

First insight into the diversity of snakes in the Pleistocene of Cuba
ELENA SYROMYATNIKOVA, ERNESTO ARANDA, and SORAIDA FIOL GONZÁLEZ
Syromyatnikova, E., Aranda, E., and Fiol González, S. 2021. First insight into the diversity of snakes in the Pleistocene of Cuba. Acta Palaeontologica Polonica 66 (2): 395–407.
The herpetofaunal biodiversity of West Indies suffered a significant change during the last few million years that is well documented for some squamate reptilies (lizards). However, almost nothing is known about past biodiversity of snakes, which are active predators and important component of terrestrial ecosystems. Here we describe the fossil remains of snakes (Reptilia: Serpentes) from the late Pleistocene of El Abrón Cave, Cuba. This is the first representative assemblage of fossil snakes from Cuba. It allows us to evaluate the taxonomic diversity of snakes in the Pleistocene of the island for the first time. The material includes eight taxa from the four snake families: cf. Cubatyphlops (Typhlopidae), Tropidophis melanurus, Tropidophis sp., Cubophis cf. cantherigerus, Arrhyton sp., cf. Caraiba andreae, Dipsadidae indet., and Natricidae indet. (Natricidae). Two (Dipsadidae indet. and Natricidae indet.) are not known in the modern fauna of Cuba. The assemblage from El Abrón Cave shows that ophidian Pleistocene assemblage was different from modern snake fauna of Cuba and was probably more diverse at genus level than it is now. Most of taxa revealed in El Abrón Cave were not previously known in the fossil record.
Key words: Reptilia, Serpentes, insular biodiversity, extinction, Pleistocene, Cuba.
Elena Syromyatnikova [esyromyatnikova@gmail.com], A.A. Borissiak Paleontological Institute, Russian Academy of Sciences, Profsoyuznaya str., 123, Moscow, 117647 Russia.
Ernesto Aranda [earanda@mnhnc.inf.cu] and Soraida Fiol González [sory@mnhnc.inf.cu], Museo Nacional de Historia Natural de Cuba, Obispo 61, Plaza de Armas, Habana Vieja, La Habana, Cuba.
Received 9 May 2021, accepted 13 October 2020, available online 2 June 2021.
Copyright © 2021 E. Syromyatnikova et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Cuba, the largest island in the Caribbean region, has a diverse living herpetofauna, which contains about 50 snake species grouped into five families: Typhlopidae, Boidae, Tropidophiidae, Dipsadidae, and Natricidae (Powell and Henderson 2012; Torres López et al. 2017). Almost all Cuba’s snakes (92%) are endemic. The most diverse are Tropidophiidae (about 16 species of Tropidophis, all endemic) and Typhlopidae (about 12 endemic species of Cubatyphlops). The Cuban boa (Chilabothrus angulifer (Bibron, 1840 in Ramón de la Sagra 1838–1843)) is the only boid species found in Cuba. This largest snake and largest land predator in the island plays a key role in the Cuban ecosystem. Dipsadidae in Cuba are represented by about 12 species from four genera of slender and typically fast-moving snakes. Natricidae are represented by the sole extant species and genus, Nerodia clarkii (Baird and Girard, 1853), inhabiting estuarine mangrove forests in northern coastal Cuba.
The origin and evolution of modern snake biodiversity can be only investigated with knowledge on its past biodiversity. Understanding past insular snake communities is critical for the quantification of various impacts on biota and setting conservation efforts for modern indigenous species. The Cuban Archipelago together with other West Indian islands support extremely fragile ecosystems which are subject to various and stressing agents, such as climate and sea level changes, ecological and human disturbances. During the Quaternary period large-scale extinctions in mammal and bird communities have occurred in Cuba (e.g., Morgan and Woods 1986; Suárez 2005; MacPhee et al. 2007; MacPhee 2009; Orihuela 2019; Orihuela et al. 2020), which led to changes in species composition and disappearance of taxa and/or populations. However, nearly nothing is known about the past Cuban biodiversity of snakes. Meanwhile, some newly published papers that focused on the fossil snakes of the Bahamas and the Lesser Antilles (Mead and Steadman 2017; Bochaton et al. 2019; Bochaton and Bailon 2018) showed the effect of different disturbances on snake biodiversity.
The primary source of information about past biodiversity are well-preserved fossils. In Cuba fossil snakes were only mentioned (without description and illustration) in the literature from late Pleistocene and Holocene caves and tar pits. To date, the only late Pleistocene snake fossil assemblage was reported from the Las Llanadas locality which provided three taxa: Chilabothrus sp., Cubophis sp., and Tropidophis sp. (Aranda et al. 2017), but those bone remains were not described. In addition, Chilabothrus angullifer and Cubophis cantherigerus (Bibron, 1840 in Ramón de la Sagra 1838–1843) were reported from the Holocene deposit of Cuevas Blancas (Jiménez et al. 2005). Other Cuban fossil snakes occurrences are almost exclusively records of Chilabothrus angulifer (as Epicrates angulifer (Bibron, 1840 in Ramón de la Sagra 1838–1843 or cf. E. angulifer) reported from: Cueva 2 (Koopman and Ruibal 1955), Solapa de Silex (Crespo Díaz and Jiménez Vázquez 2004), Solapa del Megalocnus (Arredondo and Villavicencio 2004), Cueva del Indio (Brattstrom 1958; Rojas-Consuegra et al. 2012), Luis Lago and Cueva del Rancho (Brattstrom 1958), and other localities (Varona and Arredondo 1979). The only archaeological occurrences of snake remains correspond to the mentions of Chilabothrus angulifer from Solapa de Silex and Solapa del Megalocnus. Boid remains were reported from Las Breas De San Felipe (as “Squamata Fam. Boidae”; Iturralde-Vinent et al. 2000) and from Cueva del Mono Fósil and Cueva Alta (as Boidae; Salgado et al. 1992), but it is possible that these records may also belong to Chilabothrus angulifer. In addition “fragmentary remains of snakes” were mentioned from the Ciego Montero locality (Matthew 1919). Obviously, the fossil snake record of Cuba is still mostly undescribed and nearly entirely restricted to the remains of a Cuban boa, Chilabothrus angulifer. This strong representation of Chilabothrus may be due to a recovery bias as the Cuban Boa is the largest snake in the West Indies (>400 cm in snout-vent length; Tolson and Henderson 1993). Its skeletal remains are thus easily identified and distinguished from other typically much smaller squamate fossils. The skeletal elements of other snakes (e.g., dwarf boas, Tropidophiidae), which are noticeably smaller in size, are often remain unrecognised and possibly not recovered when large mesh sizes are used with smaller bones simply gone with sediment matrix. This incomplete and occasional fossil record of Cuban snakes precludes a study of past snake diversity and its evolution on the island.
Here we describe the fossil snake assemblage from El Abrón Cave. It includes eight taxa, two of which are unknown in the modern fauna of Cuba. This paper continues a series of publications on the fossil biota from the Pleistocene of El Abrón Cave (Syromyatnikova et al. 2020; Zelenkov and Fiol-Gonzalez 2020).
Institutional abbreviations.—MNHNCu, Museo Nacional de Historia Natural de Cuba, Havana, Cuba; PIN (PIN H), Paleontological Institute of the Russian Academy of Sciences, Moscow, Russia.
Other abbreviations.—CL/NAW, centrum length/width of interzygapophyseal constriction; SVL, snout-vent length.
Material and methods
The remains described below come from El Abrón Cave, Sierra de la Güira, in the Pinar del Río Province of Cuba (Fig. 1). Data on the geology of El Abrón Cave along with some of the mammal, bird, and squamate remains from this locality have been partly published (Suárez and Díaz-Franco 2003; Suárez 2004a, b; Díaz-Franco 2001, 2002; Orihuela 2019; Syromyatnikova et al. 2020; Zelenkov and Fiol-Gonzalez 2020). The snake remains from El Abrón were not revealed and published before. New excavations were carried out in 2019 by members of the Department of Paleogeography and Paleobiology of the Museo Nacional de Historia Natural de Cuba (Havana, Cuba) and the Paleontological Institute of the Russian Academy of Sciences (Moscow, Russia) and produced the snake remains. The excavations were carried out in the area adjoining to the older section and explored sediments of Pleistocene age (reaching the depth of 2.85 m). Nine layers (numbered from top to bottom) of different thicknesses were recognised. The stratigraphy is identical with the section studied and published before. Only layer VII (0.80–1.72 m) has a radiocarbon date, of 17 406 ± 161 14C BP (20 050–21 474 cal BP), obtained using the bone material of an extinct owl (Tyto noeli) (Suárez and Díaz-Franco 2003). El Abrón Cave is richly fossiliferous, and all layers revealed abundant fossil bone material of exceptional preservation. Most of the snake remains described here come from layers V–VII. The layers V–VI, are presumably of the late Pleistocene age based on roughly homogeneous communities of small mammals. Other layers (III and IV) contain some snake remains, but they are too scarce and poorly preserved for any identification. The age of more upper layers (I and II) is uncertain. The fossil snake assemblage from El Abrón Cave is represented only by vertebrae, and no skull bones were recovered. In this study, we only describe precloacal vertebrae, which are the most suitable for diagnostic purposes and for investigating of taxonomic diversity.
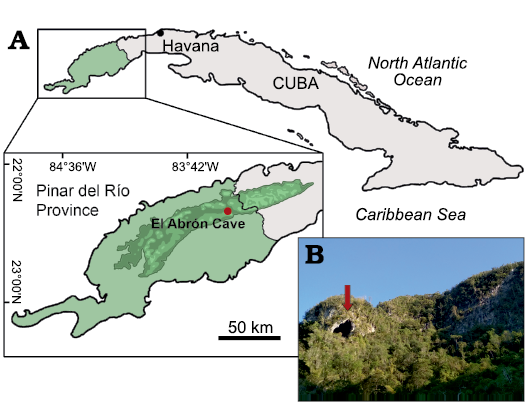
Fig. 1. A. Geographic location of the studied area. B. The locality El Abrón Cave, Cuba (arrowed).
