

New specimen of the rare requiem shark Eogaleus bolcensis from the Bolca Lagerstätte, Italy
GABRIELE LAROCCA CONTE, ENRICO TREVISANI, PAOLO GUASCHI, and FEDERICO FANTI
Larocca Conte, G., Trevisani, E., Guaschi, P., and Fanti, F. 2020. New specimen of the rare requiem shark Eogaleus bolcensis from the Bolca Lagerstätte, Italy. Acta Palaeontologica Polonica 65 (3): 547–560.
A rare carcharhinid specimen (slab and counter-slab, MSNPV 24625-24626) from the world-renowned Eocene Bolca locality was recently rediscovered during a restoration project started in 1989 by the Museo di Storia Naturale di Pavia. The individual, the largest Eogaleus bolcensis known from Bolca, is disarticulated and lies in a massive limestone matrix, suggesting its provenience from the Monte Postale site. While assessing its taxonomic status, multiple morphological affinities and ontogenetic trends within the Bolca Carcharhiniformes assemblage where documented. Eogaleus bolcensis is here distinguished from the school shark Galeorhinus cuvieri exclusively according to dermal denticle morphology, suggesting partial overlap of ecologic and trophic niches between the two species. Further, measurements and meristic counts taken on different traits of E. bolcensis (two individuals) and G. cuvieri (five individuals) specimens show high degree of similarities. The ratios “trunk length/total length” and “sum of vertebral centra (head region)/total length” of four complete individuals of the fossil assemblage were averaged and employed to estimates the total length of MSNPV 24625-24626. Here, the total length of MSNPV 24625-24626 is estimated in about 172.1±0.1 cm. The same approach is applied to MCSNV T.311 (E. bolcensis, holotype) and MNHN F.Bol.516 (G. cuvieri, holotype), two partially-preserved fossil individuals from Bolca locality. To support the ontogenetic variability among the Bolca shark assemblage, the age of the fossil individuals was estimated following the Von Bertalanffy Growth Function, using the modern chondrichthyans growth parameters as a reference. Data presented here suggest that all G. cuvieri specimens are juvenile individuals, whereas the E. bolcensis specimens were young-adult.
Key words: Chondrichthyes, Carcharhinidae, Triakidae, Eogaleus, Galeorhinus, Von Bertallanfy Growth Function, age classes, Eocene, Europe.
Gabriele Larocca Conte [glaroccaconte@ucmerced.edu], Environmental System, University of California Merced, 5200 North Lake Rd., 95343, Merced, California, United States of America; and Museo Geologico Giovanni Capellini, Alma Mater Studiorum, Università di Bologna, Via Zamboni 63, 40126, Bologna, Italy.
Enrico Trevisani [trevisani.enrico@comune.fe.it], Museo di Storia Naturale di Ferrara, Via De Pisis 24, 44121, Ferrara, Italy.
Paolo Guaschi [paolo.guaschi@unipv.it], Museo di Storia Naturale dell’Università di Pavia, Piazza Botta 9-10, 27100, Pavia, Italy.
Federico Fanti [federico.fanti@unibo.it], Dipartimento di Scienze Biologiche, Geologiche e Ambientali, Alma Mater Studiorum, Università di Bologna, Via Zamboni 67, 40126, Bologna, Italy; and Museo Geologico Giovanni Capellini, Alma Mater Studiorum, Università di Bologna, Via Zamboni 63, 40126, Bologna, Italy.
Received 10 January 2020, accepted 13 April 2020, available online 22 July 2020.
Copyright © 2020 G. Larocca Conte et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
In 1989, the Museo di Storia Naturale of the Pavia University in Italy started a complex renovation project with the goal of a complete census of the diverse items housed in the main building, including specimens pertaining to the historic paleontological collections. The need for a complete and detailed catalogue provided a unique opportunity for restoring, photographing and properly labeling several neglected specimens. Among those was an uncatalogued, exquisitely preserved individual of the requiem shark Eogaleus bolcensis from the Eocene of Bolca, Italy, one of the least represented taxa within the rich assemblage of this world-renowned “Fossil-Lagerstätte”.
The Ypresian Bolca fossil-bearing beds are among the few Eocene fossiliferous sites where partial and complete shark fossils have been collected. The taxonomy of these specimens has been discussed since the 1860’s (Molin 1861; Lioy 1865; De Zigno 1874; Jaekel 1894; De Beaumont 1960; Cappetta 1975; Fanti et al. 2016; Marramà et al. 2017a, b, 2018). A variety of research has been conducted on fossil sharks from the Bolca site, and includes detailed descriptions of specimens housed in Paris (MNHN), Bologna (MGGC), London (NHMUK), Padova (MGD-PD), and Verona (MCSNV) (Cappetta 1975; Fanti et al. 2016; Marramà et al. 2017a, b, 2018). To date, only 9 individuals and 16 isolated teeth have been collected from the Bolca site. These fossils belong to the carcharhinid E. bolcensis, the triakid Galeorhinus cuvieri, and the odontaspid lamniform Brachycarcharias lerichei.
The aim of this paper is twofold: (i), we provide a detailed anatomical description of the new E. bolcensis individual and we estimate its total length (TL) using a comparative dataset of shark centrum diameter and total length; (ii) we compare the fossil individuals of the Bolca site to highlight remarkable anatomical and ecological similarities between the taxa E. bolcensis and G. cuvieri.
Institutional abbreviations.—MCSNV, Museo Civico di Storia Naturale di Verona, Italy; MGGC, Museo Geologico Giovanni Capellini, Bologna, Italy; MGP-PD, Museo Geologico-Paleontologico dell’Università di Padova, Italy; MNHN, Muséum National d’Histoire naturelle of Paris, France; MSNPV, Museo di Storia Naturale dell’Università di Pavia, Italy.
Other abbreviations.—MC, Monte Postale site; TL, total length; VBGF, Von Bertalanffy Growth Function.
Material and methods
The investigation into the historic archive of the Pavia Museum yielded important information about the historical specimen (MSNPV 24625-24626, slab and counter-slab). In 1825, the Museum Director, Gian Maria Zendrini, purchased nine specimens collected from the Bolca locality (Jucci 1939; Rovati 1999; Galeotti 1999) including the slabs of the single specimen. As no original illustration or photographic material of the acquisition has been uncovered, the description provided by Gironi et al. (1831), following Zendrini’s annotations, represented the sole reliable source for identifying the shark material among the acquired material. Gironi et al. (1831) first identified the specimen, most likely based on the sole description of the Bolca vertebrates available at the time provided by Volta (1796–1808: 224, pl. 55) and Blainville (1818: 94), and assigning the specimen to the swamp eel Synbranchus immaculatus (now a synonym of S. marmoratus Bloch, 1795). The historical archive of the museums where the chondrichthyans from Bolca are housed does not report an accurate locality where shark samples from Bolca were excavated. Similarly, the stratigraphic context of Bolca Carcharhiniformes individuals is not mentioned. However, shark specimens from Bolca locality are restricted to two of the five fossiliferous sites: the Pesciara quarry and the Monte Postale site. These sites are roughly correlated stratigraphically and chronologically. Although such official documentation lacks detailed locality information, samples can be assigned to peculiar sites based on lithological and taphonomic characteristics (Papazzoni and Trevisani 2006; Cerato 2011; Trevisani 2015; Marramà et al. 2016). Broadly, the Monte Postale succession includes massive, low organic content limestone beds with the occurrence of benthic mollusks and bioturbations (Papazzoni and Trevisani 2006; Cerato 2011; Trevisani 2015). Fossils recovered from the Monte Postale site are often disarticulated, exposing single or multiple concave distortions of the vertebral column (Marramà et al. 2016). Following these geological and taphonomic characteristics, we assign the Monte Postale succession as the fossiliferous site from Bolca locality where the individual MSNPV 24625-24626 was originally collected (Massimo Cerato, personal communication 2019).
The slabs MSNPV 24625-24626 were cleaned and restored using soft brushes and silicone (CTS© silica 110). Several fossil elements including dermal denticles, vertebral centra, and teeth were covered with mortar glue which was probably applied during collection of the slabs. Selected areas of both slabs were prepared to expose the fossil. Following the methodology outlined by Fanti et al. (2016), the specimen was carefully analyzed under natural and UV-light. We collected samples of dermal denticles from the head region preserved in slab MSNPV 24625 and from the distal-dorsal part of the trunk preserved in slab MSNPV 24626. Scales were examined with a Scanning Electron Microscope (SEM; Tescan Mira3; Voltage = 20.0 kV) at the University of Pavia. Close examination of the specimen using UV light allowed us to accurately discriminate preserved cartilaginous tissues from areas where the pigmented mortar glue was employed.
Body-size terminology and measurements follow Cappetta (1975; Fig. 1A). Teeth are described using combined terminologies of Ebert and Stehmann (2013) and Adnet and Cappetta (2008; Fig. 1B). Finally, the terminology for dermal denticles follows Dillon et al. (2017) and Ferrón and Botella (2017). Measurements were taken with a digital caliper to the nearest 0.5 mm for teeth and 1 mm for vertebral centra. Eogaleus bolcensis (MGP-PD 8869C-8870C, MCSNV T.311) and Galeorhinus cuvieri (MGP-PD 8871-8872, MCSNV T.1124, MCSNV VII.B.96-VII.B.97, MGGC 1976, MNHN F.Bol.516) individuals were analyzed by following the adopted methodological approach.
