


Re-evaluation of pachycormid fishes from the Late Jurassic of Southwestern Germany
ERIN E. MAXWELL, PAUL H. LAMBERS, ADRIANA LÓPEZ-ARBARELLO, and GÜNTER SCHWEIGERT
Maxwell, E.E., Lambers, P.H., López-Arbarello, A., and Schweigert G. 2020. Re-evaluation of pachycormid fishes from the Late Jurassic of Southwestern Germany. Acta Palaeontologica Polonica 65 (3): 429–453.
Pachycormidae is an extinct group of Mesozoic fishes that exhibits extensive body size and shape disparity. The Late Jurassic record of the group is dominated by fossils from the lithographic limestone of Bavaria, Germany that, although complete and articulated, are not well characterized anatomically. In addition, stratigraphic and geographical provenance are often only approximately known, making these taxa difficult to place in a global biogeographical context. In contrast, the late Kimmeridgian Nusplingen Plattenkalk of Baden-Württemberg is a well-constrained locality yielding hundreds of exceptionally preserved and prepared vertebrate fossils. Pachycormid fishes are rare, but these finds have the potential to broaden our understanding of anatomical variation within this group, as well as provide new information regarding the trophic complexity of the Nusplingen lagoonal ecosystem. Here, we review the fossil record of Pachycormidae from Nusplingen, including one fragmentary and two relatively complete skulls, a largely complete fish, and a fragment of a caudal fin. These finds can be referred to three taxa: Orthocormus sp., Hypsocormus posterodorsalis sp. nov., and Simocormus macrolepidotus gen. et sp. nov. The latter taxon was erected to replace “Hypsocormus” macrodon, here considered to be a nomen dubium. Hypsocormus posterodorsalis is known only from Nusplingen, and is characterized by teeth lacking apicobasal ridging at the bases, a dorsal fin positioned opposite the anterior edge of the anal fin, and a hypural plate consisting of a fused parhypural and hypurals. The holotype specimen contributes additional palaeobiological information, with small teleosteans preserved as gastric contents and ribs showing signs of callus formation. These new findings extend our knowledge of the anatomy and diversity of Pachycormidae, and represent an important first step in understanding factors controlling their distribution and morphological variation in the Late Jurassic of Europe.
Key words: Actinopterygii, Pachycormidae, Lagerstätte, Mesozoic, Kimmeridgian, Bavaria, Nusplingen.
Erin E. Maxwell [erin.maxwell@smns-bw.de] and Günter Schweigert [guenter.schweigert@smns-bw.de], Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany.
Paul H. Lambers [P.H.Lambers@uu.nl], Universiteitsmuseum Utrecht, Lange Nieuwstraat 106, 3512 PN Utrecht, The Netherlands.
Adriana López-Arbarello [a.lopez-arbarello@lrz.uni-muenchen.de], Department of Earth and Environmental Sciences, Paleontology and Geobiology, Ludwig-Maximilians-Universität München, Richard-Wagner-Str. 10, 80333 München, Germany.
Received 24 March 2020, accepted 15 May 2020, available online 19 August 2020.
Copyright © 2020 E.E. Maxwell et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Pachycormidae is a clade of extinct fishes on the teleost stem exhibiting high size and body shape disparity (Friedman et al. 2010; Friedman 2012). Two sub-groups have been recovered in phylogenetic analyses; a clade consisting mainly of planktivorous taxa, and a macrocarnivorous clade (Friedman 2012). Pachycormids first appeared in the Early Jurassic (Toarcian) of Europe, and their diversity in both the Early and Late Jurassic is concentrated in Europe (Wretman et al. 2016). Whether this is attributable to historical biases in research and collecting effort or biological signal remains to be determined.
Pachycormids from the Late Jurassic Plattenkalks of Germany are moderately diverse, and include five named genera (Table 1): the large (>2 m standard length), edentulous Asthenocormus, belonging to the planktivorous clade, and four genera with robust dentition (Pseudoasthenocormus, Hypsocormus, Orthocormus, and Sauropsis; Arratia and Schultze 2015). The genera differ substantially from each other in size, shape, and thus in inferred palaeoecology; however, poor stratigraphic and locality data associated with historical collections makes temporal and geographic range difficult to assess.
Table 1. Diversity, stratigraphic, and geographic range of pachycormid fishes from the Late Jurassic of Central Europe.
|
Taxon |
Stratigraphic range |
Geographic range |
References |
|
Sauropsis |
Toarcian–Tithonian |
Germany, ?Cuba |
|
|
Sauropsis longimanus Agassiz, 1833 |
|
Bavaria (Germany) |
|
|
Sauropsis depressus Eastman, 1914 |
Tithonian |
Bavaria (Germany) |
EM personal observation |
|
Sauropsis curtus Eastman, 1914 |
|
Bavaria (Germany) |
|
|
Asthenocormus |
Tithonian |
Bavaria (Germany) |
|
|
Asthenocormus titanius (Wagner, 1863) |
Tithonian |
Bavaria (Germany) |
|
|
Pseudoasthenocormus |
Tithonian |
Bavaria (Germany) |
|
|
Pseudoasthenocormus retrodorsalis (Eastman, 1914) |
Tithonian |
Bavaria (Germany) |
|
|
Hypsocormus |
Callovian–Tithonian |
Germany, France, UK |
|
|
Hypsocormus insignis Wagner, 1860 |
Tithonian |
Bavaria (Germany) |
EM personal observation |
|
Hypsocormus
macrodon Wagner, 1863 |
Kimmeridgian–Tithonian |
Bavaria (Germany) |
EM personal observation |
|
Orthocormus |
Kimmeridgian–Tithonian |
Germany, France, UK, Poland |
|
|
Orthocormus roeperi Arratia and Schultze, 2013 |
Kimmeridgian |
Bavaria (Germany) |
|
|
Orthocormus teyleri Lambers, 1988 |
Kimmeridgian |
Cerin (France), Bavaria (Germany) |
|
|
Orthocormus cornutus Weitzel, 1930 |
Tithonian |
Bavaria (Germany) |
Recent research effort has emphasized understanding the planktivorous pachycormid clade (e.g., Schumacher et al. 2016; Cawley et al. 2019; Dobson et al. 2019). Much less attention has been given to the macrocarnivorous radiation, despite its richer fossil record. The monophyly of many genera is in doubt (Hypsocormus, Sauropsis; Mainwaring 1978; Lambers 1992), and only rather cursory descriptions are available for many species. The latter issue is exacerbated by taphonomic factors typical of the Bavarian plattenkalk deposits where, due to spectacular soft-tissue preservation but extensive crushing, many osteological details are not visible externally or are obscured by damage.
Although less famous than the Bavarian Plattenkalks to the east, vertebrate fossils from the Nusplingen Plattenkalk of Baden-Württemberg, Germany, have been known since the 19th Century (e.g., Fraas 1854, 1855, 1878; Quenstedt 1856–1857). The Staatliches Museum für Naturkunde Stuttgart has been conducting scientific excavations at the locality uninterruptedly since 1993, resulting in a significant collection (~350 documented species of fossil plants, animals and ichnofossils; Dietl and Schweigert 2011; Schweigert 2015). Despite substantial new fish finds, pachycormids from Nusplingen remain exceedingly rare, with only a few fragmentary specimens described in the early 1900s (Heineke 1906). These finds have not been included in recent revisions of the group, making it difficult to assess variation in these fishes across central European basins. Moreover, the recent generation of explicit palaeobiogeographical hypotheses regarding the distribution of marine reptiles in the Late Jurassic European basins (Tyborowski and Błażejowski 2019) makes such a fine-scale evaluation of the fish fauna desirable.
Institutional abbreviations.—GPIT, Palaeontological Collection of Tübingen University, Tübingen, Germany; MB, Museum für Naturkunde, Berlin, Germany; NHMUK, Natural History Museum, London, UK; NRM, Naturhistoriska Riksmuseet Stockholm, Sweden; SMNS, Staatliches Museum für Naturkunde Stuttgart, Germany; SNSB-BSPG, Bavarian State Collection for Palaeontology and Geology, Munich, Germany; SNSB-JME, Jura-Museum Eichstätt, Germany.
Other abbreviations.—SL, standard length; so, suborbital.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:C20B1AC2-CC72-426E-8382-D1AD41937329
Geological setting
The Nusplingen locality is located in the southwestern part of the Swabian Alb (Baden-Württemberg, Germany; Fig. 1). The up to 15-metre-thick section consists of several intervals of finely laminated, partly bituminous limestone, interrupted by a few bioturbated limestone beds and occasional turbidite layers (Dietl et al. 1998), of late Kimmeridgian age (Lithacoceras ulmense Subzone: Schweigert 1998, 2007, 2015). The laminated limestone was deposited in a less than 100-metre-deep restricted lagoonal environment (Dietl and Schweigert 2004). Anoxia or dysoxia was limited to the seafloor, whereas the upper parts of the water column were permanently oxygenated (Stevens et al. 2014; Hättig et al. 2019). Preservation of the ichthyofauna differs from the type generally observed at Solnhofen: soft tissues are mostly not preserved, and the fishes tend to show a higher degree of disarticulation (Schweigert et al. 2003; Chellouche 2016).
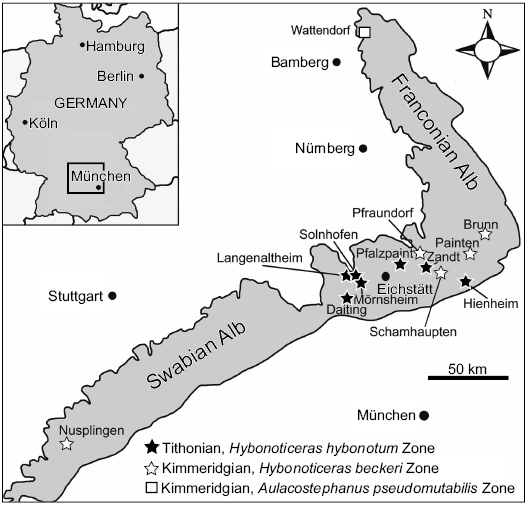
Fig. 1. Nusplingen and other localities of fossiliferous Late Jurassic Plattenkalks in SW Germany (modified from Fürsich et al. 2007 based on Schweigert 2007).
Material and methods
Four specimens referred to Pachycormidae have been reported from Nusplingen: two caudal fin fragments, of which one was figured by Quenstedt (1856–1857: pl. 100: 6); the second was mentioned by Heineke (1906) and could not be relocated. A “suboperculum” (GPIT/OS/1303) was figured by Heineke (1906: fig. 9), this specimen includes a lower jaw and some cranial elements; and lastly a relatively complete skull was fully described and figured (Heineke 1906: pl. 3: 1). In addition to these published reports, two additional specimens from Nusplingen referable to Pachycormidae were identified during the course of this study: a complete, articulated skeleton (GPIT/OS/00836), and a skull and pectoral fin in ventrolateral view (SMNS 96988/4).
Osteological nomenclature follows conventional actinopterygian terminology (Gardiner and Schaeffer 1989), with the following clarification. Some authors (Woodward 1908; Lambers 1992) have identified a bone situated between the posttemporal (= suprascapular of Mainwaring 1978) and parietal, which they labeled as the supratemporal. According to Gardiner and Schaeffer (1989), the actinopterygian supratemporal is positioned anterior to the supratemporal commissure and fuses with the dermopterotic, making the pachycormid “supratemporal” more consistent in position with an extrascapular. However, the extrascapulars are hypothesized to have been lost in pachycormids based on the position of the supratemporal commissure within the posttemporal (Mainwaring 1978). Therefore we refer to this element as a ?extrascapular, to denote its uncertain homologies.
In addition, we use the following pachycormid-specific anatomical nomenclature:
Pachycormids are characterized by the presence of a raised dorsomedial prominence on the skull, which has been hypothesized to serve as a cutwater (Weitzel 1930). In Pachycormus, this structure is formed primarily by the frontal and parietal, and has been termed the cranial boss, or frontoparietal boss (Mainwaring 1978). However, in Hypsocormus and some species of Orthocormus, the boss begins further posteriorly and rises at a steeper angle, with the bones of the dermal pectoral girdle (posttemporal and ?extrascapular) forming the largest part (Woodward 1908). For this reason, Lambers (1992) considered “temporal boss” a more appropriate name for this structure, and we follow this nomenclature here.
We use “small” to describe flank scales that occur in a 2:1 ratio with vertebral elements/myomeres when preserved (e.g., Hypsocormus insignis, Sauropsis longimanus). Taxa with “large” scales (1:1 ratio) include Simocormus macrolepidotus gen. et sp. nov., Sauropsis depressus, and Pseudoasthenocormus retrodorsalis. Orthocormus cornutus and O. teyleri have “very small” scales (4:1 ratio with vertebral elements). This nomenclatural decision addresses problems with scaling absolute measurements over a wide range of body sizes.
Hypsocormus.—Hypsocormus historically comprises two species from the Late Jurassic of Germany, Hypsocormus insignis and “Hypsocormus macrodon” (see below for discussion and revision). NRM P425, consisting of braincase and pectoral material previously described as Hypsocormus (Holmgren and Stensiö 1936; Rayner 1948; Jessen 1972), is more consistent with Orthocormus (following Lambers 1992), and so we use the latter generic attribution for these remains throughout this manuscript. Hypsocormus tenuirostris, from the Middle Jurassic of the UK, has been recovered as the sister group to Orthocormus + Protosphyraena in phylogenetic analyses (Friedman 2012), and anatomically is most similar to Orthocormus (Lambers 1992). However, because this fragmentary taxon does not form a sister-group to Orthocormus, we refer to it with open nomenclature as Orthocormus? tenuirostris following the syntax proposed by Bengtson (1988). Hypsocormus leedsi, also from the Middle Jurassic of the UK, is very fragmentary (Woodward 1895), and its generic affinities cannot be assessed. Thus, throughout this manuscript, we use the generic epithet Hypsocormus to refer exclusively to the type species, Hypsocormus insignis.
Hypsocormus macrodon.—According to the description of the holotype (Wagner 1863) and measurements thereof subsequently published by Heineke (1906), in Hypsocormus macrodon the dorsal fin was positioned mid-way between the pelvic and anal fins. The holotype of H. macrodon has been lost (Mainwaring 1978; Arratia and Schultze 2013), and the dorsal fin in material referred to this species originates posterior to the anal fin (e.g., Lambers 1992). Skeletons referred to H. macrodon are often twisted around the long axis, and it is possible that Wagner (1863) misinterpreted the dorsal and anal fins for taphonomic reasons. However, without even a photo or drawing of the holotype available, H. macrodon in modern usage may differ from H. macrodon as originally envisioned. We consider H. macrodon to be a nomen dubium, and erect a new species to include the material currently referred to “H. macrodon”. We consider the specimen NHMUK PV P 6011 (Fig. 2) to be a suitable holotype, as this specimen has been widely discussed, figured, and included in phylogenetic analyses as an example of “H. macrodon” (e.g., Woodward 1895; Heineke 1906; Lambers 1992; Friedman 2012; Liston et al. 2019).
