

First North American occurrence of hairy cicadas discovered in the Cenomanian (Late Cretaceous) of Labrador, Canada
ALEXANDRE V. DEMERS-POTVIN, JACEK SZWEDO, CASSIA P. PARAGNANI, and HANS C.E. LARSSON
Demers-Potvin, A.V., Szwedo, J., Paragnani, C.P., and Larsson, H.C.E. 2020. First North American occurrence of hairy cicadas discovered in the Cenomanian (Late Cretaceous) of Labrador, Canada. Acta Palaeontologica Polonica 65 (1): 85–98.
We report the discovery of Maculaferrum blaisi gen. et sp. nov, the first occurrence of the family Tettigarctidae, informally known as hairy cicadas, in North America. Maculaferrum blaisi is part of a new collection assembled during recent fieldwork in the Redmond Formation, Labrador, Canada, near Schefferville. It consists in a single isolated forewing whose venational characters allow a classification to Tettigarctinae at the subfamily level. Classification at a higher level remains uncertain since it displays a combination of characters supposedly unique to tribes Protabanini, Meunierini, and Tettigarctini. Thus, this discovery adds credence to suggestions of a revision of the definitions of these tribes since they seem to be based on many convergent or plesiomorphic characters. Remnants of a spotted pattern on the wing membrane and probable setae along some veins are also preserved. Observations of the holotype’s fine anatomical characters have been facilitated by the use of Reflectance Transformation Imaging (RTI), an emerging method for the visualization of compression and impression fossils. Considering that the estimated age of the Redmond Formation is the Cenomanian (Late Cretaceous), the discovery of M. blaisi contributes to a very recent expansion of the tettigarctid fossil record that fills a gap between Early Cretaceous and Cenozoic genera. It suggests that hairy cicadas maintained a global distribution and thrived in a variety of climate regimes well into the Late Cretaceous, and that their competitive exclusion by singing cicadas occurred definitely closer to the end of the Cretaceous, or even during the Cenozoic. This discovery is only the start of a thorough description of the recently expanded entomofauna in the Cretaceous of Labrador.
Key words: Insecta, Cicadoidea, Tettigarctidae, Cretaceous, Cenomanian, Redmond Formation, North America.
Alexandre Demers-Potvin [alexandre.demers-potvin@mail.mcgill.ca] and Hans C.E. Larsson [hans.ce.larsson@mcgill.ca], Redpath Museum, McGill University, 859 Sherbrooke St. W., H3A 0C4, Montreal, QC, Canada.
Jacek Szwedo [jacek.szwedo@ug.edu.pl], Laboratory of Evolutionary Entomology and Museum of Amber Inclusions, Department of Invertebrate Zoology and Parasitology, Faculty of Biology, University of Gdańsk, 59, Wita Stwosza St., 80-308, Gdańsk, Poland.
Cassia P. Paragnani [cassia.paragnani@gmail.com], Archaeology At Tardis, 4/46-50 Old Princes Highway, Beaconsfield, Victoria 3807, Australia.
Received 10 August 2019, accepted 8 November 2019, available online 21 February 2020.
Copyright © 2020 A. Demers-Potvin et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Tettigarctidae (hairy cicadas) were the dominant family within Cicadoidea for much of the Mesozoic (Shcherbakov 2009; Moulds 2018). They are distinguished from sister family Cicadidae (modern singing cicadas) by a greatly expanded pronotum covering much of the mesonotum, forewings with a conspicuous nodal line, a posterior radial sector (RP) emerging closer to the wing base than to the node, all anal veins running separately, male genitalia with styles, hind coxae overhanging the abdomen, the presence of tarsal empodia, absence of tympana, and a nervous system with separated thoracic ganglia (Evans 1941; Moulds 1990, 2005). Another distinctive tettigarctid character is the presence of rudimentary tymbals that produce low-intensity, substrate-transmitted acoustic signals, instead of well-developed tympanal auditory organs that lead to loud airborne calls characteristic of singing cicadas (Claridge et al. 1999).
Today, Cicadidae number nearly 2900 species worldwide (Bartlett et al. 2018) as a result of a radiation that probably occurred in the Palaeogene (Kaulfuss and Moulds 2015), while Tettigarctidae are represented by only two species of Tettigarcta White, 1845: T. crinita Distant, 1883 in southeastern Australia, and T. tomentosa White, 1845 in Tasmania. Both species are restricted to cool subalpine forests to which they are adapted with dense insulating hairs, and have a nocturnal habit unique among extant cicadoids (Claridge et al. 1999; Shcherbakov 2009). This is a very specialized lifestyle in a far more restricted range than that occupied by this once diverse family in the Mesozoic (Boulard and Nel 1990; Zeuner 1944). While the diet of tettigarctids shifted from gymnosperm to angiosperm herbivory, likely in response to the floral turnover that occurred in the middle of the Cretaceous, cicadids seem to have appeared at the peak of this angiosperm radiation and retained them as hosts throughout their history (Labandeira 2014). This may have conferred cicadids a competitive advantage resulting in the displacement of tettigarctids from most of their original range until a single lineage remained in biogeographical isolation in southern Australia (Zeuner 1944; Wang and Zhang 2009).
Until recently, the tettigarctid fossil record contained 29 genera and 46 species spread into subfamilies Cicadoprosbolinae and Tettigarctinae (Moulds 2018; Fu et al. 2019; Jiang et al. 2019; Lambkin 2019). The oldest members of the family are Mesodiphthera grandis Tillyard, 1919, Tardilly prosboloides (Tillyard, 1922) and Tardilly dunstani (Tillyard, 1922) from the Norian (Late Triassic) of Dinmore, Queensland, Australia. The youngest is Paratettigarcta zealandica Kaulfuss and Moulds, 2015 from the early Miocene of Hindon Maar, New Zealand. The Jurassic has the highest tettigarctid diversity of any period, although it is restricted to Laurasia and represented largely by species from the Yanliao (Daohugou) Biota (Moulds 2018). Some of these reached far larger body sizes than modern species (Chen and Wang 2016), while others had already evolved patterns of disruptive colouration (Chen et al. 2016; Zheng et al. 2016) and dense body hairs that were initially thought to be unique to modern Tettigarcta (Liu et al. 2016). The known family diversity decreases in the Early Cretaceous, but has a more global distribution, with the first occurrences in former Gondwanan landmasses: Architettix compacta Hamilton, 1990 and Tettagalma striata Menon, 2005 from the Crato Formation of Brazil, and Magrebarcta africana Nel, Zarbout, Barale, and Philippe, 1998 from the Duriet Formation of Tunisia. Not a single hairy cicada was known from the Late Cretaceous until the discovery of three new species in the earliest Cenomanian amber deposits of Kachin, Myanmar: Cretotettigarcta burmensis Fu, Cai, and Huang, 2019, Vetuprosbole parallelica Fu, Cai, and Huang, 2019, and Hpanraais problematicus Jiang, Chen, Jarzembowski, and Wang, 2019. Their unique location and relatively ancestral character states supported the hypothesis of southeast Asia as a Late Cretaceous tropical refuge based on the occurrence of other rare insect taxa.
In this paper, we report the discovery of Maculaferrum blaisi gen. et sp. nov., the first tettigarctid known from North America. It was found during a recent expedition to the Redmond no.1 mine, located in Labrador, Canada, near Schefferville. This abandoned iron ore mine contains lacustrine deposits that represent the only known exposures of Cretaceous rocks in the entire Quebec-Labrador Peninsula (Blais 1959; Dorf 1967). The expedition led to the discovery of more fossil leaf morphotypes and potentially new insect species. Maculaferrum is the first of these newly discovered insects to be formally described.
Institutional abbreviations.—MNHN, Muséum national d’histoire naturelle, Paris, France; MPE, Musée de paléontologie et de l’évolution, Montreal, Canada; NMV, National Museums Victoria, Melbourne, Australia; RMIP, Redpath Museum, Invertebrate Palaeontology, Montreal, Canada.
Other abbreviations.—A, anal vein; a, apical cell; av, ambient vein; bc, basal cell; C, costa; Cu, cubitus; CuA, cubitus anterior; CuP, cubitus posterior; M, media; M1+2, two anterior branches of M; M3+4, two posterior branches of M; m, medial cross vein; mc, medial cell; m-cua, mediocubital cross vein; RA, radius anterior; RP, radius posterior; r, radial cross vein; r-m, radio-medial cross vein; Sc, subcosta; u, ulnar cell.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:914D1FF0-AA2F-4BBB-945F-0 BDD5E822AAA
Geological setting
The Redmond no.1 mine is located at coordinates of 54°41’ N and 66°45’ W, Labrador, Canada, 16 km south southeast of Schefferville (Fig. 1). This site remains the only known exposure of the Redmond Formation, a basin surrounded by Palaeoproterozoic rocks that was 1524 m long, 508 m wide, and up to 183 m deep when first surveyed in 1957 before becoming out of geological context due to mining activities (Blais 1959). Apart from carbonized wood fragments of cupressacean affinity, the fossils found in this basin were restricted to impressions in a 1.5 m thick bed composed of a hard, very fine-grained, evenly laminated ferruginous argillite of umber colour (Blais 1959; Dorf 1967). The uncovered palaeoflora comprised a few fern, conifer, and lycopod specimens, dominated by angiosperm tree leaves that enabled palaeobotanist Erling Dorf to produce a Cenomanian age estimate based on biostratigraphic correlation and to suggest a warm temperate and fully humid climate for the region (Dorf 1959). The insect discoveries were scarce, but still led to the description of five unique and well-preserved specimens: the raphidiopteran Alloraphidia dorfi Carpenter, 1967, the hodotermitid Cretatermes carpenteri Emerson, 1967, the protocoleopteran Labradorocoleus carpenteri Ponomarenko, 1969, the (possibly) myrmeleontid Palaeoleon ferrogeneticus Rice, 1969, and the phasmatodean Palaeopteron complexum Rice, 1969. In addition, a few isolated blattodean wings (Dorf 1967), some elytra assigned to water beetles (Schizophoridae), and two elytra assigned respectively to Cupedidae and to the haliplid Peltodytes sp. (Ponomarenko 1969) have also been mentioned. Despite this meagre collection, the occurrence of taxa rarely preserved in the insect fossil record suggested that many more awaited discovery in the Redmond Formation.
