

A large pterosaur femur from the Kimmeridgian, Upper Jurassic of Lusitanian Basin, Portugal
FILIPPO BERTOZZO, BRUNO CAMILO DA SILVA, DAVID MARTILL, ELSA MARLENE VORDERWUELBECKE, TITO AURELIANO, REMMERT SCHOUTEN, and PEDRO AQUINO
Bertozzo, F., Camilo da Silva, B., Martill, D., Vorderwuelbecke, E.M., Aureliano, T., Schouten, R., and Aquino, P. 2021. A large pterosaur femur from the Kimmeridgian, Upper Jurassic of Lusitanian Basin, Portugal. Acta Palaeontologica Polonica 66 (4): 815–825.
The pterosaur fossil record in Portugal is scarce, comprising mainly isolated teeth and rare postcranial material. Here, we describe a well-preserved right proximal femur of a pterodactyloid pterosaur from the Kimmeridgian, Upper Jurassic Praia da Amoreira–Porto Novo Formation of Peniche, Portugal. It is noteworthy for its relatively large size, compared to other Jurassic pterosaurs. It shows affinities with dsungaripteroids based on a combination of features including the bowing of the shaft, the mushroom-like cap of the femoral head, and the distinctly elevated greater trochanter. The femur has a relatively thinner bone wall compared to dsungaripterids, and is more similar to basal dsungaripteroids. A histological analysis of the bone cortex shows it had reached skeletal maturity. The preserved last growth period indicates fast, uninterrupted growth continued until the final asymptotic size was reached, a growth pattern which could best be compared to pterodactyloid femora from the Early Cretaceous. The specimen is the second confirmed report of a dsungaripteroid from the Jurassic, and it is the first record of this group from the Iberian Peninsula.
Key words: Pterosauria, Dsungaripteroidea, histology, Late Jurassic, Kimmeridgian, Portugal.
Filippo Bertozzo [fbertozzo01@qub.ac.uk], School of Natural and Built Environment, Queen’s University Belfast, Belfast, UK; CI2Paleo, Sociedade de Historia Natural, Travessa Florêncio Augusto Chagas nº8B, R/C, 2560-230 Torres Vedras, Portugal.
Bruno Camilo da Silva [laboratorio@alt-shn.org], CI2Paleo, Sociedade de Historia Natural, Travessa Florêncio Augusto Chagas nº8B, R/C, 2560-230 Torres Vedras, Portugal; European Centre of Paleontology, Institute of Biology, Laboratory of Paleobiology, University of Opole, ul.Oleska 48, 45-052 Opole, Poland; Associação Geoparque Oeste. Rua João Luis de Moura nº95, 2530-158 Lourinhã, Portugal.
David Martill [david.martill@port.ac.uk], School of the Environment, Geography and Geosciences, University of Portsmouth, PO1 3QL, UK.
Elsa Marlene Vorderwuelbecke [emvorderwuelbecke@gmail.com], Division of Paleontology, Institute of Geosciences, University of Bonn, Bonn, Germany.
Tito Aureliano [aureliano.tito@gmail.com], Institute of Geosciences, University of Campinas, Campinas, Brazil; Dinosaur Ichnology and Osteohistology Laboratory, Federal University of Rio Grande do Norte, Natal, Brazil; CI2Paleo, Sociedade de Historia Natural, Travessa Florêncio Augusto Chagas nº8B, R/C, 2560-230 Torres Vedras, Portugal.
Remmert Schouten [remmertschouten@gmail.com], CI2Paleo, Sociedade de Historia Natural, Travessa Florêncio Augusto Chagas nº8B, R/C, 2560-230 Torres Vedras, Portugal.
Pedro Aquino [pedro@micronsense.com], Micronsense, Metrologia Industrial, Ida Doroana Park, Ponte da Pedra, Leiria, Portugal.
Received 9 November 2020, accepted 20 may 2021, available online 5 November 2021.
Copyright © 2021 F. Bertozzo et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Pterosaur remains are extremely rare in Portugal, despite an extensive area of highly fossiliferous Mesozoic outcrops and a long history of fossil collecting. Pterosaur remains are limited to isolated teeth and rare postcranial material from the Kimmeridgian Alcobaça Formation in the Guimarota coal mine assigned to Pterodactyloidea and Rhamphorhynchidae (e.g., Rhamphorhynchus) (Wiechmann and Gloy 2000), respectively. In the northern Lusitanian Basin, isolated pterosaur teeth have been briefly described from the Andrés fossil site (Alcobaça Formation, Kimmeridgian) and referred to simply as Pterosauria indet. (Malafaia et al. 2010). So far, pterosaur remains in the Central Lusitanian Basin sector are represented by some undescribed isolated, highly slender teeth tentatively assigned to Gnathosaurus and housed at the CI2Paleo from the Sociedade de História Natural (Torres Vedras) collections (BC personal observation), from the Sobral Formation (late Kimmeridgian–lower Tithonian) of Praia Azul, in Torres Vedras. Pterosaur tracks are reported from the Upper Jurassic of Zambujal de Baixo (Azóia Formation, Kimmeridgian) in Sesimbra and Porto das Barcas (Sobral Formation, late Kimmeridgian–lower Tithonian) in Lourinhã, both tentatively assigned to Pteraichnus (Mateus and Milán 2010). Other material is restricted to isolated teeth from the Lower Cretaceous (Papo Seco Formation, lower Barremian) of Cabo Espichel and putatively assigned to Ornithocheiridae and Ctenochasmatoidea (Figueiredo et al. 2020).
Here we describe the osteology and the taphonomic context of a new pterosaur specimen found at Praia da Almagreira (Peniche, Portugal) during Spring 2019 and now accessioned in the collection of the Sociedade de Historia Natural (SHN, Torres Vedras, Portugal), specimen number SHN.013. The specimen is an exquisitely 3D preserved proximal femur and is here compared with other Late Jurassic pterosaurs. In addition, we provide an insight into the skeletal maturity through histological analysis and comment on the paleoecology of the Upper Jurassic of Portugal.
Institutional abbreviations.—BYU, Brigham Young University, Provo, USA; DFMMh, Dino-Park Münchehagen/Verein zur Förderung der niedersächsischen Paläontologie e.V., Germany; HMNS, Houston Museum of Natural Science, Houston, USA; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Science, Beijing, China; MN, Museu Nacional, Universidade Federal do Rio de Janeiro, Brazil; SHN, CI2Paleo at Sociedade de Historia Natural, Torres Vedras, Portugal; SMNK, Staatliches Museum für Naturkunde Karlsruhe, Germany.
Other abbreviations.—EFS, external fundamental system; ICD, cortico-diametral index; LAGs, lines of arrested growth.
Material and methods
The new specimen SHN.013 was found by FB and BCS while prospecting the Paleontological Site PENFER08, registered in the SHN´s Geographical Information System Applied to Paleontology (SIGAP). This site is located in a coastal exposure north of Baleal, in the municipality of Peniche, west Portugal (Fig. 1A–C). The bone was identified in situ, with the proximal portion of the femoral head protruding from the matrix (Fig. 1D). After removing the block from the main site, it was transported to the laboratory of the SHN for further analysis. The specimen was prepared by RS, using an air scribe, and several fragments were glued back together using Paraloid B72. The specimen was photographed using a Sony a5100 mirrorless camera using standard directions. SHN.013 is officially housed at the CI2Paleo from the Sociedade de História Natural (Torres Vedras).
