


First aphidiine wasp from the Sakhalinian amber
ELENA M. DAVIDIAN, MARYNA O. KALIUZHNA, and EVGENY E. PERKOVSKY
Davidian E.M., Kaliuzhna M.O., and Perkovsky E.E. 2021. First aphidiine wasp from the Sakhalinian amber. Acta Palaeontologica Polonica 66 (Supplement to 3): 59–65.
The first ichneumonoid aphidiine wasp species from Sakhalinian amber (middle Eocene) is described. Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. іs the oldest named aphidiine female, the first fossil aphidiine from Asia, and the oldest named species of the Ephedrus. Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. and the two fossil species of Ephedrus, i.e., Ephedrus primordialis from Baltic amber (late Eocene) and Ephedrus mirabilis from Camoins-les-Bains (early Oligocene), presumably belong to the Ephedrus plagiator species group of the subgenus Ephedrus sensu stricto, and new species differs from them in having a longer petiole and a rather long 3M vein that does not reach the forewing margin. It additionally differs from E. primordialis by having longer ovipositor sheaths. The new species is most similar to the extant Ephedrus validus and Ephedrus carinatus, from which it differs by the less elongated F1, absence of notauli, and by ovipositor sheaths that are 3.0 times as long as wide.
Key words: Hymenoptera, Ichneumonoidea, Braconidae, Aphidiinae, Eocene, Oligocene, Baltic amber, Sakhalinian amber.
Elena M. Davidian [GDavidian@yandex.ru; ORCID: https://orcid.org/0000-0003-3804-4618], All-Russian Institute of Plant Protection (FSBSI VIZR), Podbelskogo, 3, St. Petersburg – Pushkin, 196608 Russian Federation.
Maryna O. Kaliuzhna [kaliuzhna.maryna@gmail.com; ORCID: https://orcid.org/0000-0002-9265-0195], I.I. Schmalhausen Institute of Zoology of NAS of Ukraine, B. Khmelnytskogo Str. 15, 01030 Kyiv, Ukraine.
Evgeny E. Perkovsky [perkovsk@gmail.com; ORCID: https://orcid.org/0000-0002-7959-4379], I.I. Schmalhausen Institute of Zoology of NAS of Ukraine, B. Khmelnytskogo Str. 15, 01030 Kyiv, Ukraine; A.A. Borissiak Paleontological Institute of the Russian Academy of Sciences, Profsoyuznaya Str. 123, 117997 Moscow, Russian Federation.
Received 15 October 2020, accepted 8 January 2021, available online 11 June 2021.
Copyright © 2021 E.M. Davidian et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Middle Eocene Sakhalinian amber (43–47 Ma) is the typical rumanite from the Dolinsk District (south of Sakhalin Island). It is much older than European succinites (Kodrul 1999; Perkovsky et al. 2007; Baranov et al. 2015), and its biota is poorly studied (Simutnik 2014, 2015, 2020; Fedotova and Perkovsky 2016; Radchenko and Perkovsky 2016; Marusik et al. 2018; Dietrich and Perkovsky 2019; Azar and Maksoud 2020; Batelka et al. 2020; Perkovsky et al. 2021; Tykhonenko et al. 2021). Aphids are extremely abundant in Sakhalinian amber. Only Late Cretaceous Baeomorpha Realm faunas display similar abundance of aphids (Gumovsky et al. 2018). Sakhalinian amber fauna is unique for Cenozoic amber in the rarity of ants (Kazantsev and Perkovsky 2019, and references therein), with only a single species of cantharine beetles as an aphid predator (Kazantsev and Perkovsky 2019). Thus, there is a high abundance of aphid parasitoids in Sakhalinian amber, unknown from any other amber fauna (Rasnitsyn 1980).
Aphidiinae is a small, globally distributed subfamily of specialized aphid parasitoids belonging to Braconidae, Hymenoptera (Yu et al. 2016; Chen and van Achterberg 2019), but it was once considered a separate family within Ichneumonoidea (Starý 1970; Tobias and Chiriac 1986; Davidian 2007, 2018, 2019). According to different estimates, there are 505 (Žikić et al. 2017) to 619 (Yu et al. 2016) extant species of aphidiines recognized worldwide, and the process of generic revision and new species description continues (Rakhshani et al. 2017; Čkrkić et al. 2019; Kocić et al. 2019, 2020; Tomanović et al. 2020). More than half of all species are known from the Palaearctic Region (Yu et al. 2016). Aphidiines are an essential part of the aphidophagous guild, and due to their practical importance, this group is well studied (Žikić et al. 2017; Chen and van Achterberg 2019); however, questions remain regarding the evolution and phylogeny of the group (Belshaw and Quicke 1997; Belshaw et al. 2000; Sanchis et al. 2000; Ortega-Blanco et al. 2009; Chen and van Achterberg 2019) and genera therein (Gӓrdenfors 1986; Kocić et al. 2019, 2020; Čkrkić et al. 2020). The study of fossil material may contribute to resolving these problems.
