


Palaeoneurology and palaeobiology of the dinocephalian therapsid Anteosaurus magnificus
JULIEN BENOIT, ASHLEY KRUGER, SIFELANI JIRAH, VINCENT FERNANDEZ, and BRUCE S. RUBIDGE
Benoit, J., Kruger, A., Jirah, S., Fernandez, V., and Rubidge, B.S. 2021. Palaeoneurology and palaeobiology of the dinocephalian therapsid Anteosaurus magnificus. Acta Palaeontologica Polonica 66 (1): 29–39.
Dinocephalians (Therapsida), some of the earliest amniotes to have evolved large body size, include the carnivorous Anteosauria and mostly herbivorous Tapinocephalia. Whilst the palaeoneurology of the Tapinocephalia has been investigated in Moschognathus whaitsi, that of the Anteosauria remains completely unknown. Here we used X-ray micro-Computed Tomography to study, for the first time, the palaeoneurology of Anteosaurus magnificus. Compared to Moschognathus, we reconstruct Anteosaurus as an agile terrestrial predator based on the enlarged fossa for the floccular lobe of the cerebellum and semicircular canals of the inner ear. A major difference between the two genera resides in the orientation of the braincase, as indicated by the angle between the long axis of the skull and the plane of the lateral semicircular canal. This angle is 25° in Anteosaurus, whereas it is 65° in Moschognathus, which suggests that the braincase of the latter was remodelled as an adaptation to head-butting. This is consistent with less cranial pachyostosis and the retention of a large canine in Anteosauria, which suggests that dentition may have been used for intraspecific fighting and display in addition to trophic interactions. The evolution of a thick skull, horns, and bosses in tapinocephalids parallels the evolutionary reduction of the canine, which lead to a shift of the agonistic function from the mouth to the skull roof, as observed in extant social ungulates. Similarly, tapinocephalians may have developed complex social behaviour.
Key words: Therapsida, Dinocephalia, head-butting, carnivory, trigeminal nerve, bony labyrinth.
Julien Benoit [julien.benoit@wits.ac.za; ORCID: https://orcid.org/0000-0001-5378-3940], Sifelani Jirah [sifelani.jirah@wits.ac.za; ORCID: https://orcid.org/0000-0002-6747-4388], and Bruce S. Rubidge [bruce.rubidge@wits.ac.za; ORCID: https://orcid.org/0000-0003-2477-1873], Evolutionary Studies Institute (ESI); School of Geosciences, University of the Witwatersrand, PO WITS, 2050, Johannesburg, South Africa.
Ashley Kruger[ashleykruger@gmail.com; ORCID: https://orcid.org/0000-0003-1196-8693], Department of Palaeobiology, Swedish Museum of Natural History, Post Office Box 50007, SE-104 05 Stockholm, Sweden.
Vincent Fernandez [v.fernandez@nhm.ac.uk; ORCID: https://orcid.org/0000-0002-8315-1458], European Synchrotron Radiation Facility, 71 Avenue des Martyrs, 38000 Grenoble, France; Imaging and Analysis Centre, Natural History Museum, Cromwell Road, SW7 5BD, London, UK.
Received 13 August 2020, accepted 28 September 2020, available online 19 February 2021.
Copyright © 2021 J. Benoit et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Head-butting (wrestling and ramming) is a widespread behaviour among modern ungulate mammals. Taxa that regularly engage in this behaviour are usually characterised by a suite of specialized anatomical traits that protect the brain (e.g., extensive pachyostosis or pneumatization) and conspicuous cranial ornamentation such as bosses and horns that are often sexually dimorphic (Geist 1966; Emlen 2008). Combat is usually associated with complex behavioural traits such as sexual display, territoriality, and hierarchical social behaviour (Geist 1966; Schaffer and Reed 1972; Emlen 2008; Cabrera and Stankowich 2018), and intraspecific contests are undertaken to secure mating partners and territory (Geist 1966). Accordingly, the suggestion of intraspecific fighting in extinct species implies a certain degree of behavioural complexity.
Dinocephalians were a diverse and abundant group of mostly large-bodied therapsids that are restricted to the middle Permian (265–260 Ma) (Rubidge and Sidor 2001; Kemp 2005; Angielczyk and Kammerer 2018). They are classified in the carnivorous Anteosauria and the primarily herbivorous Tapinocephalia, including the family Tapinocephalidae (Angielczyk and Kammerer 2018; Fraser-King et al. 2019). Tapinocephalid dinocephalians are characterised by extensive pachyostosis of their skull roof, remodelling of their braincase (complete ossification of the ventral aspect of the braincase and mesethmoid, anterior shift of the occipital condyle, and posterior tilting of the braincase) and the presence of often grotesque cranial adornments (horns and bosses), which have been interpreted as adaptations to use the head as a weapon (Brink 1958; Barghusen 1975; Benoit et al. 2016b, 2017c). In contrast, the Anteosauria and other non-tapinocephalid dinocephalians display a lesser degree of cranial pachyostosis (Kammerer 2011) and the bones of their braincase are thinner (Boonstra 1968). In addition, the vertical orientation of the occiput in Anteosaurus indicates that the cranial roof did not align with the neck during head-butting, in contrast to the situation in tapinocephalids, which increased the risk of injuries to the skull, neck articulation, and spinal cord while fighting (Barghusen 1975). This suggests that non-tapinocephalid dinocephalians either did not engage in head-butting or practiced a low energy version of it, such as flank-butting (Barghusen 1975). Among anteosaurs in particular, if intraspecific agonistic behaviours occurred (combat and display), they would likely have involved the oversized canines, as in modern sabre-toothed ungulates (Geist 1966; Emlen 2008; Cabrera and Stankowich 2018). Pachyostosis of the cranial roof and circum-orbital regions in Anteosaurus and Titanophoneus could have evolved to absorb the load generated by the powerful external adductor musculature during bites, rather than as an adaptation for head-butting (Kammerer 2011).
Although head-butting behaviour is well supported for tapinocephalids (Barghusen 1975; Benoit et al. 2016b, 2017c; Angielczyk and Kammerer 2018), relatively little research on this subject has been undertaken on non-tapinocephalids and understanding the polarity of the tapinocephalid palaeoneurological features proves challenging as no other dinocephalian nervous system has yet been studied. The current study utilises X-ray micro Computed Tomography (XµCT) to elucidate the paleoneurology of the anteosaurian Anteosaurus magnificus Watson, 1921, the best known and most abundant carnivorous dinocephalian from the South African Karoo (Kammerer 2011; Kruger et al. 2018). Comparison with the internal neurological structures of a non-head-butting dinocephalian with that of a head-butting tapinocephalid will improve our understanding of cranial adaptations for head-to-head combat in the tapinocephalid lineage and its implications for the palaeobiology of all dinocephalians.
Institutional abbreviations.—AM, Albany Museum (Grahamstown, South Africa); BP, Evolutionary Studies Insitute (formerly Bernard Price Institute, Johannesburg, South Africa); MB.R, Museum für Naturkunde, Fossil Reptile Collection (Berlin, Germany); PIN, Paleontological Institute of Moscow (Russia).
Material and methods
The disarticulated skull (BP/1/7074) of a juvenile Anteosaurus magnificus from the middle Permian of the South African Karoo (from mudstone L of the Moordenaars Member of the Abrahamskraal Formation, farm Bullekraal, Beaufort West district (Jirah and Rubidge 2014; Day and Rubidge 2014, 2020) was XµCT scanned to be digitally re-articulated some years ago (Kruger et al. 2018) but, due to the presence of numerous metallic inclusions, the contrast was not sufficient to distinguish internal structures well, particularly the delicate maxillary canal for the trigeminal nerve and the osseous capsule of the inner ear (bony labyrinth). As a result, the left maxilla and complete occiput were scanned again at the Evolutionary Studies Institute (ESI) using Nikon Metrology XTH 225/320 LC with a 225 kV reflection target, to obtain workable resolution and contrast. The following parameters were used: 140 kV, 150 µA, and an isotropic voxel size of 0.089 mm for the occiput, and 120 kV, 150 µA, and an isotropic voxel size of 0.102 mm for the maxilla. Both scans were performed with a 2.5 mm copper filter.
The recently scanned maxilla and occiput were digitally re-positioned onto the 3D model of the complete skull compiled by Kruger et al. (2018). The 3D reconstruction of BP/1/7074 was accomplished using VSG’s Avizo® Fire (FEI VSG, Hillsboro OR, USA). Each element was exported as a PLY file and was read into memory as a surface file. The surface files were combined as separate elements manually by rotation, translation, and alignment. The skull reconstruction was accomplished by reconstructing the separate areas of the skull roof, palate, and occiput. Once the translation and rotation of the individual elements were as close to real-life as possible, translation was applied. A total of 22 elements that were missing or damaged were replaced by mirroring the opposing complete element and then added to the reconstruction. Much of the right side of specimen BP/1/7074 was reconstructed from the left.
