E2) short smooth terminal teeth that merge into the free margin and a smooth median groove; the median elements may appear denticulate or locellate in poorly preserved material and in some specimens the posterior element appears loculate or dentate. Internally the valves show depressions that match the positions of StPC externally and the numerous tubuli are clearly seen (Fig. 7D6–D8, E2). StPC-M show values similer to exterior view (Table 1). Number of tubuli counted on one StPC (Fig. 7D6) 57, certainly an under estimate because of preservation. StPC-m diameter smaller than on exterior and in one pore 12 tubuli counted (Table 1).
Table 1. Characterization of sieve-type normal pore canals (StPC) of Minyocythere macroporosa sp. nov. Abbreviations: StPC-M, macro sieve-type pore canals; StPC-m, micro sieve-type pore canals; Ex/In, view of the trait on the external/internal side of the valve; SI, Size Index (ratio of the pore diameter to the valve’s length); SeP, setal pore of the StPC; SeP-SI, Setal Pore Size Index (ratio of the SeP diameter to the StPC diameter); diameter (in µm), expressed either as individual value or through the median statistics and min-max values; No, number of observations; v, variate (single reading).
|
Properties |
StPC-M sample statistics |
StPC-m sample statistics |
|||
|
Morphological trait |
Specification |
No |
Median (min-max) |
No |
Median (min-max) |
|
StPC-size (Ex) |
diameter |
12 |
11.36 (7.45–16.24) |
6 |
6.66 (5.5–7.1) |
|
SI |
0.020 (0.013–0.030) |
0.011 (0.010–0.012) |
|||
|
StPC-size (In) |
diameter |
14 |
10.78 (8.86–15.22) |
5 |
4.21 (3.16–4.43) |
|
SI |
0.018 (0.016–0.025) |
0.007 (0.006–0.008) |
|||
|
SeP-size (Ex) |
diameter |
12 |
1.01 (0.45–1.30) |
5 |
2.33 (1.76–2.6) |
|
SeP-SI |
0.09 (0.06–0.12) |
0.33 (0.32–0.37) |
|||
|
SeP-size (In) |
diameter |
3 |
0.98 (0.90–1.0) |
– |
– |
|
SeP-SI |
0.09 (0.08–0.09) |
– |
|||
|
StPC-tubuli (Ex) |
number of tubuli |
3 |
76 (59–91) |
v |
~20 |
|
StPC-tubuli (In) |
number of tubuli |
v |
~57 |
v |
~12 |
Remarks.—The Brand and Fahrion material of “Lophodentina”? sp. 99 contains both Minyocythere macroporosa sp. nov. (Brand and Fahrion 1962: pl. 20: 6) and M. maculosa (Brand and Fahrion 1962: pl. 20: 25). Luppold (2012: pl. 4: 5–8) interpreted our M. macroporosa sp. nov. as juveniles of his new species Dolocythere tuberculata, which is understandable given that M. macroporosa has relatively thinly calcified valves compared to the well calcified and strongly ornamented M. tuberculata; herein M. tuberculata (Luppold, 2012) is considered to belong to Minyocythere.
The material includes a few specimens that are similar in shape to juveniles of M. macroporosa sp. nov. but which appear to be adults in size (female: L = 0.525–0.600, H = 0.313–0.375; male: L = 0.550–0.575, H = 0.313–0.324) with a developed marginal zone and hingement and we have designated them as M. sp. cf. M. macroporosa sp. nov. (Figs. 5F, 6C, 8, 10, 11C, 21). Compared to M. macroporosa, M. sp. cf. M. macroporosa has a less irregular surface and the postero-ventral swelling is less developed; the growth series of juveniles and females of both forms is similar (regression lines in Fig. 8A, B) as opposed to the males (Fig. 8C). M. sp. cf. M. macroporosa occurs in Hambühren WA2 in the Upper Aalenian (9 C, 7 V) and in Rodewald WA12 in the Lower Bajocian (1 C, 18 V). M. macroporosa and M. sp. cf. M. macroporosa occur together in Hambühren WA2 but not in Rodewald WA12. As sizes and gender overlap (Fig. 8) and M. sp. cf. M. macroporosa is generally less well-preserved, there is no justification for recognising it as sp. nov. or subsp. nov.
Comparing NPC of M. macroporosa sp. nov. and M. sp. cf. M. macroporosa for completeness, we note that internally the former shows smaller StPC-M than the latter; median diameter 10.84 μm (n = 14) as opposed to 12.27 μm (n = 11) in the latter. This difference appears to be preservational.
The difference between the StPC-M of M. macroporosa sp. nov. and M. angulata sp. nov. is also of comparative value, as for equivalent surface areas in M. macroporosa and M. angulata almost all pores were round in the former whereas in the latter eleven were oblong and only four round (Fig. 6A2, B2).
Stratigraphic and geographic range.—Aalenian–Bajocian, Middle Jurassic; NW Europe.
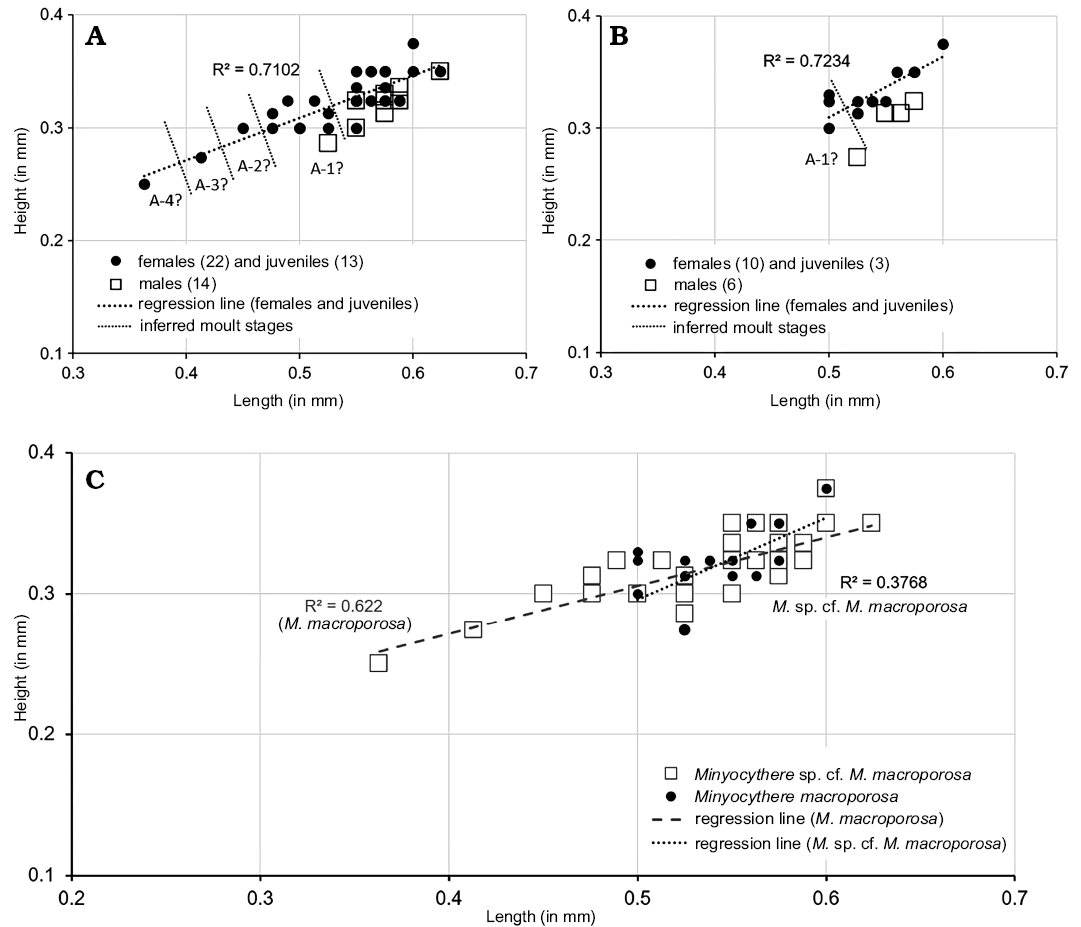
Fig. 8. Length versus height plots for Minyocythere macroporosa sp. nov. (A) and Minyocythere sp. cf. M. macroporosa sp. nov. (B), comparison (C). A-1–A-4, estimated growth (moult) stages.
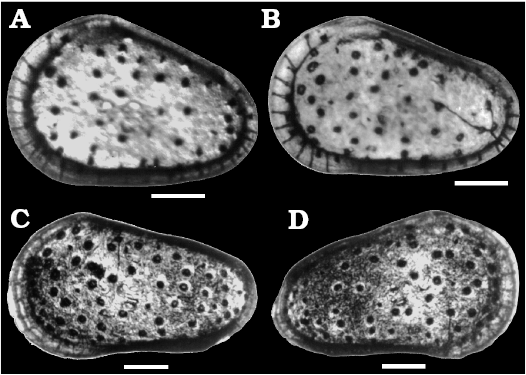
Fig. 9. Marginal pore canals of cytherid ostracods Minyocythere macroporosa sp. nov. and Minyocythere angulata sp. nov. A, B. Minyocythere angulata sp. nov. from Borehole Hambühren WA2, 203 m, NW Germany, Upper Aalenian (Braun Jura β). A. SMF Xe 23742 (specimen broken), female right valve in internal view. B. Unnumbered (specimen missing), male right valve in internal view. C, D. Minyocythere macroporosa sp. nov. from Hambühren WA2, 166 m, NW Germany, Upper Aalenian (Braun Jura β). C. Holotype, SMF Xe 23721, male left valve in external view. D. Paratype, SMF Xe 23722, female right valve in external view. Light micrographs by Erich Triebel. Scale bars 100 µm.
Minyocythere angulata sp. nov.
Figs. 4B, 5G, 6B, 9A, B, 12, 13, 21.
Zoobank LCID: urn:lsid:zoobank.org:act:3462B034-52DC-45C7-AB 35-0E42983B7F42
Etymology: From Latin angulus, angled.
Type material: Holotype, SMF Xe 23737, LV male (Fig. 12F). Paratypes: SMF Xe 23732, C female (Fig. 12A); SMF Xe 23733, RV female (Fig. 12B); SMF Xe 23734, LV female (Fig. 12C); SMF Xe 23735, C female (Fig. 12D); SMF Xe 23736, C female (Fig. 12E); SMF Xe 23738, LV female (Figs. 4B, 12G); SMF Xe 23739, RV juvenile, A-2? (Fig. 12I); SMF Xe 23740, C male (Fig. 12H); SMF Xe 23741, LV male (Fig. 5G).
Type locality: Borehole Hambühren WA2, NW Germany.
Type horizon: Borehole Hambühren WA2, 203 m, Upper Aalenian (Braun Jura β).
Other material.—21 C, 59 V, 7 C juvenile, 40 V juvenile, plus 3 C, 2 C [cf.], collective number SMF Xe 23767, from Borehole Oldenzaal 1, 246–251 m depth, The Netherlands; Upper Aalenian (Braun Jura β).
Diagnosis.—A species of Minyocythere characterized by its strongly tapering almost triangular carapace with strong cardinal angles and weak ornament of foveolae and punctae within which NPC are located.
Dimensions (in mm).—Females: L = 0.476–0.575, H = 0.324–0.350 (SMF Xe 23732, L = 0.475, H = 0.324; SMF Xe 23733, L = 0.500, H = 0.300; SMF Xe 23734, L = 0.475, H = 0.324; SMF Xe 23735, L = 0.500, H = 0.324; SMF Xe 23736, L = 0.500, H = 0.324; SMF Xe 23738, L = 0.500, H = 0.350). Males: L = 0.476–0.550, H = 0.300–0.324 (SMF Xe 23737, L = 0.525, H = 0.324; SMF Xe 23740, L = 0.476, H = 0.274; SMF Xe 23741, L = 0.490, H = 0.310). Juvenile: SMF Xe 23739, L = 0.400, H = 0.274.
Description.—Exterior: Quadrate to triangular in lateral view, greatest height at anterior cardinal angle, tapering strongly posteriorly, greatest length just below mid-height; anterior margin broadly rounded with weak marginal rims on both valves, slight concavity on anterior margin below anterior cardinal angle on RV; dorsal margin straight to weakly concave medianly especially on LV; posterior low and symmetrically rounded in LV, slightly inclined ventrally in RV; ventral margin straight to convex; LV and RV almost identical in shape and size, LV slightly larger than RV especially at anterior and posterior margins; in dorsal view the female valves are uniformly curved around mid-length. Sexual dimorphism present but relatively weakly expressed, with males less high and appearing relatively longer in lateral view than females. Juveniles tend to be more triangular in lateral view and relatively smoother than adults. Dimorphism is recognisable in “pre-adult” moult stages (Fig. 13). Ocular structures not evident externally or internally. Surface relatively smooth with shallow foveolae and punctae within some of which StPC are located (Fig. 12B2, B3, C2, F2, F3). Simple NPC rarely visible, round, diameter c. 2 μm (Fig. 6B1). StPC-M located in shallow foveolae, most oblong (Figs. 6B, 12C2), a few round (Fig. 12B3), eight oblong and four round analysed. Median diameter c. 10 μm and SI 0.02 (Table 2) for round pores. Setal pore round with diameter median value 1.5 μm. Round StPC-M the SePSI c. 0.2. Number of tubuli in both oblong and round forms high, median value 61, tubuli diameter in range 0.3–0.4 μm. Density “low” (Fig. 6B1), 48 entities counted, from which 87.5% oblong. The pore dispersion is “wide” (Fig. 6B). The DI varies between 30–60 µm. On the antero-dorsal area presence of the two StPC-M typical of the genus (Figs. 6B1, 12B1, D, F1). StPC-m round (Fig. 12B2, F3) and with small diameter of 5 μm and SI of 0.01 (Table 2). Setal pore large, c. 3 μm and SePSI around 0.6. Number of tubuli visible c. 16 disposed in 2–3 rows. Density of StPC-m pores similar to the one in M. macroporosa sp. nov. (12 entities, Fig. 6B1).
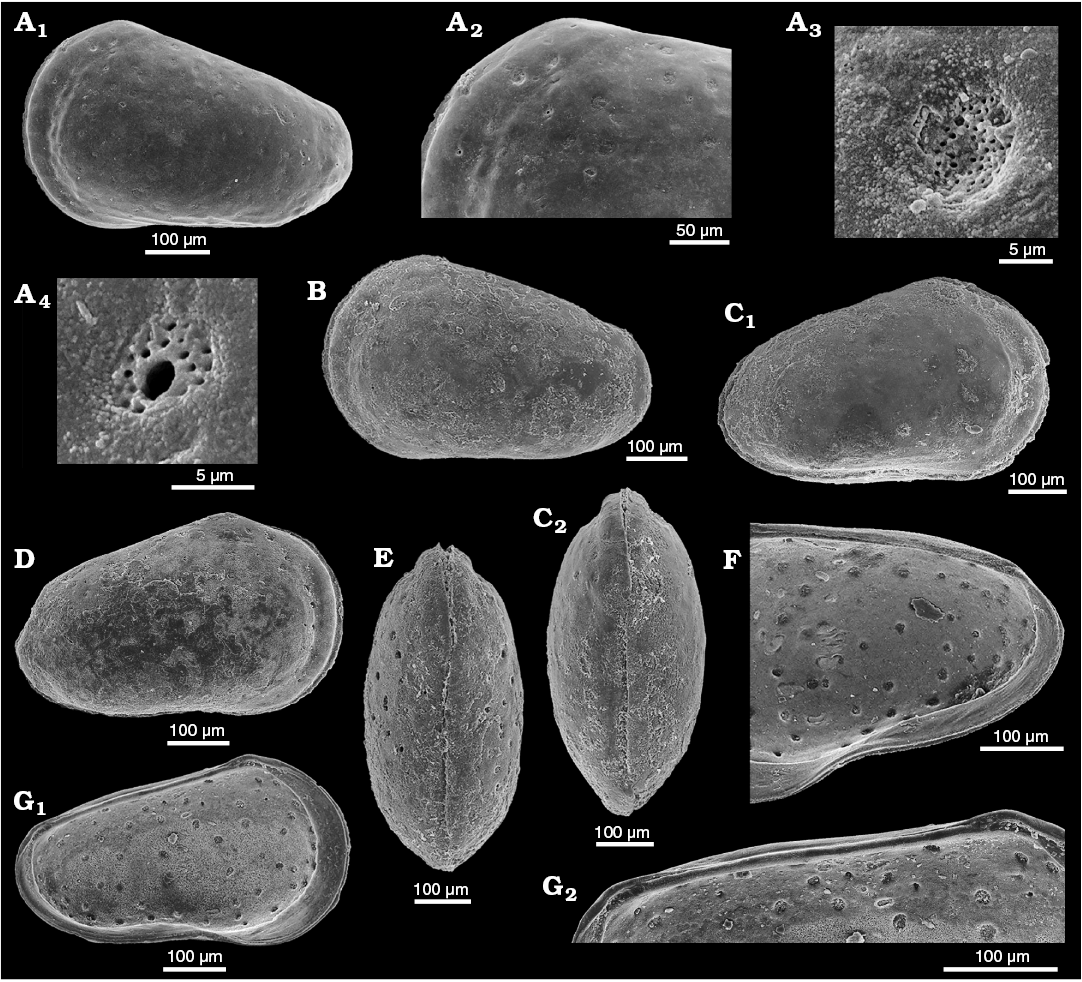
Fig. 10. Cytherid ostracod Minyocythere sp. cf. M. macroporosa sp. nov. from Borehole Rodewald WA12, 404.5 m (A), 386 m (B–E), 404.5 m (F, G), NW Germany, Witchellia laeviuscula Zone (Braun Jura γ upper), Lower Bajocian. A. SMF Xe 23725, male left valve in external view (A1), antero-dorsal area (A2), macro sieve-type normal pore canals (StPC-M) (A3), and micro sieve-type normal pore canals (StPC-m) (A4). B. SMF Xe 23726, female left valve in external view. C. SMF Xe 23727, female carapace in right (C1) and dorsal (C2) views. D. SMF Xe 23728, female right valve in external view. E. SMF Xe 23729, male carapace in dorsal view. F. SMF Xe 23730, fragment of female right valve in internal view. G. SMF Xe 23731, male left valve, internal view (G1), hinge (G2).