The fossil fauna of Aphidiinae includes 14 genera and 26 species (Ortega-Blanco et al. 2009). The oldest fossil genus and species, Archephedrus stolamissus Ortega-Blanco, Bennett, Delclòs, and Engel, 2009, was described from a single male specimen from the late Albian (Early Cretaceous) Álava amber of Spain (Ortega-Blanco et al. 2009), and this species was assigned to Ephedrini (Yu et al. 2016). The majority of fossil aphidiines have been described from the early Rupelian (Oligocene) Anna pit in the Alsace potash field (Quilis 1940; Starý 1973; Berger et al. 2005; Ortega-Blanco et al. 2009). Aphidiinae are also found in the late Eocene Baltic (Brues 1933; Starý 1970, 1973) and Rovno ambers (MOK and EEP unpublished data). Aphidiines are prevalent in middle Eocene Sakhalinian amber (Rasnitsyn 1980); however, detailed study is in its infancy, with short reports on Ephedrus Haliday, 1833, specimens (Kaliuzhna et al. 2019) and the differentiation of possible new species (Kaliuzhna et al. 2020).
Ephedrus contains about 50 living and extinct species altogether, most of which are known from the Palaearctic Region (Yu et al. 2016; Kocić et al. 2019; Tomanović et al. 2020). Among Ephedrini, the Ephedrus is the only genus with rich extant fauna and includes two fossil species described from Europe (Oligocene of France and Baltic amber; Yu et al. 2016). Diagnostic morphological characters of the genus are 11-segmented antennae in both sexes (an exception is E. antennalis Tomanović, 2020, described from a single female with 12-segmented antennae), complete venation of the forewing, with present 2RS and r-m veins, and also seven complete cells (marginal, 1st and 2nd submarginal, 1st discal, basal, subbasal, and 1st subdiscal). The ovipositor sheaths are more or less elongate, straight or slightly curved upward, usually with sparse setae.
According to the review by Kocić et al. (2019), the extant fauna of the Ephedrus is represented by three subgenera: Ephedrus sensu stricto, Breviephedrus Gӓrdenfors, 1986, and Fovephedrus Chen, 1986. The monotypic subgenus Lysephedrus Starý, 1958, is assigned by the same authors as a junior synonym of Ephedrus sensu stricto, according to the results of molecular analysis (Kocić et al. 2019). The fossil species Ephedrus primordialis Brues, 1933, from Baltic amber and Е. mirabilis Timon-David, 1944, from early Oligocene Camoins-les-Bains Marls near Marseille presumably belong to the subgenus Ephedrus sensu stricto as far as we can conclude from the original descriptions (Brues 1933; Timon-David 1944).
Institutional abbreviations.—FSBSI VIZR, All-Russian Institute of Plant Protection, St. Petersburg-Pushkin, Russian Federation; PIN, A.A. Borissiak Paleontological Institute of the Russian Academy of Sciences, Moscow, Russian Federation; SIZK, I.I. Schmalhausen Institute of Zoology of NAS of Ukraine, Kyiv, Ukraine.
Other abbreviations.—F1–F9, antennal flagellomeres; Pt, pterostigma; r, cross vein connecting pterostigma and radial sector; RS, radial sector; 3RSa, the first section of 3rd abscissa of radial sector; 3M, 3rd abscissa of media; 2RS, 2nd abscissa of radial sector; r-m, cross vein connecting radius and media.
Nomenclatural acts.—This published work and the nomenclatural act it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:CBF2F8F0-B33C-4A4E-A6CA-0FAA15FD3084.
Material and methods
Amber insects were collected in the southern part of Sakhalin Island, Russian Far East, by an expedition of the Paleontological Institute of Academy of Science of USSR in 1972 (Dietrich and Perkovsky 2019, and references therein).
In total, 36 amber specimens were studied under Axio Imager M1 Carl Zeiss microscope in FSBSI VIZR and under Leica Z16 APO microscope equipped with a Leica DFC 450 camera and LAS V3.8 software in SIZK. Photos were made using Axio Imager M1 Carl Zeiss microscope in FSBSI VIZR. The examined material housed in PIN.
Classification of aphids is given after Heie and Węgierek (2009). The morphological terminology used in this paper follows Sharkey and Wharton (1997) and Hymenoptera Anatomy Ontology Project, the latter is an illustrated glossary of morphological terms (Hymenoptera Anatomy Consortium available online at http://glossary.hymao.org).
Systematic palaeontology
Order Hymenoptera Linnaeus, 1758
Superfamily Ichneumonoidea Latreille, 1802
Family Braconidae Nees, 1811
Subfamily Aphidiinae Haliday, 1833
Genus Ephedrus Haliday, 1833
Type species: Bracon plagiator Nees, 1811; Sickershausen, Germany (destroyed); Hermanovce, Preśovské hory, Slovakia (neotype), extant.
Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov.
Figs. 1, 2.
2008 Aphidiinae (Braconidae); Zherikhin et al. 2008: 197, text-fig. 76.
Zoobank LSID: urn:lsid:zoobank.org:act:7C703563-7681-41BC-AA73- 824655228560
Etymology: The species named after famous paleoentomologist Alexandr Pavlovich Rasnitsyn.
Holotype: PIN 3387/79, single female imago.
Type locality: Starodubskoye, Dolinsk District, Sakhalin Province, Sakhalin Island, Russian Federation.