The left maxillary canal, bony labyrinths, and brain endocast were segmented manually using Avizo® 9 (FEI VSG, Hillsboro OR, USA). The maxillary canal was segmented following the method described by Benoit et al. (2016a) as the canal running through the maxilla that carries the maxillary branch of the trigeminal nerve in modern amniotes. It extends from the maxillary sinus posteriorly and internally to the “nutrient” foramina of the snout anteriorly and externally. The branches of the maxillary canal in Anteosaurus were identified and named after the corresponding branches of the trigeminal nerve in modern mammals, as their homology in non-mammalian therapsids and modern mammals is now well supported (Benoit et al. 2016a, b, 2017c, d, 2019; Laaß and Kaestner 2017; Wallace et al. 2019; Pusch et al. 2019, 2020). It should be noted that in extant species the maxillary canal also carries blood vessels and some segments of the facial nerve (Watkinson 1906; Bellairs 1949; Abdel-Kader et al. 2011).
Table 1. Measurements of the bony labyrinth of Anteosaurus magnificus Watson, 1921 (BP/1/7074) and Moschognathus whaitsi Broom, 1914 (AM4950, from Benoit et al. 2017b, d).
|
Measurement |
Anteosaurus |
Moschognathus |
|
Skull length (mm) |
280 |
340 |
|
Body mass (g) |
71031 |
327367 |
|
Length bony labyrinth (mm) |
38 |
36 |
|
Vestibule length (mm) |
21 |
22 |
|
Anterior semicircular canal radius (mm) |
6.16 |
5.73 |
|
Anterior semicircular canal length (mm) |
20.01 |
20.28 |
|
Anterior semicircular canal eccentricity |
0.42 |
0.52 |
|
Anterior semicircular canal lumen diameter (mm) |
1.63 |
1.58 |
|
Lateral semicircular canal radius (mm) |
4.41 |
4.27 |
|
Lateral semicircular canal length (mm) |
13.64 |
12.08 |
|
Lateral semicircular canal eccentricity |
0.44 |
0.02 |
|
Lateral semicircular canal lumen diameter (mm) |
1.96 |
3.09 |
|
Posterior semicircular canal radius (mm) |
4.89 |
4.06 |
|
Posterior semicircular canal length (mm) |
18.56 |
12.30 |
|
Posterior semicircular canal eccentricity |
0.36 |
0.58 |
|
Posterior semicircular canal lumen diameter (mm) |
1.54 |
1.31 |
|
Average semicircular canal radius (mm) |
5.15 |
4.69 |
|
Coefficient of agility (Spoor et al. 2007) |
5 |
4 |
|
Angle between the anterior and lateral semicircular canals (°) |
78 |
90 |
|
Angle between the posterior and lateral semicircular canals (°) |
93 |
74 |
|
Angle between the anterior and posterior semicircular canals (°) |
73 |
76 |
|
Angle between the long axis of the skull and the plane of the lateral semicircular canal (°) |
25 |
65 |
The bony labyrinths were segmented out from the occiput fragment. As the right bony labyrinth is better preserved, the description and measurements are mostly based on the morphology of this side. Measurements of the bony labyrinth (Table 1) were taken digitally using Avizo® 9 and following the protocol of Benoit et al. (2017b) (see SOM, Supplementary Online Information available at http://app.pan.pl/SOM/app66-Benoit_etal_SOM.pdf). Semicircular canal eccentricity and lumen diameter were measured following Araújo et al. (2018). The values in Table 1 were averaged from the measurements taken on the right and left bony labyrinths, except for the semicircular canal radii and eccentricity, which could be measured only on the right side. The angle between the main axis of the skull and the plane of the lateral semicircular canal could only be measured on the right side as the prootic and opisthotic are displaced on the left side of the occiput. The coefficient of agility was calculated using the equation of Spoor et al. (2007) and Silcox et al. (2009) using the average semicircular canal radius and an estimation of the body mass of BP/1/7074 (Table 1). The body mass of BP/1/7074 was estimated using Benoit et al’s (2017a) equations based on a cranial length of 280 mm (Kruger et al. 2018).
The brain endocast was reconstructed using the Scan Surface to Volume function of Avizo® 9 on the digitally re-articulated skull of BP/1/7074. This enabled the conversion of the 3D model into a series of stacked images on which manual segmentation could be performed. It should be noted that even though BP/1/7074 is an almost complete skull, it lacks the orbitosphenoids and the digitally articulated bones do not fit perfectly onto each other (Kruger et al. 2018). As such, the so-obtained endocast only gives a rough idea of the outlines of the endocast and no description or measure of the endocast volume could be safely performed. However, this reconstruction shows the orientation of the braincase inside the skull, which is an important character while discussing adaptations to head-butting in dinocephalians (Boonstra 1968; Benoit et al. 2017c).
Specimen BP/1/7074 is here compared to AM4950 (erroneously referred to as AM6556 in Benoit et al. 2016b), a sub-adult tapinocephalid skull (skull length 340 mm, see Table 1), whose palaeoneurology has been thoroughly studied using synchrotron radiation micro-computed tomography (Benoit et al. 2016b, 2017a, c). This specimen was previously identified as Moschops capensis, but was recently tentatively re-assigned to Moschognathus whaitsi Broom, 1914 (Neumann 2020). Specimen AM4950 was scanned in two parts at the European Synchrotron Radiation Facility (Grenoble France) on the ID17 beamline: first the snout in 2007, and then the braincase in 2015 (see Benoit et al. 2016b, 2017c for more detail). The temporary export of the material for synchrotron scanning was enabled by the South African Heritage Resources Agency (cases 8090 and 8560).
Results
Bony labyrinth.—The bony labyrinth of Anteosaurus specimen BP/1/7074 is a cavity within the occiput fragment that molds the shape of the membranous inner ear. It is divided between a ventral vestibular part (the auditory organ) and the semicircular canals dorsally (the balance organ) (Graf and Klam 2006; Ekdale 2016). The total length of the bony labyrinth from the fenestra vestibuli to the dorsalmost end of the anterior semicircular canal is 38 mm, which is 1.5 mm longer than that of Moschognathus (Benoit et al. 2017b, c). This suggests the two specimens are comparable in terms of bony labyrinth development stages, despite the skull of BP/1/7074 being about 20% smaller than that of AM4950, and only one-third the maximum size for Anteosaurus (Kruger et al. 2018). The total volume of the labyrinth is 1.27 cm3.
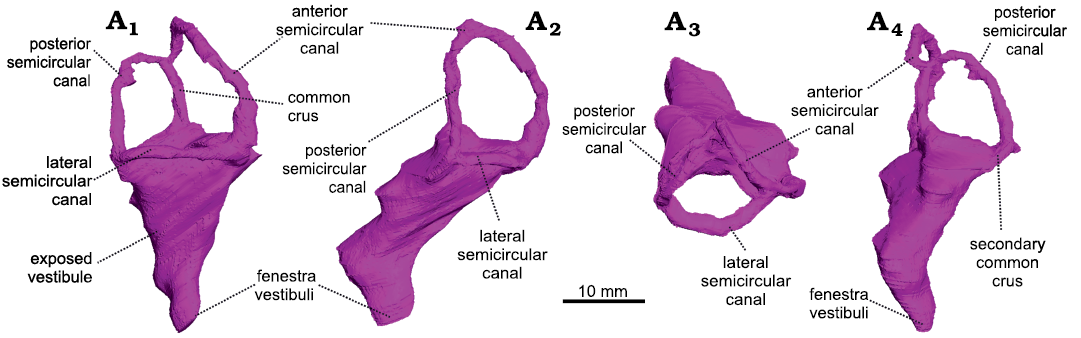
Fig. 1. The right bony labyrinth of Anteosaurus magnificus Watson, 1921 (BP/1/7074) from the middle Permian of farm Bullekraal, South Africa. In lateral view (A1), posterolateral view parallel to the plane of the anterior semicircular canal (A2), dorsal view (A3), posterior view perpendicular to the plane of the anterior semicircular canal (A4).
