

A new Late Triassic dipteridacean fern from the Paso Flores Formation, Neuquén Basin, Argentina
SILVIA CRISTINA GNAEDINGER and ANA MARÍA ZAVATTIERI
Gnaedinger, S.C. and Zavattieri, A.M. 2021. A new Late Triassic dipteridacean fern from the Paso Flores Formation, Neuquén Basin, Argentina. Acta Palaeontologica Polonica 66 (4): 885–900.
Sterile and fertile fronds of dipteridacean ferns from the Paso Flores Formation (late Norian–Rhaetian) at Cañadón de Pancho area, south of the Neuquén Province, Patagonia (Neuquén Basin), Argentina, are described. The Paso Flores Formation specimens comprise an interesting mixture of features showing a unique combination that does not fit in the diagnosis of any of the known fossil genera of Dipteridaceae. Characters such as the number and shape of primary segments in each rachial arm, the coalescence of the primary segments at the base of the frond, the distribution of sori and the number of sporangia per sori allow to differentiate the Paso Flores Formation specimens from the other genera of the family. The new genus and species, Patagoniapteris artabeae is proposed. The specimens share some frond morphological features with the fossil genera Clathropteris, Digitopteris, Thaumatopteris, Sewardalea and with some species of the Dictyophyllum, as well as the characteristic of the sori with the living species Dipteris lobbiana. The Paso Flores Formation environments developed on the western margin of Gondwana under seasonal temperate-warm and humid to sub-humid climates with a marine influence from the west. The Cañadón de Pancho assemblages are late Norian–Rhaetian in age, being the youngest fossil flora recorded from Argentinian Triassic basins to date.
Key words: Gleicheniales, Dipteridaceae, Norian, Rhaetian, Paso Flores Formation, Neuquén Basin, Patagonia, Gondwana.
Silvia Cristina Gnaedinger [scgnaed@hotmail.com ], Área de Paleontología, Centro de Ecología Aplicada del Litoral, Consejo Nacional de Investigaciones Científicas y Técnicas (CECOAL-CCT CONICET Nordeste, UNNE), Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste (FaCENA-UNNE). Casilla de Correo 291, 3400 Corrientes, Argentina.
Ana María Zavattieri [amz@mendoza-conicet.gob.ar], Departamento de Paleontología, Instituto Argentino de Nivología, Glaciología y Ciencias Ambientales (IANIGLA-CONICET), Av. Adrián Ruiz Leal s/n, Parque General San Martín, M5002IRA, Mendoza, Argentina.
Received 5 December 2020, accepted 21 May 2021, available online 29 November 2021.
Copyright © 2021 S.C. Gnaedinger and A.M. Zavattieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Dipteridaceae (order Gleicheniales) comprises two extant genera, Dipteris Reinwardt, 1828, and Cheiropleuria Presl, 1851 (Smith et al. 2006; Taylor et al. 2009; Zhang et al. 2013). Currently, they are distributed in warm tropical to sub-tropical regions of Asia, Australia, and Polynesia (Corsin and Waterlot 1979; Kramer et al. 1990; Zhang et al. 2013), where they are generally found at higher altitudes in open exposed areas (Kramer et al. 1990; Choo et al. 2016). Dipteris includes a species Dipteris lobbiana (Hooker, 1853) Moore, 1857, which grows on stream banks in open canopy, and other species, such as Dipteris conjugata Reinwardt, 1828, a colonizer of disturbed sites and exposed ridges (Cantrill 1995).
The fossil records of this group of ferns are distributed worldwide and they were diverse in the Southern Hemisphere during the Late Triassic to Early Jurassic interval when they reached their greatest generic diversity (Corsin and Waterlot 1979; Tidwell and Ash 1994; Bomfleur and Kerp 2010; Zhou et al. 2015; Bodnar et al. 2018). The earliest fossils with the distinctive venation pattern of Dipteridaceae were recorded from Middle Triassic strata (Webb 1982; Tidwell and Ash 1994; Cantrill 1995; Kustatscher and van Konijnenburg-van Cittert 2011; Bodnar et al. 2018) suggesting that the origin of the family took place in the Early Triassic or even earlier, during the late Palaeozoic (Tidwell and Ash 1994; Choo et al. 2016; Choo and Escapa 2018). This fern family decreased its diversity during Late Jurassic and Cretaceous (Choo and Escapa 2018). Cretaceous and Paleogene records of Dipteridaceae are scarce and consist only of various species of Hausmannia (Choo et al. 2016). In Argentina, the Dipteridaceae have been recorded in the late Anisian–early Ladinian and late Norian–Rhaetian intervals (Bodnar et al. 2018).
Smith et al. (2006) and Zhang et al. (2013) described the morphology of the extant Dipteridaceae characterized by a long stipe that bears fan-shaped fronds with toothed margins, bilobated and often deeply dissected lamina with a typical reticulate venation with veinlets inside the meshes. Exindusiate, discrete, compital sori are scattered over the abaxial surface of fertile fronds; sporangia maturation simultaneous or maturation mixed, with vertical or slightly oblique annulus.
Fossil fronds assigned to the Dipteridaceae are represented by several species included in the genera Clathropteris Brongniart, 1828, Dictyophyllum Lindley and Hutton, 1834, Digitopteris Pott and Bomfleur in Pott et al., 2018, Goeppertella Ôishi and Yamasita, 1936 emend. Arrondo and Petriella, 1982, Hausmannia Dunker, 1846, Sewardalea Choo and Escapa, 2018, and Thaumatopteris Goeppert, 1841 (Herbst 1992a, b; Choo and Escapa 2018).
Dipteridaceaen genera were established based on the gross morphology of fronds and their venation patterns (Ôishi and Yamasita 1936; Arrondo and Petriella 1982; Herbst 1992a, b; Rees 1993; Rees and Cleal 2004). In Argentina, Stipanicic and Menéndez (1949) described specimens of Dipteridaceae according to Ôishi and Yamasita’s concept (1936), although they remarked that there are controversial criteria in the dipteridaceaean classification among different authors. The taxonomic classification proposed by Herbst (1992a, b), in agreement with that of Ôishi and Yamasita (1936), considers morphological features, such as the dissection of the frond lamina, and the disposition and torsion of the primary veins originated from the division of the stipe. Based on these criteria, Herbst (1992a, b) established three subgenera within the genus Dictyophyllum: Dictyophyllum, Thaumatopteris, and Clathropteris.
Choo and Escapa (2018) contributed to the first verifiable phylogenetic hypothesis of the evolution of the Dipteridaceae, analyzing extinct as well as extant taxa in a single cladistic study and concluded that the evolutionary trend in this family has been toward increasing complexity in the venation pattern and laminal fusion. They recognized only five fossil genera: Goeppertella, Thaumatopteris, Clathropteris, Digitopteris, and Sewardalea. Choo and Escapa (2018) considered that fossil genera such as Dictyophyllum, Kenderlykia Tururanova-Ketova, 1962, Hausmannia, and Protorhipis Andrae, 1853, are ambiguously placed on the tree and are recognized as possibly unnatural morphogenera.
Three dipteridacean species from the Paso Flores Formation were previously described: Dictyophyllum tenuifolium (Stipanicic and Menéndez, 1949) Bonetti and Herbst, 1964, Thaumatopteris rothi Frengüelli, 1941, and Goeppertella stipanicicii Herbst, 1993 (Frengüelli 1941; Herbst 1964, 1993; Stipanicic and Menéndez 1949; Bonetti and Herbst 1964; Herbst 1992a, b) (Table 1).
The Triassic species with fertile fronds described in Argentina are: Dictyophyllum tenuifolium which has sori irregularly arranged along the primary veins and at the base of the secondary veins, as well as on the lamina occasionally, and Thaumatopteris tenuiserrata Menéndez, 1951, which has sori irregularly arranged among the secondary veins (Menéndez 1951; Herbst 1992a, b). In specimens reported from the Jurassic strata of Argentina sori are arranged on the lamina in quite a distinct manner. Thaumatopteris rocablanquesis Herbst, 1965, has the sori grouped over the whole abaxial surface; Clathropteris obovata Ôishi, 1932, has tetra-hexasporangiate sori located in each areole of third order, and Clathropteris meniscioides (Brongniart, 1825) Brongniart, 1828, has the sori scattered across the abaxial surface (Herbst 1965, 1966, 1992a, b; Choo et al. 2016) (Table 2).
In this contribution we describe a new taxon of Dipteridaceae fertile and sterile fronds, Patagoniapteris artabeae gen. and sp. nov. from the uppermost part of the Paso Flores Formation of late Late Triassic age, in the Cañadón de Pancho area, Neuquén Province, Patagonia.
Institutional abbreviations.—MCF-PBPH, Palaeontological Collection of the Museo Municipal “Carmen Funes”, Plaza Huincul city, Neuquén Province, Argentina.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in Plant Fossil Names Registry (PFNR): urn:lsid:plantfossilnames.org:ref:972.
Geological setting
The Neuquén Basin is located on the eastern side of the Andes and central Chile, between 30–41º S latitude (Fig. 1). It originated during the Late Triassic–Early Jurassic on the western edge of Gondwana by continental extension (D’Elía et al. 2012). Syn-rift depocenters developed during the initial stages of the basin formation as isolated troughs linked with profuse magmatic activity (Legarreta and Uliana 1996; Spalletti et al. 1999; Howell et al. 2005). They were filled by a complex variety of clastic and volcaniclastic deposits associated with extensive lava flows known as the Precuyano Cycle (Gulisano et al. 1984; Legarreta and Gulisano 1989). The southern part of the Neuquén Basin is characterized by reactivated normal faults and reverse faults related to the evolution of the Andes Cordillera resulting in well exposed Mesozoic successions of the initial stage of the basin (D’Elía et al. 2012).
