

Sexually dimorphic ornamentation in modern spinicaudatans and the taxonomic implications for fossil clam shrimps
XIAOYAN SUN and JINHUI CHENG
Sun, X. and Cheng, J. 2022. Sexually dimorphic ornamentation in modern spinicaudatans and the taxonomic implications for fossil clam shrimps. Acta Palaeontologica Polonica 67 (2): 475–492.
The phylogenetic studies of clam shrimps (Branchiopoda, Crustacea) demonstrated that the significance of several morphological characters for classification of branchiopod shells should be critically re-evaluated. Such a venture is particularly important for integrating the taxonomy of fossil and extant branchiopods. One of the shell characters widely used in the branchiopod classification is the carapace ornamentation pattern. This character might, however, be significantly influenced by intraspecific variability and in particular the sexual dimorphism. In this study we investigate the pattern of ornamentation in extant branchiopods—including differences resulting from sexual dimorphism—in order to assess its value for branchiopod taxonomy. We examined 184 individuals representing 10 living species belonging to 7 genera, 5 families, and 2 suborders from China, and compared with the results of previous studies. Although some differences in ornamentation were related to reproductive modes, the basic ornamentation patterns or combinations were stable within each extant species. We found out that some taxa indeed display sexually dimorphic ornamentations, but their basic ornamentation patterns or combinations are stable within each species so they do not significantly influence the taxonomic identification. Integration of data on fossil and extant taxa indicates that similar ornamentation patterns can be observed on familial level of fossil spinicaudatan branchiopods and indicates therefore that characteristic ornamentation patterns can help to identify these taxa in the fossil record. In light of the new molecular phylogeny, we re-evaluated the phylogenetic relationship between fossil and extant spinicaudatan taxa. The resulting tree suggests: (i) paraphyly of the traditional Eosestherioidea, (ii) an affinity between Ozestheria and Triglypta, and (iii) an affinity between Cyzicus and Diestheria or Aquilonoglypta.
Key words: Branchiopoda, Spinicaudata, ornamentation, sexual dimorphism, systematics, taxonomy.
Xiaoyan Sun [xysun@nigpas.ac.cn] and Jinhui Cheng [jhcheng@nigpas.ac.cn] (corresponding author), State Key laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Palaeoenvironment, Chinese Academy of Sciences, 39 Beijing Eastroad, Nanjing, 210008, China.
Received 30 March 2021, accepted 15 October 2021, available online 29 March 2022.
Copyright © 2022 X. Sun and J. Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Clam shrimps (Crustacea: Branchiopoda: Laevicaudata, Spinicaudata, Cyclestherida) are a paraphyletic group of bivalved crustaceans (Astrop et al. 2020). The suborder Cyclestherida is thought to be represented by a single extant species only, has a problematic fossil record in the Middle Devonian and may represent a sister group to Cladocera (Hegna and Astrop 2020). The suborder Laevicaudata is a basal clade of Diplostraca (Branchiopoda: Phyllopoda) with low phylogenetic diversity and poor fossil record. The earliest laevicaudatans may date back to the Permian but the earliest soft-body fossils are known from the Jurassic (Shen and Chen 1984; Hegna and Astrop 2020). The suborder Spinicaudata is a morphologically distinctive and geographically widespread group that comprises three families (Cyzicidae, Leptestheriidae, and Limnadiidae). As pioneer arthropods in freshwater environments during the colonization of terrestrial environments, the known history of Spinicaudata dates back to the Early Devonian (Zhang et al. 1976; Chen and Shen 1985; Hegna and Astrop 2020). They were diverse and abundant in most lacustrine depositional settings during the Mesozoic.
Spinicaudatans have bivalved carapaces composed mainly of chitin or chitin-mineral complex (Astrop et al. 2015; Hegna et al. 2020), with the latter easier to preserve as fossils than the soft parts. There are only few sites with exceptionally preserved fossil spinicaudatans from the Late Devonian to Early Cretaceous times, but morphological details of soft body for particular species delimitation, that are available in living spinicaudatans, were not preserved (Hegna and Astrop 2020). Thus, the classification of fossil spinicaudatans is based on the carapace ornamentation and morphology (Scholze and Schneider 2015; Hegna and Astrop 2020) following several contributions, which attempted at clarifying the taxonomy of fossil members of this group (e.g., Novojilov 1954; Tasch 1969; Zhang et al. 1976; Chen and Shen 1985; Gallego 2010; Gallego and Caldas 2001; Astrop and Hegna 2015). Represented by near 200 genera of 30 fossil families, spinicaudatans exhibit considerable variations in shape, size and carapace ornamentation (Zhang et al. 1976). However, the taxonomic diversity of fossil spinicaudatans was probably overestimated due to extremely variable carapace morphology related to phenotypic differences (Rogers et al. 2012), and distinctive sexual variation known from extant families, genera, and species (Astrop et al. 2012, 2020; Hegna and Rogers 2020). It is still debatable whether the carapace features, especially the ornamentation on growth bands, are of taxonomic importance. Rogers et al. (2012) revised the extant genera of Limnadiidae and stated that the carapace morphology of Limnadiidae is not as informative as egg or telson morphology for species delimitation (Hegna and Rogers 2020). Nevertheless, the carapace ornamentation is still used as a key characteristic for inferring the phylogenetic relationships between extant and fossil species (Wang 1989; Konstans et al. 2019; Li and Teng 2019; Hegna and Rogers 2020) though it remains uncertain whether the ornamentation is of genetic species-specific character or rather a manifestation of phenotypic plasticity. Already Mattox (1957) proposed to abandon the use of carapace features based on different ornamentation patterns co-occurring in a single specimen and since then the ornamentation had not been considered as the principal diagnostic criterion for taxonomic classification in living species (Tasch 1969; Scholze and Schneider 2015), albeit some taxonomists continued to use them (e.g., Stigall and Hartmann 2008; Orlova and Sadovnikov 2009). These changes in ornamentation in particular species were interchangeably considered to represent different responses to environmental factors in subsequent ontogenetic phases or fixed genetic differences, thus reflecting a phylogenetic pattern (Zhang et al. 1976; Chen and Shen 1985; Wang 1989; Astrop and Hegna 2015; Hegna 2021). In spite of this long-lasting controversy the growth-band ornamentation has seldom been described for extant species to address this problem (Barnard 1929; Kobayashi 1954; Ghosh 1982; Timms 2018; Konstans et al. 2019). So far, morphological differentiation of ornamentation patterns caused by sexual dimorphism has not been examined. More data from extant taxa, including comprehensive descriptions of carapace ornamentation, genetic, developmental, and ecological data should be used to evaluate these hypotheses at both generic and infrageneric levels.
Different amount of information on the carapace morphological features known from fossil and extant species results in a serious impediment in the integration of these taxa and low credibility of the taxonomy of the entire group. Reducing this discrepancy in the amount of taxonomic information known from these two groups is essential in deciphering the evolutionary history of the clade. A starting point might be to select and focus on the most appropriate set of taxonomic characters integrating fossil and extant taxonomy that could generate a resolved phylogeny for major clam shrimp groups. This is, however, challenging due to the incomplete fossil record of spinicaudatans to the extent that even distinguishing crown versus stem groups with the available characters may prove to be impossible (Hegna and Astrop 2020; Hegna and Rogers 2020). The investigations of intraspecific variation, such as sexual dimorphism of carapace shape or ontogenetic changes, have received even less interest, leading to overestimation of species diversity (Astrop and Hegna 2015; Hegna and Rogers 2020). Since the descriptions of carapace morphology and carapace ornamentation of Recent taxa are only rarely provided, it is crucial for integration of taxonomy and phylogeny of extant and fossil taxa that such data are appended.
The integration of molecular phylogenetic studies and morphological analyses of fossil and extant taxa already allowed to propose some scenarios for the evolutionary history of the suborder Spinicaudata. Astrop and Hegna (2015) first proposed a phylogenetic hypothesis integrating living and fossil spinicaudatan families based on the molecular phylogeny of Schwentner et al. (2009) and re-evaluated the evolutionary diagram of Zhang et al. (1976). However, the phylogenetic relationships between Eocyzicus (Cyzicidae), Leptestheriidae and Limnadiidae reomained not well resolved. Phylogenetic analyses by Schwentner et al. (2020) recovered the paraphyly of Cyzicidae and Leptestheriidae as a sister group to Limnadiidae. The phylogenetic hypothesis needs to be re-evaluated in light of the new phylogeny of Schwentner et al. (2020) (Hegna and Astrop 2020).
In this study we examined several individuals and taxa of clam shrimps from China. To clarify the stability of their carapace ornamentation characters, we focused on the differentiation of the ornamentation of extant species and demonstrated the differences of carapace ornamentation that resulted from sexual dimorphism. We proposed several patterns in the evolution of carapace ornamentation. We further investigated taxonomic value of ornamentation and discussed its implications for taxonomy of the fossil suborder Spinicaudata based on the integrated tree incorporating fossil families into the molecular framework. This study invites further studies and discussions on the evolutionary pattern of carapace ornamentation in Spinicaudata.
Institutional abbreviations.—NIGP, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China.
Other abbreviations.—D, diameter of reticulation; H, height of carapace; L, length of carapace.
Material and methods
We examined 184 individuals representing 10 living species belonging to 7 genera, 5 families, 2 suborders (Table 1). Morphological observations were conducted on 12–20 specimens (males, females, whole body, carapace and dissected soft body) per species and different populations from natural ponds, reservoirs and rice fields. In order to assess the taxonomic signal of ornamentation, we analysed the morphology of extant species combining with results from previous studies (e.g., Zhang et al. 1976; Chen and Shen 1985; Wang 1989; Astrop 2014). The reported ornamentation patterns on growth bands of carapace were briefly summarized into 8 common patterns and 10 combinational patterns (Table 1). We also summarized the general ornamentation patterns in different families and the implications for integrating fossil and extant taxa. The terminology was mainly adapted from Scholze and Schneider (2015) and improved based on information from Chen and Shen (1985) and Astrop and Hegna (2015). Voucher specimens are stored in 96% ethanol and deposited in Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. Specimens were dissected in glycerine and observed under a light microscope (Zeiss Stereo Discovery V20). Specimens were critical point dried and coated with gold, and examined in a stereomicroscope (SEM, LEO 1530 VP) for ornamentation on growth bands. Details regarding studied taxa can be found in SOM: table S1, Supplementary Online Material available at http://app.pan.pl/SOM/app67-Sun_Cheng_SOM.pdf.
Table 1. Summary of the reported ornamentation types on growth bands of carapace. Note: The terminology is mainly adapted from the literature of Scholze and Schneider (2015) and improved by information from Astrop and Hegna (2015), Chen and Shen (1985). The fossil data are merged from Zhang et al. (1976), Wang (1985), Chen and Shen (1985), Wang et al. (2004), and Liao et al. (2017a). Data of extant species are in bold type and collected from Astrop (2014), Rogers et al. (2013), Vannier et al. (2003), and this study. Names in bold indicate extant species. Upper: growth bands in the upper part of the carapace, including larval valve; middle: growth bands in the central part of the carapace; lower: growth bands in the lower part of the carapace. Pictures of ornamentations are drawn after Scholze and Schneider (2015) and Chen and Shen (1985). D, diameter of ornamentation.
