


Bone histology of the graviportal dinocephalian therapsid Jonkeria from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa
MOHD SHAFI BHAT, CHRISTEN D. SHELTON, and ANUSUYA CHINSAMY
Bhat, M.S., Shelton, C.D., and Chinsamy, A. 2021. Bone histology of the graviportal dinocephalian therapsid Jonkeria from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. Acta Palaeontologica Polonica 66 (4): 705–721.
Here we examine the bone histology of multiple skeletal elements of three individuals of Jonkeria from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. Our histological results reveal a predominance of highly vascularized, uninterrupted fibrolamellar bone tissue, which suggests rapid periosteal bone deposition and an overall fast growth. However, in a rib, the periosteal bone deposition periodically stops abruptly, resulting in the deposition of several lines of arrested growth. The absence of bone growth marks in the limb bones (except for an annulus in a radius) suggests a young ontogenetic status for all specimens of the studied sample. All the skeletal elements are characterized by thick bone walls, extensive secondary reconstruction and the complete infilling of the medullary cavity by bony trabeculae. The latter condition is different to observations of contemporaneous graviportal terrestrial pareiasaurs, but similar to the observations in the modern semi-aquatic Hippopotamus, and suggests a possible semi-aquatic lifestyle for Jonkeria. On the basis of our histological findings, we assert that during early ontogeny Jonkeria experienced rapid sustained rates of growth, whereas later in ontogeny they experienced cyclical rates of growth.
Key words: Therapsida, Synapsida, bone microstructure, middle Permian, Beaufort Group, Abrahamskraal Formation, South Africa.
Mohd Shafi Bhat [shafialig@gmail.com] and Anusuya Chinsamy [anusuya.chinsamy-turan@uct.ac.za], Department of Biological Sciences, University of Cape Town, Private Bag X3, Rhodes Gift 7701, South Africa.
Christen D. Shelton [cshelton@rsu.edu] Department of Biological Sciences, University of Cape Town, Private Bag X3, Rhodes Gift 7701, South Africa; current address: Biology/Mathematics & Physical Science Departments, Rogers State University, Claremore, OK 74017-3252, USA and Natural History Department, New Jersey State Museum, Trenton, NJ 08625-0530, USA.
Received 5 January 2021, accepted 3 June 2021, available online 15 October 2021.
Copyright © 2021 M.S. Bhat et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Jonkeria is an omnivorous dinocephalian therapsid belonging to the family Titanosuchidae (Van Hoepen 1916; Boonstra 1936, 1963a, b; Colbert 1969), whose remains have been found in the middle Permian of the Tapinocephalus Assemblage Zone of the Beaufort Group, Karoo Supergroup of South Africa (Boonstra 1969). The Tapinocephalus Assemblage Zone (sensu Smith and Keyser 1995) is the second oldest tetrapod biozone of the Beaufort Group, sandwiched biostratigraphically between the underlying Eodicynodon Assemblage Zone characterized by the small- to medium- sized dicynodont Eodicynodon oosthuizeni and the overlying Endothiodon Assemblage Zone characterized by Endothiodon bathystoma (Day and Rubidge 2020; Day and Smith 2020). The Karoo Basin is world renowned for its extensive record of non-mammalian synapsids (e.g., Boonstra 1963a; Fordyce et al. 2012) and dinocephalians form one of the most successful therapsid lineages which flourished during the middle Permian between 265 and 260 million years ago (Boonstra 1963a, 1968). They were the most diverse and dominant group of the time, but became extinct by the end of the middle Permian Tapinocephalus Assemblage Zone, leaving no known descendants (Boonstra 1971; Kemp 1982, 2012; Day and Rubidge 2020). Dinocephalians are characterized by their pachyostotic skulls and pleisiomorphic cranial features similar to the ancestral late Carboniferous and early Permian pelycosaur-grade synapsids (Boonstra 1963b, 1971; King 1988; Rubidge and Sidor 2001). Although they retained their basic therapsid structure (Kemp 1982, 2012), dinocephalians were large-bodied animals, adapted to both carnivory (e.g., Anteosauridae) and herbivory (e.g., Tapinocephalidae, Boonstra 1963a, b). The most common dinocephalians found in the Tapinocephalus Assemblage Zone (Kemp 1982, 2005, 2012) are members of the Titanosuchidae (namely, Jonkeria and Titanosuchus), which may have been omnivores (Shelton et al. 2019).
Jonkeria was first named by Van Hoepan (1916) based on a well-preserved skull and associated postcranial skeleton recovered from the lower Karoo beds. Subsequently, a number of species were attributed to the Jonkeria, however, some of the species were synonymized by Boonstra (1969) after re-examining the Tapinocephalus Assemblage Zone material. Morphologically, Jonkeria appears to be closely related to Titanosuchus with the most obvious difference between the two being Jonkeria’s shorter limbs (Kemp 1982, 2012). Based on the presence of a long snout and a heavily built skull equipped with sharp incisors and fang-like canines, Colbert (1969) suggested that Jonkeria was a fierce carnivore that preyed on large animals. However, the presence of numerous, small, leaf-shaped postcanine teeth that bear serrated edges suitable for grasping/plucking plant material are suggestive of a herbivorous diet for titanosuchids (Kemp 1982, 2005; King 1988). Given this mixed dentition, it is likely that Jonkeria may have been omnivorous (Kemp 1982; Shelton et al. 2019) and may have used its large body size to intimidate and confiscate the prey of small carnivores (Colbert 1969). Such behaviour is commonly seen in modern opportunistic omnivores like grizzly bears (Ursus arctos) (Bastille-Rousseau et al. 2011; Gunther et al. 2014). In contrast to other therapsid clades, such as anomodonts, therocephalians and non-mammalian cynodonts, dinocephalians have received relatively little palaeobiological attention (Barghusen 1975; Kammerer 2011; Chinsamy-Turan 2012a). Compared to other dinocephalians, fossils of Jonkeria are relatively scarce and there are only a few palaeobiological studies of this genus (Boonstra 1969; Shelton et al. 2019).
Bone histology and skeletochronology are valuable tools for inferring biological information about age, lifestyle and sexual maturity of extant (e.g., Woodward et al. 2014; Montoya-Sanhueza and Chinsamy 2018; Nacarino-Meneses and Köhler 2018; Legendre and Botha-Brink 2018; Bhat et al. 2019; Chinsamy and Warburton 2020) and extinct animals (e.g., de Ricqlès 1969, 1972; Francillon-Vieillot et al. 1990; Chinsamy 1997; Ray et al. 2004, 2005; Chinsamy-Turan 2005; Chinsamy et al. 2013, 2019, 2020; Woodward et al. 2020). Such techniques have been applied to study various vertebrate groups including dinosaurs (e.g., Chinsamy and Dodson 1995; Chinsamy et al. 1994, 1998; Horner et al. 1999, 2000; Klein and Sander 2008; Woodward et al. 2015; Handley et al. 2016; Angst et al. 2017; Cerda et al. 2017; Krupandan et al. 2018; Cullen et al. 2020), basal turtles (e.g., Scheyer and Sánchez-Villagra 2007; Scheyer and Sander 2007; Pereyra et al. 2020), mammals and their relatives (e.g., Botha and Chinsamy 2000; Ray et al. 2004; Chinsamy and Hurum 2006; Chinsamy and Abdala 2008; Hurum and Chinsamy-Turan 2012; Jasinoski and Chinsamy 2012; Shelton et al. 2013; Huttenlocker and Botha-Brink 2014; Lambertz et al. 2016; Shelton and Sander 2017; Botha-Brink et al. 2018; Huttenlocker and Shelton 2020; Botha 2020). Despite the host of information that can be revealed by vertebrate bone histology, information on dinocephalian histology has been scarce (de Ricqlès 1972; Chinsamy-Turan 2012a; Shelton et al. 2019), which has led to limited information about their biology. Although, Enlow and Brown (1956, 1957) conducted the seminal study on the bone microstructure of therapsids, de Ricqlès (1972) provided the earliest and systematic description of bone histology of a variety of therapsids including Titanosuchidae. De Ricqlès (1972) briefly described richly vascularized, dense Haversian bone in Titanosuchus ferox with numerous secondary osteons partially dispersed over each other, whereas the interstitial tissue is comprised of lamellar bone with poor vascularity. The most recent work on titanosuchid bone histology led to the identification of osteomyelitis in a femur of Jonkeria parva (Shelton et al. 2019). This pathology is characterized by the growth of radial bony spicules perpendicular to the normal unaffected fibrolamellar bone tissue of the cortex. In addition, Shelton et al. (2019) also noted an increase in localized vascularization and substantial remodeling in the vicinity of the puncture marks that may have been the cause of infection.
In the current study, we apply bone histological techniques to Jonkeria parva and J. ingens, as well as an undetermined Jonkeria species (Table 1). The main objectives of the current research are (i) to describe the inter-elemental and intraspecific bone histology of the genus Jonkeria; (ii) to shed light on the histological variation and life history information of different species of Jonkeria; and (iii) to expand our knowledge about their palaeobiology.
Institutional abbreviations.—BP, Evolutionary Sciences Institute (previously Bernard Price Institute), University of the Witwatersrand, Johannesburg, South Africa; SAM, Iziko South African Museums, Cape Town, South Africa.
Material and methods
Jonkeria specimens studied here were excavated from the Tapinocephalus Assemblage Zone of the South African Karoo Basin (Boonstra 1969), and are housed in the Iziko Museums of Cape Town, and the Evolutionary Studies Institute (formerly the Bernard Price Institute) at the University of the Witwatersrand, Johannesburg, South Africa. For our analysis, five skeletal elements, including a femur, humerus, radius, tarsal, and partial rib, were selected to investigate both inter-elemental and intraspecific histological variability (Table 1). The femur, radius, and tarsal (SAM-PK-12233a–c) belong to Jonkeria parva and were collected by Lieuwe D. Boonstra and colleagues (1969) at Deesweesfontein Farm in Laingsburg, South Africa. The specimens bearing the same specimen numbers but differentiated by a/b/c indicate different elements of the same individual. It should be noted that the femur (SAM-PK-12233a) was studied by Shelton et al. (2019) wherein a pathology was reported. In the current study, several transverse sections at different levels of the femoral shaft were studied to assess histological variation along the bone. The distal part of an isolated humerus (SAM-PK-11994) recovered from Welgemoed, Prince Albert, South Africa (Boonstra 1955; King 1988) was also studied and is referred to as Jonkeria ingens (previously known as Dinophoneus ingens; Broom 1923; Boonstra, 1969; King 1988). The species description was based on a complete skull (Broom 1923) that was previously collected by M.J. van Wyk on his farm Kookfontein in Prince Albert, Tapinocephalus Assemblage Zone, South Africa. The final specimen included in our study is BP/1/5409, which is an isolated partial rib collected from Banksdrif (Koornplaas, 41), Tapinocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup), and is designated as cf. Jonkeria sp. All the studied specimens were obtained either from the Iziko South African Museums, Cape Town, or the Evolutionary Studies Institute (formerly the Bernard Price Institute) at the University of the Witwatersrand, Johannesburg, South Africa (see Table 1). Permission to section the fossils was obtained from the South African Heritage Resources Agency (SAHRA: permits 2076, 3752-4658).
