

A new Early Cretaceous flea from China
YANJIE ZHANG, CHUNGKUN SHIH, ALEXANDR P. RASNITSYN, DONG REN, and TAIPING GAO
Zhang, Y., Shih, C., Rasnitsyn, A.P., Ren, D., and Gao, T. 2020. A new Early Cretaceous flea from China. Acta Palaeontologica Polonica 65 (1): 99–107.
Fleas are highly specialized holometabolic insects. So far, only 16 species of fossil fleas in five families have been reported due to the rare fossil records. At present, the earliest flea fossils are reported from the Middle Jurassic Jiulongshan Formation of Northeastern China. The descriptions of these earliest species pushed back the origin of Siphonaptera by at least 40 million years. It is generally accepted that saurophthirids are the “transitional” taxa from stem Jurassic fleas to living crown groups. Herein, we described a new “transitional” flea species, Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov., from the Lower Cretaceous Yixian Formation of Northeastern China, assigned to Saurophthiridae. The new species provides new evidence to support saurophthirids as a “transitional” group. Sexual dimorphism suggests significant differences in biology of opposite sexes in Saurophthirus. Analysis of described Mesozoic species demonstrates the body size reduction from the Middle Jurassic to the Early Cretaceous. Smaller body size was likely advantageous in reducing the probability of being detected and removed by the host and in minimizing flea’s demand for blood intake and energy input, indicating the adaptation of the ectoparasitic lifestyle of fleas in their early stage of evolution.
Key words: Insecta, Siphonaptera, Saurophthiridae, Saurophthirus, ectoparasitic insects, compression fossils, Mesozoic, Asia.
Yanjie Zhang [15011010331@163.com], Chungkun Shih [chungkun.shih@gmail.com], Dong Ren [rendong@mail.cnu.edu.cn], and Taiping Gao [tpgao@cnu.edu.cn] (corresponding author),College of Life Sciences, Capital Normal University, 105 Xisanhuanbeilu, Haidian District, Beijing 100048, China; Academy for Multidisciplinary Studies, Capital Normal University, 105 Xisanhuanbeilu, Haidian District, Beijing 100048, China.
Chungkun Shih [chungkun.shih@gmail.com], Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012, USA.
Alexandr P. Rasnitsyn [alex.rasnitsyn@gmail.com], Palaeontological Institute, Russian Academy of Sciences, Moscow 117997, Russia; Natural History Museum, Cromwell Road, London SW7 5BD, UK.
Received 20 September 2019, accepted 12 November 2019, available online 21 February 2020.
Copyright © 2020 Y. Zhang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Siphonaptera, commonly known as fleas, are one of the widely known blood-sucking ectoparasitic insects, comprising more than 2500 described species in 16 extant families (Grimaldi and Engel 2005; Whiting et al. 2008; Krasnov 2008). Up to now, the fossil Siphonaptera contain 16 species in five families from Cenozoic amber and Mesozoic compression fossils (Ren et al. 2019). All Cenozoic fleas have been placed in two families, Ctenophthalmidae Rothschild, 1915 and Pulicidae Billberg, 1820, while all Mesozoic fleas, grouped in three families, Pseudopulicidae Gao, Shih, and Ren, 2012, Saurophthiridae Ponomarenko, 1986, and Tarwiniidae Huang, Engel, Cai, and Nel, 2013, form an extinct superfamily Saurophthiroidea Ponomarenko, 1986 comprising nine species within five genera as shown in Table 1.
In fact, the only true (crown group) flea fossils are those from Eocene Baltic amber (Peus 1968; Beaucournu and Wunderlich 2001) and Miocene Dominican amber (Lewis and Grimaldi 1997; Perrichot et al. 2012; Poinar 2015). These amber species have some similar characters as those of the extant fleas, e.g., small body sizes, distinct ctenidia on the tibiae and the entirely retracted male genitalia. Fleas of the Cenozoic are generally considered to have common ancestor with the ancestral forms of the modern lineages of fleas (Poinar 1995; Lewis and Grimaldi 1997).
The earliest flea-like fossils from the Middle Jurassic were reported by Huang et al. (2012) and Gao et al. (2012). The majority of these huge Mesozoic basal fleas, with piercing-sucking stylets from the Middle Jurassic Daohugou Biota and the Early Cretaceous Jehol Biota, were assigned to the family Pseudopulicidae, among which Pseudopulex (Gao et al. 2012) represents a series of distinctive basal fleas. Huang et al. (2013) established Tyrannopsylla and Hadropsylla of Pseudopulicidae. Then, Gao et al. (2014) described a female flea, Pseudopulex tanlan of Pseudopulicidae from the Early Cretaceous of China, which has an extremely distended abdomen suggesting that it might have consumed its last meal before its demise.
Table 1. Fossil records of Siphonaptera. Abbreviations: E3, late Eocene (Priabonian); J2, Middle Jurassic; K1, Early Cretaceous; N1, Miocene.