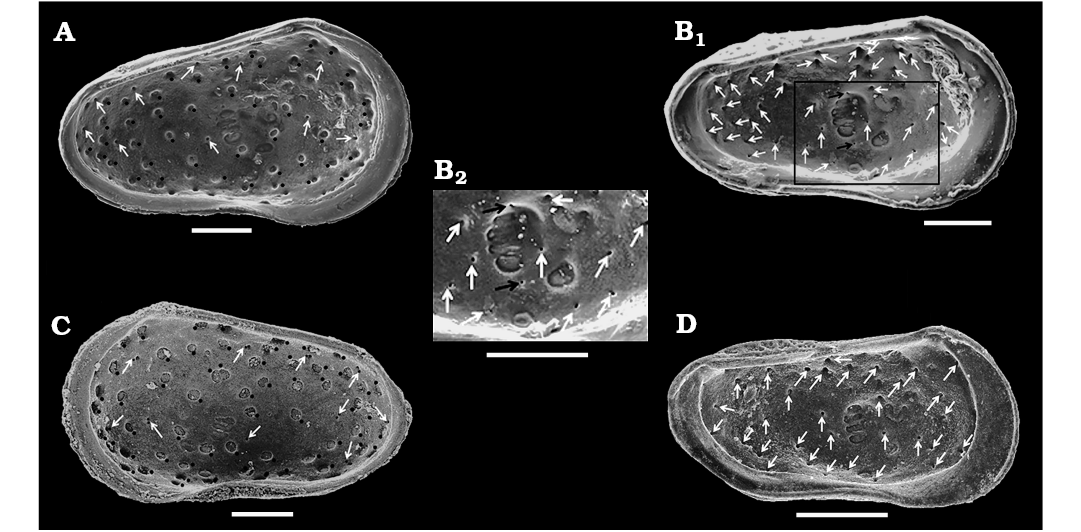
Fig. 11. Distribution of macro sieve-type normal pore canals (StPC-M), micro sieve-type normal pore canals (StPC-m), and simple normal pore canals (NPC) on the inner side of cytherid ostracods Minyocythere gen. nov. and Dolocythere Mertens, 1956. A. Minyocythere macroporosa sp. nov., holotype, SMF Xe 23721 from Borehole Hambühren WA2, 166 m, NW Germany, Witchellia laeviuscula Zone (Braun Jura γ), Lower Bajocian; male left valve. B. Dolocythere rara Mertens, 1956, SMF Xe 23756 from Borehole Rodewald WA6, 206 m, NW Germany, Lower Albian; male left valve (B1), detail (B2). C. Minyocythere sp. cf. M. macroporosa sp. nov., SMF Xe 23724 from Borehole Rodewald WA12, 386 m, NW Germany, Upper Aalenian (Braun Jura β upper); male right valve. D. Dolocythere amphistiela sp. nov., paratype, SMF Xe 23765 from Borehole Rodewald WA4, 187 m, NW Germany, Leymeriella tradefurcata Zone, Lower Albian; male left valve. In A and C, black dots refer to StPC-M, white arrows point to StPC-m; in B1, B2, D white arrows point to NPC; black arrows in B1, B2 indicate the peculiar pores of D. rara (which do not exist in D. amphistiela). Scale bars 100 µm.
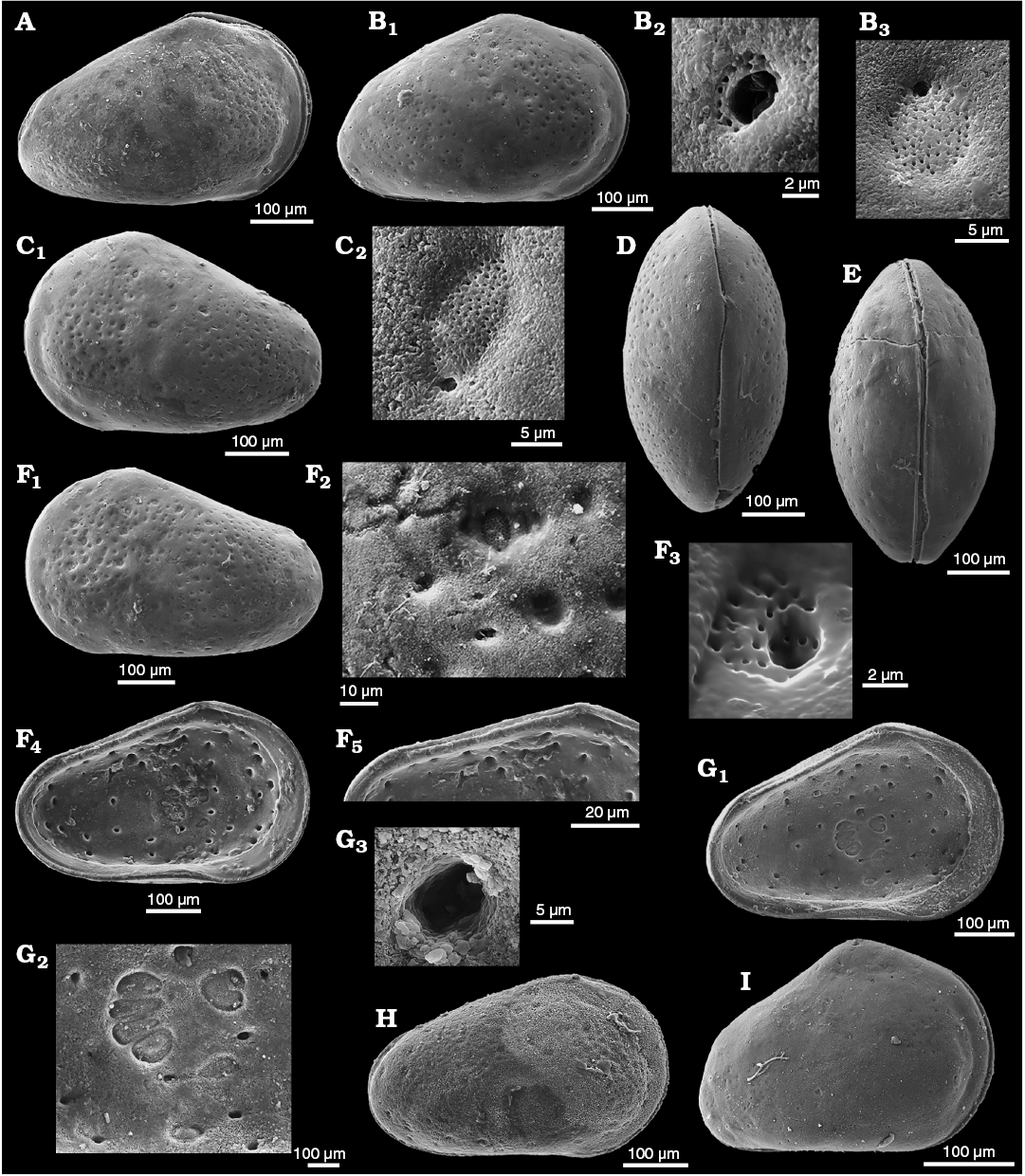
Fig. 12. Cytherid ostracod Minyocythere angulata sp. nov. from Borehole Hambühren WA2, 177–180 m (A, H), 203–206 m (B–E), 203 m (F, G), NW Germany, Upper Aalenian (Braun Jura β). A. Paratype, SMF Xe 23732, female carapace in right view. B. Paratype, SMF Xe 23733, female right valve in external view (B1), micro sieve-type pore canals (StPC-m) (B2), and round macro sieve-type normal pore canals (StPC-M) (B3). C. Paratype, SMF Xe 23734, female left valve in external view (C1), elongate StPC-M (C2). D. Paratype, SMF Xe 23735, female carapace in dorsal view. E. Paratype, SMF Xe 23736, female carapace in ventral view. F. Holotype, SMF Xe 23737, male left valve in external (F1) and internal (F4) views, ornamentation and StPC-M and StPC-m (F2), StPC-m (F3), hinge (F5). G. Paratype, SMF Xe 23738, female left valve in internal view (G1), muscle scars (G2), StPC-m (G3). H. Paratype, SMF Xe 23740, male carapace in right view. I. Paratype, SMF Xe 23739, A-2? juvenile right valve in external view.
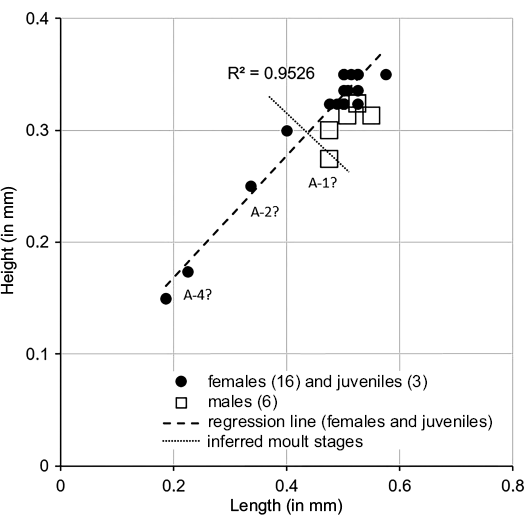
Fig. 13. Length versus height plots for Minyocythere angulata sp. nov. A-1, A-2, A-4, estimated growth (moult) stages.
Table 2. Characterization of sieve-type normal pore canals (StPC) of Minyocythere angulata sp. nov. Abbreviations: StPC-M, macro sieve-type pore canals; StPC-m, micro sieve-type pore canals; Ex/In, view of the trait on the external/internal side of the valve; SI, Size Index (ratio of the pore diameter to the valve’s length); SeP, setal pore of the StPC; SeP-SI, Setal Pore Size Index (ratio of the SeP diameter to the StPC diameter); diameter (in µm), expressed either as individual value or through the median statistics and min-max values; No, number of observations; v, variate (single reading).
|
Properties |
StPC-M sample statistics |
StPC-m sample statistics |
|||
|
Morphological trait |
Specification |
No |
Median (min-max) |
No |
Median (min-max) |
|
StPC (Ex) |
diameter |
6 |
9.97 (8.5–11.18) |
6 |
5.06 (3.9–6.0) |
|
SI |
0.020 (0.17–0.020) |
0.01 (0.008–0.010) |
|||
|
StPC (In) |
diameter |
6 |
10.7 (7.0–14.6) |
5 |
5.0 (2.4–8.0) |
|
SI |
0.021 (0.014–0.030) |
0.01 (0.005–0.016) |
|||
|
SeP (Ex) |
diameter |
3 |
1.4 (0.57–1.6) |
3 |
2.8 (2.22–3.2) |
|
SeP-SI |
– |
– |
0.57 (0.37–0.82) |
||
|
StPC-tubuli (Ex) |
number of tubuli |
3 |
61 (55–85) |
3 |
16 (12–23) |
Interior: Marginal zone well developed anteriorly, inner margin and line of concrescence coincide; marginal pore canals short, straight, widely spaced and arranged in a fan anteriorly, 10–11 anteriorly and 4–5 posteriorly (Fig. 9A, B). Muscle scars (Fig. 5G) consist of a semi-vertical curved row of four AMS, the dorsal and ventral ones rounded and the central ones more elongate, with two frontal scars one large and kidney-shaped and one small and round located centrally above, with ventrally two oval mandibular scars; between the vertical row of scars and the frontal scars there is a round depression that represents the articulation point (median depression = fulcral point) of the mandible. Hinge tripartite, relatively weakly developed, modified lophodont: LV (Fig. 12F4, F5, G1) short terminal sockets and long smooth median bar, RV short smooth terminal teeth that merge into the free margin and a smooth median groove; the median elements may appear denticulate or locellate in poorly preserved material and in some specimens the posterior element appears loculate or dentate.
Internally the valves show tubular apertures (depressions) that are typical of StPC-M or StPC-m. The apertures were most easily matched with external StPC in the centre of the valve interior.
Remarks.—Minyocythere angulata sp. nov. differs from M. macroporosa sp. nov. in its more triangular lateral outline, smoother surface with weak depressions corresponding to StPC, in number of StPC-M, and a secondary punctate ornament. M. angulata differs from M. maculosa and especially from M. tuberculata in strength of ornament and in lateral shape where M. maculosa is more rectangular and M. tuberculata more quadrate.
Especially interesting is comparison between the high frequency of oblong pores in M. angulata (Fig. 6B2) and those of M. macroporosa previously described (Fig. 6A2). For approximately equivalent surface areas we counted in M. angulata eight oblong pores and only four round ones, while in M. macroporosa practically all pores in the equivalent valve area are of round-type.
Stratigraphic and geographic range.—Aalenian–Bajocian, Middle Jurassic; NW Europe.
Minyocythere maculosa (Bate, 1963)
Figs. 5C, D, 6D, 14, 15, 16, 21.
1941 Leptocythere? sp.; Triebel 1941: 333, pl. 7: 71, 72a, b.
?1949 “Ostr. 99”; Brand 1949: table 14.
1962 “Lophodentina”? sp. 99; Brand and Fahrion 1962: 136–137, pl. 20: 25; table 9.
1963 Dolocythere maculosa sp. nov.; Bate 1963: 205–206, text-figs. 8, 9, pl. 12: 1–11.
Type material: Holotype BMNH Io. 609; paratypes BMNH Io. 610–613 and Io. 856–875, for original description see Bate (1963: 205–206).
Type locality: Eastfield Quarry, South Cave, East Yorkshire, UK.
Type horizon: “Cave Oolite”, Bajocian.
Material.—UK: 9 C, 35 V, 1 V juvenile (near topotypic), collective number SMF Xe 23767, Everthorpe Quarry, South Cave, “Basement Beds”, Bajocian. NW Germany: 29 V including 2 V (SMF Xe1227 of Triebel 1941), Borehole Rodewald WA2, 390–395 m, Lower Bajocian, Witchellia laeviuscula Zone (Braun Jura γ); 2 C, 51 V, 2 V juvenile, Borehole Hambühren WA2 171–174 m, uppermost Aalenian (Braun Jura β); 2 C, 2 V, Borehole Lingen 330, 1014–1020 m, Lower Bajocian, Witchellia laeviuscula Zone (Braun Jura γ); 1 V, Borehole Fuhrberg 26, 178.75–179.15 m, Sonninia “sowerbyi” Zone, Lower Bajocian.
Original diagnosis.—A species of Dolocythere, slightly constricted in mid-dorsal/mid-ventral region and ornamented with large circular pits scattered over carapace (Bate 1963: 205).
Remarks.—The British type material is not very well preserved but Bate (1963: 205–206) provides a full description including details of hingement and marginal pores that match the definition herein of Minyocythere and its type species. He noted (Bate 1963: 206) “Surface of carapace covered with large, circular, rather shallow pits, within each of which is a large normal pore canal.” The nature of the normal pore canals as sieve-type was not mentioned, doubtless due to preservation. Our South Cave material revealed two types of StPC. StPC-M are visible on the exterior of the valve (Fig. 14A1), majority of round type. There are few oblong pores especially at the periphery of the valve. The valve in Fig. 14A1 shows approximately 70 round pores and 3–4 oblong ones. These pores are sunken in slight depressions (Fig. 14A2). The median diameter is 15.5 µm (n = 12) and the SI is 0.023 (15.5/650). Diameter of the setal pore is in range of 1.2 µm and is peripherally located (Fig. 14A2). The DI varies between 25–45 µm (n = 5), i.e. a “widely” dispersed pore pattern. On the inner side of the valve the StPC-M display a smaller size as compared to the outer side, namely less than 10 µm diameter. Figure 14B (UK material) displays for the inner side of the female valve the positions of the pore apertures around the central AMS; their diameter is less than 10 µm (cf. Fig. 5D, female, German material). The StPC-m on the valve exterior (Fig. 14A3) display a diameter in the range 5–6 µm. The setal pore has a subcentral position and is larger than those of the StPC-M. For example, in Fig. 14A3 the diameter of the StPC-m is 5.41 µm and the setal pore is 2.33 µm. Around the setal pore 16 tubuli with diameters between 0.3–0.4 µm are disposed in two incomplete rows.
