


First Miocene megafossil of arrowhead, alismataceous plant Sagittaria, from South America
JUAN M. ROBLEDO, SILVINA A. CONTRERAS, JOHANNA S. BAEZ, and CLAUDIA I. GALLI
Robledo, J.M., Contreras, S.A., Baez, J.S., and Galli, C.I. 2021. First Miocene megafossil of arrowhead, alismataceous plant Sagittaria, from South America. Acta Palaeontologica Polonica 66 (Supplement to 3): 111–122.
The first pre-Quaternary representative of Alismataceae from South America is reported based on achenes of Sagittaria montevidensis from the Palo Pintado Formation (upper Miocene) in the south of Salta Province, Argentina. Achenes are laterally compressed, have a lateral beak and a single recurved seed inside them. The fruits were found both in the base (10 Ma) and the top of the formation (~5 Ma), suggesting similar environmental conditions during this time period. A cursory review of the Alismataceae family in the fossil record, with a special interest in those South American reports is given. During the Oligocene–Miocene Sagittaria may have arrived from tropical Africa to South America and thence to North America.
Key words: Alismataceae, Sagittaria, achene, aquatic plants, fossil fruits, Neogene, Argentina.
Juan M. Robledo [robledomanuel182@gmail.com] and Silvina A. Contreras [sailcontreras11@gmail.com], Laboratorio de Paleobotánica y Palinología desde el Neógeno hasta la Actualidad en el Norte de Argentina, Centro de Ecología Aplicada del Litoral (CONICET-UNNE), Ruta 5, km 2.5. W3400, Corrientes, Argentina and Facultad de Ciencias Exactas, Naturales y Agrimensura-Universidad Nacional del Nordeste. Av. Libertad 5450, W3400. Corrientes, Argentina.
Johanna S. Baez [johannasbaez@gmail.com], Laboratorio de Xilotafofloras del Neopaleozoico y Triásico de Sudamérica y Neógeno del Noroeste Argentino, Centro de Ecología Aplicada del Litoral (CONICET-UNNE), Ruta 5, km 2.5. W3400. Corrientes, Argentina.
Claudia I. Galli [cgalli@unsa.edu.ar], Instituto de Ecorregiones Andinas (INECOA-UNJu), Av. Bolivia 1661, S.S. de Jujuy, Universidad Nacional de Salta, Facultad de Ciencias Naturales. Av Bolivia 5150, Salta, Argentina.
Received 12 October 2020, accepted 2 February 2021, available online 6 August 2021.
Copyright © 2021 J.M. Robledo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The monocot family Alismataceae sensu lato (including Limnocharitaceae Takhtajan ex Cronquist, 1981) contains about 12 genera and approximately 100 species (Dahlgren 1980; APG III 2009; Chen et al. 2012). The family has a cosmopolitan distribution (Haynes and Holm-Nielsen 1994; Costa and Forni-Martins 2003; Soltis et al. 2005; Lehtonen 2009) and includes aquatic or semi-aquatic herbs with erect or floating leaves (Haynes and Holm-Nielsen 1994). Fossils of this family have been reported from the Lower and Upper Cretaceous and the Paleocene in North America, Europe, Siberia, and Africa (Berry 1925; Brown 1962; Golovneva 1997; Riley and Stockey 2004; Coiffard and Mohr 2018), but researchers disagree about whether all these fossil reports actually represent Alismataceae (Chandler 1963; Haggard and Tiffney 1997; Haynes and Les 2004; Chen et al. 2012).
The alismataceous genus Sagittaria has been considered to include up to 139 named species, but only about 20 of them are currently considered as legitimate (www.tropicos.org; Zepeda and Lot 2005). Species of Sagittaria, commonly named arrowheads, are mostly distributed in America, from Canada to Argentina and Chile, although four species are reported in Europe, Asia, and Oceania (Haynes and Holm-Nielsen 1994; Adair et al. 2012). Three species of Sagittaria are recorded from Argentina (Rataj 1970, 1972; Matias and Irgang 2006). The fruits of Sagittaria consist of aggregates of achenes. These achenes are laterally compressed (flattened) and have different shapes, from sub-circular to lanceolate and oblanceolate. Among the most striking morphological characters, a lateral beak (persistent style) stands out, which has different degrees of development, and a strongly recurved seed. Chen (1989) suggested that Sagittaria originated in tropical wetlands and lake areas of Africa, during the Late Cretaceous. Then, it might have dispersed to South America and subsequently to North America (Chen et al. 2012). Data concerning the biogeographic history of Alismataceae family in South America are still scarce.
Recently, Neogene impressions of Sagittaria fruits (described in this paper) were found in the Palo Pintado Formation from northwestern Argentina. This formation has been studied since the 1980-ies and nowadays discoveries still occur. Deposits of the Palo Pintado Formation indicate water bodies of low energy, as a wandering fluvial system that in rainfall periods overflowed from the channel, generating lagoons and swamps during late Miocene to Pliocene (Herbst et al. 1987; Galli et al. 2011).
In order to contribute to the history of the Alismataceae in Argentina and South America, in this paper the following objectives are proposed: (i) to describe the first record of Sagittaria from the Palo Pintado Formation; (ii) to perform a review of the Alismataceae in South America; (iii) and associate these data with stratigraphic, sedimentological and paleontological features.
Institutional abbreviations.—CTES-PB, Colección Paleontológica de la UNNE Dr. Rafael Herbst, Paleontological Collection of the Universidad Nacional del Nordeste, Corrientes, Argentina.
Geological setting
The study area is located in the Eastern Cordillera, from 25°30’ S to 66°15’ W and 25°45’ S to 66°00’ W, approximately 200 km south of the city of Salta in northwestern Argentina (Fig. 1). Cenozoic sedimentary strata crop out in the Calchaquí Valleys as part of the regional Andean foreland basin that extended into the Eastern Cordillera.
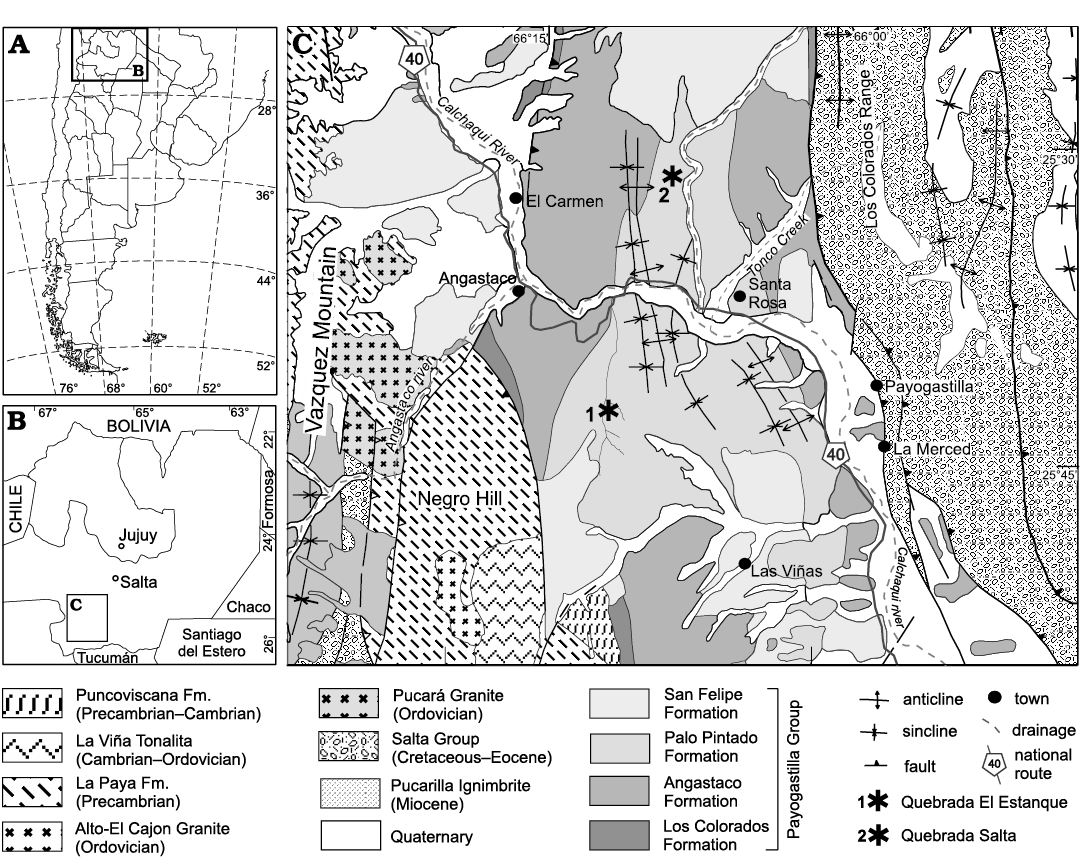
Fig. 1. A, B. Map showing location of the study area within Argentina. C. Geological map with indicated fossil localities of Quebrada El Estanque and Quebrada Salta.