The vestibule is bordered by the basisphenoid medially, the prootic anteriorly, and the opisthotic posteriorly. As in Moschognathus, the vestibule looks like a long cone in lateral view that tapers ventrally towards the fenestra vestibuli (Fig. 1A1). In posterior and posterolateral views, the vestibule bends ventrolaterally at its midlength (Fig. 1A2, A4) as in gorgonopsians and some dicynodonts (Sigogneau 1974; Araújo et al. 2017; Bendel et al. 2018; Araújo et al. 2018; Benoit et al. 2017b), whereas the vestibule of Moschognathus is straight (Benoit et al. 2017b, c). No cochlear canal is present. The vestibule is 21 mm long, 13 mm (right side) to 14 mm (left side) large, and its maximum mediolateral thickness is 6 mm (Table 1). The stapedial ratio (length-to-width ratio of the fenestra vestibuli) is 1.1 on the right side and 1.0 on the left side, which indicates a rounded fenestra vestibuli. As in Moschognathus (AM4950) and Syodon (PIN 2505/1 and PIN 157/1047; Ivakhnenko 2008), the dorsalmost part of the vestibule of Anteosaurus is exposed medially in the brain cavity (Fig. 2). The vestibule and jugular foramen are located in two distinct recesses inside the brain cavity (Fig. 2) as in gorgonopsians and other dinocephalians (Boonstra 1968; Kemp and Parrington 1969; Sigogneau 1974; Ivakhnenko 2008).
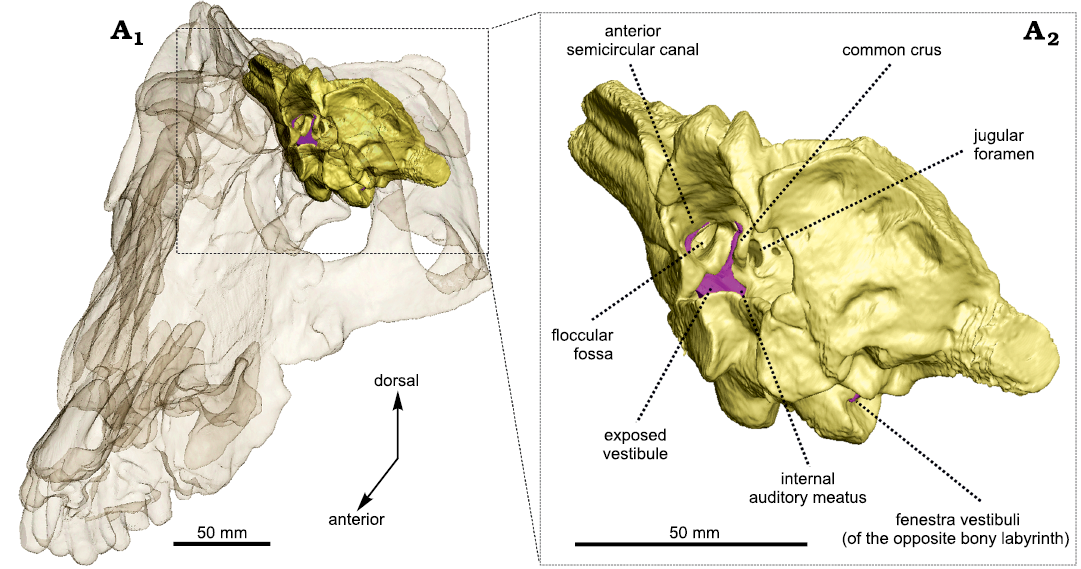
Fig. 2. Occiput fragment of Anteosaurus magnificus Watson, 1921 (BP/1/7074) from the middle Permian of farm Bullekraal, South Africa. Internal view showing the exposure of the bony labyrinth (purple) in the braincase (A1), enlarged view (A2).
The semicircular canals are bordered by the prootic (anterior semicircular canal and ampullar limb of the lateral semicircular canal) and opisthotic (posterior semicircular canal and posterior limb of the lateral semicircular canal). The anterior canal may contact the supraoccipital dorsally, although the suture is obscured by the iron-rich matrix in XµCT data. Medially, the anterior semicircular canal and the common crus are exposed in the braincase (Fig. 2). In Syodon the ampullae of all three semicircular canals are largely exposed in the braincase, but the anterior semicircular canal is completely enclosed in an osseous tube (Ivakhnenko 2008). Unlike in Moschognathus (Benoit et al. 2017c), the space delineated by the anterior semicircular canal is pierced by a deep and broad floccular fossa in Anteosaurus. Among non-mammalian therapsids, such a deep floccular fossa is also present in Syodon, gorgonopsians, and cynodonts (Sigogneau 1974; Hopson 1979; Ivakhnenko 2008; Araújo et al. 2017; Benoit et al. 2017b; Pusch et al. 2019). All three semicircular canals have a roughly oval outline (Fig. 1) and are more slender than in Moschognathus (Benoit et al. 2017b, c). As in Moschognathus (Benoit et al. 2017b, c), the ampullae are inconspicuous (Fig. 1). The anterior canal has the largest radius of curvature and is the longest of all three canals, whereas the lateral one is the smallest and shortest (Table 1), as is usual in therapsids, including mammals (Ivakhnenko 2008; Ekdale 2013; Castanhinha et al. 2013; Benoit et al. 2017b; Araújo et al. 2017, 2018; Pusch et al. 2019). The anterior canal is also taller than the posterior one as it reaches higher dorsally (Fig. 1A1). Even though the skull of BP/1/7074 is smaller than that of AM4950, the radius of curvature of all three semicircular canals is larger in BP/1/7074 than in AM4950, and the average radius of curvature is 0.47 mm larger in BP/1/7074 than in AM4950 (Table 1). BP/1/7074 also has longer posterior and lateral semicircular canals than AM4950, whereas their anterior canals are equivalent in length (Table 1). BP/1/7074 and AM4950 also differ by the angle between the plane of the lateral semicircular canal and the main axis of the skull. This angle is about 25° in BP/1/7074, whereas it is much larger (about 65°) in AM4950 (Fig. 3). The angles between the anterior and lateral semicircular canals and between the anterior and posterior semicircular canals are smaller in Anteosaurus than in Moschognathus. In contrast, the angle between the posterior and lateral semicircular canals is larger in Anteosaurus (Table 1). The posterior and lateral semicircular canals are fused into a secondary common crus (Fig. 1A4), as is usual in non-mammalian therapsids and early mammals (Ekdale 2013; Benoit et al. 2017b). Unlike in biarmosuchians (Benoit et al. 2017b), the posterior canal does not project ventrally below the level of the plane of the lateral semicircular canal, although it is evident that the posterior canal enters the vestibule slightly below the plane of the lateral semicircular canal (Fig. 1A4).
Maxillary canal.—The maxillary canal appears to be a morphologically conservative structure in dinocephalians, judging by its similarity in the two genera documented so far, Anteosaurus and Moscognathus. Compared to that of other non-prozostrodontian synapsids (Benoit et al. 2016a, b, 2017d, 2018, 2019; Laaß and Kaestner 2017; Wallace et al. 2019; Pusch et al. 2019, 2020) the maxillary canal of Anteosaurus is relatively short anteroposteriorly, as in Moschognathus (Fig. 4). Caudally, the maxillary canal of Anteosaurus emerges from the maxillary sinus above the level of the second postcanine tooth. The maxillary sinus could not be completely reconstructed digitally because of the fragmentary nature of the maxilla of BP/1/7074. Between the level of the first and second postcanines, almost immediately after leaving the maxillary sinus, the caudal alveolar canal branches off the maxillary canal ventrally (Fig. 4A). As in Moschognathus, it ramifies further distally into two branches oriented ventrally and three branches oriented posteriorly (Fig. 4A). Three branches of unknown identification are located immediately dorsally to the alveolar canal and are positioned dorsolaterally (Fig. 4A). Similar branches are present in Moschognathus (Fig. 4B) as well as in the therocephalians Bauria and Olivierosuchus (Benoit et al. 2017d) and in the anomodont Patranomodon (Benoit et al. 2018), in which they were identified as belonging to the external nasal branch of the maxillary canal. We prefer to remain cautious about the identity of these rami as they do not stem from the same trunk as the external nasal branch (Fig. 4). Anterior to the caudal alveolar branch, the rostral and medial alveolar branches diverge anteroventrally from a common trunk, as in Moschognathus (Fig. 4). They remain simple in Anteosaurus, although an isolated branch may belong to either the rostral alveolar or the superior labial branches (Fig. 4A). In contrast, the rostral alveolar branch is extremely ramified (as is the superior labial branch) in Moschognathus, most likely to innervate and supply the soft tissue associated with the elaborate rostral dentition in this tapinocephalid (Fig. 4B) (Benoit et al. 2017a). The external nasal ramus is the most ramified branch of the maxillary nerve in Anteosaurus. It branches off the maxillary canal at the level of the functional canine (Fig. 4A) and comprises five branches directed dorsally and slightly caudally. This departs from the condition in Moschognathus in which the external nasal ramus is dominated by one long and thick branch oriented dorsocaudally (Fig. 4B). A similar condition is encountered in the therocephalian Lycosuchus (Pusch et al. 2020) and the anomodont Patranomodon (Benoit et al. 2018). The condition in Anteosaurus looks more similar to that in other non-prozostrodontian therapsids (Benoit et al. 2016a, b, 2017d, 2018, 2019; Laaß and Kaestner 2017; Pusch et al. 2019). Even more rostrally in Anteosaurus, at the level of the replacement canine, the maxillary canal divides into two branches oriented rostrally (Fig. 4A). The dorsalmost branch is the internal nasal ramus, which is itself divided into a dorsal and ventral branch. The ventralmost branch is the superior labial ramus, which appears to bear no ramification. Three isolated canals oriented anteroposteriorly might belong to one of the superior labial or internal nasal branches, or may represent purely vascular, “nutrient” canals (Fig. 4A).
