

A new clupeid fish from the upper Miocene of Greece: A possible Hilsa relative from the Mediterranean
CHARALAMPOS KEVREKIDIS, GLORIA ARRATIA, NIKOS BACHARIDIS, and BETTINA REICHENBACHER
Kevrekidis, C., Arratia, G., Bacharidis, N., and Reichenbacher, B. 2021. A new clupeid fish from the upper Miocene of Greece: A possible Hilsa relative from the Mediterranean. Acta Palaeontologica Polonica 66 (3): 605–621.
Much remains to be learned about the past diversity and evolutionary history of the Clupeidae (herrings, shads and allies), owing to the frequently subtle differences between modern taxa and the moderate preservational quality of some fossils. In this study, new clupeid fossils are described from a new locality from the upper Miocene of the Serres Basin, Northern Greece. The fossils are well-preserved articulated skeletons, exhibiting features such as a small size (<150 mm in standard length), slender body, two pairs of bullae, at least six parietal–postparietal striae, two supramaxillae, five branchiostegal rays, 10 supraneurals, 40–42 vertebrae, eight or nine pelvic fin rays, 17 rays in the dorsal and 16–19 rays in the anal fin, last two fin rays of the anal fin not elongate, and belly fully scuted. The new fossils cannot be attributed to any modern genus, though they most closely resemble the monotypic genus Hilsa, which today inhabits the Indo-Western Pacific. Detailed comparisons with all fossil clupeid taxa from the Cenozoic indicate that the new fossils constitute a new species, which is tentatively attributed to the fossil genus Pseudohilsa, as Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. Clupeids reportedly similar to the modern-day tropical Hilsa have been previously described from the Pliocene of the Black Sea and the middle Miocene of the Caspian Sea. This is, however, the first time that a possible fossil Hilsa relative has been described from the Mediterranean.
Key words: Teleostei, Pseudohilsa, Kelee shad, osteology, Miocene, Paratethys, Tethys, Serres Basin, Aegean Sea.
Charalampos Kevrekidis [ch.kevrekidis@campus.lmu.de] and Bettina Reichenbacher [b.reichenbacher@lrz.uni-muenchen.de], Ludwig-Maximilians-Universität München, Department für Geo- und Umweltwissenschaften, Paläontologie & Geobiologie, GeoBio-Center, Richard-Wagner-Str. 10, 80333, Munich, Germany.
Gloria Arratia [garratia@ku.edu ], Biodiversity Institute and Department of Systematic and Evolutionary Biology, The University of Kansas, Dyche Hall, Lawrence, Kansas, 66045, USA.
Nikos Bacharidis [nbaharidis@gmail.com], Epidavrou 90, Pylaia, 54454, Thessaloniki, Greece.
Received 23 December 2020, accepted 19 February 2021, available online 26 August 2021.
Copyright © 2021 C. Kevrekidis et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The teleost cohort Otomorpha comprises almost a third of all living fish species (Fricke et al. 2021). They are divided into the Clupeomorpha (e.g., anchovies and herrings, >400 modern species) and Ostariophysi (>10 000 modern species), plus the deep-sea Alepocephaliformes (ca. 140 modern species) (Betancur-R. et al. 2017; Arratia 2018; Straube et al. 2018; Fricke et al. 2021). Both fossil and extant representatives of the Ostariophysi have attracted considerable scientific attention regarding their diversity, biogeographic history and evolution (Briggs 2005; Chen et al. 2013; Nelson et al. 2016; Betancur-R. et al. 2017; Arratia 2018). In contrast, the Clupeomorpha has received less attention, and as research from the 2000’s showed, there is still much to be learned by studying their morphology (e.g., Di Dario 2002, 2009; Di Dario and De Pinna 2006). The Clupeomorpha includes the extinct Ellimmichthyiformes and the Clupeiformes, the latter including fossil as well as living representatives (e.g., Grande 1985; Murray and Wilson 2013; Nelson et al. 2016). The largest family within the Clupeiformes, in terms of species, is the Clupeidae (Fricke et al. 2021).
The fishes of the family Clupeidae (e.g., herrings, sardines, shads and menhadens) are some of the most intensely commercially exploited fishes worldwide (FAO 2020). Clupeids, which comprise almost 200 species (Fricke et al. 2021) and are distributed globally, are medium-sized fishes, usually in the 150–250 mm length range (e.g., Whitehead 1985). The majority of clupeids are coastal pelagic fishes, forming schools and feeding on plankton (e.g., Whitehead 1985; Nelson et al. 2016). Their tolerance to low salinities allows several species to be anadromous and about 10% of all species are predominantly freshwater forms (Nelson et al. 2016). Some features that characterize, but are not limited to, this family (see “Systematic discussion to the level of family” below) are: an otophysic connection, which links the swimbladder to the inner ear, forming one or two pairs of bullae in the neurocranium; the presence of one or more scutes along the ventral midline; single unpaired fins with soft rays, the dorsal fin located approximately at the middle of the body; near or complete lack of a lateral line on the body and the possession of two elongate postcleithra (e.g., Grande 1985).
Little is known about the evolutionary history of clupeids, even though dozens of fossil species have been described to date (e.g., Grande 1985, Jerzmańska 1991; Murray et al. 2005; Baykina 2013; Marramà and Carnevale 2015, 2016, 2018; Baykina and Schwarzhans 2017a, b; Kovalchuk et al. 2020). There are several reasons for this apparent antithesis; clupeids are not only numerous, many are also morphologically similar to each other (Whitehead 1985). As a result, quite often few characters are available to differentiate between modern taxa. Additionally, several fossil clupeid taxa have been described based on poorly preserved fossils whose anatomical details are not discernible (Grande 1985).
In this paper, new clupeid fossil material is described from the new site “Aidonochori A”, from the upper Miocene deposits of the Serres Basin, northern Greece. The fossils from this site are mostly preserved as complete and well-preserved skeletons, exhibiting many details of their anatomy. The main goals of this study are (i) to describe these new fossil specimens and (ii) to examine their relationships with other modern and fossil clupeids.
Institutional abbreviations.—LGPUT, Laboratory and Museum of Geology and Palaeontology of the Aristotle University of Thessaloniki.
Other abbreviations.—HL, head length; SL, standard length; pu, preural centrum.
Nomenclatural acts.—This publication, and the nomenclatural act it contains, are registered in ZooBank: urn:lsid:zoobank.org:act:B658C5B4-585A-4F1B-BD2A-CB269E8F5 EEB
Geographic and geological setting
The Serres Basin (Fig. 1A) in northern Greece, which is part of the broader Struma-Serres Basin in Bulgaria and Greece, has a NW-SE direction and is drained by the Strymonas River (Struma in Bulgarian). In its lower reaches, the Strymonas River is joined by the Angitis River and flows into the Northern Aegean Sea (Fig. 1B). The Serres Basin is located within the Serbo-Macedonian Massif and is filled with ca. 2000 to 3000-m thick, middle–upper Miocene to Quaternary sediments (Psilovikos and Karistineos 1986; Karistineos and Ioakim 1989; Zagorchev 2007; Tranos 2011). The Neogene deposits of the Strymonas–Serres Basin contain marine, terrestrial and brackish sediments (Psilovikos and Karistineos 1986; Syrides 1995, 1998; Ioakim et al. 2005). Because of the complex paleogeographical history and uncertainties with regards to dating, the stratigraphy and paleoecology of the Serres Basin are matters of ongoing research (Syrides 1995, 2000; Ioakim et al. 2005; Pimpirev and Beratis 2010).
Fig. 1. Location of the Serres Basin in northern Greece (B). Detailed view (A), showing location of the fossiliferous locality “Aidonochori A”. Images from https://www.google.de/maps, 2020TerraMetrics, Kartendaten; Google, Mapa GIsrael.
The fossil-bearing locality “Aidonochori A” (40°50’29” N, 23°43’12” E) lies close to the village of Aidonochori and is situated at the southwest margin of the Serres Basin, at the foothills of the Kerdilio Mountain. The fossils have been found in several superimposed layers within a 0.5-m thick silty, mica-containing marl. The fossiliferous marl is exposed on two outcrops, which cross each other; one is a roadcut and the second is the slope of a gorge. The fossils were collected in 1997 by the private researcher and collector Nikos Bacharidis. Only fossils of clupeid fishes have been recovered so far from this locality. A study of the stratigraphy and age of the locality is pending, but according to George Syrides (School of Geology, Aristotle University of Thessaloniki, Thessaloniki, Greece) the geological context of the area indicates an upper Miocene age (George Syrides 2020, personal communication).
Material and methods
The studied material is part of the collection of the Laboratory and Museum of Geology and Palaeontology of the Aristotle University of Thessaloniki (LGPUT). A total of five fossil individuals were examined from the “Aidonochori A” locality. The specimen LGPUT ADS 003 is on the same slab as LGPUT ADS 002 and consists of an isolated neurocranium, seen in ventral view. Four specimens (LGPUT ADS 001, 002, 004, and 005) are preserved as complete or partial skeletons in anatomical connection. These skeletons were split in half during sampling, roughly along the midline. The slab which bears (mostly) the left side of the fish’s body was recovered in each case, except for LGPUT ADS 005 for which both slabs, left and right, are available.
The specimens were prepared with fine carbide needles (0.17–0.5 mm in diameter) and consolidated with the acrylic resin Paraloid B-72. Microscopic observations were performed with a stereomicroscope Leica M165 C connected to a digital camera Leica M170 HD. Measurements were taken with digital sliding callipers and rounded to the nearest 0.1 mm.
For anatomical comparisons, the osteological descriptions and drawings of Phillips (1942), Grande (1985), Sato (1994), Segura and Díaz de Astarloa (2004), and Di Dario (2009) were used. Regarding the bones of the skull roof, we follow the terminology for the actinopterygians based on morphological (Schultze 2008 and references therein) and genetic evidence (Teng et al. 2019). In these studies, the “frontal” bone of the traditional terminology was found to be homologous to the parietal, and the “parietal” bone to the postparietal. To facilitate comparison with the traditional terminology, the corresponding terms are given in parentheses both in the text and in the figures.
The terminology for fins and different fin rays follows that in Arratia (2008), whereas Arratia (1997), Schultze and Arratia (2013), Wiley et al. (2015), and Cumplido et al. (2020) were used for the terminology of caudal endoskeletal structures. Fin-ray counts include all rays, except for the two last rays of the dorsal and anal fins, which are each supported by a single pterygiophore and therefore were counted as one. The principal caudal rays are the segmented and branched rays plus one segmented but unbranched ray for the upper and lower lobes; the rest of the caudal fin rays are the procurrent rays (sensu Arratia 2008).
The caudal vertebrae were identified by the presence of a closed haemal arch and ventrally projecting haemapophyses. Vertebra counts include the last terminal centrum (= preural vertebra 1), which is fused to the pleurostyle. Preural vertebrae are those whose neural and/or haemal spines are associated with caudal fin rays. The polyural terminology is followed regarding the numbering of the ural centra; it reflects a one-to-one relationship between the number of ural centra and hypurals (Schultze and Arratia 2013); for example, hypural 2 is fused to ural centrum 2, not to ural centrum 1, as in the terminology used in older literature.
Systematic palaeontology
Class Actinopteri Cope, 1871
Superorder Clupeomorpha Greenwood, Rosen, Weitzman, and Myers, 1966
Order Clupeiformes Bleeker, 1859
Suborder Clupeoidei Bleeker, 1859
Family Clupeidae Cuvier, 1816
Genus Pseudohilsa Menner, 1949
Type species: Pseudohilsa brevicauda Menner, 1949; middle Miocene of Absheron Peninsula, Azerbaijan.
Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov.
Figs. 2–6, Tables 1, 2.
Etymology: Named in honor of Nikos Bacharidis, the private researcher, fossil collector and visual artist who discovered the locality and collected the material presented here, in recognition of his longstanding contribution to the development of palaeontology in Greece.
