

Femoral histology and growth patterns of the ceratopsian dinosaur Psittacosaurus sibiricus from the Early Cretaceous of Western Siberia
PAVEL P. SKUTSCHAS, SEMYON S. MOROZOV, ALEXANDER O. AVERIANOV, SERGEY V. LESHCHINSKIY, STEPAN V. IVANTSOV, ALEXEY V. FAYNGERTS, OLGA A. FEOFANOVA, OLGA N. VLADIMIROVA, and DMITRY A. SLOBODIN
Skutschas, P.P., Morozov, S.S., Averianov, A.O., Leshchinskiy, S.V., Ivantsov, S.V., Fayngerts, A.V., Feofanova, O.A., Vladimirova, O.N., and Slobodin, D.A. 2021. Femoral histology and growth patterns of the ceratopsian dinosaur Psittacosaurus sibiricus from the Early Cretaceous of Western Siberia. Acta Palaeontologica Polonica 66 (2): 437–447.
The early ceratopsian dinosaur Psittacosaurus sibiricus from the Early Cretaceous of Western Siberia, Russia, is one of the most advanced and largest (up to 2.5 m) members of the genus. Here we present a description of ontogenetic changes in the long-bone histology of this species. Analysis of a growth series of femora demonstrates significant histological maturation during ontogeny, expressed by the progressive appearance of signs of bone remodeling (erosion bays, secondary bone formation), decreasing of vascularity, changing of the orientation of vascular canals from reticular to longitudinal, and appearance of parallel-fibred bone in the outer part cortex. These ontogenetic changes in the long-bone histology of P. sibiricus are generally similar to those of another relatively advanced species, P. lujiatunensis from China. The basal P. mongoliensis from Mongolia shows less mature long-bone histology during late ontogeny (e.g., the late appearance of signs of remodeling and the predominance of reticular vascularization at later stages). We suggest that the earlier achievement of histological maturity is an evolutionary trend of the genus Psittacosaurus.
Key words: Dinosauria, Ceratopsia, Psittacosaurus, bone histology, Cretaceous, Siberia, Russia.
Pavel P. Skutschas [skutchas@mail.ru; p.skutschas@spbu.ru] and Semyon S. Morozov [semyon-morozow7@yandex.ru], Vertebrate Zoology Department, Saint Petersburg State University, Universitetskaya nab. 7/9, 199034 Saint Petersburg, Russia.
Alexander O. Averianov [dzharakuduk@mail.ru], Zoological Institute of the Russian Academy of Sciences, Universitetskaya nab. 1, 199034 Saint Petersburg, Russia.
Stepan V. Ivantsov [stepan_ivantsov@mail.ru], Laboratory of Micropaleontology, Tomsk State University, Prospekt Lenina 36, 634050, Tomsk, Russia.
Sergey V. Leshchinskiy [sl@ggf.tsu.ru] and Alexey V. Fayngerts [alex@ggf.tsu.ru], Laboratory of Mesozoic and Cenozoic Continental Ecosystems, Tomsk State University, Prospekt Lenina 36, 634050, Tomsk, Russia.
Olga A. Feofanova [feofanova42@yandex.ru], Olga N. Vladimirova [ovlad-5@mail.ru], and Dmitry A. Slobodin [d.a.slobodin@mail.ru], Kuzbass State Museum of Local Lore, Prospekt Sovetskiy 51, 650000, Kemerovo, Russia.
Received 17 September 2020, accepted 15 December 2020, available online 2 June 2021.
Copyright © 2021 P.P. Skutschas et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Representatives of the genus Psittacosaurus are relatively small (about 2–2.5 m) bipedal (as an adult) basal ceratopsian dinosaurs (You and Dodson 2004). The abundant remains of Psittacosaurus, including numerous complete skeletons, have been found in the Lower Cretaceous deposits in many parts of Asia including modern-day Mongolia, China, Siberian Russia, and Thailand (Lucas 2006). To date, Psittacosaurus is one of the largest genera among non-avian dinosaurs by the number of species. It includes at least 10 species (Napoli et al. 2019), in particular, the object of this study, Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000 (Averianov et al. 2006).
The large number of fossil remains of Psittacosaurus belonging to different age individuals makes it possible to establish the life histories and growth patterns based on paleohistological studies. Two species of Psittacosaurus, basal P. mongoliensis Osborn, 1923, and relatively advanced P. lujiatunensis Zhou, Gao, Fox, and Chen, 2006, were histologically studied in details and ontogenetic changes in their bone histology were described. These studies revealed differences in bone histology of these two species during ontogeny (Erickson and Tumanova 2000; Zhao et al. 2013, 2016, 2019).
Psittacosaurus sibiricus from the Lower Cretaceous Ilek Formation of Western Siberia, Russia is remarkable in several aspects: (i) it is one of the largest members of the genus (up to 2.5 m in length; PPS personal observations); (ii) it is one of the most advanced members of the genus (see Sereno 2010), and (iii) it is known by abundant material that provides an excellent opportunity to document the ontogenetic changes in bone histology of this species. The main goals of this study were to describe ontogenetic changes in the long-bone histology of Psittacosaurus sibiricus, to compare growth patterns of different Psittacosaurus species, and to discuss evolutionary trends in bone histology within the genus.
Institutional abbreviations.—KOKM, Kuzbass State Museum of Local Lore, Western Siberia, Kemerovo, Russia; PM TSU, Paleontological Museum of Tomsk State University, Tomsk State University, Western Siberia, Tomsk, Russia.
Other abbreviations.—LAGs, lines of arrested growth.
Material and methods
A total of nine specimens representing isolated complete and fragmentary femora of ceratopsian Psittacosaurus sibiricus from the Early Cretaceous vertebrate locality Shestakovo 3 (for geological settings and age of this locality see Skutschas et al. 2019; Averianov et al. 2018) of the Ilek Formation in Kemerovo Province (Western Siberia, Russia) were histologically studied to assess ontogenetic changes in long-bone histology. For the present study, transverse thin-sections were prepared based on the methodology outlined in Chinsamy and Raath (1992) and Lamm (2013).
It was impossible to take all thin-sections from a standard level (midshaft region), because mostly broken femora were used and, to minimize further damage to the material, sections were taken at the different levels of the shafts, always close to the broken surfaces (Fig. 1, Table 1).
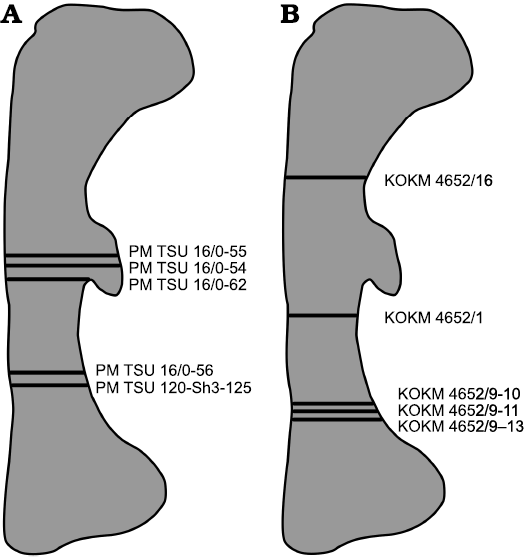
Fig. 1. Diagrammatic outline of the femur of Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia, indicating the relative positions of sections taken for histological examination. A. Sections taken from specimens from Paleontological Museum of Tomsk State University (PM TSU). B. Sections taken from specimens from Kuzbass State Museum of Local Lore (KOKM).