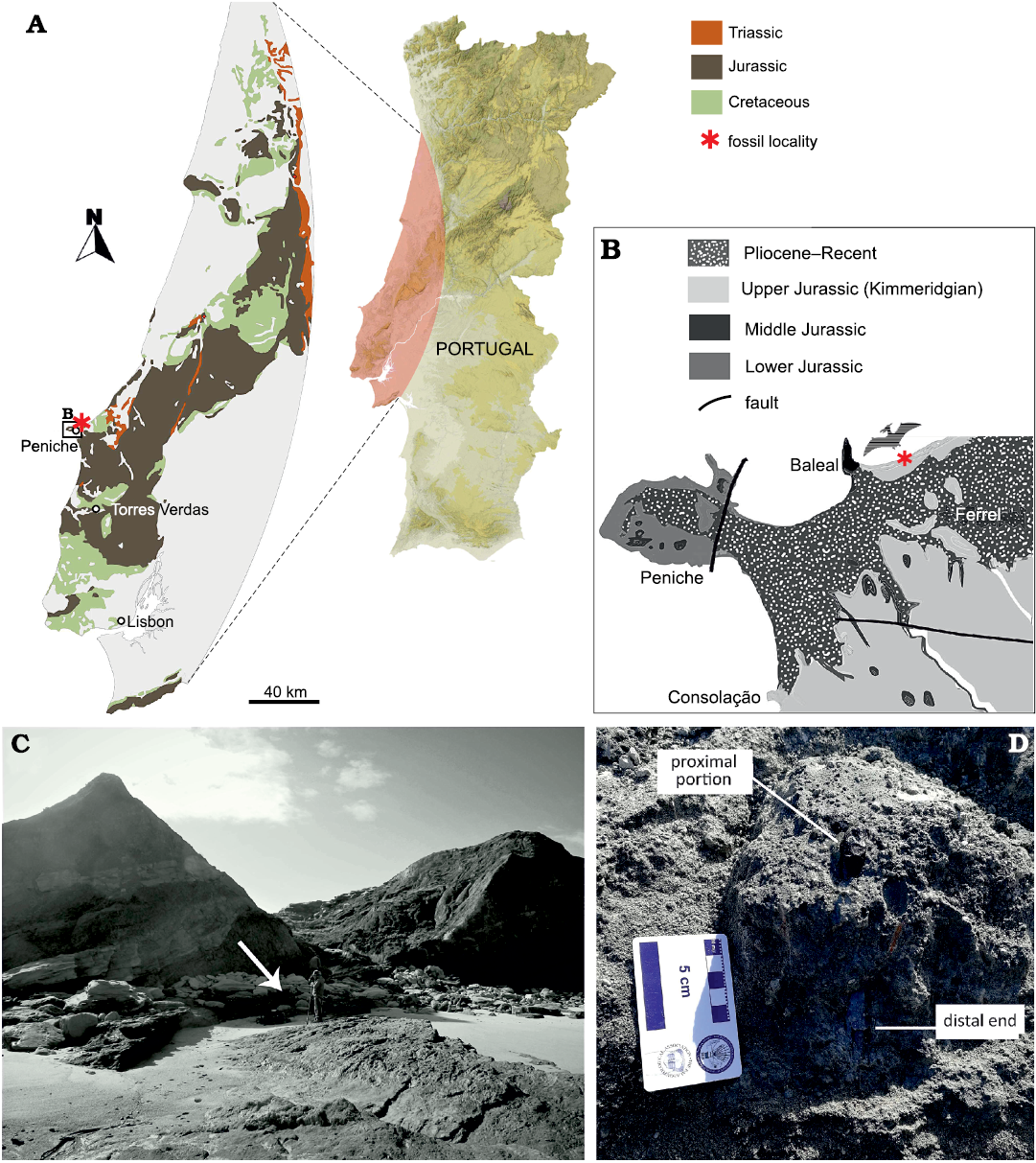
Fig. 1. Geological overview of the locality of SHN.013 in Portugal. A. Map of Portugal, with a focus on the geology of the Peniche area. B. Close-up of the Atlantic coast near Peniche, showing the age of the sediments. C. Specimen excavation site, the arrow points towards the location of the specimen. D. SHN.013 at the moment of discovery, after removing the loose sediment to identify the extension of the bone at its distal end.
A µCT scan of SHN.013 was performed at Micronsense (Leiria, Portugal), to create a 3D model of the specimen for a 3D-print to evaluate sites for histological sampling, and to analyze the internal structure. The µCT scan of the specimen was obtained using a GE VtomeX M 240 system industrial scanner with more than 2400 slices and a voxel size of about 83 µm at Micronsense (Leiria, Portugal) and an acceleration voltage of 200 kV in a current of 500 µA. We followed the workflow published by Aureliano et al. (2020) to generate a three-dimensional reconstruction with the open-source software 3D Slicer v4.10 (Fedorov et al. 2012) and CloudCompare 2.9.1 (CloudCompare 2018). Interested readers should request the 3D model file from the corresponding author.
Histological analysis was performed at the laboratory of the Division of Paleontology, University of Bonn, Germany. Following µCT-rendering, the specimen was sectioned in its mid-shaft region. The section was prepared following standard procedures (see Stein and Sander 2009 and Lambertz et al. 2018). The resulting slide was analyzed using a Leica DLMP polarizing microscope. The pictures were taken under normal (PPL) and cross-polarized (XPL) light using a Leica DFC420 camera mounted on the microscope, using ImageAccess EasyLab (Imagic Bildverarbeitung AG).
Femur length reconstruction was based on the complete correspondent skeletal element of presumed closely related similar-sized taxa to preserve allometric features of the clade. These include the Dsungaripteroidea specimens Dsungaripterus weii IVPP V.2777 (Young 1964), Dungaripteridae indet. DFMMh/FV 500 (Fastnacht 2005), and Mesadactylus ornithosphyos BYU 17214 (McLain and Bakker 2018). We could not apply the regression from Benson et al. (2014) to estimate the wingspan, since the specimen lacks any anterior appendicular elements. Instead, minimum and maximum wingspan estimates were obtained from the linear scaling relationship between femur/wingspan from Dsungaripterus IVPP V.2777 (Young 1964; Witton 2008). Measurements were taken with ImageJ v1.4 software (Schneider et al. 2012). Morphometric results are presented in SOM: tables 1 and 2 in the Supplementary Online Material available at http://app.pan.pl/SOM/app66-Bertozzo_etal_SOM.pdf.
Geological and geographical setting
SHN.013 was found in the Praia da Amoreira–Porto Novo Formation (following the scheme proposed by Manupella et al. 1999) or the Porto Novo member of the Lourinhã Formation (following Hill 1989), latest Kimmeridgian in age. The lithostratigraphic nomenclature of this sector is not well established. Portugal possesses important continental to transitional marine Upper Jurassic stratigraphic units including the Alcobaça Formation (Rauhut 2001), Bombarral and Lourinhã formations (Antunes and Mateus 2003), located within the Lusitanian Basin. This is the most extensive sequence of Mesozoic strata in Portugal. It developed along the Western Iberian Margin and is limited to the east by the Paleozoic basement and by the Berlenga Horst in the west (Rasmussen et al. 1998; Kullberg et al. 2006), corresponding to one of the proto-Atlantic basins formed by crustal extension associated with the fragmentation of Pangaea. A revision of the tectonic evolution of the basin is beyond the scope of this work, and is discussed in detail by others (e.g., Ravnås et al. 1997; Leinfelder 1993; Stapel et al. 1996; Rasmussen et al. 1998; Reis et al. 2000; Kullberg et al. 2006; Schneider et al. 2009; Martinius and Gowland 2011; Taylor et al. 2014). In the Central Lusitanian Basin, the sequence is present in several sub-basins (Consolação to the west, Bombarral-Alcobaça to the northeast, and the Turcifal in the southeast), but is mainly exposed on the coast. In general, the sequence reflects increasing continental sedimentation from the base (upper Kimmeridgian) to the top (uppermost Tithonian–?lowermost Cretaceous). These deposits represent well-vegetated floodplains and sandy fluvial channels, with some coastal plains in the southwest, adjacent to a shallow tropical sea. A well-suited environment for Mesozoic vertebrates, represented by theropods (e.g., Malafaia 2017; Malafaia et al. 2020), sauropods (e.g., Mocho et al. 2017), thyreophorans (e.g., Escaso et al. 2007; Mateus et al. 2009), ornithopods (e.g., Escaso et al. 2014; Rotatori et al. 2020), crocodyliforms (e.g., Russo et al. 2017; Guillaume et al. 2019), choristoderes, amphibians, lepidosauromorphs (Ortega et al. 2009; Malafaia et al. 2010; Guillaume 2018), turtles (e.g., Pérez-García and Ortega 2011, 2013) and mammaliamorphs (e.g., Martin 2005, 2013; Guillaume 2018).
The outcrop where SHN.013 was found constitutes mainly sandstone alternations of variable grain size, mudstone, and siltstone (Fig. 1C, D), and is interpreted as a fluvial to alluvial plain with sandy meanders with reduced sinuosity distributaries (Hill 1989; Martinius and Gowland 2011). The specific silty layer with the specimen contains pyrite nodules and plant debris and is highly bioturbated. This level is interpreted as a pond/swamp environment and represents a lateral margin slide event, which explains the vertical position of the bone. It was found in the same level as crocodylomorphs (e.g., Goniopholidae), turtles (Pleurosternidae), theropods (e.g., Allosauroidea; BC personal observation), and a relatively complete specimen of the dryosaurid ornithopod dinosaur cf. Eousdryosaurus (Camilo da Silva, 2019).
Systematic palaeontology
Pterosauria Kaup, 1834
Monofenestrata Lü, Unwin, Jin, Liu, and Ji, 2009
Pterodactyloidea Plieninger, 1901
Dsungaripteroidea Young, 1964 (sensu Unwin 2003)
Dsungaripteroidea indet.
Fig. 2.
Material.—SHN.013 is a fragmentary femur of 151 mm preserved length, missing its distal portion (Fig. 2). The proximal region is well preserved, although two slightly crushed areas are visible in posterior and lateral views; from Praia da Amoreira–Porto Novo Formation (Upper Jurassic, upper Kimmeridgian) of Praia da Almagreira, Peniche, Portugal.
