

A new representative of the “orthopteroid” insect family Cnemidolestidae from the lower Permian of Germany
ANDRE NEL and MARKUS J. POSCHMANN
Nel, A. and Poschmann, M.J. 2021. A new representative of the “orthopteroid” insect family Cnemidolestidae from the lower Permian of Germany. Acta Palaeontologica Polonica 66 (3): 641–646.
Palatinarkema prokopi gen. et sp. nov., the third German representative of the late Carboniferous–early Permian archaeorthopteran clade Cnemidolestidae is described and figured. It is compared to the other known genera. We discuss putative aspects of the paleoecology of these stick-insect-like “orthopteroids”, based on their body and leg structures, plus the presence of a high disparity of color patterns in their forewings, suggesting an important diversity of biology and habitats for these insects. Their elongate bodies and legs show some similarities with the extant but distantly related stick insects. Cnemidolestids possibly expressed cryptic behaviours among the vegetation, using disruptive colors but also ovale eye-like spots, as in the extant insects.
Key words: Insecta, Polyneoptera, Archaeorthoptera, cryptic behavior, insect predation, Palaeozoic, Germany.
André Nel [anel@mnhn.fr, https://orcid.org/0000-0002-4241-7651], Institut de Systématique, Evolution, Biodiversité (ISYEB), Muséum national d’Histoire naturelle, CNRS, Sorbonne Université, EPHE, Université des Antilles, CP 50, 57 rue Cuvier, 75005 Paris, France.
Markus J. Poschmann [markus.poschmann@gdke.rlp.de, https://orcid.org/0000-0001-9710-1673], Generaldirektion Kulturelles Erbe RLP, Direktion Landesarchäologie/Erdgeschichte, Niederberger Höhe 1, D-56077 Koblenz, Germany.
Received 11 February 2021, accepted 12 April 2021, available online 8 September 2021.
Copyright © 2021 A. Nel and M.J. Poschmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The superorder Archaeorthoptera is one of the major polyneopteran insect groups with remarkable morphological and species diversity in ecosystems recorded since the late Carboniferous. Among their most “basal” subclades, the order Cnemidolestodea Handlirsch, 1937, even in the restricted sense of Béthoux (2005), is one of the most diverse with 22 genera in the family Cnemidolestidae Handlirsch, 1906, from the upper Carboniferous and lower Permian of Europe, North America, Brazil, China, and possibly Madagascar (Aristov 2014; Gu et al. 2014). The clade Cnemidolestidae is supported by strong synapomorphies in their forewing venation, but it also shows an important diversity of venation and coloration patterns.
Here we describe one further new genus and species from the early Permian. It is the third representative of this family from Germany (Dvořák et al. 2021) and the first record from the Carboniferous–Permian of the Saar-Nahe Basin.
Institutional abbreviations.—PE, Naturhistorisches Museum Mainz/Landessammlung für Naturkunde Rheinland-Pfalz, Germany.
Other abbreviations.—C, costa; CuA, cubitus anterior; CuP, cubitus posterior; CuPa, most anterior branch of CuP; CuPb, posterior branch of CuP; MA, median vein anterior; MP, median vein posterior; ma-mp, specialized crossvein between MA and MP+CuA+CuPa; R, radius; RA/P, radius anterior/posterior; ScP, subcostal posterior.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in urn:lsid: zoobank.org:pub:96EB973D-87B2-4086-A26F-C35E93 FD12B0
Material and methods
The fossil was observed using a Leica MZ 7.5 stereomicroscope both dry and immersed in isopropanol. Photographs were taken with specimen immersed in isopropanol using a Canon EOS 600D SLR camera equipped with a Canon EFS 60 mm macro lens. Original photographs were processed using the image-editing software Adobe Photoshop. Drawings were made from enlarged photographs using Inkscape. The wing venation nomenclature generally follows Kukalová-Peck (1991) and Béthoux and Nel (2002).
Systematic palaeontology
Superorder Archaeorthoptera Béthoux and Nel, 2002
Order Cnemidolestodea Handlirsch, 1937
Family Cnemidolestidae Handlirsch, 1906
Genus Palatinarkema nov.
