

New cranial material of the acanthothoracid placoderm Palaeacanthaspis vasta from the Lower Devonian of Podolia—phylogenetic and taxonomic significance
VINCENT DUPRET, HUBERT SZANIAWSKI, MAREK DEC, and PIOTR SZREK
Dupret, V., Szaniawski, H., Dec, M., and Szrek, P. 2021. New cranial material of the acanthothoracid placoderm Palaeacanthaspis vasta from the Lower Devonian of Podolia—phylogenetic and taxonomic significance. Acta Palaeontologica Polonica 66 (2): 337–347.
The Early Devonian vertebrates of Podolia in Ukraine are well known thanks to the material of the basal arthrodire placoderm genus Kujdanowiaspis, originally mentioned by Brotzen in 1934. The anatomical fame of Kujdanowiaspis brought by Erik Stensiö almost eclipsed the presence of the acanthothoracid placoderm Palaeacanthapsis vasta in the underlying beds, with the original material of P. vasta being less well preserved and abundant than that of Kujdanowiaspis. Here we describe a newly discovered specimen of the acanthothoracid P. vasta from the Lochkovian of Podolia (Ukraine). The specimen, although incomplete, is very well preserved in three dimensions and allows a thorough description of its external morphology, which is compared to that of Romundina stellina and other well-known Acanthothoraci. A phylogenetic analysis is performed and the acanthothoracid nature of Palaeacanthaspis is confirmed. However, the position of Palaeacanthaspis within the Acanthothoraci remains uncertain, and its resemblance with Romundina could be due to either synapomorphies or symplesiomorphies. Similarities and differences between the two forms are exposed, and lead to the lack of synonymy at the specific level. Generic synonymy is also questioned for the first time but remains equivocal.
Key words: Placodermi, Acanthothoraci, neurocranium, Lochkovian, Devonian, Podolia, Ukraine.
Vincent Dupret [vincent.dupret@ebc.uu.se], Evolutionsbiologiskt centrum (EBC), Uppsala Universitet, Norbyvägen 18A-752 36 Uppsala, Sweden; and Department of Applied Mathematics, Research School of Physics, College of Sciences, Australian National University, Mills Road, Canberra ACT 2601, Australia.
Hubert Szaniawski [szaniaw@twarda.pan.pl], Institute of Palaeobiology, Polish Academy of Sciences, Twarda 51/55, 00-818 Warsaw, Poland.
Marek Dec [mdec@wp.pl] and Piotr Szrek [PSZR@pgi.gov.pl], Polish Geological Institute-National Research Institute, Rakowiecka 4, 00-975 Warsaw, Poland.
Received 6 November 2020, accepted 2 February 2021, available online 23 March 2021.
Copyright © 2021 V. Dupret et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Placoderms (armoured fishes) were the first gnathostomes (jawed vertebrates). They appeared during the Early Silurian, diversified through the Devonian and yet became extinct at the end of this epoch (Janvier 1996; Young 2010). Because of their basal and likely paraphyletic phylogenetic status, the study of their fossils allows us to understand the circumstances of the origin and successful evolution of gnathostomes (Brazeau 2009; Davis et al. 2012; Zhu et al. 2013, 2016; Dupret et al. 2014; but see King et al. 2016 for a possible monophyletic resolution). Among them, the group of “acanthothoracids” consists in a heteroclite and diverse ensemble of basal and primitive forms from which other orders of placoderms branch (e.g., antiarchs, arthrodires; Janvier 1996).
Podolia is a region in eastern Europe well known for its Early Devonian vertebrate fossils, especially the basal arthrodire genus Kujdanowiaspis Stensiö, 1942, which was extensively studied by the Stockholm school as early as the 1930s (Zych 1927, 1930, 1931, 1937; Brotzen 1933, 1934; Stensiö 1934, 1969, 1942, 1945, 1959, 1963a, b, 1969; Brotzen 1936; Karatajūtė-Talimaa 1968, 1997; Janvier 1988, 1995; Voichyshyn 1994, 1997, 1998, 1999, 2001, 2011; Dupret 2003a, b, 2004, 2010; Dumbrava and Blieck 2005; Dupret et al. 2007; Dupret and Blieck 2009; Drygant and Szaniawski 2012; Voichyshyn and Szaniawski 2012, 2018). While arthrodire material is abundant and mostly comes from the “Old Red Sandstones” strata ranging from the upper Lockhovian to upper Lochkovian–Pragian and lower Pragian Dnister series (see Dupret and Blieck 2009 for details), some more scarce acanthothoracid material was recovered from a lower stratigraphical position (middle Lochkovian Chortkov Horizon; see Brotzen 1934; Dupret and Blieck 2009; Drygant and Szaniawski 2012).
Among this material, Palaeacanthapis vasta Brotzen, 1934, was named and described on the basis of a single specimen from Podolia corresponding to an incomplete thoracic armour by Brotzen (1934). This specimen is currently curated in the fossil vertebrate collections of the Museum für Naturkunde in Berlin, Germany, under the number MB.f.3676. Later, Stensiö (1944) described additional Podolian material which he attributed to P. vasta, including a poorly preserved skull roof (NRM.P.5003), a dubious isolated nuchal plate (NRM.P.5012), median dorsal plates (NRM.P.5004, 5005, 5006), dermal scapular girdle (NRM.P.5007, 5008, 5009, 5010, 5011, 5013), curated in the collections of Naturhistoriska riksmuseet, Stockholm, Sweden. In the same publication, Stensiö worked out a classification of placoderms (see Discussion for further details), and erects the order Acanthothoraci and the family Palaeacanthaspididae for the new taxa Palaeacanthapis vasta and Dobrowliana podolica Stensiö, 1944. The latter was thought to have more arthrodiran characteristics, it was placed with Acanthothoraci on the sole basis of its stratigraphic co-occurrence with P. vasta; D. podolica is based on a single isolated median dorsal plate very similar to that of Kujanowiaspis podolica (Brotzen, 1934) (see Dupret 2003a, 2010; Dupret et al. 2007). The most recent discovery of Palaeacanthaspis aff. P. vasta was surprisingly made in the Lochkovian of Spain (Celtiberia Palaeoblock) which interestingly was of Gondwanan affinities (Dupret et al. 2011: fig. 3O). New material was more recently discovered by one of the authors (HS) during a field trip in Podolia and is described in this article.
Institutional abbreviations.—MB, Museum für Naturkunde, Berlin, Germany; NRM, Naturhistoriska riksmuseet, Stockholm, Sweden; ZPAL, Institute of Palaeontology, Polish Academy of Sciences, Warsaw, Poland.
Other abbreviations.—a.pop, anterior postorbital process; a.pr.br, articular facet for the dorsal elements of the branchial basket; av.myo, anteroventral myodome; C, central plate; c.VI, canal for the abducens nerve; c.VII, canal for the (main trunk of the) facial nerve; c.VIII, canal for the acoustic nerve; c.IX, canal for the glossopharyngeal nerve; c.pit.v, canal for the pituitary vein; c.X, canal for the vagus nerve; cc.a.g, common carotid artery groove; d.end.e, external opening for the endolymphatic duct; d.myo, dorsal myodome; e.c, cranial cavity; e.s, eyestalk; ehy+op.art, articular facet for the epihyal and opercular cartilages; eth.sh, ethmoidal dermal capsule (Stensiö 1944: fig. 1); f.III.1–2, foramina for the oculomotor nerves; f.IV, foramen for the trochlear nerve; f.V.1, foramen for the first branch of the trigeminal nerve; f.V.2–3, foramen for the second and third branches of the trigeminal nerve; f.VI, foramen for the abducens nerve; f.VII.hm, foramen for the hyomandibular branch of the facial nerve; f.X, foramen for the vagus nerve; f.ic.a, foramen for the internal carotid artery; f.j.v, foramen for the jugular vein; f.pit.v, foramen for the pituitary vein; g.II, groove for the optic nerve; g.V.2–3, groove for the second and third branches of the trigeminal nerve; g.ac.v, groove for the anterior cerebral vein; g.eff.a, groove for the efferent artery; g.ehy.a, groove for the epihyal artery; g.ic.a, groove for the internal carotid artery; g.op.a, groove for the opercular artery; g.rn.cap, groove for the insertion of the ventral part of the rostronasal capsule; ins.rn.cap, insertion slit for the dorsolateral portion of the rostronasal caspule; ioc, infraorbital sensory line groove; lc, cephalic part of the main lateral sensory line groove; M,marginal plate; mpt.art, articulation facet for the metapterygoid; m.cr, median crest of the nuchal plate; N, nuchal plate; occ, occipital cross commissure; orb, orbit (Stensiö 1944: fig. 1); pmc, postmarginal sensory line groove; p.pop, posterior postorbital process; PaN.a, anterior paranuchal plate; PaN.m, median paranuchal plate; PaN.p, posterior paranuchal plate; pfc, profundus sensory line groove; pmc, postmarginal sensory line groove; PPi, postpineal plate; ppl, posterior pitline; PRM, premedian plate; PrO, preorbital plate; PtO, postorbital plate; pv.myo, posteroventral myodome; RoNa, rostronasal capsule; sac, saccula of the inner ear; soc, supraorbital sensory line groove; vdl, “probable position of a ventrally descending lamina of dermal bone which formed the posterior boundary of the ethmoidal caspule” (Stensiö 1944: fig. 1); v.myo, ventral myodome; VII.pal.c, canal for the palatine ramus of the facial nerve.