The studied fossil materials are curated in the paleoherpetological collection of the Paleontological Institute of the Russian Academy of Sciences (Russia) under collection no. 5782. All fossil specimens are catalogued separately under their single unique catalogue number (e.g., 5782/1). The first number (5782) refers to the number of locality and the second number stands for the number of specimen. The specimens were photographed using the scanning electron microscope (Tescan Vega-II XMU) in the Paleontological Institute of the Russian Academy of Sciences in Moscow (Russia). The osteological terminology follows Szyndlar (1991a, b). Modern skeletons of Tropidophis melanurus Schlegel, 1837, Arrhyton taeniatum Günther, 1858, Caraiba andreae (Reinhardt and Lütken, 1862), Cubophis cantherigerus, and Nerodia clarkii (Baird and Girard, 1853) from the comparative osteological collection of the Paleontological Institute (PIN H) were used for comparative purpose along with literature data from Auffenberg (1963), Szyndlar (1991a, b), and Holman (1979, 1981, 2000). Colubridae, Dipsadidae and Natricidae are considered here as separate families according to Vidal et al. (2007). The Neotropic representatives of Colubridae were separated into a family Dipsadidae (former Xenodontinae) based on molecular data (Vidal et al. 2007). Because osteological identification criteria were not provided for Dipsadidae, here we apply the characteristics of Colubridae (the trunk vertebrae without hypapophysis; sensu Holman 1981) to sort Dipsadidae from other Cuba snake families out. Only North American and Central American “xenodontines” (now included in Dipsadidae) were partly characterized in vertebral morphology (Holman 1979; Whistler and Wright 1989).
Systematic palaeontology
Order Squamata Oppel, 1811
Suborder Serpentes Linnaeus, 1758
Family Typhlopidae Gray, 1825
Genus Cubatyphlops Hedges, Marion, Lipp, Marin and Vidal, 2014
Type species: Typhlops biminiensis Richmond, 1955; Recent, Bahamas.
cf. Cubatyphlops
Fig. 2A.
Material.—Four precloacal vertebrae (PIN 5782/1–4), V and VII layers, late Pleistocene, El Abrón Cave, Cuba.
Description.—All vertebrae are nearly complete. They are small and dorsoventrally compressed, their centrum length ranging 1.3–2 mm (Fig. 2A; PIN 5782/1). In dorsal view, the interzygapophyseal constriction is moderately deep (Fig. 2A1). The zygosphene is widely concave anteriorly. Posteriorly, the neural arch is widely notched. The prezygapophyseal facets are oval, oriented anteriorly, but the prezygapophyseal process is long and directed almost laterally. In ventral view, the centrum lacks a haemal keel (Fig. 2A2). Subcentral foramina are present in variable size. In some specimens only one subcentral foramen is present. In lateral view, the neural arch is depressed and devoid of a neural spine (Fig. 2A3). The synapophyses have a spherical shape and are undivided. The lateral foramina are present. In anterior view, the neural canal is relatively large, having almost the same height as the cotyle (Fig. 2A4). The zygosphene is slightly convex dorsally in anterior view. The cotyle is depressed dorsoventrally. The prezygapophyses are slightly tilted upward. No paracotylar foramina are present. In posterior view, the neural canal is large and high (wider and higher than the condyle) (Fig. 2A5). The neural arch is depressed posteriorly and nearly horizontal dorsally. The condyle is depressed dorsoventrally.
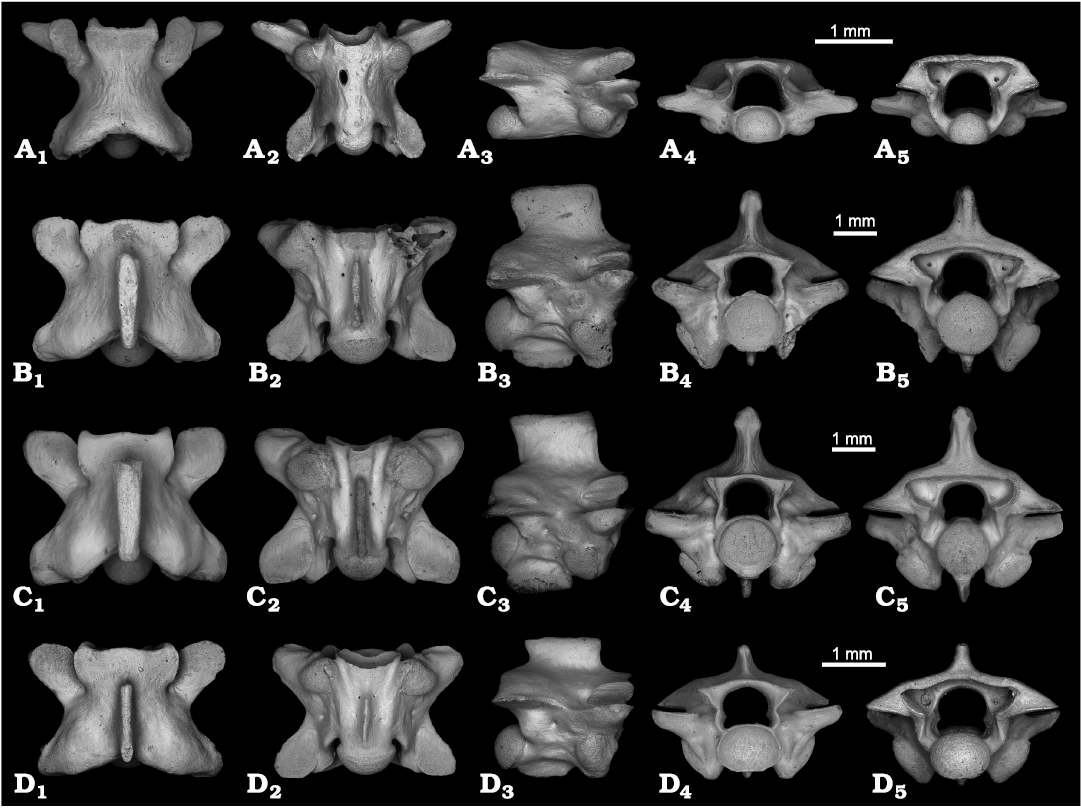
Fig. 2. Precloacal vertebrae of Typhlopidae (A) and Tropidophiidae (B–D) from Cuba, Recent (C) and the Late Pleistocene of El Abrón Cave (A, B, D). A. cf. Cubatyphlops, PIN 5782/1, in dorsal (A1), ventral (A2), lateral (A3), anterior (A4), and posterior (A5) views. B. Tropidophis melanurus (Schlegel, 1837), PIN 5782/5, in dorsal (B1), ventral (B2), lateral (B3), anterior (B4), and posterior (B5) views. C. Tropidophis melanurus Schlegel, 1837, PIN H 103, precloacal vertebra, in dorsal (C1), ventral (C2), lateral (C3), anterior (C4), and posterior (C5) views. D. Tropidophis sp., PIN 5782/12, in dorsal (D1), ventral (D2), lateral (D3), anterior (D4), and posterior (D5) views.
Remarks.—All vertebrae display the characteristic morphological features of scolecophidian snakes: depressed neural arch devoid of neural spine, undivided synapophyses, lacking haemal keel, cotyle and condyle dorso-ventrally depressed (Szyndlar 1991a). Most Cuban blindsnakes are assigned to the genus Cubatyphlops, which includes 12 species (Hedges et al. 2014); consequently, we provisionally refer scolecophidians from El Abrón Cave to that genus. We realize that our fossils may actually represent other scolecophidian taxa and we hope in future excavations to use a finer-mesh screen to recover diagnostic cranial elements that may prove useful for more refined identifications.
Tropidophiidae Brongersma, 1951
Genus Tropidophis Bibron, 1840 in Ramón de la Sagra 1838–1843
Type species: Boa melanura Schlegel, 1837; Recent and Pleistocene of West Indies and N South America, île de Cuba [Greater Antilles].
Tropidophis melanurus (Schlegel, 1837)
Fig. 2B.
Material.—Seven precloacal vertebrae (PIN 5782/5–11), VI and VII layers, late Pleistocene, El Abrón Cave, Cuba.
Description.—The vertebrae are massively built, short, and wide (Fig. 2B; PIN 5782/5). The centrum length ranges 2.5–3.1 mm with a CL/NAW ratio of about 1. In dorsal view, the interzygapophyseal constriction is poorly developed (Fig. 2B1). The zygosphene is three-lobed with a wide central lobe. The prezygapophyseal facets are large and nearly subsquare in shape. The neural spine is swollen and flattened at the apex. The prezygapophyseal processes are short and almost invisible in dorsal view. In ventral view, the hypapophysis is long, and extends for most of the centrum length (Fig. 2B2). The subcentral foramina are small but distinct, located at the bottom of deep subcentral grooves. The subcentral ridges are well developed and almost parallel. In lateral view, the neural spine is high, but its length exceeds its height, and it presents a straight dorsal margin (Fig. 2B3). The hypapophysis is squarish in shape and markedly projects ventrally. The synapophyses are undivided, robust, and rounded. In anterior view, the zygosphene is dorsally convex (Fig. 2B4). The prezygapophyseal facets are clearly oblique. The cotyle is large and circular-shaped. The paracotylar foramina are present and positioned at the bottom of deep paracotylar grooves. In posterior view, the neural arch is depressed (Fig. 2B5). As with the cotyle, the condyle is large and circular-shaped.
