

Body mass estimation in Triassic cynodonts from Argentina based on limb variables
FLORENCIA S. FILIPPINI, FERNANDO ABDALA, and GUILLERMO H. CASSINI
Filippini, F.S., Abdala, F., and Cassini, G.H. 2022. Body mass estimation in Triassic cynodonts from Argentina based on limb variables. Acta Palaeontologica Polonica 67 (2): 543–557.
Body mass estimations for extinct taxa are fundamental in palaeobiological reconstructions, but little work has been done on this topic for non-mammaliaform cynodonts (NMC), the diverse and abundant Permo-Cretaceous forerunners of mammals. Here, we estimated the body mass of five species of NMC cynognathians by linear measurements and circumferences of postcranial elements (humeri and femora) from 14 specimens from Triassic units of the Ischigualasto-Villa Union Basin located between San Juan and La Rioja provinces, and the Cuyo and San Rafael basins, both in Mendoza province, Argentina. For this purpose, we used predictive formulas available in the literature based on variables on appendicular skeleton of different extant groups of mammals and reptiles. Geometric similarity using skull length was applied to provide an estimation of adult masses for species with only samples of juvenile and subadult limb bones. A broad body mass range was recorded. Small traversodontids such as Andescynodon mendozensis and Pascualgnathus polanskii were between 1 to 3.5 kg. Medium-sized traversodontids include adult Massetognathus pascuali with masses from 20 to 40 kg, and the adult forms of large-sized cynognathians like Cynognathus crateronotus and Exaeretodon argentinus reached or surpassed 100 kg. The morphological variations in the skull and the different body sizes observed between traversodontids are interpreted as reflecting different types of diets where small-sized traversodontids had a generalist diet, and the medium/large-sized traversodontids were herbivorous. Finally, palaeoecological working hypotheses regarding cranial and dental morphology, body mass, and their possible relation with diet in non-mammaliaform cynodonts of South America are offered.
Key words: Cynodontia, Gomphodontia, Traversodontidae, allometric equations, femur, humerus, paleobiology, Triassic, Argentina.
Florencia S. Filippini [flor.s.filippini@gmail.com], Laboratorio de Becarios, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Av. Ángel Gallardo 470, C1405DJR, Ciudad Autónoma de Buenos Aires, Argentina; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Fernando Abdala [nestor.abdala@wits.ac.za], Unidad Ejecutora Lillo, Conicet-Fundación Miguel Lillo, Miguel Lillo 251, Tucumán, Argentina; Evolutionary Studies Institute, University of the Witwatersrand, 1 Jan Smuts Avenue, WITS 2050, Johannesburg, South Africa; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Guillermo H. Cassini [gcassini@macn.gov.ar], División Mastozoología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Av. Ángel Gallardo 470, C1405DJR, Ciudad Autónoma de Buenos Aires, Argentina; Departamento de Ciencias Básicas, Universidad Nacional de Luján, Ruta 5 y Av. Constitución s/n, Luján (6700), Buenos Aires, Argentina; Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Received 22 June 2021, accepted 29 November 2021, available online 11 April 2022.
Copyright © 2022 F.S. Filippini et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The evolution of mammalian biology is well documented in the fossil record by interrelated lineages thriving from the Carboniferous and leading to the rise of crown mammals in the Jurassic (Kemp 2005; Martin 2018). Two main lineages are recognized in this transition, the paraphyletic “pelycosaurs”, which include, among others, the sail-backed carnivore Dimetrodon, and the younger therapsids, which include the extant mammals (Sidor and Hopson 1998; Kemp 2005; Angielczyk and Kammerer 2018). Therapsids are represented by a series of groups known from the middle Permian; one of them, Cynodontia, which includes Mammalia, is also represented by a series of stem taxa that document the emergence of several important mammalian characters (e.g., complex postcanine dentition, osseous secondary palate, double occipital condyle; Angielczyk and Kammerer 2018). These fossil forms, known as non-mammaliaform cynodonts (NMC), were well represented in the entire Triassic, and some late members even reached the Cretaceous (Abdala 2021). Triassic NMC have a characteristic and relatively large head compared to the body, and short limbs relative to the trunk length that gives a “low-slung” appearance to the body (Jenkins 1970). They show a morphological diversity that includes small-medium sized and slender forms (e.g., Massetognathus, Pascualgnathus, and Andescynodon), and large sized robust forms (e.g., Exaeretodon and Cynognathus). Non-mammaliaform cynodonts include sectorial toothed species, presumed to have been omnivorous/carnivorous, which are similar in body size to forms like civets, foxes, quolls and thylacine, and presumed herbivorous forms varying in body size from those of caviomorph rodents, to pudus, wombats and tapirs.
Non-mammaliaform cynodonts have a particularly rich fossil record in South America, being represented in Triassic deposits of southern Brazil and western Argentina (Schultz et al. 2020; Abdala et al. 2020). The Argentinean deposits with NMC are the Middle Triassic Cerro de las Cabras Formation, Cuyo Basin, Mendoza province; the Upper Triassic Chañares, Ischigualasto, and Los Colorados formations in the Ischigualasto-Villa Union Basin, La Rioja, and San Juan provinces; the Upper Triassic Quebrada del Barro Formation in the Marayes-El Carrizal depocenter, San Juan province; and the ?Middle–?Upper Triassic Rio Seco de la Quebrada Formation, San Rafael depocenter, Mendoza province (Abdala et al. 2020).
Two main lineages of advanced cynodonts (Eucynodontia) are represented in the Argentinean Triassic: the mostly carnivorous Probainognathia, that include mammals, and the mostly herbivorous and extinct clade Cynognathia (Abdala et al. 2020). The latter includes a basal form with an entirely sectorial dentition, and the remaining forms with labio-lingually expanded crowns are included in Gomphodontia (Hendrickx et al. 2020; Fig. 1). This clade has a tooth-to-tooth occlusion because of the expanded crowns and a palinal movement of the mandible limited by the presence of transverse crests in upper and lower postcanines (Crompton 1972; Hopson 2014). The most successful Triassic cynodonts are the Traversodontidae, a lineage of gomphodonts with deep occlusal basins. The Cynognathia are well represented in Argentina, being found in four different geological units. The most basal and only member of the group with exclusively sectorial toothed postcanines, Cynognathus crateronotus, is documented in association with the diademodontid Diademodon tetragonus, and the small-sized traversodontid Pascualgnathus polanskii in the Rio Seco de la Quebrada Formation (Bonaparte 1966; Bonaparte 1969a; Martinelli et al. 2009). The small-sized traversodontid Andescynodon mendozensis is the most abundant taxon of the endemic fauna from the Cerro de las Cabras Formation. Traversodontids are also key representatives in the Chañares Formation where the medium-sized Massetognathus pascuali is the most abundant member, and in the Ischigualasto Formation with a similarly well-represented large-sized traversodontid Exaeretodon argentinus (Bonaparte 1962, 1963a, b, 1969b; Romer 1967, 1972; Hopson 1984, 1985; Abdala 1996, Martinelli et al. 2009; Martinelli 2010; Liu and Powell 2009; Liu and Abdala 2014; Abdala et al. 2020).
Size is a highly variable feature in Cynognathia. Large and small animals experience different advantages/disadvantages: the largest have fewer predators but are more sensitive to environmental changes that can produce fluctuations in available resources; small ones can survive with less food in hard times (at least in an absolute sense), but have more potential predators (see Vizcaíno et al. 2016). Assessing size in terms of body mass (bm) constitutes a meaningful measure and allows size to be compared more readily, and, along with feeding behaviour and substrate preference and use, constitutes a basic biological attribute to characterize extinct vertebrate life habits (Brown and West 2000; Polly 2007; Hopkins and Davis 2009; Vizcaíno et al. 2016).
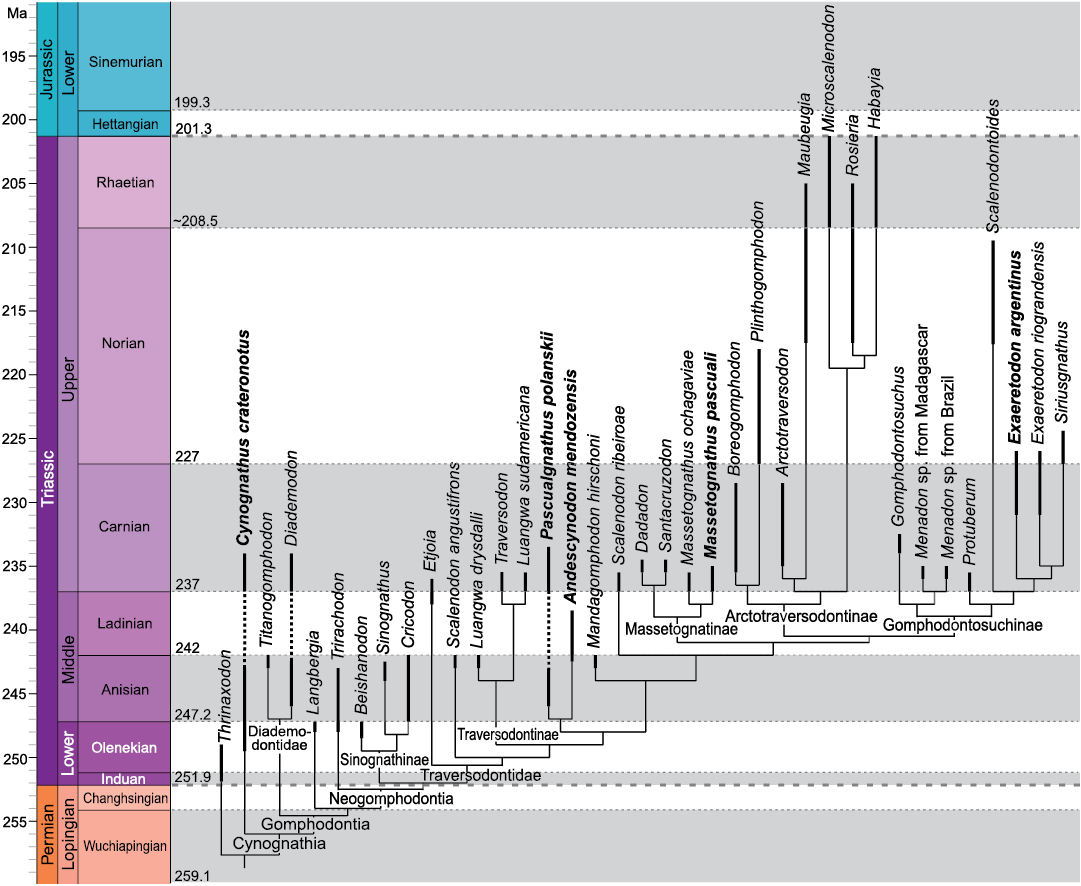
Fig. 1. Cynognathia phylogeny plotted onto a stratigraphic scale showing the known observed temporal ranges of taxa. Taxa studied in this contribution are in bold. Modified from Hendrickx et al. (2020). Thick dashed lines indicate separation between periods; thin dotted lines indicate separation between ages.
Body mass estimation in extinct taxa is the first step in the application of Radinsky’s (1987) form-function correlation paradigm to predict non-preserved biological attributes and to propose palaeoecological hypotheses (Andrews et al. 1979; Reed 1998; Vizcaíno et al. 2006; Hopkins and Davis 2009; Vizcaíno and Bargo 2021). Although some attempts have been made to estimate the bm of the NMC, they are only based on cranial length and restricted to a few taxa providing a rough idea of size (e.g., Mancuso et al. 2014; Fahn-Lai et al. 2020; Hendrickx et al. 2020).
