

The paleoecology of the Late Miocene mammals from the Optima Local Fauna of Oklahoma, USA
JOSEPH A. FREDERICKSON, JOSHUA E. COHEN, MICHAEL H. ENGEL, TYLER C. HUNT, GREG A. WILBERT, OLGA S. CASTAÑEDA, and NICHOLAS J. CZAPLEWSKI
Frederickson, J.A., Cohen, J.E., Engel, M.H., Hunt, T.C. Wilbert, G.A., Castañeda, O.S., and Czaplewski, N.J. 2022. The paleoecology of the Late Miocene mammals from the Optima Local Fauna of Oklahoma, USA. Acta Palaeontologica Polonica 67 (1): 221–238.
The Optima Local Fauna represents an important glimpse into the ecological transition between savannah and grassland during the late Miocene (Hemphillian) of what is now the southcentral Great Plains of North America. Though dominated by horses, herbivores from the Optima are morphologically diverse, bearing adaptations for both browsing and grazing lifestyles. Likewise, the carnivorans show similar ranges of size and presumed dietary behavior. In this study, we used carbonate isotope, mesowear, and tooth breakage and wear analyses to investigate the dietary complexity of mammals from a single site collected by the Oklahoma Museum of Natural History. Seventeen taxa were analyzed, including five perissodactyls (Teleoceras hicksi, Dinohippus interpolatus, Neohipparion eurystyle, Nannippus ingenuus, and Astrohippus ansae), four artiodactyls (Texoceros guymonensis, Pediomeryx hemphillensis, Megatylopus matthewi, and Platygonus sp.), a single proboscidean (Mammut sp.), two rodents (Dipoides indet. and Umbogaulus monodon), and five carnivorans (Agriotherium schneideri, Amphimachairodus coloradensis, Borophagus secundus, Eucyon davisi, Pliotaxidea cf. nevadensis). Both stable isotope analysis and dental mesowear indicate a broad dietary partitioning occurred among the Optima herbivores, where the artiodactyls were identified as mixed feeders and the perissodactyls were recovered as grazers. In the carnivorans, the large felid Amphimachairodus coloradensis was a hypercarnivore with limited tooth breakage and an enriched δ13C signature, indicating low carcass utilization and a prey preference for horses. The canids had a more generalized diet, with B. secundus showing a greater proportional consumption of carcasses through a higher tooth breakage rate. The large ursid Agriotherium schneideri is here interpreted as an omnivore based on depleted δ13C values. Overall, we found evidence for a diversity of dietary niches in both carnivores and herbivores during the late Hemphillian in Oklahoma, likely driven by the expansion of grasslands in the region.
Key words: Mammalia, grassland, mesowear, savanna, stable isotopes, tooth breakage, Neogene, North America.
Joseph A. Frederickson [fredericksoj@uwosh.edu], Weis Earth Science Museum, University of Wisconsin Oshkosh Fox Cities Campus, 1478 Midway Rd, Menasha, WI 54952, USA; Oklahoma Museum of Natural History, 2401 Chautauqua Ave., University of Oklahoma, Norman, OK 73072, USA.
Joshua E. Cohen [jcohen7@pace.edu] (corresponding author), Oklahoma Museum of Natural History, 2401 Chautauqua Ave., University of Oklahoma, Norman, OK 73072, USA; Department of Biology, Pace University, One Place Plaza, New York, NY 10038, USA.
Michael H. Engel [ab1635@ou.edu], School of Geosciences, Mewbourne College of Earth and Energy, University of Oklahoma, 100 E. Boyd St, SEC 710, Norman, OK 73019, USA.
Tyler C. Hunt [thunt@bio.fsu.edu], Department of Biological Sciences, Florida State University, 319 Stadium Drive, Tallahassee, FL 32304, USA.
Greg A. Wilbert [greg.a.wilbert-1@ou.edu] and Nicholas J. Czaplewski [nczaplewski@ou.edu], Oklahoma Museum of Natural History, 2401 Chautauqua Ave., University of Oklahoma, Norman, OK 73072, USA.
Olga S. Castañeda [castanedao@student.swosu.edu], Department of Biology, Southwestern Oklahoma State University, 100 W Campus Drive, Weatherford, OK 73096, USA.
Received 6 September 2021, accepted 25 January 2022, available online 30 March 2022.
Copyright © 2022 J.A. Frederickson et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The history of grasslands in North America is closely linked to the evolution of grazing vertebrates, mostly ungulates, across the continent, and especially in the Great Plains physiographic region (e.g., Webb 1977; Jacobs et al. 1999; MacFadden 2000; Janis et al. 2002; Semprebon et al. 2016; Pérez-Crespo et al. 2016). As Cenozoic climates changed and Paleogene forests morphed into Neogene savannas and grasslands, vertebrate faunas consequently changed too. The Great Plains preserve abundant sedimentary deposits containing vertebrate fossils of late Paleogene and Neogene vertebrates. These fossils reflect large numbers of herbivorous mammals and other vertebrates as well as their predators. They provide clues to some of the environmental changes that were happening, plus series of evolving faunas forming the basis for a biochronological framework for the region (Janis et al. 1998, 2008; Tedford et al. 2004; Woodburne 2004). Among the Neogene faunas contributing to this sequence, yielding a typical late Miocene vertebrate fauna, is the Optima Local Fauna (LF) of western Oklahoma originating from the widespread Ogallala Formation.
Table 1. Vertebrate taxa list for the Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA), updated from Hesse (1936), Savage (1941), Hirschfeld and Webb (1968), Harrison (1979, 1981), Baskin (1982), Werdelin (1985), Janis et al. (1998, 2008), Janis and Manning (1998), Lambert and Shoshani (1998), Wright (1998), Wang et al. (1999), Gordon and Czaplewski (2000), Korth (2000, 2002), Schultz (2002), Tedford et al. (2004, 2009), Prothero (2005), Prothero and Liter (2007), Flynn and Jacobs (2008), Antón et al. (2013), and others listed in references. * taxon not available in OMNH collection.
|
Chelonia |
|
|
Testudinidae |
Hesperotestudo sp. |
|
Crocodylia |
|
|
Alligatoridae |
cf. Alligator sp. |
|
Aves |
|
|
Gruiformes |
|
|
*Rallidae |
Aves indet. |
|
Mammalia |
|
|
Xenarthra |
|
|
Megalonychidae |
Megalonyx sp. |
|
Carnivora |
|
|
Canidae |
Borophagus
secundus |
|
Ursidae |
Agriotherium schneideri |
|
Felidae |
Amphimachairodus
coloradensis |
|
Mustelidae |
Plesiogulo
marshalli |
|
Procyonidae |
*Arctonasua fricki |
|
Proboscidea |
|
|
Mammutidae |
Mammut sp. |
|
Perissodactyla |
|
|
Equidae |
Dinohippus
interpolatus |
|
Rhinocerotidae |
Teleoceras
hicksi |
|
Artiodactyla |
|
|
Tayassuidae |
Platygonus sp. |
|
Camelidae |
Megatylopus
matthewi |
|
Palaeomerycidae |
Pediomeryx hemphillensis |
|
Antilocapridae |
Texoceros
guymonense |
|
Lagomorpha |
|
|
Leporidae |
Hypolagus cf. vetus |
|
Rodentia |
|
|
Mylagaulidae |
Umbogaulus monodon |
|
Castoridae |
Dipoides sp. |
|
Sciuridae |
Marmota sp. |
The Optima (sometimes known as Guymon) LF represents a well-studied, diverse assemblage of Miocene vertebrates from the Messinian, late Hemphillian North American Land Mammal Age in the panhandle of Oklahoma (Table 1). Dominated by horses (relative abundance up to 80% of the assemblage; Schultz 2002), the Optima LF and the contemporaneous Coffee Ranch Local Fauna of Texas were originally thought to represent broad, short grasslands (Savage 1941). The presence of multiple low-crowned herbivores and alligators suggests a more heterogeneous ecosystem with nearby forested areas along floodplains and deeper, slow-moving water bodies than historically existed in western Oklahoma. To date, the Optima LF has not received the scientific attention that the nearby Coffee Ranch Fauna has but given their overlapping mammal species and their temporal and geographic proximity (Schultz 2002), environmental interpretations for Coffee Ranch likely apply equally to both sites (Table 2).
Table 2. Ecomorphological attributes of mammals of the Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA) inferred from actualism and by authors of the references: Antón et al. (2013); Baskin (1982); Hoppe et al. (1999); Joeckel and Tucker (2013); Kohn et al. (2005); MacFadden (2000, 2005); McDonald (2005); Mihlbachler et al. (2011); Passey et al. (2002); Perez-Crespo et al. (2016); Prothero (1998); Rivals et al. (2012); Rybczynski (2007, 2008); Semprebon and Rivals (2007, 2010); Semprebon et al. (2004, 2016); Voorhies and Thomasson (1979); Wang (2016). * taxon not available in OMNH collection.
|
Optima Local Fauna taxon |
Body size |
Habitat |
Dietary |
Locomotion/habits |
|
Megalonyx sp. |
large |
terrestrial |
herbivore, browser |
plantigrade |
|
Borophagus secundus |
medium |
terrestrial |
carnivore, bone-eating scavenger |
digitigrade cursor, potential social group hunters |
|
Eucyon davisi |
medium |
terrestrial |
carnivore/omnivore |
digitigrade cursor |
|
*Canis ferox |
medium |
terrestrial |
carnivore/omnivore |
digitigrade cursor |
|
Vulpes stenognathus |
medium |
terrestrial |
carnivore/omnivore/insectivore |
digitigrade cursor |
|
Agriotherium schneideri |
large |
terrestrial |
omnivore |
plantigrade |
|
Amphimachairodus coloradensis |
large |
terrestrial |
hypercarnivore |
digitigrade cursor |
|
Lynx proterolyncis |
medium |
terrestrial |
hypercarnivore |
digitigrade cursor |
|
*Pseudailurus or Adelphailurus |
medium |
terrestrial |
hypercarnivore |
digitigrade cursor |
|
Plesiogulo marshalli |
medium |
terrestrial |
carnivore/scavenger |
digitigrade |
|
Pliotaxidea cf. nevadensis |
medium |
fossorial |
insectivore/carnivore |
digitigrade |
|
*Arctonasua fricki |
medium |
terrestrial? |
omnivore? |
plantigrade? |
|
Mammut sp. |
large |
terrestrial |
browser, hindgut fermenter |
graviportal, forested environments, migrator |
|
Dinohippus interpolatus |
large |
terrestrial |
grazer/mixed feeder, hindgut fermenter |
unguligrade cursor |
|
Astrohippus ansae |
large |
terrestrial |
grazer, hindgut fermenter |
unguligrade cursor |
|
Neohipparion eurystyle |
large |
terrestrial |
grazer, hindgut fermenter |
unguligrade cursor |
|
Nannippus lenticularis |
large |
terrestrial |
grazer or mixed feeder, hindgut fermenter |
unguligrade cursor |
|
Teleoceras hicksi |
large |
terrestrial, semiaquatic? |
grazer, hindgut fermenter |
unguligrade/graviportal, dry open habitats |
|
*Teleoceras guymonensis |
large |
terrestrial, semiaquatic? |
grazer/browser, hindgut fermenter |
unguligrade/graviportal |
|
*Aphelops muticus |
large |
terrestrial |
grazer/browser, hindgut fermenter |
unguligrade/graviportal cursor |
|
Platygonus sp. |
large |
terrestrial |
herbivore/omnivore, nonruminant foregut fermenter |
unguligrade cursor |
|
Megatylopus matthewi |
large |
terrestrial |
leaf browser, nonruminant foregut fermenter |
digitigrade cursor |
|
Alforjas taylori |
large |
terrestrial |
leaf browser, nonruminant foregut fermenter |
digitigrade cursor |
|
*Hemiauchenia / Pleiolama sp. |
large |
terrestrial |
leaf browser, nonruminant foregut fermenter |
digitigrade cursor |
|
Pediomeryx hemphillensis |
large |
terrestrial |
mixed feeder/grazer, ruminant? foregut fermenter |
unguligrade cursor |
|
Texoceros guymonensis |
medium |
terrestrial |
grazer/mixed feeder, ruminant foregut fermenter |
unguligrade cursor |
|
Hypolagus cf. vetus |
small |
terrestrial |
herbivore, hindgut fermenter |
digitigrade cursor |
|
Umbogaulus monodon |
small |
fossorial |
herbivore, hindgut fermenter |
plantigrade?, fossorial |
|
Dipoides sp. |
small |
semiaquatic |
browser (cambium feeder), hindgut fermenter |
plantigrade, fossorial/natatorial |
|
Marmota sp. |
small |
semifossorial |
herbivore, hindgut fermenter |
plantigrade, fossorial |
Coffee Ranch LF (and thus the shared taxa of Optima) has produced evidence of early C4 herbivory in North American horses (Wang et al. 1994; Sharp and Cerling 1998; Passey et al. 2002) and is dated to approximately 6.62 Ma (Passey et al. 2002; Lukens et al. 2017). Pedogenic analysis suggests an understory of ≤25% C4 biomass (Fox and Koch 2003, 2004) in a climate that was considerably wetter than today (900–1150 mm/yr) and at least moderately warmer (Lukens et al. 2017). These results are consistent with ecosystem reconstructions by Fraser and Theodor (2013), who estimated a mean annual precipitation (MAP) of 992 mm/yr based on the proportion of hypsodont ungulates in the assemblage. Though grasses were undoubtedly present, the high degree of hypsodonty in the Coffee Ranch and Optima faunas is thought to be a result of increased eolian sand and silt upon brushland and woody savannah vegetation (Fox and Koch 2004; Fraser and Theodor 2013; Lukens et al. 2017). Elsewhere, hypsodonty has also been considered more closely related to open habitat foraging than to grass consumption, at least in eastern hemisphere ungulates (Mendoza and Palmqvist 2007). Indeed, Fraser and Theodor (2013) called into question a significant C4 expansion in the Great Plains during the late Hemphillian, suggesting that small sample sizes from Texas fossil sites provide an exaggerated appearance of C4 as a major constituent of the diet in horses from Coffee Ranch LF. Given the shared geographical and temporal similarities between the Optima LF and Coffee Ranch LF, an ecological comparison between sites can help address this possible sampling bias. Here we use multiple proxies to test for the presence of C4 diet in Optima LF horses as compared to other members of the Optima LF. If C4 grasses were significantly present to be used as a dietary source in the Optima LF horses, then isotopic and mesowear analyses should show enriched values in species with heavily worn teeth. The highly diverse mammalian carnivore fauna is also of interest here, as multiple medium and large-bodied predators coexisted in the Optima LF. By testing stable isotopes, tooth wear, and breakage, we can differentiate large predators in the ecosystem based on the type of prey and degree of skeletal processing. Taken together, this study presents a hypothetical reconstruction of the ecology of Optima LF mammals.