|
Taxon |
Age |
Location |
Taphonomy |
References |
|
Ctenophthalmidae |
|
|||
|
Palaeopsylla |
|
|||
|
P. klebsiana Dampf, 1911 |
E3 |
Germany |
Baltic amber |
|
|
P. dissimilis Peus, 1968 |
E3 |
Germany |
Baltic amber |
|
|
P. baltica Beaucounrnu and Wunderlich, 2001 |
E3 |
Germany |
Baltic amber |
|
|
P. groehni Beaucourun, 2003 |
E3 |
Germany |
Baltic amber |
|
|
Pulicidae |
|
|||
|
Tribe Pulicini |
|
|||
|
Pulex |
|
|||
|
P. larimerius Lewis and Grimaldi, 1997 |
N1 |
Dominican Republic |
amber |
|
|
Tribe Spilopsyllini |
|
|||
|
Eospilopsyllus |
|
|||
|
E. kobberti Beaucournu and Perrichot, 2012 |
N1 |
Dominican Republic |
amber |
|
|
Tribe Atopopsyllini |
|
|||
|
Atopopsyllus |
|
|||
|
A. cionus Poinar, 2015 |
N1 |
Dominican Republic |
amber |
|
|
Pseudopulicidae |
|
|||
|
Pseudopulex |
|
|||
|
P. jurassicus Gao, Shih, and Ren, 2012 |
J2 |
Ningcheng, Inner |
compression fossil |
|
|
P. magnus Gao, Shih, and Ren, 2012 |
K1 |
Duolun, Inner |
compression fossil |
|
|
P. tanlan Gao, Shih, Rasnitsyn, and Ren, 2014 |
K1 |
Beipiao, Liaoning, China |
compression fossil |
|
|
P. wangi Huang, Engel, Cai, and Nel, 2013 |
J2 |
Ningcheng, Inner |
compression fossil |
|
|
Tyrannopsylla |
|
|||
|
T. beipiaoensis Huang, Engel, Cai, and Nel, 2013 |
K1 |
Beipiao, Liaoning, China |
compression fossil |
|
|
Hadropsylla |
|
|||
|
H. sinica Huang, Engel, Cai, and Nel, 2013 |
J2 |
Ningcheng, Inner |
compression fossil |
|
|
Saurophthiridae |
|
|||
|
Saurophthirus |
|
|||
|
S. longipes Ponomarenko, 1976 |
K1 |
Baissa, Russia |
compression fossil |
|
|
S. exquisitus Gao, Shih, Rasnitsyn, and Ren, 2013 |
K1 |
Beipiao, Liaoning, China |
compression fossil |
|
|
S. laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov. |
K1 |
Lingyuan, Liaoning, China |
compression fossil |
this paper |
|
Tarwiniidae |
|
|||
|
Tarwinia |
|
|||
|
T. australis Jell and Duncan, 1986 |
K1 |
Koonwarra, Australia |
compression fossil |
|
Tarwinia australis was the first fossil flea from the Lower Cretaceous Koonwarra fossil bed of Australia reported and attributed by Riek (1970) to Siphonaptera, but its taxonomy has been controversial until Huang (2015), transferred Tarwinia to a new family, namely Tarwiniidae. Huang (2015) revised the morphological characters of T. australis according to the elongate siphonate mouthparts with the siphon pointing apically. By comparing the structures of the body bristles, tibiae ctenidia and the terminalia with the other groups, Huang (2015) demonstrated that T. australis belongs to the basal flea group and is supposed to come from a common ancestor with other Mesozoic fleas in Northeastern China.
Saurophthirus longipes, an unusual insect from the Lower Cretaceous Zaza Formation of Baissa in Siberia, was described by Ponomarenko (1976). He reported that its similarities with fleas are piercing-sucking proboscis and soft distensible abdomen. A family of Saurophthiridae was established by Ponomarenko (1986) ten years later. Another species of this family, Saurophthirus exquisitus, was described by Gao et al. (2013) based on three key specimens and they considered saurophthirids as a “transitional” group from basal to extant fleas. These fossil fleas display some features seen in crown fleas but are still considerably different from extant fleas in many morphological characters, e.g., the absence of pronotal and genal ctenidia on body (except for Tungidae), lack of the uniquely modified jumping hind legs, distinct ctenidia on the tibiae, more developed eyes, antennae with more than 15 segments, absence of laterally compressed abdomen, presence of medium body size, swollen hind coxae and partially extended male genitalia. These features indicate that Saurophthirus is more closely related to modern fleas than to Pseudopulex and Tarwinia (Gao et al. 2012, 2013; Huang et al. 2013; Huang 2015). Recently, Rasnitsyn and Strelnikova (2017, 2018) revealed that female S. longipes has a hypertrophied air storing tracheal system and simplified, larval looking digestive system, and supposed that it was a paedomorphic gematophagous insect with a complex gonotrophic cycle which used aquatic retreats while digesting imbibed blood and developing respective egg batch. Similar hypertrophied spiracles and tracheal trunks are also visible in female S. exquisitus (Rasnitsyn and Strelnikova 2017, 2018).
To sum up, these Mesozoic fossil flea taxa exhibit lots of common characters, e.g., wingless body covered with stiff setae, short and beaded antennae, reduced eyes, mouthparts with serrated stylets, scythe-like pretarsal claws and at least partially exposed genitalia. In addition, these species share with extant fleas some similar characters, especially with the most basal extant flea lineage. Aneuretopsychidae have been suggested as a sister group of Siphonaptera by the presence of a long proboscis with siphonate stylet and mouthparts with serrate laciniae (Huang et al. 2012).
Pseudopulicidae are considered to be the basal flea group due to, among others, the large body size and the robust mouthpart (Gao et al. 2012). Tarwiniidae are known as closer to the basal fleas by morphological analysis (Huang 2015), and Saurophthiridae have many unique features as a transitional group from the stem fossil flea group to extant fleas. More importantly, according to the early morphological characters of ectoparasitic insects, e.g., large body size (up to 22.8 mm) and long serrated stylets (up to 5.15 mm), the earliest hosts of ectoparasitic insects might have been feathered dinosaurs or pterosaurs and then transitioned to mammals and birds (Zhou et al. 2003; Xu et al. 2005; Huang 2014).
Herein we describe a new species, Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov., based on one nearly completely preserved male flea from the Lower Cretaceous Yixian Formation at Dawangzhangzi, Lingyuan City, Liaoning Province. We compared the new species with other known members of Siphonaptera in the Mesozoic, and discussed the body size reduction and male genitalia change of these rare ectoparasitic insect fossils. This new flea provides important and supplemental morphological characters of the family of Saurophthiridae.