The most common approach for predicting bm of extinct species is the application of allometric functions (e.g., Fariña et al. 1998; Millien and Bovy 2010; De Esteban-Trivigno et al. 2008; Campione and Evans 2012). Cranial remains are frequently recovered in the fossil record and are therefore the source of most bm estimations, particularly in mammals (Mendoza et al. 2006; Ercoli and Prevosti 2011; Cassini et al. 2012a, b; Perry et al. 2017; Tarquini et al. 2017). However, some authors argue that postcranial elements (e.g., humerus and femur) are more accurate estimators of bm as they provide body support (Damuth and MacFadden 1990; Campione and Evans 2012). These estimations are especially appropriate when the studied group is entirely extinct, or if these estimations fall outside the size ranges of extant species (e.g., White 1993; De Esteban-Trivigno et al. 2008; Toledo et al. 2014). In mammals a taxonomically accurate bm estimation is produced when the identity of the assessed taxon is based on cranial features due to the predominance of cranial/dental characteristics for taxonomic identification, whereas postcranial elements usually have to be associated with cranial elements for safer identification. Taxonomy of NMC in the fossil record is, as in the case of mammals, usually based on cranial and/or dental features. In addition, the postcranial skeleton of our sample is mostly represented by subadults and juvenile forms. These issues make the estimation of an average for adult size or maximum size for a given species difficult, and will also cause insurmountable difficulties when proposing palaeobiological hypotheses that usually necessitate adult body mass.
In this contribution we provide the first approach to the body mass estimation of cynognathian cynodonts from Argentina based on postcranial interspecific allometric equations of extant mammals and non-avian reptiles. Due to taphonomic and representation bias, the estimation of maximum body mass needs to be extrapolated to the largest skull known for a given taxon by using the geometric similarity of skulls. Finally, we propose palaeobiological working hypotheses as bm is one of the main attributes that allow an approximation of the palaeoecology of extinct taxa. In this sense, we apply the Principle of Actualism (Cassini et al. 2011; Vizcaino et al. 2016; Kay et al. 2021), which assumes that the same biomechanical laws relating to body support in extant species have constrained the evolution of cynognathians.
Institutional abbreviations.—MCZ, Museum of Comparative Zoology, Harvard University; MLP, Museo de La Plata, La Plata, Argentina; NHMUK, Natural History Museum, London, UK; PULR, Universidad Nacional de La Rioja, Argentina; PVL, Colección de Paleontología de Vertebrados, Instituto Miguel Lillo, Universidad Nacional de Tucumán, Argentina.
Other abbreviations.—bm, body mass; BSL, basal skull length; CV, coefficient of variation; NMC, non-mammaliaform cynodonts; % PE, percent prediction error; R2, coefficient of determination; WM, weighted mean; WSD, weighted standard deviation.
Material and methods
Specimens.—Fourteen specimens of Cynognathia from Argentina, housed in MLP and PVL paleontological collections, with little or no apparent deformation, were analysed. They include 13 humeri (six left; seven right) and seven femora (four left; three right), where five specimens have humerus and femur associated (see below).
Cynognathus crateronotus Seeley, 1895, horizon: Río Seco de la Quebrada Formation (Puesto Viejo Group); age: recent absolute dating assigns a lower Carnian age (Upper Triassic) to this unit (Ottone et al. 2014), although there is no total agreement with the proposed age (see Peecook et al. 2018; Schneider et al. 2020; Abdala et al. 2020); material studied: right humerus from PVL 3859.
Andescynodon mendozensis Bonaparte, 1969, horizon: Cerro de las Cabras Formation; age: upper Anisian (Middle Triassic); material studied: right humerus and left femur from PVL 3890; left humerus and femur from PVL 3894-1; right humerus from PVL 4424; left humerus from PVL 4425; right humerus from PVL 4426; and right humerus from PVL 4427.
Pascualgnathus polanskii Bonaparte, 1966, horizon: Río Seco de la Quebrada Formation (Puesto Viejo Group); age: ?lower Carnian (see above); material studied: right and left humerus, and right femur from MLP 65-VI-18-1.
Massetognathus pascuali Romer, 1967, horizon: Chañares Formation; age: lower Carnian (Upper Triassic); material studied: right humerus from PVL 4613, left humerus and femur from PVL 5444, right femur from PVL 5445.
Exaeretodon argentinus Cabrera, 1943, horizon: Ischigualasto Formation; age: upper Carnian (Upper Triassic); material studied: left humerus from PVL 2467; left humerus and femur from PVL 2554; right humerus from PVL 2565.
Measurements.—Sixteen measurements of the humerus and femur, corresponding to lengths, diameters and circumferences of the stylopodium bones that account for size and robusticity (Fig. 2) were selected based on availability in the specimens. They were obtained as the distances between landmarks placed with Landmark v.3.0.0.6 (Institute for Data Analysis and Visualization, University of California; Wiley et al. 2005) from scaled 3D models built by photogrammetry using Agisoft® Metashape v.1.6.5 (see Otero et al. 2020 for a review on three-dimensional image surface acquisition in vertebrate palaeontology). Measurements were chosen based on Toledo et al. (2014) with the addition of the circumferences of the stylopodium bones based on Campione and Evans (2012). In line with the chosen equations (see below), each measurement was taken in millimetres and transformed to base-10 logarithm.
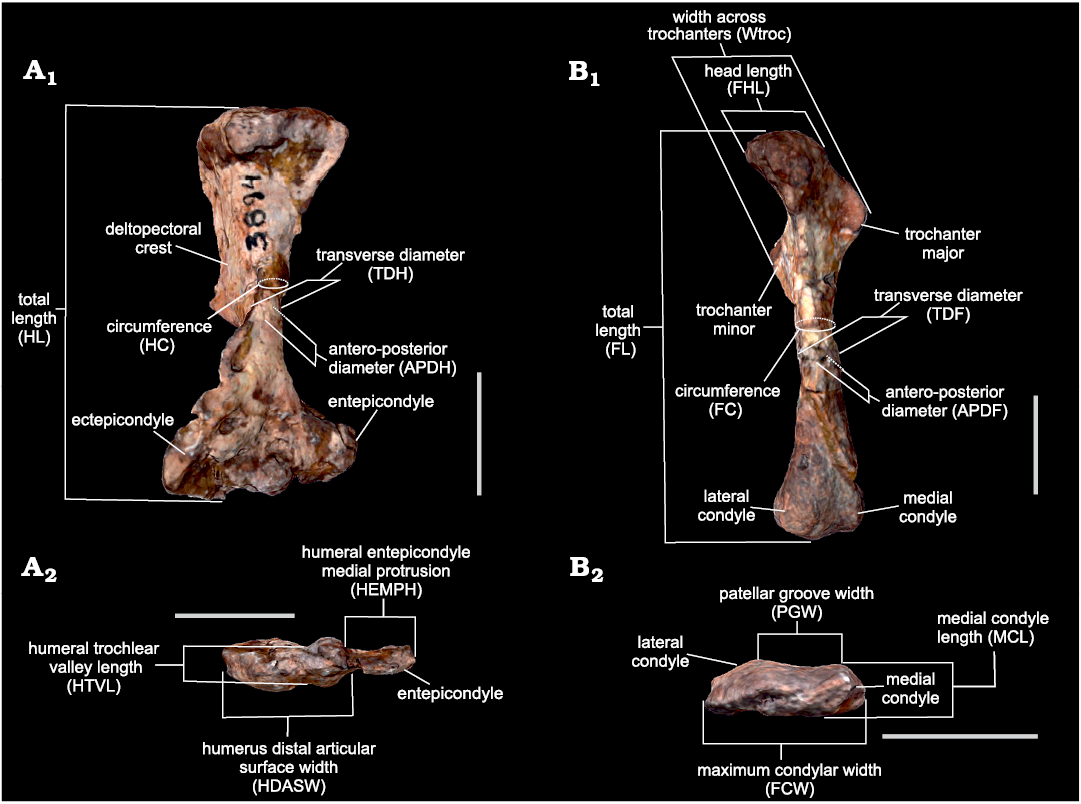
Fig. 2. Measurements used in this work based on Toledo et al. (2014), as illustrated using the 3D model of the left humerus and femur of Andescynodon mendozensis Bonaparte, 1969 (PVL 3894-1) from the Cerro de las Cabras Formation (upper Anisian), Villa de Potrerillos, Mendoza province, Argentina. A. Humerus in anterior (A1) and distal (A2) views. B. Femur in anterior (B1) and distal (B2) views. Scale bars 10 mm.
Allometric equations.—Body masses estimations of NMC were obtained using stylopodium interspecific allometric simple regression equations of extant mammals proposed by Scott (1990), Anyonge (1993), Biknevicius et al. (1993), and Figuerido et al. (2011) and the multivariate equation of Toledo et. al. (2014). Also included is the bivariate equation based on crocodiles from Farlow (2005), and simple and multivariate regression equations of extant tetrapods by Campione and Evans (2012). Using these equations, we intended to include a great diversity of extant forms (covering different limb proportions and postures) that allows us to calculate a grand mean as a conservative hypothesis of bm estimation (Fariña et al. 1998), given that NMC anatomy is not exactly represented in any extant group.
We chose equations based on the value of the percentage of variance explained (R2), the percent prediction error (% PE), and the range of size of the different extant analogues (SOM 1: tables S1 and S2, Supplementary Online Material available at http://app.pan.pl/SOM/app67-Filippini_etal_SOM.pdf). In addition, using Campione and Evans’s (2012) database we obtained four simple regressions (and their corresponding % PE) based on length and circumference of the stylopodium used as references for both mammals and non-avian reptiles (SOM 1: table S1).
The weighted mean (WM) and weighted standard deviation (WSD) of each set of regression equations (simple, multivariate) per specimen and stylopodium element were calculated using the regular weighted formulas (Cohen and Cohen 2008). We constructed a weight factor resulting in equations with lower % PE having a high contribution to the WM. In doing so, a sum of % PE of the applied equations by element was first obtained; second, the weight factor was calculated as the ratio of this sum (numerator) and each % PE (denominator). In addition, we calculated the percent coefficient of variation (% CV) by dividing the WSD by WM, multiplied by 100 (Christiansen and Harris 2005). Finally, the mean body mass by element (humerus and femur) and by taxon for adult specimens was calculated.
Postcranial remains are preferable for bm estimation over cranial and dental pieces (Gingerich 1990; White 1993; De Esteban-Trivigno et al. 2008); however, they are usually scarce and, in general, do not reflect the known size range of NMC species. Therefore, to provide a more reliable bm range based on known specimens we calculated it using a geometric similarity among different sizes, using our estimations of body mass scaled to the maximum skull lengths as follows: Mx = (Lx/Lg)3 × Mg, where Mx is the bm of the problem specimen; Mg is the bm of the known specimen (bm from this work); Lx, skull length of the problem specimen (in this work it is the largest know specimen of a species and we obtained the measurements from literature); and Lg, the same skull length measured in the known specimen. This method assumes an isometric relationship (i.e., lack of allometry) between skull length and body mass, and was found reliable to obtained body mass estimations (see Vizcaíno et al. 2006, 2011a, 2016; Vezzosi 2012).
For classification purposes, we follow Cassini et al. (2012a, b) and Toledo et al. (2014) for their three base-10 logarithmic body size ranges: small-size forms (1–10 kg), medium-size forms (10–100 kg), and large-size forms (100–1000 kg).
Results
Postcranial body mass estimations.—The weighted mean (WM), weighted standard deviation (WSD), and percent of coefficient of variation (% CV) of bm estimations obtained for each specimen discriminated by humeri, femora, and a combination of humerus plus femur equations, as well as the mean values for species, are shown in Table 1. Single regression equations allowed a more inclusive sample only in humeri (see bm estimations by equation for each specimen in SOM 1: tables S7 and S8). The values can be estimated from the humerus and femur of the same specimen in Andescynodon mendozensis (PVL 3890 and 3894-1), Massetognathus pascuali (PVL 5444), Exaeretodon argentinus (PVL 2554), and Pascualgnathus polanskii (MLP 65-VI-18-1). Body mass values obtained from these two bones are close to each other in most specimens, except for E. argentinus, with a much higher estimation from the humerus.
