

A phylogenetic analysis of the heterostracan jawless vertebrate family Cyathaspididae
DAVID K. ELLIOTT, LINDA S. LASSITER, and ALAIN BLIECK
Elliott, D.K., Lassiter, L.S., and Blieck, A. 2021. A phylogenetic analysis of the heterostracan jawless vertebrate family Cyathaspididae. Acta Palaeontologica Polonica 66 (3): 631–640.
The Heterostraci are a subclass of armored jawless vertebrates that were widespread in marginal marine environments around the Old Red Sandstone continent during the late Silurian to Middle Devonian. Although a number of clades have long been recognized, further analysis has been limited by lack of morphological information beyond that afforded by the armor, thus impeding understanding of early vertebrate evolution. Phylogenetic analysis of several heterostracan clades has been carried out previously and we here show an analysis of the family Cyathaspididae in which we prioritize the feature of a single branchial plate as a defining character of the family and reject a number of taxa previously included in analyses of this taxon. This analysis resolves to a single consensus tree showing that the Cyathaspididae is composed of a series of clades that are congruent with the subfamily groupings erected previously: Tolypelepidinae, Irregulareaspidinae, Poraspidinae, Anglaspidinae, and Boothiaspidinae. A trend can be seen from earlier members of the family with a dorsal shield divided into four epitega or growth areas (Asketaspis, Tolypelepis) to the most derived members (Poraspis, Faberaspis) in which the epitega are lost entirely. In addition, the earliest taxa are shown to have possessed shields composed of scale-like elements, which are lost and replaced by continuous ridges in the more derived members. This result supports the hypothesis that the earliest members of the Heterostraci may also have been scale-covered.
Key words: Pteraspidomorphi, Heterostraci, Cyathaspididae, taxonomy, phylogenetics, Palaeozoic.
David K. Elliott [David.Elliott@nau.edu], Geology Division, SES, Northern Arizona University, Flagstaff, Arizona 86011-4099, USA.
Linda S. Lassiter [lsl5@nau.edu], Department of Biological Sciences, Northern Arizona University, Flagstaff, Arizona 86011-5640, USA.
Alain Blieck [alain.blieck@yahoo.fr], 38 rue Paul Doumer, F-59320 Haubordin, France.
Received 7 September 2020, accepted 22 January 2021, available online 6 September 2021.
Copyright © 2021 D.K. Elliott et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Cyathaspididae are a family within the Heterostraci, which is a widespread subclass of armored jawless vertebrates currently known as “agnathans” and comprising more than 300 species. Characteristically the Heterostraci have a cephalothorax encased in a carapace consisting of a variable number of bony plates. They are associated with marine and proximal environments of the Old Red Sandstone continent from the Wenlock (middle Silurian) to the Frasnian (Late Devonian) (Elliott et al. 2015). The Heterostraci were first identified as fish by Huxley (1858), and Lankester (1864, 1868) subsequently recognized that they possess the unique character of a pair of common branchial openings. Usually they are placed in a basal position on the vertebrate stem together with the arandaspids and astraspids and are, therefore, in an important position to shed light on early vertebrate history and the origins of the gnathostome body plan (Forey 1984; Donoghue et al. 2000).
Although recent computer based phylogenetic analyses have been carried out on other armored agnathan groups: anaspids (Blom and Märss 2010), thelodonts (Wilson and Märss 2004, 2009), and osteostracans (Sansom 2008, 2009), the Heterostraci as a complete clade has yet to be resolved. However, a number of clades have been established and phylogenetic analysis has been carried out on three of them, the Pteraspididae (Pernègre and Elliott 2008; Randle and Sansom 2016, 2017; Elliott et al. 2020), the Cyathaspididae (Lundgren and Blom 2013; Randle and Sansom 2017; Elliott and Lassiter 2018), and the Psammosteoidei (Glinskiy 2017). We are here reanalyzing the Cyathaspididae as part of an ongoing study of heterostracan relationships as it is generally considered to include the basal members of the Heterostraci and is important to an understanding of development of that taxon. The analyses of Lundgren and Blom (2013) and Randle and Sansom (2017) were based on Denison (1964) and did not attempt to address extensive changes in the composition of the family proposed by later workers (e.g., the exclusion of the ctenaspids by Janvier 1996). Our analysis was conducted using maximum parsimony and a revised character matrix limited to discrete, unordered, unweighted characters and taxa of the Cyathaspididae only.
Phylogeny of the Cyathaspididae
Heterostraci.—All heterostracans have a cephalothorax completely enclosed by dermal armor, which also encloses the canals of the sensory canal system (Fig. 1). Usually a set of large median ventral and median dorsal plates are separated by branchial plates enclosing the branchial opening, orbital and cornual plates. Anteriorly the mouth is covered by small oral plates of varying pattern; in some taxa only one large plate is present, in others there is a more complex set of elongated finger-like plates (Elliott 2016 and references therein). In some taxa the cephalothorax is covered entirely by small platelets termed tesserae (see e.g., Blieck et al. 2018). The posterior body and tail are covered by small scales, which may be rhombic or elongated. Few specimens show the structure of the tail; where known it varies in outline in different taxa but it is generally paddle shaped. Heterostracans did not have paired pectoral or pelvic fins or dorsal or anal fins and so lacked mobile control surfaces. In many heterostracans outgrowths of the bony plates provided rigid control surfaces, such as the cornual plates and dorsal spine in pteraspids or the enlarged branchial plates in psammosteids (Janvier 1996).
