


Morphology and relationships of the enigmatic stenothecoid pan-brachiopod Stenothecoides—new data from the middle Cambrian Burgess Shale Formation
PAUL A. JOHNSTON and MICHAEL STRENG
Johnston, P.A. and Streng, M. 2021. Morphology and relationships of the enigmatic stenothecoid pan-brachiopod Stenothecoides—new data from the middle Cambrian Burgess Shale Formation. Acta Palaeontologica Polonica 66 (4): 723–751.
Bulk sampling of middle Cambrian carbonate units in the lower Burgess Shale Formation (Wuliuan) and the upper Wheeler Formation (Drumian) in Utah have yielded abundant silicified stenothecoids. Previously unreported from the Burgess Shale, stenothecoids discovered include at least two species: Stenothecoides cf. elongata and Stenothecoides rasettii sp. nov. The Utah material is assigned to Stenothecoides elongata. The new stenothecoid material confirms some earlier observations including a set of interior grooves and ridges forming nested chevrons across the midline and a finer set disposed around the interior shell margin. The chevroned grooves are interpreted here as mantle canals and the peripheral furrows as setal grooves. A prominent boss occurs at the valve apex in both valves. An apparent socket receiving the boss in the opposite valve described in earlier studies we show to be an artefact of preservation. Consequently, the bosses were juxtaposed when the valves were conjoined and so must have had some function other than valve articulation. Most extraordinary in Stenothecoides is an embayment at the shell apex, which likely represents a rudimentary pedicle foramen. This and other features including apparent articulate brachiopod-like calcitic fibrous shell microstructure replicated in silica, indicate phylogenetic propinquity of the Stenothecoida is with the Brachiopoda, not the Mollusca. However, phylogenetic proximity of the Stenothecoida relative to any of the brachiopod crown groups is unclear. Stenothecoids may represent a pan-brachiopod stem group derived from organocalcitic, multisclerite, eccentrothecimorph tommotiids via sclerite reduction to two opposing mitral sclerites. Discovery of stenothecoids in carbonate debris aprons in the Burgess Shale suggests transport of shelly biota downslope from the adjacent platform. However, their absence in siliciclastic units of the Burgess Shale preserving both shelly and soft-bodied biota indicates these units lack significant input of transported elements from the adjacent platform.
Key words: Stenothecoida, Brachiopoda, Mollusca, Cambrian, Burgess Shale Formation.
Paul A. Johnston [pajohnston@mtroyal.ca], Department of Earth and Environmental Sciences, Mount Royal University, Calgary, Canada.
Michael Streng [michael.streng@geo.uu.se], Department of Earth Sciences, Palaeobiology, Uppsala University, Villavägen 16, SE-75236, Uppsala, Sweden.
Received 16 July 2021, accepted 16 October 2021, available online 8 December 2021.
Copyright © 2021 P.A. Johnston and M. Streng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Cambrian Explosion included the appearance of diverse small skeletal elements, the so-called Small Shelly Fossils (SSF) in the geologic record. Many of the SSFs, whether whole animal skeletons or parts, have proved difficult to place systematically (Bengtson et al. 1990; Pratt et al. 2001). Among these are the enigmatic stenothecoids. The oldest reported occurrences of the group are from pre-trilobite strata of western Hubei, China (Yu 1996) and western Mongolia (Voronin et al. 1982), of which the latter might be as old as early Cambrian Age 2 (Kouchinsky et al. 2012; but see Smith et al. 2016 and Landing and Kouchinsky 2016 for even older estimated ages of the stenothecoid-bearing Mongolian strata). While common in the later early Cambrian and early middle Cambrian (e.g., Resser 1938; Rasetti 1954; Horný 1957; Koneva 1976, 1979a, b; Peel 1988), the stenothecoids failed to survive the middle Cambrian (Miaolingian). The odd combination of an inequivalved bivalved shell with inequilateral valves, but lacking undoubted features of either bivalves (such as adductor scars and ligament) or brachiopods (notably bilateral symmetry perpendicular to the commissure), has made systematic placement of the group problematic. Most authors have regarded stenothecoids as molluscs, although a few have suggested brachiopod affinities, and at least one proposed a new phylum (summarized by Pel’man 1985; see Historical background below).
The term “stenothecoids” did not come about until Yochelson (1968, 1969) proposed the new class Stenothecoida within the phylum Mollusca, previous works referring to these organisms by individual generic names or, following Horný (1957), as cambridiids. To date, twelve genera have been assigned to the class including: Cambridium Horný, 1957; Bagenovia Radugin, 1937; Stenothecoides Resser, 1938; Bagenoviella Aksarina, 1968; Sulcocarina Aksarina, 1968; Kaschkadakia Aksarina, 1968; Makarakia Aksarina, 1968; Stenothecella Aksarina in Aksarina and Pel’man, 1978; Sargaella Aksarina in Aksarina and Pel’man, 1978; Katunioides Aksarina in Aksarina and Pel’man, 1978; Serioides Pel’man, 1985; and Dignus Pel’man, 1985. Most of the genera have been assigned to the class’ sole family Cambridiidae, but it seems likely that more than one family level taxon is represented given the variety of shell shapes (e.g., rhombic in Makarakia, Katunioides), ornament (e.g., strong divaricate ribs in Bagenovia, Bagenoviella, Sulcocarina), and the variety of internal shell features (longitudinal, pinnate, circular, and elliptical depressions on the valve floor). Already Aksarina and Pel’man (1978) only questionably assigned Kaschkadakia to the family Cambridiidae, and they considered Makarakia and Katunioides to belong to an as yet unestablished family. Certainly Pel’man’s (1985) Mongolian taxa, Serioides, Dignus, and a species of Cambridium, differ significantly in internal apical structure from Stenothecoides species described herein and warrant at least family-level distinction. However, revision of the group Stenothecoida is beyond the scope of the present study, especially as such an undertaking would, in our opinion, require examination of original material at multiple institutions.
The discovery of silicified stenothecoids in carbonate units of the middle Cambrian Burgess Shale Formation (Wuliuan), together with new material from the middle Cambrian of Utah, provides new morphological information, especially about the apical region and the inner shell surface, which allows a more definitive statement regarding the systematic position of this group, one that favours phylogenetic proximity with the Brachiopoda, not the Mollusca (Johnston et al. 2017). Key features newly recognized include a posterior median opening and rhynchonelliform-like fibrous calcite secondary shell microstructure (replicated in silica). Other features, including inequilateral valves, a prominent internal apical boss in both valves, and a possible anterior gape in adult shells, are without homologs in crown-group brachiopods, although the latter feature is known in the stem-group taxa Apistoconcha and Micrina (Parkhaev 1998; Holmer et al. 2008). While stenothecoids can now be allied more closely with the Brachiopoda than with the Mollusca, their relationship with stem- and crown- group taxa remains uncertain.
Institutional abbreviations.—ROMIP, Royal Ontario Museum, Invertebrate Palaeontology, Toronto, Canada; TMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Canada.
Nomenclatural acts.—This published work and the nomenclatural acts it contains have been registered in ZooBank: urn:lsid:zoobank.org:pub:17C07FF2-6ECF-4ECE-97EB-FCBFAA04C76B
Historical background
Morphological understanding of the stenothecoids since their discovery nearly 140 years ago has evolved slowly, being punctuated from time to time by newly discovered material showing new and key features. These advances were often accompanied by re-assessment of the systematic position of the group, although with persistent uncertainties. As evident in Fig. 1, interpretations of the affinities of the group have varied considerably, with most authors, especially in recent decades, placing it somewhere in or near the phylum Mollusca.
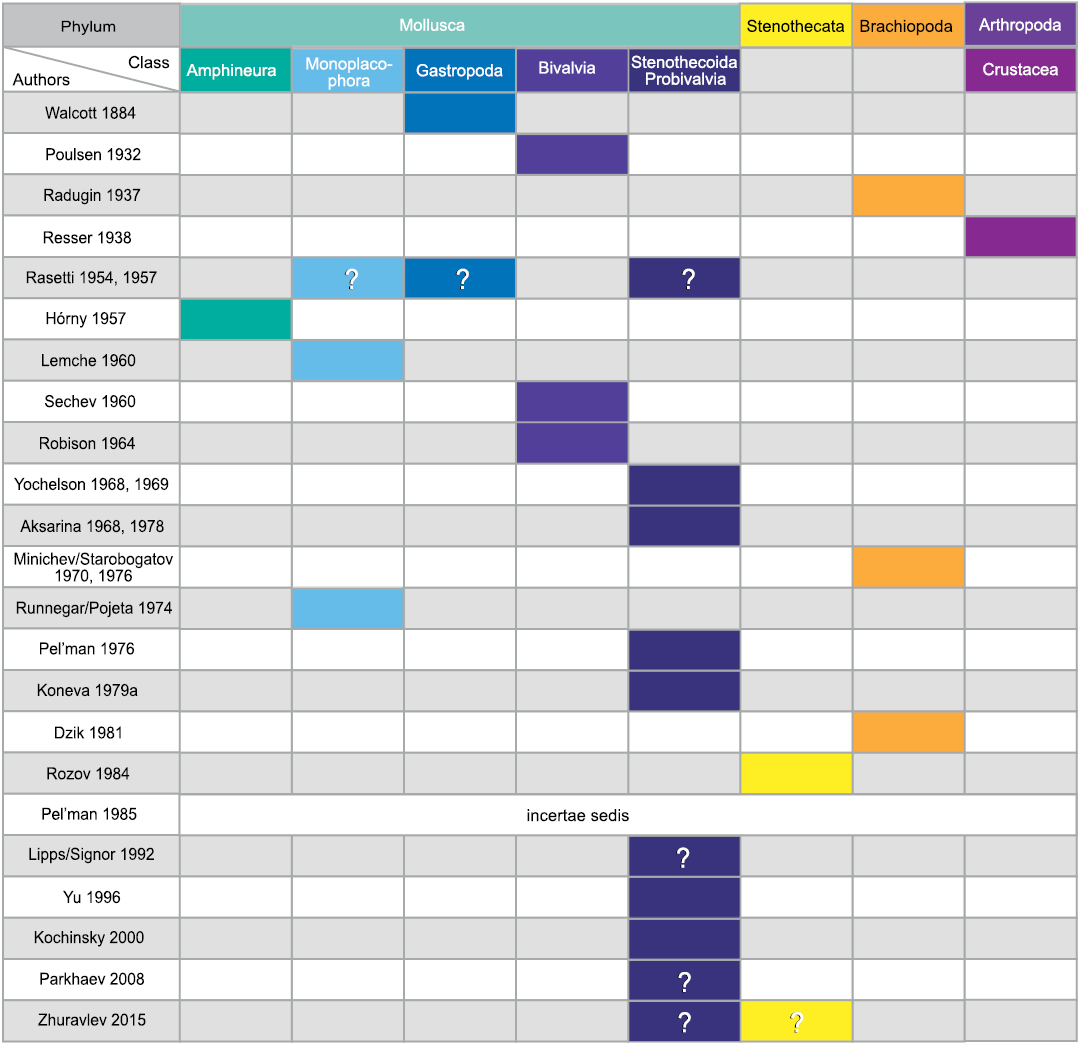
Fig. 1. Historical interpretations of the systematic position of stenothecoids (updated from Pel’man 1985).
Study of the stenothecoids began with Walcott’s (1884) discovery of rather innocuous small shells in the middle Cambrian Prospect Mountain Limestone (= Eldorado Limestone of subsequent authors, e.g., Walcott 1912; Resser 1954; Robison 1964) of central Nevada, which he named Stenotheca elongata Walcott, 1884. The shells are tear-drop-shaped with a few commarginal growth lines but preserve no internal features. Presuming these to be univalves, Walcott (1884) assigned them to Stenotheca, a genus of helcionelloid molluscs then thought to be gastropods. He figured a single incomplete valve in plan and lateral views. Asymmetry of the valve, so characteristic of the stenothecoids as later understood, was not evident to Walcott (1884) as he made no such comment, nor is it obvious in his figures. However, two years later, Walcott (1886: pl. 12: 4) figured a complete valve from the lower Cambrian of Labrador, which he regarded as likely conspecific with the Nevada species and noted asymmetry of the aperture, even remarking on the superficial similarity with juvenile shells of the mussel Mytilus.
More than 40 years later, in a study of early Cambrian faunas of east Greenland, Poulsen (1932) figured several specimens of stenothecoids. Although working only with isolated valves, he assumed these organisms were bivalved and assigned them to an indeterminate genus and species of the molluscan class Lamellibranchiata (i.e., Bivalvia). Poulsen (1932) did not recognize, or at least did not comment on, any affinities with Walcott’s (1884, 1886) Stenotheca elongata. Radugin (1937) figured two rather poor stenothecoid specimens from the Cambrian of Siberia, on which he erected the new genus Bagenovia. Departing from earlier interpretations of stenothecoids, Radugin (1937) assigned Bagenovia to the Brachiopoda, not the Mollusca. There is nothing to suggest he made any systematic connection with Walcott’s (1884, 1886) or Poulsen’s (1932) taxa, and he provided neither morphologic analysis nor explanation for his brachiopod interpretation, only that Bagenovia bore “a peculiar fir tree and not radial ribbed pattern” of the external costae (Radugin 1937: 301 [free translation]). Like Poulsen (1932), Radugin (1937) suspected from isolated valves that the animal was bivalved, correctly as it turned out.
Resser (1938) rejected all earlier interpretations and regarded stenothecoids as carapaces of crustaceans, a conclusion that received zero support from subsequent systematists. Resser’s only service here was to extract Walcott’s (1884) species Stenotheca elongata from the helcionelloid genus Stenotheca and place it as the type species in a new genus Stenothecoides Resser, 1938. He also assigned Poulsen’s (1932) Greenland specimens to a new species Stenothecoides poulseni Resser, 1938, and Walcott’s (1886) Labrador specimens to Stenothecoides labradorica Resser, 1938.
A major advance in understanding the morphology of stenothecoids came with Rasetti’s (1954, 1957) discovery of specimens from the middle Cambrian Mount Whyte Formation, in the vicinity of Field, British Columbia (coincidently in the same area as the specimens described herein from the stratigraphically younger Burgess Shale Formation). These included internal molds that showed, for the first time, internal features, notably ridges and furrows in a rather broad zone extending around the perimeter of the shell; the furrows and ridges intersect the shell margin perpendicularly or at a high angle, with some extending internally to nearly the midline of the valve. Rasetti (1954) also noted substantial intraspecific variation in the shell outline and that the apex of the valve, in plan view, is inclined to the left or to the right, or more rarely, is orthocline. Rasetti (1954) summarily dismissed Resser’s (1938) interpretation of Stenothecoides as a crustacean. He also rejected Poulsen’s (1932) notion of a bivalved shell and with it his lamellibranch assignment and instead followed Walcott’s (1884, 1886) view that Stenothecoides was a univalved mollusc(?), which he interpreted to be limpet-like in habit. Radugin’s (1937) specimens of Bagenovia are so unlike Stenothecoides in ornament and outline, that there would have been no reason for Rasetti (1954) at that time to connect these genera taxonomically, if indeed he was aware of the Siberian material. At the same time as Rasetti’s (1957) second study, Horný (1957) described important new stenothecoid material from the lower Cambrian of eastern Siberia that included internal molds showing subtransverse furrows and ridges similar to those in Rasetti’s (1954) Canadian species.
Horný (1957) likewise regarded stenothecoids as univalved molluscs, noting some similarities with the Monoplacophora, which at the time he included as an order, along with the Polyplacophora (chitons), in the molluscan class Amphineura. However, the peculiar internal furrows and ridges suggested to him body segmentation unlike any mollusc known and so he assigned stenothecoids only tentatively to the Amphineura in an unspecified order. Horný (1957) was the first to recognize a possible systematic connection of Resser’s (1938) genus Stenothecoides and Radugin’s (1937) genus Bagenovia, for which he provided a formal diagnosis. He erected a new family Cambridiidae to accommodate Bagenovia and a newly named genus Cambridium. However, while acknowledging affinities with Cambridium, he left Stenothecoides in a separate, undesignated family, being troubled by the intraspecific variability of its shell outline, the lack of longitudinal structures on the internal midline, and the apparent restriction of the internal transverse ridges and furrows largely to the shell periphery. The latter concern was soon alleviated as additional specimens of Stenothecoides described that same year (Rasetti 1957) show furrows and ridges extending to the midline as in Cambridium, a detail confirmed in Burgess Shale Stenothecoides described herein.
Knight and Yochelson (1958, 1960) questionably included the stenothecoids in the Monoplacophora (earlier elevated to a class within the Mollusca; Lemche 1957). These authors followed Horný (1957) in recognizing three genera of stenothecoids, viz. Cambridium, Stenothecoides, and Bagenovia in the Cambridiidae, which they placed as a monotypic family in a newly erected order Cambridioidea. Whereas Horný (1957) doubted the inclusion of Stenothecoides in the family, Knight and Yochelson (1958) questioned the inclusion of Bagenovia. A critical point made by the latter authors, ignored in several subsequent works (e.g., Zhuravlev 2015), is that the transverse furrows and ridges of stenothecoids are not muscle scars, as they lack the abrupt edges of muscle scars in undoubted tryblidiacean monoplacophorans (Knight and Yochelson 1958).
Lemche (1960), much intrigued by Horný’s (1957) specimens of Cambridium showing apparent metameric muscle scars (also noted by Rasetti 1954), redrew Horný’s (1957: pl. 4) figure of such and seems to have accepted an ancestral position for the stenothecoids within the Monoplacophora and argued, bizarrely from a modern viewpoint, for an even more basal position in the evolution of the Metazoa, complete with comparisons of stenothecoid internal ridges and the septa of rugose corals.
That same year, Sytchev (1960) provided critical material demonstrating that stenothecoids were bivalved organisms, not univalves. The bivalved shell, together with asymmetry of the valves, and what he interpreted to be traces of a ligament at the apex, prompted Sytchev (1960) to classify the stenothecoids as lamellibranch molluscs (i.e., Bivalvia), as had Poulsen (1932); however, Sytchev’s (1960) work had little impact at the time as a monoplacophoran interpretation of stenothecoids persisted (Rozanov and Missarzhevsky 1966; Termier and Termier 1968).
New morphologic information came with Robison’s (1964) description of silicified middle Cambrian specimens, which he considered to be conspecific with Stenothecoides elongata (Walcott, 1884). For the first time, details of potential hinge and articulation structures were available, which included a tooth-like boss below the valve apex, and an apparent gap or socket on the opposing valve, structures that seemed to support assignment of at least Stenothecoides to the lamellibranch molluscs (Robison 1964).
Subsequent studies provided few new morphologic data but expanded the known diversity of genera and species and included varying commentary on the systematic position of the group. In a probably overly influential paper, Yochelson (1969) concluded that the internal transverse furrows and ridges of stenothecoids were unlike any lamellibranchs, or any other molluscs, and so assigned the stenothecoids to a new extinct molluscan class, Stenothecoida. Noting Rowell’s (1965: H864) rejection of Bagenovia as a brachiopod, Yochelson (1969) provisionally included it, along with the more confidently placed Stenothecoides and Cambridium, in the Stenothecoida.
About the same time, and independently, Aksarina (1968) erected the class Probivalvia within the Mollusca for these same genera and for several new genera named in that work, including Bagenoviella, Sulcocarina, Kaschkadakia, and Makarakia. She also provided a formal diagnosis for the class, in contrast to Yochelson’s (1969) more informal description of the group. Yochelson (1968) earlier used the term Stenothecoida in an abstract but later (1969) advised that any priority for the name should be established on his 1969 paper. Nonetheless, subsequent usage, including later work by Aksarina and Pel’man (1978), favoured the term Stenothecoida and that usage, rather than Probivalvia, is followed herein. An important contribution of Aksarina (1968) was expansion of the known morphologic limits of the group, which includes, for example, extreme apical–abapical elongation of the shell in Bagenoviella. In his study on early Paleozoic monoplacophorans, Starobogatov (1970) commented briefly on the stenothecoids and concluded from his reading of Aksarina (1968) that if the shell is bivalved as well as equivalved and equilateral, then they should be excluded from the monoplacophorans, but he did not suggest an alternative placement. His statement about the symmetry was surprising at the time in view of Aksarina’s (1968) diagnosis of the Probivalvia, which stated plainly that the vertical axis of each valve is arcuate (the valves are therefore inequilateral) and that the valves of at least some stenothecoids are slightly unequal in convexity. Nonetheless, a more definitive statement came later from Minichev and Starobogatov (1976). They proposed that stenothecoids should be placed in their own class near the brachiopods because the principal line of symmetry appears to be perpendicular to the commissure (notwithstanding the arcuate axis in many species) and therefore unlike the bivalve molluscs. Dzik (1981), in a brief comment, independently came to a similar conclusion, suggesting that stenothecoids were essentially calcareous inarticulate brachiopods, a notion that seems to have received neither support nor even acknowledgment from subsequent authors, until the present study.