Table 1. Skeletal elements of Jonkeria that were studied. All the material was recovered from the Tapinocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. Source of information: Boonstra (1955, 1969); King (1988). SAM-PK-12233a/b/c are different elements of the same individual.
|
Specimen number |
Skeletal element |
Taxon |
Locality |
Section type |
|
SAM-PK-12233a |
femur |
Jonkeria parva Boonstra, 1955 |
Deesweesfontein Farm in Laingsburg, Tapinocephalus Assemblage Zone of the Karoo Basin, South Africa. |
transverse |
|
SAM-PK-12233b |
radius |
|||
|
SAM-PK-12233c |
tarsal |
|||
|
SAM-PK-11994 |
humerus |
Jonkeria ingens Broom, 1923 |
Welgemoed, Prince Albert, Tapinocephalus Assemblage Zone, South Africa. |
transverse core |
|
BP/1/5409 |
rib |
cf. Jonkeria sp. |
Banksdrif (Koornplaas, 41), Tapinocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup, South Africa. |
transverse, |
Since vertebrates display a wide range of variation in histological characteristics (e.g., Horner et al. 1999, 2000; Ray and Chinsamy 2004; Ray et al. 2004), as well as bone depositional rates (e.g., Amprino 1947; de Margerie et al. 2002, 2004; Starck and Chinsamy 2002; Botha-Brink and Angielczyk 2010), multi-element studies of individuals provide a better assessment of their growth patterns, life habits and evolutionary history (Botha and Chinsamy 2004, 2005; Chinsamy-Turan 2005, 2012b; Ray et al. 2009). The destructive nature of histological analyses and the scarcity of the complete specimens prohibited sectioning a large number of bones; however, an optimal sample was obtained by selecting partial skeletal elements from available species. Transverse sections were prepared from midshaft levels wherever possible as these are the regions of the bone that undergo the least secondary remodeling (Enlow 1963; Chinsamy 1995; Chinsamy-Turan 2005) and limb bones were mostly selected because they represent the best track record of growth (Francillon-Vieillot et al. 1990; Chinsamy-Turan 2005; Bhat et al. 2019; Chinsamy and Worthy 2021). On the other hand, the rib was sectioned towards the proximal end as this region is considered to maintain a better growth mark record than weight bearing long bones (Stein and Sander 2009; Waskow and Sander 2014). In addition, several longitudinal sections were also prepared to record additional information about the organisation of collagen fibrils (Stein and Prondvai 2014).
Thin sections were petrographically prepared using cutting and grinding techniques following Chinsamy and Raath (1992). In addition, some of the bones were either sampled by the hydraulic coring method using a drill with a diamond encrusted coring bit or cut using a Dremel Precision Tool, following the procedures outlined in Stein and Sander (2009). Core drilling was carried out preferentially in an area that was anatomically unimportant for taxonomic identification, but yet ideal for histological analyses. After the 1 cm cores were obtained, the holes were infilled with plaster to preserve the overall morphology. The cores were embedded in an epoxy resin (EpoxAcast 690 and/or Struers Epofix; Chinsamy and Raath 1992; Chinsamy-Turan 2005). Coring, sectioning, embedding and thin sectioning, as well as microscopy thereafter were performed in the thin sectioning laboratory of the Palaeobiology Research Group at the Biological Sciences Department at the University of Cape Town. The embedded bones were mounted on frosted glass slides, thin sectioned using a Struers Accutom-50 and thereafter ground and polished using carborundum (silicon carbide) discs of various grit sizes (400–1200 μm). This is followed by a final polish on a lap wheel with a velvet cloth using aluminium oxide (Al₂O₃) solution. The final thickness of the thin section was 50–45 μm, which proved to be optimum for our analyses. More than two sections from a single bone were prepared in case of slide breakages. All the prepared bones were studied and photographed using digital compact cameras Nikon DS-Fi1 and Axiocam 208 color mounted on a Carl Zeiss Axio Lab A1 polarizing microscope. The histological nomenclature follows Francillon-Vieillot et al. (1990) and Chinsamy-Turan (2005, 2012b). Chinsamy-Turan (2005) noted that although the orientation of canals in the bone can be used as a proxy to describe the extent and orientation of the vascular canals present in the bone, their orientation does not reflect the orientation of the actual blood vessels (Starck and Chinsamy 2002; Chinsamy and Warburton 2020) as these canals/lumens are often occupied by multiple blood vessels, as well as nerves and various other connective tissues (Chinsamy-Turan 2005). However, the estimation of canal area is comparable in different skeletal elements within a single taxon or in different taxa, as well as through ontogeny (Chinsamy 1993; Chinsamy et al. 2016; Botha and Chinsamy 2004) and therefore provides a maximum estimation of vascularization in each section (Chinsamy 1993).
Results
Our observations revealed that, in general, the bone histology of the specimens is well preserved (Figs. 1–5), although, diagenesis has substantially changed the original orientation of the crystallites in some bones and in these cases obscured some of their histological characteristics. A reddish-brown staining was noticed in a few sections and has been previously attributed to fungal activity or infiltration of humic factors as seen in most archeological settings (Piepenbrink 1986; Jans et al. 2002; Nacarino-Meneses et al. 2021). However, such staining generally occurs in the outer cortex or in the zone of the bone lying in contact with the sediment and therefore suggests a physico-chemical aetiology for the sections studied here (Garland 1987; Lyman 1994; Huculak and Rogers 2009). Our results show that each Jonkeria species has unique histological features, which are described separately in the following section.
Jonkeria parva (Boonstra, 1955)
Femur.—Here we studied a series of cross-sections of the femoral (SAM-PK-12233a) shaft proximal to the bite marks observed on the bone surface (previously described by Shelton et al. 2019). In the current study, a detailed histological analysis was performed to assess the limit of the infection and the variation in histology at different levels. All the cross-sections of the femur show a thick compact bone wall and medullary spongiosa. The medullary region is entirely infilled by struts of bony trabeculae in which the pore spaces/openings and resorption cavities are mostly infilled with sedimentary matrix. The primary bone is composed of highly vascularized uninterrupted fibrolamellar bone. Numerous primary osteons are embedded in the interstitial woven bone matrix. Secondary osteons and growth marks are not present.
In addition to the histological features described in Shelton et al (2019), the cross-section more proximal to the bite marks (see Shelton et al. 2019: 1094, fig. 1c) shows woven bone and rich vascularization (Fig. 1A, B). The density of osteocyte lacunae within the woven bone increases towards the bite marks within the same diaphyseal section (Fig. 1B). Vascular canals have a predominantly circumferential arrangement in the inner and mid-cortex whereas reticular vascular canals dominate towards the peripheral cortex (Fig. 1A), although local patches of simple longitudinal and radial canals are visible in the compacta. Large erosion spaces are present and extensive secondary reconstruction is visible which extend to the interior of the cross-section with some of these erosion spaces enlarged enough to reach cancellous dimensions (Fig. 1A1). Resorption cavities show varying degrees of centripetal deposition of lamellar bone (Fig. 1A1).
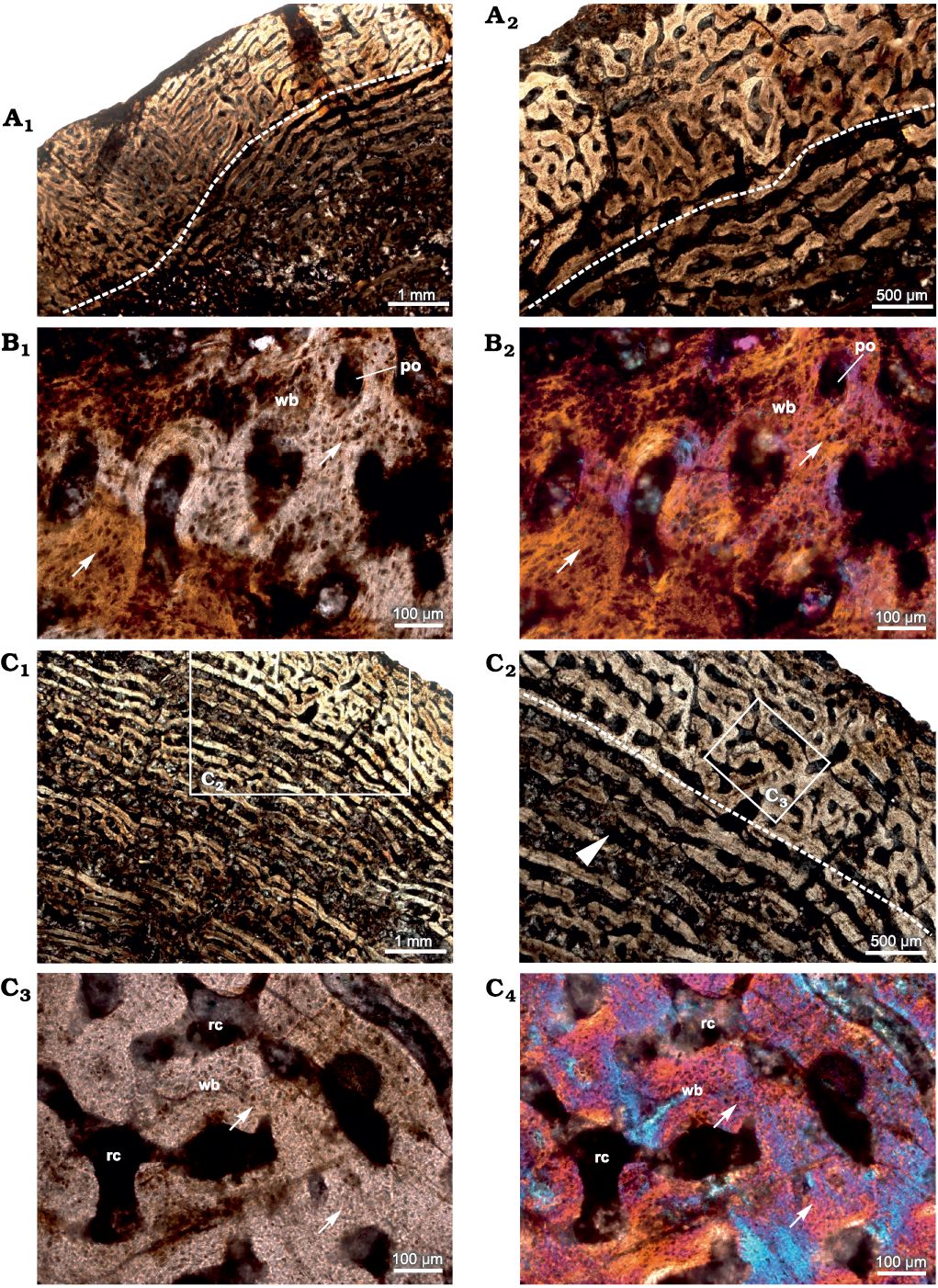
Fig. 1. Transverse sections of the femur (SAM-PK-12233a) of Jonkeria parva (Boonstra, 1955) from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. A. Histological features of diaphyseal cross-section closest to bite marks; note: change in vascular pattern (dotted line) from circumferential to reticular FLB towards the outer cortex. B. Fibrolamellar bone with highly dense osteocyte lacunae (arrows). C. Histological features of diaphyseal cross-section away from the bite marks showing less dense osteocyte lacunae (arrows) in the woven bone matrix; note: change in vascular pattern (dotted line) from circumferential to reticular; canals are erosionally enlarged (arrowhead in C2) and these canals result from extensive resorption and become cancellous towards the inner cortex. Abbreviations: po, primary osteon; rc, resorption cavity; wb, woven bone. Photographs under ordinary light (A1, A2, B1, C1–C3) and cross-polarized light with lambda compensator (B2, C4).