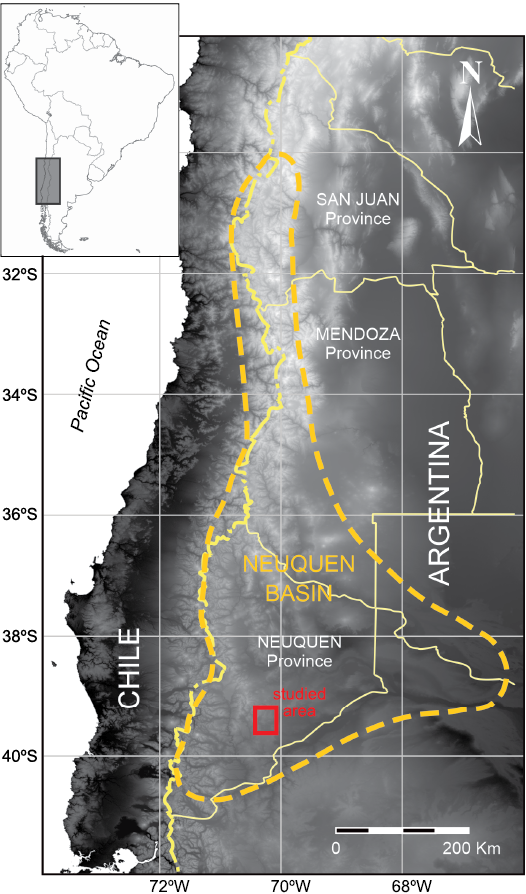
Fig. 1. Generalized outline of the Neuquén Basin, showing the location of the studied area of the Paso Flores Formation, Neuquén Province, Patagonia, Argentina (modified from Vergani et al. 1995). Image taken by NASA, July 2004.
One of the initial syn-rift Upper Triassic siliciclastic continental units of the basin, is the Paso Flores Formation that crops out as isolated sections at the south of the Neuquén Basin, northern Patagonia, Argentina (Figs. 1, 2). In this region the basement is constituted by metamorphic and igneous rocks of the Late Palaeozoic age and by the Choiyoi magmatic-volcanic complex (upper Permian–Middle Triassic). Locally, the formation is overlain unconformably by an Lower Jurassic coastal marine siliciclastic sequence of the Nestares Formation (Zavattieri and Volkheimer 2001; Zavattieri et al. 2008) and/or by Cenozoic volcanic and sedimentary rocks (Fig. 2).
The Paso Flores strata were studied by Nullo (1979), González Díaz (1982), Lapido et al. (1984), Spalletti et al. (1988), Ganuza et al. (1995), and references therein. The type section of the unit is exposed at Cerro Mariana and surrounding areas at the Estancia Manantiales de Paso Flores, on the south-eastern margin of the Limay river along the boundary between the Río Negro and Neuquén provinces (Fig. 2). The outcrops of the Paso Flores Formation on either side of the Limay River (more than 3 km east of the Alicurá Dam), and those of the Lomas and Cañadón de Ranquel Huao area (Fig. 2) are the thickest sections, representing alluvial fan deposits and gravelly braided systems described in detail by Spalletti et al. (1988, 1990). These sections represent the lower to middle parts of the unit. The thinnest section of the Paso Flores Formation crops out at the Cañadón de Pancho area, on the western side of the Collón Curá and south of the Quemquemtreu rivers, in the south-west of the Neuquén Province (González Díaz 1982; Ganuza et al. 1995) (Figs. 2–3). That section has been considered to constitute the formation’s upper part (Zavattieri and Mego 2008).
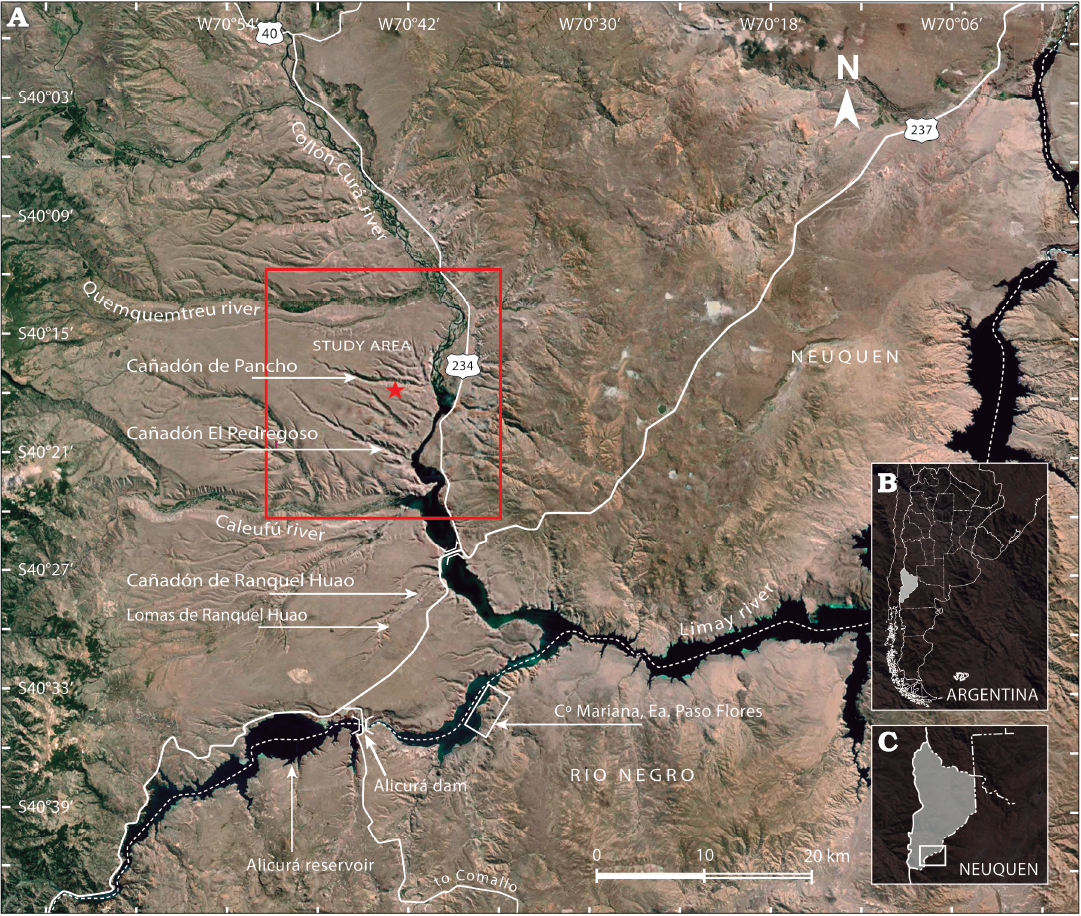
Fig. 2. Location of the study area within Argentina and southern region of the Neuquén Province and north-western region of the Río Negro Province (B, C). Aerial view of the study area showing the main outcropping sections of the Paso Flores Formation (A) (based on Spalletti et al. 1988 and González Díaz 1982). The star indicates the location of the studied fossil flora at Cañadón de Pancho described herein. Image taken from Google Earth (2016).
Cañadón de Pancho section.—In this area, the Paso Flores Formation crops out in an extensive area between the Quemquemtreu and Caleufú rivers (Figs. 2, 3). González Díaz (1982), for the first time, referred to the outcrops of the western margin of the Collón Curá River as belonging to the Paso Flores Formation. Here, it lies unconformably on granitic rocks (Huechulafquen Formation) and on the metamorphic complex (Cushamen Formation and equivalents) of the late Palaeozoic age (Varela et al. 2005), and, in turn, is covered discordantly by light-grey tuffitic continental sedimentary strata of the Collón Curá Formation (middle Miocene age) (Fig. 3). The Paso Flores Formation at the Cañadón de Pancho section is composed of four successive sedimentary facies associations described in detail by Ganuza et al. (1995): (i) lenticular beds of medium to fine clast-supported conglomerate, interbedded with lenticular coarse sandstone and conglomeratic sandstone deposited in a braided fluvial system; (ii) laterally persistent laminated and rippled mudstone and siltstone with scares and thin coal layers (marginal lacustrine facies) that upwards change to coarse and thick sandstone deposits (progradational mouth bars of deltaic system); (iii) interbedded tabular mudstone and lenticular sandstone beds, interpreted as deposits of a low-sinuosity meandering fluvial system; and (iv) lenticular coarse-grained sandstone and fine conglomerate formed in a braided fluvial system revealing renewed higher energy. The well-preserved megafloral remains and microfloral assemblages studied were recovered from low-sinuosity meandering fluvial systems, deltaic and marginal lacustrine siltstone, mudstone, and fine-grained sandstone deposits.
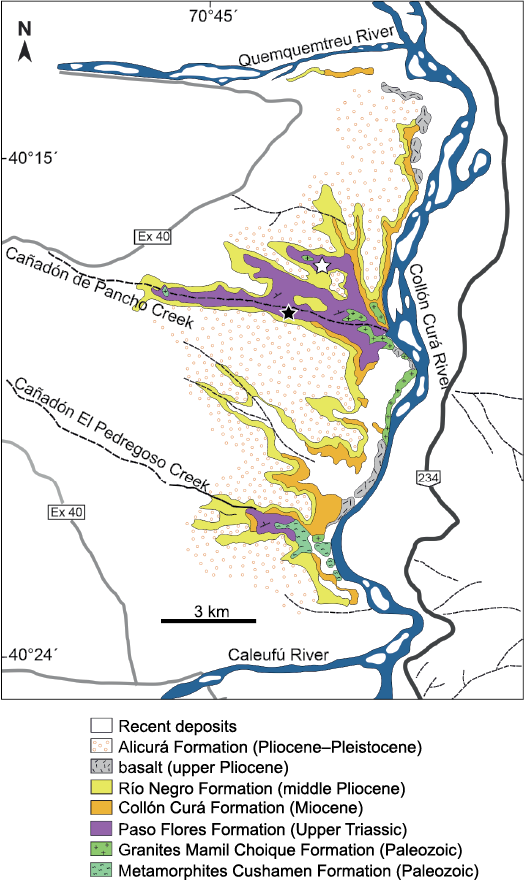
Fig. 3. Geologic map showing the outcrops of the Paso Flores Formation at Cañadón de Pancho area; white star shows the location of fossil flora recorded and studied previously (Spalletti et al. 1988; Arrondo et al. 1991; Ganuza et al. 1995; Artabe et al. 1994; Morel et al. 1999), black star shows the location of the studied plant remains described herein (adapted from González Díaz 1982).