Runnegar and Pojeta (1974) and Pojeta and Runnegar (1976) acknowledged the bivalved condition of the stenothecoids but remained convinced of their close affinities with the Monoplacophora and suggested they evolved a bivalved shell independently of other bivalved molluscs (but see Pojeta 1980). About this time, morphologic similarities of Bagenovia and Stenothecoides were convincingly demonstrated from well preserved material of the former (Koneva 1976), thus allaying Knight and Yochelson’s (1958) earlier doubts about the stenothecoid affinities of Bagenovia. Pel’man (1976a) described some poorly preserved material from the same region as Horný’s (1957) material, which he assigned to Cambridium and Stenothecoides. However, his figured valves appear fully equilateral; this, along with the absence of preserved internal features leave these specimens less convincing as stenothecoids. Soon after, Aksarina and Pel’man (1978) described additional material of previously described stenothecoid genera and added three new genera to the class roster: Stenothecella, Sargaella, and Katunioides. Internal details in some of their taxa (Stenothecella, Sargaella) include petal-like lobes and closed oval impressions on either side of the apical axis, not simply the subtransverse troughs and ridges described earlier in Stenothecoides and Cambridium (Rasetti 1954; Horný 1957). Koneva (1979a, b) followed with descriptions of new material from Kazakhstan and Uzbekistan including eighteen species, fifteen new, assigned to Stenothecoides. As noted by Peel (1988), marked intraspecific variability of the valve outline is well known for Stenothecoides and may account for the plethora of named species.
Rozov (1984) provided a helpful summary of stenothecoid morphologies described to date and, for purposes of systematic descriptions, attempted to standardize the orientation of the stenothecoid shell, which had varied in earlier works. He was critical of studies proposing left and right valves as in bivalve molluscs (e.g., Sytchev 1960; Aksarina 1968; Aksarina and Pel’man 1978; Koneva 1979b). Instead, he maintained that the apical and abapical ends be considered anterior and posterior, respectively. The valve curving to the right apically in external plan view he considered to be the dorsal valve, and the opposing valve, which curves left apically, the ventral valve. Rozov (1984) doubted the brachiopod affinities proposed by Minichev and Starobogatov (1976), pointing out the common if not ubiquitous occurrence of inequilateral valves in the stenothecoids. He further argued that signs of metamerism evident in at least some stenothecoids are unknown in brachiopods. However, drawing on the wealth of specimens, some preserving details of internal shell morphology, available especially from the earlier Aksarina and Pel’man (1978) and Koneva (1979b) studies, Rozov (1984) considered the stenothecoids as sufficiently remote morphologically from both the Mollusca and Brachiopoda to warrant placement in their own phylum Stenothecata (note that Rozanov and Zhuravlev 1992 state that Rozov 1984 considered stenothecates to be closer to brachiopods than to molluscs, although this is not clear from our reading of Rozov’s 1984 study).
Skepticism regarding the molluscan affinities of the Stenothecoida continued with Pel’man (1985), who described some extraordinary silicified specimens from the lower Cambrian of western Mongolia, including two new genera, Serioides and Dignus. Unlike any previously described stenothecoids, these, and his new species Cambridium dentatum, show complex interlocking structures on the hinge, somewhat mimicking the taxodont dentition of palaeotaxodont bivalves. Pel’man (1985) was convinced that the Mongolian specimens showed unequivocal evidence for attachment of a ligament near the apex of the shell. None of his specimens, as he explained, were sufficiently preserved to show the transverse ridges and furrows on the valve floor characteristic of many earlier described stenothecoid genera. Apparent ligament notwithstanding, Pel’man (1985) was not inclined to follow a lamellibranch or even a mollusc assignment proposed by earlier workers and considered stenothecoids as phylum incertae sedis.
Like Pel’man (1985), subsequent authors were either non-committal on the phylum level assignment of the Stenothecoida (e.g., Missarzhevsky and Mambetov 1981; Parkhaev 1998; Pratt et al. 2001; Li et al. 2014), or included them within the phylum Mollusca, often with reservation (e.g., Yu 1996; Yochelson 2000; Kouchinsky 2000; Skovsted 2006; Varlamov et al. 2008), or regarded them more informally as simply mollusc-like or molluscoid organisms (e.g., Rozanov and Zhuravlev 1992; Valentine 2004). Hence, it might not be entirely surprising that recent studies on the early evolution and diversification of the molluscs tend to exclude stenothecoids (e.g., Parkhaev 2008; Vinther 2015).
Zhang (1980) described various new species of bivalve molluscs from the early Cambrian of China, which he assigned to his new genera Cycloconchoides, Hubeinella, Praelamellodonta, and Xianfengoconcha. Runnegar and Pojeta (1992) rejected the bivalve interpretation and suggested the taxa might represent stenothecoids. However, it appears more likely that the species represent distorted inarticulate brachiopods or, in the case of Cycloconchoides, have affinity to arthropod carapaces (Geyer and Streng 1998; Pojeta 2000).
Geological setting
The material described herein was recovered from the Burgess Shale Formation (some authors restrict the term “Burgess Shale” to the type area and refer to lateral equivalents as “thick” Stephen Formation, e.g., Streng et al. [2016]) at three localities in Yoho National Park, southeastern British Columbia, Canada (Fig. 2): Locality 1 on the north limb of Mount Field; Locality 2, 2.4 km southeast of Locality 1 across the Kicking Horse River on the northwest shoulder of Mount Stephen; Locality 3 about 10 km farther to the southeast from Locality 2 on the southeastern slope of Odaray Mountain (Fig. 2B). All three localities occur in basinal facies in close proximity to the so-called Cathedral Escarpment, an abrupt, fault-controlled, northeast to southwest facies change from predominantly platform carbonates to basinal siliciclastics and deeper water carbonates (Johnston et al. 2017). Samples from localities 1 and 2, were collected from the Yoho River Limestone Member, the lowest carbonate member of the Burgess Shale Formation (Fletcher and Collins 1998). The Yoho River Limestone is a wedge-shaped unit, interpreted to be a debris-apron that varies greatly in thickness, depending on the proximity to the Cathedral escarpment (McIlreath 1977).
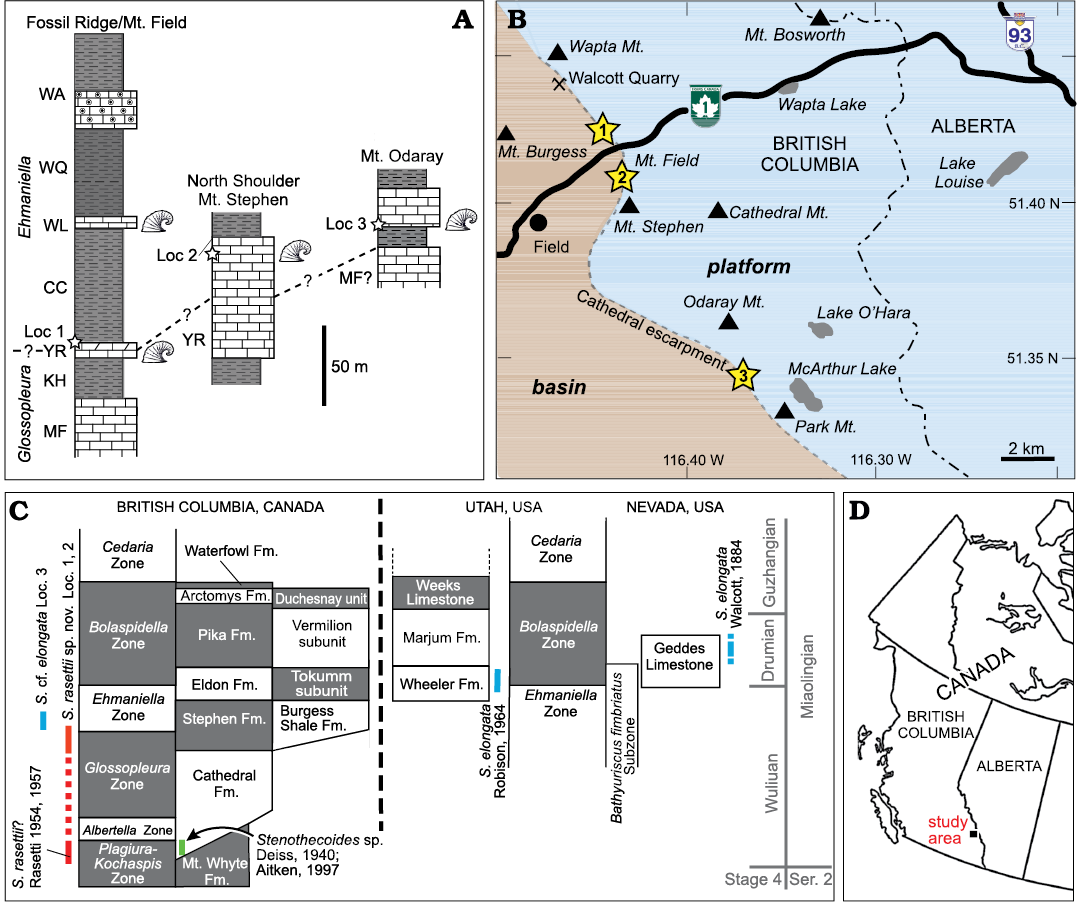
Fig. 2. Stratigraphic and geographic distribution of sample localities. A. Stratigraphic position of localities 1–3 indicated with stars. Thicknesses of units at Fossil Ridge/Mount Field from Fletcher and Collins (1998) and Mount Stephen northwest shoulder from Christopher J. Collom and PAJ (unpublished data); stratigraphic units after Collom et al. (2009), Odaray Mountain section from Streng et al. (2016). Helcionellid icons show known silicified assemblages. B. Geographic distribution of localities 1–3 relative to the Cathedral escarpment. C. Known stratigraphic distribution of stenothecoids in British Columbia, Utah, and Nevada. Stratigraphy modified from Johnston et al. (2009a). D. General location of study area in western Canada. Abbreviations: CC, Campsite Cliff Shale Member; Fm., Formation; KH, Kicking Horse Shale Member; Loc, Locality; MF, Monarch Formation; Mt., Mount or Mountain; S., Stenothecoides; WA, Wapta Member; WL, Wash Limestone Member; WQ, Walcott Quarry Shale Member; YR, Yoho River Limestone Member.
At Locality 1, collections were made on a tree-covered slope from planar thin–medium bedded limestone and from interbedded limestone-clast conglomeratic channels in the upper 3–4 metres of a 12 metre-thick carbonate unit (Fig. 2A), about 200 m down the mountain slope and along strike from the type section of the Yoho River Limestone Member. Some uncertainty remains around stratigraphic correlation of this section; Collom et al. (2009) interpreted this carbonate unit as equivalent to the Wash Limestone Member elsewhere, which occurs stratigraphically higher in the Burgess Shale Formation.
At Locality 2, the Yoho River Limestone Member (= “Bench Facies” of the Boundary Limestone of authors) is much thicker (100 m, McIlreath 1977; 47 m, Fletcher and Collins 1998; 76 m, Christopher J. Collom and PAJ, unpublished data); samples were collected from thin, planar-bedded carbonates 10.5 m below the upper contact (Fig. 2A). Samples from Locality 3 were recovered from a ca. 0.6 m thick carbonate bed at the base of a 24 m thick carbonate unit and 13 m above the base of the Burgess Shale Formation (Streng et al. 2016).
The Burgess Shale Formation varies markedly in thickness through its outcrop distribution, from 270 m thick in the type area (including localities 1 and 2) (Fletcher and Collins 1998) to only 80 m thick 60 km to the southwest at The Monarch (Johnston et al. 2009b). Thickness at Locality 3 is 150 m (Streng et al. 2016). Fletcher and Collins (1998) defined several members in the Burgess Shale Formation in the type area around Field, British Columbia, the four lowest members including, from oldest to youngest, the Kicking Horse Shale, Yoho River Limestone, Campsite Cliff Shale, and the Wash Limestone. However, these cannot be distinguished to the southeast (Johnston et al. 2009b), including the section at Locality 3 on Odaray Mountain.
The boundary of the Glossopleura Zone and the overlying Ehmaniella Zone in the type area has not been determined but is thought to be within the Yoho River Limestone (Fletcher and Collins 1998; Collom et al. 2009). At Odaray Mountain, the Glossopleura Zone is apparently not represented in the basinal facies, the equivalents of the Burgess Shale Formation here lying entirely in the Ehmaniella Zone (Streng et al. 2016), a pattern also noted in the type area at the Paradox Section at Fossil Ridge (Fletcher and Collins 1998). In adjacent platformal facies, Stenothecoides cf. elongata and Stenothecoides spp. are known from the Mount Whyte Formation (Walcott 1917a; Rasetti 1954; Fletcher and Collins 2003) and Stenothecoides? sp. and Stenothecoides sp. occur in the basal Cathedral Formation (Deiss 1940; Aitken 1997). Consequently, the known distribution of stenothecoids in the Chancellor Basin of southeastern British Columbia now extends from the Plagiura–Kochaspis Zone to the early Ehmaniella Zone (Fig. 2C).
Comparative material figured herein was collected from the stratotype of the Drumian Stage in the Drum Mountains of western Utah, from Locality 8 of Robison (1964). The material comes from near the top of the middle carbonate member between the FAD of P. atavus and the shales of the upper Wheeler Formation (Babcock et al. 2007).
Locality 3 yielded mostly articulated shells (>160) and only five isolated valves. By contrast, localities 1 and 2 yielded only a single articulated shell and several hundred isolated valves. No articulated shells were recovered from the processed Utah samples.
Material and methods
Material in the present study from the Burgess Shale Formation was collected during expeditions of the Royal Tyrrell Museum in 2001–2003 and Mount Royal University in 2006 (led by PAJ) and the Royal Ontario Museum in 2010 and 2012 (led by Jean-Bernard Caron). Material from the Wheeler Formation was collected by PAJ during fieldwork organized by Robert Gaines (Pomona College, Claremont, USA) in 2005. All specimens studied are silicified and were obtained by dissolution of limestone samples in 10% acetic acid (localities 1 and 2 and Utah samples) or 10% formic acid (Locality 3). Light microscope images were made with a stereo microscope and the imaging program Stream Start; these include some extended focus images (EFIs), which are produced using multiple images taken at different Z-levels and merged to into a single image, for increased depth of field. All light microscope images are of specimens coated with ammonium chloride sublimate. Selected specimens were coated with gold-palladium alloy and imaged using field emission scanning electron microscopes at Uppsala University (Zeiss Supra 35VP) and the University of Calgary (Philips XL-30). The studied collections are reposited at the Royal Tyrrell Museum (TMP) (localities 1 and 2, and Utah samples) and at the Royal Ontario Museum (ROMIP) (Locality 3).
Orientation and measurement: To aid description and measurement, Rozov (1984) provided a template for orienting stenothecoid valves, much of which is followed here; however, our new evidence for brachiopod affinities necessitates adjustments of Rozov’s scheme (Fig. 3). Most importantly, the apical end of the shell is here regarded as posterior and the abapical end anterior, as in brachiopods, and opposite that advocated by Rozov (1984), who followed the monoplacophoran-mollusc-orientation proposed by earlier authors. For measurement, we orient the valves following Pel’man (1985), with the apical end uppermost and the distal-most point on the anterior margin centered directly under, as in brachiopods (Williams et al. 1997). Valve length is the distance from the summit of the umbo to the distal-most point on the axial keel where it meets the anterior shell margin (Fig. 3A). The line defined by these two points is the “axial line” of Rozov (1984), and “valve axis” here. In some specimens, the tip of the right auricle (defined below) extends posteriorly to, or slightly beyond, the umbo (Figs. 4A2, 5E1, 6A). Valve width is the maximum distance measured perpendicular to valve length, and valve height is maximum inflation measured perpendicular to the commissural plane (Fig. 3A; Rozov 1984; Pel’man 1985).

Fig. 3. Orientation, measurements, and ridge zones in Stenothecoides rasettii sp. nov. A. Ventral valve, exterior, showing orientation for measurements. B. Ventral valve, interior, showing peripheral and axial ridge zones, and approximated body cavity. Abbreviations: ax, auricular axis; az, axial ridge zone; bc, body cavity; L, valve length; ll, left lobe; lx, lobe axis; pz, peripheral ridge zone; rl, right lobe; vx, valve axis; W, valve width.
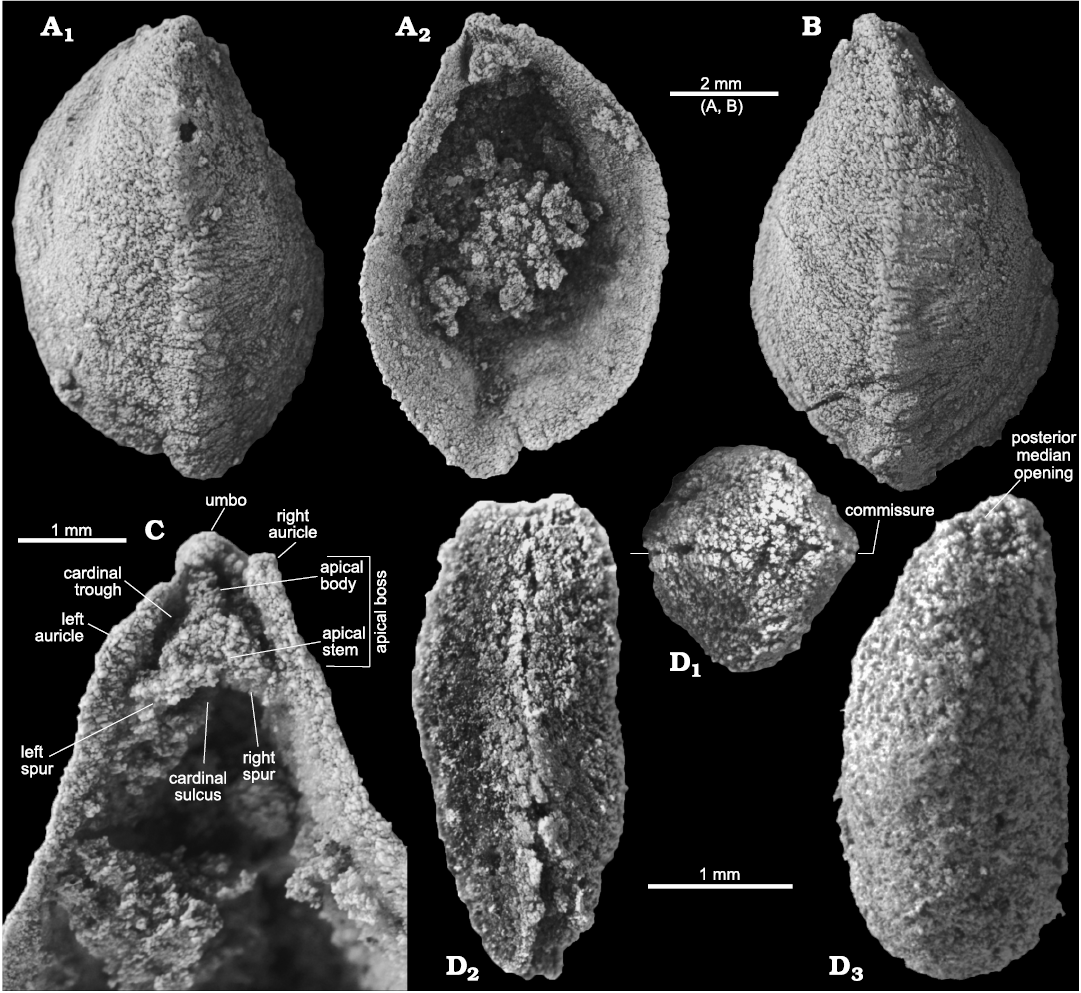
Fig. 4. Stenothecoid pan-brachiopod Stenothecoides rasettii sp. nov., middle Cambrian, Burgess Shale Formation, Yoho National Park, Canada, Locality 2 (A, B, D) and Locality 1 (C, D). A. TMP 2002.083.0176 (holotype), dorsal valve in exterior (A1) and interior (A2) views. B. TMP 2002.083.0177, ventral valve in exterior view. C. TMP 2008.024.1147, dorsal valve, internal apical area. D. TMP 2008.024.1122, articulated juvenile, dorsal/ventral (uncertain) valve in posterior (D1), lateral (D2), and oblique planar (D3) views; D1 and D3 show posterior median opening, D2 shows slightly sinusoidal commissure.