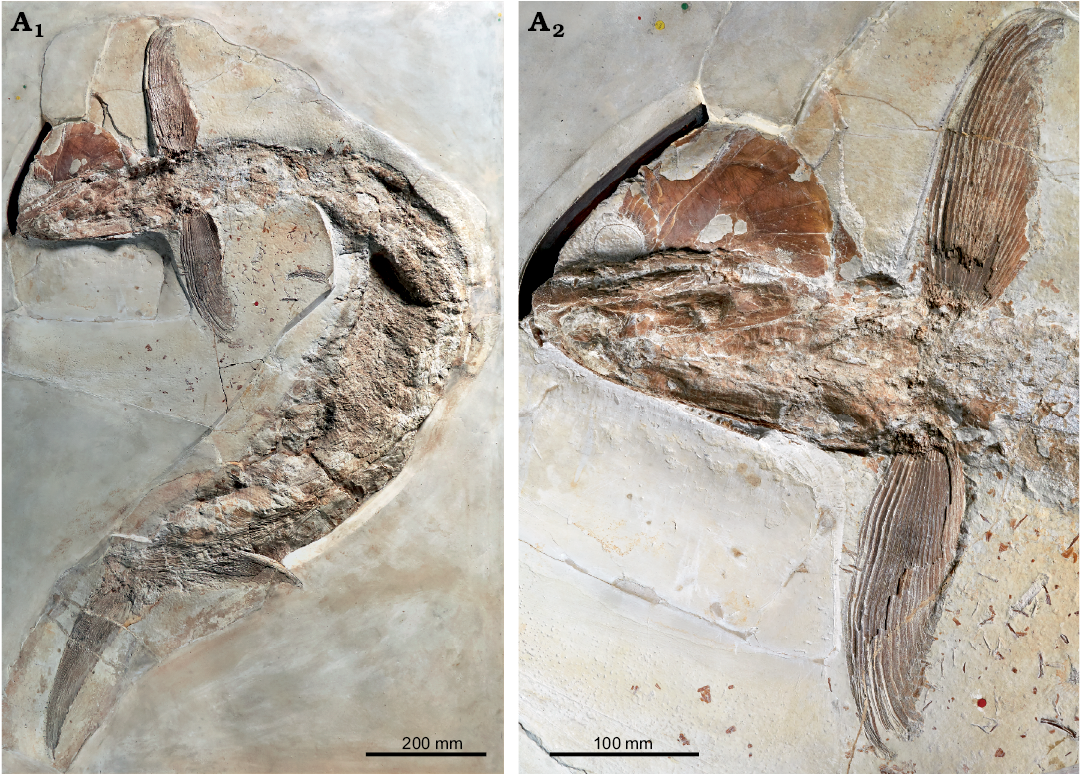
Fig. 2. Pachycormid fish Simocormus macrolepidotus gen. et sp. nov. (holotype NHMUK PV P 6011) from the Late Jurassic of Solnhofen, Bavaria, Germany. Overview (A1), detail of skull and pectoral fins illustrating the prominent ridged ornamentation on suborbital 2 (A2). © The Trustees of the Natural History Museum, London, photographer A. McArdle, used with permission.
A second taxonomic issue affecting “Hypsocormus macrodon” (modern usage) is that of referral to the genus Hypsocormus. There are few morphological features uniting material referred to H. macrodon with H. insignis, with the diagnosis for Hypsocormus proposed by Woodward (1895) applying almost exclusively to the type species (Lambers 1992). This has been subsequently supported by phylogenetic analysis, in which the two species fail to form a monophyletic group (Friedman 2012). Thus, we consider the referral of “H. macrodon” to Hypsocormus as improbable, and erect a new genus.
Systematic palaeontology
Actinopterygii sensu Goodrich 1930
Pachycormiformes Berg, 1937
Pachycormidae Woodward, 1895
Genus Simocormus nov.
ZooBank LSID: urn:lsid:zoobank.org:act:3A5ADFC0-37A2-4A8A-9981-D59499A8D8F5
Type species: Simocormus macrolepidotus sp. nov.; see below.
Etymology: From Greek simo, snub-nosed, in reference to the profile of the rostrodermethmoid in lateral view and cormus, tree-trunk, here used to indicate affinities to Pachycormidae.
Diagnosis.—As for the type and only known species.
Simocormus macrolepidotus sp. nov.
Figs. 2, 3.
1894 Hypsocormus Wagner, 1863; Woodward 1894: 511.
1895 Hypsocormus macrodon (Wagner, 1863); Woodward 1895: 305.
1992 Hypsocormus macrodon (Wagner, 1863); Lambers 1992: 262: fig. 17.
2012 “Hypsocormus” macrodon (Wagner, 1863); Friedman 2012: 947–948, fig. 2.
2019 Hypsocormus Wagner, 1893; Liston et al. 2019: 6.
ZooBank LSID: urn:lsid:zoobank.org:act: 3D8F19DE-D08E-4B30-AF7B-BB7CBE2E5EE1
Etymology: From Greek macrolepidotus, large scales; present in a 1:1 ratio with the axial elements.
Holotype: NHMUK PV P 6011, an articulated fish preserving the skull in ventrolateral view (Fig. 2).
Type locality: Germany, Bavaria.
Type horizon: Late Jurassic.
Material.—Abundant referred material is present in collections around the world (see Table 2 for some examples), including SMNS 96988/4 (described below). Simocormus macrolepidotus gen. et sp. nov. comprises material previously referred to Hypsocormus macrodon.
Table 2. Comparative Late Jurassic pachycormid material examined. All localities are in Bavaria, Germany. Age correlations for localities from Schweigert (2007). “–” age or exact locality unknown.
|
Specimen |
Taxon |
Locality |
Age |
|
JME SOS 3571a/b |
Orthocormus cornutus Weitzel, 1930 |
Birkhof |
early Tithonian |
|
JME SOS 3460 |
Orthocormus cornutus Weitzel, 1930 |
Blumenberg |
early Tithonian |
|
JME 2181 a/b |
Orthocormus cornutus? Weitzel, 1930 |
Schernfeld |
early Tithonian |
|
JME-Scha 2418 |
Orthocormus teyleri? Lambers, 1988 |
Schamhaupten |
latest Kimmeridgian |
|
BSPG AS VI 4 a/b |
Hypsocormus insignis (holotype) |
– |
– |
|
JME SOS 3557 |
Hypsocormus insignis Wagner, 1860 |
Obereichstätt |
early Tithonian |
|
JME SOS 3915 |
Hypsocormus insignis Wagner, 1860 |
– |
– |
|
MB.f.1547 |
Hypsocormus insignis Wagner, 1860 |
Solnhofen |
early Tithonian |
|
SMNS 56650 |
Hypsocormus insignis Wagner, 1860 |
Solnhofen region |
early Tithonian |
|
JME SOS 3574 |
Simocormus macrolepidotus gen. et sp. nov. |
Breitenhill |
late Kimmeridgian |
|
JME SOS 3394a/b |
Simocormus macrolepidotus gen. et sp. nov. |
Wintershof |
early Tithonian |
|
MB.f.1539 |
Simocormus macrolepidotus gen. et sp. nov. |
Solnhofen |
early Tithonian |
|
BSPG AS VII 1089 |
Sauropsis longimanus (holotype) Agassiz, 1833 |
“Eichstätt” |
– |
|
JME 3046 |
Sauropsis longimanus? Agassiz, 1833 |
Blumenberg |
early Tithonian |
|
JME SOS 03459a/b |
Sauropsis depressus Eastman, 1914 |
Blumenberg |
early Tithonian |
|
BSPG 1964 XXIII 525 |
Sauropsis depressus Eastman, 1914 |
Schernfeld |
early Tithonian |
|
BSPG 1977 XI 1 |
Sauropsis depressus Eastman, 1914 |
?Eichstätt |
– |
|
BSPG 1964 XXIII |
Sauropsis depressus Eastman, 1914 |
Schernfeld |
early Tithonian |
|
BSPG 1956.I.361 |
Pseudoasthenocormus retrodorsalis (Eastman, 1914) |
Langenaltheim |
early Tithonian |
Diagnosis (modified from Woodward 1895 and Wagner 1863).— Large fish with an elongate body shape, up to 1.5 m in length, with skull approximately 20% SL; snout short and obtuse, with large rostrodermethmoidal teeth directed almost vertically; second suborbital (so2) element much larger than the first suborbital (so1), and ornamented with prominent radiating ridges; external bones, scales, and fin rays ornamented with tubercles; scales present in a 1:1 ratio with the axial skeleton; small dorsal fin originating posterior to the anal fin; caudal fin with strong development of lepidotrichial segmentation.
Description (SMNS 96988/4 from Nusplingen, Fig. 3A1).—General appearance: SMNS 96988/4 (Fig. 3A1) consists of a skull preserved in right ventrolateral view, pectoral girdles and left pectoral fin. The specimen as preserved measures 28 cm long and 33 cm deep. The skull length (anteriormost point of rostrodermethmoid to posteriormost point of the opercle) is 21 cm, corresponding to a fish of ~105 cm SL.
Skull roof and snout: The rostrodermethmoid forms the anterodorsal border of the mouth. It does not project anterior to the mandibular symphysis (Fig. 3A1–A3). As preserved in lateral view, the rostrodermethmoid bears a single large, procumbent tooth (“tusk” or “fang”), with an apicobasal height of 12.2 mm. Posterior to the fang, the rostrodermethmoid bears some small teeth (ca. 1.5 mm apicobasal height). The anterior rostrodermethmoid is ornamented with tubercles. While sensory pits are present, poor preservation and complex ornamentation prevent tracing the course of the sensory canal.
The premaxilla is approximately oval in shape and tapering anteriorly to form a process parallel to the oral margin. It is a short, narrow, dentigerous bone articulating with the rostrodermethmoid anteriorly and the maxilla posteriorly (Fig. 3A1–A3). Its lateral surface is ornamented with tubercles, which gradually grade into larger denticles and small teeth ventrally. The premaxilla supported at minimum two moderately large teeth. The more posterior of these has an apicobasal height of 4.4 mm, the anteriormost appears to have been larger.
The maxilla is elongate and narrow (dentigerous edge = 82 mm, max. depth = 9 mm), gently bowed with a slightly concave dentigerous margin. The external surface of the maxilla is ornamented with tubercles, which gradually grade into larger denticles and small teeth ventrally (Fig. 3A3). The larger maxillary teeth are located lingual to these denticles. The maxillary teeth are largest at approximately the mid-point of the element, but are in general 2–3 mm in apicobasal height. The marginal dentition extends the entire length of the maxilla. Some larger teeth (up to 9 mm in apicobasal height) are scattered between the maxilla and dentary, but it is unclear whether these originate from the dentary or a more medially positioned element. The posterodorsal maxilla is overlapped by the supramaxilla.
The supramaxilla is relatively large (38 mm long and 14 mm deep at its posterior end), overlapping the posterior maxilla and ventral edge of so2 (Fig. 3A1, A2). The supramaxilla is roughly triangular in shape, and ornamented with tubercles, which are best developed on its anteroventral edge.
The antorbital is situated posterior to the rostrodermethmoid, dorsal to the premaxilla, and overlies a bone of uncertain identity. The antorbital is not well preserved, but its clearest characteristic is a posterodorsally directed process with a series of dorsally oriented grooves extending along its anterior edge (Fig. 3A1–A3).
It is likely that some fragments posterodorsal to the rostrodermethmoid and anterior to the dermosphenotic correspond to the nasal; however, these are poorly preserved. The dermosphenotic forms at minimum the posterodorsal edge of the orbit. Its lateral edge is dorsally convex, curving ventrally. Sensory pits are present in the dermosphenotic at the posterodorsal corner of the orbit.
The right side of the skull roof is preserved, consisting of the frontal, parietal, and dermopterotic (Fig. 3A1, A2, A4). It is crushed anteriorly, medial to the dermosphenotic, but was at least 107.1 mm in length (anterior rostrodermethmoid to posterior end of ornamented surface of the parietal, measured along the midline) and 25.6 mm in posterior width (excluding the lateral articular facet for so1). The skull roof is covered with tubercular ornamentation (Fig. 3A4). The supraorbital sensory canal extends from the medial dermosphenotic posteriorly through the lateral dermopterotic, parallel to the edge of the skull roof. The lateral dermopterotic forms an unornamented facet, this one edged laterally with a feathered bone structure (Fig. 3A4). The facet is interpreted as the articular facet for so1, and is oriented anteroposteriorly and is roughly straight. The frontal and parietal appear to have contacted their antimeres via a straight suture. A mediolaterally oriented linear feature in the ornamentation bordered by ridges, parallel to the posterior edge of the parietal, presumably corresponds to a sensory canal. Posteriorly, the dermopterotic, with a small medial contribution from the parietal, forms a large, unornamented concave flange interpreted as the articular facet for the posttemporal.
A small sagittal eminence was present, extending as far forward as the dermosphenotics. The frontals and parietals appear to be primarily involved, although comparative development of this eminence is difficult to evaluate because the specimen is quite flat. Based on the morphology of the posterior parietals (not thickened or divergent), the temporal boss is unlikely to have projected over the parietal region (Gouiric-Cavalli and Cione 2015).
Posteroventral to the dermopterotic is an oval fragment of very thin bone traversed by a prominent sensory canal (Fig. 3A1, A2, A4), interpreted as a fragment of the presupracleithrum.
An element preserved anterior to the mandible is possibly a vomer based on position and approximate shape. The visible part of the tooth preserved overlying this bone is not ankylosed to it, and it is unclear if the tooth and bone were originally associated.
The sclerotic ring has been displaced dorsally, and is partially overlying the skull roof (Fig. 3A1, A2). It is well ossified. The external surface of the ring is convex. Around the edge of the aperture, the bone of the sclerotic ring is weakly ornamented with a similar roughened- to tubercular texture as the other cranial elements. Away from the aperture, the external surface of the ring is smooth.
Cheek and opercular series: The posterolateral surface of the skull is dominated by a greatly enlarged so2 (Fig. 3A1, A2). so1 is not preserved; however, it was certainly much smaller than so2. Both the right and the left suborbitals are preserved in external view; that on the right has been displaced ventral to the skull. so2 is roughly triangular in shape, with a concave ventral edge and a convex posterior edge. The posterior third of the ventral edge is more strongly deflected ventrally, and a small facet on the ventral edge immediately anterior to this deflection point would have been overlapped by the supramaxilla. The anteriormost corner of the suborbital has been invaded by sensory canals, suggesting that the ventralmost infraorbital may be fused to the suborbital. The suborbital has a roughened ornamentation, becoming more noticeably tubercular towards the ventral edge. However, the most obvious aspect of the ornamentation are five ridges, beginning at approximately 1/3 of the length of the suborbital and extending to the posterior edge. A single long, narrow infraorbital is preserved between the braincase and skull roof (Fig. 3A1, A2).
The preopercle (Fig. 3A1, A2) is preserved at the posteroventral edge of the subopercle. The dorsal ramus has been displaced internal to the suborbital, and is visible only in the deformation of the latter. The preopercle broadens ventrally, and is exposed externally near the opercle-subopercle contact, curving anteriorly to the angular. It is ornamented with tubercles, and carries the preopercular sensory canal. No interopercle is preserved. The opercle (Fig. 3A1, A2) is a large bone (68 mm deep, ventral edge 45 mm long), much larger than the subopercle. Its posterior dorsal edge is broken; however, based on preserved growth lines it is clear that the dorsal edge was narrower than the ventral edge. The opercle-subopercle suture is oriented anteroposteriorly, and slightly dorsally. The opercle is ornamented with tubercles. The subopercle (Fig. 3A1, A2) is small and quadrangular in external view (externally exposed portion 23 mm deep, 48 mm long). The externally exposed portion is ornamented with tubercles. The dorsal edge underlies the opercle, and is anteriorly drawn up into a dorsal process. The subopercle contacts the preopercle anteriorly.
