

A new genus of chemosymbiotic vesicomyid bivalves from the Oligocene of western North America
FRIDA HYBERTSEN, JAMES L. GOEDERT, and STEFFEN KIEL
Hybertsen, F., Goedert, J.L., and Kiel, S. 2022. A new genus of chemosymbiotic vesicomyid bivalves from the Oligocene of western North America. Acta Palaeontologica Polonica 67 (3): 703–709.
We describe a new genus of the chemosymbiotic bivalve family Vesicomyidae, Squiresica, for two Oligocene species, previously assigned to Archivesica, from western North America. Squiresica is characterized by a small and weakly inflated shell, a small to nearly absent pallial sinus, an Archivesica-like hinge dentition, with an indistinct to well incised lunular incision. Two species are assigned to this new genus: the type species, S. knapptonensis from western Washington State, USA, and S. marincovichi from Oligocene strata of Alaska, USA. Squiresica knapptonensis had previously been described from the upper Oligocene of the Lincoln Creek Formation; further specimens are here reported from a newly discovered seep deposit in the lower Oligocene part of the Lincoln Creek Formation.
Key words: Bivalvia, Vesicomyidae, cold-seep, deep-water, Oligocene, North America.
Frida Hybertsen [frida.hybertsen@nrm.se] and Steffen Kiel [steffen.kiel@nrm.se], Department of Palaeobiology, Swedish Museum of Natural History, Box 50007, 10405 Stockholm, Sweden.
James L. Goedert [jamesgoedert@outlook.com], Burke Museum of Natural History and Culture, University of Washington, Seattle, Washington 98195, USA.
Received 8 March 2022, accepted 17 May 2022, available online 31 August 2022.
Copyright © 2022 F. Hybertsen et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (for details please see http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
The Vesicomyidae is a species-rich family of chemosymbiotic marine bivalves found worldwide in deeper water, especially in reducing habitats such as hydrothermal vents, methane-seeps, and decaying whale carcasses (Boss and Turner 1980; Sasaki et al. 2005; Cosel 2006; Krylova and Sahling 2010). The larger vesicomyids of the subfamily Pliocardiinae all live in a symbiotic association with sulfur-oxidizing bacteria housed in their hypertrophied gills, from which they derive most, if not all, of their nutrition (Felbeck et al. 1981; Arp et al. 1984; Childress et al. 1993; Fisher 1995). Most species live half buried in soft substrates and simultaneously mine hydrogen sulfide from sediment pore water and extract oxygen from the water column (Dubilier et al. 2008; Krylova and Sahling 2010). The only exceptions are the hydrothermal vent species Turneroconcha magnifica (Boss and Turner, 1980), which lives on pillow lavas and extents its foot into the crevices between pillows to access the diffusing hydrogen sulfide (Krylova and Sahling 2020) and Laubiericoncha puertodeseadoi Signorelli and Pastorino, 2015, of which empty shells have been found at active hydrothermal sites in the South Atlantic Ocean (Linse et al. 2020).
The family has an extensive fossil record, extending back to the middle Eocene, with the oldest record coming from Washington State (Goedert and Squires 1990; Amano and Kiel 2007). Many fossil species have been described, though the generic assignments of some of them remain contentious because their shell characters are insufficiently known. One particularly troublesome genus is Archivesica Dall, 1908, based on the large, extant Archivesica gigas Dall, 1896. Numerous fossil species of Eocene to Pleistocene age have been assigned to it (Amano and Kiel 2007, 2010; Amano and Suzuki 2010; Kiel and Taviani 2017; Hansen et al. 2017; Kiel et al. 2020), at some point even the as-yet oldest vesicomyid, though that species was subsequently considered as more likely belonging to Pliocardia (Amano and Kiel 2007, 2012). In molecular phylogenetic studies, species clustering with Archivesica gigas are often referred to as the “gigas”-group and include species assigned in other studies to the genera Archivesica, Phreagena Woodring, 1938, Laubiericoncha Cosel and Olu, 2008, Ectenagena Woodring, 1938, and Akebiconcha Kuroda, 1943 (Audzijonyte et al. 2012; Decker et al. 2012; Johnson et al. 2017).
Ongoing phylogenetic work on the Vesicomyidae using morphologic characters, by us, indicates that two small-sized, fossil species previously assigned to Archivesica, A. knapptonensis Amano and Kiel, 2007 and A. marincovichi Kiel and Amano, 2010, are closely related to each other, but are quite distant to the type species Archivesica gigas. A re-examination of the shell characters of these two species indeed revealed several features inconsistent with those of A. gigas, including their small size, slender shape, and often the presence of a lunular incision. A new genus is here introduced for these two species, and additional material of A. knapptonensis from a recently discovered late early Oligocene seep deposit in western Washington State, USA, is reported.