The sections were observed under polarized light using an optical microscope (Leica 4500, Leica Microsystems, Wetzlar, Germany) in the Saint Petersburg State University Research Centre for X-ray Diffraction Studies, Russia. Histological terminology follows Francillon-Vieillot et al. (1990) and Prondvai et al. (2014). To avoid misidentifications of interpreting vascular canal types, we follow recommendations by Zhao et al. (2019).
We estimated the total length of the fragmentary femora by using maximal length/width of the distal end ratio (measured on complete femora PM TSU 16/0-62 and KОKМ 4652/4-5, 4880) that varies in P. sibiricus between 3.3 and 3.7.
The four femoral specimens from Shestakovo 3 are housed in the Paleontological Museum of Tomsk State University, Tomsk State University, Western Siberia, Tomsk, Russia, and five specimens are housed in the Kuzbass State Museum of Local Lore, Western Siberia, Kemerovo, Russia (Table 1). The thin-sections used in the study are housed in the histological collection of the Department of Vertebrate Zoology of Saint Petersburg State University.
Table 1. List of specimens sampled for this study.
|
Specimen |
Width of the distal end (mm) |
Estimated femur length (mm) |
% of maximal femoral size |
Stage |
|
PM TSU 120-Sh3-125 |
12 |
39–40 |
<20% |
hatchling |
|
PM TSU 16/0-56 |
22 |
72–73 |
~ 30% |
juvenile |
|
PM TSU 16/0-55 |
23 |
75–77 |
~ 30% |
juvenile |
|
PM TSU 16/0-54 |
25 |
82–83 |
30–35% |
juvenile |
|
KOKМ 4652/16 |
35 |
115–117 |
40–45% |
juvenile |
|
KOKМ 4652/11 |
50 |
164–167 |
~ 63% |
subadult? |
|
KOKМ 4652/1 |
57 |
187–190 |
~ 70% |
subadult |
|
KOKМ 4652/9-10 |
75 |
246–250 |
~90% |
adult |
|
KOKМ 4652/13 |
80 |
262–267 |
100% |
adult |
Results
Hatchling stage.—The microanatomical/histological structure of the smallest femur PM TSU 120-SH3-125 (<20% of maximal femoral size; Fig. 2, Table 1) corresponds to that of hatchling individuals of Psittacosaurus lujiatunensis (e.g., initial stages of formation of fibrolamellar complex; abundance of primary vascular canals, no remodeling and growth marks; see Zhao et al. 2019) and it was referred to the hatchling stage.
The femur PM TSU 120-SH3-125 has a relatively thick cortex (maximal cortical thickness at the trochanter area is about 2 mm) entirely composed of primary bone tissue and does not present any signs of bone remodeling (e.g., erosion bays, secondary osteons, and endosteal bone deposition) (Fig. 2A1). The cortex surrounds a well-defined medullary cavity (maximal diameter of the medullary cavity about 3.7 mm).
The primary bone of the cortex is highly vascularized and contains mainly reticular primary vascular canals and primary osteons that have only just begun to form (thin lamellar bone surroundings are present in vascular canals) (Fig. 2A2). Besides reticular vascular canals, the cortex contains longitudinally oriented (throughout the cortex) and radially oriented canals at the trochanter area where the thickness of the cortex is maximal. The primary bone organization of the cortex is an incipient fibrolamellar complex (primary osteons only start to form) (Fig. 2A3, A4).
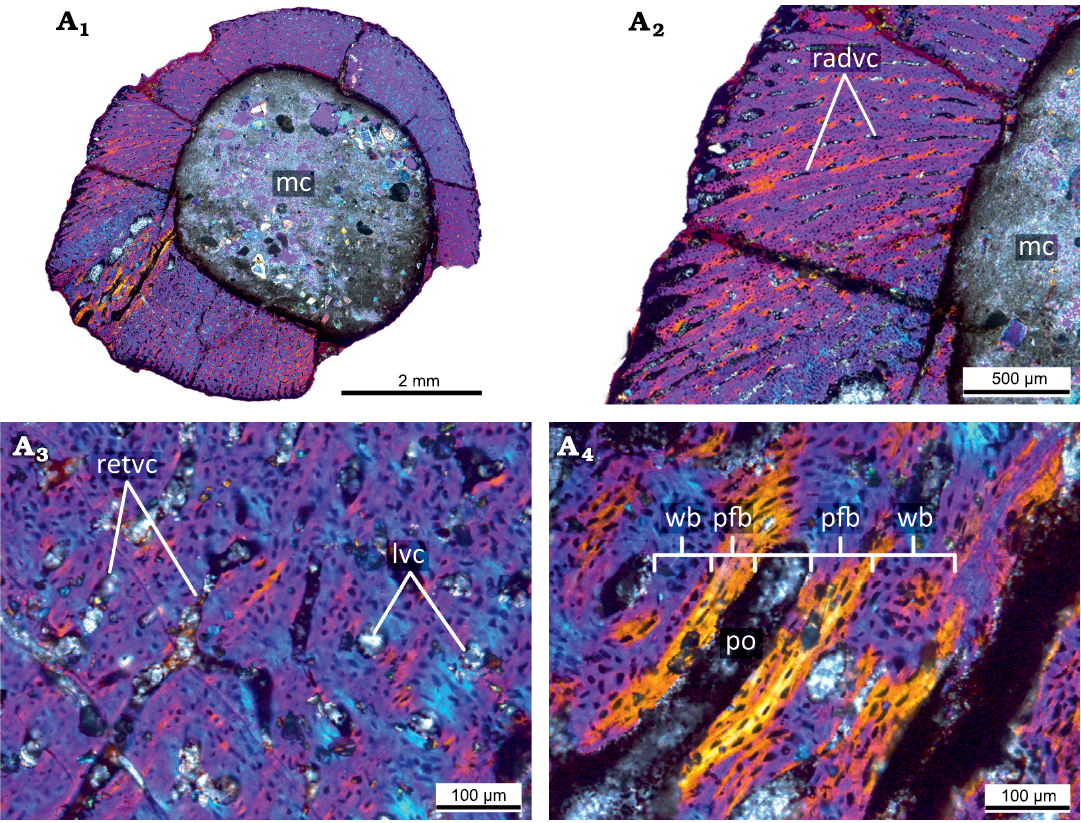
Fig. 2. Histological sections of the smallest femur PM TSU 120-Sh3-125 (<20% of maximal femoral size) of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; hatchling stage, under polarized light with lambda waveplate. Microanatomical (A1) and histological (A2) overview of the cortex showing primary highly vascularized bone. Note the presence of radial primary osteons and primary vascular canals. Close-up of the cortex, showing predominantly reticular orientation of the primary osteons and primary vascular canals (A3). Details of the composition of the primary bone tissues of the cortex (A4). Note the presence of the incipient fibrolamellar complex. Abbreviations: lvc, longitudinal vascular canals; mc, medullary cavity; pfb, parallel-fibered bone; po, primary osteon; radvc, radial vascular canals; retvc, reticular vascular canals; wb, woven bone.
