

Stromatoporoids from a Middle Devonian reef in South China and their palaeoecological implication
JIAYUAN HUANG, KUN LIANG, YUE WANG, STEPHEN KERSHAW, JUWAN JEON, YUE LI, and WENKUN QIE
Huang, J., Liang, K., Wang, Y., Kershaw, S., Jeon, J., Li, Y., and Qie, W. 2022. Stromatoporoids from a Middle Devonian reef in South China and their palaeoecological implication. Acta Palaeontologica Polonica 67 (3): 711–736.
Stromatoporoids are the major constructors of a Givetian (Middle Devonian) fossil reef in shallow marine facies, in the Jiwozhai Member of the Dushan Formation, at Dahekou, near Dushan, Guizhou Province, South China. Stromatoporoids, together with other reef building and dwelling components (rugose corals, tabulates, chaetetids and others), form a high diversity community, making the Jiwozhai reef a palaeobiodiversity hotspot. In this study 11 species belonging to nine genera and four orders are identified, including Gerronostromaria grossum (Clathrodictyida), Pseudotrupetostroma porosum, and Salairella buecheliensis (Stromatoporida), Clathrocoilona spissa, Stictostroma saginatum, and Synthetostroma actinostromoides (Stromatoporellida) and ?Habrostroma laminosum, Parallelopora sp., Stachyodes costulata, Stachyodes fasciculata and Stachyodes sp. (Syringostromatida). Among them, Clathrocoilona spissa and Gerronostromaria grossum are the most abundant stromatoporoid taxa. Stromatoporoid growth form, size, substrate and growth interruption are considered to be key autecological parameters to evaluate their growth behaviour and contribution in reef formation. Skeletons of laminar Clathrocoilona spissa are commonly smaller (up to 40 mm in basal dimension and less than 2 mm in thickness) than other stromatoporoid taxa and frequently encrusted on other organisms. In contrast, Gerronostromaria grossum dominates the assemblage, with its larger laminar growth form (up to 500 mm in basal dimension and 40 mm in thickness) expanding both on bioclastic and clay-rich micritic substrate and shows repeated growth interruptions, altogether evidence that this taxon was resilient to environmental pressure and may have pioneered the reef development. The variation of growth preference among stromatoporoid taxa therefore indicates a different growth strategy of each stromatoporoid in this reef environment.
Key words: Stromatoporoidea, reef, morphology, palaeoecology, Givetian, Guizhou, South China Block.
Jiayuan Huang [jyhuang@nigpas.ac.cn], Kun Liang [kliang@nigpas.ac.cn], Juwan Jeon [jjeon@nigpas.ac.cn], Yue Li [yueli@nigpas.ac.cn], and Wenkun Qie [wkqie@nigpas.ac.cn] (corresponding author), State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Palaeoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; University of Chinese Academy of Sciences (UCAS), Beijing 100049, China.
Yue Wang [gzyuewang@126.com], School of Resources and Environments, Guizhou University, Guiyang 550003, China.
Stephen Kershaw [Stephen.Kershaw@brunel.ac.uk], Department of Life Sciences, Brunel University, Kingston Lane, Uxbridge UB8 3PH, UK; Earth Sciences Department, Natural History Museum, Cromwell Road, London, SW7 5BD, UK.
Received 28 October 2021, accepted 17 January 2022, available online 21 July 2022.
Copyright © 2022 J. Huang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Devonian shallow marine environments are exceptionally highlighted for the worldwide proliferation of reefs in Earth history (Kiessling et al. 1999; Copper 2002; Copper and Scotese 2003). Stromatoporoids were the major reef-building organisms that became highly diverse during the Middle Devonian (Stearn 2015a; Kershaw et al. 2018). The highest Palaeozoic stromatoporoid generic diversity was in the Eifelian (early Middle Devonian); this study focuses on stromatoporoids of Givetian age (late Middle Devonian) with the second-highest diversity, represented in traditional taxonomy by seven orders and 45 genera (Stearn 2015a). As the main reef-building organism, stromatoporoids play a significant role during the colonisation stage of reef development (Jakubowicz et al. 2019) and constitute a large portion of metazoan frameworks especially in Middle to Late Devonian fossil reefs (Cockbain 1984; Cook 1999; Salerno 2008; Da Silva et al. 2011a, b; Wolniewicz 2021). On the South China Block, stromatoporoids achieved their highest diversity (37 genera) and abundance during the Givetian (Dong 2001) and constructed a reef realm extending from eastern Yunnan to northern Guangdong provinces. Stromatoporoids attracted much attention of palaeontologists in South China for over 100 years, including some representative works in Guizhou, Guangxi, Hunan, and Yunnan provinces (e.g., Yabe and Hayasaka 1920; Chi 1940; Yabe and Sugiyama 1941; Yang and Dong 1963, 1979; Wang and Huang 1985; Dong and Liu 1992).
Although the studies of Givetian stromatoporoids in South China are numerous, most previous works were poorly illustrated and written in Chinese, thus unfavourable for interpretation and a worldwide correlation. Many established taxa were limited to simple systematic descriptions with poorly preserved specimens (e.g., Yang and Dong 1979; Dong et al. 1989; Dong and Liu 1992). Also their palaeoecological implications in reef ecosystems were not fully exploited (e.g., Liu and Dong 1991; Dong and Song 1992), thereby limiting a precise reconstruction of the stromatoporoid biodiversity and reef-building habits in the various palaeoenvironments in which they occur.
A recent comprehensive study of the Jiwozhai reef in the Dushan Formation, at Dahekou, Guizhou Province revealed that stromatoporoids are the main reef component (Huang et al. 2020). This study complements the previous work of Huang et al. (2020), providing details of stromatoporoid taxonomy and their palaeoenvironmental aspects in the reef formation. The aims of this study are (i) to provide a systematic treatment of the Chinese Givetian stromatoporoid fauna from Guizhou in relation to global occurrence; this includes revision of these Givetian stromatoporoids according to the updated taxonomic classification provided by Stearn (2015b); and (ii) to interpret the palaeoecological habits of stromatoporoids in this low-relief reef structure.
Institutional abbreviations.—NIGP, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China.
Geological setting
During the Givetian Epoch, the South China Block was separated from north-eastern Gondwana and extended across the palaeoequator in the eastern Palaeo-Tethys Ocean (Xian et al. 2019). Many carbonate platforms and interplatform depressions formed within the interpreted heart-shaped sea across the South China Block, covering a large area of several provinces ranging from the present-day eastern Yunnan, southern Guizhou, Guangxi, middle-southern Hunan, to northwestern Guangdong (Jin and Ju 1998). Over 60 sites in South China preserve coral-stromatoporoid reefs, witnessing the Palaeozoic acme in reef development in the South China epicontinental sea (Huang et al. 2020), probably due to the suitable climate (related to palaeolatitude) and the ongoing transgression (Joachimski et al. 2009; Liao and Ma 2011).
The Dahekou section in Dushan, Guizhou Province, represents one of the most well-known shallow-water stratotype sections of the Devonian in South China (Fig. 1A, B). Palaeogeographically it was part of the Qian-Gui Platform interior, adjacent to the Yangtze landmass (Jin and Ju 1998). Dushan Formation cropping out therein can be divided into three lithological units in ascending order, the Jipao Member (mainly limestone), the Songjiaqiao Member (mainly sandstone) and the Jiwozhai Member (limestone) (Fig. 1C). The formation is generally consistent with the brachiopod Stringocephalus Zone, approximately corresponding to the Givetian Stage (Liao 2003). The Jiwozhai Member, consists mainly of reefal limestone, marly bedded limestone, is partially dolomitized; the member contains the rugose coral Endophyllum–Sunophyllum–Argutastrea assemblage (Liao 2003; Liao and Ma 2011) (Fig. 1C), which corroborates the Givetian age.
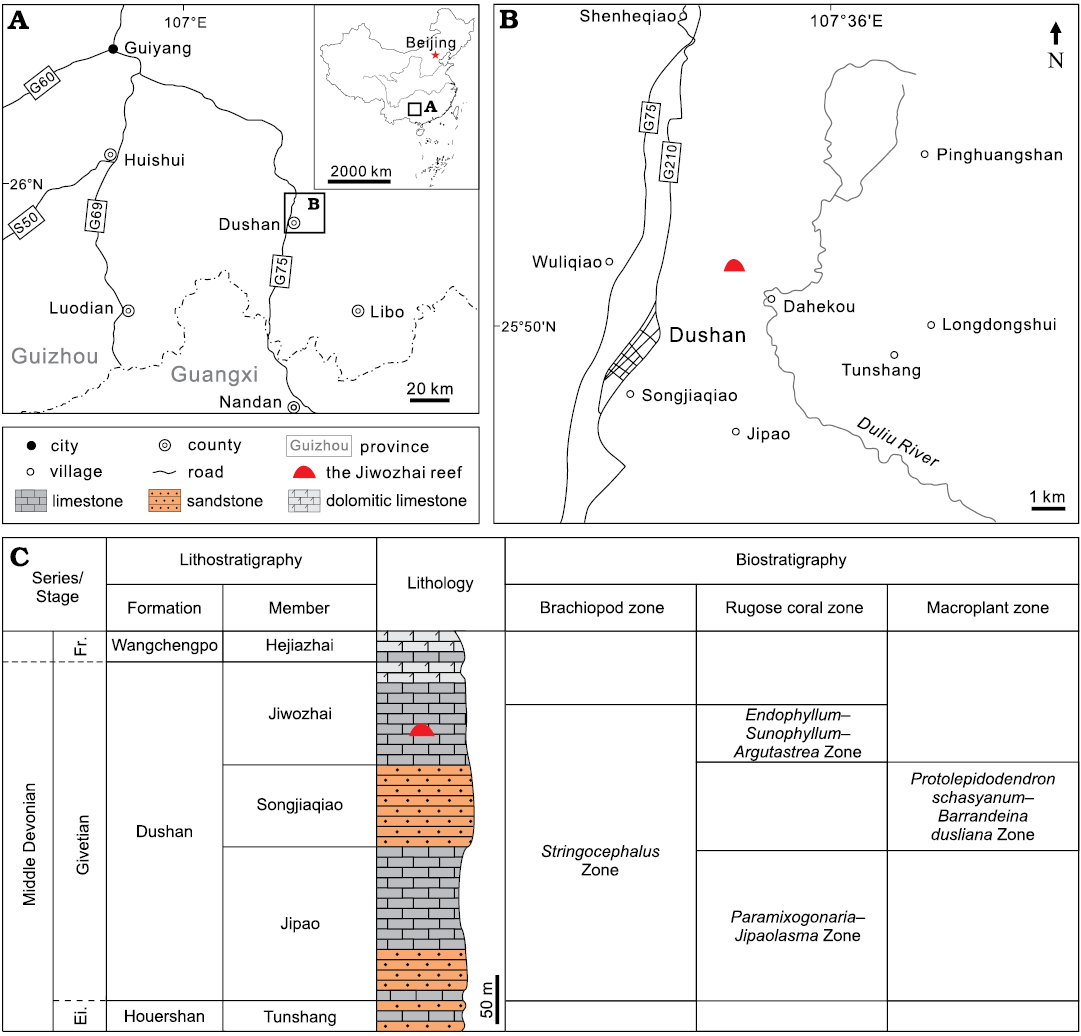
Fig. 1. Location and stratigraphy of the Jiwozhai reef in Dushan County, Guizhou, South China. A. Map showing southern Guizhou and northern Guangxi and the studied area in China. B. Locality of the Jiwozhai reef in Dushan County. C. Depositional successions and fossil assemblages of the supposed uppermost Eifelian to lowermost Frasnian in Dushan, Guizhou province (modified after Liao et al. 1979; Liao 2003). Abbreviations: Ei, Eifelian; Fr., Frasnian.
The Jiwozhai reef (GPS coordinates: 25°50’43.01” N, 107°34’46.51” E), located in the lower part of Jiwozhai Member, is orientated east-west, its exposure extending only 100 meters due to the limitation of a reverse fault in the east, and the strata slope gently towards the cliff in the west (Fig. 2A). The reef is 4.5 m thick (Fig. 2B), with underlying massive argillaceous coral floatstone, mudstone and overlying bedded coral-stromatoporoid floatstone-rudstone (Fig. 2A). The reef is thus consistent with classification as a biostrome, but the limited outcrop means that this cannot be verified at present. A large number of benthic organisms was reported from the reef unit including stromatoporoids, rugose corals, tabulate corals, chaetetid sponges, brachiopods, bryozoans, tubeworms, calcified cyanobacteria, ostracods, gastropods, echinoderms and others (e.g., Huang et al. 2020). Among them, stromatoporoids occur as mainly laminar forms, and are the most significant organisms (Fig. 2B, C). Dendroid and bulbous forms played minor roles for reef building (Fig. 2D). Furthermore, complicated bio-ecological interactions, such as encrustation, spatial competition and endosymbiotic intergrowth, have been recognized among stromatoporoids, tabulates, rugose corals, chaetetid sponges and tubeworms (Huang et al. 2020).

Fig. 2. Field photographs of the Jiwozhai reef.
A. A panoramic view of the
whole reef unit, with a white rectangle indicating the studied area.
Note the reverse fault in the east. B.
A general view shows the studied area of the reef unit. C. Enlarged field photo of the reef
fragment marked in B, showing the thick or thin laminar
stromatoporoids (undulating dashed line) covered on branching
tabulate corals (triangles), solitary rugose corals (crosses),
dendroid stromatoporoids (squares), bulbous stromatoporoid (circled
line), and clay-rich micrites (fine grained sediments between the
metazoan skeletons). D. An
example of polished block of the Jiwozhai reef limestone showing
toppled dendroid or laminar Stachyodes
fasciculata Dong in Dong et al., 1989, and encrusting
Stictostroma saginatum
(Lecompte, 1951) in the upper area. Note the conspicuous digitation
of mamelons of Stictostroma saginatum (Lecompte,
1951) in the lower right corner. Sti,
Stictostroma saginatum (Lecompte,
1951); Sta, Stachyodes fasciculata Dong in Dong et
al., 1989; Tha, Thamnopora cf. pansiensis
Tchi, 1966.
Material and methods
All stromatoporoid samples were collected from the Jiwozhai reefal boundstone in the lower part of the Jiwozhai Member (Dushan Formation) in April 2018 (Fig. 2A). 28 quadrats (0.5×0.5 m for each one), occupying 7 m2 altogether, were chosen from the vertically exposed outcrop of the reef unit (Fig. 2B) for quantitative statistics of abundance, diversity and ecological parameters of reef fauna (Huang et al. 2020). 15 to 45 samples per quadrat were randomly numbered and collected in situ, with detailed photographs showing sample locations (e.g., Fig. 2C). The growth form (including basal dimension and thickness) of each stromatoporoid was measured in the field, and details collected from polished blocks.
A total of 517 samples (some samples contain more than one stromatoporoid) of different sizes (10–150 mm in diameter) were collected from the Jiwozhai Member, and prepared into longitudinal and tangential thin sections for stromatoporoid taxonomy. Based on 1740 thin sections, 773 skeletons of stromatoporoids were identified, belonging to 11 species in nine genera and four orders. Latilaminae and associated substrates were preferentially observed with the thin sections. Well-preserved portions of stromatoporoid skeletons were measured for taxonomic identification. Considering that the skeletal thickness of many thin stromatoporoids is not more than 2 mm, the data of numbers of pachystromes (horizontal skeletal elements) per 2 mm were assembled to provide comparisons between samples and applied in palaeoecological interpretations. Growth form and skeletal terminologies in this paper follow the usage of Kershaw and Riding (1978) and Stearn (2015b), respectively.
Statistics of ecological information of the studied stromatoporoids are presented in the SOM (Supplementary Online Material available at http://app.pan.pl/SOM/app67-Huang_etal_SOM.pdf).
Results
Stromatoporoid composition and spatial distribution.—In total, 11 species belonging to nine genera are identified from the Jiwozhai reef, including Clathrocoilona spissa (Lecompte, 1951), Gerronostromaria grossum Dong, 1974, ?Habrostroma laminosum (Lecompte, 1952), Parallelopora sp., Pseudotrupetostroma porosum (Yang and Dong, 1979), Salairella buecheliensis (Bargatzky, 1881), Stachyodes costulata Lecompte, 1952, Stachyodes fasciculata Dong in Dong et al., 1989, Stachyodes sp., Stictostroma saginatum (Lecompte, 1951), and Synthetostroma actinostromoides Lecompte, 1951. Stromatoporellids are the most abundant stromatoporoid group with three species in three genera. Among them, Clathrocoilona spissa is numerically the most common species, whereas Stictostroma saginatum and Synthetostroma actinostromoides are less common. Syringostromatids include five species of three genera. Species of Stachyodes are relatively common, especially in the upper part of the reef, with two fully identified species and one species in open nomenclature. ?Habrostroma laminosum and Parallelopora sp. are relatively rare. Only one clathrodictyid species, Gerronostromaria grossum is identified, and this species is also abundant. Stromatoporids Salairella buecheliensis and Pseudotrupetostroma porosum are identified, and these stromatoporoid species are relatively rare compared to the other stromatoporoid groups.