In the cross-section most distal from the bite marks (Fig. 1C), the density of osteocyte lacunae within the primary bone tissue appears lower. Vascular canals are primarily circumferential and laminar in pattern but are widely spaced (Fig. 1C1, C2) compared to the cross-section close to the bite marks (Fig. 1A). However, all the cross-sections have reticular organization of periosteal vascular canals. Radial bony spicules present in the more proximal cross-sections closer to the bite marks (Shelton et al. 2019: 1095, fig. 2a, b) were not observed. The inner cortex is highly porous due to extensive erosional cavities (Fig. 1C1, C2) interconnected by laminar vascular canals which result in the formation of cancellous bone.
Radius.—The diaphyseal section of the radius (SAM-PK-12233b; Fig. 2) reveal that the cortex is entirely composed of fibrolamellar bone tissue with a reticular to plexiform arrangement of primary osteons. Reticular vascular canals are dominant in the inner-most cortex and become plexiform containing both radial and circumferential canals towards the subperiosteal cortex (Fig. 2A1). The vascularization pattern varies within the same diaphyseal section (Fig. 2). The primary matrix consists of woven bone with a random arrangement of collagen fibers (Fig. 2A1, B1). An additional distinct feature of this bone is the narrow band of avascular lamellar tissue identified here as an annulus in the mid-cortical region that indicates a slowing down of bone deposition (Fig. 2A2, A3) despite the fact that the overall tissue is fibrolamellar associated with numerous primary osteons. Near the inner cortex, large resorption cavities are surrounded by lamellar bone tissue (Fig. 2A1). The medullary region is occluded with secondary trabeculae (Fig. 2A1).
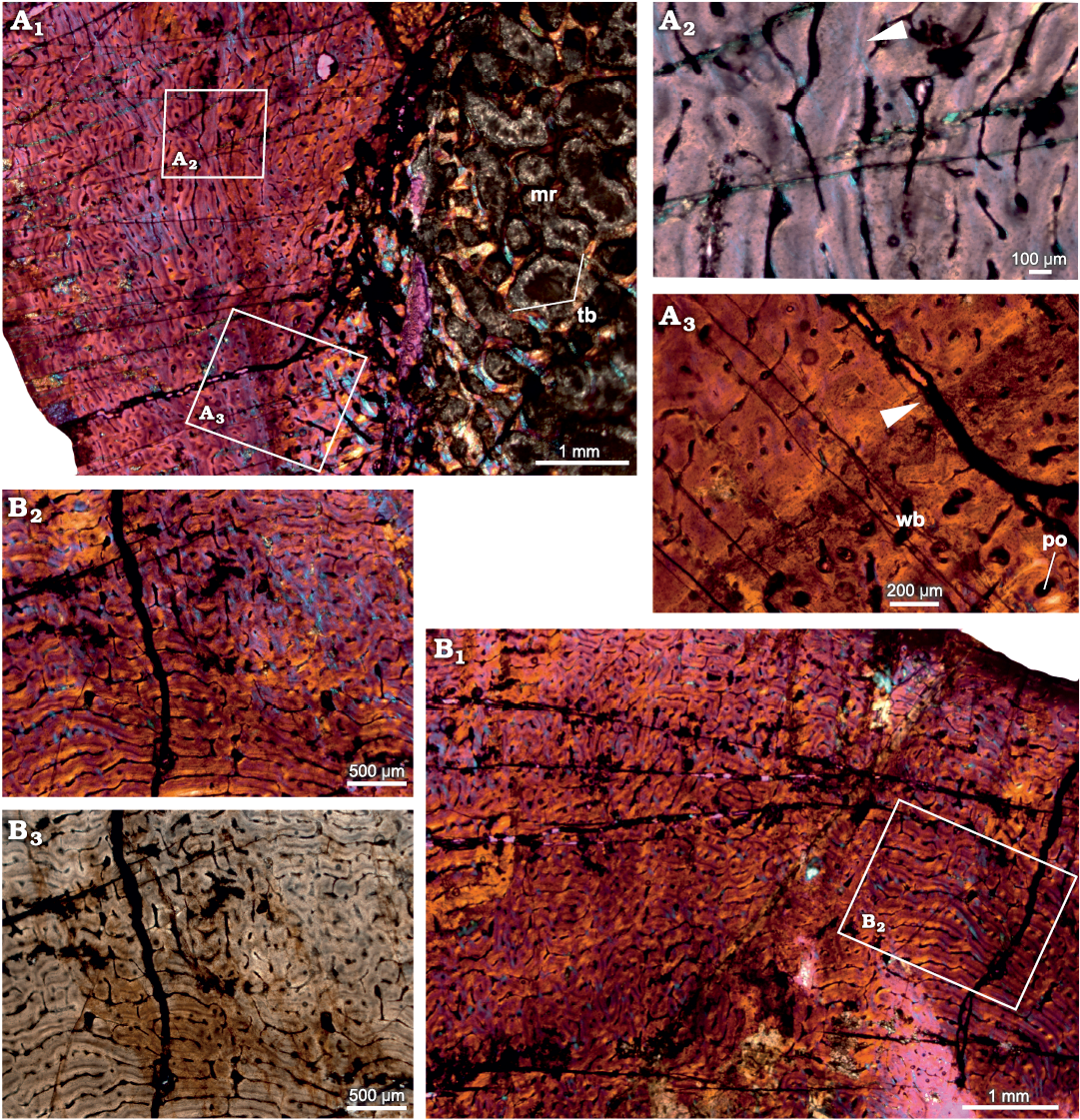
Fig. 2. Transverse sections of the radius (SAM-PK-12233b) of Jonkeria parva (Boonstra, 1955) from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. A1, diaphyseal cross-section showing highly vascularized fibrolamellar bone tissue in the outer cortex and the medullary cavity filled with bony trabeculae; note: the numerous enlarged resorption cavities in the perimedullary region. A2, detail showing an annulus with lamellar bone (arrowhead). A3, detail showing extension of an annulus in the cortex (arrowhead). B1, fibrolamellar bone tissue with woven matrix in the outer cortex showing circumferential and reticular organization of the vascular canals; B2, B3, detail showing the change to a reticular organization of the vascular canals. Abbreviations: mr, medullary region; po, primary osteon; tb, trabeculae; wb, woven bone. Photographs under cross-polarized light with lambda compensator (A1–A3, B1, B2) and ordinary light (B3).
Tarsal.—The cross-section of the tarsal (SAM-PK-12233c; Fig. 3) comprises a thick layer of compacted bone that surrounds a central cancellous region; the latter appears to be the secondarily infilled medullary cavity (Fig. 3A1). The primary bone tissue is highly vascularized, and consists of uninterrupted fibrolamellar bone tissue. Secondary reconstruction occurs in the perimedullary regions of the cross-section, and several large erosion cavities with a dense network of bony trabeculae are visible (Fig. 3A1). Vascularization varies throughout the cross-section and decreases in density from the inner to outer cortex (Fig. 3A2). Simple longitudinal and reticular vascular canals are observed throughout the cortex (Fig. 3A3–A6). Vascular canals are largely reticular laterally (Fig. 3A5, A6) and longitudinal canals occur dorsally (Fig. 3A3, A4).
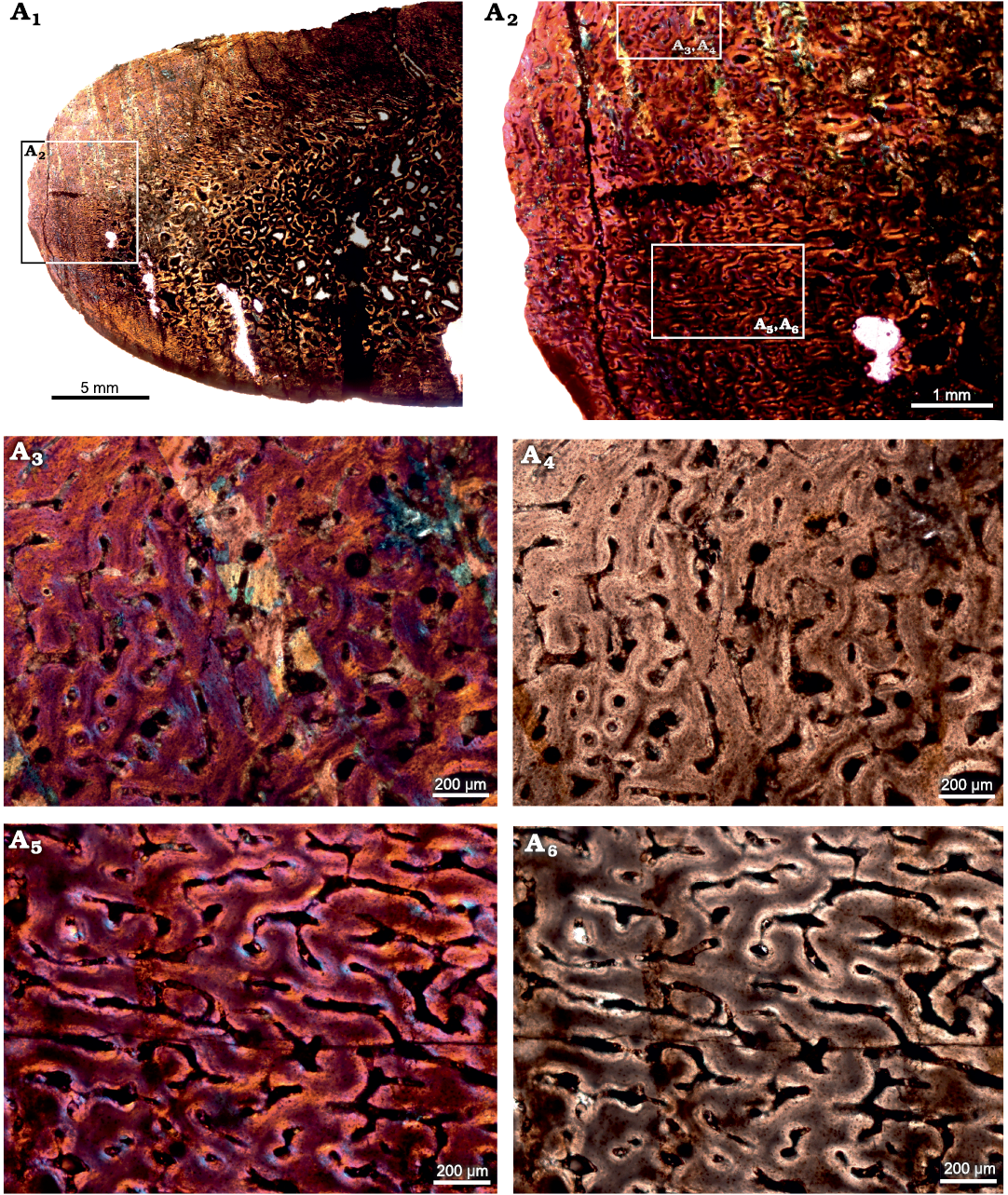
Fig. 3. Transverse sections of the tarsal (SAM-PK-12233c) of Jonkeria parva (Boonstra, 1955) from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. A1, diaphyseal cross-section showing highly vascularized fibrolamellar bone tissue in the outer cortex and cancellous bone tissue with extensively developed bony trabeculae in the medullary cavity; note: numerous enlarged resorption cavities in the perimedullary region. A2, detail showing highly vascularized reticular FLB. A3, A4, detail showing simple longitudinal vascular canals. A5, A6, detail showing reticular vascular canals. Photographs under cross-polarized light with lambda compensator (A1–A3, A5) and ordinary light (A4, A6).