Palaeobotanical records of the Paso Flores Formation.—The fossil plant content of the Paso Flores Formation has been studied by several authors (Frengüelli 1937; Bonetti and Herbst 1964; Spalletti et al. 1988; Arrondo et al. 1991; Morel et al. 1992, 1999; Ganuza et al. 1992; Herbst 1993; Artabe et al. 1994; Zamuner and Artabe 1994; Ganuza et al. 1995; Gnaedinger and Zavattieri 2017a, b), who recorded abundant and diverse megaflora assemblages. This fossil flora includes species of the following genera: Asterotheca, Marattiopsis, Cladophlebis, Coniopteris, Dictyophyllum, Thaumatopteris, Goeppertella, Dicroidium, Zuberia, Johnstonia, Xylopteris, Scleropteris, Pachydermophyllum, Heidiphyllum, Rissikia, Ginkgoites, Baiera, Sphenobaiera, Nilssonia, Pseudoctenis, Yabeiella, Taeniopteris, Kurtziana, Solenites, Czekanowskia, Cycadocarpidium, Linguifolium, Cordaicarpus, Umkomasia, Lutanthus, Rissikistrobus, and Protocircoporoxylon.
Age of the Paso Flores Formation.—It is based on its stratigraphic relationships and its floristic content (megaflora and microflora). Its plant assemblages are characterized by components of typical Southern Hemisphere Triassic “Dicroidium Flora”, together with the incoming morphotypes with strong Jurassic affinity. Thus, Spalletti et al. (1999, 2003), Artabe et al. (2003), and Morel et al. (1999, 2003) assigned the fossil flora of the Paso Flores Formation to the third Florian Stage (Florian floras) or Dictyophyllum tenuiserratum–Linguifolium arctum–Protocircoporoxylon marianensis Biozone of the Late Triassic (Norian–Rhaetian) age.
Zamuner et al. (2001), Zavattieri (2002), and Zavattieri and Volkheimer (2001) chronostratigraphically analyzed the distribution of the 25 species recorded for the first time in Paso Flores palynoflora at the Cañadón de Pancho locality. They assigned this microflora assemblage to the late Norian–Rhaetian age based on the co-occurrence of typical Triassic palynomorphs (Alisporites–Falcisporites microflora) together with Classopollis (= Corollina) simplex (Danzé-Corsin and Laveine, 1963) Reiser and Williams, 1969, and other Rhaetian species like Foveogleicheniidites atavus Raine in de Jersey and Raine, 1990, Foveosporites moretonensis de Jersey, 1964, Dictyophillidites atraktos Stevens, 1981 (Dipteridaceae dispersed spores), Retitriletes rosewoodensis (de Jersey, 1959) McKellar, 1974, among other species of Early Jurassic distribution (de Jersey and Raine 1990). The Classopollis (= Corollina) (Cheirolepidiaceae pollen) has been recorded previously in the type locality of the Paso Flores Formation at the Limay river (Zavattieri and Mego 2008) and in other Norian to Rhaetian units of Argentina (i.e., Tronquimalal Group, Malargüe depocenter). These Cheirolepidiaceae pollen grains are frequent to dominant in Jurassic strata of Argentina and Gondwana (Gnaedinger and Zavattieri 2017b, 2020, and references therein).
Therefore, the palaeobotanical content (macroflora and microflora) of the Paso Flores Formation is considered the youngest Triassic flora known in Argentina.
Material and methods
Eleven well-preserved medium to quite large adult frond fragment impressions were recovered. They were studied using a Leitz M50 stereoscopic microscope with an attached EC2 (LM) camera, and Nikon Coolpix P100 camera and scanning electron microscope (SEM Jeol 5800LV) at the Universidad Nacional del Nordeste (Corrientes Province, Argentina). For the description, we follow the terminology of Choo et al. (2016) and Choo and Escapa (2018).
The palaeobotanical material is housed in the Palaeontological Collection of the Museo Municipal “Carmen Funes”, Plaza Huincul city, Neuquén Province, Argentina, under the prefix MCF-PBPH.
Systematic palaeontology
Class Polypodiopsida Cronquist, Takhtajan, and Zimmerman, 1966
Order Gleicheniales Schimper, 1869
Family Dipteridaceae Seward and Dale, 1901
Genus Patagoniapteris nov.
PFNR: PFN002691
Etymology: In reference to Patagonia the geographical region situated in south Argentina and Chile to which the Neuquén Province belongs, and pteris refers to ferns.
Type species: Patagoniapteris artabeae sp. nov., monotypic.
Diagnosis.—Fan-shaped fronds dissected in two equal and opposite rachial arms have numerous primary segments (more than 18). Frond lamina between the primary segments fused at the base, the remaining part free, lanceolate, with undulated to deeply dissected margins. Primary and secondary veins simple, tertiary veins dichotomize and form irregularly polygonal meshes. Exindusiate sori arranged on either side of the primary veins, and of the base of secondary veins. Circular sori have more than 45 sporangia. Annulus oblique and complete.
Patagoniapteris artabeae sp. nov.
Figs. 4–8.
PFNR: PFN002692
Etymology: In honour of Analia B. Artabe, a recognized Argentinian palaeobotanist, for her important contributions mainly to the knowledge of Triassic and Jurassic floras of Argentina.
Type material: Holotype: MCF-PBPH 066, fertile frond fragment impression. Paratype: MCF-PBPH 415, fertile frond fragment impression; from the type locality and horizon.
Type locality: Cañadón de Pancho area, south-west of the Neuquén Province, Patagonia, Argentina (Figs. 2–3).
Type horizon: Upper part of the Paso Flores Formation, Late Triassic (late Norian–Rhaetian).
Material.—MCF-PBPH 067, 069, 074, 076, 077, 078, 079, 413, 416. Sterile and fertile frond fragment impressions from the type locality and horizon.
Diagnosis.—Fan-shaped fronds dissected in two equal and opposite rachial arms, each one having more than 18 primary segments. The primary segments fused up to 1/3 of the total length of the preserved lamina, and the remaining part free, lanceolate, showing undulated to deeply dissected margins (less than 2/3 length of the secondary veins). Primary and secondary veins simple, whereas tertiary veins dichotomized and forming irregular polygonal meshes. Exindusiate sori arranged in two regular rows on side of the primary veins, and on both sides of the base of secondary veins. Circular sori with 45–60 sporangia. Annulus oblique and complete.
Description.—Sterile and fertile frond fragments bilaterally symmetrical, large in size, maximum preserved length ca. 20 cm. Stipe up to 7 mm in width, unknown length; apex divided into two equal rachial arms on both sides, each one is 3–4 mm wide and has at least 18 primary segments (Figs. 4A1, A2, 5A, C). Primary segments attach directly the stipe, which is twisted forming a fan-structure in conjunction with the veins of successive primary segments. The lamina frond is fused up to 1/3 of the total length preserved in an extension of at least 6.5 cm long. This fused part has polygonal venation meshes (Figs. 4A1, A2, 5B, C). The free part of the frond is dissected (Figs. 4A3, 5B). The primary segments are lanceolate, showing undulated to deeply dissected margins (less than 2/3 in length of the secondary veins) (Figs. 4B1, B2, C, D1, D2, 5C). The lobes are triangular up to 2–2.5 cm long × 1.5 cm wide with acute apex (Figs. 4B1, B2, C, D1, D2, 5B). Primary and secondary veins are simple, tertiary veins are dichotomized forming irregular polygonal meshes (Figs. 4B–D, 5, 6A, B). Primary veins are up to 1.4 mm wide in the base and up to 0.5 mm wide in the preserved apical portion. The primary veins show scalariform pitting in the primary xylem tracheids (Fig. 8A1, A3). Secondary veins of the deep lobes are subopposite to alternate, and depart at angles between 45–70º and are 0.2–0.3 mm in wide. Between two successive secondary veins, there are other veins of the same thickness, reaching up to each interlobe sinus (Figs. 4B–D, 5, 6B, C). Tertiary veins are opposite, 0.1–0.3 mm, and departing from secondary veins at 50–60º, and dichotomize twice towards the margin of the lobes, and between them are horizontal veins forming a net (Fig. 6B). Sori are exindusiate and arranged on both sides of the primary veins, sometimes at the base on both sides of the secondary veins (Fig. 6C, D). Sori are circular, 1.22–1.45 mm; in each sorus 45–60 sporangia (preserved), although larger amount of sporangia is not excluded (Figs. 6C, D, 7, 8A2–A4). The sporangia are 142–197 µm in diameter and have a ring-like annulus. The annulus is oblique, complete, and composed of more than 12 cells (Fig. 8A5–A7).

Fig. 4. Dipteridacean fern Patagoniapteris artabeae gen. and sp. nov. from the upper Norian–Rhaetian, Upper Triassic Paso Flores Formation, Neuquén Province, Argentina. A. MCF-PBPH 066, part of the frond showing fused and dissected portions. A1, A2, basal portion of a rachial arm with the lamina of the primary segments fused. A3, detail showing primary segments with the dissected portion of the lamina. B. MCF-PBPH 415, primary segments of fertile frond; B1, B2, deeply dissected lobes. C, D. Primary segments of sterile frond. MCF-PBPH 067d (C) and MCF-PBPH 069 (D). C, D1, deeply dissected lobes. D2, detail of a lobe of the primary segment, showing secondary and tertiary veins. Scale bars 10 mm.