Sexual dimorphism was not recognized by Bate (1963), but it is less well developed in our material than in M. macroporosa sp. nov or M. angulata sp. nov.
The British material is larger than the German specimens presumably for reasons of a more favourable or stable environment or nutrients (Fig. 15).
Triebel’s (1941) discussion of his “Leptocythere? sp.”, which first drew our attention to the early record of StPC, is translated in Appendix 2. We have analysed NPC in Triebel’s material (Figs. 5D, 6D, 14C–E, 16, Table 3) to compare with the British topotype material of M. maculosa. Figure 6D shows a simple, peripherally located NPC with a diameter of about 2.5 µm. The StPC-M in most cases are round and large and located in slightly depressed alveoles (Fig. 14C2, C3). There are a few oblong pores peripherally. The median diameter of the StPC-M apertures on both sides of the valve is about 12 µm and those of the SI 0.02 (Table 3). Setal pores (Fig. 14C2, C3) display median diameter values between 1.3–1.5 µm (Table 3). The StPC-M on the inner side of the valves of Triebel’s north German material are much larger than those of the UK topotype valves. The StPC-M display numerous tubuli with a diameter of 0.3–0.4 µm; median values are in the range 90 to 106 depending on the side of the valve observed (Table 3). Density of StPC-M on the lateral side of the valve is “low”, namely in the range of 50 to 65 pores, e.g., the male valve in Fig. 14C1 displays 55 pores, while the female (Fig. 14D1) has 52. The pores are widely spaced, with DI values similar to those of the topotype material. The two characteristic StPC-M in the antero-dorsal area (Fig. 6D) are visible on both sides of the valve. The StPC-m display a small size with median values for the diameters between 6–4 µm, depending of the side of the valves observed (Table 3). The small sieve plate displays between 12–23 visible tubuli around a large setal pore, with a median value of about 2.5 µm. The median value of SeP-SI is 0.45 (Table 3). We identified on the various valves between 8 and possibly 14 StPC-m with very stable location. Figure 6D shows this pattern of “stable” location as compared to the apparently “random” distribution of the StPC-M type.
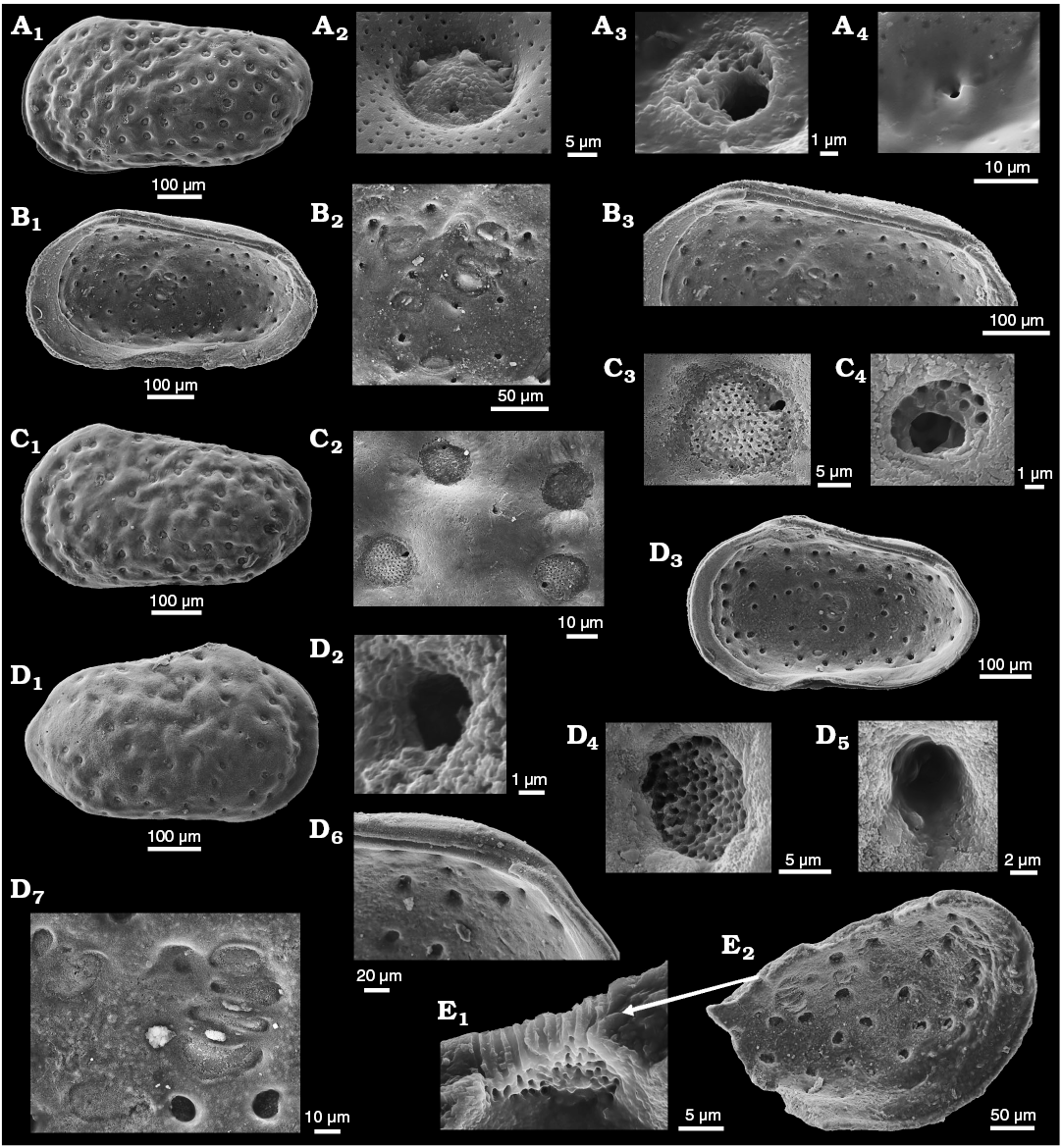
Fig. 14. Cytherid ostracod Minyocythere maculosa (Bate, 1963) from Everthorpe Quarry, South Cave, UK, Basement Beds, Bajocian (A, B) and Borehole Rodewald WA12, 395 m (C, D), 390 m (E), NW Germany, Witchellia laeviuscula Zone (Braun Jura γ), Lower Bajocian. A. Topotype, SMF Xe 23744, male left valve in external view (A1), macro sieve-type pore canals (StPC-M) (A2), micro sieve-type normal pore canals (StPC-m) (A3), and simple pore (A4). B. Topotype, SMF Xe 23745, female right valve in internal view (B1), muscle scars (B2), hinge (B3). C. SMF Xe 23746, male left valve in external view (C1), StPC-M (C2, C3), StPC-m (C4). D. SMF Xe 23747, female right valve in external (D1) and internal (D3) views, simple pore (D2), StPC-M (D4), StPC-m (D5), hinge, posterior part (D6), muscle scars (D7). E. SMF Xe 23748, interior of StPC-M (E1), fragment of a left valve in internal view (E2).
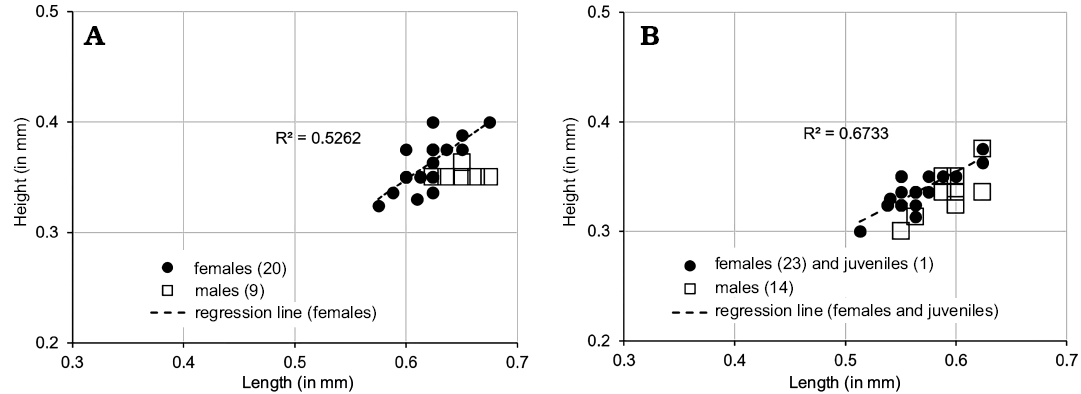
Fig. 15. Length versus height plots for Minyocythere maculosa (Bate, 1963) from UK (A) and Germany (B).
Table 3. Characterization of sieve-type normal pore canals (StPC) of Minyocythere maculosa (Bate, 1963), data only from German material. Abbreviations: StPC-m, small sieve-type normal pore canals with a large subcentral setal pore and reduced sieve plate; StPC-M, large round or rarely elongate sieve-type normal pore canals with a small excentric setal pore; Ex/In, view of the trait on the external/internal side of the valve; SI, Size Index (ratio of the pore diameter to the valve’s length); SeP, Setal pore of the StPC; SeP-SI, Setal Pore Size Index (ratio of the SeP diameter to the StPC diameter); diameter (in µm), expressed either as individual value or through the median statistics and min-max values; No, number of observations; Nt, number of tubuli; v, variate (single reading).
|
Properties |
StPC-M sample statistics |
StPC-m sample statistics |
|||
|
Morphological trait |
Specification |
No |
Median (min-max) |
No |
Median (min-max) |
|
StPC-size (Ex) |
diameter |
12 |
12.20 (8.10–17.40) |
6 |
6.35 (5.50–7.10) |
|
SI |
0.020 (0.013–0.029) |
0.010 (0.009–0.012) |
|||
|
StPC-size (In) |
diameter |
16 |
12.13 (8.63–15.01) |
10 |
4.06 (3.01–5.08) |
|
SI |
0.021 (0.015–0.027) |
0.007 (0.006–0.009) |
|||
|
SeP-size (Ex) |
diameter |
5 |
1.3 (0.90–1.89) |
4 |
2.56 (2.0–3.66) |
|
SeP-SI |
0.09 (0.08–0.12) |
0.45 (0.36–0.57) |
|||
|
SeP-size (In) |
diameter |
v |
1.51; 1.27 |
– |
– |
|
SeP-SI |
0.11; 0.12 |
– |
|||
|
StPC-tubuli (Ex) |
Nt |
4 |
106 (70–115) |
3 |
16 (12–23) |
|
StPC-tubuli (In) |
Nt |
3 |
90 (80–92) |
– |
– |
Figure 16 illustrates the development of juvenile valves of M. maculosa, the stage A-2 (L = 0.378), A-3 (L = 0.290), and A-4 (L = 0.240), using Triebel’s SMF material (Fig. 16A1, B1, C1). On these valves we analysed the size and the number of tubuli for StPC-M and StPC-m. In the A-2 stage (Fig. 16A2–A5), the StPC-m (Fig. 16A2) displays a size of about 3.7 µm with a setal pore of about 2 µm and 12 tubuli with a diameter of 0.3 µm. In this respect the StPC-m of the A-2 juvenile resemble those of the adult type (Figs. 14C2, C4, Table 3). The StPC-M in the Fig. 16A3, A4 display a sieve-type much smaller than in the adult, diameter in Fig. 16A4 is 5.5 µm and number of tubuli is about 40 with diameters of about 0.3 µm. The setal pore has a diameter close to those of the adults, namely 1.15 µm. The number of tubuli on the inner side of the A-2 (Fig. 16A5) valve is apparently in the range of those of the outer side seen in Fig. 16A4. In the A-4 valve we note that the StPC-m (Fig. 16C2) has a diameter of 3.6 µm, the setal pore has 1.5 µm diameter. We noted 12 tubuli with a diameter of 0.3 µm. The StPC-M (Fig. 16C3) displays a larger size than the StPC-m, with diameter 4.6 µm and number of tubuli about 26. The A-3 details in Fig. 16B2, B3 are not very significant but they contribute to the following interpretation of the development of the two types of StPC, namely the StPC-m approaches full development in A-2 for its size, the setal pore diameter and number of tubuli, whereas the StPC-M in the A-2 displays a size and number of tubuli which represents about the half of the dimension and the number seen in the adult pore.
Stratigraphic and geographic range.—Bajocian, Middle Jurassic; NW Europe. Bate (1967: Table 4) summarises the occurrence of M. maculosa in a number of Bajocian age units in Lincolnshire and eastern Yorkshire, UK, but the age range in terms of ammonite biostratigraphy is not well-defined as a consequence of variable marine connections (Cope et al. 1980; Rawson and Wright 1995); Lower Bajocian Witchellia laeviuscula–Otoites sauzei zones is a best estimate. Our German material from boreholes is also not closely age-constrained but the material studied is from the Upper Aalenian to Lower Bajocian, although “Lophodentina”? sp. 99 of Brand and Fahrion (1962) is reported as restricted to the Sonninia “sowerbyi” Zone, Lower Bajocian.
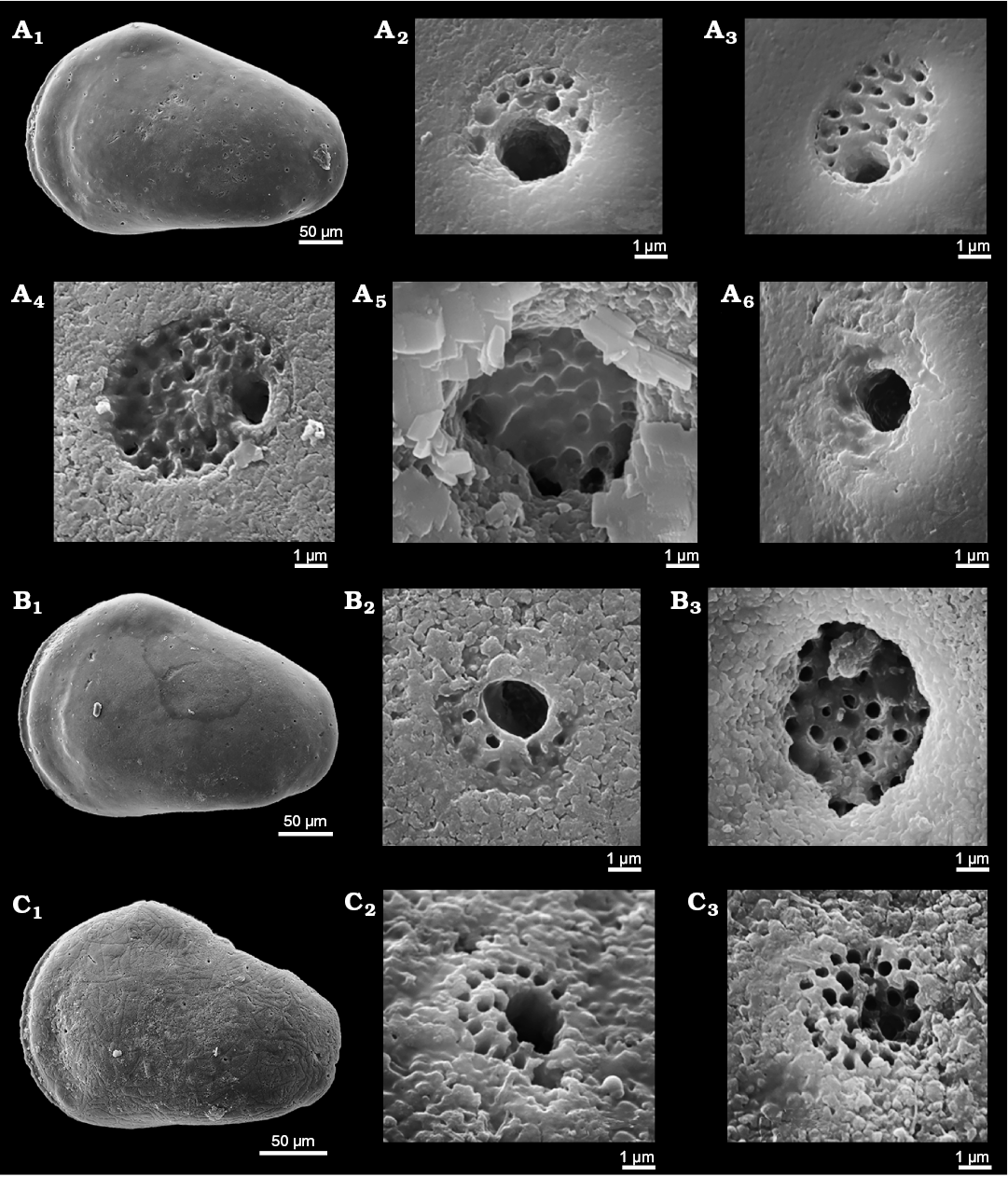
Fig. 16. Normal pore canals (NPC) on juvenile left valves (A, stage A-2; B, stage A-3; C, stage A-4) in cytherid ostracod Minyocythere maculosa (Bate, 1963) from Borehole Rodewald WA6, 390 m, NW Germany, Lower Bajocian (Braun Jura γ). A. SMF Xe 23751, general view (A1), micro sieve-type pore canals (StPC-m) on the outer side of the valve (A2), macro sieve-type normal pore canals (StPC-M) on the outer side of the valve (A3, A4), StPC-M on the inner side of the valve (A5), and simple NPC on the outer side of the valve (A6). B. SMF Xe 23752, general view (B1), StPC-m on the outer side of the valve (B2), StPC-M on the inner side of the valve (B3). C. SMF Xe 23753, general view (C1), StPC-m on the outer side of the valve (C2), StPC-M on the outer side of the valve (C3).
