


Identifying the oldest larva of a myrmeleontiformian lacewing—a morphometric approach
ANDRÉS F. HERRERA-FLÓREZ, FLORIAN BRAIG, CAROLIN HAUG, CHRISTIAN NEUMANN, JÖRG WUNDERLICH, MARIE K. HÖRNIG, and JOACHIM T. HAUG
Herrera-Flórez, A.F., Braig, F., Haug, C., Neumann, C., Wunderlich, J., Hörnig, M.K., and Haug, J.T. 2020. Identifying the oldest larva of a myrmeleontiformian lacewing—a morphometric approach. Acta Palaeontologica Polonica 65 (2): 235–250.
Neuroptera is one of the smaller ingroups of Holometabola, the ingroup of Insecta characterised by “complete” metamorphosis. Neuroptera comprises about 6000 species in the modern fauna, but appears to have been more diverse in the past. While adults distantly resemble certain moths or damselflies, the larval forms of Neuroptera are mostly fierce predators with prominent venom-injecting stylets. The most well-known of these larvae are probably those of antlions. Antlions and their closer relatives (silky lacewings, split-footed lacewings, ribbon-winged lacewings, spoon-winged lacewings, and owlflies) form a distinct monophyletic ingroup of Neuroptera, Myrmeleontiformia, hence the antlion-like forms. The fossil record of antlion-like larvae dates back far into the Cretaceous; many forms are known by exceptionally well-preserved specimens entrapped in amber. The oldest fossil record of a neuropteran larva (not an antlion-like form) comes from Lebanese amber. Interestingly, the supposedly oldest record of an antlion-like larva is preserved in rock and comes from the famous Lower Cretaceous Crato Formation. We re-evaluate this fossil based on high-resolution composite photography. Due to the non-availability of many key characters, standard procedures for identifying the specimen to a more narrow ingroup remains challenging. Therefore, we used a morphometric approach. A combination of non-metric multidimensional scaling (NMDS), parallel coordinate plots and discriminant function analysis indicates that the fossil is a representative of the group Ascalaphidae (owlflies) + Myrmeleontidae (antlions). We discuss implications of this result for the fossil record of neuropteran larvae. These include the rather derived morphology of the oldest fossil larva of Myrmeleontiformia in contrast to previous expectations. Furthermore, fossils from soil dwellers can not only be expected to be found in amber, but also as compression fossils.
Key words: Insecta, Myrmeleontidae, Ascalaphidae, compression fossil, fossil larva, Cretaceous, Crato Formation, Brazil.
Andrés F. Herrera-Flórez [andresfhf@gmail.com] and Florian Braig [f.braig@campus.lmu.de], Ludwig-Maximilians-Universität München (LMU), Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany.
Carolin Haug [carolin.haug@palaeo-evo-devo.info] and Joachim T. Haug [joachim.haug@palaeo-evo-devo.info], Ludwig-Maximilians-Universität München (LMU), Biocenter, Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany and GeoBio-Center, Ludwig-Maximilians-Universität München, Richard-Wagner-Straße 10, 80333 München, Germany.
Christian Neumann [christian.neumann@mfn-berlin.de], Museum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Invalidenstr. 43, 10115 Berlin, Germany.
Jörg Wunderlich [joergwunderlich@t-online.de], Oberer Häuselbergweg 25, 69493 Hirschberg, Germany.
Marie K. Hörnig [marie.hoernig@palaeo-evo-devo.info], University of Greifswald, Zoological Institute and Museum, Cytology and Evolutionary Biology, Soldmannstr. 23, 17489 Greifswald, Germany.
Received 7 August 2019, accepted 3 December 2019, available online 24 March 2020.
Copyright © 2020 A.F. Herrera-Flórez et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Neuroptera (formerly also known as Planipennia) is an ingroup of Holometabola, the truly hyper-diverse ingroup of Insecta. If compared to Coleoptera, Hymenoptera, Lepidoptera, and Diptera in terms of species numbers, Neuroptera might be considered less important. However, regarding its evolutionary history and morphological diversity, Neuroptera should be considered as important as the hyper-diverse groups from an ecological point of view (Engel et al. 2018). Megaloptera and Raphidioptera, two closely related groups of Neuroptera (all together forming Neuropterida), are even less diverse in both number of species and morphologies than Neuroptera.
The representatives of Neuroptera show quite a number of distinct morphotypes; these morphotypes are generally equivalent to family-ranked monophyletic groups (though at least partly challenged by phylogenomic studies, e.g., Winterton et al. 2018, see also below; yet, note that ranks are highly subjective, see e.g., Ereshefsky 2002; Laurin 2010). Depending on the chosen scheme, slightly less than 20 extant and more than 10 fossil groups are differentiated. Particularly the extant groups can be well recognised especially on distinct larval features.
Many aspects of the internal relationships of Neuroptera are currently in flux (e.g., Winterton et al. 2018; Jandausch et al. 2018) and many traditionally recognised sister-group relationships have been questioned in recent years (Garzón-Orduña et al. 2018). A major ingroup of Neuroptera that has remained stable and well accepted is Myrmeleontiformia (but note that the composition and internal relationships of Myrmeleontiformia differ to a certain extent between studies with different systematic methods). Five major extant ingroups are generally differentiated: Psychopsidae, silky lacewings; Nemopteridae, that includes two ingroups with quite distinct morphologies (Crocinae, thread-winged lacewings; and Nemopterinae, ribbon- or spoon-winged lacewings); Nymphidae, split-footed lacewings; Ascalaphidae, owlflies, and Myrmeleontidae, antlions (e.g., Badano et al 2017).
Furthermore, nine groups exclusively known from fossils have been suggested to represent distinct separate lineages within Myrmeleontiformia: Aetheogrammatidae (Ren and Engel 2008), Kalligrammatidae (Fang et al. 2010; Ren and Oswald 2002; Yang et al. 2014), Osmylopsychopidae (Shcherbakov 2008), Panfiloviidae (Yang et al. 2013; Khramov and Vasilenko 2018), Prohemerobiidae (Tillyard 1919; Martynov 1937), Araripeneuridae (Martins-Neto 1994; Makarkin et al. 2018; treated as an ingroup of Myrmeleontidae by some authors: Huang et al. 2016; Lu et al. 2019), Babinskaiidae (Martins-Neto 2000; Lu et al. 2017; Makarkin et al. 2017; Hu et al. 2018), Palaeoleontidae (Martins-Neto 1992; Myskowiak and Nel 2016; considered an ingroup of Myrmeleontidae by some authors: Stange 2004; Lu et al. 2019) and Rafaelianidae (Nel et al. 2005; Engel and Nel 2017). All these extinct groups are known only from their adult stages.
The fossil record of myrmeleontiformian larvae is therefore largely restricted to fossils interpreted as representatives of the five groups with modern-day representatives. One of the most important studies is that of MacLeod (1970), who described larval forms of Psychopsidae (Propsychopsis sp.), Ascalaphidae (Neadelphus protae MacLeod, 1970) and Nymphidae (Pronymphes sp.) all from Eocene Baltic amber. Additional specimens from Baltic amber have been figured occasionally, for example in Weitschat and Wichard (2002: fig. 56h) and Gröhn (2015: 258, fig. 1388). Also, from younger ambers there are some reports. Engel and Grimaldi (2007), for example, reported a larval representative of Myrmeleontidae (Porrerus dominicanus Poinar and Stange, 1996) from Dominican amber.
More recently, numerous larval forms from Cretaceous Burmese amber (100 mya) have been reported (Wang et al. 2016; Badano et al. 2018), among others including two representatives of Psychopsidae (Acanthopsychops triaina Badano and Engel, 2018, Aphthartopsychops scutatus Badano and Engel, 2018) and one representative of Nymphidae (Nymphavus progenitor Badano, Engel, and Wang, 2018).
Furthermore, some larvae have been reported by Badano et al. (2018) that have been interpreted as representatives of Myrmeleontiformia (although none of them were attributed to the major five extant groups): an early offshoot of the entire group (“stem-group Myrmeleontiformia”, including the two species Macleodiella electrina and Cladofer huangi), five larval forms branching off the lineage towards Ascalaphidae + Myrmeleontidae (“stem-group Myrmeleontidae + Ascalaphidae”, including Electrocaptivus xui Badano, Engel, and Wang, 2018, Burmitus tubulifer Badano, Engel, and Wang, 2018, Diodontognathus papillatus Badano, Engel, and Wang, 2018, Mesoptynx unguiculatus Badano, Engel, and Wang, 2018, and Adelpholeon lithophorus Badano and Engel, 2018), and finally one larva closely related to Myrmeleontidae (“stem-Myrmeleontidae”, Pristinofossor rictus Badano and Engel, 2018). Also recently, Haug et al. (2019) described a fossil larva from Burmese Amber resembling larval forms of Crocinae (Nemopteridae) in certain aspects.
The oldest known fossil neuropteran larvae were recently described by Pérez-de la Fuente et al. (2018, 2019) from Early Cretaceous (Barremian) Lebanese amber, interpreted as representatives of Chrysopidae (=> non-myrmeleontiformian, Tyruschrysa melqart Pérez-de la Fuente, Azar, and Engel, 2018; but note that according to some studies, e.g., Winterton et al. 2018, Chrysopidae (and their fossil representatives) is the sister group of Myrmeleontiformia).
The oldest possible larvae of Myrmeleontiformia had been briefly figured in two contributions. A rather incomplete specimen, lacking the head was figured in Rumbucher (1995: fig. 6, “Myrmelionidae, Larvenstadium”). A second, more complete specimen, possessing the head including the prominent mouth parts was figured in Martins-Neto et al. (2007: fig. 11.69d, “Neuroptera, Myrmeleontiformia, Myrmeleontidae?, larva, MB coll.”). Both specimens originate from the famous Crato Formation. This formation is of Aptian (Lower Cretaceous) age that is approximately 115 million years old. It constitutes one of the most complete records of Cretaceous biodiversity, bearing a well conserved palaeobiota (Grimaldi 1990; Menon et al. 2005; Barling et al. 2015).
We describe here the specimen figured in Martins-Neto et al. (2007) in detail. For further identifying its possible relationship we use a larger-scaled morphometric approach.
Institutional abbreviations.—MfN, Museum für Naturkunde, Berlin, Germany; ZMH, Zoologische Sammlungen am Centrum für Naturkunde (CeNak), Hamburg, Germany; ZSM, Zoological State Collection, Munich, Germany.
Other abbreviations.—AUC, area under the curve; DFA, discriminant function analysis; m1, mandible length including the curved shape (arc); m2, mandible length in straight line (chord); m3, head length; m4, head width; m5, “neck” width (located behind the end of the head); m6, widest point of the abdomen; m7, “neck” length (distance between the end of head and the fore legs); m8, distance between the fore pair of legs and the hind pair of legs; m9, total body length (head without mandible + thorax + abdomen); m10, distance between widest point of the abdomen and hind part of the abdomen; NMDS, non-metric multidimensional scaling.