Remarks.—The fossils are assigned to Tropidophis based on having vertebrae which are short and wide, with a relatively tall neural spine, depressed neural arch, reduced prezygapophyseal processes, and squarish hypapophysis (Holman 2000). They agree in size and morphology with vertebrae of Recent Tropidophis melanurus (Fig. 2C; PIN H 103), a Cuban giant dwarf boa, and assigned here to this species. Tropidophis melanurus is the only large dwarf boa (SVL ranging 800–1000 mm), whereas other species of Tropidophis are relatively small in size, with SVL ranging 300–500 mm (Powell and Henderson 2012).
Stratigraphic and geographic range.—Late Pleistocene until Recent of Cuba; Cuba.
Tropidophis sp.
Fig. 2D.
Material.—11 precloacal vertebrae (PIN 5782/12–22), VI and VII layers, late Pleistocene, El Abrón Cave, Cuba.
Description.—The vertebrae are small, short and wide (Fig. 2D; PIN 5782/12). The centrum length does not exceed 2 mm. The ratio CL/NAW is 0.9. Their morphology is generally close to that described above for Tropidophis melanurus, however the following characters are different. The neural spine is well developed, but relatively low, about two times longer than high. It is somewhat overhanging anteriorly and posteriorly. At the apex, the neural spine is not distinctly swollen in dorsal view (Fig. 2D1). The prezygapophyseal facets are only slightly oblique in anterior view (Fig. 2D4). The cotyle and condyle are flattened dorsoventrally.
Remarks.—The vertebrae PIN 5782/12–22 are assigned to Tropidophis based on the same characters as listed for Tropidophis melanurus: short and wide vertebrae, depressed neural arch, reduced prezygapophyseal processes, and squarish hypapophysis (Holman 2000). The described vertebrae differ from the Tropidophis melanurus described above in the smaller size and morphology of the neural spine, cotyle and condyle. The above-listed differences do not apparently relate to age or intra-columnar variability, and thus we suggest that Tropidophis sp. belongs to a separate small-sized species of Tropidophis. The vertebrae are morphologically more or less uniform, but some variations (proportions and shape of neural arch) were observed. We interpret that variation to reflect variation along the vertebral column rather than taxonomic differences. Because the osteological peculiarities of species of Tropidophis remain unknown in the literature, we tentatively consider all small-sized vertebrae of Tropidophis from El Abrón Cave as a single small form of Tropidophis sp.
Dipsadidae Bonaparte, 1838
Genus Cubophis Hedges and Vidal in Hedges et al., 2009
Type species: Coluber cantherigerus Bibron, 1840 in Ramón de la Sagra 1838–1843; Recent and Pleistocene of Cuba, Bahamas and Cayman Islands, Cuba.
Cubophis cf. cantherigerus (Bibron, 1840 in Ramón de la Sagra 1838–1843)
Fig. 3A, B.
Material.—16 precloacal vertebrae (PIN 5782/66–81), V–VII layers, late Pleistocene, El Abrón Cave, Cuba.
Description.—The precloacal vertebrae have a centrum length ranging 3.8–4.7 mm (Fig. 3A, B; PIN 5782/66 and 5782/67). The vertebrae are longer than wide with a CL/NAW ratio of 1.4. In dorsal view, the interzygapophyseal constriction is more (Fig. 3A1) or less (Fig. 3B1) developed, sometimes shifted posteriorly. The neural spine is thin along its dorsal margin. The zygosphene is three-lobed with the central lobe poorly developed or even reduced in some large-sized specimens. In the largest specimen (Fig. 3B1) the zygosphene is apparently narrower than in smaller specimens (Fig. 3A1). The prezygapophyseal facets are usually elongate in outline, but can be nearly circular (Fig. 3A1). The prezygapophyseal processes are well developed and oriented more laterally than anteriorly. They vary in length, reaching more or less than half the length of the prezygapophyseal facets. In ventral view, the subcentral area is wide, and in some specimens (Fig. 3B2) noticeably widened anteriorly. The haemal keel is narrow anteriorly and widened posteriorly. The subcentral grooves are wide, shallow, and confined mostly to the anterior part of the centrum. The subcentral ridges are well developed. The subcentral foramina are small and located close to the haemal keel. In lateral view, the neural spine is relatively low, about twice as long as it is high. Its dorsal margin is nearly straight or slightly inclined posteriorly. The anterior margin of the neural spine is nearly vertical, whereas the posterior margin overhangs the neural arch. The haemal keel is well visible laterally. Its ventral margin can be straight (Fig. 3A3) or slightly convex (Fig. 3B3) ventrally. In the anterior part of the subcentral area, the haemal keel is low and becomes taller in its middle and posterior parts. The synapophyses have distinct parapophyseal and diapophyseal portions of similar size. The lateral foramina are present. In anterior view, the zygosphene is convex and narrow (only slightly wider than the cotyle). The cotyle is more or less dorso-ventrally flattened. The neural canal is tunnel-like and moderately large, but its dorsoventral height is lower that the dorsoventral height of the cotyle. The prezygapophyseal facets are approximately horizontal (Fig. 3B4) or only slightly inclined (Fig. 3A4). Paracotylar foramina are present. In posterior view, the neural arch is distinctly vaulted. The condyle is spherical.
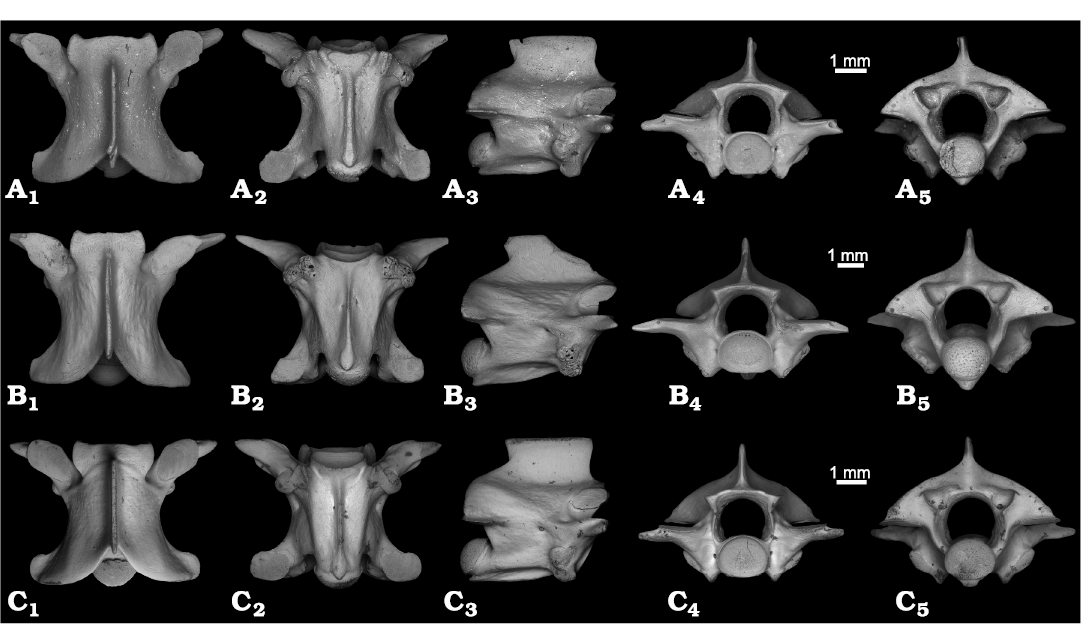
Fig. 3. Precloacal vertebrae of Cubophis spp. from Cuba, Recent (C) and the Late Pleistocene, El Abrón Cave (A, B). A. Cubophis cf. cantherigerus, PIN 5782/66, in dorsal (A1), ventral (A2), lateral (A3), anterior (A4), and posterior (A5) views. B. Cubophis cf. cantherigerus, PIN 5782/67, in dorsal (B1), ventral (B2), lateral (B3), anterior (B4), and posterior (B5) views. C. Cubophis cantherigerus (Bibron, 1840 in Ramón de la Sagra 1838–1843), PIN H 104, in dorsal (C1), ventral (C2), lateral (C3), anterior (C4), and posterior (C5) views.
Remarks.—The described vertebrae are assigned to Dipsadidae based on elongated centrum, presence of haemal keel instead of hypapophysis, synapophyses clearly divided into diapophyses and parapophyses (Holman 1979, 1981, 2000). Most West Indian snakes of the family Dipsadidae belong to the subfamily Xenodontinae and tribe Alsophiini. In Cuba, there are small (Arrhyton), moderate-sized (Caraiba), and large (Cubophis) species of alsophiines (Hedges et al. 2009). The vertebrae from El Abrón Cave are relatively large and identical in morphology and size to Recent Cubophis cantherigerus (Fig. 3C; PIN H 104). The vertebrae described are also in greatest correspondence with those of Cubophis described and figured by Mead and Steadman (2017) in having a CL/NAW ratio within the range of 1.20–1.40, a neural spine which is twice as long as high, having a slight overhang, and a horizontally positioned prezygapophyseal facet. The differences in the length of the prezygapophyseal processes, size of zygosphene, and wideness of subcentral area in the single largest specimen PIN 5782/67 (Fig. 3B) may be due to developmental or individual variation, however, it is not possible to completely exclude that the largest specimen belongs to another taxon. The species of Cubophis are medium to large-sized snakes. Оnly Cubophis cantherigerus is known in Cuba (Powell and Henderson 2012), and is nearly ubiquitous on the island (Rodríguez-Schettino et al. 2013). For this reason, as well as the morphological similarity, we provisionally assign the described vertebrae to Cubophis cf. cantherigerus.
