


A probable oligochaete from an Early Triassic Lagerstätte of the southern Cis-Urals and its evolutionary implications
DMITRY E. SHCHERBAKOV, TARMO TIMM, ALEXANDER B. TZETLIN, OLEV VINN, and ANDREY Y. ZHURAVLEV
Shcherbakov, D.E., Timm, T., Tzetlin, A.B., Vinn, O., and Zhuravlev, A.Y. 2020. A probable oligochaete from an Early Triassic Lagerstätte of the southern Cis-Urals and its evolutionary implications. Acta Palaeontologica Polonica 65 (2): 219–233.
Oligochaetes, despite their important role in terrestrial ecosystems and a tremendous biomass, are extremely rare fossils. The palaeontological record of these worms is restricted to some cocoons, presumable trace fossils and a few body fossils the most convincing of which are discovered in Mesozoic and Cenozoic strata. The Olenekian (Lower Triassic) siliciclastic lacustrine Petropavlovka Lagerstätte of the southern Cis-Urals yields a number of extraordinary freshwater fossils including an annelid. The segmented body with a secondary annulation of this fossil, a subtriangular prostomium, a relatively thick layered body wall and, possibly, the presence of a genital region point to its oligochaete affinities. Other fossil worms which have been ascribed to clitellates are reviewed and, with a tentative exception of two Pennsylvanian finds, affinities of any pre-Mesozoic forms to clitellate annelids are rejected. The new fossil worm allows tracing of a persuasive oligochaete record to the lowermost Mesozoic and confirms a plausibility of the origin of this annelid group in freshwater conditions.
Key words: Annelida, Clitellata, Oligochaeta, Mesozoic, Lagerstätte, Russia.
Dmitry E. Shcherbakov [dshh@narod.ru], Borissiak Palaeontological Institute, Russian Academy of Sciences, Profsoyuznaya St 123, Moscow 117647, Russia.
Tarmo Timm [tarmo.timm@emu.ee], Centre for Limnology, Estonian University of Life Sciences, 61117, Rannu, Tartumaa, Estonia.
Alexander B. Tzetlin [atzetlin@gmail.com], Department of Invertebrate Zoology, Faculty of Biology, Lomonosov Moscow State University, Leninskie Gory 1(12), Moscow 119234, Russia.
Olev Vinn [olev.vinn@ut.ee], Institute of Ecology and Earth Sciences, University of Tartu, Ravila 14A, 50411, Tartu, Estonia.
Andrey Y. Zhuravlev [ayzhur@mail.ru] (corresponding author), Department of Biological Evolution, Faculty of Biology, Lomonosov Moscow State University, Leninskie Gory 1(12), Moscow 119234, Russia.
Received 20 November 2019, accepted 23 March 2020, available online 20 April 2020.
Copyright © 2020 D.E. Shcherbakov et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The oligochaete Clitellata are ubiquitous in all but the driest and coldest regions. In the terrestrial fauna, they represent a principal and diverse group influential in sediment bioturbation, pedogenesis and soil profile development, mineral dissolution and clay mineral precipitation, soil fertility, topsoil and humus formation enhancing microbial activity and stimulating plant growth, feeding basis for a number of animals up to top predators and many other extremely important phenomena (Darwin 1881; Wesenberg-Lund 1939; Fisher et al. 1980; Tevesz et al. 1980; Ghilyarov 1983; Feller et al. 2003; Needham et al. 2004; Blakemore 2009; Cunha et al. 2016). Soil oligochaetes occupy even transpolar permafrost areas of eastern Siberia where they form a reliable food supply for nesting birds (Degtyarev et al. 2013; AZ personal field observation 2014).
On the contrary, the clitellate fossil record is extremely scarce and mostly represented by single incomplete specimens, the finds of which are summarised here (Table 1).
The majority of early Palaeozoic marine body fossils, which once upon a time were compared with oligochaetes (Bather 1920; Ruedemann 1925) were later ascribed to stem cycloneuralian worms (palaeoscolecidans) (Conway Morris et al. 1982; Harvey et al. 2010; Zhuravlev et al. 2011). Supposed Early and Middle Ordovician worms from Sweden, namely Hirudopsis koepingensis Moberg and Segerberg, 1906 from the Ceratopyge Limestone of Öland (Moberg and Segerberg 1906: pl. 1: 1–4) and Hammatopsis scanicus Hadding, 1913 and Stoma hians Hadding, 1913 from the Fjäcka Shale of Scania (Hadding 1913: pl. 1: 1, 2), are three-dimensionally preserved septate shelly fossils.
Of some interest are an undetermined possible annelid from the Middle Ordovician Trenton Limestone (Conway Morris et al. 1982) and two Silurian leech-like fossils from the Llandovery Waukesha Lagerstätte of Wisconsin and from the Pridoli Bertie Group of the New York State, USA, respectively (Ruedemann 1925: pl. 14: 3, 4; Mikulic et al. 1985a, b). The “Trenton worm” lacks either parapodia indicative of polychaetes or a platy phosphatic cuticle typical of palaeoscolecidans, but it does not show any diagnostic features either, except for faint transverse lines, an axially arranged probable alimentary canal and puzzling paired serial internal structures flanking a part of this canal. Thus, the affinities of this worm with the annelids, and in particular the oligochaetes, remain tenuous (Conway Morris et al. 1982). Ruedemannella obesa (Ruedemann, 1925), whose original generic name (Bertiella) has been replaced due to a preoccupation (Howell 1959, 1962), and the “Waukesha leech” possess some features in common with each other including dense prominent transverse ribbing along a relatively long (over 120 mm) plump body and a sharply rounded terminal opening resembling a rear sucker of a leech. Both fossils co-occur with rich marine faunas and due to a rigid nature of their cuticle and large size can be placed among cycloneuralian worms lacking a well-expressed introvert such as the middle Cambrian Ancalagonidae established by Conway Morris (1977), for instance.
Terrestrial Carboniferous strata of the Bohemian Massif yield more encouraging vermiform fossils, especially Pronaidites carbonarius Kušta, 1888 from the middle Pennsylvanian (Moscovian) Radnice Formation (Kušta 1888; Fritsch 1907: pl. 4: 1–3; Štamberg and Zajíc 2008). The brownish body of the holotype is about 110 mm long as preserved (incomplete at both ends) and less than 2 mm wide. It is subdivided into some 120 segments which are one third to half as long as wide. It has a sediment-filled intestine running along the body axis and chaetal rows (SOM 1: fig. 1A1–A3, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Shcherbakov_etal_SOM.pdf); according to Fritsch (1907), these rows bear chaetae of two types, one stout and several thin. Besides, this worm possesses dark paired serially arranged structures flanking the intestine and resembling those of the “Trenton worm” (SOM 1: fig. 1A2). Kušta (1888) mentioned but not figured four more, likely conspecific specimens from the same bed (yielded also a number of terrestrial arachnids), all very long (over 100 mm), narrow (0.5–2.0 mm) and multi-segmented (100–150 segments), and interpreted the worm as a freshwater oligochaete. The occurrence of Pronaidites in numbers agrees with the aquatic mode of life; its filiform body resembles both some oligochaetes (e.g., Tubificidae) and polychaetes (e.g., Capitellidae), but the presence of stout outermost chaeta in the bundle is more consistent with the polychaete nature.
Two other fossils ascribed to the genus Pronaidites by Fritsch (1907: pl. 4: 4–10) differ from the type species. Pronaidites arenivorus Fritsch, 1907 displays more similarity with polychaetes (possible appendages) while P. crenulatus Fritsch, 1907 is a trace fossil, probably conspecific with Vermites lithographus Kušta, 1888 (Kušta 1888; Štamberg and Zajíc 2008).
Another Late Palaeozoic oligochaete-like worm is pictured and briefly described by Zangerl and Richardson (1963: pl. 21: C) from the late Pennsylvanian Mecca Quarry Shale of Indiana, USA. This metalliferous shale is interpreted as deep marine deposits accumulated in a sediment-starved distal offshore setting under oxygen-depleted conditions (Coveney and Glascock 1989; Algeo and Heckel 2008). The Mecca Quarry fossil assemblage of B1 level bearing the worm is considered to be allochthonous due to a mixture of normal marine fauna (discinid brachiopods, nautiloids, acanthodians) and terrestrial plant leaves and stems (Zangerl and Richardson 1963). Thus, the primary ecotope of the vermiform fossil cannot be traced with certainty. The worm body is smooth annulated with a tapering end and bears possible transverse chaetal rows according to the authors.
Lumbricopsis permicus Fritsch, 1907 described by Fritsch (1907: pl. 4: 7) from the terrestrial strata of the Bohemian Massif, which are attributed at present to the Cisuralian (Sakmarian) lacustrine Prosečné Formation (Zajíc 2014), is a relatively long vermiform fossil. Its body is subdivided into numerous wide segments imparting the worm a platy habit and bearing short lateral paired outgrowths each (SOM 1: fig. 2A1–A3). By its overall morphology, L. permicus resembles certain polychaetes such as the freshwater Namanereidinae (Glasby 1999). Another species of the same genus, L. distinctus Fritsch, 1907 from the Asselian fluvial to lacustrine Vrchalbí Formation of the same area, is not illustrated well enough (Fritsch 1907: pl. 10: 6, 7) and is open to interpretation.
Although Pronaidites carbonarius and the “Mecca Quarry worm” display some similarities with oligochaetes, they lack undoubted clitellate features (clitellum, limited genital area) and can be compared with a number of polychaetes such as the Capitellidae, for instance (Fauchald 1977; Glasby and Timm 2008). Similarly, post-Palaeozoic oligochaete body fossils are restricted to a few finds which will be discussed in details below.
Here we report the oldest Mesozoic body fossil oligochaete which derives from the Olenekian (Lower Triassic) siliciclastic lacustrine Petropavlovka Lagerstätte of the southern Cis-Urals (Orenburg region, Russia).
Institutional abbreviations.—PIN, Borissiak Palaeontological Institute, Russian Academy of Sciences, Moscow, Russia.