Material and methods
In the centre of this study is a single specimen preserved on a limestone slab from the Brazilian Crato Formation. The specimen is deposited in the Museum für Naturkunde Berlin under the repository number MfN MB.I 2157. The label provides the following information (English information in brackets translated by the authors): Planipennia: Myrmeleontidae: gen. et sp. indet., Crato-Formation, Araripe-Becken [Araripe Basin], Brasilien [Brazil]. Untere Kreide [Lower Cretaceous], Apt-Alb, Gekauft von [purchased by] Schwickert (Nr. 150), Inv.-Nr. MB.1993/3.
For comparative purposes extant larval representatives of Neuroptera were investigated. These came from the collection of the Zoological State Collection, Munich (ZSM).
The fossil specimen was documented with a Canon EOS 70D Camera equipped with a MP-E 65 mm super-macro lens. Illumination was provided by a Canon MT-24 EX twin flash. Light was cross-polarised and undirected to avoid any artefacts caused by shadows. Images were recorded as stacks of shifting focus and later processed in CombineZP. Final optimisation was performed in Adobe Photoshop CS2. To document aspects of relief, details were documented from two different angles and then arranged to red-cyan stereo images. These protocols have proven successful for investigating fossils from the Crato Formation (e.g., Hörnig et al. 2013, 2017, 2018; Dittmann et al. 2015) and larvae of Holometabola preserved “in stone” (e.g., Haug et al. 2015).
Comparative material was documented on a Keyence VHX-6000 digital microscope, either equipped with a Keyence VH-Z 20R RZ ×20–200 lens or with a Keyence VH-ZST RZ ×20–200, ×200–2000 lens. Illumination was either provided by ring light or by coaxial cross-polarised light. All images were recorded as composite images (stacks, several adjacent image details); some were additionally recorded using the HDR function. Images were processed automatically with the built-in software (as in Haug et al. 2018, 2019).
In total, ten dimensions were measured on each specimen, m1–m10, summarised in Fig. 1 (see also above, SOM: table 2, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Herrera-Florez_etal_SOM.pdf ).
All statistical calculations and analyses were performed using the R statistics environment 3.4.3 (R Core Team 2018), utilizing the interface R-Studio 1.1.419. Packages used were car (Fox and Weisberg 2011), caret (Kuhn 2019), ggplot2 (Wickham 2016), MASS (Venables and Ripley 2002), mda (Hastie and Tibshirani 2017), nlme (Pinheiro et al. 2018), pROC (Robin et al. 2011), readxl (Wickham and Bryan 2018), reshape2 (Wickham 2007), tidyverse (Wickham 2017), and vegan (Oksanen et al. 2018).
In order to get a first impression of the dataset and to explore the relations of the different groups amongst each other, we performed a NMDS analysis (non-metric multidimensional scaling). NMDS is a multivariate ordination method that transforms distances of data points into ranks, reduces dimensionality, and plots similar data points close together (Kruskal 1964; Lee et al. 2009). Advantageous of the NMDS-approach to visualize morphospaces is that a correction of body size is not necessary. The NMDS reduces and transforms size itself, therefore, an uncorrected dataset was used for this analysis (Agarwal et al. 2007).
For a better interpretation of specimen MfN MB.I 2157, we used parallel coordinate plots (Andrienko and Andrienko 2001; Mander 2016). Hereby, we visualized nine different dimensions, each divided by body length, on the x-axis of a two-dimensional plot. The y-axis hereby depicted individual values divided by body shape for each specimen. Each specimen was therefore depicted by a continuous graph showing its values for the different measurements from left to right. This way, we plotted each group with specimen MfN MB.I 2157, to see with which group it correlates best.
To quantify the result of this analysis, we additionally performed the linear version of a discriminant function analysis (DFA). It is used to find variables that separate a given set of classes, in our case major monophyletic groups. These are then used to sort unknown objects into one of those groups (Fisher 1936; Hand and Till 2001; Sever et al. 2005). We trained a model with the size-corrected data set of representatives of all nine ingroups of Neuroptera (including six ingroups of Myrmeleontiformia and three for polarisation) and then predicted the affinity of specimen MfN MB.I 2157 in a first model. We calculated the AUC value as well as a mis-classification rate for a better assessment of the model. Then we additionally repeated the process, but with the group Ascalaphidae split into two groups, due to their separation into two distinct sub-groups in the morphospace, to create a second model.
Systematic palaeontology
Insecta Linnaeus, 1758
Neuroptera Linnaeus, 1758
Myrmeleontiformia gen. et sp. indet.
Figs. 2, 3.
Material.—MfN MB.I 2157, body organised distinctly into head and trunk (total body length 15.3 mm, including anterior projecting appendages; Fig. 2), preserved in ventral view. From the Brazilian Crato Formation, Araripe Basin, Cretaceous.
Description.—Head: Head forming enclosed capsule, presumably by 6 segments, ocular segments plus 5 post-ocular segments (Fig. 3A1). Head capsule broad, slightly wider than long (1.2× as wide as long), slightly tapering anteriorly and posteriorly, i.e., maximum width of head capsule at about half of its anterior-posterior length. Posterior rim slightly wider than anterior rim (1.1× as wide). Temple present, as a slight constriction of posterior margin of head capsule. Surface of head capsule with rough tubercles (Fig. 3B–D). These may originally represent the bases of setae.
No structures of ocular segment and post-ocular segments 1 and 2 apparent; neither eye hills (eye tubercles = elevations carrying the eyes), labrum, nor antennae observable. Post-ocular segments 3 and 4 recognisable by their appendages, mandibles and maxillae forming a pair of functional stylets (Fig. 3A2, A3). Mandibles with distinct groove, originally covered by maxillae. Stylets well separated at base, slender, clearly longer (1.4×) than head capsule; strongly curved inwards towards the apex. There are no prominent teeth, yet, there are shorter structures that may represent remains of damaged teeth (Fig. 3A2). No details of post-ocular segment 5 (e.g., labium) apparent. Neck not prominent, short, trapezoidal in ventral view, anterior rim wider than posterior rim.
Trunk: As a whole rather uniform in shape. Oval to almost circular in outline. Differentiable into anterior and posterior trunk (Fig. 2).
Anterior trunk/thorax: Anterior three trunk segments (thorax; Fig. 2A2) differentiated from further posterior ones; segments larger than further posterior ones and bearing appendages. Thorax segment 1 (prothorax) triangular to V-shaped, partly surrounding neck; only sternite apparent. Thorax segment 2 (mesothorax) significantly less V-shaped than preceding segment; only sternite apparent. In thorax segment 3 (metathorax), sternite slightly wider than that of preceding segment, almost straight. Further laterally, aspects of the tergite apparent, with a slight protrusion on each side. Each thorax segment with remains of thorax appendages (legs).
Posterior trunk/abdomen: With nine distinct units (Fig. 2A2); anterior eight corresponding to eight abdomen segments, unit nine corresponding to trunk end, most likely including several segments. Abdomen segment 1 slightly wider than preceding segment. Sternite narrower. Laterally with a small triangular sclerite (“pleural” sclerite); parts of tergite apparent. Abdomen segment 2 and 3 sub-similar to abdomen segment 1. Abdomen segment 3 slightly decreasing in width posteriorly. Tergites longer than sternites. Abdomen segments 4–7 also sub-similar to preceding segments, but progressively decreasing in width. Abdomen segment 8 narrower, no clear tergite visible. Trunk end narrow, rounded.
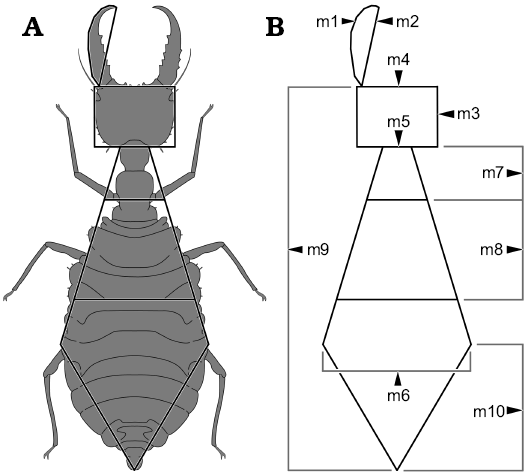
Fig. 1. Dimensions measured on the specimens. A. Example of an actual specimen (simplified from Monserrat 2008) to illustrate which dimensions were measured, resulting in a “net”. B. The naked “net”. Abbreviations: m1, mandible length including the curved shape; m2, mandible length in straight line; m3, head length; m4, head width; m5, “neck” width (located behind the end of the head); m6, widest point of the abdomen; m7, “neck” length (distance between the end of head and the fore legs); m8, distance between the fore pair of legs and the hind pair of legs; m9, total body length (head without mandible + thorax + abdomen); m10, distance between widest point of the abdomen and hind part of the abdomen.
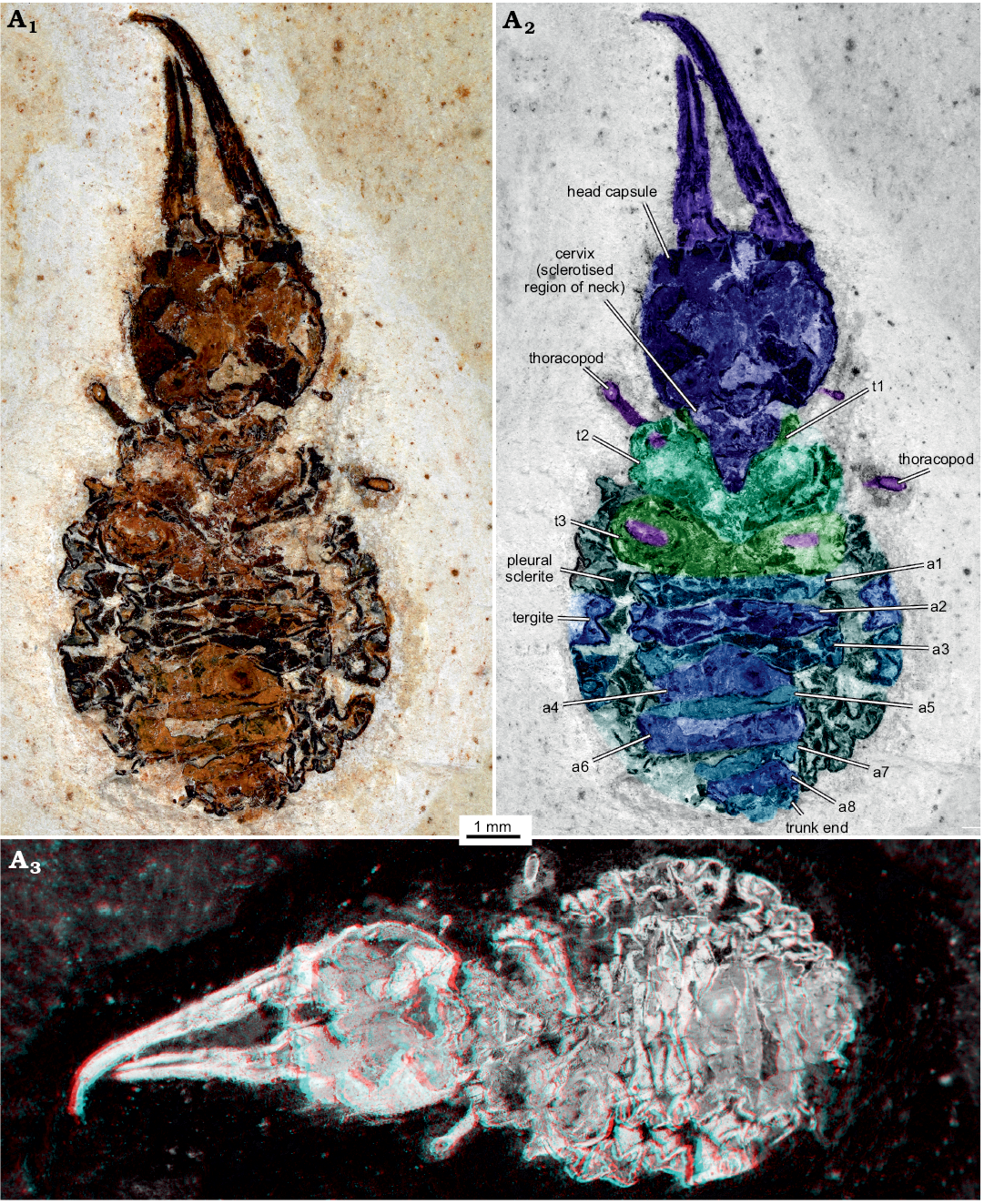
Fig. 2. Myrmeleontiformian lacewing larva (MfN MB.I 2157) from the Crato Formation, Aptian, Lower Cretaceous, Brazil. Photograph in normal light (A1), colour-marked version (A2, matrix digitally amended), stereo-image (A3), please use red-cyan glasses to view. Abbreviations: a1–a8, abdomen segment 1–8; t1–t3, thorax segment 1–3.