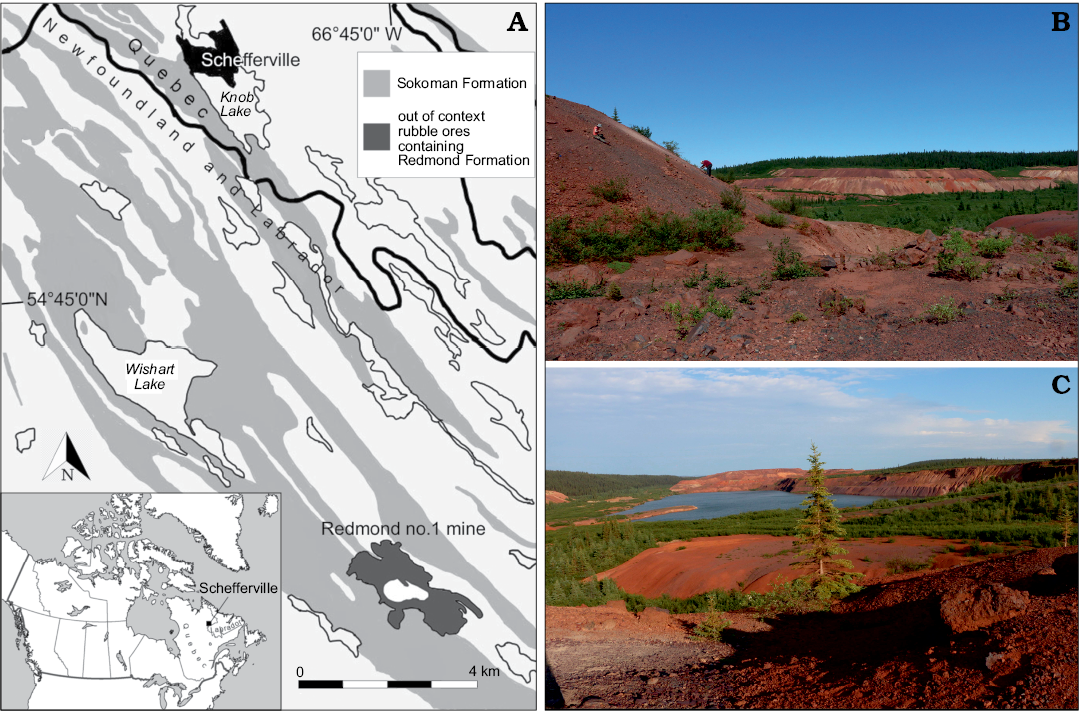
Fig. 1. Geographical location and geological setting of the Cenomanian (Late Cretaceous) Redmond Formation. A. Location of the Redmond Formation near Schefferville in Labrador, Canada (54°41’N, 66°45’W). Exposures of the Sokoman Formation based on Conliffe (2016). B. Prospecting along the spoil pile to the west of the Redmond no. 1 mine, where the specimen was discovered. C. Southeast facing view of the mine from the top of the western spoil pile. The open-pit mine is now flooded by groundwater.
The site was not explored until an expedition by the Musée de paléontologie et de l’évolution (MPE) in summer 2013, by which time mining activities had fragmented the Redmond Formation into small flat claystone rocks scattered among rubble piles (Fig. 1). In August 2018, the first author set out on a longer expedition organized jointly by the Redpath Museum and the MPE. The discovery of yet more fossil leaves in these two expeditions led to an absolute palaeoclimate estimate that encompassed the entire known angiosperm flora, and that confirmed Dorf’s (1959) initial relative palaeoclimate and dating hypotheses (Demers-Potvin and Larsson 2019). This fieldwork also led to a significant expansion of the known entomofauna from the site, including the discovery of near-complete nymphs and aquatic coleopterans that strengthened the initial hypothesis of a lacustrine depositional setting (Blais 1959; Dorf 1959; Demers-Potvin and Larsson 2019).
Among the diverse terrestrial entomofauna preserved in the Redmond Formation was a rare representative of Tettigarctidae. The sole specimen was found on a small thin slab (~6 × 10 × 0.7 cm) of argillite of burgundy colour enriched in iron oxide, among similar pieces of float. Amid this recently assembled entomofauna, we have looked for hind wings that could potentially belong to this genus or a relative within the family, but the most similar ones clearly belonged to leafhoppers (Cicadomorpha: Cicadellidae).
Material and methods
The slice of argillite in which the specimen was found was cut to a 3 × 3 cm square using a Lortone lapidary trim saw, Model FS8. The specimen was drawn and photographed at the Centre de recherche sur la Paléobiodiversité et les Paléoenvironnements (MNHN). A draft drawing was done with a microscope equipped with a camera lucida (Zeiss SteREO Discovery V8 stereomicroscope equipped with a pair of W-PL 10 × / 23 eye pieces, a Plan Apo S 1.0 × FWD objective; all from Zeiss). The drawing was finalized with Adobe Illustrator CC 2019 using the draft drawing and photographs.
A Reflectance Transformation Imaging (RTI) file was built out of a set of photographs to enhance the 3D contrasts in the impression fossil that facilitate its observation, and to produce composite photographs of superior quality. Photographs were taken using a Canon EOS 5D Mark III digital camera with a Canon MPE-65 macro lens (without polarizing filters). The photographs were taken under a Portable Light Dome, optimized and processed using Adobe Camera Raw and Adobe Photoshop CC 2019, and compiled into an RTI file using the RTI Builder software v. 2.0.2 (freely available under GNU license, using the HSH fitter; see Béthoux et al. 2016; RTI 2019 for more details). We provide the RTI file along with viewer software and instructions (see Supplementary Online Material available at http://app.pan.pl/SOM/app65-Demers-Potvin_etal_SOM.pdf). The photographs of RMIP 2018.18.24 included in this paper have all been extracted from the RTI files.
The forewing representing Maculaferrum blaisi gen. et sp. nov was also compared with those of the only two extant species of hairy cicadas, Tettigarcta crinita and Tettigarcta tomentosa. Habitus and individual forewing photographs were taken for specimens of each species (T. crinita: one male [NMV HEM5660]; T. tomentosa: one male [NMV HEM472] and one female [NMV HEM476]) with a Nikon D5300 enhanced with an AF-S Micro Nikkor 105 mm macro lens in the Entomology collections of NMV. ~20 images per view were taken and subsequently stacked and optimized using Adobe Photoshop CC 2018. The wing venation terminology follows that of Moulds (2005). Vein M in this terminology is equivalent to vein MP, as MA is always totally fused with RP, which is a synapomorphy of Hemiptera (Kukalová-Peck 1991; Nel et al. 2012, 2013; Bourgoin et al. 2015).
Systematic palaeontology
Order Hemiptera Linnaeus, 1758
Suborder Cicadomorpha Evans, 1946
Superfamily Cicadoidea Latreille, 1802
Family Tettigarctidae Distant, 1905
Subfamily Tettigarctinae Distant, 1905
Tribe Protabanini Hong, 1982 (?)
Genus Maculaferrum nov.
Zoobank LSID: urn:lsid:zoobank.org:act:6EDDA824-816B-4AE5-AE 3B-2975E12D5AA2
Type species: Maculaferrum blaisi sp. nov.; by monotypy, see below.
Etymology: From Latin macula, spot; referring to the apparent spotted pattern observed on parts of the wing membrane, and ferrum, iron; referring to the high iron content that confers the matrix a distinctive reddish colour.
Diagnosis.—As for the type species.
Maculaferrum blaisi sp. nov.
Figs. 2, 3, 4A.
Zoobank LSID: urn:lsid:zoobank.org:act:8F96A300-25A5-4F3E-BC FC-B5171A85A11D
Etymology: In reference to Roger A. Blais, who undertook the initial survey of the Redmond Formation in 1957.
Holotype: RMIP 2018.18.24 (part), impression of a single isolated forewing either ripped in half or folded onto itself so that the middle area is hidden from view. The basal part consists in the majority of the pre-nodal area, and the apical part consists in the vast majority of the post-nodal area.
Type locality: Redmond no. 1 mine, near Schefferville, Labrador, Canada.
Type horizon: Redmond Formation, Cenomanian, Late Cretaceous.
Diagnosis.—In general view, forewing similar to forewing of Protabanini fossils, Tettagalma striata Menon, 2005 from the Aptian Crato Formation of Brazil and Protabanus chaoyangensis Hong, 1982 from the Jiulongshan Formation, Liaoning, China. Costal cell narrower basally than basal cell (as in Tettagalma; in Protabanus costal cell about as wide as basal cell); single terminal RP longer than cell a6 (as in Protabanus; in Tettagalma cell u3 distinctly shorter than cell a6); apical portion of stem CuA basad of nodal line minimally curving mediad (as in Protabanus; in Tettagalma this section of stem CuA is distinctly curved mediad); CuA2 with a sharp uniform curve apically (contrary to at most faint apical curves in Protabanus and Tettagalma). Specimen also resembling some Meunierini, viz. Meuniera haupti Piton, 1936 from the Palaeocene quarry of Menat, France, and members of Tettigarctini such as extant Tettigarcta spp. from southern Australia, due to M and CuA joined by a m-cua cross vein at the apical extremity of the basal cell and CuA2 running along nodal line up to clavus apex. However, it differs from Meunierini due to stem M forking closer to nodal line than to wing base; it also differs from Tettigarctini due to forking of RA not level with cross vein r and CuA2 curving towards apex instead of base. Uniform curvature of CuA2 seems apomorphic for Maculaferrum gen. nov. Round darker markings in apical cells, less distinct rounded spots in ulnar cells; appendix with minuscule striae (corrugations) exceeding ambient vein; punctate pattern on basal portion of forewing.