There are no cyclical growth marks (e.g., annuli, LAGs) or signs of bone deposition decreasing (e.g., deposition of parallel-fibered bone). The absence of cyclical growth marks in the cortex suggests that the age of the animal was less than one year. The presence of an incipient fibrolamellar complex indicates a fast rate of growth at this ontogenetic stage.
Juvenile stage.—The femora from larger individuals (25–45% of maximal femoral size; PM TSU 16/0-54, 16/0-55, 16/0-56, KOKM 4652/16; Figs. 3–5, Table 1) display microanatomical/histological structure generally similar to that of juvenile individuals of Psittacosaurus lujiatunensis (e.g., the fibrolamellar complex, the predominance of reticular vascular canals, the appearance of LAGs, and bone remodeling; see Zhao et al. 2019) and were referred to the juvenile stage.
The PM TSU 16/0-55 (about 30% of maximal femoral size; Fig. 3A) has a relatively thick cortex (maximal cortical thickness is about 3.3 mm; thickness at the trochanter area is about 2 mm) and a well-defined medullary cavity (maximal diameter of the medullary cavity 7 mm). The cortex contains both primary and secondary bone tissues. The primary bone of the femoral cortex is formed by a fibrolamellar complex. The cortex is highly vascularized by primary osteons and vascular canals. The reticular canals are the most abundant, but longitudinal and rare radial canals are also present. The primary cortex contains three LAGs (Fig. 3A2). The innermost part of the primary cortex and perimedullary region displays remodeling leading to the formation of erosion bays and compact coarse cancellous bone (Fig. 3A).
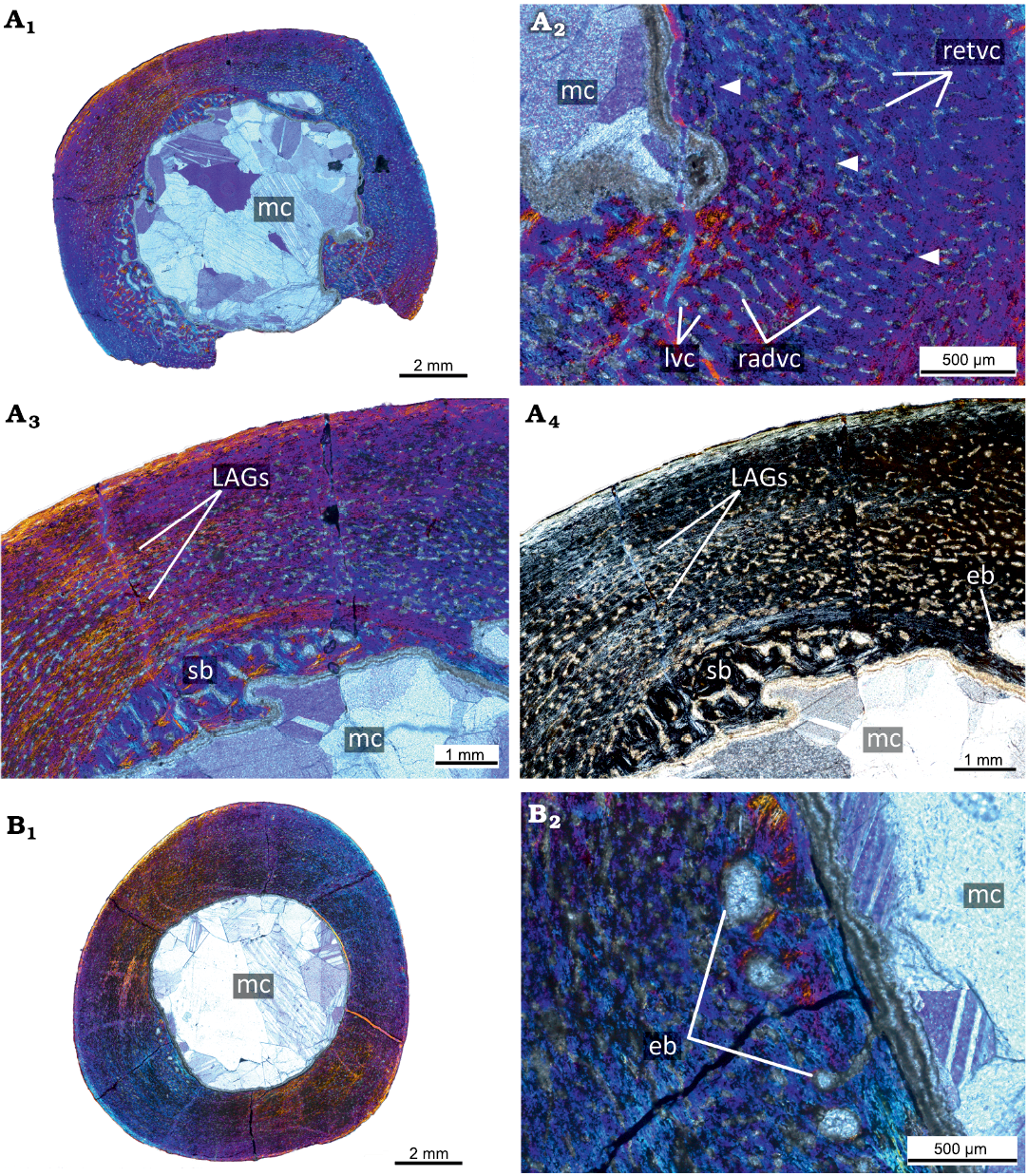
Fig. 3. Histological sections of femora PM TSU 16/0-55 (A) and PM TSU 16/0-56 (B) of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; juvenile stage; about 30% of maximal femoral size. Microanatomical overview (A1), close-up of the cortex (A2), and histological overview of the cortex under polarized light with lambda waveplate (A3) and without lambda waveplate (A4). Note the presence of erosion bays, secondary bone, and LAGs (white arrows). Microanatomical overview (B1) and close-up of the cortex under polarized light with lambda waveplate (B2). Note the presence of erosion bays. Abbreviations: eb, erosion bays; LAGs, lines of arrested growth; lvc, longitudinal vascular canals; mc, medullary cavity; pb, primary bone; radvc, radial vascular canals; retvc, reticular vascular canals; sb, secondary bone; so, secondary osteon.
The PM TSU 16/0-56 (about 30% of maximal femoral size; Fig. 3B) is not well-preserved and many details of the histological structure are not visible. Microanatomically, it is similar to the PM TSU 16/0-55 in vascularization and in the presence of erosion bays in the deep cortex.
In terms of microanatomical and histological structure, the PM TSU 16/0-54 (about 30–35% of maximal femoral size; Fig. 4) is generally similar to the PM TSU 16/0-55 by high degree of vascularization with numerous reticular and longitudinally oriented canals, the primary bone formed by a fibrolamellar complex being the dominant bone tissue type in the cortex, the presence of secondary bone (compact coarse cancellous bone) in the inner part, and the presence of growth marks (three LAGs).
