

Systematics and paleobiology of Carnivora and Hyaenodonta from the lower Miocene of Buluk, Kenya
MICHAEL MORLO, ANTHONY FRISCIA, ELLEN R. MILLER, ELLIS LOCKE, and ISAIAH NENGO
Morlo, M., Friscia, A., Miller, E.R., Locke, E., and Nengo, I. 2021. Systematics and paleobiology of Carnivora and Hyaenodonta from the lower Miocene of Kenya. Acta Palaeontologica Polonica 66 (2): 465–484.
Early Miocene carnivorous mammals from Buluk, Kenya, are described and discussed. Four taxa belonging to Hyaenodonta and four belonging to Carnivora are identified. Members of Hyaenodonta include Hyainailouros sulzeri, Hyainailouros cf. napakensis, a third taxon about the size of Leakitherium, represented only by postcranial material, and a fourth taxon represented by an edentulous jaw, in the size range of Sectisodon. Members of Carnivora include a new species of Cynelos jitu, which represents the largest species of Cynelos known. The first m2 of Cynelos macrodon is described, and the differentiation of this species from Cynelos ginsburgi and Cynelos peignei is confirmed. A third carnivoran species is represented by a mandibular fragment attributed to a viverrid similar to Mioprionodon, and a fourth taxon is represented by a feliform distal humerus, the size of that of a small cat. An ecomorphological guild structure analysis reveals that the Buluk carnivore have estimated body sizes spanning from <1 kg to >100 kg. Three very large species (>100 kg), and another two in the 30–100 kg range are present, while only two taxa are present in the 3–10 kg category. Carnivores in the 1–3 kg and the 10–30 kg categories are absent. Locomotor pattern could be obtained for only four taxa, and all are characterized by terrestrial locomotion. A minimum of three dietary classes (insectivorous, carnivorous, scavenging) are represented. The co-occurrence of multiple very large carnivores is not uncommon in early Miocene faunas, but the taphonomy of Buluk may also contribute to the favored preservation of larger and terrestrially adapted animals.
Key words: Mammalia, Carnivora, Amphicyonidae, Hyainailouridae, Viverridae, guild structure, Miocene, Kenya.
Michael Morlo [Michael.Morlo@senckenberg.de], Senckenberg Forschungsinstitut und Naturmuseum, Abt. Messelforschung und Mammalogie, Senckenberganlage 25, 60325 Frankfurt am Main, Germany.
Anthony Friscia [tonyf@ucla.edu], Department of Integrative Biology and Physiology, University of California, Los Angeles, Los Angeles, California 90095 USA.
Ellen R. Miller [millerer@wfu.edu], Wake Forest University, Department of Anthropology, Winston Salem, North Carolina 27109-7807, USA.
Ellis Locke [ellis.locke@asu.edu], Institute of Human Origins, School of Human Evolution and Social Change, Arizona State University, Tempe, Arizona 85281 USA.
Isaiah Nengo [isaiah.nengo@stonybrook.edu], Turkana Basin Institute, Social and Behavioral Sciences, Stony Brook University, Stony Brook, New York 11794 USA.
Received 20 July 2020, accepted 18 December 2020, available online 8 June 2021.
Copyright © 2021 M. Morlo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The occurrence of the early Miocene fossil mammals at Buluk, Kenya, has been known for about fifty years (Harris and Watkins 1974), but owing to the remote location of the deposits, relatively little fieldwork had been done in the area until recently. In the mid-1980’s, Richard E.F. Leakey and Alan C. Walker announced the recovery of a diverse assemblage of early Miocene mammals (ca. 20 species in 17 genera) from the site (Leakey and Walker 1985). This included the remains of primitive Old World monkeys (cercopithecoids) and apes (hominoids), which made the locality an important one for paleoanthropology (Leakey 1985; Miller et al. 2009; Locke et al. 2020). In the same publication, Leakey and Walker (1985) recognized two large carnivorous mammals in the Buluk collection: Hyainailouros nyanzae, and a second taxon identified only as a “very large amphicyonine” (Leakey and Walker 1985: 174). Twenty years later, short expeditions to Buluk in 2004 and 2009, followed by more intensive survey and excavation beginning in 2013, have recovered approximately 1000 new vertebrate fossils, including 44 specimens representing carnivorous mammals. Aside from the identification of the amphicyonid Cynelos sp. among the assemblage (Anemone et al. 2005), and the informal suggestion that the “large Amphicyonidae indet.” (Werdelin and Peigné 2010: 604, table 32.1) may represent an additional species of Cynelos (Morlo et al. 2007, 2019), this contribution presents the first systematic work on the Buluk carnivorous mammals, and the first ecomorphological guild structure analysis for an African mammalian carnivorous fauna.
Institutional abbreviations.—KNM, National Museums of Kenya, Nairobi, Kenya; NHM, Natural History Museum, London, UK; NWSW, Naturwissenschaftliche Sammlung der Stadt Winterthur, Switzerland; TBI, Turkana Basin Institute, Ileret, Kenya.
Other abbreviations.—C/c, upper/lower canine; I/i, upper/lower incisor; M/m, upper/lower molar; P/p, upper/lower premolar; RBL, relative blade length.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:CEA1AD08-302B-4578-8C87-BC8C22B71FDE
Geological setting
Buluk is located in northern Kenya (N 4.257487 E 36.59267), east of Lake Turkana (Fig. 1). Fossils at Buluk are recovered from the lower section of the Buluk Member, Bakate Formation, which is dated to the latest early Miocene (McDougall and Watkins 1985).
During the early Miocene, Buluk was occupied by a large, mature river system with a broad floodplain, and all fossil specimens are recovered from channel lag deposits associated with this fluviatile system (Watkins 1989). The presence of petrified tree trunks up to 1 m in diameter, at Buluk and elsewhere in the Bakate Formation, suggests the presence of woody cover consisting of deciduous forest or woodland (Watkins 1989; Wheeler et al. 2007; Leakey et al. 2011). Paleosol chemistry indicates a seasonal subhumid to subarid paleoclimate at the time the Buluk mammals occupied this region (Lukens et al. 2017).
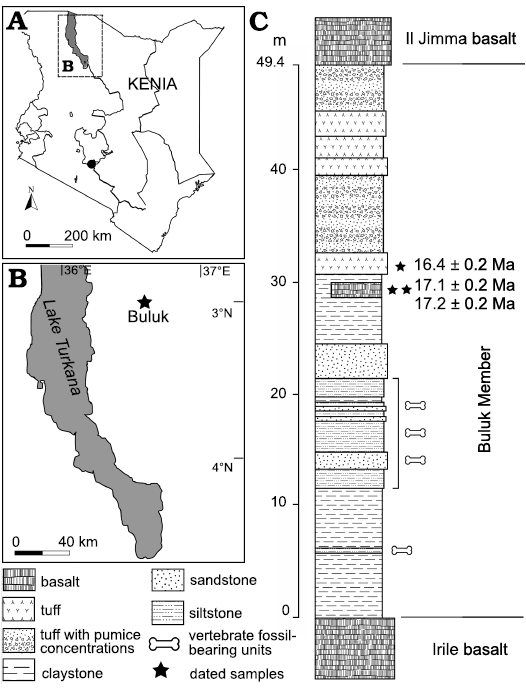
Fig. 1. A. Geographic map of Kenya with the position of studied area (A) and the Buluk (early Miocene) fossil locality (B). Simplified stratigraphic column, of the Buluk Member, Bakate Formation (C), indicating the fossil beds in relation to where radiometric dates have been obtained.
Material and methods
Fossil specimens collected at Buluk before 2013 are housed at the National Museums of Kenya, Nairobi, and specimens collected after this year are housed at the Turkana Basin Institute (TBI), Ileret.
Detailed illustrations of dental and postcranial material that cannot be assigned to a carnivore taxon are provided in the SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Morlo_etal_SOM.pdf.
Terminology and measurements.—Dental terminology is derived from Smith and Dodson (2003), and dental measurements follow Peigné and Heizmann (2003). Specimens were measured with calipers to the nearest 0.1 mm, and all measurements are given in mm. Length of teeth refers to the greatest anterior-posterior dimension of the tooth crown, and width to the greatest buccolingual breadth. If teeth were missing, the alveoli were measured and results are given in brackets.
Ecomorphology.—We use a combination of three paleobiological parameters to define the ecomorphology of a taxon: body mass, locomotor pattern, and dietary category (Morlo 1999). Since the necessary data are only partly obtainable from the mostly fragmentary remains of carnivores from Buluk, data from comparable taxa were incorporated in places.
The body mass of a carnivorous mammal defines a variety of ecological parameters often correlating with dietary preferences and behavior (see Morlo et al. 2010, 2020 and references therein). For organizing body mass data, we use the classes introduced by Morlo (1999): (i) <1 kg, (ii) 1–3 kg, (iii) 3–10 kg, (iv) 10–30 kg, (v) 30–100 kg, and (vi) >100 kg.
After body mass, locomotor pattern is the second most important factor separating different guild structures in carnivores (Morlo et al. 2010). Locomotor categories were assigned based on taxon-specific information from Ginsburg (1980), Van Valkenburgh (1987), and Argot (2010).
For classes of locomotor patterns, we follow Morlo et al. (2020) in separating: (i) arboreal, (ii) scansorial, (iii) terrestrial, (iv) cursorial, (v) semifossorial, (vi) semiaquatic, and (vii) unknown locomotor pattern.
For dietary preferences we follow Van Valkenburgh (1988) in using: (i) hypercarnivorous, (ii) bone/meat, (iii) carnivorous, and (iv) hypocarnivorous, but we add (v) insectivorous as separate from the hypocarnivorous class, based on tooth shape (Friscia et al. 2007; Nagel and Koufos 2009; Morlo et al. 2020).
Systematic palaeontology
Mammalia Linnaeus, 1758
Order Hyaenodonta Van Valen, 1967
Family Hyainailouridae Pilgrim, 1932 (sensu Borths et al. 2016)
Remarks.—Concerning higher systematics within Hyaenodonta we follow Solé et al. (2015), Borths et al. (2016), and Borths and Stevens (2019). Hyainailouridae here includes Hyainailourinae Pilgrim, 1932, and Apterodontinae Szalay, 1967, after Borths et al. (2016).
Subfamily Hyainailourinae Pilgrim, 1932 (sensu Solé et al. 2015)
Genus Hyainailouros Biedermann, 1863
Type species: Hyainailouros sulzeri Biedermann, 1863; Middle Miocene (MN 6) of Veltheim, Switzerland.
Remarks.—A revised synonymy was provided by Lewis and Morlo (2010), but following Morlo et al. (2007) we additionally recognize Megistotherium Savage, 1973, as a junior synonym of Hyainailouros. In this contribution, we focus on the African members of this genus.
Our recognition of Megistotherium as a junior synonym of Hyainailouros (Morlo et al. 2007) contrasts with the view of Borths et al. (2016) and Borths and Stevens (2019), who view the genus as the sister taxon to Leakitherium Savage, 1965. The material from Buluk does not contribute new evidence toward resolving this issue.
Hyainailouros sulzeri Biedermann, 1863
Fig. 2A–N, Table 1.
For synonymy list see Morales and Pickford (2017).
Holotype: NWSW, left maxilla with I3, C, P2–M1, and right mandible with c, p4, m2–m3.
Type locality: Veltheim, Switzerland (Biedermann 1863: pls. 4, 5; Helbing 1925: figs. 7–11, pl. 6; Beaumont 1970: figs. 1, 2; Ginsburg 1980: figs. 1, 2).
Type horizon: Middle Miocene (MN 6).
Material.—KNM-WS 12624, right maxillary fragment with P1; KNM-WS 12626, partial left P2; KNM-WS 12628, posterior fragment of a right P3; KNM-WS 12662, left P4; KNM-WS 12627, proximal left femur; KNM-WS 12620, distal part of a metatarsal III or IV; KNM-WS 65874, left p3. All from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Description.—The right P1 preserved in KNM-WS 12624 (Fig. 2A) is unicuspid with the paracone located centrally. The preparacrista curves strongly lingually and ends at the antero-lingual corner of the tooth. The postparacrista is positioned centrally and terminates at the most posterior point of the tooth. The specimen lacks a cingulum. A facet indicating horizontal abrasion is visible on the lingual aspect of the tooth, between the paracone and the tooth base.
The isolated left P2 KNM-WS 12626 (Fig. 2B) resembles P1 but is slightly larger. As in P1, the unicuspid tooth has a lingually curving preparacrista, a centrally running postparacrista, and lacks a cingulum. The posterior end of the postparacrista is obscured by breakage. The paracone has an abrasion facet.
KNM-WS 12628 (Fig. 2C) is a right, posterior fragment of a premolar, which we identify as a P3 because it has a straight cingulum rather than a curved cingulum as would be typical of a P4 (see the P4 of KNM-WS 12662 of Hyainailouros sulzeri and Simbakubwa kutokaafrika Borths and Stevens, 2019). In H. napakensis and H. ostheolastes, the lingual cingulum of P4 is also more curved than in KNM-WS 12628. Moreover, the metastyle of KNM-WS 12628 is clearly shorter than that of KNM-WS 12662. We thus interpret this fragment as a P3.