The Payogastilla Group (Díaz and Malizzia 1983) in the Calchaquí Valleys is composed of continental deposits, including in ascending order formations Los Colorados (middle Eocene to Oligocene), Angastaco (middle to upper Miocene), Palo Pintado (upper Miocene) and San Felipe (Pliocene to lower Pleistocene). The Palo Pintado Formation is ~800 m thick and contains a tuff that has been dated at 10.29 ± 0.11 Ma (K/Ar) by Galli et al. (2008). Near the top is another pyroclastic unit that was dated at 5.27 ± 0.28 Ma (206Pb/238U) by Coutand et al. (2006) and at 5.98 ± 0.32 Ma by Bywater-Reyes et al. (2010). The unit comprises thickening and coarsening-upward cycles, including matrix-supported conglomerate, fine to medium sandstone, and fine-grained sublithic sandstone that end in levels of green, brown and gray siltstone. These deposits consist of a transitional style between low- and high-sinuosity rivers that form wandering sand-gravel fluvial systems with small lakes. The geometry and the fluvial architectural characteristics are a direct consequence of allogenic controls, such as tectonic activity, under constant climatic conditions (Galli et al. 2011; Galli and Reynolds 2012). The sedimentological and paleontological evidence indicates that the sediments were deposited while the region was suffering changes regarding the hydric regimen, from a wet phase (late Miocene) to a drier one (Pliocene), due mainly to the formation of an intermountain basin (Angastaco Basin) that was bordered to the west by the Sierra de Quilmes and to the east by the uprising of the Sierras Los Colorados and León Muerto (Starck and Anzótegui 2001; Bywater-Reyes et al. 2010; Rohrmann et al. 2016; Robledo et al. 2020b).
During the late Miocene, the uplift of the basin caused an increase in the sedimentary accommodation/deposition (A/D) rate and was also associated with a change in the petrologic composition of the deposits (Galli et al. 2011, 2017). The resulting orographic barriers produced a warmer and wetter climate (Starck and Anzótegui 2001). The deposits of the San Felipe Formation at the top of the Payogastilla Group are more than 600 m thick in the southeastern Calchaquí Valley and are affected by numerous faults and folds. The transition between the Palo Pintado Formation and the San Felipe Formation is sharp and unconformable; it contains considerable clast-supported conglomerate with overlapping clasts and a lower proportion of sandstone and siltstone, which are interpreted as a gravel-braided fluvial system (Galli and Reynolds 2012). Different analyses of deposits of Palo Pintado Formation, like the presence of clay minerals in the floodplain sub-environment, or the presence of illite, smectite and kaolinite indicate generation by hydrolysis in a temperate-humid climate (Galli et al. 2011); stable isotope data from pedogenic carbonates demonstrated relatively more humid conditions between 10 to 6 Ma (Bywater-Reyes et al. 2010). Paleomagnetism studies in Palo Pintado Formation deposits indicate that at approximately 6.6 Ma there is an increase in the rate of sedimentation of 0.11–0.66 mm/y which is associated with a higher percentage of Salta Group clasts. Paleocurrents from the south to the southeast indicate the tectonic reactivation of the deposition area from the Sierra León Muerto and its continuation to the north as the Sierra Los Colorados (Galli et al. 2014). Isotope analysis of δDg recorded in volcanic glass reveal that between -6.5 to 5.3 Ma, values from the Angastaco Basin decrease by -23 ± 6‰ (absolute δDg = -95‰), which is interpreted to be the result of surface uplift in this area, with altitude and aridization in a paleoenvironment like the present-day (Pingel et al. 2016).
In the global context, the ancestor assemblage floras of the current communities already were established and diversifying during the Miocene (Bell et al. 2010). This event is also observed in South American paleofloras (Barreda et al. 2007). In turn, the Miocene is a critical moment during which the great spread of the savannas took place (Quade et al. 1989; Cerling et al. 1997; Osborne 2008).
In addition to the Sagittaria fossils described below, various other vegetal remains were found at the Quebrada Salta and Quebrada El Estanque localities, which developed in an environment with fresh or brackish permanent water. These fossils include algae, ferns, monocots, and dicots (Herbst et al. 1987; Anzótegui and Horn 2011; Horn et al. 2011; Anzótegui et al. 2015). The taxa identified reflect that at least four paleoenvironments have been developed in these localities: aquatic, marsh, riparian and open terrestrial. The fossil fauna recorded here, at the moment corresponds to fragments of a turtle shell, a mandible of Caiman latirostris Daudin, 1802 (Bona et al. 2014; Bona and Barrios 2015), a tail tube and osteoderms of two glyptodons, Pampatheriidae osteoderms, fish scales, molluscs (Herbst et al. 2000), and insect wings.
Material and methods
Two sedimentological profiles from the north and south locations of the Palo Pintado Formation in the study area were analyzed at a scale of 1: 500 (i) Quebrada El Estanque and (ii) Quebrada Salta (Figs. 1, 2), from Salta Province, Argentina. The samples described here were collected from these outcrops of the Palo Pintado Formation. The fossils are deposited at the Colecciones Paleontológicas “Dr. Rafael Herbst”, of the Universidad Nacional del Nordeste, Corrientes Province, Argentina (CTES-PB 12911–12917). The fruits analyzed here are formally considered as achenes, because those are dry fruits with a single seed inside, which does not adhere to the pericarp (Fig. 3). The specimens are well preserved, some samples containing organic remains, although others are incomplete, lacking the bases or the beaks. The fossil fruits were compared with herbarium (CTES-IBONE), field samples, and literature. The fossils were examined with a Nikon binocular stereomicroscope, model SMZ-445, and photographed with a Nikon mounted camera (model 590U). All measurements were digitally performed with the software Micrometrics. Photographs were processed with the software Corel Draw. The systematics in paper are according to APG IV 2016 (Byng et al. 2016).

Fig. 2. Stratigraphic correlation of the Quebrada Salta and Quebrada El Estanque localities (Palo Pintado Formation, upper Miocene). 1, clay; 2, limolite; 3, fine sandstone; 4, middle sandstone; 5, coarse sandstone; 6, fine conglomerate; 7, middle conglomerate; 8, coarse conglomerate; 9, ash.
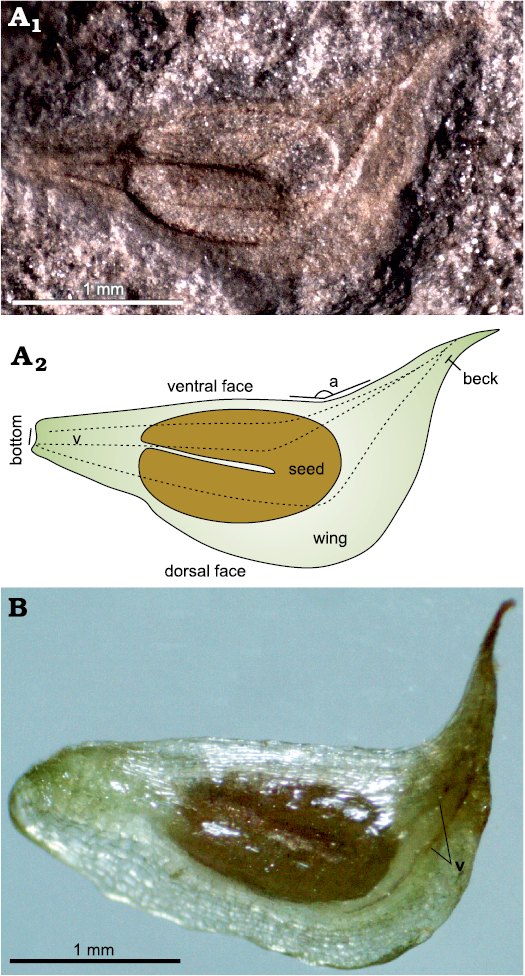
Fig. 3. Fossil and extant fruits of alismataceous plant Sagittaria montevidensis Chamisso and Schlectendal, 1827. A. CTES-PB 12911, Quebrada El Estanque (Palo Pintado Formation), upper Miocene, fossil fruit (A1), explanatory drawing (A2), dotted lines (v) represent veins present in the fossil fruit (a, angle formed by the beak and the ventral face); B. Recent fruit where the veins (v) are present, but with a slight development. The Recent material is deposited in the CTES herbarium (Corrientes City), as Sagittaria montevidensis Chamisso and Schlectendal, 1827 subsp. montevidensis.