Type material: Holotype LGPUT ADS 001. Paratypes LGPUT ADS 002–005, all from the type locality.
Type locality: Site “Aidonochori A” (40°50’ 29” N, 23°43’ 12” E) next to the village Aidonochori near Serres, Central Macedonia, Greece.
Type horizon: Upper Miocene.
Material.—Type material only.
Diagnosis.—Distinguished from the only other species in Pseudohilsa, P. brevicauda (Lednev, 1914), by the possession of more dorsal fin rays (17 vs. 10–13 in P. brevicauda), a smaller eye (ca. 25% of HL vs. ca. 33%) and the insertion of the pelvic fin under the anterior half of the dorsal fin’s base (vs. the posterior half).
Distinguished from all other extant and fossil clupeid species by the following combination of features: relatively small size (up to ca. 150 mm) with maximum depth at dorsal fin origin 24–33% SL; HL ca. 32% of SL; two pairs of bullae; at least six striae on the parietal and postparietal bones (= frontoparietal striae in older literature, see description below); mouth terminal; two supramaxillae; lower jaw articulation at about middle of orbit; five branchiostegal rays; 10 supraneurals; 40–42 vertebrae; ratio of pleural ribs to total vertebrae 0.57–0.6; pectoral fin with 15 rays; pelvic fin with eight or nine rays; anal fin with 16–19 rays; two epurals; transverse row with ca. 40 scales, no dorsal scutes; strong ventral scutes (four at gular region, 11 or 12 prepelvic associated with ribs, 10 or 11 postpelvic).
Description.—Slender-bodied fish, with a triangular head (Fig. 2). The dorsal outline is almost straight and the ventral outline is convex. In the following sections, the emphasis is placed on features or bones which are clearly discernible; structures that are badly preserved are noted as such or omitted from the description.

Fig. 2. Lateral views of the clupeid fish Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the Upper Miocene locality “Aidonochori A”, Greece. A. LGPUT ADS 001, holotype. B. LGPUT ADS 002. C. LGPUT ADS 004. D. LGPUT ADS 005. Scale bars 10 mm.
Neurocranium: The neurocranium is approximately triangular in lateral and ventral view. Some bones, particularly those behind the orbit, are crushed and their precise borders are difficult to discern. The basioccipital and first vertebra are preserved in anatomical connection. The small supraoccipital forms the posterodorsal angle of the neurocranium. Anteriorly to that is the postparietal (see Schultze 2008 and Teng et al. 2019; termed “parietal” in the traditional literature), which is separated by the oval temporal foramen from the parietal bone (see Schultze 2008 and Teng et al. 2019; termed “frontal” in the traditional literature). The temporal foramen seems not to be overlain by any flange. The parietals are large and have an almost straight to slightly convex profile in lateral view (Fig. 3A). Posteriorly, on their dorsal surface, they are ornamented with more than six prominent and reticulate striae, best seen in the specimen LGPUT ADS 005 (Fig. 3B). The parietals connect anteriorly with the mesethmoid and ventrally to the latter is the vomer, which appears toothless. Posteriorly to the vomer is the straight and slender parasphenoid. The parasphenoid projects at about the lower third of the orbit and apparently lacks teeth. It can be discerned that the orbit is limited anterodorsally by the parietal bones and lateral ethmoids, and posterodorsally by the parietal bones and sphenotics. Posteriorly to the sphenotics, the pterotics bear the pterotic bullae. Anteroventrally to the pterotic bullae, the larger prootic bullae are well preserved. Both pairs of bullae are easy to recognize owing to their round shape and their characteristic, glassy and perforated texture (Fig. 3C).
The specimen LGPUT ADS 003 is an isolated neurocranium in ventral view (Fig. 3D). In this specimen, the posterior part of the basioccipital, situated between the exoccipitals, is discernible. The parasphenoid is missing and seems to have broken off. Anterolaterally to the exoccipitals, the pterotics bear the pterotic bullae. Anteriorly to the exoccipitals and the basioccipital are the prootics with the much larger prootic bullae. Anterolaterally to the prootics, the sphenotics are recognizable. Anteriorly to the sphenotics and prootics are the large, broadly triangular parietal bones, whose anterior portion is missing.
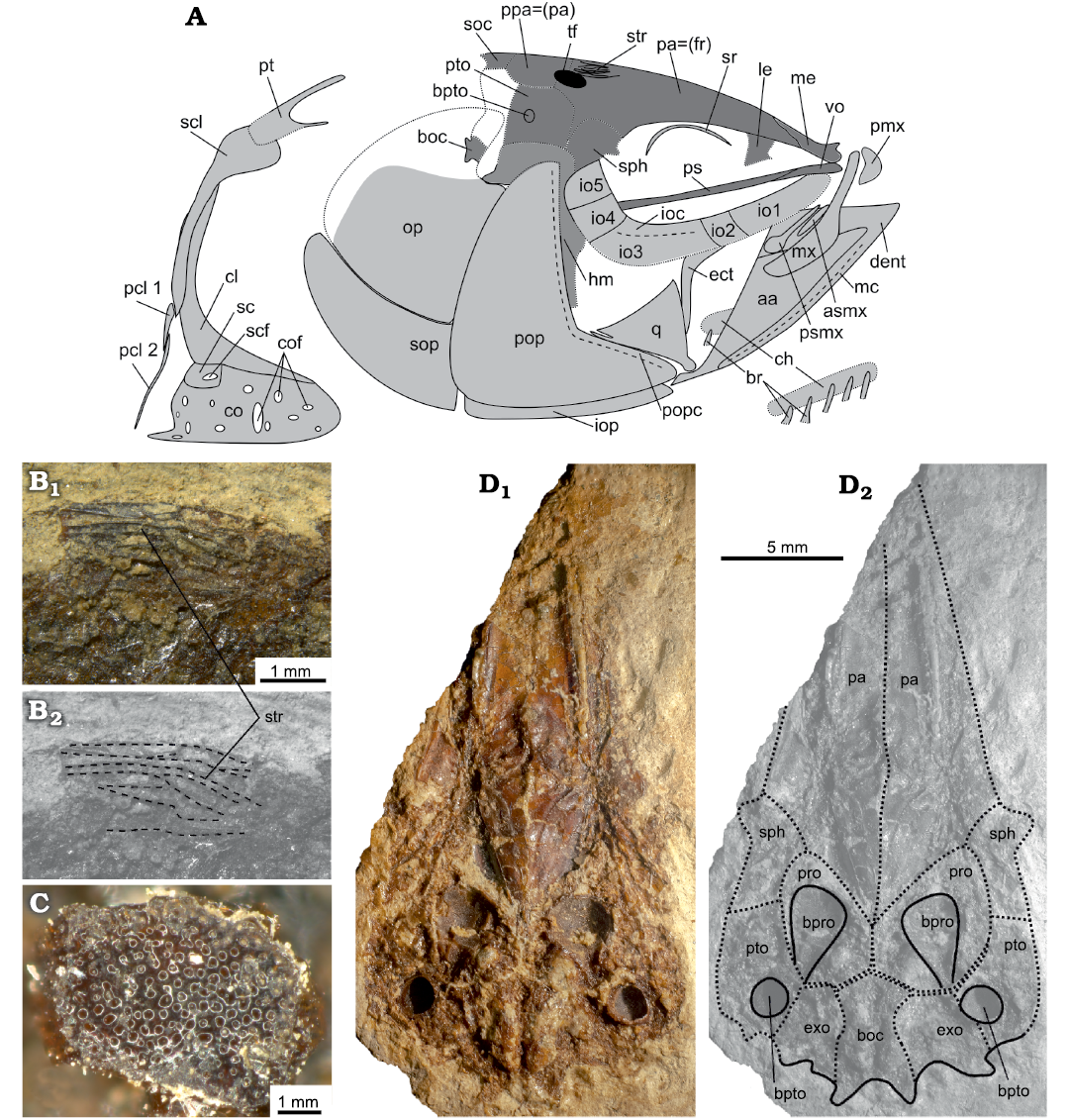
Fig. 3. The skull and pectoral girdle of the clupeid fish Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the Upper Miocene locality “Aidonochori A”, Greece. A. Explanatory drawing based on all examined specimens, lateral view. Not to scale. B. LGPUT ADS 005, the parietal-postparietal striae on the neurocranium (anterior is on the right), photograph (B1), image with parietal–postparietal striae outlined (B2). C. LGPUT ADS 004, fragment of the inner surface of the bulla prootica showing the glassy and perforated texture of this structure. D. LGPUT ADS 003, neurocranium in ventral view, photograph (D1), image with the neurocranial bones outlined (D2). Unbroken lines denote clearly defined outlines of the corresponding structures, dotted lines indicate an uncertain outline and/or a fracture, dashed lines delimit a prominent feature of the bone concerned. Medially placed structures are depicted in darker gray to facilitate viewing. Abbreviations: aa, anguloarticular; asmx, anterior supramaxilla; boc, basioccipital; bpro, bulla prootica; bpto, bulla pterotica; br, branchiostegal rays; ch, ceratohyal; cl, cleithrum; co, coracoid; cof, foramina of the coracoid; dent, dentary; ect, ectopterygoid; exo, exoccipital; io, infraorbital; ioc, infraorbital canal; iop, interopercle; le, lateral ethmoid; mc, mandibular canal; me, mesethmoid; mx, maxilla; op, opercle; pa (= fr), parietal (= frontal) bone; pcl, postcleithrum; pmx, premaxilla; pop, preopercle; popc, preopercular canal; ppa (= pa), postparietal (= parietal) bone; pro, prootic bone; ps, parasphenoid; psmx, posterior supramaxilla; pt, posttemporal; pto, pterotic; q, quadrate; sc, scapula; scf, scapular foramen; scl, supracleithrum; soc, supraoccipital; sop, subopercle; sph, sphenotic; sr, sclerotic ring; str, parietal-postparietal striae; tf, temporal foramen; vo, vomer.
Circumorbital series: The orbit is rounded and occupies ca. 25% of the HL. Five infraorbitals are discernible and traces of the infraorbital canal are visible on infraorbital 3 (Fig. 3A). Anteriorly, the first infraorbital is elongate, the second is shorter and the third is the largest of the series. The fourth and fifth infraorbitals form the postero-dorsal margin of the orbit, and they have a broadly rectangular shape (Fig. 3A). It is not clear if a sixth infraorbital (dermosphenotic) is present or not. A semi-circular sclerotic bone is preserved in the upper half of the orbit.
Jaws: There is no sign of teeth in any of the jaws. The premaxilla is subtriangular and well ossified (Fig. 3A). The maxilla has a robust, rod-like anterior articular process and a flattened and curved posterior blade; the angle between these two parts is about 150°. Of all the bones of the upper jaw, the maxilla extends farthest posteriorly, overlapping with about the anterior third of the orbit. The posterior supramaxilla is paddle-shaped and the anterior supramaxilla is smaller and elongate. There is no sign of a hypomaxilla.
The dentary is broad and robust, with a well-developed ventral arm. In specimen LGPUT ADS 004 the lateral surface of the dentary is visible; it possesses a fossa near its anterodorsal tip. The mandibular canal is positioned longitudinally near the ventral margin of the bone. The anguloarticular is preserved in anatomical connection with the dentary (Fig. 3A). The retroarticular is not recognizable. The articulation of the lower jaw with the suspensorium is positioned approximately at the level of the middle of the orbit.
Suspensorium and palatoquadrate: The dorsal part of the hyomandibula is expanded; ventrally the hyomandibular shaft is long and almost straight, and its ventral tip is separated from the quadrate by a space occupied by the symplectic, which is not preserved (Fig. 3A). The quadrate is subtriangular, with approximately equal sides. The dorsoposterior process is robust and pointed, and dorsally the quadrate’s margin is slightly concave, lacking an incision. The ectopterygoid, which appears toothless, is slender and the dorsal and ventral arms form an oblique angle (ca. 120°).