ZooBank LSID: urn:lsid:zoobank.org:act:66B028D6-D771-47A1-95 DC-873DDDBDD7E3
Etymology: From Latin palatium, origin of Palatinate, the type region, and the genus name Narkema.
Type species: Palatinarkema prokopi sp. nov., by monotypy; see below.
Diagnosis.—Forewing characters only. Posterior branch of MP+CuA+CuPa anteriorly pectinate, with small bifurcations on only two branches; stem of anterior branch of MP+CuA+CuPa very short; veinlet ma-mp very strong and not aligned with anterior branch of MP+CuA+CuPa; angle between anterior branch and posterior branch of MP+CuA+CuPa very acute; no well-defined vein in area between MA, MP+CuA+CuPa, ma-mp crossvein present; branches of anterior branch of MP+CuA+CuPa and of RP simple.
Palatinarkema prokopi sp. nov.
Fig. 1.
ZooBank LSID: urn:lsid:zoobank.org:act:D32AD59D-F659-4837-83 C8-0896E4CCFC45
Etymology: Named after our friend Jakub Prokop, specialist in fossil insects.
Holotype: PE 2020/5004-LS a, b, part and counterpart of a complete wing with parts of the basal region hidden by remains of an undetermined insect wing and/or a mineral stain.
Type locality: East of the village of Niedermoschel, Saar-Nahe Basin, Germany.
Type horizon: Niedermoschel black shale, Jeckenbach Subformation, Meisenheim Formation, Lower Rotliegend, lower Permian (sensu Schindler 1997), probably Asselian–?Sakmarian (Schneider and Werneburg 2012; Schneider et al. 2020).
Material.—Holotype only.
Diagnosis.—As for the genus; two parallel darkened bands in distal half of forewing plus some spots in mid part.
Description.—Based on forewing venation: estimated total wing length about 17.1 mm, maximum width at midwing 4.4 mm; wing membrane probably originally hyaline with oblique colored bands; concave ScP slightly curved, running parallel with costal margin, merging with RA at distal two-thirds of wing; costal and subcostal areas nearly as broad where preserved; stem of R diverging from M+CuA near base of wing; division of RA and RP proximal of midwing, 2.3 mm basal to connection of ScP with RA; strongly convex ScP+RA simple ending on costal margin basal of wing apex; numerous oblique crossveins present between RA and costal wing margin; concave RP mostly posteriorly pectinate ending with six simple branches terminating at the wing tip; neutral (neither really convex not concave) vein MA diverging from M+CuA and running parallel to stem of R/RP; MA deeply forked into two simple branches, anterior one MA1 shortly connected to RP and posterior one MA2 nearly straight; CuPa not visible at base, hidden by debris; stem of MP+CuA+CuPa elongate, 1.7 mm long, bifurcating into two branches, the posterior branch subdivided into four branches; and an anterior branch with a short stem and bifurcating into two elongate branches, defining a long but rather narrow area between it, MA and crossvein ma-mp, without well-defined and strong vein inside it; angle between anterior and posterior branches of MP+CuA+CuPa very acute, ca. 18°; both branches of anterior branch of MP+CuA+CuP simple; crossvein ma-mp between MA and anterior branch of MP+CuA+CuPa very strong, apparently convex, and not aligned with anterior branch of MP+CuA+CuPa; area between branches of RP, concave CuP basally dividing into CuPa and CuPb, CuPb running parallel to MP+CuA+CuPa towards posterior wing margin; anal area incomplete with partly preserved first and second anal veins running parallel to CuP and CuPb.
Stratigraphic and geographic range.—Type locality and horizon only.