Jonkeria ingens (Broom, 1923)
Humerus.—The core sample from the humerus (SAM-PK- 11994; Fig. 4) shows a thick, compact cortex surrounding a region of cancellous bone which appears to infill the medullary cavity. Due to intense remodeling in the inner cortex, the transition between the medullary region and cortical bone is progressive. The periosteal bone margin is crushed and is encrusted by indurated sediments (Fig. 4A1). The cortical bone is entirely composed of highly vascularized fibrolamellar bone tissues with circumferential vascular canals. These vascular canals form a laminar arrangement with their major axis approximately parallel to the cortical periphery where these canals become closely spaced (Fig. 4A2, A3). Several isolated vascular canals show centripetal deposition of lamellar bone (Fig. 4A4, A5) forming the primary osteons. The medullary region is highly cancellous in nature with large erosional cavities and mostly concentric lamellar deposits along their endosteal surfaces (Fig. 4A6, A7). The compact cortex does not exhibit any growth marks.
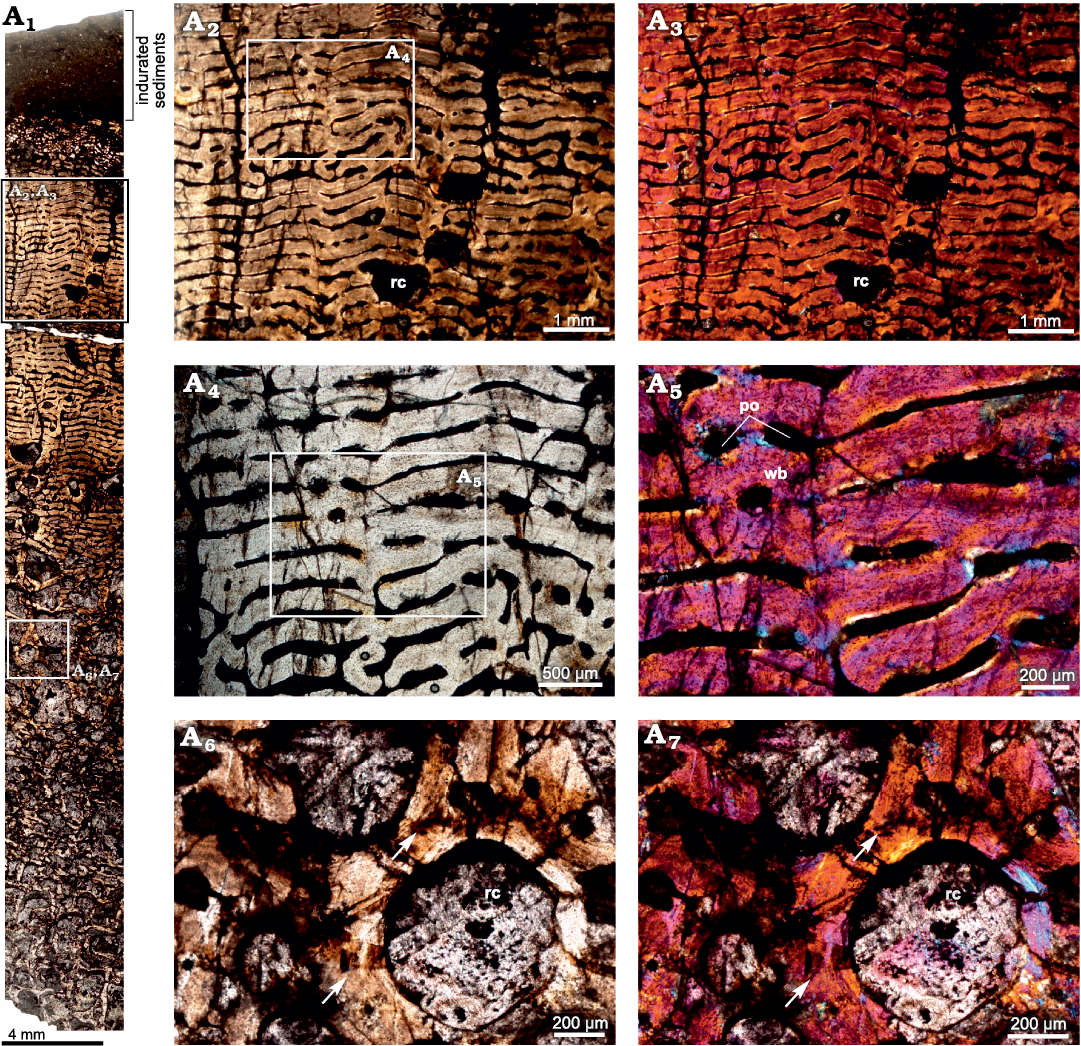
Fig. 4. Thin section of a core of the humerus (SAM-PK-11994) of Jonkeria ingens (Broom, 1923) from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. A1, overall view of humerus showing laminar fibrolamellar bone in the outer cortex and the increasingly cancellous nature of the bone towards the medullary cavity; note: numerous enlarged resorption cavities in the perimedullary cavity, and the layer of indurated sediments at the top of the section. A2, A3, highly vascularized laminar fibrolamellar bone. A4, A5, details showing a few isolated simple longitudinal vascular canals and primary osteons. A6, A7, detail showing large resorption cavities lined by narrow deposit of lamellar bone (arrows). Abbreviations: po, primary osteons; rc, resorption cavity; wb, woven bone. Photographs under ordinary light (A1, A2, A4, A6), and cross-polarized light with lambda compensator (A3, A5, A7).
cf. Jonkeria sp.
Rib.—The cross-section of the rib (BP/1/5409; Fig. 5A1) appears to have a thin outer layer of compacta and a thick inner region consisting of highly remodeled cancellous bone. The amount of cancellous bone varies within a single cross-section and at places, it penetrates the cortex leaving behind only a thin lining of compact bone wall. Overall, the cortical bone is composed of highly vascularized fibrolamellar bone tissue (Fig. 5A2, A3), although the tissue surrounding the growth marks indicates slight slowing down of bone deposition (Fig. 5A5, A6). Longitudinally organized vascular canals are prevalent, but an occasional reticular canal is observed. Secondary reconstruction is extensive throughout the cortex resulting in large resorption cavities (Fig. 5A1, A4). Many of these cavities are infilled by centripetally deposited lamellar bone (Fig. 5A4). The primary cortex exhibits five widely spaced growth marks (Fig. 5A2, A3). It should be noted here that there is a slight change in the tissue type and texture surrounding growth marks, but overall, the matrix is woven. In longitudinal sections the predominant tissue appears to be fibrolamellar with a woven texture (Fig. 6).
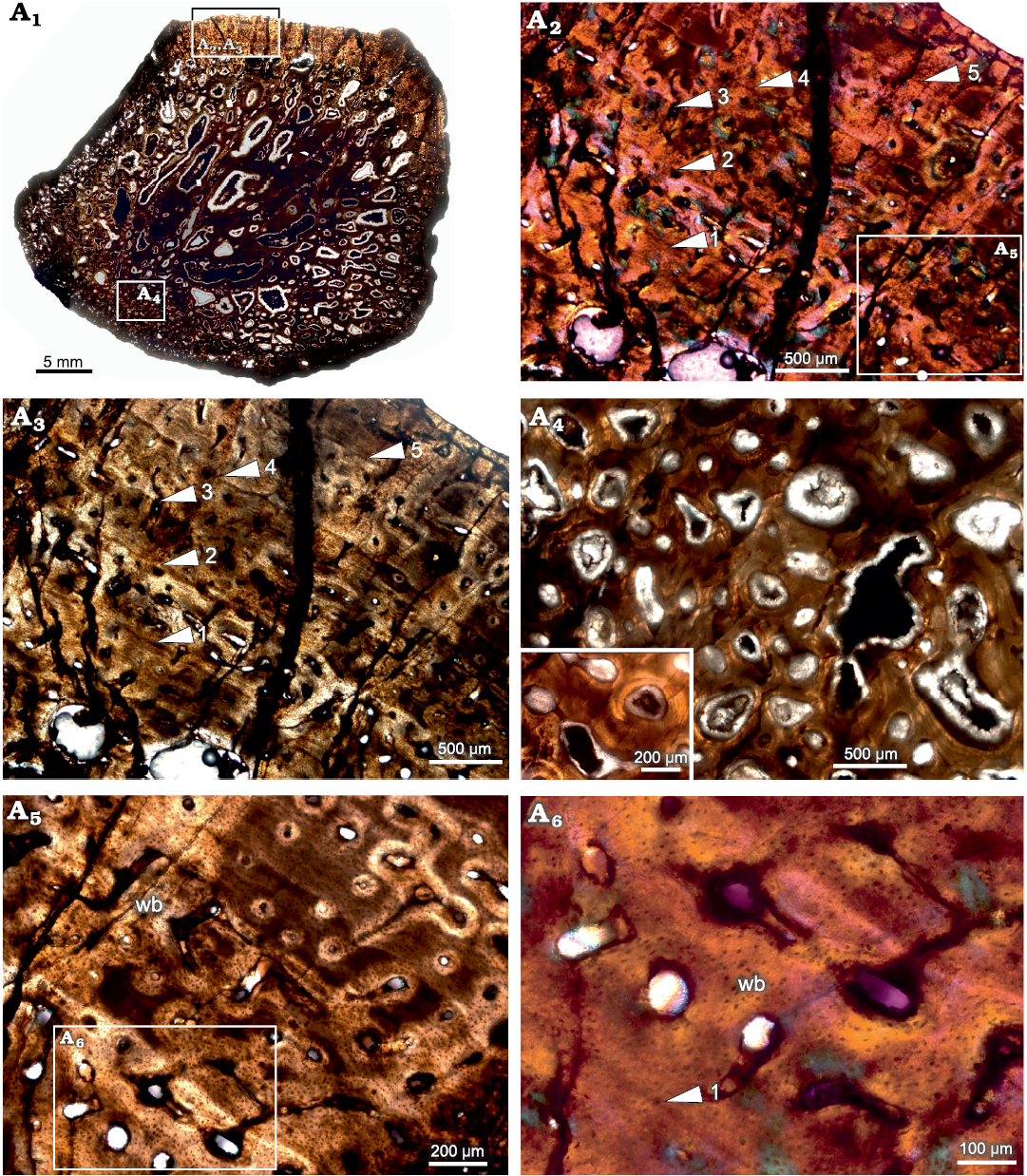
Fig. 5. Transverse section of the rib (BP/1/5409) of cf. Jonkeria sp. from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. A1, overall view of the rib showing the highly cancellous nature of the cortex; note that only the top area of the section preserves the compact cortical bone tissue. A2, A3, detail showing slight change in the tissue type around the lines of arrested growths but overall matrix is woven; note: numbers associated with arrowheads indicate lines of arrested growth (LAGs) in ascending order from the medullary region to periosteal periphery. A4, detail showing enlarged erosional cavities (inset). A5, detail showing fibrolamellar bone between lines of arrested growth. A6, detail showing dense woven matrix; note the change in the density of osteocyte lacunae around the LAG (number associated with arrowhead indicates LAG). Abbreviation: wb, woven bone. Photographs under ordinary light (A1, A3, A4, A5), and cross-polarized light with lambda compensator (A2, A6).

Fig. 6. Longitudinal section of the rib (BP/1/5409) of cf. Jonkeria sp. from the middle Permian Tapinocephalus Assemblage Zone of the Karoo Basin of South Africa. A1, overall view of the rib showing highly secondarily remodeled cortex; note: numerous enlarged resorption cavities. A2, detail showing woven bone and enlarged resorption spaces. Photographs under ordinary light (A1) and cross-polarized light with lambda compensator (A2).