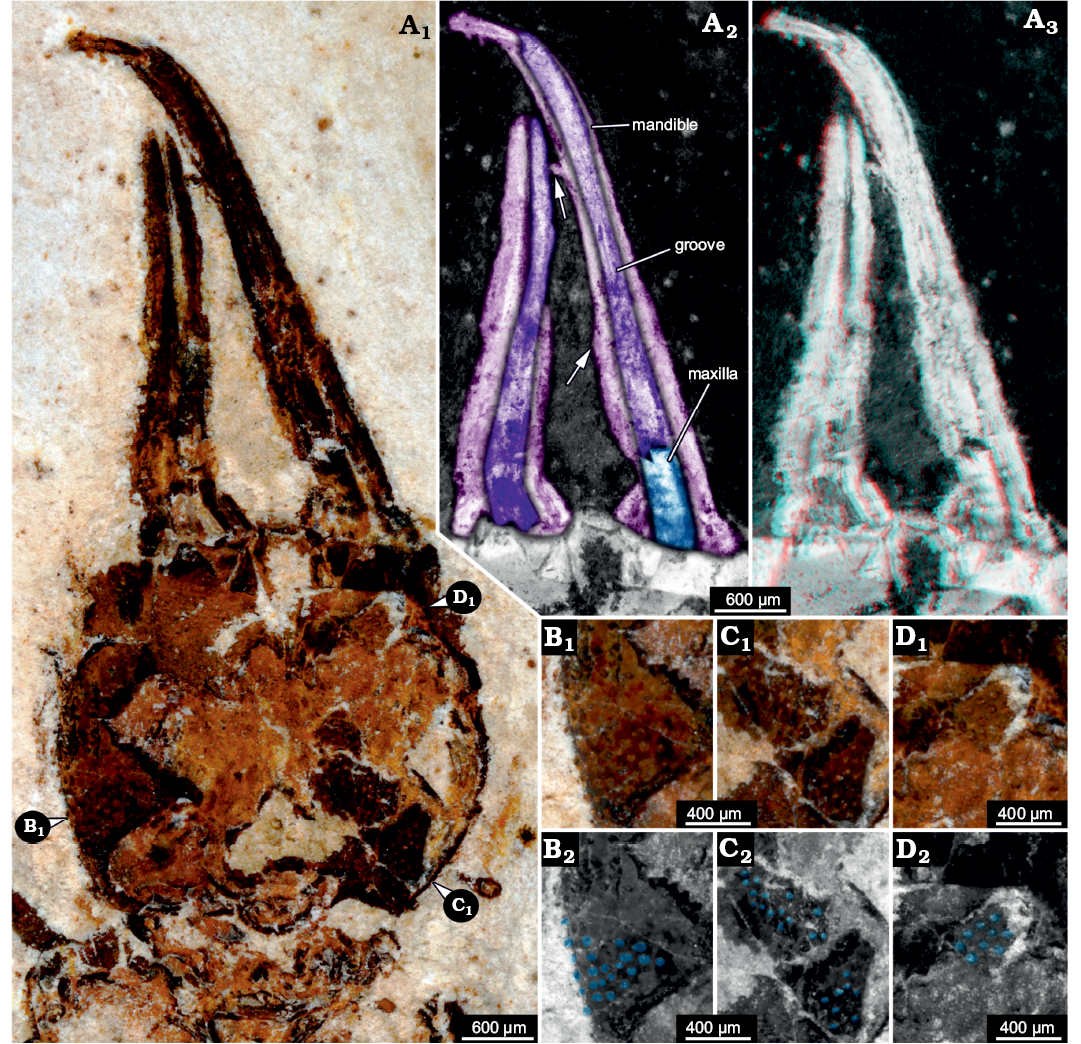
Fig. 3. Myrmeleontiformian lacewing larva (MfN MB.I 2157) from the Crato Formation, Aptian, Lower Cretaceous, Brazil. A. Head, close-up photograph in normal light (A1), colour-marked details of mouth parts (A2), arrows point to presumed remains of broken-off teeth, mouth parts stereo-image (A3), please use red-cyan glasses to view. B–D. Different areas of head capsules with tuberculate surface, positions indicated in A1. Photographs in normal light (B1–D1), colour-marked versions (B2–D2), tubercles marked in blue.
A morphometric approach
Visualization of the measurements.—In order to get a first impression of the data set based on the measurements, we performed an ordination method to visualize the entire data set in a comparative plot. To achieve this, we performed a NMDS analysis on the uncorrected data set (Fig. 4):
The representatives of the group Ascalaphidae (owlflies) are separated into two distinct clusters, one smaller cluster at the right bottom of the plot and a larger one slightly to the right and below the center of the plot. Both clusters are comparably dense and of circle to ellipsoid shape.
In the center of the plot and slightly to the top left there is a cluster representing the group of Chrysopidae (green lacewings). Despite its comparably large sample size of 29 specimens it shows a comparably small morphospace area that appears to be circle-like in shape.
The group Crocinae (thread-winged lacewings) is represented by a cluster starting in the center of the plot and extending far to the bottom left corner in an ellipsoid shape. Hereby, the individual data points are spread out, covering a larger space, although the sample size is about as large as that of Chrysopidae.
The group Hemerobiidae (brown lacewings) is represented by a cluster with a distinct shape. The seven individuals are spread out in a Y-shape in the top left corner of the plot. Also, the individual data points have comparably large distances between each other.
The group Myrmeleontidae (antlions) has the largest sample size of all groups. Yet, it does not occupy the largest area in the plot. Nevertheless, the cluster occupies a large area in the middle of the plot and extends towards the lower right corner. The individuals plot denser in the center and spread out towards the lower right.
The group Nemopterinae (spoon-winged lacewings) is represented by a cluster around the center of the plot but reaches from just underneath the center until half way up to the upper end of the plot. The group in general though is slightly off center to the right. The few specimens are strongly dispersed.
The representatives of Nymphidae (split-footed lacewings) form a cluster spreading around the center, but slightly towards the lower right of the plot. With this pattern, they largely plot in the same area as the representatives of Myrmeleontidae.
The single representative of Osmylidae (lance lacewings) plots at the top left, just between the groups Hemerobiidae and Chrysopidae.
The group Psychopsidae (silky lacewings) is represented by a cluster around the center of the plot, and the individuals plot rather close to each other. However, there are two outliers, which plot below the center. They are distinctly apart from the other representatives of Psychopsidae.
The fossil specimen MfN MB.I 2157 plots at the bottom right end of the larger cluster of Ascalaphidae.
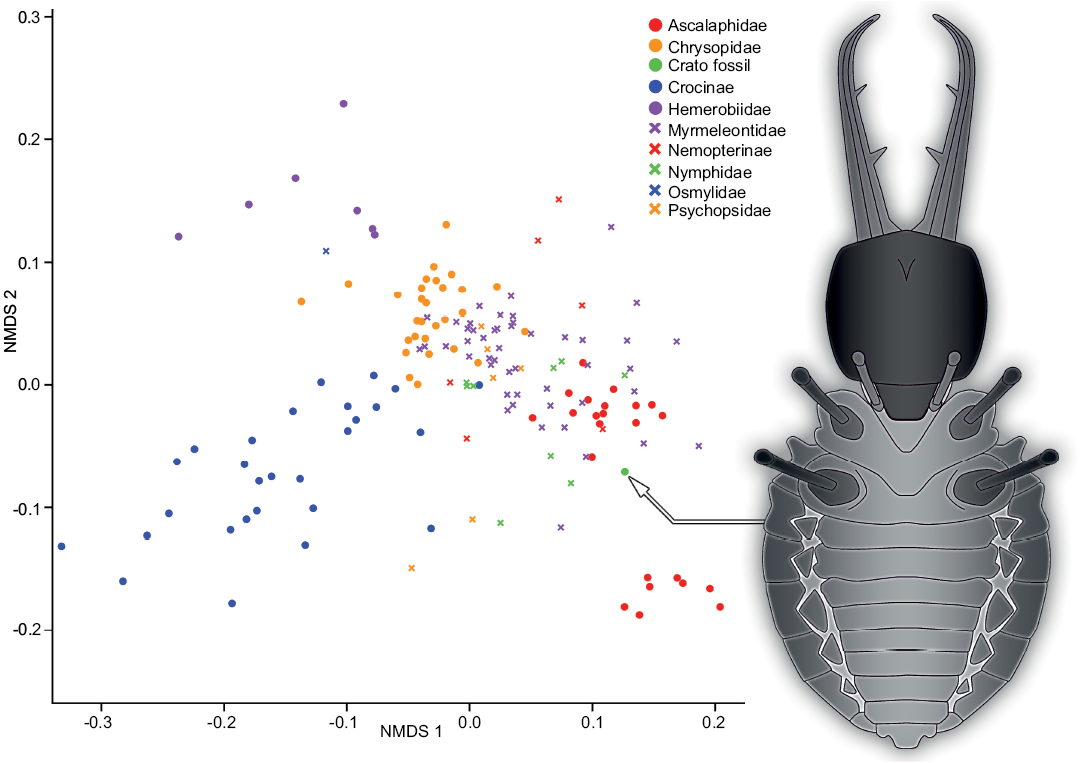
Fig. 4. Non-metric multidimensional scaling (NMDS) plot of measured dimensions. NMDS 1 and 2 are dimensions arbitrarily generated by the model in a way to best represent pairwise dissimilarity between objects (data points). On the right is a simplified restoration of MfN MB.I 2157.
Morphometric comparison of MfN MB.I 2157 to myrmeleontiformian ingroups.—To better understand possible affiliations of the specimen MfN MB.I 2157 to modern ingroups of Myrmeleontiformia, we used a parallel coordinates plot approach and quantified this with a linear discriminant function analysis. The parallel coordinates plots depicted each specimen by a line gradient that takes the correspondent values on the Y-axis for the different nine measurements on the X-axis, all divided by body size to standardize the values (Figs. 5–7).