The sectorial-toothed Cynognathus crateronotus showed an average bm of ca. 20 kg by humerus equations. Our sample included three juvenile and three adult humeri of A. mendozensis (see Table 1); the latter showed an average bm of 1.46 kg, with the smallest juvenile specimen weighing 0.67 kg and the largest adult 1.72 kg. The bm estimations of P. polanskii is 3.4 kg. For M. pascuali, our largest specimen is possibly a subadult (see discussion below), represented only by a femur, and having a bm estimation of 5.7 kg. Two large E. argentinus humeri (PVL 2467, PVL 2554) indicate a mean for the species of 86.11 kg (Table 1); the estimations based on the femur range from a smaller juvenile specimen, PVL 2565, with a weight of only 9.9 kg, to the largest, PVL 2554, of 60.56 kg.
Table 1. Weighted body mass (in kg) for specimens analysed in this work. Weighted means were calculated taking into account %PE corresponding for each equation. Only adult forms are considered for species mean. Abbreviations: n, sample size (indicated in parenthesis); sd, standard deviation; CV %, percent coefficient of variation; jv, juvenile specimen; WM, weighted mean; WSD, weighted standard deviation.
|
Taxon |
Specimen |
Humerus |
Femur |
Humerus + Femur |
||||||
|
WM |
WSD |
CV % |
WM |
WSD |
CV % |
WM |
WSD |
CV % |
||
|
Andescynodon mendozensis |
PVL 3890 |
1.09 |
0.75 |
68.70 |
1.55 |
1.11 |
71.66 |
1.28 |
1.06 |
82.66 |
|
PVL 3894-1 (jv) |
0.67 |
0.34 |
51.02 |
0.68 |
0.33 |
48.89 |
0.49 |
0.40 |
82.13 |
|
|
PVL 4424 (jv) |
1.21 |
0.73 |
60.10 |
|
|
|
|
|
|
|
|
PVL 4425 (jv) |
0.89 |
0.47 |
52.45 |
|
|
|
|
|
|
|
|
PVL 4426 |
1.56 |
0.75 |
48.26 |
|
|
|
|
|
|
|
|
PVL 4427 |
1.72 |
0.89 |
51.64 |
|
|
|
|
|
|
|
|
Species mean ± sd (n) |
1.46 ± 0.80 (3) |
1.11 ± 0.72 (2) |
|
|||||||
|
Cynognathus crateronotus |
PVL 3859 |
20.17 |
7.01 |
34.77 |
|
|
|
|
|
|
|
Exaeretodon argentinus |
PVL 2467 |
85.57 |
62.99 |
73.61 |
|
|
|
|
|
|
|
PVL 2554 |
86.65 |
56.02 |
64.66 |
60.56 |
45.13 |
74.53 |
81.89 |
68.55 |
83.71 |
|
|
PVL 2565 (jv) |
|
|
|
9.99 |
8.28 |
82.87 |
|
|
|
|
|
Species mean ± sd (n) |
86.11 ± 59.50 (2) |
|
|
|||||||
|
Massetognathus pascuali |
PVL 4613 (jv) |
1.80 |
0.77 |
42.85 |
|
|
|
|
|
|
|
PVL 5444 (jv) |
1.88 |
0.86 |
45.46 |
1.24 |
0.55 |
44.59 |
1.09 |
0.90 |
82.55 |
|
|
PVL 5445 |
|
|
|
5.72 |
3.65 |
63.86 |
|
|
|
|
|
Pascualgnathus polanskii |
MLP 65-VI-18-1 |
3.40 |
1.80 |
53.02 |
2.60 |
1.33 |
51.09 |
2.48 |
2.06 |
82.82 |
Table 2. Body mass (in kg) based on geometric similarity considering skull length (in mm) of the largest specimens known. Abbreviations: MSL, maximum skull length reported for the taxon in the literature (Abdala 1996; Abdala and Giannini 2000; Abdala et al. 2002; Liu and Powell 2009); SL, skull length; BMpe, mean body mass estimated from postcranial elements; Max. BMgs, maximum body mass estimated for the taxon by geometric similarity.
|
Taxon |
MSL |
Specimen |
SL |
BMpe present study |
Max. BMgs |
|
Andescynodon mendozensis |
137 (PVL 3900) |
PVL 3894-1 |
87 |
0.67 (humerus) |
2.62 |
|
0.68 (femur) |
2.66 |
||||
|
Cynognathus crateronotus |
360 (NHMUK R2571) |
PVL 3859 |
210 |
20.17 (humerus) |
101.61 |
|
Exaeretodon argentinus |
400 (MCZ 4486) |
PVL 2554 |
330 |
86.67 (humerus) |
154.35 |
|
60.56 (femur) |
107.85 |
||||
|
Massetognathus pascuali |
204 (PULR V02) |
PVL 4613 |
72 |
1.80 (humerus) |
40.94 |
|
PVL 5445 |
140 |
5.72 (femur) |
17.70 |
In specimens that preserve both humerus and femur, the WM of bm based on different bones differ from each other. In P. polanskii (MLP 65-VI-18-1) the values do not vary much (Table 1), with the value obtained from the femur only 0.8 kg smaller than that of the humerus (2.6 kg vs. 3.4 kg, respectively), and are among the most consistent values between estimations from different limb bones of the same individual. In the smallest A. mendozensis the body mass estimation obtained from the femur is higher by 0.46 kg than that of the humerus in PVL 3890 (1.55 kg vs. 1.09 kg, respectively), and by 0.01 kg in PVL 3894-1 (0.68 vs. 0.67 kg, respectively). In Massetognathus pascuali and Exaeretodon argentinus (PVL 5444 and PVL 2554, respectively), the estimations values obtained from the femur are smaller than that of the humerus; in M. pascuali, the estimation from the humerus is 0.64 kg higher than that of the femur (1.24 kg vs. 1.88 kg, respectively), whereas in E. argentinus this difference is 26 kg (60.56 kg vs. 86.65 kg). Using regression equations of Campione and Evans (2012) based on values of humerus and femur combined (Table 1), bm estimations obtained for specimens of A. mendozensis (PVL 3894-1; bm of 0.49 kg), M. pascuali (PVL 5444; bm of 1.09 kg), and P. polanskii (MLP 65-VI-18-1; bm of 2.48 kg) are lower when compared with the values obtained from single regression equations of single stylopodium element for the same specimen. Body mass estimation value for other specimen of A. mendozensis (PVL 3890; bm of 1.28 kg) is between the values obtained from each element (i.e., the humerus and the femur) of the same specimen; and in E. argentinus (PVL 2554; bm of 81.89 kg) the value is closer to that obtained from the humerus (86.65 kg) than the femur (60.56 kg; see Table 1).
Maximum body mass by taxon.—The maximum body mass of five genera is calculated by geometric similarity using the skull length (Table 2). Sectorial-toothed Cynognathus crateronotus shows a maximum bm for the species of approximately 100 kg. Andescynodon mendozensis (PVL 3900) has a maximum bm of nearly 3 kg, closer to the maximum bm estimation (3.77 kg) for its sister taxon, P. polanskii (PVL 4416). In M. pascuali, the largest known skull, PULR V02, is 204 mm in length, with a bm estimation between 17 kg (based on femur) and 40 kg (based on humerus). The largest E. argentinus specimen (MCZ 4486), with a skull length of 400 mm, shows a bm estimation between 107 kg and 154 kg (based on femur and humerus, respectively, from PVL 2554; Table 2).
In summary, based on known specimens and considering only adult forms, the “small-sized NMC” group (body mass 1–10 kg), is represented by A. mendozensis and P. polanskii; the “medium-sized NMC” group (10–100 kg) includes M. pascuali, and the “large-sized NMC” group (above 100 kg) includes E. argentinus and the largest C. crateronotus from South Africa (assumed adults).
Discussion
Body mass estimations in cynognathians
In order to obtain reliable predictions when applying body mass estimations based on allometric equations, it is advisable that the size of the fossil specimen under study be inside the size range of extant taxa from which the equations were built (Millien 2008). This is particularly important when extinct taxa under study have no extant relatives (see Cassini et al. 2012a, b) or have no extant related species of the same size (see Fariña et al. 1998; Candela et al. 2013; Toledo et al. 2014; Vizcaíno et al. 2018; and references therein). In these cases, choosing allometric equations on extant analogues of similar or broader size is a common solution. The body masses obtained for the cynognathians studied here are inside the body mass range of the different living tetrapods included in the datasets of the allometric equations (see SOM 1: table S2). They mainly correspond with the body mass range of species included in the dataset from Toledo et al. (2014), Campione and Evans (2012), Farlow (2005), and Figuerido et al. (2011). However, the bm estimations for Andescynodon mendozensis, Massetognathus pascuali, and Pascualgnathus polanskii based on datasets from Anyonge (1993) and Scott (1990), which do not include taxa with a body mass below 5 kg, should be taken with caution. The same applies to the predictions of bm for Exaeretodon argentinus based on Biknevicius et al. (1993), which do not cover body masses greater than 50 kg. In the present study we followed Fariña et al. (1998) and did not exclude these values, as the potential under- and overestimated values should compensate in the weighted mean from the 37 equations. As mentioned above, these equations included simple regression equations based on length, diameter and circumference for humerus or femur, and humerus plus femur from non-avian reptiles and mammals, most of them previously published (see Material and Methods section), eight new equations obtained from Campione and Evans’s (2012) supplementary dataset (SOM 1: table S1), and multivariate equations (Campione and Evans 2012 and Toledo et al. 2014; SOM 1: table S2).
Our results for the cynognathian postcranial specimens show a body mass range between 0.5 kg and 87 kg, and most of the estimation values are below 20 kg. Exaeretodon argentinus is the largest sized taxon in our sample, with a bm estimation of 60 to 86 kg (154 kg using geometric similarity with the largest known skull). The sectorial-toothed Cynognathus crateronotus has a bm estimation of 20 kg. This value was obtained from the only known individual from Argentina (PVL 3859), with its skull length (210 mm) well below the 360 mm of the largest South African skull (Abdala 1996), whereas its humerus length (135 mm) is below the 185 mm reported from a specimen of Tanzania (Wynd et al. 2018). If we consider the difference in skull length and assume a geometric similarity, the South African specimen should have a bm estimation of ca. 100 kg (Table 2). Our dataset for M. pascuali includes two small specimens (i.e., juveniles; after Abdala and Giannini 2000) with bm estimations between 1.80 and 1.88 kg, and a third subadult specimen (mandibular length ca. 125 mm) with an estimation of 6 kg (Table 1). Following geometric similarity results, the largest adult of M. pascuali should have a bm 17–40 kg (Table 2). These results are consistent with bm estimations by Mancuso et al. (2014) based on skull length (30.74 kg for a skull length of 182.5 mm) and by Fahn-Lai et al. (2020) for a juvenile (91 mm of skull length) with an estimated bm of 1.44 kg, obtained after equations of humerus and femur circumferences of Campione and Evans (2012). As for A. mendozensis, using geometric similarity for the largest specimen (with a 137 mm skull length) we find a bm estimation of 2.7 kg (Table 2), which is more than four times lower than the 12.5 kg estimated by Hendrickx et al. (2020). Finally, P. polanskii had the most consistent estimations with geometric similarity, given that the size of the skull of the largest specimen (124 mm) is very close to that of the specimen used in this study (120 mm), with a bm of near 3.7 kg (Table 2) for the largest form. This value also differs from the estimation of 9.22 kg obtained by Hendrickx et al. (2020), using the formula of Van Valkenburgh et al. (1990) based on the skull length of extant carnivores.