Institutional abbreviations.—CNU, Capital Normal University, Beijing, China; PIN, Borissiak Paleontological Institute of the Russian Academy of Sciences, Moscow.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:805F0A29-CBEC-43B1-89BE-47BA6F751D80.
Material and methods
The new material was collected from the Lower Cretaceous Yixian Formation at Dawangzhangzi Village, Lingyuan City, western Liaoning Province. Saurophthirus laevigatus (CNU-SIP-LL2015001p/c) is housed in the Key Laboratory of Insect Evolution and Environmental Changes at the Capital Normal University, Beijing. (CNUB, Dong Ren, Curator).
The Yixian Formation consists mainly of volcanic deposits with subordinate sedimentary interbeddings that are rich in fossils. The Dawangzhangzi Village is situated in the Lingyuan-Sanshijiazi Basin in the westernmost parts of Liaoning Province, and the sedimentary sequence here consists of lacustrine sediments of shale and tuff (Zhou et al. 2003; Xu et al. 2017). Fossils from Dawangzhangzi are dark gray to black color on grayish plates (Ren et al. 2019). The specimen was examined under a Leica M205C microscope, and photographed by using a Nikon SMZ 25 microscope with a Nikon DS-Ri 2 digital camera system. Lines drawings were prepared using Adobe Illustrator CC and Adobe Photoshop CC graphics software.
Systematic palaeontology
Class Insecta Linnaeus, 1758
Order Siphonaptera Latreille, 1825
Family Saurophthiridae Ponomarenko, 1986
Genus Saurophthirus Ponomarenko, 1976
Type species: Saurophthirus longipes Ponomarenko, 1976; Lower Cretaceous, Yixian Formation, Liaoning Province of China.
Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov.
Figs. 1, 2.
ZooBank LSID: urn:lsid:zoobank.org:act:9765D35B-87CD-4BFF-9 AFB-39957267FB6D
Etymology: From Latin laevigatus, smooth.
Type material: Holotype: No. CNU-SIP-LL2015001p/c, a complete male with part and counterpart.
Type locality: Dawangzhangzi Village, Lingyuan City, Liaoning Province, China.
Type horizon: Yixian Formation, Early Aptian, Lower Cretaceous.
Diagnosis.—Male body almost perfectly fusiform (not distinctly attenuate rearward), of medium size (ca. 10 mm); head length longer than width; antenna with approximately 17 segments with flagellomeres compact disc-shaped, not distinctly widened before apex; body and legs with short bristles and setae; legs nearly as long as body, hind coxae elongated, hind femur 0.35 as long as body; male genitalia partially retracted, 0.3 times as long as body.
Description.—Male (Fig. 1A1, B1), 9.8 mm long excluding antennae, almost completely-preserved ventral view, slightly dorsoventrally compressed.
Head: Helmet-like, relatively small, 1.20 mm wide, 0.89 mm high; antenna with approximately 17 segments, overall length 1.60 mm, flagellomeres uniform in shape, basal thinner, generally widening, broadest at middle section, narrow at apex (Fig. 1A2); the compact disc-shaped flagellomeres slightly compressed and the last two segments smaller. Piercing-sucking mouthparts about 0.51 mm long (as preserved), extending to pro-coxae; existing laciniae but the tip of beak invisible (Fig. 2A1, A2, B1).
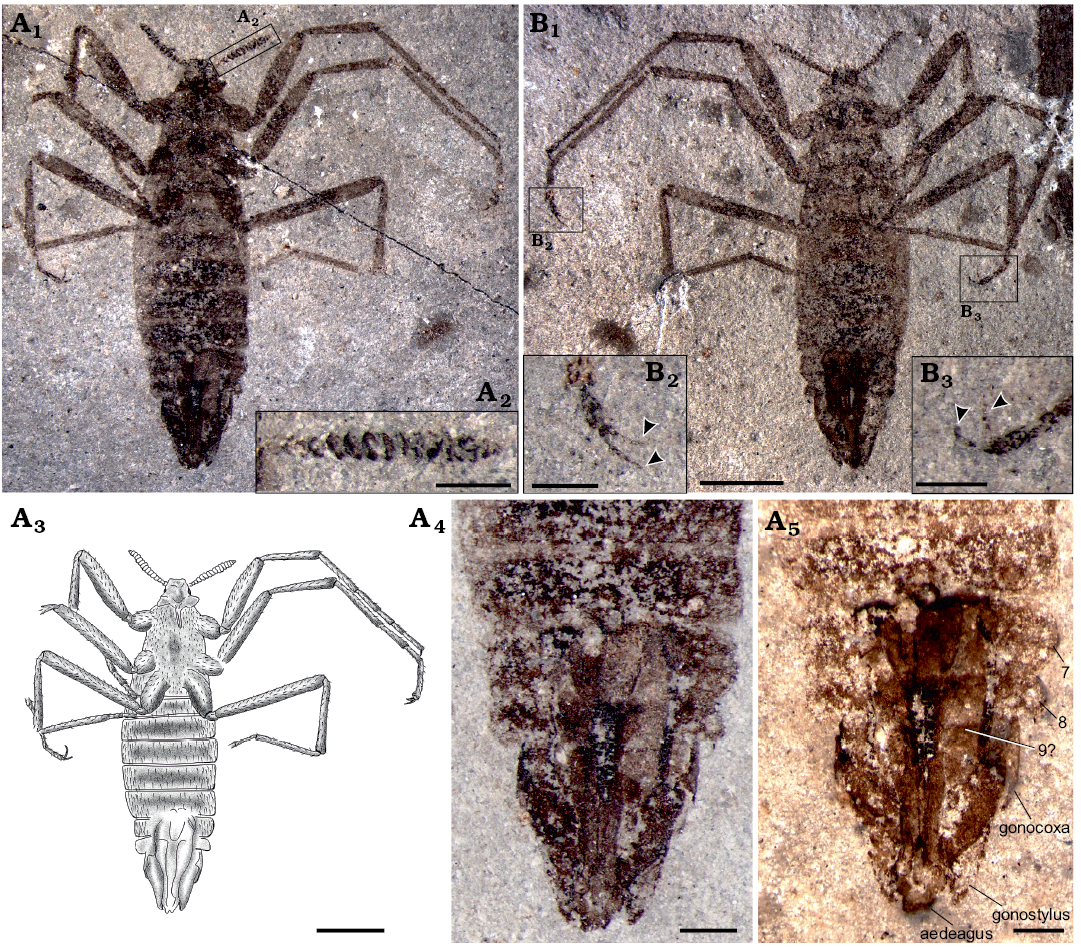
Fig. 1. Saurophthirid flea Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov., male (holotype, CNU-SIP-LL2015001) from the Lower Cretaceous Yixian Formation of Northeastern China. A. Part, habitus in general view (A1), line drawing (A3), enlargement of antenna (A2), details of genitalia (A4, A5); 7, 8, 9, the seventh to ninth abdominal segments. B. Counterpart, general view (B1), enlargements of claws (B2, B3, arrows). A5, photographed under alcohol. Scale bars: A1, B1, 1 mm; A3, 2 mm; A2, A4, A5, B2, B3, 0.5 mm.