Institutional abbreviations.—NRM, Swedish Museum of Natural History, Stockholm, Sweden; UCMP, University of California, Museum of Paleontology, Berkeley, USA; USNM, Smithsonian Museum of Natural History, Washington, DC, USA.
Other abbreviations.—H, height; L, length; W, width.
Nomenclatural acts.—This published work and the nomenclatural acts it contains, have been registered in ZooBank: urn:lsid:zoobank.org:pub:C706ED1A-0393-4414-9BDA-CC0AF38FCF56
Material and methods
The new seep deposit was discovered by JLG and SK in August 2019 in bedrock of the Lincoln Creek Formation on the east side of the West Fork Satsop River, Grays Harbor County, Washington (WGS84 datum GPS coordinates: 47.268247° N, 123.562015° W, Fig. 1). Based on Rau’s stratigraphy of the West Fork Satsop section (Rau 1966) and the magnetostratigraphy of the Canyon River section of Prothero and Armentrout (1985), the deposit was found in the upper lower Oligocene part of the Lincoln Creek Formation (at C10r, around 31 million years ago).
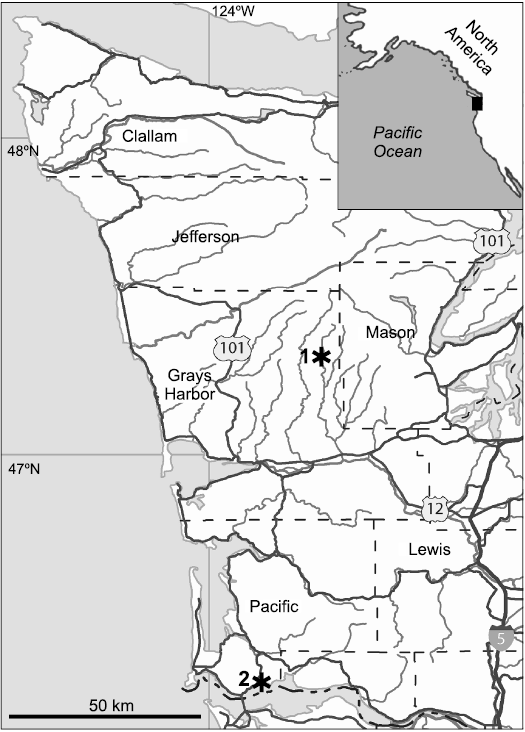
Fig. 1. Map showing location of the studied localities in North America (inset) and western Washington State, USA. 1, the new West Fork Satsop seep deposit; 2, Knappton, the type locality of Squiresica knapptonensis.
The nodular, fossiliferous limestone block measures at least 1.5 × 1.0 m. In addition to the vesicomyid species reported here, we collected two large specimens of Acharax sp. (height 30 mm), common mussels resembling Bathymodiolus (sensu lato) satsopensis Kiel and Amano, 2013 (up to 40 mm length), Conchocele sp. (30 mm long), a small Lucinoma acutilineata Conrad, 1849, a fragment probably belonging to a species of large lucinid (cf. Elliptiolucina), the naticid Polinices washingtonensis Weaver, 1916, and poorly preserved fragments possibly belonging to Provanna antiqua Squires, 1995. Specimens were mechanically extracted from the rock matrix using hammers and chisels, and pneumatic fossil preparation tools. Specimens were coated with ammonium chloride prior to photography.
Systematic palaeontology
Class Bivalvia Linnaeus, 1758
Family Vesicomyidae Dall and Simpson, 1901
Genus Squiresica nov.
Zoobank LSID: urn:lsid:zoobank.org:act:551BF127-9A67-49DA-80E1- B2FCB1A25354
Type species: Archivesica knapptonensis Amano and Kiel, 2007, Oligocene part of the Lincoln Creek Formation, western Washington State, USA.
Species included: The type species and Archivesica marincovichi Kiel and Amano, 2010, from the Oligocene Kulthieth Formation in Alaska, USA.
Etymology: In honor of Richard L. Squires (Northridge, USA), who pioneered work on fossil vesicomyids from the US West Coast, and the ending of the related vesicomyid genus Archivesica.
Diagnosis.—Shell small (L up to 50 mm), elongate, weak to moderately inflated; escutcheon narrow, lunular incision indistinct to well incised, narrow to moderately broad; umbo elevated. Pallial line impressed anteriorly; pallial sinus small and shallow, or even just a slight forward sloping of the pallial line before meeting the posterior adductor muscle scar; hinge plate moderately broad, with thick cardinal 1 radiating anteriorly, cardinal 3a thin, short and parallel to shell margin or reduced, cardinal 3b small, pointing posteroventrally, with parallel or somewhat diverging raised edges; subumbonal pit elongate or deep.