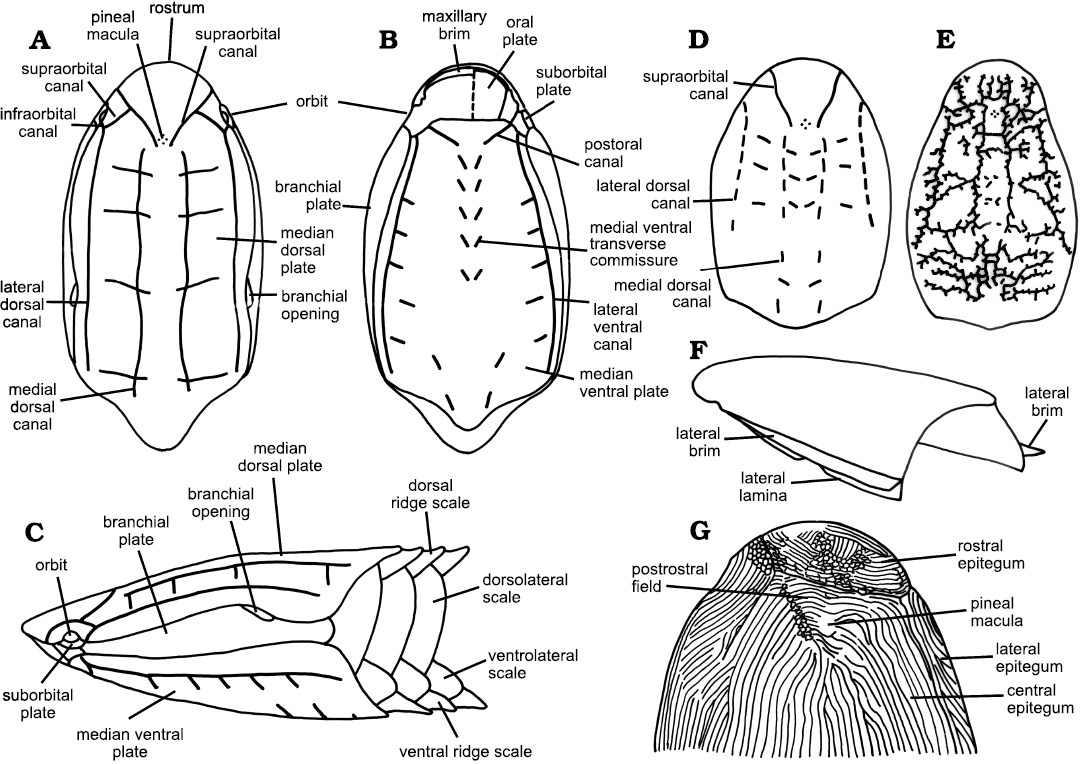
Fig. 1. Terminology for features of the cephalothorax in the Cyathaspididae. A–C. Terminology for the main plates and sensory canal system of the cephalothorax based on Poraspis, in dorsal (A), ventral (B), and lateral (C) views; after Denison (1964). D, E. Sensory canal systems in the dorsal plates of Tolypelepis (D) and Dikenaspis (E); after Denison (1964). F. Illustration of the position of the lateral brim and lateral lamina. G. Position of the epitega, postrostral field, and pineal macula in Vernonaspis bryanti; after Denison (1964).
The bone has been described as three-layered in the past (e.g., Gross 1935; Denison 1973; White 1973), however, it has recently been reinterpreted as four-layered (Keating et al. 2015). These layers comprise: (i) a superficial layer of dentine and enameloid; (ii) a layer of acellular parallel-fibred bone containing a network of vascular canals supplying the pulp canals; (iii) a trabecular layer of acellular bone consisting of intersecting radial walls; and (iv) a basal layer of isopedin. These have recently been fully illustrated in the tessellated heterostracan genus Tesseraspis (Blieck et al. 2018).
Cyathaspididae.—The Cyathaspididae are small heterostracans and the early history of their discovery has been covered in detail by Kiaer and Heintz (1935: 31–39) and is not repeated here. The taxon was initially recognized as a suborder by Kiaer (1932), who named it the Cyathaspida and included it, with the suborders Psammosteida and Pteraspida, within the order Heterostraci. He included within this taxon heterostracans in which: (i) the orbits are not surrounded by the dorsal shield but form semicircular notches in it; (ii) the large oblong branchial plate situated between the dorsal and ventral plates is detached; and (iii) the dentine ridges forming the surface of the dermal skeleton are smooth and not crenulated as in the pteraspids. At that time Kiaer (1932) also recognized two tribes within the Cyathaspida: the Poraspidei, in which the dorsal shield was not divided into epitega (areas of separate growth present on the dorsal plate); and the Cyathaspidei, in which four epitega were present on the dorsal shield. The Poraspidei included the families Poraspidae, Palaeaspidae, Dinaspidae, Anglaspidae, and Ctenaspidae, while the Cyathaspidei included the families Cyathaspidae, Tolypaspidae, Diplaspidae, and Traquairaspidae (Kiaer 1932). This remained the most complete treatment of the taxon until 1964 when Denison published a comprehensive review defining the cyathaspids as a family, the Cyathaspididae (Fig. 2C). Within the family he recognized a series of subfamilies that adhered fairly closely to the families recognized by Kiaer (1932) although several of them were amalgamated, comprising: Tolypelepidinae; Cyathaspidinae; Irregulareaspidinae; Poraspidinae; and Ctenaspidinae. The subfamilies were recognized by: (i) presence or absence of apparent scale components in the shield; (ii) presence or absence of distinct epitega; and (iii) the pattern, length, and uniformity of the superficial dentine ridges.
In 1976 Dineley and Loeffler published a monograph on ostracoderms from the Delorme and associated formations in the Mackenzie Mountains, Canada. This study included cyathaspids and broadly supported the classification of Denison (1964) with slight modifications. The Tolypelepidinae were seen as the most primitive group and a new species of Tolypelepis was added together with the new Asketaspis. Cyathaspidinae was accepted with the addition of several new species, as was Poraspidinae. The Irregulareaspidinae was enlarged by the addition of Nahanniaspis, which is preserved as completely articulated individuals. The only disagreement with the classification of Denison (1964) was in the position of Dikenaspis as a member of the Irregulareaspidinae. In Dineley and Loeffler’s (1976) view, Irregulareaspis, Dinaspidella, and Nahanniaspis share a suite of characters that indicate their close relationship, while Dikenaspis is connected only by the presence of an anastomosing lateral line system. Dineley and Loeffler (1976) showed that this is different in type from that of Irregulareaspis and suggested instead that Dikenaspis should be placed in the Cyathaspidinae, and that the Irregulareaspidinae are more closely related to the Poraspidinae.
No further attempts to develop an understanding of the relationships of the Cyathaspididae were made until 1996 when Janvier showed in cladistic form as part of a phylogeny of the Heterostraci a simple cyathaspid phylogeny in which Nahanniaspis represented basal forms and Anglaspis and Torpedaspis more derived forms, while the ctenaspids and amphiaspids formed a sister-group (Janvier 1996: fig. 4.8).
Soehn and Wilson (1990) described an important heterostracan Athenaegis chattertoni and included it within the Tolypelepidinae despite the presence of a series of small plates covering the probable branchial area. Märss (1977) had shown previously that Tolypelepis undulata had a single branchial plate.
Novitskaya (2004) reviewed fossil “agnathans and early fishes” of the former USSR and treated the cyathaspids as an order, Cyathaspidiformes, as Obruchev (1967; order Cyathaspidida) had done previously. Novitskaya (2004) recognized the families Cyathaspididae, Tolypelepididae, Irregulareaspididae, Poraspididae, Anglaspididae (Anglaspis + Liliaspis + Paraliliaspis), and Ctenaspididae. Voichyshyn (2011) reviewed Early Devonian “armoured agnathans” of Podolia, Ukraine, and also treated the group, without cladistic analysis, as order Cyathaspidiformes with the following families: Cyathaspididae, Irregulareaspididae, Poraspididae, and Ctenaspididae.
In common with the above authors we accept Denison’s (1964) definition of the Cyathaspididae but with some changes as follows.