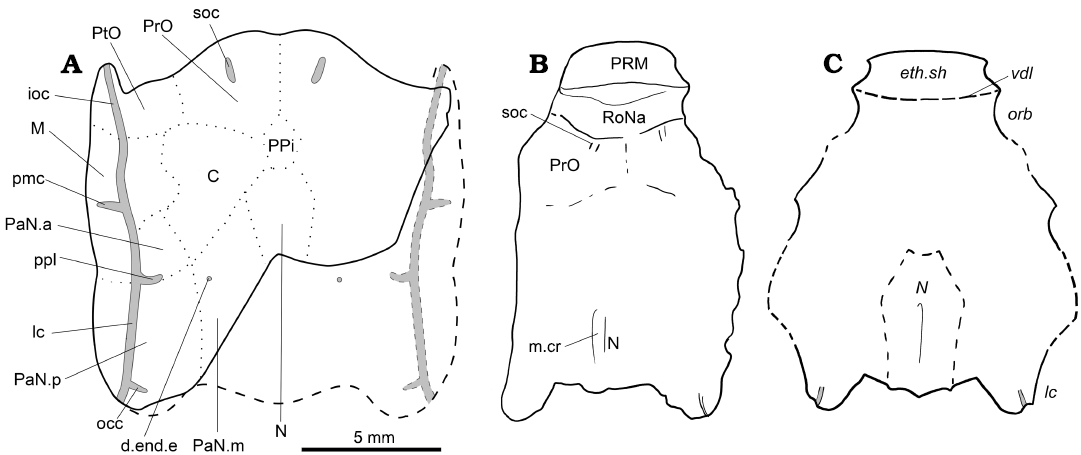
Fig. 1. The acanthothoracid placoderm Palaeacanthaspis vasta Brotzen, 1934. A. Reconstruction of the skull roof of P. vasta based on specimen ZPAL Ag. V/1 from the middle Lochkovian of Vynohradne, Podolia, Ukraine. B. Reinterpretation of NRM.P.5003 from the middle Lochkovian of Dobrivlyany, Podolia, Ukraine (based on the photograph of the specimen in Stensiö 1944: pl. 1). C. Original interpretation based NRM.P.5003 (redrawn from Stensiö 1944: fig. 1, Stensiö’s labels in italics). B, C, not to scale. Abbreviations: d.end.e, external opening for the endolymphatic duct; eth.sh, ethmoidal dermal capsule; ioc, infraorbital sensory line groove; lc, cephalic part of the main lateral sensory line groove; M, marginal plate; m.cr, median crest of the nuchal plate; N, nuchal plate; occ, occipital cross commissure; orb, orbit; pmc, postmarginal sensory line groove; PaN.a, anterior paranuchal plate; PaN.m, median paranuchal plate; PaN.p, posterior paranuchal plate; ppl, posterior pitline; PRM, premedian plate; PrO, preorbital plate; PtO, postorbital plate; RoNa, rostronasal capsule; soc, supraorbital sensory line groove; vdl, “probable position of a ventrally descending lamina of dermal bone which formed the posterior boundary of the ethmoidal caspule”.
Material and methods
The single specimen under this study is a three-dimensionally preserved skull in two parts. Originally enclosed in a limestone matrix, it was dissolved using 30% acetic acid buffered with tricalcium phosphate; some matrix remains undissolved, allowing the preservation of fine internal perichondrally ossified structures, in a similar way to what occurred in the specimen of Romundina stellina Ørvig, 1975, previously published by Dupret et al. (2014, 2017a), and thus prevented them from collapsing.
The specimen comes from Vynohradne in Podolia, western Ukraine, 8 km ESE from the type-locality of Zalishchyki (formerly known as Zaleszczyki, e.g., in Brotzen 1934). It is noteworthy that the transliterated names of various localities may change from one publication to another (e.g., Drygant and Szaniawski 2012: figs 1, 2, spelled out as Vynohradiv and Zalishchyky). Herein we use the spelling used in Dupret and Blieck (2009).
The new specimen is publicly deposited in the collections of ZPAL. All photos of the skull were produced with a Nikon D800 camera appended with a Nikon micro 60 unit lens.
The skull (NRM P. 5003) described and illustrated by Stensiö (1944: fig. 1, pl. 1; Fig. 1B, C) is curated at the Naturhistoriska riksmuseet in Stockholm, Sweden; a cast of this specimen (MB.f.12171) is available at the Museum für Naturkunde in Berlin, Germany.
The taxon/character matrix is built in Mesquite (Maddison and Maddison 2019) and based on the matrix by Vaškaninová et al. (2020; itself updated from Qiao et al. 2016) with the addition of P. vasta, resulting in a matrix of 108 taxa and 335 characters. 84 characters could be coded effectively for P. vasta (i.e., not taking into account “not applicable” scores). Minor modifications in the coding and the labelling of characters and states are included in the matrix file in SOM 1 (Supplementary Online Material available at http://app.pan.pl/SOM/app66-Dupret_etal_SOM.pdf). Galeaspida and Osteostraci are the outgroup. All characters are unordered and unpolarised, and of equal weights. The data were analysed in TNT (Goloboff and Catalano 2016) using the Zephyr module (Maddison and Will 2020; Maddison and Maddison 2020) in Mesquite 3.61 interface (Maddison and Maddison 2019). A New Technology search was performed (using the default sectorial search, ratchet, drift and tree fusing options, 1000 random addition sequences, random seed 1), with the most parsimonious trees found (26, of 961 steps) then used for a Traditional Search (tree bisection-reconnection algorithm), from which a strict consensus tree was calculated. The strict consensus tree is 1053 steps long, has a CI of 0.330, and a RI of 0.774; it was built from 18600 most parsimonious trees of 961 steps each. No robustness analysis (Bremer nor Bootstrap) was made.
Systematic palaeontology
Class Placodermi McCoy, 1848
Order Acanthothoraci Stensiö, 1944
Family Palaeacanthaspididae Stensiö, 1944
Palaeacanthaspis Brotzen, 1934
Type species: Palaeacanthapis vasta Brotzen, 1934; Podolia, Ukraine, Lochkovian, Devonian.
Palaeacanthaspis vasta Brotzen, 1934
Figs. 1, 2.
Material: ZPAL Ag. V/1, a subcomplete skull roof and associated neurocranial structures in two parts from Vynohradne, Dniester Valley, Podolia, Ukraine (locality 5, N 48°36’22.3” E 25°51’04.3” in Drygant and Szaniawski 2012: table 1). Chortkiv Formation and Horizon, Tyver series, Lochkovian (Lower Devonian, mainly middle Lochkovian; based on co-occurrence of conodonts; Drygant and Szaniawski 2012: fig. 3). Vynohradne is located about 8 km ESE from the type locality of Zalishchyki.