Among all these species, Gerronostromaria grossum is the most conspicuous and predominant stromatoporoid in almost all the quadrats of the reefal vertical outcrop (Fig. 3: 1). However, the most abundant species, Clathrocoilona spissa can hardly be distinguished in the field due to its small size (Fig. 3: 2). Pseudotrupetostroma porosum (Fig. 3: 5), Salairella buecheliensis (Fig. 3: 6) and Synthetostroma actinostromoides (Fig. 3: 11) rarely occur in the lower part of the reef unit with their relatively large size. Stachyodes costulata (Fig. 3: 7) and Stachyodes fasciculata (Fig. 3: 8) can be easily identified especially in the upper part of the reefal outcrop owing to their distinctive external form. The other species, ?Habrostroma laminosum (Fig. 3: 3), Parallelopora sp. (Fig. 3: 4), Stachyodes sp. (Fig. 3: 9) and Stictostroma saginatum (Fig. 3: 10), are mostly recognized only in thin sections because of their rare occurrence or small sizes.
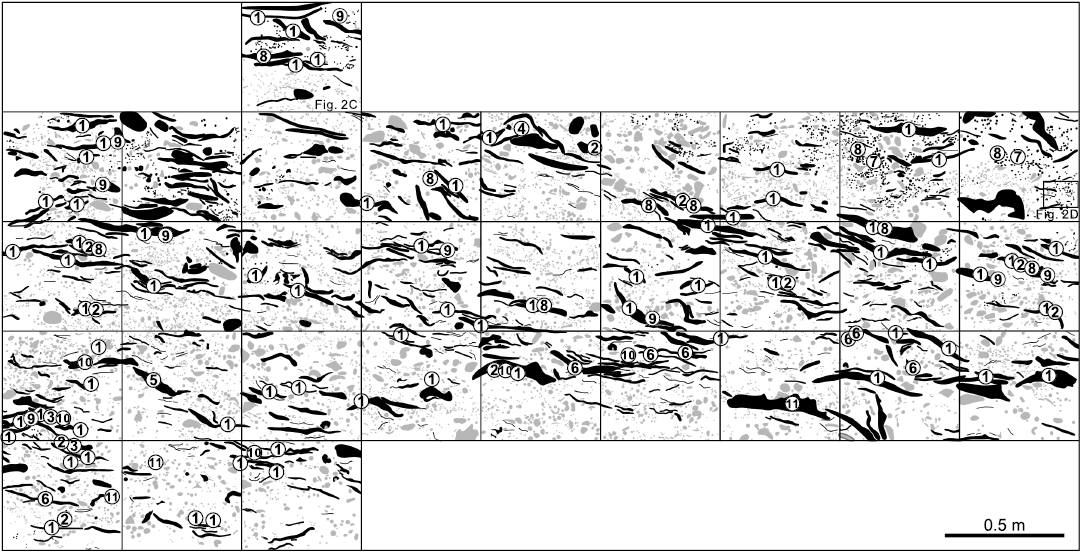
Fig. 3. Schematic vertical section of stromatoporoids in the Jiwozhai reef (Huang et al. 2020) showing the spatial distribution of different stromatoporoids, note the predominance of the Gerronostromaria grossum Dong, 1974, in the laminar form stromatoporoids. 1, Gerronostromaria grossum Dong, 1974; 2, Clathrocoilona spissa (Lecompte, 1951); 3, ?Habrostroma laminosum (Lecompte, 1952); 4, Parallelopora sp.; 5, Pseudotrupetostroma porosum (Yang and Dong, 1979); 6, Salairella buecheliensis (Bargatzky, 1881); 7, Stachyodes costulata Lecompte, 1952; 8, Stachyodes fasciculata Dong in Dong et al., 1989; 9, Stachyodes sp.; 10, Stictostroma saginatum (Lecompte, 1951); 11, Synthetostroma actinostromoides Lecompte, 1951. Black, stromatoporoids; gray, other organisms between stromatoporoids.
Palaeoecology of the stromatoporoid fauna.—Relationships between stromatoporoids, their associated organisms and sediments provide useful information for palaeoecological analysis in terms of external morphology, size, substrate, growth interruption and associated organisms. (e.g., Da Silva et al. 2011a, b; Kershaw et al. 2018; Wolniewicz 2021). We apply four attributes: (i) external morphology; (ii) size; (iii) substrate; (iv) growth interruption to statistically evaluate the palaeoecology of stromatoporoids (Fig. 4).
(i) External morphology. Laminar growth forms of stromatoporoids are most predominant in the Jiwozhai reef. 87% of the investigated stromatoporoids possess laminar growth forms (mostly in situ), and Clathrocoilona spissa is the most common taxa (Fig. 4A). Gerronostromaria grossum, Stictostroma saginatum, and Stachyodes fasciculata also possess laminar growth forms. Dendroid and bulbous growth forms also occur, but these morphologies are relatively rare (Fig. 4A). Only two Stachyodes species show dendroid growth forms, and are commonly preserved as fragments in a variety of orientations together with broken branching tabulates (e.g., Thamnopora cf. pansiensis Tchi, 1966, Cladopora fistula Tchi, 1975, solitary rugose corals). Among these Stachyodes species, S. fasciculata Dong in Dong et al., 1989, rarely shows bulbous growth form, which is approximately 1% of all the investigated stromatoporoids.
(ii) Size. The sizes of stromatoporoids were investigated by measurement of basal dimension and maximum vertical thickness of stromatoporoid skeletons. The basal dimensions generally range 10–50 cm, with some more than 50 cm and even reaching 100 cm. Thickness of stromatoporoids was divided into three groups: (a) <5 mm, (b) 5–10 mm, and (c) >10 mm (Fig. 4B). Small sizes of the skeletons are characteristic of the entire stromatoporoid fauna, 66% of which present a thickness below 5 mm, including the most common Clathrocoilona spissa and a small portion of Gerronostromaria grossum, Stictostroma saginatum, and laminar Stachyodes fasciculata. A small number of stromatoporoids range from mainly 5 mm to 10 mm in thickness, including Gerronostromaria grossum and laminar Stachyodes fasciculata. Very few stromatoporoids show relatively large thicknesses, with representative Gerronostromaria grossum exceeding 10 mm in thickness, rarely more than 20 mm.
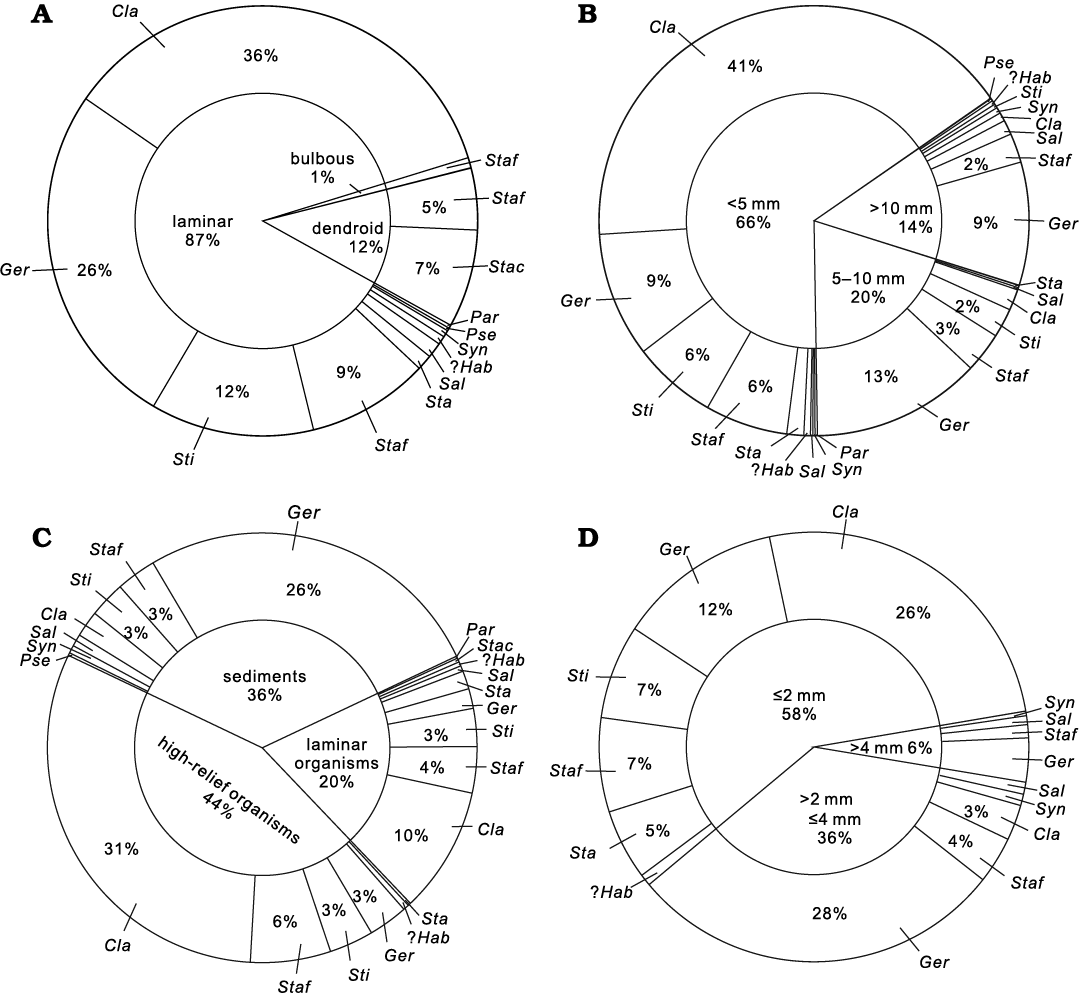
Fig. 4. Pie charts illustrating palaeoecological parameters of the stromatoporiod fauna. A. External morphology. B. Thickness. C. Substrate types. D. Thickness of growth interruption. Abbreviations: Ger, Gerronostromaria grossum Dong, 1974; Cla, Clathrocoilona spissa (Lecompte, 1951); ?Hab, ?Habrostroma laminosum (Lecompte, 1952); Par, Parallelopora sp.; Pse, Pseudotrupetostroma porosum (Yang and Dong, 1979); Sal, Salairella buecheliensis (Bargatzky, 1881); Sta, Stachyodes sp.; Stac, Stachyodes costulata Lecompte, 1952; Staf, Stachyodes fasciculata Dong in Dong et al., 1989; Sti, Stictostroma saginatum (Lecompte, 1951); Syn, Synthetostroma actinostromoides Lecompte, 1951.
(iii) Substrate. Three types of substrates are recognized in the Jiwozhai reef: (a) sediments, (b) growth surface of prior high-relief organism skeletons, and (c) prior to laminar organism skeletons (Fig. 4C). High-relief organisms (e.g., solitary rugose corals, branching and massive tabulate corals, dendroid stromatoporoids) are the most common substrates especially for the small thin laminar Clathrocoilona spissa. The relatively large skeletons of Gerronostromaria grossum generally show preference to cover a large area of sediment including bioclasts and clay-rich micrites after the initial settlement. In addition to encrusting on high-relief organisms, a number of stromatoporoids, such as Clathrocoilona spissa, overgrew different laminar organisms (e.g., chaetetids, tabulate corals or laminar stromatoporoids), and buried by sediments.
(iv) Growth interruption. Growth interruptions are common in the stromatoporoids, represented by latilaminae (prominent tangential sets of horizontal elements of the skeleton bounded by prominent lines representing growth interruptions). The thickness of latilaminae is subdivided into three grades herein: (a) ≤2 mm, (b) >2 mm and ≤4 mm, and (c) >4 mm (Fig. 4D). More than half of latilaminae (58%) are less than 2 mm, 36% of them ranges 2–4 mm, and remaining 6% of them are over 4 mm in thickness. The thickness and numbers of latilaminae are considered to be evidence that Gerronostromaria grossum and Synthetostroma actinostromoides were exceedingly resilient and recovered quickly after interruptions (which were most likely caused by sediment deposition). However, Clathrocoilona spissa, Stictostroma saginatum and laminar Stachyodes fasciculata, which frequently encrusted high-relief organisms, were presumably vulnerable to the interruption triggered by sedimentation and/or sporadic high-energy currents and could hardly keep overgrowing.
Systematic palaeontology
Phylum Porifera Grant, 1836
Class Stromatoporoidea Nicholson and Murie, 1878
Order Clathrodictyida Bogoyavlenskaya, 1969
Family Gerronostromatidae Bogoyavlenskaya, 1969
Genus Gerronostromaria Nestor, 2011
Type species: Gerronostroma elegans Yavorsky, 1931; Middle Devonian of Kuznetsk Basin, Russia.
Gerronostromaria grossum Dong, 1974
Fig. 5A.
1974 Gerronostroma grossum sp. nov.; Dong 1974: 221, pl. 102: 5, 6.
1979 Gerronostroma grossum Dong, 1974; Yang and Dong 1979: 39, pl. 14: 3, 4.
1982 Gerronostroma grossum Dong, 1974; Dong and Wang 1982: 13, pl. 4: 3, 4.
1992 Parallelopora ostiolata Bargatzky, 1881; Dong and Song 1992: 32, pl. 4: 2a.
2020 Actinostroma undulatum Yang and Dong, 1963; Huang et al. 2020: 7, text-fig. 5A.
2020 Trupetostroma dushanense Yang and Dong, 1963; Huang et al. 2020: 11, text-fig. 7G, H, 8B.
Material.—202 specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with one specimen (NIGP 177047) illustrated.
Description.—Skeletons are laminar, with thickness generally between 5–25 mm, occasionally exceeding 25 mm and up to 40 mm. Growth surface features are not visible because stromatoporoids are cemented into limestone surfaces, thus not visible. In longitudinal section, regular networks are formed by continuous laminae and pillars (Fig. 5A1), commonly with regularly spaced latilaminae (Fig. 5A1), ranging 1–6 mm in thickness. Pillars are thick, post-like or occasionally upward spreading, vertically superposed through a number of laminae (up to 22 counted), spacing 7–10 per 2 mm, ranging 0.05–0.2 mm in thickness. Laminae are thin or locally thickening, laterally extensive, slightly undulose, spacing 12–16 per 2 mm, ranging 0.02–0.05 mm in thickness. Galleries are horizontal elongate, or round. Mamelons are conspicuous, 4–6 mm wide. Dissepiments are abundant in the astrorhizal canals and occasionally common in the galleries. Spiral tubes are frequently observed. In tangential section, pillars are isolated and round (Fig. 5A2). Laminae are present as concentric circles in tangential section are due to curvature of laminae in the longitudinal plane. Astrorhizae are prominent, ranging 2–6 mm in diameter, at a distance of 4–6 mm from one to another, with central astrorhizal canals. The microstructure is essentially compact, but occasionally clinoreticular probably due to diagenetic alteration (Fig. 5A3, A4).
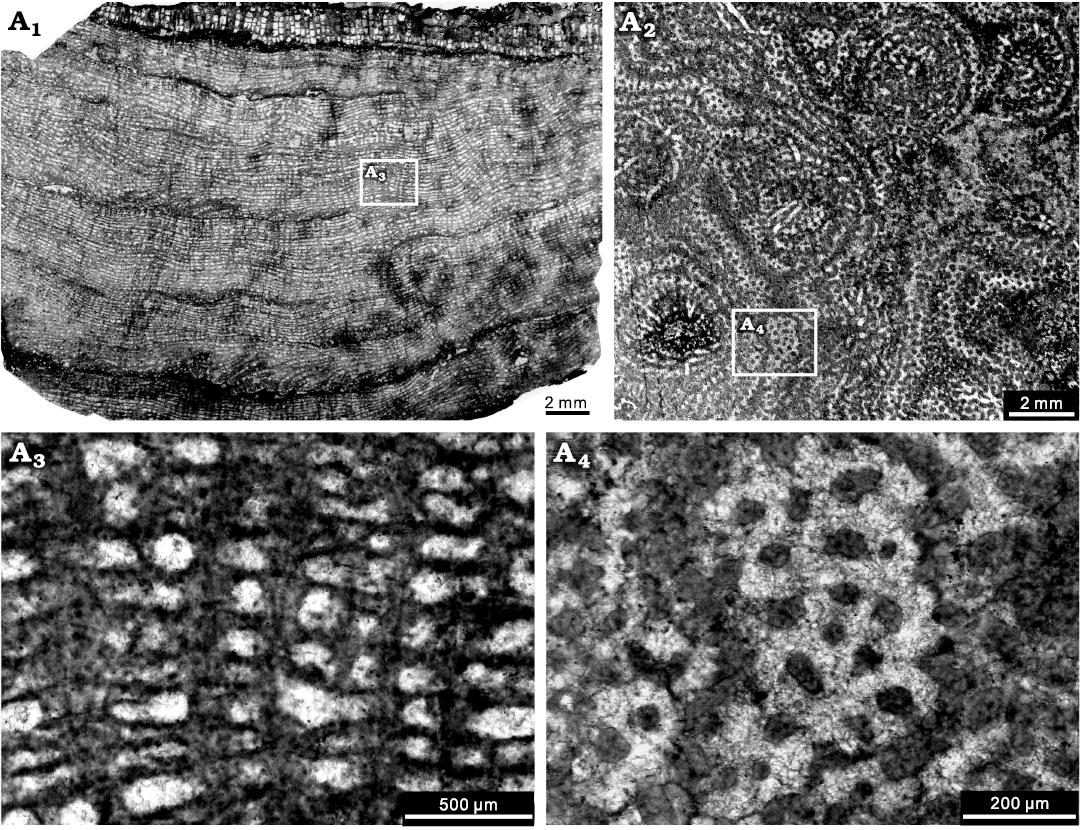
Fig. 5. Stromatoporoid Gerronostromaria grossum Dong, 1974, NIGP 177047, from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A1, longitudinal section, note the well differentiated network constructed by thin laminae and pillars; A2, tangential section showing the distinct mamelons; A3, enlargement of A1, illustrating the common occurrence of the diagenetic “clinoreticular” microstructure inside the continuous pillars; A4, enlargement of A2, showing the isolated pillars.
