


Evolution and identity of synapsid carpal bones
SUSANNA KÜMMELL, FERNANDO ABDALA, JUDYTH SASSOON, and VIRGINIA ABDALA
Kümmell, S., Abdala, F., Sassoon, J., and Abdala, V. 2020. Evolution and identity of synapsid carpal bones. Acta Palaeontologica Polonica 65 (4): 649–678.
To date there is little information on carpal bone homology in late Palaeozoic and Mesozoic Synapsida. Crucial to the understanding of homology in synapsid carpal elements is the fact that different nomenclatures are used for the carpals of non-mammaliamorph Synapsida (Gegenbauer’s canonical nomenclature) and Mammaliaformes (mammalian nomenclature). The homologies of the carpals of non-mammaliamorph synapsids and mammals were established early last century and have not been reviewed since then. Here we provide a detailed study of the carpal bones of synapsids ranging in age from the early Permian to Late Cretaceous. The mammaliamorph lunate, previously considered the homologue of the intermedium of non-mammaliamorph synapsids, is interpreted here as homologous to their lateral centrale. We interpret the single mammaliamorph centrale as a homologue of the medial centrale of non-mammaliamorph synapsids. In some synapsid specimens, we found that one or two centralia are fused to the radiale (e.g., the gorgonopsian Arctognathus and tritylodontid Bienotheroides), supporting a digging habit. A third centrale is present in the therocephalian Theriognathus, very likely an abnormal duplication. An additional medial bone in a biarmosuchian was interpreted as a prepollex/sesamoid. A cartilaginous prepollex/sesamoid may also have been present in several non-mammaliamorph synapsids, which have an open space proximal to distal carpal I. Distal carpal V is completely lost in dicynodonts and it is mainly fused to distal carpal IV in the adult stage of most other therapsid groups, but showed a delayed development in most non-mammaliamorph cynodonts. In mammaliamorphs, distal carpal V is not present. Our observations provide an updated revision of synapsid carpal homologies, mainly on the basis of position and anatomical contacts and also taking into account the results of embryological studies.
Key words: Synapsida, carpus, intermedium, lunate, manus, homology, Permian, Mesozoic.
Susanna Kümmell [susanna.kuemmell@uni-wh.de], Institute of Evolutionary Biology and Morphology, Center for Biomedical Education and Research (ZBAF), Faculty of Health, School of Medicine, University Witten/Herdecke, Stockumer Straße 10, 58454 Witten, Germany.
Fernando Abdala [1viutiabdala2@gmail.com], Unidad Ejecutora Lillo (CONICET-Fundación Miguel Lillo), Tucumán, Argentina and Evolutionary Studies Institute, University of the Witwatersrand, South Africa.
Judyth Sassoon [js7892@bristol.ac.uk], School of Earth Sciences, University of Bristol, Queen’s Road, Bristol, BS8 1RJ, UK.
Virginia Abdala [virginia@webmail.unt.edu.ar], Instituto de Biodiversidad Neotropical, CONICET-Universidad Nacional de Tucumán, Catedra de Biologia General, Facultad de Ciencias Naturales e IML, Tucumán, Argentina.
Received 2 December 2019, accepted 12 August 2020, available online 6 November 2020.
Copyright © 2020 S. Kümmell et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Figures 14B and 15 excluded, see pertinent figure captions.
Introduction
The homologies of carpal bones in late Palaeozoic and Mesozoic Synapsida have not been extensively researched. Homology is a key concept for different disciplines such as taxonomy, systematics, and morphology. In some cases, a deep understanding of homologies for the interpretation of evolutionary changes can only be provided by fossils. An example of this is the proposed homology of the amniote astragalus with four tarsal bones in anamniote tetrapods (O’Keefe et al. 2006), or the established homologies of the different phalanges in mammals and non-therapsid synapsids (Hopson 1995). Under these circumstances, providing reliable hypotheses of homology is a fundamental palaeontological task. The synapsid carpus is a complex structure consisting of many small bones, and homologizing them in fossils spanning over 170 million years is challenging. Here we investigate the homology and evolutionary change of the synapsid carpals from the early Permian to the end of the Cretaceous on the basis of currently accepted synapsid systematics.
The carpus is the proximal part of the manus (see expanded concept in SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app65-Kuemmell_etal_SOM.pdf). Traditionally, the carpals of mammals were designated using a different nomenclature than that of reptiles and amphibians. These terms were known as the “mammalian nomenclature” (sensu Shubin and Alberch 1986) and the “canonic nomenclature” (sensu Čihák 1972), respectively. The canonic nomenclature (henceforth «canonical nomenclature») is not only used for reptiles and amphibians, but also for the non-mammaliaform members of the clade Synapsida (Fig. 1A; e.g., Broom 1904; Jenkins 1971; Liu et al. 2017), whereas the carpal bones of Mesozoic Mammaliaformes are designated with the mammalian nomenclature (Fig. 1B, Table 1; e.g., Kielan-Jaworowska 1977; Ji et al. 2002; Luo et al. 2003). These different naming conventions can lead to confusion in anatomical descriptions. The use of different nomenclatures for the carpal bones of mammaliaforms and the remaining synapsids is an historical artefact. It probably arose because of the early classification of non-mammaliaform synapsids within the class Reptilia (“mammal-like reptiles”), whereas those specimens now termed Mesozoic Mammaliaformes were, as a whole, considered to be members of Mammalia in former times.
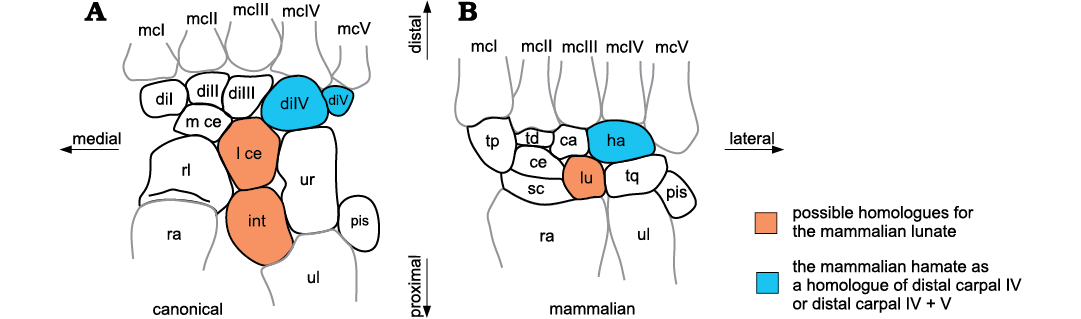
Fig. 1. Schematic diagrams of a non-therapsid synapsid (“pelycosaur”) carpus (A) and a mammaliaform carpus (B), labelled using the canonical and mammalian nomenclatures. Abbreviations: ca, capitate; ce, centrale; di, distal carpal; ha, hamate; int, intermedium; l ce, lateral centrale; lu, lunate; mc, metacarpal; m ce, medial centrale; pis, pisiform; ra, radius; rl, radiale; sc, scaphoid; td, trapezoid; tp, trapezium; tq, triquetrum; ul, ulna; ur, ulnare.
The nomenclature of the mammalian carpus was first established for the human carpals in the 17th and 18th centuries (Lyser 1653; Monro 1726; Albinus 1726; see McMurrich 1914) and later extended to describe the mammalian carpus in general. A combination of the three slightly differing nomenclature systems of Lyser (1653), Monro (1726), and Albinus (1726), together with the term central bone (centrale) are still in use to designate carpal bones of modern mammals and Mesozoic mammaliaforms (Table 1; e.g. Kielan-Jaworowska 1977; Ji et al. 2002; Luo et al. 2003). In 1864, Gegenbaur introduced the canonical nomenclature for the carpus, which was more generally applicable to all classes of vertebrates. He established homologies of the different carpal bones in all vertebrate clades and homologized the reptilian and mammalian carpals (Table 1).
Table 1. Homology of the reptilian and mammalian carpals after Gegenbaur (1864).
|
Canonical nomenclature |
Mammalian nomenclature |
|
radiale |
scaphoideum |
|
intermedium |
lunatum |
|
ulnare |
triquetrum |
|
pisiforme |
pisiforme |
|
centrale |
centrale |
|
carpale 1 |
mutungulatum majus/trapezium |
|
carpale 2 |
mutungulatum minus/trapezoides |
|
carpale 3 |
capitatum |
|
carpale 4 |
hamatum |
|
carpale 5 |
The first descriptions of the carpus in non-mammaliamorph synapsids were provided by Seeley (1888, 1895) and Bardeleben (1889). These used the mammalian nomenclature more broadly and also applied it to describe the carpals of the Permo-Triassic fossil synapsids. In contrast, Broom (1901) established a homology of the Permo-Triassic synapsids and the mammalian carpals adopting the canonical and the mammalian systems to describe the proximal and central carpals of the dicynodont “Udenodon gracilis” (= Dicynodontoides recuvidens; Angielczyk et al. 2009). However, in his later publications, he only used the canonical nomenclature to describe the carpus of Permo-Triassic synapsids (Broom 1904, 1907, 1913, 1930). Broom (1901) homologized the canonical and mammalian nomenclature, following Gegenbaur (1864), but he misinterpreted the medial centrale as distal carpal I, thus identifying only one centrale in “Udenodon”. However, later on, he described two centralia in non-mammaliamorph synapsids (Broom 1904, 1907). The carpus in non-mammaliamorph synapsids has five distal carpals and two centralia, two carpals more than extant, basal mammals, which indicates that two carpal bones were lost along the evolutionary transition to mammals (Fig. 1). Broom (1901, 1904, 1907) identified one of the lost carpals as distal carpal V and proposed the second to be a centrale when he homologized the mammalian lunate (= lunar) with the intermedium.
The homology of the canonical and mammalian nomenclature erected by Gegenbaur (1864; see Table 1) and adopted by Broom (1901, 1904) is followed up to the present day (e.g., Ihle et al. 1927; Romer and Parsons 1977; Salomon et al. 2005; Kivell 2016) and has not been revised, even in the light of new palaeontological and embryological discoveries.
From this historical perspective, the following questions arise: do the homologies proposed by Gegenbaur (1864) between the carpals of reptiles and mammals and by Broom (1901, 1904, 1907) between non-mammaliamorph synapsids and mammals still hold? Are there newer interpretations possible in the light of more recently collected specimens? Here we do not discuss Gegenbaur’s (1864) homologies between the carpals of reptiles and mammals because of the limitations of our fossil sample. Instead, we focus only on the question of homology between the carpals of non-mammaliamorph synapsids and basal mammals and the proposal put forward by Broom (1901, 1904). We studied carpal bones from Permian to Cretaceous Synapsida to understand carpal homology from a palaeontological perspective. In particular, we were interested in following the loss of the two carpal bones in the evolution towards mammals (Fig. 1).
The loss of distal carpal V in fossil synapsids was addressed by Hopson (1995). He suggested two variants of bone loss: non-ossification in Dicynodontia and gomphodont Cynodontia, and fusion to distal carpal IV in Biarmosuchia, Gorgonopsia, Therocephalia, and Mammalia. Here we provide new information on this topic based on additional material.
The loss of one centrale during the transition to mammals and the proposed homology between the intermedium and lunate (Broom 1901, 1904) can be questioned for two reasons: firstly, according to its position and bone contacts, the lunate must be interpreted as the lateral centrale of non-mammaliamorph synapsids and the intermedium as the lost carpal element (Fig. 1).
The second reason for the uncertainty of intermedium-lunate homology emerges from studies of mammalian embryology. Carpals appear as chondrogenic foci in early ontogeny and may fuse or disappear later in development, providing clues about the identity of the carpal bones. There is an ongoing debate among embryologists about the identity of the mammalian lunate. Some of them interpret the mammalian lunate as a homologue of the reptilian intermedium (Steiner 1942; Schmidt-Ehrenberg 1942; Shubin and Alberch 1986; Milaire 1978; Heppleston 2010) as did Gegenbaur (1864). Others homologize the lunate with a reptilian centrale and propose that the intermedium was lost or fused to another bone of the forelimb (Holmgren 1933, 1952; Kindahl 1941, 1942a, b, 1944; Slabý 1967, 1968; Čihák 1972). This disagreement between embryologists prompts further scrutiny of each argument.
From studies on prenatal development in modern mammals, embryologists proposed that distal carpal V fused to another manual cartilaginous anlage in early ontogeny. So the loss of this bone in some mammals is indeed related to ontogenetic fusion (Milaire 1978; Holmgren 1952; Čihák 1972; Slabý 1967). Thus, in addition to paleontological investigation, we refer to recent embryological studies for further data on carpal bone loss and putative homologies.
As well as approaching the issue of the loss of carpals in major synapsid clades, we describe carpal bone additions and losses in single species or individuals. Some synapsid fossils possess an additional central bone. This is the case in the therocephalian Theriognathus NHMUK R 5694 (Boonstra 1934: 260, fig. 34) and in a Russian biarmosuchian PIN 1758/320 (Chudinov 1983: 55–56, figs. 3–6). Boonstra (1934) and Chudinov (1983) interpreted the additional bone as a third centrale. In other cases, the most medial or preaxial bone was interpreted as a prepollex, as in the cases of Theriodesmus phylarchus NHMUK 49392 (probably a biarmosuchian, FA personal observation; Bardeleben 1889), in “Opisthoctenodon agilis” (Broom 1904; which represents most likely the dicynodont Pristerodon; Keyser 1993; Angielczyk et al. 2005) and as a probable prepollex in Zhangheotherium (Hu et al. 1998) and Asioryctes (Kielan-Jaworowska 1977; Kielan-Jaworowska et al. 2004).
Other authors described an open space medial to the central row in non-mammaliamorph synapsids (e.g., Romer and Price 1940; Case 1907), and interpreted it as a lacuna previously occupied by a medially situated, cartilaginous sesamoid. To date, the evolutionary history of the third centralia, prepollices, and sesamoids have not been systematically researched in fossil synapsids. Here, we investigated if there were more cases of synapsid fossils with more than two centralia. We also sought evidence of a prepollex, sesamoid, or an open space on the medial side of the carpus, where these elements could have been present. Because it was not possible to distinguish between prepollices and radial sesamoids in fossils, we used the term “prepollex/sesamoid” bones for such additional preaxial bones (SOM).
In this paper, we use the canonical nomenclature for both non-mammaliaform synapsids and mammaliaforms. This is also in accordance with the fundamental embryological studies of Schmidt-Ehrenberg (1942), Holmgren (1933, 1952), and Shubin and Alberch (1986). The only exception is the lunate of mammalian nomenclature whose identity within synapsids needs clarification. Thus, we continue using the term “lunate” in mammaliamorphs. The following list clarifies the terminology used here: radiale, scaphoid; ulnare, triquetrum; intermedium, intermedium of non-mammaliamorph Synapsida; lunate, lunate of Mammaliamorpha; medial and lateral central, centralia in non-mammaliamorph Synapsida; central, single centrale in Mammaliamorpha; distal carpal I, trapezium; distal carpal II, trapezoid; distal carpal III, capitate; distal carpal IV (± distal carpal V), hamate.
Institutional abbreviations.—AM, Albany Museum, Grahamstown, South Africa; AMNH, American Museum of Natural History, New York, USA; BP, Evolutionary Studies Institute, University of the Witwatersrand (formerly Bernard Price Institute for Palaeontological Research), Johannesburg, South Africa; CAGS, Chinese Academy of Geological Sciences, Beijing, China; CGS, Council for Geosciences, Pretoria, South Africa; FMNH, Field Museum of Natural History, Chicago, USA; GMV, National Geological Museum of China, Beijing, China; GPIT, Paleontology Department and Museum, Institute of Geosciences, Eberhard Karls University, Tübingen, Germany; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; MCZ, Museum of Comparative Zoology, Harvard University, Cambridge, USA; MNHN.F, Muséum national d’Histoire naturelle, collection de Paléontologie, Paris, France; NMQR, National Museum, Bloemfontein, South Africa; NHMUK, Natural History Museum, London, UK; OMNH, Oklahoma Museum of Natural History, Norman, USA; OUMNH-TSK, Oxford University Museum, T.S. Kemp Collection, Oxford, UK (material now deposited in the NHMUK); PIN, Paleontological Institute, Russian Academy of Sciences, Moscow, Russia; PVL, Colección Palaeontología de Vertebrados Lillo, Universidad Nacional de Tucumán, Argentina; RC, Rubidge Collection, Wellwood, Graaff-Reinet, South Africa; SAM, Iziko South African Museum, Cape Town, South Africa; TM, Northern Flagship Institution, Transvaal Museum, Pretoria, South Africa; TMM, Texas Memorial Museum, Austin, USA; UFRGS, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; UMZC, University Museum of Zoology, Cambridge, UK; USNM, National Museum of Natural History, Washington D.C., USA; WCW, Wucaiwan field collection, housed at the IVPP.
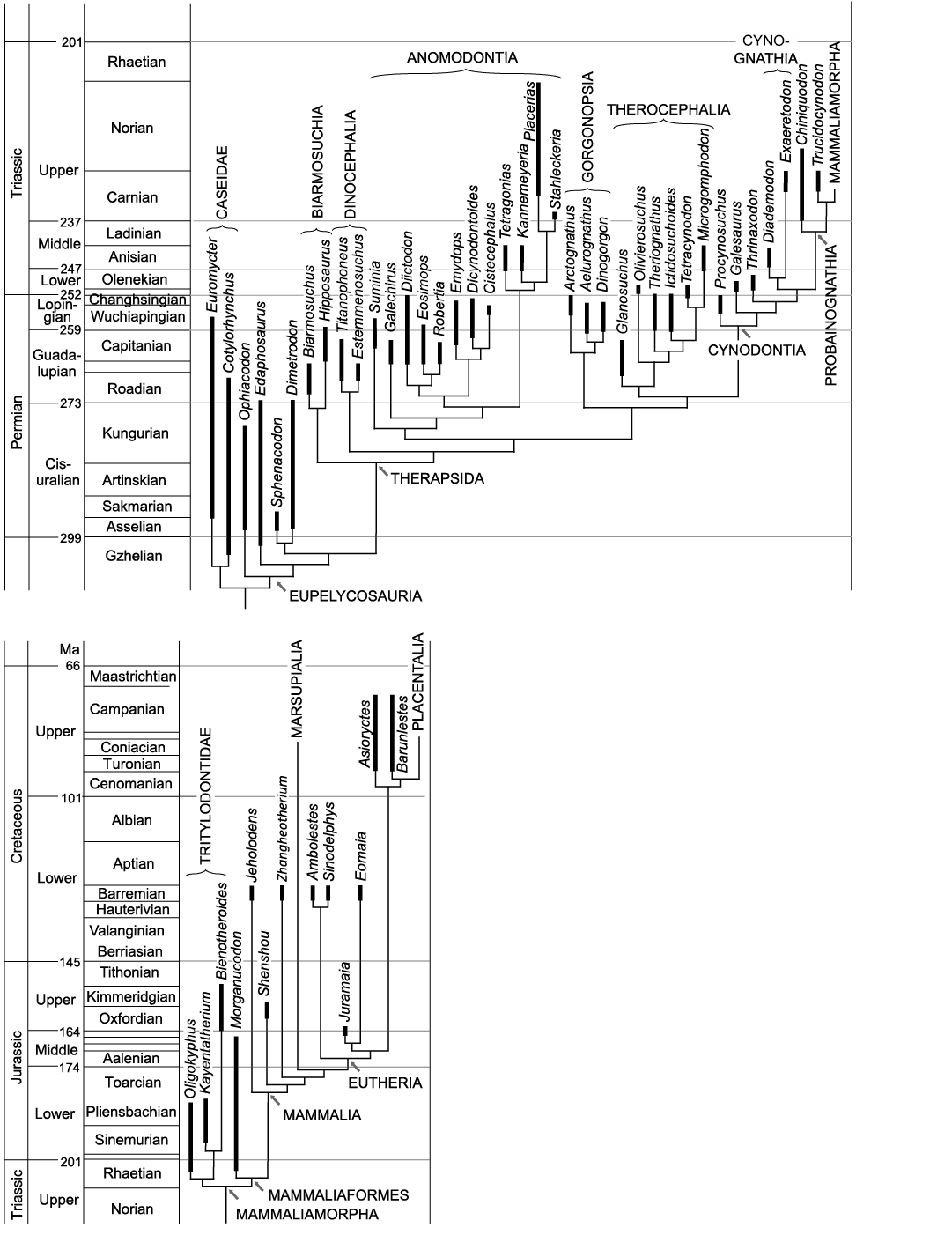
Fig. 2. Cladograms of fossil Synapsida. The cladograms are compromise trees in which different phylogenetic hypotheses were considered. Because of small space, some age names are not included in the cladogramms. These are: Wordian in the Guadalupian; Induan in the Lower Triassic; Hettangian in the Early Jurassic; Bajocian, Bathonian and Callovian in the Middle Jurassic and Santonian in the Late Cretaceous. Geological timescale after Cohen et al. (2013, updated), correlations after Schneider et al. (2020). A. Non-therapsid Synapsida (after Reisz 1986; Modesto et al. 2011; Reisz et al. 2011), Dinocephalia (after Kammerer 2011), Anomodontia (after Maisch 2001; Vega-Dias et al. 2004; Fröbisch and Reisz 2011; Kammerer et al. 2011; Angielczyk and Rubidge 2013), Gorgonopsia (after Kammerer 2016; Kammerer and Masyutin 2018; SK personal communication with Christian Kammerer 2019), Therocephalia (after Huttenlocker and Smith 2017), non-mammaliamorph Cynodontia (after Abdala 2007; Ruta et al. 2013). B. Mammaliamorpha (after Kielan-Jaworowska et al. 2004; Watabe et al. 2007; Luo et al. 2011; Ruta et al. 2013; Bi et al. 2014, 2018; Velazco et al. 2017).
Material and methods
Sixty-four synapsid specimens from 39 genera were studied. Most of the specimens, including some casts, were examined directly. The rest of the data was obtained from photographs, scans or from publications, shown in the following list. Here, we usually use the generic name for specimens. The specimen number (museum accession number) is provided, when detailed observations were made on just one specimen of a genus or species with several specimens. Accession numbers are also listed, when the identity of the fossil was uncertain. The taxonomy of the Gorgonopsia is based on the research of Christian Kammerer (SK personal communication 2019).