Systematic palaeobotany
Division: Angiospermae Linnaeus, 1753
Class: Monocotyledoneae De Candolle, 1817
Order Alismatales Brown ex von Berchtold and Presl, 1820
Family Alismataceae Ventenat, 1799
Genus Sagittaria Linnaeus, 1753
Type species: Sagittaria sagittifolia Linnaeus, 1753; Recent, Eurasia.
Sagittaria montevidensis Chamisso and Schlectendal, 1827
Fig. 4A–G.
Material.—CTES-PB 12911–12917 (Fig. 4A–C, E–G) from Miocene of Quebrada Salta and Quebrada El Estanque outcrops, Palo Pintado Formation, Salta Province, Argentina. All specimens correspond to achenes.
Description.—The studied material consists solely of fruits preserved as impressions from 1.75 mm (CTES-PB 12915) to 2.58 mm (CTES-PB 12917) in length and 0.75 mm (CTES-PB 12911) to 1.29 mm (CTES-PB 12916) in width. These fossils have a well-developed wing or keel on the dorsal face. All achenes bear an ascending beak ranging from 0.87 mm (CTES-PB 12914) to 1.40 mm (CTES-PB 12913, 12916). The angle formed by the beak and the ventral face ranges between 85° (CTES-PB 12916) and 153° (CTES-PB 12911). The curved seeds are preserved inside the achenes in most specimens. These seeds are 0.87 mm (CTES-PB 12914) to 1.40 mm (CTES-PB 12913, 12916) in length and 0.56 mm (CTES-PB 12911) to 0.85 mm (CTES-PB 12917) in width. Several specimens even preserve organic remains from the seeds (CTES-PB 12913–12916). All measurements are listed in Table 1.
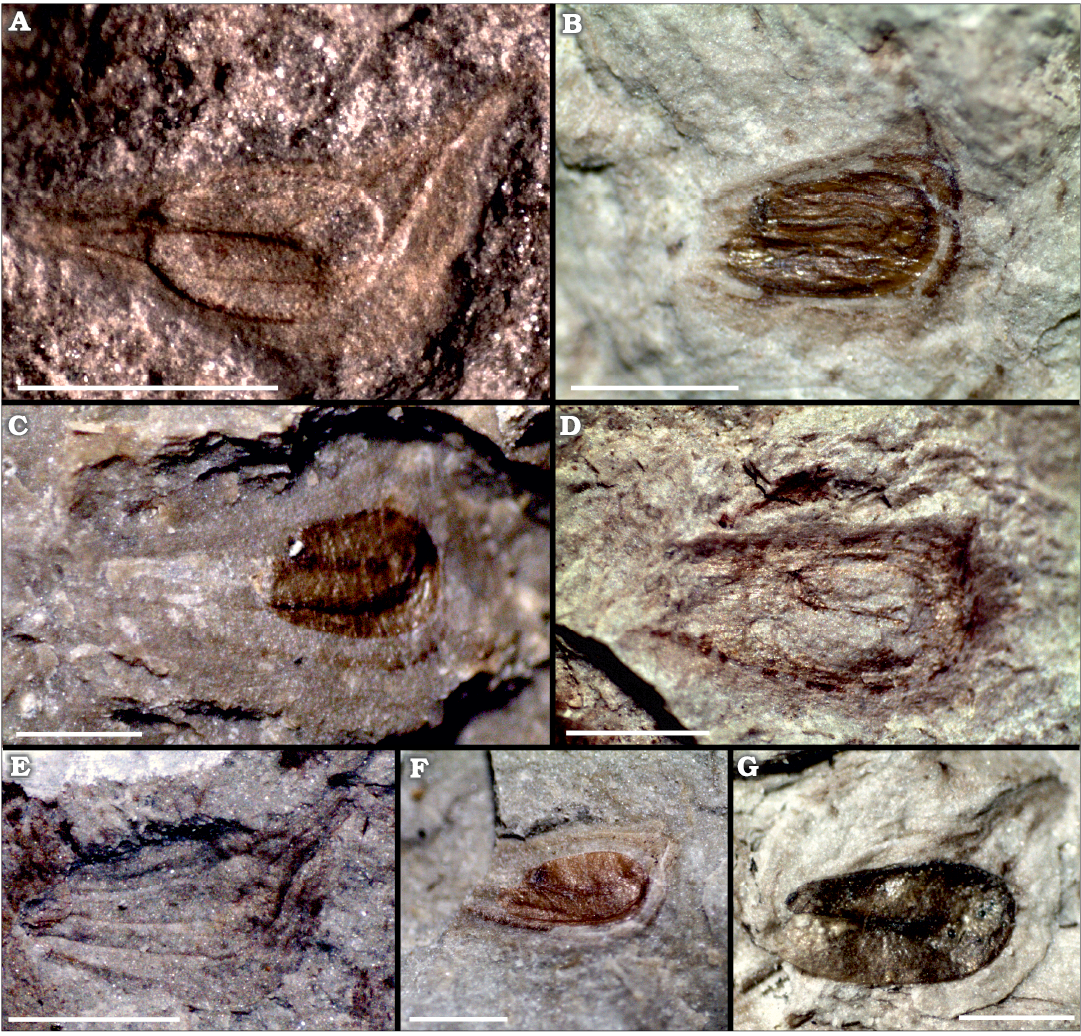
Fig. 4. Achenes of alismataceous plant Sagittaria montevidensis Chamisso and Schlectendal, 1827, from the Palo Pintado Formation, Quebrada El Estanque and Quebrada Salta localities, upper Miocene. A. Complete achene where the recurved seed and several veins are visible, CTES-PB 12911. B. Specimen showing the well-developed beak, almost in straight angle with the ventral face, CTES-PB 12916.C. Complete achene with organic remains, CTES-PB 12914. D. Fragment of achene where the beak and bottom are lacking, CTES-PB 12917. E. Complete fruit showing several veins, CTES-PB 12912. F. Incomplete achene where the bottom and beak are lacking, but the seed preserves some organic remains, CTES-PB 12913. G. Specimen showing the well-preserved seed inside the achene, CTES-PB 12915. Scale bars 1mm.
Table 1. Characters and measurements (in mm) of all specimens analyzed in this work. “–”, the structure was not observable; (f), the structure was fragmented.
|
CTES-PB |
Length |
Width |
Angle |
Beak |
Seed-length |
Seed-width |
|
12911 |
2.12 |
0.75 |
152.8° |
0.64 |
0.95 |
0.56 |
|
12912 |
2.00 |
1.01 |
135.8° |
0.37 |
– |
– |
|
12913 |
1.86 (f) |
1.07 |
123.1° |
0.48 |
1.40 |
0.76 |
|
12914 |
1.84 (f) |
0.97 |
132.5° |
0.25 (f) |
0.87 |
0.59 |
|
12915 |
1.75 |
0.96 |
140.0° |
0.36 |
1.11 |
0.57 |
|
12916 |
1.97 |
1.29 |
84.8° |
0.66 |
1.40 |
0.80 |
|
12917 |
2.58 |
1.20 |
142.1° |
0.21 |
1.11 |
0.85 |
Remarks.—According to Keener (2005), the molecular data suggest Sagittaria montevidensis is most closely related to S. intermedia Micheli, 1881, and S. calycina (Engelmann, 1867) Bogin, 1955. Based on morphological similarities, Keener (2005) also suggests Sagittaria sprucei Micheli, 1881, as a close species. Fruits of these species are different from those of S. montevidensis. S. calycina (Fig. 5A) has achenes with broadly developed dorsal wings, the beak turned into a spine and the seed is slightly longer than S. montevidensis (Fig. 5N). The achenes in S. intermedia (Fig. 5G) have faces tuberculate and the beak is shorter (0.2 mm), while S. montevidensis has achenes tuberculate and the beak can reach 1 mm in length. The fruits of S. sprucei (Fig. 5U) can be two times longer (6.0 mm) than S. montevidensis (3.0 mm), and almost three times wider (4.0 mm in S. sprucei and 1.5 mm in S. montevidensis). Seeds in S. sprucei are slightly smaller (0.9 mm), the beak is two times shorter (0.3–0.5 mm) and the angle formed by the beak and the ventral face is almost horizontal (172°), while the seeds in S. montevidensis reach 2 mm, the beak up to 1 mm and the angle is 142°.