Institutional abbreviations.—OMNH, Oklahoma Museum of Natural History, Norman, Oklahoma, USA; SMU, Shuler Museum of Paleontology, Southern Methodist University, Dallas, Texas, USA.
Other abbreviations.—DFA, discriminant function analysis; LF, local fauna; MNS, mesowear numerical score; NBS, National Bureau of Standards (for standard reference materials); VPDB, Vienna PeeDee Belemnite; WPA, Work Progress Administration.
Geological setting
During recent historic times the landscape near Optima (Beaver County, western Oklahoma) has consisted of low rolling hills mostly covered by riparian and eolian sands stabilized by vegetation, with few surface exposures of the Ogallala Formation. During the early 20th century, the site was worked by WPA-supported crews from the Museum of the University of Oklahoma (now OMNH), who dug pits and trenches into this landscape to access the vertebrate fossils of the Optima LF (Hesse 1936; Savage 1941). Walls of the pits provided a look at the local stratigraphy of the Ogallala Formation. As at other places in the high plains across the central and southern Great Plains, the Optima strata of the Ogallala Formation consist of stream channel deposits from ancient rivers draining the Rocky Mountains, and include channel gravel, sand, silt, clay, ancient soils, and caliche (Schultz 2002), and are discontinuous and thus largely unmappable as geological units. The former Optima fossil pits are now filled in and unrecognizable, but these beds also are exposed along a railroad cut nearby and in intermittent recent creeks draining the area southward to the Beaver River (Savage 1941). The local stratigraphy as exposed during the quarrying in the 1930s was fairly consistent among five quarries spread across an area of <65 hectares, and was briefly described by Savage (1941) as consisting of four units (from base to top): >0.6 m of brownish-red sand, grit and gravel; 1.2–2.1 m of white quartz sand, grading into grit and gravel in places, and yielding most of the vertebrate fossils; 2.1–2.4 m of buff to gray clay and silt, containing fossils; 1–2.1 m of soil and caliche at the surface. Quarries were also operated in the same area and time period by the University of California and the American Museum of Natural History (Hesse 1936; Schultz 2002).
Paleodietary reconstructions in mammalian communities
Estimations of the ecomorphological parameters of members of a paleofauna have been made for years using uniformitarianism/actualism. For the Optima LF, basic ecological relationships of the mammals (and other vertebrates) can be generally characterized as in Table 2. Here we apply three proxies for diet/behavioral reconstruction to the Optima LF: carbonate stable isotope analysis, tooth mesowear, and tooth breakage and wear in order to better differentiate the niches of each common mammalian species. Stable isotope analysis of carbonate has been used for a variety of paleodietary studies, often derived from the strong and non-porous enamel of vertebrate teeth (Kohn and Cerling 2002). Carbonate (CO32-) is abundant in bioapatite, substituting for the phosphate and hydroxyl. The carbon in this carbonate comes from an animal’s diet, where significant isotopic differences form at the base of the food chain caused by the photosynthetic pathways (C3 vs. C4; West-Eberhard et al. 2011) used by plants to transform CO2 into organic molecules (such as carbohydrates). The dominant vegetation in North America before the Miocene was C3 plants, which tend to vary in δ13C between -32 and -21‰. This variability reflects environmental conditions, where plants living in more arid, saline, or open landscapes tend to have more positive values, because they must close their stomata more often to preserve water, thus trapping atmospheric CO2 and forcing more of the heavy isotope to be used in the photosynthetic reactions. C4 plants, such as grasses, are substantially more enriched in 13C, with values between -14 and -10‰ (Fricke 2007). Given these large differences in plant δ13C, carbon isotopes have historically been used to map the evolution and expansion of C4 grasslands during the Cenozoic (e.g., Cerling et al. 1997). Further, fractionation occurs at each step of the food chain with variable offset between predator bioapatite carbonate and the original prey tissue. For example, Clementz et al. (2009) found an average offset between Pleistocene wolf populations and their ungulate prey items to be approximately -1.3‰. δ18O in teeth reflects ingested and atmospheric water sources (Koch 2007), varying based on climate, geography, and digestive physiology. In herbivores, fore- vs. hind-gut fermenters and browsers vs grazers both show differing degrees of water dependence leading to isotopic fractionation and meteoric water intake variability (Sponheimer and Lee-Thorp 1999; Zanazzi and Kohn 2008). In this way, species living in the same climate and geographic region can show varying δ18O values.
Another proxy for diet used in Cenozoic paleoecology is dental mesowear. The mesowear method is primarily used with ungulates and relies on the relative amount of attritional and abrasive wear in the diet. Attritional wear is due to tooth-tooth contact, actively sharpening cusps, and abrasive wear is due to tooth-food contact, actively flattening cusps (Fortelius and Solounias 2000). High levels of abrasive wear are typically related to grazing, while high levels of attritional wear are related to browsing. Abrasion is caused by phytoliths and abiotic abrasives, such as dust and grit (Kaiser and Schulz 2006; Damuth and Janis 2011; Erickson 2014). Currently, it remains unclear if phytoliths have sufficient hardness to wear enamel directly, however, phytoliths can wear softer dental tissues such as dentine and cementum. The preferential wear of these softer tissues by phytoliths forms enamel prominences that are subjected to greater contact pressures, which can result in the indirect wear of enamel (Erickson 2014). Experimental studies have found little correlation between a high amount of external grit and increased abrasion, with mesowear scores indicating an “average” diet value for a given taxon, however, small sample sizes and a limited time frame on feeding experiments provide only limited support for this relationship (Ackermans et al. 2018). Additionally, little correlation has been found between warmer and drier climates (where high amounts of dust and grit in the environment are expected) and higher abrasive mesowear scores (Kubo and Yamada 2014; DeSantis et al. 2018). Therefore, mesowear appears to be related to the internal abrasiveness of the food (i.e., phytoliths), reaffirming that specimens with blunter teeth and low relief tend to be grazers, while specimens with sharper teeth and high relief tend to be browsers. Exactly how abrasion and attrition shape occlusal tooth morphology remains poorly understood, yet a strong correlation between occlusal morphology (mesowear scores) and diet type has been clearly demonstrated (Fortelius and Solounias 2000).
Last, we investigated tooth breakage and wear in the Optima LF carnivorans to infer their dietary behavior. Tooth breakage and wear analyses in other mammalian fossil faunas have been used to compare bone consumption rates with those occurring in modern communities. For example, in the famed late Pleistocene Rancho La Brea asphalt deposits, carnivoran tooth breakage and wear is much higher than in modern carnivorans, implying a greater reliance on osteophagy, possibly during times of environmental stress (Van Valkenburgh and Hertel 1993; Van Valkenburgh 2009). Further comparisons between species can possibly help differentiate diet and prey consumption strategies in predators in the same community (Binder and Van Valkenburgh 2010), where high rates of tooth breakage and wear imply a greater reliance on harder fare.
Material and methods
Isotope analyses.—Stable isotope compositions of carbonate tooth enamel (δ13C and δ18O) were determined for each taxon in order to approximate the diet and the overall habitat of the Optima ecosystem. Specimens were sampled from the OMNH and included taxa from the orders Artiodactyla (Megatylopus matthewi, Pediomeryx hemphillensis, Playgonus sp., and Texoceros guymonensis), Carnivora (Agriotherium schneideri, Amphimachairodus coloradensis, Borophagus secundus, Eucyon davisi, and Pliotaxidea cf. nevadensis), Perissodactyla (Astrohippus ansae, Dinohippus interpolatus, Nannippus ingenuus, and Neohipparion eurystyle), Proboscidea (Mammut sp.), and Rodentia (Dipoides sp. and Umbogaulus monodon). Sample treatment followed a modified technique of Koch et al. 1997 (see Frederickson et al. 2018, 2020). All stable isotope values are presented in δ13CVPDB-LSVEC and δ18OVPDB, with comparisons made using the non-parametric Kruskal-Wallis and Tukey’s pairwise tests in PAST4 (Hammer et al. 2001).
To determine the type of habitat, and by extension the representative vegetation contributing to the δ13C of Optima LF herbivores, we followed a previously published model (Secord et al. 2008; Boardman and Secord 2013; Kita et al. 2014; Wang and Secord 2020) based on modern plant δ13C and adjusted for latitude, elevation, and modern CO2 isotopic values. Secord et al. (2008) used a ~0.3‰/10° change in latitude and ~0.65‰/km change in elevation to modify the model for differing geographical localities. The latitude and elevation of the Optima LF (located near present day Guymon, Oklahoma, USA, at 36.6° N latitude, ~950 m elevation) both differ from those in the original equation for western Nebraska (38.5° N latitude and 525 m as a mean elevation). Latitude was adjusted by -0.57‰ to that of modern Guymon, Oklahoma assuming little movement in latitude since the Miocene (Wang and Secord 2020). However, elevation was left unchanged (525 m) following the argument that uplift of the Ogallala deposits in the Great Plains was largely post-Hemphillian (Duller et al. 2012). The δ13C values of atmospheric CO2 were retained from Wang and Secord (2020) as +1.9‰ modern industrial levels, based on estimates of Tipple et al. (2010). A 14.1‰ enrichment factor for herbivores was used following Cerling and Harris (1999) based on modern ungulates. Taken together, the faunal zones are as follows: closed canopy C3 (e.g., tropical rainforests) <-14‰; open canopy C3 (e.g., woodland-savannas, seasonally dry forests, and C3 grasslands) -14 to -8.3‰; mixed C3/C4 habitat >-8.3‰; lowest pure C3 diet in water-stressed environment -7.5‰. Feeding ecology isotopic values, loosely followed below, were defined by MacFadden and Cerling (1996): -19 to -9‰ with brachydont morphology = C3 browser; -19 to -9‰ with hypsodont morphology = C3 grazer; -9 to -2‰ with intermediate or variable tooth morphology = mixed feeder; -2 to 2‰ with hypsodont morphology = C4 grazer.
In order to test carnivoran diets based on their isotopic values, a Bayesian mixed model was employed using the MixSIAR package in R v3.3.3 (Stock et al. 2018). The dietary source data were taken from the herbivore taxa sampled in this study. Three dietary source models were tested: (i) dietary sources differentiated by all four herbivore orders (Artiodactyla, Perissodactyla, Proboscidea, and Rodentia), (ii) dietary sources including all orders but differentiated into C3-browser (lighter isotopic group) and C4-grazer (heavier isotopic group) isotopic signatures, and (iii) dietary sources including only Artiodactyla (C4-poor diet) and Perissodactyla (C4-rich diet). The three models were then compared, and the highest performing model is presented below.
Mesowear analyses.—Dental mesowear was used as a dietary proxy independent of stable isotopes for the ungulate taxa. Mesowear is a method utilizing the macroscopic wear on teeth to infer relative dietary groups (Fortelius and Solounias 2000). Mesowear variables for cusp shape and relief were collected for both perissodactyls (Astrohippus ansae, Dinohippus interpolatus, Neohipparion eurystyle, Nannippus ingenuus, and Teleoceras hicksi) and artiodactyls (Pediomeryx hemphillensis, Texoceros guymonensis, and Megatylopus matthewi) from the OMNH (Fortelius and Solounias 2000). A mesowear numerical score (MNS) was calculated from the cusp and relief variables. Sharp cusps and high relief was scored as 0, round cusps and high relief was scored as 1, sharp cusps and low relief was scored as 2, round cusps and low relief was scored as 2.5, and blunt cusps with high or low relief was scored as 3, following the attrition-abrasion spectrum that mesowear represents (Cohen et al. 2021). Data collection followed Loffredo and DeSantis (2014) where five observers independently scored mesowear variables on each specimen, with the median value used in further analyses in order to reduce interobserver error. Since MNS values are non-normally distributed (Shapiro-Wilk test, p <0.0001), the non-parametric Kruskal-Wallis test and Dunn’s post hoc test were used to compare MNS values among taxa in PAST4 (Hammer et al. 2001).