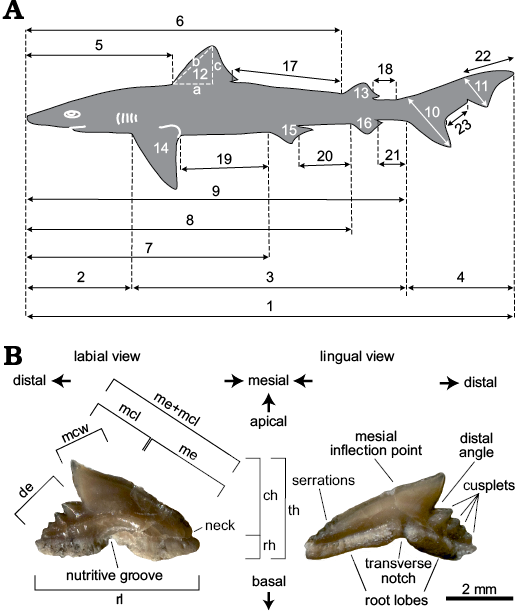
Fig. 1. A. Terminology of longitudinal measurements following Cappetta (1975). Measurements 17–23 have been added in this paper, expanding the original model. 1, total length (TL); 2, head length; 3, trunk length; 4, caudal fin length; 5, head-first dorsal fin length; 6, head-second dorsal fin length; 7, head-pelvic fin length; 8, head-anal fin length; 9, head-caudal fin length; 10, basal lobe length; 11, apical lobe length; 12a-b-c, first dorsal base-anterior edge-height; 13a-b-c, second dorsal base-anterior edge-height; 14a-b-c, pectoral fin base-anterior edge-height; 15a-b-c, pelvic fin base-anterior edge-height; 16a-b-c, anal fin base-anterior edge-height; 17, first-second dorsal fins length; 18, second dorsal-caudal fin length; 19, pectoral-pelvic fins length; 20, pelvic-anal fins length; 21, anal-caudal fins length; 22, apical lobe-caudal tip length; 23, basal-apical lobes length. B. Terminology of dental characters according to Adnet and Cappetta (2008) and Ebert and Stehemann (2013). MGGC 1976 in labial (B1) and lingual (B2) views. Measurements taken for each tooth: ch, crown height; de, distal edge; mcl, main cusp length; mcw, main cusp width; me, mesial edge; me+mcl, mesial edge + main cusp length; rh, root height; rl, root length; th, total height.
The completeness of several fossil individuals (MGP-PD 8871-8872, slab and counter-slab; MGP-PD 8869C-8870C, slab and counter-slab; MCSNV T.1124; MGGC 1976) provided a unique opportunity to compare the morphometric body measurements in terms of percentage relative to the total length (%TL) among the taxa E. bolcensis and G. cuvieri (SOM 1: table 1, Supplementary Online Material available at http://app.pan.pl/SOM/app65-LaroccaConte_etal_SOM.pdf). We used such dataset to estimate the total length (Table 1) of the partially preserved individuals (MSNPV 24625-24626, MNHN F.Bol.516, and MCSNV T.311). The errors associated with the estimates are expressed in absolute (Δa) and percentage (ɛ%) errors. We applied the Von Bertalanffy Growth Function (Bertalanffy 1938) to our body measurements dataset in order to estimate the age of the MGP-PD 8869C-8870C, MGP-PD 8871-8872, MCSNV VII.B.96-VII.B.97, MCSNV T.311, MCSNV T.1124, MSNPV 24625-24626, MGGC 1976, and MNHN F.Bol.516. We used age-growth curves of extant sharks of the families Carcharhinidae, Scyliorhinidae, Sphyrnidae, and Triakidae as a reference to compare the age-growth values of E. bolcensis and G. cuvieri individuals. Data presented here include growth parameters representative of Carcharhinus brevipinna, C. leucas, C. longimanus, Galeocerdo cuvier, Galeorhinus galeus, Galeus sauteri, Isogomphodon oxyrhynchus, Mustelus antarticus, M. californicus, M. lenticulatus, M. mustelus, Negaprion brevirostris, Prionace glauca, Rhizoprionodon porosus, R. lalandii, Sphyrna lewini, S. tiburo, S. zygaena, and Triakis semifasciata (SOM 1: table 2; Olsen 1984; Kusher 1987; Brown and Gruber 1988; Yudin and Caillet 1990; Francis and Francis 1992; Kusher et al. 1992; Moulton et al. 1992; Parsons 1993; Goosen and Smale 1997; Francis and Mulligan 1998; Lessa et al. 1999, 2000, 2009; Wintner and Dudley 2000; Skomal and Natanson 2003; Cruz-Martinez et al. 2004; Joung et al. 2005; Piercy et al. 2007; Coelho et al. 2011; Liu et al. 2011).
Systematic palaeontology
Class Chondrichthyes Huxley, 1880
Subclass Elasmobranchii Bonaparte, 1838
Order Carcharhiniformes Compagno, 1973
Family Carcharhinidae Jordan and Evermann, 1896
Genus Eogaleus Cappetta, 1975
Type species: Eogaleus bolcensis Cappetta, 1975; Bolca locality, Italy, Eocene.
Eogaleus bolcensis Cappetta, 1975
Material.—MSNPV 24625-24626, a partially articulated specimen, 120 cm TL (Figs. 2–6) most likely from Monte Postale (GPS 45°36’13.85” N, 11°13’21.41” E), Bolca locality, Vestenanova, Verona, Italy; Eocene, Ypresian, middle–late Cuisian, SBZ 11 Alveolina dainelli Biozone (Serra-Kiel et al. 1998; Trevisani 2015). The specimen is in a massive limestone matrix. The teeth are only preserved on slab MSNPV 24625, while the dermal denticles patches are preserved on both slabs. The individual is disarticulated, with an S-shaped vertebral curvature due to postmortem muscular contraction. A cluster of bivalves is preserved posterior to the head.
Description.—A partially preserved carcharhinid with the following overall features: teeth of different shape and size, of height up to 6 mm; disarticulated cartilages in the head region; 132 preserved vertebral centra, wider than high, showing an S-shaped curvature posterior to the head; clusters of fin radials run ventrally along the vertebral column; two types of dermal denticles (arrow- and teardrop-shaped) arranged in patches.
The whole individual (MSNPV 24625-24626) measures 120 cm (preserved body length; Fig. 2). The head region and the proximal section of the trunk are twisted clockwise. A bivalve cluster is preserved posteriorly to the head region (Figs. 2, 3).
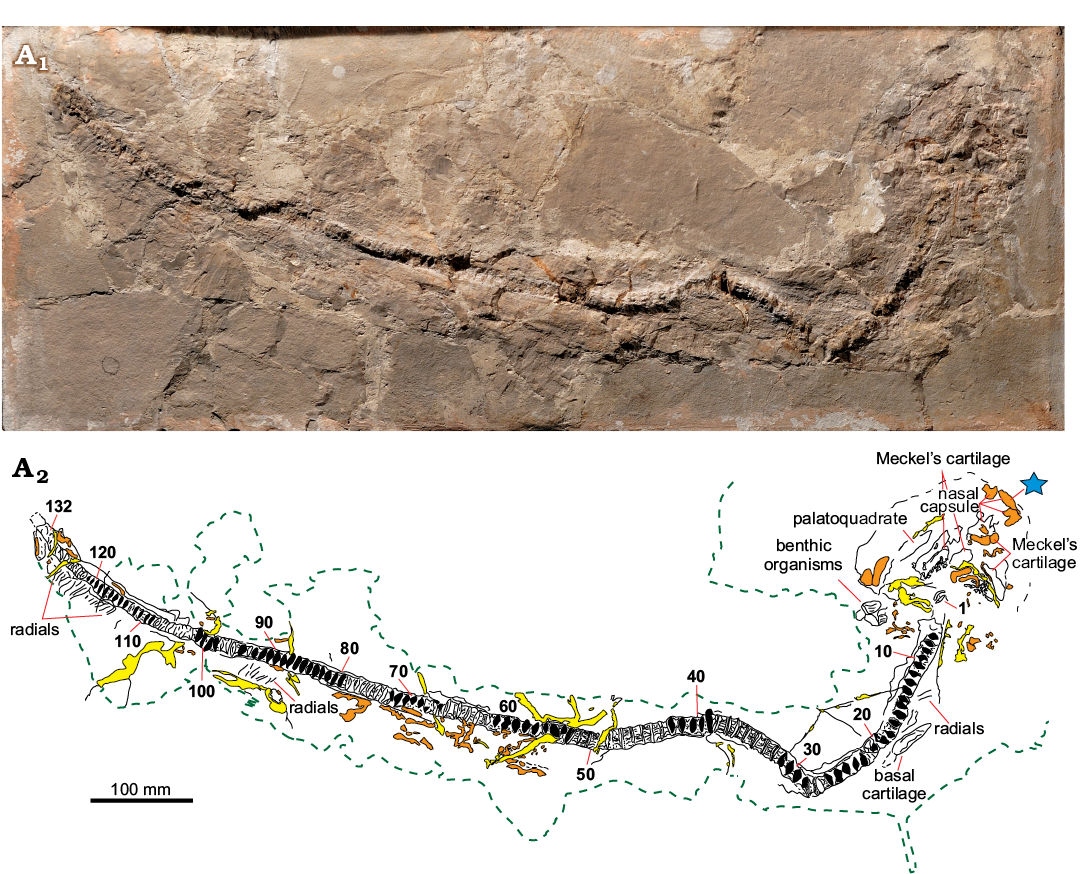
Fig. 2. Eogaleus bolcensis Cappetta, 1975, MSNPV 24625, Monte Postale, Bolca, Italy; middle–late Cuisian. The head region is exposed in dorsal view, while the trunk area is exposed in lateral view. Photograph (A1), explanatory drawing (A2). Dermal denticles are widespread arranged in patches along the body (orange). The yellow patches mark the different typologies of mortar glues employed for the assemblage of the slab. The green dashed lines separate the matrix of the specimens from the rocky blocks used for the preparation of the slabs. The star indicates the anatomical area (i.e., a fragment from identified nasal capsule) where dermal denticle samples were collected. Numerals refer to the arrangement of vertebral elements in life position. Black patches mark casts of vertebral centra.