|
Types of ornamentation |
Representative taxa |
||
|
Basic type |
smooth surface  |
Palaeolimnadia Raymond, 1946 (Palaeolimnadiidae) Palaeolimnadiopsis Raymond, 1946 (Palaeolimnadiopseidae) Eulimnadia dahli Sars, 1896 (Limnadiidae) Eulimnadia sp. (Limnadiidae) Paralimnadia badia (Wolf, 1911) (Limnadiidae) Metalimnadia serratus Mattox, 1952 (Limnadiidae) |
|
|
punctae  |
Euestheria trotternishensis (Chen and Hudson, 1991) (Euestheriidae) Aquilonoglypta clinoquadrata Wang in Wang and Liu, 1980 (Auqilonoglyptidae) Ordosestheria multicostata (Chen in Zhang et al., 1976) (Fushunograptidae) Triglypta haifanggouensis (Chen in Zhang et al., 1976) (Tryglyptidae) Imnadia yeyetta Hertzog, 1935 (Limnadiidae) Cyzicus gynecia (Mattox, 1950) (Cyzicidae) Cyzicus mexicanus (Claus, 1860) (Cyzicidae) Cyzicus morsei (Packard, 1871) (Cyzicidae) |
||
|
reticulation 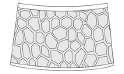 |
small reticulation |
Dictyolimnadia Shen, 1976 (Palaeolimnadiidae) Echinolimnadia Novojilov, 1965 (Palaeolimnadiidae) Cyclotuguzites Novojilov, 1958 (Palaeolimnadiidae) Sajania Novojilov, 1958 (Palaeolimnadiopseidae) Eolimnadia Chen, 1975 (Perilimnadiidae) Euestheria Depéret and Mazeran, 1912 (Euestheriidae) Loxomicroglypta Novojilov and Varentsov, 1956 (Euestheriidae) Glyptoasmussia Novojilov and Varentsov, 1956 (Euestheriidae) Cornia Lyutkevich, 1937 (Vertexiinea) Echinestheria Marliere, 1950 (Vertexiinea) Eulimnadia texana Packard, 1871 (Limnadiidae) |
|
|
medium |
Jibeilimnadia Wang, 1981 (Palaeolimnadiidae) Anyuanestheria Zhang and Chen in Zhang et al., 1976 (Loxomegaglyptidae) Diaplexa Novojilov, 1946 (Loxomegaglyptidae) Eocyzicus orientalis Daday, 1913 (Eocyzicidae) |
||
|
large |
Pseudolimnadia Novojilov, 1954 (Palaeolimnadiidae) Mesolimnadiopsis Zhang and Chen, 1976 (Palaeolimnadiopseidae) Loxomegaglypta Novojilov, 1958 (Loxomegaglyptidae) Paleoleptestheria Novojilov, 1954 (Loxomegaglyptidae) Nestoria Krasinetz, 1963 (Nestoriidae) Keratestheria Chernyshev, 1948 (Ipsiloniidae) Jiliaoestheria Wang in Wang et al., 2004 (Jiliaoestheriidae) Leptestheria brevirostris (Barnard, 1924) (Leptestheriidae) Leptestheria rubidgei (Baird, 1862) (Leptestheriidae) Maghrebestheria maroccana Thiery, 1988 (Leptestheriidae) |
||
|
isogonal |
Ulugkemia Novojilov, 1955 (Ulugkemiidae) Lynceus bioformis (Ishikawa, 1895) (Lynceidae) Lynceus sp. (Lynceidae) Cyclestheria hislopi (Baird, 1859) (Cyclestheriidae) |
||
|
radial fringes 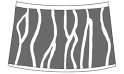 |
Howellites Bock, 1953 (Fushunograptidae) Ganestheria Bi and Xie in Chen and Shen, 1982 (Sinoestheriidae) Jiliaoestheria zhangjiawanensis Wang in Wang et al., 2004 (Jiliaoestheriidae) Jiliaoestheria nematocomperta (Wang in Wang and Liu, 1980) (Jiliaoestheriidae) Leptestheria kunmingensis Shu, Rogers, Chen, and Yang, 2015 (Leptestheriidae) Leptestheria compleximanus (Packard, 1877) (Leptestheriidae) Leptestheria kawachiensis Uéno, 1927 (Leptestheriidae) Eoleptestheria ticinensis (Balsamo-Crivelli, 1859) (Leptestheriidae) |
||
|
radial lirae 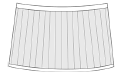 |
Orthestheria Chen in Zhang et al., 1976 (Fushunograptidae) Fushungrapta Wang in Hong et al., 1974 (Fushunograptidae) Daxingestheria Zhang and Chen in Zhang et al., 1976 (Fushunograptidae) Nemestheria Zhang and Chen, 1964 (Jilinestheriidae) Eocyzicus parooensis Richter and Timms, 2005 (Eocyzicidae) |
||
|
Basic type |
reticulation-lirae
transitional 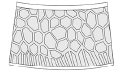 |
Eosestheria middendorfii (Jones, 1862) (Eosestheriidae) Triglypta yabraiensis Wang, 2014 (Triglyptidae) Ozestheria sp. (Cyzicidae) Cyzicus sp. (Cyzicidae) Cyzicus gifuensis (Ishikawa, 1895) (Cyzicidae) Cyzicus tetracerus (Krynicki, 1830) (Cyzicidae) Cyzicus belfragei (Packard, 1871) (Cyzicidae) |
|
|
nodules  |
Camerunograpta Novojilov,1957 (Afrograptioidea) Congestheriella Kobayashi, 1954 (Afrograptioidea) Limnadiopsis occidentalis Timms, 2009 (Limnadiidae) Eocyzicus orientalis Daday, 1913 (Eocyzicidae) Eocyzicus mongolianus Uéno, 1927 (Eocyzicidae) |
||
|
Combinatorial type |
smooth surface (upper), |
Aquilonoglypta clinoquadrata Wang in Wang and Liu, 1980 (Auqilonoglyptidae) Qaidamestheria shanshanensis Wang, 1985 (Triglyptidae) |
|
|
punctae (upper), |
Qaidamestheria dameigouensis Wang, 1983 (Triglyptidae) Junggarestheria quadrata Wang, 1985 (Polygraptidae) |
||
|
punctae (upper), |
Triglypta pingquanensis Wang, 1985 (Triglyptidae) Triglypta manasica Wang, 1985 (Triglyptidae) Triglypta tianshanensis Wang, 1985 (Triglyptidae) Ozestheria sp. (Cyzicidae) |
||
|
small reticulations (upper), |
Polygrapta Novojilov, 1946 (Polygraptidae) Huanghestheria Wang in Wang and Liu, 1980 (Vertexiinea) Yanjiestheria Chen in Zhang et al., 1976 (Eosestheriidae) Turfanograpta Novojilov, 1958 (Eosestheriidae) |
||
|
medium reticulations (upper), |
Eosestheria middendorfii Jones, 1862 (Eosestheriidae) Abrestheria Wang, 1981 (Eosestheriidae) Eosolimnadiopsis Chen in Zhang et al., 1976 (Palaeolimnadiopseidae) |
||
|
large reticulations (upper), |
Jiliaoestheria Wang in Wang et al., 2004 (Jiliaoestheriidae) Sentestheria Wang, 1981 (Sinoestheriidae) Pseudograpta Novojilov, 1954 (Loxomegaglyptidae) |
||
|
small–medium reticulations
(upper), |
Neimengolimnadiopsis Liu, 1982 (Palaeolimnadiopseidae) Shipingia Shen in Zhang et al., 1976 (Loxomegaglyptidae) Pseudestherites Chen in Zhang et al., 1976 (Loxomegaglyptidae) Jiliaoestheria Wang in Wang et al., 2004 (Jiliaoestheriidae) Eocyzicus orientalis Daday, 1913 (Eocyzicidae) |
||
|
radial lirae (upper), |
Dimorphostracus Zhang and Chen, 1964 (Dimorphostracidae) Sinoestheria Zhang, 1957 (Sinoestheriidae) |
||
|
radial fringes (upper), |
Cyzicus sp. (Cyzicidae) Cyzicus gifuensis (Ishikawa, 1895) (Cyzicidae) Cyzicus tetracerus (Krynicki, 1830) (Cyzicidae) Cyzicus belfragei Packard, 1871 (Cyzicidae) |
||
|
overlapped reticulations |
Diestheria Chen in Zhang et al., 1976 (Diestheriidae) Ozestheria pilosa (Rogers, Thaimuangphol, Saengphan, and Sanoamuang, 2013) (Cyzicidae) |
||
Results
Sexually dimorphic ornamentation was observed in the adult stages of Ozestheria sp., Cyzicus sp., Eocyzicus orientalis Daday, 1913, and Eocyzicus mongolianus Uéno, 1927.
Ozestheria sp. (suborder Spinicaudata): male and female carapaces can be recognised by conspicuous sexual dimorphism of carapace size and shape. Males are usually larger than females. The mean female carapace H/L ratios range 0.684–0.706, while the mean male carapace H/L ratios range 0.601–0.625. Males are relatively more elongate than the females. Notably, sexual dimorphism of carapace surface ornamentation is well developed in this species (Fig. 1). The ornamentation of male carapace exhibits minute punctae (D ≤ 0.01mm) restricted to the larval valves (Fig. 1A2), small reticulations (D ≤ 0.02mm) in the mid-ventral part of the carapace (Fig. 1A3), radial lirae near the ventral margin of the carapace (Fig. 1A5), and setae on the margin of the carapace (Fig. 1A4, A5). Ornamentation in the dorsal part of the carapace is similar to that on the ventral part. The change from punctae on the larval valves to reticulations in the mid-ventral part of the carapace is abrupt, while the change from reticulations to lirae is gradual. Near the ventral margin of the carapace, the edges of each mesh protuberate and merge into a larger undeveloped reticulation, and the radial lirae along the lower margin of each growth band derive from the bulge of reticulated ornamentation. The ornamentation of female carapace present punctae on the larval valves (Fig. 1B2), small reticulations in the mid-ventral part of the carapace (Fig. 1B3), and radial fringes near the ventral margin of the carapace (Fig. 1B4). Furthermore, compared with the male form, the edges of mesh along the lower margin of each growth band weakly protuberate in the females (Fig. 1B5).
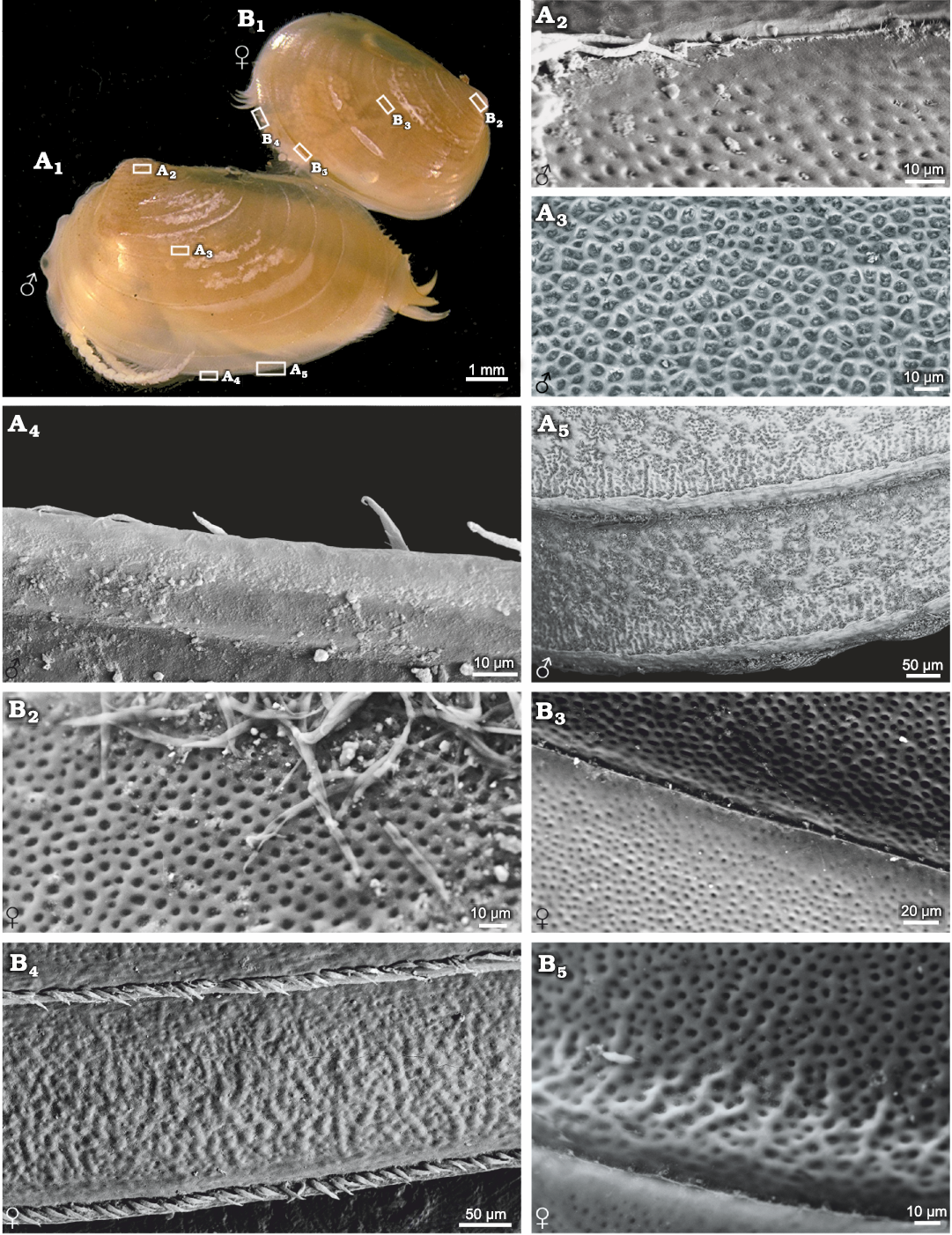
Fig. 1. Ornamentation on the growth bands of
carapaces of the extant spinicaudatan branchiopod Ozestheria
sp., from Hebei, China. A.