In order to determine average diet of each taxon, the mesowear variables were directly compared with previously published cusp sharpness and cusp relief scores from 51 modern ungulate taxa using a discriminant function analysis (DFA) (Fortelius and Solounias 2000; Rivals et al. 2007; Schulz and Kaiser 2013; Taylor et al. 2014; Jones and DeSantis 2017; Cohen et al. 2021). The modern ungulates were split into four dietary groups, including browsers, mixed feeders, non-strict grazers, and strict grazers. The dietary groups for the Miocene taxa whose diets are unknown were calculated using posterior probabilities (Díaz-Sibaja et al. 2018; Hullot et al. 2019; Cohen et al. 2021). Dietary classifications for the DFA were based on the variables % Sharp, % Round, % Blunt, and % High. Additional published mesowear scores of Miocene taxa were included in the DFA from other North American localities including Florida (Mixson’s Bone Bed, Love Bone Bed), Nebraska (Ashfall Fossil Bed, Cambridge), Kansas (Long Island Rhino Quarry), and Texas (Coffee Ranch) in order to assess dietary variability (Fraser and Theodor 2013; Mihlbachler et al. 2018). The DFA and posterior probabilities were conducted using the MASS package in R v3.3.3 (Venables and Ripley 2002).
Tooth breakage and wear.—We collected tooth breakage and wear data on carnivoran taxa available in the OMNH collections, including Amphimachairodus coloradensis, Borophagus secundus, Eucyon davisi, Plesiogulo marshalli, Pliotaxidea cf. nevadensis, and Vulpes stenognathus. While tooth breakage and wear may be correlated with an increase in age in individuals, multiple studies have shown that increased instances of tooth breakage and wear are not fully explained by age, but also related to carcass utilization, scavenging, and/or interspecific competition (Binder and Van Valkenburgh 2010; Van Valkenburgh et al. 2019). Therefore, any differences between tooth breakage and wear frequencies among species are more likely due to factors beyond age. Tooth breakage characterization followed Van Valkenburgh (2009), where a tooth was identified as broken only if there was clear damage to the tooth with subsequent wear in order to avoid counting postmortem breaks. Tooth fracture incidence was calculated on a per-tooth basis, because the vast majority of specimens are isolated teeth. Tooth wear was grouped into three categories: (i) slight, including teeth with little to no wear, (ii) moderate, including teeth with developed shear facets and slight blunting of cusps, and (iii) heavy, including teeth with strongly blunted and flattened cusps (Van Valkenburgh and Hertel 1993; Van Valkenburgh 2009; Binder and Van Valkenburgh 2010). We compared the tooth breakage and wear frequencies from members of the Optima LF to previously published tooth breakage and wear frequencies of modern and Pleistocene carnivorans (Van Valkenburgh 2009). Tooth wear frequencies were compared for taxa with adequate sample sizes (n >10) using a chi-squared test to assess differences among taxa.
Results
The major groups represented in this study were first assessed according to their five taxonomic orders: Artiodactyla, Perissodactyla, Proboscidea, Rodentia, and Carnivora, with further in-depth assessment at the species-level when differences arise. Of the presumed large-bodied herbivorous taxa, the perissodactyls are drastically different in enamel carbonate carbon and oxygen isotope composition from the artiodactyls and proboscidean. The differences between taxa in δ13C are driven by the isotopically heavier horses and the lighter mastodon (Mammut sp.), artiodactyls, and rhinoceros (Teleoceras hicksi; all comparisons p <0.01; Fig. 1). For δ18O, artiodactyls (n = 40; average = -2.43‰, SD = 0.32) and the mastodon (n = 10; average = -2.96, SD = 0.25) are significantly more enriched than perissodactyls (n = 64; average = -3.81‰, SD = 0.16) (both p <0.01; Fig. 2). In addition, enamel and dentin were compared from incisors of unidentified large equids (likely Dinohippus interpolatus) not included in the ecological data set. In both δ13C and δ18O, enamel contained a heavier average than dentin (13Ce = -6.90‰, 13Cd = -7.60‰; 18Oe = -2.94‰; 18Od = -5.69‰), though only δ18O values were significantly different (p <0.05). Similarly, the rodents show differentiation from one another with the presumably fossorial Umbogaulus monodon being significantly more enriched than the castorid Dipoides sp. (p <0.01) in δ13C and more depleted (p <0.01) in δ18O. Compared to the large-bodied herbivores, rodents are significantly different only from the perissodactyls (p <0.01).
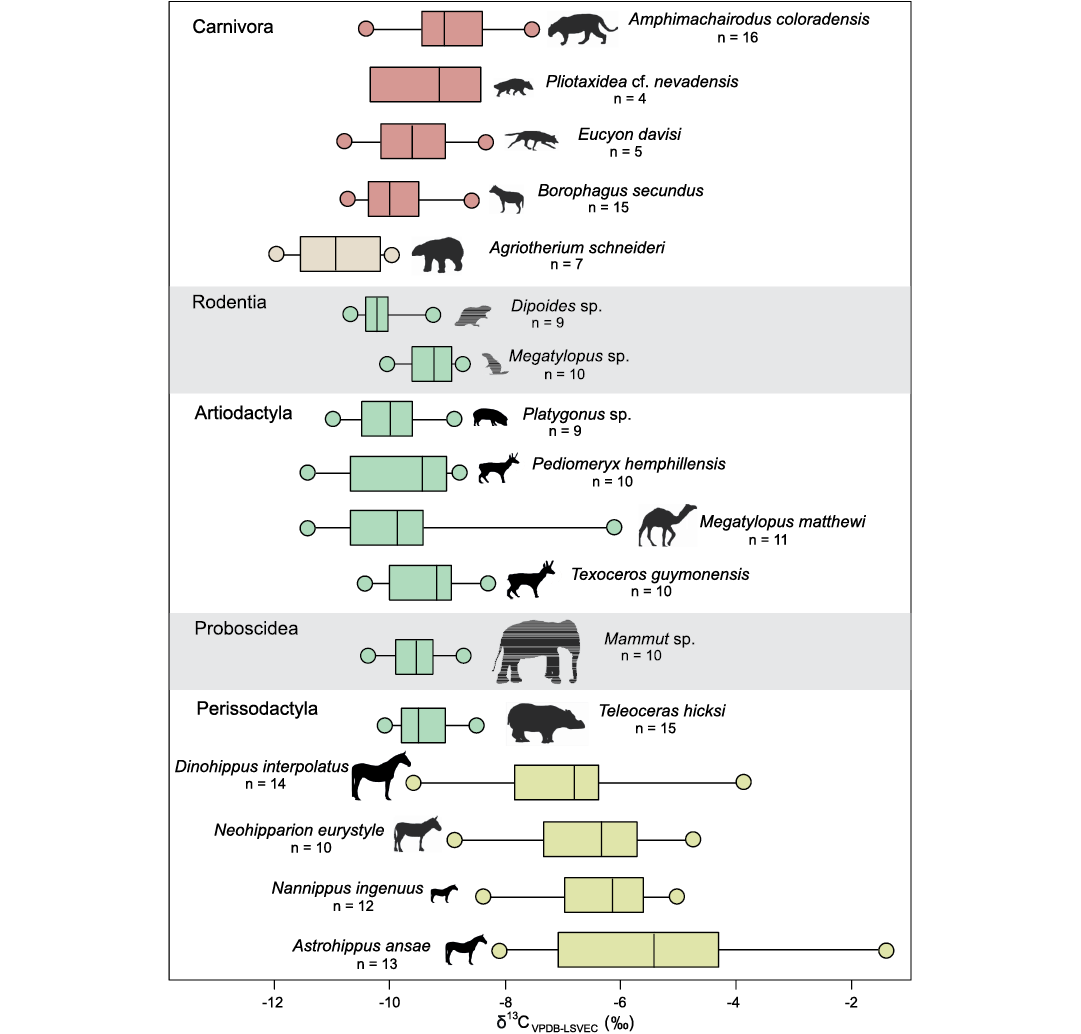
Fig. 1. δ13C values for Optima Local Fauna mammals (Miocene, late Hemphillian, Oklahoma, USA). Color differences between Agriotherium schneideri and other carnivorans (red vs. buff) and among herbivores (green vs. yellow) indicate the major statistical differences within these groups.
For carnivorans, δ13C values differ between the bear Agriotherium schneideri and all other taxa (p <0.01), and between Amphimachairodus coloradensis and Borophagus secundus (p <0.01). δ 18O values show less differentiation between taxa; a Kruskal-Wallis test of equal medians shows no significant difference between sample medians (p = 0.14). The third Bayesian mixed model was chosen, which incorporates the orders Artiodactyla (C4-poor diet) and Perissodactyla (C4-rich diet) only, based on the deviance information criterion, a best-fit measure for Bayesian analyses, where lower values have a better fit with the model (49 for the third model, 54 for the second model, and 61 for the first model) (Francois and Laval 2011). From the model, three carnivoran taxa likely fed upon both C4-rich (perissodactyls) and C4-poor (artiodactyls) sources, including Amphimachairodus coloradensis (38.7% from C4-poor and 61.3% from C4-rich sources), E. davisi (55.6% from C4-poor and 44.4% from C4-rich sources), and Pliotaxidea cf. nevadensis (44.3% from C4-poor and 55.7% from C4-rich sources). Borophagus secundus primarily fed from C4-poor sources (71.7% from C3 vs. 28.3% from C4-rich sources) and Agriotherium schneideri fed almost exclusively from C3 sources (93.0%).
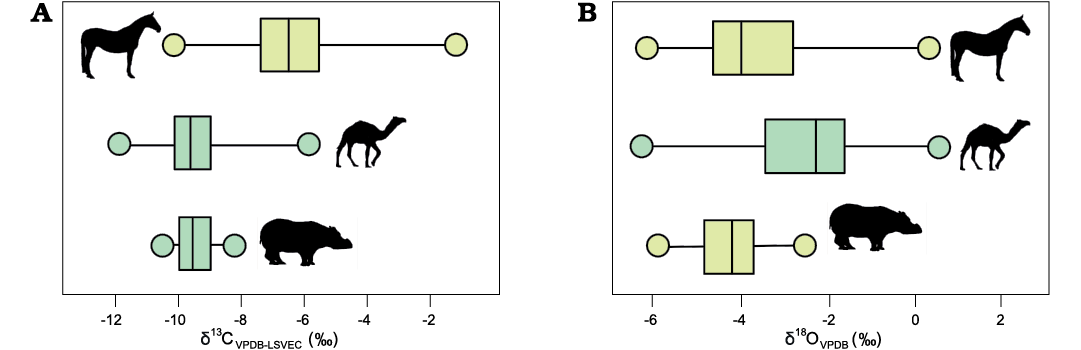
Fig. 2. Comparison of taxonomically-grouped stable isotope values for Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA) horses (top), artiodactyls plus Mammut sp. (middle), and Teleoceras hicksi (bottom). Color differences indicate groupings that are statistically significantly different from one another. A. Average δ13C values. B. Average δ18O values.
For the mesowear analysis, a Kruskal-Wallis test of MNS values was statistically significant at p <0.0001, broadly separating the eight taxa with mesowear data into two different groups. The horses and Teleoceras hicksi all had high average MNS values >2, while the artiodactyls all had significantly lower MNS values ranging 0.5–1. Within the horses and Teleoceras hicksi group, Dinohippus interpolatus (average 2.2) and Teleoceras hicksi (average 2.3) had statistically lower MNS values than Neohipparion eurostyle (average 2.9), Nannippus ingenuus (average 2.6), and Astrohippus (average 2.9). In contrast, MNS values for the artiodactyls Texoceros guymonensis (average 1.1), Pediomeryx hemphillensis (average 0.6), and Megatylopus matthewi (average 0.7) did not statistically differ from each other. The DFA correctly classified 96% of the modern taxa into their known dietary groups, with one browser and one mixed feeder incorrectly classified (Fig. 3A). Overall, the first two functions explained over 99% of the differences, with Function 1 explaining 84.2% and Function 2 explaining 15.3% (Fig. 3). Species within the same dietary group clustered together, with the four dietary groups well separated from each other. Optima LF taxa were added into the DFA along with previously published mesowear scores from Miocene mammals, and posterior probabilities were calculated to assess dietary classifications. All four species of horses from all Miocene localities (including Optima, Coffee Ranch, and Cambridge) were classified as 100% strict grazers (Fig. 3B). Teleoceras hicksi samples were highly variable, ranging from browsers to strict grazers. Teleoceras hicksi specimens were classified based on posterior probabilities from the Optima LF (Oklahoma, 6.6 Ma) as 100% strict grazer; from the Long Island Rhino Quarry (Kansas, 9.0–7.5 Ma) as 95% mixed feeder, 3% non-strict grazer, and 2% strict grazer; from the Ashfall Fossil Bed (Nebraska, 12.1–10.1 Ma) as 97% mixed feeder, 3% browser; from the Love Bone Bed (Florida, 10.1–9.0 Ma) as 100% browser; from the Mixson’s Bone Bed (Florida, 9.0–7.5 Ma) as 100% mixed feeder, and from Cambridge (Nebraska, 7.0 Ma) as 100% non-strict grazer (Fraser and Theodor 2013; Mihlbachler et al. 2018) (Fig. 3C). Megatylopus matthewi from the Optima LF was classified as 100% mixed feeder, while M. matthewi from Coffee Ranch was classified as 87% non-strict grazer and 13% mixed feeder, and from Cambridge as 100% non-strict grazer. Pediomeryx hemphillensis from Optima was classified as 100% mixed feeder and from Coffee Ranch as 80% mixed feeder and 20% non-strict grazer. Texoceros guymonensis from Optima was classified as 72% non-strict grazer and 28% mixed feeder and from Coffee Ranch as 100% strict grazer (Fig. 3D).