The gular is situated between the lower jaw rami. Preservation of this element is quite poor and little can be said regarding its morphology, however ornamentation at the anterior end is coarsely tubercular.
Numerous branchiostegal rays are present between the gular and the subopercle. This region is poorly preserved and is covered by a hash of bone fragments, scales, teeth, and pharyngeal denticles. The outward-facing surface of each branchiostegal ray is divided into a smooth posterior surface that is overlapped by the succeeding ray, and an ornamented anterior surface covered by a roughened- to tubercular ornamentation. The ornamented portion of each branchiostegal ray is a wide as the smooth portion, indicating that an individual ray had relatively limited external exposure.
Endocranium: The endocranium is preserved as disarticulated elements anterodorsal to the suborbital. The lateral ethmoid is robust, with a thinner dorsal process and an anteroposteriorly expanded base (Fig. 3A3). A ventral depression separates the anterior and posterior edges. A posterior element may represent a fragment of the anterior basisphenoid.
The basisphenoid is situated posterior to the ethmoid region. It has a complex morphology, with an anterior dorsal projection making up part of the interorbital septum (Fig. 3A3), a posterior dorsal lamina, and a posterolateral projection. The dorsal edge of the interorbital projection is thickened and curves laterally. The posterior edge of this interorbital process forms a semi-circular notch, part of the foramen for the optic nerve (II). At the midpoint of the interorbital projection anterior to the foramen is a prominent tubercle. Ventral to the foramen for cranial nerve II, the basisphenoid forms a lateral projection. The posterior dorsal lamina of the basisphenoid extends further dorsally than the interorbital projection, and contacts the pterosphenoid. The posterolateral projection lies on top of the dorsal lamina, and may represent the anterior edge of the myodome that has been displaced due to crushing.
The pterosphenoid articulates anteriorly with the posterior lamina of the basisphenoid. Its posterior surface is concave, and the posterior edge is thickened. Ventrally, the pterosphenoid forms the dorsal edge the foramen for the oculomotor nerve (III). A roughened ridge extends dorsal to the foramen. Posterodorsal to the pterosphenoid, a small fragment of the sphenotic projects ventral to the dermosphenotic. The dermal and endochondral portions are unfused, and are clearly separated by matrix. The prootic is preserved anteromedial to the hyomandibula (Fig. 3A4). The lateral surface is concave, traversed by two ridges. Anteriorly, the element is pierced by several foramina, however, the influence of lateral compression on the shape precludes more detailed interpretations.
The basi-exoccipital is preserved in condylar view (Fig. 3A4), partially overlain by the presupracleithrum. There are two condyles, consisting of oval facets lacking perichondral lining and separated by a groove, proportionately much larger than those described in Pachycormus (Mainwaring 1978).
Branchial arch: Only a portion of the dorsal end of the right hyomandibula is exposed. The articular end is convex. The right quadrate is preserved in medial view, slightly displaced from the glenoid (Fig. 3A1, A2). Its articular end is well-developed and anteroposteriorly elongate, directed anteroventrally and forming two articular facets delineated by a groove on the medial surface. There is a constriction separating the articular end of the quadrate from the fan-shaped dorsal portion, characterized by a well-developed posterior notch. A prominent tubercle is positioned at this point on the anterior half of the quadrate. Anterior to the scapulocoracoid, a semicircular bony plate-like element and an additional endochondral element are preserved; their identity is unclear but they may belong to the visceral arch.
Mandible: The lower jaw consists of three elements in external view: the angular, surangular, and dentary (Fig. 3A1, A2). In overall shape, the mandible is robust and relatively deep, deflected slightly dorsally at its anterior end. It measures 146 mm in anteroposterior length. The dentary makes the largest contribution to the external surface of the mandible. The dentary is thickened along its dorsal edge, and is ornamented with striations postero-dorsally and tubercles over most of its surface (Fig. 3A3). These become more pronounced towards the dentigerous margin and at the symphyseal end. The tubercles at the dentigerous margin lingually grade gradually into an external field of small teeth. Whether the larger lingually placed teeth are borne on the dentary itself is unclear. A posterodorsally-anteroventrally angled suture separates the posterior dentary from the angular and surangular (Fig. 3A1, A2). However, the suture between the latter two elements is largely obscured. The mandibular canal does not extend parallel to the ventral edge of the lower jaw but is roughly oriented from the posterior ventral corner of the lower jaw towards the symphysis. The posterior and ventral edges of the mandible form a ~60° angle, such that the glenoid is directed posterodorsally.
Some details of the left mandible are visible in medial view. The articular is exposed on the posterior end, and the glenoid surface is preserved. Two facets are visible, separated by a slight change in angle: an anteromedial facet, and a posterolateral facet. These correspond to the two facets of the quadrate. At the posterior end of the glenoid, the articular forms a small process. The dorsal portion of the prearticular is also exposed, but has been slightly posteriorly displaced. A posterior process of the prearticular forms the anteromedial edge of the glenoid.
Dentition: The teeth are conical, with relatively blunt acrodin caps (Fig. 3A3). The cap enameloid is smooth, but the collar enameloid bears well-developed apicobasal ridges. Most of the marginal teeth are slightly curved lingually. At least four size classes of teeth are present on the marginal jaw elements.
Numerous pharyngeal denticles are preserved. These are tiny, slender structures, much more acutely pointed than the teeth. As far as can be determined, an acrodin cap is also present.
Pectoral girdle: The right supracleithrum has been ventrally displaced (Fig. 3A1, A2). It is a large, flat bone, with a striated texture along its anterior edge and with granular ornamentation posteriorly. The anterior end is broken.
Both cleithra are exposed, the right in external view and the left in internal view. The cleithra are robust, with a well-developed slender ascending posterior process that broadens ventrally. In external view, a short, slender medially directed ventral ridge is visible anterior to the scapulocoracoid facet. The anterior edge of this ridge forms a concave lamina of bone. The external ventral portion of the cleithrum is covered by reticular ornamentation. In internal view, the ascending posterior process forms a ridge on the internal surface of the cleithrum, extending to a facet for articulation with the scapulocoracoid (Fig. 3A5). This facet is strongly ossified, forming a well-defined cup. The dorsal edge of the ventral cleithrum is convex in internal view, but recessed relative to the dorsal process. Anterior to the scapulocoracoid facet is a second anterior ridge, bordered ventrally by a concavity. Several postcleithra remain in articulation with the left cleithrum, and are exposed in medial view (Fig. 3A1, A2). These include two small dorsal postcleithra, as well as a much larger ventral postcleithrum.
The left scapulocoracoid is preserved in anterior view, slightly displaced from articulation with the cleithrum (Fig. 3A1, A2, A5). Although most of the surfaces are weakly ossified, the internal surface of the upper muscular canal is well-ossified, as is a posterior facet in articulation with the second radial. A convex facet is present for articulation with the propterygium, separated by a groove from the second radial facet. The right scapulocoracoid is exposed in external view.
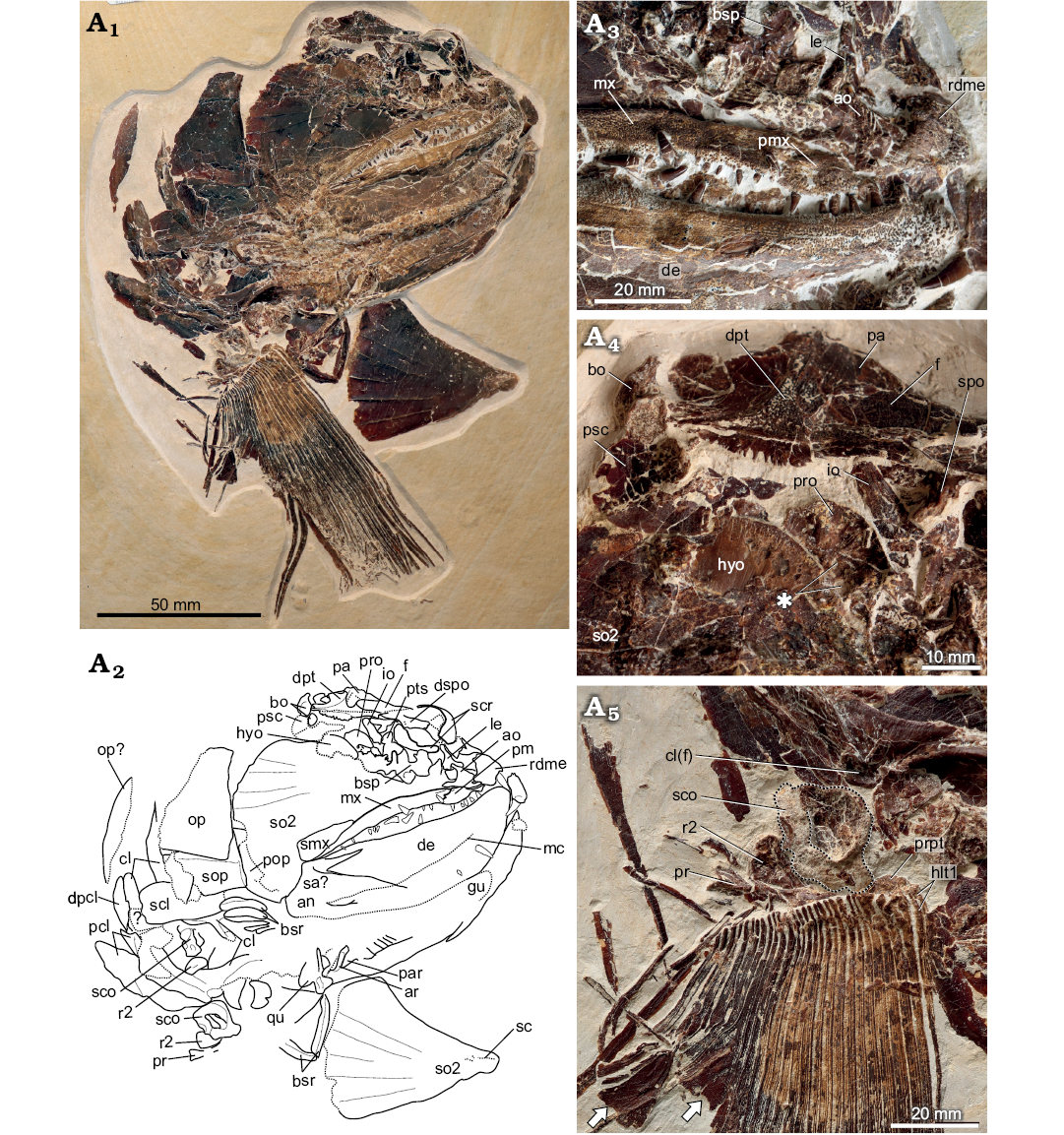
Fig. 3. Pachycormid fish Simocormus macrolepidotus gen. et sp. nov. (SMNS 96988/4) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Specimen in ventrolateral view (A1). Explanatory drawing of skull (A2). Close-up of jaws, showing ornamentation, dentition, and distribution of denticles external to the main tooth row on both the maxilla and dentary (A3). Skull roof and posterior braincase, asterisk indicates foramina of uncertain identity in the prootic (A4). Pectoral fin and girdle, scapulocoracoid outlined to better distinguish the poorly ossified endochondral component from the matrix (A5). Arrows indicate the expanded posterior hemilepidotrchia. Abbreviations: ao, antorbital; an, angular; ar, articular; bo, basi-exoccipital; bsp, basisphenoid; bsr, branchiostegal rays; cl, cleithrum; cl(f), facet on the internal cleithrum for articulation with the endochondral pectoral girdle; de, dentary; dpt, dermopterotic; dpcl, dorsal postcleithrum; dspo, dermosphenotic; f, frontal; gu, gular; hyo, hyomandibula; io, infraorbital; le, lateral ethmoid; mc, mandibular canal; mx, maxilla; hlt1, dorsal and ventral first hemilepidotrichia; op, opercle; pa, parietal; par, prearticular; pcl, postcleithra; pm, premaxilla; pop, preopercle; pr, posterior pectoral radial; pro, prootic; prpt, propterygium; psc, presupracleithrum; pts, pterosphenoid; qu, quadrate; r2, second pectoral radial; rdme, rostrodermethmoid; sa, surangular; sc, sensory canal; scl, supracleithrum; sco, scapulocoracoid; scr, sclerotic ring; smx, supramaxilla; so2, second suborbital + fused infraorbital; sop, subopercle; spo, sphenotic.
The propterygium and anteriormost fin ray are fused. The propterygium is proximodistally short, and has a concave surface anteriorly for articulation with the endochondral girdle (Fig. 3A5). The posterior end consists of a convex bulbous extension spanning lepidotrichia 2–4. Lateral to the concave surface, the opening of the propterygial canal is visible. Both the right and left second pectoral radials are preserved; these are somewhat oval in shape, and are proximally thickened. An extremely elongated, spindle-shaped posterior radial is visible on the left side, overlapped by scales and lepidotrichia.
The left pectoral fin is slightly disrupted around its edges, but consists of a minimum of 28 lepidotrichia. The fin is gladiform in overall morphology (sensu Liston et al. 2019), with a broad base and a narrow tip, and is long in proportion to the skull (preserved length = 16 cm, proximal base = minimum 5 cm). Each fin ray is ornamented with grooves and tubercles (Fig. 3A5), and becomes flattened and broadened distally. The distal half of the anteriormost ray is ornamented with a series of small ridges, similar to some specimens of Pachycormus (Wenz 1967). However, unlike in Pachycormus no separate fringing fulcra are observed. The posterior fin rays are segmented distally. The fin is composed of two distinct regions: an anterior region, with relatively narrow rays, and a small posterior region, in which the lepidotrichia branch asymmetrically with a narrow anterior ramus and a wide posterior ramus, causing the posterior edge of the fin to be deflected (Fig. 3A5). The longest rays are found along the anterior fin.
Squamation: Fragmentary scales are preserved in the area around the pectoral fin and between the mandibular rami. These show a roughened, tuberculated surface.
Remarks.—SMNS 96988/4 is referable to Pachycormidae based on the presence of a toothed rostrodermethmoid separating the premaxillae, a supramaxilla posterodorsal to the maxilla, a large, plate-like posteriorly expanded suborbital, and a pectoral fin with reduced lepidotrichial segmentation and asymmetrical branching of fin rays (Mainwaring 1978; Lambers 1992; Liston 2008). Based on the robust mandible and dentition, in particular the presence of large paramedial teeth on the rostrodermethmoid, SMNS 96988/4 is most consistent with the clade of pachycormids containing Hypsocormus and macrocarnivorous forms, and likely also Sauropsis and Pseudoasthenocormus (Liston 2008; Friedman et al. 2010).
SMNS 96988/4 differs from Orthocormus spp., Orthocormus? tenuirostris, and Protosphyraena in the absence of both an edentulous projection of the rostrodermethmoid anterior to the mandibular symphysis and laterally compressed teeth, and from Pseudoasthenocormus in the absence of an overbite. In addition, the rostrodermethmoid is not dorsally flattened in SMNS 96988/4, and the skull roof forms a steep angle with the horizontal, unlike in the aforementioned genera (Mainwaring 1978). Thus, SMNS 96988/4 is most consistent with the genera Sauropsis, Simocormus, and Hypsocormus.