The specimens studied here are mainly juveniles or subadults (see above and material and methods section), so the obtained mean values do not represent the body mass range from known adult specimens. Using the geometric similarity (i.e., assuming no allometric scaling) we were able to propose a body mass for each taxon based on the skull length of the largest known specimen. In some cases, the proposed body mass is concordant with previously published estimations based on skull length (i.e., M. pascuali; Mancuso et al. 2014), but not for small taxa (i.e., P. polanskii and A. mendozensis; Hendrickx et al. 2020). It should be noted that NMC are characterized by a robust skull with large zygomatic areas compared to the skulls of mammals, so a similar shape is not represented in extant species used to build the equations. In turn, the smaller NMC have a large and robust head in comparison to the body (skull length 80–130 mm), while the femur and humerus are very slender and short (diameter 3–7 mm and length 35–60 mm; see SOM 1: tables S3 and S4). This may explain the differences observed between estimations based on skull length and those based on postcranial elements.
Variations within WM of bm estimations for the same individual are common when different structures skeletal elements are considered (see Fariña et al. 1998). These variations in body mass estimations of the same individual are probably related to differences in shape between humerus and femur in NMC. Two main characteristics in cynognathians (and in fact most NMC) are the broad expansion of the epicondyles in the distal humerus, which is not present in the femur, and the presence of a large and projected deltopectoral flange that extends for more than half the length of the humerus (Jenkins 1970; Bonaparte 1963a; Abdala 1999; Fig. 3). In most cynognathians, these features could modify the antero-posterior diameter, but do not affect much the general slender shape of the diaphysis. However, in E. argentinus, the diaphysis is shorter and broader, with a robust dectopectoral flange that extends for almost 2/3 of the humeral length (Bonaparte 1963a; Abdala 1999), notably increasing the lateromedial diameter.
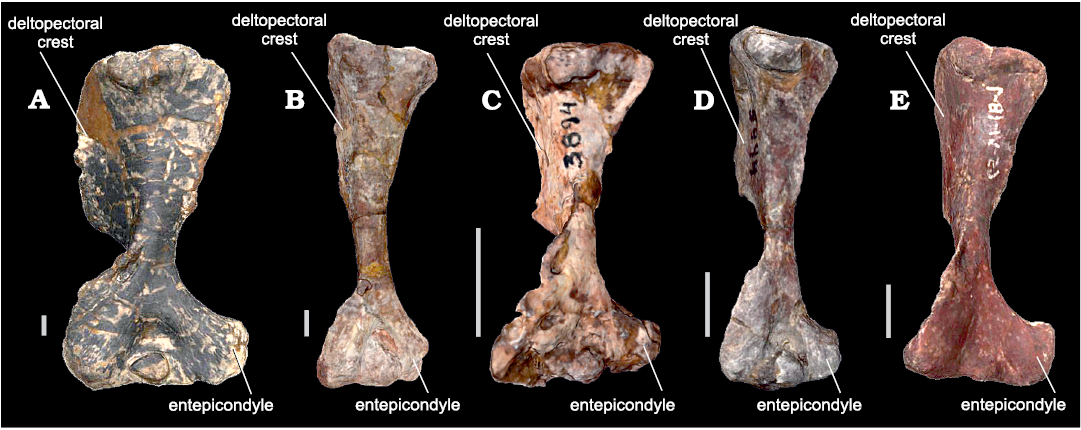
Fig. 3. Cynognathians studied in this work (all in anterior views). A. Left humerus of Exaeretodon argentinus Cabrera, 1943 (PVL 2554) from the Ischigualasto Formation (upper Carnian), Hoyada de Ischigualasto, San Juan, Argentina. B. Right humerus (mirrored) of Cynognathus crateronotus Seeley, 1895 (PVL 3859) from the Río Seco de la Quebrada Formation (lower Carnian), Puesto Viejo, Mendoza province, Argentina. C. Left humerus of Andescynodon mendozensis Bonaparte, 1969 (PVL 3894-1) from the Cerro de las Cabras Formation (upper Anisian), Villa de Potrerillos, Mendoza province, Argentina. D. Left humerus of Massetognathus pascuali Romer, 1967 (PVL 5444) from the Chañares Formation (lower Carnian), Campo de Talampaya, La Rioja province, Argentina. E. Right humerus (mirrored) from Pascualgnathus polanskii Bonaparte, 1966 (MLP 65-VI-18-1) from the Río Seco de la Quebrada Formation (lower Carnian), Puesto Viejo, Mendoza province, Argentina. Scale bars 10 mm.
These anatomical characteristics of the stylopodium are related to the position-orientation of the limbs in relation to the longitudinal body axis that determines the body stance in NMC. The forelimb posture is intermediate between classical “sprawling” and “parasagittal” limb postures, with the humerus directed posterolaterally from the body wall (Jenkins 1970; Lai et al. 2018). In contrast, the hind limb had a more or less fully mammalian mode of locomotion, a parasagittal gait, where the femur has a bulbous, inturned head and very prominent mammal-like trochanter major on the posterolateral part of the femoral head (Kemp 2005; Liu et al. 2017). Therefore, the femur is directed more anteriorly and closer to the body than the humerus. A workable solution for the limbs of NMC with differences in postural stance, and therefore different bm support in fore- and hind limbs, is to combine both stylopodial measurements in the same formula to calculate bm estimation, especially bone circumference, which is not correlated with limb posture and gait (Campione and Evans 2012). However, the humerus and femur are rarely represented in the same individual; for example, only five specimens, a third of our sample, preserved both stylopodia. For these five specimens, the estimated values from regressions of Campione and Evans (2012) lie between those obtained using only humerus or femur.
Even when comparing the estimation values obtained from different bm equations based on circumference measurements, either based on both elements (Campione and Evans 2012 equations) or on a single element (equations obtained in this work), no significant differences are found between them (see SOM 1: tables S6, S7, and S8). We propose that in order to increase the sample size, or when not having both stylopodial elements from a single specimen, it is advisable to use both circumferences and other measurements of the stylopodial element to calculate several bm estimations and report a weighted mean.
According to the adopted scale of body mass ranges (Cassini et al. 2012a, b; Toledo et al. 2014), and considering maximum body size only for the Argentinean record, E. argentinus is the only large-sized form, M. pascuali and C. crateronotus (the latter only known in Argentina by one juvenile-subadult specimen) are medium-sized, and A. mendozensis, and P. polanskii are small-sized (Fig. 4).
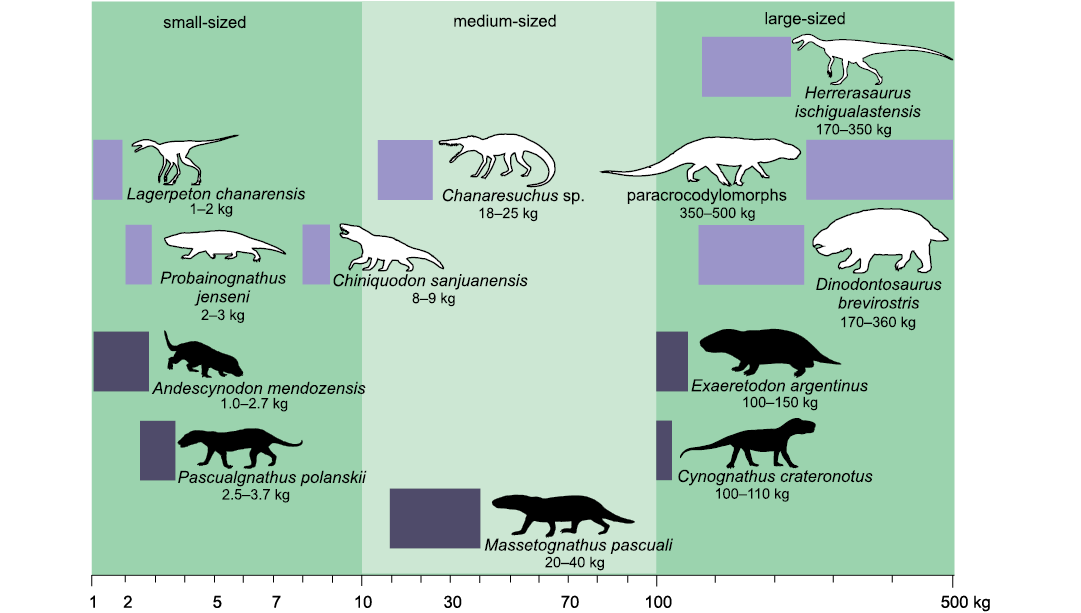
Fig. 4. Logarithmic scale representation of the body mass ranges of adult forms of amniotes Chañares and Ischigualasto formations, Triassic of Argentina. Body masses for Cynognathia obtained in this work (black silhouettes) compared with other amniotes known from these formations (white silhouettes). Cynodonts Chiniquodon sanjuanensis Martínez and Forster, 1996, and Probainognathus jenseni Romer, 1970; the dicynodont Dinodontosaurus brevirostris Cox, 1968; and archosauriforms Lagerpeton chanarensis Romer, 1971a, Chanaresuchus sp. (includes C. bonapartei Romer, 1971b, and C. ischigualastensis Trotteyn, Martínez and Alcober, 2012), paracrocodylomorphs and the dinosaur Herrerasaurus ischigualastensis Reig, 1963. The horizontal length of the rectangles represents the body mass range for the genus. The silhouettes are not to scale.
Palaeobiology of Triassic traversodontids
Traversodontid cynodont size and geological age.—Considering the traversodontid record of Argentina analysed here, the late Anisian Andescynodon mendozensis is small-sized, the early Carnian Massetognathus pascuali is medium-sized and the late Carnian–early Norian Exaeretodon argentinus is large-sized (Fig. 4). The temporal placement of the small Pascualgnathus polanskii is problematic as the Rio Seco de la Quebrada Formation, traditionally considered as Early to Middle Triassic in age, was dated as early Carnian (early Late Triassic) using the SHRIMP 238U/206Pb method (Ottone et al. 2014), suggesting that this unit was contemporaneous with the Chañares Formation. This dating, however, has not been universally accepted (e.g., Schneider et al. 2020). The base-10 log-transformed maximum body mass (kg) (Table 2) was negatively correlated with the first appearance time (Ma) of traversodontids (r -0.9523; p-value 0.04761). This result is also true for base-10 log-transformed skull lengths (r -0.9782; p-value 0.02178). For these correlations, P. polanskii was considered Anisian, but an interpretation of this taxon as Early Carnian results in traversodontid geological age not being correlated with increasing size. In any case, E. argentinus, the largest traversodontid in Argentina, is also the youngest.
In order to explore the relationship between age and traversodontid size from Triassic faunas outside Argentina, for which there are no body mass estimations available based on limb bone measurements, we will use skull length as a proxy for size.
Two of the three youngest Brazilian traversodontids, Exaeretodon riograndensis (BSL of 263 mm; Abdala et al. 2002) and Siriusgnathus niemeyerorum (BSL of 300 mm; Miron et al. 2020), are also large, with the latter taxon being the largest Brazilian traversodontid. However, we did not obtain a trend between size increase and time in Brazilian faunas (r 0.2891; p-value 0.4179), due to several early Carnian traversodontids with skull lengths of 200 mm or more. This is the case for Santacruzodon hopsoni (Melo et al. 2022), Traversodon stahleckeri, and Massetognathus (Barberena 1981; Liu et al. 2008), although in the latter, the only two really large-sized specimens (i.e., above BSL of 250 mm) previously identified as Massetognathus ochagaviae (Liu et al. 2008) were re-identified as belonging to the probainognathian Aleodon (Martinelli et al. 2017).
