

Can maxillary canal morphology inform varanopid phylogenetic affinities?
JULIEN BENOIT, DAVID P. FORD, JURI A. MIYAMAE, and IRINA RUF
Varanopidae have historically been classified as members of the basal synapsid grade “Pelycosauria”. However, recent phylogenetic works proposed that varanopids may in fact belong to the Diapsida. Here, we use µCT scanning and digital 3D reconstruction to compare the maxillary canal of the early diapsid Orovenator mayorum, the basal archosauromorph Prolacerta broomi, the ophiacodontid “pelycosaur” Varanosaurus acutrostris, and the varanopid Heleosaurus scholtzi. We find that the maxillary canals of Orovenator and Prolacerta are very similar and differ markedly from those of Heleosaurus and Varanosaurus. In the latter two, the morphology of the maxillary canal closely matches that of the Therapsida, which could support the traditional position of varanopids among the Synapsida.
Introduction
Amongst “pelycosaur” grade synapsids, the Varanopidae are remarkable for their evolutionary longevity and cosmopolitan geographic distribution. They originated in the Middle Pennsylvanian (late Carboniferous), survived to the end of the Guadalupian (middle Permian), and are the only “pelycosaurs” found in the Southern Hemisphere, which makes them the most widespread and longest-lived family of early synapsids (Dilkes and Reisz 1996; Modesto et al. 2011; Day et al. 2015; Angielczyk and Kammerer 2018; Ford and Benson 2019, 2020; Maddin et al. 2020; Modesto 2020). Varanopids also provide abundant evidence that scales were the ancestral integument of synapsids before hair evolved and multiple cases of fossilized “parental care” behavior chronicling the evolution of postnatal nurturing of young (Botha-Brink and Modesto 2007; Vickaryous and Sire 2009; Spindler et al. 2018; Maddin et al. 2020). Although they display features otherwise only found in sauropsid amniotes, such as the presence of osteoderms (Carroll 1976; Botha-Brink and Modesto 2007; Vickaryous and Sire 2009), varanopids have been traditionally classified as Synapsida (Romer and Price 1940), a position supported by most phylogenetic studies (Gauthier et al. 1988; Maddin et al. 2020; Modesto 2020; see also review in Angielczyk and Kammerer 2018). However, this was recently challenged by a series of phylogenetic analyses that place varanopids among the Diapsida instead of the Synapsida, with the pivotal taxa Archaeovenator hamiltonensis and Orovenator mayorum bridging the morphological gap between taxa with traditionally recognised diapsid and synapsid temporal fenestration (MacDougall et al. 2018; Ford and Benson 2019, 2020). A placement of varanopids among diapsids would make the condition of their integument and geographical and stratigraphic distributions unremarkable compared to other Sauropsida (Reptilia sensu Ford and Benson 2020). In contrast, this would have profound implications on the evolution of “parental care” since the earliest evidence of such behavior would shift from the mammalian to the reptilian side of the amniote phylogenetic tree (Modesto 2020). As such, this new phylogenetic proposition has encountered some opposition (Sues 2019; Maddin et al. 2020).