Description.—Total forewing length estimated at 20–23 mm; maximal forewing width 7.5 mm. Marginal membrane (appendix) present along entire margin apical of RA1 to 1A terminal apex; ambient vein perfectly visible, criss-crossed by minuscule striae, from RA2 to CuA2 (Figs. 2, 3A1, A2); postclaval membrane present, narrow. Rows of tubercles present along segments of longitudinal veins, each projecting perpendicular to vein (Fig. 3A3). Only short segment of the nodal line appears visible, parallel to CuA2 (Fig. 3B). Round to oblong patches present in apical cells a1 to a8 and postnodal portion of ulnar cells u1 to u3 and medial cell, closer to cross veins (Figs. 2, 3C). Punctate pattern observed on much of pre-nodal area but absent from post-nodal area (Figs. 2, 3D).
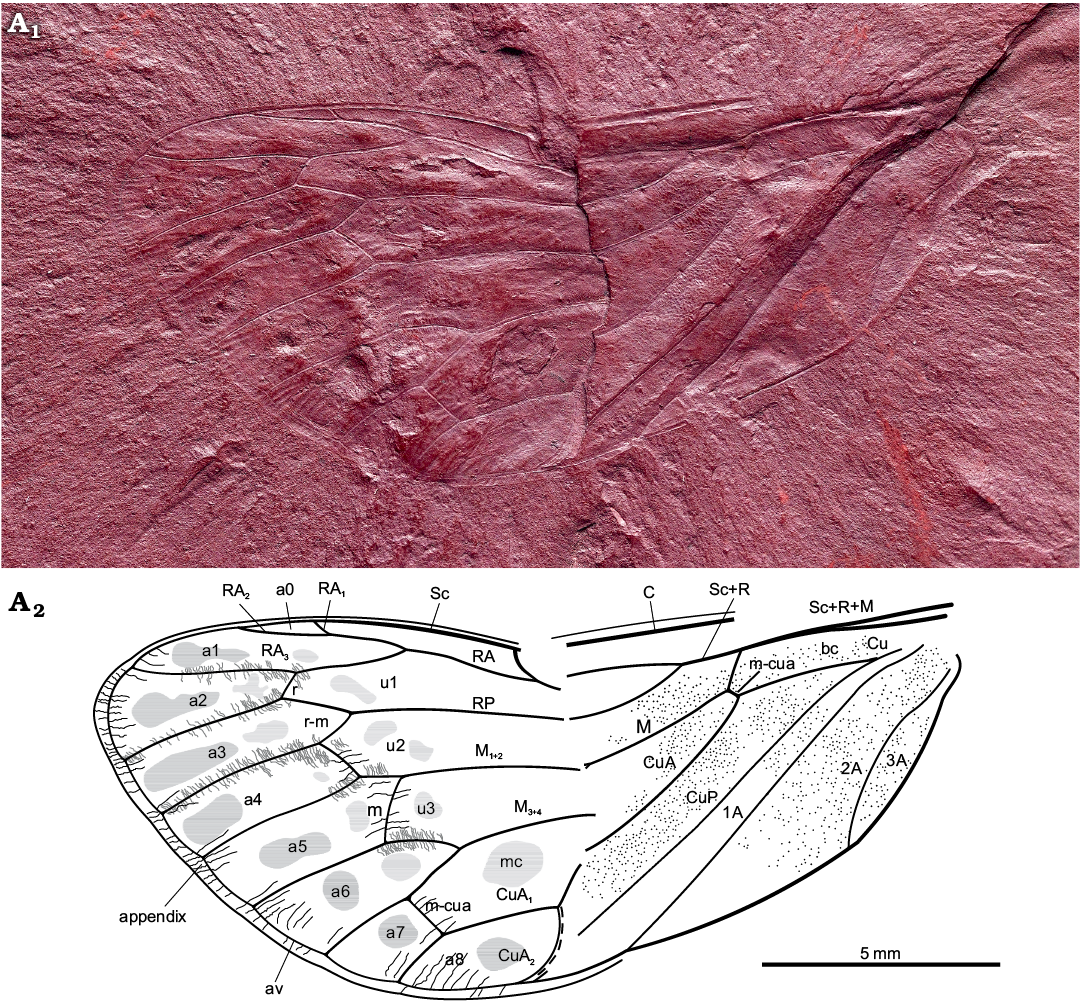
Fig. 2. Hairy cicada Maculaferrum blaisi gen. et sp. nov. (holotype RMIP 2018.18.24) from the Cenomanian (Late Cretaceous) Redmond Formation, Labrador, Canada. Habitus photograph (A1), extracted from the RTI file (downloaded from http://culturalheritageimaging.org/Technologies/RTI/ on 31 July 2019; see SOM) and interpretative line drawing (A2). Wing venation terminology after Moulds (2005). Abbreviations: A, anal vein; a, apical cell; av, ambient vein; bc, basal cell; C, costa; Cu, cubitus; CuA, cubitus anterior; CuP, cubitus posterior; M, media; M1+2, two anterior branches of M; M3+4, two posterior branches of M; m, medial cross vein; mc, medial cell; m-cua, mediocubital cross vein; RA, radius anterior; RP, radius posterior; r, radial cross vein; r-m, radio-medial cross vein; Sc, subcosta; u, ulnar cell. Black lines, striae along wing apex; grey lines, tubercles along apical vein segments; dashed line, visible portion of nodal line.
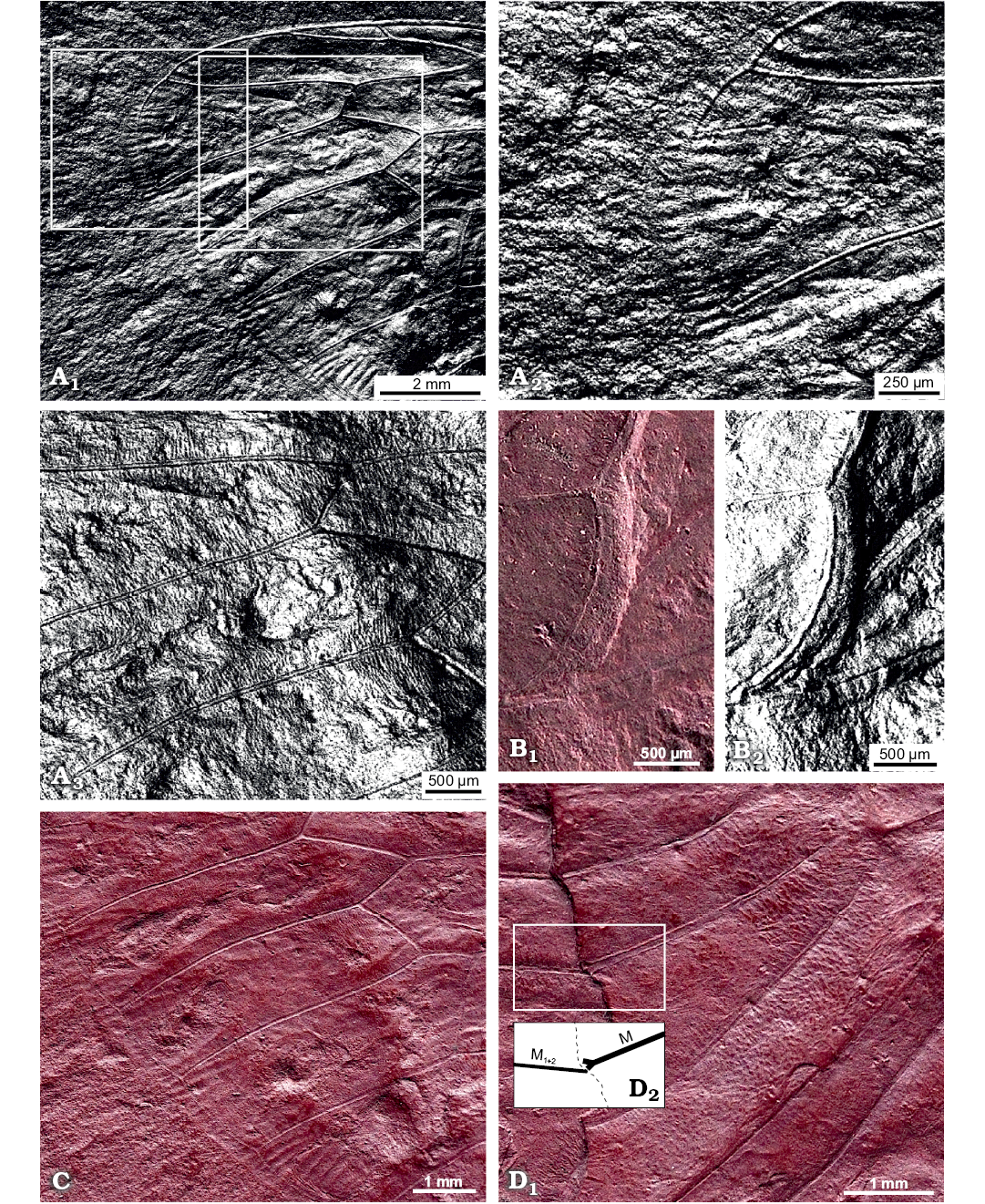
Fig. 3. Photomicrographs highlighting specific characters of the forewing of hairy cicada Maculaferrum blaisi gen. et sp. nov. (holotype, RMIP 2018.18.24) from the Cenomanian (Late Cretaceous) Redmond Formation, Labrador, Canada, extracted from the RTI file (see SOM). A. Highlights on striae along ambient vein and tubercles along longitudinal veins. Apical portion of the forewing, striae crossing ambient vein either side of apex, under specular enhancement rendering mode (A1). Emphasis on the apex, with striae particularly visible on the apicalmost segment of the ambient vein, under specular enhancement rendering mode (A2). Longitudinal veins RP, M1 and M2 (A3); note rows of tubercles emerging perpendicular to veins, under specular enhancement rendering mode. B. Posteriormost segment of nodal line between CuA2 and post-burial fracture, running subparallel to both structures (B1); image under specular enhancement rendering mode (B2). C. Round to oblong patches arranged in a row near apical edge of apical cells. Second row of smaller patches visible in basal part of apical cells. D. Emphasis on part of the pre-nodal area featuring a punctate pattern; forking point of vein M (into M1+2 and M3+4) highlighted in inset (D1); interpretative drawing (D2). Abbreviations: CuA, cubitus anterior; M, media; RP, radius posterior.