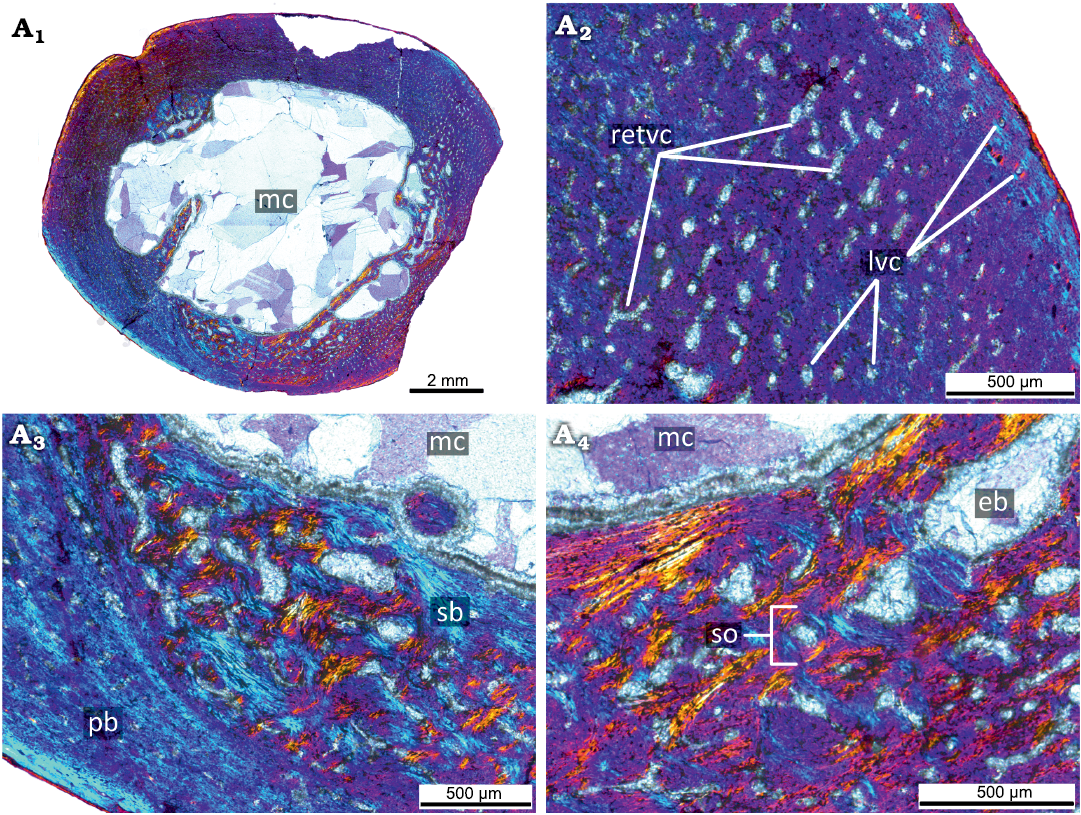
Fig. 4. Histological sections of femur PM TSU 16/0-54 of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; juvenile stage, about 30–35% of maximal femoral size. Microanatomical overview (A1) and close-ups of the cortex under polarized light with lambda waveplate (A2–A4). Abbreviations: eb, erosion bays; lvc, longitudinal vascular canals; mc, medullary cavity; pb, primary bone; retvc, reticular vascular canals; sb, secondary bone; so, secondary osteon.
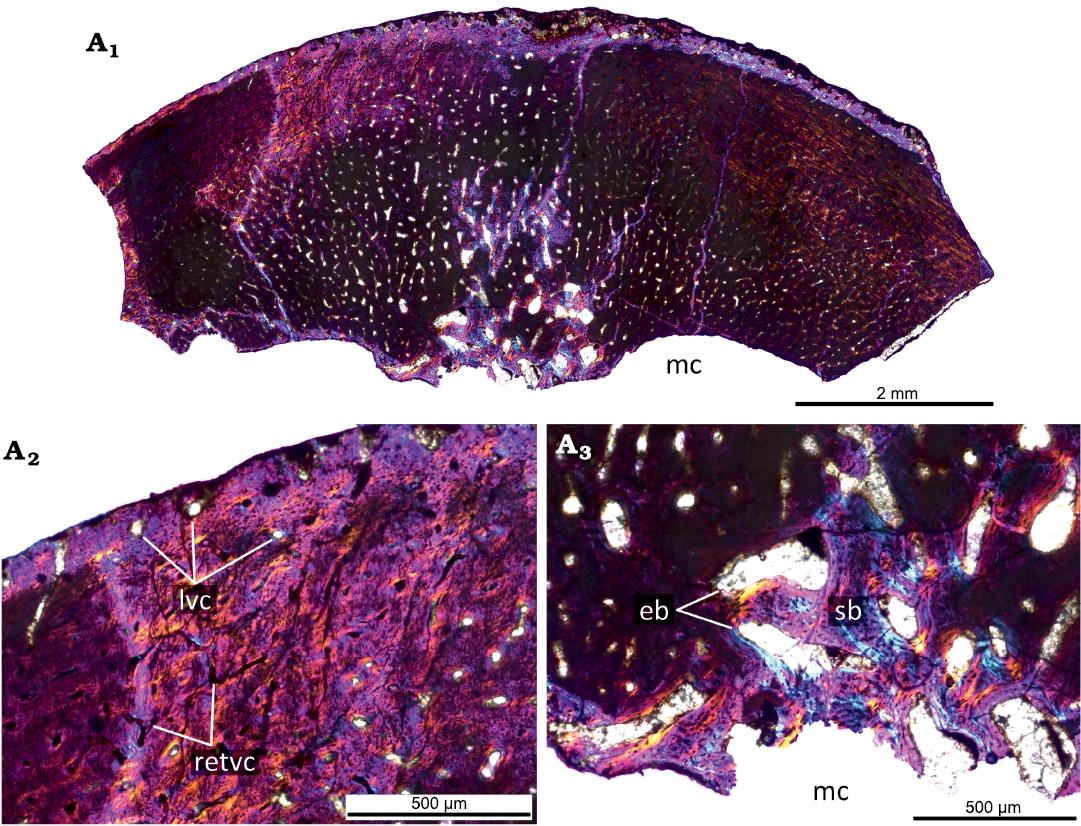
Fig. 5. Histological sections of femur KOKM 4652/16 of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; juvenile stage, about 40–45% of maximal femoral size. Microanatomical overview (A1) and close-ups of the cortex under polarized light with lambda waveplate (A2, A3) . Abbreviations: eb, erosion bays; lvc, longitudinal vascular canals; mc, medullary cavity; retvc, reticular vascular canals; sb, secondary bone.
The KOKM 4652/16 (about 40–45% of maximal femoral size; Fig. 5) is not well-preserved and many details of the histological structure are not visible. Despite this, it is characterized by a high degree of vascularization with numerous reticular and less abundant longitudinally oriented canals, by remodeling leading to formation of secondary bone.
The presence of 3 LAGs suggests that the estimated age is over three years for the specimens with about 30–35% of maximal femoral size (PM TSU 16/0-54, 16/0-55).
The fibrolamellar complex and LAGs in the femora of juveniles indicate a continuing period of fast growth with a cyclical temporary and local decrease in growth rate.