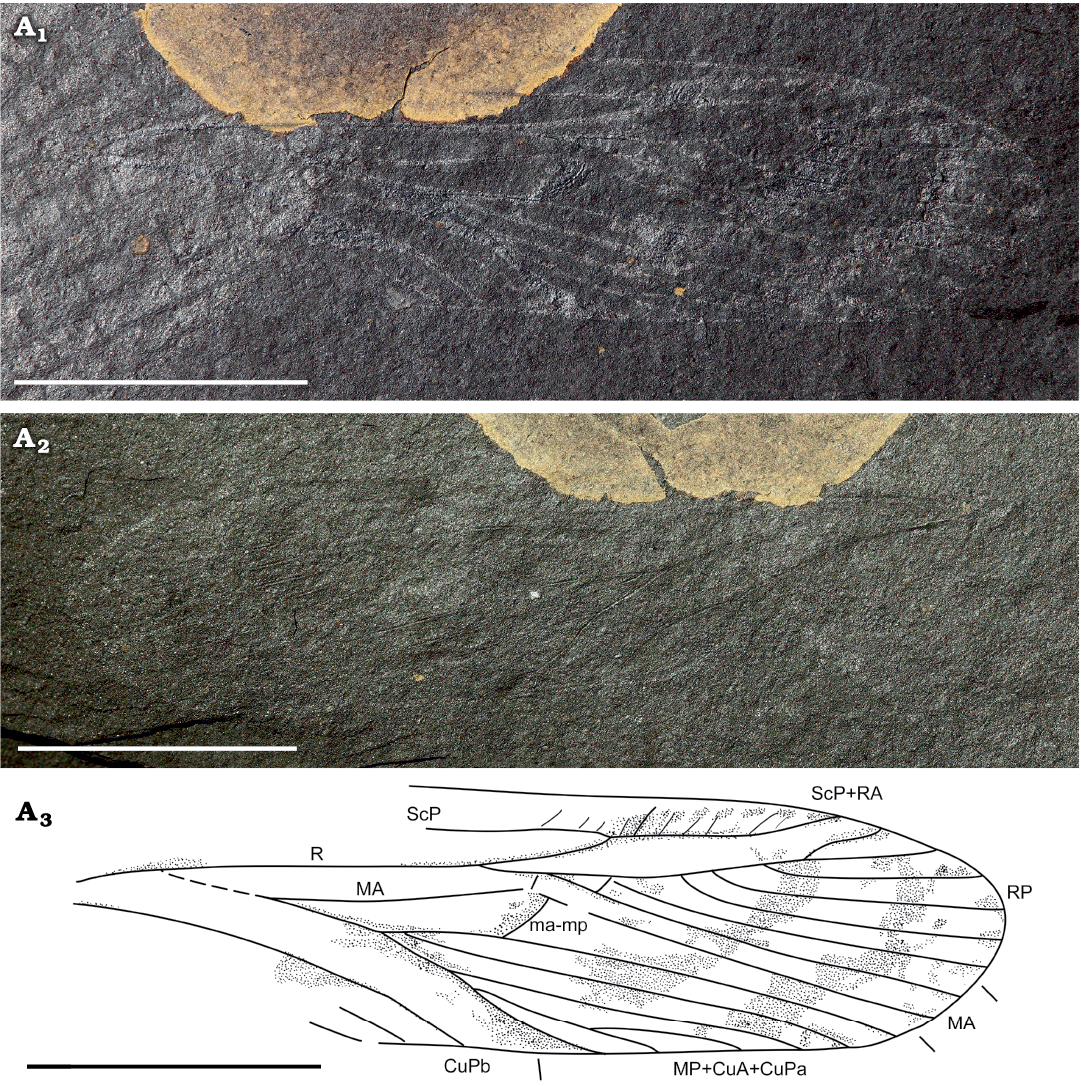
Fig. 1. Forewing of the cnemidolestid archaeorthopteran Palatinarkema prokopi gen. et sp. nov. (holotype, PE 2020/5004-LS) from Niedermoschel, Saar-Nahe Basin, Germany; Niedermoschel black shale, Lower Rotliegend, lower Permian. Photographs: part (A1), counterpart (A2), interpretive drawing (A3); small lines along wing represent the limits of the different fields. Abbreviations: CuA, cubitus anterior; CuPa, most anterior branch of cubitus posterior; CuPb, posterior branch of cubitus posterior; MA, median vein anterior; MP, median vein posterior; ma-mp, specialized crossvein between MA and MP+CuA+CuPa; R, radius; RA/P, radius anterior/posterior; ScP, subcostal posterior. Scale bars 5 mm.