Costal area of forewing straight at base, increasingly curved apically, apex relatively sharply curved, claval margin straight, apex of clavus slightly exceeding half of forewing length. Costal margin thick, veins of costal complex flattened to level of nodus. Stem vein Sc tightly adjoined and subparallel to stem R+M, fused with R+M merely apicad of apex of basal cell. Stem Sc+R short, about ¼ of length of basal cell, forked distinctly basad of nodal line and M forking; branch of Sc+R subparallel to costal margin, its prenodal section about 3 times as long as stem Sc+R; terminal Sc short, oblique, diverged slightly apically of nodal line. Branch RA forked at basal half of membrane, with three terminals: branch RA1+2 slightly sinuous, RA1 short, oblique, RA2 subparallel to forewing anterior margin, RA1 and RA2 terminal apices distinctly basad of forewing apex; RA3 reaching ambient vein slightly basad of forewing apex; RP forked from Sc+R basad of 0.3 of forewing length, reaching ambient vein with single terminal slightly basad of forewing apex. Stem M forked close to nodal line level (Fig. 3D2, not clearly preserved), branch M1+2 forked apicad of branch M3+4 forking and apicad of RA forking; M3+4 forked basad of RA forking. Stem CuA leaving basal cell distinctly curved at base, then arcuate, geniculately bent posteriad at nodal line, forked at level of nodal line; branch CuA1 much longer than branch CuA2, subparallel to branch M3+4, then bent to ambient vein, to reach it at level of RA forking. CuP and claval fold straight, reaching margin merely basad of nodal line; 1A slightly sinuate, subparallel to CuP; 2A arcuate, relatively short; 3A fused to basal margin of forewing. Cross vein r slightly apicad of terminal RA1; cross vein r-m oblique, at level of terminal RA1; cross vein m oblique, connecting M1+2 with terminal M3, slightly basad of terminal RA1; basal cross vein m-cua very short, connecting stem M with stem CuA at posterior apical corner of basal cell; apical cross vein m-cua oblique, connecting terminal M4 with CuA1 distinctly basad of terminal RA1, basad of half of M4 length, apicad of half of CuA1 length. Basal cell about 4 times as long as wide, subrectangular. Costal cell about as wide as basal cell. Prenodal portion of cell u1 shorter than postnodal portion. Prenodal portion of cell u3 short (not clearly preserved). Cell a7 significantly shorter than other apical cells. Cell a8 slightly shorter than adjoining postnodal portion of cell mc.
Remarks.—During fossilization, the forewing was broken along the nodal line, and portions partly overlap. It means that much of the area surrounding the nodal line is not clearly preserved. It is very difficult to see the position of the branching of M into M1+2 and M3+4, but it seems to be very close to the margin of the preserved basal portion of the forewing. Considering the incomplete state of the specimen, a reconstruction of the entire forewing is presented alongside wings of living relatives Tettigarcta crinita and T. tomentosa (Fig. 4).
Stratigraphic and geographical range.—Type locality and horizon only.
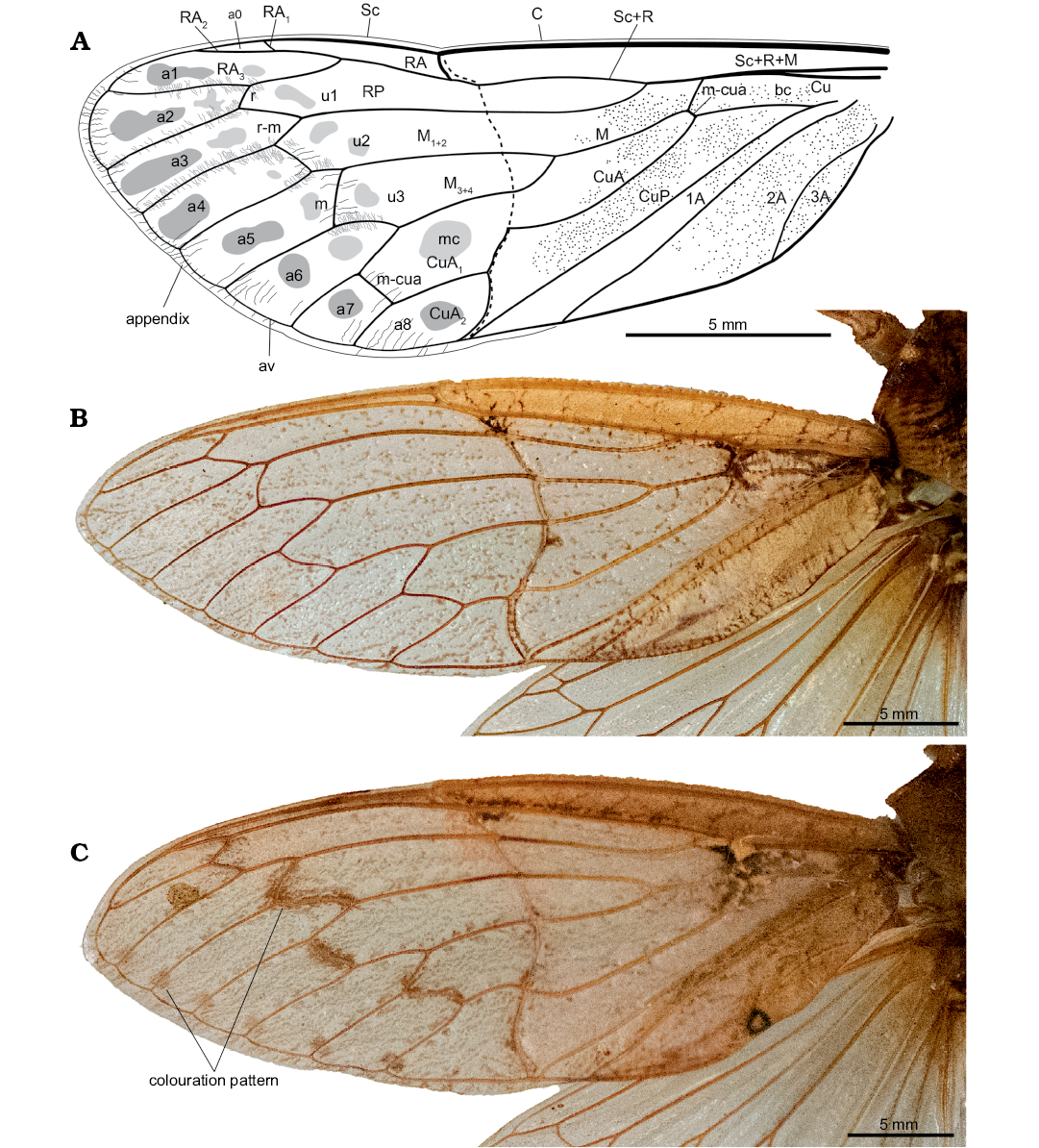
Fig. 4. Comparison of hairy cicada Maculaferrum blaisi gen. et sp. nov. (holotype RMIP 2018.18.24) from the Cenomanian (Late Cretaceous) Redmond Formation, Labrador, Canada, with forewings of extant relatives (B, C). A. Interpretative drawing of M. blaisi gen. et sp. nov. (holotype RMIP 2018.18.24) in pre-burial state, based on extant and extinct relatives. B. Left forewing of male Tettigarcta crinita Distant, 1883 from southern Victoria, Australia; NMV HEM5660 in dorsal view. C. Left forewing of female Tettigarcta tomentosa White, 1845 from Tasmania, Australia; NMV HEM476 in dorsal view. Note colouration pattern on post-nodal half: round patches near junction of terminal longitudinal veins with ambient vein, a larger darker round patch in cell a2, smaller irregular patches along more basal segments of longitudinal veins, and large oblong to irregular patches along cross veins and short segment of M1. Abbreviations: A, anal vein; a, apical cell; av, ambient vein; bc, basal cell; C, costa; Cu, cubitus; CuA, cubitus anterior; CuP, cubitus posterior; M, media; M1+2, two anterior branches of M; M3+4, two posterior branches of M; m, medial cross vein; mc, medial cell; m-cua, mediocubital cross vein; RA, radius anterior; RP, radius posterior; r, radial cross vein; r-m, radio-medial cross vein; Sc, subcosta; u, ulnar cell.