Caseidae: Euromycter rutenus MNHN.F.MCL-2 (Sigogneau-Russell and Russell 1974: fig. 18), Cotylorhynchus romeri OMNH 00655 (Stovall et al. 1966: fig. 13).
Non-therapsid Eupelycosauria: Ophiacodon retroversus FMNH UC 458, MCZ 1203, Ophiacodon mirus FMNH UC 671 (cast), Edaphosaurus boanerges NHMUK R 9204 (cast), Sphenacodon ferox CM 76895 (Henrici et al. 2005: photo, fig. 1), Dimetrodon milleri MCZ 1365 (cast).
Biarmosuchia: Biarmosuchidae indet. PIN 1758/320, Hipposaurus major SAM-PK-9081.
Dinocephalia: Titanophoneus potens PIN 157/1, Estemmenosuchus uralensis PIN 1758/23.
Anomodontia: Suminia getmanovi PIN 2212/62 (Fröbisch and Reisz 2011: figs. 9, 12), Galechirus scholtzi SAM-PK-1068, AMNH 5516, Eosimops newtoni BP/1/6674, Robertia broomiana SAM-PK-11885a, b, Diictodon feliceps CGS FL186, TM 4991, UMZC T 420, GPIT/RE/7193, SAM-PK-K10699, CGS RMS214, CGS T72, SAM-PK-K10636, Cistecephalus microrhinus BP/1/2124, BP/1/2915, Kannemeyeria simocephalus NHMUK R 3741, Stahleckeria potens MCZ 1688.
Gorgonopsia: Arctognathus curvimola SAM-PK-3329, Aelurognathus tigriceps SAM-PK-2342, cf. Cynariops robustus SAM-PK-K10000, Dinogorgon rubidgei BP/1/2190, Gorgonopsia indet. BP/1/1210.
Therocephalia: Glanosuchus macrops SAM-PK-K7809, SAM-PK-12051, CGS RS424, Olivierosuchus parringtoni BP/1/3973, BP/1/3849, Theriognathus microps NHMUK R 5694, Ictidosuchoides longiceps CGS CM86-655, Ictidosuchoides longiceps or Ictidosuchops intermedium BP/1/2294, Tetracynodon darti AM 3677, BP/1/2710, Microgomphodon oligocynus SAM-PK-K10160.
Non-mammaliamorph Cynodontia: Procynosuchus delaharpeae BP/1/591, NHMUK PV R 37054 (formerly OUMNH TSK 34), RC92, Galesaurus planiceps BP/1/2513, SAM-PK-K10465, SAM-PK-K10468, Thrinaxodon liorhinus BP/1/1737, BP/1/7199 (CT-scan), Diademodon tetragonus NHMUK R-3581, USNM 23352, Cynognathia indet. BP/1/4534, Exaeretodon argentinus PVL 2554, Trucidocynodon riograndensis UFRGS PV-1051T.
Basal Mammaliamorpha: Tritylodontidae indet. WCW-06A-34, Bienotheroides wanhsienensis IVPP V 7905, Kayentatherium wellesi TMM 43690-5.136 (scan, Eva Hoffman, see also Hoffman and Rowe 2018: supplement).
Mammaliaformes: Jeholodens jenkinsi GMV 2139a (original and cast), Zhangheotherium quinquecuspidens IVPP V7466, Eomaia scansoria CAGS01-IG-1a (cast and Luo et al. 2003: fig. 2).
Mammalian embryological studies were considered alongside paleontological observations to provide a thorough basis for testing homologies. Carpal bone homologies were identified in the articulated fossil carpi on the basis of position, relationship of elements to each other and sequential order. Other features (e.g., relative size and shape) played a subordinate role in homology assessment. In disarticulated carpi, relative size, shape and articular facets were used for bone identifications. Because of the high morphological variation of carpal elements, when possible we used articulated, complete to nearly complete carpi with elements in their original position. Using these specimens, we assessed the major evolutionary changes in carpal bones in synapsids. Fused carpals were recognized by fusion lines and/or irregular shapes with set-back angles and indentations. Open spaces in the articulated skeletons may represent unfossilised cartilaginous precursors and were also considered in our interpretations.
To assess the evolution of characters, we mapped them onto phylogenetic trees using Mesquite 3.61 (Maddison and Maddison 2019; see Discussion and SOM: figs. 1–3).
Description of the synapsid carpus
The descriptions of the bones are mostly presented in dorsal view. However, the dorsal surfaces of some of the fossils studied were not exposed. Such specimens are described in ventral view. The description is made in zero position (sensu Kümmell and Frey 2014b). In zero position the carpus is flat without a transverse arch and the rays are longitudinally aligned and not spread (ray = digit and corresponding metacarpal; Biesecker et al. 2009).
Carpal bones in synapsids sometimes show true joints with a great range of mobility, but usually they are connected by amphiarthroses, implying only a small mobility range. Joints were not exposed in all the partially prepared specimens; therefore we do not distinguish between plane contacts, real articular facets and amphiarthroses.
The carpus of Tritylodontidae resembles that of Mammaliaformes more than that of non-mammaliamorph Synapsida. Thus, we describe the tritylodontid carpus together with species of Mammaliaformes. Tritylodontidae and Mammaliaformes form the clade Mammaliamorpha (Rowe 1988; Luo 2011), and our descriptions discriminate between non-mammaliamorph synapsids and mammaliamorphs.
Non-mammaliamorph Synapsida.—Radiale: In dorsal view, the radiale appears either square, irregularly rectangular (transversely orientated) or irregularly pentagonal (Figs. 3A–8A; SOM: table 1: a). In the pentagonal radiale, the lateral and medial margins are parallel to each other, followed distally by two bevelled edges. The radiale shows a wide proximal facet, which occupies the whole distal facet of the radius (SOM: table 1: b). In some Dicynodontia, Gorgonopsia and Therocephalia, the radiale is slightly convex proximally. In species with a quadrangular radiale, the radiale contacts the medial centrale on its distal border. In species with a pentagonal radiale, the bevelled distal edges are the facets for the medial centrale distomedially to distally and the lateral centrale distolaterally (Figs. 4A, B, 6D; SOM: table 1: c–e). The lateral centrale usually lies distolaterally or laterally to the radiale (SOM: table 1: e, f) and distally to the intermedium, which usually protrudes proximally beyond the proximal border of the radiale. In non-mammaliamorph Cynodontia, the lateral centrale always contacts the lateral margin of the radiale (Fig. 7; SOM: table 1: f).
Intermedium: In non-therapsid Synapsida, the intermedium can be broad and square (e.g., Euromycter and Ophiacodon MCZ 1203; Fig. 3), rectangular with a proximodistal elongation (e.g., Ophiacodon FMNH UC 458, FMNH UC 671) or pentagonal (e.g., Sphenacodontidae; SOM: table 2: a). As reported previously, the “pelycosaurian” intermedium is thin dorsoventrally, convex dorsally and concave ventrally (Romer and Price 1940; Henrici et al. 2005). The “pelycosaurian” intermedium is larger than that of non-mammaliamorph therapsids, i.e., wider in relation to its length (square or pentagonal) and/or longer in relation to the radiale (SOM: table 2: a, e). In “pelycosaurs”, the intermedium is either longer or the same length as the radiale, whereas in therapsids, it is the same length or shorter than the radiale. The subadult therapsid Diictodon CGS FL186 is the only exception known to us where the intermedium is longer than the radiale (SOM: table 2: e). The non-mammaliamorph therapsid intermedium is lateromedially narrow and dorsoventrally deep. It is a proximodistally oriented, rectangular to hourglass-shaped slender bone (Figs. 4A–8A), or bean-shaped in Kannemeyeria and Galesaurus BP/1/2513 (Fig. 7C; SOM: table 2: a). Proximally, the intermedium articulates with the ulna (SOM: table 2: b). Distally it contacts the lateral centrale (SOM: table 2: c). Laterally, it is articulated with the ulnare and medially with the radiale and/or the lateral side of the distal end of the radius (SOM: table 2: d). In a few cases, it only contacts the radiale medially (Biarmosuchidea indet. PIN 1758/320, Fig. 4A, Estemmenosuchus, Stahleckeria, Glanosuchus SAM-PK-12051 and CGS RS424, Theriognathus and Ictidosuchoides CGS CM86-655, Fig. 6D). In Caseidae (Fig. 3A, B) and some single specimens of other groups (Edaphosaurus, Procynosuchus RC92, Fig. 7A, Exaeretodon, and probably Cistecephalus), the intermedium is situated even further proximally than in the other synapsids and lies laterally to the radius (SOM: table 2: d).
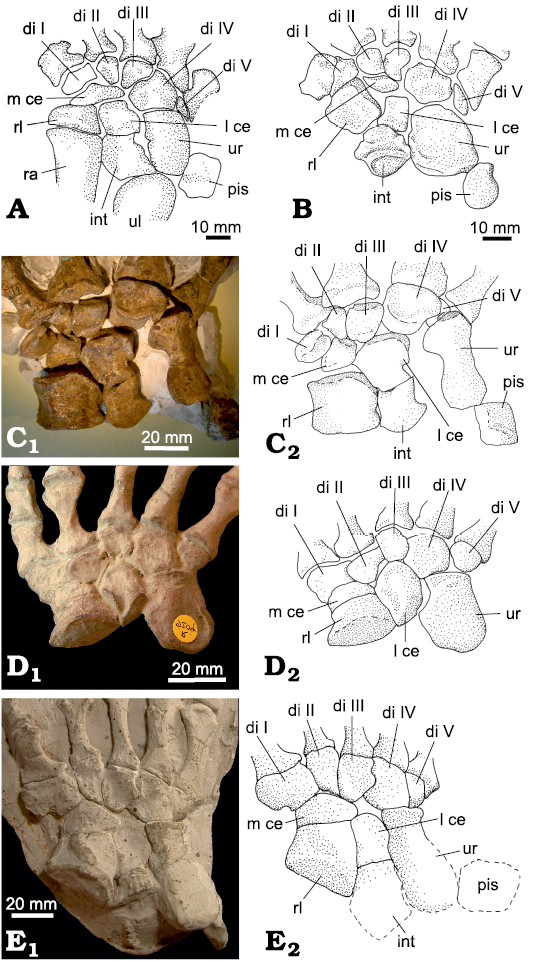
Fig. 3. Carpus of non-therapsid Synapsida. A. Euromycter rutenus (Sigogneau-Russell and Russell, 1974), MNHN.F.MCL-2, Valady, France, Sakmarian, left carpus (reversed), dorsal view (redrawn from Sigogneau-Russell and Russell 1974: fig. 18). B. Cotylorhynchus romeri Stovall, 1937, OMNH 00655, Navina, USA, Kungurian, left carpus (reversed), dorsal view (redrawn from Stovall et al. 1966: fig. 13, left). C. Ophiacodon retroversus Cope, 1878, MCZ 1203, Rattlesnake Canyon, USA, Wichita Group, Cisuralian, right carpus, dorsal view. D. Edaphosaurus boanerges Romer and Price, 1940, NHMUK R 9204 (cast), Geraldine, Archer County, USA, Wichita Group, Cisuralian, left carpus (reversed), dorsal view. E. Dimetrodon milleri Romer, 1937, MCZ 1365 (cast), Archer, USA, Putnam Formation, Cisuralian, right carpus, dorsal view. Photographs (C1–E1) and interpretative drawings (C2–E2). Abbreviations: di, distal carpal; int, intermedium; l ce, lateral centrale; m ce, medial centrale; pis, pisiform; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
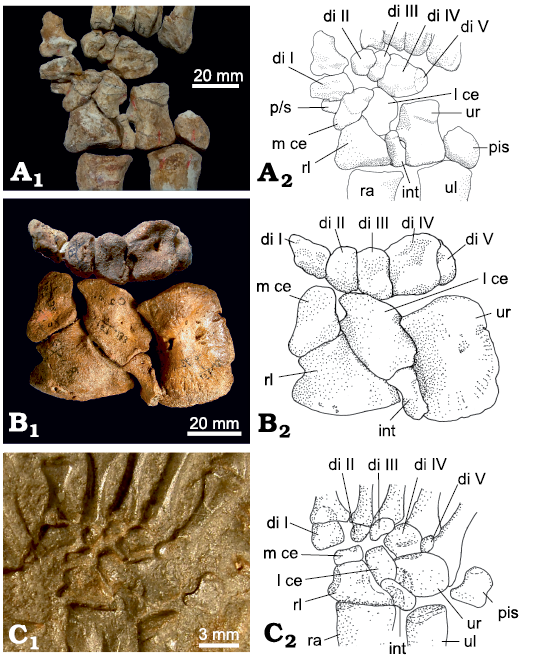
Fig. 4. Carpus of basal Therapsida. A. A biarmosuchian PIN 1758/320, Eshovo, Russia, Roadian, Guadalupian, right carpus, slightly disarticulated, dorsal view. B. Dinocephalian Titanophoneus potens Efremov, 1940, PIN 157/1, Isheevo, Russia, Capitanian, left carpus (reversed), dorsal view. C. Basal anomodontian Galechirus scholtzi Broom, 1907, SAM-PK-1068, Victoria West, South Africa, Capitanian, right carpus, dorsal view (impression). Photographs (A1–C1) and interpretative drawings (A2–C2). Abbreviations: di, distal carpal; int, intermedium; l ce, lateral centrale; m ce, medial centrale; pis, pisiform; p/s, prepollex/sesamoid; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
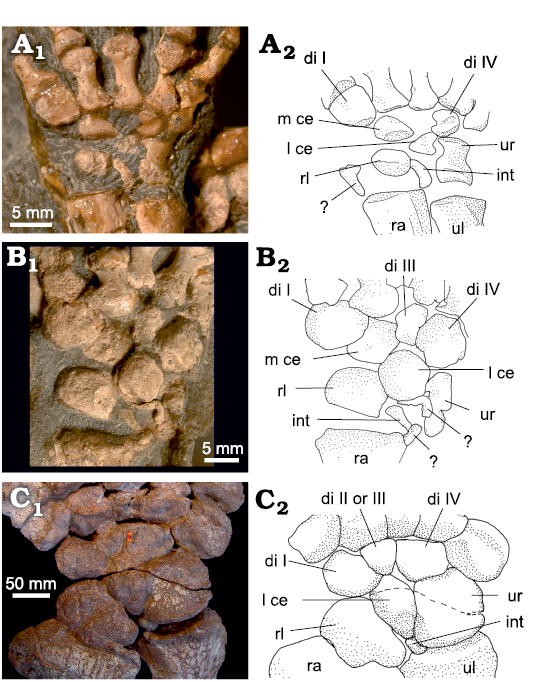
Fig. 5. Carpus of Dicynodontia. A. Diictodon feliceps (Owen 1876), CGS FL186, Jasfontein, Victoria West, South Africa, Tropidostoma Assemblage Zone, Wuchiapingian, right carpus, dorsal view. B. Eosimops newtoni Broom, 1921, BP/1/6674, Somerfontein, Philipolis District, South Africa, Pristerognathus Assemblage Zone, Capitanian–Wuchiapingian, left carpus, ventral view. C. Stahleckeria potens von Huene, 1935, MCZ 1688, Candelaria, Brazil, Santa Maria Formation, Carnian, right carpus, dorsal view. Photographs (A1–C1) and interpretative drawings (A2–C2). Abbreviations: di, distal carpal; int, intermedium; l ce, lateral centrale; m ce, medial centrale; pis, pisiform; ra, radius; rl, radiale; ul, ulna; ur, ulnare. Dotted line, fracture.
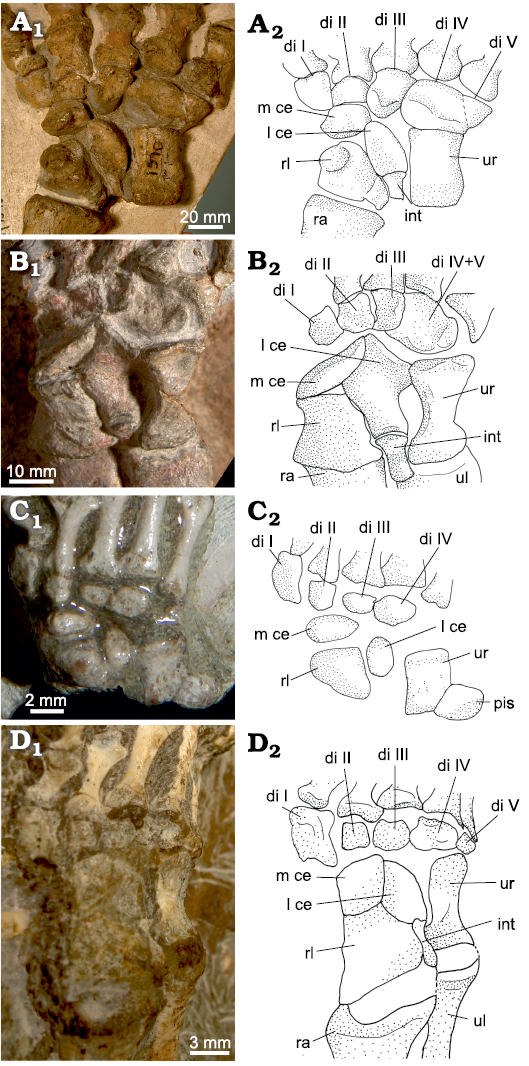
Fig. 6. Carpus of Gorgonopsia and Therocephalia. A. Gorgonopsia indet. BP/1/1210, Hoeksplaas, Murraysburg, South Africa, Daptocephalus Assemblage Zone, Changhsingian, left carpus (reversed), dorsal view. B. Basal therocephalian Glanosuchus macrops Broom, 1904, SAM-PK-K7809, La-de-da, Beaufort West, South Africa, Tapinocephalus Assemblage Zone, Capitanian, left carpus (reversed), dorsal view. C. Therocephalian Tetracynodon darti Sigogneau, 1963, AM 3677, farm Carlton Heights, Pixley ka Seme District, South Africa, Lystrosaurus Assemblage Zone, Early Triassic, right carpus, dorsal view (photo courtesy of Gabriela Fontanarrosa). D. Therocephalian Ictidosuchoides longiceps Broom, 1920, CGS CM86-655, Secretaris Kraal 19, Murraysburg, South Africa, Daptocephalus Assemblage Zone, Changhsingian, right carpus, dorsal view. Photographs (A1–D1) and interpretative drawings (A2–D2). Abbreviations: di, distal carpal; int, intermedium; l ce, lateral centrale; m ce, medial centrale; pis, pisiform; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
Ulnare: The ulnare is the longest bone of the non-mammaliamorph synapsid carpus, only in some Dicynodontia (Cistecephalus and Stahleckeria; Fig. 5C), it is approximately the same size as the radiale. In most specimens, it is approximately rectangular and proximodistally elongated (Figs. 4A–8A). Sometimes it is proximally rounded (e.g., in “pelycosaurs”, Fig. 3). In most Therocephalia it is hourglass shaped (Fig. 6B; SOM: table 3: a). The lateral margin of the ulnare is dorsoventrally thin, while the medial margin is dorsoventrally thicker and usually curved laterally. The ulnare has a complex, mainly convex articular surface on the medial side, which articulates with the intermedium and the lateral centrale. These articulations are often covered by adjacent bones or matrix, and therefore usually not visible. However, in some fossils (the biarmosuchian PIN 1758/320, Titanophoneus, Glanosuchus CGS RS424, Procynosuchus NHMUK PV R 37054, and Thrinaxodon BP/1/7199), the facet is exposed, showing a medioventrally pointing triangular process close to the mid-point of the bone’s medial margin. In Cistecephalus and one Diictodon specimen (CGS T72), the medial border of the ulnare only contacts the intermedium (SOM: table 3: c). Distally and sometimes distomedially, the contacting surface of the ulnare receives the distal carpal IV. Distally, in some cases it also receives distal carpal V (SOM: table 3d, e). Distolaterally, the ulnare contacts distal carpal V (e.g., most “pelycosaurs”, and Galechirus SAM-PK-1068, Ictidosuchoides CGS CM86-655, Procynosuchus NHMUK PV R 37054, Thrinaxodon BP/1/7199), metacarpal V (usually in Dicynodontia, Glanosuchus CGS RS424, Procynosuchus RC92), or leaves an open space between its distolateral margin and metacarpal V (many cases of non-mammaliamorph therapsids; SOM: table 3: f). Proximally the ulnare has a broad articulation area contacting the ulna (SOM: table 3: g).
Pisiform: The pisiform is often missing in synapsid fossils, especially in most Dicynodontia, Gorgonopsia and Therocephalia. From the 49 non-mammaliamorph synapsid carpi studied here, in which the typical place of the pisiform lateral of the wrist joint is well exposed, 26 lack an associated pisiform (SOM: table 4: a). However, in all synapsid groups, at least some specimens possess a pisiform, suggesting that it is usually present (Figs. 3–5, 7, 8A), but probably easily lost during fossilisation. The pisiform is a subcircular to oval bone, but can be square-shaped in some basal synapsids (Euromycter, Ophiacodon, and Dimetrodon), or sickle-shaped as in the cynodont Trucidocynodon (SOM: table 4: a). It is usually positioned close to the proximolateral border of the ulnare and the distolateral margin of the ulna (SOM: table 4: b).
Lateral centrale: The outline of the lateral centrale can be square or rectangular in proximodistal orientation, subcircular or oval and rhomboid. Nearly all forms occur in most therapsid groups (Figs. 3A–8A; SOM: table 5: a). In non-mammaliamorph Cynodontia, it is usually longer than the radiale, only in Procynosuchus, it has the same length (SOM: table 5: b). Proximally it has a facet for the intermedium, distally it contacts distal carpal III, sometimes also partly distal carpals II or IV. Only in the Caseidae Euromycter and Cotylorhynchus, it is distally articulated to the medial centrale (Fig. 3A, B; SOM: table 5: c). Distomedially, the lateral centrale articulates with the medial centrale and/or distal carpal II (SOM: table 5: d). Distolaterally it articulates with distal carpal IV (SOM: table 5: e). Proximomedially or medially, the lateral centrale is bordered by the radiale (SOM: table 1: e, f) and laterally by the ulnare (SOM: table 3: c).