Opercular series: The opercle has an almost straight anterior margin and a slightly convex ventral margin. It is smooth and bears no striations. The subopercle is ovoid to broadly triangular, with a short and pointed anterodorsal process. The preopercle is wide, L-shaped, and its upper arm is longer than the lower arm, which is tapering and rounded anteriorly (Fig. 3A). The posterior and ventral margins of the preopercle form a slightly acute angle (ca. 80°). The preopercular canal runs near the anterior margin of the preopercle. The interopercle seems to be wider posteriorly and narrower anteriorly. Its anterior tip is almost as long as the lower arm of the preopercle.
Hyoid and branchial arches: There are five branchiostegal rays attached to each ceratohyal (Fig. 3A); the border between the anterior and posterior ceratohyal is not discernible. Ceratobranchials and/or hypobranchials and epibranchials are preserved under the bones of the opercular series; they bear numerous gill rakers anteriorly. In the specimen LGPUT ADS 005, more than 20 gill rakers are recognizable in the second or third lower branchial arch, near the junction with the upper branchial arch.
Vertebral column and associated structures: There are 40–42 vertebrae, including preural centrum 1, and the caudal vertebrae start either at the 16th or 17th vertebra (Table 1). The opercle overlies the first five or five and a half vertebrae; these are included in the total count of vertebrae. The first 10 vertebrae are almost square, while the rest are longer than high. The neural arches are fused to their respective vertebral centra in the abdominal and caudal regions. In some preural vertebrae it is possible to discern that the prezygapophyses are more developed than the postzygapophyses. There are 24 pairs of ribs, starting from the third vertebra and ending on the 26th, which almost reach the ventral body margin. Therefore, the ratio of ribs to the total number of vertebrae is 0.57–0.6. Due to the state of preservation, the type of articulation of the ribs with the vertebrae and/or the parapophyses is not discernible.
Table 1. Meristic characteristics of the clupeid Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the Upper Miocene locality “Aidonochori A”, Greece. ? denotes uncertainty; + indicates that the actual number must have been greater in the living fish.
| |
LGPUT ADS 001 |
LGPUT ADS 002 |
LGPUT ADS 004 |
LGPUT ADS 005 |
|
Supraneurals |
10 |
? |
10 |
10 |
|
Branchiostegal rays |
5 |
? |
5 |
? |
|
Vertebrae total (abdominal/caudal) |
41 (16/25) |
40 (16/24) |
41 (16/25) |
42 (17/25) |
|
Pectoral fin rays |
11+ |
11+ |
10+ |
15 |
|
Pelvic fin rays |
7+ |
8 or 9 |
? |
8 |
|
Dorsal fin rays |
17 |
– |
17 |
17 |
|
First pterygiophore of dorsal fin associated with vertebra |
9 |
– |
10 |
10 |
|
Anal fin rays |
16 |
16 |
19 |
18 |
|
First pterygiophore of anal fin associated with vertebra |
26 |
27 |
27 |
27 |
|
Scutes: free prepelvic/rib associated/postpelvic scutes |
3+?/11/10 |
4/12/11 |
?/11/11 |
?/11/11 |
|
Procurrent caudal fin rays (upper/lower) |
8/8 |
-/5+ |
6+/6+ |
8/7 |
|
Principal caudal fin rays (upper/lower) |
10/9 |
10/9 |
10/9 |
10/9 |
|
Epurals |
2 |
– |
2 |
2 |
Each vertebra seems to be associated laterally with an epineural (process) and an epipleural, each very thin and elongate; the epipleurals are all free. The epineurals are processes of the lateral walls of the neural arch until ca. the 20th vertebra, and are disconnected from the wall of the neural arch posterior to that, continuing as free epineurals. In the most anterior vertebrae these structures are usually hard to discern. In the caudal region, the epineurals and epipleurals become progressively more strongly inclined towards the horizontal axis. There are 10 posterodorsally inclined supraneurals, shaped as slender wedges with an expanded dorsal tip, which become slenderer posteriorly.
Pectoral and pelvic girdles and fins: The posttemporal has a broad body and slender, rod-like dorsal and ventral arms, the dorsal arm being longer than the ventral (Fig. 3A). The supracleithrum is broad dorsally and tapers ventrally. The cleithrum is long and robust, curving anteriorly at its ventral portion. Close to the cleithrum and ventrally to the supracleithrum there are two elongate postcleithra, the upper one is short and broad, and the lower one is long, slender, and almost reaches the ventral margin of the body. Ventrally to the cleithrum and attached to it is the coracoid, which is flat, thin and highly perforated (Fig. 3A). The anterior and ventral margins of the coracoid are convex, and posteroventrally the coracoid is pointed. Posterodorsally, between the cleithrum and the coracoid, is the scapula. The scapula, which is perforated by the scapular foramen, is followed posteriorly by the pectoral radials, which are crushed in our specimens. The pectoral fin is situated close to the ventral margin and has 15 rays.
The pelvic bones are long and triangular, reaching forward the length of four or five vertebrae. The number of pelvic fin rays is eight or nine. The pelvic fin originates approximately under the 18th or 19th vertebra and under the anterior third of the base of the dorsal fin.
Dorsal and anal fins: Both median fins have the following characters in common. They are subtriangular in shape (Fig. 2), their pterygiophores decrease in size posteriorly and their fin rays increase in length until the third to fifth element and then progressively decrease in lenght (Fig. 4). The pterygiophores are formed by the proximal radials, which are interpreted as possibly fused to the middle radials (see e.g., Grande 1985: 338, 347; Fig. 4); it is not clear if the distal radials are present or fused with some other element. The first two procurrent rays are distally unbranched and the second ray may or may not be segmented. The third fin ray is segmented and unbranched = (first principal ray), and the rest of the rays are segmented and distally branched.
The dorsal fin origin is located near the middle of the body. It has 17 rays and an equal number of pterygiophores (the last two rays are borne by the same pterygiophore and are therefore counted as one). The pterygiophores of the extremities are modified; the anteriormost one is a flattened and deep, anteroventrally-facing keel, and the posteriormost pterygiophore is a slender horizontally-oriented stay (Fig. 4A). The first pterygiophore is associated with the neural spine of vertebra 9 or 10.
The distance between the end of the dorsal fin and the beginning of the anal fin corresponds to five vertebrae on the horizontal level. There are 16–19 anal fin rays supported by 15–18 pterygiophores (Fig. 4B). The first pterygiophore of the anal fin is unmodified, slender and elongate, as are the rest of the pterygiophores, and it supports two procurrent rays, which are undivided distally. The same pterygiophore is associated with the haemal spine of vertebra 26 or 27. The last pterygiophore is modified to a slender horizontally-oriented stay and bears two fin rays, counted as one (Fig. 4B).
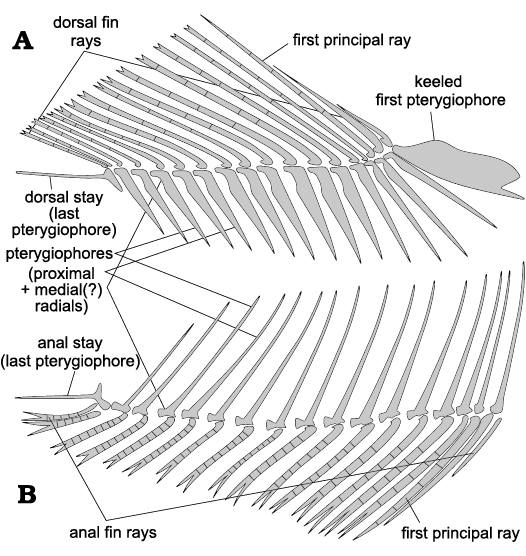
Fig. 4. Schematic representation of the unpaired fins of the clupeid fish Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the upper Miocene locality “Aidonochori A”, Greece (based on all examined specimens). Dorsal fin (A), anal fin (B). Anterior is on the right. Not to scale.
Caudal endoskeleton and fin: The caudal fin is forked and comprises ten principal and seven procurrent rays in the upper lobe and nine principal and six to seven procurrent rays in the lower lobe (Fig. 5). The longest principal rays are almost three times the length of the shortest. The uppermost principal ray is associated with the sixth hypural and the lowermost principal ray reaches the haemal spine of preural vertebra 2. All except the anteriormost procurrent rays are segmented. Anteriorly to the procurrent rays of each lobe there is a caudal scute, which appears undivided medially and also longer and flatter relative to the procurrent rays (Fig. 5). The basal segments of the two middle principal rays bear small processes. The caudal rays are additionally supported by the spines of the preural vertebrae 1–5 (Fig. 5).
Preural vertebra 1 bears a neural arch and a short neural spine and dorsoposteriorly it is fused to the elongate first uroneural, known in clupeoids as well as in other otomorphs as the pleurostyle. Between the pleurostyle and the neural spine of preural vertebra 2 there are two moderately elongate epurals. The arch of the long parhypural articulates with, and is not fused to, the preural centrum 1. The haemal spine of preural centrum 2 is long and broad, and also the haemal spine of preural centrum 3 is more expanded and robust than the haemal spines of the preceding vertebrae. Their neural and haemal arches are fused to their respective centra.
There are six hypurals, of which the first and third are expanded, the rest are narrower. The proximal end of hypural 1 tapers to a hook-shaped process, which does not reach the ural centrum 2P (polyural terminology, see Methods). Hypural 3 is posteroventrally notched, forming the hypural diastema. All hypurals are autogenous, except for hypural 2, which is fused to the ural centrum 2P. Posteriorly to the pleurostyle there is the elongate uroneural 2 and posterodorsally to that the small rod-like uroneural 3 (Fig. 5).
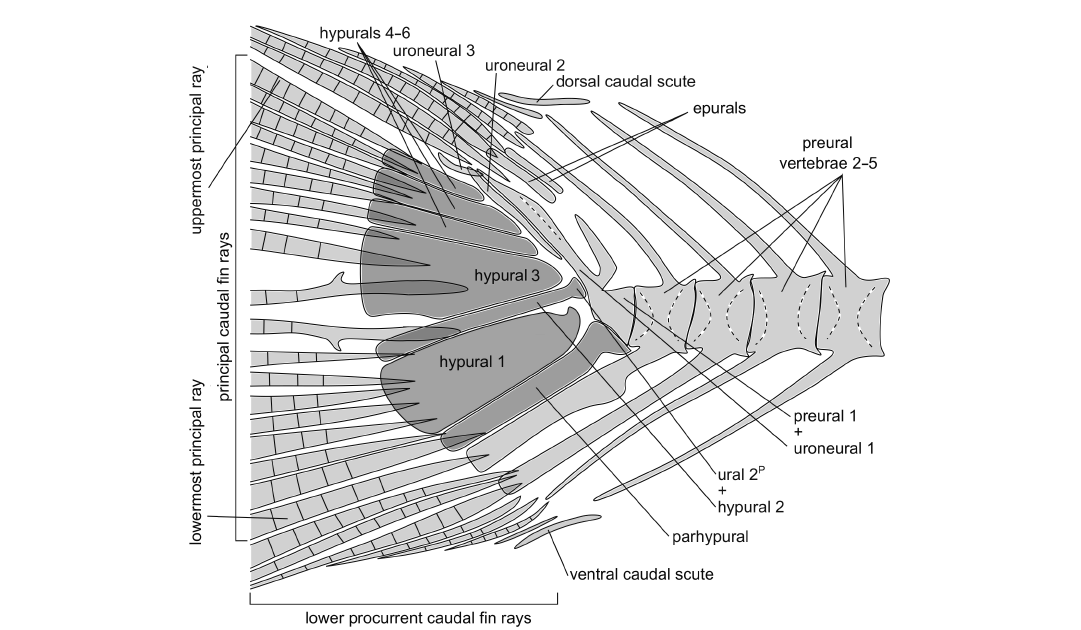
Fig. 5. Schematic representation of the caudal fin of the clupeid fish Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the upper Miocene locality “Aidonochori A”, Greece (based on all examined specimens). Unbroken lines denote clearly defined outlines of the indicated structures, and dashed lines delineate a prominent feature of the structure concerned. Medially placed structures are shown in darker gray. Anterior is on the right. The arrangement of the caudal fin rays shown here might differ slightly from the native configuration, as the fossils show minor taphonomic alterations. Not to scale.