Discussion
Maculaferrum blaisi gen. et sp. nov. belongs to family Tettigarctidae based on the following characters: costal cell present, about as wide as basal cell; branch RP arising basally, closer to base than to node; 1A separated from CuP, veins 2A and 3A separated at least at base; nodal line distinct; clavus exceeding half of forewing length; basal portion of forewing punctate. It can be placed further in subfamily Tettigarctinae based on the following characters (Shcherbakov 2009): basal cell wide, not narrowed to apex; costal cell relatively narrow (only slightly narrower than intercubital area); forewing apex sharply curved; very short basal m-cua closing basal cell; apical cell basad of CuA2 either very narrow or absent (in the case of this new genus); apex of clavus slightly exceeding half of forewing length. The latter character is also found in some taxa placed in Cicadoprosbolinae and could not be diagnostic; further analyses are necessary to assess the importance of this feature. In addition, M. blaisi seems to share with other members of Tettigarctinae a cross vein r closer to the wing apex than to the nodal line. This character cannot be observed directly in the specimen since the nodal line is not preserved for most of the wing’s width, so it is only implied here (see Remarks; Figs. 2 and 4). M. blaisi can be assigned to tribe Protabanini based on the following characters (Shcherbakov 2009): presence of very short basal m-cua closing basal cell; cross vein r reclined; anteriormost apical cell (a0, between RA1 and RA2) narrow triangular; distal section of CuA geniculately bent at nodal line, prolonged by CuA2; nodal line at or beyond mid-wing; forking of stem M far closer to nodal line than to wing base, implied due to lack of preservation of nodal line (see Fig. 4). Only the a0 cell character seems truly unique to Protabanini, while the other characters can also be observed on numerous members of Cicadoprosbolinae, such as Architettix compacta Hamilton, 1990 (Architettigini; Aptian, Crato Formation, Brazil), Turutanoviini, species of the genus Sanmai Chen, Zhang, Wang B., Zheng Y., Wang X., and Zheng X., 2016, Hirtaprosbole erromera Liu, Li, Yao, and Ren, 2016, Shuraboprosbole daohugouensis Wang and Zhang, 2009, Tianyuprosbole zhengi Chen, Wang B., Zhang, and Wang X., 2014 (all from the Callovian/Oxfordian [Middle/Late Jurassic] Daohugou Formation of northeastern China); and Cicadoprosbolinae genera unassigned to tribes, Vetuprosbole and Hpanraais (both from earliest Cenomanian [Late Cretaceous] Kachin amber of Myanmar). Maculaferrum blaisi gen. et sp. nov. also shares with the aforementioned Jurassic taxa Sc+R significantly shorter than Sc+RA, and m-cu connecting CuA1 to M4. It seems to share with members of Protabanini a stem M curving mediad just before bending geniculately along the nodal line. In this respect, M curves relatively minimally in this new genus (as in Protabanus chaoyangensis Hong, 1982 or Sunotettigarcta kudryashevae Shcherbakov, 2009) instead of curving sharply (as in Tettagalma). However, even this character is present in some Cicadoprosbolinae, most notably Hpanraais.
Several features observed on the specimen seem to exclude it from Protabanini, as originally defined (Shcherbakov 2009). These are M and CuA joined by a m-cua cross vein in the apical extremity of the basal cell (instead of cross-joining at that extremity) and CuA2 running along nodal line up to clavus apex (instead of a divergence of the distal section of CuA2 from the nodal line). These traits are more characteristic of taxa classified in Meunierini (such as Meuniera haupti Piton, 1936) and Tettigarctini (such as Tettigarcta). The new genus and species shares with Meunierini a forking of RA level with a forking of M1+2 (Shcherbakov 2009). However, it differs from Meunierini due to the forking of stem M at unequal distances from the wing base and nodal line, and it differs from Tettigarctini due to the forking of RA not level with cross vein r (Shcherbakov 2009) and CuA2 curving towards the apex instead of the base (Fig. 4). Consequently, Maculaferrum blaisi gen. et sp. nov. presents a combination of characters supposedly diagnostic of Protabanini, Meunierini, and Tettigarctini. Thus, its affinities to a particular tribe within Tettigarctinae remain obscure, and a similar condition has been noted in its contemporary Cretotettigarcta burmensis (Fu et al. 2019). Together, these recently described genera could contribute to a revision of the definitions of tribes within Tettigarctinae (such as Protabanini), since they seem based on many convergent or plesiomorphic characters.
Interpretation of spotted and filamentous patterns on the wing membrane.—The presence of minuscule tubercles along the veins suggests that setae or bristles were present on the forewing of Maculaferrum blaisi gen. et sp. nov. (Fig. 3A3). Such setae are preserved in extant relative Tettigarcta. They have also been observed in extinct relatives (not ascribed to tribes) preserved in Cenomanian (Late Cretaceous) Kachin amber of Myanmar, e.g., Hpanraais, Cretotettigarcta, and Vetuprosbole. It must also be noted that the clear delineation between a punctate pattern on the pre-nodal area and a smoother pattern on the post-nodal area is very similar to that observed in Tianyuprosbole (Chen et al. 2014).
The presence of patches on the apical portion of the forewing of Maculaferrum probably does not result from taphonomical bias. Instead, they may indicate a spotted pattern that was actually present on the wing. However, since Maculaferrum is represented by an impression fossil, the origin of this pattern is far more nebulous than in a compression fossil, and the lack of distinct pigmentation means that the wing’s original colouration pattern may not be fully preserved. This means that any interpretation of this observation must be treated with extreme caution. Spotted patterns are occasionally observed in some Tettigarcta individuals, although they are found along the veins and seem less extensive than in Maculaferrum (Fig. 4). Of the limited sample of Tettigarcta individuals available for this study, such a pattern was only found on the female T. tomentosa (Fig. 4C). A future study could aim to compare wing colouration patterns more systematically between sexes among both extant species. Similar patterns are also infrequently present among extant singing cicadas Cicadidae (Distant 1889, 1914; Emery et al. 2017). Lighter spots in cells on a dark background are present in species of the genus Ambragaeana (Gaeanini) Chou and Yao, 1985. Darker spots on a transparent background, but distributed on veins and adjoining cells, are present in Kamalata pantherina Distant, 1889 (Cicadini); more irregular patches on veins and adjoining portions of cells are also present in other Cicadini (Distant 1889, 1912). In contrast, cryptic colouration or patterns of disruptive colouration (speckles and longitudinal stripes) are frequently observed in extinct Tettigarctidae, such as in Liassocicada Bode, 1953, Sunotettigarcta kudryashevae, Sanmai, and Maculaprosbole Zheng, Chen, and Wang, 2016 from the Jurassic, or the Miocene Paratettigarcta Kaulfuss and Moulds, 2015. Cryptic and disruptive colouration are recognized as defense mechanisms for avoiding predation (Quicke 2017), and the fossil record shows that they evolved in Tettigarctidae during much of their history.
Palaeobiogeographical and palaeoclimate considerations.—The preservation state of Maculaferrum blaisi gen. et sp. nov. does not provide any insight into aspects of tettigarctid biology, such as hair density or feeding apparatus, as in other members of the family (Hamilton 1990; Li et al. 2012; Chen et al. 2014; Fu et al. 2019). However, its contribution to our understanding of tettigarctid biogeography cannot be overstated. The unique geographical distribution of Maculaferrum strongly supports its new genus status based on the anatomical diagnosis outlined above. Tettigarctids were already known to have a global distribution during the Cretaceous ranging from Eurasia to South America, and this discovery finally confirms that their range extended to North America (Fig. 5). The fact that Maculaferrum only represents the first occurrence of this family on this continent demonstrates how rare tettigarctids can be in the Cretaceous hemipteran fossil record. Most continents have only one or two known fossils representing this family (Hamilton 1990; Nel et al. 1998; Menon 2005; Kaulfuss and Moulds 2015); they are conspicuously absent from a number of well-sampled Lagerstätten, such as the Jehol biota (Fu et al. 2019), Koonwarra (Shcherbakov 2009), or the spatially and temporally close New Jersey amber (Grimaldi et al. 2000). In the Lagerstätten that do contain them (such as the Yanliao biota or the Crato Formation, see Hamilton 1990; Li et al. 2012; Chen et al. 2016), species are only ever represented by one or two specimens, and their occurrence is not clearly correlated with preservation potential. Not a single species was known in the form of amber inclusions until 2019, and these occurrences are all reported from the Hukawng Valley, a single exceptional locality in Myanmar (Fu et al. 2019; Jiang et al. 2019). In contrast, the only tettigarctids known from Africa and New Zealand, and now North America, consist in isolated fragmentary wings (Nel et al. 1998; Kaulfuss and Moulds 2015). Taphonomical bias may explain this situation more than any rarity in the Cretaceous biocoenoses since tettigarctids have a life history that would make them less likely to fossilize as part of Lagerstätten than lacustrine or riparian insects.