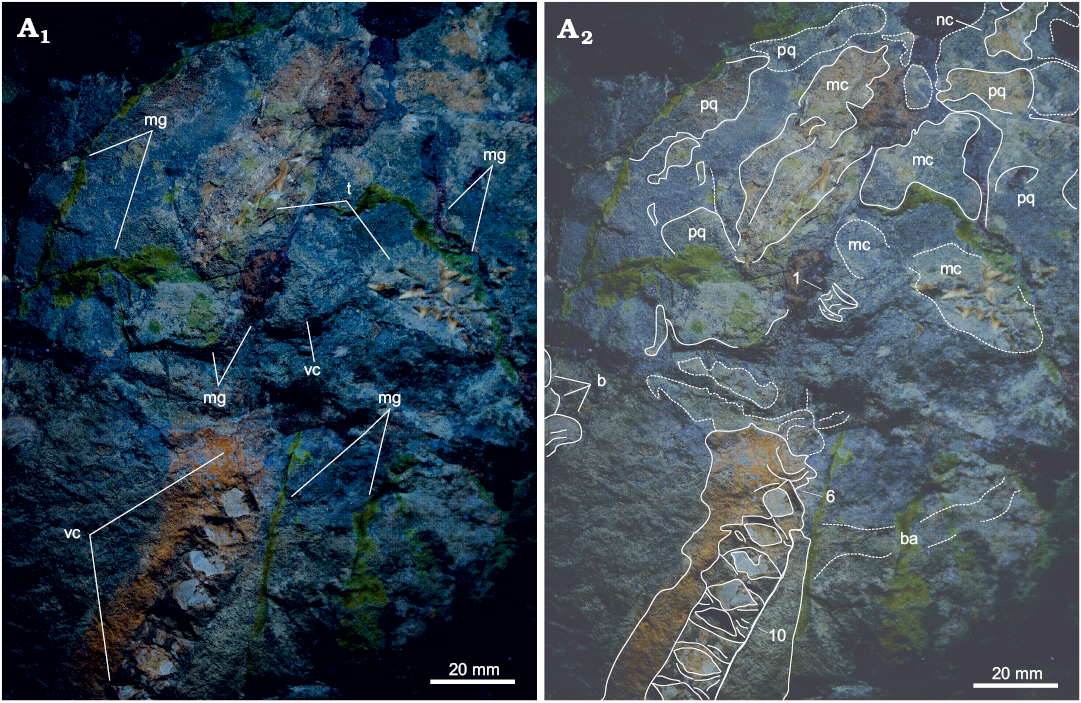
Fig. 3. Dorsal view of the head region of Eogaleus bolcensis Cappetta, 1975, MSNPV 24625, Monte Postale, Bolca, Italy; middle–late Cuisian. Photograph under UV light (A1), the hard tissues well differentiate from matrix bulk and pigmented mortar glues: photograph under natural light with explanatory drawing (A2). Numerals refer to the arrangement of vertebral elements in life position. Abbreviations: b, benthic organisms; ba, branchial arch; mc, Meckel’s cartilage; mg, mortar glue; nc, nasal capsule; pq, palatequadrate; t, teeth; vc, vertebral centra.
In the head region (Fig. 3), the palatoquadrate and the Meckel’s cartilage are fragmented and associated with patches of dermal denticles, that overall mark an arched trend. The right nasal capsule extends from the palatoquadrate to the anterior proximity of the preserved head. The first vertebral centrum is placed distal to the Meckel’s cartilage. In slab MSNPV 24625, a branchial arch is preserved posterior to the 7th and 8th centra, and is covered by dermal denticles patches (Fig. 3).
No teeth are preserved in slab MSNPV 24626. Twenty-five teeth are observed on slab MSNPV 24625 (Fig. 4). All teeth are exposed in labial view. The variation in shape and size of preserved teeth indicate high heterodonty (Fig. 4). All the measurements of teeth are provided in SOM 2. Lateral teeth are the most abundant type of the preserved tooth set (Fig. 4A2, B2). Teeth are triangular in overall shape and asymmetrical along the basal-apical axis. The total tooth height is 44% of the root length. The crown is smooth. The mesial edge is straight and has up to eight serrations. The mesial inflection point is sharp. The main cusp is approximately 20% of the root length and it is inclined distally. The distal angle (i.e., the angle set up by the intersection between the distal edge and the main cusp) is acute, approximately 45°, with a deep notch at the main cusp-distal edge intersection. The distal edge bears three secondary cusps that decrease in size distally. The distal edge is about half of the mesial one. The root is wider than high and flat on its labial surface. Several foramina run along the root surface. The anterior teeth are different in shape and size (Fig. 4B2: teeth 17, 19, and 21). The shape is overall triangular, with a mesial edge that bears up to two distally secondary cusps. The crown is smooth. The main cusp is robust or slender. The distal angle is approximately right or slightly higher than 90°. The distal edge is smooth or bears one secondary cusp. One symphyseal tooth (Fig. 4A2: tooth 12) is preserved. It is a triangular, symmetrical tooth as high as wide ( th/rl 1). Mesial and distal edges are about 25% of total height. The main cusp is approximately 3/4 of the total height and lacks serrations. The crown is smooth and convex. The distal angle is slightly higher than 90°, with a deep notch. The distal edge bears one secondary cusp.

Fig. 4. The head region of Eogaleus bolcensis Cappetta, 1975, MSNPV 24625, Monte Postale, Bolca, Italy; middle–late Cuisian, in dorsal view with preserved teeth in labial view. Close-up photos of left (A) and right (B) lateral areas of the preserved head region. Photographs under natural light (A1, B1), explanatory drawings (A2, B2), preserved teeth (orange), and tooth molds (violet). Numbers refer to teeth count on MSNPV 24625, for measurements see SOM 2. Abbreviations: mc, Meckel’s cartilage; mg, mortar glue.
The vertebral column includes 132 preserved centra and displays a strongly S-shaped bent (Fig. 2, 5; measurements provided in SOM 3). Vertebral centra are wider than high with prominent, wedge-shaped intermedial calcifications. The antero-posterior alignment of vertebral centra shows no evidence of antero-posterior compression during burial and we intepret the arangement to reflect the true anatomical positioning. The size of centra increases posteriorly, hitting a peak at the 39th centrum, the widest of the series (antero-posterior length 16 mm). The antero-posterior length declines caudally. A further peak in vertebral size is reached at the 49th centrum (antero-posterior length 16 mm; Fig. 5). The caudal decreases in the overall size of vertebral centra are herein grouped in three vertebral segments (Fig. 5): 40th–67th centrum (average ~10–11 mm), 68th–98th centrum (average ~7–8 mm), and 99th–132nd centrum (average 6 mm).
Basal cartilage is observed between the 16th and the 22nd vertebral centra (Fig. 6A1, B1). Finally, two clusters of poorly preserved ventral fin radials are preserved between the 88th and the 98th and the 115th and the 125th vertebral centra (for a total of 15 fin radials counted) (Fig. 6A2, B2).
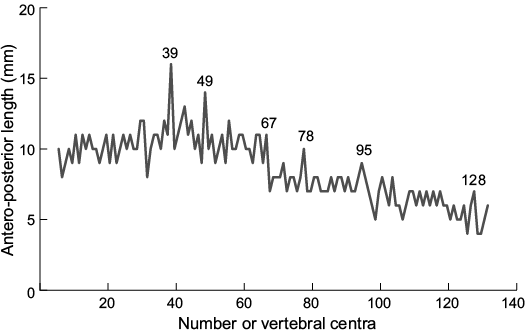
Fig. 5. Antero-posterior length of vertebral centra of Eogaleus bolcensis, MSNPV 24625. The size of centra increases rapidly the 33th–39th (16 mm). Caudal to the 39th vertebral centrum, the centrum size decreases slowly in 3 steps: (i) the 40th–67th (average: 10.6 mm reaching a peak of 14 mm at the 49th centrum); (ii) the 68th–98th (average: 7.6 mm); (iii) the 99th–132nd (average: 6.0 mm). Vertebrae are numbered from anterior (1) to posterior (132). Measurements and counts are provided in SOM 3.
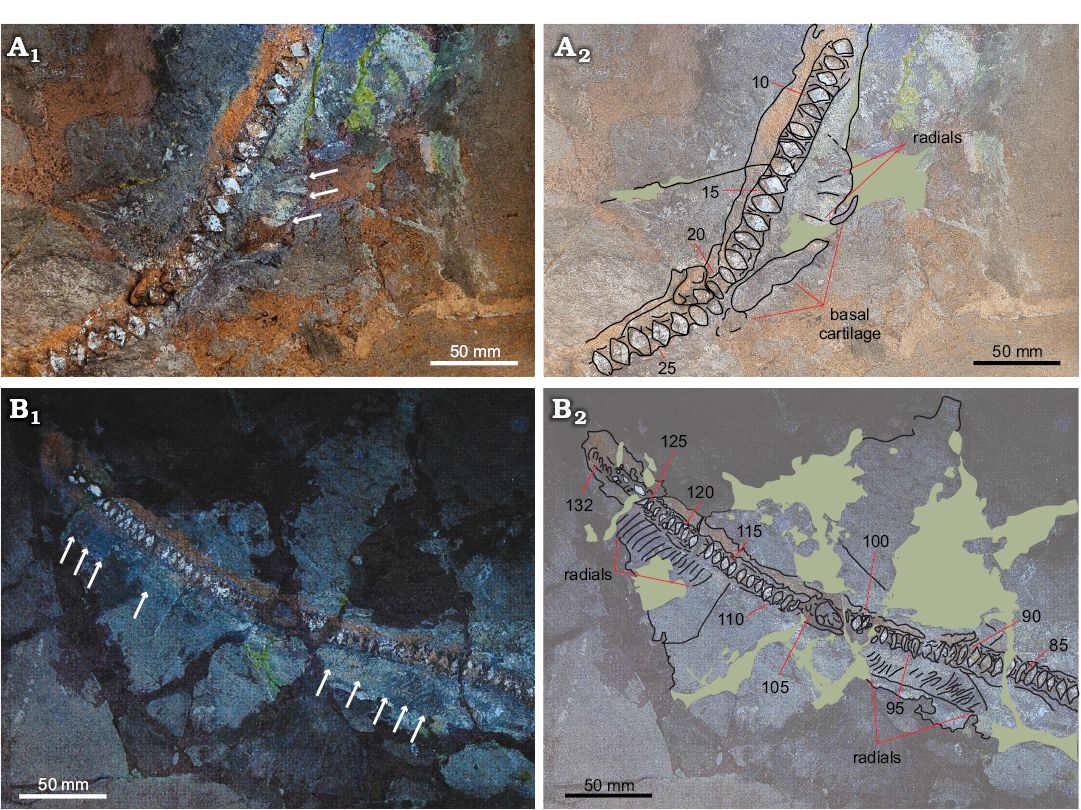
Fig. 6. The apical area (A) and distal region (B) of the trunk of Eogaleus bolcensis Cappetta, 1975, MSNPV 24625, Monte Postale, Bolca, Italy, middle–late Cuisian, in lateral view. Photographs under UV light (A1, B1) and interpretative drawings (A2, B2). The basal cartilage extends ventrally and parallel to vertebral column among the 16th–22nd centra. In B1 the clusters of fin radials ventrally extend among the 88th–98th and the 115th–125th centra. The arrows in A1 and B1 point out the preserved fin radials. Green patches mark areas covered by mortar glue. Numerals refer to the vertebral counts.