Using µCT scanning, this study investigates an overlooked aspect of early amniote anatomy, the morphology of the maxillary canal for the maxillary branch of the trigeminal nerve. Systematic studies of the maxillary canal in synapsids have demonstrated that its morphology is very conservative among “pelycosaurs”, non-mammalian therapsids, and mammals, including varanopids (represented by Heleosaurus scholtzi; Benoit et al. 2018, 2019). As a result, the six main branches of the maxillary canal in non-mammalian synapsids can be homologized with the canals for the corresponding rami of the maxillary nerve of therian mammals, namely the external and internal nasal rami, the superior labial ramus, and the rostral, median, and caudal alveolar rami (Benoit et al. 2016a, b, 2017a, b, 2018, 2019; Pusch et al. 2019, 2020; Wallace et al. 2019; monotremes are quite different and derived in their infraorbital canal anatomy, see Benoit et al. 2019 for discussion). In contrast, most modern sauropsids as well as the non-avian dinosaurs, rauisuchids, plesiosaurs, and some extinct crocodiles studied so far display a simple, long, and tubular maxillary canal that runs parallel to the dental margin of the maxilla and gives off short lateral branches directed towards small foramina that are aligned above the tooth row (Watkinson 1906; Willard 1915; Abdel-Kader et al. 2011; Leitch and Catania 2012; Foffa et al. 2014; Ibrahim et al. 2014; Porter and Witmer 2015; Benoit et al. 2016a; Lessner et al. 2016; Barker et al. 2017; Serrano-Martínez et al. 2020; see SOM: fig. S1, Supplementary Online Material available at http://app.pan.pl/SOM/app66-Benoit2_etal_SOM.pdf). As the morphology of the maxillary canal in the varanopid Heleosaurus scholtzi conforms to the general synapsid pattern (Benoit et al. 2018), it is tempting to conclude that a placement among diapsids is not supported; however, following Ford and Benson’s (2020) phylogenetic hypothesis, the condition encountered in synapsids may not be apomorphic, but plesiomorphic for amniotes or convergent in varanopids. In order to test these hypotheses, this study describes, for the first time, the morphology of the maxillary canal of two diapsids: Orovenator mayorum, an early diapsid from the lower Permian of North America, and Prolacerta broomi, an archosauromorph from the Lower Triassic of South Africa. This will present new data on the ancestral morphology of the diapsid maxillary canal and help decide whether the pattern observed in Heleosaurus scholtzi could be derived from it.
Institutional abbreviations.—CG, Council for Geoscience, Pretoria, South Africa; FMNH, Field Museum of Natural History, Chicago, USA; MCZ, Harvard Museum of Comparative Zoology, Cambridge, USA; OMNH, Sam Noble Oklahoma Museum of Natural History, Norman, USA; UCMP, University of California, Berkeley Museum of Paleontology, USA.
Material and methods
Samples.—The µCT scans of Prolacerta broomi and Orovenator mayorum (hereafter referred to as Prolacerta and Orovenator) were acquired from MorphoSource. Prolacerta broomi is represented by specimen UCMP 7151 (Media number: M63758-115171; URL: http://www.morphosource.org/Detail/MediaDetail/Show/media_id/63758) (see Modesto and Sues 2004 for details about the specimen). Orovenator mayorum is represented by specimen OMNH 74606 (Media number: M23671-49776; URL: http://www.morphosource.org/Detail/MediaDetail/Show/media_id/23671) (see Ford and Benson 2019 for details about the specimen). All the data and scan parameters are available on MorphoSource at the given URLs.
The maxillary canals of both specimens were segmented manually using Avizo 10 (FEI VSG, Hillsboro OR, USA). The structure was segmented on both sides to ensure that it is symmetrical. Only the best-preserved side is figured (Prolacerta broomi is mirrored for comparison in Fig. 1). They are compared to the maxillary canals of the middle Permian varanopid Heleosaurus scholtzi (CG-RMS353, hereafter Heleosaurus) and the early Permian ophiacodontid Varanosaurus acutirostris (FMNH PR 1670, hereafter referred to as Varanosaurus) that were illustrated and described in a previous study (see Benoit et al. 2018 for more details).
Nomenclature and homology.—The nomenclature used to identify the structures and branches of the maxillary canal in Heleosaurus and Varanosaurus is the same as in the original description (Benoit et al. 2018), but it could not be applied to Prolacerta and Orovenator due to the differences in the morphology of the canals. As such, we decided to designate the different branches and features of the maxillary canal of basal Permian–Triassic diapsids by numbers instead of attempting to homologize them with that of synapsids or to introduce new nomenclatural terms (see the description below).