Discussion
Although the early research on dinocephalian bone histology (de Ricqlès 1972) was conducted on mostly fragmented bones at a generic level, it provided an important basis for subsequent study (Chinsamy-Turan 2012b; references therein). Fibrolamellar bone tissue dominates the limb bone histology of various therapsids and implies that this fast-growing tissue appeared early in the evolution of therapsids (de Ricqlès 1972; Chinsamy and Rubidge 1993; Ray et al. 2004, 2009; Chinsamy-Turan 2012a, b). This supposition was supported by the subsequent finding of fibrolamellar tissue in the postcrania of the more basal late Carboniferous and early Permian pelycosaur-grade, non-mammalian synapsids (Shelton and Sander 2017). Classically pelycosaurs were first reported as having slowly formed, lamellar-zonal bone tissue with fibrolamellar bone reported in the extended neural spines of some species (Enlow and Brown 1956, 1957, 1958; Enlow 1969; de Ricqlès 1974; Huttenlocker et al. 2010). However, Huttenlocker et al. (2010) reported extensive parallel-fibered bone in addition to lamellar and fibrolamellar bone in the neural spines of Sphenacodon and Dimetrodon. Even though fibrolamellar bone tissue occur in the herbivorous tapinocephalids, de Ricqlès (1972) reported an overall lamellar bone matrix with a richly vascularized dense Haversian system within Titanosuchus, the sister taxon of Jonkeria. Shelton et al. (2019) first reported the presence of highly vascularized, uninterrupted fibrolamellar tissue in a femur of Jonkeria parva.
In the current study, all the sectioned Jonkeria bones exhibit highly vascularized fibrolamellar bone, which indicates rapid rates of bone deposition (Amprino 1947; Francillon-Vieillot et al. 1990; de Margerie et al. 2002; Starck and Chinsamy 2002; Chinsamy-Turan 2005). In addition, the presence of vascular canals at the peripheral margin of the bone wall indicates that periosteal growth was still ongoing at the time of death (Botha and Chinsamy 2004; de Margerie et al. 2004; Bhat et al. 2019). Except for the rib (BP/1/5409), there is no direct evidence of slowing down or termination of growth in the external cortex of the sectioned bones. This implies that skeletal elements from two individuals are from young animals (e.g., Ray et al. 2005). However, the occurrence of an annulus in the radius (SAM-PK-12233b) suggests a temporary slowing down of growth during adverse environmental conditions (Hutton 1986; Chinsamy et al. 1998, 2020; Botha and Chinsamy 2005; Ray et al. 2005; Chinsamy-Turan 2005; Köhler et al. 2012; Chinsamy and Warburton 2020; Chinsamy and Worthy 2021). A slight change in tissue type was noticed in the rib (BP/1/5409) with five lines of arrested growth (LAGs, sensu Francillon-Vieillot et al. 1990; Castanet et al. 1993; Chinsamy et al. 1995) interrupting woven bone, suggesting that this element is from an ontogenetically older individual that had experienced periodic pauses in bone deposition and hence growth. The presence of multiple growth lines/marks indicates a flexible growth strategy (Starck and Chinsamy 2002) and directly suggests that this individual grew in an interrupted manner (Chinsamy-Turan 2005). This growth strategy is plesiomorphic for extant and extinct mammals (Chinsamy and Rubidge 1993; Botha and Chinsamy 2000; Ray et al. 2004; Chinsamy and Hurum 2006; Ray et al. 2009, 2010; De Buffrénil and Lambert 2011; Hurum and Chinsamy 2012; Montoya-Sanhueza and Chinsamy 2017, 2018; Nacarino-Meneses and Orlandi-Oliveras 2021; Nacarino-Meneses et al. 2021; Chinsamy and Warburton 2021). Such LAGs have also been found in several dinosaur species (e.g., Chinsamy 1990; Starck and Chinsamy 2002; Chinsamy-Turan 2005; Klein and Sander 2008; Chinsamy and Tumarkin-Deratzian 2009; Köhler et al. 2012; Krupandan et al. 2018; Woodward 2019; Cullen et al. 2020), including fossil birds (e.g., Angst et al. 2017; Chinsamy et al. 2020; Chinsamy and Worthy 2021).
The bone histology of multiple skeletal elements of different species of Jonkeria shows a predominance of highly vascularized uninterrupted fibrolamellar bone tissue in the cortices, while the medullary regions were completely infilled by cancellous bone tissue. Based on the bone histology documented in different taxa, we conclude that Jonkeria had an overall fast growth rate. However, it appears that adverse environmental conditions may have interrupted or even ceased growth as shown by the occurrence of an annulus in the mid-cortical region of the radius, as well as multiple growth marks in the periosteal cortex of the sampled rib. It is also possible that the interrupted growth of the rib may reflect a difference in rib growth during late ontogenetic stages.
Comparative bone histology of Jonkeria.—Several taxon-specific histological variations were noticed in the skeletal elements of the studied taxa, which reflects slight differences in growth trajectories. However, it should be noted that the different bones of the different species were recovered from different localities, and individuals from these different species possibly experienced different environmental conditions. Specimens of Jonkeria parva and J. ingens are characterized by having a thick cortex of primary fibrolamellar bone with numerous primary osteons. Both lack any evidence of slowing down of growth. J. ingens has a thick cortex with a predominantly laminar fibrolamellar bone organization, whereas J. parva shows a reticular to plexiform fibrolamellar bone tissue, although the femur (SAM-PK-12233a; Fig. 1) has mostly circumferential vascular canals in the interior cortex and reticular canals in the peripheral cortex. In both species, the medullary regions are highly remodeled and completely infilled with cancellous bone tissue (Figs. 1–4). The latter has large erosional cavities which are lined by lamellar bone tissue (Fig. 4A6, A7). The amount of cancellous bone tissue varies in both species. Another similarity between the two species is the absence of bone growth marks/LAGs (Peabody 1961; Smirina 1974; Castanet 1982; Castanet and Baez 1991; Castanet et al. 1988, 1993; Francillon-Vieillot et al. 1990; Chinsamy et al. 1995). However, a narrow strip of poorly vascularized lamellar bone tissue was noticed in the radius of J. parva. Of all the specimens, the rib of cf. Jonkeria sp. was most different since it showed five LAGs in the outer cortex which represent temporary pauses in growth (Chinsamy-Turan 2005).
Histovariation.—Apart from the presence or absence of annuli and bone growth marks, differences in the degree of vascularization, secondary reconstruction and tissue organisation were observed among the different skeletal elements of a single individual. Variations in the site-specific orientation of the primary vascular canals and tissue organisation are noted within the same diaphyseal section. As bone histology is affected by phylogenetic, ontogenetic, functional and biomechanical constraints, inter-elemental and intraskeletal variations are not surprising within the single skeleton or between individuals (e.g., Starck and Chinsamy 2002; Chinsamy-Turan and Ray 2012; Bhat et al. 2019; Chinsamy and Warburton 2020).
One of the noteworthy features with regards to the pathological femur (SAM-PK-12233a) is that the density of osteocyte lacunae is higher (Fig. 1B) and there is a closer spacing between vascular canals nearer to the site of the bite marks. In addition, further away from the site of infection, such as in the midshaft region there are no radial spicules (see Shelton et al. 2019). Most of the canals within this section are erosionally enlarged; they result from an extensive resorption, become cancellous towards the inner cortex (Fig. 1C1, C2) and when present in large numbers represent haemopoesis (sensu Ray et al. 2009, 2010). Thus, based on the histological features observed in the femur shaft, we believe that the infection was localised in nature.
Although our sample size was small, inter-elemental histological variation within the same taxon were noted. A marked variation between the femur, radius and tarsal of J. parva is the degree of vascularization and orientation of vascular canals. In contrast to the femur which has laminar and reticular fibrolamellar bone in the inner and outer cortex, the radius and tarsal have a large amount of reticular fibrolamellar bone surrounding cancellous medullary regions. Isolated radial and longitudinal canals are scattered throughout the cortex. It may be also noted that the different types of organization of the vascular canals suggest slight differences in the rate at which fibrolamellar bone was deposited in the different skeletal elements. A single annulus is present in radius SAM-PK-12233b (Fig. 2A2, A3). Bone growth marks are absent in both J. parva and J. ingens. The humerus of J. ingens has circumferential vascular canals in the mid- and outer-cortex. The amount of cancellous bone tissue varies across the cross-section and it appears that the primary compact bone was resorbed to reach such cancellous proportions. The absence of lines of arrested growth and the presence of highly vascularized woven bone in the limb bones of both Jonkeria species suggests uninterrupted, rapid growth (Peabody 1961; Smirina 1974; Castanet 1982; Castanet and Baez 1991; Castanet et al. 1988, 1993; Francillon-Vieillot et al. 1990; Chinsamy et al. 1995) at least during early ontogeny. On the other hand, the rib fragment of cf. Jonkeria sp. has five equally spaced growth marks which indicate periodic cessation of growth during some adverse environmental conditions (Chinsamy-Turan 2005). This finding further suggests that the rib may have come from an older individual as compared to the other skeletal elements studied.
Lifestyle adaptations.—It is well known that bone density and cortical thickness are correlated with mode of life (Wall 1983; Chinsamy 1997; Germain and Laurin 2005; Gray et al. 2007; Laurin et al. 2011; Houssaye et al. 2016; Canoville and Chinsamy 2017). A semi-aquatic lifestyle requires an increase in bone density to counterbalance hydrological buoyancy (Chinsamy 1991; Gray et al. 2007; Hayashi et al. 2013) and often leads to a greater bone wall thickness (Wall 1983). However, burrowing/fossorial animals also have thick bone walls (Bramble 1982; Ultsch and Anderson 1988; Lips 1991; Magwene 1993; Ray and Chinsamy 2004; Botha and Chinsamy 2004, 2005; Chinsamy and Abdala 2008; Lyson et al. 2016; Montoya-Sanhueza and Chinsamy 2017; Legendre and Botha-Brink 2018; Bhat et al. 2019) as compared to terrestrial animals which have lower relative bone thickness (RBT) values (<30%; sensu Wall 1983). In this situation it is important to consider other morphological adaptations for burrowing (e.g. Cistephalus, Nasterlack et al. 2012; Bathyergus, Montoya-Sanhueza and Chinsamy 2017), as well as other morphological features that might aid in deducing their lifestyle habits (Houssaye et al. 2016). In addition to peculiar cranial and postcranial characteristics of Jonkeria (e.g., a long snout, a heavily built skull and shorter limbs), there is no record of their association with burrows; therefore, it is unlikely that Jonkeria were fossorial.
In the current study, all the bones are characterized by thick bone walls and an extensive development of cancellous bone in the medullary regions. Similar bone tissues have been reported in Lystrosaurus murrayi (Ray et al. 2005; Botha 2020). Based on this type of bone histology, Ray and Chinsamy (2005) proposed a semi-aquatic/aquatic lifestyle for Lystrosaurus. However, more recently using the same histological features, Botha (2020) proposed a fully terrestrial mode of life for Lystrosaurus. Botha (2020) further suggested biomechanical constraints to support the large body weight as the cause of the extensive development of medullary spongiosa. However, in a study of graviportal and aquatic tetrapods Houssaye et al. (2016) reported that the humeri, femora, and ribs change their shape and internal bone microanatomy (i.e., increasing bone compactness and reducing medullary cavities). In particular, ribs show stronger changes in compactness in aquatic taxa as compared to graviportal taxa, which is in accordance with a role in buoyancy and body trim control in shallow waters (Chinsamy 1991; Gray et al. 2007; Hayashi et al. 2013; Houssaye et al. 2016) rather than a role in weight-bearing on land (Ray et al. 2010; Houssaye et al. 2016), contra Botha (2020).