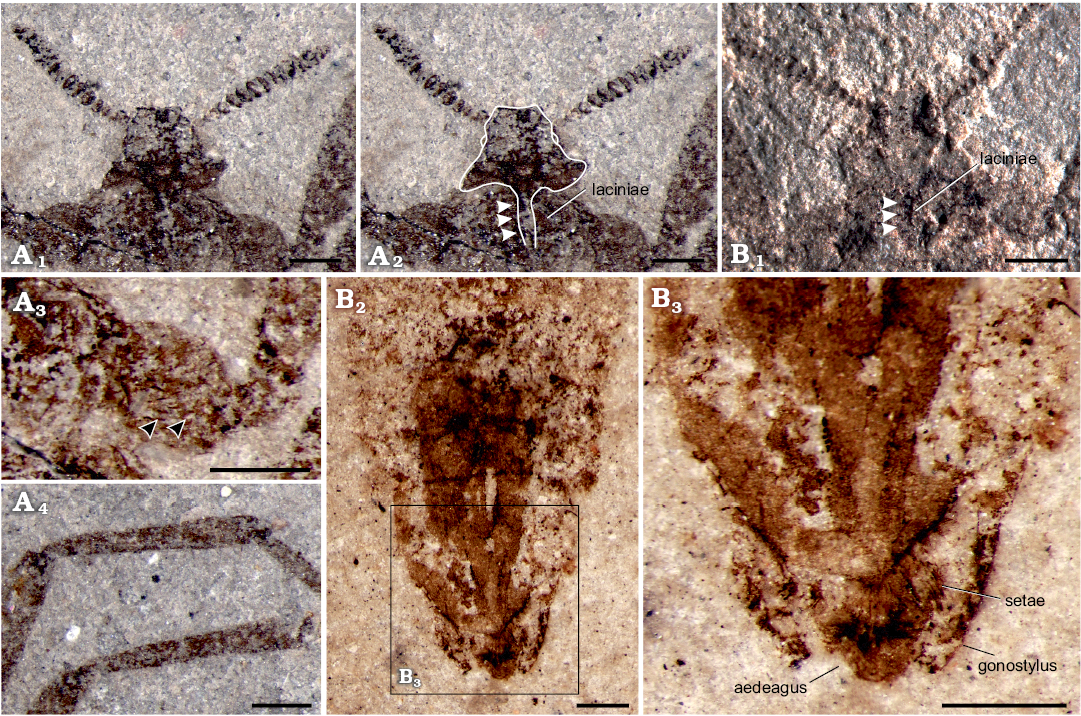
Fig. 2. Saurophthirid flea Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov., male (holotype, CNU-SIP-LL2015001) from the Lower Cretaceous Yixian Formation of Northeastern China. A. Part, head in ventral view (A1), head with position of the mouthpart indicated (A2, white arrowheads referring laciniae), bristles of the coxae (A3, black arrowheads referring bristles), tibia of the fore leg (A4). B. Counterpart, the laciniae under the unilateral light (B1), terminalia of the abdomen showing the setae (B2, B3). A3, B2, B3, photographed under alcohol. Scale bars 0.5 mm.
Thorax: Relatively small and without wings, prothorax narrower than mesothorax, mesothorax narrower than metathorax; prothorax approximately twice as long as metathorax. Legs slender, coxae slightly enlarged and with prominent bristles (Fig. 2A3), hind coxae approaching each other and the length twice as long as the fore and mid coxae; the length of each femur 1.4 times as long as tibiae, lengths obviously increasing from fore to hind femur and tibiae, particularly on femur; hind femur thinner than middle femur, much thinner than fore femur; tibiae specialized, basal narrow but gradually widened toward apex (Fig. 2A4); tarsus with five segments; basitarsus equal to the following two tarsal segments combined; fourth tarsal segment shortest, fifth tarsal segment nearly equal to the second one; pretarsus with a pair of large scythe-shaped claws.
Abdomen: Eight segments and the fourth segment widest; the width of sternites I–VI subequal in length; sternites weakly sclerotized.