Minyocythere tuberculata comb. nov.
Figs. 6E, 17, 21.
2012 Dolocythere tuberculata sp. nov.; Luppold 2012: 222–223; text-fig. 6a, ?b; pl. 4: 9–12, pl. 6: 15, 16 [non pl. 4: 5–8 = M. macroporosa sp. nov. herein).
Holotype: BGR 16172, RV.
Type locality: Klein Schöppenstedt, Braunschweig, Germany.
Type horizon: Sonninia sowerbyi Zone, Lower Bajocian.
Other material.—5 V, Borehole Rodewald WA12, 404.5 m, Lower Bajocian, Witchellia laeviuscula Zone (Braun Jura γ); 2 V Borehole Hambühren WA2 150–155 m, lowermost Bajocian (Braun Jura γ). All, collective number SMF Xe 23767.
Original diagnosis.—“A species of the genus Dolocythere covered with unoriented surface swellings” (Luppold 2012: 222).
Emended diagnosis.—A species of Minyocythere characterized by a quadrate heavily calcified carapace and strong irregular surface swellings with large sieve-pores in the depressions.
Description of NPC.—Externally large StPC-M are located in the depressions between the prominent swellings (Figs. 6E, 17A1). The median diameter for the round pores is 14.16 µm (Table 4), which is very large when expressed in terms of the length of the supporting valve (median SI 0.025) and when this latter value is compared with those of the other three Minyocythere species (median SI 0.020). The shape of the sieve plate is generally convex and surrounded by a flat ring (Fig. 17A3). Note that for comparison with the other Minyocythere species we measured only the sieve diameter leaving out the peripheral ring. The setal pore of StPC-M is commonly visible while in most cases tubuli apertures are covered with fine sediment (Fig. 17A3). The size of the setal pore with a median of 1.4 µm and a SeP-SI of 0.095 (Table 4) is similar to those for M. angulata (Table 2) and M. maculosa (Table 3). The StPC-M pores also occur with oblong shape, however, this latter shape is less frequent than the round. From the 55 pores identified on the valve in Figs. 6E and 17A1, 58% were round and distributed mainly on the central part of the valve, while the oblong type dominates in the peripheral area. Considering the total number of StPC-M pore density is “low”. These latter are “widely” dispersed on the valve. The DI is always above 10 µm, commonly between 30 and 40 µm. The typical two StPC-M in the antero-dorsal area are clearly visible (Fig. 6E).
Table 4. Characterization of sieve-type normal pore canals (StPC) of Minyocythere tuberculata (Luppold, 2012). Abbreviations: StPC-M, macro sieve-type pore canals; StPC-m, micro sieve-type pore canals; Ex/In, view of the trait on the external/internal side of the valve; SI, Size Index (ratio of the pore diameter to the valve’s length); SeP, setal pore of the StPC; SeP-SI, Setal Pore Size Index (ratio of the SeP diameter to the StPC diameter); diameter (in µm), expressed either as individual value or through the median statistics and min-max values; No, number of observations.
|
Properties |
StPC-M sample statistics |
StPC-m sample statistics |
|||
|
Morphological trait |
Specification |
No |
Median (min-max) |
No |
Median (min-max) |
|
StPC-size (Ex) |
diameter |
10 |
14.16 (12.44–16.12) |
7 |
5.57 (4.84–6.60) |
|
SI |
0.025 (0.023–0.029) |
0.010 (0.009–0.012) |
|||
|
SeP-size (Ex) |
diameter |
8 |
1.4 (0.92–1.40) |
3 |
2.76 (2.07–2.76) |
|
SeP-SI |
0.095 (0.07–0.12) |
0.50 (0.43–0.50) |
|||
StPC-m (Fig. 17A2) occur on the swellings of the valve. This is very clear in the LV of Fig. 6E. They can be recognized by the small sieve plate formed by a slightly depressed ring and by the large subcentral setal pore (Fig. 17A2). The median size of the round StPC-m with about 5.5 µm (SI 0.01) and the median of the setal pore of 2.76 µm (SeP-SI 0.5) are in the range of the other Minyocythere species (Tables 1–4). We recognize in Fig. 17A2 ten tubuli with a diameter approaching 0.3 µm. Figure 6E shows the position of 12 StPC-m.
Simple NPC were not observed on the outer sides of the valves.
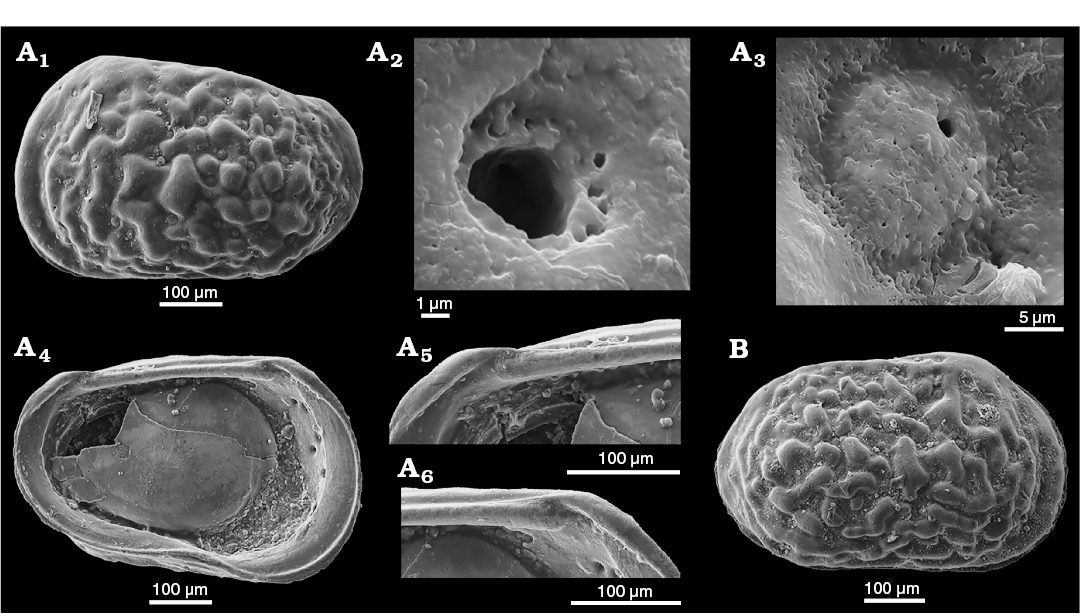
Fig. 17. Cytherid ostracod Minyocythere tuberculata (Luppold, 2012) from Borehole Rodewald WA12, 404.5 m, NW Germany, Lower Bajocian. A. SMF Xe 23754, female left valve in external (A1) and internal (A4) views, micro sieve-type pore canals (StPC-m) (A2), macro sieve-type normal pore canals (StPC-M) (A3), hinge, posterior (A5) and anterior (A6) parts. B. SMF Xe 23755, female right valve in external view.
Remarks.—We could not describe the pores on the interior of M. tuberculata because of lack of adequate clean material. However, for internal views of StPC Luppold (2012: pl. 4: 11) figures large StPC-M resembling those of other Minyocythere species. Luppold (2012: pl. 4: 5–8) figures juveniles of M. tuberculata that we consider to be adults of M. macroporosa sp. nov.
Stratigraphic and geographic range.—Lower Bajocian; NW Germany.
Genus Dolocythere Mertens, 1956
Type species: Dolocythere rara Mertens, 1956; Borehole Lingen 106, Lower Albian.
Diagnosis.—See Appendix 1.
Remarks.—Described from the Lower Albian of NW Germany, a number of other Cretaceous species have been assigned to the genus (Deroo 1966; Gründel 1966; Colin 1974; Kubiatowicz 1983; Babinot and Colin 2011) but only two Jurassic species: D. maculosa Bate, 1963 and D. tuberculata Luppold, 2012 from the Bajocian of England and NW Germany respectively, both of which we consider to belong in Minyocythere gen. nov. Dolocythere is therefore a Cretaceous genus. As we have not made a study of Dolocythere, except to determine its recognition from Minyocythere, we are not able to refine its diagnosis or discuss its species, however, in looking at Senckenberg material of D. rara we found material of a new species recognized by Erich Triebel (SMF collections) and which we describe below as D. amphistiela sp. nov. However, we have analysed the NPC in both D. rara and D. amphistiela in order to (i) compare with Minyocythere species and (ii) with each other.
Stratigraphic and geographic range.—Cretaceous, ?Western Europe.
Dolocythere rara Mertens, 1956
Figs. 4A, 5A, 11B, 18, 19.
1956 Dolocythere rara sp. nov.; Mertens 1956: 192–193; pl. 10: 33–37; pl. 13: 91–93.
?1964 Dolocythere rara Mertens; Kaye 1964: 322; pl. 55: 12, 14, 15.
?2009 Dolocythere rara Mertens; Slipper 2009: 335–336; pl. 3: 6.
Type material: Holotype BGR T.-K. 1298, C; paratypes: BGR T.-K. 1299, 1300, LV and RV female, respectively (Figs. 4A, 5A, 18A, B).
Type locality: Borehole Lingen 196, 697–704 m, Germany.
Type horizon: Lower Albian.
Material.—More than 250 C, V, juvenile, SMF collection, different localities and age.
Description of NPC.—Exterior: Minute NPC are visible on the ridges of the reticulated valve (Fig. 18A). Their apertures are less than 5 µm and can be differentiated from the larger alveolae belonging to the reticulum with diameters in the range of 6–8 µm (Fig. 18A3, A4). The solum of the alveolar reticulum display small round granules (Fig. 18A2–A4) while the punctae with a diameter of about 3 µm have a smooth bottom (Fig. 18B2). Simple normal pores have a size range of 1–2 µm (Fig. 18C3). Small StPC have diameter of about 3–4 µm with a large setal pore of about 1.5 µm (Fig. 18C2) and a reduced surrounding sieve plate represented by 1–2 rows of tubuli. In the figured example the StPC has a diameter of 1.8 µm and 5 tubuli with an inner diameter in the range of 0.3–0.4 µm. The SI for this latter StPC is 0.006 and the SeP-SI is 0.58, a value which is similar to those of Minyocythere species. Therefore, the small StPC of D. rara is in our opinion homologous to the StPC-m of Minyocythere species. The density of NPC visible on the external side of the valves is “low”. For the LV in Fig. 18C1 we counted 30 entities, without making a distinction between the simple NPC and the StPC-m. Also, the number of pores could be slightly underestimated due to the difficulty in observing the apertures on the complicated reticular surface of the valve. The DI ranges about 40 to 75 µm, pointing to the “wide” dispersion of these pores on the surface of the valve.
Interior: Figure 11B1 is the internal view of the valve in Fig. 18C1. We counted 32 small pores, and this difference to the number counted on the exterior is due to the better visibility of the apertures internally. Also, the total number of pores is slightly underestimated because the anterior peripheral area is not visible due to the inner lamella (the marginal infold of Yamada 2007). For one of the pore canals which probably relates to the outer StPC we measured a diameter of 4.4 µm (SI 0.009). An important observation is the stable position of several pore apertures around the central AMS. Figure 11B2 is an enlargement of the central section marked on Fig. 11B1 to show two apertures (black arrows, one above the AMS and one below the AMS). They can be recognized in Triebel’s material from Borehole Rodewald WA6, 206 m (Figs. 11B1 and 18C1) and also on the paratype of D. rara of Mertens (1956), Fig. 18A5, A6 here. In all these cases there are two apical pores which appear to be diagnostic for this species, because they do not appear in the same area of D. amphistiela sp. nov. (Figs. 5B2 and 11D). Interestingly, if Fig. 18A6 is enlarged for detail of the pore located posterior to the AMS of D. rara, inside the channel there is a sieve plate with 4–5 apertures, the largest having a diameter of 0.96 µm. The diameter of this pore from a specimen in the Mertens collection displays the same size as those measured on a valve from Triebel’s material, namely 4.4 µm. The DI for the pores visible on the valve interior display values similar to those for the external side, between 30 and 60 µm with an average of 42 µm. Therefore the values for density and dispersion of the pores on the interior match the data measured on the outer side of the same valve.
Remarks.—We figure here paratypic material from the Mertens collection and also specimens identified by Triebel (SMF collections) as D. rara that we consider to be conspecific. The problem with the original figures of Mertens (1956) is partly shape of outline (due to photographs cut out and mounted on a black background) and lack of definition of the surface morphology and ornament.
D. rara of Slipper (2009: pl. 3: 6) from the British Aptian has an eyespot and may represent a different species, whereas our material lacks an eyespot and ocular sinus. Two valves we figure (Fig. 18E, F) are slightly larger, appear to show eye spots and are intermediate in shape between D. rara and D. amphistiela sp. nov. and may represent another species.
Stratigraphic and geographic range.—Aptian–Lower Albian, Lower Cretaceous; NW Europe.
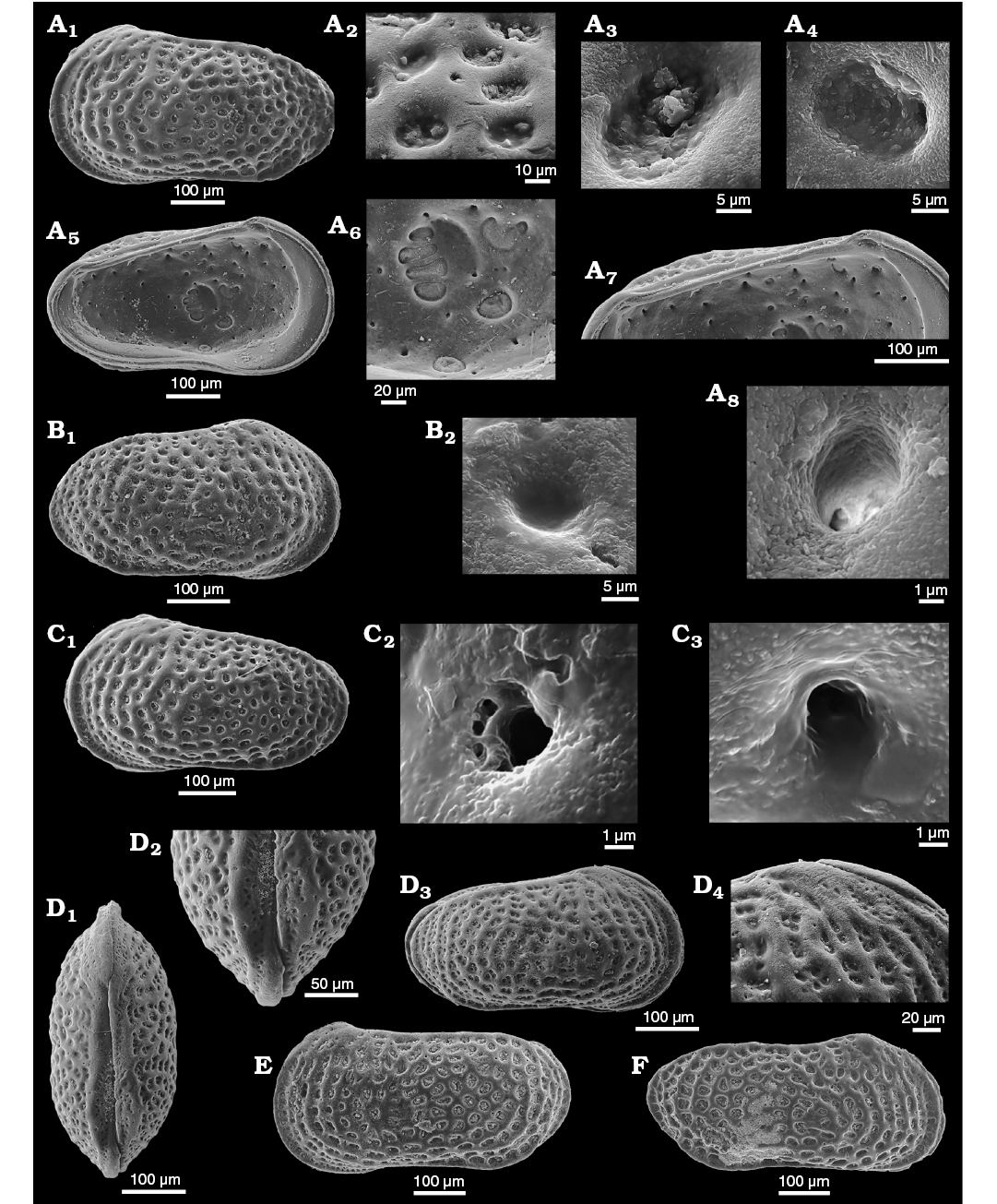
Fig. 18. Cytherid ostracod Dolocythere rara Mertens, 1956 from Borehole Lingen 196, 697–704 m (A, B), Borehole Rodewald WA6, 206 m (C), WA7, 383.7 m (E), Borehole ZW Losser 1, 256.4–258.4 m (F) from NW Germany, Lower Albian and Ziegelei Bekum (D) from NW Germany, Upper Aptian. A. Paratype, BGR T.-K. 1300 (Mertens Collection, Hannover), female left valve in external (A1) and internal (A5) views, ornamentation (A2–A4), muscle scars (A6), hinge (A7), small sieve-type pore canals (StPC) (cf. micro sieve-type normal pore canals StPC-m) (A8). B. Paratype, BGR T.-K. 1300 (Mertens Collection, Hannover), female right valve in external view (B1), ornamentation (B2). C. SMF Xe 23756, male left valve in external view (C1), small StPC (cf. StPC-m) (C2), simple pore (C3). D. SMF Xe 23757, male carapace in dorsal (D1) and right (D3) views, posterior zone (D2), antero-dorsal area (D4). E. SMF Xe 23758, female? left valve in external view, showing a form that may be a large D. rara or a different species. F. SMF Xe 23759, male right valve in external view, showing a form that may be a large D. amphistiela sp. nov. or a different species.
