

Paleoecology of the first Devonian-like sclerobiont association on Permian brachiopods from southeastern Mexico
MIGUEL A. TORRES-MARTÍNEZ, OLEV VINN, and LOURDES MARTÍN-AGUILAR
Torres-Martínez, M.A., Vinn, O., and Martín-Aguilar, L. 2021. Paleoecology of the first Devonian-like sclerobiont association on Permian brachiopods from southeastern Mexico. Acta Palaeontologica Polonica 66 (1): 131–141.
This paper describes a sclerobiont association from the Paso Hondo Formation (Roadian, middle Permian), Chiapas, Mexico. Different marine invertebrates such as hederelloids, microconchids, bryozoans, and crinoids (represented by holdfasts) encrusted brachiopod shells belonging to Athyridida and Rhynchonellida. This association is similar to those recorded in different Devonian localities, especially by the co-occurrence of microconchids, hederelloids, and bryozoans. Paleoecological analysis revealed that bryozoans were the most abundant sclerobionts, whereas crinoid holdfasts were uncommon. Likewise, hederelloids and microconchids often settled on hosts previously colonized by bryozoans. Most microconchids encrusted rhynchonellid shells. A positive correlation between the size of the hosts and abundance/diversity of sclerobionts was recorded. The distribution analysis suggests that sclerobiont colonization could have been influenced either by inhalant currents of brachiopods, time of exposure, position of hosts, or by combination of all these factors. Moreover, most of commissures and foramens of brachiopods were not covered by epibionts, suggesting that there was a live interaction. Thus, studied brachiopods were likely encrusted syn vivo, and the interaction between sclerobionts and their brachiopod hosts was likely commensal since there is no damage to the brachiopod valves in the form of malformations or borings. On the contrary, the epibiont cover might have served as a natural shield against predators and parasites. The Roadian age of the association is based on the stratigraphic distribution of host brachiopods. The studied association inhabited open waters on a homoclinal carbonate ramp in the Chicomuselo region. Although encrusted brachiopods belong to the biotic Grandian Province, similar sclerobiont communities have not been previously recorded from the Permian of North America or beyond. The described community represents the youngest record of co-occurring microconchids, hederelloids, and bryozoans, as all previously known similar communities originate from the Late Devonian.
Key words: Brachiopoda, Bryozoa, Hederelloidea, Microconchida, Permian, Roadian, Mexico, Chiapas.
Miguel A. Torres-Martínez [miguelatm@geologia.unam.mx], Departamento de Paleontología, Instituto de Geología, Circuito de la Investigación Científica, Colonia Universidad Nacional Autónoma de México, Avenida Universidad 3000, Alcaldía Coyoacán, 04510, Mexico City, Mexico.
Olev Vinn [olev.vinn@ut.ee], Department of Geology, University of Tartu, Ravila 14A, 50411, Tartu, Estonia.
Lourdes Martín-Aguilar [lourdm01@ucm.es], Máster Universitario en Paleontología Avanzada. Departamento de Paleontología, Facultad de Ciencias Geológicas, Universidad Complutense de Madrid, campus Moncloa, Calle José Antonio Novais, 12, 28040, Madrid, Spain.
Received 26 May 2020, accepted 27 August 2020, available online 25 January 2021.
Copyright © 2021 M.A. Torres-Martínez et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Paleozoic formations are widely exposed in the Chicomuselo region, Chiapas State, Mexico. The most important outcrops of the Carboniferous and Permian in southeastern Mexico which yield rich and diverse marine fossils are situated in this region. In particular, the Paso Hondo Formation represents the youngest lithostratigraphic unit with an Artinskian–Roadian age (late Cisuralian–early Guadalupian), also being the most fossiliferous of the region (Thompson and Miller 1944; Torres-Martínez et al. 2019b). The age of the formation was dated by numerous taxa identified from this unit, including the first Paleozoic sclerobionts reported in Mexico, such as hederelloids, represented by Hederella carbonaria Condra and Elias, 1944 (González-Mora et al. 2018), and the recently described microconchid Microconchus maya Heredia-Jiménez, Vinn, and Torres-Martínez, 2020 (Heredia-Jiménez et al. 2020). Even though the fauna of the Paso Hondo Formation is currently relatively well known (Torres-Martínez et al. 2019b; Heredia-Jiménez et al. 2020), the information about their depositional paleoenvironments and paleoecologic relationships is practically absent. The studied material allowed to analyse interactions of the Chiapas sclerobionts, both between each other and with their hosts, in a specific marine environment.
Similar relationships (sclerobionts-host) have previously been reported from different Paleozoic localities worldwide (Hoare and Steller 1967; Webb and Schneider 2013; Barclay et al. 2013). The Devonian sclerobiont communities are the best known and studied (Schneider 2013). In contrast, the Carboniferous (e.g., Lescinsky 1997; Schneider 2013) and Permian encruster associations are poorly known, being conspicuously less common in the late Paleozoic, probably due to the Late Devonian and end-Devonian extinctions (Schneider 2013; Taylor 2016). Therefore, the association from the Chicomuselo region is significant since it represents the only well preserved sclerobiont community from the Permian of Mexico enabling the analysis of the important paleoecological interactions in the late Paleozoic.
Webb and Schneider (2013) stated that sclerobionts could be considered as a taphonomic window to fossil ecosystems, both because of the preservation of interactions between hosts and their encrusters and between sclerobionts themselves. The epibionts are always preserved in situ with respect to their substrate and they maintain true spatial relationships. Thus, the spatial competition, ecological succession, growth orientation, and differential use of exposed versus unexposed zones of the host can be inferred (Taylor and Wilson 2003). Thereby, this work aims to describe and analyze the paleoecological interactions both between sclerobionts (bryozoans, hederelloids, microconchids, and crinoids) and between epibionts and their brachiopod hosts in the Roadian (middle Permian) of the Paso Hondo Formation from Chiapas. In addition, the settlement preference of sclerobionts on brachiopod shells is discussed. The current paper is the first paleoecological study of a fossil sclerobiont community from Mexico.
Institutional abbreviations.—IGM, Mexican Geological Institute, Mexico City, Mexico; UNAM, National Autonomous University of Mexico, Mexico City, Mexico.
Historical background
Sclerobionts can settle on a variety of hard substrates such as rocks, wood, and plants, as well as shells or skeletal remains of various animals (Taylor and Wilson 2003). Some epibionts have a preference for specific surfaces, and possible substrate preferences of other epibionts are still under discussion (Zatoń and Krawczyński 2011; Agostini et al. 2017). In general, the epibiont assemblages are composed of several groups of encrusting invertebrates and their larvae are usually transported as zooplankton by marine currents (Agostini et al. 2017). Nonetheless, epibiont taxa and their hosts have changed through the Phanerozoic, and these changes correspond to the succession of marine biota from the Paleozoic to Cenozoic (Taylor and Wilson 2003). Although there are records of sclerobionts encrusting brachiopods from the late Paleozoic, the majority of studies have been focused on the Devonian communities (Schneider 2013).
The Ordovician associations have been well described by Richards (1972), who recognized encrusting bryozoans (Cyclostomata and Trepostomata) and cornulitids on lingulid and orthid shells from Indiana and Ohio in the United States. Zhan and Vinn (2007) described Cornulites sp. on brachiopod shells of Altaethyrella zhejiangensis and Ovalospira dichotoma from the Late Ordovician of South China Palaeoplate. Smrecak and Brett (2014) analyzed the ecology of a sclerobiont community on brachiopods (Rafinesquina) composed of numerous taxa from the Late Ordovician of Cincinnati Arch region in the United States. Likewise, Hurst (1974) described an important Silurian assemblage, reporting spirorbids (i.e., microconchids), cornulitids, and cyclostome bryozoans on brachiopod shells belonging to the orders Orthida, Rhynchonellida, Atrypida, Athyridida, and Spiriferida, whereas Copper (2004) reported encrusting bryozoans on Silurian atrypid brachiopods. Both papers were based on the specimens from Gotland, Sweden.
