

Carnassiform notches improve the functional efficiency of bat molar shearing crests
NICHOLAS J. CZAPLEWSKI and CHARLES G. BAKER
Czaplewski N.J. and Baker, C.G. 2022. Carnassiform notches improve the functional efficiency of bat molar shearing crests. Acta Palaeontologica Polonica 67 (1): 257–282.
We surveyed molar surface morphology of bats of 281 extant and extinct species in 5 archaic and 19 extant families using scanning microscopy. We note the occurrence of structural features on talonid crests, the cristid obliqua, postcristid, and entocristid, and their absence in upper molars, even of the same species having them on lowers. We term the structures “carnassiform notches” (CN) for their resemblance to similar features on the carnassial teeth of carnivorans. A CN consists of a small cleft in the edge of a talonid shearing crest accompanied by an adjacent “accessory trough” on the basinward side of the notch. The CN occur in bats with tribosphenic molar morphology and insectivorous or insectivorous–omnivorous dietary habits. Of 19 extant families examined, eight include members that possess lower molars with a CN in at least the cristid obliqua: Megadermatidae, Nycteridae, Mystacinidae, Furipteridae, Thyropteridae, Phyllostomidae, Natalidae, and Vespertilionidae (Murininae and Kerivoulinae only). An extinct genus of Hipposideridae, Vaylatsia, shows CN although extant hipposiderids do not. In extinct families for which lower molar fossils are available, notches were not recognized on the talonids, indicating the condition is not plesiomorphic for bats and probably evolved convergently in different lineages. Where present, the CN or troughs are morphologically consistent within a family, and might serve in some cases as characters supporting phylogenetic analyses and clade diagnoses. CN and accessory troughs probably increase the functional efficiency at sectioning chitin by increasing the effective length of a crest while maintaining the same cusp-to-cusp distance and precise occlusal relationships, and by improving the food-capture area of the shearing blade during occlusion. The accessory troughs provide an immediately adjacent fragment-clearance area. The increased sophistication of this food-processing system might be particularly important in species that must quickly acquire, chew, and swallow their food and resume echolocating in flight. The common ancestor of bats probably did not have CN in its molars, and the presence of CN does not signal carnivory in bats.
Key words: Mammalia, Chiroptera, molars, talonid crests, dental morphology, shearing blades, bio-engineering, functional morphology, functional design.
Nicholas J. Czaplewski [nczaplewski@ou.edu] and Charles Baker [cbaker@ou.edu], Section of Vertebrate Paleontology, Oklahoma Museum of Natural History, University of Oklahoma, Norman, OK 73071, USA.
Received 18 March 2021, accepted 21 December 2021, available online 30 March 2022.
Copyright © 2022 N.J. Czaplewski and C. Baker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Complex molar teeth are a defining characteristic of the class Mammalia, and the morphology and evolution of the diverse kinds of mammal teeth have been frequent topics of study. Molar morphological features are used to identify mammals and gain insight into their dietary habits, function, and phylogenetic relationships. Bats figure prominently in research on molar structure and development, skull and feeding function, and evolution (e.g., Marshall and Butler 1966; Slaughter 1970; Menu and Sigé 1971; Phillips 1971, 2000; Freeman 1979, 1981, 1984, 1988, 1995, 1998; Koopman and MacIntyre 1980; Menu 1985, 1987; Strait 1993; Dumont 1995; Simmons and Geisler 1998; Rossina 2003; Evans 2006; Fracasso et al. 2011; Santana et al. 2011b; Horáček and Špoutil 2012; Zuercher et al. 2020; Esquivel et al. 2021; López-Aguirre et al. 2021). The tribosphenic molar is the commonest molar configuration among crown therians, and has been touted as one of the main reasons for the success of the mammalian clade because of its combination of shearing, crushing, grinding, and transporting functions with precise hemimandibular and joint kinematics during the chewing stroke (Bhullar et al. 2019). Bats are among those mammals having tribosphenic molars and also exhibiting very precise occlusion of interlocking shearing blades, providing them with “teeth of perfection” (Evans and Sanson 2003; Horáček and Špoutil 2012). In most bats, the P4 and upper molar row form a series of W-shaped ectolophs that are paired with the precisely-corresponding shearing blades of lower molars. This configuration led Freeman (1988) to liken the apparatus to a pair of pinking shears, and she remarked that each upper and lower molar pair provides “four pairs of small carnassials per molar” (Freeman 1988: 268). The geometry of the shearing blades in particular provides a nearly ideal functional design when viewed as bio-engineering models for rapid and efficient mechanical breakup of insect chitin (Evans and Sanson 1998, 2003; Evans 2005, 2006). In bats, the shearing modality overshadows the crushing-grinding modality of tribosphenic molars (Horáček and Špoutil 2012). The ubiquity and commonness of insects (as well as many other interacting factors) has no doubt contributed to the evolutionary success of bats, as well as that of many other small mammals. Important components of the shearing blades of bat molars are “crest sharpness, resistance of the crest edges to mechanical stress of attrition and the total length of the crest system of the dentition” and their highly precise alignment and interlocking (Horáček and Špoutil 2012). In this paper we focus on a similar pattern at a progressively smaller scale contributing to the effective length and functional efficiency of parts of the shearing crest system on the lower molar talonids of bats.
Carnassial notches are features of the meat-shearing cheek teeth in various groups of carnivorous mammals, usually consisting of a carnassial pair made of P4 and m1 in the Carnivora. These teeth bear a shearing blade with a median carnassial notch, which was defined as “the sharp excavation in the form of an inverted keyhole which occurs between the main shearing blades on meat-cutting teeth. In miacids [extinct early carnivorans], a carnassial notch occurs between paracone and metastyle on P4 and between protoconid and paraconid on m1. In highly specialized carnivores, the carnassial notch may be elongated into a slitlike groove, called the carnassial slit; similarly shaped but less emphatic structures occur elsewhere in carnivore dentitions and may be called carnassiform notches” (italics in original; MacIntyre 1966: 123). Similar notches have been described in the lower molars of various other extinct mammals, for example, in the paracristids and protocristids of Cretaceous and Paleocene metatherians such as Mayulestes, Allqokirus, Andinodelphys, and Kokopellia (Cifelli and de Muizon 1997; de Muizon and Lange-Badré 1997; de Muizon and Ladevèze 2020: 679–680, fig. 48) and the Miocene sparassodont Acyon (Engelman et al. 2015: fig. 5), the hyaenodont Creodonta (Paleogene–Miocene placentals, which had 2–3 carnassial pairs but usually emphasized one pair, M1 and m2 or M2 and m3), and Mesonychia (Paleogene basal ungulates in which molars had carnassiform notches on laterally compressed blades but on different tooth surfaces, no true carnassials, e.g., Dissacus and Harpagolestes) (Butler 1946; Lang et al. 2021). Peigne et al. (2009: fig. 4) described an example of an Oligocene soricomorph (Plesiosoricidae: Siamosorex) whose molars qualify as carnassial-like.
In the course of studying the teeth of fossil bats, and specifically in searching for qualitative dental morphological characters that might be useful in morphological phylogenetic analyses, Czaplewski (1997) noted certain small notches in the talonid crests of bat molars. Czaplewski et al. (2003) and Morgan and Czaplewski (1999, 2003) termed these “carnassial-like notches” and used them in systematic studies of fossil bats in the families Thyropteridae, Furipteridae, and Natalidae. More appropriately called carnassiform notches (following the usage of MacIntyre 1966 noted above), these are tiny structures that occur on the lower molars of certain bats and other small mammals. When present, these notches are developed on the cristid obliqua and postcristid of the lower molars, especially m1 and m2, occasionally in m3. The notches are sometimes accompanied by an accessory trough that runs basinward from the crests either as a round-bottomed or V-bottomed valley into the talonid basin, and sometimes as a shallow or deep oval hollow immediately adjacent to the crest on the talonid basin side. The term carnassiform notch points at their resemblance to the central notch in the sectorial blade, the true carnassial notch of the carnassial teeth of many carnivorans. In mammalian teeth where shearing blades such as these function in opposition to one another during shearing, the occluding crest edges, especially where they are concave, have been called the “capture area” or “food capture area,” while the adjoining basins, the protofossa (trigon basin), parafossa, and metafossa of upper molars and the trigonid basin and talonid basin of lower molars, provide channels for “fragment clearance” where food particles can flow away from the crests (Evans 2005, 2006; Evans and Sanson 2005). The morphology and functional significance of these notches and their distribution among bat families have not previously been investigated. The goals of the present study were to make an initial attempt to describe and classify the types of carnassiform notches in bat molars and to determine their occurrence in extant and extinct bat families. In addition, we considered the implications of the familial distribution of carnassiform notches in bat evolution relative to published phylogenies of bats.
Diet and foraging styles are profoundly tied to the evolution of morphology, flight, echolocation, and many other aspects of the behavior, physiology, and ecology of bats. Many previous studies on the diet of bats have documented how various bats have adopted diverse feeding strategies as insectivores, carnivores, piscivores, sanguinivores, nectarivores, and omnivores, which are correlated with tooth morphology (Phillips 2000; Aguirre et al. 2003). Because of the differing or variable behavioral and physical properties of certain types of food, such as its size, its ability to evade predators, and its resistance to mechanical breakdown, bat teeth can differ even among members of the same trophic guild consuming outwardly similar types of prey (Aguirre et al. 2003).
Dental morphology reflects the diet of bats and other mammals and their dental complexity is strongly related to functional aspects of feeding efficiency (Kay and Hiiemae 1974; Kay and Sheine 1979; Santana et al. 2011a, b), as are cranial and jaw shape, musculature, and other factors (Dumont 1999; Aguirre et al. 2003; Dumont et al. 2012). The molar teeth of insectivorous bats strongly resemble molars of many other mammalian insectivores; insectivory is considered the plesiomorphic condition in bats and other Laurasiatherian outgroups that are phylogenetically close to bats. Most extant insectivorous bats are adapted to eat only certain types of insects and other arthropods, with factors such as the thickness of the chitinous exoskeleton influencing prey selection (Freeman 1979). Miller (1907: 34–35) and Freeman (1998) described the chewing action of chiropteran molar teeth, which resembles that in many other mammals having tribosphenic and modified tribosphenic teeth (e.g., Crompton 1971; Crompton and Hiiemae 1969, 1970; Butler 1972; Kay and Hiiemae 1974; Kay 1975, 1984; Rose and Simons 1977; Davis 2011). The refinements made by these authors in their descriptions of the functional components of mammalian molars, including Phase I and Phase II stages of mastication and the attendant wear facets that are produced by shearing, crushing, and grinding actions applied to the food being processed (Kay and Hiiemae 1974; Schultz et al. 2020), can readily be applied to insectivorous bat molars. In bats, tooth development and use throughout ontogeny is characterized by a type of teeth that functions best when the enamel covering is intact and wear facets are parallel to the enamel surface (other types in other mammal taxa function best when dentin is exposed; Koenigswald 2020).
Freeman (2000) noted that carnivory evolved at least three times in bats and in each case it includes the largest members of the respective families: Nycteris grandis in Nycteridae, Macroderma gigas in Megadermatidae, and Vampyrum spectrum in Phyllostomidae. Bats that are primarily meat eaters (Gual-Suárez and Medellín 2021) show a tendency in their lower molars to develop an enlarged trigonid having a paracristid with a long shearing blade and a shortened protocristid and talonid to produce a tooth reminiscent of the m1 of Canidae. Associated with the elongated paracristid on the lower molars of these bats is an elongated postmetacrista on upper molars.
Behavior and morphological adaptations for feeding, along with locomotor specializations that allowed adaptations for certain environments, account for much of the adaptive radiation in mammals (Gillette 1975). Adaptations in bats have stemmed from the evolution of, and variations in, their flying ability (Gillette 1975; Simmons 1998; Storch and Habersetzer 1988). As bats diversified, feeding strategies other than insectivory evolved (Gillette 1975).
Herein we present preliminary observations and a list of occurrences of carnassiform notches and associated structures in the lower molar talonids of diverse taxa of bats; however, these morphological features should be more intensively studied in order to be functionally (and phylogenetically) informative (sensu Santana et al. 2011a, b; Dávalos et al. 2012), Significantly, the diets of many species of bats must also be much better known in order for these kinds of morphological characters to become meaningful in understanding their role in bat evolution.
Institutional abbreviations.—AMNH, American Museum of Natural History, New York, USA; FMNH, Field Museum, Chicago, USA; OMNH, Oklahoma Museum of Natural History, Norman, USA; USNM, United States National Museum of Natural History, Washington, D.C., USA.
Other abbreviations.—CN, carnassiform notch or notches. We follow standard convention in abbreviating tooth families as I, C, P, and M, with upper and lower case letters referring to upper and lower teeth, respectively.
Material and methods
We borrowed and examined specimens of extant species from four institutions: AMNH, FMNH, OMNH, and USNM. Classification follows Simmons and Cirranello (2020) for extant bats and Smith et al. (2012) for archaic bats.
We examined the gross molar surface morphology of 1–4 representative specimens from each of about 23 families, 174 genera, and 288 species of extant and extinct bats (see list of specimens studied in Table 1 and SOM, Supplementary Online Material available at http://app.pan.pl/SOM/app67-Czaplewski_Baker_SOM.pdf). Specimens with unworn teeth were not always available in museum collections, and specimens with as little tooth wear as possible were used. Members of the rare extant families Rhinonycteridae (Foley et al. 2014), Craseonycteridae, and Cistugidae (Lack et al. 2010) were unavailable for study. Published images often do not provide enough detail in the teeth to show CN. Bats without normal tribosphenic, dilambdodont lower molars were eliminated from further consideration. The molars of these bats underwent evolutionary changes that resulted in modifications obliterating the basic tribosphenic molar type. As such they lack typical trigonids/talonids and talonid crests in the lower molars. As a result, most Pteropodidae (flying foxes) and several subfamilies of Phyllostomidae with derived teeth were excluded from our study. (A modified-tribosphenic lower molar attributed to the Megachiroptera [Pteropodidae] from the early to middle Eocene of Namibia [Pickford 2018] does not show carnassiform notches.) Among the Phyllostomidae, we studied representative members of all subgroups but many have dentitions derived for frugivorous and nectarivorous feeding, while the basal taxa Macrotinae, Micronycterinae, Phyllostominae, Lonchorhininae, and Glyphonycterinae (Dumont et al. 2012) have more generalized molars. Therefore, only bats with a relatively plesiomorphic molar morphology (tribosphenic molars) were examined in detail.
Table 1. List of bat families and species studied and the occurrence among them of carnassiform notches in unworn or little-worn lower molars. † indicates extinct taxa; AT, accessory trough; CO, cristid obliqua; PC, postcristid; “unknown” indicates condition for specimens in which the lower molars were too worn, occluded with debris, or otherwise unsuitable for scoring; “derived”, indicates species whose lower molars are specialized and lack the primitive tribosphenic molar pattern.