Description.—Despite being incomplete, the femur is very well preserved and in good overall condition, with only very slight compaction damage. The preserved total length of the specimen is 151 mm. It has a slightly bowed diaphyseal shaft that has a subcircular cross-section for much of its preserved length (Fig. 2A7), The diaphysis maintains a near-constant diameter along all its preserved length, different from pterosaurs like Pteranodon longiceps Marsh, 1876 (Bennett 2001), Dorygnathus banthensis Theodori, 1830, Germanodactylus cristatus Wiman, 1925 (Unwin 2003), and Pterodaustro guinazui Bonaparte, 1970 (Wellnhofer 1978) where the shaft is gently tapered distally. The proximal articular region is well preserved, while the distal end is missing. The femoral head is offset from the main shaft at an angle of 126º, a similar value to DFMMh/FV 500 (Fastnacht 2005), and other pterosaurs (Unwin 2003) except for ornithocheroids (Unwin 2003). The articular surface of the femoral head is separated from the smooth surface of the neck by a well-defined ridge (Fig. 2A2), resulting in a mushroom-like structure as in the dsungaripterid DFMMh/FV 500 (Fastnacht 2005) and in the indet. Dsungaripteroidea HMNS/BB 5027 (McLain and Bakker 2018). The attachment site for the fovea capitis (“terete ligament” in McLain and Bakker 2018) occupies only a small portion of the proximal surface of the femoral head, and it seems to have a smooth surface. In posterior view, below the neck and above the fourth trochanter, a deep crushed area extends diagonally from the proximal region of the femur. However, since its cranial margin is smooth, we hypothesize the presence of a small depression receiving M. pubofemoralis (Witton 2013). The trochanteric rim caps the proximal limit of the greater trochanter, although it seems narrower than that of HMNS/BB 5027. In lateral view, the ridge projects dorsocranially with the cranialmost margin higher than the caudal margin, unlike the evenly curved ridge of HMNS/BB 5027. However, it is similar to DFMMh/FV 500, although the greater trochanter seems shorter in SHN.013. The precise location and nature of the trochanteric fossa are difficult to ascertain due to crushing in the dorsolateral region. The fourth trochanter is elongated proximodistally but strongly reduced in thickness (Fig. 2A1). There is a small foramen below the fourth trochanter, and another one located dorsomedial to the ligament notch. This notch corresponds to the insertion point of M. adductor femoris (Fastnacht 2005), and muscle scars can be seen lining the notch dorsoventrally (Figs. 2A1, A4). The distal end is truncated by a clear transverse fracture, exposing the bone wall and the medullary cavity. In the last third of the shaft, a long groove extends for 25 mm towards the truncated area. It might possibly represent a bite-mark, as the groove is sinuous and it does not share similarities to the elongated muscular ridge. The surface of the bone in the groove is not whitened, as it would be in modern score marks made by digging tools. The presence of smaller, parallel grooves in the distal area (Fig. 2A2) resembles further bite-marks, however fine marks that might have been made by serrations of a theropod tooth are not detected.
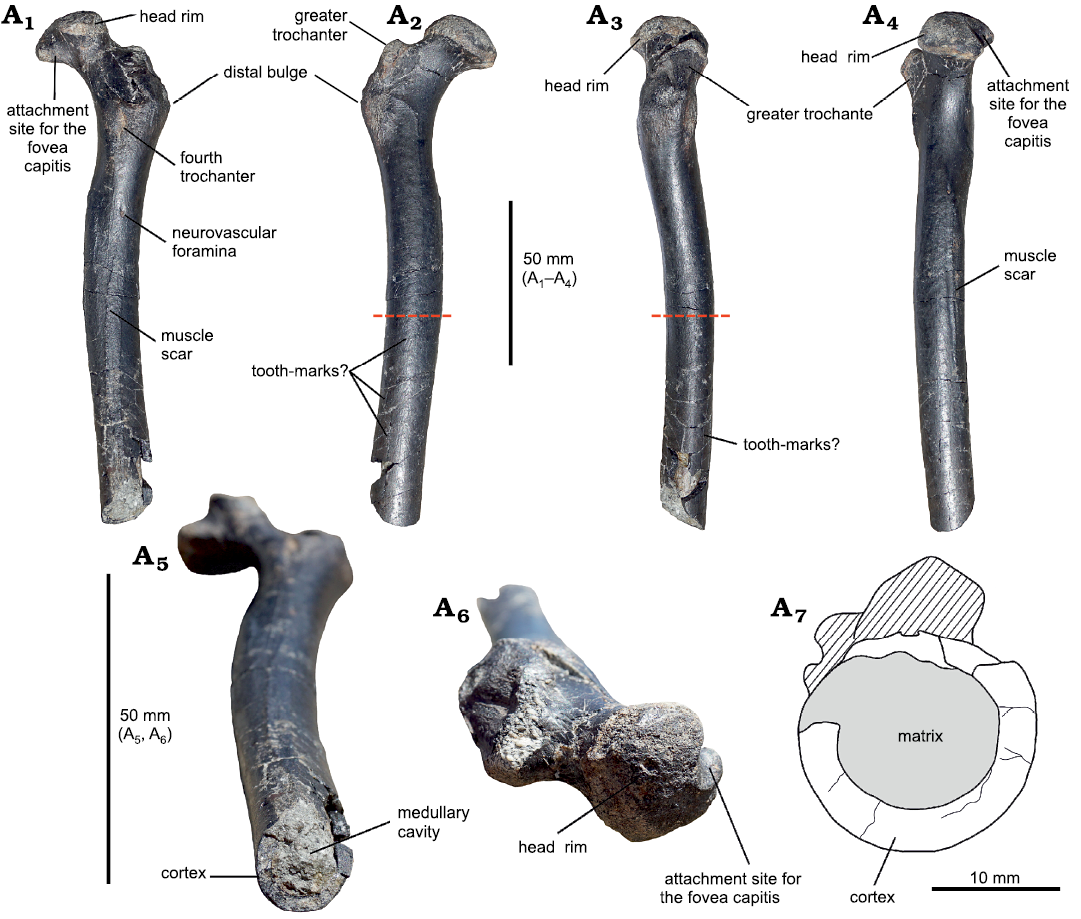
Fig. 2. A partial proximal right femur of a large indeterminate dsungaripteroid pterosaur (SHN.013) from the Kimmeridgian, Upper Jurassic, Praia da Armoreira-Porto Novo Formation, Peniche, Portugal. In ventral (posterior) (A1), dorsal (anterior) (A2), medial (A3), lateral (A4), distal (A3), and proximal (A6) views; A7, drawing of the distal end showing the thickness of the cortex compared to the lumen. Note that the sketch is about twice the scale of the images. The red dashed lines in A2 and A3 indicate the site where the histological section was taken.
Morphometry.—Although only 151 mm long, as preserved, the reconstructed maximum length of the femur is about 244 mm (see SOM: table 1), based on the dsungaripterids Dsungaripterus weii (IVPP V.2777) (Young 1964), Dungaripteridae indet. DFMMh/FV 500 (Fastnacht 2005), Mesadactylus ornithosphyos BYU 17214 (McLain and Bakker 2018), and Noripterus complicidens IVPP RV 73001 (Hone et al. 2017). The reconstructed wingspan of SHN.013 ranges between 240–314 cm (see SOM: table 2).
Histology.—The thin section of the bone, cut orthogonal to the length of the diaphysis, shows a slightly oval outline (ratio of diameters = 1/1.2). The external surface is smooth, but two small protuberances are present (Fig. 3A1, A2). The ratio between the cortex thickness and the total diameter of the bone shaft is 0.29 (cortico-diametral index or ICD, after de Ricqlès et al. 2000; mean cortical thickness ~2 mm). The section reveals a large, distinct medullary cavity which is lined by a thin, continuous layer of lamellar bone, here referred to as the “endosteal lamellar layer”. The rest of the cortex is comprised of primary fibrolamellar bone tissue in a mixture of parallel-fibered and woven bone. Fine Sharpey’s fibers are present at both protuberances (Fig. 3A1, A2). Vascularization is dense and consists of plexiform oriented primary osteons, decreasing in abundance towards the periosteal margin. There are no secondary osteons or other signs of remodeling. There are also no growth marks present except for a few lines of arrested growth (LAGs), concentrated towards the periosteal surface, building an external fundamental system (EFS). In this case, the EFS is also distinguishable by its co-occurring diagenetically induced darker color compared to the rest of the cortex. Diagenesis, in the form of iron staining, seems to have emphasized this layer as seen in the thin section. It is continuous in thickness except for the two regions where the protuberances occur and it consists of approximately five closely spaced LAGs (the amount may vary locally) which have formed around a few of the primary osteons within this region. For a better comparison with data from the literature, we additionally calculated the relative thickness of the specimen measured as the ratio of the cortical thickness to the bone circumference at the midshaft region (~0.046) in SOM: table 3 (Mitchell and Sander 2014).