Description.—General features: The ZPAL Ag. V/1 corresponds to an incomplete skull preserved in two parts separated by an oblique crack at the level of the right infraorbital sensory line groove. The anterior and medial posterior portions of the skull are missing. It is common in placoderms to have the rostronasal capsule detached from the rest of the skull at the level of the optic fissure (Goujet 1984a, b; Stensiö 1963a), which is the case for the present specimen. There is no information regarding the presence of premedian elements in front of this rostronasal capsule, but considering the high degree of similarity with the acanthothoracid Romundina, it is likely that a protrusion made of a premedian plate in front of the rostronasal capsule indeed occurred. As for the missing median posterior portion of the skull, it is located behind the canal for the vagus nerve. Consequently, only a small number of structures can be considered lost.
The dermal skull roof is covered by stellate tubercles of various sizes. The grooves for the sensory line system are well pronounced and easy to identify. The plate boundaries are much more difficult to discern. As far as what can be identified, the skull roof pattern is very similar to that observed in Romundina (see Discussion).
Dermal plates of the skull roof: Sutures between plates are not observed on the specimen; however, the ossification centres are quite recognisable. The radiation centres of each plate are slightly elevated. The boundary between preorbital plates is traceable (Fig. 1A: PrO). The preorbital plate does not seem to contact the nuchal plate posteriorly (Fig. 1A: N); a small elevation between the identified domains of the preorbital plates and nuchal plates could indicate the radiation centre of an unpaired post-pineal plate (Fig. 1A: PPi). Only an absence of tubercles indicates the boundary between the preorbital and postorbital plates, thus making this assumption disputable (Fig. 1A: PtO). A similar absence points out the limit between the postorbital and the central plates (Fig. 1A: C). A tiny portion of the median paranuchal plate may be visible, in which the external foramen for the endolyphatic duct opens (Fig. 1A: PaN.m, d.end.e). The central plates have well visible boundaries with the marginal and the anterior paranuchal plate (Fig. 1A: M, PaN.a). The connection between the anterior and posterior paranuchal plates (Fig. 1A: PaN.a, PaN.p), as well as between the marginal and the anterior paranuchal plates, are also recognisable. The suture runs towards the notch at the lateral edge of the skull roof. Other boundaries are very difficult to trace, especially the one between the postorbital and marginal plates and the one between the median and posterior paranuchal plates.
The sensory line system consists of grooves pierced by pores. A pair of short supraorbital sensory line grooves is observed on each of the preobital plates, although none reaches the anterior margin of the skull roof (Fig. 1A: soc). A profundus sensory line groove (Fig. 1A: pfc) can be seen between the growth centre of the preorbital and postorbital plates; its postorbital portion is widest and deepest. The infraorbital and main cephalic sensory line grooves are visible along the lateral side of the skull roof, between the anterior postorbital process and the tip of the posterior paranuchal plate (Fig. 1A: ioc, lc). The postmarginal sensory line groove shows a short lateral course from the infraorbital sensory line groove without reaching the lateral edge of the marginal plate (Fig. 1A: pmc). The single posterior pit line (Fig. 1A: ppl) runs between anterior and posterior paranuchal plates either near or through the suture between these plates. The occipital cross-commissure canal (Fig. 1A: occ) runs from the posterior part of the posterior paranuchal plate to the radiation centre. The central sensory line groove and the median pit line are not observed.
Neurocranium: Only the postethmoid part of the neurocranium is preserved. The endocranial cavity (Fig. 2: e.c) opens anteriorly into the rostronasal space. It is bounded dorsally by the groove for the anterior cerebral vein (Fig. 2: g.ac.v) and laterally by the groove for the optic nerve (Fig. 2: g.II). A gutter below the endocranial cavity accommodated the ventral part of the perichondral component of the rostronasal capsule (Fig. 2: g.rn.cap); additionally, two slits are visible on either side of the dorsal part of the groove for the optic nerve to accommodate the dorsolateral edge of the perichondral component of the rostronasal capsule (Fig. 2: ins.rn.cap).
In lateral view, the eyestalk is located just behind the optic fissure (Fig. 2: e.s, g.II), and is immediately surrounded by a number of depressions, identified as (clockwise from the top): the dorsal myodome and the foramen for the upper branch of the oculomotor nerve (Fig. 2: d.myo, f.III.1), the foramen for the lower branch of the oculomotor nerve posteriorly (Fig. 2: f.III.2), and the anteroventral myodome ventrally (Fig. 2: av.myo). A ventral myodome is identified laterally from the anteroventral myodome (Fig. 2: v.myo). The opening for the pituitary vein is located on the mesial wall of the orbit, at the level of the eyestalk, and between the eyestalk and the opening for the second and third branches of the trigeminal nerve, and is hardly visible because it is very deep (Fig. 2: f.pit.v, f.V.2–3). In front and above the foramen for the first branch of the trigeminal nerve, two small holes give the misleading impression that some nerves or vessels would exit there, but are just small breaks in the thin perichondral bone layer and do not represent any biological structure.
In anterior view, the foramen for the jugular vein is small and opens anteriorward in the lateral edge of the posteroventral myodome and below the groove for the second and third branches of the trigeminal nerve (Fig. 2: f.j.v, pv.myo, g.V.2–3). Dorsomesially from it, the foramina for the first branch of the trigeminal nerve and the trochlear nerve are best visible in the left orbit (Fig. 2: f.IV and f.V1).
The suborbital shelf is bounded posterolaterally by the anterior postorbital process (Fig. 1: a.pop), at the distal tip of which the foramen for the hyomandibular branch of the facial nerve opens laterally (Fig. 2: f.VII.hm). The metapterygoid articulation facet is visible on the anterior side of the tip of the anterior postorbital process, anteroventrally from the foramen for the hyomandibular branch of the facial nerve (Fig. 2: mpt.art). The epihyal and opercular articulation facets face laterally as a vertical peanut shaped slit (Fig. 2: ehy+op.art) posteriorly to the opening for the hyomandibular branch of the facial nerve. The groove for the epihyal artery is the only one that runs laterally and almost perpendicularly from the midline on the ventral surface of the neurocranium; it opens laterally at the tip of the anterior postorbital process just between the opening for the articulation facets for the metapterygoid anteriorly, and for the epihyal and opercular cartilages posteriorly (Fig. 2: g.ehy.a). The groove for the opercular artery diverges posteriorly from the epyhyal artery groove, just mesially from the lateral end of the course of the epihyal artery groove (Fig. 2: g.op.a).
The ventral view reveals the floor of the neurocranium, as well as some of the internal structures, because parts of the neurocranial floor are broken. Anteriorly, a wide groove surrounded by, and connecting, two larger holes is visible. As the two foramina opened for a branch of the internal carotid artery (Fig. 2: f.ic.a), the groove may have hosted the anterior connection for the left and right parts of this blood vessel. Alternatively, the groove may have hosted a parasphenoid. Posteriorly, a hole in the floor of the neurocranium, certainly a preservation artefact, reveals the light of the canal for the pituitary vein (Fig. 2: c.pit.v). The posterior postorbital process is bifid and is bordered mesially by the groove for the efferent artery on the ventral side of the neurocranium (Fig. 2: p.pop, g.eff.a). The base of each branch of the posterior postorbital process show a small articulation facet for the dorsal part of the branchial basket apparatus (Fig. 2: a.pr.br). On the lateral wall between each branch, a foramen opens for a branch of the vagus nerve (Fig. 2: f.X).
The right portion where the palate floor was destroyed reveals several structures: the canals for the pituitary vein, the posteroventral myodome and the abducens nerve, the facial, acoustic, glossopharyngeal and vagus nerves, as well as part of the saccula of the inner ear (Fig. 2: c.pit.v, pv.myo, c.VI, c.VII, c.VIII, c.IX, c.X, sac). The light of the endocranial cavity is also clearly visible (Fig. 2: e.c). No canal for the notochord could be identified.