The Cyathaspididae are small Heterostraci whose carapace consists of: dorsal, ventral and paired branchial plates; oral cover composed of a variable number of oral and oral lateral plates; and paired suborbital plates (Fig. 1). There may be a post-branchial lobe but there is no cornual plate. Lateral brims and laminae may be present. No separate dorsal spine is present but there may be a posterior dorsal ridge. No projection of the rostral area, and the ventrally placed mouth is sub-terminal. The orbits are bounded above by the dorsal median plate and ventrally by the suborbital plates. The branchial openings lie between the dorsal median plate and the branchial plates. Scales may be small and diamond-shaped or more commonly are of relatively large size, taller than long, and consist of median dorsal, dorso-lateral, ventro-lateral, and median ventral rows. The dorso-lateral scales are frequently the largest and tallest scales and may cover most of the flanks of the animal. The external surface is covered by dentine ridges that have smooth or gently scalloped edges. Lateral line canals are situated in the cancellous layer and open to the surface by pores (Denison 1964: 325, 351; Kiaer and Heintz 1935: 48). The pattern of the lateral line sensory canals is generally a simple one consisting of two pairs of longitudinal canals dorsally and ventrally, transverse commissures, infraorbital and supraorbital lines (Fig. 1). Pores of lateral line sensory canals have also been noted in branchial plates (Denison 1964; Märss 2019) and oral plates (Denison 1964; Elliott 2016 and references therein). Although Denison (1964) includes the probable presence of seven pairs of gills in his definition this feature is incompletely known across the Heterostraci and so is not used as part of the definition here.
Modifications to the Cyathaspididae of Denison (1964).—In their analysis, Lundgren and Blom (2013) did not modify the composition of the Cyathaspididae from that used by Denison (1964) beyond adding some taxa described since that analysis. However, there are good reasons for excluding some of the taxa included by Denison (1964) in the Cythaspididae. One of the characters listed is the presence of paired branchial plates (Denison 1964: 350) and as this was also one of the three characters used by Kiaer (1932) to identify his Cyathaspidida, we consider this feature an important one in defining a cyathaspid. However, although present in almost all of the taxa Denison (1964) lists within the family, this character is not present in a number of them. Allocryptaspis was included by Denison (1964) who assumed the branchial plates had fused to the dorsal plate resulting in a branchial opening bounded by notches in the lateral margins of the dorsal and ventral plates; however, Elliott et al. (2004) showed that such a process would have resulted in a branchial opening completely enclosed by the dorsal plate and suggested instead that this taxon had never possessed branchial plates. In the ctenaspids (Ctenaspis, Arctictenaspis, and Zaphoctenaspis), the branchial opening is posteriorly directed and there is no evidence for the presence of branchial plates. Elliott and Blieck (2010) removed them from the Cyathaspididae and included them within the new family Ctenaspididae. The same is true for Ariaspis for which Elliott and Swift (2010) showed the presence of a continuous lateral lamina with no notch for the branchial opening and a posterior opening for the branchial duct. A branchial plate was identified in Listraspis by Denison (1964), however a review of the original and some additional material indicates that this is a lateral lamina not separated from the rest of the dorsal shield (DKE personal observation). This taxon requires further study and description.
It is also noted here that several other taxa that have traditionally been included within the Cyathaspididae cannot unequivocally be identified as such. This is generally true of the taxa with scale-like elements on all or part of the dorsal shield, Tolypelepis, Asketaspis, and Ptomaspis. Of these only Tolypelepis undulata has an identified branchial plate or definite location for the branchial opening (Märss 1977), although Dineley and Loeffler (1976: 57) note the presence of postbranchial lobes in Asketaspis, implicitly indicating the presence of lateral branchial openings.
There are also a number of taxa described since 1964 that should be included within the analysis. Alainaspis and Boothiaspis form a group of related heterostracans from the Canadian Arctic (Broad 1973; Elliott and Dineley 1985, 1991). Although Boothiaspis was considered to be an amphiaspid by Broad (1973), it was shown by Elliott and Dineley (1985) to be a poraspidinid cyathaspid. A new group of cyathaspids related to the boothiaspids has recently been recognized from the Lower Devonian of the western United States (Elliott 2016; Phyllonaspis spp.) and branchial plates have been identified for them, suggesting that Boothiaspis would also have had them. A reexamination of the Alainaspis material also indicates that the large lateral opening is most likely a space that was filled by the branchial plate rather than constituting the entire branchial opening (Elliott and Dineley 1991). These species have been recognized as a distinct new subfamily, the Boothiaspidinae Elliott (2016). The new taxa described by Dineley and Loeffler (1976), Asketaspis and Nahanniaspis, should also be included. Since the publication of Lundgren and Blom (2013) the new cyathaspids Capitaspis giblingi (Elliott 2013) and Faberaspis elgae (Elliott et al. 2017) have been described from the upper Silurian of the Canadian Arctic. Although Lechraspis patula has been described from the Lower Devonian Water Canyon Formation of Utah (Elliott and Petriello 2011), the incomplete nature of the one dorsal shield, which is also crushed, limits the information that can be obtained from it and so we have not included it in this analysis.
Lundgren and Blom (2013).—In an attempt to quantify the paraphyletic grouping of the Cyathaspididae Lundgren and Blom (2013) used Denison (1964) and other published descriptions to resolve a consensus tree that has few taxonomically explainable clades (Fig. 2A). However, a review of the 61 characters used by these authors shows that some have indirectly weighted traits by duplication while in other cases taphonomic alteration has not been recognized when selecting characters. Also, 12 of the 37 included taxa from Denison (1964) no longer fit the revised taxonomic diagnosis for the Cyathaspididae (as cited earlier). For this report, the hypothesized phylogenetic trees were produced by using their published matrix and methods in PAUP* with factory-default settings (Fig. 2A).
There is a considerable amount of disagreement between the classification presented by Denison (1964; Fig. 2C) and the hypothesized phylogeny of Lundgren and Blom (2013; Fig. 2A). Asketaspis is in a polytomy with the outgroup, Athenaegis. The tolypelepids are paraphyletic and form a basal group. The ctenaspids are shown as monophyletic group in a clade that also includes Allocryptaspis and Alainaspis. Although the rest of the tree is well resolved it shows little similarity to the subfamilies developed by Denison (1964). For example, Homalaspidella and Pionaspis species appear in different parts of the tree although there have been no previous indications that they are incorrectly attributed.