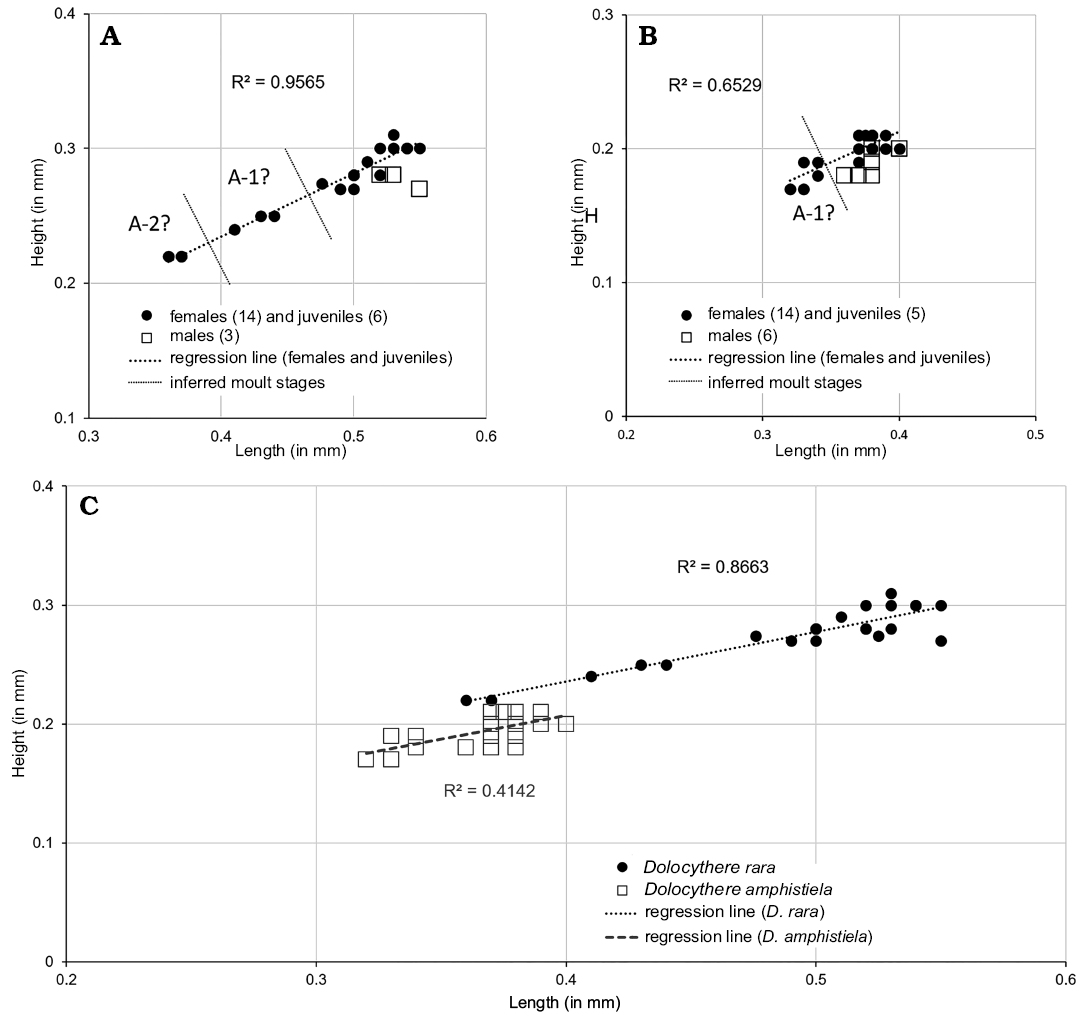
Fig. 19. Length versus height plots for Dolocythere rara Mertens, 1956 (A) and Dolocythere amphistiela sp. nov. (B), comparison (C). A-1, A-2, estimated growth (moult) stages.
Dolocythere amphistiela sp. nov.
Figs. 5B, 11D, 19, 20.
?1964 Dolocythere rara Mertens; Kaye 1964: 322; pl. 55: 12.
Zoobank LSID: urn:lsid:zoobank.org:act:3987244A-FA91-48F1-983B- C098CD0E358A
Etymology: From Ancient Greek ἀμφί (amphi), on both sides and stiela, in reference to ancient Greek coins.
Type material: Holotype, SMF Xe 23761, LV male (Fig. 20B). Paratypes: SMF Xe 23760, RV male (Fig. 20A); SMF Xe 23762, LV female (Fig. 20C); SMF Xe 23763, RV female (Fig. 20D); SMF Xe 23764, RV female (Fig. 20E); SMF Xe 23765, LV male (Fig. 20F); SMF Xe 23766, LV male (Fig. 20G); from type locality and horizon.
Type locality: Borehole Rodewald WA4, 187 m depth, Germany.
Type horizon: Leymeriella tardefurcata Zone, Lower Albian.
Other material.—140 V, 74 V juvenile, collective number SMF Xe 23767, NW Germany: Borehole Rodewald WA4, 187 m; Borehole Rodewald WA6, 203–236 m; Borehole Rodewald WA7, 245–311 m; Borehole Rodewald WA11, 184–222 m; Borehole Rodewald WA13, 211–227 m; Leymeriella tardefurcata Zone, Lower Albian.
Diagnosis.—A species of Dolocythere characterized by a strongly reticulate ornament in which an irregular rib runs diagonally from near the posterior cardinal angle to the mid antero-ventral area.
Dimensions (in mm).—Females: L = 0.370–0.400, H = 0.190–0.210 (SMF Xe 23762, L = 0.390, H = 0.210; SMF Xe 23763, L = 0.380, H = 0.210; SMF Xe 23764, L = 0.370, H = 0.200). Males: L = 0.360–0.400, H = 0.180–0.200 (SMF Xe 23761, L = 0.380, H = 0.200; SMF Xe 23760, L = 0.380, H = 0.190; SMF Xe 23765, L = 0.400, H = 0.200; SMF Xe 23766, L = 0.370, H = 0.190).
Description.—Exterior: Elongate in lateral view, greatest height at anterior cardinal angle, greatest length at mid-height; anterior margin broadly and symmetrically rounded with wide marginal rims on both valves, dorsal margin concave medianly on both valves which coincides with a ventral margin concavity giving the valves a “waisted” appearance in lateral view especially in males; posterior symmetrically rounded in LV or slightly inclined ventrally in RV; ventral margin weakly concave; small marginal rim on postero-ventral margin; LV and RV similar in shape and size, overlap difficult to determine as only valves found. Sexual dimorphism present and strongly expressed, with males longer and less high in lateral view than females (Fig. 19), sexes similar in dorsal view. Juveniles weakly ornamented compared to adults. Surface of adults with a strong reticulum with rounded sola containing small granules, ornament strongly developed in mid-valve area but weakens towards anterior and posterior margins with smaller sola; an irregular rib runs diagonally from near the posterior cardinal angle to the mid antero-ventral area.
Normal pores occur on the muri: small StPC-m (Fig. 20D2, D3) display a diameter of about 3 µm. The sieve is minimally visible. In Fig. 20D3 the StPC-m displays a small peripheral plate with 4 tubuli visible. The diameter of the setal pore is 1.6µm and those of tubuli 0.2 µm. Smaller pores of a diameter in the range of 1.5 µm represent simple NPC (Fig. 20D4). The SI for the pore of Fig. 20D3 belonging to a valve of 0.380 mm (Fig 20D1) is 0.008 and the SeP-SI is 0.5. The density and the dispersion of the pores could not be counted with accuracy, because of poor preservation of the apertures. We recognized 12 pores, this number compared to those identified on the inner side of the valve underestimates the real number.
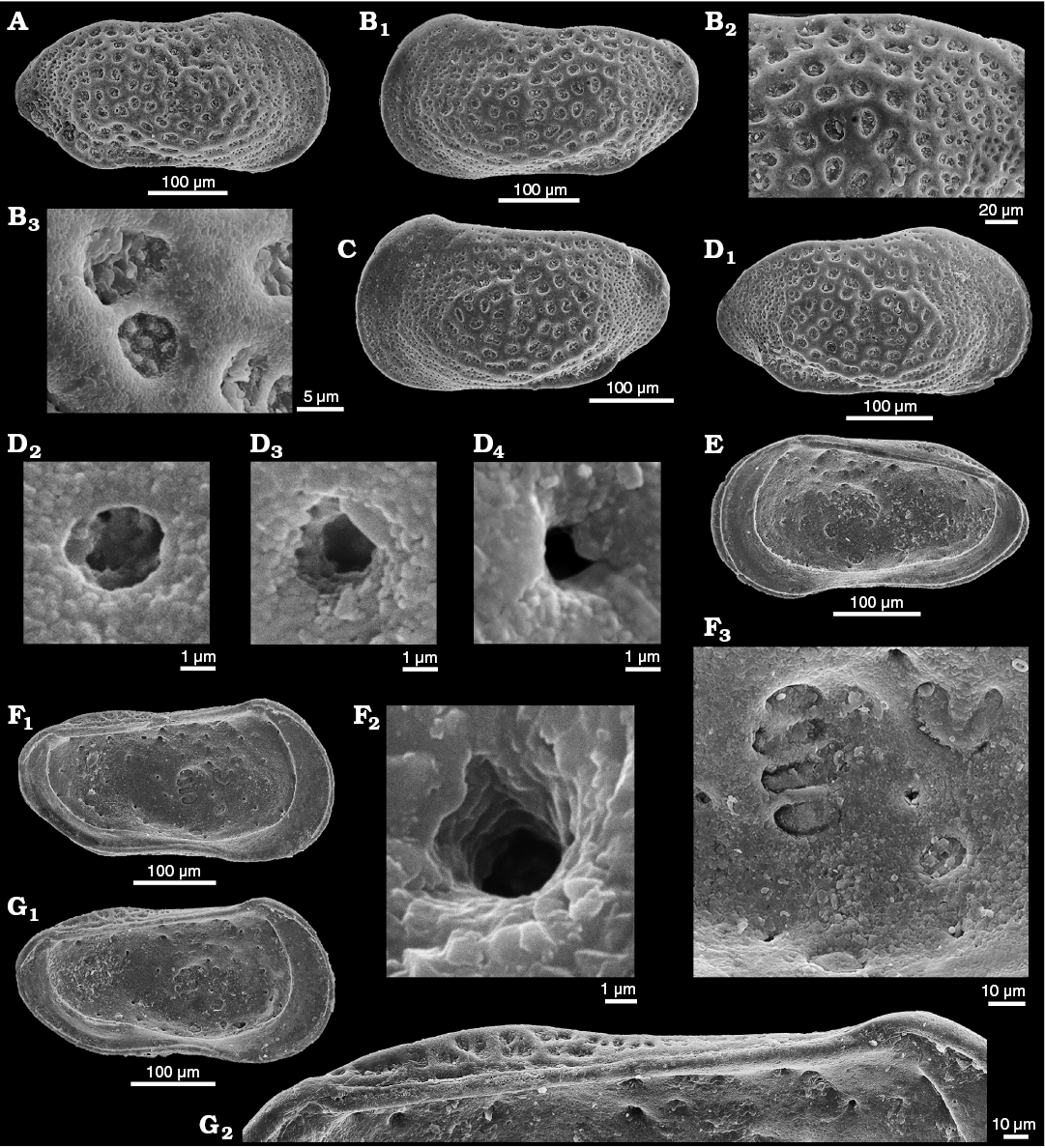
Fig. 20. Cytherid ostracod Dolocythere amphistiela sp. nov. from Borehole Rodewald WA4, 187 m, NW Germany Leymeriella tardefurcata Zone, Lower Albian. A. Paratype, SMF Xe 23760, male right valve in external view. B. Holotype, SMF Xe 23761, male left valve in external view (B1), dorsal-posterior area (B2), ornamentation (B3). C. Paratype, SMF Xe 23762, female left valve in external view. D. Paratype, SMF Xe 23763, female right valve in external view (D1), small StPC (cf. micro sieve-type normal pore canals StPC-m) (D2, D3), simple pore (D4). E. Paratype, SMF Xe 23764, female right valve in internal view. F. Paratype, SMF Xe 23765, male left valve in internal view (F1), small StPC (cf. StPC-m) (F2), muscle scars (F3). G. Paratype, SMF Xe 23766, male left valve in internal view (G1), hinge (G2).
Interior: Internally the valves show depressions that match the positions of StPC externally. Marginal zone well developed anteriorly and posteriorly, inner margin and line of concrescence coincide; marginal pore canals straight, 12 anteriorly and 6 posteriorly. AMS (Fig. 5B) consist of a vertical row of four rounded-elongate scars, with a U-shaped frontal scar that may comprise two scars one large and U-shaped with an accompanying small rounded scar anteriorly, with ventrally two rounded mandibular scars; between the vertical row of scars and the frontal scars there is an elongate depression that represents the articulation point (median depression = fulcral point) of the mandible. Hinge tripartite, relatively strongly developed lophodont: LV short terminal sockets and long smooth median bar, RV short smooth terminal peg-like teeth that merge distally into the free margin and a smooth median groove.
NPC can be observed in Figs. 5B, 11D, and 20F. In Fig. 11D the white arrows indicate the apertures of the pores. Importantly, no apical pores exist at the extremity of the AMS, namely the pores observed in D. rara are not present in D. amphistiela (Figs. 20F1, F3, 11D). Small pores (n = 31) occur on the inner side of the valve in Fig. 11D. Therefore, pore density for D. amphistiela is of the “low” type. The diameter of a NPC inner aperture is 2.86 µm (Fig. 20F2), SI for this pore is 0.007. These values correspond closely with those measured on the exterior of the valve, and even if we cannot see the sieve plate we can consider the aperture as belonging to a StPC-m. The DI values are typical for “widely” spaced pores, between about 25 and 45 µm with an average of 33.4 µm (n = 5). To compare the degree of dispersion of NPC on D. amphistiela with D. rara, we expressed the data as a ratio of the average of the values for the DI to the length of the valve on which we measured the pore dispersion. For D. amphistiela valve length is 400 µm (cf. Fig. 20F1) and the scaled value is 0.0835 (33.4/400). For D. rara the average of the DI is 42 µm (n = 5) and the valve length is 500 µm (Fig. 11B1), hence the scaled value is 0.084. Therefore the degree of dispersion of the NPC on the interior of the valves of the two Dolocythere species is surprisingly similar. This result is a good example of the value of analysing data using both interval and ratio scales.
Remarks.—D. amphistiela sp. nov. differs from D. rara in strength and arrangement of the surface reticulate ornament, in lateral outline and in the strength of the postero-ventral marginal rim. Both D. amphistiela and D. rara have postero-ventral rims or keels which seems to be a generic feature (cf. D. cristata Colin, 1974). Additionally, D. amphistiela lacks the two apical points at the extremities of the AMS in the valve interior.