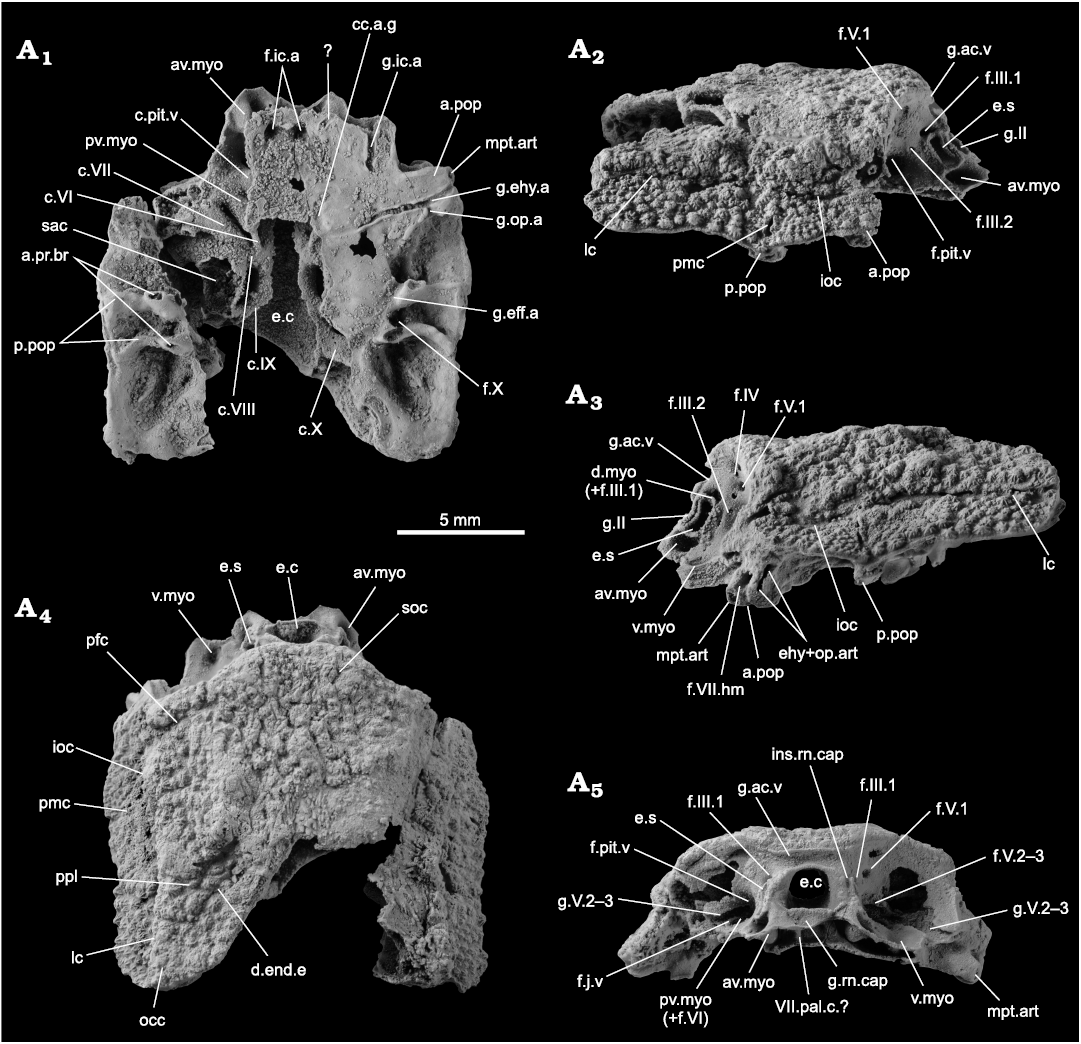
Fig. 2. Incomplete skull of the acanthothoracid placoderm Palaeacanthaspis vasta Brotzen, 1934 (ZPAL Ag. V/1) from the middle Lochkovian of Vynohradne, Podolia, Ukraine, in ventral (A1), left (A3) and right (A2) lateral, dorsal (A4), and anterior (A5) views. Abbreviations: a.pop, anterior postorbital process; a.pr.br, articular facet for the dorsal elements of the branchial basket; av.myo, anteroventral myodome; c.VI, canal for the abducens nerve; c.VII, canal for the (main trunk of the) facial nerve; c.VIII, canal for the acoustic nerve; c.IX, canal for the glossopharyngeal nerve; c.pit.v, canal for the pituitary vein; c.X, canal for the vagus nerve; cc.a.g, common carotid artery groove; d.end.e, external opening for the endolymphatic duct; d.myo, dorsal myodome; e.c, cranial cavity; e.s, eyestalk; ehy+op.art, articular facet for the epihyal and opercular cartilages; f.III.1–2, foramina for the oculomotor nerves; f.IV, foramen for the trochlear nerve; f.V.1, foramen for the first branch of the trigeminal nerve; f.V.2–3, foramen for the second and third branches of the trigeminal nerve; f.VI, foramen for the abducens nerve; f.VII.hm, foramen for the hyomandibular branch of the facial nerve; f.X, foramen for the vagus nerve; f.ic.a, foramen for the internal carotid artery; f.j.v, foramen for the jugular vein; f.pit.v, foramen for the pituitary vein; g.II, groove for the optic nerve; g.V.2–3, groove for the second and third branches of the trigeminal nerve; g.ac.v, groove for the anterior cerebral vein; g.eff.a, groove for the efferent artery; g.ehy.a, groove for the epihyal artery; g.ic.a, groove for the internal carotid artery; g.op.a, groove for the opercular artery; g.rn.cap, groove for the insertion of the ventral part of the rostronasal capsule; ins.rn.cap, insertion slit for the dorsolateral portion of the rostronasal caspule; ioc, infraorbital sensory line groove; lc, cephalic part of the main lateral sensory line groove; mpt.art, articulation facet for the metapterygoid; occ, occipital cross commissure; pmc, postmarginal sensory line groove; p.pop, posterior postorbital process; pfc, profundus sensory line groove; pmc, postmarginal sensory line groove; ppl, posterior pitline; pv.myo, posteroventral myodome; sac, saccula of the inner ear; soc, supraorbital sensory line groove; v.myo, ventral myodome; VII.pal.c, canal for the palatine ramus of the facial nerve.
Remarks.—The systematic attribution of the new material to the group “Acanthothoraci” and to the species Palaeacanthaspis vasta relies on the following converging considerations: (i) the stellate ornamentation being common in Acanthothoraci (although not exclusive to this group); (ii) the only acanthothoracid hitherto known in Podolia being P. vasta, although described solely on the basis of few thoracic materials by Brotzen (1934); and (iii) attribution to this species being obviously the most parsimonious solution.
Discussion
Remarks on naming groups and plates through times.—It is important to keep in mind that taxonomical concepts (i.e., group content, concept, and name) evolved dramatically between early publications on placoderms and our current time. It especially concerns the groups not covered by the ICZN (i.e., ranks higher than family group). Stensiö’s (1944: 75) earlier work used taxonomy and classification, which could seem confusing for present day readers. The Woodward’s (1891) clade Arthrodira sensu Stensiö (1944) is roughly synonymous with the concept of Placodermi we adopted herein. It should be, however, kept in mind that the concept of Arthrodira has evolved over time and its current interpretation does not fully corresponds to the concept of Stensiö (1944). The latter contained three divisions: Euarthrodira, Rhenanida, and Antiarchi. Euarthrodira sensu Stensiö (1944) included seven orders: Brachythoraci, Dolichothoraci, Acanthothoraci, Petalichthyida, Stegoselachii, Phyllolepida, and Ptyctodontida. It is noteworthy that Stensiö (1944: 2, footnote 2, 75) renamed the group Acanthaspida used by Brotzen (1934 ) into Dolichothoraci; the group Dolichothoraci was later subdivided by Dineley and Liu (1984) into Actinolepidoidei and Phlyctaenii. Arthrodira in its current understanding contains Brachythoraci and Dolichothoraci (themselves divided into Actinolepidoidei and Phlyctaenii; Goujet 1984a; Dineley and Liu 1984; Janvier 1996), and Phyllolepida are derived Actinolepidoidei (Dupret et al. 2007, 2009, 2017b; Dupret and Zhu 2008). Petalichthyida, Ptyctodontida, and Acanthothoraci are separate orders of Placodermi, and Stegoselachii are excluded (if valid at all).