Remarks.—The present species was previously assigned to Actinostroma undulatum Yang and Dong, 1963, by Huang et al. (2020) due to the suspected colliculi and regular network, but it essentially resembles Gerronostromaria grossum Dong, 1974, described by Dong and Wang (1982) in relation to the dense laminae and thick pillars. Also it was formerly classified as Trupetostroma dushanense Yang and Dong, 1963, by Huang et al. (2020) according to the regular network formed by thin laminae and superposed pillars, but Trupetostroma Parks, 1936, possesses tripartite laminae that are not present in Gerronostromaria Nestor, 2011. Dong and Song (1992) identified this species as Parallelopora ostiolata Bargatzky, 1881, by the appearance of unclear microreticulate microstructure, but the regular networks formed by continuous laminae and pillars in longitudinal sections of Gerronostromaria grossum Dong, 1974, are totally different from the dominant pachysteles of Parallelopora Bargatzky, 1881, and the supposed microreticulate microstructure may not represent the original structure.
Stratigraphic and geographic range.—Givetian (Middle Devonian) of Yunnan, Guizhou, Guangxi, and Hunan provinces, South China.
Order Stromatoporellida Stearn, 1980
Family Stromatoporellidae Lecompte, 1951
Genus Clathrocoilona Yavorsky, 1931
Type species: Clathrocoilona abeona Yavorsky, 1931; Middle Devonian of Kuznetsk Basin, Russia.
Clathrocoilona spissa (Lecompte, 1951)
Fig. 6.
1951 Stromatoporella spissa sp. nov.; Lecompte 1951: 187, pl. 27: 1–4.
1968 Stromatoporella spissa Lecompte, 1951; Flügel and Flügel-Kahler 1968: 399.
1971 Stromatopora spissa (Lecompte, 1951); Kaźmierczak 1971: 92, pl. 21: 2.
1971 Clathrocoilona spissa (Lecompte, 1951); Zukalová 1971: 56, pl. 15: 1, 2.
1974 Clathrocoilona spissa (Lecompte, 1951); Flügel 1974: 165, pl. 24, 26, 27.
1980 Clathrocoilona spissa (Lecompte, 1951); Mistiaen 1980: 196, pl. 7: 3–9.
1984 Clathrocoilona spissa (Lecompte, 1951); Cockbain 1984: 25, pl. 11: 1–4.
1985 Clathrocoilona spissa (Lecompte, 1951); Mistiaen 1985: 96, pl. 6: 6–8.
1988 Clathrocoilona spissa (Lecompte, 1951); Mistiaen 1988: 174.
1993 Clathrocoilona (Clathrocoilona) solidula spissa (Lecompte, 1951); May 1993: 38, pl. 7: 2; pl. 8: 1, 2.
1999 Clathrocoilona (Clathrocoilona) solidula spissa (Lecompte, 1951); May 1999: 127.
1999 Clathrocoilona spissa (Lecompte, 1951); Cook 1999: 507, text-fig. 28C, 29.
2005 Clathrocoilona (Clathrocoilona) solidula spissa (Lecompte, 1951); May 2005: 180, pl. 13: 2, 3.
2008 Clathrocoilona spissa (Lecompte, 1951); Salerno 2008: 74, pl. 11: 2.
2020 Clathrocoilona spissa (Lecompte); Huang et al. 2020: 7, text-fig. 5B; 11, text-fig. 8C, F.
2020 Clathrocoilona crassitexta (Lecompte); Huang et al. 2020: 8, text-fig. 6A, G, I, 11, text-fig. 8A, D, F.
2020 Clathrocoilona obliterata (Lecompte); Huang et al. 2020: 11, text-fig. 8F.
Material.—275 specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with two representative specimens (NIGP 177048 and NIGP 177049) illustrated.
Description.—Skeletons are thin laminar in growth form, commonly below 2 mm in thickness, with only a few exceeding 5 or 10 mm, in almost all samples encrusting high-relief organisms, such as tabulate corals (e.g., Thamnopora cf. pansiensis Tchi, 1966), solitary rugose corals (e.g., Mictophyllum cf. shawoziense He, 1978), or other laminar organisms, such as bryozoans (e.g., Fistuliporella hemispheroidea Yang, 1954), and other species of stromatoporoids. Surface features cannot be viewed because the stromatoporoids are enclosed in cemented limestone. Latilaminae are conspicuous, ranging 0.2–6 mm in thickness, mostly within 2 mm. In longitudinal section, the skeletons show distinct skeletal variations (Fig. 6A). In some areas, the structures are generally amalgamated, with laminae and pillars being hardly differentiated (Fig. 6A2). Laminae and pillars are irregularly thick with regard to both their thickness and width; they range, respectively, from 0.2 to 0.4 mm thickness, and spacing is 4–6 per 2 mm. Gallery shapes are variable, round or irregular, with or without abundant dissepiments. In the other areas, skeletons are constructed by regular laminae and pillars (Fig. 6A3), such as the terminal part of latilaminae (Fig. 6B1). Laminae are continuous, formed by the lighter axial zone and the coated dark layer, spacing 8–14 per 2 mm, 0.02–0.06 mm in thickness, with frequent single layers in a thickness of 0.2 mm (Fig. 6A3). Pillars are spool-shaped, confined to the interlaminar spaces, with thinner ones 0.02–0.04 mm in thickness, thicker ones 0.1–0.2 mm in thickness, spacing 8–12 per 2 mm. In tangential section of the regular areas, pillars appear isolated, 0.05–0.1 mm in thickness (Fig. 6B2). Laminae are occasionally tripartite with conspicuously light axial zone in regular area.
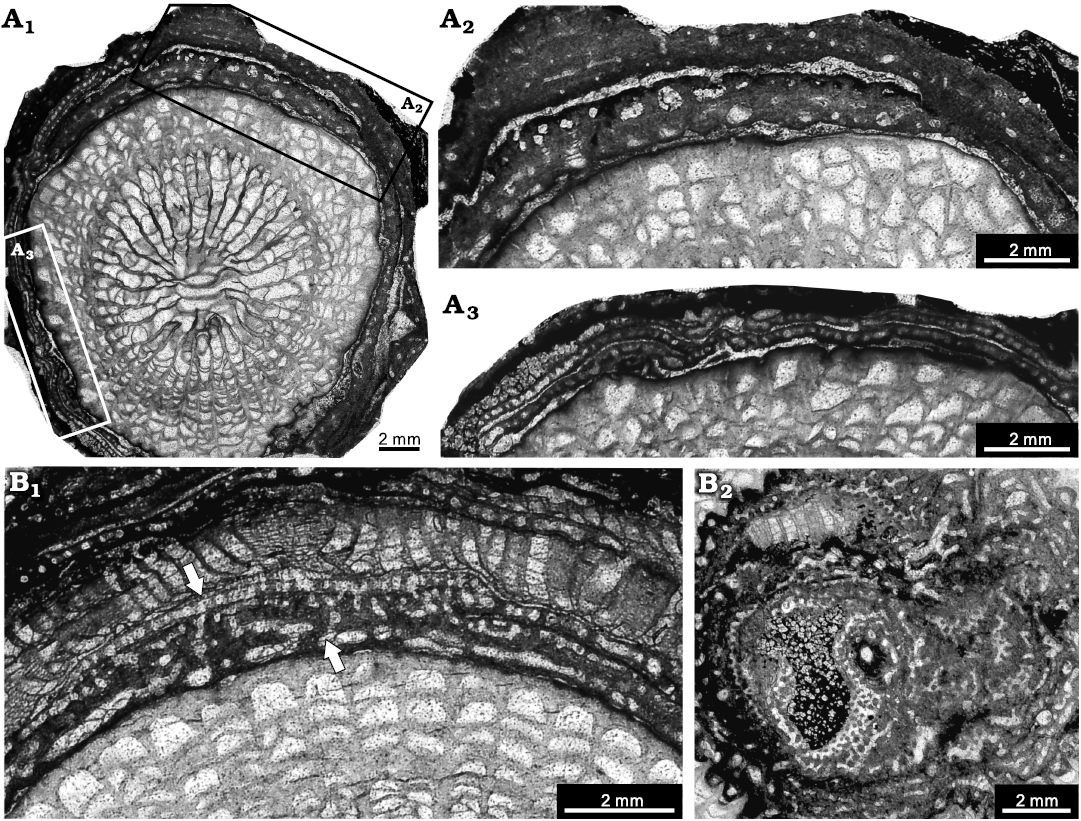
Fig. 6. Stromatoporoid Clathrocoilona spissa (Lecompte, 1951), from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A. NIGP 177048; A1, longitudinal section showing the contemporarily encrusting layers, with black and white rectangles illustrating details; A2, A3, enlargement of A1, note the obvious discrepancy between different areas in the same stromatoporoid: the condensed skeletons of A2 are similar to Clathrocoilona spissa (Lecompte, 1951) and thin laminae of A3 are consistent with Clathrocoilona obliterata (Lecompte, 1951). B. NIGP 177049; B1, longitudinal section, note the intra-specific variations (white arrows) of the lower irregular layers similar to Clathrocoilona crassitexta (Lecompte, 1951) and the upper regular layers identical to Clathrocoilona obliterata (Lecompte, 1951); B2, tangential section.
Remarks.—The present species resembles Clathrocoilona crassitexta (Lecompte, 1951), C. obliterata (Lecompte, 1951), and C. spissa (Lecompte, 1951) from the Dinant Basin, southern Belgium. Thus, this species was preliminarily assigned to Clathrocoilona crassitexta, C. obliterata and C. spissa, respectively, in Huang et al. (2020) using different samples. However, detailed study reveals intra-specific variations in specimens of the Jiwozhai reef. The irregular areas show close similarity to Clathrocoilona crassitexta because of their densely irregular skeletons, and the regular areas retain a high degree of consistency with C. obliterata in terms of prominent tripartite structure and regular thin laminae and short pillars. Therefore, all these characteristics are present within the same specimens, and this may indicate that Clathrocoilona spissa, C. obliterata, and C. crassitexta are synonymous species with intra-specific variations. Here, we temporarily assign this species to Clathrocoilona spissa due to the dominant irregular skeletons.
Stratigraphic and geographic range.—Lower Givetian (Middle Devonian) of Eifel, Germany, and central Bohemia, Czech Republic; lower to middle Givetian (Middle Devonian) of Guangxi and Guizhou provinces, South China, and Queensland, Australia; Givetian to Frasnian (Middle–Upper Devonian) of Avesnois and Boulonnais, France, Dinant Basin, Belgium, Holy Cross Mountains, Poland; lower Frasnian (Upper Devonian) of Moravian Karst, Czech Republic; Frasnian (Upper Devonian) of Canning Basin, Australia.
Genus Stictostroma Parks, 1936
Type species. Stictostroma gorriense Stearn, 1995; Emsian (Lower Devonian) of Gorrie, Ontario, Canada.
Stictostroma saginatum (Lecompte, 1951)
Fig. 7A, B.
1951 Stromatoporella saginata sp. nov.; Lecompte 1951: 171, pl. 22: 5–7; pl. 23: 1–3.
1968 Clathrocoilona saginata (Lecompte, 1951); Flügel and Flügel-Kahler 1968: 373.
1983 Clathrocoilona cf. saginata (Lecompte, 1951); Stearn 1983: 549, text-fig. 5G–H.
1984 Clathrocoilona saginata (Lecompte, 1951); Cockbain 1984: 25, pl. 10: A–D.
1985 Stictostroma saginatum (Lecompte, 1951); Mistiaen 1985: 115, pl. 8: 6–11.
1988 Stictostroma saginatum (Lecompte, 1951); Mistiaen 1988: 176, pl. 21: 5–9.
1999 Stictostroma saginatum (Lecompte, 1951); Mistiaen 1999: 36, pl. 3: 9–12; pl. 4: 1, 2.
2000 Stictostroma saginatum (Lecompte, 1951); Mistiaen and Gholamalian 2000: 83, pl. 6: 6–8.
2008 Stictostroma saginatum (Lecompte, 1951); Salerno 2008: 82, pl. 12: 2.
2020 Stictostroma saginatum (Lecompte, 1951); Huang et al. 2020: 7, text-fig. 5C.
Material.—95 specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with two typical specimens (NIGP 177050 and NIGP 177051) illustrated.
Description.—Skeletons are laminar, with common prominent digitation of mamelons forming columns (a maximum of 25 mm in height) (Figs. 2D, 7A), occasionally encrusting on solitary rugose corals or branching tabulate corals (e.g., Thamnopora sp., solitary rugose corals) (Fig. 7B). In longitudinal section, the skeleton consists of extensive laminae and restricted pillars. Laminae are extensive, gently undulating but sharply increased around the mamelons, spacing 8–12 per 2 mm and 0.05–0.2 mm in thickness. Pillars are spool-shaped, commonly confined to one interlaminar space, irregularly spacing about 7–10 in 2 mm and 0.05–0.2 mm in thickness. Galleries are circular, oval, or horizontally elongate. Mamelons are protruding, with central astrorhizal canals, generally 2–20 mm in height. In tangential section, individual pillars form round dots, 0.05–0.1 mm in diameter; laminae present as concentric circles. Mamelons are distinct, showing a round central astrorhizal canals, with separated by distances of 2–4 mm (Fig. 7A1). The microstructures are compact or ordinicellular (Fig. 7A3).
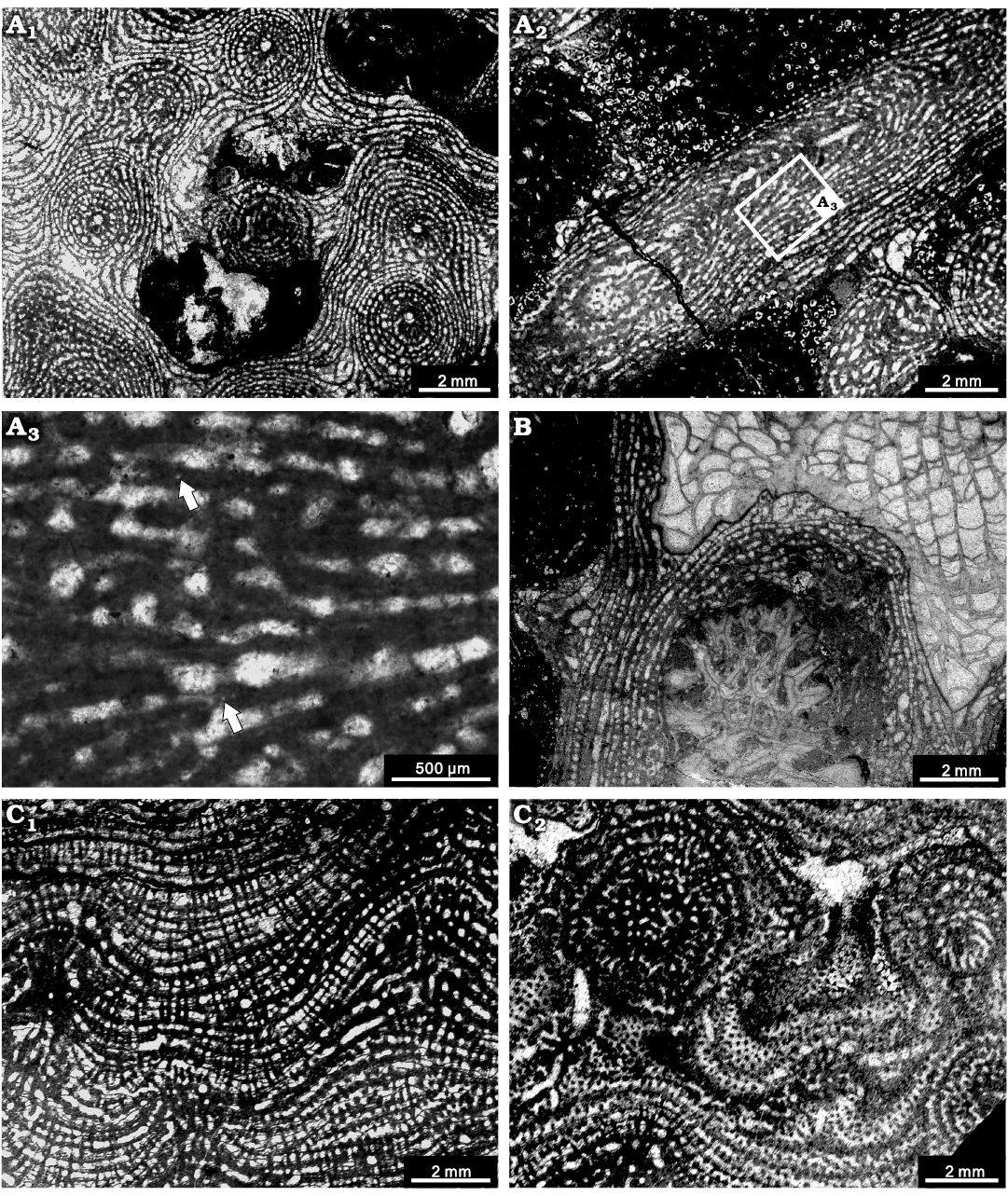
Fig. 7. Stromatoporoids from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A, B. Stictostroma saginatum (Lecompte, 1951) A. NIGP 177050; A1, tangential section showing the isolated pillars and locally irregular skeleton; A2, longitudinal section, note the prominently vertically erected mamelon; A3, enlargement of A2, note the coarsely ordinicellular microstructure (arrows). B. NIGP 177051; longitudinal section, note the encrusting skeleton over Thamnopora sp. debris and a solitary rugose coral. C. Synthetostroma actinostromoides Lecompte, 1951, NIGP 177052; C1, longitudinal section; C2, tangential section.