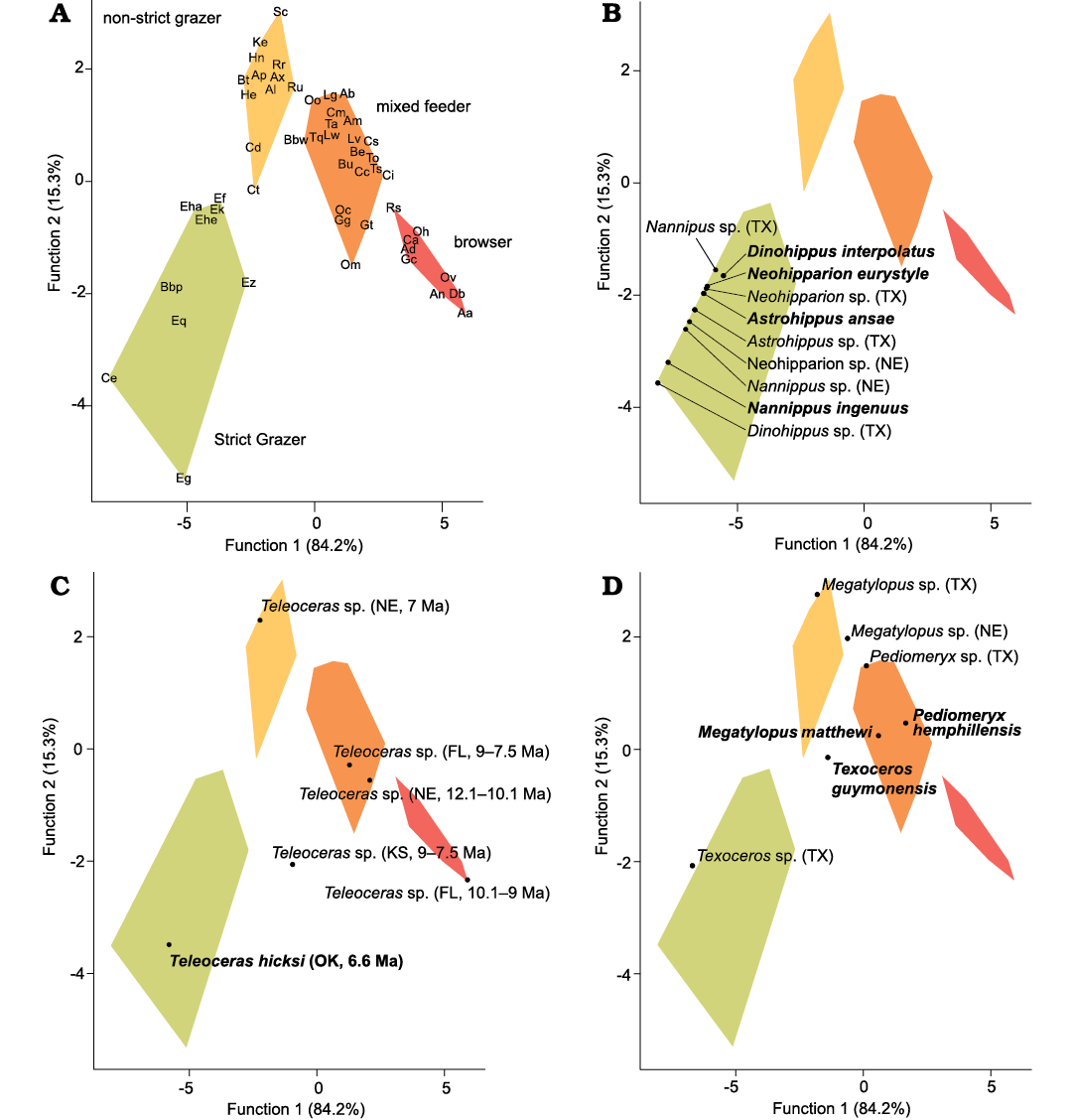
Fig. 3. Mesowear discriminant function analysis for Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA) artiodactyls and perissodactyls compared to closely-related taxa from other Miocene sites from North America and modern taxa (see Material and methods). A. Modern taxa (data from Fortelius and Solounias 2000; Rivals et al. 2007; Fraser and Theodor 2013; Schulz and Kaiser 2013; Taylor et al. 2014; Jones and DeSantis 2017; Mihlbachler et al. 2018). B. Equidae. C. Teleoceras. D. Artiodactyla. Abbreviations: Aa, Alces alces; Ab, Alcelaphus buselaphus; Ad, Antidorcas marsupialis; Al, Alcelaphus lichtensteinii; Am, Aepyceros melampus; An, Antilocapra americana; Ap, Axis porcinus; Ax, Axis axis; Bbp, Plains bison Bison bison; Bbw, Wood bison Bison bison; Be, Boocercus euryceros; Bt, Boselaphus tragocamelus; Bu, Budorcas taxicolor; Ca, Capreolus capreolus; Cc, Cervus canadensis; Cd, Cervus duvauceli; Ce, Ceratotherium simum; Ci, Capra ibex; Cm, Camelus dromedarius; Cs, Capricornis sumatraensis; Ct, Connochaetes taurinus; Db, Diceros bicornis; Ef, Equus ferus przewalski; Eg, Equus grevyi; Eha, Equus hartmannae; Ehe, Equus hemionus; Ek, Equus kiang; Eq, Equus quagga; Ez, Equus zebra; Gc, Giraffa camelopardalis; Gg, Gazella granti; Gt, Gazella thomsoni; He, Hippotragus equinus; Hn, Hippotragus niger; Ke, Kobus ellipsiprymnus; Lg, Lama glama; Lv, Lama vicugna; Lw, Litocranius walleri; Oc, Ovis canadensis; Oh, Odocoileus hemionus; Om, Ovibos moschatus; Oo, Ourebia ourebi; Ov, Odocoileus virginianus; Rr, Redunca redunca; Rs, Rhinoceros sondaicus; Ru, Rhinoceros unicornis; Sc, Syncerus caffer; Ta, Tragelaphus angasi; To, Taurotragus oryx; Tq, Tetracerus quadricornis; Ts, Tragelaphus scriptus; FL, Florida; KS, Kansas; NE, Nebraska; TX, Texas.
Among the carnivorans, three taxa exhibited premortem tooth breakage, including Borophagus secundus, Amphimachairodus coloradensis, and Eucyon davisi. For B. secundus (n = 888) 49 teeth (5.5%) had premortem breakage. Both Amphimachairodus coloradensis (n = 27; 3.7%) and E. davisi (n = 5; 20%) had a single instance of premortem breakage. The high tooth breakage rate in E. davisi is attributed to the small available sample size and without further evidence, most likely does not reflect an extreme incidence of tooth breakage. All other carnivoran taxa had no tooth breakage, including Vulpes stenognathus (n = 29), Plesiogulo marshalli (n = 13), Pliotaxidea cf. nevadensis (n = 3), and Aelurodon sp. (n = 4). Only four taxa had sufficient sample size to assess tooth wear, including Amphimachairodus coloradensis (n = 27), B. secundus (n = 888), Plesiogulo marshalli (n = 13), and V. stenognathus (n = 29), The chi-squared test recovered significant differences among these taxa (p <0.0001), with Amphimachairodus coloradensis with fewer instances of heavily worn teeth, V. stenognathus with fewer instances of moderately worn teeth, and Plesiogulo marshalli with higher instances of moderate and heavily worn teeth.
Discussion
Taphonomy and diagenesis
Carbonate isotopes could be affected by post-mortality modification of the original tissues. Fricke and Pearson (2008) outlined three comparisons to test for the extent of diagenetic alteration of carbonate isotopes in vertebrate tissues: between enamel and dentin, among vertebrate taxa, and between different localities. All three comparisons could be made with data obtained here or through comparison with the primary literature. In general, enamel had higher δ18O and δ13C values than dentin and the two dental tissues were not matching in correlation coefficients (-0.23 for dentin and 0.71 for enamel; Fig. 4). Since dentin is more porous and thus more easily altered by diagenetic factors, we would expect an offset between the two tissues if the tooth carbonate is not entirely altered. The second line of evidence against substantial diagenesis comes from the comparison between taxa. There are significant differences between results from multiple herbivorous groups along taxonomic or ecologic boundaries; for examples, horses vs all other herbivores for 13C and perissodactyls vs all others for 18O. Finally, the carbon isotope results were consistent for each taxon from similar-aged sites in the Great Plains, falling generally in-between isotope results derived from the Serravallian and Tortonian, Clarendonian North American Land Mammal Age and from the latest Hemphillian species (discussed below). Taken together, the three lines of evidence strongly suggest that the ecological and behavioral signals in our results are not completely obscured by secondary isotopic alteration.
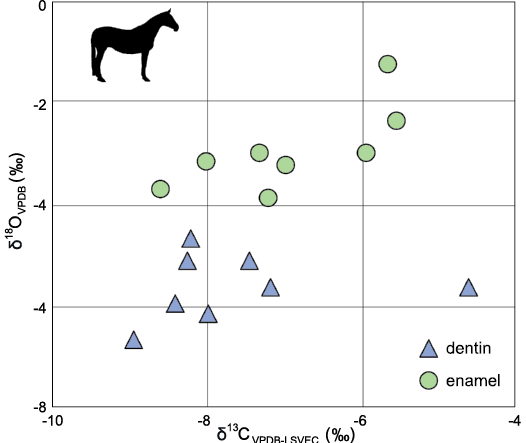
Fig. 4. Comparison of dental tissues (dentin versus enamel) and their d13C and d18O isotopes in unidentified horse teeth from the Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA). R2 = 0.0002 for dentin, 0.5054 for enamel.
Dietary ecology
Proboscidea.—The Optima proboscidean sample was limited to one genus, Mammut (family Mammutidae). The average -8.84‰ (n = 10) and relatively small range of δ13C values (-9.65 to -8‰) indicate a variable browsing lifestyle in an open C3-dominated environment (Table 3). These values are consistent with similar studies from other North American proboscideans. Fox and Fisher (2001) found that Gomphotherium productum (family Gomphotheriidae) from the early Hemphillian (~7.5 Ma) Port of Entry Pit in western Oklahoma possessed an average δ13C of -10.3‰. The authors interpreted these relatively depleted values as a diet of either 100% C3 plants in an open, xeric habitat, or a mixed diet of less than 40% C4 plants in a closed-canopy environment. In a study of samples throughout the late Miocene of Florida, MacFadden and Cerling (1996) found that proboscidean isotopic values became more enriched in δ13C through time. The gomphotheriid proboscidean Amebelodon sp. from the late Clarendonian (~9 Ma) Love Bone Bed had relatively depleted values of -12.2 and -11.9‰; a single sample of Amebelodon sp. in the “middle” Hemphillian (~7 Ma) Moss Acres deposits showed an increased δ13C value of -6.8‰. Proboscideans from the latest Hemphillian (~4.5 Ma) Bone Valley yielded even more enriched values (-6.2, -5.9, -1.9‰). This shift is interpreted as a changing diet from C3 browsing to a mixed diet, and finally exclusive C4 grazing through time. The values obtained for the Optima LF Mammut sp. are consistent with this overall, though admittedly broad, trend, showing a slight enrichment from the gomphotheriid from the older Port of Entry Pit, implying either an increased C4 component among proboscideans in general, or a shift to more covered ecosystems. Given the sampling (molar vs. tusk), species, and geographic differences, we assume the modest distinctions and overall trend are likely a result of behavioral differences in food preference, but we cannot confidently determine the precise cause.
Perissodactyla.—All four species of horses tested here had a significantly heavier δ13C signature in their enamel than all other herbivorous taxa. The largest species, Dinohippus interpolatus, was the most depleted on average (average -6.85‰; range -9.68 to -3.89‰). The other member of the equine clade, Astrohippus ansae, was the isotopically heaviest (average -5.49‰; range -8.11 to -1.35‰). The two members of the hipparionine clade, Neohipparion eurystyle (average -6.36‰; range -8.87 to -4.7‰) and Nannippus ingenuus (mean -6.25‰; range -8.34 to -4.99‰), fall between the equines (Table 3). For all four taxa, there appears to be a boundary between horses and all other herbivores at approximately -8‰; all but one of the 45 isotopically heaviest specimens were horses. These results are consistent with a single equid specimen from Coffee Ranch LF (SMU 70533; -6.1‰; Wang et al. 1994) and with the results of Passey et al. (2002) who found 41% of Hemphillian horses tested from the High Plains of Texas had δ13C values above the -7‰ threshold for drought-stressed environments composed entirely of C3 plants, meaning C4 grasses were sufficiently present to differentiate grazing horses from the other Optima LF herbivores. The mesowear analyses from all four horses exhibited high percentages of low relief with either rounded or blunt cusps, indicating a strong grazing propensity (Cohen et al. 2021). The horses were recovered as strict grazers in the DFA at 100%. Dinohippus interpolatus had the lowest MNS values, indicating a potentially more varied diet. Overall, the mesowear analysis corroborates the findings from the δ13C enamel, with D. interpolatus having a slightly different dietary component than the other horses in the Optima LF.