Remarks.—The new genus Squiresica has an Archivesica-like hinge dentition, but differs from Archivesica by (i) being much smaller, (ii) having an elevated umbo with strongly coiled beaks, (iii) its pallial sinus, which is only a small indentation, whereas Archivesica in general, and the type species A. gigas in particular, has a large and broad pallial sinus, (iv) possessing an escutcheon and typically a lunular incision, both of which are absent from Archivesica gigas. Archivesica redwoodia Kiel and Amano, 2010, a small species from Alaska, shares the small pallial sinus with the species of Squiresica, but differs by having a long anterior cardinal 3a parallel to the dorsal shell margin, a more ventrally pointing cardinal 3b, and a much broader and deeper pallial sinus (see Table 1 for comparison to other pliocardiin genera).
Table 1. Comparison of diagnosable features of the pliocardiin genera discussed herein. Shell size describes the length (L) of the shell: small (S) L <50 mm, medium (M) L 50–120 mm, large (L) L >120 mm. Cardinal 3a has three different character states, it can be separated from cardinal 3b, fused with 3b or reduced which refers to an absent state.
|
Genus |
Shell size |
Umbo |
Inflation |
Posterior ridge |
Pallial sinus |
Escutcheon |
Lunular incision |
Cardinal 3a |
Cardinal 3b |
|
Archivesica Dall, 1908 |
M–L |
low |
strong |
absent |
present |
absent |
absent |
separate |
semi-triangular shape |
|
Austrogena Krylova, Sellanes, Valdés and D’Elía, 2014 |
S |
elevated |
moderate |
present |
absent |
present |
present |
reduced |
broad |
|
Hubertschenckia Takeda, 1953 |
M |
elevated |
moderate |
absent |
small indentation |
present |
absent |
fused |
oblique state |
|
Isorropodon |
S |
elevated |
strong |
absent |
absent |
present |
present |
fused |
semi-triangular shape |
|
Pleurophopsis
|
M |
low |
moderate |
absent |
absent |
absent |
absent |
reduced |
semi-triangular shape |
|
Pliocardia Woodring, 1925 |
S |
elevated |
strong |
present |
small indentation |
present |
present |
fused |
semi-triangular shape |
|
Squiresica gen. nov. |
S |
elevated |
strong |
absent |
small indentation |
present |
present |
separate |
oblique state |
Austrogena nerudai Krylova, Sellanes, Valdés, and D’Elía, 2014, type species of Austrogena from the South-eastern Pacific, shares the presence of an escutcheon, a lunular incision, a subumbonal pit and an elongate cardinal 4b with species of Squiresica; however, it can be distinguished from these by the complete lack of a pallial sinus (Krylova et al. 2014). The oldest vesicomyid, “Archivesica cf. tschudi” from the middle Eocene of western Washington (Amano and Kiel 2007) is very small and Archivesica-like, but it has a low umbo and lacks a pallial sinus. Hubertschenckia Takeda, 1953, reaches 90 mm in length and is thus much larger than Squiresica. Further differences include the cardinal 3b, which points downwards or slightly anteriorly in Hubertschenckia, but posteriorly in Squiresica, and Hubertschenckia has a posterior nymphal ridge, absent in Squiresica (Takeda 1953; Amano and Kiel 2007). Other pliocardiins sharing the small sized shell with Squiresica are Pliocardia Woodring, 1925, and Isorropodon Sturany, 1896. Both genera have an elevated umbo and a lunular incision, but Isorropodon lacks a pallial sinus and Pliocardia has a posterior ridge (Sturany 1896; Woodring 1925; Cosel and Salas 2001; Krylova and Janssen 2006; Amano and Kiel 2007). Isorropodon is less elongate than Squiresica, and all cardinal teeth of Isorropodon are thin and more or less parallel to the dorsal shell margin (e.g., Cosel and Salas 2001), in contrast to the radiating teeth of Squiresica. Pliocardia is more rounded than Squiresica, it features a posterior ridge on the outside of the shell, lacking in Squiresica, and its cardinal 3b is shorter and more compressed than that of Squiresica. The Pleistocene Arctic species Archivesica arctica Hansen, Hoff, Sztybor, and Rasmussen, 2017, has stronger and more radiating teeth than the species of Squiresica, a very distinct, pointed pallial sinus, and reaches much larger size (55–67 mm compared to 40 mm in the species of Squiresica).
A further species that could potentially belong to Squiresica is Pleurophopsis lithophagoides Olsson, 1931, from the lower Oligocene of northern Peru (Olsson 1931). That species is notably smaller than most other species of Pleurophopsis Van Winkle, 1919 (see Kiel et al. 2020: table 1) but is in the size range of the species of Squiresica. It also has a similar elongate shape and is rather flat like Squiresica knapptonensis. The cardinal 3a of Pleurophopsis lithophagoides remains unknown, as well as the presence or absence of a pallial sinus (Olsson 1931; Kiel et al. 2020), and hence a potential assignment to Squiresica remains unresolved.