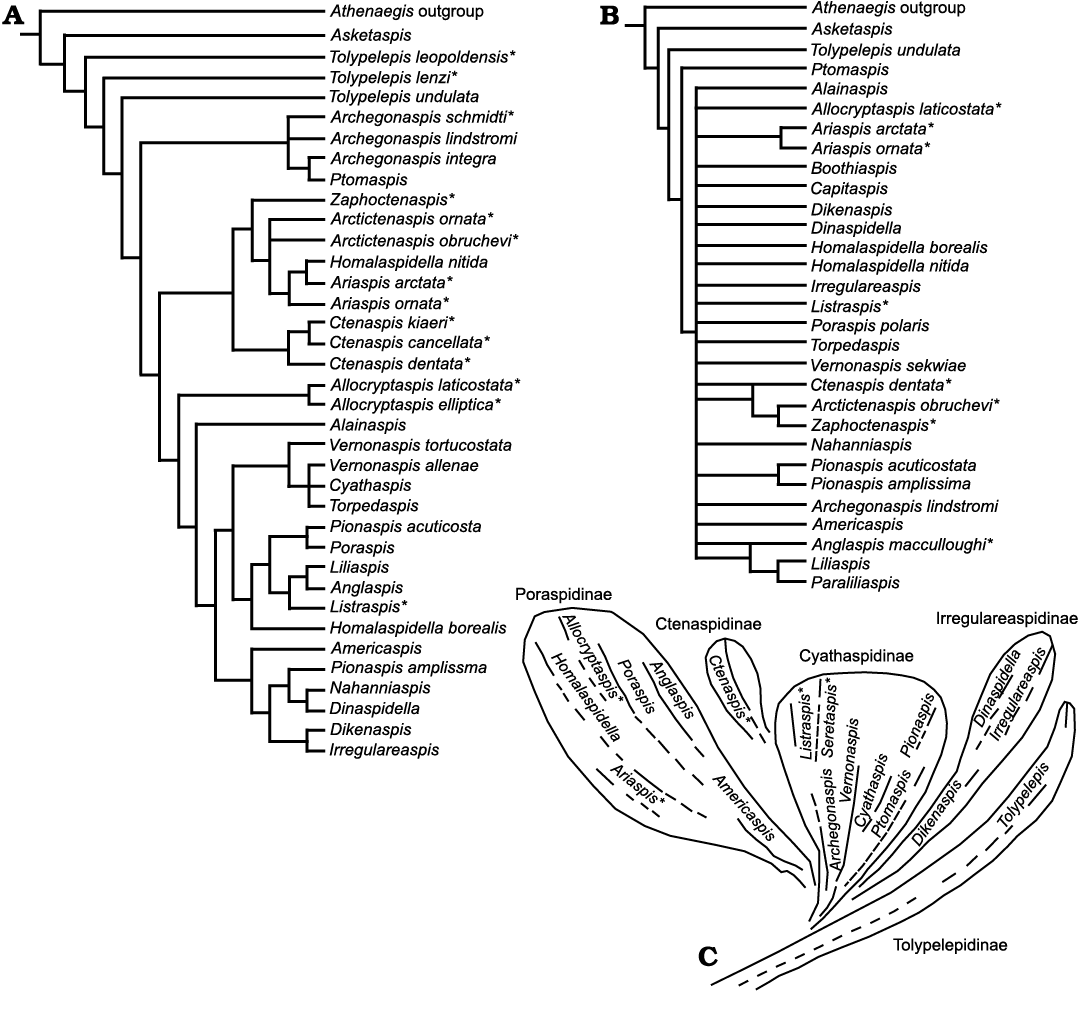
Fig. 2. Consensus trees for the Cyathaspididae. A, after Lundgren and Blom (2013); B, after Randle and Sansom (2017); C, after Denison (1964). Taxa not used in the analysis in this paper are denoted by an asterisk (*).
Randle and Sansom (2017).—Randle and Sansom (2017) used many of the same characters and taxa as Lundgren and Blom (2013) and presented multiple hypothesized phylogenies from 105 to 131 characters for the 30 taxa they identified as cyathaspids. To produce a resolved tree for the Cyathaspididae, Randle and Sansom (2017: fig. 4), relied upon weighting, ordering, and inclusion of continuous data from unreported raw values. The resulting phylogeny of only discrete characters is a large polytomy (Randle and Sansom 2017: fig. 3; Fig. 2B) for the bulk of the Cyathaspididae, which is understandable given that this is a consensus of tens of thousands of most-parsimonious trees with data included from the Pteraspidiformes. Once again, this analysis, like Lundgren and Blom (2013), included taxa (six) that do not meet the criterion of paired branchial plates as Cyathaspididae (Ctenaspidinae, Ariaspis, and Allocryptaspis). These taxa are noted with asterisks in Fig. 2B. The raw data of the measurements of adult features of the cephalothorax used by Randle and Sansom (2017) to generate about 25 characters of ratios could be informative for future analyses of this complex group of taxa. However, their resulting hypotheses (Randle and Sansom 2017: figs. 4, 5) are not explained relative to the long, historical record of other heterostracan workers (see Novitskaya 1970, 1973; Denison 1964; Dineley and Loeffler 1976; Elliott 2016 for references).
New analysis
As neither of the previous attempts at a phylogenetic analysis of this family appeared to us to be satisfactory a new analysis of the phylogeny of the family was performed. In order to delineate the boundaries of the study we deal first here with some key morphological definitions, and then review the constitution of the taxonomic groups.
Morphological terms.—Although morphological descriptions of heterostracans have usually followed the usage of Denison (1964), there has been sufficient variability in some areas to cause difficulty in precise understanding of what is being described. This is particularly the case in Lundgren and Blom (2013) and Randle and Sansom (2017), the most recent cladistic analyses of the Cyathaspididae. In order to clarify the precise meaning of some terms and elucidate our view of their correct usage, we have listed some definitions as follows (see also Fig. 1).
Dorsal shield: The dorsal part of the carapace comprising the dorsal median plate, and the branchial and suborbital plates.
Ventral shield: All ventral plates, comprising the ventral median plate and oral plates.
Anterior and posterior median carinae: A narrow vertical prominence aligned longitudinally in the midline of the dorsal plate. Anterior carinae are present from the midpoint of the median plate and finish before the posterior margin. Posterior carinae are present in the posterior quarter of the plate.
Lateral laminae: Dorsal lateral laminae. Paired, downwardly directed, relatively narrow, rim-like structures that extend from immediately behind the orbits to, or very near to, the posterolateral corners of the dorsal median plate, and are separated from the central, greater part of the plate by sharp angulations.
Lateral brims: Paired, laterally directed, relatively narrow, ledge-like laminae, that occur along the angulations marking the junctions of the central, greater part of the dorsal median plate with downwardly directed lateral laminae.
Pineal macula and pineal ornament: The pineal macula is a relatively large, single, generally round to oval dentine tubercle, positioned above the pineal organ. It is surrounded by an intercostal groove and set apart from the surrounding ornament. The pineal ornament occupies a relatively greater area than a pineal macula and is often not well defined. It constitutes a group of dentine ridges in the pineal area, often radiating or erratic in orientation.
Data matrix.—Twenty-nine cyathaspid taxa and one outgroup taxon were selected to compile a data matrix for phylogenetic analysis. The criteria for selection of Athenaegis chattertoni as the outgroup taxon was based upon three key points. First, Athenaegis chattertoni lacks a pair of single branchial plates and thus does not fit a primary trait of the cyathaspid ingroup. Second, although some workers have placed it within the Tolypelepidinae (Soehn and Wilson 1990) together with Asketaspis (Dineley and Loeffler 1976; Randle and Sansom 2017), the sister taxon relationship of Athenaegis with Tolypelepidinae has consistently been reported in earlier analyses (Lundgren and Blom 2013; Randle and Sansom 2017). Third, Athenaegis chattertoni is an articulated, nearly-completely known taxon allowing for a complete set of data for comparison with all other considered taxa.