Table 3. δ13C and δ18O values for Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA), mammalian taxa included in this study.
|
Taxa |
Order |
Number of samples |
Avg‰ ± 1σ δ13CVPDB-LSVEC(‰) |
Avg‰ ± 1σ δ18OVPDB (‰) |
|
Borophagus secundus |
Carnivora |
15 |
-10.03 ± 0.54 |
-4.98 ± 1.15 |
|
Eucyon davisi |
5 |
-9.60 ± 0.93 |
-6.31 ± 1.32 |
|
|
Agriotherium schneideri |
7 |
-10.90 ± 0.66 |
-4.10 ± 1.54 |
|
|
Amphimachairodus coloradensis |
16 |
-8.96 ± 0.79 |
-5.33 ± 1.11 |
|
|
Pliotaxidea cf. nevadensis |
4 |
-9.81 ± 0.90 |
-4.13 ± 1.16 |
|
|
Mammut sp. |
Proboscidea |
10 |
-8.84 ± 0.48 |
-2.96 ± 0.81 |
|
Dinohippus interpolatus |
Perissodactyla |
14 |
-6.85 ± 1.45 |
-3.75 ± 1.11 |
|
Astrohippus ansae |
13 |
-5.49 ± 1.89 |
-3.55 ± 1.70 |
|
|
Neohipparion eurystyle |
10 |
-6.36 ± 1.19 |
-4.01 ± 1.00 |
|
|
Nannippus ingenuus |
12 |
-6.25 ± 0.96 |
-3.43 ± 1.63 |
|
|
Teleoceras hicksi |
15 |
-9.41 ± 0.51 |
-4.29 ± 0.85 |
|
|
Platygonus sp. |
Artiodactyla |
9 |
-9.95 ± 0.59 |
-2.53 ± 1.15 |
|
Megatylopus matthewi |
11 |
-9.79 ± 1.42 |
-2.04 ± 1.91 |
|
|
Pediomeryx hemphillensis |
10 |
-9.81 ± 0.90 |
-2.63 ± 1.98 |
|
|
Texoceros guymonensis |
10 |
-9.45 ± 0.67 |
-2.58 ± 2.86 |
|
|
Umbogaulus monodon |
Rodentia |
10 |
-9.29 ± 0.43 |
-6.81 ± 0.78 |
|
Dipoides sp. |
9 |
-10.16 ± 0.40 |
-4.13 ± 1.15 |
|
|
Matrix |
|
3 |
-3.32 ± 3.99 |
-5.75 ± 1.75 |
The other common perissodactyl in the Optima LF is the rhinoceros Teleoceras hicksi. Fifteen Teleoceras hicksi specimens tested were depleted in δ13C relative to the Optima LF horses, averaging -9.41‰ (range -10.08 to -8.49‰) (Table 3). These values are not significantly different than any of the non-perissodactyl herbivores included in the study, implying a diet isotopically more similar to that of the proboscidean and artiodactyls. Wang and Secord (2020) similarly found Teleoceras sp. from the Great Plains of 6.7–5.9 Ma to be isotopically light (mean -8.8‰; range -11.8 to -7.3‰). MacFadden (1998) also found that Teleoceras sp. from 7 Ma deposits in Florida were substantially depleted in the heavy isotope (average -12.8‰, n = 2), though by 4.5 Ma rhinos became more enriched (average -7‰, n = 7), hypothetically due to increased C4 grasses in the ecosystem and diet.
Our analysis found the Optima LF horses and Teleoceras hicksi to be significantly more depleted in δ18O than the artiodactyls and Mammut sp. Comparisons of modern taxa have shown mammalian apatite oxygen isotope ratios are a product of ingested meteoric water and diet (Kohn et al. 1996). In general, browsers and mix-feeders are enriched relative to grazers (Kohn et al. 1996; Cerling et al. 1997; Sponheimer and Lee-Thorp 1999). This pattern is due to the evapotranspiration of leaf water (Gonfiantini et al. 1965; Dongmann et al. 1974; Epstein et al. 1977; Sternberg 1989; Yakir 1992), which tends to be enriched relative to meteoric water sources. Further, trees and shrubs have deeper roots than grasses, allowing them to retain greater water content than grasses during droughts (Goldstein and Sarmiento 1987). Thus, grazers ingest a larger percentage of water from meteoric sources than from food, leading to more-depleted isotopic signals in their tissues. These results are largely consistent with the carbon isotopes from our analysis, with Teleoceras hicksi being the notable exception. Teleoceras hicksi has the lowest average δ18O values, normally indicative of an animal that derives more water from meteoric sources than from food, and thus indirectly indicating a grazing habit; however, the δ13C levels for this species are also relatively depleted not enriched, matching browsing and mixed-feeding artiodactyls and proboscideans rather than grazing horses. Short-legged rhinoceroses (Teleoceras spp.) have sometimes been proposed as ecomorphologically similar to extant eastern hemisphere hippopotamuses (Hippopotamus) as amphibious grazers (e.g., Webb 1983; Prothero 1992). Bocherens et al. (1996) found that Hippopotamus amphibius tend to be depleted in δ18O compared with other herbivores, providing a possible extant model to diagnose semi-aquatic lifestyles in extinct large mammals. Previous studies of oxygen isotopes in Teleoceras sp. found insufficient support for a Hippopotamus-like lifestyle and instead suggested a terrestrial grazing habit (MacFadden 1998; Wang and Secord 2020). Similarly, the Optima LF Teleoceras hicksi data set showed a low δ18O value on average, but the low end of its range (OMNH 13162; -5.76‰) is somewhat greater than that of either artiodactyls (OMNH 12339; -6.26‰) or horses (OMNH 16636; -5.91‰).
In contrast to the δ13C results but consistent with the δ18O results, Teleoceras hicksi mesowear data from the Optima LF indicate a strict grazing diet, more similar to the other perissodactyls in the fauna than to the proboscidean and artiodactyls (Fig. 5). Published mesowear values for Teleoceras sp. rom other local faunas in the Great Plains region (encompassing Nebraska, Kansas, Oklahoma, and Texas) and Florida through the Miocene were also included in the DFA. In both Florida and the Great Plains region, an increase in the abrasiveness of the diet was seen from 12.1 Ma to 7 Ma. In Florida, there is a shift from browsing to mixed feeding during this interval, while in the Great Plains region, there is a shift from mixed feeding to non-strict grazing in Nebraska and to strict grazing in Oklahoma. This shift in mesowear scores likely corresponds to an expansion of grasslands 12.1–7 Ma. There is also a shift in mesowear scores latitudinally from the Optima LF to the Cambridge LF, with Teleoceras hicksi from the Optima LF (Oklahoma; ~36°45’ N) exhibiting higher grazing scores than those from Cambridge LF (Nebraska; ~40°15’ N). The Optima LF and the Cambridge LF are penecontemporaneous, and the difference in mesowear indicates fewer abrasives in the diet of Teleoceras sp. from Cambridge LF than from Optima LF. The decrease in abrasives supports a limited northward expansion of grasslands by 7 Ma. However, the Optima LF is slightly younger than the Cambridge LF, so a time-related difference cannot be fully discounted.
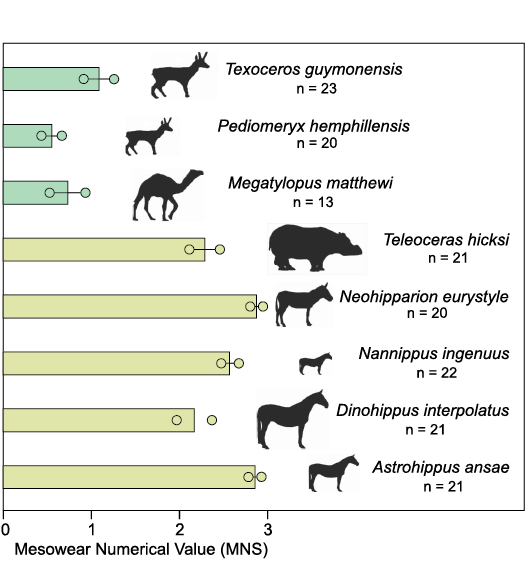
Fig. 5. Mesowear numerical values (MNS) for artiodactyls (green) and perissodactyls (yellow) from the Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA). Color differences indicate groupings that are statistically significantly difference from one another.
The degree of mesowear in the Optima LF perissodactyls indicates the presence of higher abrasives in their diet, which may vary based on the proportion and types of grasses eaten (endogenous grit) and feeding height (exogenous grit). Based on the combined lines of evidence presented here, the horses and rhino likely occupied an open, C4- and C3-rich grassland or savanna ecosystem. Feeding height varies between the species, with the largest taxa, D. interpolatus and Teleoceras hicksi, also showing the lowest mesowear scores and δ13C averages for the order. These differences could be related to less exogenous grit on hypothetically taller grasses (here presumed to be C3) if they were preferred by the larger species.
Artiodactyla.—The Optima LF artiodactyls are more depleted on average in δ13C than the horses or proboscidean. The lowest δ13C values for artiodactyls in this study belong to the peccary Platygonus sp. with an average value of -9.95‰ (n = 9), ranging -10.98 to -8.87‰ (Table 3). This result falls close to the mean average (-8.8‰) for Miocene–Pleistocene peccaries from Florida (MacFadden and Cerling 1996). Samples from Miocene sites in Florida appear more depleted than the Optima LF sample (-11.3‰ for the ~9.5 Ma Love Bone Bed and -11.8‰ for the ~4.5 Ma Bone Valley site), though Platygonus sp. from the Rancholabrean (late Pleistocene) Cutler site yielded a value (-8.3‰) much closer to the Optima LF Platygonus average. This relative difference could be related to regional vegetation or environmental differences between the Gulf Coast and southern Great Plains or to the behavioral differences between the tayassuids included in the Florida sites and our study.
Camelids are represented in our analysis by Megatylopus matthewi, with an average δ13C signature of -9.79‰ (n = 11) and a range -11.42 to -6.08‰ (Table 3). The -6.08‰ value was anomalous as no other data point was more enriched than -8.8‰. Megatylopus sp. (and cf. Megatylopus sp.) from the latest Hemphillian of Florida yielded values of -14.8 and -11.7‰, closer to the low end of the readings from the Optima LF species. The family Palaeomerycidae, represented in the Optima LF by Pediomeryx hemphillensis, yielded an average of -9.8‰ (n = 10; range -11.44 to -8.94‰) (Table 3). This average and range are more depleted than a Pediomeryx specimen (-8.9‰; UF 69945) from the ~7 Ma Moss Acres site (MacFadden and Cerling 1996). These results point to a diet heavier in C3 plants or a more closed-canopy preference for the Optima LF Pediomeryx hemphillensis. Texoceros guymonensis is the only representative of the family Antilocapridae in this study. The average δ13C value for this species is -9.45‰ with a range -10.39 to -8.3‰. Using the 12–14‰ dietary offset for wild ungulates (Cerling and Harris 1999; Howland et al. 2003; Jim et al. 2004; Passey et al. 2005), all artiodactyls in this study were consuming plants with an average δ13C value of approximately -25 to -18‰, as compared to the horses (discussed above), which had a relatively heavier diet of -23 to -10‰. Though overlapping, the large differences in vegetation isotopic ratios indicate a dietary contrast between these groups.
Based on the mesowear analysis, all three artiodactyls tested (Pediomeryx hemphillensis, Texoceros guymonensis, and M. matthewi) were mixed feeders. Overall, these taxa had teeth with high relief and rounded cusps, indicating a more attritional diet. Pediomeryx from the Coffee Ranch LF was also recovered as a mixed feeder, indicating little geographic variation. Texoceros guymonensis exhibited modest variation between the Optima and Coffee Ranch local faunas, appearing as a non-strict grazer to mixed feeder in the Optima LF and as a strict grazer in the Coffee Ranch LF. In both localities, Texoceros incorporated the greatest degree of abrasives in their diet of all the artiodactyls. Megatylopus matthewi also exhibited modest variation, ranging from a mixed feeder at Optima to a non-strict grazer at Coffee Ranch and Cambridge.
Rodentia.—The two relatively large-bodied rodents in this study were isotopically different, which supports the previously hypothesized diets and lifestyles for these creatures based on morphological and phylogenetic data. Dipoides sp., an extinct beaver, is thought to have processed wood and built dams or lodges, similar to, but less efficiently than, the modern species of Castor (Rybczynski 2008; Plint et al. 2020). Based on the distant relationship between Dipoides and modern beavers, it is thought that semi-aquatic, wood-cutting behavior likely evolved at the start of the Miocene over 24 million years ago (Rybczynski 2007). The other rodent, Umbogaulus monodon, is a fossorial scuiromorph ecologically similar to the modern burrowing prairie dog Cynomys sp. (Gobetz 2006). We interpret the differing δ13C values between these rodents as reflecting differences in their respective diets: woody plants and freshwater macrophytes in Dipoides (Plint et al. 2020) and a mixed diet of seeds, roots, grasses, and possibly arthropods in Umbogaulus. Interestingly, the modern Umbogaulus-analog Cynomys has a diet rich in grasses (e.g., Uresk 1984), while the data presented here indicate that grasses were a proportionally lesser food source for Umbogaulus in the Optima LF.