Genitalia: Male genitalia long, extending far beyond the body, with gonocoxa and gonostylus; the width of abdominal I–VI segments equal, VIII segment distinct shortened to 1/2 of I–VI segments, the width of abdominal IX–X segments nearly equal to abdominal I–VI segments; gonostylus and external part of gonocoxa equal to each abdominal segment (Fig. 1A4, A5); visible (external) part of aedeagus wide and with long setae, gonostylus and the external part of the gonocaxa with subequal lengths, the apex of gonostylus closed inward and almost complete package the aedeagus (Fig. 1A4, A5). The setae of body lost, only a few short and stout bristles preserved on the basal section of the fore leg, and the relatively sparse setae attached to the aedeagus, probably due to poor preservation (Fig. 1B2, B3).
Remarks.—The new species is distinguished from male S. longipes Ponomarenko, 1976 (Fig. 3) never described before (except for male genitalia) (Rasnitsyn 1992) and S. exquisitus Gao, Shih, Rasnitsyn, and Ren 2013 by fusiform body not distinctly attenuate rearward, short vestiture of legs and body and relatively short legs (hind femur 0.35 rather than 0.45–0.48 times as long as body) and genitalia (0.3 rather than 0.35–0.4 times as long as body). Additionally, it differs from S. longipes by 17-segmented antenna (not more than 15 in S. longipes), from S. exquisitus by antenna widest well before apex (distinctly widest near apex in S. exquisitus), with compact disc-shaped flagellomeres (more elongate in S. exquisitus).
Discussion
The extant flea displays peculiar has morphological characters including relatively small body-size, laterally compressed body, the uniquely modified jumping middle and hind legs (Smit 1972; Medvedev 2017). However, the morphological characters of the stem-group fleas are primitive compared to the crown fleas. The fossil flea documented in the Mesozoic possess the following features to support their flea affinity: the piercing-sucking mouthparts with serrate stylets to penetrate thick and body coverings; relatively large body with long but thin legs and scythe-shaped claws for living on a large surface; body and legs with stiff spines and setae all directed backward imply that they adapted to fix and move on a surface covered with hairs or feathers. Each of the above features separately can be probably found in some other insect groups. However, for insects having all these characters combined, they were most likely adapted to blood sucking of vertebrate host with outgrowths like hairs or feathers (Gao et al. 2016; Ren et al. 2019). Saurophthirids have been suggested to resemble crown-group fleas. The new species has the “transitional” characters of Saurophthiridae and particularly genus Saurophthirus, including the medium body size, short piercing-sucking stylet mouthparts, slender legs (enlarged hind coxae) and the half-retracted male genitalia (Gao et al. 2013). A combination of characters listed in diagnosis confirms its distinctness at the species level.

Fig. 3. Saurophthirid flea Saurophthirus longipes Ponomarenko, 1976, male (PIN 3064/2379, after Rasnitsyn 1992) from Lower Cretaceous of Baissa, Siberia, Transbaikalia, Russia. Part (A) and counterpart (B, photographed under alcohol), note the details of genitalia (A). 7, 8, 9, the seventh to ninth abdominal segments. Scale bars 2 mm.
We provide details of male genitalia information for Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov. (Figs. 1, 2) and S. longipes (Fig. 3), in addition to the previously described male of S. exquisitus (Gao et al. 2013), to highlight the considerable extent of the sexual dimorphism displayed by Saurophthiridae. The males are universally smaller than females and differ, in having well segmented abdomen (vs. externally non-segmented, soft and distensible in females), lack of cerci, transverse bands of short thick spines on thoracic and basal abdominal terga characteristic of females. Of particular interest is, even though not unquestionably established, that the three known males hitherto display no clear indication of inflated tracheal trunks nor hypertrophied spiracles eight which raised a far reaching hypothesis about saurophthirid biology (Rasnitsyn and Strelnikova 2017, 2018). Of course, sclerotized male abdomen makes more difficult to identify these structures in fossils, but we are not able to notice tracheae even at intersegmental gaps, and hypertrophied spiracles eight were expectable to be visible providing their size being comparable to that in females. This makes more likely that males are less deeply adapted to parasitic mode of life and particularly so toward underwater survival. Unfortunately, our knowledge is insufficient yet to propose more details about biology of saurophthirid males.
Up to now, three families, four genera with seven fossil species of basal flea insects have been reported from the Cretaceous of Australia, Russia, and the Northeastern China. In contrast to the species from the Jurassic, the taxa from the Cretaceous have a higher degree of richness, suggesting the Cretaceous is an important stage of evolutionary radiation after the origination of basal fleas in the Jurassic.
A key of the known genera of flea insects from the Mesozoic:
1. Tibiae with comb-like ctenidia armed on apical and all outer edges . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . .Tarwinia Jell and Duncan, 1986 (Tarwiniidae)
Tibiae with comb-like ctenidia armed on apical or not existing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
2. Legs as long as or longer than body, male genitalia internal for half its length or so . . . . . . . . . . . . . . . . . Saurophthirus Ponomarenko, 1976 (Saurophthiridae)
Legs shorter than the body, male genitalia external (Pseudopulicidae) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Antenna with 14–16 compact
flagellomeres; mouthparts at least extending to mesocoxae;
whole abdomen covered with posteriorly-directed
setae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . Pseudopulex
Gao, Shih, and Ren, 2012 (Pseudopulicidae)
Antenna with more than 17 compact
flagellomeres; mouthparts at least extending to the second sternite;
each abdominal segment covered with a band of transverse
rows of setae . . . . . . . . . . . . . . . . . . . . . . . . . 4
4. Tarsus longer than femur and tibia together, abdomen poorly sclerotized . . . . . . . . . . . . . . . . . . . . . . . . . Hadropsylla Huang, Engel, Cai, and Nel, 2013 (Pseudopulicidae)
Tarsus as long as femora and tibia together. abdomen sclerotized obviously . . . . . . . . . . . . . . . . . . . . . . Tyrannopsylla Huang, Engel, Cai, and Nel, 2013 (Pseudopulicidae)
We analyzed and measured all known Mesozoic flea species and list the data in Table 2 (detailed data in SOM: table S1, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Zhang_etal_SOM.pdf): Measurements (in mm) of all fossil flea insect specimens from the Mesozoic. But we excluded two incompletely preserved females, i.e., Pseudopulex tanlan (Gao et al. 2014) and Tyrannopsylla beipiaoens (Huang et al. 2013) and one male Saurophthyrus longipes (Rasnitsyn 1992). The result shows that the Mesozoic fleas have body lengths ranging 6.9–22.8 mm, and for the same species, the females are always longer than males. Comparing the species from different periods, the taxa from the Middle Jurassic have larger body and longer mouthparts.