As mentioned above, the Devonian records of sclerobionts-brachiopods are well-represented by numerous works, which are detailed in Table 1. It is worth noting that before the 21st century, the different authors only mentioned the occurrence of spirorbids rather than microconchids in the Paleozoic (Table 1). Nowadays, it is known that the spirally coiled serpulid polychaetes appeared in the late Permian (Ippolitov et al. 2014), and the earliest record of the genus Spirorbis is from the Late Cretaceous (Jäger 2004).
Table 1. Devonian records of the interaction sclerobionts/brachiopods. * represents the same taxon according to Zatoń et al. (2017); ** algae from the genus Rothpletzella according to Zatoń and Jarochowska (2020).
|
References |
Sclerobionts |
Hosts |
Age |
Region |
|
hederelloids, bryozoans, cornulitids, and craniids |
spiriferids |
Givetian |
Ohio, USA |
|
|
hederelloids, sponges, cornulitids, and craniids |
spiriferids |
Givetian |
Ohio, USA |
|
|
spirorbids, hederelloids, bryozoans, holdfast of crinoids, corals, and brachiopods |
Paraspirifer bownockeri |
Middle |
Ohio, USA |
|
|
spirorbids, hederelloids, bryozoans, and corals |
Anathyris phalaena |
Emsian |
Northeastern Spain |
|
|
spirorbids, hederelloids, bryozoans, cornulitids, craniids, and pelmatozoans |
orthids, strophomenids, and terebratulids |
Middle |
New York, USA |
|
|
microconchids, hederelloids,
bryozoans, cornulitids, foraminifera, Ascodictyon,
rugose corals, |
Productella
sp., |
late Frasnian–early Famennian |
Central Devonian Field, Russia |
|
|
microconchids, hederelloids, bryozoans, cornulitids, and rugose corals |
orthids, atrypids, and |
Givetian and Frasnian |
Western Canadian Sedimentary Basin, Canada |
|
|
microconchids, hederelloids, bryozoans, craniids, rugose corals, and cornulitids |
Desquamatia |
Givetian |
Iowa, USA |
|
|
microconchids, hederelloids, bryozoans, cornulitids, Ascodictyon, productids, and problematic discs* |
Cyrtospirifer zadonicus and Ripidiorhynchus huotinus |
early |
Central Devonian Field, Russia |
|
|
microconchids, hederelloids, bryozoans, cornulitids, Ascodictyon, rugose corals, productids, algae**, Aulopora, and Sphenothallus* |
Cyrtospirifer, Theodossia,
Ripidiorhynchus, Variatrypa,
Donalosia,
Schuchertella, |
late Frasnian–early |
Central Devonian Field, Russia |
In contrast to the Devonian, the late Paleozoic sclerobiont communities are poorly known. An important Carboniferous association was described by Lescinsky (1997), who analyzed specimens collected from the USA and Canada. He described several ecological interactions that took place between productid, orthotetid, athyridid, and spiriferid brachiopods and rugose corals, cornulitids, spirorbids (i.e., microconchids), craniids, hederelloids, bryozoans and barnacles. The latter sclerobiont community is in many ways similar to those found in the Devonian strata; however, only the Mississippian associations contain hederelloids.
Previously known encrusters on Permian shells include cyclostome bryozoans (Taylor 1985), “serpulids”, productacean brachiopods, and ‘‘oyster-like bivalves’’ (Newell and Boyd 1970). The current work contains the first detailed paleoecological analysis of a Permian epibiont community.
Geological setting
The specimens were collected from rocks of the Paso Hondo Formation, the type section of which is exposed along the Comalapa river, near the town of Comalapa, southeastern Chiapas, Mexico. This particular study site is located northeast of the Monte Redondo town, to the north side of El Manguito hill, Municipality of Frontera Comalapa, Chiapas state, about at the coordinates N 15º39’–15º 38’ and W 92º02’–92º01’ (Fig. 1). The Paleozoic succession of Chiapas is widely outcropping in the Chicomuselo region, limited with Guatemala frontier. The succession begins with the Santa Rosa Formation of Carboniferous age (late Mississippian–early Pennsylvanian), containing conglomerate, sandstone, argillaceous shale, and slate (Gutiérrez-Gil 1956; Weber et al. 2006). Unconformably overlaying the Santa Rosa Formation is the Grupera Formation, composed of shale, calcareous sandstone, and limestone of Asselian–Sakmarian age (early Cisuralian) (Gutiérrez-Gil 1956; Torres-Martínez et al. 2020). Unconformably above of the Grupera Formation is the La Vainilla limestone which mainly constitutes crystalline limestone of the Sakmarian–Artinskian age (middle Cisuralian) (Gutiérrez-Gil 1956). The Paleozoic succession ends with the Paso Hondo Formation of Artinskian–Roadian age (late Cisuralian–early Guadalupian), which is mainly composed of massive limestone, and semi-stratified limestone, as well as silicified or argillaceous shale at its base (Gutiérrez-Gil 1956; Torres-Martínez et al. 2017, 2019b). The Paleozoic succession is overlain by the Todos Santos Formation of the Triassic-Jurassic age, composed of red sandstone intercalated with thin layers of shale (Gutiérrez-Gil 1956).
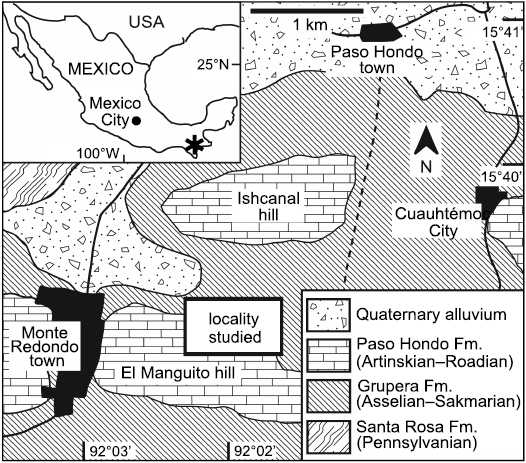
Fig. 1. Map of Mexico and geological map of the studied locality.
Material and methods
The analyzed brachiopods are mainly preserved as calcitic articulated shells. Their valves are covered with different sclerobionts, also preserved as calcitic skeletons. In this paper, we selected two different study groups, based on the taxonomic order of the hosts; one group is represented by athyridids (14 specimens), and the other group by rhynchonellids (12 specimens). The brachiopods were discarded if they were disarticulated or damaged by diagenesis. Each specimen was observed with a binocular microscope in order to locate and count all sclerobionts, following the method mentioned by Webb and Schneider (2013). This scheme consists of dividing all brachiopod valves, both ventral and dorsal, into different regions, establishing how sclerobionts were distributed in each sector (Fig. 2). The specimens illustrated are deposited at the Colección Nacional de Paleontología from the Instituto de Geología of UNAM. The material is organized with the prefix IGM and its corresponding number.
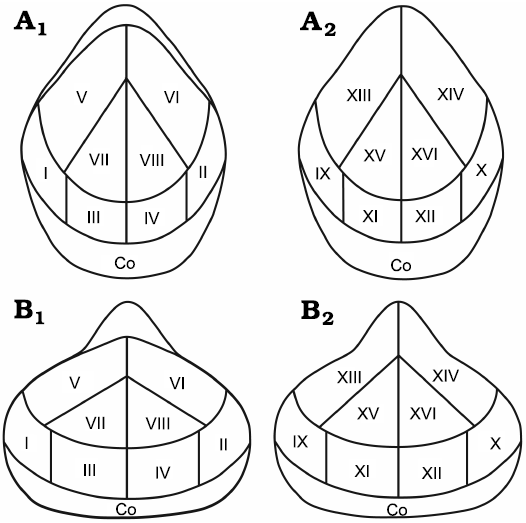
Fig. 2. Sector divisions of hosts for the encrusted abundance and distributional patterns. Athyridids (A) and rhynchonellids (B), in dorsal (A1, B1) and ventral (A2, B2) views. Co, commissure.