Type horizon: Middle Eocene.
Diagnosis.—The complete pubescence of the elongate ovipositor sheaths (Fig. 1A4) distinguishes the new species from all other species of the Ephedrus and places it closer to the extant E. validus (Haliday, 1833) and E. carinatus Tomanović, 2020. From these two species E. rasnitsyni Davidian and Kaliuzhna sp. nov. differs in following characters: first flagellomere (F1) less elongate, 2.5 times as long as wide (Fig. 1A3), notauli absent, ovipositor sheaths 3.0 times as long as wide (Fig. 1A4) and overall smaller body size. Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. differs from known fossil species E. mirabilis and E. primordialis by a longer petiole, and rather long 3M vein that, however, does not reach the forewing margin (Figs. 1A1, A2, 2). The new species additionally differs from the E. primordialis by more elongate ovipositor sheaths.
Description.—Description is based on a single female, male forms are unknown.
Head (Fig. 1A1–A3) distinctly densely pubescent. Maxillary palp with three visible palpomeres; labial palp with two palpomeres. Maxillary palpomere oval; two times as long as wide, completely pubescent, covered with short setae and with two–three long setae apically. Antenna with 11 antennomeres, short, barely reaching the apex of thorax, covered with dense setae that are slightly shorter than the width of F1; each flagellomere also with two semi-erected longer setae apically. F1–F3 parallel-sided (Fig. 1A3); other flagellomeres beginning with F4 are strongly widened towards apex, possibly flattened in amber. The apical flagellomeres tightly jointed to each other, forming a club. F1 is broken in basal third, approximately equal in length to F2, 2.5 times as long as wide in the middle; F3 and F4 2.0 times as long as wide, F5 and F6 1.5 times as long as wide; F7 and F8 almost square, i.e., the same length and width; F9 1.3 times as long as wide at base (Fig. 1A1, A2). F1 and F2 with one longitudinal placode; other flagellomeres with two placodes each.

Fig. 1. Aphidiine wasp Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov., holotype PIN 3387/79, Starodubskoye, Sakhalinian amber (Russia), middle Eocene. Specimen in lateral view (A1), explanatory morphology (A2), anterolateral part of the specimen (A3), ovipositor sheaths inlateral view (A4). Abbreviations: body: c, coxa; em+cl, empodium and claws; f, femur; F1–F9, flagellomeres 1–9; mp, maxillary palp; MS5-6, metasomal sternites 5 and 6; MsP, mesopleuron; MsSc, mesoscutum; MsSl, mesoscutellum; MT1 (petiole), first metasomal tergite, petiole; MT2–MT8, metasomal tergites 2–8; MtN, metanotum; MtP, metapleuron; OS, ovipositor sheaths; p, pedicellus; PN, pronotum; PPd, propodeum; PPl, propleuron; s, scape; ti, tibia; tr, trochanter; 1–5, tarsomeres; venation: Pt, pterostigma; R1, metacarp, anterior branch of radius; r, cross vein connecting pterostigma and radial sector; Pt, pterostigma; RS, radial sector; 3RSa, 3RSb, a and b sections of 3rd abscissa of radial sector; 2M, 3M, 2nd, and 3rd abscissae of media; Cu, cubitus.
Mesosoma densely pubescent (Fig. 1A1–A3). Propodeum with central areola.
The venation (Figs. 1A1, A2, 2) of the forewing complete, including 2RS and r-m veins and seven closed cells, however, only marginal, 1st and 2nd submarginal and basal cells are clearly visible. Pterostigma approximately two times as long as wide. 3RSa slightly longer than 2RS; 3M not reaching wing margin. Hind wing with complete basal cell. The surface of both wings densely covered with long setae. The setae along the wing edge are longer than on the wing surface.
Legs densely pubescent. First fore tarsomere 2.0 times as long as second tarsomere, first hind tarsomere 2.7 times as long as second tarsomere.
Metasoma (Fig. 1A1, A2, A4) elongate, lanceolate, densely pubescent. Petiole inverted and its shape is difficult to observe; approximately two times as long as wide at the level of the spiracular tubercles. Eight metasomal tergites clearly visible. Hypopygium and elongate ovipositor sheaths completely covered with short dense setae. Ovipositor sheaths elongate, 3.0 times as long as wide in the middle; dorsal margin of ovipositor sheaths straight, apex rounded, ventral margin slightly curved upwards. Left ovipositor sheath broken at midlength (Fig. 1A4).
Coloration of the body is brown, antennae and legs are slightly lighter; palpi are light yellow.
Body length 1.2 mm, the length of antennae 0.5 mm.