Articulated specimens (Figs. 4D, 7A, B) usually show one valve slightly more inflated than the other, which is here taken to be ventral by comparison with the typically more inflated pedicle (ventral valve) of brachiopods. Yochelson (1969) came to the same conclusion based on an inferred pleurothetic epibenthic habit with the more inflated valve undermost. However, isolated valves cannot be readily identified as dorsal or ventral from relative convexity alone. Here, the asymmetry of the valves is useful. Assuming consistent asymmetry of the opposing valves in Stenothecoides, and with reference to figured articulated specimens in Yochelson (1969), Peel (1988) found that in external plan view the dorsal valve curves anticlockwise during growth and the ventral valve curves clockwise. Growth direction is evident from the arcuate path of the keel extending from the umbo to the anteriormost margin. In a few specimens the keel follows a somewhat sinusoidal path (also noted by Rozov 1984), but growth direction of the anterior two-thirds seems consistent with the inferred valve side (Fig. 6D2). In some specimens the keel is more or less straight (Fig. 6E, G) and/or weakly developed, and consequently the valve is not easily sided, although other shell features can be helpful (see below). By analogy with terminology used for bivalved molluscs when describing the curvature of the beak (proso-, ortho-, opisthogyrate; e.g., Cox 1969), the term orthogyrate is used in species descriptions herein to indicate a beak of stenothecoids that is neither pointing left nor right, and sinistrogyrate is introduced to indicate a curved beak that points in the direction of the anatomical left side of the shell.
In external plan view, the keel divides the valve into right and left sides, or lobes, which, in the majority of Burgess Shale specimens, are asymmetrically disposed, with the left lobe positioned slightly more anteriorly than the right. A line drawn through the lateral points of maximum curvature on the valve outline is termed the lobe axis (Fig. 3A). It intersects the valve axis and forms an obtuse angle on the left lobe. The lobe axis is inclined in the same direction, but usually less steeply than a line connecting the posterior tips of the right and left auricles (auricular axis, Fig. 3A). The auricular and lobe axes therefore provide a ready clue as to the valve side represented—the axes dip to the right in the dorsal valve and to the left in the ventral valve, in external plan view.
As noted by Peel (1988), intraspecific variability of stenothecoid species can be considerable, and the Burgess Shale stenothecoids are no exception. Characterization of the Burgess Shale stenothecoids is complicated by slight to moderate tectonic deformation of many specimens recovered from Locality 1. Deformation there is demonstrable from co-occurring brachiopod valves, many of which show some degree of asymmetry. At that locality, deformation is easily recognized in some stenothecoids owing to conspicuous creases in the shell, or that the commissure departs significantly out of a single plane. However, for other specimens (Fig. 6A, D, E, G), it is difficult to know whether the shell outline has been tectonically altered. Consequently, morphometric characterization of the Burgess Shale stenothecoids, discussed below, is restricted to specimens from localities 2 and 3 where deformation is not evident.
Systematic palaeontology
Clade Pan-Brachiopoda Carlson and Cohen, 2020
Class? Stenothecoida Yochelson, 1969
Diagnosis (emended from Aksarina 1968 for class Probivalvia).—Shell organocalcitic, bivalved, slightly or moderately ventribiconvex. Valves moderately to strongly inequilateral; length typically exceeding width, rarely subequal. Commissure planar to gently sinusoidal in lateral view. Beaks orthogyrate to sinistrogyrate; growth hemiperipheral to mixoperipheral. Interior surface of valves with a serial set of furrows or elliptical to circular depressions on both sides of the valve midline, and typically with a second finer set of transverse alternating furrows and ridges around the valve perimeter.
Remarks.—Yochelson (1968, 1969) proposed the class Stenothecoida but without a formal diagnosis. Aksarina (1968) provided a diagnosis for class Probivalvia (= class Stenothecoida), which is revised here based on subsequent published studies and on new information from the Burgess Shale specimens described herein. Assignment of stenothecoids to the rank of class is provisional pending cladistic analyses with other pan-brachiopods.
Emendation of Aksarina’s (1968) diagnosis of the class Probivalvia (a synonym of Stenothecata), is necessary here because: (i) Aksarina (1968) assumed the anatomical orientation of the stenothecoid shell was like that of the Bivalvia and so characterized the beaks as prosogyrate (i.e., inclined toward the shell anterior); (ii) that diagnosis did not include the internal peripheral ridge zone, which is widespread, and possibly ubiquitous among stenothecoid taxa; and, (iii) Aksarina (1968) assumed the internal axial ridges and grooves were muscle scars, an interpretation deemed unlikely here, as noted above.
Order Cambridioidea Horný in Knight and Yochelson, 1958
Family Cambridiidae Horný, 1957
Genus Stenothecoides Resser, 1938
Type species: Stenotheca elongata Walcott, 1884, from the upper beds of the Geddes Limestone, Eureka district, Nevada; middle Cambrian. Walcott (1884: 23) described the stratigraphic occurrence of the type material as “Prospect Mountain Group, in the limestone just beneath the Secret Cañon shale on the west side of Secret Cañon, Eureka District, Nevada.” Later, in the same publication, he states the unit below the Secret Canyon Shale as Prospect Mountain Limestone (which contains Stenotheca elongata in its upper beds) (Walcott 1884: 284–285). This unit is the Eldorado Limestone of Walcott (1912: 140) and the Eldorado Formation of Walcott (1917b: 6). Rasetti (1954) and Robison (1964) cite the Eldorado Limestone for the type material. However, the Eldorado Limestone had earlier been divided into the lower Eldorado Dolomite and the upper Geddes Limestone (Wheeler and Lemmon 1939). The Eldorado Dolomite is unfossiliferous (Nowlan et al. 1956) and as Walcott (1884) stated “just beneath” the Secret Canyon Shale, and “upper beds” of the Eldorado Limestone, the Geddes Limestone is the most plausible unit for the occurrence of the type species. According to McCollum and Miller (1991), the unit is early Bolaspidella Zone in age (Fig. 2C).
Diagnosis (emended from Robison 1964 and Koneva 1979b).—Shell outline elongate suboval, subrhombic, or pear-shaped; tapered and arcuate posteriorly; moderately to strongly inequilateral; typically with weak to strong keel extending from umbo to extremity of anterior shell margin. Apical area with prominent apical boss developed internally in both valves. Both valves with semicircular to somewhat irregular emargination (posterior median opening) of the commissure below the apex forming an exit for a presumed pedicle. Shell exterior with commarginal growth lines, growth rugae, and ultra-fine radial elements.
Remarks.—The posterior median opening below the apex is well developed in species of Stenothecoides discussed below. This structure may also be present in some other genera of stenothecoids including Cambridium and Bagenovia (e.g., Koneva 1979b: pl. 1: 7d, pl. 3: 7c), but cannot be certainly established from published figures and descriptions.
Stenothecoides rasettii sp. nov.
Figs. 4–6, 11, 12A–C.
Zoobank LSID: urn:lsid:zoobank.org:act:5AAA3CC9-4799-4AF2-BE D6-7EFA273897CC
?1954 Stenothecoides cf. S. elongata (Walcott); Rasetti 1954: 63, pl. 11: 6–10, pl. 12: 1–4.
?1957 Stenothecoides cf. S. elongata (Walcott); Rasetti 1957: 972, pl. 122: 1, 2.
Etymology: After Franco Rasetti (1901–2001), physicist and paleontologist.
Type material: Holotype, dorsal valve (TMP 2002.083.0176, Fig. 4A, B). Paratypes, 14 isolated valves (TMP 2002.083.0177–0190) from the type locality and horizon.
Type locality: Northwest shoulder of Mount Stephen (Locality 2), near Field, British Columbia, Canada.
Type horizon. Carbonate bed about 10.5 m below upper boundary of Yoho River Limestone Member, Burgess Shale Formation, middle Cambrian.
Referred specimens.—Type material and 30 specimens (isolated valves and one articulated specimen) (TMP 2008.024.1121–1150) from localities 1 and 2, near Field, British Columbia, Canada, Yoho River Limestone Member, Burgess Shale Formation, middle Cambrian.
Diagnosis.—Shell outline suboval to pear-shaped, slightly to strongly inequilateral; valve length/width averages 1.6. Anteroposterior keel weakly to strongly developed. Lobe axis typically 95–100°.
Description.—As noted earlier, specimens from Locality 1 may show slight to moderate tectonic strain and consequently description of the shell outline is based on Locality 2 samples unless stated otherwise.
Shell exterior: The shell outline varies from pear-shaped to elongate-suboval. Rare specimens show a constriction of the shell outline posteriorly (Fig. 6D, G). Most specimens bear a median keel that runs the length of the valve. The keel typically follows an arcuate path early in ontogeny and commonly straightens in later growth stages. A slight protrusion of the valve outline may occur where the keel meets the anterior margin (Fig. 4A), but in some specimens the keel fades anteriorly, the anterior margin being broadly rounded (Fig. 6B, C). Rare specimens show a sulcus accompanying the keel (Fig. 6A). Adult valves, viewed anteriorly, commonly show a sinus where the keel meets the anterior valve margin; the sinus extends well outside the plane of the commissure and produces a configuration reminiscent of the fold in rhynchonelliform brachiopods (Fig. 5D2). However, the sinus occurs in both dorsal and ventral valves (Fig. 5E2, I). Strangely, no specimen is available that shows a corresponding protrusion of the shell in opposition at this position, raising the interesting possibility that a permanent gape was produced. The single known articulated juvenile, though damaged anteriorly, appears not to have a gape (Fig. 4D2). While a gape may have been produced in adult specimens of S. rasettii sp. nov., it was not shared by all members of the family or even the genus. Articulated specimens of Stenothecoides cf. elongata are abundant at Locality 3, but none show an anterior gape, nor do articulated specimens of Bagenovia kazakhstanica Koneva, 1976, and Stenothecoides bella Koneva, 1979b (Koneva 1979b: pl. 3: 1 and pl. 5: 1; note that the grammatical gender of many of Koneva’ s [1979a, b] species epithets are corrected herein, i.e., from masculine to feminine, e.g., Stenothecoides dubia Koneva, 1979b [nom. correct. pro S. dubius] in accordance with the International Code of Zoological Nomenclature, Article 30.1.4.4.).
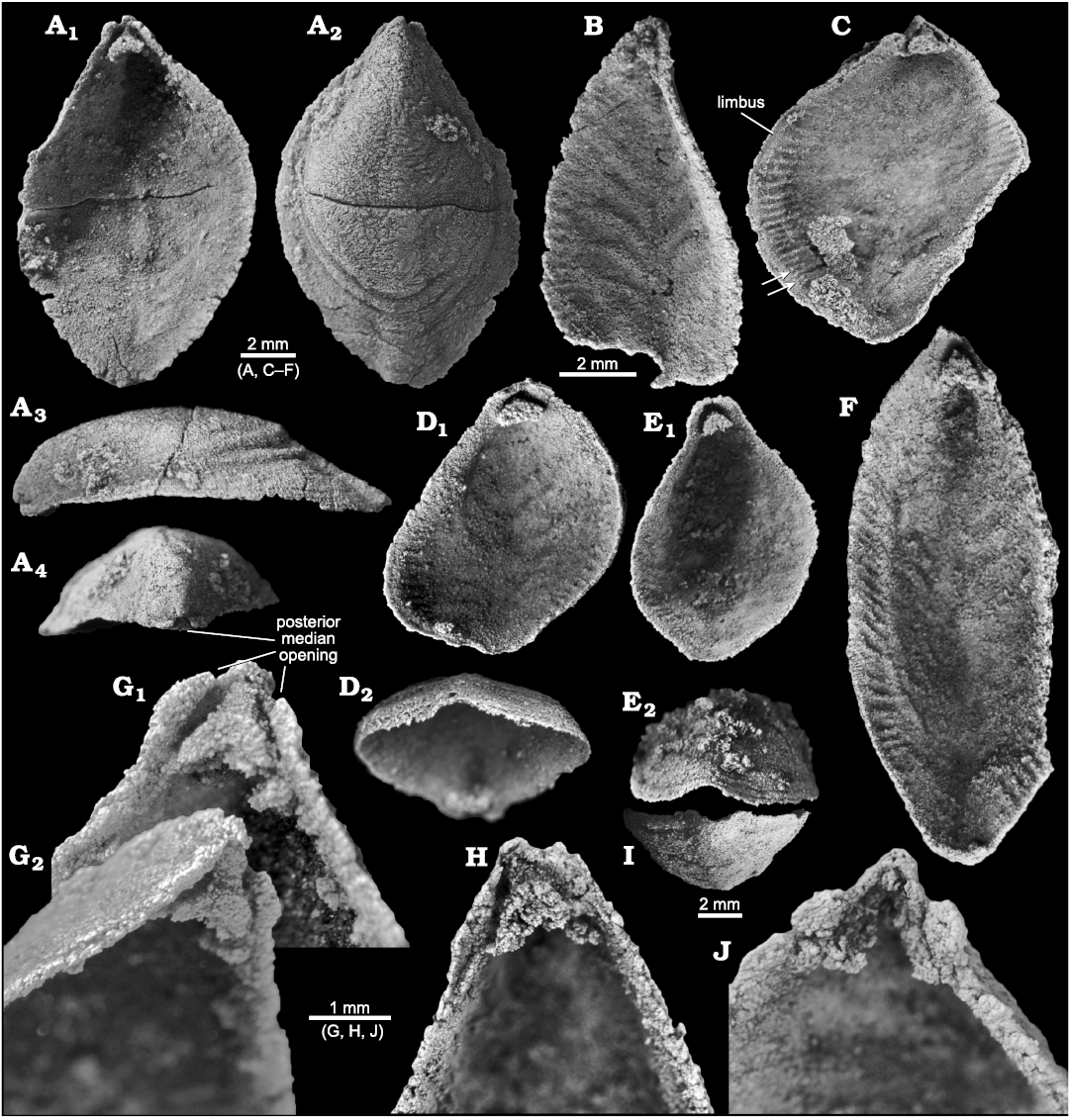
Fig. 5. Stenothecoid pan-brachiopod Stenothecoides rasettii sp. nov., middle Cambrian, Burgess Shale Formation, Yoho National Park, Canada, Locality 2 (A, G) and Locality 1 (B–F, H–J). A. TMP 2002.083.0178, dorsal valve in interior (A1), exterior (A2), right lateral (A3), and posterior (A4) views. B. TMP 2008.024.1138, ventral valve in interior view. C. TMP 2008.024.1133, dorsal valve in interior view, arrows show possible bifurcated peripheral ridges. D. TMP 2008.024.1143, dorsal valve in interior (D1) and anterior oblique (D2) views. E. TMP 2008.024.1144, dorsal valve in interior (E1) and anterior (E2) views. F. TMP 2008.024.1134 , ventral valve, interior view. G. TMP 2002.083.0177 (same specimen as Fig. 4B), ventral valve in interior (G1, magnified) and oblique (G2) views. H. TMP 2008.024.1135, dorsal valve in interior view. I. TMP 2008.024.1121, ventral valve, anterior view. J. TMP 2008.024.1136, ventral valve in interior view, showing detached apical boss and remnant apical stem and cardinal troughs.

Fig. 6. Stenothecoid pan-brachiopod Stenothecoides rasettii sp. nov., middle Cambrian, Burgess Shale Formation, Yoho National Park, Canada, Locality 1 (A, D, E, G, H) and Locality 2 (B, C, F). A. TMP 2008.024.1140, ventral valve in exterior view. B. TMP 2002.083.0179, ventral valve in exterior (B1) and interior (B2) views. C. TMP 2002.083.0180, dorsal valve in exterior (C1) and interior (C2) views. D. TMP 2008.024.1145, dorsal valve in interior (magnified, D1) and exterior (D2) views. E. TMP 2008.024.1141, ventral valve in interior (E1) and exterior (E2) views. F. TMP 2002.083.0181, dorsal valve in exterior (F1) and interior (F2) views. G. TMP 2008.024.1146, dorsal valve in posterior (G1) and exterior (G2) views. H. TMP 2008.024.1139, ventral valve in interior view.
The lobe axis in S. rasettii sp. nov. intersects the valve axis to form an obtuse angle on the left lobe, commonly about 95–100° (Fig. 3A). Where the angle of the lobe axis and the valve axis departs significantly from 95–100°, post-burial deformation is suspected, as evident in many specimens from Locality 1.
The highly variable shell outline of S. rasettii sp. nov. could be taken as evidence for multiple species in the Locality 2 sample; however, superimposed shell outlines and shell measurements show gradational variation and provide no logical means to distinguish subsets. Peel (1988) documented similarly wide variation in shell outline in Stenothecoides groenlandica Peel, 1988, from the middle Cambrian of North Greenland, which differs from S. rasettii sp. nov. in having its maximum width set more posteriorly. Stenothecoides elongata (Walcott, 1884) is proportionately longer relative to width (Figs. 8–10), with a more equilateral shell and, correspondingly, a lobe axis that approaches 90°. Lower Cambrian Stenothecoides knightii Yochelson, 1969, is more rounded posteriorly, and Stenothecoides poulseni Resser, 1938, more rhombic in outline. Koneva (1979a, b) named several new species of Stenothecoides from the lower–middle Cambrian of Kazakhstan and Uzbekistan. These differ from the type series of S. rasettii sp. nov. in having a narrower posterior margin and umbo (Stenothecoides bella Koneva, 1979b, Stenothecoides carinata Koneva, 1979b, Stenothecoides obliqua Koneva, 1979b, Stenothecoides rigida Koneva, 1979b, Stenothecoides dubia Koneva, 1979b, Stenothecoides rara Koneva, 1979a, Stenothecoides triangulata Koneva, 1979a) and/or more inflated and/or more consistently orthocline valves, typically with a weaker keel (Stenothecoides bicarinata Koneva, 1979b, Stenothecoides curva Koneva, 1979b, Stenothecoides nedovisini Koneva, 1979b, Stenothecoides media Koneva, 1979a, Stenothecoides proxima Koneva, 1979a, Stenothecoides tamdensis Koneva, 1979a, Stenothecoides variabilis Koneva, 1979a). Some specimens from Locality 1 assigned to Stenothecoides rasettii sp. nov. (e.g., Fig. 6E) show a narrow posterior margin as in, for example, Koneva’s (1979b) Stenothecoides bella, and Rasetti’s (1954) Stenothecoides labradorica Resser, 1938 (lower Cambrian, Labrador, Canada), but as noted earlier, Locality 1 shows tectonic strain and so shell outlines there are unreliable. Stenothecoides rasettii sp. nov. is most similar in outline to Rasetti’s (1954, 1957) Stenothecoides cf. elongata from the Mount Whyte Formation (Fig. 9) and the two are questionably regarded here as conspecific, hinge structure in the latter being unknown. Rasetti’s (1954, 1957) material is similarly variable in outline and there may be more than one species present. Specimens from the Mount Whyte Formation assigned to Stenothecoides spp. in Fletcher and Collins (2003) are more nearly circular to broadly elliptical in outline (Fig. 10) and are excluded from synonymy here.
Posterior margin: The shell of S. rasettii sp. nov. tapers posteriorly with a narrow posteromedian area owing to the acute apical angle. The beak occurs above the plane of the commissure, although it is well below the point of maximum inflation of the valve, which occurs much farther anteriorly, about one third to one half the distance from the apex to the posterior margin (Fig. 5A3). Valve growth was mixoperipheral.
Both valves show an emargination of the posterior margin below the beak that, when juxtaposed in articulated specimens, produces a circular opening, here interpreted as a probable egress for the pedicle (S. rasettii sp. nov., Figs. 4D3, 5A4, G1, 6B2; and S. cf. elongata, Fig. 7B2, C2). We term this opening a “posterior median opening” following Holmer et al. (2018), who described a similar configuration in the primitive rhynchonelliformean Nisusia. The posterior median opening is more or less symmetrically disposed below the beak in most specimens of S. rasetti (Fig. 5A4), but in some it is irregular and displaced slightly to the anatomical right of the beak (Fig. 6B2, F1, H), and more rarely to the anatomical left (Fig. 6D1). The opening may be obvious in internal plan view, but in most specimens it is evident only in posterior view, where the posteriormost valve margin can be seen to depart from the commissural plane to form the margin of the opening. The opening appears to be about equally shared by both valves and is developed even in the smallest specimens available, presumably juveniles (Fig. 4D). Rare specimens seem to lack a posterior median opening (Fig. 6G). In these, the posteriormost margin is extended slightly beyond the beak, in a way comparable to the “lip” in the stenothecoid Katunioides akbashinensis (Pel’man 1985: fig. 6). A growth line at this position in the Burgess Shale example (Fig. 6G1), may mark the former margin of the opening, which was secondarily closed with shell material. We speculate that such specimens may represent individuals that became detached from their pedicle holdfast, perhaps early in ontogeny, and the posterior median opening was secondarily sealed with shell material.