All brachiopods were measured (length, width, and height) to obtain proxies for surface areas in each host (mm2) to estimate the potential space that could be colonized by sclerobionts in both brachiopod groups. The sclerobionts were classified into four groups: microconchids, hederelloids, bryozoans, and crinoids. The occurrence of sclerobionts was studied as following: (i) all quadrants occupied by colonial forms were counted, (ii) in individual taxa, only the quadrant with the majority of the body was counted. Then, brachiopods were divided in two clusters, athyridids and rhynchonellids. We obtained the number of sclerobiont occurrences per quadrant, the relative abundance of each epibiont group on dorsal and ventral valves, and the total occupation percentage per region. This information allowed to analyze the frequencies of co-occurrence among sclerobionts. In addition, the ranges of size and area of the hosts were analyzed. With the number of sclerobionts occurred on each host, the values of both diversity and abundance were obtained. The statistical analyses applied to athyridids or rhynchonellids included a c2 Dispersion Test to describe the distribution pattern of sclerobionts; Goodness of fit of c2 Test to determine the preference of valve; Shapiro-Wilk Test to establish if abundance and diversity had a normal distribution; and Pearson’s correlation to know the relationship between size/abundance and size/diversity. Finally, rhynchonellids and athyridids were considered as a unique group, using Goodness of fit of c2 Test to identify if there was a preference for some order (Athyridida or Rhynchonellida), as well as to establish if there was a preference for ornamented or smooth brachiopods. The significance (α-level) considered in all tests was 0.05.
Results
Biotic association.—A total of 26 sclerobiont occurrences were recorded on brachiopods from the Roadian (middle Permian) of Chiapas, 14 on five athyridid species (Composita sp., Composita enormis, C. parasulcata, C. hapsida, and Hustedia connorsi) and 12 on four taxa of the order Rhynchonellida (Tautosia transenna, Pontisia sp., Wellerella lemasi, and Phrenophoria ventricosa) (Fig. 3). The sclerobiont assemblage is composed by microconchids (Microconchus maya), hederelloids (Hederella carbonaria), bryozoans (order Trepostomata) and holdfasts of crinoids (Fig. 4). The studied association is dominated by filter feeders, mostly lophophorates, such as brachiopods, bryozoans, and probably hederelloids and microconchids (Taylor and Vinn 2006; Taylor and Wilson 2008). The dominant epizoans were bryozoans which occurred on all hosts, followed by microconchids, hederelloids, and crinoids. Except for bryozoans, all other taxa were always accompanied by specimens from the other groups. The presence of two different epibionts per host was usual, but on one brachiopod (C. hapsida) we found all groups together. The maximum number of encrusting epibionts on one shell was five, represented by two rhynchonellid specimens (T. transenna and W. lemasi) and two athyridid shells (C. enormis) (Fig. 5).
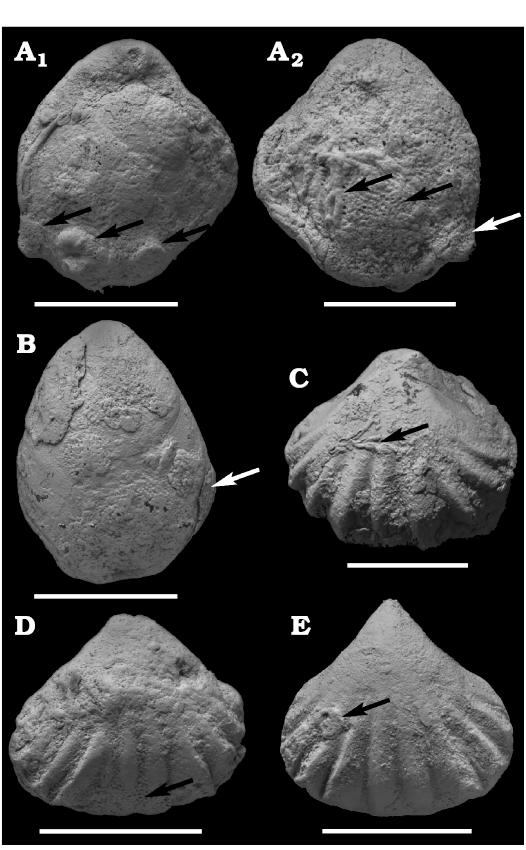
Fig. 3. Encrusting sclerobionts (arrowed) on different brachiopod Roadian specimens from Monte Redondo locality, Chiapas, Mexico. A. Composita hapsida Stehli and Grant, 1970, IGM 11150, A1 with holdfast of crinoid and two microconchids (left to right arrows); A2 with hederelloids (left black arrow), bryozoans (right black arrow), and holdfast of crinoid (white arrow). B. Composita enormis Cooper and Grant, 1976, IGM 11143 with bryozoans. C–E. Tautosia transenna Cooper and Grant, 1976. C. IGM 11140 with hederelloids. D. IGM 11138 with bryozoans. E. IGM 11139 with microconchid. Scale bars 10 mm.
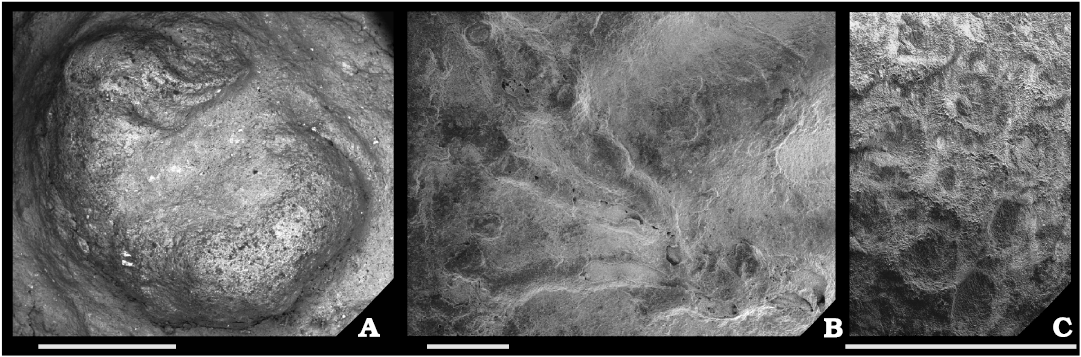
Fig. 4. Sclerobionts of the studied Roadian community from Monte Redondo locality, Chiapas, Mexico. A. Microconchus maya Heredia-Jiménez, Vinn and Torres-Martínez, 2020. B. Hederella carbonaria Condra and Elias, 1944. C. Encrusting bryozoans. Scale bars 1 mm.
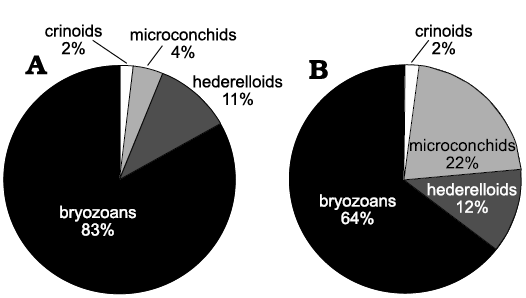
Fig. 5. Percentage of all sclerobiont groups: athyridids (A) and rhynchonellids (B) per brachiopod order.