NIGP Cr. 41, male, carapace in lateral view (A1);
ornamentation in the larval valve (A2)
and in the ventral part of the carapace (A3),
bottom view of spines on the edge of carapace (A4),
large reticulation derived from the edges of mesh and the radial
lirae along the lower margin of each growth band (A5).
B. NIGP Cr. 42, female,
carapace in lateral view (B1),
ornamentation in the larval valve (B2)
and in the ventral part of the carapace (B3),
radial fringes near the ventral margin of carapace and the dense
pilosity on the growth line (B4),
undeveloped reticulation derived from the edges of mesh along the
lower margin of each growth band (B5);
A1, B1 after Huang and Cai (2016: fig. 5-136d).
Cyzicus sp. (suborder Spinicaudata): radial fringe ornamentation is present on the larval valves, similar to Ozestheria pilosa (Rogers et al. 2013) and species of Leptestheriidae. Small reticulations (D ≤0.02 mm) and radial lirae are present in the ventral part of the carapace (Fig. 2). The male carapace has relatively distinct ornamentation on the entire carapace, including reticulation and reticulate–lirae transition along the lower margin of the growth band (Fig. 2A2, A3). However, the female form has an extensive, weak reticulation ornamented area near the ventral margin (Fig. 2B3).
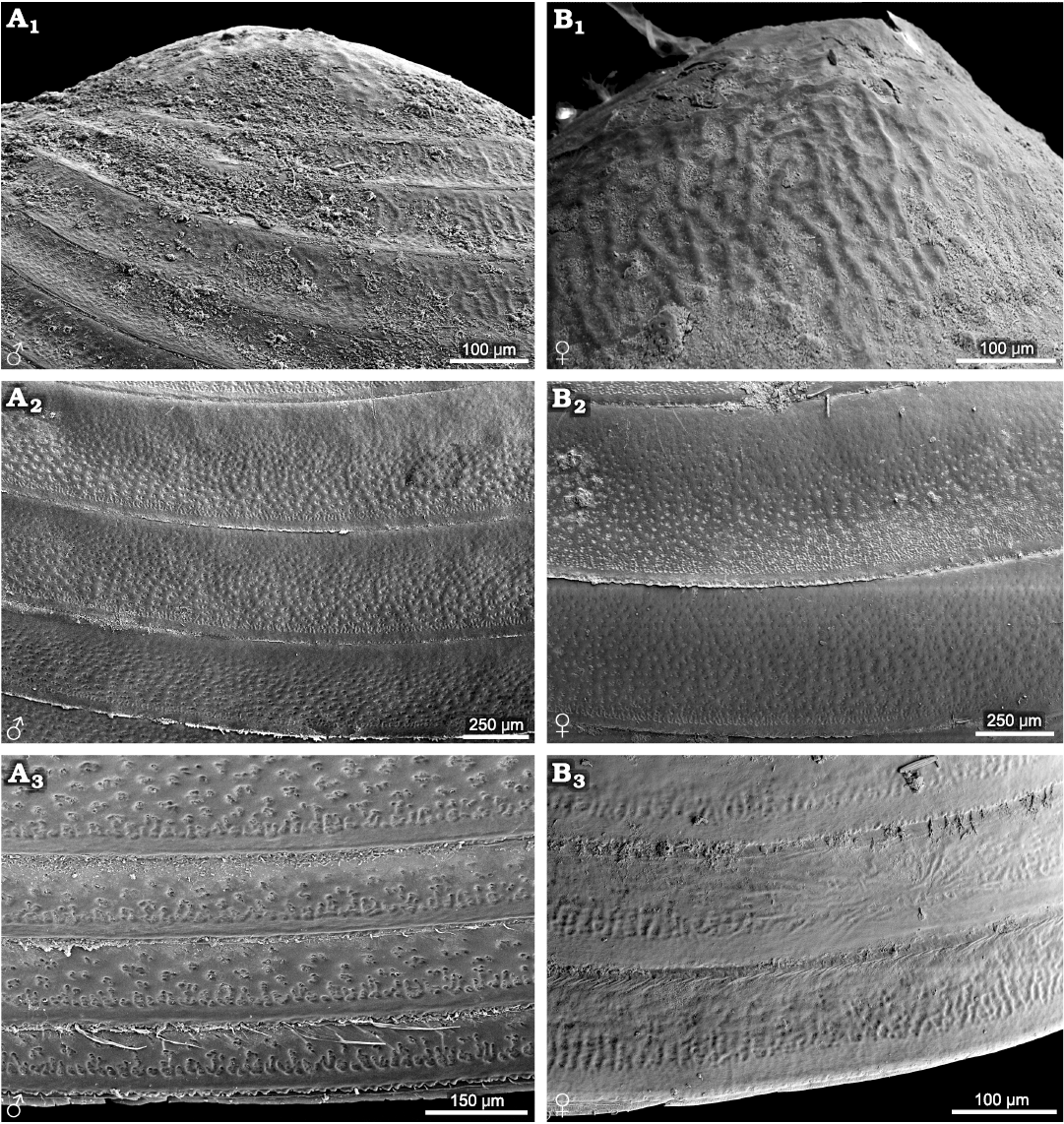
Fig. 2. Ornamentation on the growth bands of carapaces of the extant spinicaudatan branchiopod Cyzicus sp., from Jilin, China. A. NIGP Cr. 141, male, ornamentation in the larval valve (A1), in the middle part of the carapace (A2), large reticulation and the radial lirae along the lower margin of the growth band (A3). B. NIGP Cr. 142, female, ornamentation in the larval valve (B1) and in the middle part of the carapace (B2), weakly ornamented area near the ventral margin (B3).
Eocyzicus orientalis and Eocyzicus mongolianus (suborder Spinicaudata): growth bands in the upper to middle parts of carapace are mainly ornamented with small to medium reticulations. The female carapace has rows of nodular ornamentation on growth bands in the ventral part of the carapace, absent in the male carapace (Fig. 3). Such sexually dimorphic ornamentations are not observed in Leptestheria kunmingensis Shu, Rogers, Chen, and Yang, 2015, Leptestheria kawachiensis Uéno, 1927, Eoleptestheria ticinensis (Balsamo-Crivelli, 1859), Lynceus sp., and Eulimnadia sp.
Eulimnadia sp. (suborder Spinicaudata): The carapace surfaces of Eulimnadia sp. are smooth (Fig. 4A, B).
Lynceus sp. (suborder Laevicaudata): The species possesses isogonal reticulate ornamentation on the entire carapace (Fig. 4C, D), and this pattern is considered as the basal condition of carapace ornamentation in Spinicaudata, reflecting a basic reprinting of the underlying epidermal layer’s cellular structure (Astrop and Hegna 2015).
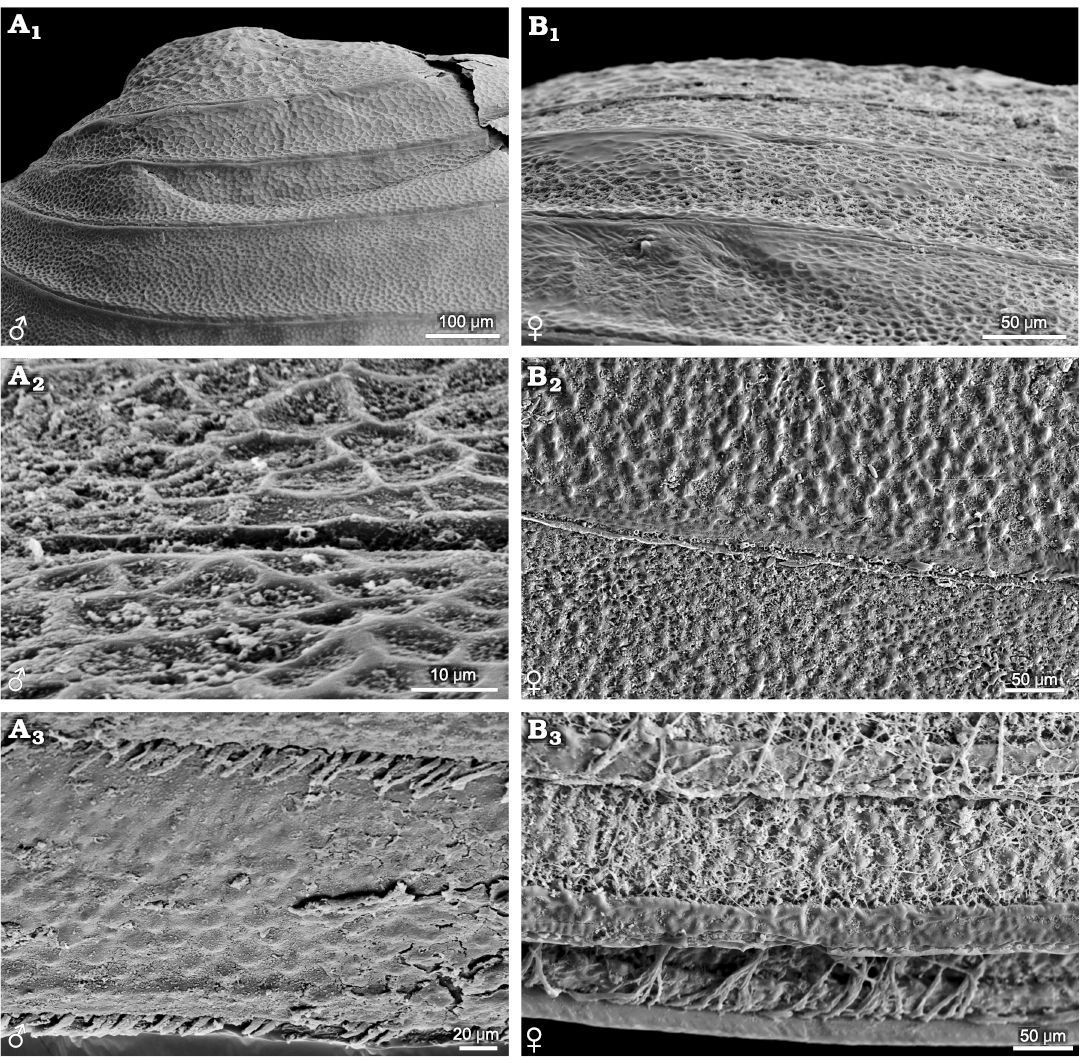
Fig. 3. Ornamentations on the growth bands of carapace of the extant spinicaudatan branchiopod Eocyzicus orientalis Daday, 1913, from Xinjiang, China. A. NIGP Cr. 1, male, ornamentation in the upper to middle parts of the carapace (A1), reticulate ornaments in the ventral part of the carapace (A2), dense pilosity on the growth lines near the edge of the carapace (A3). B. NIGP Cr. 2, female, ornamentation in the upper to middle parts of the carapace (B1), rows of nodular ornaments in the ventral part of the carapace (B2), stout setae on the growth lines near the edge of the carapace (B3).