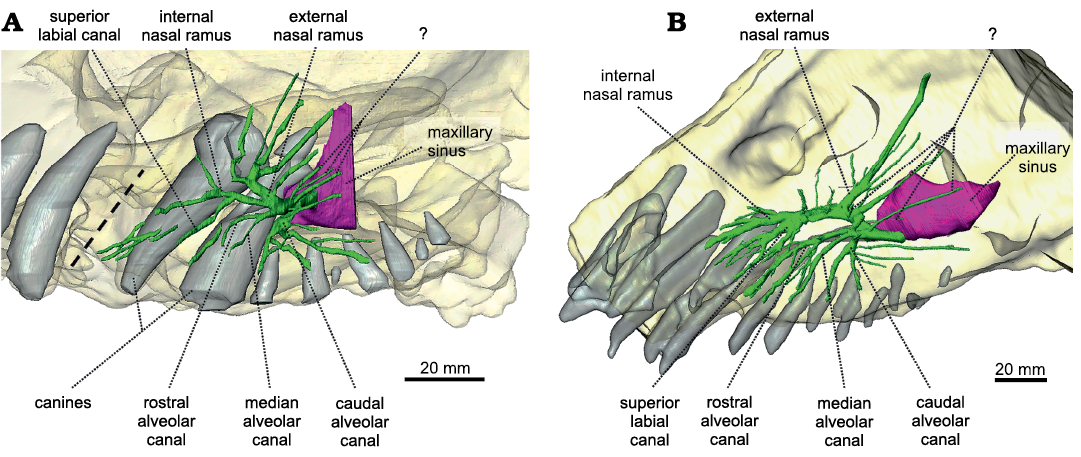
Fig. 4. The maxillary canal system of Anteosaurus magnificus Watson, 1921 (A, BP/1/7074) from the middle Permian of farm Bullekraal, South Africa and Moschognathus whaitsi Broom, 1914 (B, AM4950) from the middle Permian of farm The Grant 39, South Africa. The dashed line indicates the position of a crack in BP/1/7074. Green, maxillary canal; purple, maxillary sinus; light grey, teeth.
Discussion
Adaptations to carnivory.—Because of its recurved canines, bone-crushing dentition, prominent pterygoid processes, and enlarged temporal fenestrae that provide large points of insertion for powerful jaw adductor musculature, Anteosaurus has been unquestionably interpreted as a carnivorous animal (Boonstra 1954b; Kammerer 2011). These adaptations to carnivory are expected to be reflected in the nervous system as well (Witmer and Ridgely 2009; Grohé et al. 2018; King et al. 2020). The bony labyrinth of Anteosaurus is remarkable for its large semicircular canals compared to those of Moschognathus, both in terms of length and radii of curvature (Table 1), even though the Anteosaurus skull studied here (BP/1/7074) is 20% smaller than that of AM4950. The average radius of curvature of all three semicircular canals is 5.15 mm in BP/1/7074, which is greater than that of all non-mammalian therapsids studied so far (Castanhinha et al. 2013; Rodrigues et al. 2013; Benoit et al. 2017b; Araújo et al. 2017, 2018; Pusch et al. 2019, 2020). Few non-mammalian therapsids are large enough to be compared to Anteosaurus, but Pusch et al. (2020) reported an average semicircular canal radius of 3.44 mm in the contemporaneous carnivorous therocephalian Lycosuchus (specimen MB.R.995) with similar skull length (estimated to 260 mm). As Anteosaurus has semicircular canals larger than both Moschognathus and Lycosuchus, it is safe to conclude that its semicircular canals are larger than expected for a middle Permian therapsid of this size. This is supported by the agility score calculated for BP/1/7074, which is five (given an estimated body mass of 71 kg for this individual, Table 1). This corresponds to a medium-to-fast-moving animal and is equivalent to that of a mountain lion (Felis concolor) among modern carnivores (Spoor et al. 2007). In comparison, the agility score of Moschognathus and Lycosuchus is four (using body masses estimated from skull lengths using the equation of Benoit et al. 2017b) which corresponds to less agile animals (Spoor et al. 2007). As the semicircular canals are involved in synchronizing eye, head, and neck movements, larger semicircular canals compared to body size are usually associated with enhanced tracking abilities, agility, and fast locomotion in carnivores (Spoor et al. 2007; Silcox et al. 2009; Witmer and Ridgely 2009; Ryan et al. 2012; Grohé et al. 2018; King et al. 2020). The skull of BP/1/7074, which is a juvenile, is only one third the maximum size for Anteosaurus magnificus (Kruger et al. 2018) and it is not yet known whether the proportions of the semicircular canals would be conserved in adulthood. Current data on the ontogeny of the bony labyrinth in non-mammalian therapsids are scarce, and it is difficult to assess if their bony labyrinth reached their adult morphology early in ontogeny as in mammals (e.g., in primates [MacPhee 1981; Daniel et al. 1982; Jeffery and Spoor 2004], rodents and rabbits [Hoyte 1961; Lindenlaub and Burda 1993], carnivorans [Curthoys et al. 1982], afrotherians [Klaauw 1931; Fischer 1989], artiodactyls [Mennecart and Costeur 2016], and marsupials [Ekdale 2010]), or whether it continued growing throughout ontogeny as is plesiomorphic for amniotes (Bullar et al. 2019; Neenan et al. 2019). In the dataset published by Benoit et al. (2017b), the bony labyrinths of two specimens of the cynodont Cynosaurus suppostus of very different skull lengths were measured. The smallest skull measures 49.70 mm, slightly less than half the size of the adult one, which measures 115.83 mm. The bony labyrinths of the two specimens are 6.22 mm and 12.58 mm long, respectively. Although this is not enough data to suggest an isometric scaling of the bony labyrinth to cranial size, it nevertheless indicates that preservation of size proportions of the semicircular canals relative to skull length during ontogeny cannot be excluded in Permian therapsids.
The hypothesized enhanced locomotory and tracking abilities in Anteosaurus are also supported by the presence of a deep and broad floccular fossa compared to a very shallow one in Moschognathus (Fig. 3). The contrasting dimensions of the fossa in the closely related Anteosaurus and Moschognathus support the notion that this trait is not constrained by phylogeny in dinocephalians. Moreover, in mammals and non-mammalian therapsids, a deep floccular fossa is more typically encountered in small taxa (Hopson 1979; Gannon et al. 1988; Schmitt and Gheerbrant 2016), whereas Anteosaurus is one of the largest Anteosauria, which suggests that its deep floccular fossa was functionally significant. This structure housed the floccular lobe of the cerebellum, a region of the brain that monitors the vestibuloocular and vestibulocollic reflexes during motion, and enhances gaze stability while tracking moving prey (Sánchez-Villagra 2002; King et al. 2020). Sight stabilization would have been particularly important if anteosaurids were not hunting in broad daylight as suggested for Titanophoneus by using the dimensions of the sclerotic ring (Angielczyk and Schmitz 2014). Although the dimensions of the floccular fossa do not perfectly reflect the volume of the floccular lobe (Walsh et al. 2013), it is expected that agile, active terrestrial predators would have an enlarged floccular fossa (Hopson 1979; Witmer and Ridgely 2009; King et al. 2020; Schade et al. 2020; but see Ferreira-Cardoso et al. 2017 for a critical reappraisal of this hypothesis). Even though enlarged semicircular canals or floccular fossa alone are weak indicators of agility (David et al. 2016; Ferreira-Cardoso et al. 2017), taken together, the presence of both features in Anteosaurus gives a consistent signal supporting the idea that the latter was likely more agile than its herbivorous close relative Moschognathus. Overall, the palaeoneurology of Anteosaurus thus corresponds to that of a more agile and rapidly moving predator than previously considered based on its postcranial anatomy (Boonstra 1955), and far removed from the sluggish amphibious lifestyle that has been proposed for anteosaurids by some authors (Olson 1962; King 1988; Ivakhnenko 2008).