Genus Arrhyton Günther, 1858
Type species: Arrhyton taeniatum Günther, 1858; Recent of Cuba.
Arrhyton sp.
Fig. 4A.
Material.—13 precloacal vertebrae (PIN 5782/23–35), V and VII layers, late Pleistocene, El Abrón Cave, Cuba.
Description.—The vertebrae are small, with a centrum length ranging 1.6–2.2 mm (Fig. 4A; PIN 5782/23). They are slightly longer than wide (ratio CL/NAW is 1.1). In dorsal view, the interzygapophyseal constriction is moderately developed and anteroposteriorly short, positioned close to the prezygapophyseal facets (Fig. 4A1). The neural spine is thickened along its dorsal edge, sometimes with an indistinct bifurcation at its anterior tip. Posteriorly, the thickening of the neural spine is slightly decreased, and the posterior tip of the spine is pointed. The zygosphene is clearly three-lobed in dorsal view. Posteriorly, the neural arch bears a wide triangular notch. The prezygapophyseal facets are elongate in outline. The prezygapophyseal processes are relatively small, rounded at the tips, and anterolaterally oriented. They do not extend beyond the level of the prezygapophyseal facets in dorsal view. In some specimens the prezygapophyseal processes tend to be the conical shaped. In ventral view, the haemal keel is spatulate shaped, and can be more or less widened (Fig. 4A2). The subcentral area is relatively narrow and slightly concave ventrally. The subcentral ridges are poorly developed. The subcentral foramina are present, but small. In lateral view, the neural spine is longer than high (Fig. 4A3). Its anterior end is somewhat sloping and slightly overhangs anteriorly, whereas the posterior end is acute and has a stronger overhang posteriorly. The haemal keel is clearly visible in lateral view, and its ventral margin is straight. The synapophyses are massive, clearly separated into diapophyses and parapophyses, the latter located more posteriorly. The lateral foramina are relatively large, about twice the size of the subcentral foramina. In anterior view, the neural canal is large and wide, being higher and wider than the cotyle (Fig. 4A4). The zygosphene is distinctly convex dorsally with the shape of an inverted “V”. The cotyle is deep and slightly flattened dorsoventrally. The prezygapophyseal facets are approximately horizontal. The paracotylar foramina are present. In some specimens a pair of relatively large foramina are positioned above the paracotylar foramina. In posterior view, the neural arch is vaulted, with a triangular shape (Fig. 4A5). The neural canal is large and wide, as in anterior view, and is higher and wider than the condyle. At the bottom the neural canal is markedly widened, looking tetragonal in outline. The condyle is circular in shape.
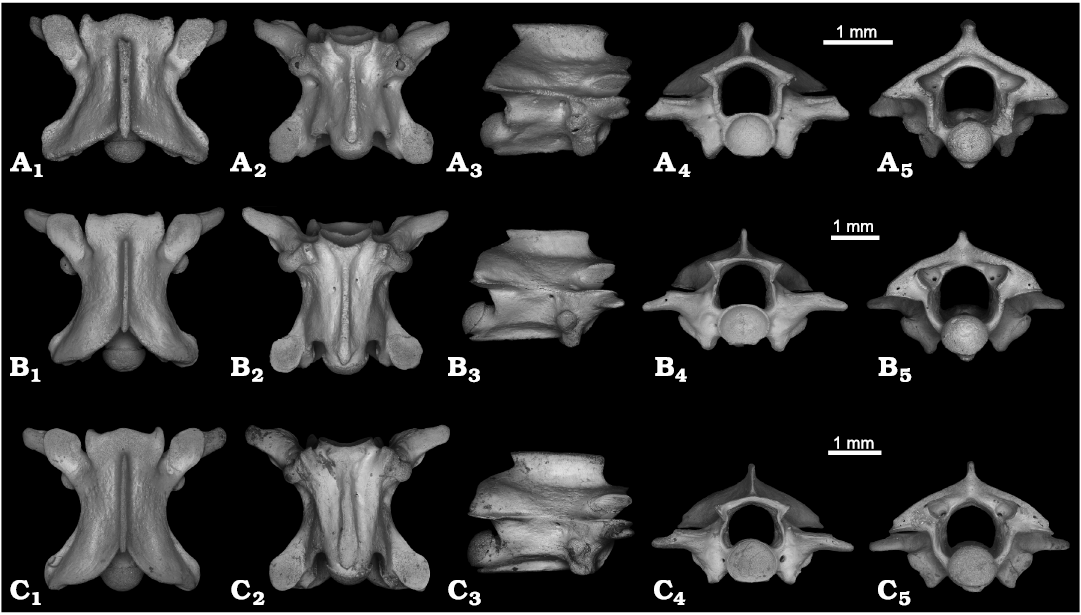
Fig. 4. Precloacal vertebrae of Dipsadidae from Cuba, Recent (C) and the Late Pleistocene, El Abrón Cave (A, B). A. Arrhyton sp., PIN 5782/23, in dorsal (A1), ventral (A2), lateral (A3), anterior (A4), and posterior (A5) views. B. cf. Caraiba andreae, PIN 5782/36, in dorsal (B1), ventral (B2), lateral (B3), anterior (B4), and posterior (B5) views. C. Caraiba andreae (Reinhardt and Lütken, 1862), PIN H 105, in dorsal (C1), ventral (C2), lateral (C3), anterior (C4), and posterior (C5) views.
Remarks.—The described vertebrae can be assigned to Dipsadidae based on relatively elongated centrum, presence of haemal keel instead of hypapophysis, and synapophyses clearly divided into diapophyses and parapophyses (Holman 1979, 1981, 2000). The vertebrae described from El Abrón Cave are relatively small and correspond in size to species of Arrhyton, showing SVL <450 mm. It is also similar to species of Arrhyton in general proportions, and the shape of the prezygapophyseal processes and zygosphene (EVS, personal observation based on the Recent species Arrhyton taeniatum, PIN H 101). Arrhyton has eight species which are endemic to Cuba (Powell and Henderson 2012). According to Rodríguez-Schettino et al. (2013), only two species (A. taeniatum and A. vittatum) are widely distributed in Cuba, including the Pinar del Río Province. The species of Arrhyton from El Abrón Cave differs from Arrhyton vittatum in having a higher neural spine and moderately vaulted triangular neural arch (EVS, personal observation based on the specimen Arrhyton vittatum (Gundlach in Peters, 1861), PIN H 102). Comparison with other species of Arrhyton from El Abrón Cave was not possible given the limited available sample of Recent specimens. Because no osteological characteristics are provided for Arrhyton, we assign the described specimens to Arrhyton sp.
Genus Caraiba Zaher, Grazziotin, Cadle, Murphy, Moura-Leite, and Boanatto, 2009
Type species: Liophis andreae Reinhardt and Lütken, 1862; Recent of Cuba, Havanna, Cuba.
cf. Caraiba andreae (Reinhardt and Lütken, 1862)
Fig. 4B.
Material.—30 precloacal vertebrae (PIN 5782/36–65), VI and VII layers, late Pleistocene, El Abrón Cave, Cuba.
Description.—The vertebrae are relatively small, with a centrum length ranging 1.8–2.5 mm (Fig. 4B; PIN 5782/36). They are elongated (longer than wide, ratio CL/NAW is 1.5) and lightly built. In dorsal view, the interzygapophyseal constriction is well developed (Fig. 4B1). The neural spine is thin along its dorsal margin. The zygosphene is three-lobed, with lobes which are relatively poorly developed. The prezygapophyseal facets are elongate in outline. The prezygapophyseal processes are well developed and anterolaterally oriented with rounded tips, their length reaches more than half the length of the prezygapophyseal facets. In ventral view, the haemal keel is prominent and relatively thin, only slightly widened posteriorly (Fig. 4B2). It is accompanied by shallow subcentral grooves. The subcentral ridges are low. Two small subcentral foramina are present on each side of the haemal keel. In lateral view, the neural spine is low, with a straight dorsal margin (Fig. 4B3). Its anterior end only slightly overhangs anteriorly, but the posterior end clearly overhangs posteriorly. The haemal keel is clearly visible, straight ventrally and pointed posteriorly. The synapophyses have distinct parapophyseal and diapophyseal portions of nearly similar size. The lateral foramina are clearly visible. In anterior view, the zygosphene is somewhat convex dorsally (Fig. 4B4). The neural canal is relatively large and nearly the same height as the height of the cotyle. It is wider at the bottom than at the top. The prezygapophyseal facets are nearly horizontal, whereas the prezygapophyseal processes are often directed slightly ventrally. The cotyle is dorsoventrally compressed. The paracotylar foramina are present. In posterior view, the neural canal is relatively large and high, and the neural arch is vaulted (Fig. 4B5). The condyle is more rounded than the cotyle.
Remarks.—The described vertebrae are assigned to Dipsadidae based on elongated centrum, presence of haemal keel instead of hypapophysis, and clearly divided synapophyses (Holman 1979, 1981, 2000). The vertebrae can be distinguished from those assigned to Arrhyton (see above) based on having larger size, elongated proportions, long prezygapophyseal processes, low and thin neural spine, poorly convex zygosphene, and more vaulted posteriorly neural arch. They are identical in morphology and size to Recent Caraiba andreae (Fig. 4C; PIN H 105). They differ from the large Cubophis by having a low neural spine (see Mead and Steadman 2017). Caraiba is a moderately-sized racer of Cuba. Only C. andreae is known (Powell and Henderson 2012), and it is distributed widely across the territory of the island (Rodríguez-Schettino et al. 2013). It is highly probable that the described fossils belong to this snake.
Dipsadidae indet.
Fig. 5A.