Squamation: The scales are visible only from their medial side, and therefore their anterior fields are exposed to the observer rather than the posterior fields that one would see in a live specimen. Consequently, the sculpture on the lateral surface of the scales is not visible, except for some patches that are transparent enough to allow one to see the fine circuli on the lateral surface of the anterior field of the scales (Fig. 6A). A transverse row along the body, from behind the head to the end of the hypural plates, comprises ca. 40 scales and there are ca. 11 horizontal scale rows over the pelvic fin. The scales are imbricate and of similar size (Fig. 6A), with the posterior scales being somewhat smaller than the anterior ones. There are no lateral line scales.
There is a series of scutes along the ventral midline of the fishes’ body. There are ca. four free prepelvic scutes along the gular region, 11 or 12 prepelvic scutes each associated with the ventral portion of a pair of ribs and 11 postpelvic scutes, also associated with an equal number of pairs of ribs. The scutes are robust, with a sharp median keel which deepens posteriorly (Fig. 6B, C). The pelvic scute, i.e. the scute which is directly in front of the insertion of the pelvic fin, is shaped similarly to the rest. All the scutes, with the possible exception of the one directly below the pelvic fin, bear ascending arms. These arms are robust and long; the arms of the rib-associated prepelvic scutes extend to about 40% or more of the body cavity and the pelvic scute has the most prominent arms. In general, the ascending arms are placed closer to the anterior end of the scute, except for the posteriormost postpelvic scutes in which the ascending arms are placed closer to the posterior end. Predorsal scutes are absent.
Stratigraphic and geographic range.—Upper Miocene, Serres Basin, Greece.
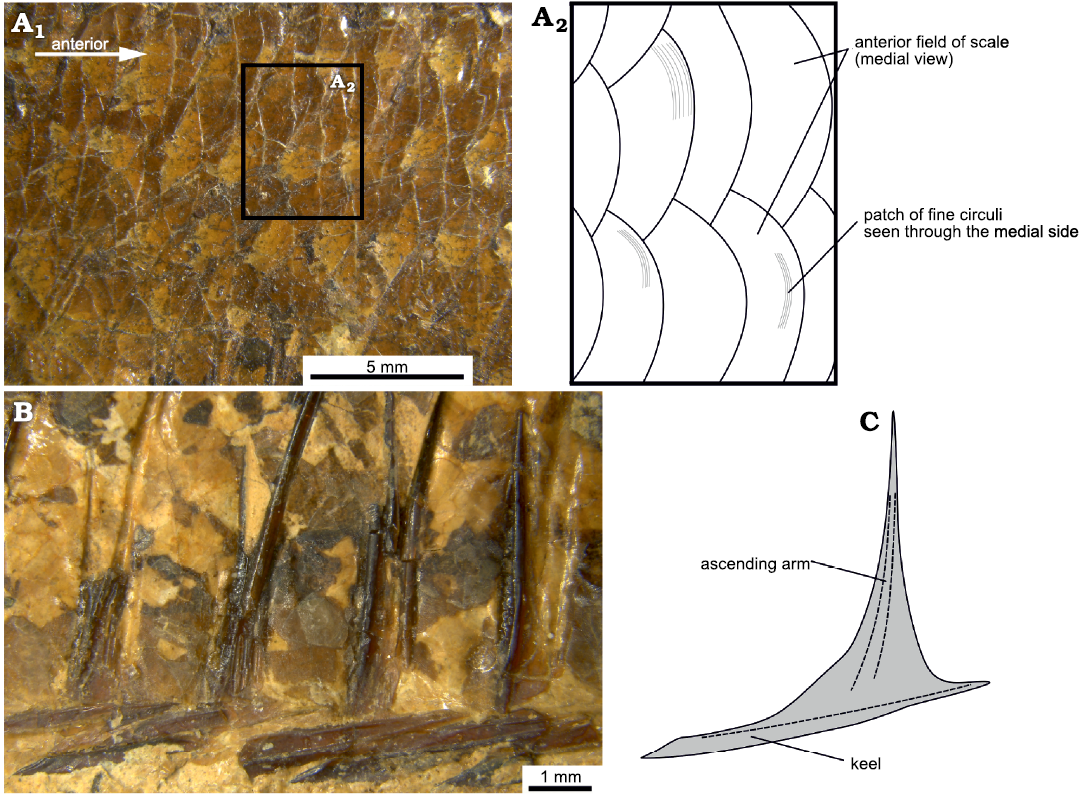
Fig. 6. Squamation of the clupeid fish Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the upper Miocene locality “Aidonochori A”, Greece. Anterior is to the right. A. LGPUT ADS 004, flank scales in medial view, photograph (A1), detail in a schematic representation (A2), showing the circuli of the scales. B. LGPUT ADS 002, postpelvic abdominal scutes in lateral view. C. Schematic representation of a scute based on all examined specimens. Dashed lines depict prominent features of the bone.
Discussion
Systematic discussion down to the level of family.—Some characters which are typical for most Otomorpha are clearly discernible in “Aidonochori A” fossil fishes, such as the fusion of haemal arches to centra anterior to preural centrum 2, a hypural 2 that is fused to ural centrum 2P (polyural terminology, see Material and methods here and Schultze and Arratia 2013) and the presence of a pleurostyle (Arratia 1999, 2018; Wiley and Johnson 2010; Straube et al. 2018). Furthermore, the fossil fishes from the site “Aidonochori A” possess some typical characteristics of the superorder Clupeomorpha, namely the presence of bullae in the skull, ventral scutes and an autogenous hypural 1 (Grande 1985; Wiley and Johnson 2010). Moreover, the lack of a lateral line, the fusion of the preural centrum 1 to the first uroneural or pleurostyle but not to the parhypural, a temporal foramen which does not seem to be overlaid by any flange, and a hypural 1 with a hooked or irregular anterior end which does not reach the base of the ural centrum 2P, together indicate that the fossils can be assigned to the suborder Clupeoidei of the order Clupeiformes (Grande 1985; Fujita 1990; Di Dario and de Pinna 2006; Wiley and Johnson 2010).
Based on anatomical characters, the Clupeoidei have been divided into the families Engraulidae (anchovies), Chirocentridae (wolf herrings), Pristigasteridae (longfin herrings) and Clupeidae (herrings and allies) (Nelson 1970; Grande 1985; Whitehead 1985). We follow this classification for the purposes of our study. It should, however, be emphasized that there is no consensus regarding the relationships between these families based either on morphological (e.g., Grande 1985; Di Dario 2009; Patterson and Johnson 1995; Miyashita 2010) or molecular evidence (e.g., Li and Ortí 2007; Lavoué et al. 2013; Bloom and Egan 2018). Moreover, the monophyly of the Clupeidae is contested, e.g., because some molecular studies place Chirocentrus Cuvier, 1816, within the Clupeidae (Wilson et al. 2008; Li and Ortí 2007; Lavoué et al. 2007, 2010, 2013; Queiroz et al. 2020).
That the fossils described here belong to the Engraulidae can be excluded, because in that family the tip of the maxilla and the lower jaw articulation are positioned well behind the eye (vs. at the level of the eye in the specimens from the site “Aidonochori A” ), the snout is projecting anteriorly (vs. snout not projecting) and there is a larger number of branchiostegals (7–19 vs. 5) (Ridewood 1904; Whitehead 1962; McAllister 1968; Grande 1985; Nelson et al. 2016). Additionally, the ratio of ribs to the total number of vertebrae exceeds that found in Engraulidae (up to 0.54 vs. 0.57–0.6, see Grande 1985: 263–264; Di Dario 2009). Chirocentrus is the only genus in the family Chirocentridae and is characterized by fang-like oral teeth (vs. absent), six to eight branchiostegals (vs. 5), anal fin with many rays (>30 vs. <20), pelvic fin with six or seven rays (vs. 8 or 9), lack of abdominal scutes (vs. fully scuted), about 70 or more vertebrae (vs. 40–42), and small scales (vs. normal) (McAllister 1968; Grande 1985; Whitehead 1985; Nelson et al. 2016). Pristigasteridae clearly differ from the specimens from the site “Aidonochori A”, because they too have more than 30 rays in the anal fin (vs. <20), the supraneurals are inclined vertically or anterodorsally (vs. posterodorsally), the postzygapophyses are larger than the prezygapophyses (vs. prezygapophyses larger, see Fig. 5 and Di Dario 2002) and they lack a notch on the third hypural (vs. notch present) (Wongratana 1980; Grande 1985; Nelson et al. 2016). The Clupeidae is the only family that matches the fishes from the site “Aidonochori A” in all the above-mentioned characters, and it is additionally characterized by the presence of two long, rod-like postcleithra (Grande 1985), which are also found in the fossils (Fig. 3A).
Systematic discussion to the level of subfamily.—There is currently no consensus regarding the systematics of Clupeidae at the subfamily level, as different molecular phylogenies have suggested different groupings (e.g., Wilson et al. 2008; Bloom and Lovejoy 2014; Lavoué et al. 2014). The classification presented in Nelson et al. (2016) is followed here, in which the Clupeidae are divided into the subfamilies Dussumieriinae, Ehiravinae, Pellonulinae, Dorosomatinae, Clupeinae, and Alosinae.
The Dussumieriinae are excluded because they possess a single, W-shaped, abdominal scute (vs. abdomen fully scuted in the specimens from the site “Aidonochori A”) (Grande 1985). Fishes of the subfamily Ehiravinae are small and paedomorphic (up to 50–60 mm vs. up to ca. 150 mm SL) (Nelson et al. 2016). The Ehiravinae and the Pellonulinae have lost the anterior supramaxilla (vs. present) and their second ural centrumP (polyural terminology, see Material and methods here and Schultze and Arratia 2013) is fused not only to hypural 2, but also to the compound preural centrum 1 plus the pleurostyle (vs. second ural centrum only fused to hypural 2) (Grande 1985). In Dorosomatinae, the last dorsal fin ray is elongate and filamentous (vs. short and lacking a filament) and/or the mouth is inferior to subterminal (vs. terminal) (Grande 1985; Whitehead 1985). The remaining two subfamilies, Clupeinae and Alosinae, are not distinguished by unambiguous synapomorphies that can be easily observed in fossils (Grande 1985; Whitehead 1985; Nelson et al. 2016). For this reason, all modern and fossil genera attributed to either of these subfamilies are included in the discussion below.
Comparison with modern genera.—As noted above, molecular phylogenies have yet to reach agreement on which genera should be included in either of the subfamilies Clupeinae or Alosinae (e.g., Lavoué et al. 2014; Bloom and Lovejoy 2014). Here the classifications presented in Whitehead (1985) and Nelson et al. (2016) are followed, which were mostly based on morphological characters and assigned the most genera to these subfamilies. According to Whitehead’s (1985) list, there are 16 genera in Clupeinae (Amblygaster Bleeker, 1849; Clupea Linnaeus, 1758; Clupeonella Kessler, 1877; Escualosa Whitley, 1940; Harengula Valenciennes in Cuvier and Valenciennes, 1847); Herklotsichthys Whitley, 1951; Lile Jordan and Evermann, 1896; Opisthonema Gill, 1861; Platanichthys Whitehead, 1968; Ramnogaster Whitehead, 1964; Rhinosardinia Eigenmann, 1912; Sardina Antipa, 1904; Sardinella Valenciennes in Cuvier and Valenciennes, 1847); Sardinops Hubbs, 1929; Sprattus Girgensohn, 1846; Strangomera Whitehead, 1964) and seven in Alosinae (Alosa Linck, 1790; Brevoortia Gill, 1861; Ethmalosa Regan, 1917; Ethmidium Thompson, 1916; Gudusia Fowler, 1911; Hilsa Regan, 1917; Tenualosa Fowler, 1933). Unless otherwise stated, the generic features described in Whitehead (1985) are used.