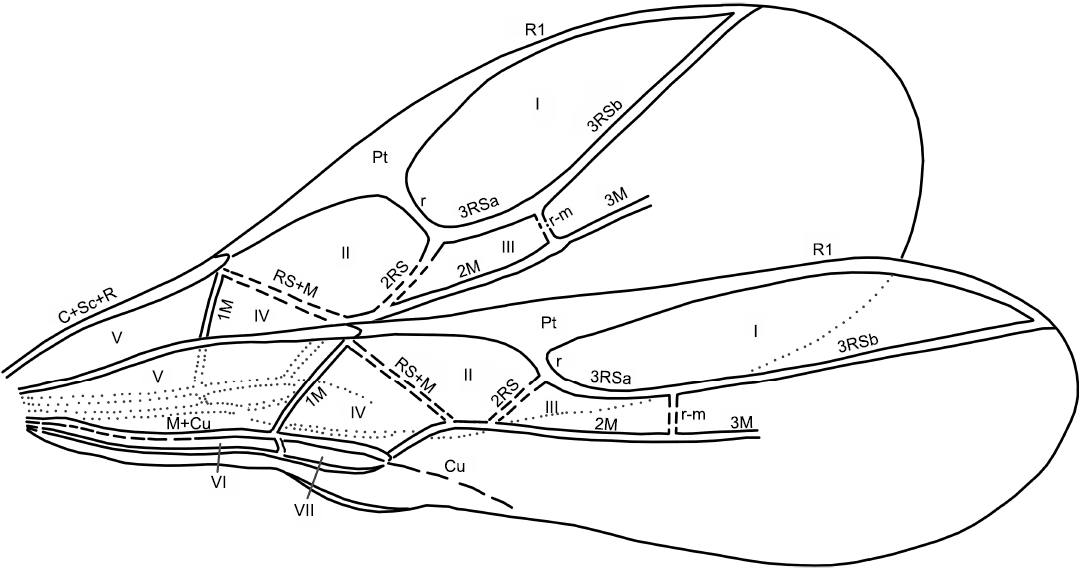
Fig. 2. Schema of forewing venation of aphidiine wasp Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov., holotype PIN 3387/79, Starodubskoye, Sakhalinian amber (Russia), middle Eocene. Abbreviations: Veins: C+Sc+R, costa+subcosta+radius; Pt, pterostigma; R1, metacarp, anterior branch of radius; r, cross vein connecting pterostigma and radial sector; Pt, pterostigma; RS, radial sector; 3RSa, 3RSb, a and b sections of 3rd abscissa of radial sector; 1M, 2M, 3M, 1st, 2nd and 3rd abscissae of media; RS+M, radial sector+media; 2RS, 2nd abscissa of radial sector; r-m, cross vein connecting radius and media; M+Cu, media+cubitus; Cu, cubitus. Cells: I, marginal; II, 1st submarginal; III, 2nd submarginal; IV, 1st discal; V, basal; VI, subbasal; VII, subdiscal.
Remarks.—Left side of the specimen is convex towards observer. The head is strongly deformed, it is not possible to observe the eyes and clypeus. The specimen has clearly visible mesosoma, legs, metasomal tergites, ovipositor sheaths; the wings and petiole are partly visible.
Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. is the first Aphidiinae species described from Sakhalinian amber, and the oldest named female of subfamily.
Ephedrus rasnitsyni sp. nov. presumably belongs to E. plagiator species group of the subgenus Ephedrus sensu stricto (Gärdenfors 1986; Kocić et al. 2019) on the basis of following characters: 11-segmented antennae, complete venation of the forewing, with 3RSa slightly longer than 2RS, and rather long petiole (Figs. 1A1, A2, 2).
Ephedrus rasnitsyni sp. nov., as well as other studied Sakhalinian Aphidiinae, are much shorter than extant species of the E. plagiator group. This group includes 12 extant species, with only some specimens of two species, i.e., E. laevicollis (Thomson, 1895) (1.3–1.9 mm) and Ephedrus koponeni Halme, 1992 (1.4–1.9 mm), smaller than 1.5 mm, while all known specimens of six species are longer than 1.7 mm, with the largest being Ephedrus prociphili Starý, 1982 (2.5–3.5 mm; Gärdenfors 1986; Tomanović et al. 2020). Compared to fossil species, Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. is larger than E. primordialis (0.6–0.7 mm) and smaller than E. mirabilis (1.56 mm).
Stratigraphical and geographic range.—Middle Eocene, Starodubskoye, Dolinsk District, Sakhalin Province, Sakhalin Island, Russian Federation.
Concluding remarks
Among studied aphidiine specimens from Sakhalinian amber, the most abundant species are from the genus Ephedrus, tribe Ephedrini Haliday, 1833 (29 out of 36 specimens).
Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. clearly belongs to the Ephedrus. We compared this specimen with all known genera of Ephedrini according to the electronic catalog Taxapad (Yu et al. 2016): Ephedrus Haliday, 1833, Parephedrus Starý and Carver, 1971, Toxares Haliday, 1840, Diospilites Brues, 1933, Indoephedrus Samanta, Pramanik, and Raychaudhuri, 1983, and Archephedrus Ortega-Blanco, Bennet, Delclòs, and Engel, 2009.