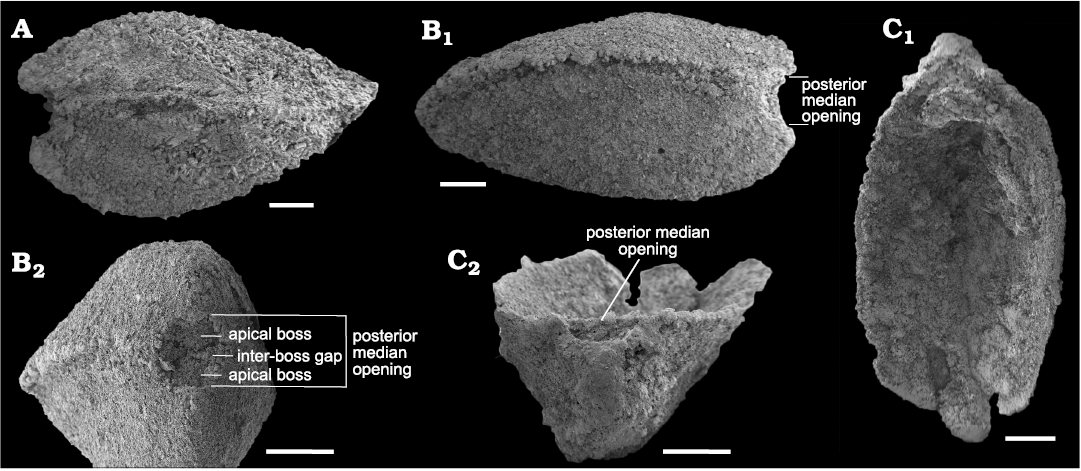
Fig. 7. SEM micrographs of stenothecoid pan-brachiopod Stenothecoides cf. elongata, middle Cambrian, Burgess Shale Formation, Kootenay National Park, Canada, Locality 3. A. ROMIP 66248, articulated shell in right lateral view. B. ROMIP 66249, articulated shell in left lateral (B1) and posterior (B2) views. C. ROMIP 66250, ventral valve in interior (C1) and posterior (C2) views. Scale bars 500 µm.
Some specimens show a finished valve edge extending across the posterior margin of the posterior median opening (Fig. 5E1), but in most, the posteriormost margin of the valve is interrupted by the opening leaving little or no shell material between the opening and the beak (Fig. 5G1, H), likely resulting from resorption around the margin of the opening to accommodate enlargement of the pedicle with growth.
Auricles are variably present on one or both sides of the apical area as slight protrusions of the shell outline that are sometimes thickened (Fig. 5A, G1, J). Some specimens lack auricles altogether (Fig. 6C). It is difficult to determine whether this variation is phenotypic or a result of varying degrees of damage on the seafloor prior to burial or to incomplete silicification.
Valve interior: Several authors have noted transverse ridges and furrows around the internal margin of the shell in Stenothecoides and in some other stenothecoid genera, and in at least some species, a second set of larger ridges and furrows is preserved emanating from the midline (Rasseti 1954, 1957; Horný 1957; Koneva 1979b). These structures are evident in both dorsal and ventral valves of S. rasettii sp. nov. (Figs. 3B, 5B–D, F). Following Rozov (1984), the outer set is here designated the peripheral ridge zone (= mantle-edge ridges of Yochelson 1969) and the inner set, the axial ridge zone (Fig. 3B). The furrows and ridges of the peripheral ridge zone are better defined and more commonly preserved than those of the axial zone, which are larger, more subtly developed, rarely preserved, and, where present, evident only with oblique lighting.
Furrows and ridges of the peripheral zone are oriented normal to, or at a high angle to, the valve margin and diminish before reaching the valve margins, leaving a slightly flattened valve edge reminiscent of the limbus in inarticulate brachiopods (Fig. 5C, D1). In some specimens (Fig. 5C), peripheral furrows can be traced well into the valve interior but are weak there and presumably represent the growth tracts of furrows at earlier grow stages. In the posterior quarter of the valve, the peripheral zone weakens but can be traced almost to the apical area. Anteriorly the peripheral zone terminates before reaching the junction of the keel and the anterior valve margin (Fig. 5F). No specimens are sufficiently preserved to provide an exact count of ridges and furrows in these zones. Extrapolating from well-preserved segments of the peripheral zone, we estimate about 30–35 furrow-ridge pairs in each of the anatomical left and right sides of the peripheral zone in adult shells (Fig. 5F). We estimate a similar number in Utah specimens of S. elongata (Fig. 8A), although none is complete enough for an exact count. Rasetti (1954) recorded 15–18 in one specimen of S. cf. elongata. Illustrations in Horný (1957: pl. 4: 1) show more than 50 per side in Cambridium nikiforovae Koneva, 1979b.
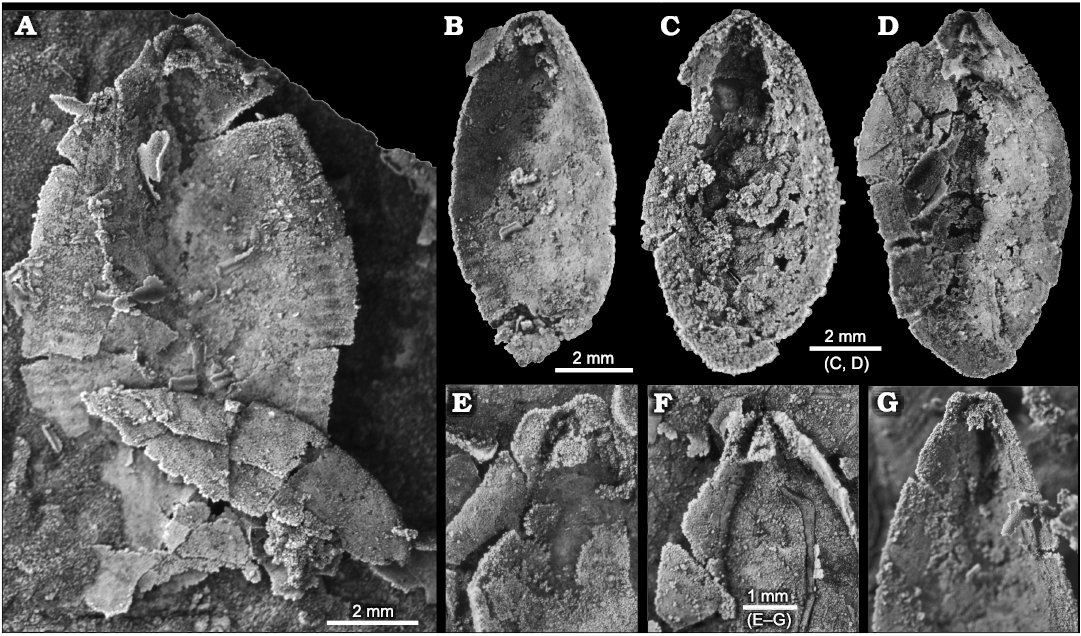
Fig. 8. Stenothecoid pan-brachiopod Stenothecoides elongata (Walcott, 1884), middle Cambrian, Drumian Stage stratotype, Drum Mountains, western Utah (USA), Locality 8 (Robison 1964), internal shell features. Dorsal vs. ventral valves uncertain. A, B. TMP 2021.022.0001 (A) and TMP 2021.022.0002 (B), valves preserving peripheral furrows; note in A a prominent cardinal sulcus. C. TMP 2021.022.0003, valve interior, showing a cardinal pseudosocket. D. TMP 2021.022.0004, valve interior, preserving a nearly symmetrical apical area and conspicuous posterior median opening. E–G. Variation of apical areas of valve interiors. TMP 2021.022.0005 (E), TMP 2021.022.0006 (F), TMP 2021.022.0007 (G).
In S. rasettii sp. nov., furrows in the left and right axial zones together form a pinnate pattern, with the individual “leaves” apparently opposing although some seem slightly offset (Fig. 5B). Pairs of leaves on opposite sides of the midline diverge posteriorly producing chevrons with the point of the chevrons directed anteriorly. Chevrons in the posterior half of the valve form lower angles with the valve axis than those anteriorly. We estimate six or seven chevrons produced in the axial zone. Bagenovia spp. show a similar number (Koneva 1979b: pls. 2, 4).
The axial zone was not observed in the posterior quarter of the valve floor. Furrows and ridges of the axial zone arise from the valve midline, which corresponds to the external keel. The keel is typically expressed internally as a weak medial trough (Fig. 5B; rarely with central ridge, Fig. 5A1), though often obscure, and where present does not extend into the posterior third of the valve (Fig. 6E1).
Internal apical area: Except for brief comments in Robison (1964), internal features of the apical area of Stenothecoides have not previously been described. Consequently, new morphologic terms are introduced for new details revealed in the Burgess Shale material (Fig. 4C).
A prominent feature of the apical area of S. rasettii sp. nov. is the tooth-like trigonal structure, here termed the apical boss, near the valve apex. The apical boss expands anteriorly (the apical body) and narrows posteriorly (the apical stem), attaching to the valve below the beak and along its lateral sides (Fig. 4C). A break in slope typically occurs at the junction of the apical stem and body, with the apical stem more recessed than the apical body (Figs. 4C, 5G2, 6H). The anatomical left anterior corner (left spur) of the apical boss typically extends further anteriorly than the anatomical right anterior corner (right spur) such that the apical boss appears tilted to the right in ventral valves and to the left in dorsal valves, when viewed internally in plan view (Figs. 4C, 5G, H). A narrow trough (cardinal trough, Fig. 4C) is developed on either side of the apical boss, separating the boss from the valve margin. The left and right troughs are commonly interrupted across the apex by the apical stem, but in some specimens the apical stem descends to the valve floor rather than to the apex, with the result that the troughs are joined across the posterior edge of the apical boss to form an arcuate groove (Fig. 5D1, E1). The anterior edge of the apical boss usually shows a broad embayment, here termed the cardinal sulcus (Figs. 4C, 5A1), although in some specimens the anterior edge is relatively straight (Fig. 4A2), irregular (Fig. 6F1, H) or convex (Fig. 6C2). It is unclear to what extent differing degrees of silicification are responsible for these variances. Some specimens of S. elongata also show a cardinal sulcus (Fig. 8A). In S. rasettii sp. nov., the apical boss generally appears more robust than in S. elongata.
Remarks.—Although stenothecoids are commonly affiliated with molluscs (e.g., Yochelson 1969), they have not appeared in the many studies of shell microstructure in early molluscs (e.g., Runnegar 1985; Kouchinsky 2000; Vendrasco et al. 2010). To date, details of shell structure in stenothecoids are limited to an inferred composition of low Mg-calcite (Zhuravlev and Wood 2008). Some specimens of S. rasettii sp. nov. display newly observed microstructural details replicated in silica that are reminiscent of the secondary shell layer of organocalcitic brachiopods.
Silicification of stenothecoids at the study localities typically produces a grainy and/or fibrous texture on the valve exterior with growth lines and rugae poorly expressed (Figs. 4A, B, 5A2, 6E2). Of particular interest is the fibrous structure comprising subparallel silica crystallites, which are best developed in specimens from Locality 1 (Fig. 11). Locality 2 preserves only rare specimens having aligned crystallites, and specimens of S. cf. elongata from Locality 3 are mostly coarsely silicified and lack aligned crystallites. Articulate brachiopods in the same beds show comparable preservational variants. At localities 1 and 2, individual brachiopods show silica crystallites arranged in what appears to be a siliceous facsimile of the originally calcitic fibres of the secondary shell layer (Fig. 12F, G). In these specimens, the crystallites are slightly inclined relative to the commissural plane with the result that the crystallites are imbricated. Viewed externally, crystallites overlap their more proximal neighbour, and viewed internally they overlap their more distal neighbour, as is the habit for secondary shell calcite fibres (Williams 1997). S. rasettii sp. nov. specimens with fibrous texture commonly show a similar arrangement, with the distal tips of radially to obliquely arranged acicular crystallites inclined or subparallel to the outer shell surface (Figs. 11A3, C, 12C).
Seven crystallites measured in a specimen of the articulate brachiopod, Tomteluva sp., from Locality 1 (Fig. 12F), ranged in diameter from 24.7–38.95 μm with a mean of 31.76 μm. A specimen of S. rasettii sp. nov. (Fig. 11C) from the same beds showed crystallite diameters (n = 12) varying from 14.84–41.92 μm and averaging 27.02 μm. In both instances, average crystallite diameters exceed fibre diameters in living brachiopods; Ye et al. (2018) recorded a range of maximum fibre diameter of about 5–30 μm with an average of 12.38 μm for six living species. Crystallites in S. rasettii sp. nov. and associated articulate brachiopods likely represent amalgamations of several calcite fibres. Longitudinal striae on some crystallites may represent edges of once separate fibres (Fig. 12C, F2). Five striae measured in an SEM of S. rasettii sp. nov. (Fig. 12C) ranged from 4.8–9.2 μm in width.
Silicification is normally destructive to shell microstructures; however, silica facsimiles of original fabric are sometimes preserved in brachiopods (Holdaway and Clayton 1982; Daley and Boyd 1996; Sun and Baliński 2008). In these examples, silicification may focus on the space occupied by the protein sheath that surrounded individual fibres producing a honeycomb texture, or silicification may replace the fibres themselves. In a study of silicified Mississippian brachiopods, Daley and Boyd (1996) found that, while replacement silica follows the orientation and shape of secondary shell carbonate fibres, individual replacement quartz crystals do not necessarily correspond to single calcite fibres, a pattern evidently repeated in S. rasettii sp. nov. and accompanying articulates.
These comparisons invite a simple interpretation of the fibrous microstructure in S. rasettii sp. nov. as matching the secondary shell layer in early calcareous articulates, which display, as also most of their descendants, a two-layered mineralized shell: a largely featureless outer primary layer composed of amalgamated calcite rhombs underlain by a secondary layer of either fibrous or laminar structure (Williams 1990, 1997). The primary layer, which may have been thin and loosely mineralized, is not typically preserved in fossil brachiopods (Williams 1968, 1997), whereas the secondary layer, which also forms all internal features, is frequently preserved displaying original ultrastructural details. Among these, a fibrous secondary layer with individual fibres inclined outwards by about 10° is common among many families of calcareous brachiopods including the early Cambrian chileid Kotujella and kutorginid Nisusia (Williams 1968, 1990; Popov and Williams 2000).
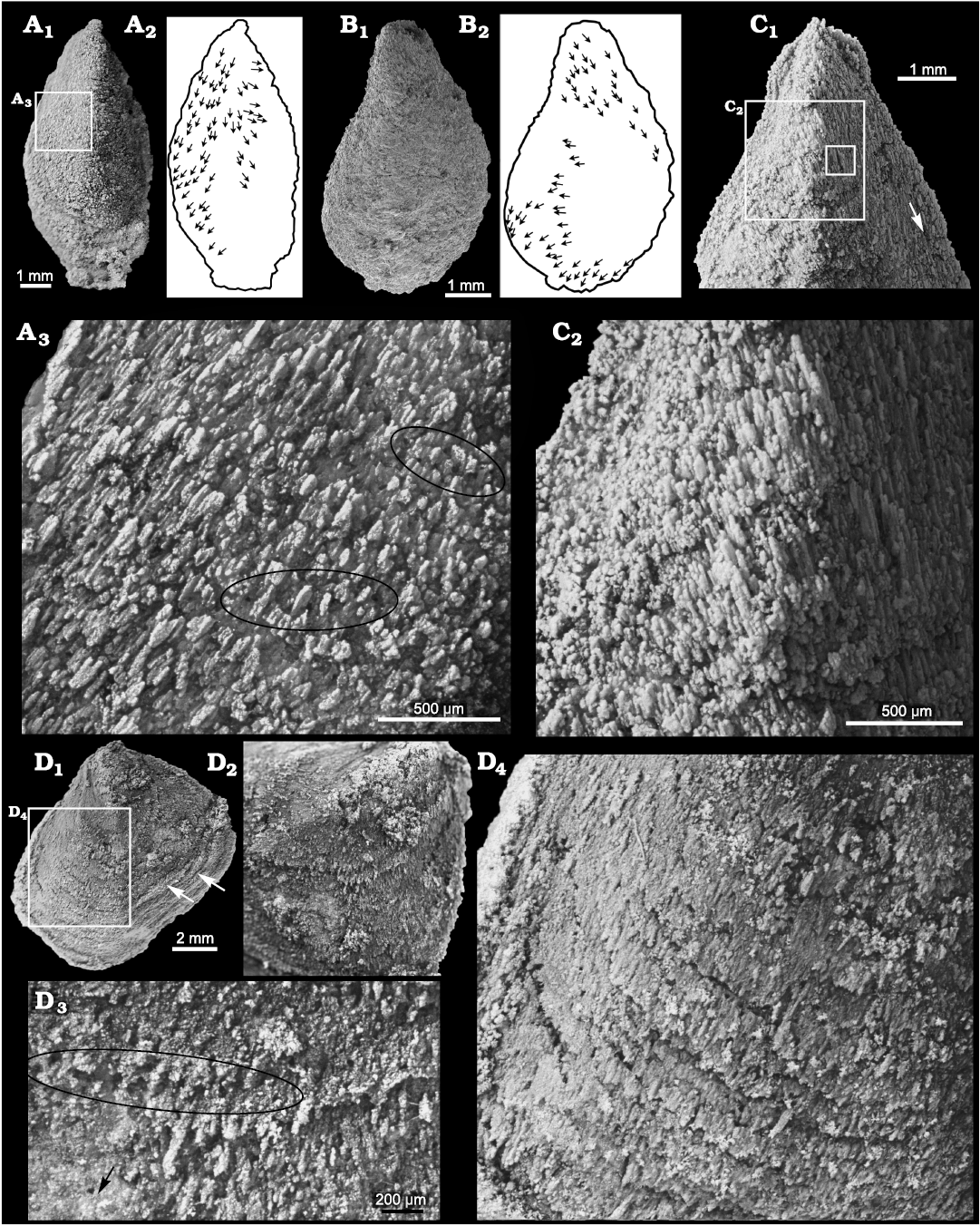
Fig. 11. Stenothecoid pan-brachiopod Stenothecoides rasettii sp. nov., middle Cambrian, Burgess Shale Formation, Yoho National Park, Canada, Locality 1. A. TMP 2008.024.1148, dorsal valve (A1) and silhouette (A2), with arrows showing long axes of silica rods; detail showing holes (some highlighted in black ellipses) and imbricated silica rods (A3). B. TMP 2008.024.1149 (SEM image), ventral valve (B1) and silhouette (B2), with arrows showing long axes of silica rods. C. TMP 2008.024.1141 (same specimen as in Fig. 6E), ventral valve in exterior view (C1), with arrow showing fibres oriented nearly parallel to valve margin; detail showing imbricated silica rods (C2); inner box is enlarged in Fig. 12C. D. TMP 2008.024.1137, a distorted ventral valve (D1), arrows show creases in shell indicating tectonic strain; umbonal area showing radiating silica rods (D2); detail of shell surface showing row of holes (ellipse) (D3), arrow shows direction of shell margin; detail showing imbricate lamellae on valve surface (D4).