Distribution of sclerobionts.—The average number of sclerobionts per athyridid was 2.93, and the number of different taxa on each brachiopod was 1.71 (Fig. 6). Bryozoans were the most common group with 28 colonies; eight occupied only one sector, 13 were extended from two to five sectors, and seven in more than five sectors. In the case of hederelloids, all colonies occurred in less than four sectors. In addition, there were sectors cohabited by more than one sclerobiont, for instance, bryozoan/hederelloid thrice, bryozoan/microconchid twice, bryozoan/bryozoan once and bryozoan/microconchid/crinoid on an exceptional sector. The XIII sector from the ventral valve was the region with most records of sclerobionts (10 occurrences), followed by sectors II, IV, X and XV with nine occurrences. Bryozoans were located in all regions, but they were more frequent in the sectors X and XIII. The distribution of sclerobionts was clumped (c2 test, p < 0.05; mean/variance = 0.7). On rhynchonellids bryozoans were also the dominant group, followed by microconchids, but much more abundant than in athyridids. The approximate number of sclerobionts on each rhynchonellid was 2.92, finding 1.83 different taxa on average per brachiopod (Fig. 6). From the 20 colonies of bryozoans, 12 occupied one sector, whereas the rest were extended on two or four sectors. In hederelloids, the colonies were located on less than three sectors. Besides, on two rhynchonellids, only one sector was occupied by more than one epibiont (bryozoans/microconchids). The total number of sclerobionts on rhynchonellids was smaller than on athyridids, with the commissure showing most of occurrences (seven), followed by the sectors XI, XII (five), VII and XV (four). Thus, the commissure and sectors XI and XII were preferred by bryozoans, whereas the sector VII was mainly colonized by microconchids (small-size individuals). Also, the distribution of sclerobionts was clumped (c2 test, p < 0.05; mean/variance = 0.9).
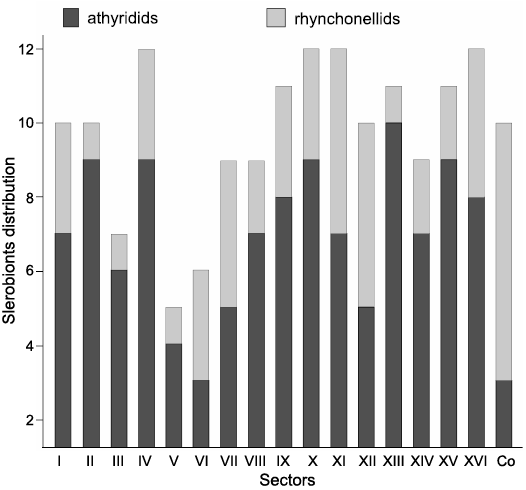
Fig. 6. Abundance of epibionts per sector on both rhynchonellids and athyridids. Co, commissure.
Preference of valve and host.—Part of this study aimed the question whether there was any preference by sclerobionts for a specific valve (ventral or dorsal) of the hosts. In athyridids, we recorded that bryozoans occupied both valves in a similar proportion, but microconchids, hederelloids, and crinoids showed probable preference for the dorsal valve. In rhynchonellids, we also noticed some differences: bryozoans were slightly more abundant on the ventral valve, microconchids, and crinoids mainly settled on the dorsal valve, whereas hederelloids had some preference for the ventral valve. Considering all sclerobiont occurrences, the dorsal valves were also preferred, although the difference was not significant. The results calculated separately for athyridids (c2 = 0.42) and rhynchonellids (c2 = 0.14) confirmed that there was no significant preference for any particular valve (α = 0.05). Both distribution data and external traits of brachiopods were used to determine if there was any host preference for settlement of sclerobionts. The smooth brachiopods are the athyridids Composita sp., C. enormis, C. parasulcata, and C. hapsida; and the ornamented hosts are the athyridid Hustedia connorsi and the rhynchonellids T. transenna, Pontisia sp., W. lemasi, and P. ventricosa. The result of c2 (0.05) validated that there was no preference for ornamented or smooth shells (α = 0.05).
Host size versus abundance and diversity of sclerobionts.—The abundance data for both athyridids and rhynchonellids showed a normal distribution. The area of athyridids ranged from 130.83 to 442.03 mm2, with 264.82 mm2 on average. In this group, a very low correlation between the host area and epibiont abundance in the dorsal valve was observed (R2 = 0.09); however, the epibionts were most abundant on brachiopods of medium size (250–350 mm2). In the ventral valves, also the correlation between the host size and sclerobiont abundance was observed but to a lesser extent (R2 = 0.05). The epibiont diversity on the dorsal valves (R2 = 0.81) and the ventral valves (R2 = 0.89) showed a positive correlation. The abundance of sclerobionts can be slightly correlated with increasing host size in athyridids (Pearson’s correlation, r = 0.42, p < 0.01), similar correlation occurs between epibiont diversity and the brachiopod size (Pearson’s correlation, r = 0.30, p < 0.01) (Fig. 7).
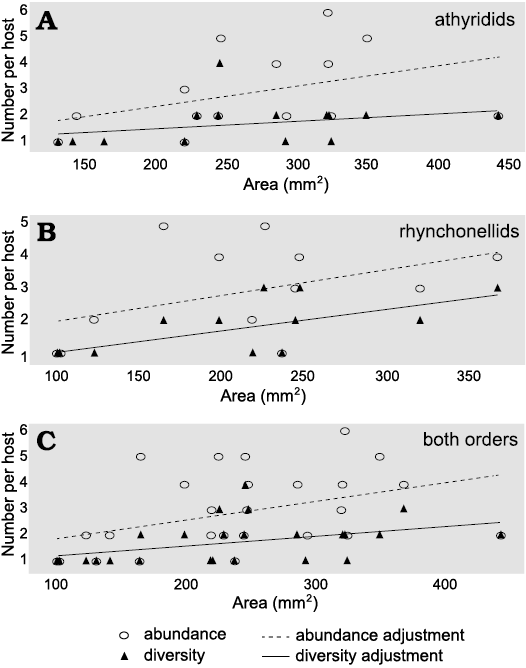
Fig. 7. Diversity and abundance of sclerobionts versus potential area of brachiopods brachiopods: athyridids (A), rhynchonellids (B), and both orders (C). The adjustments allowed us to observe low to moderate correlation in all graphs.
Encrustation patterns in rhynchonellids are similar to those in athyridids. The area of rhynchonellids ranged from 100.25–367.26 mm2, with 212.41 mm2 on average. There was an apparent linear correlation between the dorsal valve area and the abundance of sclerobionts (R2 = 0.36). Nonetheless, epibionts were most abundant on specimens ranging from 160 to 250 mm2. On the contrary, in the ventral valve, the correlation was not linear (R2 = 0.02). Diversity had similar values for the dorsal (R2 = 0.89) and ventral valves (R2 = 0.9). Our results indicate a moderate positive correlation of abundance (Pearson’s correlation, r = 0.43, p < 0.01) and diversity (Pearson’s correlation r = 0.64, p < 0.01) regarding the brachiopod size (Fig. 7).