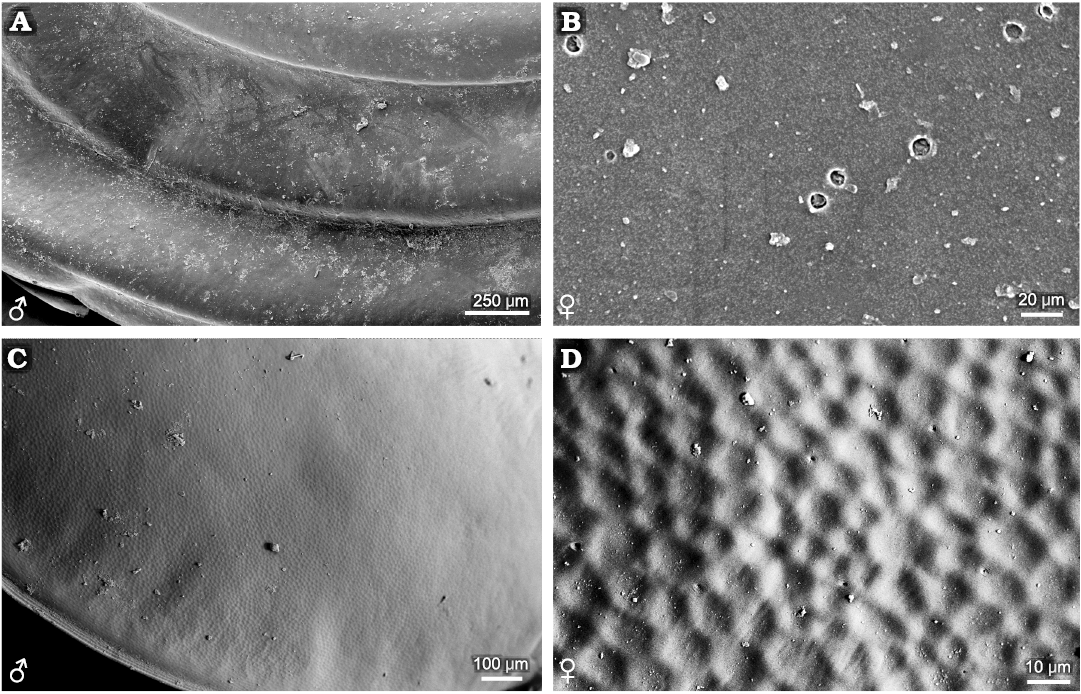
Fig. 4. Ornamentations on the growth bands in the extant spinicaudatan branchiopod Eulimnadia sp. and the extant laevicaudatan branchiopod Lynceus sp. A, B. Eulimnadia sp., from Jiangxi, China. A. NIGP Cr. 161, male, carapace in lateral view. B. NIGP Cr. 162, female, unornamented area near the ventral margin. C, D. Lynceus sp., from Heilongjiang, China. C. NIGP Cr. 173, male, carapace in lateral view. D. NIGP Cr. 174, female, isogonal reticulate ornamentation in the valve.
Discussion
Sexually dimorphic ornamentations.—Spinicaudatans grow by incomplete moulting. Sexual dimorphism can be observed on the carapace and appendages during the adult stage, and carapace shape, and size sexual dimorphism is common in spinicaudatans. As yet, no certain explanation about the function of the sexually dimorphic ornamentation of the carapace in Spinicaudata has been proposed. In this study, male and female individuals are shown to be morphologically indistinguishable for ornamentation of juvenile stage, while in the adult stage the male and female individuals exhibit sexually dimorphic ornamentation. Specifically, the male individuals have more elaborate and enhanced ornamentation in the middle part to the margin of the carapace, which is the main manifestation of sexually dimorphic ornamentation. The disorganized ornamentation near the ventral margin of the female carapace might indicate a loss or reduction rather than a gain of complexity in female individuals. Schwentner et al. (2011) proposed a “lock-and-key” mechanism between male claspers and female carapace. The first one or two pairs of trunk limbs of clam shrimps modified as clasping structures (claspers) are used to fixate the female on the carapace margin during mating and mate guarding. Schwentner et al. (2011) suggested that clasper–carapace interactions played a role in mate recognition in spinicaudatan Limnadopsis. However, Sigvardt et al. (2017) have found no clear evidence for the presence of a signal function in both sexes in some species. The current observations suggest that behavioral differences may drive the sexually dimorphic ornamentation and it is likely an example of ecological sex-trait, especially wherein males spend more time in swimming as they search for mates. In this respect, ornamentation dimorphism may be related to male investment, that is devoting a larger portion of physiological costs of investing in reproductive structures, large size or elaborate ornamentation (Hunt et al. 2017). Also, the males have stronger carapace calcification than the females due to the differential physiological costs of eggs and sperms. The ecological and evolutionary significance of this sexually dimorphic ornamentation is an unresolved topic and requires future investigation.
Taxonomic value of ornamentation.—The ornamentation pattern on carapace has significant implications for integrating fossil and extant species. As follows, we discussed the general ornamentation patterns in different families and the implications for integrating fossil and extant taxa.
Three genera, i.e., Leptestheria, Eoleptestheria, Maghrebestheria are currently included in the family Leptestheriidae (Rogers 2020). Leptestheriidae differ from Cyzicidae in the elongated carapace, broad growth bands, and slight recurvature of the dorsal carapace margin with respect to the carapace morphology. Leptestheria rubidgei (Baird, 1862) and Maghrebestheria maroccana Thiery, 1988, have been reported to display irregular reticulate ornamentations (Barnard 1929: 266, figs. d, e; Astrop 2014: 54, fig. 2.7). Eocyzicus orientalis and E. mongolianus possess similar reticulate ornamentations on the entire carapace. However, Leptestheria kunmingensis (Fig. 5), L. kawachiensis (Fig. 6A), and Eoleptestheria ticinensis (Fig. 6B) display the same ornamentation of radial fringes, which is consistent with the ornamentation possessed by L. compleximanus (Packard, 1877) (Astrop 2014: 54, fig. 2.7). Among these species, there are morphological differences in details of ornamentation. For example, in Leptestheria kunmingensis (Fig. 5A), dense punctae (D ≤ 0.01mm) are developed between the radial fringes (Fig. 5A2–A4), and delicate setae are attached to the disto-medial surfaces and margins (Fig. 5A5, A6, B). However, these features are absent in Eoleptestheria ticinensis. Instead, it exhibits a nearly smooth surface in the ventral part of the carapace, possessing shallow fringed ornamentation (Fig. 6B2–B4). The ornamentation pattern of radial fringes with dense punctae was also observed in the ventral parts of a carapace of Eocyzicus parooensis Richter and Timms, 2005 (Astrop 2014: 54, fig. 2.7). The current results indicate that reticulate and fringed ornamentations are the basic ornamentation patterns in Leptestheriidae. The presence of irregular reticulation in the larval valves and radial fringes, sometimes with dense punctae, are likely to be shared taxonomic features of Leptestheriidae and Eocyzicus, which is also supported by Astrop and Hegna (2015).
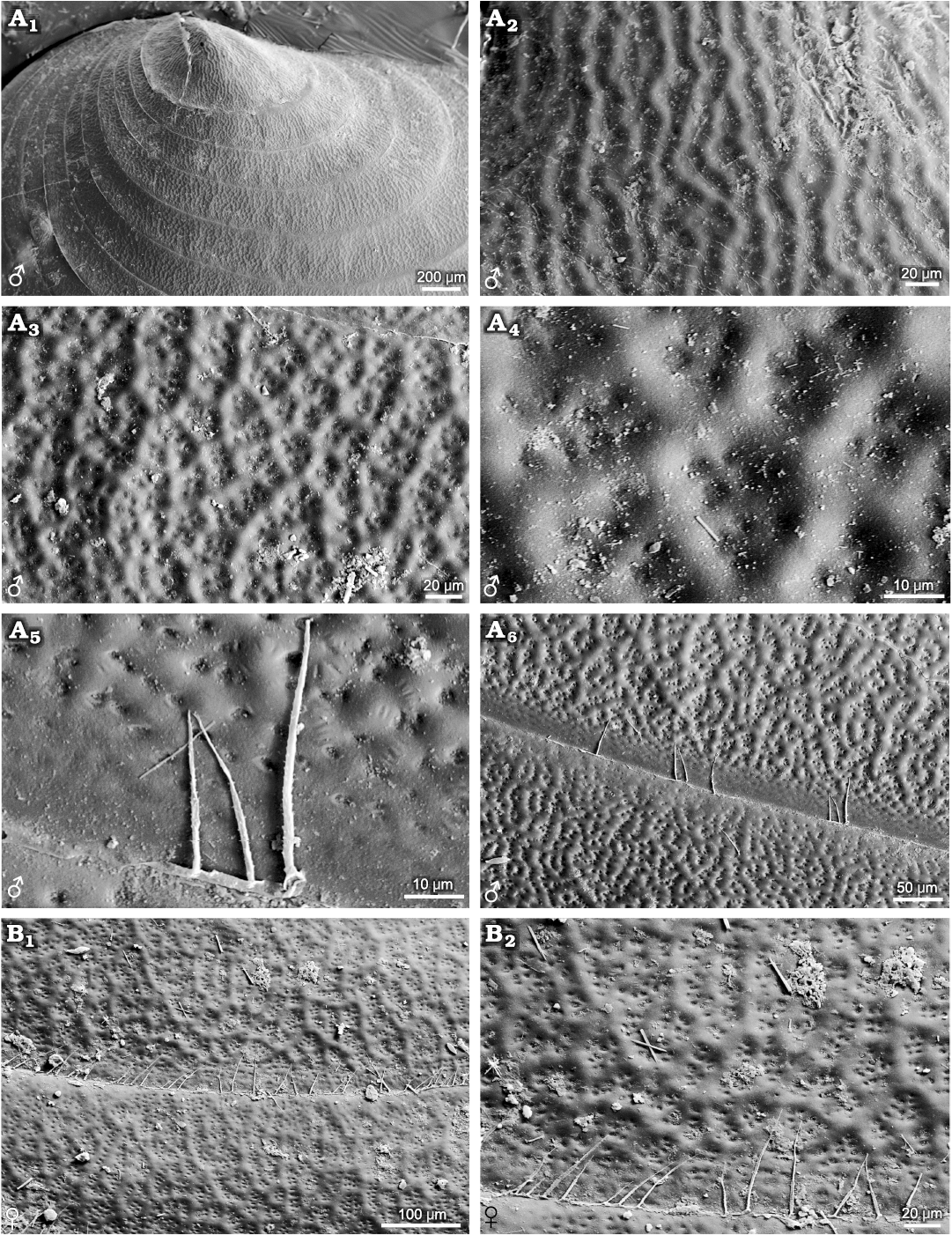
Fig. 5. Ornamentation pattern on the growth bands of carapaces in the and female of the extant spinicaudatan branchiopod Leptestheria kunmingensis Shu, Rogers, Chen, and Yang, 2015, from Sichuan, China. A. NIGP Cr. 81, male. B. NIGP Cr. 82, female. Right valve in lateral view (A1); ornamentation in the larval valve area with anastomosing radial fringes pattern (A2); growth bands ornamentation in the median-ventral valve area (A3); ornamentation pattern is formed by anastomosing radial fringes occupying the whole growth band and dense punctae developing between fringes; detail of the radial fringes, depicting dense punctae between the radial fringes (A4); details of carapace growth lines, depicting delicate setae and dense punctae between the radial fringes (A5, A6, B1, B2).
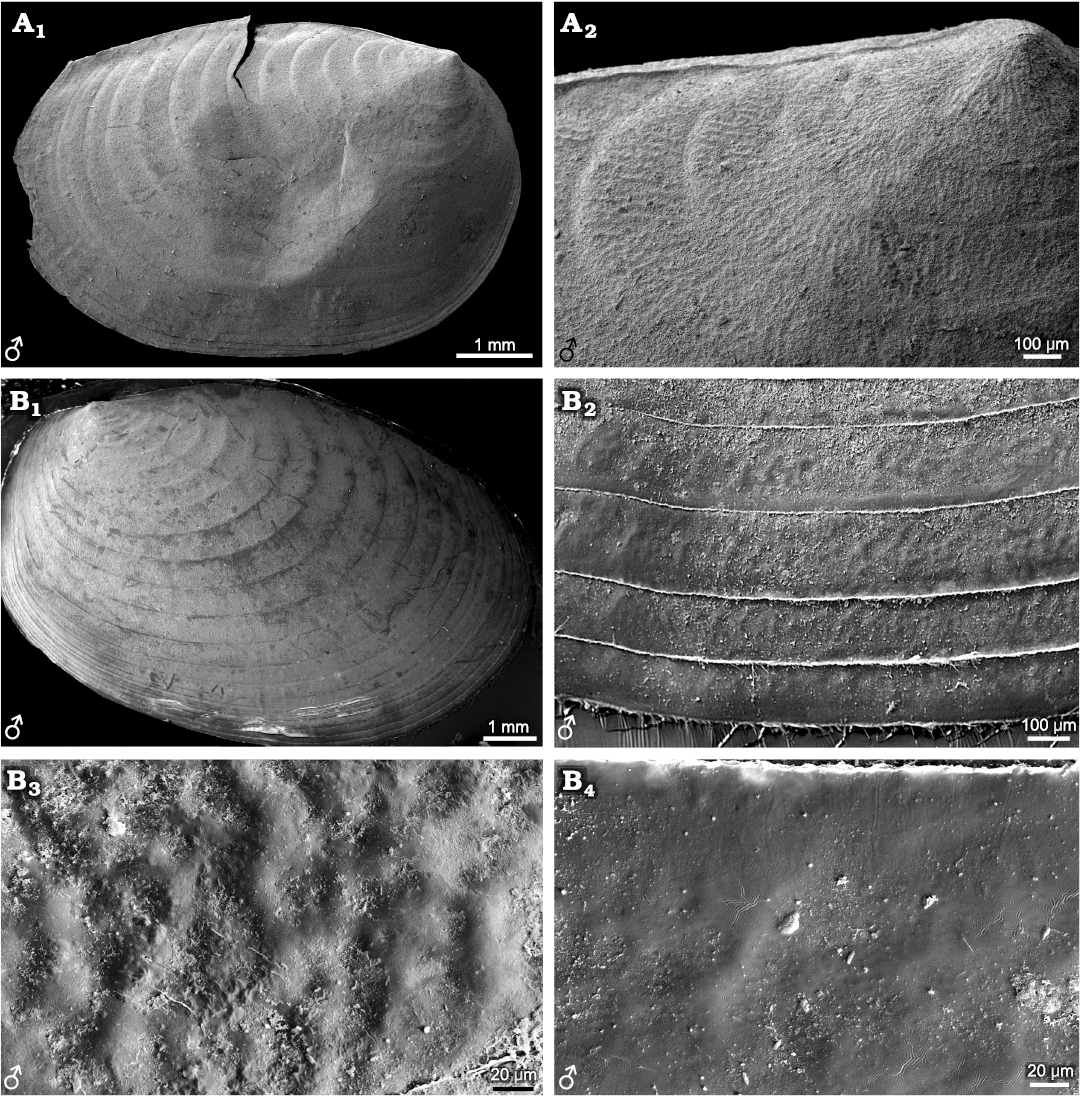
Fig. 6. Carapaces and ornamentations of representatives of the extant spinicaudatan family Leptestheriidae. A. Leptestheria kawachiensis Uéno, 1927, from Hubei, China, NIGP Cr. 101, male, lateral view; left valve, oval outline (A1); growth bands in the upper part of carapace with wide radial fringes pattern (A2). B. Eoleptestheria ticinensis (Balsamo-Crivelli, 1859), from Jiangsu, China, NIGP Cr. 61, male, lateral view; right valve, oval outline (B1); growth bands in the ventral part of carapace with shallow fringes pattern, never developing reticulation or punctae between fringes (B2); details of ventral growth bands with shallow fringes pattern separated with smooth surface (B3, B4).