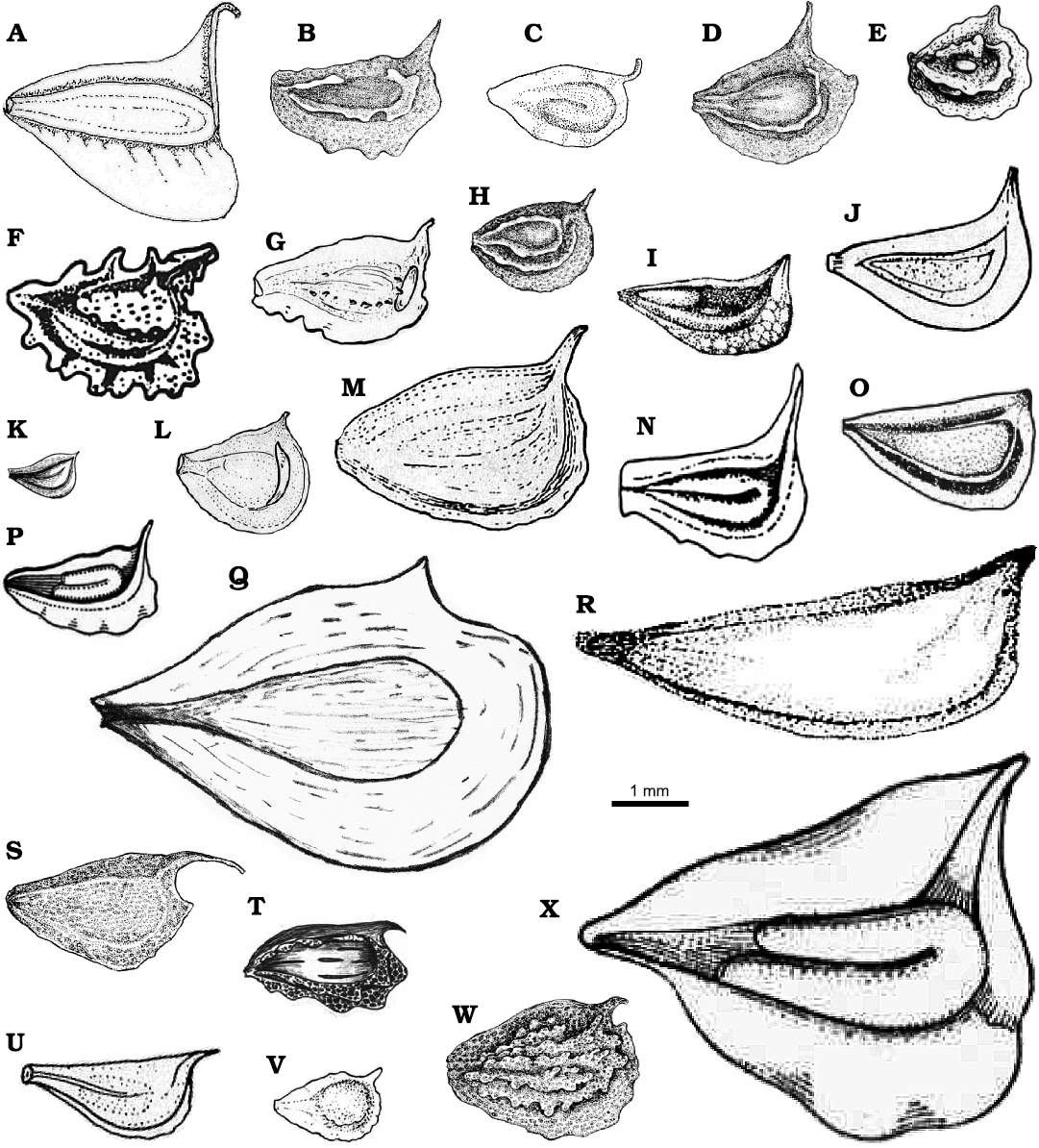
Fig. 5. Illustrations of species detailed in Table 2. A. Sagittaria montevidensis ssp. calycina (Ricketson 2018) (S. calycina following Keener 2005). B. Sagittaria cristata (Wooten 1973). C. Sagittaria demersa (Smith 1895). D. Sagittaria fasciculata (Wooten 1973). E. Sagittaria graminea ssp. graminea (Haynes and Holm-Nielsen 1994). F. Sagittaria guayanensis (Rataj 1970). G. Sagittaria intermedia (Haynes and Holm-Nielsen 1994). H. Sagittaria isoetiformis (Wooten 1973). I. Sagittaria lancifolia (Matias and Irgang 2006). J. Sagittaria latifolia (Haynes and Holm-Nielsen 1994). K. Sagittaria lichuanensis (Wang et al. 2010). L. Sagittaria longiloba (Haynes and Holm-Nielsen 1994). M. Sagittaria macrophylla (Haynes and Holm-Nielsen 1994). N. Sagittaria montevidensis ssp. montevidensis (Rataj 1978). O. Sagittaria planitiana (Matias and Irgang 2006). P. Sagittaria platyphylla (Den Hartog 1957). Q. Sagittaria potamogetonifolia (Wang et al. 2010). R. Sagittaria rhombifolia (Matias and Irgang 2006). S. Sagittaria rigida (Wooten 1973). T. Sagittaria secundifolia (Kral 1982). U. Sagittaria sprucei (Haynes and Holm-Nielsen 1994). V. Sagittaria subulate (Haynes and Holm-Nielsen 1994). W. Sagittaria teres (Wooten 1973). X. Sagittaria sagittifolia ssp. S. leucopetala (Den Hartog 1957) (S. trifolia following Lim 2015).
Five plesiomorphic species of Sagittaria are currently distributed in South America, S. guayanensis Kunth, 1816, S. intermedia, S. montevidensis, S. planitiana Agostini, 1970, and S. rhombifolia Chamisso, 1835 (Keener 2005; Chen et al. 2012). Three of them are reported from Argentina (S. guayanensis, S. montevidensis, and S. rhombifolia) (Rataj 1970). S. guayanensis (Fig. 5F) achenes have similar to those in S. montevidensis, but the first presents ribs bearing spiny ridges, both at the dorsal as the ventral faces, while the achenes of S. montevidensis have a smooth surface. In turn, S. rhombifolia (Fig. 5R) has ovobate achenes without ornaments (smooth surface), but these are from two to four times longer (5–8 mm in length) than achenes of S. montevidensis. Other species of Sagittaria also have some differences, like an absent or slightly developed beak, as observed in S. longiloba Engelmann, 1895 (Fig. 5L) and S. planitiana (Fig. 5O). Several species are considerably bigger than S. montevidensis, such as S. lancifolia Linnaeus, 1758 (Fig. 5I), S. latifolia Willdenow, 1805 (Fig. 5J), and S. trifolia Linnaeus, 1753 (Fig. 5X). Furthermore, S. lancifolia has fruits bearing conspicuous glands. S. secundifolia Kral, 1982 (Fig. 5T) presents robust and ornamented ribs. In addition, the beak in this species turned into a spine. More differences regarding measurements, development of beak and ornaments between S. montevidensis and other species of Sagittaria are listed in Table 2 and Fig. 5. Wang et al. (2010) reported another three species of Sagittaria, these are S. natans, S. pymaea, and S. tengtsungensis. The first is a synonym of S. subulata (Haynes and Holm-Nielsen 1994) (Fig. 5V), the remaining two were not illustrated in Fig. 5 because pictures or schemas of these species were not found.