Subadult stage.—The femora KOKM 4652/1 and 4652/11 from larger individual (about 63% and 70% of maximal femoral size respectively; Fig. 6, Table 1) display microanatomical/histological structure generally similar to that of subadult individuals of Psittacosaurus lujiatunensis (e.g., extensive bone remodeling; see Zhao et al. 2019) and was referred to the subadult stage.
The primary cortex of the femur KOKM 4652/1 (Fig. 6A) is formed by a fibrolamellar complex. The cortex is highly vascularized by primary osteons and vascular canals. The reticular canals are the most abundant, but rare longitudinal canals are also present. The primary cortex contains four LAGs and the estimated age is over four years. The inner part of the cortex is widely remodeled by numerous erosion bays of different sizes, few secondary osteons, and compact coarse cancellous bone is present in the inner part of the cortex. The appearance of numerous erosion bays leads to the formation of spongiosa in the perimedullary region. Most of the trabeculae are composed of the secondary lamellar bone, but the remains of primary bone could present.
The fibrolamellar complex and LAGs in the femora of subadults indicate a continuing period of fast growth with a cyclical temporary and local decrease in growth rate.
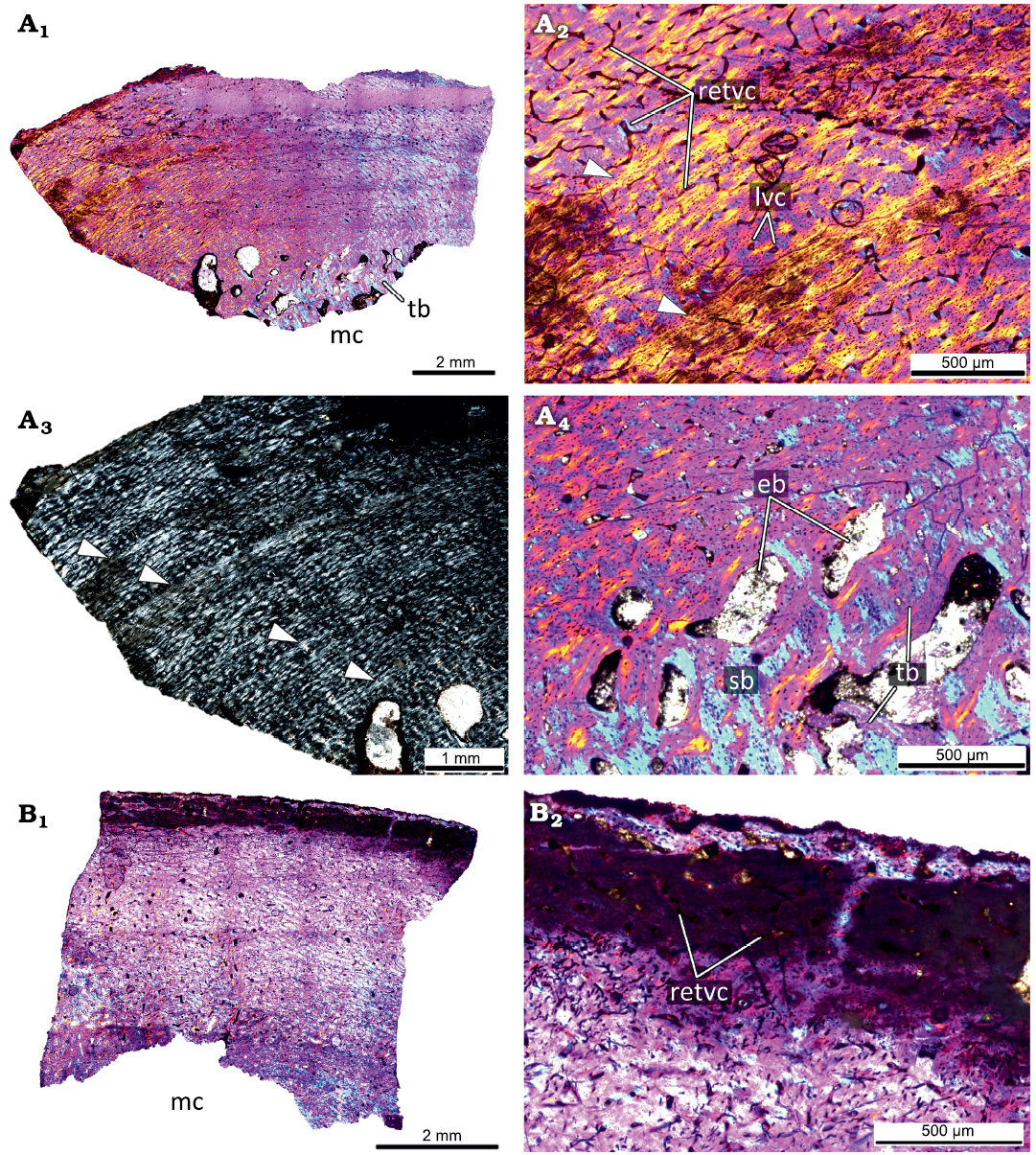
Fig. 6. Histological sections of femora KOKM 4652/1 (A) and KOKM 4652/11 (B) of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; subadult stage; about 63–70% of maximal femoral size. Histological overview of the cortex (A1, B1) under polarized light with lambda waveplate and without lambda waveplate (A3) and close-ups of the cortex (A2, A4, B2) under polarized light with lambda waveplate. Note the presence of the fibrolamellar complex and four LAGs (white arrows). Abbreviations: eb, erosion bays; lvc, longitudinal vascular canals; mc, medullary cavity; retvc, reticular vascular canals; sb, secondary bone; tb, trabeculae.
The femur KOKM 4652/11 (about 63% of maximal femoral size; Fig. 6B) is poorly preserved, but reticular canals and rare longitudinal canals are visible in some parts of the cortex. We tentatively refer this specimen to the subadult stage.
Adult stage.—The femora from the largest individuals (90–100% of maximal femoral size; KOKM 4652/9-10, 4652/13; Figs. 7, 8, Table 1) display microanatomical/histological structure generally similar to that of adult individuals of Psittacosaurus lujiatunensis (e.g, decreasing in vascularization in the outer part of the cortex, the appearance of parallel-fibred bone; see Zhao et al. 2019) and were referred to the adult stage.
The primary cortex of the KOKM 4652/9-10 (Fig. 7) is formed mostly by a fibrolamellar complex and it retains a high degree of vascularization (except the outermost part of the cortex). The orientation of vascular canals is predominantly longitudinal, but reticular canals are also present. The outermost part of the cortex is composed of parallel-fibred bone. In the KOKM 4652/9-10 growth marks were not observed, possibly due to extensive remodeling of the primary cortex. The compact coarse cancellous bone is present in the inner part of the cortex. The thick lamellar trabeculae form an extensive secondary spongiosa in the perimedullary region.