Dimension 1, the relative length of the outer edge of the stylet (m1/m9), shows overall low variation in most groups; it mostly ranges around 0.2. An exception is the group Ascalaphidae (Fig. 5) which shows two distinctly separate clusters, one located around a value of 0.3 and one around a value of 0.65. Also the group Crocinae (Fig. 5) shows a larger variation for this dimension. The value provides a certain estimation about the curvature of the stylets.
Dimension 2, the relative length of the distance between the largest width of the trunk to the trunk end (m10/m9), shows large variation in most groups and peak values compared to other measurements. Most groups show values of about 0.5 and variation around this value. Ascalaphidae again shows two distinct groups (Fig. 5), one around the value of 0.5 and one around a value of 0.3. For the group Crocinae the peak in this dimension is not as large as for other groups (Fig. 5), i.e., the value is rather low (due to the long neck region). Also for Nymphidae this value is comparably smaller (Fig. 7).
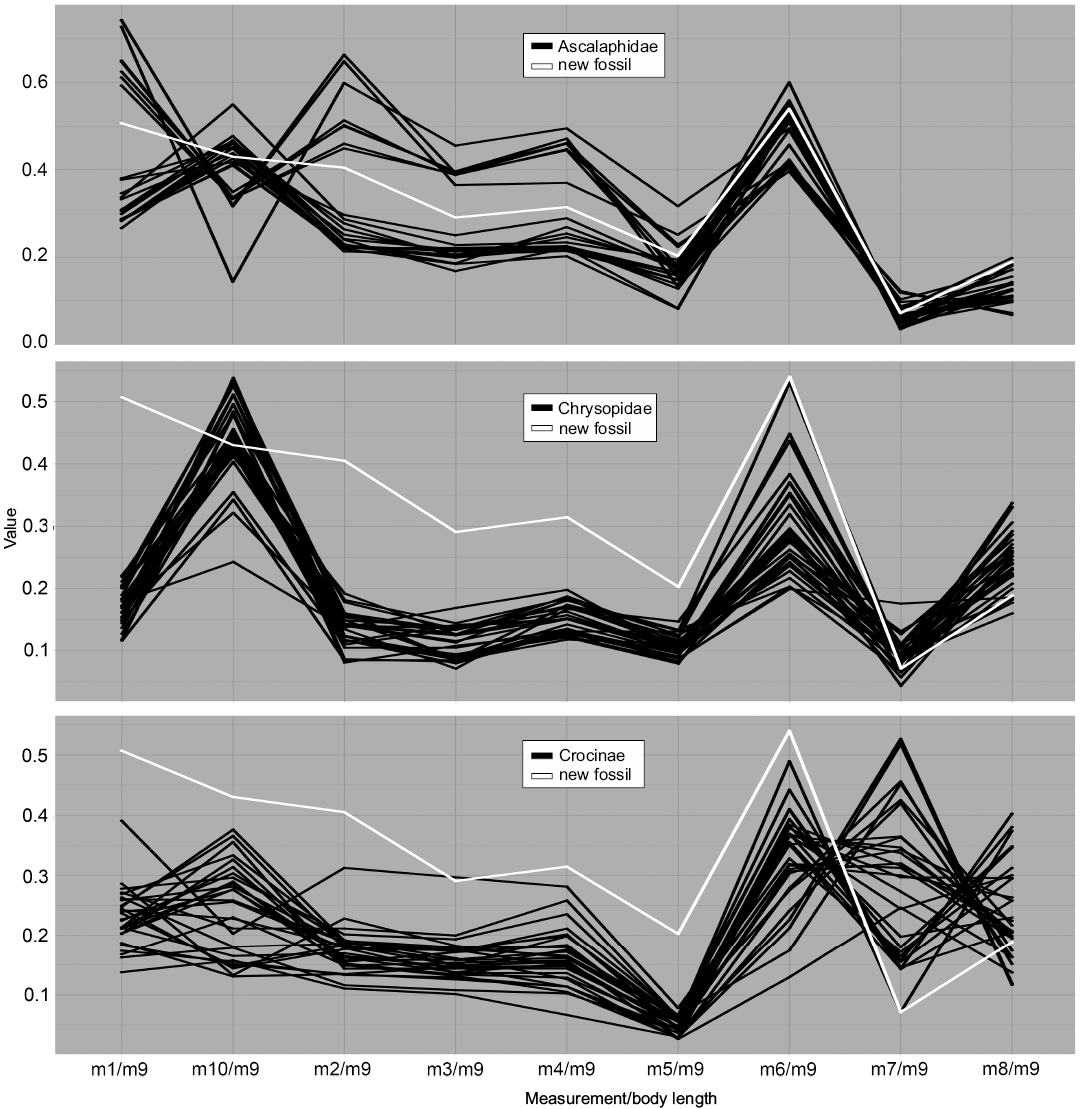
Fig. 5. Parallel coordinates plot for larvae of Ascalaphidae (owl flies), Chrysopidae (green lacewings), and Crocinae (thread-winged lacewings). White line represents MfN MB.I 2157.
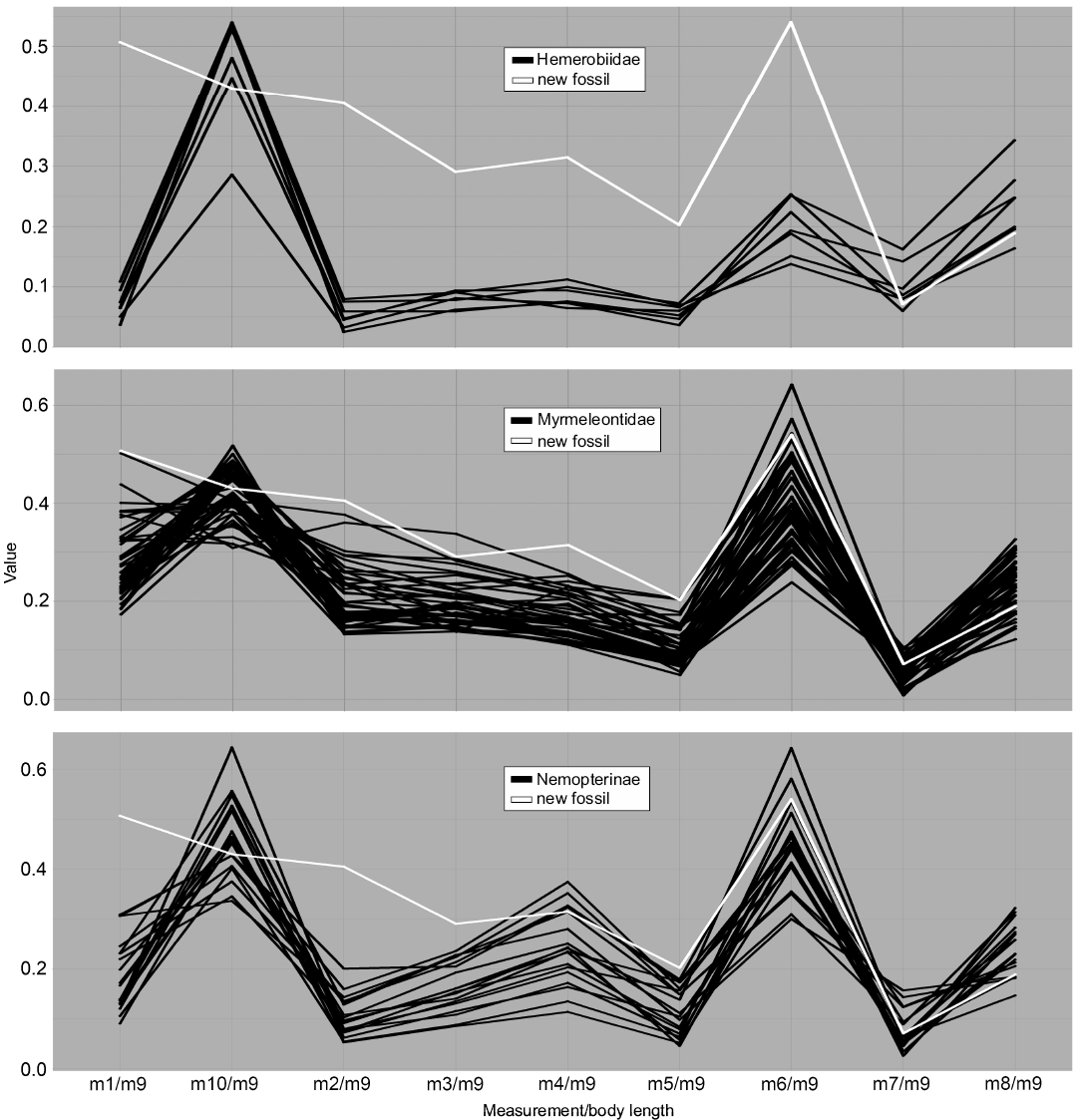
Fig. 6. Parallel coordinates plot for larvae of Hemerobiidae (brown lacewings), Myrmeleontidae (antlions), and Nemopterinae (spoon-winged lacewings). White line represents MfN MB.I 2157.