Hypsocormus historically comprises two species from the Late Jurassic of Germany, H. insignis and “H. macrodon” (= Simocormus macrolepidotus) (Table 1). Hypsocormus leedsi, from the Middle Jurassic of the UK, is very fragmentary, making meaningful comparisons with SMNS 96988/4 difficult (Mainwaring 1978, Lambers 1992). Neither Hypsocormus insignis nor Simocormus macrolepidotus gen. et sp. nov. are well described. H. insignis is a fusiform fish, with cranial elements ornamented with sparse and fine granulations, which are better developed on the dentary and maxilla, and pectoral fin rays lacking segmentation (Woodward 1895). Although it has been described as having smooth scales, personal observation (SNSB-JME SOS 3557) suggests that the scales near the dorsal midline are small and have a finely granulated ornamentation. Simocormus macrolepidotus, in contrast, is an elongate fish with a posteriorly placed dorsal fin, larger than but otherwise similar in morphology to Sauropsis depressus (see below). The well-developed longitudinal ridges ornamenting so2 appear to be unique to Simocormus macrolepidotus (Woodward 1895).
Sauropsis comprises three species from the Late Jurassic of Germany, Sauropsis longimanus, Sauropsis depressus, and Sauropsis curtus (Eastman 1914), of which Sauropsis depressus is relatively abundant. Fragmentary material referred to Sauropsis has also been identified from the Late Jurassic of Cuba (Sauropsis woodwardi; Gregory 1923), and in addition, two Early Jurassic species have been described (Sauropsis veruinalis and Sauropsis latus; White 1925). As with Hypsocormus, all species of Sauropsis are poorly known and the genus is in need of revision. The three Late Jurassic Bavarian species are all relatively small (<50 cm SL). Sauropsis longimanus and Sauropsis curtus can be differentiated from SMNS 96988/4 by the extensive external exposure of the preopercle in lateral view, as well as the very high, narrow opercle (see e.g., Lambers 1992: fig. 14). Sauropsis depressus is more problematic, as it shares many characteristics with Simocormus macrolepidotus gen. et sp. nov. (e.g., elongate body shape, posteriorly placed dorsal fin, large scales, large supramaxilla, relative size, shape, and ornamentation of the opercle and subopercle, segmentation of distal pectoral lepidotrichia). However, it appears to lack the pattern of radiating ridges on the suborbital characteristic of Simocormus macrolepidotus.
When compared to all other Late Jurassic pachycormids from Germany, SMNS 96988/4 is most consistent with Simocormus macrolepidotus gen. et sp. nov., with no observations contradicting referral to this taxon. In addition to the characteristics discussed above, the two share large body size and flattened, expanded posterior lepidotrichia.
Stratigraphic and geographic range.—Kimmeridgian–Tithonian, Bavaria and Baden-Württemberg, Germany.
Genus Hypsocormus Wagner, 1863
Type species: Hypsocormus insignis (Wagner, 1860), Late Jurassic, Bavaria, Germany (Figs. 4A1, 5B1).
Diagnosis (modified from Woodward 1895).—Fusiform fish, head small (25% SL) and length of skull less than depth of trunk, snout short and obtuse, so2 greatly enlarged at the expense of so1, opercle much larger than subopercle, robust dentition, teeth round to slightly oval in cross-section, ossifications in notochordal sheath absent, dorsal fin base opposite- to completely anterior to the anterior edge of the anal fin, leaf-like fringing fulcra on leading edge of caudal fin, pectoral fin gladiform, pelvic fins small and closer to pectoral fins than to anal fin, scales in a 2:1 ratio with axial segments, scaly caudal apparatus consisting of L-shaped scales present.
Remarks.—Under this strict definition, species previously included in Hypsocormus (H. macrodon and H. tenuirostris) are excluded; see taxonomic nomenclature section for details.
Hypsocormus posterodorsalis sp. nov.
Figs. 4B, 5A, 6–8.
Etymology: From Latin posterodorsalis, posterior on the back; in reference to the position of the dorsal fin opposite the anal.
Holotype: GPIT/OS/00836, a complete fish skeleton in right lateral view.
Type locality: Nusplingen, Baden-Württemberg, Germany.
Type horizon: Nusplingen Lithographic Limestone, Upper Jurassic, upper Kimmeridgian, Hybonoticeras beckeri Zone, Lithacoceras ulmense Subzone (Schweigert 1998).
Material.— Holotype only.
Diagnosis.—As per genus, with the following unique combination of features: Elongate-fusiform body shape with the ratio of maximum body depth to standard length approximately 23% (less elongate than Simocormus macrolepidotus gen. et sp. nov. [~15%], less deep-bodied than H. insignis [~30%]), prominent temporal boss projecting onto the parietal region, maxilla with smooth to slightly granular ornamentation (prominent tubercles in Simocormus macrolepidotus, H. insignis referred material), dentary with robust, acutely pointed teeth, supraneurals absent immediately posterior to the skull (unlike all other pachycormids), pelvic fin lepidotrichia half the length of pectoral lepidotrichia, pelvic plate with expanded posterior process and narrow, elongate anterior process (as in Simocormus macrolepidotus, Orthocormus), dorsal fin opposite anal fin (unlike Simocormus macrolepidotus, Orthocormus, Pseudoasthenocormus, H. insignis), anterior hypural plate offset from anteroventral edge and with prominent lateral foramen and hypural process (similar to Simocormus macrolepidotus).
Description.—General appearance: GPIT/OS/00836 (Fig. 4B1) consists of a mid-sized pachycormid fish (SL = 690 mm) preserved in right-lateral view. The skull has been slightly disrupted (Fig. 5A1, A2), and the scale covering on the right lateral side is absent, however the fish is largely articulated.

Fig. 4. Pachycormid fish Hypsocormus Wagner, 1863 from the Late Jurassic of Germany. A. Hypsocormus insignis (Wagner, 1860) (holotype SNSB-BSPG AS VI 4a, mirrored), Bavaria, Germany. B. Hypsocormus posterodorsalis sp. nov. (holotype GPIT/OS/00836), Nusplingen, Germany. Arrows indicate anterior insertion of median fins.
Cranium: The skull bones are for the most part very difficult to interpret. The rostrodermethmoid and premaxilla appear to be missing. The maxilla is a robust, mediolaterally thickened element with a broad, flat dorsal surface, bearing small teeth along its ventral margin. It becomes mediolaterally compressed posteriorly. The dentigerous margin is straight. The external surface of the maxilla is roughened in places, but lacks tubercular ornamentation (Fig. 5A5). A small anterior region of the dorsal skull roof is preserved in external view anterior to the orbit. It bears granular to tubercular ornamentation, and appears to carry a sensory canal, determined based on the presence of pores, suggesting possible identification as part of the anterior frontal and nasal. Otherwise, the dorsal dermatocranium has been rotated to the left, such that none of the bones of the skull roof are preserved in external view.
A prominent temporal boss is present (Fig. 5A1, A2, A7), and projects anteriorly over the parietal region. It consists primarily of the enlarged posttemporal posteriorly and the small, anteromedially positioned ?extrascapular. Ornamentation on the external surface of both elements is strongly tuberculated. The ventrolateral surface of both bones is concave and smooth. The posttemporal bears a robust descending process for articulation with the braincase; its dorsal (external) portion transmits the sensory canal.
The braincase is preserved in lateral view (Fig. 5A3, A4). It is anvil-shaped in overall form. The large basi-exoccipital makes up the posteroventral portion of the braincase. Ventrally, the basi-exoccipital is underlain by the parasphenoid, while its anterodorsal edge forms a thickened ridge, articulating with the intercalary and opisthotic. The intercalar is small and rounded, with a lumpy external surface. Its suture with the opisthotic is not clear, either anteriorly or ventrally. Ventrally, a projection of the intercalar towards the basi-exoccipital forms what we interpret as the posterior edge of the vagus foramen. A discrepancy with the state described in both Orthocormus and Pachycormus exists, in which the basi-exoccipital is excluded from the vagus foramen in lateral view by a process of the intercalar (Pachycormus; Mainwaring 1978) or a process of the opisthotic with a small contribution from the intercalar (Orthocormus; Rayner 1948). Such a process may have been present, but could have been obscured during crushing. However, in either case, the dorsal edge of the vagus foramen appears to have been formed entirely by the intercalar in GPIT/OS/00836, unlike in the other taxa.
Anterior to the intercalar is a much larger element that has been interpreted as an opisthotic (Holmgren and Stensiö 1936). The opisthotic is dorsoventrally high. Its dorsal edge is semicircular and forms the facet for the hyomandibula; the dorsolateral surface is weakly concave. Towards the anterior end of the dorsolateral surface is a small foramen not reported in other pachycormids but matching in size and position the foramen for the supratemporal branch of the glossopharyngeal nerve (IX) reported in “Aspidorhynchus” (Rayner 1948; note that this specimen is not consistent Aspidorhynchus and may actually be a caturid according to Patterson 1975); a foramen for the supratemporal branch of the glossopharyngeal has not been identified in Pachycormus. Unlike in “Aspidorhynchus” in which the foramen is dorsally directed, in GPIT/OS/00836 it is posteriorly directed. The ventral half of the opisthotic in GPIT/OS/00836 is separated from the ventral half by a ridge, which forms part of the dorsal edge of the jugular groove. A small foramen just anterior to the intercalar and above the suture with the basi-exoccipital is interpreted as the foramen for a subsidiary branch of the vagus nerve (X), as in Pachycormus (Mainwaring 1978). Both the foramen for the main and subsidiary branches of the vagus nerve lie in a groove that broadens into a deep concavity on the ventrolateral opisthotic. This fossa is confluent with the jugular groove, which becomes much broader in its posterior portion. Sutures in the anterodorsal braincase are difficult to see, in part due to fragments of the overlying hyomandibula. In overall shape, a relatively poorly developed subtemporal fossa is present immediately anterior to the dorsal opisthotic, separated from the jugular groove by a ridge. The jugular groove itself runs the length of the braincase, narrowest at its anterior point and becoming broader in the region of the vestibular fontanelle. Dorsal to the jugular canal, two additional foramina of uncertain identity are present within the prootic, the larger of which may correspond to the foramen for the facial nerve (VII). A foramen in a similar location was illustrated in the braincase reconstruction of Orthocormus presented by Holmgren and Stensiö (1936), but was not labeled.
The parasphenoid underlies the braincase, and is preserved in lateral view in articulation. The ascending process of the parasphenoid is obscured by the overlying hyomandibula. The foramen for the internal carotid artery lies posterior to the ascending process, as in Pachycormus (Rayner 1948; Mainwaring 1978), at approximately the same level as the jugular canal.
The right lower jaw is preserved in lateral view. Posterodorsally, a small surangular is present. The angular is relatively small, and weakly ornamented with ridges and tubercles posteriorly; anteriorly it is smooth. The majority of the lateral lower jaw consists of the dentary. The dentigerous margin is straight, curving dorsally only at its anteriormost end. A small groove is present ventral to the region presumably occupied by the premaxilla, suggesting a slightly larger tooth in this region. A row of small teeth is present external to the robust dentary tooth row (Fig. 5A6). The dentary teeth are large and conical, with acrodin caps and a round cross-section. The largest teeth are present in the mid-dentary region, becoming slightly smaller anteriorly. The posteriormost two tooth positions are smaller than more anterior positions, and are each made up of two small teeth, closely spaced and curving towards each other. Based on spacing and morphology, these are probably twinned (i.e., developed from a single tooth germ) (Fig. 5A6).
The palatal elements are very difficult to interpret. The right hyomandibula appears to be covering the anterior braincase as well as parts of the palate; the bones are so tightly compacted as to be difficult to differentiate (Fig. 5A1, A2). The left ectopterygoid and a dermopalatine are preserved internal to and covered by the hyomandibula. Posteriorly, these palatal elements are very difficult to distinguish from the overlying element, however at the anterior end they are fully exposed. The anterior end of the ectopterygoid is pointed, with the point overlapping the dermopalatine. A medial shelf is present. Anterior and ventral to the ectopterygoid and hyomandibula is a fragment interpreted as the entopterygoid. Its position overlapping the hyomandibula suggests that it originates from the right-hand side of the skull. It is relatively deformed, and nothing can be added with regard to its morphology.
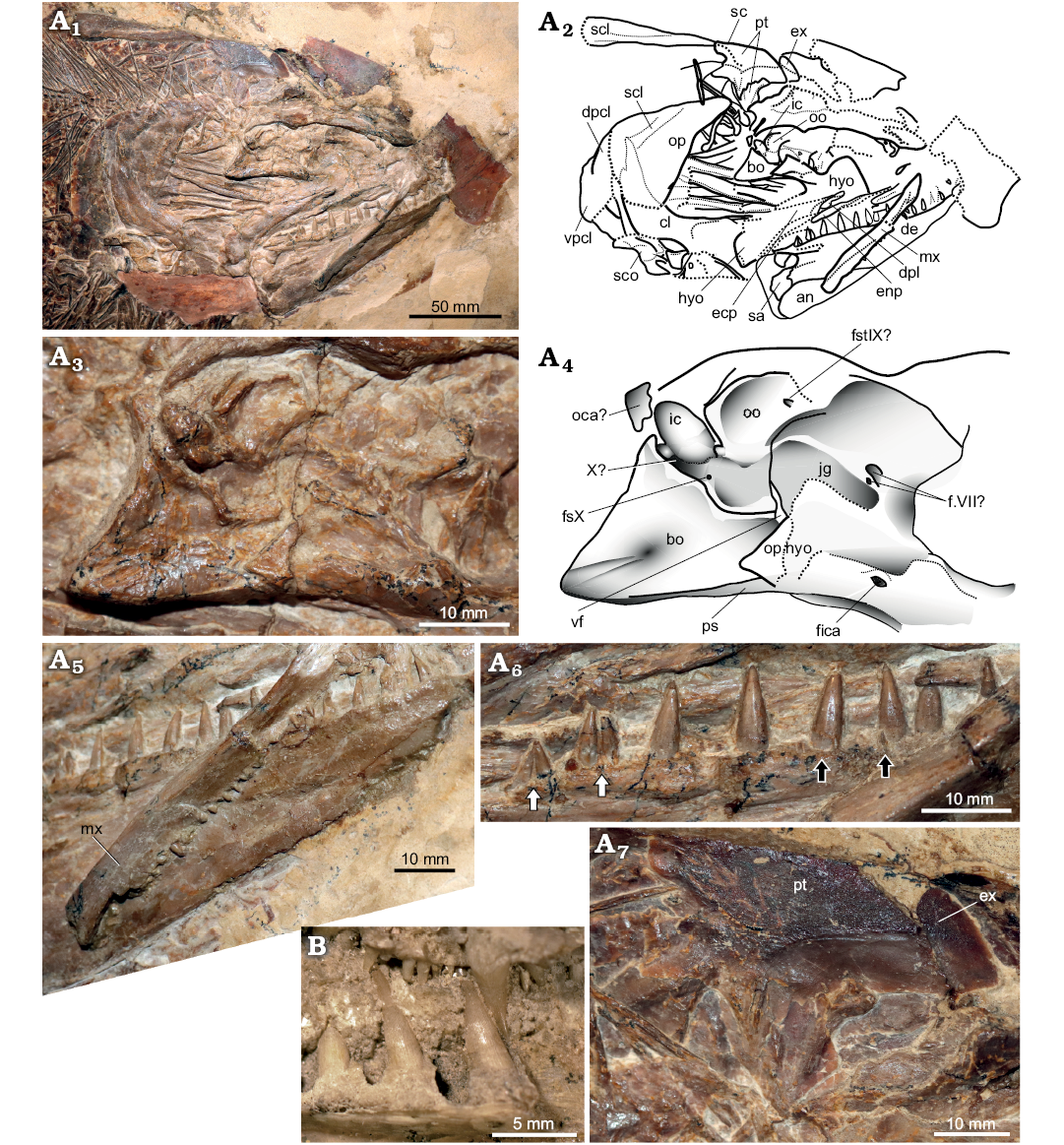
Fig. 5. Pachycormid fish Hypsocormus
Wagner, 1863 from the Late Jurassic of Germany. A. Hypsocormus
posterodorsalis sp. nov. (GPIT/OS/00836) from the
Kimmeridgian of Nusplingen. Skull preserved in right lateral view,
photograph (A1),
explanatory drawing (A2).