Specimens, published descriptions and imagery were evaluated for synapomorphic characters to distinguish the taxa. Of the 61 characters used by Lundgren and Blom (2013), we retained 18 (see SOM 1: Cyathaspididae character and transition state list, in Supplementary Online Material available at http://app.pan.pl/SOM/app66-Elliott_etal_SOM.pdf). Potential characters required the merit of increasing the resolution of changes within and between the ingroup (29 taxa). All characters were unordered, equally weighted, and informative. Contingencies between characters were coded as gaps, “–”, for reading clarity although most tools for parsimony analysis treat morphological, non-nucleotide gaps as missing data. The coding practice of using the missing state, “?”, was reserved for instances of actual missing fossil evidence.
Mesquite, Version 3.5.1 (Madison and Madison 2018) was used to convert text files into the required file formats (NEXUS, TNT) for the analysis in PAUP* (Swofford 2003) and TNT (Goloboff et al. 2003; Goloboff and Catalano 2016) phylogenetic software packages. Readers interested in replicating our work are referred to the supplemental information for this paper that includes the spreadsheet with the coded data (SOM 2), scripted files with embedded settings for TNT (SOM 4) and PAUP* (SOM 3). Heuristic searches were performed for 1000 random additional replicates using tree bisection reconnection branch swapping (TBR) and saving multiple trees per swap in TNT and PAUP*. TNT, a single-user edition provided “by the Willi Hennig Society, was used for rapid” what—if analyses of changes in the character matrix, to confirm the results from PAUP*, to test results in the “New technology search (xmult)” methods of TNT, and to run bootstrap analyses. Bootstrap support was calculated by computing 50 000 replications, with replacement, using TBR with 10 replicates in TNT. Bremer support values were calculated by saving more trees beyond the shortest length resulting trees until clade support collapsed.
Results
Previous analyses generated poor support with a substantially larger number of trees. This result resolves into only two trees, with the placement of the Tolypelepidinae as a clade or a cascade. Otherwise the cyathaspid subtrees do not vary.
The base of the strict consensus tree consistently resolves Asketaspis basal to either a clade or bifurcating cascade of the remaining subfamily members, Tolypelepis and Ptomaspis. Although paraphyletic this does equate with the Tolypelepidinae as recognized by Dineley and Loeffler (1976) and supports the hypothesis that the ancestral members of the Cyathaspididae had scale-like elements on the dorsal shield (Obruchev 1945, 1964; Denison 1964). The next group of paraphyletic clades of the difficult to resolve cyathaspids of Vernonaspis, Dikenaspis, Cyathaspis, Pionaspis, Torpedaspis, and Archegonaspis are equivalent to the Cyathaspidinae of Denison (1964) and Dineley and Loeffler (1976) with the addition of the new genus Capitaspis, although at least this hypothesized result is bifurcating and distinguishable. However, within this group there are small clades that include Torpedaspis + Vernonaspis sekwiae, Cyathaspis + Vernonaspis tortucostata, Pionaspis spp., and Archegonaspis spp. The two species of Vernonaspis do not form a clade, which might suggest that one of the species is incorrectly assigned. Torpedaspis was previously placed in the Poraspidinae by Broad and Dineley (1973), but here resolves as more closely related to the Cyathaspidinae than the Poraspidinae. This is due in part to the constriction in front of the orbits that is seen in Poraspidinae but is not present in Torpedaspis.
Taken as a whole the clade from Archegonaspis to Poraspis includes most of the taxa in the Poraspidinae and Irregulareaspidinae of Denison (1964) and Dineley and Loeffler (1976). An initial clade of Archegonaspis spp. cascades to a clade of Irregulareaspis, Dinaspidella, and Nahanniaspis forming the Irregulareaspidinae sensu Dineley and Loeffler (1976). Dikenaspis was included in this subfamily by Denison (1964) but removed to the Cyathaspidinae by Dineley and Loeffler (1976) as it retained epitega, an attribution which is supported by this tree (Fig. 3). Anglaspis and Liliaspis were recognized by Novitskaya (2004) as forming the family Anglaspididae, although Denison (1964) included Anglaspis within his Poraspidinae. This analysis supports Novitskaya’s interpretation.
Americaspis forms the sister group to the two final clades and is here separated as the subfamily Americaspidinae. The first clade consists of Alainaspis, Boothiaspis, and the two species of Phyllonaspis thus supporting their recent inclusion within the Boothiaspidinae (Elliott 2016). The second includes Homalaspidella, Faberaspis, and Poraspis, which comprise the Poraspidinae of Denison (1964) with the addition of Faberaspis (Elliott et al. 2017), which has been described since.
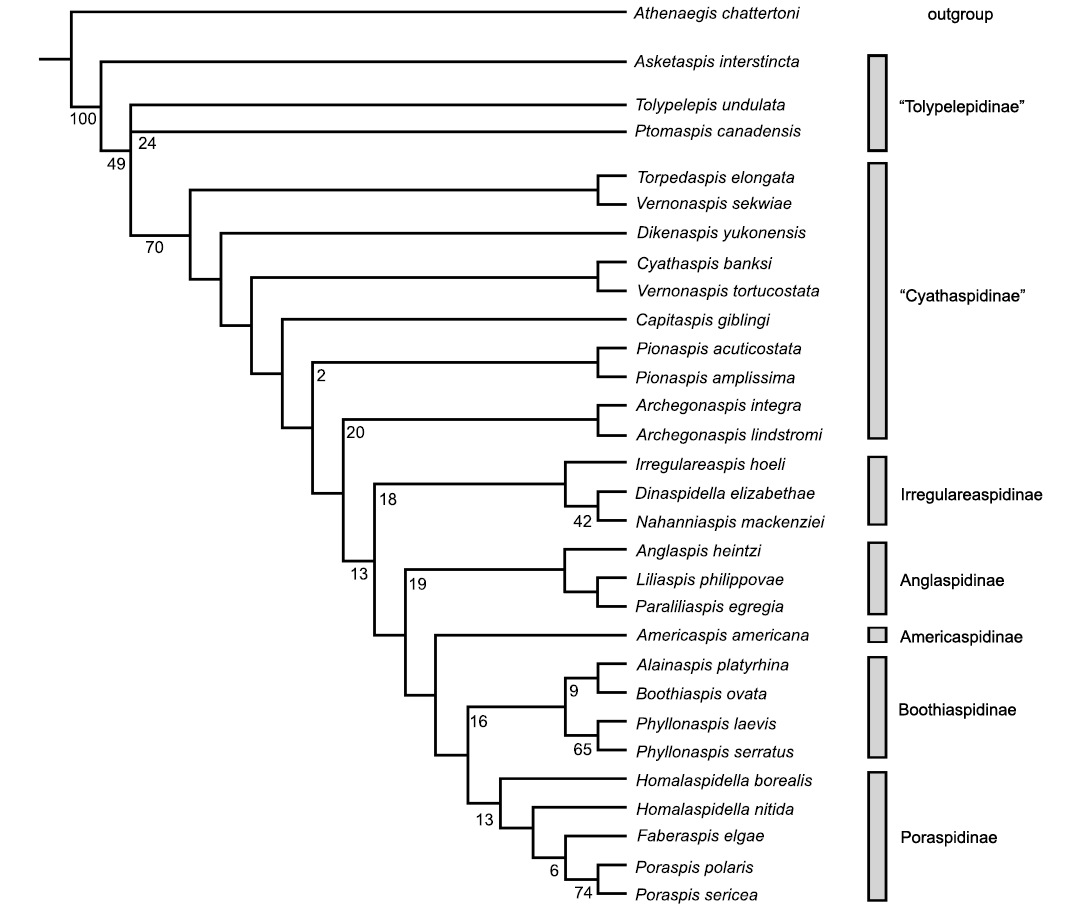
Fig. 3. Phylogeny of the Cyathaspididae as a strict consensus tree from two most parsimonious trees (Length 142, Consistency Index 0.4366, Retention Index 0.6244) using unordered and unweighted characters (35) and 29 cyathaspid taxa with one outgroup (Athenaegis chattertoni). Subline numbers represent bootstrap support values as further described in the text.