Discussion
Sclerobiont association.—The sclerobionts studied (bryozoans, hederelloids, and microconchids) are also typical taxa for the Devonian epibiont associations (e.g., Zatoń and Krawczyński 2011; Zatoń and Borszcz 2013; Webb and Schneider 2013; Barclay et al. 2013; Zatoń et al. 2017). Both hederelloids (over 80 species) and microconchids (about seven species) were diverse in the Devonian (Taylor and Wilson 2008; Zatoń and Olempska 2017). In contrast, in the Carboniferous, hederelloids were represented only by three species, most characteristic Hederella carbonaria (González-Mora et al. 2018) and microconchids by three species: Microconchus hintonensis, a non-marine microconchid (Zatoń and Peck 2013) as well as Microconchus carbonarius and Microconchus cravenensis, both marine (Zatoń et al. 2014; Zatoń and Mundy 2020). In the Permian, hederelloids were represented by H. carbonaria (a species extended from the Carboniferous) and microconchids by two different species (Taylor and Wilson 2008; Zatoń and Olempska 2017; González-Mora et al. 2018; Heredia-Jiménez et al. 2020). It is evident that diversity of hederelloids and microconchids decreased drastically in the late Paleozoic. In contrast, bryozoans were the most diverse and abundant invertebrates in late Paleozoic sclerobiont associations (Taylor and Wilson 2003). Although Devonian and late Paleozoic epibiont communities contained similar invertebrates, their abundances usually were different. For example, Álvarez and Taylor (1987) recorded hederelloids in similar proportion to trepostome bryozoans in the Devonian. According to Taylor and Wilson (2003) microconchids and hederelloids were more abundant than bryozoans in the Devonian sclerobiont associations studied. Zatoń and Krawczyński (2011) mentioned microconchids as the most common epibionts in their Devonian association, whereas Webb and Schneider (2013) determined bryozoans and microconchids as the most abundant taxa. We found that bryozoans were the most common epizoans on the Permian brachiopods, followed by microconchids, hederelloids, and crinoids, respectively. Regarding the co-occurrences, the most usual association on athyridid brachiopods was bryozoans-hederelloids association, followed by bryozoans-microconchids association while on rhynchonellids it was bryozoans-microconchids association, as well as bryozoans-hederelloids association. We have observed that some microconchids encrusted bryozoans on rhynchonellid brachiopods which could indicate some succession in the sclerobiont association, while on athyridids some hederelloids grew on bryozoan colonies. This behavior could be related to intra- or interspecific competition for space; nonetheless, it has also been proposed that settlement on previously colonized substrate is to avoid encrusting of other uninhabitable surfaces (Webb and Schneider 2013). Our results indicated a low competition for space, corroborated by the small frequency of occurrence of more than one epibiont per quadrant. Our data suggest that some sclerobionts were overlapped by other ones just accidentally. The epibionts accidentally settled on other sclerobionts only to colonize a hard substrate. On the other hand, we recorded 2.93 sclerobionts on average per host on athyridids, whereas on rhynchonellids there were 2.92 sclerobionts on average per host. We determined that the abundance of sclerobionts found on each brachiopod from the Paso Hondo Formation was similar to that reported in the Devonian community of Cedar Valley (2.97) (Webb and Schneider 2013). Moreover, the percentage of brachiopods with more than one epibiont was higher in Permian athyridids and rhynchonellids of Chiapas (85.71% and 75%, respectively) than in the Devonian brachiopods of Iowa in USA (59.7%). Our numbers show that the distribution of the encrusting fauna from the Paso Hondo Formation is similar to the Devonian community from the Givetian of the Robins Quarry (Solon Member) (Webb and Schneider 2013).
Preference of host.—The preference for substrate by sclerobionts has often been related to several factors. Sclerobionts could choose a host depending on its size, morphology, or ornamentations (Barclay et al. 2013), or even the age and growth stage (Webb and Schneider 2013). In this context, we obtained different data, varying according to the brachiopod group. In athyridids we found highest number of encrusters on the posterior zone of shells, considered to be the oldest region of the hosts while on rhynchonellids most epibionts were located near the commissure. This relative preference for one specific region of the hosts is still under discussion since there are different variables to take into account. The abundance on the anterior zone could be explained by two possibilities: (i) sclerobionts benefited from the inhalant and exhalant currents of the hosts and increased so their feeding efficiency, or (ii) encrusters colonized this area just for getting away from the seafloor and so avoiding the turbidity of the environment (Hurst 1974; Zatoń and Krawczyński 2011). The former has been the most usual explanation, since the currents produced by brachiopods, especially of spire-bearing and rhynchonellids could provide suspension feeding epibionts with their food. In this work, most epizoans occurred in anterior area, suggesting that their feeding and growth benefited from selection of this site of encrustation. It is possible that some brachiopods grew in a vertical position, and their sclerobionts benefitted from the position near the commissure because of the feeding currents in this area, such as in spiriferid Cyrtospirifer (Ager 1961; Zatoń and Krawczyński 2011). The colonization of these zones provided epibionts with important advantages: higher tier for growth to escape sedimentation on the bottom, as well as favorable feeding situation due to presence of strong feeding currents produced by brachiopods. Although these factors can explain the presence of epibionts on the anterior zone, they do not explain the epibiont occurrences in posterior regions. In the latter case, the distribution was not related to sclerobiont feeding but instead might have been associated with the settlement of epibionts in the early stages of the host growth. Nonetheless, the inhalant currents are laterally produced in athyridids, and might have supported the colonization on posterior areas.
Rodland et al. (2006) have demonstrated that the time for encrustation before shells are completely buried is really brief, inhibiting the growth of sclerobiont colonies, and hosts became more heavily encrusted with time. Webb and Schneider (2013) and Barclay et al. (2013) reported a positive correlation between host size and the number of sclerobionts encrusted. Thus, the chance to be encrusted is higher if the brachiopod surface area is large; so, if the host has a significant size, it means that it is old, and in turn, it was longer exposed to colonization. This pattern reflects how a large size and being long-time exposed fostered larval settlement that was controlled by two parameters: time and available surface area. The small brachiopods were less susceptible to colonization because they were a much smaller target (Barclay et al. 2013). We observed a similar pattern among our brachiopods: size versus abundance and diversity of sclerobionts. Figure 7 displays a positive correlation between abundance and host size, and that epibionts are slightly more common on the dorsal valve. In both brachiopod groups, there was a weak correlation between medium-large brachiopods and a higher number of encrusters.
There was a correlation between diversity and abundance of epibionts showing similar pattern to encrustation by microconchids described in Zatoń and Krawczyński (2011). The correlation coefficients in athyridids and rhynchonellids did not differ significantly (except in size-diversity of rhynchonellids); however, the close result to the critical r allows to assume that there was a positive correlation between the size and the number of sclerobionts. Besides, it highlights that athyridids are more heavily encrusted and show larger bryozoan colonies than rhynchonellids. Consequently, athyridids could have been exposed for longer time than rhynchonellids, indicated by the continuous growth of bryozoan colonies and the settlement of sclerobionts on previous layers of encrustation.
Regarding brachiopod morphology, Richards (1972), Hurst (1974), and Bordeaux and Brett (1990) found that the shape and size of ornamentation of brachiopod controlled the encrustation by sclerobionts. On the contrary, Zatoń and Krawczyński (2011) did not notice any preferences related to the substrate texture as they observed sclerobionts on brachiopod shells with costae or spines, as well as on smooth valves. In our study, all samples of rhynchonellids displayed costae, as well as the athyridid Hustedia connorsi, whereas the other athyridids were smooth. The results did not show significant differences in encrustation between ornamented or smooth brachiopods. Likewise, the frequencies of all sclerobionts observed did not display significant variance, even though there were 12 rhynchonellids and 14 athyridids with encrusting invertebrates (2.93 and 2.92, respectively). Therefore, the preference for particular host is not conclusive in both brachiopods from the Permian.
The analyses of disposition and distribution of epibionts are also useful to identify the life position of hosts. For example, the athyridid shells herein studied were encrusted in similar proportions by different epibionts. This pattern is similar to that described in atrypids by Webb and Schneider (2013), who refer that the settlement of sclerobionts on both valves was controlled by brachiopod life position (ventral or dorsal up) that changed in accordance to the host ontogeny. The behavior could have been similar in athyridids of the Paso Hondo Formation, where both valves were exposed to the colonization during their growth. In rhynchonellids, there were similar proportions of sclerobionts on both valves, most colonizing the anterior area. Even though the statistical analyses did not find any difference in occupation by sclerobionts on ventral and dorsal valves of athyridids and rhynchonellids, the dorsal valves were more frequently encrusted. This suggests that dorsal valves could have been exposed more time than the ventral valves during the life of the brachiopod and they were further away from the sediment.
Nonetheless, a similar sclerobiont distribution on both athyridid and rhynchonellid shells does not imply that all brachiopods had the same life orientation, as an analogous distribution of epibionts might also be related to different orientations of host shells. Another possibility would be a random colonization, but in this case, hosts’ specific life positions would not control the encrustation. This result is possibly supported by c2, which proved that sclerobionts settlement was mainly arbitrary on both brachiopod orders.