Table 2. Measurements (in mm) of all documented fossil flea specimens from the Mesozoic. Abbreviations: e, estimated; J2, Middle Jurassic; K1, Early Cretaceous.
| |
Sex/specimen number |
Age |
Body |
Mouthparts |
Antenna |
Fore |
Fore leg/body |
Mid leg |
Mid leg/body |
Hind leg |
Hind leg/body |
|
Pseudopulex jurassicus Gao, Shih, and Ren, 2012 |
♀2010001 |
J2 |
17 |
3.44 |
2.35 |
8.51 |
0.5 |
10.97 |
0.65 |
14.16 |
0.83 |
|
♀154244 |
J2 |
14 |
2.4 |
1.6 |
7 |
0.5 |
8.3 |
0.59 |
10.8 |
0.77 |
|
|
Pesudopulex wangi Huang, Engel, Cai, and Nel, 2013 |
♂154245 |
J2 |
8 |
0.9 |
1.5 |
|
|
4.2 |
0.53 |
|
|
|
♂154246 |
J2 |
≥7.7 |
|
≥1.3 |
|
|
|
|
|
|
|
|
Hadropsylla sinica Huang, Engel, Cai, and Nel, 2013 |
♀154247a/b |
J2 |
20.6 |
6.3 |
3 |
8.95 |
0.43 |
10.9 |
0.53 |
14.45 |
0.7 |
|
♀154248 |
J2 |
17.8 |
6.5 |
3.1 |
|
|
|
|
13.9 |
0.78 |
|
|
Average |
15.48 |
3.91 |
2.31 |
8.15 |
0.48 |
8.59 |
0.58 |
13.33 |
0.77 |
||
|
Standard deviation |
4.29 |
2.19 |
0.67 |
0.84 |
0.03 |
2.75 |
0.05 |
1.47 |
0.05 |
||
|
Sample size |
5 |
5 |
5 |
3 |
3 |
4 |
4 |
4 |
4 |
||
|
Pseudopulex magnus Gao, Shih, and Ren, 2012 |
♀2010002 |
K1 |
22.8 |
5.15 |
2.42 |
13.79e |
0.6 |
17.22 |
0.76 |
|
|
|
Pseudopulex tanlan Gao, Shih, Rasnitsyn, and Ren 2014 |
♀2013002 |
K1 |
10 |
|
1.28 |
|
|
|
|
|
|
|
♂2013003 |
K1 |
9.5 |
|
1.72 |
4.95 |
0.52 |
|
|
|
|
|
|
Tyrannopsylla beipiaoensis Huang, Engel, Cai, and Nel, 2013 |
♂154249a/b |
K1 |
14.7 |
|
2.3 |
|
|
|
|
9.9 |
0.67 |
|
♀154250 |
K1 |
16.2 |
|
2.5 |
|
|
|
|
|
|
|
|
Tarwinia australis Jell and Duncan, 1986 |
♂26202 |
K1 |
7 |
1.12 |
1.52 |
4.14 |
0.59 |
5.79 |
0.83 |
7.68 |
1.1 |
|
Saurophthyrus longipes Ponomarenko, 1976 |
♀3064/1898 |
K1 |
12 |
1.34 |
1.44 |
11.39 |
0.95 |
12.61 |
1.05 |
15.87 |
1.32 |
|
♂3064/2379 |
K1 |
10.1 |
|
1.23 |
|
|
|
|
|
|
|
|
Saurophthyrus exquisitus Gao, Shih, Rasnitsyn, and Ren, 2013 |
♀2010016p/c |
K1 |
8.46 |
1.31 |
1.41 |
9.24 |
1.09 |
11.3 |
1.34 |
13.69 |
1.62 |
|
♀2010017 |
K1 |
9.74 |
1.1 |
1.13 |
|
|
|
|
|
|
|
|
♂2010018 |
K1 |
6.9 |
0.41 |
1.22 |
6.91 |
1 |
8.58 |
1.24 |
10.59 |
1.53 |
|
|
Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov. |
♂ 2015001p/c |
K1 |
9.78 |
0.51 |
1.6 |
7.78 |
0.8 |
8.99 |
0.92 |
|
|
|
Average |
11.55 |
1.56 |
1.69 |
8.31 |
0.79 |
10.75 |
1.02 |
11.55 |
1.25 |
||
|
Standard deviation |
4.50 |
1.50 |
0.47 |
3.19 |
0.21 |
3.61 |
0.21 |
2.89 |
0.34 |
||
|
Sample size |
11 |
7 |
11 |
7 |
7 |
6 |
6 |
5 |
5 |
||
Gao et al. (2013) proposed that saurophthirids were the “transitional” group of Siphonaptera based on many transitional morphological characters from pseudopulicids to extant fleas, such as short piercing-sucking stylet mouthparts, rows of short and stiff bristles on the thorax, highly elongated legs, partially invaginated genitalia, especially the small body size. As shown in the Table 2, there is a significant difference, e.g., the body lengths of fleas in the Middle Jurassic ranging from 8.00 to 20.60 mm with an average of 15.48 ± 4.29 mm (n = 5) vs. the Early Cretaceous, from 6.90 to 22.80 mm with an average of 11.55 ± 4.50 mm (n = 11). On the other hand, the foreleg lengths increased slightly from an average of 8.15 ± 0.84 mm (n = 3) in the Middle Jurassic to 8.31 ± 3.19 mm (n = 7) in the Early Cretaceous. The length ratios of leg/body increased from an average of 0.48 ± 0.03 mm (n = 3) in the Middle Jurassic to 0.79 ± 0.21 mm (n = 7) in the Early Cretaceous. Therefore, the main reason for the change of the length ratios of leg/body is decrease in body size. This phenomenon is mainly reflected by saurophthirids, especially Saurophthirus exquisitus (Gao et al. 2013) with the body length of only 6.90 mm (male).