Although the support values are low for the resulting two most parsimonious trees both tools produced the same strict consensus, as depicted in Fig. 3. Bremer support values are only 1, 2 or 3 and are not shown. The Tolypelepidinae are still distinct from the Cyathaspidinae at a tree length of 145 (support value 3), with clade support for the poraspids, Anglaspidinae, Irregulareaspidinae, and Boothiaspidinae collapsing at a tree length of 144 (support value of 2). Bootstrapping support is shown in Fig. 3 at generally low levels of probability support for a few, not all, of the clades. The Pionaspis and Archegonaspis have weak bootstrap support (2% and 20%, respectively) as do the subfamilies (Anglaspidinae 19%, Boothiaspidinae 16%, Irregulareaspidinae 18%, and Poraspidinae 12%). There is significant support (>50%) for the Phyllonaspis and Poraspis (65% and 74%, respectively). What is notable is that the cyathaspids resolve to a single branch at 49% and beyond, Tolypelepidinae at 70% reinforcing that this group is a well-defined clade even though the single branchial pair of plates was not included as a character (uninformative for this analysis of only cyathaspids and one outgroup). The resolution by parsimony into only two trees, however, has not been achieved by other analyses for this group except in an earlier analysis by Elliott and Lassiter (2018).
Discussion
Trends that can be recognized within the presented phylogeny of the Cyathaspididae (Fig. 3) include changes in scale type and presence of epitega that conform with previously noted differences between taxa. There is a simplification of the dorsal and ventral shields by the gradual loss of scale-like elements, which are gradually replaced by longitudinal ridges. Although the development of the heterostracan shield by the gradual fusion of elements was originally supported (Traquair 1899) it was later suggested (Jaekel 1903; Stensiö 1927) that a gradual subdivision of an original continuous covering better explained the paleontological evidence. This hypothesis was accepted and further developed for the cyathaspids (Obruchev 1945; Stensiö 1958; Tarlo 1960; Ørvig 1961); however, Denison (1964: 458, 462) considered that the Tolypelepidinae included the ancestral stock of the family, that the Cyathaspidinae were derived from it by the gradual loss of the scale-like elements, and that Ptomaspis was the most primitive of the Cyathaspidinae by virtue of the retention of these elements at the posterior margin of the shield (Denison 1964: 462). This hypothesis is supported by the first part of the tree published here (Fig. 3). This feature is consistent with the hypothesis that a precursor to the Cyathaspididae would have had shields entirely composed of scale-like elements.
A second trend captured in the cladogram is the gradual loss of epitega. Although originally viewed as being merely superficial divisions (Kiaer 1932) it was later believed that in some genera the epitega formed separate plates (Obruchev 1945; Stensiö 1958). Recent work (Greeniaus and Wilson 2003) has shown that they represent areas of growth in the shield that commenced when the animal was 60% or 70% of full size, and then fused on contact. The reason for a trend from four epitega in the early members of the group (anterior, paired lateral and central; Fig. 1) to the complete loss of epitega in the most derived is not understood, but this trend is clearly illustrated in the tree (Fig. 3). Americaspis appears to represent a transitional form in which there is no longer a rostral–median epitegal boundary and the lateral epitega are not distinct posteriorly.
The oral cover is only known from a few genera in the Cyathaspididae but this tree (Fig. 3) does appear to indicate a simplification in the arrangement and number of the constituent plates. In the outgroup Athenaegis chattertoni the oral cover consists of a number of oral–lateral and lateral plates flanking a series of narrow rod-like elements (Soehn and Wilson 1990). This is assumed to be representative of the primitive arrangement in heterostracans (Janvier 1996). Only one example of the oral cover in the Cyathaspidinae (sensu Denison 1964) is known. Capitaspis giblingi (Elliott 2013) has an oral cover in which two rows of oral plates are present, a central plate being the largest, and there are no rod-like oral plates. Anglaspis heintzi as reconstructed by Blieck and Heintz (1983) has a single row of oral plates in which a small median plate is flanked by paired oral–lateral and lateral plates (Blieck and Heintz 1983: fig. 16B). Poraspis cf. P. polaris (Dineley and Loeffler 1976; subsequently renamed Poraspis elizabethae) and Poraspis thomasi (Elliott and Petriello 2011) both have a single, large, oblong oral plate possibly flanked by paired lateral oral plates. Phyllonaspis, a member of the most derived taxon, the Boothiaspidinae, has an oral cover in which a large trapezoidal central oral plate is flanked by paired oral plates (Elliott 2016: fig. 7). This may be a synapomorphy of the Poraspidinae (sensu Denison 1964) and of the two most derived clades resolved in this tree. Thus, a decreasing complexity in the oral cover appears to be present in the Cyathaspididae as opposed to the increasing complexity that is seen in the Pteraspidiformes (Blieck 1984), the only other group of Heterostraci for which there is information on this area. However, although this is an observed trend shown by the cladogram, there are too few taxa with preserved and documented oral areas to provide strong support.
Conclusions
A reassessment of the phylogeny of the Cyathaspididae, a family of armored jawless vertebrates from the late Silurian and Early Devonian was undertaken to resolve problems resulting from previous analyses. Although the character of a single pair of branchial plates had been an important part of the original definition of the taxon, additional taxa that did not fit the definition of a cyathaspid had been included in previous analyses resulting in phylogenies that distracted from resolving any explainable relationships within the family. In this analysis we established a definition of the family and developed a list of constituent taxa based on that definition, together with additional recently described taxa and a new list of heritable characters. The resulting phylogenetic tree shows a series of clades that broadly explain a number of trends previously identified within the Cyathaspididae. The three main trends are: (i) the loss of an initial armor composed of scale-like elements and its fusion to form a continuous cover through time; (ii) the gradual loss of growth areas within the dorsal shield termed epitega, from four in the earliest members of the group to none in the most derived subfamilies; and (iii) a gradual simplification of the oral area through time from many small plates to one plate only.