It is also worth noting that middle Permian pareiasaurs, which were similarly large extinct tetrapods contemporaneous with dinocephalians, exhibited spongious stylopod diaphyses and thin compact cortices, though not to the same extent as dinocephalians (Canoville and Chinsamy 2017). Isotopic analysis of the teeth of dinocephalians, pareiasaurs and therocephalians has shown that these large graviportal animals inhabited different ecological niches during middle and late Permian times, with pareiasaurs and therocephalians sharing a terrestrial habitat (Canoville et al. 2014). Furthermore, contra Botha (2020), elephants (Houssaye et al. 2016), giraffes (Smith 2020), and bison (Sander and Andrassy 2006; Houssaye et al. 2016; Canoville et al. 2016), which are large, graviportal terrestrial animals do not have medullary cavities infilled with spongious bone tissue as a biomechanical adaption for their bulk. In addition, as in Jonkeria, the ribs of the known aquatic reptile, Claudiosaurus (De Buffrénil and Mazin 1989) show a complete infilling of the medullary region by cancellous bone and, are considered pachyostotic as an adaptation for a semi-aquatic lifestyle (de Ricqlès and De Buffrénil 2001). Given these similarities, we cautiously propose that the bone histology of Jonkeria is indicative of a semi-aquatic habitat, though we note that further sampling and more data would be needed to further test this hypothesis.
Other physiological inferences.—Following earlier work (e.g., Reid 1987; Chinsamy 1990; Chinsamy-Turan 2005; Chinsamy and Hillenius 2004), we believe that bone tissue types cannot be used to differentiate between endothermic and ectothermic tetrapods. However, it is evident that the different types of tissues provide valuable information about the rate at which the bone was deposited (Amprino 1947; Reid 1987; Chinsamy 1990; Starck and Chinsamy 2002; Chinsamy-Turan 2005; Chinsamy and Hillenius 2004). Since the bone histology of Jonkeria is dominated by fibrolamellar bone with abundant primary osteons it directly suggests that this bone was laid down rapidly (Amprino 1947; Francillon-Vieillot et al. 1990; de Margerie et al. 2002; Starck and Chinsamy 2002; Chinsamy-Turan 2005). In addition, the extensive development of vascular canals is commensurate with rapid rates of bone formation irrespective of whether the animal is ectothermic or endothermic (e.g., Reid 1987, Chinsamy and Hillenius 2004). More recently, using phylogenetic eigenvector maps Faure-Brac and Cubo (2020) proposed an ectothermic metabolism for the synapsid Ophiacodon uniformis and an endothermic metabolism for the anomodonts (e.g., Lystrosaurus sp.) although both have fibrolamellar bone tissue (Shelton and Sander 2017; Botha 2020). Indeed, if Jonkeria were included in the Faure-Brac and Cubo (2020) study, it would likely also be considered endothermic. Thus, it is apparent that deciphering the thermal physiology of an extinct animal on the basis of its histology is highly contested (see for example, Chinsamy and Hillenius 2004). Furthermore, despite some suggestions of a correlation between osteocyte lacunae size and thermophysiology (Huttenlocker and Farmer 2017), more evidence is needed. On the basis of our histological findings, we can assert that during early ontogeny Jonkeria experienced rapid sustained rates of growth, whereas later in ontogeny (as seen in the rib) they experienced cyclical rates of growth. Like large sauropodomorph dinosaurs (e.g., Cerda et al. 2017), the varying rates of growth during ontogeny are probably related to the attainment of large body size, i.e., during early ontogeny they experienced sustained uninterrupted growth to reach a large body size, whereas in later stages of ontogeny (perhaps once sexual maturity was attained), they experienced slower cyclical growth patterns.
Conclusions
Authors’ contributions
AC developed the initial concept for the research. AC, CDS, and MSB devised and planned the research strategy. CDS performed the original histological sectioning. MSB polished the histological sections, performed data collection and analysis, and wrote the first draft of the manuscript. AC assisted with the sample selection and supervised the histological work and reviewed the drafts of the manuscript. CDS read and contributed to the final draft.
Acknowledgements
We are grateful to Iziko South African Museums, Cape Town and Evolutionary Studies Institute (formerly the Bernard Price Institute) at the University of the Witwatersrand, Johannesburg, South Africa, for providing access to the specimens used in the current study. Permits 2076 and 3752-4658 (for histological analyses) were provided by the South African Heritage Resources Agency (SAHRA). The constructive comments by editor Olivier Lambert (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) and two anonymous reviewers are gratefully acknowledged. The DST-NRF Centre of Excellence (CoE) in Palaeosciences is acknowledged for postdoctoral support to MSB [grants numbers COE2018-12POST, COE2019-PD09 and COE2020-PD09]. The Claude Leon Foundation is acknowledged for financial support to CDS [grant number 98813]. AC is supported by the NRF African Origins Platform [grant number 117716]. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the CoE.
References
Amprino, R. 1947. La structure du tissu osseux envisagée comme expression de différences dans la vitesse de l’accroissement. Archives de Biologie 58: 315–330.
Angst, D., Chinsamy, A., Steel, L., and Hume, J.P. 2017. Bone histology sheds new light on the ecology of the dodo (Raphus cucullatus, Aves, Columbiformes). Scientific Reports 7 (1): 7993. Crossref
Barghusen, H.R. 1975. A review of fighting adaptations in dinocephalians (Reptilia, Therapsida). Paleobiology 1: 295–311. Crossref
Bastille-Rousseau, G., Fortin, D., Dussault, C., Courtois, R., and Ouellet, J.-P. 2011. Foraging strategies by omnivores: are black bears actively searching for ungulate neonates or are they simply opportunistic predators? Ecography 34: 588–596. Crossref
Bhat, M.S., Chinsamy, A., and Parkington, J. 2019. Long bone histology of Chersina angulata: interelement variation and life history data. Journal of Morphology 280: 1881–1899. Crossref
Boontra, L.D. 1936. The cranial morphology of some titanosuchid dinocephalians. Bulletin of the American Museum of Natural History 72: 99–116.
Boonstra, L.D. 1955. The girdles and limb bones of the South African Deinocephalia. South African Journal of Science 42: 185–326.
Boonstra, L.D. 1963a. Diversity within the South African Dinocephalia. South African Journal of Science 59: 196–206.
Boonstra, L.D. 1963b. Early dichotomies in the therapsids. South African Journal of Science 59: 176–195.
Boonstra, L.D. 1968. The terrestrial reptile fauna of Tapinocephalus-Zone-age and Gondwanaland. South African Journal of Science 46: 199–204.
Boonstra, L.D. 1969. The fauna of the Tapinocephalus-Zone. Annals of the South African Museum 56: 1–73.
Boonstra, L.D. 1971. The early therapsids. Annals of the South African Museum 59: 17–46.
Botha, J. 2020. The paleobiology and paleoecology of South African Lystrosaurus. PeerJ 8: e10408. Crossref
Botha, J. and Chinsamy, A. 2000. Growth patterns from the bone histology of the cynodonts Diademodon and Cynognathus. Journal of Vertebrate Paleontology 20: 705–711. Crossref
Botha, J. and Chinsamy, A. 2004. Growth and life habits of the Triassic cynodont Trirachodon, inferred from bone histology. Acta Palaeontologica Polonica 49: 619–627.
Botha, J. and Chinsamy, A. 2005. Growth patterns of Thrinaxodon liorhinus, a non-mammalian cynodont from the Lower Triassic of South Africa. Palaeontology 48: 385–394. Crossref
Botha-Brink, J. and Angielczyk, K.D. 2010. Do extraordinarily high growth rates in Permo-Triassic dicynodonts (Therapsida, Anomodontia) explain their success before and after the end-Permian extinction? Zoological Journal of the Linnean Society 160: 341–365. Crossref
Botha-Brink, J., Soares, M.B., and Martinelli, A.G. 2018. Osteohistology of Late Triassic prozostrodontian cynodonts from Brazil. PeerJ 6: e5029. Crossref
Bramble, D.M. 1982. Scaptochelys: generic revision and evolution of gopher tortoises. Copeia 4: 852–867. Crossref
Broom, R. 1923. On the structure of the skull in the carnivorous dinocephalian reptiles. Proceedings of the Zoological Society of London 93: 661–684. Crossref
Canoville, A. and Chinsamy, A. 2017. Bone microstructure of pareiasaurs (Parareptilia) from the Karoo Basin, South Africa: implications for growth strategies and lifestyle habits. The Anatomical Record 300: 1039–1066. Crossref
Canoville, A., de Buffrénil, V., and Laurin, M. 2016. Microanatomical diversity of amniote ribs: an exploratory quantitative study. Biological Journal of the Linnean Society 118: 706–733. Crossref
Canoville, A., Thomas, D.B., and Chinsamy, A. 2014. Insights into the habitat of middle Permian pareiasaurs (Parareptilia) from preliminary isotopic analyses. Lethaia 47: 266–274. Crossref
Castanet, J. 1982. New data on cement lines in bones. Archives of Biology 92: 1–24.
Castanet, J. and Baez, M. 1991. Adaptation and evolution in Gallotia lizards from the Canary Islands: age, growth, maturity and longevity. Amphibia-Reptilia 12: 81–102. Crossref
Castanet, J., Francillon-Vieillot, H., Meunier, F.J., and de Ricqlès, A. 1993. Bone and individual aging. In: B.K. Hall (ed.), Bone. Vol. 7: Bone Growth-B, 245–283. CRC Press, Boca Raton.
Castanet, J., Newman, D.G., and Saint Girons, H. 1988. Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica 44: 25–37.
Cerda, I., Chinsamy, A., Pol, D., Apaldetti, C., Otero, A., Powell, J., and Martinez, R. 2017. Novel insight into the origin of the growth dynamics of sauropod dinosaurs, and the attainment of gigantism. PLoS One 12: e0179707. Crossref
Chinsamy, A. 1990. Physiological implications of the bone histology of Syntarsus rhodesiensis (Saurischia: Theropoda). Palaeontologia Africana 27: 77–82.
Chinsamy, A. 1991. The Osteohistology of Femoral Growth Within a Clade: a Comparison Of the Crocodile Crocodylus niloticus, the Dinosaurs Massospondylus and Syntarsus, and the Birds, Struthio and Sagittarius. 200 pp. Unpublished Ph.D. Dissertation, University of Witwatersrand, Johannesburg.
Chinsamy, A. 1993. Bone histology and growth trajectory of the prosauropod dinosaur Massospondylus carinatus Owen. Modern Geology 18: 319–329.
Chinsamy, A. 1995. Ontogenetic changes in the bone histology of the Late Jurassic ornithopod Dryosaurus lettowvorbecki. Journal of Vertebrate Paleontology 15: 96–104. Crossref
Chinsamy, A. 1997. Assessing the biology of fossil vertebrates through bone histology. Palaeontologia Africana 33: 29–35.
Chinsamy, A. and Abdala, F. 2008. Palaeobiological implications of the bone microstructure of South American traversodontids (Therapsida: Cynodontia). South African Journal of Science 104: 225–230.
Chinsamy, A. and Dodson, P. 1995. Inside a dinosaur bone. American Scientist 83: 174–180.
Chinsamy, A. and Hillenius, W.J. 2004. Physiology of nonavian dinosaurs. In: D.B. Weishampel, P. Dodson, and H. Osmólska (eds.), The Dinosauria, 2nd Edition, 643–659. University of California Press, Berkeley. Crossref
Chinsamy, A. and Hurum, J.H. 2006. Bone microstructure and growth patterns of early mammals. Acta Palaeontologica Polonica 51: 325–338.
Chinsamy, A. and Raath, M. 1992. Preparation of fossil bone for histological examination. Palaeontologia Africana 29: 39–44.