Discussion
Palatinarkema gen. nov. can be attributed to the Archaeorthoptera as it shares the main diagnostic character of this superorder, namely the basal fusion of CuA with M and subsequent connection with the anterior branch of CuP as CuPa (Béthoux and Nel 2002). Furthermore, it displays characters typical of the order Cnemidolestodea (sensu Béthoux 2005), such as ScP merging with RA, CuPa merging with MP+CuA, MP+CuA+CuPa emitting an anterior branch weakly convergent to MA, with a specialized crossvein ma-mp between it and MA, thus defining a large median area between it and MA (Béthoux 2005; Gu et al. 2014: figs. 1, 2). The archaeorthopteran family Tococladidae and “lobeattids” show some similarities in the forewing venation with Palatinarkema gen. nov. in the subcostal, radial and cubital veins, with a fundamental difference concerning the vein MP separated from CuA+CuP, resulting in the absence of a median area in the former (Béthoux et al. 2003, 2012; Chen et al. 2020).
Aristov (2014), using a different diagnosis and wing venation nomenclature for the Cnemidolestodea, proposed a key to families. On the basis of the character “MP weak, ending on CuA or MA, or absent”, Palatinarkema gen. nov. falls in the family Cnemidolestidae sensu Aristov 2014, which includes also families Ischnoneuridae Handlirsch, 1906, Aetophlebidae Handlirsch, 1906, Narkemidae Handlirsch, 1911, and Narkeminidae Pinto and Ornellas, 1991). Indeed, in Palatinarkema gen. nov., MP is clearly basally fused to CuA. Béthoux (2005) and Gu et al. (2014: fig. 2 A1, C1) supposed that the vein MP can be reduced to a weak veinlet between MA and CuA+CuP.
The family Cnemidolestidae comprises the following genera (Aristov 2014; Gu et al. 2014; Dvořák et al. 2021): Aetophlebia Scudder, 1885; Amphiboliacridites Langiaux and Parriat, 1974; Anarkemina Aristov, 2014; Argentinonarkemina Martins-Neto, Gallego, and Brauckmann, 2007; Bouleites Lameere, 1917; Carbonokata Aristov, 2013; Cnemidolestes Handlirsch, 1906; Evenkiophlebia Aristov, 2013; Irajanarkemina Martins-Neto, Gallego, and Brauckmann, 2007; Ischnoneura Brongniart, 1893; Longzhua Gu, Béthoux, and Ren, 2011; Narkema Handlirsch, 1911; Narkemina Martynov, 1930; Narkeminopsis Whalley, 1979; Narkeminuta Aristov, 2013; Narkemulla Aristov, 2013; Paranarkemina Pinto and Ornellas, 1980; Piesbergopterum Dvořák, Pecharová, Leipner, Nel, and Prokop, 2021; Protodiamphipnoa Brongniart, 1885; Tshunoptera Aristov, 2013; Velizphlebia Martins-Neto, Gallego, and Brauckmann, 2007; and Xixia Gu, Béthoux, and Ren, 2014.
Palatinarkema gen. nov. shares with Narkema and Longzhua a narrow area between MA and MP+CuA+CuPa, but differs from it in the quite longer anterior branch of MP+CuA+CuPa with a simple anterior subdivision, and a pectinate (with only a branch having a short fork) posterior branch of MP+CuA+CuPa (Béthoux 2005: fig. 3; Gu et al. 2011). Xixia has also a narrow area between MA and MP+CuA+CuPa but the crossvein ma-mp is weaker than in Palatinarkema gen. nov. and has a posterior branch of MP+CuA+CuPa simple or with two branches (Gu et al. 2014). Ischnoneura, Cnemidolestes, Aetophlebia, Carbonokata, and Anarkemina differ from Palatinarkema gen. nov. in the crossvein ma-mp aligned with the anterior branch of MP+CuA+CuPa and the presence of a well-defined sigmoidal vein in the area between MA and MP+CuA+CuPa (Béthoux and Nel 2005: figs. 18, 19; Aristov 2013: fig. 1g; 2014; Gu et al. 2014). Narkemulla has also a well-defined sigmoidal vein in the area between MA and MP+CuA+CuPa (holotype specimen PIN 3115/119), but PIN 5384/16 has a very different area between MA and MP+CuA+CuPa from that of the holotype of this genus, much narrower and without any vein (Aristov 2013: fig. 1c–e).