Braincase in lateral view, photograph (A3),
explanatory drawing (A4).
Anterior skull in ventrolateral view showing the maxillary dentition
and the absence of strong tubercular ornamentation on the lateral
surface of the maxilla (A5).
Posterior dentary teeth, white arrows indicate posterior two tooth
positions showing twinning of tooth germ, black arrows indicate
small teeth external to main dentary tooth row (A6).
Temporal boss in lateral view (A7).
B. Hypsocormus
insignis (Wagner, 1860) (holotype SNSB-BSPG AS VI 4a),
dentition in lingual view showing apicobasal ridges (anterior to the
left). Abbreviations: an, angular; bo,
basi-exoccipital; cl, cleithrum; de, dentary; dpcl, dorsal
postcleithrum; dpl, dermopalatine; ecp, ectopterygoid; enp,
entopterygoid; fica, foramen for the internal carotid artery; ex,
?extrascapular; f.VII?, foramina potentially transmitting the facial
nerve; fstIX, foramen for the supratemporal branch of the
glossopharyngeal nerve; fsX, foramen for a subsidiary branch of the
vagus nerve; hyo, hyomandibula; ic, intercalar; jg, jugular groove;
mx, maxilla; oca, occipital arch; oo, opisthotic; op, opercle;
op.hyo, opercular process of the hyomandibula; ps, parasphenoid; pt,
posttemporal; sc, sensory canal; scl, supracleithrum; sco,
scapulocoracoid; vf, vestibular fontanelle; vpcl, ventral
postcleithrum; X, formen for the vagus nerve.
The left hyomandibula is present, rotated forward and overlying the lateral braincase and palate, but is extremely poorly preserved. It is a long element with a “waisted” shape and a well-developed opercular process. The proximal articular surface in lateral view is straight, rather than semicircular. At the distal end, the hyomandibula is mediolaterally thickened, especially along the anterior edge, forming a well-developed facet for articulation with the symplectic; the latter element is not preserved.
Branchial elements are visible in the space between the anterior skull and the operculum; no associated denticles are present.
The opercle is preserved posterior to the branchial elements. It is narrow dorsally, with an anteroventral to anteriorly oriented facet for articulation with one or both suborbitals and/or preopercle. The anterior edge of the opercle forms a 150° angle, dividing the dorsal third from the ventral portion. The posterior and ventral extent of the opercle is difficult to assess. The dorsalmost subopercle is preserved in articulation with the opercle, but is ventrally incomplete and posteriorly damaged; only the anterodorsal process can be unambiguously recognized.
Postcranial axial skeleton: Approximately 105 neural arches are present anterior to the caudal fin. The dorsal fin inserts around segment 57, and the anal fin is approximately opposite. The vertebral column is aspondylous along its entire length. In the anterior abdominal region, paired neural arches and thin, elongated paired neural spines are present. Near the anterior insertion of the dorsal fin, the neural spines develop thin bony laminae anterior and posterior to the spine immediately dorsal to the arch. The antimeric neural spines are fused from this point posteriorly. At a point approximately opposite the pelvic girdle, sigmoidal supraneurals appear. The supraneurals insert between every two dorsal fin pterygiophores. Supraneurals persist until halfway along the base of the dorsal fin, at which point they cease to ossify.
In the abdominal region, well-ossified ribs are present. These are slender and elongate, with a small cup-like expansion proximally. Small, circular endochondral ossifications are irregularly present and are interpreted as basiventrals. Approximately halfway along the abdominal region, the proximal end of the ribs becomes enlarged and flattened, as if the ribs had fused with these basiventrals. At the segment corresponding to the anterior insertion of the dorsal fin, unpaired median haemal spines appear. Posterior to the mid-point of the anal fin, the haemal spines gradually become oriented increasingly parallel to the vertebral column, as do the neural spines.
Median fins (Fig. 6): The dorsal fin is supported by 27 slender, elongate pterygiophores, the anteriormost of which are laterally expanded distally, forming a broad base for the lepidotrichia (Fig. 6). The posteriormost element is anteroposteriorly expanded and ventrally bifurcating; based on comparisons with Pachycormus, this is most likely a compound element consisting of the posteriormost two pterygiophores, making the total number of dorsal pterygiophores 29. The dorsal fin lepidotrichia are segmented. Although the anterior rays are incomplete, the longest preserved fin rays are less than twice the length of the pterygiophores. Branching cannot be assessed due to preservation.
The anal fin base appears to have been slightly posteriorly displaced. It inserts approximately opposite- to at maximum a few segments posterior to the dorsal fin base. Twenty-nine anal pterygiophores are preserved in articulation (Fig. 6A2), and at least 13 more are scattered posterior to the fin, suggesting that the count was far higher. The anteriormost pterygiophore is more robust than the more posterior elements, and is extremely elongate, extending along most of the posterior edge of the abdominal cavity. The anal fin pterygiophores are similar in morphology to the dorsal pterygiophores. The anal fin lepidotrichia were segmented, with the robust lepidotrichia concentrated anteriorly. Posteriorly, the lepidotrichia become very delicate, and extend posteriorly beyond the articulated pterygiophores. The anal fin appears to be deepest anteriorly, and decreases in height rapidly posteriorly (falcate morphology).
The caudal fin is well preserved, but the distal tip of the dorsal lobe is missing, and the elements have been dissociated laterally (Fig. 6A3). This dissociation makes the number of lepidotrichia difficult to assess. The caudal fin is deeply forked, with an angle of approximately 110° formed between the lobes. The proximal lepidotrichia are unsegmented, but distally are divided into short segments. Fringing fulcra are of type B (Arratia 2009), in which small fringing fulcra are intercalated between the distal lepidotrichia along the leading edge of the fin; this is true in both the dorsal and ventral lobes (Fig. 6A7). A series of long, unpaired but deeply forked basal fulcra is present in the dorsal lobe; this character could not be assessed for the ventral lobe but appears probable based on the lack of segmentation of the anterior fin elements. The basal fulcra are much shorter than the depth of the caudal fin.
The caudal fin endoskeleton as preserved consists of at least five free thickened and robust unpaired preural haemal arches (Fig. 6A5, A6). The dorsal surface of the preural haemal arches is saddle-shaped and anteroposteriorly short. In preurals 3–6, the proximal end is laterally expanded, and the spines taper distally. The anterior surface is concave, with two lateral ridges running the length of the spine. Preurals 5–6 have an additional extremely thin, anterolaterally directed lamina slightly distal to the proximal end. Preural 2 is proximally laterally constricted ventral to the saddle-shaped dorsal surface. A prominent lip and anterior articular facet are present, analogous to the haemal process. Dorsal to preural 2 is an element interpreted as “uroneural 4” (sensu Arratia and Lambers 1996). Posterodorsal to this element, a single elongated dorsal element, interpreted as an epural, is preserved. This identification is based on the observation that this element is a median element, and quite thin posterodistally, but with a prominent lateral expansion involving the anterior and proximal portions. The hypural plate consists of a broad, fan-shaped posterior plate and an anterior “neck”. The neck bears a large lateral foramen, indicating that the hypural plate is formed by both the parhypural and hypurals (as per Arratia and Lambers 1996) (Fig. 6A5, A6). The anterior dorsal hypural plate is unfortunately poorly preserved, but forms a clear anterior projection corresponding to the hypural process (Arratia and Lambers 1996). A notch on the posteroventral surface of the hypural plate may delimit the parhypural; the total number of elements making up the plate is uncertain. The hypural plate is ornamented with radiating grooves for articulation of the lepidotrichia. An additional groove, leading to a notch on the anteroventral edge of the plate, may correspond to the path of a branch of the caudal artery (Arratia and Lambers 1996).

Fig. 6. Pachycormid fish Hypsocormus posterodorsalis sp. nov. (GPIT/OS/00836) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Unpaired fins: dorsal (A1), anal (A2), caudal (A3), arrows indicate L-shaped scales of the scaly caudal apparatus. Magnified view of scales of scaly caudal apparatus (A4). Caudal fin endoskeleton (photograph A5, explanatory drawing A6). Caudal fin, dorsal lobe, fringing fulcra (A7), arrows indicate the distal tips of the procurrent rays. Abbreviations: da, neural arches; ep, “epural”; hyp, hypural plate; pu2, 3, preural haemal arches; sc, scute; un4, “uroneural” 4.
Pectoral girdle and fin: The right cleithrum is largely covered by the opercle, which is deformed over it. The right supracleithrum appears to remain in articulation, also under the opercle. The left supracleithrum is displaced posteromedial to the right temporal boss. The dorsal process of the cleithrum is narrow, but a posterior expansion is present, making the element broader and more angular posteriorly than anteriorly. The glenoid is oriented posteroventrally, and is very poorly defined. Anterior to the glenoid, the cleithrum has a medial expansion, and anteriorly forms a concave bony lamina. At least two postcleithra are present.
The right endochondral pectoral girdle is exposed in lateroventral view, and appears to be broken. We consider the anterior piece to be the slender anterior process of the scapulocoracoid, and the posterior fragment to represent the main body of the element. The posterior element is large, three-dimensionally complex, and shows relatively weak endochondral ossification. The anterior edge bears a well-developed notch, potentially representing the posterior edge of the coracoid canal. Two short, broad radials are preserved posterior to the scapulocoracoid, and two elongate, distally flaring radials are present, one mixed with the pectoral lepidotrichia and the second posterodorsally displaced. A small subcircular element posterior to the scapulocoracoid is interpreted as a displaced distal radial. The pectoral lepidotrichia are robust and elongate, with a grooved ornamentation. No evidence of segmentation of pectoral lepidotrichia is observed.
Pelvic girdle and fin: The pelvic girdle, consisting of two robust pelvic plates, is exposed in dorsal view (Fig. 7). Each pelvic plate is formed by a medially expanded posterior lamina and an elongate anterior process, and is grooved in dorsal view. The pelvic fin lepidotrichia are robust, curved and unsegmented, located closer to the pectoral fins than to the anal fin.

Fig. 7. Pachycormid fish Hypsocormus posterodorsalis sp. nov. (GPIT/OS/00836) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Pelvic plates and lepidotrichia in internal view (photograph A1, explanatory drawing A2).
Squamation: The scales are relatively robust, and appear to have been roughly rectangular in shape. They measure ca. 3 mm along the anteroposterior axis, and appear to have a 2:1 relationship with vertebral elements. A scaly caudal apparatus (sensu Arratia and Schultze 2013) was present, but has been displaced anteroventrally. The scales of the caudal apparatus are L-shaped, narrow and rounded ventrally and becoming flattened and expanded posteriorly (Fig. 6A3, A4). There is also evidence for at least one mid-dorsal scute immediately anterior to the caudal fin (Fig. 6A3, A5, A6).
Paleobiology (Fig. 8): Many of the ribs show irregularly distributed small swellings along their lengths (Fig. 8A2). These types of swellings could be interpreted as hyperostosis, an idiopathic condition to which many lesions in the fish axial skeleton are referred, with these lesions usually occurring predictably in certain regions of the skeleton in a given species (usually the neural and haemal spines or pterygiophores, but also occasionally ribs; reviewed by Witten and Hall 2015). Hyperostosis has not previously been described in pachycormids, and hyperostotic lesions are usually much larger than those observed in GPIT/OS/00836. The alternative is that the lesions are calluses resulting from traumatic injury to the ribs. Pathologies affecting the actinopterygian axial skeleton are rarely reported, as the probability of surviving such an injury is considered relatively low. However, callus development has been described in the neural and haemal spines of farmed cod, and in the ribs of sculpin (Horton and Summers 2009; Fjelldal et al. 2018), and is superficially similar to the lesions observed in GPIT/OS/00836. We interpret the lesions on the ribs in GPIT/OS/00836 as calluses resulting from traumatic injury to the abdominal region.
GPIT/OS/00836 has at least two small teleosteans as gastric contents (Fig. 8A3), indicating a piscivorous diet.
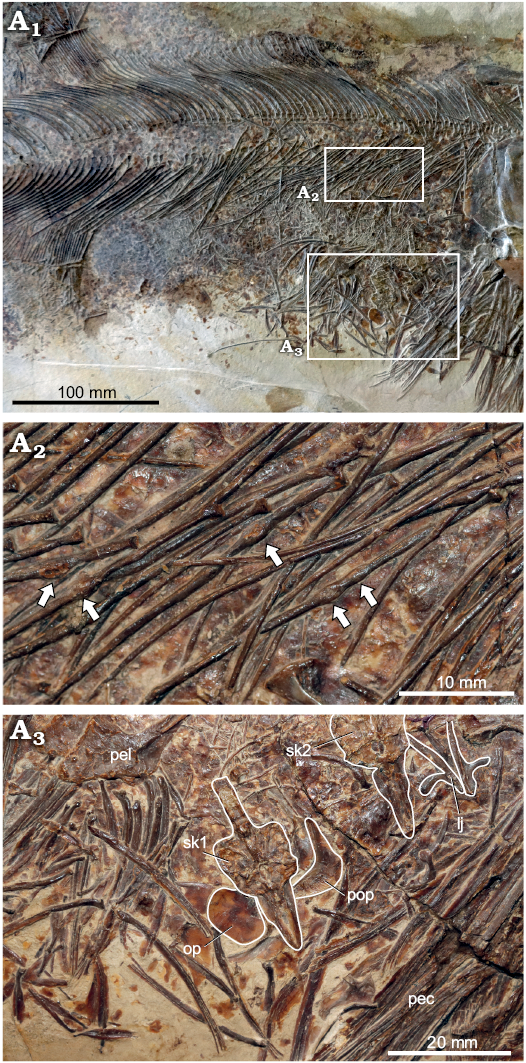
Fig. 8. Pachycormid fish Hypsocormus posterodorsalis sp. nov. (GPIT/OS/00836) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Overview of abdominal region (A1). Ribs with swellings interpreted as showing callus development (indicated by arrows) (A2). Small teleosteans in gastrointestinal cavity, with certain identifiable elements outlined to increase contrast (A3). Abbreviations. lj, prey mandible; op, prey opercle; pec, Hypsocormus pectoral lepidotrichia; pel, Hypsocormus pelvic plate; pop, prey preopercle; sk1+2, prey skulls.
Remarks.—GPIT/OS/00836 has relatively large pelvic fins, positioned closer to the pectoral fins than to the anal fins, and a robust pelvic plate with an expanded posterior plate and a slender anterior process, character states only documented in Simocormus macrolepidotus gen. et sp. nov. and Orthocormus among the toothed pachycormids (Lambers 1992). However, the scales in GPIT/OS/00836 are much larger and more heavily ossified than those of Orthocormus but smaller than those of Simocormus macrolepidotus, the dorsal fin is only slightly anterior to and largely overlapping with the anal fin base, and the posterior and ventral edges of the lower jaw form a right angle, all differing from Orthocormus and Simocormus macrolepidotus, but consistent with Hypsocormus insignis. At 69 cm SL, GPIT/OS/00836 is similar in size to H. insignis; the relative head length (at least 22% SL) and prominent temporal boss are equally consistent with the latter species. Distally expanded median fin pterygiophores and presence of scaly caudal apparatus have been used to diagnose Orthocormus (Arratia and Schultze 2013), but appear to be more widely distributed within the family, including in H. insignis (see discussion).