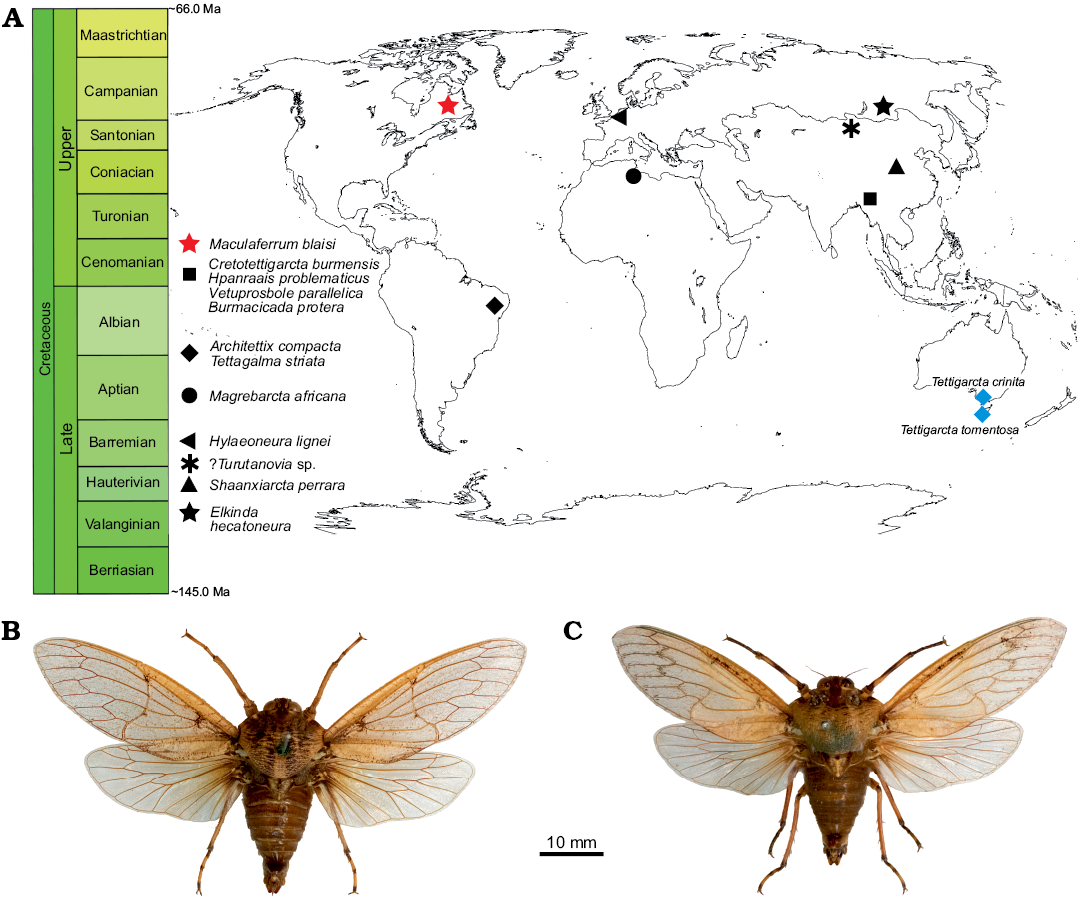
Fig. 5. A. Spatial and geographical distribution of cicadoid occurrences in the Cretaceous (red star, black shapes) compared with extant tettigarctids (blue diamonds). B. Habitus photograph of male Tettigarcta crinita Distant, 1883 from southern Victoria, Australia; NMV HEM5660 in dorsal view. C. Habitus photograph of male Tettigarcta tomentosa White, 1845 from Tasmania, Australia; NMV HEM472 in dorsal view. Fossil occurrences retrieved from the Paleobiology Database (Czaplewski 2019).
How does this discovery contribute to our understanding of cicadoid eco-evolutionary trends throughout the Cretaceous? At the time of Moulds’ (2018) review of cicadoid diversity, seven tettigarctid species were known from the Early Cretaceous, and none from the Late Cretaceous. One year later, four species (including Maculaferrum blaisi gen. et sp. nov.) are known from the Late Cretaceous, from very distant localities (Fig. 5). If the relative dating of the Redmond Formation is accurate, it would be of a similar age to the reasonably constrained Hukawng Valley of Myanmar, the only other Cenomanian locality to have produced tettigarctids (Shi et al. 2012; Fu et al. 2019; Jiang et al. 2019). These new discoveries suggest that the decline in tettigarctid diversity was not as abrupt as first envisioned. They do not completely refute the hypothesis of competitive displacement by Cicadidae (Wang and Zhang 2009), but suggest a shift in its timing towards the Cenozoic, or at least nearer the end of the Cretaceous, especially now that Burmacicada protera Poinar and Kritsky, 2012 is assigned to Tettigarctidae instead of Cicadidae (Moulds 2018; Chen et al. 2019a, b). The discovery of Maculaferrum also leads us to doubt Fu et al.’s (2019) hypothesis on southeast Asia as a tropical refuge for tettigarctids during the Cenomanian. It may be supported for more specialized taxa because of their unique occurrence in an environment that is exceptionally humid compared with localities that experienced a more seasonal tropical climate (Spicer et al. 1996; Grimaldi et al. 2002; Hay and Flögel 2012). However, our discovery demonstrates the occurrence of tettigarctids at a higher latitude that experienced a warm temperate to subtropical climate with a significant seasonality (Demers-Potvin and Larsson 2019), and suggests that the versatility demonstrated by the diverse Jurassic record in this family extended at least into the beginning of the Late Cretaceous. Conversely, it also confirms their presence in another habitat experiencing milder conditions than their extant relatives’ current refuge (Claridge et al. 1999). Considering the evidence for Mesozoic tettigarctids living in tropical to subtropical environments, this family constitutes yet another example of an insect group for which uniformitarian assumptions based on the natural history of modern species cannot be applied to extinct relatives.
At a higher taxonomical level, the discovery of Maculaferrum blaisi gen. et sp. nov. offers more insight on the biogeography of its subfamily. Jurassic taxa assigned to Tettigarctinae were distributed in Europe, as well as central and northeastern areas of Asia (Kazakhstan and northeastern China) (Martynov 1937; Bode 1953; Hong 1983). In the Early Cretaceous, members of this subfamily are only found in northern Africa and eastern South America (Nel et al. 1998; Menon 2005). The discovery of Maculaferrum extends the Late Cretaceous record of Tettigarctinae from the West Burma Block to northern areas of North America. The spatiotemporal distribution of the known fossil record of Tettigarctinae suggests a dispersal of the subfamily over its history in two possible scenarios: either an expansion of the range, or a retreat of the group from warm areas of Europe and/or central and eastern Asia towards equatorial Africa, South America and the West Burma Block, then to slightly cooler areas at higher latitudes during the Cenozoic (Fig. 6). Considering that the North Atlantic Ocean was opening during the Late Cretaceous, the presence of Eotettigarcta scotica Zeuner, 1944 in the Palaeocene Isle of Mull deposits, Scotland, could be explained by a dispersal across the Thulean Land Bridge, which is considered to have been the most important path of interchange for temperate biota in the earliest span of the Eocene (Sanmartín et al. 2001; Archibald et al. 2011). Alternatively, a vicariance hypothesis cannot be ruled out considering that the oldest known tettigarctids existed in Australia likely before the breakup of Pangaea (Lambkin 2019), and that this same landmass is now home to the family’s only extant representatives (Moulds 2005). Such a small sample size stretched over a ~200 Ma fossil record may restrict our ability to precisely elucidate the biogeographical patterns of tettigarctids.
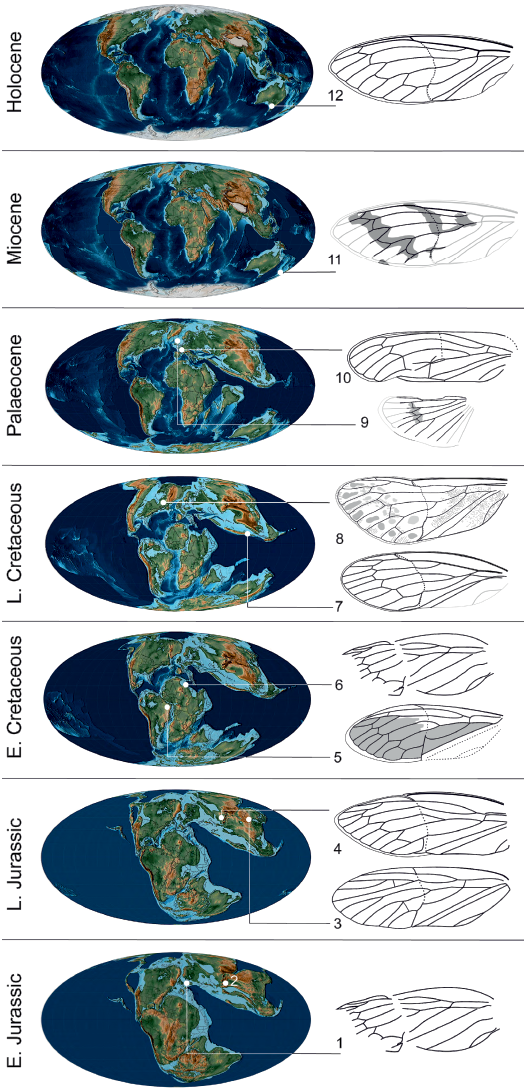
Fig. 6. Tettigarctinae occurrences through time. 1, Liassocicada antecedens Bode, 1953 from Early Jurassic Posidonia Shale Formation, Germany (drawing from Nel et al. 1998); 2, Kisylia psylloides Martynov, 1937 from Early Jurassic Kisyl-Kiya, Kyrgyzstan; 3, Protabanus chaoyangensis Hong, 1982 from the Callovian/Oxfordian (Middle/Late Jurassic) Jiulongshan Formation, China; 4, Sunotettigarcta (represented by drawing of Sunotettigarcta kudryashevae Shcherbakov, 2009) from Late Jurassic Karatau, Kazakhstan; 5, Tettagalma striata Menon, 2005 from the Aptian (Early Cretaceous) Crato Formation, Brazil; 6, Magrebarcta africana Nel, Zarbout, Barale, and Philippe, 1998 from the Aptian (Early Cretaceous) Duriet Formation, Tunisia; 7, Cretotettigarcta burmensis Fu, Cai, and Huang, 2019 from the Cenomanian (Late Cretaceous) Hukawng Valley, Myanmar; 8, Maculaferrum blaisi gen. et sp. nov. (holotype RMIP 2018.18.24) from the Cenomanian (Late Cretaceous) Redmond Formation, Labrador, Canada; 9, Eotettigarcta scotica Zeuner, 1944 from the Palaeocene Isle of Mull, UK (only known from a partial hind wing); 10, Meuniera haupti Piton, 1936 from the Palaeocene Menat quarry, France; 11, Paratettigarcta zealandica Kaulfuss and Moulds, 2005 from the Miocene Hindon Maar, New Zealand; 12, Tettigarcta crinita Distant, 1883 from southern Victoria, Australia (extant). The classification of Liassocicada ignota Brodie, 1845 within Cicadoprosbolinae introduces uncertainty about the affinities of L. antecedens to Tettigarctinae (Shcherbakov 2009). Drawings not to scale; 1, 4, 6, 7, 9–11, mirrored to facilitate comparison with Maculaferrum. Fossil occurrences retrieved from the Paleobiology Database (Czaplewski 2019). Palaeogeographical maps from Scotese (2001).