Description
Overall, the maxillary canals of Prolacerta and Orovenator are both simple and tubular, as in the terrestrial sauropsids studied so far (Watkinson 1906; Willard 1915; Porter and Witmer 2015; Benoit et al. 2016a; Lessner et al. 2016; Barker et al. 2017; Serrano-Martínez et al. 2020). In Prolacerta and Orovenator, the maxillary canal is essentially a two-dimensional structure best visible in lateral view (Fig. 1A, B), and can be divided into four main parts numbered from 1 rostrally to 4 caudally. The maxillary sinus (or medial cavity in Ford and Benson 2019) is longer and dorsoventrally taller in Orovenator than in Prolacerta (Fig. 1A, B). In this respect, Orovenator is more similar to Varanosaurus (Fig. 1D). In Orovenator, the maxillary canal begins medially by a foramen located on the ventral margin of the maxillary sinus within an anteroposteriorly elongated sulcus, whereas in Prolacerta, the medial foramen for the maxillary canal is located on the rostrolateral margin of the maxillary sinus. This anterior medial foramen likely corresponds to the point of entry of the superior alveolar nerve within the maxilla (Watkinson 1906; Willard 1915; Abdel-Kader et al. 2011). At the level of this foramen, the maxillary canal splits into two branches in Orovenator and Prolacerta, one rostral (Fig. 1: 2) and one caudal (Fig. 1: 3). Both branches run parallel to the ventral margin of the maxilla, just above the tooth row, and give off many small dorsolateral and ventrolateral branches at regular intervals, as in other terrestrial sauropsids (Watkinson 1906; Willard 1915; Porter and Witmer 2015; Benoit et al. 2016a; Lessner et al. 2016; Barker et al. 2017; Serrano-Martínez et al. 2020). Prolacerta has only a few lateral branches, whereas they are numerous in Orovenator (Fig. 1A, B). The rostralmost branch of the maxillary canal is the main trunk of the maxillary canal in Orovenator and Prolacerta (Fig. 1: 2). It extends from the maxillary sinus caudomedially to the anterior maxillary foramen rostrally. It is long, tubular, and thicker than the other parts of the maxillary canal in Prolacerta and Orovenator (Fig. 1A, B). In both Prolacerta and Orovenator, the main trunk of the maxillary canal terminates anteriorly by a large and conspicuous anterior maxillary foramen located just caudal to the anterior margin of the maxilla (Modesto and Sues 2004; Ford and Benson 2019; Fig. 1A, B). Dorsally, the maxillary canal ramifies into a tall, conical cavity oriented at an angle of about 40° from the main trunk of the maxillary canal in Orovenator, and 50° in Prolacerta (Fig. 1: 1). This conical cavity is located just rostral to the anterior margin of the maxillary sinus in Orovenator, whereas it is far removed from it in Prolacerta (Fig. 1A, B). Notably, this cavity is blind in both taxa, as it does not lead to any internal or external foramen. Instead, it vanishes into the bone trabeculae of the maxilla. This is unlike the condition in Heleosaurus, Varanosaurus, and other synapsids, in which the dorsal-most branch of the maxillary canal, called the external nasal canal, opens externally into numerous foramina (Benoit et al. 2016a, 2017a, 2018, 2019). The caudal-most part of the maxillary canal is made of two successive portions of about equal length in Orovenator and Prolacerta (Fig. 1: 3 and 4). In both taxa, the section noted 3 starts immediately caudal to the internal foramen for the superior alveolar nerve. This canal differs from the main trunk by its smaller diameter. Section 3 terminates caudally by a large foramen located medially under the anterior margin of the orbit in Orovenator (Fig. 1A). It is located more rostrally in Prolacerta (Fig. 1B). This posterior medial foramen may correspond to the entry of the infraorbital artery (maxillary artery) into the maxilla (Soliman 1964; Albrecht 1967; Porter and Witmer 2015). As the main trunk of the maxillary canal, section 3 is a tubular structure that gives off short lateral ramifications at regular intervals in Orovenator and Prolacerta. Instead of opening into an external foramen laterally, the caudal-most two or three of these smaller branches (this number varies bilaterally) bifurcate caudally and extend toward the jugal to constitute the 4th section of the maxillary canal in Orovenator and Prolacerta (Fig. 1A, B). In Orovenator, one of these small branches carries on branching off laterally at regular intervals over a short distance, which is similar to the condition visible on the caudal alveolar canal of Heleosaurus and Varanosaurus (Fig. 1C, D). Section 4 extends caudally well below the orbit in Orovenator (as in Heleosaurus and Varanosaurus), but not in Prolacerta (Fig. 1A, B). The canals of section 4 terminate caudally into small external foramina immediately followed by short sulci in both Orovenator and Prolacerta (Modesto and Sues 2004; Ford and Benson 2019).