GPIT/OS/00836 differs from Hypsocormus insignis in several details. The supraneurals do not begin immediately posterior to the skull and the pelvic plate is robust with an expanded posterior plate and a slender anterior process in GPIT/OS/00836 but not in H. insignis (Lambers 1992); however, both of these characters may be artefactual: a robust pelvic plate may be taphonomically obscured by phosphatization of soft tissues in the Bavarian material of H. insignis, and the anterior supraneurals may be lost or scattered in GPIT/OS/00836. GPIT/OS/00836 also differs from material referred to H. insignis in the lack of tubercular ornamentation on the maxilla; however, this character cannot be observed in the holotype of H. insignis. The most substantive difference exists in the morphology of the caudal endoskeleton in GPIT/OS/00836. In particular the shape of the anteroventral hypural plate and the presence of a foramen in the lateral hypural plate indicate that the parhypural is fused to the hypural plate in GPIT/OS/00836 but not in material referred to H. insignis (Arratia and Lambers 1996). This cannot be evaluated in the H. insignis holotype. A hypural plate consisting of both the parhypural and hypurals is also observed in Simocormus macrolepidotus gen. et sp. nov. (SNSB-JME SOS 3574b), as well as in some species of Protosphyraena (Arratia and Lambers 1996). GPIT/OS/00836 differs from the H. insignis holotype (SNSB-BSPG AS VI 4 a/b) in the extensive overlap of the dorsal and anal fin bases in GPIT/OS/00836, whereas the dorsal fin is almost entirely anterior to the anal fin in SNSB-BSPG AS VI 4 a/b (Fig. 4), and in the prominent apicobasal ridging of the tooth bases in SNSB-BSPG AS VI 4 a/b, but not GPIT/OS/00836 in which the tooth bases are smooth (Fig. 5A6, B1). Given these differences, we refer GPIT/OS/00836 to Hypsocormus posterodorsalis sp. nov.
Stratigraphic and geographic range.—Type locality and horizon only.
Genus Orthocormus Weitzel, 1930
Type species: Orthocormus cornutus Weitzel, 1930.
Orthocormus sp.
Material.—GPIT/OS/1302, skull and anteriormost postcranium in right lateral view, from the Late Jurassic of Nusplingen, Germany.
Description.—General appearance: This skull (GPIT/OS/ 1302), from a large fish (SL estimated at ~1.1 m), was partially figured by Quenstedt (1856–1857: pl. 97: 12, 1885: fig. 105) as Strobilodus giganteus and was later redescribed by Heineke (1906) as Hypsocormus macrodon following Wagner (1863). Lambers (1992) was unable to locate the material, but referred it to Pseudoasthenocormus retrodorsalis based on Heineke’s description and photograph. We located the specimen in the collections of the GPIT (Fig. 9), and re-evaluated its morphology and affinities. Our description emphasizes details not adequately discussed by Heineke (1906).
The rostrodermethmoid forms two distinct processes in lateral view, separated by the antorbital: a dorsomedial portion, and a ventrolateral portion, forming a ~55° angle, making the rostrodermethmoid relatively deep rather than acutely pointed (Fig. 9A1). Anteriorly, the rostrodermethmoid forms an edentulous presymphyseal rostrum, which is flattened dorsally, and ventrally is offset from the oral margin. The dorsomedial rostrodermethmoid is strongly ornamented with tubercles. In lateral view, a large paramedial tooth is visible near the ventrolateral edge of the rostrodermethmoid.
The maxilla and suborbital fragment are as described by Heineke (1906). The premaxilla has been displaced, and is preserved ventral and medial to the maxilla. It is elongated in morphology with tubercular lateral ornamentation. The premaxilla bears two mid-sized posterior teeth and smaller anterior teeth.
The antorbital is anteroposteriorly elongate, situated between the dorsomedial and ventrolateral rami of the rostrodermethmoid anteriorly, and more posteriorly between the premaxilla and nasal (Figs. 9, 10A6). The posterior edge contacts the lateral ethmoid. The antorbital lacks pronounced ornamentation.
The nasal is positioned lateral to the dorsomedial rostrodermethmoid on the skull roof. Posteromedially, it articulates with the frontal. The anterodorsal edge of the orbit is damaged and the dermosphenotic is not preserved, so it is unclear whether the nasal was excluded from the edge of the orbit.
The posterior parietal is tented upwards to form the anterior portion of the temporal boss. The posterior edges of the parietals are greatly thickened and laterally divergent, indicating a well-developed temporal boss (Gouiric-Cavalli and Cione 2015), represented by several dermal bone fragments posterior to the parietals.
The braincase is not well-preserved, but some details can be discussed (Fig. 10A1, A2). The lateral ethmoid is highly asymmetrical along its anteroposterior axis, with the dorsal process strongly inclined posteriorly and expanded, forming a posteriorly elongate articulation with the ventral surface of the skull roof. The sphenotic is present anteriorly, largely covered by the dermal skull. Slightly more detail can be added regarding the posterior braincase. The parasphenoid remains in articulation, underlying the basi-exoccipital. The latter bears a prominent posterior semicircular extension that may represent the fusion of a posteroventral arch element. The intercalar is small and externally concave, positioned posteroventral to the opisthotic (Fig. 10A1, A2). Ventrally, the intercalar forms the dorsal edge of the vagus canal. The element or fragment ventral to the intercalar is of uncertain identity. The opisthotic itself is relatively poorly preserved. It is divided into dorsal and ventral portions, separated by a ridge.
The anterior edge of the quadrate forms an obtuse angle (ca. 110°), and its lateral surface bears a prominent median spine (Fig. 9). An overlying element is interpreted as an anteriorly displaced symplectic. The symplectic is approximately rectangular, with a weakly concave ventral edge. The lateral surface is grooved, and the anterior end is thickened and convex. The hyomandibula is slightly anteriorly inclined, forming a ~50° angle with the long axis of the lower jaw. A large opercular process is present.
The dentary, angular, and surangular form the lateral lower jaw (Fig. 9). The angular is high posteriorly, and anteriorly elongated, forming a slightly concave suture with the dentary. The surangular is very small, restricted to the posterodorsal corner of the lower jaw. The mandibular sensory canal is positioned close to the ventral edge of the lower jaw, and only begins to curve dorsally near the anterior tip. The dentary bears the remnants of a large procumbent and laterally compressed tooth on its anterior edge, followed posteriorly by four to five very small teeth (Fig. 10A3); the other dentary teeth are moderate in size. The anterior dentary bears a groove on its lateral surface, extending from the oral margin ventrally. An inflated coronoid bearing a large tooth is present medial to the reduced dentary teeth (Fig. 10A3). The teeth, including the procumbent and paramedial fangs, are laterally compressed, and are oval in cross-section.
The vertebral column is aspondylous; however, neural arches and ribs are ossified. The scales, while not well preserved, are relatively large: up to 4 mm in diameter, and appear to lack ganoine. The size of the scales relative to the vertebral column is difficult to assess, but is estimated at 2:1.
The first pectoral ray is more strongly ornamented and shorter than the successive rays, and is tightly fused to the second ray, forming a compound element (Fig. 10A4, A5). Comparison of the right fin in dorsal view and the left fin in ventral view indicates strong asymmetry in the development of the compound ray (1+2), with ornamentation being much better developed on the dorsal surface (Fig. 10A4), and the first dorsal hemilepidotrichium being much broader than the first ventral hemilepidotrichium (Fig. 10A5). These observations suggest that the first “ray” is probably an unpaired, asymmetrical bony splint (see e.g., Arratia 2008: fig. 28) rather than a lepidotrichium, and the compound ray is formed by the fusion of the splint to the hemilepidotrichia of the first lepidotrichium.

Fig. 9. Pachycormid fish Orthocormus sp. (GPIT/OS/1302) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Overview (photograph A1, explanatory drawing A2). Abbreviations: an, angular; ao, antorbital; bo, basi-exoccipital; bsr, branchiostegal rays; cl, cleithrum; co, coronoid; da+ns, neural arch + spine; de, dentary; dpl, dermopalatines 1 + 2; hyo, hyomandibula; ic, intercalar; le, lateral ethmoid; mc, mandibular canal; mx, maxilla; na, nasal; pe, pre-ethmoid; pec (r/l), right and left pectoral lepidotrichia; oo, opisthotic; ps, parasphenoid; qu, quadrate; r2, second pectoral radial; rdme, rostrodermethmoid; ri, ribs, sa, surangular; sco, scapulocoracoid; spo, sphenotic; sy, symplectic; tb, temporal boss.

Fig. 10. Pachycormid fish Orthocormus sp. (GPIT/OS/1302) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Braincase in lateral view (photograph A1, explanatory drawing A2). Anterior dentary and inflated coronoid (A3), arrows indicate the “diastema” formed by tiny teeth along the lateral margin of the dentary, found only in the region lateral to the coronoids. Anterior pectoral fin lepidotrichia in ventral (A4) and dorsal (A5) views; lepidotrichia are numbered anterior to posterior; the white arrows indicate the first point of fusion between fin rays 1 and 2, the black arrows indicate the suture between the two rays. Rostrodermethmoid in anterolateral view (A6), arrow indicates the notch separating the edentulous rostrum from the portion of the bone forming the oral margin. Abbreviations: bo, basi-exoccipital; hy, hyomandibula; ic, intercalar; oo, opisthotic; op.hy, opercular process of the hyomandibula; ps, parasphenoid; spo, sphenotic; x, vagus foramen.
Remarks.—GPIT/OS/1302 is a pachycormid based on the presence of a toothed rostrodermethmoid separating the premaxillae (Liston 2008). The robust mandible and dentition, in particular the presence of large, paramedial teeth on the rostrodermethmoid, suggest GPIT/OS/1302 is most consistent with the clade of pachycormids containing Hypsocormus, Simocormus, and macrocarnivorous forms, and likely also Sauropsis and Pseudoasthenocormus (Liston 2008; Friedman et al. 2010).
GPIT/OS/1302 differs from Hypsocormus, Simocormus, and Sauropsis in the presence of an edentulous projection of the rostrodermethmoid anterior to the mandibular symphysis. Lambers (1992) suggested the referral of this specimen to Pseudoasthenocormus based on the presymphyseal extension of the rostrodermethmoid, large angle formed between the upper jaw and the dorsal skull roof, and relatively slender fin rays. However, re-evaluation of the material indicates that there are many inconsistencies with Pseudoasthenocormus. Specifically, GPIT/OS/1302 differs from P. retrodorsalis in that (i) the anterior rostrodermethmoid is edentulous; (ii) the premaxilla is elongated relative to its depth; (iii) the antorbital portion of the dermatocranium is as long as the postorbital segment; (iv) the hyomandibula is much less steeply inclined; (v) the mandible and mandibular dentition is much more robust and the teeth are laterally compressed; (vi) the angular is reduced in lateral view relative to the dentary; (vii) mandibular ornamentation is much less prominent; (viii) the first pectoral fin ray is shorter than more posterior rays, with distinctive ornamentation, and is fused to the anterior surface of the second ray; (ix) the pectoral fin rays are more robust; and (x) the scales are smaller than those of Pseudoasthenocormus. Based on these differences, GPIT/OS/1302 is inconsistent with Pseudoasthenocormus.
The only Late Jurassic genus with an edentulous presymphyseal projection of the rostrodermethmoid, as in GPIT/OS/1302, is Orthocormus. In addition to this character, GPIT/OS/1302 shares the elongate premaxilla, long antorbital region, laterally compressed teeth, robust mandible and robust and fused pectoral fin rays with Orthocormus (see Lambers 1988). Orthocormus? tenuirostris, from the Callovian of the UK, also has a pronounced edentulous rostrum, but numerous osteological differences separate it from GPIT/OS/1302, including the shape of the premaxilla and the tubercular ornamentation completely covering the lateral surface of the lower jaw (Woodward 1895).
Orthocormus currently contains three named Late Jurassic species: O. cornutus, O. teyleri, and O. roeperi (see Table 1). O. roeperi and O. teyleri are both relatively small fishes (<55 cm SL), whereas O. cornutus is much larger (~1.1 m SL); the ontogenetic status of material referred to the two smaller species is unknown. The species are differentiated primarily based on dentition and postcranial meristics. In addition, O. roeperi differs from the two other species in lacking an anterior projection of the temporal boss (Arratia and Schultze 2013), and thus is inconsistent with GPIT/OS/1302. However, GPIT/OS/1302 cannot easily be referred to either of the other species, as both O. teyleri and O. cornutus have minute scales in a 4:1 ratio with the axial skeleton, whereas the scales in GPIT/OS/1302 appear to be considerably larger. Thus, while GPIT/OS/1302 shares several key apomorphies with Orthocormus spp., we cannot assign this specimen to species.
A braincase referred to Orthocormus sp., NRM P425, differs from GPIT/OS/1302 in a few respects (Holmgren and Stensiö 1936; Rayner 1948). The braincase of GPIT/OS/1302 is proportionately much deeper, a feature possibly exacerbated by lateral compression. However, the position of the vagus foramen ventral to the intercalar is not shared with NRM P425. The opisthotic bears a notch in GPIT/OS/1302 in a similar position to the vagus foramen in NRM P425, but preservation is insufficient to assess whether this is a depression, foramen, or damage. The more posterior foramen ventral to the intercalar is absent from NRM P425 (Holmgren and Stensiö 1936; Rayner 1948). GPIT/OS/1302 and Orthocormus sp. share the shape of the braincase in lateral view (reconstruction of Rayner 1948), in which the sphenotic and posterior edge of the braincase are roughly parallel, the posterior edge of the braincase is straight with convex projections, rather than concave, and projects posteriorly substantially beyond the posterior edge of the dorsal skull roof.
The braincases of both GPIT/OS/00836 (Hypsocormus posterodorsalis sp. nov.) and GPIT/OS/1302 (Orthocormus sp.) are preserved in a similar way, permitting direct comparisons between the posterior braincases of these two genera. Both show the unexpected position of the vagus foramen ventral to the intercalar, unlike in other published descriptions. The state of preservation of both of these crania raises the possibility that the interpretation presented here may be incorrect. Both also share a well-developed opisthotic, larger than the intercalar, as do other described pachycormid braincases (Rayner 1948; Mainwaring 1978). The two differ in some specific details: the braincase of GPIT/OS/1302 projects posteriorly further beyond the posterior edge of the dermatocranium, and lacks the concave posterior edge of the basi-exoccipital seen in H. posterodorsalis. The suture between the intercalar and opisthotic is essentially vertically oriented in Orthocormus (GPIT/OS/1302, Rayner 1948), whereas in H. posterodorsalis it is strongly posterodorsally-anteroventrally inclined. The significance of these observations across genera requires less crushed material.
Pachycormidae gen. et sp. indet.
Material.—GPIT/OS/1301 (Fig. 11), a caudal fin fragment figured by Quenstedt (1856–1857: pl. 100: 6); GPIT/OS/1303, a partial skull consisting of both lower jaw rami and some poorly preserved cranial and visceral arch elements; both from the Late Jurassic of Nusplingen, Germany.

Fig. 11. Caudal fin fragment of an unidentified pachycormid fish (GPIT/OS/1301) from the Kimmeridgian (Jurassic) of Nusplingen, Germany.