Argentinonarkemina, Protodiamphipnoa, Bouleites, and Irajanarkemina share with Palatinarkema gen. nov. a narrow area between MA and MP+CuA+CuPa, but this area is also very long compared to its width and with a very short crossvein ma-mp and a very long anterior branch of MP+CuA+CuPa (Béthoux and Nel 2005; Martins-Neto et al. 2007: figs. 2, 7). Paranarkemina differs from Palatinarkema gen. nov. in the absence of the crossvein ma-mp (Martins-Neto et al. 2007: fig. 6). Narkeminuta has also a very weak crossvein ma-mp (Aristov 2013: fig. 1i).
Palatinarkema gen. nov. differs from Narkemina, Piesbergopterum, Amphiboliacridites, Evenkiophlebia, Tshunoptera, and Velizphlebia in the clearly narrower area between MA and MP+CuA+CuPa, the more acute angle between the anterior and the posterior branches of MP+CuA+CuPa, and the specialized crossvein ma-mp not aligned with the anterior branch of MP+CuA+CuPa (Martynov 1930: fig. 6; Langiaux and Parriat 1974; Martins-Neto et al. 2007: figs. 3–5; Aristov 2012, 2013: fig. 1a, f, h; Dvořák et al. 2021). Their patterns of coloration are also different.
Narkeminopsis has a narrow area between MA and MP+CuA+CuPa and a pectinate posterior branch of MP+ CuA+CuPa, but a more open angle than Palatinarkema gen. nov., and a longer stem of anterior branch of MP+CuA+CuPa (Béthoux and Nel 2005: fig. 20; Aristov 2013: fig. 1b). The second species Narkeminopsis inversa Aristov, 2013, differs from the type species Narkeminopsis eddi Whalley, 1979, and Palatinarkema prokopi gen. et sp. nov. in the presence of several branches of MP+CuA+CuPa and RP with forks.
Conclusions
The present discovery of Palatinarkema prokopi gen. et sp. nov. increases our knowledge on the diversity of the Cnemidolestidae. Unfortunately, the body structures of the Cnemidolestidae remain poorly known, thus few morpho-functional inferences can be drawn from their biology, and potentially shed light on the causes for their extinction. Some late Carboniferous representatives (e.g., Protodiamphipnoa tertrini Brongniart, 1893, Cnemidolestes woodwardi [Brongniart, 1893]) have elongate bodies and very strong and long legs (but apparently not jumping hind legs), somewhat reminiscent of those of some extant stick insects (e.g., Eurycantha calcarata Lucas, 1869; Béthoux and Nel 2005; Béthoux 2005), possibly suggesting similar cryptic behavior among leaves and plants. The shape of their hind wings remains poorly know, especially the relative size of their anal fan (see Aristov 2012: figs. 2, 3). Thus we cannot accurately establish their flight ability, but their massive bodies and large legs suggest a rather poor flight ability. The hypothesis of a cryptic lifestyle could be supported by the spectacular diversity of color patterns of their forewings. These are hyaline in some cases (in Narkemina kata Aristov, 2013), while some have a series of more or less parallel bands of colors (in Narkeminopsis eddi or Cnemidolestes woodwardi). Others have a large ovale eye-like spot situated in the middle of the wing, as in Protodiamphipnoa gaudryi (Brongniart, 1885) (Fig. 2), or a series of numerous spots distributed in the distal part of the wing (Piesbergopterum punctatum Dvořák, Pecharová, Leipner, Nel, and Prokop, 2021), or even a pattern of dark patches distributed over the entire forewing as in Xixia huban Gu, Béthoux, and