Syn vivo or post-mortem encrustation.—It is difficult to establish whether a fossil association of marine invertebrates represents a syn vivo association, as benthic invertebrates are commonly disarticulated and transported immediately after death. Even when fossils are preserved in life position, it is almost impossible to determine whether they all belong to a live association. To be sure that brachiopods were encrusted during their lives, we should control that valve interiors are free of encrustation and epibionts do not block the commissure, hinge line or pedicle foramen (Webb and Schneider 2013). In this study, we did not observe encrustation on the interior of brachiopod shells, though there were some specimens with encrusting bryozoans covering the commissure (two athyridids and eight rhynchonellids). Most likely bryozoans colonized the surface while brachiopods were alive and continued their growth after the death of the hosts, indicating that brachiopod shells were not immediately buried after their death. Although several brachiopods display bryozoan’s encrustation on the commissure, most samples did not have sclerobiont cover on the anterior aperture of shells, hinge, or foramen, indicating that brachiopods must have been encrusted during their life.
Furthermore, in all brachiopods, sclerobiont individuals and colonies of different sizes occur, indicating that both solitary and colonial encrusters settled on their hosts multiple times during the life of brachiopods. We did not notice any damage on brachiopod shells triggered by epibionts, such as borings, abrasion, or deformation, which shows that the sclerobionts did not have any negative effect on the host brachiopods. The relationship between brachiopod hosts and their sclerobionts was most likely commensal as brachiopods were used by sclerobionts mostly as a hard substrate for settlement. In addition, the epibionts might have served as a natural shield against other perpetrators. We classify this interaction as commensalism according to Schneider (2013), because we were able to identify that there was a positive gain for encrusters (i.e., substrate), but positive or negative effect on the host was undetectable.
Paleoenvironment.—As mentioned above, the settlement of sclerobionts can be influenced by different factors, such as the dynamics and physicochemical parameters of the environment (Barclay et al. 2013; Webb and Schneider 2013). In this context, the carbonate ramps are environments where the energy is usually significant in the shallow zone (Nichols 2009). The continuous movement of the water can prevent settlement of larvae and affect the survival of previously settled sclerobionts. The Paso Hondo Formation, mainly composed of calcareous rocks, represents the thickest unit of all lithostratigraphic formations of the Chicomuselo region. Throughout this unit, different changes in facies have been related to rises and falls of the sea level (Torres-Martínez et al. 2017). The studied association was deposited in calcarenite and limestone rocks, besides the host brachiopods were permineralized and mostly articulated. The studied strata are comprised of fossiliferous wackestone of gray to light brown matrix with abundant bryozoans, frequent crinoid ossicles, and rare fragments of spines and brachiopod shells, echinoderms, and ostracodes, as well as scattered organic matter. All facies features, along with the preservation of the fossil association, have allowed conclusion that the biota was deposited in open waters within a homoclinal ramp, characterized by low-medium energy, homogeneous salinity, good oxygenation, and high productivity and recurrent input of nutrients (Torres-Martínez et al. 2019b).
Age and paleobiogeography.—Regarding the age of the association we should stress that sclerobionts occurred on different host brachiopods: Wellerella lemasi, Tautosia transenna, Composita enormis, Composita hapsida, and Hustedia connorsi. These hosts along with other brachiopods associated, such as Dyoros (Tetragonetes) rectangulatus, Costispinifera rugatula, Echinosteges tuberculatus; Tropidelasma furcillatum, Neospirifer venezuelensis, and Texarina solita can be correlated with taxa from the Cherry Canyon (Getaway Member) and Road Canyon formations of Texas in the United States, establishing a Roadian age (middle Permian) for the bearing rocks (Torres-Martínez et al. 2019b).
On the other hand, during the early-middle Permian, the supercontinent Pangea had already been consolidated and marine faunas (e.g., fusulinids and brachiopods), formed different biotic provinces which composed three paleobiogeographical realms (Boreal, Paleoequatorial, and Gondwanan) (Shen et al. 2009, 2013). The presence of Pangea triggered several changes in all marine communities, favoring the generic and specific regionalization of some invertebrate groups and marine biota. In this context, it has been established that part of the biota from the upper Paleozoic of Chiapas, such as fusulinids (Thompson and Miller 1944; Kling 1960), ammonoids (Müllerried et al. 1941) and brachiopods (Torres-Martínez et al. 2016, 2018, 2019b), had a strong affinity with those faunas reported in Permian localities from New Mexico and Texas in USA, Coahuila and Sonora in Mexico, Huehuetenango in Guatemala, as well as Palmarito in Venezuela. These regions are areas where the Grandian province of North America was extended to, enduring from the Cisuralian to Guadalupian (Yancey 1975; Shen et al. 2009; Torres-Martínez et al. 2019b).
The sclerobionts on brachiopod shells from the Chicomuselo belonged to the same biotic province as their hosts. Most of encrusted brachiopods have been mentioned as typical taxa from the Roadian (early Guadalupian) of North America. Nonetheless, it is difficult to discuss the possible sclerobiont provinces as there are no previous records of a similar sclerobiont association in the Grandian province, and even there is no information on any other epibiont community from the middle Permian around the world. There are a few papers devoted to the Permian sclerobionts, they are not focused on the paleoecology of the community. For instance, Lisitsyn (1998) recorded Hederella carbonaria from Central Urals of Russia, accurately, in the right bank of the Ai River, at the base of Abdullino Reef from the Sterlitamak Formation. This material is older than our specimens since the taxon described by Lisitsyn is from the Sakmarian (early Cisuralian). The presence of the same species, in both east and west side of Pangea, could be due to the hederelloids migrated using the paleoequatorial current of Panthalassa; something previously proposed in other invertebrates, for example, brachiopods (Shen et al. 2011; Tazawa et al. 2016; Torres-Martínez et al. 2019a). Nevertheless, it is worth of noting that Condra and Elias (1944) described the holotype of this species from the Pennsylvanian of the USA. Likewise, Dunham and Stubblefield (1944) referred to Hederella cf. chesterensis (a synonym), and later, Bancroft (1986) reported to H. carbonaria, but from the Mississippian (Early Carboniferous) of Great Britain. Accordingly, this taxon had wide distribution in the late Paleozoic; however, there is still a scarcity of information of the group for the Cisuralian of North America, stressing that the only records are from the Middle Pennsylvanian (Moscovian–Kasimovian) (Condra and Elias 1944) and middle Permian (Roadian) (González-Mora et al. 2018).
Regarding microconchids, the species herein studied, recently described as Microconchus maya, is the only known Permian species of this genus (Heredia-Jiménez et al. 2020). Other species of Microconchus known from the late Paleozoic are M. hintonensis and M. cravenensis from the Mississippian of the United States (Zatoń and Peck 2013; Zatoń and Mundy 2020) and M. carbonarius from the Pennsylvanian of England and Canada (Zatoń et al. 2014). The only previous Permian record of the order Microconchida was made by Wilson et al. (2011), who described Helicoconchus elongatus from the Artinskian (late Cisuralian) of central Texas.
As mentioned above, the previous youngest record of microconchids, hederelloids, and bryozoans forming single community is from the Famennian (Late Devonian) of Russia (Zatoń and Borszcz 2013). It should be stressed that species composition of Devonian and Permian hederelloid and microconchid associations is different, though there is similarity to the late Paleozoic at a higher taxonomic level. No other association of this type had ever been recorded in any Permian region. Herewith, the Roadian association from the Paso Hondo Formation represents the first globally confirmed record of Devonian-like sclerobiont community in the Permian.
Conclusions
The sclerobionts (microconchids, hederelloids, bryozoans, and crinoids) encrust different brachiopod shells (Athyridida and Rhynchonellida) from the Paso Hondo Formation of Chiapas, Mexico. The most common associations among sclerobionts were bryozoans-hederelloids association and bryozoans-microconchids association. In some cases, both hederelloids and microconchids are encrusting bryozoans, that were previously settled on host brachiopods. The average settlement density is comparable to the Devonian associations.
Several factors could have influenced the settlement and distribution of epibionts on the host valves such as inhalant and exhalant currents of brachiopods, age of the host, and tendency of sclerobionts to settle as far as possible from the sediment-water interface. Nonetheless, there was no significant preference for some particular region of the host shell, suggesting that colonization of epibionts was rather random.