|
Taxon |
Notch absent |
Notch present |
Condition if notch present |
AT associated with cristid |
Age and locality (for fossils) |
|
†Onychonycteridae |
|
||||
|
†Ageina tobieni |
× |
|
|
|
early–middle Eocene; Europe and North America |
|
†Eppsinycteris anglica |
× |
|
|
|
early Eocene; MP8+9; England |
|
†Honrovits tsuwape |
× |
|
|
|
early Eocene; Wasatchian, Wa7; USA |
|
†”Hassianycteris” joeli |
× |
|
|
|
early Eocene; late Ypresian; Belgium |
|
†Marnenycteris michauxi |
× |
|
|
|
early Eocene; Ypresian; France |
|
†Icaronycteridae |
|
||||
|
†Icaronycteris? menui |
× |
|
|
weak |
early Eocene; France |
|
†Archaeonycteridae |
|
||||
|
†Archaeonycteris brailloni |
× |
|
|
|
early Eocene; France |
|
†Archaeonycteris? praecursor |
× |
|
|
|
earliest Eocene; Portugal |
|
†Australonycteris clarkae |
× |
|
|
|
earliest Eocene; ~54.6 Ma; Australia |
|
†Xylonycteris stenodon |
× |
|
|
|
early Eocene; MP10; France |
|
†Hassianycteridae |
|
||||
|
†Hassianycteris revilliodi |
× |
|
|
|
middle Eocene; Germany |
|
†Palaeochiropterygidae |
|
||||
|
†Anatolianycteris insularis |
× |
|
|
|
middle Eocene; Lutetian; Turkey |
|
†Palaeochiropteryx tupaiodon |
× |
|
|
weak |
middle Eocene; MP11; Germany |
|
†Cecilionycteris prisca |
× |
|
|
weak |
middle Eocene; MP13; Germany |
|
†Stehlinia sp. |
× |
|
|
× |
middle Eocene–late Oligocene; Europe |
|
†Philisidae |
|
||||
|
†Philisis sphingis |
× |
|
|
weak |
early Oligocene; Rupelian; Egypt |
|
Incertae sedis |
|
||||
|
†Necromantis adichaster |
× |
|
|
|
middle to late Eocene; France |
|
Pteropodidae? |
|
||||
|
?Megachiroptera |
× |
|
|
|
early or middle Eocene;
Ypresian– |
|
Rhinolophidae |
|
||||
|
Rhinolophus euryale |
× |
|
|
|
|
|
Rhinolophus ferrumequinum |
× |
|
|
|
|
|
Rhinolophus hipposideros |
× |
|
|
|
|
|
Hipposideridae |
|
||||
|
Hipposideros (†Pseudorhinolophus) morloti |
× |
|
|
|
late Eocene; MP17–19; Europe |
|
Hipposideros (†Pseudorhinolophus) schlosseri |
× |
|
|
|
late middle Eocene to early Oligocene; MP16–22; Europe |
|
†Vaylatsia ulmensis |
|
× |
CO + PC |
× |
late Oligocene; MP28–29; Germany |
|
Macronycteris gigas or M. vittatus |
× |
|
|
|
|
|
Megadermatidae |
|
||||
|
Cardioderma cor |
|
× |
CO only |
× |
|
|
Lavia frons |
× |
|
|
|
|
|
Lyroderma lyra |
|
× |
CO only |
× |
|
|
Macroderma gigas |
× |
|
|
|
|
|
Megaderma spasma |
|
× |
CO only |
× |
|
|
Rhinopomatidae |
|
||||
|
Rhinopoma hardwickii |
× |
|
|
|
|
|
Rhinopoma microphyllum |
× |
|
|
|
|
|
Rhinopoma muscatellum |
× |
|
|
|
|
|
Nycteridae |
|
||||
|
†Khoufechia gunnelli |
× |
|
|
|
latest early Eocene to earliest middle Eocene; Tunisia |
|
Nycteris arge |
|
× |
CO only |
× |
|
|
Nycteris aurita |
|
× |
CO only |
× |
|
|
Nycteris grandis |
|
× |
CO only |
× |
|
|
Nycteris hispida |
|
× |
CO only |
× |
|
|
Nycteris pallida |
|
× |
CO only |
× |
|
|
Nycteris thebaica |
|
× |
CO only |
× |
|
|
Emballonuridae |
|
||||
|
†Vespertiliavus kasserinensis |
× |
|
|
|
latest early Eocene to earliest middle Eocene; Tunisia |
|
†Vespertiliavus schlosseri |
× |
|
|
|
middle Eocene; France |
|
Balantiopteryx io |
× |
|
|
× |
|
|
Centronycteris centralis |
× |
|
|
× |
|
|
Centronycteris maximiliani |
|
× |
on entocristid only |
× |
|
|
Coleura afra |
|
× |
on entocristid only |
× |
|
|
Cormura brevirostris |
× |
|
|
× |
|
|
Cyttarops alecto |
|
× |
weak on entocristid |
× |
|
|
Diclidurus albus |
|
× |
weak on entocristid |
× |
|
|
Diclidurus ingens |
|
× |
weak on entocristid |
× |
|
|
Diclidurus isabella |
× |
|
|
× |
|
|
Diclidurus scutatus |
× |
|
|
× |
|
|
Emballonura alecto |
× |
|
|
× |
|
|
Mosia nigrescens |
|
× |
weak on entocristid |
× |
|
|
Peropteryx kappleri |
× |
|
|
× |
|
|
Peropteryx macrotis |
|
× |
on entocristid only |
× |
|
|
Peropteryx trinitatus |
|
× |
weak on entocristid |
× |
|
|
Rhynchonycteris naso |
× |
|
|
× |
|
|
Saccolaimus flaviventris |
× |
|
|
× |
|
|
Saccolaimus peli |
× |
|
|
× |
|
|
Saccolaimus saccolaimus |
× |
|
|
× |
|
|
Saccopteryx bilineata |
× |
|
|
× |
|
|
Saccopteryx canescens |
× |
|
|
× |
|
|
Saccopteryx gymnura |
|
× |
weak on entocristid |
× |
|
|
Saccopteryx leptura |
|
× |
weak on entocristid |
× |
|
|
Taphozous melanopogon |
× |
|
|
× |
|
|
Taphozous nudiventris |
× |
|
|
× |
|
|
Myzopodidae |
|
||||
|
†Phasmatonycteris butleri |
× |
|
|
|
late Eocene; Priabonian; Egypt |
|
†Phasmatonycteris phiomensis |
× |
|
|
|
early Oligocene; Rupelian; Egypt |
|
Myzopoda aurita |
× |
|
|
|
|
|
Myzopoda schliemanni |
× |
|
|
|
|
|
Mystacinidae |
|
||||
|
†Vulcanops jennyworthyae |
× |
|
|
|
Early Miocene; 19–16 Ma; New Zealand |
|
Mystacina tuberculata |
|
× |
CO + PC |
× |
|
|
Furipteridae |
|
||||
|
Amorphochilus schnablii |
|
× |
CO + PC + entocristid |
× |
|
|
Furipterus horrens |
|
× |
CO + PC + entocristid |
× |
|
|
Thyropteridae |
|
||||
|
Thyroptera discifera |
|
× |
CO + PC + entocristid |
× |
|
|
Thyroptera lavali |
|
× |
CO + PC + entocristid |
× |
|
|
Thyroptera tricolor |
|
× |
CO + PC + entocristid |
× |
|
|
Taxon |
Notch absent |
Notch present |
Condition if notch present |
AT associated with cristid |
Age and locality (for fossils) |
|
Noctilionidae |
|
||||
|
Noctilio †lacrimaelunaris |
× |
|
|
× |
Late Miocene; Huayquerian; Peru |
|
Noctilio albiventris |
× |
|
|
× |
|
|
Noctilio leporinus |
× |
|
|
× |
|
|
Mormoopidae |
|
||||
|
†Koopmanycteris palaeomormoops |
× |
|
|
× |
late Oligocene; early Arikareean; USA |
|
Mormoops blainvillei |
× |
|
|
× |
|
|
Pteronotus parnellii |
× |
|
|
× |
|
|
†Speonycteridae |
|
||||
|
†Speonycteris aurantiadens |
× |
|
|
weak |
late Oligocene; early Arikareean; USA |
|
Phyllostomidae |
|
||||
|
†Notonycteris magdalenensis |
× |
|
|
weak |
Late Miocene; Laventan; Colombia |
|
Macrotus californicus |
|
× |
CO + PC |
× |
|
|
Macrotus waterhousii |
|
× |
CO + PC |
× |
|
|
Lampronycteris brachyotis |
|
× |
CO + PC |
× |
|
|
Micronycteris brosseti |
|
× |
CO + PC |
× |
|
|
Micronycteris hirsuta |
|
× |
CO + PC |
× |
|
|
Micronycteris megalotis |
|
× |
CO + PC |
× |
|
|
Micronycteris microtis |
|
× |
CO + PC |
× |
|
|
Micronycteris minuta |
|
× |
CO + PC |
× |
|
|
Micronycteris schmidtorum |
|
× |
CO + PC |
× |
|
|
Diphylla ecaudata |
derived |
|
|
|
|
|
Desmodus rotundus |
derived |
|
|
|
|
|
Diaemus youngi |
derived |
|
|
|
|
|
Macrophyllum macrophyllum |
× |
|
|
weak |
|
|
Trachops cirrhosus |
× |
|
|
|
|
|
Lophostoma brasiliense |
|
× |
CO + PC |
× |
|
|
Lophostoma carrikeri |
|
× |
CO + PC |
× |
|
|
Lophostoma evotis |
|
× |
CO + PC |
× |
|
|
Lophostoma schulzi |
|
× |
CO + PC |
× |
|
|
Lophostoma silvicolum |
|
× |
CO + PC |
× |
|
|
Tonatia bidens |
|
× |
CO + PC |
× |
|
|
Tonatia saurophila |
|
× |
CO + PC |
× |
|
|
Mimon bennettii |
× |
|
|
|
|
|
Mimon cozumelae |
|
× |
CO + PC |
|
|
|
Gardnerycteris crenulatum |
|
× |
CO + PC |
× |
|
|
Phylloderma stenops |
|
× |
CO + PC |
× |
|
|
Phyllostomus discolor |
× |
|
|
× |
|
|
Phyllostomus elongatus |
|
× |
CO + PC |
× |
|
|
Phyllostomus hastatus |
|
× |
CO + PC |
× |
|
|
Chrotopterus auritus |
× |
|
|
|
|
|
Vampyrum spectrum |
× |
|
|
|
|
|
Lonchorhina aurita |
|
× |
CO + PC |
× |
|
|
Lonchorhina orinocoensis |
|
× |
CO + PC |
× |
|
|
Lionycteris spurrelli |
derived |
|
|
|
|
|
Lonchophylla handleyi |
derived |
|
|
|
|
|
Platalina genovensium |
derived |
|
|
|
|
|
Monophyllus plethodon |
derived |
|
|
|
|
|
Glossophaga longirostris |
derived |
|
|
|
|
|
Glossophaga soricina |
derived |
|
|
|
|
|
Leptonycteris curasoae |
derived |
|
|
|
|
|
Leptonycteris nivalis |
derived |
|
|
|
|
|
Brachyphylla cavernarum |
derived |
|
|
|
|
|
Erophylla sezekorni |
derived |
|
|
|
|
|
Phyllonycteris aphylla |
derived |
|
|
|
|
|
Anoura caudifer |
derived |
|
|
|
|
|
Anoura geoffroyi |
derived |
|
|
|
|
|
Anoura latidens |
derived |
|
|
|
|
|
Hylonycteris underwoodi |
derived |
|
|
|
|
|
Choeroniscus godmani |
derived |
|
|
|
|
|
Musonycteris harrisoni |
derived |
|
|
|
|
|
Lichonycteris obscura |
derived |
|
|
|
|
|
Scleronycteris ega |
derived |
|
|
|
|
|
Glyphonycteris daviesi |
|
× |
CO + PC |
× |
|
|
Glyphonycteris sylvestris |
|
× |
CO + PC |
× |
|
|
Trinycteris nicefori |
|
× |
CO + PC |
× |
|
|
Carollia perspicillata |
derived |
|
|
|
|
|
Rhinophylla pumilio |
derived |
|
|
|
|
|
Sturnira aratathomasi |
derived |
|
|
|
|
|
Chiroderma salvini |
derived |
|
|
|
|
|
Uroderma bilobatum |
derived |
|
|
|
|
|
Vampyressa thyone |
derived |
|
|
|
|
|
Vampyrodes caraccioli |
derived |
|
|
|
|
|
Platyrrhinus brachycephalus |
derived |
|
|
|
|
|
Platyrrhinus lineatus |
derived |
|
|
|
|
|
Enchisthenes hartii |
derived |
|
|
|
|
|
Ectophylla alba |
derived |
|
|
|
|
|
Artibeus jamaicensis |
derived |
|
|
|
|
|
Dermanura tolteca |
derived |
|
|
|
|
|
Ariteus flavescens |
derived |
|
|
|
|
|
Ardops nichollsi |
derived |
|
|
|
|
|
Stenoderma rufum |
derived |
|
|
|
|
|
Centurio senex |
derived |
|
|
|
|
|
Pygoderma bilabiatum |
derived |
|
|
|
|
|
Sphaeronycteris toxophyllum |
derived |
|
|
|
|
|
Ametrida centurio |
derived |
|
|
|
|
|
Phyllops falcatus |
derived |
|
|
|
|
|
Natalidae |
|
||||
|
†Primonatalus prattae |
|
× |
CO + PC; blocky |
× |
Early Miocene; early Hemingfordian; USA |
|
Chilonatalus micropus |
|
× |
CO + PC; blocky |
× |
|
|
Chilonatalus tumidifrons |
|
× |
CO + PC; blocky |
× |
|
|
Natalus lanatus |
|
× |
CO + PC; blocky |
× |
|
|
Natalus stramineus |
|
× |
CO + PC; blocky |
× |
|
|
Natalus tumidirostris |
|
× |
CO + PC; blocky |
× |
|
|
Nyctiellus lepidus |
|
× |
CO only; blocky |
× |
|
|
Molossidae |
|
||||
|
†Wallia scalopidens |
unknown |
|
|
unknown |
middle Eocene; Uintan, Ui3; Canada |
|
†Cuvierimops parisiensis |
× |
|
|
weak |
late Eocene–medial Oligocene; MP17–MP25; France |
|
†Cuvierimops legendrei |
× |
|
|
× |
early Oligocene; MP22–MP23; France |
|
Tomopeas ravus |
× |
|
|
|
|
|
Platymops setiger |
× |
|
|
weak |
|
|
Promops centralis |
× |
|
|
× |
|
|
Promops nasutus |
× |
|
|
× |
|
|
Sauromys petrophilus |
× |
|
|
× |
|
|
Nyctinomops aurispinosus |
× |
|
|
unknown |
|
|
Nyctinomops femorosaccus |
× |
|
|
× |
|
|
Taxon |
Notch absent |
Notch present |
Condition if notch present |
AT associated with cristid |
Age and locality (for fossils) |
|
Nyctinomops laticaudatus |
× |
|
|
× |
|
|
Nyctinomops macrotis |
× |
|
|
× |
|
|
Mops spurrelli |
× |
|
|
× |
|
|
Mops thersites |
× |
|
|
× |
|
|
Mormopterus †faustoi |
× |
|
|
unknown |
Oligocene; Deseadan; Brazil |
|
Mormopterus †barrancae |
× |
|
|
× |
Early Miocene; Colhuehuapian; Argentina |
|
Mormopterus kalinowskii |
× |
|
|
× |
|
|
Mormopterus phrudus |
× |
|
|
× |
|
|
Myopterus daubentonii |
× |
|
|
× |
|
|
Cabreramops aequitorianus |
× |
|
|
× |
|
|
Neoplatymops mattogrossoensis |
× |
|
|
× |
|
|
Molossops temminckii |
× |
|
|
× |
|
|
Molossus coibensis |
× |
|
|
weak |
|
|
Eumops auripendulus |
× |
|
|
|
|
|
Eumops patagonicus |
× |
|
|
|
|
|
Eumops dabbenei |
× |
|
|
× |
|
|
Eumops glaucinus |
× |
|
|
weak |
|
|
Eumops hansae |
× |
|
|
|
|
|
Eumops perotis |
× |
|
|
|
|
|
Eumops underwoodi |
× |
|
|
|
|
|
Cheiromeles parvidens |
× |
|
|
|
|
|
Cheiromeles torquatus |
× |
|
|
|
|
|
Cynomops abrasus |
× |
|
|
|
|
|
Cynomops greenhalli |
× |
|
|
weak |
|
|
Tadarida brasiliensis |
× |
|
|
× |
|
|
Otomops martiensseni |
× |
|
|
× |
|
|
Vespertilionidae |
|
||||
|
†Premonycteris vesper |
× |
|
|
× |
early Eocene; late Ypresian; MP10; France |
|
†Khonsunycteris aegyptiacus |
× |
|
|
unknown |
latest Eocene; Priabonian; Egypt |
|
†Ancenycteris rasmusseni |
× |
|
|
× |
Middle Miocene; Barstovian; USA |
|
†Hanakia agadjaniani |
|
× |
CO + PC |
× |
Early Miocene; MN3; Germany |
|
Eptesicus brasiliensis |
× |
|
|
weak |
|
|
Eptesicus fuscus |
× |
|
|
|
|
|
Histiotus montanus |
× |
|
|
|
|
|
Histiotus velatus |
× |
|
|
× |
|
|
Hesperoptenus tickelli |
× |
|
|
|
|
|
Lasiurus borealis |
× |
|
|
weak |
|
|
Lasiurus cinereus |
× |
|
|
|
|
|
Lasiurus ega |
× |
|
|
|
|
|
Nycticeinops schlieffeni |
× |
|
|
|
|
|
Nycticeius cubanus |
× |
|
|
|
|
|
Nycticeius humeralis |
× |
|
|
|
|
|
Rhogeessa mira |
× |
|
|
|
|
|
Rhogeessa tumida |
× |
|
|
|
|
|
Scotoecus pallidus |
× |
|
|
unknown |
|
|
Scotomanes ornatus |
× |
|
|
|
|
|
Scotophilus kuhlii |
× |
|
|
|
|
|
Scotophilus nux |
× |
|
|
|
|
|
Scotorepens balstoni |
× |
|
|
× |
|
|
Scotorepens greyii |
× |
|
|
× |
|
|
Nyctophilus arnhemensis |
× |
|
|
weak |
|
|
Glischropus tylopus |
× |
|
|
× |
|
|
Nyctalus azoreum |
× |
|
|
× |
|
|
Scotozous dormeri |
× |
|
|
× |
|
|
Barbastella barbastellus |
× |
|
|
unknown |
|
|
Barbastella darjelingensis |
× |
|
|
unknown |
|
|
Corynorhinus mexicanus |
× |
|
|
× |
|
|
Corynorhinus rafinesquii |
× |
|
|
× |
|
|
Corynorhinus townsendii |
× |
|
|
× |
|
|
Euderma maculatum |
× |
|
|
unknown |
|
|
Plecotus auritus |
× |
|
|
× |
|
|
Plecotus austriacus |
× |
|
|
× |
|
|
Otonycteris hemprichii |
× |
|
|
|
|
|
Chalinolobus nigrogriseus |
× |
|
|
× |
|
|
Glauconycteris argentata |
× |
|
|
× |
|
|
Ia io |
× |
|
|
weak |
|
|
Laephotis namibensis |
× |
|
|
|
|
|
Mimetillus moloneyi |
× |
|
|
|
|
|
Philetor brachypterus |
× |
|
|
|
|
|
Tylonycteris fulvida |
× |
|
|
|
|
|
Tylonycteris robustula |
× |
|
|
× |
|
|
Vespertilio murinus |
× |
|
|
unknown |
|
|
Vespertilio sinensis |
× |
|
|
× |
|
|
Antrozous pallidus |
× |
|
|
|
|
|
Bauerus dubiaquercus |
× |
|
|
|
|
|
Lasionycteris noctivagans |
× |
|
|
weak |
|
|
Myotis albescens |
× |
|
|
× |
|
|
Myotis californicus |
× |
|
|
|
|
|
Myotis nigricans |
× |
|
|
× |
|
|
Myotis velifer |
× |
|
|
× |
|
|
Myotis vivesi |
× |
|
|
weak |
|
|
Parastrellus hesperus |
× |
|
|
weak |
|
|
Perimyotis subflavus |
× |
|
|
× |
|
|
Vespertilionidae: Murininae |
|
||||
|
Harpiocephalus harpia |
derived |
|
|
|
|
|
Murina aurata |
× |
|
|
× |
|
|
Murina cyclotis |
× |
|
|
× |
|
|
Murina florium |
× |
|
|
× |
|
|
Murina suilla |
× |
|
|
× |
|
|
Murina ussuriensis |
|
× |
CO + PC |
× |
|
|
Vespertilionidae: Kerivoulinae |
|
||||
|
Kerivoula argentata |
|
× |
CO + PC + entocristid |
× |
|
|
Kerivoula hardwickii |
|
× |
CO + PC |
× |
|
|
Kerivoula javanus |
|
× |
CO + PC + entocristid |
× |
|
|
Kerivoula myrella |
|
× |
CO + PC + entocristid |
× |
|
|
Kerivoula picta |
|
× |
CO + PC |
× |
|
|
Phoniscus atrox |
|
× |
CO + PC |
× |
|
|
Phoniscus jagorii |
|
× |
CO + PC |
× |
|
|
Phoniscus papuensis |
|
× |
CO + PC |
× |
|
|
Miniopteridae |
|
||||
|
Miniopterus africanus |
× |
|
|
× |
|
|
Miniopterus australis |
× |
|
|
× |
|
|
Miniopterus fraterculus |
× |
|
|
× |
|
|
Miniopterus magnater |
× |
|
|
× |
|
|
Miniopterus natalensis |
× |
|
|
× |
|
|
Miniopterus schreibersii |
× |
|
|
× |
|
In addition, we examined the teeth of a few extinct members of extant bat families as well as members of several extinct bat families using original fossils, resin casts, or published images. Familial assignments of fossil bats follow Smith et al. (2012), Maitre (2014), and original authors of taxa. We examined the following fossil bats for which lower molars were available or sufficiently well-illustrated (references are cited only for those for which published images were used in place of three-dimensional specimens): Onychonycteridae: Ageina tobieni, Eppsinycteris anglica (Hooker 1996), Honrovits tsuwape, “Hassianycteris” joeli (Smith and Russell 1992; for inclusion of this species in Onychonycteridae see Smith et al. 2012), and Marnenycteris michauxi (Hand et al. 2015); Icaronycteridae: Icaronycteris? menui; Archaeonycteridae: Archaeonycteris brailloni (Russell et al. 1973), A.? praecursor (Tabuce et al. 2009), Australonycteris clarkae (Hand et al. 1994), and Xylonycteris stenodon (Hand and Sigé 2018); Hassianycteridae: Hassianycteris revilliodi (Russell and Sigé 1970); Palaeochiropterygidae: Anatolianycteris insularis (Jones et al. 2019), Palaeochiropteryx tupaiodon, P. spiegeli, Cecilionycteris prisca, and Stehlinia spp.; Philisidae: Philisis sphingis (Gunnell et al. 2008); family incertae sedis: Necromantis adichaster (Hand et al. 2012); Myzopodidae: Phasmatonycteris butleri and P. phiomensis (Gunnell et al. 2014: fig. 3); Mystacinidae: Vulcanops jennyworthyae (Hand et al. 2018); Mormoopidae: Koopmanycteris palaeomormoops; Speonycteridae: Speonycteris aurantiadens; Phyllostomidae: Notonycteris magdalenensis and N. sucharadeus; Natalidae: Primonatalus prattae; Molossidae: Wallia scalopidens (this species was considered a molossid by Legendre [1985] but was considered to be of uncertain family by Smith et al. [2012]; it may have been described from a composite sample of isolated teeth but the holotype M1 does appear to be chiropteran, as do some of the hypodigm lower molars), Cuvierimops parisiensis, C. legendrei (Maitre 2014: pls. 1–3), and Mormopterus faustoi; Vespertilionidae: Premonycteris vesper (Hand et al. 2016: figs. 3, 4), Khonsunycteris aegyptiacus (Gunnell et al. 2012: fig. 7.4A), Ancenycteris rasmusseni, and Hanakia agadjaniani (Rosina and Rummel 2012, 2019); Emballonuridae: Vespertiliavus kasserinensis (Ravel et al. 2016: figs. 12, 13) and V. schlosseri (Smith et al. 2012: fig. 2.14O–Q); Hipposideridae: Hipposideros (Pseudorhinolophus) morloti and H. (P.) schlosseri (Maitre 2014), and Vaylatsia ulmensis (Ziegler 2000: pl. 3: 22–24); and Nycteridae: Khoufechia gunnelli (Ravel et al. 2016).