Chinsamy, A. and Rubidge, B.S. 1993. Dicynodont (Therapsida) bone histology: phylogenetic and physiological implications. Palaeontologia Africana 30: 97–102.
Chinsamy, A. and Tumarkin-Deratzian, A. 2009. Pathologic bone tissues in a turkey vulture and a nonavian dinosaur: implications for interpreting endosteal bone and radial fibrolamellar bone in fossil dinosaurs. The Anatomical Record 292: 1478–1484. Crossref
Chinsamy, A. and Warburton, N. 2020. Ontogenetic growth and the development of a unique fibrocartilage entheses in Macropus fuliginosus. Zoology [published online, https://doi.org/10.1016/j.zool.2020.125860].
Chinsamy, A. and Worthy, T.H. 2021. Histovariability and palaeobiological implications of the bone histology of the dromornithid, Genyornis newtoni. Diversity 13 (5): 219. [published online, https://doi.org/ 10.3390/d13050219]. Crossref
Chinsamy, A., Angst, D., Canoville, A., and Göhlich, U.B. 2020. Bone histology yields insights into the biology of the extinct elephant birds (Aepyornithidae) from Madagascar. Biological Journal of the Linnean Society 130: 268–295. Crossref
Chinsamy, A., Cerda, I., and Powell, J. 2016. Vascularised endosteal bone tissue in armoured sauropod dinosaurs. Scientific Reports 6: 24858. Crossref
Chinsamy, A., Chiappe, L.M., and Dodson, P. 1994. Growth rings in Mesozoic avian bones. Physiological implications for basal birds. Nature 368: 196–197. Crossref
Chinsamy, A., Chiappe, L.M., Marugán-Lobón, J., Chunling, G., and Fengjiao, Z. 2013. Gender identification of the Mesozoic bird Confuciusornis sanctus. Nature Communications 4 (1381): 1–5. Crossref
Chinsamy, A., Hanrahan, S.A., Neto, R.M., and Seely, M. 1995. Skeletochronological assessment of age in Angolosaurus skoogi, a cordylid lizard living in an aseasonal environment. Journal of Herpetology 29: 457–460. Crossref
Chinsamy, A., Marugán-Lobón, J., Serrano, F., and Chiappe, L. 2019. Osteohistology and life history of the basal pygostylian, Confuciusornis sanctus. The Anatomical Record 303: 949–962. Crossref
Chinsamy, A., Rich, T., and Vickers-Rich, P. 1998. Polar dinosaur bone histology. Journal of Vertebrate Paleontology 18: 385–390. Crossref
Chinsamy-Turan, A. 2005. The Microstructure of Dinosaur Bone: Deciphering Biology with Fine-Scale Techniques. 216 pp. The John Hopkins University Press, Baltimore.
Chinsamy-Turan, A. 2012a. Forerunners of Mammals. Radiation, Histology, Biology. 372 pp. Indiana University Press, Bloomington.
Chinsamy-Turan, A. 2012b. The microstructure of bones and teeth of nonmammalian therapsids. In: A. Chinsamy-Turan (ed.), Forerunners of Mammals: Radiation, Histology, Biology, 65–88. Indiana University Press, Bloomington.
Chinsamy-Turan, A. and Ray, S. 2012. Bone histology of some therocephalians and gorgonopsians, and evidence of bone degradation by fungi. In: A. Chinsamy-Turan (ed.), Forerunners of Mammals: Radiation, Histology, Biology, 3–28. Indiana University Press, Bloomington.
Colbert, E.H. 1969. Evolution of the Vertebrates. 2nd Edition. 535 pp. John Wiley and Sons Inc., New York.
Cullen, T.M., Canale, J.I., Apesteguía, S., Smith, N.D., Hu, D., and Makovicky, P.J. 2020 Osteohistological analyses reveal diverse strategies of theropod dinosaur body-size evolution. Proceedings of the Royal Society B 287: 20202258. Crossref
Day, M.O. and Rubidge, B.S. 2020. Biostratigraphy of the Tapinocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology 123: 149–164. Crossref
Day, M.O. and Smith, R.M.H. 2020. Biostratigraphy of the Endothiodon Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology 123: 165–180. Crossref
De Buffrénil, V. and Lambert, O. 2011. Histology and growth pattern of the pachy-osteosclerotic premaxillae of the fossil beaked whale Aporotus recurvirostris (Mammalia, Cetacea, Odontoceti). Geobios 44: 45–56. Crossref
De Buffrénil, V. and Mazin, J-M. 1989. Bone histology of Claudiosaurus germaini (Reptilia, Claudiosauridae) and the problem of pachyostosis in aquatic tetrapods. Historical Biology 2: 311–322. Crossref
De Margerie, E., Cubo, J., and Castanet, J. 2002. Bone typology and growth rate: testing and quantifying “Amprino’s rule” in the mallard (Anas platyrhynchos). Comptes Rendus Biologies 325: 221–230. Crossref
De Margerie, E., Robin, J.-P., Verrier, D., Cubo, J., Groscolas, R., and Castanet, J. 2004. Assessing a relationship between bone microstructure and growth rate: a fluorescent labelling study in the king penguin chick (Aptenodytes patagonicus). The Journal of Experimental Biology 207: 869–879. Crossref
De Ricqlès, A. 1969. Recherches paléohistologiques sur les os longs des tétrapodes. II. Quelques observations sur la structure des os longs des Thériodontes. Annales de Paléontologie (Vertébrés) 55: 1–52.
De Ricqlès, A. 1972. Recherches paléohistologiques sur les os longs des tétrapodes. III. Titanosuchiens, Dinocéphales et Dicynodontes. Annales de Paléontologie (Vertébrés) 58: 17–60.
De Ricqlès, A.J. 1974. Evolution of endothermy: histological evidence. Evolutionary Theory 1: 51–80.
De Ricqlès, A. and de Buffrénil, V. 2001. Bone histology, heterochronies and the return of tetrapods to life in water: w[h]ere are we? In: J.-M. Mazin and V. de Buffrénil (eds.), Secondary Adaptation of Tetrapods to Life in Water, 289–306. Verlag Dr F Pfeil, München.
Enlow, D.H. 1963. Principles of bone remodeling. An account of post-natal growth and remodeling processes in long bones and the mandible. American Lecture Series in Anatomy, Monograph 531: 1–131.
Enlow, D.H. 1969. The bones of reptiles. In: C. Gans (ed.), Biology of the Reptilia, 45–80. Academic Press, New York.
Enlow, D.H. and Brown, S.O. 1956. A comparative histological study of fossil and Recent bone tissues. Part I. The Texas Journal of Science 8: 405–443.
Enlow, D.H. and Brown, S.O. 1957. A comparative histological study of fossil and Recent bone tissues. Part II. The Texas Journal of Science 9: 136–214.
Enlow, D.H. and Brown, S.O. 1958. A comparative histological study of fossil and Recent bone tissues. Part 3. The Texas Journal of Science 10: 18–230.
Faure-Brac, M.G. and Cubo, J. 2020. Were the synapsids primitively endotherms? A palaeohistological approach using phylogenetic eigenvector maps. Philosophical Transactions of Royal Society B 375: 20190138. Crossref
Fordyce, N., Smith, R., and Chinsamy, A. 2012. Evidence of a therapsid scavenger in the late Permian Karoo Basin, South Africa. South African Journal of Science 108 (11–12): 114–118. Crossref
Francillon-Vieillot, H., de Buffrénil, V., Castanet, J., Géraudie, J., Meunier, F.J., Sire, J.Y., Zylberberg, L., and de Ricqlès, A. 1990. Microstructure and mineralization of vertebrate skeletal tissues. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, 471–548. Van Nostrand Reinhold, New York. Crossref
Garland, A.N. 1987. A histological study of archaeological bone decomposition. In: A. Boddington, A.N. Garland, and R.C. Janaway (eds.), Death, Decay, and Reconstruction: Approaches to Archaeology and Forensic Science, 109–126. Manchester University Press, Manchester.
Germain, D. and Laurin, M. 2005. Microanatomy of the radius and lifestyle in amniotes (Vertebrata Tetrapoda). Zoologica Scripta 34: 335–350. Crossref
Gray, N.M. Kainec, K. Madar, S. Tomko, L., and Wolfe, S. 2007. Sink or swim? Bone density as a mechanism for buoyancy control in early cetaceans. The Anatomical Record 290: 638–653. Crossref
Gunther, K.A., Shoemaker, R.R., Frey, K.L., Haroldson, M.A., Cain, S.L., van Manen, F.T., and Fortin, J.K. 2014. Dietary breadth of grizzly bears in the Greater Yellowstone Ecosystem. Ursus 25: 60–72. Crossref
Handley, W.D., Chinsamy, A., Yates, A.D., and Worthy, T.H. 2016. Sexual dimorphism in the late Miocene mihirung Dromornis stirtoni (Aves: Dromornithidae) from the Alcoota Local Fauna of central Australia. Journal of Vertebrate Paleontology 36: 5. Crossref
Hayashi, S., Houssaye, A., Nakajima, Y., Chiba, K., Ando, T., Sawamura, H., Inuzuka, N., Kaneko, N., and Osaki, T. 2013. Bone inner structure suggests increasing aquatic adaptations in Desmostylia (Mammalia, Afrotheria). PLoS One 8 (4): e59146. Crossref
Horner, J.R., de Ricqlès, A., and Padian, K. 1999. Variation in dinosaur skeletochronology indicators: Implications for age assessment and physiology. Paleobiology 25: 295–304. Crossref
Horner, J.R, de Ricqlès, A., and Padian, K. 2000. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: Growth dynamics and physiology of an ontogenetic series of skeletal elements. Journal of Vertebrate Paleontology 20: 115–129. Crossref
Houssaye, A., Waskow, K., Hayashi, S., Cornette, R., Lee, A.H., and Hutchinson, J.R. 2016. Biomechanical evolution of solid bones in large animals: a microanatomical investigation. Biological Journal of the Linnean Society 117: 350–371. Crossref
Huculak, M.A. and Rogers, T.L. 2009. Reconstructing the sequence of events surrounding body disposition based on color staining of bone. Journal of Forensic Sciences 54: 979–84. Crossref
Hurum, J. and Chinsamy-Turan, A. 2012. The Radiation, Bone Histology and Biology of Early Mammals. In: A. Chinsamy-Turan, (ed.), Forerunners of Mammals: Radiation, Histology, Biology, 249–270. Indiana University Press, Bloomington.
Huttenlocker, A.K. and Botha-Brink, J. 2014. Bone microstructure and the evolution of growth patterns in Permo-Triassic therocephalians (Amniota, Therapsida) of South Africa. PeerJ 2:e325. Crossref
Huttenlocker, A.K. and Farmer, C.G. 2017. Bone microvasculature tracks red blood cell size diminution in Triassic mammal and dinosaur forerunners. Current Biology 27: 48–54. Crossref
Huttenlocker, A.K. and Shelton, C.D. 2020. Bone histology of varanopids (Synapsida) from Richards Spur, Oklahoma, sheds light on growth patterns and lifestyle in early terrestrial colonizers. Philosophical Transactions of the Royal Society B 375: 20190142. Crossref
Huttenlocker, A.K., Rega, E., and Sumida, S.S. 2010. Comparative anatomy and osteohistology of hyperelongate neural spines in the sphenacodontids Sphenacodon and Dimetrodon (Amniota: Synapsida). Journal of Morphology 271: 1407–1421. Crossref
Hutton, J.M. 1986. Age determination of living Nile crocodiles from the cortical stratification of bone. Copeia 2: 332–341. Crossref
Jans, M.M.E., Kars, H., Nielsen-Marsh, C.M., Smith, C.I., Nord, A.G., Arthur, P., and Earl, N. 2002. In situ preservation of archaeological bone: a histological study within a multidisciplinary approach. Archaeometry 44: 343–352. Crossref
Jasinoski, S.C. and Chinsamy, A. 2012. Mandibular histology and growth of the nonmammaliaform cynodont Tritylodon. Journal of Anatomy 220: 564–579. Crossref
Kammerer, C.F. 2011. Systematics of the Anteosauria (Therapsida: Dinocephalia). Journal of Systematic Palaeontology 9: 261–304. Crossref
Kemp, T.S. 1982. Mammal-like Reptiles and the Origin of Mammals. 363 pp. Academic Press, London.