Among these genera, Diospilites and Indoephedrus have been erroneously assigned to Aphidiinae in Taxapad (Yu et al. 2016). We agree with Tobias (1987), who redescribed the fossil Diospilites brevicornis Brues, 1933, from Baltic amber and erected for him the monotypic subfamily Diospilitinae. The Indoephedrus was erected for two parasitoids of Greenideidae from Meghalaya (northeast India): I. reticulata Samanta, Pramanik, and Raychaudhuri, 1983, and I. neoficicola Samanta, Pramanik, and Raychaudhuri, 1983. According to the description by Samanta et al. (1983), the structure of the head (oval head shape, long temples, narrow face with sparse setae, reticular region between the antennal fossae and simple eyes), venation of the forewings, long antennae (25-segmented in I. neoficicola and 33- in I. reticulata) as well as the structure of the long narrow ovipositor sheaths completely covered with setae, are similar to those of some genera of the subfamily Braconinae Nees, 1811 (Sergey A. Belokobylskij, personal communication 2020) and does not belong to Aphidiinae. This opinion is also shared by other aphidiine specialists (Ehsan Rakhshani, personal communication 2020), and the genus was not included to the list of world aphidiine parasitoids of greenideids (Starý et al. 2010).
The fossil Archephedrus stolamissus Ortega-Blanco, Bennet, Delclòs, and Engel, 2009, was described based on a single male from Early Cretaceous (late Albian) Álava amber (Peñacerrada I) from Spain. It differs from the new species by having 16-segmented antennae that clearly narrow towards the apex and by the 5-segmented maxillary palps.
The Parephedrus and Toxares are represented exclusively by extant species. The Australian Parephedrus, despite the absence of notauli, is characterized by sparse pubescence of the ovipositor sheaths, as well as having two thickened setae at the apex of the sheath, similar to species of the Praon Haliday, 1833. The Toxares occupies an isolated position in the group because it has 16–23-segmented antennae and a plow-shaped ovipositor sheath that is curved downward and widens towards the apex.
Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. has all of the plesiomorphic features of the Ephedrus: 11-segmented antennae, complete wing venation, propodeum with central areola, hind wings with a complete basal cell, a straight, triangular ovipositor sheath. The absence of notauli is characteristic to both previously described fossil Ephedrus species and modern Parephedrus (Starý 1973; Gӓrdenfors 1986). Apomorphic features of Ephedrini include posterior position of propodeal spiracles, an elongated petiole, free cuspises of male genitalia, and black colored aphid host mummies (Gӓrdenfors 1986). The only clearly visible apomorphy of the new species is an elongated petiole; other characters are hardly visible or not present in the available specimen.
The fossil species E. mirabilis and E. primordialis can be easily distinguished from the new species. The former has a short petiole and a very short 3M that is approximately equal to r (Timon-David 1944; Starý 1973). E. primordialis has a short, wide, and almost square petiole and a long 3M vein that reaches the apical margin of the wing, and rather short, narrow, triangular ovipositor sheaths (the character of pubescence is absent in the description) (Brues 1933; Starý 1973). The new species has a longer petiole, a long 3M vein that does not reach the forewing margin, and more elongated ovipositor sheaths that are completely covered with setae.
Ephedrus rasnitsyni Davidian and Kaliuzhna sp. nov. is most similar to the extant E. validus and E. carinatus in the pubescence of the ovipositor sheaths but differs from these species in the following characters: the F1 is shorter, 2.5 times as long as wide; notauli are absent; and the ovipositor sheaths are 3.0 times as long as wide. In the related extant species, the F1 is much longer (in E. validus, it is 4.2–4.7 times as long as wide, and in E. carinatus, 5.8 times as long as wide); the notauli are well developed; and the ovipositor sheaths are about two times as long as wide (Starý 1958; Tomanović et al. 2020). Interestingly, E. validus was at one time included in the monotypic subgenus Lysephedrus established based on morphological and ecological data (Starý 1958). The main diagnostic characters that differentiate Lysephedrus from the rest of the subgenera are the reticulated sculpture of the propodeum and petiole and the continuous pubescence of the ovipositor sheath. Lysephedrus was also considered a subgenus in the Ephedrus monograph by Gӓrdenfors (1986). In several other studies, Lysephedrus was considered as a genus (Mackauer 1968; Starý 2006; Davidian 2018, 2019). On the other hand, Ephedrus carinatus (Ephedrus sensu stricto) from Austria was described from a single female already in the nominative subgenus (Tomanović et al. 2020). This species, like E. validus, is characterized by pubescent ovipositor sheaths. This morphological character could be an adaptation to the parasitization of root aphids (e.g., subfamily Eriosomatinae), such as in E. validus (Starý and Schlinger 1967; Tobias and Chiriac 1986; Davidian 2007; Yu et al. 2016) and a similar host was assumed by Tomanović et al. (2020) for E. carinatus. Eriosomatidae are known from Sakhalinian amber as well (Piotr Węgierek, personal communication 2020), and because E. rasnitsyni Davidian and Kaliuzhna sp. nov. has the same character, it could also be a parasite of root aphids.
Sakhalinian amber is potentially the best source to reveal the crucial information in understanding the early stages of aphid-aphidiine coevolution. The Sakhalinian amber biota existed after the rise of ants and after the establishment of close ant-aphid relationships (Perkovsky and Węgierek 2018, and references therein), but ants are rare in this amber—four times less abundant than in Baltic and Rovno ambers (Perkovsky et al. 2007; Radchenko and Perkovsky 2016, and references therein).