Remarks.—The skeletal structures of this species are exactly the same as Stromatoporella saginata Lecompte, 1951, and Stictostroma saginatum described by Mistiaen (1988: 176–179, pl. 21: 8, 9). Although Stictostroma Parks, 1936, is highly similar to Stromatoporella Nicholson, 1886, in relation to extensive laminae, confined pillars, and ordinicellular microstructure, the latter genus is characterized by abundant ring pillars. In the absence of ring pillars, the species was assigned to Clathrocoilona Yavorsky, 1931 (Flügel and Flügel-Kahler 1968; Stearn 1983; Cockbain 1984) or Stictostroma (Mistiaen 1985, 1988, 1999; Salerno 2008). It closely resembles Clathrocoilona in terms of laterally continuous laminae and confined pillars, but they are different in microstructure. Here, we assign this species to Stictostroma due to the coarsely ordinicellular microstructure.
Stratigraphic and geographic range.—?Emsian (Lower Devonian) of Ellesmere Island, Canada; Givetian (Middle Devonian) of Eifel, Germany and Guizhou Province, South China; lower Frasnian (Upper Devonian) of Central Mountains, Afghanistan; middle Frasnian (Upper Devonian) of Boulonnais and Avesnois, France; upper Frasnian (Upper Devonian) of Dinant and Namur basins, Belgium; Frasnian (Upper Devonian) of Kerman and Esfahan provinces, Iran, and Canning Basin, Australia (Mistiaen and Gholamalian 2000).
Genus Synthetostroma Lecompte, 1951
Type species: Synthetostroma actinostromoides Lecompte, 1951; Givetian (Middle Devonian) of Surice, Belgium.
Synthetostroma actinostromoides Lecompte, 1951
Fig. 7C.
1951 Synthetostroma actinostromoides sp. nov.; Lecompte 1951: 194, pl. 20: 3, 4.
1968 Synthetostroma actinostromoides Lecompte, 1951; Flügel and Flügel-Kahler 1968: 18.
1971 Synthetostroma actinostromoides Lecompte, 1951; Zukalová 1971: 53, pl. 12: 1–5.
1978 Synthetostroma actinostromoides Lecompte, 1951; Huang 1978: 33, pl. 6: 4.
?1978 Synthetostroma actinostromoides Lecompte, 1951; Wang 1978: 35, pl. 18: 1.
1980 Synthetostroma cf. actinostromoides Lecompte, 1951; Mistiaen 1980: 196, pl. 9: 3–6.
1982 Trupetostroma doupenglingense Yang and Dong, 1979; Dong and Wang 1982: 14, pl. 5: 7, 8.
1982 Pseudoactinodictyon cf. distanum Yang and Dong, 1979; Dong and Wang 1982: 16, pl. 7: 7, 8
?1988 Synthetostroma actinostromoides Lecompte, 1951; Wang 1988: pl. 15: 1.
1992 Synthetostroma actinostromoides Lecompte, 1951; Dong and Song 1992: 29, pl. 2: 3.
1992 Trupetostroma assamptum Yang and Dong, 1979; Dong and Liu 1992: 167, pl. 1: 1.
1992 Clathrocoilona cf. variabilis Yang and Dong, 1979; Dong and Liu 1992: 169, pl. 3: 4.
1993 Clathrocoilona (Synthetostroma) actinostromoides (Lecompte, 1951); May 1993: 40, pl. 10: 1.
2008 Synthetostroma actinostromoides Lecompte, 1951; Salerno 2008: 79, pl. 11: 1.
2020 Synthetostroma actinostromoides Lecompte, 1951; Huang et al. 2020: 7, text-fig. 5D.
Material.—Four specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with one specimen (NIGP 177052) illustrated.
Description.—Skeletons are commonly laminar. Among the four specimens, two small ones preserved as fragments, 3–5 mm in thickness. The largest one shows a thickness up to 50 mm (Fig. 3). Latilaminae exist, each latilamina is 2–6 mm thick. In longitudinal section, the skeletons consist of multilamellar laminae and slender pillars (Fig. 7C1). Laminae are continuous, undulating, integrated by multiple microlaminae and abundant imbricating dissepiments, spacing 5–7 per 2 mm and 0.05–0.2 mm in thickness. Pillars are post-like, confined to interlaminar spaces but systematically superposed, penetrating 3–4 laminae, spacing 7–10 per 2 mm and 0.05–0.15 mm in thickness. The galleries are variable, rectangular to subcircular. Mamelons are conspicuous, with a height of 4–6 mm (Fig. 7C1). Astrorhizal canals and interlaminar spaces are often pervaded by dissepiments. In tangential section, isolated pillars are rounded or locally irregular in form, in diameter of 0.1–0.2 mm. Laminae show concentric circles around mamelon. Mamelons are 2–3 mm in width, with a distance of 4–8 mm from one to another (Fig. 7C2). The microstructure is compact.
Remarks.—The present specimens from Jiwozhai reef share high morphological similarities with the holotype of Synthetostroma actinostromoides Lecompte, 1951, and show well-preserved mamelons. Although Synthetostroma actinostromoides once was regarded as a junior synonym of Clathrocoilona by Kosareva (1976), the presence of superposed pillars and frequent microlaminae is not matched with the generic concept of Clathrocoilona (laminae thick, tripartite; pillars confined to the interlaminar spaces, not systematically superposed). Trupetostroma doupenglingense Yang and Dong, 1979, Pseudoactinodictyon cf. distanum Yang and Dong, 1979, described by Dong and Wang (1982) and Trupetostroma assamptum Yang and Dong, 1979, Clathrocoilona cf. variabilis Yang and Dong, 1979, described by Dong and Liu (1992) seem to be similar to the Jiwozhai reef specimens in terms of tripartite laminae and abundant dissepiments, thus further re-evaluation of these species is required.
Stratigraphic and geographic range.—Lower Givetian (Middle Devonian) of Eifel and Sauerland, Germany; middle Givetian (Middle Devonian) of Boulonnais, France; Guizhou, Hunan, and Yunnan provinces, South China, and Dinant Basin, Belgium; ?upper Givetian to lower Frasnian (Middle–Late Devonian) of Moravian Karst, Czech Republic.
Order Stromatoporida Stearn, 1980
Family Stromatoporidae Winchell, 1867
Genus Pseudotrupetostroma Khalfina and Yavorsky, 1971
Type species: Stromatopora pellucida artyschtensis Yavorsky, 1955; Givetian (Middle Devonian) of Kuznetsk Basin, Russia.
Pseudotrupetostroma porosum (Yang and Dong, 1979)
Fig. 8A.
1979 Hermatostroma porosum sp. nov.; Yang and Dong 1979: 68, pl. 38: 7, 8.
1980 Hermatostroma parksi Lecompte, 1952; Mistiaen 1980: 202: pl. 9: 7–9; pl. 10: 1, 2.
1982 Trupetostroma cellulosum Lecompte, 1952; Dong and Wang 1982: 13, pl.5: 1, 2.
1982 Hermatostroma porosum Yang and Dong, 1979; Dong and Wang 1982: 22, pl. 13: 1, 2.
1999 Hermatostroma maculatum Yang and Dong, 1979; Cook 1999: 511, text-fig. 32A–C; 33A–D.
2008 Pseudotrupetostroma cellulosum (Lecompte, 1952); Salerno 2008: 92, pl. 15: 1–3.
2020 Trupetostroma cf. colliculosum Khalfina, 1960; Huang et al. 2020: 6.
Material.—Two specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with one specimen (NIGP 177053) illustrated.
Description.—Skeletons are laminar, up to 50 mm in thickness (Fig. 3). Surface features are not visible in cemented limestone. In longitudinal section the skeleton between astrorhizae forms a regular network (Fig. 8A1). Pachystromes are laterally continuous, occasionally undulose, spacing 8–12 per 2 mm, and of 0.1 mm in thickness. Pachysteles are vertically extensive, closely spaced, spacing 6–8 per 2 mm, 0.1–0.2 mm in thickness. In the upper part of the mamelons, the skeletons are dominated by continuously thick pachysteles, with 6 per 2 mm, 0.2 mm in thickness. Pachystromes weakly occur (Fig. 8A1). Galleries are variable in sizes, 0.1–0.3 mm in diameter. Mamelons are conspicuous, horizontally extensive, with a height of 15–20 mm. Astrorhizal canals commonly contain abundant dissepiments. In tangential section, skeletons are densely compact with irregular networks or rare isolated pachysteles. Astrorhizae are predominant, up to 15 mm in diameter, with over 10 branching canals (Fig. 8A2). The microstructure was mostly affected by the alteration of diagenesis, which possesses coarsely cellular-like structure (Fig. 8A3).

Fig. 8. Stromatoporoids from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A. Pseudotrupetostroma porosum (Yang and Dong, 1979), NIGP 177053; A1, longitudinal section; A2, tangential section; A3, enlargement of longitudinal section showing coarsely cellular microstructure coated tangential elements (arrows). B. Salairella buecheliensis (Bargatzky, 1881), NIGP 177054; B1, longitudinal section, note the predominance of the pachysteles in the right standard area and amalgamated structure in the left oblique orientation of the thin section; B2, tangential section, note the autotubes in the upper middle area; B3, enlargement of B1 showing coarsely cellular microstructure (arrows) partially transformed into melanospheric microstructure; such features demonstrate the likely diagenetic character of these microstructures and therefore their unreliability as taxonomic discriminators.
Remarks.—This species was previously assigned to Trupetostroma cf. colliculosum Khalfina, 1960, but the specimens from Jiwozhai reef are quite different from Trupetostroma Parks, 1936, in terms of the atypical regular network of pachystromes and pachysteles. It closely resembles the Pseudotrupetostroma cellulosum (Lecompte, 1952) documented by Salerno (2008: 93–96, pl. 15: 3) with respect to structures near the mamelons, but the holotype of Trupetostroma cellulosum Lecompte, 1952, shows apparently confined inter-laminar pillars and different thin laminae.
Stratigraphic and geographic range.—Lower Givetian (Middle Devonian) of Eifel, Germany; lower to middle Givetian (Middle Devonian) of Guangxi, Yunnan, and Guizhou provinces, South China, and Queensland, Australia; middle Givetian (Middle Devonian) of Boulonnais, France.
Family Syringostromellidae Stearn, 1980
Genus Salairella Khalfina, 1961
Type species: Salairella multicea Khalfina, 1961; Eifelian (Middle Devonian) of Salair, Russia.
Remarks.—Genera of Stromatoporida and Syringostromatida were confused with each other for over 150 years until Stearn (1993) revised Stromatoporida in detail. The dominance of pachysteles was traditionally regarded as the diagnostic feature of the first named stromatoporoid Stromatopora Goldfuss, 1826 (e.g., Nicholson 1886; Galloway 1957; Dong 2001). Also some species belonging to Salairella were erroneously assigned to Stromatopora (e.g., Dong and Song 1992). Stearn (1993) elaborated the relationships between typical Stromatopora and its similar genera. He reaffirmed that presence of cassiculate structure is the key morphological feature of the holotype of Stromatopora from Germany. Therefore, although Salairella closely resembles Stromatopora in having tangentially amalgamated network, they are totally different in morphology of vertical sections of the skeleton.
Salairella buecheliensis (Bargatzky, 1881)
Fig. 8B.
1881 Caunopora bücheliensis sp. nov.; Bargatzky 1881: 290.
1952 Parallelopora bücheliensis (Bargatzky, 1881); Lecompte 1952: 290, pl. 50: 3, 4.
1968 Caunopora bücheliensis Bargatzky, 1881; Flügel and Flügel-Kahler 1968: 53.
1978 Stromatopora hüpschii (Bargatzky, 1881); Wang 1978: 27, pl. 13: 1.
1980 Stromatopora cf. bücheliensis (Bargatzky, 1881); Mistiaen 1980: 210, pl. 13: 7–9; pl. 14: 1–3.
1985 Salairella buecheliensis (Bargatzky, 1881); Mistiaen 1985: 145, pl. 12: 10–12; pl. 13: 1.
1992 Stromatopora hüpschii (Bargatzky, 1881); Dong and Song 1992: 30, pl. 3: 1a, b.
1999 Salairella buecheliensis (Bargatzky, 1881); May 1999: 129, pl. 1: 6.
1999 Salairella buecheliensis (Bargatzky, 1881); Cook 1999: 525, text-fig. 45A–E.
2005 Salairella buecheliensis (Bargatzky, 1881); May 2005: 204, pl. 21: 1, 2; pl. 30: 2, 3; pl. 31: 1; pl. 32: 1.
2008 Salairella buecheliensis (Bargatzky, 1881); Salerno 2005: 97, pl. 14: 2.
2020 Stromatopora huepschii (Bargatzky, 1881); Huang et al. 2020: 7, text-fig. 5F.
Material.—10 specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with one representative specimen (NIGP 177054) illustrated.
Description.—Skeletons are laminar in growth form, up to 20 mm in thickness and 500 mm in basal dimension (Fig. 3). The longitudinal section is dominated by pachysteles, partly with coarsely amalgamated networks or atypical cassiculate structures. Pachysteles are vertically continuous, commonly joining and dividing in oblique thin section (Fig. 8B1), spacing 5–8 per 2 mm, 0.1–0.2 mm in thickness. Pachystromes are not clear, oblique or horizontal. Galleries are vertically elongate or occasionally irregular, crossed by flat or oblique dissepiments, at the same width as the pachysteles. Syringoporid symbionts are abundant, with a width of 0.2–0.3 mm, spacing 2–3 per 2 mm. The tangential section shows a closed network with dominantly circular autotubes between the astrorhizae (Fig. 8B2). Width of the skeletal elements and autotubes 0.1–0.2 mm. Astrorhizae are conspicuous, generally bifurcated, 0.1–0.25 mm in diameter. Microstructure is normally cellular, 0.02 mm in diameter, but in some cases is melanospheric due to the diagenesis (Fig. 8B3).
Remarks.—The predominance of vertically elongate pachysteles was also regarded as the main diagnostic characteristics of Stromatopora Goldfuss, 1826, for many years (e.g., Yang and Dong 1979; Dong 2001). Therefore, the samples in Jiwozhai reef were previously assigned to Stromatopora huepschii (Bargatzky, 1881) due to the rare existence of inconspicuous cassiculate structure probably caused by intraspecific variation, but the dominance of vertical elongate pachysteles justifies the designation as Salairella buecheliensis.
Stratigraphic and geographic range.—Eifelian to Givetian (Middle Devonian) of Dinant Basin, Belgium; lower Givetian (Middle Devonian) of Eifel, Germany, and Bohemia, Czech Republic; lower to middle Givetian (Middle Devonian) of Boulonnais, France, Queensland, Australia, Central Mountains, Afghanistan, and Hunan and Guizhou provinces, South China.
Order Syringostromatida Bogoyavlenskaya, 1969
Family Coenostromatidae Waagen and Wentzel, 1887
Genus Habrostroma Fagerstrom, 1982
Type species: Stromatopora proxilaminata Fagerstrom, 1961; Middle Devonian of Ontario, Canada.
?Habrostroma laminosum (Lecompte, 1952)
Fig. 9A, B.
1952 Stromatopora laminosa sp. nov.; Lecompte 1952: 276, pl. 55: 3; pl. 56: 1, 2.
1968 Stromatopora laminosa Lecompte, 1952; Flügel and Flügel-Kahler 1968: 235.
1968 Parallelopora laminosa (Lecompte, 1952); Mallett 1968: 225, pl. 26: 1–6.
1978 Stromatopora laminosa Lecompte, 1952; Wang 1978: 29, pl. 14: 3.
1979 Stromatopora cf. laminosa Lecompte, 1952; Yang and Dong 1979: 55, pl. 27: 5, 6.
1982 Stromatopora laminosa Lecompte, 1952; Dong and Wang 1982: 37, pl. 9: 5, 6.
1993 Habrostroma laminosum (Lecompte, 1952); Stearn 1993: 222.
1995 Stromatopora laminosa Lecompte, 1952; Krebedünkel 1995: 110, pl. 14: 7, 8.
2005 Habrostroma laminosum (Lecompte, 1952); Stadelmaier et al. 2005: 24, pl. 9: 3.
2008 Habrostroma laminosum (Lecompte, 1952); Salerno 2008: 101, pl. 15: 4, 5.
2020 Habrostroma laminosum (Lecompte, 1952); Huang et al. 2020: 14.
Material.—Five specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with two specimens (NIGP 177055 and NIGP 177056) illustrated.
Description.—Skeletons are laminar to high domical, overgrowing on tabulate corals, solitary rugose corals, or other stromatoporoids, commonly 1–3 mm in thickness, with a maximum of 15 mm. Surface features are not visible in cemented limestone. Latilaminae are common, with individual latilaminae in a thickness of 1–3 mm. In longitudinal section, the skeletons are extremely dense (Fig. 9A, B1), composed of thin laminae and thick pillars. Laminae are continuous, sharply undulose near the mamelons, spacing 8–14 mm per 2 mm, commonly with thickness of 0.02 mm (Fig. 9A). Pillars are short and thick, spool-shaped, laterally interconnected to account for the dense structure, spacing 7–10 per 2 mm, 0.1–0.2 mm in thickness. Galleries are tiny, smaller than the pillars, round or rarely elongate, commonly 0.05–0.15 mm in diameter. Mamelons conspicuous, with height of 2–5 mm, with central and branching astrorhizal canals. Dissepiments are distinct in the astrorhizal canals. The central areas of high domical specimens show indistinctive microreticulate structure formed by melanospheres multi-microlaminae (Fig. 9B1). In tangential section, the skeletons are also densely packed and can hardly be differentiated (Fig. 9B2). The microstructure locally appears to be melanospheric or striated (Fig. 9B2, B3), probably due to diagenetic alteration.