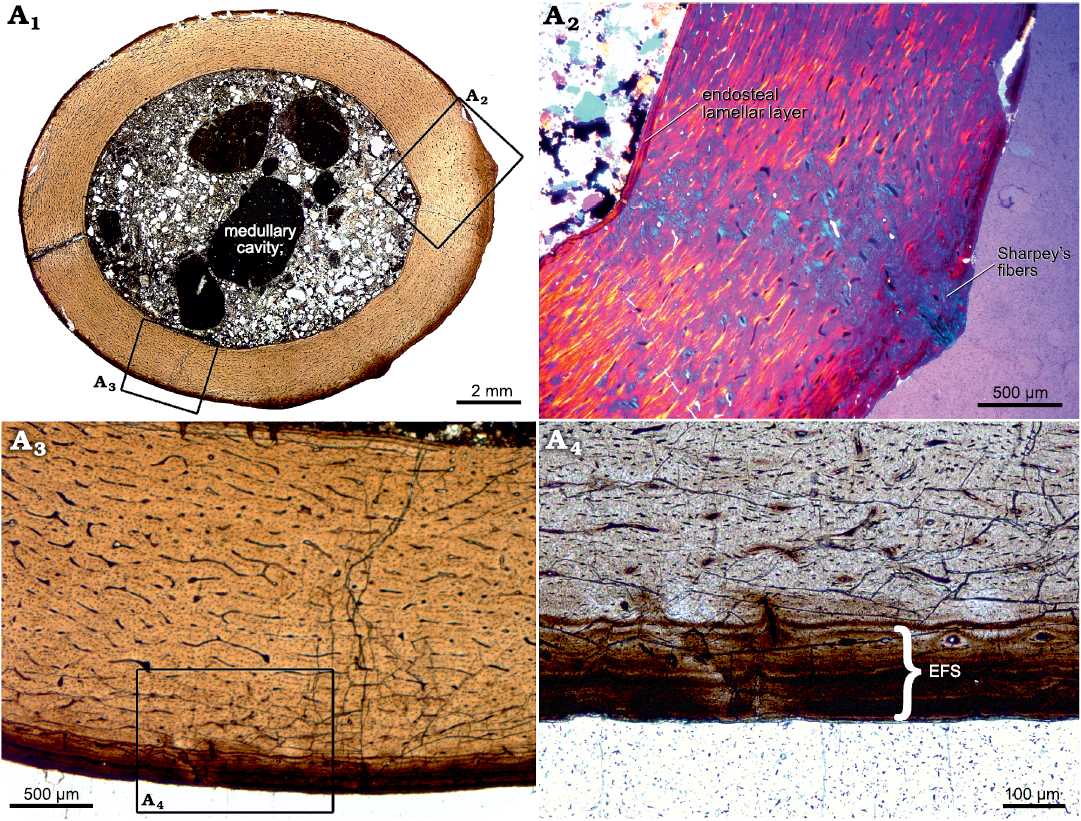
Fig. 3. Histological thin section of femur of indeterminate dsungaripteroid pterosaur species (SHN.013) from the Upper Jurassic, Praia da Armoreira-Porto Novo Formation, Peniche, Portugal. A1, overview of the complete section, revealing densely vascularized, fibrolamellar bone tissue. Growth marks (except for those forming an EFS) and secondary bone tissue are absent; A2, magnified part of the bone cortex. Here, an endosteal lamellar layer which lines the whole medullary cavity can be seen, as well as distinct Sharpey’s fibers which run across the cortex, angled at 36º; A3, magnified part of the fibrolamellar bone tissue, showing a plexiform network of primary osteons. At the periosteal margin, an EFS is clearly visible; A4, detailed picture of the EFS. Abbreviation: EFS, external fundamental system.
Discussion
Specimen SHN.013 is a tridimensional, nearly complete pterosaur femur referable to Dsungaripteroidea, but due to the lack of unambiguous diagnostic features, we regard it as Dsungaripteroidea indet. A few features provide some clues to its phylogenetic relationship with other Late Jurassic and Early Cretaceous pterosaurs. The significant bowing of the femoral shaft, the presence of a mushroom-like cap of the head on the neck, and the distinctly elevated greater trochanter are characters of Dsungaripteroidea (Unwin 2003; Fastnacht 2005; McLain and Bakker 2018). SHN.013 thus appears to have affinities with basal dsungaripteroids, and in terms of its size, with Dsungaripteridae. Similar femora from the Kimmeridgian are reported from the Langenberg Quarry of Germany (Fastnacht 2005) and the Claw Quarry of Como Bluff in the Western United States (McLain and Bakker 2018), but these authors did not establish new species because of the incomplete nature of the material. Another Upper Jurassic pterosaur, Normannognathus wellnhoferi, from the Argiles d’Oceville Formation of France was referred to Dsungaripteroidea by Buffetaut et al. (1998), although it was later considered Monofenestrata incertae sedis (Witton et al. 2015). Normannognathus wellnhoferi comprises the left front portion of the skull and the associated non-articulated lower jaws, but the lack of any postcranial material for this species precludes a direct comparison with SHN.013. In the paratype specimen of Noripterus complicidens IVPP RV 73001 (Young 1973: pl. 5; Hone et al. 2017: fig. 2) the femur appears to be continuously gently curved along its entire length, however in referred specimen IVPP V 4059, a femur broken into two pieces of approximately equal length, the proximal portion is continuously gently curved, but the distal portion appears straight. As only one view is provided, it is difficult to fully evaluate the curvature in this second specimen. In Dsungaripterus weii the femur is gently curved along its entire length (Young 1964: fig. 6), this is especially noticeable on its caudal face when seen in lateral view. In other Dsungaripteroidea, or possible Dsungaripteridae (e.g., Benguela, Domeykodactylus, Lonchognathosaurus, Ordosipterus, Puntanipterus) the femur is unknown.
In Germanodactylidae, a group that formerly was considered close to Dsungaripteridae (Vidovic and Martill 2017, although this position was not reported by Kellner 2003), the femora of both Althmuehlopterus and Germanodactylus are gently curved proximally but straighten distally (Vidovic and Martill 2017: figs. 2 and 3B, respectively).
In Azhdarchoidea, a group often found to be a sister taxon to Dsungaripteroidea (e.g., Pinheiro et al. 2011), the femur is known for several prominent taxa of Tapejaridae and Azhdarchidae. In Volgadraco bogolubovi, only the diaphysis of the femur is preserved, and it is a large, hollow, thin-walled bone, with a bowed shaft (Averianov et al. 2008). Hatzegopteryx has a slightly bowed femur with a thin wall, the fourth trochanter lacks a marked tuberosity, and a small knob is located close to the proximal ridge, perhaps corresponding to the internal trochanter (Buffetaut et al. 2003). A curved femur is recognized also in the azhdarchoids Shenzhoupterus (Lü et al. 2008) and Huaxiapterus, where the femoral head is distinct and set on a narrow neck, angled from the shaft (Lü and Yuan 2005; Lü et al. 2006). A similar condition is also found in the pteranodontian Pteranodon (Bennett 2001). In the tapejarid Sinopterus, however, the shaft is straight (Wang and Zhou 2003). Thin-walled femora are recognized also in Arambourgiania (2 mm thick; Martill and Moser 2018) and Aralazhdarcho, which also possess a well-developed greater trochanter (Averianov 2007).
In the anhanguerid Anhanguera piscator the femur is slender, and although it has a gentle anterior curve when seen laterally or medially, it is subtle (Kellner and Tomida 2000: figs. 50, 52) such that the bone looks almost straight.
The estimated wingspan of SHN.013 ranges between 240–314 cm. Consequently, the Portuguese specimen is bigger than other Late Jurassic taxa such as Harpactognathus gentryii with an estimated wingspan of 2.5 m (skull/skeleton scaling by Carpenter et al. 2003). Therefore, SHN.013 represents one of the largest pterosaurs of the Jurassic, second only to a large Swiss pterosaur, with a wingspan calculated at approximately 4 m (Meyer and Hunt 1999). Pterosaur body size evolution increased dramatically during the Cretaceous, with regression models showing a clear shift in body size by the end of the Jurassic (Benson et al. 2014), with SHN.013 corroborating these data.