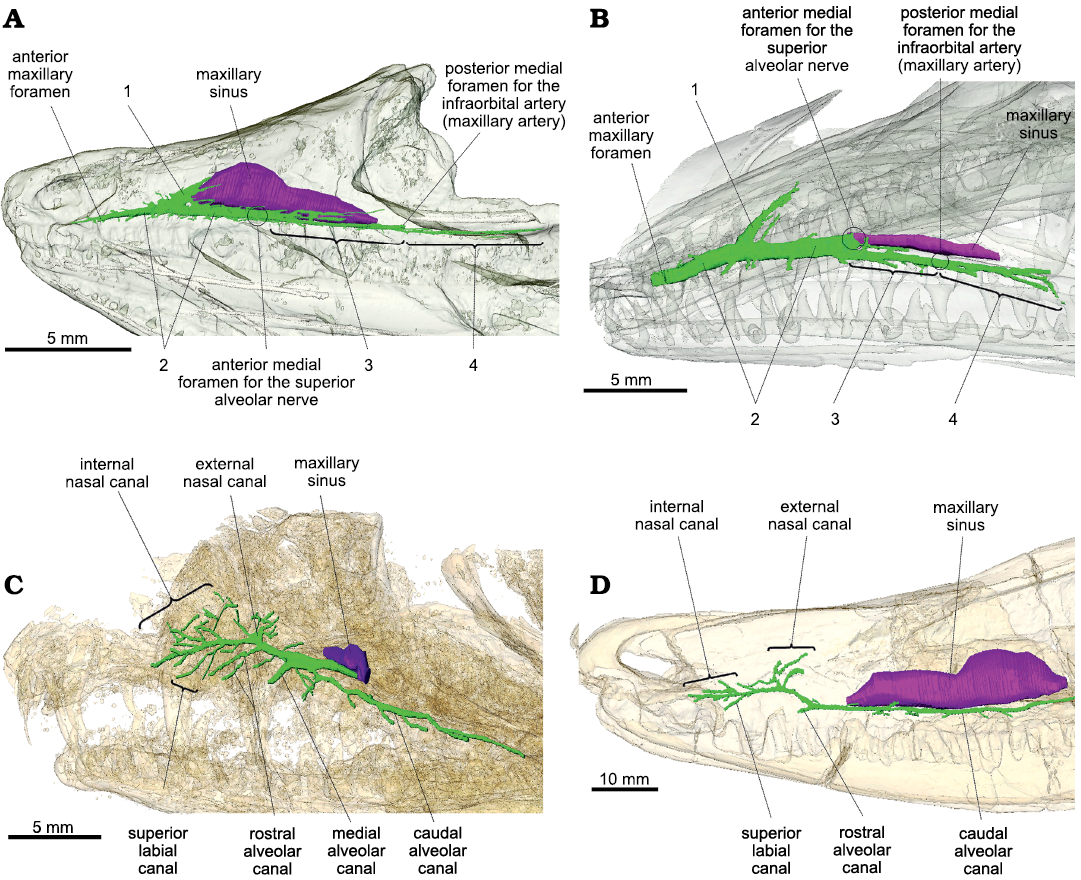
Fig. 1. Lateral view of the maxillary canal in left lateral view. A. Orovenator mayorum Reisz, Modesto, and Scott, 2011 (OMNH 74606) from the lower Permian of the Dolese Brothers Limestone Quarry (Oklahoma, USA). B. Prolacerta broomi Parrington, 1935 (UCMP 7151, mirrored for comparison) from the Lower Triassic of Big Bank (Harrismith District, South Africa). C. Heleosaurus scholtzi Broom, 1907 (CG-RMS353) from the middle Permian of South Africa. D. Varanosaurus acutirostris Broili, 1904 (FMNH PR 1670) from the lower Permian Wellington Formation (Garvin County, Oklahoma, USA). The maxillary canal is in green, the maxillary sinus is in purple, and the skull is transparent. Circles represent the position of a structure not visible in lateral view. Abbreviations: 1, conical cavity; 2, main trunk of the maxillary canal; 3, caudal section of the maxillary canal; 4, caudally extended side branches.