Acknowledgements
We thank Vadim Glinskiy (St. Petersburg State University, Russia) and Tiiu Märss (Tallinn University of Technology, Estonia) for their helpful reviews that enabled us to substantially improve this paper.
References
Blieck, A. 1984. Les Hétérostracés Pteraspidiformes, Agnathes du Silurien–Dévonien du Continent Nord Atlantique et des blocs avoisinants. Cahiers de Paléontologie, CNRS édit.: 1–199.
Blieck, A. and Heintz, N. 1983. The cyathaspids of the Red Bay Group (Lower Devonian) of Spitsbergen. The Downtonian and Devonian vertebrates of Spitsbergen. XIII. Polar Research 1: 49–74. Crossref
Blieck, A., Elliott, D.K., and Karatajūtė-Talimaa, V.N. 2018. A redescription of Tesseraspis mosaica Karatajūtė-Talimaa, 1983 (Vertebrata: †Pteraspidomorphi: Heterostraci) from the Lochkovian (Lower Devonian) of Severnaya Zemlya, Russia, with a review of the tessellated heterostracan taxa. Acta Geologica Polonica 68: 275–306.
Blom, H. and Märss, T. 2010. The interrelationships and evolutionary history of anaspid fishes. In: D.K. Elliott, J.G. Maisey, X. Yu, and D. Miao (eds.), Morphology, Phylogeny, and Paleobiogeography of Fossil Fishes, 45–58. Verlag Dr. F. Pfeil, Munich.
Broad, D.S. 1973. Amphiaspidiformes (Heterostraci) from the Silurian of the Canadian Arctic Archipelago. Geological Survey of Canada, Bulletin 222: 35–48. Crossref
Broad, D.S. and Dineley, D.L. 1973. Torpedaspis, a new Upper Silurian and Lower Devonian genus of Cyathaspididae (Ostracodermi) from Arctic Canada. Geological Survey of Canada, Bulletin 222: 53–90. Crossref
Denison, R.H. 1964. The Cyathaspididae: a family of Silurian and Devonian jawless vertebrates. Fieldiana: Geology 13: 309–473. Crossref
Denison R.H. 1973. Growth and wear of the shield in Pteraspididae (Agnatha). Palaeontographica, Abteilung A 143: 1–10.
Dineley, D.L. and Loeffler, E.J. 1976. Ostracoderm faunas of the Delorme and associated Siluro-Devonian formations, North West Territories, Canada. Special Papers in Palaeontology 18: 1–214.
Donoghue, P.C.J., Forey, P.L., and Aldridge, R.J. 2000. Conodont affinity and chordate phylogeny. Biological Review 75: 191–251. Crossref
Elliott, D.K. 2013. A new cyathaspid (Agnatha, Heterostraci) with an articulated oral cover from the late Silurian of the Canadian Arctic. Journal of Vertebrate Paleontology 33: 29–34. Crossref
Elliott, D.K. 2016. The Boothiaspidinae, a new agnathan subfamily (Heterostraci, Cyathaspididae) from the late Silurian and Early Devonian of the western United States and the Canadian Arctic. Journal of Paleontology 90: 1212–1224. Crossref
Elliott, D.K. and Blieck, A. 2010. A new ctenaspid (Agnatha, Heterostraci) from the Early Devonian of Nevada, with comments on taxonomy, paleobiology and paleobiogeography. In: D.K. Elliott, J.G. Maisey, X. Yu, and D. Miao (eds.), Morphology, Phylogeny, and Paleobiogeography of Fossil Fishes, 25–38. Verlag Dr. F. Pfeil, Munich.
Elliott D.K. and Dineley, D.L. 1985. A new heterostracan from the upper Silurian of Northwest Territories, Canada. Journal of Vertebrate Paleontology 5: 103–110. Crossref
Elliott, D.K. and Dineley, D.L. 1991. Additional information on Alainaspis and Boothiaspis, cyathaspidids (Agnatha: Heterostraci) from the upper Silurian of Northwest Territories, Canada. Journal of Paleontology 65: 308–313. Crossref
Elliott, D.K. and Lassiter, L.S. 2018. A phylogenetic analysis of the Cyathaspididae (Agnatha, Heterostraci). In: 78th Annual Meeting. Program and Abstracts, 121. Society of Vertebrate Paleontology, Albuquerque.
Elliott, D.K. and Petriello, M.A. 2011. New poraspids (Agnatha, Heterostraci) from the Early Devonian of the western United States. Journal of Vertebrate Paleontology 31: 518–530. Crossref
Elliott, D.K. and Swift, S. 2010. A new species of Ariaspis (Agnatha: Heterostraci) from the late Silurian of the Canadian Arctic. Journal of Vertebrate Paleontology 30: 1874–1878. Crossref
Elliott, D.K., Lassiter, L.S., and Blieck, A. 2017. A new species of cyathaspid (Vertebrata: Pteraspidomorphi: Heterostraci) from the Lower Devonian Drake Bay Formation, Prince of Wales Island, Nunavut, Arctic Canada. Estonian Journal of Earth Sciences 67: 88–95. Crossref
Elliott, D.K., Lassiter, L.S., and Geher, K.E. 2020. The last pteraspids (Vertebrata, Heterostraci): new material from the Middle Devonian of Alberta and Idaho. Journal of Paleontology 94 (4): 1–15. Crossref
Elliott, D.K., Reed, R.C., and Loeffler, E.J. 2004. A new species of Allocryptaspis (Heterostraci) from the Early Devonian, with comments on the structure of the oral area in cyathaspidids. In: G. Arratia, M.V.H. Wilson, and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates, 455–472. Verlag Dr. F. Pfeil, Munich.
Elliott, D.K., Schultze, H.-P., and Blieck, A. 2015. A new pteraspid (Agnatha, Heterostraci) from the Lower Devonian Drake Bay Formation, Prince of Wales Island, Nunavut, Arctic Canada, and comments on environmental preferences of pteraspids. Journal of Vertebrate Paleontology 35: e1005098. Crossref
Forey, P.L. 1984. Yet more reflections on agnathan–gnathostome relationships. Journal of Vertebrate Paleontology 4: 330–343. Crossref
Glinskiy, V. 2017. Phylogenetic relationships of psammosteid heterostracans (Pteraspidiformes), Devonian jawless vertebrates. Biological Communications 62: 219–243. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, with a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Goloboff, P.J., Farris, J., and Nixon, K. 2003. TNT: Tree Analysis Using New Technology. Program and Documentation, http://www.zmuc.dk/public/phylogeny.
Greeniaus, J.W. and Wilson, M.V.H. 2003. Fossil juvenile Cyathaspididae (Heterostraci) reveal rapid cyclomorial development of the dermal skeleton. Journal of Vertebrate Paleontology 23: 483–487. Crossref
Gross, W. 1935. Histologische studien am aussenskelett fossiler Agnathen und Fische. Palaeontographica, Abteilung A 83: 1–60.