The youngest African traversodontid, Scalenodontoides macrodontes (BSL of 280 mm; Battail 2005), from the Norian of South Africa, is also the largest from Africa (Gow and Hancox 1993; Battail 2005); there is a general trend to size increase with younger ages, but it is not significant (r 0.6009; p-value 0.08702). Finally, traversodontids have a limited record in Laurasia, only known from isolated teeth in the Norian–Rhaetian of Europe, suggesting small-size taxa (Hendrickx et al. 2020), and represented by two Carnian taxa and one Norian taxon in North America. Arctotraversodon plemmyridon, one of the Carnian taxa, is the largest traversodontid from Laurasia (Hopson 1984; Sues and Olsen 2015), whereas the other two species, including the youngest Norian species, are relatively small.
Therapsid and amniote size and age in the Argentinean Triassic.—Large-sized therapsids are best represented in the Ischigualasto fauna, the youngest fauna analysed here (Fig. 5A). It is imperative to mention that we only obtained estimation sizes for specimens recorded in Argentina. This is especially important when considering the fauna from the Rio Seco de la Quebrada Formation, where Cynognathus crateronotus and Diademodon tetragonus are represented by medium-sized specimens, while these taxa have much larger representatives in Africa (Abdala 1996; Martinelli et al. 2009).
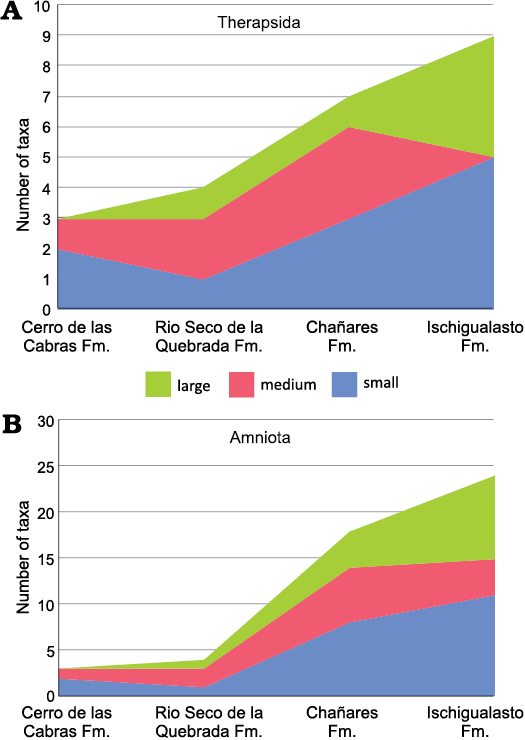
Fig. 5. Stacked area chart of animal size (after values of the skull length for Therapsida and skull or limb bone lengths, when skull is not preserved, for Archosauromorpha) from the Argentinean Triassic units: Cerro de La Cabras, Río Seco de la Quebrada, Chañares, and Ischigualasto formations. A. Therapsida (Cynodontia plus Dicynodontia). B. Amniota (Therapsida plus Archosauromorpha). Small, maximum skull length below 150 mm; medium, skull length 150–250 mm; large, maximum skull length greater than 250 mm. Fm., Formation.
Considering the Argentinean Triassic record for all amniotes, two small- and one medium-sized taxa are known from the Cerro de las Cabras Formation, whereas one large species is represented in the Rio Seco de la Quebrada Formation (Fig. 5B). A remarkable difference between the faunas of the Chañares and Ischigualasto formations is expressed in a notable increase of the number of taxa with a large skull size in the Ischigualasto, a trend also reflected in therapsids (Fig. 5A) and traversodontid cynodonts (see above). Comparing the Chañares and Ischigualasto faunas, the number of large taxa is multiplied by two in the latter unit, reflecting an increase in the number of large-sized species in both archosauromorphs, represented by five species, and therapsids, represented by four species. In addition, there is also a decrease in the number of medium-sized species in the Ischigualasto fauna. In summary, in Ischigualasto, the youngest and most diverse fauna from Argentina analysed here, where the largest traversodontid (i.e., E. argentinus) is documented, is also characterized by a general increase of the number of large amniote species (both therapsids and archosauromorphs).
Morphological variation in traversodontid cynodonts.—Argentinean traversodontids vary in skull morphology and dental features. The small-sized Andescynodon mendozensis and Pascualgnathus polanskii have a generalized skull morphology with a narrow temporal area, long and slender snout, hypertrophied canine, and simple oval or subrectangular gomphodont postcanines lacking shouldering (Liu and Powell 2009; Martinelli 2010; Liu and Abdala 2014). The transverse crest in the upper postcanines is only incipient in these taxa, resulting in the labial and lingual cusps, which are generally integrated in the crest in traversodontids, forming relatively independent structures on the crown (Goñi 1986; Liu and Powell 2009: fig. 7; Martinelli 2010: fig. 3).
Massetognathus is recovered in phylogenies as an intermediate form (member of Massetognathinae) (Hendrickx et al. 2020), with a developed temporal area, enlarged complex incisors, small canines, incipient shouldering in upper postcanines, and high and sharp transverse crest of lower postcanines (Romer 1967, 1972; Crompton 1972). The upper postcanines depict already the classical transverse crest with the addition of a central cusp located between the labial and lingual cusps. The occlusal basins in front of the upper posterior crest and the lower anterior crest of the postcanines are much better defined compared to basal forms of traversodontids, and the incipient shouldering starts to integrate adjacent teeth and makes the whole postcanine dentition more compact (Romer 1967; Hopson 1985). Exaeretodon argentinus displays a very broad zygomatic area, enlarged and procumbent lower incisors, small lower canine integrated in the incisors line, and marked shouldering in upper postcanines, clearly demarcating a lateral and a medial section of the tooth (Bonaparte 1962; Hopson 1984; Abdala et al. 2002). The accentuated shouldering results in more integrated adjacent upper postcanines in the dental series. Upper postcanines in E. argentinus do not form a transverse crest (just mesial and distal walls), and the occlusal basins in the upper postcanines are relatively more developed than in earlier traversodontids. While small forms would have fed selectively, as indicated by their small size and narrow muzzle; the large forms would have been bulk feeders as suggested by their broad muzzle (Jarman 1974; Bro-Jørgensen 2008). It must also be considered that NMC shared their habitat with other herbivorous forms, such as similar sized rhynchosaurs and large-sized dicynodonts, which were the largest animals of the Triassic terrestrial faunas analysed here (Martinez et al. 2012; Mancuso et al. 2014; SOM 2). In extant mammalian assemblages, coexistence of many herbivores implies partition of vegetation as food resources (Hirst 1975), and one of the attributes for niche differentiation is size (Jarman 1974; Owen-Smith 1988).
Another aspect to be considered is the possibility of a generalist diet for some of the gomphodont species. Small animals have a higher basal metabolic rate per gram than larger animals (Peters 1983; Calder 1996; Brown and West 2000). As we look at the total mass of the animals, larger ones have higher energetic requirements. Plant availability is considerably higher than protein-based food availability. As plant matter has lower protein content, larger quantities of food need to be ingested, and the more plants that need to be ingested, the more intraoral processing is needed (Texera 1974; Janis and Constable 1993; Janis 1995). On the one hand, it is expected that small Argentinean traversodontids fulfil their higher energetic requirements feeding on nutritious items (i.e., a more generalist diet not exclusively on plant material). On the other hand, large traversodontids could fulfil their energetic requirements by consuming a large amount of low nutritious food (e.g., plant material). Thus, when masticatory apparatus morphology and body size of the Argentinean traversodontids are integrated, we see significant variation in their feeding ecology under the changing Triassic climate of western Gondwana (Mancuso et al. 2021) and, more generally, the whole world (Scotese et al. 2021).
Environments and floras in the Argentinean Triassic.—There were intense variations in maximum temperature along the Triassic stages, from an extreme hot period in the earliest Triassic to a moderate hothouse condition switching at the end of the Middle Triassic and being well established in the Late Triassic (Scotese et al. 2021). In the Middle–Late Triassic transition the western Argentine basins were dominated by fluvial systems with flood plains and interfluves with well-developed soils, and partially closed lacustrine systems (Gibling et al. 1998; Spalletti 1997, 2001; Tanner 2000; Spalletti et al. 2003; Mancuso et al. 2021). These environments suggest semiarid conditions developed under a megamonsoonal seasonal climatic regime (dry subtropical) (Dubiel and Smoot 1994; Tanner 2000; Spalletti et al. 2003).
This climatic period coincides with the maximum diversification of the Dicroidium flora (Bomfleur et al. 2018), the second floristic event of the Cortaderitian (Spalleti et al. 2003), and the adaptive radiation of Corystospermaceae, Peltaspermaceae, and Cycadales (Anderson et al. 1999; Spalletti et al. 1999; Zamuner et al. 2001). Plant communities consisted of evergreen forests formed by corystosperms and peltasperms, with secondary elements such as conifers and ferns that characterized the fluvial areas; deciduous forests dominated by conifers and ginkgoales with an understory of ferns and shrubby pteridosperms associated with lacustrine margins; and herbaceous and shrubby vegetation with a dominance of sphenophytes forming cane thickets, gnetales, and pteridosperms growing in flooded lowlands (Spalleti et al. 2003; Artabe et al. 2001). Early Carnian deposits, particularly those of the Chañares Formation, are characterized by tuffaceous sandstones and siltstones deposited in fluvial–lacustrine environments and alluvial fans (Mancuso et al. 2014, 2021), indicating intensive volcanism (Rogers et al. 2001). Intense magmatism by extensional rifting is also interpreted for southwestern Gondwana during the Middle Triassic, which produced adverse microenvironmental conditions as a result of volcanic episodes (Cariglino et al. 2016, 2018).
As mentioned above, the Triassic had a great variety and abundance of plants available for primary consumers. Tied to the acme of the “Dicroidium flora” in Gondwana, this availability most likely triggered the diversification of large amniotes in younger Late Carnian faunas.
According to Pineda-Muñoz et al. (2016), smaller mammals are mainly insectivores, granivores, or mixed feeders, whereas larger animals are usually either carnivores or herbivores, the latter feeding on large volumes of grasses and/or leaves. The shift from a generalist to a more folivorous diet associated with body mass increase (i.e., link between size and energetic efficiency for different diets) was also reported in different mammal lineages (Jarman 1974; Janis 2007; Bro-Jørgensen 2008; and Cassini 2013 for ungulates; Barbero et al. 2020 for sigmodontine rodents; Vizcaíno and Loughry 2008 and Vizcaíno et al. 2011b for xenarthrans). According to previously published data (see for example the discussion of feeding ecology in basal gomphodonts in Hendrickx et al. 2020), we could expect that small-sized traversodontids with a generalized skull morphology had a generalist diet, feeding on leaves, roots, reproductive structures, and possibly insects, whereas the middle- and large-sized traversodontids with specialized skulls for food processing had a herbivorous diet, feeding on herbaceous and shrubby vegetation including pteridosperms, gnetales, and ferns. However, morpho-functional aspects related to dietary preferences in traversodontids remain to be further investigated.
One of our main goals in this contribution was to identify working hypotheses about NMC to be tested using ecomorphological approaches. Some general hypotheses are: (i) Small-sized traversodontids (Pascualgnathus polanskii, Andescynodon mendozensis) have a skull morphology and dentition suggestive of highly nutritious food items (generalist diet); (ii) Medium-sized traversodontids Massetognathus pascuali have a skull morphology indicative of a generalized herbivorous diet; and (iii) large-sized Exaeretodon argentinus have optimized craniodental features related to maximizing oral food processing of large amounts of low-nutrient food items (specialized herbivorous diet).