Discussion
Despite minor differences, the overall organization of the maxillary canal is essentially the same in Orovenator and Prolacerta as it can be divided into four components that are topographically and morphologically homologous (Fig. 1: 1–4). This implies that the morphology of the maxillary canal in both taxa is likely to be representative of the ancestral condition in diapsids, i.e., a simple, long, and tubular canal oriented horizontally from which regularly-spaced offshoots are sent laterally above the tooth row. However, the maxillary canal of the varanopid Heleosaurus does not match this condition as it is not tubular and sends a considerable amount of branches that ramify in various directions within the maxilla (Fig. 1C). In contrast, it matches the condition present in the ophiacodontid “pelycosaur” Varanosaurus (except for the apparent lack of a median alveolar canal in Varanosaurus, Fig. 1D). As demonstrated by Benoit et al. (2018), the branching pattern of the maxillary canal in Heleosaurus and Varanosaurus closely resembles that of Permian–Triassic therapsids, which suggests that the morphology of the maxillary canal in Heleosaurus is homologous to that of synapsids, rather than diapsids.
It may be argued that the conical cavity (Fig. 1: 1) could have evolved into the branched external nasal canal of the varanopids as this canal displays a conical base in Heleosaurus (Fig. 1C). The main trunk of the maxillary canal is also thickened between this region and the maxillary sinus in Heleosaurus, which resembles the condition of section 1 in Orovenator and Prolacerta (Fig. 1A, B). However, the conical cavity is blind in Orovenator and Prolacerta whereas it opens externally in Heleosaurus and other synapsids (Benoit et al. 2016a, b, 2017a, 2018, 2019; Pusch et al. 2019, 2020). In addition, a thickened conical base of the external nasal ramus is also present in many therapsids, particularly theriodonts (Benoit et al. 2016a, 2019; Pusch et al. 2019).
One interesting shared feature is the similarity between section 4 of the maxillary canal of Orovenator and the caudal alveolar canal of Heleosaurus and Varanosaurus, which are very long and send off short lateral branches at regular intervals above the ventral margin of the maxilla (Fig. 1A, C, D), resulting in the presence of aligned supralabial foramina. Such a long caudal alveolar canal is also found in the basal-most therapsid Raranimus dashoukensis (Duhamel et al. 2019), but it becomes very short in more derived taxa (Benoit et al. 2016a). Supralabial foramina are also found in basal diapsids, such as Petrolacosaurus and other Araeoscelidia, as well as in pelycosaurs, including the Varanosaurus and Heleosaurus specimens studied here, and the varanopids Apsisaurus and Archaeovenator (Reisz 1977; Carroll 1988; Reisz and Dilkes 2003; Sues 2019; DPF personal obserwations of MCZ 1474). This suggests that the presence of supralabial foramina is a plesiomorphic feature for amniotes and it is possible that section 4 of the maxillary canal in diapsids and the caudal alveolar canal of synapsids is homologous. It is hypothesized that the maxillary canal in Permian–Triassic synapsids predominantly carried the maxillary branch of the trigeminal nerve (which includes the infraorbital nerve) (Benoit et al. 2016a), whereas in modern sauropsids the maxillary canal (or superior alveolar canal) carries a nerve designated as the superior alveolar branch of the infraorbital nerve (Watkinson 1906; Willard 1915; Bellairs 1949; Abdel-Kader et al. 2011; Leitch and Catania 2012). It is thus unclear if these parts of the maxillary canal of Permian–Triassic synapsids and sauropsids can be homologized to each other as this would imply an (at least partial) homology of the mammalian caudal alveolar nerve and sauropsid superior alveolar nerve (and their accompanying vessels). Although this may account for the almost ubiquitous presence of supralabial foramina among early amniotes (Carroll 1988), this hypothesis will have to be tested in the future by studying the maxillary nerve of modern tetrapods and the maxillary canal of other “pelycosaurs” and early sauropsids (e.g., caseasaurs, varanopids, neodiapsids, parareptiles, and captorhinids).