Huxley, T.H. 1858. On Cephalaspis and Pteraspis. Quarterly Journal of the Geological Society of London 14: 267–280. Crossref
Jaekel, O. 1903. Die Organisation und systematische Stellung der Asterolepiden. Zeitschrift der Deutschen geologischen Gesellschaft 55: 41–60.
Janvier, P. 1996. Early Vertebrates. Oxford Monographs on Geology and Geophysics 33. 393 pp. Oxford Science Publications and Clarendon Press, Oxford.
Keating, J.N., Marquart, C.L., and Donoghue, P.C.J. 2015. Histology of the heterostracan dermal skeleton: insight into the origin of the vertebrate mineralized skeleton. Journal of Morphology 276: 657–680. Crossref
Kiaer, J. 1932. The Downtonian and Devonian vertebrates of Spitsbergen. Suborder Cyathaspidida. Skrifter om Svalbard og Ishavet 52: 6–26.
Kiaer, J. and Heintz, A. 1935. The Downtonian and Devonian vertebrates of Spitsbergen. V. Suborder Cyathaspidida. Part I, Tribe Poraspidei, Family Poraspididae. Skrifter om Svalbard og Ishavet 40: 4–138.
Lankester, E.R. 1864. Scales of Pteraspis. Quarterly Journal of the Geological Society 20: 194. Crossref
Lankester, E.R. 1868. On some new cephalaspidean fishes. Reports of the British Association for the Advancement of Science London 37: 63.
Lundgren, M. and Blom, H. 2013. Phylogenetic relationships of the cyathaspidids (Heterostraci). Geologiska föreningens i Stockholm förhandlingar (GFF) 135: 74–84. Crossref
Märss, T. 1977. Structure of Tolypelepis from the Baltic upper Silurian. Proceedings of the Academy of Sciences of the Estonian SSR. Chemistry and Geology 26: 57–69.
Märss, T. 2019. Silurian cyathaspidids of Northern Eurasia. Estonian Journal of Earth Sciences 68: 113–146. Crossref
Maddison, W.P. and Maddison, D.R. 2018. Mesquite: a Modular System for Evolutionary Analysis. Version 3.51,
Novitskaya, L.I. 1970. Late Silurian Archegonaspis (Agnatha) on Vaygach Island. Paleontology Journal 4: 388–396.
Novitskaya, L.I. 1973. Liliaspis – ein Poraspide aus dem Unterdevon des Urals und einige Bemerkungen über die Phylogenie der Poraspiden (Agnatha). Palaeontographica Abt. A 143: 25–34.
Novitskaya, L.I. [Novitskaâ, L.I.] 2004. Subclass Heterostraci. Fossil vertebrates of Russia and adjacent countries [in Russian]. In: L.I. Novitskaâ and O.B. Afanassieva (eds.), Beŝelûstnye i drevnie ryby – Spravočnik dlâ paleontologov, biologov i geologov, 69–207. Rossijskaâ Akademiâ Nauk, Paleontologičeskij Institut, Moskva.
Obruchev, D.V. [Obručev, D.V.] 1945. The evolution of Agnatha [in Russian]. Zoologičeskij žurnal 24: 257–272.
Obruchev, D.V. 1964. Agnatha, Pisces. In: Y.A. Orlov (ed.), Fundamentals of Palaeontology 11, 1–825. Israel Program for Scientific Translations, Jerusalem.
Obruchev, D.V. 1967. On the evolution of the Heterostraci. Problemes actuels de Paleontologie 167: 37–43.
Ørvig, T. 1961. Notes on some early representatives of the Drepanaspida (Pteraspidomorphi, Heterostraci). Arkiv för Zoologi 12: 515–535.
Pernègre, V.N. and Elliott, D.K. 2008. Phylogeny of the Pteraspidiformes (Heterostraci), Silurian–Devonian jawless vertebrates. Zoological Scripta 37: 391–403. Crossref
Randle, E. and Sansom, R.S. 2016. Exploring phylogenetic relationships of Pteraspidiformes heterostracans (stem–gnathostomes) using continuous and discrete characters. Journal of Systematic Palaeontology 15: 583–599. Crossref
Randle, E. and Sansom, R.S. 2017. Phylogenetic relationships of the “higher heterostracans” (Heterostraci: Pteraspidiformes and Cyathaspididae), extinct jawless vertebrates. Zoological Journal of the Linnean Society 20: 1–17. Crossref
Sansom, R.S. 2008. The origin and early evolution of the Osteostraci: a phylogeny for the Thyestiida. Journal of Systematic Palaeontology 6: 317–332. Crossref
Sansom, R.S. 2009. Phylogeny,
classification and character polarity of the Osteostraci
(Vertebrata). Journal of Systematic
Palaeontology 7: 95–115. Crossref
Soehn, K. and Wilson, M.H.V. 1990. A complete, articulated heterostracan from Wenlockian (Silurian) beds of the Delorme Group, Mackenzie Mountains, Northwest Territories, Canada. Journal of Vertebrate Paleontology 10: 405–419. Crossref
Stensiö, E.A. 1927. The Downtonian and Devonian vertebrates of Spitsbergen. Part I. Family Cephalaspidae. Skrifter Svalbard Nordishavet 12: 1–391.
Stensiö, E.A. 1958. Les cyclostomes fossils ou ostracodermes. In: P.P. Grassé (ed.), Traité de Zoologie, 173–425. Masson édit., Paris.
Swofford, D.L. 2003. PAUP* ver 4.0 a168.PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods). Sinauer Associates, Sunderland, http://phylosolutions.com/paup-test
Tarlo, L.B. 1960. The Downtonian ostracoderm Corvaspis kingi Woodward, with notes on the development of dermal plates in the Heterostraci. Palaeontology 3: 217–226.
Traquair, R.H. 1899. Report on fossil fishes collected by the Geological Survey of Scotland in the Silurian rocks of the south of Scotland. Transactions of the Royal Society of Edinburgh 39: 827–864. Crossref
Voichyshyn, V. 2011. The Early Devonian armored agnathans of Podolia, Ukraine. Palaeontologia Polonica 66: 1–211. Crossref
White, E.I. 1973. Form and growth in Belgicaspis (Heterostraci). Palaeontographica Abteilung A 143: 11–24.
Wilson, M.V.H. and Märss, T. 2004.Toward a phylogeny of the thelodonts. In: G. Arratia, M.V.H. Wilson, and R. Cloutier (eds), Recent Advances in the Origin and Early Radiation of the Vertebrates, Honoring Hans-Peter Schultze, 95–108. Verlag Dr. Friedrich Pfeil, Munich.
Wilson, M.V.H. and Märss, T. 2009. Thelodont phylogeny revisited, with inclusion of scale-based taxa. Estonian Journal of Earth Sciences 58: 297–310.Crossref
Acta Palaeontol. Pol. 66 (3): 631–640, 2021
https://doi.org/10.4202/app.00811.2020