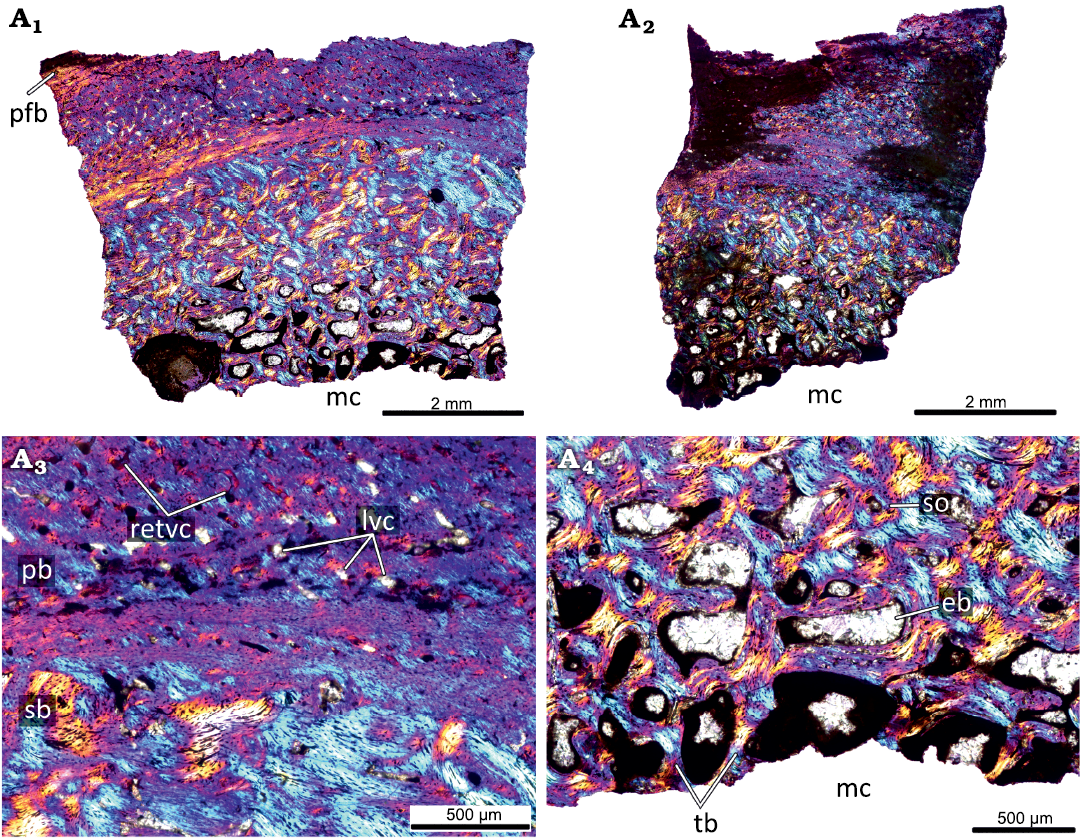
Fig. 7. Histological sections of femur KOKM 4652/9-10 of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; adult stage, about 90% of maximal femoral size. Histological overview of the cortex (A1, A2) and close-ups of the cortex (A3, A4) under polarized light with lambda waveplate. Note the parallel-fibred bone in the outermost part of the cortex. Abbreviations: eb, erosion bays; lvc, longitudinal vascular canals; mc, medullary cavity; pb, primary bone; pfb, parallel-fibred bone; retvc, reticular vascular canals; sb, secondary bone; so, secondary osteon; tb, trabeculae.
The largest femur KOKM 4652/13 (Fig. 8) has a moderately vascularized cortex that is predominantly composed of parallel-fibred bone. Like in KOKM 4652/9-10, the orientation of vascular canals is predominantly longitudinal. Growth marks were not observed, possibly due to poor preservation or extensive remodelling of the primary cortex. The deep cortex is represented by dense secondary bone tissue (compact coarse cancellous bone).
The appearance of parallel-fibred bone and changing of the dominant type of vascular canals from reticular to longitudinal indicates a decreasing in growth rate.
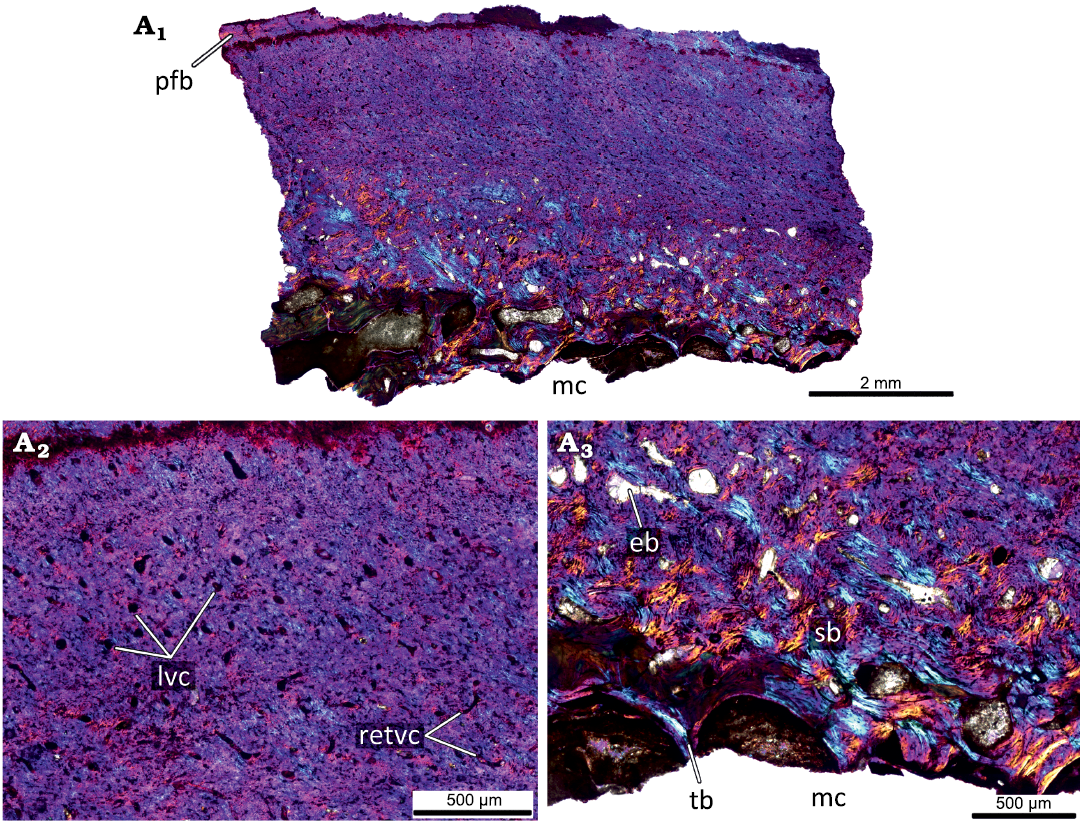
Fig. 8. Histological sections of femur KOKM 4652/13 of the ceratopsian dinosaur Psittacosaurus sibiricus Voronkevich and Averianov in Leshchinskiy et al., 2000, from the Lower Cretaceous Ilek Formation (Shestakovo 3 locality) of Western Siberia, Russia; adult stage, about 100% of maximal femoral size. Histological overview of the cortex (A1) and close-ups of the cortex under polarized light with lambda waveplate (A2, A3). Note moderately vascularized cortex that is predominantly composed of parallel-fibred bone and the predominantly longitudinal orientation of vascular canals. Abbreviations: eb, erosion bays; lvc, longitudinal vascular canals; mc, medullary cavity; pfb, parallel-fibred bone; retvc, reticular vascular canals; sb, secondary bone; tb, trabeculae.