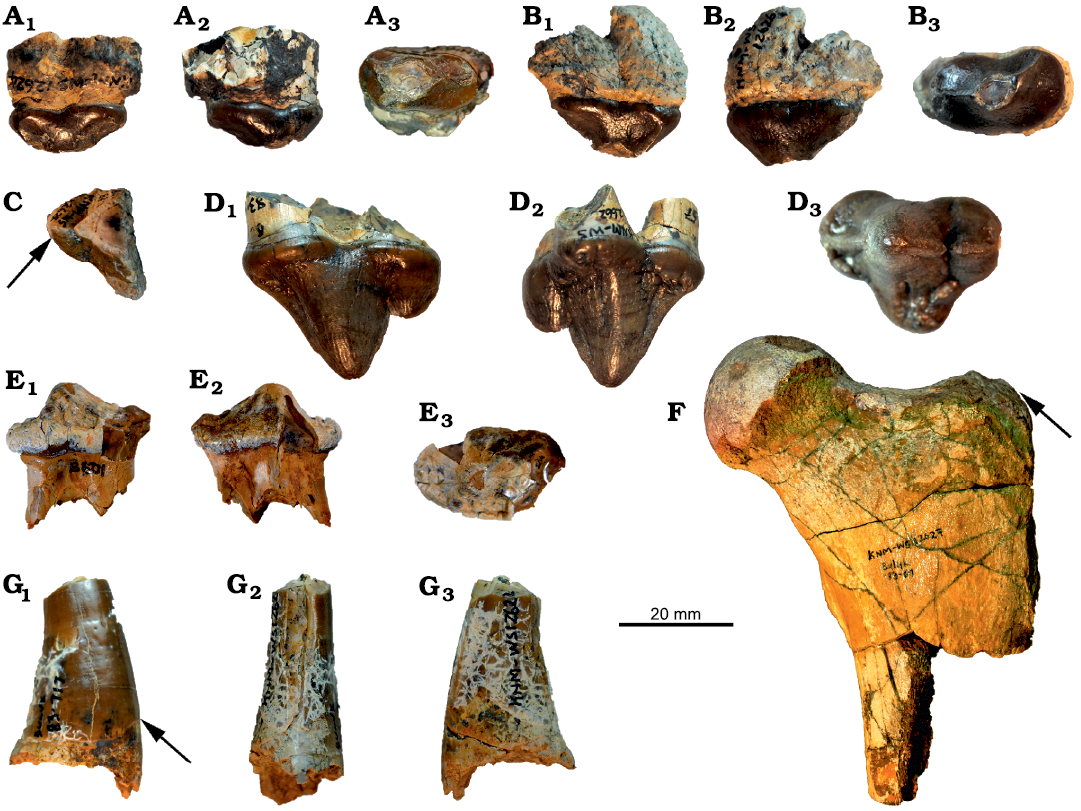
Fig. 2. Hyaenodonts Hyainailouros sulzeri Biedermann, 1863 (A–F) and Hyainailouros cf. napakensis (G), from the lower Miocene of Buluk, Kenya. A. KNM-WS 12624, isolated right P1 in buccal (A1), lingual (A2), and occlusal (A3) views. B. KNM-WS 12626, isolated left P2 in buccal (B1), lingual (B2), and occlusal (B3) views. C. KNM-WS 12628, posterior fragment of a right P3 in occlusal view. D. KNM-WS 12662, isolated left P4 in buccal (D1), lingual (D2), and occlusal (D3) views, notice size difference between P3 and P4 and different shape of lingual cingulum indicated by the arrow. E. KNM-WS 65874, isolated left p3 in in buccal (E1), lingual (E2), and occlusal (E3) views. F. KNM-WS 12627, proximal left femur in anterior view, notice low condyle, indicated by the arrow, being about as high as femoral head. G. KNM-WS 12622, right lower canine in buccal (G1), posterior (G2), and mesial (G3) views, notice basal enamel border, indicated by the arrow, concave towards the tip of the tooth.
Table 1. Dental measurements (in mm) of Hyaenodonta from the lower Miocene of Buluk, Kenya. Abbreviations: L, length; W, width; (), measurements inferred from alveoli.
|
Taxon |
Specimen |
|
P1 |
P2 |
P4 |
c |
p3 |
m2 |
m3 |
|
Hyainailouros sulzeri Biedermann, 1963 |
KNM-WS 12624 |
L |
19.9 |
|
|
|
|
|
|
|
W |
10.0 |
|
|
|
|
|
|
||
|
KNM-WS 12626 |
L |
|
22.9 |
|
|
|
|
|
|
|
W |
|
13.3 |
|
|
|
|
|
||
|
KNM-WS 12662 |
L |
|
|
|
|
26.0 |
|
|
|
|
W |
|
|
|
|
16.2 |
|
|
||
|
KNM-WS 65874 |
L |
|
|
31.4 |
|
|
|
|
|
|
W |
|
|
23.3 |
|
|
|
|
||
|
Hyainailouros cf. napakensis |
KNM-WS 12622 |
L |
|
|
|
12.4 |
|
|
|
|
W |
|
|
|
22.7 |
|
|
|
||
|
Hyaenodonta indet. B, size of Sectisodon |
KNM-WS 65730 |
L |
|
|
|
|
|
(9.8) |
(10.2) |
The P4 KNM-WS 12662 (Fig. 2D) is slightly larger than the P4 in the holotypes of S. kutokaafrika and H. sulzeri, but the Buluk specimen shares the high P4 length/width index present in these two taxa. The Buluk P4 also resembles other specimens of H. sulzeri (Helbing 1925; Ginsburg 1980: fig. 3), in exhibiting a characteristic symmetry, produced by having the parastyle nearly as long as the metastyle. In S. kutokaafrika the parastyle is less pronounced. The metastyle in KNM-WS 12662 is separated from the paracone by a deep notch, and the short protocone is located directly below the paracone. The protocone in H. ostheolastes and H. napakensis is longer than that in H. sulzeri. A strong cingulum surrounds the tooth. The dental enamel is rugose with small horizontal striations.
The isolated left p3 KNM-WS 65874 (Fig. 2E) is low crowned, unicuspid, and surrounded by a strong cingulid, which curves slightly lingually directly lingual to the paraconid.
The proximal left femur KNM-WS 12627 (Fig. 2F) resembles, and is similar in size to, a femur of H. sulzeri known from Europe (Ginsburg 1980: fig. 33). In particular, the size and placement of the third trochanter on KNM-WS 12627 is identical to that in the European specimen, and the proximal end, in both the Buluk and European specimens, resembles Amphicyon major (Blainville, 1841) (see Argot 2010: fig. 8E) in having the greater trochanter only slightly lower than the femoral head. In addition, the overall size of KNM-WS 12627 suggests that it represents an animal larger than a lion, which places the Buluk specimen in a size class exceeding what would be expected for even the very large species of Cynelos from Buluk.
KNM-WS 12620 preserves the distal part of metatarsal III or IV, and the specimen resembles the metatarsals of European H. sulzeri. The Buluk metatarsal has a width of 27.3 mm, which more closely approximates the size of metatarsal III than metatarsal IV (see Ginsburg 1980: fig. 42), and KNM-WS 12620 is also much larger than what would be expected for either African species of Amphicyon Lartet in Michelin, 1836 (Morales et al. 2003) or the very large species of Cynelos from Buluk.
Remarks.—Specimens from Buluk attributed to H. sulzeri are directly comparable to the European holotype material in both size and morphology (see Ginsburg 1980: fig. 1). In particular, the P4 from Buluk, KNM-WS 12662, resembles H. sulzeri and differs from S. kutokaafrika (Borths and Stevens 2019: fig 6) in having a stronger parastyle, longer metastyle, and a narrower paracone. The P3 fragment, KNM-WS 12628, is also reminiscent of the H. sulzeri type material, but it is possible that KNM-WS 12628 represents a P4 of H. napakensis. This alternative interpretation is less likely, because the metastyle in the Buluk specimen seems to be less well developed than in H. napakensis, and the lingual cingulum is less curved (see Borths and Stevens 2019: fig. 6B). The postcranial remains are attributed to H. sulzeri on the basis of their similarity to European specimens of this species, and because their size exceeds that of African Amphicyon and Cynelos from Buluk.
The Buluk material of H. sulzeri further documents the broad geographic range of this genus in Africa (Morlo et al. 2007; Lewis and Morlo 2010; Morales and Pickford 2017), especially if the North African “Megistotherium” osteothlastes is regarded as Hyainailouros osteothlastes (after Morlo et al. 2007 and Morales and Pickford 2017). The P4 parastyle of the Buluk specimen is as long as it is in Simbakubwa. P4 parastyle length, however, differs intra- and interspecifically within the species of Hyainailouros, and it may be large or small in H. sulzeri (see Ginsburg 1980: figs. 3, 4). The P4 parastyle is also large in H. “fourtaui” (Morlo et al. 2007, = H. osteothlastes after Morales and Pickford 2017) from Moghra, Egypt, while it is short in both the holotype of H. ostheolastes and in H. napakensis. As all other large hyaenodontid specimens from Buluk can be assigned to H. sulzeri, we also assign the P4 to this species. As for a possibly varying orientation of the protocone in the upper molars, the Buluk material provides no new information.
Stratigraphic and geographic range.—Lower to middle Miocene of Europe and Africa (Lewis and Morlo 2010).
Hyainailouros cf. napakensis Ginsburg, 1980
Fig. 2G, Table 1.
Material.—KNM-WS 12622, right lower canine fragment, with the tip and root broken; from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Description.—The canine fragment KNM-WS 12622 (Fig. 2G) bears a serrated crest running from the point where the tip is broken to the inferior enamel border. Posterior to this crest, a groove is present, which runs vertically from the base towards the tip. Mesially, the enamel-dentine juncture curves up towards the tip of the tooth, which is a typical feature of hyaenodonts. A vertical attrition facet is present. In all these features, the tooth resembles the lower canines of H. sulzeri (Ginsburg 1980) and S. kutokaafrika (Borths and Stevens 2019), but KNM-WS 12622 is smaller than the canines in either of these two taxa. The Buluk specimen is also narrower buccolingually than would be expected for an amphicyonid, and shorter antero-posteriorly than would be expected for an early Miocene felid. The Buluk canine fragment is also too large to belong to any species of Anasinopa Savage, 1965, the second largest hyainailourid from Rusinga Island, Kenya, after Hyainailouros (Morales and Pickford 2017). We thus assign the tooth to H. cf. napakensis, as the smallest known species of Hyainailouros. The assignment of an isolated canine at the species level, however, can only be tentative.
Remarks.—The partial canine KNM-WS 12622 is the only specimen that suggests a second, smaller species of Hyainailouros may be present at Buluk. The tooth presents a typical hyainailourid morphology but is much smaller than in H. sulzeri. The occurrence of a second and smaller species of Hyainailouros at Buluk is not considered surprising, as the co-occurrence of two species of Hyainailouros, one large and one small has been documented previously in the Kenyan lower Miocene (Lewis and Morlo 2010; Friscia et al. 2020), mostly involving H. nyanzae, a species recently synonymized with H. napakensis (Morales and Pickford 2017).
Hyaenodonta indet. A (size of Leakitherium Savage, 1965)
Fig. 3A, B, Table 2.
Material.—KNM-WS 12619, proximal left ulna fragment; KNM-WS 12584, distal left tibia; from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Description.—KNM-WS 12619 (Fig. 3A) is a left proximal ulna fragment, with a maximum diameter of 25.8 mm, which is comparable in size to a large leopard (<100 kg) (Stein and Hayssen 2013). The length of the olecranon cannot be determined due to breakage, but morphologically the olecranon appears to bend strongly posteriorly as indicated by the strong posteriorly running anterior olecranon ridge. This is reminiscent of specimens of H. sulzeri (Ginsburg 1980: fig. 25).
KNM-WS 12584 (Fig. 3B) preserves the distal end of a left tibia, which, based on size alone, may belong to the same taxon as KNM-WS 12619. The morphology of the tibia is felid-like in being more similar to extant species of Panthera Oken, 1816, than Amphicyon (Argot 2010), but as in H. sulzeri, the medial malleolus is strong and more symmetrical than in members of Carnivora (see Ginsburg 1980: fig. 34). Moreover, no felid of this size is known from the lower Miocene of Africa. We thus refer this specimen to Hyaenodonta and suggest that the ulnar and tibial fragments may belong to the same species.

Fig. 3. Hyaenodonts Hyaenodonta indet. A, size of Leakitherium Savage, 1965 (A, B) and Hyaenodonta indet. B, size of Sectisodon Morales and Pickford, 2017 (C), from the lower Miocene of Buluk, Kenya. A. KNM-WS 12619, fragment of left ulna in anterior (A1) and lateral (A2) views, arrows point to very low anterior olecranon ridge. B. KNM-WS 12584, distal end of left tibia in anterior (B1), posterior (B2), and distal (B3) views. C. KNM-WS 65730, right edentulous jaw with broken alveoli of m1, alveoli of m2, and roots of m3 in buccal (C1) and occlusal (C2) views, notice length of molars increasing distally.