Species belonging to Cyzicus and Ozestheria often have complex ornamentations. For example, Cyzicus sp. exhibits radial fringe ornamentation on the larval valves, with an ornamentation combination of small reticulations (D ≤0.02mm) and radial lirae in the ventral part of the carapace. The radial fringe ornamentation on the larval valves can be observed in Leptestheria kunmingensis and Ozestheria pilosa (Fig. 7A; Rogers et al. 2013: fig. 3A). In the mid-ventral part of the carapace of Cyzicus sp., the edges of mesh protuberate into linearly arranged lirae along the lower margin of each growth band (Fig. 7B). This ornamentation pattern was also observed in Cyzicus tetracerus (Krynicki, 1830) (Vannier et al. 2003: fig. 3B), Cyzicus belfragei (Packard, 1871) (Astrop 2014: fig. 2.5B), and fossil species Diestheria longinqua Chen in Zhang et al., 1976 of the Early Cretaceous Jehol Biota (Li et al. 2017: fig. 2.2). The current results using the dioecious Cyzicus sp. showed a similar tendency of variability in reticulation-lirae transition (Fig. 2). Thus, this ornamentation pattern could be a general morphological character in the dioecious Cyzicus. The similarities in ornamentation patterns and carapace shape might suggest a close relationship between the dioecious Cyzicus and the fossil Diestheria. For hermaphroditic Cyzicus gynecia (Mattox, 1950), growth bands of carapace were ornamented with punctae or independent pits (Astrop 2014), which also appeared in some fossil species, such as Aquilonoglypta clinoquadrata Wang in Wang and Liu, 1980, Euestheria trotternishensis Chen and Hudson, 1991, Triglypta haifanggouensis (Chen in Zhang et al., 1976) and Ordosestheria multicostata (Chen in Zhang et al., 1976). The pitted ornamentation also occurred in Cyzicus morsei (Packard, 1871) and androdioecious Cyzicus mexicanus (Claus, 1860) (Astrop 2014) and some fossil species of Palaeorthothemos, Orthothemos, and Estherites (Zhang et al. 1976). Triglyptids were considered to derive from the puncta-bearing Aquilonoglyptidae (Wang 2014). The similarity in ornamentations might suggest a close affinity between hermaphroditic Cyzicus and Aquilonoglypta as suggested by Astrop and Hegna (2015).
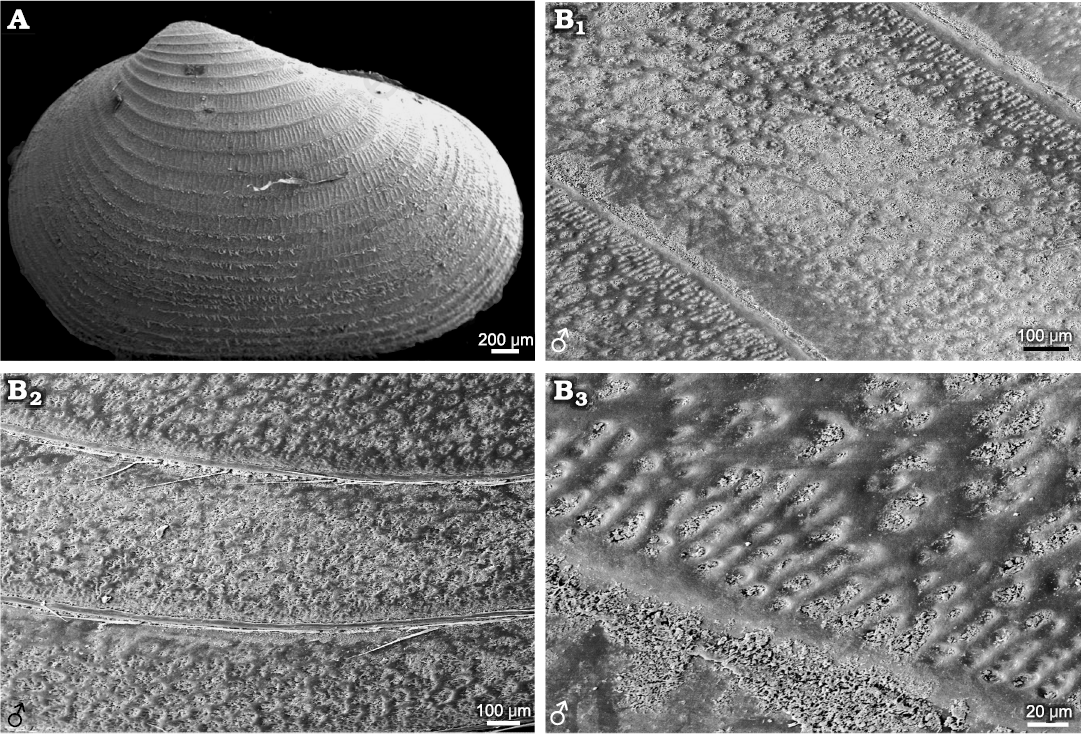
Fig. 7. Ornamentations on the growth bands in extant spinicaudatans species of Cyzicus Audouin, 1837, Ozestheria Schwentner, Just, and Richter, 2015, and Diestheria longinqua Chen in Zhang et al., 1976. A. Carapace of Ozestheria pilosa (Rogers, Thaimuangphol, Saengphan, and Sanoamuang, 2013), from Thailand (after Rogers et al. 2013: fig. 3A). B. Cyzicus gifuensis (Ishikawa, 1895), from Anhui, China, NIPG Cr.121, male; ornamentation in the ventral part of the carapace (B1) and near the ventral margin of carapace (B2); radial lirae along the lower margin of each growth band (B3).
The transition pattern from reticulation to lirae in the ventral part of the carapace in the Ozestheria differs from the Cyzicus which has the large undeveloped reticulation. Australian species of Ozestheria had reticulation, granulated ornaments, or a combination of punctae and lirae (Timms 2018). The ornamentation pattern of O. pilosa was similar to species of Diestheriidae, in which transversely enlarged reticulation overlapped on the lirae ornamentation of each growth band of the carapace (Rogers et al. 2013). The larger secondary reticulation was likely originated from the intra-cuticular layer rather than the reticulation from procuticle (Astrop 2014). The ornamentation pattern in Ozestheria sp. (males, Fig. 1A5), including punctae-reticulation-lirae combination, the transition from reticulation to lirae, and the larger undeveloped reticulation, is in line with that of fossil species Triglypta yabraiensis Wang, 2014 (Wang 2014: pl. 2: 2). The close morphological resemblance of ornamentations and carapace shape suggests that Ozestheria might be closely related to Triglypta or Tianzhuestheria.
The carapaces of the family Limnadiidae are thin and lightly mineralized, which commonly resulted in a reticulate depression on the carapace surface, such as Eulimnadia texana Packard, 1871 (Astrop 2014). However, the carapace surfaces of most species of Eulimnadia are unornamented (smooth surface pattern). This pattern also occurs in Metalimnadia serratus Mattox, 1952, Paralimnadia badia (Wolf, 1911) and some Triassic fossil species of Paleolimnadiidae (Table 1). The fossil family Palaeolimnadiopsidae is characterized by the recurvature of growth lines to form carinate at the posterior-dorsal marginal junction of the carapace. This feature has also been observed in living species of Limnadopsis. The ornamentation documented for Palaeolimnadiopsidae ranged from reticulation to reticulation-lirae combination. However, the ornamentation possessed by Limnadopsis occidentalis Timms, 2009, is nodular (Astrop 2014). Imnadia yeyetta Hertzog, 1935, was reported to exhibit punctae ornamentation (Astrop 2014). Nevertheless, this pattern was not mentioned in the original descriptions of the fossil families Paleolimnadiidae, Palaeolimnadiopsidae or Perilimnadiidae.
The phenotypic differentiation of ornamentation pattern is a model to investigate morpho-functional adaptation to some aquatic environments. Moreover, it might be influenced by many factors including sexual dimorphism, ecophenotype and preservation differences. Changes in ornamentation pattern may be related to changes in reproductive modes within the Cyzicus. For example, the ornamentation pattern of dioecious Cyzicus is composed of fringes and reticulation-lirae transitional type (Figs. 2, 7), while the ornamentation pattern of androdioecious C. mexicanus consists of fringes and punctae type (Mattox 1957), and hermaphroditic C. gynecia consists of punctae (Astrop 2014). Although sexual dimorphism is an essential source of intraspecific morphological variation, the influences on characteristic ornamentation patterns appear complex.
The basic ornamentation patterns or combinations are stable within each extant species, even for dioecious species possessing sexually dimorphic ornamentations. In general, in animals where speciation process is mainly driven by sexual and/or natural selection, characters under the influence of any of these forces will be more informative than those known to evolve independently of such selection (Padial et al. 2010). Although differences in ornamentation patterns are related to changes in reproductive modes within Cyzicus, such variation will not pose significant problems for species discrimination.
Some researchers questioned the applicability of ornamentations because of co-occurrence of different ornamentations in a single specimen (Mattox 1957; Scholze and Schneider 2015). Hegna (2021) suggested that ornamentations did not vary as wildly and randomly as Scholze and Schneider (2015) implied. The spinicaudatan carapace is formed by partial moulting during ecdysis and the outer surface of the carapace is not shed (Astrop 2014; Hegna and Astrop 2020). Therefore, the ontogenetic history of each individual is preserved in the carapace. Ontogenetic differentiation of carapace shape has been observed in all living species of Spinicaudata (Astrop et al. 2012; Brown et al. 2014). It is necessary to define such ontogenetic variation of ornamentation within a complete growth series by comparing within and between modern species. Ornamentation pattern would be helpful for interpreting the taxonomic significance of inter-generic variation in the fossil taxa, once the stability of ornamentation patterns throughout the ontogenetic trajectory has been determined. For example, Gallego et al. (2020b) studied complex ornamentation patterns in successive growth bands as a part of detailed description of fossil clam shrimp species. Our results have shown that the shared conservative ontogenetic ornamentation patterns existed within different lineages, such as fringed-reticulated pattern in Leptestheriidae, reticulate pattern in Eocyzicidae, punctated or reticulated-lirate pattern in Cyzicidae. Konstans et al. (2019) suggested that the intraspecific variation was minor and ornamentation could be used for inferring phylogenetic relationships between living and fossil taxa. They also highlighted the need for sampling more extant taxa to better understand the evolutionary pattern of ornamentation.