The external morphology of MSNPV 24625-24626 is interpreted using the following criteria: vertebral elements included in the head region extends to the 15th centrum for a total length of 12.4 cm. The preserved head length (i.e., from the 15th centrum to the anterior edge of the nasal capsule) is approximately 18 cm; the basal cartilage preserved between the 16th and the 22nd is referred to as the pectoral fin. The 16th centrum marks the beginning of the trunk region. Fin radials clustered among the 88th–98th centra are considered as components of the anal fin. The 114th centrum marks the end of the trunk area (total centra of the trunk 99). According to vertebral centra length, the trunk is 89.3 cm long. The ventral fin radials between the 115th–125th centra are interpreted as components of the caudal fin. The 115th centrum is the first of the caudal series, which measures 10.1 cm.
Patches of dermal denticles occur discontinuously along the entire body of the shark (Fig. 7). SEM imaging of skin samples collected from the head region and the distal part of the trunk revealed two different morphotypes. The first morphotype (here referred to as Morphotype A; Fig. 7A) includes arrow-shaped scales. This type of denticles is taller than wide, running along the distal area of the trunk. The number of ridges per scale varies from 5 to 8; the edges of peaks (i.e., apical termination of ridges) are rounded. The crown measures approximately 420 μm in average. The overall thickness is about 100 μm. Hexagonal, micro-cells cover the bottom outline of the scale. In several individual scales, the surface is medio-distally thickened by ridges, developing an upward-pointing spine. Ridges diverge apically and are well separated, arranging subparallel to each other. The interspace of ridges ranges between 100 and 120 μm. The second morphotype (here referred as to Morphotype B; Fig. 7B) includes teardrop-shaped scales, taller than wide (about 75 μm thick), and they are arranged on the head region. The crown size is about 450 μm. Six subparallel ridges develop on the crown surface, in which the medial ones converge apically. Ridges decrease progressively in thickness apically until they merge with the surface. The ridge interspace average is approximately 74 μm. The apical edge is smooth. The surface is distally covered by hexagonal micro-structures. Medial spines are absent.
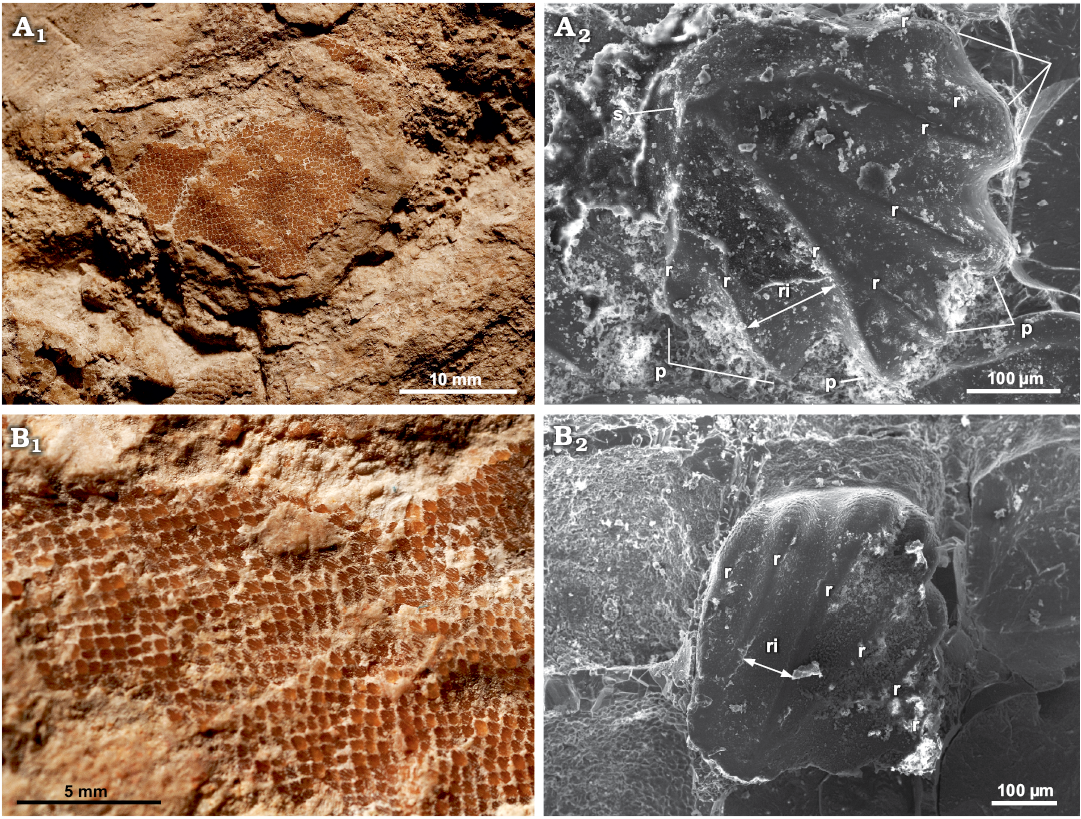
Fig. 7. Dermal denticles at different scales of head and trunk regions (in dorsal view) of Eogaleus bolcensis Cappetta, 1975, Monte Postale, Bolca, Italy; middle–late Cuisian. A. MSNPV 24625, details of a skin patch preserved in the head region (A1), SEM imaging of a Morphotype A denticle (A2); the scale is thick and well ridged, an upward-pointing spine occurs in dorsal view, thickened by the distal convergence of ridges. B. MSNPV 24626, patches of dermal denticles of arranged ventrally to vertebral column, along the distal area of the trunk (B1), SEM imaging of a denticle in dorsal view from the trunk region, where dermal denticles are teardrop-shaped (Morphotype B) (B2); the ridges end apically, without forming peaks, medial-distal upward spines are absent. Abbreviations: p, peak; r, ridge; ri, ridge interspace; s, spine.
Discussion
Taxonomic identification.—To date, the species Eogaleus bolcensis (Cappetta, 1975) is differentiated from the other Bolca carcharhiniforms Galeorhinus cuvieri (Agassiz, 1835) by distinct anatomical features, including overall body morphology and proportions, number of vertebral centra, tooth morphology and size, and dermal denticles shape and size (Cappetta 1975; Fanti et al. 2016; Marramà et al. 2018). Analysis of dental characteristics in the new specimens suggests similarities with tooth types of E. bolcensis. However, dentition, as well as external morphology, cannot provide enough information to properly assess MSNPV 24625-24626 due to taphonomic artifacts (i.e., the individual is partially preserved, and few well-preserved teeth are exposed in the head region). Dermal denticles represent a reliable anatomical feature for comparison, and assign the individual described herein to the taxon E. bolcensis. Dermal scales of E. bolcensis display systematic differences in shape and size with those of G. cuvieri. Morphotype A has morphological and size affinities with placoid scales collected from the trunk region of MCSNV VII.B.94 (E. bolcensis; Cappetta 1975: fig. 4c; Marramà et al. 2018: fig. 9D), and the diagnosis of Morphotype B matches with the irregular-shaped scales found in the head, pelvic and caudal region of the same individual (Cappetta 1975: fig. 4e).
Galeorhinus vs. Eogaleus: taphonomic artifacts and affinities.—The shark assemblage from Bolca offers a rare opportunity to study a framework of the diversity of carcharhiniforms during the early Eocene. The completeness of the fossil shark material from this assemblage allows for an in-depth comparative assessment.
Tooth morphology represents the most common source for the identification of extinct shark species (Cappetta 2012; Marramà and Kriwet 2017). Dental analysis of the fossil taxa Eogaleus and Galeorhinus provided by Fanti et al. (2016), Marramà et al. (2018), and this study highlights differences in the taxonomic position of the two genera. Unlike G. cuvieri, the anterior teeth of E. bolcensis are bladelike and taller than they are wide, with the main cusp weakly expanded along its mesio-distal axis. The labial face of the crown is thickened by a bulge that partially overshadows the root basally (for the comparison see Marramà et al. 2018: fig. 7a, b; Fanti et al. 2016: fig. 5e–g). Furthermore, no mesial heel and upturned crown tip are displayed in lateral and antero-lateral teeth of E. bolcensis and the roots are generally thicker than those of G. cuvieri (for the comparison see Marramà et al. 2018: fig. 7a, b; Fanti et al. 2016: fig. 5e–g). However, except for the tooth size, lateral and antero-lateral teeth of E. bolcensis have overall shape and dental characteristics quite similar to those of G. cuvieri, such as the cutting-type, incised distal heel, cusplets that decrease in size distally (i.e., up to four cusplets in E. bolcenses, whereas up to five cusplets could occur in G. cuvieri dental types), occurrence of serrations on the mesial edge (for the comparison see Marramà et al. 2018: figs. 7c, d, 8a–f; Fanti et al. 2016: figs. 5a, b, h, i).