Table 2. Postcranial measurements (in mm) of Hyaenodonta and Carnivora from the lower Miocene of Buluk, Kenya. Abbreviations: L, length; W, width; La, anteroposterior length, Lo, olecranon length.
|
Taxon |
Specimen |
Skeletal element |
|
Measurement |
|
Hyaenodonta indet. A, size of Leakitherium |
KNM-WS 12584 |
distal end of left tibia |
L |
27.5 |
|
W |
36.9 |
|||
|
Cynelos jitu sp. nov. |
KNM-WS 65651 |
distal phalanx |
L |
6.4 |
|
W |
>31.0 |
|||
|
Feliformia indet. |
KNM-WS 65365 |
right distal humerus |
La |
8.25 |
|
W |
16.4 |
|||
|
Hyaenodonta or Carnivora indet. |
KNM-WS 31036 |
fragment of right astragalus |
L |
25.5 |
|
W |
22.7 |
|||
|
Carnivora indet. |
KNM-WS 65395 |
phalanx |
L |
24.4 |
|
KNM-WS 65733 |
fragment of left proximal ulna |
Lo |
~15.0 |
|
|
KNM-WS 101029 |
fragment of left proximal ulna |
Lo |
~20.0 |
Remarks.—The only leopard-sized hyaenodonts known from the lower Miocene of Kenya are Leakitherium hiwegi Savage, 1965, and Isohyaenodon andrewsi Savage, 1965 (Lewis and Morlo 2010). However, no postcranial material is included in the hypodigm of either of these taxa, and no corresponding dental remains are known from Buluk.
Hyaenodonta indet. B (size of Sectisodon occultus Morales and Pickford, 2017)
Fig. 3C, Table 1.
Material.—KNM-WS 65730, right edentulous mandible fragment, with alveoli for m1 and m2, and roots of m3; from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Description.—For its size, KNM-WS 65730 (Fig. 3C) is a fairly deep mandible. A tiny mental foramen is present below m1. Only the posterior alveolus of m1 is intact; the anterior one is broken anteriorly. However, what can be seen suggests that m1 was slightly shorter mesiodistally than m2. The alveoli for m3 suggest that the tooth was much larger than either m1 or m2. The m3 anterior alveolus is ovoid, points slightly lingually, and is wider than the posterior alveolus, suggesting that the m3 trigonid was larger than in m2.
Remarks.—Although KNM-WS 65730 is edentulous, the alveoli preserved clearly indicate that molar size increases posteriorly. We interpret these tooth positions to represent m1–m3 and note that the size gradient of m1<m2<m3 is a common pattern for African hyaenodonts but not for African carnivorans. Alternatively, the alveoli could represent p3–m1 of a carnivoran with m2 lacking. The only carnivorans lacking m2 known from the early Miocene of Africa are the barbourofelids Afrosmilus and Ginsburgsmilus, and the felids Diamantofelis and Namafelis. However, the mandibular corpus of barbourofelids tends to be curved (Morlo et al. 2004) and less deep, and all of these barbourofelids and felids are much larger than KNM-WS 65730 with the exception of Namafelis minor, which is much smaller than the mandible from Buluk. Also, in N. minor p4 is longer relative to its width compared to KNM-WS 65730. Absolute and relative alveolar lengths of KNM-WS 65730 molars are close to those observed for the early Miocene hyaenodonts Sectisodon occultus Morales and Pickford, 2017, from Napak (Morales and Pickford 2017: text-fig. 3) and Buhakia moghraensis Morlo, Miller, and El-Barkooky, 2007, from Moghra (Morlo et al. 2007), while most other early and middle Miocene hyaenodonts, e.g., Dissopsalis Pilgrim, 1910, have the m3 much larger than m2 (see Morales and Pickford 2017: text-fig. 3). We suggest that this specimen represents a hyaenodont with the m3 being much more trenchant than m2, but not much longer as in other hyaenodonts. However, due to lack of other comparable features, we refer the specimen only to Hyaenodonta. At present, KNM-WS 65730 is the only specimen representing a small-sized hyaenodont from Buluk.
Order Carnivora Bowdich, 1821
Family Amphicyonidae Trouessart, 1885
Subfamily Amphicyoninae Hunt, 1998
Genus Cynelos Jourdan, 1862 = Hecubides Savage, 1965
Type species: Cynelos lemanensis (Pomel, 1846), originally described as Amphicyon lemanensis. Early Miocene (MN 2) of Billy (Allier, France).
Diagnosis.—Emended after Peigné and Heizmann (2003), Werdelin and Peigné (2010), and Morlo et al. (2019): small to large sized amphicyonids with low, slender mandibles; diastemata between anterior premolars; premolars widest distally; p4 with strong postprotocuspid; the p4 is larger in relation to m1 and to m2 than in Amphicyon, the tip of the main cusp of p4 does not project posteriorly, and the p4 talonid is wider; m1 with low metaconid and tall hypoconid crest, entoconid crest distinct but low, talonid wider than trigonid; m2 mesiodistal length about two thirds the length of m1, m2 lacking the paraconid, with a long and wide talonid, protoconid lacking a distal crest; P4 with small protocone; M1 rectangular; M2 slightly more reduced than M1, with paracone slightly larger than metacone, and v-shaped hypocone crests in African species.
Cynelos differs from Afrocyon and African Amphicyon in having a diastema between p3 and p4 and further differs from Afrocyon in having a single-rooted m3. African Cynelos differs from Myacyon Sudre and Hartenberger, 1992, in having a much longer m2 talonid and having a swelling at the posterobuccal corner of m2 (see Tsujikawa 2005: fig. B, D; discussion in Morales et al. 2016; Morlo et al. 2019).
Remarks.—In this contribution, we focus on the African occurrences of Cynelos. The composition of Cynelos has been discussed previously (e.g., Morlo et al. 2007, 2019; Morales et al. 2016; Adrian et al. 2018; Jiangzuo et al. 2018). We follow Adrian et al. (2018) and Morlo et al. (2007, 2019) in recognizing “Hecubides” Savage, 1965, as a junior synonym of Cynelos, and Myacyon, as a middle Miocene descendant of Cynelos, rather than referring small amphicyonid specimens to Hecubides and large ones to Myacyon (e.g., Morales et al. 2016). For a detailed discussion see Morlo et al. (2019).
Cynelos macrodon (Savage, 1965)
Fig. 4, Tables 3, 4.
Holotype: Left M1 (NHM M 19086); Savage (1965).
Type locality: Site 31, Rusinga Island, Kiahera Formation, Kenya; Early Miocene.
Type horizon: Early Miocene.
Material.—KNM-WS 49476, right m2; KNM-WS 49485, left M1; KNM-WS 65315, left M3; KNM-WS 65407, right m2; KNM-WS 65418, right m2; KNM-WS 65465, left m2. All from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Diagnosis.—As in Morlo et al. (2019). See also Savage (1965), Morales et al. (2016), and Adrian et al. (2018).
Description.—The protoconid, metaconid, and hypoconid of the m2 specimen KNM-WS 49476 (Fig. 4F) are heavily abraded, but there appears to be no notch separating the metaconid from the anterior cingulid. The base of the tooth is enlarged at its posterobuccal corner, and the tooth is slightly longer than the m2 of Cynelos anubisi Morlo, Miller, and El-Barkooky, 2007, from the lower Miocene of North Africa (Morales et al. 2010; Morlo et al. 2019), and Cynelos cf. macrodon from the middle Miocene Muruyur Formation, Kenya (Morlo et al. 2019). The talonid of the Buluk specimen is also wider than that observed for the similar sized Cynelos ginsburgi (Morales, Pickford, Soria, and Fraile, 1998), and the hypoconid is smaller. The morphology of three other second lower molars (Fig. 4A–D) of this species from Buluk are similar in morphology to KNM-WS 49476, except that they are smaller in size, with the smallest specimen, KNM-WS 65418, being about 20% shorter and 13% narrower than KNM-WS 49476 (Table 4).
The M1 KNM-WS 49485 (Fig. 4E) is close in morphology to the holotype of Cynelos macrodon from Rusinga Island, especially in their shared triangular occlusal outline (Savage 1965; Morales et al. 2016: fig. 9/2A). The cusps of the trigon are heavily abraded and the same is true for the hypocone. As in the holotype, the hypoconule and entoconule are not clearly demarcated, a feature that separates C. macrodon from C. ginsburgi. The lingual cingulum is smaller than in the holotype.
The M3 KNM-WS 65315 (Fig. 4F) is the first recovered M3 of C. macrodon. Morphologically, the tooth closely resembles the M3 of the larger species of Cynelos present at Buluk, but KNM-WS 65315 is much smaller.
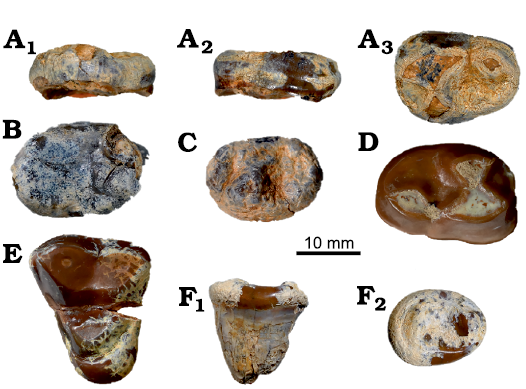
Fig. 4. Amphicyonid Cynelos macrodon (Savage, 1965) from the lower Miocene of Buluk, Kenya. A. KNM-WS 65465, right m2 in: buccal (A1), occlusal (A2), and lingual (A3) views. B. KNM-WS 65407, left m2 in occlusal view. C. KNM-WS 65418, left m2 in occlusal view. D. KNM-WS 49476, right m2 in occlusal view. E. KNM-WS 49485, left M1 in occlusal view. F. KNM-WS 65315, left M3 in buccal (F1) and occlusal (F2) views.
Remarks.—Although the contribution to the C. macrodon hypodigm from Buluk consists of only seven teeth, the collection represents the largest sample of C. macrodon described from a single locality. The four m2 specimens confirm the morphology of this tooth, as suggested by the tentative assignment of an m2 from the middle Miocene Muruyur Formation, Kenya, to C. cf. macrodon (Morlo et al. 2019). However, the Buluk m2s differ from the middle Miocene specimen of C. cf. macrodon in being slightly longer anteroposteriorly, and in having a smaller hypoconid. The four Buluk m2 specimens also vary in absolute size as well as in relative length. Given the general lack of comparable specimens from other sites, it is not clear how to interpret this large size variation, as such variation has only been documented previously for the upper canines of Cynelos (Peigné and Heizmann 2003).
Stratigraphic and geographic range.—Lower and middle Miocene of East Africa.
Cynelos jitu sp. nov.
Figs. 5–8, Tables 2–4.
Zoobank LSID: zoobank.org:act:E00245FD-76DB-403F-B125-1253E 68380EF
Etymology: From Swahili jitu, giant; as the taxon represents the largest known species of African Cynelos.
Holotype: KNM-WS 12663, a partially crushed cranium preserving partial right and left maxillary dentitions, and an associated snout fragment. Right maxilla in two fragments, with the first fragment bearing the roots of I1, I2, I3 fragment, C fragment, root of single-rooted P1, P2–P3 alveoli, anterior root and buccal portion of P4, and the second fragment bearing the root of right M2 and the talon of M3. Left maxilla with roots of I1–I3, C alveolus, root of single-rooted P1, alveoli of P2, P3, and anterior root and buccal part of lingual alveolus of P4. Associated isolated fragments of right P4, left and right M1 talons, left and right M1 paracones.
Type locality: Buluk, Kenya.
Type horizon: Early Miocene.
Material.—Hypodigm: KNM-WS 2, left lower canine; KNM-WS 12621, left upper canine fragment; KNM-WS 12625, left m2; KNM-WS 12632, associated partial mandibles with root of left p2, roots of left and right p3–m3, and left m2 trigonid; KNM-WS 12661, left mandible in two pieces with i2–i3, c, root of p2, p3, roots of p4, m1 talonid, roots of m2, and alveolus of m3; KNM-WS 12870, right M3; KNM-WS 49461, right mandibular fragment with roots of p4 and complete m1; KNM-WS 49470, right mandible fragment with m1 fragment; KNM-WS 49481, left m3; KNM-WS 49495, left P2; KNM-WS 49497, talon of right M1; KNM-WS 49509, right m2; KNM-WS 65385, right m1; KNM-WS 65625, left c fragment; KNM-WS 101033, right m3; KNM-WS 65651, distal phalanx. All from the type locality and horizon.
Diagnosis.—Cynelos jitu sp. nov. differs from all other species of Cynelos in being much larger (Tables 2–4), even though the North American C. sinapius is only slightly smaller. C. jitu sp. nov. differs from other species of African Cynelos (C. macrodon, C. anubisi, and C. ginsburgi) in having a taller m1 hypoconid, and the m2 metaconid separated from the anterior cingulid by a small notch (see Morlo et al. 2007). Further differs from C. macrodon and C. anubisi in having an m1 paraconid that protrudes more anteriorly, and further differs from C. macrodon in having a taller m2 paraconid, taller M1 hypocone, and stronger M1 hypoconule and entoconule.
Description.—Skull and upper dentition: The skull KNM-WS 12663 (Fig. 5) is partly crushed laterally, and this, coupled with some distortion, obscures nearly all sutures and key landmarks. Only maximum length of the cranium without snout (410 mm), and maximum width across the jugals (225 mm) can be measured with confidence. As the palatines and occipital condyles are broken, measurements of the basicranium can also only be estimated. Although the dimensions of the occiput cannot be fully assessed, the remnant of a pronounced sagittal crest is preserved, indicating a large attachment area for the temporalis muscle. The skull is broken such that the snout is not attached to the rest of the skull (Fig. 6). As measured along the basicranium and including the separately measured snout, the type skull of C. jitu sp. nov. is about 450 mm long, which is longer than the basilar length of 390–440 mm reported for the North American C. sinapius (Hunt and Stepleton 2015).