Conclusions
Resolving the phylogenetic position of varanopids is crucial for understanding the early radiation of basal synapsids and sauropsids and the evolution of many important defining soft tissue features of amniotes, which has implications beyond the field of palaeontology. Here, we demonstrate that the morphology of the maxillary canal in the varanopid Heleosaurus more closely resembles that of the ophiacodontid “pelycosaur” Varanosaurus than that of the diapsids Orovenator and Prolacerta. Although this does not disprove a close relationship between varanopids and diapsids, this highlights that a comprehensive knowledge of the internal skeletal structures across a broad range of early sauropsids, synapsids, and stem amniotes is essential for resolving the amniote phylogenetic tree as they may provide a valuable phylogenetic signal. We suggest that palaeoneurological structures should be taken into account in future works that will address the affiliations of early amniotes.
Acknowledgements.—The authors thank Jessica Anderson Maisano (University of Texas at Austin, USA) for enabling access to the µCT data of Prolacerta. Thanks also go to Kenneth Angielczyk (FMNH) and Sean P. Modesto (Cape Breton University, Sydney, Canada) whose reviews helped to improve the manuscript. This study was made with the financial support of the DST-NRF Centre of Excellence in Palaeosciences (CoE in Palaeosciences) and the Palaeontological Scientific Trust and its Scatterlings program (PAST), and the NRF Postdoctoral Research Fellowship (DPF).
References
Abdel-Kader, T.G., Ali, R.S., and Ibrahim, N.M. 2011. The cranial nerves of Mabuya quinquetaeniata III: nervus trigeminus. Life Science Journal 8: 650–669.
Albrecht, P.W. 1967. The cranial arteries and cranial arterial foramina of the turtle genera Chrysemys, Sternotherus, and Trionyx: a comparative study with analysis of possible evolutionary implications. Tulane Studies in Zoology 14: 81–99.
Angielczyk, K.D. and Kammerer, C.F. 2018. Non-mammalian synapsids: the deep roots of the mammalian family tree. In: F. Zachos and R. Asher (eds.), Mammalian Evolution, Diversity and Systematics, 117–198. De Gruyter, Berlin. Crossref
Barker, C.T., Naish, D., Newham, E., Katsamenis, O.L., and Dyke, G. 2017. Complex neuroanatomy in the rostrum of the Isle of Wight theropod Neovenator salerii. Scientific Reports 7 (1): 3749. Crossref
Bellairs, A.D. 1949. Observations on the snout of Varanus, and a comparison with that of other lizards and snakes. Journal of Anatomy 83: 116–146.1.
Benoit, J., Angielczyk, K.D., Miyamae, J.A., Manger, P., Fernandez, V., and Rubidge, B. 2018. Evolution of facial innervation in anomodont therapsids (Synapsida): insights from X-ray computerized microtomography. Journal of Morphology 279: 673–701. Crossref
Benoit, J., Manger, P.R., and Rubidge, B.S. 2016a. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Scientific Reports 6 (1): 25604. Crossref
Benoit, J., Manger, P.R., Fernandez, V., and Rubidge, B.S. 2016b. Cranial bosses of Choerosaurus dejageri (Therapsida, Therocephalia): earliest evidence of cranial display structures in eutheriodonts. PLoS ONE 11 (8): e0161457. Crossref
Benoit, J., Manger, P.R., Norton, L., Fernandez, V., and Rubidge, B.S. 2017a. Synchrotron scanning reveals the palaeoneurology of the head-butting Moschops capensis (Therapsida, Dinocephalia). PeerJ 5: e3496. Crossref
Benoit, J., Norton, L.A., Manger, P.R., and Rubidge, B.S. 2017b. Reappraisal of the envenoming capacity of Euchambersia mirabilis (Therapsida, Therocephalia) using μCT-scanning techniques. PLoS ONE 12 (2): e0172047. Crossref
Benoit, J., Ruf, I., Miyamae, J.A., Fernandez, V., Rodrigues, P.G., and Rubidge, B.S. 2019. The evolution of the maxillary canal in Probainognathia (Cynodontia, Synapsida): reassessment of the homology of the infraorbital foramen in mammalian ancestors. Journal of Mammalian Evolution 27: 329–348. Crossref
Botha-Brink, J. and Modesto, S.P. 2007. A mixed-age classed “pelycosaur” aggregation from South Africa: earliest evidence of parental care in amniotes? Proceedings of the Royal Society B: Biological Sciences 274: 2829–2834. Crossref
Carroll, R.L. 1976. Eosuchians and the origin of archosaurs. In: C.S. Churcher (ed.), ATHLON, Essays on Palaeontology in Honour of Loris Shano Russell, 58–79. Royal Ontario Museum, Toronto.