Carnivora.—Of the five tested carnivorans, Amphimachairodus coloradensis was the isotopically most-enriched at an average d13C value of -9‰ (n = 16). Comparable values for the others are progressively more depleted in δ13C: the badger Pliotaxidea cf. nevadensis, -9.2‰ (n = 4); the canids Eucyon davisi, -9.6‰ (n = 5) and Borophagus secundus, -10.0‰ (n = 15); and the bear Agriotherium schneideri, -10.9‰ (n = 7). Unlike in this study, Domingo et al. (2016) found similar isotopic values between the large ursid Indarctos arctoides and the predatory felid Machairodus aphanistus from the late Miocene Cerro de los Batallones fossil complex, Spain. These authors also found high levels of interspecific competition between different-sized predators for the preferred prey, hipparion horses. The Optima LF isotopic data indicate less competition between species, with the two largest taxa Amphimachairodus coloradensis and Agriotherium schneideri having the largest difference in δ13C value.
The carnivorans can be distinguished by hypothetical diet, between the hypercarnivorous Amphimachairodus coloradensis, the small mesocarnivorous dogs and mustelid, and possibly omnivorous Agriotherium schneideri. Indeed, the lack of tooth breakage, low tooth-wear rates, and enriched δ13C values indicate that the large felid, Amphimachairodus coloradensis was feeding on soft tissue with relatively 13C enriched isotopic values. The tooth breakage data for this large scimitar-toothed cat compare closely with that for modern pumas (Puma concolor) and gray wolves (Canis lupus) (Fig. 6). Correcting for predator isotopic-offset using Clementz et al. (2009; +1.3‰), a d13C value of -7.6‰ would be intermediate between the C3-consuming herbivores and C4-consuming horses, supporting a diet consisting of both prey types. This is consistent with the Bayesian mixed model, which reconstructs a diet composed of 61.4% C4-consuming herbivores (horses in this data set).
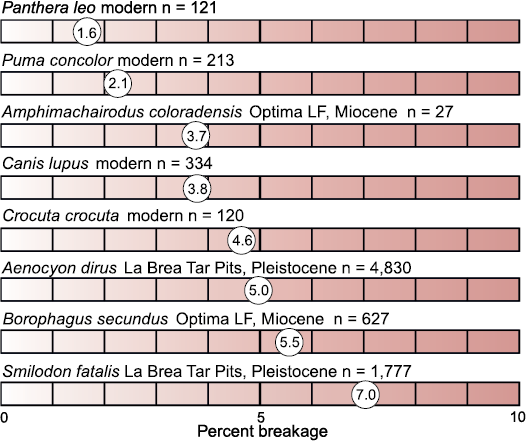
Fig. 6. Tooth breakage percentages for Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA), Pleistocene, and modern felids and canids. Higher percent tooth breakage corresponds with darker shade. Pleistocene and modern data from Van Valkenburgh (2009).
In comparison, the other large-bodied carnivoran, Agriotherium schneideri, shows the opposite pattern with low δ13C values (tooth breakage and wear not reported due to the small sample available). Consistent with these results, Sorkin (2006) found Agriotherium schneideri to be poorly suited for a hunting lifestyle when compared to other ursids and instead likely subsisted upon scavenged remains and dense plant fare. The negative shift in the Optima LF Agriotherium schneideri carbon values as compared to a contemporaneous large felid matches patterns seen in other extinct ursids, such as Ursus spelaeus (Richards et al. 2008), which was previously used as evidence for a herbivorous or omnivorous lifestyle, as also shown by most modern ursids. Because the most common herbivore remains at the Optima site are those of horses (Schultz 2002), if Agriotherium schneideri was a scavenger we would expect horses to make up a considerable part of its diet simply due to their availability. However, even corrected for predator offset values, the isotopic results (average -9.6‰) do not far exceed those of the C3-consuming herbivores. This value for Agriotherium schneideri can be explained in many ways including a diet less carnivorous than expected, a dietary bias towards prey/scavenged items found in closed environments, a preference for larger-bodied scavenged items, or increased consumption of bone compared to other tissues.
The isotopic values for the remaining carnivorans fall between those for the ursid and felid and the taxa are mesocarnivores based on their smaller size. Pliotaxidea cf. nevadensis is smaller than, but hypothetically analogous to, and potentially ancestral to, the modern American badger, Taxidea taxus, a fossorial carnivore that deals primarily with struggling rodent prey (Long 1975; Collins et al. 2007 and citations therein). The high rate of heavily worn teeth in Pliotaxidea cf. nevadensis suggests a high degree of bone crushing, consistent with modern badgers (Fig. 7; Long 1975; Nelson 1990). The borophagine dog, Borophagus secundus, is the most abundant carnivore in the Optima LF and almost certainly represents a predator capable of a significant degree of osteophagy, corroborated by the high tooth breakage rate (5.5%). Recent reports from purported B. secundus coprolites show consumption of both large-bodied and small-bodied prey (Wang et al. 2018). Their abundance and the presence of large prey remains may imply pack hunting behavior in these canids. The other canid, Eucyon davisi, shows fewer adaptations for osteophagy, is much rarer than B. secundus and approximately overlaps with its range of δ13C values in this study. Bartolini Lucenti and Rook (2021) found E. davisi to fall morphologically closer to generalist (omnivorous) feeders, with a diet consisting largely of meat likely from small prey. Taken together, size, carbon isotope data, and dental morphology appear to support broad differences in diet indicative of relatively low competition between predators at this site.
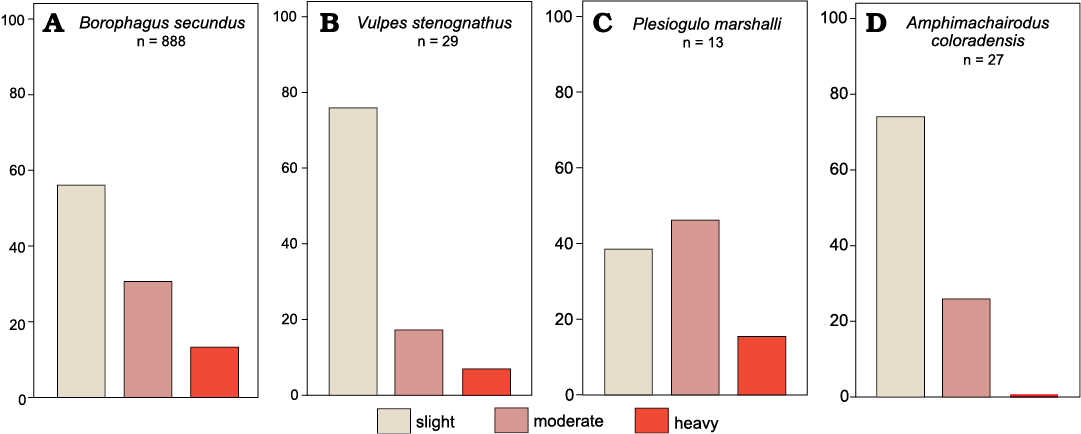
Fig. 7. Tooth wear frequencies for Optima Local Fauna (Miocene, late Hemphillian, Oklahoma, USA) carnivorans. Borophagus secundus (A), Vulpes stenognathus (B), Plesiogulo marshalli (C); Amphimachairodus coloradensis (D).
Environmental significance of the Optima Local Fauna
The late Hemphillian Optima LF is similar in age and faunal composition to those from multiple deposits in neighboring Texas and Kansas (Schultz 2002). These contemporaneous deposits have long drawn comparisons especially in reference to the temporal and geographic expansion of C4 grasses during the late Neogene (e.g., Fox and Koch 2003). As in Passey et al. (2002) and Cerling et al. (1998), both Coffee Ranch and Optima local faunas equids show evidence of C4 grazing in the form of 13C-enriched carbon isotope ratios. Indeed, at both sites three of the four species tested for carbon isotopes possess values above -6.5‰, a likely threshold for positive amounts of C4 consumption by these animals (Cerling et al. 1998). These data differ from those obtained for Nebraska, where horses show generally lower δ13C values in late Hemphillian deposits (e.g., Passey et al. 2002; Kita et al. 2014). Fraser and Theodor (2013) argued that, based on paleosol isotopes, phytolith data, and mammalian faunal structure, differences between Texas and Kansas are not likely related to environmental expansion of C4 at this specific time. Instead, they reasoned that isotopic differences in horses between Texas and Kansas sites were a result of either an over reliance on the data from Coffee Ranch and/or dietary behavioral differences between the sites. Though agnostic towards the greater questions regarding C4 expansion during this time, this study demonstrates that the Optima LF is in congruence with the Coffee Ranch fauna, supporting C4 grasses as a dietary staple of horses in the region. Given the aforementioned geographic and taphonomic similarities between the sites, these results are not surprising but provide a larger data set demonstrating C4 grasses as a regionally important flora for the diet of horses, and also as a dietary signature used to differentiate prey preferences in predators during the Hemphillian in the southern plains.
Conclusions
The Late Miocene Optima LF from the Great Plains region of the panhandle of Oklahoma, North America, reflects a diverse ecosystem that is composed of both open (mixed C3–C4) grasslands and closed forests. Through multiple analyses (stable carbon and oxygen analysis, mesowear analysis, and tooth breakage and wear analyses) we were able to show that the herbivores are largely composed of mixed feeders, among which horses possess the chemical signatures of more 13C-enriched foods (reflecting open habitats and/or C4 grasses) and higher mesowear scores, while all other herbivores exhibit signs of consuming more 13C-depleted vegetation (closed habitats and/or C3 plants) with lower mesowear scores in most taxa scored (except for the rhinoceros Teleoceras hicksi). These differences are followed up the food chain, where the large felid Amphimachairodus coloradensis has an isotopic signature most consistent with a diet of mostly horses. The relatively low tooth breakage rate in this species reflects a predator that primarily consumed soft flesh and little bone. The canids were more generalized carnivores; with higher tooth breakage in Borophagus secundus consistent with increased osteophagy. Our study demonstrates the Optima LF was a diverse ecosystem during the Hemphillian with evidence for a heterogeneous environment in which C4 grasses fundamentally pervaded the food web relative to that of earlier Miocene times.
Acknowledgements
We thank Beth Tweedy (University of Oklahoma, Norman, USA) for assisting with preparation of fossils for the isotopic analysis, Lucy Taylor (University of Oxford, UK) for providing raw mesowear scores on modern rhinocerotids, and Blaire Van Valkenburgh (University of California Los Angeles, USA) for her helpful insight and comments on measuring tooth breakage and wear. Gratitude to Jen Larsen (University of Oklahoma, Norman, USA) for help with the OMNH database. The authors also thank Brian Davis (University of Louisville, USA), Matt Wedel (Western University of Health Sciences, Pomona, USA), and Brooke Haiar (University of Lynchburg, USA) (and many others) for their work on this edition. We are also indebted to William Lukens (James Madison University, Harrisonburg, USA) and an anonymous reviewer for helpful comments and edits that greatly improved this paper. Finally, we thank Rich Cifelli (University of Oklahoma, Norman, USA) for his role in shaping our careers as a mentor and colleague.
References
Ackermans, N.L., Winkler, D.E., Schulz-Kornas, E., Kaiser, T.M., Müller, D.W., Kircher, P.R., Hummel, J., Clauss, M., and Hatt, J.M. 2018. Controlled feeding experiments with diets of different abrasiveness reveal slow development of mesowear signal in goats (Capra aegagrus hircus). Journal of Experimental Biology 221 (21): jeb186411. Crossref
Antón, M., Salesa, M.J., and Siliceo, G. 2013. Machairodont adaptations and affinities of the Holarctic Late Miocene homotherin Machairodus (Mammalia, Carnivora, Felidae): the case of Machairodus catocopis Cope, 1887. Journal of Vertebrate Paleontology 33: 1202–1213. Crossref
Bartolini Lucenti, S. and Rook, L. 2021. “Canis” ferox revisited: Diet ecomorphology of some long gone (Late Miocene and Pliocene) fossil dogs. Journal of Mammalian Evolution 28: 285–306. Crossref
Baskin, J.A. 1982. Tertiary Procyoninae (Mammalia: Carnivora) of North America. Journal of Vertebrate Paleontology 2: 71–93. Crossref
Binder, W.J. and Van Valkenburgh, B. 2010. A comparison of tooth wear and breakage in Rancho La Brea sabertooth cats and dire wolves across time. Journal of Vertebrate Paleontology 30: 255–261. Crossref
Boardman, G.S. and Secord, R. 2013. Stable isotope paleoecology of White River ungulates during the Eocene–Oligocene climate transition in northwestern Nebraska. Palaeogeography, Palaeoclimatology, Palaeoecology 375: 38–49. Crossref
Bocherens, H., Koch, P.L., Mariotti, A., Geraads, D., and Jaeger, J.J. 1996. Isotopic biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene hominid sites. Palaios: 306–318. Crossref
Cerling, T.E. and Harris, J.M. 1999. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120: 347–363. Crossref
Cerling, T.E., Harris, J.M., and MacFadden, B.J. 1998. Carbon isotopes, diets of North American equids, and the evolution of North American C4 grasslands. In Stable Isotopes. In: H. Griffiths (ed.), Stable Isotopes, 363–379. Garland Science, London.