The bone histology of SHN.013 corresponds well with previous histological analyses of pterosaurs in general, which are primarily defined by rapid growth and thin bone walls, the latter being a result of high resorption rates in the early stages of growth (de Ricqlès et al. 2000; Steel 2008). Here, fast growth is inferred from the exclusive presence of fibrolamellar bone tissue with dense vascularization (de Margerie et al. 2002; Padian and Lamm 2013). The presence of an EFS indicates that this individual attained skeletal maturity (de Ricqlès et al. 2000; Woodward et al. 2011; Andrade et al. 2015), therefore growth is considered to have terminated. This is relevant since it indicates that the histological sections record the final growth period of the specimen, to which we can assign the displayed histological properties accordingly. An EFS and correspondingly a determinate growth strategy has been reported for some pterodactyloid pterosaurs (i.e., Padian et al. 1995; de Ricqlès et al. 2000; Sayão 2003; Steel 2008; Kellner et al. 2013; Aureliano et al. 2014, Kellner et al. 2019), but it has also been considered that some pterosaurs may have had a form of indeterminate growth (e.g., see Prondvai et al. 2012; Dalla Vecchia 2018). In this context, the term indeterminate growth is specified by the lack of a known asymptote, e.g., because organisms die before an asymptote could ever be reached and is therefore termed determinate growth type III sensu Sebens (1987). Before reaching any kind of skeletal maturity, a comprehensive decreasing growth rate in the later stages of ontogeny is observed in pterosaurs. Such a decrease has been described for more basal taxa like Eudimorphodon, Dimorphodon, and Rhamphorhynchus, i.e., expressed by a change to parallel-fibered bone tissue, a decrease in vascularization, or by an increasing number of LAGs (de Ricqlès et al. 2000; Padian et al. 2004; Prondvai et al. 2012). In contrast, SHN.013 mostly displays a rapid growth rate in the last growth period, a transitional slow down before the onset of the EFS is only indicated by a slight decrease in vascularization. Since pterosaur skeletons are known to show histovariability within different bones of one individual (Chinsamy et al. 2009; Sayão 2003; Prondvai et al. 2012), it is reasonable to compare the same skeletal elements. However, histological descriptions of pterosaur femora are rare (Steel 2008). A femoral thin section of a female, egg-bearing specimen Kunpengopterus sp. was described histologically and, unlike the continuous growth pattern of SHN.013, it shows three different layers of bone tissue (Wang et al. 2015). While it resembles our sample in exhibiting an inner layer of circumferential, lamellar bone, it shows a clear extent of remodeling in the subsequent layer. This latter feature was explained by the metaphyseal region from which the sample was taken. The outer layer was described as containing a “reduced number of vascular channels”, but an EFS, as well as LAGs, are absent and the specimen was accordingly interpreted as a late juvenile to subadult female. Among adult pterodactyloid femora, there is some variability in growth patterns but the similarities with our sample are considerable. Chinsamy et al. (2009) described three femora of Pterodaustro guinazui from the Early Cretaceous of Argentina, one of which constitutes an adult individual (V382) which can therefore best be compared with our adult specimen. Its histology resembles that of SHN.013 in its overall fibrolamellar bone tissue. However, the presence of at least 4 distinct growth marks contrasts the uninterrupted growth in the late growth period of our specimen. In addition, the thin section of the adult Pterodaustro guinazui specimen exhibits a significant decline in the growth rate, indicated by a change to a lamellar bone organization after the outermost growth mark. In contrast, MN 4809-V, the femur of a putative anhanguerid pterosaur from the Early Cretaceous of Brazil, does not show any interruptions within the growth period recorded in the preserved cortex and it shares the fast-growing woven bone matrix seen in SHN.013 (Sayão 2003). Since an EFS has also been described for this specimen, it is highly comparable in terms of skeletal maturity. The only significant difference between MN 4809-V and SHN.013 is the amount of secondary bone tissue, which has been described as extensive in the former and could indicate an even older ontogenetic age (Mitchell and Sander 2014). The growth record of a femur of the Early Cretaceous pterodactyl Prejanopterus curvirostris (Pereda-Superbiola et al. 2012) shares this overall growth dynamic. Its highly vascular fibrolamellar bone tissue extends to the periosteal margin, where a decrease in vascularity was described. The lack of LAGs or other growth marks corresponds to the uninterrupted growth period recorded in SHN.013. Due to the lack of an EFS in this specimen, Pereda-Superbiola et al. (2012) concluded that it had not reached asymptotic size. Accordingly, possible later changes in bone tissue with increasing age cannot be considered. Summarizing the observed growth patterns, SHN.013 shows many similarities to the bone histology of other pterodactyloid femora of variable sizes, although possible taxonomically based differences in growth dynamics can be registered too. Comparing the histology of SHN.013 to femora of non-pterodactyloid taxa, the observed differences in growth patterns increase, since these taxa tend to exhibit a somewhat slower growth expressed by a tendency towards a proportional increase in parallel-fibered bone compared to woven bone (cf. Jenkins et al. 2001; Padian et al. 2004; Prondvai et al. 2012; EMV personal observation).
Outside Portugal, pterosaurs from the Late Jurassic are known from the USA (Galton 1981; Harris and Carpenter 1996; Czerkas and Mickelson 2002; Carpenter et al. 2003), France (Buffetaut et al. 1998; Jouve 2004; Barrett et al. 2008), Germany (Frey et al. 2011), England (Barrett et al. 2008; Martill and Etches 2012), Poland (Barrett et al. 2008), Switzerland (Barrett et al. 2008; Meyer and Hunt 1999) and Spain (Barrett et al. 2008). Dsungaripterids are medium-sized pterosaurs that make their first appearance in the Late Jurassic of Europe (Buffetaut et al. 1998; Fastnacht 2005) but are best known from the Early Cretaceous of China; (Young 1964, 1973; Hone et al. 2017), and to a lesser degree from South America (Montanelli 1987; Martill et al. 2000; Codorniú et al. 2006). Basal dsungaripteroids are well known from Europe (Bennett 2006; Vidovic and Martill 2017). The remains of dsungaripterids occur almost exclusively in continental deposits (Fastnacht 2005), therefore it has been hypothesized that dsungaripterids were likely terrestrial (Witton 2013). The notable characteristic of derived dsungaripterids are the thick bone walls (Unwin et al. 1996; Unwin 2003), a condition which implies enhanced quadrupedal terrestrial locomotion. The ICD of SHN.013 (0.29) is similar to the dsungaripteroid HMNS/BB 5027 (ICD = 0.2) and slightly lower than the tibia of Dsungaripteridae indet. DFMMh/FV 500 (ICD = 0.47–0.5). This suggests that basal dsungaripteroids tend to have a slightly thinner cortex than dsungaripterids, although still thicker than other non-dsungaripteroid pterosaurs, such as the juvenile azhdarchoid SMNK PAL 3985 (ICD = 0.19) and the Anhangueridae indet. MN 4809 V (ICD = 0.18).
In SHN.013, the slight elongation of the fourth trochanter and the bowing of the shaft might suggest a similar terrestrial lifestyle to dsungaripterids, an assumption reinforced by the sedimentology of the fossiliferous layer showing a continental floodplain and a swamp, a more typical habitat for these later forms. The moderately lower thickness value of the femur compared to other dsungaripterids might indicate a small ecological relationship to more aerial and soaring-based lifestyle, however, without a more complete skeleton, it is impossible to deduce the exact type of habitat of this taxon.
Inhabiting terrestrial environments exposes organisms to a wide range of predators. When SHN.013 was prepared in the field, we noticed that the lack of the distal portion of the femur was not the result of diagenesis, erosion or due to excavation work. The proximal portion and the shaft were perfectly preserved yet with superficial weathering and the distal part was not exposed to the surface. Therefore, distal region truncation must have occurred before the burial of the bone. A long groove and a set of smaller sulci are visible on the shaft, resembling tooth-marks left by a smaller scavenger (Fig. 2A2, A3).
Conclusions
The new pterosaurian femur described here displays a combination of features including the bowing of the shaft, the mushroom-like cap of the femoral head, and the distinctly elevated greater trochanter that suggest an affinity with dsungaripteroids. Although these pterosaurs are better known from the Cretaceous, many likely basal forms were present in the Late Jurassic. The slightly thinner bone wall of the diaphysis and the isolated nature of the specimen limit placing it within a specific dsungaripteroid clade. The bone histology of SHN.013 shows that it is a skeletally mature specimen with continuously fast growth within the latest, recorded ontogenetic window, which was maintained until the skeletal maturity was reached. Comparisons to other taxa from the literature show that SHN.013 can best be compared to femora from pterodactyloid taxa from the Early Cretaceous, which adds to our hypothesis of a taxonomic proximity to dsungaripteroids. The presence of putative tooth marks (and possibly the abruptly cut distal end) suggests the animal/carcass was chewed by scavengers before burial (Fig. 2A2, A3). The discovery of a large-sized pterosaur in the Bombarral Formation shows a diversified flying fauna in the Late Jurassic of Europe, stimulating more research on these fossiliferous strata.