Description.—GPIT/OS/1301 is rather large fragment, ca. 9.5 cm in preserved length. The lepidotrichia are thin and the segments are relatively elongated, slightly expanded at the proximal and distal ends. The fin rays bifurcate distally. The anterior edge of the fin is damaged, making the morphology of the fringing fulcra impossible to determine. The posterior lepidotrichia are posteriorly curved and very slender. Based on the height to length of the fragment, it originates from a high aspect-ratio fin.
GPIT/OS/1303 (Fig. 12) was described by Heineke (1906), but only a single element, the “suboperculum”, was figured. This element is in fact the articulated remains of the left so1 and so2. The right suborbitals are also present, but are not as well preserved. so2 is dorsally concave, and so1 is ventrally narrow, expanding dorsally and roughly triangular (Fig. 12A3). so2 is greatly enlarged and the anteriormost end of which is invaded by a sensory canal (Fig. 12A3). The presence of the sensory canal suggests that the ventralmost infraorbital is fused with the anterior end of so2. A small facet is present on the ventral edge of so2 for articulation with the supramaxilla. The surface of so2 is ornamented with tubercles, which are better-developed close to the ventral edge. Ventral to the suture with so1, the lateral surface of so2 is ornamented short lines radiating from its posterior margin, partially preserved as impressions on the matrix. These suggest a ridged ornamentation, more weakly developed than that of Simocormus macrolepidotus gen. et sp. nov. (Figs. 2, 3A1, A2).
The lower jaws are 117 mm (right) and 120 mm (left) in length, indicating a fish of around 75 cm SL. The lateral surface of the dentary is smooth; the angular shows light tubercular ornamentation. The angular-dentary suture is clearly visible on the right. The mandibular sensory canal is visible, forming a 90° angle within the angular, continuing anteriorly parallel to the ventral edge of the angular and trending gradually dorsally once it enters the dentary.
The sharply pointed teeth appear to be conical in cross-section, and the cap enameloid is smooth. The collar enameloid is covered in fine striations (Fig. 12A4). Two dentary tooth rows are present, a tiny external row and a larger internal row. The dentary teeth in the larger tooth row are relatively small, but the anterior dentary bears two enlarged teeth, the anteriormost of which is procumbent.
The right anterior ceratohyal is preserved medial to the right mandibular ramus; the left is preserved under the left ramus and is only visible due to damage to the external surface of the latter. The left hypohyal with a foramen for the afferent hyoid artery remains in articulation with the corresponding anterior ceratohyal (Fig. 12A1, A2).
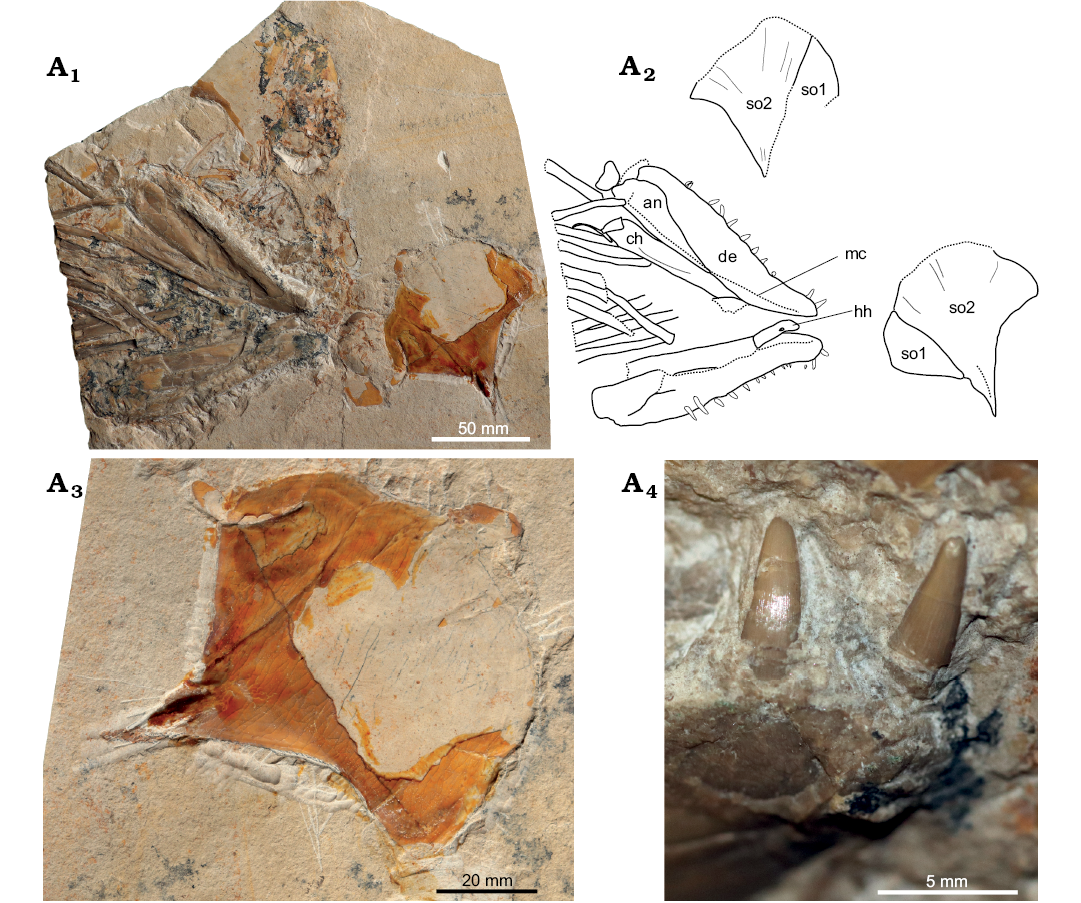
Fig. 12. Fragmentary skull attributed to Pachycormidae gen. et sp. indet. (GPIT/OS/1303) from the Kimmeridgian (Jurassic) of Nusplingen, Germany. Overview with mandibles and suborbitals in external view (photograph A1, explanatory drawing A2). Left first and second suborbitals in external view (A3). Anterior teeth of the right dentary (A4), note the procumbency of the mesial tooth, as well as the smooth apical and striated collar enameloid. Abbreviations: an, angular; ch, ceratohyal; de, dentary; hh, hypohyal; mc, mandibular canal; so1/so2, first and second suborbitals + fused infraorbital.
Remarks.—GPIT/OS/1301 is not amenable to synapomorphy-based diagnosis, its large size, long, slender lepidotrichial segments, and the inferred high aspect-ratio shape of the fin are most consistent with Pachycormidae among the fishes reported from the Late Jurassic plattenkalks. Many of the filter-feeding pachycormids, including Asthenocormus, have unsegmented caudal fin rays (Liston et al. 2013); however, lepidotrichial segmentation of the type observed in GPIT/OS/1301 is widely distributed among the toothed pachycormids.
GPIT/OS/1303 is referable to Pachycormidae based on the size, shape and arrangement of the suborbital elements (see argumentation in Lambers 1992). The well-developed mandibular dentition and procumbent dentary tooth place GPIT/OS/1303 within the toothed pachycormid clade. Generic referral is not possible due to the fragmentary nature of the specimen; however, the conical cross-section of the dentary teeth suggests that this specimen cannot be attributed to Orthocormus. The ridged ornamentation of so2 is shared with Simocormus macrolepidotus gen. et sp. nov. (Woodward 1895; Lambers 1992), but is more weakly developed in GPIT/OS/1303. The small size, open sutures between the mandibular elements, and possibly also the lack of ornamentation on the lower jaw suggest that, despite the relatively large estimated size, GPIT/OS/1303 might represent a juvenile.
Discussion
New anatomical information
Partly due to limitations of existing descriptions, this revision of the Nusplingen pachycormid material produced some new morphological observations broadly relevant for the interpretation of pachycormid anatomy.
Suborbitals.—Two large, plate-like and posteriorly expanded suborbitals are diagnostic for Pachycormidae (Lambers 1992). Invasion of the anterior end of so2 by sensory canals has not previously been documented in the clade, but here was observed in two individuals, both from Nusplingen (Figs. 3A1, A2, 12A3). The presence of the sensory canal in so2 suggests that it is most likely a compound element, fused with the posteroventralmost infraorbital (so2 + infraorbital) in these specimens. Whether this fusion is diagnostic of Simocormus macrolepidotus gen. et sp. nov., however, or whether it is more widely distributed within Pachycormidae is uncertain, since although it has not been previously reported the course of the infraorbital sensory canal is rarely mentioned.
Scale size in Pachycormidae.—One of the diagnostic characteristics of Pachycormidae is the small size of the scales, with mineralized scales lost entirely in the filter feeding taxa (Lambers 1992; Friedman 2012). However, there is extensive variation in absolute scale size, with Orthocormus and Sauropsis said to have particularly small scales (Lambers 1992). In order to control for body size differences between taxa, we standardize scale size relative to the vertebral column and myomeres (Fig. 13). One scale per axial segment is present in the flank region of Euthynotus, Simocormus macrolepidotus gen. et sp. nov. (Fig. 13A), Sauropsis depressus, and Pseudoasthenocormus retrodorsalis. While these scales may be very shallow, their length is the characteristic of interest. Flank scales present in a 2:1 ratio with vertebral elements (or myomeres when preserved) occur in Pachycormus, Saurostomus, Hypsocormus insignis, and Sauropsis longimanus (Fig. 13B). Orthocormus cornutus and O. teyleri have extremely reduced scales (4:1 ratio with vertebral elements; Fig. 13C).

Fig. 13. Variation in relative scale size in Pachycormidae from the Late Jurassic of Germany. A. Simocormus macrolepidotus gen. et sp. nov. (SNSB-JME SOS 3574), “large” scales. B. Sauropsis longimanus Agassiz, 1833 (SNSB-BSPG AS VII 1089), “small” scales. C. Orthocormus cornutus? (SNSB-JME 2181), “very small” scales. White arrows frame five axial segments; black arrows frame five vertical scale rows. Anterior is to the left.
Based on the topology of Friedman (2012), the primitive condition for Pachycormidae is one scale row per axial segment, but the number of scale rows per axial segment has increased multiple times independently, resulting in repeated reductions in scale length. The first increase occurred at the node separating Euthynotus from all other pachycormids (1:1 → 2:1). In the filter-feeding clade, scales were eventually lost entirely (2:1 → 0:1), whereas in the toothed clade at least one reversal occurred on the branch leading to Simocormus macrolepidotus gen. et sp. nov. (2:1 → 1:1), with further reduction in relative scale length in the lineage leading to Orthocormus (2:1 → 4:1).
A second character related to scale reduction and potentially to locomotion in pachycormids is the loss of the ganoine layer on the body scales. Ganoine is absent in Pachycormus based on histological study (Meunier and Brito 2004), and was noted as present only in Hypsocormus insignis and Simocormus macrolepidotus gen. et sp. nov. among the Late Jurassic pachycormid species (Schultze 1966). The relationship of histology to scale size is intriguing; however, we could not assess the presence of ganoine based on macroscopic examination.
Scaly caudal apparatus.—The scaly caudal apparatus, a series of highly modified scales projecting laterally at the base of the caudal lepidotrichia, was first described by Arratia and Schultze (2013) in Orthocormus roeperi. Similar scales can be observed in situ in Orthocormus cornutus? (SNSB-JME 2181), Hypsocormus insignis (SNSB-JME SOS 3557, MB.f.1547, SMNS 56650), H. posterodorsalis sp. nov. (Fig. 6A3, A4), and as impressions on the matrix in Sauropsis longimanus (SNSB-BSPG AS VII 1089). A scaly caudal apparatus, if present at all in Pachycormus and Sauropsis depressus, appears to be somewhat different in structure (Arratia and Schultze 2013).
The scaly caudal apparatus is closely correlated with the presence of a robust, elongated dorsal scute preceding the caudal fin (sensu Arratia and Schultze 2013). This scute was only noted in taxa in which the scaly caudal apparatus was well-developed (i.e., Orthocormus spp., Hypsocormus insignis, H. posterodorsalis sp. nov. (Fig. 6A3, A5, A6), and Sauropsis longimanus). Whether the presence of this scute is related to the same selective pressures leading to a well-developed scaly caudal apparatus (and thus was convergently developed in these taxa), phylogenetic relatedness, or chance of preservation is at present unclear.
Fusion of propterygium and anterior lepidotrichia.—SMNS 96988/4 shows fusion of the propterygium to the anterior lepidotrichia. The propterygium has been described, figured, and scored as fused to the anterior lepidotrichia in large specimens of Pachycormus, Hypsocormus insignis, Orthocormus? tenuirostris, Orthocormus sp., and Protosphyraena (Woodward 1895; Loomis 1900; Jessen 1972; Friedman et al. 2010; EM personal observation). This character has previously been cited as a teleostean synapomorphy shared by pachycormids (Patterson 1977; Friedman et al. 2010). However, some authors describe the propterygium as unfused to the lepidotrichia in Pachycormus and O.? tenuirostris (Arratia and Lambers 1996; Arratia and Schultze 2013), creating confusion in the literature. The basis for this difference in observation is unclear, but may centre around whether the anterior elements in the pectoral fin are treated as fin rays or basal fulcra (Arratia 2008). In the case of SMNS 96988/4, the element fused to the propterygium is identified as a ventral hemilepidotrichium; the dorsal hemilepidotrichium may also be fused to the propterygium but this is uncertain. Whether convergent or homologous with the teleostean condition, it seems likely that some degree of fusion of the propterygium and anterior lepidotrichia or compound anterior ray is widely distributed within the toothed pachycormids, at least in large individuals. In conjunction with the fusion of the lepidotrichia to a bony splint along the leading edge of the fin to form a compound ray (e.g., GPIT/OS/1302, Fig. 10A4, A5), fusion of the lepidotrichia and propterygium would have functioned to strengthen and stabilize the anterior edge of the pectoral fin.
Assessment of pachycormid diversity from the Nusplingen locality
Although pachycormid remains are very uncommon at the Nusplingen locality, diversity is relatively high, with at least three taxa present. These genera (Orthocormus, Hypsocormus, and Simocormus gen. nov.) are also present in presumed sympatry in the Late Jurassic Bavarian Plattenkalk basins to the East. The oldest record of Orthocormus can tentatively be considered to be O.? tenuirostris, from the Callovian (Middle Jurassic) of the UK. By the Late Jurassic, Orthocormus was widely distributed across Europe, including Cerin (France), southern Germany, and as far North as Poland (Lambers 1988; Arratia and Schultze 2013; Tyborowski 2017). Hypsocormus is both less distinctive based on fragmentary remains, and has historically been used as a catchall genus. Both of these factors make tracing its geographic and stratigraphic range highly problematical. The oldest confirmed occurrences of both Hypsocormus insignis and Simocormus macrolepidotus are from the south German Plattenkalks, with no diagnostic material having been reported from elsewhere.
Piscivory is widely assumed for toothed pachycormids (Gouiric-Cavalli and Cione 2015); however, evidence thereof is relatively sparse. Piscivory has been confirmed for Hyposcormus insignis from the Late Jurassic of Bavaria based on gut contents; small teleosteans appear to have be more common as prey items, possibly due to relative abundance (Viohl 1990). In addition to informing diversity, the gastrointestinal contents noted here for GPIT/OS/00836 confirm piscivorous feeding in H. posterodorsalis sp. nov. (Fig. 8A2), suggesting that this dietary preference for Hypsocormus can be extended back into the Kimmeridgian, and may be typical of the genus.