Ren, 2014 (Béthoux and Nel 2005; Aristov 2013; Gu et al. 2014; Dvořák et al. 2021). Palatinarkema gen. nov. has a pattern of colored parallel bands, probably with a disruptive function during flight or at rest, which has been observed rather frequently among Palaeozoic insects, e.g. also in Palaeodictyoptera (Jarzembowski 2005; Li et al. 2013). Stevens et al. (2006) noticed that this strategy is efficient for insects to escape flying predators such as birds. Large ovale eye-like spots are clearly less frequent during the same period, but become more common during the Jurassic and Cretaceous with the kalligrammatid lacewings, and later with the Lepidoptera Saturniidae and Nymphalidae Satyrinae. The eyespots can have multiple functions, such as intimidation of a predator, deflection of the attack to a non-vital zone of the body, and in sexual selection (Stevens 2005, Collins 2013; Crees et al. 2021). It is nearly impossible to determine the exact function of the eyespots of Protodiamphipnoa gaudryi, but their large size and concentric ellips of different colors would suggest a function of intimidation as in some Recent Lepidoptera (Blest 1957), or sexual selection. In the case of Piesbergopterum punctatum, the small spots could have had a function of deflection of attacks as they are small and mainly distributed in the non-vital distal part of the forewing. In conclusion, such a disparity in the wing patterns strongly suggests an outstanding diversity with regards to the biology of the Cnemidolestidae.

Fig. 2. Habitus of the cnemidolestid archaeorthopteran Protodiamphipnoa gaudryi (Brongniart, 1885) (MNHN.F.R51393) from Commentry, France; Gzhelian, Carboniferous. Photographs: part (A1) and counterpart (A2). Scale bars 10 mm.
The “giant” griffenflies (Odonatoptera Meganeuridae) were the major flying predators during the late Carboniferous and until the middle Permian (Nel et al. 2018), before the emergence of gliding or flying vertebrates. However, they were probably not able to capture insects hidden among the vegetation. The development of various strategies of cryptic behavior among the late Carboniferous and early Permian Cnemidolestidae suggests that the predation pressure of the small terrestrial vertebrates greatly increased at that time. The balance between the costs/benefit was favorable for the development of cryptic coloration in some of their potential prey.
Acknowledgements
MJP thanks Olivier Béthoux (Muséum national d’Histoire naturelle, Paris, France) for offering generous help with software. We also thank Andrew Ross (Museum of Scotland, Edinburgh, UK) and an anonymous referee for their very useful remarks on the first version of the paper.
References
Aristov, D.S. 2012. Members of genus Narkemina Martynov (Insecta; Eoblattida: Cnemidolestidae) from the Carboniferous of Siberia [in Russian]. In: A.Û. Rozanov, A.V. Lopatin, and P.Û. Parhaev (eds.), Sovremennaâ paleontologiâ: klassičeskie i novejšie metody, 37–48. Paleontologičeskij Institut im. A.A.Borisâka Rossijskoj Akademii Nauk, Moskva.
Aristov, D.S. 2013. New and little-known Eoblattida (Insecta) from the Paleozoic of Russia. Paleontological Journal 47: 272–282. Crossref
Aristov, DS. 2014. Classification of the order Cnemidolestida (Insecta: perlidea) with descriptions of new taxa. Far Eastern Entomologist 277: 1–46.