Stratigraphic and geographic range.—Oligocene, Pacific Coast of North America, from Alaska to Washington State.
Squiresica knapptonensis (Amano and Kiel, 2007) comb. nov.
Figs. 2, 3.
1993 Calyptogena (Calyptogena) chinookensis Squires and Goedert, 1991; Goedert and Squires 1993: 74, fig. 4.
2007 Archivesica knapptonensis sp. nov.; Amano and Kiel 2007: 284, figs. 30–43.
Type material: Fragment of right valve with preserved hinge dentition, length 13.5 mm+, height 7.0 mm, USNM 534954.
Type locality: LACMIP loc. 5843, beach terrace of the Columbia River near Knappton, Washington State, USA.
Type horizon: Upper Oligocene part of the Lincoln Creek Formation.
Material.—Numerous specimens (figured: NRM PAL Mo 195149–195155) from the West Fork Satsop seep deposit, lower Oligocene, Lincoln Creek Formation, western Washington, USA.
Description.—Elongated, weakly inflated, small shell, reaching little over 20 mm in length, H/L-ratio 0.46–0.60, W/H-ratio 0.61–0.65; umbo slightly elevated and moderately coiled, positioned at anterior 20–25% of shell length; external ligament opisthodetic, occupying >60% of postero-dorsal margin; anterior adductor scar D-shaped, its posterior margin thick; posterior adductor scar nearly round; pallial line impressed anteriorly, commarginal, forming a small indentation right beneath posterior adductor scar.
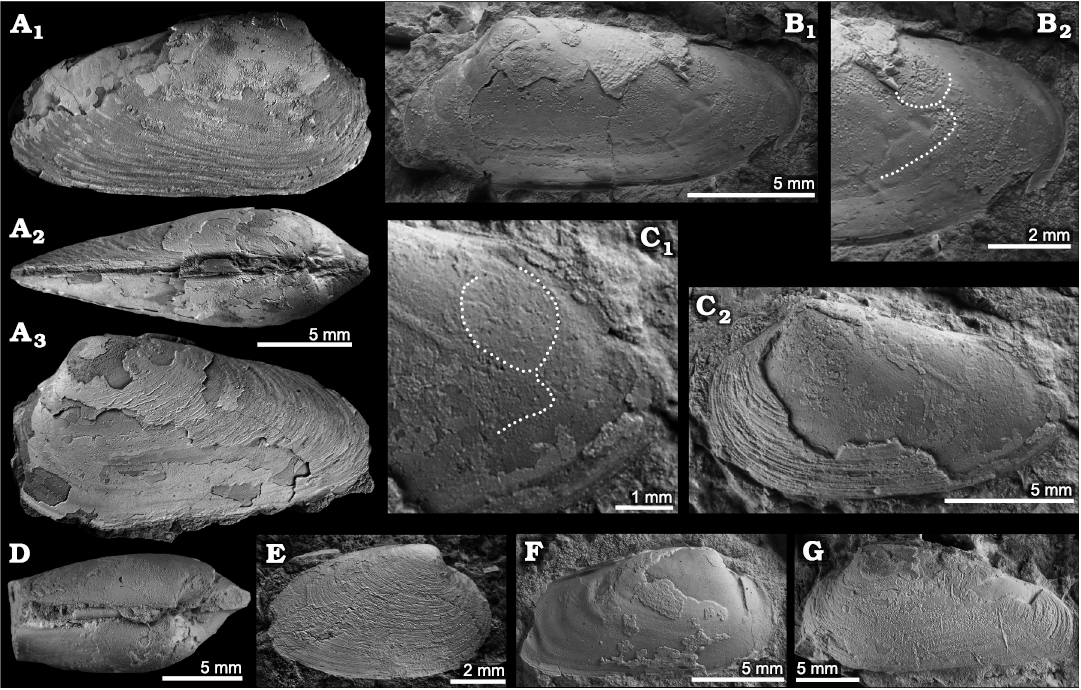
Fig. 2. The vesicomyid bivalve Squiresica knapptonensis (Amano and Kiel, 2007) from the West Fork Satsop seep deposit, lower Oligocene, Lincoln Creek Formation, western Washington, USA. A. NRM PAL Mo 195149, articulated specimen with partially preserved shell, in lateral (A1, A3) and dorsal (A2) views. B. NRM PAL Mo 195150, left valve embedded in matrix, with partially preserved shell; B2, close-up showing pallial line and anterior adductor muscle scar (dotted line). C. NRM PAL Mo 195151, left valve embedded in matrix, with partially preserved shell (C2) and with posterior adductor muscle scar barely visible (dotted line in C1). D. NRM PAL Mo 195153, articulated specimen showing inflation and ligament. E. NRM PAL Mo 195152, small specimen with fully preserved shell, view on outer shell surface. F. NRM PAL Mo 195154, right valve embedded in matrix, with partially preserved shell, showing pallial line and anterior adductor muscle scar. G. NRM PAL Mo 195155, left valve with mostly preserved shell.