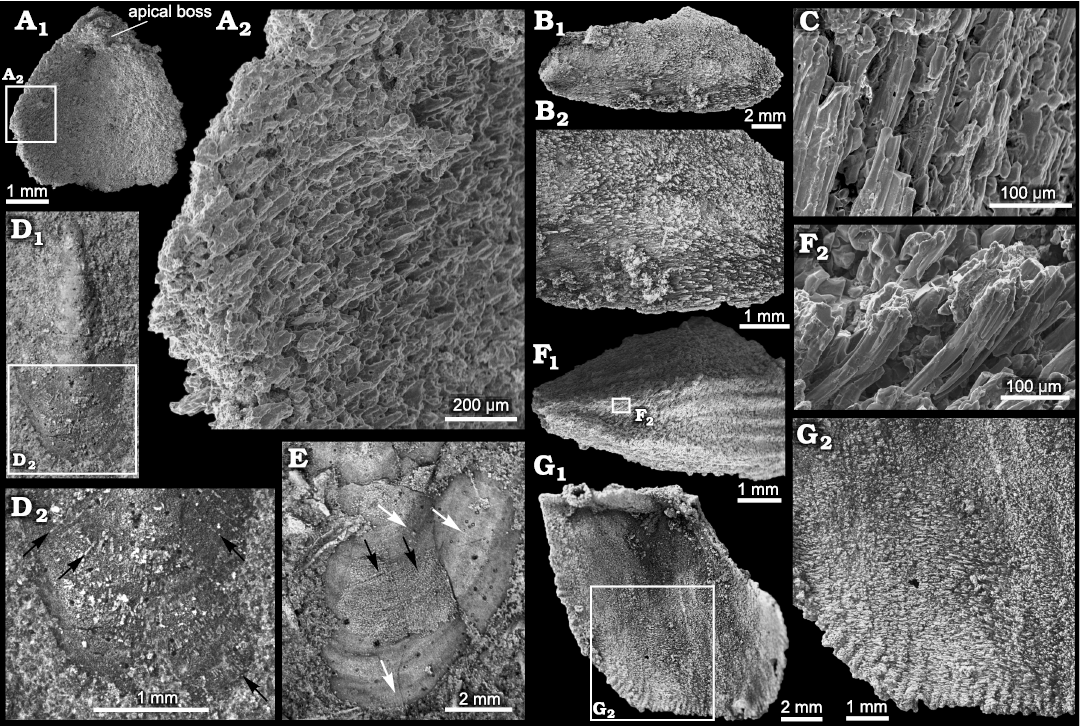
Fig. 12. Microstructure in the stenothecoid pan-brachiopod Stenothecoides spp. and co-occurring rhynchonelliformean brachiopods. A–C. Stenothecoides rasettii sp. nov., middle Cambrian, Burgess Shale Formation, Yoho National Park, Canada, Locality 1. A. TMP 2008.024.1142, fragmentary valve in interior view (A1), detail showing silica rods imbricated and inclined toward valve margin (A2). B. TMP 2008.024.1150, ventral valve in exterior view (B1), showing silica rods oriented with proximal ends of rods overlapping distal ends of preceding rods in posterior third of valve, but seemingly reversing orientation in posterior third of valve (B2). C. TMP 2008.024.1141 (same specimen and valve area as in Fig. 11C1, inner box) detail showing silica rods. D, E. Stenothecoides elongata (Walcott, 1884), Drumian Stage stratotype, Drum Mountains, western Utah (USA), Locality 8 (Robison 1964), external shell features. D. TMP 2021.022.0008, ventral(?) valve in exterior view (D1), detail of anterior end (D2), arrows show incompletely silicified radial rods. E. TMP 2021.022.0009, dorsal(?) valve, anterior end showing silicified outer shell surface with fine radial elements extending across growth varices (black arrows) and apparently shorter radial rods in valve sublayers (white arrows). F, G. Rhynchonelliformean brachiopods, silicified microstructure, Burgess Shale Formation, Canada, Locality 1. F. Tomteluva sp., TMP 2008.024.1151, silicified shell in exterior view (F1), posterior end is broken; anterior is to the right; detail showing orientation of silica rods (F2), anterior is to the left. G. Fragmentary nisusiid, TMP 2008.024.1152, silicified shell in interior view (G1), detail showing imbricated silica rods (G2). A, C, F, SEM images and B, D, E, G, light microscope images.
However, an important caveat here is that some specimens of S. rasettii sp. nov. show some or most crystallites inclined in apparently the opposite direction, with crystallites on the shell exterior overlapping their distal neighbour (Fig. 12B2). It is difficult to envisage a mode of secretion by a mantle template whereby calcite fibres would orient in a direction reversed relative to that in articulate brachiopods. Consequently, a diagenetic overprint, possibly tectonic, influencing the observed microstructure cannot be ruled out, especially as the fibrous structure is best developed at Locality 1 where asymmetrical brachiopods indicate tectonic strain. Alternatively, secretory style may have differed between shell layers (cf., Ye et al. 2018: fig. 4), resulting in variable crystallite alignment, patterns not resolvable from silicified material. Additionally, advances of shell secreting epithelium followed by mantle retraction and then resumption of secretion produces complex patterns of fibre relationships in which older fibres in places overlap younger formed fibres (cf., Williams and Rowell 1965: fig. 66; Williams 1997: fig. 280). Regardless, it is unlikely that the fibrous structure in S. rasettii sp. nov. is wholly an artefact of diagenetic processes because: (i) helcionellid molluscs and echinoderm ossicles in the same beds at Locality 1 do not show this fabric, but articulate brachiopods and stenothecoids do; (ii) if the silica fibres simply reflect tectonic strain, we might expect them to be all aligned within any one specimen, yet as shown in Fig. 11A, B, the orientation of the fibres varying considerably within individual specimens, with some fibres nearly parallel with the lateral margins as occurs in articulate brachiopods (Williams 1997: figs. 243, 244); (iii) in the umbonal area of at least some specimens, the fibres seem to radiate toward the shell margin in a biologically sensible way (Fig. 11D); (iv) the fibrous structure is unlike previously described diagenetic silicification textures (patterns I–V of Schmitt and Boyd 1981); and (v) a few specimens of S. elongata, in the Utah samples show radiating crystallites on the valve exterior (Fig. 12D, E), indicating that this fabric is not unique to the Burgess Shale localities.
One specimen of S. rasettii sp. nov., though tectonically distorted, shows a particularly well preserved exterior with imbricated growth laminae composed of radial to subradial silica crystallites (Fig. 11D). At the junction of successive lamellae, proximal crystallites appear to overlap those on the next distal lamella, but within any one lamella, the crystallites conform to an articulate pattern with distal crystallites overlapping proximal neighbours, a pattern also noted in S. elongata from Utah (Fig. 12D2). Of additional interest on this specimen, and others from Locality 1 and more rarely from Locality 2, are small pore-like structures between the crystallites reminiscent of brachiopod punctae or setal canals. The structures, typically measuring ca. 30–40 µm in diameter, are mostly randomly distributed but occur in places in commarginal rows (Fig. 11A3, D3). Some specimens show crystallites emarginated by the pores suggesting a biologic rather than diagenetic origin. If these pores are indeed terminations of canals and not simply diagenetic artefacts, silicification is too coarse to determine their orientation, whether inclined like setal canals or perpendicular like punctae. If the pores are biogenic, they are unlikely punctae, as punctae are unknown in Cambrian brachiopods, impunctate shells being likely primitive for Brachiopoda (Carlson 1995). Jin et al. (2007) found in Ordovician orthides that openings for seta-bearing epipunctae were anterior-facing, located mostly along the anterior slope of fine growth lines, reminiscent of pores in some S. rasettii sp. nov. (Fig. 11D3). The pore-like structures in S. rasettii sp. nov. are also visible on the inner valve surface near the valve margin in some specimens. Pores are not evident in S. elongata at hand nor have they been reported in other stenothecoids (e.g., Yochelson 1969), leaving uncertainty whether these are indeed biogenic or simply a diagenetic artefact.
Stratigraphic and geographic range.—Yoho River Limestone Member, Burgess Shale Formation, and questionably, Mount Whyte Formation, Yoho National Park, British Columbia, Canada.
Stenothecoides elongata (Walcott, 1884)
Figs. 8, 12D, E.
1884 Stenotheca elongata n. sp.; Walcott 1884: 23, pl. 9: 2, 2a.
part 1886 Stenotheca ? elongata Walcott; Walcott 1886: 129, pl. 12: 4a, b (not fig. 4).
1938 Stenothecoides elongata (Walcott); Resser 1938: 24.
1954 Stenothecoides elongata (Walcott); Rasetti 1954: 63, pl. 11: 3, 4.
1964 Stenothecoides elongata (Walcott); Robison 1964: 562, pl. 92: 18–21.
Material.—Total 9 valves (TMP 2021.022.0001–0007, acid-etched on single limestone slab and TMP 2021.022.0008, 2021.022.0009, not acid-prepared on second limestone slab) from Drum Mountains, Utah, USA, Locality 8 of Robison (1964).
Diagnosis (modified from Robison 1964).—Shell suboval with lobe axis near 90°, and length to width averaging 2:1. Exterior with growth lines and growth rugae, and, in at least some specimens, ultrafine radial fabric. Axial keel weakly developed, evanescing anteriorly. Peripheral ridge zone well developed. Axial ridge zone not observed. Auricles typically well developed adjacent to relatively broad posterior median opening. Cardinal boss suborthocline to weakly sinistrogyrate. Posterior median opening well developed.
Remarks.—Some specimens in the Utah samples figured here show fine radial structures (Fig. 12D, E) not previously noted for this species. One specimen, incompletely silicified, bears radial siliceous crystallites like those in S. rasettii sp. nov. (cf., Figs. 11D4, 12D2). A second specimen shows a thin siliceous layer externally with radial lirae (Fig. 12E).
Comparative shell outlines show S. elongata is proportionately less tapered posteriorly than S. rasettii sp. nov., an exception being Walcott’s (1884) type specimen (Fig. 10), which approaches S. labradorica in posterior shape. However, a paratype and lectotype figured in Rasetti (1954) fall within the range of outlines of the Utah material, and so Walcott’s (1884) type is provisionally accepted here as an extreme variant in posterior width and conspecfic with the Utah material here and in Robison (1964). The axial keel is better developed in S. labradorica and the valves more conspicuously inequilateral than in S. elongata and so these species are regarded here as distinct as proposed by Resser (1938) and Robison (1964) (contra Walcott 1886 and Horný 1957). Specimens assigned to S. elongata in Koneva (1979b: pl. 6: 10–14) appear narrower posteriorly than Robison’s (1964) material and likely represent a different species.
Stratigraphic and geographic range.—Geddes Limestone, Eureka district, Nevada, and Wheeler Formation, Drum Mountains, western Utah, USA.
Stenothecoides cf. elongata (Walcott, 1884)
Fig. 7.
Material.—Total 3 specimens (two articulated shells and one isolated valve), (ROMIP 66248–66250) and more than 160 uncatalogued specimens from Locality 3, Odaray Mountain, Yoho National Park, Canada, middle Cambrian.
Description.—Specimens from Locality 3 are closely similar to S. elongata in shell outline (Fig. 10) but are much smaller than the largest of the Utah specimens (Fig. 9) and so may represent juveniles or a separate species. Most specimens are articulated and show valve inequality varies from moderate (Fig. 7A) to scarcely detectable. None show any radial fabric but may be too coarsely silicified to retain such details. Auricles in the few isolated valves recovered seem less well developed than is typical for S. elongata.
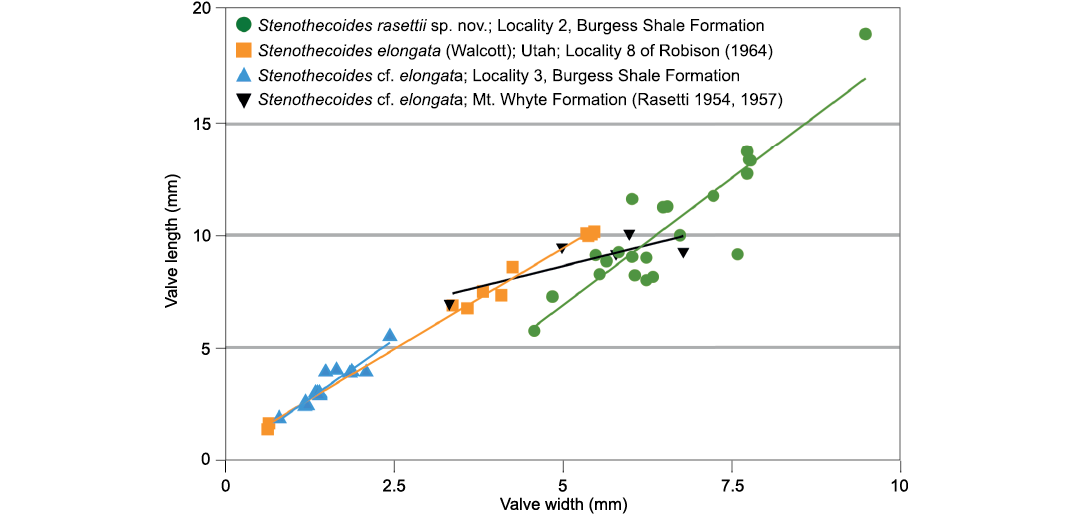
Fig. 9. Bivariate plots of length and width and best fit lines of Stenothecoides rasettii sp. nov., Burgess Shale Formation, Locality 2 (tectonic strain is evident in some specimens at Locality 1, which are excluded from this plot); Stenothecoides elongata (Walcott, 1884), Wheeler Formation, Drum Mountains, Utah, USA, Locality 8 of Robison (1964); Stenothecoides cf. elongata, Burgess Shale Formation, Locality 3; Stenothecoides cf. elongata, Mount Whyte Formation, Ross Lake, Mount Stephen, and Mount Field, Yoho National Park, Canada (Rasetti 1954, 1957).
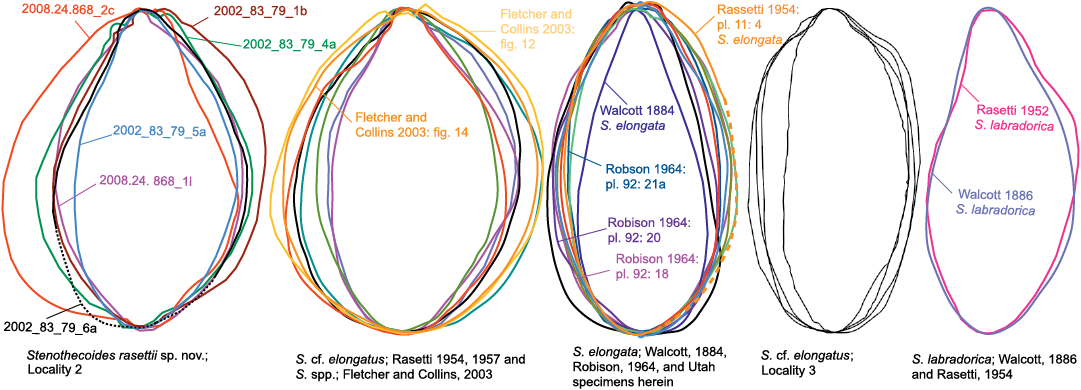
Fig. 10. Shell outlines of species discussed herein.
Discussion
Posterior median opening and pedicle.—Although unrealized at the time, definitive clues to the brachiopod affinities of the stenothecoids began with Sytchev (1960). Between the apices, he noted what seemed to be “traces of a ligament which ran from one valve to the other as a narrow band. ” There was, apparently, an internal ligament: “traces of a small ligament hole were preserved on the inner side of the key edge under the crowns” (Sytchev 1960: 256, free translation). Sytchev’s (1960) “ligament hole” is surely a posterior median opening as understood here, and the “traces of ligament” are likely sediment-filling or cement within the opening. This is poorly shown in Sytchev’s (1960) figures but is most evident in his plate 16: 6 (apical view). The same is evident in Yochelson (1969: fig. 2C), where a bivalved specimen, in fact the type of Stenothecoides knighti Yochelson, 1969, in apical view shows a cement- or sediment-filled posterior median opening. Yochelson (1969: fig. 2, caption) interpreted this as shell damage. A posterior median opening is visible in species that Koneva (1979b) assigned to Cambridium spp. and to Stenothecoides nedovisini (1979b: pl. 1: 5c, 7c, 7d, 8c and pl. 7: 1c, 2d, respectively).
If stenothecoids housed a bivalve-like ligament for opening the valves, as Sytchev (1960) and others (Aksarina and Pel’man 1978; Koneva 1979b; Pel’man 1985) have suggested, then the apical boss and adjacent troughs would seem the most likely structures for ligament attachment. We consider such a function for these structures unlikely for several reasons: (i) no analogues with this configuration occur in the Bivalvia; (ii) no growth lines are evident on the apical boss or troughs, unlike ligament areas in the Bivalvia; and, (iii) other morphologic features described herein indicate affinities with the Brachiopoda, which lack a ligament mechanism.
It seems likely that the posterior median opening of Stenothecoides was for egress of a pedicle-like structure. No difference in size of the emarginature of the opposing valves was detected and so the egress was evidently shared equally by both valves, a condition unknown in living brachiopods in which the pedicle exits mostly or entirely from the ventral valve.
In living inarticulate brachiopods the pedicle is an extension of the posterior body wall of the ventral valve and, in lingulids, features a central coelomic-fluid filled cavity that is continuous with mantle canals; in living articulates, by contrast, the posterior body wall of both valves is continuous with the pedicle, which lacks a coelomic cavity (Williams et al. 1997). Some fossil brachiopods with preserved pedicles correspond to neither. Notably, the middle Cambrian kutorginate Nisusia sulcata Rowell and Caruso, 1985, shows a thick pedicle emerging from a posterior median opening formed by both a delthyrium (ventral valve) and notothyrium (dorsal valve) (Holmer et al. 2018). Unlike other kutorginates, N. sulcata lacks evidence of an apical foramen for extrusion of a juvenile pedicle (Holmer et al. 2018). The beaks of Stenothecoides show no evidence of an apical foramen. Equally developed posterior emarginations on the opposing valves of Stenothecoides suggest a pedicle derived from both valves as in N. sulcata, for which Holmer et al. (2018) inferred a muscled, flexible, externally chitinous, movable pedicle, possibly with a central coelomic cavity. If a coelomic cavity was present in the pedicle of Stenothecoides, connection with the body cavity through the narrow gap left between opposed apical bosses (Fig. 7B2) is constrained. Connection of a coelomic cavity via the more spacious apical troughs is possible, although achieving a split configuration unknown in fossil or extant brachiopods. The well preserved pedicle of the primitive chileate(?) rhynchonelliform brachiopod, Longtancunella chengjiangensis Hou, Bergstrom, Chen, Feng, and Wang, 1999, shows no evidence of a central coelom (Zhang et al. 2011), which is, however, present in the stem-group brachiopod-like Yuganotheca elegans Zhang, Li, and Holmer in Zhang et al., 2014. Given Stenothecoides’ apparent stem-group position, it is here tentatively reconstructed with a narrow pedicle coelom (Fig. 13A).
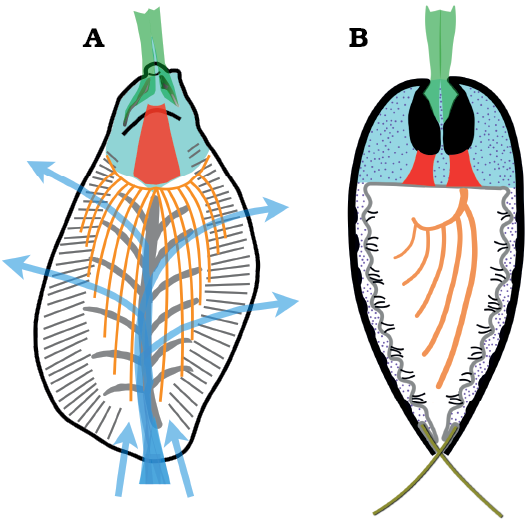
Fig. 13. Stylized anatomical reconstruction of the stenothecoid pan-brachiopod Stenothecoides rasettii sp. nov. A. Dorsal valve, plan view. B. Longitudinal section slightly off the midline of the valves and normal to commissure. Colors: black in A, shell outline; black in B, valves and juxtaposed apical bosses; green, pedicle, inserting on posterior surface of apical boss and in cardinal troughs; red, muscles originating on anterior surface of apical boss and attaching to the anterior body wall; turquoise, visceral mass; orange, lophophore; grey, peripheral and axial furrow zones; short black lines, cilia in grooves between mantle canals; blue arrows, inferred inhalant and exhalant water currents; dotted areas, coelomic fluid; olive green, setae (omitted in A). Lophophore modelled after Heliomedusa (Chen et al. 2007).
Apical boss.—Robison (1964: 562) noted that some specimens of Stenothecoides elongata in his collection of silicified valves lack an apical boss and instead show “a depression or dental socket” at this position. He suggested that together these functioned as an articulating tooth and socket as in bivalve molluscs. In the Burgess Shale collections at hand, about 20% of isolated valves show a gap instead of a boss. We interpret these mostly as specimens in which the apical boss became detached before burial or that are incompletely silicified, with damage occasionally occurring during sample preparation, all these instances leaving a pseudo-socket below the valve apex (Figs. 5J, 6D1, 8C).