We use the standard mammalian terminology for gross surface morphology, crests, cusps, and basins of tribosphenic teeth, the carnassial and carnassiform terms of MacIntyre (1966), the functional wear facets as numbered by Crompton (1971), and also the functional morphological terminology of Evans (2005) and Evans and Sanson (2005). The term carnassiform notch (CN) is used herein to indicate a small structure present on the crests of the talonids of the lower molars developed as a tiny interruption or indentation about midway along the length of the cristid obliqua and the postcristid on the edges of these crests (Fig. 1). In some bats (e.g., Furipteridae, Thyropteridae, and some Emballonuridae) there may also be a notch developed at the apex of the angled or strongly curved entocristids (as seen in occlusal view) with an accessory trough on the lingual (i.e., not the basinward) side. Carnassiform notches sometimes also occur on the trigonid crests (paracristid and protocristid) of bat molars. The notches in the trigonid along the edge of the paracristid and protocristid of many kinds of tribosphenic mammals are present primitively and are largely excluded from our discussion.
We selected specimens with lower jaws having little to no wear and containing CN or accessory troughs, making molds in RTV silicone and casts in resin. Teeth with moderate to heavy wear could not be used for this study, because advancing tooth wear eventually obliterates the notches. Casts were sputter-coated with gold palladium and examined at magnifications 10–150 × on a LEO® 1450VP Digital Scanning Electron Microscope. Optimal viewing angles of CN varied among the taxa and molars under SEM were rotated through various angles to find the best angle for image capture. We characterized CN according to their occurrence by taxon and by their appearance in occlusal and labial views. The notches, where present, are best seen in profile in anterolabial view for the cristids obliqua and posterolabial view for the postcristids. Accessory troughs are best seen in occlusal views. Stereopairs of some specimens provide more informative occlusal views.
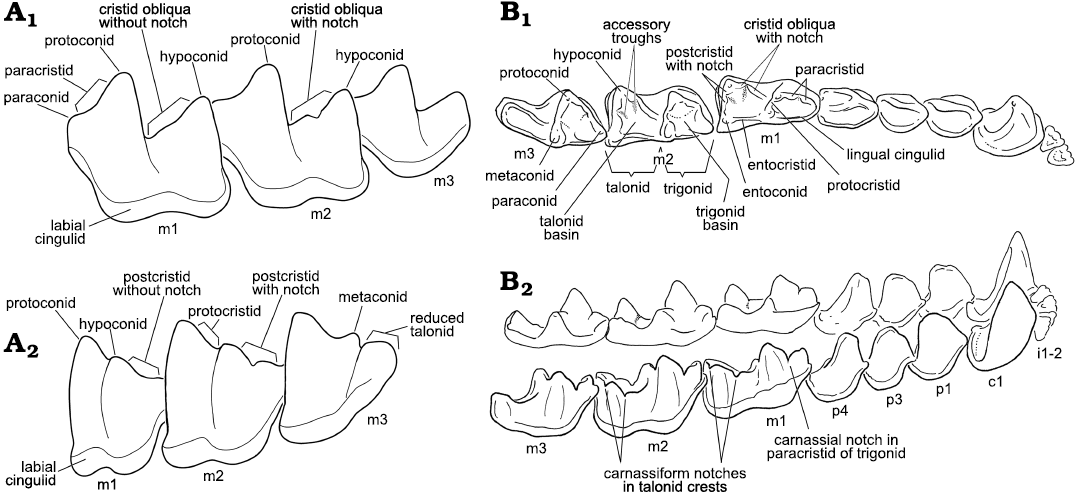
Fig. 1. Dental terminology of bat teeth used in this paper emphasizing the appearance of carnassiform notches in the lower molars. A. Lower molars in anterolabial (A1) and posterolabial (A2) views. B. Example bat (Glyphonycteris sylvestris Thomas, 1896); B1, left lower toothrow with strong carnassiform notches on the talonid crests of the molars, in occlusal view (note also deep carnassial notch in paracristids); B2, entire mandibular dentition (both right and left toothrows) as seen from the right side of the mandible. Not to scale.
Results
Carnassiform notches and accessory troughs in lower molars of the bat taxa examined vary in the degree of development from weak or slight to deep or wide. Thus, our descriptions of the structures are preliminary and somewhat arbitrary; examining larger samples of individual species might help to clarify some of these problems. We judged CN to be absent in members of a family such as Molossidae and most Vespertilionidae in which the cristid obliqua and postcristid of a lower molar have an edge with a relatively smooth shallow or open curve when seen in a profile view (anterolabial for the cristid obliqua or posterolabial for the postcristid; see for example, Figs. 8B1, 11A). By comparison, when the same talonid crests have an edge profile showing an abrupt emargination along their length, we called that emargination a carnassiform notch (e.g., Figs. 3A1, 13A1). Some species had talonid crests which showed no abrupt within-crest emargination but their crest edges exhibited a deeper edge curve (e.g., Figs. 12A1, 13B); although these may act similarly to abruptly-notched crests, we judged them as lacking notches for purposes of our study. In occlusal view the notch crosses the edge of the talonid crest at a right angle to the crest. From a labial aspect the notches change the profile of the crest, which is normally a sinuous or continuous smooth curve in those species without notches, to a curve with a V-shaped or U-shaped cutout in the middle. In profile the notch may also range in form from a low, wide (obtuse) angle to a narrow, deep (acute to ~90°) angle. We observed that notches at either end of this range can change profile with progressive normal tooth wear, usually deepening and widening or rounding the apex at the bottom, or show loss of the distinctiveness of notch shape to one with no definitive angular appearance. These shallow wear notches can form in species with abrupt CN as well as species with no CN when unworn, possibly due to breakage.
Accessory troughs appear within the talonid basin as small foveas or “divots,” small hollows alongside the talonid crests on the basinward side immediately adjacent to the CN in the cristid obliqua or the postcristid, more generally both (Fig. 1B1). Accessory troughs are centered directly below the apex of the CN in the crest. They may appear: (i) as shallow to deep, oval depressions, as though a scoop shape has been taken out of the slope of the talonid basin near each crest, or (ii) as more elongated troughs or sulci running from just below the notches in the crests down into the talonid basin, or (iii) as deep and flaring pockets, rather like the open area in carnivoran carnassials adjacent to the carnassial slit. In bat molars the accessory troughs are usually separate from one another (i.e., the trough adjacent to the cristid obliqua is not confluent with the trough adjacent to the postcristid) with a swollen or columnar area between them supporting the hypoconid. The swollen area probably has more to do with crushing and grinding functions of the talonid rather than shearing functions. Importantly, the troughs can occur in bat molars in which there are no CN (in which they are usually shallow) as well as in bats with CN (in which the troughs are often deep). They can also vary in depth in different species of the same genus, and even differ in depth between m1 and m2 (and sometimes m3) talonids within a species. The troughs probably function in concert with the shearing crests and CN, if present. With wear, accessory troughs can merge with talonid crests even when no CN are present, creating an edge that will appear in labial views to have rough, open notches. In some species, accessory troughs seem to affect progressive tooth wear in such a way as to enhance the development of a crude CN as seen in labial profiles of the talonid crests, at least temporarily or until continued wear effaces the notch. In our view, the CN and accessory troughs simply appear to provide an additional series of food capture edges and fragment clearance areas, respectively, smaller than those described by Evans (2006) and Evans and Sanson (2005), and inserted onto the larger crests and basins.
The earliest known bats were likely insectivorous (Simmons and Giesler 1998; Simmons et al. 2008) and most contemporary species still are. The dentally most primitive forms show incomplete dilambdodonty of the upper molar ectoloph or necromantodonty (primitive condition for bats in which the hypoconulid is medially situated between the hypoconid and entoconid; Sigé et al. 2012) of the lower molars (see Smith et al. 2012; Hand et al. 2016: table 2), and all archaic bats examined by us lack CN. In our sample, the late Oligocene hipposiderid Vaylatsia ulmensis (Ziegler 2000: pl. 3: 22–24) is the earliest fossil bat to show weak or incipient CN. We found carnassiform notches in bats of eight extant families (Table 1), but the occurrence of CN was not necessarily ubiquitous across all members of a family. Notches occurred in tribosphenic molars in extant genera and species examined within the families Megadermatidae (3 of 5 genera), Nycteridae (all 6 examined species of the single genus), Mystacinidae (1 of 1 genus and species), Furipteridae (both of the 2 genera and species), Thyropteridae (2 of 3 species in the single genus), Phyllostomidae (13 of 17 genera and 27 of 33 species), and Natalidae (3 of 3 genera and 6 of 6 species), and Vespertilionidae (only in the subfamilies Kerivoulinae and possibly Murininae). Talonid notches in each of these are described below.
Within the families that possess CN on the talonids, the notches are consistently present on the cristid obliqua or on both the cristid obliqua and the postcristid. On most bat species in which CN are present, the CN occurs on both the cristid obliqua and the postcristid. Exceptions to this trend were the Megadermatidae and Nycteridae, in which CN occur on the cristid obliqua only. CN are often present only on the m1 and m2, although in Furipteridae, Natalidae, Nycteridae, Mystacinidae, and Thyropteridae, as well as some genera of Phyllostomidae, CN are also developed on the m3 but usually weakly so because of reduction in size of the m3 talonids. In many taxa of bats the entoconid is tall and in some of these the entocristid can be long and bears a curved edge (concave upward occlusal edge), providing yet another shearing crest that runs at a different angle than the cristid obliqua and postcristid. In addition, the entocristid is sometimes angular or strongly curved (concave lingually) in several taxa, and may even have a carnassiform notch. CN on the cristid obliqua and postcristid are absent in at least the families Emballonuridae, Rhinopomatidae, Rhinolophidae, Hipposideridae, Myzopodidae, Noctilionidae, Mormoopidae, Molossidae, and Miniopteridae. The occurrence of CN is unknown in Craseonycteridae, Rhinonycteridae, and Cistugidae, which we were unable to examine.
Megadermatidae.—Few specimens of this family with little-worn or unworn molars were available for examination. The most carnivorous megadermatid, Macroderma gigas, lacks CN on the talonid crests of its lower molars. However, we found a very weak CN on the edge of the cristid obliqua in Cardioderma cor, Lyroderma lyra, and Megaderma spasma (Fig. 2), and none on the postcristid. A relatively well-developed accessory trough occurs on the basinward slope of the notch, and there is no analogous pit adjacent to the postcristid. A late Oligocene species Megaderma herrlingensis illustrated by Ziegler (2000: pl. 1: 1, 4, 5) clearly shows accessory troughs adjacent to the cristids obliqua, although the specimens may be too worn to determine whether CN were present. The condition in the extant megadermatids is rather similar to the condition in Nycteridae (see below), but the cleft in the cristid obliqua is much less developed in the megadermatids studied. No other extant members of Yinpterochiroptera were found to have CN, although (Ziegler 2000) illustrated fossils of extinct late Oligocene hipposiderids Vaylatsia spp. that show accessory troughs which are better developed next to the cristid obliqua than those next to the postcristid (Ziegler 2000: pls. 1–3). The newly named megadermatid Eudiscoderma thongareeae Soisook, Prajakjitr, Karapan, Francis, and Bates, 2015 lacks CN (Soisook et al. 2015).
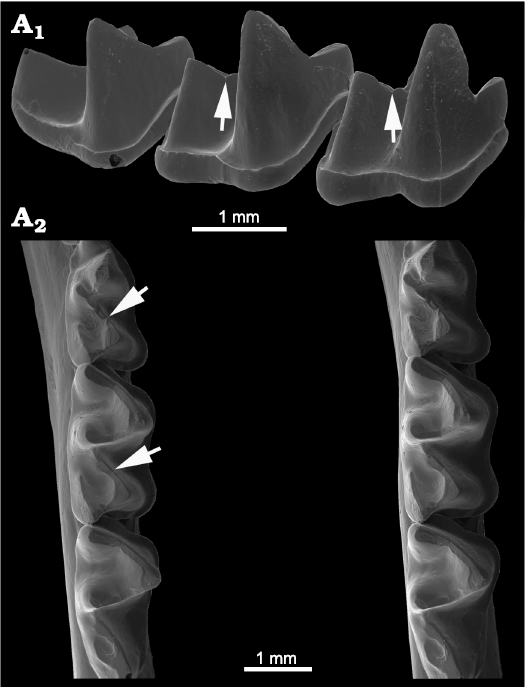
Fig. 2. Megadermatid bat Megaderma spasma (Linnaeus, 1758) (AMNH 216806) from Malaysia, Recent. Lower molars showing weak carnassiform notches in the cristids obliqua of m1 and m2 (indicated by arrows) in anterolabial view (A1), note that the entocristids are partly visible in the immediate background behind the cristid obliqua in m1 and m2; occlusal view (stereopair, A2), arrows indicate notches in the cristids obliqua that are developed to a lesser degree than those in the trigonid crests. Note entocristids with indistinct entoconids.
Emballonuridae.—Emballonurids do not show CN on the cristid obliqua or postcristid, but the basinward edges of these crests show shallow accessory troughs adjacent to the crests, deeper adjacent to the cristid obliqua than the postcristid. However, most emballonurids bear an entocristid that is angular and concave lingually, and often encloses a small lingual pit or basin. With wear, the angled entocristid develops a shear facet that is inclined medially (lingually) with a V-shaped cutting edge. Among the extant emballonurids, Centronycteris maximiliani, Coleura afra, and Peropteryx macrotis have a distinct CN on the angular entocristid of m1 and m2, while Cyttarops alecto, Diclidurus albus, Diclidurus ingens, Mosia nigrescens, Peropteryx trinitatus, Saccopteryx gymnura, and S. leptura have weak but incipient notches on at least the m1, even weaker on the m2. Of the taxa we sampled, Saccolaimus flaviventris and S. peli differ in having a short, low, straight entocristid; in these taxa it lacks a notch and extends mesially from a stout entoconid. Taphozous nudiventris and T. melanopogon are similar but the entoconid is thinner and the entocristid is higher and slightly curved or straight. Species of the Paleogene European genus Vespertiliavus have angular entocristids that are not notched (Smith et al. 2012; Maitre 2014; Ravel et al. 2016). Two unpublished new genera and three new species from the late Paleogene–early Neogene of Florida (being currently in description by Gary Morgan and NJC) have no notch in the angular entocristid, but show the small fovea or basin lingual to the entocristid.