The incisors are only represented by roots with the exception of a fragmentary right I3 that does not reveal any morphological details. Root size increases only moderately from I1–I3. Only a part of the right upper canine alveolus is preserved, and judging from the size of the alveolus, this would have been a very large tooth (Table 3). An additional isolated upper canine, KNM-WS 12621, is assigned here to C. jitu sp. nov. on the basis of its large size, and because it differs from the upper canine of H. sulzeri (Morales et al. 2003), the only possible alternative assignment, by possessing an anterior ridge rather than a strong anterior cusplet.
There is a diastema between P1 and P2 of about 10–11 mm, and a shorter diastema between P2 and P3 of 3–5 mm length (Table 3).
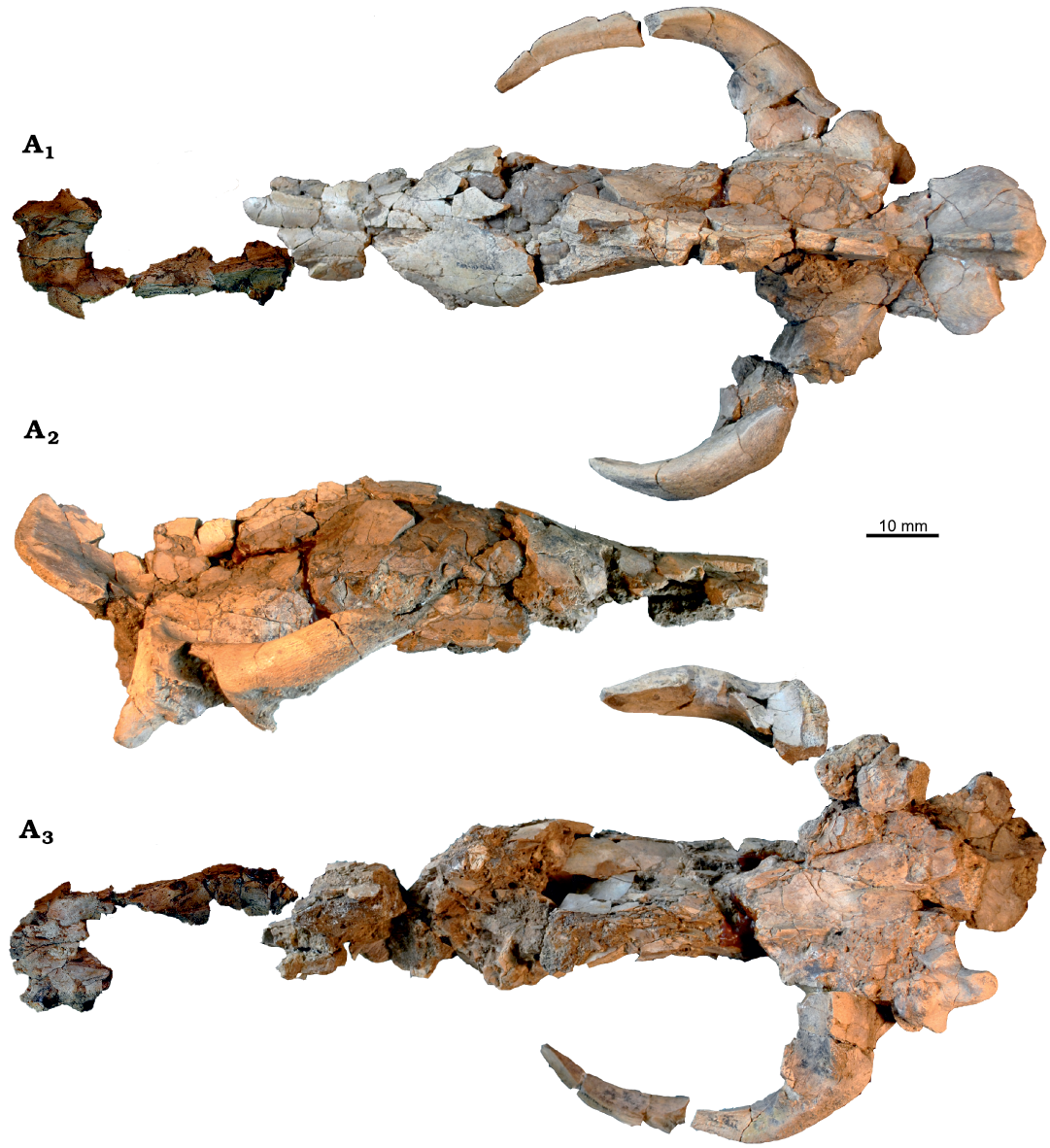
Fig. 5. Amphicyonid Cynelos jitu sp. nov. from the lower Miocene of Buluk, Kenya. A. KNM-WS 12663, holotype, skull in dorsal (A1), lateral (A2), and ventral (A3) views, notice separate snout fragment only shown in A1 and A3.

Fig. 6. Amphicyonid Cynelos jitu sp. nov. from the lower Miocene of Buluk, Kenya. A. KNM-WS 12663, upper dentition of holotype, isolated fragment with root of right M2 and talon of M3 in occlusal (A1) and buccal (A2) views; isolated fragment of the snout and parts of the right maxilla (left and right [I1–I2], [left I3], fragment of right I3, [right C], root of right P1, alveoli of P2, P3, and anterior root and buccal wall of lingual alveoli of P4) in occlusal view (A3). Brackets indicate alveoli (see Methods).
Table 3. Upper dental measurements (in mm) of Cynelos spp. from the lower Miocene of Buluk, Kenya. Note: no upper second molars were measured. Abbreviations: h, mesiodistal width of tooth across the hypocone; L, length; p, mesiodistal length of the paracone; t, talon measures; W, width; (), measurements inferred from alveoli.
|
Taxon |
Specimen |
|
C |
P1 |
P1–P2 diastema |
P2 |
P2–P3 diastema |
P3 |
M1 |
M3 |
|
Cynelos macrodon (Savage, 1965) |
KNM-WS 49485 (left) |
L |
|
|
|
|
|
|
19.7 |
|
|
W |
|
|
|
|
|
|
23.6 |
|
||
|
KNM-WS 65315 (left) |
L |
|
|
|
|
|
|
|
10.8 |
|
|
W |
|
|
|
|
|
|
|
15.5 |
||
|
Cynelos jitu sp. nov. |
KNM-WS 12663 (right) |
L |
|
(10.0) |
10.2 |
(12.4) |
4.4 |
14.5 |
14.0p |
(13.5)t |
|
W |
|
(6.0) |
|
(6.4) |
|
7.1 |
15.2h |
|
||
|
KNM-WS 12663 (left) |
L |
|
7.6 |
10.8 |
(12.2) |
3.6 |
17.0 |
14.0p |
|
|
|
W |
|
5.4 |
|
6.6) |
|
7.4 |
15.4h |
|
||
|
KNM-WS 12870 (right) |
L |
|
|
|
|
|
|
|
14.9 |
|
|
W |
|
|
|
|
|
|
|
20.7 |
||
|
KNM-WS 49497 (right) |
L |
|
|
|
|
|
|
(19.5)t |
|
|
|
W |
|
|
|
|
|
|
(19.3)t |
|
||
|
KNM-WS 49481 (left) |
L |
|
|
|
|
|
|
|
15.0 |
|
|
W |
|
|
|
|
|
|
|
21.1 |
||
|
KNM-WS 12621 (left) |
L |
21.2 |
|
|
|
|
|
|
|
|
|
W |
27.2 |
|
|
|
|
|
|
|
||
|
KNM-WS 49495 (left) |
L |
|
|
|
7.8 |
|
|
|
|
|
|
W |
|
|
|
6.5 |
|
|
|
|
Both right and left P1 are preserved in the holotype, and P1 is a single-rooted and peg-like tooth. A low crest runs centrally from the anteriorly placed tip of the main cusp to the posterior edge of the crown. A strong cingulum surrounds the tooth.
KNM-WS 49495 is a left P2 (Fig. 7D). The tooth resembles P1, but the postprotocrista is longer. Although KNM-WS 49495 is an isolated specimen, its two diverging roots fit perfectly into the left P2 alveoli of the holotype.
The alveoli for right and left P3 are present but the teeth are not. The P3 alveoli are located directly anterior to P4, with no diastema separating these two teeth. Due to breakage and abrasion, little information is available from the one preserved right P4 fragment. What is clear is that a low but distinct protocone is placed anterolingually, and the protocone is connected to the paracone by a faint crest. The protocone has a strong buccal cingulum, and although the metastyle is broken, it is separated from the protocone by a notch. No information about the paracone or parastyle is available, owing to the severe abrasion of the crown.
The upper first molars in the holotype skull are fragmentary, but the talons of both right and left M1s show the nearly symmetrical configuration typical of Cynelos, with a large hypocone, strong hypoconule and entoconule, and a strong lingual cingulum. The same features are also found in the partial upper molar KNM-WS 49497, but the talon is longer than in the holotype (Table 3), suggesting some size variability in the species. In both preserved M1s, the trigon is much longer than the talon, a characteristic that is also present in the much smaller upper first molars of C. macrodon from Buluk (KNM-WS 49485) and C. ginsburgi from Namibia (Morales et al. 2016).
No information is available for M2, because the tooth is represented by only a single maxillary fragment preserving an M2 posterior root. One M3, KNM-WS 12870, is known from Buluk, in addition to the partial M3 preserved in the holotype. Both are similar in size, single-rooted, with symmetrical crowns, a strong lingual cingulum, and a small cusp at the lingual margin of the cingulum. The specimens differ only in that the hypocone in KNM-WS 12870 is taller than in the other specimen. The M3 trigon basin is low. The lingual wall has some small cuspules.
KNM-WS 12632 preserves associated right and left partial mandibles, with the right one having a length of 350 mm, while the length of the left one cannot be measured as it lacks the incisor region. The mandibular symphysis extends posteriorly to below the anterior root of p3. However, where it can be measured, the absolute length of the symphysis varies with the size of the jaw. The distance from the posterior margin of the lower canine to the anterior margin of p3 may vary between individuals (KNM-WS 12661 vs. right mandible of KNM-WS 12632) by up to 25% (Table 3). The height of the mandible also varies between the individuals.
The ascending ramus rises directly behind m3, at about 45°, and a large area for insertion of the M. temporalis is present, as would be expected given the pronounced sagittal crest on the holotype skull.
On all mandibles, diastemata separate p2, p3, and p4, but the lengths of these diastemata appear to vary as much as 25% even within the same individual (KNM-WS 12632, Table 4). Contrastingly, mandibular height below m2 varies not within but only between individuals (KNM-WS 12632 and KNM-WS 12661, Table 4) but also by about 25%.
The lower incisors are represented in KNM-WS 12661 only by their roots. Judging from the size of the alveoli, there is a slight increase in size from i1–i3.
The KNM-WS 12661 mandible also contains a well-preserved canine, although this tooth is slightly broken lingually (Fig. 8). The KNM-WS 2 is a nearly identical isolated lower canine. Both teeth are very robust and are oval in cross-section. The enamel border surrounds the tooth at about the same height, which differs from the morphology in hyaenodonts.
None of the Buluk specimens preserve the p1 crown, but a small p2 is present in the left mandible of KNM-WS 12632, and this tooth differs from the single-rooted p2 in C. anubisi (Morlo et al. 2019) in being double-rooted. Details of the crown morphology of p3 are unknown, as this tooth is fragmentary in KNM-WS 12661 (Fig. 8). The p4 is also represented only by roots. There is no diastema between p4 and m1.
The m1 is well-preserved in KNM-WS 49461 (Fig. 7A), slightly damaged in KNM-WS 65385, and represented by partial talonids in KNM-WS 12661 and 49470; in KNM-WS 12632 only the roots remain. The m1 is characterized by a relatively short but anteriorly projecting paraconid, and a small metaconid, which is positioned close to the protoconid, and is located slightly posterior of the protoconid. The m1 talonid is dominated by a very high hypoconid reaching the height of the paraconid (KNM-WS 12661). Lingually, a strong entocristid is present, and in KNM-WS 49461, a faint buccal cingulid reaches from the posterobuccal corner of the tooth to the tooth’s anterior tip.
The m2 crown is preserved in three specimens (KNM-WS 12625, 12632, and 49509, Fig. 7B), and the roots of m2 are present in one (KNM-WS 12661). All of the m2s show a slight swelling at the posterobuccal corner of the tooth, and all have a high protoconid and metaconid, both of similar height, and the paraconid is absent. The talonid on m2 displays a very high hypoconid, while the entoconid is much lower and is nearly merged into the buccal ridge. Distally on the talonid a small trough is present, and this feature reaches almost to the posterior cingulid. In contrast to other African Cynelos, the metaconid is separated from the anterior cingulid by a small notch.
Three m3 are known, within the right mandible of KNM-WS 12632, and two isolated specimens, KNM-WS 101033 (Fig. 7C) and KNM-WS 65651 (Fig. 7F). Although there is some size variability within this small sample (Table 4), all three teeth are single-rooted, low and ovoid, with a low but broad cingulid surrounding the tooth. The only cuspid recognizable on this broad cingulid is a very low protoconid, which is connected to the lingual side of the tooth by a low crest that separates the trigonid from the talonid.
One postcranial element is attributed here to the species. This is a very large terminal phalanx (KNM-WS 65651, Fig. 7F), which is not fissured and thus cannot to belong to a hyainailourid.