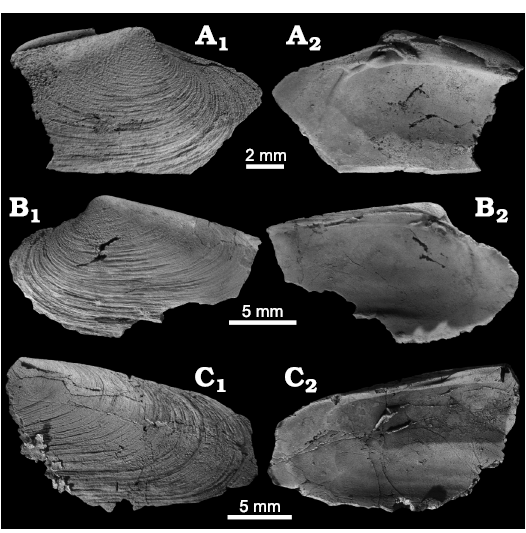
Fig. 3. The vesicomyid bivalve Squiresica knapptonensis (Amano and Kiel, 2007) from the upper Oligocene part of the Lincoln Creek Formation at Knappton, along the Columbia River, western Washington, USA. A. Holotype USNM 534954, left valve showing external surface (A1) and hinge (A2). B. Paratype USNM 534956, left valve showing external surface (B1) and hinge (B2). C. USNM 534955, posterior part of a left valve showing external surface (C1) and the posterior end of the nymphal ridge (C2). All images (except C2) from Amano and Kiel (2007).
Remarks.—Goedert and Squires (1993) reported Calyptogena chinookensis Squires and Goedert, 1991 from the Pysht and Makah formations along the northern coast of Washington state, and from the upper Oligocene part of the Lincoln Creek Formation on the southern side of Washington state. Among their illustrated specimens, that from the Lincoln Creek Formation (Goedert and Squires 1993: fig. 4) shows a lunular incision and a different hinge morphology than C. chinookensis and instead belongs to Squiresica knapptonensis (i.e., Amano and Kiel 2007). The newly collected material from the West Fork of Satsop River shows all characters possessed by specimens from the upper Oligocene type locality of S. knapptonensis and thus extends the stratigraphic range of this species down into the lower Oligocene.
The second species assigned here to the new genus, Squiresica marincovichi (Kiel and Amano 2010: 80–81, figs. 17–25) from the Oligocene Kulthieth Formation in Alaska, differs from S. knapptonensis by being less elongated, having a broader escutcheon, lacking irregular growth lines on the outer surface, and a less distinct subumbonal pit (see Fig. 4 for a re-illustration of the type material). Squiresica marincovichi reaches almost 40 mm in length and it thus notably larger than S. knapptonensis.
Stratigraphic and geographic range.—Oligocene, Lincoln Creek Formation, western Washington State, USA.
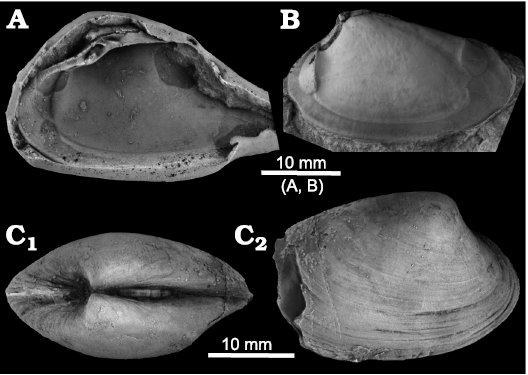
Fig. 4. The vesicomyid bivalve Squiresica marincovichi (Kiel and Amano, 2010), from the Oligocene Kulthieth Formation, Alaska, USA. A. Rubber peel of holotype UCMP 555221 showing internal features including hinge dentition of the right valve. B. Paratype UCMP 555223, internal mold of a left valve, showing adductor muscle scars and small pallial sinus. C. Paratype UCMP 555224, an articulated specimen showing inflation, lunular incision, and escutcheon (C1) and external sculpture of right valve (C2). All images from Kiel and Amano (2010).