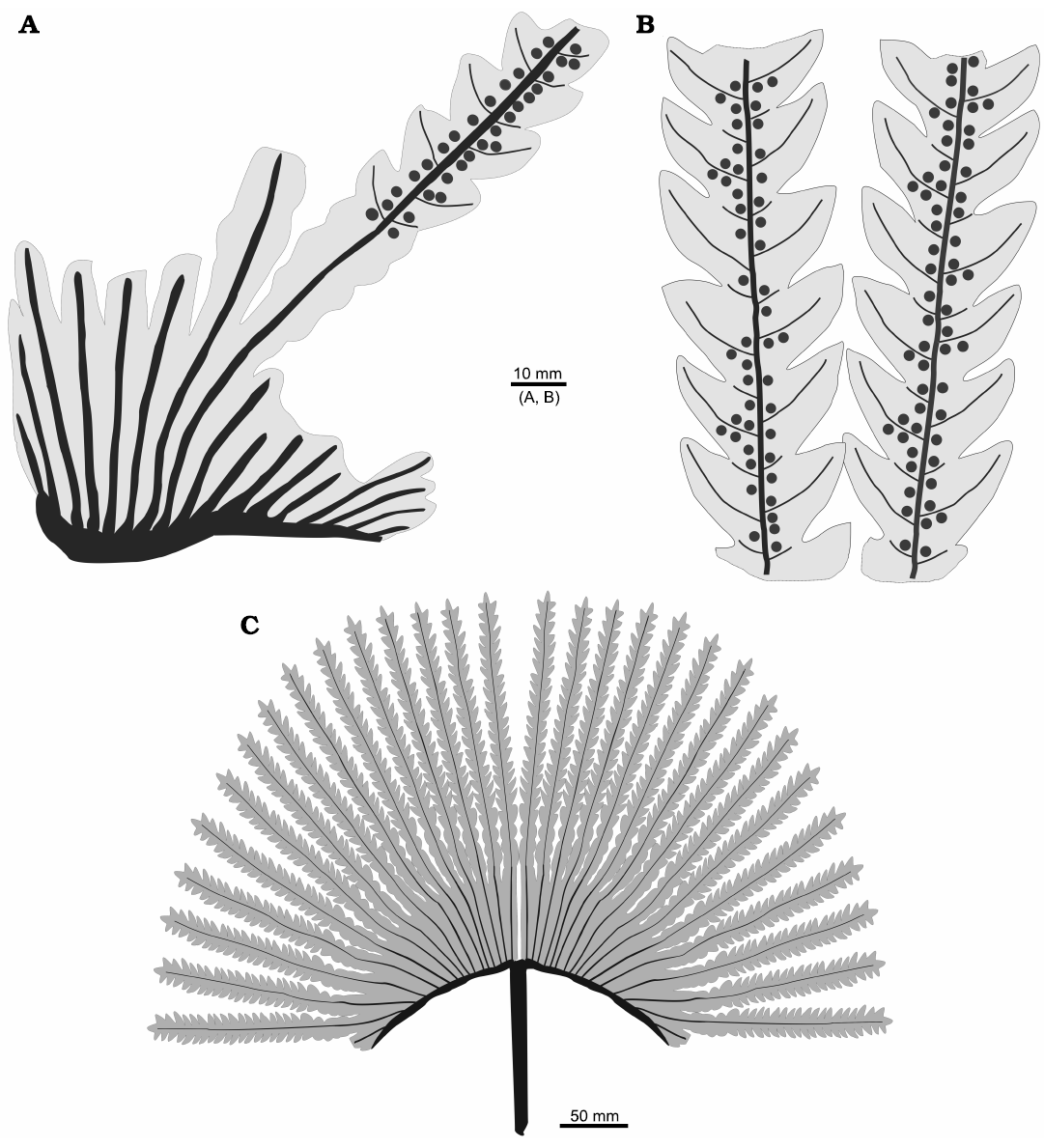
Fig. 5. Drawing of the dipteridacean fern Patagoniapteris artabeae gen. and sp. nov. from the upper Norian–Rhaetian, Upper Triassic Paso Flores Formation, Neuquén Province, Argentina. A. MCF-PBPH 066, basal portion of a rachial arm with the primary segments fused lamina. B. MCF-PBPH 415, primary segments of fertile frond with deeply dissected lobes. C. Hypothetical reconstruction of the frond.

Fig. 6. Dipteridacean fern Patagoniapteris artabeae gen. and sp. nov. from the upper Norian–Rhaetian, Upper Triassic Paso Flores Formation, Neuquén Province, Argentina. A. MCF-PBPH 074, detail of polygonal pattern venation of the primary segment fused lamina. B. MCF-PBPH 415, polygonal pattern of venation. B1, B2, details of the secondary and tertiary veins and polygonal meshes. B3, details of the dichotomized tertiary veins and polygonal meshes shown. C, D. Shape and distribution of sori. MCF-PBPH 076 (C) and MCF-PBPH 066 (D). View of the sori on both side of the primary veins, as well as at the base of the deep lobes on both sides of the secondary veins (arrows). Scale bars 5 mm.
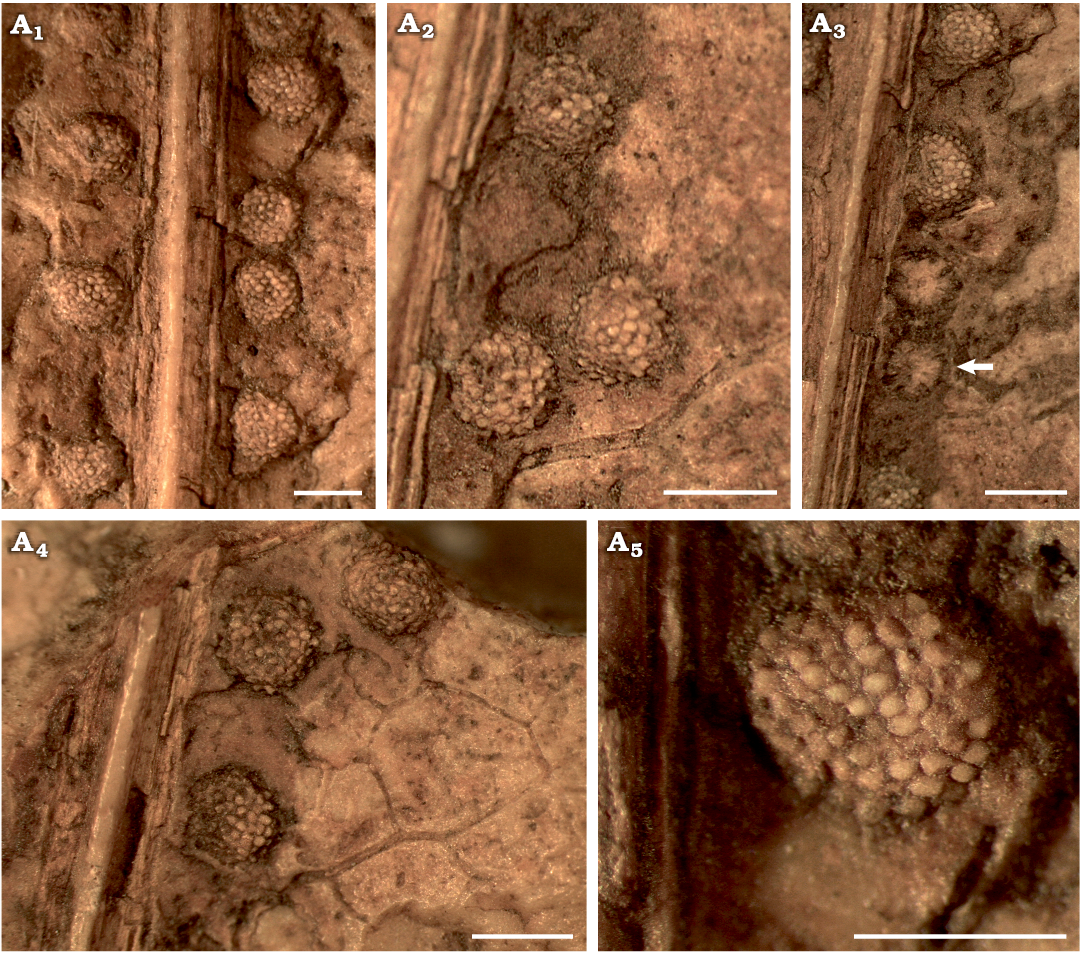
Fig. 7. Dipteridacean fern Patagoniapteris artabeae gen. and sp. nov. from the upper Norian–Rhaetian, Upper Triassic Paso Flores Formation, Neuquén Province, Argentina. A. MCF-PBPH 066, view of the sori. A1–A5, details of the sori with and without sporangia (arrow). Scale bars 1.5 mm.
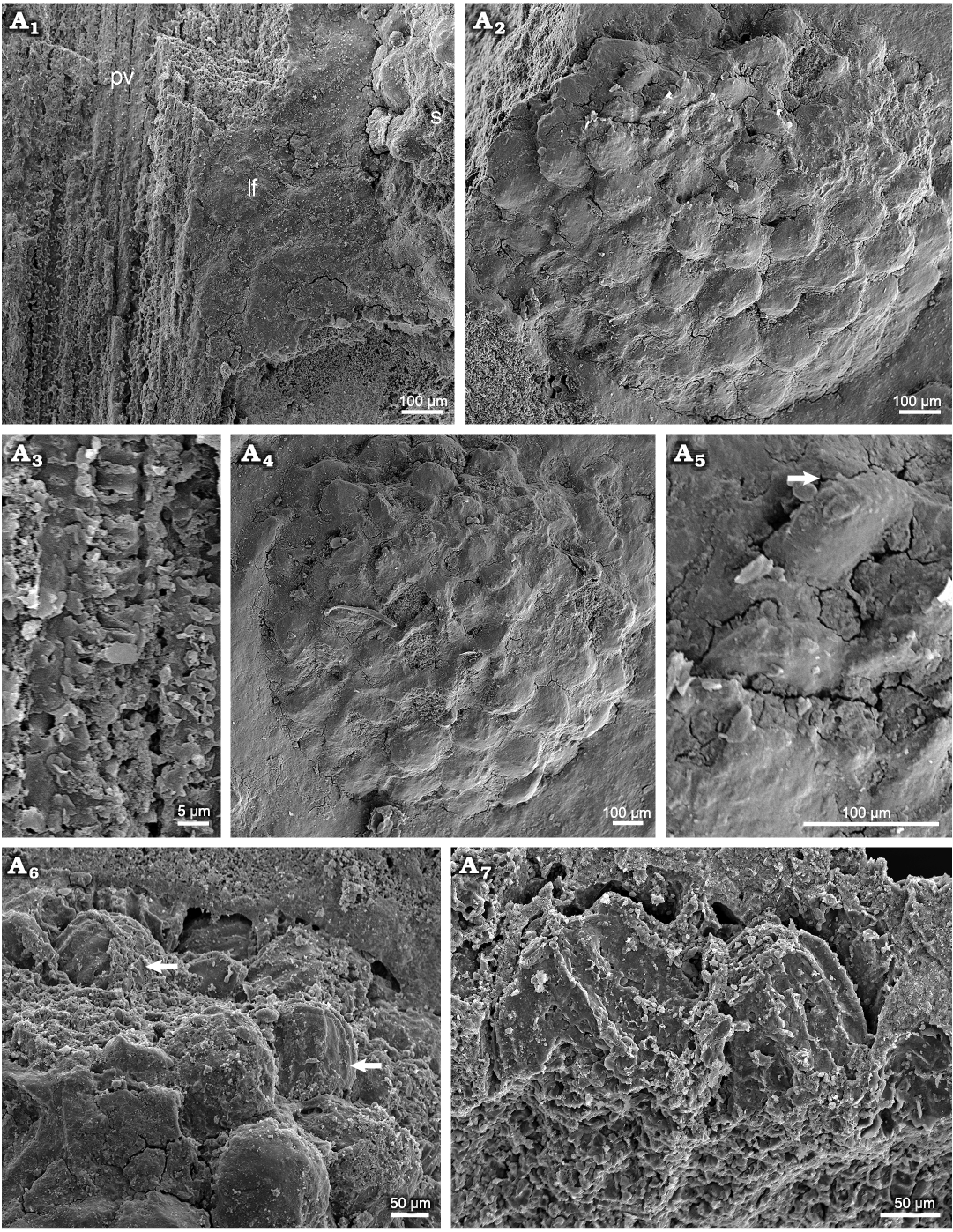
Fig. 8. Dipteridacean fern Patagoniapteris artabeae gen. and sp. nov. from the upper Norian–Rhaetian, Upper Triassic Paso Flores Formation, Neuquén Province, Argentina. MCF-PBPH 076, SEM images of part of the frond. A1, detail of the primary vein (pv), lamina of the frond (lf), and sorus (s); A2, A4, details of the sporangia of a sorus; A3, detail of the scalariform pitting of primary xylem tracheids in radial section of the primary vein; A5, detail of the annulus of a sporangia; A6, A7, details of the cells of the annulus of the sporangia.