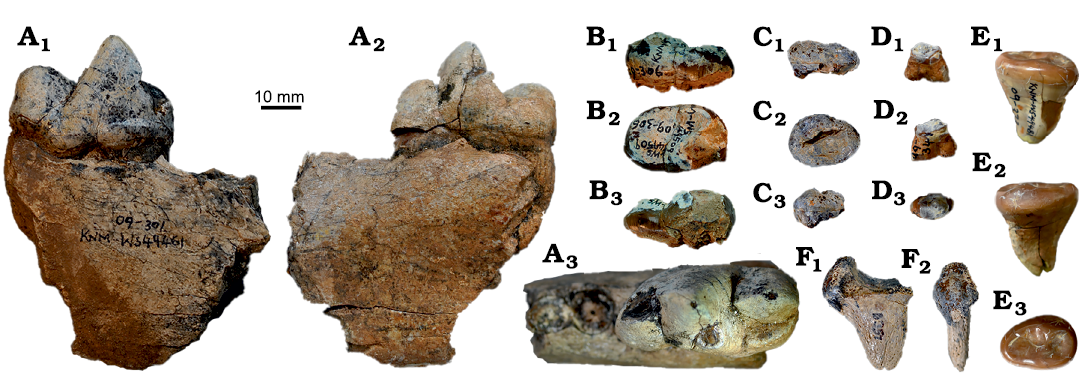
Fig. 7. Amphicyonid Cynelos jitu sp. nov. from the lower Miocene of Buluk, Kenya. A. KNM-WS 49461, right mandibular fragment with m1 in buccal (A1), occlusal (A2), and lingual (A3) views. B. KNM-WS 49509, right m2 in buccal (B1), occlusal (B2), and lingual (B3) views. C. KNM-WS 101033, isolated right m3 in buccal (C1), occlusal (C2), and lingual (C3) views. D. KNM-WS 49495, isolated left P2 in D1 buccal (D1), occlusal (D2), and lingual (D3) views. E. KNM-WS 49481 isolated left m3 in buccal (E1), occlusal (E2), and lingual (E3) views. F. KNM-WS 65651, distal phalanx in lateral (F1) and ventral (F2) views.
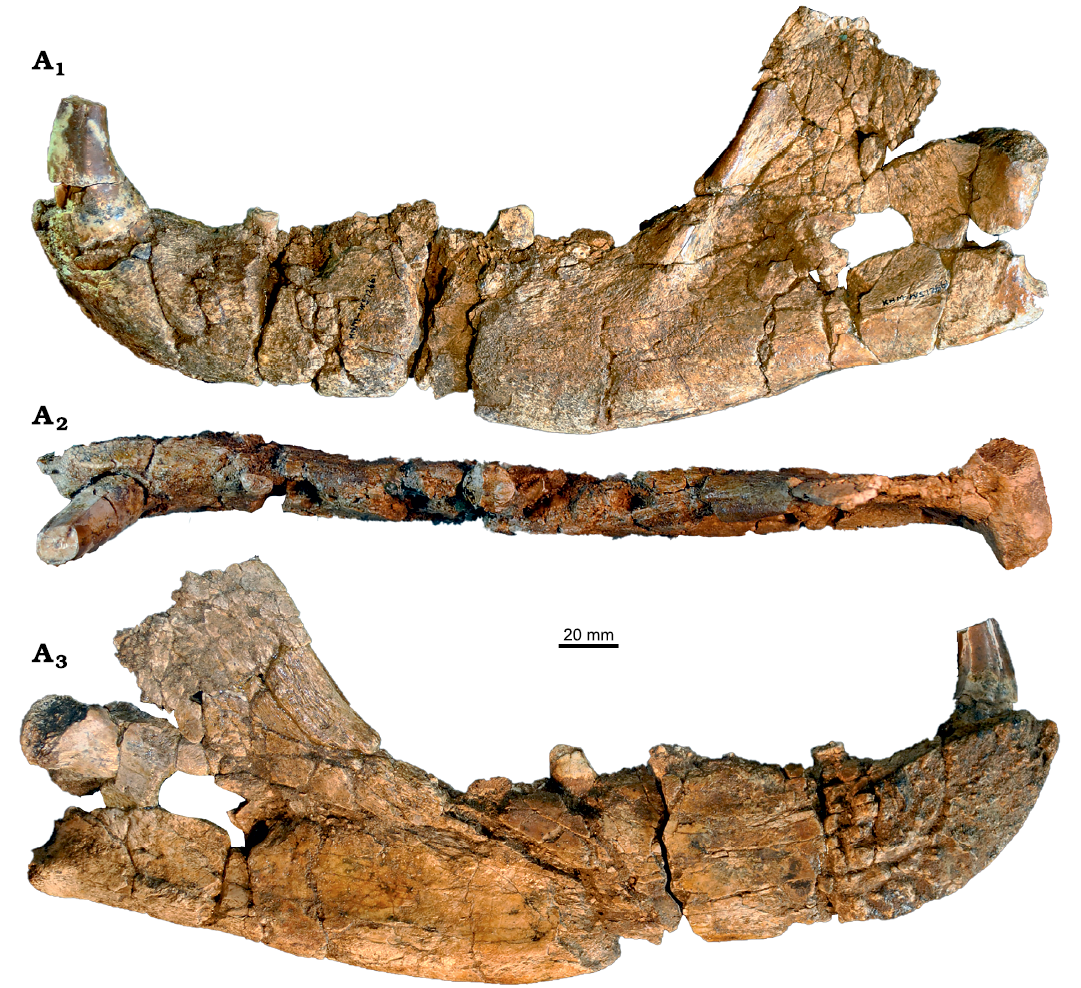
Fig. 8. Amphicyonid Cynelos jitu sp. nov. from the lower Miocene of Buluk, Kenya. A. KNM-WS 12661, left mandible in two pieces well connecting on the lingual side ([i2–i3], c, root of p2, p3, roots of p4, m1-talonid, roots of m2, and [m3]) in buccal (A1), occlusal (A2), and lingual (A3) views.
Table 4. Lower dental measurements (in mm) of carnivorans from the lower Miocene of Buluk, Kenya. Abbreviations: L, length; W, width; (), measurements inferred from alveoli; >,the tooth was broken so the measurement is a minimum.
|
Taxon |
Specimen |
|
c |
p2 |
p2–p3 diastema |
p3 |
p3–p4 diastema |
p4 |
m1 |
m2 |
m3 |
|
Cynelos
macrodon |
KNM-WS 49476 (right) |
L |
|
|
|
|
|
|
|
23.3 |
|
|
W |
|
|
|
|
|
|
|
15.3 |
|
||
|
KNM-WS 65407 (right) |
L |
|
|
|
|
|
|
|
20.0 |
|
|
|
W |
|
|
|
|
|
|
|
13.7 |
|
||
|
KNM-WS 65418 (right) |
L |
|
|
|
|
|
|
|
18.1 |
|
|
|
W |
|
|
|
|
|
|
|
13.1 |
|
||
|
KNM-WS 65465 (left) |
L |
|
|
|
|
|
|
|
19.5 |
|
|
|
W |
|
|
|
|
|
|
|
14.2 |
|
||
|
KNM-WS 65705 (right) |
L |
|
|
|
|
|
|
>22.5 |
|
|
|
|
W |
|
|
|
|
|
|
13.1 |
|
|
||
|
Cynelos
jitu |
KNM-WS 12661 (left) |
L |
|
|
|
11.2 |
|
|
|
|
|
|
W |
|
|
|
7.6 |
|
|
|
|
|
||
|
KNM-WS 12632 (right) |
L |
|
|
|
|
11.9 |
22.1 |
39.8 |
28.5 |
15.8 |
|
|
W |
|
|
|
|
|
10.4 |
16.7 |
14.0 |
11.5 |
||
|
KNM-WS 12632 (left) |
L |
|
(1.0) |
15.3 |
(15.7) |
16.0 |
23.7 |
38.2 |
(28.9) |
|
|
|
W |
|
(6.0) |
|
(6.7) |
|
9.8 |
16.2 |
17.6 |
|
||
|
KNM-WS2 (left) |
L |
18.0 |
|
|
|
|
|
|
|
|
|
|
W |
25.1 |
|
|
|
|
|
|
|
|
||
|
KNM-WS 12625 (left) |
L |
|
|
|
|
|
|
|
26.0 |
|
|
|
W |
|
|
|
|
|
|
|
16.8 |
|
||
|
KNM-WS 49509 (right) |
L |
|
|
|
|
|
|
|
26.1 |
|
|
|
W |
|
|
|
|
|
|
|
17.0 |
|
||
|
KNM-WS 49461 (right) |
L |
|
|
|
|
|
(21.5) |
39.9 |
|
|
|
|
W |
|
|
|
|
|
(11.2) |
20.1 |
|
|
||
|
KNM-WS 49470 (right) |
L |
|
|
|
|
|
|
>33.0 |
|
|
|
|
W |
|
|
|
|
|
|
19.0 |
|
|
||
|
KNM-WS 65385 (right) |
L |
|
|
|
|
|
|
36.7 |
|
|
|
|
W |
|
|
|
|
|
|
19.0 |
|
|
||
|
KNM-WS 101033 (right) |
L |
|
|
|
|
|
|
|
|
17.5 |
|
|
W |
|
|
|
|
|
|
|
|
12.5 |
||
|
?Mioprionodon sp. |
KNM-WS 49500 (right) |
L |
|
|
|
|
|
|
>5.2 |
|
|
|
W |
|
|
|
|
|
|
2.3 |
|
|
Remarks.—The presence at Buluk of an undescribed amphicyonine of outstanding size has been known for more than thirty years (Leakey and Walker 1985; Anemone et al. 2005; Morlo et al. 2007, 2019; Werdelin and Peigné 2010). The species currently represents the largest amphicyonid known from Africa, exceeding in size even Amphicyon giganteus Schinz, 1825, from South Africa (Fig. 9). As all Cynelos, C. jitu sp. nov. further differs from A. giganteus (Kuss 1965; Bastl et al. 2018; Siliceo et al. 2020) in having a diastema between p3 and p4, a more reduced ovoid m2, and a much lower and more reduced m3.
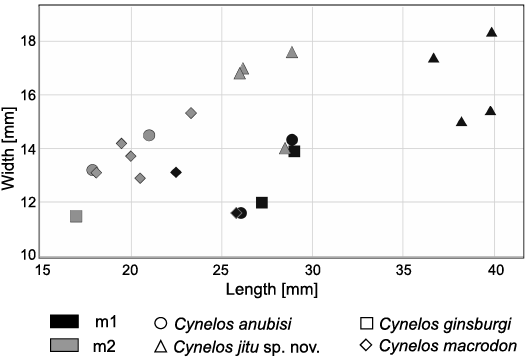
Fig. 9. Bivariate plot of comparative m1 and m2 sizes of large African species of Cynelos Jourdan, 1862. Data for specimens not from Buluk were taken from Morlo et al. (2019).
The hypodigm of C. jitu sp. nov. comprises the largest assemblage of individuals of an African species of Cynelos and may offer a first insight into variation in this species. However, the damage to some specimens complicates separating taphonomic distortion from individual variation, sexual dimorphism, and important functional and phylogenetic signals. There are noteworthy differences in overall size, mandibular height, and m1 metaconid height. Moreover, there are differences in the presence or absence of p2, and length of the c–p3 and p3–p4 diastemata, features varying even intra-individually in the KNM-WS 12632 mandibles, in which the length of the p3–p4 diastema is different on the right versus left sides (Table 4). Taken together, variation in these features suggests that the premolar region may be functionally and taxonomically irrelevant, owing to the fact that upper and lower premolars do not contact each other during chewing in this species.
Among the different species of Cynelos, members of C. jitu sp. nov. most closely resemble material attributed to C. ginsburgi, due to the shared presence in these taxa of a strong anteriorly protruding m1 paraconid. In addition, both C. jitu sp. nov. and C. ginsburgi show a distinct M1 hypoconule and entoconule, in contrast to C. macrodon, which has only very weak conules.
Stratigraphic and geographic range.— Type locality and horizon only.
Family Viverridae Gray, 1821
Genus Mioprionodon Schmidt-Kittler, 1987
Type species: Mioprionodon pickfordi Schmidt-Kittler, 1987, from the early Miocene of Songhor, Kenya.
?Mioprionodon sp.
Fig. 10, Table 4.
Material.—KNM-WS 49500, right mandible fragment with damaged m1 crown and alveolus of m2 (Fig. 10A, Table 4); from Buluk, east of Lke Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Description.—The mandible fragment KNM-WS 49500 (Fig. 10A) has a slender body. The lingual portion of the m1 paraconid is broken, as is the cusp tip of the protoconid. Both the paraconid and metaconid are slightly shorter than the height of the protoconid. Judging by the size of the alveoli, the m1 in this specimen is likely slightly longer than in the holotype of Mioprionodon pickfordi Schmidt-Kittler, 1987, from the early Miocene of Songhor, Kenya. The short talonid basin has a single cusp, a high hypoconid, which is perched on the edge of the talonid. The second lower molar is represented only by a single root, which has a length of about 1.0 mm.
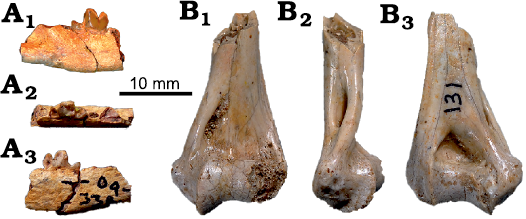
Fig. 10. Feliformia from the lower Miocene of Buluk, Kenya. A. KNM-WS 49500, ?Mioprionodon sp., right mandible fragment with damaged m1 and alveolus of m2 in buccal (A1), occlusal (A2), and lingual (A3) views. B. KNM-WS 65365, Feliformia indet., fragment of right distal humerus in anterior (B1), lateral (B2), and posterior (B3) views.