Table 2. Comparative chart (measurements in mm) of current Sagittaria fruits. Data obtained from: Smith (1895); Den Hartog (1957); Godfrey and Adams (1964); Rataj (1970, 1978); Wooten (1973); Kral (1982); Haynes and Holm-Nielsen (1994); Zepeda and Lot (1999); Matias and Irgang (2006); Moreira and Bove (2010); Wang et al. (2010); Matias and Sousa (2011); Lim (2015); Hall and Gil (2016); Canalli and Bove (2017); Ricketson (2018). *, authors differ in this character; –, the dimension could not to be established.
|
Species |
Length |
Width |
Angle |
Seed length |
Seed width |
Beak |
Presence/development of keels and/or ornaments |
Illustration on Fig. 5 |
|
S. calycina |
2.6 |
1.9 |
118 |
2.4 |
0.7 |
0.9 |
dorsal wing broadly developed |
A |
|
S. cristata |
2.1 |
1.2 |
120 |
1.4 |
0.5 |
0.6 |
crenate wings |
B |
|
S. demersa |
1.5–2.0 |
1.0 |
135–178 |
1.0 |
0.6 |
1.1 |
without keel |
C |
|
S. fasciculata |
2.1 |
1.3 |
116 |
1.0 |
0.6 |
0.6 |
dorsal wing broadly developed |
D |
|
S. graminea ssp. graminea |
1.5–2.8 |
1.1–1.5 |
115 |
1.1 |
0.5 |
0.2 |
without keel; 1–2 conspicuous glands |
E |
|
S. guayanensis |
1.5–2.7 |
1.2–2.0 |
155 |
1.7 |
1.0 |
0.2–0.5 |
keeled; margins echinate |
F |
|
S. intermedia |
1.5–2.2 |
1.0–1.4 |
111 |
1.1 |
0.6 |
0.2 |
without keel, face tuberculate |
G |
|
S. isoetiformis |
1.5 |
1.1 |
120 |
0.9 |
0.5 |
0.3 |
keeled; horizontal beak |
H |
|
S. lancifolia |
1.2–2.5 |
0.7–1.1 |
139–145 |
0.6–1.6 |
0.1–0.5 |
0.3–0.7 |
keeled |
I |
|
S. latifolia |
2.5–3.5 |
1.9–2.0 |
108–131 |
2.0 |
0.9 |
1.0–2.0 |
without keel |
J |
|
S. lichuanensis |
0.8–1.0 |
0.4–0.6 |
130 |
0.6 |
0.3 |
0.1 |
narrowly winged |
K |
|
S. longiloba |
1.2–2.5 |
0.8–1.6 |
148 |
1.0 |
0.7 |
0.1–0.6 |
keeled |
L |
|
S. macrophylla |
3.1–3.5 |
1.8–2.4 |
122–132 |
1.8–1.9 |
0.9 |
0.6–1.2 |
without keel |
M |
|
S. montevidensis |
2.0–3.0 |
1.0–1.5 |
142 |
1.1–2.0 |
0.69 |
0.3–1.0 |
without keel; smooth surface |
N |
|
S. planitiana |
1.5–2.4 |
1.0–1.44 |
142–144 |
1.5 |
0.6 |
0.1–0.3 |
keeled* |
O |
|
S. platyphylla |
1.2–2.2 |
0.8–1.3 |
118–158 |
0.8–1.4 |
0.4–0.7 |
0.3–0.6 |
without keel; 2-ribbed |
P |
|
S. potamogetonifolia |
5.0–7.0 |
4.5–6.0 |
105 |
3.0 |
1.5 |
0.5 |
dorsal wing undulated |
Q |
|
S. rhombifolia |
4.0–7.0 |
2.0–3.0 |
130–135 |
4.9 |
1.5 |
0.7–1.2 |
keeled |
R |
|
S. rigida |
2.4 |
1.5 |
165 |
1.6 |
0.7 |
1.0 |
horizontal beak |
S |
|
S. secundifolia |
2.1 |
1.1 |
172 |
1.5 |
0.6 |
0.3 |
beak turned in a spur; margins crested |
T |
|
S. sprucei |
2.1–6.0 |
0.9–4.0 |
172 |
0.9 |
0.4 |
0.3–0.5 |
without keel |
U |
|
S. subulata |
1.4–2.0 |
0.9–1.5 |
143 |
0.8 |
0.6 |
0.2–0.4 |
keeled, crenate wings |
V |
|
S. teres |
2.4 |
1.7 |
173 |
– |
– |
0.4 |
keeled; horizontal beak; irregularly crenate dorsal wing |
W |
|
S. trifolia |
3.0–5.0 |
1.5–3.0 |
150 |
1.5 |
0.6 |
0.5 |
dorsal wing broadly developed |
X |
Some species of Echinodorus (Alismataceae) have similar achenes to those in Sagittaria, but their fruits are mainly terete (cylindrical), rather than flat. The achenes of Echinodorus berteroi (Spreng, 1825) Fassett, 1955 and E. paniculatus Micheli, 1881, are similar in shape (Lehtonen 2009), although these are almost straight from the base to the beak, whereas in Sagittaria montevidensis, the beak and the ventral face form an angle that can even reach 90°. Moreover, the achenes of E. berteroi and E. paniculatus are proportionally bigger than the achenes of Sagittaria montevidensis, also they present well-developed ribs that in some cases are dichotomized (E. paniculatus), while S. montevidensis is not ribbed.
Stratigraphic and geographic range.—Miocene of Argentina and Recent from North and South America.
Discussion
The fossil record of Alismataceae.—The fossil fruits of Sagittaria montevidensis are the first Neogene report of Alismataceae from South America. In one previous work, pollen grains corresponding with Alismataceae were reported from Paso Otero locality (late Pleistocene–Holocene), in the Pampean region of Argentina (Gutiérrez et al. 2011). The specimens were dated between 9900–7700 BP, although the authors only mentioned the finding of Alismataceae pollen and do not provide descriptions or illustrations permitting the comparison with other palynological records.
After an analysis of the current distribution of its Recent species Chen (1989) suggested Late Cretaceous age for the origin of Sagittaria. Later Chen et al. (2012) interpreted late Eocene and the Miocene time of origination from DNA analysis. This suggestion is consistent with the oldest records of Sagittaria accepted by Haggard and Tiffney (1997) who rejected the age proposed by Chen (1989). Furthermore, Chen et al. (2012) suggested an African origin of Sagittaria and four potential dispersion routes during the Oligocene–Miocene: (i) the first route suggests that Sagittaria has migrated from tropical Africa to South America and later to North America; (ii) the second one proposes an initial dispersal to Europe, followed by dispersal to North America, and then to South America. The remaining routes hypothesize the arrival in (iii) Madagascar and (iv) Asia. Considering the great dispersal capacity of Sagittaria, including possible large distances by water in streams and rivers, both by asexual (tubers) as sexual reproduction (achenes) (Gordon 1996; Zhang et al. 2010), and since in the late Oligocene–late Miocene range, about 20 million years have elapsed, we consider that both routes (i) and (ii) would explain the presence of Sagittaria montevidensis in South America during the late Miocene. The first route seems more plausible, because besides of Sagittaria montevidensis, other four plesiomorphic species have a current South American distribution (S. guayanensis, S. intermedia, S. planitiana, and S. rhombifolia) (Keener 2005; Chen et al. 2012).
In addition to the South American occurrences reported herein, a large number of fossil records of Alismataceae from the Cretaceous and Cenozoic times have been reported in the Northern Hemisphere (Berry 1925; Teixeira 1948; Brown 1962; Doyle 1973; Muller 1981; Cevallos-Ferriz and Ramírez 1998; Retallack 2004). Before the year 2000, most of the authors agreed that the oldest remains of Alismataceae were the genera Alisma and Caldesia, from the Oligocene of Europe and early Miocene of the United States respectively (Daghlian 1981; Friis 1985; Haggard and Tiffney 1997). Recently, other authors proposed again that the Alismataceae have originated at the end of Mesozoic, most likely around the mid-Cretaceous (Chen et al. 2012; Smith 2013; Coiffard and Mohr 2018). Among the reports that undoubtedly correspond to Alismataceae, Riley and Stockey (2004) described the species Cardstonia tolmanii Riley and Stockey, 2004, from St. Mary River Formation (Campanian–Maastrichtian of Canada). Originally the species was included in Limnocharitaceae, but this family was later included into Alismataceae (APG III 2009; Chen et al. 2012). Furthermore, Smith (2013) suggested that Haemantophyllum Budantsev, 1983, a genus from Cretaceous of Russia (Golovneva 1997), correspond to the Alismataceae family. More recently, Coiffard and Mohr (2018) described an Alismataceae (Alismataceae gen. et sp. indet.), which corresponds to an almost complete leaf from the Upper Cretaceous of Egypt and firstly assigned to Echinodorus cf. cordifolius by Kahlert et al. (2009).