Data archiving statement.—Data for this study (additional images) are available in the SOM as well as Dryad Digital Repository: http://doi.org/10.5061/dryad.95x69p8gg
Geological setting
In general, the Permian and Triassic of the southern Cis-Urals are well known for diverse fossil vertebrates—the tetrapod faunas of this region are essential for regional stratigraphy and allow a precise correlation of Triassic strata of eastern Euramerica and Gondwana (Ochev and Shishkin 1989; Ochev and Surkov 2000; Shishkin et al. 2000; Shishkin and Novikov 2017). The succession of fossiliferous horizons in the Cis-Urals embracing a significant Permian–Triassic interval provides a reliable basis for a detailed study of changes in climate, landscapes, vegetation, insect and vertebrate communities across the Permian/Triassic boundary (Benton et al. 2004; Gomankov 2005; Shcherbakov 2008b; Benton and Newell 2014).
The Petropavlovka Formation (Petropavlovskaya Svita) comprising a part of this succession is ascribed to the upper Olenekian (Lower Triassic) judging by the Parotosuchus tetrapod fauna, lungfish Ceratodus multicristatus Vorobyeva and Minikh, 1968, miospore assemblages rich in Densoisporites nejburgii (Schulz, 1964) Balme, 1970 associated with the lycophyte Pleuromeia, and magnetostratigraphy (Fig. 1; Shishkin et al. 1995; Tverdokhlebov et al. 2003; Novikov 2018).
In the Olenekian, orogenic movements were renewed in the Ural Mountains and the Peri-Caspian Depression was inundated by a transgression of the Palaeotethys, which led to increased rates of siliciclastic deposition in the Cis-Ural area (Tverdokhlebov 1987). In the Cis-Ural Trough and on the nearby southeastern slope of the Volga-Ural Anteclise, a vast lacustrine-deltaic floodplain was formed, framing the Peri-Caspian marine basin of the Palaeotethys from the north. The Petropavlovka area was a part of this floodplain accumulating grey and reddish-grey siliciclastics, mostly a rhythmic alternation of cross-laminated coarse-grained polymictic sandstone, parallel-bedded fine-grained sandstone, reddish-yellow, reddish-brown, or grey subparallel-layered clay, siltstone, and fine-grained clayey sandstone of 400–800 m in total thickness (Tverdokhlebov 1987; Shishkin et al. 1995). In addition, conglomerate lenses are common with igneous and metamorphic rock pebbles originated from the Urals. Mud cracks and rhizoliths are basically restricted to finer parallel-bedding lithologies; the coarser varieties represent alluvial deposits while finer ones are shallow water lacustrine sediments (Tverdokhlebov et al. 2007). These facies characterise delta floodplain and delta front complexes of the Petropavlovka Formation.
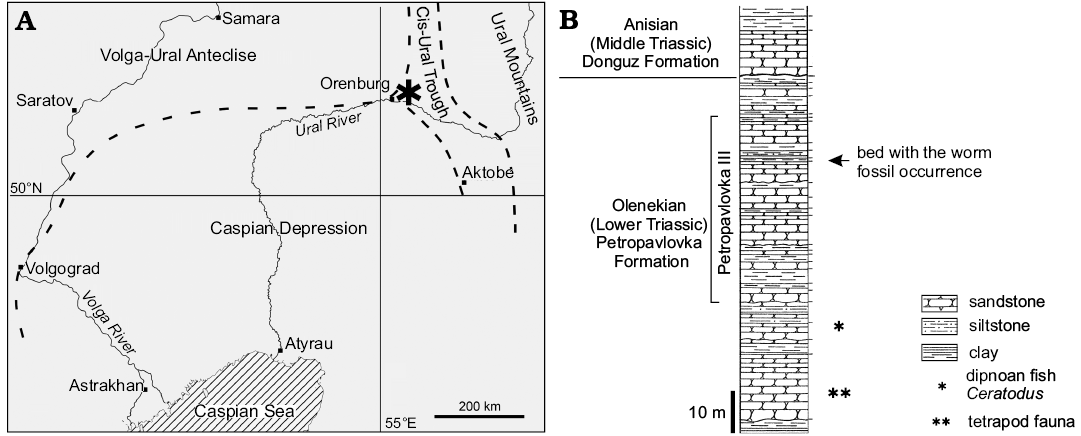
Fig. 1. A. Map showing the Lower Triassic locality Petropavlovka III (asterisk) on the Sakmara River valley bank near the village of Petropavlovka ca. 45 km north-east of the town of Orenburg, Russia, in the tectonic context (dashed lines, boundaries of tectonic regions; modified from Minikh and Minikh 1997). B. Combined stratigraphic log of Petropavlovka II–IV sections (modified from Tverdokhlebov 1967).
In a ravine occurring along the Sakmara River valley near the village of Petropavlovka ca. 45 km north-east of the town of Orenburg (coordinates N 52°02’, E 55°38’), fossiliferous coarse-grained red beds yield an 1-m-thick lens of grey fine-grained micro-wavy to parallel-laminated polymictic siltstone to sandstone (locality Petropavlovka III, bed 43; Tverdokhlebov 1967: 119). Thin section studies of the rock sample bearing the fossil worm show that it represents a greenish-grey siltstone consisting of angular grains of uneven size and comprising essentially feldspars, micas, and iron oxides and some plant material (SOM 2: fig. 1). A geochemical analysis does not reveal a significant content of elements indicative of low oxic, euxinic or any other specific conditions and fits the mineralogical composition listed above (SOM 2: fig. 2, tables 1, 2). The plant and animal fossils themselves are not restricted to certain bedding planes but are randomly distributed in the rock, thus, preserving some three-dimensionality of their bodies. Such a sediment likely was accumulated in an ephemeral pond during a flood event. The lens contains abundant plant megafossils including sphenophytes (Equisetites sp. and Neocalamites sp.), gymnosperms (Carpolithus sp. seeds, and Voltziopsis sp. conifer ovuliferous scales) (Dobruskina 1994; Shishkin et al. 1995). The fossil vertebrate coenosis represented by lungfishes Ceratodus (Minikh and Minikh 1997), dipnoan aestivation burrows (Sennikov 2018) and diverse temnospondyl amphibians with specific adaptations for feeding on aquatic animals (Shishkin et al. 1995; Novikov 2018; Sennikov and Novikov 2018) characterising the entire Petropavlovka Formation points to freshwater conditions of the lens genesis. Besides, the red beds yield clam shrimps (spinicaudatans, formerly in conchostracans), ostracods and crayfish burrows (Tverdokhlebov 1967; Sennikov and Novikov 2018).
In 2018, numerous insect wings and fragments including various roaches, beetles, and hemipterans, rare dragonflies, grylloblattids, and orthopterans, as well as several millipedes were discovered there (Shcherbakov et al. 2019). Further ostracods, clam shrimps and fish scales as well as a few horseshoe crabs were excavated. In addition, in 2019 microconchid Spirorbis-like tentaculitoid tubeworms encrusting horseshoe crab head shields and terrestrial plant remains were detected. This fossil assemblage represents a common Early Triassic freshwater fauna while insects and plants constitute a shore community proliferated in a floodplain environment (Kozur and Weems 2010; Zatoń et al. 2012; Kustatscher et al. 2014; Haig et al. 2015; Lamsdell 2016; Feng et al. 2018). Nowadays, the Petropavlovka III locality can be rated as a Lagerstätte preserving the richest definitely Early Triassic insect fauna world wide, more diverse than that of the Lower Olenekian Kockatea Shale in Australia (Haig et al. 2015) or the uppermost Olenekian–lowermost Anisian Pałęgi clay-pit in Poland (Żyła et al. 2013); several Eurasian faunas formerly regarded Early Triassic are now suggested to be the latest Permian (Shcherbakov 2008a, 2015).
Material and methods
A single incomplete worm specimen was collected in the Lower Triassic Petropavlovka Formation from the locality Petropavlovka III in 2018 during field work of the Arthropoda Laboratory (PIN). The fossil is a single fragment of three-dimensionally preserved worm body wall on a bedding surface of a greenish-grey polymictic micro-wavy-laminated siltstone.
The images of the fossil are obtained with a Leica M165C stereomicroscope coupled to a Leica DFC425 digital camera, and a TESCAN VEGA variable-pressure and environmental SEM using backscattered electron detector in PIN. An elemental analysis of uncoated and unpolished sample including the fossil and adjacent matrix was performed with a quantitative energy dispersive X-ray Inca microanalyser coupled to a TESCAN VEGA SEM, at an accelerating voltage of 20 keV, in PIN. Besides, quantitative elemental composition data were obtained from the host sediment powder by a wavelength dispersive X-ray fluorescence SPEKTROSKAN-MAKS-GV spectrometer, using high resolution LiF(200) analysing diffraction crystal at an accelerating voltage of 40 keV, in the Lomonosov Moscow State University (MSU).
For a comparison, extant Tubifex tubifex (Tubificidae) individuals were caught in the polluted Khripan’ River of the Moscow region (Russia), critical point dried and studied under the same SEM.
Fossil worm description and interpretation
The small cylindrically convex annulated fossil preserves an elongated worm portion of ca. 7 mm long and 1–1.3 mm wide reaching the edge of the rock slab (Fig. 2A). All the transverse ornamentation is not equally well developed, some rings are more prominent than others and form raised annuli at somewhat irregular interval (Figs. 2A, 3A, 4A1). It is possible to count 12 or less likely 13 fairly regularly spaced, wider, prominently raised annuli along the body (Fig. 2A). At least 5–6 finer annulets visible under polarised light and SEM are counted between these prominent structures (Fig. 2A). One end of the fossil is terminated with a subtriangular median projection (Fig. 3A1), the opposite end is marked by a slightly W-shaped (bisinuate) transverse depression, ca. 100 µm wide, extending over the entire visible worm diameter and having an anteriorly directed inflection along its midline (Figs. 2A2, 3A2). The entire body wall is 10 µm deep and consists of multiple micron-thick layers (Fig. 4A).
Four irregular folds, two arched sublongitudinal, one subtransverse and one Z-shaped occurring in the middle part of the fossil, are observed (Fig. 2A2, dash-and-dot lines). Although two arched folds, in places, run along the axis of the fossil and can fit to a position of some longitudinal organs such as an intestine, a blood vessel or a nerve cord, the discontinuity and irregularity of all these structures are merely indicative of a later post-mortem deforming and fracturing of the worm body.