Nycteridae.—The Eocene nycterid Khoufechia gunnelli of northern Africa (Ravel et al. 2016) lacks CN. Otherwise, all extant species of Nycteris we examined share a unique condition of a deep CN occurring only on the cristid obliqua, never on the postcristid, including in the seasonally carnivorous Nycteris grandis (Gual-Suárez and Medellín 2021) (see also an illustration of a lower molar of Nycteris thebaica in Horáček and Špoutil 2012: fig. 12.15B showing the deep cristid obliqua notch). Of the sixteen living species of Nycteris (Simmons and Cirranello 2020), we examined 6 species. All 6 possess a very distinct and sharp V-shaped carnassiform notch on the cristid obliqua of m1 and m2, and even on the reduced talonid of m3 (Fig. 3). The notch is always associated with a deep accessory trough on the basinward side of the cristid obliqua. Entoconids and entocristids are thin, straight, and notchless, with a lingual opening of the basin either anteriorly (adjacent to the metaconid) or posteriorly (adjacent to the hypoconulid).
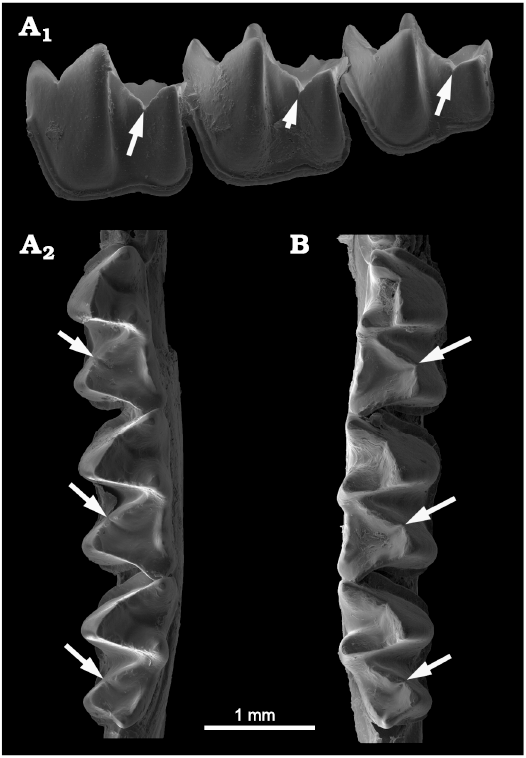
Fig. 3. Nycterid bat Nycteris aurita (Andersen, 1912) (AMNH 187310) from Kenya, Recent; showing deep carnassiform notches (arrows) in the cristids obliqua and adjacent accessory troughs in the talonid basins of m1–m3. A. Left m1–m3 in anterolabial (A1) and occlusal (A2) view. B. Right m1–m3 in occlusal view.
Mystacinidae.—Talonid CN are absent in the extinct Vulcanops jennyworthyae from the Miocene of New Zealand, although there is a shallow accessory trough on the basin side of the cristid obliqua judging from the images of lower molars (Hand et al. 2018: fig. 1). We examined only one specimen of the extant species Mystacina tuberculata, which has rather weak, low-angle CN on the cristid obliqua and postcristid, each accompanied by an accessory trough in the talonid basin.
Furipteridae.—Furipterids possess CN on the major talonid crests, cristid obliqua and postcristid, and they bear a strongly angular entocristid that sometimes shows a slight notch (Fig. 4).
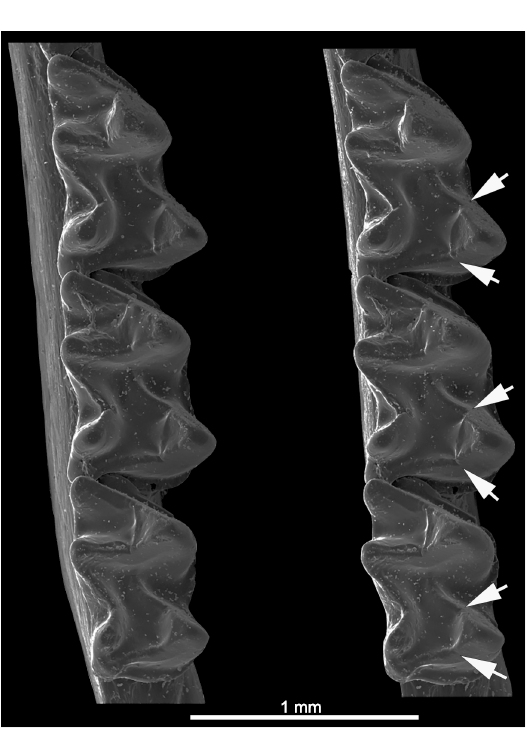
Fig. 4. Furipterid bat Furipterus horrens (Cuvier, 1828) (USNM 549505) from Brazil, Recent. Right lower molars in occlusal view (stereopair), showing angular entocristids and carnassiform notches (arrows) with accessory troughs in the cristids obliqua and postcristids in m1–m3.
Thyropteridae.—Among the Thyropteridae, the extant species Thyroptera lavali and T. tricolor show postcristid and cristid obliqua CN, as do 12 million-year-old fossils of the same species (Czaplewski 1997; Czaplewski et al. 2003). In all Thyroptera species the entocristid is angular to curved with the concave side facing lingually, and there is also a notch within the entocristid.
Mormoopidae.—Both extant genera in this family, Pteronotus and Mormoops, lack notches in the talonid crests but have shallow accessory troughs that run alongside the cristid obliqua and postcristid in the talonid basin on m1–m3. The entocristids are straight to slightly curved and also lack notches. The extinct late Oligocene Koopmanycteris palaeomormoops from Florida is similar in these features to the extant mormoopids.
Phyllostomidae.—The diversity among phyllostomids’ foraging style is vast and includes diets of fruit, nectar, pollen, blood, vertebrates, and insects. In past studies, it has been shown that several subgroups of Phyllostomidae (traditionally placed in the subfamily Phyllostominae, but recently split into several subfamilies; Baker et al. 2016; Cirranello et al. 2016) eat insects, other vertebrates, and occasionally some fruits, and have molars with conservative tribosphenic morphology. Other subfamilies of phyllostomids dietarily specialized on nectar, fruit, and blood have moderately to highly modified teeth, which lack the basic tribosphenic tooth shape. The Miocene phyllostomid genus Notonycteris is represented by two fossil species, one of which (N. magdalenensis) lacks CN; the other species (N. sucharadeus) is yet known only by heavily worn lower molars in which the pristine talonid crests are obliterated (Savage 1951; Czaplewski 1997; Czaplewski et al. 2003). Notonycteris magdalenensis lacks carnassiform notches but bears weak accessory troughs (Table 1). The lower molars of this Miocene species were originally interpreted as showing dental adaptations for carnivory relative to the lower molars of known carnivorous phyllostomids (Savage 1951). The fossils were recently re-studied using multivariate dental topography analysis and determined not to show specializations for carnivory but instead the species was interpreted as probably insectivorous or omnivorous (López-Aguirre et al. 2021).
Carnassiform notches and accessory troughs are found in the cristid obliqua and postcristid of lower molars throughout most of the insectivorous–omnivorous phyllostomids, including the Macrotinae, Micronycteridae, Lonchorhininae, Phyllostominae, (except tribe Macrophyllini), and Glyphonycterinae. The notches are present and mostly well developed in all these taxa except the Phyllostominae tribes Macrophyllini (Macrophyllum and Trachops), in which they are essentially absent, and Vampyrini (Chrotopterus, Mimon, and Vampyrum) in which they are absent or weakly developed (in some Mimon species but not others). Although Mimon cozumelae and Gardnerycteris crenulatum showed low-angle CN with accessory troughs, Mimon bennettii had no notches and no accessory troughs, and its cristid obliqua stops short of reaching the distal wall of the trigonid; this condition is autapomorphic for M. bennettii. The paracristid blades of the phyllostomids Vampyrum spectrum and Chrotopterus auritus show carnassiform notches similar to those on the trigonid crests of many insectivorous bats. However, these specialized carnivorous bats lack notches in the talonid crests.
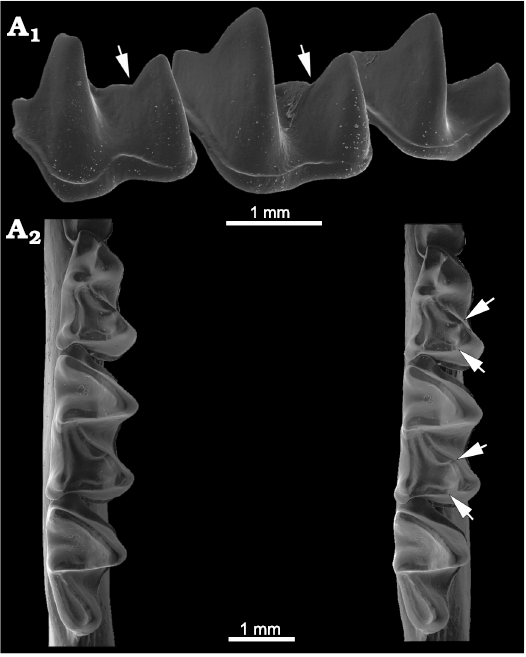
Fig. 5. Phyllostomid bat Macrotus waterhousii Gray, 1843 (OMNH 10653) from Mexico, Recent. Lower molars showing carnassiform notches in the cristid obliqua (arrows) and postcristid and accompanying accessory troughs in the talonid basins of m1–m2, in anterolabial view (A1) and occlusal (A2, stereopair) views, notches indicated by arrows.
CN and accessory troughs are moderately developed in Macrotinae (Macrotus; Fig. 5) and most strongly expressed in Micronycterinae (Micronycteris and Lampronycteris; Fig. 6), Lonchorhininae (Lonchorhina; Fig. 7), Phyllostomini (Gardnerycteris, Lophostoma, Phylloderma, Phyllostomus, and Tonatia; Fig. 8), and Glyphonycterinae (Glyphonycteris, Neonycteris, and Trinycteris; Fig. 9). Regarding the micronycterines, Micronycteris has been shown to exhibit a high degree of dietary flexibility; Santana et al. (2011a) showed that Micronycteris microtis, a small (5–7 g) species, ate a wide variety of insects, spiders, and a tiny lizard making them the smallest bat known to exhibit rare carnivory. Much of the species’ feeding behavior involved chewing motions involving the premolars and molars.
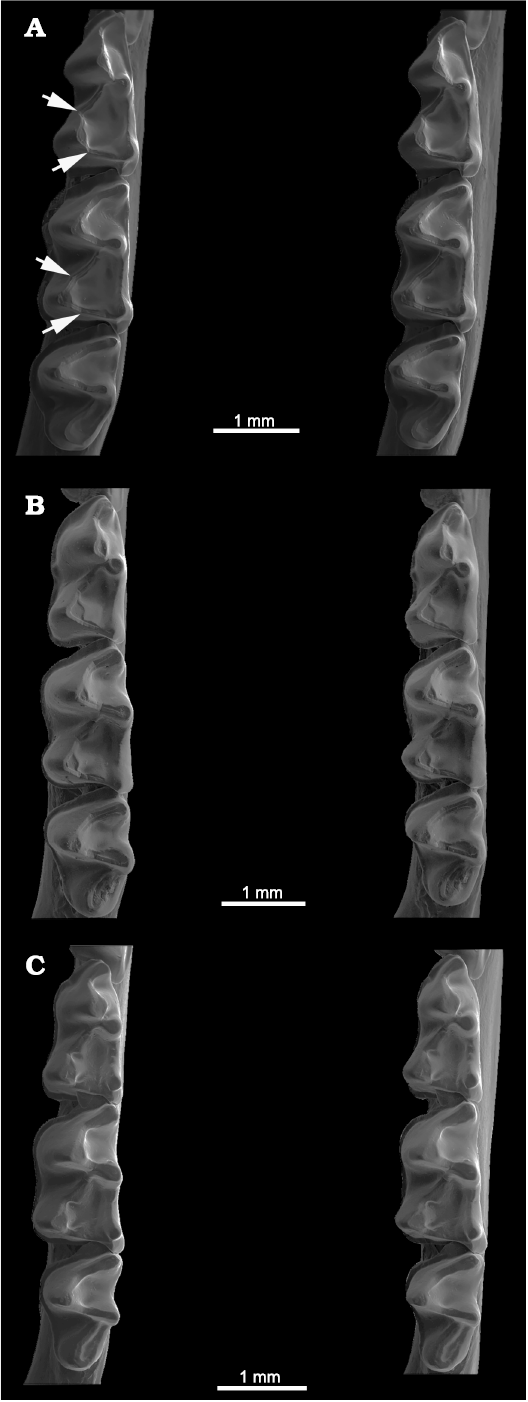
Fig. 6. Lower molars of Recent phyllostomid bats in occlusal view, showing carnassiform notches in the talonid crests and accompanying accessory troughs in the m1–m2 (indicated by arrows). A. Lampronycteris brachyotis (Dobson, 1878a) (USNM 306546) from Panama. B. Micronycteris hirsuta (Peters, 1869) (AMNH 139441) from Costa Rica. C. Micronycteris megalotis (Gray, 1842) (OMNH 6194). All stereopairs.
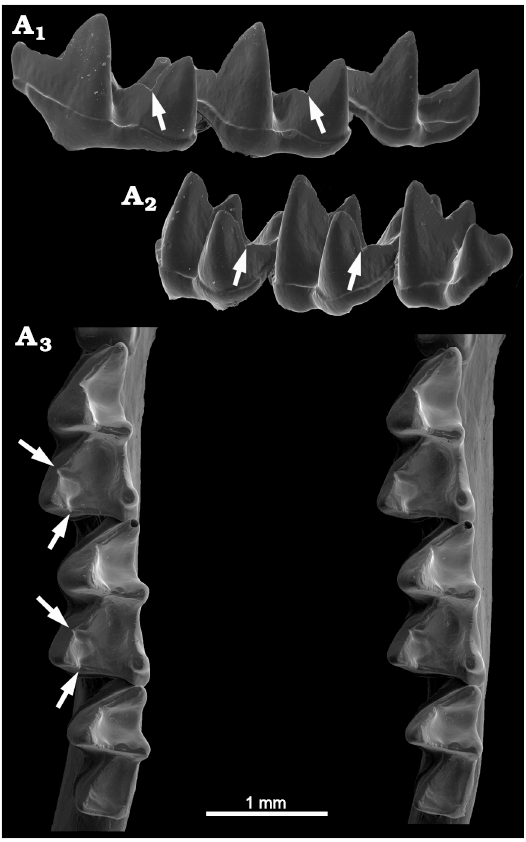
Fig. 7. Phyllostomid bat Lonchorhina orinocoensis Linares and Ojasti, 1971 (USNM 373254) from Venezuela, Recent. Left lower molars showing deep carnassiform notches in the talonids and accessory troughs in the talonid basins of m1–m2; A1, anterolabial view showing cristid obliqua notches (arrows); A2, posterolabial view showing postcristid notches (arrows); A3 (stereopair), occlusal view showing the talonid notches and troughs (arrows).
Based on molecular evidence, Glyphonycterinae was recently recognized as a distinct subfamily of Phyllostomidae within a radiation of omnivorous and frugivorous bats (the Nullicauda, including Carolliinae, Glyphonycterinae, Rhinophyllinae, and Stenodermatinae; Cirranello et al. 2016), and contains the genera Glyphonycteris, Neonycteris, and Trinycteris (Baker et al. 2016). As far as known, glyphonycterines are primarily gleaning arthropod-feeders but capable of eating fruit (Gardner 1977; Pine et al. 1996) and possibly small bird eggs or nestlings (Glyphonycteris sylvestris; Perrella et al. 2019). Such a diet was either present in the ancestor of the phyllostomid clade Nullicauda and retained in Glyphonycteris and Trinycteris (Freeman 2000; Rojas et al. 2011; Baker et al. 2012; Rossoni et al. 2017), or else the primary insectivory of glyphonycterines was secondarily convergently derived from nullicaudan ancestral frugivory (Wetterer et al. 2000; Dávalos et al. 2020). Unfortunately, glyphonycterines are difficult to capture (Simmons and Voss 1998) and relatively rare (Neonycteris is known from only two specimens collected in 1929; Sanborn 1949); thus, many aspects of their biology remain poorly known. Even for Glyphonycteris and Trinycteris species, precious few empirical dietary data are available (Gardner 1977; Pine et al. 1996; Solari et al. 1999) that were not conflated with those data for true Micronycteris as currently defined (and the systematics of the genus are still being sorted out; Morales-Martínez et al. 2021). These glyphonycterine bats have small canines and unusually broad, flattened upper premolars (Sanborn 1949; Baker et al. 2016; Cirranello et al. 2016). In the dentary Glyphonycteris daviesi has a low coronoid process (intermediate height in Glyphonycteris sylvestris and Trinycteris nicefori) but a relatively high condyloid process about twice the height of the molars. Their molars have rarely been mentioned in the literature, yet also are distinctive and unusual (but still recognizably tribosphenic) among bats and among insectivorous–omnivorous phyllostomids. Their lower molars possess carnassiform notches on the talonid crests (Fig. 9) and a carnassial notch on the paracristid of the m1. The paracristid is elongated and mesiodistally-oriented on the m1 and also has a reduced metaconid, strongly resembling the trigonid of a carnivoran m1 and that of the carnivorous phyllostomids Chrotopterus and Vampyrum except that the trigonid cusps are lower. These taxa (Glyphonycteris daviesi, Chrotopterus, and Vampyrum but not the Vampyrini genus Mimon) are also the only carnivorous bats we examined that have a slight carnassial slit at the bottom of the V-shaped paracristid notch (absent in megadermatids and Nycteris grandis). In Glyphonycteris daviesi, these slits are present on all three lower molar trigonids. Glyphonycteris daviesi and Trinycteris nicefori lower molars are also lower crowned and less exodaenodont than other insectivorous-omnivorous phyllostomids. They have a very thin but more extensive cingulum that is nearly continuous around the base of m1 including lingually, but may be absent beneath the entoconid, entocristid, and sometimes the metaconid in m2 and m3. These molars reflect a specialized structure of unknown functional significance in the processing of food, along with the other distinctive teeth.