Remarks.—The typical viverrid morphology of this mandibular fragment, combined with its small size, invites comparison with the other very small African early Miocene viverrids: Leptoplesictis Major, 1903, Mioprionodon Schmidt-Kittler, 1987, and Ugandictis Morales, Pickford, and Soria, 2007. The two similarly sized species of Leptoplesictis, L. rangwai Schmidt-Kittler, 1987, and L. mbitensis Schmidt-Kittler, 1987, both have a wider and more basin-like talonid, and the hypoconid is shorter than in the Buluk specimen. In Ugandictis, the metaconid and the talonid basin are larger than in the Buluk specimen. In our view, KNM-WS 49500 most closely resembles Mioprionodon pickfordi from Songhor. Even so, the m1 metaconid of M. pickfordi is located more anteriorly than in the Buluk specimen, and the m2 of M. pickfordi is larger. The same characters also distinguish KNM-WS 49500 from Mioprionodon hodopeus Rasmussen and Gutierrez, 2009. Due to the fragmentary nature of the specimen we refrain from a specific allocation. Better material may reveal whether KNM-WS 49500 represents a new species of Mioprionodon or a new genus of viverrid.
Feliformia indet.
Fig. 10, Table 2.
Material.—KNM-WS 65365, fragment of a right distal humerus; from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene.
Description.—The humeral fragment KNM-WS 65365 (Fig. 10B, Table 2) shows typical feliform characteristics, in being narrow, with the ulnar condyle more asymmetrical in shape than in Canis (see Argot 2010: fig. 4C, D) or in hyainailourids (see, e.g., Ginsburg 1980: fig. 22; Borths and Seiffert 2017: fig. 13). In both size and morphology, the Buluk specimen closely resembles the fossil humerus of Leptofelis vallesiensis Salesa, Antón, Morales, and Peigné, 2012 (see Salesa et al. 2019: fig. 12), or among extant taxa, Felis silvestris lybica Forster, 1780, the African wild cat. The Buluk specimen appears to be too small to belong to Afrosmilus Kretzoi, 1929, known from the lower Miocene of the Kenyan localities Rusinga, Moruorot, and Songhor (Schmidt-Kittler 1987).
Remarks.—Although KNM-WS 65365 clearly belongs to a feliform, it is unknown whether the specimen represents a very small feline, a barbourofelid, or one of the numerous viverrid species known from the lower Miocene of Africa. The fact that the humeral morphology of KNM-WS 65365 closely resembles that observed for L. vallesiensis and F. silvestris suggests that a similar terrestrial locomotor pattern can be inferred for the Buluk taxon, an adaptation that has not been documented among Miocene African viverrids. As no dental remains are associated with this feliform humerus, we leave the taxonomic assignment of this specimen open below the subordinal level.
Hyaenodonta or/and Carnivora indet.
Tables 2, 5, SOM: figs. S1, S2.
Material.— All from Buluk, east of Lake Turkana, Kenya; lower section of the Buluk Member, Bakate Formation, uppermost lower Miocene. Teeth (SOM: fig. S1): KNM-WS 12623, isolated incisor; KNM-WS 12630, tooth fragments; KNM-WS 12631, broken tooth cusp; KNM-WS 12664, incisor fragment; KNM-WS 65236, isolated incisor; KNM-WS 65465, right p3?; KNM-WS 65591, isolated left P2, possibly of a hyaenodont; KNM-WS 65726, nasal and maxillary fragment with possible left I3 in the size range of H. sulzeri or C. jitu sp. nov.; KNM-WS 101012, isolated premolar.
Postcranial material (SOM: fig. S2): KNM-WS 31036 is a right astragalus fragment comparable in size with Crocuta. The articulation for the tibia is very narrow, much more so than that documented for species in Amphicyon and Panthera. The articulation is not deeply inflated like in Amphicyon, but resembles Panthera in this respect in being less inflated. KNM-WS 65395, phalanx; KNM-WS 65472, fragment of distal metapodial; KNM-WS 65597, scapholunare of Cynelos or Hyainailouros; KNM-WS 65731, fragment of distal metapodial; KNM-WS 65733, fragment of left proximal ulna. The latter presumably represents a carnivoran, as the anterior crest of olecranon runs straight proximally. KNM-WS 101029 is also a left proximal ulna fragment. This specimen is slightly larger than KNM-WS 65733, but is otherwise very similar in morphology. KNM-WS 65766, fragment of a large right calcaneum; KNM-WS 65797, large sacrum and last lumbar vertebra; KNM-WS 101031, large right scapholunare, unlike Hyainailouros.
Remarks.—Several specimens from Buluk represent members of Hyaenodonta or Carnivora, but the remains are too fragmentary to be attributed to a specific taxon. None of these specimens indicate the presence of an additional taxon at Buluk.
Table 5. Measurements (in mm) of isoalted teeth of Hyaenodonta or Carnivora indet. from the lower Miocene of Buluk, Kenya.
|
Taxon |
Specimen |
|
left nasal and maxillary |
I/i? |
p3? right |
P2? left |
P/p? |
|
Hyaenodonta or Carnivora indet. |
KNM-WS 12623 |
L |
|
6.7 |
|
|
|
|
W |
|
10.1 |
|
|
|
||
|
KNM-WS 65236 |
L |
|
6.3 |
|
|
|
|
|
W |
|
9.9 |
|
|
|
||
|
KNM-WS 65465 |
L |
|
|
16.5 |
|
|
|
|
W |
|
|
9.0 |
|
|
||
|
KNM-WS 101012 |
L |
|
|
|
|
21.5 |
|
|
W |
|
|
|
|
13.5 |
||
|
KNM-WS 65726 |
L |
12.2 |
|
|
|
|
|
|
W |
16.3 |
|
|
|
|
||
|
?Hyaenodonta indet. |
KNM-WS 65591 |
L |
|
|
|
11.3 |
|
|
W |
|
|
|
8.1 |
|
Ecomorphological analysis
Due to the fragmentary nature of most specimens, only limited information can be given on the guild structure of Buluk carnivores. Nonetheless, using the parameters outlined in Morlo et al. (2010), an ecomorphological analysis provides some insight into the distribution of body mass, locomotor category, and dietary preference. Body mass was estimated for all eight taxa, while a diet preference class could be assigned for five taxa, and locomotor pattern class for three.
Details of the guild structure assignments are as follows (Tables 6–8):
(i) Hyainailouros sulzeri. Body mass of H. sulzeri was estimated to be over 1000 kg (Borths and Stevens 2019) which is why we place it into the >100 kg body mass class. As the femur and metatarsal fragments from Buluk are very close to European H. sulzeri, we follow Ginsburg (1980) in regarding H. sulzeri as terrestrial. Concerning diet preference class, we follow Morlo et al. (2010) in interpreting H. sulzeri as bone/meat-eater.
(ii) Hyainailouros cf. napakensis. Due to lack of data for this species, we use the same diet preference (bone/meat) and locomotor category (terrestrial) as for H. sulzeri, but rely for body mass on Borths and Stevens (2019), who estimated a body mass of about 250 kg for H. napakensis. We therefore assign the taxon to the >100 kg body mass class.
(iii) Hyaenodonta indet. A, size of Leakitherium. Due to the leopard-size of the ulnar and tibial fragments from Buluk, we assign this taxon to the 30–100 kg body mass class, and due to their similarities to the olecranon and tibial medial malleus of Hyainailouros, we regard the taxon as terrestrial. No diet preference class can be identified.
(iv) Hyaenodonta indet. B, size of Sectisodon. Body mass can only be estimated by alveoli size. As size of the m1 is unclear, the regression of Morlo (1999) cannot be used. Instead, we follow Borths and Stevens (2019) in applying the felid regression of Van Valkenburgh (1990) to m3, resulting in an assignment to the 3–10 kg body mass class (Table 6). Diet preference and locomotor pattern cannot be assigned to a specific class.
(v) Cynelos macrodon. Body mass is estimated using the regressions for m1 length provided in Van Valkenburgh (1990). For maximum length we rely on NHM M 34303, because it was not possible to measure this dimension in KNM-WS 65407, the m1 from Buluk. The results (Table 6) vary between 22.3 and 89.5 kg, depending on which regression is used (Ursidae, Canidae or Carnivora). As no amphicyonid exists today, we assign C. macrodon to the 30–100 kg body mass class based on the Carnivora regression. For diet preference class, we use the index relative blade length at m1 (RBL, Table 7), as established by Van Valkenburgh (1988). Cynelos macrodon is assigned to the carnivorous diet preference class. Due to lack of postcranial specimens, we assign no locomotor pattern class.
Table 6. Body mass estimation of carnivores from the lower Miocene of Buluk, Kenya, based on regressions out of Van Valkenburgh (1990), with the family specific regression and the equation result. * length of the m3 alveoli was measured. Empty cells indicate estimations based on the same specimen with different equations.
|
Order/family |
Taxon |
Specimen |
Length of m1 [mm] |
Body mass [kg] |
Equation result |
Equation for |
|
Hyaenodonta |
Hyaenodonta sp. indet. B, size of Sectisodon |
KNM-WS 65730 |
10.2* |
8.437825194 |
0.9262305 |
Felidae |
|
Amphicyonidae |
Cynelos macrodon |
NHM M 34303 |
25.8 |
83.65858732 |
1.9225105 |
Carnivora |
|
89.47334129 |
1.9516937 |
Ursidae |
||||
|
22.3433282 |
1.3491479 |
Canidae |
||||
|
Cynelos jitu sp. nov. |
KNM-WS 12632 |
39.8 |
303.1466156 |
2.4816527 |
Carnivora |
|
|
KNM-WS 12632 |
38.2 |
268.3664293 |
2.4287282 |
Carnivora |
||
|
KNM-WS 49461 |
39.9 |
305.4143933 |
2.4848895 |
Carnivora |
||
|
KNM-WS 5385 |
36.7 |
238.2638806 |
2.3770582 |
Carnivora |
||
|
Viverridae |
?Mioprionodon sp. |
KNM-WS 49500 |
5.5 |
0.848940373 |
-0.0711228 |
Carnivora |
|
1.282644204 |
0.1081062 |
Felidae |
Table 7. Diet preference estimation of Carnivora from lower Miocene of Buluk, Kenya, based on Relative Blade Length (Van Valkenburgh 1988) of m1.
|
Family |
Taxon |
Specimen |
RBL |
Diet category |
|
Amphicyonidae |
Cynelos macrodon |
NHM M 34303 |
0.65 |
carnivorous |
|
Amphicyonidae |
Cynelos jitu sp. nov. |
KNM-WS 49461 |
0.64 |
carnivorous |
|
Viverridae |
?Mioprionodon sp. |
KNM-WS 49500 |
0.68 |
carnivorous or insectivorous |
Table 8. Designations of ecomorphological classes of carnivores from lower Miocene of Buluk, Kenya. Empty cells indicate class designations that cannot be inferred from the Buluk specimens.
|
Taxon |
Body mass [kg] |
Locomotor pattern |
Diet preference |
|
Hyainailouros sulzeri |
>100 |
terrestrial |
bone/meat |
|
Hyainailouros cf. nakapaensis |
>100 |
terrestrial |
bone/meat |
|
Hyaenodontida indet. A, size of Leakitherium |
30–100 |
terrestrial |
|
|
Hyaenodontida indet. B, size of Sectisodon |
3–10 |
|
|
|
Amphicyon macrodon |
30–100 |
|
carnivorous |
|
Amphicyon jitu sp. nov. |
>100 |
|
carnivorous |
|
?Mioprionodon sp. |
<1 |
|
insectivorous |
|
Feliformia indet. size of Leptofelis |
3–10 |
terrestrial |
|
(vi) Cynelos jitu sp. nov. As in C. macrodon, body mass is estimated by using the Carnivora regression for m1 length (Van Valkenburgh 1990). The results (Table 6) vary between 238.3 and 305.4 kg. We thus assign C. jitu to the >100 kg body mass class. For dietary preference, we use the index relative blade length at m1 (RBL, Table 7), as established by Van Valkenburgh (1988). As with C. macrodon, C. jitu sp. nov. is assigned to the carnivorous dietary class. With the distal phalanx KNM-WS 65651 being the only postcranial specimen yet attributed to C. jitu sp. nov., we assign no locomotor pattern class.
(vii) ?Mioprionodon sp. Body mass is estimated by using the regressions for m1 length for Carnivora and Felidae of Van Valkenburgh (1989). However, as the paraconid of KNM-WS 49500 is anteriorly broken, we estimate a length of 5.5 mm for these equations. This implies a body mass between 0.85 and 1.3 kg (Table 6). As ?Mioprionodon sp. is not a felid, we assign it to the 0–1 kg body mass class obtained from using the Carnivora regression. For diet preference class, we use the index relative blade length at m1 (RBL, Table 7), as established by Van Valkenburgh (1988), but took into account its pointed cusps (Nagel and Koufos 2009) and assign it to the insectivorous diet preference class. Due to lack of postcranial specimens, we assign no locomotor pattern class.
(viii) Feliformia indet. As this humeral fragment is of the same size and morphology as Leptofelis, we assign the taxon to the 3–10 kg body mass class, and to the terrestrial locomotor pattern class suggested for Leptofelis (Salesa et al. 2012). As we have no dentition, we do not assign a diet preference class, but Feliformia indet. might represent a hypercarnivorous taxon as it largely resembles Leptofelis.