For ectoparasitic insects living in feathers of feathered dinosaurs, pterosaurs, or birds or in hairs of mammals, their small body size would have provided advantage for concealment in the host and reduced probability for being detected and removed by the host. Furthermore, we consider that the reason for decreasing body size of basal fleas was to reduce the blood intake and minimize flea’s demand for food and energy inputs. According to Hu et al. (2001), the blood consumption of the flea is positively correlated with its weight, and the blood consumption of female fleas are significantly higher than males. Besides, as early as 1992, there were reports that the blood consumption of the female cat fleas, Ctenocephalides felis Bouché, 1835 per day, which was equivalent to 15.15 times of their body weight. So, we believe that the Early Cretaceous fleas, especially the transitional fleas, became smaller in order to avoid host detection and reduce blood intake, which is the adaptation to ectoparasitic life in the early stage of evolution (Dryden and Gaafar 1991; Patterson 1991; Hu et al. 2001).
In addition, the evolution of the male genitalia is clearly indicated in fossils. The basal taxa from the Jurassic have entirely exposed genitalia with broad gonostylus articulated at apex to form a wide clasping organ, in contrast to the half-retracted genitalia from the Early Cretaceous. Therefore, we believe that the new species with more retracted genitalia might have provided concealment and protection.
Conclusions
A new flea, Saurophthirus laevigatus Zhang, Shih, Rasnitsyn, and Gao sp. nov., represented by a male specimen, is described from the Early Cretaceous of China. It is regarded as a “transitional” form between stem and crown fleas. This report, by describing a male specimen from the Early Cretaceous, not only increased the diversity of Mesozoic fleas, but also provided new evidence for saurophthirids as a “transitional” group. Furthermore, we summarized all documented fossil flea insects and prepared a key of the known genera of flea insects from the Mesozoic. In addition, we measured key body parts for all fossil flea insects reported from the Mesozoic. The analysis of published Mesozoic species indicates the body lengths of these fleas became smaller from the Middle Jurassic to the Early Cretaceous to provide advantage for concealment in the host, reducing probability for being detected and removed by the host, reducing the blood intake, and minimizing flea’s demand for food and energy inputs. It clearly suggests the adaptation of the ectoparasitic lifestyle in the early stage of flea evolution. Examined morphology of the male Saurophthiridae is found less sophisticated than in females and suggests that they had less extravagant, even though parasitic, biology than is hypothesized for females.
Acknowledgements
We express our gratitude to George Poinar (Oregon State University, Corvallis, US) and Sergei Medvedev (Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia) for their critical but valuable review and improvement of the manuscript. We are grateful to Hongru Yang (CNU) for constructive suggestion and the revision of the article. We appreciate Nan Yang and Yimo Yang (CNU) for their helpful advices. TPG was supported by the National Natural Science Foundation of China (31872277), the Program for Changjiang Scholars and Innovative Research Team in University (IRT-17R75), and Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (IDHT20180518). DR was supported by grants from the National Natural Science Foundation of China (31730087, 41688103, and 31672323).
References
Beaucournu, J.C. 2003. Palaeopsylla groehni n. sp., quatrième espèce de puce connue de l’ambre de la Baltique (Siphonaptera, Ctenophthalmidae). Bulletin de la Société entomologique de France 108: 217–220.
Beaucournu, J.C. and Wunderlich, J. 2001. A third species of Palaeopshylla Wagner, 1903, from Baltic amber (Siphonaptera: Ctenophthalmidae). Deutsche Entomologische Zeitschrift 11: 296–298.
Dampf, A. 1911. Palaeopsylla klebsiana n. sp., ein fossiler Floh aus dem baltischen Bernstein. Schriften der PhysikalischÖkonomischen Gesellschaft zu Königsberg 51: 248–259.
Dryden, M.W. and Gaafar, S.M. 1991. Blood consumption by the cat flea, Ctenocephalides felis (Siphonaptera: Pulicidae). Journal of Medical Entomology 28: 394–400. Crossref
Gao, T.P., Rasnitsyn, A.P., Shih, C.K., Xu, X, Wang, S., and Ren, D. 2016. On the probability of dinosaur fleas. BMC Evolutionary Biology 16: 9. Crossref
Gao, T.P., Shih, C.K., Rasnitsyn, A.P., Xu, X., Wang, S., and Ren, D. 2013. New transitional fleas from China highlighting diversity of Early Cretaceous Ectoparasitic insects. Current Biology 23: 1261–1266. Crossref
Gao, T.P., Shih, C.K., Rasnitsyn, A.P., Xu, X., Wang, S., and Ren, D. 2014. The first flea with fully distended abdomen from the Early Cretaceous of China. BMC Evollutionary Biology 14: 168–174. Crossref
Gao, T.P., Shih, C.K., Xu, X., Wang, S., and Ren, D. 2012. Mid-Mesozoic flea-like ectoparasites of feathered or haired vertebrates. Current Biology 22: 732–735. Crossref
Grimaldi, D.A. and Engel, M.S. 2005. Evolution of the Insects. 710 pp. Cambridge University Press, New York.