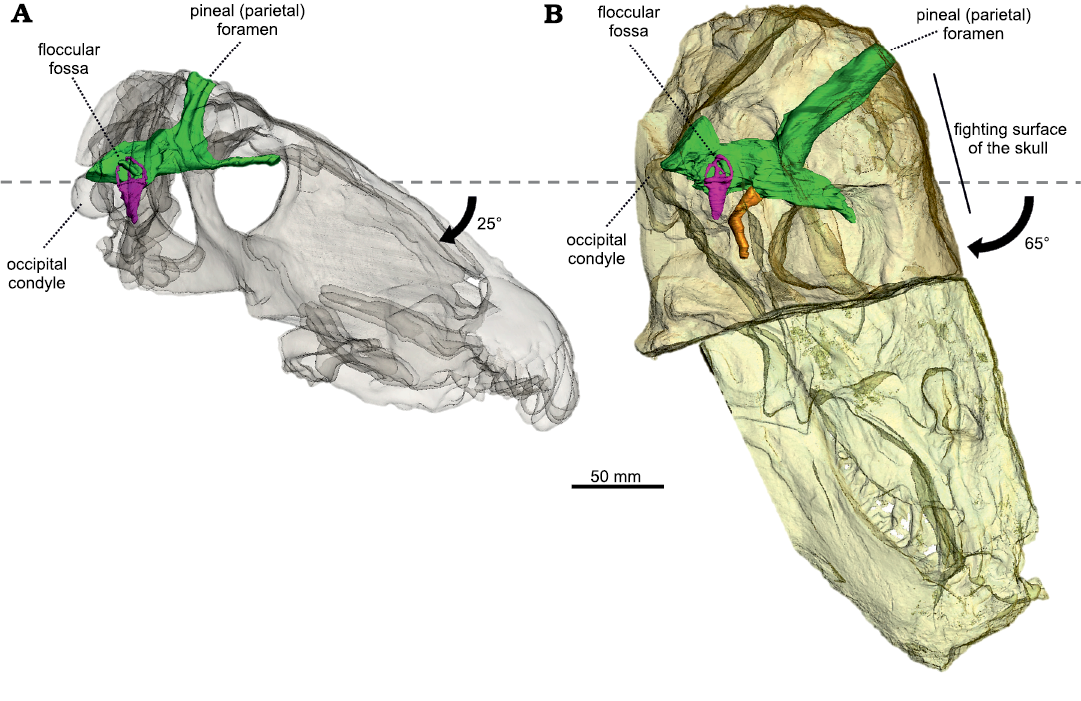
Fig. 3. The semi-transparent skulls of Anteosaurus magnificus Watson, 1921 (A, BP/1/7074) from the middle Permian of farm Bullekraal, South Africa and Moschognathus whaitsi Broom, 1914 (B, AM4950) from the middle Permian of farm The Grant 39, South Africa, aligned on the plane of their lateral semicircular canal. The black arrow indicates the tilting of the long axis of the skull compared to the plane of the lateral semicircular canal. The dashed line represents the plane of the lateral semicircular canal. Green, endocranial cast; purple, bony labyrinth; orange, pituitary fossa.
Re-organisation of the braincase and head-butting in tapinocephalids.—Anteosaurus and Moschognathus differ greatly in the orientation of the braincase compared to the rest of the skull (Fig. 3). In Anteosaurus, the long axis of the braincase and the skull are almost parallel, as in the other non-tapinocephalid dinocephalians Syodon and Jonkeria (Boonstra 1968; Ivakhnenko 2008), which illustrates that their braincase retains the plesiomorphic orientation for therapsids. In contrast, in Moschognathus the snout points distinctly downward when the braincase is horizontal (Benoit et al. 2017c). The same downward tilting of the snout is also observed when aligning the braincase to the horizontal in the tapinocephalids Mormosaurus, Moschops, Keratocephalus, and Struthiocephalus (Brink 1958; Boonstra 1968; Fig. 5). This orientation of the braincase can be quantified by measuring the orientation of the plane of the lateral semicircular canal compared to the long axis of the skull. This angle is 25° in Anteosaurus, whereas it is 65° in Moschognathus (Benoit et al. 2017c; Fig. 3). This demonstrates a dramatic re-organisation of the braincase in tapinocephalid dinocephalians (Brink 1958; Benoit et al. 2017c) which was probably driven by an adaptation to using the head as a weapon in this lineage as hypothesized by Barghusen (1975) and Benoit et al. (2017c) (Fig. 5).
Such misalignment of the braincase and snout is termed klinorhynchy or cyptocephaly in modern mammals and is usually interpreted as an adaptation to grazing or head butting in ungulates, or as a consequence of the enlargement of the braincase in Great Apes (Schaffer and Reed 1972; Lieberman et al. 2000; Solounias 2007). As the braincase of tapinocephalid dinocephalians is relatively small (Benoit et al. 2017a), the last hypothesis is unlikely. The downward pitching of the snout relative to the braincase could be an adaptation to feed on low vegetation (Solounias 2007), as hypothesized for the dinosaur Nigersaurus (Sereno et al. 2007) and modern Rhinocerotidae (Schellhorn 2018). However, this hypothesis is not supported by the data published by Benoit et al. (2017b), who documented the angle between the long axis of the skull and the plane of the lateral semicircular canal in 29 non-mammalian therapsids and found no difference between herbivores and carnivores. Diet is thus unlikely to account for the cyptocephaly of the tapinocephalid skull and adaptation to head-butting seems more likely.
Most authors accept that tapinocephalids were head-butting taxa (Geist 1972; Barghusen 1975; Rubidge and Sidor 2001; Kemp 2005; Emlen 2008; Benoit et al. 2016b, 2017c). Tapinocephalid skulls have numerous traits (such as ossified braincase, inner ear, and cranial architecture) that are interpreted as adaptations for head-butting and are absent or less pronounced in other dinocephalian taxa (Barghusen 1975; Benoit et al. 2016b, 2017c). The skull roof of tapinocephalids, as indicated in Moschognathus AM4950, comprises two histologically distinct layers (Benoit et al. 2016b, 2017c). The outer bony layer (15–20 mm thick) has dense, osteosclerotic cortical bone that covers a 40 mm thick layer of trabeculated, spongy bone. In some tapinocephalids cranial thickness can reach up to 310 mm around the pineal foramen (Boonstra 1954a; Day et al. 2015). These bone layers were likely covered by an external keratinous sheath since blood vessels supplied the surface of the hypothesized fighting surface in Moschognathus (Barghusen 1975; Benoit et al. 2016b, 2017c). The thickened and histologically differentiated cranial vault of tapinocephalids presumably aided in absorbing shocks and dissipating stresses as in pachycephalosaurid dinosaurs (Snively and Cox 2008; Snively and Theodor 2011). In addition, the ossified braincase in tapinocephalids was also an important adaptation to head-butting as the braincase walls comprise thick trabeculated bone that, coupled with thick meninges, would have protected the central nervous system against mechanical stress (Boonstra 1968; Benoit et al. 2016b, 2017c). The re-orientation of the braincase is also consistent with head-butting behaviour as it results in the posterior shifting of the parietal foramen in tapinocephalids compared to that of Anteosaurus (Fig. 5). This served to remove the delicate pineal eye from the top of the skull where it would have been directly exposed to blows (Benoit et al. 2017c) and to align the fighting surface, braincase, and occipital condyle with the neck, which enhanced the transmission and dissipation of energy to the body (Barghusen 1975; Benoit et al. 2017c) This cyptocephalic reorganisation of the braincase is similar to that of modern high energy head-butting mammals (Schaffer and Reed 1972).
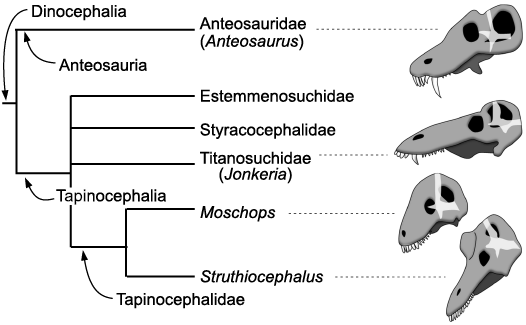
Fig. 5. Phylogeny of the Dinocephalia (after Fraser-King et al. 2019). Skulls and braincase (transparent white) redrawn after Boonstra (1968). The skulls are positioned so that their braincases are oriented the same way.