Borehole material in the Senckenberg Forschungsinstitut suggests that a number of Dolocythere morphotypes occur in the Albian. No carapaces observed which is probably a taphonomic feature.
Stratigraphic and geographic range.—Lower Albian, Lower Cretaceous; NW Germany.
Discussion
In describing the new genus Minyocythere we find ourselves again looking at Jurassic material, supposedly rather well-known, from historical collections where much new is still to be discovered (e.g., Cabral et al. 2014). The material is all from cored boreholes and the only limitation is that the lithostratigraphic succession is generalised and there is little biostratigraphic control. All four (?five) Minyocythere species occur in Borehole Hambühren WA2 from NW Germany in the sequence: Minyocythere angulata (206–177 m), M. sp. cf. M. macroporosa (198–192 m), M. macroporosa (174–158 m), M. maculosa (174–171 m), and M. tuberculata (154–150 m) (Upper Aalenian to Lower Bajocian), which corresponds to a sequence of surface ornamental change from almost smooth to strongly ridged (Fig. 21). This trait may be environmentally cued, for example in relation to availability of calcium carbonate or varying environmental stress, but with few sedimentological data available it is difficult to tell. Other records are patchy in NW Germany, from one borehole in The Netherlands and outcrops in eastern Britain. Only in Borehole Rodewald WA12 do we have three of the taxa together: Minyocythere sp. cf. M. macroporosa, M. maculosa, and M. tuberculata, but in a different stratigraphical sequence. Luppold (2012) as reinterpreted here records M. macroporosa and M. tuberculata from the Lower Bajocian near Braunschweig, NW Germany. It is therefore difficult to make meaningful discussion of the evolutionary pattern of Minyocythere and its species in time and space beyond NW Europe in the Aalenian and Bajocian. The genus may be related to Aphelocythere, with which it shares StPC, by simplification of hinge type but we have not observed intermediate forms. Similarly, Minyocythere may be ancestral to Dolocythere but we have as yet no direct evidence.
In the introductory parts we focused on NPC, especially StPC as seen in Minyocythere gen. nov., the discussion below enlarges to include other groups in the superfamily Cytheroidea and more specifically the family Cytheridae.
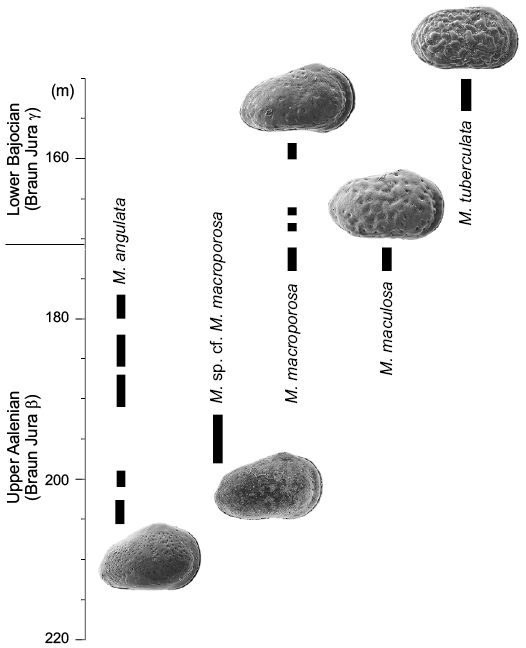
Fig. 21. Stratigraphic distribution of Minyocythere angulata sp. nov., Minyocythere sp. cf. M. macroporosa sp. nov., Minyocythere macroporosa sp. nov., Minyocythere maculosa (Bate, 1963), and Minyocythere tuberculata (Luppold, 2012) in Borehole Hambühren WA2, NW Germany.
Implications for comparative morphology.—We have demonstrated that StPC of Minyocythere species are visible on both sides of the valves and it is important to document this in all NPC investigations as it brings useful complementary information. In Minyocythere StPC are two distinct types of morphological entities, characterized as StPC-M and StPC-m, not only reflecting their size but also their distinctive structure and in one case (M. maculosa) their different developmental trajectories. A synthesis of these characteristics in Minyocythere is presented in Table 5.
Table 5. Synthesis of sieve-type pore canals-Macro (StPC-M) and sieve-type pore canals-micro (StPC-m) of Minyocythere species. Abbreviations: SI, Size Index; SeP, setal pore of the StPC; SeP-SI, Setal Pore Size Index (ratio of the SeP diameter to the StPC diameter).
|
Properties |
StPC-M |
StPC-m |
|||
|
Trait No |
Morphological trait |
Specification |
Characteristics and approximate values |
||
|
1 |
StPC-size |
diameter |
large (~ 10–14 µm) |
small (~ 5–7 µm) |
|
|
2 |
SI |
~ 0.02 |
~ 0.01 |
||
|
3 |
SeP-size |
diameter |
small (~ 1–1.5 µm) |
large (~ 2–3 µm) |
|
|
4 |
SeP-SI |
~ 0.1 |
~ 0.3–0.6 |
||
|
5 |
SeP within StPC |
position |
peripheral position |
subcentral position |
|
|
6 |
StPC-tubuli |
N° |
large (~ 50–>100) |
small (~ <25) |
|
|
7 |
position |
covering whole area |
2–3 incomplete rows |
||
|
8 |
StPC-shape |
round/oblong |
round and oblong |
round |
|
|
9 |
StPC-density |
N° / valve |
~ 60–70 |
~ 8–13 |
|
In the case of M. maculosa the developmental trajectory for the StPC-m of the A-2 juvenile stage resembles those of the A-1 and the adult. The situation is different for the StPC-M which in the A-2 stage displays an unfinished, undeveloped sieve-plate with a reduced number of tubuli, about 50, whereas in adults we note higher values close to 100 (cf. Table 3).
The difference of the aperture size of the setal pore between StPC-M and StPC-m resembles those observed by Danielopol et al. (2018) in the case of the pores A1 and A2 of Gomphocythere besni Külköylüoğlu, Yavuzatmaca, Cabral and Colin, 2015 (Limnocytheridae, Timiriaseviinae).
The presence of two types of StPC seen in Minyocythere species also occurs in the species belonging to the slightly older Jurassic genus Camptocythere Triebel, 1950, namely C. praecox Triebel, 1950 and C. media Triebel, 1950 (cf. Fig. 3B, D) and the clearly older Jurassic genus Phraterfabanella Whatley and Boomer in Boomer et al., 2001 (cf. Fig. 2A3, A4; P. tridentinensis Whatley and Boomer in Boomer et al., 2001).
The Cretaceous Dolocythere species described here display only simple NPC and small StPC which resemble the StPC-m of Minyocythere species. These pores in the case of the reticulated species of Dolocythere are located on the tectum of the reticular muri. This position resembles those of Minyocythere tuberculata where generally only the StPC-m are located on the surface swellings.
The Neogene to Recent Cythere species were documented by Japanese workers (Tsukagoshi and Ikeya 1987, 1991; Ikeya and Tsukagoshi 1988; Tsukagoshi 1990). Tsukagoshi and Ikeya (1991) recognize five types of NPC: (i) and (ii) simple with different setae, (iii) funnel-type with a well-developed rim, (iv) sieve pore, round plate with a simple stout seta, and (v) sieve pore, elliptical plate with a branched seta. The spatial distribution of StPC-m on the valves of Minyocythere species with approximately stable positions, resembles that of the funnel and rimmed pores of the Cythere species described by Tsukagoshi and Ikeya (1987, 1991) and Tsukagoshi (1990, 1998) as well as the similar pore system of Schizocythere ikeyai Tsukagoshi and Briggs, 1998. This similarity of location between the StPC-m of Minyocythere and those of the above mentioned cytherids is interpreted here as a possible case of homology. If we accept this view then we could interpret the stable positions of the StPC-m of Minyocythere as a morphological trait which could have evolved into rimmed type NPC as seen in the living Cythere and Schizocythere taxa mentioned above and as described for the A2-type pores of the Timiriaseviinae (Danielopol et al. 2018). Therefore we hypothesize that the StPC-m could be considered an old morpho-functional trait.
Implications for systematics.—We understand the term “systematics” to mean the search for a coherent pattern of relationships between taxonomic entities, which should reflect as far as possible phylogenetic relationships, and which is achieved by careful comparative morphological descriptions leading to robust taxonomies.
NPC, simple or StPC, have been used as presence/absence features to discriminate taxa at various systematic levels which demonstrates that some workers have considered them of value, e.g., Hartmann (1963), Hanai (1970), followed by later workers including Puri (1974) and Keyser (1980). Tsukagoshi and Ikeya (1987) and Tsukagoshi (1990) studying species of Cythere mapped the distribution of StPC as a criterion for species differentiation in Cythere. Tsukagoshi (1990) traced the ontogenetic development of NPC which allowed him to reconstruct the phylogeny of eleven living and three extinct species of Cythere. Similarly, Kamiya (1989) and Kamiya and Hazel (1992) mapped NPC distribution patterns in Loxoconcha. Martens (1989) used StPC as a diagnostic character for Ovambocythere Martens, 1989.
For NPC to have a practical usage in the systematics of, say, an ostracod family, in this case the Cytheridae, a necessary assumption is that they display a stable genetic and developmental expression. Solid evidence for such an assumption is in the case of fossil ostracods generally difficult to obtain, other than via the limited carapace characteristics. Therefore, morphological traits for the systematics of fossil ostracod taxa, in many cases, take on the status of “risky hypothesis” (in the meaning of Godfrey-Smith 2003). This means that we accept them under the premise that additional investigations will bring firmer confirmation or alternatively will be “falsified” (unconfirmed).
The following arguments derived from the present study favour the usage of NPC for systematics of Jurassic and Cretaceous ostracods:
(i) At the species level the differences of NPC morphologic traits are not always very strong. The position of StPC-m on the surface of M. macroporosa is not related to the positive ornamental features on the exterior of the valve, while in the case of M. maculosa most of the StPC-m are located on the top of the positive ornamental features. In the case M. tuberculata the position of the StPC-m is always on the top of the positive features. Therefore these differences in position of the StPC-m in relation to the presence or absence of swollen ridges of different species of Minyocythere could be added to the other diagnostic traits which differentiate the four (or possible five) new species.
(ii) Minyocythere angulata differs from M. macroporosa and M. maculosa by its high number of oblong StPC-M distributed over the whole external surface of the valve. The latter two species display on the central part of the valves mainly round-shaped StPC-M and only at the margins of the valve do oblong entities commonly occur.
(iii) At generic level we note interesting differences between the structural type of the StPC of Minyocythere and Dolocythere, namely species of the former display both types of pores, StPC-M and StPC-m, while the latter has only small StPC-m. Cythere and Schizocythere species (cited above) display instead of StPC-m only NPC surrounded by a rim (Type A” of Puri and Dickau 1969).
(iv) Considering only the spatial distribution of the StPC-M on the external antero-dorsal part of the valve it is possible to differentiate at a generic level Cythere from Minyocythere. The former displays several StPC-M in the antero-dorsal area while the latter lacks such pores. Minyocythere species display in the antero-dorsal area two StPC-M, while the taxa of Cythere generally display three such pores (see Tsukagoshi and Ikeya 1987). Therefore Minyocythere, with its four (?five) species, displays several morphological peculiarities expressed by the NPC, which strengthens the diagnosis of the genus proposed. Minyocythere appears to us as a well characterized systematic unit within which the species we described reflect phylogenetic affinities.
(v) The genus Camptocythere Triebel, 1950 was considered, following the original description of six species from the Middle Jurassic of Europe by Triebel (1950), as belonging to the family Loxoconchidae Sars, 1926. This view was accepted by later workers (Van Morkhoven 1962; Hartmann 1975), however, recent investigations by Ozawa and Ishii (2008), Ozawa and Kamiya (2013) and Lee and Tsukagoshi (2014, 2018) show that loxoconchids possess only StPC-M and not StPC-m. Therefore Camptocythere taxa for which we document here two types of StPC, similar to those existing in Minyocythere species, are probably not loxoconchids. The taxonomic status of this genus requires review.
Implications for palaeobiology.—The paleobiological information to be gained from deeptime fossil material is necessarily limited. We discuss below the basis for posing questions on the origin and function of the NPC of ostracods.
We start with the question why in the case of Minyocythere, and by extension also in the case of Camptocythere species, more than one kind of NPC exists in one animal, namely the presence of StPC-M and StPC-m? What exactly are the StPC-m, are they just a poorly developed variant of the more common StPC-M? Given that the calcified valves represent, from the ecdysis process, a frozen moment in the growth/development of the animal, the small StPC might be the expression of an arrested growth stage. We figure (Fig. 7F) a juvenile valve with two different kinds of StPC, however, this argument cannot apply to the final growth stage. Therefore, for the moment we can only speculate that the diversity of NPC on the valves is an aspect of the animals structural complexity. This latter concept is understood with the meaning given by McShea and Brandon (2010: 2), that it “is a function only of the amount of differentiation among parts within an individual. Or in the special case in which variation is discontinuous, complexity is the number of part types”. Considering the disparity of NPC we note that these structural entities integrate not only the part of the valve we observe “frozen” in the case of the fossil material but also the sensory part represented by a sensillum which traverses the normal pore. To get a better perception of the disparity of NPC of the family Cytheridae (to which most of our studied material belongs) we refer again to the data on living Cythere of Ikeya and Tsukagoshi (1988), Tsukagoshi and Ikeya (1991) and Tsukagoshi (1990, 1998). Tsukagoshi and Ikeya (1991) showed that there is a relationship between the shape of StPC and the structure of the sensorial seta which traverse them, namely the round-shaped StPC is traversed by a uniramous seta while the elliptical StPC displays a biramous seta.
In the case of Minyocythere species we note that the degree of structural complexity is represented by three types of NPC (one of them presenting two shape types). Unfortunately we cannot asssess the functional difference between the StPC-M and the StPC-m of Minyocythere, although it was clearly important as the two types of pores display clear differences as shown in the descriptions of the new Minyocythere species.
The structural complexity of the Minyocythere pore types is greater than those of the Dolocythere species which do not display StPC-M. Did this difference of structural (and most probably functional) complexity between these ostracod groups play a role in the way species perceived their surrounding environment? This question has a biological meaning for which we do not have an answer at present.
Implications for environmental interpretation.—StPC occur in both marine and non-marine taxa, but what is the common function, if any?
We have referred to the numerous studies on Cyprideis torosa following the work of Rosenfeld and Vesper (1977) and subsequent authors on StPC and salinity. All these works are interesting and may be useful but there can be no convincing application to environmental interpretation, modelling or monitoring without an understanding of the causal relationship between StPC shape and, in this case, salinity. It is difficult to see how real progress can be made in applying normal and marginal pore canals to systematics or to environmental matters until fundamental work is done on the function(s) of the canals and their setae.
Danielopol et al. (2018) provide a descriptive model that the total surface area and number of tubuli belonging to a StPC could be related to the ionic concentration of the aquatic environment where the ostracods develop their carapaces. It is not the external shape of the StPC that is the decisive trait, that could be used as a finger-print for salinity or ionic concentration of the host water, but the total surface of the tubuli’s apertures through which the aqueous solution is transported. This model needs to be tested on other ostracod species.
Conclusions
In the Introduction we posed the following questions: (i) When did StPC first evolve? (ii) What was their adaptive significance? (iii) Why did not all ostracods develop this feature? (iv) What is the taxonomic importance of StPC? Our conclusions are: (i) StPC apparently first appeared in the Carboniferous in podocopids, Ordovician in palaeocopids (but are all palaeocopids ostracods?). (ii) Adaptive significance of StPC not clear until function(s) known in living animals. (iii) Why are StPC not present in all ostracods is not clear and may reflect life style, habitat and habitat stability. (iv) Taxonomic importance of StPC depends on the taxonomic unit and is not uniformly applicable. The same conclusion applies to contact grooves versus simple versus complex hingement or to complex marginal zones with vestibules, which must be significant but it is not yet clear how or why.
In defining Minyocythere gen. nov. we recognize four (?five) constituent species of which two (?three) are new: M. macroporosa, M. sp. cf. M. macroporosa, and M. angulata. In the process of defining Minyocythere we have looked at the type material of Dolocythere Mertens, 1956 and refigured its type species D. rara and described M. amphistiela sp. nov. We conclude that Dolocythere is a Cretaceous genus and Minyocythere a Jurassic genus that may be ancestral to the former. The species assigned to Dolocythere in the literature require reevaluation in order to redefine the genus in modern terms.
This study reveals that by the Toarcian (late Early Jurassic) StPC were not only rather common in cytheroid ostracods but present in certainly three forms (namely in terms of size, shape and structure) in addition to simple NPC, a phenomenon connected to the evolutionary explosion of the cytheroids following the extinction of ostracods of the Suborder Metacopina in the early Toarcian.