Medial centrale: The outline of the medial centrale is mostly irregularly oval, rectangular or rhomboid (Figs. 3–7). In Trucidocynodon, it is triangular (Fig. 8A; SOM: table 6: a). Proximally, or occasionally proximolaterally, it is articulated with the radiale, proximolaterally or laterally with the lateral centrale. Only caseids show a proximal contact to the lateral centrale (SOM: table 6: b–d). In non-mammaliamorph cynodonts, the radiale is always proximal to the medial centrale as is also the case with the single centrale in mammaliamorphs (SOM: table 6: b; see below). The medial centrale is usually adjacent to the distal carpals II and I in non-mammaliamorph synapsids, but often extends to meet distal carpal III at its distolateral edge (SOM: table 6: e).
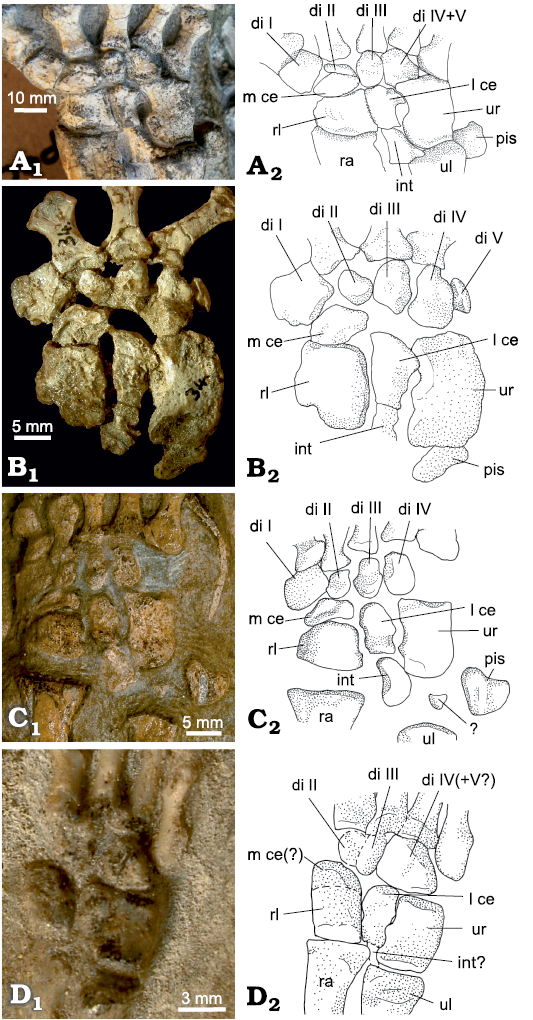
Fig. 7. Carpus of non-mammaliamorph Cynodontia. A. Procynosuchus delaharpeae Broom, 1937, RC92, Doornkloof, Graaff-Reinet, South Africa, Cistecephalus Assemblage Zone, Lopingian, right carpus, dorsal view. B. Procynosuchus delaharpeae Broom, 1937, NHMUK PV R 37054, Middle Luangwa Valley, Zambia, Daptocephalus Assemblage Zone, Changshingian, right carpus, dorsal view. C. Galesaurus planiceps Owen, 1860, BP/1/2513, Honingkrans, Burgersdorp, South Africa, Lystrosaurus Assemblage Zone, Early Triassic, left carpus (reversed), dorsal view. D. Cynognathia indet. BP/1/4534, Hugoskop 620, Roxville, South Africa, Cynognathus Assemblage Zone, Olenekian–Anisian, left carpus (reversed), dorsal view. Photographs (A1–D1) and interpretative drawings (A2–D2). Abbreviations: di, distal carpal; int, intermedium; l ce, lateral centrale; m ce, medial centrale; pis, pisiform; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
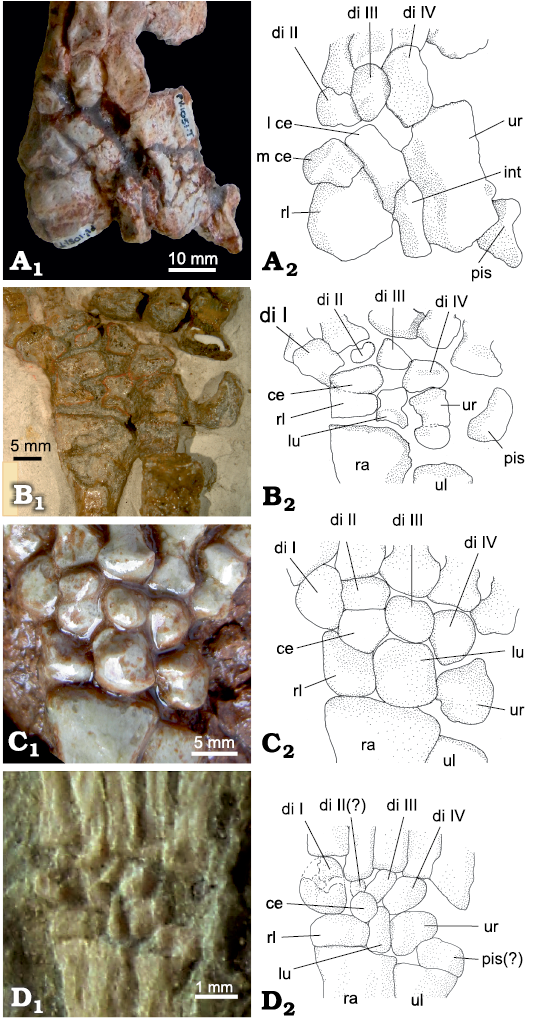
Fig. 8. Carpus of a non-mammaliamorph cynodont and three Mammaliamorpha. A. Trucidocynodon riograndensis Oliveira, Soares, and Schultz, 2010, UFRGS PV-1051T, Sítio Janner, Rio Grande do Sul, Brazil, Santa Maria Formation, Carnian, right carpus, dorsolateral view. B. Bienotheroides wanhsienensis Young, 1982, IVPP V 7905, Sichuan, China, Middle to Late Jurassic, right carpus, dorsal view. C. Tritylodontidae indet. WCW-06A-34, Wucaiwan, Junggar Basin, northwestern China, Shishugou Formation, probably Oxfordian, right carpus, dorsal view. D. Jeholodens jenkinsi Ji, Luo, and Ji, 1999, GMV 2139a, Sihetun, Liaoning Province, China, late Barremian, left carpus (reversed), dorsal view. Photographs (A1–D1) and interpretative drawings (A2–D2). Abbreviations: di, distal carpal; int, intermedium; l ce, lateral centrale; m ce, medial centrale; pis, pisiform; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
Three centralia: Besides the two centralia, a third bone is present in the central row of the therocephalian Theriognathus microps NHMUK R 5694 (Fig. 9). We do not know if this condition is an individual variant or common also in other specimens of the species Theriognathus microps. The lateral centrale is located at its common position, proximal to distal carpal III and distal to the intermedium. Medial to the lateral centrale are two small bones, closely connected to each other with tight fitting articular surfaces (Fig. 9). Together, both medially situated central bones have the general oval outline of the medial centrale and occupy the same position distal/distomedial of the radiale and proximal to distal carpals II and to a small portion of distal carpal I. All three central bones are in articulation with the radiale. There is an empty space medial to the medialmost central bone, between the medial half of the proximal margin of distal carpal I and the distomedial corner of the radiale.
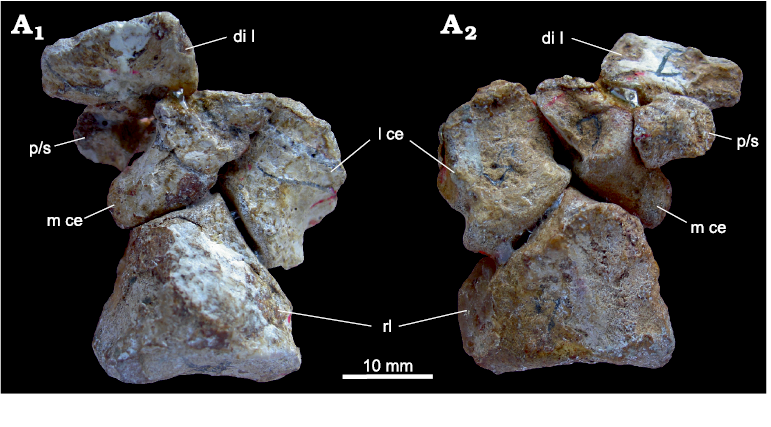
Fig. 9. Medial part of the carpus of Theriognathus microps Owen, 1876, NHMUK R 5694, Thaba ‘Nchu, South Africa, Cistecephalus Assemblage Zone, Lopingian, with three centralia. Dorsal (A1) and distoventral (A2) views. Abbreviations: int, intermedium; l ce, lateral centrale; m ce, medial centrale; rl, radiale.
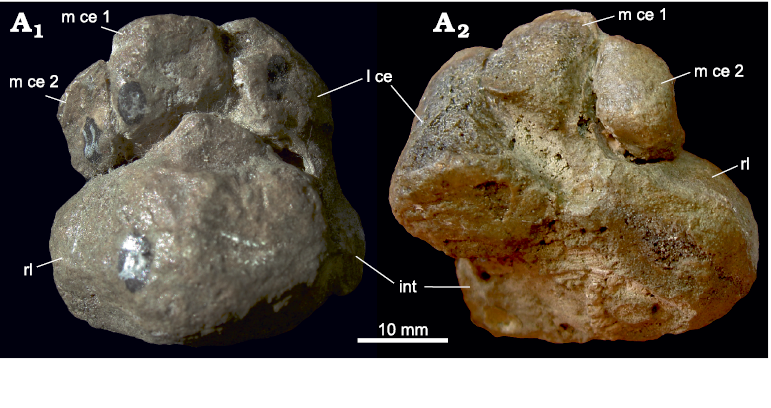
Fig. 10. Prepollex/sesamoid on the carpus of the biarmosuchian PIN 1758/320, Eshovo, Russia, Roadian. Dorsal (A1) and ventral (A2) views. Abbreviations: di I, distal carpal I; l ce, lateral centrale; m ce, medial centrale; rl, radiale; p/s, prepollex/sesamoid.
Prepollex/sesamoid: There are three central bones present in the biarmosuchian PIN 1758/320. As in Theriognathus NHMUK R 5694, the lateral centrale is in the usual position, between distal carpal III distally and the intermedium proximally. The position of the two medially situated central bones is, however, different from that in Theriognathus NHMUK R 5694. Adjacent to the lateral centrale is a normal medial centrale occupying the whole distal/distomedial facet of the radiale. It is distally connected to distal carpal II and the lateral part of the proximal margin of distal carpal I. The third central bone in PIN 1758/320 is oval with an approximate lateromedial orientation of its long axis. It does not articulate with the radiale, but lies on the junction of distal carpal I and the medial centrale, slightly ventral to both, so that its lateral and distolateral border underlies these carpal bones (Figs. 4A, 10). The medial section shows a free ending. In our view, this represents a prepollex/sesamoid, interpreted as additional preaxial bone (see SOM).
In many synapsid fossils, there is an open space proximal/proximomedial to distal carpal I. Usually only the proximolateral side of distal carpal I is articulated with the medial centrale (see section “Distal carpal I” below). The open space is at the position of the prepollex/sesamoid in the biarmosuchian PIN 1758/320, leaving open the possibility that this space was occupied by a cartilaginous prepollex/sesamoid in these species.
Loss or fusion of centralia: In the dicynodont Stahleckeria MCZ 1688, only one centrale is present (Fig. 5C). This centrale contacts the intermedium proximally and is placed distolateral to the radiale and medial to the ulnare. Distally, distolaterally and distomedially it is connected to distal carpal I, the central distal carpal (II or III) and distal carpal IV and can be unequivocally identified as the lateral centrale. The distal border of the radiale is connected to distal carpal I, leaving no space for the medial centrale.
In the gorgonopsian Arctognathus curvimola SAM-PK-3329, the centralia are fused to the radiale (Fig. 11). The fusion lines between the centralia and the radiale are clearly present and there are small set-back angles in the junctions between the different fused bones. In dorsal view, the centralia are proximodistally short, but widen ventrally (compare Fig. 11A1 and A2).
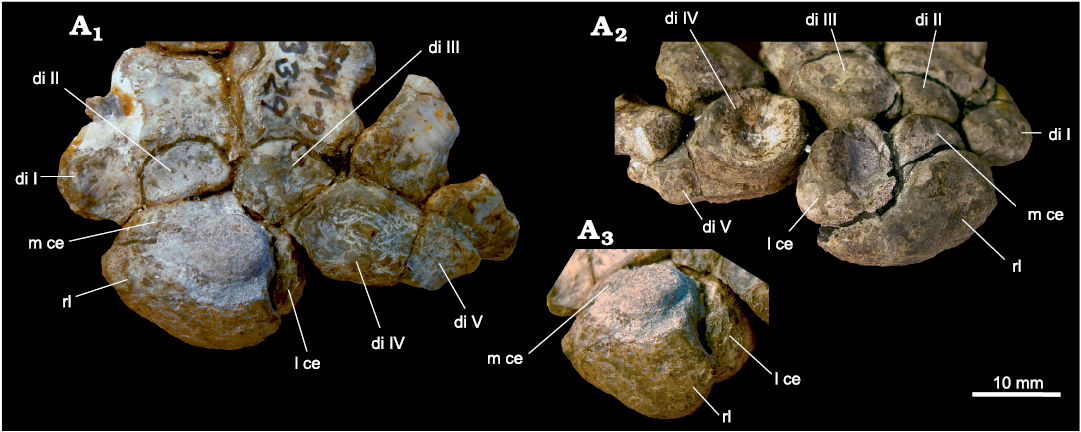
Fig. 11. Part of the carpus of Arctognathus curvimola (Owen, 1876), SAM-PK-3329, Oudeberg, Graaff-Reinet, South Africa, Cistecephalus Assemblage Zone, Lopingian. Dorsal (A1), ventral (A2), and dorsolateral (A3) views, showing the fusion lines between the radiale and the centralia. Abbreviations: di, distal carpal; l ce, lateral centrale; m ce, medial centrale; rl, radiale.
In an unidentified cynognathian cynodont BP/1/4534, only the lateral centrale is present as a separate bone (Fig. 7D). The medial centrale is probably fused to the radiale. This fusion is visible as a faint lateromedially orientated line of coalescence. The radiale (in probable fusion with the medial centrale), is approximately the same length as the ulnare. This is uncommon among non-mammaliamorph Cynodontia, where the ulnare is usually longer than the radiale (Fig. 7). This is an additional suggestion that the medial centrale is fused to the radiale in this specimen. In Thrinaxodon BP/1/1737, the medial centrale was also probably fused to the radiale. However, in Thrinaxodon BP/1/7199, the bones were seperate in both manus. So, the fusion in BP/1/1737 could represent an individual variant.
Distal carpal I: The first distal carpal is usually square, oval or rectangular, with a mostly mediolaterally orientated long axis (Figs. 3–7; SOM: table 7: a). In non-therapsid Synapsida and in the anomodont Galechirus, distal carpal I is aligned with the row of distal carpals. In some therapsids, however, it lies more distally, medial to the proximal portion of the row of metacarpals as in the dicynodonts Robertia and Diictodon (Fig. 5A; SOM: table 7: b). In most other non-mammaliamorph Synapsida, distal carpal I has an intermediate position. Its proximal portion is aligned with the row of distal carpals and the bone protrudes distally on the medial side of metacarpal II (SOM: table 7: b; Kümmell and Frey 2014b; Fontanarrosa et al. 2019). The distal carpal I connects the first ray to the carpus. Proximolaterally, or proximally, distal carpal I articulates with the distomedial border of the medial centrale (SOM: table 7: c, d). In Stahleckeria, where the medial centrale is absent, distal carpal I articulates with the radiale and lateral centrale instead. Proximomedially and sometimes also proximally, the bone is usually free of connections. There is an empty space between the junction of the distal carpal I and the medial centrale, often extending to the radiale in the carpi with articulated carpals of most non-mammaliamorph synapsids (SOM: table 7: e). In a few fossils, the contact area of distal carpal I to the medial centrale is very short and sometimes nearly absent as in some Gorgonopsia (Arctognathus, Aelurognathus, and gorgonopsian BP/1/1210; Figs. 6A, 11) and Therocephalia (Theriognathus, Ictidosuchoides CGS CM86-655, and Tetracynodon; Fig. 6C, D). In these cases, there is a large empty space proximal/proximomedial to the distal carpal I. In a few specimens, distal carpal I shows an extensive proximal contact to one or two carpal bones (e.g., Edaphosaurus, where the whole proximal side of the bone is articulated with the medial centrale or in the biarmosuchian PIN 1758/320 where it contacts the medial centrale and a prepollex/sesamoid).
Distal carpals II and III: Distal carpal II is usually the shortest of the first four distal carpals. However, in many non-therapsid Synapsida, distal carpal I is shorter than distal carpal II (Fig. 3A, C, E) and in a few Therapsida, distal carpal III is the shortest (e.g., Olivierosuchus BP/1/3973 and Tetracynodon AM 3677; SOM: table 8: a). Distal carpal II is usually oval to quadrangular or triangular (Figs. 3A–8A; SOM: table 8: b). It forms the base for the articulation of ray II with the carpus (SOM: table 8: c). Distal carpal III is mostly oval, sometimes quadrangular or triangular, or wedge shaped (SOM: table 9: a). It is associated with the third ray (SOM: table 9: b).
Generally, the carpometacarpal joints II–V form the carpometacarpal line, which is straight or slightly convex distally. In some specimens, however, the carpometacarpal line corresponding to the carpometacarpal joint II is slightly more proximal than the line through the lateral carpometacarpal joints, e.g., in Edaphosaurus, Galesaurus BP/1/2513 and most Gorgonopsia (Figs. 6A, 7C; SOM: table 8: d).
In Diictodon specimens, distal carpal II is usually absent and occasionally distal carpal III is also missing (Fig. 5A, Table 2), having an open space in the articulated carpi at the typical position of these bones, which suggests that these elements were present in cartilage. Distal carpal III is absent in the three smallest Diictodon specimens. Distal carpal II is either completely absent or only present as a very small bony nodule in two or three Diictodon specimens (Table 2). In Stahleckeria, only three distal carpals are visible in dorsal view, but in this case, there is no open space in any position (Fig. 5C). The second distal carpal of Stahleckeria is connected to the second ray and the medial side of ray III and the largest, third distal bone connects to rays III, IV and the medial side of V. We are unsure of the identity of the second element, which could be either distal carpal II or III. The position of this bone at the base of ray II and its partial contact with ray III suggests it is more likely distal carpal II. The size and position of the third distal bone suggest it is distal carpal IV. A possible fusion of distal carpal III to distal carpal IV cannot be excluded.
Table 2. State and shape of distal carpals II and III in different Diictodon specimens. The specimens are ordered (top to bottom) according to the increasing length of their skulls and/or their long bones.
|
Diictodon feliceps |
Distal carpal II |
Distal carpal III |
|
CGS FL186 |
absent |
absent |
|
TM4991 |
absent |
absent |
|
UMZC T 420 |
absent |
absent |
|
GPIT/RE/7193 |
very small nodule |
oval |
|
SAM-PK-K10699 |
absent |
oval |
|
CGS RMS214 |
very small nodule? |
oval |
|
CGS T72 |
absent |
oval |
|
SAM-PK-K10636 |
small oval nodule |
|
Distal carpals IV and V: Distal carpal IV is usually the longest distal carpal in non-therapsid synapsids and dinocephalians, whereas in dicynodonts, therocephalians and cynodonts, it is usually distal carpal I that is the longest (SOM: table 10: a). Distal carpal IV is pentagonal, especially in many non-therapsid synapsids and gorgonopsians. It is usually quadrangular or trapezoidal in biarmosuchians and anomodonts and mostly ovoid in therocephalians and non-mammaliamorph cynodonts (Figs. 3A–8A; SOM: table 10: b). Distal carpal V is small in relation to other carpals and is ovoid, triangular, quadrangular or trapezoidal in outline (SOM: table 10: c).
Five distal carpals is the plesiomorphic condition in synapsids, but in many non-mammaliamorph therapsids, distal carpal V is lost or fused to distal carpal IV, where a fusion line may be visible (SOM: table 10: d, e).
In “pelycosaurs”, distal carpals IV and V articulate with the fourth and fifth rays, respectively (SOM: table 10: f, g). In non-mammaliamorph therapsids, distal carpal IV sometimes extends laterally to articulate with ray V (SOM: table 10: f), especially when distal carpal V is fused to IV. In some specimens with distal carpal V, e.g., Thrinaxodon, a lateral extension of distal carpal IV also contacts metacarpal V.
The following states for distal carpal V can be distinguished: (i) Distal carpal V is a separate bone adjacent to distal carpal IV, e.g., in non-therapsid synapsids (Fig. 3), dinocephalians (Fig. 4B), anomodont Galechirus (Fig. 4C), and therocephalian Ictidosuchoides CGS CM86-655 (Fig. 6D). In a few cases, distal carpal V appears as a small nodule located within a space between metacarpal V and ulnare. This is the case in the gorgonopsian cf. Cynariops SAM-PK-K10000, the cynodonts Procynosuchus BP/1/591, NHMUK PV R 37054 (Fig. 7B; but not in RC92, Fig. 7A), Thrinaxodon and Diademodon NHMUK R-3581. (ii) Distal carpal V is not present in the fossil like in the smallest Diictodon CGS FL186 (Fig. 5A), therocephalians Tetracynodon AM 3677 (Fig. 6C) and Olivierosuchus BP/1/3973, cynodonts Galesaurus (Fig. 7C), cynognathian BP/1/4534 (Fig. 7D), Exaeretodon and Trucidocynodon (Fig. 8A, SOM: table 10: h), and there is an open space between metacarpal V and ulnare. (iii) Distal carpals IV and V are fused with a visible fusion line: gorgonopsians Arctognathus, Dinogorgon and the gorgonopsid BP/1/1210 (Fig. 6A and 11), Hipposaurus (see also Boonstra 1965), the biarmosuchian PIN 1758/320 (very faint line dorsally and an indentation denoting the fusion ventrally; see also Chudinov 1983) and therocephalians Theriognathus and Microgomphodon (SOM: table 10: e). (iv) Distal carpal V is absent or fused with no apparent fusion line and no space between metacarpal V and ulnare: in dicynodonts such as adult specimens of Diictodon, in Stahleckeria (Fig. 5C) and probably Cistecephalus, therocephalians Glanosuchus SAM-PK-K7809 and ?Ictidosuchoides BP/1/2294 and cynodont Procynosuchus RC92 (Fig. 7A). However, in Ictidosuchoides CGS CM86-655 and in Procynosuchus BP/1/591 and NHMUK PV R 37054, the distal carpal V is separated (SOM: table 10: d, e).