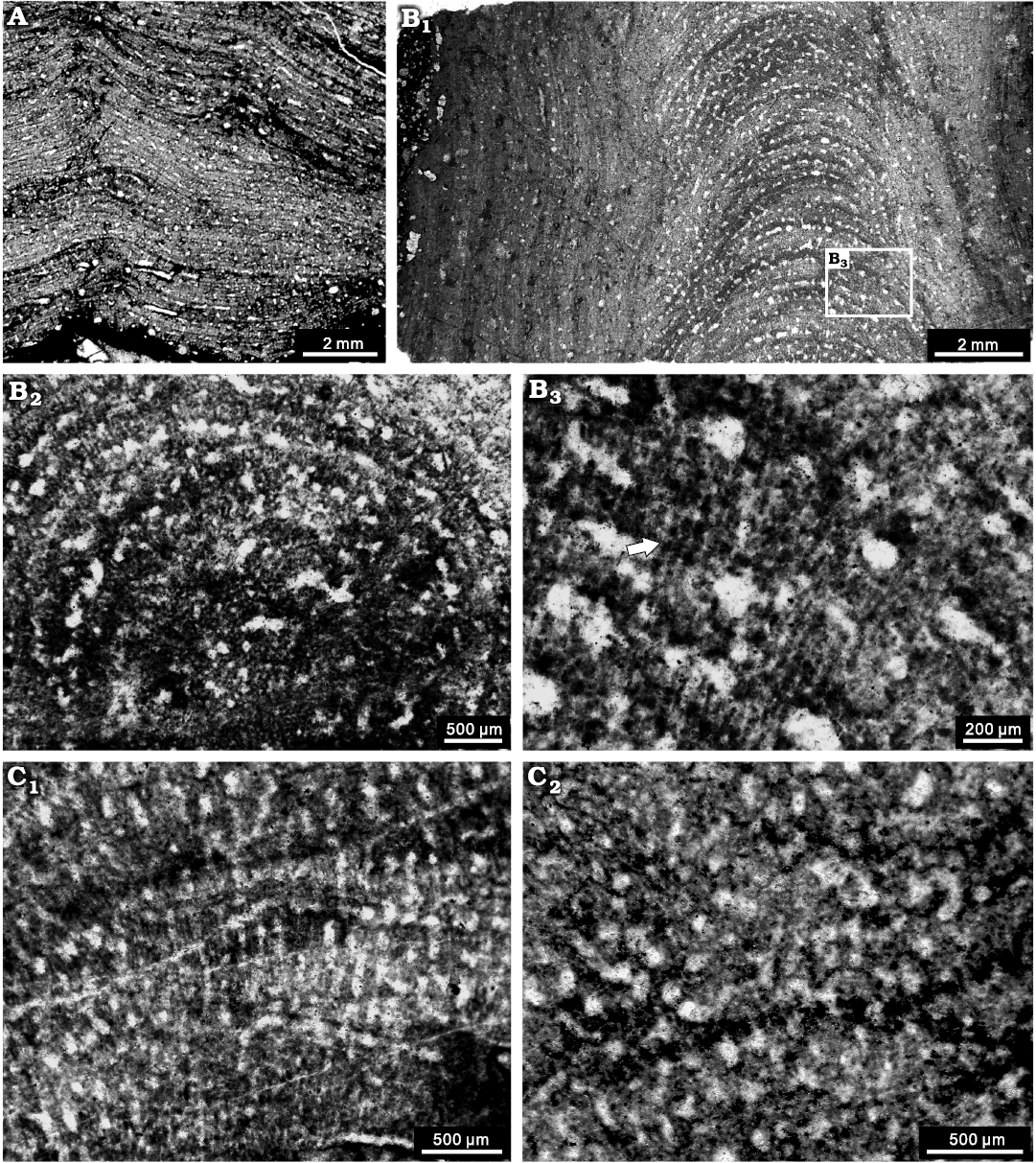
Fig. 9. Stromatoporoids from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A, B. ?Habrostroma laminosum (Lecompte, 1952) A. NIGP 177055; longitudinal section of the laminar form; B. NIGP 177056; B1, longitudinal section of the high domical form; B2, poor preservation of tangential section showing the dense structure; B3, enlargement of B1, showing possible microreticulate microstructure (arrow). C. Parallelopora sp., NIGP 177057; C1, longitudinal section, note the microreticulate microstructure; C2, tangential section showing the autotubes.
Remarks.—The present specimens resemble Stromatopora laminosa Lecompte, 1952 due to the indistinctive microreticulate structure and diagenetically melanospheric or striated microstructure. This species was assigned by many palaeontologists to Stromatopora Goldfuss, 1826, for over four decades, but it does not have the typical cassiculate structure. Stearn (1993) revised Stromatopora laminosa into Habrostroma laminosum and we agree with this decision. Although Webby and Zhen (2008), Stearn (2015b), and Stock and Burry-Stock (2019) generally use pachystele and pachystrome to describe the skeleton of Habrostroma Fagerstrom, 1982, the holotype presented by Fagerstrom (1982) apparently shows isolated longitudinal elements, which are totally different from the vertically interconnected pachysteles. Therefore, we tend to use “laminae” and “pillars” to describe the skeleton of this genus. However, rare specimens in Jiwozhai reef show poor preservation of tangential section, so are not reliably assigned to Habrostroma laminosum.
Stratigraphic and geographic range.—Lower Givetian (Middle Devonian) of Eifel Germany, and Guizhou Province, South China; lower to middle Givetian (Middle Devonian) of Queensland, Australia; Givetian to Frasnian (Middle–Late Devonian) of Dinant Basin, Belgium.
Family Parallelostromatidae Bogoyavlenskaya, 1984
Genus Parallelopora Bargatzky, 1881
Type species: Parallelopora ostiolata Bargatzky, 1881; Middle Devonian of Eifel, Germany.
Parallelopora sp.
Fig. 9C.
2020 Parallelopora ostiolata Bargatzky, 1881; Huang et al. 2020: 3.
Material.—One fragmental skeleton (NIGP 177057) from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China.
Description.—Skeleton appears to be laminar. Surface features are not visible in cemented limestone. In longitudinal section, the skeleton is made up of conspicuous pachysteles and indistinct pachystromes (Fig. 9C1). Pachysteles are slender, vertically straight, densely distributed, spacing 15–16 per 2 mm, 0.05–0.075 mm in thickness. Pachystromes are thin, rarely exist, commonly replaced by dissepiments. Galleries are extremely narrow, with a width of 0.025–0.075 mm, infilled with concave dissepiments. In tangential section, the pachysteles interconnect, forming a closed network enclosing autotubes (Fig. 9C2). Autotubes are circular, 0.025–0.05 mm in diameter. The microstructure is coarsely orthoreticulate.
Remarks.—The present species was preliminarily assigned to Parallelopora ostiolata Bargatzky, 1881, by Huang et al. (2020) according to the coarsely orthoreticulate microstructure, but the latter shows more thick and sparsely distributed pachysteles, commonly spacing 5–6, or 8–10 per 2 mm, and with a width of 0.07–0.18 mm (Lecompte 1952; Galloway and St. Jean 1957; Galloway 1960). However, the poorly preserved single specimen cannot justify the assignment to a new species.
Family Stachyoditidae Khromykh, 1967
Genus Stachyodes Bargatzky, 1881
Type species: Stachyodes verticillata (M’Coy, 1850); Middle Devonian of Eifel, Germany.
Stachyodes costulata Lecompte, 1952
Fig. 10.
1952 Stachyodes costulata sp. nov.; Lecompte 1952: 309, pl. 64: 3; pl. 65: 1–4.
1959 Stachyodes costulata Lecompte, 1952; Gogolczyk 1959: 372, pl. 4: 3; pl. 5: 1–3.
1963 Stachyodes costulata Lecompte, 1952; Stearn 1963: 660, pl. 86: 4, 5.
1966 Stachyodes costulata Lecompte, 1952; Klovan 1966: 31, pl. 9: 1–6.
1970 Stachyodes costulata Lecompte, 1952; Stearn and Mehrotra 1970: 18, pl. 4: 3, 4.
1971 Stachyodes costulata Lecompte, 1952; Zukalová 1971: 101, pl. 34: 5, 6.
1974 Stachyodes costulata Lecompte, 1952; Khromykh 1974: 62, pl. 16: 1; pl. 17: 2
1979 Stachyodes costulata Lecompte, 1952; Yang and Dong 1979: 81, pl. 46: 9, 10.
1984 Stachyodes costulata Lecompte, 1952; Cockbain 1984: 28, pl. 19: a–d; pl. 20: a.
1984 Stachyodes costulata Lecompte, 1952; Dong and Wang 1984: 268, pl. 20: 3a, b.
1988 Stachyodes costulata Lecompte, 1952; Khromykh and Hung 1988: 34, pl. 16: 6
1989 Stachyodes costulata Lecompte, 1952; Dong in Dong et al. 1989: 174, pl. 2: 3a–d.
1992 Stachyodes costulata Lecompte, 1952; Dong and Liu 1992: 172, pl. 4: 5a, b.
1999 Stachyodes costulata Lecompte, 1952; Mistiaen 1999: 39.
2020 Stachyodes costulata Lecompte, 1952; Huang et al. 2020: 7, text-fig. 5E.
Material.—57 specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with two typical specimens (NIGP 177058 and NIGP 177059) illustrated.
Description.—Skeletons are dendroid, with some in situ specimens growing from laminar into erect columnar (Fig. 10A1), but preserved as fragmented branches in most specimens. The branches are commonly 4–5 mm in diameter, up to 14 mm. In longitudinal section, the skeleton consists of dark thin pachystromes and thick pachysteles (Fig. 10A). In the central zone, the structure is relatively undifferentiated, with astrorhizal canal, 0.4 mm in diameter (Fig. 10A1). In the peripheral zone, the structure is well differentiated. Pachysteles radiate from the central area and emerge perpendicularly to the surface, with a width of 0.2 mm. Pachystromes distributed as arches, spacing 7–8 per 2 mm, 0.02 mm in thickness (Fig. 10A2, A3). Galleries are circular, perpendicular to the surface, 0.1–0.2 mm in diameter (Fig. 10A3). In tangential section, pachysteles interconnect to form a regular closed network in the central zone, and gradually radiate to the peripheral zone (Fig. 10B1). In the marginal zone, pachystromes distributed as concentric circles. The microstructure is apparently striated (Fig. 10A2, A3, B2).
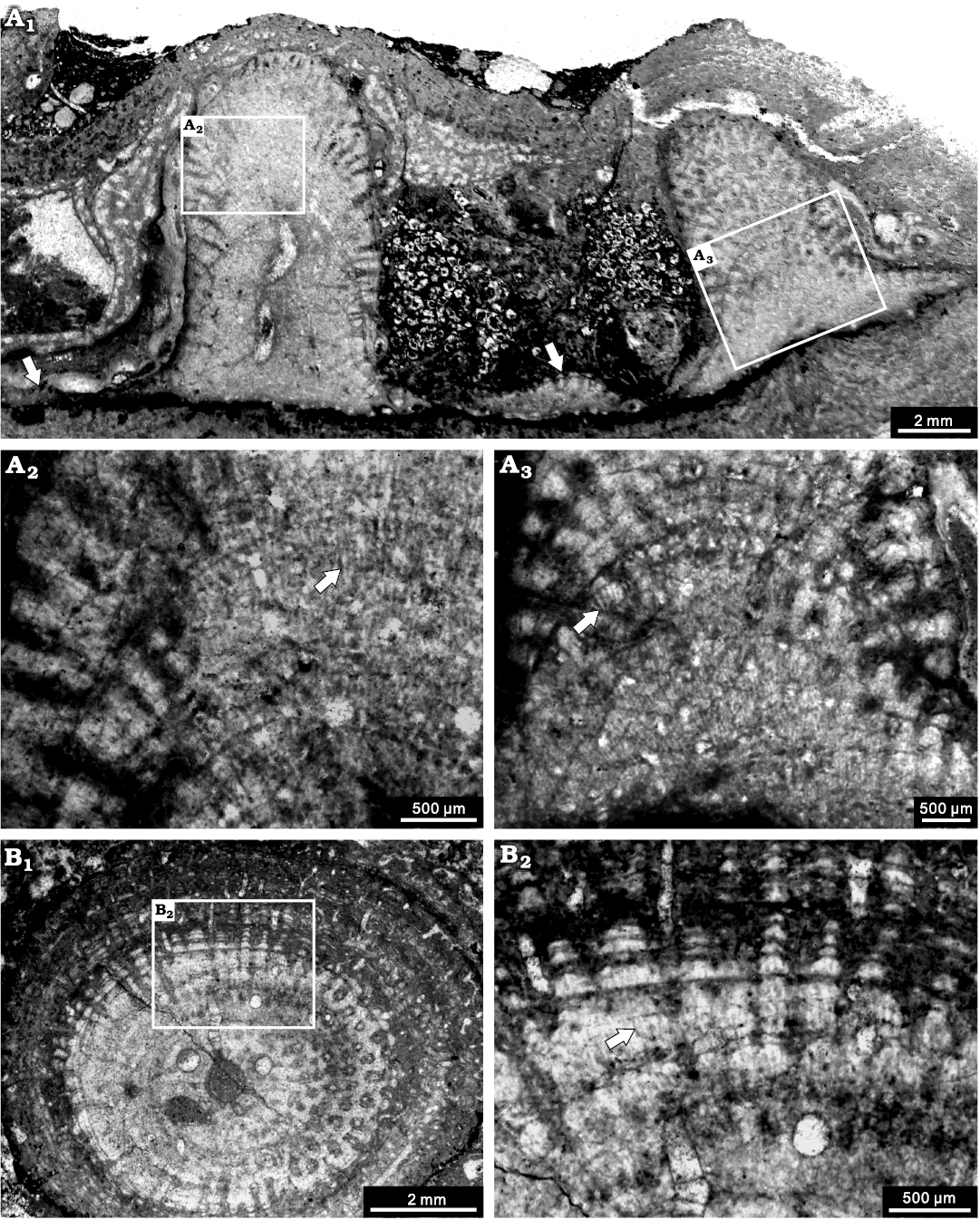
Fig. 10. Stromatoporoid Stachyodes costulata Lecompte, 1952, from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A. NIGP 177058; A1, longitudinal section of the whole thin section showing the columnar skeleton interconnected through laminar skeleton in the bottom (arrows); A2, enlargement of A1, note the striated microstructure (arrow); A3, enlargement of A1, showing autotubes and striated microstructure (arrow). B. NIGP 177059; B1, tangential section showing the regular autotubes; B2, enlargement of B1, note the striated microstructure (arrow).
Remarks.—The present species is consistent with Stachyodes costulata Lecompte, 1952, due to the dense distributed of pachysteles. It differs from Stachyodes radiata Lecompte, 1952, in having a relatively undifferentiated structure in the central zone.
Stratigraphic and geographic range.—Givetian (Middle Devonian) of Queensland, Australia and Yukon, Canada; Givetian to lower Frasnian (Middle–Upper Devonian) of the Holy Cross Mountains, Poland; Givetian to Frasnian (Middle–Upper Devonian) of Yunnan, Guangxi, and Guizhou provinces, South China and Central Mountains, Afghanistan; Middle–Upper Devonian of Alberta, Canada; lower Frasnian (Upper Devonian) of Moravian Karst, Czech Republic; upper Frasnian (Upper Devonian) of the Dinant Basin, Belgium; Frasnian (Upper Devonian) of Namur Basin, Belgium; Frasnian (Upper Devonian) of Omolon, Russia; Frasnian (Upper Devonian) of Kerman Province, Iran, Canning Basin, Australia; Upper Devonian of northern and central Vietnam and Ulachan Mountains, Russia (Mistiaen 1999).
Stachyodes fasciculata Dong in Dong et al., 1989
Fig. 11.
1989 Stachyodes fasciculata sp. nov.; Dong in Dong et al. 1989: 273, pl. 17: 2a, b.
1989 Stachyodes bifurca (Stearn, 1962); Dong et al. 1989: 275, pl. 16: 1a–c.
1992 Stachyodes fasciculata Dong in Dong et al., 1989; Dong and Liu 1992: 172, pl. 4: 4a, b.
2020 Stachyodes radiata Lecompte, 1952; Huang et al. 2020: 13.
Material.—112 specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with three specimens (NIGP 177060–62) illustrated.
Diagnosis.—Skeleton dendroid, laminar, and bulbous, formed by extremely well differentiated pachystromes and pachysteles. Pachystromes thin, in a thickness of 0.02 mm. Pachysteles thick, radiated from the central zones of the skeleton, interconnected to build the closed networks. Axial canal prominent in the dendroid forms, but branching canals common in laminar and bulbous forms.