Dermal denticles are commonly used in living taxa as a tool to detect spatial and temporal patterns to survey seasonal movements and habitat preferences of coral-reef shark communities (Dillon et al. 2017; Ferrón and Botella 2017). Denticle morphotypes vary along the body of a single individual in shape and size, and their morphology is strictly linked to (i) the function that a single scale plays; (ii) the ecological niche occupied by the shark species. Except for a few documented cases, dermal denticle morphology is usually identified at the family level (Reif 1982, 1985a, b; Raschi and Tabit 1992; Dillon et al. 2017; Ferrón and Botella 2017). However, dermal denticle morphologies found on Bolca individuals (Cappetta 1975: figs. 4, 9c–e; Fanti et al. 2016: fig. 7c–h; Marramà et al. 2018: fig. 9; this study) definitively discriminate the individuals among G. cuvieri and E. bolcensis. Following the classification of Dillon et al. (2017) and Ferrón and Botella (2017), the denticle morphotypes of G. cuvieri described by Fanti et al. (2016) can be included into the generalized function-type (i.e., dermal denticles that exposes: no peaks on its anterior edge; smooth crown or crown with moderately low developed, ridges and furrows; an overall rounded or teardrop-shape) since none of the ridges, when present, run parallel to each other along the apical-distal axis of the dorsal face of each scale. Accordingly, G. cuvieri was probably a schooling species of low-moderate speed inhabiting warm or tropical waters (e.g., enclosed bays or lagoons), similar to the extant school shark G. galeus (Fanti et al. 2016; Dillon et al. 2017; Ferrón and Botella 2017). In contrast, Morphotype A and B of MSNPV 24625-24626 belong to the defense and generalized functions types, respectively. Defense-type scales (i.e., dermal denticles with the following characters: star- or arrow-shaped denticle, with the crown that bears thick ridges, joining into an upward spine posteriorly; furrows are low deepened or absent; the anterior edge of the crown is shaped by developed peaks) are also found on MCSNV VII.B.94; Marramà et al. (2018: fig. 9D) included these denticles into the drag-reduction type (i.e., crowned- or oval-shaped scales with high developed ridges that run parallel to each other and cross the whole crown surface; the ridges are well separated by deep furrows; the peaks are prominent in the anterior edge of the crown) although we consider such denticles as defense-type since ridges merge posteriorly on the crown surface. Furthermore, this morphotype is virtually indistinguishable to arrow-shaped scales of the living tiger shark Galeocerdo cuvier (see Dillon et al. 2017: fig. 2a, denticle “r”). Thus, denticle morphology suggests E. bolcensis was a moderate-swimming speed taxon with costal-pelagic behaviors similar to the modern tiger shark (Dillon et al. 2017; Ferrón and Botella 2017). Marammà et al. (2018: fig. 10) compared the ecological role of E. bolcensis and modern sharks based on the correlation between ridge spacing and crown width, inferring E. bolcensis as a near-shore moderate swimmer. Such statistical analysis is consistent with the ecological behaviors of E. bolcensis inferred in this study.
Meristic characters are used to evaluate inter- and intra-specific variation (Jawad 2001). Vertebral elements maintain a central role in swimming. As Porter et al. (2016) recently argued, the biomechanism of vertebral bending acts as spring where stiffness and bending properties depend on the degree of the vertebral calcification. Either shape, size, and number of vertebral centra are active controllers of the body curvature during the swimming (Porter et al. 2009). Vertebral counts performed on Bolca carcharhiniforms is not a reliable anatomical character to discriminate G. cuvieri from E. bolcensis. Cappetta (1975: pl. 2) and Marramà et al. (2018: fig. 2a) considered the individual MCSNV T.311 (E. bolcensis, holotype; the locality of the specimen is unclear) as a nearly complete specimen counting 139 vertebral centra. MGP-PD 8869C-8870C, an E. bolcensis individual from the Pesciara quarry (Cappetta 1975; Marramà et al. 2018), preserves 202 centra, considerably higher than MCSNV T.311 and the range proposed by Cappetta (1975) and Marramà et al. (2018) for the genus (see SOM 3 for measurements of centra). Figure 8 shows the centra size profiles of Bolca fossil specimens. All specimens show an increase in size on the anterior part of the vertebral column. All the individuals display a dramatic increase in the antero-posterior size of vertebral centra around the 40th centrum, which corresponds to the trunk area close to the first dorsal fin. The length of centra decreases in several steps toward the caudal fin. The graph outlines close similarities of counts and size trends between MGP-PD 8869C-8870C (classified as E. bolcensis) and G. cuvieri specimens. Thus, affinities in vertebral elements and arrangements may suggest a similar swimming performance among the two fossil taxa. In comparison to the examined specimens, the plotted axial arrangements of MSNPV 24625-24626 and MCSNV T.311 lack the distal decreasing steps, interrupting abruptly through the caudal region (Fig. 8). Such data support incompleteness of the holotype MCSNV T.311 and similar taphonomic remarks to those of MSNPV 24625-24626. Finally, the individual MCSNV T.311 may be addressed as an E. bolcensis specimen from the Monte Postale locality, as well as MSNPV 24625-24626.
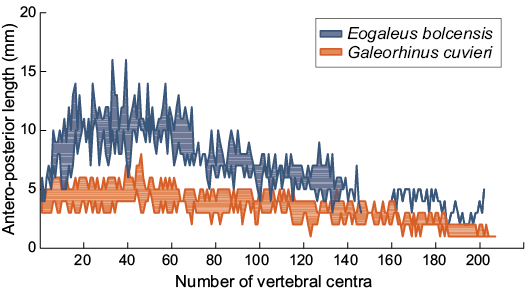
Fig. 8. Trends in antero-posterior length of vertebra of Galeorhinus cuvieri and Eogaleus bolcensis. Each area is given by the minimum and the maximum antero-posterior length of a single centrum from the same anatomical position of different individuals. All specimens show a slowly increase in size on the anterior area of the trunk. The largest size of centra occurs around the 40th centrum, close to the first dorsal. The length of centra decreases in several steps towards the caudal fin. Centra are numbered according to their anterior-posterior longitudinal arrangements. Measurements and counts are provided in SOM 3.
Longitudinal measurements of the external morphology are commonly used to investigate morphometric differences at the species level and species-specific variation between shark communities. Body measurements of fossil shark specimens from Bolca (SOM 1: table 1) display consistent affinities with living taxa, thus supporting negligible morphological variations among Carcharhiniformes body proportion from the Eocene and onward (Compagno and Garrik 1983; Compagno 1984; Compagno and Stevens 1993; Compagno et al. 1996, 2008; Choi et al. 1998; Sato et al. 1999; Nakaya and Séret 2000; White and Last 2006; Séret and Last 2007; Schaaf-Da Silva and Ebert 2008; White and Ebert 2008; Iglésias 2012; McCosker et al. 2012; Weigmann 2012; White and Harris 2013; White and Weigmann 2014; Famhi and White 2015).
Body-size estimates and growth curves.—As Galeorhinus cuvieri and Eogaleus bolcensis display no significant differences in their body ratios, all specimens have been included on the same plots. To estimate the body length of MSNPV 24625-24626, the ratios of the trunk and the centra of the head region were plotted against each body segment. Estimates were calculated using four well-preserved Bolca carcharhiniforms as standards (MCSNV T.1124, VII.B.97; MGGC 1976; MGP-PD 8869C-8870C, 8871-8872). We used the averages of trunk length/total length and length of vertebral centra of head region/total length, computed from well-preserved Bolca carcharhinids, to estimate the total length of MSNPV 24625-24626. The Total length of MSNPV 24625-24626 is here estimated in about 172.1±0.1 cm (absolute error (Δa) 0.1 cm, percentage error (ɛ%) 0.03%; Table 1, Fig. 9A). This approach has been consequently extended to MCSNV T.311 and MNHN F.Bol.516, predicting the lengths in 152 cm and 72.5 cm, respectively (Table 1). MCSNV VII.B.96-VII.B.97 measures 83 cm, lacking the proximal area of the caudal fin (preserved caudal fin length 23 cm). The total length of the specimen is estimated as 89 cm according to the apical lobe-caudal tip/trunk length ratio (0.17).
Estimates provided here suggest that MSNPV 24625-24626 could be one of the largest individuals of the Bolca elasmobranch fossil assemblage (Table 1, Fig. 9A).
Suggested ontogenetic calibrations are shown in Fig. 10 and Table 2. The trend lines of both diagrams display consistent linearity between specimens (Fig. 10). Specimens of G. cuvieri and E. bolcensis cluster in discernible groups. These trends suggest small ontogenetic variations among triakid individuals. On the contrary, the specimens of E. bolcensis cluster on both diagrams, although displaying relatively high variations among individuals (Fig. 10; see SOM 3 for vertebral centra counts and measurements). The centra length averages of E. bolcensis specimens proportionally vary according to the size of body segments, in the same way as Bolca triakids (Fig. 10A, B; Table 2). Nevertheless, diagrams (Fig. 10) indicate three main ontogenetic classes according to proportional increasing of size among the individuals.
Table 1. Measurements, body ratios, and estimated total length of Galeorhinus cuvieri and Eogaleus bolcensis. In italics estimates based on longitudinal measurements and ratios against total length of four well-preserved fossil specimens. * trunk length/(average trunk length/total length) ×100; ° head vertebral centra/(average head vertebral centra/total length) ×100. The accuracy of the estimates is expressed as absolute (Δa) and percentage (ɛ%) errors.
| |
Galeorhinus cuvieri |
Eogaleus bolcensis |
Average |
|||||
|
MGP-PD |
MCSNV T.1124 |
MGGC 1976 |
MNHN F.Bol.516 |
MGP-PD 8869C-8870C |
MSNPV 24625-24626 |
MCSNV T.311 |
||
|
Trunk length (cm) |
35.5 |
46 |
48.3 |
37 |
73 |
89.3 |
85.7 |
|
|
Head vertebral centra (cm) |
5 |
6.7 |
6.5 |
5.3 |
9.7 |
12.4 |
10 |
|
|
Trunk length/total length (%TL) |
51.15 |
50 |
52.48 |
|
54.07 |
|
|
51.93 |
|
Head vertebral centra/total length (%TL) |
7.2 |
7.28 |
7.07 |
|
7.19 |
|
|
7.2 |
|
Total length (cm)* |
|
|
|
71.2 |
|
172 |
165 |
|
|
Total length (cm)° |
|
|
|
73.6 |
|
172.2 |
138.9 |
|
|
TL average (cm) |
|
|
|
72.5 |
|
172.1 |
152 |
|
|
TL Δa |
|
|
|
1.2 |
|
0.1 |
13.1 |
|
|
TL ɛ% |
|
|
|
1.7 |
|
0.03 |
8.6 |
|
Table 2. Antero-posterior measurements (in mm) of Galeorhinus cuvieri and Eogaleus bolcensis trunk and head regions according to the sum of vertebral centra length and the average of centrum size of the trunk and the head regions.