Acknowledgments
We thank Olaf Dülfer and Pia Schucht (both Institute of Geosciences, Bonn, Germany) for their help in preparing the thin sections of the femur, and Artur Mateus (Polytechnical Institute of Leiria, Portugal) for advising on CTScan and 3D modelling. We thank Joana Ferreira, Francisco Rodrigues and Pedro Bonifácio (both SHN) for their help during fieldwork. We are grateful to Alexander Kellner (MN) and Edina Prondvai (Universiteit Gent, Belgium) for their criticisms and comments that greatly improved the manuscript.
References
Andrade, de R.C., Bantim, R.A.M., de Lima, F.J., dos Santos Campos, L., de Souza Eleutério, L.H., and Sayão, J.M. 2015. New data about the presence and absence of the external fundamental system in archosaurs. Cadernos de Cultura e Ciência 14: 200–211. Crossref
Antunes, M.T. and Mateus O. 2003. Dinosaurs of Portugal. Comptes Rendus Palevol 2: 77–95. Crossref
Aureliano, T., Ghilardi, A.M., Duque, R.R., and Barreto, A.M. 2014. On the occurrence of Pterosauria in Exu, Pernambuco (Lower Cretaceous Romualdo Formation, Araripe Basin), Northeastern Brazil. Estudos Geológicos 24: 15–27. Crossref
Aureliano, T., Ghilardi, A.M., Silva-Junior, J.C.G., Martinelli, A.G., Ribeiro, L.C.B., Marinho, T., Fernandes, M.A., Ricardi-Branco, F., and Sander, P.M. 2020. Influence of taphonomy on histological evidence for vertebral pneumaticity in an Upper Cretaceous titanosaur from South America. Cretaceous Research 108: 104337. Crossref
Averianov, A.O. 2007. New records of azhdarchids (Pterosauria, Azhdarchidae) from the Late Cretaceous of Russia, Kazakhstan, and Central Asia. Paleontological Journal 41: 189–197. Crossref
Averianov, A.O., Arkhangelsky, M.S., and Pervushov, E.M. 2008. A new Late Cretaceous azhdarchid (Pterosauria, Azhdarchidae) from the Volga Region. Paleontological Journal 42: 634–642. Crossref
Barrett, P.M., Butler, R.J., Edwards, N.P., and Milner, A.R. 2008. Pterosaur distribution in time and space: an atlas. Zitteliana B 28 61–107.
Bennett, S.C. 2001. The osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon Part I. General description of osteology. Palaeontographica Abteilung A 260: 1–112.
Bennett, S.C. 2006. Juvenile specimens of the pterosaur Germanodactylus cristatus, with a review of the genus. Journal of Vertebrate Paleontology 26: 872–878. Crossref
Benson, R.B., Frigot, R.A., Goswami, A., Andres, B., and Butler, R.J. 2014. Competition and constraint drove Cope’s rule in the evolution of giant flying reptiles. Nature Communications 5: 1–8. Crossref
Bonaparte, J.F. 1970. Pterodaustro guinazui gen. et sp. nov. Pterosaurio de la Formacion Lagarcito, Provincia de San Luis, Argentina y su significado en la geologia regional (Pterodactylidae). Acta Geologica Lilloana 10: 209–225.
Buffetaut, E., Grigorescu, D., and Csiki, Z. 2003. Giant azhdarchid pterosaurs from the terminal Cretaceous of Transylvania (western Romania). Geological Society, London, Special Publications 217: 91–104. Crossref
Buffetaut, E., Lepage, J.J., and Lepage, G. 1998. A new pterodactyloid pterosaur from the Kimmeridgian of the Cap de La Hève (Normandy, France). Geological Magazine 135: 719–22. Crossref
Camilo da Silva, B. 2019. Long Bone Histology of Late Jurassic Dryosaurids from Portugal, with Emphasis on Eousdryosaurus nanohallucis. 61 pp. M.Sc. Thesis, University of Opole, Opole.
Carpenter, K., Unwin, D., Cloward, K., Miles, C., and Miles, C. 2003. A new Scaphognathine pterosaur from the Upper Jurassic Morrison Formation of Wyoming, USA. In: E. Buffetaut, and J.M. Mazin (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society of London, Special Publications 217: 45–54. Crossref
Chinsamy, A., Codorniú, L. and Chiappe, L. 2009. Palaeobiological implications of the bone histology of Pterodaustro guiñazui. The Anatomical Record 292:1462–1477. Crossref
CloudCompare 2018. Version 2.9.1 [GPL software]. Available at http://www.cloudcompare.org/.
Codorniú, L., Gasparini, Z., and Paulina-Carabajal, A. 2006. A Late Jurassic pterosaur (Reptilia, Pterodactyloidea) from northwestern Patagonia, Argentina. Journal of South American Earth Sciences 20: 383–389. Crossref
Czerkas, S.A. and Mickelson, D.L. 2002. The first occurrence of skeletal pterosaur remains in Utah. In: S.J. Czerkas (ed.), Feathered Dinosaurs and the Origin of Flight, 3–13. The Dinosaur Museum, Blanding.
Dalla Vecchia, F.M. 2018. Comments on Triassic pterosaurs with a commentary on the “ontogenetic stages” of Kellner (2015) and the validity of Bergamodactylus wildi. Rivista Italiana di Paleontologia e Stratigrafia 124: 317–341.
Escaso, F., Ortega, F., Dantas, P., Malafaia, E., Pimentel, N.L., Pereda-Suberbiola, X., Sanz, J.L., Kullberg, J.C., Kullberg, M.C., and Barriga, F. 2007. New evidence of shared dinosaur across Upper Jurassic proto-North Atlantic: Stegosaurus from Portugal. Naturwissenschaften 94: 367–374. Crossref
Escaso, F., Ortega, F., Dantas, P., Malafaia, E., Silva, B., Gasulla, J. M., Mocho, P., Narvaez, I., and Sanz, J.L. 2014. A new dryosaurid ornithopod (Dinosauria, Ornithischia) from the Late Jurassic of Portugal. Journal of Vertebrate Paleontology 34: 1102–1112. Crossref
Fastnacht, M. 2005. The first dsungaripterid pterosaur from the Kimmeridgian of Germany and the biomechanics of pterosaur long bones. Acta Palaeontologica Polonica 50: 273–288.
Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J.C., Pujol, S., Bauer, C., Jennings D., Fennessy F., Sonka M., Buatti, J., Aylward S., Miller, J.V., Pieper, S., and Kikinis R. 2012. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magnetic Resonance Imaging 30: 1323–1341. Crossref
Figueiredo, S.D., Rosina, P., Strantzali, I.B., Antunes, V., and Figueiredo, S. 2020. Paleoenvironmental approach on the Lower Cretaceous sequences of Areia do Mastro (Cabo Espichel, Southern Portugal). Journal of Environmental Science and Engineering A 9: 66–71. Crossref
Frey, E., Meyer, C.A., and Tischlinger, H. 2011. The oldest azhdarchoid pterosaur from the Late Jurassic Solnhofen Limestone (early Tithonian) of southern Germany. Swiss Journal of Geosciences 104: 35–55. Crossref
Galton, P.M. 1981. A rhamphorhynchoid pterosaur from the Upper Jurassic of North America. Journal of Paleontology 55: 1117–1122. Crossref
Guillaume, A.R.D. 2018. Microvertebrates of the Lourinhã Formation (Late Jurassic, Portugal). 163 pp. M.Sc. Thesis, Universidade Nova de Lisboa, Lisboa.
Guillaume, A.R.D., Moreno-Azanza, M., Puértolas-Pascual, E., and Mateus, O. 2019. Palaeobiodiversity of crocodylomorphs from the Lourinhã Formation based on the tooth record: insights into the palaeoecology of the Late Jurassic of Portugal. Zoological Journal of the Linnean Society 189: 549–583. Crossref
Harris, J.D. and Carpenter, K. 1996. A large pterodactyloid from the Morrison Formation (Late Jurassic) of Garden Park, Colorado. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1996 (8): 473–484. Crossref
Hill, G. 1989. The Sedimentology and Lithostratigraphy of the Upper Jurassic Lourinha Formation, Lusitanian Basin, Portugal. 42 pp. Ph.D. Thesis, The Open University, London (unpublished).
Hone, D.W.E., Jiang, S., and Xu, X. 2017. A taxonomic revision of Noripterus complicidens and Asian members of the Dsungaripteridae. Geological Society, London, Special Publications 455: 149–157. Crossref
Jenkins, F.A., Shubin, N.H., Gatesy, S.M., and Padian, K. 2001. A diminutive pterosaur (Pterosauria: Eudimorphodontidae) from the Greenlandic Triassic. Bulletin of the Museum of Comparative Zoology 156: 151–170.