Conclusions
The equations with a higher taxonomic (and therefore morphological) representation of amniotes turned out to be the best body mass estimators of extinct animals with no modern relatives, such as cynognathians. The inclusion of all the values from all the considered equations in the weighted mean compensates for potential over- and underestimations in individual values.
There are different degrees of congruence between bm estimations for species having specimens with both the humerus and femur. In Pascualgnathus polanskii the estimation values based on the humerus and femur equations are close; in Andescynodon mendozensis values from the femur equations are higher; and in Massetognathus pascuali and Exaeretodon argentinus bm estimations obtained from the femur equations are lower. These variations in body mass estimations for a single individual are probably related to differences in shape between the humerus and femur in cynodonts due to limb posture. The forelimb posture is intermediate between “sprawling” and “parasagittal”, whereas the hind limbs have a parasagittal limb posture. A solution to this particular issue is to gauge bm estimations based on both stylopodial elements using circumference measurements, which are not correlated with limb posture and gait. However, when both elements from the same individual are not available, we propose the use of several equations and calculate a weighted mean.
Some previous bm estimations based on skull length measurements are greater than estimations obtained here after measurements of postcranial elements. These differences could be related to the anatomy of cynodonts, which have large and robust skulls compared to the body, and very short and slender humeri and femora.
The “small-sized NMC” group (1–10 kg) includes A. mendozensis and P. polanskii, with a bm range 1–3.7 kg. The “medium-sized NMC” group (10–100 kg) includes M. pascuali with a bm range 17–40 kg. The “large-sized NMC” group (above 100 kg) includes E. argentinus from Argentina and Cynognathus crateronotus, whose largest specimens are known from South Africa, with a bm range of 100–154 kg. The only known C. crateronotus specimen from Argentina is interpreted as a subadult and its bm is estimated at 20 kg.
Traversodontids show a tendency to increase in body size through the Triassic in Argentinean faunas and reach their largest body sizes at the time of the acme of the “Dicroidium flora”. The largest traversodontids are also the youngest in Brazil and African Triassic faunas, but a size increase trend through time is not supported by our data. The morphological variations in the skull and the different body sizes observed between traversodontids are interpreted as reflecting different types of diets, such as a generalist diet (i.e., not exclusively plant material), generalized hervibory, and specialist hervibory.
This contribution presents the first results of a research project aimed at specifically exploring bm in NMC. As previously mentioned, the skull and mandibles comprise the most abundant skeletal elements for Triassic cynognathians, and are the elements providing the most information on size variation in the group (and NMC in general). Hence, a morphogeometric approach under development, using specifically cranial data for bm estimations, will allow for a better comparison within a more inclusive sample. This approach will enable a significant enlargement of the studied sample and a comparison with the results presented here.
Acknowledgements
The authors thank the following persons and institutions for access to specimens in their care: Rodrigo González and Pablo Ortiz (Colección Paleontología de Vertebrados Lillo, Universidad Nacional de Tucumán, Argentina) and Marcelo Reguero (Museo de La Plata, Universidad Nacional de La Plata, Argentina). We wish to acknowledge the assistance provided by Lucinda Backwell (Instituto Superior de Estudios Sociales, Conicet- UNT, Tucumán, Argentina) with the English and insightful comments by Jun Liu (Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China) and an anonymous reviewer. This research was financed by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) doctoral grant to FSF; projects PUE 0098 to the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, PICT 2016-2665 Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and CDD-CB 014/19 Universidad Nacional de Lujan. FA is financed by CONICET and the National Research Foundation of South Africa.
References
Abdala, F. 1996. Redescripción del cráneo y reconsideración de la validez de Cynognathus minor (Eucynodontia–Cynodonthidae) del Triásico Inferior de Mendoza. Ameghiniana 33: 115–126.
Abdala, F. 1999. Elementos postcraneanos de Cynognathus (Synapsida–Cynodontia) del Triásico Inferior de la provincia de Mendoza, Argentina. Consideraciones sobre la morfología del humero en cinodontes. Revista Española de Paleontología 14: 13–24. Crossref
Abdala, F. 2021. Permo-Jurassic cynodonts: the early road to mammalness. Reference Module in Earth Systems and Environmental Sciences. [published online, htpps://doi.org/10.1016/B978-0-12-409548-9.12020-2] Crossref
Abdala, F. and Giannini, N.P. 2000. Gomphodont cynodonts of the Chañares Formation: the analysis of an ontogenetic sequence. Journal of Vertebrate Paleontology 20: 501–506. Crossref
Abdala, F., Barberena, M.C., and Dornelles, J. 2002. A new species of the traversodontid cynodont Exaeretodon from the Santa Maria Formation (Middle/Late Triassic) of southern Brazil. Journal of Vertebrate Paleontology 22: 313–325. Crossref
Abdala, F., Gaetano, L.C., Martinelli, A.G., Soares, M.B., Hancox, P.J., and Rubidge, B.S. 2020. Non-mammaliaform cynodonts from western Gondwana and the significance of Argentinean forms in enhancing understanding of the group. Journal of South American Earth Sciences 104: 102884. Crossref
Anderson, J.M., Anderson, H.M., Archangelsky, S., Bamford, H., Chandra, S., Dettmann, M., Hill, R., McLoughlin, S., and Rosler, O. 1999. Patterns of Gondwana plant colonization and diversification. Journal of African Earth Sciences 28: 145–167. Crossref
Andrews, P., Lord, J.M., and Evans, E.M.N. 1979. Patterns of ecological diversity in fossil and modern mammalian faunas. Biological Journal of the Linnean Society 11: 177–205. Crossref
Angielczyk, K.D. and Kammerer, C.F. 2018. 5. Non-mammalian synapsids: the deep roots of the mammalian family tree. In: F. Zachos and R. Asher (eds.), Mammalian Evolution, Diversity and Systematics, 117–198. De Gruyter, Berlin. Crossref
Anyonge, W. 1993. Body mass in large extant and extinct carnivores. Journal of Zoology 231: 339–350. Crossref
Artabe, A.E., Morel, E.M., and Spalletti, L.A. 2001. Paleoecologia de las floras triásicas argentinas. In: A.E. Artabe, E.M. Morel, and A.B. Zamuner (eds.), El Sistema Triásico en la Argentina, 199–225. Fundación Museo de La Plata “Francisco Pascasio Moreno”, La Plata.
Barberena, M.C. 1981. Uma nova espécie de Massetognathus (Massetognathus ochagaviae, sp. nov.) da Formação Santa Maria, Triássico do Rio Grande do Sul. Pesquisas 14: 181–195. Crossref
Barbero, S., Teta, P., and Cassini, G.H. 2020. An ecomorphological comparative study of extant and Late Holocene Sigmodontinae (Rodentia, Cricetidae) assemblages from central-eastern Argentina. Journal of Mammalian Evolution 27: 697–711. Crossref
Battail, B. 2005. Late Triassic traversodontids (Synapsida, Cynodontia) in South Africa. Palaeontologia Africana 41: 67–80.
Biknevicius, A.R., McFarlane, A.D., and MacPhee, R.D.E. 1993. Body size in Amblyrhiza inundata (Rodentia: Caviomorpha), an extinct megafaunal rodent from the Anguilla Bank, West Indies: estimates and implications. American Museum Novitates 3079: 1–25.
Bomfleur, B., Blomenkemper, P., Kerp, H., and McLoughlin, S. 2018. Polar regions of the Mesozoic–Paleogene greenhouse world as refugia for relict plant groups. In: M. Krings, C.J. Harper, N.R. Cúneo, and G.W. Rothwell (eds.), Transformative paleobotany, 593–611. Academic Press, London. Crossref
Bonaparte, J.F. 1962. Descripción del cráneo y mandíbula de Exaeretodon frenguellii, Cabrera, y su comparación con Diademodontidae, Tritylodontidae y los cinodontes sudamericanos. Publicaciones del Museo Municipal de Ciencias Naturales y Tradicional de Mar del Plata 1: 135–202.
Bonaparte, J.F. 1963a. Descripción del esqueleto postcraneano de Exaeretodon (Cynodontia–Traversodontidae). Acta Geologica Lilloana 4: 5–53.
Bonaparte, J.F. 1963b. Un nuevo cinodonte gonfodonte del Triásico Medio Superior de San Juan, Proexaeretodon vincei n. gen., n. sp. (Cynodontia–Traversodontidae). Acta Geológica Lilloana 4: 129–133.
Bonaparte, J.F. 1966. Una nueva “fauna” Triásica de Argentina. (Therapsida: Cynodontia–Dicynodontia). Consideraciones filogenéticas y paleobiogeográficas. Ameghiniana 4: 243–296.
Bonaparte, J.F. 1969a. Cynognathus minor n. sp. (Therapsida–Cynodontia), nueva evidencia de la vinculación faunística afro-sudamericana a principios del Triásico. Gondwana Stratigraphy, I.U.G.S. Coloquio Mar del Plata 1967 (2): 273–281.
Bonaparte, J.F. 1969b. Dos nuevas “faunas” de reptiles triásicos de Argentina. Gondwana Stratigraphy, I.U.G.S., Coloquio Mar del Plata 2: 283–302.
Brown, J.H. and West, G.B. 2000. Scaling in Biology. 352 pp. Oxford University Press, New York.
Bro-Jørgensen, J. 2008. Dense habitats selecting for small body size: a comparative study on bovids. Oikos 117: 729–737. Crossref
Cabrera, A. 1943. El primer hallazgo de terápsidos en la Argentina. Notas del Museo de La Plata 8: 317–331.
Calder, W.A. 1996. Size, Function, and Life History. 431 pp. Dover Publications, New York.
Candela, A.M., Cassini, G.H., and Nasif, N.L. 2013. Fractal dimension and cheek teeth crown complexity in the giant rodent Eumegamys paranensis. Lethaia 46: 369–377. Crossref
Campione, N.E. and Evans, D.C. 2012. A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biology, 10:60. Crossref
Cariglino, B., Monti, M., and Zavattieri, A.M. 2018. A Middle Triassic macroflora from southwestern Gondwana (Mendoza, Argentina) with typical northern hemisphere elements: biostratigraphic, palaeogeographic and palaeoenvironmental implications. Review of Palaeobotany and Palynology 257: 1–18. Crossref
Cariglino, B., Zavattieri, A.M., Gutiérrez, P.R., and Balarino, M.L. 2016. The paleobotanical record of the Triassic Cerro de las Cabras Formation at its type locality, Potrerillos, Mendoza (Uspallata Group): an historical account and first record of fossil flora. Ameghiniana 53: 184–204. Crossref
Cassini, G.H. 2013. Skull geometric morphometrics and paleoecology of Santacrucian (late early Miocene; Patagonia) native ungulates (Astrapotheria, Litopterna, and Notoungulata). Ameghiniana 50: 193–216. Crossref
Cassini, G.H., Cerdeño, M.E., Villafañe, A.L., and Muñoz, N.A. 2012a. Paleobiology of Santacrucian native ungulates (Meridiungulata: Astrapotheria, Litopterna and Notoungulata). In: S.F. Vizcaíno, R. Kay, and M.S. Bargo (eds.), Early Miocene Paleobiology in Patagonia: High-latitude Paleocommunities of the Santa Cruz Formation, 243–286. Cambridge University Press, Cambridge. Crossref
Cassini G.H., Mendoza M., Vizcaíno S.F., and Bargo, M.S. 2011. Inferring habitat and feeding behaviour of early Miocene notoungulates from Patagonia. Lethaia 44 (2): 153–165. Crossref
Cassini, G.H., Vizcaíno, S.F., and Bargo, M.S. 2012b. Body mass estimation in Early Miocene native South American ungulates: a predictive equation based on 3D landmarks. Journal of Zoology 287: 53–64. Crossref
Christiansen, P. and Harris, J.M. 2005. Body size of Smilodon (Mammalia: Felidae). Journal of Morphology 266: 369–384. Crossref
Cohen, Y. and Cohen, J.Y. 2008. Statistics and Data with R: An Applied Approach Through Examples. First Edition, 618 pp. John Wiley and Sons, Chichester, West Sussex.