The specimen exhibits minor plastic deformations including longitudinal and posterior transverse (W-shaped) depressions and a relative linear displacement of right and left areas of some segments along each other, which are indicative of the originally relatively flexible integument. An absence of a difference in the elemental composition between the fossil surface and the host rock (high content of aluminium, silicon and oxygen and a detectable amount of magnesium, potassium and iron) suggests a soft tissue replication with clay minerals (SOM 2: fig. 2). As a result, some fine details including possible musculature are preserved (see below). A similar process was suggested for a number of soft-bodied fossils (Gámez Vintaned et al. 2009; Wilson and Butterfield 2014; McMahon et al. 2016) and observed on invertebrates experimentally fossilised in fine-grained sediment (Naimark et al. 2016).
The presence of segment boundaries is marked by relatively regular transverse constrictions occurring approximately in the middle between each pair of prominently raised annuli (Figs. 2A, 3A, 4A1). Such body constrictions can be indicative of the presence of transverse dissepiments. As a part of the fossil is missing, the total number of body segments is undetermined, but twelve segments are counted judging by the number of raised rings and their presumably regular arrangement and denoted here as II to XIII (Fig. 2A2). However, a presence of some more segments is not entirely excluded. An inferred anterior end of the specimen is rounded, with a subtriangular median projection, without visible appendages or sensory organs (Figs. 2A, 3A1). This projection does not differ from following segments either by texture or by a nature of its boundary with the first segment of a regular width. By its position and overall shape, the projection matches closely to the prostomium morphology of microdriles such as, for instance, Nais longidentata Cui, He, Peng, and Wang, 2015 and Tubifex tubifex (Müller, 1774) (Fig. 3B1). In Nais longidentata illustrated by Cui et al. (2015) the head shape is especially similar to that of the fossil, it is almost trilobate with a subtriangular prostomium.
The chaetae themselves are not preserved, but the presence of chaetal bundles on the most prominent annuli is inferred judging by the sublateral elevations of these annuli (Fig. 4A1). Similar sublateral elevations support chaetal bundles in extant microdriles (e.g., Shain et al. 2000: fig. 6A, B; Cui et al. 2015: fig. 5B). Besides, each raised presumably chaetigerous ring is restricted to the median area of each segment, and the anteriormost ring abutting the worm front end is likely confined to the segment II, which is typical of oligochaetes and supports the interpretation of the terminal triangular element as the prostomium.
Following the prostomium location, the opposite end of the fossil is interpreted as its incomplete rear part, possibly, preserving the worm genital region. Here, several segments at the edge of the slab are marked by a W-shaped depression (Figs. 2A2, 3A2) alike that of some extant oligochaetes. Indeed, a similar W-shaped depression is observed in the genital region of critical point dried specimens of Tubifex, posterior to male pores of the segment XI, which seems to be resulted after shrinkage of inflated genital segments (Figs. 2B, 3B2). Thus, the inferred genital region marked with a W-shaped depression embracing the 10th–11th chaetigerous segments of the fossil, possibly, corresponds to the segments XI–XII in oligochaetes (Figs. 2B, 3B2).
The natural cross section of the worm body wall consists of multiple ca. 1 µm thick layers (Fig. 4A), similar to the body wall cross section of extant oligochaetes (Fig. 4B). The entire depth of the laminated structure reaches 10 µm. Due to the overall thickness, this structure does not represent a cuticle, the depth of which is 2 µm or less in different oligochaetes, but it is comparable in size to the circular and longitudinal muscle layers underlying the epidermis, in which comparatively thick muscles are lying parallel to the body wall surface (Richards 1977; Jamieson 1992; Gustavsson 2001; De Wit et al. 2011). Thin sections revealed the absence of any microbial films or other organic coatings from the fossil host sediment, thus, the layering pattern observed here was not imparted to the fossil by microbial mat structures.
In summary, the presence of a small triangular prostomium, the absence of prominent chaetae on the anterior segments, and the relatively thick body wall point to the oligochaete rather than polychaete affinities of the fossil. In the Polychaeta, the chaetal bundles can occur already in the peristomium (I) while in the Oligochaeta the peristomium is always devoid of chaetae and usually smaller.
Early Triassic worm systematic inference
Neither parapodia, nor gills, nor any other external appendages suggestive for the polychaete nature of the Petropavlovka fossil are present. Freshwater polychaetes, although being relatively simplified in their morphology in comparison to their marine relatives, can be distinguished from the Clitellata by at least the presence of segmental parapodia and in the majority of cases by prominent sensory appendages on the head (Glasby and Timm 2008).
By its size and overall shape and the prostomium outlines, segmentation and annulation and the body wall structure, the fossil worm resembles “microdriles” (which are an informal but practical grouping of mostly aquatic diminutive oligochaetes). There are four major microdrile families: Naididae sensu stricto, Tubificidae (a part of Naididae sensu lato in a phylogenetical system according to Erséus et al. 2008), Lumbriculidae and Enchytraeidae. Significant external differences of them are observed in the position of chaetae, chaetal morphology and in the location of the genital region (Brinkhurst 1986; Timm 2012). While chaetal morphology is not recognisable in the fossil, an inferred position of its genital region, which embraces male pores in segment XI (or XII) in most extant microdriles, is indicative of either tubificids or enchytraeids.
The Tubificidae have, in a typical case, spermathecal pores in segment X, and male pores in XI; the Enchytraeidae possess spermathecal pores much ahead, between segments IV and V and male ones in XII; the Naididae sensu stricto differ drastically by the location of the corresponding pores in segments V and VI, respectively (Brinkhurst 1971; Caramelo and Martínez-Ansemil 2012; Timm and Martin 2015). However, there are deviations of these basic body plans in some extant species. The microdrile clitellum always covers the segment with male pores and usually one or two neighbouring segments: typically, XI–XII in tubificids and XII–XIII in enchytraeids. If the W-shaped transverse depression on the fossil (Figs. 2A2, 3A2) is situated behind male pores and the pores themselves correspond to the segment XI, their location would be typical of the Tubificidae. On the contrary, if this is the segment XII, the fossil worm would appear more similar to an enchytraeid. The naidid genitals would lie much more forward than those of the fossil. The thick body wall points to burrowing tubificids and enchytraeids but is atypical of naidids (De Wit et al. 2011). Alternatively, this worm can represent an extinct group combining features of different extant microdrile taxa.

Fig. 2. Microdrile oligochaete PIN 5640/212 (A) from Petropavlovka Formation, Olenekian (Lower Triassic), Petropavlovka III section, Russia and extant Tubifex tubifex (Müller, 1774) (B) from Khripan’ River, Moscow region, Russia. A. Photograph under polarised light (A1) and SEM image depicting main features of the specimen (А2): general outlines (continuous line), W-shaped depression (long dashed line), prominent annuli (dashed line), dissepiments (dotted line), and post-mortem fractures (dash-and-dot line). B. SEM image. Dissepiments (asterisks), segments are numbered, depression in posterior part of genital region (arrow).
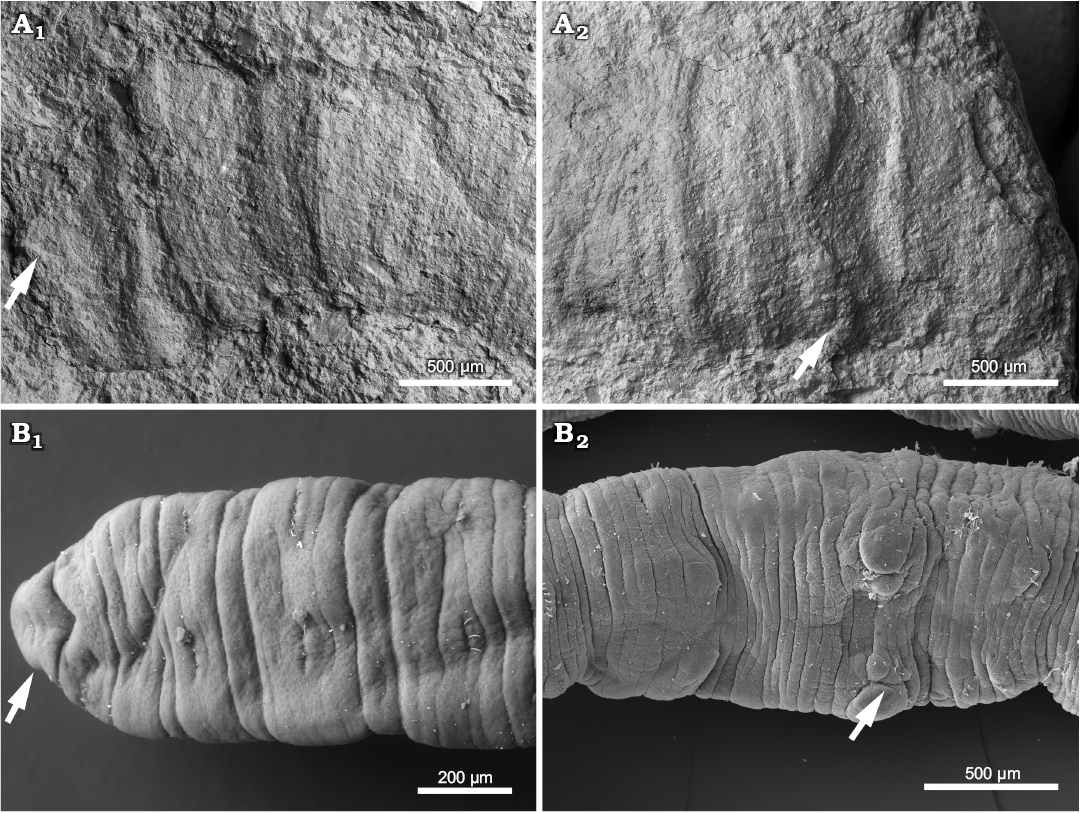
Fig. 3. Microdrile oligochaete PIN 5640/212 (A) from the Petropavlovka Formation, Olenekian (Lower Triassic), Petropavlovka III section, Russia and extant Tubifex tubifex (Müller, 1774) (B) from the Khripan’ River, Moscow region, Russia, SEM. A. Anterior part with possible prostomium (arrowed) (A1). Posterior part of specimen with W-shaped depression (arrowed) and possible genital region (A2). B. Anterior part showing prostomium (arrowed) and arrangement of chaetae (B1). Genital region depicting male pores (arrowed) (B2).