Mammaliamorpha.—In contrast to the non-mammaliamorph Synapsida, the carpus of Mammaliamorpha is short and more compact in relation to the whole manus. In non-mammaliamorph cynodonts, the ratio of the carpus to the whole manus (measured as carpus + ray III) is approximately 1:3. In tritylodontids it is about 1:4 and in Mesozoic mammaliaforms it is about 1:6–8. This reduction in relative size of the carpus is mainly due to a shortening of the ulnare and the loss of one bone of the proximal or central row and a concomitant elongation of metacarpals and phalanges.
Radiale: The radiale is triangular to rectangular in outline in the tritylodontid Bienotheroides and the tritylodontid WCW-06A-34, with its apex pointing distomedially and similar, but more rectangular in Jeholodens (Fig. 8B–D). It is trapezoid in Kayentatherium and short and rectangular in Zhangheotherium and Eomaia (SOM: table 1: a). In contrast to non-mammaliamorph synapsids, where the proximal side of the radiale contacts the entire distal end of the radius, the mammaliamorph radiale contacts the medial half of the distal facet of the radius (Fig. 8B–D; SOM: table 1: b). The distal or distolateral border of the radiale articulates with the single centrale (SOM: table 1: d, e). The radiale is fused to the centrale in Bienotheroides, with a fusion line present between the two bones and a wedge-shaped angle (set-back angle) between the outline of both bones (Fig. 8B). Laterally, the radiale contacts the lunate (SOM: table 1: f). In contrast to most non-mammaliamorph synapsids, there is no empty space between the radiale and distal carpal I, except in Kayentatherium (SOM: table 7: e). In the tritylodontid WCW-06A-34 and Bienotheroides, the distomedial top of the triangular radiale forms a short process, which articulates slightly with the proximomedial end of distal carpal I (Fig. 8B, C). In Zhangheotherium, however, a small nodular bone interpreted as a probable fragment of a prepollex/sesamoid (Hu et al. 1998), intercalates between the distomedial border of the radiale and distal carpal I (Fig. 12; SOM: table 1: c).
Ulnare: The mammaliamorph ulnare is pentagonal (tritylodontid WCW-06A-34), rectangular (Bienotheroides) or irregularly triangular (Zhangheotherium; Figs. 8B, C, 12; SOM: table 3: a). In relation to metacarpal III, it is short compared with the ulnare of non-mammaliamorph cynodonts (ulnare length as a percentage of the length of metacarpal III: non-mammaliamorph cynodonts 70–111% (except cynognathian BP/1/4534 with 57%), tritylodontids 60–62% and Mesozoic mammals 18–29%; SOM: table 3: b). In Zhangheotherium, the ulnare is wider than long (Fig. 12). Proximally, the ulnare is articulated with the ulna (SOM: table 3: g). Medially, it contacts the lunate and in the tritylodontid Bienotheroides, the unidentified tritylodontid WCW-06A-34 and Zhangheotherium, it also contacts the uppermost end of the lateral side of the radius (SOM: table 3: c). It receives distal carpal IV distomedially or distally (SOM: table 3: d, e). Distolaterally there is an open space between metacarpal V, distal carpal IV and the ulnare, and only in Zhangheotherium, the ulnare probably articulates with metacarpal V (SOM: table 3: f).
Pisiform: The pisiform is sickle-shaped in Bienotheroides, subcircular to oval in Jeholodens and proximodistally rectangular in Zhangheotherium (Figs. 8B, D, 12; SOM: table 4: a). It is relatively long in mammaliamorphs. The pisiform is usually fossilised on the lateral/proximolateral border of the ulnare, but in Zhangheotherium, it lies mainly lateral to the ulna and articulates with the proximal to proximolateral side of the ulnare (Fig. 12; SOM: table 4: b).
Lunate: The lunate is square to sub-oval, subcircular or triangular (Figs. 8B–D, 12; SOM: table 5: a) and is longer than the radiale (SOM: table 5: b). The central constriction of the lunate in Bienotheroides is unique in this species. It is prominent and compact in the tritylodontid WCW-06A-34. The lunate articulates distally with distal carpal III, distomedially with the centrale and distolaterally with distal carpal IV (SOM: table 5: c–e). These contacts are identical to those of the lateral centrale in non-mammaliamorph cynodonts, assuming that the single centrale of mammaliamorphs is homologous with the medial centrale of non-mammaliamorph cynodonts. Between the lunate and distal carpal III, a (small) open space is found in the tritylodontids Bienotheroides and Kayentatherium, not present in the tritylodontid WCW-06A-34 (Fig. 8B, C). A similar space is also visible distal to the lateral centrale in the cynodonts Diademodon NHMUK R-3581 and Galesaurus SAM-PK-K10465. In Diademodon USNM 23352 it is very small and is absent in Galesaurus BP/1/2513 (Fig. 7C), probably becoming obscured by the further growth of the bones. Proximally, the lunate articulates with the lateral portion of the distal facet of the radius (SOM: table 1: b).
Centrale: The centrale is irregularly oval or pentagonal (Figs. 8B–D, 12; SOM: table 6: a). Distally, distomedially and -laterally, it contacts distal carpals II, I, and III (SOM: table 6: e). It contacts the radiale proximally and the lunate proximolaterally or laterally (SOM: table 6: b–d). In Kayentatherium TMM 43690-5.136, distal carpal II is lost or fused to distal carpal III and the centrale is articulated with distal carpal I and the medial part of the centrally located distal carpal. In Jeholodens, the interpretation is difficult, because the presumed distal carpal II, a small bone proximal to metacarpal II, has indistinct edges. A bone approximately the size of distal carpal III lies proximal to this inconspicuous bone, which we interpret here as the centrale (Fig. 8D).
Prepollex/sesamoid: A small nodular bone lies medial to the centrale in Zhangheotherium and intercalates between the distomedialmost border of the radiale and the proximal part of distal carpal I (Fig. 12). This is likely to be a fragment of a prepollex/sesamoid, as proposed by Hu et al. (1998).
Distal carpal I: Distal carpal I is the longest of the distal carpals in the tritylodontid WCW-06A-34 and in Bienotheroides (Fig. 8B, C), whereas in Kayentatherium TMM 43690-5.136 and Zhangheotherium it is about the same length to distal carpal IV (Fig. 12; SOM: table 10: a). It is square to subcircular in most specimens and rectangular with a proximodistal orientation in Eomaia (SOM: table 7: a). In Jeholodens, the bone proximal to metacarpal I is damaged and was interpreted as two bones, a distal carpal I and a centrale in Kümmell and Frey (2014b). Here we interpret this bone as a broken distal carpal I in accordance with Ji et al. (2002). A centrale at this position is unlikely because the position of the (medial) centrale in fossil synapsids is very conserved (Fig. 8D). In the tritylodontids WCW-06A-34, Bienotheroides and Kayentatherium TMM 43690-5.136, distal carpal I extends from the row of distal carpals into the row of metacarpals. In the other mammaliamorphs, distal carpal I is situated at the medial end of the row of distal carpals (Fig. 8B, C; SOM: table 7: b). Note that in Mesozoic mammals, the carpometacarpal joint II is shifted slightly proximally (see below), and the distal carpal I is in line with distal carpals III–V, even when distal carpal I is slightly displaced distally compared to ray II (Figs. 8D, 12).
Proximally, distal carpal I contacts the radiale in most mammaliamorph species, but connects to a small questionable prepollex/sesamoid in Zhangheotherium. In Kayentatherium, distal carpal I shows an open space proximally as most non-mammaliamorph synapsids (Fig. 12; SOM: table 7: c, e).
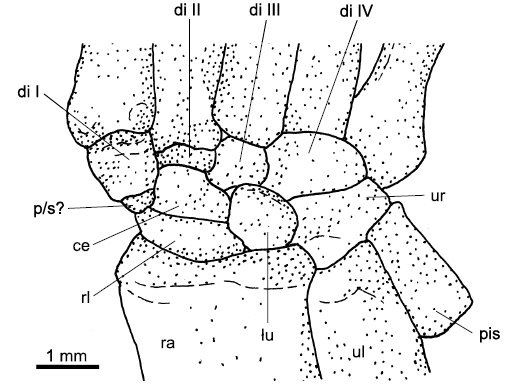
Fig. 12. Carpus of Zhangheotherium quinquecuspidens Hu, Wang, Luo, and Li, 1997, IVPP V7466, Jianshangou Valley, Liaoning Province, China, Barremian, right carpus, dorsal view. Abbreviations: di, distal carpal; ce, centrale; lu, lunate; p/s, prepollex/sesamoid, probably a fragment; pis, pisiform; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
Distal carpals II and III: Distal carpal II is the shortest of the four distal carpals. It is an oval bone articulating with metacarpal II (Figs. 8B–D, 12; SOM: table 8: b, c). Distal carpal III is also mostly oval, but triangular in Bienotheroides (SOM: table 9: a). It articulates with metacarpal III (SOM: table 9: b). The carpometacarpal joint II is shifted proximally in Mesozoic mammals (compared to tritylodontids and most non-mammaliamorph cynodonts), so that the carpometacarpal line curves slightly proximally at the position of ray II (Figs. 8D, 12; SOM: table 8: d). In Kayentatherium TMM 43690-5.136, only three distal carpals are present. We identified the medial-most distal carpal as distal carpal I and the lateral-most as distal carpal IV. The middle distal carpal is probably slightly turned and is interpreted as distal carpal III because of its size. However, it could also be a fusion of distal carpals II and III. The very small bony structure proximal to metacarpal II of Jeholodens is interpreted here as distal carpal II (Fig. 8D).
Distal carpal IV: Distal carpal IV is variable in shape: pentagonal, oval or irregularly triangular (Figs. 8B–D, 12; SOM: table 10: b). It articulates with metacarpal IV and usually with the medial part of metacarpal V. In Zhangheotherium, it also articulates with the lateral part of metacarpal III (SOM: table 10: f). Between metacarpal V and the ulnare is an open space in the tritylodontids WCW-06A-34 and Bienotheroides and also in the eutriconodont Jeholodens. There is a small space between distal carpal IV and ulnare in Eomaia (SOM: tables 3: f, 10: h).
Discussion
This large-scale review of synapsid carpals shows that the position and contacts of single carpal bones are relatively conserved from the Permian to Late Cretaceous, with few exceptions. The different carpal elements usually maintain their positions, with only slight changes in the length or width of individual bones. In the evolution of carpals, not only position and contacts are conserved, but also the relative sizes and width to length proportions of bones. However, the general outlines of the carpal elements are rather variable, with the exception of the mainly rectangular ulnare. Here we assessed homologies according to bone element position, contacts, sequential order and relative size.
Besides the morphological variations of carpal bones related to different locomotory modes, other reasons for shape variation are changes during ontogenetic development (Luo et al. 2003; Stafford and Thorington 1998). Changes in the outline of the carpals do not appear only in early ontogeny, but can develop later, even between subadult and early adult stages, because carpal ossification can occur right up to the latest part of the growing phase (Nesslinger 1956; Oliveira et al. 1998; Stafford and Thorington 1998; Prochel and Sánchez-Villagra 2003; Gilsanz and Ratib 2005; Fröbisch 2008; Wilson et al. 2010). As a general rule, suboval to round shapes occur in the early ontogeny of carpals, whereas complex shapes are found in the mature stages. When other elements of the carpus have a complex outline, round to oval shapes of individual carpal bones suggest a delayed ossification of these elements.
In fossil specimens, fusion lines are important indicators of ontogenetic carpal bone coalescence, whereas open spaces between elements can suggest the persistence of cartilaginous precursors of the bones, which never ossified. However, spaces can also arise from taphonomic distortion of the original bone contacts, e.g., by flattening of the transverse arch during fossilisation.
There are also instances of sudden changes in carpal position and relative size during synapsid evolution, identified using the traditional homology of carpal bones. An example of this is the dimensional change of the intermedium in the transition from “pelycosaurs” to therapsids and from non-mammaliamorph cynodonts to mammaliamorphs. These sudden changes can arise for several reasons: e.g., a gap in the fossil record or in the sampling used in this study or an inaccurate homologization of the specific carpal bone in the groups before and after the change.
There are significant gaps in the fossil record of synapsids preserving a complete carpus. Besides the poorly sampled “pelycosaurs”, an extensive information gap is found between basal Sphenacodontidae and the first appearance of therapsids, which cover a time span of around 30 Ma, during the Cisuralian and early Roadian (Sidor and Hopson 1998; Abdala et al. 2008). There is another temporal information gap of about 10 Ma between the first record of Therocephalia and Cynodontia from Wordian to Wuchiapingian (Fig. 2A; Sidor and Hopson 1998). A notable information gap in our sample is present before the emergence of mammaliamorphs (about 30 Ma, during the Late Triassic; Fig. 2A, B). These information gaps have to be considered when discussing the sudden changes in bone position, contacts, shape and relative size during carpal evolution.
Position and evolution of radiale and ulnare and the stiffness of the digger’s carpus.—The radiale and ulnare are easily recognized in non-mammaliamorph synapsids, because the radiale occupies the entire distal facet of the radius, and the ulnare articulates with the ulna. The connections of the radiale to the two centralia and the connection of the ulnare to distal carpal IV are both highly conserved. There is a trend towards a shortening of the ulnare in relation to the more elongated metapodium, which emerged in basal mammaliamorphs (tritylodontids) and became even more pronounced in Mesozoic mammals. The mammaliamorph radiale is narrower in relation to the width of the distal radial facet than in non-mammaliamorph synapsids. It occupies only the medial half of the distal facet of the radius, whereas the lateral half of the distal facet of the radius contacts the lunate. Distally, besides the connection of the radiale to the single centrale of mammaliamorphs, a new connection of the mammaliamorph radiale to distal carpal I appeared in several specimens of our sample (tritylodontid WCW-06A-34, Bienotheroides, probably Jeholodens) and some other Mesozoic mammaliaforms such as the docodont Agilodocodon and the haramiyid Shenshou lui (Bi et al. 2014; Meng et al. 2015).
In some specimens, the radiale is fused to the centralia. In the gorgonopsian Arctognathus curvimola, both centralia are fused to the radiale. Arctognathus curvimola SAM-PK-3329 is interpreted as a highly fossorial animal (Kümmell 2009). This can be deduced from its putative long ungual phalanges in relation to the whole digital length and the compact, stiff carpus (Fig. 11; the tips of the ungual phalanges are broken and their lengths were estimated). Further hints for a digging lifestyle in this species are the short and stout metacarpals and basal and middle phalanges. In addition, the strong basal and middle joints of the digits show near quadrangular facets with low mobility ranges. According to these features, the manus of Arctognathus curvimola is midway between that of the scratch diggers Vombatus and Lasiorhinus and those of the shovel diggers Talpa and Tachyglossus (Kümmell 2009). Hildebrand and Goslow (2004) suggest that in diggers the carpus is protected against dislocations of the single carpals either by a structural unity of the bones or by the presence of very strong ligaments. In Talpa, for example, radiale, lunate and ulnare, though unfused, are tightly bound, so that there is no mobility between them (Yalden 1966). In Arctognathus curvimola the structural unity of the carpus is evident, not only from the fusion of the centralia to the radiale, but also from the compact arrangement of the carpal bones and the very close contact of the distal carpals with the corresponding metacarpals (Fig. 11).
In the undescribed cynognathian cynodont BP/1/4534, the medial centrale probably fused to the radiale as well (Fig. 7D). In contrast to Arctognathus, where the structure of the manus suggests that it was an equipped digger, the manual struture of BP/1/4534 suggests it was mainly terrestrial, but could dig sporadically. The length to width-index of the basal phalanx IV was similar (after size corrections) to that of the scratch digger cynodonts Procynosuchus and Chiniquodon and lower (that means more robust) than that of the extant scratch digger gerbil rodent Meriones (Kümmell 2009). Scratch digging therefore, appears to have been possible for the cynognathian BP/1/4534. The fusion of the medial centrale and the radiale would have stabilized the carpus during digging, as in Arctognathus. Other Permo-Triassic therapsids may have strengthen their carpus by ligaments rather than by bone fusion, because they show unfused carpals (e.g., Procynosuchus and Diictodon; Kümmell 2009; Kümmell and Frey 2012).
Fusion of the radiale to other carpal bones also occurred in mammaliamorphs: a fusion to the centrale in the tritylodontid Bienotheroides and to the lunate in the zalambdalestid Barunlestes (Kielan-Jaworowska 1978). It is likely that Bienotheroides was also capable of scratch digging. The digits are not well preserved; however, the ossified olecranon process of the ulna is very long, 40% of the distal segment of the ulna and the deltopectoral crest of the humerus is prominent and long. These features are thought to be associated with digging abilities in the tritylodontid Kayentatherium (Sues and Jenkins 2006).
Position of the pisiform and its probable sesamoid identity.—The location of the pisiform in a position approximately distolateral of the ulna and proximolateral of the ulnare is stable throughout fossil synapsids. Slight variations or dislocations are present, so the pisiform is occasionally fossilised just laterally to the ulna or ulnare or (partly) ventrally. The pisiform is not strongly interconnected with other carpal bones, and articulates with the ulna and/or ulnare with short, simple articular surfaces. It is free of contacts laterally, distally and proximally. The contacts to the carpus and its positioning close to the joint between ulna and ulnare, makes the pisiform easy to identify.
The pisiform is often absent in fossil synapsids. However, it is known from specimens of every major lineage of synapsids. Because of this, we consider its frequent absence as taphonomic, arising from the minimal intercalation of the pisiform in the structure of the carpus.
In most placentals for which data are available as well as in the marsupials Didelphis and Monodelphis (Prochel and Sánchez-Villagra 2003), the pisiform together with the prepollex/sesamoid are the last carpals to ossify during ontogeny. The late onset of ossification and the minimal contact to the rest of the carpals support the hypothesis that the pisiform is a sesamoid, embedded in the tendon of m. flexor carpi ulnaris (Haines 1969; Fabrezi et al. 2007; Fontanarrosa and Abdala 2014, 2016; Amador et al. 2018). Other authors argue for its nature as a true carpal bone (Gillies 1929; Kivell 2016; Diaz and Trainor 2015; Kjosness et al. 2014; Reno et al. 2016; see SOM for further information).
Because of its minimal contacts to the other carpal bones and its free endings distally, laterally and proximally, the synapsid fossil record suggests a sesamoid identity for the pisiform.
The homology of the mammaliamorph centrale.—In nearly all non-mammaliamorph synapsids, the medial centrale is articulated distally, distomedially, and distolaterally with distal carpals II and I, often also with distal carpal III. It contacts the radiale proximally or proximolaterally. In some cases it is fused to the radiale (see above). The same connections, with distal carpals I, II, and III and proximally with the radiale, are observed in the single centrale of mammaliamorphs. Because of the relative position towards the medial side of the manus and its anatomical contacts, we interpret the single centrale of mammaliamorphs as homologous to the medial centrale of non-mammaliamorph synapsids.
The mammaliamorph centrale is absent in Monotremata and Marsupialia (Flower 1885; Holmgren 1952; Grassé 1955; Szalay 1994; Flores and Diaz 2009). It is also reported to be lost in some basal eutherians such as Ambolestes and Sinodelphys (Sinodelphys was originally interpreted as metatherian by Luo et al. 2003, but most recently as eutherian by Bi et al. 2018). It is likely that the centrale persisted in the stem lineage of Mesozoic Eutheria (as in Barunlestes and probably Asioryctes; Kielan-Jaworowska et al. 2004: figs. 13.15 and 13.12), because it appears in many extant placentals, e.g., in Tupaia (e.g., Schmidt-Ehrenberg 1942; Stafford and Thorington 1998).
The question of the lunate homology.—Palaeontological and morphological evidence: In previous anatomical work and textbooks, the intermedium of reptiles and non-mammaliamorph synapsids is homologized with the lunate of mammals (Fig. 13I, A2–D2; e.g., Gegenbaur 1864; Broom 1901; Ihle et al. 1927; Romer and Parsons 1977; Starck 1979; Salomon et al. 2005; Kivell 2016). Also, Sun and Li (1985) in their description of the basal mammaliamorph tritylodontid Bienotheroides designated the bone in the position of the lunate as an intermedium. We argue for the homology of the mammaliamorph lunate with the lateral centrale of non-mammaliamorph synapsids (Fig. 13II, A2–D3) for the following two reasons. (i) Position: The mammaliamorph lunate articulates distally with the distal carpal III, distolaterally with the distal carpal IV and distomedially with the centrale. It contacts the radiale medially and the ulnare laterally. These contacts are identical to those of the lateral centrale in non-mammaliamorph cynodonts (SOM: tables 1: f, 3: c, 5: c–e). Proximally, the lunate contacts the lateral half of the distal articular surface of the radius. This contact to the radius resembles neither the proximal contact of the lateral centrale, which articulates with the intermedium, nor that of the intermedium, which articulates proximally with the medial section of the distal ulnar facet. So, the articulation of the lunate with the radius is an apomorphy of mammaliamorphs. Thus, in position and contacts, the lunate of mammaliamorphs resembles the lateral centrale of non-mammaliamorph cynodonts more than their intermedium (Fig. 13II, A2–D3). (ii) In terms of relative size, the mammaliamorph lunate resembles the lateral centrale of non-mammaliamorph cynodonts, as well. The lunate is longer than the corresponding radiale and relatively wide. That is also the case for the lateral centrale and radiale of non-mammaliamorph cynodonts (except the most basal form Procynosuchus), whereas the intermedium of non-mammaliamorph cynodonts is very slender, and the same length or shorter than the corresponding radiale (Fig. 13, SOM: tables 2: e, 5: b).