The Sprattus and Clupeonella are readily excluded, because they lack a pterotic bulla (vs. pterotic bulla present in the fishes from the site “Aidonochori A”, Fig. 3A, D). The presence of one or more dorsal scutes further excludes Herklotsichthys, Harengula, Ethmalosa, Ethmidium, Tenualosa, and Opisthonema (vs. no dorsal scutes, see Grande 1985: tables 9a, 10a). In Amblygaster and Sardinella, the last two anal fin rays are elongated (vs. equal or shorter to the penultimate ray). Sardina and Sardinops have distinct bony ridges radiating downward on their opercle (vs. opercle smooth). The last dorsal fin ray in Opisthonema is elongate and filamentous (vs. equal or shorter to the penultimate ray). Rhinosardinia possesses a sharp spine on the anteroposterior part of the maxilla (vs. smooth, Fig. 3A). Among the remaining genera, the pelvic fin has fewer than eight rays in Escualosa, Platanichthys, Ramnogaster, Lile, and Brevoortia (vs. 8 or 9).
Gudusia differs from the fossil fishes from the site “Aidonochori A” because its pelvic fin inserts just before the dorsal fin (vs. slightly behind), and also because it has a deep body (vs. slender), a preorbital length that is much shorter than the horizontal diameter of the eye (vs. roughly the same; Table 2) and many more scales in the lateral series (77–91 vs. ca. 40). Clupea and Strangomera have higher counts of supraneurals (15–19 and 15 respectively vs. 10), branchiostegal rays (8–9 and 7 respectively vs. 5, see also McAllister 1968) and vertebrae (>50 and 44 respectively vs. 40–42). Alosa also possesses a larger number of branchiostegal rays (7–8 vs. 5, see also McAllister 1968) and vertebrae (ca. 50 or more vs. 40–42) and often has a striated opercle (see also Svetovidov 1963: 233). Hilsa has fewer supraneural bones (7 vs. 10), and a deep body (vs. slender).
Therefore, the fishes from the site “Aidonochori A” described here cannot be attributed to any of the modern genera. They do, however, seem to have the least pronounced differences with Hilsa. Hilsa is a genus that includes a single species, Hilsa kelee (Cuvier, 1829), also known as the Kelee shad.
Table 2. Morphometric characteristics of the clupeid Pseudohilsa nikosi Kevrekidis, Arratia, and Reichenbacher sp. nov. from the upper Miocene locality “Aidonochori A”, Greece. Measurements are given in mm and as a percentage of the standard length (in parentheses). + denotes that the actual number must have been greater in the living fish.
| |
LGPUT ADS 001 |
LGPUT ADS 002 |
LGPUT ADS 004 |
LGPUT ADS 005 |
|
Standard length |
99.3 |
119+ (estimate ca. 150) |
111.6 |
88.3 |
|
Total length |
118.6 (119%) |
148+ (estimate ca. 180) |
128+ |
102.3 (116%) |
|
Head length |
31.5 (32%) |
– |
34.6 (31%) |
28.8 (33%) |
|
Head depth |
24.9 (25%) |
– |
33.8 (30%) |
22.7 (26%) |
|
Preorbital distance |
8.7 (9%) |
– |
8.6 (8%) |
7.5 (8%) |
|
Horizontal eye diameter |
7.9 (8%) |
– |
8.1 (7%) |
7.7 (9%) |
|
Predorsal distance |
50.7 (51%) |
– |
54 (48%) |
44.7 (51%) |
|
Postdorsal distance |
36.3 (37%) |
– |
40.1 (36%) |
31.9 (36%) |
|
Prepelvic distance |
52 (52%) |
– |
61 (55%) |
46.1 (52%) |
|
Postpelvic distance |
46.8 (47%) |
59.1 |
49.7 (45%) |
41.9 (47%) |
|
Preanal distance |
75.8 (76%) |
– |
84.1 (75%) |
65 (74%) |
|
Postanal distance |
10.8 (11%) |
14 |
24.35 (22%) |
11 (12%) |
|
Body depth at dorsal fin origin |
27.5 (28%) |
– |
36.4 (33%) |
21 (24%) |
|
Body depth at pelvic fin origin |
24.9 (25%) |
32.5 |
34.7 (31%) |
20 (23%) |
|
Body depth at anal fin origin |
20.9 (21%) |
– |
27 (24%) |
15.9 (18%) |
|
Minimum body depth at the level of the caudal peduncle |
11.2 (11%) |
– |
13.6 (12%) |
8.9 (10%) |
Fossils attributed to Hilsa.—Danil’chenko (1980) reassigned four species formerly placed in Alosa to Hilsa, namely H. elegans (Gabelaia, 1976), H. oblonga (Gabelaia, 1976), H. torosa (Gabelaia, 1976), H. lata (Gabelaia, 1976). According to Danil’chenko (1980) they come from the early to middle Pliocene deposits of Abkhazia, on the eastern coast of the Black Sea, in the South Caucasus region. The delimitation of these species was based on meristic (e.g., numbers of vertebrae and rays of unpaired fins) and morphometric characters (e.g., body depth) (Gabelaia 1976; Danil’chenko 1980). These characters of the fossil “Hilsa” species are generally similar to those of the fossils from the site “Aidonochori A”, but other taxonomically important characters are missing from the description of these species (e.g., presence/absence of parietal–postparietal striae, perforation of the scales, number of pelvic fin rays). It is therefore not possible to compare the fossils from the site “Aidonochori A” directly with these “Hilsa” specimens, or to be certain to which genus the latter belong. Moreover, the differences between these fossils from the region of Abkhazia appear small, and the validity of some of these species needs to be reconsidered.
Comparison of the herrings from the site “Aidonochori A” with extinct genera.—In this section, the fishes from the site “Aidonochori A” are compared with extinct clupeid genera from the Cenozoic which are sufficiently well described to allow comparisons with our specimens. Only genera attributed to the subfamilies Clupeinae and Alosinae, or to the Clupeidae without specific classification at subfamily level are considered. The differences between the material from “Aidonochori A” and these fossil genera are described below. Unless otherwise stated, the data on the fossil genera were compiled from the original descriptions. The fossil genera are presented from the oldest to the youngest.
Primisardinella Danil’chenko, 1968; upper Paleocene of Turkmenistan; larger size (ca. 600 mm vs. up to ca. 180 mm total length in the fossils from the site “Aidonochori A”), last two anal rays elongated (vs. equal to, or shorter than the penultimate fin ray) (see also Danil’chenko 1980).
Horaclupea Borkar, 1973; upper Paleocene or lower Eocene (or Maastrichian, Upper Cretaceous, see Arratia et al. 2004) of Gujarat, India; fewer vertebrae (30–35 vs. 40–42), and fewer dorsal fin rays (8 vs. 16–17).
Eoalosa Marramà and Carnevale, 2018; Eocene marine sediments of Monte Bolca, Italy; larger number of vertebrae (47 vs. 40–42), fewer pelvic fin rays (7 vs. 8 or 9).
Bolcaichthys Marramà and Carnevale, 2015; Eocene marine sediments of Monte Bolca, Italy; “pelvic-fin origin slightly in front of or behind the posterior end of the dorsal fin” (Marramà and Carnevale 2015: 2) (vs. under the anterior third of the dorsal fin base), lower number of supraneurals (8 vs. 10), higher number of epurals (3 vs. 2).
Gosiutichthys Grande, 1982; middle Eocene lacustrine sediments of Wyoming, USA; smaller fish (up to ca. 40 mm vs. up to ca. 150 mm), dorsal scutes present (6–13 vs. none), lower number of vertebrae (34–36 vs. 40–42), supraneural bones (6 or 7 vs. 10), pelvic fin rays (6 or 7 vs. 8 or 9), dorsal fin rays (9 or 10 vs. 16 or 17) and anal fin rays (9–12 vs. 16–19), higher number of branchiostegal rays (8 vs. 5).
Marambionella Jerzmańska, 1991; upper Eocene–lower? Oligocene of Seymour Island/Marambio, Antarctica; higher numbers of vertebrae (ca. 49 vs. 40–42) and ventral scutes (19 prepelvic and 13 postpelvic vs. ca. 16 and 10 or 11).
Chasmoclupea Murray, Simons, and Attia, 2005; lower Oligocene lacustrine sediments of Fayum, Egypt; fewer pelvic fin rays (7 vs. 8 or 9), dorsal fin rays (12 vs. 16 or 17), higher number of supraneurals (13? vs. 10). In the original description of Murray et al. (2005: figs. 1 and 2) it is obvious that the ascending arms of the ventral scutes are very broad (vs. slender).
Rupelia Baykina and Kovalchuk in Kovalchuk et al., 2020; lower Oligocene (Rupelian) of northern Caucasus and Transcaucasia; last two anal rays elongated (vs. equal to, or shorter than the penultimate fin ray), ventral scutes poorly developed (vs. robust), pelvic fin at posterior third of dorsal fin base (vs. under the anterior third of the dorsal fin base), higher number of branchiostegals (7 vs. 5) and vertebrae (48–50 vs. 40–42).
Maicopiella (Menner, 1949); Oligocene (Rupelian and Chattian) and lower Miocene of northern Caucasus, Transcaucasia, Central and Eastern Europe; parietals smooth (vs. striated), last two anal rays elongated (vs. equal to, or shorter than the penultimate fin ray), ventral scutes poorly developed (vs. robust), and higher number of branchiostegals (7 vs. 5).
Clupeops Sauvage, 1880; Miocene of Drome, France; prominent teeth on premaxilla (vs. no teeth), 55 vertebrae (vs. 40–42).
Karaganops Baykina and Schwarzhans, 2017a; middle Miocene of Tambov, Russia; last two anal fin rays elongated (vs. equal to, or shorter than the penultimate fin ray), higher number of branchiostegals (7 vs. 5) and vertebrae (44–46 vs. 40–42).
Moldavichthys Baykina and Schwarzhans, 2017b; middle Miocene of Moldova; premaxilla and dentary toothed (vs. premaxilla and dentary toothless), opercle with radial ridges (vs. smooth), higher number of branchiostegals (7–8 vs. 5).
Pseudohilsa Menner, 1949; middle Miocene of Absheron Peninsula, Azerbaijan; lower number of dorsal fin rays (10–13 vs. 16 or 17) (see also Danil’chenko 1980).
Ganolytes Jordan, 1919 (including its junior synonyms Diradias Jordan, 1924 and Xenothrissa Jordan, 1925, according to David 1943); upper Miocene marine sediments of California, USA; opercle strongly striated (vs. smooth), higher number of vertebrae (45–52 vs. 40–42) (see also David 1943).
Eosardinella Sato, 1966; upper Miocene marine sediments of NE Japan; a pair of “very pronounced” ridges on the operculum (vs. smooth), higher number of vertebrae (47 vs. 40–42).
Quisque Jordan, 1920; upper Miocene marine sediments of California, USA; probably 9–10 dorsal scutes present (vs. none), lower number of vertebrae (30–32 vs. 40–42), dorsal fin rays (12 vs. 16 or 17) and anal fin rays (10 vs. 16–19), higher number of pelvic fin rays (10 vs. 8 or 9) (see also Jordan 1921).
Sarmatella Menner, 1949; upper Miocene deposits of Croatia; last two anal fin rays elongated (vs. equal to or shorter than the penultimate fin ray), higher number of vertebrae (44–54 vs. 40–42) and branchiostegal rays (7 vs. 5) (see also Baykina 2013).