Fig. 4. Microdrile oligochaete PIN 5640/212 (A) from the Petropavlovka Formation, Olenekian (Lower Triassic), Petropavlovka III section, Russia and extant Tubifex tubifex (Müller, 1774) (B) from the Khripan’ River, Moscow region, Russia. A. Oblique view of posterior end emphasizing body wall layering (arrowed), prominent raised annuli and finer annulets (A1). Detail of body wall layers (А2). B. Body wall layers (arrowed; B1, B2).
On the evolution of aquatic oligochaetes
It is possible that aquatic oligochaetes appeared already in the Palaeozoic, when the terrestrial vegetation began to produce organic sediments accumulating in freshwater bodies (Timm et al. 2016). However, there is no undisputed fossil record to support this assumption, and doubtless pre-Mesozoic oligochaetes are currently unknown. The studied Early Triassic annelid provides us with the first information on the anatomy of early aquatic oligochaetes and enables us to make some evolutionary conclusions. The new annelid fossil has similar size to microdriles, which usually measure 1–2 mm or less in diameter (Timm et al. 2016). This could indicate that the size of such oligochaetes was a conservative character in their evolution as Early Triassic and extant representatives of the group do not differ in the body dimensions. The occurrence of an annulated clitellate thick-walled body in the Triassic oligochaete is not surprising as these are likely plesiomorphic characters for the aquatic non-marine oligochaetes.
Currently, the existence of oligochaetes in the Early Triassic is inferred by the presence of some microburrows, the identification of exact producers of which is equivocal. Although oligochaete burrowing activity can be intense, dense, rapid and seize wide areas and large volumes of sediment, these worms produce relatively simple burrow traces and faecal pellets only. Aquatic oligochaetes feed in a conveyor-belt mode, more or less in vertical position, with the head downward and the anus at or below the sediment-water interface; surface defecation may leave small faecal mounds (Tevesz et al. 1980; White and Miller 2008). As a result, narrow irregular endostratal trails in seemingly random patterns are appeared, which make their producers indistinguishable from those of aquatic non-biting midge larvae, as well as of some polychaetes (Schlirf et al. 2001; Voigt and Hoppe 2010). Oligochaete, primarily tubificid, burrows were commonly mentioned earlier, but these were records mostly discussing extant forms (Olsen 1991; Walker and James 1992; Bromley 1996). Among rare reports of more convincing oligochaete burrows in palaeosols, the oldest are of the Early Triassic age and are represented by fine open deeply folded tubules filled with elliptical faecal pellets and pelletoid masses, and the burrow fill commonly differs in the quantitative mineral grain composition from the host sediment (Retallack 1976, 1997; Bown and Kraus 1983; Chin et al. 2013).
The earliest body fossil microdriles were mentioned from the Lower Cretaceous and later strata only, namely from the terrestrial Koonwarra Fossil Bed of Victoria, Australia (Jell and Duncan 1986; Dettmann et al. 1992), the Helvetiafjellet Formation of Spitsbergen (Manum et al. 1991; Poinar 2007), the La Huérguina Formation of the Las Hoyas Lagerstätte, Spain (Timm et al. 2016) and the Yixian Formation of north-eastern China (Hethke et al. 2019), the Upper Cretaceous Perucer Schichten of the Bohemian Massif, Czech Republic (Fritsch 1910: pl. 3: 12), the Palaeocene Fort Union Formation of Wyoming, USA (Hazen 1937) and the Pliocene Willershausen Lagerstätte of Lower Saxony, Germany (Straus 1970) (Table 1).
Almost three dozen of specimens from the Las Hoyas Lagerstätte were attributed tentatively to the aquatic family Tubificidae judging by their minor sizes, general habitus and partly papillated cuticular surface; a sharp prostomium, a digestive tract and a longitudinal blood vessel are observed in a single fossil only (Timm et al. 2016). Some other Early Cretaceous oligochaetes, preserved in freshwater lacustrine deposits of the Gippsland Basin in Victoria (Jell and Duncan 1986: fig. 79A–C; Dettmann et al. 1992: fig. 19h), are characterised by a small, sharp-tipped prostomium on the relatively narrow segment I (peristomium) followed by the broader segment II, by a clear external and internal segmentation in the anterior body half and, probably, by an abrupt beginning of the dark cover (chloragogen tissue) on its digestive tube in VI, thus, by a set of features typical of tubificids. Their relatively short and slender body without any prominent features in the potential genital segments (X–XII), narrowing and faint external segmentation of the caudal half, which consists of very numerous short, still developing segments (a growth zone) are indicative of juveniles.
Table 1. Possible body fossil clitellates and their reinterpretation. Distinct trace fossils and cycloneuralian palaeoscolecidans are not included.
|
Original formal |
Age |
Provenance |
Environment |
References |
Current status |
|
Hirudopsis koepingensis |
Ceratopyge
Limestone; |
Öland, Sweden |
marine |
shelly fossil? |
|
|
“Trenton worm” |
Trenton Limestone; |
Quebec, Canada |
marine |
Annelida? (Conway |
|
|
Hammatopsis scanicus |
Fjäcka Shale; |
Scania, Sweden |
marine |
shelly fossil? |
|
|
Stoma hians |
Fjäcka Shale; |
Scania, Sweden |
marine |
shelly fossil? |
|
|
“Waukesha leech” |
Brandon Bridge strata; |
Wisconsin, USA |
marine |
Cycloneuralia? |
|
|
Bertiella obesa
|
Bertie Group; |
New York State, USA |
marine |
Cycloneuralia? |
|
|
Pronaidites carbonarius |
Radnice Formation; |
Bohemia, |
continental |
Annelida |
|
|
Pronaidites arenivorus |
Radnice Formation; |
Bohemia, |
continental |
Annelida |
|
|
“Mecca Quarry worm” |
Mecca Quarry Shale; |
Indiana, USA |
marine? |
Annelida? |
|
|
Lumbricopsis permicus |
Prosečné Formation; |
Bohemia, |
continental |
Annelida? |
|
|
Lumbricopsis distinctus |
Vrchlabí Formation; |
Bohemia, |
continental |
Annelida? |
|
|
Hirudella angusta |
Solnhofen Plattenkalk; |
Bavaria, |
marine? |
Annelida? |
|
|
Epitrachys rugosus |
Solnhofen Plattenkalk; |
Bavaria, |
marine? |
Hirudinea? |
|
|
Epitrachys granulatus |
Solnhofen Plattenkalk; |
Bavaria, |
marine |
Sabellidae? |
|
|
Palaeohirudo eichstaettensis |
Solnhofen Plattenkalk; |
Bavaria, |
marine? |
Hirudinea? |
|
|
oligochaetes |
Kashpir Oil Shales Formation; Upper Jurassic |
Ul’yanovsk, Russia |
marine |
undetermined microfossils and coprolites |
|
|
Tubificidae morphotypes 1–6 |
La Huérguina Limestone Formation;
|
Las Hoyas, Castilla-La Mancha, Spain |
continental |
Oligochaeta, Tubificidae |
|
|
Stylaria-like naidid |
Yixian Formation; |
Liaoning, |
continental |
Oligochaeta, Naididae |
|
|
Captivonema cretacea |
Helvetiafjellet |
Spitsbergen, Norway |
continental |
Oligochaeta, Capilloventridae? |
|
|
oligochaetes |
Strzelecki Group; |
Victoria, |
continental |
Oligochaeta, Tubificidae |
|
|
oligochaete? |
Perucer Schichten; |
Bohemia, |
continental |
Oligochaeta |
|
|
“fossil earthworm” |
Fort Union Formation; |
Wyoming, USA |
continental |
Oligochaeta, |
|
|
Enchytraeus
sepultus |
Baltic amber; |
Kaliningrad, Russia |
continental |
Oligochaeta, |
|
|
enchytraeid oligochaete |
Baltic amber; |
Kaliningrad, Russia |
continental |
Nematoda |
|
|
enchytraeid oligochaete |
Baltic amber; |
Kaliningrad, Russia |
continental |
Oligochaeta, Enchytraeidae? (Larsson 1978) |
|
|
Oligochaeta, Enchytraeidae |
Baltic amber; |
Kaliningrad, Russia |
continental |
Oligochaeta, Enchytraeidae (Ulrich and Schmelz 2001) |
|
|
Palaeoenchytraeus dominicanus |
Dominican amber; |
Dominican Republic |
continental |
Oligochaeta, Enchytraeidae (Poinar 2007) |
|
|
oligochaete |
Mezhygorje Formation; |
Rivne, Ukraine |
continental |
Oligochaeta |
|
|
oligochaetes and hirudineans |
Ústí Formation; |
Bohemia, Czech Republic |
continental |
possible worms |
|
|
“Lumbriculus” sp. |
Willershausen Lagerstätte; |
Lower Saxony, Germany |
continental |
Oligochaeta, Lumbricidae? (Straus 1970) |
Although chaetae and many other fine details are not visible in two Lower Cretaceous vermiform fossils from the lacustrine Jianshangou Member of the Yixian Formation in Liaoning, China, they are surprisingly similar to the extant naidid oligochaete Stylaria having a thin prostomial tentacle (Hethke et al. 2019: fig. 5E, F). Judging by the presence of several thickened anterior segments (presumably V–VII) with internal genitalia and a thick clitellum, the individuals are sexually mature. Here oligochaetes are thought to form a dominant epifaunal component in several lacustrine benthic palaeocommunities (Hethke et al. 2019).
The worm from the Helvetiafjellet Formation was found associated with a clitellate cocoon (Manum et al. 1991: fig. 12) but, later on, ascribed to a new nematode genus and species Captivonema cretacea Boström in Manum et al., 1994 due to lack of the cuticle segmentation and the irregular distribution of bristles (Manum et al. 1994). The presence of long paired chaetae and faint segmentation allowed Poinar (2007) to suggest affinities of this worm to the oligochaete family Naididae. However, the appearance of Captivonema is not so naidid-like, but rather has more in common with the relatively recently described miniature aquatic Capilloventridae. This family is considered by some researchers to be the most ancient among extant clitellates (Erséus 2005).
A number of body fossil oligochaetes were reported from Eocene–Miocene ambers of the Baltic coast (Menge 1866; Michaelsen 1928; Larsson 1978; Ulrich and Schmelz 2001; Weitschat and Wichard 2002: fig. 7g, h), north-western Ukraine (Perkovsky et al. 2010), and Dominican Republic (Poinar 2007). All these worms were ascribed to the family Enchytraeidae, except for an “enchytraeid” illustrated by Bachofen-Echt (1949: fig. 33), which was covered with a thick milky layer and co-occurred with a chironomid midge. It was recognised as a mermithid nematode after further re-examination (Poinar 2007). Enchytraeid species are mostly soil-dwellers inhabiting also leaf litter and can be carried into the tree resin by predaceous flies (Dolichopodidae), which are associated with them in amber drops (Ulrich and Schmelz 2001).