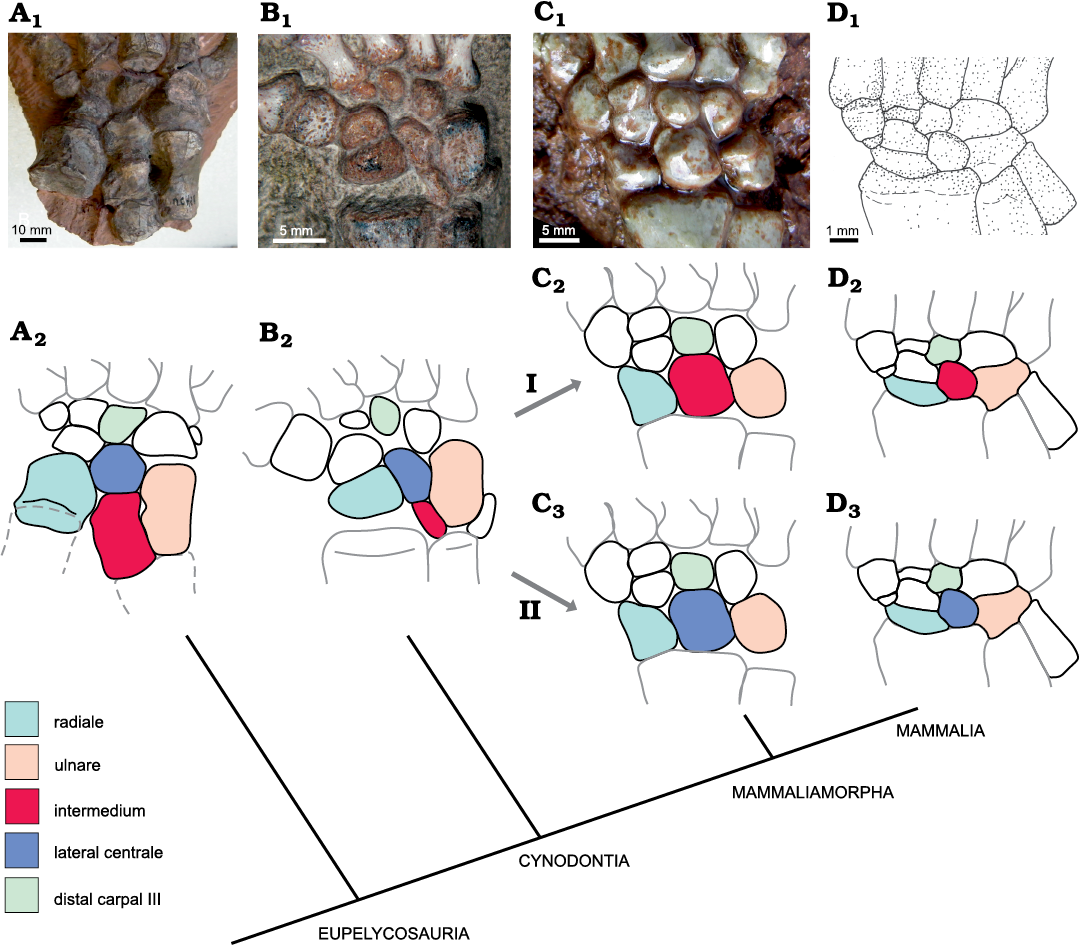
Fig. 13. Homologization of the mammaliamorph lunate with the intermedium or lateral centrale of non-mammaliamorph synapsids. I. The homologue of the lunate is the intermedium (C2, D2); this is the traditional view (Gegenbaur 1864; Romer and Parsons 1977; Kivell 2016) and was put forward by Broom (1901) for non-mammaliamorph therapsids and extant mammals. II. The homologue of the lunate is the lateral centrale (C3, D3) as suggested here. A. Ophiacodon (FMNH UC 458). B. Galesaurus (SAM-PK-K10465). C. Tritylodontid (WCW-06A-34). D. Zhangheotherium (IVPP V7466). Photographs (A1–C1), drawing (D1), and explanatory drawings (A2–D2, C3, D3). The figures show the right manus in dorsal view, except for A, where the left manus has been lateromedially reversed.
There are two arguments that can be brought against our proposal, which should be discussed here. First, one can argue that during the transition from “pelycosaurs” to therapsids, the intermedium altered significantly in form and size (Fig. 13). This would suggest considerable evolutionary plasticity, which could also account for the proposed changes in form at the transition from non-mammaliamorph cynodonts to mammaliamorphs. In our data set, there are temporal information gaps in both transitions: from non-therapsid synapsids to therapsids and from non-mammaliamorph cynodonts to mammaliamorphs (Fig. 2A, B; see above). This leaves open the possibility that large evolutionary changes could have taken place during that time. However, in the transition from non-therapsid synapsids to therapsids, the position of the intermedium and its contacts did not change, but the relative width and dorsoventral depth did change. In non-therapsid synapsids, it is mostly broad, square or pentagonal (Fig. 3A–C) except in two Ophiacodon specimens (FMNH UC 671 and FMNH UC 458), where it is longer than in other non-therapsid synapsids (Figs. 3A–C, 13). In therapsids, the intermedium is considerably narrower in dorsal view. The intermedium is dorsoventrally shallow in non-therapsids and deep in therapsids. Because the anatomical position and the relevant contacts remain the same, we propose that the intermedia in both groups are homologous. The change in proportions of the intermedium may be related to the slight rotation of the elbow posteriorly, producing a semi-sprawled posture on the transition to therapsids, which altered the geometry of the wrist (Colbert 1948; Jenkins 1971). However, in the transition from non-mammaliamorph cynodonts to mammaliamorphs, the situation would be different, if the lunate derived from the intermedium, as suggested previously. In this case, the intermedium of non-mammaliamorph cynodonts would not only have changed in relative size and form, but also in position. Such a transformation is unlikely, especially given the fact that this shift must have occurred in the midst of tight anatomical contacts of the carpal bones. Thus, we argue that the intermedium was lost during the transition from non-mammaliamorph synapsids to mammaliamorphs or otherwise fused to the lateral centrale or another carpal or zeugopodial bone (see below) and that the lunate is the homologue of the lateral centrale.
Secondly, Kayentatherium MCZ 8812, which belongs to Tritylodontidae, one of the most basal mammaliamorph clades, appears to contradict our proposal at first glance. Sues and Jenkins (2006) described two centralia and one intermedium for each of the partly articulated right and left manus of the specimen. With its square outline, the bone designated as intermedium resembles the mammaliamorph lunate rather than the non-mammaliamorph intermedium. Because the tritylodontid Kayentatherium is one of the earliest mammaliamorphs, the situation in this fossil could indicate that the intermedium changed its form and relative width prior to the evolutionary loss of one carpal bone. In this case, the lunate of mammaliamorphs would be homologous to the non-mammaliamorph synapsid intermedium. But the presence of two centralia and one intermedium in each manus of Kayentatherium MCZ 8812 is questionable, because both intermedia are out of place. Another Kayentatherium TMM 43690-5.136 was recently described, which show a carpus fossilised in articulation (Hoffman and Rowe 2018). In this specimen, two centralia or one centrale and one lunate, respectively, are present with no intermedium (according to supplement video 1 in Hoffman and Rowe 2018, and images of scans sent by Eva Hoffman to SK). This articulated carpus of Kayentatherium resembles the carpi of the tritylodontid WCW-06A-34 and Bienotheroides (Figs. 8B, C, 13). In Kayentatherium TMM 43690-5.136, the distal carpal I is comparatively large and resembles the previously designated intermedium in Kayentatherium MCZ 8812 (Sues and Jenkins 2006). If the latter were to be distal carpal I, the situation in Kayentatherium would not be different from that in other mammaliamorphs. A thorough comparison of the two specimens of Kayentatherium is necessary to solve the identity of the bones completely.
Embryological evidence: An investigation of the early ontogeny of extant mammals may shed light on the question of lunate identity and help to identify the bones that were lost in the transition to mammaliamorphs. Despite differing views on lunate identity (intermedium versus centrale), there is general agreement that a mesenchyme string (intermedial string; sensu Schmidt-Ehrenberg 1942) forms in early mammalian ontogeny, which detaches from the ulna, and gives rise to the lunate (Fig. 14A, B; Steiner 1935; Schmidt-Ehrenberg 1942; Holmgren 1933, 1952; Čihák 1972; Shubin and Alberch 1986). While Steiner (1942), Schmidt-Ehrenberg (1942), and Shubin and Alberch (1986) interpret the lunate as homologous to the intermedium, Holmgren (1933, 1952), Kindahl (1941, 1942a, b, 1944), Čihák (1972) and Slabý (1967, 1968) interpret parts of the intermedial mesenchyme string as the homologue of the intermedium and the lunate as homologous to a centrale.
In some mammals (Tupaia, Elephantulus, Tarsius, among many others), Holmgren (1933, 1952) and Kindahl (1942b) observed the chondrification of the mesenchymal remnant (intermedium anlage) of the connection of the lunate anlage to the ulna. This chondrified remnant, interpreted as the intermedium, can persist in these species even when the precursor of the lunate chondrifies (Fig. 14B, C). Holmgren (1952) described that in later ontogenetic stages of mammalian embryos, the intermedium fused to other chondrogenic anlagen: usually to the ulnare (= centrale 4 of Holmgren), sometimes to the lunate (= centrale 1 of Holmgren), and very occasionally to the ulna or the radiale.
The mesenchymal connection between the distomedial corner of the ulna and the lunate, distal to the radius, is the secondary digital arch (sensu Shubin and Alberch 1986), along which the lunate and centrale develop. The proximal part of this axis resembles the intermedium of non-mammaliamorph synapsids in shape and position (Fig. 14) and may thus represent the homologue of this bone. In mammaliamorphs, it is plausible that the anlage of the intermedium persists through early ontogeny, even though the intermedium in the adult stage is lost. This is because the anlage constitutes the developmental axis of the lunate and centrale in early development.
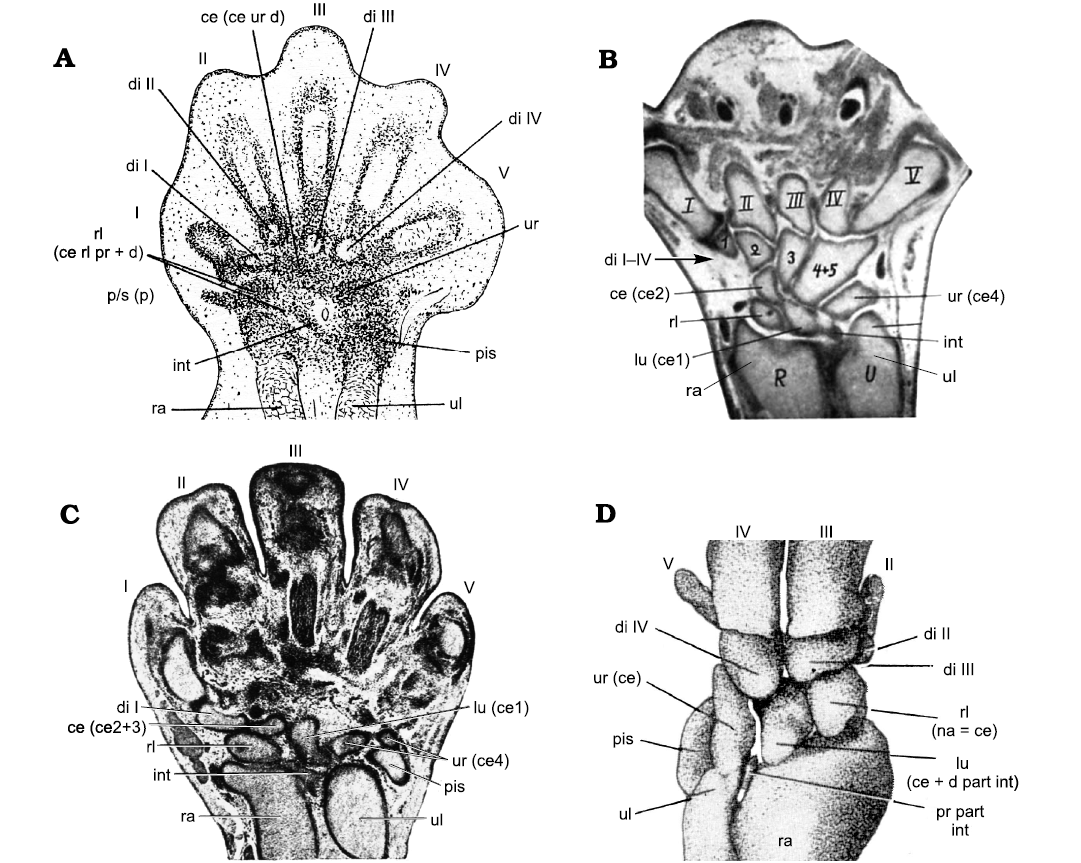
Fig. 14. Interpretation of the cartilaginous anlagen of the embryological carpus of different mammals by different authors. Whenever the radiale, ulnare or lunate have been interpreted previously as homologues of a centrale, the details are given in brackets following the abbreviations in the figures. A. Microcebus myoxinus Peters, 1852, 10.2 mm neck-rump length (from Schmidt-Ehrenberg 1942: 61, fig. 10, courtesy of Revue Suisse de Zoologie). B. Tarsius spectrumgurskyae Shekelle, Groves, Maryanto, and Mittermeier, 2017, 20.5 mm body length, (from Holmgren 1952: 80, fig. 93, reproduced with permission of Acta Zoologica, © The Royal Swedish Academy of Sciences, all rights reserved). C. Talpa europaea Linnaeus, 1758, 15 mm body length (from Kindahl 1942b: 270, fig. 4, courtesy of Sächsisches Staatsarchiv, Staatsarchiv Leipzig). D. Ovis aries Linnaeus, 1758, 38 mm body length, three-dimensional reconstruction of the left carpus in dorsal aspect (from Slabý 1967: pl. 6: 30). Abbreviations: ce, centrale; d, distal; di, distal carpal; int, intermedium; lu, lunate; na, naviculare; p, prepollex; p/s, prepollex/sesamoid; pis, pisiform; pr, proximal; ra, radius; rl, radiale; ul, ulna; ur, ulnare.
The embryological studies of Holmgren (1952), Kindahl (1942b), Čihák (1972), and Slabý (1976) present strong evidence that the lunate of mammaliamorphs is best homologized with a centrale of non-mammaliamorph synapsids. Mammaliamorphs thus resemble modern tetrapod taxa such as lizards, crocodiles, and anurans, in which the intermedium is frequently absent in the adult stage (Fabrezi and Alberch 1996; Fabrezi and Barg 2001; Fabrezi et al. 2007). The intermedium may appear as an isolated element in early ontogeny but can also become fused to the radiale in later stages, as in Crocodylia (Müller and Alberch 1990; Müller 1991).
Using the evidence derived from our paleontological investigations and the embryological studies of those mammals, in which both the lunate and the intermedium anlage chondrified, we homologized the mammaliamorph lunate with the lateral centrale of non-mammaliamorph synapsids. Thus, the mammaliamorph lunate should be called “lateral centrale” and the single centrale of mammaliamorphs would be the “medial centrale”. For traditional reasons and considering the uncertainty surrounding a probable early ontogenic fusion of the intermedium to the lateral centrale of mammaliamorphs, we argue for the continued use of the term “lunate” and “centrale” in mammaliamorphs.
Third centrale—an abnormal duplication of one centrale?—Three centralia have been described in the therocephalian Theriognathus NHMUK R 5694 (Boonstra 1934: 260, fig. 34). The two medial bones are in the same position as the medial centrale of many Therapsida. The bones are tightly connected and were previously interpreted as fused (Boonstra 1934). Our study shows they are separate bones, with tight fitting articular surfaces (Fig. 9). We also interpret the three elements as centralia in accordance with Boonstra (1934).
Angielczyk and Rubidge (2013) tentatively identified three centralia in the right carpus of the dicynodont Eosimops BP/1/6674, lying lateral and distolateral of the radiale. We interpret the proximalmost of these three bones as the lateral centrale. The medial centrale is, in our opinion, in its normal location distal to the radiale (an element interpreted as the distal carpal II by Angielczyk and Rubidge 2013). The bones of the distal row of carpals are displaced from their natural position and the two distally located bones, interpreted by Angielczyk and Rubidge (2013) as centralia, could also be interpreted as distal elements out of place. The left carpus of the same specimen, however, is fossilised in articulation with only some disturbance at the ulnare. Here, the lateral and medial centralia are present in their usual position. The form, size and proximal articulation of the medial centrale in Eosimops is typical for Dicynodontia (Fig. 5B, SOM: table 6: a, b).
A third centrale could arise from “de novo” duplication of an early ontogenetic cartilaginous condensation. Such a case is known from an abnormal duplication of centrale 3 in the turtle Phrynops hilarii (Fabrezi et al. 2009). “De novo” duplication probably occurred in the condensation of the medial centrale in Theriognathus NHMUK R 5694, because the two medial centralia occupy the same position as the medial centrale of the other non-mammaliamorph synaspids.
Has there been a prepollex/sesamoid in synapsids?—Prepollices occur in a wide range of tetrapods. They were shown to be present in anurans as a complex of skeletal elements (Fabrezi 2001). They occasionally appear in reptiles (Wagner et al. 2000), birds (Starck 1979) and frequently in mammals (Salesa et al. 2006; Echeverria et al. 2019). However, in many cases the so-called prepollex of mammals was identified as a radial sesamoid (see SOM), so here we designate the prepollex of mammals as “prepollex/sesamoid”. A prepollex/sesamoid in extant mammals usually differs from most carpals in being incompletely integrated in the carpus. This bone is often situated at the medial border of the carpus, between radiale and distal carpal I (scaphoid and trapezium) oriented towards the palmar surface, with a free ending pointing medially or distally (Salton and Sargis 2008).
Previously, the three central bones of the Russian biarmosuchian PIN 1758/320 were interpreted as centralia (Chudinov 1983: 55–56). Two of these bones occupy the position of the medial and lateral centrale, whereas the medialmost third central bone lies in the junction of distal carpal I and the medial centrale, in a slightly ventral position. The medial half of this bone shows a free medial margin. This position and orientation do not support the interpretation as a third centrale and is more consistent with the interpretation of a prepollex/sesamoid instead.
Other non-mammaliamorph synapsids show small bones of uncertain identity close to distal carpal I. These include Chiniquodon PVL 3820, Galesaurus SAM-PK-K10468 (Kümmell and Frey 2014b), Suminia 2212/62 (Fröbisch and Reisz 2011: fig. 9), “Opisthoctenodon agilis” (Broom 1904; most likely Pristerodon; Keyser 1993, Angielczyk et al. 2005) and Theriodesmus NHMUK 49392 (probably a biarmosuchian, FA personal observation; Bardeleben 1889). These bones may also be prepollices/sesamoids.
In most non-mammaliamorph synapsids there is an open space proximal to distal carpal I at the junction of this bone with the medial centrale, sometimes extending to the distomedial rim of the radiale (SOM: table 7: e). This space is at the same position in relation to distal carpal I as the prepollex/sesamoid of the biarmosuchian PIN 1758/320. The proximal contact area of distal carpal I to the medial centrale is usually very short or nearly absent as in some Therocephalia and Gorgonopsia. In the latter, the first digit is opposable and very manoeuvrable (Kümmell and Frey 2014b), so a strong articular connection to the carpus is likely. Because the free space proximal to distal carpal I appears in both juveniles and adults, its interpretation as representing an unossified, cartilaginous medial extension of the medial centrale is not guaranteed. It is more likely that distal carpal I was supported by a cartilaginous unfossilised prepollex/sesamoid.
Earlier authors proposed the presence of a sesamoid, medial to the medial centrale. Thus, a large sesamoid was proposed to be present proximal to distal carpal I at the junction of distal carpal I and the medial centrale in Dimetrodon incisivus (Case 1907) and Dimetrodon grandis (Gilmore 1919), and a small sesamoid between the medial centrale and the radiale in non-therapsid Eupelycosauria (Romer and Price 1940: 160). However, recent criticism of these interpretations arose from Henrici et al. (2005), stating that there is no indication of articular surfaces for sesamoids on the medial carpals in the eupelycosaurian Sphenacodon ferox. Like Henrici et al. (2005), we did not find any articular structures for a prepollex/sesamoid, similar to those between the other carpals on distal carpal I, medial centrale and radiale in synapsids where the medial portion of the carpus is exposed. However, because of the slightly ventral position of the prepollex/sesamoid in the biarmosuchian PIN 1758/320, its articular structures do not resemble the articular surfaces in other carpal bones. In the biarmosuchian PIN 1758/320, only slight indentations with no special articular surfaces are visible on the first distal carpal and medial centrale (Fig. 10A2). Flat, medioventrally facing indentations at the proximal margin of distal carpal I and the distomedial margin of the medial centrale are also present in Titanophoneus and Galesaurus SAM-PK-K10468, similar to that of the biarmosuchian PIN 1758/320. These indentations do not have a different surface texture that would allow their identification as true facets. Therefore, a prepollex/sesamoid may have been present in Titanophoneus and Galesaurus SAM-PK-K10468, but cannot be completely verified. An extensive analysis of these structures in synapsid skeletons was not possible in this study, because the first distal carpal and the medial centrale were not exposed from the ventral side in most fossil Synapsida. Further preparation or CT-scanning would clarify this in the future.
In early non-synapsid amniotes and amphibians, a prepollex/sesamoid often appears in the tracks of these early tetrapods. It was found in the ichnofossil Amphisauropus, which presumably represents a track of a seymouriomorph trackmaker (Fig. 15) and also in the earliest diadectomorph tracks Ichniotherium praesidentis and the putative tracks of the temnospondyl Eryops (Voigt and Ganzelewski 2010; SK personal communication 2016 with Sebastian Voigt; Voigt and Lucas 2017). Despite not being preserved in the fossilised skeleton of Eryops, a prepollex was reconstructed by some authors on the basis of the facets of the medialmost centrale and distal carpal I, combined with data from extant amphibians (Steiner 1921; Gregory et al. 1923). In contrast to Gregory et al. (1923), Dilkes (2015) did not reconstruct a prepollex in Eryops. To date, a prepollex/sesamoid-like structure was not found in the tracks of synapsids (SK personal communication 2016 with Sebastian Voigt). However, because of the small size of the prepollex/sesamoid found in the biarmosuchian PIN 1758/320, a visible separate prepollex/sesamoid structure within the imprint of a manus would be improbable.