Kemp, T.S. 2005. The Origin and Evolution of Mammals. 331 pp. Oxford University Press, Oxford. Crossref
Kemp, T.S. 2012. The origin and radiation of therapsids. In: A. Chinsamy-Turan (ed.), Forerunners of Mammals: Radiation, Histology, Biology, 3–28. Indiana University Press, Bloomington.
King, G.M. 1988. Anomodontia. In: P. Wellnhofer (ed.), Encyclopaedia of Paleoherpetology Volume 17C, 1–174. Gustav Fischer, Stuttgart.
Klein, N. and Sander, M. 2008. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology 34: 247–263. Crossref
Köhler, M., Marín-Moratalla, N., Jordana, X., and Aanes, R. 2012. Seasonal bone growth and physiology in endotherms shed light on dinosaur physiology. Nature 487: 358–361. Crossref
Krupandan, E., Chinsamy-Turan, A., and Pol, D. 2018. The long bone histology of the sauropodomorph, Antetonitrus ingenipes. The Anatomical Record 301: 1506–1518. Crossref
Lambertz, M., Shelton, C.D., Spindler, F., and Perry, S.F. 2016. A caseian point for the evolution of a diaphragm homologue among the earliest synapsids. Annals of the New York Academy of Science 1385: 3–20. Crossref
Laurin, M., Canoville, A., and Germain, D. 2011. Bone microanatomy and lifestyle: a descriptive approach. Comptes rendus Palevol 10: 381–402. Crossref
Lips, K.R. 1991. Vertebrates associated with tortoise (Gopherus polyphemus) burrows in four habitats in south-central Florida. Journal of Herpetology 25: 477–481. Crossref
Legendre, L.J. and Botha-Brink, J. 2018. Digging the compromise: investigating the link between limb bone histology and fossoriality in the aardvark (Orycteropus afer). PeerJ 6:e5216. Crossref
Lyman, R. 1994. Vertebrate Taphonomy (Cambridge Manuals in Archaeology). 549 pp. Cambridge University Press, Cambridge.
Lyson, T.R., Rubidge, B.S., Scheyer, T.M., de Queiroz, K., Schachner, E.R., Smith, R.M.H., Botha-Brink, J., and Bever, G.S. 2016. Fossorial origin of the turtle shell. Current Biology 26: 1887–1894. Crossref
Magwene, P.M. 1993. What’s Bred in the Bone: Histology and Cross-sectional Geometry of Mammal-like Reptile Long Bones-evidence of Changing Physiological and Biomechanical Demands. 54 pp. M.Sc. Dissertation, Harvard University, Cambridge.
Montoya-Sanhueza, G. and Chinsamy, A. 2017. Long bone histology of the subterranean rodent Bathyergus suillus (Bathyergidae): ontogenetic pattern of cortical bone thickening. Journal of Anatomy 230: 203–233. Crossref
Montoya-Sanhueza, G. and Chinsamy, A. 2018. Cortical bone adaptation and mineral mobilization in the subterranean mammal Bathyergus suillus (Rodentia: Bathyergidae): effects of age and sex. PeerJ 6: e4944. Crossref
Nacarino-Meneses, C. and Köhler, M. 2018. Limb bone histology records birth in mammals. PLoS One 13: 9–12. Crossref
Nacarino-Meneses, C. and Orlandi-Oliveras, G. 2021 The life history of European middle Pleistocene equids: first insights from bone histology. Historical Biology 33: 672–682. Crossref
Nacarino-Meneses, C., Chinsamy, A., Mayda, S., Kaya, T., and Erismis, U. 2021. Bone histology, palaeobiology, and early diagenetic history of extinct equids from Turkey. Quaternary Research 100: 240–259. Crossref
Nasterlack, T., Canoville, A., and Chinsamy, A. 2012. New insights into the biology of the Permian genus Cistecephalus (Therapsida, Dicynodontia). Journal of Vertebrate Paleontology 32: 1396–1410. Crossref
Peabody, F.E. 1961. Annual growth zones in vertebrates (living and fossil). Journal of Morphology 108: 11–62. Crossref
Piepenbrink, H. 1986. Two examples of biogenous bone decomposition and their consequences for taphonomic interpretation. Journal of Archaeological Science 13: 417–30. Crossref
Pereyra, M.E., Bona, P., Cerda, I.A., Jannello, J.M., De la Fuente, M.S., and Desántolo, B. 2020. Growth dynamics and body size evolution of South American long-necked chelid turtles: A bone histology approach. Acta Palaeontologica Polonica 65: 535–545. Crossref
Ray, S. and Chinsamy, A. 2004. Diictodon feliceps (Therapsida, Dicynodontia): bone histology, growth and biomechanics. Journal of Vertebrate Paleontology 24: 180–194. Crossref
Ray, S., Bandyopadhyay, S., and Appana, R. 2010. Bone histology of a kannemeyriid dicynodont Wadiasaurus: palaeobiological implications. In: S. Bandyopadhyay (ed.), New Aspects of Mesozoic Biodiversity, Lecture Notes in Earth Sciences 132, 73–89. Springer, Berlin. Crossref
Ray, S., Botha, J., and Chinsamy, A. 2004. Bone histology and growth patterns of some non-mammalian therapsids. Journal of Vertebrate Paleontology 24: 634–648. Crossref
Ray, S., Chinsamy, A., and Bandyopadhyay, S. 2005. Lystrosaurus murrayi (Therapsida, Dicyndontia): bone histology, growth and lifestyle adaptations. Palaeontology 48: 1169–1185. Crossref
Ray, S., Mukherjee, D., and Bandyopadhyay, S. 2009. Growth patterns of fossil vertebrates as deduced from bone microstructure: case studies from India. Journal of Biosciences 34: 661–672. Crossref
Reid, R.E.H. 1987. Bone and dinosaurian “endothermy”. Modern Geology 11: 133–154.
Rubidge, B.S. and Sidor, C.A. 2001. Evolutionary patterns among Permo-Triassic therapsids. Annual Reviews of Ecology and Systematics 32: 449–480. Crossref
Sander, P.M and Andrassy, P. 2006. Lines of arrested growth and long bone histology in Pleistocene large mammals from Germany: what do they tell us about dinosaur physiology? Palaeontographica Abteilung A 277: 143–159. Crossref
Scheyer, T.M. and Sánchez-Villagra, M.R. 2007. Carapace bone histology in the giant pleurodiran turtle Stupendemys geographicus: Phylogeny and function. Acta Palaeontologica Polonica 52: 137–154.
Scheyer, T.M. and Sander, P.M. 2007. Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proceedings of the Royal Society B: Biological Sciences 274: 1885–1893. Crossref
Shelton, C.D. and Sander, P.M. 2017. Long bone histology of Ophiacodon reveals the geologically earliest occurrence of fibrolamellar bone in the mammalian stem lineage. Comptes Rendus Palevol 16: 397–424. Crossref
Shelton, C.D., Chinsamy, A., and Rothschild, B.M. 2019. Osteomyelitis in a 265-million-year-old titanosuchid (Dinocephalia, Therapsida). Historical Biology 31: 1093–1096. Crossref
Shelton, C.D., Sander, P.M., Stein, K., and Winkelhorst, H. 2013. Long bone histology indicates sympatric species of Dimetrodon (lower Permian, Sphenacodontidae). Transactions of the Royal Society of Edinburgh Earth Science 103: 217–236. Crossref
Smirina, E.M. 1974. Prospects of age determination by bone layers in Reptilia [in Russian]. Zoologičeskij žurnal 53: 111–117.
Smith, C.C.D. 2020. Giraffa camelopardalis: Limb Bone Histology Through Ontogeny. 96 pp. M.Sc. Dissertation, University of Cape Town.
Smith, R.M.H. and Keyser, A.W. 1995. Biostratigraphy of the Tapinocephalus Assemblage Zone. In: B.S. Rubidge (ed.), Biostratigraphic Series, Biostratigraphy of the Beaufort Group (Karoo Supergroup). South African Committee for Stratigraphy Biostratigraphic Series 1, 8–12. Council for Geoscience, Pretoria.
Starck, J.M. and Chinsamy, A. 2002. Bone microstructure and developmental plasticity in birds and other dinosaurs. Journal of Morphology 254: 232–246. Crossref
Stein, K. and Prondvai, E. 2014. Rethinking the nature of fibrolamellar bone: an integrative biological revision of sauropod plexiform bone formation. Biological Reviews 89: 24–47. Crossref
Stein, K. and Sander, P.M. 2009. Histological core drilling: a less destructive method for studying bone histology. Methods in fossil preparation. In: M.A. Brown, J.F. Kane, and W.G. Parker (eds.), Proceedings of the First Annual Fossil Preparation and Collections Symposium, 69–80. Petrified Forest National Park, Arizona.
Ultsch, G.R. and Anderson, J.F. 1988. Gas exchange during hypoxia and hypercarbia of terrestrial turtles: a comparison of a fossorial species (Gopherus polyphemus) with a sympatric nonfossorial species (Terrapene carolina). Physiological Zoology 61: 142–152. Crossref
Van Hoepen, E.C.N. 1916. De ouderdom der Transvaalsche Karroolagen. Verhandelingen van het Geologisch-Mijnbouwkundig Genootschap voor Nederland en Kolonien Geologische serie 3: 107–117.
Wall, W.P. 1983. The correlation between high limb bone density and aquatic habits in recent mammals. Journal of Paleontology 57: 197–207.
Waskow, K. and Sander, P.M. 2014. Growth record and histological variation in the dorsal ribs of Camarasaurus sp. (Sauropoda). Journal of Vertebrate Paleontology 34: 852–869. Crossref
Woodward, H.N. 2019. Maiasaura (Dinosauria: Hadrosauridae) tibia osteohistology reveals non-annual cortical vascular rings in young of the year. Frontiers in Earth Science 7: 50. Crossref
Woodward, H.N., Freedman Fowler, E.A., Farlow, J.O., and Horner, J.R. 2015. Maiasaura, a model organism for extinct vertebrate population biology: a large sample statistical assessment of growth dynamics and survivorship. Paleobiology 41: 503–527. Crossref
Woodward, H.N., Horner, J.R., and Farlow, J.O. 2014. Quantification of intraskeletal histovariability in Alligator mississippiensis and implications for vertebrate osteohistology. PeerJ 2: e422. Crossref
Woodward, H.N., Tremaine, K., Williams, S.A., Zanno, L.E., Horner, J.R. and Myhrvold, N. 2020. Growing up Tyrannosaurus rex: Osteohistology refutes the pygmy “Nanotyrannus” and supports ontogenetic niche partitioning in juvenile Tyrannosaurus. Science Advances 6: eaax6250. Crossref
Acta Palaeontol. Pol. 66 (4): 705–721, 2021
https://doi.org/10.4202/app.00872.2021