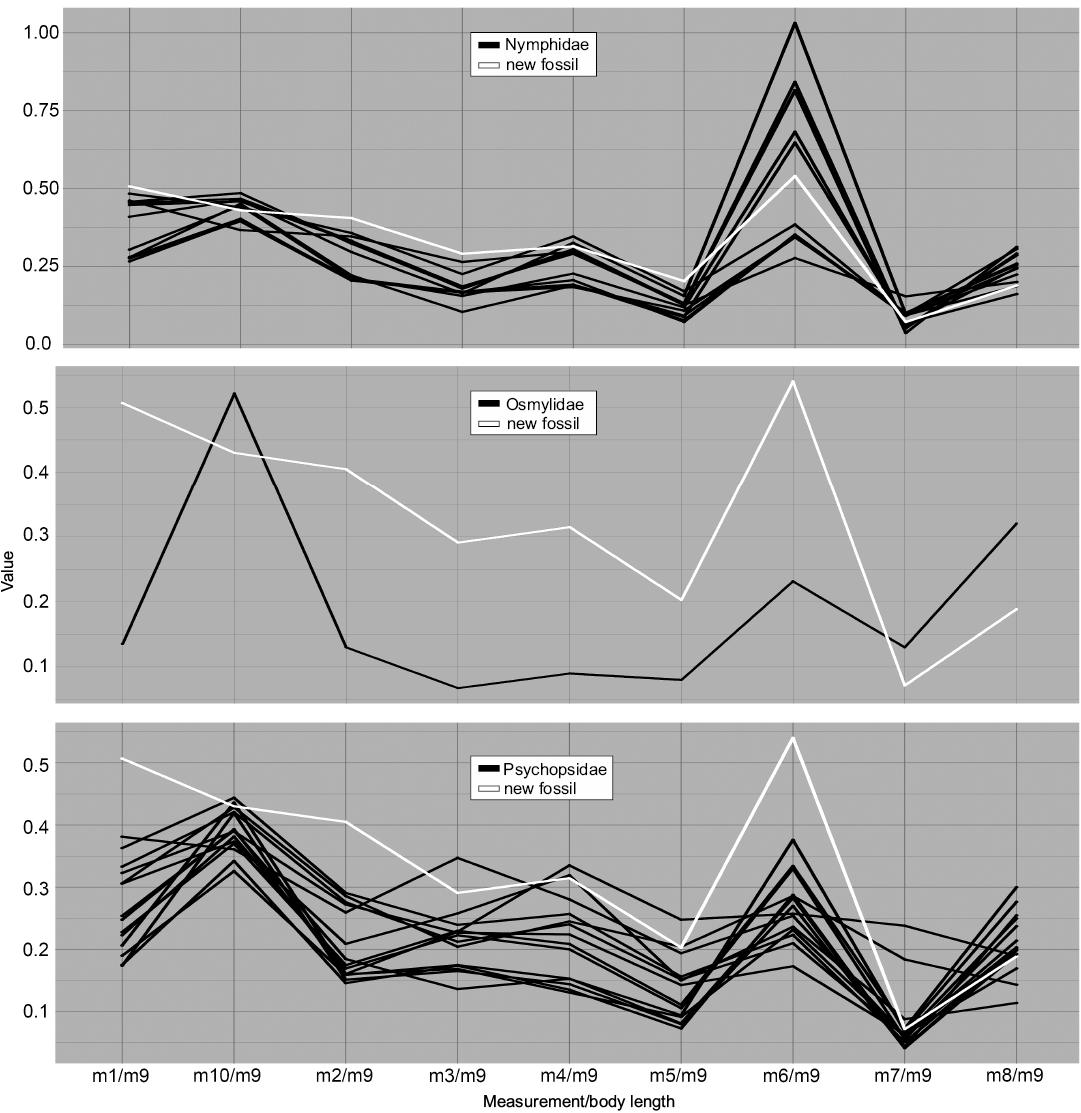
Fig. 7. Parallel coordinates plot for larvae of Nymphidae (split-footed lacewings), Osmylidae (lance lacewings), and Psychopsidae (silky lacewings). White line represents MfN MB.I 2157.
Dimension 3, the relative length of the stylet measured directly from proximal joint to tip (m2/m9), again shows low values with low variation for most groups, taking values around 0.1 to 0.2. Ascalaphidae again represents an exception as there are two distinct clusters around the values of 0.25 and 0.5 (Fig. 5).
Dimension 4, the relative length of the head (m3/m9), shows a comparable pattern. The groups show overall low values and low variation, with values around 0.1 to 0.2. Ascalaphidae shows two distinct groups, one around a value of 0.2 and one around a value of 0.4 (Fig. 5). In Psychopsidae, a larger variation occurs, up to 0.35 (Fig. 7).
Dimension 5, the relative width of the head (m4/m9), shows low values with low variation in most groups. Ascalaphidae forms again two sub-groups, one around a value of 0.2 and one around a value of 0.4 (Fig. 5). However, the two groups, Crocinae (Fig. 5) and Nemopterinae (Fig. 6), show a comparably larger variation in this dimension than for the previous two dimensions. In Psychopsidae, a similar variation as in the previous dimension occurs (Fig. 7).
Dimension 6, the relative width of the neck (m5/m9), shows an even lower variation around lower values for most groups, mostly ranging between 0.05 and 0.2. An exception is Psychopsidae, which shows also in this dimension considerable variation (Fig. 7). Ascalaphidae does not show two distinct sub-groups in this dimension (Fig. 5).
Dimension 7, the relative maximum width of the trunk (m6/m9), on the contrary, has the largest peaks for most groups and overall large variation in values. Ascalaphidae only shows a single group and also rather low variation in comparison to other groups (Fig. 5). Hemerobiidae (Fig. 6) and Nemopterinae show a weak peak in this dimension, as well as Psychopsidae (Fig. 7). Nymphidae has a very dominant peak with values above 1.0 (Fig. 7), which is the only dimension in which this group steps out.
Dimension 8, the relative distance between the back of the head to the anterior insertion of the first walking leg (m7/m9), again shows a drop in both values and variation for most groups. The only exception is Crocinae, which shows large variation of values between 0.1 and 0.5 (Fig. 5).
Dimension 9, the relative distance between the insertions of the first pair of walking legs and those of the last pair of walking legs (m8/m9), shows larger values and variation than the previous measurement among most groups. The group Crocinae again shows a larger variation than all other groups (Fig. 5).
The results of the DFA for the first model (with Ascalaphidae as one group) shows a high correlation of MfN MB.I 2157 to Ascalaphidae (above 0.8; SOM: table 1) and a low correlation between MfN MB.I 2157 and Myrmeleontidae (above 0.1; SOM: table 1). Due to the prominent separation of two sub-groups of Ascalaphidae in the morphospaces, we decided to split the group in two sub-groups based on those characters and performed a second analysis with those new parameters. The first sub-group of Ascalaphidae represents possibly a stage 1 specimen, the second a stage 3 specimen. This time, the model showed a medium correlation between MfN MB.I 2157 and Myrmeleontidae (above 0.5; SOM: table 1) and also a medium correlation between the second sub-group of Ascalaphidae (about 0.4; SOM: table 1). AUC values for both models are sufficient, but tested misclassification rates were rather high for both models.
Discussion
The morphology of Myrmeleontiformia.—Before further interpreting the results of the morphometric analysis for the ingroups of Myrmeleontiformia, a few points of the statistical accuracy of this analysis need to be addressed. The data points (measurements) originate from both literature and specimens documented by the authors. While we were able to use high-resolution images for our analysis of the latter, the former provided us often with reconstruction drawings and images of varying quality (notable exceptions are papers by Badano and co-workers, e.g., Badano and Pantaleoni 2014a, b; Badano et al. 2017, which feature high quality photographs). Measuring of distances could therefore be influenced by imprecisions in the source material.
Furthermore, the sample sizes are different for each of the groups. While we have a comparably vast record for Myrmeleontidae in our analysis, groups such as Hemerobiidae or Osmylidae have very few representatives. This becomes apparent especially in the parallel coordinates plots.
With that being said, our different approaches to compare outer body morphology of myrmeleontiformian ingroups had comparable results. The parallel coordinates plots are a great tool for comparing the morphometry of different groups in a direct way. They emphasize points of interest in the measurements, which all depict real outer body morphology. The NMDS helps to explore the data set and to find interesting relations between the groups. Lastly, the DFA showed a possible affiliation of MfN MB.I 2157 with some extant groups of Myrmeleontiformia.
In both visual methods, the group Ascalaphidae showed an interesting pattern, namely being separated into two distinct sub-groups in many dimensions (Figs. 4, 5). This mostly concerns measurements around the head, like head length and width, and is best explained by the fact that possibly only stage 1 and stage 3 specimens have been used as material for this study. If some stage 2 specimens would have been included, they would most likely have fallen in between the two clusters for both analyses and therefore might have filled up the space. This pattern, observed both in the parallel coordinates plot and the NMDS, also led us to distinguish the two sub-groups for a second model in the DFA.
The group Chrysopidae shows a centered position in the NMDS plot (Fig. 4) as well as a pattern in the parallel coordinates plot that is similar to most other groups (Fig. 5). The comparably higher variation within Chrysopidae most likely originates from the comparably larger sample size.
The group Crocinae shows large variation in the NMDS plot as well as a significant distribution, distinct from other groups (Fig. 4). This is mirrored in the parallel coordinates plot, where it shows high variation especially in the neck length (m7/m9, Fig. 5). This reflects the partly extreme morphologies with very elongated necks of Crocinae (see discussion in Herrera-Flórez et al. in press).
The group Hemerobiidae does not really show interesting patterns, except maybe the comparably slender body, represented by m6 (Fig. 6). The group shows a quite distinct position in the NMDS plot (Fig. 4), yet its sample size is rather small.
The group Myrmeleontidae is significant in that it has the largest sample size, apparent in both the NMDS and the parallel coordinates plot (Figs. 4, 6). Apart from that, it shows lower variation and a similar pattern to most other myrmeleontiformian groups.
The group Nemopterinae shows higher variation in their head width (m4, Fig. 6) but apart from that they show a rather similar parallel coordinates plot pattern compared to the other groups.
The group Nymphidae is remarkable in that its representatives show wide bodies, which is reflected in m6 (Fig. 7) where some specimens possess bodies that are even wider than long. Also, they show high variation in the NMDS plot compared to their small sample size (Fig. 4).
The group Osmylidae was only represented by a single specimen, equalling little more than a test shot. The group is most similar in many aspects to Chrysopidae and in some aspects to Hemerobiidae.
Finally, the group Psychopsidae shows comparably higher variation in the parallel coordinates plot as in the NMDS plot, except for the two outliers that are shown in the latter (Fig. 4).
Possible identity of MfN MB.I 2157.—MfN MB.I 2157 does not preserve many of the characters that could be used for identifying it as a representative of any of the major modern lineages of Myrmeleontiformia, or as closely related to one of these, as for example used in Badano et al. (2017, 2018) or Jandausch et al. (2018). In this aspect the preservation is clearly less detailed compared to the average preservation in amber (Badano et al. 2018). Therefore, we will use here body shape as the main line of argumentation. Although this is not a strict phylogenetic approach, it in fact resembles in many aspects a phenetic one; aspects of the body shape may indeed represent true apomorphies that can only be revealed by such an approach. As laid out above, body shape of the larvae, proxied by the dimensions measured, allows to identify the major modern lineages.
When comparing MfN MB.I 2157 (Fig. 8C) to the modern groups, it becomes obvious that it plots outside the areas occupied by most groups. The only two exceptions, which are supported by all three types of analysis, are the two groups Ascalaphidae (Fig. 8B) and Myrmeleontidae (Fig. 8A, D); also there is only little deviation from the area occupied by Nymphidae. The specimen falls well into the occupied area of Ascalaphidae and Myrmeleontidae in the parallel coordinates plot (Figs. 5, 6). The NMDS (Fig. 4) shows the same pattern. The DFA analysis shows a stronger support for a connection between MfN MB.I 2157 and Ascalaphidae in the first model. In the second model, it shows stronger affiliation to Myrmeleontidae, but the difference to the second group of Ascalaphidae (stage 3) is only about 15% (SOM: table 1). Also, the statistical power of the second model is lower, compared to the first. The DFA would therefore support an interpretation of MfN MB.I 2157 as representative of Ascalaphidae, but also offer an option as being interpreted as representative of Myrmeleontidae. However, the visual analyses show a better match of specimen MfN MB.I 2157 with Myrmeleontidae.
The results could therefore be seen as a support for the suggestion of Martins-Neto et al. (2007: fig. 11.69d), interpreting the here presented fossil as a possible larva of Myrmeleontidae. Yet, the case of Ascalaphidae is more complicated. We see two very distinct sub-groups within Ascalaphidae. One group has rather long stylets and short trunks, the other group shorter stylets and longer trunks. The first sub-group most likely corresponds to stage 1 specimens, the second group to stage 3 specimens. The fossil specimen is right in between the two in many aspects. We could therefore speculate that the fossil is a stage 2 larva of Ascalaphidae. Most neuropterans develop through three larval stages. Mostly figured in the literature we find stage 3 specimens, more rarely stage 1 specimens; stage 2 specimens appear most uncommon. It is hence not easily testable whether the here described specimen could represent such a stage and it cannot be easily falsified. We tentatively conclude that the specimen is most likely either a representative of Myrmeleontidae, of Ascalaphidae, or of the direct lineage towards the node of Myrmeleontidae + Ascalaphidae. Also the similarity of MfN MB.I 2157 to Nymphidae may not be surprising as the latter is one of the possible sister groups to Myrmeleontidae + Ascalaphidae (but see, e.g., Winterton et al. 2018). It seems that the body shape, used as proxy here, also has a phylogenetic signal. We therefore plan to further expand the data set and explore this aspect.