Hypsocormus is characterized by closely spaced teeth, relatively homogenous in size, whereas Orthocormus spp. has anterior caniniform dentary teeth, followed by a block of small teeth, and then additional large teeth in the posterior part of the dentary (e.g., Tyborowski 2017: fig. 6; note that the coronoid fang has been erroneously labeled as associated with the dentary). The latter pattern is termed back-fanged macrodont, and has been hypothesized to be related to the post-capture processing of prey which are large in proportion to the gape (Mihalitis and Bellwood 2019). These two divergent patterns of dentition hint at potential differences in prey preference between these two pelagic predators.
Conclusions
Three genera referable to Pachycormidae are present in the Kimmeridgian sediments of Nusplingen, in southern Germany: Orthocormus, Simocormus gen. nov., and Hypsocormus. The latter is represented by a specimen distinct enough to merit the erection of a new species, Hypsocormus posterodorsalis sp. nov., so far known only from Nusplingen. All three genera are members of the toothed clade of pachycormids, and indeed, Hypsocormus is represented by a specimen containing small teleosteans as gastric contents. Although moderately diverse, pachycormid fishes are uncommon finds from Nusplingen, and thus are unlikely to have constituted a major part of the resident fauna, potentially having been occasional visitors to the lagoon. Improved understanding of the anatomy of this clade is crucial in reconstructing the interrelationships of Pachycormidae, and will be critical in rigorously assessing rates of morphological evolution in these pelagic fishes.
Acknowledgements
Thanks to Emma Bernard (NHMUK) for providing photographs of NHMUK PV P 6011, Ingmar Werneburg (GPIT), Martin Ebert and Martina Köbl-Ebert (both SNSB-JME), Oliver Rauhut (SNSB-BSPG) and Florian Witzmann (MB) for collections access, and to Martin Kapitzke for preparation of SMNS 96988/4. Thanks also to reviewers Jeff Liston (Vivacity Peterborough Museum & Art Gallery, Peterborough, UK) and Matt Friedman (University of Michigan, Ann Arbor, USA), whose thoughtful comments improved the manuscript.
References
Agassiz, L. 1833-1843 Recherches sur les Poissons fossiles. 188 pp. Neuchatel et Soleure.
Arratia, G. 2008. Actinopterygian postcranial skeleton with special reference to the diversity of fin ray elements, and the problem of identifying homologies. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 4—Homology and Phylogeny, 49–101. Dr. Friedrich Pfeil, München.
Arratia, G. 2009. Identifying patterns of diversity of the actinopterygian fulcra. Acta Zoologica 90 (Supplement 1): 220–235. Crossref
Arratia, G. and Lambers, P. 1996. The caudal skeleton of Pachycormiformes: parallel evolution? In: G. Arratia and G. Viohl (eds.), Mesozoic Fishes—Systematics and Paleoecology, 191–218. Verlag Dr. Friedrich Pfeil, München.
Arratia, G. and Schultze, H.-P. 2013. Outstanding features of a new Late Jurassic pachycormiform fish from the Kimmeridgian of Brunn, Germany and comments on current understanding of pachycormiforms. In: H.-P. Schultze and M.V.H. Wilson (eds.), Mesozoic Fishes 5—Global Diversity and Evolution, 87–120. Verlag Dr. Friedrich Pfeil, Munich.
Arratia, G. and Schultze, H.-P. 2015. Teleosteomorpha, oder: die Stammgruppe der Teleosteer. In: G. Arratia, H.-P. Schultze, H. Tischlinger, and G. Viohl (eds.), Solnhofen: Ein Fenster in die Jurazeit, 381–389. Verlag Dr. Friedrich Pfeil, Munich.
Bengtson, P. 1988. Open nomenclature. Palaeontology 31: 223–227.
Cawley, J.J., Kriwet, J., Klug, S., and Benton, M.J. 2019. The stem group teleost Pachycormus (Pachycormiformes: Pachycormidae) from the Upper Lias (Lower Jurassic) of Strawberry Bank, UK. PalZ 93: 285–302. Crossref
Chellouche, P. 2016. Comparative Actinopterygian Fish Taphonomy of Laminated Fossil Lagerstätten: Case Studies from the late Mesozoic of Eurasia. 360 pp. Friedrich-Alexander-Universität, Erlangen-Nürnberg.
Dietl, G. and Schweigert, G. 2004. The Nusplingen Lithographic Limestone—a “fossil lagerstaette” of Late Kimmeridgian age from the Swabian Alb (Germany). Rivista Italiana di Paleontologia e Stratigrafia 110: 303–309.
Dietl, G. and Schweigert, G. 2011. Im Reich der Meerengel—Fossilien aus dem Nusplinger Plattenkalk. 144 pp. Verlag Dr. Friedrich Pfeil, Munich.
Dietl, G., Schweigert, G., Franz, M., and Geyer, M. 1998. Profile des Nusplinger Plattenkalks (Oberjura, Schwäbische Alb). Stuttgarter Beiträge zur Naturkunde B 265: 1–37.
Dobson, C., Giles, S., Johanson, Z., Liston, J., and Friedman, M. 2019. Cranial osteology of the Middle Jurassic (Callovian) Martillichthys renwickae (Neopterygii, Pachycormiformes) with comments on the evolution and ecology of edentulous pachycormiforms. Papers in Palaeontology [published online, https://doi.org/10.1002/spp2.1276] Crossref
Eastman, C.R. 1914. Catalog of the fossil fishes in the Carnegie Museum. Part IV. Descriptive catalog of fossil fishes from the lithographic stone of Solenhofen, Bavaria. Memoirs of the Carnegie Museum 6 (7): 389–423.
Fjelldal, P.G., van der Meeren, T., Fraser, T.W.K., Sambraus, F., Jawad, L., and Hansen, T.J. 2018. Radiological changes during fracture and repair in neural and haemal spines of Atlantic cod (Gadus morhua). Journal of Fish Diseases 41: 1871–1875. Crossref
Fraas, O. 1854. Squatina acanthoderma. Die Meerengel von Nusplingen. Zeitschrift der Deutschen Geologischen Gesellschaft 6: 782–799.
Fraas, O. 1855. Beiträge zum obersten weissen Jura in Schwaben. Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg 11: 76–107.
Fraas, O. 1878. Über Pterodactylus suevicus Qu. von Nusplingen. Palaeontographica 25: 163–174.
Friedman, M. 2012. Parallel evolutionary trajectories underlie the origin of giant suspension-feeding whales and bony fishes. Proceedings of the Royal Society B 279 (1730): 944–951. Crossref
Friedman, M., Shimada, K., Martin, L.D., Everhart, M.J., Liston, J., Maltese, A., and Triebold, M. 2010. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science 327: 990–993. Crossref
Fürsich, F.T., Mäuser, M., Schneider, S., and Werner, W. 2007. The Wattendorf Plattenkalk (Upper Kimmeridgian)—a new conservation lagerstätte from the northern Franconian Alb, southern Germany. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 245: 45–58. Crossref
Gardiner, B.G. and Schaeffer, B. 1989. Interrelationships of lower actinopterygian fishes. Zoological Journal of the Linnean Society 97: 135–187. Crossref
Gouiric-Cavalli, S. and Cione, A.L. 2015. Notodectes is the first endemic pachycormiform genus (Osteichthyes, Actinopterygii, Pachycormiformes) in the Southern Hemisphere. Journal of Vertebrate Paleontology 35 (4): e933738. Crossref
Gregory, W.K. 1923. A Jurassic fish fauna from western Cuba, with an arrangement of the families of holostean ganoid fishes. Bulletin of the American Museum of Natural History 48: 223–242.
Hättig, K., Stevens, K., Thies, D., Schweigert, G., and Mutterlose, J. 2019. Evaluation of shark tooth diagenesis-screening methods and the application of their stable oxygen isotope data for palaeoenvironmental reconstructions. Journal of the Geological Society 176: 482–491. Crossref
Heineke, E. 1906. Die Ganoiden und Teleostier des lithographischen Schiefers von Nusplingen. Geologische und palaeontologische Abhandlungen, neue Folge 8 (3): 159–214.
Holmgren, N. and Stensiö, E. 1936. B. Kranium und Visceralskelett der Akranier, Cyclostomen und Fische. In: L. Bolk, E. Göppert, E. Kallius, and W. Lubosch (eds.), Handbuch der vergleichenden Anatomie der Wirbeltiere, 233–500. Urban & Schwarzenberg, Berlin.
Horton, J.M. and Summers, A.P. 2009. The material properties of acellular bone in a teleost fish. Journal of Experimental Biology 212: 1413–1420. Crossref
Jessen, H. 1972. Schultergürtel und Pectoralflosse bei Actinopterygiern. Fossils and Strata 1: 1–101.
Lambers, P. 1988. Orthocormus teyleri nov. spec., the first pachycormid (Pisces, Actinopterygii) from the Kimmeridge lithographic limestone at Cerin (Ain), France; with remarks on the genus Orthocormus Weitzel. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. Series B 91 (4): 369–391.
Lambers, P. 1992. On the Ichthyofauna of the Solnhofen Lithographic Limestone (Upper Jurassic, Germany). 336 pp. Rijksuniversiteit, Groningen.
Liston, J. 2008. A review of the characters of the edentulous pachycormiforms Leedsichthys, Asthenocormus and Martillichthys nov. gen. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 4—Homology and Phylogeny, 181–198. Verlag Dr. Friedrich Pfeil, Munich.
Liston, J., Maltese, A.E., Lambers, P.H., Delsate, D., Harcourt-Smith, W.E.H., and van Heteren, A.H. 2019. Scythes, sickles and other blades: defining the diversity of pectoral fin morphotypes in Pachycormiformes. PeerJ 7: e7675. Crossref
Liston, J., Newbrey, M.G., Challands, T.J., and Adams, C.E. 2013. Growth, age and size of the Jurassic pachycormid Leedsichthys problematicus (Osteichthyes: Actinopterygii). In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 5—Global Diversity and Evolution, 145–175. Verlag Dr. Friedrich Pfeil, Munich.
Loomis, F.B. 1900. Die Anatomie und die Verwandtschaft der Ganoid- und Knochen-Fische aus der Kreide-Formation von Kansas. Palaeontographica 46: 213–283.
Mainwaring, A.J. 1978. Anatomical and Systematic Review of the Pachycormidae, a Family of Mesozoic Fossil Fishes. 162 pp. University of London, London.
Meunier, F.J. and Brito, P.M. 2004. Histology and morphology in some extinct and extant teleosts. Cybium 28: 225–235.
Mihalitis, M. and Bellwood, D.R. 2019. Functional implications of dentition-based morphotypes in piscivorous fishes. Royal Society Open Science 6: 190040. Crossref
Patterson, C. 1975. The braincase of pholidophorid and leptolepid fishes, with a review of the actinopterygian braincase. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 269 (899): 275–579. Crossref
Patterson, C. 1977. The contribution of paleontology to teleostean phylogeny. In: M.K. Hecht, P.C. Goody, and B.M. Hecht (eds.), Major Patterns in Vertebrate Evolution. NATO Advanced Study Institutes Series, 579–643. Plenum Press, New York. Crossref
Quenstedt, F.A. 1856–1857. Der Jura. VI + 842 pp. H. Laupp, Tübingen.
Quenstedt, F.A. 1885. Handbuch der Petrefaktenkunde, 3. Auflage. 1230 pp. H. Laupp’sche Buchhandlung, Tübingen. Crossref
Rayner, D.H. 1948. The structure of certain Jurassic holostean fishes with special reference to their neurocrania. Philosophical Transactions of the Royal Society London Series B. 601 (233): 287–345. Crossref
Schultze, H.-P. 1966. Morphologische und histologische Untersuchungen an Schuppen mesozoischer Actinopterygier (Übergang von Ganoid- zu Rundschuppen). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 126: 232–314.
Schumacher, B.A., Shimada, K., Liston, J., and Maltese, A. 2016. Highly specialized suspension-feeding bony fish Rhinconichthys (Actinopterygii: Pachycormiformes) from the mid-Cretaceous of the United States, England, and Japan. Cretaceous Research 61: 71–85. Crossref
Schweigert, G. 1998. Die Ammonitenfauna des Nusplinger Plattenkalks (Ober-Kimmeridgium, Beckeri-Zone, Ulmense-Subzone, Schwäbische Alb). Stuttgarter Beiträge zur Naturkunde B 267: 1–61.
Schweigert, G. 2007. Ammonite biostratigraphy as a tool for dating Upper Jurassic lithographic limestones from South Germany—first results and open questions. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 245: 117–125. Crossref
Schweigert, G. 2015. Der Nusplinger Plattenkalk. In: G. Arratia, H.-P. Schultze, H. Tischlinger, and G. Viohl (eds.), Solnhofen: ein Fenster in die Jurazeit, 536–540. Dr. Friedrich Pfeil, Munich.
Schweigert, G., Böttcher, R., and Dietl, G. 2003. Erhaltung und Einbettung von Fischen im Nusplinger Plattenkalk (Oberjura, Schwäbische Alb). Terra Nostra 2003 (5): 153–154.
Stevens, K., Mutterlose, J., and Schweigert, G. 2014. The environment of the Nusplingen Plattenkalk (Upper Jurassic, southern Germany)—evidence from belemnite stable isotope data. Lethaia 47: 512–523. Crossref
Tyborowski, D. 2017. Large predatory actinopterygian fishes from the Late Jurassic of Poland studied with X-ray microtomography. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 283: 161–172. Crossref
Tyborowski, D. and Błażejowski, B. 2019. New marine reptile fossils from the Late Jurassic of Poland with implications for vertebrate faunas palaeobiogeography. Proceedings of the Geologists’ Association 130: 741–751. Crossref
Viohl, G. 1990. Piscivorous fishes of the Solnhofen Lithographic limestone. In: A.J. Boucot (ed.), Evolutionary Paleobiology of Behavior and Coevolution, 287–303. Elsevier, New York.
Wagner, A. 1863. Monographie der fossilen Fische aus den lithographischen Schiefern Bayern’s. Abhandlungen der königlich bayerischen Akademie der Wissenschaften II 9: 613–748.
Weitzel, K. 1930. Drei Riesenfische aus den Solnhofener Schiefern von Langenaltheim. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 42: 85–113.
Wenz, S. 1967. Compléments a l’étude des poissons actinoptérygiens du Jurassique français. Cahiers de Paléontologie 1967: 1–276.
White, E. 1925. Additions to the Upper Liassic fish-fauna of Holzmaden. Annals and Magazine of Natural History, Series 9 15: 601–611. Crossref
Witten, P.E. and Hall, B.K. 2015. Teleost skeletal plasticity: modulation, adaptation, and remodelling. Copeia 103: 727–739. Crossref
Woodward, A.S. 1894. On the affinities of the Cretaceous fish Protosphyraena. Annals and Magazine of Natural History, Series 6 13: 510–512. Crossref
Woodward, A.S. 1895. Catalogue of the Fossil Fishes in the British Museum (Natural History). Part III. 544 pp. British Museum (Natural History), London. Crossref
Woodward, A.S. 1908. On some remains of Pachycormus and Hypsocormus from the Jurassic of Normandy. Mémoires de la Société Linnéenne de Normandie 23: 29–34.
Wretman, L., Blom, H., and Kear, B.P. 2016. Resolution of the Early Jurassic actinopterygian fish Pachycormus and a dispersal hypothesis for Pachycormiformes. Journal of Vertebrate Paleontology 36: e1206022. Crossref
Acta Palaeontol. Pol. 65 (3): 429–453,
2020 https://doi.org/10.4202/app.00749.2020