Discussion
Ontogenetic changes in the long-bone histology of Psittacosaurus sibiricus.—The histology of the smallest individual (hatchling stage; <20% of maximal femoral size; Fig. 2) of P. sibiricus is characterized by the presence of only primary bone tissues, a high degree of vascularity, the presence of incipient fibrolamellar complex, numerous reticular vascular and few longitudinally and radially oriented canals, the absence of remodeling process and growth mark formation.
In larger individuals (juvenile stage, 25–45% of maximal femoral size; Figs. 3–5, Table 1), the following main changes in bone histology were observed: (i) appearance of remodeling and the secondary bone tissues; (ii) formation of the developed fibrolamellar complex; (iii) longitudinal canals become more abundant; and (iv) appearance of growth marks (LAGs). According to the presence of three LAGs, the estimated age is over three years for individuals with about 30–35% of the maximal femoral size.
In the larger individual (subadult stage, about 70% of maximal femoral size; Fig. 6C–F, Table 1) is characterized by progressive remodeling of the cortex with numerous erosion bays formation and by an expansion of compact coarse cancellous bone and secondary spongiosa.
In contrast to subadult stage individuals, the largest individuals (adult stage, 90–100% of maximal femoral size; Figs. 7, 8, Table 1) are characterized by decreasing vascularity and predominantly longitudinal orientation of vascular canals, and the presence of parallel-fibred bone in the outer cortex.
Growth patterns of Psittacosaurus sibiricus.—Initial growth of P. sibiricus was fast based on the presence of fast-growing primary bone (namely incipient fibrolamellar complex) in hatchling (<20% of the maximal femoral size). The fast growth continued later in ontogeny—juvenile (25–45% of the maximal femoral size) and subadult individuals (about 70% of the maximal femoral size) retained fibrolamellar complex as the main type of bone tissue in the cortex. The appearance of a parallel-fibered bone in the primary cortex of adult (90–100% of the maximal femoral size) individuals indicates a change in growth regime and a decrease in growth rate during late ontogeny. P. sibiricus shows a cyclical cessation of growth during ontogeny, with the general growth rate decrease being reflected in the appearance of LAGs.
Comparisons of long-bone histology of Psittacosaurus sibiricus and other Psittacosaurus species.—Before this study, long-bone histology of P. lujiatunensis and P. mongoliensis were described among Psittacosaurus species. According to the recent phylogenetic analysis by Sereno et al. (2010: fig.2.23E), P. sibiricus and P. lujiatunensis are species within two different advanced clades (P. lujiatunensis is a member of the P. major-clade, and P. sibiricus–P. sinensis-clade), while P. mongoliensis is the most basal species.
The primary cortex of all three species is formed predominantly by the fibrolamellar complex through ontogeny (Erickson and Tumanova 2000; Zhao et al. 2013, 2016, 2019). The hatchling individuals of P. sibiricus and P. lujiatunensis are similar in the presence of an incipient fibrolamellar complex in long bones (Zhao et al. 2019). The presence of incipient primary osteons in the studied hatchling individual of P. sibiricus and only primary vascular canals in hatchling individuals of P. lujiatunensis (Zhao et al. 2019) can be explained by slightly younger individual age of the latter. It should be noted that in the histological study on P. mongoliensis (Erickson and Tumanova 2000) the hatchling individuals were not studied directly; the information on histology of individuals of this stage is an extrapolation of histological data of the internal areas of the cortex of later stages. Therefore, it is not known exactly what type of bone matrix formed the primary cortex of the hatchling individuals of P. mongoliensis.
The vascular organization of the femora of the hatchling individuals of P. lujiatunensis and P. sibiricus is different: it is dominated by longitudinal canals in P. lujiatunensis (Zhao et al. 2016, 2019), while reticular canals are more abundant in P. sibiricus. Other ontogenetic stages of these two species demonstrate similar vascular patterns in the femora: prevalence of reticular vascular canals in juvenile and subadult individuals and prevalence of longitudinal vascularization in adults. The femora of P. mongoliensis show a different pattern of vascularization: starting from the age of three (juvenile stage), the main types of vascular canals are reticular canals; this type of canals prevails in the late stages of development and in other bones of the limbs (Erickson and Tumanova 2000).
The first signs of primary bone remodeling, the erosion bays, appear in the femur and fibula of three-year-old P. lujiatunensis (Zhao et al. 2019). Erosion bays in the femur of P. sibiricus appear at the juvenile stage: in the femur bone of PM TSU 16/0-55 (judging by the three LAG’s, this bone probably belongs to individuals older than three years) and the femur PM TSU 16/0-56 (due to poor preservation there are no data on the number of LAG’s). In P. mongoliensis, erosion bays are present only in the tibia of an eight-year-old individual (Erickson and Tumanova 2000).
Secondary bone formation in P. lujiatunensis and P. sibiricus begins at the juvenile stage, with P. lujiatunensis being the first to begin endosteal bone formation (due to centripetal growth), followed by erosion bays in the primary cortex and formation of secondary osteons (Zhao et al. 2019); in P. sibiricus there is no active endosteal bone formation, secondary bone is formed due to remodeling of the primary cortex. The absence of active endosteal bone formation in P. sibiricus could be the result of the fact that sections were made at the different levels of the shafts. For P. mongoliensis, the only thing known is that endosteal ossification occurs locally in different thin sections (Erickson and Tumanova 2000).
The ontogenetic changes in the long-bone histology of P. sibiricus and P. lujiatunensis are similar in many aspects (predominance of reticular vascularization in juvenile and subadult individuals and longitudinal in adults, early start of primary bone remodeling), which may be explained by their rather advanced phylogenetic positions (Sereno et al. 2010). The basal P. mongoliensis differs in ontogenetic changes of long-bone histology from P. sibiricus and P. lujiatunensis: predominance of reticular vascularization (a sign of active growth) and absence of well-developed secondary bone at late stages, as well as later appearance of remodeling signs, indicate that P. mongoliensis reached histological maturity later than P. sibiricus and P. lujiatunensis. The largest known femur of P. mongoliensis (210 mm) belongs to a nine-year-old individual and is histologically immature (Erickson and Tumanova 2000); in comparison, the largest femur of P. lujiatunensis (202 mm) is an eleven-year-old (Erickson et al. 2009). This means that P. mongoliensis probably had a longer growth period, and representatives of this species could reach even larger sizes. Thus, the earlier achievement of histological maturity may be one of the evolutionary trends within the genus Psittacosaurus.
Conclusions
The histological structure of the femora of Psittacosaurus sibiricus changed significantly during ontogeny. The hatchling stage is characterized by a high degree of vascularization and absence of signs of bone remodeling; at subadult and adult stages a gradual decrease of vascularization degree is observed and the appearance of signs of bone remodeling (erosion bays, secondary bone formation). The adult stage is characterized by a low degree of vascularization (the dominant type of vascularization is the longitudinal vascular canals) and well developed secondary bone tissue.