Carroll, R.L. 1988. Vertebrate Paleontology and Evolution. 698 pp. W.H. Freeman and Company, New York.
Day, M.O., Ramezani, J., Bowring, S.A., Sadler, P.M., Erwin, D.H., Abdala, F., and Rubidge, B.S. 2015. When and how did the terrestrial mid-Permian mass extinction occur? Evidence from the tetrapod record of the Karoo Basin, South Africa. Proceedings of the Royal Society B: Biological Sciences 282: 20150834. Crossref
Dilkes, D.W. and Reisz, R.R. 1996. First record of a basal synapsid (“mammal-like reptile”) in Gondwana. Proceedings of the Royal Society of London. Series B: Biological Sciences 263: 1165–1170. Crossref
Duhamel, A.E., Benoit, J., Rubidge, B.S., and Liu, J. 2019. Filling the Olson’s Gap? A Re-appraisal of Raranimus dashankouensis (Synapsida, Therapsida) using CT-scanning technologies. Journal of Morphology 280 (S1): S111–S112.
Foffa, D., Sassoon, J., Cuff, A.R., Mavrogordato, M.N., and Benton, M.J. 2014. Complex rostral neurovascular system in a giant pliosaur. Naturwissenschaften 101: 453–456. Crossref
Ford, D.P. and Benson, R.B.J. 2019. A redescription of Orovenator mayorum (Sauropsida, Diapsida) using high‐resolution μ CT , and the consequences for early amniote phylogeny. Papers in Palaeontology 5: 197–239. Crossref
Ford, D.P. and Benson, R.B.J. 2020. The phylogeny of early amniotes and the affinities of Parareptilia and Varanopidae. Nature Ecology & Evolution 4: 57–65. Crossref
Gauthier, J., Kluge, A.G., and Rowe, T. 1988. Amniote phylogeny and the importance of fossils. Cladistics 4: 105–209. Crossref
Ibrahim, N., Sereno, P.C., Dal Sasso, C., Maganuco, S., Fabbri, M., Martill, D.M., Zouhri, S., Myhrvold, N., and Iurino, D.A. 2014. Semiaquatic adaptations in a giant predatory dinosaur. Science 345: 1613–1616. Crossref
Leitch, D.B. and Catania, K.C. 2012. Structure, innervation and response properties of integumentary sensory organs in crocodilians. Journal of Experimental Biology 215: 4217–4230. Crossref
Lessner, E.J., Stocker, M.R., Smith, N.D., Turner, A.H., Irmis, R.B., and Nesbitt, S.J. 2016. A new rauisuchid (Archosauria, Pseudosuchia) from the Upper Triassic (Norian) of New Mexico increases the diversity and temporal range of the clade. PeerJ 4: e2336. Crossref
MacDougall, M.J., Modesto, S.P., Brocklehurst, N., Verrière, A., Reisz, R.R., and Fröbisch, J. 2018. Commentary: a reassessment of the taxonomic position of mesosaurs, and a surprising phylogeny of early amniotes. Frontiers in Earth Science [published online, https://doi.org/10.3389/feart.2018.00099]. Crossref
Maddin, H.C., Mann, A., and Hebert, B. 2020. Varanopid from the Carboniferous of Nova Scotia reveals evidence of parental care in amniotes. Nature Ecology & Evolution 4: 50–56. Crossref
Modesto, S.P. 2020. Rooting about reptile relationships. Nature Ecology & Evolution 4: 10–11. Crossref
Modesto, S.P. and Sues, H.-D. 2004. The skull of the Early Triassic archosauromorph reptile Prolacerta broomi and its phylogenetic significance. Zoological Journal of the Linnean Society 140: 335–351. Crossref
Modesto, S.P., Smith, R.M.H., Campione, N.E., and Reisz, R.R. 2011. The last “pelycosaur”: a varanopid synapsid from the Pristerognathus Assemblage Zone, middle Permian of South Africa. Naturwissenschaften 98: 1027–1034. Crossref