Cox, C.B. 1968. The Chañares (Argentina) Triassic reptile fauna. IV. The dicynodont fauna. Breviora 295: 1–27.
Crompton, A.W. 1972. Postcanine occlusion in cynodonts and tritylodontids. Bulletin of the British Museum (Natural History), Geology 21: 29–71.
Damuth, J. and MacFadden, B.J. 1990. Introduction: body size and its estimation. In: J. Damuth and B.J. MacFadden (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 1–10. Cambridge University Press, Cambridge.
De Esteban-Trivigno, S., Mendoza, M., and De Renzi, M. 2008. Body mass estimation in Xenarthra: A predictive equation suitable for all quadrupedal terrestrial placentals. Journal of Morphology 269: 1276–1293. Crossref
Dubiel, R.F. and Smoot, J.P. 1994. Criteria for interpreting paleoclimate from red beds—a tool for Pangean reconstructions. In: A.F. Embry, B. Beauchamp, and D.J. Glass (eds.), Pangea: Global Environments and Resources. Canadian Society of Petroleum Geologists, Memoir 17: 295–310.
Ercoli, M.D. and Prevosti, F.J. 2011. Estimación de masa de las especies de Sparassodonta (Mammalia, Metatheria) de la edad Santacrucense (Mioceno temprano) a partir del tamaño del centroide de los elementos apendiculares: inferencias paleoecológicas. Ameghiniana 48: 462–479. Crossref
Fahn-Lai P., Biewener A.A., and Pierce, S.E. 2020. Broad similarities in shoulder muscle architecture and organization across two amniotes: implications for reconstructing non-mammalian synapsids. PeerJ 8: e8556 [published online, https://doi.org/10.7717/peerj.8556]. Crossref
Fariña, R.A., Vizcaíno, S.F., and Bargo, M.S. 1998. Body size estimations in Lujanian (Late Pleistocene–Early Holocene of South America) mammal megafauna. Mastozoología Neotropical 5: 87–108.
Farlow, J.O., Hurlburt, G.R., Elsey, R.M., Britton, A.R.C., and Langston, W. Jr. 2005. Femoral dimensions and body size of Alligator mississippiensis: estimating the size of extinct mesoeucrocodylians. Journal of Vertebrate Paleontology 25: 354–369. Crossref
Figueirido, B., Pérez-Claros, J.A., Hunt, R.M. Jr., and Palmqvist, P. 2011. Body mass estimation in amphicyonid carnivoran mammals: a multiple regression approach from the skull and skeleton. Acta Palaeontologica Polonica 56: 225–246. Crossref
Gibling, M.R., Nanson, G.C., and Maroulis, J.C. 1998. Anastomosing river sedimentation in the Channel Country of Central Australia. Sedimentology 45: 595–619. Crossref
Gingerich, P.D. 1990. Prediction of body mass in mammalian species from long bone lengths and diameters. Contributions from the Museum of Paleontology, University of Michigan 38: 79–92.
Goñi, R. 1986. Reemplazo de dientes postcaninos en Andescynodon mendozensis Bonaparte (Cynodontia, Traversodontidae). Actas del IV Congreso Argentino de Paleontología y Bioestratigrafía, Mendoza 2: 7–14.
Gow, C.E. and Hancox, P.J. 1993. First complete skull of the Late Triassic Scalenodontoides (Reptilia, Cynodontia) from southern Africa. New Mexico Museum of Natural History and Science Bulletin 3: 161–168.
Hendrickx, C., Gaetano, L.C., Choiniere, J.N., Mocke, H., and Abdala, F. 2020. A new traversodontid cynodont with a peculiar postcanine dentition from the Middle/Late Triassic of Namibia and dental evolution in basal gomphodonts. Journal of Systematic Palaeontology 18: 1669–1706. Crossref
Hirst, S.M. 1975. Ungulate-habitat relationships in South African woodland/savanna ecosystem. Wildlife Monographs 44: 1–60.
Hopkins, S.S.B. and Davis, E.B. 2009. Quantitative morphological proxies for fossoriality in small mammals. Journal of Mammalogy 90: 1449–1460. Crossref
Hopson, J.A. 1984. Late Triassic traversodont cynodonts from Nova Scotia and southern Africa. Palaeontologia Africana 25: 181–201.
Hopson, J.A. 1985. Morphology and relationships of Gomphodontosuchus brasiliensis von Huene (Synapsida, Cynodontia, Tritylodontoidea) from the Triassic of Brazil. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1985 (5): 285–299. Crossref
Hopson, J.A. 2014. The traversodontid cynodont Mandagomphodon hirschsoni from the Middle Triassic of the Ruhuhu Valley, Tanzania. In: C.F. Kammerer, K.D. Angielczyk, and J. Fröbisch (eds.), Early Evolutionary History of the Synapsida, 233–253. Springer, Dordrecht. Crossref
Janis, C.M. 1995. Correlations between craniodental morphology and feeding behavior in ungulates: reciprocal illumination between living and fossil taxa. In: J. Thomason (ed.), Functional Morphology in Vertebrate Palaeontology, 76–98. Cambridge University Press, Cambridge.
Janis, C.M. 2007. Artiodactyl paleoecology and evolutionary trends. In: D.R. Prothero and S.E. Foss (eds.), The Evolution of Artiodactyls, 292–302. John Hopkins University Press, Baltimore.
Janis, C.M. and Constable, E. 1993. Can ungulate craniodental features determine digestive physiology? Journal of Vertebrate Paleontology 13: 43A.
Jarman, P.J. 1974. The social organization of antelope in relation to their ecology. Behaviour 48: 215–267. Crossref
Jenkins, F.A. Jr. 1970. The Chañares (Argentina) Triassic reptile fauna VII. The postcranial skeleton of the traversodontid Massetognathus pascuali (Therapsida, Cynodontia). Breviora 352: 1–28.
Kay, R.F., Vizcaíno, S.F., Bargo, M.S., Spradley, J.P., and Cuitiño, J.I. 2021. Paleoenvironments and Paleoecology of the Santa Cruz Formation (Early–Middle Miocene) along the Río Santa Cruz, Patagonia. Journal of South American Earth Sciences 109: 103296. Crossref
Kemp, T.S. 2005. The Origin and Evolution of Mammals. 331 pp. Oxford University Press, Oxford. Crossref
Lai, P.H., Biewener, A.A., and Pierce, S.E. 2018. Three-dimensional mobility and muscle attachments in the pectoral limb of the Triassic cynodont Massetognathus pascuali (Romer, 1967). Journal of Anatomy 232: 383–406. Crossref
Liu, J. and Abdala, F. 2014. Phylogeny and taxonomy of the Traversodontidae. In: C.F. Kammerer, K.D. Angielczyk, and J. Fröbisch (eds.), Early Evolutionary History of the Synapsida, 255–279. Springer, Dordrecht. Crossref
Liu, J. and Powell, J. 2009. Osteology of Andescynodon (Cynodontia, Traversodontidae) from the Middle Triassic of Argentina. American Museum Novitates 3674: 1–19. Crossref
Liu, J., Schneider, V.P., and Olsen, P.E. 2017. The postcranial skeleton of Boreogomphodon (Cynodontia: Traversodontidae) from the Upper Triassic of North Carolina, USA and the comparison with other traversodontids. PeerJ 5: e3521. Crossref
Liu, J., Soares, M.B., and Reichel, M. 2008. Massetognathus (Cynodontia, Traversodontidae) from the Santa Maria Formation of Brazil. Revista Brasileira de Paleontologia 11: 27–36. Crossref
Mancuso, A.C., Gaetano, L.C., Leardi, J.M., Abdala, F., and Arcucci, A.B. 2014. The Chañares Formation: a window to a Middle Triassic tetrapod community. Lethaia 47: 244–265. Crossref
Mancuso, A.C., Horn, B.L.D., Benavente, C.A., Schultz, C.L., and Irmis, R.I. 2021. The paleoclimatic context for South American Triassic vertebrate evolution. Journal of South American Earth Sciences 110: 10321. Crossref
Martin, T. 2018. Mesozoic mammals—early mammalian diversity and ecomorphological adaptations. In: F.E. Zachos and R.J. Asher (eds.), Mammalian Evolution, Diversity, and Systematics, 199–299. De Gruyter, Berlin. Crossref
Martinelli, A.G. 2010. On the postcanine dentition of Pascualgnathus polanskii Bonaparte (Cynodontia, Traversodontidae) from the Middle Triassic of Argentina. Geobios 43: 629–638. Crossref
Martinelli, A.G., de la Fuente, M., and Abdala, F. 2009. Diademodon tetragonus Seeley, 1894 (Therapsida: Cynodontia) in the Triassic of South America and its biostratigraphic implications. Journal of Vertebrate Paleontology 29: 852–862. Crossref
Martinelli, A.G., Kammerer, C.F., Melo, T.P., Neto, V.D.P., Ribeiro, A.M., Da-Rosa, A.A., Schultz, C.L., and Soares, M.B. 2017. The African cynodont Aleodon (Cynodontia, Probainognathia) in the Triassic of southern Brazil and its biostratigraphic significance. PLoS ONE 12: e0177948. Crossref
Martínez, R.N. and Forster, C.A. 1996. The skull of Probelesodon sanjuanensis, sp. nov., from the Late Triassic Ischigualasto Formation of Argentina. Journal of Vertebrate Paleontology 16: 285–291. Crossref
Martínez, R.N., Apaldetti, C., Alcober, O., Colombi, C.E., Sereno, P.C., Fernández, E., Santi Malnis, P., Correa, G.A., and Abelin, D. 2012. Vertebrate succession in the Ischigualasto Formation. Journal of Vertebrate Paleontology 32: 10–30. Crossref
Melo, T.P., Martinelli, A.G., and Soares, M.B. 2022. New occurrences of massetognathine traversodontids and chiniquodontids (Synapsida, Cynodontia) from the early Late Triassic Santacruzodon Assemblage Zone (Santa Maria Supersequence, southern Brazil): Geographic and biostratigraphic implications. Journal of South American Earth Sciences 115 [published online, https://doi.org/10.1016/j.jsames.2022.103757]. Crossref
Mendoza, M., Janis, C.M., and Palmqvist, P. 2006. Estimating the body mass of extinct ungulates: a study on the use of multiple regression. Journal of Zoology 270: 90–101. Crossref
Millien, V. 2008: The largest among the smallest: the body mass of the giant rodent Josephoartigasia monesi. Proceedings of the Royal Society B 275: 1953–1955. Crossref
Millien, V. and Bovy, H. 2010. When teeth and bones disagree: body mass estimation of a giant extinct rodent. Journal of Mammalogy 91: 11–18. Crossref
Miron, L.R., Pavanatto, A.E.B., Pretto, F.A., Müller, R.T., Dias-da-Silva, S., and Kerber, L. 2020. Siriusgnathus niemeyerorum (Eucynodontia: Gomphodontia): the youngest South American traversodontid? Journal of South American Earth Sciences 97: 102394. Crossref
Otero, A., Moreno, A.P., Falkingham, P.L., Cassini, G., Ruella, A., Militello, M., and Toledo, N. 2020. Three-dimensional image surface acquisition in vertebrate paleontology: a review of principal techniques. Publicación Electrónica de la Asociación Paleontológica Argentina 20: 1–14. Crossref
Ottone, E.G., Monti, M., Marsicano, C.A., de la Fuente, M.S., Naipauer, M., Armstrong, R., and Mancuso, A.C. 2014. A new Late Triassic age for the Puesto Viejo Group (San Rafael depocenter, Argentina): SHRIMP U-Pb zircon dating and biostratigraphic correlations across southern Gondwana. Journal of South American Earth Sciences 56: 186–199. Crossref
Owen-Smith, N. 1988. Megaherbivores. The Influence of Very Large Body Size on Ecology. 388 pp. Cambridge University Press, Cambridge Crossref
Peecook, B.R., Steyer, J.S., Tabor, N.J., and Smith, R.M.H. 2018. Updated geology and vertebrate paleontology of the Triassic Ntawere Formation of northeastern Zambia, with special emphasis on the archosauromorphs. In: C.A. Sidor and S.J. Nesbitt (eds.), Vertebrate and Climatic Evolution in the Triassic Rift Basins of Tanzania and Zambia. Society of Vertebrate Paleontology Memoir 17. Journal of Vertebrate Paleontology 37 (Supplement 6): 8–38. Crossref
Perry, J.M.G., Cooke, S.B., Runestad Connour, J.A., Burgess, M.L., and Ruff, C.B. 2017. Articular scaling and body mass estimation in platyrrhines and catarrhines: modern variation and application to fossil anthropoids. Journal of Human Evolution 115: 20–35. Crossref
Peters, R.H. 1983. The Ecological Implications of Body Size. xii + 329 pp. Cambridge University Press, Cambridge. Crossref
Pineda-Muñoz, S., Evans, A.R., and Alroy, J. 2016. The relationship between diet and body mass in terrestrial mammals. Paleobiology 42: 659–669. Crossref
Polly, P.D. 2007. Limbs in mammalian evolution. In: B.K. Hall (ed.), Fins into Limbs: Evolution, Development, and Transformation, 245–268. University Chicago Press, Chicago.