Description.—Skeletons are dendroid, laminar, or rarely bulbous, but fragmented or overturned in most specimens (Fig. 11A). The dendroid branches are commonly 3–5 mm in diameter, sporadically up to 15 mm (Fig. 11A). The laminar forms 3–20 mm in thickness (Fig. 11B). The bulbous one shows a height of 40 mm and width of 30 mm (Fig. 11C1). In longitudinal section, the skeleton consists of conspicuous pachystromes and pachysteles (Fig. 11A, B, C1). Pachystromes are thin, parabolic in the dendroid forms, relatively closely spaced, spacing 10–13 per 2 mm, 0.02 mm in thickness. Pachysteles formed a closed network in the skeleton surface, 0.2 mm thick. The galleries are divided by the thin pachystromes, with a width of 0.2 mm. Axial canal is prominent in the dendroid forms, 0.4–0.5 mm in diameter, infilled with dissepiments, while branching canals are more common in the laminar and bulbous forms (Fig. 11C1–C3). In tangential section, axial canal is circular, pachysteles clearly radiate from the axial to peripheral zone and pachystromes distributed as concentric circles in the dendroid forms (Fig. 11A). Pachysteles apparently interconnect to form a closed network in the bulbous forms (Fig. 11C3). The microstructure is coarsely striated (Fig. 11C4).
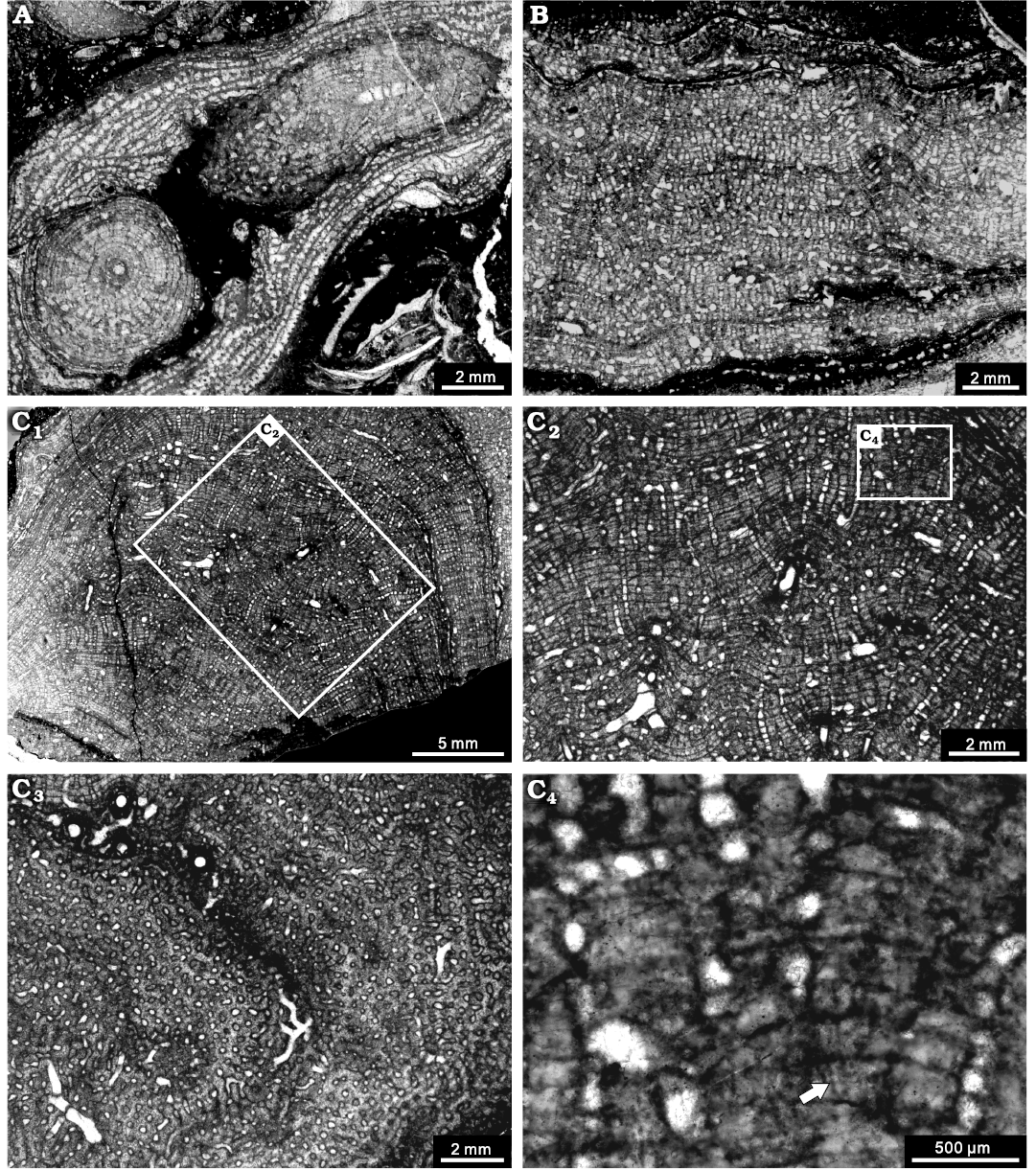
Fig. 11. Stromatoporoid Stachyodes fasciculata Dong in Dong et al., 1989, from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A. NIGP 177060; longitudinal section and tangential section of the dendroid form. B. NIGP 177061; longitudinal section showing the thick pachysteles and thin pachystromes. C. NIGP 177062; C1, longitudinal section of the bulbous form; C2, enlargement of C1, illustrating regular skeleton; C3, tangential section showing the autotubes and astrorhizal canals; C4, enlargement of C2, showing the coarsely striated microstructure (arrow).
Remarks.—The dendroid forms of the present species were preliminarily assigned to Stachyodes radiata Lecompte, 1952, by Huang et al. (2020), but the latter shows more irregular and thicker pachysteles. Further study indicates the species is compatible with both Stachyodes bifurca (Stearn, 1962) and Stachyodes fasciculata as defined by Dong in Dong et al. (1989), and thus determination is problematic. Although the two samples are different in size, the close similarity of the skeleton morphology justifies the assignment to the same species. However, the relationships between Syringostroma bifurcum Stearn, 1962, and Stachyodes bifurca (Stearn, 1962) are not fully clear due to the preservation quality of the samples from Canada. Here, we assign these specimens to Stachyodes fasciculata Dong in Dong et al., 1989, in relation to the high consistency of the skeleton. The laminar forms of Stachyodes are not common in the Devonian, e.g., Stachyodes australe (Wray, 1967) and Stachyodes jonelrayi Stearn, 1975. The difference between the three species lies in the absence of two layers in the present species. The laminar forms of this species were partly assigned to Salairella hybridina Yang and Dong, 1979, by Huang et al. (2020) according to the closed network in the tangential section, but they are totally different in relation to microstructure and laterally extensive thin pachystromes in longitudinal section. Moreover, some of the laminar forms were assigned to Trupetostroma dushanense Yang and Dong, 1963, T. regulamellatum Yang and Dong, 1963, or T. sublamellatum Lecompte, 1952, in terms of thin pachystromes and closed network formed by pachysteles, but Trupetostroma Parks, 1936, is fundamentally featured by isolated pillars rather than interconnected pachysteles, thus these species do not belong to Trupetostroma.
Stratigraphic and geographic range.—Givetian (Middle Devonian) of Guizhou, Guangxi, and Hunan provinces, South China.
Stachyodes sp.
Fig. 12A.
Material.—Nine specimens from the Givetian (Middle Devonian) Jiwozhai reef, Dushan Formation, Dushan, Guizhou, South China, with one best preserved specimen (NIGP 177063) illustrated.
Description.—Skeletons are laminar forms, with a height up to 4 mm, commonly covering the other stromatoporoids. Latilaminae are exceptionally conspicuous and laterally undulating, 1.5–2 mm in thickness. In longitudinal section, the skeleton consists of two zones (Fig. 12A1). In the basal zone, the structure is weakly differentiated, with branching astrorhizal canals irregularly distributed, 0.1–0.4 mm in diameter. In the upper zone, short pachysteles are dominant, spacing 10 per 2 mm, 0.1 mm in thickness (Fig. 12A1). Pachystromes cannot be recognized clearly. Mamelons are not apparent. In tangential section, the skeleton shows tight network formed by the interconnected pachysteles (Fig. 12A2). Astrorhizal canals conspicuous, common with two branching canals (Fig. 12A1, A2). The microstructure is striated (Fig. 12A3–A5).
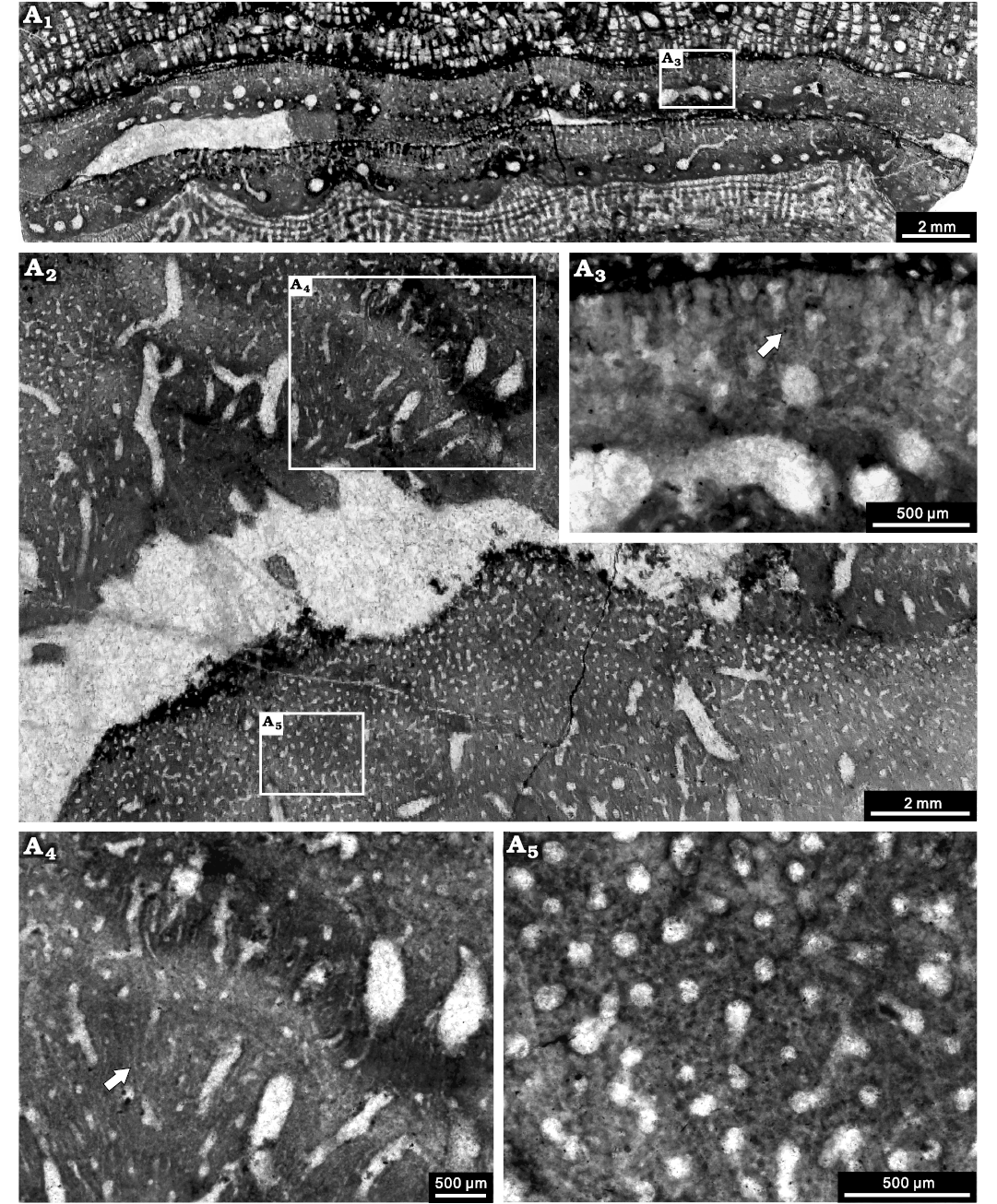
Fig. 12. Stromatoporoid Stachyodes sp. NIGP 177063, from the Jiwozhai reef, Jiwozhai Member, Dushan Formation, Givetian, Middle Devonian; Dushan, Guizhou, South China. A1, longitudinal section; A2, tangential section, note the autotubes and conspicuous astrorhizal canals; A3, enlargement of A1, note the coarsely striated microstructure (arrow); A4, enlargement of the upper middle part of A2, note the probably striated microstructure (arrow); A5, enlargement of the lower left part of A2, note the black dots in the tangential section.
Remarks.—This species apparently differs from the other dendroid species and it corresponds very well to the laminar Stachyodes species demonstrated by Wolniewicz (2021: fig. 9G) in the Frasnian of Poland. It differs from the laminar Stachyodes australe (Wray, 1967) in terms of the two special different zones and lacking crescent growth zone in the basal layer. There is some similarity with a key species from Alberta, Stachyodes jonelrayi Stearn, 1975, that is characterised by possessing a basal layer in the skeleton; this taxon also shows basal layers in the lower portions of successive individual latilaminae after growth interruptions. Nevertheless we are not confident that the species from Jiwozhai reef is necessarily the same as Stachyodes jonelrayi and prefer to leave it in open nomenclature in this study. Further work may be able to finalise the species-level taxon.
Discussion
Reef-building implications of stromatoporoids.—Laminar stromatoporoid fossils are common in shallow marine carbonates as well as reef environments (Kershaw 2015) and presumably contributed to stabilize soft, muddy substrates (Jakubowicz et al. 2019). The interactions between stromatoporoids and their substrates can be divided into two patterns: (i) ambitopic growth pattern that expanded on unconsolidated substrates after initial settlement, and (ii) encrusting growth on hard substrates during their entire life without lateral expansion (Webby and Kershaw 2015: fig. 305). A few Devonian stromatoporoids adopted encrusting habits, such as Clathrocoilona spissa (Cook 1999; Wolniewicz 2021), while many Devonian stromatoporoids show ambitopic lifestyles through most of their life history (Webby and Kershaw 2015).
In the Jiwozhai reef, Clathrocoilona spissa, with thickness 0.5–5.0 mm, is the most abundant laminar stromatoporoid. Its initial growth stages commonly encrusted on hard surfaces of high-relief organisms forming a spherical skeleton. Only a few examples show ambitopic habits. It seems that Clathrocoilona spissa rarely sustained itself to grow large or expand laterally, and could hardly further stabilize the substrates. In contrast, Gerronostromaria grossum generally presents thick and wide skeletons (Fig. 3), possibly reflecting vertically and laterally growth abilities, which were conducive to preventing sediment winnowing by episodic water currents, thus further stabilizing substrates. Pseudotrupetostroma porosum, Salairella buecheliensis, and Synthetostroma actinostromoides are quantitatively less than the other stromatoporoids, but their laterally extensive and vertically thick skeletons account for their abilities in covering sediments (Fig. 3). Dendroid but fragmented Stachyodes costulata and Stachyodes fasciculata presumably form hard substrates for the settlement of laminar taxa.
Individual thin laminar stromatoporoids (e.g., Clathrocoilona spissa) are interpreted to have been extremely vulnerable under episodic or sudden environmental pressures, and developed complex overgrowth skeletons, attached by other stromatoporoids, tabulate corals, chaetetids, bryozoans and calcified cyanobacteria (Figs. 6B1, 10A1, 12A1). Such features are common in the Jiwozhai reef facies (Huang et al. 2020); they are significant for stabilization of the underlying substrates (dendroid stromatoporoids, massive and branching tabulate corals, solitary rugose corals, brachiopods, and micrites) and further development of the reef framework.
Comparisons with other laminar stromatoporoid communities.—Laminar stromatoporoid-dominated patch reefs, biostromes or mud mounds are common in shallow marine environments from the Eifelian to Frasnian in Europe, North America, North Africa, and Australia (Cook 1999; Mabille and Boulvain 2007; Da Silva et al. 2011a, b; MacNeil and Jones 2016), especially in the initial stage of reef formation (Cook 1995; Boulvain et al. 2009; Jakubowicz et al. 2019).
A Givetian barrier reef tract, which extended from Glageon of North France to Hotton of South Belgium, was initiated by laminar stromatoporoids, solitary and fasciculate rugose corals, branching and massive tabulate corals over the crinoidal mats (Boulvain et al. 2009). Although the systematic palaeontology and diversity of the stromatoporoids was studied by Lecompte (1951, 1952) and Hubert (2008), detailed palaeoecological studies of the laminar stromatoporoids and their reef-building habits are not well-developed. According to Hubert (2008), laminar stromatoporoids in the reef facies of Trois-Fontaines Formation in Glageon were identified as Clathrocoilona spissa, Stictostroma saginatum, Stictostroma sp. 2, Hermatoporella sp. 4 and Stromatoporella granulata. This stromatoporoid assemblage is similar to the Jiwozhai reef with common occurrence of Clathrocoilona spissa and Stictostroma saginatum, but the dominant stromatoporoid taxa are unclear.
A series of Givetian biostromes, reefs and reef complexes have been reported from the Fanning River Group, north Queensland, Australia (Cook 1995, 1999). Reefs in the Fletcherview–Burdekin Downs area were constructed mainly by the Gerronostromaria hendersoni–Hermatostroma maculatum stromatoporoid community (Cook 1999). This community is characterized by Gerronostromaria hendersoni, Hermatostroma maculatum (constructors), Stachyodes costulata (bafflers), with minor role of Clathrocoilona abeona (encrusters), Salairella buecheliensis and some other stromatoporoids and corals. The common occurrence of coverstone in patch reefs is presumed to have resulted from fast growth expansion of laminar stromatoporoids upon muddy substrate for support (Cook 1999). A few stromatoporoids show extremely thin forms in the lagoonal pavements, in width of 20 to 80 cm and below a height of 10 cm (Cook 1999: fig. 3b). Also compound skeletons, formed by stromatoporoids and other organisms, are notable in the nearshore, fringing biostromes, probably indicating environmental stress under periodical depositional events or salinity variations (Cook 1999). The Givetian reef-building stromatoporoids in eastern Australia closely resemble the Jiwozhai reef stromatoporoids in terms of growth forms (thin laminar dominated), taxonomic compositions (Gerronostromaria, Stachyodes, Salairella) and lateral growth habit for the formation of reefal coverstone.