The ontogenetic changes in P. sibiricus long-bone histology are similar to those in P. lujiatunensis expressed in the early signs of bone remodeling and similar patterns of vascularization. P. mongoliensis shows different ontogenetic changes (late appearance of signs of remodeling, the predominance of reticular vascularization at later stages); considering the basal position of this species within the genus, we can suggest that the earlier achievement of histological maturity is a possible evolutionary trend within the genus Psittacosaurus. To confirm this assumption, further paleohistological studies of the differently sized bones of other Psittacosaurus species are needed.
Acknowledgements
For assistance in the field, we thank Vladimir Saev (Tomsk State University, Tomsk, Russia), who was one of the leaders of the field works, Alexey Voronkevich, Lyubov Shihovtseva, Stanislav Gromov, and Irina Sevastyanova (students of the Faculty of Geology and Geography, Tomsk State University in 1995–1996), and Elena Burkanova (student of Tomsk State Pedagogical University in 1995–1996) who took part in the field training in 1995–1996 and all participants of excavations at Shestakovo 3 in 2014–2020. We thank the government of Kuzbass and Governor Sergey Tsivilev personally for supporting and facilitating the excavations. We thank the staff of the Saint Petersburg State University Research Centre for X-ray Diffraction Studies for their help with using the Leica 2500P microscope. We thank Roman Bapinaev and Valentina Markova (Saint Petersburg State University, Saint Petersburg, Russia) for preparing images of the histological sections and Veniamin Kolchanov (Saint Petersburg State University, Saint Petersburg, Russia) for preparing some figures. The authors thank anonymous reviewers and Qi Zhao (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China) for providing helpful comments that improved the quality of the manuscript. Financial support was provided by the Russian Science Foundation (project 19-14-00020). AOA was supported by the Zoological Institute, Russian Academy of Sciences (project АААА-А19-119032590102-7). SVI was supported by the State Assignment of the Ministry of Education and Science of the Russian Federation (project no. 0721-2020-0041). SVL and AVF were supported by the State Assignment of the Ministry of Education and Science of the Russian Federation (project no. 5.4217.2017/4.6).
References
Averianov, A.O., Voronkevich, A.V., Leshchinskiy, S.V., and Fayngertz, A.V. 2006. A ceratopsian dinosaur Psittacosaurus sibiricus from the Early Cretaceous of West Siberia, Russia and its phylogenetic relationships. Journal of Systematic Palaeontology 4: 359–395. Crossref
Averianov, A.O., Ivantsov, S.V., Skutschas, P.P., Faingertz, A.V., and Leshchinskiy, S.V. 2018. A new sauropod dinosaur from the Lower Cretaceous Ilek Formation, Western Siberia, Russia. Geobios 51: 1–14. Crossref
Chinsamy, A. and Raath, M.A. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Erickson, G.M. and Tumanova, T.A. 2000. Growth curve of Psittacosaurus mongoliensis Osborn (Ceratopsia: Psittacosauridae) inferred from long bone histology. Zoological Journal of the Linnean Society 130: 551–566. Crossref
Francillon-Vieillot, H., Buffrénil, V. de, Castanet, J., Géraudie, J., Meunier, F.J., Sire, J.Y., Zylberberg, L., and Ricqlès, A. de 1990. Microstructure and mineralization of vertebrate skeletal tissues. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. Vol. 1, 471–530. Van Nostrand Reinhold, New York. Crossref
Lamm, E.-T. 2013. Preparation and sectioning of specimens. In: K. Padian and E.-T. Lamm (eds.), Bone Histology of Fossil Tetrapods—Advancing Methods, Analysis and Interpretation, 55–160. University of California Press, Berkeley. Crossref
Leshchinskiy, S.V. [Leŝinskij, S.V.], Fayngertz, A.V., Voronkevich, A.V. [Voronkevič, A.V.], Maschenko, E.N. [Maŝenko, E.N.], and Averianov, A.O. 2000. Preliminary results of the investigation of the Shestakovo localities of Early Cretaceous vertebrates [in Russian]. In: A.V. Komarov (ed.), Predvaritel’nye rezul’taty izučeniâ mestonahoždenij Ŝestakovskogo kompleksa rannemelovih pozvonočnih, 363–366. GalaPress, Tomsk.
Lucas, S.G. 2006. The Psittacosaurus biochron, Early Cretaceous of Asia. Cretaceous Research 27: 189–198. Crossref
Napoli, J.G., Hunt, T., Erickson, G.M., and Norell, M.A. 2019. Psittacosaurus amitabha, a new species of ceratopsian dinosaur from the Ondai Sayr locality, Central Mongolia. American Museum Novitates 3932: 1–36. Crossref
Osborn, H.F. 1923. Two Lower Cretaceous dinosaurs of Mongolia. American Museum Novitates 95: 1–10.
Prondvai, E., Stein, K., de Ricqlès, A., and Cubo, J. 2014. Development-based revision of bone tissue classification: the importance of semantics for science. Biological Journal of the Linnean Society 112: 799–816. Crossref
Sereno, P.C. 2010. Taxonomy, cranial morphology, and relationships of parrot-beaked dinosaurs (Ceratopsia: Psittacosaurus). In: M.J. Ryan, B.J. Chinnery-Allgeier, and D.A. Eberth (eds.), New Perspectives on Horned Dinosaurs, 21–58. Indiana University Press, Bloomington.
Skutschas, P.P., Markova, V.D., Boitsova, E.A., Leshchinskiy, S.V., Ivantsov, S.V., Maschenko, E.N., and Averianov, A.O. 2019. The first dinosaur egg from the Lower Cretaceous of Western Siberia, Russia. Historical Biology 31: 836–844. Crossref
You, H. and Dodson, P. 2004. Basal Ceratopsia. In: D.B. Weishampel, P. Dodson, H. Osmólska (eds.), The Dinosauria, 2nd. Edition, 478–493. University of California Press, Berkeley. Crossref
Zhao, B., Hedrick, B.P., Gao, C., Tumarkin-Deratzian, A.R., Zhang, F., Shen, C., and Dodson, P. 2016. Histologic examination of an assemblage of Psittacosaurus (Dinosauria Ceratopsia) juveniles from the Yixian Formation (Liaoning, China). The Anatomical Record 299: 601–612. Crossref
Zhao, Q., Benton, M.J., Hayashi, S., and Xu, X. 2019. Ontogenetic stages of ceratopsian dinosaur Psittacosaurus in bone histology. Acta Palaeontologica Polonica 64: 323–334. Crossref
Zhao, Q., Benton, M.J., Sullivan, C., Sander, M.P., and Xu, X. 2013. Histology and postural change during the growth of the ceratopsian dinosaur Psittacosaurus lujiatunensis. Nature Communications 4: 2079. Crossref
Zhou, C.-F., Gao, K.-Q., Fox, R.C., and Chen, S.-H. 2006. A new species of Psittacosaurus (Dinosauria: Ceratopsia) from the Early Cretaceous Yixian Formation, Liaoning, China. Palaeoworld 15: 100–114. Crossref
Acta Palaeontol. Pol. 66 (2): 437–447, 2021
https://doi.org/10.4202/app.00819.2020