Conclusions
The discovery of Maculaferrum blaisi gen. et sp. nov. finally confirms the presence of the once-widespread cicada family Tettigarctidae in North America and contributes to filling a Late Cretaceous gap in the fossil record of hairy cicadas. The erection of a new genus is strongly supported by distinctive forewing venational characters and a unique geographical distribution. This is the most recently described member of Tettigarctinae to display a combination of venational characters supposedly diagnostic of tribes Protabanini, Meunierini and Tettigarctini. Consequently, it supports the hypothesis according to which the definitions of these taxa are based on many convergent or plesiomorphic characters, and that they should be revised accordingly. It also leads us to propose the hypothesis that tettigarctids were still thriving at least at the start of the Late Cretaceous, and that their competitive displacement by singing cicadas occurred at least later than the Cenomanian. Additionally, the relatively high palaeolatitude of the locality of this new genus supports a hypothesis of shifts in the extent of the world distribution of Tettigarctinae throughout the lineage’s history. More fossil discoveries are necessary to refine these ecological and biogeographical hypotheses. Together with a more thorough study of wing venation patterns and variability among the extant Tettigarcta species, they should also contribute to refining the taxonomy of this family. The small number of known Late Cretaceous, and more precisely Cenomanian, sites means that it remains difficult to determine whether the abundance of rare occurrences in the Redmond entomofauna is caused by taphonomical bias or a biogeographically significant phenomenon. Maculaferrum is the first hemipteran described from Labrador’s Redmond Formation, and represents the beginning of a renewed scientific interest in this unique and remote Cretaceous locality. Further descriptions of this recently expanded palaeocommunity, and its eventual comparison with the relatively spatially and temporally close assemblage in New Jersey amber, may provide insight on this matter on a more continental scale.
Acknowledgements
The authors wish to thank Olivier Béthoux (Muséum national d’histoire naturelle, Paris, France) for his invaluable assistance in the imaging of the specimen, including the use of his lab’s camera lucida and Portable Light Dome. Thanks are extended to Mario Cournoyer and Michel Chartier (both Musée de paléontologie et de l’évolution, Montreal, Canada), and Noemie Sheppard (McGill University, Montreal, Canada), who initially discovered the specimen, for their assistance in fieldwork alongside AVD-P. We also thank Elaine Anderson and Ken Walker (both NMV) for making the photographs of extant Tettigarcta specimens possible, along with Bo Wang (Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China) for providing advice on the classification of this new genus. As for museum collection visits, we thank Étienne Normandin-Leclerc, manager of the Ouellet-Robert insect collection (Université de Montréal, Montreal, Canada), for his support in diagnosing the specimen, as well as Paul Nascimbene, Courtney Richenbacher and David Grimaldi (American Museum of Natural History, New York City, USA) for granting access to Architettix specimens from the Crato Formation to the first author. We also wish to thank Jean-Pierre Guilbault, Pierre Bédard, and Jacques Letendre (all Musée de paléontologie et de l’évolution, Montreal, Canada) for their initial collection efforts alongside Mario Cournoyer (Musée de paléontologie et de l’évolution, Montreal, Canada) at the Redmond no.1 mine back in 2013. This collection was made available to the first author from the start of this project. Finally, the authors wish to thank Jun Chen (Linyi University, Linyi, China), Ryan McKellar (Royal Saskatchewan Museum, Regina, Canada) and an anonymous reviewer for their constructive comments that greatly improved this paper. This research was supported by funding from the Fonds de recherche Nature et technologies Québec (FRQNT), a National Geographic Society Early Career Grant, the Northern Scientific Training Program (NSTP), a Redpath Museum Class of 66 Award, and a NSERC Discovery Grant to HCEL.
References
Archibald, S.B., Johnson, K.R., Mathewes, R.W., and Greenwood, D.R. 2011. Intercontinental dispersal of giant thermophilic ants across the Arctic during early Eocene hyperthermals. Proceedings of the Royal Society of London B: Biological Sciences 278: 3679–3686. Crossref
Bartlett, C.R., Deitz, L.L., Dmitriev, D.A., Sanborn, A.F., Soulier-Perkins, A., and Wallace, M.S. 2018. The diversity of the true hoppers (Hemiptera: Auchenorrhyncha). In: R.G. Foottit and P.H. Adler (eds.), Insect Biodiversity: Science and Society, II, 501–590. Wiley-Blackwell, Hoboken. Crossref
Béthoux, O., Llamosi, A., and Toussaint, S. 2016. Reinvestigation of Protelytron permianum (Insecta; Early Permian; USA) as an example for applying reflectance transformation imaging to insect imprint fossils. Fossil Record 20: 1–7. Crossref
Blais, R.A. 1959. L’origine des minerais crétacés du gisement de fer de Redmond, Labrador. Le Naturaliste Canadien 86: 265–299.
Bode, A. 1953. Die Insektenfauna des Ostniedersachsischen Oberen Lias. Palaeontographica Abteilung 1: 1–47.
Boulard, M. and Nel, A. 1990. Sur deux cigales fossiles des terrains tertiaires de la France. Revue française d’Entomologie 12: 37–45.
Bourgoin, T., Wang, R.-R., Asche, M., Hoch, H., Soulier-Perkins, A., Stroiński, A., Yap, S., and Szwedo, J. 2015. From micropterism to hyperpterism: recognition strategy and standardized homology-driven terminology of the forewing venation patterns in planthoppers (Hemiptera: Fulgoromorpha). Zoomorphology 134: 63–77. Crossref
Brodie, P.B. 1845. A History of the Fossil Insects in the Secondary Rocks of England: Accompanied by a Particular Account of the Strata in which They Occur, and of the Circumstances Connected with Their Preservation. 130 pp. John Van Voorst, London. Crossref
Carpenter, F.M. 1967. Cretaceous insects from Labrador 2. A new family of snake-flies (Neuroptera: Alloraphidiidae). Psyche: A Journal of Entomology 74: 270–275. Crossref
Chen, J. and Wang, B. 2016. A giant tettigarctid cicada from the Mesozoic of northeastern China. Spixiana 39: 119–124.
Chen, J., Wang, B., Zhang, H., and Wang, X. 2014. A remarkable new genus of Tettigarctidae (Insecta, Hemiptera, Cicadoidea) from the Middle Jurassic of northeastern China. Zootaxa 3764: 581–586. Crossref
Chen, J., Wang, B., Zhang, H., Jiang, H., Jiang, T., Zheng, Y., and Wang, X. 2019a. New discovery of Minlagerrontidae in mid-Cretaceous Burmese amber (Hemiptera, Cicadomorpha, Clypeata). Cretaceous Research 106: 1–6. Crossref
Chen, J., Wang, B., Zheng, Y., Jiang, H., Jiang, T., Zhang, J., and Zhang, H. 2019b. A new sinoalid froghopper in mid-Cretaceous Burmese amber, with inference of its phylogenetic position (Hemiptera, Cicadomorpha). Cretaceous Research 95: 121–129. Crossref
Chen, J., Zhang, H., Wang, B., Zheng, Y., Wang, X., and Zheng, X. 2016. New Jurassic tettigarctid cicadas from China with a novel example of disruptive coloration. Acta Palaeontologica Polonica 61: 853–862. Crossref
Chou, I. and Yao, W. 1985. Studies on the Gaeanini tribe in Chinese (Homoptera: Cicadoidea) [in Chinese]. Entomotaxonomia 7: 123–140.
Claridge, M.F., Morgan, J.C., and Moulds, M.S. 1999. Substrate-transmitted acoustic signals of the primitive cicada, Tettigarcta crinita Distant (Hemiptera Cicadoidea, Tettigarctidae). Journal of Natural History 33: 1831–1834. Crossref
Conliffe, J. 2016. Geology and Geochemistry of High-Grade Iron-Ore Deposits in the Kivicic, Timmins and Ruth Lake Areas, Western Labrador. In Current Research. 26 pp. Newfoundland and Labrador Department of Natural Resources Geological Survey, St. John’s.
Czaplewski, J.J. 2019. Subfamily = Tettigarctinae. Downloaded from https://paleobiodb.org/navigator/ on 10 October 2019.
Demers-Potvin, A.V. and Larsson, H.C.E. 2019. Palaeoclimatic reconstruction for a Cenomanian-aged angiosperm flora near Schefferville, Labrador. Palaeontology 62: 1027–1048. Crossref
Distant, W.L. 1883. Contributions to a proposed monograph of the homopterous family cicadidae-Part I. Proceedings of the Zoological Society of London 51: 187–194. Crossref
Distant, W.L. 1889. Descriptions of a new genus and some new species of Cicadidæ belonging to the Oriental region. Annals and Magazine of Natural History 6: 49–53. Crossref
Distant, W.L. 1905. Cicadidae and Fulgoridae. Biologia Centrali Americana 1: 140–146.
Distant, W.L. 1912. Homoptera, Fam. Cicadidae, Subfam. Cicadinae. In: P. Wystman and L.H. Townsend (eds.), Genera Insectorum, 142, 1–64. Desmet-Verteneuil, Bruxelles.
Distant, W.L. 1914. Homoptera, Fam. Cicadidae, Subfam. Gaeaninae. In: P. Wystman and L.H. Townsend (eds.), Genera Insectorum, 158, 1–38. Desmet-Verteneuil, Bruxelles.
Dorf, E. 1959. Cretaceous flora from beds associated with rubble iron-ore deposits in the Labrador Trough. Bulletin of the Geological Society of America 70: 1591.
Dorf, E. 1967. Cretaceous insects from Labrador I. Geologic occurrence. Psyche: A Journal of Entomology 74: 267–269. Crossref
Emerson, A.E. 1967. Cretaceous insects from Labrador 3. A new genus and species of termite (Isoptera: Hodotermitidae). Psyche: A Journal of Entomology 74: 276–289. Crossref
Emery, D.L., Lee, Y.J., and Pham, H.-T. 2017. Descriptions of four new species of Semia Matsumura (Hemiptera: Cicadidae: Psithyristriini) from Vietnam, with a key to the species of Semia. Zootaxa 4216: 153–166. Crossref
Evans, J.W. 1941. The morphology of Tettigarcta tomentosa White, (Homoptera, Cicadidae). Papers and Proceedings of the Royal Society of Tasmania 1940: 35–49.