Trends in dinocephalian behavioural evolution.—Among dinocephalians, the Anteosauria are distinguished from derived Tapinocephalia by their very large upper canines and their relatively small cranial adornments (Kammerer 2011; Angielczyk and Kammerer 2018; Kruger et al. 2018). The presence of a large canine and limited cranial pachyostosis is likely plesiomorphic for dinocephalians (Kammerer 2011; Fraser-King et al. 2019). The oversized canine was likely used as a weapon and/or a display tool during intraspecific contests for mates and territory to impress and stab rivals, as in the walrus and modern saber-toothed ruminants (Geist 1966, 1972; Emlen 2008). A low energy form of agonistic behaviour too cannot be excluded for Anteosaurus given the pachyostosis of its skull (Barghusen 1975; Benoit et al. 2016b), although the pachyostosis of the circum-orbital region may have enabled the skull to withstand the action of the powerful adductor musculature (Kammerer 2011) and the nasal ridge may have strengthened the snout against a large struggling prey. As such, head-butting is not a necessary condition to account for cranial pachyostosis in Anteosaurus. Tapinocephalia, on the other hand, show a transition from basal taxa that have both enlarged canines and pachyostotic cranial bosses and horns, such as the Titanosuchidae, Estemmenosuchidae, and Styracocephalidae, to the derived Tapinocephalidae that have a reduced or no visible caniniform but conspicuous cranial adornments and horns (Geist 1972; Angielczyk and Kammerer 2018; Fraser-King et al. 2019; Fig. 5). Such a displacement of the display/combat function from the mouth to the skull roof occurred numerous times in ruminants (e.g., cervids, giraffids) (Geist 1966; Cabrera and Stankowich 2018), rhinocerotids (Heissig 1973), and perhaps pachycephalosaurs (Dieudonné et al. 2020) and correlates significantly with a shift from solitary to social behaviour in ungulates (Cabrera and Stankowich 2018). Agonistic behaviour involving stabbing by canines is mostly found in solitary taxa as it would be counter-selected in gregarious species since individuals with open wounds would attract predators, thus threatening the security of the herd (Cabrera and Stankowich 2018). Also, living in herds would increase the probability of agonistic intraspecific encounters and thus the risk of individuals being fatally wounded (Geist 1966). In gregarious species, it is expected that an evolutionary shift toward more dissuasive and less harmful cranial adornments, such as the massive yet blunt cranial roof of tapinocephalids, would lead to more ritualized and less dangerous combat (Geist 1966, 1972; Emlen 2008; Cabrera and Stankowich 2018). Head-butting results in a ranking of individuals and implies social bonds and a hierarchical structure of the group that are two complex behavioural traits (Geist 1966; Schaffer and Reed 1972; Emlen 2008; Cabrera and Stankowich 2018). There is no direct evidence for gregariousness in tapinocephalids (i.e., parallel trackways), but group death assemblages comprising 5–12 individuals have been anecdotally reported (Gregory 1926; Boonstra 1955; Rubidge et al. 2019; Neumann 2020), which would support the hypothesis that tapinocephalids might have lived in small groups, at least intermittently.
Additionally (or alternatively), the evolutionary change from an omnivorous diet in basal tapinocephalians toward a more specialized herbivorous diet in tapinocephalids imposed a strong selection toward homodonty (i.e., the talon and heel morphology of tapinocephalid dentition) (Rubidge and Sidor 2001; Angielczyk and Kammerer 2018). This selected against the presence of a caniniform tooth, hence facilitating the displacement of the display/combat function to the cranial roof. A combination of a shift from carnivory to herbivory, and from biting contest to head-butting could well account for the re-organisation of the braincase in tapinocephalid dinocephalians (Fig. 5).
Conclusions
The study of the bony labyrinth, braincase, and maxillary canal of Anteosaurus magnificus enables, for the first time, comparison between the tapinocephalids and their non-head-butting relatives. The maxillary canals of Anteosaurus and Moschognathus are very similar, suggesting that the morphology of the trigeminal nerve was conservative in dinocephalians. The bony labyrinth of the two genera differs mostly in the larger dimensions of the semicircular canals in Anteosaurus, which, along with the presence of an enlarged floccular fossa, suggests that Anteosaurus was a comparatively more agile predator than hitherto envisioned. Comparisons of the orientation of the braincase and lateral semicircular canal between tapinocephalid and non-tapinocephalid dinocephalians confirm that the former were cyptocephalic, probably as a result of adaptations to withstand head-butting. Anteosaurids likely used their canines for display and face biting during agonistic intraspecific behaviours, whereas tapinocephalids were using their thickened, pachyostotic skulls for display and fighting. Accordingly, we hypothesize a displacement of the display/combat function during the evolutionary history of dinocephalians. This could be further tested by a systematic survey of fighting injuries and bite punctures on well-preserved skulls of dinocephalians, an approach that has already proven fruitful for many other taxonomic groups (Boucot 1990; Boucot and Poinar 2011; Peterson and Vittore 2012).
Acknowledgements
We thank the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities and Paul Tafforeau (European Synchrotron Radiation Facility, Grenoble, France) for assistance in using beamline ID17. We would like to thank Taahirah Mangera (School of Mechanical, Industrial and Aeronautical Engineering, University of the Witwatersrand, Johannesburg, South Africa), Sandra C. Jasinoski (Evolutionary Studies Institute, University of the Witwatersrand, South Africa), and Safiyyah Iqbal (School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, South Africa) for their insight on some parts of the discussion, and the reviewers Ricardo Araújo (Universidade de Lisboa, Portugal), Christian Kammerer (North Carolina Museum of Natural Sciences, Raleigh, USA), and Luisa Pusch (Museum für Naturkunde, Berlin, Germany) for their comments. This research was conducted with financial support from the Palaeontological Scientific Trust (PAST) and its scatterlings projects; the National Research Foundation of South Africa (NRF) African Origins Platform; and the DST-NRF Centre of Excellence in Palaeosciences (CoE in Palaeosciences).
References
Abdel-Kader, T.G., Ali, R.S., and Ibrahim, N.M. 2011. The cranial nerves of Mabuya quinquetaeniata III: nervus trigeminus. Life Science Journal 8: 650–669.
Angielczyk, K.D. and Kammerer, C.F. 2018. 5. Non-mammalian synapsids: the deep roots of the mammalian family tree. In: F. Zachos and R. Asher (eds.), Mammalian Evolution, Diversity and Systematics, 117–198. De Gruyter, Berlin. Crossref
Angielczyk, K.D. and Schmitz, L. 2014. Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proceedings of the Royal Society B: Biological Sciences 281 (1793): 20141642. Crossref
Araújo, R., Fernandez, V., Polcyn, M.J., Fröbisch, J., and Martins, R.M.S. 2017. Aspects of gorgonopsian paleobiology and evolution: insights from the basicranium, occiput, osseous labyrinth, vasculature, and neuroanatomy. PeerJ 5: e3119. Crossref
Araújo, R., Fernandez, V., Rabbitt, R.D., Ekdale, E.G., Antunes, M.T., Castanhinha, R., Fröbisch, J., and Martins, R.M.S. 2018. Endothiodon cf. bathystoma (Synapsida: Dicynodontia) bony labyrinth anatomy, variation and body mass estimates. PLoS ONE 13 (3): e0189883. Crossref
Barghusen, H.R. 1975. A review of fighting adaptations in dinocephalians (Reptilia, Therapsida). Paleobiology 1: 295–311. Crossref
Bellairs, A.D. 1949. Observations on the snout of Varanus, and a comparison with that of other lizards and snakes. Journal of Anatomy 83: 116–146.
Bendel, E.-M., Kammerer, C.F., Kardjilov, N., Fernandez, V., and Fröbisch, J. 2018. Cranial anatomy of the gorgonopsian Cynariops robustus based on CT-reconstruction. PLoS ONE 13 (11): e0207367. Crossref
Benoit, J., Angielczyk, K.D., Miyamae, J.A., Manger, P., Fernandez, V., and Rubidge, B. 2018. Evolution of facial innervation in anomodont therapsids (Synapsida): Insights from X-ray computerized microtomography. Journal of Morphology 279: 673–701. Crossref
Benoit, J., Fernandez, V., Manger, P.R., and Rubidge, B.S. 2017a. Endocranial casts of pre-mammalian therapsids reveal an unexpected neurological diversity at the deep evolutionary root of mammals. Brain, Behavior and Evolution 90: 311–333. Crossref
Benoit, J., Manger, P.R., and Rubidge, B.S. 2016a. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Scientific Reports 6 (1): 25604. Crossref
Benoit, J., Manger, P.R., Fernandez, V., and Rubidge, B.S. 2016b. Cranial bosses of Choerosaurus dejageri (Therapsida, Therocephalia): Earliest evidence of cranial display structures in eutheriodonts. PLoS ONE 11 (8): e0161457. Crossref
Benoit, J., Manger, P.R., Fernandez, V., and Rubidge, B.S. 2017b. The bony labyrinth of late Permian Biarmosuchia: palaeobiology and diversity in non-mammalian Therapsida. Palaeontologia Africana 52: 58–77.