Minyocythere species are remarkable for the presence of two types of morphological entities named here StPC-M and StPC-m, clearly differentiated by several morphological traits belonging to the sieve-plate, to the setal pore, and to different patterns of spatial distribution on the valve. The comparative study of StPC of Minyocythere and Dolocythere species demonstrates that NPC can be used for taxonomic purposes at both species and genus levels. It is probably possible to use StPC characteristics for discrimination of suprageneric taxa, which should be useful for the development of more comprehensive systematics of the family Cytheridae integrating living and fossil taxa like Minyocythere species from the Jurassic and Dolocythere from the Cretaceous.
From the palaeobiological point of view the Minyocythere species display a higher structural complexity of the NPC as compared to the limited number of other Jurassic and Cretaceous species we have examined: Dolocythere rara, D. amphistiella, Phraterfabanella tridentinensis, Camptocythere praecox, C. media, and Aphelocythere perforata. However, the exact adaptive function of the different types of normal pores remains unexplained.
Recognition of well-developed and impressive StPC in early to middle Jurassic age ostracods prompted this study. We have not followed the NPC terminology of Puri and Dickau (1969) and Puri (1974) because this was not adequate to describe the range and the structural characteristics of StPC encountered. Further documentation of NPC in living and fossil material is necessary, but to make real progress with our understanding, interpretation and application of NPC, and marginal pore canals, their function(s) must be understood via biophysical and biochemical methods with living animals.
Acknowledgements
We are indebted to the pioneer work of Erich Triebel (1894–1971) who, were he alive today, should be the lead author. David Horne (Queen Mary, University of London, UK) made very helpful comments on a draft version of the manuscript for which we are grateful. Friedrich Luppold (BGR) kindly arranged the loan of type and comparative material from the collections of the Bundesanstalt für Geowissenschaften und Rohstoffe/Landesamt für Bergbau, Energie und Geologie, Hannover. Ian Boomer (University of Birmingham, UK) generously provided material of Phraterfabanella tridentinensis. SEM images were made by Claudia Franz (Senckenberg Forschungsinstitut, Frankfurt, Germany) and Telmo Nunes (University of Lisbon, Portugal) and the plates and figures assembled with care and skill by Vera Lopes (University of Lisbon, Portugal). We are indebted to Mihai Nasta (Brussels, Belgium) for advice on latin names, and to Claudia Franz and Eberhard Schindler (Senckenberg Forschungsinstitut, Frankfurt, Germany) for translations from original German. We are also grateful for the helpful comments of reviewers Ian Boomer (University of Birmingham, UK), Ewa Olempska (Institute of Paleobioolgy, PAS, Warsaw, Poland), and Akira Tsukagoshi (Shizuoka University, Japan). DLD received logistical support from the Department of Geology and Palaeontology, Karl-Franzens University, Graz. MCC was supported by FCT-project UID/GEO/50019/2019 (Instituto Dom Luiz, University of Lisbon).
References
Aladin, N.V. and Potts, W.T.W. 1996. The osmoregulatory capacity of Ostracoda. Journal of Comparative Physiology B 166: 215–222. Crossref
Athersuch, J. 1976. The genus Xestoleberis (Crustacea: Ostracoda) with particular reference to Recent Mediterranean species. Pubblicazioni della Stazione Zoologica di Napoli 40: 282–343.
Athersuch, J., Horne, D.J., and Whittaker, J.E. 1989. Marine and brackish water ostracods (superfamilies Cypridacea and Cytheracea). In: D.M. Kermack and R.S.K. Barnes (eds.), Synopses of the British Fauna, Vol. 43, 1–343. Brill, Leiden.
Babinot, J.-F. and Colin, J.-P. 2011. Barremian ostracods from the Serre de Bleyton (Drôme, SE France). Annales Naturhistorisches Museum Wien A113: 735–775.
Baird, W. 1835. List of Entomostraca found in Berwickshire. Transactions of the Berwickshire Naturalists’ Club 1: 95–100.
Baird, W. 1838. The natural history of the British Entomostraca: Part IV. Magazine of Zoology and Botany 2: 132–144.
Baird, W. 1850. The Natural History of the British Entomostraca. 364 pp. The Ray Society, London. Crossref
Bate, R.H. 1963. Middle Jurassic Ostracoda from North Lincolnshire. Bulletin of the British Museum (Natural History). Geology 8: 173–219.
Bate, R.H. 1967. Stratigraphy and palaeogeography of the Yorkshire Oolites and their relationships with the Lincolnshire Limestone. Bulletin of the British Museum (Natural History), Geology 14: 111–141.
Bate, R.H. 1968. Praeschuleridea ventriosa ventriosa (Plumhoff) and Paraschuleridea ornata Bate [Ostracoda] from the Bajocian of N.E. England. Journal of Natural History 2: 205–214. Crossref
Bate, R.H. 1978. The Jurassic Part II. Aalenian to Bathonian. In: R. Bate and E. Robinson (eds.), A Stratigraphical Index of British Ostracoda. British Micropalaeontological Society, Special Publications, 213–258. Seel House Press, Liverpool.
Błaszyk, J. 1959. Two new Bathonian ostracods of the genus Progonocythere. Acta Palaeontologica Polonica 4: 431–448.
Boomer, I., Whatley, R., Bassi, D., Fugagnoli, A., and Loriga, A. 2001. An Early Jurassic oligohaline ostracod assemblage within the marine carbonate platform sequence of the Venetian Prealps, N.N. Italy. Palaeogeography, Palaeoclimatology, Palaeoecology 166: 331–344. Crossref
Brady, G.S. 1868. A monograph of the Recent British Ostracoda. Transactions of the Linnean Society of London 26: 353–495. Crossref
Brady, G.S., Crosskey, H.W., and Robertson, D. 1874. A monograph of the post-Tertiary Entomostraca of Scotland including species from England and Ireland. Monographs of the Palaeontographical Society 28: 1–232. Crossref
Brand, E. 1949. Neue Ergebnisse zur mikropaläontologische Gliederung des nordwestdeutschen Dogger und Valendis. In: A. Bentz (ed.), Erdöl und Tektonik in Nordwestdeutschland, 335–348. Amt für Bodenforschung, Hannover-Celle.
Brand, E. and Fahrion, H. 1962. Dogger NW-Deutschlands. In: W. Simon and H. Bartenstein (eds.), Arbeitskreis Deutscher Mikropaläontologen, Leitfossilien der Mikropaläontologie, 123–158. Gebrüder Borntraeger, Berlin.
Brand, E. and Malz, H. 1962. Ostracoden-Studien im Dogger, 5: Glyptocythere n. g.. Senckenbergiana lethaea 43: 433–435.
Brayer, R.C. 1952. Salem Ostracoda of Missouri. Journal of Paleontology 26: 162–174.
Cabral, M.C., Colin, J.-P., Azerêdo, A.C., Silva, R.L, and Duarte, L.V. 2015. Brackish and marine ostracode assemblages from the Sinemurian of western Portugal, with descriptions of new species. Micropaleontology 61: 3–24.
Cabral, M.C., Lord, A.R., Boomer, I., Loureiro, I.M., and Malz, H. 2014. Tanycythere new genus and its significance for Jurassic ostracod diversity. Journal of Paleontology 88: 519–530. Crossref
Colin, J.-P. 1974. Contribution a l’étude des ostracodes du Crétacé supérieur de Dordogne. Géobios 7: 19–42. Crossref
Colin, J.-P. and Carbonel, P. 1990. Phylogenetical affinities of Cytherissa with other Cytherideinae (Vernoniella, Fabanella, Neocyprideis, Cyprideis) a paleontological approach. Bulletin de l’Institut de Geologie du Bassin d’Aquitaine, Bordeaux 47: 83–95.
Cope, J.C.W., Duff, K.L., Parsons, C.F., Torrens, H.S., Wimbledon, W.A., and Wright, J.K. 1980. A correlation of Jurassic rocks in the British Isles. Part Two. Middle and Upper Jurassic. The Geological Society, London, Special Report 15: 1–109.
Danielopol, D.L., Cabral, M.C., Lord, A., Carbonel, P., Gross, M., Stoica, M., Humphreys, W.F., Namiotko, T., Mohr, E., Külköylüoğlu, O., Piller, W., and Nunes, T. 2018. Sieve-type pore canals in the Timiriaseviinae—A contribution to the comparative morphology and the systematics of Limnocytheridae (Ostracoda). Zootaxa 4495: 1–64. Crossref
Danielopol, D.L., Olteanu, R., Lete, C., and Carbonel, P. 1990. Carapace morphology of Cytherissa lacustris (Cytherideidae): its interest for the systematics and phylogeny of the group. Bulletin de l’Institut de Geologie du Bassin d’Aquitaine, Bordeaux 47: 27–53.
De Deckker, P. and Lord, A. 2017. Cyprideis torosa: a model organism for the Ostracoda? Journal of Micropalaeontology 36: 3–6. Crossref
Deroo, G. 1966. Cytheracea (Ostracodes) du Maastrichtian de Maastricht (Pays-Bas) et des regions voisines; resultats stratigraphiques et paleontologiques de leur étude. Mededelingen van de Geologische Stichting C, V 2 (2): 1–197.
Donze, P., Colin, J.-P., Damotte, R., Oertli, H.J., Peypouquet, J.-P., and Said, R. 1982. Les Ostracodes du Campanien terminal à l’Éocène inférieur de la coupe du Kef, Tunisie nord-occidentale. Bulletin des Centres de Recherches Exploration-Production Elf-Aquitaine 6: 273–335.
Frenzel, P., Ewald, J., and Pint, A. 2017. Salinity-dependent sieve pore variability in Cyprideis torosa: an experiment. Journal of Micropalaeontology 36: 57–62. Crossref
Godfrey-Smith, P. 2003. An Introduction to the Philosophy of Science: Theory and Reality. 272 pp. University of Chicago Press, Chicago. Crossref
Gramm, M.N. 1977. Sieve-type pores in Palaeozoic Editia [in Russian]. Paleontologičeskij žurnal 1: 151–154.
Gramm, M.N. and Egorov, G.I. 1986. The early Carboniferous Editiidae (Ostracoda) and comments on the phylogeny of the Cytheracea [in Russian]. Paleontologičeskij žurnal 2: 50–60.
Gründel, J. 1966. Taxonomische, biostratigraphische und variationsstatistische untersuchungen an den Ostracoden der Unterkreide in Deutschland. Freiberger Forschungshefte C 200: 1–105.
Gründel, J. 1967. Zur Grossgliederung der Ordnung Podocopida G.W. Müller, 1894. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1967 (6): 321–332.
Hanai, T. 1959. Studies on the Ostracoda from Japan: 5. Subfamily Cytherinae Dana, 1852 (emend.). Journal of the Faculty of Science, University of Tokyo, Section 2 11: 409–418.
Hanai, T. 1970. Studies on the ostracod Subfamily Schizocytherinae Mandelstam. Journal of Paleontology 44: 693–729.
Hanai, T. 1982. Introductory note on the studies on Japanese Ostracoda. In: T. Hanai (ed.), Studies on Japanese Ostracoda. The University Museum, The University of Tokyo Bulletin 20: 1–14.
Hanai, T., Abe, K., Kamiya, T., and Tabuki, R. 1985. A few problems occurred through analyzing the Quaternary palaeoenvironments by fossil Ostracoda (in Japanese). In: K. Kajiyama (ed.), Ocean Characteristics and Their Changes, 424–429. Koseisha-Koseikaku, Tokyo.
Hartmann, G. 1963. Zur Phylogenie und Systematik der Ostracoden. Zeitschrift für zoologischen Systematische Evolutionsforschung 1: 1–154. Crossref
Hartmann, G. 1964. Neontological and Paleontological classification of Ostracoda. Pubblicazioni della Stazione Zoologica di Napoli 33 (Supplement): 550–587.
Hartmann, G. 1975. Ostracoda. In: H.G. Bronns (ed.), Klassen und Ordnung des Tierreichs: 5 (Arthropoda), I (Crustacea), Buch 2, 4 (Ostracoda), 569–786. Gustav Fischer Verlag, Jena.
Horne, D.J. 1982. The ostracod fauna of an intertidal Sabellaria reef at Blue Anchor, Somerset, England. Estuarine, Coastal and Shelf Science 15: 671–678. Crossref
Horne, D.J. 2005. Homology and homeomorphy in ostracod limbs. In: N. Ikeya, A. Tsukagoshi, and D.J. Horne (eds.), Evolution and Diversity of Ostracoda. Hydrobiologia 538: 55–80. Crossref
Horne, D.J., Smith, R.J., Whittaker, J.E., and Murray, J.W. 2004. The first British record and a new species of the superfamily Terrestricytheroidea (Crustacea, Ostracoda): morphology, ontogeny, lifestyle and phylogeny. Zoological Journal of the Linnean Society 142: 253–288. Crossref
Ikeya, N. and Tsukagoshi, A. 1988. The interspecific relations between three close species of the genus Cythere O.F. Müller, 1785. In: T. Hanai (ed.), Evolutionary Biology of Ostracoda, its Fundamentals and Applications, 891–917. Kodansha Scientific Co., Tokyo. Crossref
Ishizaki, K. 1973. Discovery of the family Punciidae, Ostracoda (Crustacea), from Okinawa Island, Japan. Tohoku University, Scientific Reports, 2nd Series (Geology), Special Volume 6: 403–405.
Ishizaki, K. and Gunther, F.J. 1974. Ostracoda of the family Cytheruridae from the Gulf of Panama. Tohoku University, Scientific Reports, 2nd Series, (Geology) 45: 1–50.
Jones, T.R. 1850. Description of the Entomostraca of the Pleistocene Beds of Newbury, Copford, Clacton and Greys. Annals and Magazine of Natural History (Series 2) 6: 25–28.
Jones, T.R. 1857. A monograph of the Tertiary Entomostraca of England. Monographs of the Palaeontographical Society 9 (for 1855): 1–68. Crossref
Kamiya, T. 1989. Differences between the sensory organs of phytal and bottom-dwelling Loxoconcha (Ostracoda, Crustacea). Journal of Micropalaeontology 8: 37–47. Crossref
Kamiya, T. and Hazel, J.E. 1992. Shared versus derived characters in the pore-system of Loxoconcha (Ostracoda, Crustacea). Journal of Micropalaeontology 11: 159–166. Crossref
Kaye, P. 1964. Revision of the Ostracoda from the Bargate Beds in Surrey. Palaeontology 7: 317–330.
Kesling, R.V. 1956. The Ostracod. A neglected little crustacean. Turtox News 34: 1–10.
Keyser, D. 1980. Auftreten und Konstanz von Poren und Borsten auf der Schale von Podocopa (Ostracoda, Crustacea). Verhandlungen des naturwissenschaftlichen Vereins Hamburg, NF 23: 175–193.
Keyser, D. 1981. Aufbau der Tastsinnesorgane in den Schalen von Ostracoden (Crustacea). Beiträge zur elektronenmikroskopischen Direktabbildungen von Oberflächen 14: 603–610.
Keyser, D. 1982. Development of the sieve pores in Hirschmammia viridis (O.F. Müller, 1785). In: R.H. Bate, E. Robinson, and L.M. Sheppard (eds.), Fossil and Recent Ostracods, 51–60. Ellis Horwood Limited for The British Micropalaeontological Society, Chichester.
Keyser, D. 1983. Ultrastructure of carapace-sensilla in Aurila convexa. In: R.F. Maddocks (ed.), Applications of Ostracoda, 649–658. University of Houston Geosciences, Houston.
Kubiatowicz, W. 1983. Upper Jurassic and Neocomian ostracodes from central Poland. Acta Geologica Polonica 33: 1–72.
Külköylüoğlu, O., Yavuzatmaca, M., Cabral, M.C., and Colin, J.-P. 2015. Gomphocythere besni n. sp. (Crustacea, Ostracoda) from a man-made pool (Adıyaman, Turkey). Zootaxa 3937: 456–470. Crossref
Latreille, P.A. 1806. Genera crustaceorum et insectorum, secundum ordinem naturalem et familias disposita. xviii + 302 pp. Amand Koenig, Paris. Crossref
Lee, D.D. and Tsukagoshi, A. 2014. Three new species of the genus Loxoconcha (Crustacea, Ostracoda, Podocopida) from the Okinawa Islands, southern Japan. Zootaxa 3796: 147–165. Crossref
Lee, D.D. and Tsukagoshi, A. 2018. Three new species of the genera Loxoconcha and Xestoleberis (Crustacea, Ostracoda, Podocopida) from central and southern Vietnam. Zootaxa 4472: 111–126. Crossref
Lewontin, R. 2001. Foreword. In: G.P. Wagner (ed.), The Character Concept in Evolutionary Biology, xvii–xxiii. Academic Press, San Diego. Crossref
Luppold, F.W. 2012. Ostracod assemblages from the Middle Jurassic of NW Germany with special reference to the Sowerbyi ammonite Zone (Early Bajocian, Jurassic). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 266: 217–238. Crossref
Maddocks, R.F. and Steineck, P.L. 1987. Ostracoda from experimental wood-island habitats in the deep sea. Micropaleontology 33: 318–355. Crossref
Malz, H. 1975. Ostracoden-Studien im Dogger: Die arten der gattung Lophocythere, ihre stratigraphische und regionale verbreitung. Senckenbergiana lethaea 56: 123–145.