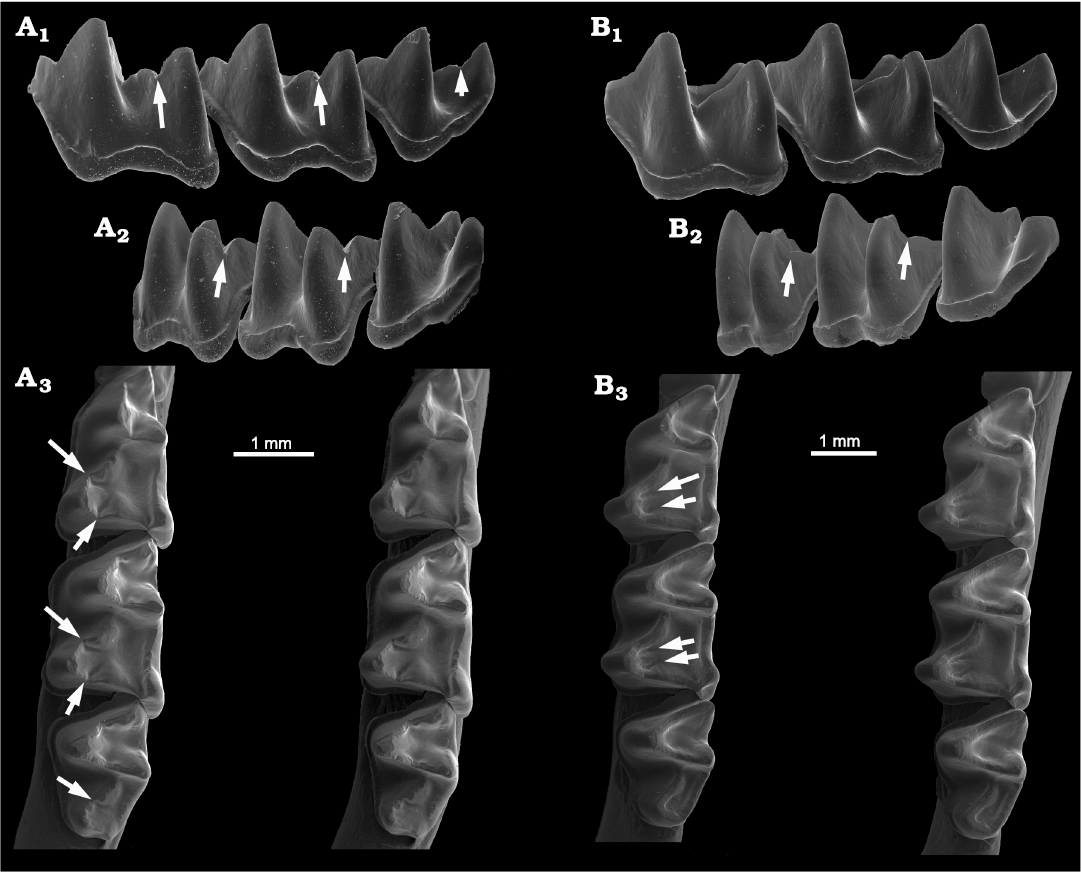
Fig. 8. Lower molars of Recent phyllostomid bats. A. Lophostoma silvicolum D’Orbigny, 1836, (USNM 335113) from Panama. Lower molars in anterolabial view (A1), showing deep carnassiform notches in the cristids obliqua of m1–m2 and a weaker notch in m3 (arrows); in posterolabial view (A2), showing deep carnassiform notches in the postcristids of m1–m2 (arrows); in occlusal view (A3, stereopair), showing notches and associated accessory troughs in the talonid basins (arrows). B. Phyllostomus elongatus (Geoffroy, 1810) (USNM 388796) from Venezuela. Lower molars in anterolabial view (B1), showing absent to incipient cristid obliqua notches on m1–m2; posterolabial view (B2), showing weak carnassiform notches on the postcristids in m1–m2 (arrows); occlusal view (B3, stereopair), showing the deep accessory troughs adjacent to both the cristids obliqua and postcristids in the talonid basins of m1–m2 (arrows).
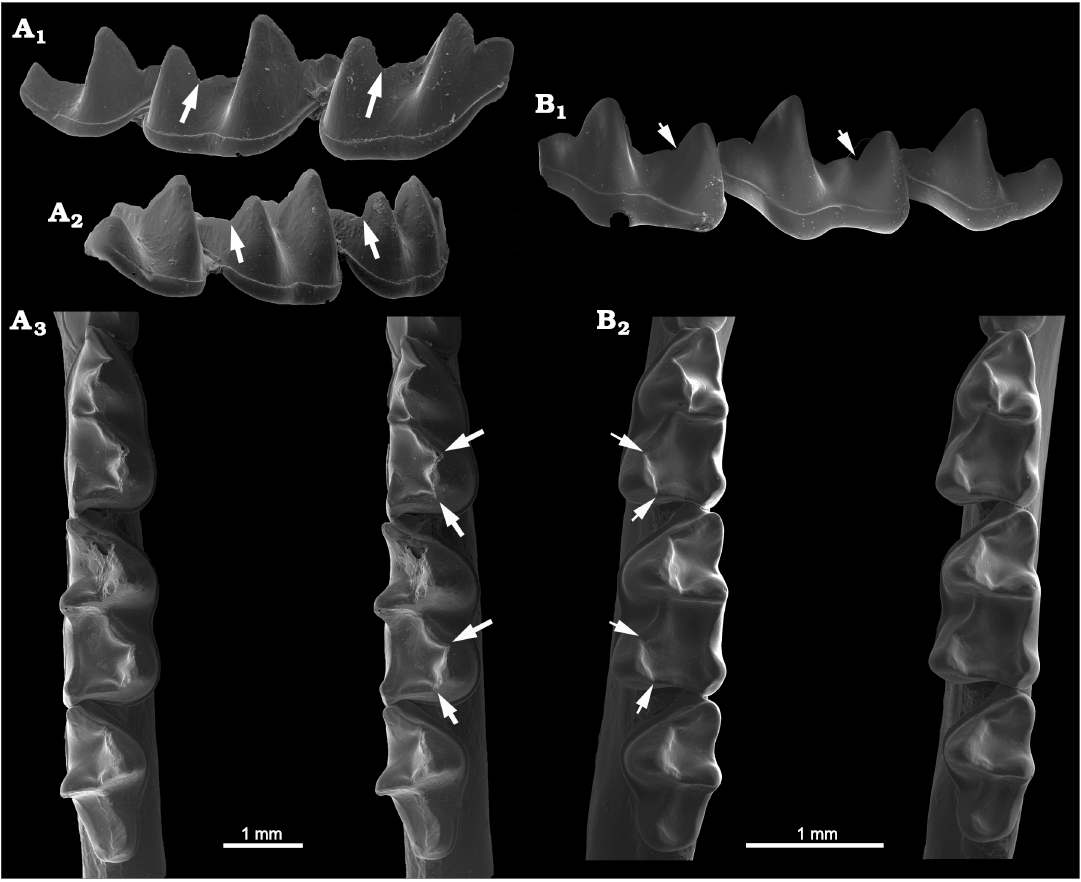
Fig. 9. Molars of Recent phyllostomid bats, showing carnassiform notches and accessory troughs in the talonid basins of m1 and m2. A. Glyphonycteris daviesi (Hill, 1964) (USNM 364266) from Peru. Right lower molars in anterolabial view (A1) showing the notches in the cristids obliqua (arrows; note that the entoconids are partly visible in the background immediately behind the cristid obliqua notch in each molar); posterolabial view (A2) showing the notches in the postcristids (arrows), and occlusal view (A3, stereopair) showing the talonid notches and adjacent troughs (arrows). Note also the carnassial notch and slit in the mesiodistally-oriented paracristid of the m1 trigonid. B. Trinycteris nicefori (Sanborn, 1949) (AMNH 184558) from Panama. Left lower molars in anterolabial view (B1) showing carnassiform notches in the cristids obliqua of m1–m2, entoconid in background partly obscures the notch in m1, and occlusal view (B2, stereopair) showing carnassiform notches and accessory troughs in m1–m2 (arrows).
Natalidae.—According to Morgan and Czaplewski (2003), the three genera and then-recognized eight living species of natalids all share distinctive blocky or step-like carnassiform notches (Fig. 10). Morgan and Czaplewski (2003) used the synapomorphic blocky notches in lower molars of Natalidae as a phylogenetic character in describing the Miocene natalid Primonatalus prattae. However, in this study we note that, while the blocky CN are present on both the cristid obliqua and postcristid in Chilonatalus, Natalus, and Primonatalus, closer inspection reveals that they occur only on the cristid obliqua in Nyctiellus. The accessory trough on the basinward slopes of each of the cristids become confluent with one another across the talonid basin in Chilonatalus, Natalus (Fig. 10), and Primonatalus. In Nyctiellus the accessory trough adjacent to the cristid obliqua notch angles down across the talonid basin. This is the only family in which the blocky CN are expressed in the upper molars (postparacrista and premetacrista, the crests that occlude with the cristid obliqua and postcristid of lowers), although very weakly, of at least Natalus tumidirostris. Tejedor (2011) recently revised the extant Natalidae to 12 species, and the molars of the additional taxa should be checked for CN.
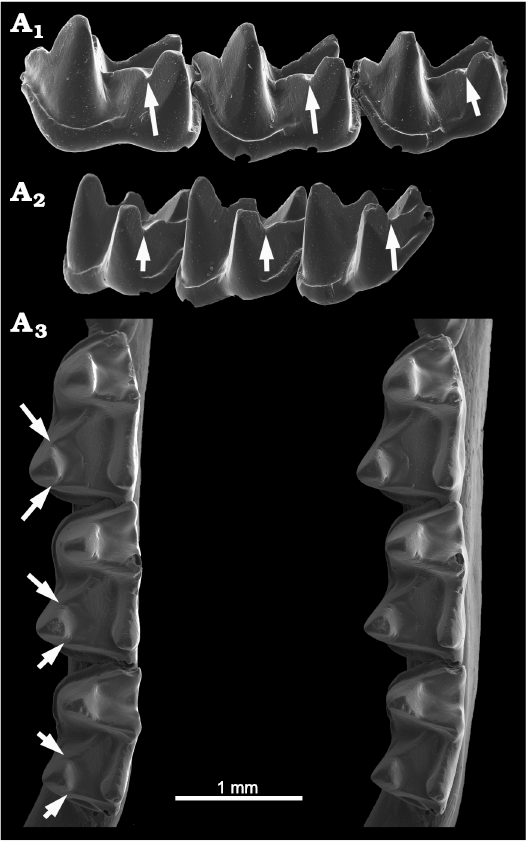
Fig. 10. Natalid bat Natalus tumidirostris Miller, 1900 (USNM 455974) from Venezuela, Recent. Left lower molars showing blocky or step-like carnassiform notches on m1–m3; anterolabial view (A1) showing the notches in the cristids obliqua (arrows); posterolabial view (A2) showing the notches in the postcristids (arrows); occlusal view (A3, stereopair) showing the carnassiform notches (arrows). Note how the rather angular accessory troughs accompanying the notches join within the talonid basins to form a V-shaped trench.
Molossidae.—The molossids we examined do not show CN on the talonid crests. All molossids have tall entoconids with relatively high and curved (concave lingually) entocristids except species of Tomopeas, Eumops, Myopterus, and some species of Mormopterus, in which the entocristids are straight to slightly curved. The entocristid in the Oligocene South American Mormopterus faustoi is straight. No member of the family has notches in the entocristids.
Miniopteridae.—Miniopterids have broad shallow accessory troughs adjacent to the cristid obliqua and postcristid on m1–m3 (Fig. 11), but no distinct notch fitting our characterization is present. However, the accessory troughs seem to contribute to the formation or maintenance of the deeper curves in the cristids as they become increasingly worn. Similarly, the entocristids are curved to angular, forming another shearing crest but without a distinct notch in the species we examined.
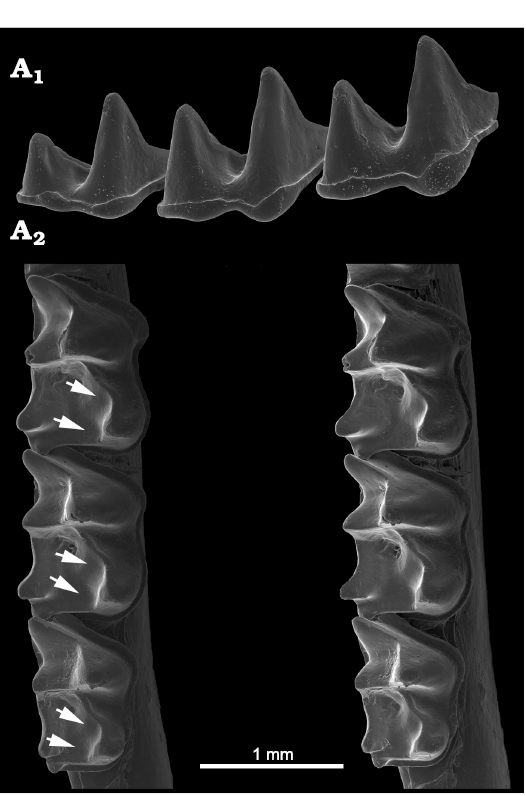
Fig. 11. Miniopterid bat Miniopterus australis Tomes, 1858 (USNM 590280) from Malaysia, Recent. Right lower molars in anterolabial view (A1) showing no carnassiform notches in the cristids obliqua; note that the entoconids are partly visible in the background immediately behind the cristid obliqua in each molar); and in occlusal view (A2, stereopair) showing well-developed accessory troughs (arrows) in m1–m3.
Vespertilionidae.—The late early Eocene European Premonycteris vesper illustrated by Hand et al. (2016) lacks CN on the talonids, as do most Vespertilionidae; instead, P. vesper and many other vespertilionids have relatively deeply excavated cristids obliqua and postcristids with shallow accessory troughs. In the Lower Miocene (MN 4) of Germany the extinct vespertilionid Hanakia agadjaniani shows CN and accessory troughs as illustrated by Rosina and Rummel (2019: fig. 4). Among the extant Vespertilionidae, only members of the Murininae and Kerivoulinae have CN.
In the subfamily Murininae we examined five species of Murina and one of Harpiocephalus (we did not examine specimens of Harpiola). Harpiocephalus shows an intriguing, derived dental morphology similar to that of zalambdodont mammals (Miller 1907; Asher et al. 2002), but the heavy, reduced talonids lack CN. The general curve of the cristid obliqua and postcristid edges in Murina species runs low to form a deep, nearly right-angled curve (Fig. 12A1). A few specimens of Murina species show CN (e.g., M. ussuriensis) but most Murina species lack distinct within-crest notches. All specimens show accessory troughs adjacent to the talonid crests (Fig. 12A2) that potentially augment the development of crest notches or contribute to the development of deeper crest curves like those described above for miniopterids. Entocristids are curved but do not display notches.
Among the Kerivoulinae studied, species of the genus Kerivoula show an approximately right-angled CN in the cristid obliqua and postcristid combined with accessory troughs (Fig. 13A1), while Phoniscus species have no or much weaker notches and shallower accessory troughs (Fig. 13A2). The cristids of Phoniscus appear similar to the deeply curved cristid edges in Miniopteridae. The accessory troughs are sometimes confluent with one another in Kerivoula but not in Phoniscus. The entocristids are angled in Kerivoula and sometimes bear a minute notch (in K. argentata, K. javanus, and K. myrella, but not in K. hardwickii and K. picta) in m1–m3, whereas in Phoniscus the entocristids are straight or slightly angled and lack a notch. The entocristid notch in Kerivoula species is present at the apex of the angle in this crest and nearer the metaconid, while the entocristid lingual trough is nearer the entoconid. This differs from the entocristids in Emballonuridae, in which the entocristid angle, notch, and lingual trough are aligned with one another.
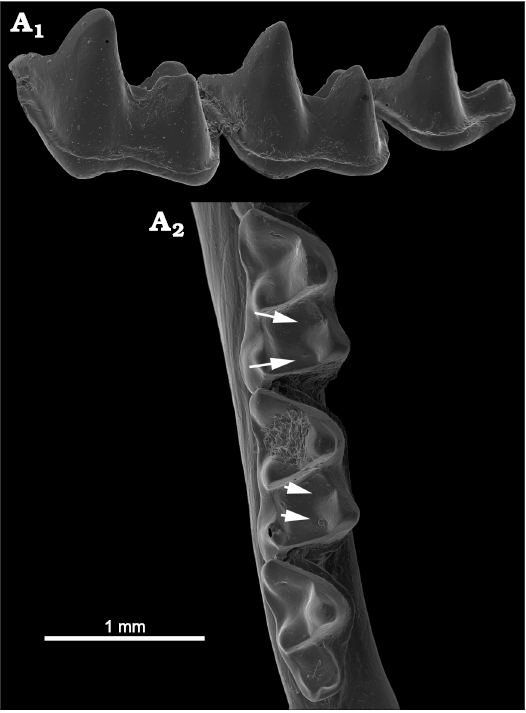
Fig. 12. Vespertilionid bat Murina suilla (Temminck, 1840) (AMNH 217012) from Malaysia, Recent. Left lower molars in anterolabial view (A1) showing deeply excavated cristids obliqua in m1–m2 but no within-crest notches; and in occlusal view (A2) showing accessory troughs (arrows) in m1–m2.