Ornamentation pattern on carapace is of different taxonomic importance for superfamilies of clam shrimps. For example, in the superfamilies Eosestherioidea and Estheriteoidea, ornamentation patterns have been used extensively as a key diagnostic character for high-level taxonomy. The ornamentation patterns on growth bands in Eosestherioidea and Estheriteoidea exhibited a wide morphological diversity, which is much richer than the described ornamentation patterns of living species of Leptestheriidae, Eocyzicidae, and Cyzicidae. The systematics of Vertexioidea (including Limnadiidae) mainly relies on a number of diagnostic carapace characters, including carapace outline, size of the larval shell, growth-line pattern, protrusion above the adductor muscle scars and the recurvature of growth lines at the postero-dorsal margin, while ornamentation pattern is less important for taxonomy. Multiple types of ornamentation were mentioned in the original description of this superfamily (e.g., reticulation, radial fringes, pit, smooth surface and reticulate-lirae combinational type; Zhang et al. 1976). These types have been observed in living species of Limnadiidae, except the types of fringes and reticulate-lirae combination. The number of and angles between concentric ribs are the most important diagnostic criteria for taxonomic determinations of Leaniina and Estheriella (Zhang et al. 1976; Chen and Shen 1985; Scholze and Schneider 2015).
The evolutionary trends of the characteristic ornamentation patterns may be interpreted as a stabilizing solid selection (both natural and sexual). As mentioned above, ornamentation pattern is an important character used in the fossil taxonomy of Spinicaudata. The application of ornamentation pattern for taxonomy needs to be undertaken with caution. It is necessary to consider the intraspecific variation due to sexual dimorphism, and other carapace features as well. To integrate fossil and extant lineages in a phylogenetic and macroevolutionary framework, a comprehensive data set of extant and fossil clam shrimp together with a rich diversity of detailed carapace features is needed for further analysis.
Integrated taxonomy of fossil and extant Spinicaudata.—Without an integrated phylogenetic hypothesis of fossil and extant taxa, it is not easy to discuss the evolution and differentiation of carapace ornamentation patterns. Phylogenetic analyses provide comprehensive insights into the evolutionary history of Spinicaudata. Zhang et al. (1976) presented the first hypothesis of the relationship between spinicaudatan fossil and extant lineages based on stratigraphic occurrence and carapace morphology (e.g., carapace shape, umbo position, size of larval valves, number and angle of radial ribs and many sculptures on carapace). Astrop and Hegna (2015) addressed objections to this hypothesis and proposed a phylogenetic hypothesis incorporating extant Spinicaudata with re-evaluated fossil families. They suggested that Limnadiidae was a sister of Perilimnadiidae, which meant that the changes of carapace characters were parallel with acquisitions of the traits unique to Limnadiidae and their related fossil lineages, such as one pair of carinae on the anterior margin in Metalimnadia and the fossil lineage. Paleolimnadiidae originated in the Late Devonian when they were represented by some species ornamented with minute reticulation (Zhang et al. 1976). The overall evolutionary trend of ornamentation within this family in the Mesozoic was towards reticulation with different sizes, smooth carapace, and a combination of reticulation and lirae. This family was regarded as closely allying with Limnadia (Tasch 1956; Zhang et al. 1976). Perilimnadiidae that originated in the late Permian was very similar to Paleolimnadiidae. The ornamentations documented for Perilimnadiidae ranged from reticulate to punctate type. The ancient Eulimnadia lineage seems to be associated with Paleolimnadiidae rather than with Perilimnadiidae in terms of smooth ornamentation pattern. However, divergence time estimates based on the Bayesian relaxed methods suggested that the earliest limnadiid divergence occurred around 190.8 Ma and a common ancestor of Eulimnadia and Metalimnadia around 50 Ma (Bellec and Rabet 2016). Based on the molecular data, the Eulimnadia lineage might have derived from the Cenozoic representatives of Perilimnadiidae. Zhang et al. (1976) and Chen and Shen (1985) suggested that Eulimnadia originated from a Paleocene perilimnadiid ancestor based on preserved adductor muscle attachment scars and larger larval valve than that of Paleolimnadiidae. We accepted this proposal and placed Eulimnadia as a sister to Perilimnadiidae (Fig. 8). Several molecular analyses demonstrated that either Limnadia or Imnadia was basal for extant clade of Limnadiidae and Paralimnadia was a sister group to Limnadopsis (Bellec and Rabet 2016; Schwentner et al. 2020). In this sense, Paleolimnadiidae might be polyphyletic because of the pervasive morphological homoplasy of carapace. Schwentner et al. (2020) reconstructed the phylogeny of Spinicaudata using four molecular loci. In the related analyses of basal Limnadiidae, Imnadia was basal in Limnadiidae, either as the sole sister group of all other taxa or as the sister to Limnadia. However, the support values for these alternative topologies were low (value of Bayesian posterior probability 0.62). Currently, the phylogenetic relationships of Imnadia and Limnadia are not fully understood.
Leptestheriidae has been considered to derive from the fossil family Loxomegaglyptidae (Shen 1994; Astrop and Hegna 2015). Loxomegaglyptidae exhibit large to medium reticulations (D≥0.02mm) or complex reticulate ornamentation. Combinations of reticulation and dendritic fringes with dense punctae are dominant in some genera, such as Pseudograpta and Defretinia (Chen and Shen 1985). Molecular phylogenetic analyses by Schwentner et al. (2020) revealed the paraphyly of Cyzicidae and concluded Leptestheriidae as a sister group to Limnadiidae. Cyzicidae sensu stricto is the basal clade of Spinicaudata. Schwentner et al. (2020) established a new family Eocyzicidae. However, phylogenomic analyses based on 864 molecular loci data tended to strongly support a sister group relationship between Leptestheriidae and Eocyzicidae and Limnadiidae as their closest relative (Schwentner et al. 2018). A sister group relationship between Leptestheriidae and Eocyzicidae determined with a much larger set of molecular loci was more phylogenetically convincing (Schwentner et al. 2020). Considering the similarities in ornamentation patterns and carapace morphology, we agree with the hypothesis of Astrop and Hegna (2015) that the monophyletic clade Leptestheriidae + Eocyzicidae probably show affinity with Loxomegaglyptidae. When combined with the sister group relationship between Leptestheriidae + Eocyzicidae and Limnadiidae (Schwentner et al. 2018), the traditional Eosestherioidea is paraphyletic (Fig. 8).
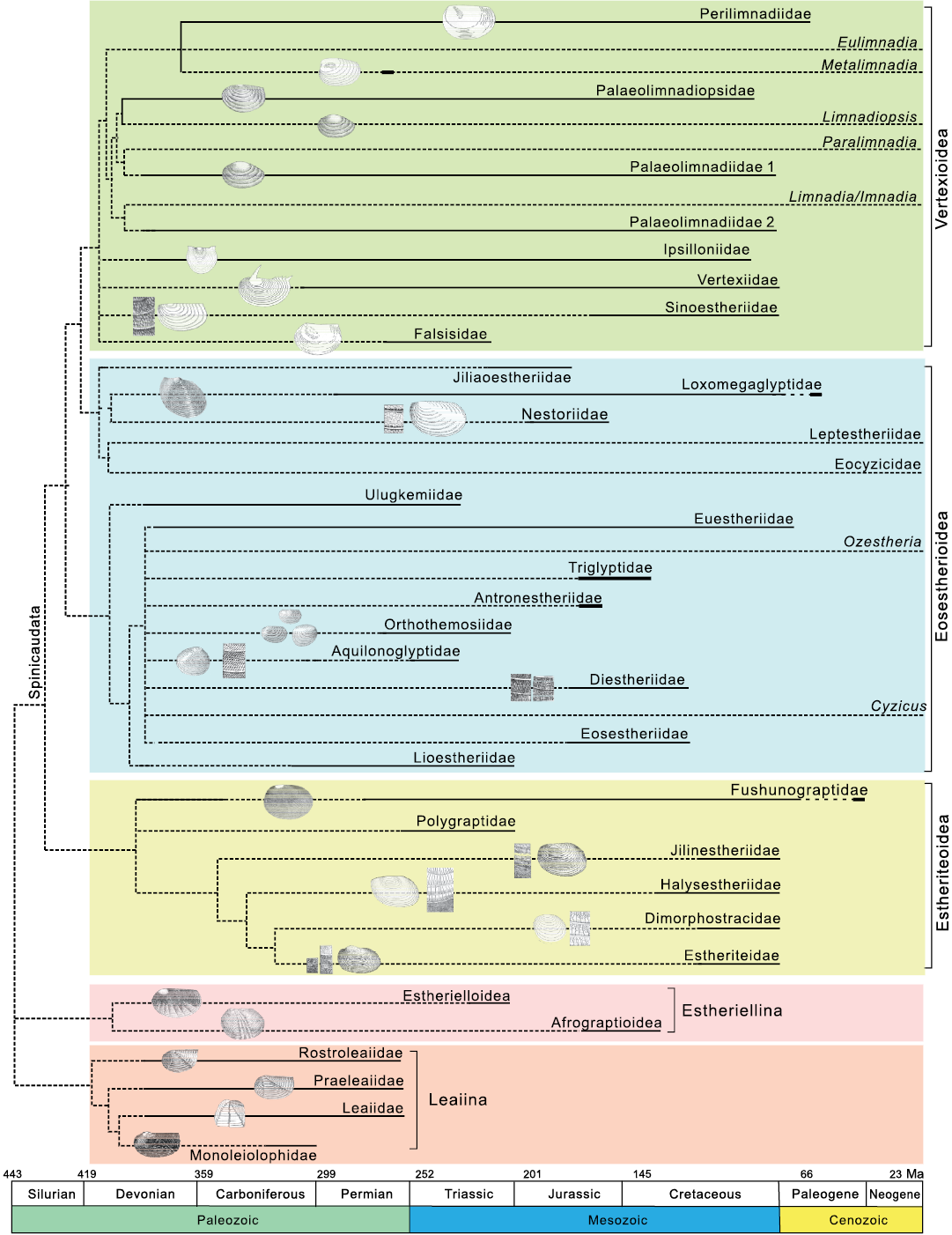
Fig. 8. Hypothesis of the phylogenetic relationships between fossil and extant taxa of Spinicaudata incorporating molecular framework of Schwentner et al. (2018) with fossil families sensu Zhang et al. (1976), Chen and Shen (1985), Shen (2003), and Astrop and Hegna (2015). The molecular topology of extant spinicaudatan taxa from Schwentner et al. (2018) was used as a backbone phylogenetic constraint to construct a framework for fossil taxa. Living taxa were associated with 11 nodes in the phylogeny of Spinicaudata by carapace morphological characters. The small images of clam shrimps were adapted from Chen and Shen (1985).
The family Triglyptidae was characterized by three types of ornamentations: minute punctae, small reticulation and radial lirae. Six genera Triglypta, Tianzhuestheria, Neopolygrapta, Skyestheria, Dendrostracus, and Carapacestheria were included in this family (Wang 2014). Wang (1983) assigned Qaidamestheria to the family Aquilonoglyptidae based on the uniform punctae ornamentation. The carapace shape was defined by the quotient of height/length of the whole valve and the position of the umbo. The changes of this feature in intraspecific variation during ontogeny have been described and quantified, and there was often an overlap between species (Straškraba 1965; Tippelt and Schwentner 2018). We agree with Wang (1983) in placing the Qaidamestheria in the family Aquilonoglyptidae. Antronestheriidae differed from Triglyptidae in lacking lirae ornamentation. Euestheriidae possessing small reticulation (D≤0.02mm) was considered paraphyletic encompassing an ancestral lineage that gave rise to Ozestheria (Chen and Shen 1985). The current results suggest the affinity between Ozestheria and Triglyptidae. Diestheriidae has been considered to derive from Eosestheriidae. The transition of ornamentation from reticulation to lirae and overlapped reticulation observed in dioecious Cyzicus suggested the affinity to Eosestheriidae and Diestheriidae, and uniform punctae ornamentations observed in hermaphroditic Cyzicus suggested the affinity to Aquilonoglyptidae or Orthothemosiidae. The family Orthothemosiidae is controversial because it is considered part of Eosestherioidea (Zhang et al. 1976; Chen and Shen 1985; Astrop and Hegna 2015; Gallego et al. 2020a). These four families might be closely related (Fig. 8).
There are some other modifications in the systematic classification of fossil clam shrimps at the high level of taxonomic rank, such as the placement of Afrograptioidea. This superfamily was recently revised by Shen (2003) and Liao et al. (2017a) and placed in the suborder Estheriellina based on the multi-radiating costae and stout tubercles. The leaiid specimens with soft-tissue body outlines were analysed by Shen and Schram (2014). The leaiids were promoted to the suborder Leaiina and placed in the branchiopodan Diplostraca, based on the ribbed valves and structure of soft parts. Shen and Schram (2014) also suggested that radial ribs on the valves probably facilitated a burrowing habbit in addition to strengthen the valves, which implies the functional significance of ribs. The phylogenitic relationships among the suborders Spinicaudata, Estheriellina, and Leaiia still need further investigation (Fig. 8).