Jouve, S. 2004. Description of the skull of a Ctenochasma (Pterosauria) from the latest Jurassic of eastern France, with a taxonomic revision of European Tithonian Pterodactyloidea. Journal of Vertebrate Paleontology 24: 542–554. Crossref
Kaup, J.J. 1834. Ersuch Einer Eintheilung Der Saugethiere in 6 Stämme Und Der Amphibien in 6 Ordnungen. Isis 3: 311–324.
Kellner, A.W.A. 2003. Pterosaur phylogeny and comments on the evolutionary history of the group. Geological Society, London, Special Publications 217:105–137. Crossref
Kellner, A.W.A. and Tomida, Y. 2000. Description of a new species of Anhangueridae (Pterodactyloidea) with comments on the pterosaur fauna from the Santana Formation (Aptian–Albian), northeastern Brazil. National Science Museum Monographs 17: 1–135.
Kellner, A.W.A., Campos, D.A., Sayão, J.M., Saraiva, A.A., Rodrigues, T., Oliveira, G., Cruz, L.A., Costa, F.R., Silva, H.P., and Ferreira, J.S. 2013. The largest flying reptile from Gondwana: a new specimen of Tropeognathus cf. T. mesembrinus Wellnhofer, 1987 (Pterodactyloidea, Anhangueridae) and other large pterosaurs from the Romualdo Formation, Lower Cretaceous, Brazil. Anais da Academia Brasileira de Ciências 85: 113–135. Crossref
Kellner, A.W.A., Rodrigues, T., Costa, F.R., Weinschütz, L.C., Figueiredo, R.G., Souza, G.A., Brumi, A.S., Eleutério, L.H.S., Mueller, C.W., and Sayao, J.M. 2019. Pterodactyloid pterosaur bones from Cretaceous deposits of the Antarctic Peninsula. Anais da Academia Brasileira de Ciências 91 [published online, https://doi.org/10.1590/0001-3765201920191300] Crossref
Kullberg, J.C., Rocha, R.B., Soares, A.F., Rey, J., Terrinha, P., Callapez, P., and Martins, L. 2006. A Bacia Lusitaniana: estratigrafia, paleogeografia e tectónica. Geologia de Portugal no Contexto da Ibéria 317–368.
Lambertz, M., Bertozzo, F., and Sander, M.P. 2018. Bone histological correlates for air sacs and their implications for understanding the origin of the dinosaurian respiratory system. Biology Letters 14: 20170514. Crossref
Leinfelder, R. 1993. A sequence stratigraphic approach to the Upper Jurassic mixed carbonate-siliciclastic succession of the central Lusitanian Basin, Portugal. Profil 5: 119–140.
Lü, J. and Yuan, C. 2005. New tapejarid pterosaur from western Liaoning, China. Acta Geologica Sinica 79: 453–458. Crossref
Lü, J., Jin, X., Unwin, D.M., Zhao, L., Azuma, Y., and Ji, Q. 2006. A new species of Huaxiapterus (Pterosauria: Pterodactyloidea) from the Lower Cretaceous of Western Liaoning, China with comments on the systematics of tapejarid pterosaurs. Acta Geologica Sinica 80: 315–326. Crossref
Lü, J., Unwin, D.M., Xu, L., and Zhang, X. 2008. A new azhdarchoid pterosaur from the Lower Cretaceous of China and its implications for pterosaur phylogeny and evolution. Naturwissenschaften 95: 891–897. Crossref
Lü, J.C., Azuma, Y., Dong, Z.M., Barsbold, R., Kobayashi, Y., and Lee, Y.N. 2009. New material of dsungaripterid pterosaurs (Reptilia: Pterosauria) from Western Mongolia and its paleoecological implications. Geological Magazine 146: 690–700. Crossref
Malafaia, E. 2017. Phylogenetic Analysis, Paleoenvironmental and Paleobiogeographic Interpretation of Theropod Dinosaurs from the Upper Jurassic of the Lusitanian Basin. 388 pp. Ph.D. Thesis, Universidade de Lisboa, Lisboa.
Malafaia, E., Mocho, P., Escaso, F., and Ortega, F. 2020. A new carcharodontosaurian theropod from the Lusitanian Basin: evidence of allosauroid sympatry in the European Late Jurassic. Journal of Vertebrate Paleontology 40: 1–8. Crossref
Malafaia, E., Ortega, F., Escaso, F., Dantas, P., Pimentel, N., Gasulla, J.M., Ribeiro, B., Barriga, F., and Sanz, J.L. 2010. Vertebrate fauna at the Allosaurus fossil-site of Andrés (Upper Jurassic), Pombal, Portugal. Journal of Iberian Geology 36: 193–204. Crossref
Manupella, G. (coord.), Antunes, M.T., Pais, J., Ramalho, M.M., and Rey, J. 1999. Carta Geológica de Portugal à escala 1:50 000. Noticia Explicativa da Folha 30-A, Lourinhã. 83 pp. Instituto Geológico e Mineiro, Lisboa.
de Margerie, E., Cubo, J., and Castanet, J. 2002. Bone typology and growth rate: testing and quantifying “Amprino’s rule” in the mallard (Anas platyrhynchos). Comptes Rendus Biologies 325: 221–230. Crossref
Marsh, O.C. 1876. Notice of a new sub-order of Pterosauria. American Journal of Science. Series 3 11: 507–509. Crossref
Martill, D.M. and Etches, S. 2012. A new monofenestratan pterosaur from the Kimmeridge Clay Formation (Kimmeridgian, Upper Jurassic) of Dorset, England. Acta Palaeontologica Polonica 58: 285–294. Crossref
Martill, D.M. and Moser, M. 2018. Topotype specimens probably attributable to the giant azhdarchid pterosaur Arambourgiania philadelphiae (Arambourg 1959). Geological Society, London, Special Publications 455: 159–169. Crossref
Martill, D.M., Frey, E., Diaz, G.C. and Bell, C.M., 2000. Reinterpretation of a Chilean pterosaur and the occurrence of Dsungaripteridae in South America. Geological Magazine 137: 19–25. Crossref
Martin, T. 2005. Postcranial anatomy of Haldanodon exspectatus (Mammalia, Docodonta) from the Late Jurassic (Kimmeridgian) of Portugal and its bearing for mammalian evolution. Zoological Journal of the Linnean Society 145: 219–248. Crossref
Martin, T. 2013. Mammalian postcranial bones from the Late Jurassic of Portugal and their implications for forelimb evolution. Journal of Vertebrate Paleontology 33: 1432–1441. Crossref
Martinius, A.W. and Gowland, S. 2011. Tide-influenced fluvial bedforms and tidal bore deposits (Late Jurassic Lourinhã Formation, Lusitanian Basin, Western Portugal). Sedimentology 58: 285–324. Crossref
Mateus, O. and Milán J. 2010. First records of crocodyle and pterosaur tracks in the Upper Jurassic of Portugal. New Mexico Museum of Natural History and Science Bulletin 51: 83–87.
Mateus, O., Maidment, S.C., and Christiansen, N.A. 2009. A new long-necked “sauropod-mimic” stegosaur and the evolution of the plated dinosaurs. Proceedings of the Royal Society B: Biological Sciences 276: 1815–1821. Crossref
McLain, M.A. and Bakker, R.T. 2018. Pterosaur material from the uppermost Jurassic of the uppermost Morrison Formation, Breakfast Bench Facies, Como Bluff, Wyoming, including a pterosaur with pneumatized femora. Geological Society, London, Special Publications 455: 105–124. Crossref
Meyer, C.A. and Hunt, A.P. 1999. The first pterosaur from the Late Jurassic of Switzerland: evidence for the largest Jurassic flying animal. Oryctos 2: 111–116.
Mitchell, J. and Sander, P.M. 2014. The three-front model: a developmental explanation of long bone diaphyseal histology of Sauropoda. Biological Journal of the Linnean Society 112: 765–781. Crossref
Mocho, P., Royo-Torres, R., Escaso, F., Malafaia, E., de Miguel Chaves, C., Narváez, I., Pérez-García, A., Pimentel, N., Silva, B., and Ortega, F. 2017. Upper Jurassic sauropod record in the Lusitanian Basin (Portugal): Geographical and lithostratigraphical distribution. Palaeontologia Electronica 20: 1–50. Crossref
Montanelli, S.B. 1987. Presencia de Pterosauria (Reptilia) en la formación La Amarga (Hauteriviano–Barremiano), Neuquén, Argentina. Ameghiniana 24: 109–113.
Ortega, F., Malafaia, E., Escaso, F., and Dantas, P. 2009. Faunas de répteis do Jurássico Superior de Portugal. Paleolusitana 1: 43–56.
Padian, K. and Lamm, E.T. 2013. Bone Histology of Fossil Tetrapods: Advancing Methods, Analysis, and Interpretation. 298 pp. University of California Press, Berkeley. Crossref
Padian, K, de Ricqlès, A.J. and Horner, J.R. 1995. Bone histology determines identification of a new taxon of pterosaur (Reptilia: Archosauria). Comptes Rendus à l’Académie des Sciences 320: 77–84.