Béthoux, O. 2005. Cnemidolestodea (Insecta): an ancient order reinstated. Journal of Systematic Palaeontology 3: 403–408. Crossref
Béthoux, O. and Nel, A. 2002. Venation pattern and revision of Orthoptera sensu nov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthoptera sensu nov. Zootaxa 96: 1–88. Crossref
Béthoux, O. and Nel, A. 2005. Some Palaeozoic “Protorthoptera” are “ancestral” orthopteroids: major wing braces as clues to a new split among the “Protorthoptera”. Journal of Systematic Palaeontology 2: 285–309. Crossref
Béthoux, O., Gu, J.-J., Yue, Y.-L., and Ren, D. 2012. Miamia maimai n. sp., a new Pennsylvanian stem-orthopteran insect, and a case study on the application of cladotypic nomenclature. Fossil Record 15: 103–113. Crossref
Béthoux, O., Nel, A., Galtier, J., Lapeyrie, J., and Gand, G. 2003. A new species of Tococladidae Carpenter, 1966 from the Permian of France (Insecta: Archaeorthoptera). Geobios 36: 275–283. Crossref
Blest, A.D. 1957. The function of eyespot patterns in the Lepidoptera. Behaviour 11: 209–258. Crossref
Brongniart, C. 1885. Les insectes fossiles des terrains primaires. Coup d’oeil rapide sur la faune entomologique des terrains paléozoïques. Bulletin de la Société des Amis des Sciences naturelles de Rouen 1885: 50–68. Crossref
Brongniart, C. 1893. Recherches pour servir à l’histoire des insectes fossiles des temps primaires précédées d’une étude sur la nervation des ailes des insectes. Bulletin de la Société d’Industrie Minérale de Saint-Etienne (3) 7: 1–491. Crossref
Chen, L., Ren, D., and Béthoux, O. 2020. A new, rare and small “lobeattid” species (Insecta: Archaeorthoptera) found at Xiaheyan (Pennsylvanian; Ningxia, China). Fossil Record 23: 71–74. Crossref
Collins, M.M. 2013. Interpretation of wing pattern elements in relation to bird predation on adult Hyalophora (Saturniidae). Journal of the Lepidopterists’ Society 67: 49–55. Crossref
Crees, L.D., DeVries, P., and Penz, C.M. 2021. Do hind wing eyespots of Caligo butterflies function in both mating behavior and antipredator defense? (Lepidoptera, Nymphalidae). Annals of the Entomological Society of America [published online, https://doi-org.eres.qnl.qa/10.1093/aesa/saaa050] Crossref
Dvořák, T., Pecharová, M., Leipner, A., Nel, A., and Prokop, J. 2021. New archaeorthopteran insects from the Pennsylvanian of Piesberg reveal unexpected mosaic of morphological traits and colouration pattern of the tegmina. Historical Biology [published online, https://doi.org/10.1080/08912963.2020.1867127] Crossref
Gu, J.-J., Béthoux, O., and Ren, D. 2011. Longzhua loculata n. gen. n. sp., one of the most completely documented Pennsylvanian Archaeorthoptera (Insecta; Ningxia, China). Journal of Paleontology 85: 303–314. Crossref
Gu, J.-J., Béthoux, O., and Ren, D. 2014. A new cnemidolestodean stem-orthopteran insect from the late Carboniferous of China. Acta Palaeontologica Polonica 59: 689–696.
Handlirsch, A. 1906. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch für Paläontologen und Zoologen. i–vi + 1–640 pp. Engelman, V.W., Leipzig.
Handlirsch, A. 1911. New Paleozoic insects from the vicinity of Mazon Creek, Illinois. American Journal of Science 31: 297–326, 353–377. Crossref
Handlirsch, A. 1937. Neue Untersuchungen über die fossilen Insekten mit Ergänzungen und Nachträgen sowie Ausblicken auf phylogenetische, palaeogeographische und allgemein biologische Probleme. Teil 1. Annalen des Naturhistorischen Museums in Wien 48: 1–140.
Jarzembowski, E.A. 2005. Colour and behaviour in late Carboniferous terrestrial arthropods. Zeitschrift der Deutschen Gesellschaft für Geowissenschaften 156: 381–386. Crossref
Kukalová-Peck, J. 1991. Fossil history and the evolution of hexapod structures. In: CSIRO (eds.). The Insects of Australia. A Textbook for Students and Research Workers, 141–179. Melbourne University Press, Melbourne.
Langiaux, J. and Parriat, H. 1974. Faune entomologique du bassin de Blanzy-Montceau. La Physiophile 81: 62–74.
Lameere, A. 1917. Révision sommaire des insectes fossiles du Stéphanien de Commentry. Bulletin du Muséum National d’Histoire Naturelle de Paris 23: 141–200.