Concluding remarks
Regarding shell characters, the species of Squiresica share more with the geochronologically oldest vesicomyid, “Pliocardia cf. tschudi” (formerly identified as Archivesica cf. tschudi) than with the type species of Archivesica, A. gigas. Presently, Squiresica is restricted to Oligocene strata of the northeast Pacific region, with a potential extension to northern Peru (Pleurophopsis lithophagoides). Notably, no species assignable to Squiresica is known from the well-investigated fossil record of Japan, where the dominant vesicomyid at Oligocene seep deposits is the much larger (up to 90 mm long) genus Hubertschenckia (Majima et al. 2005; Amano and Kiel 2007, 2010, 2011; Amano et al. 2013, 2019). This emphasizes the differences among vesicomyids between eastern and western Pacific seep communities during Cenozoic times (Amano et al. 2019), contrary to the present-day situation with several vesicomyid species known from both sides of the North Pacific Ocean (Kojima et al. 2004; Audzijonyte et al. 2012). This is in remarkable contrast to the bathymodiolin mussels, the other major group of seep-inhabiting bivalves. At least one bathymodiolin species, “Bathymodiolus” inouei Amano and Jenkins, 2011, occurred in both Japan and Washington during the Oligocene (Amano and Jenkins 2011; Kiel and Amano 2013), but no bathymodiolin species is shared between the two regions today (Duperron 2010; Lorion et al. 2013; McCowin et al. 2020). Reasons for these contrasting patterns remain to be investigated.
Acknowledgements
We thank Green Diamond Resources for continuing to allow access to localities on their land, and Mark Golliet (Green Diamond, Shelton, Washington office) for facilitating this access. We are grateful for Columbia Land Trust for allowing access to the Knappton localities. We also thank Kazutaka Amano (Joetsu University of Education, Japan) and an anonymous reviewer for their constructive comments.Financial support was provided by Vetenskapsrådet (Swedish Research Council) through grant 2016-03920 to SK.
References
Amano, K. and Jenkins, R.G. 2011. New fossil Bathymodiolus (s. l.) (Mytilidae, Bivalvia) from Oligocene seep-carbonates in eastern Hokkaido, Japan—with remarks on the evolution of Bathymodiolus (s. l.). The Nautilus 125: 29–35.
Amano, K. and Kiel, S. 2007. Fossil vesicomyid bivalves from the North Pacific region. The Veliger 49: 270–293.
Amano, K. and Kiel, S. 2010. Taxonomy and distribution of fossil Archivesica (Vesicomyidae, Bivalvia) in Japan. The Nautilus 124: 155–165.
Amano, K. and Kiel, S. 2011. Fossil Adulomya (Vesicomyidae, Bivalvia) from Japan. The Veliger 51: 76–90.
Amano, K. and Kiel, S. 2012. Two Neogene vesicomyid species (Bivalvia) from Japan and their biogeographic implications. The Nautilus 126: 79–85.
Amano, K. and Suzuki, A. 2010. Redescription of “Calyptogena” shiretokensis Uozumi (Bivalvia: Vesicomyidae) from the Miocene Rusha Formation on the Shiretoko Peninsula, Eastern Hokkaido, Japan. Venus 68: 165–171.
Amano, K., Jenkins, R.G., Sako, Y., Ohara, M., and Kiel, S. 2013. A Paleogene deep-sea methane-seep community from Honshu, Japan. Palaeogeography, Palaeoclimatology, Palaeoecology 387: 126–133. Crossref
Amano, K., Miyajima, Y., Jenkins, R.G., and Kiel, S. 2019. The Miocene to Recent biogeographic history of vesicomyid bivalves in Japan, with two new records of the family. The Nautilus 133: 48–56.
Arp, A.J., Childress, J.J., and Fisher, C.R. 1984. Metabolic and blood gas transport characteristics of the hydrothermal vent bivalve, Calyptogena magnifica. Physiological Zoology 57: 648–662. Crossref
Audzijonyte, A., Krylova, E.M., Sahling, H., and Vrijenhoek, R.C. 2012. Molecular taxonomy reveals broad trans-oceanic distributions and high species diversity of deep-sea clams (Bivalvia: Vesicomyidae: Pliocardiinae) in chemosynthetic environments. Systematics and Biodiversity 10: 403–415. Crossref
Boss, K.J. and Turner, R.D. 1980. The giant white clam from the Galapagos Rift, Calyptogena magnifica species novum. Malacologia 20: 161–194.
Childress, J.J., Favuzzi, J.A., Arp, A.J., and Oros, D.R. 1993. The role of a zinc-based, serum-borne sulphide-binding component in the uptake and transport of dissolved sulphide by the chemoautotrophic symbiont containing clam Calyptogena elongata. Journal of Experimental Biology 179: 131–158. Crossref
Conrad, T.A. 1849. Appendix I. Descriptions of fossils. In: J.D. Dana (Ed.), Geology. With a Folio Atlas of twenty-one Plates. United States Exploring Expedition during the Years 1838, 1839, 1840, 1841, 1842. Under the Command of Charles Wilkes, U.S.N., 681–734. Putnam, New York.