Padian, K, Horner, J.R., and de Ricqlès, A.J. 2004. Growth in small dinosaurs and pterosaurs: the evolution of archosaurian growth strategies. Journal of Vertebrate Paleontology 24: 555–571. Crossref
Pereda-Suberbiola, X., Knoll, F., Ruiz-Omeñaca, J.I., Company, J., and Torcida Fernández-Baldor, F. 2012. Reassessment of Prejanopterus curvirostris, a basal pterodactyloid pterosaur from the Early Cretaceous of Spain. Acta Geologica Sinica 86: 1389–1401. Crossref
Pérez-García, A. and Ortega, F. 2011. Selenemys lusitanica, gen. et sp. nov., a new pleurosternid turtle (Testudines: Paracryptodira) from the Upper Jurassic of Portugal. Journal of Vertebrate Paleontology 31: 60–69. Crossref
Pérez-García, A. and Ortega, F. 2013. A new species of the turtle Hylaeochelys (Eucryptodira) outside its known geographic and stratigraphic ranges of distribution. Comptes Rendus Palevol 13: 183–188. Crossref
Pinheiro, F.L., Fortier, D.C., Schultz, C.L., Andrade, J.A.F.G., and Bantim, R.A.M. 2011. New information on the pterosaur Tupandactylus imperator, with comments on the relationships of Tapejaridae. Acta Palaeontologica Polonica 56: 567–580. Crossref
Plieninger, F. 1901. Beiträge Zur Kenntnis Der Flugsaurier. Paläontographica 48: 65–90.
Prondvai, E., Stein, K., Ösi, A., and Sander, P.M. 2012. Life history of Rhamphorhynchus inferred from bone histology and the diversity of pterosaurian growth strategies. PLoS One 7: e31392. Crossref
Rauhut, O.W. 2001. Herbivorous dinosaurs from the Late Jurassic (Kimmeridgian) of Guimarota, Portugal. Proceedings of the Geologists’ Association 112: 275–283. Crossref
Rasmussen, E.S., Lomholt, S., Andersen, C., and Vejbæk, O.V. 1998. Aspects of the structural evolution of the Lusitanian Basin in Portugal and the shelf and slope area offshore Portugal. Tectonophysics 300: 199–225. Crossref
Ravnås, R., Windelstad, J., Mellere, D., Nøttvedt, A., Stuhr Sjøblom, T., Steel, R.J., and Wilson, R.C.L. 1997. A marine Late Jurassic syn-rift succession in the Lusitanian Basin, western Portugal—tectonic significance of stratigraphic signature. Sedimentary Geology 114: 237–266. Crossref
de Ricqlès, A.J., Padian, K., Horner, J.R., and Francillon-Vieillot, H. 2000. Palaeohistology of the bones of pterosaurs (Reptilia: Archosauria): anatomy, ontogeny, and biomechanical implications. Zoological Journal of the Linnean Society 129: 349–385. Crossref
Reis, R.P.B.P., Cunha, P.P., Dinis, J.L., and Trincao, P.R. 2000. Geologic evolution of the Lusitanian Basin (Portugal) during the Late Jurassic. Forum American Bar Association 6: 345–357.
Rotatori, F.M., Moreno-Azanza, M., and Mateus, O. 2020. New information on ornithopod dinosaurs from the Late Jurassic of Portugal. Acta Palaeontologica Polonica 65: 35–57. Crossref
Russo, J., Mateus, O., Marzola, M., and Balbino, A. 2017. Two new ootaxa from the Late Jurassic: the oldest record of crocodylomorph eggs, from the Lourinhã Formation, Portugal. PloS One 12: e0171919. Crossref
Sayão, J.M. 2003. Histovariability in bones of two pterodactyloid pterosaurs from the Santana Formation, Araripe Basin, Brazil: preliminary results. In: E. Buffetaut and J.M. Mazin (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society of London, Special Publication 217: 335–342. Crossref
Schneider, S., Fürsich, F.T., and Werner, W. 2009. Sr-isotope stratigraphy of the Upper Jurassic of central Portugal (Lusitanian Basin) based on oyster shells. International Journal of Earth Sciences 98: 1949–1970. Crossref
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. Crossref
Sebens, K.P. 1987. The ecology of indeterminate growth in animals. Annual Review of Ecology and Systematics 18: 371–407. Crossref
Stapel, G., Cloetingh, S., and Pronk, B. 1996. Quantitative subsidence analysis of the Mesozoic evolution of the Lusitanian Basin (western Iberian margin). Tectonophysics 266: 493–507. Crossref
Steel, L. 2008. The palaeohistology of pterosaur bone: an overview. Zitteliana B 28: 109–125.
Stein, K. and Sander, P.M. 2009. Histological core drilling: a less destructive method for studying bone histology. In: M.A. Brown, J.F. Kane, and W.G. Parker (eds.), Methods in Fossil Preparation. Proceedings of the First Annual Fossil Preparation and Collections Symposium, 69–80. Petrified Forest, AZ.
Taylor, A.M., Gowland, S., Leary, S., Keogh, K.J., and Martinius, A.W. 2014. Stratigraphical correlation of the Late Jurassic Lourinhã Formation in the Consolação sub-basin (Lusitanian Basin), Portugal. Geological Journal 49: 143–162. Crossref
Theodori, C. 1830. Knochen vom Pterodactylus aus der Liasformation von Banz. Frorieps Notizen für Natur- und Heilkunde 632: 1–101.
Unwin, D.M. 2003. On the phylogeny and evolutionary history of pterosaurs. In: E. Buffetaut and J.-M. Mazin (eds.), Evolution and Palaeobiology of Pterosaurs. Geological Society of London, Special Publications 217: 139–190. Crossref
Unwin, D., Manabe, M.M., Shimizu, K., and Hasegawa, Y. 1996. First record of pterosaurs from the Early Cretaceous Tetori Group: A wing-phalange from Amagodani Formation in Shokawa, Gifu Prefecture, Japan. Bulletin-National Science Museum Tokyo Series C 22: 37–46.
Vidovic, S.U. and Martill, D.M. 2017. The taxonomy and phylogeny of Diopecephalus kochi (Wagner, 1837) and “Germanodactylus rhamphastinus” (Wagner, 1851). Geological Society of London, Special Publications 455: 125–147. Crossref
Wang, X. and Zhou, Z. 2003. A new pterosaur (Pterodactyloidea, Tapejaridae) from the Early Cretaceous Jiufotang Formation of western Liaoning, China and its implications for biostratigraphy. Chinese Science Bulletin 48: 16–23. Crossref
Wang, X., Kellner, A.W., Cheng, X., Jiang, S., Wang, Q., Sayao, J.M., Rodrigues, T., Costa, F.R., Li, N., Meng, X., and Zhou, Z. 2015. Eggshell and histology provide insight on the life history of a pterosaur with two functional ovaries. Anais da Academia Brasileira de Ciências 87 (3): 1599–1609. Crossref
Wellnhofer, P. 1978. Pterosauria. In: P. Wellnhofer (ed.), Handbuch der Paläoherpetologie, 1–82. Gustav Fischer, Stuttgart.
Wiechmann, M.F. and Gloy, U. 2000. Pterosaurs and urvogels from the Guimarota mine. In: T. Martin and B. Krebs (eds.), Guimarota: A Jurassic Ecosystem, 83–86. Verlag Dr. Friedrich Pfeil, Munich.
Wiman, C. 1925. Über Pterodactylus westmanni und andere Flugsaurier. Bulletin of the Geological Institution of the University of Uppsala 20: 1–38.
Witton, M.P. 2008. A new approach to determining pterosaur body mass and its implications for pterosaur flight. Zitteliana B 28: 143–158.
Witton, M.P. 2013. Pterosaurs: Natural History, Evolution, Anatomy. 304 pp. Princeton University Press, Princeton. Crossref
Witton, M.P., O’Sullivan, M., and Martill, D.M. 2015. The relationships of Cuspicephalus scarfi Martill and Etches, 2013 and Normannognathus wellnhoferi Buffetaut et al., 1998 to other monofenestratan pterosaurs. Contributions to Zoology 84: 115–127. Crossref
Woodward, H.N., Horner, J.R., and Farlow, J.O. 2011. Osteohistological evidence for determinate growth in the American alligator. Journal of Herpetology 45: 339–343. Crossref
Young, C.C. 1964. On a new pterosaurian from Sinkiang, China. Vertebrata PalAsiatica 8: 221–255.
Young, CC. 1973. Wuerho pterosaurs. Special Publication of the Institute of Vertebrate Paleontology and Paleoanthropology Academia Sinica 11: 18–34.
Acta Palaeontol. Pol. 66 (4): 815–825, 2021
https://doi.org/10.4202/app.00858.2020