Acknowledgements
The authors are grateful to Alexandr P. Rasnitsyn and Irina D. Sukatsheva (both PIN), who provided the amber material; to Andranik R. Manukian (Senior Researcher of the Kaliningrad Regional Amber Museum, Kaliningrad, Russian Federation) who kindly helped in separating inclusions and polishing amber; to Ehsan Rakhshani (University of Zabol, Zābol, Iran), Dmitri V. Logunov (Curator of Arthropods at the Manchester Museum, the University of Manchester, UK), and Hirotsugu Ono (National Museum of Nature and Science, Tokyo, Japan), who provided a copy of the Indoephedrus description. Piotr Węgierek (Silesian University, Katowice, Poland) is thanked for discussion and Sarah C. Crews (California Academy of Sciences, San Francisco, USA) for editing of the English text. Authors are grateful to reviewers of this article Sergey A. Belokobylskij (Zoological Institute of Russian Academy of Sciences, St. Petersburg, Russian Federation) and Michael Sharkey (University of Kentucky, Lexington, USA) for useful recommendations and corrections.
References
Azar, D. and Maksoud, S. 2020. New psychodid flies from the Upper Cretaceous Yantardakh amber and Eocene Sakhalin amber (Diptera: Psychodidae: Psychodinae). Palaeoentomology 3: 500‒512. Crossref
Baranov, V., Andersen, T., and Perkovsky, E.E. 2015. Orthoclads from Eocene amber from Sakhalin (Diptera: Chironomidae, Orthocladiinae). Insect Systematics & Evolution 46: 359–378. Crossref
Batelka, J., Perkovsky, E.E., and Prokop, J. 2020. Diversity of Eocene Ripiphoridae with descriptions of the first species of Pelecotominae and larva of Ripidiinae (Coleoptera). Zoological Journal of the Linnean Society 188: 412–433. Crossref
Belshaw, R. and Quicke, D.L.J. 1997. A molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae). Molecular Phylogenetics and Evolution 7: 281–293. Crossref
Belshaw, R., Dowton, M., Quicke, D.L.J., and Austin, A.D. 2000. Estimating ancestral geographical distributions: a Gondwanan origin for aphid parasitoids? Proceedings of the Royal Society of London, Series B, Biological Sciences 267: 491–496. Crossref
Berger, J.P., Reichenbacher, B., Becker, D., Grimm, M., Grimm, K., Picot, L., Storni, A., Pirkenseer, C., and Schaefer, A. 2005. Eocene–Pliocene time scale and stratigraphy of the Upper Rhine Graben (URG) and the Swiss Molasse Basin (SMB). International Journal of Earth Sciences (Geologische Rundschau) 94: 711–731. Crossref
Brues, C.T. 1933. The parasitic Hymenoptera of the Baltic amber: Part I. In: K. Andrée (ed.), Bernstein-Forschungen (Amber Studies) 3, 4–178. Walter de Gruyter & Co., Berlin.
Chen, X. and van Achterberg, C. 2019. Systematics, phylogeny, and evolution of braconid wasps: 30 years of progress. Annual Review of Entomology 64: 335–358. Crossref
Čkrkić, J., Petrović, A., Kocić, K., Mitrović, M., Kavallieratos, N.G., van Achterberg, C., Hebert, P.D.N., and Tomanović, Ž. 2020. Phylogeny of the subtribe Monoctonina (Hymenoptera, Braconidae, Aphidiinae). Insects 11 (3): 160 [published online, https://doi.org/10.3390/insects11030160] Crossref
Davidian, E.M. [David’ân, E.M.] 2007. Family Aphidiidae [in Russian]. In: A.S. Leley (ed.), Opredelitel’ nasekomyh Dal’nego Vostoka Rossii, 192–254. Dal’nauka, Vladivostok.
Davidian, E.M. 2018. Check-list of the aphidiid-wasp subfamily Ephedrinae (Hymenoptera, Aphidiidae) from Russia and adjacent countries. Entomological Review 98: 1091–1104. Crossref
Davidian, E.M. [David’ân, E.M.] 2019. Family Aphidiidae [in Russian]. In: S.A. Belokobyl’skij, K.G. Samartsev, and A.S. Il’inskaya (eds.), Annotated Catalogue of the Hymenoptera of Russia. Volume II. Apocrita: Parasitica. Trudy Zoologičeskogo instituta Rossijskoj akademii nauk (Supplement 8): 329–340.
Dietrich, C.H. and Perkovsky, E.E. 2019. First record of Cicadellidae (Insecta, Hemiptera, Auchenorrhyncha) from Eocene Sakhalinian amber. ZooKeys 886: 127–134. Crossref
Fedotova, Z.A. and Perkovsky, E.E. 2016. A new genus and species of gall midges of the supertribe Heteropezidi (Diptera, Cecidomyiidae) found in Eocene amber from Sakhalin. Paleontological Journal 50: 1033–1037. Crossref
Gärdenfors, U. 1986.Taxonomic and biological revision of Palearctic Ephedrus Hal. (Hymenoptera, Braconidae, Aphidiinae). Entomologica Scandinavica, Supplement 27: 1–95.