The fossil record of Sagittaria in the late Miocene context.—The Sagittaria fossils studied here were recovered from late Miocene units ranging from approximately 10–5 Ma. According to Herbert et al. (2016) between 7–5.4 Ma, both hemispheres were affected by a drought, an increase in seasonality, and a restructuring of terrestrial plant and animal communities. These events were especially observed in the subtropical areas, but they were typically attributed to regional tectonic forces (Herbert et al. 2016). During the Neogene, the uplift of the Andes Mountains occurred in discrete periods, progressing from south to north and from west to east (Amarilla et al. 2015). The region experienced a deformation and compartmentalization of the basin, which extended during the Pliocene. These processes modified the environment, from a humid foreland to an elevated and hydrologically restricted semi-arid intermontane basins (Pingel et al. 2016). The Andes Mountains became the sole barrier to atmospheric circulation in the Southern Hemisphere (Amarilla et al. 2015). However, the fossil assemblages and the sedimentological evidence from the Palo Pintado Formation are not consistent with dry environments. During the deposition of this formation, a warm climate with seasonal rainfall is observed without significant climatic changes. Bywater-Reyes et al. (2010) suggested relatively more humid conditions between 10 to 6 Ma, although with seasonal conditions (Galli et al. 2011; Anzótegui et al. 2019). Changes to more arid environments started about 5 Ma until reaching the current conditions to 1 Ma ago (Pingel et al. 2016). Other Neogene locations close to the studied sites, such as the Quebrada del Toro locality (Miocene–Pliocene; Salta Province), also reflect similar conditions, with the presence of plant remains associated with marshy environments (Robledo et al. 2020a). This evidence suggests that the changes to a drier environment in the Palo Pintado Formation would have been gradual.
The achenes of Sagittaria montevidensis were found in different sections of two outcrops of the Palo Pintado Formation (see Fig. 2). The Quebrada El Estanque locality yielded a smaller number of Sagittaria fruits but they were the most complete fossils. In turn, the Quebrada Salta locality yielded a greater number of Sagittaria montevidensis impressions, including both complete and incomplete fruits. In both localities, these fossils are related to the marsh and lacustrine environments and they would have developed under a warm and humid climate with dry seasonality suggested for the Palo Pintado Formation (Galli et al. 2011). These conditions are some of the characteristic environments where Sagittaria currently develops. The arrowheads like Sagittaria montevidensis are rooted aquatic plants that grow in freshwater and slow current wetlands (i.e., swamps and marshes), wet or flooded soils, alongside streams or in drainage channels (Costa and Forni-Martins 2003; Haynes and Les 2004; Lehtonen 2009, 2018). Their presence in the outcrops studied would suggest these types of environments during the Neogene in the study area. Additionally, Demetrio et al. (2014) suggested that this species could morphologically modify its corm as an adaptive response to environmental stress, as flood periods or dry seasons, both events proposed for Palo Pintado Formation. Sagittaria montevidensis coexisted with other aquatic or marsh plants (for example, Cabomba, Salvinia, Azolla, Mayaca, and Equisetum), previously recorded from sediments of the Palo Pintado Fm., attesting to the great diversity of aquatic plants preserved in these wetland deposits (Anzótegui et al. 2017, 2019).
Conclusions
The Miocene Alismataceae record introduced here is the first Neogene fossil belonging to this family in South America and the most well substantiated fossil record of Sagittaria found of the continent.
The finding of Sagittaria in these Neogene sediments supports the possibility of a migratory route from Africa to South America and thence to North America.
This record constitutes another evidence that the environment during the deposition of the Palo Pintado Formation was warm and humid, in the interval of 10–5 Ma. During the late Miocene these Sagittaria would have inhabited a fluvial paleoenvironment in a warm climate with seasonal rainfall and without significant climatic changes, very different from the current dry conditions of the place, where it is now part of the South American Arid Diagonal.
Acknowledgements
We would like to thank the reviewers Steven Manchester (Florida Museum of Natural History, Gainesville, USA) and Adam T. Halamski (Institute of Paleobiology, Polish Academy of Sciences, Warszawa, Poland) for insightful comments and suggestions. Samuli Lehtonen (Biodiversity Unit, University of Turku, Finland) for helping in the interpretation of the fossil structures. We thank Steven Manchester also for providing bibliography. This research received support from project PI 16F008 (Secretaría General de Ciencia y Técnica de la Universidad Nacional del Nordeste) to Lilia R. Mautino and PICT 2017-1010 to Claudia Galli.
References
Adair, R.J. Keener, B.R., Kwong, R.M., Sagliocco, J.L., and Flower, G.E. 2012. The Biology of Australian weeds. Sagittaria platyphylla (Engelmann) J.G. Smith and Sagittaria calycina Engelmann. Plant Protection Quarterly 27: 47–58.
Agostini, G. 1970. Notes on Alismataceae. Phytologia 20: 1–5. Crossref
Amarilla, L.D., Anton, A.M., Chiapella, J.O., Manifesto, M.M., Angulo, D.F., and Sosa, V. 2015. Munroa argentina, a grass of the South American Transition Zone, survived the Andean uplift, aridification and glaciations of the Quaternary. PLoS ONE 10 (6): e0128559. Crossref
Anzótegui, L.M. and Horn, M.Y. 2011. Megaflora de la Formación Palo Pintado (Mioceno Superior) Salta, Argentina. Parte II. Revista Brasileira de Paleontologia 14: 239–254. Crossref
Anzótegui, L.M., Horn, M.Y., Robledo, J.M., and Galli, C.I. 2015. Novedades paleoflorísticas en la Localidad Quebrada Salta, Formación Palo Pintado (Mioceno Tardío/Plioceno), Salta, Argentina. In: J. Bodnar and G. Márquez (eds.), XVI Simposio Argentino de Paleobotánica y Palinología (Abstract Book), 40. Facultad de Ciencias Naturales y Museo (UNLP), La Plata.
Anzótegui, L.M., Mautino, L.R., Garralla, S.S., Herbst, R., Robledo, J.M., and Horn, M.Y. 2017. Paleovegetación cenozoica del Noroeste Argentino. In: C. Muruaga and P. Grosse (eds.), Ciencias de la Tierra y Recursos Naturales del NOA, Relatorio del Congreso Geológico Argentino, No. 20, 767–781. Asociación Geológica Argentina, Tucumán.
Anzótegui, L.M., Mautino, L.R., Horn, M.Y., Garralla, S.S., and Robledo, J.M., 2019. Paleovegetación del Mioceno tardío del Noroeste de Argentina. Opera Lilloana 52: 109–130.
APG III. 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society 161: 105–121. Crossreff
APG IV. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society 181: 1–20. Crossref
Barreda, V., Anzótegui, L.M., Prieto, A.R., Aceñolaza, P., Bianchi, M.M., Borromei, A.M., Brea, M., Caccavari, M., Cuadrado, G.A., Garralla, S.S., Grill, S.G., Guerstein, R., Lutz, A.I., Mancini, M.V., Mautino, L.R., Ottone, E.G., Quattrocchio, M., Romero, E.J., Zamaloa, M.C., and Zucol, A. 2007. Diversificación y cambios de las Angiospermas durante el Neógeno en Argentina. Ameghiniana 11: 173–191.
Bell, C.D., Soltis, D.E., and Soltis, P.S. 2010. The age and diversification of the angiosperms re-revisited. American Journal of Botany 97: 1296–1303. Crossref
Berchtold, F.G. von and Presl, J.S. 1820. O Přirozenosti Rostlin. 502 pp. K.W. Endersa, Prague.
Berry, E.W. 1925. The flora of the Ripley Formation. US Geological Survey Professional Paper 136: 1–94. Crossref
Bogin, C. 1955. Revision of the genus Sagittaria (Alismataceae). Memoirs of the New York Botantical Garden 9: 179–233.
Bona, P., Starck, D., Galli, C.I., Gasparini, Z., and Reguero, M. 2014. Caiman cf. latirostris (Alligatoridae, Caimaninae) in the late Miocene Palo Pintado Formation, Salta province, Argentina: Paleogeographic and Paleoenvironmental Considerations. Ameghiniana 51: 26–36. Crossref
Bona, P. and Barrios, F. 2015. The alligatoroidea of Argentina: An update of its fossil record. Publicación Electrónica de la Asociación Paleontológica Argentina 15: 143–158. Crossref
Brown, R.W. 1962. Paleocene flora of the Rocky Mountains and Great Plains. US Geological Survey Professional Paper 275: 1–119. Crossref
Budantsev, L.J. [Budancev, L.J.] 1983. Istoriâ arktičeskoj flory epoki rannevo kainofita. Nauka, Moskva.
Byng, J.W., Chase, M., Christenhusz, M., Fay, M.F., Judd, W.F., Mabberley, D., Sennikov, A., Soltis, D.E., Soltis, P.S., and Stevens, P. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. Crossref
Bywater-Reyes, S., Carrapa, B., Clementz, M., Clementz, M., and Schoenbohm, L. 2010. Effect of late Cenozoic aridification on sedimentation in the Eastern Cordillera of north‐west Argentina (Angastaco basin). Geology 38: 235–238. Crossref
Canalli, Y.M. and Bove, C.P. 2017. Flora of Rio de Janeiro: Alismataceae. Rodriguésia 68: 17–28. Crossref
Cerling, T.E., Harris, J.M., MacFadden, B.J., Leakey, M.G., Quade, J., Eisenmann, V., and Ehleringer, J.R. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389: 153–158. Crossref
Cevallos-Ferriz, S. and Ramírez, J.L. 1998. Las plantas con flores en el registro fósil. Ciencias 52: 46–57.