Cosel, R. von 2006. Vesicomyidae. Denisia 18: 142–148. Crossref
Cosel, R. von and Bouchet, P. 2008. Tropical deep-water lucinids (Mollusca: Bivalvia) from the Indo-Pacific: essentially unknown, but diverse and occasionally gigantic. In: V. Héros et al. (eds.), Tropical Deep-Sea Benthos, Volume 25. Mémoires du Muséum national d’Histoire naturelle 196: 115–213.
Cosel, R. von and Olu, K. 2008. A new genus and new species of Vesicomyidae (Mollusca, Bivalvia) from cold seeps on the Barbados accretionary prism, with comments on other species. Zoosystema 30: 929–944.
Cosel, R. von and Salas, C. 2001. Vesicomyidae (Mollusca: Bivalvia) of the genera Vesicomya, Waisiuconcha, Isorropodon and Callogonia in the eastern Atlantic and the Mediterranean. Sarsia 86: 333–366. Crossref
Dall, W.H. 1896. Diagnoses of new species of mollusks from the west coast of America. Proceedings of the U.S. National Museum of Natural History 18: 7–20. Crossref
Dall, W.H. 1908. The Mollusca and the Brachiopoda. Bulletin of the Museum of Comparative Zoology, Harvard University 43: 205–487. Crossref
Dall, W.H. and Simpson, C.T. 1901. The Mollusca of Porto Rico. United States Fishery Commission, Bulletin 20: 351–524.
Decker, C., Olu, K., Cunha, R.L., and Arnaud-Haond, S. 2012. Phylogeny and diversification patterns among vesicomyid bivalves. PLoS ONE 7: e33359. Crossref
Dubilier, N., Bergin, C., and Lott, C. 2008. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nature Reviews Microbiology 6: 725–740. Crossref
Duperron, S. 2010. The diversity of deep-sea mussels and their bacterial symbioses. In: S. Kiel (ed.), The Vent and Seep Biota, 137–167. Springer, Heidelberg. Crossref
Felbeck, H., Childress, J.J., and Somero, G.N. 1981. Calvin-Benson cycle and sulphide oxidising enzymes in animals from sulphide-rich habitats. Nature 293: 291–293. Crossref
Fisher, C.R. 1995. Toward an appreciation of hydrothermal-vent animals: their environment, physiological ecology, and tissue stable isotope values. In: S.E. Humphris, R.A. Zierenberg, L.S. Mullineaux, and R.E. Thomson (eds.), Geophysical Monograph Series, Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geochemical Interactions, 297–316. Blackwell, Washington, DC. Crossref
Goedert, J.L. and Squires, R.L. 1990. Eocene deep-sea communities in localized limestones formed by subduction-related methane seeps, southwestern Washington. Geology 18: 1182–1185. Crossref
Goedert, J.L. and Squires, R.L. 1993. First Oligocene record of Calyptogena (Bivalvia: Vesicomyidae). The Veliger 36: 72–77.
Hansen, J., Hoff, U., Sztybor, K., and Rasmussen, T.L. 2017. Taxonomy and palaeoecology of two Late Pleistocene species of vesicomyid bivalves from cold methane seeps at Svalbard (79°N). Journal of Molluscan Studies 83: 270–279. Crossref
Johnson, S.B., Krylova, E.M., Audzijonyte, A., Sahling, H., and Vrijenhoek, R.C. 2017. Phylogeny and origins of chemosynthetic vesicomyid clams. Systematics and Biodiversity 15: 346–360. Crossref
Kiel, S. and Amano, K. 2010. Oligocene and Miocene vesicomyid bivalves from the Katalla district in southern Alaska, USA. The Veliger 51: 76–84.