Gumovsky, A., Perkovsky, E., and Rasnitsyn, A. 2018. Laurasian ancestors and “Gondwanan” descendants of Rotoitidae (Hymenoptera: Chalcidoidea): what a review of Late Cretaceous Baeomorpha revealed. Cretaceous Research 84: 286–322. Crossref
Heie, O.E. and Węgierek, P. 2009. A classification of the Aphidomorpha (Hemiptera: Sternorrhyncha) under consideration of the fossil taxa. Redia 92: 69–77.
Kaliuzhna, М.О., Davidian, E.M., and Perkovsky, E.E.2019. Fossil aphidiine wasps (Hymenoptera: Braconidae, Aphidiinae): an overview of the known records and new ones from Sakhalinian amber. In: M.Yu. Proshchalykin (executive editor), A.S. Lelej, V.M. Loktionov, A.G. Radchenko, S.V. Triapitsyn, and A.V. Fateryga (eds.), IV Euroasian Symposium on Hymenoptera. Book of Abstracts, 13–14. FSC Biodiversity FEB RAS, Vladivostok.
Kaliuzhna, М.О., Davidian, E.M., and Perkovsky, E.E. 2020. Fossil Ephedrus species (Hymenoptera, Braconidae, Aphidiinae): from Sakhalinian amber. In: 2nd Palaeontological Virtual Congress. Book of Abstracts. Palaeontology in the Virtual Era, 36. Published by Evangelos Vlachos, Esther Manzanares, Vicente D. Crespo, Carlos Martínez-Pérez, Humberto G. Ferrón, José Luis Herráiz, Arturo Gamonal, Fernando Antonio M. Arnal, Francesc Gascó, and Paolo Citton.
Kazantsev, S.V. and Perkovsky, E.E. 2019. A new genus of soldier beetles (Insecta: Coleoptera: Cantharidae: Cantharinae) from Sakhalinian amber. Paleontological Journal 53: 300–304. Crossref
Kocić, K., Petrović, A., Čkrkić, J., Kavallieratos, N.G., Rakhshani, E., Arnó, J., Aparicio, Y., Hebert, P.D.N., and Tomanović, Ž. 2020. Resolving the taxonomic status of potential biocontrol agents belonging to the neglected genus Lipolexis Förster (Hymenoptera, Braconidae, Aphidiinae) with descriptions of six new species. Insects 11 (10): 667. Crossref
Kocić, K., Petrović, A., Čkrkić, J., Mitrović, M., and Tomanović, Ž. 2019. Phylogenetic relationships and subgeneric classification of European Ephedrus species (Hymenoptera, Braconidae, Aphidiinae). ZooKeys 878: 1–22. Crossref
Kodrul, T.M. 1999. Paleogene phytostratigraphy of the South Sakhalin [in Russian]. Trudy Geologičeskogo Instituta Rossijskoj Akademii Nauk 519: 1–150.
Mackauer, M. 1968. Aphidiidae. In: C. Ferrière and J. van der Vecht (eds.), Hymenopterorum Catalogus 3, 1–103. Dr. W. Junk N.V., The Hague.
Marusik, Y.M., Perkovsky, E.E., and Eskov, K.Y. 2018. First records of spiders (Arachnida: Aranei) from Sakhalinian amber with description of a new species of the genus Orchestina Simon, 1890. Far Eastern Entomologist 367: 1–9. Crossref
Ortega-Blanco, J., Bennett, D.J., Delclòs, X., and Engel, M.S. 2009. Primitive aphidiine wasp in Albian amber from Spain and a Northern Hemisphere origin for the subfamily (Hymenoptera: Braconidae: Aphidiinae). Journal of the Kansas Entomological Society 82: 273–282. Crossref
Perkovsky, E.E. and Węgierek, P. 2018. Aphid-Buchnera-ant symbiosis, or why are aphids rare in the tropics and very rare further south? Earth and Environmental Science Transactions of the Royal Society of Edinburgh 107: 297–310. Crossref
Perkovsky, E.E., Háva, J., and Zaitsev, A.A. 2021. The first finding of a skin beetle (Coleoptera, Dermestidae) from Sakhalinian amber. Paleontological Journal 55: 184–192. Crossref
Perkovsky, E.E., Rasnitsyn, A.P., Vlaskin, A.P., and Taraschuk, M.V. 2007. A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. African Invertebrates 48: 229–245.
Quilis, P.M. 1940. Los Aphidiidae fósiles de Wittenheim (Haut-Rhin, Francia) (Hym. Brac.). Eos, Revista Española de Entomología 14: 23–61.
Radchenko, A.G. and Perkovsky, E.E. 2016. The ant Aphaenogaster dlusskyana sp. nov. (Hymenoptera, Formicidae) from the Sakhalin amber—the earliest described species of an extant genus of Myrmicinae. Paleontological Journal 50: 936–946. Crossref
Rakhshani, E., Pons, X., Lumbierres, B., Havelka, J., Pérez Hidalgo, N., Tomanović, Ž., and Starý, P. 2017. A new parasitoid (Hymenoptera: Braconidae: Aphidiinae) of the invasive bamboo aphids Takecallis spp. (Hemiptera: Aphididae) from Western Europe. Journal of Natural History 51: 1237–1248. Crossref
Rasnitsyn, A.P. [Rasnicyn, A.P.] 1980. Origin and evolution of Hymenoptera [in Russian]. Trudy Paleontologičeskogo instituta Akademii Nauk SSSR 174: 1–191.