Widespread are fossil clitellate cocoon shells, which, being resilient toward thermal, chemical, and proteolytic decay, commonly occur in lacustrine and purely terrestrial palynological samples from the Late Triassic onwards (Fritsch 1910: pl. 3: 5; Manum et al. 1991; Jansson et al. 2008; Tosolini and Pole 2010; Bomfleur et al. 2012; Steinthorsdottir et al. 2015; McLoughlin et al. 2016). The morphology and structure of these sac-like organic, acid resistant mesoobjects allow attributing some fossil cocoons to leeches. Mostly they are of a limited taxonomic value despite of some remarkable anatomical content such as fossilized spermatozoa with a conspicuous helical “drill-bit” structure, which is comparable to the branchiobdellid acrosome (Bomfleur et al. 2015).
In addition, several putative leech impressions, representing two species and characterised by dense transverse ribbing and possible suction discs, were reported from the Upper Jurassic Solnhofen lithographic slates of Bavaria (Münster 1842; Ehlers 1869: pl. 36; Kozur 1970). Still, sabellid and sipunculan origins for some of these peanut-like fossils are not excluded (Schweigert et al. 1998; Muir and Botting 2007). Lagoonal marine conditions were inferred for the Solnhofen Plattenkalk accumulation, but a number of animals were probably either delivered by freshwater streams or swept by hurricanes into the lagoon from the nearby archipelago (Barthel et al. 1990; Röper 2005).
A single Cenozoic fossil was compared with earthworms. It is a sand filled cast of a cavity formerly occupied by an animal from the alluvial Fort Union Formation (Hazen 1937; Reynolds et al. 2009).
Earlier, Upper Jurassic (Tithonian), suggested oligochaetes from a combustible shale of the Middle Volga River (Zalessky 1928), unfortunately, were not properly described. The organic rich shale (at present the Kashpir Oil Shales Formation, Ul’yanovsk region) itself was accumulated under marine eutrophic conditions and yielded numerous ammonoids and calcareous nanoplankton (Riboulleau et al. 2003; Gavrilov et al. 2008). The original somewhat imaginative drawings from thin sections represent two groups of elongated fossils. Of these, larger (up to 3 mm long) randomly twisted and irregularly constricted forms resemble coprolites rather than worms (Zalessky 1928: pl. 5: 3–5), while the second group (less than 0.1 mm in length) with transverse septation merely represents sections of microfossils that are abundant in this facies (Zalessky 1928: pl. 1: 4, pl. 2: 3, pl. 6: 3). Similarly, Oligocene featureless curved vermiform siliceous moulds from diatomites of Bohemian Bechlejovice, which have been referred to oligochaetes and hirudineans (Zigler 1992), elude proper interpretation. Some of them are terminated by blunt ends as well as twisted and overlapped, thus, lacking signs of a self-avoiding behaviour typical of trace fossil producers. The diatomites themselves represent lacustrine deposits of the mostly volcanic Ústí Formation yielding frogs, fish and rich terrestrial flora (Kvaček and Walther 2004).
In general, the Mesozoic–Cenozoic clitellate fossil record demonstrates a relatively rapid diversification of this group including tubificids sensu stricto, naidids and enchytraeids as well as hirudineans and even branchiobdellidans in terrestrial environments including freshwater lacustrine, edaphic, and probably arboreal conditions (such an inferred worm body occurring within a leaf blade was illustrated by Fritsch 1910: pl. 3: 12).
Conclusions
The monophyly of the Clitellata is strongly supported by their common morphology (Brinkhurst 1994; Jenner 2006; Martin et al. 2008; Nielsen 2012; Purschke et al. 2014), spermatozoon ultrastructure (Ferraguti 2000), molecular data (Rousset et al. 2008; Struck et al. 2011, 2015; Andrade et al. 2015; Weigert et al. 2016) and developmental peculiarities, probably related to the loss of the planktonic larval stage (Kuo and Hsiao 2018), but their origins from a particular polychaete group are still unresolved (Westheide et al. 1999; Christoffersen 2012). The last common ancestor of the living Clitellata was likely a freshwater species while soil-inhabiting and marine clitellates are generally considered to be secondarily evolved from freshwater predecessors (Timm 1981; Erséus 2005; Rousset et al. 2008).
The loss of the larval stage and development of cocoon-excreting glands in the clitellum were certainly connected with adaptations to life in ephemeral non-marine basins. The appearance of clitellum in some marine polychaetes seems to be a convergence (Timm 2012). In turn, the reduction of appendages could be either inherited from tube-dwelling ancestors or evolved due to a burrowing life style in pond sediments. Of interesting features of the new Early Triassic worm is its circular and longitudinal musculature, which is relatively thick and well developed by comparison with other oligochaetes, and consists of multiple layers forming a continuous entity. Such a body wall structure is directly related to the locomotory performance of burrowing oligochaetes in peristalsis through a dense medium (Jamieson 1992; De Wit et al. 2011). It is not excluded that sediment burrowing was originally another way to escape problems of desiccation on the bottom of seasonally drying ponds. Although the Early Triassic clitellate fossil is larger than typical sediment-dwelling annelids, its find further supports the suggestion that miniaturisation is important evolutionary process in the Annelida (Struck et al. 2015).
The unique oligochaete fossil from the Petropavlovka Formation described here indicates that taxa which are externally similar to the extant “microdriles” existed at least by the Early Triassic but hardly earlier than in the Carboniferous. Despite taphonomic oddities (oligochaete body fossils are mostly restricted to lacustrine Lagerstätten and ambers), the fossil record confirms that the Clitellata, even including doubtful Carboniferous forms, are a derived annelid group. Thus, earlier views suggesting their basal position among annelids (Rouse and Fauchald 1995, 1998) can be ruled out by palaeontological data in addition to molecular evidence. The terrestrial freshwater origin of clitellates as a whole and the Oligochaeta sensu stricto is more plausible and supported here.
Acknowledgements
We thank Olga P. Yaroshenko (GIN RAS, Geological Institute, Russian Academy of Sciences, Moscow, Russia) for valuable consultation on palynology, Alexey V. Gomankov (Botanical Institute, Russian Academy of Sciences, St. Petersburg, Russia) and Eugeny V. Karasev (PIN) for comments on plant megafossils, Valentin P. Tverdokhlebov (Saratov State University, Saratov, Russia) and Andrey G. Sennikov (PIN) for providing the data on fossil localities, Anna E. Zhadan for the help with preparing Tubifex specimens, Eugeny N. Samarin for the assistance with an elemental analysis of the rock sample (both Lomonosov Moscow State University, Russia), Roman A. Rakitov (PIN) for the superb SEM images, Vojtěch Turek and Lenka Váchová (both National Museum, Prague, Czech Republic), Rolf Schmidt and Thomas Rich (both Museum Victoria, Melbourne, Australia) and Patricia Vickers-Rich (Monash University, Melbourne, Australia) for their friendly help with new images of the fossil worm types, Alexei P. Ippolitov (GIN RAS) and Rüdiger M. Schmelz (Universidade da Coruña, La Coruña, Spain) for extremely scrupulous, comprehensive and practicable journal reviews. The work was supported by the Russian Foundation for Basic Research, RFBR projects 16-04-01498 and 19-04-00501. Financial support to O.V. was provided by Estonian Research Council Project IUT20-34.
References
Algeo, T.J. and Heckel, P.H. 2008. The late Pennsylvanian Midcontinent Sea of North America: A review. Palaeogeography, Palaeoclimatology, Palaeoecology 268: 205–221. Crossref
Andrade, S.C.S., Novo, M., Kawauchi, G.Y., Worsaae, K., Pleijel, F., Giribet, G., and Rouse, G. W. 2015. Articulating “archiannelids”: Phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Molecular Biology and Evolution 32: 2860–2875. Crossref
Bachofen-Echt, A. 1949. Der Bernstein und Seine Einschlüsse. 204 pp. Springer-Verlag, Wien. Crossref
Balme, B.E. 1970. Palynology of Permian and Triassic strata in the Salt Range and Surghar Range, West Pakistan. In: B. Kummel and C. Teichert (eds.), Stratigraphic Boundary Problems: Permian and Triassic of West Pakistan. University of Kansas, Department of Geology Special Publication 4: 306–453.
Barthel, K.W., Swinburne, N.H.M., and Conway Morris, S. 1990. Solnhofen. A study in Mesozoic Palaeontology. 236 pp. Cambridge University Press, Cambridge.
Bather, F.A. 1920. Protoscolex latus, a new “Worm” from Lower Ludlow Beds. The Annals and Magazine of Natural History, 9th Series 5: 124–132. Crossref
Benton, M.J. and Newell, A.J. 2014. Impacts of global warming on Permo-Triassic terrestrial ecosystems. Gondwana Research 25: 1308–1337. Crossref
Benton, M.J., Tverdokhlebov, V.P., and Surkov, M.V. 2004. Ecosystem remodelling among vertebrates at the Permian–Triassic boundary in Russia. Nature 432: 97–100. Crossref
Blakemore, R.J. 2009. Cosmopolitan earthworms—a global and historical perspective. In: D.H. Shain (ed.), Annelids in Modern Biology, 257–283. John Wiley and Sons, Hoboken. Crossref
Bomfleur, B., Kerp, H., Taylor, T.N., Moestrup, Ø., and Taylor, E.L. 2012. Triassic leech cocoon from Antarctica contains fossil bell animal. Proceedings of National Academy of Sciences 109: 20971–20974. Crossref
Bomfleur, B., Mörs, T., Ferraguti, M., Reguero, M.A., and McLoughlin, S. 2015. Fossilized spermatozoa preserved in 50-Myr-old annelid cocoon from Antarctica. Biology Letters 11: 20150431. Crossref
Bown, T.M. and Kraus, M.J. 1983. Ichnofossils of the alluvial Willwood Formation (Lower Eocene), Bighorn Basin, northwest Wyoming, U.S.A. Palaeogeography, Palaeoclimatology, Palaeoecology 43: 95–128. Crossref
Brinkhurst, R.O. 1971. A guide for the identification of British aquatic Oligochaeta. Freshwater Biological Association Scientific Publication 22: 1–55.