Material.—One precloacal vertebra (PIN 5782/82), VII layer, late Pleistocene, El Abrón Cave, Cuba.
Description.—The vertebra PIN 5782/82 (Fig. 5A) is relatively small and elongated, but strongly built, with a centrum length of 3.1 mm and CL/NAW ratio of 1.5. In dorsal view, the interzygapophyseal constriction is clear and anteroposteriorly long (Fig. 5A1). The zygosphene is narrow and bears a crenate anterior margin with two small lateral lobes, and wide, but poorly protruding anteriorly median lobe. The neural spine is markedly thickened along the dorsal margin. Its posterior end is rounded, and although the anterior one is slightly damaged, it can be reconstructed as bifurcated. The prezygapophyseal facets are oval in outline. The prezygapophyseal processes (only the left one is preserved) are massive, greatly expanded dorsoventrally and obtuse distally. Its length is less than half that of the facets. The prezygapophyseal processes are directed anterolaterally, extend beyond the level of facets. In ventral view, the subcentral area is moderately wide (Fig. 5A2). It bears a haemal keel which is flattened ventrally, spatulate-shaped, and extremely widened posteriorly where it is almost as wide as the condyle. Two wide, paired tubercles are present below the cotylar rim. The subcentral grooves and subcentral ridges are poorly developed. The subcentral foramina are small and positioned a distance from a haemal keel. In lateral view, the neural spine is low with a slightly rounded dorsal margin (Fig. 5A3). The anterior and posterior margins of the neural spine overhangs the cotyle and condyle. The haemal keel is poorly protruded ventrally, and even looks concave in its ventral margin. The synapophysis on the left side is elongated and has distinct parapophyseal and diapophyseal portions, whereas the synapophysis on the right side is greatly enlarged and robust, its parapophyseal and diapophyseal portions are fused (probably due to pathology). The lateral foramina are small. In anterior view, the zygosphene is convex. Its dorsolateral parts are thickened and bend dorsally (Fig. 5A4, marked by arrow). The neural canal is tunnel-like, and its dorsoventral height is nearly the same as the dorsoventral height of the cotyle. The prezygapophyseal facets are approximately horizontal. The cotyle is flattened. Two tubercles are visible below the cotylar rim. The paracotylar foramina are minute. In posterior view, the neural arch is moderately vaulted (Fig. 5A5). The neural canal is the same shape as it is in anterior view. The condyle is slightly flattened.
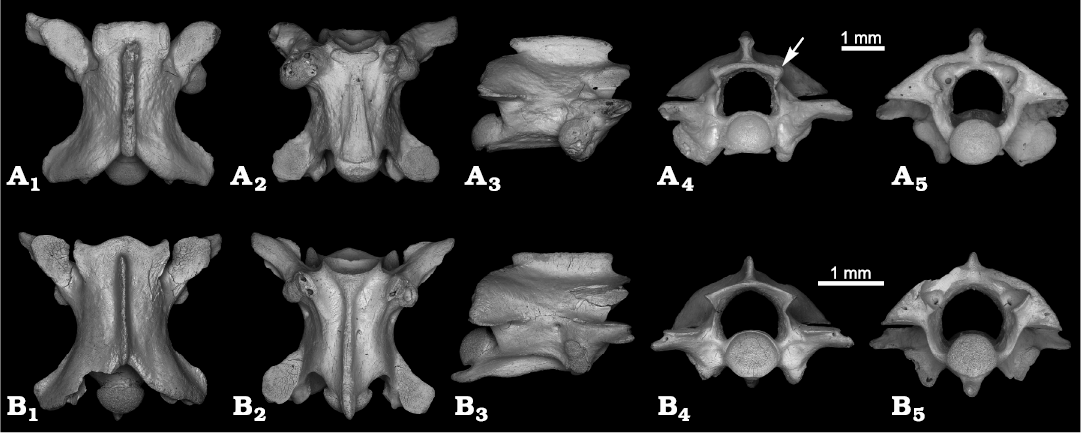
Fig. 5. Precloacal vertebrae of Dipsadidae indet. and Natricidae indet. from the Late Pleistocene, El Abrón Cave, Cuba. A. Dipsadidae indet., PIN 5782/82, in dorsal (A1), ventral (A2), lateral (A3), anterior (A4), and posterior (A5) views. Arrow marked the thickened and bend dorsally dorsolateral parts of the zygosphene. B. Natricidae indet., PIN 5782/83, in dorsal (B1), ventral (B2), lateral (B3), anterior (B4), and posterior (B5) views.
Remarks.—PIN 5782/82 is elongated and has a haemal keel and divided synapophysis, and thus, it can be assigned to Dipsadidae. It is similar to the group of North American “xenodontines” in having a wide haemal keel (sensu Holman 1979). The combination of its other characters (extremely widened posteriorly haemal keel, dorsoventrally expanded prezygapophyseal processes, rounded and widened dorsally neural spine overhanging anteriorly and posteriorly) was not observed in other taxa described above and in other known Cuban snakes. The thickened and dorsally bent dorsolateral part of the zygosphene was not mentioned in other known extinct and extant colubrids. The shape of haemal keel and low neural spine of PIN 5782/82 are somewhat similar to Carphophis Gervais in d’Orbigny, 1843 (see Holman 2000), however, in other features (general proportions, shape of zygosphene, thickened neural spine, etc.) PIN 5782/82 differs from that snake. In some characters (massive, dorsoventrally expanded and obtuse distally prezygapophyseal processes and rounded and dorsally widened neural spine overhanging anteriorly and posteriorly) PIN 5782/82 is similar to some Lampropeltis spp. (Holman 2000: 170; Jim Mead, personal communication 2020). The broad and flattened haemal keel may also suggest that PIN 5782/82 belongs to the posterior precloacal portion of the vertebral column. As this peculiar vertebral morphology is observed only in the single specimen, its systematic allocation cannot be fully demonstrated. Pending the discovery of additional material and a better comprehension of the vertebral morphology of Dipsadidae, the described specimen is herein tentatively assigned to Dipsadidae indet.
Natricidae Boettger, 1883
Natricidae indet.
Fig. 5B.
Material.—One precloacal vertebra (PIN 5782/83), VI layer, late Pleistocene, El Abrón Cave, Cuba.
Description.—The vertebra PIN 5782/83 (Fig. 5B) is almost complete and has a slightly damaged posterior margin on the neural arch and synapophyses. It is small and elongated, with a centrum length of 2.2 mm and ratio CL/NAW of 1.7. In dorsal view, the interzygapophyseal constriction is well developed (Fig. 5B1). The zygosphene bears a protruding median lobe, delimited laterally with two smaller but distinct lobes. The neural spine is long, reaching the roof of the zygosphene, but it does not approach its anterior border. Its dorsal margin is thin. The prezygapophyseal facets are roundish and the prezygapophyseal processes are short (about 1/3 of the length of the prezygapophyseal facets), anterolaterally directed and distally pointed. In ventral view, the centrum has a fully preserved hypapophysis (Fig. 5B2). It is laterally compressed, and homogeneous in width along its entire length. The subcentral grooves and subcentral ridges are shallow. The subcentral foramina are moderate in size. In lateral view, the neural spine is low, with a straight dorsal margin (Fig. 5B3). The anterior and posterior margins of the neural spine are only slightly inclined. The hypapophysis protrudes beyond the level of the condyle and is directed posteriorly rather than ventrally. Along the ventral border it is slightly sinusoidal. The tip of the hypapophysis is rather acute. Although damaged, the parapophysis and diapophysis have a nearly similar diameter. The lateral foramina are well developed. In anterior view, the zygosphene is wide (wider than cotyle) and arched dorsally (Fig. 5B4). The neural canal is nearly subcircular. Its dorsoventral height is less that the dorsoventral height of the cotyle. The prezygapophyseal facets are horizontally oriented. The paracotylar foramina are indistinct. In posterior view, the neural arch is moderately vaulted (Fig. 5B5). The neural canal is slightly higher than it is in anterior view. The condyle is similar to the cotyle in outline.
Remarks.—PIN 5782/83 is somewhat similar to anterior precloacal vertebrae (= cervicals) of Caraiba andreae in general proportions and presence of the hypapophysis. Within the anterior precloacal vertebrae of C. andreae, the most anterior ones have a ventrally directed hypapophysis, whereas the most posterior vertebrae have a posteriorly directed hypapophysis, similar to those observed in PIN 5782/83. However, PIN 5782/83 differs from all cervicals of Caraiba in low and long neural spine, distally pointed prezygapophyseal processes, and not anteriorly directed parapophyseal processes. PIN 5782/83 comes from the middle precloacal region rather than from anterior precloacal region. Based on the presence of a hypapophysis, PIN 5782/83 can be assigned to Natricidae, Viperidae, or Elapidae (Szyndlar 1991b). In Viperidae, however, the longer hypapophysis is directed more ventrally than posteriorly, the neural arch is typically more depressed, the parapophyseal processes distinctly longer, and vertebral centrum is shorter. PIN 5782/83 shares some traits of Elapidae, sharing the low neural spine and the presence of a non-sigmoid hypapophysis. In PIN 5782/83 the axis of the hypapophysis lies at an acute angle to the vertebral axis, which is typical for small-sized Elapidae such as the species of Micrurus Wagler in Spix, 1824 (see Auffenberg 1963; Rage and Holman 1984). As in Micrurus, PIN 5782/83 has small dimensions, an elongate centrum, neural spine that tends to be undercut both anteriorly and posteriorly, a slender and compressed hypapophysis projecting posteriorly beyond the tip of the condyle, and wide neural canal in comparison with the cotyle (e.g., Auffenberg 1963; Rage and Holman 1984; Szyndlar and Schleich 1993). The hypapophysis in PIN 5782/83 has an unusual shape because of its sinusoidal ventral border, whereas in elapids the hypapophysis is straight ventrally. The presence of Elapidae in Cuba would also be unexpected because there are no fossil or Recent elapids in the Greater Antilles.