Laminar stromatoporoid biostromes also occur in Frasnian limestone of southern Belgium (e.g., Da Silva et al. 2011b; Poty and Chevalier 2007). The middle Frasnian laminar stromatoporoid biostromes mainly in the outer intermediate zone of the Tailfer and Villers sections are dominated by species of Stachyodes, Stictostroma, with few encrusting Clathrocoilona. Species of Stictostroma occur mostly in carbonate mudstone and Stachyodes australe (Wray, 1967) commonly grew on carbonate mud substrates, shells or encrusted by other stromatoporoids (Da Silva et al. 2011b). Moreover, three middle Frasnian carbonate mud mounds with laminar stromatoporoids were also described in La Boverie–Rochefort quarry of southern Belgium (Da Silva et al. 2011a). Laminar stromatoporoids were documented in the base of core mound, mound flank and adjacent level-bottom environments, and were featured by species of Stictostroma, Stachyodes and Salairella, similar to the stromatoporoid fauna in the Tailfer and Villers sections. Species of Stictostroma and Salairella mostly grew on mud and Stachyodes australe (Wray, 1967) also occurred on mud, shells or encrusted other stromatoporoids (Da Silva et al. 2011a). The laminar stromatoporoid fauna in the Frasnian of Belgium is slightly different from the Givetian Jiwozhai reef due to the special occurrence of Stachyodes australe (Wray, 1967) and differences in the most abundant stromatoporoid taxa; nevertheless their ecological implications for the stabilization of substrate are similar.
The middle Frasnian Alexandra Reef System, situated in the Northwest Territories, Canada, displays well-preserved stromatoporoids in different reef-related facies (MacNeil and Jones 2016). A large number of in situ laminar and anastomosing laminar stromatoporoids are concentrated in the reef front, including the species of Stachyodes, Actinostroma and some other stromatoporoids Stromatopora, Hermatostroma, and Clathrocoilona. These laminar stromatoporoids were interpreted to live in a water depth of 20–25 m, and generally associated with sediment, or encrusted by microbial organisms (MacNeil and Jones 2016). Compared with the stromatoporoid accumulation in relatively deeper setting of the Alexandra Reef System, laminar stromatoporoid framestones constructed by species of Stromatopora are also common in the shallow belt. Species of Stromatopora show intensive lateral and vertical growth habits to encrust the underlying stromatoporoids and further contributed to the thick framestone (MacNeil and Jones 2016). The Frasnian large reef complex in Canada shows distinctive ecological difference of the stromatoporoid assemblages, which are manifested by the species of thin laminar Stachyodes and Actinostroma in the deeper water coverstone and thick laminar Stromatopora in the shallow water framestone. Compared with the large reef complex, the small Givetian Jiwozhai reef was particularly characterized by the thin laminar Gerronostromaria grossum Dong, 1974, in the stromatoporoid fauna of this particular palaeoenvironment.
Therefore, the Jiwozhai stromatoporoid reef shows similarities to the Givetian and Frasnian reefs in South Belgium, North America, and East Australia in terms of stromatoporoid assemblages and depositional environment (Cook 1995, 1999; Boulvain et al. 2009; Da Silva et al. 2011a, b; MacNeil and Jones 2016). These stromatoporoid faunas are mainly characterized by species of Stictostroma, Clathrocoilona, Salairella, laminar Stachyodes, and dendroid Stachyodes, but their most abundant taxa possibly vary between different time periods and various geographical positions (Fig. 13) (Cockbain 1984; Cook 1999; Mistiaen and Gholamalian 2000; Da Silva et al. 2011a, b; MacNeil and Jones 2016; Jakubowicz et al. 2019). In this study, Gerronostromaria grossum chiefly accounts for the formation of the Jiwozhai reef, noting that Gerronostromaria hendersoni–Hermatostroma maculatum dominated the laminar communities of the Givetian reefs in East Australia. However, laminar species of Stachyodes characterized the laminar communities of the Frasnian reefs and mud mounds in North America and South Belgium. The laminar species of Stictostroma and Salairella, which mostly grew on muddy substrate in the mid-Frasnian platform of South Belgium, also show high abundance in the development of biostromes. These thin laminar stromatoporoids generally show strong abilities stabilizing the soft, muddy substrate. Therefore, although Clathrocoilona spissa in the Jiwozhai reef commonly encrusted on high-relief organisms, it does not present the same positive effect on the construction of the reef as the laterally extensive Gerronostromaria grossum.
Overall, stromatoporoids in the Jiwozhai reef tend to show paleoecological preferences in terms of growth form indicating different growth strategies of stromatoporoid taxa, which corresponds with other previous studies (e.g., May 1993; Salerno 2008; Da Silva et al. 2011a, b; Wolniewicz 2021). Although the stromatoporoid taxa vary depending on the palaeogeographic distribution through the continents and time, it should be noted that low profile (i.e., laminar) stromatoporoids likely acted as important sediment stabilizers, thus triggering expansion of the carbonate factory (Da Silva et al. 2011b). Similarly, extensive development of Middle Devonian reefs and carbonate platforms throughout South China, which were favourable environments for benthic organisms including stromatoporoids, was presumably pioneered by growth variability of diverse stromatoporoid taxa. A combination of taxonomic investigation and palaeoenvironmental interpretation, as performed in this study, allow developments in interpretations of stromatoporoid biology and aids understanding of carbonate platform development in South China.
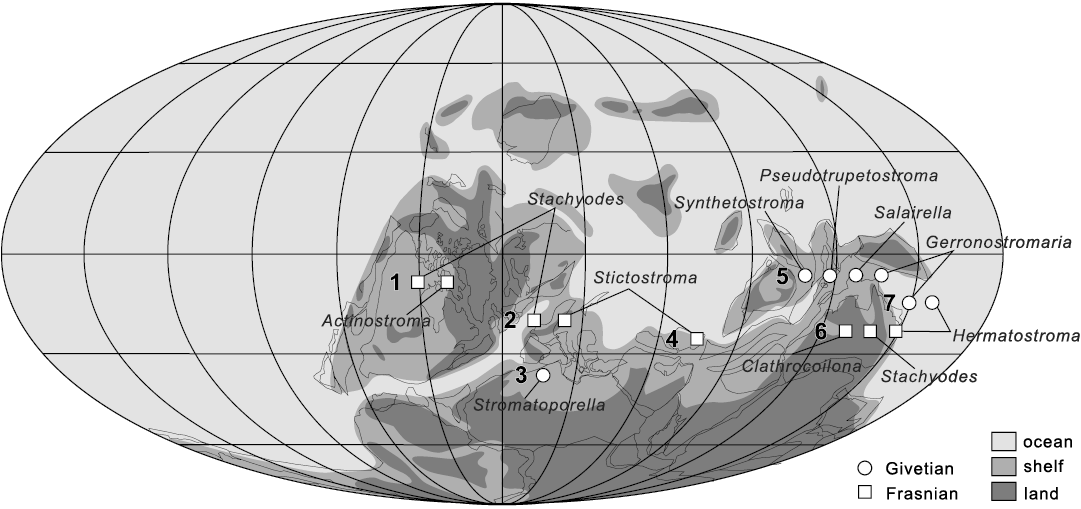
Fig. 13. Palaeogeographic distribution of the reef-building laminar stromatoporoid genera during the Givetian and Frasnian (Middle–Late Devonian). Localities: 1, Northwest Territories, Canada (MacNeil and Jones 2016); 2, Dinant Basin, southern Belgium (Da Silva et al. 2011a, b); 3, Anti‐Atlas, Morocco (Jakubowicz et al. 2019); 4, Esfahan, central Iran (Mistiaen and Gholamalian 2000); 5, Guizhou, South China; 6, Canning Basin, western Australia (Cockbain 1984); 7, Queensland, eastern Australia (Cook 1999). Palaeogeographic map is based on Golonka (2002).
Conclusions
– Revision of the stromatoporoids from Jiwozhai reef, Dushan, Guizhou, South China documents 11 species belonging to nine genera. The stromatoporoid assemblage is characterized by diverse syringostromatids, abundant stromatoporellids and clathrodictyids, and a small number of other stromatoporids.
– The stromatoporoid fauna was dominated by laminar stromatoporoids (Clathrocoilona spissa, Gerronostromaria grossum, Stictostroma saginatum, Synthetostroma actinostromoides, Pseudotrupetostroma porosum, Salairella buecheliensis, ?Habrostroma laminosum, Parallelopora sp., Stachyodes fasciculata and Stachyodes sp.), which pioneered the formation of the reef coverstone by stabilizing large bioclasts and micrite sediment. Dendroid stromatoporoids (Stachyodes costulata and Stachyodes fasciculata) are also common, but generally present fragmented skeletons mixed in the matrix. Bulbous forms (Stachyodes fasciculata) are rare. The variation of stromatoporoid growth form indicates different growth strategies of stromatoporoid taxa.
– The morphology, substrate, size and minimal growth interruption features indicate that Gerronostromaria grossum is the main reef-building stromatoporoid in relation to its absolute predominance of growth advantage in stabilizing sediments. By contrast, Clathrocoilona spissa contributed less to the formation of the Jiwozhai reef. Advantages of these laminar stromatoporoids are manifested by sediment stability that presumably pioneered development of Middle Devonian carbonate platform in South China.
– Devonian laminar stromatoporoid communities are widespread in Europe, North America, North Africa, Australia, and South China. The laminar stromatoporoid faunas generally show similarities in relation to stromatoporoid composition, but the dominating species vary among different localities and periods.
Acknowledgements
The authors are immensely thankful to the reviewers Paweł Wolniewicz (Adam Mickiewicz University, Poznań, Poland) and Michał Zatoń (Faculty of Natural Sciences, University of Silesia, Sosnowiec, Poland) for their constructive comments that helped to improve this manuscript. We would also like to show our gratitude to Andreas May (Unna, Germany) for providing stromatoporoid references. The assistance provided by Junjun Song and Wen Guo (both NIGP) in the field work is also deeply appreciated. This study is financially supported by the Chinese Academy of Sciences (XDB26000000) and National Natural Science Foundation of China (grant No. 41772004).
References
Bargatzky, A. 1881. Die Stromatoporen des rheinischen Devons. Verhandlungen des naturhistorischen Vereins des preußischen Rheinlandes und Westfalens 38: 233–304.
Bogoyavlenskaya, O.V. [Bogoâvlenskaâ, O.V.] 1969. Revision of Silurian actinostromatids of Podolia [in Russian]. Paleontologičeskij žurnal 1969 (2): 15–20.
Bogoyavlenskaya, O.V. [Bogoâvlenskaâ, O.V.] 1984. Stromatoporaty paleozoâ: Morfologiâ, sistematičeskoe položenie, klassifikaciâ i puti razvitiâ [in Russian]. 92 pp. Nauka, Moskva.
Boulvain, F., Mabille, C., Poulain, G., and Da Silva, A.-C. 2009. Towards a palaeogeographical and sequential framework for the Givetian of Belgium. Geologica Belgica 12: 161–178.
Chi, Y.S. 1940. On some Silurian and Devonian Stromatoporoids of South-Western China. Bulletin of the Geological Survey of China 20: 283–322. Crossref
Cockbain, A.E. 1984. Stromatoporoids from the Devonian reef complexes, Canning Basin, Western Australia. Geological Survey of Western Australia Bulletin 129: 1–108.
Cook, A.G. 1995. Sedimentology and depositional environments of the Middle Devonian lower Fanning River Group (Big Bend Arkose and Burdekin Formation), Burdekin Subprovince, north Queensland, Australia. Memoirs of the Queensland Museum 38: 53–91.
Cook, A.G. 1999. Stromatoporoid palaeoecology and systematics from the Middle Devonian Fanning River Group, north Queensland. Memoirs of the Queensland Museum 43: 463–552.
Copper, P. 2002. Silurian and Devonian reefs: 80 million years of global greenhouse between two ice ages. In: W. Kiessling, E. Flügel, and J. Golonka (eds.), Phanerozoic Reef Patterns. SEPM Special Publication 72: 181–238. Crossref
Copper, P. and Scotese, C.R. 2003. Megareefs in Middle Devonian supergreenhouse climates. Special Paper of the Geological Society of America 370: 209–230. Crossref
Da Silva, A.-C., Kershaw, S., and Boulvain, F. 2011a. Sedimentology and stromatoporoid paleoecology of Frasnian (Upper Devonian) carbonate mounds in southern Belgium. Lethaia 44: 255–274. Crossref
Da Silva, A.-C., Kershaw, S., and Boulvain, F. 2011b. Stromatoporoid palaeoecology in the Frasnian (Upper Devonian) Belgian platform, and its applications in interpretation of carbonate platform environments. Palaeontology 54: 883–905. Crossref
Dong, D.Y. 1974. Devonian stromatoporoids [in Chinese]. In: Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (ed.), A Handbook of Stratigraphy and Paleontology in Southwest China, 221–223. Science Press, Beijing.
Dong, D.Y. 2001. Stromatoporoids of China [in Chinese, with English abstract]. 423 pp. Science Press, Beijing.
Dong, D.Y. and Liu, L. 1992. Middle Devonian stromatoporoids from the Chitzechiao Formation of Shaodong, Hunan and their ecological environment [in Chinese, with English abstract]. Acta Micropalaeontologica Sinica 9: 165–176.
Dong, D.Y. and Song, Y.F., 1992. Stromatoporoids from Devonian Chitzechiao (Qiziqiao) Formation of Jukoupu in Xinshao, Hunan and their reef-building characteristics [in Chinese, with English abstract]. Acta Micropalaeontologica Sinica 9: 25–36.
Dong, D.Y. and Wang, C.Y. 1982. Devonian stromatoporoids of eastern Yunnan [in Chinese, with English abstract]. Bulletin of Nanjing Institute of Geology and Palaeontology, Academica Sinica 4: 1–40.
Dong, D.Y. and Wang, B.Y. 1984. Paleozoic stromatoporoids from Xinjiang and their stratigraphical significances [in Chinese, with English abstract]. Bulletin of Nanjing Institute of Geology and Palaeontology, Academica Sinica 7: 237–286.
Dong, D.Y., Wang, S.B., Zhou, H.L., Zhang, Z.X., Luo, Q.H., Fu, J.H., and Huang, T.Y. 1989. Devonian stromatoporoid biota of northern Guangxi and mountlike superimposed bioherm of Huanjiang county —with remarks on the distribution of the Devonian and sedimentary paleogeography in this area [in Chinese, with English abstract]. Memoir of Nanjing Institute of Geology and Palaeontology, Academia Sinica 26: 235–290.
Fagerstrom, J.A. 1961. The fauna of the Middle Devonian Formosa reef limestone of southwestern Ontario. Journal of Paleontology 35: 1–48.
Fagerstrom, J.A. 1982. Stromatoporoids of the Detroit River Group and adjacent rocks in the vicinity of the Michigan Basin. Geological Survey of Canada Bulletin 339: 1–81. Crossref
Flügel, E. 1974. Stromatoporen aus dem Schwelmer Kalk (Givet) des Sauerlandes (Stromatoporen aus dem deutschen Paläozoikum 1). Paläontologische Zeitschrift 48: 149–187. Crossref
Flügel, E. and Flügel-Kahler, E. 1968. Stromatoporoidea (Hydrozoa palaeozoica). Fossilium Catalogus I: Animalia 115: 1–416; 116: 417–681.
Galloway, J.J. 1957. Structure and classification of the Stromatoporoidea. Bulletins of American Paleontology 37: 345–480.
Galloway, J.J. 1960. Devonian stromatoporoids from the Lower Mackenzie Valley. Journal of Paleontology 34: 620–636.
Galloway, J.J. and St. Jean, J. Jr. 1957. Middle Devonian Stromatoporoidea of Indiana, Kentucky and Ohio. Bulletins of American Paleontology 37: 25–308.
Gogolczyk, W. 1959. Rodzaj Stachyodes (Stromatoporoidea) w dewonie Polski. Acta Palaeontologica Polonica 4: 353–388.
Goldfuss, G.A. 1826. Petrefacta Germaniae, Band I, Heft I. 76 pp. Arnz and Co. Dusseldorf.
Golonka, J. 2002. Plate-tectonic maps of the Phanerozoic. In: W. Kiessling, E. Flügel, and J. Golonka (eds.), Phanerozoic Reef Patterns. SEPM Special Publication 72: 21–75. Crossref
Grant, R.E. 1836. Animal Kingdom. In: R.B. Todd (ed.), The Cyclopaedia of Anatomy and Physiology 1, 107–118. Sherwood, Gilbert, and Piper, London.
He, Y.X. 1978. Rugosa [in Chinese]. In: Southwest Institute of Geosciences (ed.), Paleontological Atlas of Southwest China, Sichuan, 1, (Sinian–Devonian), 98–178. Geological Publishing House, Beijing.