Based on the assumption that MfN MB.I 2157 is most likely an ingroup of Myrmeleontidae + Ascalaphidae, we can also better understand some morphological aspects of the larva. Most importantly: the two possible indications of teeth probably represent damaged (broken) teeth, as larvae of Myrmeleontidae have one to four teeth (Fig. 8E), extant larvae of Ascalaphidae have three teeth, and fossil representatives closely related to these two groups have two teeth (Fig. 8G; Wang et al. 2016; Badano et al. 2018); the latter seems to be also the case in the here described fossil. Also, the protrusion of the metathorax can in this frame be well understood as a partly preserved scolus (structures possibly used for carrying camouflage).
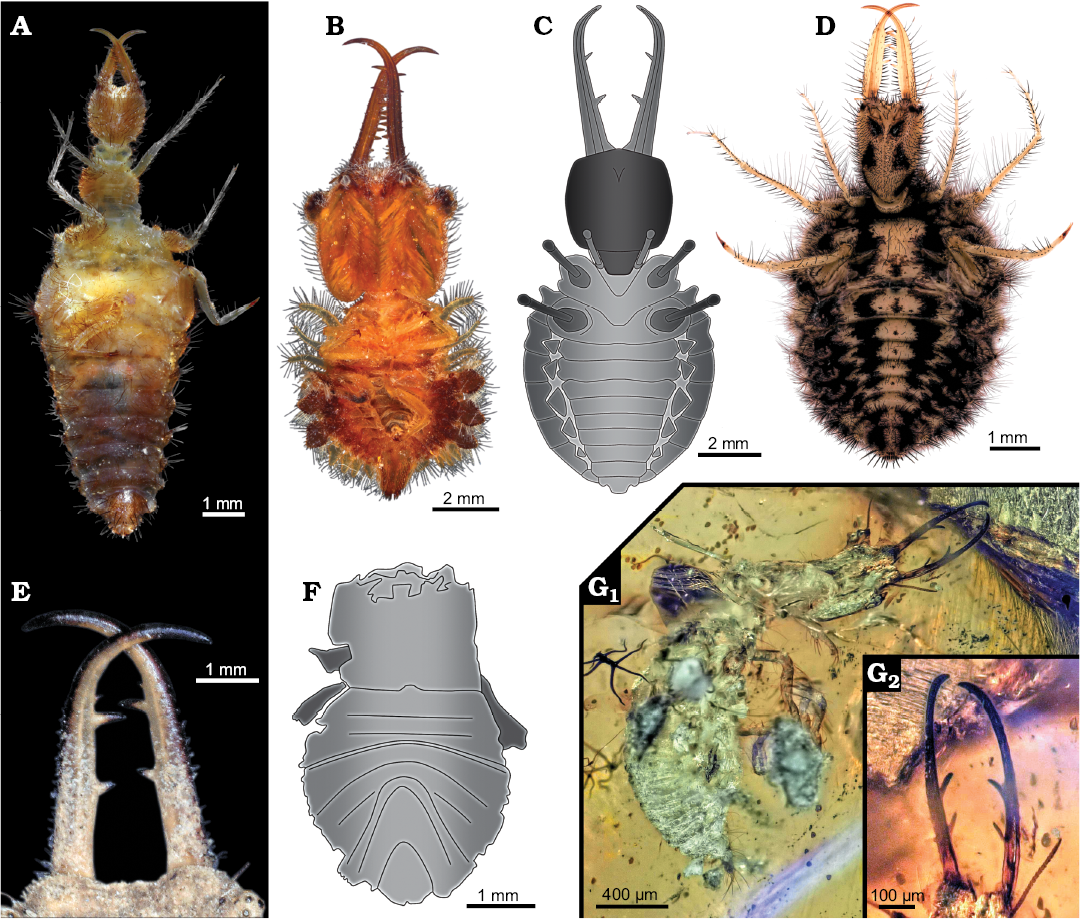
Fig. 8. Examples of extant and fossil myrmeleontiformian larvae. A. Extant Myrmeleontidae (ZMH 62891), note set off anterior trunk. B. Extant Ascalaphidae (ZMH 62880), note very round trunk. C. Drawing of MfN MB.I 2157 from Crato Formation, Aptian, Lower Cretaceous, Brazil. D. Extant Myrmeleontidae (ZSM unnumbered), note very round trunk. E. Close-up on extant myrmeleontiformian larva (ZSM unnumbered) with two prominent teeth and one very tiny tooth. F. Drawing of unnumbered specimen from Crato Formation, Aptian, Lower Cretaceous, Brazil, simplified from Rumbucher 1995. G. Larva with two prominent teeth, Burmese amber, Cenomanian, Upper Cretaceous, Myanmar (formerly collection Jörg Wunderlich under F3199_BU_CJW, deposited in the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-University Munich, Germany). Overview image (G1), close-up on stylet region (G2).
A second specimen?—While the here described specimen was already briefly mentioned in Martins-Neto et al. (2007), this represents not the first record of a possible myrmeleontiformian larva from the Crato deposits. Rumbucher (1995: 54, fig. 6) figured a small specimen, about 5 mm, that he labelled “Myrmelionidae, Larvenstadium”. According to the label and text the specimen should have been figured in Maisey (1991). Yet, there seems to be no corresponding specimen figured in Maisey (1991). We therefore do not have information where this specimen is located. Hence our data basis for understanding the specimen is the image provided by Rumbucher (1995: fig. 6; Fig. 8F).
The specimen lacks the head, only the trunk appears to be preserved, including some remains of the thorax appendages, i.e., the legs. As the length of the specimen was reported to be 5 mm it was smaller than the here described specimen in which the corresponding body region measures 8 mm. One could speculate that Rumbucher’s (1995) specimen is an earlier instar of the same species as the here described specimen. The size increase of about factor 1.6 is comparable to an increase from a stage 1 larva to a stage 2 larva in some extant species, such as Solter ledereri Navás, 1912 with a factor of 1.7 (based on Satar et al. 2014) or Myrmeleon bore (Tjeder, 1941) with a factor of almost 1.8 (Nicoli Aldini 2007).
Yet, there are some major differences between the two specimens that make it unlikely that they are conspecific. Firstly, the shape of the trunk is quite different. In the here described specimen the trunk is of overall circular to oval shape as in many larvae of Ascalaphidae (e.g., Badano and Pantaleoni 2014a). In Rumbucher’s (1995) specimen, the anterior region of the trunk, corresponding to either the thorax or at least the anterior two thorax segments, is more set off from the posterior trunk. The anterior trunk is more or less rectangular, only the posterior region appears more rounded. Such a basic trunk arrangement is known from some larvae of Myrmeleontidae, but also for example from certain larvae of Crocinae (e.g., Monserrat 2008).
A second major difference is the arrangement of the individual trunk segments. In the here described larva the sternites appear rather straight as in many larvae of Myrmeleontidae and Ascalaphidae (e.g., Badano and Pantaleoni 2014a) and in fact many other larvae of Neuroptera (e.g., Monserrat 2008). Rumbucher’s (1995) specimen, on the other hand, has strongly U-shaped segments. Such an arrangement is found only in some neuropteran larvae (e.g., Monserrat 2008; Badano and Pantaleoni 2014a, b). A drastic change from strongly U-shaped segments to straight ones has so far not been reported during the post-embryonic ontogeny of neuropterans. It is therefore highly unlikely that the two specimens are conspecific.
For Rumbucher’s (1995) specimen we cannot even clearly demonstrate that it is indeed a representative of Myrmeleontidae. Neither can we conclude a position within Myrmeleontiformia. In fact it is, based on the available image, not easy to find strong characters indicating an ingroup position of Neuroptera as the head is missing. Yet, there are also no strong characters excluding such an interpretation.
The early fossil record of Myrmeleontiformia.—The compression fossil that we present here is the oldest larva known for Myrmeleontiformia, possibly together with Rumbucher’s (1995) specimen. All fossil larvae of Neuroptera older than that have been interpreted as representatives of the lineage of Chrysopidae.
It is partly surprising that the oldest fossil larva of Myrmeleontiformia is most likely a representative of the group Ascalaphidae + Myrmeleontidae. The morphology of this group is rather derived. This partly indicates that the origin of Myrmeleontiformia is far older, but not represented in the fossil record. It seems in general presumed that many aspects of the larvae of Psychopsidae are a kind of proxy for the early larvae of Myrmeleontiformia. They lack teeth in the stylets, which might represent a plesiomorphy (but see discussion in Badano et al. 2018 and Haug et al. 2019). Also, they still possess empodia, but these seem to have been lost in various lineages repeatedly. It is therefore partly unclear whether larvae of Psychopsidae are indeed a good proxy for those of the stem species of Myrmeleontiformia. Fossils that might be further candidates for such proxy (Badano et al. 2018; Haug et al. 2019) differ significantly from the larvae of Psychopsidae. In any case, the oldest fossil larva of Myrmeleontiformia clearly possesses a quite derived morphology and not a rather plesiomorphic one.
Further implications: preservation of neuropteran larvae.—Besides providing an important minimum age for the occurrence of the specific larval morphology, the new data on MfN MB.I 2157 have further implications. The present fossil partly challenges the assumption that fossils from soil dwellers are only expected to be preserved in amber (Badano et al. 2018). So far, most fossil larvae of Neuroptera have been reported from amber. Yet, as demonstrated by the here described fossil, there is obviously the possibility that such organisms are also preserved as compression fossils. It is in fact quite astonishing that we so far lack aquatic neuropteran larvae from compression-type preservation. Larvae of Megaloptera, the likely sister group to Neuroptera, are likewise aquatic and are well known as compression fossils (e.g., Ponomarenko 1976; Wang and Zhang 2010). Quite on the contrary, we have larvae in amber of which we know the modern counterparts to be aquatic, which is usually considered to be more unusual, including Nevrorthidae (e.g., Weitschat and Wichard 2002; Gröhn 2015), in some cases with several specimens in a single amber piece (Wichard et al. 2009), Sisyridae (Weitschat and Wichard 2002; Wichard et al. 2009), and also the partly aquatic larvae of Osmylidae (Wichard et al. 2009). It may be worthwhile to inspect collections of compression fossils that contain other aquatic larvae of Insecta specifically for those of Neuroptera.