|
ID |
Galeorhinus cuvieri |
Eogaleus bolcensis |
||||||
|
MGP-PD 8871-8872 |
MCSNV T.1124 |
MCSNV |
MGGC 1976 |
MNHN F.Bol.516 |
MCSNV T.311 |
MGP-PD 8869C-8870C |
MSNPV 24625-24626 |
|
|
Trunk length |
355 |
460 |
420 |
483 |
414 |
857 |
730 |
893 |
|
Centra length average (trunk region) |
3.5 |
4.6 |
4.1 |
4.5 |
4.4 |
8.9 |
8.5 |
9 |
|
Head vertebral centra |
64 |
62 |
60 |
65 |
53 |
100 |
97 |
124 |
|
Centra length average (head region) |
4.7 |
4.8 |
4.3 |
4.3 |
3.8 |
7 |
6.5 |
9.3 |
In support of ontogenetic variability found within the Bolca carcharhiniform assemblage, the fossil individuals were compared with extant ground sharks by superimposing size dataset of G. cuvieri and E. bolcensis with growth parameters of modern carcharhiniforms populations. Curves plotted by using the VBGF are performed according to the total length or head-caudal fin length of Bolca specimens as Lt, whereas the remaining parameters of the equations refer to extant populations (SOM 1: table 2, Fig. 9B). When growth parameters of several representatives of the families Carcharhinidae and Triakidae are used, the Bolca individuals distribute along logarithmic trends in accordance with the growth model (R2 values range 0.92–1.00; SOM 1: table 2, Fig. 9B). Moreover, age estimates and computed fits cluster the fossil individuals in three ontogenetic classes according to their size, though specimens belong to different taxa. Fits provided herein separate young individuals (G. cuvieri specimens), from young-adult individuals (E. bolcensis specimens). This datum is consistent with previous age estimates of G. cuvieri individuals, using the VBGF for living relatives, and vertebral bands count of MCSNV T.1124 provided by Fanti et al. (2016). The estimated values for MCSNV T.311, and in particular for MSNPV 24265, display a significant variation probably reflecting the decreasing of growth rate during the life of the individuals within the population (SOM 1: table 2, Fig. 9B). The total length of MSNPV 24265 is close to the plateau level of fits (i.e., the total length is similar to the L∞ computed for the surrogated populations).
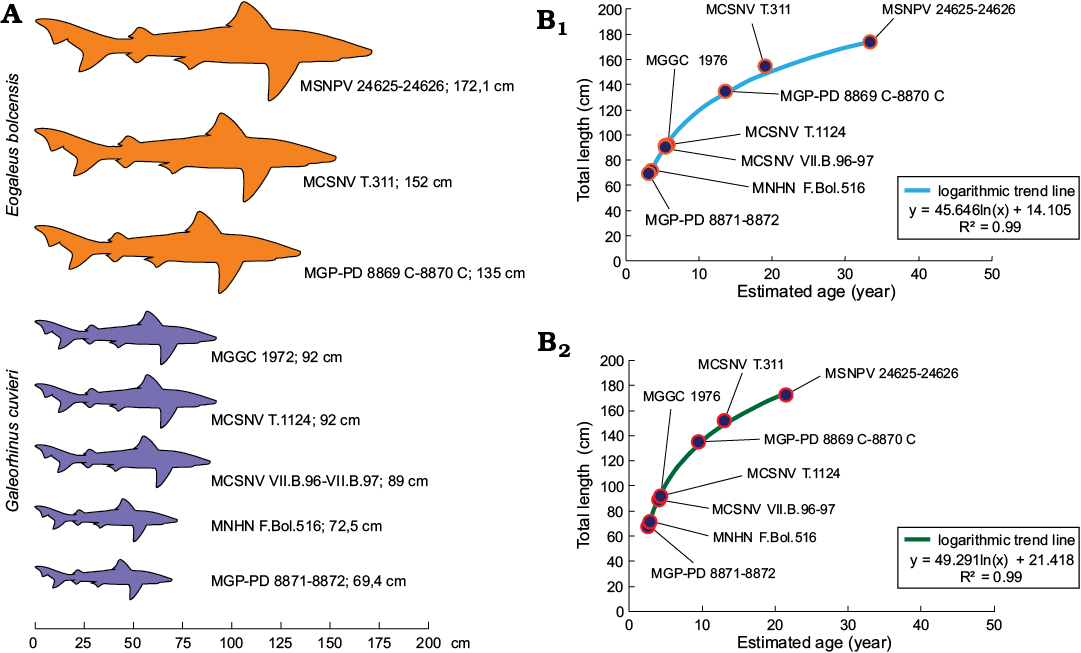
Fig. 9. A. Size comparison between Galeorhinus cuvieri and Eogaleus bolcensis specimens. The total length of MSNPV 24625-24626, MCSNV T.311, and MNHN F.Bol.516 refers to estimated values discussed herein. MSNPV 24625-24626 is the largest of the series. B. Examples of total length-age fits performed with growth parameters of Galeorhinus galeus populations from New Zealand (B1, female growth parameters; Francis and Mulligan 1998) and Australian (B2, combined growth parameters; Moulton et al. 1992) by using VBGF (Bertalanffy 1938; see Table 2 for estimated age values). Both curves discriminate fossil individuals in three main ontogenetic classes: G. cuvieri specimens cluster close to each other on the bottom of the logarithmic trend line. Thus, G. cuvieri specimens were young individuals. On the contrary, E. bolcensis specimens arranged more spaced to each other toward the top of the trend line. Accordingly, E. bolcensis specimens were young or adult individuals, where MSNPV 24625-24626 is the largest of the series.
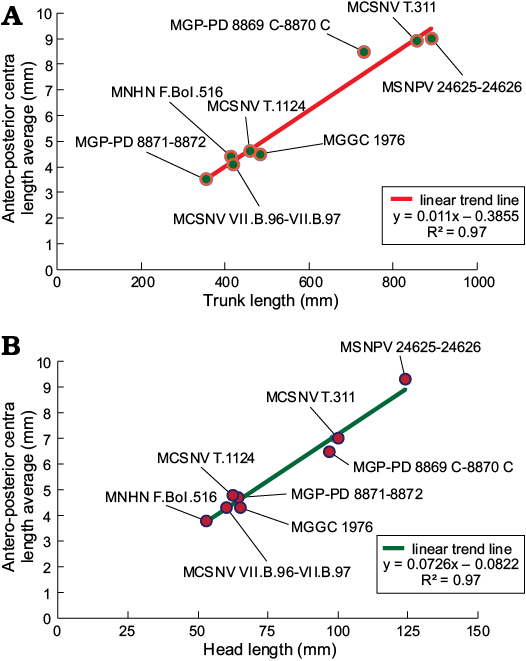
Fig. 10. Correlation between length of trunk (calculated as the sum of antero-posterior vertebral centra) (A), length of the head vertebral centra (B), and centra length averages of Bolca specimens. All the individuals arrange along a robust linear distribution in each diagram (R2 = 0.97), showing three distinct ontogenetic clusters. Length averages of centra are listed in Table 3.
Conclusions
SEM imaging of dermal denticles patches allowed us to identify MSNPV 24625-24626 as Eogaleus bolcensis Cappetta, 1975. The morphometric dataset of centrum to total body length suggests that all Galeorhinus cuvieri specimens collected from Bolca are juvenile individuals, whereas the E. bolcensis specimens were young-adults. MSNPV 24625-24626 represented probably the largest (and therefore probably the oldest) specimen among G. cuvieri and E. bolcensis individuals from Bolca.
Paleoecological, paleoenvironmental, and anatomical features agree with a partial overlap of trophic niches between the two species (i.e., costal-pelagic habits for E. bolcensis and lagoonal-costal for G. cuvieri; see also Fanti et al. 2016; Marramà et al. 2018). Therefore, the analog characters discussed here could reflect the sharing of similar habitat areas. Modern representatives of the families Carcharhinidae and Triakidae inhabit a wide range of marine environments, including enclosing bays and coral reef settings (Compagno 1984). The Bolca Carcharhiniformes assemblage, therefore, highlights conservative ecological habit preferences between carcharinid and triakid species through geological time. Cases of convergent evolution involving Carcharhiniformes evolutionary history are well documented in the fossil records (Cappetta 1992, 2012). As previously discussed by Cachera and Le Loc’h (2017), Mouquet et al. (2012) and Srivastava et al. (2012), similarities in habitat traits infer weak phylogenetic signal between ecologically different species, in particular when the evolutionary history of a taxon crosses a wide temporal range, such as Elasmobranchii (Cappetta 2012).
Acknowledgements
We are grateful to Director Giorgio Mellerio (MSNPV), curators Mariagabriella Fornasiero and Letizia Del (both MGP-PD), and curator Roberto Zorzin (MCSNV), for access to the specimens in their care. We are also grateful to Pietro Galinetto and Ilenia Tredici (both “Centro interdipartimentale di studi e ricerche per la conservazione del patrimonio culturale dell’Università di Pavia”, CISRiC, Pavia, Italy) for SEM imaging. Photographic imaging of Paolo Ferrieri (MGGC) provided excellent outcomes for the study. We are grateful to Giuseppe Marramà (Universität Wien, Vienna, Austria) and Robin B. Trayler (University of California, Merced, CA, USA) for the insightful comments regarding this study. We are extremely grateful to the reviewers, Mohamad Bazzi (Uppsala University, Uppsala, Sweden) and Allison Bronson (Humboldt State University, Arcata, CA, USA), for the valuable comments and contribution to the publishing of the manuscript.
References
Adnet, S. and Cappetta, H. 2008. New fossil triakid sharks from the early Eocene of Prémontré, France, and comments on fossil record of the family. Acta Palaeontologica Polonica 53: 433–448. Crossref
Agassiz, L. 1833–1844. Recherches sur les poissons fossiles. Tome IV: 296 pp.; Tome V, Pt 1: 122 pp. Petitpierre, Neuchâtel.