Alongside his first description of the cranial material of Palaeacanthaspis vasta, Stensiö (1944) also erected the order Acanthothoraci, which he considered as belonging to his division Euarthrodira and did not constitute a separate order. He based his conclusions on the morphology of the dermal shoulder girdle, although he admitted himself (see Stensiö 1944: 75–76) that the knowledge about this feature was deficient. He also believed that the Acanthothoraci morphologically linked Dolichothoraci and Petalichthyida.
Our perception of the group Acanthothoraci has changed a lot since its erection: it is now an order distinct from either Arthrodira and Petalichthyida. However, the consistency of Acanthothoraci as a homogenous group is challenged. If it has been suggested that Acanthothoraci is not a monophyletic group for approximately 25 years (Janvier 1996), its paraphyletic vs. polyphyletic nature still remains uncertain.
It is among other things remarkable that the presence of a premedian plate, homologous with that of Antiarchi, is found in some Acanthothoraci. Ørvig (1975: 45) refers to this plate as a median prerostral plate in Romundina and Radotina Gross, 1950, and only cautiously homologises it to the premedian plate of Antiarchi, following Moy-Thomas and Miles’s hypothesis (1971), who are the first authors to propose this interpretation. Ørvig (1975) however, denied the homology with the “prerostral plate Ra” of Stensiö (1963a, 1969) in Arthrodira sensu stricto (i.e., their Eurarthrodira); this “prerostral plate Ra” is now considered as a weak or vestigial premedian plate (Dupret et al. 2017a: 34). To summarize, a plate homologous and named as the premedian plate of Antiarchi was unknown in Acanthothoraci before Moy-Thomas and Miles work (1971).
The term “Acanthothoraci” is still vastly used today, although more by commodity and tradition rather than as a monophyletic ensemble. In other words, Acanthothoraci is used to assess, in a practical way, a placoderm taxon that is neither arthrodire, petalichthyid, ptyctodont, rhenanid, antiarch, nor maxillate placoderm. The discovery of new acanthothoracid material and/or the possibility to use new technologies such as synchrotron micro-CT scanning sheds more and more lights on the diversity and the anatomical complexity of this order. Precise phylogenetic relationships within the group and among placoderms are still debated.
A new interpretation of Stensiö’s (1944) description.—Being unaware in 1944 of the diversity of forms and the cranial anatomy of the Acanthothoraci, and likely because the general shape resembles more of an Arthrodira than an Antiarchi, Stensiö (1944) interpreted the most anterior dermal element of the skull of his specimen of P. vasta NRM.P5003 as the ethmoid capsule. The discovery of a new specimen attributed to this species invites us to propose herein another interpretation. The attribution of new material to Palaeacanthaspis by Stensiö (1944) rested on very few features such as a ratio of the anterolateral plate more similar to that of Brotzen’s (1934) material rather than any other “acanthaspid” (i.e., “Dolichothoraci”; see above for the history of these groups), and same “Dittonian” in age from the Chortkov series (Stensiö 1944: 3–4, 16, 26).
The skull of P. vasta ZPAL Ag. V/1 is shorter than that of NRM.P.5003 because the former lacks its ethmoid and pre-ethmoid components (i.e., rostropineal capsule and premedian plates). The tubercules of NRM.P.5003 are as wide as long, indicating that the specimen was not laterally compressed. One supraorbital sensory line groove is identified in NRM.P.5003 (Fig. 1B), indicating the location of the preorbital plate. A boundary between the preorbital plates is also identified near the midline of the specimen. An even clearer boundary delimits the preorbital plates from a more anterior element which is here interpreted as the rostronasopineal capsule (Fig. 1B: RoNa). The latter extends anteriorly until another demarcation with another more anterior plate, which we interpret as the premedian plate (Fig. 1B: PRM). The boundary between the rostropineal capsule and the premedian is clear and corresponds to what Stensiö (1944: 9, fig. 2) interpreted as the “probable position of a ventrally descending lamina of dermal bone which formed the posterior boundary of the ethmoidal caspule”, but that we interpret as the anterior edge of the rostronasal capsule.
This new anatomical interpretation of the skull roof conforms more to that of an Acanthothoraci such as Romundina than that of an Arthrodira such as Kujdanowiapsis which for Stensiö (1944) was a model animal for this group. This updated pattern is taken into account for the completion of the characters related to Palaeacanthaspis in the data matrix.
Comparison between Palaeacanthaspis and other well known “Acanthothoraci”.—The ZPAL Ag. V/1 of Palaeacanthaspis vasta also differs mainly from Romundina stellina (see for example Goujet and Young 2004: fig. 2) by the contact between the two preorbital plates, and its much shorter postpineal plate. Few other minor differences, possibly attributed to individual or specific variations are discussed in the next section (Phylogenetic analysis).
The skull roof of Sudaspis chlupaci Vaškaninová and Ahlberg, 2017, is only known in its posteriormost portion, which is mostly lacking in the present material of Palaeacanthaspis. The two forms seem to differ, however, by the position of the external foramen for the endolymphatic duct on the posterior paranuchal plate which is more mesial in Sudaspis and more lateral in Palaeacanthaspis (see Vaškaninová and Ahlberg 2017: fig. 7A, B).
The central plates of Tlamaspis inopinatus Vaškaninová and Ahlberg, 2017, are much longer and more polygonal than those of the present Palaeacanthaspis, its nuchal plate appears much shorter, and its supraorbital sensory line grooves are very long, and meet in the midline posteriorly to the post pineal plate and on the nuchal plate (see Vaškaninová and Ahlberg 2017: fig. 4).
The skull roof of Radotina being substantially tessellated is hardly comparable to that of Palaeacanthaspis and other forms (Gross 1958, 1959; Vaškaninová and Ahlberg 2017).
The current specimen of P. vasta differs from what is observed in Arabosteus variabilis Olive, Lelièvre, Goujet, and Janjou, 2011, by the absence in the former of a posterior central plate (but some specimens of A. variabilis show a unique large central plate rather than two smaller), very short supraorbital sensory line grooves, the presence of a post pineal plate, a more slender nuchal plate (see Olive et al. 2011).
As a conclusion, P. vasta seems closer morphologically to the Canadian species of Romundina rather than to the European species of Radotina, Tlamaspis, and Sudaspis. The Acanthothoraci from the Prague Basin appear to be slightly younger (late Lochkovian and early Pragian; Vaškaninová and Ahlberg 2017: fig. 1) than Palaeacanthaspis vasta from Podolia (mainly middle Lochkovian; Drygant and Szaniawski 2012: fig. 3) and Romundina stellina (middle Gedinian = middle Lochkovian after Ørvig 1975; Denison 1978; Carr 1995). Arabosteus variabilis from the Emsian Jauf Formation of the then Gondwanan Saudi Arabia (Lelièvre et al. 1994, 1995) is even younger.
Phylogenetic analysis.—The consensus tree obtained from the new data set (Fig. 3, SOM 1) presents a quite different resolution of “Placodermi” from the one obtained by Vaškaninová et al. 2020 (SOM 1: fig. 1). We present here only a simplified version of the tree and more complete information is available in SOM. Most of the previously resolved relationships within the [Brindabellaspis–Entelognathus] bracket collapsed in iterative polytomies in our analysis. Within this bracket, the Antiarchi remain the most basal sister-group for all other gnathostomes. Palaeacanthaspis, Romundina, a clade composed of Radotina, Kosoraspis, Tlamaspis, and CPW 9 are in polytomy with a clade containing completely unresolved Arthrodira, monophyletic Phyllolepida, a monophyletic ensemble composed of paraphyletic Petalichthyida and monophyletic Ptyctodontida, and a clade containing monophyletic Rhenanida themselves sister-group to Entelognathus of Zhu et al. 2013, and all crown gnathostomes.