Evidence that an apical boss was developed in both valves, and that a gap at this position is an artefact, includes: (i) specimens with an apical gap typically show remnants of the apical troughs on either side of the gap, some specimens preserve a remnant of the apical stem below the beak (Fig. 5J), and a few specimens preserve a remnant of the apical body (Fig. 6D1); (ii) the apical boss and pseudo-socket show no preference for dorsal or ventral valves (Fig. 5G1, H); (iii) unlike the teeth of bivalve molluscs, the apical boss does not extend across the commissural plane and therefore could not have inserted into a corresponding socket to articulate the valves (Figs. 5A4, 7C2; cf. Johnston 1993: fig. 70B); (iv) an articulated specimen of S. cf. elongata shows what is likely the two apical bosses in apposition, as viewed through the posterior median opening (Fig. 7B2); (v) abundant silicified stenothecoids collected from the middle Cambrian of New South Wales (not part of the present study) invariably show an apical boss, never a gap or socket (Bruce Runnegar, personal communication 2018).
Assuming the posterior median opening in Stenothecoides species decribed herein is for the extrusion of a pedicle, then the only available surfaces for attachment of the pedicle to the shell (assuming a rhynchonelliformean-like pedicle, but see below) would seem to be the posterior surfaces of the apical bosses, the apical stems, and the apical troughs. In well preserved specimens, there is a textural change, with the apical body having a more rugose surface than does the apical stem (Fig. 6H). It may be that pediculate connective tissue attached to the posterior surface of the apical body and pedicle adjustor muscles in the apical troughs on either side where they would have mechanical advantage as in articulate brachiopods (cf., Williams and Rowell 1965: fig. 25c). The anterior surface of the apical body may have supported insertion of other muscles (Fig. 13), as explained below.
The function of the cardinal sulcus is unclear. The sulcate apical boss of stenothecoids is remarkably similar to the cardinal pivotal tooth in the left valve of schizodid bivalves. In the latter, the sulcus facilitates passage of an umbonal elevator muscle across the plane of the hinge plate to attach to the foot. Although the umbonal cavity narrows to a point in Stenothecoides, a discrete muscle scar is not developed at this position, although generally coarse silicification might prohibit its recognition. In stenothecoid steinkerns described by other authors, there is no offset projection at the apex, unlike the prominent structure at this position on steinkerns of schizodid bivalves (Johnston 1993). In fact, no structures can be recognized in stenothecoids from the Burgess Shale, or elsewhere, that can be convincingly demonstrated to be muscle insertions. Consequently, the cardinal sulcus cannot be related to attachment of a foot-like structure or for passage of related muscles. Nor could the sulcus have acted as a site for pedicle attachment as there would be limited room if any for passage of pedicle muscle to the anterior surface of the apical boss. It is more likely that the sulcus (and the general anterior surface of the apical boss) was associated with attachment of some structure more anteriorly. For brachiopods such structures are likely to be either diductor muscles, the lophophore, or the anterior body wall. We exclude diductors because there is no obvious rotational axis anterior to the sulcus. Lophophore attachment likewise seems unlikely as the lophophore is attached to the anterior body wall in lingulid brachiopods, and only to the brachial valve in articulates (Williams and Rowell 1965).
The cardinal sulcus is developed in both valves in Stenothecoides. If the sulcus had a function—it may be simply an artefact of extension of the left and right spurs and the associated apical troughs—then it seems best suited for insertion of muscles attached to the anterior body wall (Fig. 13). These muscles, upon contraction, may have acted to compress coelomic fluid behind the anterior body wall, thus opening the valves. A difficulty with this suggestion is that in inarticulate brachiopods muscles attached to the anterior body wall insert on the valve floor (e.g., anterior lateral muscles and oblique lateral muscle, Williams and Rowell 1965). If such muscles arose from the cardinal sulcus in Stenothecoides, they have no equivalent in brachiopods.
Axial and peripheral ridges and furrows.—The axial and peripheral ridge zones are conspicuous internal shell features of Stenothecoides and several authors have commented on their structure and possible function. Interpretations of axial zone ridges and furrows as tracts of muscle attachments (e.g., Aksarina 1968) are rejected as discussed earlier. Rozanov and Zhuravlev (1992) noted similarity of the loop-like structures in the axial zone of Stenothecella with orthothecid hyolith intestinal loops, which, unlike muscle scars, are staggered along the shell axis, but we are unaware of any examples of gut tracts in either molluscs or brachiopods leaving impressions on the shell interior. Yochelson (1969) and Pojeta and Runnegar (1976), working within a molluscan concept for stenothecoids, suggested the furrows of the axial and peripheral zones mark sinuses or tubes/canals within the mantle. Yochelson (1969), presuming axial furrows occurred only in the ventral valve, suggested they supplied cilia associated with transport and evacuation of pseudofaeces. However, this scenario is unlikely as the present material shows axial furrows developed in both valves. Dzik (1981), alleging similarity of the furrow patterns in stenothecoids with the mantle canals of brachiopods, suggested stenothecoids are simply calcareous inarticulates. Certainly, the furrow patterns, at least peripherally, are broadly similar in the two groups and warrant comparison.
Inarticulates commonly show one pair of mantle canals (left and right vascula lateralia) in the ventral valve and two pairs (left and right vascula lateralia and vascula media) in the dorsal valve (Williams et al. 1997). Furrows seem equally developed in the dorsal and ventral valves of Stenothecoides (Fig. 5B, F), and in this respect, Stenothecoides is without a match among Cambrian inarticulates, but resembles the later lingulids (Family Lingulidae), which lack vascula media in the dorsal valve (Williams et al. 1997). While the furrows of the axial zone in Stenothecoides are vaguely similar with the medially directed side branches of the main canal of the vascula lateralia in lingulids (cf. Williams et al. 1997: figs. 384.2, 384.6), this would require emanation of the axial zone, not from the valve midline, but from the junction with the peripheral ridge zone. Rare specimens of S. rasettii sp. nov. show a commarginal ridge (Fig. 5F) or a moderate break-in-slope (Fig. 5C, D1) at this position, which could be taken to mark a main canal of the vascula lateralum, but others show none (Fig. 5B). Also, other stenothecoids preserving an axial ridge zone show no evidence of a main canal at the medial edge of the peripheral zone (Horný 1957; Rasetti 1957; Koneva 1979b). Articulate brachiopods possess a pair of branching medial canals (vascula media) that have no obvious counterpart in Stenothecoides.
Unlike the mantle canal pattern in lingulids, or any other brachiopods, the axial furrows in Stenothecoides converge on the midline to form chevrons straddling the medial trough (also in Bagenovia, Koneva, 1979b and Cambridium, Horný, 1957). This orientation seems well suited to brachiopod-like feeding currents (Fig. 13A). Inhalant flow in most brachiopods at adult stages is along the lateral margins and exhalant flow is medial on the anterior margin. However, in trocholophe- and schizolophe-bearing brachiopods, including many small species and all juvenile brachiopods, inhalant flow is antero-medial and exhalant flow lateral (Rudwick 1970), a probable plesiomorphic condition for the Brachiopoda (Emig 1992). Accordingly, inhalant flow at the anterior margin is also inferred for Yuganotheca, a stem-group brachiopod-like lophophorate (Zhang et al. 2014). Given the small size and probable stem-group position of the stenothecoids, water flow here is reconstructed as posteriorly directed through the anterior margin as in trocho/schizolophous brachiopods (Fig. 13A). The axial furrows may have been subtended by mantle canals that supplied ciliated areas of the mantle cavity in the axial zone (Fig. 13B). The mantle canal systems of modern lingulids and terebratulids approximately parallel feeding currents, which are significantly enhanced by ciliary pumping on the mantle surface, with cilia most concentrated on mantle grooves between the canals but absent at the mantle periphery (Westbroek et al. 1980).
The possibility that the ridges of the axial zone are brachial ridges induced by a lophophore is unlikely. The valve floor of brachiopods is unmarked by the lophophore except on the dorsal valve of various strophomenates (Carlson 2016), whereas axial zone ridges occur in both valves of Stenothecoides. Were the chevrons in Stenothecoides lophophore-related, it would suggest a complex, probably spirolophe-like structure, which seems unlikely for a presumed stem-group.
Some authors state that the axial furrows in stenothecoids split to produce the peripheral furrows (Horný 1957; Sytchev 1960; Pojeta and Runnegar 1976; Koneva 1979b). While individual axial furrows seem aligned with the peripheral furrows in some specimens of S. rasettii sp. nov. (Fig. 5B), in others, most axial furrows, though faint, appear to extend toward the margins at a much steeper angle and are not obviously contiguous with the peripheral furrows (Fig. 5D1, F), a pattern also described in Bagenovia spp. and Stenothecoides spp. from Kazakhstan (Koneva 1979b).
The peripheral furrows in Stenothecoides are positionally similar to the vascula terminalia in lingulids, which branch toward the valve perimeter (Williams et al. 1997). In Stenothecoides specimens at hand, bifurcation of peripheral furrows is difficult to detect. Where questionably present (Fig. 5C), the resulting branches remain parallel, as in the stem-group brachiopod Micrina (Holmer et al. 2008: fig. 1C), and not divergent as, for example, in kutorginids (cf. Skovsted and Holmer 2005: pl. 6: 5). In at least articulate brachiopods, the distal ends of mantle canals terminate short of the mantle edge and connect with setal follicles (Williams et al. 1997). Setae (= chaetae) are ubiquitous in brachiopod larvae and widespread in adult forms (Williams et al. 1997) and are likely plesiomorphic for the Lophotrochozoa (Thomas et al. 2020). Accordingly, Stenothecoides is reconstructed here with marginal follicular setae (Fig. 13B) that were seated in the peripheral furrows. Assuming that the original extent of marginal setae is reflected in the distribution of peripheral furrows, the entire shell periphery was setigerous except the posteriormost margin and the anterior inhalant area (Fig. 5F). Interestingly, the angle and distribution of the peripheral furrows in Stenothecoides is comparable to the arrangement and distribution of peripheral setae in the stem brachiopod Mickwitzia, which shows no setae in the center of the anterior margin and setae are less perpendicular to the shell margin at the lateral posterior margins (Butler et al. 2015: fig. 14). However, unlike Mickwitzia and Micrina, the presence of penetrative setae in Stenothecoides cannot be certainly established owing to preservational vagaries of the pores and will not likely be resolved without well preserved calcareous shells.
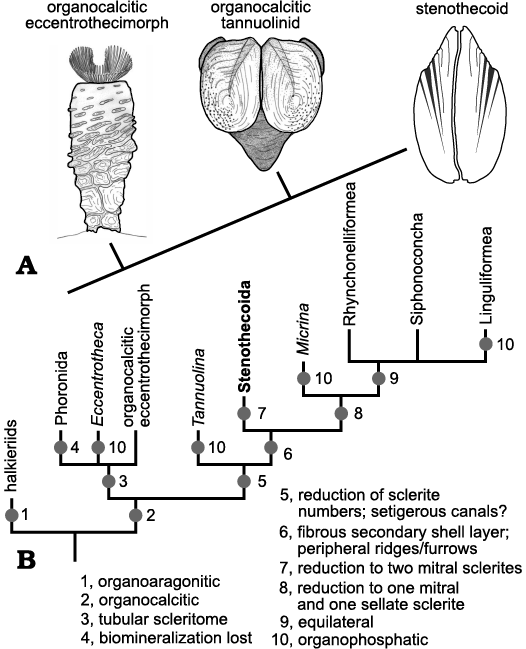
Fig. 14. A. Hypothesized morphogenetic pathway for derivation of the bivalved stenothecoid scleritome from a multisclerite, tubular, organocalcitic, eccentrothecimorph ancestor via a hypothetical organocalcitic tannuolinid. Eccentrothecimorph slightly modified from Skovsted et al. (2011: text-fig. 17). Tannuolinid modified from Skovsted et al. (2014). B. A possible cladogram that assumes an organocalcitic scleritome is primitive for the Pan-Brachiopoda. In this view, organophosphatic mineralogy evolved independently several times and the Stenothecoida evolved a bivalved scleritome independently of the Brachiopoda + Micrina clade.
The lack of axial ridges and furrows internally in the umbonal area of Stenothecoides may mark a body cavity, as in brachiopods (Fig. 3B). However, this is not a consistently developed feature among stenothecoids. In Bagenovia, axial furrows and ridges are replaced in the umbonal area by rows of pits oriented less acutely than the axial furrows (Koneva 1979b), which suggests some anatomical change umbonally and so does not rule out a body cavity. Cambridium likewise shows a change in the posterior third of internal molds (Horný 1957: pl. 4) and Stenothecella in the posterior fifth (Aksarina and Pel’man 1978: pl. 17: 4a; Rozov 1984: fig. 7F). However, in Sargaella, internal molds show longitudinal axial structures and small closed loops on either side extending to the apex without interruption, although the peripheral zones seem to diminish umbonally (Aksarina and Pel’man 1978: 18, figs. 1–3; Rozov 1984: fig. 7B).
Valve asymmetry.—Functional reasons for valve asymmetry in stenothecoids are not obvious. The only conspicuous anatomical asymmetries of brachiopods that might offer clues are the dispositions of the anus and digestive diverticulae in inarticulates. In lingulid and discinid brachiopods the anus opens at the right body wall; in craniids the anus empties posteriorly, but just right of the midline (Williams and Rowell 1965: H19–H20). Of four digestive diverticulae in lingulid brachiopods, the right posterodorsal diverticulum is much larger than the rest (Williams and Rowell 1965). A much expanded right posterodorsal diverticulum together with a right-exiting anus in stenothecoids might account for the inequilateral valves, with a more posteriorly positioned right lobe. However, why these same anatomical asymmetries in lingulid brachiopods (though presumably less pronounced than in stenothecoids) are not expressed in at least slight valve asymmetry is unclear. Alternatively, valve asymmetry in stenothecoids may be a plesiomorphic feature inherited from asymmetric ancestors (see below).
Systematic relationships.—While a few authors have suggested that stenothecoids lie somewhere near or within Brachiopoda (Radugin 1937; Minichev and Starobogatov 1976) rather than Mollusca, only Dzik (1981) ventured to suggest a relationship within that group, that being with Inarticulata, although without citing any synapomorphies, and none is evident from the present study for this association. Certainly, it can be reasonably argued that stenothecoids fall within the total clade Pan-Brachiopoda (sensu Carlson and Cohen 2020), especially given the new evidence for a pedicle, while relationships with constituent members of the clade remain unclear. Prominent features of Stenothecoides, including the apical boss and axial furrow zone are without obvious equivalents among Pan-Brachiopoda. However, a few features of Stenothecoides occur in stem-group members and are noteworthy here, although they provide few clues to phylogenetic propinquity.
Peripheral furrows and ridges of Stenothecoides, reminiscent of vascula terminalia but more probably setal grooves, appear in the early Cambrian organophosphatic eccentrothecimorph tommotiid Micrina (Holmer et al. 2008) and so are an archaic feature within the Pan-Brachiopoda, although perhaps not plesiomorphic, being so far unreported in other eccentrothecimorphs, which are likely stem-group Brachiopoda (Balthasar et al. 2009; note that Skovsted et al. 2015, and references therein, recognized two distinct clades of tommotiids, the camenellans and eccentrothecimorphs, with only the latter having brachiopod affinities). Micrina also shows a putative pedicle egress from a posterior median opening between the valves (Holmer et al. 2008) as in Stenothecoides and certain kutorginate articulates (see above), rather than from an apical foramen. Beyond this, other features in common with Micrina, excepting possible setigerous canals, are not evident, and those identified do not constitute synapomorphies, being variously distributed in the total group.
Parkhaev (1998) suggested stenothecoids may be allied with another problematic early Cambrian group, the Tianzhushanellidae (class Siphonoconcha), in particular the genus Apistoconcha Conway Morris in Bengtson et al. 1990, citing shared calcareous biomineralization and inferred dorso-ventral rather than left-right disposition of the opposing valves. However, new morphologic details revealed in the Burgess Shale stenothecoids offer only meagre support for this association. Unlike Stenothecoides, Apistoconcha shows symmetrical valves, dental ridges, unique muscle scars, and lacks evidence for a pedicle, although it is still regarded as a likely stem-group brachiopod (Conway Morris in Bengtson et al. 1990). Nonetheless, a possible connection of these taxa cannot be wholly dismissed. Pel’man (1985) described unusual specimens with somewhat intermediate morphology from the lower Cambrian of Mongolia, which he assigned to Cambridium and Serioides in the Stenothecoida. These show dental platforms in the same relative position as the dental ridges in Apistoconcha but with prominent taxodont teeth; Apistoconcha shows no equivalents except perhaps incipiently in the form of ribs on the dental ridges of the dorsal(?) valve (Parkhaev 1998; Conway Morris in Bengtson 1990: 174). Also noteworthy in the Mongolian material is valve obliquity as well as an apical appendage with flanking depressions below the apex, features that recall the apical boss and troughs in Stenothecoides, but developed in only the ventral valve and with an opposing groove in the dorsal valve (Pel’man 1985). This configuration is similar to the boss-like structure and opposing pit in the tianzhushanellid Aroonia (Bengtson in Bengtson et al. 1990).
Both Apistoconcha and Aroonia show fibrous calcite microstructure (Conway Morris and Bengtson in Bengtson et al. 1990; Skovsted 2006) reminiscent of that in S. rasettii sp. nov. and the secondary shell layer of articulates. However, fibrous microstructure is widespread in calcareous small shelly fauna including not only some stem-group brachiopods (Apistoconcha; Aroonia) but also some problematic taxa (e.g., Tunudiscus Skovsted, 2006) and even certain molluscs (Runnegar 1985), although in some of these the fibres appear much finer than in Stenothecoides and articulate brachiopods; more detailed comparisons of microstructure in these forms is required before any links can be made. On the preserved exterior of Apistoconcha from Greenland (Skovsted 2006), distal ends of fibres overlap their distal neighbours (Christian Skovsted, personal communication 2020) as on the exterior of some Stenothecoides described above, and opposite the orientation of the secondary shell fibres in articulates. As in Stenothecoides, fibres on the flanks of Apistoconcha intersect the valve margin at a much lower angle than at the anterior margin (Conway Morris in Bengtson et al. 1990).
The morphology of paterinids, the earliest occurring brachiopods, indicates no close affinities with stenothecoids. The paterinid shell is organophosphatic, equilateral, strophic (with interareas), and with two (ventral valve) or four (dorsal valve) distinct muscle insertions on the valve floor, and, at least in Paterina, saccate vascula genitalia in the ventral valve (Laurie 1987; Williams et al. 1998), features generally characteristic of the brachiopod crown group and without parallel in stenothecoids. The vascula media in paterinids are finely divided anteriorly (i.e., vascula terminalia of Williams et al. 1998) and therefore quite unlike the chevroned mantle canals of stenothecoids, but they do show at least superficially similarity to the stenothecoid peripheral setal groove array except that they extend across the anteromedial portion of the valves, where a gap occurs in stenothecoids. The medial branches of the vascula media in Paterina are elongate and parallel the mid-line in both valves and so are reminiscent of the axial groove in Stenothecoides and the longitudinal structures in other stenothecoids, such as Cambridium (Horný, 1957). However, unlike paterinids, the axial groove(s) of stenothecoids extends to the anterior valve margin and lacks obvious connection to the peripheral setal grooves.
Further difficulties assigning stenothecoids to any Pan-Brachiopoda subgroups arise from uncertainties regarding polarity of some fundamental features in the latter (Carlson and Cohen 2020). Monophyly of the bivalved scleritome is uncertain (Wright 1979; Carlson 1995), and even if monophyletic, mineralization may have been acquired separately in major groups (Zhang et al. 2014). As for shell composition, some authors (Skovsted et al. 2008; Balthasar et al. 2009; Larsson et al. 2014; Zhao et al. 2017; Li et al. 2019) regard organophosphatic biomineralization as plesiomorphic and calcitic biomineralization derived for Pan-Brachiopoda (= total clade Brachiopoda), while others (Carlson 1995) see calcite as plesiomorphic, and organophosphate, derived. If the latter holds, then calcite mineralogy in stenothecoids is plesiomorphic, indicating only that they are unlikely to be derived from any organophosphatic stem-group taxa. If the former is true, then there is presently no way to determine whether calcite mineralogy in stenothecoids is a synapomorphy with other calcareous groups within Pan-Brachiopoda or independently derived.