Brinkhurst, R.O. 1986. Guide to the freshwater aquatic microdrile oligochaetes of North America. Canadian Special Publication of Fisheries and Aquatic Sciences 84: 1–259.
Brinkhurst, R.O. 1994. Evolutionary relationships within the Clitellata: an update. Megadrilogica 5: 109–112.
Bromley, R.G. 1996. Trace Fossils—Biology, Taphonomy and Applications. 361 pp. Chapman and Hall, London. Crossref
Caramelo, C. and Martínez-Ansemil, E. 2012. Microscopic anatomy of aquatic oligochaetes (Annelida, Clitellata): a zoological perspective. In: A. Méndez-Vilas (ed.), Current Microscopy Contributions to Advances in Science and Technology, 21–27. Formatex Research Center, Badajoz.
Chin, K., Pearson, D., and Ekdale, A. A. 2013. Fossil worm burrows reveal very early terrestrial animal activity and shed light on trophic resources after the end-Cretaceous mass extinction. PLoS ONE 8 (8): e70920. Crossref
Christoffersen, M.L. 2012. Phylogeny of basal descendants of cocoon-forming annelids (Clitellata). Turkish Journal of Zoology 36: 95–119.
Conway Morris, S. 1977. Fossil priapulid worms. Special Papers in Palaeontology 20: 1–155.
Conway Morris, S., Pickerill, R.K., and Harland, T.L. 1982. A possible annelid from the Trenton Limestone (Ordovician) of Quebec, with a review of fossil oligochaetes and other annulate worms. Canadian Journal of Earth Sciences 19: 2150–2157. Crossref
Coveney, R.M., Jr. and Glascock, M.D. 1989. A review of the origins of metal-rich Pennsylvanian black shales, central USA, with an inferred role for basinal brines. Applied Geochemistry 4: 347–367. Crossref
Cui, Y., He, X., Peng, Y., and Wang, H. 2015. Records of Naididae and Lumbriculidae (Clitellata) from Tibet, China, with description of a new species of Nais. Zootaxa 3956: 513–530. Crossref
Cunha, L., Brown, G.G., Stanton, D.W.G., Da Silva, E., Hansel, F.A., Jorge, G., McKey, D., Vidal-Torrado, P., Macedo, R.S., Velasquez, E., James, S.W., Lavelle, P., Kille, P., and Terra Preta de Indio Network. 2016. Soil animals and pedogenesis: The role of earthworms in anthropogenic soils. Soil Science 181: 110–125. Crossref
Darwin, C. 1881. The Formation of Vegetable Mould, Through the Action of Worms, With Observations on Their Habits. 326 pp. John Murray, London. Crossref
Degtyarev, V.G. [Degtârev, V.G.], Sleptsov, S.M. [Slepcov, S.M.], and Pshennikov, A.E. [Pšennikov, A.E.] 2013. Piscivory in eastern population of Siberian crane (Grus leucogeranus) [in Russian with English abstract]. Zoologičeskij žurnal 92: 588–595. Crossref
Dettmann, M.E., Molnar, R.E., Douglas, J.G., Burger, D., Fielding, C., Clifford, H.T., Francis, J., Jell, P., Rich, T., Wade, M., Rich, P.V., Pledge, N., Kemp, A., and Rozefelds, A. 1992. Australian Cretaceous terrestrial faunas and floras: biostratigraphic and biogeographic implications. Cretaceous Research 13: 207–262. Crossref
De Wit, P., Erséus, C., and Gustavsson, L.M. 2011. Ultrastructure of the body wall of three species of Grania (Annelida: Clitellata: Enchytraeidae). Acta Zoologica 92: 1–11. Crossref
Dobruskina, I.A. 1994. Triassic Floras of Eurasia. Schriftenreihe der Erdwissenschaftlichen Kommissionen/Österreichische Akademie der Wissenschaften 10. 422 pp. Springer, Wien.
Ehlers, E. 1869. Ueber fossile Würmer aus dem lithographischen Schiefer in Bayern. Palaeontographica 17: 145–175.
Erséus, C. 2005. Phylogeny of oligochaetous Clitellata. Hydrobiologia 535/536: 357–372. Crossref
Erséus, C., Wetzel, M.J., and Gustavsson, L. 2008. ICZN rules—a farewell to Tubificidae (Annelida, Clitellata). Zootaxa 1744: 66–68.
Fauchald, K. 1977. The polychaete worms. Definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles County, Science Series 28: 1–188.
Feller, C., Brown, G.G., Blanchart, E., Deleporte, P., and Chernyanskii, S.S. 2003. Charles Darwin, earthworms and the natural sciences: various lessons from past to future. Agriculture, Ecosystem and Environment 99: 29–49. Crossref
Feng, Z., Wei, H., Guo, Y., and Bomfleur, B. 2018. A conifer-dominated Early Triassic flora from Southwest China. Science Bulletin 63: 1462–1463. Crossref
Ferraguti, M. 2000. Euclitellata. In: B.J.M. Jamieson (ed.), Reproductive Biology of Invertebrates. Volume IX, Part B, Progress in Male Gamete Ultrastructure and Phylogeny, 125–182. Oxford and IBH Publishing, New Delhi.
Fisher, J.B., Lick, W.J., McCall, P.L., and Robbins, J.A. 1980. Vertical mixing of lake sediments by tubificid oligochaetes. Journal of Geophysical Research 85: 3997–4006. Crossref
Fritsch, A. 1907. Miscellanea palaeontologica. I. Palaeozoica. 23 pp. Fr. Řivnáč, Prag.
Fritsch, A. 1910. Miscellanea palaeontologica. II. Mesozoica. 25 pp. Fr. Řivnáč, Prag.
Gámez Vintaned, J.A., Liñán, E., Zhuravlev, A.Y., Bauluz, B., Gozalo, R., Zamora, S., and Esteve, J. 2009. The preservation of Cambrian Murero biota in the Mesones Group, Cadenas Ibéricas, Spain. In: M.R. Smith, L.J.O’Brien, and J.-B. Caron (eds.), International Conference on the Cambrian Explosion, Abstract Volume, 32–33. Burgess Shale Consortium, Toronto.
Gavrilov, Y.O., Shchepetova, E.V., Rogov, M.A., and Shcherbinina, E.A. 2008. Sedimentology, geochemistry, and biota of Volgian carbonaceous sequences in the northern part of the Central Russian Sea (Kostroma region). Lithology and Mineral Resources 43: 354–379. Crossref
Ghilyarov, M.S. 1983. Darwin’s formation of vegetable mould—its philosophical basis. In: J.E. Satchell (ed.), Earthworm Ecology: From Darwin to Vermiculture, 1–5. Chapman and Hall, London. Crossref
Glasby, C.J. 1999. The Namanereidinae (Polychaeta: Nereididae). Part 1, taxonomy and phylogeny. Records of the Australian Museum, Supplement 25: 1–129. Crossref
Glasby, C.J. and Timm, T. 2008. Global diversity of polychaetes (Polychaeta; Annelida) in freshwater. Hydrobiologia 595: 107–115. Crossref
Gomankov, A.V. 2005. Floral changes across the Permian–Triassic boundary. Stratigraphy and Geological Correlation 13 (2): 74–83.
Gustavsson, L.M. 2001. Comparative study of the cuticle in some aquatic oligochaetes (Annelida: Clitellata). Journal of Morphology 248: 185–195. Crossref
Hadding, A. 1913. Undre Dicellograptusskiffern i Skåne jämte några därmed ekvivalenta bildingar. Lunds Universitets Årsskrift, Ny följd, Afdeling 2 9: 1–90.
Haig, D.W., Martin, S.K., Mory, A.J., McLoughlin, S., Backhouse, J., Berrell, R.W., Kear, B.P., Hall, R., Foster, C.B., Shi, G.R., and Bevan, J.C. 2015. Early Triassic (early Olenekian) life in the interior of East Gondwana: mixed marine-terrestrial biota from the Kockatea Shale, Western Australia. Palaeogeography, Palaeoclimatology, Palaeoecology 417: 511–533. Crossref
Harvey, T.H.P., Dong, X., and Donoghue, P.C.J. 2010. Are palaeoscolecids ancestral ecdysozoans? Evolution & Development 12: 177–200. Crossref
Hazen, B.M. 1937. A fossil earthworm(?) from the Paleocene of Wyoming. Journal of Paleontology 11: 250.
Hethke, M., Fürsich, F.T., Jiang, B., Wang B., Chellouche, P., and Weeks, S.C. 2019. Ecological stasis in Spinicaudata (Crustacea, Branchiopoda)? clam shrimp of the Yixian Formation of north-east China occupied a broader realized ecological niche than extant members of the group. Palaeontology 62: 483–513. Crossref
Howell, B.F. 1959. Three notes on Silurian worm genera. Journal of Paleontology 33: 487.
Howell, B.F. 1962. Worms. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part W, Miscellanea, Conodonts, Conoidal Shells of Uncertain Affinities, Worms, Trace Fossils and Problematica, W144–W177. Geological Society of America, New York and University of Kansas Press, Lawrence.
Jamieson, B.G.M. 1992. Oligochaeta. In: F.W. Harrison and S.L. Gardiner (eds.), Microscopic Anatomy of Invertebrates. Volume 7, Annelida, 217–322. Wiley-Liss, New York.
Jansson, I.-M., McLoughlin, S., and Vajda, V. 2008. Early Jurassic annelid cocoons from eastern Australia. Alcheringa 32: 285–296. Crossref
Jell, P.A. and Duncan, P.M. 1986. Invertebrates, mainly insects, from the freshwater Lower Cretaceous Koonwarra Fossil Beds (Korumburra Group), South Gippsland, Victoria. Memoirs of the Association of Australasian Palaeontologists 3: 111–205.