PIN 5782/83 rather belongs to Natricidae, based on the posteriorly vaulted neural arch, short parapophyseal processes, and long centrum. Of the natricid snakes, only Nerodia clarkii is known in the present-day fauna of Cuba, but the vertebrae of N. clarkii clearly differ from PIN 5782/83 in having a shorter centrum, high neural spine, and more ventrally directed hypapophysis (Mead and Steadman 2017: fig. 4.1). PIN 5782/83 cannot be assigned to Neonatrix Holman, 1973, due to the long hypapophysis extending beyond the condyle (Holman 2000). In contrast to Nerodia and Neonatrix, the vertebrae of Thamnophis Fitzinger, 1843, are characterised by their small size, elongated centra, and low and long neural spine (Parmley 1988). The vertebrae of Thamnophis, however, usually bear a slightly higher neural spine (Auffenberg 1963; Holman 2000). According to Auffenberg (1963), PIN 5782/83 is similar to the snakes of Group I (i.e., vertebrae with a long centrum and a low, long neural spine) of the New World natricid snakes, which includes Storeria Baird and Girard, 1853, Tropidoclonion Cope, 1860, and Virginia Baird and Girard, 1853. The generic differentiation of these taxa is difficult because these small snakes are similar in vertebral morphology (Parmley and Hunter 2010). For this reason, and because only one specimen was found, we tentatively assign PIN 5782/83 to Natricidae indet.
Discussion
The fossil material of El Abrón Cave provides the first representative assemblage of fossil snakes in Cuba. We identified the following eight taxa from the four snake families in El Abrón Cave: cf. Cubatyphlops (Typhlopidae), Tropidophis melanurus, Tropidophis sp. (Tropidophiidae), Cubophis cf. cantherigerus, Arrhyton sp., cf. Caraiba andreae, Dipsadidae indet. (Dipsadidae), and Natricidae indet. (Natricidae). Most (except Cubophis cf. cantherigerus and Tropidophis melanurus) have not been found before in the fossil record of Cuba. Most of the revealed taxa are endemic to the West Indies, but two taxa (Dipsadidae indet. and Natricidae indet.) are unknown in the present-day snake fauna of Cuba.
Scolecophidia, a group of primitive, blind, burrowing snakes, are represented in assemblage of El Abrón Cave by only a few remains of cf. Cubatyphlops, but it is difficult to estimate the real abundance of these snakes in the Pleistocene fauna because they are diminutive in size and can rarely be collected in the fossil material. The Caribbean islands have one of the largest blindsnake faunas in the world (Hedges et al. 2014), but as fossils they are known only from the Pleistocene of the Bahamas (Pregill 1982; Steadman et al. 2015; Mead and Steadman 2017), Puerto Rico (Pregill 1981), the Guadeloupe Archipelago (Bochaton et al. 2015), the Cayman (Morgan 1994), Jamaica (Morgan 1993), and Barbuda (Auffenberg 1958) islands, as well as from the Holocene of Antigua (Pregill et al. 1988). In Cuba blindsnakes have a relatively sporadic distribution (Thomas and Hedges 2007). The remains of cf. Cubatyphlops were revealed in Pleistocene deposits of El Abrón Cave, but no blindsnakes are currently known in the vicinity of the locality (see Hedges 2018). This could be related to different ecological conditions of the research area in the Pleistocene.
Two species of Tropidophis (T. melanurus and Tropidophis sp.) were found in El Abrón Cave, which can be clearly differentiated, first of all, by their size. Given their other differences (morphology of the neural spine, cotyle and condyle), they do not seem to be associated with an ontogenetic age. The large vertebrae are morphologically similar to T. melanurus, a Cuban giant dwarf boa. That species is the largest among the Tropidophis spp. (with SVL up to 1 m), and is found throughout Cuba. Fossil Tropidophis is mentioned only from the Las Llanadas locality (Aranda et al. 2017; Martínez-López 2019), where two vertebrae of this snake have been recovered. Due to the relatively large size of the one figured vertebra (Aranda et al. 2017: fig. 1G), it most likely belongs to T. melanurus. The small vertebrae are morphologically more or less uniform and assigned here to Tropidophis sp., however, 17 species of Tropidophis inhabit present-day Cuba and most (except T. melanurus) are relatively small in size, with SVL ranging 300–500 mm (Powell and Henderson 2012). Thus, we cannot exclude the presence of several small species of Tropidophis in El Abrón Cave. No fossil records of small Tropidophis have previously been reported from Cuba. The species of this genus occur widely in South America and the West Indies, but Cuba is the centre of its diversity (Hedges 2002). The monophyly of the Cuban species of Tropidophis supports the impressive radiation of this genus on the island, rather than multiple colonisations by different members of the genus (Reynolds et al. 2014). The presence of at least two species of Tropidophis in El Abrón Cave is the first fossil evidence of the genus diversity in the Pleistocene.
Four of the eight recognised taxa in El Abrón Cave (Cubophis cf. cantherigerus, Arrhyton sp., cf. Caraiba andreae, and Dipsadidae indet.) belong to the family Dipsadidae. It is one of the largest families of snakes (~700 species), and distributed throughout the West Indies. About 12 species of Dipsadidae currently inhabit Cuba (Torres López et al. 2017). Almost all dipsadid genera currently known in Cuba are represented in the Pleistocene deposits of El Abrón Cave (except Tretanorhinus Duméril, Bibron and Duméril, 1854), but specific attribution is not possible due to the limited available sample of Recent dipsadids for osteological comparison. Cubophis was reported from the Las Llanadas and Cuevas Blancas localities (Jiménez et al. 2005; Aranda et al. 2017), but no species of Arrhyton and Caraiba have been found in the fossil record. Cubophis cantherigerus was reported from Quaternary cave deposits on the Cayman Islands (as Alsophis cantherigerus; Morgan 1994), but those remains were not described. Morgan (1993) mentioned Arrhyton from Jamaica, based on the literature, however there are no references to Arrhyton in the original papers. Arrhyton from Jamaica may belong to Hypsirhynchus Günther, 1858. Pregill (1981) described a fossil cf. Arrhyton exiguum from Puerto Rico, but that taxon is currently classified as Magliophis exiguus (Mead and Steadman 2017). Caraiba is a recently described monospecific genus based on Antillophis andreae (Zaher et al. 2009). This species was not previously reported in the fossil record. The described remains provide the first fossil evidence for the presence of Arrhyton and Caraiba in the late Pleistocene.
The vertebrae of Caraiba and Cubophis (both fossil and Recent) are similar and differ mostly in size, and in the height of the neural spine. At the same time, they both clearly differ from the vertebrae of Arrhyton, which are wider and have a dorsally thickened neural spine, small prezygapophyseal processes, and a neural arch in the shape of an inverted “V”. These morphological differences of taxa generally agree with the hypothesized phylogeny and divergence timing of Alsophiini (Hedges et al. 2009; Burbrink et al. 2011), by which Caraiba and Cubophis are positioned as sister groups which diverged at the end of the Miocene (about 6 Ma). The Arrhyton clade split from other alsophiines much earlier, in the late middle Miocene (about 11 Ma).
Two snake records (Dipsadidae indet. and Natricidae indet.) from the El Abrón Cave assemblage cannot be assigned to any known genus/species of the modern fauna of Cuba. These records represent an extinct or currently non-occurring genus on the island, but because each is represented by a single specimen, we refrain from naming them as new taxa. Moreover, Dipsadidae indet. has an asymmetry of the synapophyses. This pathological condition is not considered as taxonomically diagnostic, however it could potentially also impact the morphology of other structures. Nevertheless, some of other features of this specimen (see Remarks for Dipsadidae indet.) which most likely appeared independently were not observed in other taxa described in this paper and in other known Cuban snakes. Dipsadidae indet. and Natricidae indet. were apparently not widespread on the study area during the Pleistocene. The effect of the Pleistocene/Holocene transition has been documented for dipsadid snakes of the Guadeloupe Islands (Bailon et al. 2015; Boudadi-Maligne et al. 2015; Bochaton et al. 2019). In Cuba we currently cannot estimate the precise time of the extinction event for Dipsadidae indet. and Natricidae indet. between the Pleistocene and Recent. The record of Natricidae indet. could indicate the presence of more than one natricid snake in the Pleistocene of Cuba, however, presence of Nerodia clarkii in the Cuban Pleistocene is still not established. Barbour and Ramsden (1919) reported that N. clarkii “must be a recent arrival in Cuba” (Mead and Steadman 2017). Both records of Dipsadidae indet. and Natricidae indet. indicate that snake fauna of the Pleistocene was different from modern snake fauna of Cuba and was probably more diverse at genus level than it is now. This cannot be estimated at the species level until osteological diagnostic features can be identified for all modern species of Cuban snakes.
The snake assemblage of El Abrón Cave differs from the Pleistocene snakes of other known Cuban caves. A large boa Chilabothrus angulifer, nearly the only previously known fossil Cuban snake, is totally absent in the snake assemblage of El Abrón Cave. The absence of Chilabothrus angulifer in El Abrón Cave can be explained by food preferences and the size of the predators (owls), which generally contributed to the accumulation of the cave deposits (e.g., Pregill 1981; Pregill et al. 1994). The absence of remains of other large animals in the fossil vertebrate assemblage of El Abrón supports this hypothesis. The snake material shows the traces of digestion (e.g., Fig. 3B), that also confirms that the bone accumulation was produced mainly by avian predators. The remains of Chilabothrus angulifer may be common in other cave deposits of Cuba (see Introduction) due to the presence of larger predators, or because the habitat of this boa was near the entrance of caves in order to capture bats (Barbour and Ramsden 1919; Dinets 2017).