Huang, J.Y., Liang, K., Wang, Y., Liao, W.H., Guo, W., Kershaw, S., Jeon, J, Qiao, L., Song, J.J., Ma, J.Y., Li, Y., Tu, B., Tian, Y., Wang, Y.J., Wang, Y., Ma, J.X., Luo, M., and Qie, W.K. 2020. The Jiwozhai patch reef: A palaeobiodiversity hotspot in middle Givetian (Devonian) of South China. Palaeogeography, Palaeoclimatology, Palaeoecology 556: 109895. Crossref
Huang, Y.M. 1978. Stromatoporoidea [in Chinese]. In: Guizhou Stratigraphic Paleontological Team (ed.), Paleontological Atlas of Southwest China, Guizhou, 1, (Cambrian–Devonian), 18–35. Geological Publishing House, Beijing.
Hubert, L.B. 2008. Les stromatopores givétiens et frasniens de l’Ardenne méridionale et du Boulonnais (France et Belgique): sédimentologie, paléobiodiversité et paléobiogéographie. 368 pp. Unpublished PhD thesis, Université Catholique de Lille, Université des Sciences et Technologies de Lille, Lille and Université de Liège, Liège.
Jakubowicz, M., Król, J.J., Zapalski, M.K., Wrzołek, T., Wolniewicz, P., and Berkowski, B. 2019. At the southern limits of the Devonian reef zone: Palaeoecology of the Aferdou el Mrakib reef (Givetian, eastern Anti-Atlas, Morocco). Geological Journal 54: 10–38. Crossref
Jin, S.Y. and Ju, T.Y. 1998. Distribution Characteristics, Genesis and Reservoir Property of Sinian–Triassic Reefs in South China [in Chinese]. 152 pp. Shanghai Scientific and Technical Press, Shanghai.
Joachimski, M.M., Breisig, S., Buggisch, W., Talent, J.A., Mawson, R., Gereke, M., Morrow, J.R., Day, J., and Weddige, K. 2009. Devonian climate and reef evolution: Insights from oxygen isotopes in apatite. Earth and Planetary Science Letters 284: 599–609. Crossref
Kaźmierczak, J. 1971. Morphogenesis and systematics of the Devonian Stromatoporoidea from the Holy Cross Mountains, Poland. Palaeontologia Polonica 26: 1–150.
Kershaw, S. 2015. Paleoecology of the Paleozoic Stromatoporoidea. In: P.A. Selden (ed.), Treatise on Invertebrate Paleontology, Part E, Porifera 4–5 (Revised), 631–651. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Kershaw, S. and Riding, R. 1978. Parameterization of stromatoporoid shape. Lethaia 11: 233–242. Crossref
Kershaw, S., Munnecke, A., and Jarochowska, E. 2018. Understanding Palaeozoic stromatoporoid growth. Earth-Science Reviews 187: 53–76. Crossref
Khalfina, V.K. [Halfina, V.K.] 1960. Order Stromatoporoidea: Devonian System [in Russian]. In: L.L. Halfin (ed.), Biostratigrafiâ paleozoâ Saâio-Altajskoj gornoj oblasti, 1, Srednij paleeozoj 19: 370–373.
Khalfina, V.K. [Halfina, V.K.] 1961. Order Stromatoporoidea: Devonian System [in Russian]. In: L.L. Halfin (ed.), Biostratigrafiâ paleozoâ Saâio-Altajskoj gornoj oblasti, 2, Srednij paleeozoj 20: 323–349.
Khalfina, V.K. [Halfina, V.K.] and Yavorsky V.I. [Âvorskij, V.I.] 1971. New groups of stromatoporoids [in Russian]. Geologiâ i geofizika 8: 118–121.
Khromykh, V.G. [Hromyh V.G.] 1967. Systematic position of the dendroid stromatoporoids [in Russian]. In: Siberian Branch, Transactions of the Institute of Geology and Geophysics (ed.), Transactions of the Conference of Young Students and Postgraduates, 67. Nauka, Moskva.
Khromykh, V. G. [Hromyh V.G.] 1974. Devonian stromatoporoids of the north-eastern USSR. [in Russian]. Geologiâ i geofizika 68: 1–104.
Khromykh, V.G. [Hromyh V.G.] and Hung. N.H. 1988. Stromatoporoidea [in Russian]. In: V.N. Dubatalov (ed.), Stratigafiâ i celenteraty devona V’etnama, Vol. 2, 6–37, 157–162, 173–189. Nauka, Novosybirsk.
Kiessling, W., Flügel, E., and Golonka, J. 1999. Paleoreef maps: evaluation of a comprehensive database on Phanerozoic reefs. American Association of Petroleum Geologists Bulletin 83: 1552–1587. Crossref
Klovan, J.E. 1966. Upper Devonian stromatoporoids from the Redwater Reef Complex, Alberta. Bulletin of Geological Survey of Canada 133: 1–33.
Kosareva, E.G. 1976. On the revision of the genera Clathrocoilona and Synthetostroma (Stromatoporoidea) [in Russian]. Paleontologičeskij žurnal 1976 (1): 19–26.
Krebedünkel, J. 1995. Stromatoporen aus dem Givet und Frasne des Bergischen Landes. Sonderveröffentlichungen, Geologisches Institut der Universität zu Köln 106: 1–182.
Lecompte, M. 1951. Les stromatoporoides du Dévonien moyen et supérieur du bassin de Dinant. Part 1. Mémoires de l’Institut Royal des Sciences Naturelles de Belgique 116: 1–215.
Lecompte, M. 1952. Les stromatoporoides du Dévonien moyen et supérieur du bassin de Dinant. Part 2. Mémoires de l’Institut Royal des Sciences Naturelles de Belgique 117: 216–359.
Liao, W.H. 2003. Devonian biostratigraphy of Dushan, southern Guizhou and its coral extinction events [in Chinese, with English abstract]. Acta Palaeontologica Sinica 42: 417–427.
Liao, W.H. and Ma, X.P. 2011. On the Givetian transgression in South China and the Endophyllum-bearing beds—with a comparison of Endophyllum from S-China and “Endophyllum” from N-Xinjiang [in Chinese, with English abstract]. Acta Palaeontologica Sinica 50: 64–76.
Liao, W.H., Xu, H.K., Wang, C.Y., Cai, C.Y., Ruan, Y.P., Mu, D.C., and Lu, L.C. 1979. Discussion on several basic Devonian sections of Southwest China [in Chinese]. In: Nanjing Institute of Geology and Palaeontology (ed.), Carbonate Biostratigraphy of Southwest China, 221–249. Science Press, Beijing.
Liu, J.R. and Dong, D.Y. 1991. Middle Devonian stromatoporoids from mountlike superimposed bioherms along carbonate platform margin from Liuzhai, Nandan, Guangxi [in Chinese, with English abstract]. Acta Micropalaeontologica Sinica 8: 309–324.
M’Coy, F. 1850. Description of three new Devonian zoophytes. Annals and Magazine of Natural History (Series 2) 6: 377–378.
Mabille, C. and Boulvain, F. 2007. Sedimentology and magnetic susceptibility of the Couvin Formation (Eifelian, South Western Belgium): Carbonate platform initiation in a hostile world. Geologica Belgica 10: 47–67.
MacNeil, A.J. and Jones, B. 2016. Stromatoporoid growth forms and Devonian reef fabrics in the Upper Devonian Alexandra Reef System, Canada—insight on the challenges of applying Devonian reef facies models. Sedimentology 63: 1425–1457. Crossref
Mallett, C.W. 1968. Devonian Stromatoporoids from Pandanus Creek Station, North Queensland. 284 pp. Unpublished M.Sc. Thesis, University of Queensland, Brisbane.
May, A. 1993. Stratigraphie, Stromatoporen-Fauna und Palökologie von Korallenkalken aus dem Ober-Eifelium und Unter-Givetium (Devon) des nordwestlichen Sauerlandes (Rheinisches Schiefergebirge). Geologie und Palaeontologie in Westfalen 24: 3–93.
May, A. 1999. Die Stromatoporen-Fauna des Mitteldevons von Zentral-Böhmen. Münstersche Forschungen zur Geologie und Paläontologie 88: 121–134.
May, A. 2005. Die Stromatoporen des Devons und Silurs von Zentral-Böhmen (Tschechische Republik) und ihre Kommensalen. Zitteliana B 25: 117–250.
Mistiaen, B. 1980. Stromatopores du Givétien de Ferques (Boulonnais, France). Bulletin du Musé National d’Histoire Naturelle, 4e série 2C: 167–257.
Mistiaen, B. 1985. Phénomenes recifaux dans le Dévonien d Afghanistan (Montagnes Centrales). Analyse et systématique des Stromatopores. Publication, Société Géologique du Nord 11: 1–245.
Mistiaen, B. 1988. Stromatopores du Givetien et du Frasnien de Ferques (Boulonnais-France). In: D. Brice (ed.), Le Dévonien de Ferques, Bas-Boulonnais (N. France). Biostratigraphie du Paléozoique 7: 163–195.
Mistiaen, B. 1999. On some Devonian (Frasnian) stromatoporoids from Kerman Province, Eastern Iran. Annales de la Société Géologique du Nord (2ème série) 7: 33–44.
Mistiaen, B. and Gholamalian, H. 2000. Stromatoporoids and some Tabulate Corals from Chariseh Area (Esfahan Province, Central Iran). Annales de la Société Géologique du Nord (2ème série) 8: 81–91.
Nestor, H. 2011. Clathrodictyida. Part E, Revised, Volume 4, Chapter 16C. Treatise Online 26: 1–15.
Nicholson, H.A. 1886. A monograph of the British stromatoporoids. Part 1, General Introduction. Palaeontographical Society, London 39: 1–130. Crossref
Nicholson, H.A. and Murie, J. 1878. On the minute structure of Stromatopora and its allies. Zoological Journal of the Linnean Society 14: 187–246. Crossref
Parks, W.A. 1936. Devonian stromatoporoids of North America, Part 1. University of Toronto Studies, Geological Series 39: 1–125.
Poty, E. and Chevalier, E. 2007. Late Frasnian phillipsastreid biostromes in Belgium. Geological Society, London, Special Publications 275: 143–161. Crossref
Salerno, C. 2008. Stromatoporen-Fauna, Fazies und Paläoökologie von Plattformkarbonaten aus dem Unter-Givetium der Eifel (Devon, Rheinisches Schiefergebirge). Zitteliana B 27: 1–129.
Stadelmaier, M., Nose, M., May, A., Salerno, C., Schröder, S., and Leinfelder R.R. 2005. Ästige tabulate Korallen-Gemeinschaften aus dem Mitteldevon der Sötenicher Mulde (Eifel): Faunenzusammensetzung und fazielles Umfeld. Zitteliana B 25: 5–38.
Stearn, C.W. 1962. Stromatoporoid fauna of the Waterways Formation (Devonian) of northeastern Alberta. Geological Survey of Canada Bulletin 92: 1–23.
Stearn, C.W. 1963. Some stromatoporoids from the Beaverhill Lake Formation (Devonian) of the Swan Hills area, Alberta. Journal of Paleontology 37: 651–668.
Stearn, C.W. 1975. Stromatoporoid assemblages, Ancient Wall reef complex (Devonian), Alberta. Canadian Journal of Earth Sciences 12: 1631–1667. Crossref
Stearn, C.W. 1980. Classification of the Paleozoic stromatoporoids. Journal of Paleontology 54: 881–902.
Stearn, C.W. 1983. Stromatoporoids from the Blue Fiord Formation (Lower Devonian) of Ellesmere Island, Arctic Canada. Journal of Paleontology 57: 539–559.
Stearn, C.W. 1993. Revision of the order Stromatoporida. Palaeontology 36: 201–229.
Stearn, C.W. 1995. The type species of Stictostroma Parks, 1936 (Porifera, Stromatoporoidea). Journal of Paleontology 69: 20–27. Crossref
Stearn, C.W. 2015a. Diversity trends of the paleozoic stromatoporoidea. In: P.A. Selden (ed.), Treatise on Invertebrate Paleontology, Part E, Porifera. 4–5 (Revised), 593–597. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Stearn, C.W. 2015b, Stromatoporellida, Stromatoporida, Syringostromatida, Amphiporida, and genera with uncertain affinities: systematic descriptions. In: P.A. Selden (ed.), Treatise on Invertebrate Paleontology, Part E, Porifera. 4–5 (Revised), 781–829. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Stearn, C.W. and Mehrotra, P.N. 1970. Lower and Middle Devonian stromatoporoids from Northwestern Canada. Geological Survey of Canada Paper 70-13: 1–43. Crossref
Stock, C.W. and Burry-Stock, J.A. 2019. The stromatoporoid Habrostroma in the upper Silurian (uppermost Pridoli)–Lower Devonian (Lochkovian) of North America, and the paleobiogeographic significance of H. centrotum (Girty, 1895). Journal of Paleontology 94: 11–27. Crossref
Tchi, Y.Y. 1966. Late Middle Devonian tabulate corals from shuitouzhai, Panxi, Eastern Yunnan [in Chinese]. Acta Palaeontologica Sinica 14: 110–123.
Tchi, Y.Y., 1975. Middle Devonian tabulate corals in Guangxi Zhuang Autonomous Region [in Chinese]. Papers on Stratigraphy and Paleontology 2: 98–121.
Waagen, W. and Wentzel, J. 1887. Class Hydrozoa. In: W. Waagen (ed.), Salt Range fossils, VI. Productus Limestone Fossils. Memoirs of the Geological Survey of India, Palaeontologia Indica (Series 13): 925–962.
Wang, S.B. 1978. Stromatoporoidea [in Chinese]. In: Geological Institute of South-West China (ed.), Paleontological Atlas of Southwest China, Sichuan, 1, (Sinian–Devonian), 11–36. Geological Publishing House, Beijing.
Wang, S.B. 1988. Stromatoporoidea [in Chinese]. In: Chengdu Institute of Geology and Mineral Resources and Institute of Geology, Chinese Academy of Geological Sciences (ed.), Devonian Stratigraphy, Paleontology and Sedimentary Facies of Longmenshan, Sichuan, 159–165. Geological Publishing House, Beijing.
Wang, S.B. and Huang, Y.Q. 1985. Middle Devonian stromatoporoids from Qinjia, Debao, Guangxi [in Chinese, with English abstract]. Acta Micropalaeontologica Sinica 2: 409–412.
Webby, B.D. and Kershaw, S. 2015. External morphology of the Paleozoic Stromatoporoidea: shapes and growth habits. 419–486. In: P.A. Selden (ed.), Treatise on Invertebrate Paleontology, Part E, Porifera. 4–5 (Revised), 419–486. Geological Society of America, Boulder and University of Kansas Press, Lawrence.
Webby, B.D. and Zhen, Y.Y. 2008. Devonian syringostromatid stromatoporoids from the Broken River region, North Queensland. Records of the Australian Museum 60: 215–236. Crossref
Winchell, A.N. 1867. Stromatoporidae: Their structure and zoological affinities. Proceedings of the American Association for the Advancement of Science 15: 91–99.
Wolniewicz, P. 2021. From lagoons to mud mounds: palaeoecology of the Givetian to Frasnian stromatoporoids from the Holy Cross Mountains, Poland. Lethaia 54: 378–398. Crossref
Wray, J.L. 1967. Upper Devonian calcareous algae from the Canning Basin, Western Australia. Professional Contributions of the Colorado School of Mines 3: 1–76.
Xian, H.B., Zhang, S.H., Li, H.Y., Xiao, Q.S., Chang, L.X., Yang, T.S., and Wu, H.C. 2019. How did South China connect to and separate from Gondwana: New paleomagnetic constraints from the Middle Devonian red beds in South China. Geophysical Research Letter 46: 7371–7378. Crossref
Yabe, H. and Hayasaka, T. 1920. Palaeontology of Southern China. Geographical Research in China, Reports 3: 1–221.
Yabe, H. and Sugiyama, T. 1941. Tienodictyon zonatum, a new stromatoporoid from Eastern Yunnan, China. Proceedings of the Imperial Academy 17: 139–141. Crossref
Yang, J.Z. 1954. Middle Devonian bryozoans in Yongchun, Guangxi [in Chinese]. Acta Palaeontologica Sinica 2: 207–226.
Yang, J.Z. and Dong, D.Y. 1963. Stromatoporoids from the Jiwozhai Member, upper part of the Middle Devonian of Dushan District, Guizhou (Kueichow) [in Chinese, with English abstract]. Acta Palaeontologica Sinica 11: 147–177.
Yang, J.Z. and Dong, D.Y. 1979. Devonian stromatoporoids from central and eastern parts of Guangxi, China [in Chinese, with English abstract]. Palaeontologia Sinica (New Series B) 157: 1–89.
Yavorsky, V.I. [Âvorskij, V.I.] 1931. Some Devonian Stromatoporoidea from the margins of the Kuznetsk Basin, the Urals, and other localities [in Russian]. Izvestiâ Vsesoûznogo Geologo-Razvedočnogo Ob’edineniâ 94: 1387–1415.
Yavorsky, V.I. [Âvorskij, V.I.] 1955. Stromatoporoidea of the Soviet Union, Part 1 and Supplement to Part 1 [in Russian]. Trudy Vsesoûznogo Naučno-Issledovatel’skogo Geologičeskogo Instituta (VSEGEI), Novaâ Seriâ 8: 1–173.
Zukalová, V. 1971. Stromaporoidea from the Middle and Upper Devonian of the Moravian Karst. Rozpravy Ústředniho ústavu Geologickeho 37: 1–143.
Acta Palaeontol. Pol. 67 (3): 711–736, 2022
https://doi.org/10.4202/app.00954.2021