Fig. 15. The track Amphisauropus Haubold, 1970, NMMNH P-37922, central New Mexico, USA, Abo Formation, Cisuralian, left manus and pes imprint, convex hyporelief, presumably from a seymouriamorph trackmaker. Photo made by Sebastian Voigt, published with the permission of Sebastian Voigt, all rights reserved (see also Voigt and Lucas 2017: fig. 3F).
The few instances described here suggest the possibility that in non-mammaliamorph synapsids a cartilaginous prepollex/sesamoid-like structure was present. It probably supported distal carpal I by filling the space on its proximal and proximomedial side. In Mesozoic mammaliamorphs, two putative prepollices have been reported: one in Asioryctes (Kielan-Jaworowska 1977; Kielan-Jaworowska et al. 2004) and a fragmentary nodular bone proximal to distal carpal I in Zhangheotherium (Hu et al. 1998). Salton and Sargis (2008: fig. 8) reported on a very small rudiment of a prepollex in a specimen of the otter shrew Potamogale, intercalated between distal carpal I and the radiale. This bone resembles the nodule in Zhangheotherium in both shape and position.
In Mesozoic mammaliamorphs, with the exception of Kayentatherium TMM 43690-5.136, no free space is present between the junctions of centrale and distal carpal I, centrale and radiale or in the junction of all three bones. Instead, the proximal rim of distal carpal I articulates about its whole width with fossilised bones. Either it articulates with the centrale and the probable prepollex/sesamoid as in Zhangheotherium and Asioryctes or with the centrale and the radiale. The radiale developed a distomedial process, to connect to distal carpal I in some Mesozoic mammaliamorphs without a prepollex/sesamoid. This process is present and short in the tritylodontid WCW-06A-34 and in Bienotheroides and is very long in the haramiyidian Shenshou lui (Bi et al. 2014). This additional contact between distal carpal I and the radiale of many mammaliamorphs integrates distal carpal I further into the carpus adding greater stability and reducing mobility (Kümmell and Frey 2014b).
The prepollex/sesamoid is present in many extant mammals and in some synapsid fossils. The configuration of the fossil carpals suggests it might also have been present in other synapsid taxa. Prepollex/sesamoid impressions in some early tetrapod tracks further suggest that it was fairly widespread among early tetrapods. The systematic framework of the occurrence of prepollex/sesamoid-like structures in most extant mammals and in early tetrapods makes it very likely that such structures were present in synapsids of the Permian and Mesozoic. Indeed, a prepollex/sesamoid was also proposed for stem eutherians by Szalay (1994).
Position of the distal carpals and their evolutionary loss or fusion.—In the early ontogenetic development of mammals, the distal carpals and the metacarpals I–IV separate from each other by one of two possible processes. Either the metacarpals segment from their corresponding distal carpal (Shubin and Alberch 1986) or the distal carpal detach from the proximal border of the metacarpal blastemata (Holmgren 1952; Milaire 1978; Johanson et al. 2007). In both cases, the distal carpal and metacarpal are developmentally associated with each other. This is also the case for ray V, where distal carpal V detaches separately from the corresponding metacarpal blastemata in early ontogeny. In later stages, the cartilaginous foci of distal carpals IV and V fuse either to each other (Mus, Milaire 1978; Perameles, Dasyupus, Holmgren 1952) or distal carpal V fuses to metacarpal V (Homo, Čihák 1972). Only Slabý (1976, investigating Procavia capensis, Sus scrofa, Ovis aries, and Bos taurus) interpreted distal carpal V as ontogenetically derived from the ulnare. The different manual rays are connected to the carpus by a joint between the single ray and the corresponding distal carpal, only rays IV and V are both connected to the distal carpal IV of modern mammals, which probably represents a fusion of distal carpals IV and V. The connected developmental derivation and the functional relation through ontogeny makes the association of the distal carpals and the corresponding manual rays very stable in ontogeny. This is also probably the case for phylogeny. Thus, the identity of a single distal carpal is relatively easy to determine by its position at the base of a manual ray.
Distal carpal I: Distal carpal I is always the base for ray I, but shows some proximodistal positional changes within fossil synapsids. In “pelycosaurs”, the anomodont Galechirus and in Mesozoic mammaliaforms, it lies in the row of distal carpals, whereas in some non-mammalian therapsids (e.g., Robertia, Diictodon, Microgomphodon, Procynosuchus RC92, Exaeretodon), it is aligned with the row of metacarpals. In all other analyzed non-mammaliamorph therapsids, it shows an intermediate position (SOM: table 7: b). In non-mammaliamorph synapsids, the distal shift of distal carpal I is connected to the change of the autopodial rolling mode with increasing parasagittal posture, e.g., in Permian dicynodonts (Kümmell and Frey 2014a, b). The autopodia rolled medially to the medial digits in early forms and to the central digits in later forms, facilitated by an increasing length of digit I. During the transition to Mesozoic Mammaliaformes and the tritylodontid Oligokyphus, metacarpal I (metacarpal or metatarsal I in Oligokyphus) changed its function. In non-mammaliamorph synapsids (with the exception of Galechirus), metacarpal I functioned as a basal phalanx, whereas in Mesozoic mammaliaforms and Oligokyphus it functioned as a true metacarpal. Accordingly, distal carpal I of non-mammaliamorph synapsids was more mobile than that of mammaliaformes and Oligokyphus, where it is completely integrated in the carpus (Kümmell and Frey 2014b). The situation of the other tritylodontids needs further investigation.
Distal carpal II and III: In Dicynodontia, distal carpals II and III are irregularly absent (Fig. 5A) or present. Both bones are absent in the articulated fossil carpus of many specimens of Diictodon feliceps, where an empty space occupies their usual position, but they are sometimes present in other specimens of the species. Considering this situation, we interpret the absence of distal carpal II and/or III as due to a heterochronically delayed ossification (paedomorphosis; sensu McNamara 2002) of cartilaginous precursors. Distal carpal III is apparently absent in the smaller, very likely younger forms, but present in the larger, presumably older forms of Diictodon feliceps. The ossification of distal carpal II is even more delayed than that of distal carpal III. It is either not present even in the larger and very likely older forms or it is present as a very small bony nodule, as in Diictodon GPIT/RE/7193 and in the largest and probably most mature specimen SAM-PK-K10636 (Table 2).
The delayed ossification of distal carpals II and III in Diictodon is remarkable, since the animal is known to have been a burrow-dweller that dug its own burrow (Smith 1987; Ray and Chinsamy 2003). Diggers usually have a strong and stiff, well ossified carpus (see above). As judged from the digits of the manus, Diictodon was able to scratch dig (Kümmell 2009). In the manus the second digit is longer than the fourth, a condition typical in scratch diggers for example the extant marsupial Lasiorhinus latifrons. The ungual phalanges are relatively short in relation to those of other dicynodonts (the length of the ungual phalanges III in relation to the corresponding digital length is 31%, 34%, 41%, and 49% in four Diictodon specimens). These values are typical in extant scratch diggers, as Meriones shawi (38%), Oryctolagus cuniculus (35%), and Meles meles (46%). The robustness of basal phalanx III (median value of the ratio of length to width is 1.65) is similar in other dicynodonts. It is slightly lower (i.e., the phalanges are more robust) than in terrestrial mammals and in the placental scratch digger Meles meles (2.48) and resembles that of the marsupial scratch digger Bettonia pinicillata (1.78). Diictodon shows well ossified joints. It must have been a scratch digger that dug in relatively soft soil (Ray and Chinsamy 2003; Kümmell 2009). The absence of wear facets on the tusks or keratinized frontal part of the snout suggest that these did not play a major role in the digging process of Diictodon (Ray and Chinsamy 2003). The digging method of Diictodon needs further investigation to explain the paedomorphic evolution of distal carpals II and III in this species.
Stahleckeria MCZ 1688 has only three distal carpals. In contrast to the situation in Diictodon, there is no empty space in the articulated carpus of Stahleckeria, so the distal carpals were lost in evolution or fused to an adjacent bone. The distal carpals lost were distal carpal V (see below) and one of the central distal carpals (II or III), probably III judging by the position of the central distal carpal mainly at the base of ray II. The absence of one central distal carpal or its fusion to another distal carpal was probably associated with the evolution of large sole cushions in that animal, which correlates with reduced carpus mobility (Kümmell 2009). It has also been suggested that distal carpal II was cartilaginous and did not ossify in Stahleckeria MCZ 1688 (Romer and Price 1944).
The tritylodontid Kayentatherium TMM 43690-5.136 possess only three distal carpals instead of the usual four of mammaliamorphs. Distal carpal II is probably absent or fused to distal carpal III. We analyzed this fossil from images of scans, so a more detailed study of the specimen comparing it with Kayentatherium MCZ 8812 (Sues and Jenkins 2006) is needed to investigate the question of the identity of the distal carpals.
Distal carpal V: The presence of distal carpal V is plesiomorphic in therapsids and is found in the “pelycosaur” taxa analysed here. During therapsid evolution, it is lost in nearly every group. Hopson (1995) described two processes of losses in therapsids. The first was non-ossification of distal carpal V in Dicynodontia and gomphodont Cynodontia (with the absence of distal carpal V as an individual variant in some early cynodonts where it probably failed to ossify). The second was fusion to distal carpal IV in Biarmosuchia, Gorgonopsia, Therocephalia, and Mammalia.
Here we propose that the processes leading to the loss of distal carpal V were even more subtle. In the most basal therapsids, the biarmosuchians, distal carpals IV and V are fused. The fusion line of the biarmosuchian PIN 1758/320 is very faint, but clear in Hipposaurus. In dinocephalians, distal carpal V is still present. In dicynodonts, distal carpal V is completely lost, as can be inferred from the compact form of distal carpal IV without indentations or set-back angles in its outline and the contact of the proximal rim of metacarpal V with the ulnare in many Diictodon specimens and Stahleckeria (Fig. 5B, C; SOM: table 3: f). In gorgonopsians, therocephalians and cynodonts, the following states of distal carpal V are present. State 1: An open space is present instead of distal carpal V. Here, distal carpal V was not ossified, but remained in a cartilaginous form (Hopson 1995; Fontanarrosa et al. 2019). This state appears in the therocephalians Olivierosuchus BP/1/3973 (Fig. 16A), Tetracynodon AM 3677 (Figs. 6C, 16B) and the cynodonts Galesaurus (Fig. 7C), Exaeretodon and Trucidocynodon (Figs. 8A, 16C). State 2: Ossification of distal carpal V had begun, appearing as a small nodule within an open space between metacarpal V and ulnare, present in the gorgonopsian cf. Cynariops SAM-PK-K10000 and the cynodonts Procynosuchus BP/1/591 and NHMUK PV R 37054 (Figs. 7B, 16D, E), Thrinaxodon and Diademodon NHMUK R-3581. State 3: The space is nearly completely filled by the distal carpal V. This state is found in the therocephalian Ictidosuchoides CGS CM86-655 (Figs. 6D and 16F). State 4: Distal carpals IV and V are fused with a visible fusion line, which is the case in the gorgonopsians Arctognathus, Dinogorgon and the gorgonopsid BP/1/1210 (Figs. 6A, 11, 16G, I) and the therocephalians Theriognathus (Fig. 16H) and Microgomphodon. State 5: Distal carpal IV and V are fused, but no fusion line is present. This state is present in the therocephalians Glanosuchus SAM-PK-K7809 (Figs. 6B, 16J; Fontanarrosa et al. 2019), ?Ictidosuchoides BP/1/2294 (Fig. 16K) and in the cynodont Procynosuchus RC92 (Figs. 7A, 16L). The fusion of distal carpal V is deduced from the shape of distal carpal IV with a distal indentation showing the point of fusion of the two bones. A further suggestion advocating a fused state rather than the loss of distal carpal V, is that distal carpal V is also found in other specimens of Procynosuchus (state 2).
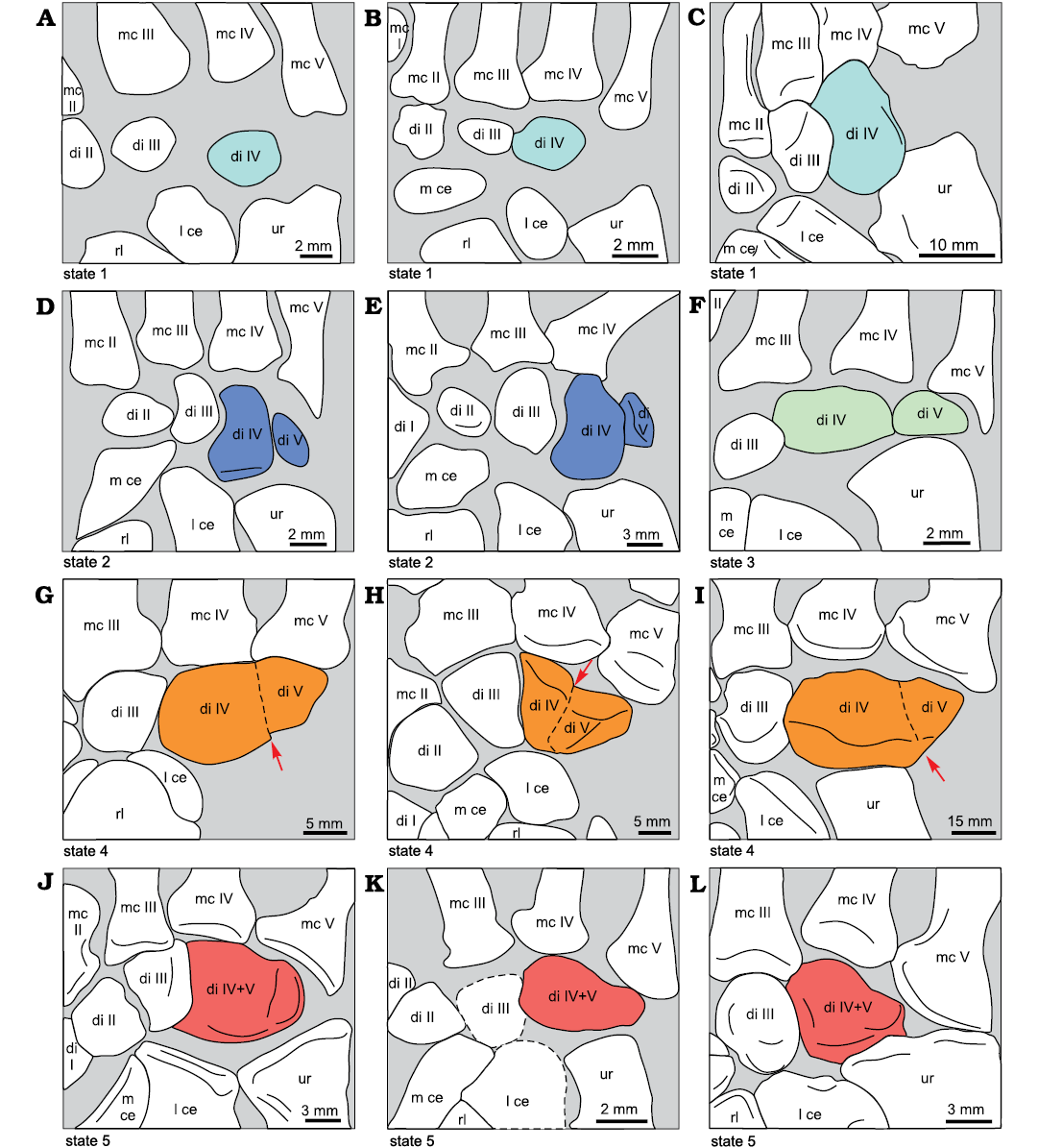
Fig. 16. Selected specimens showing the different states of distal carpal V in gorgonopsians, therocephalians, and cynodonts, all in dorsal view (except A, ventral view). A. Olivierosuchus parringtoni (Brink, 1965), BP/1/3973, left carpus. B. Tetracynodon darti Sigogneau, 1963, AM 3677, right carpus. C. Trucidocynodon riograndensis Oliveira, Soares, and Schultz, 2010, UFRGS PV-1051T, right carpus. D. Procynosuchus delaharpeae Broom, 1937, BP/1/591, right carpus. E. Procynosuchus delaharpeae Broom, 1937, NHMUK PV R 37054, right carpus. F. Ictidosuchoides longiceps Broom, 1920, CGS CM86-655, right carpus. G. Arctognathus curvimola (Owen, 1876), SAM-PK-3329, right carpus. H. Theriognathus microps Owen, 1876, NHMUK R 5694, right carpus. I. Gorgonopsian BP/1/1210, left carpus (reversed). J. Glanosuchus macrops Broom, 1904, SAM-PK-K7809, left carpus (reversed). K. Ictidosuchoides longiceps Broom, 1920, or Ictidosuchops intermedium (Broom, 1938), BP/1/2294, right carpus (dashed lines are shown where the bone margins are uncertain). L. Procynosuchus delaharpeae Broom, 1937, RC92, right carpus. Colours show the different states of distal carpal IV and V. Arrows show lines of fusion (dashes) between distal carpals IV and V. Abbreviations: mc, metacarpals, di, distal carpals; l ce, lateral centrale; m ce, medial centrale; rl, radiale; ur, ulnare.
Thus, in gorgonopsians, therocephalians, and cynodonts, distal carpal V is always present, but appears in different forms: as a cartilaginous precursor, a separate bone or in a fused state. A fused state, in which a fusion line is present or in which there is other evidence suggestive of the fusion of two different bones, provides evidence of two ossification centres, from which two bones grow and fuse during ontogeny. Because carpals usually ossify relatively late compared to the long bones and other bones of the manus (Nesslinger 1956; Oliveira et al. 1998; Stafford and Thorington 1998; Prochel and Sánchez-Villagra 2003; Gilsanz and Ratib 2005; Fröbisch 2008; Wilson et al. 2010), the fusion of two ossified carpals may appear in a juvenile or subadult stage. An example of this in extant mammals is found in bats, where the radiale, lunate and centrale fuse in a postnatal stage (Stafford and Thorington 1998). As in any endochondral bone, the ossification of carpals is preceded by a cartilaginous precursor (Holmgren 1952; Stafford and Thorington 1998). So, in early ontogeny (prenatally or postnatally or before or after hatching), there is only a cartilaginous precursor present instead of an ossified distal carpal V. So, it is very likely that individuals with fusion of distal carpals IV and V showed two prior states of distal carpal V earlier in ontogeny: a separate ossified distal carpal V in an older ontogenetic stage and an open space representing the cartilaginous precursor of distal carpal V in a younger ontogenetic stage.
Within our sample, specimens of Procynosuchus delaharpeae show such an ontogenetic change in distal carpal V. Two specimens show state 2 and one state 5 (Fig. 16D, E, L). The cynodont Procynosuchus BP/1/591 is considered a subadult (see also Brink and Kitching 1951) after the immature growth of carpals, which are more rounded and partly surrounded by open spaces, whereas carpals of adult specimens are not surrounded by open spaces and are more edged and angular. The skull and the long bones of BP/1/591 show smaller sizes than the other fossils of the same species (Table 3). In Procynosuchus NHMUK PV R 37054, the carpals are not fully grown. It can be verified as being slightly smaller than Procynosuchus RC92 (Table 3). NHMUK PV R 37054 might have been close to being mature, but were probably still developing at the time of death. In contrast, RC92 is considered to be an adult (in fact it is one of the largest specimens of Procynosuchus).
Within the Procynosuchus specimens, the subadult and the nearly mature form (BP/1/591 and NHMUK PV R 37054) show an immature ossification state in distal carpal V, whereas the mature Procynosuchus RC92 shows a fused state without fusion line. That means that fusion of distal carpal IV and V would have occurred close to the adult stage.
An alternative explanation is that the observed differences in distal carpal V in these species are individual variations. Individual variants are known from the fusion of the middle and terminal phalanges in the human fifth toe, which do not appear in all individuals, but in 73% and 77% of the Japanese and Chinese (Hong Kong) populations and in 38–46% of Europeans (Nakashima et al. 1995; Chan et al. 2019). Here fused and unfused phalanges are present in adults. However, in Procynosuchus, the different ontogenetic stages are clearly apparent (Table 3), so the variation is likely to be ontogenetic.
Table 3. Length (in mm) of skull and long bones of Procynosuchus specimens and the percentual size differences between the specimens.
|
Procynosuchus |
BP/1/591 |
NHMUK PV R37054 |
RC92 |
Length difference (%) |
|
|
subadult |
slightly immature |
adult |
BP/1/591 and RC92 |
NHMUK PV R37054 and RC92 |
|
|
Basal skull |
82 |
111 |
144 |
43 |
23 |
|
Humerus |
48 |
67 |
80 |
40 |
16 |
|
Radius |
41 |
|
61 |
33 |
|
|
Ulna |
46 |
|
72 |
36 |
|
Ictidosuchoides CGS CM86-655 and ?Ictidosuchoides BP/1/2294 also show different states of distal carpal V (states 3 and 5). The identity of BP/1/2294 is not fully established, and that specimen could also belong to Ictidosuchops (the taxonomic identity of Ictidosuchoides and Ictidosuchops needs reevaluation; FA personal observation). BP/1/2294 is slightly bigger than CGS CM86-655 (humerus length difference 8%), but in both specimens the carpals are not completely developed. These specimens show distal carpal V in states 3 and 5 and demonstrate the high variability of that character in immature therocephalians.