The majority of fossil snake material from El Abrón Cave is assigned to cf. Caraiba andraea. As does Cubophis cantherigerus, which is also relatively common in the Pleistocene deposits, it has a wide altitudinal and ecological distribution. The presence of the species of Tropidophis and Arrhyton suggest different types of habitats, with species of Tropidophis preferring wet habitats, whereas species of Arrhyton being more fossorial snakes, inhabiting open grassland. Such different ecological conditions may reflect the palaeoclimatic and palaeoenvironment differences during the period of the deposition of fossiliferous horizons, from which the material is described here (layers V–VII).
Conclusions
The first representative assemblage of fossil snakes from Cuba described here includes eight taxa from the four snake families. The identification of two taxa in the El Abrón Cave assemblage not currently known in Cuba gives a first insight into the probably more diverse snake assemblage of the Pleistocene than it is today. These data are an important addition to our understanding of the palaeobiogeography of West Indian ophidians and ecological impacts on insular biodiversity.
Acknowledgements
We thank fellow members of the expeditions to the El Abrón Cave for collecting specimens. We also thank Esther Pérez Lorenzo, Jesus Pajon, and Reinaldo Rojas Consuegra (all MNHNCu) who provided kind assistance during the work at MNHNCu. We very grateful to Jim Mead (The Mammoth Site, Hot Springs, USA) for his kind assistance during the work with the paper and valuable comments to the text. We thank Christopher Bell (The University of Texas at Austin, USA) and Corentin Bochaton (Muséum national d’Histoire naturelle, Paris, France) for their very constructive reviews of the manuscript, valuable comments and English correction. Roman Rakitov (PIN) is thanked for assistance with SEM microscopy. The reported study was funded by RFBR (Russian Foundation for Basic Research) and CITMA (Ministerio de Ciencia, Tecnología y Medio Ambiente) under the research project no. 18-54-34004.
References
Aranda, E., Martínez-López, J.G., Jiménez, O., Alemán C., and Viñola, L.W. 2017. Nuevos registros fósiles de vertebrados terrestres para Las Llanadas, Sancti Spíritus, Cuba. Novitates Caribaea 11: 115–123. Crossref
Arredondo, C.A. and Villavicencio, R. 2004. Tafonomía del depósito arqueológico solapa del Megalocnus en el noroeste de Villa Clara, Cuba. Revista de Biología 18: 160–170.
Auffenberg, W. 1958. A small fossil herpetofauna from Barbuda, Leewards Islands, with the description of a new species of Hyla. Quarterly Journal of the Florida Academy of Sciences 21: 248–254.
Auffenberg, W. 1963. The fossil snakes of Florida. Tulane Studies in Zoology 10: 131–216. Crossref
Bailon, S., Bochaton, C., and Lenoble, A. 2015. New data on Pleistocene and Holocene herpetofauna of Marie Galante (Blanchard Cave, Guadeloupe Islands, French West Indies): insular faunal turnover and human impact. Quaternary Science Reviews 128: 127–137. Crossref
Baird, S.F. and Girard, C. 1853 Catalogue of North American Reptiles in the Museum of the Smithsonian Institution. Part I. Serpents. xvi + 172 pp. Smithsonian Institution, Washington. Crossref
Barbour, T. and Ramsden, C.T. 1919. The Herpetology of Cuba. Memoirs of the Museum of Comparative Zoology 47: 71–213.
Bochaton, C. and Bailon, S. 2018. A new fossil species of Boa Linnaeus, 1758 (Squamata, Boidae), from the Pleistocene of Marie-Galante Island (French West Indies). Journal of Vertebrate Paleontology 38: e1462829. Crossref
Bochaton, C., Boistel, R., Grouard, S., Ineich, I., Tresset, A., and Bailon, S. 2019. Fossil dipsadid snakes from the Guadeloupe Islands (French West-Indies) and their interactions with past human populations. Geodiversitas 41: 501–523. Crossref
Bochaton, C., Grouard, S., Cornette, R., Ineich, I., Tresset, A., and Bailon, S. 2015. Fossil and subfossil herpetofauna from Cadet 2 Cave (Marie-Galante, Guadeloupe Islands, F.W.I.): evolution of an insular herpetofauna since the late Pleistocene. Comptes Rendus Palevol 14: 101–110. Crossref
Boettger, O. 1883. Herpetologische Mittheilungen. Berichte des Offenbacher Vereins für Naturkunde 22–23: 147–156.
Bonaparte, C.L. 1838. Iconografia della Fauna Italica per le quattro Classi degli Animali Vertebrati. Tomo II. Amfibi. 65 pp. Tipografie Salviucci, Roma.
Boudadi-Maligne, M., Bailon, S., Bochaton, C., Casagrande, F., Grouard, S., Serrand, N., and Lenoble, A. 2015. Evidence for historical human-induced extinctions of vertebrate species on La Désirade (French West Indies). Quaternary Research 85: 54–65. Crossref
Brattstrom, B.H. 1958. More fossil reptiles from Cuba. Herpetologica 13: 278.
Brongersma, L.D. 1951. Some notes upon the anatomy of Tropidophis and Trachyboa (Serpentes). Zoologische Mededelingen 3: 107–124.
Burbrink, F.T., Ruane, S., and Pyron, R.A. 2011. When are adaptive radiations replicated in areas? Ecological opportunity and unexceptional diversification in West Indian dipsadine snakes (Colubridae: Alsophiini). Journal of Biogeography 39: 465–475. Crossref
Crespo Díaz, R. and Jiménez Vázquez, O. 2004. Arqueología precolombina del municipio Boyeros. Revista de Gabinete de Arqueología 3: 67–74.
Díaz-Franco, S. 2001. Estructura dental interna y modificación del diseño oclusal inferior en Boromys offella (Rodentia: Echimyidae). Revista Biologia Havana 15: 152–157.
Díaz-Franco, S. 2002. La variación del diseño oclusal inferior en Boromys torrei (Rodentia: Echimyidae). Revista Biologia Havana 16: 60–65.
Dinets, V. 2017. Coordinated hunting by Cuban boas. Animal Behavior and Cognition 4 (1): 24–29. Crossref
Fitzinger, L.J.F.J. 1843. Systema reptilium. Fasciculus primus. Amblyglossae. vi + 106 pp. Braumüler et Seidel Bibliopolas, Vindobonae.
Gray, J.E. 1825. A synopsis of the genera of reptiles and Amphibia, with a description of some new species. Annals of Philosophy Series 2: 193–217.
Günther, A.C.L.G. 1858. Catalogue of Colubrine Snakes in the Collection of the British Museum. xvi + 281 pp. British Museum (Natural History), London.
Hedges, S.B. 2002. Morphological variation and the definition of species in the snake genus Tropidophis (Serpentes, Tropidophiidae). Bulletin of the Natural History Museum London (Zoology) 68: 83–90. Crossref
Hedges, S.B. 2018. Caribherp: West Indian Amphibians and Reptiles (www.caribherp.org). Temple University, Philadelphia.
Hedges, S.B., Couloux, A., and Vidal, N. 2009. Molecular phylogeny, classification, and biogeography of West Indian racer snakes of the tribe Alsophiini (Squamata, Dipsadidae, Xenodontinae). Zootaxa 2067: 1–28. Crossref
Hedges, S.B., Marion, A.B., Lipp, K.L., Marin, J., and Vidal, N. 2014. A taxonomic framework for typhlopid snakes from the Caribbean and other regions (Reptilia, Squamata). Caribbean Herpetology 49: 1–61. Crossref
Holman, J.A. 1979. A review of North American Tertiary snakes. Publications of the Museum, Michigan State University, Paleontological Series 1: 200–260.
Holman, J.A. 1981. A review of North American Pleistocene snakes. Michigan State University Publications of the Museum, Paleontological Series 1: 261–306.
Holman, J.A. 2000. Fossil Snakes of North America: Origin, Evolution, Distribution, Paleoecology. 357 pp. Indiana University Press, Bloomington.
Iturralde-Vinent, M.A., MacPhee, R.D.E., Díaz-Franco, S., Rojas-Consuegra, R., Suárez, W., and Lomba, A. 2000. Las Breas de San Felipe, a Quaternary fossiliferous asphalt seep near Martí (Matanzas Province, Cuba). Caribbean Journal of Science 36: 300–313.
Jiménez, O., Condis, M.M., and García, E. 2005. Vertebrados post-glaciales en un residuario fósil de Tyto alba scopoli (Aves: Tytonidae), en el occidente de Cuba. Revista Mexicana de Mastozoología 9: 85–112. Crossref
Koopman, K.F. and Ruibal, R. 1955. Cave-fossil vertebrates from Camagiuey, Cuba. Breviora Museum of Comparative Zoology 46: 1–8.
Linnaeus, C. 1758. Systema Naturae per Regna Tria Naturae, secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Vol. 1, 10th ed. 823 pp. Laurentii Salvii, Stockholm. Crossref
MacPhee, R.D.E. 2009. Insulae infortunatae: Establishing a chronology for Late Quaternary mammal extinctions in the West Indies. In: G. Haynes (ed.), American Megafaunal Extinctions at the End of the Pleistocene, 169–193. Springer, Dordrecht. Crossref
MacPhee, R.D.E., Iturralde-Vinent, M., and Jiménez-Vázquez, O. 2007. Prehistoric sloth extinctions in Cuba: implications of a new “last” occurrence date. Caribbean Journal of Science 43: 94–98. Crossref
Martínez-López, J.G. 2019. Natural and anthropogenic factors as taphonomic agents in the differential preservation of paleontological remains from the fossil deposit “Las Llanadas”, Central Cuba. Novitates Caribaea 13: 92–114. Crossref
Matthew, W.D. 1919. Recent discoveries of fossil vertebrates in the West Indies and their bearing on the origin of the Antillean fauna. Proceedings of the American Philosophical Society 58: 161–181.