Conclusions
The ornamentation pattern is an important character for the fossil taxonomy of Spinicaudata, especially Eosestherioidea and Estheriteoidea. Sexual dimorphism is a fundamental aspect for studies of clam shrimp taxonomy. In this study, we described the sexually dimorphic ornamentation patterns in extant species of Spinicaudata and made a preliminary investigation on taxonomic values assessment of ornamentations. The results indicate that certain sexually dimorphic ornamentations occur in some species of Spinicaudata, but the basic ornamentation patterns or combinations are stable within each extant species, even for dioecious species possessing sexually dimorphic ornamentations. When integrated with the sister group relationship between Leptestheriidae + Eocyzicidae and Limnadiidae in light of the new molecular phylogeny of Schwentner et al. (2018), the traditional Eosestherioidea is paraphyletic.
Extracting stable taxonomic signal from the external carapace morphology still presents a major challenge. The application of ornamentation patterns for taxonomy needs to be undertaken with caution considering the intraspecific variation generated by sexual dimorphism. The evolutionary relationship between fossil and extant spinicaudatan lineages is still an open question. To establish a comprehensive taxonomic system of clam shrimps, more extensive sampling of extant taxa and integrative analyses are needed. Further investigation into both characteristic ornamentation patterns and quantitative measurements used for taxonomic identification and taxonomic diagnosis is pending.
Acknowledgements
The authors are grateful to Yanbin Shen (NIGP) for his encouragement and valuable discussion. The authors extend their appreciation to Gengo Tanaka (Kumamoto University, Kumamoto, Japan), Oscar Gallego (National University of the Northeast, Corrientes, Argentina) and Thomas Hegna ( State University of New York at Fredonia, USA) for their valuable comments and suggestions that significantly improved this paper. The authors are also thankful to Yongqiang Mao and Zhouqing Chen (both NIGP) for their technical support in scanning electron microscope and light microscope photography. This work was supported by National Natural Science Foundation of China (41730317), by the research grant of State Key Laboratory of Palaeobiology and Stratigraphy at Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, and by Chinese Academy of Geological Sciences (DD20190009). It is also a contribution to UNESCO-IUGS IGCP Project 679.
References
Astrop, T.I. 2014. The Evolutionary Dynamics of Sexual Systems in Deep Time: An Integrated Biological and Paleontological Approach. 233 pp. PhD thesis, University of Akron, Akron.
Astrop, T.I. and Hegna, T.A. 2015. Phylogenetic relationships between living and fossil spinicaudatan taxa (Branchiopoda Spinicaudata): reconsidering the evidence. Journal of Crustacean Biology 35: 339–354. Crossref
Astrop, T.I., Park Boush, L., and Weeks, S.C. 2020. Testing Weissman’s lineage selection model for the maintenance of sex: the evolutionary dynamics of clam shrimp reproduction over geologic time. Zoological Studies 59: 1–16.
Astrop, T.I., Park Boush, L., Brown, B., and Weeks, S.C. 2012. Sexual discrimination at work: Spinicaudatan “clam shrimp” (Crustacea: Branchiopoda) as a model organism for the study of sexual system evolution. Palaeontologia Electronica 15: 1–15. Crossref
Astrop, T.I., Sahni, V., Blackledge, T.A., and Stark, A.Y. 2015. Mechanical properties of the chitin-calcium-phosphate “clam shrimp” carapace (Branchiopoda: Spinicaudata): implications for taphonomy and fossilization. Journal of Crustacean Biology 35: 123–131. Crossref
Audouin, V. 1837. Seance du 15 Fevrier 1837, Presidence de m. Audouin. Annales de la Société entomologique de France, Serie 1 6: 12–14, 124.
Baird, W. 1862. Description of seven new species of phyllopodous crustaceans, belonging to the genera Estheria and Limnetis. Proceedings of the Zoological Society of London 1862: 147–149.
Baird, W. 1859. Description of some new Recent Entomostraca from Nagpur, collected by the Rev. S. Hislop. Proceedings of the Royal Society of London Series B-Biological Sciences 63: 231–234.
Barnard, K.H. 1924. Contributions to a knowledge of the fauna of southwest Africa. II. Crustacea Entomostraca, Phyllopoda. Annals of the South African Museum 20: 213–228.
Barnard, K.H. 1929. Contributions of the crustacean fauna of South Africa. Annals of South African Museum 28: 181–272.
Bellec, L. and Rabet, N. 2016. Dating the Limnadiidae family suggests an American origin of Eulimnadia. Hydrobiology 773: 149–161. Crossref
Balsamo-Crivelli, G. 1859. Di un nuovo crostaceo delle famiglia dei Branchiopodi filopodi riscontrato nella provincia di Pavia e considerazioni sovra i generi affini. Memorie Istituto Lombardo di Lettere Scienze Morale e Storiche 7: 113–120.
Bock, W. 1953. American Triassic estherids. Journal of Paleontology 27: 62–76.
Brown, B.P., Astrop, T.I., and Weeks, S.C. 2014. Post-larval developmental dynamics of the spinicaudatan (Branchiopoda: Diplostraca) carapace. Journal of Crustacean Biology 34: 611–617. Crossref
Chen, P.J. 1975. Tertiary Conchostraca from China [in Chinese]. Scientia Sinica 6: 618–630.
Chen, P.J. and Hudson, J. 1991. The conchostracan fauna of the Great Estuarine Group, Middle Jurassic, Scotland. Palaeontology 15: 515–545.
Chen, P.J. and Shen, Y.B. 1982. Late Mesozoic conchostracans from Zhejiang, Anhui and Jiangsu provinces [in Chinese, with English summary]. Palaeontologia Sinica 161 (New Series B, 17): 1–116.
Chen, P.J. and Shen, Y.B. 1985. An Introduction to Fossil Conchostracans [in Chinese]. 241 pp. Science Press, Beijing.
Chernyshev, B.I. [Černyšev, B.I. ] 1948. Conchostraca from the coal-field in the vicinity of Bukachacha (eastern Transbaikalia) [in Ukrainian]. Zbirnik prats z paleontologii ta stratigrafii. Instytut geologičnyh nauk, Akademiâ Nauk Ukrains’koj SSR 1 (2): 9–14.
Claus, C. 1860. Über die Estherien, insbesondere über Estheria mexicana. Beiträge zur Kenntnis der Entomostraken 4: 12–25.
Daday, D.D. 1913. Deux aberrations intéressantes dans le sous-ordre Phyllopoda Conchostraca. Annales des Sciences Naturelles, Zoologie, Serie 9 17: 195–206.
Depéret C. and Mazeran P. 1912. Les Estheria du Permien d’Autun. Bulletin de la Société d’Histoire naturelle d’Autun 25: 165–173.
Gallego, O.F. 2010. A new crustacean clam shrimp (Spinicaudata: Eosestheriidae) from the Upper Triassic of Argentina and its importance for “conchostracan” taxonomy. Alcheringa 34: 179–195. Crossref
Gallego, O.F. and Caldas, E.B. 2001. Critical revision of the family Afrograptidae Novojilov, 1957 (Conchostraca), its validity and probable South American members. In: L.M. Barros, P.C. Nuvens, and J.B.M. Filgueira (eds.), Comunicações do I e II Simpósios Sobre a Bacia do Araripe e Bacias Interiores do Nordeste, 1990 e 1997, Crato, 164–177. Universidade Regional do Cariri e Sociedade Brasileira de Paleontologia, Crato.
Gallego, O.F., Monferran, M.D., Stigall, A.L., Zacariás, I.A., Hegna, T.A., Jiménez, V.C., Bittencourt, J.S., Li, G., and Calathaki, H.G.B. 2020a. The Devonian–Cretaceous fossil record of “conchostracans” of Africa and their paleobiogeographic relationships with other Gondwanan faunas. Journal of African Earth Sciences 161: 103648. Crossref
Gallego, O.F., Monferran, M.D., Zacarías, I.A., Jiménez, V.C., Buscalioni, A., and Liao, H. 2020b. Clam shrimp fauna (Diplostraca–Spinicaudata and Estheriellina) from the Lower Cretaceous of Las Hoyas, Cuenca (Spain). Cretaceous Research 110: 104389. Crossref
Ghosh, S.C. 1982. Significance of carapace ornamentation of conchostracans: living and fossil forms. In: 14th Annual Conference of Electron Microscope Society of India, Abstracts, 23–24. I.A.C.S., Jadavpur.
Hegna, T.A. 2021. A redescription of the clam shrimp, Orthestheria shupei (Stephenson, in Stephenson & Stenzel, 1952) comb. nov., from the Cenomanian (Cretaceous) of Texas. Cretaceous Research 125: 104864. Crossref
Hegna, T.A. and Astrop, T I. 2020. The fossil record of the clam shrimp (Crustacea; Branchiopoda). Zoological Studies 59: 43.
Hegna, T.A. and Rogers, D.C. 2020. The world’s first clam shrimp symposium: drawing paleontology and biology together. Zoological Studies 59: 46.
Hegna, T., Czaja, A., and Rogers, D.C. 2020. Raman spectroscopic analysis of the composition of the clam-shrimp carapace (Branchiopoda: laevicaudata, Spinicaudata, Cyclestherida): a dual calcium phosphatecalcium carbonate composition. Journal of Crustacean Biology 40 (6): 756–760. Crossref
Hertzog, L. 1935. Notes faunistiques de Camargue, I Crustacea. Bulletin de la Societe Zoologique Francaise 60: 265–281.
Hong, Y.C., Yang, T.Q., Wang, S.T., Wang, S.E., Li, Y.G., Sun, M.R., Sun, X.J., and Du, N.Q. 1974. Stratigraphy and palaeontology of Fushun Coal-field, Liaoning province [in Chinese, with English abstract]. Acta Geologica Sinica 48: 113–158.
Huang, D.Y. and Cai, C.Y. 2016. The arthropods. In: D.Y. Huang (ed.), The Daohugou Biota, 84–211. Shanghai Scientific and Technical Publishers, Shanghai.
Hunt, G., Martins, M., Puckett, T., Lockwood, R., Swaddle, J., Hall, C., and Stedman, J. 2017. Sexual dimorphism and sexual selection in cytheroidean ostracodes from the Late Cretaceous of the U.S. Coastal Plain. Paleobiology 43: 620–641. Crossref
Ishikawa, C. 1895. Phyllopod Crustacea of Japan. The Zoological Magazine 7: 1–154.
Jones, T.R. 1862. A Monograph of the Fossil Estheriae. 132 pp. Palaeontographical Society, London. Crossref
Kobayashi, T. 1954. Fossil estherians and allied fossils. Journal of the Faculty of Science, Tokyo University, Section 2 9 (1): 107–110.
Konstans, N., Hegna, T.A., and Rogers, D.C. 2019. The utility of clam shrimp (Branchiopoda: Laevicaudata, Spinicaudata, Cyclestherida) carapace ornamentation patterns: A Rosetta Stone for understanding modern and fossil clam shrimp phylogeny. In: Abstract booklet of The Crustacean Society Mid-Year Meeting, 137. The Chinese University of Hong Kong, Hongkong.
Krasinetz, S.S. [Krasinec, S.S.] 1963. On the significance of bivalved phyllopod crustaceans (Conchostraca) for the stratigraphy of upper Mesozoic freshwater continental beds of eastern Transbaikalia [in Russian]. Materialy po Geologii i Poleznym Iskopaemym, Chitinskoj Oblasti 1: 32–63.
Krynicki, J. 1830. Des Limnadies. Bulletin de la Société impériale des naturalistes de Moscou 2: 173–182.
Li, G. and Teng, X. 2019. A hope for an integrated taxonomy of fossil and extant clam shrimps. Open Journal of Geology 9: 609–612. Crossref
Li, Y.L., Teng, X., Matsuoka, A., and Li, G. 2017. SEM morphological study of clam shrimp Diestheria (spinicaudatan) of the Jehol Biota of China. Science Report of Niigata University (Geology) 31: 69–74.