In gorgonopsians and therocephalians, the fused state of the distal carpal V, either with or without fusion line, is the most common state. The fossils with fused distal carpal IV and V (states 4 and 5) in both lineages show fully mature carpals with the exception of ?Ictidosuchoides BP/1/2294. Also, the basal cynodont Procynosuchus with a fused distal carpal IV and V (RC92) is interpreted as fully adult. Considering the widespread occurrence of the fused state in adults of gorgonopsians and therocephalians and in the basal adult cynodont Procynosuchus, the fusion of distal carpal IV and V is very likely a synapomorphy of Theriodontia. This is even more likely because the basal members of these lineages show fusion (therocephalian Glanosuchus, gorgonopsians Arctognathus and Viatkogorgon (SK personal observation on PIN 2212/61) and adult cynodont Procynosuchus). However, some later theriodont adult or nearly mature specimens do not exhibit fusion, but rather an immature state of distal carpal V. This is the case for instance in the gorgonopsian cf. Cynariops SAM-PK-K10000 and most non-mammaliamorph cynodonts. Cf. Cynariops SAM-PK-K10000 and the cynodont Thrinaxodon possess a distal carpal V as a small bone within an open space (state 2) and in the cynodonts Galesaurus (Fig. 7C), Exaeretodon and Trucidocynodon (Fig. 16C) distal carpal V was cartilaginous (state 1). Cf. Cynariops SAM-PK-K10000 is an adult judging by the form of its carpals (see also Ray et al. 2004). Both Thrinaxodon specimens (BP/1/1737 and BP/1/7199) and Galesaurus BP/1/2513 are thought to be adults by Jasinoski et al. (2015), Jasinoski and Abdala (2017), and Brink (1965). The carpals of Thrinaxodon BP/1/7199 and Galesaurus BP/1/2513 are nearly mature, but probably not completely ossified. The carpal condition of Exaeretodon and Trucidocynodon indicates that they are fully mature. Variations of the states of distal carpal V in the adult stage of gorgonopsians, therocephalians and cynodonts may be due to changes in the timing of the distal carpal V ontogenetic stages. A delay in the development of distal carpal V could result in an unfused variant of distal carpal V or the presence of a cartilaginous precursor in an adult stage, which shows up as an open space in the fossil. This delayed development of distal carpal V is likely to have occurred in the evolution of the gorgonopsian cf. Cynariops SAM-PK-K10000 and most cynodonts. Thus, the development of distal carpal V is heterochronically delayed in many non-mammaliamorph Cynodontia (i.e., the distal carpal V evolved paedomorphically, sensu McNamara 2002).
Only the state of cynognathian BP/1/4534 (Fig. 7D) is ambigious and similar to many mammaliamorphs (see below). It has an open space distolateral to the ulnare, but also a relatively wide distal carpal IV. This bone shows no fusion line, no set-back angle or indentation in the outline and resembles the unfused wide distal carpal IV of Thrinaxodon, although it is wider than in Galesaurus in which distal carpal V was cartilaginous. So, distal carpal IV could be unfused and distal carpal V could have been cartilaginous. However, it is also possible that distal carpals IV and V were fused in the species represented by this specimen.
In mammaliamorphs, distal carpal V is not present. Distal carpal IV often extends towards the medial part of metacarpal V, but leaves a space between the lateral part of metacarpal V and the ulnare. Distal carpal V is either not fossilised and stays cartilaginous or is fused to another forelimb bone, most probably to distal carpal IV. In the case of fusion, it is possible that the cartilaginous precursor became fused to another anlage of forelimb bones in early ontogeny, a situation known to occur in some modern mammals (Milaire 1978; Holmgren 1952; Čihák 1972; Slabý 1976).
Synopsis on character evolution.—The key transformations in carpal anatomy of synapsids were a morphological change and reduction in size of the intermedium, the loss of intermedium and distal carpal V, the evolution of the medial and lateral centralia, a positional change of distal carpal I and the overall shortening of the carpal elements, especially of the proximal carpals.
In therapsids, the morphology of the intermedium differs markedly from that of “pelycosaurs”, and it is much reduced in size (in length and/or width) in relation to the radiale (Fig. 17: character 1, SOM: fig. 1, table 2: a, e).
Another putative therapsid synapomorphy is the distal shift of distal carpal I, which became aligned partly with the row of remaining distal carpals and partly with the proximal end of the row of metacarpals (Fig. 17: character 2; SOM: fig. 2, table 7: b). In the anomodont Galechirus and the mammals analysed here, the distal carpal I was again aligned in the row of distal carpals (SOM: fig. 2). A further distal shift of distal carpal I producing a complete alignment to the row of metacarpals occurs homoplastically in some dicynodonts (Diictodon and Robertia), one therocephalian (Microgomphodon) and some non-mammaliaform cynodonts (Exaeretodon and Procynosuchus RC92; SOM: fig. 2, table 7: b). In caseids, one synapomorphy seems to be a distal contact of the lateral centrale to the medial centrale (Fig. 17: character 3; SOM: table 5: c), whereas the placement of the lateral centrale lateral to the radiale is a synapomorphy of cynodonts (SOM: table 1: f). In mammaliamorphs, the intermedium is completely lost or fused to an adjacent carpal (Fig. 17: character 4; SOM: fig. 1). This loss was linked to some important changes in the proximal row of the carpus: the lateral centrale (lunate) became proximally connected to the lateral part of the distal facet of the radius and the radiale became narrower in relation to the radius width, contacting only to the medial side of the radial facet (Fig. 17: character 5; SOM: table 1: b). Simultaneously with these changes, there was a reduction of the relative length of the ulnare (Fig. 17: character 6; SOM: table 3: b). The structure of the proximal row of carpals as a whole, therefore, was reorganized on the transition to mammaliamorphs. The size of proximal carpals was further reduced on the line to mammals (SOM: table 3: b), which resulted in a compact and short carpus in Mesozoic mammals.
There is a high degree of plasticity in the condition of the medial centrale, resulting in the loss of the bone by fusion to the radiale, observed in specimens of unrelated lineages: the gorgonopsian Arctognathus, the tritylodont Bienotheroides, probably in the unnamed cynognathian BP/1/4534 and in one Thrinaxodon specimen (BP/1/1737), in which this condition would be an intraspecific variation (SOM: fig. 3). Loss of the medial centrale with no evidence of fusion to the radiale is also demonstrating a plastic condition observed in the dicynodont Stahleckeria, in the evolutionary lines of both monotremes and marsupials, and also in the eutherian Sinodelphys (Fig. 17: character 7; SOM: table 3).
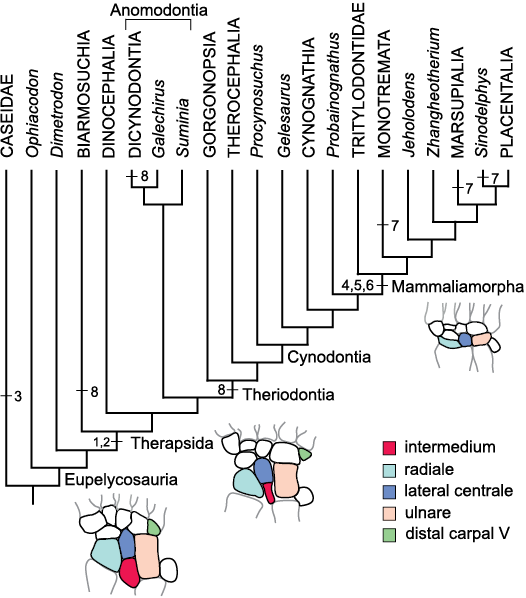
Fig. 17. Phylogenetic tree showing the major evolutionary changes in the carpus during synapsid evolution. For explanation, see text. The phylogeny is based on the same references as Fig. 2A and B.
The loss of the distal carpal V is an important step towards the reduction of the number of carpal bones, but the record of this loss is widely scattered in punctual records of different therapsids (SOM: table 10: d, e). Absence of distal carpal V or its fusion to distal carpal IV is represented independently in biarmosuchians, dicynodonts and in most adults of theriodonts (as in the basal forms Viatkogorgon, Arctognathus, Glanosuchus, and Procynosuchus; Fig. 17: character 8). The condition in theriodonts is, however, complicated by the presence of a separate distal carpal V in the adult gorgonopsian cf. Cynariops SAM-PK-K10000, and in the adult non-mammaliaform cynodont Thrinaxodon.
With the reduction of two to three carpal bones (intermedium, distal carpal V and in some lineages the medial centrale) and the shortening of the carpus, there is an overall trend of skeletal simplification in the synapsid carpus on the line to mammals, a trend also reported for the cranium of synapsids (Sidor 2001). While the absence or fusion of the medial centrale and the distal carpal V present a highly homoplastic distribution in synapsid phylogeny, the loss of the intermedium occurred only once, as far as can be judged from our sample.
Conclusions
We analyzed the homology of the carpal bones in fossil representatives of the clade Synapsida from the early Permian to the Late Cretaceous. Our interpretation is in accordance with the traditional view (e.g., Broom 1901, 1904) for the following carpal bones of non-mammaliamorph synapsids and mammaliaforms: radiale/scaphoid, ulnare/triquetrum (= cuneiform), pisiform, distal carpal I/trapezium, distal carpal II/trapezoid, distal carpal III/capitate. We homologize the medial centrale of non-mammaliamorph synapsids with the single centrale of mammaliamorphs. Traditionally, the mammaliamorph lunate is considered as a homologue of the intermedium of non-mammaliamorph synapsids. We interpret the lunate as the homologue of the lateral centrale of non-mammaliamorph synapsids, because of its position and relative proportions, and because of patterns in the embryonal cartilaginous foci of some extant mammals. The proximal contact of the lunate with the radius is an apomorphy of mammaliamorphs and the intermedium is lost in this clade. Despite the homologies with the lateral and medial centrale of non-mammaliamorph synapsids, we argue for the continued use of the terms “lunate” and “centrale” for the relevant bones in mammaliamorph anatomy.
Different carpal elements can be fused to the radiale: in Arctognathus both centralia, in Bienotheroides the single mammaliamorph centrale and in the unidentified cynognathian BP/1/4534 probably the medial centrale. Arctognathus was a fossorial gorgonopsid and the other two taxa also show digging abilities. A digging habit in these taxa would have been facilitated by the stiffening of the carpus through carpal fusion.
In regard to three central bones in Theriognathus NHMUK R 5694, we follow Boonstra (1934) and designate them as three centralia on the basis of their positions. In the biarmosuchian PIN 1758/320, we interpret the medialmost of the three central bones as a prepollex/sesamoid, because of its unusual position for a centrale. The open space proximal to distal carpal I in articulated carpi of most non-mammaliamorph synapsids, suggests the presence of cartilaginous prepollices/sesamoids at this position during life. The presence of prepollex/sesamoid-like structures in modern mammals and in the tracks of early tetrapods, is a further suggestion that a prepollex/sesamoid could have been present in synapsids during the Permian and Mesozoic.
Distal carpals and metacarpals developmentally derive from each other in ontogeny and remain connected by distinct joints during life, making this connection very stable in ontogeny and phylogeny. Some exceptional situations are: distal carpals II and III highly delayed in development observed in Diictodon feliceps, where they evolved paedomorphically. Distal carpal V is lost in Dicynodontia and fused in Biarmosuchia and also in most adults of Gorgonopsia, Therocephalia (including the basal forms of the latter two) and in the adult basal cynodont Procynosuchus. In most non-mammaliamorph Cynodontia, distal carpal V show a delayed development compared to Procynosuchus and basal therocephalians, suggesting distal carpal V evolved paedomorphically. In Mesozoic mammaliamorphs, distal carpal V is either cartilaginous or fused, very likely to distal carpal IV.
Acknowledgements
Many thanks go to Marissa Fabrezi (Instituto de Bio y Geociencias, Universidad Nacional de Salta, Salta, Argentina), who improved the manuscript substantially and contributed to supplement 1 and most discussions. We thank the reviewers Christian Sidor (Department of Biology, University of Washington, USA) and Kenneth Angielczyk (Field Museum, Chicago, USA) for valuable comments and suggestions. Thanks go also to Christian Kammerer (North Carolina Museum of Natural Science, Raleigh, USA) for providing information on the taxonomy of some gorgonopsian species. We are very grateful to the following persons for their warm hospitality during visit to institutions and collections: Rob Gess (AM), Susan Bell, Carl Mehling (both AMNH), Ellen De Kock, Johann Neveling, Linda Karny (all CGS), Luo Zhe-Xi (Carnegie Museum of Natural History, Pittsburgh, USA), Bruce Rubidge, Michael Raath (both Evolutionary Studies Institute, University of the Witwatersrand, Johannesburg, South Africa), Ken Angielczyk, Bill Simpson, Adrienne Stroup (all FMNH), Lu Liwu (GMV), Philipe Havlik, Michael Maisch, Andreas Matzke, Franziska Großmann, Eva Gebauer (all GPIT), Gabriele Gruber (Hessisches Landesmuseum Darmstadt, Germany), Liu Jun, Xu Xing, Chen Jin, Corwin Sullivan (all IVPP), Charles Schaff, Farish Jenkins (both MCZ), Sandra Chapman, Jerry Hooker, Lorna Steel, Philippa Brewer (all NHMUK), Tom Kemp, Małgosia Nowak-Kemp (both OUMNH), Valery Golubev, Andrey Kurkin (both PIN), Pablo Ortiz, Rodrigo González (both PVL), Sheena Kaal, Roger Smith (both SAM), Heidi Fourie, Stephany Potze (both TM), Cesar Schultz, Marina Bento Soares (both UFRGS), Jenny Clack, Matthew Allinson, Ray Symonds, Michael Akam (all UMZC) Hans Sues, Matt Carrano (USNM). We are very thankful to Xu Xing (IVPP) for giving us access to his field collecion of Wucaiwan, Beijing. We are also grateful to the staff of the ID17 and ID19 beamlines, especially to V. Fernandez and P. Tafforeau at the European Synchrotron Radiation Facility for microtomographic experiments on BPI/1/7199. We thank Gabriela Fontanarrosa (National Scientific and Technical Research Council, Tucumán, Argentina) for providing photos of Tetracynodon darti AM 3677, Eva Hoffman (University of Texas, Austin, USA) for scans of Kayentatherium, and Sebastian Voigt (Geoskop, Umweltmuseum, Thallichtenberg, Germany) for photos on the track Amphisauropus. This project was possible thanks to the financial support of the Rudolf Steiner-Fonds, Mahle-Stiftung, GLS-Gemeinschaftsbank and Evidenz Stiftung (SK); PIP CONICET 389, Agencia Nacional PICT 2016-2772 and PICT 2018-832 (VA); National Research Foundation, South Africa, CONICET and Agencia Nacional PICT 2015-2389 (FA).
References
Abdala, F. 2007. Redescription of Platycraniellus elegans (Therapsida, Cynodontia) from the Lower Triassic of South Africa, and the cladistic relationships of eutheriodonts. Palaeontology 50: 591–618.Crossref
Abdala, F., Rubidge, B.S., and Van den Heever, J.A. 2008. The oldest therocephalians (Therapsida, Eutheriodontia) and the early diversification of Therapsida. Palaeontology 51: 1011–1024. Crossref
Albinus, B.S. 1726. De ossibus corporis humani. 309 pp. Leidae Batavorum: Apud Henricum Mulhovium, Leiden.
Amador, L.I., Giannini, N.P., Simmons, N.B., and Abdala, V. 2018. Morphology and evolution of sesamoid elements in bats (Mammalia: Chiroptera). American Museum Novitates 3905: 1–38. Crossref
Angielczyk, K.D. and Rubidge, B.S. 2013. Skeletal morphology, phylogenetic relationships and stratigraphic range of Eosimops newtoni Broom, a pylaecephalid dicynodont (Therapsida, Anomodontia) from the Middle Permian of South Africa. Journal of Systematic Palaeontology 11: 191–231. Crossref
Angielczyk, K.D., Fröbisch, J., and Smith, R.M.H. 2005. On the stratigraphic range of the dicynodont taxon Emydops (Therapsida: Anomodontia) in the Karoo Basin, South Africa. Palaeontologia Africana 41: 23–33.
Angielczyk, K.D., Sidor, C.A., Nesbitt, S.J., Smith, R.M.H., and Tsuji, L.A. 2009. Taxonomic revision and new observations on the postcranial skeleton, biogeography, and biostratigraphy of the dicynodont genus Dicynodontoides, the senior subjective synonym of Kingoria (Therapsida, Anomodontia). Journal of Vertebrate Paleontology 29: 1174–1187. Crossref
Bardeleben, K. 1889. On the prepollex and prehallux, with observations on the carpus of Theriodesmus phylarchus. Proceedings of the Zoological Society of London 18: 259–262.
Bi, S., Wang, Y., Guan, J., Sheng, X., and Meng, J. 2014. Three new Jurassic euharamiyidan species reinforce early divergence of mammals. Nature 514: 579–584. Crossref
Bi, S., Zheng, X., Wang, X., Cignetti, N.E., Yang, S., and Wible, J.R. 2018. An early Cretaceous eutherian and the placental–marsupial dichotomy. Nature 558: 390–395. Crossref
Biesecker, L.G., Aase, J.M., Clericuzio, C., Gurrieri, F., Temple, I.K., and Toriello, H. 2009. Defining morphology: Hands and feet. American Journal of Medical Genetics Part A 149A: 93–127. Crossref
Boonstra, L.D. 1934. A contribution to the morphology of the mammal-like reptiles of the suborder Therocephalia. Annals of the South African Museum 31: 215–267.
Boonstra, L.D. 1965. The girdles and limbs of the Gorgonopsia of the Tapinocephalus Zone. Annals of the South African Museum 48: 237–249.
Brink, A.S. 1965. On two new specimens of Lystrosaurus-Zone cynodonts. Palaeontologica Africana 9: 107–122.
Brink, A.S. and Kitching, J.W. 1951: On Leavachia, a procynosuchid cynodont from the Middle Cistecephalus Zone. South African Journal of Science 1951: 342–347.
Broom, R. 1901. On the structure and affinities of Udenodon. Proceedings of the Zoological Society of London 901: 162–190.
Broom, R. 1904. The origin of the mammalian carpus and tarsus. Transactions of the South African Philosophical Society 15: 89–94. Crossref
Broom, R. 1907. On the origin of the mammal-like reptiles. Proceedings of the Zoological Society of London 1907: 1047–1061. Crossref
Broom, R. 1913. On the origin of the mammalian digital formula. Anatomischer Anzeiger 43: 230–232.
Broom, R. 1930. On the structure of the mammal-like reptiles of the suborder Gorgonopsia. Philosophical Transactions of the Royal Society of London B 218: 345–371. Crossref
Case, E.C. 1907. Revision of the Pelycosauria of North America. Carnegie Institution of Washington Publication 55: 1–176. Crossref
Chan, Y.L.C., Dao, W.H., Yeung, T., and Chow, E.C.S. 2019. Prevalence of toe symphalangism in Hong Kong Chinese population. Journal of Orthopaedics, Trauma and Rehabilitation 26 (1): 56–60. Crossref
Chudinov, P.K. [Čudinov, P.K.] 1983. Early therapsids [in Russian]. Trudy Palaeontologičeskij Institut, Akademiâ Nauk SSSR 202: 1–229.
Čihák, R. 1972. Ontogenesis of the skeleton and intrinsic muscles of the human hand and foot. Advances in Anatomy, Embryology and Cell Biology 46: 1–194. Crossref
Cohen, K.M., Finney, S.C., Gibbard, P.L., and Fan, J.-X. 2013 (updated). The ICS International Chronostratigraphic Chart. Episodes 36: 199–204. [published online, http://www.stratigraphy.org/ICSchart/Chrono stratChart2020-01.pdf] Crossref
Colbert, E.H. 1948. The mammal-like reptile Lycaenops. Bulletin American Museum of Natural History 89: 357–404.
Diaz, R.E. and Trainor, P.A. 2015. Hand/foot splitting and the “re-evolution” of mesopodial skeletal elements during the evolution and radiation of chameleons. BMC Evolutionary Biology 15: 184. Crossref
Dilkes, D. 2015. Carpus and tarsus of Temnospondyli. Vertebrate Anatomy Morphology Palaeontology 1: 51‒87. Crossref
Echeverria, A.I., Abdala, V., Longo, M.V., and Vassallo, A. 2019. Functional morphology and identity of the thenar pad in the subterranean genus Ctenomys (Rodentia, Caviomorpha). Journal of Anatomy 235: 940–952. [published online, https://doi.org/10.1111/joa.13049] Crossref
Fabrezi, M. 2001. A survey of prepollex and prehallux variation in anuran limbs. Zoological Journal of the Linnean Society 131: 227–248. Crossref
Fabrezi, M. and Alberch, P. 1996. The carpal elements of anurans. Herpetologica 52: 188–204.
Fabrezi, M. and Barg, M. 2001. Patterns of carpal development among anuran amphibians. Journal of Morphology 249: 210–220. Crossref
Fabrezi, M., Abdala, V., and Martínez Oliver, M.I. 2007. Developmental basis of limb homology in lizards. Anatomical Record 290: 900–912. Crossref
Fabrezi, M., Manzano, A., Abdala, V., and Zaher, H. 2009. Developmental basis of limb homology in pleurodiran turtles, and the identity of the hooked element in the chelonian tarsus. Zoological Journal of the Linnean Society 155: 845–866. Crossref
Flores, D.A. and Diaz, M.M. 2009. Postcranial skeleton of Glironia venusta (Didelphimorphia, Didelphidae, Caluromyinae): Description and functional morphology. Zoosystematics and Evolution 85: 311–339. Crossref
Flower, W.H. 1885. An Introduction to the Osteology of the Mammalia. 375 pp. Macmillan and Co., London. Crossref
Fontanarrosa, G. and Abdala, V. 2014. Anatomical analysis of the lizard carpal bones in the terms of skilled manual abilities. Acta Zoologica 95: 249–263. Crossref
Fontanarrosa, G. and Abdala, V. 2016. Bone indicators of grasping hands in lizards. PeerJ 4:e1978. Crossref
Fontanarrosa, G., Abdala, F., Kümmell, S.B., and Gess, R. 2019. The manus of Tetracynodon (Therapsida: Therocephalia) provides evidence for survival strategies following the Permotriassic extinction. Journal of Vertebrate Paleontology 38: 4. Crossref
Fröbisch, J. and Reisz, R.R. 2011. The postcranial anatomy of Suminia getmanovi (Synapsida: Anomodontia), the earliest known arboreal tetrapod. Zoological Journal of the Linnean Society 162: 661–698. Crossref
Fröbisch, N.B. 2008. Ossification patterns in the tetrapod limb—conservation and divergence from morphogenetic events. Biological Reviews 83: 571–600. Crossref
Gegenbaur, C. 1864. Untersuchungen zur vergleichenden Anatomie der Wirbelthiere. Erstes Heft: Carpus und Tarsus. 127 pp. Verlag von Wilhelm Engelmann, Leipzig. Crossref
Gillies, C.D. 1929. The origin of the os pisiform. Journal of Anatomy 63: 380–383.