Results of the ecomorphological analysis (Table 8) verify the presence of a wide range of body sizes, from the <1 kg of ?Mioprionodon sp. to the >100 kg of H. sulzeri, H. cf. napakensis, and C. jitu sp. nov. However, while the very large bodied category (taxa >100 kg) is represented by three species, taxa of intermediate size (1–30 kg) are rare or absent, and the very small category (<1 kg) is occupied only by ?Mioprionodon sp. Locomotor pattern is obtainable for only four of eight taxa (Feliformia indet., Hyaenodonta indet. A, size of Leakitherium, H. sulzeri). All four taxa are assigned to a terrestrial locomotor pattern, with the small feliform being very close in morphology to small felines like the late Miocene Leptofelis (see Salesa et al. 2012). No hypocarnivore or omnivore has been found among the eight carnivore taxa of Buluk. Instead, insectivorous ?Mioprionodon sp., carnivorous Cynelos, and bone/meat eating Hyainailouros represent the remaining dietary classes. The two remaining hyaenodonts cannot be assigned to a diet class. In general, omnivorous and hypocarnivorous early Miocene hyaenodonts appear to be rare, with Teratodon being the only possible exception among the early to middle Miocene African hyaenodonts (Morlo et al. 2010).
Discussion
Faunal relationships of hyaenodonts and carnivorans of Buluk.—A diverse array of eight carnivores is known from Buluk; four are members of Hyaenodonta (Hyainailouros sulzeri, H. cf. napakensis, and two indeterminate species), and four represent Carnivora (Cynelos macrodon, C. jitu sp. nov., ?Mioprionodon sp., and an indeterminate feliform). This fauna largely resembles that from other African early Miocene sites (Fig. 11), except that two large species of Cynelos co-occur at Buluk, while only one species appears to be present at other early Miocene localities. In contrast, the Buluk carnivore fauna is very different from African middle Miocene carnivore faunas (Morales and Pickford 2005, 2008; Lewis and Morlo 2010; Werdelin and Peigné 2010; Werdelin 2019). This is perhaps not unexpected given the well-known faunal turnover event that occurred at the early to middle Miocene boundary (16–15 Ma; Zachos et al. 2001). However, as Buluk is the youngest of the early Miocene sites, continued work on the Buluk fauna may contribute to understanding why some lineages, including some hyaenodonts and carnivorans, thrived across the early–middle Miocene transition, while others underwent near total replacement. For example, among the early Miocene hyaenodonts, neither of the taxa in Hyainailouros recovered from Buluk are known from African middle Miocene faunas. Hyainailouros osteothlastes is recorded from middle Miocene deposits in East and North Africa (Morales and Pickford 2005, 2008; Lewis and Morlo 2010), but is not present at Buluk. Among the members of Carnivora represented at Buluk, only Cynelos macrodon is known from the middle Miocene, at the site of Kipsaramon in western Kenya (Morales and Pickford 2008; Morlo et al. 2019).
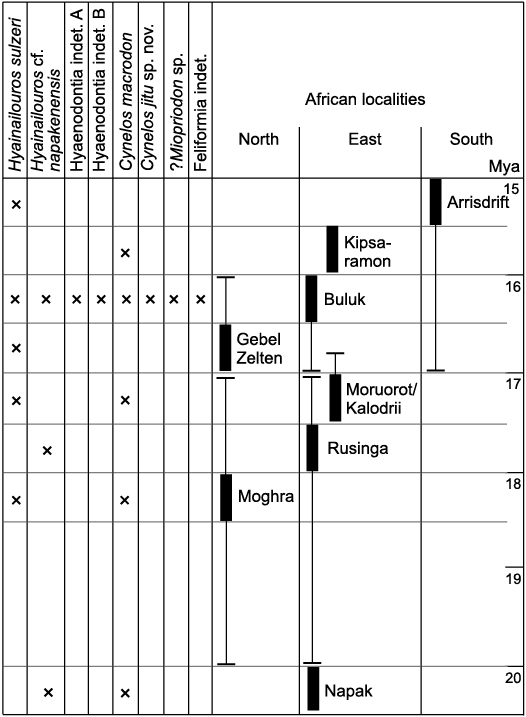
Fig. 11. African occurrences of Hyaenodonta and Carnivora species known from the lower Miocene of Buluk, Kenya. An “x” indicates the taxon is present at the corresponding fossil site (thick bar). Temporal ranges of fossil localities are indicated by bracketed thin lines. Full locality temporal data from Adrian et al. (2018) for Moruorot and Kalodirr; Behrensmeyer et al. (2002) for Kipsaramon; Hassan et al. (2012) for Moghra; McDougall and Watkins (1985) for Buluk; Morales et al. (2016) for Napak; Peppe et al. (2017) for Rusinga; Werdelin (2010) for Arrisdrift and Gebel Zelten.
It has been suggested that the middle Miocene taxon Myacyon is derived from one of the species in Cynelos (see Tsujikawa 2005: figs. B, D; Morales and Pickford 2005, 2008; discussion in Morales et al. 2016). Currently, the fragmentary nature of both the Buluk Cynelos and Myacyon hypodigms does not allow us to clarify how the large early and middle Miocene amphicyonids of Africa are related to each other. All that can be noted at present is that the m2 of C. macrodon from Buluk differs from the Kipsaramon specimen of this taxon in being slightly longer and having a smaller hypoconid, and the m1 of C. macrodon is shorter in relation to its width than that assigned to Myacyon kiptalami Morales and Pickford, 2005, from the Kenyan Ngorora Formation and the Kenyan locality of Cheparawa (Morales and Pickford 2005, 2008). With regard to ?Myacyon peignei Werdelin, 2019, from the middle Miocene site of Fort Ternan, Kenya, this species differs from all early Miocene Cynelos, except the small Cynelos euryodon (Savage, 1965), in being smaller, bearing a small entoconid in m1, and having the base of the m1 metaconid less voluminous (Werdelin 2019). If additional fossils of C. macrodon and C. jitu sp. nov. are recovered from Buluk or other late early Miocene localities, they may contribute to untangling the origin of the genus Myacyon.
Ecomorphology of hyaenodonts and carnivorans of Buluk.—The carnivore guild structure at Buluk (Table 8) accommodated the co-occurrence of three species with reconstructed body masses exceeding 100 kg (H. sulzeri, H. cf. napakensis, C. jitu sp. nov.). This community of apex predators is unlike the guild structure of any modern carnivore community, but it is not an uncommon pattern in the early and middle Miocene. In Europe, the co-occurrence of multiple large predators has been documented at Sansan, France, and Steinheim and Eppelsheim, Germany (Morlo et al. 2010, 2020). No other analyses specifically addressing African early Miocene carnivore guild structures have yet been conducted, but published faunal lists suggest that the presence of several large contemporaneous predators is fairly common. For example, faunas containing one or two very large species of Hyainailouros, sometimes along with one or two very large amphicyonids, have been documented at Moghra, Egypt (Morlo et al. 2007, 2019), Nakwai, Kenya (Rasmussen and Gutierrez 2009; Friscia et al. 2020), Kalodirr, Kenya (Leakey et al. 2011), Gebel Zelten, Libya (Morales et al. 2010), and Arrisdrift, South Africa (Morales et al. 2003, 2008).
The pattern observed for large carnivores contrasts with the lack of diversity documented for small (0–3 kg) carnivores. One reason for this disparity may be that the Buluk fauna is taphonomically size-sorted large. Fossils at Buluk are retrieved from channel lag deposits, which favored the preservation of larger-bodied animals. Most specimens are recovered as surface finds, and dry screening of productive localities tends to yield smaller elements representing large and medium-sized animals (e.g., isolated teeth) rather than microfauna. In general, animals such as proboscideans and rhinoceroses are common, while animals such as rodents are rare (n = 3 incisors), and insectivores are thus far absent. One possible additional explanation for the fact that carnivores in the 0–3 kg size class remain elusive is that the Buluk collection also favors the recovery of terrestrial animals. At present, with the exception of some primate taxa, all of the mammals known from the locality, including the large hyaenodonts and carnivorans, are terrestrial species, that is, none are demonstrably semiaquatic, scansorial, or committed arborealists. It is also noteworthy that at Buluk, as well as at other African early Miocene sites, the diets of the very large predators, i.e., Hyainailouros, Simbakubwa, Cynelos, and Amphicyon, are reconstructed as carnivorous or as bone/meat eater (Borths and Stevens 2019; Morlo et al. 2007, 2010, 2019), with other dietary categories, e.g., hypocarnivory and omnivory, less well represented.
In summary, there is a taphonomic bias at Buluk towards larger fossil specimens, and within the carnivoran guild this bias may be compounded by the preservation of terrestrial and carnivorous or scavenging adaptations, among the broad repertoire of other possible locomotor (e.g., scansorial, semiaquatic) and dietary regimes (e.g., hypocarnivory, omnivory) utilized by carnivores. One avenue for future work would be a comparison between the carnivore guild structure at Buluk with that from other Miocene sites of Africa. This has the potential to elucidate whether the pattern observed at Buluk is solely the result of taphonomic biases, or whether it reflects a true scarcity. Understanding this would greatly enhance our interpretation of early Miocene carnivore behavior.
Conclusions
In this contribution we analyze the systematics and paleobiology of 44 fossil specimens of carnivorous mammals, from the upper lower Miocene of Buluk, Kenya. Eight carnivorous taxa are recognized, four hyaenodonts and four carnivorans. One of these taxa represents a new and very large species of Cynelos, C. jitu sp. nov., and a possibly new viverrid species, ?Mioprionodon sp. also occurs at Buluk, while the other taxa have been documented previously at other African lower Miocene localities (Fig. 11).
Understanding guild structures of carnivorous taxa is critical for interpreting mammalian predator-prey relationships, and the first such analysis of an African Miocene carnivore guild reveals that a range of body sizes are present at Buluk (1 kg to >100 kg), but there is a predominance of large to very large taxa (>30 kg). There is also a greater representation of species with terrestrial, and carnivorous or scavenging adaptations, among the wider range of carnivore niches potentially available.
The taphonomy of Buluk plays a role in the structure of this ecomorphological profile, but it is notable that the co-occurrence of multiple large carnivores has been observed at other early Miocene sites in Africa and Europe, although not for any middle Miocene assemblages, or any extant faunas.
Acknowledgements
We thank the National Commission for Science, Technology, and Innovation for permission to conduct paleontological research in Kenya, and The Director General of the National Museums of Kenya, Nairobi, and the Turkana Basin Institute, Ileret, for affiliations with their institutions, and for access to specimens in their care. We particularly appreciate the support of Job Kibii, Frederik K. Manthi, Rose Nyaboke, and Justice Erus Edung (KNM). Special thanks are owed to the fossil hunters of Team Buluk, Martin Kirinya (Ileret, Kenya), and the staff at TBI-Ileret, whose professionalism makes field and lab work in such a remote location possible. Comments from reviewers Jorge Morales (Spanish National Research Council, Madrid, Spain) and Matthew Borths (Duke University, Durham, USA) greatly improved this manuscript. Katharina Bastl (Medical University of Vienna, Austria) helped with Fig. 9. The scanning was done by Timothy Gichunge and his team (TBI). Funding for work at Buluk is supported by De Anza Community College Foundation, the Leakey Foundation, National Geographic Society, and the Turkana Basin Institute.
References
Adrian, B., Werdelin, L., and Grossman, A. 2018. New Miocene Carnivora (Mammalia) from Moruorot and Kalodirr, Kenya. Palaeontologia Electronica 21.1.10a: 1–19. Crossref
Anemone, R., Grossman, A., Miller, E.R., and Watkins, R. 2005. Biochronology and paleoecology of the Buluk fauna, early Miocene of northern Kenya. Journal of Vertebrate Paleontology 25 (3 Supplement): 32A.
Argot, C. 2010. Morphofunctional analysis of the postcranium of Amphicyon major (Mammalia, Carnivora, Amphicyonidae) from the Miocene of Sansan (Gers, France) compared to three extant carnivores: Ursus arctos, Panthera leo, and Canis lupus. Geodiversitas 32: 65–106. Crossref
Bastl, K., Nagel, D., Morlo, M., and Göhlich, U. B. 2018. The Carnivora (Mammalia) from the middle Miocene locality of Gračanica (Bugojno Basin, Gornji Vakuf, Bosnia and Herzegovina). Palaeobiodiversity and Palaeoenvironments 100: 307–319. Crossref
Beaumont, G. de 1970. Le problème de la position taxonomique de Hyainailouros Biedermann (Mammalia). Bulletin de Société vaudoise de Sciences naturelles 70 (8), No. 333: 357–363.
Behrensmeyer, A.K., Deino, A.L., Hill, A., Kingston, J.D., and Saunders, J.J. 2002. Geology and geochronology of the middle Miocene Kipsaramon site complex, Muruyur Beds, Tugen Hills, Kenya. Journal of Human Evolution 42: 11–38. Crossref
Biedermann, W.G.A. 1863. Petrefacten aus der Umgegend von Wintherthur. 23 pp. S. Bleuler-Hausheer, Wintherthur.