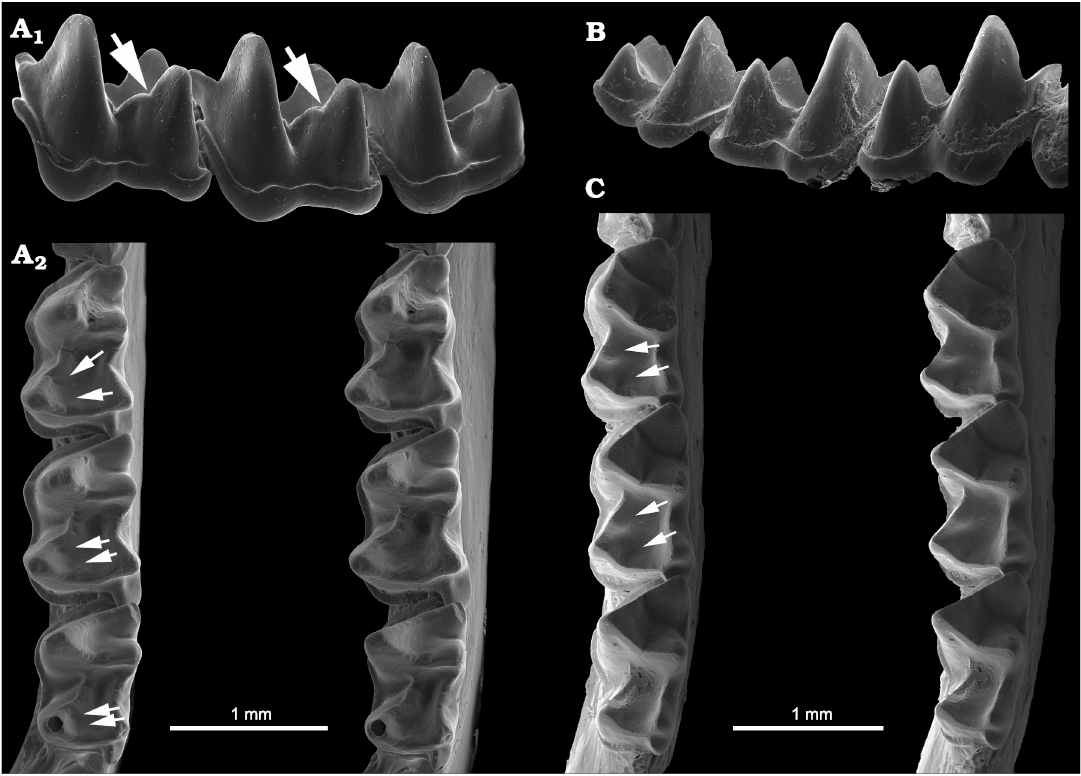
Fig. 13. Molars of Recent vespertilionid bats. A. Kerivoula argentata Tomes, 1861 (AMNH 89177) from Zambia. Left lower molars in anterolabial view (A1) showing carnassiform notches (arrows) in the cristids obliqua of m1–m2, and in occlusal view (A2, stereopair) showing accessory troughs (arrows) in m1–m3. B, C. Phoniscus papuensis (Dobson, 1878b) (AMNH 157475) from Papua New Guinea. B. Right lower molars in anterolabial view showing deeply excavated cristids obliqua in m1-m3 but no within-crest carnassiform notches. C. Left lower molars in occlusal view (stereopair) showing well-developed accessory troughs (arrows) in m1–m2.
Discussion
Despite many helpful treatises and investigations dealing partly or wholly with mammalian and bat teeth (Miller 1907; Osborn 1907; Slaughter 1970; Koopman and MacIntyre 1980; Thenius 1989; Freeman 1979, 1981, 1984, 1988, 1992, 1995, 1998, 2000; Strait 1993; Dumont 1995, 1999; Dumont et al. 2012; Evans 2005, 2006; Evans and Sanson 1998, 2003, 2005; Horáček and Špoutil 2012; Ravel et al. 2015; Berkovitz and Shellis 2018; Lang et al. 2021), the carnassiform notches of the lower molar talonids of bats have rarely been mentioned previously and have not been investigated functionally, or were absent in the species under study. Koopman and MacIntyre (1980: figs. 4, 8) illustrated bat teeth bearing carnassiform notches without noting or discussing them (e.g., Natalus major and Micronycteris [=Lampronycteris] brachyotis). The structures might nevertheless be important in the astounding evolutionary success of bats, along with many other important aspects of their natural history (Irving et al. 2021) that continue bringing us new lessons about bats.
The carnassial notch in the molar trigonid (paracristid) of mammals has traditionally been interpreted as an adaptation for carnivory, and a paracristid notch is often present in bat trigonids, too, but not only in the carnivorous species. In fact, while paracristid notches are present in carnivorous bats, carnassiform notches on the molar talonids (cristid obliqua and postcristid) are absent in the most carnivorous phyllostomids Trachops, Chrotopterus, and Vampyrum, but are present in many insectivorous and omnivorous phyllostomids. These most carnivorous phyllostomids and certain other carnivorous bats such as the megadermatid Macroderma actually show reduction of the talonid portions of their lower molars, as occurs in other, non-chiropteran carnivorous mammals (Solé and Ladevèze 2017; Lang et al. 2021). The widespread occurrence of carnassiform notches in bat molar talonids, most of which are insectivorous or insectivorous–omnivorous, suggests that the notches in bats and certain other mammals do not necessarily indicate carnivory.
Fenton et al. (1981) described the feeding habits of the bat Nycteris grandis (Nycteridae) in one particular colony along the Zambezi River in Zimbabwe. These bats clearly made heavy use of vertebrates as well as arthropods as food (Fenton et al. 1981). For some bat species that eat vertebrates, they are mainly a supplementary or circumstantial food (Gillette 1975). Other species of nycterids are primarily insectivorous but occasionally take small vertebrates such as frogs and fishes (e.g., N. thebaica, Seamark and Bogdanowicz 2002).
Because the stem bats lack CN and were probably insectivorous, then the common ancestor of all bats probably had no notches. Nature selects for shapes and strategies that improve the conservation of energy. Evans provided a model addressing functional aspects of the shearing blades on bat upper molar crests from a tool design standpoint (Evans 2005, 2006; Evans and Sanson 2003). Evans (2006) described “food capture areas” as the space between occluding blades (such as each of the major upper molar ectoloph crests), and “fragment clearance” areas (the protofossa, parafossa, and metafossa of upper molars), which allow food particles to flow away from shearing blades and their rake surfaces so they do not interfere with the edge. Cutting efficiency is improved as the “approach angle” of at least one of occluding blades differed from zero degrees, providing “one point cutting” (Evans 2006).
Relative to the Evans model (Evans and Sanson 2003; Evans 2005, 2006), we believe CN further improve the energetic efficiency of shearing crests. If the notches were neutral in the face of selection, or only conveyed a slight evolutionary advantage, some lineages could still survive without CN. The slightly different types of CN and accessory troughs in different taxa may reflect differences to be expected in independently and convergently evolved structures in different phylogenetic lineages, or may reflect different foods with differing physical properties to be overcome when eaten, or some other evolutionary-developmental phenomena. Of course, the study of the functioning of these notches in conjunction with the upper molars during chewing could shed light on their purpose and appropriateness for certain physical qualities of the food being eaten.
No bat families had CN on only the postcristids. This could be due to the fact that the cristid obliqua is the first crest of the talonid to interact with the food during chewing (Crompton 1971). However, it might be more advantageous to have CN on both talonid crests because it would further increase the total length of crests.
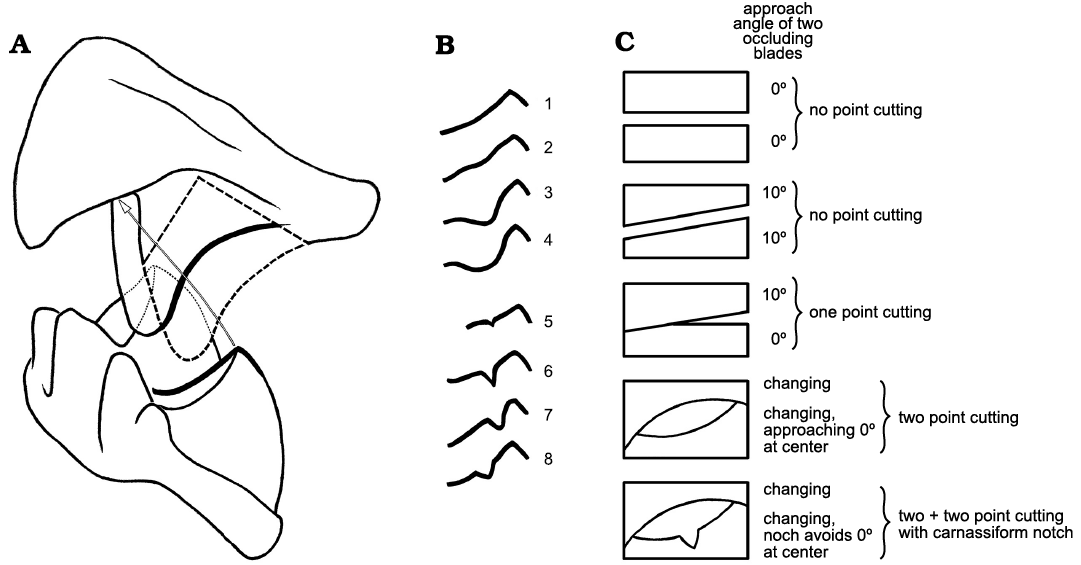
Fig. 14. A. Diagram of a bat M2 and m2 in posterolingual view showing the relationship of the shearing crests (postparacrista of M2 with cristid obliqua of m2) of tribosphenic bat molars during occlusion. Dotted line indicates portion of m2 hidden by M2; dashed line indicates metacone “removed” to reveal the paracone and postparacrista of M2; thin arrow indicates trajectory of hypoconid into the protofossa (not visible) of M2 during occlusion; boldest line on M2 is edge of postparacrista; boldest line on m2 is tip of hypoconid and cristid obliqua. B. Outline representations of the hypoconid and cristid obliqua observed in this study, without and with in-crest carnassiform notches. Types 1–4 without carnassiform notches: 1 and 2 are seen for example, in Hipposideridae, Rhinolophidae, Rhinopomatidae, Emballonuridae, Mormoopidae, Phyllostomidae, Molossidae, Vespertilionidae; 3 in Miniopteridae, Murininae; 4 in Phoniscus. Types 5–8 with carnassiform notches: 5 in Megadermatidae; 6 in Nycteridae; 7 in Natalidae; 8 in Mystacinidae, Furipteridae, Thyropteridae, Phyllostomidae, Kerivoulinae. C. Evans model showing effect of approach angles of two occluding blades on point cutting: With both blades at 0° and both at 10° no point cutting occurs; with one blade at 10° and one blade at 0°, one point cutting is enabled (Evans 2006: fig. 5.1c). Our application of Evans model to bat postparacrista and cristid obliqua as the upper and lower blades, respectively: with two curved blades without carnassiform notches, two point cutting occurs at opposite end of blades until center is reached and approach angles reach 0°; with emplacement of a carnassiform notch in lower blade at the point at which changing blade angles approach 0°, notch enables a second change in approach angles.
Interestingly, notches in the paracristids of lower molars occlude with notchless postmetacristas of upper molars in some of the other mammals mentioned earlier, such as the sparassodont family Hathliacynidae, predatory metatherians of the South American Miocene (Marshall 1981). For example, the hathliacynid Acyon myctoderos has pronounced carnassial notches on lowers m2–m4, each of which is functionally paired with a notchless (but vertically concave) elongated postmetacrista on M1–M3 (Forasiepi et al. 2006; Engelman et al. 2015). These extinct metatherians were large-bodied and probably carnivorous. Even in some Carnivora the P4 has no carnassial notch (Mustelidae, Mephitidae). In contrast to the larger-bodied sparassodonts and carnivorans noted, many bats discussed herein that have carnassiform notches on the talonid crests as well as on the paracristid (and sometimes the protocristid) are small-bodied and insectivorous–omnivorous–carnivorous, and none have notches on the corresponding upper molar crests, the postparacrista and premetacrista. However, as noted in bat molars by Miller (1907), the edges of the shearing crests are often concave. The concavities in the upper molar crests are sufficient to capture food when working jointly with concave lower molar crests (or enhanced by notched lower crests; Fig. 14). The dietarily most carnivorous bats such as Macroderma and Vampyrum (Bonato et al. 2004; Gual-Suárez and Medellín 2021) have reduced central crests (postparacrista and premetacrista) on the ectoloph of upper molars (Freeman 1998). For insectivorous–omnivorous bats, CN in the cristid obliqua and postcristid may take advantage of the extra length of the central crests of the W-shaped ectoloph, increasing the efficiency in sectioning and chopping chitinous invertebrates and small vertebrates in those species that need to quickly process food in flight in order to empty their oral cavity and return to echolocating, while others temporarily perch to process captured food. Notches might improve chewing efficiency in insectivorous and insectivorous–omnivorous bats by increasing the effective length of a crest or the total length of crests in a molar while the position of, and distance between, cusps remain the same. The curved cutting edges of bat molar shearing crests provide changing approach angles and improved point cutting. A carnassiform notch in one of the occluding blades avoids the problem of losing point cutting by again changing the approach angle just as it approaches zero degrees in both occluding blades. Accessory troughs adjacent to carnassiform notches in the cristids obliqua, postcristids, and sometimes even in entocristids probably provide improved clearance of the resulting chitin fragments. Yet questions remain. Why don’t the notches appear in the corresponding crests of the upper molars, when they presumably evolve together as functional units in occlusion? Precise mandibular motion is a critical component of the highly integrated development and functioning of mammalian teeth (Horáček and Špoutil 2012; Polly 2012; Smits and Evans 2012). Might it be because the crests of mandibular teeth move while the maxillary teeth are relatively immobile?
Phylogenetic distribution of CN in bat molars
Carnassiform notches were absent from almost all of the archaic Paleogene bats we examined, suggesting that CN were absent in the ancestral bats. When the occurrence of CN on cristids obliqua and postcristids that we observed among extant bat families is considered relative to available recent phylogenies of bats (e.g., Simmons et al. 2008; Ravel et al. 2015, 2016; Amador et al. 2016; Shi et al. 2018; Teeling et al. 2018), CN have probably evolved multiple times independently during the radiation of crown bats as well as in a few extinct taxa. They occur in at least two yinpterochiropteran clades, Hipposideridae (only in the extinct Paleogene genus Vaylatsia) and Megadermatidae. They are absent in at least Rhinolophidae and Rhinopomatidae (two yinpterochiropteran families, Rhinonycteridae and Craseonycteridae, were not studied). Notches appear in three clades within Yangochiroptera: Nycteridae, two of five or six families (depending on whether Myzopodidae belongs in Vespertilionoidea or Noctilionoidea) of the Vespertilionoidea (Natalidae and Vespertilionidae), and four of six or seven families of the Noctilionoidea (Mystacinidae, Furipteridae, Thyropteridae, and Phyllostomidae). They are absent in at least Emballonuridae (except on entocristids of a few), Mormoopidae, Noctilionidae, Myzopodidae, Miniopteridae, and Molossidae (Cistugidae were not studied). Moreover, the notches and accessory troughs vary in their shape details and combinations where they are present. For example, the most common configuration is a V-shaped or U-shaped notch in both the cristid obliqua and postcristid in those noctilionoids and most vespertilionoids in which notches occur (Mystacinidae, Furipteridae, Thyropteridae, Macrotinae, Micronycterinae, Phyllostominae, Lonchorhininae, Glyphonycterinae, Murininae, and Kerivoulinae); in Nycteridae and Megadermatidae notches occur only on the cristid obliqua, are very small in megadermatids and deep in nycterids; in natalids they are uniquely blocky; and in several families accessory troughs occur without the presence of notches. These differences suggest the notches arose convergently or in parallel.
Conclusions
Preliminary observations on the occurrence of carnassiform notches and associated accessory troughs in the shearing crests of the lower molar talonids of diverse taxa of bats suggest that these notches have arisen in several families having tribosphenic molars. The notches and troughs appear to provide both phylogenetic and functional data. Where present, these structures are morphologically consistent within a family except for Phyllostomidae in which they are mostly consistent within certain subfamilies or tribes. In at least the species we examined they occur in Emballonuridae (in only a few species, on entocristid only), Nycteridae (all species, on cristid obliqua only), Megadermatidae (few species, weak on cristid obliqua only), Mystacinidae (on cristid obliqua and postcristid), Furipteridae (all species, on cristid obliqua and postcristid), Thyropteridae (all species, on all crests), Phyllostomidae (in Macrotinae, Micronycterinae, most Phyllostominae, Lonchorhininae, and Glyphonycterinae, on cristid obliqua and postcristid), and Natalidae (all species, distinctive blocky or step-like type, on cristid obliqua and postcristid in Chilonatalus species and Natalus species; on cristid obliqua in Nyctiellus). In addition, some members of Vespertilionidae (especially Murininae and Kerivoulinae) and Miniopteridae bear accessory troughs in the talonid basins but lack notches in the crests. The notches are mostly absent from the corresponding shearing crests of upper molars except weakly so in some Natalidae.