Considering the evidence for pan-brachiopod affinities of the Stenothecoida, asymmetry in the form of inequilateral valves remains an unusual and contrasting feature with inarticulates, articulates and some stem-group taxa such as Yuganotheca (Zhang et al. 2014). However, other stem-group members, notably the tommotiids, show some asymmetric scleritome elements that may offer clues to stenothecoid ancestry. Presumed primitive tommotiids, the eccentrothecids, show a complex tubular scleritome comprising multiple stacked sclerites (Skovsted et al. 2008). Proposals for the origin of the bivalved brachiopod scleritome include paedomorphic retention of a bivalved tommotiid larval shell (Holmer et al. 2011), or specialization and reduction of sclerite numbers from a multi-sclerite eccentrothecid-like ancestor to a bivalved scleritome as in Micrina via Tannuolina-like intermediates (Skovsted et al. 2014).
Tannuolina and Micrina, both organophosphatic and with setal canals, constitute the tommotiid family Tannuolinidae (Skovsted et al. 2014). While Micrina, the most derived known tommotiid, shows a presumed bivalved scleritome with anterior gape (Holmer et al. 2008), Tannuolina has a complex scleritome comprising multiple sclerites that come in two forms: sellate, which are compressed and usually symmetrical, and; mitral, which are pyramidal and asymmetric (Skovsted et al. 2014). Asymmetrical paired sclerites may be primitive for the tommotiids (Skovsted et al. 2014). The bivalved scleritome of Micrina is formed by a single sellate and opposing mitral sclerite, both of which are not quite symmetrical (Holmer et al. 2008).
Reconstruction of a Moroccan species, Tannuolina maroccana Skovsted and Clausen in Skovsted et al., 2014, shows a multisclerite scleritome with an enlarged pair of asymmetrical sellate sclerites, one dextral and the other sinistral, fused along their decrescent margins, with the remainder of the tube-like scleritome composed of two rows of asymmetrical sellate sclerites and a single symmetric sella at the base (Skovsted et al. 2014). While a reduction of sclerites to a single sella and opposing mitral could account for a Micrina-like descendent, we tentatively suggest that a similar reduction of sclerites, but to two opposing asymmetric mitrals, could produce a stenothecoid-like scleritome, as shown in Fig. 14A. Skovsted et al. (2014) show the mitrals with the more expansive carinate ends growing toward the attached end of the scleritome, but there seems no compelling reason that the mitrals could not be reversed in their orientation, with growth of these sclerites most pronounced away from the attachment surface, as in Micrina, and Stenothecoides.
A difficulty deriving stenothecoids from a tommotiid ancestry is the organophosphatic mineralogy assumed to be ubiquitous for the latter (Balthasar et al. 2009; Larsson et al. 2014), a problem also noted for deciphering the relationship of tommotiids with stem and crown-group calcareous brachiopods generally (Li et al. 2014). However, Skovsted (2016) recently described, from the lower Cambrian of Greenland, a silicified eccentrothecid tommotiid and convincingly argued for an originally organocalcitic scleritome for this animal. This would displace the origin of organocalcitic scleritomes to somewhere deeper in the Pan-Brachiopoda clade and opens interesting possibilities for the origin of stenothecoids and other calcitic Pan-Brachiopoda from calcitic tommotiids. A tentative phylogeny that assumes an originally calcareous mineralogy for Pan-Brachiopoda is given in Fig. 14B.
Paleoecology.—Pedicle attachment as benthic suspension feeders seems a likely life mode for Stenothecoides given the conspicuous posterior median opening and a presumed lophophore. Zhuravlev (1996: 82, 87) regards at least some stenothecoids as important facultative reef-builders. Stenothecoids were evidently broad in preferred habitats as those found in the Burgess Shale are not obviously associated with reef facies. At localities 1 and 2, Stenothecoides occurs in planar laminated limestone and debrites at the type section of the Yoho River Limestone Member (see Fletcher and Collins 1998 for description of the section). McIlreath (1977) interpreted the Yoho River Limestone (his Boundary Limestone) as a debris apron abutting the Cathedral escarpment. Fletcher and Collins (1998) regarded the planar bedded component as likely autochthonous but interpreted the debrites as shelf-derived. If so, stenothecoids and their associated biota in at least the debrites may represent allochthonous components originating from shelf facies up paleoslope. At Locality 3, stenothecoids were collected from packstone that may represent “an amalgamation of several slumping events” (Streng et al. 2016: 270) and so, may too include allochthonous components from shallower facies, although the preponderance of articulated stenothecoid shells compared to localities 1 and 2, suggests more limited or a more gentle transport.
A persistent debate concerning the paleoecology of the Burgess Shale fossil assemblages, at least those occurring in fine-grained siliciclastic facies (e.g., the Phyllopod Bed at the Walcott Quarry), centers on the inferred degree of transport of the preserved biota. Some authors maintain these assemblages are autochthonous with little or no transport, and thus provide a “snapshot” of seafloor Cambrian communities (e.g., Caron and Jackson 2006, Johnston et al. 2009b, 2017), while others (e.g., Conway Morris 1986; Gaines 2014; Enright et al. 2021) argue for significant transport of the biota in sediment slurries that produced mixed assemblages including biota from adjacent shelf environments.
The fossil assemblages of the Yoho River Limestone are germane to this discussion (Virmani and Johnston 2017). Co-occurring elements with stenothecoids at localities 1 and 2 include helcionellid molluscs, rhynchonelliformean brachiopods (including the distinctive coral-shaped brachiopod Tomteluva, Streng et al. 2016), echinoderm plates, phosphatic tubes and rare linguliformean brachiopods; trilobites and hyoliths are absent (Virmani and Johnston 2017). A similar assemblage occurs at Locality 3, but includes trilobites, sponge spicules, and rare bradoriid arthropods (Streng et al. 2016).
Despite the collections of thousands of specimens from multiple sites in the non-carbonate facies of the Burgess Shale, none has yielded stenothecoids or Tomteluva. If sites like the Phyllopod Bed include substantial introduction of shallow water organisms transported from platform environments to the basin as some authors propose (e.g., Conway Morris 1986), it seems extraordinary that two of the most abundant, robust, and easily distinguished elements, namely Stenothecoides and Tomteluva, from presumably adjacent platform environments were omitted. Consequently, the presumed platform-source for biotas in limestone units of the lower Burgess Shale Formation provides indirect evidence that the famous fossil assemblages in the fine-grained siliciclastic components of the unit do not likely include significant input of elements transported from adjacent platform environments.
Conclusions
Silicified specimens of Stenothecoides recovered from basal carbonate units of the Burgess Shale, and from the middle Cambrian of Utah, provide new morphologic information that indicates stenothecoids are phylogenetically more closely related to Brachiopoda than to Mollusca. The Burgess Shale material includes at least two species, Stenothecoides rasettii sp. nov. and Stenothecoides cf. elongata. Stenothecoides rasettii sp. nov. differs from S. elongata in shell outline and in shell length to shell width ratio. Stenothecoides rasettii sp. nov. is regarded as questionably conspecific with S. cf. elongata that Rasetti (1954) described from the older Mount Whyte Formation, but not conspecific with S. cf. elongata from the Burgess Shale described herein. In the Chancellor Basin (which includes the Burgess Shale), Stenothecoides is now known from the upper Plagiura–Kochaspis (Rasetti 1954) to the lower Ehmaniella (this study) zones (Wuliuan).
An apical boss is documented in both valves of Stenothecoides. These bosses abutted when the valves were articulated and therefore did not likely function in valve articulation. An opposing socket that was thought to receive the boss upon articulation, described in an earlier study of Stenothecoides (Robison 1964), is shown to be an artefact of shell damage or incomplete silicification. Besides Stenothecoides, the internal apical area is poorly known in other stenothecoid genera, and so it is unclear whether the apical boss is widespread in the group. It was apparently not ubiquitous, because apical areas preserved in Mongolian specimens assigned to Cambridium and Serioides, do not show an apical boss (Pel’man 1985).
A second major feature of the apical area of Stenothecoides is a posterior median opening that emarginated the posterior-most margin of both valves and likely functioned as an exit for a pedicle. Some features previously described at this position in stenothecoids as shell damage (Yochelson 1969) or as a ligament suture (e.g., Stychev 1960) are more likely a posterior median opening, although published figures and descriptions are vague on details.
Fibrous microstructure identified in S. rasettii sp. nov. and the Utah specimens indicates replication in silica of an originally calcite microstructure that is reminiscent of the secondary shell layer of articulate brachiopods, although in S. rasettii sp. nov. the preserved microstructure may have a tectonic overprint. In S. rasettii sp. nov., minute subcircular openings in the shell, sometimes in commarginal rows, suggest penetrative setal canals, but these cannot be established with certainty owing to coarseness of silicification. Confirmation or rejection of shell pores, and of a fibrous microstructure, may come with studies of well-preserved calcitic shells of other stenothecoids.
On the valve interior of Stenothecoides, axial chevrons (axial ridge zone) are interpreted as mantle canals and peripheral furrows and ridges as setal grooves with interspersed ridges. Our reconstruction of the soft part anatomy shows a posterior pedicle, a lophophore, peripheral setae, mantle canals and accompanying ciliated mantle troughs. A mechanism for adduction and diduction of the valves is unknown, although we tentatively suggest muscles extending from the apical boss to the anterior body wall may have facilitated diduction by increasing hydrostatic pressure in the coelomic fluid of the body cavity. Inhalant and exhalant water flow is reconstructed as anteromedial and lateral, respectively.
The morphology of Stenothecoides as now understood, particularly the newly recognized posterior median opening, places the Stenothecoida within the Pan-Brachiopoda, likely as a stem-group of the brachiopod crown group. A peculiar feature of stenothecoids relative to pan-brachiopods are the inequilateral valves (equilateral in all crown-group brachiopods). We suggest a possible morphologic pathway for derivation of the stenothecoids was by sclerite reduction from a multi-sclerite, tubular, organocalcitic, eccentrothecid ancestor, via a hypothesized calcitic tannuolinid intermediate, leaving two opposing asymmetric mitral plates comprising the stenothecoid scleritome. This contrasts with the crown-group brachiopods where the bivalved scleritome may have evolved from sclerite reduction to one mitral sclerite and an opposing sellate sclerite (Holmer et al. 2008; Skovsted et al. 2014). Recognition of stenothecoids as having pan-brachiopod-like characters allows extraction of this group from the Mollusca where they have been a complicating taxon in phylogenic studies (Parkhaev 2008) and expands the known morphologic disparity of pan-brachiopods to include organocalcitic bivalved forms with inequilateral valves and unique internal apical structure.
Stenothecoids are now known from abundant specimens in basal carbonates of the Burgess Shale, which are thought to include sediments and biota mass transported from adjacent platformal environments to the basin. The absence of stenothecoids and some accompanying shelly fauna from the famous biotic assemblages preserved in fine-grained siliciclastic units (e.g., Phyllopod Bed) indicates that these assemblages are mostly autochthonous, having received little or no input of biota transported from shallower environments.
Note added in proof
A recent paper (Peel 2021) published after the current paper was accepted for publication describes new species of stenothecoids from the Cambrian of Greenland. Information therein does not alter the conclusions in the present work.
Acknowledgements
We thank Chris Collom (McCallum Geological Consulting, Calgary, Canada) for valuable discussion and field assistance, and for measured sections at localities 1 and 2 as part of the PAJ field teams. The late Kimberley J. Johnston (1961–2015, formerly Imperial College London, UK) ably assisted with field work. Parks Canada, Lake Louise Office supplied research permits (YPN-2001-0031, -0032, LLYK02-16, and YPN-2006-516) and logistical support to PAJ. Specimens of Stenothecoides from Odaray Mountain were collected during fieldwork led by the Royal Ontario Museum in 2010 and 2012 under Parks Canada Collection and Research Permits to Jean-Bernard Caron (ROM) (YNP-2010-6139 and YNP-2012-12054). Robert Gaines (Pomona College, Claremont, USA) generously provided stratigraphic information for the Utah samples, and Jean-Bernard Caron kindly allowed access to Royal Ontario Museum specimens. PAJ thanks Yuanlin Sun (Peking University, Bejing, China) and Bruce Runnegar (University of California, Los Angeles, USA) for helpful discussion, Pavel Kabanov (Geological Survey of Canada, Calgary, Canada) and Lida Goldchteine (Calgary, Canada) for advice on Russian translations, and Wendy Witczak (Mount Royal University, Calgary, Canada) for interlibrary loan services. MS acknowledges financial support from the Swedish Research Council (Vetenskapsrådet, grant number 621-2011-4961). PAJ thanks the Natural Sciences and Engineering Research Council of Canada (Grant #244503), Mount Royal University Faculty of Science and Technology, and the Royal Tyrrell Museum of Palaeontology for research funding and/or logistical support. Pavel Parkhaev (Borissiak Paleontological Institute of Russian Academy of Sciences, Moscow, Russia) and Christian Skovsted (Swedish Museum of Natural History, Stockholm, Sweden) provided helpful comments in their reviews of the manuscript.
References
Aitken, J.D. 1997. Stratigraphy of the Middle Cambrian Platformal Succession, Southern Rocky Mountains. Geological Survey of Canada Bulletin 398: 1–322. Crossref
Aksarina, N.A. 1968. Probivalvia—a new class of the oldest mollusks [in Russian]. Novye dannye po geologii i poleznym iskopaemym zapadnoj Sibiri 3: 77–86.
Aksarina, N.A. and Pel’man, Y.L. 1978. Cambrian brachiopods and bivalve molluscs of Siberia [in Russian]. Trudy Akademii Nauk SSSR, Sibirskoj otdelenie 362: 1–179.
Babcock, L.E., Robison, R.A., Rees, M.N., Peng, S., and Saltzman, M.R. 2007. The Global boundary Stratotype Section and Point (GSSP) of the Drumian Stage (Cambrian) in the Drum Mountains, Utah, USA. Episodes 30: 84–94. Crossref
Balthasar, U., Skovsted, C.B., Holmer, L.E., and Brock, G.A. 2009. Homologous skeletal secretion in tommotiids and brachiopods. Geology 37: 1143–1146. Crossref
Bengtson, S., Conway Morris, S., Cooper, B.J., Jell, P.A., and Runnegar, B.N. 1990. Early Cambrian fossils from South Australia. Association of Australasian Palaeontologists Memoir 9: 1–364.
Butler, A.D., Streng, M., Holmer, L.E., and Babcock, L.E. 2015. Exceptionally preserved Mickwitzia from the Indian Springs Lagerstätte (Cambrian Stage 3), Nevada. Journal of Paleontology 89: 933–955. Crossref
Carlson, S.J. 1995. Phylogenetic relationships among extant brachiopods. Cladistics 11: 131–197. Crossref
Carlson, S.J. 2016. The evolution of Brachiopoda. Annual Review of Earth and Planetary Sciences 44: 409–438. Crossref
Carlson S.J., and Cohen B.L. 2020. Brachiopoda, Pan-Brachiopoda, Neoarticulata, Pan-Neoarticulata. In: K. de Queiroz, P.D. Cantino, and J.A. Gauthier (eds.), Phylonyms: A Companion to the PhyloCode, 521–526. CRC Press, Boca Raton.
Caron, J.-B. and Jackson, D.A. 2006. Taphonomy of the Greater Phyllopod Bed community, Burgess Shale. Palaios 21: 451–465. Crossref
Chen, J.-Y., Huang, D.-Y., and Chuang, S.-H. 2007. Re-interpretation of the lower Cambrian brachiopod Heliomedusa orienta Sun and Hou, 1987A as a discinid. Journal of Paleontology 81: 38–47. Crossref
Collom, C.J., Johnston, P.A., and Powell, W.G. 2009. Reinterpretation of “Middle” Cambrian stratigraphy of the rifted western Laurentian margin: Burgess Shale Formation and contiguous units (Sauk II Megasequence); Rocky Mountains, Canada. Palaeogeography, Palaeoclimatology, Palaeoecology 277: 63–85. Crossref
Conway Morris, S. 1986, The community structure of the Middle Cambrian phyllopod bed (Burgess Shale). Palaeontology 29: 423–467.
Cox, L.R. 1969. General features of Bivalvia. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, N, Mollusca 6, N2–N129. University of Kansas, Lawrence.
Daley, R.L. and Boyd, D.W. 1996. The role of skeletal microstructure during selective silicification of brachiopods. Journal of Sedimentary Research 66: 155–162. Crossref
Deiss, C. 1940. Lower and Middle Cambrian stratigraphy of southwestern Alberta and southeastern British Columbia. Geological Society of America Bulletin 51: 731–793. Crossref
Dzik, J. 1981. Larval development, musculature, and relationships of Sinuitopsis and related Baltic bellerophonts. Norsk Geologisk Tidsskrift 61: 111–121.
Emig, C.C. 1992. Functional disposition of the lophophore in living Brachiopoda. Lethaia 25: 291–302. Crossref
Enright, O.G.B., Minter, N.J., Sumner, E.J., Mángano, M.G., and Buatois, L.A. 2021. Flume experiments reveal flows in the Burgess Shale can sample and transport organisms across substantial distances. Nature Communications Earth & Environment 2 (104): 1–7. Crossref
Fletcher, T.P. and Collins, D.H. 1998. The Middle Cambrian Burgess Shale and its relationship to the Stephen Formation in the southern Canadian Rocky Mountains. Canadian Journal of Earth Sciences 35: 413–436. Crossref
Fletcher, T.P. and Collins, D.H. 2003. The Burgess Shale and associated Cambrian formations west of the Fossil Gully Fault Zone on Mount Stephen, British Columbia. Canadian Journal of Earth Sciences 40: 1823–1838. Crossref
Gaines, R.R. 2014. Burgess Shale-type preservation and its distribution in space and time. Paleontological Society Papers 20: 123–146. Crossref
Geyer, G. and Streng, M. 1998. Middle Cambrian pelecypods from the Anti-Atlas, Morocco. Revista Española de Paleontología no extr. Homenaje al Prof. Gonzalo Vidal: 83–96.
Holdaway, H.K. and Clayton, C.J. 1982. Preservation of shell microstructure in silicified brachiopods from the Upper Cretaceous Wilmington Sands of Devon. Geological Magazine 119: 371–382. Crossref
Holmer, L.E., Popov, L.E., Ghobadi Pour, M., Claybourn, T., Zhang, Z., Brock, G.A., and Zhang, Z. 2018. Evolutionary significance of a middle Cambrian (Series 3) in situ occurrence of the pedunculate rhynchonelliform brachiopod Nisusia sulcata. Lethaia 51: 424–432. Crossref
Holmer, L.E., Skovsted, C.B., Brock, G.A., Valentine, J.L. and Paterson, J.R. 2008. The Early Cambrian tommotiid Micrina, a sessile bivalved stem group brachiopod. Biological Letters 4: 724–728. Crossref
Holmer, L.E., Skovsted, C.B., Larsson, C., Brock, G.A., and Zhang, Z. 2011. First record of a bivalved larval shell in Early Cambrian tommotiids and its phylogenetic significance. Palaeontology 54: 235–239. Crossref
Horný, R. 1957. Problematic molluscs (?Amphineura) from the Lower Cambrian of south and east Siberia (USSR). Sborník Ústředního ústavu geologického, Oddíl paleontologický 23: 297–432.
Jin, J., Zhan, R., Copper, P., and Caldwell, W.G.E. 2007. Epipunctae and phosphatized setae in Late Ordovician plaesiomyid brachiopods from Anticosti Island, Eastern Canada. Journal of Paleontology 81: 666–683. Crossref
Johnston, K.J., Johnston, P.A., and Powell, W.G. 2009a. A new Middle Cambrian, Burgess Shale-type biota, Bolaspidella Zone, Chancellor Basin, southeastern British Columbia: Palaeogeography, Palaeoclimatology, Palaeoecology 277: 106–126. Crossref
Johnston, P.A. 1993. Lower Devonian Pelecypoda from southeastern Australia. Association of Australasian Palaeontologists Memoir 14: 1–134.
Johnston, P.A., Collom, C.J., and Desjardins, P. 2017. Lower to Middle Cambrian of the southern Canadian Rockies. In: J.C.C. Hsieh (ed.), Geologic Field Trips of the Canadian Rockies, 2017 Meeting of the GSA Rocky Mountain Section. Geological Society of America Field Guide 48: 71–121.