Cerling, T.E., Harris, J.M., MacFadden, B.J., Leakey, M.G., Quade, J., Eisenmann, V., and Ehleringer, J.R. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389: 153–158. Crossref
Clementz, M.T., Fox-Dobbs, K., Wheatley, P.V., Koch, P.L., and Doak, D.F. 2009. Revisiting old bones: coupled carbon isotope analysis of bioapatite and collagen as an ecological and palaeoecological tool. Geological Journal 44: 605–620. Crossref
Cohen, J.E., DeSantis, L.R., Lindsey, E.L., Meachen, J.A., O’Keefe, F.R., Southon, J.R., and Binder, W.J. 2021. Dietary stability inferred from dental mesowear analysis in large ungulates from Rancho La Brea and opportunistic feeding during the late Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology 570: 110360. Crossref
Collins, D.P., Harveson, L.A., and Ruthven, D.C. 2007. Food habits of the American badger (Taxidea taxus) in southern Texas: an observation. Texas Journal of Agriculture and Natural Resources 20: 28–31.
Damuth, J. and Janis, C.M. 2011. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biological Reviews 86: 733–758. Crossref
DeSantis, L.R., Alexander, J., Biedron, E.M., Johnson, P.S., Frank, A.S., Martin, J.M., and Williams, L. 2018. Effects of climate on dental mesowear of extant koalas and two broadly distributed kangaroos throughout their geographic range. PloS One 13 (8): e0201962. Crossref
Díaz-Sibaja, R., Jiménez-Hidalgo, E., Ponce-Saavedra, J., and García-Zepeda, M.L. 2018. A combined mesowear analysis of Mexican Bison antiquus shows a generalist diet with geographical variation. Journal of Paleontology 92: 1130–1139. Crossref
Domingo, M.S., Domingo, L., Abella, J., Valenciano, A., Badgley, C., and Morales, J. 2016. Feeding ecology and habitat preferences of top predators from two Miocene carnivore-rich assemblages. Paleobiology 42: 489–507. Crossref
Dongmann, G., Nürnberg, H.W., Förstel, H., and Wagener, K. 1974. On the enrichment of H218O in the leaves of transpiring plants. Radiation and Environmental Biophysics 11: 41–52. Crossref
Duller, R.A., Whittaker, A.C., Swinehart, J.B., Armitage, J.J., Sinclair, H.D., Bair, A., and Allen, P.A. 2012. Abrupt landscape change post—6 Ma on the central Great Plains, USA. Geology 40: 871–874. Crossref
Erickson, K.L. 2014. Prairie grass phytolith hardness and the evolution of ungulate hypsodonty. Historical Biology 26: 737–744. Crossref
Epstein, S., Thompson, P., and Yapp, C.J. 1977. Oxygen and hydrogen isotopic ratios in plant cellulose. Science 198: 1209–1215. Crossref
Flynn, L.J. and Jacobs, L.L. 2008. Aplodontoidea. In: C.M. Janis, G.F. Gunnell, and M.D. Uhen (eds.), Evolution of Tertiary Mammals of North America, Volume 2: Small Mammals, Xenarthrans, and Marine Mammals, 377–390. Cambridge University Press, Cambridge. Crossref
Fortelius, M. and Solounias, N. 2000. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. American Museum Novitates 3301: 1–36. Crossref
Fox, D.L. and Fisher, D.C. 2001. Stable isotope ecology of a late Miocene population of Gomphotherium productus (Mammalia, Proboscidea) from Port of Entry Pit, Oklahoma, USA. Palaios 16: 279–293. Crossref
Fox, D.L. and Koch, P.L. 2003. Tertiary history of C4 biomass in the Great Plains, USA. Geology 31: 809–812. Crossref
Fox, D.L. and Koch, P.L. 2004. Carbon and oxygen isotopic variability in Neogene paleosol carbonates: constraints on the evolution of the C4-grasslands of the Great Plains, USA. Palaeogeography, Palaeoclimatology, Palaeoecology 207: 305–329. Crossref
Fraser, D. and Theodor, J.M. 2013. Ungulate diets reveal patterns of grassland evolution in North America. Palaeogeography, Palaeoclimatology, Palaeoecology 369: 409–421. Crossref
Frederickson, J.A., Engel, M.H., and Cifelli, R.L. 2018. Niche partitioning in theropod dinosaurs: Diet and habitat preference in predators from the Uppermost Cedar Mountain Formation (Utah, USA). Scientific Reports 8 (1): 1–13. Crossref
Frederickson, J.A., Engel, M.H., and Cifelli, R.L. 2020. Ontogenetic dietary shifts in Deinonychus antirrhopus (Theropoda; Dromaeosauridae): Insights into the ecology and social behavior of raptorial dinosaurs through stable isotope analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 552: 109780. Crossref
Francois, O. and Laval, G. 2011. Deviance information criteria for model selection in approximate Bayesian computation. Statistical Applications in Genetics and Molecular Biology 10 (1) [published online, https://doi.org/10.2202/1544-6115.1678] Crossref
Fricke, H. 2007. Stable isotope geochemistry of bonebed fossils: reconstructing paleoenvironments, paleoecology, and paleobiology. In: R.R. Rogers, D.A. Eberth, and A.R. Fiorillo (eds.), Bonebeds, 437–490. University of Chicago Press, Chicago. Crossref
Fricke, H.C. and Pearson, D.A. 2008. Stable isotope evidence for changes in dietary niche partitioning among hadrosaurian and ceratopsian dinosaurs of the Hell Creek Formation, North Dakota. Paleobiology 34: 534–552. Crossref
Gobetz, K.E. 2006. Possible burrows of mylagaulids (Rodentia: Aplodontoidea: Mylagaulidae) from the late Miocene (Barstovian) Pawnee Creek Formation of northeastern Colorado. Palaeogeography, Palaeoclimatology, Palaeoecology 237: 119–136. Crossref
Goldstein, G. and Sarmiento, G. 1987. Water relations of trees and grasses and their consequences for the structure of savanna vegetation. In: B.H. Walker (ed.), Determinants of Tropical Savannas. IUBS Monograph Series 3: 13–38.
Gonfiantini, R., Gratziu, S., and Tongiorgi, E. 1965. Oxygen isotopic composition of water in leaves. Isotopes and Radiation in Soil-Plant Nutrition Studies 405: 410.
Gordon, C.L. and Czaplewski, N.J. 2000. A fossil marmot from the late Miocene of western Oklahoma. Oklahoma Geology Notes 60: 28–32.
Hammer, Ø., Harper, D.A., and Ryan, P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4 (1): 1–9.
Harrison, J.A. 1979. Revision of the Camelinae (Artiodactyla, Tylopoda) and description of the new genus Alforjas. University of Kansas Paleontological Contributions 95: 1–28.
Harrison, J.A. 1981. A review of the extinct wolverine, Plesiogulo (Carnivora: Mustelidae), from North America. Smithsonian Contributions to Paleobiology 46: 1–27. Crossref
Hesse, C. J. 1936. A Pliocene vertebrate fauna from Optima, Oklahoma. University of California Publications, Bulletin of the Department of Geological Sciences 24: 57–70.
Hirschfeld, S.E. and Webb, S.D. 1968. Plio-Pleistocene megalonychid sloths of North America. Bulletin of the Florida State Museum 12: 213–296.
Hoppe, K.A., Koch, P.L., Carlson, R.W., and Webb, S.D. 1999. Tracking mammoths and mastodons: Reconstruction of migratory behavior using strontium isotope ratios. Geology 27: 439–442. Crossref
Howland, M.R., Corr, L.T., Young, S.M., Jones, V., Jim, S., Van Der Merwe, N.J., Mitchell, A.D., and Evershed, R.P. 2003. Expression of the dietary isotope signal in the compound-specific δ13C values of pig bone lipids and amino acids. International Journal of Osteoarchaeology 13: 54–65. Crossref
Hullot, M., Antoine, P.O., Ballatore, M., and Merceron, G. 2019. Dental microwear textures and dietary preferences of extant rhinoceroses (Perissodactyla, Mammalia). Mammal Research 64: 397–409. Crossref
Jacobs, B.F., Kingston, J.D., and Jacobs, L.L. 1999. The origin of grass-dominated ecosystems. Annals of the Missouri Botanical Garden 86: 590–643. Crossref
Janis, C.M. and Manning, E.M. 1998. Dromomerycidae. In: C.M. Janis, K.M. Scott, and L. Jacobs (eds.), Tertiary Mammals of North America, Volume 1: Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals, 477–490. Cambridge University Press, Cambridge. Crossref
Janis, C.M., Damuth, J., and Theodor, J. M. 2002. The origins and evolution of the North American grassland biome: the story from the hoofed mammals. Palaeogeography, Palaeoclimatology, Palaeoecology 177: 183–198. Crossref
Janis, C.M., Gunnell, G.F., and Uhen, M.D. (eds.) 2008. Evolution of Tertiary Mammals of North America, Volume 2: Small Mammals, Xenarthrans, and Marine Mammals. 802 pp. Cambridge University Press, Cambridge. Crossref
Janis, C.M., Scott, K.M., and Jacobs, L.L. (eds.) 1998. Tertiary Mammals of North America, Volume 1: Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals. 691 pp. Cambridge University Press, Cambridge.
Jim, S., Ambrose, S.H., and Evershed, R.P. 2004. Stable carbon isotopic evidence for differences in the dietary origin of bone cholesterol, collagen and apatite: implications for their use in palaeodietary reconstruction. Geochimica et Cosmochimica Acta 68: 61–72. Crossref
Joeckel, R.M. and Tucker, S.T. 2013. Exceptionally well preserved latest Miocene (Hemphillian) rodent burrows from the eastern Great Plains, United States, and a review of the burrows of North American rodents. Palaios 28: 793–824. Crossref
Jones, D.B. and Desantis, L.R. 2017. Dietary ecology of ungulates from the La Brea tar pits in southern California: a multi-proxy approach. Palaeogeography, Palaeoclimatology, Palaeoecology 466: 110–127. Crossref
Kaiser, T.M. and Schulz, E. 2006. Tooth wear gradients in zebras as an environmental proxy—a pilot study. Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 103: 187–210.
Kita, Z.A., Secord, R., and Boardman, G.S. 2014. A new stable isotope record of Neogene paleoenvironments and mammalian paleoecologies in the western Great Plains during the expansion of C4 grasslands. Palaeogeography, Palaeoclimatology, Palaeoecology 399: 160–172. Crossref
Koch, P.L. 2007. Isotopic study of the biology of modern and fossil vertebrates. Stable Isotopes in Ecology and Environmental Science 2: 99–154. Crossref
Koch, P.L., Tuross, N., and Fogel, M.L. 1997. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. Journal of Archaeological Science 24: 417–429. Crossref
Kohn, M.J. and Cerling, T.E. 2002. Stable isotope compositions of biological apatite. Reviews in Mineralogy and Geochemistry 48: 455–488. Crossref
Kohn, M.J., McKay, M.P., and Knight, J.L. 2005. Dining in the Pleistocene—who’s on the menu? Geology 33: 649–652. Crossref
Kohn, M.J., Schoeninger, M.J., and Valley, J.W. 1996. Herbivore tooth oxygen isotope compositions: effects of diet and physiology. Geochimica et Cosmochimica Acta 60: 3889–3896. Crossref
Korth, W.W. 2000. Review of Miocene (Hemingfordian to Clarendonian) mylagaulid rodents (Mammalia) from Nebraska. Annals of Carnegie Museum 69: 227–280.
Korth, W.W. 2002. Comments on the systematics and classification of the beavers (Rodentia, Castoridae). Journal of Mammalian Evolution 8: 279–296.
Kubo, M.O. and Yamada, E. 2014. The inter-relationship between dietary and environmental properties and tooth wear: comparisons of mesowear, molar wear rate, and hypsodonty index of extant sika deer populations. PloS One 9 (3): e90745. Crossref
Lambert, W.D., and Shoshani, J. 1998. Proboscidea. In: C.M. Janis, K.M. Scott, and L. Jacobs (eds.), Tertiary Mammals of North America, Volume 1: Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals, 606–621. Cambridge University Press, Cambridge. Crossref
Loffredo, L.F. and DeSantis, L.R. 2014. Cautionary lessons from assessing dental mesowear observer variability and integrating paleoecological proxies of an extreme generalist Cormohipparion emsliei. Palaeogeography, Palaeoclimatology, Palaeoecology 395: 42–52. Crossref
Long, C.A. 1975. Growth and development of the teeth and skull of the wild North American badger, Taxidea taxus. Transactions of the Kansas Academy of Sciences 77: 106–120. Crossref
Lukens, W.E., Driese, S.G., Peppe, D.J., and Loudermilk, M., 2017. Sedimentology, stratigraphy, and paleoclimate at the late Miocene Coffee Ranch fossil site in the Texas Panhandle. Palaeogeography, Palaeoclimatology, Palaeoecology 485: 361–376. Crossref
MacFadden, B.J. 1998. Equidae. In: C.M. Janis, K.M. Scott, and L. Jacobs (eds.), Tertiary Mammals of North America, Volume 1: Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals, 537–559. Cambridge University Press, Cambridge. Crossref
MacFadden, B.J. 2000. Cenozoic mammalian herbivores from the Americas: reconstructing ancient diets and terrestrial communities. Annual Review of Ecology and Systematics 31: 33–59. Crossref
MacFadden, B.J. 2005. Diet and habitat of toxodont megaherbivores (Mammalia, Notoungulata) from the late Quaternary of South and Central America. Quaternary Research 64: 113–124. Crossref
MacFadden, B.J. and Cerling, T.E. 1996. Mammalian herbivore communities, ancient feeding ecology, and carbon isotopes: a 10 million-year sequence from the Neogene of Florida. Journal of Vertebrate Paleontology 16: 103–115. Crossref
McDonald, H.G. 2005. Paleoecology of extinct xenarthrans and the Great American Biotic Interchange. Bulletin of the Florida Museum of Natural History 45: 313–333.