Radinsky, L.B. 1987. The Evolution of Vertebrate Design. 188 pp. University of Chicago Press, Chicago. Crossref
Reed, K.E. 1998. Using large mammal communities to examine ecological and taxonomic structure and predict vegetation in extant and extinct assemblages. Paleobiology 24: 384–408.
Reig, O.A. 1963. La presencia de dinosaurios saurisquios en los “Estratos de Ischigualasto” (Mesotriásico superior) de las Provincias de San Juan y La Rioja (República Argentina). Ameghiniana 3: 3–20.
Rogers, R.R., Arcucci, A.B., Abdala, F., Sereno, P.C., Forster, C.A. and May, C.L. 2001. Paleoenvironment and taphonomy of the Chañares Formation tetrapod assemblage (Middle Triassic), northwestern Argentina: spectacular preservation in volcanogenic concretions. Palaios 16: 461–481. Crossref
Romer, A.S. 1967. The Chañares (Argentina) Triassic reptile fauna. III. Two new gomphodonts, Massetognathus pascuali and Massetognathus teruggii. Breviora 264: 1–25.
Romer, A.S. 1970. The Chañares (Argentina) Triassic reptile fauna VI: a chiniquodontid cynodont with an incipient squamosal-dentary jaw articulation. Breviora 344: 1–18.
Romer, A.S. 1971a. The Chañares (Argentina) Triassic reptile fauna. X. Two new but incompletely known long-limbed pseudosuchians. Breviora 378: 1–10.
Romer, A. 1971b. The Chañares (Argentina) Triassic reptile fauna. XI. Two new long-snouted thecodonts. Chanaresuchus and Gualosuchus. Breviora 379, 1–22.
Romer, A.S. 1972. The Chañares (Argentina) Triassic reptile fauna. The Chañares gomphodonts. Breviora 396: 1–9.
Schneider, J.W., Lucas, S.G., Scholze, F., Voigt, S., Marchetti, L., Klein, H., Opluštil, S., Werneburg, R., Golubev, V.K., Barrick, J.E., Nemyrovska, T., Ronchi, A., Day, M.O., Silantiev, V.V., Rößler, R., Saber, H., Linnemann, U., Zharinova, V. and Shen, S.-Z. 2020. Late Paleozoic–early Mesozoic continental biostratigraphy—links to the Standard Global Chronostratigraphic Scale. Palaeoworld 29: 186–238. Crossref
Schultz, C.L., Martinelli, A.G., Soares, M.B., Pinheiro, F.L., Kerber, L., Horn, B.L.D., Pretto, F.A., Müller, R.T., and Melo, T.P. 2020. Triassic faunal successions of the Paraná Basin, southern Brazil. Journal of South American Earth Sciences 104: 102846. Crossref
Scotese, C.R., Song, H., Mills, B.J.W., and van der Meer, D.G. 2021. Phanerozoic paleotemperatures: The earth’s changing climate during the last 540 million years. Earth-Science Reviews 215: 103503. Crossref
Scott, K. 1990. Postcranial dimensions of ungulates as predictors of body mass. In: J. Damuth and B.J. MacFadden (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 301–335. Cambridge University Press, Cambridge.
Seeley, H.G. 1895. Researches on the structure, organization, and classification of the fossil Reptilia. On the skeleton in new Cynodontia from the Karroo rocks. Philosophical Transactions of the Royal Society B 186: 59–148. Crossref
Sidor, C.A. and Hopson, J.A. 1998. Ghost lineages and “mammalness”: assessing the temporal pattern of character acquisition in the Synapsida. Paleobiology 24: 254–273.
Spalletti, L.A. 1997. Sistemas deposicionales fluvio-lacustres en el rift Triásico de Malargue (sur de Mendoza, República Argentina). Anales de la Academia Nacional de Ciencias Exactas Fisicas y Naturales 49: 109–124.
Spalletti, L.A. 2001. Modelo de sedimentación fluvial y lacustre en la rampa de un hemigraben: el Triásico de la Precordillera Occidental de San Juan, República Argentina. Revista de la Asociación Geológica Argentina 56: 189–210.
Spalletti, L.A., Artabe, A., and Morel, E. 2003. Geological factors and evolution of southwestern Gondwana Triassic Plants. Gondwana Research 6: 119–134. Crossref
Spalletti, L., Artabe, A., Morel, E.M., and Brea, M. 1999. Biozonación paleoflorística y cronoestratigrafía del Triásico Argentino. Ameghiniana 36: 419–451.
Sues, H.D. and Olsen, P.E. 2015. Stratigraphic and temporal context and faunal diversity of Permian–Jurassic continental tetrapod assemblages from the Fundy rift basin, eastern Canada. Atlantic Geology 51: 139–205. Crossref
Tanner, L.W. 2000. Palustrine-lacustrine and alluvial facies of the (Norian) Owl Rock Formation (Chinle Group), Four Corners Region, southwestern USA: implications for late Triassic paleoclimate. Journal of Sedimentary Research 7: 1280–1289. Crossref
Tarquini, J., Toledo, N., Soibelzon, L.H., and Morgan, C.C. 2017. Body mass estimation for †Cyonasua (Procyonidae, Carnivora) and related taxa based on postcranial skeleton. Historical Biology 30: 496–506. Crossref
Texera, W. 1974. Algunos aspectos de la biología del huemul (Hippocamelus bisulcus) (Mammalia: Artiodactyla, Cervidae) en cautividad. Anales del Instituto de la Patagonia 5: 155–188.
Toledo, N., Cassini, G.H., Vizcaíno, S.F., and Bargo, M.S. 2014. Mass estimation of Santacrucian sloths from the Early Miocene Santa Cruz Formation of Patagonia, Argentina. Acta Palaeontologica Polonica 59: 267–280.
Trotteyn, M.J., Martínez, R.N. and Alcober, O.A. 2012. A new proterochampsid Chanaresuchus ischigualastensis (Diapsida, Archosauriformes) in the early Late Triassic Ischigualasto Formation, Argentina. Journal of Vertebrate Paleontology 32: 485–489. Crossref
Van Valkenburgh, B., Damuth, J., and MacFadden, B.J. 1990. Skeletal and dental predictors of body mass in carnivores. In: J. Damuth and B.J. MacFadden (eds.), Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 181–205. Cambridge University Press, Cambridge.
Vezzosi, R.I. 2012. Tamaño y estimación de la masa corporal en Procariama simplex Rovereto, 1914 (Aves: Phorusrhacidae: Psilopterinae). Ameghiniana 49: 401–408. Crossref
Vizcaíno, S.F. and Bargo, M.S. 2021. Views on the form-function correlation and biological design. Journal of Mammalian Evolution 28(1): 15–22. Crossref
Vizcaíno, S.F. and Loughry, W.J. 2008. Xenarthran biology: past, present and future. In: S.F. Vizcaíno and W.J. Loughry (eds.), The Biology of the Xenarthra, 1–7. University Press of Florida, Gainesville.
Vizcaíno, S.F., Bargo, M.S., Cassini, G.H., and Toledo, N. 2016. Forma y función en paleobiología de vertebrados. 268 pp. Editorial de la Universidad Nacional de La Plata (EDULP), La Plata. Crossref
Vizcaíno, S.F., Bargo, M.S., Kay, R.F., and Milne, N. 2006. The armadillos (Mammalia, Xenarthra, Dasypodidae) of the Santa Cruz Formation (early–middle Miocene): an approach to their paleobiology. Palaeogeography, Palaeoclimatology, Palaeoecology 237: 255–269. Crossref
Vizcaíno, S.F., Blanco, R.E., Bender, J.B., and Milne, N. 2011a. Proportions and function of the limbs of glyptodonts (Mammalia, Xenarthra). Lethaia 44: 93–101. Crossref
Vizcaíno, S.F., Cassini, G.H., Fernicola, J.C., and Bargo, M.S. 2011b. Evaluating habitats and feeding habits through ecomorphological features in glyptodonts (Mammalia, Xenarthra). Ameghiniana 48: 305–319. Crossref
Vizcaíno, S.F., Toledo, N., and Bargo, M.S. 2018. Advantages and limitations in the use of extant xenarthrans (Mammalia) as morphological models for paleobiological reconstruction. Journal of Mammalian Evolution 25: 495–505. Crossref
Wiley, D.F., Amenta, N., Alcantara, D.A., Ghosh, D., Kil, Y.J., Delson, E., Harcourt-Smith, W., Rohlf, K.St., and Hamann, B. 2005. Evolutionary morphing. In: Visualization Conference (VIS 05), Piscataway: IEEE, 431–432, Minneapolis.
White, J.L. 1993. Indicators of locomotor habits in xenarthrans: evidence for locomotor heterogeneity among fossil sloths. Journal of Vertebrate Paleontology 13: 230–242. Crossref
Wynd, B.M., Peecook, B.R., Whitney, M.R., and Sidor, C.A. 2018. The first occurrence of Cynognathus crateronotus (Cynodontia: Cynognathia) in Tanzania and Zambia, with implications for the age and biostratigraphic correlation of Triassic strata in southern Pangea. In: C.A. Sidor and S.J. Nesbitt (eds.), Vertebrate and Climatic Evolution in the Triassic Rift Basins of Tanzania and Zambia. Society of Vertebrate Paleontology Memoir 17, Journal of Vertebrate Paleontology 37 (Supplement 6): 228–239. Crossref
Zamuner, A.B., Zavattieri, A.M., Artabe, A.E., and Morel, E.M. 2001. Paleobotánica. In: A.E. Artabe, E.M. Morel, and A.B. Zamuner (eds.), El Sistema Triásico en la Argentina. Fundación Museo La Plata “Francisco P. Moreno” 8: 143–184.
Acta Palaeontol. Pol. 67 (2): 543–557, 2022
https://doi.org/10.4202/app.00919.2021