The encrustation of brachiopods by sclerobionts took place syn vivo, deduced by a similar number of epibionts on both valves and the absence of sclerobionts covering either on the commissure or foramen of brachiopods. The host shells did not display any kind of damage produced by sclerobionts.
A positive correlation between brachiopod size and diversity or abundance of epibionts was observed, as the dorsal valves were slightly more colonized than the opposite valves. On the other hand, we did not detect a significant difference in the encrustation between ornamented and smooth brachiopods, as well as between athyridids and rhynchonellids.
The studied association was deposited in open waters on a homoclinal ramp, characterized by low-middle energy, good oxygenation, high productivity, and normal salinity. The age of the encrusting community is corroborated by the stratigraphic distribution of hosts and other associated brachiopods, dated as the Roadian (early Guadalupian) in age.
Despite the host taxa were recorded from the Grandian Province, biogeography of the studied sclerobiont association (microconchids, hederelloids, and bryozoans on brachiopods) is unknown. This association appears to be the youngest occurrence of this type of sclerobiont community because the previously described similar associations are known from the Famennian (Late Devonian) of Russia.
Acknowledgements
We thank Daniela Heredia-Jiménez and Sergio González-Mora (both Posgrado en Ciencias Biológicas, UNAM) for their assistance in identifying specimens, as well as Paola Porras-López (freelance photographer, Mexico City, Mexico) for brachiopods’ photos, and Margarita Reyes-Salas and Sonia Ángeles García (both Laboratorio de Microscopía Electrónica y Microanálisis, LANGEM-UNAM) for SEM images. Equally, we are grateful to Andrej Ernst (University of Hamburg, Germany), Michał Zatoń (University of Silesia, Katowice, Poland), Mark Wilson (College of Wooster, Ohio, USA), and Przemysław Gorzelak (Institute of Paleobiology PAS, Warsaw, Poland) for all comments and suggestions which widely improved the original manuscript. MATM thanks to projects PAPIIT IA102618 and IA103920 (DGAPA-UNAM) which supported this work. Likewise, MATM thanks to project PAPIIT IA102618 (DGAPA-UNAM) which supported the bachelor’s degree thesis of LMA whence this manuscript was partially derived. Financial support to OV was provided by Estonian Research Council Project IUT20-34.
References
Ager, D.V. 1961. The epifauna of a Devonian spiriferid. Quarterly Journal of the Geological Society of London 465: 1–10. Crossref
Agostini, V.O., do Nascimento Ritter, M., Macedo, A.J., Muxagata, E., and Erthal, F. 2017. What determines sclerobiont colonization on marine mollusk shells? PLoS ONE 12: e0184745. Crossref
Álvarez, F. and Taylor, P.D. 1987. Epizoan ecology and interactions in the Devonian of Spain. Palaeogeography, Palaeoclimatology, Palaeoecology 61: 17–31. Crossref
Bancroft, A.J. 1986. Hederella carbonaria Condra and Elias, a rare ?bryozoan from the Carboniferous of Great Britain. Proceedings of the Geologistsʼ Association 97: 243–248. Crossref
Barclay, K.M., Schneider, C.L., and Leighton, L.R. 2013. Palaeoecology of Devonian sclerobionts and their brachiopod hosts from the Western Canadian Sedimentary Basin, Palaeogeography, Palaeoclimatology, Palaeoecology 383–384: 79–91. Crossref
Bordeaux, Y.L. and Brett, C.E. 1990. Substrate specific associations of epibionts on Middle Devonian brachiopods: implications for paleoecology. Historical Biology 4: 203–220. Crossref
Condra, G.E. and Elias, M.K. 1944. Hederella and Corynotrypa from the Pennsylvanian. Journal of Paleontology 18: 535–539.
Cooper, G.A. and Grant, R.E. 1976. Permian brachiopods of West Texas IV. Smithsonian Contributions to Paleobiology 21: 1923–2607. Crossref
Copper, P. 2004. Silurian (late Llandovery-Ludlow) Atrypid Brachiopods from Gotland, Sweden, and the Welsh Borderlands, Great Britain. 220 pp. National Research Council of Canada, Ottawa. Crossref
Dunham, K.C. and Stubblefield, C.J. 1944. The stratigraphy, structure and mineralization of the Greenhow mining area, Yorkshire. Quarterly Journal of the Geological Society 100: 209–268. Crossref
González-Mora, S., Wyse Jackson, P.N., Torres-Martínez, M.A., Buitrón-Sánchez, B.E., Barragán, R., and Sour-Tovar, F. 2018. Hederella carbonaria Condra & Elias, 1944 from the Roadian (Middle Permian) of Mexico. Bulletin of Geosciences 93: 457–461. Crossref
Gutiérrez-Gil, R. 1956. Bosquejo Geológico del estado de Chiapas. In: M. Maldonado-Koerdell (ed.), XX Congreso Geológico Internacional, Geología del Mesozoico y estratigrafía pérmica del Estado de Chiapas, Libreto guía de la excursión C-15, 9–32, Servicio Geológico Mexicano, Ciudad de México.
Heredia-Jiménez, D.P., Vinn, O., Buitrón-Sánchez, B.E., and Torres-Martínez, M.A. 2020. A new middle Permian microconchid from Chiapas, Mexico, and its paleoecological implications. Palaeobiodiversity and Palaeoenvironments 100: 975–983. Crossref
Hoare, R.D. and Steller, D.L. 1967. A Devonian brachiopod with epifauna. Ohio Journal of Science 67: 291–297.
Hurst, J.M. 1974. Selective epizoan encrustation of some Silurian brachiopods from Gotland. Palaeontology 17: 423–429.
Ippolitov, A.P., Vinn, O., Kupriyanova, E.K., and Jäger, M. 2014. Written in stone: history of serpulid polychaetes through time. Memoirs of Museum Victoria 71: 123–159. Crossref
Jäger, M. 2004. Serpulidae und Spirorbidae (Polychaeta sedentaria) aus Campan und Maastricht von Norddeutschland, den Niederlanden, Belgien und angrenzenden Gebieten. Geologisches Jahrbuch A 157: 121–249.
Kesling, R.V., Hoare, R.D., and Sparks, D.K. 1980. Epizoans of the Middle Devonian brachiopod Paraspirifer bownockeri: their relationships of one another and to their host. Journal of Paleontology 54: 1141–1154.
Kling, S.A. 1960. Permian fusulinids from Guatemala. Journal of Paleontology 34: 637–655.
Lescinsky, H.L. 1997. Epibiont communities: Recruitment and competition on North American Carboniferous brachiopods. Journal of Paleontology 71: 34–53. Crossref
Lisitsyn, D.V. 1998. The first Permian find of the genus Hederella (Bryozoa). Paleontological Journal 32: 589–591.
Müllerried, F.K.G., Miller, A.K., and Furnish, W.M. 1941. The middle Permian of Chiapas, southernmost Mexico, and its fauna. American Journal of Science 239: 397–406. Crossref
Newell, N.D. and Boyd, D.W. 1970. Oyster-like Permian Bivalvia. Bulletin of the American Museum of Natural History 143: 217–282.
Nichols, G. 2009. Sedimentology and Stratigraphy. 419 pp. Wiley-Blackwell, Oxford.
Richards, R.P. 1972. Autecology of Richmondian brachiopods (Late Ordovician of Indiana and Ohio). Journal of Paleontology 46: 386–405.