Xyne Jordan and Gilbert, 1919 (Xyrinius Jordan and Gilbert, 1919, referred to this genus, see David 1943); upper Miocene marine sediments of California, U.S.A.; pelvic fin inserted slightly before dorsal fin (vs. under the anterior third of the dorsal fin base), preopercle with longitudinal ridges (vs. smooth), higher number of vertebrae (46 or 47 vs. 40–42) (see also David 1943).
Austroclupea Bardack, 1961; Miocene or Pliocene freshwater sediments of Argentina; large orbit corresponding to one third of HL (vs. about one quarter), lower numbers of vertebrae (35–37 vs. 40–42) and pelvic fin rays (7 vs. 8 or 9).
Paleopiquitinga de Figueiredo 2010; Pliocene of NE Brazil; higher number of branchiostegal rays (7 vs. 5), lower number of pelvic fin rays (7 vs. 8 or 9), supraneural bones (8 vs. 10), epurals (1 vs. 2) and postpelvic scutes (8 vs. 10–11).
The genus to which the fossils from the site “Aidonochori A” resemble the most is Pseudohilsa (misspelled as “Pseudochilsa” in Grande 1985). Menner (1949) erected this genus to accommodate the species Diplomystus brevicaudus, described by Lednev (1914). Lednev (1914) assigned this species to Diplomystus even though it lacked some typical characteristics of that genus, e.g., dorsal scutes. Apart from the lower number of dorsal fin rays, few other differences distinguish Pseudohilsa brevicauda from the material described here, e.g., the eye is ca. 33% of HL (vs. ca. 25% in the fossils from the site “Aidonochori A”) and the pelvic fin is positioned under the posterior half of the dorsal fin’s base (vs. the anterior half) (Lednev 1914; Danil’chenko 1980). We therefore tentatively place the fossils from the site “Aidonochori A” in the genus Pseudohilsa.
Pseudohilsa brevicauda shares several similarities with the fossils from the site “Aidonochori A”, including a similar standard length (ca. 100–150 mm), a slender body, a smooth opercle, absence of teeth, similar number of vertebrae (36–40 vs. 40–42 at the fossils from the site “Aidonochori A”), 10 or 11 supraneurals, last two anal rays not enlarged, ca. 40 transverse scale rows, ca. 22 ventral scutes, half of which are postpelvic (it is not clear if the scutes of the throat were taken into account) (Lednev 1914; Danil’chenko 1980). However, some important features of Pseudohilsa brevicauda are not described in the available literature, such as the presence or absence of parietal–postparietal striae, the number of epurals, the number of branchiostegals and pelvic rays. For the reasons listed above, the specimens from the site “Aidonochori A” are placed tentatively in the genus Pseudohilsa but in a separate species, Pseudohilsa nikosi sp. nov.
Other fossil Clupeomorpha from Greece.—The oldest clupeomorph fossil from Greece is Scombroclupea sp. from the Upper Cretaceous (Maastrichtian) near Karpenisi (Koch and Nicholaus 1969). The only record of a clupeomorph fossil fish from northern Greece is a clupeid from the Pliocene of the Serres Basin (Weiler 1943). This fossil has ca. 50 vertebrae (vs. 40–42 in Pseudohilsa nikosi sp. nov.) and a striated opercle (vs. smooth) and is therefore clearly different from those of Pseudohilsa nikosi sp. nov. Based primarily on these characters, Weiler (1943) attributed this fossil to “Caspialosa nordmanni” (Antipa, 1904), a junior synonym of the extant Alosa tanaica (Grimm, 1901). Other fossil clupeomorphs from Greece have been described from the upper Miocene to Pliocene of central Greece and Crete. They have been assigned to the extant genera Alosa, Sardina and Spratelloides (Gaudant 2001, 2004, 2014; Gaudant et al. 1994, 1997, 2005, 2006, 2010; Argyriou and Theodorou 2011; Argyriou in press).
Paleoecological remarks.—Systematic excavations of the fossil site “Aidonochori A” are still pending, but some preliminary remarks regarding the paleoecology of the site can be made. The sediment is fine-grained, and contains mica and carbonate. These characteristics, combined with the good state of preservation of the fish skeletons, point to a low-energy environment and anoxic conditions at the bottom. Possible environments may have been a lagoon or a lake. It is not possible to infer the salinity of this environment, as the clupeids are known to be euryhaline, but the occurrence of a single fish species may hint at a brackish environment, where species diversity usually is low (see Reichenbacher 1993).
The modern clupeid which seems to resemble Pseudohilsa nikosi sp. nov. most closely, Hilsa kelee, is a marine pelagic fish, which, like many other clupeids, lives in shoals and can tolerate very low salinities (Whitehead 1985). The aforementioned characteristics are congruent with what is known of Pseudohilsa nikosi sp. nov. from the site “Aidonochori A”, where multiple individuals in close proximity to each other have been recovered from the sediments.
Paleobiogeographical remarks.—The only occurrence of Pseudohilsa so far has been from the middle Miocene of Absheron Peninsula, Azerbaijan, near the Caspian Sea (Menner 1949; Danil’chenko 1980). Therefore, the fossils from the site “Aidonochori A” may help expand the known range of this genus. It is also notable that these two occurrences are not very far apart, either in space or geological time.
Hilsa kelee is found in the Indian Ocean and the Western Pacific (Whitehead 1985). The fossils of Pseudohilsa come from the Caspian Sea (Pseudohilsa brevicauda; see Menner 1949; Danil’chenko 1980) and the Eastern Mediterranean (Pseudohilsa nikosi sp. nov.), and the fossils attributed to Hilsa were recovered in the region of the Black Sea (H. elegans, H. oblonga, H. torosa and H. lata; see Gabelaia 1976; Danil’chenko 1980). This distribution hints at the existence of Hilsa look-alikes in the Tethys and the Eastern Paratethys during the middle Miocene to Pliocene. Local extinction of these fishes in these regions may have taken place at some point during the late Neogene or Quaternary.
A similar biogeographical scenario involving the Mediterranean and Indo-Pacific regions seems to apply to other clupeiform fishes, such as Spratelloides or Etrumeus. These genera were present in the Neogene of the Mediterranean (e.g., Grande 1985; Gaudant et al. 1994, 2010; Gaudant 2004; Landini and Sorbini 2005; Argyriou and Theodorou 2011; Argyriou in press), but subsequently became extinct in this region. These two genera were, however, among the first fishes to re-invade the Mediterranean Basin after the opening of the Suez Canal (Por 1978; Golani 1998), an invasion which might be facilitated by the current climatic trend (Por 2009).
Nevertheless, with regard to Pseudohilsa nikosi sp. nov., such biogeographical scenarios ought to be treated with caution until the affinities of this species are interpreted with confidence. In order to elucidate the possible relationships of the fossil Hilsa look-alikes with each other and modern Hilsa, further systematic studies are necessary.
Conclusions
The clupeid fossil fishes from the upper Miocene site “Aidonochori A” are attributed to a new species, Pseudohilsa nikosi sp. nov., owing to their unique morphology among fossil and extant clupeids. They are placed provisionally in the genus Pseudohilsa, which is known from the middle Miocene of the Caspian Sea. Among extant clupeids, they seem morphologically most similar to Hilsa kelee, which has a tropical distribution in the Indo-West Pacific. The fossils attributed to Pseudohilsa, as well as some fossils attributed to Hilsa from the middle Pliocene of the Black Sea, need to be revised, but it seems that clupeids resembling Hilsa were present in the Mediterranean and Eastern Paratethys during the Neogene. Future systematic studies of the fossils should help clarify the relationships between the aforementioned taxa and establish their biogeographical links.
Acknowledgements
We thank Evangelia Tsoukala, Director of the Museum of Geology and Palaeontology of the Aristotle University of Thessaloniki, Greece, for her collaboration. This study benefited from discussions with, and the suggestions of George Syrides (Aristotle University of Thessaloniki, Greece). We thank Ulrich Schliewen and Dirk Neumann for providing access to the X-ray source and Bernhard Ruthensteiner for performing the μCT (all SNSB-Bavarian State Collection of Zoology, Munich, Germany). We thank the reviewers of this paper, Oleksandr Kovalchuk (University of Wrocław, Poland and National Museum of Natural History, Kyiv, Ukraine) and Giorgio Carnevale (Università degli Studi di Torino, Italy) for their constructive comments as well as the editor, Daniel E. Barta (Oklahoma State University College of Osteopathic Medicine at the Cherokee Nation, Tahlequah, USA) for the processing of this manuscript. Finally, we thank Paul Hardy (Düsseldorf, Germany) for critical reading of the manuscript. This project was funded by a grant from the Deutsche Forschungsgemeinschaft (Grant No. RE 1113/24-1).
References
Antipa, G. 1904. Die Clupeinen des westlichen Teiles des Schwarzen Meeres und der Donaumündungen. Anzeiger der Akademie für Wissenschaftlichen, Wien 41: 299–303.
Argyriou, T. (in press). The fossil record of ray-finned fishes (Actinopterygii) in Greece. In: E. Vlachos (ed.), Fossil Vertebrates of Greece, Vol. 1. Springer International Publishing.
Argyriou, T. and Theodorou, G. 2011. New findings from the Pliocene (Zanclean) ichthyofauna of Aegina island, Greece. In: A. van der Geer and A. Athanassiou (eds.), Program and Abstracts, European Association of Vertebrate Paleontologists, 9th Annual Meeting, 13. Heraklion.
Arratia, G. 1997. Basal teleosts and teleostean phylogeny. Palaeo Ichthyologica 7: 5–168.
Arratia, G. 1999. The monophyly of Teleostei and stem-group teleosts. Consensus and disagreements. In: G. Arratia and H.-P. Schultze (eds.), Mesozoic Fishes 2, 265–334. Verlag Dr. Friedrich Pfeil, München.
Arratia, G. 2008. Actinopterygian postcranial skeleton with special reference to the diversity of fin ray elements, and the problem of identifying homologies. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 4—Homology and Phylogeny, 49–101. Verlag Dr. Friedrich Pfeil, München.
Arratia, G. 2018. Otomorphs (= otocephalans or ostarioclupeomorphs) revisited. Neotropical Ichthyology 16: e180079. Crossref
Arratia, G., López-Arbarello, A., Prasad, G.V., Parmar, V., and Kriwet, J. 2004. Late Cretaceous–Paleocene percomorphs (Teleostei) from India—early radiation of Perciformes. In: G. Arratia, W. M.V.H., and R. Cloutier (eds.), Recent Advances in the Origin and Early Radiation of Vertebrates, 635–663. Dr. Friedrich Pfeil, München.
Bardack, D. 1961. New tertiary teleosts from Argentina. American Museum Novitates 2041: 1–27.
Baykina, E.M. 2013. A revision of Clupea doljeana Kramberger and Sarmatella vukotinovici (Kramberger) (Pisces, Clupeidae) from the Sarmatian of Croatia. Paleontological Journal 47: 523–532. Crossref
Baykina, E.M. and Schwarzhans, W.W. 2017a. Description of Karaganops n. gen. perratus (Daniltshenko 1970) with otoliths in situ, an endemic Karaganian (middle Miocene) herring (Clupeidae) in the Eastern Paratethys. Swiss Journal of Palaeontology 136: 129–140. Crossref
Baykina, E.M. and Schwarzhans, W.W. 2017b. Review of “Clupea humilis” from the Sarmatian of Moldova and description of Moldavichthys switshenskae gen. et sp. nov. Swiss Journal of Palaeontology 136: 141–149. Crossref
Betancur-R., R., Wiley, E.O., Arratia, G., Acero, A., Bailly, N., Miya, M., Lecointre, G., and Ortí, G. 2017. Phylogenetic classification of bony fishes. BMC Evolutionary Biology 17 (162): 1–40.
Bleeker, P. 1849. Contribution to the knowledge of the ichthyological fauna of Celebes. The Journal of Indian Archipelago and Eastern Asia 3: 65–74.