Gilmore, C.W. 1919. A mounted skeleton of Dimetrodon gigas in the United States National Museum with notes on the skeletal anatomy. Proceedings of the United States National Museum 56: 525–539. Crossref
Gilsanz, V. and Ratib, O. 2005. Hand bone age. A digital Atlas of Skeletal Maturity. 106 pp. Springer, Berlin.
Grassé, P.P. 1955. Traité de Zoologie. Anatomie. Systèmatique. Biologie. Mammifére. Les ordres: anatomie, éthologie, systématique. Tome XVII, 1147–1447. Masson and Co., Paris.
Gregory, W.K., Miner, R.W., and Noble G.K. 1923. The carpus of Eryops and the structure of the primitive chiropterygium. Bulletin of the American Museum of Natural History 48: 279–288.
Haines, R.W. 1969. Epiphyses and sesamoids. In: C. Gans, A.d’A. Bellairs, and T.S. Parsons, (eds.), Biology of the Reptilia. Volume 1. Morphology A, 81–115. Academic Press, London.
Henrici, A.C., Berman, D.S., Lucas, S.G., Heckert, A.B., Rinehart, L.F., and Zeigler, K.E. 2005. The carpus and tarsus of the early Permian synapsid Sphenacodon ferox (Eupelycosauria: Sphenacodontidae). The nonmarine Permian. New Mexico Museum of Natural History and Science Bulletin 30: 106–110.
Heppleston, A.C. 2010. Patterns and Processes of Digit Number Reduction. Ph.D. Thesis, McGill University, Montreal. Available at http://digitool.library.mcgill.ca/R/?func=dbin-jump-full&object_id=92163&local_base=GEN01-MCG02
Hildebrand, M. and Goslow, G.E. 2004. Vergleichende und funktionelle Anatomie der Wirbeltiere. 709 pp. Springer, Berlin. Crossref
Hoffman, E.A. and Rowe, T.B. 2018. Jurassic stem-mammal perinates and the origin of mammalian reproduction and growth. Nature 561: 104–108. Crossref
Holmgren, N. 1933. On the origin of the tetrapod limb. Acta Zoologica 14: 185–295. Crossref
Holmgren, N. 1952. An embryological analysis of the mammalian carpus and its bearing upon the question of the origin of tetrapod limb. Acta Zoologica 33: 1–115. Crossref
Hopson, J.A. 1995. Patterns of evolution in the manus and pes of non-mammalian therapsids. Journal of Vertebrate Paleontology 15: 615–639. Crossref
Hopson, J.A. and Barghusen, H. 1986. An analysis of therapsid relationships. In: N. Hotton, P.D. MacLean, J.J. Roth, and E.C. Roth (eds.), The Ecology and Biology of the Mammal-like Reptiles, 83–106. Smithsonian Institution Press, Washington.
Hu, Y.M., Wang, Y.Q., Li, C.K., and Luo, Z.X. 1998. Morphology of dentition and forelimb of Zhangheotherium. Vertebrata Palasiatica 36: 102–125.
Huttenlocker, A.K. and Smith, R.M.H. 2017. New whaitsioids (Therapsida: Therocephalia) from the Teekloof Formation of South Africa and therocephalian diversity during the end-Guadalupian extinction. PeerJ 5: e3868. Crossref
Ihle, J.E.W., Kampen, P.N. van, Nierstrasz, H.F., and Versluys, J. 1927. Vergleichende Anatomie der Wirbeltiere. 906 pp. Verlag von Julius Springer, Berlin.
Jasinoski, S.C., Abdala, F., and Fernandez, V. 2015. Ontogeny of the Early Triassic cynodont Thrinaxodon liorhinus (Therapsida): cranial morphology. The Anatomical Record 298: 1440–1464. Crossref
Jasinoski, S.C. and Abdala, F. 2017. Cranial ontogeny of the Early Triassic basal cynodont Galesaurus planiceps. The Anatomical Records 300 (2): 353–381. Crossref
Jenkins, F.A., 1971. The postcranial skeleton of African cynodonts. Peabody Museum of Natural History, Yale University, Bulletin 36:1–216.
Ji, Q., Luo, Z.X., Wible, J.R., Zhang, J.P., and Georgi, J.A. 2002. The earliest known eutherian mammal. Nature 416: 816–822. Crossref
Johanson, Z., Josse, J., Boisvert, C.A., Ericsson, R., Sutija, M., and Ahlberg, P.E. 2007. Fish fingers: Digit homologues in sarcopterygian fish fins. Journal of Experimental Zoology Part B 308: 757–768. Crossref
Kammerer, C.F. 2011. Systematics of the Anteosauria (Therapsida: Dinocephalia). Journal of Systematic Palaeontology 9: 261–304. Crossref
Kammerer, C.F. 2016. Systematics of the Rubidgeinae (Therapsida: Gorgonopsia). PeerJ 4: e1608. Crossref
Kammerer, C.F. and Masyutin,V. 2018. Gorgonopsian therapsids (Nochnitsa gen. nov. and Viatkogorgon) from the Permian Kotelnich locality of Russia. PeerJ 6: e4954. Crossref
Kammerer, C.F., Angielczyk, K.D., and Fröbisch, J. 2011. A comprehensive taxonomic revision of Dicynodon (Therapsida, Anomodontia) and its implications for dicynodont phylogeny, biogeography, and biostratigraphy. Journal of Vertebrate Paleontology 31 (Supplement to 6): 1–158. Crossref
Keyser, A.W. 1993. A re-evaluation of the smaller Endothiodontidae. Memoirs of the Geological Survey of South Africa 82: 1–53.
Kielan-Jaworowska, Z. 1977. Evolution of the therian mammals in the Late Cretaceous of Asia. Part II. Postcranial skeleton in Kennalestes and Asioryctes. In: Z. Kielan-Jaworowska (ed.), Results Polish Mongolian Palaeontological Expeditions. Palaeontologica Polonica 37: 65–84.
Kielan-Jaworowaka, Z. 1978. Evolution of therian mammals in the Late Cretaceous of Asia. Part III. Postcranial skeleton in Zalambdalestidae. In: Z. Kielan-Jaworowska (ed.), Results Polish Mongolian Palaeontological Expeditions. Palaeontologica Polonica 38: 5–41.
Kielan-Jaworowska, Z., Cifelli, R.L., and Luo, Z.X. 2004. Mammals from the Age of Dinosaurs. Origins, Evolution, and Structure. 630 pp. Columbia University Press, New York. Crossref
Kindahl, M. 1941. Untersuchungen einiger Entwicklungsstadien der Hand und des Fußes von Erinaceus europaeus. Zeitschrift für mikroskopisch-anatomische Forschung 50: 458–464.
Kindahl, M. 1942a. Beitrag zur Kenntnis der Entwicklung des Extremitätenskelets bei Centetes ecaudatus und Ericulus setosus. Zeitschrift für mikroskopisch-anatomische Forschung 51: 322–333.
Kindahl, M. 1942b. Einige Mitteilungen über die Entwicklung der Hand und des Fußes bei Talpa europaea L. Zeitschrift für mikroskopisch-anatomische Forschung 52: 267–273.
Kindahl, M. 1944: On the development of the hand and the foot of Tarsius tarsius and Microcebus myoxinus. Acta Zoologica 25: 49–58. Crossref
Kivell, T.L. 2016. The primate wrist. In: T.L. Kivell, P. Lemelin, B.G. Richmond, and D. Schmitt (eds.), The Evolution of the Primate Hand. Anatomical, Developmental, Functional, and Paleontological Evidence, 17–54. Springer, New York. Crossref
Kjosness, K.M., Hines, J.E., Lovejoy, C.O., and Reno, P.L. 2014. The pisiform growth plate is lost in humans and supports a role for Hox in growth plate formation. Journal of Anatomy 225: 527–538. Crossref
Kümmell, S.B. 2009. Die Digiti der Synapsida: Anatomie, Evolution und Konstruktionsmorphologie. 424 pp. Ph.D. Thesis, Fakultät für Biowissenschaften der Universität Witten/Herdecke. Shaker Verlag, Aachen.
Kümmell, S.B. and Frey, E. 2012. What digits tell us about digging, running and climbing in recent and fossil Synapsida. Fundamental 20: 117–119.
Kümmell, S.B. and Frey, E. 2014a. Autopodial rotation as a measure for stance and gait in Synapsida from early Permian to later Cretaceous. In: M. Delfino, G. Carnevale, and M. Pavia (eds.), EAVP XII Annual Meeting, Abstract Book and Field Trip Guide, 91. Museo Regionale di Scienze Naturali, Torino.
Kümmell, S.B. and Frey, E. 2014b. Range of movement in ray I of manus and pes and the prehensility of the autopodia in the early Permian to Late Cretaceous non-anomodont Synapsida. PLoS One 9: e113911. Crossref
Liu, J., Schneider, V.P., and Olsen, P.E. 2017. The postcranial skeleton of Boreogomphodon (Cynodontia: Traversodontidae) from the Upper Triassic of North Carolina, USA and the comparison with other traversodontids. PeerJ 5: e3521. Crossref
Luo, Z.X. 2011. Developmental patterns in Mesozoic evolution of mammal ears. Annual Review of Ecology, Evolution, and Systematics 42: 355–80. Crossref
Luo, Z.X., Ji, Q., Wible, J.R., and Yuan, C.X. 2003. An early Cretaceous tribosphenic mammal and metatherian evolution. Science 302: 1934–1940. Crossref
Luo, Z.X., Yuan, C.X., Meng, Q.J., and Ji, Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476: 442–445. Crossref
Lyser, M. 1653. Culter anatomicus. 217 pp. Lamprecht, Copenhagen.
Maddison, W.P. and Maddison, D.R. 2019. Mesquite: a Modular System for Evolutionary Analysis. Version 3.61 http://www.mesquiteproject.org
Maisch, M.W. 2001. Observations on Karoo and Gondwana vertebrates. Part 2: A new skull-reconstruction of Stahleckeria potens von Huene, 1935 (Dicynodontia, Middle Triassic) and a reconsideration of kannemeyeriiform phylogeny. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 220: 127–152. Crossref
McNamara, K.J. 2002. What is heterochrony. In: N. Minugh-Purvis, K.J. McNamara (eds.), Human Evolution through Developmental Change, 1–4. The Johns Hopkins University Press, Baltimore.
McMurrich, J.P. 1914. The nomenclature of the carpal bones. Anatomical Record 8: 173–182. Crossref
Meng, Q.J., Ji, Q., Zhang, Y.G., Liu, D., Grossnickle, D.M., and Luo, Z.X. 2015. An arboreal docodont from the Jurassic and mammaliaform ecological diversification. Science 347: 764–768. Crossref
Milaire, J. 1978. Étude morphologique, histochemique et autoradiographique de développement du squelette des membres chez l’embryon de souris. I. Membres anterieurs. Archives Biologiques (Brussels) 89: 169–216.
Modesto, S.P., Smith, R.M.H., Campione, N.E., and Reisz, R.R. 2011. The last “pelycosaur”: a varanopid synapsid from the Pristerognathus Assemblage Zone, Middle Permian of South Africa. Naturwissenschaften 98: 1027–1034. Crossref
Monro, A. 1726. The Anatomy of the Humane Bones. 352 pp. Ruddiman, Edinburgh.
Müller, G.B. 1991. Evolutionary transformation of limb pattern: heterochrony and secondary fusion. In: J.R. Hinchliffe, J.M. Hurlé, and D. Summerbell (eds.), Developmental Pattering of the Vertebrate Limb, 395–405. Plenum Press, New York. Crossref
Müller, G.B. and Alberch, P. 1990. Ontogeny of the limb skeleton in Alligator mississippiensis: developmental invariance and change in the evolution of the archosaur limbs. Journal of Morphology 203: 151–164. Crossref
Nakashima, T., Hojo, T., Suzuki, K., and Ijichi, M. 1995. Symphalangism (two phalanges) in the digits of the Japanese foot. Annals of Anatomy 177: 275–278. Crossref
Nesslinger, C.L. 1956. Ossification centers and skeletal development in the postnatal virginia opossum. Journal of Mammalogy 37: 382–394. Crossref
O’Keefe, F.R., Sidor, C.A., Larsson, H.C.E., Maga, A., and Ide, O. 2006. Evolution and homology of the astragalus in early amniotes: New fossils, new perspectives. Journal of Morphology 267: 415–425. Crossref
Oliveira, C.A. de, Nogueira, J.C., and Bohórquez Mahecha, G.A. 1998. Sequential order of appearance of ossification centers in the opossum Didelphis albiventris (Didelphidae) skeleton during development in the Marsupium. Annals of Anatomy 180: 113–121. Crossref
Prochel, J., and Sánchez-Villagra, M.R. 2003. Carpal ontogeny in Monodelphis domestica and Caluromys philander (Marsupialia). Zoology 106: 73–84. Crossref
Ray, S. and Chinsamy, A. 2003. Functional aspects of the postcranial anatomy of the Permian dicynodont Diictodon and their ecological implications. Palaeontology 46: 151–183. Crossref
Ray, S., Botha, J., and Chinsamy, A. 2004. Bone histology and growth patterns of some nonmammalian therapsids. Journal of Vertebrate Paleontology 24: 634–648. Crossref
Reisz, R.R. 1986. Pelycosauria. Encyclopedia of Paleoherpetology. Part 17A: 1–102. Gustav Fischer Verlag, Stuttgart.
Reisz, R.R., Maddin, H.C., Fröbisch, J., and Falconnet, J. 2011. A new large caseid (Synapsida, Caseasauria) from the Permian of Rodez (France), including a reappraisal of “Casea” rutena Sigogneau-Russell & Russell, 1974. Geodiversitas 33: 227–246. Crossref
Reno, P.L., Kjosness, K.M., and Hines, J.E. 2016. The role of Hox in pisiform and calcaneus growth plate formation and the nature of the zeugopod/autopod boundary. Journal of Experimental Zoology Part B 326: 303–332. Crossref
Romer, A.S. and Parsons, T.S. 1977. The Vertebrate Body. 624 pp. Saunders College Publishing, Philadelphia.
Romer, A.S. and Price, L.I. 1940. Review of the Pelycosauria. Special Paper Geological Society of America 28: 1–538. Crossref
Romer, A.S. and Price, L.I. 1944. Stahleckeria lenzii, a giant Triassic Brazilian dicynodont. Bulletin Museum of Comparative Zoology 93: 465–491.
Rowe, T. 1988. Definition, diagnosis and origin of mammalia. Journal of Vertebrate Paleontology 8: 241–264. Crossref
Ruta, M., Botha-Brink, J., Mitchell, S.A., and Benton, M.J. 2013. The radiation of cynodonts and the ground plan of mammalian morphological diversity. Proceedings of the Royal Society B: Biological Sciences 280: 20131865. Crossref
Salesa, M.J., Antón, M., Peigné, S., and Morales, J. 2006. Evidence of a false thumb in a fossil carnivore clarifies the evolution of pandas. Proceedings of the National Academy of Sciences 103: 379–382. Crossref
Salomon, F.V., Geyer, H., and Gille, U. 2005. Anatomie für die Tiermedizin. 790 pp. Enke Verlag, Stuttgart.
Salton, J.A., and Sargis, E.J. 2008. Evolutionary morphology of the Tenrecoidea (Mammalia) carpal complex. Biological Journal of the Linnean Society 93: 267–288. Crossref
Schmidt-Ehrenberg, E.C. 1942. Die Embryogenese des Extremitätenskelettes der Säugetiere. Revue Suisse de Zoologie 49: 33–131.
Schneider, J.W., Lucas, S.G., Scholze, F., Voigt, S., Marchetti, L., Klein, H., Opluštil, S., Werneburg, R., Golubev, V.K., Barrick, J.E., Nemyrovska, T., Ronchi, A., Day, M.O., Silantiev, V.V., Rößler, R., Sabero, H., Linnemann, U., Zharinova, V., and Shen, S.Z. 2020. Late Paleozoic–early Mesozoic continental biostratigraphy. Links to the Standard Global Chronostratigraphic Scale. Palaeoworld 29 (2): 186–238. Crossref
Seeley, H.G. 1888. Researches on the structure, organization and classification of the fossil Reptilia. III. On parts of the skeleton of a mammal from Triassic rocks of Klipfontein, Fraserberg, South Africa (Theriodesmus phylarchus, Seeley), illustrating the reptilian inheritance in the mammalian hand. Philosophical Transactions of the Royal Society, Series B 179: 141–155. Crossref
Seeley, H.G. 1895. Researches on the structure, organization and classification of the fossil Reptilia. Part IX, Section 4. On the Gomphodontia. Philosophical Transactions of the Royal Society, Series B 186: 1–57. Crossref
Shubin, N.H. and Alberch, P. 1986. A morphogenetic approach to the origin and basic organization of the tetrapod limb. Evolutionary Biology 20: 319–387. Crossref
Sidor, C.A. 2001. Simplification as a trend in synapsid cranial evolution. Evolution 55: 1419–1442.
Sidor, C.A. and Hopson, J.A. 1998. Ghost lineages and “mammalness”: assessing the temporal pattern of character acquisition in the Synapsida. Paleobiology 24: 254–273. Crossref
Sigogneau-Russell, D. and Russell, D.E. 1974. Étude du premier caséidé (Reptilia, Pelycosauria) d’Europeoccidentale. Bulletin du Muséum national d’Histoire naturelle 230: 145–215.
Slabý, O. 1967. Die Morphogenese und phylogenetische Morphologie des Carpus der Paarhufer. 55 pp. Academia Nakladatelstvi Československé Akademie Vĕd, Praha.
Slabý, O. 1968. Wege und Gesetzmäßigkeiten der Evolution in Bezug auf die phylogenetische Entwicklung der Extremitäten. Acta Universitatis Carolinae Medica. Monographia 35: 1–137.
Smith, R.M. 1987. Helical burrow casts of therapsid origin from the Beaufort Group (Permian) of South Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 60: 155–170. Crossref
Stafford, B.J. and Thorington, R.W. 1998. Carpal development and morphology in archontan mammals. Journal of Morphology 235: 135–155. Crossref
Starck, D. 1979. Vergleichende Anatomie der Wirbeltiere, auf evolutionsbiologischer Grundlage. Vol. II: Das Skeletsystem. Allgemeines, Skeletsubstanzen, Skelet der Wirbeltiere einschließlich Lokomotionstypen. 776 pp. Springer Verlag, Berlin. Crossref
Steiner, H. 1921. Hand und Fuss der Amphibien, ein Beitrag zur Extremitätenfrage. Anatomischer Anzeiger 53: 513–542.
Steiner, H. 1935. Beiträge zur Gliedmaßentheorie. Die Entwicklung des Chiropterygium aus dem Ichthyopterygium. Revue Suisse de Zoologie 42: 715–729.
Steiner, H. 1942. Der Aufbau des Säugetier-Carpus und -Tarsus nach neueren embryologischen Untersuchungen. Revue Suisse de Zoologie 49: 217–223. Crossref
Stovall, J.W., Price, L.I., and Romer, A.S. 1966. The postcranial skeleton of the giant Permian pelycosaur Cotylorhynchus romeri. Bulletin of the Museum of Comparative Zoology 135: 1–30.
Sues, H.D. and Jenkins, F.A. 2006. The postcranial skeleton of Kayentatherium wellesi from the Lower Jurassic Kayenta Formation of Arizona and the phylogenetic significance of postcranial features in tritylodontid cynodonts. In: M.T. Carrano, T.J. Gaudin, R.W. Blob, and J.R. Wible (eds.), Amniote Paleobiology. Perspectives on the Evolution of Mammals, Birds, and Reptiles, 114–152. The University of Chicago Press, Chicago.
Sun, A. and Li, Y. 1985. The postcranial skeleton of the late tritylodont Bienotheroides [in Chinese with English summary]. Vertebrata PalAsiatica 23: 133–151.
Szalay, F.S. 1994. Evolutionary History of the Marsupials and an Analysis of Osteological Characters. 481 pp. Cambridge University Press, Cambridge. Crossref
Vega-Dias, C., Maisch, M., and Schultz, C.L. 2004. A new phylogenetic analyses of Triassic dicynodonts (Therapsida) and the systematic position of Jachaleria candelariensis from the Upper Triassic of Brazil. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 231: 145–166. Crossref
Velazco, P.M., Buczek, A.J., and Novacek, M.J. 2017. Two new tritylodontids (Synapsida, Cynodontia, Mammaliamorpha) from the Upper Jurassic, Southwestern Mongolia. American Museum Novitates 3874: 1–35. Crossref
Voigt, S. and Ganzelewski, M. 2010. Toward the origin of amniotes: Diadectomorph and synapsid footprints from the early late Carboniferous of Germany. Acta Palaeontologica Polonica 55: 57–72. Crossref
Voigt, S. and Lucas, S.G. 2017. Early Permian tetrapod footprints form central New Mexico. New Mexico. New Mexico Museum of Natural History and Science Bulletin 77: 333–352.
Wagner, G.P., Chiu, C.H., and Laubichler, M. 2000. Developmental evolution as a mechanistic science: The inference from developmental mechanisms to evolutionary processes. American Zoologist 40: 819‒831. Crossref
Watabe, M., Tsubamoto, T., and Tsogtbaatar, K. 2007. A new tritylodontid synapsid from Mongolia. Acta Palaeontologica Polonica 52: 263–274.
Wilson, L.A.B., Schradin, C., Mitgutsch, C., Galliari, F.C., Mess, A., and Sánchez-Villagra, M.R. 2010. Skeletogenesis and sequence heterochrony in rodent evolution, with particular emphasis on the African striped mouse, Rhabdomys pumilio (Mammalia). Organisms Diversity & Evolution 10: 243–258. Crossref
Yalden, D.W. 1966. The anatomy of mole locomotion. Journal of Zoology 149: 55–64. Crossref
Acta Palaeontol. Pol. 65 (4): 649–678,
2020 https://doi.org/10.4202/app.00709.2019