Mendoza, M. and Palmqvist, P. 2007. Hypsodonty in ungulates: an adaptation for grass consumption or for foraging in open habitat? Journal of Zoology 2007: 1–9.
Mihlbachler, M.C., Campbell, D., Chen, C., Ayoub, M., and Kaur, P. 2018. Microwear–mesowear congruence and mortality bias in rhinoceros mass-death assemblages. Paleobiology 44: 131–154. Crossref
Mihlbachler, M.C., Rivals, F., Solounias, N., and Semprebon, G.M. 2011. Dietary change and evolution of horses in North America. Science 331: 1178–1181. Crossref
Nelson, M.E. 1990. Holocene predation of the Uinta ground squirrel by a badger. Great Basin Naturalist 50: 385.
Passey, B.H., Cerling, T.E., Perkins, M.E., Voorhies, M.R., Harris, J.M., and Tucker, S.T. 2002. Environmental change in the Great Plains: an isotopic record from fossil horses. Journal of Geology 110: 123–140. Crossref
Passey, B.H., Robinson, T.F., Ayliffe, L.K., Cerling, T.E., Sponheimer, M., Dearing, M.D., Roeder, B.L., and Ehleringer, J.R. 2005. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. Journal of Archaeological Science 32: 1459–1470. Crossref
Perez-Crespo, V.A., Ferrusquía-Villafranca, I., Bravo-Cuevas, V.M., Morales-Puente, P., and Ruiz-González, J.E. 2016. Dietary analysis of late Cenozoic Mexican equids from three different geographic/geologic settings using stable carbon isotopes: coincidences, differences and paleobiologic significance. Journal of South American Earth Sciences 66: 97–109. Crossref
Plint, T., Longstaffe, F.J., Ballantyne, A., Telka, A., and Rybczynski, N. 2020. Evolution of woodcutting behaviour in Early Pliocene beaver driven by consumption of woody plants. Scientific Reports 10 (1): 1–16. Crossref
Prothero, D.R. 1992. Fifty million years of rhinoceros evolution. In: O.A. Ryder (ed.), Rhinoceros Biology and Conservation, 82–91. Zoological Society, San Diego.
Prothero, D.R. 1998. Rhinocerotidae. In: C.M. Janis, K.M. Scott, and L. Jacobs (eds.), Tertiary Mammals of North America, Volume 1: Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals, 595–605. Cambridge University Press, Cambridge. Crossref
Prothero, D.R. 2005. The Evolution of North American Rhinoceroses. 228 pp. Cambridge University Press, Cambridge.
Prothero, D.R. and Liter, M.R. 2007. Family Palaeomerycidae. In: D.R. Prothero and S.E. Foss (eds.), The Evolution of Artiodactyls, 241–248. Johns Hopkins University Press, Baltimore.
Richards, M.P., Pacher, M., Stiller, M., Quilès, J., Hofreiter, M., Constantin, S., Zilhão, J., and Trinkaus, E. 2008. Isotopic evidence for omnivory among European cave bears: Late Pleistocene Ursus spelaeus from the Peştera cu Oase, Romania. Proceedings of the National Academy of Sciences 105: 600–604. Crossref
Rivals, F., Semprebon, G., and Lister, A. 2012. An examination of dietary diversity patterns in Pleistocene proboscideans (Mammuthus, Palaeoloxodon, and Mammut) from Europe and North America as revealed by dental microwear. Quaternary International 255: 188–195. Crossref
Rivals, F., Solounias, N., and Mihlbachler, M.C. 2007. Evidence for geographic variation in the diets of late Pleistocene and early Holocene Bison in North America, and differences from the diets of recent Bison. Quaternary Research 68: 338–346. Crossref
Rybczynski, N. 2007. Castorid phylogenetics: implications for the evolution of swimming and tree-exploitation in beavers. Journal of Mammalian Evolution 14: 1–35. Crossref
Rybczynski, N. 2008. Woodcutting behavior in beavers (Castoridae, Rodentia): estimating ecological performance in a modern and a fossil taxon. Paleobiology 34: 389–402. Crossref
Savage, D.E. 1941. Two new middle Pliocene carnivores from Oklahoma with notes on the Optima fauna. American Midland Naturalist 25: 692–710. Crossref
Schultz, G.E. 2002. Clarendonian and Hemphillian vertebrate faunas from the Ogallala Formation (late Miocene–early Pliocene) of the Texas panhandle and adjacent Oklahoma. In: R. Burkhalter, N. Czaplewski, and R. Lupia (eds.), Society of Vertebrate Paleontology Field Trip Guidebook, 62nd Annual Meeting, Norman, Oklahoma, 2–38. SVP, Norman.
Schulz, E. and Kaiser, T.M. 2013. Historical distribution, habitat requirements and feeding ecology of the genus Equus (Perissodactyla). Mammal Review 43: 111–123. Crossref
Secord, R., Wing, S.L., and Chew, A. 2008. Stable isotopes in early Eocene mammals as indicators of forest canopy structure and resource partitioning. Paleobiology 34: 282–300. Crossref
Semprebon, G. and Rivals, F. 2007. Was grass more prevalent in the pronghorn past? An assessment of the dietary adaptations of Miocene to recent Antilocapridae (Mammalia: Artiodactyla). Palaeogeography, Palaeoclimatology Palaeoecology 253: 332–347. Crossref
Semprebon, G. and Rivals, F. 2010. Trends in the paleodietary habits of fossil camels from the Tertiary and Quaternary of North America. Palaeogeography, Palaeoclimatology, Palaeoecology 295: 131–145. Crossref
Semprebon, G., Janis, C., and Solunias, N. 2004. The diets of the Dromomerycidae (Mammalia: Artiodactyla) and their response to Miocene vegetational change. Journal of Vertebrate Paleontology 24: 427–444. Crossref
Semprebon, G., Rivals, F., Solounias, N., and Hulbert, R.C., Jr. 2016. Paleodietary reconstruction of fossil horses from the Eocene through Pleistocene of North America. Palaeogeography, Palaeoclimatology, Palaeoecology 442: 110–127. Crossref
Sharp, Z.D. and Cerling, T.E. 1998. Fossil isotope records of seasonal climate and ecology: straight from the horse’s mouth. Geology 26: 219–222. Crossref
Sorkin, B. 2006. Ecomorphology of the giant short-faced bears Agriotherium and Arctodus. Historical Biology 18: 1–20. Crossref
Sponheimer, M. and Lee-Thorp, J.A. 1999. Oxygen isotopes in enamel carbonate and their ecological significance. Journal of Archaeological Science 26: 723–728. Crossref
Sternberg, L.D.S.L. 1989. Oxygen and hydrogen isotope ratios in plant cellulose: mechanisms and applications. Stable Isotopes in Ecological Research 68: 124–141. Crossref
Stock, B.C., Jackson, A.L., Ward, E.J., Parnell, A.C., Phillips, D.L., and Semmens, B.X. 2018. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6: e5096. Crossref
Taylor, L.A., Muller, D.W., Schwitzer, C., Kaiser, T., Codron, D., Schulz, E., and Clauss, M. 2014. Tooth wear in captive rhinoceroses (Diceros, Rhinoceros, Ceratotherium: Perissodactyla) differs from that of free-ranging conspecifics. Contributions to Zoology 83: 107–117. Crossref
Tedford, R.H., Albright III, L.B., Barnosky, A.D., Ferrusquía-Villafranca, I., Hunt, R.M., Jr., Storer, J.E., Swisher III, C.C., Voorhies, M.R., Webb, S.D., and Whistler, D.P. 2004. Mammalian biochronology of the Arikareean through Hemphillian interval (late Oligocene through early Pliocene epochs). In: M.O. Woodburne (ed.), Late Cretaceous and Cenozoic Mammals of North America: Geochronology and Biostratigraphy, 169–231. University of California Press, Berkeley. Crossref
Tedford, R.H., Wang, X., and Taylor, B.E. 2009. Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae). Bulletin of the American Museum of Natural History 325: 1–218. Crossref
Tipple, B.J., Meyers, S.R., and Pagani, M. 2010. Carbon isotope ratio of Cenozoic CO2: A comparative evaluation of available geochemical proxies. Paleoceanography 25 (3): 1–11. Crossref
Uresk, D.W. 1984. Black-tailed prairie dog food habits and forage relationships in western South Dakota (Cynomys ludovicianus). Rangeland Ecology & Management/Journal of Range Management Archives 37: 325–329. Crossref
Van Valkenburgh, B. 2009. Costs of carnivory: tooth fracture in Pleistocene and Recent carnivorans. Biological Journal of the Linnean Society 96: 68–81. Crossref
Van Valkenburgh, B. and Hertel, F. 1993. Tough times at La Brea: tooth breakage in large carnivores of the late Pleistocene. Science 261: 456–459. Crossref
Van Valkenburgh, B., Peterson, R.O., Smith, D.W., Stahler, D.R., and Vucetich, J.A. 2019. Tooth fracture frequency in gray wolves reflects prey availability. eLife 8: e48628. Crossref
Venables, W.N. and Ripley, B.D. 2002. Modern Applied Statistics with S. Fourth Edition. 495 pp. Springer, New York. Crossref
Voorhies, M.R. and Thomasson, J.R. 1979. Fossil grass anthoecia within Miocene rhinoceros skeletons: diet in an extinct species. Science 206: 331–333. Crossref
Wang, B. 2016. Stable isotope ecology of rhinoceroses (Mammalia: family Rhinocerotidae) in the Hemphillian land-mammal age of the Great Plains, North America. Geological Society of America Annual Meeting, Denver, Colorado, USA, paper 162-19. [published online, https://gsa.confex.com/gsa/2016AM/webprogram/Paper281895.html] Crossref
Wang, B. and Secord, R. 2020. Paleoecology of Aphelops and Teleoceras (Rhinocerotidae) through an interval of changing climate and vegetation in the Neogene of the Great Plains, central United States. Palaeogeography, Palaeoclimatology, Palaeoecology 542: 109411. Crossref
Wang, X., Tedford, R.H., and Taylor, B.E. 1999. Phylogenetic systematics of the Borophaginae (Carnivora: Canidae). Bulletin of the American Museum of Natural History 243: 1–391.
Wang, X., White, S.C., Balisi, M., Biewer, J., Sankey, J., Garber, D., and Tseng, Z.J. 2018. First bone-cracking dog coprolites provide new insight into bone consumption in Borophagus and their unique ecological niche. eLife 7: e34773. Crossref
Wang, Y., Cerling, T.E., and MacFadden, B.J. 1994. Fossil horses and carbon isotopes: new evidence for Cenozoic dietary, habitat, and ecosystem changes in North America. Palaeogeography, Palaeoclimatology, Palaeoecology 107: 269–279. Crossref
Webb, S.D. 1977. A history of savanna vertebrates in the New World. North America. Annual Review of Ecology and Systematics 8: 355–380. Crossref
Webb, S.D. 1983. The rise and fall of the late Miocene ungulate fauna in North America. In: M. H. Nitecki (ed.) Coevolution, 267–306. University of Chicago Press, Chicago.
Werdelin, L. 1985. Small Pleistocene felines of North America. Journal of Vertebrate Paleontology 5: 194–210. Crossref
West-Eberhard, M.J., Smith, J.A.C., and Winter, K. 2011. Photosynthesis, reorganized. Science 332: 311–312. Crossref
Woodburne, M.O. (ed.) 2004. Late Cretaceous and Cenozoic Mammals of North America: Geochronology and Biostratigraphy. 400 pp. University of California Press, Berkeley. Crossref
Wright, D.B. 1998. Tayassuidae. In: C.M. Janis, K.M. Scott, and L. Jacobs (eds.), Tertiary Mammals of North America, Volume 1: Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals, 389–401. Cambridge University Press, Cambridge. Crossref
Yakir, D. 1992. Variations in the natural abundance of oxygen-18 and deuterium in plant carbohydrates. Plant, Cell & Environment 15: 1005–1020. Crossref
Zanazzi, A. and Kohn, M.J. 2008. Ecology and physiology of White River mammals based on stable isotope ratios of teeth. Palaeogeography, Palaeoclimatology, Palaeoecology 257: 22–37. Crossref
Acta Palaeontol. Pol. 67 (1): 221–238, 2022
https://doi.org/10.4202/app.00941.2021