Samanta, A.K., Pramanik, D.R., and Raychaudhuri, D. 1983. Some new aphid parasitoids (Hymenoptera: Aphidiidae) from Meghalaya, North East India. Akitu 54: 1–8.
Sanchis, A., Amparo, L., González-Candelas, F., and Michelena, J.M. 2000. An 18S rDNA-based molecular phylogeny of Aphidiinae (Hymenoptera: Braconidae). Molecular Phylogenetics and Evolution 14: 180–194. Crossref
Sharkey, M.J. and Wharton, R.A. 1997. Morphology and terminology. In: R.A. Wharton, P.M. Marsh, and M.J. Sharkey (eds.), International Society of Hymenopterists Special Publication I, Manual of the New World Genera of the Family Braconidae, 19–37. The International Society of Hymenopterists, Washington.
Simutnik, S.A. 2014. First record of Encyrtidae (Hymenoptera, Chalcidoidea) from the Sakhalin amber. Paleontological Journal 48: 621–623. Crossref
Simutnik, S.A. 2015. Description of two new monotypic genera of encyrtid wasps (Hymenoptera, Chalcidoidea: Encyrtidae), based on males from the middle Eocene Sakhalin amber. Entomological Review 95: 937–940. Crossref
Simutnik, S.A. 2020. The earliest Encyrtidae (Hymenoptera, Chalcidoidea). Historical Biology [published online, https://doi.org/10.1080/08912963.2020.1835887]. Crossref
Starý, P. 1958. A taxonomic revision of some aphidiine genera with remarks on the subfamily Aphidiinae (Hymenoptera, Braconidae). Acta Faunistica Entomologica Musei Nationalis Pragae 3: 53–96.
Starý, P. 1970. Biology of Aphid Parasites (Hymenoptera: Aphidiidae) With Respect to Integrated Control. Series Entomologica 6. 643 pp. Dr. W. Junk B.V., Hague.
Starý, P. 1973. A revision of the fossil Aphidiidae (Hymenoptera). Annotationes Zoologicae et Botanicae 87: 1–22.
Starý, P. and Schlinger, E.I. 1967. A revision of the Far East Asian Aphidiidae (Hymenoptera). Series Entomologica 3: 1–204. Dr. W. Junk Publisher, The Hague. Crossref
Starý, P. 2006. Aphid Parasitoids of the Czech Republic (Hymenoptera: Braconidae, Aphidiinae). 430 pp. Academia, Praha.
Starý, P., Rakhshani, E., Havelka, J., Tomanović, Ž., Kavallieratos N.G., and Sharkey, M. 2010. Review and key to the world parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Greenideinae aphids (Hemiptera: Aphididae), including notes on invasive pest species. Annals of the Entomological Society of America 103: 307–321. Crossref
Timon-David, J. 1944. Insectes fossiles de l’Oligocène inférieur des Camoins (Bassin de Marseille). II. Hyménoptères. Bulletin de la Société Entomologique de France 49: 40–45.
Tobias, V.I. 1987. New taxa of Braconidae from Baltic amber (Hymenoptera) [in Russian]. Entomologičeskoe obozrenie 66 (4): 845–859.
Tobias, V.I. and Chiriac, I.G. [Kiriâk I.G.] 1986. Family Aphidiidae [in Russian]. In: G.S. Medvedev (ed.), Opredelitel’nasekomyh evropejskoj časti SSSR 3 (5), 232–308. Nauka, Lenindrad.
Tomanović, Ž., Petrović, A., Kocić, K., Čkrkić, J., and Žikić, V. 2020. Two new morphologically interesting species of the genus Ephedrus Haliday (Hymenoptera, Braconidae, Aphidiinae). Journal of Hymenoptera Research 77: 167–174. Crossref
Tykhonenko, Y.Y., Hayova, V.P., Sukhomlyn, M.M., Ignatov, M.S., Vasilenko, D.V., and Perkovsky, E.E. 2021. The first record of the rust fungus spores (Pucciniales) from middle Eocene Sakhalinian amber. Paleontological Journal 55: 105–110. Crossref
Yu, D.S., van Achterberg, C., and Horstmann, K. 2016. World Ichneumonoidea 2015: Taxonomy, Biology, Morphology and Distribution. CD/DVD. Taxapad, Vancouver.
Zherikhin, V.V. [Žerihin V.V.], Ponomarenko, A.G. [Ponomarenko, A.G.], and Rasnitsyn, A.P. [Rasnicyn, A.P.] 2008. Vvedenie v paleoèntomologiû. 371 pp. KMK, Moskva.
Žikić, V., Lazarević, M., and Milosević, D. 2017. Host range patterning of parasitoid wasps Aphidiinae (Hymenoptera: Braconidae). Zoologischer Anzeiger 268: 75–83. Crossref
Acta Palaeontol. Pol. 66 (Supplement to 3): 59–65, 2021
https://doi.org/10.4202/app.00843.2020