Chamisso, L.K.A. von 1835. Spicilegium Alismacearum. Linnaea 10: 219–220.
Chamisso, L.K.A. von and Schlechtendal, D.F.L. von. 1827. De plantis in expeditione speculatoria Romanzoffiana observatis. Linnaea 2: 145–233.
Chandler, M.E.J. 1963. The Lower Tertiary Floras of Southern England. III. Flora of the Bournemouth Beds, the Boscombe and the Highcliff Sands. 169 pp. British Museum (Natural History), London.
Chen, J.K., 1989. Systematic and Evolutionary Botanical Studies on Chinese Sagittaria. 141 pp. National Natural Science Foundation of China, Wuhan University Press, Wuhan.
Chen, L.-Y., Chen, J.-M., Gituru, R.W., Temam, T.D., and Wang, Q.-F. 2012. Generic phylogeny and historical biogeography of Alismataceae, inferred from multiple DNA sequences. Molecular Phylogenetics and Evolution 63: 407–416. Crossref
Coiffard, C. and Mohr, B.A.R. 2018. Cretaceous tropical Alismatales in Africa: diversity, climate and evolution. Botanical Journal of the Linnean Society 188: 117–131. Crossref
Coutand, I., Carrapa, B., Deeken, A., Schmitt, A.K., Sobel, E., and Strecker, M. 2006. Orogenic plateau formation and lateral growth of compressional basins and ranges: Insights from sandstone petrography and detrital apatite fission‐track thermochronology in the Angastaco Basin, NW Argentina. Basin Research 18: 1–26. Crossref
Costa, J.I. and Forni-Martins, E.R. 2003. Karyology of some Brazilian species of Alismataceae. Botanical Journal of the Linnean Society 143: 159–164. Crossref
Cronquist, A. 1981. An Integrated System of Classification of Flowering Plants. 1262 pp. Columbia University Press, New York
Daghlian, C.P. 1981. A review of the fossil record of monocotyledons. The Botanical Review 47: 517–555. Crossref
Dahlgren, R.M.T. 1980. A revised system of classification of the angiosperms. Botanical Journal of the Linnean Society 80: 91–124. Crossref
Daudin, F.M. 1802. Histoire Naturelle, Générale et Particulière des Reptiles, Vol. 2. 452 pp. F. Dufart, Paris.
De Candolle, A.P. 1817. Regni vegetabilis systema naturale, sive Ordines, Genera et Species Plantarum Secundum Methodi Naturalis Normas Digestarum et Descriptarum. 564 pp. Treuttel et Würtz, Paris. Crossref
Demetrio, G.R., Barbosa, M.E.A., and Coelho, F.F. 2014. Water level-dependent morphological plasticity in Sagittaria montevidensis Cham. and Schl. (Alismataceae). Brazilian Journal of Biology 74: 199–206. Crossref
Den Hartog, C. 1957. Alismataceae. In: C.G.G.J. Van Steenis (ed.), Flora Malesiana ser. 1, Vol. 5, 317–334. Jakarta, Indonesia.
Díaz, J.I. and Malizzia, D.C. 1983. Estudio geológico y sedimentológico del Terciario Superior del valle Calchaquí (departamento de San Carlos, provincia de Salta). Boletín Sedimentológico 2: 8–28.
Doyle, J.A. 1973. Fossil evidence on early evolution of the monocotyledons. Quarterly Review of Biology 48: 399–413. Crossref
Engelmann, G. 1895. Sagittaria longiloba. In: J.G Smith (ed.), A Revision of the North American Species of Sagittaria and Lophotocarpus, Vol. 16, 16. Missouri Botanical Garden Press, Saint Louis. Crossref
Fassett, N.C. 1955. Echinodorus in the American tropics. Rhodora 57: 133–156. Crossref
Friis, E.M. 1985. Angiosperm fruits and seeds from the middle Miocene of Jutland (Denmark), Alismataceae. Danske Videnskabernes Selskab, Copenhagen. Biologiske Skrifter 24: 71–73.
Galli, C.I. and Reynolds, J. 2012. Evolución paleoambiental del Grupo Payogastilla (Eoceno–Plioceno) en el valle Calchaquí-Tonco, provincia de Salta, Argentina. In: R. Marquillas, C. Sanchez, and J. Salfity (eds.), Aportes Sedimentológicos a la Geología del Noroeste Argentino. XIII Reunión Argentina de Sedimentología, 67–80, Salta.
Galli, C.I., Alonso, R.N., and Coira, L.B. 2017. Integrated Stratigraphy of the Cenozoic Andean Foreland Basin (Northern Argentina), In: G. Aiello (ed.), Seismic and Sequence Stratigraphy and Integrated Stratigraphy—New Insights and Contributions, 129–156. IntechOpen, Budapest. Crossref
Galli, C.I., Anzótegui, L.M., Horn, M.Y., and Morton, L.S. 2011. Paleoambiente y paleocomunidades de la Formación Palo Pintado (Mioceno‐Plioceno, Provincia de Salta, Argentina. Revista Mexicana de Ciencias Geológicas 28: 161–174.
Galli, C.I., Coira, L.B., Alonso, R.N., Matteini, M., and Hauser, N. 2014. Evolución tecto-sedimentaria del Grupo Payogastilla y su relación con el arco volcánico del Cenozoico, en los valles Calchaquí, Tonco y Amblayo, provincia de Salta, Argentina. Acta Geológica Lilloana 26: 30–52. Crossref
Galli, C.I., Ramirez, A., Barrientos, C., Reynolds, J., Viramonte, J.G., and Idleman, B. 2008. Estudio de proveniencia de los depósitos del Grupo Payogastilla (Mioceno Medio‐Superior) aflorantes en el río Calchaquí, provincia de Salta, Argentina. 17° Congreso Geológico Argentino, Jujuy 1: 353–354.
Godfrey, R.K. and Adams, P. 1964. The identity of Sagittaria isoetiformis. SIDA, Contributions to Botany 1: 269–273.
Golovneva, L.B. 1997. Morphology, systematics and distribution of the genus Haemanthophyllum in the Paleogene floras of the Northern Hemisphere. Paleontological Journal 31: 197–207.
Gordon, E. 1996. Tipo de dispersión, germinación y crecimiento de plántulas de Sagittaria latifolia (Alismataceae). Fragmenta Floristica et Geobotanica 41: 657–668.
Gutiérrez, M.A., Martínez, G.A., Luchsinger, H., Grill, S., Zucol, A.F., Hassan, G.S., Barros, M.P., Kaufmann, C.A., and Álvarez, M.C. 2011. Paleoenvironments in the Paso Otero locality during late Pleistocene–Holocene (Pampean region, Argentina): an interdisciplinary approach. Quaternary International 245: 37–47. Crossref
Haggard, K.K. and Tiffney, B. H. 1997. The flora of the early Miocene Brandon lignite, Vermont, USA. VIII. Caldesia (Alismataceae). American Journal of Botany 84: 239–252. Crossref
Hall, C.F. and Gil, A.S.B. 2016. Flora das cangas da Serra dos Carajás, Pará, Brasil: Alismataceae. Rodriguésia 67: 1195–1199. Crossref
Haynes, R.R. and Holm-Nielsen, L.B. 1994. The Alismataceae. Flora Neotropica 64: 1–112.
Haynes, R.R. and Les, D.H. 2004. Alismatales (water plantains). In: Encyclopedia of Life Sciences, 1–4. John Wiley & Sons Ltd, Chichester. Crossref
Herbst, R., Anzótegui, L.M., and Jalfin, G. 1987. Estratigrafía, paleoambientes y dos especies de Salvinia Adanson (Filicopsida) del Mioceno superior de Salta, Argentina. Revista de la Facultad de Ciencias Exactas y Naturales y Agrimensura 7: 15–42.
Herbert, T.D., Lawrence, K.T., Tzanova, A., Peterson, L.C., Caballero-Gill, R., and Kelly, C.S. 2016. Late Miocene global cooling and the rise of modern ecosystems. Nature Geoscience 9: 843–847. Crossref
Herbst, R., Anzótegui, L.M., Esteban, G., Mautino, L.R., Morton, S., and Nassif, N. 2000. Síntesis paleontológica del Mioceno de los valles Calchaquíes, noroeste argentino. In: F. Aceñolaza and R. Herbst (eds.), El Neógeno de Argentina, 263–288. Instituto Superior de Correlación Geológica, Serie Correlación Geológica N° 14, Consejo Nacional de Investigaciones Científicas y Técnicas Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, Argentina.