Porter, W.R. and Witmer, L.M. 2015. Vascular patterns in iguanas and other squamates: blood vessels and sites of thermal exchange. PLoS ONE 10 (10): e0139215. Crossref
Pusch, L.C., Kammerer, C.F., and Fröbisch, J. 2019. Cranial anatomy of the early cynodont Galesaurus planiceps and the origin of mammalian endocranial characters. Journal of Anatomy 234: 592–621. Crossref
Pusch, L.C., Ponstein, J., Kammerer, C.F., and Fröbisch, J. 2020. Novel endocranial data on the early therocephalian Lycosuchus vanderrieti underpin high character variability in early theriodont evolution. Frontiers in Ecology and Evolution 7: 464. Crossref
Reisz, R.R. 1977. Petrolacosaurus, the oldest known diapsid reptile. Science 196: 1091–1093. Crossref
Reisz, R.R. and Dilkes, D.W. 2003. Archaeovenator hamiltonensis, a new varanopid (Synapsida: Eupelycosauria) from the upper Carboniferous of Kansas. Canadian Journal of Earth Sciences 40: 667–678. Crossref
Romer, A.S. and Price, L.W. 1940. Review of the Pelycosauria. Geological Society of America Special Papers 28: 1–534. Crossref
Serrano-Martínez, A., Knoll, F., Narváez, I., Lautenschlager, S., and Ortega, F. 2020. Neuroanatomical and neurosensorial analysis of the Late Cretaceous basal eusuchian Agaresuchus fontisensis (Cuenca, Spain). Papers in Palaeontology [published online, https://doi.org/10.1002/spp2.1296]. Crossref
Soliman, M.A. 1964. Die Kopfnerven der Schildkröten. Zeitschrift für Wissenschaftliche Zoologie 1969: 216 –312.
Spindler, F., Werneburg, R., Schneider, J.W., Luthardt, L., Annacker, V., and Rößler, R. 2018. First arboreal “pelycosaurs” (Synapsida: Varanopidae) from the early Permian Chemnitz Fossil Lagerstätte, SE Germany, with a review of varanopid phylogeny. Paläontologische Zeitschritf 92: 315–364. Crossref
Sues, H.-D. 2019. The Rise of Reptiles: 320 Million Years of Evolution. 385 pp. Johns Hopkins University Press, Baltimore.
Vickaryous, M.K. and Sire, J.-Y. 2009. The integumentary skeleton of tetrapods: origin, evolution, and development. Journal of Anatomy 214: 441–464. Crossref
Wallace, R.V.S., Martínez, R., and Rowe, T. 2019. First record of a basal mammaliamorph from the early Late Triassic Ischigualasto Formation of Argentina. PLoS ONE 14 (8): e0218791. Crossref
Watkinson, G.B. 1906. The cranial nerves of Varanus bivittatus. Gegenbaurs Morphologisches Jahrbuch 35: 450–472.
Willard, W.A. 1915. The cranial nerves of Anolis carolinensis. Bulletin of the Museum of Comparative Zoology 59: 15–116.
Julien Benoit [Julien.benoit@wits.ac.za] and David P. Ford [dpford@btinternet.com], Evolutionary Studies Institute (ESI) and School of Geosciences, University of the Witwatersrand, PO Wits, 2050, Johannesburg, South Africa.
Juri A. Miyamae [curious.sengi@gmail.com], Department of Geology & Geophysics, Yale University, New Haven, Connecticut, USA.
Irina Ruf [irina.ruf@senckenberg.de], Abteilung Messelforschung und Mammalogie, Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Senckenberganlage 25, 60325 Frankfurt am Main, Germany.
Received 11 September 2020, accepted 26 October 2020, available online 23 April 2021.
Copyright © 2021 J. Benoit et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Acta Palaeontol. Pol. 66 (2): 389–393, 2021
https://doi.org/10.4202/app.00816.2020