Rodland, D.L., Kowalewski, M., Carroll, M., and Simoes, M.G. 2006. The temporal resolution of sclerobiont assemblages: are they ecological snapshots or overexposures? Journal of Geology 114: 313–324. Crossref
Schneider, C.L. 2013. Epibiosis across the Late Devonian biotic crisis: a review. Proceedings of the Geologists’ Association 124: 893–909. Crossref
Shen, S.Z., Tazawa, J., and Miyake, Y. 2011. A Kungurian (early Permian) Panthalassan brachiopod fauna from Hatahoko in the Mino belt, Central Japan. Journal of Paleontology 85: 553–566. Crossref
Shen, S.Z., Xie, J.F., Zhang, H., and Shi, G.R. 2009. Roadian–Wordian (Guadalupian, Middle Permian) global palaeobiogeography of brachiopods. Global and Planetary Change 65: 166–181. Crossref
Shen, S.Z., Zhang, H., Shi, G.R., Li, W.Z., Xie, J.F., Mu, L., and Fan, J.X. 2013. Early Permian (Cisuralian) global brachiopod palaeobiogeography. Gondwana Research 24: 104–124. Crossref
Smrecak, T.A. and Brett, C.E. 2014. Establishing patterns in sclerobiont distribution in a Late Ordovician (Cincinnatian) depth gradient: toward a sclerobiofacies model. Palaios 29: 74–85. Crossref
Stehli, F.G. and Grant, R.E. 1970. Permian brachiopods from Huehuetenango, Guatemala. Journal of Paleontology 44: 23–36.
Steller, D.L. 1965. The Epifaunal Elements on the Brachiopoda of the Silica Formation. 79 pp. Unpublished Ph.D. Thesis, Bowling Green State University, Ohio.
Taylor, P.D. 1985. Carboniferous and Permian species of the cyclostome bryozoan Corynotrypa Bassler, 1911 and their clonal propagation. Bulletin of the British Museum, Natural History, Geology Series 38: 359–372.
Taylor, P.D. 2016. Competition between encrusters on marine hard substrates and its fossil record. Palaeontology 59: 481–497. Crossref
Taylor, P.D. and Vinn, O. 2006. Convergent morphology in small spiral worm tubes (“Spirorbis”) and its palaeoenvironmental implications. Journal of the Geological Society 163: 225–228. Crossref
Taylor, P.D. and Wilson, M.A. 2003. Palaeoecology and evolution of marine hard substrate communities. Earth Science Reviews 62: 1–103. Crossref
Taylor, P.D. and Wilson, M.A. 2008. Morphology and affinities of hederelloid “bryozoans”. In: S.J. Hageman, M.M. Key, Jr., and J.E. Winston (eds.), Bryozoan Studies 2007, Proceedings of the 14th IBA Conference, 301–309. Virginia Museum of Natural History, North Carolina.
Tazawa, J., Okumura, Y., Miyake, Y., and Mizuhara, T. 2016. A Kungurian (early Permian) brachiopod fauna from Ogama, Kuzu area, central Japan, and its palaeobiogeographical affinity with the Wolfcampian–Leonardian (early Permian) brachiopod fauna of West Texas, USA. Paleontological Research 20: 367–384. Crossref
Thompson, M.L. and Miller, A.K. 1944. The Permian of southernmost Mexico and its fusulinid faunas. Journal of Paleontology 18: 481–504.
Torres-Martínez, M.A., Barragán, R., Sour-Tovar, F., and González-Mora, S. 2017. Depositional paleoenvironments of the Lower Permian (upper Cisuralian) carbonate succession of Paso Hondo Formation in Chiapas State, southeastern Mexico. Journal of South American Earth Sciences 79: 254–263. Crossref
Torres-Martínez, M.A., Heredia-Jiménez, D.P., Quiroz-Barroso, S.A., Navas-Parejo, P., Sour-Tovar, F., and Quiroz-Barragán, J. 2019a. A Permian (late Guadalupian) brachiopod fauna from northeast Mexico and their paleobiogeographic affinities. Journal of South American Earth Sciences 92: 41–55. Crossref
Torres-Martínez, M.A., Heredia-Jiménez, D.P., Sour-Tovar, F., Buitrón-Sánchez, B.E., and Barragán, R. 2019b. Permian brachiopods from Chiapas, Mexico: new stratigraphical and paleobiogeographical insights. Paläontologische Zeitschrift 93: 607–624. Crossref
Torres-Martínez, M.A., Sour-Tovar, F., and Barragán, R. 2016. Permian (Leonardian) brachiopods from Paso Hondo Formation, Chiapas, southern Mexico. Paleobiogeographical implications. Journal of South American Earth Sciences 71: 71–81. Crossref
Torres-Martínez, M.A., Sour-Tovar, F., and Barragán, R. 2018. Kukulkanus, a new genus of buxtoniin brachiopod from the Artinskian-Kungurian (Early Permian) of Mexico. Alcheringa 42: 268–275. Crossref
Torres-Martínez, M.A., Villanueva-Olea, R., and Sour-Tovar, F. 2020. Columnar ossicles of Permian crinoids, including two new genera, from the Grupera Formation (Asselian‒Sakmarian) of Chiapas, Mexico. Boletín de la Sociedad Geológica Mexicana 72: 1–17. Crossref
Webb, A.E. and Schneider, C.L. 2013. Ecology of an encrusting fauna on Desquamatia (Atrypida, Brachiopoda) from Cedar Valley Formation (Givetian, Devonian) of Iowa, USA. Palaeogeography, Palaeoclimatology, Palaeoecology 377: 102–109. Crossref
Weber, B., Schaaf, P., Valencia, V.A., Iriondo, A., and Ortega-Gutierrez, F. 2006. Provenance ages of late Paleozoic sandstones (Santa Rosa Formation) from the Maya block, SE Mexico. Implications on the tectonic evolution of western Pangea. Revista Mexicana de Ciencias Geológicas 23: 262–276.
Wilson, M.A., Vinn, O., and Yancey, T.E. 2011. A new microconchid tubeworm from the Artinskian (lower Permian) of central Texas, U.S.A. Acta Palaeontologica Polonica 56: 785–791. Crossref
Yancey, T.E. 1975. Permian marine biotic provinces in North America. Journal of Paleontology 49: 758–766.
Zatoń, M. and Borszcz, T. 2013. Encrustation patterns on post-extinction early Famennian (Late Devonian) brachiopods from Russia. Historical Biology 25: 1–12. Crossref
Zatoń, M. and Jarochowska, E. 2020. Enigmatic encrusting fossils from the Upper Devonian of Russia: probable Rothpletzella microproblematica preserved in three dimensions. Historical Biology 32: 837–847. Crossref
Zatoń, M. and Krawczyński, W. 2011. Microconchid tubeworms across the upper Frasnian–lower Famennian interval in the Central Devonian Field, Russia. Palaeontology 54: 1455–1473. Crossref
Zatoń, M. and Mundy, D.J.C. 2020. Microconchus cravenensis n. sp.: a giant among microconchid tubeworms. Journal of Paleontology [published online, http://dx.doi.org/10.1017/jpa.2020.45]. Crossref
Zatoń, M. and Olempska, E. 2017. A family-level classification of the Order Microconchida (Class Tentaculita) and the description of two new microconchid genera. Historical Biology 29: 885–894. Crossref
Zatoń, M. and Peck, R.L. 2013. Morphology and palaeoecology of new, non-marine microconchid tubeworm from Lower Carboniferous (Upper Mississippian) of West Virginia, USA. Annales Societatis Geologorum Poloniae 83: 37–50.
Zatoń, M., Borszcz, T., and Rakociński, M. 2017. Temporal dynamics of encrusting communities during the Late Devonian: a case study from the Central Devonian Field, Russia. Paleobiology, 43: 550–568. Crossref
Zatoń, M., Grey, M., and Vinn, O. 2014. Microconchid tubeworms (Class Tentaculita) from the Joggins Formation (Pennsylvanian), Nova Scotia, Canada. Canadian Journal of Earth Sciences 51: 669–676. Crossref
Zhan, R. and Vinn, O. 2007. Cornulitid epibionts on brachiopod shells from the Late Ordovician (middle Ashgill) of East China. Estonian Journal of Earth Sciences 56: 101–108.
Acta Palaeontol. Pol. 66 (1): 131–141, 2021
https://doi.org/10.4202/app.00777.2020