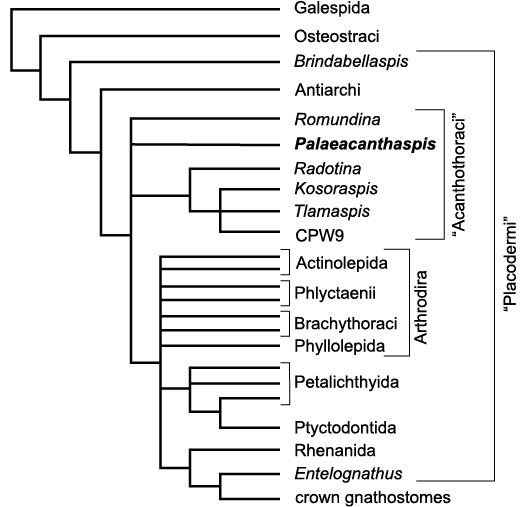
Fig. 3. Simplified strict consensus tree of gnathostomes of n = 18600 most parsimonious trees of L = 961 steps each; strict consensus tree length is Lcs = 1053, its consistency index is CIcs = 0.330 and its retention index is RIcs=0.774. Complete tree with 108 taxa can be consulted in SOM.
As far as the topic of the current article is concerned, it should be retained that Palaeacanthaspis does not have a definite phylogenetic position. This is obviously due to a completeness of 25.08% only for this taxon (84 out of 335 characters could be coded effectively, i.e., without taking into account non-applicable data which are treated as unknown). Its similar looking morphology to Romundina differs in the coding by three characters of weak phylogenetic value (24: presence/absence of a pineal foramen in the skull roof; 194 and 272: posterior extension and commissure of the supraorbital sensory groove). These three characters rely a lot on the amount of specimens at hand, and unfortunately only 2 specimens of Palaeacanthaspis are available, one being incomplete anteriorly and the other poorly preserved. The generalised arthrodire characteristics of Palaeacanthaspis, allied with its 74.92% of specific characters optimisable, probably led to the complete collapse of the Arthrodira clade and its content. It remains, however, certain that Palaeacanthaspis and Romundina belong to an “Acanthothoraci” bracket, most likely paraphyletic.
Poor tree resolution, similarities between some Arthrodira and Palaeacanthaspis that we regard as either a consequence of a lack of available specimens, evolutionary convergence, or characters of weak phylogenetic significance do not preclude a discussion regarding a probable synonymy between the Romundina and Palaeacanthaspis groups.
Could Romundina be a junior synonym of Palaeacanthaspis?—Romundina was erected by Ørvig in 1975, based on cranial and thoracic armour material from the Lochkovian (Siegenian) of the Canadian Arctic Archipelago. Until now, the genus has remained monospecific, with Romundina stellina as sole species. More recent works reveal that more material has since been discovered, and that more than one species could occur (Daniel Goujet, personal communication 2020).
Palaeacanthaspis was erected by Brotzen in 1934, based on fragmentary thoracic armour material from the Lower Devonian of Podolia. This material, owing to the observable plate boundaries can be referred to as an acanthothoracid, despite weathered tubercles not showing their typical stellate aspect. Later, Stensiö (1944) published more material, including a skull roof. This cranial material is, however, quite weathered and only its outline can be distinguished; the plate boundaries or sensory line system cannot be defined. We agree that this material might be included to P. vasta Brotzen, 1934, and it is certainly different in its proportions and ornamentation from that of Dobrowlania podolica Stensiö, 1944, which is also much scarcer, less well preserved, and of uncertain affinity, as already assessed by Stensiö’s work (1944: 70–73; see also Denison 1978: 35).
The outline of the preserved portion of the skull roof of the new specimen of P. vasta offers similarities with those of P. vasta from Podolia illustrated by Stensiö (1944) and R. stellina from the Canadian Arctic Archipelago (see Stensiö 1944; Ørvig 1975; Goujet and Young 2004; Dupret et al. 2010, 2014, 2017a).
The dermal ornamentation of the new Podolian specimen of P. vasta consists of stellate turbercules. The plate boundaries are difficult to discern, but when visible are similar to those observed in R. stellina. Major differences occur in the much smaller post pineal plate not precluding contact between the preorbital plates, the presence of a profundus sensory line groove, and the absence of a central sensory line groove in P. vasta. This anatomical attribution relies on the anteromedially rather than posteromedially direction of the groove.
It seems that the two central plates are separated by a postpineal and nuchal plate as it occurs in R. stellina, the exact contact between the two latter plates being however unclear. As mentioned above, the postpineal of P. vasta seems much shorter than the one of R. stellina.
The general organisation of the orbital part of the neurocranium is similar between the new specimen of P. vasta and that of R. stellina. However, some differences occur. The opening for the jugular vein is very small and located under the path of the second and third branches of the trigeminal nerve and on the lateral edge of the posteroventral myodome in P. vasta, while it is much bigger and located over a V3+VII common trunk in R. and disconnected from the same myodome (Dupret et al. 2014, 2017a).
The presence of a postmarginal sensory line groove in P. vasta compared to its absence in R. stellina constitutes another difference between the two taxa. It is unsure, however, if this difference reflects an individual variation or a preservation problem rather than a reliable morphological marker and hence is of limited importance on a taxonomic point of view. It is nevertheless noteworthy that among “Acanthothoraci” such a postmarginal sensery line groove may also be visible in some forms of Arabosteus variabilis only (Olive et al. 2011: fig. 2A, B).
The comparison between the subethmoid area of the neurocranium does not bring more information because its anterior part is lacking in the new specimen of P. vasta. The only striking difference would consist in the median connection between the paired internal carotid grooves in P. vasta, unknown in R. stellina, assuming that this homology is correct.
Based on these observations, we consider that the differences listed above are sufficient to assess that Palaeacanthaspis vasta Brotzen, 1934, is not synonymous with Romundina stellina Ørvig, 1975. We nevertheless do not entirely rule out the possibility that these two genera still could be synonymous; testing this hypothesis will, however, require an extensive study of yet unpublished acanthothoracid material from the Canadian Arctic Archipelago (Daniel Goujet, personal communication 2020) or new material of Palaeacanthaspis.
Conclusions
A new specimen of the acanthothoracid species Palaeacanthaspis vasta Brotzen, 1934, from the Lochkovian (Lower Devonian) of Podolia is described, and its position within an Acanthothoraci phylogenetic bracket is confirmed. Despite general similarities, the synonymy between P. vasta and Romundina stellina Ørvig, 1975, is ruled out. This specimen invites for an implementation of the growing library on early and lower vertebrate neurocrania. An analysis of the internal structures of this specimen is hence in progress.
Authors’ contributions
VD: description, interpretations, discussion, phylogenetic analysis, first and final draft, writing, figures; HS: found the specimen and established its stratigraphic age based on the co-occurring conodonts, remarks to the manuscript; PS: description, writing, discussion; MD: description, interpretation.
Aknowledgements
The authors thank Florian Witzmann (Museum für Naturkunde, Berlin, Germany) and Thomas Mörs (Naturhistoriska riksmuseet, Stockholm, Sweden) for sending information about the material in their collections during the difficult access period of 2020. We are also grateful to Daniel Goujet (Museum national d’Histoire naturelle, Paris, France) who discussed with us about the Romundina material from the Canadian Arctic Archipelago, and to Andrzej Baliński (ZPAL) who took the photographs of the new specimen. This work was funded by grants from the Polish National Science Centre attributed to Hubert Szaniawski (2016/21/B/ST10/02334) and Michał Ginter–Piotr Szrek (2019/35/B/ST10/01505). We deeply thank Sébastien P. Olive (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) and Philippe Janvier (CNRS-MNHN-Sorbonne Université, Paris, France) for their reviews and constructive comments, and Hannah Byrne (Uppsala University, Sweden) for her English language expertise.
References
Brazeau, M.D. 2009. The braincase and jaws of a Devonian “acanthodian” and modern gnathostome origins. Nature 457: 305–308. Crossref
Brotzen, F. 1933. Die silurischen und devonischen Fischvorkommen in Westpodolien I. Palaeobiologica 5: 423–466.