Li, Y., Ren D., Pecharová, M., and Prokop, J. 2013. A new palaeodictyopterid (Insecta: Palaeodictyoptera: Spilapteridae) from the upper Carboniferous of China supports a close relationship between insect faunas of Quilianshian (northern China) and Laurussia. Alcheringa 37: 487–495. Crossref
Lucas, H. 1869. Orthoptères de l’ile San-Georges, Archipel Salomon. Bulletin de la Société Entomologique de France 9: 25–26.
Martins-Neto, R.G., Gallego, O.F., Brauckmann, C., and Cruz, J.L. 2007. A review of the South American Palaeozoic entomofauna. Part I: the Ischnoneuroidea and Cacurgoidea, with description of new taxa. African Invertebrates 48: 87–101.
Martynov, A.V. 1930. On the Paleozoic insects of the Kuznetsk Basin [in Russian]. Izvetiâ Glavnogo Geologorazvedočnogo Upravleniâ 49: 73–100.
Nel, A., Prokop, J., Pecharová, M., Engel, M.S., and Garrouste, R. 2018. Palaeozoic giant dragonflies were hawker predators. Scientific Reports 8: 12141. Crossref
Pinto, I.D. and Ornellas, L. 1980. Upper Carboniferous insects from Argentina. 2. Familia Narkemocacurgidae (Parapleocoptera). Boletin de la Academia Nacional de Ciencias de Cordoba Argentina 53: 287–291.
Pinto, I.D. and Ornellas, L. 1991. Substitute names for the extinct insecta families Narkemocacurgidae Pinto et Ornellas, 1978 and Cacurgonarkemidae Pinto, 1990. Pesquisas, (Zoologia) 18: 93. Crossref
Schindler, T. 1997. Neue lithostratigraphische Leithorizonte im unteren Rotliegend des Saar-Nahe-Beckens (U. Perm, SW Deutschland). 1. Leithorizonte der lithostratigraphischen Einheit Lauterecken-bis Odernheim-Schichten L-O 5 (Boy & Fichter). Mainzer geowissenschaftliche Mitteilungen 26: 37–44.
Schneider, J.W. and Werneburg, R. 2012. Biostratigraphie des Rotliegend mit Insekten und Amphibien. In: Deutsche Stratigraphische Kommission (eds.), Stratigraphie von Deutschland X. Rotliegend. Teil I: Innervariscische Becken. Schriftenreihe der Deutschen Gesellschaft für Geowissenschaften 61: 110–142. Crossref
Schneider, J.W., Lucas, S.G., Scholze, F., Voigt, S., Marchetti, L., Klein, H., Opluštil, S., Werneburg, R., Golubev, V.K., Barrick, J.E., Nemyrovska, T., Ronchi, A., Day, M.O., Silantiev, V.V., Rößler, R., Saber, H., Linnemann, U., Zharinova, V., and Shen, S.-Z. 2020. Late Paleozoic–early Mesozoic continental biostratigraphy—links to the Standard Global Chronostratigraphic Scale. Palaeoworld 29: 186–238. Crossref
Scudder, S.H. 1885. Palaeodictyoptera: or the affinities and classification of Paleozoic Hexapoda. Memoirs of the Boston Society of Natural History 3: 319–351.
Stevens, M. 2005. The role of eyespots as anti-predator mechanisms, principally demonstrated in the Lepidoptera. Biological Review 80: 573–588. Crossref
Stevens, M., Cuthill, I.C., Parraga, C.A., and Troscianko, T. 2006. The effectiveness of disruptive coloration as a concealment strategy. In: J.-M. Alonso, S. Macknik, L. Martinez, P. Tse, and S. Martinez-Conde (eds.), Progress in Brain Research, 49–64. Elsevier, Amsterdam. Crossref
Whalley, P.E.S. 1979. New species of Protorthoptera and Protodonata (Insecta) from the upper Carboniferous of Britain, with a comment on the origin of wings. Bulletin of the British Museum of Natural History, Geology 32: 85–90.
Acta Palaeontol. Pol. 66 (3): 641–646, 2021
https://doi.org/10.4202/app.00879.2021