Hu, X.L., He, J.H., Zhang, H.Y., Yang, Z.M., Zhao, W.H., Liang, Y., Wu, M.T., and Gao, Z.H. 2001. Body weight and quantity of blood-sucking of six species of fleas in Yunnan Province, China. Chinese Journal of Pest Control 17: 393–395.
Huang, D.Y. 2014. Trace back the origin of recent insect orders—evidence from the Middle Jurassic Daohugou Biota. Science Foundation in China 22: 34–42.
Huang, D.Y. 2015. Tarwinia australis (Siphonaptera: Tarwiniidae) from the Lower Cretaceous Koonwarra fossil bed: morphological revision and analysis of its evolutionary relationship. Cretaceous Research 52: 507–515. Crossref
Huang, D.Y., Engel, M.S., Cai, C.Y., and Nel, A. 2013. Mesozoic giant fleas from Northeastern China (Siphonaptera): taxonomy and implications for palaeodiversity. Chinese Science Bulletin 58: 1682–1690. Crossref
Huang, D.Y., Engel, M.S., Cai, C.Y., Wu, H., and Nel, A. 2012. Diverse transitional giant fleas from the Mesozoic era of China. Nature 483: 201–204. Crossref
Jell, P.A. and Duncan, P.M. 1986. Invertebrates, mainly insects, from the freshwater, Lower Cretaceous, Koonwarra fossil bed (Korumburra Group), South Gippsland, Victoria. Memoirs of the Association of Australasian Palaeontologists 3: 111–205.
Krasnov, B.R. 2008. Functional and Evolutionary Ecology of Fleas: A Model for Ecological Parasitology. 593 pp. Cambridge University Press, New York. Crossref
Lehane, M.J. 2005. The Biology of Blood-Sucking in Insects. 337 pp. Cambridge University Press, New York. Crossref
Lewis, R.E. and Grimaldi, D.A. 1997. A pulicid flea in Miocene amber from the Dominican Republic (Insecta: Siphonaptera: Pulicidae). American Museum Novitates 3205: 1–9.
Medvedev, S.G. 2017. Adaptations of fleas (Siphonaptera) to parasitism. Entomological Review 97: 1023–1030. Crossref
Patterson, R.S. 1991. Hematophagous strategies of the cat flea (Siphonaptera, Pulicidae). Florida Entomologist 74: 377–385. Crossref
Perrichot, V., Beaucournu, J.C., and Velten, J. 2012. First extinct genus of a flea (Siphonaptera: Pulicidae) in Miocene amber from the Dominican Republic. Zootaxa 3438: 54–61. Crossref
Peus, F. 1968. Über die beiden Bernstein-Flöhe (Insecta, Siphonaptera). Paläontologische Zeitschrift 42: 62–72. Crossref
Poinar, G.O. Jr. 2015. A new genus of fleas with associated microorganisms in Dominican amber. Journal of Medical Entomology 52: 1234–1240. Crossref
Poinar, G.O. Jr. 1995. Fleas (Insecta, Siphonaptera) in Dominican amber. Medical Science Research 23: 789.
Ponomarenko, A.G. 1976. A new insect from the Cretaceous of Transbaikalia USSR a possible parasite of Pterosaurians. Paleontological Journal 3: 102–106.
Ponomarenko, A.G. 1986. Insects in the Early Cretaceous ecosystems of Western Mongolia. Joint Soviet-Mongolian Geological and Paleontological Expedition 28: 110–112.
Rasnitsyn, A.P. 1992. Strashila incredibilis, a new enigmatic mecopteroid insect with possible siphonateran affinities from the Upper Jurassic of Siberia. Psyche 99: 319–329. Crossref
Rasnitsyn, A.P. and Strelnikova, O.D. 2017. Tracheal system and biology of the Early Cretaceous Saurophthirus longipes Ponomarenko, 1976 (Insecta, ?Aphaniptera, Saurophthiroidea stat. nov.). Paleontological Journal 51: 171–182. Crossref
Rasnitsyn, A.P. and Strelnikova, O.D. 2018. Digestive system of the Early Cretaceous Saurophthirus longipes Ponomarenko, 1976 (Insecta, ?Aphaniptera, Saurophthiroidea stat. nov.). Paleontological Journal 52: 146–154. Crossref
Ren, D., Shih, C.K., Gao, T.P., Wang, Y.J., and Yao, Y.Z. 2019: Rhythms of Insect Evolution—Evidence From the Jurassic and Cretaceous in Northern China. 710 pp. Wiley Blackwell, New York. Crossref
Riek, E.F. 1970. Lower Cretaceous fleas. Nature 227: 746–747. Crossref
Smit, F.G. 1972. On some adaptive structures in Siphonaptera. Folia parasitologica 19: 5–17.
Whiting, M.F., Whiting, A.S., Hastriter, M.W., and Dittmar, K. 2008. A molecular phylogeny of fleas (Insecta: Siphonaptera): Origins and host associations. Cladistics 24: 677–707. Crossref
Xu, X. and Zhang, F.C. 2005. A new maniraptoran dinosaur from China with long feathers on the metatarsus. Naturwissenschaften 92: 173–177. Crossref
Xu, X., Zhou, Z.H., Sullivan, C., and Wang, Y. 2017. The Yanliao Biota: a trove of exceptionally preserved Middle–Late Jurassic terrestrial life forms. In: N.C. Fraser and H.-D. Sues (eds.), Terrestrial Conservation Lagerstatten: Window into the Evolution of Life on Land, 131–168. Dunedin Academic Press, London.
Zhou, Z.H., Barrett, P.M., and Hilton, J. 2003. An exceptionally preserved Lower Cretaceous ecosystem. Nature 421: 807–814. Crossref
Acta Palaeontol. Pol. 65 (1): 99–107, 2020
https://doi.org/10.4202/app.00680.2019