Remarks.—Comparisons with fossil genera: In the Dipteridaceae, the morphological and reproductive features commonly used for the diagnoses of genera and species include: the number, size and shape of primary segments (Ôishi 1932; Frengüelli 1941; Ash 1970; Choo and Escapa 2018), tooth shape (Ôishi 1932), the angle of insertion of the secondary, tertiary and quaternary veins (Heer 1877; Ôishi 1940; Schweitzer et al. 2009), and the size and number of sporangia per sori (Ash 1970). The appearance of tertiary veins is an additional feature sporadically mentioned in the discussions of species boundaries, but rarely used as a diagnostic generic feature (Herbst 1966; Kon’no 1968; Choo et al. 2016).
Table 1 shows that the Paso Flores specimens share morphological features with those established for the Dipteridaceae: (i) with Clathropteris and Digitopteris they share frond lamina between primary segments fused up to 1/3 of the total length of the primary veins and the free apical portion; (ii) with Thaumatopteris and with some species of the Dictyophyllum they share the presence of deep lobes between secondary veins (more than 2/3 of the length of them); (iii) with Sewardalea they share the presence of numerous primary segments (more than 12); (iv) with Thaumatopteris, Dictyophyllum, and Digitopteris they share the polygonal venation meshes.
Table 1. Comparison of the morphology fronds and reproductive organ features between fossil and current genera of the Dipteridaceae (data taken from Seward and Dale 1901; Herbst 1992a, b; Smith et al. 2006; Choo and Escapa 2018; Zhang et al. 2013). Grey areas indicate features of different genera shared with the Paso Flores Formation specimens.
| |
Thaumatopteris |
Sewardalea |
Clathropteris |
Digitopteris |
Dictyophyllum |
Hausmannia |
Dipteris |
Patagoniapteris |
|
|
Initial stipe dichotomy |
anisotomous |
stipe with an isotomous initial dichotomy |
|||||||
|
Rachial arms |
two unequal |
two equal and opposite |
|||||||
|
Frond lamina |
dissected; |
dissected; primary segments linear and free |
primary segments fused up to 1/3 of the total length of the primary veins; apical portion dissected |
primary segments basally |
dissected; |
entire or slightly dissected, lobed and/or crenate margins |
deeply dissected primary segments |
primary segments fused up to 1/3 of the total length of the primary veins, the apical portion is free; primary segment margins are undulated to deeply dissected between secondary veins (more than 2/3 length of the secondary veins) |
|
|
primary |
|||||||||
|
apical portion free, linear and entire-margins |
primary |
||||||||
|
Primary segments per rachial arm |
3–6 |
more than 12, but up to over 100 |
3–12 |
less than 10 |
less than 15 |
bilobulate |
4–5 (1 or more times
dichotomized |
more than 18 |
|
|
Venation |
Primary and secondary veins |
simple |
simple |
simple |
primary veins simple; secondary veins dichotomized |
simple |
primary veins dichotomized |
primary veins dichotomized |
simple |
|
Meshes |
irregularly polygonal |
irregularly polygonal |
regular orthogonal |
polygonal |
irregularly |
quadrangular to polygonal |
polygonal |
irregularly polygonal |
|
|
Sori distribution |
scattered |
no data |
in the rectangular areole |
the complete lamina is covered by sori, one in each areole |
either side of primary and secondary veins, |
the complete lamina is covered
by sori, grouped |
the complete lamina is covered by sori, |
a single row on each side of the primary veins, as well as on both sides of the base of the secondary veins |
|
|
and crowded on the lamina |
except D. lobbiana in a single row on each side of the primary veins |
||||||||
|
Sporangia |
1–4 |
– |
few |
– |
see Table 2 |
4–6 |
see Table 2 |
>45 |
|
|
Age |
Middle Triassic–Early Jurassic |
Late Triassic–Early Jurassic |
Late Triassic–Early Jurassic |
Late |
Middle |
Middle Triassic–Early Cretaceous |
Extant |
Late Triassic |
|
The presence of sori in the Dipteridaceae classification, has different considerations. Harris (1931) showed an evolutionary sequence from well-defined sori of acrosticoid disposition, whereas Ôishi and Yamasita (1936) considered that: (i) the shape of sori are basically the same, being circular or rounded, and the size of the sori decrease in the following order of the genera: Hausmannia–Thaumatopteris–Dictyophyllum; (ii) the number of sporangia is not constant, even in one species, however, it decreases according to the following approximate genera order: Thaumatopteris–Clathropteris–Hausmannia–Dictyophyllum–Camptopteris; (iii) the size of the sporangia decreases according to the increase in size of sori; (iv) the annulus is sometimes oblique and mostly complete and each annulus usually has up to 30 cells. On the other hand, in the systematic review of the family, Herbst (1992a, b) remarked that he does not use the characteristics of the sori (or sporangia) because they are similar in all Dipteridaceae. In genera such as Dictyophyllum and Thaumatopteris (see Tables 1 and 2) the distribution of the sporangia can be: in acrosticoid form (regularly or irregularly distributed) or grouped in sori (also regularly or irregularly) (Ôishi and Yamasita 1936).
So far, there are several species described in the literature that provide some descriptions of reproductive structures (Table 2). The species that have sori distributed in the primary veins are: Dictyophyllum davidii Walkom, 1917, and D. bremerense Shirley, 1898. The first mentioned species differs from the material of Argentina in the elongated shape of the sori, and from the second one by the number of sporangia per sori (Table 2).
Based on the morphological features of the fronds and the distribution of sori and number of sporangia, the Paso Flores Formation specimens are clearly distinct from other dipteridacean genera, justifying the establishment of Patagoniapteris artabeae gen. and sp. nov. (Tables 1, 2).
Table 2. Comparison of sori among Dipteridaceae taxa (updated from Ôishi and Yamasita 1936; Webb 1982; Herbst 1992a, b; Guignard et al. 2009).
|
Species |
Shape |
Arrangement |
Number of sporangia |
|
Dictyophyllum bremerense Shirley, 1898 |
elongated or circular |
elongated on either side of primary and secondary veins; circular well-spaced or crowded on abaxial surface |
at least 30 |
|
Dictyophyllum davidi Walkom, 1917 |
elongated or circular |
elongated on either side of primary and secondary veins; circular scattered over abaxial surface |
at least 25 |
|
Dictyophyllum ellenbergii Fabre and Greber, 1960 |
elongated or circular |
elongated on either side of
primary veins; circular |
– |
|
Dictyophyllum rugosum Lindley and Hutton, 1834 |
circular |
densely crowded over whole abaxial surface |
1–4 |
|
Dictyophyllum tenuifolium (Stipanicic and Menéndez, 1949) Bonetti and Herbst, 1964 |
circular |
irregularly distributed along the primary veins and at the base of the secondary veins, as well as on the abaxial surface occasionally |
5–7 |
|
Thaumatopteris brauniana Popp, 1863 |
circular |
well-spaced over whole abaxial surface; rarely slightly elongated along primary veins |
3–30 (average 12–15) |
|
Thaumatopteris rocablanquensis Herbst, 1965 |
circular |
very crowded over whole abaxial surface |
20–40 |
|
Thaumatopteris tenuiserrata Menéndez, 1951 |
circular |
well-spaced, over whole abaxial surface and 2 to 3 between the secondary veins |
– |
|
Sewardalea nathorstti (Zeiller, 1903) Choo and Escapa, 2018 (= Dictyophyllum nathorstii Zeiller, 1903) |
circular |
well-spaced over whole abaxial surface predominantly near primary and secondary veins |
5–8 |
|
Sewardalea exile (Brauns, 1862) Choo and Escapa, 2018 (= Dictyophyllum exile (Brauns, 1862) Nathorst, 1978) |
circular |
very crowded over whole abaxial surface between tertiary veins |
4–7 or 3–5 |
|
Sewardalea falcata (Naito in Kon’no, 1968) Choo and Escapa, 2018 (= Dictyophyllum falcatum Naito in Kon’no, 1968) |
circular |
crowded over whole abaxial surface |
6–7 |
|
Clathropteris meniscioides (Brongniart, 1825) Brongniart, 1828 |
circular |
scattered on the abaxial surface |
8–15 |
|
Clathropteris obovata Ôishi, 1832 |
circular |
scattered on the abaxial surface |
10–20 |
|
Dipteris conjugata Reinwardt, 1828 |
variable |
crowded over whole abaxial surface |
12–17 |
|
Dipteris lobbiana (Hooker, 1853) Moore, 1857 |
circular |
a single row on either side of the primary veins |
average 30 |
|
Patagoniapteris artabeae gen. and sp. nov. |
circular |
a single row on each side of the primary veins, as well as on both sides of the base of the secondary veins |
45–60 |
Dictyophyllum tenuifolium (Stipanicic and Menéndez, 1949) Bonetti and Herbst, 1964, and Dictyophyllum (Thaumatopteris) rothi (Frengüelli, 1941) Bonetti and Herbst, 1964, were previously described from the Paso Flores Formation. Patagoniapteris artabeae gen. and sp. nov., differs from the first mentioned species in the number of sporangia per sori (Table 2). It differs from the second species because this new taxon herein described has partially fused lamina fronds and more than 15 rachial arms (Table 1).