Bleeker, P. 1859. Enumeratio specierum piscium hucusque in Archipelago indico observatarum, adjectis habitationibus citationibusque, ubi descriptiones earum recentiores reperiuntur, nec non speciebus Musei Bleekeriani Bengalensibus, Japonicis, Capensibus Tasmanicisque. 276 pp. Typis Langii & soc, Batavia (Jakarta).
Bloom, D.D. and Egan, J.P. 2018. Systematics of Clupeiformes and testing for ecological limits on species richness in a trans-marine/freshwater clade. Neotropical Ichthyology 16: e180095. Crossref
Bloom, D.D. and Lovejoy, N.R. 2014. The evolutionary origins of diadromy inferred from a time-calibrated phylogeny for Clupeiformes (herring and allies). Proceedings of the Royal Society B: Biological Sciences 281: 20132081. Crossref
Borkar, V. 1973. Fossil fishes from the inter-trappean beds of Surendranagar district, Saurashtra. Proceedings of the Indian Academy of Sciences-Section B 78 (4): 181–193. Crossref
Briggs, J.C. 2005. The biogeography of otophysan fishes (Ostariophysi: Otophysi): a new appraisal. Journal of Biogeography 32: 287–294. Crossref
Chen, W.J., Lavoué, S., and Mayden, R.L. 2013. Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution 67: 2218–2239. Crossref
Cope, E.D. 1871. Contribution to the ichthyology of the Lesser Antilles. Transactions of the American Philosophical Society (new series) 14: 445–483. Crossref
Cumplido, N., Allende, M.L., and Arratia, G. 2020. From Devo to Evo: patterning, fusion and evolution of the zebrafish terminal vertebra. Frontiers in zoology 17: 1–17. Crossref
Cuvier, G. 1816. Le Règne Animal: distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Les reptiles, les poissons, les mollusques et les annélides. 532 pp. Déterville, Paris.
Cuvier, G. 1829. Le Règne Animal: distribué d’après son organisation, pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Les reptiles, les poissons, les mollusques et les annélides. 406 pp. Déterville, Paris.
Cuvier, G. and Valenciennes, A. 1847. Histoire naturelle des poissons. Livre vingt et unième. De la famille des Clupéoïdes. 606 pp. Bertrand, Paris.
Danil’chenko, P. [Danil’čenko, P.] 1968. Upper Paleocene fishes of Turkmenia [in Russian]. In: D.V. Obručev (ed.), Očerki po filogenii i sistematike iskopaemyh ryb i bezčelûstnyh, 113–156. Nauka, Moskva.
Danil’chenko, P. [Danil’čenko, P.] 1980. Order Clupeiformes [in Russian]. In: L.I. Novitskaâ (ed.), Iskopaemye kostistye ryby SSSR. Trudy Paleontologičeskogo Instituta Akademii Nauk SSSR 178: 7–26.
David, L.R. 1943. Miocene Fishes of Southern California. 193 pp. Geological Society of America, Baltimore. Crossref
De Figueiredo, F.J. 2010. Morphological and systematic reassessment of †Knightia brasiliensis Woodward, 1939 (Teleostei: Clupeiformes) from the Pliocene of Parnaíba Basin, northeastern Brazil. Zootaxa 2440: 1–17. Crossref
Di Dario, F. 2002. Evidence supporting a sister-group relationship between Clupeoidea and Engrauloidea (Clupeomorpha). Copeia 2002: 496–503. Crossref
Di Dario, F. 2009. Chirocentrids as engrauloids: evidence from suspensorium, branchial arches, and infraorbital bones (Clupeomorpha, Teleostei). Zoological Journal of the Linnean Society 156: 363–383. Crossref
Di Dario, F. and De Pinna, M.C. 2006. The supratemporal system and the pattern of ramification of cephalic sensory canals in Denticeps clupeoides (Denticipitoidei, Teleostei): additional evidence for monophyly of Clupeiformes and Clupeoidei. Papéis Avulsos de Zoologia 46 (10): 107–123. Crossref
Eigenmann, C.H. 1912. The Freshwater Fishes of British Guiana, Including a Study of the Ecological Grouping of Species, And the Relation of the Fauna of the Plateau to That of the Lowlands. 578 pp. Carnegie Institute, Pitttsburgh. Crossref
FAO 2020. The State of World Fisheries and Aquaculture, 2020. Sustainability in Action. 206 pp. Rome.
Fowler, H.W. 1911. Notes on clupeoid fishes. Proceedings of the Academy of Natural Sciences of Philadelphia 63: 204–221.
Fowler, H.W. 1933. Descriptions of new fishes obtained 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Proceedings of the Academy of Natural Sciences of Philadelphia 85: 233–367.
Fricke, R., Eschmeyer, W.N., and van der Laan, R. (eds.) 2021. Eschmeyer’s Catalog of Fishes: Genera, Species, References. accessed 27.01.2021.
Fujita, K. 1990. The Caudal Skeleton of Teleostean Fishes. 897 pp. Tokai University Press, Tokyo.
Gabelaya, T.D. [Gabelaâ, T.D.] 1976. Ryby pliotsenovyh otloženij. 111 pp. Metsniereba, Tiflis.
Gaudant, J. 2001. Amnissos: un gisement clé pour la connaissance de l’ichthyofaune du Pliocène supérieur de Crete. Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie 102: 131–187.
Gaudant, J. 2004. Additions à l’ichthyofaune tortonienne du bassin de Ierapetra (Crète orientale, Grèce). Annalen des Naturhistorischen Museums in Wien. Serie A für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie 105: 257–285.
Gaudant, J. 2014. Fish mass mortality in the upper Miocene laminated gypsum of western Crete (Hania Province, Greece). Palaeodiversity 7: 39–45.
Gaudant, J., Courme-Rault, M., and Saint-Martin, S. 2010. On the fossil fishes, diatoms, and foraminifera from Zanclean (lower Pliocene) diatomitic sediments of Aegina Island (Greece): a stratigraphical and palaeoenvironmental study. Palaeodiversity 3: 141–149.
Gaudant, J., Delrieu, B., Dermitzakis, M.D., and Symeonidis, N.K. 1994. Découverte d’une ichthyofaune marine dans les diatomites du Pliocène supérieur (Plaisancien) des environs d’Héraklion (Crète centrale, Grèce). Comptes rendus de l’Académie des sciences. Série 2. Sciences de la terre et des planètes 319 (5): 589–596.
Gaudant, J., Fourtanier, E., Lauriat-Rage, A., Tsagaris, S., Vénec-Peyré, M.-T., and Zorn, I. 1997. Découverte d’une ichthyofaune marine dans le Messinien préévaporitique de la Messara (Crète centrale, Grèce): interprétation paléoécologique. Géologie méditerranéenne 24 (3): 175–195. Crossref
Gaudant, J., Tsaparas, N., Antonarakou, A., Drinia, H., and Dermitzakis, M.D. 2005. The Tortonian fish fauna of Gavdos island (Greece). Comptes Rendus Palevol 4: 687–695. Crossref
Gaudant, J., Tsaparas, N., Antonarakou, A., Drinia, H., Saint-Martin, S., and Dermitzakis, M.D. 2006. A new marine fish fauna from the pre-evaporitic Messinian of Gavdos Island (Greece). Comptes Rendus Palevol 5: 795–802. Crossref
Gill, T.N. 1861. Synopsis of the subfamily Clupeinae, with descriptions of new genera. Proceedings of the Academy of Natural Sciences of Philadelphia 13: 33–38.
Girgensohn, O.G. 1846. Anatomie und Physiologie des Fisch-Nervensystems. Mémoires de l’Académie des Sciences de St. Pétersbourg 5 (5): 275–589.
Golani, D. 1998. Distribution of Lessepsian migrant fish in the Mediterranean. Italian Journal of Zoology 65 (S1): 95–99. Crossref
Grande, L. 1982. A revision of the fossil genus Knightia, with a description of a new genus from the Green River Formation (Teleostei, Clupeidae). American Museum Novitates 2731: 1–22.
Grande, L. 1985. Recent and fossil clupeomorph fishes with materials for revision of the subgroups of clupeoids. Bulletin of the American Museum of Natural History 181: 231–372.
Greenwood, P.H., Rosen, D.E., Weitzman, S.H., and Myers, G.S. 1966. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bulletin of the American Museum of Natural History 131: 339–456.
Grimm, O. von 1901. The herrings of the Sea of Azov [in Russian]. Vestnik Rybopromyšlennosti, St. Petersburg 16 (2): 57–70.
Hubbs, C.L. 1929. The generic relationships and nomenclature of the California sardine. Proceedings of the California Academy of Sciences, fourth Series 18: 261–265.
Ioakim, C., Rondoyanni, T., and Mettos, A. 2005. The Miocene basins of Greece (Eastern Mediterranean) from a palaeoclimatic perspective. Revue de Paléobiologie 24: 735.
Jerzmańska, A. 1991. First articulated teleost fish from the Paleogene of West Antarctica. Antarctic Science 3: 309–316. Crossref
Jordan, D.S. 1919. I. Fossil fishes of the soledad deposits. In: L. Stanford Jr. (ed.), Fossil Fishes of Southern California, 3–12. Stanford University Press, Stanford. Crossref
Jordan, D.S. 1920. The Genera of Fishes Part IV. From 1881 to 1920, Thirty-nine Years, with the Accepted Type of Each. A Contribution to the Stability of Scientific Nomenclature. 415–576 pp. Stanford University Press, Stanford. Crossref
Jordan, D.S. 1921. The Fish Fauna of the California Tertiary. 300 pp. Stanford University Press, Stanford.
Jordan, D.S. 1924. Description of Miocene fishes from southern California. Bulletin of the Southern California Academy of Sciences 23 (2): 42–50.
Jordan, D.S. 1925. The Fossil Fishes of the Miocene of Southern California. 51 pp. Stanford University Press, Stanford.
Jordan, D.S. and Evermann, B.W. 1896. The Fishes of North and Middle America: A Descriptive Catalogue of the Species of Fish-like Vertebrates Found in the Waters of North America, North of the Isthmus of Panama. 1240 pp. US Government Printing Office, Washington. Crossref
Jordan, D.S. and Gilbert, J.Z. 1919. II. Fossil fishes of the (Miocene) Monterey Formations of Southern California. In, Fossil fishes of southern California, 13–60. Stanford University Press, California. Crossref
Karistineos, N. and Ioakim, C. 1989. Palaeoenvironmental and palaeoclimatic evolution of the Serres Basin (N. Greece) during the Miocene. Palaeogeography, Palaeoclimatology, Palaeoecology 70: 275–285. Crossref
Kessler, K.T. 1877. Trudy aralo-kaspіjskoj èkspedicіi. IV. Ryby vodâŝіâsâ i vstrechaûŝіâsâ v Aralo-kaspіjsko-pontіjskoj ihtіologičeskoj oblasti. 360 pp. Stasûleviča, St. Petersburg.
Koch, K.E. and Nikolaus, H. 1969. Zur Geologie des Ostpindos—Flyschbeckens und seiner Umrandung. 190 pp. Institute for Geology and subsurface research, Athens.