Liao, H.Y., Gallego, O.F., Shen, Y.B., Jarzembowski, E., and Huang, D.Y. 2017a. A new afrograptid (Diplostraca: Estheriellina) from the Lower Cretaceous of southern England. Cretaceous Research 71: 79–84. Crossref
Liao, H.Y., Shen, Y.B., and Huang, D.Y. 2017a. Conchostracans of the Middle–Late Jurassic Daohugou and Linglongta beds in NE China. Palaeoworld 26: 317–330. Crossref
Liao, H.Y., Shen, Y.B., and Huang, D.Y. 2017b. Conchostracans of the Middle–Late Jurassic Daohugou and Linglongta beds in NE China. Palaeoworld 26: 317–330. Crossref
Liu, S.W. 1982. Early Jurassic Palaeolimnadiopseoidea (Conchostraca) of China [in Chinese, with English abstract]. Acta Palaeontologica Sinica 21 (4): 383–390.
Lyutkevich E.M. 1937. On some Phyllopoda from the USSR. Summary. The Triassic Estheriae of the Permian Platform. Annuaire de las Societe Paleontologique de Russie 11, 59–70.
Marliére, R. 1950. Ostracodes et phyllopodes du système du Karroo au Congo belge et les régions avoisinantes. Annales du Musée du Congo Belge, Sciences géologiques, Série 8 6: 1–43.
Mattox, N.T. 1950. Notes on the life history and description of a new species of conchostracan phyllopod, Caenestheriella gynecia. Transactions American Microscopical Society 69: 50–53. Crossref
Mattox, N.T. 1952. A new genus and species of Limnadiidae from Venezuela (Crustacea: Conchostraca). Journal of the Washington Academy of Sciences 42: 23–26.
Mattox, N.T. 1957. A new estheriid conchostracan with a review of the other North American forms. American Midland Naturalist 58: 367–377. Crossref
Novojilov, N.I. 1954. Upper Jurassic and Cretaceous conchostracans from Mongolia [in Russian]. Transactions of the Palaeontological Institute, USSR Academy of Sciences 48: 7–124.
Novojilov, N.I. 1955. The new genus of the bivalved phyllopods crustaceans Ulugkemia and its stratigraphy meaning [in Russian]. Voprosy Geologii Azii Akademiâ Nauk SSSR 2: 773–782.
Novojilov, N.I. 1957. Crustacés bivalves de l’ordre des conchostracés du Crétacé inférieur Chinois et Africain. Annales de la Société Géologique du Nord 77: 235–243.
Novojilov, N.I. 1958. Recueil d’articles sur les phyllopodes Conchostracés. Annales du Service d’Information Geologique du Bureau de Recherches Géologiques Géophysiques et Minières, Paris 26: 1–135.
Novojilov, N.I. 1946. New Phyllopoda from the Permian and Triassic deposits of the Nordwick-Khatanga region [in Russian, with English abstract]. Nedra Arctiki 1: 172–202.
Novojilov N.I. 1965. Nouveaux conchostraces de Siberie. Annales de paleontologie (Invertebres) 54: 67–69.
Novojilov, N. and Varentsov, I.M. [Varencov, I.M.] 1956. New Conchostraca from the Givetien of Tuva [in Russian]. Doklady Adademii Nauk SSSR 110: 670–673.
Orlova, E.F. and Sadovnikov, G.N. 2009. Distribution and microsculpture of Limnadiidae, Falsiscidae and Glyptoasmussiidae (Conchostraca) of the terminal Permian of Siberia. Paleontological Journal 43: 631–639. Crossref
Packard, A.S. 1871. Preliminary notice of new North American Phyllopoda. The American Journal of Science and Arts, Third Series 2: 108–113. Crossref
Packard, A.S. 1877. Descriptions of new Phyllopod Crustacea from the West. Bulletin of the United States Geological and Geographical Survey of the Territories, Washington 3: 171–179.
Padial, J.M., Miralles, A., De la Riva, I., and Vences, M. 2010. The integrative future of taxonomy. Frontiers in Zoology 7: 16. Crossref
Raymond, P.E. 1946. The genera of fossil Conchostraca—an order of bivalved Crustacea. Bulletin of the Museum of Comparative Zoology 96: 217–307.
Richter, S. and Timms, B.V. 2005. A list of the recent clam shrimps (Crustacea: Laevicaudata, Spinicaudata, Cyclestherida) of Australia, including the description of a new species of Eocyzicus. Records Australian Museum 57: 341–354. Crossref
Rogers, D.C. 2020. Spinicaudata Catalogus (Crustacea: Branchiopoda). Zoological Studies 59: 45.
Rogers, D.C., Rabet, N., and Weeks, S.C. 2012. Revision of the extant genera of Limnadiidae (Branchiopoda: Spinicaudata). Journal of Crustacean Biology 32: 827–842. Crossref
Rogers, D.C., Thaimuangphol, W., Saengphan, N., and Sanoamuang, L. 2013. Current knowledge of the South East Asian large branchiopod Crustacea (Anostraca, Notostraca, Laevicaudata, Spinicaudata, Cyclestherida). Journal of Limnology 72 (s2): 69–80. Crossref
Sars, G.O. 1896. Description of two new Phyllopoda from North Australia. Archiv for Mathematik og Naturvidenskab 18: 1–34.
Scholze, F. and Schneider, J.W. 2015. Improved methodology of conchostracan (Crustacea: Branchiopoda) classification for biostratigraphy. Newsletters on Stratigraphy 48: 287–298. Crossref
Schwentner M., Just., F., and Richter, S. 2015. Evolutionary systematics of the Australian Cyzicidae (Crustacea, Branchiopoda, Spinicaudata) with the description of a new genus. Zoological Journal of the Linnean Society 173: 271–295. Crossref
Schwentner, M., Rabet, N., Richter, S., Giribet, G., Padhye, S., Cart, J.-F., Bonillo, C., and Rogers, D.C. 2020. Phylogeny and Biogeography of Spinicaudata (Crustacea: Branchiopoda). Zoological Studies 59: 44.
Schwentner, M., Richter, S., Rogers, D.C., and Giribet, G. 2018. Tetraconatan phylogeny with special with special focus on Malacostraca and Branchiopoda: highlighting the strength of taxon-specific matrices in phylogenomics. Proceedings of the Royal Society B 285: 20181524. Crossref
Schwentner, M., Timms, B.V., and Richter, S. 2011. An integrative approach to species delineation incorporating different species concepts: A case study of Limnadopsis (Branchiopoda: Spinicaudata). Biological Journal of Linnean Society 104: 575–599. Crossref
Schwentner, M., Timms, B.V., Bastrop, R., and Richter, S. 2009. Phylogeny of Spinicaudata (Branchiopoda, Crustacea) based on three molecular markers—an Australian origin for Limnadopsis. Molecular Phylogenetics and Evolution 53: 716–725. Crossref
Shen, Y.B. 1994. A new conchostracan genus (Loxomegaglyptidae) from Lower Carboniferous of Britain [in Chinese, with English abstract]. Acta Palaeontologica Sinica 33: 156–165.
Shen, Y.B. 2003. Review of the classification of the family Afrograptidae (Crustacea: Conchostraca) [in Chinese, with English abstract]. Acta Palaeontologica Sinica 42: 590–597.
Shen, Y.B. and Chen, P.J. 1984. Late Middle Jurassic conchostracans from the Tuchengzi Formation of W. Liaoning, NE China [in Chinese, with English abstract]. Bulletin of Nanjing Institute of Geology and Palaeontology, Academia Sinica 9: 309–326.
Shen, Y.B. and Schram, F.R. 2014. Soft-body preservation in the leaiid clam shrimp (Branchiopoda, Diplostraca) and its palaeoecological implications. Crustaceana 87: 1338–1350. Crossref
Shu, S.S., Rogers, D.C., Chen, X.Y., and Yang, J.X. 2015. Two new species of Clam Shrimp (Branchiopoda: Spinicaudata) from Yunnan province, China. Journal of Crustacean Biology 35: 454–460. Crossref
Sigvardt, Z.M.S., Rogers, D.C., and Olesen, J. 2017. Functional morphology of amplexus (clasping) in spinicaudatan clam shrimps (Crustacea, Branchiopoda) and its evolution in bivalved branchiopods: a video-based analysis. Journal of Morphology 278: 523–546. Crossref
Stigall, A.L. and Hartmann, J.H. 2008. A new spinicaudatan genus (Crustacea: “Conchostraca”) from the Late Cretaceous of Madagascar. Palaeontology 51: 1053–1067. Crossref
Straškraba, M. 1965. Taxonomic studies on Czechoslovak Conchostraca, I. Family Limndadiidae. Crustaceana 9: 263–273. Crossref
Tasch, P. 1956. Three general principles for a system of classification of fossil conchostracans. Journal of Paleontology 30: 1248–1257.
Tasch, P. 1969. Branchiopoda. In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part R, Arthropoda 4 (1), R128–R191. Geological Society of America and University of Kansas, Boulder and Lawrence.
Thiery, A. 1988. Maghrebestheria maroccana n. gen., n. sp., nouveau représentant des Leptestheriidae au Maroc (Conchostraca). Crustaceana 54: 43–56. Crossref
Timms, B.V. 2009. Revision of the Australian endemic clam shrimp Limnadopsis Spencer and Hall (Crustacea: Branchiopoda: Spinicaudata: Limnadiidae). Records of the Australian Museum 61: 49–72. Crossref
Timms, B.V. 2018. Keys to the Australian clam shrimps (Crustacea: Branchiopoda: Laevicaudata, Spinicaudata, Cyclestherida). Museum Victoria Science Reports 20: 1–25. Crossref
Tippelt, L. and Schwentner, M. 2018. Taxonomic assessment of Australian Eocyzicus species (Crustacea: Branchiopoda: Spinicaudata). Zootaxa 4410: 401–452. Crossref
Uéno, M. 1927. On some freshwater branchiopods from China. Annotationes Zoologicae Japonenses 11: 157–164.
Vannier, J., Thiéry, A., and Racheboeuf, P.R. 2003. Spinicaudatans and ostracods (Crustacea) from the Montceau lagerstätte (late Carboniferous, France): morphology and palaeoenvironmental signifcance. Palaeontology 46: 999–1030. Crossref
Wang, S.E. 1981. On Upper Jurassic phyllopods (Conchostraca) from northern Hebei and Daxing’anling and their significance [in Chinese, with English abstract]. Bulletin of Chinese Academy of Geological Sciences 3: 97–117.
Wang, S.E. 1983. Some Jurassic–Cretaceous conchostracans from Qinghai [in Chinese, with English abstract]. Acta Palaeontologica Sinica 22: 461–470.
Wang, S.E. 1985. The Jurassic and Cretaceous conchostracans from Sinkiang Uigur Autonomous region, China [in Chinese, with English abstract]. Professional Papers of Stratigraphy and Palaeontology 12: 1–21.
Wang, S.E. 1989. Mechanics and the evolution and functional morphology of the conchostracan carapace. Science in China Series B 32: 631–640.
Wang, S.E. 2014. Triglyptidae fam. nov. and its significance in evolution and biostratigraphy [in Chinese, with English abstract]. Acta Palaeontologica Sinica 53: 486–496.
Wang, S.E. and Liu, S.W. 1980. Fossil clam shrimps [in Chinese]. In: Institute of Geology, Chinese Academy of Geological Sciences (ed.), The Mesozoic Stratigraphy and Palaeontology of Shaan-Gan-Ning Basin (Part Two), 84–110. Geological Publishing House, Beijing.
Wang, W.L., Zhang, H., Zhang, L.J., Zheng, S.L., Yang, F.L., Li, Z.T., Zheng, Y.J., and Ding, Q.H. 2004. Standard Sections of Tuchengzi Stage and Yixian Stage and Their Stratigraphy, Palaeontology and Tectonic-volcanic Actions [in Chinese, with English abstract]. 514 pp. Geological Publishing House, Beijing.
Wolf, E. 1911. Phyllopoda. In: W. Michaelsen and R. Hartmeyer (eds.), Die Fauna Sudwest-Australiens. Ergebnisse der Hamburger sudwest-australischen Forschungsriese 1905 3: 353–276.
Zhang, W.T. 1957. Some Cretaceous conchostracans from Tsaidam Basin [in Chinese, with English abstract]. Acta Palaeontologica Sinica 5 (4): 503–511.
Zhang, W.T. and Chen, P.J. 1964. New Cretaceous Conchostraca from Jilin and Heilongjiang [in Chinese and English]. Acta Palaeontologica Sinica 12 (1): 1–25.
Zhang, W.T., Chen, P.J., and Shen, Y.B. 1976. Fossil Conchostracans of China [in Chinese]. 325 pp. Science Press, Beijing.
Acta Palaeontol. Pol. 67 (2): 475–492, 2022
https://doi.org/10.4202/app.00892.2021