Bertalanffy, L. von 1938. A quantitative theory of organic growth. Human Biology 10: 181–213.
Blainville, H.D. de 1818. Sur les ichthyolites ou les poissons fossiles. Nouveau Dictionnaire d’Histoire Naturelle 27: 310–391.
Bloch, M.E. 1795. Naturgeschichte der ausländischen Fische. Vol. 9. ii+192 pp. Published at the expense of the author and in commission with the bookseller Mr. Hesse, Berlin.
Bonaparte, C.L.J.L. 1838. Selachorum tabula analytica. Nuovi Annali delle Scienze Naturali 2: 195–214.
Brown, C.A. and Gruber, S. 1988. Age assessment of the lemon shark, Negaprion brevirostris, using tetracycline validated vertebral centra. Copeia 3: 747–753. Crossref
Cachera, M. and Le Loc’h, F. 2017. Assessing the relationships between phylogenetic and functional singularities in sharks (Chondrichthyes). Ecology and Evolution 7 (16): 1–12. Crossref
Cappetta, H. 1975. Les Selaciens Eocenes du Monte Bolca. I – Les Carcharhinidae. In: L. Sorbini (ed.), Miscellanea Paleontologica, Volume 3, 279–305. Museo Civico di Storia Naturale, Verona.
Cappetta, H. 1992. New Carcharhiniformes (Chondrichthyes, Neoselachii) from the Ypresian of the Paris Basin. Geobios 25: 639–646. Crossref
Cappetta, H. 2012. Chondrichthyes. Mesozoic and Cenozoic Elasmobranchii: teeth. In: H.-P. Schultze (ed.), Handbook of Paleoichthyology, Vol. 3E, 1–512. Verlag Dr. Friedrich Pfeil, Munich.
Cerato, M. 2011. Cerato. I pescatori del tempo. 178 pp. Grafica Alpone Srl, San Giovanni Ilarione, Verona.
Choi, Y., Kim, I., and Nakaya, K. 1998. A taxonomic revision of the genus Carcharhinus (Pisces: Elasmobranchii) with description of two new records in Korea. The Korean Journal of Systematic Zoology 14: 43–49.
Coelho, R., Fernandez-Carvalho, J., Amorim, S., and Santos, M.N. 2011. Age and growth of the smooth hammerhead shark, Sphyrna zygaena, in the Eastern Equatorial Atlantic Ocean, using vertebral sections. Aquatic Living Resources 24: 351–357. Crossref
Compagno, L.J.V. 1973. Interrelationships of living elasmobranchs. Zoological Journal of the Linnean Society 53: 15–61.
Compagno, L.J.V. 1984. Sharks of the world. An annotated and illustrated catalogue of sharks species known to date. Part 2, Carcharhiniformes. In: W. Fisher and C.E. Nauen (eds.), FAO Species Catalogue 125: 251–655. FAO, Rome.
Compagno, L.J.V. and Garrick, J.R.F. 1983. Nasolamia, new genus, for the shark Carcharhinus velox Gilbert, 1898 (Elasmobranchii: Carcharhinidae). Zoology Publications from Victoria University of Wellington 76: 1–16.
Compagno, L.J.V. and Stevens, J.D. 1993. Hemitriakis falcata n. sp. and H. abdita n. sp., two new Houndsharks (Carcharhiniformes: Triakidae) from Australia. Records of the Australian Museum 45: 195–220. Crossref
Compagno, L.J.V., Krupp, F., and Kent, C.E. 1996. New Weasel Shark of the genus Paragaleus from the Northwestern Indian Ocean and the Arabian Gulf (Carcharhiniformes: Hemigaleidae). Fauna of Saudi Arabia 15: 391–401.
Compagno, L.J.V., White, W.T., and Last, P.R. 2008. Glyphis garricki sp. nov., a new species of River Shark (Carcharhiniformes: Carcharhinidae) from northern Australia and Papua New Guinea, with a redescription of Glyphis (Müller and Henle, 1839). CSIRO Marine and Atmospheric Research Paper 22: 203–225.
Cruz-Martinéz, A., Chiappa-Carrara, X., and Arenas-Fuentes, V. 2004. Age and growth of the bull shark, Carcharhinus leucas, from southern Gulf of Mexico. Journal of Northwest Atlantic Fishery Science 35: 367–374. Crossref
De Beaumont, G. 1960. Un Notidianus de l’Eocène du Mont Bolca. Abandlungen der Schweizerische Paläontologische Gesellschaft 53 (1): 308–314.
De Zigno, A. 1874. Catalogo ragionato dei pesci fossili del calcare eoceno di M. Bolca e M. Postale. 215 pp. Stabilimento Tipografia Grimaldo E.C., Venezia.
Dillon, E.M., O’Dea, A., and Norris, R.D. 2017. Dermal denticles as a tool to reconstruct shark communities. Marine Ecology Progress Series 566: 117–134. Crossref
Ebert, D.A. and Stehmann, M.F.W. 2013. Sharks, batoids, and chimaeras of the North Atlantic. In: D.A. Ebert, M.F.W. Stehmann, F. Fisher and E. Mostarda (eds.), FAO Species Catalogue for Fishery Purposes 7: i–x + 1–523.
Famhi, F. and White, W.T. 2015. Atelomycterus erdmanni, a new species of catshark (Scyliorhinidae: Carcharhiniformes) from Indonesia. Journal of the Ocean Science Foundation 14: 14–27.
Fanti, F., Minelli, D., Larocca Conte, G., and Miyashita, T. 2016. An exceptionally preserved Eocene shark and the rise of modern predatory-prey interaction in the coral reef food web. Zoological Letters 2, article number 9. Crossref
Ferrón, H.G. and Botella, H. 2017. Squamation and ecology of thelodonts. PLoS ONE 12 (2): e0172781. Crossref
Francis, M.P. and Francis, R.I.C.C. 1992. Growth rate estimates for New Zealand Rig (Mustelus lenticulatus). Australian Journal of Marine and Freshwater Research 43: 1157–1176. Crossref
Francis, M.P. and Mulligan, K.P. 1998. Age and growth of the New Zealand school shark, Galeorhinus galeus. New Zealand Journal of Marine and Freshwater Research 32: 427–440. Crossref
Galeotti, P. 1999. Il Museo di Storia Naturale dell’Imperial Regia Università di Pavia. In: C. Rovati and P. Galeotti (eds.), Il Museo di Lazzaro Spallanzani 1771–1799: una camera delle meraviglie tra l’Arcadia e Linneo, 42–55. Greppi Editore, Pavia.
Gironi, R., Carlini, F., Fumagalli, I., and Brugnatelli, G. 1831. Biblioteca Italiana o sia Giornale di Letteratura, Scienze ed Arti compilato da vari letterati. Giornale di Letteratura, Scienze ed Arti 62: 421–422.
Goosen, A.J.J. and Smale, M.J. 1997. A preliminary study of age and growth of the smooth-hound shark Mustelus (Triakidae). South African Journal of Marine Science 18: 85–91. Crossref
Huxley, T.H. 1880. On the application of the laws of evolution to thearrangement of the Vertebrata and more particularly of the Mammalia. Proceedings of the Zoological Society of London 43: 649–662.
Iglésias, S.P. 2012. Apristurus nakayai sp. nov., a new species of deepwater catshark (Chondrichthyes: Pentanchidae) from New Caledonia. Cybium 36: 511–519.
Jaekel, O. 1894. Die eocänen Selachier vom Monte Bolca: ein Beitrag zur Morphogenie der Wirbelthiere. 176 pp. J. Springer, Berlin. Crossref
Jawad, L.A. 2001. Variation in meristic characters of a tilapian fish, Tilapia zilli (Gervais, 1848) from the inland water bodies in Libia. Acta Ichthyologica et Piscatoria 31: 159–164. Crossref
Jordan, D.S. and Evermann, B.W. 1896. The fishes of North and Middle America, a descriptive catalogue of the species of fish-like vertebrates foundin the waters of North America, north of the isthmus of Panama. Part I. Bullettin of the United States National Museum 47: 1–1240. Crossref
Joung, S.J., Liao, Y.Y., Liu, K.M., Chen, C.T., and Leu, L.C. 2005. Age, growth, and reproduction of the Spinner Shark, Carcharhinus brevipinna, in the Northeastern Waters of Taiwan. Zoological Studies 44: 102–110.
Jucci, C. 1939. L’Istituto di Zoologia “Lazzaro Spallanzani” della R. Università di Pavia. Cenno sulla storia dell’Istituto – sulla sua organizzazione e sulla attività svolta durante il quinquennio 1934–1938. 152 pp. Tipografia già Cooperativa di Bortolo Bianchi, Pavia.
Kusher, D.I. 1987. Age and Growth of the Leopard Shark, Triakis semifasciata, from Central California. 36 pp. MLML/S.F. State Thesis, San Francisco State University, California.
Kusher, D.I., Smith, S.E., and Caillet, G.M. 1992. Validated age and growth of the leopard shark, Triakis semifasciuta, with comments on reproduction. Environmental Biology of Fishes 35: 187–203. Crossref
Lessa, R., Santana, F.M., and Almeida, Z. 2009. Age and growth of the Brazilian sharpnose shark, Rhizoprionodon lalandii and Caribbean sharpnose shark, R. porosus (Elasmobranchii, Carcharhinidae) on the northern coast of Brazil (Maranhão). Pan-American Journal of Aquatic Sciences 4: 532–544.
Lessa, R., Santana, M.F., and Renato, P. 1999. Age, growth and stock structure of the oceanic whitetip shark, Carcharhinus longimanus, from the southwestern equatorial Atlantic. Fisheries Research 42: 21–30. Crossref
Lessa, R., Santana, F.M., Batista, V., and Almeida, Z. 2000. Age and growth of the daggernose shark, Isogomphodon oxyrhynchus, from northern Brazil. Marine and Freshwater Research 51: 339–347. Crossref
Lioy, P. 1865. Sopra alcuni avanzi di plagiostomi fossili del Vicentino e specialmente sull’ Alopiopsis plejodon Lioy (Galeus cuvieri Ag.). Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 8: 398–405.