Kovalchuk, O., Baykina, E., Świdnicka, E., Stefaniak, K., and Nadachowski, A. 2020. A systematic revision of herrings (Teleostei, Clupeidae, Clupeinae) from the Oligocene and early Miocene from the Eastern Paratethys and the Carpathian Basin. Journal of Vertebrate Paleontology 40: e1778710. Crossref
Landini, W. and Sorbini, C. 2005. Evolutionary dynamics in the fish faunas of the Mediterranean basin during the Plio-Pleistocene. Quaternary International 140: 64–89. Crossref
Lavoué, S., Konstantinidis, P., and Chen, W.-J. 2014. Progress in clupeiform systematics. In: K. Ganias (ed.), Biology and Ecology of Sardines and Anchovies, 3–42. CRC Press, Boca Raton. Crossref
Lavoué, S., Miya, M., and Nishida, M. 2010. Mitochondrial phylogenomics of anchovies (family Engraulidae) and recurrent origins of pronounced miniaturization in the order Clupeiformes. Molecular Phylogenetics and Evolution 56: 480–485. Crossref
Lavoué, S., Miya, M., Musikasinthorn, P., Chen, W.-J., and Nishida, M. 2013. Mitogenomic evidence for an Indo-west Pacific origin of the Clupeoidei (Teleostei: Clupeiformes). PLOS ONE 8: e56485. Crossref
Lavoué, S., Miya, M., Saitoh, K., Ishiguro, N.B., and Nishida, M. 2007. Phylogenetic relationships among anchovies, sardines, herrings and their relatives (Clupeiformes), inferred from whole mitogenome sequences. Molecular Phylogenetics and Evolution 43: 1096–1105. Crossref
Lednev, N. 1914. Die Fauna der Fischschichten der Halbinsel Apscheron. Mémoires du Comité Géologique. Nouvelle serie 80: 64.
Li, C. and Ortí, G. 2007. Molecular phylogeny of Clupeiformes (Actinopterygii) inferred from nuclear and mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 44: 386–398. Crossref
Linck, H. 1790. Versuch einer Entheilung der Fische nach den Zahnen. Magazin für das Neueste aus der Physik und Naturgeschichte 6 (3): 28–38.
Linnaeus, C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Holmia. Crossref
Marramà, G. and Carnevale, G. 2015. The Eocene sardine †Bolcaichthys catopygopterus (Woodward, 1901) from Monte Bolca, Italy: osteology, taxonomy, and paleobiology. Journal of Vertebrate Paleontology 35: e1014490. Crossref
Marramà, G. and Carnevale, G. 2016. An Eocene anchovy from Monte Bolca, Italy: The earliest known record for the family Engraulidae. Geological Magazine 153: 84–94. Crossref
Marramà, G. and Carnevale, G. 2018. Eoalosa janvieri gen. et sp. nov., a new clupeid fish (Teleostei, Clupeiformes) from the Eocene of Monte Bolca, Italy. PalZ 92: 107–120. Crossref
McAllister, D.E. 1968. Evolution of the branchiostegals and classification of teleostome fishes. Bulletin of the National Museum of Canada (Biology Series) 77: 1–239. Crossref
Menner, V.V. 1949. The class Pisces [in Russian]. In: A.G. Zabelin (ed.), Atlas rukovodâŝih form iskopaemyh fanny SSSR, Tom 13, Neogen, 348–352. Gosgeolitizdat, Moskva.
Miyashita, T. 2010. Unique occipital articulation with the first vertebra found in pristigasterids, chirocentrids, and clupeids (Teleostei: Clupeiformes: Clupeoidei). Ichthyological Research 57 (2): 121–132. Crossref
Murray, A.M., Simons, E.L., and Attia, Y.S. 2005. A new clupeid fish (Clupeomorpha) from the Oligocene of Fayum, Egypt, with notes on some other fossil clupeomorphs. Journal of Vertebrate Paleontology 25: 300–308. Crossref
Murray, A.M. and Wilson, M.V. 2013. Two new paraclupeid fishes (Clupeomorpha: Ellimmichthyiformes) from the Upper Cretaceous of Morocco. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 5—Global Diversity and Evolution, 267–290. Verlag Dr. Friedrich Pfeil, Munich.
Nelson, G.J. 1970. The hyobranchial apparatus of teleostean fishes of the families Engraulidae and Chirocentridae. American Museum Novitates 2410: 1–30.
Nelson, J.S., Grande, T.C., and Wilson, M.V.H. 2016. Fishes of the World. 707 pp. John Wiley & Sons, Hoboken, New Jersey.
Patterson, C. and Johnson, G.D. 1995. The Intermuscular Bones and Ligaments of Teleostean Fishes. 87 pp. Smithsonian Institution Press, Washington, D.C. Crossref
Phillips, J.B. 1942. Osteology of the sardine (Sardinops carulea). Journal of Morphology 70: 463–500. Crossref
Pimpirev, C. and Beratis, I. 2010. Lithostratigraphy of the Miocene sedimentary sequences in Strymon Basin, northern Greece. Comptes rendus de l’Académie bulgare des Sciences 63: 1177–1190.
Por, F.D. 1978. Lessepsian Migration: The Influx of Red Sea Biota Into the Mediterranean by Way of the Suez Canal. 228 pp. Springer Verlag, Berlin. Crossref
Por, F.D. 2009. Tethys returns to the Mediterranean: success and limits of tropical re-colonization. BioRisk 3: 5–19. Crossref
Psilovikos, A. and Karistineos, N. 1986. A depositional sedimentary model for the Neogene uraniferous lignites of the Serres graben, Greece. Palaeogeography, Palaeoclimatology, Palaeoecology 56: 1–16. Crossref
Queiroz, C.d.C.S., Souza, R.F.C., Silva, S.S., de Mattos Dias, C.A.G., Fecury, A.A., de Oliveira, E., da Cunha, D.B., Schneider, H., and Sampaio, I. 2020. Molecular phylogeny of Clupeiformes and the placement of some Western Atlantic and Amazonian taxa. Biota Amazônia 10 (2): 14–19.
Regan, C.T. 1917. A revision of the Clupeoid fishes of the genera Pomolobus, Brevoortia and Dorosoma, and their allies. Annals and Magazine of Natural History, Series 8 19: 297–316. Crossref
Reichenbacher, B. 1993. Mikrofaunen, Paläogeographie und Biostratigraphie der miozänen Brack- und Süßwassermolasse in der westlichen Paratethys unter besonderer Berücksichtigung der Fisch-Otolithen. Senckenbergiana lethaea 73: 277–374.
Ridewood, W. 1904. On the cranial osteology of the clupeoid fishes. Proceedings of the Zoological Society of London 74: 448–493. Crossref
Sato, J. 1966. A new genus and species of sardine from the Miocene Hishinai Formation, northeastern Japan. Japanese Journal of Ichthyology 13 (4/6): 112–125.
Sato, Y. 1994. Cladistic-Phenetic Relationships and the Evolutionary Classification of the Suborder Clupeoidei (Pisces, Teleostei). 85 pp. Tokyo Metropolitan University, Tokyo.
Sauvage, M.H. 1880. Note sur les Poissons Fossiles d’Eurre Drome. In: C.-F. Fontannes (ed.), Études Stratigraphiques et Paléontologiques pour Servir a l’Histoire de la Période Tertiaire dans le Bassin du Rhone 6, 205–210. Pitrat Ainé, Lyon.
Schultze, H.-P. 2008. Nomenclature and homologization of cranial bones in actinopterygians. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 4—Homology and Phylogeny. Proceedings of the International Meeting Miraflores de la Sierra, 2005, 23–48. Verlag Dr. Friedrich Pfeil, München.
Schultze, H.-P. and Arratia, G. 2013. The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. In: G. Arratia, H.-P. Schultze, and M.V.H. Wilson (eds.), Mesozoic Fishes 5—Global Diversity and Evolution, 187–246. Verlag Dr. Friedrich Pfeil, München.
Segura, V. and Díaz de Astarloa, J.M. 2004. Análisis osteológico de la saraca Brevoortia aurea (Spix) (Actinopterygii: Clupeidae) en el Atlántico suroccidental. Revista de Biología Marina y Oceanografía 39 (2): 37–52. Crossref
Straube, N., Li, C., Mertzen, M., Yuan, H., and Moritz, T. 2018. A phylogenomic approach to reconstruct interrelationships of main clupeocephalan lineages with a critical discussion of morphological apomorphies. BMC Evolutionary Biology 18 (158): 1–17. Crossref
Svetovidov, A. 1963. Fauna of USSR: Fishes, Clupeidae. 428 pp. Israel Program for Scientific Translations, Jerusalem.
Syrides, G.E. 1995. Neogene mollusk faunas from Strymon Basin, Macedonia, Greece. First results for biochronology and palaeoenvironment. Geobios 28: 381–388. Crossref
Syrides, G.E. 1995. Paratethyan mollusc faunas from the Neogene of Macedonia and Thrace, Northern Greece. Romanian Journal of Stratigraphy 78: 171–180.
Syrides, G.E. 2000. Neogene marine cycles in Strymon Basin, Macedonia, Greece. In: G.D. Koufos and Ch. Ioakim (eds.), Mediterranean Neogene Cyclostratigraphy in Marine-Continental Palaeoenvironments. Geological Society of Greece, Special Publications 9: 217–225.
Teng, C.S., Cavin, L., Jnr, R.E.M., Sánchez-Villagra, M.R., and Crump, J.G. 2019. Resolving homology in the face of shifting germ layer origins: Lessons from a major skull vault boundary. Elife 8: e52814. Crossref
Thompson, W.F. 1916. Fishes collected by the United States Bureau of Fisheries steamer” Albatross” during 1888, between Montevideo, Uruguay, and Tome, Chile, on the voyage through the Straits of Magellan. Proceedings of the United States National Museum 50: 401–476. Crossref
Tranos, M.D. 2011. Strymon and Strymonikos Gulf basins (Northern Greece): Implications on their formation and evolution from faulting. Journal of Geodynamics 51 (4): 285–305. Crossref
Weiler, W. 1943. Über einen pliozänen Fischrest aus Mazedonien. Zeitschrift der Deutschen Geologischen Gesellschaft 95: 210–213.
Whitehead, P.J. 1962. A contribution to the classification of clupeoid fishes. Journal of Natural History 5: 737–750. Crossref
Whitehead, P.J. 1964. A new genus and subgenus of clupeid fishes and notes on the genera Clupea, Sprattus and Clupeonella. Annals and Magazine of Natural History, Series 13 7: 321–330. Crossref
Whitehead, P.J. 1968. A new genus for the South American clupeid fish, Lile platana Regan. Journal of Natural History 2: 477–486. Crossref
Whitehead, P.J. 1985. Clupeoid Fishes of the World (Suborder Clupeoidei). An Annotated and Illustrated Catalogue of the Herrings, Sardines, Pilchards, Sprats, Anchovies and Wolf-Herrings. Part 1—Chirocentridae, Clupeidae and Pristigasteridae. 303 pp. United Nations Development Programme—Food and Agriculture Organization of the United Nations, Rome.
Whitley, G.P. 1940. Illustrations of some Australian fishes. Australian Zoologist 9: 397–428.
Whitley, G.P. 1951. New fish names and records. Proceedings of the Royal Zoological Society of New South Wales 50: 61–68.
Wiley, E. and Johnson, G.D. 2010. A teleost classification based on monophyletic groups. In: J.S. Nelson, H.-P. Schultze, and M.V. Wilson (eds.), Origin and Phylogenetic Interrelationships of Teleosts, 123–182. Dr. Friedrich Pfeil, Munich.
Wiley, E.O., Fuiten, A.M., Doosey, M.H., Lohman, B.K., Merkes, C., and Azuma, M. 2015. The caudal skeleton of the zebrafish, Danio rerio, from a phylogenetic perspective: a polyural interpretation of homologous structures. Copeia 103: 740–750. Crossref
Wilson, A.B., Teugels, G.G., and Meyer, A. 2008. Marine incursion: the freshwater herring of Lake Tanganyika are the product of a marine invasion into West Africa. PLOS ONE 3: e1979. Crossref
Wongratana, T. 1980. Systematics of Clupeoid Fishes of the Indo-Pacific Region. 432 pp. University of London, London.
Zagorchev, I. 2007. Late Cenozoic development of the Strouma and Mesta fluviolacustrine systems, SW Bulgaria and northern Greece. Quaternary Science Reviews 26: 2783–2800. Crossref
Acta Palaeontol. Pol. 66 (3): 605–621, 2021
http://dx.doi.org/10.4202/app.00871.2020