Comparisons with extant genera: Seward and Dale (1901) proposed that the genus Dipteris should be representative of the family Dipteridaceae. Morphologically, the leaf architecture of Dipteris is quite characteristic. The fronds branch dichotomously with veins that split forming a mesh pattern. Sori are exindusiate and arranged following the primary veins or distributed on abaxial surface on both sides of the primary veins. The annulus of the sporangia is oblique.
The distribution of the sori in Dipteris conjugata Reinwardt, 1828, covers the whole abaxial surface of the large frond, although they are frequently concentrated on either side of the primary veins with 12–17 sporangia (Table 2). In Dipteris lobbiana (Hooker, 1853) Moore, 1857, the sori are situated on the abaxial surface of the narrow lamina in two regular rows on either side of the primary veins, which contains an average of 35 sporangia (Armour 1907). The other species of Dipteris either resemble one of these two species or form intermediates between them (Armour 1907; Webb 1982) (Table 2).
Webb (1982) interpreted that in modern species, this division appears to be mainly a function of the width of the lamina on either side of the primary veins (wide in Dipteris conjugata, narrow in Dipteris lobbiana). This is not the case in Patagoniapteris artabeae gen. and sp. nov. because it has a broad lamina like Dipteris conjugata and sori distribution and number of sporangia similar to Dipteris lobbiana (Tables 1, 2).
Stratigraphic and geographic range.—Type horizon and locality only.
Discussion
In the recent phylogenetic analysis, Choo and Escapa (2018) provided an enlightening analysis into the evolution of Gleicheniales as a whole, and of Dipteridaceae in particular. Patagoniapteris artabeae gen. and sp. nov. could be considered a new transitional form among Dipteridacae because it shares some morphological and reproductive features with several fossil genera and the current Dipteris (see Table 1 and 2): (i) with Thaumatopteris it has a common deep lobes between secondary veins, and with Sewardalea it has in common numerous primary segments; but neither genera have the fused base of the lamina; (ii) Clathropteris, many Dictyophyllum and Digitopteris species have primary segments up to 1/3 fused at the base. However, the first genus is noted for its orthogonal venation and the second has less than 15 rachial arms, while the third has apical portion segments with whole margins. Dipteris, on the other hand, has a sori distribution and a number of sporangia similar to Patagoniapteris artabeae gen. and sp. nov., but differs from this new taxon because the frond is deeply dissected and the primary veins are dichotomized (Fig. 5C, Tables 1, 2; Choo and Escapa 2018: fig. 3).
Bodnar et al. (2018) pointed out that during the Triassic, the Dipteridaceae ferns had a restricted spatio-temporal distribution in Argentina related to humid conditions: (i) the earliest occurrence was recorded in the Barreal Formation of late Anisian–early Ladinian age, Barreal-Calingasta Depocenter, San Juan Province, Cuyana Basin, were less diverse and their frond size smaller, and (ii) in the late Norian–Rhaetian interval these ferns had their maximum diversification with the development of individuals with large fronds, registered at the north-eastern end of the Neuquén Basin, in the Malargüe Depocenter (Tronquimalal Group), Mendoza Province (Gnaedinger and Zavattieri 2020, and references therein), and in the south-southwestern region of the basin, at Paso Flores depocenter (Gnaedinger and Zavattieri 2017b, and references therein). Such diversity and morphological conditions of the Dipteridacean family could indicate that at the end of the Triassic, the humidity regimen was more benign than in the Middle Triassic (Bodnar et al. 2018).
Spalletti et al. (2003) and Artabe et al. (2003) analyzed the palaeogeographical reconstructions and the distribution of plants within the phytogeographic Triassic provinces of south-southwestern Gondwana. Towards the end of the Triassic, in Argentina, the third recognized floristic event, the Florian Stage (late Norian–Rhaetian) (Spalletti et al. 1999) was characterized by an important change in plant composition, when the long-lasting endemic Triassic Dicroidium-dominated communities declined and the incoming of taxa that persisted during the Jurassic occurred. At this time, the basins located on the western margin of southern South America had a seasonal temperate-warm and subtropical humid to sub-humid climate with marine influence from the west, allowing for more humid conditions, characterized by monsoonal climates (Artabe et al. 2001, 2003; Spalletti et al. 2003).
The Dipteridaceae grew in herb-shrub and tree communities developed on floodplains of braided and meandering fluvial systems (Artabe et al. 2001). The record of the new taxon, Patagoniapteris artabeae gen. and sp. nov. in the uppermost section of the Paso Flores Formation in the Cañadón de Pancho area, is congruent with above the mentioned pattern of distribution and climatic and environmental conditions.
Conclusions
A new taxon, Patagoniapteris artabeae gen. and sp. nov. is established based on sterile and fertile fronds showing affinities with fossil and extant Dipteridaceae from the Paso Flores Formation, late Norian–Rhaetian, Neuquén Basin, Argentina.
Patagoniapteris artabeae gen. and sp. nov. shows a unique combination of morphological and reproductive features that do not belong to any described genera of Dipteridaceae. It comprises an interesting combination because it shares some morphological frond features with the fossil genera Clathropteris, Digitopteris, Thaumatopteris, Sewardalea, and with some species of Dictyophyllum. It also has some reproductive features in common with the extant species Dipteris lobbiana (e.g., sori distribution and number of sporangia).
Patagoniapteris artabeae gen. and sp. nov. represents a new transitional form which shows an increasing complexity in the venation patterns and laminal fusion. In addition, it shows an increase in the number of sporangia per sori, only observed in the Recent genus Dipteris. The Cañadón de Pancho plant assemblages are late Norian–Rhaetian in age, being the youngest fossil flora recorded from Argentinian Triassic basins to date. The record of the new taxon, Patagoniapteris artabeae gen. and sp. nov. in the uppermost section of the Paso Flores Formation at Cañadón de Pancho area, is consistent with the higher diversity of Dipteridaceae at the end of the Triassic in Argentina. In the western margin of southern South America, during “Florian Stage” (late Norian–Rhaetian), the climate was temperate-warm seasonal subtropical with humid conditions influenced by the penetration of maritime air masses from the west (Spalletti et al. 2003). These humid to sub-humid regimen favoured the development of the Dipteridaceae with large fronds.
Acknowledgements
We wish to thank Rodolfo Coria (CONICET-Neuquén, Argentina), who led the Project “Searching for primitive dinosaurs in the Late Triassic of Northern Patagonia, Neuquén, Argentina”, which was financed by The Dinosaur Society (1996–1997) and in the frame of which the plant material herein described was collected. Furthermore, as Managing Director R. Coria allowed us to access to the Paleontological Collection of the Museo Municipal “Carmen Funes”, Plaza Huincul, Argentina, to study the plant assemblages housed therein. We also extend our appreciation and gratitude to Flavio Bellardini (Paleontological Curator of the Museo Municipal “Carmen Funes”), for his constant encouragement and assistance with the materials and repository. Grateful acknowledgement is extended to Eugenia Zavattieri (illustrator and graphic designer, Puerto Madryn, Argentina) for the improvement of the photo-micrographic figures and plates. Thanks are due to an anonymous reviewer and Maria Barbacka (Hungarian Natural History Museum, Budapest, Hungary) for their insightful comments and corrections that improved the manuscript. The manuscript greatly benefited from thorough review by Roslyn Lacey Wallace (Houston, USA) who made extensive English language and stylistic improvements. This research has been partially funded by the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (research grants ANPCYT–FONCyT, Argentina, PICT 2011-2546 to AMZ). Parts of the research that led to the study of this material were financially supported by the Secretaría General de Ciencia y Técnica, SGCYT-UNNE, Corrientes Province (PI 2018-2022, F013 to SCG) and CONICET (PIP 2014-2016, 112 201301 00317 to SCG-AMZ).
References
Andrae, K.J. 1853. Beitrage zur Kenntnis der fossilen Flora Siebenburgens und des Banates. Abhandlungen der Kaiserlich-Königlichen Geologischen Reichanstalt 2: 1–48.
Armour, H.M. 1907. On the sorus of Dipteris. New Phytologist 6: 238–244. Crossref
Arrondo, O.G. and Petriella, B. 1982. Revisión del género Goeppertella Ôishi y Yamasita emend (Goeppertelloideae–Dipteridaceae). Ameghiniana 19: 67–78.
Arrondo, O.G., Spalletti, L.A., Morel, E., and Ganuza, D.G. 1991. The sedimentological and paleobotanical characteristics of an Upper Triassic–Lower Liassic basin in northwestern Patagonia (Argentina). In: H. Ulbrich and A.C. Rocha-Campos (eds.), Gondwana Symposium 7º Proceedings, Instituto de Geociencias, Universidade de Sao Paulo 714: 517–532.
Artabe, A.E., Morel, E.M., and Spalletti, L.A. 2001. Paleoecología de las floras triásicas argentinas. In: A.E. Artabe, E.M. Morel, and A.B. Zamuner (eds.), El Sistema Triásico de Argentina, 199–225. Fundación Museo de La Plata “Francisco Pascasio Moreno”, La Plata.
Artabe, A.E., Morel, E.M., and Spalletti, L.A. 2003. Caracterización de las provincias fitogeográficas triásicas del Gondwana Extratropical. Ameghiniana 40: 387–405.
Artabe, A.E., Morel, E.M., and Zamuner, A.B. 1994. Estudio paleobotánico y taxonómico en la Formación Paso Flores (Triásico Superior), en el Cañadón de Pancho, Neuquén, Argentina. Ameghiniana 31: 153–160.