Brotzen, F. 1934. Die silurischen und devonischen Fischvorkommen in Westpodolien II. Paleobiologica 6: 111–131.
Brotzen, F. 1936. Beiträge zur Vertebratenfauna des Westpodolischen Silurs und Devons. I. Protaspis arnelli n. sp., und Brachipteraspis n. gen. latissima Zych. K. Svenska Vetenskapsakad. Arkiv för zoologi 28A (22): 1–52.
Carr, R.K. 1995. Placoderm diversity and evolution. Bulletin du Muséum National d’Histoire Naturelle de Paris, 4ème série 17: 85–125.
Davis, S.P., Finarelli, J.A., and Coates, M.I. 2012. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature 486: 247–251. Crossref
Denison, R.H. 1978. Placodermi. 128 pp. Gustav Fischer Verlag, Stuttgart.
Dineley, D.L. and Liu, Y.-H. 1984. A new actinolepid arthrodire from the Lower Devonian of Arctic Canada. Palaeontology 27: 875–888.
Drygant, D. and Szaniawski, H. 2012. Lochkovian conodonts from Podolia, Ukraine,and their stratigraphic significance. Acta Palaeontologica Polonica 57: 833–861. Crossref
Dumbrava, M. and Blieck, A. 2005. Review of the pteraspidiform heterostracans (Vertebrata, Agnatha) from the Devonian of Podolia, Ukraine, in the Theodor Vascautanu collection, Bucharest, Romania. Acta Palaeontologica Romaniae 5: 163–171.
Dupret, V. 2003a. Etude anatomique des genres Kujdanowiaspis Stensiö 1942 et Erikaspis nov. gen. (Placodermi Arthrodira “Actinolepida”) du Dévonien inférieur de Podolie (Ukraine). Nouvelle proposition de la phylogénie des Arthrodira. 403 pp. Muséum national d’Histoire naturelle, Paris.
Dupret, V. 2003b. The genus Kujdanowiaspis Stensiö, 1942. Contribution in the phylogeny of actinolepids (Placodermi: Arthodira). In: H.P. Schultze, E. Luksevics, and D. Unwin (eds.), The Gross Symposium 2: Advances in Palaeoichthyology & IGCP 491 Project: Middle Palaeozoic Vertebrate Biogeography, Palaeogeography, and Climate, 21. Faculty of Geographical and Earth Sciences, University of Latvia, Riga.
Dupret, V. 2004. The phylogenetic relationships between actinolepids (Placodermi: Arthrodira) and other arthrodires (phlyctaeniids and brachythoracids). Fossils and Strata 50: 40–55.
Dupret, V. 2010. Revision of the genus Kujdanowiaspis Stensiö, 1942 (Placodermi, Arthrodira, “Actinolepida”) from the Lower Devonian of Podolia (Ukraine). Geodiversitas 32: 5–63. Crossref
Dupret, V. and Blieck, A. 2009. The Lochkovian–Pragian boundary in Podolia (Lower Devonian, Ukraine) based upon placoderm vertebrates. Comptes Rendus Geoscience (Paris) 341: 63–70. Crossref
Dupret, V. and Zhu, M. 2008. The earliest phyllolepid (Placodermi, Arthrodira), Gavinaspis convergens, from the late Lochkovian (Lower Devonian) of Yunnan (South China). Geological Magazine 145: 257–278. Crossref
Dupret, V., Carls, P., Martinez-Pérez, C., and Botella, H. 2011. First Perigondwanan record of actinolepids (Vertebrata: Placodermi: Arthrodira) from the Lochkovian (Early Devonian) of Spain and its palaeobiogeographic significance. Palaeogeography, Palaeoclimatology, Palaeoecology 310: 273–282. Crossref
Dupret, V., Goujet, D., and Mark-Kurik, E. 2007. A new genus of placoderm (Arthrodira: “Actinolepida”) from the Lower Devonian of Podolia (Ukraine). Journal of Vertebrate Paleontology 27: 266–284. Crossref
Dupret, V., Sanchez, S., Goujet, D., and Ahlberg, P.E. 2017a. The internal cranial anatomy of Romundina stellina Ørvig, 1975 (Vertebrata, Placodermi, Acanthothoraci) and the origin of jawed vertebrates. Anatomical atlas of a primitive gnathostome: new insights on the evolution of gnathostomes and early vertebrate structures. PLOS ONE 12 (2): e0171241. Crossref
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P., and Ahlberg, P. 2010. Bone vascularization and growth in placoderms (Vertebrata): the example of the premedian plate of Romundina stellina Ørvig, 1975. Comptes Rendus Palevol 9: 369–375. Crossref
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P., and Ahlberg, P.E. 2014. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature 507: 500–503. Crossref
Dupret, V., Zhu, M., and Wang, J.-Q. 2009. The morphology of Yujiangolepis liujingensis (Placodermi, Arthrodira) from the Pragian of Guangxi (South China) and its phylogenetic significance. Zoological Journal of The Linnean Society 157: 70–82. Crossref
Dupret, V., Zhu, M., and Wang, J.-Q. 2017b. Redescription of Szelepis Liu, 1981 (Placodermi, Arthrodira), from the Lower Devonian of China. Journal of Vertebrate Paleontology 37 (2): e1312422. Crossref
Goloboff, P.A. and Catalano, S.A. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32: 221–238. Crossref
Goujet, D. 1984a. Les poissons placodermes du Spitsberg. Arthrodires Dolichothoraci de la Formation de Wood Bay (Dévonien inférieur). 284 pp. Editions du CNRS, Paris.
Goujet, D. 1984b. Placoderm interrelationships: a new interpretation, with a short review of placoderm classifications. Proceedings of the Linnean Society of New South Wales 107: 211–243.
Goujet, D. and Young, G.C. 2004. Placoderm anatomy and phylogeny: new insights. In: G. Arratia, M.V.H. Wilson, and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates, 109–126. Verlag Dr. Friedlich Pfeil, München.
Gross, W. 1950. Die päleontologische und stratigraphische Bedeutung der Wirbeltierfaunen des Old Reds und der marinen altpaläozoischen Schichten. Abhandlungen der Deutschen Akademie der Wissenschaften zu Berlin, Mathematisch-naturwissenschaftliche Klasse 1: 1–130.
Gross, W. 1958. Über die älteste Arthrodiren-Gattung. Notizblatt des Hessischen Landesamtes für Bodenforschung zu Wiesbaden 86: 7–30.
Gross, W. 1959. Arthrodiren aus dem Obersilur der Prager Mulde. Palaeontographica A 113: 1–135.
Janvier, P. 1988. Un nouveau céphalaspide (Osteostraci) du Dévonien inférieur de Podolie (R.S.S. d’Ukraine) [in French with Polish summary]. Acta Palaeontologica Polonica 33: 353–358.
Janvier, P. 1995. Preliminary description of Lower Devonian Osteostraci from Podolia (Ukrainian S.S.R.). Bulletin British Museum (Natural History) Geology 38: 309–334.
Janvier, P. 1996. Early Vertebrates. 393 pp. Oxford Science Publications, Oxford.
Karatajūtė-Talimaa, V.N. 1968. New thelodonts, heterostracans and arthrodires from the Chortkov horizon of Podolia [in Russian]. Nauka 1: 33–42.
Karatajūtė-Talimaa, V.N. 1997. Chondrichthyan scales from Lochkovian (Lower Devonian) of Podolia (Ukraine). Geologija 22: 5–17.
King, B., Qiao, T., Lee, M.S.Y., Zhu, M., and Long, J.A. 2016. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Systematic Biology 6: 499–516. Crossref
Lelièvre, H., Janjou, D., Halawani, M.A., Janvier, P., Al-Muallem, M.S., Wyns, R., and Robelin, C. 1994. Nouveaux vertébrés (Placodermes, Acanthodiens, Chondrichtyens et Sarcoptérygiens) de la formation de Jauf (Dévonien inférieur, région de Al Huj, Arabie Saoudite). Comptes Rendus de l’Académie des Sciences, Paris, (II) 319: 1247–1254.