Fu, Y., Cai, C., and Huang, D. 2019. First hairy cicadas in mid-Cretaceous amber from northern Myanmar (Hemiptera: Cicadoidea: Tettigarctidae). Cretaceous Research 93: 285–291. Crossref
Grimaldi, D.A., Shedrinsky, A., and Wampler, T.P. 2000. A remarkable deposit of fossiliferous amber from the Upper Cretaceous (Turonian) of New Jersey. In: D.A. Grimaldi (ed.), Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey, 1–76. Backhuys Publishers, Leiden.
Grimaldi, D.A., Engel, M.S., and Nascimbene, P.C. 2002. Fossiliferous Cretaceous amber from Myanmar (Burma): its rediscovery, biotic diversity, and paleontological significance. American Museum Novitates 3361: 1–71. Crossref
Hamilton, K.G.A. 1990. Chapter 6. Homoptera. In: Grimaldi, D.A. (ed.), Insects from the Santana Formation, Lower Cretaceous, Brazil. Bulletin of the American Museum of Natural History 195: 40.
Hay, W.W. and Flögel, S. 2012. New thoughts about the Cretaceous climate and oceans. Earth-Science Reviews 115: 262–272. Crossref
Hong, Y.C. 1982. Mesozoic Fossil Insects of Jiuquan Basin in Gansu Province [in Chinese]. 10 pp. Geological Publishing House, Beijing.
Hong, Y.C. 1983. Middle Jurassic Fossil Insects in North China [in Chinese, with English abstract and summary]. 17 pp. Geological Publishing House, Beijing.
Jiang, H., Chen, J., Jarzembowski, E., and Wang, B. 2019. An enigmatic fossil hairy cicada (Hemiptera, Tettigarctidae) from mid-Cretaceous Burmese amber. Cretaceous Research 96: 14–18. Crossref
Kaulfuss, U. and Moulds, M. 2015. A new genus and species of tettigarctid cicada from the early Miocene of New Zealand: Paratettigarcta zealandica (Hemiptera, Auchenorrhyncha, Tettigarctidae). ZooKeys 484: 83–94. Crossref
Kukalová-Peck, J. 1991. Fossil history and the evolution of Hexapod structures. In: I.D. Naumann (ed.), The Insects of Australia 1, 141–179. Melbourne University Press, Melbourne.
Labandeira, C.C. 2014. Why did terrestrial insect diversity not increase during the angiosperm radiation? Mid-Mesozoic, plant-associated insect lineages harbor clues. In: P. Pontarotti (ed.), Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life, 61–99. Springer International Publishing, Cham. Crossref
Lambkin, K.J. 2019. Mesodiphthera Tillyard, 1919, from the Late Triassic of Queensland, the oldest cicada (Hemiptera: Cicadomorpha: Cicadoidea: Tettigarctidae). Zootaxa 4567: 358–366. Crossref
Latreille, P.A. 1802. Sectio Secunda. Familia quarta. Cicadariae. Cicadaires. In: Genera crustaceorum et insectorum: secundum ordinem natrualem in familias disposita, iconibus exemplisque plurimis explicata. 258 pp. Parisiis, Argentorati, Armand Koenig, Paris.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. 824 pp. Editio decima, reformata. Laurentii Salvii, Holmiae. Crossref
Li, S., Wang, Y., Ren, D., and Pang, H. 2012. Revision of the genus Sunotettigarcta Hong, 1983 (Hemiptera, Tettigarctidae), with a new species from Daohugou, Inner Mongolia, China. Alcheringa: An Australasian Journal of Palaeontology 36: 501–507. Crossref
Liu, X.-H., Li, Y., Yao, Y.-Z., and Ren, D. 2016. A hairy-bodied tettigarctid (Hemiptera: Cicadoidea) from the latest Middle Jurassic of northeast China. Alcheringa: An Australasian Journal of Palaeontology 40: 383–389. Crossref
Martynov, A.V.E. 1937. Liassic insects from Shurab and Kisyl-Kiya [in Russian, with English abstract and summary]. Trudy Paleontologičeskovo Instituta Akademii nauk SSSR 7: 1–232.
Menon, F. 2005. New record of Tettigarctidae (Insecta, Hemiptera, Cicadoidea) from the Lower Cretaceous of Brazil. Zootaxa 1087: 53–58. Crossref
Moulds, M.S. 1990. A Guide to Australian Cicadas. 217 pp. New South Wales University Press, Kensington.
Moulds, M.S. 2005. An appraisal of the higher classification of cicadas (Hemiptera: Cicadoidea) with special reference to the Australian fauna. Records of the Australian Museum 57: 375–446. Crossref
Moulds, M.S. 2018. Cicada fossils (Cicadoidea: Tettigarctidae and Cicadidae) with a review of the named fossilised Cicadidae. Zootaxa 4438: 443–470. Crossref
Nel, A., Prokop, J., Nel, P., Grandcolas, P., Huang, D.-Y., Roques, P., Guilbert, E., Dostál, O., and Szwedo, J. 2012. Traits and evolution of wing venation pattern in paraneopteran insects. Journal of Morphology 273: 480–506. Crossref
Nel, A., Roques, P., Nel, P., Prokin, A.A., Bourgoin, T., Prokop, J., Szwedo, J., Azar, D., Desutter-Grandcolas, L., Wappler, T., Garrouste, R., Coty, D., Huang, D., Engel, M.S., and Kirejtshuk, A.G. 2013. The earliest known holometabolous insects. Nature 503: 257–261. Crossref
Nel, A., Zarbout, M., Barale, G., and Philippe, M. 1998. Liassotettigarcta africana sp. n. (Auchenorrhyncha: Cicadoidea: Tettigarctidae), the first Mesozoic insect from Tunisia. European Journal of Entomology 95: 593–598.
Piton, L.E. 1936. Les hémiptères homoptères de l’Éocène de Menat (P.-de.-D.). Miscellanea Entomologica 37: 93–94.
Poinar, G.O., Jr., and Kritsky, G. 2012. Morphological conservatism in the foreleg structure of cicada hatchlings, Burmacicada protera n. gen., n. sp. in Burmese amber, Dominicicada youngi n. gen., n. sp. in Dominican amber and the extant Magicicada septendecim (L.) (Hemiptera: Cicadidae). Historical Biology 24: 461–466. Crossref
Ponomarenko, A.G. 1969. Cretaceous insects from Labrador. 4. A new family of beetles (Coleoptera: Archostemata). Psyche: A Journal of Entomology 76: 306–310. Crossref
Quicke, D.L.J. 2017. Mimicry, Crypsis, Masquerade and other Adaptive Resemblances. 576 pp. Wiley-Blackwell, Hoboken.
Rice, H.M.A. 1969. An antlion (Neuroptera) and a stonefly (Plecoptera) of Cretaceous age from Labrador, Newfoundland. Geological Survey of Canada, Department of Energy, Mines and Resources, Paper 68–65: iv + 1–11. Crossref
Sanmartín, I., Enghoff, H., and Ronquist, F. 2001. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biological Journal of the Linnean Society 73: 345–390. Crossref
Scotese, C.R. 2001. Atlas of Earth History. Vol. 1. Paleogeography. 58 pp. PALEOMAP Project, Arlington.
Shcherbakov, D. 2009. Review of the fossil and extant genera of the cicada family Tettigarctidae (Hemiptera: Cicadoidea). Russian Entomological Journal 17: 343–348.
Shi, G., Grimaldi, D.A., Harlow, G.E., Wang, J., Wang, J., Yang, M., Lei, W., Li, Q., and Li, X. 2012. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research 37: 155–163. Crossref
Spicer, R.A., Rees, P.M., and Herman, A.B. 1996. The Cretaceous vegetation and climate of Asia: some insights. Cretaceous stratigraphy and palaeoenvironments. Memoirs of the Geological Society of India 37: 405–433.
Tillyard, R.J. 1919. Mesozoic insects of Queensland. No.7. Hemiptera Homoptera; with a note on the phylogeny of the suborder. Proceedings of the Linnean Society of New South Wales 44: 857–896.
Tillyard, R.J. 1922. Mesozoic insects of Queensland. No.9. Orthoptera, and additions to the Protorthoptera, Odonata, Hemiptera and Planipennia. Proceedings of the Linnean Society of New South Wales 47: 447–470.
Wang, B. and Zhang, H. 2009. Tettigarctidae (Insecta: Hemiptera: Cicadoidea) from the Middle Jurassic of Inner Mongolia, China. Geobios 42: 243–253. Crossref
White, A. 1845. Descriptions and figures of four new species of Australian insects. In: E.J. Eyre (ed.), Journals of Expeditions of Discovery into Central Australia, and Overland from Adelaide to King George’s Sound in the Years 1840–1; Sent by the Colonists of South Australia, with the Sanction and Support of the Government: Including an Account of the Manners and Customs of the Aborigines and the State of Their Relations with Europeans. I. Appendix. 3 pp. D.T. and W. Boone, London.
Zeuner, F.E. 1944. Notes on Eocene Homoptera from the Isle of Mull, Scotland. Annals and Magazine of Natural History 11: 110–117. Crossref
Zheng, Y., Chen, J., and Wang, X. 2016. A new genus and species of Tettigarctidae from the Mesozoic of northeastern China (Insecta, Hemiptera, Cicadoidea). ZooKeys 632: 47–55. Crossref
Acta Palaeontol. Pol. 65 (1): 85–98, 2020
https://doi.org/10.4202/app.00669.2019