Further implications: the Crato Formation.—For the Crato Formation, the fauna composition and abundance of different groups of Insecta are remarkable. Most preserved terrestrial forms of Insecta are winged adult forms and, to our knowledge, no terrestrial larvae or nymphs are known so far from the Crato Formation (Martill et al. 2007; personal observation of JTH). It has been assumed, that the original area of the formation was a lake or lagoon, with at least partly high freshwater entry. Aquatic nymphs (“naiads”) are accordingly preserved in comparatively high numbers, such as immatures of dragonflies and damselflies (Odonata) and especially mayflies (Ephemeroptera; Grimaldi 1990; Martill et al. 2007). Interestingly, there are also findings of spiders (e.g., Dunlop and Barov 2005; Selden et al. 2006), some scorpions (Carvalho and Lourenço 2001; Menon 2007) and centipedes (e.g., Martill and Barker 1998; Wilson 2001; Menon et al. 2003) and also some scattered representatives of Diplura and Collembola (Bechly 1998; Wilson and Martill 2001; Martill et al. 2007). In these cases, an airborne fallout, as to be expected for winged forms, is excluded. These non-flying terrestrial organisms must have been transported, e.g., from temporarily inundated areas to the lake or lagoon system. It remains unclear why representatives of these non-winged organisms obviously were transported somehow, whereas terrestrial nymphs and holometabolan larvae were not. It seems rather plausible for groups which live in more hidden habitats, such as barks of trees or in nests, but many of these groups are missing in general (adult and immature forms) in the Crato Formation. Yet, also nymphs of the very abundant groups of Dictyoptera and Orthoptera are entirely missing; this is rather surprising.
The here presented specimen, possibly together with the specimen described by Rumbucher (1995), presents the only non-aquatic immatures of Insecta from the Crato Formation. These specimens are so far also the only cases of holometabolan larvae of the Crato Formation.
Conclusions
For this study, we applied quantitative methods to evaluate the character composition of the oldest known myrmeleontiformian larva. With this morphometric approach, we were able to draw conclusions about the phylogenetic position of this specimen (i.e., an ingroup of Myrmeleontidae + Ascalaphidae). Additionally, we could show that the morphology of this old specimen is derived and resembles modern representatives. With its preservation as compression fossil instead of an amber inclusion, it provides hints to further interesting finds of holometabolan larvae outside amber. Furthermore, it shows that also non-aquatic immatures of Insecta can be found in the Crato Formation. All in all, this single specimen provides several important and unexpected new hints for future research on holometabolan larvae.
Acknowledgements
We thank Anneke van Heteren (ZSM) and an anonymous reviewer for helpful comments. Original data acquisition was during a student trip to the Museum für Naturkunde (Berlin). Martin Schwentner and Martin Husemann (both ZMH) are thanked for their support during a student trip to Hamburg. We thank all students helping during these trips as well as the Faculty of Biology, Ludwig-Maximilians-University Munich (Germany) and the Lehre@LMU program for support. We thank Lars Hendrich and Katja Neven (Section Insecta Varia-ZSM) for providing access to comparative material. We also thank J. Matthias Starck (Ludwig-Maximilians-University Munich) for long-term support. Joshua Gauweiler (Ludwig-Maximilians-University Munich) kindly prepared the drawing used in Fig. 1. This is LEON publication #11. The study was kindly supported by the Volkswagen Foundation in the course of a Lichtenberg professorship for JTH. CH received funding via LMUexcellent. We thank all people providing free or low cost software for their general support of science.
References
Agarwal, S., Wills, J., Cayton, L., Lanckriet, G., Kriegman, D. and Belongie, S. 2007. Generalized Non-metric Multidimensional Scaling. Proceedings of Machine Learning Research 2: 11–18.
Andrienko, G. and Andrienko, N. 2001. Constructing parallel coordinates plot for problem solving. In: A. Butz, A. Krueger, P. Olivier, S. Schlechtweg and M. Zhou (eds.), Proceedings of the 1st International Symposium on Smart Graphics, 9–14. ACM, New York
Badano, D. and Pantaleoni, R.A. 2014a. The larvae of European Ascalaphidae (Neuroptera). Zootaxa 3796: 287–319. Crossref
Badano, D. and Pantaleoni, R.A. 2014b. The larvae of European Myrmeleontidae (Neuroptera). Zootaxa 3762: 1–71. Crossref
Badano, D., Aspöck, U., Aspöck, H., and Cerretti, P. 2017. Phylogeny of Myrmeleontiformia based on larval morphology (Neuropterida: Neuroptera). Systematic Entomology 42: 94–117. Crossref
Badano, D., Engel, M. S., Basso, A., Wang B., and Cerretti P. 2018. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Communications 9: 1–14. Crossref
Barling, N., Martill, D.M., Heads, S.W., and Gallien, F. 2015. High fidelity preservation of fossil insects from the Crato Formation (Lower Cretaceous) of Brazil. Cretaceous Research 52: 605–622. Crossref
Bechly, G. 1998. Santana-Schatzkammer fossiler Insekten. Fossilien 2: 95–99.
Carvalho, M.G.P. de and Lourenço, W.R. 2001. A new family of fossil scorpions from the Early Cretaceous of Brazil. Comptes Rendus de l’Académie des Sciences-Series IIA-Earth and Planetary Science 332: 711–716. Crossref
Dittmann, I.L., Hörnig, M.K., Haug, J.T., and Haug, C. 2015. Raptoblatta waddingtonae n. gen. et n. sp. an Early Cretaceous roach-like insect with a mantodean-type raptorial foreleg. Palaeodiversity 8: 103–111.
Dunlop, J.A. and Barov, V. 2005. A new fossil whip spider (Arachnida: Amblypygi) from the Crato Formation of Brazil. Revista Ibérica de Aracnología 12: 53–62.
Engel, M.S. and Grimaldi, D.A. 2007. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). American Museum Novitates 3587: 1–58. Crossref
Engel, M.S. and Nel, A. 2017. A replacement family-group name among fossil Neuroptera (Insecta). Novitates Paleoentomologicae 19: 1–3. Crossref
Engel, M.S., Winterton S.L., and Breitkreuz L.C. 2018. Phylogeny and evolution of Neuropterida: where have wings of lace taken us? Annual Review of Entomology 3: 531–551. Crossref
Ereshefsky, M. 2002. Linnaean ranks: Vestiges of a bygone era. Philosophy of Science 69: 305–315. Crossref
Fang, S.W., Zhang, X., Yang, Q., Guan, X.Y., Gao, T.P., and Ren, D. 2010. Mimicry and extinction mechanism of kalligrammatid lacewings during Mesozoic (Neuroptera, Kalligrammatidae): Acta Zootaxonomica Sinica 35: 165–172.
Fisher, R.A. 1936. The use in multiple measurements in taxonomic problems. Annals of Human Genetics 7: 179–188. Crossref
Fox, J. and Weisberg, S. 2011. An R Companion to Applied Regression. Second Edition. Thousand Oaks CA, Sage. Accessed at http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. Crossref
Garzón-Orduña, I.J., Winterton, S.L., Jiang, Y.-L., Breitkreuz, L.C.V., Duelli, P., Engel, M.S., Penny, N.D., Tauber, C.A., Mochizuki, A., and Liu, X.-Y. 2018. Evolution of green lacewings (Neuroptera: Chrysopidae): a molecular supermatrix approach. Systematic Entomology 44: 499–513. Crossref
Grimaldi, D.A. 1990. Insects from the Santana Formation, Lower Cretaceous, of Brazil. Bulletin of the American Museum of Natural History 195: 1–193.
Gröhn, C. 2015. Einschlüsse im baltischen Bernstein. 424 pp. Wachholtz Verlag-Murmann Publishers, Kiel.
Hand, D.J. and Till, R.J. 2001. A simple generalisation of the area under the ROC curve for multiple class classification problems. Machine Learning 45: 171–186. Crossref
Hastie, T. and Tibshirani, R. Original R port by F. Leisch, K. Hornik and B. D. Ripley 2017. mda: Mixture and Flexible Discriminant Analysis. R package version 0.4-10. Accessed at https://CRAN.R-project.org/package=mda Crossref
Haug, C., Herrera-Flórez, A.F., Müller, P., and Haug, J.T. 2019. Cretaceous chimera—an unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity 12: 1–11 [published online, https://doi.org/10.18476/pale.v12.a1]. Crossref
Haug, J.T., Labandeira, C.C., Santiago-Blay, J.A., Haug, C., and Brown, S. 2015. Life habits, hox genes, and affinities of a 311 million-year-old holometabolan larva. BMC Evolutionary Biology 15: 1–10. Crossref
Haug, J.T., Müller, P., and Haug, C. 2018. The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters 4: 1–8. Crossref
Herrera-Flórez, A.F., Haug, C, Burmeister, E.-G. and Haug, J.T. in press. A neuropteran insect with the relatively longest prothorax: the “giraffe” among insects is the larva of a Necrophylus species from Libya. Spixiana.
Hörnig, M.K., Haug, J.T., and Haug, C. 2013. New details of Santanmantis axelrodi and the evolution of the mantodean morphotype. Palaeodiversity 6: 157–168.
Hörnig, M.K., Haug, J.T., and Haug, C. 2017. An exceptionally preserved 110 million years old praying mantis provides new insights into the predatory behaviour of early mantodeans. PeerJ 5: 1–19. Crossref
Hörnig, M.K., Haug, C., Schneider, J.W., and Haug, J. T. 2018. Evolution of reproductive strategies in dictyopteran insects—clues from ovipositor morphology of extinct roachoids. Acta Palaeontologica Polonica 63: 1–24. Crossref
Hu, J., Lu, X., Wang, B., and Liu, X. 2018. Taxonomic notes on Babinskaiidae from the Cretaceous Burmese amber, with the description of a new species (Insecta, Neuroptera). Zookeys: 748: 31–46. Crossref
Huang, D., Azar, D., Engel, M.S., Garrouste R., Cai, C., and Nel, A. 2016. The first araripeneurine antlion in Burmese amber (Neuroptera: Myrmeleontidae). Cretaceous Research 63: 1–6. Crossref
Jandausch, K., Beutel, R.G., Pohl, H., Gorb, S.N., and Büsse, S. 2018. The legs of “spider associated” parasitic primary larvae of Mantispa aphavexelte (Mantispidae, Neuroptera)—Attachment devices and phylogenetic implications. Arthropod Structure and Development 47: 449–456. Crossref
Khramov, A.V. and Vasilenko, D.V. 2018. New records of Grammolingiidae, Saucrosmylidae, and Panfiloviidae (Insecta: Neuroptera) from the Jurassic of Mongolia and Kyrgyzstan. Paleontological Journal 52: 1391–1400. Crossref
Kruskal, J.B. 1964. Nonmetric Multidimensional Scaling: A numerical approach. Psychometrika 29: 115–129. Crossref
Kuhn, M. (contributions from Wing, J., Weston, S., Williams, A., Keefer, C., Engelhardt, A., Cooper, T., Mayer, Z., Kenkel, B., the R Core Team, Benesty, M., Lescarbeau, R., Ziem, A., Scrucca, L., Tang, Y., Candan, C., and Hunt, T. 2019. caret: Classification and Regression Training. R package version 6.0-84. Accessed at https://CRAN.R-project.org/package=caret Crossref
Laurin, M. 2010. The subjective nature of Linnaean categories and its impact in evolutionary biology and biodiversity studies. Contributions to Zoology 79: 131–146. Crossref
Lee, C., Kim, J., Kwanghyun, H., O’Flaherty, V., and Hwang, S. 2009. Quantitative analysis of methanogenic community dynamics in three anaerobic batch digesters treating different wastewaters. Water Research 43: 157–165. Crossref
Lu, X., Hu, J., Wang, B., Zhang, W., Ohl, M., and Liu, X. 2019. New antlions (Insecta: Neuroptera: Myrmeleontidae) from the mid-Cretaceous of Myanmar and their phylogenetic implications. Journal of Systematic Palaeontology 17: 995–1012. Crossref
Lu, X., Zhang, W., and Liu, X. 2017. Discovery of the family Babinskaiidae (Insecta: Neuroptera) in mid-Cretaceous amber from Myanmar. Cretaceous Research 71: 14–23. Crossref
MacLeod, E.G., 1970. The Neuroptera of the Baltic Amber. I. Ascalaphidae, Nymphidae, and Psychopsidae. Psyche 77: 147–180. Crossref
Maisey, J.G. 1991. Santana Fossils: An Illustrated Atlas. 459 pp. T.F.H Publications, Neptune City.