Jenner, R.A. 2006. Challenging received wisdoms: Some contributions of the new microscopy to the new animal phylogeny. Integrative and Comparative Biology 46: 93–103. Crossref
Kozur, H. 1970. Fossile Hirudinea aus dem Oberjura von Bayern. Lethaia 3: 225–232. Crossref
Kozur, H. and Weems, R.E. 2010. The biostratigraphic importance of conchostracans in the continental Triassic of the northern hemisphere. In: S.G. Lucas (ed.), The Triassic Timescale. Geological Society, London, Special Publications 334: 315–417. Crossref
Kuo, D.-H. and Hsiao, Y.-H. 2018. Duplicated FoxA genes in the leech Helobdella: Insights into the evolution of direct development in clitellate annelids. Developmental Dynamics 247: 763–778. Crossref
Kušta, J. 1888. Příspĕvek k seznání zvířeny kamenouhelné u Rakovníka. Sitzungsberichte der königlich-böhemischen Gesellschaft der Wissenschaften, Mathematisch-naturwissenschaftliche Classe 1887: 561–564.
Kustatscher, E., Franz, M., Heunisch, C., Reich, M., and Wappler, T. 2014. Floodplain habitats of braided river systems: depositional environment, flora and fauna of the Solling Formation (Buntsandstein, Lower Triassic) from Bremke and Fürstenberg (Germany). Palaeobiodiversity and Palaeoenvironments 94: 237–270. Crossref
Kvaček, Z. and Walther, H. 2004. Oligocene flora of Bechlejovice at Dĕčín from neovolcanic area of the České středohoří Mountains, Czech Republic. Acta Musei Nationalis Pragae, Series B, Natural History 60: 9–60.
Lamsdell, J.C. 2016. Horseshoe crab phylogeny and independent colonizations of fresh water: ecological invasion as a driver for morphological innovation. Palaeontology 59: 181–194. Crossref
Larsson, S.G. 1978. Baltic Amber—A Palaeobiological Study. Entomonograph Volume 1. 192 pp. Scandinavian Science Press, Klampenborg.
Manum, S.B., Bose, M.N., and Sawyer, R. T. 1991. Clitellate cocoons in freshwater deposits since the Triassic. Zoologica Scripta 20: 347–366. Crossref
Manum, S.B., Bose, M.N., Sawyer, R.T., and Boström, S. 1994. A nematode (Captivonema cretacea gen. et sp. n.) preserved in a clitellate cocoon wall from the Early Cretaceous. Zoologica Scrtipta 23: 27–31. Crossref
Martin, P., Martínez-Ansemil, E., Pinder, A., Timm, T., and Wetzel, M.J. 2008. Global diversity of oligochaetous clitellates (“Oligochaeta”; Clitellata) in freshwater. Hydrobiologia 595: 117–127. Crossref
McLoughlin, S., Bomfleur, B., Mörs, T., and Reguero, M. 2016. Fossil clitellate annelid cocoons and their microbiological inclusions from the Eocene of Seymour Island, Antarctica. Palaeontologia Electronica 19.1.11A: 1–27. Crossref
McMahon, S., Anderson, R.P., Saupe, E.E., and Briggs, D.E.G. 2016. Experimental evidence that clay inhibits bacterial decomposers: implications for preservation of organic fossils. Geology 44: 867–870. Crossref
Menge, A. 1866. Ueber ein Rhipidopteron und einige andere im Bernstein eingeschlossenne Thiere. Schriften der Naturforschenden Gesselschaft in Danzig, neue Folge 1: 1–8.
Michaelsen, W. 1928. Clitellata = Gürtelwürmer. Dritte Klasse der Vermes Polymera (Annelida). In: W.G. Kükehthal and T. Krumbach (eds.), Handbuch der Zoologie: eine Naturgeschichte der Stämme des Tierreiches 2 (8), 1–112. Walter de Gruyter, Berlin.
Mikulic, D.G., Briggs, D.E.G., and Kluessendorf, J. 1985a. A new exceptionally preserved biota from the Lower Silurian of Wisconsin, U.S.A. Philosophical Transactions of the Royal Society of London B 311: 75–85. Crossref
Mikulic, D.G., Briggs, D.E.G., and Kluessendorf, J. 1985b. A Silurian soft-bodied biota. Science, New Series 228: 715–717. Crossref
Minikh, M.G. and Minikh, A.V. 1997. Ichthyofaunal correlation of the Triassic deposits from the northern Cis-Caspian and southern Cis-Urals regions. Geodiversitas 19: 279–292.
Moberg, J.C. and Segerberg, C.O. 1906. Bidrag till kännedomen om Ceratopygeregionen med särskild hänsyn till dess utveckling i Fogelsångstrakten. Lunds Universitets Årsskrift, Ny följd, Afdeling 2, Band 2 7: 1–116.
Muir, L.A. and Botting, J.P. 2007. A Lower Carboniferous sipunculan from the Granton Shrimp Bed, Edinburgh. Scottish Journal of Geology 43: 51–56. Crossref
Müller, O.F. 1774. Vermium terrestrium et fluviatilium, seu animalium infusorium, helminthicorum et testaceorum, non marinorum, succinta historia, 1 (2). 214 pp. Heinek et Faber, Havnia. Crossref
Münster, G. 1842. Ueber einige neue fossile schalenlose Cephalopoden und eine neue Gattung Ringelwürmer (Anneliden). Beiträge zur Petrefacten-Kunde 5: 95–99.
Naimark, E.B., Kalinina, M.A., Shokurov, A.V., Markov, A.V., and Boeva, N.M. 2016. Decaying of Artemia salina in clay colloids: 14-month experimental formation of subfossils. Journal of Paleontology 90: 472–484. Crossref
Needham, S.J., Worde, R.H., and McIlroy, D. 2004. Animal-sediment interactions: the effect of ingestion and excretion by worms on mineralogy. Biogeosciences 1: 113–121. Crossref
Nielsen, C. 2012. Animal Evolution: Interrelationships of the Living Phyla. Third edition. 402 pp. Oxford University Press, Oxford. Crossref
Novikov, I.V. 2018. Early Triassic amphibians of Eastern Europe: Evolution of dominant groups and peculiarities of changing communities [in Russian]. Trudy Paleontologičeskogo Instituta Rossijskoj Akademii Nauk 296: 1–358.
Ochev, V.G. and Shishkin, M.A. 1989. On the principles of global correlation of the continental Triassic on the tetrapods. Acta Palaeontologica Polonica 34: 149–173.
Ochev, V.G. and Surkov, M.V. 2000. The history of excavation of Permo-Triassic vertebrates from Eastern Europe. In: M.J. Benton, M.A. Shishkin, D.M. Unwin, and E.N. Kurochkin (eds.), The Age of Dinosaurs in Russia and Mongolia, 1–16. Cambridge University Press, Cambridge.
Olsen, P.E. 1991. Tectonic, climatic, and biotic modulation of lacustrine ecosystems—examples from Newark Supergroup of eastern North America. In: B. Katz (ed.), Lacustrine Basin Exploration: Case Studies and Modern Analogs. American Association Petroleum Geologists Memoir 50: 209–224. Crossref
Perkovsky, E.E., Zosimovich, V.Y., and Vlashkin, A.P. 2010. Rovno amber. In: D. Penney (ed.), Biodiversity of Fossils in Amber From the Major World Deposits, 80–100. Siri Scientific Press, Manchester.
Poinar, G.O., Jr. 2007. Enchytraeidae (Annelida: Oligochaeta) in amber. Megadrilogica 11: 53–57.
Purschke, G., Bleidorn, C., and Struck, T. 2014. Systematics, evolution and phylogeny of Annelida—a morphological perspective. Memoirs of Museum Victoria 71: 247–269. Crossref
Retallack, G.J. 1976. Triassic palaeosols in the upper Narrabeen Group of New South Wales. Part I: Features of palaeosols. Journal of the Geological Society of Australia 23: 383–399. Crossref
Retallack, G.J. 1997. Palaeosols in the upper Narrabeen Group of New South Wales as evidence of Early Triassic palaeoenvironments without exact modern analogues. Australian Journal of Earth Sciences 44: 185–201. Crossref
Reynolds, J.W., Reeves, W.K., and Spence, R.M. 2009. The earthworms (Oligochaeta: Lumbricidae) of Wyoming, USA, revisited. Megadrilogica 13: 25–35.
Riboulleau, A., Baudin, F., Deconinck, J.-F., Derenne, S., Largeau, C., and Tribovillard, N. 2003. Depositional conditions and organic matter preservation pathways in an epicontinental environment: the Upper Jurassic Kashpir Oil Shales (Volga Basin, Russia). Palaeogeography, Palaeoclimatology, Palaeoecology 197: 171–197. Crossref
Richards, K.S. 1977. Structure and function of oligochaete epidermis (Annelida). Symposium of the Zoological Society of London 39: 171–193.
Röper, M. 2005. Field trip B: East Bavarian Plattenkalk—different types of upper Kimmeridgian to lower Tithonian Plattenkalk deposits and facies. Zitteliana B 26: 57–70.
Rouse, G.W. and Fauchald, K. 1995. The articulation of annelids. Zoologica Scripta 24: 269–301. Crossref
Rouse, G.W. and Fauchald, K. 1998. Recent views on the status, delineation and classification of Annelida. American Zoologist 38: 953–964. Crossref
Rousset, V., Plaisance, L., Erséus, C., Siddall, M.E., and Rouse, G.W. 2008. Evolution of habitat preference in Clitellata (Annelida). Biological Journal of the Linnean Society 95: 447–464. Crossref
Ruedemann, R. 1925. Some Silurian (Ontarian) faunas of New York. Bulletin of the New York State Museum 265: 5–134.
Schlirf, M., Uchman, A., and Kümmel, M. 2001. Upper Triassic (Keuper) non-marine trace fossils from the Haßberge area (Franconia, south-eastern Germany). Paläontologische Zeitschrift 75: 71–96. Crossref
Schulz, E. 1964. Sporen und Pollen aus dem Mittleren Buntsandstein des germanischen Beckens. Monatsberichte der deutschen Akademie der Wissenschaften zu Berlin 6: 597–606.
Schweigert, G., Dietl, G., and Röper, M. 1998. Muensteria vermicularis Sternberg (Vermes, Sabellidae) aus oberjurassischen Plattenkalken Süddeautchlands. Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Histor, Geologie 38: 25–37.