Ash, S.R. 1970. Ferns From the Chinle Formation (Upper Triassic) in the Fort Wingate Area, New Mexico. 72 pp. United States Government Printing Office, Washington D.C. Crossref
Bodnar, J., Drovandi, J.M., Morel, E.M., and Ganuza, D.G. 2018. Middle Triassic dipterid ferns from west-central Argentina and their relationship to palaeoclimatic changes. Acta Palaeontologica Polonica 63: 397–417. Crossref
Bomfleur, B. and Kerp, H. 2010. The first record of the dipterid fern leaf Clathropteris Brongniart from Antarctica and its relation to Polyphacelus stormensis Yao, Taylor and Taylor nov. emend. Review of Palaeobotany and Palynolology 160: 143–153. Crossref
Bonetti, M.I.R. and Herbst, R. 1964. Dos especies de Dictyophyllum del Triásico de Paso Flores. Provincia del Neuquén, Argentina. Ameghiniana 3: 273–279.
Brauns, D. 1862. Der Sandstein bei Seinstedt unweit desFallsteins und die in ihm vorkommenden Pflanzenreste. Palaeontographica 9: 47–62.
Brongniart, A. 1825. Observations sur les végétaux fossiles renfermés dans les Grès de Hoer en Scanie. Annales des Sciences Naturelles 4: 200–224.
Brongniart, A. 1828. Prodrome d’une histoire des végétaux fossiles. viii + 223 pp. Levrault, Paris. Crossref
Cantrill, D.J. 1995. The occurrence of the fern Hausmannia Dunker (Dipteridaceae) in the Cretaceous of Alexander Island, Antarctica. Alcheringa 19: 243–254. Crossref
Choo, T.Y.S. and Escapa, I.H. 2018. Assessing the evolutionary history of the fern family Dipteridaceae (Gleicheniales) by incorporating both extant and extinct members in a combined phylogenetic study. American Journal of Botany 105: 1–14. Crossref
Choo, T.Y.S., Escapa, I.H., and Bomfleur, B. 2016. Monotypic colonies of Clathropteris meniscioides (Dipteridaceae) from the Early Jurassic of central Patagonia, Argentina: implications for taxonomy and palaeoecology. Palaeontographica B 294: 85–109. Crossref
Corsin, P. and Waterlot, M. 1979. Palaeobiogeography of the Dipteridaceae and Matoniaceae of the Mesozoic. In: B. Laskar and C.S.R. Rao (eds.), Fourth International Gondwana Symposium Papers, 51–70. Hindustan Publishing, Delhi.
Cronquist, A., Takhtajan, A., and Zimmerman, W. 1966. On the higher taxa of Embryobionta. Taxon 15: 129–134. Crossref
Danzé-Corsin, P. and Laveine, J.P. 1963. Microflore. In: P. Briche, P. Danzé-Corsin, and J.P. Laveine (eds.), Flore infraliassique du Boulonnais. Société Géologique du Nord Mémoire 13: 57–143.
D’Elía, L., Muravchik, M., Franzese, J.R., and López, L. 2012. Tectonostratigraphic analysis of the Late Triassic–Early Jurassic syn-rift sequence of the Neuquén Basin in the Sañicó depocentre, Neuquén Province, Argentina. Andean Geology 39: 133–157. Crossref
De Jersey, N.J. 1959. Jurassic spores and pollen grains from the Rosewood Coalfield. Queensland Government Mining Journal 60: 346–366.
De Jersey, N.J. 1964. Triassic spores and pollen from the Bundamba Group. Geological Survey of Queensland Publication 321: 1–21.
De Jersey, N.J. and Raine, J.I. 1990. Triassic and earliest Jurassic miospores from the Murihiku Supergroup, New Zealand. New Zealand Geological Survey Paleontological Bulletin 62: 1–164.
Dunker, W. 1846. Monographie der norddeutschen Wealdenbildung. 85 pp. Oehme, Braunschweig.
Fabre, J. and Greber, C. 1960. Presence d’un Dictyophyllum dans la flore Molteno du Basutoland (Afrique Australe). Bulletin de la Société Géologique de France S7-II: 178–182. Crossref
Frengüelli, J. 1937. La flórula Jurásica de Paso Flores en el Neuquén, con referencias a la de Piedra Pintada y otras floras jurásicas argentinas. Revista del Museo de La Plata (Nueva Serie) 1. Paleontología 3: 67–108.
Frengüelli, J. 1941. Las Camptopterídeas del Lias de Piedra Pintada en el Neuquén (Patagonia). Revista del Museo de La Plata, nueva serie, Paleontología 27: 27–57.
Ganuza, D., Morel, E.M., Spalletti, L.A., and Arrondo, O.G. 1992. Las plantas fósiles triásicas en pelitas lacustres del Cañadón de Pancho (Formación Paso Flores) Provincia del Neuquén. VIII Simposio Argentino de Paleobotánica y Palinología. Asociación Paleontológica Argentina. Publicación Especial 2: 55–58.
Ganuza, D.G., Spalletti, L.A., Morel, E.M., and Arrondo, O.G. 1995. Paleofloras y sedimentología de una sucesión lacustre-fluvial del triásico tardío: La Formación Paso Flores en Cañadón de Pancho, Neuquén, Argentina. Ameghiniana 32: 3–18.
Gnaedinger, S. and Zavattieri A.M. 2017a. A new name for Baiera taeniata Geinitz, Ginkgo taeniata (Geinitz) Frengüelli and Sphenobaiera taeniata (Geinitz) Morel, Ganuza and Zúñiga. Ameghiniana 54: 252–254. Crossref
Gnaedinger, S. and Zavattieri A.M. 2017b. First record of Voltzialean male cone (Lutanthus) and podocarpacean female cone (Rissikistrobus) from the Late Triassic of Argentina, including new plant remains from the Paso Flores Formation. Ameghiniana 54: 224–246. Crossref
Gnaedinger, S. and Zavattieri A.M. 2020. Coniferous woods from the Upper Triassic of southwestern Gondwana, Tronquimalal Group, Neuquén Basin, Mendoza Province, Argentina. Journal of Paleontology 94: 387–416. Crossref
Goeppert, H.R. 1841–1846. Die Gattungen der fossilen Pflanzen verglichen mit denen der Jetztwelt und durch Abbildungen erläutert. 151 pp. Henry and Cohen, Bonn.
González Díaz, E.F. 1982. Sedimentitas del Triásico superior continental en el valle del Río Collón Curá, entre los arroyos Quemquemtreu y Caleufú, Provincia de Neuquén. Revista de la Asociación Geológica Argentina 37: 214–220.
Guignard, G., Wang, Y., Ni, Q., Tian, N., and Jiang, Z. 2009. A dipteridaceous fern with in situ spores from the Lower Jurassic in Hubei, China. Review of Palaeobotany and Palynology 156: 104–115. Crossref
Gulisano, C.A., Gutiérrez Pleimling, A.R., and Digregorio, R.E. 1984. Esquema estratigráfico de la secuencia jurásica del oeste de la provincia del Neuquén. 9º Congreso Geológico Argentino, San Carlos de Bariloche, Actas 1: 236–259.
Harris, T.M. 1931. The fossil flora of Scoresby Sound East Greenland. Part 1: Cryptogams (exclusive of Lycopodiales). Meddelelser om Grønland 85: 1–104.
Heer, O. 1877. Flora Fossilis Helvetiae die Vorweltliche Flora der Schweiz. vi + 182 pp. Verlag von J. Wurster & Co., Zürich.
Herbst, R. 1964. La flora liásica de la zona del río Atuel, Mendoza, Argentina. Revista de la Asociación Geológica Argentina 19: 108–131.
Herbst, R. 1965. La flora fósil de la formación Roca Blanca, provincia de Santa Cruz, Patagonia. Con consideraciones geológicas y estratigráficas. Opera Lilloana 12: 1–101.
Herbst, R. 1966. Revisión de la flora liásica de Piedra Pintada provincia de Neuquén Argentina. Revista del Museo de La Plata (Nueva Serie) 9 Paleontología 30: 27–53.
Herbst, R. 1992a. Propuesta de clasificación de las Dipteridaceae, con un Atlas de las especies argentinas. D’Orbignyana 6: 1–71.
Herbst, R. 1992b. Propuesta de clasificación de las Dipteridaceae. Publicación Especial de la Asociación Paleontológica Argentina 2: 69–72.
Herbst, R. 1993. Dipteridaceae (Filicales) del Triásico del Arroyo Llantenes (provincia de Mendoza) y de Paso Flores (provincia del Neuquén), Argentina. Ameghiniana 30: 155–162.
Hooker, W.J. 1853. Hooker’s Journal of Botany and Kew Garden Miscellany. Vol. 5. 416 pp. Lovell, Reeve, Henrietta Street. Covent Garden, London.
Howell, J.A., Schwarz, E., Spalletti, L.A., and Veiga, G.D. 2005. The Neuquén Basin: an overview. In: G.D. Veiga, L.A. Spalletti, J.A. Howell, and E. Schwarz (eds.), The Neuquén Basin, Argentina: a Case Study in Sequence Stratigraphy and Basin Dynamics. Geological Society, London, Special Publications 252: 1–14. Crossref
Kon’no, E. 1968. Some Upper Triassic species of Dipteridaceae from Japan and Borneo. Journal of the Proceedings of the Linnean Society (Botany) 61: 93–105. Crossref
Kramer, K.U., Green, P.S., and Gotz, E. 1990. Pteridophytes and Gymnosperms. Vol. 1. The Families and Genera of Vascular Plants. 424 pp. Springer-Verlag, New York.
Kustatscher, E. and van Konijnenburg-van Cittert, J.H.A. 2011. The ferns of the Middle Triassic flora from Thale. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 261: 209–248. Crossref
Lapido, O.R., Lizuaín, A., and Núñez, E. 1984. La cobertura sedimentaria Mesozoica. In: V. Ramos (ed.), Geología y recursos naturales de la Provincia de Río Negro. Relatorio 9º Congreso Geológico Argentino 1: 139–162.
Legarreta, L. and Gulisano, C.A. 1989. Análisis estratigráfico secuencial de la Cuenca Neuquina (Triásico superior–Terciario inferior). In: G. Chebli and L. Spalletti (eds.), Cuencas Sedimentarias Argentinas. Serie Correlación Geológica 6, 221–243. Universidad Nacional de Tucumán, Tucumán.
Legarreta, L. and Uliana, M.A. 1996. The Jurassic succession in west-central Argentina: stratal pattern, sequences and paleogeographic evolution. Palaeogeography, Palaeoclimatology and Palaeoecology 120: 303–330. Crossref
Lindley, J. and Hutton, W. 1834. The Fossil Flora of Great Britain. 223 pp. James Ridgway and Sons, London.