Borths, M.R. and Seiffert, E.R. 2017. Craniodental and humeral morphology of a new species of Masrasector (Teratodontinae, Hyaenodonta, Placentalia) from the late Eocene of Egypt and locomotor diversity in hyaenodonts. PLoS ONE 12 (4): e0173527. Crossref
Borths, M.R. and Stevens, N.J. 2019. Simbakubwa kutokaafrika, gen. et sp. nov. (Hyainailourinae, Hyaenodonta, “Creodonta”, Mammalia), a gigantic carnivore from the earliest Miocene of Kenya. Journal of Vertebrate Paleontology 39 (1): e1570222. Crossref
Borths, M.R., Holroyd P.A., and Seiffert, E.R. 2016. Hyainailourine and teratodontine cranial material from the late Eocene of Egypt and the application of parsimony and Bayesian methods to the phylogeny and biogeography of Hyaenodonta (Placentalia, Mammalia). PeerJ 4: e2639. Crossref
Friscia, A.R., Macharwas, M., Muteti, S., Ndiritu, F., and Rasmussen, D.T. 2020. A transitional mammalian carnivore community from the Paleogene–Neogene boundary in northern Kenya. Journal of Vertebrate Paleontology 40 (5): e1833895. Crossref
Friscia, A.R., Van Valkenburgh, B., and Biknevicius, A. 2007. An ecomorphological analysis of extant small carnivorans. Journal of Zoology, London 272: 82–100. Crossref
Ginsburg, L. 1980. Hyainailouros sulzeri, mammifère créodonte du Miocène d’Europe. Annales de Paléontologie, Vertébrés 66 (1): 19–73.
Harris, J. and Watkins, R. 1974. New early Miocene vertebrate locality near Lake Rudolf, Kenya. Nature 252: 567–577. Crossref
Hassan, S.M., Steel, R.J., El Barkooky A., Hamdan M., Olariu C., and Helper, M.A. 2012. Stacked, lower Miocene tide-dominated estuary deposits in a transgressive succession, Western Desert, Egypt. Sedimentary Geology 282: 241–255. Crossref
Helbing, H. 1925. Das Genus Hyaenaelurus Biedermann. Eclogae geologicae Helvetiae 19: 214–245.
Hunt, R.M. Jr. and Stepleton, E. 2015. A skull of the immigrant eurasian beardog Cynelos (Carnivora, Amphicyonidae) from the early Miocene of Southern California. Journal of Vertebrate Paleontology 35 (1): e891229. Crossref
Jiangzuo, Q., Chunxiao, L., Zhang, X., Wang S., Ye, L., and Li, Y. 2018. Diversity of Amphicyonidae (Carnivora, Mammalia) in the middle Miocene Halamagai formation in Ulungur River area, Xinjiang, Northwestern China. Historical Biology 32: 187–202. Crossref
Jourdan, C. 1862. Des terrains sidérolitiques. Revue des sociétés savantes. Sciences mathématiques, physiques et naturelles Paris 1: 130–133.
Kuss, S. E. 1965. Revision der europäischen Amphicyoninae (Canidae, Carnivora, Mamm.) ausschliesslich der voroberstampischen Formen. Sitzungsberichte der Heidelberger Akademie der Wissenschaften 1: 5–168. Crossref
Leakey, M.G. 1985. Early Miocene cercopithecoids from Buluk, northern Kenya. Folia Primatologica 44: 1–14. Crossref
Leakey, R.E.F. and Walker, A. 1985. New higher primates from the early Miocene of Buluk, Kenya. Nature 318: 173–175. Crossref
Leakey, M., Grossman, A., Gutièrrez, M. and Fleagle, J.G. 2011. Faunal change in the Turkana Basin during the Late Oligocene and Miocene. Evolutionary Anthropology 20: 238–253. Crossref
Lewis, M.E. and Morlo, M. 2010. “Creodonta”. In: L. Werdelin and W.J. Sanders (eds.), Cenozoic Mammals of Africa, 543–560. Cambridge University Press, Cambridge. Crossref
Locke, E.M., Benefit, B.R., Kimock, C., Miller, E.R., and Nengo, I.O. 2020. New dentognathic fossils of Noropithecus bulukensis (Primates, Victoriapithecidae) from the late early Miocene of Buluk, Kenya. Journal of Human Evolution 148 [published online https://doi.org/10.1016/j.jhevol.2020.102886]. Crossref
Lukens, W.E., Peppe, D.J., Locke, E., Miller, E.R., Deino, A.L., Oginga, K.O., and Nengo, I. 2017. Paleoenvironments and mammalian fauna of the early Miocene fossil site at Buluk, Kenya. American Journal of Physical Anthropology 162 (S64): 268–269.
Major, C.I.F. 1903. New Carnivora from the middle Miocene of La Grive Saint Alban, Isère, France. Geological Magazine 10: 534–538. Crossref
McDougall, I. and Watkins, R.T. 1985. Age of hominoid-bearing sequence at Buluk, northern Kenya. Nature 318: 175–178. Crossref
Miller, E.R., Benefit, B.R., McCrossin, M.L., Plavcan, J.M., Leakey, M.G., El-Barkooky, A.N., Hamdan, M.A., Gawad, M.A., Hassan, S.M., and Simons, E.L. 2009. Systematics of early and middle Miocene Old World monkeys. Journal of Human Evolution 57: 195–211. Crossref
Morales, J. and Pickford, M. 2005. Carnivores from the middle Miocene Ngorora Formation (13–12 Ma), Kenya. Estudios Geologicos 61: 271–284. Crossref
Morales, J. and Pickford, M. 2008. Creodonts and carnivores from the middle Miocene Muruyur Formation, Kipsaramon and Cheparawa, Baringo District, Kenya. Comptes Rendus Palevol 7: 487–497. Crossref
Morales, J. and Pickford, M. 2017. New hyaenodonts (Ferae, Mammalia) from the early Miocene of Napak (Uganda), Koru (Kenya) and Grillental (Namibia). Fossil Imprint 73: 332–359. Crossref
Morales, J., Pickford, M., and Salesa, M.J. 2008. Creodonta and Carnivora from the early Miocene of the Northern Sperrgebiet, Namibia. Memoir of the Geological Survey of Namibia 20: 291–310.
Morales, J., Pickford, M., and Soria, D. 2007. New carnivoran material (Creodonta, Carnivora and Incertae sedis) from the early Miocene of Napak, Uganda. Paleontological Research 11(1): 71–84. Crossref
Morales, J., Pickford, M., and Valenciano, A. 2016. Systematics of African Amphicyonidae, with descriptions of new material from Napak (Uganda) and Grillental (Namibia). Journal of Iberian Geology 42 (2): 131–150.
Morales, J., Pickford, M., Fraile, S., Salesa, M.J., and Soria, D. 2003. Creodonta and Carnivora from Arrisdrift, early Miocene of Southern Namibia. Memoirs of the Geological Survey of Namibia 19: 177–194.
Morlo, M. 1999. Niche structure and evolution in creodont (Mammalia) faunas of the European and North American Eocene. Géobios 32: 297–305. Crossref
Morlo, M., Gunnell, G.F., and Nagel, D. 2010. Ecomorphological analysis of carnivore guilds in the Eocene through Miocene of Laurasia. In: A. Goswami and A. Friscia (eds.), Carnivoran Evolution: New Views on Phylogeny, Form, and Function, 269–310. Cambridge University Press, Cambridge. Crossref
Morlo, M., Miller, E.R., and El-Barkooky, A.N. 2007. Creodonta and Carnivora from Wadi Moghra, Egypt. Journal of Vertebrate Paleontology 27 (1): 145–159. Crossref
Morlo, M., Miller, E.R., Bastl, K., Gawad, M.A., Hamdan, M., El-Barkooky, A.N., and Nagel, D. 2019. New amphicyonids (Mammalia, Carnivora) from Moghra, early Miocene, Egypt. Geodiversitas 41: 731–745. Crossref
Morlo, M., Nagel, D., and Bastl, K. 2020. Evolution of the carnivoran (Carnivora, Mammalia) guild structure across the middle/upper Miocene boundary in Germany. Palaeogeography, Palaeoclimatology, Palaeoecology 553: 109801. Crossref
Morlo, M., Peignè, S., and Nagel, D. 2004. A new species of Prosansanosmilus: Implications for the systematic relationships of the family Barbourofelidae new rank (Carnivora, Mammalia). Zoological Journal of the Linnean Society 140: 43–61. Crossref
Nagel, D. and Koufos, G.D. 2009. The upper Miocene mammal faunas of the Mytilinii Basin, Samos Island, Greece: New Collection. 15. Carnivore Guild Structure. Beiträge zur Paläontologie 31: 391–396.
Peigné, S. and Heizmann, E.P.J. 2003. The Amphicyonidae (Mammalia: Carnivora) from Ulm-Westtangente (MN 2, early Miocene), Baden-Württemberg, Germany: Systematics and Ecomorphology. Staatliches Museum für Naturkunde, Serie B 343: 1–133.
Peppe, D.J., Deino, A.L., McNulty, K.P., McCollum, M.S., Mitchell, A.L., Driese, S.G., Dunsworth, H.M., Fox, D.L., Harcourt-Smith, W.E., Jenkins, K., Lehmann, T., and Michel, L.A. 2017. Revised geochronology of the early Miocene faunas from Rusinga Island and Mfangano Island (Lake Victoria, Kenya): implications for Miocene hominoid evolution and faunal succession. American Journal of Physical Anthropology 162: 313.
Pilgrim, G.E. 1932. The fossil Carnivora of India. Memoirs of the Geological Survey of India. Palaeontologia Indica, New Series 18: 1–232.
Pomel, A. 1846. Mémoire pour servir à la géologie paléontologique des terrains tertiaires du département de l’Allier. Bulletin de la Société géologique de France 3 (2): 353–373.
Rasmussen, D.T. and Gutierrez, M. 2009. A mammalian fauna from the late Oligocene of Northwestern Kenya. Palaeontographica A 288: 1–52. Crossref
Salesa, M., Siliceo, G., Antón, M., Peigné, S. and Morales, J. 2019. Functional and systematic implications of the postcranial anatomy of a late Miocene feline (Carnivora, Felidae) from Batallones-1 (Madrid, Spain). Journal of Mammalian Evolution 26: 101–131. Crossref
Savage, R.J.G. 1965. Fossil mammals of Africa: the Miocene Carnivora of East Africa. Bulletin of the British Museum of Natural History (Geology) 10 (8): 239–316.
Schmidt-Kittler, N. 1987. The Carnivora (Fissipedia) from the lower Miocene of East Africa. Palaeontographica A 197: 85–126.
Siliceo, G., Morales, J., Antón, M., and Salesa, M.J. 2020. New fossils of Amphicyonidae (Carnivora) from the middle Miocene (MN6) site of Carpetana (Madrid, Spain). Geodiversitas 42 (15): 223–238. Crossref
Smith, J.B. and Dodson, P. 2003. A proposal for a standard terminology of anatomical notation and orientation in fossil vertebrate dentitions. Journal of Vertebrate Paleontology 23:1–12. Crossref
Solé, F., Amson, E., Borths, M., Vidalenc, D., Morlo, M., and Bastl, K. 2015. A new large Hyainailourine from the Bartonian of Europe and its bearings on the evolution and ecology of massive hyaenodonts (Mammalia). PLoS ONE 10(9): e0135698. Crossref
Stein, A.B. and Hayssen, V. 2013. Panthera pardus (Carnivora: Felidae). Mammalian Species 45: 30–48. Crossref
Sudre, J. and Hartenberger, J.-L. 1992. Oued Mya 1, nouveau gisement de mammifères du Miocène supérieur dans le sud algérien. Géobios 25: 553–565. Crossref
Szalay, F.S. 1967. The Affinities of Apterodon (Mammalia, Deltatheridia, Hyaenodontidae). American Museum Novitates 2293: 1–17.
Tsujikawa, H. 2005. The updated late miocene large mammal fauna from Samburu Hills, Northern Kenya. African Study Monographs. Supplementary Issue 32: 1–50.
Van Valkenburgh, B. 1987. Skeletal indicators of locomotor behavior in living and extinct carnivores. Journal of Vertebrate Paleontology 7: 162–182. Crossref
Van Valkenburgh, B. 1988. Trophic diversity in past and present guilds of large predatory mammals. Paleobiology 14: 155–173. Crossref
Van Valkenburgh, B. 1990. Skeletal and dental predictors of body mass in carnivores. In: J. Damuth and B.J. MacFadden (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 181–205. Cambridge University Press, Cambridge.
Watkins, R.T. 1989. The Buluk Member, a fossil hominoid-bearing sedimentary sequence of Miocene age from northern Kenya. Journal of African Earth Sciences 8: 107–112. Crossref
Werdelin, L. 2010. Chronology of Neogene mammal localities. In: L.Werdelin and W.J. Sanders (eds.), Cenozoic Mammals of Africa, 27–43. Cambridge University Press, Cambridge Crossref
Werdelin, L. 2019. Middle Miocene Carnivora and Hyaenodonta from Fort Ternan, western Kenya. Geodiversitas 41 (6): 267–283. Crossref
Werdelin, L. and Peigné, S. 2010. Carnivora. In: L. Werdelin and W.J. Sanders (eds.), Cenozoic Mammals of Africa, 543–560. Cambridge University Press, Cambridge. Crossref
Wheeler, E.A., Wiemann, M.C., and Fleagle, J.G. 2007. Woods from the Miocene Bakate Formation, Ethiopia: Anatomical characteristics, estimates of original specific gravity and ecological inferences. Review of Paleobotany and Palynology 146: 193–207. Crossref
Zachos, J., Pagani, M., Sloan, L., Thomas, E., and Billups, K. 2001. Trends, rhythms and aberrations in global climate 65 Ma to present. Science 292: 686–693 Crossref.
Acta Palaeontol. Pol. 66 (2): 465–484, 2021
https://doi.org/10.4202/app.00794.2020