Martens, K. 1989. Ovambocythere milani gen. n., spec. n. (Crustacea, Ostracoda), an African limnocytherid reared from dried mud. Revue de Zoologie Africaine 103: 379–387.
McShea, D.W. and Brandon, R.N. 2010. Biology’s First Law. The Tendency for Diversity and Complexity to Increase in Evolutionary Systems. 170 pp. University of Chicago Press, Chicago. Crossref
Meisch, C. 2000. Freshwater Ostracoda of Western and Central Europe. 520 pp. Spektrum Akademischer Verlag, Heidelberg.
Meisch, C. and Wouters, C. 2004. Valve surface structure of Candona neglecta Sars, 1887 (Crustacea, Ostracoda). Studia Quaternaria 21: 15–18.
Mertens, E. 1956. Zur Grenzziehung Alb/Cenoman in Nordwestdeutschland mit Hilfe von Ostracoden. Geologisches Jahrbuch 72: 173–230.
Müller, G.W. 1890. Neue Cypridiniden. Zoologische Jahrbücher, Abteilung für Systematik, Geographie und Biologie der Tiere 5: 211–252.
Müller, G.W. 1894. Die Ostracoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Herausgegeben von der Zoologischen Station zu Neapel 21: 1–404.
Müller, O.F. 1785. Entomostraca seu insecta testacea, quae in aquis Daniae et Norvegiae reperit, descripsit et iconibus illustravit. 135 pp. Sumtibus J.G. Mülleriani, Lipsiae. Crossref
Namiotko, T., Wouters, K., Danielopol, D.L., and Humphreys, W.F. 2004. On the origin and evolution of a new anchialine stygobitic Microceratina species (Crustacea: Ostracoda: Loxoconchidae) from Christmas Island (Indian Ocean). Journal of Micropalaeontology 23: 49–59. Crossref
Oertli, H.J. 1957. Ostracodes. In: F. Bernard, J.-J. Bizon, and H.J. Oertli (eds.), Ostracodes lacustres du Bathonien du Poitou (Bassin de Paris). Bulletin de la Société Géologique de France, Serie 6 6: 753–770. Crossref
Okada, R., Tsukagoshi, A., Smith, R.J. and Horne, D.J. 2008. The ontogeny of the platycopid Keijcyoidea infralittoralis (Ostracoda: Podocopida). Zoological Journal of the Linnean Society 153: 213–237. Crossref
Okada, Y. 1982. Ultrastructure and pattern of the carapace of Bicornucythere bisanensis (Ostracoda, Crustacea). In: T. Hanai (ed.), Studies on Japanese Ostracoda. The University Museum, The University of Tokyo, Bulletin 20: 229–255.
Okada, Y. 1983. Ultrastructure and functions of pores of ostracodes. In: R.F. Maddocks (ed.), Applications of Ostracoda, 640–648. University of Houston, Geosciences, Houston.
Okubo, I. 1975. Recent marine Ostracoda in the Inland Sea, Japan. 1. Callistocythere pumila Hanai, 1957 and Leguminocythereis bisanensis sp. nov. in the Inland Sea, Japan. Proceedings of the Japanese Society of Systematic Biology 11: 23–31. Crossref
Olempska, E. 2008. Soft body-related features of the carapace and the lifestyle of Paleozoic beyrichioidean ostracodes. Journal of Paleontology 82: 717–736. Crossref
Omatsola, M.E. 1970. On structure and morphologic variation of normal pore system in Recent cytherid ostracoda (Crustacea). Acta Zoologica 51: 115–124. Crossref
Ozawa, H. and Ishii, T. 2008. Taxonomy and sexual dimorphism of a new species of Loxoconcha (Podocopida, Ostracoda) from the Pleistocene of the Japan Sea. Zoological Journal of the Linnean Society 153: 239–251. Crossref
Ozawa, H. and Kamiya, T. 2013. A new species of Sagmatocythere (Crustacea: Ostracoda: Loxoconchidae) from the Pleistocene Kawachi Formation in Central Japan. Bulletin of National Museum of Natural Sciences C 39: 7–15.
Peypouquet, J.-P. 1979. Ostracodes et paléoenvironnements. Méthodologie et application aux domaines profonds du Cénozoïque. Bulletin Bureau Recherches Géologiques et Minières 4: 3–79.
Plusquellec, P.L. and Sandberg, P.A. 1969. Some genera of the ostracode subfamily Campylocytherinae. Micropaleontology 15: 427–480. Crossref
Plumhoff, F. 1963. Die Ostracoden des Oberaalenium und tiefen Unterbajocium (Jura) des Gifhorner Troges, Nordwestdeutschland. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 503: 1–99.
Puri, H.S. 1974. Normal pores and the phylogeny of Ostracoda. Geoscience and Man 6: 137–151.
Puri, H.S. and Dickau, B.E. 1969. Use of normal pores in taxonomy of Ostracoda. Transactions Gulf Coast Association of Geological Societies 19: 353–367.
Rawson, P.F. and Wright, J.K. 1995. Jurassic of the Cleveland Basin, North Yorkshire. In: P.D. Taylor (ed.), Field Geology of the British Jurassic, 1–286. The Geological Society, London.
Rome, R. 1944. Note sur la présence de scolopoïdes chez les ostracodes. Bulletin du Musee Royal d’Histoire naturelle de Belgique 20: 1–8.
Rome, R. 1947. Herpetocypris reptans (ostracode), étude morphologique et histologique—1: Morphologie externe et système nerveux. La Cellule 51: 49–152.
Rosenfeld, A. and Vesper, B. 1977. The variability of the sieve-pores in Recent and fossil species of Cyprideis torosa (Jones, 1850) as an indicator for salinity and palaeosalinity. In: H. Löffler and D.L. Danielopol (eds.), Aspects of Ecology and Zoogeography of Recent and Fossil Ostracoda, 55–67. Dr W. Junk, Hague.
Sandberg, P.A. 1964. The ostracod genus Cyprideis in the Americas. Stockholm Contributions in Geology 12: 1–178.
Sandberg, P.A. and Plusquellec, P.L. 1969. Structure and polymorphism of normal pores in cytheracean Ostracoda (Crustacea). Journal of Paleontology 43: 517–521.
Sars, G.O. 1866. Oversigt af Norges marine ostracoder. Forhandlinger i Videnskabs-selskebet i Christiania 1865: 1–130.
Sars, G.O. 1887. Nye bidrag til kundskaben om Middelhavets invertebratfauna: 4. Ostracoda Mediterranea (Sydeuropaeiske Ostracoder). Archiv for Mathematik og Natutvidenskab 12: 173–324. Crossref
Sars, G.O. 1925. An account of the Crustacea of Norway with short descriptions and figures of all the species. Ostracoda 9–10: 137–176.
Sars, G.O. 1926. An Account of the Crustacea of Norway with short descriptions and figures of all the species. Ostracoda 13–14: 209–240.
Schallreuter, R.E.L. 1966. Drepanellacea (Ostracoda, Beyrichiida) aus Mittelordovizischen backsteinkalkgeschieben—1: Klimphores planus g.n.sp.n. und Vaivanovia hiddenseensis g.n.sp.n.. Berichte der Deutschen Gesellschaft für Geologische Wissenschaften, A (Geologie und Paläontologie) 11: 393–402.
Schallreuter, R.E.L. 1977. On Miehlkella cribroporata Schallreuter gen. et. sp. nov.. A Stereo-Atlas of Ostracod Shells 4 (2): 9–16.
Schallreuter, R.E.L. 1980. On Klimphores planus Schallreuter. A Stereo-Atlas of Ostracod Shells 7 (2): 9–16.
Schallreuter, R.E.L. 1983. On some special morphological features of Ordovician ostracodes and their palaeoecological implications. In: R.F. Maddocks (ed.), Applications of Ostracoda, 659–666. University of Houston Geosciences, Houston.
Schneider, D.C. 1994. Quantitative Ecology—Spatial and Temporal Scaling. 395 pp. Academic Press, San Diego. Crossref
Schornikov, E.I. [Ŝornikov, E.I.] 1969. A new family of Ostracoda from the supralittoral zone of the Kuril Islands [in Russian with English abstract]. Zoologičeskij žurnal 48: 494–498.
Slipper, I.J. 2009. Marine Lower Cretaceous. In: J.E. Whittaker and M.B. Hart (eds.), Ostracods in British Stratigraphy. The Micropalaeontological Society, Special Publications, 309–344. The Geological Society, London. Crossref
Swanson, K.M. 1980. Five new species of Ostracoda from Port Pegasus, Stewart Island. New Zealand Journal of Marine and Freshwater Research 14: 205–211.
Swanson, K.M. 1989. Ostracod phylogeny and evolution—a manawan perspective. Courier Forschungs-Institut Senckenberg 113: 11–20. Crossref
Sylvester-Bradley, P.C. 1948. Bathonian ostracods from the boueti Bed of Langton Herring, Dorset. Geological Magazine 85: 185–204. Crossref
Sylvester-Bradley, P.C. and Benson, R.H. 1971. Terminology for surface features in ornate ostracodes. Lethaia 4: 249–286. Crossref
Triebel, E. 1941. Zur Morphologie und Ökologie der fossilen Ostracoden. Mit Beschreibung einiger neuer Gattungen und Arten. Senckenbergiana 23: 294–400.
Triebel, E. 1950. Camptocythere, eine neue Ostracoden-Gattung aus dem Dogger Norddeutschlands. Senckenbergiana 31: 197–208.
Triebel, E. and Klingler, W. 1959. Neue Ostracoden-Gattungen aus dem deutsche Lias. Geologisches Jahrbuch 76: 335–372.
Tsukagoshi, A. 1990. Ontogenetic change of distributional patterns of pore systems in Cythere species and its phylogenetic significance. Lethaia 23: 225–241. Crossref
Tsukagoshi, A. 1998. On Cythere hanaii Tsukagoshi and Ikeya. A Stereo-Atlas of Ostracod Shells 24: 5–12.
Tsukagoshi, A. and Briggs, W.M. 1998. On Schizocythere ikeyai sp. nov. A Stereo-Atlas of Ostracod Shells 25 (10): 43–52.
Tsukagoshi, A. and Ikeya, N. 1987. The ostracod genus Cythere O.F. Müller, 1785 and its species. Transactions and Proceedings of the Palaeontological Society of Japan, New Series 148: 197–222.
Tsukagoshi, A. and Ikeya, N. 1991. A redescription of Cythere japonica Hanai, 1959 (Podocopida, Ostracoda). Zoological Journal of the Linnean Society 103: 129–143. Crossref
Tsukagoshi, A., Okada, R., and Horne, D.J. 2006. Appendage homologies and the first record of eyes in platycopid ostracods, with the description of a new species of Keijcyoidea (Crustacea, Ostracoda) from Japan. Hydrobiologia 559: 255–274. Crossref
Van Morkhoven, F.P.C.M. 1962. Post-Palaeozoic Ostracoda. Volume 1 General. vii + 204 pp. Elsevier, Amsterdam.
Whatley, R.C. and Stephens, J.M. 1977. Precocious sexual dimorphism in fossil and Recent Ostracoda. In: H. Löffler and D.L. Danielopol (eds.), Aspects of Ecology and Zoogeography of Recent and Fossil Ostracoda, 69–91. Dr W. Junk, Hague.
Whittaker, J.E. 1975. On Hirschmannia viridis (O.F. Müller). A Stereo-Atlas of Ostracod Shells 2: 149–156.
Whittaker, J.E. and Hart, M.B. (eds.) 2009. Ostracods in British Stratigraphy. The Micropalaeontological Society, Special Publications, viii + 1–485. The Geological Society, London.
Wienholz, E. 1967. Neue Ostracoden aus dem Norddeutschen Callov. Freiberger Forschungshefte, C (Paläontologie) 213: 23–51.
Yamada, S. 2007. Ultrastructure of the carapace margin in the Ostracoda (Arthropoda: Crustacea). Hydrobiologia 585: 201–211. Crossref
Diagnoses translated from the original German of the generic diagnoses of Camptocythere Triebel, 1950, Aphelocythere Triebel and Klingler, 1959, and Dolocythere Mertens, 1956. [ ] = our additions.
Camptocythere Triebel, 1950 (see Triebel 1950: 198)
Type species: Camptocythere praecox Triebel, 1950, Aalenian (Dogger α), Borehole Hambühren WA2 (depth not given), NW Germany.
Diagnosis: Genus with the muscle scars of the Cytheridae and the following particularities: Right valve with a bulge dorsally which is longer than the straight hinge margin of the left valve which is posteriorly inclined. Ventrally the left valve overlaps the right, so that from the end view they are offset. Anterior marginal zone with simple widely spaced pore canals. Hinge on left side without teeth, on the right side with two flatly triangular incompletely crenulate teeth.
Aphelocythere Triebel and Klingler, 1959 (see Triebel and Klingler 1959: 338–339)
Type species: Aphelocythere undulata Triebel and Klingler, 1959, Toarcian (Lias ζ), Borehole Hambühren WA2, 354 m, NW Germany.
Diagnosis: Sexual dimorphism very evident, males larger and more elongate than females. Carapace middle sized, in side view four-sided maximum height just before mid-length. LV slightly larger than RV, dorsal and upper edge of the margin overreach. Anterior part broadly rounded, posterior part at half-height and below middle line narrowly rounded to broadly angular, in the upper half almost straight inclined downwards. Dorsal rim [margin] in female almost straight to slightly convex.
The upper surface close to anterior margin with broad ridge [Wulst], which starts at the antero-dorsal [cardinal] angle and a little below middle line transitions [merges] into curve of shell. Middle part of lateral surface without strong sculpture, the posterior part with some curved vertical ridges which cause a more-or-less pronounced wavelike sculpture. Rest of the upper surface spotted, with small, rounded and not always clearly visible small pits distributed. Distinctive eye spot or inner eye groove lacking.
Inner rim and amalgamation line coincident [IM = LoC]. Marginal pore canals not divided, widely distributed and not numerous. True submarginal canals lacking. Lateral canals large and sieve-like.
The four central muscle scars form a slightly curved vertical row. The lower scar is bigger than the others and has an almost triangular shape. Frontal group of scars with two different big scars which may or may not be amalgamated to each other.
Hinge threefold, right side [RV] with narrowly low and dentate tooth plates and a slim narrowly crenulate middle furrow.
[IM = LoC] Inner Margin coincides with Line of Concresence.
Dolocythere Mertens, 1956 (see Mertens 1956: 191)
Type species: Dolocythere rara Mertens, 1956, Lower Albian, Borehole Lingen 106, 697–704 m, NW Germany.
Diagnosis: A genus with the characteristics of the Cytheridae with the following peculiarities: Carapace rounded four-sided with long margins [dorsal and ventral] weakly convergent towards posterior. Surface reticulated but without bulges [ridges] or knots [nodes]. Hinge in LV is constructed from two terminal smooth depressions [Gruben = rounded holes], which are closed by the overhang of the anterior and posterior margins; betweeen them there is a smooth ridge. The RV wears a smooth sulcus [Furche, interpreted as median groove]. On their ends the upper parts of the anterior and posterior margins are made stronger [strengthened] and form elongated false teeth. All hinge elements are not located on a straight line, but the terminal elements are strongly curved ventrally. Accommodation groove is missing. Inner margin and line of concrescence coincide. Anteriorly about 8–12 and posteriorly about 6 straight unfanned marginal pore canals. Sexual dimorphism present.
Translation of Triebel’s (1941: 333, second paragraph) first description of sieve-type pore canals in fossil material. [ ] = our additions.
G.W. Müller (1894: 105; 1927: 413) suspects that some of the sieve-like pore canals of the ostracod valve can be interpreted as organs of light sensitivity—in other words eyes. These strange sieve-like canals of the valve can also be detected easily on fossil material if the preservation is favourable. First I found them on Tertiary species (Triebel 1941: pl. 2: fig. 9 [on Paracyprideis]), but recently also down to the Jurassic. The form figured on pl. 7: fig. 72a from the Dogger [Middle Jurassic] of North Germany shows well the difference between the close standing forms near the edge [of the valve] and the pore canals on the surface area which stand out as distinctive bright spots; the sieve structure of the second [type] is shown on fig. 72b. As the many close sieve-tubes of such a canal offer a large surface to attacking agents during weathering it happens that instead of pore canals holes are found on the otherwise rather well preserved valves (fig. 71).
Triebel figured one well preseved valve (pl. 7: fig. 72a) with well preserved sieve-type pore canals (fig. 72b) and one etched valve with holes where the sieve-type pore canals are located (fig. 71); all catalogue number SMF Xe 1227 under the name Leptocythere? sp.
Acta Palaeontol. Pol. 65 (2): 313–349, 2020
https://doi.org/10.4202/app.00632.2019