Johnston, P.A., Johnston, K.J., Collom, C.J., Powell, W.P., and Pollock, R.J. 2009b. Paleontology and depositional environments of ancient brine seeps in the Middle Cambrian Burgess Shale at The Monarch, British Columbia, Canada. Palaeogeography, Palaeoclimatology, Palaeoecology 277: 86–105. Crossref
Johnston, P.A., Streng, M., and Virmani, N. 2017. New observations on the enigmatic Cambrian Stenothecoida. 26th Canadian Paleontology Conference, Calgary, Proceedings 14: 18.
Knight, J.B. and Yochelson, E.L. 1958. A reconsideration of the Monoplacophora and the primitive Gastropoda. Proceedings of the Malacological Society of London 33: 37–48. Crossref
Knight, J.B. and Yochelson, E.L. 1960. Monoplacophora. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, I, Mollusca 1, 177–184. University of Kansas Press, Lawrence.
Koneva, S.P. 1976. New members of the class Stenothecoida from the Lower Cambrian of central Kazakhstan. Paleontologičeskij žurnal 1976 (2): 230–233. [in Russian]
Koneva, S.P. 1979a. Cambrian stenothecoids from Maly Karatau and Tamdytia [in Russian]. Paleontologičeskij žurnal 1: 44–51.
Koneva, S.P. 1979b. Stenothecoids and inarticulate brachiopods of the Lower Cambrian and basal Middle Cambrian of Central Kazakhstan [in Russian]. Trudy Instituta Geologii, AN SSR Kazakhstana, Alma Ata 1979: 1–124.
Kouchinsky, A. 2000. Shell microstructures in early Cambrian molluscs. Acta Palaeontologica Polonica 45: 119–150.
Kouchinsky, A., Bengtson, S., Runnegar, B., Skovsted, C., Steiner, M., and Vendrasco, M. 2012. Chronology of early Cambrian biomineralization. Geological Magazine 149: 221–251. Crossref
Landing, E. and Kouchinsky, A. 2016. Correlation of the Cambrian Evolutionary Radiation: geochronology, evolutionary stasis of earliest Cambrian (Terreneuvian) small shelly fossil (SSF) taxa, and chronostratigraphic significance. Geological Magazine 153 (4): 750–756. Crossref
Larsson, C.M., Skovsted, C.B., Brock, G.B., Balthasar, U., Topper, T.P., and Holmer, L.E. 2014. Paterimitra pyramidalis from South Australia: Scleritome, shell structure and evolution of a lower Cambrian stem group brachiopod. Palaeontology 57: 417–446. Crossref
Laurie, J.R. 1987. The musculature and vascular systems of two species of Cambrian Paterinida (Brachiopoda). BMR Journal of Australian Geology & Geophysics 10: 261–265.
Lemche, H. 1957. A new living deep-sea mollusc of the Cambro-Devonian class Monoplacophora. Nature 179: 413–416. Crossref
Lemche, L. 1960. A possible central place for Stenothecoides Resser, 1939 and Cambridium Horny, 1957 (Mollusca Monoplacophora) in invertebrate phylogeny. Reports of the International Geological Congress, XXl Session, Norden 22: 92–101.
Li, G., Zhang, Z., Hua, H., and Yang, H. 2014. Occurrence of the enigmatic bivalved fossil Apistoconcha in the Lower Cambrian of Southeast Shaanxi, North China Platform. Journal of Paleontology 88: 359–366. Crossref
Li, L., Zhang, X., Skovsted, C.B., Yun, H., Bing, P., and Li, G. 2019. Homologous shell microstructures in Cambrian hyoliths and molluscs. Palaeontology 62: 515–532.
McCollum, L.B. and Miller, D.M. 1991. Cambrian stratigraphy of the Wendover area, Utah and Nevada. United States Geological Survey, Bulletin 1948: 1–43.
McIlreath, I.A. 1977. Accumulation of a Middle Cambrian, deepwater limestone debris apron adjacent to a vertical, submarine carbonate escarpment, southern Rocky Mountains, Canada. In: H.E. Cook and P. Enos (eds.), Deep-water Carbonate Environments. Society of Economic Paleontologists and Mineralogists, Special Publication 25: 113–124. Crossref
Minichev, Y.S. [Miničev, Y.S.] and Starobogatov, Y.I. 1976. About the phylogenetic relationship of classes within the molluscs [in Russian]. In: L.A. Nevesskaâ (ed.), Sostoânie izučennosti grup organičeskogo mira dvustvorčatye mollûski, 205–274. Paleontologičeskij Institut RAN, Moskva.
Missarzhevsky, V.V. [Missarževskij, V.V.] and Mambetov, A.M. 1981. Stratigraphy and fauna of the Precambrian–Cambrian boundary beds of the Malyi Karatau Range [in Russian]. Trudy Instituta Geologii, Akademia Nauk SSSR 326: 1–92.
Nowlan, T.P., Merriam, C.W., and Williams, J.S. 1956. The stratigraphic section in the vicinity of Eureka, Nevada. Geological Survey Professional Paper 276: 1–77. Crossref
Parkhaev, P.Y. 1998. Siphonoconcha—a new class of Early Cambrian bivalved organisms. Paleontological Journal 32: 1–15.
Parkhaev, P.Y. 2008. The Early Cambrian radiation of Mollusca. In: W.F. Ponder and D.R. Lindberg (eds.), Phylogeny and Evolution of the Mollusca, 33–69. University of California Press, Berkeley. Crossref
Peel, J.S. 1988. Molluscs of the Holm Del Formation (late Middle Cambrian), central North Greenland. Meddeleser om Grønland. Geoscience 20: 145–168.
Peel, J.S. 2021. Ontogeny, morphology and pedicle attachment of stenothecoids from the middle Cambrian of North Greenland (Laurentia). Bulletin of Geosciences 96: 381–399. Crossref
Pel’man, Y.L. 1985. New stenothecoids from the Lower Cambrian of western Mongolia [in Russian]. Trudy, Institut Geologii i Geofiziki, Sibirskoe otdelenie, Akademiâ Nauk SSSR 632: 103–114.
Pojeta, J. Jr. 1980. Molluscan phylogeny. Tulane Studies in Geology and Paleontology 16: 55–80.
Pojeta, J. Jr. 2000. Cambrian Pelecypoda (Mollusca). American Malacological Bulletin 15: 157–166.
Pojeta, J. Jr. and Runnegar, B. 1976. The palaeontology of rostroconch molluscs and the early history of the phylum Mollusca. United States Geological Survey, Professional Paper 968: 1–88. Crossref
Popov, L.E. and Williams, A. 2000. Class Kutorginata. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H, Brachiopoda Revised, Vol. 2, 208–215. Geological Society of America and University of Kansas Press, Boulder and Lawrence.
Poulsen, C. 1932. The Lower Cambrian faunas of East Greenland. Meddelelser om Grønland 87 (6): 1–66.
Pratt, B.R., Spincer, B.R., Wood, R.A., and Zhuravlev A.Y. 2001. Ecology and evolution of Cambrian reefs. In: A.Y. Zhuravlev and R. Riding (eds.), The Ecology of the Cambrian Radiation, 254–274. Columbia University Press, New York. Crossref
Radugin, K.V. 1937. On the relations of the Cambrian and Precambrian in the Gornaya Shoriya [in Russian]. Problemy Sovetskoj Geologii 7 (4): 295–317.
Rasetti, F. 1954. Internal shell structures in the Middle Cambrian gastropod Scenella and the problematic genus Stenothecoides. Journal of Paleontology 28: 59–66.
Rasetti, F. 1957. Additional fossils from the Middle Cambrian Mt. Whyte Formation of the Canadian Rocky Mountains. Journal of Paleontology 31: 955–972.
Resser, C.E. 1938. Fourth contribution to nomenclature of Cambrian fossils. Smithsonian Miscellaneous Collections 97 (l0): 1–43.
Robison, R.A. 1964. Late Middle Cambrian faunas from western Utah. Journal of Paleontology 38: 510–566.
Rowell, A.J. 1965. [Listing in] Generic names erroneously ascribed to Brachiopoda. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part H, Vol. 2, Brachiopoda, H864. Geological Society of America, New York.
Rowell, A.J. and Caruso, N.E. 1985. The evolutionary significance of Nisusia sulcata, an early articulate brachiopod. Journal of Paleontology 59: 1227–1242.
Rozov, S.N. 1984. Morphology, terminology, and systematic affinity of stenothecoids [in Russian]. Trudy, Institut Geologii i Geofiziki, Sibirskoe otdelenie, Akademiâ Nauk SSSR 597: 117–133.
Rozanov, A.Y. and Missarzhevsky, V.V. [Missarževskij, V.V.] 1966. Biostratigraphy and fauna of lower Cambrian horizons [in Russian]. Trudy Geologičeskogo Instituta AN SSSR 148: 1–119.
Rozanov, A.Y. and Zhuravlev, A.Y. 1992. The Lower Cambrian fossil record of the Soviet Union. In: J.H. Lipps and P.W. Signor (eds.), Origin and Early Evolution of the Metazoa, 205–282. Plenum Press, New York. Crossref
Rudwick, M.J.S. 1970. Living and Fossil Brachiopods. 199 pp. Hutchinson & Co. Ltd., London,
Runnegar, B. 1985. Shell microstructures of Cambrian mollusks replicated by phosphate. Alcheringa 9: 245–257. Crossref
Runnegar, B. and Pojeta, J. Jr. 1974. Molluscan phylogeny: The paleontological viewpoint. Science 186: 311–317. Crossref
Runnegar, B. and Pojeta, J. Jr. 1992. The earliest bivalves and their Ordovician descendants. American Malacological Bulletin 9: 117–122.
Schmitt, J.G. and Boyd, D.W. 1981. Patterns of silicification in Permian pelecypods and brachiopods from Wyoming. Journal of Sedimentary Research 51: 1297–1308. Crossref
Skovsted, C.B. 2006. Small shelly fauna from the late Early Cambrian Bastion and Ella Island Formations, North-East Greenland. Journal of Paleontology 80: 1087–1112. Crossref
Skovsted, C.B. 2016. A silicified tommotiid from the lower Cambrian of Greenland. Bulletin of Geosciences 91: 553–559. Crossref
Skovsted, C.B. and Holmer, L.E. 2005. Early Cambrian brachiopods from north-east Greenland. Palaeontology 48: 325–345. Crossref
Skovsted, C.B., Betts, M.J., Topper, T.P., and Brock, G.A. 2015. The early Cambrian tommotiid genus Dailyatia from South Australia. Memoirs of the Association of Australasian Palaeontologists 48: 1–117.
Skovsted, C.B., Brock, G.A., Paterson, J.R., Holmer, L.E., and Budd, G.E. 2008. The scleritome of Eccentrotheca from the Lower Cambrian of South Australia: lophophorate affinities and implications for tommotiid phylogeny. Geology 36: 171–174. Crossref
Skovsted, C.B., Brock, G.A., Topper, T.P., Paterson, J.R., and Holmer, L.E. 2011. Scleritome construction, biofacies, biostratigraphy and systematics of the tommotiid Eccentrotheca helenia sp. nov. from the Early Cambrian of South Australia. Palaeontology 5: 253–286. Crossref
Skovsted, C.B., Clausen, S., Álvaro, J.J., and Ponlevé, D. 2014. Tommotiids from the Early Cambrian (Series 2, Stage, 3) of Morocco and the evolution of the tannuolinid scleritome and setigerous shell structures in stem group brachiopods. Palaeontology 57: 171–192. Crossref
Smith, E.F., Macdonald, F.A., Petach, T.A., Bold, U., and Schragg, D.P. 2016. Integrated stratigraphic, geochemical, and paleontological late Ediacaran to early Cambrian records from southwestern Mongolia. Geological Society of America Bulletin 128: 442–468. Crossref
Starobogatov, Y.I. 1970. A contribution to the systematics of the Early Paleozoic Monoplacophora. Paleontologičeskij žurnal 1970 (3): 6–16.
Streng, M., Butler, A.D., Peel, J.S., Garwood, R.J., and Caron, J.-B. 2016. A new family of Cambrian rhynchonelliformean brachiopods (Order Naukatida) with an aberrant coral-like morphology. Palaeontology 59: 269–293. Crossref
Sun, Y. and Baliński, A. 2008. Silicified Mississippian brachiopods from Muhua, southern China: Lingulids, craniids, strophomenids, productids, orthotetids, and orthids. Acta Palaeontologica Polonica 53: 485–524. Crossref
Sytchev, L.A. [Sytčev, L.A.] 1960. Phylum Mollusca: Mollusks. Class Lamellibranchiata: Pelecypods [in Russian]. In: L.L. Halfina (ed.), Biostratigrafiâ paleozoâ Saâno-Altajskoj Gornoj oblasti. Trudy SNIIGGIMS 19: 253–256.
Termier, H. and Termier, G. 1968. Evolution et biocinèse. 241 pp. Masson, Paris.
Thomas, R.D.K., Runnegar, B., and Matt, K. 2020. Pelagiella exigua, an early Cambrian stem gastropod with chaetae: lophotrochozoan heritage and conchiferan novelty. Palaeontology 63: 1–27. Crossref
Varlamov, A.I., Rozanov, A.Y., Khomentovsky, V.V. [Komentovskij, V.V.], Shabanov, Y.Y. [Šabanov, Y.Y.], Abaimova, G.P., Demidenko, Y.E., Karlova, G.A., Korovnikov, I.V., Luchinina, V.A. [Lučinina, V.A.], Malakhovskaya, Y.E. [Malahovskaâ, Y.E.], Parkhaev, P.Y. [Parhaev, P.Y.], Pegel, T.V., Skorlotova, N.A., Sundukov, V.M., Sukhov, S.S. [Suhov, S.S.], Fedorov, A.B., and Kipriyanova, L.D. [Kipriânova, L.D.] 2008. Kembrii Sibirskoj platformy. Kniga 1: Aldano-Lenskij region]. 232 pp. PIN RAS, Moskva.
Valentine, J.W. 2004. On the Origin of Phyla. 613 pp. University of Chicago Press, Chicago.
Vendrasco, M., Porter,
S.M., Kouchinsky, A.V., Li, G., and Fernandez, C.Z. 2010. Shell
microstructures in early mollusks. The
Festivus 42 (4): 43–53. Crossref
Vinther, J. 2015. The origins of mollusks. Palaeontology 58: 19–34. Crossref
Virmani, N. and Johnston, P.A. 2017. A middle Cambrian small shelly fauna from carbonate members of the Burgess Shale Formation, British Columbia. Geological Society of America, Rocky Mountain Section, 69th Annual Meeting Calgary, Canada, Abstract with Programs 49 (5): Paper No. 6-1. Crossref
Voronin, Y.I., Voronova, L.G., Grigoreva, N.V., Drozdova, N.A., Zhegallo, E.A. [Žegallo, E.A.], Zhuravlev, A.Y. Žuravlev, A.Y.], Ragozina, A.L., Rozanov, A.Y., Sayutina, T.A. [Saûtina, T.A.], Sysoev, V.A., and Fonin, V.D. 1982. The Precambrian–Cambrian boundary in the geosynclinal areas (the reference section of Salany Gol, MPR) [in Russian]. Trudy Sovmestnoj Sovetsko-Mongol’skoj Paleontologičeskoj Ekspedicii 18: 1–150.
Walcott, C.D. 1884. Paleontology of the Eureka district. United States Geological Survey Monograph 8: i–xiv + 1–298. Crossref
Walcott, C.D. 1886. Second contribution to the studies on the Cambrian faunas of North America. United States Geological Survey Bulletin 30: 1–369. Crossref
Walcott, C.D. 1912. Cambrian Brachiopoda. United States Geological Survey Monograph 51: 1–872.
Walcott, C.D. 1917a. Fauna of the Mount Whyte formation. Cambrian geology and paleontology. Smithsonian Miscellaneous Collections 67 (3): 61–115.
Walcott, C.D. 1917b. Nomenclature of some Cordilleran formations. Cambrian geology and paleontology IV. Smithsonian Miscellaneous Collections 67 (1): 1–8.
Westbroek, P., Yanagida, J., and Isa, Y. 1980. Functional morphology of brachiopod and coral skeletal structures supporting ciliated epithelia. Paleobiology 6: 313–330. Crossref
Wheeler, H. E., and Lemmon, D. M., 1939. Cambrian formations of the Eureka and Pioche districts, Nevada. Nevada University Bulletin 33 Geology and Mining Series 3: 1–57.
Williams, A. 1968. Evolution of the shell structure of articulate brachiopods. Special Papers in Palaeontology 2: 1–55.
Williams, A. 1990. Biomineralization in the lophophorates. In: J.G. Carter (ed.), Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. Volumes 1 and 2, 67–82. Van Nostrand Reinhold, New York. Crossref
Williams, A. 1997. Shell structure. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H (Revised), Vol. 1, Brachiopoda, H267–H320. University of Kansas, Lawrence.
Williams, A. and Rowell, A.J. 1965. Brachiopod anatomy. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part H, Vol. 1, Brachiopoda, H6–H57. University of Kansas Press, Lawrence.
Williams, A., Brunton, H.C., and MacKinnon, D.I. 1997. Morphology. In: R.L. Kaesler (ed.), Treatise on Invertebrate Paleontology, Part H (Revised), Vol. 1, Brachiopoda, H321–H440. University of Kansas, Lawrence. Crossref
Williams, A., Popov, L.E., Holmer, L.E., and Cusack, M. 1998. The diversity and phylogeny of the paterinate brachiopods. Palaeontology 41: 221–262.
Wright, A.D. 1979. Brachiopod radiation. In: M.R. House (ed.), The Origin of Major Invertebrate Groups, 235–252. Academic Press, London.
Yochelson, E.L. 1968. Stenothecoida, a proposed new class of Cambrian Mollusca. International Palaeontological Union, Prague, Czechoslovakia, August 20–27, 1968, Abstracts, 34.
Yochelson, E.L. 1969. Stenothecoida, a proposed new class of Cambrian Mollusca. Lethaia 2: 49–62. Crossref
Yochelson, E.L. 2000. Stenothecoida. In: R. Singer (ed.). Encyclopedia of Paleontology, Volume 2, 767–768. Fitzroy Dearborn, New York. Crossref
Ye, F., Crippa, G., Angiolini, L., Brand, U., Capitani, G., Cusack, M., Garbelli, C., Greisshaber, E., Harper, E., and Schmahl, W. 2018. Mapping of recent brachiopod microstructure: A tool for environmental studies. Journal of Structural Biology 201: 221–236. Crossref
Yu, W. 1996. Early Cambrian stenothecoid molluscs from China. Records of the Western Australian Museum 18: 209–217.
Zhang, R. 1980. The earliest bivalve fauna; bivalves from the Lower Cambrian Tianheban Formation, Xianfen, Hubei [in Chinese with English abstract]. Bulletin of the Yichang Institute of Geology and Mineral Resources, Chinese Academy of Geological Sciences 1 (1): 1–17.
Zhang, Z., Holmer, L.E., Ou, Q., Han, J., and Shu, D. 2011. The exceptionally preserved Early Cambrian stem rhynchonelliform brachiopod Longtancunella and its implications. Lethaia 44: 490–495. Crossref
Zhang, Z.F., Li, G.X., Holmer, L.E., Brock, G.A., Balthasar, U., Skovsted, C.B., Fu, D.J., Zhang, X.L., Wang, H.Z., Butler, A., Zhang, Z.L., Cao, C.Q., Han, J., Liu, J.N., and Shu, D.G. 2014. An early Cambrian agglutinated tubular lophophorate with brachiopod characters. Scientific Reports 4: 4682. Crossref
Zhao, F., Smith, M.R., Yin, Z., Zeng, H., Li, G., and Zhu, M. 2017. Orthrozanclus elongata n. sp. and the significance of sclerite-covered taxa for early trochozoan evolution. Scientific Reports 7 (1): 16232. Crossref
Zhuravlev, A.Y. 1996. Reef ecosystem recovery after the Early Cambrian extinction. In: M.B. Hart (ed.), Biotic Recovery from Mass Extinction Events. Geological Society of London Special Publication 102: 79–96. Crossref
Zhuravlev, A.Y. 2015. The early history of the Metazoa—a paleontologist’s viewpoint. Biology Bulletin Reviews 5: 415–461. Crossref
Zhuravlev, A.Y. and Wood, R.A. 2008. Eve of biomineralization: controls on skeletal mineralogy. Geology 36: 923–926. Crossref
Acta Palaeontol. Pol. 66 (4): 723–751, 2021
https://doi.org/10.4202/app.00928.2021