Sennikov, A.G. 2018. Lungfish (Dipnoi) burrows from the Triassic of the southern Cis-Urals. Paleontological Journal 52 (12): 1408–1411. Crossref
Sennikov, A.G. and Novikov, I.V. 2018. On possible trophic adaptations of some Rhytidosteidae (Amphibia, Temnospondyli). Paleontological Journal 52 (12): 1412–1418. Crossref
Shain, D.H., Carter, M.R., Murray, K.P., Maleski, K.A., Smith, N.R., McBride, T.R., Michalewicz, L.A., and Saidel, W.M. 2000. Morphologic characterization of the ice worm Mesenchytraeus solifugus. Journal of Morphology 246: 192–197. Crossref
Shcherbakov, D.E. 2008a. Insect recovery after the Permian/Triassic crisis. Alavesia 2: 125–131.
Shcherbakov, D.E. 2008b. On Permian and Triassic insect faunas in relation to biogeography and the Permian/Triassic crisis. Paleontological Journal 42 (1): 15–31.
Shcherbakov, D.E. 2015. Permian and Triassic ancestors of webspinners. Russian Entomological Journal 24 (3): 187–200. Crossref
Shcherbakov, D.E. [Ŝerbakov, D.E.], Bashkuev, A.S. [Baškuev, A.S.], Vasilenko, D.V., Karasev, E.V., Lukashevich, E.D. [Lukaševič, E.D.], Tarasenkova, M.M., Strelnikova, O.D. [Strel’nikova, O.D.], and Felker, A.S. [Fel’ker, A.S.] 2019. A new locality of Early Triassic insects—Petropavlovka [in Russian]. In: A.S. Alekseev (ed.), Paleostrat-2019, Moskva, 28–30 ânvarâ 2019, Programma i tezisy, 68–69. Paleontologičeskij Institut RAN, Moskva.
Shishkin, M.A. and Novikov, I.V. 2017. Early stages of recovery of the East European tetrapod fauna after the end-Permian crisis. Paleontological Journal 51: 612–622. Crossref
Shishkin, M.A., Ochev, V.G., Lozovskii, V.R., and Novikov, I.V. 2000. Tetrapod biostratigraphy of the Triassic of Eastern Europe. In: M.J. Benton, M.A. Shishkin, D.M. Unwin, and E.N. Kurochkin (eds.), The Age of Dinosaurs in Russia and Mongolia, 120–139. Cambridge University Press, Cambridge.
Shishkin, M.A. [Šiškin, M.A.], Ochev, V.G. [Očev, V.G.], Tverdokhlebov, V.P. [Tverdohlebov, V.P.], Vergay, I.F. [Vergaj, I.F.], Gomankov, A.V. [Goman’kov, A.V.], Kalandadze, N.N., Leonova, E.M., Lopato, A.Y., Makarova, I.S., Minikh, M.G. [Minih, M.G.], Molostovskiy, E.M. [Molostovskij, E.M.], Novikov, I.V., and Sennikov, A.G. 1995. Biostratigrafiâ kontinental’nogo triasa užnogo Predural’â. 205 pp. Nauka, Moskva.
Štamberg, S. and Zajíc, J. 2008. Carboniferous and Permian Faunas and Their Occurrence in the Limnic Basins of the Czech Republic. 224 pp. Museum of Eastern Bohemia, Hradec Králové.
Steinthorsdottir, M., Tosolini, A.-M.P., and McElwain, J.C. 2015. Evidence for insect and annelid activity across the Triassic–Jurassic transition of East Greenland. Palaios 30: 597–607. Crossref
Straus, A. 1970. “Lumbriculus” sp. nov. (?), ein Wurm (Annelida) aus dem Pliozän von Willershausen. Berichte der Naturhistorischen Gesellschaft zu Hannover 114: 75–76.
Struck, T., Golombek, A., Weigert, A., Franke, F.A., Westheide, W., Purschke, G., Bleidorn, C., and Halanych, K.M. 2015. The evolution of annelids reveals two adaptive routes to the interstitial realm. Current Biology 25: 1993–1999. Crossref
Struck, T., Pasul, C., Hill, N., Hartmann, S., Hösel, C., Kube, M., Lieb, B., Meyer, A., Tiedemann, R., Purschke, G., and Bleidorn, C. 2011. Phylogenomic analyses unravel annelid evolution. Nature 471: 95–98. Crossref
Tevesz, M.J.S., Soster, F.M., and McCall, P.L. 1980. The effects of size-selective feeding by oligochaetes on the physical properties of river sediments. Journal of Sedimentary Petrology 50: 0561–0568. Crossref
Timm, T. 1981. On the origin and evolution of aquatic Oligochaeta. Eesti NSV Teaduste Akadeemia Toimetised, Bioloogia 30: 174–181.
Timm, T. 2012. Life forms in Oligochaeta: a literature review. Zoology in the Middle East 58 (Supplementum 4): 71–82. Crossref
Timm, T. and Martin, P.G. 2015. Chapter 21. Clitellata: Oligochaeta. In: J. Thorp and D.C. Rodgers (eds.), Ecology and General Biology, 4th Edition: Thorp and Covich’s Freshwater Invertebrates, 529–549. Academic Press, San Diego. Crossref
Timm, T., Vinn, O., and Buscalioni, Á.D. 2016. Soft-bodied annelids (Oligochaeta) from the Lower Cretaceous (La Huérguina Formation) of the Las Hoyas Konservat-Lagerstätte, Spain. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 280: 315–324. Crossref
Tosolini, A.-M.P. and Pole, M. 2010. Insect and clitellate annelid traces in mesofossil assemblages from the Cretaceous of Australia. Alcheringa 34: 397–419. Crossref
Tverdokhlebov, V.P. [Tverdohlebov, V.P.] 1967. 19.20. Petropavlovka, Berezovyy [in Russian]. In: N.S. Morozov (ed.), Putevoditel’ èkskursii po verhnepermskim i triasovym kontinental’nym obrazovaniâm ûgo-vostoka Russkoj platformy i Priural’â, 109–148. Saratovskij Gosudarstvennyj Universitet imeni N.G. Černyševskogo, Orenburgskoe Geologičeskoe Upravlenie, Saratov.
Tverdokhlebov, V.P. [Tverdohlebov, V.P.] 1987. Triassic lakes of the southern Cis-Urals [in Russian]. In: G.G. Martinson and I.Y. Neustrueva (eds.), Istoriâ ozer pozdnego paleozoâ i rannego mezozoâ, 235–242. Nauka, Leningrad.
Tverdokhlebov, V.P., Tverdokhlebova, G.I., Surkov, M.V., and Benton, M.J. 2003. Tetrapod localities from the Triassic of the SE of European Russia. Earth-Science Reviews 60: 1–66. Crossref
Tverdokhlebov, V.P. [Tverdohlebov, V.P.], Surkov, M.V., and Tverdokhlebova, G.I. [Tverdohlebova, G.I.] 2007. Continental palaeoecosystems of the Palaeozoic and Mesozoic boundary. Post-crisis stage. Paper 4. The Early and Middle Triassic, the Southwest of the East-European Platform [in Russian]. Izvestiâ vysših učebnyh zavedenij, Geologiâ i razvedka 2007 (4): 3–11.
Ulrich, H. and Schmelz, R.M. 2001. Enchytraeidae as prey of Dolichopodidae, Recent and in Baltic amber (Oligochaeta; Diptera). Bonner zoologische Beiträge 50: 89–101.
Voigt, S. and Hoppe, D. 2010. Mass occurrence of penetrative trace fossils in Triassic lake deposits (Kyrgyzstan, Central Asia). Ichnos 17: 1–11. Crossref
Vorobyeva, E.I. and Minikh, M.G. 1968. Experimental application of biometry to the study of ceratodontid dental plates. Paleontological Journal 2: 217–227.
Walker, R.G. and James, N.P. (eds.) 1992. Facies Models: Response to Sea Level Change. 409 pp. Geological Association of Canada, Stittsville.
Weigert, A., Golombek, A., Gerth, M., Schwarz, F., Struck, T., and Bleidorn, C. 2016. Evolution of mitochondrial gene order in Annelida. Molecular Biology and Evolution 94: 196–206. Crossref
Weitschat, W. and Wichard, W. 2002. Atlas of Plants and Animals in Baltic Amber. 256 pp. Dr. Friedrich Pfeil, München.
Wesenberg-Lund, C. 1939. Biologie der Süsswassertiere. 817 pp. Verlag von Julius Springer, Wien. Crossref
Westheide, W., McHugh, D., Purschke, G., and Rouse, G. W. 1999. Systematization of the Annelida: different approaches. Hydrobiologia 402: 291–307. Crossref
White, D.S. and Miller, M.F. 2008. Benthic invertebrate activity in lakes: linking present and historical bioturbation pattern. Aquatic Biology 2: 269–277. Crossref
Wilson, L.A. and Butterfield, N.J. 2014. Sediment effects on the preservation of Burgess Shale-type compression fossils. Palaios 29: 145–154. Crossref
Zajíc, J. 2014. Permian faunas of the Krkonoše Piedmont Basin (Bohemian Massif, central Europe). Sborník Národního Muzea v Praze 70: 131–142. Crossref
Zalessky, M.D. [Zalesskij, M.D.] 1928. First microscopic studies of a lower Volgian combustible shale [in Russian]. Izvestiâ Sapropelevogo Komiteta 4: 1–28.
Zangerl, R. and Richardson, E.S., Jr. 1963. The paleoecological history of two Pennsylvanian black shales. Fieldiana: Geology Memoirs 4: 1–239.
Zatoń, M., Vinn, O., and Tomescu, A.M.F. 2012. Invasion of freshwater and variable marginal marine habitats by microconchid tubeworms—an evolutionary perspective. Geobios 45: 603–610. Crossref
Zhuravlev, A.Y., Gámez Vintaned, J.A., and Liñán, E. 2011. The Palaeoscolecida and the evolution of the Ecdysozoa. In: P.A. Johnston and K.J. Johnston (eds.), International Conference on the Cambrian Explosion, Proceedings. Palaeontographica Canadiana 31: 177–204.
Zigler, V. 1992. Imprints of the bodies of oligochaete worms and leeches in Oligocene diatomites from Bechlejovice near Dĕčín. Acta Universitatis Carolinae, Geologica 1–2: 155–158.
Żyła, D., Wegierek, P., Owocki, K., and Niedźwiedzki, G. 2013. Insects and crustaceans from the latest Early–early Middle Triassic of Poland. Palaeogeography, Palaeoclimatology, Palaeoecology 371: 136–144. Crossref
Acta Palaeontol. Pol. 65 (2): 219–233, 2020
https://doi.org/10.4202/app.00704.2019