Benoit, J., Manger, P.R., Norton, L., Fernandez, V., and Rubidge, B.S. 2017c. Synchrotron scanning reveals the palaeoneurology of the head-butting Moschops capensis (Therapsida, Dinocephalia). PeerJ 5: e3496. Crossref
Benoit, J., Norton, L.A., Manger, P.R., and Rubidge, B.S. 2017d. Reappraisal of the envenoming capacity of Euchambersia mirabilis (Therapsida, Therocephalia) using μCT-scanning techniques. PLoS ONE 12 (2): e0172047. Crossref
Benoit, J., Ruf, I., Miyamae, J.A., Fernandez, V., Rodrigues, P.G., and Rubidge, B.S. 2019. The evolution of the maxillary canal in Probainognathia (Cynodontia, Synapsida): Reassessment of the homology of the infraorbital foramen in mammalian ancestors. Journal of Mammalian Evolution 27: 329–348. Crossref
Boonstra, L.D. 1954a. The cranial morphology and taxonomy of the Tapinocephalid genus Struthiocephalus. Annals of the South African Museum 42: 32–53.
Boonstra, L.D. 1954b. The cranial structure of the titanosuchian: Anteosaurus. Annals of the South African Museum 42: 108–148.
Boonstra, L.D. 1955. The girdles and limbs of the South African Deinocephalia. Annals of the South African Museum 42: 185–326.
Boonstra, L.D. 1968. The braincase, basicranial axis and median septum in the Dinocephalia. Annals of the South African Museum 50: 195–273.
Boucot, A.J. 1990. Evolutionary Paleobiology of Behavior and Coevolution. 725 pp. Elsevier, New York.
Boucot, A.J. and Poinar, G.O. Jr. 2011. Fossil Behavior Compendium. 424 pp. CRC Press, Boca Raton. Crossref
Brink, A.S. 1958. Struthiocephalus kitchingi sp. nov. Palaeontologia Africana 5: 39–56.
Bullar, C.M., Zhao, Q., Benton, M.J., and Ryan, M.J. 2019. Ontogenetic braincase development in Psittacosaurus lujiatunensis (Dinosauria: Ceratopsia) using micro-computed tomography. PeerJ 7: e7217. Crossref
Cabrera, D. and Stankowich, T. 2018. Stabbing slinkers: Tusk evolution among artiodactyls. Journal of Mammalian Evolution 27: 265–272. Crossref
Castanhinha, R., Araújo, R., Júnior, L.C., Angielczyk, K.D., Martins, G.G., Martins, R.M.S., Chaouiya, C., Beckmann, F., and Wilde, F. 2013. Bringing dicynodonts back to life: Paleobiology and anatomy of a new emydopoid genus from the upper Permian of Mozambique. PLoS ONE 8 (12): e80974. Crossref
Curthoys, I.S., Blanks, R.H.I., and Markham, C.H. 1982. Semicircular canal structure during postnatal development in cat and guinea pig. Annals of Otology, Rhinology & Laryngology 91: 185–192. Crossref
Day, M.O. and Rubidge, B.S. 2014. A brief lithostratigraphic review of the Abrahamskraal and Koonap formations of the Beaufort Group, South Africa: towards a basin-wide stratigraphic scheme for the middle Permian Karoo. Journal of African Earth Sciences 100: 227–242. Crossref
Day, M.O. and Rubidge, B.S. 2020. Biostratigraphy of the Tapinocephalus Assemblage Zone. South African Journal of Geology 123: 149–164. Crossref
Day, M.O., Güven, S., Abdala, F., Jirah, S., Rubidge, B., Almond, J., and Natura Viva, Cape Town, South Africa. 2015. Youngest dinocephalian fossils extend the Tapinocephalus Zone, Karoo Basin, South Africa. South African Journal of Science 111: 78–82. Crossref
Daniel, H.J., Schmidt, R.T., Olshan, A.F., and Swindler, D.R. 1982. Ontogenetic changes in the bony labyrinth of Macaca mulatta. Folia Primatologica 38 (1–2): 122–129. Crossref
David, R., Stoessel, A., Berthoz, A., Spoor, F., and Bennequin, D. 2016. Assessing morphology and function of the semicircular duct system: introducing new in-situ visualization and software toolbox. Scientific Reports 6 (1): 32772. Crossref
Dieudonné, P.-E., Cruzado-Caballero, P., Godefroit, P., and Tortosa, T. 2020. A new phylogeny of cerapodan dinosaurs. Historical Biology [published online, https://doi.org/10.1080/08912963.2020.1793979]. Crossref
Ekdale, E.G. 2010. Ontogenetic variation in the bony labyrinth of Monodelphis domestica (Mammalia: Marsupialia) following ossification of the inner ear cavities. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 293: 1896–1912. Crossref
Ekdale, E.G. 2013. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS ONE 8 (6): e66624. Crossref
Ekdale, E.G. 2016. Form and function of the mammalian inner ear. Journal of Anatomy 228: 324–337. Crossref
Emlen, D.J. 2008. The evolution of animal weapons. Annual Review of Ecology, Evolution, and Systematics 39: 387–413. Crossref
Ferreira-Cardoso, S., Araújo, R., Martins, N.E., Martins, G.G., Walsh, S., Martins, R.M.S., Kardjilov, N., Manke, I., Hilger, A., and Castanhinha, R. 2017. Floccular fossa size is not a reliable proxy of ecology and behaviour in vertebrates. Scientific Reports 7 (1): 2005. Crossref
Fischer, M.S. 1989. Zur Ontogenese der Tympanalregion der Procaviidae (Mammalia: Hyracoidea). Gegenbaurs Morphologisches Jahrbuch 135: 795–840.
Fraser-King, S.W., Benoit, J., Day, M.O., and Rubidge, B.S. 2019. Cranial morphology and phylogenetic relationship of the enigmatic dinocephalian Styracocephalus platyrhynchus from the Karoo Supergroup, South Africa. Palaeontologia Africana 54: 14–29.
Gannon, P.J., Eden, A.R., and Laitman, J.T. 1988. The subarcuate fossa and cerebellum of extant primates: comparative study of a skull-brain interface. American Journal of Physical Anthropology 77: 143–164. Crossref
Geist, V. 1966. The evolution of horn-like organs. Behaviour 27: 175–214. Crossref
Geist, V. 1972. An ecological and behavioural explanation of mammalian characteristics and their implication to therapsid evolution. Zeitschrift für Säugetierkunde: im Auftrage der Deutschen Gesellschaft für Säugetierkunde e.V. 37: 1–15.
Graf, W. and Klam, F. 2006. Le système vestibulaire : anatomie fonctionnelle et comparée, évolution et développement. Comptes Rendus Palevol 5: 637–655. Crossref
Gregory, W.K. 1926. The skeleton of Moshops capensis Broom, a dinocephalian reptile from the Permian of South Africa. Bulletin of the American Museum of Natural History 56: 179–251.
Grohé, C., Lee, B., and Flynn, J.J. 2018. Recent inner ear specialization for high-speed hunting in cheetahs. Scientific Reports 8 (1): 2301. Crossref
Heissig, K. 1973. Die Unterfamilien und Tribus der rezenten und fossilen Rhinocerotidae (Mammalia). Säugetierkundliche Mitteilungen 21: 25–30.
Hopson, J.A. 1979. Paleoneurology. In: C. Gans, R.G. Northcutt, and P. Ulinski (eds.), Biology of the Reptilia, Vol. 9: Neurobiology A, 39–146. Academic Press, San Diego.