McKellar, J.L. 1974. Jurassic miospores from the upper Evergreen Formation, Hutton Sandstone and basal Injune Creek Group, northeastern Surat Basin. Geological Survey of Queensland Publication 361, Palaeontological Papers 35: 1–89.
Menéndez, C.A. 1951. La flora mesozoica de la Formación Llantenes (provincia de Mendoza). Revista del Instituto Nacional de Investigaciones en Ciencias Naturales (Botánica) 2: 147–261.
Moore, T. 1857. Index Filicum. 204 pp. William Pamplin, London.
Morel, E.M., Ganuza, D.G., and Zúñiga, A. 1999. Revisión paleoflorística de la Formación Paso Flores, Triásico superior de Río Negro y del Neuquén. Revista de la Asociación Geológica Argentina 54: 389–406.
Morel, E.M., Spalletti, L.A., Arrondo, O.G., and Ganuza, D.G. 1992. Los estratos plantíferos de la Formación Paso Flores (Triásico Superior) de las Lomas y Cañadón de Ranquel Huao, provincia del Neuquén, Argentina. Revista del Museo de La Plata (Nueva Serie) 9 Paleontología 58: 199–221.
Nathorst, A.G. 1878. Floran vid Höganäs och Helsingborg. Kungliga Svenska Vetenskapsakademiens Handlingar 16: 1–53.
Nullo, F.E. 1979. Descripción geológica de la Hoja 39c, Paso Flores, Provincia de Río Negro. Boletín Servicio Geológico Nacional 167: 1–70.
Ôishi, S. 1932. The Rhaetic plants from the Nariwa District Prov. Bithcu (Okayama Prefecture), Japan. Journal of the Faculty of Science, Hokkaido Imperial University Series 4, Geology and Mineralogy 1: 257–379.
Ôishi, S. 1940. The Mesozoic floras of Japan. Journal of the Faculty of Science, Hokkaido University. Series 4, Geology and Mineralogy 5: 123–480.
Ôishi, S. and Yamasita, K. 1936. On the fossil Dipteridaceae. Journal of the Faculty of Science, Hokkaido Imperial University Series 4, Geology and Mineralogy 3: 135–184.
Popp, O. 1863. Der Sandstein von Jägersberg bei Forchheim und die in ihm vorkommende Pflanzen. Neues Jahrbuch für Mineralogie und Geologie 1863: 399–417.
Pott, C., Bouchal, J.M., Choo, T.Y.S., Yousif, R., and Bomfleur, B. 2018. Ferns and fern allies from the Carnian (Upper Triassic) of Lunz am See, Lower Austria: A melting pot of Mesozoic fern vegetation. Palaeontographica, Abteilung B: Palaeobotany – Palaeophytology 297 (1–6): 1–101. Crossref
Presl, C.B. 1851. Epimeliae Botanicae Abhandlungen der Böhmischen Gesellschaft der Wissenschaften 6: 361–624.
Rees, P.M. 1993. Dipterid ferns from the Mesozoic of Antarctica and New Zealand and their stratigraphical significance. Palaeontology 36: 637–656.
Rees, P.M. and Cleal, C.J. 2004. Lower Jurassic floras from Hope Bay and Botany Bay, Antarctica. Special Papers in Palaeontology 72: 1–90.
Reinwardt, C.G.C. 1828. Nova Plantarum indicarum genera. In: C.F. Hornschuch (ed.), Sylloge Plantarum Novarum itemque minus cognitarum, Tomus secundus, 3. Regensburgische Botanische Gesellschaft, Regensburg.
Reiser, R.F. and Williams, A.J. 1969. Palynology of the lower Jurassic sediments of the northern Surat Basin. Geological Survey of Queensland 399, Palaeontological Papers 15: 1–24.
Schimper, W.P. 1869–1874. Traité de paléontologie végétale, ou, la flore du monde primitif dans ses rapports avec les formations géologiques et la flore du monde actuel. Vol. 1: iv+738 pp.; Vol. 2: 996 pp.; Vol. 3: 896 pp. Bailliere, Paris. Crossref
Schweitzer, H.J., Schweitzer, U., Kirchner, M., Van Konijnenburg-van Cittert, J.H.A., Van der Burgh, J., and Ashraf, R.A. 2009. The Rhaeto–Jurassic flora of Iran and Afghanistan. 14. Pteridophyta–Leptosporangiatae. Palaeontographica Abteilung B 279: 1–108. Crossref
Seward, A.C. and Dale, E. 1901. On the structure and affinities of Dipteris, with notes on the geological history of Dipteridinae. Philosophical Transactions of the Royal Society of London 194: 487–513. Crossref
Shirley, J. 1898. Additions to the flora of Queensland. Mainly from the Ipswich Formation, Trias–Jura System. Bulletin Geological Survey of Queensland 7: 1–25.
Smith, A.R., Pryer, K.M., Schuettpelz, E., Korall, P., Schneider, H., and Wolf, P.G. 2006. A classification for extant ferns. Taxon 55: 705–731. Crossref
Spalletti, L.A., Arrondo, O.G., Morel, E.M, and Ganuza, D.G. 1988. Estudio sedimentológico y paleoflorístico de la Formación Paso Flores – Triásico superior – en el sector occidental del Macizo Nordpatagónico, Argentina. Actas 5º Congreso Geológico Chileno 2: 395–413.
Spalletti, L.A., Arrondo, O.G., Morel, E.M, and Ganuza, D.G. 1990. Los depósitos fluviales de la cuenca Triásica superior en el sector noroeste del Macizo Nordpatagónico. Revista de la Asociación Geológica Argentina 43 (for 1988): 544–557.
Spalletti, L.A., Artabe, A.E. and Morel, E.M. 2003. Geological factors and evolution of southwestern Gondwana Triassic plants. Gondwana Research 6: 119–134. Crossref
Spalletti, L., Artabe, A.E., Morel, E.M., and Brea, M. 1999. Biozonación paleoflorística y cronoestratigrafía del Triásico Argentino. Ameghiniana 36: 419–451.
Stevens, J. 1981. Palynology of the Callide Basin, east-central Queensland. University of Queensland Papers, Department of Geology 9: 1–35.
Stipanicic, P.N. and Menéndez, C.A. 1949. Contribución al conocimiento de la flora fósil de Barreal (provincia de San Juan). I. Dipteridaceae. Boletín de Informaciones Petroleras, Buenos Aires 24: 44–73.
Taylor, E.L., Taylor, T.N., and Krings, M. 2009. Paleobotany: The Biology and Evolution of Fossil Plants. 2nd Edition. 1230 pp. Academic Press, Amsterdam,
Tidwell, W.D. and Ash, S.R. 1994. A review of selected Triassic to Early Cretaceous ferns. Journal Plant Research 107: 417–442. Crossref
Turutanova-Ketova, A.I. 1962. New genus of ferns from the Mesozoic sediments of Kazakhstan [in Russian]. Paleontologičeskij žurnal 1962 (2): 145–148.
Varela, R., Basei, M.A.S., Cingolani, C.A., Siga J.O., and Passarelli, C.R. 2005. El basamento cristalino de los Andes norpatagónicos en Argentina: geocronología e interpretación tectónica. Revista Geológica de Chile 32: 167–187. Crossref
Vergani, G.D., Tankard, J., Belotti, J., and Welsink, J. 1995. Tectonic evolution and paleogeography of the Neuquén Basin, Argentina. In: A.J. Tankard, R. Suárez, and H.J. Welsink (eds.), Petroleum Basins of South America. American Association of Petroleum Geologists, Memoir 62: 383–402. Crossref
Walkom, A.B. 1917. Mesozoic floras of Queensland. Part 1: The Flora of the Ipswich and Walloon Series (c), Filicales. Geological Survey of Queensland 257: l–46.
Webb, J.A. 1982. Triassic species of Dictyophyllum from eastern Australia. Alcheringa 6: 79–91. Crossref
Zamuner, A.B. and Artabe, A.E. 1994. Estudio de un leño fossil, Protocircoporoxylon marianaensis n. sp., de la Formación Paso Flores (Neotriásico), Provincia de Río Negro, Argentina. Ameghiniana 31: 203–207.
Zamuner, A.B., Zavattieri, A.M., Artabe, A.E., and Morel, E.M. 2001. Paleobotánica. In: A.E. Artabe, E.M. Morel, and A.B. Zamuner (eds.), El Sistema Triásico en Argentina, 143–184. Fundación Museo de La Plata “Francisco Pascasio Moreno”, La Plata.
Zavattieri, A.M. 2002. Anexo 4: Microfloras. In: P.N. Stipanicic and C.A. Marsicano (eds.), Léxico Estratigráfico de la Argentina. Vol. 8: Triásico. Asociación Geológica Argentina, Serie “B” (Didáctica y Complementaria) 26: 318–321.
Zavattieri, A.M. and Mego, N. 2008. Palynological record of the Paso Flores Formation (Late Triassic) on the southeastern side of the Limay River, Patagonia, Argentina. Ameghiniana 45: 483–502.
Zavattieri, A.M. and Volkheimer, W. 2001. Palynologic and paleoenvironmental characterization of strata near the Triassic–Jurassic boundary in northern Patagonia, Argentina. In: 34th Annual Meeting of the American Association of Stratigraphic Palynologists, Abstracts: 51, San Antonio, Texas.
Zavattieri, A.M., Rosenfeld, U., and Volkheimer, W. 2008. Palynofacies analysis and sedimentary environment of Early Jurassic coastal sediments at the southern border of the Neuquén Basin, Argentina. Journal of South American Earth Sciences 25: 227–245. Crossref
Zeiller, R. 1903. Flore fossile des gîtes de charbon du Tonkin. 316 pp. Ministère des Travaux Publications, Paris Crossref
Zhang, X.C., Kato, M., and Nooteboom, H.P. 2013. Dipteridaceae. In: Z.Y. Wu, P.H. Raven, and D.Y. Hong (eds.), Flora of China, Vols. 2–3 (Pteridophytes), 116–117. Science Press, Beijing.
Zhou, N., Wang, Y., Li, L., and Zhang, X. 2015. Diversity variation and tempo-spatial distributions of the Dipteridaceae ferns in the Mesozoic of China. Palaeoworld 25: 263–286. Crossref
Acta Palaeontol. Pol. 66 (4): 885–900, 2021
https://doi.org/10.4202/app.00864.2020