We assert that carnassiform notches on the talonid crests of mammals serve to increase the functional efficiency of molar crests in the chewing and sectioning of certain kinds of food material such as vertebrate muscle tissue and arthropod chitinous exoskeletons. Carnassiform notches and accessory troughs probably cause the shearing crests to be functionally more efficient by increasing the effective length of a crest while maintaining the same cusp-to-cusp distance and precise occlusal relationships, and by improving the food-capture area of the shearing blade during occlusion. The carnassiform notches and accessory troughs appear to provide an additional series of food capture edges and fragment clearance areas, respectively, smaller than those typically considered in studies of tribosphenic molars, and fractally inserted onto the larger crests and basins. The increased sophistication of this food-processing system might be particularly important in those species that must quickly acquire, chew, and swallow their food and resume echolocating to avoid obstacles, while others such as above-canopy open-air flyers such as molossids lack talonid notches. The conclusion that CN have evolved multiple times among bats seems inescapable because of their phylogenetic distribution and differing structure. However, these morphological features should be more intensively and extensively studied in order to be phylogenetically and functionally informative. They could benefit insectivorous mammals as well as carnivorous mammals.
Acknowledgements
We greatly appreciate the help of curators and collection managers in making us loans and allowing us to use and mold the lower jaws of Recent specimens: Richard W. Thorington Jr. (deceased), Michael D. Carleton, and David F. Schmidt (all USNM); Nancy B. Simmons and Eileen Westwig (both AMNH); William Stanley, Lawrence R. Heaney, and John D. Phelps Jr. (all FMNH); Janet K. Braun, Marcia A. Revelez, and Jennifer M. Larsen (all OMNH), especially for the time-consuming process of selecting specimens with little-worn teeth for our study. We are grateful to K. Christopher Beard and Matthew F. Jones (both University of Kansas Museum of Natural History, USA), Donald E. Savage (deceased, formerly University of California Museum of Paleontology, Berkeley, USA), Bernard Sigé (Université Claude Bernard-Lyon 1, France), and John E. Storer (Royal Saskatchewan Museum, Regina, Canada) for providing casts of fossil bats. We extend our sincere gratitude to Alison Baker, Kyle L. Davies, Brian M. Davis, Jennifer M. Larsen, Richard Lupia, and Stephen R. Westrop (University of Oklahoma, Norman, USA) for various kinds of support in the completion of this project. Helpful reviews by Nancy B. Simmons (AMNH) and Camilo López-Aguirre (University of Toronto, Canada) significantly improved an earlier version of the manuscript. Finally, with this contribution in the journal he has long edited, we thank and esteem Richard L. Cifelli for his support, humor, and role-modeling scholarship during CGB’s graduate work and NJC’s research over many years.
References
Aguirre, L.F., Herrel, A., Van Damme, R., and Matthysen, E. 2003. The implications of food hardness for diet in bats. Functional Ecology 17: 201–212. Crossref
Amador, L.I., Moyers Arévalo, R.L., Almeida, F.C., Catalano, S.A., and Giannini, N.P. 2018. Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. Journal of Mammalian Evolution 25: 37–70. Crossref
Andersen, K. 1912. Brief diagnoses of eight new Petalia, with a list of the known forms of the genus. Annals and Magazine of Natural History Series 8 10: 546–550. Crossref
Asher, R.J., McKenna, M.C. Emry, R.J. Tabrum, A.R., and Kron, D.G. 2002. Morphology and relationships of Apternodus and other extinct, zalambdodont, placentaal mammals. Bulletin of the American Museum of Natural History 273: 1–117. Crossref
Baker, R.J., Bininda-Emonds, O.R.P. Mantilla-Meluk, H., Porter, C.A., andVan Den Bussche, R.A. 2012. Molecular time scale of diversification of feeding strategy and morphology in new world leaf-nosed bats (Phyllostomidae): a phylogenetic perspective. In: G.F. Gunnell and N.B. Simmons (eds.), Evolutionary History of Bats: Fossils, Molecules and Morphology, 385–409. Cambridge University Press, Cambridge. Crossref
Baker, R.J., Solari, S., Cirranello, A., and Simmons, N.B. 2016. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chiropterologica 18: 1–38. Crossref
Berkovitz, B. and Shellis, P. 2018. The Teeth of Mammalian Vertebrates. 334 pp. Academic Press, New York.
Bhullar, B.-A.S., Manafzadeh, A.R., Miyamae, J.A., Hoffman, E.A., Brainerd, E.L., Musinsky, C., and Crompton, A.W. 2019. Rolling of the jaw is essential for mammalian chewing and tribosphenic molar function. Nature 566: 528–532. Crossref
Bonato, V., Gomes Facure, K., and Uieda, W. 2004. Food habits of bats of subfamily Vampyrinae in Brazil. Journal of Mammalogy 85: 708–713. Crossref
Butler, P.M. 1946. The evolution of carnassial dentitions in the Mammalia. Proceedings of the Zoological Society of London 116: 198–220. Crossref
Butler, P.M. 1972. Some functional aspects of molar evolution. Evolution 26:474–483. Crossref
Cifelli, R.L. and de Muizon, C. 1993. Dentition and jaw of Kokopellia juddi, a primitive marsupial or near-marsupial from the medial Cretaceous of Utah. Journal of Mammalian Evolution 4: 241–258.
Cirranello, A., Simmons, N.B., Solari, S., and Baker, R.J. 2016. Morphological diagnoses of higher-level phyllostomid taxa (Chiroptera: Phyllostomidae). Acta Chiropterologica 18: 39–71. Crossref
Crompton, A.W. 1971. The origin of the tribosphenic molar. In: D.M. Kermack and K.A. Kermack (eds.), Early Mammals. Zoological Journal of the Linnean Society 50 (Supplement 1): 65–87.
Crompton, A.W. and Hiiemae, K.M. 1969. Functional occlusion in tribosphenic molars. Nature 222: 678–679. Crossref
Crompton, A.W. and Hiiemae, K.M. 1970. Functional occlusion and mandibular movements during occlusion in the American opossum, Didelphis marsupialis L. Zoological Journal of the Linnean Society 49: 21–47. Crossref
Cuvier, F. 1828. Description d’un nouveau genre de chauve-souris sous le nom de Furie. Mémoires du Muséum d’Histoire Naturelle, Paris 16: 149–155.
Czaplewski, N.J. 1997. Chiroptera. In: R.F. Kay, R.H. Madden, R.L. Cifelli, and J.J. Flynn (eds.), Vertebrate Paleontology in the Neotropics: the Miocene Fauna of La Venta, Colombia, 410–431. Smithsonian Institution Press, Washington, D.C.
Czaplewski, N.J., Takai, M., Naeher, T.M., Shigehara, N., and Setoguchi, T. 2003. Additional bats from the Middle Miocene La Venta fauna of Colombia. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 27: 263–282.
Dávalos, L.M., Cirranello, A.L. Geisler, J.H., and Simmons, N.B. 2012. Understanding phylogenetic incongruence: lessons from phyllostomid bats. Biological Reviews 87: 991–1024. Crossref
Dávalos, L.M., Cirranello, A.L., Dumont, E.R., Rossiter, S.J., and Rojas, D. 2020. Adapt or live: adaptation, convergent evolution, and plesiomorphy. In: T. Fleming, L.M. Dávalos, and M.A.R. Mello (eds.), Phyllostomid Bats: A Unique Mammalian Radiation, 105–121. University of Chicago Press, Chicago.
Davis, B.M. 2011. Evolution of the tribosphenic molar pattern in early mammals, with comments on the “dual-origin” hypothesis. Journal of Mammalian Evolution 18: 227–244. Crossref
de Muizon, C. and Lange-Badré, B. 1997. Carnivorous dental adaptations in tribosphenic mammals and phylogenetic reconstruction. Lethaia 30: 353–366. Crossref
de Muizon, C. and Ladevèze, S. 2020. Cranial anatomy of Andinodelphys cochabambensis, a stem metatherian from the early Palaeocene of Bolivia. Geodiversitas 42: 597–739. Crossref
Dobson, G.E. 1878a. Notes on recent additions to the collection of Chiroptera in the Muséum d’Histoire Naturelle at Paris, with descriptions of new and rare species. Proceedings of the Zoological Society of London Part III 1878: 873–880. Crossref
Dobson, G.E. 1878b. Catalogue of the Chiroptera in the collection of the British Museum. xlii + 567 pp. British Museum (Natural History), London.
D’Orbigny, A. 1836. Lophostoma sylvicolum. In: A. D’Orbigny and P. Gervais (eds.), Voyage dans l’Amérique Méridionale, Vol. 4, Part 2 Mammifères. 11 pp. P. Bertrand, Paris.
Dumont, E.R. 1995. Enamel thickness and dietary adaptation among extant primates and chiropterans. Journal of Mammalogy 76: 1127–1136. Crossref
Dumont, E.R. 1999. The effect of food hardness on feeding behaviour in frugivorous bats (Phyllostomidae): an experimental study. Journal of Zoology, London 248: 219–229. Crossref
Dumont, E.R., Dávalos, L.M., Goldberg, A., Santana, S.E., Rex, K., and Voigt, C.C. 2012. Morphological innovation, diversification and invasion of a new adaptive zone. Proceedings of the Royal Society B 279: 1797–1805. Crossref
Engelman, R.K., Anaya, F., and Croft, D.A. 2015. New specimens of Acyon myctoderos (Metatheria, Sparassodonta) from Quebrada Honda, Bolivia. Ameghiniana 52: 204–225. Crossref
Esquivel, D.A., Maestri, R., and Santana, S.E. 2021. Evolutionary implications of dental anomalies in bats. Evolution 75: 1087–1096. Crossref
Evans, A.R. 2005. Connecting morphology, function and tooth wear in microchiropterans. Biological Journal of the Linnean Society 85: 81–96. Crossref
Evans, A.R. 2006. Quantifying relationships between form and function and the geometry of the wear process in bat molars. In: A. Zubaid, G.F. McCracken, and T.H. Kunz (eds.), Functional and Evolutionary Ecology of Bats, 93–109. Oxford University Press, Oxford.
Evans, A.R. and Sanson, G.D. 1998. The effect of tooth shape on the breakdown of insects. Journal of Zoology, London 246: 391–400. Crossref
Evans, A.R. and Sanson, G.D. 2003. The tooth of perfection: functional and spatial constraints on mammalian tooth shape. Biological Journal of the Linnean Society 78: 173–191. Crossref
Evans, A.R. and Sanson, G.D. 2005. Correspondence between tooth shape and dietary biomechanical properties in insectivorous microchiropterans. Evolutionary Ecology Research 7: 453–478.
Fenton, M.B., Thomas, D.W., and Sasseen, R. 1981. Nycteris grandis (Nycteridae): an African carnivorous bat. Zoological Society of London 194: 461–465. Crossref
Foley, N.M., Thong, V.D., Soisook, P., Goodman, S.M., Armstrong, K.N., Jacobs, D.S., Puechmaille, S.J., and Teeling, E.C. 2014. How and why overcome the impediments to resolution: lessons from rhinolophid and hipposiderid bats. Molecular Biology and Evolution 32: 313–333. Crossref
Forasiepi, A.M., Sánchez-Villagra, M.R., Goin, F.J., Takai, M., Shigehara, N., and Kay, R.F. 2006. A new species of Hathliacynidae (Metatheria, Sparassodonta) from the Middle Miocene of Quebrada Honda, Bolivia. Journal of Vertebrate Paleontology 26: 670–684. Crossref
Fracasso, M.P.A., Salles, L.O., and Araújo Perini, F. 2011. Upper molar morphology and relationships among higher taxa in bats. Journal of Mammalogy 92: 421–432. Crossref
Freeman, P.W. 1979. Specialized insectivory: beetle-eating and moth-eating molossid bats. Journal of Mammalogy 60: 467–479. Crossref
Freeman, P.W. 1981. Correspondence of food habits and morphology in insectivorous bats. Journal of Mammalogy 62: 166–173. Crossref
Freeman, P.W. 1984. Functional cranial analysis of large animalivorous bats (Microchiroptera). Biological Journal of the Linnean Society 21: 387–408. Crossref
Freeman, P.W. 1988. Frugivorous and animalivorous bats (Microchiroptera): dental and cranial adaptations. Biological Journal of the Linnean Society 33: 249–272. Crossref
Freeman, P.W. 1992. Canine teeth of bats (Microchiroptera): size, shape and role in crack propagation. Biological Journal of the Linnean Society 45: 97–115. Crossref
Freeman, P.W. 1995. Nectarivorous feeding mechanisms in bats. Biological Journal of the Linnean Society 56: 439–463. Crossref
Freeman, P.W. 1998. Form, function, and evolutioin in skulls and teeth of bats. In: T.H. Kunz and P.A. Racey (eds.), Bat Biology and Conservation, 140–156. Smithsonian Institution Press, Washington, D.C.
Freeman, P.W. 2000. Macroevolution in Microchiroptera: recoupling morphology and ecology with phylogeny. Evolutionary Ecology Research 2: 317–335.
Gardner, A.L. 1977. Feeding habits. In: R.J. Baker and D.C. Carter (eds.), Biology of the Bats of the New World family Phyllostomatidae, Part 2. Special Publication Museum Texas Tech University 16: 293–350.
Geoffroy Saint-Hilaire, E. 1810. Sur les Phyllostomes et les Mégadermes, deux genres de la famille des chauve-souris. Annales du Muséum d’Histoire Naturelle Paris 15: 157–198.
Gillette, D.D. 1975. Evolution of feeding strategies in bats. Tebiwa, Journal of the Idaho State University 18: 39–48.
Gray, J.E. 1842. Descriptions of some new genera and fifty unrecorded species of Mammalia. Annals and Magazine of Natural History Series 1 10: 255–267. Crossref
Gray, 1843. [A letter from J.E. Gray, Esq., addressed to Mr. Waterhouse, was read, containing an account of two new species of bats, a species of the family Hystricidae, and a new Manis.] Proceedings of the Zoological Society of London 11: 20–22.
Gual-Suárez, F. and Medellín, R.A. 2021. We eat meat: a review of carnivory in bats. Mammal Review 2021: 1–19. Crossref
Gunnell, G.F., Eiting, T.P., and Simons, E.L. 2012. African Vespertilionoidea (Chiroptera) and the antiquity of Myotinae. In: G.F. Gunnell and N.B. Simmons (eds.), Evolutionary History of Bats: Fossils, Molecules and Morphology, 252–266. Cambridge University Press, Cambridge. Crossref
Gunnell, G.F., Simmons, N.B., and Seiffert, E.R. 2014. New Myzopodidae (Chiroptera) from the late Paleogene of Egypt: emended family diagnosis and biogeographic origins of Noctilionoidea. PLoS One 9: e86712. Crossref
Gunnell, G.F., Simons, E.L., andSeiffert, E.R. 2008. New bats (Mammalia: Chiroptera) from the late Eocene and early Oligocene, Fayum Depression, Egypt. Journal of Vertebrate Paleontology 28: 1–11. Crossref
Hand, S.J. and Sigé, B. 2018. A new archaic bat (Chiroptera: Archaeonycteridae) from an early Eocene forest in the Paris Basin. Historical Biology 30: 227–236. Crossref
Hand, S.J., Beck, R.M.D. Archer, M., Simmons, N.B., Gunnell, G.F., Scofield, R.P., Tennyson, A.J.D., De Pietri, V.L., Salisbury, S.W., and Worthy, T.H. 2018. A new, large-bodied omnivorous bat (Noctilionoidea: Mystacinidae) reveals lost morphological and ecological diversity since the Miocene in New Zealand. Scientific Reports 8: 235. Crossref
Hand, S.J., Novacek, M., Godthelp, H., and Archer, M. 1994. First Eocene bat from Australia. Journal of Vertebrate Paleontology 14: 375–381. Crossref
Hand, S.J., Sigé, B., and Maitre, E. 2012. Necromantis Weithofer, 1887, large carnivorous middle and late Eocene bats from the French Quercy Phosphorites: new data and unresolved relationships. In: G.F. Gunnell and N.B. Simmons (eds.), Evolutionary History of Bats: Fossils, Molecules and Morphology, 210–251. Cambridge University Press, Cambridge. Crossref
Hand, S.J., Sigé, B., Archer, M., and Black, K.H. 2016. An evening bat (Chiroptera: Vespertilionidae) from the late early Eocene of France, with comments on the antiquity of modern bats. Palaeovertebrata 40 (2): e2. Crossref
Hand, S.J., Sigé, B., Archer, M., Gunnell, G.F., and Simmons, N.B. 2015. A new early Eocene (Ypresian) bat from Pourcy, Paris Basin, France, with comments on patterns of diversity in the earliest chiropterans. Journal of Mammalian Evolution 22: 343–354. Crossref
Hill, J.E. 1964. Notes on bats from British Guiana, with the description of a new genus and species of Phyllostomidae. Mammalia 28: 553–572. Crossref
Hooker, J.J. 1996. A primitive emballonurid bat (Chiroptera, Mammalia) from the earliest Eocene of England. Palaeovertebrata 25: 287–300.
Horáček, I. and Špoutil F. 2012. Why tribosphenic? On variation and constraint in developmental dynamics of chiropteran molars. In: G.F. Gunnell and N.B. Simmons (eds.), Evolutionary History of Bats: Fossils, Molecules and Morphology, 410–455. Cambridge University Press, Cambridge. Crossref
Irving, A.T., Ahn, M., Goh, G., Anderson, D.E., and Wang, L.-F. 2021. Lessons from the host defences of bats, a unique viral reservoir. Nature 589: 363–370. Crossref
Jones, M.F., Coster, P.M.C., Licht, A., Métais, G., Ocakoglu, F., Taylor, M.H., and Beard, K.C. 2019. A stem bat (Chiroptera: Palaeochiropterygidae) from the late middle Eocene of northern Anatolia: implications for the dispersal and palaeobiology of early bats. Palaeobiodiversity and Palaeoenvironments 99: 261–269. Crossref
Kay, R.F. 1975. The functional adaptations of primate molar teeth. American Journal of Physical Anthropology 43: 195–216. Crossref
Kay, R.F. 1984. On the use of anatomical features to infer foraging behavior in extinct primates. In: P.S. Rodman and J.G.H. Cant (eds.), Adaptations for Foraging in Non-human Primates: Contributions to an Organismal Biology of Prosimians, Monkeys and Apes, 21–53. Columbia University Press, New York. Crossref
Kay, R.F. and Hiiemae, K.M. 1974. Jaw movement and tooth use in recent and fossil primates. American Journal of Physical Anthropology 40: 227–256. Crossref
Kay, R.F. and Sheine, W.S. 1979. On the relationship between chitin particle size and digestibility in the primate Galago senegalensis. American Journal of Physical Anthropology 50: 301–308. Crossref
Koenigswald, W. von 2020. Construction and wear of mammalian teeth in terms of heterochrony. In: T. Martin and W. von Koenigswald (eds.) Mammalian Teeth—Form and Function, 171–186. Verlag Dr Friederich Pfeil, München.