Liu, K.M., Lin, C.P., Joung, S.J., and Wang, S.B. 2011. Age and Growth Estimates of the Blacktip Sawtail Catshark Galeus sauteri in Northeastern Waters of Taiwan. Zoological Studies 50: 284–295.
Marramà, G. and Kriwet, J. 2017. Principal component and discriminant analyses as powerful tools to support taxonomic identification and their use for functional and phylogenetic signal detection of isolated fossil shark teeth. Plos One 12 (11): 1–22. Crossref
Marramà, G., Bannikov, A.F., Tyler, J.C., Zorzin, R., and Carnevale, G. 2016. Controlled excavations in the Pesciara and Monte Postale sites provide new insights about the palaeoecology and taphonomy of the fish assemblages of the Eocene Konservat-Lagerstätte, Italy. Palaeogeography, Palaeoclimatology, Palaeoecology 454: 228–245. Crossref
Marramà, G., Carnevale, G., and Kriwet, J. 2018. New observations on the anatomy and paleobiology of the Eocene requiem shark †Eogaleus bolcensis (Carcharhiniformes, Carcharhinidae) from Bolca Lagerstätte, Italy. Comptes Rendus Palevol 17: 442–459. Crossref
Marramà, G., Carnevale, G., Engelbrecht, A., Claeson, K.M., Zorzin, R., Fornasiero, M., and Kriwet, J. 2017a. A synoptic review of the Eocene (Ypresian) cartilaginous fishes (Chondrichthyes: Holocephali, Elasmobranchii) of the Bolca Konservat Lagerstatte, Italy. Paläontologische Zeitschrift 92: 283–313. Crossref
Marramà, G., Engelbrecht, A., Carnevale, G., and Kriwet, J. 2017b. Eocene sand tiger sharks (Lamniformes, Odontaspididae) from the Bolca Konservat-Lagerstätte, Italy: palaeobiology, palaeobiogeography and evolutionary significance. Historical Biology 31: 102–116. Crossref
McCosker, J.E., Long, D.J., and Baldwin, C.C. 2012. Description of a new species of deepwater catshark, Bythaelurus giddingsi sp. nov., from the Galápagos Islands (Chondrichthyes: Carcharhiniformes: Scyliorhinidae). Zootaxa 3221: 48–59. Crossref
Molin, R. 1861. De Rajidis tribus bolcanis. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften (Mathematisch-naturwissenschaftliche Klasse) 42: 576–582.
Moulton, P.L., Walker, T.I., and Saddlier, S.R. 1992. Age and growth studies of gummy shark, Mustelus antarcticus Giinther, and school shark, Galeorhinus galeus (Linnaeus), from Southern Australian Waters. Australian Journal of Marine and Freshwater Research 43: 1241–1267. Crossref
Mouquet, N., Devictor, D., Meynard, C.N., Munoz, F., Bersier, L.-F.Chave, J., Couteron, P., Dalecky, A., Fontaine, C., Gravel, D., Hardy, O.J., Jabot, F., Lavergne, S., Leibold, M., Mouillot, D., Münkemüller, T., Pavoine, S., Prinzing, A., Rodrigues, A.S.L., Rohr, R.P., Thébault, E., and Wilfried, W. 2012. Ecophylogenetics: Advances and perspectives. Biological Reviews 87: 769–785. Crossref
Nakaya, K. and Séret, B. 2000. Re-description and taxonomy of Pentanchus profundicolus (Smith and Radcliff), based on a second specimen from the Philippines (Chondrichthyes, Carcharhiniformes, Scyliorhinidae). Ichthyological Research 47: 373–378. Crossref
Olsen, A.M. 1984. Synopsis of biological data on the school shark Galeorhinus australis (Macleay 1881). FAO Fisheries Synopsis 139: 1–42.
Papazzoni, C.A. and Trevisani, E. 2006. Facies analysis, palaeoenvironmental reconstruction, and biostratigraphy of the “Pesciara di Bolca” (Verona, northern Italy): An early Eocene Fossil-Lagerstätte. Palaeogeography, Palaeoclimatology, Palaeoecology 242: 21–35. Crossref
Parsons, G.R. 1993. Geographic variation in reproduction between two populations of the bonnethead shark, Sphyrna tiburo. Environmental Biology of Fishes 38: 25–35. Crossref
Piercy, A.N., Carlson, J.K., Sulikowski, J.A., and Burgess, G.H. 2007. Age and growth of the scalloped hammerhead shark, Sphyrna lewini, in the north-west Atlantic Ocean and Gulf of Mexico. Marine and Freshwater Research 58: 34–40. Crossref
Porter, M.E., Ewoldt, R.H., and Long, J.H., Jr. 2016. Automatic control: the vertebral column of dogfish sharks behaves as a continuously variable transmission with smoothly shifting functions. Journal of Experimental Biology 219: 2908–2919. Crossref
Porter, M.E., Roque, C.M., and Long, J.H., Jr. 2009. Turning maneuvers in sharks: predicting body curvature from axial morphology. Journal of Morphology 270: 954–965. Crossref
Raschi, W. and Tabit, C. 1992. Functional aspects of placoid scales: a review and update. Australian Journal of Marine and Freshwater Research 43: 123–147. Crossref
Reif, W.E. 1982. Morphogenesis and function of the squamation in sharks. I. Comparative functional morphology of shark scales, and ecology of sharks. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 164: 172–183. Crossref
Reif, W.E. 1985a. Functions of scales and photophores in mesopelagic luminescent sharks. Acta Zoologica 66: 111–118. Crossref
Reif, W.E. 1985b. Squamation and ecology of sharks. Courier Forschungsinstitut Senckenb Band 78: 1–255.
Rovati, C. 1999. Storia del Museo dall’ottocento a oggi. In: C. Rovati and P. Galeotti (eds.), Il Museo di Lazzaro Spallanzani 1771–1799 una camera delle meraviglie tra l’Arcadia e Linneo, 106–116. Greppi Editore, Pavia.
Sato, K., Nakaya, K., and Stewart, A.L. 1999. A new species of the deep-water catshark genus Apristurus from New Zealand waters (Chondrichthyes: Scyliorhinidae). Journal of the Royal Society of New Zealand 29: 325–335. Crossref
Schaaf-Da Silva, J.A. and Ebert, D.A. 2008. A revision of the western North Pacific swellsharks, genus Cephaloscyllium Gill 1862 (Condrichthyes: Carcharhiniformes: Scyliorhinidae), including descriptions of two new species. Zootaxa 1872: 1–28. Crossref
Séret, B. and Last, P.R. 2007. Four new species of deep-water catsharks of the genus Parmaturus (Carcharhiniformes: Scyliorhinidae) from New Caledonia, Indonesia and Australia. Zootaxa 1657: 23–39. Crossref
Serra-Kiel, J., Hottinger, L., Caus, E., Drobne, K., Ferràndez, C., Jauhri, A.K., Less, G., Pavlovec, R., Pignatti, J., Samsó, J.M., Schaub, H., Sirel, E., Strougo, A., Tambareau, Y., Tosquella, J., and Zakrevskaya, E. 1998. Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bulletin de la Société Géologique de France 169 (2): 281–299.
Skomal, G.B. and Natanson, L.J. 2003. Age and growth of the blue shark (Prionace glauca) in the North Atlantic Ocean. Fishery Bulletin 101: 627–639.
Srivastava, D.S., Cadotte, M.W., MacDonald, A.A.M., Marushia, R.G., and Mirotchnick, N. 2012. Phylogenetic diversity and the functioning of ecosystems. Ecology Letter 15: 637–648. Crossref
Trevisani, E. 2015. Upper Cretaceous–Lower Eocene succession of the Monte Postale and its relationship with the “Pesciara di Bolca” (Lessini Mountains, northern Italy): deposition of a fossilfish lagerstätte. Facies 61 [published online, https://doi.org/10.1007/s10347-015-0431-y] Crossref
Volta, S. 1796–1808. Ittiolitogia Veronese del Museo Bozziano ora annesso a quello del Conte Giovambattista Gazola e di altri gabinetti di fossili veronesi. 323 pp. Tipografia Giuliari, Verona. Crossref
Weigmann, S. 2012. Contribution to
the taxonomy and distribution of six shark species (Chondrichthyes,
Elasmobranchii) from the Gulf of Thailand. International
Scholarly Research Notices 2012 [published
online, https://doi.org/10.5402/2012/860768] Crossref
White, W.T. and Ebert, D.A. 2008. Cephaloscyllium hiscosellum sp. nov., a new swellshark (Carcharhiniformes: Scyliorhinidae) from northwestern Australia. In: P.R. Last, W.T. White, and J.J. Pogonoski (eds.), Descriptions of New Australian Chondrichthyans, No. 022, 171–178. CSIRO Marine and Atmospheric Research, Hobart.
White, W.T. and Harris, M. 2013. Redescription of Paragaleus tengi (Chen, 1963) (Carcharhiniformes: Hemigaleidae) and first record of Paragaleus randalli Compagno, Krupp and Carpenter, 1996 from the western North Pacific. Zootaxa 3752: 172–184. Crossref
White, W.T. and Last, P.R. 2006. Description of two new species of smooth-hounds, Mustelus widodoi and M. ravidus (Carcharhiniformes: Triakidae) from the western central Pacific. Cybium 30: 235–246.
White, W.T. and Weigmann, S. 2014. Carcharhinus humani sp. nov., a new whaler shark (Carcharhiniformes: Carcharhinidae) from the western Indian Ocean. Zootaxa 3821: 71–87. Crossref
Wintner, S.P. and Dudley, S.F.J. 2000. Age and growth estimates for the tiger shark, Galeocerdo cuvier, from the east coast of South Africa. Marine and Freshwater Research 51: 43–53. Crossref
Yudin, K.G. and Caillet, G.M. 1990. Age and Growth of the Gray Smoothhound, Mustelus californicus, and the Brown Smoothhound, M. henlei, Sharks from Central California. Copeia 1: 191–204. Crossref
Acta Palaeontol. Pol. 65 (3): 547–560,
2020 https://doi.org/10.4202/app.00725.2020