Mead, J.I. and Steadman, D.W. 2017. Late Pleistocene snakes (Squamata: Serpentes) from Abaco, The Bahamas. Geobios 50: 431–440. Crossref
Morgan, G.S. 1993. Quaternary land vertebrates of Jamaica. In: R.M. Wright and E. Robinson, (eds.), Biostratigraphy of Jamaica. Geological Society of America Memoir 182, 417–442. Geological Society of America, Boulder, Colorado. Crossref
Morgan, G.S. 1994. Late Quaternary fossil vertebrates from the Cayman Islands. In: M.A. Brunt and J.E. Davies (eds.), The Cayman Islands: Natural History and Biogeography, 465–508. Kluwer Academic, Boston. Crossref
Morgan, G.S. and Woods, C.A. 1986. Extinction and the zoogeography of West Indian land mammals. Biological Journal of the Linnean Society 28: 167–203. Crossref
Oppel, M. 1811. Die Ordnungen, Familien und Gattungen der Reptilien, als Prodrom einer Naturgeschichte derselben. 87 pp. Joseph Lindauer, Munich. Crossref
d’Orbigny, C. 1843. Dictionnaire universel d’histoire naturelle: résumant et complétant tous les faits présentés par les encyclopédies, les anciens dictionnaires scientifiques, les Oeuvres complètes de Buffon, et les meilleurs traités spéciaux sur les diverses branches des sciences nautrelles; donnant la description des etres et des divers phénomènes de la nature, l’étymologie et la définition des noms scientifiques, et les principales applications des corps organiques et inorganiques à l’agriculture, à la médecine, aux arts industriels, etc. Tome roisiéme (CAA–CLA). 744 pp. Bureau Principal des Éditeurs, Paris. Crossref
Orihuela, J. 2019. An annotated list of late Quaternary extinct birds of Cuba. Ornitología Neotropical 30: 57–67.
Orihuela, J., Viñola, L.W., and Viera, R.A. 2020. Nuevas localidades y registros de murciélagos para Cuba, con énfasis en la provincia de Matanzas. Novitates Caribaea 15: 96–116. Crossref
Parmley, D. 1988. Early Hemphillian (late Miocene) snakes from the Higgins Local Fauna of Lipscomb County, Texas. Journal of Vertebrate Paleontology 8: 322–327. Crossref
Parmley, D. and Hunter, K.B. 2010. Fossil snakes of the Clarendonian (Late Miocene) Pratt Slide Local Fauna of Nebraska, with the description of a new natricine colubrid. Journal of Herpetology 44: 526–543. Crossref
Powell, R. and Henderson, R.W. 2012. Island lists of West Indian amphibians and reptiles. Bulletin of the Florida Museum of Natural History 51: 85–166.
Pregill, G.K. 1981. Late Pleistocene herpetofaunas from Puerto Rico. University of Kansas Museum of Natural History Miscellaneous Publications 71: 1–72. Crossref
Pregill, G.K. 1982. Fossil amphibians and reptiles from New Providence Island, Bahamas. Smithsonian Contributions to Paleobiology 48: 8–21.
Pregill, G.K., Steadman, D.W., and Watters, D.R. 1994. Late Quaternary vertebrate faunas of the Lesser Antilles: Historical components of Caribbean biogeography. Bulletin of Carnegie Museum of Natural History 30: 1–51.
Pregill, G.K., Steadman, D.W., Olson, S.L., and Grady, F.V. 1988. Late Holocene fossil vertebrates from Burma Quarry, Antigua, Lesser Antilles. Smithsonian Contributions to Zoology 463: 1–27. Crossref
Rage, J.C. and Holman, J.A. 1984. Des Serpents (Reptilia, Squamata) de type Nord-Américain dans le Miocene francais. Évolution parallele ou dispersion? Geobios 17: 89–104. Crossref
Ramon de la Sagra, D. 1838–1843. Historia física, política y natural de la isla de Cuba. Tomo II. Segunda parte – eptiles y peces. Reptiles. Atlas. i + 143 pp. Arthus Bertrand, Paris.
Reinhardt, J.T. and Lütken, C.F. 1862. Bidrag til det vestindiske öriges og navnligen til de dansk-vestindiske öers Herpetologie. Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjöbenhavn (1862–1863) 24 (10–18): 153–291.
Reynolds, G.R., Niemiller, M.L., and Revell, L.J. 2014. Toward a Tree-of-Life for the boas and pythons: multilocus species-level phylogeny with unprecedented taxon sampling. Molecular Phylogenetics and Evolution 71: 201–213. Crossref
Richmond, N.D. 1955. The blind snakes (Typhlops) of Bimini, Bahama Islands, British West Indies, with description of new species. American Museum Novitates 1734: 1–7.
Rodríguez-Schettino, L., Mancina, C.A., and Rivalta, V. 2013. Reptiles of Cuba: checklist and geographic distributions. Smithsonian Herpetological Information Service 144: 1–96. Crossref
Rojas-Consuegra, R., Jiménez, O., Condis-Fernández, M.M., and Díaz-Franco, S. 2012. Tafonomia y paleoecologia de un yacimiento paleontológico del cuaternario en la Cueva del Indio, La Habana, Cuba. Espelunca Digital 12: 15.
Salgado, E.S.J., Calvache, D.G., Macphee, R.D.E., and Gould, G.C. 1992. The Monkey caves of Cuba. Cave Science 19: 25–28.
Schlegel, H. 1837. Essai sur la physionomie des serpents. xxviii + 606 pp. M.H. Schonekat, Amsterdam. Crossref
Steadman, D.W., Albury, N.A., Kakuk, B., Mead, J.I., Soto-Centeno, J.A., Singleton, H.M., and Franklin, J. 2015. Vertebrate community on an ice-age Caribbean island. Proceedings of the National Academy of Sciences of the United States of America 112: E5963–E5971. Crossref
Suárez, W. 2004a. The enigmatic snipe Capella sp. (Aves: Scolopacidae) in the fossil record of Cuba. Caribbean Journal of Science 40: 155–157.
Suárez, W. 2004b. Biogeografía de las aves fósiles de Cuba. In: M. Iturralde-Vinent (ed.), Origen y Evolución del Caribe y sus biotas Marinas y Terrestres. Centro Nacional de Información Geológica, La Habana.
Suárez, W. and Díaz-Franco, S. 2003. A new fossil bat (Chiroptera: Phyllostomidae) from a Quaternary cave deposit in Cuba. Caribbean Journal of Science 39: 371–377.
Syromyatnikova, E., Aranda, E., and Fiol González, S. 2020. The first fossil record of Cadea (Amphisbaenia, Cadeidae) and other amphisbaenian remains from the upper Pleistocene of Cuba. Journal of Vertebrate Paleontology [published online, https://doi.org/10.1080/02724634.2019.1729167]. Crossref
Szyndlar, Z. 1991a. A review of Neogene and Quaternary snakes of Central and Eastern Europe. Part I: Scolecophidia, Boidae, Colubrinae. Estudios Geológicos 47: 103–126. Crossref
Szyndlar, Z. 1991b. A review of Neogene and Quaternary snakes of Central and Eastern Europe. Part II: Natricinae, Elapidae, Viperidae. Estudios Geológicos 47: 237–266. Crossref
Szyndlar, Z. and Schleich, H.H. 1993. Description of Miocene snakes from Petersbuch 2 with comments on the Lower and Middle Miocene Ophidian faunas of Southern Germany. Stuttgarter Beiträge für Naturkunde B 192: 1–47.
Thomas, R. and Hedges, S.B. 2007. Eleven new species of snakes of the genus Typhlops (Serpentes: Typhlopidae) from Hispaniola and Cuba. Zootaxa 1400: 1–26. Crossref
Tolson, P.J. and Henderson, R.W. 1993. The Natural History of West Indian Boas. 125 pp. R&A Publishing Ltd., Tauton.
Torres López, J., Rodríguez-Cabrera, T.M., and Marrero Romero, R. 2017. Reptiles. In: C.A. Mancina and D.D. Cruz (eds.), Diversidad Biológica de Cuba: Métodos de Inventario, Monitoreo y Colecciones Biológicas, 376–411. Editorial AMA, La Habana.
Varona, L.S. and Arredondo, O. 1979. Nuevos táxones fósiles de Capromyidae (Rodentia: Caviomorpha). Poeyana 195: 1–51.
Vidal, N., Delmas, A.-S., David, P., Cruaud, C., Couloux, A., and Hedges, S.B. 2007. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. Comptes Rendus Biologies 330: 182–187. Crossref
Whistler, D.P. and Wright, J.W. 1989. A late Miocene rear-fanged colubrid snake from California with comments on the phylogeny of North American snakes. Herpetologica 45: 350–367.
Zaher, H., Grazziotin, F.G., Cadle, J.E., Murphy, R.W., Moura-Leite, J.C., and Bonatto, S.L. 2009. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papeis Avulsos de Zoologia 49: 115–153. Crossref
Zelenkov, N.V. and Gonzalez, S.F. 2020. The first fossil tody (Aves: Todidae) from Cuba. Paleontological Journal 54: 414–419. Crossref
Acta Palaeontol. Pol. 66 (2): 395–407, 2021
https://doi.org/10.4202/app.00766.2020