Hongn, F., and Seggiaro, R. 2001. Hoja Geológica 2566-III, Cachi (Escala 1:250 000). Instituto de Geología y Recursos Minerales, Servicio Geológico Minero Argentino, Boletín 248: 1–87.
Horn, M.Y., Galli, C.I., Mautino, L.R., and Anzótegui, L.M. 2011. Palinología y litofacies de la Formación Palo Pintado (Mioceno Superior) en las localidades Río Calchaquí y Quebrada El Estanque, Salta, Argentina. Ameghiniana (Abstract book) 48: R15.
Kahlert, E., Rüffle, L., and Gregor D.H.J. 2009. Die Oberkreide Flora (Campanian) von Baris (Ägypten) und ihre ökologische geographischen Beziehungen unter plattentektonischen Aspekten. Documenta Naturae 178: 1–71.
Keener, B.R. 2005. Molecular Systematics and Revision of the Aquatic Monocot Genus Sagittaria (Alismataceae). 168 pp. Unpublished Ph.D. Thesis. The University of Alabama, Tuscaloosa.
Kral, R. 1982. A new phyllodial-leaved Sagittaria (Alismaceae) from Alabama. Brittonia 34: 12–17. Crossref
Kunth, C.S. 1816. Alismataceae. In: A. von Humboldt, A. Bonpland, and C.S. Kunth (eds.), Nova genera et species plantarum, Vol. 4, 250. Chez N. Maze Libraire, Paris.
Lehtonen, S. 2009. Systematics of the Alismataceae—a morphological evaluation. Aquatic Botany 91: 279–290. Crossref
Lehtonen, S. 2018. Alismataceae. In: L. Ramella (ed.), Flora del Paraguay 49, 1–48. Conservatoire et Jardin botaniques de la Ville de Genève, Geneva.
Lim, T.K. 2015. Sagittaria trifolia. In: T.K. Lim (ed.), Edible Medicinal and Non Medicinal Plants, 96–102. Springer, Dordrecht. Crossref
Linnaeus, C. 1753. Species plantarum, exhibentes plantas rite cognitas ad genera relatas cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. Tomus II. 783 pp. Laurentii Salvius, Stokholm. Crossref
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus II. Editio decima, reformata. 824 pp. Holmiæ, Stockholm. Crossref
Matias, L.Q. and Irgang, B.E. 2006. Taxonomy and distribution of Sagittaria (Alismataceae) in north-eastern Brazil. Aquatic Botany 84: 183–190. Crossref
Matias, L.Q. and Sousa, D.J.L. 2011. Alismataceae no estado do Ceará, Brasil. Rodriguésia 62: 887–900. Crossref
Micheli, M. 1881. Alismacaea. In: A.C.P De Candolle and A.P. De Candolle (eds.), Monographiae phanerogamarum, Vol. 3, 29–83. S.G. Masson, Paris.
Moreira, A.D.R. and Bove, C.P. 2010. Flórula do Parque Nacional da Restinga de Jurubatiba, Rio de Janeiro, Brasil: Alismataceae. Arquivos do Museu Nacional 68: 163–165.
Muller, J. 1981. Fossil pollen records of extant angiosperms. The Botanical Review 47: 1–142. Crossref
Osborne, C.P. 2008. Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands? Journal of Ecology 96: 35–45. Crossref
Pingel, H., Mulch, A., Alonso, R.N., Cottle, J., Scott, A., Jacob, P., Rohrmann, A., Schmitt, K., Stockli, D., and Strecker, M.R. 2016. Surface uplift and convective rainfall along the southern Central Andes (Angastaco Basin, NW Argentina). Earth and Planetary Science Letters 440: 33–42. Crossref
Quade, J., Cerling, T.E., and Bowman, J.R. 1989. Development of the Asian monsoon revealed by marked ecological shift during the latest Miocene in northern Pakistan. Nature 342: 163–166. Crossref
Rataj, K. 1970. Las Alismataceae de la República Argentina. Darwiniana 16: 9–39.
Rataj, K. 1972. Revision of the genus Sagittaria Part. II. Annotations Zoologicae et Botanicae 70: 1–61.
Rataj, K. 1978. Alismataceae of Brazil. Acta Amazonica 8: 5–53. Crossref
Retallack, G.J. 2004. Late Miocene climate and life on land in Oregon within a context of Neogene global change. Palaeogeography, Palaeoclimatology, Palaeoecology 214: 97–123. Crossref
Ricketson, J.M. 2018. Vascular plants of Arizona: Alismataceae. Canotia 14: 10–21.
Riley, M.G. and Stockey, R.A. 2004. Cardostonia tolmanii gen. et sp. nov. (Limnocharitaceae) from the Upper Cretaceous of Alberta, Canada. International Journal of Plant Sciences 165: 897–916. Crossref
Robledo, J.M., Anzótegui, L.M., Martínez, O.G., and Alonso, R.N. 2020a. Flora and insect trace fossils from the Mio-Pliocene Quebrada del Toro locality (Gobernador Solá, Salta, Argentina). Journal of South American Earth Sciences 100: 102544. Crossref
Robledo, J.M., Horn, M.Y., Galli, C.I., and Anzótegui, L.M. 2020b. Inferencias paleoclimáticas para el Mioceno tardío en la cuenca de Angastaco basadas en el análisis fisionómico foliar: Formación Palo Pintado, Salta, Argentina. Andean Geology 47: 418–429. Crossref
Rohrmann, A., Sachse, D., Mulch, A., Pingel, H., Tofelde, S., Alonso, R.N., and Strecker, M.R. 2016. Miocene orographic uplift forces rapid hydrological change in the southern central Andes. Scientific Reports 6: 35678. Crossref
Salfity, J. and Monaldi, C. 2006. Hoja Geológica 2566-IV, Metán, provincia de Salta. Servicio Geológico Minero Argentino, Boletín Nº 352, Buenos Aires.
Smith, J.G. 1895 A Revision of the North American Species of Sagittaria and Lophotocarpus. Missouri Botanical Garden Annual Report 1895: 27–64. Crossref
Smith, S.Y. 2013. The fossil record of noncommelinid monocots. In: P. Wilkin and S.J. Mayo (eds.), Early Events in Monocot Evolution, 29–59. Cambridge University Press, Cambridge. Crossref
Soltis, D.E., Soltis, P.S., Endress, P.K., and Chase, M.W. 2005. Phylogeny and Evolution of Angiosperms. 370 pp. Sinauer Associates, Sunderland.
Starck, D. and Anzótegui L.M. 2001. The late Miocene climatic change persistence of a climatic signal through the orogenic stratigraphic record in north‐western of Argentina. Journal of South American Earth Sciences 14: 763–774. Crossref
Teixeira, C. 1948. Flora Mesozoica Portuguesa. Parte 1. 118 pp. Direção Geral da Minas e Serviços Geológicos de Portugal, Lisboa.
Ventenat, E.P. 1799. Tableau du Règne Végétal, Selon de Méthode de Jussieu, Vol. 2. 607 pp. J. Drisonnier, Paris. Crossref
Wang, Q.F., Haynes, R.R., and Hellquist, C.B. 2010. Alismataceae. In: Z.Y. Wu, P.H. Raven, and D.Y. Hong (eds.), Flora of China (Acoraceae through Cyperaceae), 84–89. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis.
Willdenow, C.L. 1805. Species Plantarum. Vol. 4 (1). 629 pp. G.C. Nauk, Berlin.
Wooten, J.W. 1973. Taxonomy of seven species of Sagittaria from eastern North America. Brittonia 25: 64–74. Crossref
Zepeda, C. and Lot, A. 1999. Acuitlacpalli or Sagittaria macrophylla (Alismataceae): A Mexican endemic hydrophyte and a threatened food resource. Economic Botany 53: 221–223.
Zepeda, C. and Lot, A. 2005. Distribución y uso tradicional de Sagittaria macrophylla Zucc. y S. latifolia Willd. en el Estado de México. CIENCIA ergo-sum, Revista Científica Multidisciplinaria de Prospectiva 12: 282–290.
Zhang, Y.W., Huang, S.J., Zhao X.N., Liu F., and Zhao, J.M. 2010. New record of an invasive species, Sagittaria graminea, in Yalu river estuary wetland. Journal of Wuhan Botanical Research 28: 631–633.
Acta Palaeontol. Pol. 66 (Supplement to 3): 111–122, 2021
https://doi.org/10.4202/app.00835.2020