Kiel, S. and Amano, K. 2013. The earliest bathymodiolin mussels: Evaluation of Eocene and Oligocene taxa from deep-sea methane seep deposits in western Washington State, USA. Journal of Paleontology 87: 589–602. Crossref
Kiel, S. and Taviani, M. 2017. Chemosymbiotic bivalves from Miocene methane-seep carbonates in Italy. Journal of Paleontology 91: 444–466. Crossref
Kiel, S., Hybertsen, F., Hyžný, M., and Klompmaker, A.A. 2020. Mollusks and a crustacean from early Oligocene methane-seep deposits in the Talara Basin, northern Peru. Acta Palaeontologica Polonica 65: 109–138. Crossref
Kojima, S., Fujikura, K., and Okutani, T. 2004. Multiple trans-Pacific migrations of deep-sea vent/seep-endemic bivalves in the family Vesicomyidae. Molecular Phylogenetics and Evolution 32: 396–406. Crossref
Krylova, E.M. and Janssen, R. 2006. Vesicomyidae from Edison Seamount (South West Pacific: Papua New Guinea: New Ireland fore-arc basin). Archiv für Molluskenkunde 135: 231–261. Crossref
Krylova, E.M. and Sahling, H. 2010. Vesicomyidae (Bivalvia): current taxonomy and distribution. PLoS ONE 5: e9957. Crossref
Krylova, E.M., Sellanes, J., Valdés, F., and D’Elía, G. 2014. Austrogena: a new genus of chemosymbiotic bivalves (Bivalvia; Vesicomyidae; Pliocardiinae) from the oxygen minimum zone off central Chile described through morphological and molecular analyses. Systematics and Biodiversity 12: 225–246. Crossref
Kuroda, T. 1943. Akebiconcha, a new pelecypod genus. Venus 13: 14–18.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. 824 pp. Laurentius Salvius, Stockholm. Crossref
Linse, K., Sigwart, J.D., Chen, C., and Krylova, E.M. 2020. Ecophysiology and ecological limits of symbiotrophic vesicomyid bivalves (Pliocardiinae) in the Southern Ocean. Polar Biology 43: 1423–1437. Crossref
Lorion, J., Kiel, S., Faure, B.M., Kawato, M., Ho, S.Y.W., Marshall, B.A., Tsuchida, S., Miyazaki, J.-I., and Fujiwara, Y. 2013. Adaptive radiation of chemosymbiotic deep-sea mussels. Proceedings of the Royal Society B 280: 20131243. Crossref
Majima, R., Nobuhara, T., and Kitazaki, T. 2005. Review of fossil chemosynthetic assemblages in Japan. Palaeogeography, Palaeoclimatology, Palaeoecology 227: 86–123. Crossref
McCowin, M.F., Feehery, C., and Rouse, G.W. 2020. Spanning the depths or depth-restricted: Three new species of Bathymodiolus (Bivalvia, Mytilidae) and a new record for the hydrothermal vent Bathymodiolus thermophilus at methane seeps along the Costa Rica margin. Deep-Sea Research I 164: 103322. Crossref
Olsson, A.A. 1931. Contributions to the Tertiary paleontology of northern Peru: Part 4, The Peruvian Oligocene. Bulletins of American Paleontology 17: 97–264.
Prothero, D.R. and Armentrout, J.M. 1985. Magnetostratigraphic correlation of the Lincoln Creek Formation, Washington: implications for the age of the Eocene/Oligocene boundary. Geology 13: 208–211. Crossref
Rau, W.W. 1966. Stratigraphy and Foraminifera of the Satsop River area, southern Olympic Peninsula, Washington. State of Washington Division of Mines and Geology Bulletin 53: 1–66.
Sasaki, T., Okutani, T., and Fujikura, K. 2005. Molluscs from hydrothermal vents and cold seeps in Japan: A review of taxa recorded in twenty recent years. Venus 64: 87–133.
Signorelli, J.H. and Pastorino, G. 2015. A new species of Laubiericoncha (Bivalvia: Vesicomyidae) from deep waters off Argentina. Malacologia 58: 349–360. Crossref
Squires, R.L. 1995. First fossil species of the chemosynthetic-community gastropod Provanna: Localized cold-seep limestones in Upper Eocene and Oligocene rocks, Washington. The Veliger 38: 30–36.
Squires, R.L. and Goedert, J.L. 1991. New Late Eocene mollusks from localized limestone deposits formed by subduction-related methane seeps, Southwestern Washington. Journal of Paleontology 65: 412–416. Crossref
Sturany, R. 1896. Mollusken I (Prosobranchier und Opisthobranchier; Scaphopoden; Lamellibranchier) gesammelt von S.M. Schiff “Pola” 1890–94. Denkschriften der Mathematisch-Naturwissenschaftlichen Klasse der Kaiserlichen Akademie der Wissenschaften 63: 1–36.
Takeda, H. 1953. The Poronai Formation (Oligocene Tertiary) of Hokkaido and South Sakhalin and its fossil fauna. Studies on Coal Geology 3: 1–103.
Van Winkle, K. 1919. Remarks on some new species from Trinidad. In: K. van Winkle and G.D. Harris (eds.), New or otherwise interesting Tertiary Molluscan species from the east coast of America. Bulletins of American Paleontology 8: 19–27.
Weaver, C.E. 1916. Tertiary faunal horizons of western Washington. University of Washington Publications in Geology 1: 1–67. Crossref
Woodring, W.P. 1925. Miocene Mollusca from Bowden, Jamaica, pelecypods and scaphopods. Carnegie Institution of Washington Publications 336: 1–564. Crossref
Woodring, W.P. 1938. Lower Pliocene mollusks and echinoids from the Los Angeles basin, California, and their inferred environment. U.S. Geological Survey Professional Paper 190: 1–67. Crossref
Acta Palaeontol. Pol. 67 (3): 703–709, 2022
https://doi.org/10.4202/app.00992.2022