Lelièvre, H., Janvier, P., Janjou, D., and Halawani, M. 1995. Nefudina qalibahensis nov. gen., nov. sp. Un rhénanide (Vertebrata, Placodermi) du Dévonien Inférieur de la formation Jauf (Emsien) d’Arabie Saoudite. Geobios MS 19: 109–115. Crossref
Maddison, D.R. and Maddison, W.P. 2020. Zephyr: a Mesquite package for interacting with external phylogeny inference programs. Version 3.11. http://zephyr.mesquiteproject.org.
Maddison, D.R. and Will, K.R. 2020. TNT Tree Searcher. In: D.R. Maddison and W.P. Maddison (eds.), Zephyr: A Mesquite Package for Interacting with External Phylogeny Inference Programs. Version 3.1. http://zephyr.mesquiteproject.org/TNTOverview.html.
Maddison, W.P. and Maddison, D.R. 2019. Mesquite: A Modular System for Evolutionary Analysis. Version 3.61 http://www.mesquiteproject.org.
McCoy, F. 1848. On some new fossil fish of the Carboniferous period. Annals and Magazine of Natural History 2 (2): 1–10. Crossref
Moy-Thomas, J.A. and Miles, R.S. 1971. Paleozoic fishes (deuxième édition). 259 pp. Chapman & Hall, London. Crossref
Olive, S., Goujet, D., Lelievre, H., and Janjou, D. 2011. A new placoderm fish (Acanthothoraci) from the Early Devonian Jawf Formation (Saudi Arabia). Geodiversitas 33: 393–409. Crossref
Ørvig, T. 1975. Description, with special reference to the dermal skeleton, of a new radotinid arthrodire from the Gedinnian of Arctic Canada. Extrait des Colloques internationaux du Centre National de la Recherche Scientifique. Problèmes actuels de Paléontologie. Evolution des Vertébrés 218: 41–71.
Qiao, T., King, B., Long, J.A., Ahlberg, P.E., and Zhu, M. 2016. Early gnathostome phylogeny revisited: multiple method consensus. PLOS ONE 11 (9): e0163157. Crossref
Stensiö, E. 1934. On the heads of certain Arthrodires. Kunglica Svenska Vetenskapsakademiens Handlingar, Ser. 3 13 (5): 1–79.
Stensiö, E. 1942. On the snout of Arthrodires. Kungliga Svenska Vetenskaps Akademiens Handlingar 20 (3): 1–32.
Stensiö, E. 1944. Contributions to the knowledge of the vertebrate fauna of the Silurian and Devonian of Podolia. II. Note on two Arthrodires from the Downtonian of Podolia. Arkiv för Zoologi 35 (9): 1–83.
Stensiö, E. 1945. On the heads of certain arthrodires. II. On the cranium and cervical joint of the Dolichothoracids (Acanthaspida). Kungliga Svenska Vetenskapsakademiens Handlingar 22 (1): 1–70.
Stensiö, E. 1959. On the pectoral fin and shoulder girdle of the Arthrodires. Kungliga Svenska Vetenskapsakademiens Handlingar, Ser. 4 8 (1): 1–229.
Stensiö, E. 1963a. Anatomical studies on the arthrodiran head. Part 1. Preface, geological and geographical distribution, the organisation of the arthrodires, the anatomy of the head in the Dolichothoraci, Coccosteomorphi and Pachyosteomorphi. Taxonomic appendix. Kungliga Svenska Vetenskapsakademiens Handlingar, Ser. 4 9 (2): 1–419.
Stensiö, E. 1963b. The brain and cranial nerves in fossil, lower craniate vertebrates. Skrifter utgitt av Det Norske Videnskaps-Akademi i Oslo 13: 1–120.
Stensiö, E. 1969. Arthrodires. In: J. Piveteau (ed.), Traité de Paléontologie, 71–693. Masson, Paris.
Vaškaninová, V. and Ahlberg, P.E. 2017. Unique diversity of acanthothoracid placoderms (basal jawed vertebrates) in the Early Devonian of the Prague Basin, Czech Republic: A new look at Radotina and Holopetalichthys. PLOS ONE 12 (4): e0174794. Crossref
Vaškaninová, V., Chen, D., Tafforeau, P., Johanson, Z., Ekrt, B., Blom, H., and Ahlberg, P.E. 2020. Marginal dentition and multiple dermal jawbones as the ancestral condition of jawed vertebrates. Science 369: 211–216. Crossref
Voichyshyn, V.K. [Voičyšyn, V.K.] 1994. New species of the genus Mimetaspis (Agnatha) from the Lower Devonian of Podolia [in Russian]. Paleontologičnyj Zbirnik 30: 19–24.
Voichyshyn, V.K. [Voičyšyn, V.K.] 1997. Taphoichthyologic assemblages of the Lower Devonian of Podolia [in Russian]. Vestnik zoologii 31 (3): 33–39.
Voichyshyn, V. and Szaniawski, H. 2012. Acanthodian jaw bones from Lower Devonian marine deposits of Podolia, Ukraine. Acta Palaeontologica Polonica 57: 879–896. Crossref
Voichyshyn, V. and Szaniawski, H. 2018. New ischnacanthiform jaw bones from the Lower Devonian of Podolia, Ukraine. Acta Palaeontologica Polonica 63: 327–339.
Voichyshyn, V.K. [Voičyšyn, V.K.] 1998. A new specimen of the genus Zychaspis (Agnatha) from the Dnister Series (Lower Devonian) of Podolia [in Russian]. Paleontologičnyj Zbirnik 32: 25–29.
Voichyshyn, V.K. 1999. The new forms of pteraspids (Agnatha, Heterostraci) from Podolian Early Devonian. Vestnik zoologii 33 (3): 47–56.
Voichyshyn, V.K. [Voičyšyn, V.K.] 2001. Localities with ichthyofaunas from the Lower Devonian of Podolia [in Russian]. Paleontologičnyj Zbirnik 33: 134–143.
Voichyshyn, V.K. 2011. The Early Devonian armoured agnathans of Podolia, Ukraine. Palaeontologia Polonica 66: 3–211. Crossref
Woodward, A.S. 1891. Catalogue of the Fossil Fishes in the British Museum of Natural History. Part II. Containing the Elasmobranchii (Acanthodii), Holocephali, Ichthyodorulites, Ostracodermi, Dipnoi, and Teleostomi (Crossopterygii), and Chondrostean Actinopterygii. 567 pp. British Museum of Natural History, London. Crossref
Young, G.C. 2010. Placoderms (armored fish): dominant vertebrates of the Devonian period. Annual Review of Earth and Planetary Sciences 38: 523–550. Crossref
Zhu, M., Ahlberg, P.E., Pan, Z.-H., Zhu, Y.-A., Qiao, T., Zhao, W.-J., Jia, L.-T., and Lu, J. 2016. A Silurian maxillate placoderm illuminates jaw evolution. Science 354: 334–336. Crossref
Zhu, M., Yu, X.-B., Ahlberg, P., Choo, B., Lu, J., Qiao, T., Qu, Q.-M., Zhao, W.-J., Jia, L.-T., Blom, H., and Zhu, Y.-A. 2013. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502: 188–193. Crossref
Zych, W. 1927. Old-Red podolski. Prace Polskiego Instytutu Geologicznego 2 (1): 1–65.
Zych, W. 1930. Sprawozdanie z badań na Podolu w r. 1929. Posiedzenia Naukowe Państwowego Instytutu Geologicznego 27: 67–68.
Zych, W. 1931. Fauna ryb dewonu i downtonu Podola. Pteraspidomorphi: Heterostraci. 92 pp. Drukarnia Słowa Polskiego, Lwów.
Zych, W. 1937. Cephalaspis kozlowskii n. sp. from the Downtonian of Podole (Poland). Archiwum Towarzystwa Naukowego we Lwowie 9: 46–96.
Acta Palaeontol. Pol. 66 (2): 337–347, 2021
https://doi.org/10.4202/app.00857.2020