Hoyte, D.A.N. 1961. The postnatal growth of the ear capsule in the rabbit. American Journal of Anatomy 108: 1–16. Crossref
Ivakhnenko, M.F. 2008. Cranial morphology and evolution of Permian Dinomorpha (Eotherapsida) of eastern Europe. Paleontological Journal 42: 859–995. Crossref
Jeffery, N. and Spoor, F. 2004. Prenatal growth and development of the modern human labyrinth. Journal of Anatomy 204: 71–92. Crossref
Jirah, S. and Rubidge, B.S. 2014. Refined stratigraphy of the Middle Permian Abrahamskraal Formation (Beaufort Group) in the southern Karoo Basin. Journal of African Earth Sciences 100: 121–135. Crossref
Kammerer, C.F. 2011. Systematics of the Anteosauria (Therapsida: Dinocephalia). Journal of Systematic Palaeontology 9: 261–304. Crossref
Kemp, T.S. 2005. The Origin and Evolution of Mammals. 331 pp. Oxford University Press, Oxford. Crossref
Kemp, T.S. and Parrington, F.R. 1969. On the functional morphology of the gorgonopsid skull. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 256 (801): 1–83. Crossref
King, G.M. 1988. Handbuch der Paläoherpetologie 17C, Anomodontia. 174 pp. Gustav Fischer Verlag, New York.
King, J.L., Sipla, J.S., Georgi, J.A., Balanoff, A.M., and Neenan, J.M. 2020. The endocranium and trophic ecology of Velociraptor mongoliensis. Journal of Anatomy [published online, https://doi.org/10.1111/joa.13253] Crossref
Klaauw, C.J. van der 1931. The auditory bulla in some fossil mammals : with a general introduction to this region of the skull. Bulletin of the American Museum of Natural History 62: 1–352.
Kruger, A., Rubidge, B.S., and Abdala, F. 2018. A juvenile specimen of Anteosaurus magnificus Watson, 1921 (Therapsida: Dinocephalia) from the South African Karoo, and its implications for understanding dinocephalian ontogeny. Journal of Systematic Palaeontology 16: 139–158. Crossref
Laaß, M. and Kaestner, A. 2017. Evidence for convergent evolution of a neocortex-like structure in a late Permian therapsid. Journal of Morphology 278: 1033–1057. Crossref
Lieberman, D.E., Ross, C.F., and Ravosa, M.J. 2000. The primate cranial base: ontogeny, function, and integration. Yearbook of Physical Anthropology 43: 117–169. Crossref
Lindenlaub, T. and Burda, H. 1993. Morphometry of the vestibular organ in neonate and adult African mole-rats Cryptomys species. Anatomy and Embryology 188: 159–162. Crossref
MacPhee, R.D.E. 1981. Auditory regions of primates and eutherian insectivores. Contributions to Primatology 18: 1–282.
Mennecart, B. and Costeur, L. 2016. Shape variation and ontogeny of the ruminant bony labyrinth, an example in Tragulidae. Journal of Anatomy 229: 422–435. Crossref
Neenan, J.M., Chapelle, K.E.J., Fernandez, V., and Choiniere, J.N. 2019. Ontogeny of the Massospondylus labyrinth: implications for locomotory shifts in a basal sauropodomorph dinosaur. Palaeontology 62: 255–265. Crossref
Neumann, S. 2020. Taxonomic Revision of the Short-Snouted Tapinocephalid Dinocephalia (Amniota–Therapsida) The Key to Understanding Middle Permian Tetrapod Biodiversity. 411 pp. Ph.D. Thesis, University of the Witwatersrand, Johannesburg.
Olson, E.C. 1962. Late Permian terrestrial vertebrates, USA and USSR. Transactions of the American Philosophical Society 52 (2): 1–224. Crossref
Peterson, J.E. and Vittore, C.P. 2012. Cranial pathologies in a specimen of Pachycephalosaurus. PLoS ONE 7 (4): e36227. Crossref
Pusch, L.C., Kammerer, C.F., and Fröbisch, J. 2019. Cranial anatomy of the early cynodont Galesaurus planiceps and the origin of mammalian endocranial characters. Journal of Anatomy 234: 592–621. Crossref
Pusch, L.C., Ponstein, J., Kammerer, C.F., and Fröbisch, J. 2020. Novel endocranial data on the early therocephalian Lycosuchus vanderrieti underpin high character variability in early theriodont evolution. Frontiers in Ecology and Evolution 7: 464. Crossref
Rodrigues, P.G., Ruf, I., and Schultz, C.L. 2013. Digital reconstruction of the otic region and inner ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. Journal of Mammalian Evolution 20: 291–307. Crossref
Rubidge, B.S. and Sidor, C.A. 2001. Evolutionary patterns among Permo-Triassic therapsids. Annual Review of Ecology and Systematics 32: 449–480. Crossref
Rubidge, B.S., Govender, R., and Romano, M. 2019. The postcranial skeleton of the basal tapinocephalid dinocephalian Tapinocaninus pamelae (Synapsida: Therapsida) from the South African Karoo Supergroup. Journal of Systematic Palaeontology 17: 1767–1789. Crossref
Ryan, T.M., Silcox, M.T., Walker, A., Mao, X., Begun, D.R., Benefit, B.R., Gingerich, P.D., Köhler, M., Kordos, L., McCrossin, M.L., Moyà-Solà, S., Sanders, W.J., Seiffert, E.R., Simons, E., Zalmout, I.S., and Spoor, F. 2012. Evolution of locomotion in Anthropoidea: the semicircular canal evidence. Proceedings of the Royal Society B: Biological Sciences 279 (1742): 3467–3475. Crossref
Sánchez-Villagra, M.R. 2002. The cerebellar paraflocculus and the subarcuate fossa in Monodelphis domestica and other marsupial mammals—ontogeny and phylogeny of a brain-skull interaction. Acta Theriologica 47: 1–14. Crossref
Schade, M., Rauhut, O.W.M., and Evers, S.W. 2020 Neuroanatomy of the spinosaurid Irritator challengeri (Dinosauria: Theropoda) indicates potential adaptations for piscivory. Scientific Reports 10: 9259. Crossref
Schaffer, W.M. and Reed, C.A. 1972. The Co-Evolution of Social Behavior and Cranial Morphology in Sheep and Goats (Bovidae, Caprini). 112 pp. Field Museum of Natural History, Chicago. Crossref
Schellhorn, R. 2018. A potential link between lateral semicircular canal orientation, head posture, and dietary habits in extant rhinos (Perissodactyla, Rhinocerotidae). Journal of Morphology 279: 50–61. Crossref
Schmitt, A. and Gheerbrant, E. 2016. The ear region of earliest known elephant relatives: new light on the ancestral morphotype of proboscideans and afrotherians. Journal of Anatomy 228: 137–152. Crossref
Sereno, P.C., Wilson, J.A., Witmer, L.M., Whitlock, J.A., Maga, A., Ide, O., and Rowe, T.A. 2007. Structural extremes in a Cretaceous dinosaur. PLoS ONE 2 (11): e1230. Crossref
Sigogneau, D. 1974. The inner ear of Gorgonops (Reptilia, Therapsida, Gorgonopsia). Annals of the South African Museum 64: 53–69.
Silcox, M.T., Bloch, J.I., Boyer, D.M., Godinot, M., Ryan, T.M., Spoor, F., and Walker, A. 2009. Semicircular canal system in early primates. Journal of Human Evolution 56: 315–327. Crossref
Snively, E. and Cox, A. 2008. Structural mechanics of pachycephalosaur crania permitted head-butting behavior. Palaeontologia Electronica 11 (1): 3A.
Snively, E. and Theodor, J.M. 2011. Common functional correlates of head-strike behavior in the Pachycephalosaur Stegoceras validum (Ornithischia, Dinosauria) and combative artiodactyls. PLoS ONE 6 (6): e21422. Crossref
Solounias, N. 2007. Family Bovidae. In: D.R. Prothero and S.E. Foss (eds.), The Evolution of Artiodactyls, 278–291. University of The Johns Hopkins Press, Baltimore.
Spoor, F., Garland, T., Krovitz, G., Ryan, T.M., Silcox, M.T., and Walker, A. 2007. The primate semicircular canal system and locomotion. Proceedings of the National Academy of Sciences 104: 10808–10812. Crossref
Wallace, R.V.S., Martínez, R., and Rowe, T. 2019. First record of a basal mammaliamorph from the early Late Triassic Ischigualasto Formation of Argentina. PLoS ONE 14 (8): e0218791. Crossref
Walsh, S.A., Iwaniuk, A.N., Knoll, M.A., Bourdon, E., Barrett, P.M., Milner, A.C., Nudds, R.L., Abel, R.L., and Sterpaio, P.D. 2013. Avian cerebellar floccular fossa size is not a proxy for flying ability in birds. PLoS ONE 8 (6): e67176. Crossref
Watkinson, G.B. 1906. The cranial nerves of Varanus bivittatus. Gegenbaurs Morphologisches Jahrbuch 35: 450–472.
Witmer, L.M. and Ridgely, R.C. 2009. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 29: 1266–1296. Crossref
Acta Palaeontol. Pol. 66 (1): 29–39, 2021
https://doi.org/10.4202/app.00800.2020