Koopman, K.F. and MacIntyre, G.T. 1980. Phylogenetic analysis of chiropteran dentition. In: D.E. Wilson and A.L. Gardner (eds.), Proceedings, Fifth International Bat Research Conference, 279–288. Texas Tech Press, Lubbock.
Lack, J.B., Roehrs, Z.P., Stanley, C.E., Ruedi, M., and Ven Den Bussche, R.A. 2010. Molecular genetics of Myotis indicate family-level divergence for the genus Cistugo (Chiroptera). Journal of Mammalogy 91: 976–992. Crossref
Lang, A.J., Engler, T., and Martin, T. 2022. Dental topographic and three-dimensional geometric morphometric analysis of carnassialization in different clades of carnivorous mammals (Dasyuromorpha, Carnivora, Hyaenodonta). Journal of Morphology 283 (1): 911–108. Crossref
Legendre, S. 1985. Molossidés (Mammalia, Chiroptera) cénozoiques de l’Ancien et du Nouveau Monde: statut systématique; intégration phylogéniquede données. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 170: 205–227.
Linares, O. and Ojasti, J. 1971. Una nueva especie de murciélago del género Lonchorhina (Chiroptera: Phyllostomidae) del sur de Venezuela. Novedades Científicas Serie Zoológica Contribuciones Ocasionales del Museo de Historia Natural La Salle 36: 1–8.
Linnaeus, C. 1758. Systema naturae per regnia tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Vol. 1, Editio decima, reformata. 824 pp. Laurentii Salvii, Stockholm. Crossref
López-Aguirre, C., Czaplewski, N.J., Link, A., Takai, M., and Hand, S.J. 2021. Dietary and body-mass reconstruction of the Miocene neotropical bat Notonycteris magdalenensis (Phyllostomidae) from La Venta, Colombia. Paleobiology: 1–17 [published online, https://doi.org/10.1017/pab.2021.21]. Crossref
MacIntyre, G.T. 1966. The Miacidae (Mammalia, Carnivora) Part 1. The systematics of Ictidopappus and Protictis. Bulletin of the American Museum of Natural History 131: 115–210.
Maitre, E. 2014. Western European middle Eocene to early Oligocene Chiroptera: systematics, phylogeny, and palaeoecology based on new material from the Quercy (France). Swiss Journal of Palaeontology 133: 141–242. Crossref
Marshall, L.G. 1981. Review of the Hathlyacyninae, an extinct subfamily of South American “dog-like” marsupials. Fieldiana Geology new series 7: 1–120. Crossref
Marshall, P.M. and Butler, P.M. 1966. Molar cusp development in the bat, Hipposideros beatus, with reference to the ontogenetic basis of occlusion. Archives of Oral Biology 11: 949–965, IN1. Crossref
Menu, H. 1985. Morphotypes dentaires actuels et fossiles des chiroptères vespertilioninés. 1e partie: Étude des morphologies dentaires. Palaeovertebrata 15: 71–128.
Menu, H. 1987. Morphotypes dentaires actuels et fossiles des chiroptères vespertilioninés. 2ème partie:implications systématiques et phylogéniques. Palaeovertebrata 17: 77–150.
Menu, H. and Sigé, B. 1971. Nyctalodontie et myotodontie, importants caractères de grades évolutifs chez les chiroptères entomophages. Comptes rendu de l’Academie des Sciences, Paris 272: 1735–1738.
Miller, G.S., Jr. 1900. A second collection of bats from the island of Curaçao. Proceedings of the Biological Society of Washington 13: 159–162.
Miller, G.S., Jr. 1905. A new genus of bats from Sumatra. Proceedings of the Biological Society of Washington 18: 229–230.
Miller, G.S., Jr. 1907. The families and genera of bats. Bulletin of the United States National Museum 57: 1–282. Crossref
Morales-Martínez, D.M., López-Arévalo, H.F., and Vargas-Ramírez, M. 2021. Beginning the quest: phylogenetic hypothesis and identification of evolutionary lineages inbats of the genus Micronycteris (Chiroptera, Phyllostomidae). ZooKeys 1028: 135–159. Crossref
Morgan, G.S. and Czaplewski, N.J. 1999. First fossil record of Amorphochilus schnablii (Chiroptera: Furipteridae), from the late Quaternary of Peru. Acta Chiropterologica 1 (1): 75–79.
Morgan, G.S. and Czaplewski, N.J. 2003. A new bat (Chiroptera: Natalidae) from the Early Miocene of Florida, with comments on natalid phylogeny. Journal of Mammalogy 84 (2): 729–752. Crossref
Osborn, H.F. 1907. Evolution of Mammalian Molar Teeth to and from the Triangular Type. 250 pp. The MacMillan Company, New York.
Peigne, S., Chaimanee, Y., Yamee, C., Marandat, B., Srisuk, P., and Jaeger, J.-J. 2009. An astonishing example of convergent evolution toward carnivory: Siamosorex debonisi n. gen., n. sp. (Mammalia, Lipotyphla, Soricomorpha, Plesiosoricidae) from the latest Oligocene of Thailand. Geodiversitas 31: 973–992. Crossref
Perrella, D.F., Zima, P.V.Q., Ribeiro-Silva, L,. Biagolini, C.H., Jr., Carmignotto, A.P., Galetti, P.M., Jr, and Francisco, M.R. 2019. Bats are predators at the nests of tropical forest birds. Journal of Avian Biology 2020: e02277. Crossref
Peters, W. 1869. [Hr. W. Peters las bemerkungen über neue oder weniger bekannte flederthiere, besonders des Pariser Museums.] Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin 1869: 391–408.
Phillips, C.J. 1971. The dentition of glossophagine bats: development, morphological characteristics, variation, pathology, and evolution. Miscellaneous Publications, University of Kansas Natural History Museum 54: 1–138.
Phillips, C.J. 2000. A theoretical consideration of dental morphology, ontogeny, and evolution in bats. In: R.A. Adams and S.C. Peterson (eds.), Ontogeny, Functional Ecology, and Evolution of Bats, 247–274. Cambridge University Press, Cambridge. Crossref
Pickford, M. 2018. Fossil fruit bat from the Ypresian/Lutetian of Black Crow, Namibia. Communications of the Geological Survey of Namibia 18: 64–71.
Pine, R.H., LaVal, R.K., Carter, D.C., and Mok, W.-Y. 1996. Notes on the graybeard bat, Micronycteris daviesi (Hill) (Mammalia: Chiroptera: Phyllostomidae), with the first records from Ecuador and Brazil. Contributions in Mammalogy, Museum of Texas Tech University 7: 183–190.
Polly, P.D. 2012. Movement adds bite to the evolutionary morphology of mammalian teeth. BMC Biology 10: 69. http://www.biomedcentral.com/1741-7007/10/69 Crossref
Ravel, A., Adaci, M., Bensalah, M., Charruault, A.-L., Essid, E.M., Ammar, H. K., Marzougui, W., Mahboubi, M., Mebrouk, F., Merzeraud, G., Vianey-Liaud, M., Tabuce, R., and Marivaux, L. 2016. Origine et radiation initiale des chauves-souris modernes: nouvelles découvertes dans l’Éocène d’Afrique du Nord. Geodiversitas 38: 355–434. Crossref
Ravel, A., Adaci, M., Bensalah, M., Mahboubi, M., Mebrouk, F., Mebrouk Essid, E., Marzougui, W., Khayati Ammar, H., Charruault, A.-L., Lebrun, R., Tabuce, R., Vianey-Liaud, M., and Marivaux, L. 2015. New philisids (Mammalia, Chiroptera) from the Early–Middle Eocene of Algeria and Tunisia: new insight into the phylogeny, palaeobiogeography and palaeoecology of the Philisidae. Journal of Systematic Palaeontology 13 (8): 691–709. Crossref
Rojas, D., Vale, Á., Ferrero, V., and Navarro, L. 2011. When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Molecular Ecology 20: 2217–2228. Crossref
Rose, K.D. and Simons, E.L. 1977. Dental function in the Plagiomenidae: origin and relationships of the mammalian order Dermoptera. Contributions from the Museum of Paleontology, the University of Michigan 24: 221–236.
Rossina, V.V. [Rosina, V.V.] 2003. Murinodonty—a special type of structure crowns lower molars of bats [in Russian]. Plecotus et al. 6: 3–6.
Rosina, V.V. and Rummel, M. 2012. The bats (Chiroptera, Mammalia) from the Early Miocene of Petersbuch (Bavaria, southern Germany). Geobios 45: 463–478. Crossref
Rosina, V.V. and Rummel, M. 2019. The Early Miocene bats (Chiroptera, Mammalia) from the karstic sites of Erkertshofen and Petersbuch 2 (southern Germany). Fossil Imprint 75: 412–437. Crossref
Rossoni, D.M., Assis, A.P.A., Giannini, N.P., and Marroig, G. 2017. Intense natural selection preceded the invasion of new adaptive zones during the radiation of new world leaf-nosed bats. Scientific Reports 7: 11076. Crossref
Russell, D.E. and Sigé, B. 1970. Révision des chiroptères lutétiens de Messel (Hesse, Allemagne). Palaeovertebrata 3: 83–182. Crossref
Russell, D.E., Louis, P., and Savage, D.E.1973. Chiroptera and Dermoptera of the French early Eocene. University of California Publications in Geological Sciences 95: 1–57.
Sanborn, C.C. 1949. Bats of the genus Micronycteris and its subgenera. Fieldiana Zoology 31: 215–233. Crossref
Santana, S.E., Geipel, I., Dumont, E.R., Kalka, M.B., and Kalko, E.K.V. 2011a. All you can eat: high performance capacity and plasticity in the common big-eared bat, Micronycteris microtis (Chiroptera: Phyllostomidae). PLoS One 6 (12): e28584. Crossref
Santana, S.E., Strait, S.G., and Dumont, E.R. 2011b. The better to eat you with: functional correlates of tooth structure in bats. Functional Ecology 25: 839–847. Crossref
Savage, D.E. 1951. A Miocene phyllostomatid bat from Colombia, South America. University of California Publications Bulletin of the Department of Geological Sciences 28: 357–366.
Schultz, J.A., Anders, U., Braune, C., Brinkötter, J.J., Calandra, I., Engels, S., Findeisen, E., Gailer, J.-P., Hummel, J., Jäger, K.R.K., Kaiser, T.M., Kalthoff, D.C., Koenigswald, W. von, Kullmer, O., Landwehr, C., Mau, M., Menz, U., Ruf, I., Schubert, A.M., Schulz-Kornas, E., Schwermann, A.H., Schwermann, L.C., Skiba, M., Steuer, P., Südekum, K.-H., Winkler, D. E., and Martin, T. 2020. A new wear facet terminology for mammalian dentitions. In T. Martin and W. v. Koenigswald (eds.) Mammalian Teeth—Form and Function, 11–24. Verlag D. Friedrich Pfeil, München.
Seamark, E.C.J. and Bogdanowicz, W. 2002. Feeding ecology of the common slit-faced bat (Nycteris thebaica) in KwaZulu-Natal, South Africa. Acta Chiropterologica 4: 49–54. Crossref
Shi, J.J., Westeen, E.P., and Rabosky, D.L. 2018. Digitizing extant bat diversity: an open-access repository of 3D mCT-scanned skulls for reserch and education. PLoS One 13: e0203022. Crossref
Sigé, B., E. Maitre, and S. Hand. 2012. Necromantodonty, the primitive condition of lower molars among bats. In: G. F. Gunnell and N. B. Simmons (eds.) Evolutionary History of Bats: Fossils, Molecules and Morphology, 456–469. Cambridge University Press, New York. Crossref
Simmons, N.B. 1998. A reappraisal of interfamilial relationships of bats. In: T.H. Kunz and P.A. Racey (eds.), Bat Biology and Conservation, 3–26. Smithsonian Institution Press, Washington, D.C.
Simmons, N.B. and Cirranello, A.L. 2020. Bat Species of the World: A taxonomic and geographic database. www.batnames.org [accessed on 18 January 2021].
Simmons, N.B. and Giesler, J.H. 1998. Phylogenetic relationships of Icaronycteris, Archaeonycteris, Hassianycteris, and Palaeochiropteryx to extant bat lineages, with comments on the evolution of echolocation and foraging strategies in Microchiroptera. Bulletin of the American Museum of Natural History 235: 4–182.
Simmons, N.B. and Voss, R.S. 1998. The mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna, Part 1. Bats. Bulletin of the American Museum of Natural History 237: 1–219.
Simmons, N.B., Seymour, K.L., Habersetzer, J., and Gunnell, G.F. 2008. Primitive early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451: 818–821. Crossref
Slaughter, B.H. 1970. Evolutionary trends of chiropteran dentitions. In: B.H. Slaughter and D.W. Walton (eds.), About Bats: A Chiropteran Biology Symposium, 51–83. Southern Methodist University Press, Dallas.
Smith, R. and Russell, D.E.1992. Mammiferes (Marsupialia, Chiroptera) de l’Ypresien de la Belgique. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de La Terre 62: 223–227.
Smith, T., Habersetzer, J., Simmons, N.B., and Gunnell, G.F. 2012. Systematics and paleobiogeography of early bats. In: G.F. Gunnell and N.B. Simmons (eds.), Evolutionary History of Bats: Fossils, Molecules and Morphology, 23–66. Cambridge University Press, New York. Crossref
Smits, P.D. and Evans, A.R. 2012. Functional constraints on tooth morphology in carnivorous mammals. BMC Evolutionary Biology 12: 146. Crossref
Soisook, P., Prajakjitir, A., Karapan, S., Frrancis, C.M., and Bates, P. J.J. 2015. A new genus and species of false vampire (Chiroptera: Megadermatidae) from peninsular Thailand. Zootaxa 3931 528–550. Crossref
Solari, S., Pacheco, V., and Vivr, E. 1999. New distribution records of Peruvian bats. Revista Peruviana de Biología 6: 152–159.
Solé, F. and Ladevèze, S. 2017. Evolution of the hypercarnivorous dentition in mammals (Metatheria, Eutheria) and its bearing on the development of tribosphenic molars. Evolution and Development 19: 56–68. Crossref
Storch, G. and Habersetzer, J. 1988. Archaeonycteris pollex (Mammalia, Chiroptera), eine neue fledermaus aus dem Eozan der Grube Messel bei Darmstadt. Courier Forschungs-Institut Senckenberg 107: 249–261.
Strait, S.G. 1993. Molar morphology and food texture among small-bodied insectivorous mammals. Journal of Mammalogy 74: 391–402. Crossref
Tabuce, R., Antunes, M.T., and Sigé, B. 2009. A new primitive bat from the earliest Eocene of Europe. Journal of Vertebrate Paleontology 29: 627–630. Crossref
Teeling, E.C., Vernes, S.C., Dávalos, L.M., Ray, D.A., Gilbert, M.T.P., Myers, E., and Bat1K Consortium. 2018. Bat biology, genomes, and the Bat1K Project: to generate chromosome-level genomes for all living bat species. Annual Review of Animal Biosciences 6: 23–46. Crossref
Tejedor, A. 2011. Systematics of funnel-eared bats (Chiroptera: Natalidae). Bulletin of the American Museum of Natural History 353: 1–140. Crossref
Temminck, C.J. 1840. Monographies de mammalogie, ou description de quelques genres de mammifères, dont les espèces ont été observées dans les différens musées de l’Europe. Tome 2. 392 pp. C.C. Vander Hoek, Leiden.
Thenius, E. 1989. Zähne und Gebiss der Säugetiere. Bd. 8, Mammalia, Teilband 56. Handbuch der Zoologie. Eine naturgeschichte der Stämme des Tierreichen. 513 pp. Walter de Gruyter, Berlin. Crossref
Thomas, O. 1896. On new small mammals from the Neotropical region. Annals and Magazine of Natural History series 6 18: 301–314. Crossref
Tomes, R.F. 1858. A monograph of the genus Miniopteris [sic]. Proceedings of the Zoological Society of London 1858 (part 26): 115–128. Crossref
Tomes, R.F. 1861. Notes on a collection of bats made by Mr. Anderson in the Damara Country, south-western Africa, withnotices of some other African species. Proceedings of the Zoological Society of London 1861: 31–40.
Wetterer, A.L., Rockman, M.V., and Simmons, N.B. 2000. Phylogeny of phyllostomid bats (Mammalia: Chiroptera): data from diverse morphological systems, sex chromosomes, and restriction sites. Bulletin of the American Museum of Natural History 248: 1–200. Crossref
Ziegler, R. 2000. The bats (Chiroptera, Mammalia) from the late Oligocene fissure fillings Herrlingen 8 and Herrlingen 9 near Ulm (Baden-Württemberg). Senckenbergiana Lethaea 80: 647–683. Crossref
Zuercher, M.E., Monson, T.A., Dvoretzky, R.R., Ravindramurthy, S., and Hlusko, L.J. 2020. Dental variation in megabats (Chiroptera: Pteropodidae): tooth metrics correlate with body size and tooth proportions reflect phylogeny. Journal of Mammalian Evolution 28: 543–558. Crossref
Acta Palaeontol. Pol. 67 (1): 257–282, 2022
https://doi.org/10.4202/app.00889.2021