Makarkin, V.N., Heads, S.W., and Wedmann S. 2017. Taxonomic study of the Cretaceous lacewing family Babinskaiidae (Neuroptera: Myrmeleontoidea: Nymphidoidae), with description of new taxa. Cretaceous Research 78: 149–160. Crossref
Makarkin, V.N., Wedmann, S., and Heads, S.W. 2018. A systematic reappraisal of Araripeneuridae (Neuroptera: Myrmeleontoidea), with description of new species from the Lower Cretaceous Crato Formation of Brazil. Cretaceous Research 84: 600–621. Crossref
Mander, L. 2016. A combinatorial approach to angiosperm pollen morphology. Proceedings of the Royal Society of London B [Biological Sciences] 283: 1–10. Crossref
Martill, D.M. and Barker, M.J. 1998. A new centipede (Arthropoda, Chilopoda) from the Crato Formation (Lower Cretaceous, Aptian) of NE Brazil. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 207: 395–404. Crossref
Martill, D.M., Bechly, G., and Loveridge, R.F. 2007. The Crato Fossil Beds of Brazil: Window into an Ancient World. 608 pp. Cambridge University Press, Cambridge. Crossref
Martins-Neto, R.G. 1992. Neurópteros (Insecta, Planipennia) da Formação Santana (Cretáceo Inferior), Bacia do Araripe, Nordeste do Brasil. VII—Palaeoleontinae, Nova Subfamlia de Myrmeleontidae e descrição de novos Taxons. Revista Brasileira de Entomologia 36: 803–815.
Martins-Neto, R.G. 1994. Neurópteros (Insecta, Planipennia) da Formaçao Santana (Cretáceo Inferior) Bacia do Araripe, Nordeste do Brasil. IX—Primeiros resultados da composiçao da fauna e descriçao de novos taxons. Acta Geologica Leopoldensia 17: 269–288.
Martins-Neto, R.G. 2000. Remarks on the neuropterofauna (Insecta, Neuroptera) from the Brazilian Cretaceous, with keys for the identification of the known taxa. Acta Geológica Hispanica 35: 97–118.
Martins-Neto, R.G., Heads, S.W., and Bechly, G. 2007. Neuropterida: snakeflies, dobsonflies and lacewings. In: D. Martill, G. Bechly, and R. Loveridge (eds.), The Crato Fossil Beds of Brazil: Window into an Ancient World, 328–340. Cambridge University Press, Cambridge. Crossref
Martynov, A.V. 1937. Liassic insects from Shurab and Kisyl-Kiya [in Russian]. Trudy Paleontologičeskogo Instituta Akademii Nauk SSSR 7: 1–232.
Menon, F. 2007. Higher systematics of scorpions from the Crato Formation, Lower Cretaceous of Brazil. Palaeontology 50: 185–195. Crossref
Menon, F., Heads, S.W., and Martill, D.M. 2005. New Palaeontinidae (Insecta: Cicadomorpha) from the Lower Cretaceous Crato Formation of Brazil. Cretaceous Research 26: 837–844. Crossref
Menon, F., Penney, D., Selden, P.A., and Martill, D.M. 2003. A new fossil scolopendromorph centipede from the Crato Formation of Brazil. Bulletin of the British Myriapod and Isopod Group 19: 62–66.
Monserrat, V. J. 2008. Nuevos datos sobre algunas especies de Nemopteridae y Crocidae (Insecta: Neuroptera). Heteropterus Revista de Entomologia 8: 1–33. Crossref
Myskowiak, J. and Nel, A. 2016. New antlion species (Insecta, Neuroptera, Palaeoleontidae) from the Lower Cretaceous Crato Formation in northeastern Brazil. Cretaceous Research 59: 278–284. Crossref
Nel, A., Bechly, G., Garrouste, R., Pohl, B., and Escuillie, F. 2005. A new extraordinary neuropterid family from the Lower Cretaceous Crato Formation of Brazil: a new insect order? (Insecta, Neuropterida). Cretaceous Research 26: 845–852. Crossref
Nicoli Aldini, R. 2007. Observation on the larval morphology on the Antlion Myrmeleon bore (Tjelder, 1941) (Neuroptera Myrmeleontidae) and its life cycle in the Po Valley (northern Italy). Annali del Museo civico di Storia naturalae di Ferrara 8: 59–66.
Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos P., Stevens, M.H., Szoecs, E., and Wagner, H. 2018. Vegan: Community Ecology Package. R package version 2.5-3. Accessed at https://CRAN.R-project.org/package=vegan Crossref
Pérez-de la Fuente, R., Engel, M.S., Azar, D., and Peñalver, E. 2019. The hatching mechanism of 130-million-year-old insects: an association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology 62: 547–559. Crossref
Pérez-de la Fuente, R., Peñalver, E., Azar, D., and Engel, M.S. 2018. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Scientific Reports 8: 1–12. Crossref
Pinheiro, J., Bates D., DebRoy S., Sarkar D., and R Core Team 2018. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-137. Acessed at https://CRAN.R-project.org/package=nlme. Crossref
Ponomarenko, A.G. 1976. Corydalidae (Megaloptera) from Cretaceous deposits of Northern Asia [in Russian]. Entomologičeskoe obozrenie USSR 60: 425–433.
R Core Team 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Accessed at https://www.R-project.org/ Crossref
Ren, D. and Engel, M.S. 2008. Aetheogrammatidae, a new family of lacewings from the Mesozoic of China (Neuroptera: Myrmeleontiformia). Journal of the Kansas Entomological Society 81: 161–167. Crossref
Ren, D. and Oswald, D. 2002. A new genus of kalligrammatid lacewings from the Middle Jurassic of China (Neuroptera: Kalligrammatidae). Stuttgarter Beiträge zur Naturkunde Serie B (Geologie und Paläontologie) 317: 1–8.
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J.-C., and Mueller, M. 2011. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 1–17. Crossref
Rumbucher, K. 1995. Hemerobiidae (Insecta, Planipennia), eine bisher noch nicht entdeckte Familie der Santana Formation aus der brasilianischen Unterkreide. Bericht der Naturforschenden Gesellschaft, Augsburg 55: 46–61.
Satar, A., Tusun, S., and Aykut, M. 2014. Morphology and surface structure of third instar larvae of Solter ledereri Navás, 1912 (Neuroptera: Myrmeleontidae) from Turkey. Entomological News 124: 67–73. Crossref
Shcherbakov, D.E. 2008. Madygen, Triassic Lagerstätte number one, before and after Sharov. Alavesia 2: 113–124.
Selden, P.A., Da Costa
Casado, F., and Vianna Mesquita, M. 2006. Mygalomorph spiders
(Araneae: Dipluridae) from the Lower Cretaceous Crato lagerstätte,
Araripe Basin, north-east Brazil. Palaeontology
49: 817–826. Crossref
Sever, M., Lajovic, J., and Rajer, B. 2005. Robustness of the Fisher’s discrimination function to skew-curved normal distribution. Metodološki zvezki 2: 231–242.
Stange, L.A. 2004. A systematic catalog, bibliography and classification of the world antlions (Insecta: Neuroptera: Myrmeleontidae). Memoirs of the American Entomological Institute 74: 1–565.
Tillyard, R.J. 1919. The panorpoid complex. Part 3: The wing-venation. Proceedings of the Linnean Society of New South Wales 44: 533–718.
Venables, W.N. and Ripley, B.D. 2002. Modern Applied Statistics with S. Fourth Edition. 495 pp. Springer, New York. Crossref
Wang, B. and Zhang, H. 2010. Earliest evidence of fishflies (Megaloptera: Corydalidae): an exquisitely preserved larva from the Middle Jurassic of China. Journal of Paleontology 84: 774–780. Crossref
Wang, B., Xia, F., Engel, M.S., Perrichot, V., Shi, G., Zhang, H., Chen, J., Jarzembowski, E.A., Wappler, T., and Rust, J. 2016. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Science Advances 2: 1–8. Crossref
Weitschat, W. and Wichard, W. 2002. Atlas of Plants and Animals in Baltic Amber. 256 pp. Verlag Dr. Friedrich Pfeil, München.
Wichard, W., Gröhn, C., and Seredszus, F. 2009. Aquatic Insects in Baltic Amber. 336 pp. Kessel, Remagen.
Wickham, H. 2007. Reshaping Data with the reshape Package. Journal of Statistical Software 21: 1–20. Accessed at http://www.jstatsoft.org/v21/i12/ Crossref
Wickham, H. 2016. ggplot2: Elegant Graphics for Data Analysis. 268 pp. Springer-Verlag, New York. Crossref
Wickham, H. 2017. Tidyverse: Easily Install and Load the “Tidyverse”. R package version 1.2.1. Accessed at https://CRAN.R-project.org/package=tidyverse Crossref
Wickham, H. and Bryan, J. 2018. Readxl: Read Excel Files. R package version 1.1.0. Accessed at https://CRAN.R-project.org/package=readxl Crossref
Wilson, H.M. 2001. First Mesozoic scutigeromorph centipede, from the Lower Cretaceous of Brazil. Palaeontology 44: 489–495. Crossref
Wilson, H.M. and Martill, D.M. 2001. A new japygid dipluran from the Lower Cretaceous of Brazil. Palaeontology 44: 1025–1031. Crossref
Winterton, S.L., Lemmon, A.R., Gillung, J.P., Garzon, I.J., Badano, D., Bakkes, D.K., Breitkreuz, L.C.V., Engel, M.S., Moriarty Lemmon, E., Liu, X., Machado, R.J.P., Skevington, J.H., and Oswald, J.D. 2018. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Systematic Entomology 43: 330–354. Crossref
Yang, Q., Makarkin, V.N., and Ren, D. 2013. A new genus of the family Panfiloviidae (Insecta, Neuroptera) from the Middle Jurassic of China. Palaeontology 56: 49–59. Crossref
Yang, Q., Wang, Y.J., Labandeira, C.C., Shih, C.K., and Ren, D. 2014. A new extraordinary neuropterid family from the Lower Cretaceous Crato Formation of Brazil: a new insect order (Insecta, Neuropterida). Cretaceous Research 26: 845–852. Crossref
Acta Palaeontol. Pol. 65 (2): 235–250, 2020
https://doi.org/10.4202/app.00662.2019